- Hunan Clinical Research Center of Oral Major Diseases and Oral Health, Xiangya Stomatological Hospital, Xiangya School of Stomatology, Central South University, Changsha, Hunan, China
The investigation of bone defect repair has been a significant focus in clinical research. The gradual progress and utilization of different scaffolds for bone repair have been facilitated by advancements in material science and tissue engineering. In recent times, the attainment of precise regulation and targeted drug release has emerged as a crucial concern in bone tissue engineering. As a result, we present a comprehensive review of recent developments in responsive scaffolds pertaining to the field of bone defect repair. The objective of this review is to provide a comprehensive summary and forecast of prospects, thereby contributing novel insights to the field of bone defect repair.
1 Introduction
Bone tissue defects present a significant health risk to individuals (Buza and Einhorn, 2016). Approximately four million surgical procedures are performed annually to address bone loss, utilizing grafts and/or substitutes, thereby establishing it as the second most commonly transplanted tissue worldwide (Greenwald et al., 2001). Although bone grafting serves as the preferred method for repairing extensive defects resulting from congenital anomalies, tumor removal, and traumatic fractures, it is accompanied by challenges such as limited availability, morbidity at the donor site, and inflammation, among others (Brydone et al., 2010; Tang et al., 2016).
The primary objective of bone tissue engineering is to develop bone-graft substitutes that can overcome the limitations associated with natural bone grafts (Shrivats et al., 2014). Scaffolds, which serve as a potential approach for treating bone defects, are currently being explored. The selection of appropriate biomaterials is of utmost importance in the fabrication of these scaffolds, and various techniques and materials are under investigation. The ideal bone graft substitutes should possess biocompatibility, biodegradability, and ease of production. Additionally, they should facilitate cell infiltration, stimulate bone growth, and provide biomechanical support during the regeneration of bone by osteoblasts (Bose et al., 2012).
Researchers have employed diverse scaffold materials to facilitate endogenous regeneration. Conventional scaffold materials comprise organic polymers such as collagen and hyaluronic acid, artificial polymers like polylactic acid, and biologically active inorganic materials like calcium phosphate, which enhance bone regeneration (Khan et al., 2008; Collins et al., 2021). However, conventional scaffolds exhibit a deficiency in controlled release capabilities, rendering them incapable of effectively regulating chronic inflammation as required (Ye et al., 2022). As a result, scholars have redirected their focus towards the advancement of responsive scaffolds.
Responsive scaffolds are considered to be groundbreaking in the field of bone repair. These scaffolds are comprised of materials that possess the capability to be activated and respond to various external stimuli, such as light, magnetism, and pH, or internal stimuli, including cytokines, enzymes, and biological signals. Responsive scaffolds demonstrate the ability to react to triggers originating from external regulatory equipment and internal microenvironment alterations, thereby enabling them to deliver drugs in a timely manner in response to a diverse array of circumstances (Wei et al., 2022). Moreover, they exhibit the capacity to react to both external and internal triggers, enabling them to deliver drugs as needed in response to a wide range of situations.
The occurrence of bone loss can lead to injury in both hard and soft tissues, with the microenvironment of diseased tissue exhibiting notable distinctions from that of healthy tissue. These disparities are believed to exploit various stimuli, including lower pH levels, elevated concentrations of reactive oxygen species (ROS), and heightened enzyme and osteoclast activities, thereby promoting bone resorption. By utilizing a responsive scaffold, it becomes possible to activate and target these specific stimuli, facilitating the precise delivery of drugs to modify the microenvironment and effectively repair the injury. For instance, researchers have developed a modified-scaffold composed of an electrospun asymmetric double-layer membrane made of polycaprolactone and collagen (PCL/Col) to address the low pH environment in bone defect sites. This composite scaffold exhibited the release of approximately 93% of Zn2+ ions from the PCL/Col/ZIF-8 membrane within 12 h under acidic conditions (pH 5.5). The pH-sensitive structure of the scaffold provides a favorable environment for the proliferation of osteoblasts, thereby presenting a promising approach for bone regeneration (Xue et al., 2021). Responsive scaffolds have demonstrated potential and approval in the treatment of bone injuries.
This review primarily examines the recent advancements in responsive biomaterials and scaffolds utilized in bone tissue engineering. It specifically delves into their application, material selection, scaffold design, and their efficacy in addressing bone defects. Furthermore, the review explores the current limitations and potential prospects for bone defect restoration, drawing upon substantial evidence that substantiates the favorable outcomes achieved through the implementation of functionalized responsive scaffolds.
2 The categories of responsive scaffolds
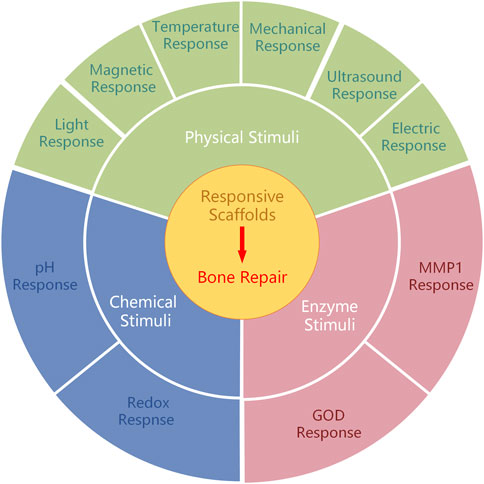
This study primarily encompasses three primary categorizations of stimulus-responsive scaffolds based on the source of stimuli: physical stimuli (e.g., light, temperature change, electric field, magnet, and ultrasound), chemical stimuli (e.g., pH level and ROS), and enzyme stimuli (Figure 1) (Table 1).
2.1 Physical stimuli
Physical-responsive scaffolds are predominantly comprised of materials that are sensitive to physical stimuli. These materials possess a structure that can be reconfigured when exposed to various factors, including light, magnetism, temperature, ultrasonic waves, and magnetic fields. Consequently, these alterations in structure facilitate the delivery of drugs. For instance, temperature-responsive scaffolds exhibit stability in healthy tissue, but undergo degradation in diseased tissue. In the initial phases of bone defects, inflammation induces a localized increase in temperature. This change in temperature can serve as an endogenous stimulus for the scaffold to respond and subsequently release the drug (Zhang et al., 2013; Karimi et al., 2016b).
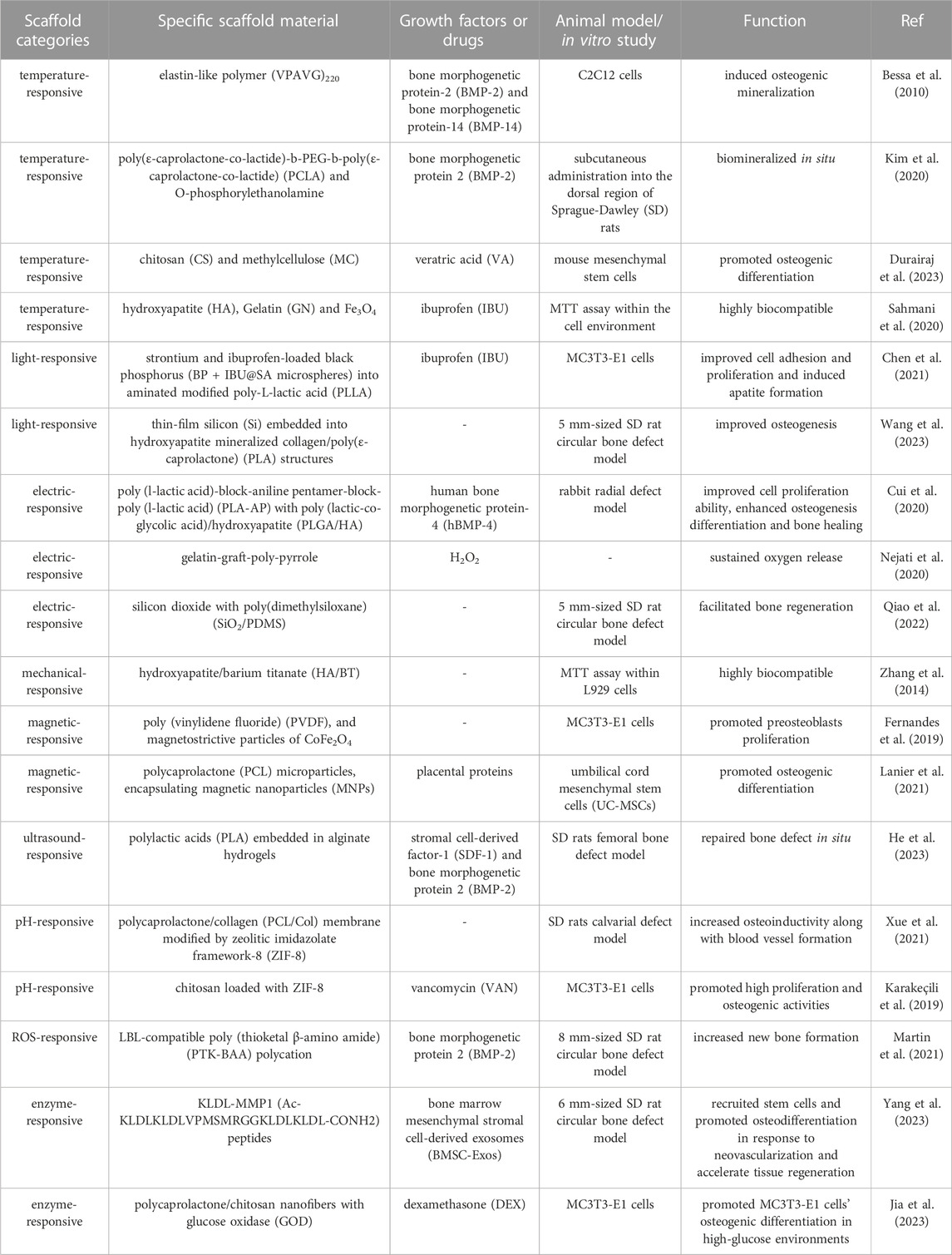
Under specific circumstances, elastin-like polypeptides (ELPs) exhibit a lower critical solution temperature (LCST) in contrast to other synthetic polymers (Tamburro et al., 2005). Upon surpassing this transition temperature, ELPs undergo a first-order phase transition, resulting in the formation of a peptide and water-rich phase (Krishna et al., 2012; Zhao et al., 2016). This distinctive characteristic has sparked considerable enthusiasm in the advancement of biomaterials capable of reacting to external stimuli. In a recent study, researchers have successfully synthesized elastin-like self-assembly nanoparticles with thermos-responsive characteristics. These nanoparticles were employed for the controlled release of bone morphogenetic protein-2 (BMP-2) and bone morphogenetic protein-14 (BMP-14), exploiting the reverse temperature transition of bio-generated polymer(VPAVG) 220 (Bessa et al., 2010). This distinctive property can be harnessed to mitigate inflammation and facilitate bone regeneration under specific conditions. In the field of bone tissue engineering, a wide range of physical-responsive scaffolds are frequently utilized to modulate drug delivery.
2.1.1 Temperature response
Temperature-responsive scaffolds can undergo structural reconstruction and release drug payloads when the desired temperature is achieved, as they are triggered by changes in the surrounding environment. Thermo-responsive polymers, employed in the fabrication of these scaffolds, may experience phase transitions above the LCST (Bordat et al., 2019) or expedite degradation when exposed to elevated temperatures. For instance, certain thermo-responsive polymers undergo a transition from a liquid state to a stable viscoelastic gel, while certain thermo-sensitive polymers exhibit accelerated degradation at elevated temperatures, thereby facilitating the controlled release of drugs.
In a recent study, a cohort of alginate bioconjugates comprising micrografted poly(ε-caprolactone-co-lactide)-b-PEG-b-poly(ε-caprolactone-co-lactide) (PCLA) and o-phosphorylethanolamine were synthesized with the aim of facilitating bone regeneration (Kim et al., 2020). These bioconjugated salts have the ability to undergo a conversion into durable viscoelastic gels when administered in vivo and subjected to physiological temperature, surpassing the LCST threshold. This conversion enhances the mechanical characteristics and fosters bone regeneration, thereby suggesting their potential utility in promoting bone formation. Methylcellulose is a cellulose polysaccharide known for its biocompatibility, biodegradability, and hydrophilicity. It demonstrates gelation properties, resulting in gel formation at specific temperatures (Lioubavina-Hack et al., 2005; Kim et al., 2018). Composite hydrogels, comprising chitosan and methylcellulose, encapsulate veratric acid and exhibit desirable biocompatibility. These hydrogels gelatinize at 37°C, making them suitable for use as a restorative agent to enhance osteoblast differentiation (Durairaj et al., 2023).
Endogenous temperature stimulation is elicited by fluctuations in temperature conditions at the site of the lesion. The lesion tissue undergoes pathological deformation and hyperthermia, which stem from trauma and tumors, thereby triggering the release of inflammatory factors and evaluation of the local environmental temperature. Consequently, the scaffold structure in pathological sites undergoes alterations when the temperature is elevated. During the initial phase of bone defect, the localized temperature elevation can serve as a stimulus for scaffold response and/or drug release (Zhang et al., 2013; Karimi et al., 2016b).
Nanocarriers engineered for thermal responsiveness have the potential to maintain stability at the physiological temperature of the human body. Upon exposure to external heat or assessment of the local environmental temperature, these carriers can efficiently release therapeutic agents either promptly upon heating or in a controlled manner at the site of disease. In a recent study, Fe3O4 nanoparticles were employed by researchers within a magnetic field to induce heat generation and eradicate infected cells (Abdellahi et al., 2018a). The magnetite nanoparticles (MNPs) possess the ability to elevate temperature upon exposure to an alternating magnetic field, thus facilitating the degradation of the drug carrier for drug release (Abdellahi et al., 2018b; Sahmani et al., 2018). In this particular scenario, hydroxyapatite (HA) and gelatin (GN) were integrated with MNPs to fabricate bio-nanocomposite scaffolds, which subsequently underwent degradation under magnetothermal conditions. The results indicate that the prepared scaffold exhibits promising potential for utilization in bone tissue engineering for both biological and thermal applications (Sahmani et al., 2020).
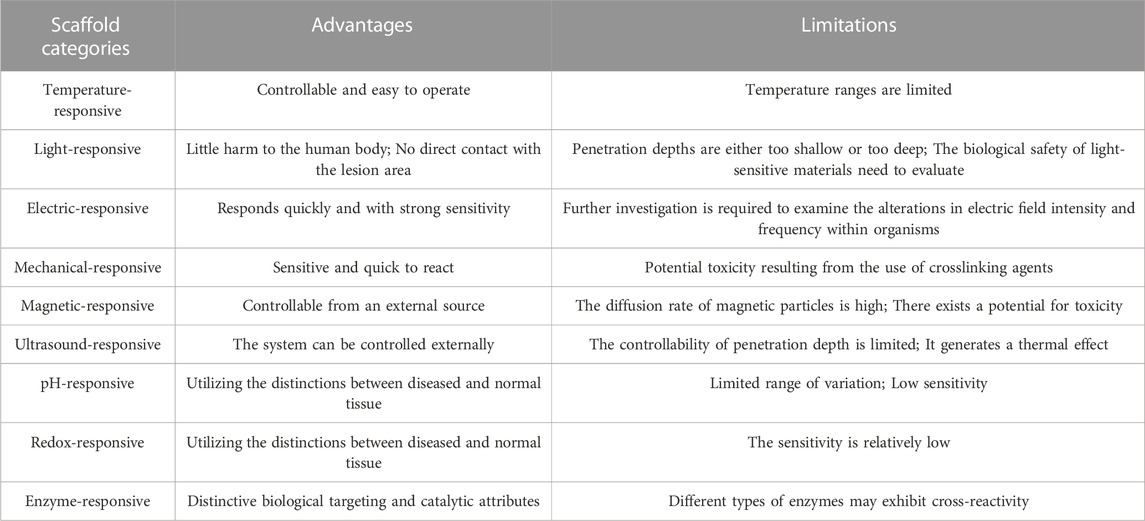
The achievement of thermal specificity in temperature-responsive systems poses a significant obstacle due to the restricted variability observed in pathological tissues within living organisms. Consequently, future research endeavors should prioritize the development of scaffold materials that are more responsive to lower temperatures, possess enhanced stability in normal tissues, and ensure greater safety.
2.1.2 Light response
Various light-responsive materials exhibit different responses to various wavelengths of light, thereby facilitating the identification of suitable materials for diverse clinical needs. Upon exposure to light, light-responsive scaffolds undergo changes in their physical properties, thereby enabling efficient drug delivery. This responsiveness of scaffolds is primarily attributed to the degradation of materials containing light-sensitive components or the modification of light-sensitive molecules. Consequently, when scaffolds are exposed to light, the drug bound or encapsulated within them is released (Mayer and Heckel, 2006; Chen and Zhao, 2018).
Black phosphorus (BP), a novel nanomaterial characterized by its two-dimensional framework, exhibits remarkable biosafety, inherent biocompatibility, and photosensitivity (Pandey et al., 2020). In a recent study, a multifunctional nanofiber scaffold was developed by incorporating ibuprofen black phosphorus (BP + IBU@SA microspheres) and sodium alginate microspheres onto aminated poly-L-lactic acid (PLLA) nanofibers. This scaffold demonstrates exceptional near-infrared light-responsive release capabilities and anti-inflammatory properties. BP was employed to induce the destruction of polymeric shells through the utilization of near-infrared (NIR)-mediated photothermal performance, thereby achieving controlled drug release. By subjecting the scaffolds to NIR light, the adverse effects of rapid drug release can be mitigated, while maintaining the drug concentration at an optimal level to meet the specific requirements of bone repair. The conducted investigations have demonstrated that the incorporation of functionalized scaffolds enhances cell adhesion, proliferation, and apatite formation, rendering it a viable and promising approach for bone tissue engineering (Chen et al., 2021).
Silicon (Si) is widely employed as a semiconductor material in bio-implantation devices (Kang et al., 2016; Parameswaran et al., 2018). When subjected to near-infrared illumination, Si structures produce electrical signals that depolarize cell potentials and trigger intracellular calcium activation. Consequently, these optoelectronic signals play a role in directing hBMSCs towards osteogenic differentiation (Wang et al., 2023). Recently, a three-dimensional (3D) biomimetic scaffold utilizing thin-film Si microstructures has been developed. Through the utilization of NIR light, researchers have discovered that the Si film facilitates the attachment and growth of cells. The Si-based hybrid scaffold offers a 3D hierarchical structure that effectively governs cell growth and regulates cell behavior via light-responsive electrical signals. These silicon structures are remotely manipulated by infrared radiation to regulate the depolarization of stem cell membranes, resulting in heightened Ca2+ activities for hBMSCs, as well as improved potential and intracellular calcium dynamics. Consequently, this process promotes both cell proliferation and differentiation. The utilization of silicone scaffolds resulted in enhanced bone formation when subjected to light stimulation (Wang et al., 2023) (Figure 2).
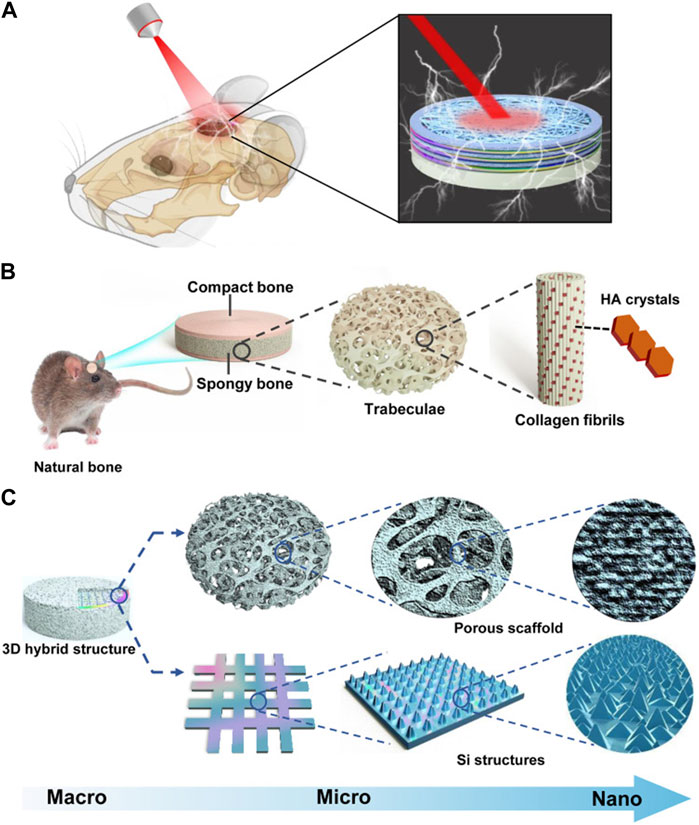
FIGURE 2. Bioregenerative 3D optoelectronic scaffold with Si nanostructures for bone regeneration. (A) An illustration of an implantable scaffold in concept. (B) Native bone hierarchical structure. (C) Structural design of the 3D hybrid scaffold. Reproduced from (Wang et al., 2023) with permission. Copyright 2023 AAAS.
However, the advancement of light-responsive systems continues to encounter various obstacles. In numerous applications, the ability of radiation below 650 nm to penetrate tissue beyond a depth of 1 cm is limited, while NIR light within the range of 650–900 nm (as water absorbs wavelengths longer than 900 nm) can penetrate up to 10 cm. However, these penetration depths are not considered clinically significant due to being either too shallow or too deep in vitro (Weissleder, 2001; Fomina et al., 2012). Additionally, further quantitative investigation is necessary to evaluate the biological safety of light-sensitive materials and ascertain the optimal duration and intensity of light exposure.
2.1.3 Electric response
Electrical stimulation (EStim) has undergone extensive research and has proven to be an effective intervention in medical settings for the purpose of enhancing bone healing (Bhavsar et al., 2020), as it exerts influence on the migration (Yuan et al., 2014), proliferation (Ercan and Webster, 2008), differentiation (Eischen-Loges et al., 2018) of bone cells. Presently, the integration of electrostimulation therapy with electric-responsive stents is regarded as a compelling approach in clinical practice (Palza et al., 2019; Vaca-González et al., 2019).
Endogenous electrical currents exert a substantial influence on diverse physiological processes in the human body. These naturally occurring electrical fields possess the capacity to induce either depolarization or hyperpolarization of the membrane potential in living tissues, thereby eliciting the activation of signaling factors that facilitate cell proliferation and migration, including those of bone cells (Funk, 2015). Exogenous electrical stimulation including alternating current (AC), which reverses direction periodically and direct current (DC) which flows in one direction both have effects on bone tissue and scaffold (Chen et al., 2013). Electric-responsive scaffolds possess inherent bioactivity and can facilitate tissue formation with or without the need for external electrical stimulation. These scaffolds are capable of responding to electrical fields in living tissues, thereby expediting drug release. Consequently, electric-responsive scaffolds have been employed in various studies within the field of bone tissue engineering.
Conductive polymers, namely polyaniline, poly-pyrrole, polythiophene, and their derivatives (Cui et al., 2012; Xie et al., 2015) have been found to augment cellular activities, including cell adhesion, proliferation, differentiation, migration, and protein secretion, at the interface between the polymer and tissue, regardless of electrical stimulation (Hardy et al., 2013). These polymers demonstrate favorable biocompatibility in both in vivo and in vitro settings, while also exhibiting high conductivity under physiological conditions. Polyaniline (PA) is a conductive polymer that exhibits the ability to undergo transference when subjected to pulsed EStim (Wang et al., 2017). Additionally, polylactide (PLA) is a polymer known for its favorable biodegradability (Huang et al., 2007). In light of these aforementioned attributes, a novel electric-responsive scaffold has been developed, comprising a main chain composed of poly (l-lactic acid)-block-aniline pentamer-block-poly (l-lactic acid) (PLA-AP) and a triblock copolymer of poly (lactic-co-glycolic acid)/hydroxyapatite (PLGA/HA). The composite scaffold (PLGA/HA/PLA-AP/phBMP-4) underwent degradation upon electrical stimulation (Cyclic voltammograms (CV), scanning rate of 100 mVs−1) to control the release of phBMP-4 and regulate gene expression of doxycycline (Dox). In an experimental model involving rabbit radius defects, the electric-responsive scaffold demonstrated enhanced cell proliferation, improved osteogenic differentiation, and influenced the process of bone healing (Cui et al., 2020).
Poly-pyrrole has garnered significant attention in academic research due to its exceptional conductivity. In this study, H2O2-loaded polylactic acid microparticles were manufactured, and gelatin-graft-poly-pyrrole with varying pyrrole contents and periodate-oxidized pectin were synthesized to create an injectable conductive hydrogel/microparticle scaffold. This scaffold demonstrated the ability to sustain oxygen release for a duration of 14 days. The conductivity of the scaffold can enhance the bone healing process when responding to electrical stimuli, making it a promising candidate for bone tissue engineering applications (Nejati et al., 2020).
Electret materials, known for their enduring polarization properties (Zhang et al., 2023), have the ability to generate intrinsic electrical stimulation when subjected to an external electric field (Guo et al., 2022; Lin et al., 2022; Qiao et al., 2022). In tissue engineering, electret materials commonly employed include inorganic compounds like silicon dioxide (SiO2) (Qiao et al., 2022), zinc oxide (ZnO) (Zhu et al., 2018), HA (Nakamura et al., 2009), as well as biopolymers such as proteins (e.g., collagen), polysaccharides (e.g., chitin), and polynucleotides (e.g., DNA), also demonstrate the phenomenon of the electron effect (Zheng et al., 2020). SiO2, a material with electret properties, exhibits favorable biocompatibility and charge retention ability (Li et al., 2015). In order to enhance its electroactive properties, researchers developed a composite membrane by integrating silicon dioxide with poly(dimethylsiloxane) (SiO2/PDMS). The composite membranes underwent polarization through the application of an external electric field, resulting in the retention of residual charge for a duration of up to 6 weeks. The electreted SiO2/PDMS membranes demonstrated a favorable electrical microenvironment, leading to enhanced osteogenic differentiation of BMSCs in vitro and accelerated bone defect healing in vivo (Qiao et al., 2022) (Figure 3).
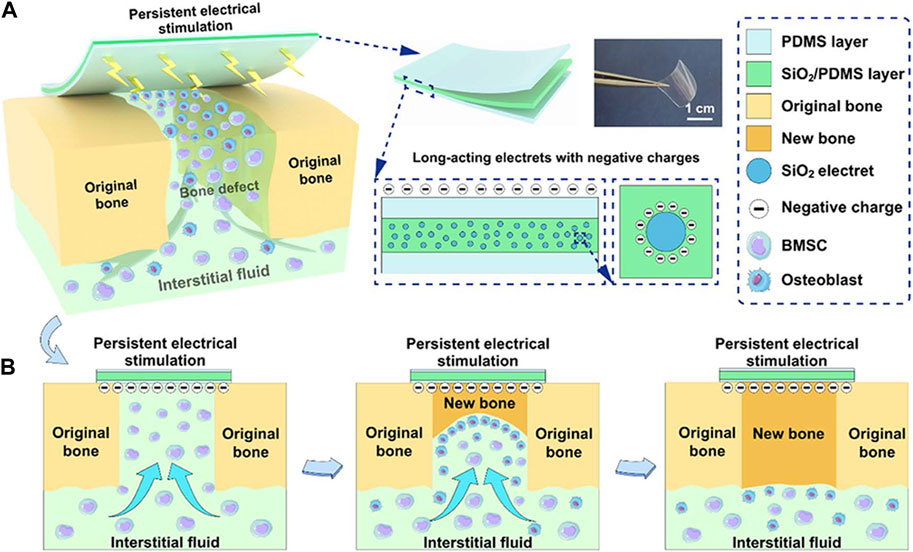
FIGURE 3. The schemes of electreted sandwich membranes. (A) Illustration of persistent electrical stimulation provided by electreted sandwich-like SiO2/PDMS composite membranes. (B) Implanted composite membranes act as native periosteum covering the bone defect region to enhance well-integrated bone formation and regeneration. Reproduced from (Qiao et al., 2022) with permission. Copyright 2022 American Chemical Society.
The potential application of electrical stimuli-responsive scaffolds in bone repair shows promise. However, the controllability of electric field changes in organisms remains uncertain, necessitating further investigation into the application of telephony stents.
2.1.4 Mechanical response
Mechanical-responsive materials possess the ability to promptly alter their physiochemical attributes when subjected to mechanical force or deformation (Shabani and Bodaghi, 2023). Piezoelectric biomaterials are a class of intelligent materials capable of producing electrical activity in response to mechanical stimulation, independent of the need for external electrical devices (Zheng et al., 2020). Piezoelectricity arises from the inherent crystal or chemical structure of materials, leading to the development of a net dipole or charge during mechanical deformation. Additionally, piezoelectric materials possess the ability to modulate cellular behavior by generating surface charges in response to deformation caused by cellular interaction. This characteristic offers novel avenues for biomechanical simulation, bone regeneration, and bone defect repair (Tandon et al., 2018; Khare et al., 2020).
Piezoelectric materials can be classified into various categories including polymers (such as PLLA and poly (vinylidene fluoride) (PVDF)), ceramics [such as HA and barium titanate (BT)], natural materials like collagen, and composite polymers. An example of such composites is the aligned porous BT/HA composites, which have been developed to possess high piezoelectric coefficients owing to their exceptional piezoelectric property. These composites serve as a charge supplier, thereby stimulating the bone healing process, and exhibit similar charge supply properties and stress-generated potentials as natural collagen bone (Baxter et al., 2010; Zhang et al., 2014).
Collagen, a naturally occurring protein and integral component of bone, exhibits piezoelectric properties that render it well-suited for tissue engineering applications. Specifically, the piezoelectric nature of collagen within bone induces the generation of a streaming potential when subjected to stress, leading to a decrease in hydraulic permeability and an augmentation in stiffness (Ahn and Grodzinsky, 2009; Ferreira et al., 2012). The suitability of the collagen-HA piezoelectric composite scaffold for cellular growth and bone healing has been demonstrated in previous research (Silva et al., 2001). Nevertheless, this scaffold is subject to certain limitations, including low mechanical stiffness, rapid degradation, and potential toxicity resulting from the use of crosslinking agents.
Despite facing challenges related to material stability, biocompatibility, and the need to balance mechanical properties, the investigation of piezoelectric materials in the realm of bone tissue engineering presents promising opportunities for the treatment of bone defects and the regeneration of bone.
2.1.5 Magnetic response
The utilization of magnetic nanoparticles in bone tissue engineering has gained attention due to their inherent magnetism and the magnetocaloric effect, among other factors. These nanoparticles demonstrate a responsive characteristic towards magnetic fields, including both alternating magnetic field (AMF) that periodically change direction, and constant magnetic field (CMF) that remain in one direction (Goharkhah et al., 2015). Moreover, they possess the potential to augment the osteoinductive, osteoconductive, and angiogenic properties of scaffolds (Dasari et al., 2022).
A magnetic-responsive scaffold comprising of a piezoelectric polymer, PVDF, and magnetostrictive particles of CoFe2O4 has been successfully fabricated, with nylon template structures utilized to facilitate the solvent casting process. The investigation revealed that the PVDF component of the scaffold undergoes crystallization into the electroactive β-phase when subjected to magnetic and/or electromagnetic stimulation (permanent magnets, frequency of 0.3 Hz), thereby enhancing the proliferation of preosteogenic cells. This observed phenomenon can be attributed to the interplay between the magnetic and electromagnetic properties of the magnetic nanoparticles upon stimulation (Fernandes et al., 2019) (Figure 4). The magnetomechanical and magnetoelectric response of the scaffolds is believed to be a valuable resource.
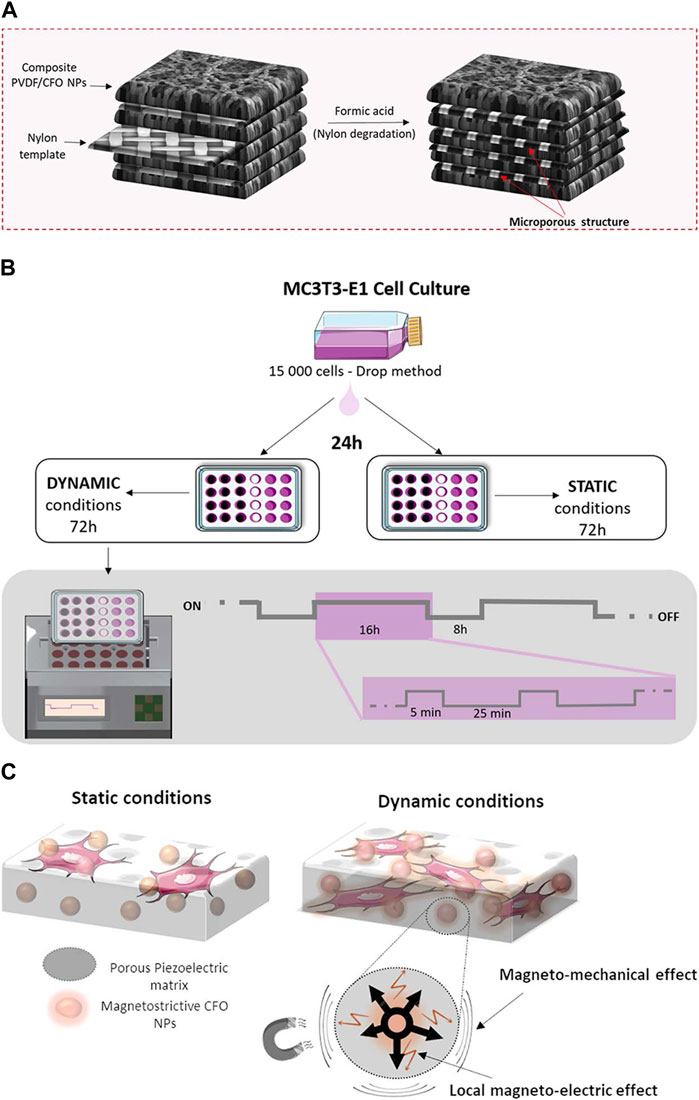
FIGURE 4. The schemes of 3D magnetoactive scaffolds for bone tissue engineering. (A) Schematic representation for the 3D scaffold development. (B) Schematic representation of the cell culture assays and stimulation profile. (C) Schematic representation of magnetomechanical and local magnetoelectrical properties of 3D scaffolds upon the magnetic stimuli. Reproduced from (Fernandes et al., 2019) with permission. Copyright 2019 American Chemical Society.
In a separate investigation, the magnetic-responsive scaffold is comprised of PCL microparticles that enclose MNPs and placental proteins. The MNPs, due to their magnetocaloric effect, induce heating and subsequent melting of the PCL upon exposure to AMF (strength from−1 to 1 T), thereby facilitating the diffusion of proteins from the microparticles to stimulate bone formation. Upon deactivation of the magnetic field, the PCL solidifies once again, potentially enabling repeated administration of drugs in a cyclic manner (Lanier et al., 2021). This present study introduces a magnetic-responsive delivery system designed for localized drug release, with potential applications in bone regeneration.
The magnetic field presents a superior setting for external stimuli-responsiveness in comparison to light and temperature due to its ability to fully penetrate human tissue and initiate release, while also allowing for complete external control. Nevertheless, magnetic nanoparticle could face drawbacks of diffusion out within one or 2 days, thus preventing a continuous release (Veres et al., 2022), during the bone defect treatment and Iron oxide magnetic nanoparticles may mediate ROS generation and have an impact on other cells (Hohnholt et al., 2011; Sruthi et al., 2018).
2.1.6 Ultrasound response
Ultrasound, a mechanical wave with a high frequency (≥20 kHz), possesses the ability to be concentrated and transmitted within a particular medium, thereby finding utility in various clinical domains including in vivo imaging and physical therapy (Wheatley and Cochran, 2013). Furthermore, ultrasound exhibits potential in addressing bone defects as it can influence the biological aspects and drug administration characteristics of materials (Wei et al., 2021; He et al., 2023).
In order to obtain biomimetic scaffold composites (BSCs), researchers fabricated acoustically responsive hydrogel scaffolds (ARSs) that were developed and incorporated with stromal cell derived factor-1 (SDF-1) and BMP-2. The alginate hydrogel scaffold was degraded through pulsed ultrasound (p-US) irradiation, resulting in the exposure of ARSs to BMSCs due to its thermal effect. Subsequently, sinusoidal continuous wave ultrasound (s-US) irradiation was applied to stimulate the intrinsic resonance of ARSs, thereby facilitating the capture of endogenous BMSCs on the scaffolds and significantly enhancing their adhesion and growth for the in situ repair of bone defects (He et al., 2023).
The ultrasound-responsive scaffold facilitates the precise release of drugs and recruitment of cells in a spatiotemporal manner, while minimizing adverse effects through a non-toxic pathway (Pitt et al., 2004; Zardad et al., 2016). Nonetheless, the uncontrolled depth of ultrasound penetration and the potential thermal effect necessitate further investigation. In contrast to ultrasound, shockwave, which is a prevalent mechanical wave, exhibits greater shock amplitude and energy (Smallcomb et al., 2022). It is commonly employed to facilitate the biological healing processes of bones. Although shockwave is seldom reported as a stimulus source for responsive scaffolds, it offers a promising and innovative avenue for the repair of bone defects (Cheng and Wang, 2015).
2.2 Chemical stimuli
Chemically-responsive scaffolds are primarily constructed using materials that demonstrate sensitivity to specific variations in environmental concentration. When exposed to changes in the pH value, ROS concentration, ion concentration, and other conditions, the drug-encapsulated scaffold undergoes stimulation, leading to the rupture of responsive chemical bonds or modification of the functional group structure within the scaffold. Consequently, this process triggers the release of the drug. For example, ROS concentration can be activated by phagocytes (such as granulocytes and macrophages) under inflammatory conditions after trauma, and phenylborate pinanol ester (PBAP), a compound that can be combined on scaffold, break chemical bonds and fracture under high ROS, which lead to the quickly drug releasing on the certain site (Zhang et al., 2017; Yuan et al., 2021). Therefore, the development of chemical-responsive materials holds potential in facilitating targeted drug delivery at the site of injury to promote bone repair. Here are several common types of chemical-responsive scaffolds on bone regeneration.
2.2.1 pH response
pH-responsive scaffolds are specifically engineered to react to alterations in pH levels, which are induced by the release of inflammatory factors from injured tissues. Extensive research has demonstrated that the pH value can decrease to 6.5 within a span of 60 h following the onset of inflammation (Caliceti, 2011). Furthermore, various organelles exhibit distinct pH values, such as lysosomes (4.5–5), endosomes (5.5–6), golgi apparatus (6.4), and cytosol (7.4) (Karimi et al., 2016a). Consequently, it becomes feasible to incorporate pH-responsive chemical groups into scaffold materials, thereby empowering the scaffold to regulate the release of drugs within the affected tissue.
Zeolitic imidazolate framework-8 (ZIF-8) belongs to the class of metal-organic frameworks (MOFs), which are formed through the connection of metal ions or clusters with organic ligands. Its remarkable pH-sensitivity has led to its application as a bone substitute and drug carrier (Zheng et al., 2016). Research has demonstrated that ZIF-8 is capable of releasing Zn2+ ions in acidic environments, thereby displaying a favorable osteogenic impact (Liu et al., 2022). To facilitate the promotion of vascularized bone regeneration, electrospun polycaprolactone/collagen (PCL/Col) membranes were modified with ZIF-8. The ZIF-8 structure experienced collapse and subsequent release of Zn2+ ions at a pH value of 5.5. Within a 12-h timeframe, approximately 93% of Zn2+ ions were discharged from the PCL/Col/ZIF-8 composite membrane under acidic conditions (pH 5.5). Utilizing this pH-responsive scaffold, concurrent restoration of blood vessels and bone was achieved in a rat model with calvarial defects (Xue et al., 2021).
In a separate study, ZIF-8 nanocrystals were employed as a carrier for vancomycin in order to achieve a delivery profile that responds to changes in pH. These nanocrystals were incorporated into chitosan fiber-scaffolds to create a potential substitute for bone tissue, which also possessed antimicrobial properties and facilitated interaction with osteoblast cells. Following a 48-h period at a pH of 5.4, the release of vancomycin reached a plateau at 77%, subsequent to the increased dissolution of ZIF-8 under acidic conditions. This dissolution served to diminish the activity of S. aureus and promote the differentiation of preosteoblasts into osteoblasts (García-González et al., 2018; Karakeçili et al., 2019).
The pH-responsive system presents a captivating approach for drug delivery, leveraging the pH discrepancies observed in various tissues within the living organism. Nevertheless, the exclusive reliance on pH reaction systems may encounter limitations in terms of specificity and sensitivity, given the inconsistent magnitude of pH disparity between the target tissue and healthy tissue.
2.2.2 Redox response
ROS, encompassing highly reactive ions, free radicals or molecular compounds such as superoxide (O2-), hydroxyl radicals (·OH), hypochlorite ion (ClO−), and hydrogen peroxide (H2O2), play a significant role as signaling molecules in the progression of inflammatory disorders. Given their close relationship with bone growth and remodeling, ROS hold particular appeal for augmenting material responsiveness (Martin et al., 2021).
At the site of a bone defect caused by inflammation, polymorphonuclear neutrophils (PMNs) produce an excessive amount of ROS, leading to endothelial dysfunction and tissue damage, which is detrimental to the process of bone repair. In comparison to healthy tissues, inflamed tissues exhibit ROS concentrations that are 10–100 times higher (Liu et al., 2016). Consequently, the development of a scaffold that is responsive to ROS for the purpose of regulating drug release in inflammatory sites and other afflicted tissues represents a promising approach for enhancing bone repair (Mura et al., 2013).
A critically-sized bone defect refers to a clinical situation wherein bone loss or removal occurs as a result of trauma, infection, tumor, or other factors, and is unable to undergo spontaneous healing (Huang et al., 2022). In such circumstances, the defect lacks the ability to self-repair and necessitates external interventions. A recent investigation has documented a study on a polycation that exhibits compatibility with the Layer-by-Layer (LBL) technique and is exclusively degraded by ROS produced by cells. When the concentrations of ROS increase in the surrounding environment, the thioketal-based polymers containing a scaffold structure can be activated and broken down by physiological levels of ROS. Additionally, these polymers enable the controlled release of therapeutic BMP-2 upon oxidation. The findings of this study suggest a direct correlation between ROS-responsive scaffolds and the promotion of bone growth in critically-sized bone defects (Martin et al., 2021).
In the context of pathological tissues, the maintenance of a stable structure by the ROS response system assumes critical importance. Nevertheless, the development of a ROS-responsive system that operates optimally under specific conditions presents a persistent challenge owing to the intricate and heterogeneous in vivo microenvironment. Despite continuous endeavors, there persist unresolved fundamental concerns that impede its attainment of perfection.
2.3 Enzyme stimuli
Enzymes have found application in the realm of nanotechnology, particularly in the development of nano-drug carriers, owing to their distinctive biological targeting and catalytic attributes. In the context of lesion tissue, enzyme levels undergo alterations within the local microenvironment as a consequence of injury and inflammation. By employing this approach, enzymes can be directed towards specific biochemical signals within the area of bone defect, facilitating the regulation of active ingredient release.
Neovascularized bone, for example, expresses high levels of matrix metalloproteinase-1 (MMP1) (Quintero-Fabián et al., 2019). MMP1 can degrade extracellular matrix proteins by cleaving specific amino acid sequences, which can promote the migration of vascular endothelial cells by decomposing the extracellular matrix (Quintero-Fabián et al., 2019). The KLDL-MMP1 (Ac-KLDLKLDLVPMSMRGGKLDLKLDL-CONH2) peptides were synthesized by the researchers, as they can be degraded by MMP1. To develop a microfluidic chip, the researchers utilized an injectable MMP1-sensitive hydrogel microsphere (KGE), which was created by combining self-assembling peptide (KLDL-MMP1), gelatin methacryloyl (GelMA), and bone marrow mesenchymal stromal cell-derived exosomes (BMSC-Exos). The Exo-release material, which is sensitive to enzymes, exhibits a specific response and degradation towards MMP1 originating from neovascularization during the angiogenesis phase subsequent to bone injury. This degradation process facilitates the release of exosomes within scaffolds, thereby facilitating the recruitment of cells for the purpose of bone defect repair (Yang et al., 2023) (Figure 5).
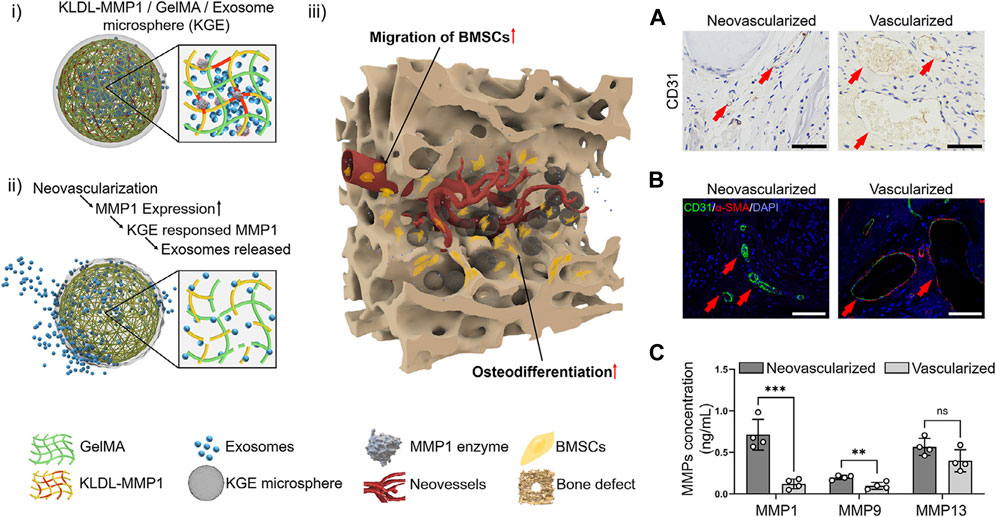
FIGURE 5. Structural design of enzyme-responsive KGE microspheres. (i) KGE microspheres encapsulated Exos before enzymatic hydrolysis. (ii) MMP1 degrades KGE, releasing Exos after injection. (iii) Through neovessels, Exos diffuse into bone defects to promote BMSCs migration and osteodifferentiation. (A–C) The MMPs expression in neovascularized and vascularized bone tissues. (A) CD31 immunohistochemical staining of SD rat skull defect on day 14, scale = 100 μm. (B) CD31/α-SMA Immunofluorescent staining of SD rat skull defect on day 14, scale = 100 μm. (C) Detection of MMPs concentration by ELISA, ∗∗∗: p < 0.001, ∗∗: p < 0.01, ns: p > 0.05. Reproduced from (Jia et al., 2023) with permission. Copyright 2023 Elsevier.
A novel enzyme-responsive scaffold has been developed utilizing glucose oxidase (GOD), an enzyme capable of selectively catalyzing the degradation of glucose. In individuals with diabetes, the process of osteogenesis is frequently hindered due to the presence of elevated glucose levels in the body, which in turn leads to inflammation that inhibits osteogenesis. In a high-glucose environment, glucose can undergo specific catalysis by glucose oxidase, resulting in the production of gluconic acid. Consequently, the researchers incorporated glucose oxidase into the nanofiber scaffold to construct a glucose oxidase responsive scaffold. As the glucose concentration increased, the nanofiber scaffolds gradually expanded, leading to the subsequent release of dexamethasone (DEX), which possesses anti-inflammatory properties and promotes bone formation. Thus, these glucose-sensitive nanofiber scaffolds present a promising therapeutic approach for individuals with diabetes and alveolar bone defects (Jia et al., 2023).
Despite the extensive development of enzymatic reaction systems, they still possess several drawbacks within an academic context. One such limitation pertains to the variability in enzyme expression levels observed among patients, thereby raising concerns regarding the adequacy of enzyme expression within the target population. Additionally, the lack of specificity poses another challenge, as different types of matrix metalloproteinases (MMPs) may exhibit cross-reactivity. For example, all of MMP1, MMP8, and MMP13 can cleave glycine–isoleucine or glycine–leucine bond (Williams and Olsen, 2009). These limitations ultimately impede the progress of enzyme-reactive scaffolds in the field of bone engineering.
3 Conclusion and discussion
Stimuli-responsive scaffolds have emerged as a promising class of intelligent biomaterials in recent years. The advantages and limitations of responsive scaffold categories are shown in Table 2. They possess the ability to detect various physical stimuli, including light, temperature, electric field, magnetic field, and ultrasound, as well as chemical stimuli such as pH and redox response, and enzyme stimuli. Upon encountering specific stimuli, these scaffolds facilitate cell adhesion, migration proliferation, and differentiation. Consequently, they hold great potential for the repair of bone defects. Despite the notable progress made in biomaterial advancements for bone tissue engineering over the past few decades, there remains a considerable amount of work to be done, particularly in three specific areas that warrant further investigation in the future: 1) Responsive scaffolds for bone tissue engineering must possess specific biocompatibility and exhibit targeted responses to particular stimuli. However, achieving a singular response is challenging due to the intricate nature of the human physiological environment and the diverse conditions found at injury sites. Consequently, the development of multi-response scaffolds is gradually gaining momentum as a means to attain optimal therapeutic outcomes; 2) Further in-vivo experiments are necessary to ascertain the interactions between biomaterials and the local microenvironment. The implantation of biomaterials can induce substantial alterations in the microenvironment, thereby exerting a significant influence on osteogenesis. Consequently, it is imperative to continuously monitor the dynamic changes of substances within the body; 3) Furthermore, the challenges pertaining to the precision and specificity of responsive tissue engineering scaffolds persist. The accurate identification of the lesion site and the implementation of targeted responses necessitate additional attention and research. Continued advances in bone tissue engineering are anticipated to facilitate the rapid development of stimuli-responsive scaffolds, offering additional treatment options for the clinical management of bone defects, and ultimately influencing clinical outcomes.
Author contributions
TZ: Writing–original draft, Funding acquisition. HZ: Supervision, Writing–original draft. XC: Writing–review and editing, Supervision. YZ: Funding acquisition, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Natural Science Foundation for Youth of Hunan Province of China (Grant No. 2021JJ40909); Fundamental Research Funds for the Central Universities of Central South University (Grant No. 1053320213327).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdellahi, M., Karamian, E., Najafinezhad, A., Ranjabar, F., Chami, A., and Khandan, A. (2018a). Diopside-magnetite; A novel nanocomposite for hyperthermia applications. J. Mech. Behav. Biomed. Mater 77, 534–538. doi:10.1016/j.jmbbm.2017.10.015
Abdellahi, M., Najfinezhad, A., Saber-Samanadari, S., Khandan, A., and Ghayour, H. (2018b). Zn and Zr co-doped M-type strontium hexaferrite: synthesis, characterization and hyperthermia application. Chin. J. Phys. 56, 331–339. doi:10.1016/j.cjph.2017.11.016
Ahn, A. C., and Grodzinsky, A. J. (2009). Relevance of collagen piezoelectricity to "Wolff's Law": a critical review. Med. Eng. Phys. 31, 733–741. doi:10.1016/j.medengphy.2009.02.006
Baxter, F. R., Bowen, C. R., Turner, I. G., and Dent, A. C. (2010). Electrically active bioceramics: a review of interfacial responses. Ann. Biomed. Eng. 38, 2079–2092. doi:10.1007/s10439-010-9977-6
Bessa, P. C., Machado, R., NüRNBERGER, S., Dopler, D., Banerjee, A., Cunha, A. M., et al. (2010). Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J. Control Release 142, 312–318. doi:10.1016/j.jconrel.2009.11.003
Bhavsar, M. B., Han, Z., Decoster, T., Leppik, L., Costa Oliveira, K. M., and Barker, J. H. (2020). Electrical stimulation-based bone fracture treatment, if it works so well why do not more surgeons use it? Eur. J. Trauma Emerg. Surg. 46, 245–264. doi:10.1007/s00068-019-01127-z
Bordat, A., Boissenot, T., Nicolas, J., and Tsapis, N. (2019). Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 138, 167–192. doi:10.1016/j.addr.2018.10.005
Bose, S., Roy, M., and Bandyopadhyay, A. (2012). Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 30, 546–554. doi:10.1016/j.tibtech.2012.07.005
Brydone, A. S., Meek, D., and Maclaine, S. (2010). Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. H. 224, 1329–1343. doi:10.1243/09544119jeim770
Buza, J. A., and Einhorn, T. (2016). Bone healing in 2016. Clin. Cases Min. Bone Metab. 13, 101–105. doi:10.11138/ccmbm/2016.13.2.101
Caliceti, P. (2011). “Targeted delivery of small and macromolecular drugs,”. Editors S. Ajit,, and I. Narang and Ram, 6, 2323–2325. ChemMedChem.
Cheng, J. H., and Wang, C. J. (2015). Biological mechanism of shockwave in bone. Int. J. Surg. 24, 143–146. doi:10.1016/j.ijsu.2015.06.059
Chen, H., and Zhao, Y. (2018). Applications of light-responsive systems for cancer theranostics. ACS Appl. Mater Interfaces 10, 21021–21034. doi:10.1021/acsami.8b01114
Chen, Q., Cordero-Arias, L., Roether, J. A., Cabanas-Polo, S., Virtanen, S., and Boccaccini, A. R. (2013). Alginate/Bioglass® composite coatings on stainless steel deposited by direct current and alternating current electrophoretic deposition. Surf. Coatings Technol. 233, 49–56. doi:10.1016/j.surfcoat.2013.01.042
Chen, S., Guo, R., Liang, Q., and Xiao, X. (2021). Multifunctional modified polylactic acid nanofibrous scaffold incorporating sodium alginate microspheres decorated with strontium and black phosphorus for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 32, 1598–1617. doi:10.1080/09205063.2021.1927497
Collins, M. N., Ren, G., Young, K., Pina, S., Reis, R. L., and Oliveira, J. M. (2021). Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 31, 2010609. doi:10.1002/adfm.202010609
Cui, H., Liu, Y., Deng, M., Pang, X., Zhang, P., Wang, X., et al. (2012). Synthesis of biodegradable and electroactive tetraaniline grafted poly(ester amide) copolymers for bone tissue engineering. Biomacromolecules 13, 2881–2889. doi:10.1021/bm300897j
Cui, L., Zhang, J., Zou, J., Yang, X., Guo, H., Tian, H., et al. (2020). Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials 230, 119617. doi:10.1016/j.biomaterials.2019.119617
Dasari, A., Xue, J., and Deb, S. (2022). Magnetic nanoparticles in bone tissue engineering. Nanomater. (Basel) 12, 757. doi:10.3390/nano12050757
Durairaj, K., Balasubramanian, B., Arumugam, V. A., Easwaran, M., Park, S., Issara, U., et al. (2023). Biocompatibility of veratric acid–encapsulated chitosan/methylcellulose hydrogel: biological characterization, osteogenic efficiency with in silico molecular modeling. Appl. Biochem. Biotechnol. 195, 4429–4446. doi:10.1007/s12010-023-04311-5
Eischen-Loges, M., Oliveira, K. M. C., Bhavsar, M. B., Barker, J. H., and Leppik, L. (2018). Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ 6, e4959. doi:10.7717/peerj.4959
Ercan, B., and Webster, T. J. (2008). Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation. Int. J. Nanomedicine 3, 477–485. doi:10.2147/ijn.s3780
Fernandes, M. M., Correia, D. M., Ribeiro, C., Castro, N., Correia, V., and Lanceros-Mendez, S. (2019). Bioinspired three-dimensional magnetoactive scaffolds for bone tissue engineering. ACS Appl. Mater Interfaces 11, 45265–45275. doi:10.1021/acsami.9b14001
Ferreira, A. M., Gentile, P., Chiono, V., and Ciardelli, G. (2012). Collagen for bone tissue regeneration. Acta Biomater. 8, 3191–3200. doi:10.1016/j.actbio.2012.06.014
Fomina, N., Sankaranarayanan, J., and Almutairi, A. (2012). Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Deliv. Rev. 64, 1005–1020. doi:10.1016/j.addr.2012.02.006
Funk, R. H. (2015). Endogenous electric fields as guiding cue for cell migration. Front. Physiol. 6, 143. doi:10.3389/fphys.2015.00143
GarcíA-GonzáLEZ, C. A., Barros, J., Rey-Rico, A., Redondo, P., GóMEZ-Amoza, J. L., Concheiro, A., et al. (2018). Antimicrobial properties and osteogenicity of vancomycin-loaded synthetic scaffolds obtained by supercritical foaming. ACS Appl. Mater Interfaces 10, 3349–3360. doi:10.1021/acsami.7b17375
Goharkhah, M., Salarian, A., Ashjaee, M., and Shahabadi, M. (2015). Convective heat transfer characteristics of magnetite nanofluid under the influence of constant and alternating magnetic field. Powder Technol. 274, 258–267. doi:10.1016/j.powtec.2015.01.031
Greenwald, A. S., Boden, S. D., Goldberg, V. M., Khan, Y., Laurencin, C. T., and Rosier, R. N. (2001). Bone-graft substitutes: facts, fictions, and applications. J. Bone Jt. Surg. Am. 83-A (Suppl. 2 Pt 2), 98–103. doi:10.2106/00004623-200100022-00007
Guo, Z. F., Patil, Y., Shinohara, A., Nagura, K., Yoshida, M., and Nakanishi, T. (2022). Organic molecular and polymeric electrets toward soft electronics. Mol. Syst. Des. Eng. 7, 537–552. doi:10.1039/d1me00180a
Hardy, J. G., Lee, J. Y., and Schmidt, C. E. (2013). Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 24, 847–854. doi:10.1016/j.copbio.2013.03.011
He, Y., Li, F., Jiang, P., Cai, F., Lin, Q., Zhou, M., et al. (2023). Remote control of the recruitment and capture of endogenous stem cells by ultrasound for in situ repair of bone defects. Bioact. Mater 21, 223–238. doi:10.1016/j.bioactmat.2022.08.012
Hohnholt, M. C., Geppert, M., and Dringen, R. (2011). Treatment with iron oxide nanoparticles induces ferritin synthesis but not oxidative stress in oligodendroglial cells. Acta Biomater. 7, 3946–3954. doi:10.1016/j.actbio.2011.06.052
Huang, E. E., Zhang, N., Ganio, E. A., Shen, H., Li, X., Ueno, M., et al. (2022). Differential dynamics of bone graft transplantation and mesenchymal stem cell therapy during bone defect healing in a murine critical size defect. J. Orthop. Transl. 36, 64–74. doi:10.1016/j.jot.2022.05.010
Huang, L., Hu, J., Lang, L., Wang, X., Zhang, P., Jing, X., et al. (2007). Synthesis and characterization of electroactive and biodegradable ABA block copolymer of polylactide and aniline pentamer. Biomaterials 28, 1741–1751. doi:10.1016/j.biomaterials.2006.12.007
Jia, Y., Liu, J., Tan, Z., Liu, J., Meng, X., Luo, D., et al. (2023). Preparation and characterization of a biocompatible glucose-sensitive electrospun nanofibers scaffolds containing dexamethasone with enhanced osteogenic properties in vitro high glucose environment. Biomed. Mater. 18, 045006. doi:10.1088/1748-605x/acd314
Kang, S. K., Murphy, R. K., Hwang, S. W., Lee, S. M., Harburg, D. V., Krueger, N. A., et al. (2016). Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76. doi:10.1038/nature16492
KarakeçILI, A., Topuz, B., Korpayev, S., and Erdek, M. (2019). Metal-organic frameworks for on-demand pH controlled delivery of vancomycin from chitosan scaffolds. Mater Sci. Eng. C Mater Biol. Appl. 105, 110098. doi:10.1016/j.msec.2019.110098
Karimi, M., Ghasemi, A., Sahandi Zangabad, P., Rahighi, R., Moosavi Basri, S. M., Mirshekari, H., et al. (2016a). Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 45, 1457–1501. doi:10.1039/c5cs00798d
Karimi, M., Sahandi Zangabad, P., Ghasemi, A., Amiri, M., Bahrami, M., Malekzad, H., et al. (2016b). Temperature-responsive smart nanocarriers for delivery of therapeutic agents: applications and recent advances. ACS Appl. Mater Interfaces 8, 21107–21133. doi:10.1021/acsami.6b00371
Khan, Y., Yaszemski, M. J., Mikos, A. G., and Laurencin, C. T. (2008). Tissue engineering of bone: material and matrix considerations. J. Bone Jt. Surg. Am. 90 (Suppl. 1), 36–42. doi:10.2106/jbjs.g.01260
Khare, D., Basu, B., and Dubey, A. K. (2020). Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 258, 120280. doi:10.1016/j.biomaterials.2020.120280
Kim, M. H., Kim, B. S., Park, H., Lee, J., and Park, W. H. (2018). Injectable methylcellulose hydrogel containing calcium phosphate nanoparticles for bone regeneration. Int. J. Biol. Macromol. 109, 57–64. doi:10.1016/j.ijbiomac.2017.12.068
Kim, S. H., Thambi, T., Giang Phan, V. H., and Lee, D. S. (2020). Modularly engineered alginate bioconjugate hydrogel as biocompatible injectable scaffold for in situ biomineralization. Carbohydr. Polym. 233, 115832. doi:10.1016/j.carbpol.2020.115832
Krishna, O. D., Wiss, K. T., Luo, T., Pochan, D. J., Theato, P., and Kiick, K. L. (2012). Morphological transformations in a dually thermoresponsive coil-rod-coil bioconjugate. Soft Matter 8, 3832–3840. doi:10.1039/c2sm07025a
Lanier, O. L., Ficarrotta, J. M., Adjei, I., Wable, D., Lewis, C., Nacea, C., et al. (2021). Magnetically responsive polymeric microparticles for the triggered delivery of a complex mixture of human placental proteins. Macromol. Biosci. 21, e2000249. doi:10.1002/mabi.202000249
Lin, S. Z., Xu, Z. S., Wang, S. T., Cao, J. L., Zhong, J. W., Li, G. L., et al. (2022). Multiplying the stable electrostatic field of electret based on the heterocharge-synergy and superposition effect. Adv. Sci. 9, e2203150. doi:10.1002/advs.202203150
Lioubavina-Hack, N., Karring, T., Lynch, S. E., and Lindhe, J. (2005). Methyl cellulose gel obstructed bone formation by GBR: an experimental study in rats. J. Clin. Periodontol. 32, 1247–1253. doi:10.1111/j.1600-051x.2005.00791.x
Liu, D., Yang, F., Xiong, F., and Gu, N. (2016). The smart drug delivery system and its clinical potential. Theranostics 6, 1306–1323. doi:10.7150/thno.14858
Liu, Y., Li, T., Sun, M., Cheng, Z., Jia, W., Jiao, K., et al. (2022). ZIF-8 modified multifunctional injectable photopolymerizable GelMA hydrogel for the treatment of periodontitis. Acta Biomater. 146, 37–48. doi:10.1016/j.actbio.2022.03.046
Li, X., Wang, N., Fan, G., Yu, J., Gao, J., Sun, G., et al. (2015). Electreted polyetherimide-silica fibrous membranes for enhanced filtration of fine particles. J. Colloid Interface Sci. 439, 12–20. doi:10.1016/j.jcis.2014.10.014
Martin, J. R., Howard, M. T., Wang, S., Berger, A. G., and Hammond, P. T. (2021). Oxidation-responsive, tunable growth factor delivery from polyelectrolyte-coated implants. Adv. Healthc. Mater 10, e2001941. doi:10.1002/adhm.202001941
Mayer, G., and Heckel, A. (2006). Biologically active molecules with a "light switch. Angew. Chem. Int. Ed. Engl. 45, 4900–4921. doi:10.1002/anie.200600387
Mura, S., Nicolas, J., and Couvreur, P. (2013). Stimuli-responsive nanocarriers for drug delivery. Nat. Mater 12, 991–1003. doi:10.1038/nmat3776
Nakamura, S., Kobayashi, T., Nakamura, M., and Yamashita, K. (2009). Enhanced in vivo responses of osteoblasts in electrostatically activated zones by hydroxyapatite electrets. J. Mater Sci. Mater Med. 20, 99–103. doi:10.1007/s10856-008-3546-7
Nejati, S., Karimi Soflou, R., Khorshidi, S., and Karkhaneh, A. (2020). Development of an oxygen-releasing electroconductive in-situ crosslinkable hydrogel based on oxidized pectin and grafted gelatin for tissue engineering applications. Colloids Surf. B Biointerfaces 196, 111347. doi:10.1016/j.colsurfb.2020.111347
Palza, H., Zapata, P. A., and Angulo-Pineda, C. (2019). Electroactive smart polymers for biomedical applications. Mater. (Basel) 12, 277. doi:10.3390/ma12020277
Pandey, A., Nikam, A. N., Fernandes, G., Kulkarni, S., Padya, B. S., Prassl, R., et al. (2020). Black phosphorus as multifaceted advanced material nanoplatforms for potential biomedical applications. Nanomater. (Basel) 11, 13. doi:10.3390/nano11010013
Parameswaran, R., Carvalho-De-Souza, J. L., Jiang, Y., Burke, M. J., Zimmerman, J. F., Koehler, K., et al. (2018). Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol. 13, 260–266. doi:10.1038/s41565-017-0041-7
Pitt, W. G., Husseini, G. A., and Staples, B. J. (2004). Ultrasonic drug delivery--a general review. Expert Opin. Drug Deliv. 1, 37–56. doi:10.1517/17425247.1.1.37
Qiao, Z. G., Lian, M. F., Liu, X. Z., Zhang, X., Han, Y., Ni, B., et al. (2022). Electreted sandwich membranes with persistent electrical stimulation for enhanced bone regeneration. Acs Appl. Mater. Interfaces 14, 31655–31666. doi:10.1021/acsami.2c06665
Quintero-FabiáN, S., Arreola, R., Becerril-Villanueva, E., Torres-Romero, J. C., Arana-ArgáEZ, V., Lara-Riegos, J., et al. (2019). Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 9, 1370. doi:10.3389/fonc.2019.01370
Sahmani, S., Khandan, A., Saber-Samandari, S., and Mohammadi Aghdam, M. (2020). Effect of magnetite nanoparticles on the biological and mechanical properties of hydroxyapatite porous scaffolds coated with ibuprofen drug. Mater Sci. Eng. C Mater Biol. Appl. 111, 110835. doi:10.1016/j.msec.2020.110835
Sahmani, S., Saber-Samandari, S., Shahali, M., Joneidi Yekta, H., Aghadavoudi, F., Montazeran, A. H., et al. (2018). Mechanical and biological performance of axially loaded novel bio-nanocomposite sandwich plate-type implant coated by biological polymer thin film. J. Mech. Behav. Biomed. Mater 88, 238–250. doi:10.1016/j.jmbbm.2018.08.030
Shabani, M., and Bodaghi, M. (2023). “Mechanical-responsive materials: properties, design, and applications,” in Stimuli-responsive materials for biomedical applications (American Chemical Society).
Shrivats, A. R., Mcdermott, M. C., and Hollinger, J. O. (2014). Bone tissue engineering: state of the union. Drug Discov. Today 19, 781–786. doi:10.1016/j.drudis.2014.04.010
Silva, C. C. S., Thomazini, D., Pinheiro, A. G., Aranha, N., Figueiró, S. D., GóES, J. C., et al. (2001). Collagen–hydroxyapatite films: piezoelectric properties. Mater. Sci. Eng. B-advanced Funct. Solid-state Mater. 86, 210–218. doi:10.1016/s0921-5107(01)00674-2
Smallcomb, M., Khandare, S., Vidt, M. E., and Simon, J. C. (2022). Therapeutic ultrasound and shockwave therapy for tendinopathy: a narrative review. Am. J. Phys. Med. Rehabil. 101, 801–807. doi:10.1097/phm.0000000000001894
Sruthi, S., Maurizi, L., Nury, T., Sallem, F., Boudon, J., Riedinger, J. M., et al. (2018). Cellular interactions of functionalized superparamagnetic iron oxide nanoparticles on oligodendrocytes without detrimental side effects: cell death induction, oxidative stress and inflammation. Colloids Surf. B Biointerfaces 170, 454–462. doi:10.1016/j.colsurfb.2018.06.041
Tamburro, A. M., Pepe, A., Bochicchio, B., Quaglino, D., and Ronchetti, I. P. (2005). Supramolecular amyloid-like assembly of the polypeptide sequence coded by exon 30 of human tropoelastin. J. Biol. Chem. 280, 2682–2690. doi:10.1074/jbc.m411617200
Tandon, B., Blaker, J. J., and Cartmell, S. H. (2018). Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 73, 1–20. doi:10.1016/j.actbio.2018.04.026
Tang, D., Tare, R. S., Yang, L. Y., Williams, D. F., Ou, K. L., and Oreffo, R. O. (2016). Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials 83, 363–382. doi:10.1016/j.biomaterials.2016.01.024
Vaca-GonzáLEZ, J. J., Guevara, J. M., Moncayo, M. A., Castro-Abril, H., Hata, Y., and GarzóN-Alvarado, D. A. (2019). Biophysical stimuli: a review of electrical and mechanical stimulation in hyaline cartilage. Cartilage 10, 157–172. doi:10.1177/1947603517730637
Veres, T., Voniatis, C., MolnáR, K., Nesztor, D., FehéR, D., Ferencz, A., et al. (2022). An implantable magneto-responsive poly(aspartamide) based electrospun scaffold for hyperthermia treatment. Nanomater. (Basel) 12, 1476. doi:10.3390/nano12091476
Wang, H., Tian, J., Jiang, Y., Liu, S., Zheng, J., Li, N., et al. (2023). A 3D biomimetic optoelectronic scaffold repairs cranial defects. Sci. Adv. 9, eabq7750. doi:10.1126/sciadv.abq7750
Wang, Z., Crandall, C., Sahadevan, R., Menkhaus, T. J., and Fong, H. (2017). Microfiltration performance of electrospun nanofiber membranes with varied fiber diameters and different membrane porosities and thicknesses. POLYMER 114, 64–72. doi:10.1016/j.polymer.2017.02.084
Wei, H. P., Cui, J. J., Lin, K. L., Xie, J., and Wang, X. D. (2022). Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 10, 17. doi:10.1038/s41413-021-00180-y
Wei, P., Cornel, E. J., and Du, J. (2021). Ultrasound-responsive polymer-based drug delivery systems. Drug Deliv. Transl. Res. 11, 1323–1339. doi:10.1007/s13346-021-00963-0
Weissleder, R. (2001). A clearer vision for in vivo imaging. Nat. Biotechnol. 19, 316–317. doi:10.1038/86684
Wheatley, M. A., and Cochran, M. (2013). Ultrasound contrast agents. J. Drug Deliv. Sci. Technol. 23, 57–72. doi:10.1016/s1773-2247(13)50007-4
Williams, K. E., and Olsen, D. R. (2009). Matrix metalloproteinase-1 cleavage site recognition and binding in full-length human type III collagen. Matrix Biol. 28, 373–379. doi:10.1016/j.matbio.2009.04.009
Xie, M., Wang, L., Ge, J., Guo, B., and Ma, P. X. (2015). Strong electroactive biodegradable shape memory polymer networks based on star-shaped polylactide and aniline trimer for bone tissue engineering. ACS Appl. Mater Interfaces 7, 6772–6781. doi:10.1021/acsami.5b00191
Xue, Y., Zhu, Z., Zhang, X., Chen, J., Yang, X., Gao, X., et al. (2021). Accelerated bone regeneration by MOF modified multifunctional membranes through enhancement of osteogenic and angiogenic performance. Adv. Healthc. Mater 10, e2001369. doi:10.1002/adhm.202001369
Yang, Y., Zheng, W., Tan, W., Wu, X., Dai, Z., Li, Z., et al. (2023). Injectable MMP1-sensitive microspheres with spatiotemporally controlled exosome release promote neovascularized bone healing. Acta Biomater. 157, 321–336. doi:10.1016/j.actbio.2022.11.065
Ye, J., Gong, M., Song, J., Chen, S., Meng, Q., Shi, R., et al. (2022). Integrating inflammation-responsive prodrug with electrospun nanofibers for anti-inflammation application. Pharmaceutics 14, 1273. doi:10.3390/pharmaceutics14061273
Yuan, J., Li, L., Yang, Q., Ran, H., Wang, J., Hu, K., et al. (2021). Targeted treatment of ischemic stroke by bioactive nanoparticle-derived reactive oxygen species responsive and inflammation-resolving nanotherapies. ACS Nano 15, 16076–16094. doi:10.1021/acsnano.1c04753
Yuan, X., Arkonac, D. E., Chao, P. H., and Vunjak-Novakovic, G. (2014). Electrical stimulation enhances cell migration and integrative repair in the meniscus. Sci. Rep. 4, 3674. doi:10.1038/srep03674
Zardad, A. Z., Choonara, Y. E., Du Toit, L. C., Kumar, P., Mabrouk, M., Kondiah, P. P. D., et al. (2016). A review of thermo- and ultrasound-responsive polymeric systems for delivery of chemotherapeutic agents. Polym. (Basel) 8, 359. doi:10.3390/polym8100359
Zhang, Q., Zhang, F., Chen, Y., Dou, Y., Tao, H., Zhang, D., et al. (2017). Structure–property correlations of reactive oxygen species-responsive and hydrogen peroxide-eliminating materials with anti-oxidant and anti-inflammatory activities. Chem. Mater. 29, 8221–8238. doi:10.1021/acs.chemmater.7b02412
Zhang, X., Yang, P., Dai, Y., Ma, P. A., Li, X., Cheng, Z., et al. (2013). Multifunctional up-converting nanocomposites with smart polymer brushes gated mesopores for cell imaging and thermo/pH dual-responsive drug controlled release. Adv. Funct. Mater. 23, 4067–4078. doi:10.1002/adfm.201300136
Zhang, X., Zhao, J., Xie, P., and Wang, S. (2023). Biomedical applications of electrets: recent advance and future perspectives. J. Funct. Biomaterials, 14. [Online]. doi:10.3390/jfb14060320
Zhang, Y., Chen, L., Zeng, J., Zhou, K., and Zhang, D. (2014). Aligned porous barium titanate/hydroxyapatite composites with high piezoelectric coefficients for bone tissue engineering. Mater Sci. Eng. C Mater Biol. Appl. 39, 143–149. doi:10.1016/j.msec.2014.02.022
Zhao, B., Li, N. K., Yingling, Y. G., and Hall, C. K. (2016). LCST Behavior is Manifested in a Single Molecule: elastin-Like polypeptide (VPGVG)n. Biomacromolecules 17, 111–118. doi:10.1021/acs.biomac.5b01235
Zheng, H., Zhang, Y., Liu, L., Wan, W., Guo, P., NyströM, A. M., et al. (2016). One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 138, 962–968. doi:10.1021/jacs.5b11720
Zheng, T., Huang, Y., Zhang, X., Cai, Q., Deng, X., and Yang, X. (2020). Mimicking the electrophysiological microenvironment of bone tissue using electroactive materials to promote its regeneration. J. Mater Chem. B 8, 10221–10256. doi:10.1039/d0tb01601b
Keywords: responsive scaffolds, bone tissue engineering, stimuli, inflammatory, targeted drug delivery
Citation: Zhu T, Zhou H, Chen X and Zhu Y (2023) Recent advances of responsive scaffolds in bone tissue engineering. Front. Bioeng. Biotechnol. 11:1296881. doi: 10.3389/fbioe.2023.1296881
Received: 19 September 2023; Accepted: 09 November 2023;
Published: 17 November 2023.
Edited by:
Wenjie Zhang, Shanghai Jiao Tong University, ChinaReviewed by:
Wanting Niu, Regis College, United StatesGaizhen Kuang, University of Chinese Academy of Sciences, China
Jiulong Zhao, Naval Medical University, China
Copyright © 2023 Zhu, Zhou, Chen and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanjing Zhu, emh1eWoxMTVAY3N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Tongyu Zhu
Tongyu Zhu Hongbo Zhou
Hongbo Zhou Xiaojing Chen
Xiaojing Chen Yuanjing Zhu
Yuanjing Zhu