- 1Research Institute of the Diabetes Academy Mergentheim (FIDAM), Diabetes Center Mergentheim (DZM), Bad Mergentheim, Germany
- 2German Center for Diabetes Research (DZD), Neuherberg, Germany
- 3Department of Clinical Psychology and Psychotherapy, University of Bamberg, Bamberg, Germany
Aims: Measurement tools to evaluate self-management behavior are useful for diabetes research and clinical practice. The Diabetes Self-Management Questionnaire (DSMQ) was introduced in 2013 and has become a widely used tool. This article presents a revised and updated version, DSMQ-R, and evaluates its properties in assessing self-management practices in type 1 diabetes (T1D) and type 2 diabetes (T2D).
Methods: The DSMQ-R is a multidimensional questionnaire with 27 items regarding essential self-management practices for T1D and T2D (including diabetes-adjusted eating, glucose testing/monitoring, medication taking, physical activity and cooperation with the diabetes team). For the revised form, the original items were partially amended and the wording was updated; eleven items were newly added. The tool was applied as part of health-related surveys in five clinical studies (two cross-sectional, three prospective) including a total of 1,447 people with T1D and T2D. Using this data base, clinimetric properties were rigorously tested.
Results: The analyses showed high internal and retest reliability coefficients for the total scale and moderate to high coefficients for the subscales. Reliability coefficients for scales including the new items were consistently higher. Correlations with convergent criteria and related variables supported validity. Responsiveness was supported by significant short to medium term changes in prospective studies. Significant associations with glycemic outcomes were observed for DSMQ-R-assessed medication taking, glucose monitoring and eating behaviors.
Conclusions: The results support good clinimetric properties of the DSMQ-R. The tool can be useful for research and clinical practice and may facilitate the identification of improvable self-management practices in individuals.
Introduction
Diabetes mellitus is a chronic metabolic disease characterized by elevated blood glucose levels due to absolute [type 1 diabetes (T1D)] or relative [type 2 diabetes (T2D)] insulin deficiency (1). The International Diabetes Federation estimates that 537 million adult people (20–79 years) are currently living with diabetes worldwide; the number is expected to rise to 643 million by 2030 (2). Diabetes care aims to help people with diabetes achieve near-normal glycemic levels in order to reduce the risk of long-term (e.g., vascular) complications of diabetes while avoiding acute metabolic risks and preserving best possible quality of life (3).
The key factor to achieving good glycemic levels is the person with diabetes’s self-management of their condition. People with diabetes may need to control carbohydrate intake via their selection of foods, adapt eating behaviors with regard to glycemic load, fats and healthy nutrition, manage blood glucose using glucose-lowering medications, monitor glucose levels using blood tests or sensors, engage in sufficient physical exercise (to optimize glycemia, manage weight or maintain good health) and arrange their activities around current glycemic levels and treatment requirements, as recommended by current guidelines (4–6). Where rapid acting insulin is used (to cover glucose rises after meals), estimating carbohydrate loads of the meals, dose-adjusting insulin doses and correcting elevated glucose levels are additional required practices of daily diabetes self-management.
Persistent or recurrent hyperglycemia increases the risk for developing serious long-term complications of diabetes such as diabetic retinopathy, neuropathy, nephropathy and foot syndrome; further, suboptimal glycemic management is associated with increased risks of acute metabolic complications such as severe hypoglycemia or severe hyperglycemia with the risk of ketoacidosis or hyperosmolar coma (7–9). Therefore, the adoption and maintenance of functional self-management behaviors to achieve good glycemic outcome is decisive for maintaining good health and preventing complications and morbidity (10). However, evidence supports that people with diabetes’ self-management practices and overall performance are often improvable (11, 12); this may be particularly true for people with comorbid mental conditions such as depression and diabetes-specific distress (13–15).
Since self-management is the decisive determinant of the course of diabetes, reflecting/monitoring relevant behaviors in individuals to identify areas of potential improvement and offer suitable education and support may be useful for routine clinical practice. The assessment and evaluation of diabetes self-management behaviors may be of particular interest in people with persistent suboptimum diabetes outcomes where possible problems and barriers are to be detected. Furthermore, measuring self-management may be required as part of research where facilitators and barriers to optimal diabetes care, including mental factors, shall be analyzed [e.g. (15, 16)] or effects of interventions (e.g., diabetes self-management education) are to be evaluated. Thus, suitable measurement tools are required.
Several systematic reviews of available measurement tools for diabetes self-management confirm that many different tools have been developed; however, most instruments have been applied in limited numbers of studies and the testing of measurement properties was often limited, with few scales meeting rigorous appraisal criteria, according to the reviewers’ conclusions (17–20). These problems may limit the available tools’ usability for research and practice.
In 2013, the Diabetes Self-Management Questionnaire [DSMQ (21)] was introduced to provide a multidimensional measure of diabetes self-management behaviors relevant for the control of glycemia in both major types of diabetes and to overcome limitations of contemporary questionnaires [e.g. (22)]. In direct comparisons, the DSMQ explained significantly more glycemic variation than an established standard self-care scale (21, 23). Since then, it has been translated into diverse languages and used in many studies, supporting its potential value for research and practice. A recent systematic review listed the DSMQ as one of only three scales on diabetes self-management which met the COSMIN (COnsensus-based Standards for the selection of health Measurement Instruments) guidelines for measurement tools that can be recommended for use and results obtained with can be trusted (20).
However, technological innovations such as continuous glucose monitoring and automatic insulin delivery have changed terms and expressions in diabetes care. Furthermore, a shift in diabetes-related language has taken place (24). Also, some specific self-management aspects should be better covered by the tool. For these reasons, a revision of the DSMQ was needed. The present article presents a revised and updated version of the tool and rigorous testing of its clinimetric properties and functions. Experiences with the tool’s use within five clinical studies provides a broad evidence base to inform about its characteristics and potentials.
Materials and Methods
Diabetes Self-Management Questionnaire (DSMQ)
The DSMQ is a multidimensional questionnaire consisting of self-descriptive statements from the person’s point of view (Table 1). Respondents are asked to reflect their self-management behaviors over the past weeks and rate to which extent each statement applies to them. An eight-week reference period was chosen to cover behaviors explaining present HbA1c; however, a shorter period (e.g., four weeks) might support the reflection of short-term changes, thus adaption of the instruction, where needed, might be considered. Responses are given on a four-point scale (from 0–’does not apply to me’ to 3–’applies to me very much’). Item scores are summed to scale scores reflecting the following specific activities: adjusting one’s diet towards diabetes (subscale ‘eating behavior’), taking medications consistently (subscale ‘medication taking’), testing/monitoring blood glucose or interstitial glucose (subscale ‘glucose monitoring’), being physically active to improve diabetes and health (subscale ‘physical activity’) and interacting with one’s diabetes-treating physician/healthcare professionals (subscale ‘cooperation with diabetes team’). A total score as a global measure of diabetes self-management can be calculated. Raw sum scores are transformed to a range from 0–10 for better interpretability and comparability (by dividing the raw sum score by the maximum possible sum of the scale [i.e., item number * 3] and multiplying with 10; details on scoring in Supplementary Table 1). The tool contains positively and negatively keyed items for greater validity and reliability (e.g., avoidance of one-sided, biased responses); negatively keyed items are reverse-scored before summing, thus higher scale scores reflect more optimal behavior. Since its introduction in 2013, the tool has been widely adopted and used for research and practice across countries and languages (Supplementary Table 2).
Original Version
The original version of the DSMQ consists of 16 items (Table 1) which were developed and selected in a systematic, iterative process: A set of newly developed and qualitatively piloted items were initially tested on a sample of 110 people and successively excluded until only those with good properties remained (21). The resulting questionnaire was then administered to 261 people with T1D or T2D to evaluate measurement properties against a convergent standard measure; results supported reliability and validity (21). A subsequent study yielded further supportive evidence (23).
Revision
Reasons for the revision were: i) wording considered as improvable in single items, ii) findings suggesting limited reliability for the ‘cooperation with diabetes team’ subscale in some studies and iii) practices of dose-adjusting insulin injections and correcting glucose levels (where intensive insulin treatment applies) being insufficiently covered. The original scale was amended accordingly, that is: i) items were updated to conform with new technologies such as continuous glucose monitoring (CGM) and data management software; the potentially misleading term ‘blood sugar levels’ was replaced with ‘glucose levels’, referring to both blood and interstitial glucose; some items were revised to avoid compliance-oriented expressions (e.g., ‘strictly follow’ or ‘as prescribed’); ii) the ‘cooperation with diabetes team’ items were harmonized and one additional item was added to improve reliability; iii) seven items covering practices of intensive insulin treatment were added as an optional extra. Item-level amendments are given in detail in Supplementary Table 3; old and new items are compared in Table 1. In summary, two items remained unchanged, seven items were slightly revised and seven items were significantly altered but the essential meaning was kept (Table 1). The original item order was kept, except for item 16 which was repositioned as number 20. A total of eleven items were newly added, thereof four regarding general behaviors (no. 16–19) and seven (no. 21–27) regarding intensive insulin treatment practices specifically (e.g., adjusting insulin; correcting glucose levels), the latter given in a separate section with specific instruction.
The DSMQ-R thus contains a total of 27 items, 20 on general behaviors relevant for most people with diabetes and seven on specific insulin treatment behaviors. A total score is estimated using the 20 general items; where applicable, a 27-item total score including the optional items can be calculated. The subscale ‘eating behavior’ contains now six items and the subscale ‘cooperation with diabetes team’ four; ‘medication taking’, ‘glucose monitoring’ and ‘physical activity’ remain unchanged with two, three and three items, respectively; two of the 20 general items request global statements and are included in the total scale only (Table 1).
Study Design and Data Collection
This evaluation of the DSMQ-R includes T1D and T2D. The analyzed data were acquired as part of five clinical studies, three cross-sectional, two prospective, conducted between 2015 and 2021. All studies were ethically approved and carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent.
● Study 1 was a multi-center, cross-sectional survey to evaluate person-reported outcome measures for diabetes, conducted in 2015–16; details of the study are reported elsewhere (25). Ethical approval was obtained from the Ethics Committee of the German Psychological Society (file no. NH 032015). N=606 participants were surveyed using questionnaires including the DSMQ-R, DAS, PAID-5, PHQ-9, DTSQ (explained below); 606 people participated; data of n=588 (56.6% T1D) could be used for this evaluation.
● Study 2 (‘Depression and Diabetes Control Trial’) was a randomized controlled trial testing a diabetes-specific treatment program for people with depressive symptoms (CES-D ≥16) and hyperglycemia (HbA1c >7.5%/59 mmol/mol) against diabetes care as usual. It was approved by the Ethics Committee of the State Medical Chamber of Baden-Wuerttemberg (file no. F-2015-056) and is registered at ClinicalTrials.gov (ID no. NCT02675257). Participants were enrolled from 1/2016–3/2017. The first follow-up (FU) assessment after six months was used for retest analysis in this evaluation. Questionnaire assessments included the DSMQ-R, SDSCA, PAID, DDS, DAS and PHQ-9 (below). HbA1c was assessed in a central laboratory. N=213 were enrolled and 198 (66.2% T1D) provided suitable data for this evaluation.
● Study 3: a cross-sectional FU survey of the ‘DIAMOS’ and ‘ECCE HOMO’ trial participants was conducted in 2017–18, on average five years after participating in the original trials. Participants had been enrolled using equivalent inclusion and exclusion criteria, enabling aggregation to one cohort; all had elevated depressive symptoms (CES-D ≥16) at baseline [study details accessible elsewhere (26–28)]. The FU was approved by the Ethics Committee of the State Medical Chamber of Baden-Wuerttemberg (file no. F-2017-071). A total of 323 people (68.1% of the total cohort) could be followed up using questionnaires including the DSMQ-R, PAID, DDS, DAS and PHQ-9; HbA1c was estimated. N=298 people (64.0% T1D) provided sufficient data for this evaluation.
● Study 4 (‘DIA-LINK1’) was a prospective observational study analyzing links between mental health and glycemic outcomes in T1D (ClinicalTrials.gov ID no. NCT03811132); participants were enrolled from 3/2019–3/2020 and followed over three months. Measurements comprised repeated surveys (including DSMQ-R, PAID, T1-DDS, CES-D), HbA1c estimation, 17-day ecological momentary assessment (EMA) with daily diabetes-related questions and 4-week continuous glucose monitoring (CGM) (29). The study was approved by the Ethics Committee of the German Psychological Society (file no. NH 082018). N=203 participants were enrolled.
● Study 5: the ‘DIA-LINK2’ study (2020–21) is a prospective observational study regarding mental health and glycemia with the same design as DIA-LINK1 but regarding T2D (ClinicalTrials.gov ID no. NCT04438018). Ethical approval was obtained from the Ethics Committee of the German Psychological Society (file no. HermannsNorbert2020-03-05AM). A total of 190 people with T2D have been enrolled, and n=180 provided suitable data for this evaluation.
Variables and Measurements
Besides the DSMQ-R, the following variables were assessed as part of the studies:
Glycemic outcome: Glycated hemoglobin (HbA1c) was estimated from venous blood samples taken at the same time as the questionnaire assessments in all studies. HbA1c was usually estimated in a central laboratory (at the Diabetes Center Mergentheim) using high performance liquid chromatography (performed with the Bio-Rad Variant II Turbo analyzer in studies 2 and 3 and the Tosoh Automated Glycohemoglobin Analyzer HLC-723G11 in studies 4 and 5), meeting IFCC standard [laboratory normal range 4.3–6.1% (24–43 mmol/mol)]; study 1 included four different laboratory cites.
Study 4 additionally assessed glycemic levels over four weeks using intermittently scanned CGM. The following CGM-derived parameters were calculated: mean sensor glucose (in mg/dl), time in range (% values between 70–180 mg/dl, 3.9–10 mmol/l), time below range (% values <70 mg/dl, <3.9 mmol/l), time above range (% values >180 mg/dl, >10 mmol/l), and glucose variability [coefficient of variation (CV)].
Diabetes self-care activities: The 10-item Summary of Diabetes Self-Care Activities Measure [SDSCA (22, 30)] was applied as a convergent measure of diabetes self-management in study 2. The tool requests on how many days of the past week the person engaged in healthy eating, exercising, blood sugar testing and foot care. Responses are averaged to scales (e.g., Diet, Exercise, Blood Sugar Testing) with scores ranging from 0–7 and higher values reflecting more frequent activity.
Diabetes distress and diabetes-specific problems: The 20-item Problem Areas in Diabetes Scale (PAID) measuring diabetes-related distress (31) was applied in all studies. The questionnaire requests ratings of diabetes-specific emotional problems on a five-point scale (0–’not a problem’ to 4–’serious problem’). The item scores are summed and transformed to a total score ranging from 0–100; higher scores reflect higher distress; scores ≥40 suggest meaningful distress (32). In study 1, the 5-item short form [PAID-5 (33)] was used.
In studies 2–5, the Diabetes Distress Scale [DDS (34)] or T1-Diabetes Distress Scale [T1-DDS (35)] was administered in addition to the PAID. The DDS/T1-DDS items address a range of diabetes-specific problems; however, it also includes items and scales whose relations to the construct of diabetes distress have been questioned (14, 32, 36). Therefore, we did not estimate a total score but rather selected specific items whose contents regarding self-management-related problems could be used for the correlation analysis (i.e., DDS items 6, 8 and 12 on ‘not testing blood sugars frequently enough’, ‘often failing with diabetes routine’ and ‘not sticking closely enough to a good meal plan’, and T1-DDS items 2, 8, 12, 23 and 28 on ‘not eating as carefully as one should’, ‘not taking as much insulin as one should’, ‘not checking blood glucose as often as one should’, ‘eating being out of control’ and ‘not giving diabetes as much attention as one should’); these aspects were assessed as convergent criteria for corresponding DSMQ-R scales. Items regarding doctor-related problems (i.e., DDS item 15 on ‘not having a doctor who one can see regularly about diabetes’ and T1-DDS items 7 and 18, ‘can’t tell diabetes doctor what is really on my mind’, ‘diabetes doctor doesn’t really understand what it’s like to have diabetes’) were used for correlation with the DSMQ-R scale ‘cooperation with diabetes team’. Responses in the DDS/T1-DDS are given on a six-point scale (1–’not a problem’ to 6–’a very serious problem’), thus higher scores reflect greater problems.
Diabetes acceptance, a measure of psychological adjustment to living with diabetes, was assessed using the Diabetes Acceptance Scale (DAS); in studies 1–3, the full 20-item version was used, in studies 4–5, the 10-item short form (25). The items request aspects of acceptance and integration (e.g., ‘I accept diabetes as part of my life’) versus avoidance, neglect and demotivation (e.g., ‘I avoid dealing with topics related to diabetes’). Responses are given on a four-point scale (0–’never true for me’ to 3–’always true for me’). Item scores are summed so that higher scores reflect higher acceptance (range 0–60). Higher acceptance scores have been associated with more optimal self-management (25, 37). Besides the total score, items specifically related to treatment motivation (e.g., ‘I have difficulties to motivate myself to perform good diabetes self-care’) and treatment neglect (e.g., ‘I neglect diabetes self-care because I want to avoid topics related to diabetes’) were aggregated to subscales (Cronbach’s α=0.71 and 0.83, respectively).
Diabetes treatment satisfaction was measured using the Diabetes Treatment Satisfaction Questionnaire (DTSQ) in study 1, including six satisfaction-related items and a 7-point scale (0–’very dissatisfied’ to 6–’very satisfied’). Items are summed to a total score from 0–64; higher scores reflect higher satisfaction (38). Higher treatment satisfaction was expected to be associated with more optimal treatment behavior (DSMQ-R).
Depressive symptoms were assessed in all studies due to their high prevalence in diabetes as well as the studies focusing on depression and mental health. Studies included either the Patient Health Questionnaire-9 (PHQ-9) or the Center for Epidemiologic Studies Depression Scale (CES-D); both have excellent properties (39). The PHQ-9 assesses the nine symptoms of major depression according to DSM-5 during the past two weeks. Responses are given on a four-point scale (0–’not at all’ to 3–’nearly every day’). Total score range is 0–27; higher scores indicate more symptoms. The CES-D assesses 20 depressive symptoms during the past week; responses are given on a four-point scale (0–’rarely or none of the time’ to 3–’most or all of the time’), resulting in a total score from 0–60 (higher scores=more symptoms). Depressive symptoms have been consistently associated with less optimal self-management across behaviors [e.g. (13)].
Daily diabetes problems/burdens: The DIA-LINK studies included a smartphone-based EMA with daily diabetes-related questions over 17 days (29). Items constituting likely correlates of the DSMQ-R were used as convergent criteria (e.g., ‘How much have you felt guilty when neglecting your diabetes treatment today?’; full item details in Supplementary Table 4). Responses were given on a scale from 0–’not at all’ to 10–’very much’. Daily responses were averaged.
Demographic and person-related variables comprised sex, age, BMI, diabetes type, diabetes duration and treatment regimen. Long-term and acute complications of diabetes (study 1) were based on medical examinations, laboratory assessments and interviews (assessed were diabetic retinopathy, neuropathy, nephropathy, foot syndrome; treated ketoacidosis, past 12 months). Mean numbers of daily insulin injections (where applicable) and daily glucose tests or scans/readings as well as frequencies of diabetologist visits per past six months were assessed in face-to-face interviews.
Statistical Analyses
Statistical analyses were performed using SPSS 26.0.0 (IBM SPSS Statistics). P values < 0.05 (two-tailed) were considered to indicate statistical significance. For the DSMQ-R, total and subscale scores were calculated as per scoring instruction (Supplementary Table 1) with scores ranging between 0 and 10. Negatively-keyed items were reverse-scored so that higher scale scores suggest more optimal behavior. Where applicable, a 27-item total score was calculated in addition to the 20-item total; yet the optional items were not included in subscale scores to warrant comparisons of scores between subgroups. Measurement functions were analyzed according to clinimetric criteria (40). Internal reliability was analyzed using Cronbach’s α; since potential preference of McDonald’s ω over α has been discussed (41), ω was additionally estimated [using Hayes’ OMEGA macro for SPSS (41)]. Reproducibility was tested using retest correlations in the prospective studies. Construct validity was evaluated via correlations with convergent measures and related variables to develop a nomological network. Since adjusting eating behaviors towards diabetes, taking medications consistently and checking glucose levels regularly can be expected to result in better glycemic levels, associations between the corresponding DSMQ-R scales and glycemic outcomes were analyzed as indicators of validity. Similarly, associations with acute and long-term complications were assessed in study 1. Further, associations between the DSMQ-R scales and convergent measures of self-care activities, treatment satisfaction, treatment motivation and neglect as well as diabetes acceptance, diabetes distress and depressive symptoms were analyzed. Structural validity was assessed using confirmatory factor analyses (AMOS 26.0.0, IBM SPSS Statistics). Model fit was evaluated according to Comparative Fit Index (CFI) ≥ 0.95, Tucker Lewis Index (TLI) ≥ 0.95, Standardized Root Mean Square Residual (SRMR) ≤ 0.08 and Root Mean Square Error of Approximation (RMSEA) ≤ 0.06. Responsiveness, the ability to detect change, was assessed via changes of the DSMQ-R scales in prospective studies, given as Cohen’s d. Where applicable, changes were compared between treatment groups (i.e., study 2, with participants randomized to either depression treatment or diabetes care as usual).
Results
Sample Characteristics
The sample characteristics are given in Table 2. Studies 1–3 had mixed samples including people with T1D and T2D (T1D being overrepresented in line with secondary and tertiary care enrolment), study 4 and 5 assessed only T1D or T2D, respectively. Sample sizes varied between 180 and 588. Study 1 contained a more general sample, whereas other studies overrepresented people with specific mental aspects: study 2 contained people with current depressive symptoms, study 3 contained people with a history of depressive symptoms and studies 4 and 5 included majorities with either depressive symptoms or diabetes distress. All samples had a wide age range with a mean age between 45 and 53 years, except for study 4 (T1D only) whose sample’s mean age was 39 years. The mean diabetes duration reflected relatively long-standing diabetes throughout. HbA1c levels were generally elevated with mean values around 7.8 to 9.3% (62 to 78 mmol/mol) across the studies.
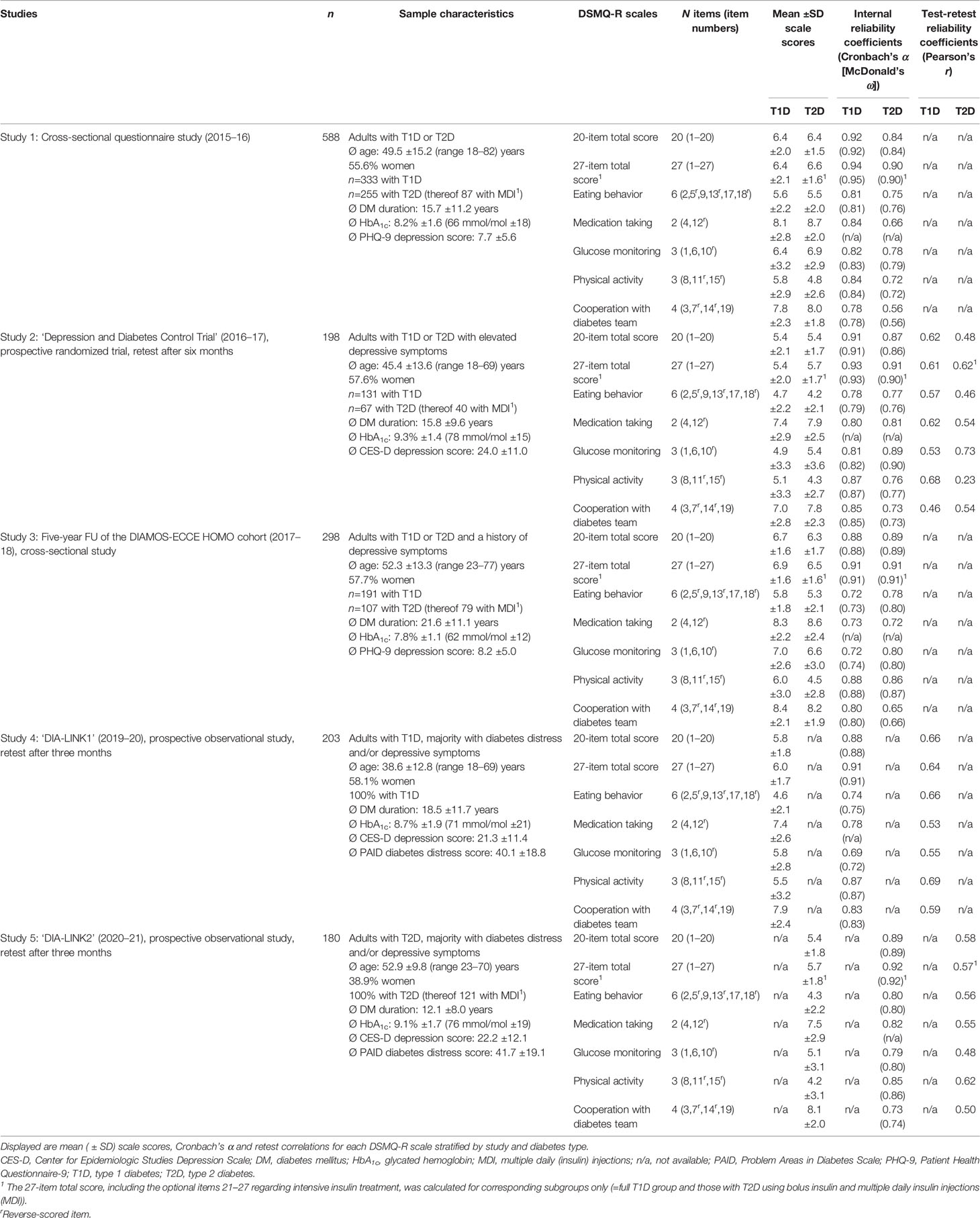
Table 2 Study sample characteristics and normative data and reliability indices for the DSMQ-R scales by study and diabetes type.
Internal Reliability
Cronbach’s α of the 20-item total scale varied from 0.88–0.92 (mean=0.90) in T1D and from 0.84–0.89 (mean=0.87) in T2D across studies. Coefficients were slightly higher for the 27-item total scale, where applicable (Table 2). For the subscales, mean coefficients α for T1D (T2D) were: ‘eating behavior’=0.76 (0.78), ‘medication taking’=0.79 (0.75), ‘glucose monitoring’=0.76 (0.82), ‘physical activity’=0.87 (0.80) and ‘cooperation with diabetes team’=0.82 (0.67). McDonald’s ω yielded consistent results (Table 2). Direct comparisons of scale reliabilities estimated including the newly added items versus original ones only yielded consistently higher reliability coefficients for the new scales (Supplementary Table 5).
Reproducibility
Retest correlations over three to six months reflected sufficient intra-individual stability of the measurement over time. Mean correlations for 20-item total scale were 0.64 in T1D and 0.53 in T2D; mean correlations for the subscales were from 0.53–0.69 in T1D and from 0.43–0.61 in T2D (Table 2).
Construct Validity
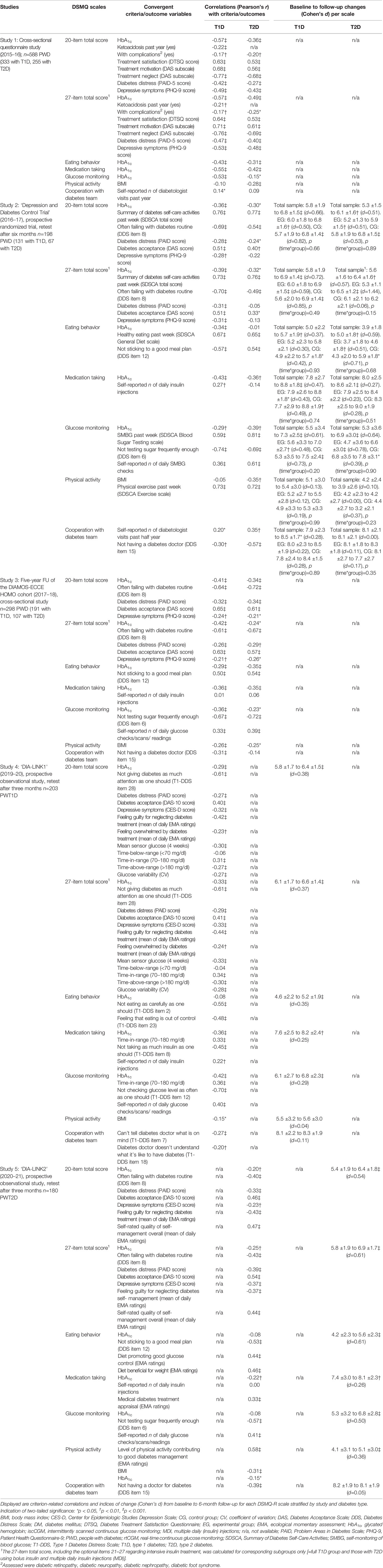
Correlations with convergent criteria were generally in line with expectations towards validity of the measurement and a meaningful nomological network.
Total scale: Higher DSMQ-R total scores (suggesting more optimal self-management) were consistently associated with better HbA1c values across studies and diabetes types; however, the sizes of associations varied (e.g., from -0.29 to -0.57, mean = -0.41, in T1D and from -0.20 to -0.36, mean=-0.30, in T2D; 20-item total). Higher DSMQ-R total scores were also associated with lower mean sensor glucose, more time in range, less time above range and lower glucose variability in T1D (study 4). Further, higher DSMQ-R total scores were associated with lower rates of long-term complications and less events of ketoacidosis (T1D). DSMQ-R total scores were highly positively associated with convergent measures of treatment motivation, treatment satisfaction and self-management performance according to the SDSCA questionnaire and corresponding DDS/T1-DDS items (Table 3); and highly negatively with items reflecting suboptimal treatment behavior. In studies 4 and 5, significant correlations with EMA items reflecting self-management were observed. Finally, higher DSMQ total scores were seen in people with better mental health, lower diabetes distress and less depressive symptoms.
Subscales: The subscales ‘eating behavior’, ‘medication taking’ and ‘glucose monitoring’ showed significant associations with corresponding convergent criteria for diabetes-adjusted eating (e.g., SDSCA scale on healthy eating, DDS/T1-DDS items regarding sticking to a good meal plan and eating carefully), medication taking (e.g., T1-DDS item on insulin taking, mean number of daily insulin injections in T1D), glucose monitoring (e.g., SDSCA scale on blood sugar testing, DDS/T1-DDS items on glucose checking, mean number of daily glucose checks/scans). Each of the scales showed significant associations with better HbA1c in several studies, however not all. The subscale ‘physical activity’ showed high correlations with the convergent SDSCA scale on past-week physical exercise and small-to-moderate associations with BMI. The subscale ‘cooperation with diabetes team’ showed significant correlations with self-reported frequencies of diabetologist visits as well as corresponding DDS/T1-DDS items on doctor-related problems. ‘Eating behavior’, ‘medication taking’ and ‘physical activity’ were also significantly associated with corresponding EMA ratings in studies 4 and 5.
Structural Validity
Confirmatory factor analyses supported a five-factor structure representing the five subscales with excellent fit to the data for both T1D and T2D (Supplementary Figures 1–2). One-factor models representing the total scale showed good fit as well; however, with slightly lower fit indices and lower factor loadings (Supplementary Figures 3–6).
Responsiveness
The ability to detect change was supported by significant changes over time in the total score and most subscale scores in the prospective studies. Greater changes were seen in the total scale and ‘eating behavior’ and ‘glucose monitoring’ subscales, while changes in ‘medication taking’ were modest and changes in ‘physical activity’ and ‘cooperation with diabetes team’ were small or lacking (Table 3). Between-group comparisons for people receiving depression treatment versus diabetes care as usual in study 2 suggested similar changes in DSMQ-R scores without significant differences between the groups at six-month follow-up.
Discussion
Main Findings
The evaluation of the DSMQ-R using data from diverse studies suggests very good properties in measuring diabetes self-management behavior in both T1D and T2D according to clinimetric criteria (40). Results suggest that the tool has good reliability, validity and responsiveness to change. The terms and expressions used in the questionnaire were updated to conform with modern diabetes-related language. The revised scales with newly added items showed higher internal reliability than the original version’s item sets.
The DSMQ-R total scale constitutes a reliable and valid measure of overall self-management. Yet it is a global measure; thus assessing the specific behaviors using the subscales may be preferred and even necessary for understanding individual aspects. For the subscales, however, differential properties and options should be considered: First, the numbers of items per scale differ which may affect reliability of the measurement. In this evaluation, most subscales yielded satisfactory to good reliability estimates; however, lower reliability coefficients were seen for subscales with fewer items (e.g., medication taking) in some of the studies. Furthermore, coefficients varied across studies and patient groups, suggesting that the utilization of subscales in research might benefit from affirming reliability within a given study data set. Notably, despite specific revisions and improved internal reliability, the ‘cooperation with diabetes team’ subscale still showed subthreshold reliability coefficients in two of five studies for T2D; yet not for T1D.
Reliability coefficients were mostly slightly higher in T1D subsamples compared to T2D which is in line with previous findings (21). This might be explained by more diverse treatment regimens and practices in T2D; for instance, prescribed medications may be diverse (oral drugs, insulin and/or incretin mimetics), glucose testing may or may not be required and dietary recommendations may vary in relevance and function. This might also explain higher associations between the DSMQ-R scales and HbA1c in T1D [consistent with previous findings (23)], where glycemic outcomes directly depend on the consistent coordination and adjustment of carbohydrate intake, activities and insulin doses; whereas in T2D, glycemic control may rely more on diet and activity and less on glucose checking and meal-specific decisions (depending on the treatment regimen); also, residual insulin action may stabilize glycemic levels and reduce hyperglycemia.
It should be noted that two-sided questioning (using both positively and negatively keyed items) may lower internal consistency as observed in some DSMQ-R subscales; at the same time, higher validity is achieved and response bias is prevented. From a clinimetric perspective, a varied assessment using items covering different aspects from different sides is more important than a highly homogeneous measurement (40).
Validity of the scale measurement was supported by high correlations with convergent scores and items from other questionnaires. However, as self-report is prone to bias, associations with objective measures constitute another important source of information. Thus, the widely consistent associations between DSMQ-R scales and HbA1c (as well as CGM-derived glucose parameters) across studies may be seen as extra evidence favoring validity.
Relatively good explanation of variation in HbA1c was already observed in our previous studies for both T1D and T2D (21, 23). This might be explained by i) the reflection of behaviors over a broader, more representative reference period and ii) the items requesting behavioral evaluations (e.g., ‘with care and attention’) rather than behavior frequency (e.g., ‘on how many days…?’ as in the SDSCA). On the other hand, three studies using the DSMQ with non-Western samples (42–44) and one Hungarian study (45) have reported lower associations with HbA1c, suggesting caution against generalization across cultures.
Validity of the measurement was also supported by the structural representation of assessed contents (i.e., items and scales) in the factor analyses with good model fit for both T1D and T2D.
Change scores reflecting improvements in DSMQ-R-assessed behaviors supported good responsiveness of the measurement. In study 4, similar changes were seen for people randomized to depression treatment versus diabetes care as usual; this could be explained by both groups receiving treatment with beneficial effects on self-management behavior. The tool’s ability to detect change is also supported by findings from international studies using the DSMQ which found significant self-management improvement over time and between-group differences in randomized trials (46–49); notably, observed changes in DSMQ scores by group were often accompanied by parallel changes in HbA1c, which might be taken as evidence supporting validity of the changes (46, 48, 50). With regard to responsiveness and the tool’s reference period (eight weeks), a shorter period might facilitate the detection of short-term changes, thus adapting the instruction (e.g., four weeks), where needed, may be considered.
In terms of item amendments (e.g., revised wording), the DSMQ-R probably constitutes a relevant improvement. However, since most revisions were minor and item concepts were kept equivalent, the original 16-item version is basically included in the revised form. Estimation of scales as for the original version, where needed (e.g., to compare scores with former study results), would still be possible.
Limitations and Strengths
The inferences drawn from this research are qualified by the following limitations: first, four of the studies whose data were analyzed here focused on diabetes-comorbid mental conditions, thus rates of depressive symptoms and/or diabetes distress were elevated and the samples may not be representative for the general population with diabetes (i.e., risk of spectrum bias). Second, we assessed cross-sectional associations between self-reported behavior and diabetes outcomes, thus inferences towards causation are not possible; in fact, associations with glycemic outcomes might be bidirectional; for instance, knowing of glycemic levels (e.g., last HbA1c) might influence self-management self-appraisal in the questionnaire. Third, the study samples were recruited within secondary or tertiary care, thus samples may not represent the primary care population; based on this, people with T2D assessed here used advanced medical treatments often including insulin and even basal-bolus therapy with multiple daily injections, whereas people with diet-and-exercise regimens and/or oral antidiabetic treatment alone were less represented.
The strengths of the evaluation may be seen in the standardized assessment using validated scales and items, temporal coincidence of questionnaire self-reports, interviews and laboratory measures and the inclusion of multiple methods including CGM and EMA for the assessment of convergent criteria. Furthermore, the stratified analyses for T1D and T2D using sufficiently large samples support evaluation for both major types of diabetes. Due to potential advantages of McDonald’s ω over Cronbach’s α (41), we calculated both estimates, yielding highly consistent results. Finally, the evaluation across different study samples, both general and specific, yields a more comprehensive and representative total evidence base; the fact that indices of reliability and validity, including associations with clinical criteria, were relatively consistent across studies may favor generalizability.
Conclusions
In summary, the results support good clinimetric properties of the DSMQ-R. The tool can be used for research and clinical practice. It may help understand barriers and facilitators of functional self-management in T1D and T2D, facilitate the identification improvable practices in individuals and monitor behavior change following treatment in practical care or research trials.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the German Psychological Society or the Ethics Committee of the State Medical Chamber of Baden-Wuerttemberg. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AS: developed and revised the DSMQ; planned and designed study 1; co-planned and designed studies 2–5; collected the data; performed the evaluation, analyzed and interpreted the data; wrote the manuscript. BK and NH: planned and designed studies 2–5; discussed the findings and revised the manuscript. DE: planned and designed studies 4–5; co-planned and designed studies 2–3; discussed the findings and revised the manuscript. TH: discussed the findings and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Study 1 was supported by the German Diabetes Foundation (DDS) (grant number 375.10.15). Studies 2–3 were supported by the German Center for Diabetes Research (DZD) (grant number 82DZD01102). Studies 4–5 were supported by the German Center for Diabetes Research (DZD) [grant number 82DZD11A02]. The funders were not involved in decisions regarding study design; collection, analysis and interpretation of data; writing of the report; and submission of the article for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the persons who kindly participated in the studies enabling this research. We acknowledge the valuable contributions to participant enrolment and data collection of André Reimer (studies 1–3), Paula Rubertus (study 4) and Fabienne Schmid (study 5).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2021.823046/full#supplementary-material
Abbreviations
CES-D, Center for Epidemiologic Studies Depression Scale; CG, control group; CV, coefficient of variation; DAS, Diabetes Acceptance Scale; DDS, Diabetes Distress Scale; DM, diabetes mellitus; DSMQ, Diabetes Self-Management Questionnaire; DTSQ, Diabetes Treatment Satisfaction Questionnaire; EG, experimental group; EMA, ecological momentary assessment; HbA1c, glycated hemoglobin; iscCGM, intermittently scanned continuous glucose monitoring; MDI, multiple daily (insulin) injections; PAID, Problem Areas in Diabetes Scale; PHQ-9, Patient Health Questionnaire-9; PWD, people with diabetes; rtCGM, real-time continuous glucose monitoring; SDSCA, Summary of Diabetes Self-Care Activities; SMBG, self-monitoring of blood glucose; T1-DDS, Type 1 Diabetes Distress Scale; T1D, type 1 diabetes; T2D, type 2 diabetes.
References
1. World Health Organization (WHO). Diabetes. Available at: https://www.who.int/health-topics/diabetes#tab=tab_1 (Accessed on 10.11.2021).
2. International Diabetes Federation. IDF Diabetes Atlas. 10th edn. Brussels, Belgium (2021). Available at: https://www.diabetesatlas.org.
3. American Diabetes Association. Facilitating Behavior Change and Well-Being to Improve Health Outcomes: Standards of Medical Care in Diabetes-2021. Diabetes Care (2021) 44:S53–72. doi: 10.2337/dc21-S005
4. American Association of Diabetes Educators (AADE). AADE7 Self-Care Behaviors. Diabetes Educ (2008) 34:445–9. doi: 10.1177/0145721708316625
5. American Diabetes Association. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. Clin. Diabetes (2020) 38:10–38. doi: 10.2337/cd20-as01
6. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of Hyperglycaemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia (2018) 61:2461–98. doi: 10.1007/s00125-018-4729-5
7. Akalin S, Berntorp K, Ceriello A, Das AK, Kilpatrick ES, Koblik T, et al. Intensive Glucose Therapy and Clinical Implications of Recent Data: A Consensus Statement From the Global Task Force on Glycaemic Control. Int J Clin Pract (2009) 63:1421–25. doi: 10.1111/j.1742-1241.2009.02165.x
8. Yu PC, Bosnyak Z, Ceriello A. The Importance of Glycated Haemoglobin (HbA(1c)) and Postprandial Glucose (PPG) Control on Cardiovascular Outcomes in Patients With Type 2 Diabetes. Diabetes Res Clin Pract (2010) 89:1–9. doi: 10.1016/j.diabres.2009.12.009
9. Sina M, Graffy J, Simmons D. Associations Between Barriers to Self-Care and Diabetes Complications Among Patients With Type 2 Diabetes. Diabetes Res Clin Pract (2018) 141:126–31. doi: 10.1016/j.diabres.2018.04.031
10. Houle J, Beaulieu M-D, Chiasson J-L, Lespérance F, Côté J, Strychar I, et al. Glycaemic Control and Self-Management Behaviours in Type 2 Diabetes: Results From a 1-Year Longitudinal Cohort Study. Diabetes Med (2015) 32:1247–54. doi: 10.1111/dme.12686
11. Coyle ME, Francis K, Chapman Y. Self-Management Activities in Diabetes Care: A Systematic Review. Aust Health Rev (2013) 37:513–22. doi: 10.1071/AH13060
12. Alexandre K, Vallet F, Peytremann-Bridevaux I, Desrichard O. Identification of Diabetes Self-Management Profiles in Adults: A Cluster Analysis Using Selected Self-Reported Outcomes. PloS One (2021) 16:e0245721. doi: 10.1371/journal.pone.0245721
13. Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, et al. Depression and Diabetes Treatment Nonadherence: A Meta-Analysis. Diabetes Care (2008) 31:2398–403. doi: 10.2337/dc08-1341
14. Gonzalez JS, Shreck E, Psaros C, Safren SA. Distress and Type 2 Diabetes-Treatment Adherence: A Mediating Role for Perceived Control. Health Psychol (2015) 34:505–13. doi: 10.1037/hea0000131
15. Schmitt A, Bendig E, Baumeister H, Hermanns H, Kulzer B. Associations of Depression and Diabetes Distress With Self-Management Behavior and Glycemic Control. Health Psychol (2021) 40:113–24. doi: 10.1037/hea0001037
16. Osborn CY, Egede LE. Validation of an Information-Motivation-Behavioral Skills Model of Diabetes Self-Care (IMB-DSC). Patient Educ Couns (2010) 79:49–54. doi: 10.1016/j.pec.2009.07.016
17. Eigenmann CA, Colagiuri R, Skinner TC, Trevena L. Are Current Psychometric Tools Suitable for Measuring Outcomes of Diabetes Education? Diabetes Med (2009) 26:425–36. doi: 10.1111/j.1464-54912009.02697.x
18. Caro-Bautista J, Martín-Santos FJ, Morales-Asencio JM. Systematic Review of the Psychometric Properties and Theoretical Grounding of Instruments Evaluating Self-Care in People With Type 2 Diabetes Mellitus. J Adv Nurs (2014) 70:1209–27. doi: 10.1111/jan.12298
19. Lu Y, Xu J, Zhao W, Han H-R. Measuring Self-Care in Persons With Type 2 Diabetes: A Systematic Review. Eval Health Prof (2016) 39:131–84. doi: 10.1177/0163278715588927
20. Wee PJL, Kwan YH, Loh DHF, Phang JK, Puar TH, Østbye T, et al. Measurement Properties of Patient-Reported Outcome Measures for Diabetes: Systematic Review. J Med Internet Res (2021) 23:e25002. doi: 10.2196/25002
21. Schmitt A, Gahr A, Hermanns N, Kulzer B, Huber J, Haak T. The Diabetes Self-Management Questionnaire (DSMQ): Development and Evaluation of an Instrument to Assess Diabetes Self-Care Activities Associated With Glycaemic Control. Health Qual Life Outcomes (2013) 11:138. doi: 10.1186/1477-7525-11-138
22. Kamradt M, Bozorgmehr K, Krisam J, Freund T, Kiel M, Qreini M, et al. Assessing Self-Management in Patients With Diabetes Mellitus Type 2 in Germany: Validation of a German Version of the Summary of Diabetes Self-Care Activities Measure (SDSCA-G). Health Qual Life Outcomes (2014) 12:185. doi: 10.1186/s12955-014-0185-1
23. Schmitt A, Reimer A, Hermanns N, Huber J, Ehrmann D, Schall S, et al. Assessing Diabetes Self-Management With the Diabetes Self-Management Questionnaire (DSMQ) Can Help Analyse Behavioural Problems Related to Reduced Glycaemic Control. PloS One (2016) 11:e0150774. doi: 10.1371/journal.pone.0150774
24. Speight J, Conn J, Dunning T, Skinner TC, Diabetes Australia. Diabetes Australia Position Statement. A New Language for Diabetes: Improving Communications With and About People With Diabetes. Diabetes Res Clin Pract (2012) 97:425–31. doi: 10.1016/j.diabres.2012.03.015
25. Schmitt A, Reimer A, Kulzer B, Icks A, Paust R, Roelver KM, et al. Measurement of Psychological Adjustment to Diabetes With the Diabetes Acceptance Scale. J. Diabetes Complications (2018) 32:384–92. doi: 10.1016/j.jdiacomp.2018.01.005
26. Hermanns N, Schmitt A, Gahr A, Herder C, Nowotny B, Roden M, et al. The Effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for Patients With Diabetes and Subclinical Depression: Results of a Randomized Controlled Trial. Diabetes Care (2015) 38:551–60. doi: 10.2337/dc14-1416
27. Schmitt A, Kulzer B, Reimer A, Herder C, Roden M, Haak T, et al. Evaluation of a Stepped Care Approach to Manage Depression and Diabetes Distress in Patients With Type 1 Diabetes and Type 2 Diabetes: Results of a Randomized Controlled Trial (ECCE HOMO Study). Psychother Psychosom (2021). doi: 10.1159/000520319
28. Herder C, Schmitt A, Budden F, Reimer A, Kulzer B, Roden M, et al. Association Between Pro- and Anti-Inflammatory Cytokines and Depressive Symptoms in Patients With Diabetes-Potential Differences by Diabetes Type and Depression Scores. Transl Psychiatry (2018) 7:1. doi: 10.1038/s41398-017-0009-2
29. Ehrmann D, Schmitt A, Priesterroth L, Kulzer B, Hermanns N. Time in Diabetes Distress and Glycaemia-Specific Distress: New Patient-Reported Outcome Measures for Psychosocial Burden of Diabetes Using Ecological Momentary Assessment in an Observational Study. Diabetes Care (2022).
30. Toobert DJ, Hampson SE, Glasgow RE. The Summary of Diabetes Self-Care Activities Measure: Results From 7 Studies and a Revised Scale. Diabetes Care (2000) 23:943–50. doi: 10.2337/diacare.23.7.943
31. Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of Diabetes-Related Distress. Diabetes Care (1995) 18:754–60. doi: 10.2337/diacare.18.6.754
32. Schmitt A, Reimer A, Kulzer B, Haak T, Ehrmann D, Hermanns N. How to Assess Diabetes Distress: Comparison of the Problem Areas in Diabetes Scale (PAID) and the Diabetes Distress Scale (DDS). Diabetes Med (2016) 33:835–43. doi: 10.1111/dme.12887
33. McGuire BE, Morrison TG, Hermanns N, Skovlund S, Eldrup E, Gagliardino J, et al. Short-Form Measures of Diabetes-Related Emotional Distress: The Problem Areas In Diabetes Scale (PAID)-5 and PAID-1. Diabetologia (2010) 53:66–9. doi: 10.1007/s00125-009-1559-5
34. Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing Psychosocial Distress in Diabetes: Development of the Diabetes Distress Scale. Diabetes Care (2005) 28:626–31. doi: 10.2337/diacare.28.3.626
35. Fisher L, Polonsky WH, Hessler DM, Masharani U, Blumer I, Peters AL, et al. Understanding the Sources of Diabetes Distress in Adults With Type 1 Diabetes. J Diabetes Complications (2015) 29:572–7. doi: 10.1016/j.jdiacomp.2015.01
36. Fenwick EK, Rees G, Holmes-Truscott E, Browne JL, Pouwer F, Speight J. What is the Best Measure for Assessing Diabetes Distress? A Comparison of the Problem Areas in Diabetes and Diabetes Distress Scale: Results From Diabetes MILES–Australia. J Health Psychol (2018) 23:667–80. doi: 10.1177/1359105316642006
37. Schmitt A, Reimer A, Kulzer B, Haak T, Gahr A, Hermanns N. Assessment of Diabetes Acceptance can Help Identify Patients With Ineffective Diabetes Self-Care and Poor Diabetes Control. Diabetes Med (2014) 31:1446–51. doi: 10.1111/dme.12553
38. Bradley C. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. In: Bradley C, editor. Diabetes Treatment Satifaction Questionnaire (DTSQ). London: Overseas Publishers Association (1994). p. 111–32.
39. van Dijk SEM, Adriaanse MC, van der Zwaan L, Bosmans JE, van Marwijk HWJ, van Tulder MW, et al. Measurement Properties of Depression Questionnaires in Patients With Diabetes: A Systematic Review. Qual Life Res (2018) 27:1415–30. doi: 10.1007/s11136-018-1782-y
40. Carrozzino D, Patierno C, Guidi J, Berrocal Montiel C, Cao J, Charlson ME, et al. Clinimetric Criteria for Patient-Reported Outcome Measures. Psychother Psychosom (2021) 90:222–32. doi: 10.1159/000516599
41. Hayes AF, Coutts JJ. Use Omega Rather Than Cronbach’s Alpha for Estimating Reliability. But… Commun Methods Meas (2020) 14:1–24. doi: 10.1080/19312458.2020.1718629
42. Hammad S, Darawad M, Hourani E, Demeh W. Predictors of Glycated Hemoglobin Among Jordanian Diabetic Patients. Iran J Public Health (2015) 44:1482–91.
43. Bukhsh A, Khan TM, Nawaz MS, Ahmed HS, Chan KG, Lee L-H, et al. Association of Diabetes-Related Self-Care Activities With Glycemic Control of Patients With Type 2 Diabetes in Pakistan. Patient Prefer Adherence (2018) 12:2377–85. doi: 10.2147/PPA.S177314
44. Totesora D, Ramos-Rivera MI, Villegas-Florencio MQ, Reyes-Sia PN. Association of Diabetes-Related Emotional Distress With Diabetes Self-Care and Glycemic Control Among Adult Filipinos With Type 2 Diabetes Mellitus at a Tertiary Hospital in Manila, Philippines. J ASEAN Fed Endocr Soc (2019) 34:189–96. doi: 10.15605/jafes.034.02.10
45. Vincze A, Losonczi A, Stauder A. The Validity of the Diabetes Self-Management Questionnaire (DSMQ) in Hungarian Patients With Type 2 Diabetes. Health Qual Life Outcomes (2020) 18:344. doi: 10.1186/s12955-020-01595-7
46. Eroglu N, Sabuncu N. The Effect of Education Given to Type 2 Diabetic Individuals on Diabetes Self-Management and Self-Efficacy: Randomized Controlled Trial. Prim Care Diabetes (2021) 15:451–58. doi: 10.1016/j.pcd.2021.02.011
47. Fearon-Lynch JA, Sethares KA, Asselin ME, Batty K, Stover CM. Effects of Guided Reflection on Diabetes Self-Care: A Randomized Controlled Trial. Diabetes Educ (2019) 45:66–79. doi: 10.1177/0145721718816632
48. Sayin Kasar K, Duru Asiret G, Kutmec Yilmaz C, Canlar Ş. The Effect of Model-Based Telephone Counseling on HbA1c and Self-Management for Individuals With Type 2 Diabetes: A Randomized Controlled Trial. Prim Care Diabetes (2021) 10. doi: 10.1016/j.pcd.2021.09.005
49. Schnell O, Klausmann G, Gutschek B, Garcia-Verdugo RM, Hummel M. Impact on Diabetes Self-Management and Glycemic Control of a New Color-Based SMBG Meter. J Diabetes Sci Technol (2017) 11:1218–25. doi: 10.1177/1932296817706376
Keywords: diabetes, treatment behavior, self-managament, health behavior, clinimetric, measurement instrument, questionnaire, evaluation
Citation: Schmitt A, Kulzer B, Ehrmann D, Haak T and Hermanns N (2022) A Self-Report Measure of Diabetes Self-Management for Type 1 and Type 2 Diabetes: The Diabetes Self-Management Questionnaire-Revised (DSMQ-R) – Clinimetric Evidence From Five Studies. Front. Clin. Diabetes Healthc. 2:823046. doi: 10.3389/fcdhc.2021.823046
Received: 26 November 2021; Accepted: 17 December 2021;
Published: 13 January 2022.
Edited by:
Kirsty Winkley, King’s College London, United KingdomReviewed by:
Bogdan Timar, Victor Babes University of Medicine and Pharmacy, RomaniaAndrea Lukács, University of Miskolc, Hungary
Copyright © 2022 Schmitt, Kulzer, Ehrmann, Haak and Hermanns. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Schmitt, c2NobWl0dEBkaWFiZXRlcy16ZW50cnVtLmRl
 Andreas Schmitt
Andreas Schmitt Bernhard Kulzer1,2,3
Bernhard Kulzer1,2,3 Dominic Ehrmann
Dominic Ehrmann Norbert Hermanns
Norbert Hermanns