- 1Department of Health and Sport Sciences, Graduate School of Medicine, Osaka University, Toyonaka, Japan
- 2Department of Orthopaedic Biomaterial Science, Graduate School of Medicine, Osaka University, Suita, Japan
- 3Department of Orthopaedic Surgery, Graduate School of Medicine, Osaka University, Suita, Japan
- 4Graduate School of Health Sciences, Morinomiya University of Medical Sciences, Osaka, Japan
Three-dimensional preoperative surgical simulation of the medial open-wedge high tibial osteotomy (OWHTO), simplified as the rigid rotation around the hinge axis, has been performed to predict postoperative realignment. However, the practicality of this highly simplified simulation method has not been verified. This study aimed to investigate the validity of realignment simulation simplified as a rotation around a hinge axis compared with a postoperative CT model. A three-dimensional surface model of the tibia and femur was created from preoperative computed tomography (CT) images (preoperative model) of three patients. The simulation of medial OWHTO created sixty computer simulation models in each patient simplified as the rigid rotation of the proximal part of the tibia relative to the distal part from 1° to 20° around three types of hinge axes. The simulation models were compared with the actual postoperative model created from postoperative CT images to assess the reality of the simulation model. The average surface distance between the two models was calculated as an index representing the similarity of the simulation model to the postoperative model. The minimum value of average surface distances between the simulation and postoperative CT models was almost 1 mm in each patient. The rotation angles at which the minimum value of average surface distances was represented were almost identical to the actual correction angles. We found that the posterior tibial tilt and the axial rotation of the proximal tibia of the simulation model well represented those of the postoperative CT model, as well as the valgus correction. Therefore, the realignment simulation of medial OWHTO can generate realistic candidates for postoperative realignment that includes the actual postoperative realignment, suggesting the efficacy of the preoperative simulation method.
Introduction
The medial open-wedge high tibial osteotomy (OWHTO) aims to realign the femur and tibia (Lobenhoffer and Agneskirchner, 2003). The medial OWHTO corrects the varus deformity by shifting the weight-bearing axis from the medial compartment to the lateral compartment (valgus correction), thereby reducing the excessive load on the medial compartment (Agneskirchner et al., 2007). In addition to valgus correction, control of the posterior tibial slope (PTS) and internal/external rotation are crucial factors associated with the postoperative result (Jang et al., 2016; Lee et al., 2017). Three-dimensional (3D) preoperative surgical realignment simulation of medial OWHTO has been performed to predict the postoperative change in the PTS and internal/external rotation as well as the valgus correction (Song et al., 2007; Chernchujit et al., 2019; Kang et al., 2022). In addition, 3D preoperative surgical realignment simulation has been used to develop a patient-specific instrument for accurate osteotomy (Victor and Premanathan, 2013; Donnez et al., 2018; Jörgens et al., 2022). Muscle skeletal simulation has been performed using a postoperative realignment simulation model to predict the change in the intra-articular load after medial OWHTO (Kuriyama et al., 2019; Kuriyama et al., 2020b).
The postoperative realignment simulation of medial OWHTO was performed using preoperative computed tomography (CT) images and a 3D model. In the postoperative realignment simulation of medial OWHTO, the position and orientation of the hinge axis and the osteotomy plane through that axis are defined, and the postoperative realignment is predicted by cutting the 3D model at the osteotomy plane and realigning it around the hinge axis (Moon et al., 2015; Lee et al., 2017; Teng et al., 2021; Jörgens et al., 2022; Thürig et al., 2022). Combining the hinge axis and the rotation angle around the axis makes it possible to create various postoperative simulation models systematically. The simplified simulation has been verified for the preoperative simulation of the upper extremity (Murase et al., 2008; Murase, 2018). However, the realistic practicality of this highly simplified simulation model of a rotation axis and rotation around that axis has not been verified for OWHTO. To verify whether the virtual realignment generation realistically reflects the postoperative realignment, it is necessary to compare it with the actual postoperative realignment. Therefore, this study aimed to investigate the validity of realignment simulation, in which medial OWHTO is simplified as a rotation around a hinge axis, compared with a postoperative CT model.
Materials and methods
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Osaka University Hospital (19027-2). All participants provided written informed consent. A male patient (75 years, 167.0 cm, 69 kg) and two female patients (71 years 151.2 cm, 50.8 kg and 69 yrs 146 cm, 72 kg) who were scheduled for medial OWHTO were included in this study. CT images of the three patients were acquired using a 3D-CT imaging system (Optima CT660Pro Advance; GE Healthcare, Milwaukee, WI), under the following conditions: slice thickness (1.25 mm), tube voltage (120 kV), tube current (440 mA), and acquisition matrix (512 × 512). The contours of the cortical bone in the tibia, fibula, and femur were semi-automatically segmented from the CT images using a 3D image analysis workstation (Volume Analyzer Synapse Vincent, Fujifilm Corporation, Tokyo, Japan). A 3D surface model of the bones was reconstructed from the segmented images (preoperative CT model) (Figure 1A).

FIGURE 1. Definition of bony landmarks and axis of rotation for realignment simulation. (A) A preoperative 3D surface model was developed using CT images, and the preoperative CT-driven model was spatially normalized by the anatomical coordinate system. (B) Cutting plane was defined by rotating the horizontal plane around the y-axis passing the fibula head and M0 (35 mm inferior to the medial edge of the articular surface). Additional landmarks (L0, L1, L2, and L3) were identified along with the cutting plane for simulating variation of clinically observed hinge axes in the medial OWHTO (C). (D) Simulation of medial OWHTO rotating the proximal tibia around the given axis of rotation and (E) variation of 60 simulated shapes (3 axes
Three anatomical landmarks of the tibia (insertion of the anterior cruciate ligament and posterior cruciate ligament, and the center of the distal articular surface) were identified on the preoperative CT model to define the tibial anatomical coordinate system (Tajima et al., 2009; Kusano et al., 2017). The preoperative CT model of the tibia and femur was transformed into the tibial coordinate system for spatial normalization. Five additional landmarks were identified to determine the cutting plane (Figure 1B), and three axes of rotation (hinge axes) were defined (Figure 1C). The variations in the hinge axis can represent the clinically observed type of osteotomy (Ogawa et al., 2017). The variations are set within the “safe zone” of medial OWHTO (Han et al., 2013). Vertices of the preoperative CT model located above the osteotomy plane were translated by the matrix transformation (Figure 1D), which was determined by the rotation angle (
The simulation models were compared with the actual postoperative model to assess the reality of the simulation of medial OWHTO simplified as the rotation around the hinge axis. The postoperative tibial model was created with a semiautomatic segmentation from the postoperative CT images. The inserted metal fixation plate was deleted manually. The postoperative CT model was cut below the osteotomy plane, where the shape was not changed before and after medial OWHTO. Surface registration of the distal part of the tibia was performed to align the simulation and postoperative CT models using the Image Registration Tool Kit (Rueckert et al., 1999). Afterward, the surface distance of the proximal tibia between the simulation models and postoperative CT models was evaluated. The average surface distance was calculated between each simulation model and postoperative CT model as an index representing the similarity of the simulation model to the postoperative model (Figure 2).
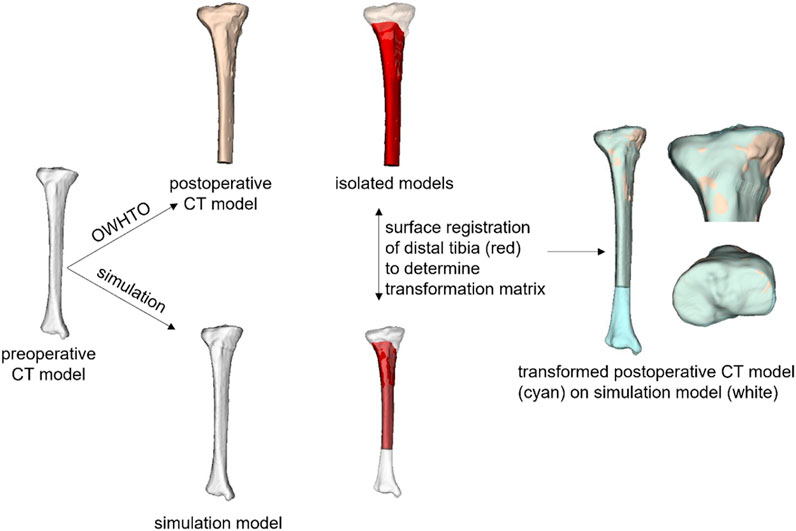
FIGURE 2. Comparison of simulation model and postoperative CT model. After the simulation and postoperative CT models were aligned by a surface registration of the distal part of the tibia, the average surface distance between the two models was calculated as an index representing the similarity of the simulation model to the postoperative model.
Results
Figure 3 demonstrates the results of patient 1, who underwent an average correction angle by medial OWHTO (10.3° by the postoperative X-ray evaluation). When the axis of rotation was set to axis 1 or axis 2, the average surface distance was smallest when it was rotated by 10°. The rotation angle was consistent with the postoperative correction angle recorded by the postoperative X-ray evaluation. On the other hand, when the axis of rotation was set to axis 3, the average surface distance was smallest when it was rotated by 13° (Figure 3A). Graphics show the overlay of the simulation model generated by three different hinge axes (wire model in colors) on the postoperative CT model. The rotation angle of the model displayed in the graphics was set to the angle with the smallest surface distance, and −2° and +2° to it. The simulation model representing the smallest surface distance in each hinge axis (center column) demonstrated that the valgus angle of the tibial plane was more consistent with the postoperative CT model on the backward view (Figure 3B). The simulation model of −2° and +2° demonstrated the under (left column) and overcorrection (right column) of medial OWHTO (Figure 3B). When compared within the model in the center column, the model generated by axis 2 was more fitted to the postoperative CT model than axis 1 and axis 3 in the posterior tibial slope (Figure 3C). The models generated by axis 2 or axis 3 were more fitted to the postoperative CT model than axis 1 in the axial rotation of the tibial plane (Figure 3D). The simulation model could also represent the postoperative CT model in patients 2 and 3 who underwent relatively small and large correction angles (Figures 4, 5).

FIGURE 3. The results of patient 1 who underwent an average correction angle (10.3° by the postoperative x-ray evaluation). (A) The average surface distance of each simulation model, generated by the combination of hinge axis and rotation angle, relative to the postoperative CT model. (B–D) Backward, medial, and proximal viewed images of overlay of the simulation model generated by three different hinge axes (wire model in colors) on the postoperative CT model. The number on the bottom right shows the rotation angle around each hinge axis.
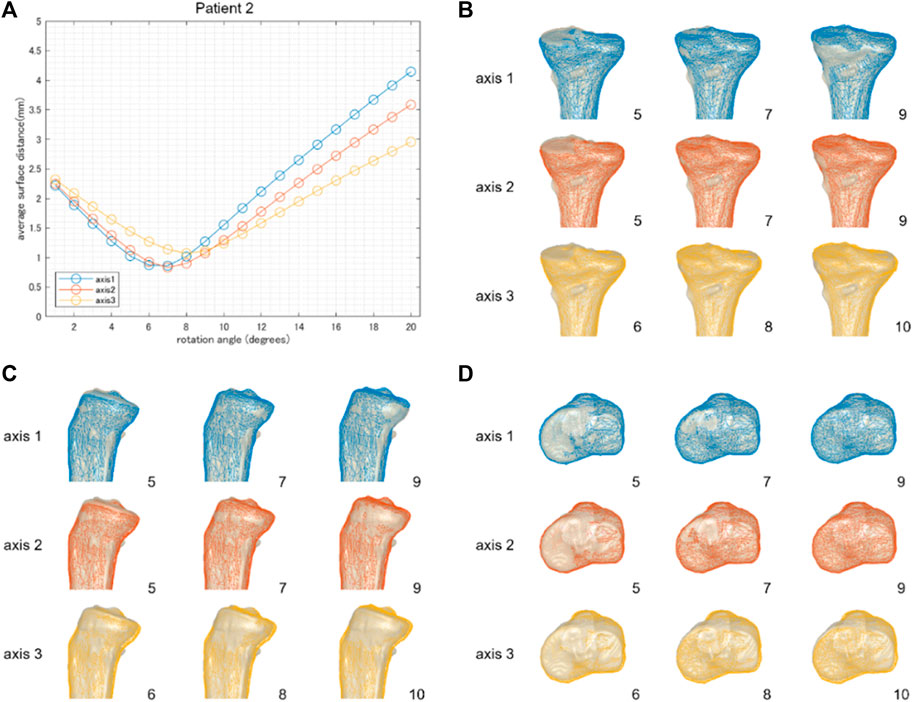
FIGURE 4. The result of patient 2 underwent a relatively small correction angle by medial OWHTO (8.2° by postoperative x-ray evaluation). The average surface distance was smallest when rotated by 7 or 8° (A). The rotation angle was consistent with the postoperative correction angle recorded by the postoperative X-ray evaluation. The simulation model (wire model in colors) representing the smallest surface distance in each hinge axis (center column) demonstrated that the valgus angle of the tibial plane is more consistent with the postoperative CT model on the backward view (B). When compared within the model in the center column, the models generated by axis 1 and axis 2 were more fitted to the postoperative CT model than axis 3 in the posterior tibial slope (C) and axial rotation of the tibial plane (D). The number on the bottom right shows the rotation angle around each hinge axis.
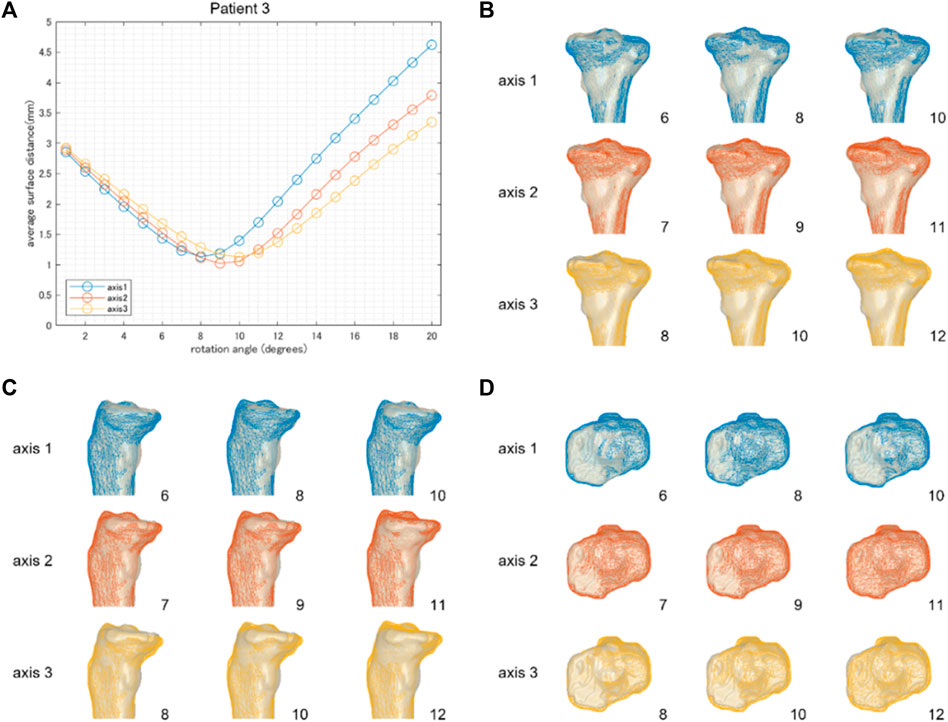
FIGURE 5. The results of patient 3 underwent a relatively large correction angle by medial OWHTO (12.2° by postoperative x-ray evaluation). The average surface distance was smallest when it was rotated by 8, 9, and 10° around hinge axes 1, 2, and 3, respectively (A). The rotation angle was inconsistent with the postoperative correction angle recorded by the postoperative X-ray evaluation. The simulation model (wire model in colors) representing the smallest surface distance in each hinge axis (center column) demonstrated that the valgus angle of the tibial plane is more consistent with the postoperative CT model on the backward view (B). When compared within the model in the center column, the model generated by axis 2 was more fitted to the postoperative CT model than axis 1 and axis 3 in the posterior tibial slope (C) and axial rotation of the tibial plane (D). The number on the bottom right shows the rotation angle around each hinge axis.
Discussion
We aimed to investigate the validity of the realignment simulation of the medial OWHTO simplified as the rigid rotation around the hinge axis compared to a postoperative CT model. The minimum average surface distance between the simulation model generated by the rotation around three hinge axes and the postoperative CT model was almost 1 mm. The rotation angle at which the minimum average surface distance was represented on each hinge axis was almost identical to the actual correction angle. Overlaying the 3D surface models of the simulation and the postoperative CT, we found that the posterior tibial tilt (Figures 3, 4, 5) and the axial rotation of the proximal tibia (Figures 3, 4, 5) of the simulation model well represented that of the postoperative CT model as well as the valgus correction (Figures 3, 4, 5). Therefore, the realignment simulation of medial OWHTO simplified as the rigid rotation around the hinge axis can generate realistic candidates of postoperative realignment that include the actual postoperative realignment, suggesting the efficacy of the preoperative simulation method.
A significant advantage of realignment simulation in which medial OWHTO is simplified as a hinge axis and rotation around that axis is a systematic generation of the candidates of the postoperative realignment. The 60 generated simulation models have great variability; however, some of the simulation models may show an unrealistic realignment. The acceptable simulations in the 60 simulations were selected based on the clinical criteria for femorotibial alignment, as shown in Figure 6A. The medial and lateral edges of the articular surface of the proximal tibia were identified on the frontal view of the simulation model and were defined as 0% and 100%, respectively. A femorotibial alignment where the mechanical line (the line connecting the center of the hip joint center and the center of the distal tibia) passes through 62.5%, called the Fujisawa point, has been recognized as a clinically ideal alignment (Fujisawa et al., 1979). In this study, the acceptable range was set at
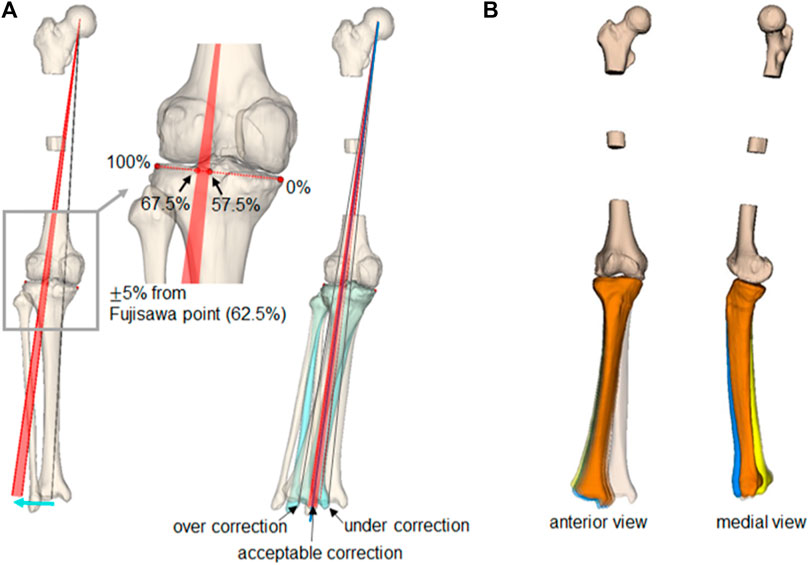
FIGURE 6. Selection of clinically acceptable simulations and simulated postoperative realignment. (A) The gray line shows the preoperative mechanical line, and the red arc shows the clinically acceptable target alignment after the medial OWHTO. The simulation models in which the mechanical line passes within the arc were selected as the clinically acceptable simulation. (B) Three-dimensional realignment variation of clinically acceptable simulation models with three types of hinge axes (axis 1: blue, axis 2: orange, axis 3: yellow).
A disadvantage of this simulation method was that the simplified simulation ignored the bone deformability and the biplane medial OWHTO process. The simulation model was created by rigid rotation around the set hinge axis between the proximal and distal tibial parts. In actual intraoperative situations, the tibia can be deformed manually at the preserved cortex when inserting a fixation plate in addition to rotation around the hinge axis. The vertices of the surface model located superior to the osteotomy plane were transformed along the rotation around the hinge axis; however, the tuberosity is preserved with the distal tibial parts in the biplane medial OWHTO. Although the present simulation models the medial OWHTO as the rigid rotation around the hinge axis, the simulation model well represents the postoperative CT model.
A limitation of this study is the small sample size, which may affect the generalizability of our findings. However, it is important to note that, as this study primarily aimed to propose a methodological approach, the small sample size does not fundamentally impact the validity of our results. Further studies with larger sample sizes may be needed to validate our results and to explore the full potential and limitations of the realignment simulation method in the medial OWHTO.
Conclusion
The realignment simulation of the medial OWHTO simplified as the rigid rotation around the hinge axis can generate realistic candidates of postoperative realignment that include the actual postoperative realignment, suggesting the efficacy of the preoperative simulation method. We believe the simplified realignment simulation can be used for preoperative planning of the medial OWHTO.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee of Osaka University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. TI: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing–review and editing. MT: Data curation, Investigation, Supervision, Writing–review and editing. TT: Conceptualization, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by JSPS KAKENHI Grant Number JP80598227.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agneskirchner, J. D., Hurschler, C., Wrann, C. D., and Lobenhoffer, P. (2007). The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthrosc. J. Arthrosc. Relat. Surg. 23, 852–861. doi:10.1016/j.arthro.2007.05.018
Chernchujit, B., Tharakulphan, S., Prasetia, R., Chantarapanich, N., Jirawison, C., and Sitthiseripratip, K. (2019). Preoperative planning of medial opening wedge high tibial osteotomy using 3D computer-aided design weight-bearing simulated guidance: technique and preliminary result. J. Orthop. Surg. Hong. Kong) 27, 230949901983145. doi:10.1177/2309499019831455
Donnez, M., Ollivier, M., Munier, M., Berton, P., Podgorski, J. P., Chabrand, P., et al. (2018). Are three-dimensional patient-specific cutting guides for open wedge high tibial osteotomy accurate? An in vitro study. J. Orthop. Surg. Res. 13, 171. doi:10.1186/s13018-018-0872-4
Dugdale, T. W., Noyes, F. R., and Styer, D. (1992). Preoperative planning for high tibial osteotomy. The effect of lateral tibiofemoral separation and tibiofemoral length. Clin. Orthop. Relat. Res. 274, 248–264. doi:10.1097/00003086-199201000-00025
Fujisawa, Y., Masuhara, K., and Shiomi, S. (1979). The effect of high tibial osteotomy on osteoarthritis of the knee. Orthop. Clin. North Am. 10, 585–608. doi:10.1016/s0030-5898(20)30753-7
Han, S. B., Lee, D. H., Shetty, G. M., Chae, D. J., Song, J. G., and Nha, K. W. (2013). A "safe zone" in medial open-wedge high tibia osteotomy to prevent lateral cortex fracture. Knee Surg. Sports Traumatol. Arthrosc. 21, 90–95. doi:10.1007/s00167-011-1706-7
Jang, K. M., Lee, J. H., Park, H. J., Kim, J. L., and Han, S. B. (2016). Unintended rotational changes of the distal tibia after biplane medial open-wedge high tibial osteotomy. J. Arthroplasty 31, 59–63. doi:10.1016/j.arth.2015.08.010
Jörgens, M., Keppler, A. M., Degen, N., Bachmeier, A. T., Bergstraesser, M., Sass, J., et al. (2022). Reliability of 3D planning and simulations of medial open wedge high tibial osteotomies. J. Orthop. Surg. 30, 102255362211016. doi:10.1177/10225536221101699
Kang, B. Y., Lee, D. K., Kim, H. S., and Wang, J. H. (2022). How to achieve an optimal alignment in medial opening wedge high tibial osteotomy? Knee Surg. Relat. Res. 34, 3. doi:10.1186/s43019-021-00130-2
Kuriyama, S., Morimoto, N., Shimoto, T., Takemoto, M., Nakamura, S., Nishitani, K., et al. (2019). Clinical efficacy of preoperative 3D planning for reducing surgical errors during open-wedge high tibial osteotomy. J. Orthop. Res. 37, 898–907. doi:10.1002/jor.24263
Kuriyama, S., Watanabe, M., Nakamura, S., Nishitani, K., Sekiguchi, K., Tanaka, Y., et al. (2020a). Classical target coronal alignment in high tibial osteotomy demonstrates validity in terms of knee kinematics and kinetics in a computer model. Knee Surg. Sports Traumatol. Arthrosc. 28, 1568–1578. doi:10.1007/s00167-019-05575-3
Kuriyama, S., Watanabe, M., Nakamura, S., Nishitani, K., Tanaka, Y., Sekiguchi, K., et al. (2020b). Large medial proximal tibial angles cause excessively medial tibiofemoral contact forces and abnormal knee kinematics following open-wedge high tibial osteotomy. Clin. Biomech. 80, 105190. doi:10.1016/j.clinbiomech.2020.105190
Kusano, M., Yonetani, Y., Mae, T., Nakata, K., Yoshikawa, H., and Shino, K. (2017). Tibial insertions of the anterior cruciate ligament and the anterior horn of the lateral meniscus: a histological and computed tomographic study. Knee 24, 782–791. doi:10.1016/j.knee.2017.04.014
Lee, B. H., Ha, C. W., Moon, S. W., Chang, M., Kim, H. Y., Park, S. H., et al. (2017). Three-dimensional relationships between secondary changes and selective osteotomy parameters for biplane medial open-wedge high tibial osteotomy. Knee 24, 362–371. doi:10.1016/j.knee.2016.11.010
Lobenhoffer, P., and Agneskirchner, J. D. (2003). Improvements in surgical technique of valgus high tibial osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 11, 132–138. doi:10.1007/s00167-002-0334-7
Miniaci, A., Ballmer, F. T., Ballmer, P. M., and Jakob, R. P. (1989). Proximal tibial osteotomy. A new fixation device. Clin. Orthop. Relat. Res. 246, 250–259. doi:10.1097/00003086-198909000-00035
Moon, S. W., Park, S. H., Lee, B. H., Oh, M., Chang, M., Ahn, J. H., et al. (2015). The effect of hinge position on posterior tibial slope in medial open-wedge high tibial osteotomy. Arthroscopy 31, 1128–1133. doi:10.1016/j.arthro.2015.01.009
Murase, T. (2018). Morphology and kinematics studies of the upper extremity and its clinical application in deformity correction. J. Orthop. Sci. 23, 722–733. doi:10.1016/j.jos.2018.05.013
Murase, T., Oka, K., Moritomo, H., Goto, A., Yoshikawa, H., and Sugamoto, K. (2008). Three-dimensional corrective osteotomy of malunited fractures of the upper extremity with use of a computer simulation system. J. Bone Jt. Surg. Am. 90, 2375–2389. doi:10.2106/jbjs.g.01299
Ogawa, H., Matsumoto, K., and Akiyama, H. (2017). The prevention of a lateral hinge fracture as a complication of a medial opening wedge high tibial osteotomy: a case control study. Bone Jt. J. 99-B, 887–893. doi:10.1302/0301-620x.99b7.bjj-2016-0927.r1
Rueckert, D., Sonoda, L. I., Hayes, C., Hill, D. L., Leach, M. O., and Hawkes, D. J. (1999). Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. imaging 18, 712–721. doi:10.1109/42.796284
Song, E. K., Seon, J. K., and Park, S. J. (2007). How to avoid unintended increase of posterior slope in navigation-assisted open-wedge high tibial osteotomy. Orthopedics 30, S127–S131.
Tajima, G., Nozaki, M., Iriuchishima, T., Ingham, S. J., Shen, W., Smolinski, P., et al. (2009). Morphology of the tibial insertion of the posterior cruciate ligament. J. Bone Jt. Surg. Am. 91, 859–866. doi:10.2106/jbjs.h.00991
Teng, Y., Mizu-Uchi, H., Xia, Y., Akasaki, Y., Akiyama, T., Kawahara, S., et al. (2021). Axial but not sagittal hinge Axis affects posterior tibial slope in medial open-wedge high tibial osteotomy: a 3-dimensional surgical simulation study. Arthroscopy 37, 2191–2201. doi:10.1016/j.arthro.2021.01.063
Thürig, G., Korthaus, A., Frings, J., Berninger, M. T., Frosch, K.-H., and Krause, M. (2022). Influence of medial osteotomy height and hinge position in open wedge high tibial osteotomy: a simulation study. Diagnostics 12, 2546. doi:10.3390/diagnostics12102546
Keywords: knee, osteoarthritis, medial OWHTO, preoperative surgical simulation, hinge axis
Citation: Konda S, Ishibashi T, Tamaki M and Tomita T (2023) A preoperative simulation of medial open-wedge high tibial osteotomy for predicting postoperative realignment. Front. Bioeng. Biotechnol. 11:1278912. doi: 10.3389/fbioe.2023.1278912
Received: 25 August 2023; Accepted: 12 December 2023;
Published: 21 December 2023.
Edited by:
Jukka Hirvasniemi, Erasmus Medical Center, NetherlandsReviewed by:
Jay Trivedi, Rhode Island Hospital, United StatesAsa Barber, City University of London, United Kingdom
Copyright © 2023 Konda, Ishibashi, Tamaki and Tomita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tetsuya Tomita, dGV0c3V5YV90b21pdGFAbW9yaW5vbWl5LXUuYWMuanA=; Shoji Konda, c2tvbmRhLm1lZEBvc2FrYS11LmFjLmpw
 Shoji Konda
Shoji Konda Teruya Ishibashi
Teruya Ishibashi Masashi Tamaki3
Masashi Tamaki3