
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol. , 11 March 2025
Sec. Synthetic Biology
Volume 13 - 2025 | https://doi.org/10.3389/fbioe.2025.1556516
This review explores the advancements, application potential, and challenges of microbial metabolic engineering strategies for sustainable organic acid production. By integrating gene editing, pathway reconstruction, and dynamic regulation, microbial platforms have achieved enhanced biosynthesis of key organic acids such as pyruvate, lactic acid, and succinic acid. Strategies including by-product pathway knockout, key enzyme overexpression, and improved CO2 fixation have contributed to higher production efficiency. Additionally, utilizing non-food biomass sources, such as lignocellulose, algal feedstocks, and industrial waste, has reduced reliance on conventional carbon sources, supporting sustainability goals. However, challenges remain in substrate inhibition, purification complexity, and metabolic flux imbalances. Addressing these requires omics-driven metabolic optimization, stress-resistant strain development, and biorefinery integration. Future research should focus on system-level design to enhance cost-effectiveness and sustainability, advancing industrial bio-manufacturing of organic acids.
Organic acids are essential in serving as intermediates for a wide range of industrial processes and find utility in various sectors including food and drink, pharmaceuticals, agriculture, and biochemistry. Among them, fumaric acid, lactic acid, butyric acid, succinic acid, malic acid, alpha-ketoglutaric acid, citric acid, and isocitric acid have received much attention due to their multifunctional effects and economic importance. Widely employed in the food sector, lactic acid a type of hydroxy acid serves to conserve freshness and amplify taste. It is also employed in pharmaceutical formulations and biotechnology to manufacture environmentally friendly plastics that are biodegradable. Recent progress in the domain of metabolic engineering has concentrated on enhancing biosynthetic routes and examining alternative carbon substrates to augment efficiency and yield, with gene suppression techniques emerging as a promising avenue for regulating enzyme activity.
Butyric acid plays a crucial role in food, pharmaceutical, and chemical industries, and is primarily generated through the microbial fermentation of substances like glucose and xylose. Current research is focused on utilizing low-cost alternative substrates and improving strain tolerance to increase yields and reduce costs. Succinate, an up-and-coming platform chemical, is being more and more generated through microbial fermentation using sustainable materials like lignocellulosic and citrus waste. The primary goal is to enhance strain effectiveness, fine-tune the fermentation procedure, and boost the use of pentose in order to minimize expenses and address environmental concerns. Its primary synthesis originates from the citric acid cycle, and the generation of α-ketoglutarate relies on essential catalysts like pyruvate oxidoreductase and α-ketoglutarate oxidoreductase. Metabolic engineering aims to optimize output by maximizing the performance of these pivotal enzymes and metabolic routes. Fumaric acid is produced through three metabolic pathways. TCA reduction cycle, TCA oxidation cycle and glyoxylic acid pathway. Each pathway has different efficiencies and limitations, and current research is focused on increasing yields and addressing inhibition in high-sugar environments. The primary method of production is through microbial fermentation, with microorganisms like Aspergillus niger being utilized to produce citric acid. Research advances include optimizing genetic engineering strategies, fermentation conditions, and adding precursors to improve yield and efficiency. Referred to as isocitric acid, this compound, while not as plentiful in the natural world, finds use in the fields of food production, pharmaceuticals, and biotechnology. The synthesis of it through the TCA cycle and glyoxylate cycle requires important enzymes like citrate synthase and isocitrate dehydrogenase. The direction of research work is genetic modification, fermentation optimization and cost effective utilization of raw materials. Malic acid is synthesized by TCA cycle and glyoxylic acid pathway, and is widely used in industry. Improving its production involves optimizing metabolic pathways and fermentation conditions to increase yield and reduce production costs.
These organic acids are key to driving a wide range of industrial applications, and continued research into microbial metabolic engineering is essential to increase production efficiency, reduce costs, and promote sustainable practices. In the pursuit of more environmentally friendly and economical options, it is crucial to focus on creating new types of microbial strains, improving fermentation methods, and investigating different raw materials in order to progress the manufacturing of these important organic acids.
In this paper, the metabolic engineering strategies and industrial applications of monocarboxylic organic acids, dicarboxylic organic acids and tricarboxylic organic acids are discussed according to the number of carboxylic groups. The important application prospect of organic acid metabolism in metabolic engineering is presented, and the current research progress of organic acid metabolism engineering is summarized and analyzed. In this paper, the production and significance of nine organic acids are introduced, with emphasis on the latest research trends and technical progress of microbial fermentation and metabolic engineering.
Pyruvate and its related compounds are extensively used across healthcare, beauty, cosmetics, food, and multiple sectors. In manufacturing, pyruvate is mainly used for producing amino acids like L-tyrosine and L-tryptophan, as well as bioactive molecules such as N-acetylneuraminic acid (sialic acid), 3,4-dihydroxyphenylalanine (DOPA), and levodopa (Yuan et al., 2022).
To optimize metabolic engineering for microorganisms used in pyruvate biosynthesis, it is crucial to understand their metabolic regulatory mechanisms. At present, pyruvate is primarily generated via the glycolytic pathway of glucose metabolism occurring in the cytoplasm (Figure 1) (Wang et al., 2015). The glycolysis process ends with a final step, and the reactions of pyruvate can result in the production of various chemicals depending on oxygen levels (Wang et al., 2005). When oxygen is available, pyruvate is subjected to an oxidative decarboxylation process, resulting in the release of carbon dioxide and the formation of acetyl-CoA. Reactions between acetyl-CoA and oxaloacetate result in the production of citrate, allowing it to enter the TCA cycle. The efficacy of this process is contingent upon the activity of the pyruvate dehydrogenase complex. Conversely, devoid of oxygen, pyruvate is converted to α-acetolactate by the action of α-acetolactate synthase (ALS), which then leads to the 2,3-butanediol pathway or is transformed into acetate via pyruvate oxidase (POX). Simultaneously, surplus pyruvate is acted upon by pyruvate formate-lyase (PFL) to undergo conversion.
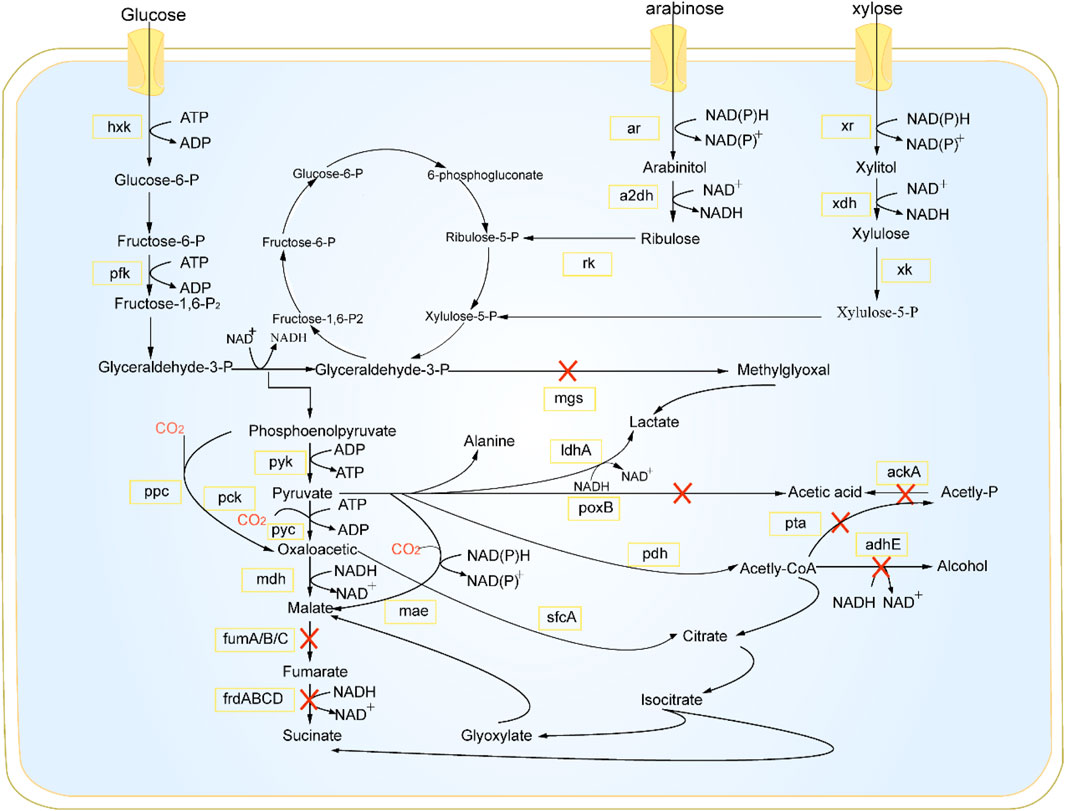
Figure 1. Pyruvate synthesis pathway. hxk, hexokinase; pfk, phosphofructokinase; ar, alcohol dehydrogenase; a2dh, aldehyde dehydrogenase; rk, kinase; xr, reductase; mdh, malate dehydrogenase; xk, xylulose kinase; mgs, methylglyoxal synthase; pyk, pyruvate kinase; ppc, pyruvate carboxylase; pck, phosphoenolpyruvate carboxykinase; pyc, pyruvate carboxylase; fumA/B/C, catalyzing the conversion of fumarate to malate; frdABCD, enzymes that reduce fumarate to succinate; mae, malic enzyme; ldhA, lactate dehydrogenase A; poxB, pyruvate oxidase; pdh, pyruvate dehydrogenase; sfcA, succinyl - CoA synthetase A; ackA, acetate kinase A; pta, phosphotransacetylase; adhE, aldehydedehydrogenase E.
In the absence of oxygen, most bacteria, such as Escherichia coli, employ ldh to transform pyruvate into lactate (Xiong et al., 2024). In yeast, the enzyme pyruvate decarboxylase facilitates the transformation of pyruvate into acetaldehyde. Pyruvate has the ability to be converted into alanine by alanine transaminase (ALT) in both aerobic and anaerobic environments. The activity of key enzymes in the pyruvate biosynthesis pathway is influenced by feedback metabolites, and some enzymes are dynamically regulated by cofactors (Figure 1) (Cybulski et al., 2019). Zhang and his team disrupted the gene responsible for pyruvate decarboxylase (KmPDC1) and the glycerol-3-phosphate dehydrogenase gene (KmGPD1) in Kluyveromyces marxianus YZJ051. They then performed overexpression of mth1 and its variants to improve the growth of KmPDC-null strains. Strain YZB053 was obtained through overexpressing the (SsXYL2-ARS) gene, resulting in an increase in the pentose phosphate pathway and xylitol dehydrogenase activity. This led to the production of 24.62 g/L of pyruvate from 80 g/L xylose at a temperature of 42°C, with a productivity rate of 0.51 g/L/h (Zhang et al., 2017). Cao et al. utilized a acid-resistant, pyruvate-tolerant strain of Klebsiella oxytoca PDL-0 and integrated the nox (NADH oxidase gene) into the ldhD locus to inhibit lactic acid production and regenerate NAD (Cao et al., 2020). Through deletion of the cstA and yjiY genes, the modified K. oxytoca PDL-YC strain achieved a yield of 71.0 g/L pyruvate from glucose (Cao et al., 2020). Wu et al. deleted several genes essential for by-product synthesis in Vibrio natriegens. Through the expression of ppc gene, 54.22 g/L of pyruvate was produced to balance cell growth and pyruvate synthesis (Wu et al., 2023).
Currently, E. coli and yeast are the primary microorganisms used for pyruvate fermentation (Wang et al., 2022). These organisms are favored for their capacity to gather and release substantial amounts of pyruvate, their quick growth, efficient conversion rates, and non-harmful nature to humans and animals. Novel strategies in metabolic engineering are increasingly focusing on suppressing gene expression rather than removing genes entirely. One example includes the suppression of the aceE gene, which affects pyruvate dehydrogenase and retains some level of activity in this enzyme, enabling glucose to be the sole carbon source (Moxley et al., 2021). Current research directions explore the use of alternative substrates, including whey, alginate (Kawai et al., 2014), mannitol (Yoshida et al., 2015), and lactic acid (Gao et al., 2010), to produce pyruvate. These efforts indicate that future studies will likely prioritize the use of low-cost and unconventional carbon sources in producing pyruvate (Gao et al., 2010).
Lactic acid, a prevalent hydroxy acid in the natural world, engages in numerous reactions that yield an array of its derivatives (Ren et al., 2022). It is particularly noted for its role in the creation of polylactic acid, which stands as the most valuable biodegradable plastic raw material in commerce (Abdel-Rahman et al., 2013). This chemical entity is pivotal in the manufacture of eco-friendly polymers, oxygenated compounds, sustainable solvents, and plant growth promoters (Nwamba et al., 2021).
At present, advancements in lactic acid manufacturing are contingent upon the utilization of metabolic engineering and conventional fermentation methods (Eş et al., 2018). In the cytoplasm of eukaryotic cells, glycolysis breaks down a single glucose molecule into two pyruvate molecules, which are subsequently transformed into lactic acid through the lactic fermentation pathway by lactate dehydrogenase (Ren et al., 2022). Lactic acid fermentation in prokaryotes is divided into two types. Homologous fermentation is similar to lactic acid fermentation in eukaryotes, both of which produce L-lactic acid. Opposite-sex fermentation will produce D-lactic acid, but also ethanol, acetic acid and other by-products (Tian et al., 2021a).
Traditional metabolic engineering methods focus on pathway redirection and heterologous gene expression. Following the consumption of glucose by certain host microorganisms, they may utilize the resulting end product for growth. By disrupting the genes responsible for D-lactate dehydrogenase and monocarboxylate transporter, the utilization of D-lactic acid can be effectively eradicated. Furthermore, specific microorganisms possess the capability to break down heterozygosis and produce substances such as ethanol. These byproducts can be removed by inactivating the gpd1 and gpd2 genes, which encode glycerol-3-phosphate dehydrogenase (Liu et al., 2023b). The low acid tolerance of host cells in LA producers is not favorable for industrial production (Liu et al., 2023b). Therefore, the use of acid-resistant strains for metabolic engineering is a good method. Liu et al. utilized a S. cerevisiae TAM strain that demonstrated tolerance to pH 2.4 as the initial strain. Subsequent adjustments in energy supply and redox balancing led to an increase in L-LA titer, reaching 72.7 g/L during shake-flask fermentation without the use of a neutralizer, with a yield of 0.66 g/L/h (Liu et al., 2023b). In another example, heterologous ldh gene was expressed in acid-resistant S. cerevisiae CEN. PK2 and ethanol-producing pathways pdc1 and adh1 were knocked out. The yield of E. coli acetyl-CoA synthesis pathway reached 142 g/L (Juodeikiene et al., 2016).
In recent years, CRISPR-Cas9 technology has been used to successfully develop high optical purity of Lactobacillus paraceo strains, in order to achieve an optical purity of over 99.1% for L-lactic acid in the fermentation liquid (Tian et al., 2021b). At the same time, low-cost green substrates such as lignocellulose, glycerin (Jodłowski and Strzelec, 2021), matrine residue (Ma et al., 2020), municipal household waste (Acedos et al., 2022) and sugarcane molasses (Sun et al., 2019) were developed. This suggests that future lactic acid production research will focus on improving optical purity and developing low-cost substrates.
Butyric acid is extensively employed in the creation of industrial chemicals, food items, pharmaceuticals, and additives for animal feed (Fu et al., 2022). It holds the capacity to act as a precursor for specific cellulosic organic acid esters utilized in the coating industry, providing superior protection against light, heat, and moisture (Jiang et al., 2018). Within the food and beverage sector, butyric acid is used to intensify buttery flavors, often supplemented to enhance the taste of fruits, and is a crucial component in the manufacture of aromatic compounds for flavoring (Fu et al., 2017).
The microbiological research primarily focuses on several Gram-positive bacteria, including Butyribacterium, Butyrivibrio, Clostridium, and Eubacterium. Glucose is converted into pyruvate in the cytosol through the glycolytic pathway, which is then decarboxylated by pyruvate-ferredoxin oxidoreductase to generate acetyl-CoA (Guo et al., 2024).
Although the cost of fermentation to produce butyric acid is no advantage compared to chemical synthesis, it is necessary in industries such as beauty (Mariën et al., 2023). In a research experiment, the increased expression of acid-resistant Class I heat shock protein (hsg) had significant effects on strains in conjunction with the increased expression of dnaK and groE operons. The strain’s acid tolerance was greatly improved by groESL, while dnaK had a detrimental effect on the strain’s ability to tolerate acid. Compared with the control group, the modified strain increased by 15%, and the titer reached 52.2 g/L (Suo et al., 2017). Part of the phosphotransacetylase gene (pta) from C. tyrosine was integrated into C. butyricum by homologous substitution, which disrupted the acetic acid formation pathway. Part of the pta (phosphotransacetylase gene) from Clostridium tyrosine was integrated into Clostridium butyricum by homologous substitution, which disrupted the acetic acid formation pathway. Compared with the original strain, the modified strain had a titer of 32.5 g/L (Guo et al., 2024). Due to the shortage of tools and the difficulty of transformation in the genetic engineering of Clostridium difficile. In a particular research, the native fadR, aceF, ldhA, and pta genes in E. coli were inactivated, resulting in a modified strain that yielded 3.3 g/L of butyric acid in a medium containing 10 g/L of glucose (Seregina et al., 2010).
Due to the presence of carbon decomposition metabolite inhibition (CCR), several clostridium bacteria used for butyric acid fermentation are strongly inhibited by glucose while utilizing xylose. In one study, replacement xylA (xylose isomerase gene), xylB (xylokinase gene) and xyylt (xylose proton homology gene) were co-expressed in Clostridium tyrobutyricum. The modified strain can produce butyric acid from xylose and glucose at the same time, and the final titer reaches 46.4 g/L (Fu et al., 2017).
The study of butyric acid fermentation through traditional strain breeding and metabolic engineering strategies will be the focus. Currently, most studies use agricultural derivatives such as glucose and xylose as substrates, which are not only expensive but also compete with the food industry, which is not only economically but also resource-wise unsustainable. However, the use of cheap lignocellulosic substrates would result in high pretreatment costs and inhibition of the microbial strain (Kelbert et al., 2024). The high cost of production is due to the low yield of butyric acid and the difficulties in separating and purifying the co-product, acetic acid (Wang et al., 2019). In the future, research on butyric acid can be conducted by adopting alternative inexpensive substrates to avoid excessive substrate costs and to avoid competition with the food industry (Guo et al., 2024; Mariën et al., 2023). The utilization of underutilized substrates in industrial and agricultural sectors for fermentation to produce butyric acid has become a focus of interest for researchers (Wang et al., 2019).
It is anticipated that succinic acid will soon become one of the most significant platform chemicals, alongside malic acid. Furthermore, it will serve as a plasticizer within the food industry. In recent years, various engineering approaches have been established to produce succinic acid through microbial fermentation. Due to their ability to withstand low pH levels, microorganisms like Saccharomyces cerevisiae (Liu et al., 2021) and E. coli are being considered (Chiang et al., 2021), as they can play a crucial role in reducing downstream expenses.
At present, there are three distinct methods for catalyzing the synthesis of succinic acid: oTCA, rTCA, and the glyoxylate pathway. The rTCA pathway to form succinic acid is currently a hot spot, and the ability to fix a molecule of carbon dioxide is very consistent with the current environmental protection concept. The yield of succinic acid can be improved by boosting the activity of pivotal enzymes within the TCA cycle, such as succinate dehydrogenase (SDH), fumarate reductase (FRD), and malate dehydrogenase (MDH) (Ahn et al., 2020). Intracellular succinic acid needs to be transported outside the cell as soon as possible, avoiding key enzyme activity that affects intracellular pH and the synthesis pathway. The succinic acid transporter SucE was found by Li et al. to have a titer of 68.66 g/L encoded by NCgl2130 (Figure 2) (Li et al., 2017).
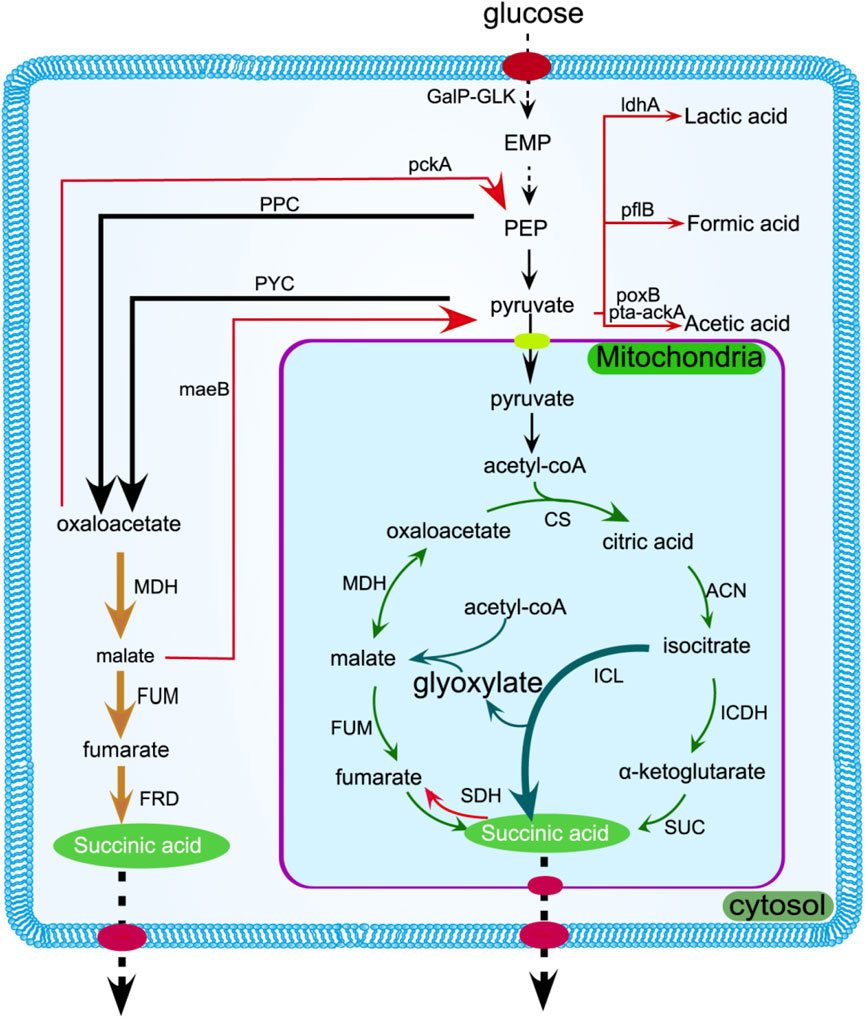
Figure 2. Succinic acid is produced in the cytoplasm and mitochondria through the rTCA pathway (yellow arrow) and oTCA pathway (green arrow), respectively. The enzymes can be enhanced to increase metabolic flux, as indicated by the bold arrows. The glyoxylate pathway is represented by the dark green arrow, while gene suppression or deletion is denoted by the red arrow. Transmembrane transport is shown by dotted arrows. GalP-GLK,It refers to a transport system in Escherichia coli (E. coli) that involves two components: galactose penetrase (GalP) and glucokinase (GlK); pflB, pyruvate formate lyase; cs, citrate synthetase; ldhA, lactate dehydrogenase A; can, aconitase; sdh, succinate dehydrogenase; frd, fumarate reductase.
The main carbon source of commercially produced succinic acid is a variety of common sugars, such as sucrose (Lee et al., 2016) and glucose (Babaei et al., 2019). Serving as a crucial source of energy for the metabolic processes of living organisms, glucose also generates numerous chemical compounds and essential substances for conversion during the catabolic process (Figure 2). It is the most commonly used carbon source for producing chemicals derived from biological sources. Reaching elevated levels of succinic acid via the glucose metabolism is equally attainable with the efficiency of succinic acid (Ferone et al., 2019). The focus on using sustainable raw materials for producing succinic acid is increasing, with a goal of capturing carbon and reaching carbon neutrality. In order to make use of lignocellulose, a metabolically engineered strain of E. coli Suc260 (pTbglA) was used to overexpress the bglA gene, which encodes β-glucosidase. This led to the generation of 25.06 g/L of succinic acid using cellobiose as the carbon source (Dong et al., 2017). Related to the environmental concept, it is reported that citrus peel waste is distilled and extracted by dilute acid hydrolysis to obtain pectin (30.53%) and essential oil (0.43%, including 17 compounds, mainly D-limonene). At the same time, 22.4 g/L of succinic acid was produced through fermentation (Patsalou et al., 2020). Succinic acid can be produced from residual biomass hydrolysates obtained by direct biomass esterification from microalgae (Sorokina et al., 2020). Bagasse (Shaji et al., 2021) can also be used as a carbon source for succinic acid biosynthesis. In the process of hydrolysis, an acid or base is commonly employed to eliminate lignin and break down cellulose and hemicellulose, resulting in the production of cellulase and xylanase for saccharification. However, the concentration of succinic acid remains comparatively low, ranging from 20 to 60 g/L, due to the high concentration of pentoses in the hydrolysates of lignocellulosic biomass like olive pits, bagasse, and straw (Lee et al., 2022).
Although biobased processes are more energy efficient and have less environmental impact compared to petrochemical processes, it is important to acknowledge that they also come with their own set of limitations. Despite lower raw material costs, the expensive, laborious, and intricate downstream processes make it less accessible. Much of the downstream cost comes from crystallizing succinic acid and removing various impurities or byproducts by lowering the pH of the fermentation solution. Given this context, the current focus of research is on enhancing the strain’s production efficiency, refining the fermentation process, and minimizing raw material expenses (Thangarasu et al., 2018).
L- Malate, also known as 2-hydroxysuccinic acid, molecular formula C4H6O5, its powder is white crystalline, easily soluble in water and ethanol and other solvents (Wei et al., 2021).
Malic acid, recognized for its distinctive and agreeable taste, is extensively employed in the food and beverage sectors to boost flavor profiles. Additionally, it plays a role in the production of unsaturated polyester resins and coatings (Ding and Ye, 2023). Sunitinib malate, which acts as a tyrosine kinase inhibitor, exhibits both anti-angiogenic and anti-tumor properties and is approved by the FDA for treating renal cell carcinoma and gastrointestinal stromal tumor (Wu et al., 2022).
In the past 20 years, different strains have been created to produce malic acid, with each strain having its own set of strengths and weaknesses. For example, E. coli, which has been widely studied among bacteria, has rapid growth and reproduction and simple genetic manipulation, but it is non-food safe, has low substrate tolerance concentration and limited acid production capacity (Zhu et al., 2020). S. cerevisiae can withstand high-sugar and high-acid fermentation environment (Kang et al., 2021), but the reported malic acid production intensity is low, and the level of heteroacid is high. So far, the highest yield of malic acid production strain reported is Ustilago trichophora (Zambanini et al., 2017), but it is a plant pathogen, and the fermentation cycle is about 10–12 days. Some natural strains, such as Aspergillus oryzae (Ji et al., 2021), can accumulate a large amount of malic acid when cultured with high glucose concentration, appropriate nitrogen source, inorganic salt and CaCO3.
The rTCA pathway is the most efficient among L-malic acid metabolic pathways in terms of carbon conversion rate. Therefore, enhancing or building the rTCA pathway has consistently been the favored approach for metabolic engineering aimed at increasing L-malic acid production, as seen in Figure 3 (Wei et al., 2021). The rTCA pathway produces oxaloacetic acid by fixing CO2, which is further converted into malic acid, and has high carbon efficiency (Wu et al., 2022). In addition, the combination expression of L-malic acid transporters, the directed evolution of key enzymes, and the regulation of coenzyme regeneration are also important methods to improve the yield of L-malic acid (Jiang et al., 2021). The production process initiates with the carboxylation of phosphoenolpyruvate or pyruvate and the by-products of glycolysis. Consequently, managing the metabolic fluxes of glycolysis, the reductive tricarboxylic acid (rTCA) cycle, and CO2 fixation is crucial for the efficient biosynthesis of malic acid, which is shown in Figure 3 (Chen et al., 2023a).
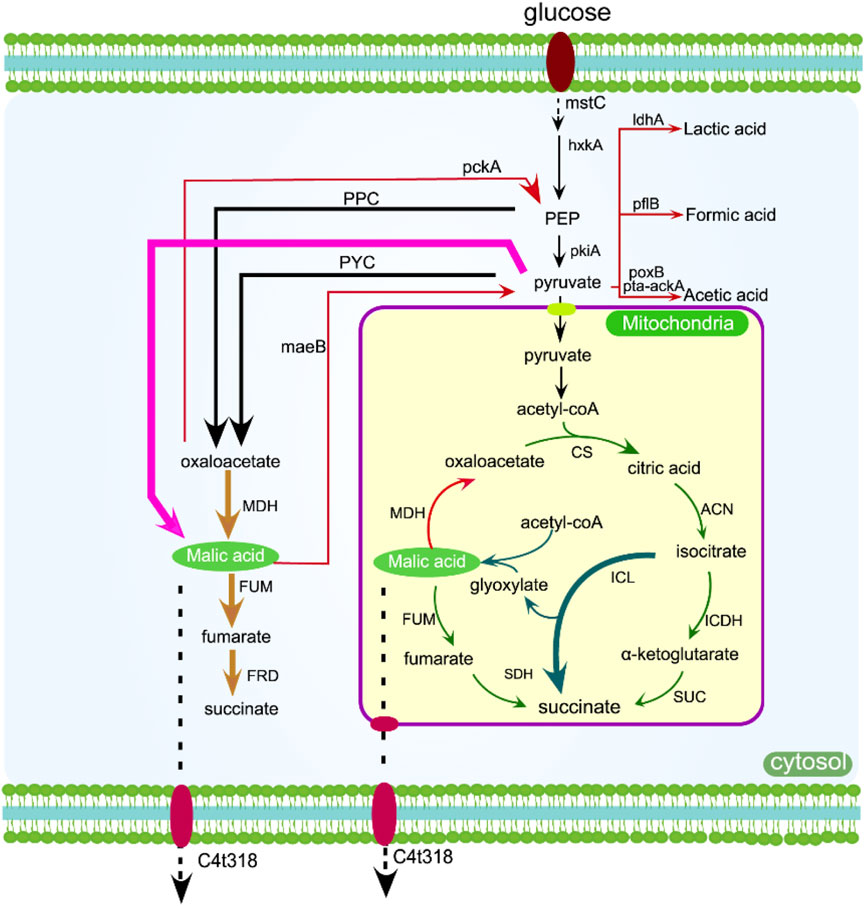
Figure 3. The production of malic acid can be increased by overexpressing enzymes in the rTCA pathway (yellow arrow), oTCA pathway (green arrow), glyoxylic acid pathway (dark green arrow), and one-step pathway (pink arrow). Genes that may inhibit or delete the metabolic flux are indicated by red arrows, while transmembrane transport is shown with dotted arrows. C4t318, C4-dicarboxylate transporter; mstC, glucose low-affinity transporter; hxkA, hexokinase A; pckA, phosphoenolpyruvate carboxykinase A; mdh, malate dehydrogenase.
Xu et al. developed a genome editing system, Cre-loxP, to modify the metabolic flux of organic-producing strains for malic acid production. They utilized this system to delete oahA and integrate pyc, mdh3, and c4t318 overexpressed in A. niger in order to enhance the metabolic flux of the rTCA pathway and increase malic acid output. After 9 days of fed-batch fermentation, they achieved a titer of 201.24 g/L (Xu et al., 2020). In the rTCA pathway of filamentous fungi, malate dehydrogenase is dependent on the coenzyme NADH in the cytoplasm. To address this limitation, E. coli stha is an energy-independent FAD-containing enzyme. The catalyzed reversible reaction of NAD (H) and NADP (H) leads to a significant increase in the production of L-malic acid in A. niger (Yang et al., 2024). Chen et al. achieved a titer of 235.8 g/L MA from the final engineered strain by overexpressing trmae1, a natural MA transporter gene in Trichoderma reesei. The yield was 1.48 mol MA per mole of glucose and the productivity reached 1.23 g/L/h (Chen et al., 2023b). This represents the maximum titer that has been documented. In general, improving the metabolic flux and rTCA pathways within glycolysis is an optimal approach for boosting malic acid output.
The employment of microbial metabolism to convert it into valuable products has emerged as a prominent area in the biological field lately (Wei et al., 2021). Although L-malic acid fermentation technology’s industrialization has been reported, its production rate and purity are not high, subsequent separation and purification are challenging, and costs are significant. Studies on filamentous fungal fermentation for L-malic acid production have only reached laboratory stages without any industrialization yet (Li et al., 2023).
In the field of medicine, it acts as an important precursor for creating different medications and can be used to generate antioxidants (Zhang et al., 2020), thus improving antioxidant levels (Li et al., 2016). Within the food industry, α-ketoglutaric acid functions as a nutritional component in sports beverages, offering energy and preventing ammonia toxicity while also serving as an antimicrobial agent (Legendre et al., 2020).
Reproduction of alpha-ketoglutarate is achievable through chemical synthesis, enzymatic processes, and microbial fermentation. While the industrial production currently relies on chemical synthesis, this method presents challenges such as a complex process, difficult separation, and environmental pollution (Liu et al., 2023b). In contrast, microbial fermentation offers advantages including low cost, high efficiency, and environmental friendliness, making it a promising option for industrial production (Yovkova et al., 2014).
The biosynthesis pathway of α-KG was improved in E. coli by reorganizing the TCA cycle to increase pyruvate production, and additional improvements were achieved by adjusting the levels of gene expression. Furthermore, enhancing the supply of acetyl-CoA can alleviate the constraints in the synthesis of α-ketoglutarate (α-KG) (Chen et al., 2020). The metabolic intermediate α-KG is essential for the Tricarboxylic acid cycle (TCA cycle) in microbial cells (Chen et al., 2020). Carbon source materials from the environment are transported into cells and converted to pyruvate through glycolysis. The tricarboxylic acid cycle utilizes carbon sources with the help of pyruvate dehydrogenase complex, citrate synthase, aconitase, and isocitrate dehydrogenase to produce α-KG. Subsequently, α-KG is further metabolized into succinyl-CoA by α-ketoglutarate dehydrogenase (KGDH), accompanied by electron transfer and energy generation. This process provides both carbon source material and energy for cell growth and reproduction. Furthermore, α-KG participates in nitrogen metabolism by transamination to form L-glutamate, thus serving as a vital metabolic intermediate that links carbon metabolism with nitrogen metabolism (Song et al., 2016).
A variety of metabolic engineering tactics have been devised for the biosynthesis of α-ketoglutaric acid, employing Clostridium glutamicum as the production host (Tenhaef et al., 2021). Through overexpression of pyc or pdh complexes (Yin et al., 2012), the carbon pathway of pyruvate into the TCA cycle is enhanced to produce α-KG. Nevertheless, the build-up of secondary substances like pyruvate poses challenges and expenses for the subsequent separation process (Yin et al., 2012). Increased expression of genes that encode glycerol kinase, methylcitrate synthase, and transporters for organic acids in the mitochondria. Alpha-KG of 53.1 g/L was obtained with a productivity of 0.35 g/L/h (Tomaszewska-Hetman et al., 2021). The overexpression of NADP-dependent isocitrate dehydrogenase (IDH) and pyc1 in fatty acid degrading yeast significantly enhanced the synthesis of α-ketoglutaric acid, resulting in a remarkable increase in the yield to 186 g/L after fermentation for 117 h. Bovine et al. successfully expressed L-glutamate oxidase (LGOXStr) and catalase (KatGEsc) from Streptomyces virusosporus R111 in E. coli H736. Through the addition of sfGFP tags, they were able to anchor L-glutamate oxidase (KatGEsc) and catalase (KatGEsc) to the outer membrane of E. coli cells, allowing for one-step whole-cell catalysis of alpha-ketoglutaric acid with a conversion efficiency of up to 75% (Niu et al., 2024).
Microbial fermentation can significantly reduce the overall production cost of α-KG. To achieve this goal, subsequent improvements will primarily focus on fine-tuning the metabolic pathway by lowering nodes in the tricarboxylic acid cycle and dehydrogenases such as kgdh and icl, while increasing the activity of pyruvate dehydrogenase (PDH) to balance the host cell metabolism, enhance production efficiency, and design optimized cell phenotypes to enhance substrate utilization and environmental tolerance (Zhou et al., 2023).
Commonly known as an essential substance, fumaric acid is of significant value in a range of sectors such as food and beverage, cleaning products, animal nutrition, pharmaceuticals, and other industrial goods (Sebastian et al., 2019). With the increasing emphasis on protecting the environment and promoting sustainable development, new challenges and opportunities are emerging for the production of fumaric acid. The traditional petrochemical route has problems such as high energy consumption and high pollution emissions. Therefore, more and more companies are turning to “green production” and environmental protection technologies. During the implementation of “green production,” there is a strong focus on utilizing inexpensive raw materials for the biotechnological production of fumaric acid (Sebastian et al., 2021). By utilizing microorganisms or other biological pathways containing or capable of obtaining fumaric acid raw materials, combined with modern process technology for extraction and purification can effectively reduce costs and reduce reliance on traditional resources. At the same time, this method also has good environmental friendliness, meeting the urgent needs of today’s society for sustainable development and resource conservation (Sebastian et al., 2019).
Fumaric acid is generated via three distinct metabolic routes. The reductive arm of the citric acid cycle is pivotal in cellular energy metabolism, transforming pyruvate into oxaloacetate and enabling ATP production. This metabolic sequence boasts a theoretical maximum yield of 2 moles of ATP per mole of glucose and necessitates ATP and CO2 for the carboxylation of pyruvic acid into oxaloacetic acid. Oxaloacetic acid is then converted into fumarate via malate dehydrogenase (MDH) catalyzed decarboxylation reaction, which includes an NADH reduction step (Guo et al., 2020).
The alternative route includes the oxidative TCA cycle, which is essential for producing fumarate. In this process, pyruvate is initially transformed into acetyl-CoA through the pyruvate dehydrogenase complex. Acetyl-CoA is involved in the tricarboxylic acid cycle, leading to the formation of succinate. Subsequently, succinate is acted upon by succinate dehydrogenase (SDH) to form fumarate, achieving a theoretical maximum yield of 1 mole per mole of glucose due to the release of CO2 during the reaction (Xu et al., 2013).
The potential application of the glyoxylate shunt in the production of fumaric acid is currently being evaluated. Isocitric acid, produced in the TCA cycle, is transformed into succinic acid and glyoxylic acid through the activity of isocitrate lyase (Johannsen et al., 2018). Subsequently, malic acid is formed through the reaction of glyoxylic acid and acetyl-CoA with the help of malate synthase. Despite having a lower potential yield (1 mole/mole glucose) compared to the reductive TCA cycle, the glyoxylate pathway demonstrates potential due to its more efficient metabolic pathway (Guo et al., 2020). However, in high-sugar environments, this key metabolic pathway is strongly inhibited and difficult to activate when glucose is used as a substrate. The suppression is caused by phosphoenolpyruvate (PEP), a product of glucose metabolism that acts as an inhibitor for isocitrate lyase (Sebastian et al., 2019).
At present, the main research object of fumaric acid production of A. oryzae has increased the yield of fumaric acid by 26% through overexpression of ppc (Li et al., 2014). A prevalent approach is to focus on E. coli to amplify the expression of genes associated with the citric acid cycle, like those for fumarase (fum) and fumarate reductase (frd). Moreover, augmenting the flow through the glyoxylate cycle can significantly boost the production of fumaric acid. Additionally, interrupting the primary side-product formation pathway is also a widely adopted strategy (Xu et al., 2013).
In the manufacture of industrial organic acids, the cost of substrates accounts for roughly 30%–40% of total costs (Jimenez-Quero et al., 2020). While glucose is commonly used as a carbon source for fumaric acid production, alternative sources including glycerol, xylose, and sucrose have been explored as well. However, these alternative carbon sources generally have lower fermentation efficiency compared to glucose due to the need for additional pathways to connect them to glycolysis and the TCA cycle. In light of growing economic and environmental pressures, there is a growing interest in exploring renewable raw materials that are abundant in starch or lignocellulose as potential alternatives (Ilica et al., 2019).
The future focus of metabolic engineering for fumaric acid production should prioritize global regulation rather than solely concentrating on individual genes or specific pathways. Novel techniques such as mitochondrial manipulation, scaffold modification, and cofactor adjustment will provide valuable knowledge for improving the procedure (Pairazaman et al., 2024). Utilizing waste biomass or co-substrate fermentation may present a more economical approach to enhancing the feasibility of biological production procedures. Additionally, optimization strategies should not only aim to increase yields at the laboratory scale but also consider industrial-scale fermentation feasibility to enhance economic competitiveness (Deng and Aita, 2018).
Citric acid is mainly extracted from various sources of carbohydrates such as molasses and starch-based culture media, and produced by deep fermentation of A. niger (Rakicka et al., 2019). In order to facilitate extensive production, it is essential to guarantee that the manufacturing process is eco-friendly by making use of readily available and cost-effective agro-industrial residues, all while sustaining high levels of output (Mores et al., 2021).
A.niger is commonly used as a microorganism in the manufacturing process of citric acid. By means of fermentation, A. niger transforms glucose or alternative carbon sources into citric acid through the EMP (glycolytic pathway) and TCA cycles (Angumeenal and Venkappayya, 2013). Crucial enzymes involved in this process include Citrate synthase, which aids in the formation of citric acid from acetyl-CoA and oxaloacetate, and ATP-citrate lyase, which plays a vital role in citric acid synthesis (Ozdal and Kurbanoglu, 2018). Yarrowia lipolytica is another significant producer of citric acid, particularly when utilizing raw materials such as petroleum and ethanol. Similar to A. niger, Y. lipolytica converts carbon sources into citric acid through a comparable metabolic pathway with high conversion efficiency (Carsanba et al., 2019). In industrial production settings, various approaches are employed to enhance the yield and efficiency of citric acid production. These methods include genetic engineering to strengthen the biosynthetic pathway of citric acid, optimization of fermentation conditions, and inhibition or bypassing of metabolic pathways unrelated to or competing with the accumulation of citric acid (Yuzbasheva et al., 2019). For instance, genetic engineering can be used to enhance the expression of citrate synthase or optimize fermentation conditions such as pH level, temperature control, oxygen supply regulation for improved production efficiency (Angumeenal and Venkappayya, 2013).
The production of citric acid can be increased by knocking out the by-product generating gene. For example, when corn starch is used as carbon source, deletion of isomaltose synthesis gene A-glucosidase coding gene agdA can effectively reduce isomaltose concentration and increase citric acid production (Wang et al., 2016). Enhancing the production of citric acid via precursor engineering can be achieved by supplementing the reaction with acetyl-CoA and oxaloacetic acid, the precursors necessary for citric acid synthesis. Several enzymatic systems contribute to the generation of acetyl-CoA, including pyruvate dehydrogenase (PDH) and cytoplasmic acetyl-CoA synthetase (ACS) (Kamzolova, 2023). Overexpression of mdh2 (malate dehydrogenase), fumarate reductase (FumR) and fumarate reductase (Frds1) can increase the synthesis of oxaloacetic acid, shown in Figure 4. The strain that inhibited the engineered mutant phosphofructokinase pfk1 by feedback produced 70% more citric acid than the control strain (Figure 4) (Hu et al., 2019). Murchitine synthetase gene (chsC) interferes with RNA. Following the silencing of the chsC gene, A. niger mutant strains form compact mycelial pellets, which reduce the viscosity of the medium and enhance the mass transfer of oxygen. Consequently, this leads to a 42.6% increase in citric acid yield compared to the wild-type strain (Hu et al., 2019).
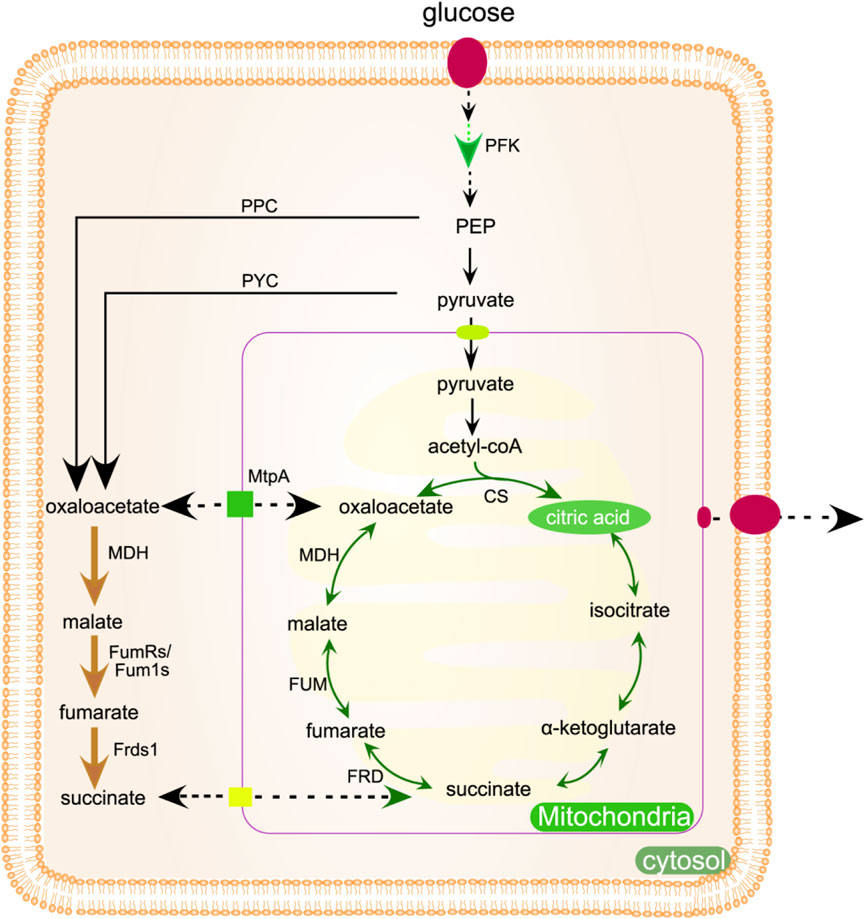
Figure 4. Metabolic engineering methods can be used to improve the production of citric acid, which is produced in mitochondria. Enzymes indicated by bold arrows may be increased or altered to boost metabolic flux. The rTCA pathway is represented by the yellow bold arrow, while the PFK can be modified to alleviate feedback inhibition as shown by the green bold arrow. Dotted arrows indicate transmembrane transport. Pfk, 6-phosphofructokinase; MtpA, mitochondrial transport protein A; mdh, malate dehydrogenase; FumRs, fumarate reductase; Fum1s, fumarase; ppc, pyruvate carboxylase; pckA, phosphoenolpyruvate carboxykinase A; Frds1, fumarate reductase d1; pyc, pyruvate carboxylase.
The selection of carbon source greatly influences citric acid production, with monosaccharides and disaccharides being the most suitable options for carbon sources (Kadooka et al., 2020). The decreased enzyme activity in the fermentation medium is responsible for the slow rate of polysaccharide hydrolysis, leading to changes in pH value (Ajala et al., 2020). Sucrose is superior to glucose, fructose and lactose in increasing citric acid yield. Sucrose can be rapidly hydrolyzed at low pH. The presence of nitrogen has been shown to significantly impact the synthesis of citric acid, as nitrogen is essential for both cellular protein formation and metabolism (Morgunov et al., 2020). The synthesis of citric acid and fungal growth were influenced by the type of nitrogen source. It is essential to restrict the supply of nitrogen, as concentrations exceeding 0.25% result in urea accumulation and a reduction in citric acid production. High nitrogen concentration will increase carbon source consumption and fungal growth while reducing citric acid production (Rzechonek et al., 2019).
The presence of oxygen is crucial for the biological synthesis of citric acid, as it significantly influences the production process (Zhang et al., 2022). Receiving varying levels of aeration will negatively impact the fermentation process and overall production output. With an elevated aeration rate, the partial pressure of dissolved carbon dioxide in the medium was found to decrease. The substrate for pyruvate carboxylase (PC) is carbon dioxide, which serves as a replacement for the oxaloacetic acid needed by citrate synthase (CS). Pyruvate decarboxylation is catalyzed by pyruvate decarboxylase (PDC) to produce carbon dioxide, but extreme aeration conditions will cause certain losses (Figure 4). Increased carbon dioxide levels can damage final biomass and citric acid concentrations.
Currently, microbial fermentation is the dominant method for producing citric acid, and it is highly developed. However, there is still room for improvement in the purification process downstream. In the future, with the continuous progress of science and technology, it is expected to shift from mature production technology to new cheap raw materials and new recovery methods of citric acid. This will greatly promote the improvement of resource utilization efficiency in the production process of citric acid, and also have a positive significance for environmental protection (Rzechonek et al., 2019).
However, although the technology is quite mature at this stage, it still needs to continue in-depth research to reach a higher level. For example, in the process of microbial fermentation, how to improve the production of citric acid, reduce energy consumption, reduce waste emissions and other aspects need to continue to explore and improve. At the same time, in the application of new materials and new equipment also need to continue to innovate and improve.
In short, although the microbial fermentation production of citric acid has made remarkable achievements, in the future there is still a need to continue to promote scientific research and practice in related fields, in order to achieve a more sustainable, efficient and environmentally friendly production of citric acid.
Isocitric acid, a less common isomer of citric acid, has various industrial applications despite its lower natural abundance (Rzechonek et al., 2019). It can be used as an acid agent in food and beverages, enhancing the taste of food and extending the shelf life. In pharmaceutical manufacturing, it can be used in drug synthesis or as an auxiliary ingredient in some drugs, such as pH regulators or stabilizers (Kamzolova et al., 2016). In addition, it can also be used as a detergent ingredient to remove stains and improve cleaning effect; In the field of biotechnology, it can be used as metabolic intermediates in the process of microbial fermentation to produce other biological chemicals (Kamzolova et al., 2021). In the agricultural field, it can be used as a plant growth regulator or some pesticide formulations. Simultaneously, it has the potential to serve as a starting material for the production of various organic compounds in the chemical industry and holds significance in research, such as its use as a possible precursor material for biofuel production (Kamzolova et al., 2016).
The isocitrate molecule has four different forms, but only one of them, threo-DS-isocitric acid, is important for the TCA cycle in aerobic organisms (Kamzolova et al., 2018). In recent studies, ICA has been examined for its potential as a natural agent for prevention and treatment. Specifically, its effectiveness in treating iron deficiency anemia and in absorbing blood clots has been documented (Kamzolova and Morgunov 2019). Trimethylcitrate is currently being investigated as a novel treatment for Parkinson’s disease associated with dj-1 gene dysfunction (Kamzolova et al., 2018). It has been hypothesized that membrane-permeable trimethyl isocitrate prevents dopamine production from disrupting DNA structure in mitochondrial neurons. ICA, which exists in the form of lactones with chiral properties, has great potential in the chemical and pharmaceutical industries (Morgunov et al., 2020).
The synthesis of isocitric acid is a complex biochemical process, mainly completed through the TCA cycle and glyoxylate cycle (Kamzolova and Morgunov 2019). In this process, citrate synthase, isocitrate dehydrogenase, and isocitrate lyase play important roles. Among them, citrate synthase participates in the synthesis of isocitric acid; while isocitrate dehydrogenase and isocitrate lyase are involved in the decomposition process of isocitric acid (Kamzolova and Morgunov 2019). It is worth noting that due to their low activity, these two key catalysts play a balancing role in maintaining normal metabolic pathways. The mitochondrial succinic acid-fumaric acid carrier YlSfc1 is the main control point of isocitric acid in Y. lipolytica. By overexpressing YlSfc1 and knocking out the citric acid transporter YlYHM2, the modified strain produced 136.7 g/L isocitric acid (Yuzbasheva et al., 2021). Overexpression of citric acid synthase cit1 and cit2 in Y. lipolytica significantly increased isocitric acid synthesis. Compared with the original strain, the synthesis of overexpressed cit1 isocitrate increased by 9.5 times, and the synthesis of overexpressed cit2 isocitrate increased by 6.8 times (Hapeta et al., 2020). By overexpressing glycerol kinase (GUT1) and glycerol-3-phosphate dehydrogenase (GUT2) in Y. lipolytica, 42.5 g/L isocitric acid was produced from crude glycerol (Rzechonek et al., 2019).
Further efforts in the research of citral production could include improving the strains that produce ICA, for example, by using genetic engineering techniques to modify and optimize the strains to enhance their efficiency and stability in producing ICA (Morgunov et al., 2020); at the same time, research on utilizing the diversity of microorganisms to screen for more suitable strains for ICA production could also be conducted (Kamzolova et al., 2021). In the fermentation process, in addition to optimizing the existing fermentation processes, exploring the introduction of new reactors or adjusting fermentation conditions to increase the yield and purity of ICA could also be pursued (Yuzbasheva et al., 2021).
Furthermore, using new inexpensive raw materials is an important direction, such as using agricultural waste and industrial by-products as sources of raw materials for ICA production to reduce costs and achieve sustainable development. Additionally, in the development of new methods for separating and purifying ICA (Schlembach et al., 2024), advanced separation techniques such as ultrafiltration and ion exchange chromatography should be combined with continuous exploration of innovative technologies to improve the quality and purity of ICA products (Kamzolova et al., 2016). In summary, there are still many worthwhile directions for further research and exploration in the field of ICA production.
Currently, the most common method for biological fermentation of organic acids is the use of calcium salts. This method involves using CaCO3 to neutralize the organic acids in the fermentation liquid (Figure 5). The process includes several important steps such as fermentation, acid hydrolysis, purification, evaporation, crystallization, and drying. Acidolysis occurs when calcium isocitrate is used under acidic conditions with an increase in hydrogen ion concentration. In the presence of sulfuric acid, a double decomposition reaction takes place resulting in an insoluble gypsum precipitate and release of a weak acid (Mores et al., 2021). This reaction leads to irreversible precipitation of calcium sulfate (Figure 5). As a result, both consumed sulfuric acid and citric acid can be completely decomposed into citric acid and calcium sulfate (Kamzolova et al., 2013).
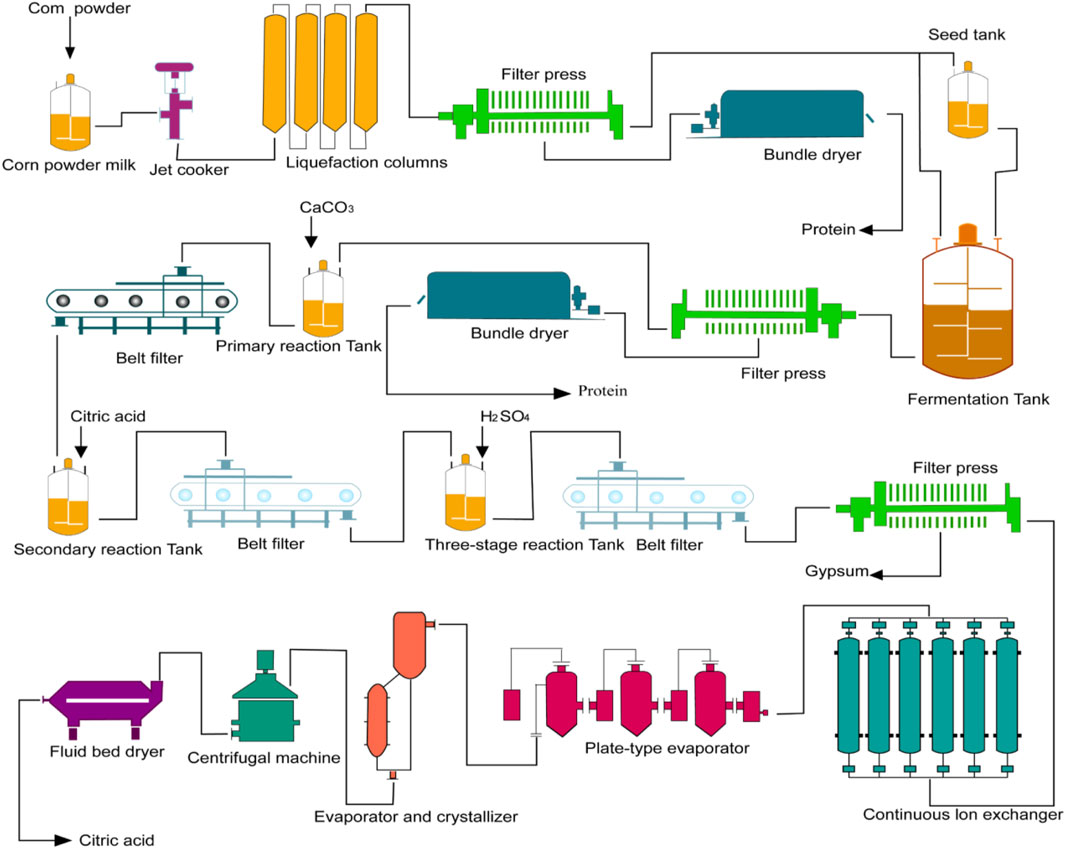
Figure 5. Citric acid production process. Including raw material treatment, culture, fermentation, mash treatment, extraction process, refining process, drying and packaging.
Metabolic engineering in microorganisms seeks to enhance the efficiency of biosynthetic routes, boost production yields, and reduce expenses in the manufacture of organic acids such as lactic acid, butyric acid, succinic acid, α-ketoglutaric acid, fumaric acid, citric acid, and isocitric acid. In the field of microbial metabolic engineering, efforts are directed towards enhancing biosynthetic pathways, improving production efficiency and cutting down on expenses in the manufacturing of lactic acid, butyric acid, succinic acid, α-ketoglutaric acid, fumaric acid, citric acid and isocitric acid.
Acid resistance is the core trait of industrial organic acid producing bacteria. The accumulation of organic acids leads to a sharp drop in pH, triggering an increase in intracellular H levels, undermining membrane potential, inhibiting enzyme activity and DNA stability. Acid-tolerant bacteria maintain pH homeostasis by enhancing proton efflux (e.g., H-atpase), synthesizing alkaline substances or modifying cell membranes, thereby reducing the cost of neutralizers, increasing product concentrations (e.g., A. niger produces citric acid at pH < 2.0) and inhibiting bacterial contamination (Hu et al., 2019).
Adaptive evolution (ALE) induces the accumulation of multigene mutations in strains through progressive pressurization (gradual reduction of pH) or dynamic stimulation (pH oscillations), including membrane structure strengthening, proton pump optimization, stress protein activation, and metabolic flux reprogramming. Combined with high-throughput screening and omics analysis, key genes can be located. Compared to metabolic engineering, ALE does not need to predict the target, but it takes a longer time. The collaborative strategy combines ALE base strains with CRISPR editing to accelerate tolerance optimization (Xu et al., 2025).
Methods for improving production efficiency focus on genetic modification of microbial strains, optimization of fermentation conditions, and the utilization of cost-effective raw materials. To sum up, the production and research of various organic acids are constantly advancing, improving production efficiency through various optimization measures, reducing costs, exploring new raw materials and new technologies, and achieving sustainable development.
TW: Writing–review and editing, Funding acquisition, Writing–original draft. HX: Conceptualization, Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. HL: Formal Analysis, Project administration, Supervision, Writing–review and editing. HY: Conceptualization, Formal Analysis, Methodology, Validation, Writing–review and editing. DH: Data curation, Methodology, Project administration, Visualization, Writing–review and editing. YJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Shandong Province (ZR2021QB113, ZR2021QB074), the Key Research and Development Program of Shandong Province (2022CXGC010506), the National Natural Science Foundation of China (22308180), and the Key Research and Development Program of Zibo (2021XCYF0085).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Rahman, M. A., Tashiro, Y., and Sonomoto, K. (2013). Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 31 (6), 877–902. doi:10.1016/j.biotechadv.2013.04.002
Acedos, M. G., Gómez-Pérez, P., Espinosa, T., Abarca, C., Ibañez, B., and Ruiz, B. (2022). New efficient meta-fermentation process for lactic acid production from municipal solid waste. Microb. Cell Factories 21 (1), 233. doi:10.1186/s12934-022-01960-9
Ahn, J. H., Seo, H., Park, W., Seok, J., Lee, J. A., Kim, W. J., et al. (2020). Enhanced succinic acid production by Mannheimia employing optimal malate dehydrogenase. Nat. Commun. 11 (1), 1970. doi:10.1038/s41467-020-15839-z
Ajala, A. S., Adeoye, A. O., Olaniyan, S. A., and Fasonyin, O. T. (2020). A study on effect of fermentation conditions on citric acid production from cassava peels. Sci. Afr. 8, e00396. doi:10.1016/j.sciaf.2020.e00396
Angumeenal, A. R., and Venkappayya, D. (2013). An overview of citric acid production. LWT - Food Sci. Technol. 50 (2), 367–370. doi:10.1016/j.lwt.2012.05.016
Babaei, M., Rueksomtawin Kildegaard, K., Niaei, A., Hosseini, M., Ebrahimi, S., Sudarsan, S., et al. (2019). Engineering Oleaginous Yeast as the host for fermentative succinic acid production from glucose. Front. Bioeng. Biotechnol. 7, 361. doi:10.3389/fbioe.2019.00361
Cao, M., Jiang, T., Li, P., Zhang, Y., Guo, S., Meng, W., et al. (2020). Pyruvate production from whey powder by metabolic engineered Klebsiella oxytoca. J. Agric. Food Chem. 68 (51), 15275–15283. doi:10.1021/acs.jafc.0c06724
Carsanba, E., Papanikolaou, S., Fickers, P., and Erten, H. (2019). Screening various Yarrowia lipolytica strains for citric acid production. Yeast 36 (5), 319–327. doi:10.1002/yea.3389
Chen, X., Dong, X., Liu, J., Luo, Q., and Liu, L. (2020). Pathway engineering of Escherichia coli for alpha-ketoglutaric acid production. Biotechnol Bioeng. 117 (9), 2791–2801. doi:10.1002/bit.27456
Chen, Y., Han, A., Wang, M., Wei, D., and Wang, W. (2023a). Metabolic engineering of Trichoderma reesei for L-malic acid production. J. Agric. Food Chem. 71 (9), 4043–4050. doi:10.1021/acs.jafc.2c09078
Chen, Y., Wang, J., Wang, M., Han, A., Zhao, X., Wang, W., et al. (2023b). Engineering the metabolism and morphology of the filamentous fungus Trichoderma reesei for efficient L-malic acid production. Bioresour. Technol. 387, 129629. doi:10.1016/j.biortech.2023.129629
Chiang, C.-J., Hu, R.-C., Huang, Z.-C., and Chao, Y.-P. (2021). Production of succinic acid from amino acids in Escherichia coli. J. Agric. Food Chem. 69 (29), 8172–8178. doi:10.1021/acs.jafc.1c02958
Cybulski, K., Tomaszewska-Hetman, L., Rakicka, M., Juszczyk, P., and Rywińska, A. (2019). Production of pyruvic acid from glycerol by Yarrowia lipolytica. Folia Microbiol. 64 (6), 809–820. doi:10.1007/s12223-019-00695-2
Deng, F., and Aita, G. M. (2018). Fumaric acid production by Rhizopus oryzae ATCC® 20344™ from lignocellulosic syrup. BioEnergy Res. 11 (2), 330–340. doi:10.1007/s12155-018-9899-y
Ding, Q., and Ye, C. (2023). Recent advances in producing food additive L-malate: chassis, substrate, pathway, fermentation regulation and application. Microb. Biotechnol. 16 (4), 709–725. doi:10.1111/1751-7915.14206
Dong, W., Xue, M., Zhang, Y., Xin, F., Wei, C., Zhang, W., et al. (2017). Characterization of a β-glucosidase from Paenibacillus species and its application for succinic acid production from sugarcane bagasse hydrolysate. Bioresour. Technol. 241, 309–316. doi:10.1016/j.biortech.2017.05.141
Eş, I., Mousavi Khaneghah, A., Barba, F. J., Saraiva, J. A., Sant'Ana, A. S., and Hashemi, S. M. B. (2018). Recent advancements in lactic acid production - a review. Food Res. Int. 107, 763–770. doi:10.1016/j.foodres.2018.01.001
Ferone, M., Ercole, A., Raganati, F., Olivieri, G., Salatino, P., and Marzocchella, A. (2019). Efficient succinic acid production from high-sugar-content beverages by Actinobacillus succinogenes. Biotechnol. Prog. 35 (5), e2863. doi:10.1002/btpr.2863
Fu, H., Yu, L., Lin, M., Wang, J., Xiu, Z., and Yang, S.-T. (2017). Metabolic engineering of Clostridium tyrobutyricum for enhanced butyric acid production from glucose and xylose. Metab. Eng. 40, 50–58. doi:10.1016/j.ymben.2016.12.014
Fu, H., Yue, Z., Feng, J., Bao, T., Yang, S.-T., Cai, Y., et al. (2022). Consolidated bioprocessing for butyric acid production from raw cassava starch by a newly isolated Clostridium butyricum SCUT620. Industrial Crops Prod. 187, 115446. doi:10.1016/j.indcrop.2022.115446
Gao, C., Xu, X., Hu, C., Zhang, W., Zhang, Y., Ma, C., et al. (2010). Pyruvate producing biocatalyst with constitutive NAD-independent lactate dehydrogenases. Process Biochem. 45 (12), 1912–1915. doi:10.1016/j.procbio.2010.05.029
Guo, F., Wu, M., Dai, Z., Zhang, S., Zhang, W., Dong, W., et al. (2020). Current advances on biological production of fumaric acid. Biochem. Eng. J. 153, 107397. doi:10.1016/j.bej.2019.107397
Guo, X., Li, X., Feng, J., Yue, Z., Fu, H., and Wang, J. (2024). Engineering of Clostridium tyrobutyricum for butyric acid and butyl butyrate production from cassava starch. Bioresour. Technol. 391, 129914. doi:10.1016/j.biortech.2023.129914
Hapeta, P., Rakicka-Pustułka, M., Juszczyk, P., Robak, M., Rymowicz, W., and Lazar, Z. (2020). Overexpression of citrate synthase increases isocitric acid biosynthesis in the Yeast Yarrowia lipolytica. Sustainability 12 (18), 7364. doi:10.3390/su12187364
Hu, W., Li, W. J., Yang, H. Q., and Chen, J. H. (2019). Current strategies and future prospects for enhancing microbial production of citric acid. Appl. Microbiol. Biotechnol. 103 (1), 201–209. doi:10.1007/s00253-018-9491-6
Ilica, R. A., Kloetzer, L., Galaction, A. I., and Cascaval, D. (2019). Fumaric acid: production and separation. Biotechnol. Lett. 41 (1), 47–57. doi:10.1007/s10529-018-2628-y
Ji, L., Wang, J., Luo, Q., Ding, Q., Tang, W., Chen, X., et al. (2021). Enhancing L-malate production of Aspergillus oryzae by nitrogen regulation strategy. Appl. Microbiol. Biotechnol. 105 (8), 3101–3113. doi:10.1007/s00253-021-11149-6
Jiang, L., Fu, H., Yang, H. K., Xu, W., Wang, J., and Yang, S.-T. (2018). Butyric acid: applications and recent advances in its bioproduction. Biotechnol. Adv. 36 (8), 2101–2117. doi:10.1016/j.biotechadv.2018.09.005
Jiang, Y., Ye, X., Zheng, T., Dong, W., Xin, F., Ma, J., et al. (2021). Microbial production of L-malate from renewable non-food feedstocks. Chin. J. Chem. Eng. 30, 105–111. doi:10.1016/j.cjche.2020.10.017
Jimenez-Quero, A., Pollet, E., Averous, L., and Phalip, V. (2020). Optimized bioproduction of itaconic and fumaric acids based on solid-state fermentation of lignocellulosic biomass. Molecules 25 (5), 1070. doi:10.3390/molecules25051070
Jodłowski, G. S., and Strzelec, E. (2021). Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: state of the art in Poland. Open Chem. 19 (1), 998–1008. doi:10.1515/chem-2021-0073
Johannsen, J., Fieg, G., and Waluga, T. (2018). Synthese von spezialchemikalien durch multienzymkaskaden im zweiphasen-system. Chem. Ing. Tech. 90 (9), 1272. doi:10.1002/cite.201855307
Juodeikiene, G., Zadeike, D., Bartkiene, E., and Klupsaite, D. (2016). Application of acid tolerant Pedioccocus strains for increasing the sustainability of lactic acid production from cheese whey. LWT - Food Sci. Technol. 72, 399–406. doi:10.1016/j.lwt.2016.05.023
Kadooka, C., Nakamura, E., Mori, K., Okutsu, K., Yoshizaki, Y., Takamine, K., et al. (2020). LaeA controls citric acid production through regulation of the citrate exporter-encoding cexA gene in Aspergillus luchuensis mut. kawachii. Appl. Environ. Microbiol. 86 (5), e01950. doi:10.1128/AEM.01950-19
Kamzolova, S. V. (2023). A review on citric acid production by Yarrowia lipolytica Yeast: past and present challenges and developments. Processes 11 (12), 3435. doi:10.3390/pr11123435
Kamzolova, S. V., Allayarov, R. K., Lunina, J. N., and Morgunov, I. G. (2016). The effect of oxalic and itaconic acids on threo-Ds-isocitric acid production from rapeseed oil by Yarrowia lipolytica. Bioresour. Technol. 206, 128–133. doi:10.1016/j.biortech.2016.01.092
Kamzolova, S. V., Dedyukhina, E. G., Samoilenko, V. A., Lunina, J. N., Puntus, I. F., Allayarov, R. L., et al. (2013). Isocitric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl. Microbiol. Biotechnol. 97 (20), 9133–9144. doi:10.1007/s00253-013-5182-5
Kamzolova, S. V., and Morgunov, I. G. (2019). Microbial production of (2 R,3 S)-isocitric acid: state of the arts and prospects. Appl. Microbiol. Biotechnol. 103 (23-24), 9321–9333. doi:10.1007/s00253-019-10207-4
Kamzolova, S. V., Samoilenko, V. A., Lunina, J. N., and Morgunov, I. G. (2021). Isocitric acid production from ethanol industry waste by Yarrowia lipolytica. Fermentation-Basel 7 (3), 146. doi:10.3390/fermentation7030146
Kamzolova, S. V., Shamin, R. V., Stepanova, N. N., Morgunov, G. I., Lunina, J. N., Allayarov, R. K., et al. (2018). Fermentation conditions and media optimization for isocitric acid production from ethanol byYarrowia lipolytica. BioMed Res. Int. 2018, 1–9. doi:10.1155/2018/2543210
Kang, N. K., Lee, J. W., Ort, D. R., and Jin, Y. S. (2021). L-malic acid production from xylose by engineered Saccharomyces cerevisiae. Biotechnol. J. 17 (3), e2000431. doi:10.1002/biot.202000431
Kawai, S., Ohashi, K., Yoshida, S., Fujii, M., Mikami, S., Sato, N., et al. (2014). Bacterial pyruvate production from alginate, a promising carbon source from marine brown macroalgae. J. Biosci. Bioeng. 117 (3), 269–274. doi:10.1016/j.jbiosc.2013.08.016
Kelbert, M., Machado, T. O., Araújo, P. H. H., Sayer, C., de Oliveira, D., Maziero, P., et al. (2024). Perspectives on biotechnological production of butyric acid from lignocellulosic biomass. Renew. Sustain. Energy Rev. 202, 114717. doi:10.1016/j.rser.2024.114717
Lee, J.-S., Lin, C.-J., Lee, W.-C., Teng, H.-Y., and Chuang, M.-H. (2022). Production of succinic acid through the fermentation of Actinobacillus succinogenes on the hydrolysate of Napier grass. Biotechnol. Biofuels Bioprod. 15 (1), 9. doi:10.1186/s13068-022-02106-0
Lee, J. W., Yi, J., Kim, T. Y., Choi, S., Ahn, J. H., Song, H., et al. (2016). Homo-succinic acid production by metabolically engineered Mannheimia succiniciproducens. Metab. Eng. 38, 409–417. doi:10.1016/j.ymben.2016.10.004
Legendre, F., MacLean, A., Appanna, V. P., and Appanna, V. D. (2020). Biochemical pathways to α-ketoglutarate, a multi-faceted metabolite. World J. Microbiol. Biotechnol. 36 (8), 123. doi:10.1007/s11274-020-02900-8
Li, B., Li, B., Wang, P., Feng, Y., Xu, X., Zhang, Y., et al. (2023). Bio-refinery of xylose processing wastes for green polymalic acid production and l-malic acid recovery by engineered Aureobasidium pullulans in a non-waste-disposal system. Chem. Eng. J. 454, 140533. doi:10.1016/j.cej.2022.140533
Li, N., Zhang, B., Wang, Z., Tang, Y. J., Chen, T., and Zhao, X. (2014). Engineering Escherichia coli for fumaric acid production from glycerol. Bioresour. Technol. 174, 81–87. doi:10.1016/j.biortech.2014.09.147
Li, X., Diffenderfer, J., and Zhu, J. (2017). Construction of a full row-rank matrix system for multiple scanning directions in discrete tomography. J. Comput. Appl. Math. 311, 529–538. doi:10.1016/j.cam.2016.08.039
Li, Y., Sun, L., Feng, J., Wu, R., Xu, Q., Zhang, C., et al. (2016). Efficient production of α-ketoglutarate in the gdh deleted Corynebacterium glutamicum by novel double-phase pH and biotin control strategy. Bioprocess Biosyst. Eng. 39 (6), 967–976. doi:10.1007/s00449-016-1576-y
Liu, K., Liu, Y., Li, X., Zhang, X., Xue, Z., and Zhao, M. (2023a). Efficient production of α-ketoglutaric acid using an economical double-strain cultivation and catalysis system. Appl. Microbiol. Biotechnol. 107 (21), 6497–6506. doi:10.1007/s00253-023-12757-0
Liu, T., Sun, L., Zhang, C., Liu, Y., Li, J., Du, G., et al. (2023b). Combinatorial metabolic engineering and process optimization enables highly efficient production of L-lactic acid by acid-tolerant Saccharomyces cerevisiae. Bioresour. Technol. 379, 129023. doi:10.1016/j.biortech.2023.129023
Liu, Y., Esen, O., Pronk, J. T., and van Gulik, W. M. (2021). Uncoupling growth and succinic acid production in an industrial Saccharomyces cerevisiae strain. Biotechnol. Bioeng. 118 (4), 1557–1567. doi:10.1002/bit.27672
Ma, X., Gao, M., Yin, Z., Zhu, W., Liu, S., and Wang, Q. (2020). Lactic acid and animal feeds production from Sophora flavescens residues by Rhizopus oryzae fermentation. Process Biochem. 92, 401–408. doi:10.1016/j.procbio.2020.01.030
Mariën, Q., Regueira, A., and Ganigué, R. (2023). Steerable isobutyric and butyric acid production from CO2 and H2 by Clostridium luticellarii. Microb. Biotechnol. 17 (1), e14321. doi:10.1111/1751-7915.14321
Mores, S., Vandenberghe, L. P. S., Magalhães Júnior, A. I., de Carvalho, J. C., de Mello, A. F. M., Pandey, A., et al. (2021). Citric acid bioproduction and downstream processing: status, opportunities, and challenges. Bioresour. Technol. 320, 124426. doi:10.1016/j.biortech.2020.124426
Morgunov, I. G., Kamzolova, S. V., Karpukhina, O. V., Bokieva, S. B., Lunina, J. N., and Inozemtsev, A. N. (2020). Microbiological production of isocitric acid from biodiesel waste and its effect on spatial memory. Microorganisms 8 (4), 462. doi:10.3390/microorganisms8040462
Moxley, W. C., Eiteman, M. A., and Atomi, H. (2021). Pyruvate production by Escherichia coli by use of pyruvate dehydrogenase variants. Appl. Environ. Microbiol. 87 (13), e0048721. doi:10.1128/aem.00487-21
Niu, S., Liu, F., Wang, Y., Rao, B., and Wang, Y. (2024). A study on the efficient preparation of α-Ketoglutarate with L-Glutamate oxidase. Molecules 29 (8), 1861. doi:10.3390/molecules29081861
Nwamba, M. C., Sun, F., Mukasekuru, M. R., Song, G., Harindintwali, J. D., Boyi, S. A., et al. (2021). Trends and hassles in the microbial production of lactic acid from lignocellulosic biomass. Environ. Technol. and Innovation 21, 101337. doi:10.1016/j.eti.2020.101337
Ozdal, M., and Kurbanoglu, E. B. (2018). Citric acid production by Aspergillus niger from agro-industrial by-products: molasses and chicken feather peptone. Waste Biomass Valorization 10 (3), 631–640. doi:10.1007/s12649-018-0240-y
Pairazaman, O. D., Woiciechowski, A. L., Zevallos, L. A., Tanobe, V. O. A., Zandona, A., and Soccol, C. R. (2024). Fumaric acid production by Rhizopus species from acid hydrolysate of oil palm empty fruit bunches. Braz. J. Microbiol. 55 (2), 1179–1187. doi:10.1007/s42770-024-01322-0
Patsalou, M., Chrysargyris, A., Tzortzakis, N., and Koutinas, M. (2020). A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 113, 469–477. doi:10.1016/j.wasman.2020.06.020
Rakicka, M., Wolniak, J., Lazar, Z., and Rymowicz, W. (2019). Production of high titer of citric acid from inulin. BMC Biotechnol. 19 (1), 11. doi:10.1186/s12896-019-0503-0
Ren, Y., Wang, X., Li, Y., Li, Y.-Y., and Wang, Q. (2022). Lactic acid production by fermentation of biomass: recent achievements and perspectives. Sustainability 14 (21), 14434. doi:10.3390/su142114434
Rzechonek, D. A., Dobrowolski, A., Rymowicz, W., and Mirończuk, A. M. (2019). Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 271, 340–344. doi:10.1016/j.biortech.2018.09.118
Schlembach, I., Bardl, B., Regestein, L., and Rosenbaum, M. A. (2024). Nonengineered fungus provides a shortcut from cellulose to bulk erythro-isocitric acid. ACS Sustain. Chem. and Eng. 12 (9), 3408–3418. doi:10.1021/acssuschemeng.3c04664
Sebastian, J., Dominguez, K. V., Brar, S. K., and Rouissi, T. (2021). Fumaric acid production using alternate fermentation mode by immobilized Rhizopus oryzae-a greener production strategy. Chemosphere 281, 130858. doi:10.1016/j.chemosphere.2021.130858
Sebastian, J., Hegde, K., Kumar, P., Rouissi, T., and Brar, S. K. (2019). Bioproduction of fumaric acid: an insight into microbial strain improvement strategies. Critical Rev. Biotechnol. 39 (6), 817–834. doi:10.1080/07388551.2019.1620677
Seregina, T. A., Shakulov, R. S., Debabov, V. G., and Mironov, A. S. (2010). Construction of a butyrate-producing E. coli strain without the use of heterologous genes. Appl. Biochem. Microbiol. 46 (8), 745–754. doi:10.1134/s000368381008003x
Shaji, A., Shastri, Y., Kumar, V., Ranade, V. V., and Hindle, N. (2021). Economic and environmental assessment of succinic acid production from sugarcane bagasse. ACS Sustain. Chem. and Eng. 9 (38), 12738–12746. doi:10.1021/acssuschemeng.1c02483
Song, Y., Li, J., Shin, H.-D., Liu, L., Du, G., and Chen, J. (2016). Biotechnological production of alpha-keto acids: current status and perspectives. Bioresour. Technol. 219, 716–724. doi:10.1016/j.biortech.2016.08.015
Sorokina, K. N., Samoylova, Y. V., Gromov, N. V., Ogorodnikova, O. L., and Parmon, V. N. (2020). Production of biodiesel and succinic acid from the biomass of the microalga Micractinium sp. IC-44. Bioresour. Technol. 317, 124026. doi:10.1016/j.biortech.2020.124026
Sun, Y., Xu, Z., Zheng, Y., Zhou, J., and Xiu, Z. (2019). Efficient production of lactic acid from sugarcane molasses by a newly microbial consortium CEE-DL15. Process Biochem. 81, 132–138. doi:10.1016/j.procbio.2019.03.022
Suo, Y., Luo, S., Zhang, Y., Liao, Z., and Wang, J. (2017). Enhanced butyric acid tolerance and production by Class I heat shock protein-overproducing Clostridium tyrobutyricum ATCC 25755. J. Industrial Microbiol. Biotechnol. 44 (8), 1145–1156. doi:10.1007/s10295-017-1939-7
Tenhaef, N., Kappelmann, J., Eich, A., Weiske, M., Briess, L., Brusseler, C., et al. (2021). Microaerobic growth-decoupled production of alpha-ketoglutarate and succinate from xylose in a one-pot process using Corynebacterium glutamicum. Biotechnol. J. 16 (9), e2100043. doi:10.1002/biot.202100043
Thangarasu, S., Siva, V., Athimoolam, S., and Bahadur, S. A. (2018). Molecular structure, spectroscopic and quantum chemical studies on benzoic acid and succinic acid co-crystals of 2-aminopyrimidine. J. Theor. Comput. Chem. 17 (04), 1850021. doi:10.1142/s0219633618500219
Tian, X., Chen, H., Liu, H., and Chen, J. (2021a). Recent advances in lactic acid production by lactic acid bacteria. Appl. Biochem. Biotechnology193 193 (12), 4151–4171. doi:10.1007/s12010-021-03672-z
Tian, X., Liu, X., Zhang, Y., Chen, Y., Hang, H., Chu, J., et al. (2021b). Metabolic engineering coupled with adaptive evolution strategies for the efficient production of high-quality L-lactic acid by Lactobacillus paracasei. Bioresour. Technol. 323, 124549. doi:10.1016/j.biortech.2020.124549
Tomaszewska-Hetman, L., Rywinska, A., Lazar, Z., Juszczyk, P., Rakicka-Pustulka, M., Janek, T., et al. (2021). Application of a new engineered strain of Yarrowia lipolytica for effective production of calcium ketoglutarate dietary supplements. Int. J. Mol. Sci. 22 (14), 7577. doi:10.3390/ijms22147577
Wang, D., Wang, L., Hou, L., Deng, X., Gao, Q., and Gao, N. (2015). Metabolic engineering of Saccharomyces cerevisiae for accumulating pyruvic acid. Ann. Microbiol. 65 (4), 2323–2331. doi:10.1007/s13213-015-1074-5
Wang, L., Cao, Z., Hou, L., Yin, L., Wang, D., Gao, Q., et al. (2016). The opposite roles of agdA and glaA on citric acid production in Aspergillus niger. Appl. Microbiol. Biotechnol. 100 (13), 5791–5803. doi:10.1007/s00253-016-7324-z
Wang, L., Chauliac, D., Moritz, B. E., Zhang, G., Ingram, L. O., and Shanmugam, K. T. (2019). Metabolic engineering of Escherichia coli for the production of butyric acid at high titer and productivity. Biotechnol. Biofuels 12 (1), 62. doi:10.1186/s13068-019-1408-9
Wang, Q., He, P., Lu, D., Shen, A., and Jiang, N. (2005). Metabolic engineering of Torulopsis glabrata for improved pyruvate production. Enzyme Microb. Technology36 36 (5-6), 832–839. doi:10.1016/j.enzmictec.2005.01.015
Wang, S., Yang, Y., Yu, K., Xu, S., Liu, M., Sun, J., et al. (2022). Engineering of Yarrowia lipolytica for producing pyruvate from glycerol. 3 Biotech. 12 (4), 98. doi:10.1007/s13205-022-03158-7
Wei, Z., Xu, Y., Xu, Q., Cao, W., Huang, H., and Liu, H. (2021). Microbial biosynthesis of l-malic acid and related metabolic engineering strategies: advances and prospects. Front. Bioeng. Biotechnol. 9, 765685. doi:10.3389/fbioe.2021.765685
Wu, F., Wang, S., Peng, Y., Guo, Y., and Wang, Q. (2023). Metabolic engineering of fast-growing Vibrio natriegens for efficient pyruvate production. Microb. Cell Factories 22 (1), 172. doi:10.1186/s12934-023-02185-0
Wu, N., Zhang, J., Chen, Y., Xu, Q., Song, P., Li, Y., et al. (2022). Recent advances in microbial production of L-malic acid. Appl. Microbiol. Biotechnol. 106 (24), 7973–7992. doi:10.1007/s00253-022-12260-y
Xiong, T., Gao, Q., Zhang, J., Zhang, J., Zhang, C., Yue, H., et al. (2024). Engineering Escherichia coli with a symbiotic plasmid for the production of phenylpyruvic acid. RSC Adv. 14 (36), 26580–26584. doi:10.1039/d4ra03707c
Xu, G., Chen, X., Liu, L., and Jiang, L. (2013). Fumaric acid production in Saccharomyces cerevisiae by simultaneous use of oxidative and reductive routes. Bioresour. Technol. 148, 91–96. doi:10.1016/j.biortech.2013.08.115
Xu, Y., Zhou, Y., Cao, W., and Liu, H. (2020). Improved production of malic acid in Aspergillus niger by abolishing citric acid accumulation and enhancing glycolytic flux. ACS Synth. Biol. 9 (6), 1418–1425. doi:10.1021/acssynbio.0c00096
Xu, Z., Sha, Y., Li, M., Chen, S., Li, J., Ding, B., et al. (2025). Adaptive evolution and mechanism elucidation for ethanol tolerant Saccharomyces cerevisiae used in starch based biorefinery. Int. J. Biol. Macromol. 284, 138155. doi:10.1016/j.ijbiomac.2024.138155
Yang, D., Xu, Y., Mo, L., Shi, M., Wu, N., Lu, L., et al. (2024). Enhancing l-malic acid production in Aspergillus niger via natural activation of sthA gene expression. J. Agric. Food Chem. 72 (9), 4869–4879. doi:10.1021/acs.jafc.3c09321
Yin, X., Madzak, C., Du, G., Zhou, J., and Chen, J. (2012). Enhanced alpha-ketoglutaric acid production in Yarrowia lipolytica WSH-Z06 by regulation of the pyruvate carboxylation pathway. Appl. Microbiol. Biotechnol. 96 (6), 1527–1537. doi:10.1007/s00253-012-4192-z
Yoshida, S., Tanaka, H., Hirayama, M., Murata, K., and Kawai, S. (2015). Production of pyruvate from mannitol by mannitol-assimilating pyruvate decarboxylase-negative Saccharomyces cerevisiae. Bioengineered 6 (6), 347–350. doi:10.1080/21655979.2015.1112472
Yovkova, V., Otto, C., Aurich, A., Mauersberger, S., and Barth, G. (2014). Engineering the alpha-ketoglutarate overproduction from raw glycerol by overexpression of the genes encoding NADP+-dependent isocitrate dehydrogenase and pyruvate carboxylase in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 98 (5), 2003–2013. doi:10.1007/s00253-013-5369-9
Yuan, W., Du, Y., Yu, K., Xu, S., Liu, M., Wang, S., et al. (2022). The production of pyruvate in biological technology: a critical review. Microorganisms 10 (12), 2454. doi:10.3390/microorganisms10122454
Yuzbasheva, E. Y., Agrimi, G., Yuzbashev, T. V., Scarcia, P., Vinogradova, E. B., Palmieri, L., et al. (2019). The mitochondrial citrate carrier in Yarrowia lipolytica: its identification, characterization and functional significance for the production of citric acid. Metab. Eng. 54, 264–274. doi:10.1016/j.ymben.2019.05.002
Yuzbasheva, E. Y., Scarcia, P., Yuzbashev, T. V., Messina, E., Kosikhina, I. M., Palmieri, L., et al. (2021). Engineering Yarrowia lipolytica for the selective and high-level production of isocitric acid through manipulation of mitochondrial dicarboxylate–tricarboxylate carriers. Metab. Eng. 65, 156–166. doi:10.1016/j.ymben.2020.11.001
Zambanini, T., Hosseinpour Tehrani, H., Geiser, E., Sonntag, C. K., Buescher, J. M., Meurer, G., et al. (2017). Metabolic engineering of Ustilago trichophora TZ1 for improved malic acid production. Metab. Eng. Commun. 4, 12–21. doi:10.1016/j.meteno.2017.01.002
Zhang, B., Zhu, Y., Zhang, J., Wang, D., Sun, L., and Hong, J. (2017). Engineered Kluyveromyces marxianus for pyruvate production at elevated temperature with simultaneous consumption of xylose and glucose. Bioresour. Technol. 224, 553–562. doi:10.1016/j.biortech.2016.11.110
Zhang, L., Bryan, S. J., and Selão, T. T. (2022). Sustainable citric acid production from CO2 in an engineered cyanobacterium. Front. Microbiol. 13, 973244. doi:10.3389/fmicb.2022.973244
Zhang, Z.-R., Shen, J.-T., Dai, J.-Y., Sun, Y.-Q., Dong, Y.-S., and Xiu, Z.-L. (2020). Separation and purification of Klebsiella phage by two-step salting-out extraction. Sep. Purif. Technol. 242, 116784. doi:10.1016/j.seppur.2020.116784
Zhou, S., Ding, N., Han, R., and Deng, Y. (2023). Metabolic engineering and fermentation optimization strategies for producing organic acids of the tricarboxylic acid cycle by microbial cell factories. Bioresour. Technol. 379, 128986. doi:10.1016/j.biortech.2023.128986
Keywords: pyruvate derivatives, metabolic engineering, microbial fermentation, carbon sources, organic acids
Citation: Wang T, Xue H, Liu H, Yuan H, Huang D and Jiang Y (2025) Advancements in metabolic engineering: unlocking the potential of key organic acids for sustainable industrial applications. Front. Bioeng. Biotechnol. 13:1556516. doi: 10.3389/fbioe.2025.1556516
Received: 07 January 2025; Accepted: 24 February 2025;
Published: 11 March 2025.
Edited by:
Jose Ruben Morones-Ramirez, Autonomous University of Nuevo León, MexicoReviewed by:
Zhaoxian Xu, Nanjing University of Science and Technology, ChinaCopyright © 2025 Wang, Xue, Liu, Yuan, Huang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Jiang, eWlqaWFuZ0BxbHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.