- 1Department of Biotechnology, College of Natural and Computational Science, Wolkite University, Wolkite, Ethiopia
- 2Regulatory Science Group, International Centre for Genetic Engineering and Biotechnology, Trieste, Italy
Genome editing and gene drive technologies are increasingly gaining attraction in Africa, with researchers exploring their potential applications in agriculture, health and the environment. Acknowledging that robust regulatory frameworks are crucial in facilitating the development and utilization of these technologies, informed decision-making is, however, being impeded by the fragmented information availability and readiness of regulatory authorities on the continent.
Objectives: This study investigates the regulatory frameworks governing genome editing and gene drive technologies in African countries, identifies common regulatory challenges and proposes actionable solutions.
Methods: Primary data were collected through questionnaires and complemented by analysing existing biosafety regulations from online databases and scientific literature.
Results: Our findings suggest that while a few African countries have recently updated their regulatory frameworks, many are still under discussion. Challenges to development and implementation include limited resources, expertise, awareness, and public resistance.
Conclusion: The findings underscore the urgent need for further development in regulatory capacities. By shedding light on these challenges, our study could provide African regulators with valuable insights to guide the formulation of effective regulatory frameworks. Such frameworks are essential for harnessing the potential of genome editing and gene drive technologies while safeguarding human health and the environment in Africa.
1 Introduction
Genome editing is the ability to make precise changes to DNA sequences within organisms using engineered nuclease enzymes to cut and replace existing DNA segments (Fridovich-Keil, 2024). Among the numerous genome-editing methods now available, CRISPR/Cas-based genome editing stands out as the most convenient, efficient, precise and widely used genome editing tool (Knott and Doudna, 2018; Li et al., 2022; Matsumoto and Nomura, 2023). Furthermore, CRISPR/Cas-based gene drive systems enable the manipulation of self-propagating genetic elements, which are passed on to offspring at frequencies surpassing Mendelian inheritance (Bier, 2022). Thus, gene drives bias inheritance patterns by increasing their prevalence in successive generations (Alphey et al., 2020).
In Africa, genome editing and gene drive technologies hold promise for addressing pressing challenges in agriculture, health and the environment. For instance, the increasing food demand, exacerbated by factors such as climate change, diseases, and limited access to fertilizers and agrochemicals, necessitates innovative solutions. Genome editing technology is another approach that offers avenues to enhance agricultural productivity by, for instance, developing drought tolerance (Osakabe et al., 2016; Sami et al., 2021; Shelake et al., 2022), disease resistance (Karmakar et al., 2022), salt tolerance (Saradadevi et al., 2021; Shelake et al., 2022), and nutritional improvement (Ku and Ha, 2020; Nagamine and Ezura, 2022).
Likewise, the escalating prevalence of insect-borne diseases like malaria in tropical and subtropical regions of Africa underscores the urgency of novel public health interventions. Despite various control strategies, African countries continue to grapple with the highest malaria burden globally (Ombogo, 2023). In pursuit of the ambitious goal of malaria elimination by 2030, the World Health Organization (WHO) has prioritized gene drive mosquitoes as a transformative technology (Wamba, 2023), which represents a promising new tool for the elimination of malaria and other mosquito-borne diseases (North et al., 2020; Metchanun et al., 2022).
Researchers are increasingly drawn to gene drive technologies due to their potential as highly effective, cost-efficient, and enduring solutions (Bier, 2022; James et al., 2018; James et al., 2023). However, the adoption of genome editing and gene drive technologies highly depends on government regulation in each country (Jenkins et al., 2021). Such regulation plays a crucial role in determining whether approval is necessary and thus given for the development and commercialization of these products (Entine et al., 2021). Moreover, regulatory frameworks serve to safeguard human health and the environment while fostering public trust and legal certainty for research institutions and industries (Zawedde et al., 2018; DiversityS. O. T. C. O. B., 2000).
The global regulatory landscape for genome-edited products is evolving rapidly (Tripathi et al., 2022). While some countries have swiftly adapted legislation or regulatory frameworks to support genome editing, others remain in the policy formulation stages (Jenkins et al., 2021), and some still classify these products as Genetically Modified Organisms (GMOs) (Hundleby and Harwood, 2022). Whether genome-edited products are exempt from GMO regulations often depends on the specific genome-editing techniques used. For instance, certain gene editing methods, such as site-directed nucleases (SDNs), including SDN-1, which induces gene disruptions through insertions or deletions, and SDN-2, which uses homologous templates for gene correction or modification, are fully exempted in some countries (Wolt et al., 2015). In contrast, SDN-3, which involves inserting larger DNA elements or foreign genes, is typically treated as a GMO (Vora et al., 2023). Countries like Argentina, Australia, Brazil, Chile, India, Kenya, Nigeria, Paraguay, Russia, and the USA have exempted genome-edited plants from GMO regulations, while China and the UK follow simplified GMO regulations. The EU, New Zealand and South Africa, however, regulate genome-edited products as GMOs, and in many countries, proper regulations or discussions are still lacking (Friedrichs et al., 2019; Schmidt et al., 2020; Vora et al., 2023).
Although regulatory frameworks for GMOs have been established in many African countries following their adoption of the Cartagena Protocol on Biosafety (Akinbo et al., 2021; Quemada, 2022), several countries are still working to effectively manage modern biotechnology and implement national biosafety frameworks for GMOs (Komen et al., 2020). Furthermore, most African countries do not have adequate regulatory frameworks specifically tailored to regulate genome editing and gene drive technologies regulatory oversight (Masehela and Barros, 2023). A well-established GMO regulatory framework can be a logical departure point when contemplating genome-editing governance (Abkallo et al., 2024). In this regard, efforts have been made to incorporate genome editing products into the existing GMO biosafety regulatory frameworks on a case-by-case basis; however, concerns remain about the overly restrictive nature of regulations concerning the introduction and development of genome editing and gene drive products in Africa (Ongu et al., 2023). Additionally, existing laboratory biosafety and biosecurity review processes may not effectively address the unique challenges posed by gene drive research and its components (Millett et al., 2022). Uncertainties such as limited access to laboratories, equipment and reagents, a shortage of trained professionals for molecular biology work, and a low rate of returnees among the trained professionals working internationally have significantly negatively impacted genome editing research and development in Africa (Abkallo et al., 2024), which, in turn, has also affected the development of regulatory frameworks for genome editing and gene drive technologies.
Concerns also arise regarding the potential dispersion and persistence of gene drive transgenes beyond the release area, posing challenges to their regulation under existing GMO regulatory frameworks (Gene Drives on the Horizon, 2016; Programme, 2021). Furthermore, the lack of mitigation and traceability strategies (Noble et al., 2018), together with limited experience in risk assessment, exacerbates regulatory uncertainties (AGBC, 2022). Responsible field-based gene drive research also raises significant concerns (Thizy et al., 2020), further complicating regulatory efforts. As a result, the regulatory status of genome editing and gene drive technologies in Africa remains uncertain, raising questions about the adequacy of existing frameworks and the need for new or updated regulatory frameworks (Asquer and Morrison, 2022). However, a few African countries have initiated efforts to incorporate genome editing products into their biosafety regulatory frameworks, such as Nigeria and Kenya (Boluwade and Smith, 2021; Tripathi et al., 2022), while other African countries like Burkina Faso, Mali and Uganda plan to start field trials of gene drive mosquitoes within the next 5–10 years (Hartley et al., 2021).
Given these challenges, an African Union policy consultation advocates for a more enabling and science-based regulatory approach in order to leverage genome editing and gene drive technologies (Komen et al., 2020). Dolezel et al. (2020) suggested a need for a comprehensive examination of current GMO regulatory frameworks to determine their suitability for addressing the potential risks and challenges posed by gene drive applications. A comprehensive analysis is required to shed light on the existing regulatory frameworks that govern these technologies in African countries. The aim of our study was, therefore, to analyze the status of regulatory frameworks for genome editing and gene drive technologies and identify gaps in their development and implementation in African nations. To this end, the regulatory approaches of various African countries were compared and contrasted, common trends and differences were identified, the adaptability of the current regulatory frameworks to emerging technologies was assessed and recommendations for improving biosafety regulations in African countries were formulated.
This serves as a comprehensive update on the current status and challenges facing biosafety regulatory frameworks in African countries concerning genome-edited and gene drive technologies, contributing to the advancement of these technologies on the continent. This information can equip African regulators, policymakers and researchers with valuable insights into the establishment of robust regulatory frameworks for genome editing and gene drive products.
2 Methodology
This exploratory study adopted a qualitative approach to investigate the regulatory environment for genome editing and gene drive technologies in Africa. A survey was designed with five distinct sections, combining both closed-ended and open-ended questions, thereby allowing for in-depth insights into the aspects of biosafety regulations. The survey was deployed between 22 July and 07 August 2023. The initial section gathered demographic information and general insights. Subsequently, the survey enquired into core aspects, including the status of biosafety regulatory frameworks for genome editing and gene drive technologies, international collaborations and harmonization efforts, identified gaps and challenges in existing regulations and explored public perceptions along with ethical considerations. The survey targeted Cartagena Protocol national focal points and national competent authorities across 54 African countries, aiming to discern the presence and effectiveness of regulatory frameworks. Additionally, data on national biosafety regulatory status was cross-verified through the database of Biosafety Clearing-House (BCH) and the one curated by the African Biosafety Network of Expertise (ABNE). To enhance the comprehensiveness of our findings, a literature search was conducted to supplement and triangulate the information gathered from the questionnaire and databases, ensuring a robust and multifaceted analysis of the regulatory landscape in Africa.
3 Results
3.1 The authorization for genetic engineering applications in agriculture
The survey findings reveal varying degrees of authorization for genetic engineering applications across African countries at different developmental stages. For example, some African countries may have authorized only research and development (R&D) and confined field trials (CFTS) activities, as exhibited in DRC, Tunisia and Uganda, while others may have authorized GM crops, including the importation and cultivation for feed and food applications (Table 1). Notably, Eswatini, Kenya and Nigeria have authorized genetic engineering applications at nearly all developmental stages. In contrast, countries such as Ethiopia, Ghana, Mozambique, Uganda and Zambia have limited their authorization to laboratory research and confined field trials. While in countries such as Benin, Côte d'Ivoire, DRC, Gambia, Mali, Nambia, Senegal, Togo, Tunisia and Zimbabwe, authorizations for genetic engineering applications at various stages of development are still lagging compared to other African countries.
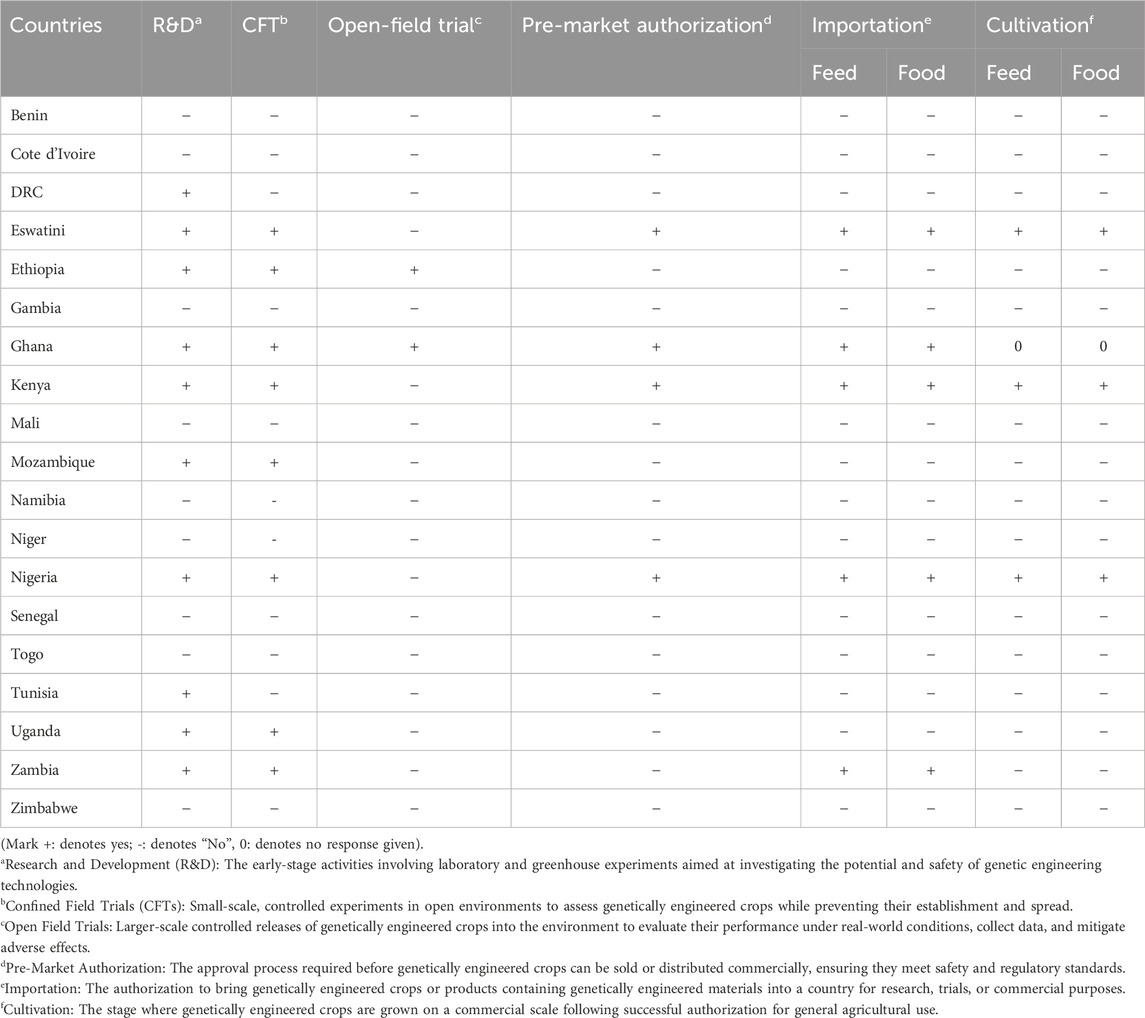
Table 1. The status of authorization for biotechnology applications in African countries according to the survey respondents. Data was collected between 22 July and 07 August 2023.
3.2 Status of regulatory framework for genome editing and gene drive technologies in Africa
According to survey respondents, Benin, Eswatini, Kenya, Nigeria and Uganda have established suitable regulatory frameworks to regulate genome editing and gene drive technologies and their products. In Benin, Eswatini and Uganda, existing biosafety regulations can be applied to regulate genome-edited and gene drive products. Kenya and Nigeria have implemented specific regulations tailored to oversee these technologies. In this sense, Nigeria has amended existing biosafety regulations to encompass genome editing products within governmental safety review and approval procedures. Kenya has also developed a new genome editing-specific regulatory framework that oversees technology use within the safety review and requisite governmental approval. For gene drive technology, the Kenyan respondents replied that existing biosafety regulations would be applied. Survey results further highlight that Eswatini, Nigeria and Uganda adopt a process-based approach to regulating these technologies, while Benin and Kenya employ a product-based approach to regulating genome editing technology (Table 2). In Zambia, on the other hand, the approach to regulation remains under development.
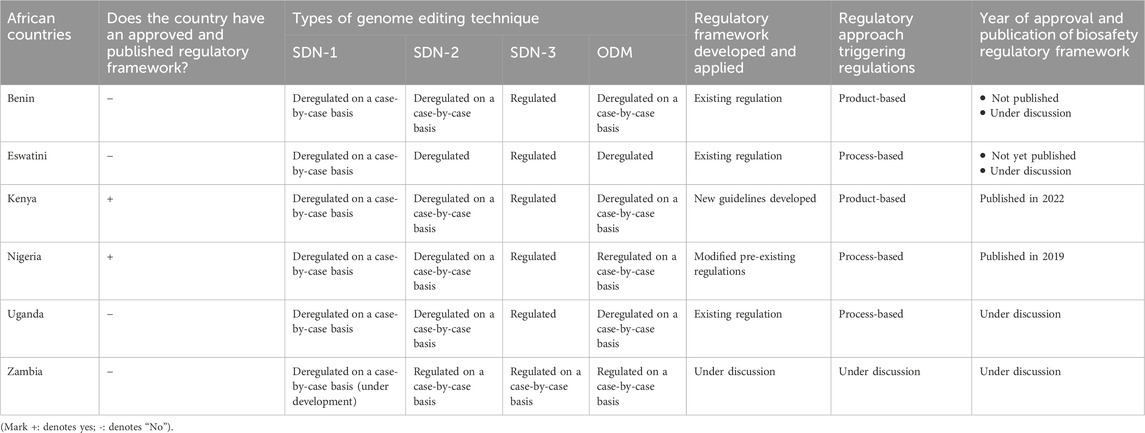
Table 2. Details status of the regulatory framework for genome editing and gene drive technologies in six African countries.
Regarding genome editing techniques, responses from Benin, Kenya, Nigeria and Uganda indicate that those techniques incorporating site-directed nucleases-3 (SDN-3) fall under regulatory oversight, while those utilising site-directed nucleases-1 (SDN-1), site-directed nucleases 2 (SDN-2) and Oligonucleotide-directed mutagenesis (ODM) are exempted from existing regulation oversight on a case-by-case basis (Table 2; Figure 1). Additionally, respondents from Eswatini indicated that SDN-1, SDN-2 and SDN-3(with cis-insert) are exempted from GMO regulations on a case-by-case approach, while SDN-3 (with trans-insert) are regulated under existing GMO regulations. Similarly, in Zambia, in which the regulatory framework is under development, SDN-2 and SDN-3 categories are expected to fall under existing regulations, while SDN-1 and ODM will be deregulated from GMO regulation on a case-by-case basis.
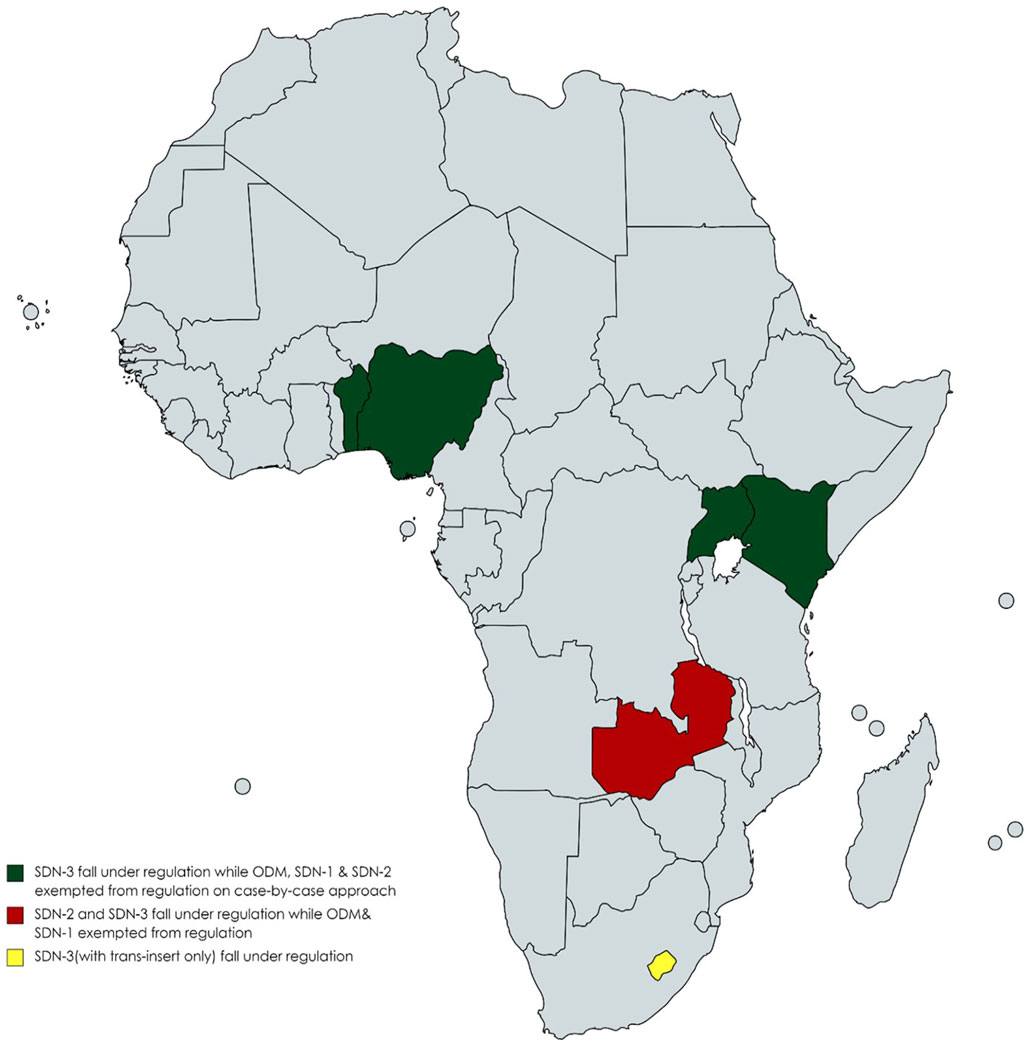
Figure 1. Regulation of genome edited products according to the genome editing technique used by country.
3.3 Challenges
3.3.1 Identified gaps in the implementation of regulatory frameworks
Respondents highlighted significant challenges in the implementation of national biosafety regulations for genome editing and gene drives across most African countries. Top among these challenges are limited resources and expertise, coupled with a lack of awareness or understanding of the technologies (Figure 2A). Additionally, the implementation of these regulations faces hurdles in certain countries due to public resistance or scepticism. Notably, respondents from The Gambia and the Democratic Republic of Congo (DRC) indicated gaps in their regulatory framework, with the DRC notably lacking a biosafety law for these technologies. The response from Benin underscores that although lack of awareness or understanding, limited resources and expertise, and public resistance or scepticism exist as gaps in the implementation of the national legal framework for genome editing and gene drive technologies, the issues are so dynamic and difficult to address, suggesting the need for a holistic, comprehensive approach to implementing the framework.
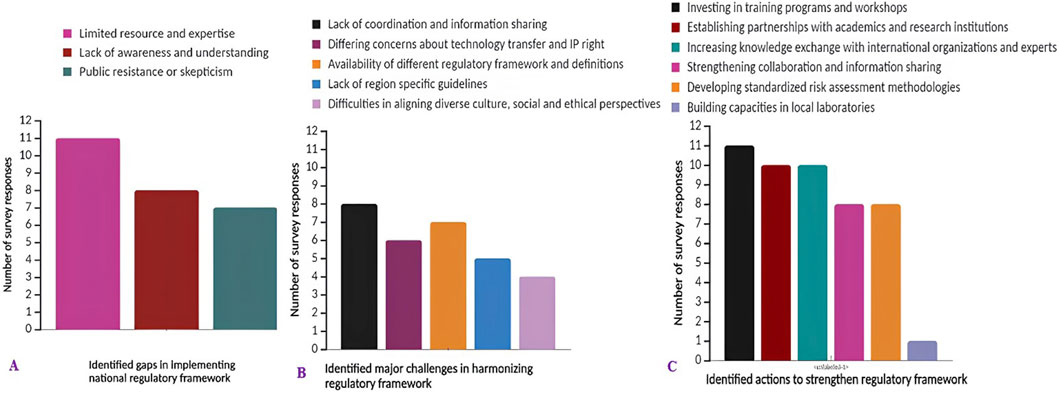
Figure 2. The survey response analysis on: (A) identified gaps in the implementation of the national biosafety regulatory framework; (B) major challenges or barriers in harmonizing biosafety regulations for genome editing at a regional or international level; (C) action needs to strengthen the biosafety regulatory framework for genome editing and gene drive technologies.
3.3.2 The major challenges in harmonizing biosafety regulatory framework in African countries
The survey findings indicate significant obstacles to aligning the biosafety regulatory framework for genome editing and gene drive technology at a regional or international level in African countries. Foremost among these harmonization challenges is the lack of coordination and information sharing between regulatory authorities. Additionally, differing concerns about technology transfer and intellectual property rights, as well as variations in regulatory frameworks and definitions among African countries, pose considerable hurdles. Aligning diverse cultural, social and ethical perspectives also presents challenges. However, respondents identified this as the least significant barrier to harmonizing the biosafety regulatory frameworks (Figure 2B). Thus, unharmonized regulatory frameworks could potentially hinder the application of genome editing and gene derive technologies and future international trade.
3.3.3 Strengthening regulatory frameworks for genome editing and gene drive technologies
This investigation, drawing input from African biosafety authorities, experts, scientists and civil society, underscores several crucial actions necessary for the development and enhancement of biosafety regulatory frameworks for genome editing and gene drive technologies and products (Figure 2C). Foremost among these actions is the investment in training programmes and workshops aimed at regulators and stakeholders. Furthermore, the analysis identified pivotal actions such as the establishment of partnerships with academic institutions for research and capacity building, along with increasing knowledge-sharing with international organizations and experts. Additionally, strengthening collaboration among African countries and the development of specific, standardized risk assessment methodologies for genome editing and gene drive was indicated as an additional crucial action.
3.3.4 Public perception, resistance and ethical issues
Responses from nine countries, including Benin, the DRC, Ethiopia, Kenya, Mozambique, Nigeria, Tunisia, Uganda, and Zambia, underline significant public resistance and controversies surrounding genome editing and gene drive technologies and products. The reasons cited for this opposition relate to the early stage of development of these technologies and public concerns regarding potential harm to human health and the environment (Table 3). Moreover, respondents from five countries indicated concerns about the potential for unintended consequences or misuse of these technologies. Social media activism emerged as a key driver of resistance and controversies against these technologies. Notably, respondents from Eswatini reported no controversy or public opposition to genome editing or gene drive, while in Nigeria, although controversies exist, there is no apparent public resistance raised through social media activism to prevent activities with the technologies.
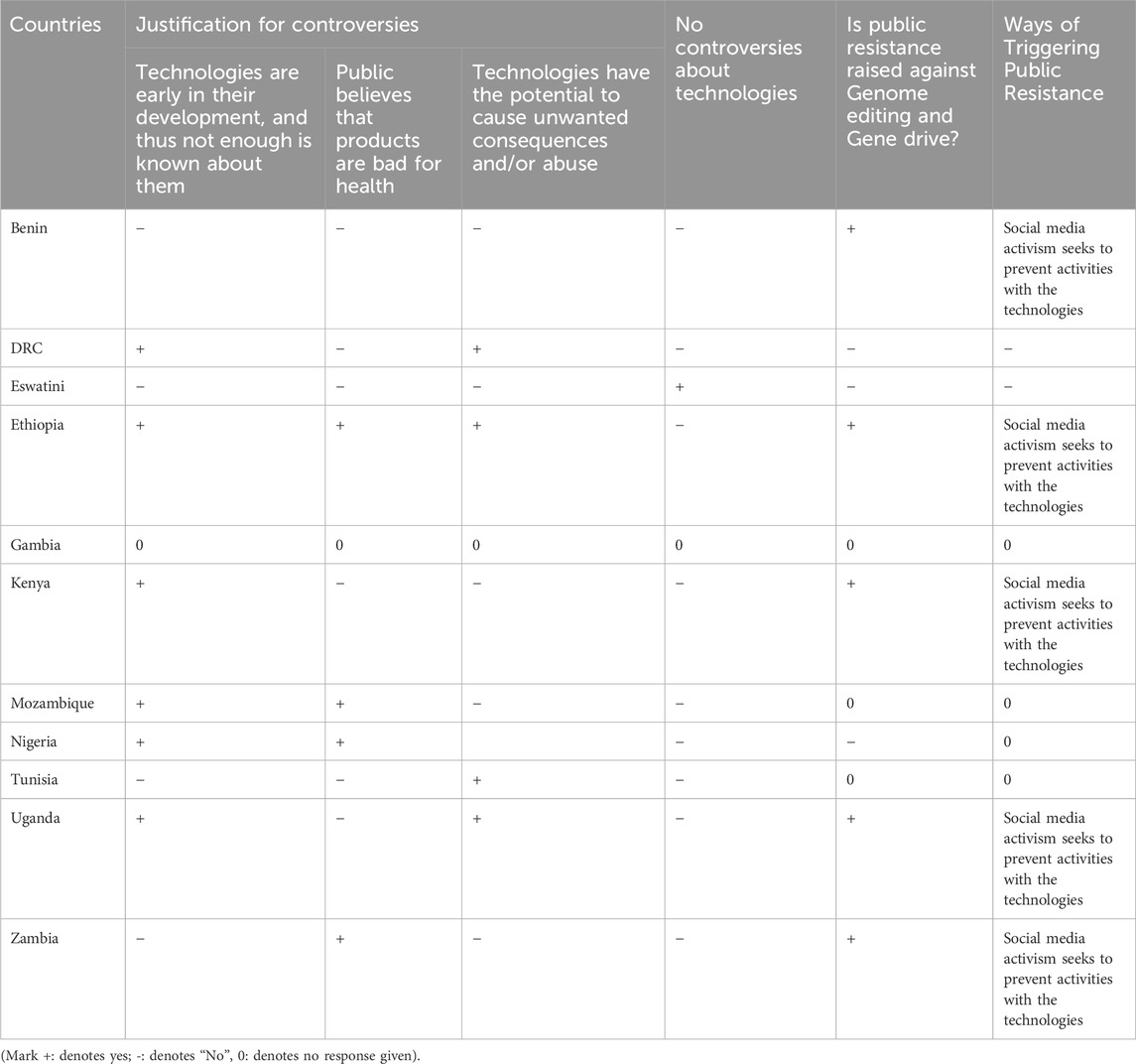
Table 3. Survey analysis on the types of public resistance against genome editing and gene drive technologies.
3.3.5 Improving public perception and attitude
Survey responses indicate that public perception can be improved by involving the public more in the decision-making process regarding the release of genome-edited and gene drive products (Figure 3A). Furthermore, providing unbiased information and easily understandable information about these technologies to non-specialists is seen as crucial for improving public perception. Only a few countries mentioned the importance of identifying and defining ethical issues, increasing public confidence in the regulatory systems, and requiring informed consent from recipient communities to support the improvement of public perceptions and attitudes towards genome editing and gene drive technologies.
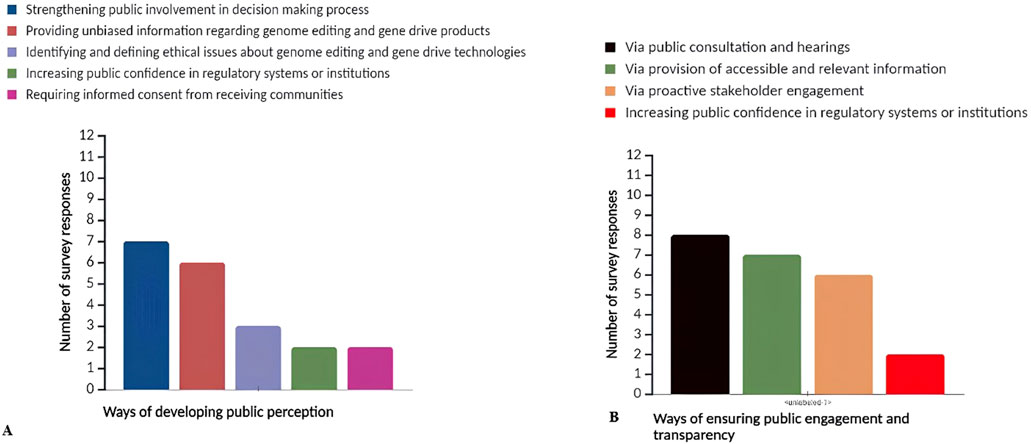
Figure 3. The survey response analysis on: (A) ways of public perception and attitude development towards genome editing and gene drive technologies/products; (B) ways to ensure public engagement and transparency in the decision-making process towards genome editing and gene drive technologies and products.
3.3.6 Ensuring public engagement and transparency
This investigation highlights the importance of public consultation and hearings in the decision-making process regarding genome editing and gene drive technologies (Figure 3B). Additionally, ensuring accessible information about these technologies and proactive stakeholder engagement and transparency in the decision-making process are seen as essential for improving public perception.
3.4 Prospective toward developing/drafting the biosafety regulatory framework in African countries
The current findings highlight the growing interest among several African countries in developing and implementing regulatory frameworks to regulate the use of genome editing and gene drive technologies (Table 4). This includes the formulation of regulatory frameworks aimed at ensuring the safe and ethical use of these technologies within respective national contexts. In countries like the DRC, Eswatini, Ethiopia, Mozambique and Uganda, discussions and drafting efforts are underway to establish modern biosafety regulatory regimes. These countries recognize the need for a regulatory system tailored to address the specific challenges and opportunities presented by these technologies.
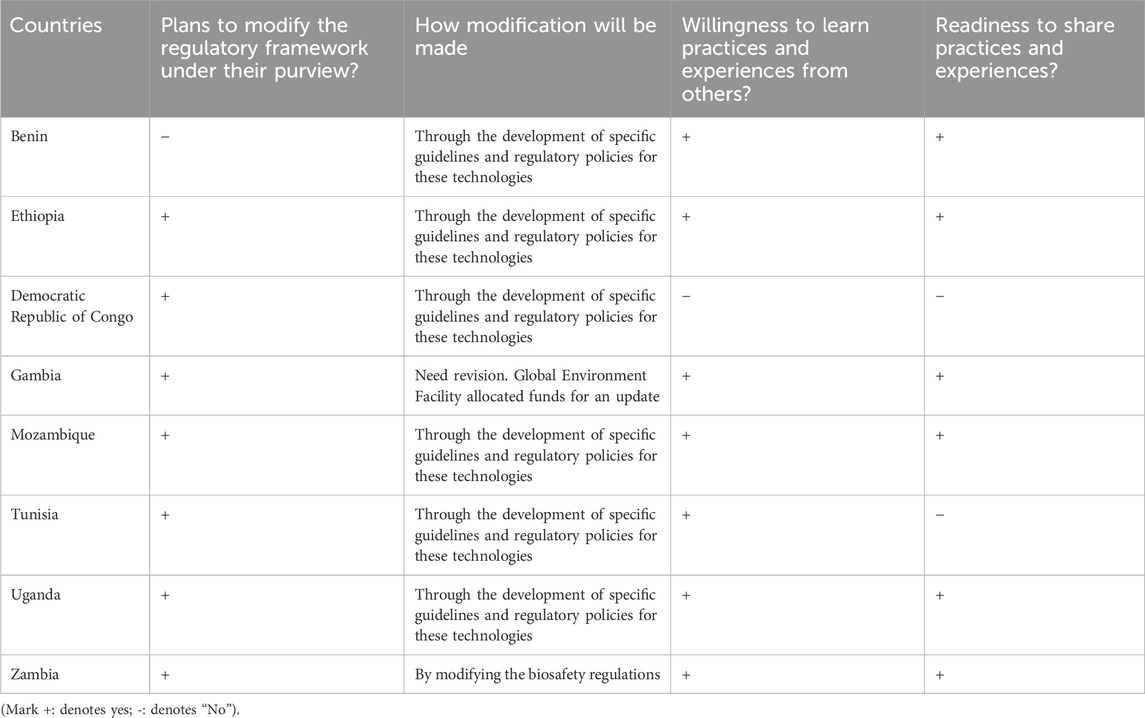
Table 4. The survey results on some African countries plan to draft or modify their existing regulations for genome editing and gene drive technologies.
3.5 Literature search
3.5.1 Status approval of genetic engineering applications
The scientific literature provides insights into the heterogeneous landscape of approval processes for genetic engineering applications across African countries. While some nations have established regulatory frameworks for authorizing GMOs, including for research and development, CFTs, open field trials activities, and importing for the direct use of GM products, others are still in the developmental stages of such frameworks (Table 5).
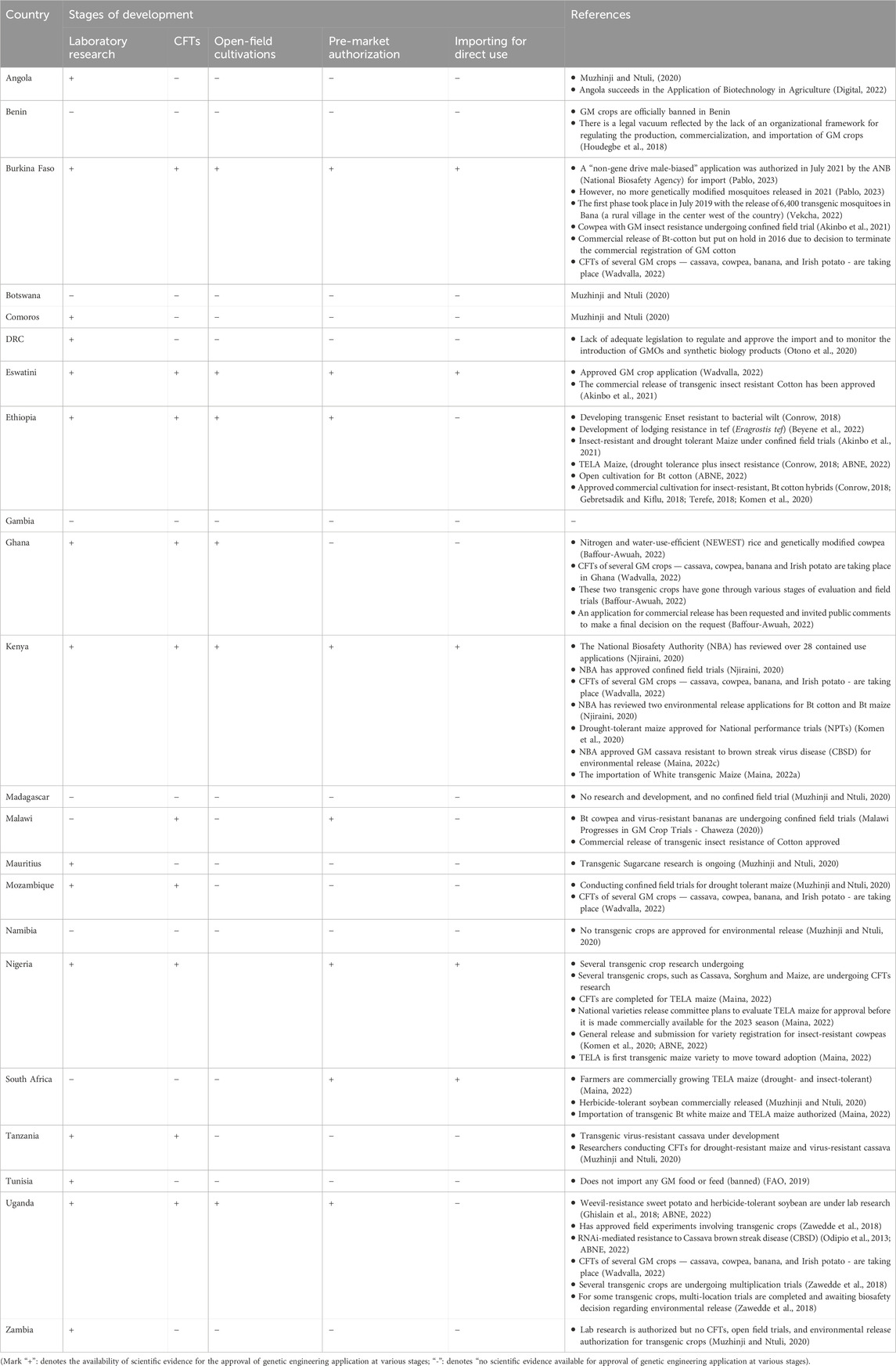
Table 5. Status on authorization for genetic engineering applications in African countries according to scientific literature.
3.5.2 The literature finding on the regulatory landscape of genome editing and gene drive in Africa
Literature findings concerning the regulatory frameworks surrounding genome editing and gene drives in African countries are critical for understanding the various laws, policies and guidelines governing these technologies on the continent. Recent studies have shed light on how different African countries approach genome editing and gene drive regulation. African countries have developed various types of regulatory approvals for genome-edited product usage, i.e., laboratory research and development, confined/small scale field trials, open scale field releases, and multiplication field trials for agronomic performance evaluation and commercialization in African countries (Table 6).
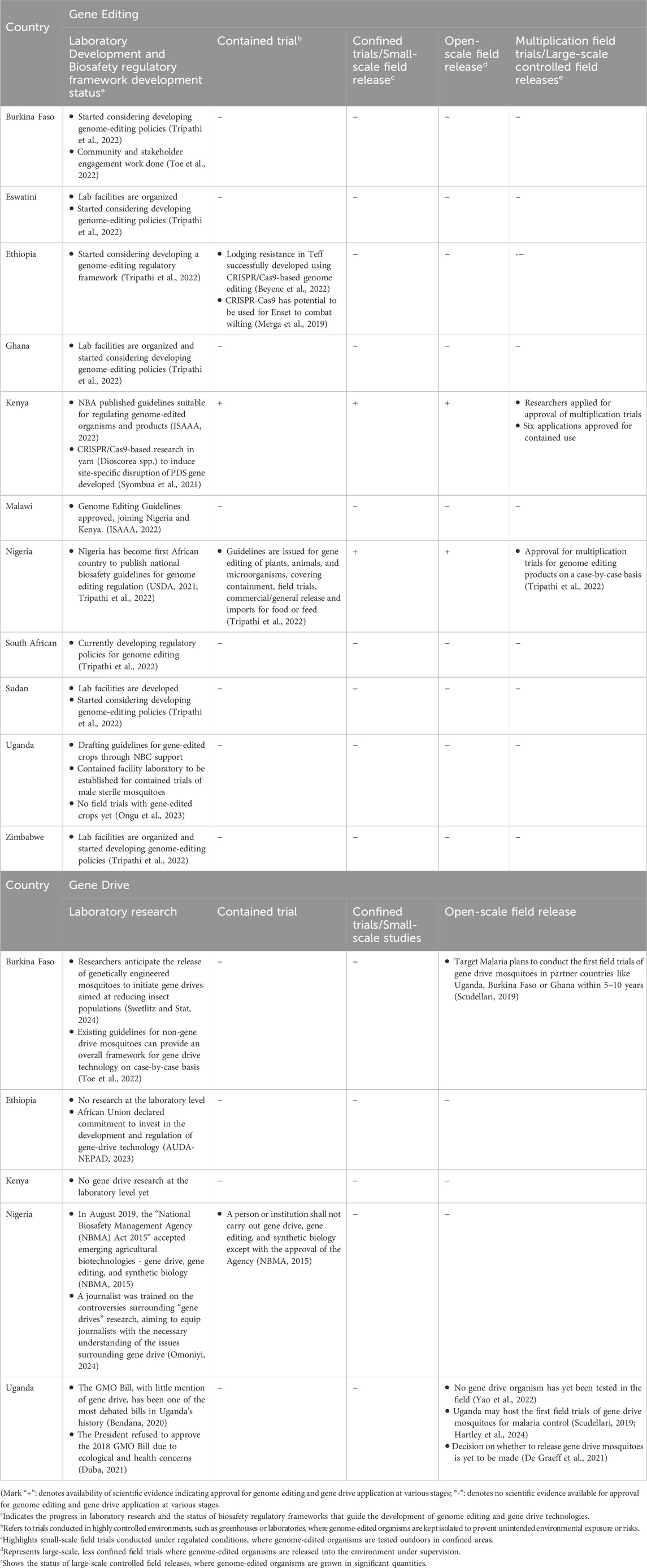
Table 6. The literature findings analyzed from academic journals on the status of genome editing and gene drive technologies in African countries.
4 Discussion
In light of the diverse regulatory landscapes, approval processes and approaches toward genome editing and gene drive, the discussion section explores the implications and broader significance of these findings. By synthesizing the results of the literature search and survey analyses, this section aims to elucidate key insights, identify challenges, and propose avenues for future research and policy development concerning genetic engineering applications and biotechnology regulation across African countries.
4.1 Authorization of genetic engineering applications in Africa
The regulatory framework landscape for genetic engineering applications in Africa varies significantly between countries. While some countries have enacted comprehensive regulations for the use of GMOs, others have more outdated regulatory frameworks. Several African countries have biosafety regulatory frameworks that can approve genetic engineering applications in CFTs, open field trials, commercialization and importation for use as food or feed, while many African countries have no experience in authorizing CFTs or even have a biosafety regulatory framework at all (ABNE, 2023).
The survey findings (Table 1) and literature review (Table 5) reveal both consistent patterns and new insights. In several countries, such as Eswatini, the Democratic Republic of Congo (DRC), Kenya, Mozambique, and Nigeria, the approval processes for genetic engineering applications appear to be interrelated and mutually supportive. However, the survey also introduces new information not previously documented in the literature. For instance, Ethiopia and Ghana have authorized the full spectrum of genetic engineering applications, including the importation of GM plants for direct use. Additionally, Zambia has approved genetic engineering for R&D, CFTs, and the importation of GM crops for food and feed—details that have not been reported in earlier studies. In cases where survey data were unavailable, such as Burkina Faso, Malawi, and Mauritius, the literature review helped fill gaps and validate the survey results.
Eleven countries (Burkina Faso, Egypt, Eswatini, Ethiopia, Ghana, Kenya, Malawi, Nigeria, South Africa, Sudan, and Zambia) have approved GM crop applications, although Zambia has yet to establish biosafety laws regulating GMOs (ABNE, 2022; ISAAA, 2024 as reviewed in Mmbando, 2024). The other ten countries have conducted field trials and approved the commercial cultivation of GM crops (Mmbando, 2024). Despite several field trials and commercial release authorizations, African countries continue to bear the reputation of being slow adaptors of GM technologies, mainly attributed to overly restrictive biosafety regulatory frameworks (Ongu et al., 2023) and political challenges (Komen et al., 2020) often exacerbated by anti-GMO activism which slowed or halted the adoption of biosafety legislation (Entine, 2022). These challenges hinder the testing and adoption of new crop varieties, including those developed by genome editing or other advanced technologies, which aim to enhance income and reduce the environmental impact of agriculture (Komen et al., 2020). Notably, African researchers are working tirelessly to develop biotechnology products so that Africa is no longer a battleground for adopting GMOs (Muthie, 2022).
According to data retrieved from ABNE in August 2023, countries such as Ghana, Tanzania, Mozambique, and Uganda have experience approving CFTs. Additionally, Burkina Faso, Nigeria, Sudan, Ethiopia, Kenya, Malawi, and Eswatini have approved commercial cultivation of GM crops. In particular, Eswatini, Ghana, Kenya and Nigeria are the front liners in the development and maturation of national biosafety laws and, hence, they have authorized the genetic engineering applications for several activities (Table 1). In contrast, Benin has not approved any commercial release or importation of GMOs. Despite the existence of relevant institutions in Benin, a legal vacuum appears to persist, reflected in the absence of an organizational framework for regulating the production, commercialization and importation of GMOs (IOBC-WPRS, 2023).
CTFs have been approved for various crops in several African countries: maize, cotton, sorghum, cassava and sweet potato in Kenya; cassava, cotton, cowpea, rice and soybean in Nigeria; banana, cassava, maize, potato and rice in Uganda, and; cotton, cowpea, banana and plantains in Malawi (Komen et al., 2020) (Table 5). Additionally, CFTs for GM crops — such as cassava, cowpea, banana and Irish potato are taking place in Mozambique, Kenya, Uganda, Ghana, Burkina Faso and Rwanda (Wadvalla, 2022). In contrast, Zambia, Tunisia and the DRC have not yet authorized CFTs, according to ABNE. This lack of authorization could be due to the lack of appropriate legislation to regulate the importation of GMOs and to monitor their introduction (Otono et al., 2020) (Table 5). The DRC is currently considering revising or strengthening its biosafety framework (Otono et al., 2020).
Ghana stands out as a West African country that have approved genetic engineering applications for laboratory research, CFTs, open-field trials and commercial cultivation. In 2012, the National Biosafety Committee in Ghana approved multi-locational trials for insect-resistant GM cotton after accepting data from CFTs conducted previously in Burkina Faso (Komen et al., 2020). Genetically modified nitrogen- and water-efficient rice and pod borer-resistant cowpeas have not been commercialized yet, although they have undergone various stages of evaluation and field trials (Baffour-Awuah, 2022).
In general, most African countries have not yet fully overcome the challenges associated with GMOs. These hurdles may pose difficulties in adapting to new regulatory frameworks and governance of emerging technologies such as genome editing and gene drive (Masehela and Barros, 2023).
4.2 Status of genome editing and gene drive regulatory frameworks in Africa
Genome editing technology is increasingly attracting the interest of African researchers due to its potential to address agricultural, health and environmental issues. To fully benefit from genome editing, there are calls that products developed through this technology must be subjected to reasonable, science-based regulation (Entine et al., 2021). Globally, while some countries have quickly adapted or amended their legislation to support the use of genome editing, others are in the initial stages of policy development (Jenkins et al., 2021). However, some countries still categorize all organisms derived through genome editing as GMOs (Hundleby and Harwood, 2022).
In Africa, the regulatory landscape is varied. Some countries have enacted regulatory frameworks despite not yet utilizing crop genetic engineering technology, while others have already conducted field trials and are beginning to authorize GM crops for cultivation (Komen et al., 2020; ABNE, 2022). This study found that few African countries have developed specific regulatory approaches tailored to genome-editing. For instance, the survey analysis (Table 2) highlights that countries like Benin, Eswatini, Kenya, Nigeria, and Uganda have established governance frameworks for genome editing, an observation also supported in the literature (Meeme, 2021; Genetic Literacy Project, 2021; Wadvalla, 2022; Buchholzer and Frommer, 2023). Additionally, Burkina Faso and Ghana have begun considering developing policies for genome editing (Tripathi et al., 2022) (Table 6).
Nigeria was the first African country to publish its regulatory frameworks on genome editing in December 2020, followed by Kenya, which published its frameworks in February 2022, an important milestone regulatory development for genome editing in Africa (ISAAA, 2022). Rather than amending its biosafety act, Kenya’s National Biosafety Authority (NBA) opted to develop a guideline on genome editing (Komen et al., 2020).
Malawi also approved its regulatory frameworks for genome editing in August 2022 (ISAAA, 2022), and Burkina Faso has validated the national regulatory frameworks, which are awaiting final approval and publication (AUDA-NEPAD, 2023). Burkina Faso is, therefore, expected to become the fourth African country to approve and publish national biosafety regulatory frameworks for genome editing. Eswatini, too, has made significant progress in establishing regulations for genome editing (Meeme, 2021).
Although Benin, Eswatini and Uganda have not yet formally published their regulatory frameworks for genome editing and gene drive, their existing biosafety regulations could be applied to oversee these technologies. This approach is similar to the EU regulatory framework, where existing biosafety regulations are applied without amendments, requiring prior government safety approval, which is the most stringent regulation for genome editing products (Tachikawa and Matsuo, 2023). Furthermore, the survey findings show that the regulatory frameworks in Benin, Eswatini, and Uganda include provisions for genome editing and gene drive technologies, deregulating SDN-1 and SDN-2 techniques while regulating SDN-3 on a case-by-case basis (Table 2). However, a detailed investigation into the regulatory status of genome editing and gene drive technologies in these African countries remains largely unexplored in the scientific literature. On the other hand, in Burkina Faso, Ethiopia, Ghana, South Africa, Sudan, and Zimbabwe, lab facilities have been developed and initiated developing genome editing policies (Tripathi et al., 2022) (Table 6). Moreover, survey results from Burkina Faso, Cameroon, Kenya, Liberia, Mali, Nigeria, South Africa, and Uganda claimed that there are ongoing research activities on Gene Drive Modified Mosquito (GDMM) in the laboratory phase and emphasized the importance of a regulatory framework (Finda et al., 2023). Several African countries, including the DRC, Ethiopia, Gambia, Ghana, Mozambique, Tanzania, Tunisia, and Zambia, lack biosafety regulatory frameworks governing genome editing and gene drive technologies. However, Ethiopia, Ghana, Sudan, South Africa and Zimbabwe have begun considering the development of such frameworks (Jenkins et al., 2021; Tripathi et al., 2022; Africa, 2022). The absence of clear regulatory frameworks in these countries poses a challenge to the development, adoption and implementation of genome editing and gene drive technologies. In response, several African countries have shown interest in revising or adapting their existing regulatory frameworks to accommodate these emerging biotechnologies (Meeme, 2021).
The survey found that biosafety experts and regulators in the DRC, Gambia, Ghana, Mozambique, Zambia and Tanzania are interested in amending their biosafety regulatory frameworks to regulate genome editing and gene drive products effectively. By doing so, these countries aim to fully leverage the potential of genome editing and gene drive technologies.
4.3 Challenges in implementing and harmonizing biosafety regulatory frameworks in Africa
African countries face significant challenges in developing and implementing regulations for genome editing and gene drive technologies. Addressing these gaps is crucial for ensuring the responsible and ethical use of these technologies. Our survey analysis identified several key challenges, including a lack of public trust, opposition, and moral concerns regarding genome editing and gene drive technologies. Public trust is critical to the successful adoption and realization of the benefits of these technologies on the African continent (Masehela and Barros, 2023).
The survey revealed that limited resources, a lack of trained and skilled expertise, insufficient public awareness, and public resistance or scepticism had hindered the implementation of national biosafety regulations for genome editing and gene drive technologies. Masehela and Barros (2023) also noted that the lack of trained technical expertise, inadequate intellectual property rights infrastructure and various concerns on genome editing products and unsupportive government leadership remain prevalent on the African continent. Similarly, Maruta et al. (2023) highlighted that insufficient infrastructure, training, capacity building and financing affect efforts to strengthen biosafety in African nations. Enhancing expertise for the development of regulatory frameworks for genome editing or gene drive technology could increase the legitimacy of the overall assessment process (Adenle et al., 2013).
Our survey analysis further indicates that negative public perception is a significant barrier, primarily due to the perceived lack of relevant data about the technologies as they are still in their early stages of development, together with fears about their potential unintended consequences or misuse. Abkallo et al. (2024) reported that mistrust surrounding genome editing technology arises from ethical concerns, fears of unintended consequences and a lack of transparency in communication, which hinder its widespread acceptance and implementation in African nations. Public perception can significantly impact gene editing technology implementation; thus, both public and stakeholder acceptance is crucial for favorable regulations and deployment (Strobbe et al., 2023).
Unharmonized regulations, especially with respect to definitions and risk assessment approaches, can hinder the applications of these technologies and future trade between countries. Therefore, harmonizing the regulatory climate is essential for equitable access and international trade in technology products (Hundleby and Harwood, 2022). However, several challenges impede the harmonization of the biosafety regulatory framework in African countries. Our survey found that the lack of coordination and information-sharing among regulatory agencies, issues related to technology transfer, concerns about intellectual property rights, the absence of region-specific regulatory frameworks for genome editing, the existence of different regulatory frameworks and definitions, and difficulties in aligning different cultural, social, and ethical perspectives were cited as major obstacles to harmonizing biosafety regulatory frameworks at the regional level. This finding is supported by a report (Africa, 2016) highlighting that significantly different legislation, definitions, and regulatory approaches among countries hinder regional or international harmonization. The report emphasized the importance of cooperation and harmonization among regulators to prevent arbitrary decisions, regional and international asymmetries in scientific and technical development, and trade barriers.
To address these challenges, it is crucial for African countries to collaborate, discuss, debate and attempt to harmonize their science-based regulatory frameworks. According to the chief executive of Kenya’s National Biosafety Authority (NBA), many countries in the Global North have adopted a common approach to regulating these new technologies, so there is an opportunity for the Global South to learn from them in developing approaches for these new technologies, by promoting greater international collaboration in knowledge sharing and figuring out how to best utilize new technologies that are being developed or used in their own countries (Ladenheim, 2023). This will prepare them for the eventual approval and use of products developed using genome editing approaches (Masehela and Barros, 2023). Regulatory diplomacy can play a critical role by facilitating dialogue and collaboration, ensuring that diverse perspectives are captured in cohesive regulatory approaches (Warner and Pink, 2024). Such collaborative efforts can foster a more cohesive regulatory environment, facilitating the responsible development and deployment of genome editing technologies across the continent.
4.4 Challenge-driven opportunities for developing biosafety regulatory framework in Africa
Africa faces an urgent need to close the yield gap in staple crops and increase food production to feed the growing population (Tripathi et al., 2022). The challenge of producing more food with the same or less land and water, improving nutrition and helping farmers adapt to climate change is particularly acute (Searchinger et al., 2024). Climate change is predicted to negatively impact the African food system, as agriculture in the region is largely dependent on rain-fed farming and subsistence agriculture (Manners et al., 2021). Additionally, climate change may drive the expansion of disease vectors and vector-borne pathogens like dengue, Zika, and Chikungunya viruses (De Souza and Weaver, 2024), creating suitable temperature conditions for transmission beyond current limits (Samy et al., 2016; Minigan et al., 2018; Kraemer et al., 2019; Ryan et al., 2019). These challenges underscore the need to scale up research, build capacity, and develop genome editing and gene drive technologies within appropriate regulatory frameworks. Realizing the full potential of new breeding tools, such as genome editing, alongside conventional technologies is essential (Pixley et al., 2019).
To leverage these advancements, African countries must develop new regulatory frameworks or adjust existing biosafety regulatory frameworks to accommodate genome editing and gene drive technologies, perhaps drawing on models of Nigeria (USDA, 2021) and Kenya (ISAAA, 2022). In this context, adopting adaptive regulatory frameworks will enable African countries to respond more effectively to the rapid developments in genome editing and gene drive technologies while addressing public concerns and ethical considerations (Greer and Trump, 2019).
In regions with low awareness and high public resistance to genome editing and gene drive technologies, effective regulation can assure society that these technologies will not be misused. Building public trust is critical to the success and acceptance of these technologies (Masehela and Barros, 2023) while addressing concerns related to safety (Trump et al., 2023).
The regulatory challenges encountered with GM crops provide valuable lessons that can inform the development of regulatory frameworks for genome editing and gene drive technologies (Rock et al., 2023). These experiences offer an opportunity to create more robust and informed regulatory frameworks.
4.5 Strengthening the regulatory landscape in Africa
Our survey analysis highlights the importance of investing in training programmes and workshops for regulators and stakeholders to strengthen the regulatory framework for genome editing and gene drive technologies. Establishing partnerships with academic institutions for research and capacity building, along with increasing knowledge-sharing with international organizations and experts, emerged as key strategies to enhance the regulatory framework in African nations.
Enhancing research and development capacities through international collaboration has already shown promising results. For instance, Nigeria, Kenya and Uganda have significantly advanced their plant genome editing technology capacities through partnerships with the International Institute of Tropical Agriculture (IITA) and the Swedish University of Agricultural Sciences (SLU) (Ongu et al., 2023). Such collaborations provide a blueprint for other African countries to strengthen their regulatory landscape.
A critical element in this process is the proactive engagement of diverse stakeholders, including policymakers, scientists, the public and educators, in open and accessible dialogues concerning the scientific principles, potential benefits and risks associated with genome editing (Abkallo et al., 2024) may ensure a more inclusive and transparent regulatory process. Public participation, transparency and accountability are crucial elements in policy development and agenda-setting, playing a significant role throughout the regulatory pathway (Nielsen et al., 2021). Without the bottom-up regulatory structures involving public engagement and the pursuit of people-centered, measures to hold researchers accountable for unethical research practices could be weak (Barbosa et al., 2021). Incorporating this approach can help address public concerns, build trust and ensure that the regulatory frameworks are comprehensive and well-informed by a wide range of perspectives and expertise.
5 Conclusion
The regulatory landscape for genome editing and gene drive technologies in Africa is diverse and evolving. Our study has highlighted significant disparities in the development and implementation of biosafety frameworks across different countries. While nations such as Nigeria and Kenya have made notable strides in establishing regulatory frameworks specific to genome editing, others are still in the preliminary stages of policy formulation. The findings indicate that the lack of clear, harmonized regulations poses challenges to the adoption and application of these technologies. Additionally, issues such as limited resources, inadequate technical expertise and public scepticism further complicate the regulatory environment.
Despite these challenges, there is a strong interest among African countries to harness the potential of genome editing and gene drive technologies to address pressing agricultural, health and environmental issues. The successful implementation of these technologies will require comprehensive regulatory frameworks that are science-based, context-specific and adaptable to emerging innovations. It is also evident that building public trust through transparent communication and stakeholder engagement is crucial for the acceptance and success of these technologies.
6 Actionable recommendations
Based on the findings of this study, the following recommendations are proposed to strengthen the regulatory frameworks for genome editing and gene drive technologies in Africa:
1. Build Capacity and Provide Training: Continue offering comprehensive training programmes and workshops for regulators and stakeholders. While traditional programmes remain important, also explore innovative approaches such as inter-agency consultations and internships in countries with established practices. These initiatives will equip local regulators and technical advisors with the practical experience and knowledge needed to contribute to informed decision-making and effective regulatory oversight.
2. Foster International Collaboration: Promote partnerships with international organizations, academic institutions and experts to facilitate knowledge transfer and technical assistance. By fostering international cooperation, countries can benefit from shared expertise in developing robust regulatory frameworks. Emphasize regulatory diplomacy to navigate global advancements, ensuring Africa’s regulatory systems remain aligned with global developments.
3. Adopt Best Practices: Incorporate best practices from countries with established regulatory frameworks, such as Nigeria and Kenya. While leveraging these successful models, tailoring them to the unique contexts and needs of other African nations is crucial. This ensures that the regulatory process becomes more relevant and adaptable to each country’s specific challenges and opportunities.
4. Develop Context-Specific Regulatory Frameworks: Formulate flexible regulatory frameworks that can accommodate ongoing technological advancements. These frameworks should address local ethical, social, and environmental considerations. This approach can help empower African countries to develop regulations that reflect their national aspirations together with their specific context and challenges.
5. Harmonize Approaches that Underpin Regulatory Decision-Making: Align key regulatory decision-making components, such as definitions and risk assessment methodologies, across African countries. This alignment can reduce inconsistencies and asymmetries between national regulatory systems while preserving flexibility for local adaptations. Additionally, recognize technical data, analyses, and assessments from foreign or neighboring regulatory authorities, reducing duplicative work and increasing the efficiency of regulatory authorizations. This will help lower costs and create smoother, faster approval processes.
6. Encourage Stakeholder Involvement: Engage a broad range of stakeholders, including scientists, policymakers, industry representatives and the public (including indigenous people and local communities). Ensuring their active participation in the regulatory process can lead to more comprehensive, widely accepted regulatory frameworks. Inclusive consultations help bring diverse perspectives to the table, improving the quality and transparency of regulations.
7. Engage the Public and Educate: Implement innovative education campaigns to raise public awareness about the potential benefits and risks of genome editing and gene drive technologies. Transparent and accessible communication can mitigate public skepticism, foster trust in the regulatory process, and support informed decision-making. Ensuring that the public understands the science and the risk management in place would lead to greater acceptance of these technologies.
By implementing these recommendations, African countries can develop robust and adaptive regulatory frameworks that support the safe, ethical and beneficial use of genome editing and gene drive technologies. This proactive approach will help enable the continent to address critical challenges in agriculture, health and environmental sustainability, ultimately contributing to improved livelihoods and economic development.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study involved the collection of data through surveys and did not require formal ethical review or approval in accordance with relevant legislation and institutional guidelines. Participation in the survey was voluntary, and participants provided their informed consent by completing the survey. No personally identifiable information was collected, and all responses were anonymized to ensure privacy and confidentiality in line with relevant regulations and institutional requirements.
Author contributions
TR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. FM-B: Conceptualization, Formal Analysis, Investigation, Methodology, Writing–review and editing, Data curation. WC: Conceptualization, Methodology, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The International Centre for Genetic Engineering and Biotechnology has supported TR through sponsoring a fellowship program for the research work.
Acknowledgments
We thank the biosafety competent national authorities of the African countries, as well as the experts and other stakeholders who generously participated in our survey. Tilahun Rabuma is very grateful to the Regulatory Science Group for providing all necessary facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abkallo, H. M., Arbuthnot, P., Auer, T. O., Berger, D., Burger, J. T., Chakauya, E., et al. (2024). Making genome editing a success story in Africa. Nat. Biotechnol. 42, 551–554. doi:10.1038/s41587-024-02187-2
ABNE (2023). African biosafety Network of expertise (ABNE). - AUDA-NEPAD. Available at: https://www.nepad.org/programme/african-biosafety-network-of-expertise-abne.
Adenle, A. A., Morris, E. J., and Parayil, G. (2013). Status of development, regulation and adoption of GM agriculture in Africa: views and positions of stakeholder groups. Food Policy 43, 159–166. doi:10.1016/j.foodpol.2013.09.006
Africa, A. O. S. O. S. (2016). The regulatory implications of new breeding techniques. Available at: https://research.assaf.org.za/items/acc72a68-1fe4-452f-95af-9788e525b876.
African Biosafety Network of Expertise (ABNE), (2022). Building functional biosafety regulatory systems in Africa.
AGBC (2022). COP 15 side event: capacity strengthening for risk assessment of new biotechnologies in Africa – building on experiences with genetic biocontrol - the african genetic biocontrol consortium. Available at: https://www.genbioconsortium.africa/cop-15-side-event-capacity-strengthening-for-risk-assessment-of-new-biotechnologies-in-africa-building-on-experiences-with-genetic-biocontrol/.
Akinbo, O., Obukosia, S. D., Ouedraogo, J. T., Sinebo, W., Savadogo, M., Timpo, S. E., et al. (2021). Commercial release of genetically modified crops in Africa: interface between biosafety regulatory systems and varietal release systems. Front. Plant Sci. 12, 605937. doi:10.3389/fpls.2021.605937
Alphey, L., Crisanti, A., Randazzo, F. F., and Akbari, O. S. (2020). Standardizing the definition of gene drive. Proc. Natl. Acad. Sci. U. S. A. 117 (49), 30864–30867. doi:10.1073/pnas.2020417117
Asquer, A., and Morrison, M. (2022). Editorial: regulation and governance of gene editing technologies (CRISPR, etc.). Front. Political Sci. 4. doi:10.3389/fpos.2022.1027410
AUDA-NEPAD (2023). Gene drives for malaria control and elimination - health Tech. Health Tech. - Health Tech. Available at: https://healthtechafrica.org/technologies/gene-drives-for-malaria-control-and-elimination/.
AUDA-NEPAD (2023). Genome Editing at center of discussions in Burkina Faso. Available at: https://www.nepad.org/news/genome-editing-center-of-discussions-burkina-faso.
Baffour-Awuah, D. (2022). Ghana’s first GMO food crop: all you need to know. Available at: https://allianceforscience.org/blog/2022/03/ghanas-first-gmo-food-crop-all-you-need-to-know/#:∼:text=In%20Ghana%2C%20local%20scientists%20from,as%20the%20pod%20borer%2Dresistant%20.
Barbosa, S., Toe, L. P., Thizy, D., Vaz, M., and Carter, L. (2021). Engagement and social acceptance in genome editing for human benefit: reflections on research and practice in a global context. Wellcome Open Res. 5, 244. doi:10.12688/wellcomeopenres.16260.2
Bendana, C. (2020). Ugandan president wants GMO bill. Alliance Sci. Available at: https://allianceforscience.org/blog/2020/03/ugandan-president-wants-gmo-bill-passed/.
Beyene, G., Chauhan, R. D., Villmer, J., Husic, N., Wang, N., Gebre, E., et al. (2022). CRISPR/Cas9-mediated tetra-allelic mutation of the ‘Green Revolution’ SEMIDWARF-1 (SD-1) gene confers lodging resistance in tef (Eragrostis tef). Plant Biotechnol. J. 20 (9), 1716–1729. doi:10.1111/pbi.13842
Bier, E. (2022). Gene drives gaining speed. Nat. Rev. Genet. 23 (1), 5–22. doi:10.1038/s41576-021-00386-0
Boluwade, E., and Smith, G. (2021). “Government of Nigeria approved national biosafety guideline on gene editing,” in Government of Nigeria approved national biosafety Guideline on gene editing (biotechnology and other new production technologies No. NI2021-0001) (Lagos, Nigeria: USDA). Available at: https://www.aatf-africa.org/.
Buchholzer, M., and Frommer, W. B. (2022). An increasing number of countries regulate genome editing in crops. New Phytol. 237 (1), 12–15. doi:10.1111/nph.18333
Chaweza, K. (2020). Malawi progress in GM crop trail-Alliance for science Available at: https://allienceforscienec.org/blog/2017/04/malawi-progresses-in-gm-crop-trials/.
Conrow, J. (2018). Ethiopia progresses with GMO crops. Alliance For Sci. Available at: https://allianceforscience.org/blog/2018/06/ethiopia-progresses-gmo-crops/.
De Graeff, N., Jongsma, K. R., Lunshof, J. E., and Bredenoord, A. L. (2021). Governing gene drive technologies: a qualitative interview study. AJOB Empir. Bioeth. 13 (2), 107–124. doi:10.1080/23294515.2021.1941417
De Souza, W. M., and Weaver, S. C. (2024). Effects of climate change and human activities on vector-borne diseases. Nat. Rev. Microbiol. 22, 476–491. doi:10.1038/s41579-024-01026-0
Digital, G. (2022). “Angola succeeds in application of biotechnology in agriculture,” in Genetic literacy Project. Available at: https://geneticliteracyproject.org/2013/10/03/angola-succeeds-in-application-of-biotechnology-in-agriculture.
Diversity, S. O. T. C. O. B. (2000). Cartagena Protocol on biosafety to the convention on biological diversity: text and annexes. Available at: https://www.cbd.int/doc/legal/cartagena-protocol-en.pdf.
Dolezel, M., Lüthi, C., and Gaugitsch, H. (2020). Beyond limits – the pitfalls of global gene drives for environmental risk assessment in the European Union. BioRisk 15, 1–29. doi:10.3897/biorisk.15.49297
Duba, M. (2021). Can Museveni’s renewed commitment to eliminate Malaria in Uganda be the glimmer of hope for legal reforms needed to pave way for testing gene drives? Nairobi, Kenya: African Institute for Development Policy - AFIDEP. African Institute for Development Policy - AFIDEP. Available at: https://www.afidep.org/can-musevenis-renewed-commitment-to-eliminate-malaria-in-uganda-be-the-glimmer-of-hope-for-legal-reforms-needed-to-pave-way-for-testing-gene-drives/.
Entine, J. (2022). “Uganda’s anti-GMO activists blamed for stonewalling nation’s new biosafety rules,” in Genetic literacy Project. Available at: https://geneticliteracyproject.org/2019/05/06/ugandas-anti-gmo-activistsblamed-for-stonewalling-nations-new-biosafety-rules/.
Entine, J., Felipe, M. S. S., Groenewald, J., Kershen, D. L., Lema, M. A., McHughen, A., et al. (2021). Regulatory approaches for genome edited agricultural plants in select countries and jurisdictions around the world. Transgenic Res. 30 (4), 551–584. doi:10.1007/s11248-021-00257-8
FAO (2019). Food safety and quality: country page. Available at: https://www.fao.org/food/food-safety-quality/gm-foods-platform/browse-information-by/country/country-page/en/?cty=TUN.
Finda, M. F., Juma, E. O., Kahamba, N. F., Mthawanji, R. S., Sambo, M., Emidi, B., et al. (2023). Perspectives of African stakeholders on gene drives for malaria control and elimination: a multi-country survey. Malar. J. 22 (1), 384. doi:10.1186/s12936-023-04787-w
Fridovich-Keil, J. L. (2024). Gene editing. Encyclopedia Britannica, 19 Available at: https://www.britannica.com/science/gene-editing.
Friedrichs, S., Takasu, Y., Kearns, P., Dagallier, B., Oshima, R., Schofield, J., et al. (2019). An overview of regulatory approaches to genome editing in agriculture. Biotechnol. Res. Innovation 3 (2), 208–220. doi:10.1016/j.biori.2019.07.001
Gebretsadik, K., and Kiflu, A. (2018). Challenges and opportunities of genetically modified crops production; future perspectives in Ethiopia, review. ˜the œOpen Agric. J. 12 (1), 240–250. doi:10.2174/1874331501819010240
Gene drives on the horizon (2016). Washington, DC: National Academies Press eBooks. doi:10.17226/23405
Genetic Literacy Project (2021). 3 pioneering African nations embrace gene editing to boost food security, farmer incomes. Available at: https://geneticliteracyproject.org/2021/01/29/three-african-nations-take-the-lead-in-agricultural-use-of-genome-editing/.
Ghislain, M., Byarugaba, A. A., Magembe, E., Njoroge, A., Rivera, C., Román, M. L., et al. (2018). Stacking three late blight resistance genes from wild species directly into African highland potato varieties confers complete field resistance to local blight races. Plant Biotechnol. J. 17 (6), 1119–1129. doi:10.1111/pbi.13042
Greer, S. L., and Trump, B. (2019). Regulation and regime: the comparative politics of adaptive regulation in synthetic biology. Policy Sci. 52 (4), 505–524. doi:10.1007/s11077-019-09356-0
Hartley, S., Smith, R., Kokotovich, A., Opesen, C., Habtewold, T., Ledingham, K., et al. (2021). Ugandan stakeholder hopes and concerns about gene drive mosquitoes for malaria control: new directions for gene drive risk governance. Malar. J. 20 (1), 149. doi:10.1186/s12936-021-03682-6
Hartley, S., Stelmach, A., Opesen, C., Openjuru, G. L., and Neema, S. (2024). Talking about gene drive in Uganda: the need for science communication to underpin engagement. Sci. Commun. 46, 431–457. doi:10.1177/10755470241234048
Houdegbe, C. A., Tchokponhoue, D. A., and Sogbohossou, E. O. D. (2018). Current status andFuture of genetically modified crops in Benin. GMOs Integr. Plant Prod. 131, 33–40. Available at: https://www.researchgate.net/publication/336881278_Resistance_to_legume_pod_borer_Maruca_vitrata_Fabricius_in_cowpea_genetic_advances_challenges_and_future_prospects (Accessed May 24, 2024).
Hundleby, P., and Harwood, W. (2022). Regulatory constraints and differences of Genome-Edited crops around the globe. Springer eBooks, 319–341. doi:10.1007/978-3-031-08072-2_17
IOBC-WPRS (2023). Current status and future of genetically modified crops in Benin -IOBC-WPRS. Available at: https://iobc-wprs.org/product/current-status-and-future-of-genetically-modified-crops-in-benin/.
ISAAA (2022). “Kenya publishes genome editing regulations becoming second African country to do so,” in Crop biotech update.
James, S., Collins, F. H., Welkhoff, P. A., Emerson, C., Godfray, H. C. J., Gottlieb, M., et al. (2018). Pathway to deployment of gene drive mosquitoes as a potential biocontrol tool for elimination of malaria in Sub-Saharan Africa: recommendations of a scientific working group. ˜the œAmerican J. Trop. Med. Hyg. 98 (6_Suppl. l), 1–49. doi:10.4269/ajtmh.18-0083
James, S., Dass, B., and Quemada, H. (2023). Regulatory and policy considerations for the implementation of gene drive-modified mosquitoes to prevent malaria transmission. Transgenic Res. 32 (1–2), 17–32. doi:10.1007/s11248-023-00335-z
Jenkins, D., Dobert, R. C., Atanassova, A., and Pavely, C. (2021). Impacts of the regulatory environment for gene editing on delivering beneficial products. Vitro Cell. and Dev. Biol. Plant 57 (4), 609–626. doi:10.1007/s11627-021-10201-4
Karmakar, A., Taufiqa, S., Baig, M. J., and Molla, K. A. (2022). Increasing disease resistance in host plants through genome editing. Proc. Indian Natl. Sci. Acad. Part a, Phys. Sci. 88 (3), 417–429. doi:10.1007/s43538-022-00100-6
Knott, G. J., and Doudna, J. A. (2018). CRISPR-Cas guides the future of genetic engineering. Science 361 (6405), 866–869. doi:10.1126/science.aat5011
Komen, J., Tripathi, L., Mkoko, B., Ofosu, D. O., Oloka, H. K., and Wangari, D. (2020). Biosafety regulatory reviews and Leeway to operate: case studies from Sub-Sahara Africa. Front. Plant Sci. 11, 130. doi:10.3389/fpls.2020.00130
Kraemer, M. U. G., Reiner, R. C., Brady, O. J., Messina, J. P., Gilbert, M., Pigott, D. M., et al. (2019). Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 4 (5), 854–863. doi:10.1038/s41564-019-0376-y
Ku, H., and Ha, S. (2020). Improving nutritional and functional quality by genome editing of crops: status and Perspectives. Front. Plant Sci. 11, 577313. doi:10.3389/fpls.2020.577313
Ladenheim, A. (2023). “Countries from Global South urged to harmonize regulatory frameworks on biotechnology,” in Genetic literacy Project. Available at: https://geneticliteracyproject.org/2023/08/30/countries-from-global-south-urged-to-harmonize-regulatory-frameworks-on-biotechnology/?action=genpdf&id=2472613.
Li, G., Li, X., Zhuang, S., Wang, L., Zhu, Y., Chen, Y., et al. (2022). Gene editing and its applications in biomedicine. Sci. China. Life Sciences/Science China. Life Sci. 65 (4), 660–700. doi:10.1007/s11427-021-2057-0
Maina, J. (2022a). Kenya approves GMOs after 10-year ban-Alliance for Science Available at: https://allianceforscience.org/blog/2022/10/kenya-approves-gmos-after-10-year-ban/.
Maina, J. (2022b). Nigeria to Commence National Trials on TELA Maize. Science Africa Available at: https://scienceafrica.co.ke/2022/04/13/nigeria-to-commence-national-trials-on-telamaize/.
Maina, J. (2022c). South Africa has reaped major benefits from GM maize, study finds. Alliance Sci. Available at: https://allianceforscience.org/blog/2021/06/south-africa-has-reaped-major-benefits-from-gm-maize-study-finds/.
Manners, R., Vandamme, E., Adewopo, J., Thornton, P. K., Friedmann, M., Carpentier, S., et al. (2021). Suitability of root, tuber, and banana crops in Central Africa can be favoured under future climates. Agric. Syst. 193, 103246. doi:10.1016/j.agsy.2021.103246
Maruta, T., Kenfack, J. A. N., Tebeje, Y. K., Bangure, D., and Ouma, A. E. O. (2023). Regional approach to strengthening biosafety and biosecurity systems in Africa. Glob. Secur. Health, Sci. Policy 8 (1). doi:10.1080/23779497.2023.2257766
Masehela, T., and Barros, E. (2023). The African continent should consider a harmonized consultative and collaborative effort towards coordinated policy and regulatory guidelines across the fields of biotechnology. Front. Bioeng. Biotechnol. 11, 1211789. doi:10.3389/fbioe.2023.1211789
Matsumoto, D., and Nomura, W. (2023). The history of genome editing: advances from the interface of chemistry and biology. Chem. Commun. 59 (50), 7676–7684. doi:10.1039/d3cc00559c
Meeme, V. (2021). Three African nations take the lead in agricultural use of genome editing -. Alliance Sci. Available at: https://allianceforscience.org/blog/2021/01/three-african-nations-take-the-lead-in-agricultural-use-of-genome-editing/.
Merga, I. F., Tripathi, L., Hvoslef-Eide, A. K., and Gebre, E. (2019). Application of genetic engineering for control of bacterial wilt disease of Enset, Ethiopia’s sustainability crop. Front. Plant Sci. 10, 133. doi:10.3389/fpls.2019.00133
Metchanun, N., Borgemeister, C., Amzati, G., Von Braun, J., Nikolov, M., Selvaraj, P., et al. (2022). Modeling impact and cost-effectiveness of driving-Y gene drives for malaria elimination in the Democratic Republic of the Congo. Evol. Appl. 15 (1), 132–148. doi:10.1111/eva.13331
Millett, P., Alexanian, T., Palmer, M. J., Evans, S. W., Kuiken, T., and Oye, K. A. (2022). IGEM and Gene Drives: a case study for governance. Health Secur. 20 (1), 26–34. doi:10.1089/hs.2021.0157
Minigan, J. N., Hager, H. A., Peregrine, A. S., and Newman, J. A. (2018). Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick-borne Dis. 9 (2), 354–362. doi:10.1016/j.ttbdis.2017.11.012
Mmbando, G. S. (2024). The adoption of genetically modified crops in Africa: the public’s current perception, the regulatory obstacles, and ethical challenges. GM Crops and Food 15 (1), 1–15. doi:10.1080/21645698.2024.2345401
Muthie, J. (2022). Africa is not a battle ground for GMO adoption, say experts - AATF. AATF. Available at: https://www.aatf-africa.org/africa-is-not-a-battle-ground-for-gmo-adoption-say-experts/.
Muzhinji, N., and Ntuli, V. (2020). Genetically modified organisms and food security in Southern Africa: conundrum and discourse. GM Crops and Food 12 (1), 25–35. doi:10.1080/21645698.2020.1794489
Nagamine, A., and Ezura, H. (2022). Genome editing for improving crop nutrition. Front. Genome Ed. 4, 850104. doi:10.3389/fgeed.2022.850104
NBMA (2015)). National biosafety management agency (NBMA) act. Available at: https://r.search.yahoo.com/_ylt=Awr9zNZyuk9mQOADIIFXNyoA;_Ylu=Y29sbwNncTEEcG9zAzcEdnRpZAMEc2VjA3Ny/RV=2/RE=1717710706/RO=10/RU=https%3a%2f%2fnbma.gov.ng%2fwp-content%2fuploads%2f2022%2f03%2fNATIONAL-GENE-EDITING-GUIDELINE.pdf/RK=2/RS=V2oMyDfuHJgCk4lSxM.OBQ4PeHY.
Nielsen, J., Eckstein, L., Nicol, D., and Stewart, C. (2021). Integrating public participation, transparency and accountability into governance of marketing authorisation for genome editing products. Front. Political Sci. 3. doi:10.3389/fpos.2021.747838
Njiraini, J. (2020). The state of GMOs in Kenya. Agri-Business Glob. Direct. Available at: https://www.agribusinessglobal.com/genetics/the-state-of-gmos-in-kenya/.
Noble, C., Adlam, B., Church, G. M., Esvelt, K. M., and Nowak, M. A. (2018). Current CRISPR gene drive systems are likely to be highly invasive in wild populations. eLife 7, e33423. doi:10.7554/elife.33423
North, A., Burt, A., and Godfray, H. C. J. (2020). Modelling the suppression of a malaria vector using a CRISPR-Cas9 gene drive to reduce female fertility. BMC Biol. 18 (1), 98. doi:10.1186/s12915-020-00834-z
Odipio, J., Ogwok, E., Taylor, N. J., Halsey, M., Bua, A., Fauquet, C. M., et al. (2013). RNAi-derived field resistance to Cassava brown streak disease persists across the vegetative cropping cycle. GM Crops Food 5 (1), 16–19. doi:10.4161/gmcr.26408
Ombogo, G. (2023). Eleven countries in Africa have approved GM crops but only seven have regulatory legislation- Alliance for Science. Available at: https://allianceforscience.org/blog/2023/08/eleven-countries-in-africa-have-approved-gm-crops-but-only-seven-have-regulatory-legislation/.
Omoniyi, T. (2024). Group trains Nigerian journalists on “gene drives” controversies. Premium Times Nigeria. Available at: https://www.premiumtimesng.com/news/top-news/680075-group-trains-nigerian-journalists-on-gene-drives-controversies.html.
Ongu, I., Olayide, P., Alexandersson, E., Zawedde, B. M., and Eriksson, D. (2023). Biosafety regulatory frameworks in Kenya, Nigeria, Uganda and Sweden and their potential impact on international R&D collaborations. GM Crops and Food 14 (1), 1–17. doi:10.1080/21645698.2023.2194221
Osakabe, Y., Watanabe, T., Sugano, S. S., Ueta, R., Ishihara, R., Shinozaki, K., et al. (2016). Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci. Rep. 6 (1), 26685. doi:10.1038/srep26685
Pablo, . Congo—GMOS/Synthetic Biology Rules/Regulations and Biodiversity: a Legal Perspective Results from months of monitoring following the first release of non gene drive genetically modified mosquitoes in Africa - target Malaria. Target Malar., 475–479. doi:10.1007/978-3-030-53183-6_22Pablo
Pixley, K., Falck-Zepeda, J. B., Giller, K. E., Glenna, L., Gould, F., Mallory-Smith, C., et al. (2019). Genome editing, gene drives, and synthetic biology: will they contribute to Disease-Resistant crops, and who will benefit? Annu. Rev. Phytopathology 57 (1), 165–188. doi:10.1146/annurev-phyto-080417-045954
Programme, G. M. (2021). Global technical strategy for malaria 2016-2030, 2021 update. Available at: https://www.who.int/publications/i/item/9789240031357.
Quemada, H. (2022). Lessons learned from the introduction of genetically engineered crops: relevance to gene drive deployment in Africa. Transgenic Res. 31 (3), 285–311. doi:10.1007/s11248-022-00300-2
Rock, J., Schnurr, M. A., Kingiri, A., Glover, D., Stone, G. R., Ely, A., et al. (2023). Beyond the genome: genetically modified crops in Africa and the implications for genome editing. Dev. Change 54 (1), 117–142. doi:10.1111/dech.12750
Ryan, S. J., Carlson, C. J., Mordecai, E. A., and Johnson, L. R. (2019). Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Neglected Trop. Dis. 13 (3), e0007213. doi:10.1371/journal.pntd.0007213
Sami, A., Zhao, X., Tazein, S., Arshad, A., Zhu, Z., Chen, Y. P., et al. (2021). CRISPR–Cas9-based genetic engineering for crop improvement under drought stress. Bioengineered 12 (1), 5814–5829. doi:10.1080/21655979.2021.1969831
Samy, A. M., Elaagip, A. H., Kenawy, M. A., Ayres, C. F. J., Peterson, A. T., and Soliman, D. E. (2016). Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of west nile virus and lymphatic filariasis. PloS One 11 (10), e0163863. doi:10.1371/journal.pone.0163863
Saradadevi, G. P., Das, D., Mangrauthia, S. K., Mohapatra, S., Chikkaputtaiah, C., Roorkiwal, M., et al. (2021). Genetic, epigenetic, genomic and microbial Approaches to Enhance salt tolerance of Plants: a Comprehensive review. Biology 10 (12), 1255. doi:10.3390/biology10121255
Schmidt, S. M., Belisle, M., and Frommer, W. B. (2020). The evolving landscape around genome editing in agriculture: many countries have exempted or move to exempt forms of genome editing from GMO regulation of crop plants. EMBO Rep. 21 (6), e50680. doi:10.15252/embr.202050680
Scudellari, M. (2019). Self-destructing mosquitoes and sterilized rodents: the promise of gene drives. Nature 571 (7764), 160–162. doi:10.1038/d41586-019-02087-5
Searchinger, T., Waite, R., Hanson, C., Ranganathan, J., and Matthews, E. (2024). Creating a sustainable food future. Washington, DC: World Resources Institute.
Shelake, R. M., Kadam, U. S., Kumar, R., Pramanik, D., Singh, A. K., and Kim, J. (2022). Engineering drought and salinity tolerance traits in crops through CRISPR-mediated genome editing: targets, tools, challenges, and perspectives. Plant Commun. 3 (6), 100417. doi:10.1016/j.xplc.2022.100417
Strobbe, S., Wesana, J., Van Der Straeten, D., and De Steur, H. (2023). Public acceptance and stakeholder views of gene edited foods: a global overview. Trends Biotechnol. 41 (6), 736–740. doi:10.1016/j.tibtech.2022.12.011
Swetlitz, I., and Stat, (2024). Researchers to release genetically engineered mosquitoes in Africa for first time. Sci. Am.
Syombua, E. D., Zhang, Z., Tripathi, J. N., Ntui, V. O., Kang, M., George, O. O., et al. (2021). A CRISPR/Cas9-based genome-editing system for yam (Dioscorea spp.). Plant Biotechnol. J. 19 (4), 645–647. doi:10.1111/pbi.13515
Tachikawa, M., and Matsuo, M. (2023). Divergence and convergence in international regulatory policies regarding genome-edited food: how to find a middle ground. Front. Plant Sci. 14, 1105426. doi:10.3389/fpls.2023.1105426
Terefe, M. (2018). Biosafety issues of Genetically modified crops: addressing the potential risks and the status of GMO crops in Ethiopia. Cloning and Transgenesis 07 (02). doi:10.4172/2168-9849.1000164
Thizy, D., Coche, I., and De Vries, J. (2020). Providing a policy framework for responsible gene drive research: an analysis of the existing governance landscape and priority areas for further research. Wellcome Open Res. 5, 173. doi:10.12688/wellcomeopenres.16023.1
Toe, L. P., Dicko, B., Linga, R. R., Barry, N., Drabo, M., Sykes, N., et al. (2022). Operationalizing stakeholder engagement for gene drive research in malaria elimination in Africa—translating guidance into practice. Malar. J. 21 (1), 225. doi:10.1186/s12936-022-04241-3
Tripathi, L., Dhugga, K. S., Ntui, V. O., Runo, S., Syombua, E. D., Muiruri, S., et al. (2022). Genome editing for sustainable agriculture in Africa. Front. Genome Ed. 4, 876697. doi:10.3389/fgeed.2022.876697
Trump, B. D., Cummings, C., Klasa, K., Galaitsi, S., and Linkov, I. (2023). Governing biotechnology to provide safety and security and address ethical, legal, and social implications. Front. Genet. 13, 1052371. doi:10.3389/fgene.2022.1052371
USDA (2021). Nigeria: government of Nigeria approved national biosafety guideline on gene editing. USDA Foreign Agricultural Service.
Vekcha (2022). Burkina Faso – the Target Malaria project continues despite irregularities – inf’OGM. Available at: https://infogm.org/burkina-faso-the-target-malaria-project-continues-despite-irregularities/.
Vora, Z., Pandya, J., Sangh, C., and Vaikuntapu, P. R. (2023). The evolving landscape of global regulations on genome-edited crops. J. Plant Biochem. Biotechnol. 32 (4), 831–845. doi:10.1007/s13562-023-00863-z
Wadvalla, B. (2022). Advancing biotechnology to solve Africa’s food challenges. Nat. Afr. doi:10.1038/d44148-022-00106-8
Wamba, A. (2023). Gene drive: emerging mosquito threats in Africa call for new technologies to fight malaria. Alliance Sci. Available at: https://allianceforscience.org/blog/2023/03/fighting-malaria-emerging-mosquito-threats-in-africa-necessitate-new-technologies/.
Warner and Pink (2024). Regulatory diplomacy: the practitioner’s path to agility and foresight | ANZSOG. Available at: https://anzsog.edu.au/news/regulatory-diplomacy-the-practitioners-path-to-agility-and-foresight/.
Wolt, J. D., Wang, K., and Yang, B. (2015). The regulatory status of genome-edited crops. Plant Biotechnol. J. 14 (2), 510–518. doi:10.1111/pbi.12444
Yao, F. A., Millogo, A., Epopa, P. S., North, A., Noulin, F., Dao, K., et al. (2022). Mark-release-recapture experiment in Burkina Faso demonstrates reduced fitness and dispersal of genetically-modified sterile malaria mosquitoes. Nat. Commun. 13 (1), 796. doi:10.1038/s41467-022-28419-0
Keywords: biosafety regulations, emerging biotechnologies, regulatory frameworks, Africa, genome editing, gene drive
Citation: Rabuma T, Moronta-Barrios F and Craig W (2024) Navigating biosafety regulatory frameworks for genetic engineering in Africa: a focus on genome editing and gene drive technologies. Front. Bioeng. Biotechnol. 12:1483279. doi: 10.3389/fbioe.2024.1483279
Received: 19 August 2024; Accepted: 08 October 2024;
Published: 24 October 2024.
Edited by:
Clara Rubinstein, Institute for Scientific Cooperation in Environment and Health (ICCAS), ArgentinaReviewed by:
Dalia Marcela Lewi, Ministerio de Agricultura, ArgentinaKaren Hokanson, Agriculture and Food Systems Institute, United States
Copyright © 2024 Rabuma, Moronta-Barrios and Craig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wendy Craig, Y3JhaWdAaWNnZWIub3Jn; Tilahun Rabuma, dGlsZWZpcmVAZ21haWwuY29t
 Tilahun Rabuma
Tilahun Rabuma Felix Moronta-Barrios
Felix Moronta-Barrios Wendy Craig
Wendy Craig