Corrigendum: The possibility of clinical bonding between metal/ceramic brackets to zirconia: in vitro study
- 1School and Hospital of Stomatology, China Medical University, Shenyang, China
- 2Department of Dental Materials Science, The Second Department of Prosthodontics, School and Hospital of Stomatology, China Medical University, Shenyang, China
- 3Department of Restorative Dentistry, Division of Oral Health Science, Faculty of Dental Medicine, Hokkaido University, Sapporo, Japan
- 4Department of Oral Biological and Medical Sciences, Division of Biomaterials, Faculty of Dentistry, University of British Columbia, Vancouver, BC, Canada
Objective: The present study aimed to assess the bond strength and durability of six bonding agents concerning their application to metal or ceramic brackets and zirconia.
Materials and Methods: Six resin cement bonding agents (XT, XTS, RSBU, RGBU, SBPM, and GMP) were chosen for this investigation. Specimens were either stored in distilled water at 37°C for 24 h or subjected to 5,000 thermocycles before conducting a Shear Bond Strength (SBS) test. Statistical analysis of the SBS data was performed using three-way ANOVA and Games-Howell tests (α = 0.05). The Adhesive Remnant Index was examined, and the debonding surface details on brackets and zirconia were observed.
Results: For metal brackets, all groups demonstrated clinically acceptable bond strength, irrespective of storage conditions, except for the XT group. Regarding ceramic brackets, all groups displayed acceptable bond strength after 24 h of water storage. However, following thermocycling, a significant decrease in SBS was noted across all groups (p < 0.05), with SBPM exhibiting a higher bond strength. Three-way ANOVA analysis indicated that SBS values were notably influenced by each factor, and an interaction among the three independent variables was observed (p = 0.000).
Conclusion: The reliable bond strength between ceramic brackets and zirconia was significantly lower after thermocycling compared to that of metal brackets and zirconia. SBPM exhibited consistent and robust bond strength between ceramic/metal brackets and zirconia across various storage conditions. Furthermore, the HEMA-free adhesive demonstrated a potentially more consistent bonding performance compared to the HEMA-containing adhesive employed in this study.
1 Introduction
Zirconia (ZrO2) has gained widespread use in dentistry for fixed dental prostheses (FDPs), single crowns, bridge restorations, and implant abutments (Ju et al., 2020). Its exceptional mechanical strength sets it apart from other conventional ceramic materials (Zhang and Lawn, 2018). Additionally, Zirconia exhibits favorable biocompatibility, aesthetic properties, and high resistance to corrosion (Gautam et al., 2016; Zarone et al., 2019). The popularity of zirconia restorations has surged due to their convenient milling from prefabricated disks using CAD/CAM devices (Ju et al., 2019; Goracci et al., 2022).
Zirconia crystals exist in various patterns: monoclinic (M), cubic (C), and tetragonal (T) structures (Nistor et al., 2019). At ambient temperature, the monoclinic phase is most stable but transforms into tetragonal and cubic phases upon heating (Hanawa, 2020). Yttrium-stabilized zirconia (YSZ), also known as tetragonal zirconia polycrystal (TZP), achieves enhanced molecular stability by combining ZrO2 with Y2O3(Nistor et al., 2019; Hanawa, 2020). The typical yttria content in dental YSZ ranges between 3 and 5 mol% (Vult von Steyern et al., 2022). Increasing the yttria content enhances zirconia’s translucency for anterior teeth restoration, but it compromises strength and toughness (Vult von Steyern et al., 2022). In clinical practice, zirconia is predominantly used for porcelain-fused-to-zirconia crowns in anterior teeth, while full zirconia crowns are favored for posterior teeth (Makhija et al., 2016; Rauch et al., 2021). Despite zirconia’s microstructural characteristics, chemical inertness, and biocompatibility, establishing reliable bonding between zirconia and resin cement remains challenging (Lima et al., 2019). Silica-based porcelains are preferred due to superior translucency and their ability to be acid-etched and silanized, enhancing adhesion and reinforcing resin bonding (Zhang and Lawn, 2018).
The rising demand for dental aesthetics has led to an increased number of individuals seeking orthodontic treatment, including adults with a history of fixed prosthetic treatments (Babaee Hemmati et al., 2022). Orthodontic brackets are categorized as metal or ceramic brackets. Ceramic brackets were developed to meet the demand for improved aesthetics (Urichianu et al., 2022). Despite their aesthetic appeal, ceramic brackets exhibit lower bond strength to enamel, acrylic, and porcelain surfaces compared to traditional metal brackets (Pinho et al., 2020). Moreover, ceramic brackets are prone to fracture and may cause irreversible tooth damage during debonding (Alexopoulou et al., 2020). The demographic of adult patients seeking orthodontic treatment is on the rise (Jawad et al., 2015; Hellak et al., 2016).
As ceramic restorations, including zirconia restorations, are frequently performed in adults, the bonding strategy for different brackets and restoration surfaces becomes a pertinent concern (Pinho et al., 2020). Numerous chair-side challenges can arise when orthodontic brackets are bonded to zirconia ceramic surfaces. These challenges include insufficient familiarity with bonding techniques, lack of knowledge about resin cement or bonding products, and inconvenient treatment methods. For instance, clinicians may suggest replacing a ceramic crown with a CAD/CAM resin crown during orthodontic treatment to achieve better bonding performance, subsequently remaking the ceramic crown (Blakey and Mah, 2010). Enhancing the bonding performance between zirconia and metal or ceramic brackets to cater to orthodontic treatment needs could significantly benefit patients. However, scarce previous studies have explored the durability of various bonding agents between different brackets and zirconia.
Hence, this study aims to evaluate the bonding performance and durability of six different bonding agents concerning their application to metal or ceramic brackets and zirconia. The null hypotheses were as follows: (1) no significant difference exists in the bond strength of different bonding agents; (2) the storage condition does not affect the bond strength of the six bonding agents; and (3) metal/ceramic brackets achieve equivalent bonding durability on zirconia.
2 Materials and methods
2.1 Bonding agents and brackets
CAD/CAM-produced zirconia cube specimens (48 in total, Aidite Technology, Qinhuangdao, China) with a length of 2 cm were acquired for the study. These zirconia samples were randomly divided into six experimental groups based on the use of specific bonding agents:
• Group 1 (XT): Transbond™ XT Light Cure adhesive paste (XTL) + Transbond™ XT Light Cure Orthodontic Adhesive Primer (XTP)
• Group 2 (XTS): Transbond™ XT Light Cure adhesive paste (XTL) + Single Bond Universal (SBU)
• Group 3 (RSBU): Rely X™ Ultimate Clicker Adhesive Resin Cement (RUC) + Single Bond Universal (SBU)
• Group 4 (RGBU): Rely X™ Ultimate Clicker Adhesive Resin Cement (RUC) + Gluma Bond Universal (GBU)
• Group 5 (SBPM): Superbond C&B (SB) + Porcelain liner M (PLM)
• Group 6 (GMP): GC G-CEM ONE (GCO) + G-Multi Primer (MP)
Please refer to Table 1 for detailed information on the chemical composition and application procedures of the bonding agents and cleaning paste used in this study.
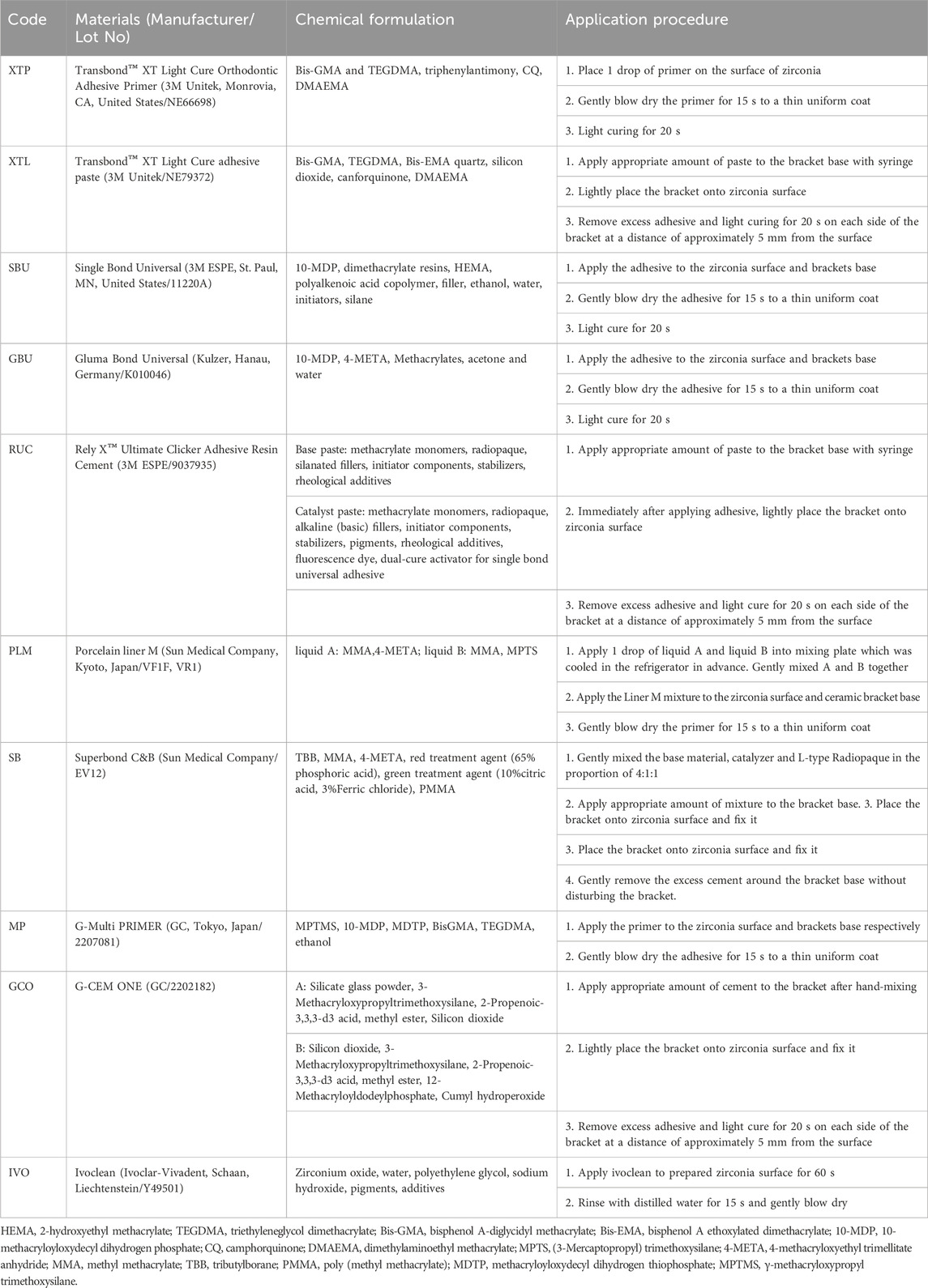
Table 1. The chemical composition and application procedure of the bonding agents and cleaning paste used in present study.
The zirconia cube specimens in each group were divided into two subgroups, each accommodating two types of brackets: metal brackets (Victory Series, 3M Unitek, United States) and ceramic brackets (Maia Series, Protect, Zhejiang, China). A total of 216 metal brackets and 216 ceramic brackets were utilized. The mean base surface areas of the metal and ceramic brackets were 11.94 mm2 and 14.82 mm2.
2.2 Surface preparation
To prepare the bonding surfaces on the zirconia cube, sandblasting with 125 μm aluminum oxide particles was conducted for 60 s at a distance of 1 cm and 2.8 bar pressure. Subsequently, all specimens underwent ultrasonic cleaning in distilled water for 5 min and gentle air drying for 15 s. The application of Ivoclean (IVO) followed the manufacturer’s instructions.
2.3 Application procedure
The same pressure (5 N) was applied to each bracket using a consistent clamp across all groups. The application procedures for light curing (Kerr Demi Plus, Orange, CA, United States) and cement removal were standardized and detailed in Table 1.
Each group comprised a total of 72 bracket specimens, divided into two subgroups (metal and ceramic brackets) with 36 specimens each.
2.4 Shear bond strength (SBS) test
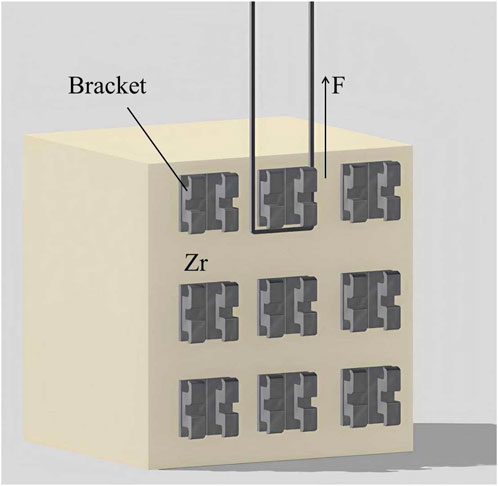
Half of the brackets in each subgroup were tested after storing in distilled water at 37°C for 24 h, while the rest underwent 5,000 cycles of thermocycling between 5°C and 55°C in a thermocycling device (SD Mechatronik, Feldkirchen-Westerham, Germany). The SBS test was conducted using a universal testing machine (WD-200 Weidu, Wenzhou, China) with a crosshead speed of 1 mm/min (Figure 1). The SBS formula used was P (MPa) = F (N)/S (mm2). Out of 18 results in each group, the highest 4 and lowest 4 data points were discarded, and the remaining 10 were considered for analysis (n = 10).
2.5 Adhesive remnant index (ARI) score
The ARI scoring was performed based on the amount of remaining cement on the zirconia surface with the help of a dental digital camera (EyeSpecial C-IV, shofu, Koyoto, Japan). The score is represented by a scale with 5 levels (Score A to Score E) as follows:
Score A: almost all the cement remained on the zirconia surface;
Score B: more than 90% of the cement remained on the zirconia surface;
Score C: more than 10% but less than 90% of the cement remained on the zirconia surface;
Score D: less than 10% of the cement remained on the zirconia surface;
Score E: no cement remained on the zirconia surface.
Images were scored by three calibrated examiners, and a majority opinion was adopted in cases of disagreement. Figure 2 demonstrates the ARI score on the samples.
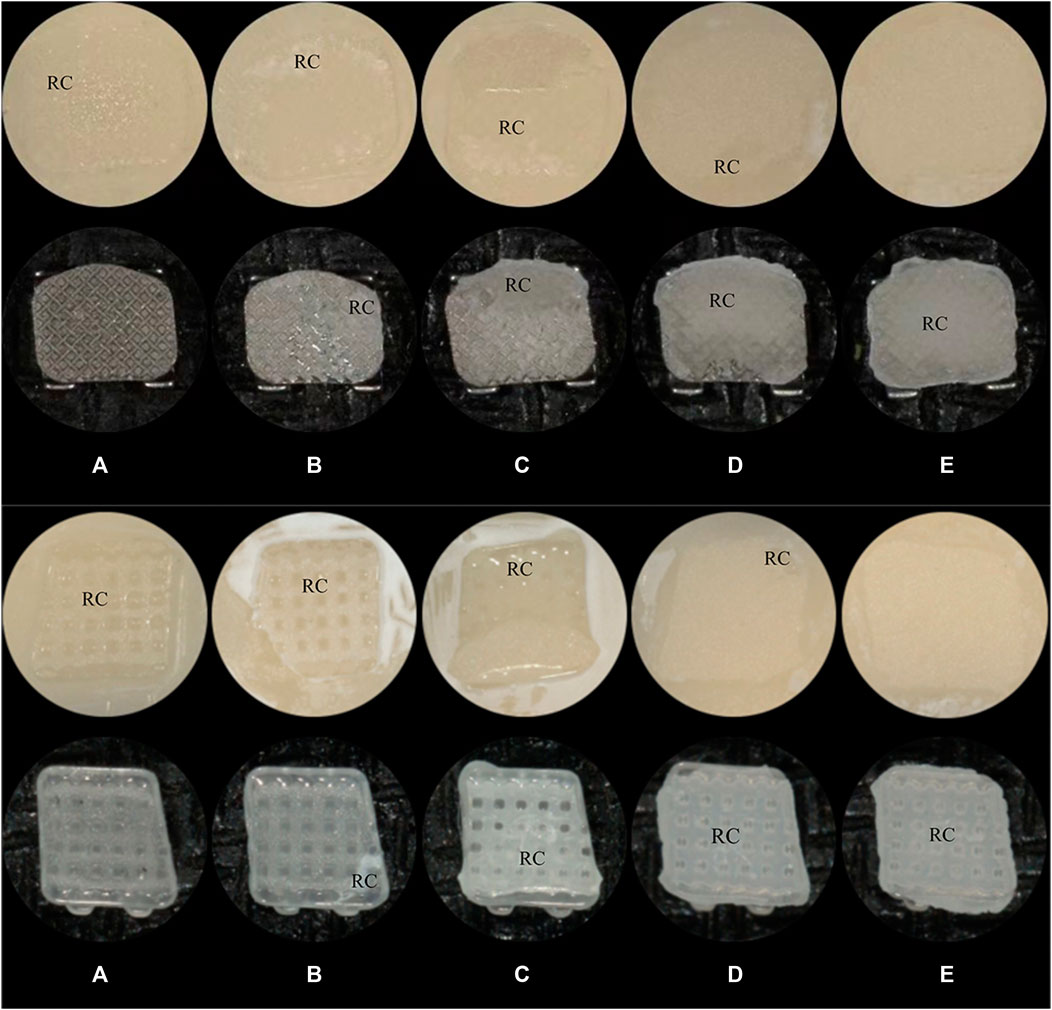
Figure 2. The ARI scores(A): score A; (B): score B; (C): score C; (D): score D; (E): score E; RC: Resin Cement. (A) Metal Brackets (B) Ceramic Brackets.
2.6 Statistical analysis
Statistical analysis involved three-way ANOVA (bonding agents, storage conditions, and brackets) and the Games-Howell test using SPSS version 26.0, with a significance level of α = 0.05.
2.7 Surface evaluation
The debonding bracket base surface was analyzed using field emission scanning electron microscopy (FE-SEM) and energy dispersive X-ray spectrometry (EDS; Phenom Pharos G2, Netherlands) to determine elemental composition and distribution.
3 Results
3.1 Shear bond strength (SBS)
The SBS mean values and standard deviations for each group are summarized in Table 2. Notably, RSBU, SBPM, and GMP exhibited higher SBS for metal bracket groups after 24 h of water storage, with no significant difference among these groups (p > 0.05). However, RSBU’s SBS decreased significantly after thermocycling, while SBPM and GMP remained unchanged.
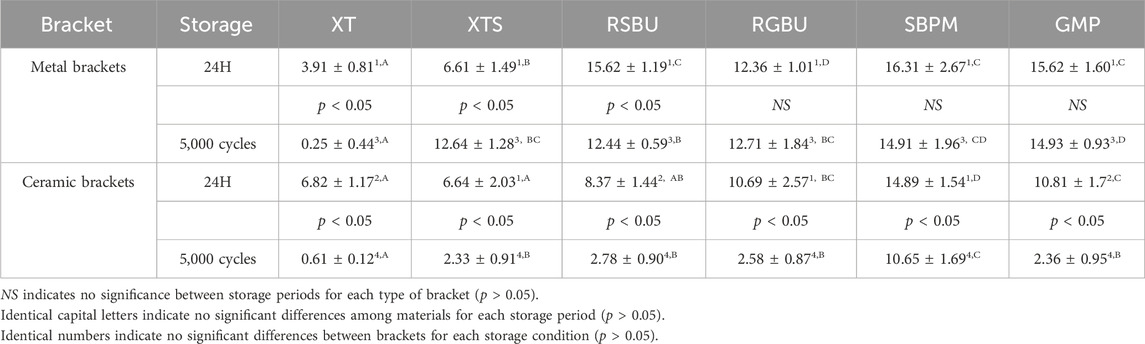
Table 2. SBS values (MPa) for six bonding agents using different brackets in two different storage conditions (mean ± SD).
For ceramic brackets, SBPM displayed higher SBS after 24 h of water storage but significantly declined post-thermocycling, akin to other bonding agents (p < 0.05). Comparatively, ceramic brackets demonstrated a more significant decrease in SBS after thermocycling in contrast to metal brackets. Generally, the SBS of ceramic brackets was lower than that of metal brackets after both 24 h of water storage (p > 0.05) and thermocycling (p < 0.05), except for XTS group results (p > 0.05) post-24 h of water storage and XT group outcomes under both storage conditions (p < 0.05).
SBPM showcased higher SBS values after thermocycling, irrespective of bracket type, while XT exhibited the lowest SBS in the metal bracket group. XTS displayed a significant increase in SBS for metal brackets post-thermocycling (p < 0.05).
The SPSS analysis (Table 3) revealed significant influences on SBS values by brackets, bonding agents, and storage conditions (p < 0.001). Interactions were observed among brackets, bonding agents, and storage conditions (p < 0.001).
Regarding the SBS results in Tables 4, 5, metal brackets displayed significantly higher SBS than ceramic brackets after thermocycling (p < 0.05). Also, RSBU, RGBU, SBPM, and GMP exhibited significantly higher SBS after 24 h of water storage compared to XT and XTS (p < 0.05). XT demonstrated notably lower SBS than other groups post-thermocycling (p < 0.05). SBPM consistently demonstrated higher SBS irrespective of storage conditions.
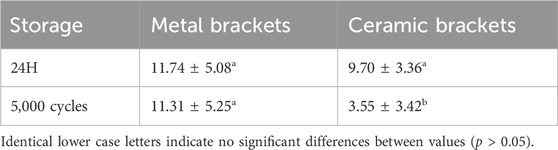
Table 4. The mean SBS values (MPa) of all six bonding agents in the different experimental groups (mean ± SD).
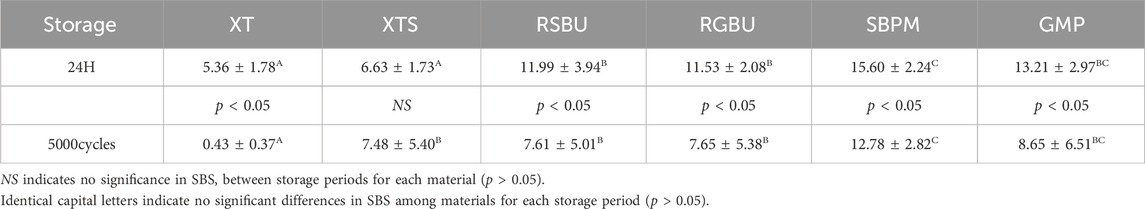
Table 5. The mean shear bond strength values (MPa) of all brackets in the different experimental groups (mean ± SD).
3.2 Failure modes
The distribution of failure modes for metal and ceramic bracket groups are visualized in Figures 3, 4, respectively. Under 24 h water storage, ARI scores for various groups were concentrated within different categories. After thermocycling, shifts in ARI scores were observed across groups.
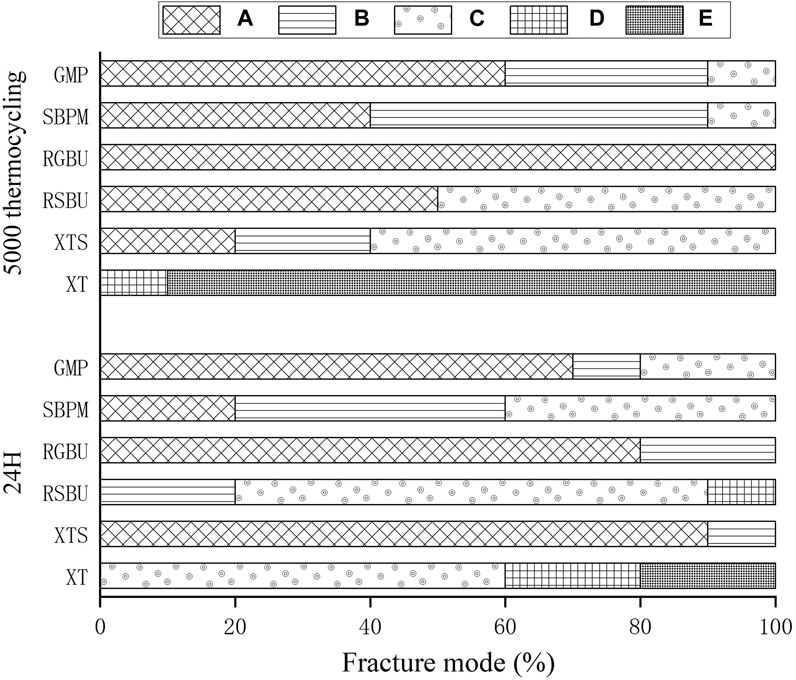
Figure 3. Percentages (%) of the different failure modes after SBS test of metal brackets bonded to zirconia. A to E correspond to Score A to Score E.
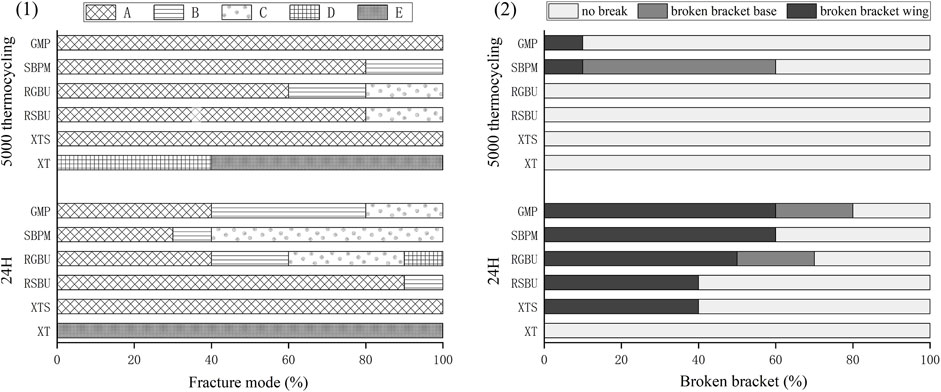
Figure 4. Results of ARI and breakage of ceramic brackets. (1) Percentages (%) of the different failure modes after shear bond strength test of ceramic brackets bonded to zirconia. (2) Percentages (%) of breakage of different ceramic brackets.
For ceramic brackets under both conditions, XT showed a tendency toward E in ARI scores, while GMP and SBPM exhibited a notable increase in A and a decrease to 0 in C after thermocycling.
Typical bracket fractures were observed in ceramic bracket groups after 24 h water storage, except for XT, predominantly in bracket wings with fewer base fractures in RGBU and GMP. After thermocycling, fractures were observed only in SBPM and GMP, with SBPM showing bracket base fractures. Figure 5 displays the two modes of bracket fractures.
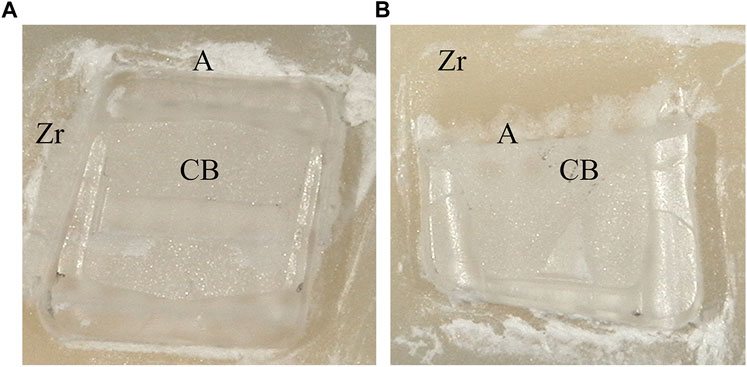
Figure 5. Typical bracket fractures including wings breakage (A) and base breakage (B): Zr (zirconia), A (Adhesive), CB (ceramic bracket base).
3.3 Surface characterization
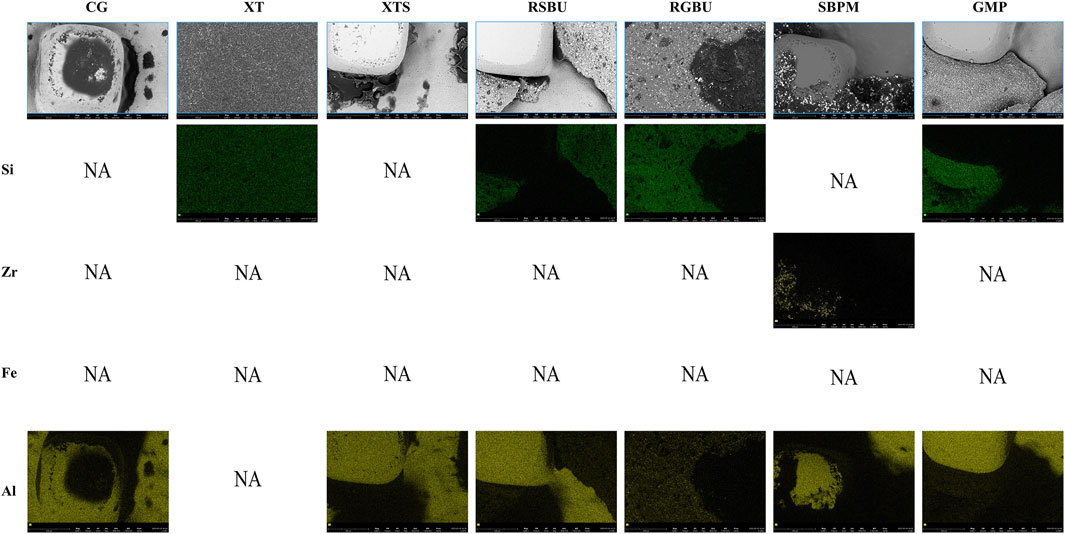
FE-SEM images (Figure 6) and EDS results (Figures 7, 8) indicated the presence of Si on bracket bases of XT, XTS, RSBU, RGBU, and GMP groups. Zr particles were detected in RSBU and RGBU metal bracket bases. SBPM showed Zr particles in both ceramic and metal bracket bases. Fe and Al were detected on metal bracket bases, whereas Fe was absent on ceramic bracket bases.
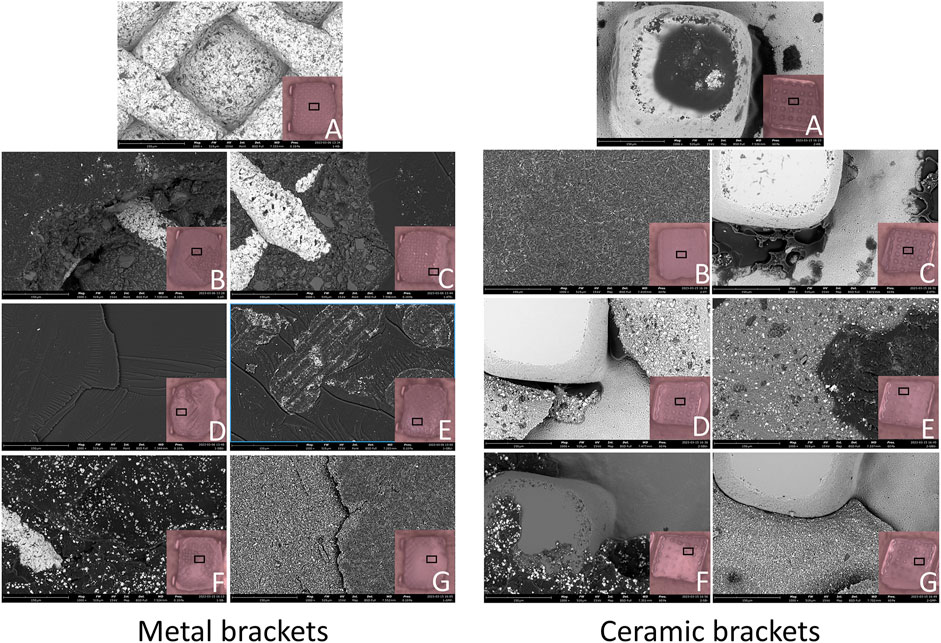
Figure 6. SEM photographs (1,000 × original magnification) of bracket bases: (A) Control Group; (B) XT Group; (C) XTS Group; (D) RSBU Group; (E) RGBU Group; (F) SBPM Group; (G) GMP Group.
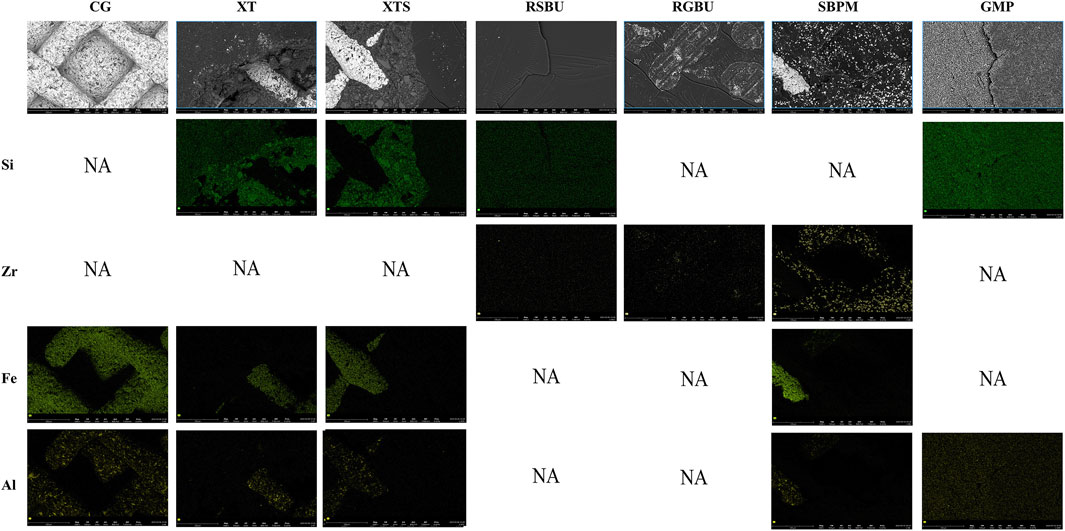
Figure 7. Distribution of elemental composition on the debonding base of metal brackets and ceramic brackets. CG indicates Control Group; NA indicates no applicable.
These observations provide insights into the elemental composition and characteristics of the bracket bases across different bonding agents and brackets used in the study.
4 Discussion
4.1 Comparison of bonding agents
The study aimed to evaluate the bond strength and durability of six bonding agents with metal or ceramic brackets on zirconia. Table 5 highlighted significant differences in shear bond strength (SBS) among bonding agents, rejecting the null hypothesis that no significant difference exists in the bond strength of different bonding agents. Typically, for optimal orthodontic efficacy, the bond strength of orthodontic brackets should ideally be within the range of 6–8 MPa at least (Uysal et al., 2004; Tecco et al., 2005). According to Table 5, except for the XT group, all metal bracket groups achieved long-term bond strengths deemed clinically acceptable. In contrast, among the ceramic bracket groups, while acceptable strength was achieved in the short term by all groups, only the SBPM group exhibited sustained durability.
Using XT as a control group for bonding brackets to enamel, it demonstrated higher SBS in ceramic brackets after 24 h of water storage compared to metal brackets. This outcome suggests the potential influence of ceramic translucence in enhancing XT resin polymerization, contributing to improved short-term bonding (Al-Hity et al., 2012; Reginato et al., 2013). However, XT’s SBS significantly decreased after thermocycling, potentially due to mismatched coefficients of shrinkage/expansion between XT resin and brackets, failing to meet the required clinical bond strength.
The XTS group, employing SBU, an universal adhesive, exhibited improved bonding performance attributed to 10-MDP’s presence, facilitating chemical bonding to zirconia via phosphate groups (Khan et al., 2017; Nagaoka et al., 2017). This bond was indicated by ARI scores concentrated on A, consistent with SBU’s ability to strengthen XTL resin-zirconia bonds.
4.2 Hydrophilic monomer HEMA’s effect
The absence of HEMA in GBU aimed to evaluate HEMA’s impact on bracket-zirconia bonding. RSBU (containing HEMA) for metal brackets demonstrated significantly higher SBS than HEMA-free RGBU after 24 h water storage. HEMA’s role in enhancing component miscibility and forming a uniform adhesive layer could explain RSBU’s superiority (Toledano et al., 2001; Moszner et al., 2005; Van Landuyt et al., 2005), despite its decreased SBS post-thermocycling. However, RGBU’s hydrophobic nature prevented water absorption, resulting in more stable bonding durability, aligning with earlier findings (Hu et al., 2022).
4.3 Impact of resin water absorption on durability
Studies have shown that HEMA-containing adhesives, due to continuous water absorption, lead to decreased bond strength post-polymerization (Ito et al., 2005; Takahashi et al., 2011). RSBU demonstrated significant SBS reduction after 5,000 cycles of thermocycling, attributed to water absorption. Conversely, RGBU’s SBS remained stable in metal brackets group, indicating its resistance to water-induced degradation. Therefore, Three-way ANOVA demonstrated a statistically significant interaction among bonding agents, storage conditions and brackets (p < 0.001), which was not detected in previous experiment (Hu et al., 2022).
4.4 Specific bonding agents’ performance
GMP, containing a self-adhesive resin cement, showed comparable SBS to SBPM in the metal bracket group after 5,000 thermocycles, suggesting GMP’s stability post-thermal stress.
SBPM exhibited significantly higher SBS for ceramic brackets, despite a notable 60% bracket breakage rate. This could be attributed to MMA resin’s water resistivity (Ikemura and Endo, 2010; Aoki et al., 2011) and the absence of silicon components in EDS analysis. Due to the absence of inorganic fillers, SBPM is typically polymerized in a linear form, resulting in better toughness compared to cross-linked polymers. On the other hand, SBPM can promote free radical polymerization of the resin using oxygen and water in the presence of TBB, a polymerization initiator, which can significantly improve the bond strength and long-term durability of ceramic brackets to zirconia porcelain (Tanaka et al., 2004; Meguro et al., 2006; Shinagawa et al., 2019). The presence of Zr in the EDS results also reveals the viewpoint. This finding is consistent with those of Shimoe et al., who also observed an increase in the [C] and [O] intensity peaks when the zirconia surface was treated with 4-META, indicating that 4-META chemically adheres to the zirconia surface effectively (Shimoe et al., 2018).
4.5 Influence of storage conditions
While most groups demonstrated decreased SBS after thermocycling, XTS displayed a significant SBS increase, possibly due to radical mobility enhancement in high-temperature conditions during resin solidification (Al Jabbari et al., 2014). This variance partially rejected the null hypothesis of storage conditions not affecting bonding agents’ strength.
4.6 Comparison of metal vs. ceramic brackets
Metal and ceramic brackets showcased comparable SBS after 24 h water storage, but ceramic brackets exhibited significantly lower SBS post-thermocycling, rejecting the null hypothesis of similar bonding durability between metal and ceramic brackets on zirconia.
4.7 Discussion limitations
Limitations include in vitro conditions not entirely mimicking oral temperatures, potentially impacting XTS’s clinical bond strength, and bracket fractures affecting measured SBS accuracy in ceramic brackets. Meanwhile, the study solely applied sandblasting treatment to zirconia surfaces, without comprehensive assessment of other factors capable of zirconia surface modification, such as laser treatment (Hou et al., 2020) and silica coating (Galvão Ribeiro et al., 2018), which have been demonstrated to have a positive impact on the bonding efficacy between zirconia and resin. The subsequent phase of research may encompass the effects of diverse surface modification treatments between orthodontic brackets and zirconia and investigate their clinical practicality.
5 Conclusion
1. Ceramic brackets displayed significantly lower bond strength on zirconia compared to metal brackets after thermocycling.
2. SBPM exhibited stable and sufficient bond strength between ceramic/metal brackets and zirconia under diverse storage conditions.
3. HEMA-free adhesive presented more stable bonding performance compared to HEMA-containing adhesive used in the study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YH: Formal Analysis, Investigation, Project administration, Visualization, Writing–original draft. JG: Investigation, Supervision, Writing–original draft. XH: Investigation, Visualization, Writing–original draft. YL: Investigation, Writing–original draft. ZC: Investigation, Writing–original draft. DZ: Supervision, Writing–review and editing. HS: Supervision, Writing–review and editing. RC: Funding acquisition, Supervision, Writing–review and editing. JF: Conceptualization, Methodology, Resources, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the support of Minnesota Mining and Manufacturing Company (3M, St.Paul, MN, United States) and Zhejiang Protect Medical Equipment Co., Ltd. (Protect, Zhejiang, China) for providing Unitek Gemini™ metal brackets (2022-ISR-000369) and Maia ceramic brackets (KYHZ20221212-1). The support of Research and Development Center, Aidite (Qinhuangdao) Technology Co., Ltd. during the surface evaluation stages of this research is also appreciated. Additionally, this research was organized and carried out by the Student Innovation and Entrepreneurship Training Program of China Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alexopoulou, E., Polychronis, G., Konstantonis, D., Sifakakis, I., Zinelis, S., and Eliades, T. (2020). A study of the mechanical properties of as-received and intraorally exposed single-crystal and polycrystalline orthodontic ceramic brackets. Eur. J. Orthod. 42, 72–77. doi:10.1093/ejo/cjz024
Al-Hity, R., Gustin, M.-P., Bridel, N., Morgon, L., and Grosgogeat, B. (2012). In vitro orthodontic bracket bonding to porcelain. Eur. J. Orthod. 34, 505–511. doi:10.1093/ejo/cjr043
Al Jabbari, Y. S., Al Taweel, S. M., Al Rifaiy, M., Alqahtani, M. Q., Koutsoukis, T., and Zinelis, S. (2014). Effects of surface treatment and artificial aging on the shear bond strength of orthodontic brackets bonded to four different provisional restorations. Angle Orthod. 84, 649–655. doi:10.2319/090313-649.1
Aoki, K., Kitasako, Y., Ichinose, S., Burrow, M. F., Ariyoshi, M., Nikaido, T., et al. (2011). Ten-year observation of dentin bonding durability of 4-META/MMA-TBB resin cement--a SEM and TEM study. Dent. Mater J. 30, 438–447. doi:10.4012/dmj.2011-003
Babaee Hemmati, Y., Neshandar Asli, H., Falahchai, M., and Safary, S. (2022). Effect of different surface treatments and orthodontic bracket type on shear bond strength of high-translucent zirconia: an in vitro study. Int. J. Dent. 2022, 1–8. doi:10.1155/2022/9884006
Blakey, R., and Mah, J. (2010). Effects of surface conditioning on the shear bond strength of orthodontic brackets bonded to temporary polycarbonate crowns. Am. J. Orthod. Dentofac. Orthop. 138, 72–78. doi:10.1016/j.ajodo.2008.08.030
Galvão Ribeiro, B. R., Galvão Rabelo Caldas, M. R., Almeida, A. A., Fonseca, R. G., and Adabo, G. L. (2018). Effect of surface treatments on repair with composite resin of a partially monoclinic phase transformed yttrium-stabilized tetragonal zirconia. J. Prosthet. Dent. 119, 286–291. doi:10.1016/j.prosdent.2017.02.014
Gautam, C., Joyner, J., Gautam, A., Rao, J., and Vajtai, R. (2016). Zirconia based dental ceramics: structure, mechanical properties, biocompatibility and applications. Dalton Trans. 45, 19194–19215. doi:10.1039/c6dt03484e
Goracci, C., Di Bello, G., Franchi, L., Louca, C., Juloski, J., Juloski, J., et al. (2022). Bracket bonding to all-ceramic materials with universal adhesives. Mater. (Basel) 15, 1245. doi:10.3390/ma15031245
Hanawa, T. (2020). Zirconia versus titanium in dentistry: a review. Dent. Mater J. 39, 24–36. doi:10.4012/dmj.2019-172
Hellak, A., Ebeling, J., Schauseil, M., Stein, S., Roggendorf, M., and Korbmacher-Steiner, H. (2016). Shear bond strength of three orthodontic bonding systems on enamel and restorative materials. Biomed. Res. Int. 2016, 1–10. doi:10.1155/2016/6307107
Hou, Y., Yi, J., Huang, Y., Gao, J., Chen, Y., and Wang, C. (2020). Effect of Er:YAG laser etching on the shear bond strength and microleakage of self-glazed zirconia ceramics. Photobiomodul Photomed. Laser Surg. 38, 289–294. doi:10.1089/photob.2019.4658
Hu, B., Hu, Y., Li, X., Gao, J., Sun, R., Zhan, D., et al. (2022). Shear bond strength of different bonding agents to orthodontic metal bracket and zirconia. Dent. Mater J. 41, 749–756. doi:10.4012/dmj.2022-028
Ikemura, K., and Endo, T. (2010). A review of our development of dental adhesives--effects of radical polymerization initiators and adhesive monomers on adhesion. Dent. Mater J. 29, 109–121. doi:10.4012/dmj.2009-057
Ito, S., Hashimoto, M., Wadgaonkar, B., Svizero, N., Carvalho, R. M., Yiu, C., et al. (2005). Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 26, 6449–6459. doi:10.1016/j.biomaterials.2005.04.052
Jawad, Z., Bates, C., and Hodge, T. (2015). Who needs orthodontic treatment? Who gets it? And who wants it? Br. Dent. J. 218, 99–103. doi:10.1038/sj.bdj.2015.51
Ju, G.-Y., Lim, B.-S., Moon, W., Park, S.-Y., Oh, S., and Chung, S. H. (2020). Primer-treated ceramic bracket increases shear bond strength on dental zirconia surface. Mater. (Basel) 13, 4106. doi:10.3390/ma13184106
Ju, G.-Y., Oh, S., Lim, B.-S., Lee, H.-S., and Chung, S. H. (2019). Effect of simplified bonding on shear bond strength between ceramic brackets and dental zirconia. Mater. (Basel) 12, 1640. doi:10.3390/ma12101640
Khan, A. A., Al Kheraif, A. A. A., Jamaluddin, S., Elsharawy, M., and Divakar, D. D. (2017). Recent trends in surface treatment methods for bonding composite cement to zirconia: a reveiw. J. Adhes. Dent. 19, 7–19. doi:10.3290/j.jad.a37720
Lima, R. B. W., Barreto, S. C., Alfrisany, N. M., Porto, T. S., De Souza, G. M., and De Goes, M. F. (2019). Effect of silane and MDP-based primers on physico-chemical properties of zirconia and its bond strength to resin cement. Dent. Mater 35, 1557–1567. doi:10.1016/j.dental.2019.07.008
Makhija, S. K., Lawson, N. C., Gilbert, G. H., Litaker, M. S., McClelland, J. A., Louis, D. R., et al. (2016). Dentist material selection for single-unit crowns: findings from the national dental practice-based research network. J. Dent. 55, 40–47. doi:10.1016/j.jdent.2016.09.010
Meguro, D., Hayakawa, T., Kawasaki, M., and Kasai, K. (2006). Shear bond strength of calcium phosphate ceramic brackets to human enamel. Angle Orthod. 76, 301–305. doi:10.1043/0003-3219(2006)076[0301:SBSOCP]2.0.CO;2
Moszner, N., Salz, U., and Zimmermann, J. (2005). Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent. Mater 21, 895–910. doi:10.1016/j.dental.2005.05.001
Nagaoka, N., Yoshihara, K., Feitosa, V. P., Tamada, Y., Irie, M., Yoshida, Y., et al. (2017). Chemical interaction mechanism of 10-MDP with zirconia. Sci. Rep. 7, 45563. doi:10.1038/srep45563
Nistor, L., Grădinaru, M., Rîcă, R., Mărășescu, P., Stan, M., Manolea, H., et al. (2019). Zirconia use in dentistry - manufacturing and properties. Curr. Health Sci. J. 45, 28–35. doi:10.12865/CHSJ.45.01.03
Pinho, M., Manso, M. C., Almeida, R. F., Martin, C., Carvalho, Ó., Henriques, B., et al. (2020). Bond strength of metallic or ceramic orthodontic brackets to enamel, acrylic, or porcelain surfaces. Mater. (Basel) 13, 5197. doi:10.3390/ma13225197
Rauch, A., Schrock, A., Schierz, O., and Hahnel, S. (2021). Material selection for tooth-supported single crowns-a survey among dentists in Germany. Clin. Oral Investig. 25, 283–293. doi:10.1007/s00784-020-03363-9
Reginato, C. F., Oliveira, A. S., Kaizer, M. R., Jardim, P. S., and Moraes, R. R. (2013). Polymerization efficiency through translucent and opaque fiber posts and bonding to root dentin. J. Prosthodont Res. 57, 20–23. doi:10.1016/j.jpor.2012.05.003
Shimoe, S., Hirata, I., Otaku, M., Matsumura, H., Kato, K., and Satoda, T. (2018). Formation of chemical bonds on zirconia surfaces with acidic functional monomers. J. Oral Sci. 60, 187–193. doi:10.2334/josnusd.17-0160
Shinagawa, J., Inoue, G., Nikaido, T., Ikeda, M., Burrow, M. F., and Tagami, J. (2019). Early bond strengths of 4-META/MMA-TBB resin cements to CAD/CAM resin composite. Dent. Mater J. 38, 28–32. doi:10.4012/dmj.2017-438
Takahashi, M., Nakajima, M., Hosaka, K., Ikeda, M., Foxton, R. M., and Tagami, J. (2011). Long-term evaluation of water sorption and ultimate tensile strength of HEMA-containing/-free one-step self-etch adhesives. J. Dent. 39, 506–512. doi:10.1016/j.jdent.2011.04.008
Tanaka, Y., Sugaya, T., Tanaka, S., and Kawanami, M. (2004). Long-term durability of root-end sealing with 4-MEtA/MMA-TBB resin. Dent. Mater J. 23, 453–456. doi:10.4012/dmj.23.453
Tecco, S., Traini, T., Caputi, S., Festa, F., de Luca, V., and D’Attilio, M. (2005). A new one-step dental flowable composite for orthodontic use: an in vitro bond strength study. Angle Orthod. 75, 672–677. doi:10.1043/0003-3219(2005)75[672:ANODFC]2.0.CO;2
Toledano, M., Osorio, R., de Leonardi, G., Rosales-Leal, J. I., Ceballos, L., and Cabrerizo-Vilchez, M. A. (2001). Influence of self-etching primer on the resin adhesion to enamel and dentin. Am. J. Dent. 14, 205–210.
Urichianu, M., Makowka, S., Covell, D., Warunek, S., and Al-Jewair, T. (2022). Shear bond strength and bracket base morphology of new and rebonded orthodontic ceramic brackets. Mater. (Basel) 15, 1865. doi:10.3390/ma15051865
Uysal, T., Sari, Z., and Demir, A. (2004). Are the flowable composites suitable for orthodontic bracket bonding? Angle Orthod. 74, 697–702. doi:10.1043/0003-3219(2004)074<0697:ATFCSF>2.0.CO;2
Van Landuyt, K. L., De Munck, J., Snauwaert, J., Coutinho, E., Poitevin, A., Yoshida, Y., et al. (2005). Monomer-solvent phase separation in one-step self-etch adhesives. J. Dent. Res. 84, 183–188. doi:10.1177/154405910508400214
Vult von Steyern, P., Bruzell, E., Vos, L., Andersen, F. S., and Ruud, A. (2022). Sintering temperature accuracy and its effect on translucent yttria-stabilized zirconia: flexural strength, crystal structure, tetragonality and light transmission. Dent. Mater 38, 1099–1107. doi:10.1016/j.dental.2022.04.023
Zarone, F., Di Mauro, M. I., Ausiello, P., Ruggiero, G., and Sorrentino, R. (2019). Current status on lithium disilicate and zirconia: a narrative review. BMC Oral Health 19, 134. doi:10.1186/s12903-019-0838-x
Keywords: shear bond strength (SBS), ceramic bracket, zirconia, resin cement, metal bracket, storage condition
Citation: Hu Y, Gao J, Huang X, Li Y, Chen Z, Zhan D, Sano H, Carvalho RM and Fu J (2024) The possibility of clinical bonding between metal/ceramic brackets to zirconia: in vitro study. Front. Bioeng. Biotechnol. 12:1354241. doi: 10.3389/fbioe.2024.1354241
Received: 12 December 2023; Accepted: 04 January 2024;
Published: 15 January 2024.
Edited by:
Hongye Yang, Wuhan University, ChinaCopyright © 2024 Hu, Gao, Huang, Li, Chen, Zhan, Sano, Carvalho and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiale Fu, ZnVsbGVyc0AxMjYuY29t
 Yichun Hu1
Yichun Hu1 Yutong Li
Yutong Li Jiale Fu
Jiale Fu