- 1Department of Ophthalmology, Huaian Hospital of Huaian City, Huaian, China
- 2Department of Ophthalmology, Shenzhen Eye Hospital, Jinan University, Shenzhen, China
- 3Department of Ophthalmology, Third Affiliated Hospital, Wenzhou Medical University, Ruian, China
Retinal blood vessels are the only directly observed blood vessels in the body; changes in them can help effective assess the occurrence and development of ocular and systemic diseases. The specificity and efficiency of retinal vessel quantification technology has improved with the advancement of retinal imaging technologies and artificial intelligence (AI) algorithms; it has garnered attention in clinical research and applications for the diagnosis and treatment of common eye and related systemic diseases. A few articles have reviewed this topic; however, a summary of recent research progress in the field is still needed. This article aimed to provide a comprehensive review of the research and applications of retinal vessel quantification technology in ocular and systemic diseases, which could update clinicians and researchers on the recent progress in this field.
1 Introduction
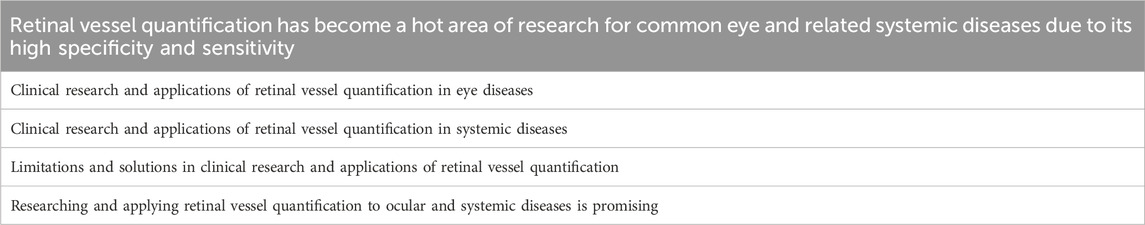
Recent advancements in ophthalmic imaging technology have led to challenges in monitoring disease progression during early and advanced stages, largely due to its limited use for quantitative analysis of diseases; it has been mainly used for qualitative analysis of diseases. The rise in computational power, imaging data expansion, and ethical system improvement has led to a rapid development of AI in medicine (Ting et al., 2021). The retinal vessel quantification technology has been advanced, providing new opportunities for quantitative analysis of diseases. The retinal blood vessels are the only blood vessels that can be directly observed without using an invasive approach; their structures and functions are similar to those of the systemic vascular system. Quantitative research on retinal blood vessels could provide insights into the conditions of cardiovascular, cerebrovascular, and systemic blood vessels because they are components of the body’s circulatory system (Dumitrascu and Koronyo-Hamaoui, 2020). The AI-based retinal vessel quantification technology can help clinicians and researchers study ocular and systemic diseases, benefiting disease prevention, diagnosis, and treatment (Gadde et al., 2016; Fuchs et al., 2022; Fu et al., 2023). A few articles have reviewed this technology; however, summarizing its recent progress is still needed. This article aimed to provide a comprehensive review of the research and applications of retinal vessel quantification technology in ocular and systemic diseases. As shown in Table 1.
2 Retinal vessel quantification technology
Retinal vessel quantification is a method to measure and analyze retinal blood vessels in the fundus through imaging technology, and extensive research has been conducted in clinical Retinal vessel quantification based on AI using optical coherence tomography angiography (OCTA). OCTA utilizes specific algorithms to image only moving red blood cells in the vessels, providing three-dimensional (3D) visualization and quantitative analysis of the blood flow status across different vascular membrane layers of the retina and choroid (Lommatzsch, 2020; Koutsiaris et al., 2023). With the continual advancement in AI algorithms and ophthalmic imaging technology, several quantitative models for retinal blood vessels have been established, with high levels of sensitivity and specificity (Nguyen et al., 2013; Nunez do Rio et al., 2020; Comin et al., 2021; Liefers et al., 2021; Zhou et al., 2022; Nardini et al., 2023). Retinal vessel quantification is crucial in studying ocular and systemic diseases. Repeated quantitative analyses of vascular parameters such as vascular diameter, vascular fractal dimension (FD), vascular angle, vascular density (VD), retinal non-perfusion (RNP), and foveal avascular zone (FAZ) can assist in the comprehension of changes in disease occurrence, progression, and treatment (Pournaras and Riva, 2013; Lee et al., 2018; Ramos et al., 2019; Alibhai et al., 2020; Kadomoto et al., 2021).
Quantitative analysis of the normal retina allows for an in-depth examination of the retinal vascular structure, benefiting the diagnosis and analysis of vascular abnormalities. The density of the deep retinal vascular plexus is higher than that of the superficial retinal vascular plexus. The retina is divided into four sectors centered on the FAZ. The vascular density of the inferior region, whether deep or superficial, is higher than that of the other regions (temporal, superior, and nasal), and age has no significant effect on VD (Gadde et al., 2016). Macular vascular parameters are related to sex and age, and the FD and VD of blood vessels in males are significantly higher than those in females; in contrast, no significant differences exist in vessel curvature, and the entire macular region’s FD, VD, and average vascular diameter exhibit negative correlations with age (Feng et al., 2021). Based on the measurement of the retinal vascular parameters of individuals aged 50 and over, the greater the FAZ area and non-circular index, the lower the average capillary density and superficial vascular density, the poorer visual acuity. These vascular parameters might be used as biomarkers for predicting the visual acuity of those aged 50 and over (Li et al., 2023).
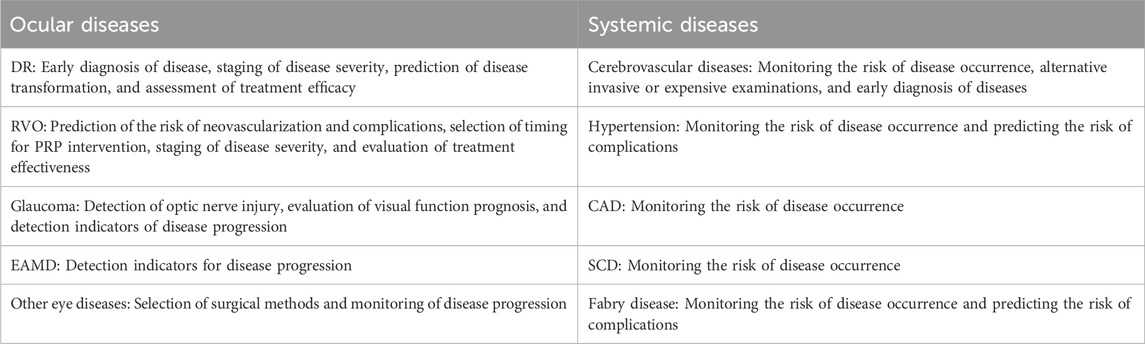
Retinal vessel quantification technology has the potential to foster research on relevant ailments and provide novel methods for managing systemic and ocular health. Precise quantitative analysis of the retinal vasculature can provide insight into the pathophysiological mechanisms of ocular diseases and early warning and monitoring of systemic diseases as shown in Table 2.
3 Clinical research and applications in ocular disorders
3.1 Diabetic retinopathy
Diabetic retinopathy (DR) is a prevalent microvascular complication of diabetes and a frequent cause of blindness; the disease triggers vascular variations by damaging capillary cells like the vascular endothelial cells and pericytes, leading to local ischemia. DR progression leads to an increase in the number of microaneurysms (Wu et al., 2014; Jiang et al., 2020), vascular curvature (Lee et al., 2018), RNP area (Baxter et al., 2019; Alibhai et al., 2020; Kim et al., 2021), FAZ area (Mihailovic et al., 2019; Ratra et al., 2021; Meng et al., 2022), and non-circular index and a decrease in VD (Durbin et al., 2017). The RNP area shows a positive correlation with the emergence of neovascularization (Yu et al., 2020). These vascular parameters can function as reference indicators for the onset and progression of DR and predictive indicators for disease transformation. Certain parameters, including VD, vascular length, and FAZ area, could undergo changes prior to the onset of visual impairment in individuals with DR, and they offer substantial value in facilitating the detection of DR lesions at an early stage (Zhu et al., 2019). Among all parameters, there was a statistically significant difference in vascular curvature between patients with non-DR and mild non-proliferative diabetic retinopathy (NPDR), especially within the 1.5-mm area of the superficial layer of the retina (Lee et al., 2018). Quantitative indicators of retinal blood vessels can serve as potential biomarkers for DR staging (Xu et al., 2019; Chua et al., 2020; Boned-Murillo et al., 2021; Xu et al., 2021). Borrelli and colleagues attempted to utilize 3D analysis of OCTA to create a 3D image of the retinal vasculature and used a global threshold algorithm to procure two vascular parameters, 3D vascular volume, and 3D perfusion density, to assess the status of macular ischemia in patients with NPDR; the smaller the 3D vascular volume and 3D perfusion density, the more severe the macular ischemia (Borrelli et al., 2020). After thorough examinations, the reliability of 3D analysis for evaluating the state of retinal blood vessels has been confirmed, indicating broad potential applications (Borrelli et al., 2020). Retinal vessel quantification assesses the treatment prognosis of patients with DR, and it quantifies the alterations in retinal neovascularization pre- and post-treatment with anti-vascular endothelial growth factor (anti-VEGF) therapy to evaluate the susceptibility of patients with DR to VEGF (Hu et al., 2019); helping informed decisions for follow-on treatment. Pan-retinal photocoagulation (PRP) can effectively reverse retinal DR-caused ischemia while maintaining the integrity of macular microvascular structure. The treatment effect can be effectively evaluated by quantifying the retinal VD and FAZ areas after PRP in patients with DR (Abdelhalim et al., 2022; Sariyildiz et al., 2023).
3.2 Retinal vein occlusion
Retinal vein occlusion (RVO) is the second most common retinal vascular disease that can cause blindness following DR. It happens when one or more veins in the retina are blocked or obstructed. The retina, located at the posterior of the eye, detects light and transmits signals to the brain for visual perception. Venous obstruction interferes with normal blood flow on the retina, potentially resulting in vision impairments. The impact of RVO on the deep capillary plexus (DCP) of the retina is 1.77–1.84 times that of the shallow capillary plexus (SCP) (Kim et al., 2020). The larger the area of RNP, the greater the risk of neovascularization. Quantifying the area of RNP can effectively assess the risk of neovascularization (Kadomoto et al., 2021). Neovascularization and associated neovascular glaucoma are common complications of RVO that can cause serious damage to a patient’s vision and the eyeball itself (Hayreh, 2021). PRP can effectively prevent and treat neovascularization, which could benefit from quantifying the area of the RNP to select the timing of PRP use. The severity of macular ischemia increases as the VD in the macular area reduces and the RNP area expands, accurately quantifying the VD and the RNP area can be used to grade macular ischemia in RVO patients, and accurately quantifying the VD and the RNP areas can be used to grade macular ischemia in patients with RVO and assess disease severity and prognosis (Ouederni et al., 2019; Tang et al., 2021; Yeung et al., 2021). Huang et al., 2022 classified patients with branch vein occlusion (BRVO) into reactive and refractory groups based on their responses to anti-VEGF treatment, which was determined by semi-automatic quantitative fluorescein angiography to measure the amount of fluorescein leakage around and near the fovea of the macular area in patients with BRVO. The refractory group demonstrated more severe leakage than the reactive group; thus, this technique could effectively predict the efficacy of anti-VEGF treatment for evaluating treatment feasibility (Huang et al., 2022).
3.3 Glaucoma
Glaucoma is an irreversible condition that can cause blindness. Primary angle closure glaucoma is the most common type of glaucoma in China, which occurs when the angle between the iris and cornea in the front chamber of the eye becomes narrow or even closes completely, preventing the flow of aqueous humor. If the anterior chamber angle is narrow, the normal outflow of aqueous humor is impeded, causing an elevation in intraocular pressure, resulting in optic nerve damage and eventual vision loss. Based on the quantification of the retinal blood vessels in patients suffering from primary angle closure, the microvessel density around the optic disc reduces, despite the absence of any changes in the thickness of the retinal nerve fiber layer and the ganglion cell complex, the microvascular density around the optic papilla is a sensitive indicator of changes in intraocular pressure and a predictive and monitoring parameter for the onset of glaucoma nerve changes (Miguel et al., 2021; Nascimento E Silva et al., 2021; Wang et al., 2021). These observations highlight the potential utility of adjusting target intraocular pressure based on changes in microvessel density. Van Melkebeke et al., 2018 showed that the microvascular density surrounding the optic disc is a prognostic indicator of visual function in patients with glaucoma. Kromer et al., 2019 used OCTA to measure macular blood flow density in patients with open-angle glaucoma and found that macular blood flow density was significantly decreased in glaucoma patients compared to the healthy population. A noteworthy correlation exists between the density of blood flow in the macula and the visual field (Yarmohammadi et al., 2017), presenting an opportunity to repeatedly evaluate glaucoma progression by measuring macular blood flow density.
3.4 Wet age-related macular degeneration
Wet age-related macular degeneration (wAMD) is a common cause of irreversible visual impairment, typically affecting the central vision of the eye. It is usually caused by abnormal vascular growth beneath the retina, resulting in macular region damage and fluid exudation; the incidence rate of wAMD has increased significantly in recent years (Stahl, 2020). Gao et al. quantitatively analyzed the RNP areas of the extrafoveal superficial vascular complex (SVC), intermediate capillary plexus (ICP), and deep capillary plexus (DCP) in patients with wAMD and healthy individuals, ruling out related interfering factors. They found that these patients had larger areas of RNP in SVC, ICP, and DCP (Gao et al., 2022). Hence, it is evident that the area of the RNP is closely related to the onset and progression of wAMD; measuring the area of the RNP can be used in clinical practice to predict and monitor the onset and progression of wAMD.
Quantitative analysis of retinal effusion secondary to retinal angiopathy facilitates evaluating disease progression and treatment responses in patients with retinal effusion like RVO, wAMD, and diabetes macular edema (Farinha et al., 2020; Schmidt-Erfurth et al., 2020; Fuchs et al., 2022; Michl et al., 2022; Muste et al., 2022; Reiter and Schmidt-Erfurth, 2022; Coulibaly et al., 2023). Wu et al., 2021 proposed a new optimized segmentation and quantification algorithm for neovascularization based on OCTA, which has higher accuracy and effectively monitors changes in neovascularization. It can be useful in follow-up monitoring during diagnosing and treating ischemic ophthalmopathy and systemic diseases.
3.5 Other ocular diseases
Iris VD is decreased shortly after refractive surgery, and the densities of superficial and deep retinal blood vessels do not recover within 3 months of surgery (Olcay et al., 2015). The small incision corneal stromal lenticule extraction (SMILE) is more significantly reduced than femtosecond laser in situ keratomileusis (FS-LASIK), which might be related to an abnormal elevation in intraocular pressure during the surgical process (Olcay et al., 2015). Thus, it is necessary to consider the impact of vascular changes and the selection of surgical approaches for patients requiring refractive surgery in clinical practice (Cui et al., 2022). Retinal vessel quantification can monitor and analyze vascular changes in the occurrence, development, and treatment of eye diseases, such as retinitis pigmentosa (Wang et al., 2019; Lu et al., 2022), type 2 macular telangiectasia (Chidambara et al., 2016; Pauleikhoff et al., 2019; Pauleikhoff et al., 2022), familial retinal arteriolar tortuosity (Saraf et al., 2019), Behcet’s disease (Türkcü et al., 2020), optic disc drusen (Leal-González et al., 2020), and retinopathy of prematurity (Cabrera et al., 2021).
4 Clinical research and applications in systemic diseases
4.1 Cerebrovascular diseases
Retinal and cerebral blood vessels come from the internal carotid artery and interconnect and influence each other. Thus, abnormalities in retinal blood vessels are often accompanied by abnormalities in cerebral blood vessels, and abnormal changes in retinal blood vessels are associated with stroke, vascular cognitive impairment, and dementia (Frost et al., 2017; Cabrera DeBuc et al., 2018; Cabrera DeBuc et al., 2020; Dumitrascu and Koronyo-Hamaoui, 2020). Widespread retinal arteriolar stenosis is linked to a high risk of disabling dementia (Jinnouchi et al., 2017), which could serve as an effective biomarker for populations with disabling dementia and one of the traditional screening indicators. The APOE ε4 allele is among the genetic factors most closely linked with Alzheimer’s disease, and individuals carrying the APOE ε4 allele have a greater risk of developing Alzheimer’s disease. Carriers of the APOE ε4 allele have significantly higher vascular width ratios than normal individuals, and measuring the ratio of retinal vessel width is an alternative approach to the invasive examination of APOE ε4 (Frost et al., 2017). Brain white matter volume reduction and enlargement of the inferior lateral ventricle adversely affect brain function, potentially leading to cognitive impairments, motor dysfunction, epilepsy, and visual and speech issues, depending on the extent, location, and cause of the reduction. Brain white matter volume is typically assessed and evaluated using MRI, and a larger diameter of retinal venules is associated with a smaller brain white matter volume (Ikram et al., 2013). Decreased densities of retinal SCP and perfusion are associated with the enlargement of the inferior lateral ventricle (Yoon et al., 2019). As an alternative to MRI, assessing and monitoring changes in brain white matter volume and the inferior lateral ventricle can be achieved by measuring retinal venule diameter, retinal SCP density, and perfusion density. Early abnormalities in brain microvasculature, that cannot be detected by head MRI, are closely associated with changes in retinal vasculature. Therefore, quantifying retinal vascular changes can be used to assess brain vascular function, achieving early diagnosis and intervention (London et al., 2013; Wardlaw et al., 2013).
4.2 Hypertension
Hypertension is a common cardiovascular disease. As blood pressure becomes unstable or consistently rises, the risk of developing diseases, such as heart, retinal, stroke, and kidney diseases, also increases. Hypertension typically results in an elevated ratio of small artery length to diameter and decreased terminal branch arteries. Alterations in the retina often signal potential disease risks in other target organs; thus, quantifying retinal vascular parameters could help evaluate the risk of hypertension complications (Hughes et al., 2006; Leclaire et al., 2021). High blood pressure elevates the pressure on blood vessels, leading to weakened and hardened elasticity of retinal arterioles, compressing the veins, and resulting in decreased blood flow and gradual thinning of the veins, known as Gunn’s sign (Wigdahl et al., 2015). Furthermore, the pressure of arterioles at the intersection can be transmitted to the veins, resulting in the characteristic S-shaped appearance; the Salus sign is a term used to describe a phenomenon at the intersection of retinal arteries and veins, and quantifying the Gunn and Salus signs could predict hypertensive retinopathy and RVO development (Wigdahl et al., 2015). Bringing together deep learning and OCTA could more precisely quantify the retinal vascular structure, it is feasible to precisely forecast the onset and development risk of hypertension and its complications based on the changes in retinal vascular structure. (Tan et al., 2022).
4.3 Coronary artery disease
Coronary artery disease (CAD) is a cardiovascular disease occurring when the coronary artery (one of the main blood vessels supplying the heart) narrows or becomes blocked. CAD can trigger angina (chest pain) and myocardial infarction (severe damage to heart muscles), endangering the patient’s life. The high incidence and mortality rates of CAD imply early detection, and intervention are imperative. Fu et al. used deep learning technology to quantify retinal vascular parameters from color fundus photography of 57,947 participants without CAD; after approximately 11 years of follow-up, the FD of blood vessels had decreased, and the reduction in the number of arterial and small venous segments and arterial and venous bone densities was closely associated with an increased risk of subsequent CAD (Fu et al., 2023). Hence, detecting retinal vascular parameters could help predict CAD (Shokr et al., 2021).
4.4 Sickle cell disease
Sickle cell disease (SCD), also called sickle cell anemia, is a prevalent hereditary blood disease characterized by the deformation of red blood cells into sickle or curved shapes, different from the round shape of normal red blood cells. Such abnormal red blood cells are susceptible to adhesion and blockage within blood vessels, leading to ischemic damage. When OCTA was used to determine retinal capillary perfusion, dynamic changes were observed in retinal capillary perfusion in patients with SCD compared with healthy individuals (Zhou et al., 2021). Retinal ischemia and hypoxia can result in various complications. Non-invasive dynamic monitoring of retinal capillary perfusion in patients with SCD allows for effective evaluation of the state of systemic blood vessels, benefiting the early detection and treatment of the disease and assessing treatment efficacy.
4.5 Other systemic diseases
Fabry disease is a rare genetic disorder where glycolipids accumulate in multiple tissues and cells due to deficiency or reduced activity of the enzyme cleavage lipase, resulting in damage to multiple organs and systems. Quantifying retinal blood vessels demonstrate a negative correlation between retinal VD and myocardial damage associated with fabry disease (Cennamo et al., 2020). Primary nephrotic syndrome (PNS) is typically linked to dysfunction in glomerular filtration. The VD and blood flow perfusion density in the macular areas of patients with PNS significantly decreased compared with healthy individuals and negatively correlated with urinary protein levels (Yao et al., 2022). Thus, retinal blood vessels can be quantified to monitor and analyze the onset and progression of associated systemic illnesses and their complications and to assess disease alterations throughout treatment.
5 Limitations and solutions
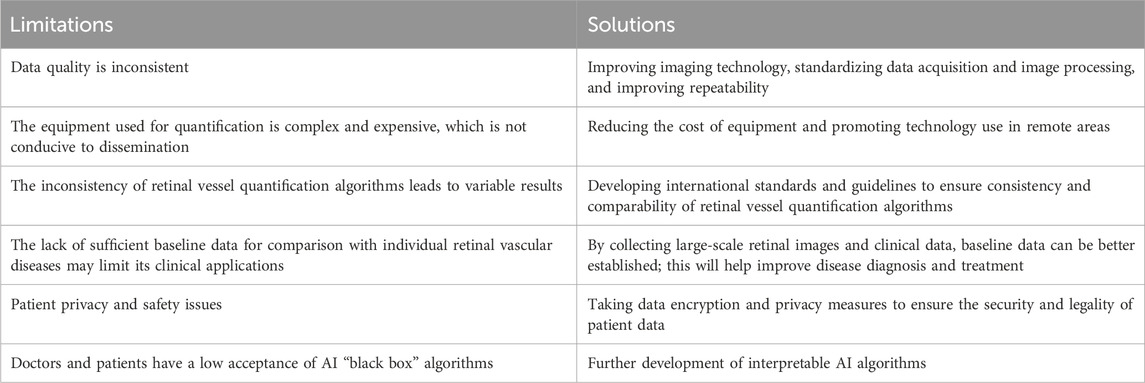
Limitations exist with the retinal vessel quantification technology. This technology has limited generalization for large clinical applications, and trust and acceptance of algorithm outcomes vary among physicians and patients (Ting et al., 2019). Retinal vessel quantification techniques have some limitations, including inconsistent data quality, high equipment costs, inconsistent algorithms, and insufficient baseline data. To address these issues, we propose the following improvements: Firstly, we suggest improving reproducibility and reducing costs by enhancing imaging techniques and standardizing data acquisition and image processing processes. Secondly, we recommend developing international standards and guidelines to ensure algorithmic consistency and comparability. Thirdly, we propose establishing better baseline data through big data analytics to support clinical applications. Lastly, we suggest adopting data encryption and privacy protection measures to ensure the security of patient data. To enhance the acceptance of AI algorithms among physicians and patients, it is important to further develop explainable AI algorithms., Limitations and solutions of retinal vessel quantification are shown in Table 3. Fostering inter-team cooperation, improving technology, and increasing collaboration with clinicians and patients would help overcome these limitations, increasing its clinical applications and benefiting disease diagnosis and treatment.
6 Application trends and prospects
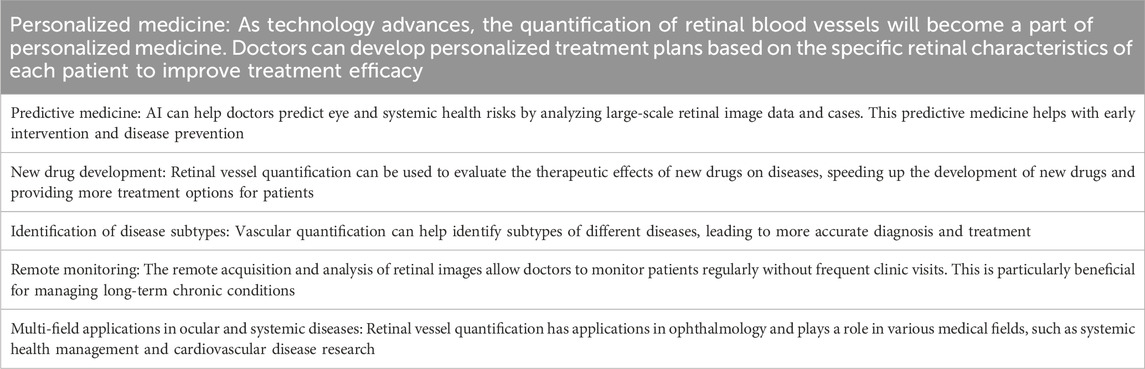
The development of modern technologies, dataset expansion, improvement in doctor-patient acceptance, data privacy and ethics, and increased financial support will render retinal vessel quantification to have broader application prospects in disease research. By observing changes in the retinal blood vessels of diverse populations, researchers evaluate the effectiveness of disease treatment protocols and study pathophysiologic changes in disease to more accurately diagnose and treat disease and develop drugs (Al-Shabrawey, 2023; Middel et al., 2023; Yucel Gencoglu et al., 2023). Prospects and trends of retinal vessel quantification in clinical disease are shown in Table 4.
Retinal vessel quantification will continue to play an important role in clinical research. It would improve the effectiveness of disease diagnosis, treatment, and prevention and promote the further development of medical science by combining advanced technologies and data analysis methods.
7 Conclusion
The quantification of retinal vessels has important clinical research and application values, mainly reflected in the following aspects. First, it can be used for early diagnosis of diseases. Doctors can detect early signs of DR, glaucoma, CAD, and other diseases by quantifying retinal vessels, allowing for early intervention and treatment to reduce permanent damage. Second, it can be used to monitor disease progression. Retinal vessel quantification can be used to monitor disease progression, determine the effectiveness of treatment, and adjust treatment plans in a timely manner in patients with eye and related systemic diseases. Third, it can be used for personalized treatment. By understanding each patient’s retinal vascular status, physicians can develop more personalized treatment plans to improve treatment efficacy and reduce side effects. Fourth, it can assist in replacing relevant clinical examinations. Retinal vessel quantification can effectively assist in replacing some traumatic and costly clinical examinations with repeatability, helping clinicians evaluate disease occurrence, development, and treatment effectiveness. Fifth, it can be used for studying disease mechanisms and pathogenesis, providing a foundation for new treatment methods and drug development.
Overall, retinal vessel quantification has broad research application prospects in the diagnosis, treatment, and research of ocular and systemic diseases, helping prevent disease onset and development and better protect and maintain the physical health of patients (Keskinbora and Güven, 2020).
Author contributions
NC: Writing–original draft. ZZ: Writing–review and editing. WY: Writing–review and editing. QW: Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Public Benifit Technology Research Program of Zhejiang Science and Technology Department (GC21H120001), Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP014) and Sanming Project of Medicine in Shenzhen (SZSM202311012).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhalim, A. S., Abdelkader, M. F. S. O., Mahmoud, M. S. E., and Mohamed Mohamed, A. A. (2022). Macular vessel density before and after panretinal photocoagulation in patients with proliferative diabetic retinopathy. Int. J. Retina Vitr. 8, 21. doi:10.1186/s40942-022-00369-1
Alibhai, A. Y., De Pretto, L. R., Moult, E. M., Or, C., Arya, M., McGowan, M., et al. (2020). Quantification of retinal capillary nonperfusion in diabetics using wide-field optical coherence tomography angiography. Retina 40, 412–420. doi:10.1097/IAE.0000000000002403
Al-Shabrawey, M. (2023). Methods of studying retinal vessels in health and diseases. J. Vis. Exp. 192. doi:10.3791/65008
Baxter, S. L., Ashir, A., Nguyen, B. J., and Nudleman, E. (2019). Quantification of retinal nonperfusion associated with posterior segment neovascularization in diabetic retinopathy using ultra-widefield fluorescein angiography. Ophthalmic Surg. Lasers Imaging Retina 50, 86–92. doi:10.3928/23258160-20190129-04
Boned-Murillo, A., Albertos-Arranz, H., Diaz-Barreda, M. D., Orduna-Hospital, E., Sánchez-Cano, A., Ferreras, A., et al. (2021). Optical coherence tomography angiography in diabetic patients: a systematic review. Biomedicines 10, 88. doi:10.3390/biomedicines10010088
Borrelli, E., Sacconi, R., Querques, L., Battista, M., Bandello, F., and Querques, G. (2020). Quantification of diabetic macular ischemia using novel three-dimensional optical coherence tomography angiography metrics. J. Biophot. 13, e202000152. doi:10.1002/jbio.202000152
Cabrera, M. T., Chia, T., Wallace, D. K., Ulrich, J. N., Freedman, S. F., Ding, L., et al. (2021). Short-term computer-assisted quantification of plus disease after treatment of TYPE 1 retinopathy of prematurity with intravitreal bevacizumab or retinal laser photocoagulation. Retin. Cases Brief. Rep. 15, 314–319. doi:10.1097/ICB.0000000000000794
Cabrera DeBuc, D., Feuer, W. J., Persad, P. J., Somfai, G. M., Kostic, M., Oropesa, S., et al. (2020). Investigating vascular complexity and neurogenic alterations in sectoral regions of the retina in patients with cognitive impairment. Front. Physiol. 11, 570412. doi:10.3389/fphys.2020.570412
Cabrera DeBuc, D., Somfai, G. M., Arthur, E., Kostic, M., Oropesa, S., and Mendoza Santiesteban, C. (2018). Investigating multimodal diagnostic eye biomarkers of cognitive impairment by measuring vascular and neurogenic changes in the retina. Front. Physiol. 9, 1721. doi:10.3389/fphys.2018.01721
Cennamo, G., Montorio, D., Santoro, C., Cocozza, S., Spinelli, L., Di Risi, T., et al. (2020). The retinal vessel density as a new vascular biomarker in multisystem involvement in Fabry disease: an optical coherence tomography angiography study. J. Clin. Med. 9, 4087. doi:10.3390/jcm9124087
Chidambara, L., Gadde, S. G., Yadav, N. K., Jayadev, C., Bhanushali, D., Appaji, A. M., et al. (2016). Characteristics and quantification of vascular changes in macular telangiectasia type 2 on optical coherence tomography angiography. Br. J. Ophthalmol. 100, 1482–1488. doi:10.1136/bjophthalmol-2015-307941
Chua, J., Sim, R., Tan, B., Wong, D., Yao, X., Liu, X., et al. (2020). Optical coherence tomography angiography in diabetes and diabetic retinopathy. J. Clin. Med. 9, 1723. doi:10.3390/jcm9061723
Comin, C. H., Tsirukis, D. I., Sun, Y., and Xu, X. (2021). Quantification of retinal blood leakage in fundus fluorescein angiography in a retinal angiogenesis model. Sci. Rep. 11, 19903. doi:10.1038/s41598-021-99434-2
Coulibaly, L. M., Sacu, S., Fuchs, P., Bogunovic, H., Faustmann, G., Unterrainer, C., et al. (2023). Personalized treatment supported by automated quantitative fluid analysis in active neovascular age-related macular degeneration (nAMD)-a phase III, prospective, multicentre, randomized study: design and methods. Eye (Lond.). 37, 1464–1469. doi:10.1038/s41433-022-02154-8
Cui, L., Xue, W., Yao, W., Huang, X., Xue, W., Wang, Y., et al. (2022). Quantitative changes in iris and retinal blood flow after femtosecond laser-assisted in situ keratomileusis and small-incision lenticule extraction. Front. Med. (Lausanne). 9, 862195. doi:10.3389/fmed.2022.862195
Dumitrascu, O. M., and Koronyo-Hamaoui, M. (2020). Retinal vessel changes in cerebrovascular disease. Curr. Opin. Neurol. 33, 87–92. doi:10.1097/WCO.0000000000000779
Durbin, M. K., An, L., Shemonski, N. D., Soares, M., Santos, T., Lopes, M., et al. (2017). Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 135, 370–376. doi:10.1001/jamaophthalmol.2017.0080
Farinha, C., Santos, T., Santos, A. R., Lopes, M., Alves, D., Silva, R., et al. (2020). OPTICAL COHERENCE TOMOGRAPHY LEAKAGE IN neovascular AGE-RELATED MACULAR DEGENERATION: identification of choroidal neovascularization activity by location and quantification of abnormal fluid under anti-vascular endothelial growth factor therapy. Retina 40, 881–890. doi:10.1097/IAE.0000000000002470
Feng, Z., Wang, G., Xia, H., Li, M., Liang, G., Dong, T., et al. (2021). Macular vascular geometry changes with sex and age in healthy subjects: a fundus photography study. Front. Med. (Lausanne). 8, 778346. doi:10.3389/fmed.2021.778346
Frost, S., Bhuiyan, A., Offerman, D., Doecke, J. D., Macaulay, S. L., Sohrabi, H. R., et al. (2017). Modulation of retinal arteriolar central reflection by APOE genotype. Curr. Alzheimer Res. 14, 916–923. doi:10.2174/1567205014666170309115016
Fu, Y., Yusufu, M., Wang, Y., He, M., Shi, D., and Wang, R. (2023). Association of retinal microvascular density and complexity with incident coronary heart disease. Atherosclerosis 380, 117196. doi:10.1016/j.atherosclerosis.2023.117196
Fuchs, P., Coulibaly, L., Reiter, G. S., and Schmidt-Erfurth, U. (2022). Artificial intelligence in the management of anti-VEGF treatment: the Vienna fluid monitor in clinical practice. Ophthalmol. Ophthalmol. 119, 520–524. doi:10.1007/s00347-022-01618-2
Gadde, S. G., Anegondi, N., Bhanushali, D., Chidambara, L., Yadav, N. K., Khurana, A., et al. (2016). Quantification of vessel density in retinal optical coherence tomography angiography images using local fractal dimension. Invest. Ophthalmol. Vis. Sci. 57, 246–252. doi:10.1167/iovs.15-18287
Gao, L., Wang, J., You, Q., Guo, Y., Flaxel, C. J., Hwang, T. S., et al. (2022). Plexus-specific retinal capillary avascular area in exudative age-related macular degeneration with projection-resolved OCT angiography. Br. J. Ophthalmol. 106, 719–723. doi:10.1136/bjophthalmol-2020-317562
Hayreh, S. S. (2021). Photocoagulation for retinal vein occlusion. Prog. Retin. Eye Res. 85, 100964. doi:10.1016/j.preteyeres.2021.100964
Hu, Z., Su, Y., Xie, P., Chen, L., Ji, J., Feng, T., et al. (2019). OCT angiography-based monitoring of neovascular regression on fibrovascular membrane after preoperative intravitreal conbercept injection. Graefes Arch. Clin. Exp. Ophthalmol. 257, 1611–1619. doi:10.1007/s00417-019-04315-0
Huang, P. W., Lai, C. C., Hwang, Y. S., Wu, W. C., Wu, C. H., Huang, J. C., et al. (2022). Treatment responses for branch retinal vein occlusion predicted by semiautomated fluorescein angiography quantification. BMC Ophthalmol. 22, 50. doi:10.1186/s12886-022-02245-w
Hughes, A. D., Martinez-Perez, E., Jabbar, A. S., Hassan, A., Witt, N. W., Mistry, P. D., et al. (2006). Quantification of topological changes in retinal vascular architecture in essential and malignant hypertension. J. Hypertens. 24, 889–894. doi:10.1097/01.hjh.0000222759.61735.98
Ikram, M. K., de Jong, F. J., Vernooij, M. W., Hofman, A., Niessen, W. J., van der Lugt, A., et al. (2013). Retinal vascular calibers associate differentially with cerebral gray matter and white matter atrophy. Alzheimer Dis. Assoc. Disord. 27, 351–355. doi:10.1097/WAD.0b013e31829344ed
Jiang, A., Srivastava, S., Figueiredo, N., Babiuch, A., Hu, M., Reese, J., et al. (2020). Repeatability of automated leakage quantification and microaneurysm identification utilising an analysis platform for ultra-widefield fluorescein angiography. Br. J. Ophthalmol. 104, 500–503. doi:10.1136/bjophthalmol-2019-314416
Jinnouchi, H., Kitamura, A., Yamagishi, K., Kiyama, M., Imano, H., Okada, T., et al. (2017). Retinal vascular changes and prospective risk of disabling dementia: the circulatory risk in communities study (CIRCS). J. Atheroscler. Thromb. 24, 687–695. doi:10.5551/jat.37291
Kadomoto, S., Muraoka, Y., Uji, A., Tamiya, R., Oritani, Y., Kawai, K., et al. (2021). Nonperfusion AREA QUANTIFICATION IN BRANCH RETINAL VEIN OCCLUSION: a widefield optical coherence tomography angiography study. Retina 41, 1210–1218. doi:10.1097/IAE.0000000000002999
Keskinbora, K., and Güven, F. (2020). Artificial intelligence and ophthalmology. Turk. J. Ophthalmol. 50, 37–43. doi:10.4274/tjo.galenos.2020.78989
Kim, J. T., Chun, Y. S., Lee, J. K., Moon, N. J., and Yi, D. Y. (2020). Comparison of vessel density reduction in the deep and superficial capillary plexuses in branch retinal vein occlusion. Ophthalmologica 243, 66–74. doi:10.1159/000502385
Kim, K., You, J., Park, J. R., Kim, E. S., Oh, W. Y., and Yu, S. Y. (2021). Quantification of retinal microvascular parameters by severity of diabetic retinopathy using wide-field swept-source optical coherence tomography angiography. Graefes Arch. Clin. Exp. Ophthalmol. 259, 2103–2111. doi:10.1007/s00417-021-05099-y
Koutsiaris, A. G., Batis, V., Liakopoulou, G., Tachmitzi, S. V., Detorakis, E. T., and Tsironi, E. E. (2023). Optical coherence tomography angiography (OCTA) of the eye: a review on basic principles, advantages, disadvantages and device specifications. Clin. Hemorheol. Microcirc. 83, 247–271. doi:10.3233/CH-221634
Kromer, R., Glusa, P., Framme, C., Pielen, A., and Junker, B. (2019). Optical coherence tomography angiography analysis of macular flow density in glaucoma. Acta Ophthalmol. 97, e199–e206. doi:10.1111/aos.13914
Leal-González, M., Pessanha, F., Azevedo González-Oliva, M., Pérez-Fernández, E., and Gili, P. (2020). Study of peripapillary vascular flow using optical coherence tomography angiography in optic nerve head drusen. Clin. Exp. Ophthalmol. 48, 775–782. doi:10.1111/ceo.13783
Leclaire, M. D., Eter, N., and Alnawaiseh, M. (2021). Optical coherence tomography angiography and cardiovascular diseases. An overview of the current knowledge. Ophthalmol. Ophthalmol. 118, 1119–1127. doi:10.1007/s00347-021-01336-1
Lee, H., Lee, M., Chung, H., and Kim, H. C. (2018). Quantification of retinal vessel tortuosity in diabetic retinopathy using optical coherence tomography angiography. Retina 38, 976–985. doi:10.1097/IAE.0000000000001618
Li, Y. K., Fung, N. S., Chan, J. C. H., Choy, B. N. K., Chow, L. L. W., Shih, K. C., et al. (2023). Octa biomarkers in adults aged 50 and above: a prospective and cross-sectional community-based study. BMC Ophthalmol. 23, 71. doi:10.1186/s12886-023-02815-6
Liefers, B., Taylor, P., Alsaedi, A., Bailey, C., Balaskas, K., Dhingra, N., et al. (2021). Quantification of key retinal features in early and late age-related macular degeneration using deep learning. Am. J. Ophthalmol. 226, 1–12. doi:10.1016/j.ajo.2020.12.034
London, A., Benhar, I., and Schwartz, M. (2013). The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 9, 44–53. doi:10.1038/nrneurol.2012.227
Lu, B., Chao, G., and Xie, L. (2022). Optical coherence tomography angiography in retinitis pigmentosa: a narrative review. Med. (Baltim.). 101, e30068. doi:10.1097/MD.0000000000030068
Meng, Y., Lan, H., Hu, Y., Chen, Z., Ouyang, P., and Luo, J. (2022). Application of improved U-net convolutional neural network for automatic quantification of the foveal avascular zone in diabetic macular ischemia. J. Diabetes Res. 2022, 1–8. doi:10.1155/2022/4612554
Michl, M., Fabianska, M., Seeböck, P., Sadeghipour, A., Haj Najeeb, B., Bogunovic, H., et al. (2022). Automated quantification of macular fluid in retinal diseases and their response to anti-VEGF therapy. Br. J. Ophthalmol. 106, 113–120. doi:10.1136/bjophthalmol-2020-317416
Middel, C. S., Dietrich, N., Hammes, H. P., and Kroll, J. (2023). Analysis of the morphology of retinal vascular cells in zebrafish (Danio rerio). Front. Cell Dev. Biol. 11, 1267232. doi:10.3389/fcell.2023.1267232
Miguel, A., Legeai, J., and Silva, B. (2021). A software for quantification of vessel density in glaucoma: an OCT-Angiography study. J. Fr. Ophtalmol. 44, 376–381. doi:10.1016/j.jfo.2020.06.038
Mihailovic, N., Eter, N., and Alnawaiseh, M. (2019). Foveal avascular zone and OCT angiography. An overview of current knowledge. Ophthalmol. Ophthalmol. 116, 610–616. doi:10.1007/s00347-018-0838-2
Muste, J. C., Iyer, A. I., Kalur, A., Talcott, K. E., and Singh, R. P. (2022). The quantification and impact of persistent retinal fluid compartments on best-corrected visual acuity of patients with retinal vein occlusion. Ophthalmic Surg. Lasers Imaging Retina 53, 139–147. doi:10.3928/23258160-20220215-03
Nardini, J. T., Pugh, C. W. J., and Byrne, H. M. (2023). Statistical and topological summaries aid disease detection for segmented retinal vascular images. Microcirculation 30, e12799. doi:10.1111/micc.12799
Nascimento E Silva, R., Chiou, C. A., Wang, M., Devlin, J., Li, D., Lovelace, S., et al. (2021). Quantification of the peripapillary microvasculature in eyes with glaucomatous paracentral visual field loss. Ophthalmol. Glaucoma 4, 286–294. doi:10.1016/j.ogla.2020.10.009
Nguyen, U. T., Bhuiyan, A., Park, L. A., Kawasaki, R., Wong, T. Y., Wang, J. J., et al. (2013). Automated quantification of retinal arteriovenous nicking from colour fundus images. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 5865–5868. doi:10.1109/embc.2013.6610886
Nunez do Rio, J. M., Sen, P., Rasheed, R., Bagchi, A., Nicholson, L., Dubis, A. M., et al. (2020). Deep learning-based segmentation and quantification of retinal capillary non-perfusion on ultra-wide-field retinal fluorescein angiography. J. Clin. Med. 9, 2537. doi:10.3390/jcm9082537
Olcay, K., Cakir, A., Sagdic, S. K., Duzgun, E., and Yildirim, Y. (2015). Bilateral iris atrophy after the femtosecond assisted laser in situ keratomileusis surgery. Case Rep. Ophthalmol. Med. 2015, 1–3. doi:10.1155/2015/127806
Ouederni, M., Sassi, H., Nefaa, F., Kharroubi, A., Kellil, N., and Cheour, M. (2019). Anatomo-functional study in branch retinal vein occlusion using swept source optical coherence tomography angiography. J. Fr. Ophtalmol. 42, 255–261. doi:10.1016/j.jfo.2018.09.010
Pauleikhoff, D., Gunnemann, F., Book, M., and Rothaus, K. (2019). Progression of vascular changes in macular telangiectasia type 2: comparison between SD-OCT and OCT angiography. Graefes Arch. Clin. Exp. Ophthalmol. 257, 1381–1392. doi:10.1007/s00417-019-04323-0
Pauleikhoff, D., Pauleikhoff, L., and Chew, E. Y. (2022). Imaging endpoints for clinical trials in MacTel type 2. Eye (Lond.). 36, 284–293. doi:10.1038/s41433-021-01723-7
Pournaras, C. J., and Riva, C. E. (2013). Retinal blood flow evaluation. Ophthalmologica 229, 61–74. doi:10.1159/000338186
Ramos, L., Novo, J., Rouco, J., Romeo, S., Álvarez, M. D., and Ortega, M. (2019). Computational assessment of the retinal vascular tortuosity integrating domain-related information. Sci. Rep. 9, 19940. doi:10.1038/s41598-019-56507-7
Ratra, D., Dalan, D., Prakash, N., Kaviarasan, K., Thanikachalam, S., Das, U. N., et al. (2021). Quantitative analysis of retinal microvascular changes in prediabetic and diabetic patients. Indian J. Ophthalmol. 69, 3226–3234. doi:10.4103/ijo.IJO_1254_21
Reiter, G. S., and Schmidt-Erfurth, U. (2022). Quantitative assessment of retinal fluid in neovascular age-related macular degeneration under anti-VEGF therapy. Ther. Adv. Ophthalmol. 14, 251584142210833. doi:10.1177/25158414221083363
Saraf, S. S., Tyring, A. J., Chen, C. L., Le, T. P., Kalina, R. E., Wang, R. K., et al. (2019). Familial retinal arteriolar tortuosity and quantification of vascular tortuosity using swept-source optical coherence tomography angiography. Am. J. Ophthalmol. Case Rep. 14, 74–78. doi:10.1016/j.ajoc.2019.03.001
Sariyildiz, C., Çiloğlu, E., and Yetkin, E. (2023). Quantification of macular perfusion following panretinal photocoagulation for diabetic retinopathy: an optical coherence tomography angiography study. Photodiagnosis Photodyn.Ther. 41, 103233. doi:10.1016/j.pdpdt.2022.103233
Schmidt-Erfurth, U., Vogl, W. D., Jampol, L. M., and Bogunović, H. (2020). Application of automated quantification of fluid volumes to anti-VEGF therapy of neovascular age-related macular degeneration. Ophthalmology 127, 1211–1219. doi:10.1016/j.ophtha.2020.03.010
Shokr, H., Dias, I. H., and Gherghel, D. (2021). Oxysterols and retinal microvascular dysfunction as early risk markers for cardiovascular disease in normal, ageing individuals. Ageing Individ. Antioxidants (Basel) 10, 1756. doi:10.3390/antiox10111756
Stahl, A. (2020). The diagnosis and treatment of age-related macular degeneration. Dtsch. Ärztebl. Int. 117, 513–520. doi:10.3238/arztebl.2020.0513
Tan, W., Yao, X., Le, T. T., Tan, B., Schmetterer, L., and Chua, J. (2022). The New Era of retinal imaging in hypertensive patients. Asia Pac. J. Ophthalmol. (Phila). 11, 149–159. doi:10.1097/APO.0000000000000509
Tang, Z., Zhang, X., Yang, G., Zhang, G., Gong, Y., Zhao, K., et al. (2021). Automated segmentation of retinal nonperfusion area in fluorescein angiography in retinal vein occlusion using convolutional neural networks. Med. Phys. 48, 648–658. doi:10.1002/mp.14640
Ting, D. S. J., Foo, V. H., Yang, L. W. Y., Sia, J. T., Ang, M., Lin, H., et al. (2021). Artificial intelligence for anterior segment diseases: emerging applications in ophthalmology. Br. J. Ophthalmol. 105, 158–168. doi:10.1136/bjophthalmol-2019-315651
Ting, D. S. W., Pasquale, L. R., Peng, L., Campbell, J. P., Lee, A. Y., Raman, R., et al. (2019). Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 103, 167–175. doi:10.1136/bjophthalmol-2018-313173
Türkcü, F. M., Şahin, A., Karaalp, Ü., Çınar, Y., Şahin, M., Özkurt, Z. G., et al. (2020). Automated quantification of foveal avascular zone and vascular density in Behçet’s disease. Ir. J. Med. Sci. 189, 349–354. doi:10.1007/s11845-019-02051-2
Van Melkebeke, L., Barbosa-Breda, J., Huygens, M., and Stalmans, I. (2018). Optical coherence tomography angiography in glaucoma: a review. Ophthalmic Res. 60, 139–151. doi:10.1159/000488495
Wang, X., Chen, J., Kong, X., and Sun, X. (2021). Quantification of retinal microvascular density using optic coherence tomography angiography in primary angle closure disease. Curr. Eye Res. 46, 1018–1024. doi:10.1080/02713683.2020.1849728
Wang, X. N., Zhao, Q., Li, D. J., Wang, Z. Y., Chen, W., Li, Y. F., et al. (2019). Quantitative evaluation of primary retinitis pigmentosa patients using colour Doppler flow imaging and optical coherence tomography angiography. Acta Ophthalmol. 97, e993–e997. doi:10.1111/aos.14047
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi:10.1016/S1474-4422(13)70124-8
Wigdahl, J., Guimarães, P., Leontidis, G., Triantafyllou, A., and Ruggeri, A. (2015). Automatic Gunn and Salus sign quantification in retinal images. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 5251–5254. doi:10.1109/embc.2015.7319576
Wu, H., Zhang, X., Geng, X., Dong, J., and Zhou, G. (2014). Computer aided quantification for retinal lesions in patients with moderate and severe non-proliferative diabetic retinopathy: a retrospective cohort study. BMC Ophthalmol. 14, 126. doi:10.1186/1471-2415-14-126
Wu, S., Wu, S., Feng, H., Hu, Z., Xie, Y., Su, Y., et al. (2021). An optimized segmentation and quantification approach in microvascular imaging for octa-based neovascular regression monitoring. BMC Med. Imaging. 21, 13. doi:10.1186/s12880-021-00546-y
Xu, B., Chen, J., Zhang, S., Shen, S., Lan, X., Chen, Z., et al. (2021). Association between the severity of diabetic retinopathy and optical coherence tomography angiography metrics. Front. Endocrinol. (Lausanne). 12, 777552. doi:10.3389/fendo.2021.777552
Xu, X., Chen, C., Ding, W., Yang, P., Lu, H., Xu, F., et al. (2019). Automated quantification of superficial retinal capillaries and large vessels for diabetic retinopathy on optical coherence tomographic angiography. J. Biophot. 12, e201900103. doi:10.1002/jbio.201900103
Yao, T., He, Y., Huang, L., Chen, J., Zhang, Z., Yang, W., et al. (2022). Quantitative vessel density analysis of macular and peripapillary areas by optical coherence tomography angiography in adults with primary nephrotic syndrome. Microvasc. Res. 144, 104407. doi:10.1016/j.mvr.2022.104407
Yarmohammadi, A., Zangwill, L. M., Diniz-Filho, A., Saunders, L. J., Suh, M. H., Wu, Z., et al. (2017). Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology 124, 709–719. doi:10.1016/j.ophtha.2017.01.004
Yeung, L., Lee, Y. C., Lin, Y. T., Lee, T. W., and Lai, C. C. (2021). Macular ischemia quantification using deep-learning denoised optical coherence tomography angiography in branch retinal vein occlusion. Transl. Vis. Sci. Technol. 10, 23. doi:10.1167/tvst.10.7.23
Yoon, S. P., Thompson, A. C., Polascik, B. W., Calixte, C., Burke, J. R., Petrella, J. R., et al. (2019). Correlation of octa and volumetric MRI in mild cognitive impairment and Alzheimer’s disease. Ophthalmic Surg. Lasers Imaging Retina 50, 709–718. doi:10.3928/23258160-20191031-06
Yu, G., Aaberg, M. T., Patel, T. P., Iyengar, R. S., Powell, C., Tran, A., et al. (2020). Quantification of retinal nonperfusion and neovascularization with Ultrawidefield fluorescein angiography in patients with diabetes and associated characteristics of advanced disease. JAMA Ophthalmol. 138, 680–688. doi:10.1001/jamaophthalmol.2020.1257
Yucel Gencoglu, A., Ağın, A., Un, Y., and Ozturk, Y. (2023). Quantification of retinal vein and artery trajectories using second-order polynomial equation in eyes with vitreomacular traction. Photodiagnosis Photodyn. Ther. 42, 103616. doi:10.1016/j.pdpdt.2023.103616
Zhou, D. B., Castanos, M. V., Pinhas, A., Gillette, P., Migacz, J. V., Rosen, R. B., et al. (2021). Quantification of intermittent retinal capillary perfusion in sickle cell disease. Biomed. Opt. Express. 12, 2825–2840. doi:10.1364/BOE.418874
Zhou, Y., Wagner, S. K., Chia, M. A., Zhao, A., Woodward-Court, P., Xu, M., et al. (2022). AutoMorph: automated retinal vascular morphology quantification via a deep learning pipeline. Transl. Vis. Sci. Technol. 11, 12. doi:10.1167/tvst.11.7.12
Keywords: retinal vasculature, artificial intelligence, ocular diseases, systemic diseases, retinal vessel quantification
Citation: Chen N, Zhu Z, Yang W and Wang Q (2024) Progress in clinical research and applications of retinal vessel quantification technology based on fundus imaging. Front. Bioeng. Biotechnol. 12:1329263. doi: 10.3389/fbioe.2024.1329263
Received: 28 October 2023; Accepted: 12 February 2024;
Published: 22 February 2024.
Edited by:
Laura Cercenelli, University of Bologna, ItalyReviewed by:
Shujun Wang, Hong Kong Polytechnic University, Hong Kong SAR, ChinaJiong Zhang, University of Southern California, Los Angeles, United States
Copyright © 2024 Chen, Zhu, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhentao Zhu, anNoYXl5enp0QDE2My5jb20=; Weihua Yang, YmVuYmVuMDYwNkAxMzkuY29t; Qiang Wang, d2FuZ3FpYW5nMTk4MUB3bXUuZWR1LmNu
 Naimei Chen
Naimei Chen Zhentao Zhu
Zhentao Zhu Weihua Yang
Weihua Yang Qiang Wang
Qiang Wang