
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cancer Control Soc., 05 January 2024
Sec. Social Determinants in Cancer
Volume 1 - 2023 | https://doi.org/10.3389/fcacs.2023.1330410
Background: With the increasing number of cancer survivors in the US, survivorship care plans (SCP) have been promoted to improve survivorship outcomes for cancer patients. Few studies have assessed if the receipt of SCPs differs by race/ethnicity. This study evaluated if racial/ethnic disparities exist in SCP receipt among female cancer survivors living in Maryland.
Methods: Survey data were analyzed for 1,353 non-Hispanic white (NHW) and 280 non-Hispanic Black (NHB) women with a self-reported history of cancer living in Maryland who completed the Maryland Behavioral Risk Factor Surveillance Survey (BRFSS) between 2011 and 2020. Multivariable logistic regression models were used to estimate prevalence odds ratios (PORs) and 95% confidence intervals (CI) for SCP receipt by race/ethnicity. Models were further stratified by demographic, cancer-related, and lifestyle factors to examine effect modification.
Results: On average, survivors were 66.8 years of age at time of BRFSS survey and 53.5 years of age at time of cancer diagnosis. Compared with NHW survivors, NHB survivors reported higher odds of receiving a summary of cancer treatments (POR = 3.81, 95% CI: 2.27, 6.39), instructions from a doctor for follow-up check-ups (POR = 2.10, 95% CI: 1.00, 4.39), and written or printed instructions (POR = 4.74, 95% CI: 2.12, 10.61). Age at survey (<65 years vs. ≥65 years) (p-interaction term = 0.01) and income level (≤50k vs. >50k) (p-interaction term = 0.04) significantly modified the relationship between race/ethnicity and receiving SCPs.
Conclusion: Our findings indicate that NHB female cancer survivors in Maryland are more likely to receive SCP information compared to NHW survivors and this association is significantly modified by age at survey and income level. More research is needed at the patient-provider level to gain a better understanding of the impact of SCP delivery to minority cancer populations.
In 2022, the total number of cancer survivors in the US was 18 million (1) and is projected to increase to 26 million by 2040 (2). This growing population might be due to the aging population, improvement in cancer screening, early detection, and development of new and/or more effective treatments (3). In 2022, the majority of cancer survivors in the US were female survivors (54% of all cancer survivors), with breast cancer as the most prevalent cancer (42% of all female cancer survivors) (1). Women are also more likely to be long-term cancer survivors (4).
In 2006, the Institute of Medicine (IOM) published a report to promote survivorship care plans (SCPs) among patients completing primary treatment, and SCPs were defined as a combination of written treatment summaries and follow-up instructions (5). SCPs summarize information about cancer diagnosis and treatment, provide follow-up or check-up recommendations and give some general wellness tips (6). According to the cancer program standards published by the American College of Surgeon's Commission on Cancer, all cancer centers should implement SCPs (7). The potential benefits of receiving SCPs for cancer survivors include increasing contact with primary care physicians and improved patient-provider communication (8–10). Additionally, a study of 3,191 US adult cancer survivors who responded to the 2010 Behavioral Risk Factor Surveillance Survey (BRFSS) survey indicated that patients benefit from SCPs for their overall psychological wellbeing, even after 5 years, as patients who received SCP were three times less likely to experience current symptoms of depression (11).
While studies have been conducted to understand disparities in the use and impact of SCPs (12–14), there remains a knowledge gap in assessing racial/ethnic disparities in SCP receipt and utilization. Studies have examined disparities in SCP receipt among female cancer survivors using US BRFSS data (15–17). One study focusing on Alabama, Georgia, and Mississippi found that the likelihood of receiving follow-up instructions for survivors who were younger, with higher education and income levels was significantly higher compared to survivors who were older, with lower education and income levels (15). Furthermore, Timsina et al. (16) found that 7,061 cancer survivors who responded to 2016 BRFSS survey with lower educational levels, widowed/divorced/separated marital status, and without insurance were less likely to receive SCPs from their healthcare providers. Wu et al. (17) evaluated predictors associated with receiving follow-up instructions among 954 women with breast cancer and 492 women with gynecologic cancers, and the researchers found that breast cancer patients with lower income levels were less likely to receive follow-up instructions, but there was no association observed between race/ethnicity and follow-up instructions received.
In Maryland, about 30% of the total population is Black/African American (AA), which is much higher than the proportion in the United States (14%) (18). Approximately 16,000 women living in Maryland were diagnosed with cancer in 2018 and one-third of all these reported cases were AA (19). Therefore, Maryland could serve as a resource to study racial/ethnic disparities in SCP-related outcomes among female cancer survivors (20). In this study, we utilized Maryland BRFSS data collected from 2010 to 2020 to investigate racial/ethnic disparities in SCP receipt.
The BRFSS, conducted by the Centers for Diseases Control and Prevention (CDC), is an ongoing nationwide telephone survey system that has collected data on health-related behaviors, health status, and healthcare access since 1984 (21). The Maryland BRFSS samples about 15,000 non-institutionalized Maryland residents aged 18 and older per year, and the survey is conducted under guidance from the CDC but also includes state-specific modules in the questionnaires (22).
A total of 81,025 people who completed the Maryland BRFSS survey in 2011, 2013, 2015, 2017, 2019, and 2020 were identified. Figure 1 shows exclusion and inclusion criteria. People without valid cancer survivorship module records (N = 75,793) were excluded. Participants who were male (N = 2,096) or not NHB or NHW (N = 85) were also excluded. BRFSS respondents who reported having more than one type of cancer (N = 564) after first cancer diagnosis, did not report type of cancer diagnosed (N = 94), or had skin cancer except for melanoma (N = 760) were not eligible for our study. After these exclusions, a total of 1,633 female cancer survivors were included in the current analysis (1,353 NHW and 280 NHB).

Figure 1. Flowchart describing the selection process of the study population (N = 1,633) from the BRFSS participants (N = 81,025).
The exposure of interest in this study was self-reported race/ethnicity, which was categorized as White, Black/AA, American Indian or Alaskan, Asian, Other, Don't know/Not sure, and Refused. The participants were also asked if they were Hispanic or Latino. For analysis, we recategorized race/ethnicity as NHW and NHB/AA and excluded other categories due to small sample size. NHW women were designated as the reference group for the analysis.
In 2011, 2013, 2015, 2017, 2019, and 2020, the Maryland BRFSS included optional cancer survivorship modules in the BRFSS survey. If respondents answered “Yes” to the question “Ever told had any types of cancer,” they would be asked questions related to cancer survivorship. We assessed survivorship care experiences using the answers to following questions:
• “Did any doctor, nurse, or other health professional EVER give you a written summary of all the cancer treatments that you received?”
• “Have you ever received instructions from a doctor, nurse, or other health professional about where you should return or who you should see for routine cancer check-ups after completing your treatment for cancer?”
• “Were these instructions written down or printed on paper for you?”
Then the answers were recategorized as binary variables (“Yes” or “No”) for each question.
Two-sample t-tests and chi-squared tests were used to determine if the characteristics were equally distributed between NHW and NHB groups for continuous and categorical variables, respectively.
Weighted crude and multivariable logistic regression models were constructed to analyze the associations between race/ethnicity and outcomes. Fully adjusted models accounted for the following covariates (age at survey, age at cancer diagnosis, time since cancer diagnosis, education level, income level, marital status, home ownership, smoking status, alcohol consumption in last week, exercise in past 30 days, general health, diabetes, and obesity). Covariates were self-reported, and some variables were recategorized for ease of interpretation and statistical power (see Supplementary Table 1).
Stratified analyses were exploratory and intended to examine if specific covariates modified the relationship between race/ethnicity and SCP outcomes. Stratification variables were categorized and included: cancer type, with breast cancer most reported (breast cancer or other), BRFSS survey age based on Medicare eligibility (23) (<65 or ≥ 65 years), age at diagnosis based on early onset cancer diagnosis status (24) (<50 or ≥ 50 years), time since cancer diagnosis based on long-term cancer survivor status (25) (<5 or ≥5 years), education (less than college or college graduate and higher), income based on the median income the average US resident (26) (≤$50k or >$50k), body mass index (BMI) (obese or not obese), and survey year (≤2015 or >2015). Interaction terms between race/ethnicity and stratification variables were modeled to examine statistical interactions.
As there could be differences in SCP receipt between survivors with ongoing cancer treatment and survivors who have completed treatment, a sensitivity analysis was conducted by excluding BRFSS participants who responded “Yes” to the question “Are you currently receiving treatment for cancer?” (N = 169). People who refused to answer this question were also excluded from this analysis (N = 20). Multivariate logistic regression was utilized to examine the association between race/ethnicity and SCP receipt among this subpopulation.
All statistical tests were two-sided, and statistically significant main effects and interactions were indicated by p < 0.05. All analyses were conducted using R version 4.2.1 (survey package 4.1-1) to account for BRFSS survey weights.
Table 1 describes the survey characteristics of the study sample overall and by race/ethnicity. About 83% of the sample identified as NHW race/ethnicity. The average age of the female cancer survivors at BRFSS survey was 66.8 years: 67.3 years for NHW and 64.0 years for NHB women (p < 0.001). For general health status, NHW women were more likely to respond as “Very Good” (33.2%) compared with NHB women (20.4%), and more NHB survivors reported “Fair” (28.9%) than NHW survivors (15.7%) (p < 0.001). As for obesity status, 29.9% of all females were obese, with a significantly different distribution by racial/ethnic groups (NHW: 27.1% vs. NHB: 43.9%, p < 0.001). Also, more NHB females reported they have been diagnosed as having diabetes (31.1%) compared with NHW females (16.1%) (p < 0.001).
Table 1 also shows the responses overall and by race/ethnicity for the cancer related BRFSS modules. On average, women were 53.5 years old (SD = 15.6 years) when they were diagnosed with cancer with no significant difference between NHW and NHB female survivors (53.6 years vs. 52.7 years, p = 0.4). Survivors reported an average of 13.0 years (SD = 12.2 years) since cancer diagnosis, and NHW survivors (13.4 years) reported a significantly longer time from cancer diagnosis than NHB survivors (11.1 years) (p = 0.002). Breast cancer was the most common type of cancer reported, and this prevalence was higher among NHW survivors (61.8%) than NHB survivors (45.0%) (p < 0.001). Overall, 32.8% of the survivors reported that they received a summary of cancer treatments received. The proportion was much higher among NHB (48.9%) than NHW participants (29.4%) (p < 0.001). Among all survivors, 60.3% reported receiving instructions from a doctor for follow-up check-ups, and NHB survivors (64.6%) reported such experiences significantly more than NHW survivors (59.3%) (p = 0.006). Among people who received instructions for follow-up check-ups, 40.4% of them reported “Yes” they were printed/written, and this proportion differed between NHB women (54.3%) and NHW women (37.5%) (p < 0.001).
Table 2 presents the results from crude and fully adjusted models between race/ethnicity and SCP related outcomes. For NHB female cancer survivors living in Maryland, they were more likely to receive a summary of cancer treatments (POR = 3.14, 95% CI: 1.94, 5.09), instructions from a doctor for follow-up check-ups (POR = 2.09, 95% CI: 1.08, 4.04), and written or printed instructions (POR = 3.84, 95% CI: 1.92, 7.69). After adjusting for covariates, NHB cancer survivors were more likely to receive a summary of cancer treatments (POR = 3.81, 95% CI: 2.27, 6.39), instructions from a doctor for follow-up check-ups (POR = 2.10, 95% CI: 1.00, 4.39), written or printed instructions (POR = 4.74, 95% CI: 2.12, 10.61).
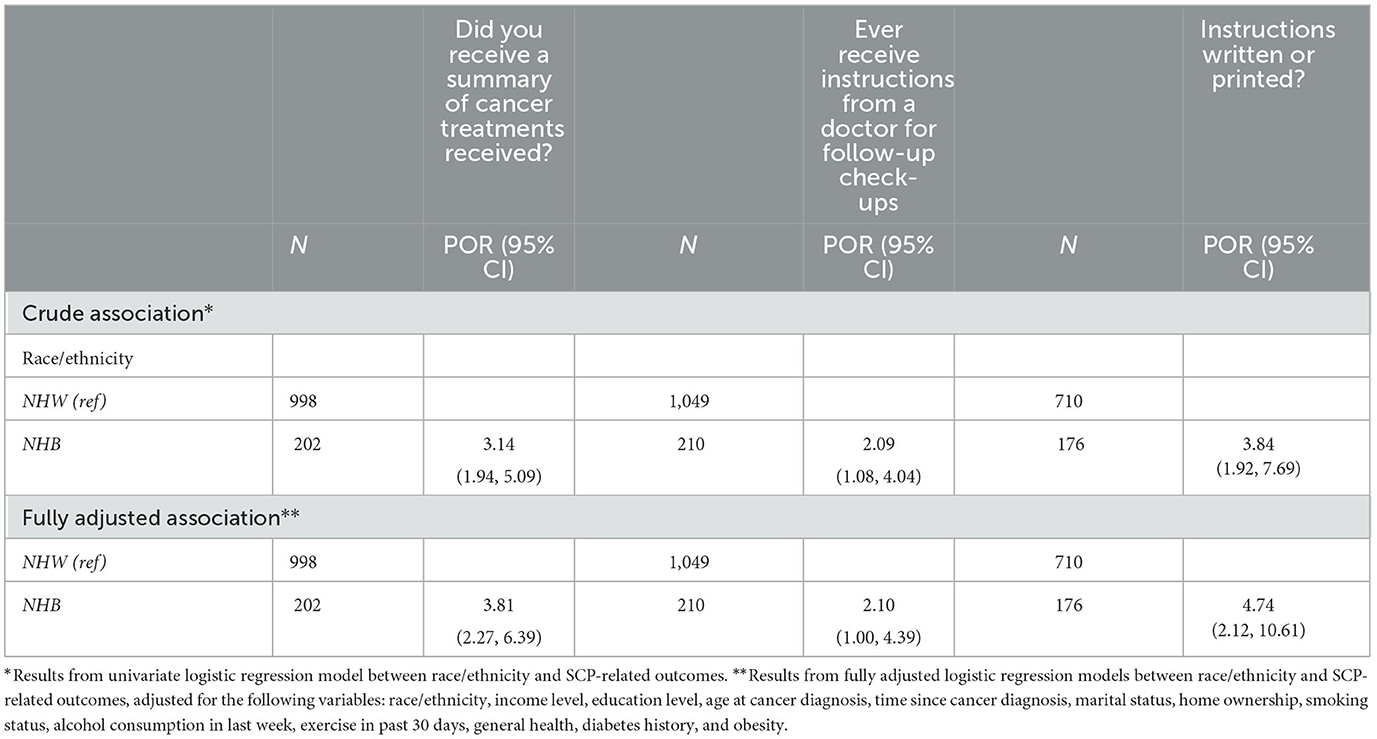
Table 2. Crude and fully adjusted associations between race/ethnicity and receipt of SCP outcomes among female cancer survivors, overall.
Table 3 illustrates the relationship between race/ethnicity and receiving SCPs by age at survey. For women who were ≥65 years of age at survey, NHB survivors had higher odds of receiving instructions than NHW survivors (POR = 4.77, 95% CI: 2.07, 11.00). However, the relationship was not significantly significant in the younger group (<65 years) (POR = 1.07, 95% CI: 0.42, 2.70) (p-interaction = 0.01).

Table 3. Associations between race/ethnicity and receipt of SCP outcomes stratified by age at survey, adjusted for all covariates.
Table 4 illustrates the association between race/ethnicity and SCP receipt stratified by household income adjusting for covariates. Among women with lower income (≤$50K), NHB survivors had almost 4 times the odds of receiving follow-up instructions than NHW survivors (POR = 4.74, 95% CI: 1.64, 13.72), but the relationship between race/ethnicity and this SCP outcome was not significant among women with higher income (>$50k) (POR = 0.85, 95% CI: 0.30, 2.38) (p-interaction = 0.04).
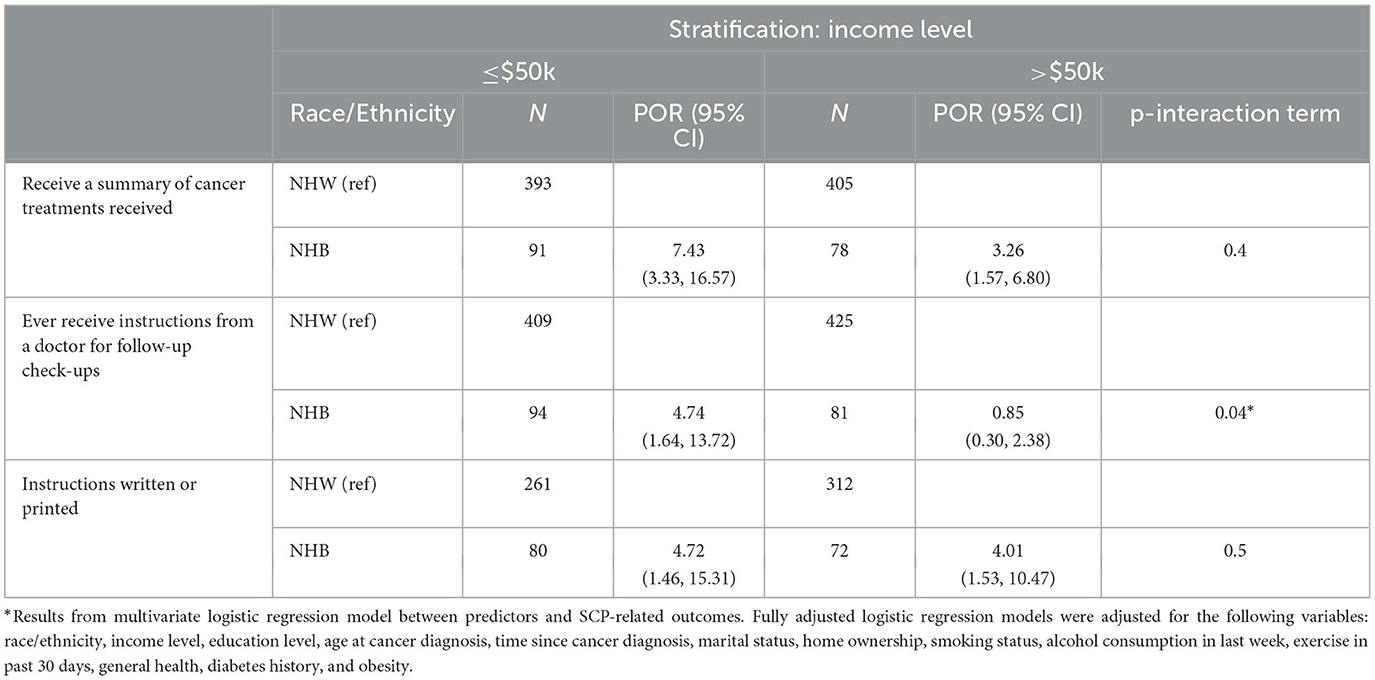
Table 4. Associations between race/ethnicity and receipt of SCP outcomes stratified by annual household income level, adjusted for covariates.
After adjusting for covariates, the association between race/ethnicity and receiving SCPs was not significantly modified by education, time since diagnosis, survey year, obesity, or cancer type (Supplementary Tables 2–6).
There were 1,444 survivors who completed treatment before their survey after excluding participants with ongoing cancer treatment or unknown status. As illustrated in Supplementary Table 7, adjusting for covariates, NHB female survivors remained more likely to receive treatment summaries (POR = 3.64, 95% CI: 2.19, 6.04) compared to NHW survivors, and were 5.21 times (95% CI: 2.51, 10.82) more likely to have their instructions written or printed compared to NHW survivors. The association between race/ethnicity and receiving follow-up instructions was no longer statistically significant.
We examined disparities in SCP receipt among female cancer survivors in Maryland. Overall, the percentage of female survivors reporting receiving a summary of cancer treatment was only about 30%, and the percentage of female survivors who have received printed or written instructions for follow-up check-ups was 40%, which indicated that delivery of SCP was still low in Maryland, despite the IOM mandated use of SCPs in 2006 (5). In a study using 2012 and 2014 BRFSS data, the rates of female survivors reporting receiving a summary of cancer treatment were somewhat similar to our findings, which were 34.0% in Alabama, 38.3% in Georgia, and 44.9% in Mississippi, while the proportions of participants who reported receiving follow-up care instructions were much higher in those three states, which were 63.6% in Alabama, 71.7% in Georgia, and 70.85% in Mississippi (15).
Our findings showed that NHB female survivors were more likely to receive SCPs compared to NHW survivors. Overall, the odds of receiving a cancer treatment summary among NHB survivors were 3.81 times the odds for NHW survivors. Also, NHB women had 2-fold odds of receiving instructions from a doctor for follow-up check-ups and almost 5-fold odds of receiving written or printed instructions in comparison with NHW survivors. In stratified analysis, we found that older NHB women were more likely to report receiving follow-up instructions than NHW women, but the association was not significant in the younger subgroup. Additionally, the odds of receiving follow-up instructions for NHB women with household lower income were more than 4 times the odds for NHW women, while the association might be inverse with higher income.
Although some studies have investigated disparities in SCP receipt (15, 16), there are few studies focusing on racial/ethnic disparities in receiving SCPs in the US. Researchers have highlighted the importance of promoting adherence to care guidelines among minority and underserved patient populations. A review of 50 studies focusing on SCPs indicated that SCPs could have a positive influence on self-reported adherence to medical recommendations among cancer survivors (27). Another study conducted by Shay et al. (28) using BRFSS data demonstrated that receiving SCPs was associated with health behaviors, such as having a recent medical appointment, exercise in the past month, non-smoking status, and up-to-date mammography among 1,855 cancer survivors. Therefore, SCP receipt was expected to improve adherence to medical recommendations, thus improving survival outcomes.
Our study may be one of the first studies to observe that NHB female survivors are more likely to receive SCPs than NHW survivors using BRFSS data. To the best of our knowledge, only a few other population-based studies have found a significant positive association between racial/ethnic minorities and receipt of SCPs, in comparison with NHW survivors. One study using the National Health Interview Survey (NHIS) also found that NHB cancer survivors had over three times the odds of receiving both a written treatment summary and written advice, compared to NHW survivors (29). Sabatino et al. (30) also utilized the NHIS data and obtained similar results, which demonstrated that NHB survivors were more likely to receive a treatment summary and written follow-up instructions. Another study conducted in California found that non-White survivors had higher odds of receiving a written summary of received treatments (31).
Our findings among NHB female cancer survivors and SCP receipt could be attributed to several factors. There has been research indicating that there are racial/ethnic disparities in the co-occurrence of chronic health conditions between NHB and NHW cancer survivors (32, 33). Therefore, it has been hypothesized that the potential reason for these positive associations among racial/ethnic minority cancer survivors and receipt of SCPs could be that NHB cancer survivors might have more comorbidities, so they are more likely to receive SCPs from their physicians. Lastly, NHB patients might have health providers that are more likely to provide SCPs.
NHB women who were ≥65 years at survey were almost five times more likely to report receiving instructions from a doctor for follow-up check-ups than NHW counterparts, although this association was not significant among survivors <65 years at survey. We hypothesize that physicians might tend to provide instructions for follow-up check-ups to older NHB survivors as they would need more check-ups to avoid experiencing severe outcomes. Interestingly, we did not observe a significant interaction by early onset of cancer diagnosis. Additional research by age should be conducted in this field to determine the impact of age and Medicare coverage.
We also found household income to be a significant effect modifier between race/ethnicity and receiving follow-up instructions. NHB race and lower socioeconomic status were studied among cancer patients to be related to higher nonadherence to medication, treatment and follow-up visits and delayed diagnosis and treatment (34–38). In research focusing on low-income females with breast cancer living in California, the researchers found that AAs had 3.55 times the odds of diagnostic delay than Caucasian patients (38), which could lead to delayed treatment and worse health outcomes. It is possible that physicians would provide more detailed follow-up instructions to patients who were more likely to be non-adherent to follow-up appointments and with worse health status or prognosis. Further research could determine if physicians providing SCPs have more targeted approaches for socioeconomically disadvantaged patients.
A major strength of this study is utilization of Maryland BRFSS data. The Maryland BRFSS questionnaires have included the cancer survivorship module for over two decades; therefore, the SCP outcomes of interest could be evaluated over time. Our study has some limitations. Cancer history and SCP outcomes were self-reported. The BRFSS did not collect clinical information or medical records to confirm diagnoses or collect stage and treatments. Women who have advanced stage, poor prognostic factors, and more aggressive treatments may be more likely to receive SCPs due to them being at-risk of treatment-related side effects and risk of recurrence; and future studies of SCPs should account for these factors particularly among Black women due to these factors being more prevalent among them (39). People who responded to the BRFSS survey may be more likely to be healthier survivors with healthcare coverage, as almost 75% of all participants reported “Good” or better for their overall health status and very few women (n < 50) reported that did not have healthcare insurance. Therefore, our results might be less generalizable to people with worse health conditions and limited healthcare coverage. The BRFSS did not assess the quality of SCP. Lastly, other racial/ethnic groups could not be adequately evaluated in our analysis because few participants identified themselves as Asian, American Indian, or Alaskan, or Hispanic.
Overall, race/ethnicity was associated with SCP receipt among Maryland female cancer survivors. NHB female cancer survivors were more likely to receive SCPs than NHW survivors. Additionally, household income and age at survey were observed to be significant effect modifiers for the relationship between race/ethnicity and SCP receipt. More research is needed at the patient-provider level to gain a better understanding of the impact of SCP delivery to minority cancer populations. Future studies could also evaluate the impact of SCP implementation on patient health outcomes specifically among minority populations to inform strategies to reduce disparities in cancer outcomes.
The data analyzed in this study is subject to the following licenses/restrictions: This publication utilizes data provided by the Maryland Department of Health, Maryland Behavioral Risk Factor Surveillance System Program; collected under guidance of the Centers for Disease Control and Prevention. Requests to access these datasets should be directed to the Maryland BRFSS program at bWRoLmJyZnNzQG1hcnlsYW5kLmdvdg==.
The studies involving humans were approved by Maryland Department of Health Institutional Review Board (IRB) and deemed not human subjects research by Johns Hopkins Bloomberg School of Public IRB. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
MJ: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MRJ: Supervision, Writing – original draft, Writing – review & editing. AC: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. AC was funded by the American Cancer Society MRSG-19-010-01-CPHPS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This publication utilizes data provided by the Maryland Department of Health, Maryland Behavioral Risk Factor Surveillance System Program, collected under guidance of the Centers for Disease Control and Prevention, and analyzed by MJ. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Maryland Department of Health or the Centers for Disease Control and Prevention.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcacs.2023.1330410/full#supplementary-material
1. American Cancer Society. Cancer Treatment and Survivorship Facts and Figures 2022-2024. Washington, DC: American Cancer Society (2022).
2. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and co-morbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. (2016) 25:1029–36. doi: 10.1158/1055-9965.EPI-16-0133
3. Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. (2016) 66:271–89. doi: 10.3322/caac.21349
4. Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. (2011) 20:1629–37. doi: 10.1158/1055-9965.EPI-11-0246
5. Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. England: National Academies Press (2006).
6. CDC. Cancer Survivorship Care Plans. Available online at: https://www.cdc.gov/cancer/survivors/life-after-cancer/survivorship-care-plans.htm#:~:text=A%20survivorship%20care%20plan%20is,and%20ideas%20for%20staying%20healthy (accessed April 30, 2023).
7. American College of Surgeons Commission on Cancer. Cancer Program Standards: Ensuring Patient-Centered Care. Washington, DC: American College of Surgeons Commission on Cancer (2011).
8. Nicolaije KAH, Ezendam NPM, Vos MC, Pijnenborg JMA, Boll D, Boss EA, et al. Impact of an automatically generated cancer survivorship care plan on patient-reported outcomes in routine clinical practice: longitudinal outcomes of a pragmatic, cluster randomized trial. J Clin Oncol. (2015) 33:3550–9. doi: 10.1200/JCO.2014.60.3399
9. Chrischilles EA, McDowell BD, Rubenstein L, Charlton M, Pendergast J, Juarez GY, et al. Survivorship care planning and its influence on long-term patient-reported outcomes among colorectal and lung cancer survivors: the CanCORS disease-free survivor follow-up study. J Cancer Surv. (2015) 9:269–78. doi: 10.1007/s11764-014-0406-y
10. Blinder VS, Norris VW, Peacock NW, Griggs JJ, Harrington DP, Moore A, et al. Patient perspectives on breast cancer treatment plan and summary documents in community oncology care. Cancer. (2013) 119:164–72. doi: 10.1002/cncr.27856
11. Oancea SC, Cheruvu VK. Psychological distress among adult cancer survivors: importance of survivorship care plan. Supp Care Cancer. (2016) 24:4523–31. doi: 10.1007/s00520-016-3291-2
12. Blaauwbroek R, Tuinier W, Meyboom-de Jong B, Kamps WA, Postma A. Shared care by paediatric oncologists and family doctors for long-term follow-up of adult childhood cancer survivors: a pilot study. Lancet Oncol. (2008) 9:232–8. doi: 10.1016/S1470-2045(08)70034-2
13. Grunfeld E, Levine MN, Julian JA, Coyle D, Szechtman B, Mirsky D, et al. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol. (2006) 24:848–55. doi: 10.1200/JCO.2005.03.2235
14. Boekhout AH, Maunsell E, Pond GR, Julian JA, Coyle D, Levine MN, et al. A survivorship care plan for breast cancer survivors: extended results of a randomized clinical trial. J Cancer Surv. (2015) 9:683–91. doi: 10.1007/s11764-015-0443-1
15. Desmond RA, Jackson BE, Waterbor JW. Disparities in cancer survivorship indicators in the deep south based on BRFSS data: recommendations for survivorship care plans. South Med J. (2017) 110:181–7. doi: 10.14423/SMJ.0000000000000617
16. Timsina LR, Zarzaur B, Haggstrom DA, Jenkins PC, Lustberg M, Obeng-Gyasi S. Dissemination of cancer survivorship care plans: who is being left out? Supp Care Cancer. (2021) 29:4295–302. doi: 10.1007/s00520-020-05915-x
17. Wu J, Blair J, Izevbigie OC, Wright NC, Arend RC. Disparities in receipt of follow-up care instructions among female adult cancer survivors: results from a national survey. Gynecol Oncol. (2018) 150:494–500. doi: 10.1016/j.ygyno.2018.06.024
18. United States Department of Housing and Urban Development. Race. 2020 Census Data. (2020). Available online at: https://data.census.gov/ (accessed April 30, 2023).
19. Maryland Health Department. 2021 Cancer Data-Cigarette Restitution Fund Program. (2021). Available online at: https://health.maryland.gov/phpa/cancer/Documents/2021CRF CancerReport_FINAL.pdf (accessed April 30, 2023).
20. Connor AE, Kaur M, Sheng JY, Hayes JH. Racial disparities in mortality outcomes among women diagnosed with breast cancer in Maryland: Impact of cardiovascular disease and clinical characteristics. Cancer. (2022) 128:727–36. doi: 10.1002/cncr.33889
21. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. (2022). Available online at: https://www.cdc.gov/brfss/index.html (accessed April 30, 2023).
22. Maryland Department of Health. Behavioral Risk Factor Surveillance System. Maryland Department of Health Surveys and Reports. (2022). Available online at: https://health.maryland.gov/phpa/ccdpc/reports/pages/brfss.aspx (accessed April 30, 2023).
23. United States Government. What's Medicare? Available online at: https://www.medicare.gov/what-medicare-covers/yourmedicare-coverage-choices/whats-medicare (accessed April 30, 2023).
24. Robbins HA, Engels EA, Pfeiffer RM, Shiels MS. Age at Cancer Diagnosis for Blacks Compared With Whites in the United States. JNCI. (2015)107:489. doi: 10.1093/jnci/dju489
25. Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. (2002) 360:1131–5. doi: 10.1016/S0140-6736(02)11199-8
26. United States Census Bureau. Income and Poverty in the United States: 2020. Suitland, MD: United States Census Bureau (2020).
27. Hill RE, Wakefield CE, Cohn RJ, Faredell JE, Brierley ME, Kothe E, et al. Survivorship care plans in cancer: a meta-analysis and systematic review of care plan outcomes. Oncologist. (2020) 25:e351–72. doi: 10.1634/theoncologist.2019-0184
28. Shay LA, Schmidt S, Dioun SI, Grimes A, Embry L. Receipt of a survivorship care plan and self-reported health behaviors among cancer survivors. J Cancer Surv. (2019) 13:180–6. doi: 10.1007/s11764-019-00740-6
29. Hinyard L, Wirth LS. Race is a strong predictor of receipt of a written survivorship care plan: results from the national health interview survey. J Commun Health. (2017) 42:1156–62. doi: 10.1007/s10900-017-0365-0
30. Sabatino SA, Thompson TD, Smith JL, Rowland JH, Forsythe LP, Pollack L, et al. Receipt of cancer treatment summaries and follow-up instructions among adult cancer survivors: results from a national survey. J Cancer Surviv. (2013) 7:32–43. doi: 10.1007/s11764-012-0242-x
31. Boehmer U, Potter J, Clark MA, Ozonoff A, Ceballos RM, Winter M, et al. Neighborhood characteristics and colorectal cancer survivors' quality of care. Health Equity. (2019) 3:619–27. doi: 10.1089/heq.2019.0062
32. Tammemagi CM. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. (2005) 294:1765. doi: 10.1001/jama.294.14.1765
33. Ashing K, Rosales M, Lai L, Hurria A. Occurrence of comorbidities among African-American and Latina breast cancer survivors. J Cancer Surv. (2014) 8:312–8. doi: 10.1007/s11764-014-0342-x
34. Zheng C, Chagpar AB. Contribution of cost to treatment nonadherence in the US breast cancer survivors: a population-based analysis. Breast Cancer Res Treat. (2022) 192:369–73. doi: 10.1007/s10549-022-06510-w
35. Costas-Muniz R, Leng J, Aragones A, Ramirez J, Roberts N, Mujawar MI, et al. Association of socioeconomic and practical unmet needs with self-reported nonadherence to cancer treatment appointments in low-income Latino and Black cancer patients. Ethn Health. (2016) 21:118–28. doi: 10.1080/13557858.2015.1034658
36. Ashing-Giwa KT, Gonzalez P, Lim J-W, Chung C, Paz B, Somlo G, et al. Diagnostic and therapeutic delays among a multiethnic sample of breast and cervical cancer survivors. Cancer. (2010) 116:3195–204. doi: 10.1002/cncr.25060
37. Elmore JG, Nakano CY, Linden HM, Reisch LM, Ayanian JZ, Larson EB. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care. (2005) 43:141–8. doi: 10.1097/00005650-200502000-00007
38. Maly RC, Leake B, Mojica CM, Liu Y, Diamant AL, Thind A. What influences diagnostic delay in low-income women with breast cancer? J Womens Health. (2011) 20:1017–23. doi: 10.1089/jwh.2010.2105
Keywords: women's health, race, cancer survivors, income, survivorship care
Citation: Jin M, Jones MR and Connor AE (2024) Racial disparities in receipt of survivorship care plans among female cancer survivors in Maryland. Front. Cancer Control Soc. 1:1330410. doi: 10.3389/fcacs.2023.1330410
Received: 30 October 2023; Accepted: 08 December 2023;
Published: 05 January 2024.
Edited by:
Jessica Yasmine Islam, Moffitt Cancer Center, United StatesReviewed by:
Vinit Nalawade, Duke University Health System, United StatesCopyright © 2024 Jin, Jones and Connor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Avonne E. Connor, YWNvbm5vcjhAamh1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.