
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Bioeng. Biotechnol., 07 September 2023
Sec. Nanobiotechnology
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1271489
 Jinming Zhao1,2
†
Jinming Zhao1,2
†
 Jingxing Yang
1,3
†
Jingxing Yang
1,3
† Jian Jiao1,2
Jian Jiao1,2
 Xiangdong Wang
1,2
Xiangdong Wang
1,2
 Yan Zhao
1,2*
Yan Zhao
1,2*
 Luo Zhang
1,2,4,5*
Luo Zhang
1,2,4,5*
Intranasal administration offers a feasible, non-invasive method of delivering therapeutic drugs to the brain, allowing therapeutic pharmaceuticals to be administered directly to the central nervous system by bypassing the blood-brain barrier. Furthermore, exosomes are naturally occurring cell-derived nanovesicles that can serve as carriers for a variety of chemical compounds. Many studies have focused on artificial exosomes as innovative medication delivery methods. As a result, trans-nasal delivery of artificial exosomes might be employed to treat brain illnesses in a novel method. This review will outline the drug delivery mechanism of artificial extracellular vesicles, emphasize its advantages as a nasal drug carrier, particularly its application as a novel nanocarriers in brain diseases, and focus on its prospective application in chronic inflammatory nose disorders. Finally, artificial exosomes may become a unique drug delivery mode for clinical therapeutic usage.
The blood-brain barrier (BBB) may be seen at the level of the cerebral microvasculature and is critical for maintaining homeostasis of the central nervous system (CNS) (Lochhead et al., 2020). The BBB significantly restricts access to all but tiny, nonpolar molecules, making medication penetration into the CNS challenging and limiting CNS illness therapy (Lochhead and Thorne, 2012). Intranasal drug administration is a feasible, noninvasive method of delivering therapeutic medications to the brain, and it has the potential to circumvent the BBB, allowing therapeutic compounds direct access to the CNS. Because it is noninvasive and may be repeated, intranasal injection of extracellular vesicles (EVs) has received a lot of attention (Kodali et al., 2019). While intranasal drug administration is an excellent method of delivering therapeutic medications, it has been demonstrated that there were some hurdles in transportation. Due to the permeability barrier in the nasal mucosa, the presence of mucociliary clearance system and the degradation of enzymes in the mucosa, multiple and complex factors contribute to many drugs into the brain is quite little by intranasal administration (Touitou and Illum, 2013; Kumar et al., 2017).
EVs are naturally occurring cell-derived nanovesicles that convey information between tissue microenvironments. They also have the ability to alter target cell activity and differentiation (Quesenberry and Aliotta, 2010; Hood and Wickline, 2012). EVs are found in most biological fluids, including blood, urine, saliva, cerebrospinal fluid, and breast milk (Zhang et al., 2019a). EVs can carry specific proteins, nucleic acid and metabolites, and the cargo in EVs retains its biological activity and can modulate recipient cells (Vázquez-Ríos et al., 2019). Although EVs are naturally derived vesicles, their surfaces can be conveniently modified, and surface engineering can confer targeted specificity to vesicles (Liang et al., 2021). The use artificial EVs is a new method for drug delivery. The nanovesicles (NVs), exosome mimics (EMs) and hybrid exosomes (HEs) are the major types of artificial EVs, which are obtained by top-down, bottom-up and biohybrid strategies, respectively (Figure 1). At present, top-down is the most widely used strategy to prepare artificial exosomes. The main advantage is that it contains being carried are from the producer cells, which mimics the biological complexity of natural exosomes. However, this method of production consumes more time and manpower, and the limited number of producer cells also limits production. The bottom-up strategy can produce artificial exosomes with pure composition and controllable characteristics, which can be used for large-scale production, significantly reducing time and labor costs. However, the biological complexity of synthetic exosomes is not as good as that of natural exosomes. The strategy of biohybrid has the advantage of including some natural components of exosomes, which may have higher delivery efficiency than liposomes and higher stability than exosomes. The limitation is that the yield will not be very high (García-Manrique et al., 2018; Li et al., 2021).
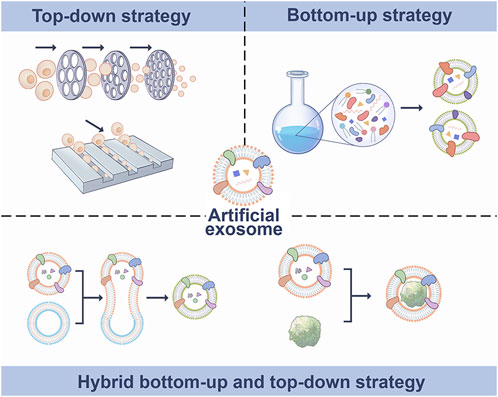
FIGURE 1. Synthetic methods of artificial exosomes. Artificial exosomes were obtained by top-down, bottom-up and biohybrid bottom-up and top-down strategies.
In recent years, a variety of artificial exosomes that can overcome the shortcomings of natural exosomes have been developed. It has been reported to be effective in the treatment of cancer (Zhang et al., 2019b; Lin et al., 2021), ischemic diseases (Aday et al., 2021) and injury (Staufer et al., 2021), and has a good prospect for clinical application. However, the research of this new biological treatment material is still in its early stage. In this review, we introduced the mechanism of artificial EVs delivery, reviewed its benefits as a drug carrier in nasal delivery, particularly its use as a new nanocarrier in brain diseases and emphasized the important potential for application in chronic rhinosinusitis and allergic rhinitis.
Stroke has a high fatality rate and a leading cause of disability (Towfighi and Saver, 2011). Ischemic stroke, a subtype of stroke, is caused by the narrowing of one or more cerebral arteries due to occlusion by emboli or thrombi. The disease is typically treated with thrombolytic drugs. However, brain injury often worsens after reperfusion. This pathological process is often called ischemia reperfusion (IR) injury (Zhou et al., 2018). In recent years, EVs produced from embryonic stem cells (ESCs) have been found to boost endogenous repair mechanisms and improve heart function after myocardial infarction and have shown therapeutic potential following stroke (Khan et al., 2015). Curcumin, as a natural polyphenol found in the rhizome of Curcuma longa (turmeric), has been extensively explored for its therapeutic effect ischemic stroke and its bioavailability is hampered by its low absorption and rapid metabolism (Li et al., 2017). One previous study created a curcumin-loaded EVs from cultured mouse embryonic stem cell (MESC) lines by a fast freeze-thawing technique and delivered nasally (Kalani et al., 2016). They found that MESC-exoscur (curcumin-loaded in MESC derived exosomes) has greater stability and solubility and MESC-exoscur therapy dramatically reduced neurological scores, cerebral edema, and infarct volume (Figure 2A).
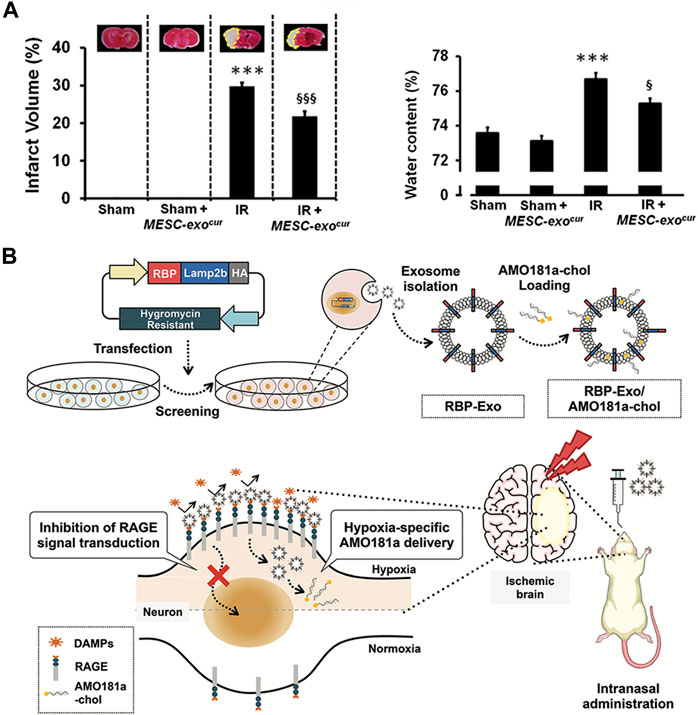
FIGURE 2. Biomedical applications of artificial exosomes for drug delivery in stroke/ischemia-reperfusion damage: (A) MESC-exocur treatment reduced infarct volume and water content in IR mice. Reproduced with permission from Kalani et al. (2016). (B) Production and anoxic-specific delivery of RBP-Exo/AMO181a-chol by intranasal administration. Reproduced with permission from Kim et al. (2021).
Furthermore, receptor for advanced glycation end-products (RAGE) has been identified as a critical element involved in the pathophysiological process of IR injury (Ramasamy and Schmidt, 2012). Recombinant RAGE-antagonist peptide (RAGE-binding peptide, RBP) could have neuroprotective effect by reducing RAGE-mediated ischemic brain inflammation (Kim et al., 2021). Another study reported that delivery of anti-microRNA oligonucleotide (AMO) against miR181a (AMO181a) attenuated ischemic neuronal damage and that knockdown of miRN181a can reduce infarct volume. Therefore, investigators engineered an artificial EVs contains the RBP-linked exosomes (RBP-exos) combined cholesterol-modified AMO181a (AMO181a-chol) by hydrophobic interactions. The nanovesicles were administered intranasally to treat a mouse model of ischemic brain injury. As a result, intranasal administration of this hypoxia-specific vector allowed AMO181a to be delivered into the ischemic brain more effectively, and the RBP moiety promoted the delivery of AMO181a in the ischemic tissue. Amo181a-chol significantly inhibited RAGE pathways, inflammatory cytokines, apoptotic cells and infarct volume (Kim et al., 2021) (Figure 2B).
Glioblastoma multiforme (GBM) is the most malignant primary tumor of the central nervous system, having a poor prognosis and a high mortality rate. Chemotherapy remains the primary therapeutic choice for GBM treatment and the existence of the BBB inhibits medication effects. Sandbhor et al. (2021) recently shown in orthotopic GBM mouse models that in situ administration of hydrogel embedded with miltefosine (HePc, a proapoptotic antitumor agent) and temozolomide (TMZ, a DNA methylating agent)-loaded targeted nanovesicles could suppress the tumor relapses. Because transferrin receptors are overexpressed on the surface of the tumor, surface engineering of vesicles can increase cellular uptake and medication internalization into tumor (Zhao et al., 2020). As a result, TMZ was given via lipid nanovesicles (LNs) that were surface transferrin-decorated and coencapsulated with HePc. In terms of tumor-bearing mouse survival, the effectiveness of the TLNs rose by 1.8-fold when compared to free medicines. Furthermore, off-target organ damage has been demonstrated in the clinic, and tailored nanovesicles in conjunction with HePc will be tolerated better than traditional systemic delivery, lowering systemic drug exposure. This study shows that LNs have the potential to boost brain medication bioavailability following intranasal delivery, and the treatment strategy is also likely to improve compliance in patients on long-term drug therapy (Sandbhor et al., 2021) (Figure 3A).
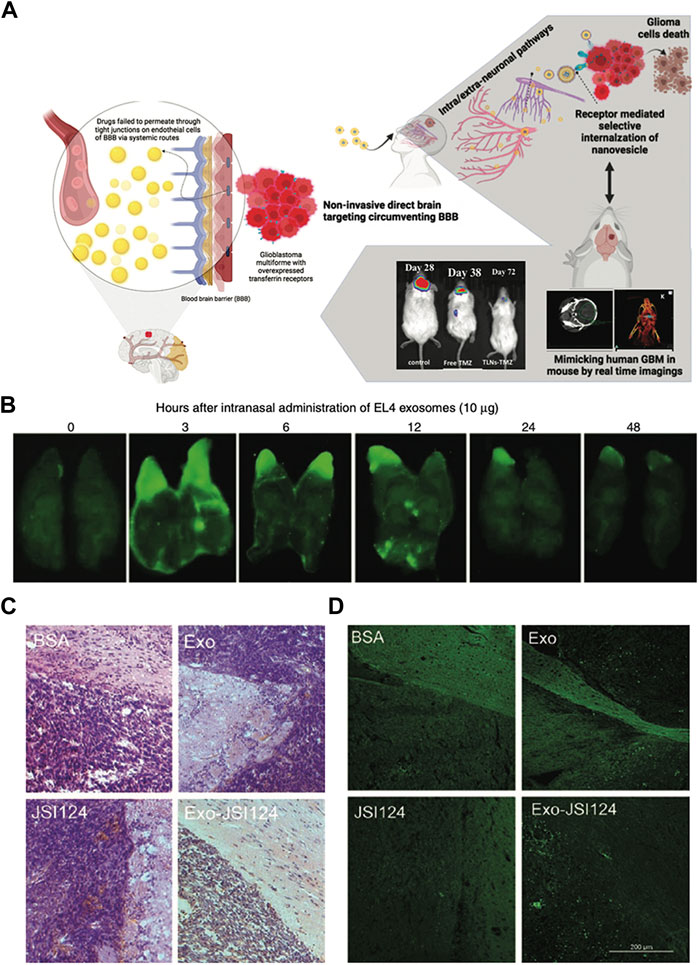
FIGURE 3. Biomedical applications of artificial exosomes for drug delivery in glioblastoma multiforme: (A) Schematic illustration of the synergistic drug-loaded transferrin-targeted nanovesicle system. The nanovesicles can bypass the blood-brain barrier and reach the brain through neurological pathways. Reproduced with permission from Sandbhor et al. (2021). (B) A total of 10 μg of IRDye 800-labeled EL-4 T cell-derived exosomes were administered intranasally into C57BL/6j mice, and the distribution pattern of exosomes in the brain was observed. The fluorescence of the brain was stronger after intranasal administration for 3 h. Reproduced with permission from Zhuang et al. (2011). (C) The results of HE-stained brain tumor tissue suggest that tumors adjacent to the olfactory region aggressively invaded the adjacent tissue of mice treated with BSA, Exo, and JSI124. In contrast, intranasally administration of Exo-JSI124 was much less invasive. Reproduced with permission from Zhuang et al. (2011). (D) The results of TUNEL staining were similar to those of HE staining. Reproduced with permission from Zhuang et al. (2011).
The signal transducer and activator of transcription 3 (STAT3) protein is constitutively active in several forms of human malignancies and is essential for tumor development, including GBM. Cucurbitacin I (JSI-124), as a potent STAT3 signaling pathway inhibitor, have antitumor properties in human breast cancer, neuroblastoma, lung cancer, murine melanoma cell lines and B cell leukemia (Ren et al., 2014). Furthermore, JSI-124 inhibits the proliferation of glioblastoma polymorphic cells by increasing apoptosis and inducing G2/M-phase cell cycle arrest (Su et al., 2008). Therefore, to investigate the therapeutic impact of artificial EVs, GL26 brain tumor model mice were treated with the exosome-encapsulated JSI-124 (Exo-JSI124). On the one hand, EVs have been observed in brain tissue to selectively target microglial cells, exhibiting potent anti-tumor capabilities (Figures 3B–D). On the other hand, Exo-JSI124-treated mice had considerably higher survival periods, with an average of 44.5 days. Furthermore, none of the mice displayed any signs of toxicity or aberrant behavior during the 15-day period after Exo-JSI124 intranasal administration (Zhuang et al., 2011).
In a recent work, researchers used a pressure-based disruption and reconstitution procedure to load miRNA (anti-miRNA 21 and miRNA 100) while managing the size of microfluidically processed EVs (mpEVs) (Wang et al., 2021). In this work, vesicles were collected from neural stem cells, designed to overexpress the CXCR4 receptor, and then engineered to have the ideal size of mpEVs. Intranasal injection of miRNA-loaded mpEVs to a GBM mouse model resulted in a consistent pattern of mpEV transport across the nasal epithelium, bypassing the BBB and entering the cerebral compartment. Such EVs have a GBM-specific tropism, and the loaded miRNA made GBM cells susceptible to TMZ. The findings revealed that the miRNA-loaded mpEVs not only reduced tumor size but also increased mouse survival.
It is evident that microglial cells, or brain-resident macrophages, play an important role in many CNS inflammation-related disorders, including meningitis, migraine headaches, Parkinson’s disease, and others. Curcumin, as previously said, not only has anticancer and preventative effects, but it is also a potent anti-inflammatory agent. After incubation, the mixture was centrifuged using a sucrose gradient to extract exosomal curcumin (Exo-cur), the size and morphology of Exo-cur were similar to those of natural exosomes (Figures 4A, B). LPS-challenged mice were treated with intranasal treatment of exosome-encapsulated curcumin. It was discovered in the brain 1 h after the fake EVs were administered, which is consistent with prior findings. The levels of cytokines decreased after treatment (Sun et al., 2010) (Figure 4C). Meningitis is a disease caused by microbial infection that can be divided into bacterial meningitis, viral meningitis, tuberculosis meningitis and cryptococcal meningitis. Bacterial meningitis responsible for 75% of all meningitis cases globally and the strong dosages of antibacterial medications are used to treat the infection (van de Beek, 2012). The key to efficient meningitis management is to keep high doses of antibiotics in the brain. Ofloxacin-loaded transfersomal nanovesicles (OFLOX-TNVs) were created for the treatment of meningitis by nasal administration to avoid the difficulties of its high-dose use. These NVs were created using the thin film hydration process with chemicals such as lecithin, edge activator (EA), and cholestasis. These findings imply that OFLOX-loaded nanometastases might be a useful colloidal delivery mechanism for brain targeting and treatment of bacterial meningitis (Hussein et al., 2019).
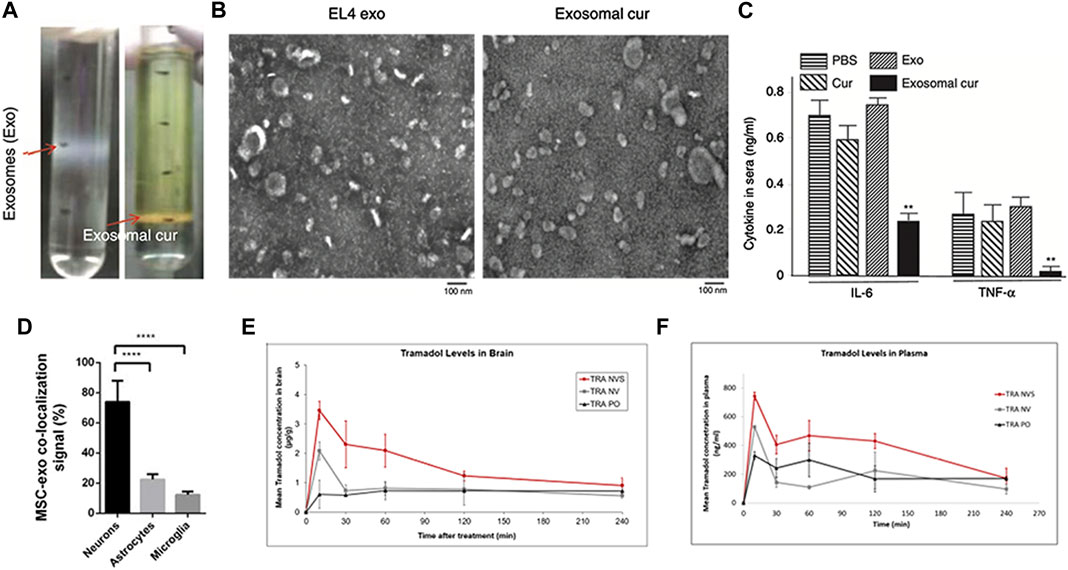
FIGURE 4. Biomedical applications of artificial exosomes for drug delivery in brain inflammatory illness and other brain diseases: (A) Exo-cur was obtained by gradient centrifugation after co-incubation of curcumin and EL-4 exosomes. (B) Electron microscopic images of EL-4 exosomes and Exo-cur. (C) Exo-cur can reduce IL-6 and TNF-α secretion after LPS stimulation. Reproduced with permission from Sun et al. (2010). (D) Nasal administration of MSC-Exo in SCI rats showed that MSC-Exo inherit MSC targeting capability, rendering them a suitable delivery system to the injured spinal cord. Reproduced with permission from Guo et al. (2019). (E) Pharmacokinetic profiles of tramadol in the brain and plasma (F) following administration of the drug in the nasal nanovesicular system (TRA NVS) compared to the nasal nonvesicular system (TRA NV) and oral administration (TRA PO). The TRA PO and TRA NV groups showed lower levels than the NVS-treated animals. Reproduced with permission from Touitou et al. (2021).
Spinal cord injury (SCI) is a crippling disorder with few treatment options and erratic recovery. The inherent obstacle to axonal development in the pathological phase of SCI is mostly phosphatase and tensin homolog (PTEN). PTEN downregulates cytoplasmic mammalian target of rapamycin (mTOR) activity and plays an important role in regulating the regeneration of corticospinal neurons (Terenzio et al., 2018). MSC-Exos may be able to cross the BBB and move to the damaged spinal cord region (Figure 4D). In another animal studies, this type of EV has been shown to greatly increase mobility, sensory recovery, and urine reflex recovery (Guo et al., 2019).
A nasal nanovesicle delivery system (NVS) for pain treatment was created and developed in a recent study. Ketoprofen (KET), butorphanol (BUT), or tramadol (TRA) were incorporated in a phospholipid nanovesicular carrier in an animal model of pain, resulting in a faster onset and improved analgesic effectiveness (Touitou et al., 2021). According to pharmacokinetic results, the blood concentration of the ketoprofen nasal nanovesicular system (KET-NVS) after administration was higher than that of the drug after oral administration, the delivery time was shorter, and it took less time to reach the highest plasma concentrations (Figures 4E, F). This phenomenon is also consistent in the tramadol nasal nanovesicular system (TRA-NVS). Taken together, the NVS not only offers a high transmission efficiency but is also safe for the nasal mucosa. In conclusion, this noninvasive, rapid, cell-free, lesion specific, and effective therapy has a lot of potential for therapeutic usage in brain disorders.
Chronic rhinosinusitis (CRS) is a common clinical inflammatory disease of the nasal cavity and sinuses that often involves multiple sinuses (Stevens et al., 2015). The pathogenesis of CRS is complicated, and many internal and external factors influence its occurrence and development. EVs have also been found to play an important role in chronic sinusitis. Nasal mucus-derived EVs (NMDEs) containing cystatin-SA (CST-2) can predict the severity and phenotype of the CRS phenotype (Miyake et al., 2019). Pappalysin-1 (PAPP-A), an EVs biomarker, can not only monitor the severity of CRS but also predict recurrence at an early stage (Mueller, 2021). Previous study revealed that the nasal microbiota in patients with CRS had increased abundance and reduced diversity (Choi et al., 2014), and that bacterial-derived EVs may be responsible for the onset of CRS and inflammation (Miyake et al., 2019). LPS exposure in vivo and in vitro induced a 2-fold increase in NMDEs secretion (Nocera et al., 2019). P-glycoprotein (P-gp) was enriched in CRSwNP NMDEs and those NMDEs are capable of rapid interepithelial protein transfer (Nocera et al., 2017) (Figures 5A–C). These EVs not only exhibit direct microbiocidal activity but also actively arm epithelial cells with immunoprotective proteins that can be used to defend against the same microorganisms in mucus.
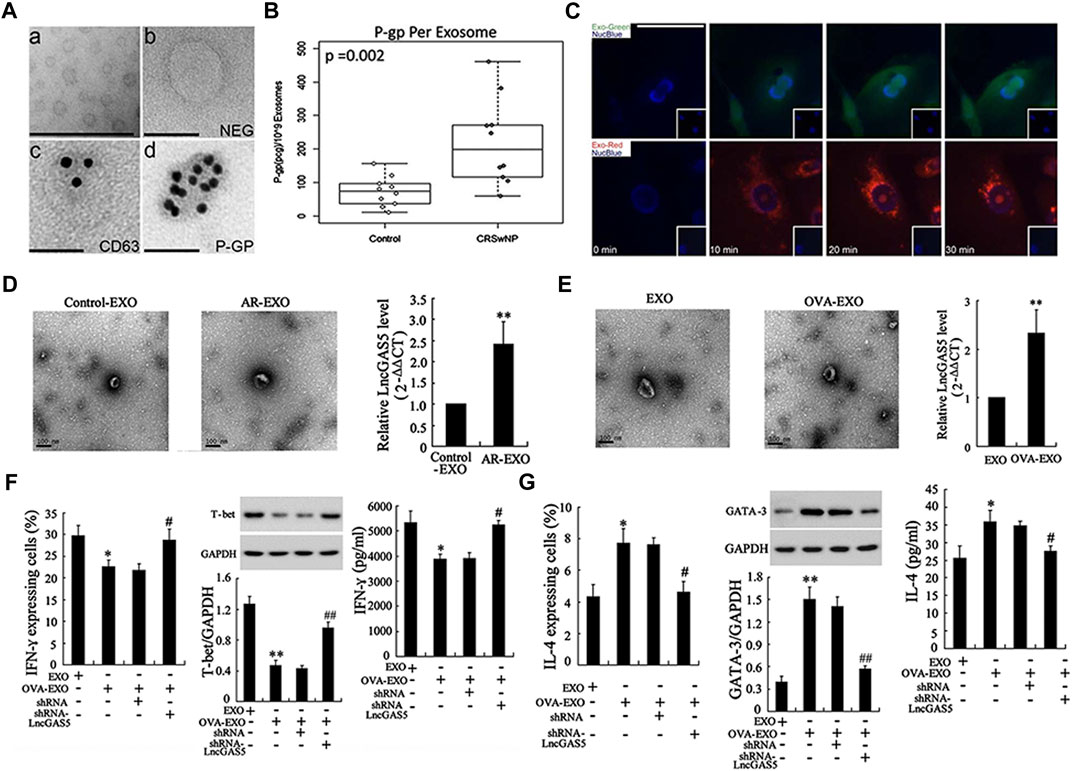
FIGURE 5. Research progress of exosomes in chronic rhinosinusitis and allergic rhinitis: (A) transmission electron microscopy images showed exosomes purified from nasal mucus (i) and negative controls (ii), immunolabeling of exosome marker CD63 (iii), and P-gp (iv) localization on exosome membranes. (B) The concentration of P-gp per exosome was significantly higher in patients with CRSwNP compared to the control group. (C) Time-lapse fluorescent images showed rapid absorption of labeled exosomal proteins and RNA within 10 min after exposure to purified exosomes. Reproduced with permission from Nocera et al. (2017). (D) LncGAS5 was upregulated in both exosomes of AR mucosa (AR-EXO) and OVA-treated RPMI 2650 cells (OVA-EXO) (E). (F) LncGAS5 in OVA-EXO suppresses Th1 differentiation via regulating EZH2 and T-bet. (G) LncGAS5 in OVA-EXO promotes Th2 differentiation via regulating EZH2 and T-bet. Reproduced with permission from Zhu et al. (2020).
Allergic rhinitis (AR) is one of the most common upper respiratory disorders worldwide, with a convoluted cause. It is a nasal mucosal inflammatory condition induced by allergen exposure. The imbalance between Th1 and Th2 differentiation is implicated in the development of AR (Eifan and Durham, 2016). Recent research suggests that EVs may have a role in immune-driven illness such as allergies. MiRNA profiles in AR patients’ EVs were significantly changed as compared to healthy controls. Furthermore, considerable enrichment of these differentially expressed miRNAs in certain biological and physiological processes, such as the B cell receptor signaling, natural killer cell-mediated cytotoxicity, and T cell receptor signaling pathway, have been established (Hovhannisyan et al., 2021). Another study found that miR-146a-loaded nasal epithelial cell (NEC)-derived EVs increased IL-10 production in monocytes, which inhibited downstream allergy reactions. IL-10+ monocytes exert immunosuppressive effects on CD4+ effector T cells and Th2 polarization in this mouse AR model (Zhou et al., 2021). GAS5, a long noncoding RNA (lncRNA), is expressed in nasal mucus-derived EVs as well as ovalbumin-stimulated NEC-derived EVs in AR (Figures 5D, E). And EVs loaded with the lncRNA GAS5 could inhibit Th1 cell development while inducing Th2 differentiation (Zhu et al., 2020) (Figures 5F, G).
EVs have the potential to overcome biological barriers by themselves, and their ability to express particular proteins, cell adhesion molecules, or ligands that cause natural target selectivity for certain recipient cells is what makes them appealing as delivery vehicles. Artificial EVs for intranasal administration have been widely used as vectors for delivering therapeutic drugs in some kinds of brain illness. However, its transmission efficiency still needs to be further improved. The intranasally administered drug enters the parenchymal space of the brain or cerebrospinal fluid (CSF) via trigeminal and olfactory nerves via axonal or endocytic routes and intra- or extra-neuronal pathways, respectively (Djupesland PG and Mahmoud, 2014). Although nasal administration bypasses the BBB, the mucosal barrier still important in delivery. The medicine must be deposited on the luminal surface of the epithelial membrane and absorbed before being removed or destroyed in the respiratory system. Controlling the drug’s release profile as it passes through different biological barriers may also be required for adequate absorption (Ghadiri et al., 2019).
Many artificial approaches, such as in situ chitosan hydrogels, mucoadhesive nanostructured lipid carriers, and chitosan nanoparticles, have been used to overcome the nasal mucosa barrier and improve the efficacy of drug therapy. However, several of these techniques may cause nasal toxicity and membrane component leaching, resulting in local irritation of the nasal mucosa. Full artificial EVs based on phospholipids have been developed for nasal delivery. This carrier has demonstrated the potential to improve drug delivery to the brain and the systemic circulation without generating nasal mucosal irritation or toxicity. Furthermore, the carrier combining magnesium ions with soft phospholipid vesicles and a mucoadhesive molecule called the phospholipid magnetosome was performed to explore a transport carrier with higher efficiency (Natsheh and Touitou, 2018). This chemically modified nasal carrier can boost pharmacological effects, probably by a combination of absorption enhancement and prolongation of mucosal contact. It seems to solve the difficulties caused by the BBB and mucosal barrier, but its safety and other aspects are still being studied as a new method.
Although several studies demonstrated that nasal administration of artificial nanovesicles had significant advantages in safety and transport efficiency on the treatment of brain illness. Collectively, the BBB is considered to be the main barrier to drug penetration into the CNS. Intranasal administration is an attractive alternative route to central nervous system administration because it bypasses the BBB more effectively than systemic administration. Artificial EVs can effectively cross the BBB. Intranasal administration of artificial EVs may be particularly appropriate for drugs that cannot achieve perfect blood concentrations in the brain due to the presence of the BBB, drugs that tend to cause adverse effects in peripheral tissues or in the blood, and drugs that are easily degraded by intravenous or oral administration. Although intranasal treatment has been shown to be effective in many brain illnesses, it has not been used in chronic inflammatory nose disorders. Only few reports on the use of artificial EVs in the treatment of chronic nasal diseases, such as chronic rhinosinusitis and allergic rhinitis. The great ability of artificial EVs in nasal disorders of the nasal cavity can be further explored in the future.
JZ: Conceptualization, Project administration, Writing–original draft. JY: Conceptualization, Writing–original draft. JJ: Conceptualization, Funding acquisition, Writing–review and editing. XW: Funding acquisition, Supervision, Writing–review and editing. YZ: Conceptualization, Funding acquisition, Supervision, Writing–original draft, Writing–review and editing. LZ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China (2022YFC2504100), the program for the Changjiang Scholars and Innovative Research Team (IRT13082), the National Natural Science Foundation of China (82000962, 82171110, and 81970852), Beijing Hospitals Authority Youth Programme (QML20230201), the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-022), Beijing Municipal Science and Technology Commission (Z211100002921057), the Capital’s funds for health improvement and research (2022-1-1091), the Beijing Natural Science Foundation (7212013 and 7222024), the Beijing New-star Plan of Science and Technology (20220484226), and the Public Welfare Development and Reform Pilot Project (2019-10).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aday, S., Hazan-Halevy, I., Chamorro-Jorganes, A., Anwar, M., Goldsmith, M., Beazley-Long, N., et al. (2021). Bioinspired artificial exosomes based on lipid nanoparticles carrying let-7b-5p promote angiogenesis in vitro and in vivo. Mol. Ther. 29 (7), 2239–2252. doi:10.1016/j.ymthe.2021.03.015
Choi, E. B., Hong, S. W., Kim, D. K., Jeon, S. G., Kim, K. R., Cho, S. H., et al. (2014). Decreased diversity of nasal microbiota and their secreted extracellular vesicles in patients with chronic rhinosinusitis based on a metagenomic analysis. Allergy 69 (4), 517–526. doi:10.1111/all.12374
Djupesland Pg, M. J., and Mahmoud, R. A. (2014). The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther. Deliv. 6 (5), 709–733. doi:10.4155/tde.14.41
Eifan, A. O., and Durham, S. R. (2016). Pathogenesis of rhinitis. Clin. Exp. Allergy 46 (9), 1139–1151. doi:10.1111/cea.12780
García-Manrique, P., Gutiérrez, G., and Blanco-López, M. C. (2018). Fully artificial exosomes: towards new theranostic biomaterials. Trends Biotechnol. 36 (1), 10–14. doi:10.1016/j.tibtech.2017.10.005
Ghadiri, M., Young, P. M., and Traini, D. (2019). Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics 11 (3), 113. doi:10.3390/pharmaceutics11030113
Guo, S., Perets, N., Betzer, O., Ben-Shaul, S., Sheinin, A., Michaelevski, I., et al. (2019). Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog sirna repairs complete spinal cord injury. ACS Nano 13 (9), 10015–10028. doi:10.1021/acsnano.9b01892
Hood, J. L., and Wickline, S. A. (2012). A systematic approach to exosome-based translational nanomedicine. Wires Nanomed Nanobi 4 (4), 458–467. doi:10.1002/wnan.1174
Hovhannisyan, L., Czechowska, E., and Gutowska-Owsiak, D. (2021). The role of non-immune cell-derived extracellular vesicles in allergy. Front. Immunol. 12, 702381. doi:10.3389/fimmu.2021.702381
Hussein, M. E., Mohammed, H. E., El Menshawe, S. F., and Salem, H. F. (2019). Transfersomal nanovesicles for nose-to-brain delivery of ofloxacin for better management of bacterial meningitis: formulation, optimization by box-behnken design, characterization and in vivo pharmacokinetic study. J. Drug Deliv. Sci. Tec. 54, 101304. doi:10.1016/j.jddst.2019.101304
Kalani, A., Chaturvedi, P., Kamat, P. K., Maldonado, C., Bauer, P., Joshua, I. G., et al. (2016). Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 79, 360–369. doi:10.1016/j.biocel.2016.09.002
Khan, M., Nickoloff, E., Abramova, T., Johnson, J., Verma, S. K., Krishnamurthy, P., et al. (2015). Embryonic stem cell–derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 117 (1), 52–64. doi:10.1161/CIRCRESAHA.117.305990
Kim, M., Lee, Y., and Lee, M. (2021). Hypoxia-specific anti-rage exosomes for nose-to-brain delivery of anti-mir-181a oligonucleotide in an ischemic stroke model. Nanoscale 13 (33), 14166–14178. doi:10.1039/d0nr07516g
Kodali, M., Castro, O. W., Kim, D. K., Thomas, A., Shuai, B., Attaluri, S., et al. (2019). Intranasally administered human msc-derived extracellular vesicles pervasively incorporate into neurons and microglia in both intact and status epilepticus injured forebrain. Int. J. Mol. Sci. 21 (1), 181. doi:10.3390/ijms21010181
Kumar, H., Mishra, G., Sharma, A. K., Gothwal, A., Kesharwani, P., and Gupta, U. (2017). Intranasal drug delivery: a non-invasive approach for the better delivery of neurotherapeutics. Pharm. Nanotechnol. 5 (3), 203–214. doi:10.2174/2211738505666170515113936
Li, W., Suwanwela, N. C., and Patumraj, S. (2017). Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of nf-κb, icam-1, mmp-9 and caspase-3 expression. Mol. Med. Rep. 16 (4), 4710–4720. doi:10.3892/mmr.2017.7205
Li, Y. J., Wu, J. Y., Liu, J., Xu, W., Qiu, X., Huang, S., et al. (2021). Artificial exosomes for translational nanomedicine. J. Nanobiotechnology 19 (1), 242. doi:10.1186/s12951-021-00986-2
Liang, Y., Duan, L., Lu, J., and Xia, J. (2021). Engineering exosomes for targeted drug delivery. Theranostics 11 (7), 3183–3195. doi:10.7150/thno.52570
Lin, Y., Li, S., Xiao, Z., Chen, S., Yang, L., Peng, Q., et al. (2021). Epigenetic inhibition assisted chemotherapeutic treatment of lung cancer based on artificial exosomes. Pharmacol. Res. 171, 105787. doi:10.1016/j.phrs.2021.105787
Lochhead, J. J., and Thorne, R. G. (2012). Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 64 (7), 614–628. doi:10.1016/j.addr.2011.11.002
Lochhead, J. J., Yang, J., Ronaldson, P. T., and Davis, T. P. (2020). Structure, function, and regulation of the blood-brain barrier tight junction in central nervous system disorders. Front. Physiol. 11, 914. doi:10.3389/fphys.2020.00914
Miyake, M. M., Workman, A. D., Nocera, A. L., Wu, D., Mueller, S. K., Finn, K., et al. (2019). Discriminant analysis followed by unsupervised cluster analysis including exosomal cystatins predict presence of chronic rhinosinusitis, phenotype, and disease severity. Int. Forum Allergy Rhinol. 9 (9), 1069–1076. doi:10.1002/alr.22380
Mueller, S. K. (2021). The role of exosomes in the pathophysiology of chronic rhinosinusitis. Front. Cell Infect. Microbiol. 11, 812920. doi:10.3389/fcimb.2021.812920
Natsheh, H., and Touitou, E. (2018). Phospholipid magnesome-a nasal vesicular carrier for delivery of drugs to brain. Drug Deliv. Transl. Res. 8 (3), 806–819. doi:10.1007/s13346-018-0503-y
Nocera, A. L., Mueller, S. K., Stephan, J. R., Hing, L., Seifert, P., Han, X., et al. (2019). Exosome swarms eliminate airway pathogens and provide passive epithelial immunoprotection through nitric oxide. J. Allergy Clin. Immunol. 143 (4), 1525–1535 e1. doi:10.1016/j.jaci.2018.08.046
Nocera, A. L., Miyake, M. M., Seifert, P., Han, X., and Bleier, B. S. (2017). Exosomes mediate interepithelial transfer of functional p-glycoprotein in chronic rhinosinusitis with nasal polyps. Laryngoscope 127 (9), E295–E300. doi:10.1002/lary.26614
Quesenberry, P. J., and Aliotta, J. M. (2010). Cellular phenotype switching and microvesicles. Adv. Drug Deliv. Rev. 62 (12), 1141–1148. doi:10.1016/j.addr.2010.06.001
Ramasamy, R., and Schmidt, A. M. (2012). Receptor for advanced glycation end products (rage) and implications for the pathophysiology of heart failure. Curr. Heart Fail Rep. 9, 107–116. doi:10.1007/s11897-012-0089-5
Ren, Y., Yu, K., Sun, S., Li, Z., Yuan, J., Han, X. D., et al. (2014). Jsi124 inhibits breast cancer cell growth by suppressing the function of b cells via the downregulation of signal transducer and activator of transcription 3. Oncol. Lett. 8 (2), 928–932. doi:10.3892/ol.2014.2221
Sandbhor, P., Goda, J., Mohanty, B., Chaudhari, P., Dutt, S., and Banerjee, R. (2021). Non-invasive transferrin targeted nanovesicles sensitize resistant glioblastoma multiforme tumors and improve survival in orthotopic mouse models. Nanoscale 14 (1), 108–126. doi:10.1039/d1nr05460k
Staufer, O., Dietrich, F., Rimal, R., Schröter, M., Fabritz, S., Boehm, H., et al. (2021). Bottom-up assembly of biomedical relevant fully synthetic extracellular vesicles. Sci. Adv. 7 (36), eabg6666. doi:10.1126/sciadv.abg6666
Stevens, W. W., Lee, R. J., Schleimer, R. P., and Cohen, N. A. (2015). Chronic rhinosinusitis pathogenesis. J. Allergy Clin. Immunol. 136 (6), 1442–1453. doi:10.1016/j.jaci.2015.10.009
Su, Y., Li, G., Zhang, X., Gu, J., Zhang, C., Tian, Z., et al. (2008). JSI-124 inhibits glioblastoma multiforme cell proliferation through G2/M cell cycle arrest and apoptosis augmentation. Cancer Biol. Ther. 7 (8), 1243–1249. doi:10.4161/cbt.7.8.6263
Sun, D. Z. X., Xiang, X., Liu, Y., Zhang, S., Liu, C., Barnes, S., et al. (2010). A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 9 (18), 1606–1614. doi:10.1038/mt.2010.105
Terenzio, M., Koley, S., Samra, N., Rishal, I., Zhao, Q., Sahoo, P. K., et al. (2018). Locally translated mtor controls axonal local translation in nerve injury. Science 359 (6382), 1416–1421. doi:10.1126/science.aan1053
Touitou, E., and Illum, L. (2013). Nasal drug delivery. Drug Deliv. Transl. Res. 3 (1), 1–3. doi:10.1007/s13346-012-0111-1
Touitou, E., Natsheh, H., Boukeileh, S., and Awad, R. (2021). Short onset and enhanced analgesia following nasal administration of non-controlled drugs in nanovesicular systems. Pharmaceutics 13 (7), 978. doi:10.3390/pharmaceutics13070978
Towfighi, A., and Saver, J. L. (2011). Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke 42 (8), 2351–2355. doi:10.1161/STROKEAHA.111.621904
van de Beek, D. (2012). Progress and challenges in bacterial meningitis. Lancet 380 (9854), 1623–1624. doi:10.1016/S0140-6736(12)61808-X
Vázquez-Ríos, A. J., Molina-Crespo, Á., Bouzo, B. L., López-López, R., Moreno-Bueno, G., and de la Fuente, M. (2019). Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J. nanobiotechnology 17, 85–15. doi:10.1186/s12951-019-0517-8
Wang, K., Kumar, U. S., Sadeghipour, N., Massoud, T. F., and Paulmurugan, R. (2021). A microfluidics-based scalable approach to generate extracellular vesicles with enhanced therapeutic microrna loading for intranasal delivery to mouse glioblastomas. ACS Nano 15 (11), 18327–18346. doi:10.1021/acsnano.1c07587
Zhang, K.-L., Wang, Y.-J., Sun, J., Zhou, J., Xing, C., Huang, G., et al. (2019b). Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy. Chem. Sci. 10 (5), 1555–1561. doi:10.1039/c8sc03224f
Zhang, Y. L. Y., Liu, H., and Tang, W. H. (2019a). Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 9 (19), 19. doi:10.1186/s13578-019-0282-2
Zhao, M., van Straten, D., Broekman, M. L. D., Preat, V., and Schiffelers, R. M. (2020). Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 10 (3), 1355–1372. doi:10.7150/thno.38147
Zhou, H., Ma, Q., Zhu, P., Ren, J., Reiter, R. J., and Chen, Y. (2018). Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J. Pineal Res. 64 (3), e12471. doi:10.1111/jpi.12471
Zhou, J., Lu, Y., Wu, W., and Feng, Y. (2021). Hmsc-derived exosome inhibited th2 cell differentiation via regulating mir-146a-5p/serpinb2 pathway. J. Immunol. Res. 2021, 1–11. doi:10.1155/2021/6696525
Zhu, X., Wang, X., Wang, Y., and Zhao, Y. (2020). Exosomal long non-coding rna gas5 suppresses th1 differentiation and promotes th2 differentiation via downregulating ezh2 and t-bet in allergic rhinitis. Mol. Immunol. 118, 30–39. doi:10.1016/j.molimm.2019.11.009
Keywords: artificial exosomes, drug delivery, intranasal administration, blood-brain barrier, biomedical applications
Citation: Zhao J, Yang J, Jiao J, Wang X, Zhao Y and Zhang L (2023) Biomedical applications of artificial exosomes for intranasal drug delivery. Front. Bioeng. Biotechnol. 11:1271489. doi: 10.3389/fbioe.2023.1271489
Received: 02 August 2023; Accepted: 21 August 2023;
Published: 07 September 2023.
Edited by:
Qing Liu, Kansai University, JapanReviewed by:
Xiaocui Fang, Chinese Academy of Sciences (CAS), ChinaCopyright © 2023 Zhao, Yang, Jiao, Wang, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luo Zhang, ZHIubHVvemhhbmdAMTM5LmNvbQ==; Yan Zhao, emhhb3lhbnJheUAxMjYuY29t
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.