- 1Jiaxing Key Laboratory of Pathogenic Microbiology, Jiaxing Center for Disease Control and Prevention, Jiaxing, Zhejiang, China
- 2Department of Research and Emergency, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang, China
- 3Department of Microbiology, Haiyan Center for Disease Control and Prevention, Haiyan, Zhejiang, China
In order to understand the laboratory biosafety status of Jiaxing from 2021 to 2023, before and after the end of the COVID-19 strict control strategy, this study used Zhejiang local standard DB33/T 2540 to conduct biosafety quality control inspection and risk identification, and used Chinese industry standard RB/T 040 for risk analysis and assessment. The results showed that the major problems in biosafety management were from organization management, laboratory housekeeping, material and label management, and facilities and equipment, accounting for 39.76%, 28.97% and 14.69% respectively. Statistical analysis showed that the significant improvement of laboratory filing requirements (χ2 = 5.84, P = 0.016) was the main reason for the decrease of organization management problems (χ2 = 5.007, P = 0.025). The problems of laboratory housekeeping, experimental material and safety label management had become increasingly prominent (χ2 = 6.192, P = 0.013), especially the nonstandard use of biosafety label (χ2 = 5.218, P = 0.022). Assessment results showed that all found problems as identified risk factors in the past 3 years were determined at the level of medium or low. These suggested that in 2021–2023, the overall laboratory biosafety risk level in Jiaxing was controllable and acceptable, and the organization management had been improved greatly. At the same time, the management of laboratory housekeeping, materials and labels, especially the use of biosafety labels, had become increasingly prominent and need to be standardized and strengthened.
1 Introduction
Biosafety (or biosecurity) has become a scientific discipline which has to be implemented to protect public health and safety and to provide a safe environment for scientific growth and protection of individuals. It is extensive and diverse, and related to the fate of a nation, a country, and even the whole mankind (Wang et al., 2022). Laboratory biosafety is a crucial aspect of biosafety management, and it has become an important component of China’s national biosafety concept (Cao, 2021; Xiao and Chen, 2020; Zhao et al., 2020; Wang, 2020). Emergency response for laboratory biosafety accidents and risk assessment and control for laboratory biosafety risks are two important aspects of laboratory biosafety management. Conducting biosafety risk assessment and control by regular internal audit and external quality control inspection is of great significance for a laboratory or institution to operate safely and securely, prevent laboratory biosafety risks and reduce potential accidents (Pavone et al., 2024; Peng et al., 2018; Humblet and Saegerman, 2023).
In China, the SARS epidemic and related biosafety accidents at the end of 2002 made people realize the importance of biosafety management (Inglesby and Cicero, 2017; Lim et al., 2006). With the promulgation of Regulations on Biosafety Management of Pathogenic Microbiology Laboratories in 2004 (State Council Bulletin - China Government Website, 2019), the biosafety management of laboratories in China embarked on a standardized process. By 2018, laboratories in China had generally established laboratory biosafety management systems, and different types and levels of biosafety quality management organizations had been found in various regions, including administrative biosafety management leading groups, advisory expert committees, and technical-guiding laboratory biosafety quality control centers (LBQCCs). With the outbreak of the COVID-19 epidemic in December 2019 and the release of the Biosafety Law in October 2020, the biosecurity work of laboratories across the country had been further strengthened and emphasized (Phyu et al., 2022; Li et al., 2021). Jiaxing, as a coastal city in the north of Zhejiang, adjacent to Shanghai and Hangzhou, less than 100 km away, was greatly affected by the epidemic, and the pressure on laboratory biosafety was highlighted (Zhou and Cen, 2020).
In December 2022, China’s COVID-19 posture was relaxed duly from strict control, and entered a state of normal prevention and control (Diao et al., 2023). Therefore, 2021–2023 became an important period before and after the change of COVID-19 control strategy in China. Additionally, since 2020, with the establishment of the biosafety quality management organization LBQCC in each county, Jiaxing had achieved full coverage of the quality control network, and actively explored and gradually formed a long-term biosafety management model characterized by the collaborative participation from administrative supervision and management, expert technical guidance, and institution quality management. This study was to analyze the problems found in the biosafety quality control inspection of the laboratory in Jiaxing region before and after the strict control of the COVID-19 epidemic in 2021–2023, and to conduct risk assessment of the laboratory biosafety status, so as to propose countermeasures and improvement measures for the biosafety management of Jiaxing in the future.
2 Materials and methods
From 2021 to 2023, a total of 40 biosafety quality control inspections of Jiaxing laboratories were carried out, consisting of routine spot inspections by county-level laboratory biosafety quality control centers, and regular spot inspections by Jiaxing Laboratory Biosafety Quality Control Center (Jiaxing LBQCC) and Zhejiang provincial quality control centers (mainly Diagnostic Center and Detection Center), according to the task from the administrative department of Zhejiang Province, more than 40% of the filed/registered laboratories be randomly inspected every year. All inspections were conducted in accordance with the Zhejiang local standard DB33/T 2540 (Zhejiang Provincial Administration for Market Regulation Intellectual Property Office, 2022a; Zhejiang Provincial Administration for Market Regulation Intellectual Property Office, 2022b). It was worth noting that the manual inspection checklists based on the unpublished version of DB33/T 2540 were used before September 2022, and then the Zhejiang Biosafety Quality Control and Evaluation System (referred to as the Evaluation System) based on the published version of the standard was used in the whole province. The system, together with the Zhejiang Pathogenic Microbiology Laboratory Management Information Network (referred to as the Filing System), the Zhejiang Pathogenic Microorganism Transportation Monitoring System, the Zhejiang “Sheng’an Eye” Laboratory Biosafety Monitoring System and the Zhejiang Laboratory Biosafety Visualization Cockpit, had become an integral part of Zhejiang “Biosafety Online” Digital Intelligence Supervision System, and jointly built a “1+4”biosafety supervision matrix in Zhejiang Province (China Reform Newspaper, 2022).
From the inspection data collected from 2021 to 2023, a total of 1,001 problems or risk factors have been identified from 437 filed laboratories in Jiaxing, covering hospitals, disease control centers, blood collection and supply institutions, customs technical service centers, food and drug testing institutes, third-party testing institutions, enterprises and so on. These problems were judged by using 67 third-level indicators, and were sorted into 40 second-level indicators and eight first-level indicators, according to the standard DB33/T 2540. The three-year trends were analyzed by the statistical method Chi-Square Tests (Linear-by-Linear Association) with the software IBM SPSS Statistics version 23 (64- bit edition), and the level of significance was set at 0.05 (P = 0.05).
All found problems were regarded as identified risk factors in Jiaxing region, and the risk assessment was carried out by using risk level matrix method, combining the occurrence likelihood of a risk-induced incident with its consequence severity, according to the Chinese industry standard RB/T 040 (CNCA, 2020; National Standards and Technical Evaluation Center of the State Administration for Market Regulation, 2024). This method divides the likelihood of incidents (occurrence frequency) into five levels, 1–5, and divides the severity of the leakage of pathogenic microorganisms and the harm to society (personnel infection, environmental pollution, property damage, and social impact) into five levels, 1–5, and combines the two into the risk matrix table to determine the risk level from four levels: low, medium, high and extremely high.
3 Results
3.1 Major problems in 2021–2023
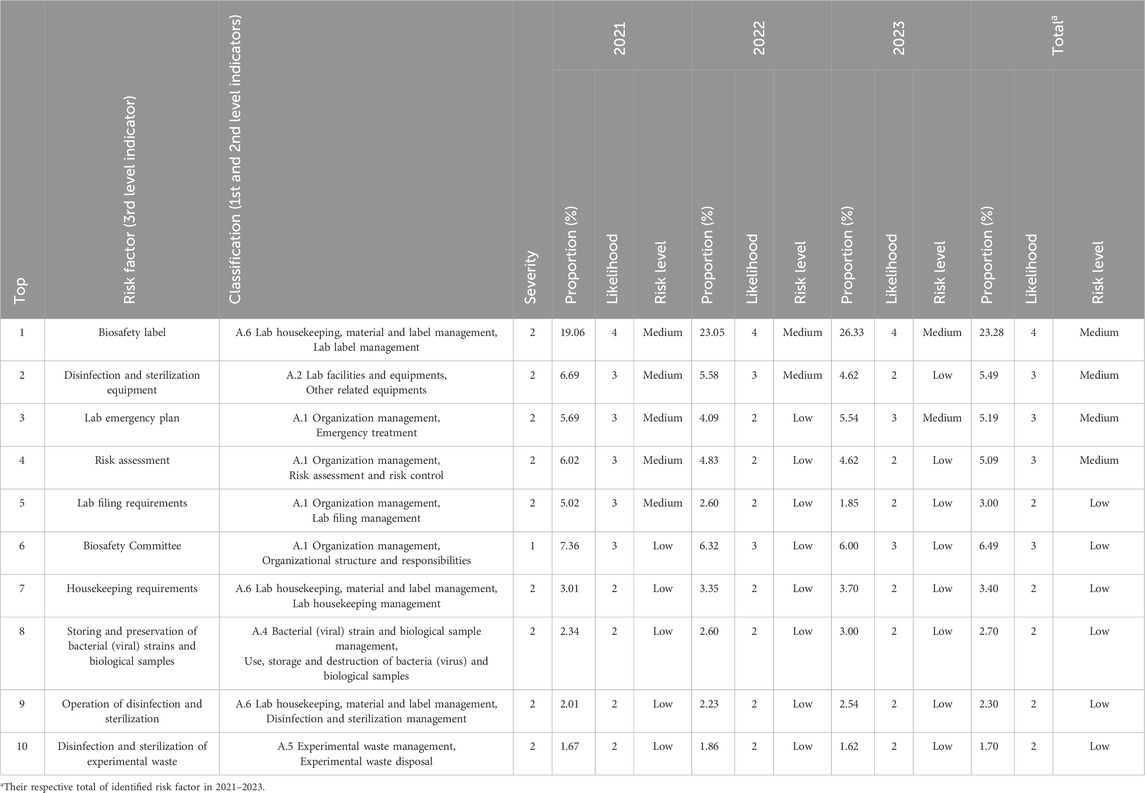
In the past 3 years, the major problems of biosafety management found in Jiaxing region came from “organizational management,” “laboratory housekeeping, material and label management,” and “laboratory facility and equipment management,” accounting for 39.76%, 28.97%, and 14.69% respectively (Figure 1).
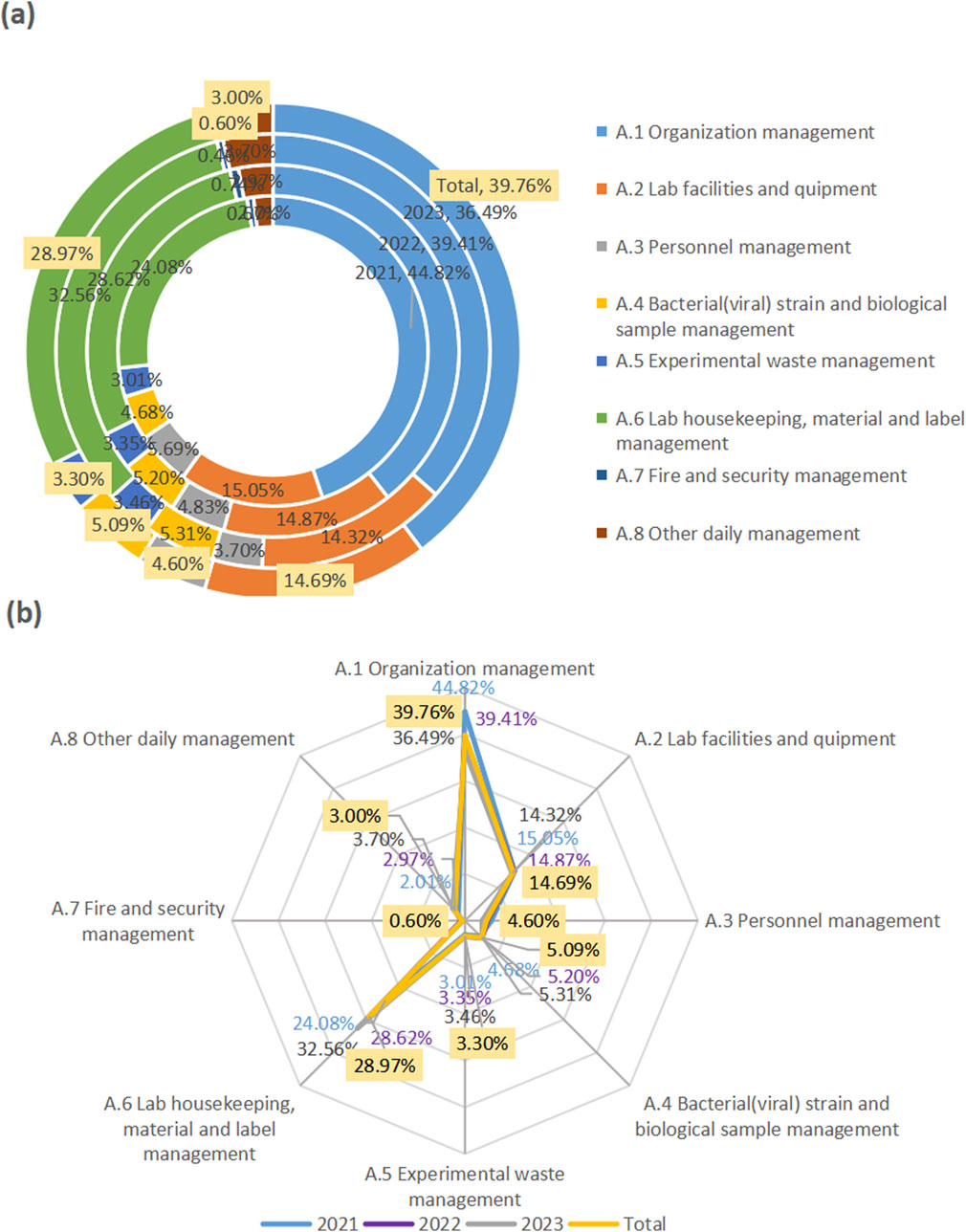
Figure 1. Major problems of biosafety management in Jiaxing in 2021–2023. The rings from inside to outside represent the problems in the years from 2021 to 2023. The “Total”with yellow labels was their respective total over the 3 years. The found problems in the first-level indicators (A1–A8) were displayed in the circular chart showing their proportion (a), and in the radar chart highlighting their contribution degree (b).
From data in the three-level indicators, in terms of organizational management, the top three were from biosafety committee (6.49%), laboratory emergency plan (5.19%), risk assessment (5.09%). In terms of “laboratory housekeeping, material and label management,” biosafety label (23.28%), laboratory housekeeping management (3.40%), and disinfection and sterilization management (2.30%) accounted for the top three. In the “laboratory facilities and equipment,” disinfection and sterilization (5.49%), hand-washing, eye-washing and sprinkler devices (4.40%), and biosafety cabinets (1.90%) were among the top three.
3.2 Asymptotic analysis at institution level
At the institution level, statistical analysis (Table 1) showed that the problems from organization management had significantly decreased over the past 3 years (χ2 = 5.007, P = 0.025), indicating that organization management had been continuously and significantly improved from 2021 to 2023, and the biosafety management work had been valued and strengthened by lab-setting institutions.
Further analysis (Table 2) showed that data from the second-level evaluation indicator of organizational management, “filing management”, and the third-level indicator “filing requirements,” had significantly decreased, and the statistical values of both were χ2 = 5.840 (P = 0.016). The values were the same because all data of the second-level indicator “filing management” were contributed by those in the third-level indicator “filing requirements,” which were 15, 7, and eight problems found in 2021, 2022, and 2023, respectively. It showed that since 2021, due to the COVID-19, Jiaxing’s administrative departments had significantly strengthened the filing work of biosafety laboratories, and made remarkable achievements in requiring the laboratories to carry out experimental activities in accordance with laws and regulations.
In addition, the “other daily management” had no statistically significant difference (χ2 = 1.723, P = 0.189) over the past 3 years, but its third-level evaluation indicators, “internal audit” (χ2 = 5.164, P = 0.023) and “management review” (χ2 = 3.123, P = 0.077), had obvious upward trends in the different significances, which suggested that laboratory-setting institutions should strengthen the internal audit and management review to improve the lab biosafety management work.
3.3 Asymptotic analysis at laboratory level
At the laboratory level, it was worth noting that there was a significant increase (Table 1) in the first-level evaluation indicator “laboratory housekeeping, material, and labeling management” in the past 3 years (χ2 = 6.192, P = 0.013). Further analysis showed that its second-level indicator “laboratory label management” (χ2 = 5.218, P = 0.022) and its third-level indicator “biosafety label” (χ2 = 5.218, P = 0.022) had been increasing from 2021 to 2023, with 57, 62, 114 problems found respectively. This indicated that in the past 3 years, the problems in the management of laboratory internal affair, experimental material and safety label, especially the use of biosafety label, had become increasingly prominent and urgently need to be standardized and strengthened.
From the data perspective, there seem to be some increase in the problems related to “bacterial (viral) strain and biological sample management” (χ2 = 0.137, P = 0.712), experimental waste management (χ2 = 0.110, P = 0.740), as well as other daily management (χ2 = 1.723, P = 0.189), though there had been no statistically significant difference over the past 3 years. This suggested that these should be the primary directions for future efforts. Risk identification, correction, prevention, and continuous improvement measures should be strengthened to achieve significant results as soon as possible.
3.4 Risk analysis at region level
All risk factors identified or problems discovered in quality control inspections in the past 3 years were determined as “general non-conformities” (according to DB33/T 2540) or level 1 - 2 of consequence severity (according to RB/T 040), due to none of pathogenic microorganism leakage, personnel infection, significant property damage or social impact. The number of medium-level risk factors was 5, 2, 2 from 2021 to 2023 respectively, and the others were at low level (Table 2).
Statistical analysis was conducted on the assessed risk levels of the top 10 identified risk factors (Table 2). The results showed no significant diffrentce in risk levels (χ2 = 2.071, P = 0.150), indicating that the overall biosafety risk levels of laboratories in Jiaxing in the past 3 years, had no significant trend change, and were acceptable and controllable, all being equal to or below the “medium.” So, all laboratory activities were allowed without stopping during inspections or assessments, and the identified risks could be reduced in a timely manner through control measures by laboratories and institutions.
4 Discussion
In this study, the local standard DB33/T 2540 of Zhejiang Province was adopted with 67 third-level indicators to identify problems or risks in laboratory biosafety management in Jiaxing, and Chinese industry standard RB/T 040 was adopted for risk assessment. In fact, DB33/T 2540 could also evaluate the found problems from the perspective of compliance with relevant regulations and standards, with a very simple evaluation process, and the possibility and severity of biosafety incidents were comprehensively considered by the evaluation experts. The results were directly provided by the evaluator at the inspection site, including inapplicability, compliance, general non-compliance, and serious non-compliance (Zhejiang Provincial Administration for Market Regulation, 2022b). Generally, a general non-compliance should be rectified (by corrective, preventive, and improvement measures) within a given period of time (usually 1 month). While a serious non-compliance refers to violations of laws, regulations, and technical standards, it means that there is a systematic deficiency in the management system, which can lead to serious biosafety incidents and must be rectified immediately.
It is worth mentioning that the WHO Laboratory Biosafety Manual also uses an assessment method based on a risk matrix (World Health Organization, 2020b; World Health Organization, 2020a), which is similar to China’s RB/T 040 that uses more simplified semi-quantitative results. The probability of an incident and the severity of its consequences are divided into three levels, the former is divided into negligible, moderate and severe, and the latter includes unlikely, possible, and likey, and the final risk level results are very low, low, medium, high and very high. Although the comparisons of methodology and matrix for risk assessment on the three standards mentioned in this study were listed in Table 3, there are no mandatory or rigid requirements for the selection of standards or methods of difficulty and complexity to carry out a biosafety risk assessment.

Table 3. Comparison of methodology and matrix for risk assessment on 3 standards mentioned in this study.
Usually, risk assessments are carried out for a specific laboratory, and reports for a regional biosafety risk assessment are rarely seen. Moreover, there are no standards and specifications for regional laboratory biosafety risk assessments. In order to systematically understand the overall laboratory biosafety risk status in Jiaxing, this study innovatively attempted to select the relatively complete and complex RB/T 040 to assess the risk status of the whole region, with the whole Jiaxing region regarded as a management body of all laboratories, the problems found in the quality control inspections as the identified risk factors, and the proportion of the problems as the occurrence frequency of risk-related incident in the inspection period. Since the probability of the occurrence of an incident and the severity of its consequences varied depending on the different risk factors, the level results were determined based on the professional experience of the industry reviewers and the actual situation of the laboratory (World Health Organization, 2020b). According to the standard RB/T 040 and from the principle of strict management, this study determined the probability of an incident on a level of 1–5: unlikely, rare, likely, very likely, and certain, with the corresponding proportion of the incident: <1%, 1%–5%, 5%–15%, 15%–30%, and >30% respectively, and determined the severity of incident consequence to 1-5 level: no impact, general impact, large impact, significant impact and particularly significant impact, depending on the degree of the personal infection, property damage and social impact caused by the leakage or release of pathogenic microorganisms. In this study, the severity of the improper use or operational risks easily leading to accidents, such as disinfection and sterilization, medical waste disposal, accident emergency disposal, personal protective equipment, biological safety cabinets and management of biological specimens of bacterial strains, were judged to be level 2, because no reports about pathogen leakage were received in Jiaxing in the past 3 years. Of course, one of the possible situations was that even if a leakage accured, with timely on-site disposal, the scope of the incident was limited into the laboratory and had not caused negative consequences, but such a incident did not need to be reported to superiors, according to Zhejiang Provincial Laboratory Biosafety Emergency Plan (Zhejiang Pathogenic Microbiology Laboratory Biosafety Quality Management Center, 2023).
From the results of this study, the overall risk level of laboratory biosafety in Jiaxing from 2021 to 2023 was at or below the medium level, indicating that the overall situation was controllable. After being standardized and strengthened, the laboratory “filing requirements” during the COVID-2019 epidemic in 2021 (medium level) had ceased to be the most main problem in 2022–2023 (low level), as a major contributor which had led to a significant improvement in organizational management over the past 3 years. While, the highest level (medium) risk factors in 2023 were biosafety label and laboratory emergency plan, and the highest level (medium) risk factors in 2021–2023 were biosafety label, disinfection and sterilization equipment, laboratory emergency plan and risk assessment, suggesting that the biosafety management organizations of Jiaxing region should follow up these risk factors or problems and pay attention to them in the near and long term. In particular, the problem of laboratory “biosafety label” had become the top risk factor, on the one hand, the non-standard use of label was indeed widespread, and on the other hand, it might be related to the subjective focus of inspection experts at different stages, that was, with the obvious improvement of organizational management in the past 3 years, the focus of inspection had shifted from organizational management at the institution level to internal management at the laboratory level.
In fact, whether a laboratory, institution, or regional biosafety management agency (administrative or technical), conducting laboratory biosafety risk assessment and risk control, should choose appropriate standards or approaches according to site-specific situation of the laboratory, the institution or neighborhood. Based on the evaluation, risk control measures, that may include but not limited to corrective, preventive and enhancement, can be implemented in line with the current situation, to minimize risk and maximize sustainability (CNCA, 2020; World Health Organization, 2020b). It is essential for the acceptable risk to determine a benchmark considering actual situations and resources such as the compliance (laws, standards and policies), security (fire, theft or loss), socioeconomic impact (environment, agriculture and public health), even the perceptions of relevant stakeholders (for example, government departments, donors, audit/oversight agencies, general public and local community) (World Health Organization, 2020b; Weng et al., 2024; Bayot and Limaiem, 2023; Bellati et al., 2022). If necessary, appropriate procedures or strategies for risk assessment and control should be developed according to the actual situation and relevant standards or manuals mentioned above, so as to better carry out relevant work (Ruoxi et al., 2018; Haque and Saha, 2020; DiGiandomenico et al., 2020). It is particularly worth mentioning that the importance of personnel training should be emphasized, although its problems did not ranking in the top 10 among all 67 three-level evaluation indicators in Jiaxing, as it plays an critical role in having a comprehensive impact on biosafety management, promoting overall systematic improvement and reducing other risks and hazards (Cornish et al., 2021; Tuncer-Göktuna et al., 2024).
5 Conclusion
From 2021 to 2023, just before and after the strict control of the COVID-19 in China, the overall laboratory biosafety level in Jiaxing had been controllable and acceptable, and the organization management had been greatly improved, especially the laboratory filing work. At the same time, new challenges have also emerged. The management of laboratory housekeeping, experimental materials and safety labels, especially the use of biosafety labels, has been becoming increasingly prominent and urgently needs to be standardized and strengthened.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YY: Funding acquisition, Methodology, Writing – original draft. CL: Resources, Writing – original draft. JX: Resources, Writing – original draft. LG: Data curation, Writing – review and editing. ZJ: Funding acquisition, Writing – review and editing. SL: Investigation, Writing – review and editing. PL: Formal Analysis, Writing – review and editing. ZX: Data curation, Writing – review and editing. PH: Validation, Writing – review and editing. GR: Investigation, Writing – review and editing. GZ: Methodology, Writing – review and editing. ZC: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The APC was funded by Zhejiang “551”Health Innovation Talent Training Project, Grant Number 2020-18-85 and Zhejiang Medical and Health Science and Technology Program (Grant No. 2022KY128).
Acknowledgments
The authors are grateful to the experts from the laboratory biosafety quality control centers at all levels for participating in Jiaxing’s quality control inspections during 2021–2023, and also thank the administrative staffs from the Science and Education Department of the Jiaxing Municipal Health Commission for their supports and helps.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1442651/full#supplementary-material
References
Bayot, M. L., and Limaiem, F. (2023). “Biosafety guidelines,” in StatPearls (Treasure Island, FL: StatPearls Publishing).
Bellati, M., Russo, V., Leone, P. A., Zito, M., and Luperini, A. (2022). Biosafety: from a traditional approach to an integrated approach. Front. Public Health 10, 956623. doi:10.3389/fpubh.2022.956623
Cao, C. (2021). China’s evolving biosafety/biosecurity legislations. J. Law Biosci. 8, lsab020. doi:10.1093/jlb/lsab020
China Reform Newspaper (2022). Building a “1+4” biosafety supervision matrix based on autonomous and controllable IoT key technologies. Available online at: http://www.cfgw.net.cn/epaper/content/202211/04/content_53217.htm (Accessed April 16, 2024).
CNCA (2020). Guidelines on risk management of biosafety in pathogenic microorganism laboratories. RB/T 040-2020. Beijing, China: Certification and Accreditation Administration of the People’s Republic of China.
Cornish, N. E., Anderson, N. L., Arambula, D. G., Arduino, M. J., Bryan, A., Burton, N. C., et al. (2021). Clinical laboratory biosafety gaps: lessons learned from past outbreaks reveal a path to a safer future. Clin. Microbiol. Rev. 16 (3), e0012618. doi:10.1128/CMR.00126-18
Diao, C., Tan, H., Wen, Y., Zhu, R., Wu, X., Zhang, S., et al. (2023). Emotions, COVID-19 related thoughts and satisfaction with life during the critical period from control to relaxation. Front. Psychol. 14, 1211614. doi:10.3389/fpsyg.2023.1211614
DiGiandomenico, K., Dunn, E., Sadowski, C., Godwin, S., Keeler, M., Preston, F., et al. (2020). Environmental health and biosafety risk assessment guidance for commercial-scale cell and gene therapy manufacturing. Appl. Biosaf. 25, 201–213. doi:10.1177/1535676020946235
Haque, M. S., and Saha, N. R. (2020). Biosafety measures, socio-economic impacts and challenges of Bt-brinjal cultivation in Bangladesh. Front. Bioeng. Biotechnol. 8, 337. doi:10.3389/fbioe.2020.00337
Humblet, M. F., and Saegerman, C. (2023). Internal audits as a tool to assess the compliance with biosecurity rules in a veterinary faculty. Front. Vet. Sci. 10, 960051. doi:10.3389/fvets.2023.960051
Inglesby, T., and Cicero, A. (2017). Protecting the nation from health security threats. Health Secur. 15, 1–5. doi:10.1089/hs.2016.0122
Li, J., Li, S., Cao, W., Wang, Z., Liang, Z., Fu, W., et al. (2021). Appraisal of China’s response to the outbreak of COVID-19 in comparison with SARS. Front. Public Health 9, 679540. doi:10.3389/fpubh.2021.679540
Lim, W., Ng, K. C., and Tsang, D. N. (2006). Laboratory containment of SARS virus. Ann. Acad. Med. Singap 35, 354–360. doi:10.47102/annals-acadmedsg.v35n5p354
National Standards and Technical Evaluation Center of the State Administration for Market Regulation (2024). Certification and accreditation of standardized information service platform. Available online at: https://hbba.sacinfo.org.cn/stdDetail/0d86937156bafe660c1e1497d4890b8e93e9f9bc6c1657fcefac62436fe91d8c (Accessed April 16, 2024).
Pavone, S., Iscaro, C., Giammarioli, M., Beato, M. S., Righi, C., Petrini, S., et al. (2024). Biological containment for african swine fever (ASF) laboratories and animal facilities: the Italian challenge in bridging the present regulatory gap and enhancing biosafety and biosecurity measures. Animals 14, 454. doi:10.3390/ani14030454
Peng, H., Bilal, M., and Iqbal, H. M. N. (2018). Improved biosafety and biosecurity measures and/or strategies to tackle laboratory-acquired infections and related risks. Int. J. Environ. Res. Public Health 15 (12), 2697. doi:10.3390/ijerph15122697
Phyu, S., Joseph, T., and Goulart, M. (2022). Strengthening biorisk management in research laboratories with security-sensitive biological agents like SARS-CoV-2. Methods Mol. Biol. 2452, 395–439. doi:10.1007/978-1-0716-2111-0_23
Ruoxi, P., Kelsey, A. H., Walther, M., Pradeep, D. U., and Benjamin, F. (2018). A biocontainment procedure for intravital microscopy of high-risk pathogens. Appl. Biosaf. 23, 211–222. doi:10.1177/1535676018785177
State Council Bulletin - China Government Website (2019). Regulations on the management of biological safety in pathogenic microbial laboratories - supplement 1, 2019. Available online at: https://www.gov.cn/gongbao/content/2019/content_5468882.htm (Accessed April 16, 2024).
Tuncer-Göktuna, P., Fontes, B. A., Çokçalışkan, C., Asar, E., and Karakaya, M. (2024). Implementing an organizational culture of biosafety and biosecurity in the ŞAP Institute. Health Secur. 22 (4), 271–280. doi:10.1089/hs.2023.0044
Wang, H., Zhu, S., Zhang, J., You, L., Yin, Z., Chu, Q., et al. (2022). “Biosafety and the change of world pattern,” in China biosafety: strategies and countermeasures. Editors H. Wang, L. Yan, H. Wang, and M. Lang 1st ed. (Beijing, China: Citic Publishing Group), 4–34.
Wang, X. L. (2020). The age of biosecurity:new biotechnology revolution and national biosecurity governance. China Biotechnol. 40 (9), 95–109. doi:10.14093/j.cnki.cn10-1132/d.2020.04.005 (Chinese).
Weng, S. T., Li, Q. W., Gao, Y. D., and Qiu, Y. F. (2024). Biosafety risk control strategies in laboratory animal research. Saf. Health Work 15, 118–122. doi:10.1016/j.shaw.2023.11.005
World Health Organization (2020a). Laboratory biosafety manual. 4th edition. Available online at: https://www.who.int/publications/i/item/9789240011311 (Accessed April 16, 2024).
World Health Organization (2020b). “Risk assessment,” in Laboratory biosafety manual. 3rd edition (Geneva: World Health Organization), 25–45.
Xiao, X., and Chen, X. (2020). Biosafety Governance under the Overall National Security Concept: Generating Logic, Practical Value, and Path Exploration [J]. Inter. Outlook, 12(5), 21. doi:10.13851/j.cnki.gjzw.202005007
Zhao, C.H., Su, D. D., Li, C., Wu, Z., Zuo, K., Xu, Y., et al. (2022). Synthetic biology risks and biosafety strategies in the view of overall national security concept. China Biotech. 42(12), 120–128. doi:10.13523/j.cb.2206014
Zhejiang Pathogenic Microbiology Laboratory Biosafety QualityManagement Center (2023). Zhejiang Province pathogenic Microbiology laboratory management health network. Available online at: https://zjsys.wsjkw.zj.gov.cn/xxinfo?uuid=1735497796005023745 (Accessed April 16, 2024).
Zhejiang Provincial Administration for Market Regulation (Intellectual Property Office) (2022a). Details of local standards_Zhejiang standards online. Available online at: https://zlzx.zjamr.zj.gov.cn/bzzx/public/std/db/view/caa2e31b96e54bafa61b3798de63af8a.html (Accessed April 16, 2024).
Zhejiang Provincial Administration for Market Regulation (2022b). Specification for the evaluation of biosafety laboratory management. DB33/T 2540-2022. Hangzhou, China: Zhejiang Provincial Administration for Market Regulation.
Keywords: biosafety, biosecurity, risk assessment, laboratory, quality control, public health
Citation: Yan Y, Li C, Xu J, Gao L, Jiang Z, Lv S, Li P, Xiang Z, He P, Ren G, Zhu G and Chen Z (2025) Biosafety status analysis and risk assessment of laboratories from 2021 to 2023 in Jiaxing, China. Front. Bioeng. Biotechnol. 13:1442651. doi: 10.3389/fbioe.2025.1442651
Received: 02 June 2024; Accepted: 27 March 2025;
Published: 16 April 2025.
Edited by:
John M. Hardham, Zoetis, United StatesReviewed by:
Furqan Kabir, Aga Khan University, PakistanShamsul Arfin Qasmi, Air University, Pakistan
Copyright © 2025 Yan, Li, Xu, Gao, Jiang, Lv, Li, Xiang, He, Ren, Zhu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoying Zhu, anhjZGN6aHVndW95aW5nQDE2My5jb20=; Zhongwen Chen, NTY0MzIzNTI4QDEzOS5jb20=
†These authors have contributed equally to this work
 Yong Yan
Yong Yan Chan Li2†
Chan Li2†