- 1Finch Research Network, Cincinnatus, NY, United States
- 2Department of Biological Sciences, University of Pittsburgh, Pittsburgh, PA, United States
- 3K. Lisa Yang Center for Conservation Bioacoustics, Cornell Lab of Ornithology, Cornell University, Ithaca, NY, United States
- 4Chemnitz University of Technology, Chemnitz, Germany
- 5Wisconsin Department of Natural Resources, Bureau of Natural Heritage Conservation, Ashland, WI, United States
- 6Pennsylvania Natural Heritage Program, Western Pennsylvania Conservancy, Pittsburgh, PA, United States
Red crossbills (Loxia curvirostra) are the archetypal example of a taxon with high infraspecific diversity in traits including bill size and especially vocal characteristics. Currently, at least 11 different call types in North America have been recognized. We hypothesize that a variant call within type 10 has been overlooked and is a distinct type. Principal component analysis showed that the inverted “V” of these calls is consistently and demonstrably different from similar calls of birds previously categorized as Type 10 variants. We argue these calls should be treated separately as a distinct type, Type 12. Due to increasingly available recordings of crossbills gathered and archived into public databases by birders, our analyses reveal that this call type is predominantly distributed across northeastern North America. Although crossbill types do not always map to formerly described subspecies, we also argue that Type 12 likely matches the historically described L. c. neogaea, the “old Northeastern subspecies”.
1 Introduction
The crossbill genus Loxia represents enigmatic but widespread birds that inhabit much of the Northern Hemisphere. Across this range, two species of wing-barred crossbills (L. leucoptera, L. megaplaga) and four species of red crossbills (L. curvirostra, L. sinesciuris, L. scotica, L. pytyopsittacus) have evolved bills with crossed mandibles and varying sizes suited for prying open the scales of different conifer cones to feed on the seeds therein (del Hoyo, 2020). Given the ephemeral and erratic pattern of conifer masting, some crossbill species are well-known for their nomadic behavior and irruptions, where they travel far beyond their expected range in search of food (Benkman, 2020; Benkman and Young, 2020).
During the late 1980s and early 1990s, the red crossbill (Loxia curvirostra) in the United States, Canada, and Mexico was recognized as consisting of several different “call types” (Groth, 1993), or ecotypes based on flight calls. Although some crossbill vocalizations are variable, flight calls have been found to reliably identify individuals as belonging to a particular call type (Groth, 1988, 1993; Benkman, 1993). Call types also correlate with slight differences in bill depth morphology which correspond to optimal feeding on preferred sized cones (Benkman, 1993; Parchman and Benkman, 2002). Groth (1988) noted that call types can display “species-like” behavior, noting that Types 1 and 2 bred side-by-side in Virginia but did not interbreed. Another study showed that females presented with males of Type 2 or 9 preferred to associate males of their own call type (Snowberg and Benkman, 2007) and Type 9 was subsequently promoted to the species level (Cassia crossbill, L. sinesciuris) due to differences in genetics, vocalizations, morphology, and a limited range (Benkman et al., 2009). Although overall the situation is complex and genetic differences between types may be small (Lovett, 2016), there is certainly more work to be done on understanding call types, their interactions, and the degree to which these populations should be considered cryptic or incipient species (Groth, 1988; Smith and Benkman, 2007; Benkman et al., 2022). Distinct flight calls are apparently a mechanism used to help to maintain differentiation in crossbills even in sympatry when flocks of red crossbill types leave their core ranges to roam the continent.
Over time, the number of recognized call types has expanded as researchers have analyzed sound recordings and other data gathered in the field, both in the Nearctic and Palearctic. As of this research, 11 call types range from Alaska to Newfoundland and south to Nicaragua in North America, and at ~20 types have been reported from the Palearctic (Martin et al., 2019).
In the period between 2012 and today, red crossbill recordings in the Macaulay Library archive increased by > 4000%, enabling much learning about the status and distribution of these birds. The impetus for the work in this paper began as we were engaged in audiospectographically classifying to type these recordings. This endeavor led to the identification of Type 11 red crossbills which inhabit Central America (Young and Spahr, 2017).
Analyses of crossbill flight call recordings over the last 60 years indicate noticeable variation in call structure, sometimes leading to the discovery of new types. For example, Irwin (2010) described Type 10 in the Pacific Northwest USA, which has a relatively restricted range and is most closely associated with Sitka spruce (Picea sitchensis) along the Pacific coast. Yet recordings from there and elsewhere across North America, especially the northeast, contained calls thought to be either odd variants of Type 10 or possibly an unrecognized call type. Such variants are present in recordings dating back to at least the 1960s.
These variant flight calls, which look like an inverted “V” in spectrograms (Figure 1A), appear distinct from typical Type 10 birds (Figure 1B). Looking back into historical recordings, a single bird making similar flight calls was recorded in Klamath County Oregon in August 1986 (aF497 in Groth, 1993, Figure 1C) and at the time was classified as Type 7, the most poorly understood type. In the early 2000s, several birds were recorded in Humbolt County California, (variants #30 through #33 in Irwin, 2010), then thought to be a rare variant of Type 10. In the last decade, research into red crossbill call types has gained momentum with the proliferation of smartphones, the relative ease of audiospectrographic analysis, and the popularization of centralized audio archives such as xeno-canto.org and ebird.org (Macaulay Library, 2024). As red crossbill recording has increased, so, too, has the detection of these unknown birds showing the inverted “V”-shaped spectra, especially in the northeast. In recent years, there has been wider recognition that these birds were not Type 10, for example, a dozen similar birds recorded in northern Wisconsin in 2017–2018 were provisionally labeled “eastern Type 10” (ML89997101 in Brady et al., 2019, Figure 1D).
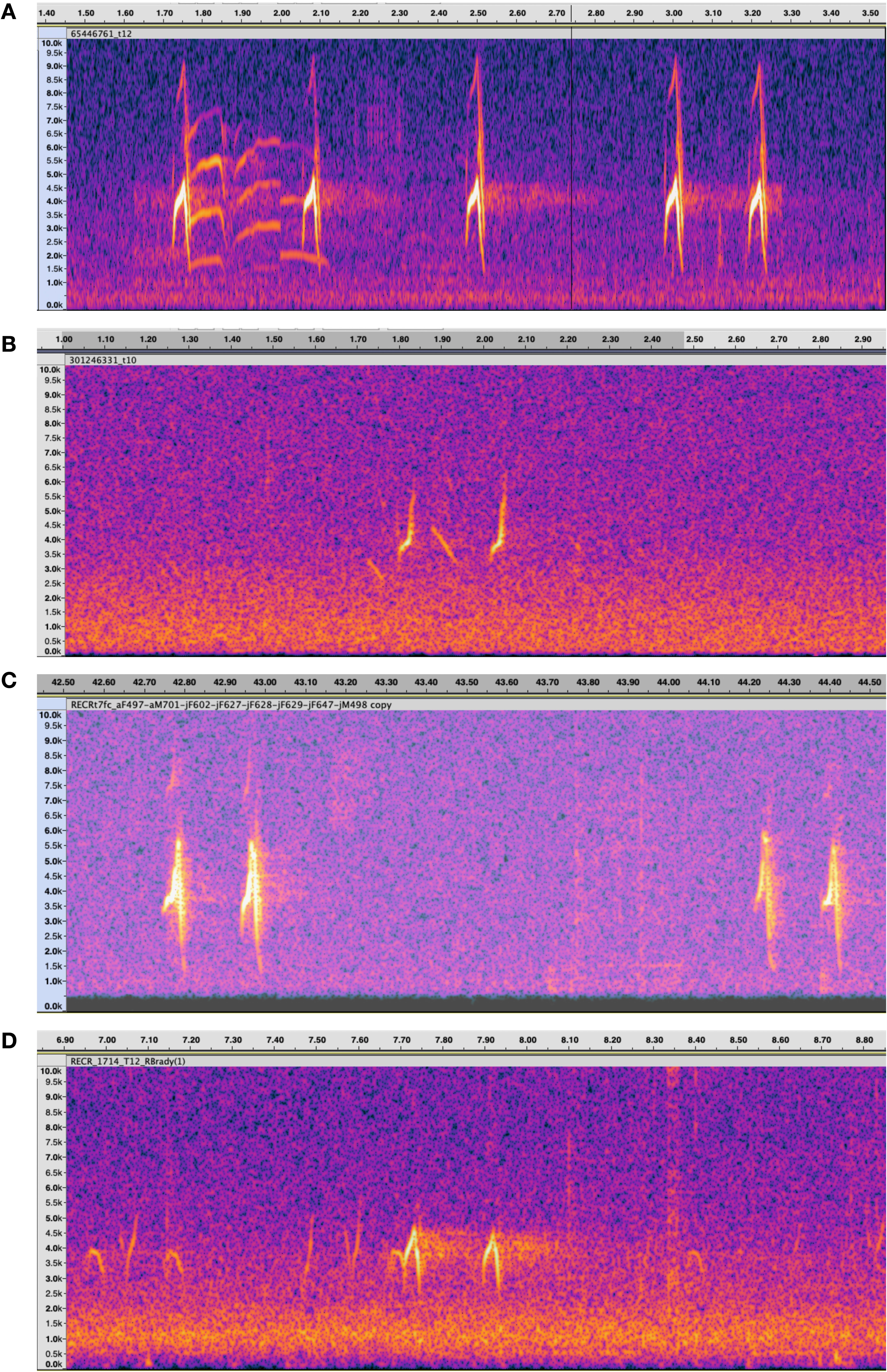
Figure 1. Spectrograms of red crossbill flight calls. Time axes are in seconds and frequency axes are in kilohertz. (A) Inverted V call, recorded in New York in 2017 (ML65446761). (B) Representative Type 10 from New Jersey in 2012 (ML301246331). (C) Recorded in Oregon in 1986 and classified at the time as Type 7 (Groth, 1993). (D) Recorded in Wisconsin in 2018 (ML89997101) and classified at the time as “eastern” Type 10 (Brady et al. 2019).
Literature reports indicate red crossbills were common across the northeastern U.S. and southern Canada approximately 100 years ago, but drastic overlogging of vast eastern white pine (Pinus strobus), eastern hemlock, (Tsuga canadensis), and red spruce (Picea rubens) forests during the late 1800s and early 1900s may have led to a decline in numbers or driven this population of crossbills from its historical core range (Dickerman, 1987; Russell et al., 1993). Griscom (1937) proposed subspecies neogaea for crossbills across this region, which was also endorsed by Dickerman (1987) who called the subspecies the “old Northeastern” red crossbill (Figure 2). Until recent decades, crossbills were considered rare to uncommon (though occasionally common locally) in the northeastern U.S. and southeastern Canada, with the most frequently recorded types 1, 2, and 3, and the birds up until now categorized as these Type 10 variants (Young, 2011; eBird, 2024).

Figure 2. All Type 12 red crossbill records reported to eBird (2024) including 3,876 records from 1,671 unique locations from January 2011 to February 2024. Dark blue points are from July and August 2023, highlighting the core range that crossbills typically retreat to in fall. Gold points include all other records. The northeastern U.S. and southern maritime provinces are clearly the core range for this type, with the mid-Atlantic, Appalachians, and Great Lakes regions getting use in some years. Hypothesized range boundaries of neogaea are approximated from the description of Griscom (1937) (green) and the map of Dickerman (1987) (brown).
Here, we hypothesize that these variant calls represent a distinct red crossbill call type that can be identified based on quantitative characteristics of its flight calls, which we term Type 12. We further hypothesize that this distinct call type is a good fit formerly proposed northeastern subspecies L. c. neogaea (Griscom, 1937). To evaluate our hypotheses, we compared call structure of recordings made across the northeastern U.S. with other birds classed as Type 10. We also use eBird data to describe the known range and compare the range of Type 12 to the hypothetical described and mapped range for neogaea (Griscom, 1937; Dickerman, 1987).
2 Methods
We focused the principal component analysis (PCA) on 57 flight call recordings from across North America from the Macaulay Library (2024). These recordings included 30 Type 10 and 27 already identified as Type 12. Type 12 was added to the eBird Taxonomy in 2022 (eBird, 2022) based on preliminary results of the work described here. Prior to the analysis described here, recordings were manually identified to type by the authors (MAY and TBS), along with thousands of other red crossbill recordings in Macaulay Library, based on audiospectrographic analysis, i.e. listening to the call while visually examining the spectrogram. To the ear, the flight call for Type 12 is significantly different than those of other North American red crossbill call types and can be described phonetically as a hard “kip kip”. Spectrographically the Type 12 call (Figures 1A, C, D), peaks over 4 kHz with a consistent downward component at the end. Type 10 (Irwin) flight calls are somewhat like the “whit” call of a least flycatcher (Empidonax minimus) call but with a sweet or pipping quality, and peaks over 5 kHz (Figure 1B). Type 10 calls can be termed as upslurred (rising) and Type 12 as overslurred (with a peak in the middle) (Pieplow, 2017). We selected the specific sound clips for this analysis because they had loud and high-quality examples of flight calls with minimal background noise and represented good variation across both call types. Type 10 was chosen for comparison to Type 12 because it is the type that most closely resembles it, and up to this point, these birds have been considered type 10 (e.g. variants #30 through #33 from Irwin, 2010).
For our analysis of these flight calls, we employed an innovative approach utilizing feature embeddings derived from a machine learning model. The application of machine learning to the study of bird vocalizations is growing (e.g., Yang et al., 2024). Within this framework, feature embeddings are vectors produced by an intermediate layer of a trained machine learning model (Stowell, 2022). These vectors capture abstract yet semantically significant features that extend beyond traditional human-engineered metrics such as note sequences or signal shapes. Despite their abstract nature, these features efficiently represent the input audio signal and can distinguish between different call types or dialects, which often vary only subtly. Additionally, feature embeddings have the capability to support cross-taxa classification, as they can generalize across various acoustic domains and events. The machine learning algorithm utilized in this study is BirdNET v2.3 (Kahl et al., 2021), a project leveraging machine learning, in particular deep neural networks, to identify over 6,500 common bird species worldwide. BirdNET feature embeddings have demonstrated effectiveness in taxonomically agnostic, fine-grained classification (Ghani et al., 2023; Kath et al., 2024; Williams et al., 2024). BirdNET, for example, is capable of separating various yellowhammer (Emberiza citrinella) dialects (Ghani et al., 2023), similar to what was performed for Type 12 in this paper.
We used the BirdNET-Analyzer GUI which is openly available online at GitHub (Kahl et al., 2024). BirdNET allows users to extract class scores (of bird species) or feature embeddings (which are independent of a specific class and thus contain information on the input audio). All the crossbill recording clips were stretched in time by a factor of 5 before being fed into the BirdNET model in order to meet the minimum required duration for the BirdNET model. We extracted feature vectors that BirdNET produced for each of our 57 recordings. The size of the embeddings vector was 420. During the process, each audio file was analyzed with BirdNET and the embedding results were saved in a .csv file which contained the embeddings vector values, which were then used to cluster the call types. These feature vectors were then fed into the standard PCA algorithm in scikit-learn, an open-source set of python tools (Pedregosa et al., 2011). One downside of using BirdNET feature embeddings is that they are not inherently human-interpretable. This lack of interpretability can make it challenging to understand and validate the specific features the model is using, and we cannot ascertain the exact feature of a spectrogram that is represented in each principal component. However, recent research has validated the quality of similar methods, confirming the ability of automated data-driven processes to capture and organize relevant latent features in underlying processes that are less apparent in traditional methods of quantifying audio data (Sainburg et al., 2019; Goffinet et al., 2021).
To evaluate whether Type 12 is a good fit for L. c. neogaea (Griscom, 1937), we plotted records that we manually identified as Type 12 and are currently in eBird (2024) to examine what is currently known about their range. We examined all records of Type 12, but we especially focused on July and August of a single year, to illustrate when crossbills tend to return to their “core” range of occupancy. We visually compared whether these were good fit to the hypothetical range boundaries of neogaea described by Griscom (1937) and drawn by Dickerman (1987) (Figure 2).
3 Results
Figure 3 shows a standard PCA plot—with component 1 on the X-axis and component 2 as the Y-axis. Types 10 and 12 are easily separated based on this analysis, with a clear demarcation between the types. Only 3 of 57 recordings differ in assignment between the PCA and the manual assignment by the authors, and those 3 had PC1 values very close to zero. The fraction of the variance explained by PC1 is 14.8%; the fraction explained by PC2 is 8.0%. These numbers are lower than one would expect from a standard PCA analysis, but we must remember that instead of using hand-selected inputs to the PCA analysis (e.g. call duration or mean frequency), we are using the embeddings from BirdNET. These embeddings are meant to capture the variability in birdsong in a broad sense and therefore are likely picking up on more variation in the calls than just the main spectrographic features that our eyes are drawn to.
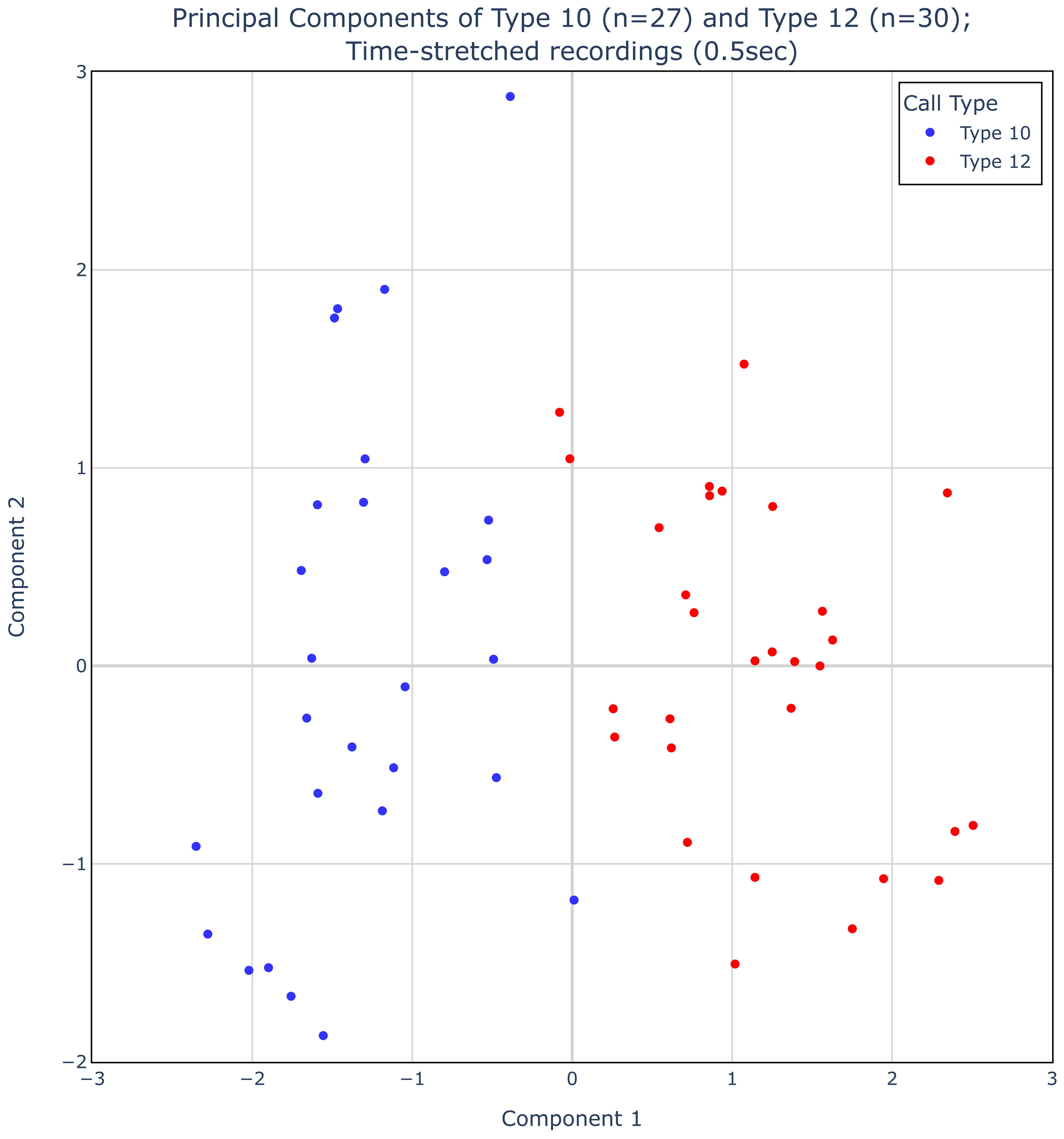
Figure 3. Principal component analysis of 27 Type 10 and 30 Type 12 recordings from the Macaulay Library. The two types separate into two unique clusters deliniated by a positive or negative component 1.
Despite the challenge of specifically assigning a feature embedding to a variable we can gain some insight into the characteristic of this first component by looking at the 57 spectrograms, ordered by their value of the first component (Figure 4). These spectrograms suggest that the first component of the PCA describes whether the tail of the spectrogram sweeps up or plunges down. There is slight overlap between these labeled groups; the three spectrograms with Component 1 values closest to zero are probably out of order. The best division between Type 10 and Type 12 in this analysis may simply be positive or negative values for PC 1.
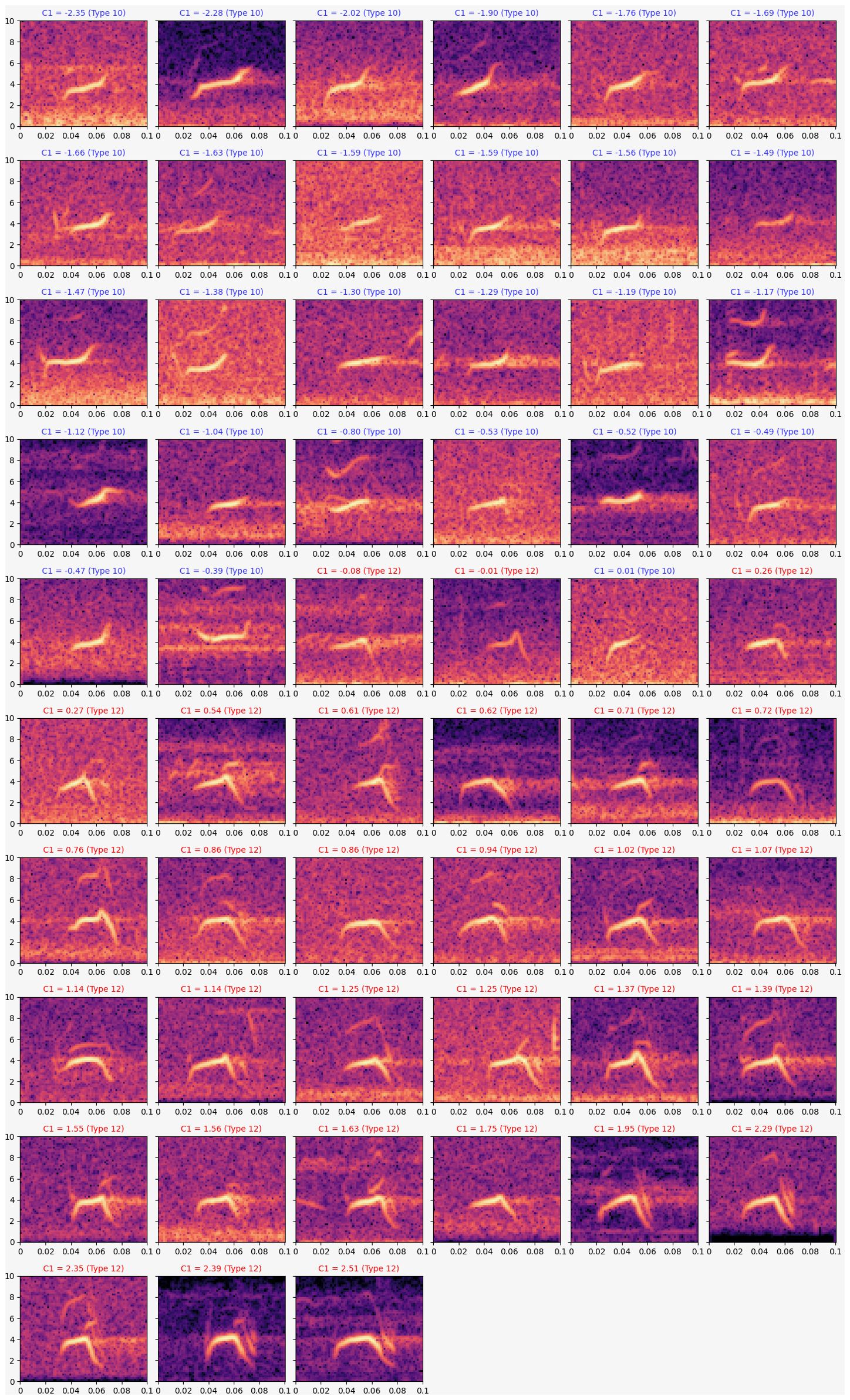
Figure 4. Spectrograms of Type 10 and Type 12 flight calls used for principal component analysis. Spectrograms are ordered by their value of component 1. Time axis is in seconds; Frequency axis is in kilohertz.
Records of Type 12 birds in the eBird and Macaulay Library archives produced a picture of their movements and locations throughout the year. Specifically, in late summer, when crossbills generally return to their primary core range, Type 12 largely overlaps the eastern range ranges mapped for neogaea (Figure 2; see Figure 5 for a generalized view). Birds were most commonly found in Massachusetts, New Hampshire, Maine, New York, Vermont, and Nova Scotia. Secondary range appears to be south to North Carolina and west to Minnesota, in some seasons. There were few records of Type 12 birds are occupying the northern and westernmost extents of the hypothesized neogaea range.
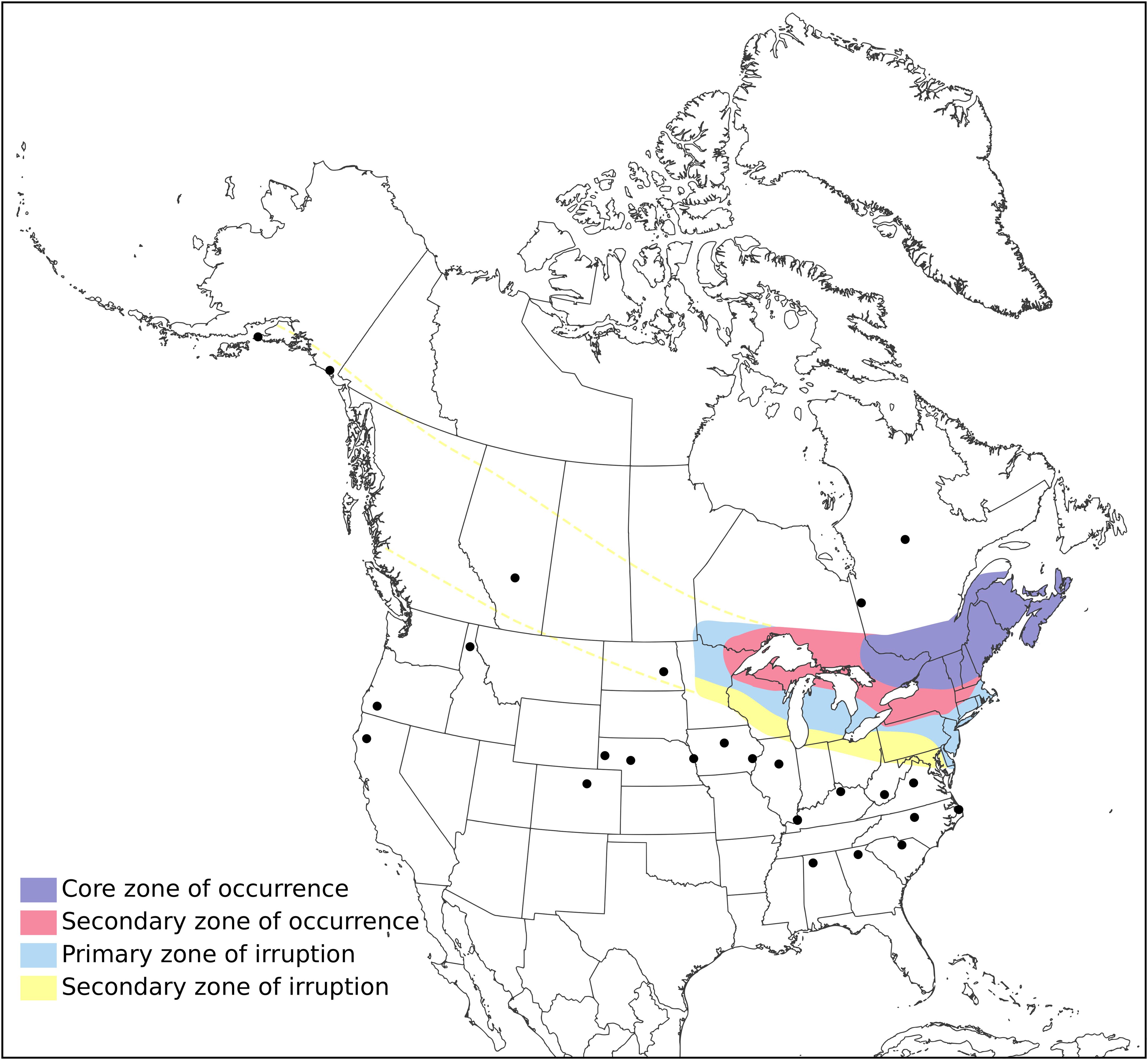
Figure 5. Hypothetical range for Type 12 red crossbill, based on known records from eBird, (2024). Core zone (purple): core area where crossbills found most years eating key conifers and breeding. Secondary zone of occurrence (pink): crossbills present in less numbers most years and small numbers breed. Primary zone of irruption (light blue): crossbills flee to these areas when key conifers in their core zone fail, may stay and nest rarely in small numbers. Secondary zone of irruption (yellow): crossbills flee to these areas when key conifers in their core zone and primary irruption zone experience widespread failure of many conifers, and they very rarely nest here. Source: The Stokes Guide to Finches of the United States and Canada by Lillian Stokes and Matthew A. Young, copyright © 2024. Reprinted by permission of Little, Brown & Company, an imprint of Hachette Book Group, Inc.
4 Discussion
Our analyses of the call structures reveal distinctive and repeatable differences leading us to propose formal recognition of this call type as North American Call Type 12, or “Northeastern” red crossbill. The flight calls are clearly separable and in 54 of 57 cases, the PCA agreed with our manual auditory and spectrographic assessment. The agreement of the PCA and manual methods supports our methods for classifying these birds auditorily and by spectrogram. The PCA also affirms that the feature of the flight calls that was most significant for separation was the presence of the downward inflection at the end of the Type 12 calls.
In July and August, Type 12 crossbills move back to their core range, which appears to be extensively the northeastern United States, and to a lesser extent, the central Atlantic states and the western Great Lakes (Figure 5; see Figure 2 for a detailed, point-by-point view). When we compare current records to historical predicted ranges of neogaea, we find a good match for the eastern part of that range. However, birds appear to be further south than predicted by Griscom (1937) and Dickerman (1987) and we did not find birds using the northern or western extents of the historical range estimates, however some areas of boreal Canada have very limited survey coverage.
The taxonomy of crossbills has long been confusing. Matching conventional subspecies determined via museum skins with call types from live birds has been a challenge with this species (or species complex) because types are not necessarily diagnosable from museum skins with simple measurements (Groth, 1993). Furthermore, there was much confusion with the initial establishment of subspecies groups—even before people recognized there were call types, people recognized that populations tended to irrupt across the continent, and this, combined with errors and differences of opinion made for a muddy picture (Griscom, 1937; Dickerman, 1987; Groth, 1993). The American Ornithologists Union (1957) did not recognize L. c. neogaea as an official subspecies, and it is currently treated as a junior synonym of L. c. bendirei (avibase-4B1E469A; Avibase, 2024), a western subspecies that is difficult to tie directly to a call type (Groth, 1993; Benkman and Young, 2020). The current understanding of crossbill taxonomy does not contain a subspecies whose range is in the northeastern United States, formerly these were called americana (which moved to minor [Ridgway, 1885], now considered Type 3, resident in the Pacific Northwest); pusilla (AOU, 1931) (now considered type 2, the most widespread species); and again in 1957 (AOU) minor was considered northeastern (Griscom, 1937; Groth, 1993; Benkman and Young, 2020; Avibase, 2024). We therefore suggest that L. c. neogaea should be resurrected as the subspecies for the Type 12 red crossbill. A type specimen exists, taken February 9, 1886 in Lake Umbagog Maine, it is unclear whether a combination of morphometrics and genetics would be able to confidently verify that specimen as a Type 12 bird or not, given our current understanding that it is challenging to identify types on simple measurements alone (Griscom, 1937; Groth, 1993).
Clearly, establishing Type 12 red crossbill as its own type is the first step to learning more about it. Bill morphology is thought to be a critical aspect of red crossbill types (Benkman, 1993), and the few preliminary measurements that exist suggest that Type 12 is a medium-billed type largely overlapping with Type 1 and 4 (MAY unpub. data). However more study is needed on morphometrics and genetics to further understand subpopulations of red crossbills.
Based on our own limited observations and conifers available in its core breeding range, we suspect important trees include red spruce, white spruce (Picea glauca), and red (Pinus resinosa), jack (Pinus banksiana), pitch pine (Pinus rigida) and white pine. We have also observed them feeding on tamarack (Larix laricina), Eastern hemlock, Japanese black pine (Pinus thunbergii), and Norway spruce (Picea abies). Based on records in eBird and patterns of other types, we would expect them to shift seasonally with cone ripening phenologies. The cone cycle year starts approximately July 1 when new cone crops are developing. Type 12 (like almost all North American crossbills) first forages on soft-coned conifers (i.e. white and red spruces) when seeds ripen. As those seeds are dropped, Type 12 may move to eastern white pine, and eventually to species that hold their seeds the longest such as the hard-coned red, jack and pitch pines.
Based on eBird records, the core zone of occurrence for Type 12 includes Maine, New Hampshire, western Massachusetts, Nova Scotia and the Adirondack region of New York. Type 12 also occurs with frequency in eastern Massachusetts, Vermont, central New York, Ontario, and Michigan, Wisconsin, and eastern Minnesota—this would be its secondary core zone of occurrence. This type apparently also sometimes migrates down the east coast to Cape Cod, Long Island, New Jersey, Delaware. In late summer 2020, Type 12 south along the coast to North Carolina and in the interior to the southern Appalachians (eBird, 2024, Figure 5). On its return flight in summer of 2021, many settled into areas of northern Pennsylvania and southern New York to nest, with many confirmed breeding from January to May 2022 (eBird, 2024). Type 12 should likely be considered a vagrant in the western United States.
5 Conclusions
We have shown that calls formerly considered as variants of types 7 and 10 found in Western North America are consistently different from calls of the already described types and are consistently associated with birds recorded in Northeastern North America. There have been over 3,800 recordings of Type 12 as of this writing (eBird, 2024), and in this paper we have summarized some preliminary information on distribution, movements, and likely important conifer species. The red crossbill complex represents an ecological puzzle for biologists and birders alike, and an opportunity for pioneering citizen-science driven fieldwork for those inclined to explore some of North America’s under-birded coniferous habitats. The value of recording crossbills for identification to type has become increasingly recognized, and as crossbills continue to move around, as do birders, new call types could be identified in the future.
More work is needed on red crossbill call types, including exploring morphological and genetic variation within and between types, and long-term persistence of flight call characteristics, and studies on assortative mating. We encourage everyone to record and archive audio of red crossbills, as they too could be part of new and exciting discoveries.
Data availability statement
The dataset presented in this study is available to the public and can be found online at Macaulay Library at the Cornell Lab of Ornithology (https://www.macaulaylibrary.org). We used the following recordings: ML52172751, ML123435191, ML146363001, ML302385451, ML166741681, ML251384271, ML291596351, ML251382691, ML104487211, ML73216801, ML284673751, ML302447281, ML261216601, ML143501131, ML309922261, ML140552761, ML257204411, ML146361771, ML254305011, ML79681201, ML68288161, ML126076831, ML184495481, ML290406691, ML255180601, ML280405751, ML146362951, ML146362991, ML255361541, ML166639471, ML125804521, ML247473351, ML278091551, ML281116501, ML321335001, ML149109121, ML266676901, ML252688821, ML256238321, ML252596091, ML84149341, ML262064191, ML102344491, ML71662361, ML281740721, ML84149391, ML209409041, ML315802411, ML93542171, ML78834791, ML290480061, ML88552971, ML146057101, ML126076841, ML285808521, ML64984631, ML23032596.
Author contributions
MY: Writing – original draft, Writing – review & editing. KM: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. TR: Writing – original draft, Writing – review & editing. SK: Formal analysis, Writing – original draft, Writing – review & editing. NA: Visualization, Writing – original draft, Writing – review & editing. RB: Writing – original draft, Writing – review & editing. DY: Writing – original draft, Writing – review & editing. RM: Writing – original draft, Writing – review & editing. TS: Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Details of all funding sources should be provided, including grant numbers if applicable. Please ensure to add all necessary funding information, as after publication this is no longer possible. The development of BirdNET is supported by Jake Holshuh (Cornell class of ‘69) and The Arthur Vining Davis Foundations). The German Federal Ministry of Education and Research is funding the development of BirdNET through the project “BirdNET+” (FKZ 01|S22072). Additionally, the German Federal Ministry of Environment, Nature Conservation and Nuclear Safety is funding the development of BirdNET through the project “DeepBirdDetect” (FKZ 67KI31040E).
Acknowledgments
We thank the Macaulay Library at the Cornell Lab of Ornithology for use of audio recordings. The authors would like to thank each recordist for providing the time and effort required to assemble a library sufficiently detailed and voluminous to allow machine learning and principal component analysis on thousands of bird species (including red crossbills). We also acknowledge pioneers in the field of call type research—Jeff Groth, Tom Hahn, and Craig Benkman. W. Douglas Robinson and three reviewers provided comments that improved this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
American Ornithologists’ Union. (1931). Check-list of North American birds. 4th ed. (Lancaster, PA: The American Ornithologists’ Union).
American Ornithologists’ Union. (1957). Check-list of North American birds. 5th ed. (Ithaca, NY: American Ornithologists’ Union).
Avibase. (2024). The world bird database. Available online at: https://avibase.bsc-eoc.org (Accessed April 12, 2024).
Benkman C. W. (1993). Adaption to single resources and the evolution of crossbill diversity. Ecol. Monogr. 63, 305–325. doi: 10.2307/2937103
Benkman C. W. (2020). “White-winged Crossbill (Loxia leucoptera), version 1.0,” in Birds of the World. Ed. Billerman S. M. (Cornell Lab of Ornithology, Ithaca, NY, USA). doi: 10.2173/bow.whwcro.01
Benkman C. W., Brock C. D., Parchman T. L., Porter C. K. (2022). Response to Hill and Powers: It is irrelevant that the mode and tempo of Cassia crossbill speciation is not typical for birds. J. Avian Biol. 2022, e02967. doi: 10.1111/jav.v2022.i5
Benkman C. W., Smith J. W., Keenan P. C., Parchman T. L., Santisteban L. (2009). A new species of the Red Crossbill (Fringillidae: Loxia) from Idaho. Condor 111, 169–176. doi: 10.1525/cond.2009.080042
Benkman C. W., Young M. A. (2020). “Red Crossbill (Loxia curvirostra), version 1.0,” in Birds of the World. Eds. Billerman S. M., Keeney B. K., Rodewald P. G., Schulenberg T. S. (Cornell Lab of Ornithology, Ithaca, NY, USA). doi: 10.2173/bow.redcro.01
Brady R. S., Anich N. M., Young M. A. (2019). Wisconsin’s Red Crossbill Irruption of 2017–18: Distribution, abundance, and breeding behavior of multiple call types. Passenger Pigeon 81, 215–240.
eBird. (2022). 2022 eBird taxonomy update (Ithaca, NY: Cornell Lab of Ornithology). Available at: https://science.ebird.org/en/use-ebird-data/the-ebird-taxonomy/2022-ebird-taxonomy-update.
eBird. (2024). An online database of bird distribution and abundance (Ithaca, NY: Cornell Lab of Ornithology). Available at: www.ebird.org.
Ghani B., Denton T., Kahl S., Klinck H. (2023). Global birdsong embeddings enable superior transfer learning for bioacoustic classification. Sci. Rep. 13, 22876. doi: 10.1038/s41598-023-49989-z
Goffinet J., Brudner S., Mooney R., Pearson J. (2021). Low-dimensional learned feature spaces quantify individual and group differences in vocal repertoires. eLife 10, e67855. doi: 10.7554/eLife.67855.sa2
Griscom L. (1937). A monographic study of the red crossbill. Proc. Boston Soc. Natural History 41, 77–209.
Groth J. G. (1988). Resolution of cryptic species in Appalachian Red Crossbills. Condor 90, 745–760. doi: 10.2307/1368832
Groth J. G. (1993). Evolutionary differentiation in morphology, vocalizations, and allozymes among nomadic sibling species in the North American Red Crossbill (Loxia curvirostra) complex Vol. 127 (Berkeley: Univ of California Press).
Kahl S., Wood C. M., Eibl M., Klinck H. (2021). BirdNET: A deep learning solution for avian diversity monitoring. Ecol. Inf. 61, 101236. doi: 10.1016/j.ecoinf.2021.101236
Kahl S., Wood C. M., Eibl M., Klinck H. (2024). BirdNet-analyzer. Available online at: https://github.com/kahst/BirdNET-Analyzer (Accessed April 12, 2024).
Kath H., Serafini P. P., Campos I. B., Gouvêa T. S., Sonntag D. (2024). Leveraging transfer learning and active learning for data annotation in passive acoustic monitoring of wildlife. Ecol. Inf. 82, 102710. doi: 10.1016/j.ecoinf.2024.102710
Lovett E. L. (2016). Population genetic structure and parasite communities in a nomadic songbird, the Red Crossbill (Loxia curvirostra). University of Western Ontario, London, ON.
Macaulay Library. (2024). Cornell lab of ornithology (Ithaca, NY). Available at: www.macaulaylibrary.org.
Martin R., Rochefort J., Mundry R., Segelbacher G. (2019). Delimitation of call types of Red Crossbill (Loxia curvirostra) in the Western Palearctic. Ecoscience 26, 177–194. doi: 10.1080/11956860.2018.1564483
Parchman T. L., Benkman C. W. (2002). Diversifying coevolution between crossbills and black spruce on Newfoundland. Evolution 56, 1663–1672. doi: 10.1111/j.0014-3820.2002.tb01478.x
Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., et al. (2011). Scikit-learn: machine learning in python. J. Mach. Learn. Res. 12, 2825–2830.
Pieplow N. (2017). Peterson Field Guide to Bird Sounds of Eastern North America. Boston: Houghton Mifflin Harcourt.
Ridgway R. (1885). Some emended names of North American birds. Proc. United States Natl. Museum. 8, 354–356. doi: 10.5479/si.00963801.524.354
Russell E. W. B., Davis R. B., Anderson R. S., Rhodes T. E., Anderson D. S. (1993). Recent centuries of vegetational change in the glaciated north-eastern United States. J. Ecol. 81, 647–664. doi: 10.2307/2261663
Sainburg T., Theilman B., Thielk M., Gentner T. Q. (2019). Parallels in the sequential organization of birdsong and human speech. Nat. Commun. 10, 3636. doi: 10.1038/s41467-019-11605-y
Smith J. W., Benkman C. W. (2007). A coevolutionary arms race causes ecological speciation in crossbills. Am. Nat. 169, 455–465. doi: 10.1086/511961
Snowberg L. K., Benkman C. W. (2007). The role of marker traits in the assortative mating within red crossbills, Loxia curvirostra complex. J. Evolutionary Biol. 20, 1924–1932. doi: 10.1111/j.1420-9101.2007.01372.x
Stowell D. (2022). Computational bioacoustics with deep learning: a review and roadmap. PeerJ 10, e13152. doi: 10.7717/peerj.13152
Williams B., van Merriënboer B., Dumoulin V., Hamer J., Triantafillou E., Fleishman A. B., et al. (2024). Leveraging tropical reef, bird and unrelated sounds for superior transfer learning in marine bioacoustics. arXiv preprint arXiv:2404.16436.
Yang J., Carstens B. C., Provost K. L. (2024). Machine learning reveals that climate, geography, and cultural drift all predict bird song variation in coastal Zonotrichia leucophrys. Ornithology 141, ukad062. doi: 10.1093/ornithology/ukad062
Young M. (2011). Red Crossbill (Loxia curvisrotra) call-types of New York: their taxonomy, flight call vocalizations, and ecology. Kingbird 61, 106–123.
Young M., Spahr T. (2017). Crossbills of North America: Species and Red Crossbill call types. Available online at: https://ebird.org/news/crossbills-of-north-america-species-and-red-crossbill-call-types/ (Accessed April 12, 2024).
Keywords: crossbill, finch, machine learning, cryptic species, conifer, red crossbill, Loxia curvirostra
Citation: Young MA, Spahr TB, McEnaney K, Rhinehart T, Kahl S, Anich NM, Brady R, Yeany D and Mandelbaum R (2024) Detection and identification of a cryptic red crossbill call type in northeastern North America. Front. Bird Sci. 3:1363995. doi: 10.3389/fbirs.2024.1363995
Received: 31 December 2023; Accepted: 26 August 2024;
Published: 06 November 2024.
Edited by:
Scott Rush, Mississippi State University, United StatesReviewed by:
Melissa Lin Grunst, Université de la Rochelle, FranceYoni Vortman, Tel-Hai College, Israel
Copyright © 2024 Young, Spahr, McEnaney, Rhinehart, Kahl, Anich, Brady, Yeany and Mandelbaum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy B. Spahr, dHNwYWhyNDRAZ21haWwuY29t
 Matthew A. Young
Matthew A. Young Timothy B. Spahr
Timothy B. Spahr Kenneth McEnaney
Kenneth McEnaney Tessa Rhinehart2
Tessa Rhinehart2 Nicholas M. Anich
Nicholas M. Anich Ryan Brady
Ryan Brady David Yeany
David Yeany