- 1Stanford University, Stanford, CA, United States
- 2Center for International Security and Cooperation, Freeman Spogli Institute for International Studies, Stanford University, Stanford, CA, United States
AI-enabled synthetic biology has tremendous potential but also significantly increases biorisks and brings about a new set of dual use concerns. The picture is complicated given the vast innovations envisioned to emerge by combining emerging technologies, as AI-enabled synthetic biology potentially scales up bioengineering into industrial biomanufacturing. However, the literature review indicates that goals such as maintaining a reasonable scope for innovation, or more ambitiously to foster a huge bioeconomy do not necessarily contrast with biosafety, but need to go hand in hand. This paper presents a literature review of the issues and describes emerging frameworks for policy and practice that transverse the options of command-and-control, stewardship, bottom-up, and laissez-faire governance. How to achieve early warning systems that enable prevention and mitigation of future AI-enabled biohazards from the lab, from deliberate misuse, or from the public realm, will constantly need to evolve, and adaptive, interactive approaches should emerge. Although biorisk is subject to an established governance regime, and scientists generally adhere to biosafety protocols, even experimental, but legitimate use by scientists could lead to unexpected developments. Recent advances in chatbots enabled by generative AI have revived fears that advanced biological insight can more easily get into the hands of malignant individuals or organizations. Given these sets of issues, society needs to rethink how AI-enabled synthetic biology should be governed. The suggested way to visualize the challenge at hand is whack-a-mole governance, although the emerging solutions are perhaps not so different either.
1 Introduction
Synthetic biology, the multidisciplinary field of biology attempting to understand, modify, redesign, engineer, enhance, or build biological systems with useful purposes (El Karoui et al., 2019; Singh et al., 2022; Plante, 2023), has the potential to advance food production, develop new therapies, regulate the environment, generate renewable energy, edit the genome, predict the structure of proteins, and invent effective synthetic biological systems, and more (Yamagata, 2023). It is arguably moving from the lab to the marketplace (Hodgson et al., 2022; Lin et al., 2023). However, the immense promise of synthetic biology has been subject to much hype and it is a paradox that it is still a nascent technology that has not scaled beyond the microscale (Hanson and Lorenzo, 2023). The next major breakthrough might relate to plants (Eslami et al., 2022) or even to mammalian systems (Yan et al., 2023). Despite the small scale, the intermediate term risks are significant, and include contaminating natural resources, aggravation of species with complex gene modifications, threats to species diversity, abuse of biological weapons, laboratory leaks, and man-made mutations, hurting workers, creating antibiotic resistant superbugs, or damaging human, animal, or plant germlines (Hewett et al., 2016; O’Brien and Nelson, 2020; Nelson et al., 2021; Sun et al., 2022). Some even claim synthetic biology produces potential existential risks from lab accidents or engineered pandemics (Ord, 2020), especially in combination with AI (Boyd and Wilson, 2020).
AI-enabled synthetic biology, while surely adding to the risk calculations, has tremendous medium term potential to provide a vehicle for scaling synthetic biology so it may finally deliver on its promise (Hillson et al., 2019; Dixon et al., 2020; Ebrahimkhani and Levin, 2021; Bongard and Levin, 2023). That being said, the prospect of AI-enabled synthetic biology significantly increases biorisks and particularly brings about a new set of dual use concerns (Grinbaum and Adomaitis, 2023). Although biorisk is subject to an established governance regime (Mampuys and Brom, 2018; Wang and Zhang, 2019), and scientists generally adhere to biosafety protocols if they receive the appropriate training and build a culture of responsibility (Perkins et al., 2019), even experimental, but legitimate use by scientists could lead to unexpected developments (O’Brien and Nelson, 2020). Additionally, recent advances in chatbots enabled by generative AI, technology capable of producing convincing real-world content, including text, code, images, music, and video, based on vast amounts of training data (Feuerriegel et al., 2024), accelerates knowledge mining in biology (Xiao et al., 2023) but has revived fears that advanced biological insight can get into the hands of malignant individuals or organizations (Grinbaum and Adomaitis, 2023). It also further blurs the boundary between our understanding of living and non-living matter (Deplazes and Huppenbauer, 2009). The picture is complicated given the vast innovations envisioned to emerge by combining emerging technologies, as synthetic biology scales up bioengineering turning it into industrial biomanufacturing. Given these sets of issues, society needs to rethink how AI-enabled synthetic biology should be governed.
The research question in this paper is: what are the most important emergent best practices on governing the risks and opportunities of AI-enabled synthetic biology? Relatedly, is stewardship or laissez-faire governance the right approach? How can humanity seek to maintain a reasonable scope for synthetic biology innovation, and integration of its potential into manufacturing, agriculture, health, and other sectors? Do we need additional early warning systems that enable prevention and mitigation of future AI-enabled biohazards from the lab, from deliberate misuse, or from the public realm?
From these questions, the following hypotheses were derived: [1] there is a nascent literature on the impact of AI-enabled synthetic biology, [2] active stewardship is emerging as a best practice on governing the risks and opportunities of AI-enabled synthetic biology, [3] to achieve proper governance, most, if not all AI-development needs to immediately be considered within the Dual Use Research of Concern (DURC) regime, [4] even with the appropriate checks and balances, with AI-enabled synthetic biology, industrial biomanufacturing can conceivably scale up beyond the microscale within a decade or so.
The paper first describes the methods used for the literature review followed by a presentation of the results. A discussion of these findings ensues, addressing the research question and support for the hypotheses, followed by a brief conclusion and suggestions for further research.
2 Methods
The purpose of this paper is to conduct a literature review of the issues surrounding AI-enabled synthetic biology and present a set of recommendations for policy and practice. The research goal is to show that a reasonable scope for innovation can be maintained even with instituting early warning systems that enable prevention and mitigation of future AI-enabled biohazards.
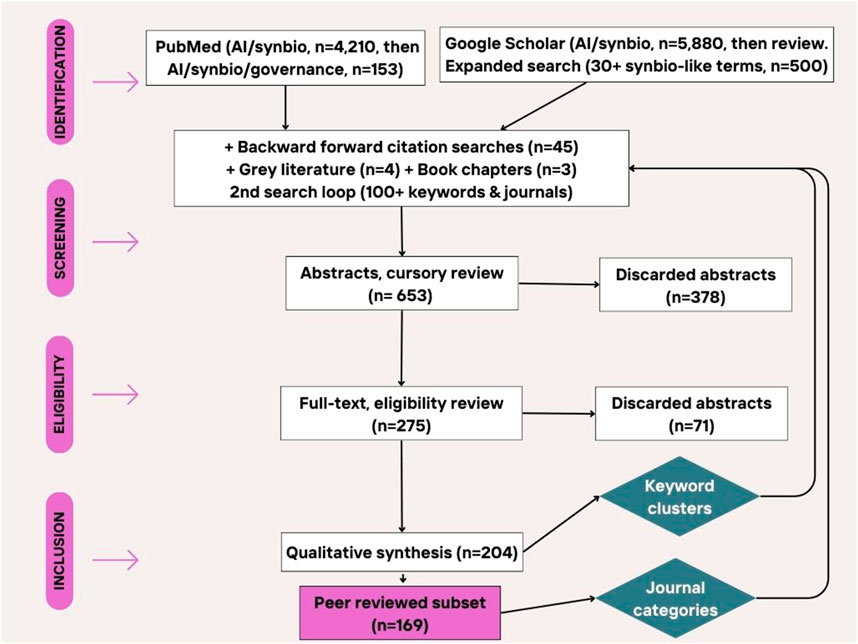
Literature review (Sauer and Seuring, 2023), a comprehensive summary of existing research on the topic, was pursued because AI’s influx into the synthetic biology field is a very recent development. There is a need to identify gaps in the knowledge that ensue from AI’s emerging impact. The goal is to align the AI literature with the synthetic biology risk literature, and develop a theoretical framework for future research. The approach broadly followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) for systematic reviews, using formal, repeatable, transparent procedures with separate steps for identification, screening, eligibility, and inclusion of papers (Moher et al., 2015). That being said, in the end a mix of search terms (clearly identified below), plus backward/forward citation searches, where used, which means attempts at replication might yield slightly different results (see Figure 1). However, because of the nascent research field, in this case, the benefit of flexibility outweighs the costs.
A literature review using the search terms “generative AI”, “synthetic biology” and “governance” in Google Scholar generated only 97 results, so the search was broadened to “AI”, which generated 5,880 results, also capturing important articles before the generative AI discoveries of 2022–2023. Similar searches in Scopus (artificial AND intelligence AND synthetic AND biology AND governance) generated only 9 documents. Using the terms “AI synthetic biology governance” in PubMed generated only 14 results of which only 1 paper (on synthetic yeast research and techno-political trends) was retained. However, removing the term governance gave 4,210 results. The search was limited to 2020–2024 publication dates, and further filtered to only review or systematic reviews to get 153 results which were screened down to 14 relevant articles.
Searching Social Sciences Citation Index (pub dates 2021–2023) searches yielded 257 results for “synthetic biology”, which were all reviewed, and 13 abstracts were selected into the sample.
Searching Business Source Complete (pub dates 2020–2023) for “synthetic biology” yielded 183 academic journal papers, 15 of which were relevant and from which 7 were retained after deduplication (this search was finalized last). Other search terms such as “bioeconomy” and “governance” performed better in this database.
The final search protocol borrows from Shapira et al. who note that papers that do not explicitly use “synthetic biology” in their title, abstract or key words could still be relevant because it is an interdisciplinary field (Shapira et al., 2017). Shapira et al. track the emergence of synthetic biology over the 2000–2015 period, first retrieving benchmark records, extract keywords from there, and then searching. With that insight, having selected 150 articles, read their abstracts, indexed their keywords, and scanned the content of all papers, I then went back, did new searches based on the keyword clusters that seemed promising, and, as a result, found additional papers to include in the sample.
The analysis was also complemented with papers that did discuss the overall impact of generative AI on biology or science, or research, using the search terms: “generative AI” AND “Science” OR “research”. Because generative AI is such a recent term, the search was expanded to preprints in the gray literature. The final research protocol included a much wider set of search terms, including a fuller set of keywords such as AI risk, bioethics, bioinformatics, biohacking, biorisk, biosafety, biosecurity, computational biology, DIY biology, Do-It-yourself laboratories, dual risk, dual-use research of concern (DURC), emerging technology, generative AI, industry, large language models (LLMs), multi-omics, risk mitigation, systems biology, AI-bio capabilities, chatGPT, biomanufacturing, biosurveillance, bioweapons (always used in combination with AI and/or risk). Separate searches for “synthetic biology” AND legislation OR “policy” OR “regulation” were also conducted. Similarly, when few papers were found on the management and industry aspects, specific searches on “synthetic biology” AND/OR “startups”, “industry”, “market”, and “economy” were pursued.
The final inclusion criteria involved any type of published scientific research or preprints (article, review, communication, editorial, opinion, etc.) as well as any high quality article (based on subjective review) published by a government agency, think tank or consulting firm. A total of 653 abstracts were considered, but only 204 sources and a subset of 169 peer reviewed papers were included in the final review (see Appendix A-papers in sample), representing 111 different journals (average impact factor: 9.94) from 4 fields. The overwhelming number of papers (114) originated from journals in Science, Engineering & Technology, 36 from interdisciplinary journals and only 9 from Social science and Humanities journals and 9 from Management journals (see Appendix B-journals in sample), as well as 4 preprints and 3 other types of publications (such as chapters in books as well as think tank white papers and memos). Once papers were identified for synthesis and analysis, 6–10 keywords were manually extracted from each article, starting with the ones identified by the authors (if any), and the diversity of journal types was recorded.
No human data was collected for this study. However, ethical considerations, such as how to discuss whether synthetic biology is significantly different from nature, were carefully addressed throughout the study.
The study’s findings may not be generalizable to biological research that does not rely on synthetic approaches or that only have limited use of AI technologies. Given that scale-up seems to be a much desired future development that industry and researchers both expect, future research could explore the complex factors influencing the scale-up of industrial biomanufacturing.
3 Results
There was no significant concentration of papers in any specific journal, instead the topic was covered broadly across journals. However, 23 percent of the journals (25 journals) were published by Elsevier, and 23 percent of the journals (25 journals) were published by Springer Nature, each highly overrepresented in the sample. The world’s two top publishers (in number of published journals) each publish nearly 3,000 journals (Curcic, 2023).) The showing of the third (Taylor & Francis, 2,508 journals total 8 in our sample), and fourth (Wiley, 1,607 journals total, 6 in our sample) was far lower, grouped with the fifth (Oxford Academic, 7 journals in sample), and sixth (MDPI, 5 journals in sample), who only publish about 500 journals total (Curcic, 2023). The country of publication provided another slight surprise compared to data presented by Shapira et al. ‘s (Shapira et al., 2017) findings of a US and UK dominance when tracking the emergence of synthetic biology over the 2000–2015 period. Our data, in contrast, shows the US a bit behind in synthetic biology publishing (the Netherlands 23 percent, UK 21 percent, US 19 percent, Germany 12 percent, and Switzerland 11 percent). One explanation might be that in several cases US professional societies use a European publisher.
At least 8 breakout papers contained especially innovative, useful, or surprising observations for scholars and policymakers alike (Camacho et al., 2018; Trump et al., 2019; Hagendorff, 2021; Eslami et al., 2022; Hanson and Lorenzo, 2023; Holzinger et al., 2023; Sundaram et al., 2023; Yan et al., 2023), each summarized in a sentence:
[1] As long as the black box issues of deep learning models are addressed they will transform insights into molecular components and synthetic genetic circuits and reveal the design principles behind so one can iterate rapidly and create complex biomedical applications (Camacho et al., 2018).
[2] An interdisciplinary approach between the physical and social sciences is necessary (and seems to be proceeding), fostering sustainable, risk-informed, and societally beneficial technological advances that are driven by safety-by-design and adaptive governance that properly reflects uncertainty (Trump et al., 2019).
[3] Machine learning for synthetic biology can yield (forbidden) knowledge with dual-use implications that needs to be governed given legitimate misuse concerns that we have seen in other areas such as nuclear energy (Hagendorff, 2021).
[4] If synthetic biology can deploy the Design-Build-Test-Learn (DBTL) cycle, bridging the cultures of bench scientists and computational scientists, and properly quantify uncertainty, it will impact every activity sector in the world (Eslami et al., 2022).
[5] For the field of synthetic biology, considering all the hype, it is high time to deliver, likely by toning down claims to have all the answers and capitalize on the achievable goals, and enlist tool builders in universities, realizing that biofoundries will not be generalized industrial factories near term but will remain fermentation plants for enzymes (Hanson and Lorenzo, 2023).
[6] AI is already ubiquitous in biotechnology (Holzinger et al., 2023).
[7] In the future, AI will be a driving force of biotechnology whether we like it or not (Sundaram et al., 2023).
[8] So far, AI in synthetic biology has been used for foresight, data collection, and analysis but in the future it will be used to design complicated systems (Yan et al., 2023).
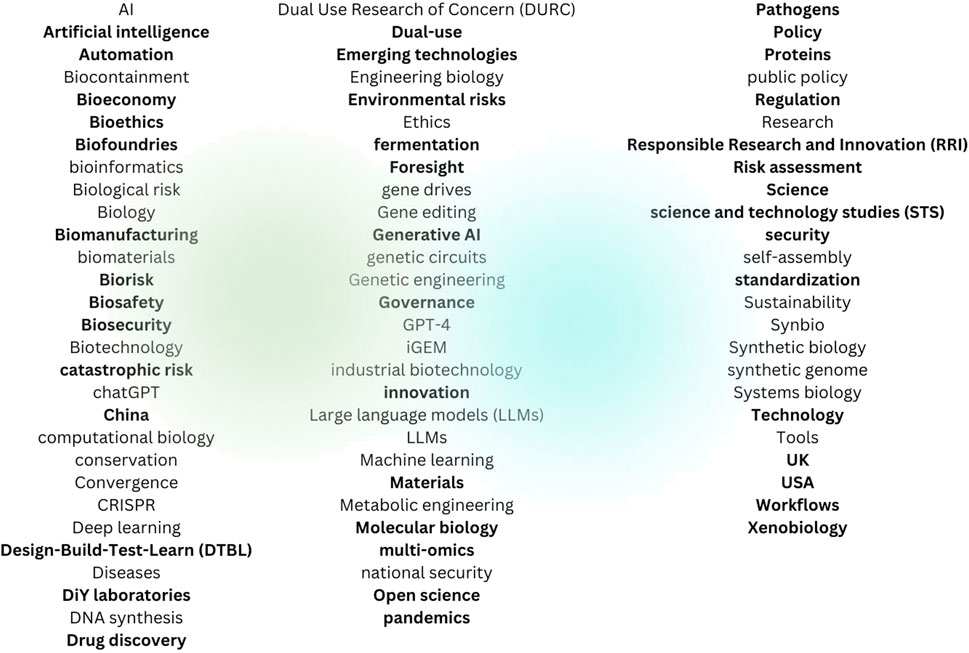
The 1,297 unique keywords found in these 204 sources were clustered into 81 broad categories, 42 of which seemed particularly important as literature search keywords (see Figure 2).
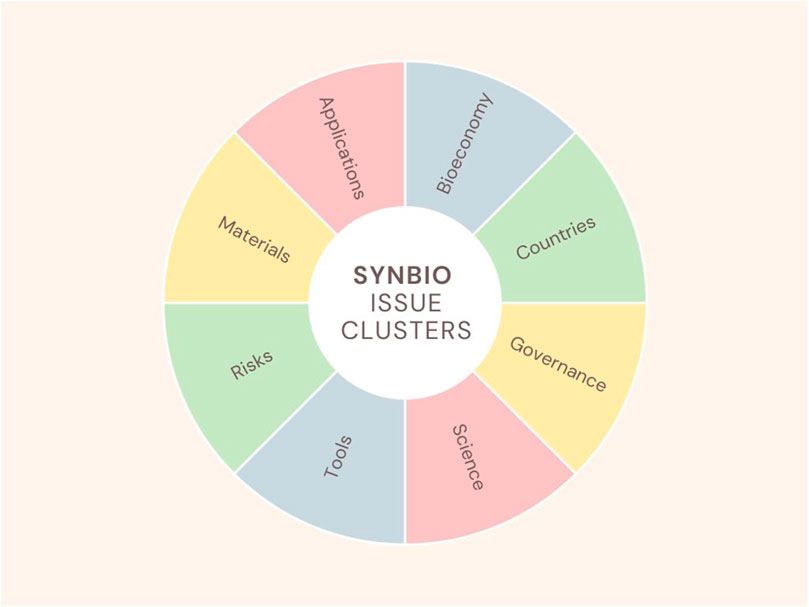
These categories were further reduced into 8 clusters representing key issues: Applications (drug discovery), Bioeconomy (biomanufacturing, innovation), Countries (China, EU, UK, US), Governance (bioethics, biosafety, dual use, risk assessment), Science (computational biology, Design-Build-Test-Learn (DTBL), materials, molecular biology, open science, RRI, STS, xenobiology), Tools (artificial Intelligence, DIY laboratories, fermentation, generative AI, biofoundries, emerging technologies, laboratories, multi-omics, technologies, xenobiology, workflows), Materials (genes, proteins), and Risks (biological, environmental, pandemics) (see Figure 3).
The presentation of results is organized according to the initial four hypotheses on: [1] the nascent literature, [2] best practices, [3] the DURC regime, and [4] scale up.
3.1 The impact of AI-enabled synthetic biology
Among the 169 peer-reviewed papers in the sample, there were 81 papers that explicitly discussed the impact of AI-enabled synthetic biology. The other 88 papers discussed risk but not explicitly from AI. Equally surprising was the exclusion of AI in all the other papers, given that several were review articles or otherwise covered state-of-the art or emerging technologies and tools for synthetic biology. There is no ready explanation for this omission, except to say that perhaps (a) those researchers are not familiar with the potential of (generative) AI for biology, (b) do not think it is as big of a deal as others do, or (3) consider it less relevant for today’s concerns in synthetic biology, or (4) consider AI (machine learning) an essential tool but prefer not to elevate it beyond its obvious place as a key research tool.
Another finding is that many papers that I found relevant to the future of synthetic biology’s governance, risk, and innovation trajectory, did not in fact use that term. Dozens and dozens of papers included in the sample happily discussed AI and the impact on their field, be it metagenomics of the microbiome (Wani et al., 2022), health and intelligent medicine (Achim and Zhang, 2022), applied microbiology (Xu et al., 2022), designer genes (Hoffmann, 2023), drug discovery (Yu et al., 2022), oncology (Wu et al., 2022), systems biology (Helmy et al., 2020) without realizing that synthetic biology is bound to intersect with it at some point soon (or at least explicitly omitting the use of the term). What could the reason be? Is the term upsetting to part of the biology community or establishment? Synbio scholars clearly frame their problems differently from the biology establishment. Perhaps there is a disparity in age, experience, and skills between patchy biological knowledge of bio-IT nerds and lacking IT skills among biologists? AI has been applied to material discovery, finding 700+ new materials so far (Merchant et al., 2023) and it is a question of time before it will be used for scalable materials design using AI-enabled synthetic biology (Tang et al., 2020; Burgos-Morales et al., 2021) although for real world applications we might first need better standardized vocabularies for biocompatibility (Mateu-Sanz et al., 2023).
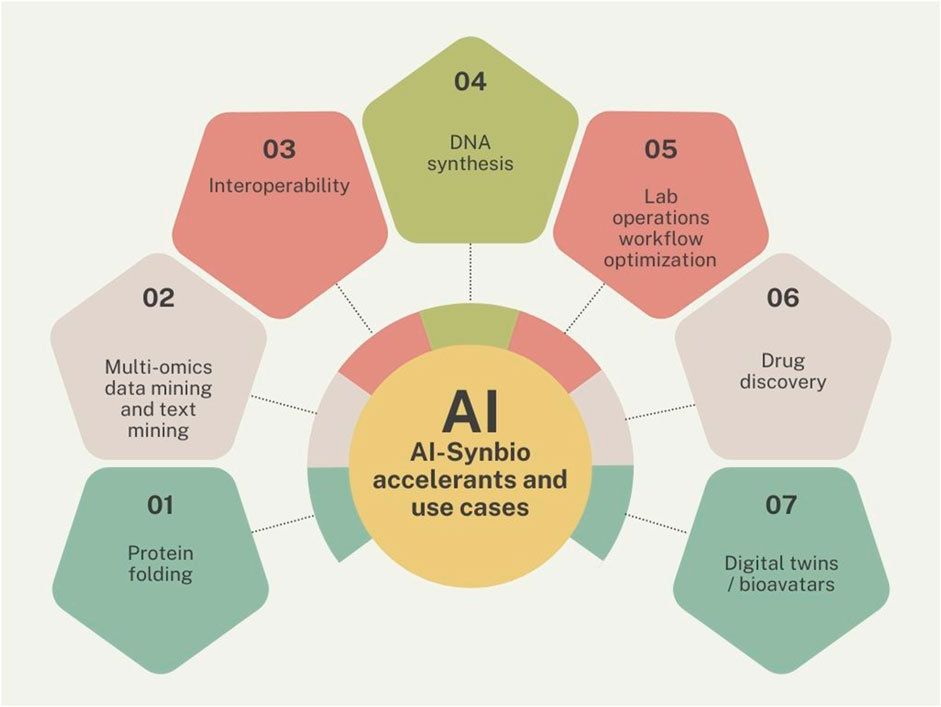
Only 5 papers (Kather et al., 2022; Grinbaum and Adomaitis, 2023; Morris, 2023; Ray, 2023; Xiao et al., 2023), a popular science article (Tarasava, 2023), and an editorial (Generating ‘smarter’ biotechnology, 2023) discussed the impact of generative AI on synthetic biology. This is expected to increase dramatically quite soon, given the success of this latest wave of AI technology and the platform aspects of its spread. However, as one paper put it, synthetic biology has a natural synergy with deep learning (Beardall et al., 2022). The use cases discussed in various papers include: as a classifying text, generic search engine, generating ideas, helping with access to scientific knowledge, coding, patient care (Clusmann et al., 2023), protein folding, proofreading, sequence analysis, summarizing knowledge, text mining of biomedical data, translation (Clusmann et al., 2023), workflow optimization, foresight of future research directions (Yan et al., 2023); collection of related synthetic biology data, and more (Beardall et al., 2022; Clusmann et al., 2023; Tarasava, 2023). The promise of AI-enabled cell-free synbio systems (Lee and Kim, 2023), which use molecular machinery extracted from cells, is particularly significant for automation and scale-up of biosensors among other things. It bears pointing out that the significant advances in cell-free synbio systems enabling the acceleration of biotechnology development, specifically its ability to enable rapid prototyping as well as the ability to conduct predictive modeling, pre-date generative AI by a decade (Moore et al., 2018; Müller et al., 2020). Even today, he the barriers seem to be limited availability of relevant data either because it does not exist yet, because data is scarce, the data set is small, because it is not publicly available, or because it is not formatted in useful ways (Rosenbush, 2023). Not all of these challenges can be immediately resolved by generative AI.
What matters most to the governance and innovation concern would be those barriers, areas, or workflows where AI could make the biggest impact, not just for the field applying it but for the shared resource that is AI-enabled synbio that would grow the pie. Based on the literature review, I’ve attempted to suggest which topics fit in that perspective (see Figure 4). For example, progress on interoperability would benefit all, as would AI-enabled lab operations workflows. Each would be a synbio building block. Big ticket items such as protein folding is in a bit of a different category. When AlphaFold achieved near-perfect protein fold predictions, it was the most important moment for AI in science so far, yet left plenty of work for structural biology (Perrakis and Sixma, 2021), including the application of coiled coils as a self-assembly building block in synthetic biology (Woolfson, 2023). Similarly, when the mRNA platform became a successful vehicle for a COVID-19 vaccine that changed the world, this happened in vitro, yet, the production of synthetic mRNA (Hınçer et al., 2023) or miRNA (Matsuyama and Suzuki, 2019) in the cell itself would be an even more significant breakthrough–and getting there might require the use of AI (Naderi Yeganeh et al., 2023).
On the other hand, there is no reason to believe that the top labs will be overtaken, quite the contrary, in fields such as consulting, it seems that generative AI accelerates the work of top teams (Moran, 2023). However, the worrying aspect is that the previous assumption from biorisk work pre-generative AI was that developing pathogens is an activity only possible in highly advanced biolabs. The increasing availability of insight, instructions, as well as wetlabs and foundries on demand (Sandberg and Nelson, 2020), would seem to be a potential issue to watch.
3.2 Best practice in AI-synbio governance
In the literature, there is ample evidence of what constitutes biosafety and biosafety governance best practice (Perkins et al., 2019; Wang and Zhang, 2019; Li et al., 2021; Mökander et al., 2022; Sandbrink, 2023b) and the emphasis is on a mix of specific training and, relatedly, developing a safety and responsibility work culture. In previous decades, the few advanced labs that existed were “compliant” biocontainment actors, for which acceptable systems were in place. However, regulating synthetic DNA comes with new challenges, including scalability, and less ability to create genetic firewalls to natural organisms, and it has become easier to circumvent oversight (Hoffmann et al., 2023). The more accessible (Wang and Zhang, 2019) and generally useful synbio potentially is regarded to be (Sun et al., 2022), the less likely it is that prohibition will remain an effective tool. Decades-old bioinformatics resources originally developed to compare gene sequences, such as BLAST, have been re-used, with mixed results, as biosafety tools to identify pathogens (Beal et al., 2023). Newer tools, such as machine learning-based topic models, enable spotting trends across a wide set of biosafety research publications (Guan et al., 2022). AI-synbio governance (Achim and Zhang, 2022; Mökander et al., 2022; Grinbaum and Adomaitis, 2023; Holland et al., 2024) is expected to be more of the above, but also requires AI skills and perspectives that go far beyond wet lab practices and will require updates to biosafety laws, regulation, governance, standardization (Pei et al., 2022). It will change the role of the state (Djeffal et al., 2022) as it will no longer be the primary norm setter or enforcer of responsibility.
AI is already contributing to the fragmentation of biology (Hassoun et al., 2022) and will challenge medical expertise among specialists (Patel et al., 2009). Generative AI, and especially other advancements in multi-modal AI, combined with better multi-omics synbio dataset interoperability (Topol, 2019) and standardization, will eventually lead to fundamentally new playing fields. Vigilance is required (Harrer, 2023) both to get us there, predict when we will get there, and decide what to do when we get there. The initial issue surrounds AI-synbio lab safety practices (D’Alessandro et al., 2023) when the “lab” suddenly is a dispersed concept, and decisions around forbidden knowledge (Hagendorff, 2021), new sets of responsibilities in the research community (Blok and von Schomberg, 2023) and among health practitioners (Achim and Zhang, 2022), avoidance of doom speak (Bray, 2023), handling the reality of malicious actors (Carter et al., 2023), and will represent an enormous challenge for reskilling and upskilling those who want to work with the topic (Xu et al., 2022).
Getting it right will mean balancing brave investments (Hodgson et al., 2022) with monitoring the effects, including developing an ethics and a taxonomy for working with AI-synbio-human hybrids and intelligence (Nesbeth et al., 2016; Damiano and Stano, 2023), dealing with new synthetic pathogens (O’Brien and Nelson, 2020), saying carefully goodbye to the natural world (Lawrence, 2019; Webster-Wood et al., 2022; Bongard and Levin, 2023) or at least radically enhancing biocontainment (Schmidt and de Lorenzo, 2016; Aparicio, 2021; Vidiella and Solé, 2022; Hoffmann, 2023), as well as developing new approaches to worker safety (Murashov et al., 2020). This leads into the issue of dual use of concern, which currently is a binary issue even though it is about to become immensely complex, requiring a more nuanced approach (Evans, 2022; Sandbrink, 2023a), given the legitimate concern with deliberate, perhaps even deliberate synthetic pandemics (Sandbrink, 2023b).
3.3 Broadening the DURC regime
Dual use is mentioned by several papers (Getz and Dellaire, 2018; Torres, 2018; DiEuliis et al., 2019; Alexander Hamilton et al., 2021; Hagendorff, 2021; Grinbaum and Adomaitis, 2023; Vaseashta, 2023). However, it can have broader meaning, for example, positively referring to open source (Esquivel-Sada, 2022) as opposed to negatively referring to non-conformant use. The idea of broadening the dual use research of concern (DURC) regime, which gained steam during COVID-19 (Sandbrink et al., 2023) is mentioned in the preprint literature (Grinbaum and Adomaitis, 2023), and alarms about dual-use involving AI are sounded in several papers (Urbina et al., 2022; D’Alessandro et al., 2023) and in Sandbrink’s Ph. D thesis (Sandbrink, 2023b).
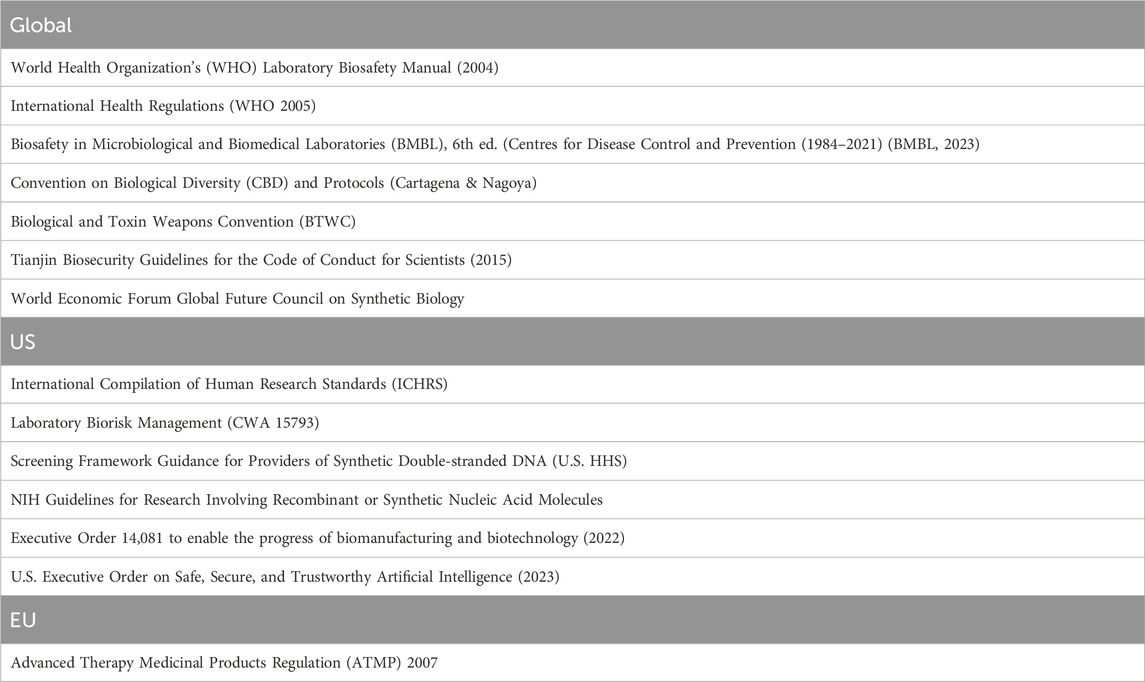
Giving a complete regulatory overview of synthetic biology is complex (Beeckman and Rüdelsheim, 2020) and is not the task of this paper, but Table 1 still lists some key standards and guidelines discussed in the sample, and relevant to DURC issues.
3.4 Scaling industrial (bio) manufacturing
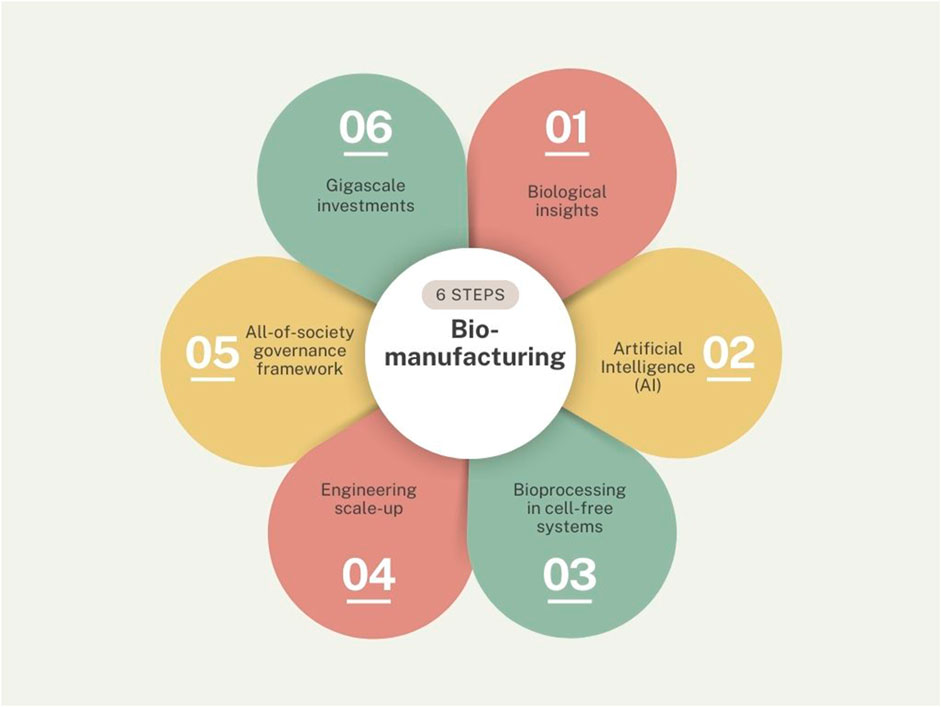
Synbio is not yet a mature engineering industry with well-understood costs and timelines (Watson, 2023) and investments fluctuate from year to year (SynBioBeta, 2023). A recent Schmidt futures report defines commercial production scale as a fermentation capacity of 100,000 L or more and states only a few U.S. companies currently have infrastructure at this scale and relatively inaccessible to small- and medium enterprises at the present moment (Hodgson et al., 2022). Achieving pilot scale is the first hurdle to pass and would cost in excess of $1 billion to build a dozen pilot facilities to fuel the U.S infrastructure alone (Hodgson et al., 2022). Synbio was not truly part of the industry 4.0 paradigm either (Jan et al., 2023). The keywords to describe the industrial aspect of synthetic biology included: “bioeconomy”, “bio-capitalism”, “biomanufacturing”, “biotech industry”. Surprisingly few path breaking peer reviewed articles were found on these topics. The six key ingredients for biomanufacturing derived from our sample are: biological insights, AI, bioprocessing, engineering scale-up, governance frameworks, and gigascale investments (see Figure 5).
Attempting to pinpoint exactly when a sci-tech paradigm will take off commercially is a fool’s errand. Exceptional growth in research communities can be tentatively forecasted from citation analysis (Klavans et al., 2020). The emergence of new industries is significantly more complex but the growth in intangible assets (Börner et al., 2018), such as generative AI, applied to an industry (manufacturing) would be a clear indicator. One article in our sample proposed a taxonomy of four innovation types specific to the bioeconomy: Substitute Products, New (bio-based) Processes, New (bio-based) Products, and New Behavior, each carries their own commercialization challenges (Bröring et al., 2020). Deriving insights from other papers, existing or emerging business models in synthetic biology would include automation, contract research, increasing crop yields in agriculture (Bhardwaj et al., 2022; Wang et al., 2022), data driven design (Freemont, 2019), efficiencies, new components, DNA synthesis (Seydel, 2023), infrastructure, licensing, manufacturing molecules for the food industry (Helmy et al., 2020), modularity, new materials, new platforms, new products, open source tools, services, or substitution, such as a new technology stack (Freemont, 2019).
That being said, despite the relatively low number of papers describing the synbio industry (van Doren et al., 2022), there are signs in the gray literature and in the consulting literature (Candelon et al., 2022) that things are changing within this decade. Arguably, the synbio startup boom in pharma and food industries will be duplicated in health and beauty, medical devices, and electronics, with cost-based competition from syn-bio alternatives in chemicals, textiles, fashion, and water industries, soon to be followed by the mining, electricity, and construction sectors (Candelon et al., 2022). The way it might happen is not necessarily only through flashy, radical innovations, but incrementally because synbio is becoming a useful tool to improve performance, quality, and sustainability of almost all types of manufacturing (Candelon et al., 2022).
Made-to-order synthetic DNA is faster and cheaper than before but is still a massive bottleneck to building scalable biological systems based on synthetic components (Seydel, 2023). The future role of synthetic biology in carbon seq uestration into biocommodities could be of major industrial importance provided the bioproduct could be commercialized (Jatain et al., 2021).
4 Discussion
One of the papers in the sample reports that synbio discourse is framed in six major ways: as science, social progress, risks and control, ethics, economics, and governance (Bauer and Bogner, 2020), which roughly matches the eight clusters identified based on the papers in the present sample: Applications, Bioeconomy, Countries, Governance, Science, Tools, Materials, and Risks. These frames tend to belong to different camps (particularly citizens, corporations, governments, nonprofits, and startups), with separate agendas and concerns, as opposed to characterizing aspects of a discussion that all actors should be having. There are signs this is changing towards more adaptive approaches to address the uncertainty surrounding the effects of novel technologies (Millett et al., 2020; Mourby et al., 2022) in parts of the system, such as in innovation communities such as iGEM (Millett et al., 2020; Kirksey, 2021; Millett and Alexanian, 2021; Vinke et al., 2022), or in entrepreneurial ecosystems (Nylund et al., 2022). However, those are not characteristic of the governance system as a whole.
4.1 Terminological and sectoral confusion, growing pains
Given the nascent state of AI-enabled synthetic biology, there is an overload of related and relatable search terms and keywords that proliferate in the scientific community and online, making it difficult to compare, find, and cluster case studies, research, and policy relevant insight. Even after considerable search efforts, we were left with 1,297 unique keywords, which were boiled down to 81 broad categories, and further to 42 literature search keywords. The situation will persist, and in all likelihood, it will get worse before it gets better. There are those hoping for a taxonomic renaissance (Bik, 2017) to remedy the problem, including a taxonomy for engineered living materials (Lantada et al., 2022). An early article attempted to do the same for the field of synthetic biology (Deplazes, 2009), but it might have been too early in the cycle.
Historically, synbio has been seen as a disruptive innovation yielding products and processes which may not be well aligned with existing business models, value chains and governance systems (Banda and Huzair, 2021), but this might now be changing and synbio approaches get integrated into traditional industries and sectors. That is exciting for industrial innovation but challenging for governance, risk and regulation.
Even though commercially available synthetic biology-derived products are already on the market that are, arguably “changing the world” (Voigt, 2020), the economics of synthetic biology (Henkel and Maurer, 2007), the biomanufacturing industry overall, is in its infancy. McKinsey might be right that it is a $4 trillion gold rush waiting to happen (Cumbers, 2020), or as BCG claims, $30 trillion by the end of the decade (Candelon et al., 2022), across food and ag, consumer products and services, materials and energy production, and human health and performance (Ang, 2022; Clay, 2023). However, the conspicuous absence of management and business articles on synbio in our sample might indicate that the business dimension is so embryonic that these visions are not yet a story worthy of sustained business school attention. The umbrella term bioeconomy (Baker, 2017; Bröring et al., 2020; Marvik and Philp, 2020; Banda and Huzair, 2021; Hodgson et al., 2022; Bröring and Thybussek, 2023; Clay, 2023; Rennings et al., 2023) is perhaps convenient, but encompasses so much that it is hard to know what it means.
4.2 Transdisciplinary barriers to growth
What seems to be missing in the literature is a clear vision for how AI-enabled synthetic biology would be truly different from previous approaches. Most of the papers imply that AI will remain only one of many technologies relevant to progress in the synthetic biology field. No papers paint a picture where there is a straightforward path to massive scale-up, with possible exception of AI for multi-omics. The shift would happen once the synbio field was able to shift from its current systems-centric approaches (requiring slow, cumbersome wet lab experiments and trial-and-error tinkering) to data-centric bioprocessing approaches (not just using AI for data processing) that are themselves digitally scalable (Owczarek, 2021; Scheper et al., 2021), and constitute automated design-build-test systems (Holland et al., 2024), supported by digital twins (Manzano and Whitford, 2023). Having said that, enormous efficiencies could be had through even much simpler process automation and operations improvements in biomanufacturing, for example, through no-code methods (Linder and Undheim, 2022).
The barriers to the field of synthetic biology’s growth are many, from (1) technical feasibility, including the scientific problems connected with the fusion of three disciplines; synthetic biology, artificial intelligence, and social science (Trump et al., 2019), via (2) various forms of risk to (3) industrial challenges, to (4) institutional challenges, or (5) social dynamics.
On the technical side, we find the challenges surrounding data quality (Patel et al., 2009) the fragmentation of knowledge (Hassoun et al., 2022) interoperability (Mateu-Sanz et al., 2023) or standardization (Endy, 2005; Hanczyc, 2020; Garner, 2021; Pei et al., 2022; Mateu-Sanz et al., 2023). For example, even though there is great need, and the desire is there, standardizing complex biological systems is difficult (Garner, 2021). As many of the papers in the sample point out, there are also scientific problems connected with the fusion of two disciplines, synthetic biology and artificial intelligence. A multi-layer technology stack is evolving (Freemont, 2019). There is transdisciplinary training and perspective required (Hammang, 2023). There is also considerable uncertainty produced when three domains (or more), and their methodologies, technologies, and tools, are merging (Trump et al., 2019).
Notably, (1) biological insight is needed to deploy AI correctly, yet cell behavior is unpredictable (Lawson et al., 2021) (2) AI insight is needed to capture what ends up retranslated as biological patterns in the data, but AI insight alone is not sufficient to identify what data might be relevant and (3) social science insight, including business models, sociotechnical issues (Marris and Calvert, 2020), social dynamics, social implications, governance, risk, ethics, and psychological reactions, is needed to assess the feasibility of R&D, product development, and commercialization of the emergent field’s output. That’s a tall order for individual researchers, teams, companies, and political institutions alike. As the field grows in importance, scale, and impact, it will entail an enormous societal reskilling effort (Hammang, 2023).
Industrial challenges include: biosafety (Pei et al., 2022), the availability of capital (Baker, 2017; Helmy et al., 2020; Hodgson et al., 2022; Sargent et al., 2022), the lack of a scalable manufacturing workflow (Hillson et al., 2019; Ataii et al., 2023), regulatory uncertainty (Huzair, 2021), innovation challenges (Banda and Huzair, 2021; Tait and Wield, 2021), intellectual property rights (Esquivel-Sada, 2022), investment risks (Hodgson et al., 2022), or worker safety (Murashov et al., 2020).
Various forms of risk will impact synbio growth, notably AI risk (O’Brien and Nelson, 2020; Grinbaum and Adomaitis, 2023), the potential for a slew of catastrophic risks (DiEuliis et al., 2019) such as new pathogens, or even the specter of existential risks (Boyd and Wilson, 2020) threatening the flourishing or survival of humanity.
On the institutional side, we cannot discount biosecurity (Millett et al., 2020), bioterrorism (Trump et al., 2020; Sheahan and Wieden, 2021; Bray, 2023), the constant challenge of existing, emerging, or evolving bioweapons (Gronvall, 2018; DiEuliis et al., 2019; Trump et al., 2020), the cost of deregulation (Sargent et al., 2022), dual use (Getz and Dellaire, 2018; Ienca and Vayena, 2018; Evans, 2022; Grinbaum and Adomaitis, 2023), global governance (Linkov et al., 2018a; Dixon et al., 2022), security (Palmer et al., 2015) startup dependency (Freemont, 2019; Nylund et al., 2022) in terms of achieving a steady stream of new innovation in the domain.
Social dynamics such as differing notions and rationales surrounding bioethics (Szocik et al., 2021; Bohua et al., 2023), social acceptance (Bauer and Bogner, 2020; Frow, 2020), also play a part.
(Endy, 2005; Patel et al., 2009; Palmer et al., 2015; Bauer and Bogner, 2020; Boyd and Wilson, 2020; Frow, 2020; Hanczyc, 2020; Murashov et al., 2020; Garner, 2021; Pei et al., 2022; Sargent et al., 2022; Mateu-Sanz et al., 2023).
4.3 Whack-a-mole governance
The most reasonable way to look at it would be: what can generative AI do within the frame of all of these barriers? From this we can wonder whether generative AI-enabled synthetic biology really would be truly different from previous approaches. We could, of course, also wonder how different the situation would be if many of those previously mentioned barriers somehow went away. Interestingly, what AI fanatics would respond is that once those barriers are gone, AIs would themselves produce such approaches that are far superior to what could be conceived by human experts. Alternatively, it is always possible that emerging, superior and multi-modal AI systems would be able to overcome enough barriers to transform the field anyway.
For now, the most prudent governance path seems to be to keep fostering a responsibility mindset in a distributed manner at global scale. Machine learning enabled digital processing is already improving diagnostic accuracy and reducing turnaround time for even complex lab tests (Undru et al., 2022). The smart laboratory, with AI-automation of biosecurity, has arguably moved from concept to reality, enabling self-control process management flows of personnel, materials, water, and air, automated operation, automated risk identification and alarms (Li et al., 2022). However, when technologies merge, uncertainties multiply (O’Brien and Nelson, 2020), cybervulnerabilities and circumvention options increase (O’Brien and Nelson, 2020).
Take the case of biosafety labs, state-of-the-art labs designed not only to protect researchers from contamination, but also to prevent microorganisms from entering the environment. After concerns about health risks from recombinant DNA technologies, scientists met in 1975 to create safety guidelines according to the risk involved. Since its initial release in 1984, Biosafety in Microbiological and Biomedical Laboratories (BMBL), a 574-page document created by 200 contributors, has served as the cornerstone of biosafety practice in the United States. BMBL, currently in its 6th edition (Hatcher et al., 2023), is issued by the Centers for Disease Control and Prevention (CDC) and created to oversee the biosafety of recombinant DNA research at all institutions receiving funding from the National Institutes of Health (NIH). Since 1983, the WHO has its own Laboratory Biosafety Manual, a 128-page document currently in its 4th edition (LBM4), which adopts an evidence-based risk approach to biosafety rather than a prescriptive approach (WHO, 2020). The WHO also has a discussion forum, the Technical Advisory Group on Biosafety (TAG-B) (Hstoday, 2022). Despite obvious cyber risks, the prevalence of publishing potential dual use research on preprint servers, as well as the fact that there might be AI-enabled pathways to pathogen gain-of-function (Urbina et al., 2022), as well as other unintended consequences of increased automation (Kulken, 2023), implied in a digitalization of core aspects of even labs mostly working on low pathogenic agents (Lentzos et al., 2022), neither BMBL nor LBM, mention AI precautions. AI-synbio integration guardrails, including technical AI safeguards, and strengthening DNA synthesis screening, even limiting the access to AI biodesign tools, might be needed (Sandbrink, 2023a; Carter et al., 2023). Even if one achieves consensus on such measures on a multilateral (or national) basis, the implementation will be complex.
International biosafety guidance and regulation is highly variable, and key factors such as awareness, safety culture (Perkins et al., 2019), capability, practice, understanding, error documentation, training, and enforcement are particularly underdeveloped across Asia (Johnson and Casagrande, 2016). In 2020, as a potentially important step forward, the International Organization for Standardization (IS) issued an international standard for biorisk management, ISO 35001:2019 (ISO, 2020), but whether and how it is being used is too early to tell (Callihan et al., 2021). In the absence of a sufficient set of internationally recognized biosafety standards and norms (Gronvall and Rozo, 2015; Silver, 2022; Hadshar, 2023), BMBL also currently sets the international de facto standard. Having said that, it is primarily an advisory document. Now, consider the economic incentives, including tax incentives, combined with the health imperatives to build lab capacity across the world in a post-pandemic world (KMPG, 2020; Field, 2023). According to the Global BioLabs Initiative (www.globalbiolabs.org/), jointly published by King’s College London, Bulletin of the Atomic Scientists, and George Mason University, the global boom in construction of BSL4 and BSL3+ labs raises biosafety and biosecurity concerns, particularly given that it is occurring in places where biorisk management oversight is weak (GBR, 2023).
If it indeed was the case that AI lifts all boats, it wouldt mean that mediocre labs can more rapidly gain the ambition to modify their facilities and work practices, and start doing work regulated by BSL-3 and BSL-4 designations. But while more lab researchers than before might potentially deploy AI to carry out experiments that should be carried out in a lab with a stricter designation (a higher BSL), this would, in most cases, be against the regulations. Having said that, in India, for example, there are no national reference standards, guidelines, or accreditation agencies for biosafety labs, so those labs that do comply, rely on the international ones (Mourya et al., 2014; 2017). China, also, lacks a comprehensive regulatory system for BSL-2 labs, and lacks trained biosafety staff (Wu, 2019). The numbers game is indeed instructional. The International Laboratory Accreditation Cooperation (ILAC) accredits over 88,000 laboratories (ILAC, 2023). If you consider that there are currently 64 BSL-4 labs, just imagine if all BSL-3 labs wanted to do BSL-4-type work, and were capable of it. There are already some 57 BSL-3+ labs (Kaiser, 2023), the safety levels of which are poorly defined (GBR, 2023). There are currently great experiments going on regarding the feasibility of rapid response mobile BSL-2 lab deployment to areas with a public health crisis, but those labs carry additional risks from rogue elements (Qasmi et al., 2023). Or, what about if all BSL-2 labs (which include most labs that work with agents associated with human diseases) suddenly started doing BSL-3 or BSL-4 type work? With AI, and without national control regimes, more BSL-2 labs might be tempted to think they can take on more advanced work, too quickly. Regardless of intent, there is also the risk that a lab leak could be a digital leak, facilitated by AI. Either way, beyond adopting a needed multilateral approach (GBR, 2023), the next best might be to increase scientists’ motivation (which might be even more important than raising skill levels) to practice good biorisk management (Greene et al., 2023) and adopting a culture of safety (Perkins et al., 2019).
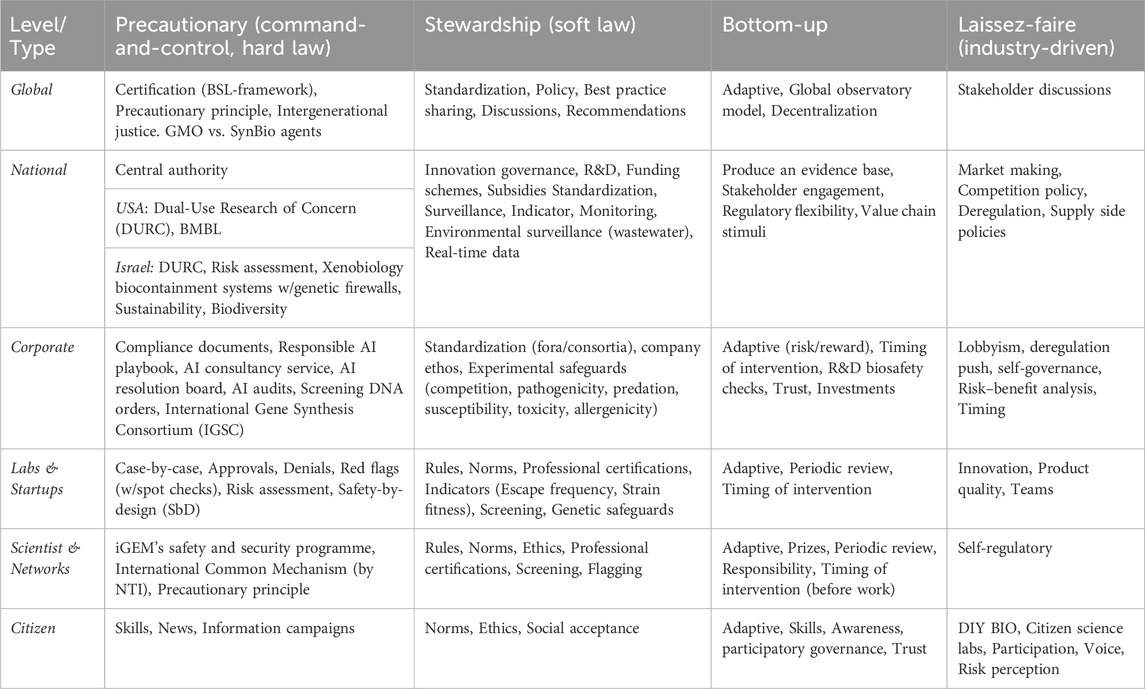
As has been pointed out, there is a need to deploy a governance continuum (Hamlyn, 2022). Based on our reworking of the issues based on the literature review (Linkov et al., 2018b), there are six governance levels (global, national, corporate, lab, scientist, citizens) and four governance types (precautionary, stewardship, bottom-up, and laissez-faire) to be considered in an emerging Framework for AI-enabled synbio governance (see Table 2). Each of these need constant monitoring and renewal based on assessing threats, hazards, and opportunities. Each governance level might prioritize one approach, but must have aspects of all governance types. Each governance type must be reflected at all governance levels. Today, we only have elements of such a framework implemented, and the skills required to make a comprehensive approach happen are formidable and require an all-of-society effort.
At any level, the process is quite complex. For example, the corporate AI governance at British biopharma AstraZeneca includes compliance documents, a responsible AI playbook and consultancy service, an AI resolution board, and AI audits–emphasizing procedural regularity and transparency–and interlinking with existing procedures, structures, tools, and methods (Mökander et al., 2022).
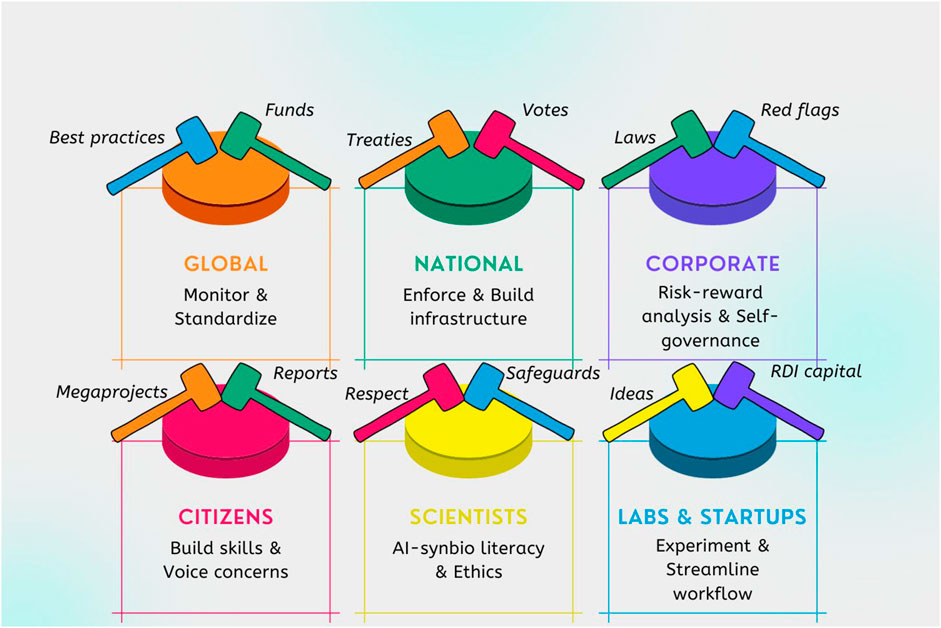
However, the literature review points to the fact that the true governance challenge is not only about the individual elements doing things right. Rather, proper governance is interactive, and adaptive, and requires working on all levels of governance simultaneously while not ignoring any one level for much time at all. One could describe the process as whack-a-mole governance (see Figure 6) where there are many actors using small rubber mallets (aka laws, rules, norms, votes) that need to hit each level simultaneously for the button (risk) to stay down.
The above schematic must be complemented by transparent approaches for each set of tools (real-time monitoring, certification, compliance documentation, standardization, prizes, rewards). The fact that some types may adversely affect others, for example, soft law might delay or undermine regulation or hard law, must be monitored and dealt with through responsible innovation (RI) approaches (Hamlyn, 2022). The entire governance structure (See Table 2) must work in a holistic way.
5 Conclusion
The research question was: what are the most important emergent best practices on governing the risks and opportunities of AI-enabled synthetic biology. Indeed, some best practices are emerging, but it is still a disjointed picture. The first hypothesis that [1] there is a nascent literature on the impact of AI-enabled synthetic biology only found partial support. In fact the literature is nascent but there is scarce evidence on whether generative AI makes a big difference, or only adds to the emergence, and we had to consult related literature on generative AI in science and research to get closer to an answer.
The second hypothesis found more support, because [2] active stewardship is emerging as a best practice on governing the risks and opportunities of AI-enabled synthetic biology. Having said that, top-down governance, especially the command-and-control flavor, is not sufficient, and the literature points to decentralized governance as a remedy. A whack-a-mole governance model was formulated to describe and possibly also to address these challenges.
Hypothesis three which said that [3] to achieve proper governance, most, if not all AI-development needs to immediately be considered within the Dual Use Research of Concern (DURC) regime has some support. The larger point is that in some ways all research is dual use (Evans, 2022) because research always has many meanings and uses and compliance with the letter of imperfect, imprecise and rapidly outdated laws can only get you so far and also limits research in undesirable ways. Whose security are we trying to protect? Whose security typically is not protected? The DURC regime itself, instigated with the Fink report in 2003, is in serious need of an update in light of generative AI-enabled synthetic biology, and dual use is understood differently internationally (Lev, 2019). The review should begin immediately, but clarity on the threat is not likely to emerge for a few years, as generative AI-ready synbio-datasets and related functionality still needs to mature.
The last hypothesis [4] that even with the appropriate checks and balances, with AI-enabled synthetic biology, industrial biomanufacturing can conceivably scale up beyond the microscale within a decade or so, found some support but the field is still largely dependent on innovations that still have not materialized such as bioprocessing workflow, standardization of multi-omic datasets, and a design-build-test cycle that would be required to enable such scale-up. In fact, delving into the impact of AI-enabled synthetic biology for industrial biomanufacturing is a fruitful direction for future research.
At the end of the day it is safe to assume that AI-enabled synthetic biology is both a catalyst for risk (through creating novel synthetic organisms) and a potential for risk reduction and mitigation (through optimizing or restoring natural organisms and detecting pathogens). Governance of the phenomenon, and any attempts to megascale the bioeconomy in short order (by the US, UK, EU, China, or others) needs to keep both perspectives firmly in mind.
In closing, the premise of the article was that it is possible to identify best practices for governance, innovation, research, or policy on AI-enabled synthetic biology, and that these issues have commonalities and are best explored together. The subtext was to be more resilient towards risks but still being able to capture the opportunities. The topics do seem related, and relatable, but it is complex both for researchers, entrepreneurs, corporations, and policymakers to do so because of the transdisciplinary efforts required (Lee and George, 2023; Taylor et al., 2023). However, in light of the revolutionary potential of AI-enabled synthetic biology, admittedly not yet fulfilled beyond single-cell microorganisms, one would have to conclude that best practices will change rather rapidly. If so, one implication might be that we chase such best practices in vain and that synthetic biology cannot deliver them (Hanson and Lorenzo, 2023).
Whack-a-mole type games seemingly are about quick reactions. However, it turns out that, according to the inventor of the version of the game with air cylinders, Aaron Fetcher, the best way to get a high score is to gaze in a relaxed way at the center of the playing field with the side moles in your peripheral vision (Brown et al., 2011). It is exactly that mix of calm focus with minimum effective effort which is needed for safe and sound AI-enabled synthetic biology scale-up. We are dealing with an environment with many possible distractions. As soon as one problem is fixed, another one will appear. Terminological and sectoral confusion, and growing pains within the industry, in the scientific establishment, and across the industries that are touched, will persist for some time. The obvious transdisciplinary barriers to growth are not easily or quickly resolved, even with a major reskilling effort. Generative AI might be a gamechanger, but biology will still be complex and surprising even to experts (and certainly surprising to machines). That’s why emerging frameworks for AI-enabled synbio governance likely should contain a mix of precautionary (command-and-control, hard law), stewardship (soft law), bottom-up, and laissez-faire (industry-driven) approaches.
Author contributions
TU: Conceptualization, Methodology, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was partially supported through grant funding from Open Philanthropy.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2024.1359768/full#supplementary-material
References
Achim, R., and Zhang, X. (2022). Exploring the social, ethical, legal, and responsibility dimensions of artificial intelligence for health-a new column in Intelligent Medicine. Intell. Med. 2, 103–109. doi:10.1016/j.imed.2021.12.002
Alexander Hamilton, R., Mampuys, R., Galaitsi, S. E., Collins, A., Istomin, I., Ahteensuu, M., et al. (2021). “Opportunities, challenges, and future considerations for top-down governance for biosecurity and synthetic biology,” in Emerging threats of synthetic biology and biotechnology. Editors B. D. Trump, M. V. Florin, E. Perkins, and I. Linkov (Springer).
Ang, C. (2022). Synthetic biology: the $3.6 trillion science changing life as we know it. Available at: https://www.visualcapitalist.com/synthetic-biology-3-6-trillion-change-life/(Accessed: January 31, 2024).
Aparicio, A. (2021). “That would break the containment”: the co-production of responsibility and safety-by-design in xenobiology. J. Responsible Innovation 8 (1), 6–27. doi:10.1080/23299460.2021.1877479
Ataii, N., Bakshi, S., Chen, Y., Fernandez, M., Shao, Z., Scheftel, Z., et al. (2023). Enabling AI in synthetic biology through construction file specification. bioRxiv. doi:10.1101/2023.06.28.546630
Baker, B. (2017). Synthetic biology and the marketplace: building the new bioeconomy. Bioscience 67 (10), 877–883. doi:10.1093/biosci/bix101
Banda, G., and Huzair, F. (2021). Introduction to special issue: innovation/governance interactions in the bioeconomy. Technol. Analysis Strategic Manag. 33 (3), 257–259. doi:10.1080/09537325.2021.1883928
Bauer, A., and Bogner, A. (2020). Let’s (not) talk about synthetic biology: framing an emerging technology in public and stakeholder dialogues. Public Underst. Sci. 29 (5), 492–507. doi:10.1177/0963662520907255
Beal, J., Clore, A., and Manthey, J. (2023). Studying pathogens degrades BLAST-based pathogen identification. Sci. Rep. 13 (1), 5390. doi:10.1038/s41598-023-32481-z
Beardall, W. A. V., Stan, G.-B., and Dunlop, M. J. (2022). Deep learning concepts and applications for synthetic biology. Gen. Biotechnol. 1 (4), 360–371. doi:10.1089/genbio.2022.0017
Beeckman, D. S. A., and Rüdelsheim, P. (2020). Biosafety and biosecurity in containment: a regulatory overview. Front. Bioeng. Biotechnol. 8, 650. doi:10.3389/fbioe.2020.00650
Bhardwaj, A., Kishore, S., and Pandey, D. K. (2022). Artificial intelligence in biological sciences. Life 12 (9), 1430. doi:10.3390/life12091430
Bik, H. M. (2017). Let’s rise up to unite taxonomy and technology. PLoS Biol. 15 (8), e2002231. doi:10.1371/journal.pbio.2002231
Blok, V., and von Schomberg, L. (2023). “Introduction,” in Putting responsible research and innovation into practice: a multi-stakeholder approach. Editor V. Blok (Cham: Springer International Publishing), 1–7.
Bohua, L., Yuexin, W., Yakun, O., Kunlan, Z., Huan, L., and Ruipeng, L. (2023). Ethical framework on risk governance of synthetic biology. J. Biosaf. Biosecurity 5 (2), 45–56. doi:10.1016/j.jobb.2023.03.002
Bongard, J., and Levin, M. (2023). There’s plenty of room right here: biological systems as evolved, overloaded, multi-scale machines. Biomimetics 8 (1), 110. doi:10.3390/biomimetics8010110
Börner, K., Rouse, W. B., Trunfio, P., and Stanley, H. E. (2018). Forecasting innovations in science, technology, and education. Proc. Natl. Acad. Sci. U. S. A. 115 (50), 12573–12581. doi:10.1073/pnas.1818750115
Boyd, M., and Wilson, N. (2020). Existential risks to humanity should concern international policymakers and more could Be done in considering them at the international governance level. Risk analysis official Publ. Soc. Risk Analysis 40 (11), 2303–2312. doi:10.1111/risa.13566
Bray, D. (2023). Artificial Intelligence and synthetic biology are not Harbingers of doom. Stimson. Available at: https://www.stimson.org/2023/artificial-intelligence-and-synthetic-biology-are-not-harbingers-of-doom/(Accessed November 27, 2023).
Bröring, S., Laibach, N., and Wustmans, M. (2020). Innovation types in the bioeconomy. J. Clean. Prod. 266, 121939. doi:10.1016/j.jclepro.2020.121939
Bröring, S., and Thybussek, V. (2023). Understanding the business model design for complex technology systems: the case of the bioeconomy. EFB Bioeconomy J. 3, 100052. doi:10.1016/j.bioeco.2023.100052
Brown, J., Fenske, M., and Neporent, L. (2011). The winner’s brain: 8 strategies great minds use to achieve success. Reprint edition. Boston: Da Capo Lifelong Books.
Burgos-Morales, O., Gueye, M., Lacombe, L., Nowak, C., Schmachtenberg, R., Hörner, M., et al. (2021). Synthetic biology as driver for the biologization of materials sciences. Mater. today. Bio 11, 100115. doi:10.1016/j.mtbio.2021.100115
Callihan, D. R., Downing, M., Meyer, E., Ochoa, L. A., Petuch, B., Tranchell, P., et al. (2021). Considerations for laboratory biosafety and biosecurity during the coronavirus disease 2019 pandemic: applying the ISO 35001:2019 standard and high-reliability organizations principles. Appl. Biosaf. J. Am. Biol. Saf. Assoc. 26 (3), 113–122. doi:10.1089/apb.20.0068
Camacho, D. M., Collins, K. M., Powers, R. K., Costello, J. C., and Collins, J. J. (2018). Next-Generation machine learning for biological networks. Cell 173 (7), 1581–1592. doi:10.1016/j.cell.2018.05.015
Candelon, F., Gombeaud, M., Stokol, G., Patel, V., Gourévitch, A., and Goeldel, N. (2022). Synthetic biology is about to disrupt your industry, BCG global. Available at: https://www.bcg.com/publications/2022/synthetic-biology-is-about-to-disrupt-your-industry (Accessed: November 30, 2023).
Carter, S. R., Wheeler, N., Chwalek, S., Isaac, C., and Yassif, J. M. (2023). The Convergence of artificial Intelligence and the life sciences, the nuclear threat initiative. NTI. Available at: https://www.nti.org/analysis/articles/the-convergence-of-artificial-intelligence-and-the-life-sciences/(Accessed November 27, 2023).
Clay, M. (2023). A rallying call for synthetic biology: challenges, opportunities, and future of the bioeconomy - SynBioBeta. Available at: https://www.synbiobeta.com/read/a-rallying-call-for-synthetic-biology-challenges-opportunities-and-future-of-the-bioeconomy (Accessed: November 30, 2023).
Clusmann, J., Kolbinger, F. R., Muti, H. S., Carrero, Z. I., Eckardt, J. N., Laleh, N. G., et al. (2023). The future landscape of large language models in medicine. Commun. Med. 3 (1), 141. doi:10.1038/s43856-023-00370-1
Cumbers, J. (2020). New McKinsey report sees A $4 trillion gold rush in this one hot sector. Who’s selling picks and shovels?’, forbes magazine. Available at: https://www.forbes.com/sites/johncumbers/2020/05/30/mckinsey-report-4-trillion-gold-rush-bioeconomy-synthetic-biology/(Accessed January 31, 2024).
Curcic, D. (2023). Academic publishers statistics. Available at: https://wordsrated.com/academic-publishers-statistics/(Accessed November 30, 2023).
D’Alessandro, W., Lloyd, H. R., and Sharadin, N. (2023). Large Language models and biorisk. Am. J. Bioeth. AJOB 23 (10), 115–118. doi:10.1080/15265161.2023.2250333
Damiano, L., and Stano, P. (2023). Explorative synthetic biology in AI: criteria of relevance and a taxonomy for synthetic models of living and cognitive processes. Artif. life 29 (3), 367–387. doi:10.1162/artl_a_00411
Deplazes, A. (2009). Piecing together a puzzle. An exposition of synthetic biology. EMBO Rep. 10 (5), 428–432. doi:10.1038/embor.2009.76
Deplazes, A., and Huppenbauer, M. (2009). Synthetic organisms and living machines: positioning the products of synthetic biology at the borderline between living and non-living matter. Syst. synthetic Biol. 3 (1-4), 55–63. doi:10.1007/s11693-009-9029-4
DiEuliis, D., Ellington, A. D., Gronvall, G. K., and Imperiale, M. J. (2019). Does biotechnology pose new catastrophic risks? Curr. Top. Microbiol. Immunol. 424, 107–119. doi:10.1007/82_2019_177
Dixon, T., C Curach, N., and Pretorius, I. S. (2020). Bio-informational futures: the convergence of artificial intelligence and synthetic biology. EMBO Rep. 21 (3), e50036. doi:10.15252/embr.202050036
Dixon, T. A., Freemont, P. S., Johnson, R. A., and Pretorius, I. S. (2022). A global forum on synthetic biology: the need for international engagement. Nat. Commun. 13 (1), 3516. doi:10.1038/s41467-022-31265-9
Djeffal, C., Siewert, M. B., and Wurster, S. (2022). Role of the state and responsibility in governing artificial intelligence: a comparative analysis of AI strategies. J. Eur. Public Policy 29 (11), 1799–1821. doi:10.1080/13501763.2022.2094987
Ebrahimkhani, M. R., and Levin, M. (2021). Synthetic living machines: a new window on life. iScience 24 (5), 102505. doi:10.1016/j.isci.2021.102505
El Karoui, M., Hoyos-Flight, M., and Fletcher, L. (2019). Future trends in synthetic biology-A report. Front. Bioeng. Biotechnol. 7, 175. doi:10.3389/fbioe.2019.00175
Endy, D. (2005). Foundations for engineering biology. Nature 438 (7067), 449–453. doi:10.1038/nature04342
Eslami, M., Adler, A., Caceres, R. S., Dunn, J. G., Kelley-Loughnane, N., Varaljay, V. A., et al. (2022). Artificial intelligence for synthetic biology. Commun. ACM 65 (5), 88–97. doi:10.1145/3500922
Esquivel-Sada, D. (2022). Responsible intellectual property rights? Untangling open-source biotech adherence to intellectual property rights through DIYbio. Technol. Soc. 70, 102005. doi:10.1016/j.techsoc.2022.102005
Evans, S. W. (2022). When all research is dual use. Issues Sci. Technol. 38 (3). Available at: https://issues.org/dual-use-research-biosecurity-social-context-science-evans/(Accessed: November 28, 2023).
Feuerriegel, S., Hartmann, J., Janiesch, C., and Zschech, P. (2024). Generative AI. Bus. Inf. Syst. Eng. 66, 111–126. doi:10.1007/s12599-023-00834-7
Field, M. (2023). Despite risk-management gaps, countries press ahead with new labs that study deadly pathogens, Bulletin of the Atomic Scientists. Available at: https://thebulletin.org/2023/01/despite-risk-management-gaps-countries-press-ahead-with-new-labs-that-study-deadly-pathogens/(Accessed: February 1, 2024).
Freemont, P. S. (2019). Synthetic biology industry: data-driven design is creating new opportunities in biotechnology. Emerg. Top. life Sci. 3 (5), 651–657. doi:10.1042/etls20190040
Frow, E. (2020). From “experiments of concern” to “groups of concern”: constructing and containing citizens in synthetic biology. Sci. Technol. Hum. values 45 (6), 1038–1064. doi:10.1177/0162243917735382
Garner, K. L. (2021). Principles of synthetic biology. Essays Biochem. 65 (5), 791–811. doi:10.1042/ebc20200059
GBR (2023). Global biolabs report 2023. Available at: https://www.kcl.ac.uk/warstudies/assets/global-biolabs-report-2023.pdf.
Generating ‘smarter’ biotechnology (2023). Generating ‘smarter’ biotechnology. Nat. Biotechnol. 41 (2), 157. doi:10.1038/s41587-023-01695-x
Getz, L. J., and Dellaire, G. (2018). Angels and devils: dilemmas in dual-use biotechnology. Trends Biotechnol. 36 (12), 1202–1205. doi:10.1016/j.tibtech.2018.07.016
Greene, D., Palmer, M. J., and Relman, D. A. (2023). Motivating proactive biorisk management. Health Secur. 21 (1), 46–60. doi:10.1089/hs.2022.0101
Grinbaum, A., and Adomaitis, L. (2023). Dual use concerns of generative AI and large language models. arXiv [cs.CY]. Available at: http://arxiv.org/abs/2305.07882.
Gronvall, G. K. (2018). Safety, security, and serving the public interest in synthetic biology. J. industrial Microbiol. Biotechnol. 45 (7), 463–466. doi:10.1007/s10295-018-2026-4
Gronvall, G. K., and Rozo, M. (2015). Addressing the gap in international norms for biosafety. Trends Microbiol. 23 (12), 743–744. doi:10.1016/j.tim.2015.10.002
Guan, R., Pang, H., Liang, Y., Shao, Z., Gao, X., Xu, D., et al. (2022). Discovering trends and hotspots of biosafety and biosecurity research via machine learning. Briefings Bioinforma. 23 (5), bbac194. Available at:. doi:10.1093/bib/bbac194
Hadshar, R. (2023). An overview of standards in biosafety and biosecurity. Effective Altruism. Available at: https://forum.effectivealtruism.org/posts/YDTgRR7Qjmj47PaTj/an-overview-of-standards-in-biosafety-and-biosecurity (Accessed February 1, 2024).
Hagendorff, T. (2021). Forbidden knowledge in machine learning reflections on the limits of research and publication. AI Soc. 36 (3), 767–781. doi:10.1007/s00146-020-01045-4
Hamlyn, O. (2022). Synthesize this: integrating innovation governance and EU regulation of synthetic biology. J. law Soc. 49 (3), 577–602. doi:10.1111/jols.12375
Hammang, A. (2023). Troubleshooting: the automation of synthetic biology and the labor of technological futures. Sci. Technol. Hum. values, 016224392211495. doi:10.1177/01622439221149524
Hanczyc, M. M. (2020). Engineering life: a review of synthetic biology. Artif. life 26 (2), 260–273. doi:10.1162/artl_a_00318
Hanson, A. D., and Lorenzo, V. D. (2023). Synthetic Biology─High time to deliver? ACS Synth. Biol. 12 (6), 1579–1582. doi:10.1021/acssynbio.3c00238
Harrer, S. (2023). Attention is not all you need: the complicated case of ethically using large language models in healthcare and medicine. EBioMedicine 90, 104512. doi:10.1016/j.ebiom.2023.104512
Hassoun, S., Jefferson, F., Shi, X., Stucky, B., Wang, J., and Rosa, E. (2022). Artificial intelligence for biology. Integr. Comp. Biol. 61 (6), 2267–2275. doi:10.1093/icb/icab188
Hatcher, B., Meechan, P. J., and Potts, J. (2023). Biosafety in microbiological and biomedical laboratories (BMBL). 6th Edition. Available at: https://www.cdc.gov/labs/BMBL.html (Accessed February 1, 2024).
Helmy, M., Smith, D., and Selvarajoo, K. (2020). Systems biology approaches integrated with artificial intelligence for optimized metabolic engineering. Metab. Eng. Commun. 11, e00149. doi:10.1016/j.mec.2020.e00149
Henkel, J., and Maurer, S. M. (2007). The economics of synthetic biology. Mol. Syst. Biol. 3, 117. doi:10.1038/msb4100161
Hewett, J. P., Wolfe, A. K., Bergmann, R. A., Stelling, S. C., and Davis, K. L. (2016). Human health and environmental risks posed by synthetic biology R&D for energy applications: a literature analysis. Appl. Biosaf. J. Am. Biol. Saf. Assoc. 21 (4), 177–184. doi:10.1177/1535676016672377
Hillson, N., Caddick, M., Cai, Y., Carrasco, J. A., Chang, M. W., Curach, N. C., et al. (2019). Building a global alliance of biofoundries. Nat. Commun. 10 (1), 2040. doi:10.1038/s41467-019-10079-2
Hınçer, A., Ahan, R. E., Aras, E., and Şeker, U. Ö. Ş. (2023). Making the next generation of therapeutics: mRNA meets synthetic biology. ACS Synth. Biol. 12 (9), 2505–2515. doi:10.1021/acssynbio.3c00253
Hodgson, A., Maxon, M. E., and Alper, J. (2022). The U.S. bioeconomy: charting a course for a resilient and competitive future. Ind. Biotechnol. 18, 115–136. doi:10.1089/ind.2022.29283.aho
Hoffmann, A. (2023). Designer genes courtesy of artificial intelligence. Genes. and Dev. 37 (9-10), 351–353. doi:10.1101/gad.350783.123
Hoffmann, S. A., Diggans, J., Densmore, D., Dai, J., Knight, T., Leproust, E., et al. (2023). Safety by design: biosafety and biosecurity in the age of synthetic genomics. iScience 26 (3), 106165. doi:10.1016/j.isci.2023.106165
Holland, C., McCarthy, A., Ferri, P., and Shapira, P. (2024). Innovation intermediaries at the convergence of digital technologies, sustainability, and governance: a case study of AI-enabled engineering biology. Technovation 129, 102875. doi:10.1016/j.technovation.2023.102875
Holzinger, A., Keiblinger, K., Holub, P., Zatloukal, K., and Müller, H. (2023). AI for life: trends in artificial intelligence for biotechnology. New Biotechnol. 74, 16–24. doi:10.1016/j.nbt.2023.02.001
Hstoday (2022). WHO seeks experts for technical advisory Group on biosafety - HS today, homeland security today. Available at: https://www.hstoday.us/subject-matter-areas/pandemic-biohazard/who-seeks-experts-for-technical-advisory-group-on-biosafety/(Accessed: February 1, 2024).
Huzair, F. (2021). Risk and regulatory culture: governing recombinant DNA technology in the UK from 1970–1980. Technol. Analysis Strategic Manag. 33 (3), 260–270. doi:10.1080/09537325.2020.1843616
Ienca, M., and Vayena, E. (2018). Dual use in the 21st century: emerging risks and global governance. Swiss Med. Wkly. 148, w14688. doi:10.4414/smw.2018.14688
ILAC (2023). Facts and figures international laboratory accreditation cooperation. Available at: https://ilac.org/about-ilac/facts-and-figures/(Accessed: November 29, 2023).
ISO (2020). Improving biosecurity with first International Standard for biorisk management, ISO. Available at: https://www.iso.org/news/ref2472.html (Accessed: February 1, 2024).
Jan, Z., Ahamed, F., Mayer, W., Patel, N., Grossmann, G., Stumptner, M., et al. (2023). Artificial intelligence for industry 4.0: systematic review of applications, challenges, and opportunities. Expert Syst. Appl. 216, 119456. doi:10.1016/j.eswa.2022.119456
Jatain, I., Dubey, K. K., Sharma, M., Usmani, Z., Sharma, M., and Gupta, V. K. (2021). Synthetic biology potential for carbon sequestration into biocommodities. J. Clean. Prod. 323, 129176. doi:10.1016/j.jclepro.2021.129176
Johnson, B., and Casagrande, R. (2016). Comparison of international guidance for biosafety regarding work conducted at biosafety level 3 (BSL-3) and gain-of-function (GOF) experiments. Appl. Biosaf. J. Am. Biol. Saf. Assoc. 21 (3), 128–141. doi:10.1177/1535676016661772
Kaiser, J. (2023). ‘Growing number of high-security pathogen labs around world raises concerns’, Science. Available at: https://www.science.org/content/article/growing-number-high-security-pathogen-labs-around-world-raises-concerns (Accessed March 17, 2023).
Kather, J. N., Ghaffari Laleh, N., Foersch, S., and Truhn, D. (2022). Medical domain knowledge in domain-agnostic generative AI. NPJ Digit. Med. 5 (1), 90. doi:10.1038/s41746-022-00634-5
Kirksey, E. (2021). Living machines go wild: policing the imaginative horizons of synthetic biology. Curr. Anthropol. 62 (S24), S287–S297. doi:10.1086/715011
Klavans, R., Boyack, K. W., and Murdick, D. A. (2020). A novel approach to predicting exceptional growth in research. PloS one 15 (9), e0239177. doi:10.1371/journal.pone.0239177
KMPG (2020). Site selection for life sciences companies in Asia. India, China, Hong Kong SAR, Singapore and the emerging markets of southeast Asia. KPMG. Available at: https://assets.kpmg.com/content/dam/kpmg/ch/pdf/site-selection-for-life-sciences-companies-in-asia.pdf.
Kulken, T. (2023). Artificial Intelligence in the biological sciences: uses, safety, security, and oversight. R47849. Congressional research service. Available at: https://crsreports.congress.gov/product/pdf/R/R47849.
Lantada, A. D., Korvink, J. G., and Islam, M. (2022). Taxonomy for engineered living materials. Cell Rep. Phys. Sci. 3 (4), 100807. doi:10.1016/j.xcrp.2022.100807
Lawrence, D. R. (2019). Advanced bioscience and AI: debugging the future of life. Emerg. Top. life Sci. 3 (6), 747–751. doi:10.1042/etls20180069
Lawson, C. E., Martí, J. M., Radivojevic, T., Jonnalagadda, S. V. R., Gentz, R., Hillson, N. J., et al. (2021). Machine learning for metabolic engineering: a review. Metab. Eng. 63, 34–60. doi:10.1016/j.ymben.2020.10.005
Lee, S. J., and Kim, D.-M. (2023). Cell-free synthetic biology: navigating the new frontiers of biomanufacturing and biological engineering. Curr. Opin. Syst. Biol. 37, 100488. doi:10.1016/j.coisb.2023.100488
Lee, T., and George, J. (2023). Future worldbuilding with synthetic biology: a case study in interdisciplinary scenario visualization. Futures 147, 103118. doi:10.1016/j.futures.2023.103118
Lentzos, F., Koblentz, G. D., and Rodgers, J. (2022). The urgent need for an overhaul of global biorisk management. CTS-Sentinel 15 (4). Available at: https://ctc.westpoint.edu/the-urgent-need-for-an-overhaul-of-global-biorisk-management/(Accessed: February 28, 2023).
Lev, O. (2019). Regulating dual-use research: lessons from Israel and the United States. J. Biosaf. Biosecurity 1 (2), 80–85. doi:10.1016/j.jobb.2019.06.001
Li, J., Wang, Z., Tao, G., Fu, Y., Jin, Y., Cheng, H., et al. (2022). Smart heightened-containment biological laboratory: technologies, modules, and aims. J. Biosaf. Biosecurity 4 (2), 89–97. doi:10.1016/j.jobb.2022.06.003
Li, J., Zhao, H., Zheng, L., and An, W. (2021). Advances in synthetic biology and biosafety governance. Front. Bioeng. Biotechnol. 9, 598087. doi:10.3389/fbioe.2021.598087
Lin, B., Bousquette, I., and Loten, A. (2023). Synthetic biology moves from the lab to the marketplace, WSJ online. Available at: https://www.wsj.com/articles/synthetic-biology-moves-from-the-lab-to-the-marketplace-3f409a87 (Accessed November 28, 2023).
Linder, N., and Undheim, T. A. (2022). Augmented lean: a human-centric framework for managing frontline operations. John Wiley and Sons.
Linkov, I., Trump, B. D., Anklam, E., Berube, D., Boisseasu, P., Cummings, C., et al. (2018a). Comparative, collaborative, and integrative risk governance for emerging technologies. Environ. Syst. Decis. 38 (2), 170–176. doi:10.1007/s10669-018-9686-5
Linkov, I., Trump, B. D., Poinsatte-Jones, K., and Florin, M. V. (2018b). Governance strategies for a sustainable digital world. Sustain. Sci. Pract. Policy 10 (2), 440. doi:10.3390/su10020440
Mampuys, R., and Brom, F. (2018). Emerging crossover technologies: how to organize a biotechnology that becomes mainstream? Environ. Syst. Decis. 38 (2), 163–169. doi:10.1007/s10669-017-9666-1
Manzano, T., and Whitford, W. (2023). AI-enabled digital twins in biopharmaceutical manufacturing. Available at: https://bioprocessintl.com/2023/july-august-2023/ai-enabled-digital-twins-in-biopharmaceutical-manufacturing/(Accessed November 30, 2023).
Marris, C., and Calvert, J. (2020). Science and technology studies in policy: the UK synthetic biology roadmap. Sci. Technol. Hum. values 45 (1), 34–61. doi:10.1177/0162243919828107
Marvik, O. J., and Philp, J. (2020). The systemic challenge of the bioeconomy: a policy framework for transitioning towards a sustainable carbon cycle economy. EMBO Rep. 21 (10), e51478. doi:10.15252/embr.202051478
Mateu-Sanz, M., Fuenteslópez, C. V., Uribe-Gomez, J., Haugen, H. J., Pandit, A., Ginebra, M. P., et al. (2023). ‘Redefining biomaterial biocompatibility: challenges for artificial intelligence and text mining’. Trends Biotechnol. S0167-7799. doi:10.1016/j.tibtech.2023.09.015
Matsuyama, H., and Suzuki, H. I. (2019). Systems and synthetic microRNA biology: from biogenesis to disease pathogenesis. Int. J. Mol. Sci. 21 (1), 132. doi:10.3390/ijms21010132
Merchant, A., Batzner, S., Schoenholz, S. S., Aykol, M., Cheon, G., and Cubuk, E. D. (2023). Scaling deep learning for materials discovery. Nature 624, 80–85. doi:10.1038/s41586-023-06735-9
Millett, P., and Alexanian, T. (2021). Implementing adaptive risk management for synthetic biology: lessons from iGEM’s safety and security programme. Eng. Biol. 5 (3), 64–71. doi:10.1049/enb2.12012
Millett, P., Isaac, C. R., Rais, I., and Rutten, P. (2020). The synthetic-biology challenges for biosecurity: examples from iGEM. Nonproliferation Rev. 27 (4-6), 443–458. doi:10.1080/10736700.2020.1866884
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 (1), 1. doi:10.1186/2046-4053-4-1
Mökander, J., Sheth, M., Gersbro-Sundler, M., Blomgren, P., and Floridi, L. (2022). Challenges and best practices in corporate AI governance: lessons from the biopharmaceutical industry. Front. Comput. Sci. 4. doi:10.3389/fcomp.2022.1068361
Moore, S. J., MacDonald, J. T., Wienecke, S., Ishwarbhai, A., Tsipa, A., Aw, R., et al. (2018). Rapid acquisition and model-based analysis of cell-free transcription-translation reactions from nonmodel bacteria. Proc. Natl. Acad. Sci. U. S. A. 115 (19), E4340–E4349. doi:10.1073/pnas.1715806115
Moran, L. (2023). Generative AI provides significant boost to knowledge workers, study finds. Available at: https://www.legaldive.com/news/harvard-business-school-study-generative-ai-boston-consulting-group/693973//(Accessed November 3, 2023).
Morris, M. R. (2023). Scientists’ perspectives on the potential for generative AI in their fields. arXiv [cs.CY]. Available at: http://arxiv.org/abs/2304.01420.
Mourby, M., Bell, J., Morrison, M., Faulkner, A., Li, P., Bicudo, E., et al. (2022). Biomodifying the “natural”: from adaptive regulation to adaptive societal governance. J. law Biosci. 9 (1), lsac018. doi:10.1093/jlb/lsac018
Mourya, D. T., Yadav, P. D., Khare, A., and Khan, A. H. (2017). Certification and validation of biosafety level-2 and biosafety level-3 laboratories in Indian settings and common issues. Indian J. Med. Res. 146 (4), 459–467. doi:10.4103/ijmr.IJMR_974_16
Mourya, D. T., Yadav, P. D., Majumdar, T. D., Chauhan, D. S., and Katoch, V. M. (2014). Establishment of Biosafety Level-3 (BSL-3) laboratory: important criteria to consider while designing, constructing, commissioning and operating the facility in Indian setting. Indian J. Med. Res. 140 (2), 171–183.
Müller, J., Siemann-Herzberg, M., and Takors, R. (2020). Modeling cell-free protein synthesis systems-approaches and applications. Front. Bioeng. Biotechnol. 8, 584178. doi:10.3389/fbioe.2020.584178
Murashov, V., Howard, J., and Schulte, P. (2020). “Synthetic biology industry: biosafety risks to workers,” in Synthetic biology 2020: Frontiers in risk analysis and governance. Editors B. D. Trump, C. L. Cummings, J. Kuzma, and I. Linkov (Cham: Springer International Publishing), 165–182.
Naderi Yeganeh, P., Teo, Y. Y., Karagkouni, D., Pita-Juárez, Y., Morgan, S. L., Slack, F. J., et al. (2023). PanomiR: a systems biology framework for analysis of multi-pathway targeting by miRNAs. Briefings Bioinforma. 24 (6), bbad418. doi:10.1093/bib/bbad418
Nelson, C., Adiguzel, I., Lentzos, F., Florin, M. V., Knutsson, R., Rhodes, C., et al. (2021). “Foresight in synthetic biology and biotechnology threats,” in Emerging threats of synthetic biology and biotechnology: addressing security and resilience issues. Editors B. D. Trump, M. V. Florin, E. Perkins, and I. Linkov (Dordrecht, DE: Springer).
Nesbeth, D. N., Zaikin, A., Saka, Y., Romano, M., Giuraniuc, C., Kanakov, O., et al. (2016). Synthetic biology routes to bio-artificial intelligence. Essays Biochem. 60 (4), 381–391. doi:10.1042/ebc20160014
Nylund, P. A., Ferràs-Hernández, X., Pareras, L., and Brem, A. (2022). The emergence of entrepreneurial ecosystems based on enabling technologies: evidence from synthetic biology. J. Bus. Res. 149, 728–735. doi:10.1016/j.jbusres.2022.05.071
O’Brien, J. T., and Nelson, C. (2020). Assessing the risks posed by the convergence of artificial intelligence and biotechnology. Health Secur. 18 (3), 219–227. doi:10.1089/hs.2019.0122
Ord, T. (2020). The precipice: existential risk and the future of humanity. New York: Hachette Books.
Owczarek, D. (2021). ‘Bioprocessing 4.0 and the benefits of introducing AI to biopharmaceutical manufacturing’, nexocode. Available at: https://nexocode.com/blog/posts/bioprocessing-4-ai-in-biopharmaceutical-manufacturing/(Accessed: November 30, 2023).
Palmer, M. J., Fukuyama, F., and Relman, D. A. (2015). A more systematic approach to biological risk. Science 350 (6267), 1471–1473. doi:10.1126/science.aad8849
Patel, V. L., Shortliffe, E. H., Stefanelli, M., Szolovits, P., Berthold, M. R., Bellazzi, R., et al. (2009). The coming of age of artificial intelligence in medicine. Artif. Intell. Med. 46 (1), 5–17. doi:10.1016/j.artmed.2008.07.017
Pei, L., Garfinkel, M., and Schmidt, M. (2022). Bottlenecks and opportunities for synthetic biology biosafety standards. Nat. Commun. 13 (1), 2175. doi:10.1038/s41467-022-29889-y
Perkins, D., Danskin, K., Rowe, A. E., and Livinski, A. A. (2019). The culture of biosafety, biosecurity, and responsible conduct in the life sciences: a comprehensive literature review. Appl. Biosaf. J. Am. Biol. Saf. Assoc. 24 (1), 34–45. doi:10.1177/1535676018778538
Perrakis, A., and Sixma, T. K. (2021). AI revolutions in biology: the joys and perils of AlphaFold. EMBO Rep. 22 (11), e54046. doi:10.15252/embr.202154046
Plante, M. (2023). Epistemology of synthetic biology: a new theoretical framework based on its potential objects and objectives. Front. Bioeng. Biotechnol. 11, 1266298. doi:10.3389/fbioe.2023.1266298
Qasmi, S. A., Ikram, A., Tariq, M., Khadim, M. T., and Ahmed Maqbool, N. (2023). Mobile biosafety level (BSL) 2 laboratories deployment: strengthening the diagnostic facilities in Pakistan with emerging public health challenges and the way forward. J. Biosaf. Biosecurity 5 (2), 79–83. doi:10.1016/j.jobb.2023.05.002
Ray, P. P. (2023). ChatGPT: a comprehensive review on background, applications, key challenges, bias, ethics, limitations and future scope. Internet Things Cyber-Physical Syst. 3, 121–154. doi:10.1016/j.iotcps.2023.04.003
Rennings, M., Burgsmüller, A. P. F., and Bröring, S. (2023). Convergence towards a digitalized bioeconomy—exploring cross-industry merger and acquisition activities between the bioeconomy and the digital economy. Bus. strategy and Dev. 6 (1), 53–74. doi:10.1002/bsd2.223
Rosenbush, S. (2023). AI accelerates ability to program biology like software. Available at: https://www.wsj.com/articles/ai-accelerates-ability-to-program-biology-like-software-9962a975 (Accessed November 28, 2023).
Sandberg, A., and Nelson, C. (2020). Who should we fear more: biohackers, disgruntled postdocs, or bad governments? A simple risk chain model of biorisk. Health Secur. 18 (3), 155–163. doi:10.1089/hs.2019.0115
Sandbrink, J. B. (2023a). Artificial intelligence and biological misuse: differentiating risks of language models and biological design tools. arXiv [cs.CY]. Available at: http://arxiv.org/abs/2306.13952.
Sandbrink, J. B. (2023b). Panoptic dual-use management: preventing deliberate pandemics in an age of synthetic biology and artificial intelligence. Oxford: University of Oxford. doi:10.5287/ORA-BPQK58EAD
Sandbrink, J. B., Musunuri, S., and Monrad, J. T. (2023). Widening the framework for regulation of dual-use research in the wake of the COVID-19 pandemic. Available at: https://absa.org/wp-content/uploads/2021/07/Sandbrink-Widening_DURC_Frameworks.pdf (Accessed November 28, 2023).
Sargent, D., Conaty, W. C., Tissue, D. T., and Sharwood, R. E. (2022). Synthetic biology and opportunities within agricultural crops. J. Sustain. Agric. Environ. 1 (2), 89–107. doi:10.1002/sae2.12014
Sauer, P. C., and Seuring, S. (2023). How to conduct systematic literature reviews in management research: a guide in 6 steps and 14 decisions. Rev. Manag. Sci. 17 (5), 1899–1933. doi:10.1007/s11846-023-00668-3
Scheper, T., Beutel, S., McGuinness, N., Heiden, S., Oldiges, M., Lammers, F., et al. (2021). “Digitalization and bioprocessing: promises and challenges,” in Digital twins: tools and concepts for smart biomanufacturing. Editors C. Herwig, R. Pörtner, and J. Möller (Cham: Springer International Publishing), 57–69.
Schmidt, M., and de Lorenzo, V. (2016). Synthetic bugs on the loose: containment options for deeply engineered (micro)organisms. Curr. Opin. Biotechnol. 38, 90–96. doi:10.1016/j.copbio.2016.01.006
Seydel, C. (2023). DNA writing technologies moving toward synthetic genomes. Nat. Biotechnol. 41 (11), 1504–1509. doi:10.1038/s41587-023-02006-0
Shapira, P., Kwon, S., and Youtie, J. (2017). Tracking the emergence of synthetic biology. Scientometrics 112 (3), 1439–1469. doi:10.1007/s11192-017-2452-5
Sheahan, T., and Wieden, H.-J. (2021). Emerging regulatory challenges of next-generation synthetic biology. Biochem. Cell Biol. = Biochimie Biol. Cell. 99 (6), 766–771. doi:10.1139/bcb-2021-0340
Silver, A. (2022). Why the world has no universal biosafety standards. BMJ 377, o954. doi:10.1136/bmj.o954
Singh, V., Bhattacharjee, G., Gohil, N., Maurya, R., Lam, N. L., and Alzahrani, K. J. (2022). “Chapter 1 - an introduction to advanced technologies in synthetic biology,” in New Frontiers and applications of synthetic biology. Editor V. Singh (Academic Press), 1–9.
Sun, T., Song, J., Wang, M., Zhao, C., and Zhang, W. (2022). Challenges and recent progress in the governance of biosecurity risks in the era of synthetic biology. J. Biosaf. Biosecurity 4 (1), 59–67. doi:10.1016/j.jobb.2022.02.002
Sundaram, L. S., Ajioka, J. W., and Molloy, J. C. (2023). Synthetic biology regulation in Europe: containment, release and beyond. Synth. Biol. 8 (1), ysad009. doi:10.1093/synbio/ysad009
SynBioBeta (2023). 2023 SynBioBeta investment report. Available at: https://www.synbiobeta.com/attend/synbiobeta-2023/2023-synbiobeta-investment-report (Accessed November 30, 2023).
Szocik, K., Shelhamer, M., Braddock, M., Cucinotta, F. A., Impey, C., Worden, P., et al. (2021). Future space missions and human enhancement: medical and ethical challenges. Futures 133, 102819. doi:10.1016/j.futures.2021.102819
Tait, J., and Wield, D. (2021). Policy support for disruptive innovation in the life sciences. Technol. Analysis Strategic Manag. 33 (3), 307–319. doi:10.1080/09537325.2019.1631449
Tang, T.-C., An, B., Huang, Y., Vasikaran, S., Wang, Y., Jiang, X., et al. (2020). Materials design by synthetic biology. Nat. Rev. Mater. 6 (4), 332–350. doi:10.1038/s41578-020-00265-w
Tarasava, K. (2023). How AI is transforming synthetic biology: reaching far beyond biopharma. Available at: https://www.synbiobeta.com/read/how-ai-is-transforming-synthetic-biology-reaching-far-beyond-biopharma (Accessed November 28, 2023).
Taylor, K., Woods, S., Johns, A., and Murray, H. (2023). Intrinsic responsible innovation in a synthetic biology research project. New Genet. Soc. 42 (1), e2232684. doi:10.1080/14636778.2023.2232684
Topol, E. J. (2019). High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25 (1), 44–56. doi:10.1038/s41591-018-0300-7
Torres, P. (2018). Who would destroy the world? Omnicidal agents and related phenomena. Aggress. violent Behav. 39, 129–138. doi:10.1016/j.avb.2018.02.002
Trump, B. D., Cegan, J., Wells, E., Poinsatte-Jones, K., Rycroft, T., Warner, C., et al. (2019). Co-evolution of physical and social sciences in synthetic biology. Crit. Rev. Biotechnol. 39 (3), 351–365. doi:10.1080/07388551.2019.1566203
Trump, B. D., Galaitsi, S., Appleton, E., Bleijs, D. A., Florin, M., Gollihar, J. D., et al. (2020). Building biosecurity for synthetic biology. Mol. Syst. Biol. 16 (7), e9723. doi:10.15252/msb.20209723
Undru, T. R., Uday, U., Lakshmi, J. T., Kaliappan, A., Mallamgunta, S., Nikhat, S. S., et al. (2022). Integrating artificial intelligence for clinical and laboratory diagnosis - a review. Maedica 17 (2), 420–426. doi:10.26574/maedica.2022.17.2.420
Urbina, F., Lentzos, F., Invernizzi, C., and Ekins, S. (2022). Dual use of artificial intelligence-powered drug discovery. Nat. Mach. Intell. 4 (3), 189–191. doi:10.1038/s42256-022-00465-9
van Doren, D., Khanagha, S., Volberda, H. W., and Caniëls, M. C. J. (2022). The external commercialisation of technology in emerging domains – the antecedents, consequences, and dimensions of desorptive capacity. Technol. Analysis Strategic Manag. 34 (3), 258–273. doi:10.1080/09537325.2021.1895103
Vaseashta, A. (2023). “Existential risks associated with dual-use technologies,” in Proceedings of the stanford existential risks conference 2023. Editors T. Undheim, and D. Zimmer (Stanford University), 156–170.
Vidiella, B., and Solé, R. (2022). Ecological firewalls for synthetic biology. iScience 25 (7), 104658. doi:10.1016/j.isci.2022.104658
Vinke, S., Rais, I., and Millett, P. (2022). The dual-use education gap: awareness and education of life science researchers on nonpathogen-related dual-use research. Health Secur. 20 (1), 35–42. doi:10.1089/hs.2021.0177
Voigt, C. A. (2020). Synthetic biology 2020-2030: six commercially-available products that are changing our world. Nat. Commun. 11 (1), 6379. doi:10.1038/s41467-020-20122-2
Wang, F., and Zhang, W. (2019). Synthetic biology: recent progress, biosafety and biosecurity concerns, and possible solutions. J. Biosaf. Biosecurity 1 (1), 22–30. doi:10.1016/j.jobb.2018.12.003
Wang, L., Zang, X., and Zhou, J. (2022). Synthetic biology: a powerful booster for future agriculture. Adv. Agrochem. 1 (1), 7–11. doi:10.1016/j.aac.2022.08.005
Wani, A. K., Roy, P., Kumar, V., and Mir, T. u. G. (2022). Metagenomics and artificial intelligence in the context of human health. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 100, 105267. doi:10.1016/j.meegid.2022.105267
Watson, E. (2023). Synthetic biology in food and ag: we’re just getting started, says SynBioBeta founder, ‘Biology isn’t easy to engineer’. Available at: https://agfundernews.com/synthetic-biology-in-food-ag-were-just-getting-started-says-synbiobeta-founder-biology-isnt-easy-to-engineer (Accessed: November 30, 2023).
Webster-Wood, V. A., Guix, M., Xu, N. W., Behkam, B., Sato, H., Sarkar, D., et al. (2022). Biohybrid robots: recent progress, challenges, and perspectives. Bioinspiration biomimetics 18 (1), 015001. doi:10.1088/1748-3190/ac9c3b
Woolfson, D. N. (2023). Understanding a protein fold: the physics, chemistry, and biology of α-helical coiled coils. J. Biol. Chem. 299 (4), 104579. doi:10.1016/j.jbc.2023.104579
Wu, G. (2019). Laboratory biosafety in China: past, present, and future. Biosaf. health 1 (2), 56–58. doi:10.1016/j.bsheal.2019.10.003
Wu, T., Duan, Y., Zhang, T., Tian, W., Liu, H., and Deng, Y. (2022). Research trends in the application of artificial intelligence in oncology: a bibliometric and network visualization study. Front. Biosci. 27 (9), 254. doi:10.31083/j.fbl2709254
Xiao, Z., Li, W., Moon, H., Roell, G. W., Chen, Y., and Tang, Y. J. (2023). Generative artificial intelligence GPT-4 accelerates knowledge mining and machine learning for synthetic biology. ACS Synth. Biol. 12 (10), 2973–2982. doi:10.1021/acssynbio.3c00310
Xu, D., Liu, B., Wang, J., and Zhang, Z. (2022). Bibliometric analysis of artificial intelligence for biotechnology and applied microbiology: exploring research hotspots and frontiers. Front. Bioeng. Biotechnol. 10, 998298. doi:10.3389/fbioe.2022.998298
Yamagata, M. (2023). SynBio: a journal for advancing solutions to global challenges. SynBio 1 (3), 190–193. doi:10.3390/synbio1030013
Yan, X., Liu, X., Zhao, C., and Chen, G. Q. (2023). Applications of synthetic biology in medical and pharmaceutical fields. Signal Transduct. Target. Ther. 8 (1), 199. doi:10.1038/s41392-023-01440-5
Keywords: AI risk, biorisk, biosafety, biosecurity, dual risk, generative AI, synthetic biology
Citation: Undheim TA (2024) The whack-a-mole governance challenge for AI-enabled synthetic biology: literature review and emerging frameworks. Front. Bioeng. Biotechnol. 12:1359768. doi: 10.3389/fbioe.2024.1359768
Received: 21 December 2023; Accepted: 05 February 2024;
Published: 28 February 2024.
Edited by:
Subhradip Karmakar, All India Institute of Medical Sciences, IndiaReviewed by:
Richard Kelwick, Imperial College London, United KingdomMario Andrea Marchisio, Tianjin University, China
Copyright © 2024 Undheim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trond Arne Undheim, dHJvbmR1bkBzdGFuZm9yZC5lZHU=
 Trond Arne Undheim
Trond Arne Undheim