- 1Eco-Environmental Protection Research Institute, Shanghai Academy of Agricultural Sciences, Shanghai, China
- 2State Key Laboratory of Pollution Control and Resource Reuse, College of Environmental Science and Engineering, Tongji University, Shanghai, China
- 3Shanghai Engineering Research Centre of Low-Carbon Agriculture, Shanghai, China
- 4National Engineering Research Center of Protected Agriculture, Shanghai Engineering Research Center of Protected Agriculture, Tongji University, Shanghai, China
- 5Soils and Water Department, Faculty of Agriculture, Soils and Water Department, Benha University, Benha, Egypt
- 6Department of Biology, Faculty of Arts and Sciences, Najran University, Najran, Saudi Arabia
- 7Soils and Water Department, Faculty of Agriculture, New Valley University, New Valley, Egypt
- 8National Committee of Soil Science, Academy of Scientific Research and Technology, Cairo, Egypt
Environmental pollution with potentially toxic elements (PTEs) has become one of the critical and pressing issues worldwide. Although these pollutants occur naturally in the environment, their concentrations are continuously increasing, probably as a consequence of anthropic activities. They are very toxic even at very low concentrations and hence cause undesirable ecological impacts. Thus, the cleanup of polluted soils and water has become an obligation to ensure the safe handling of the available natural resources. Several remediation technologies can be followed to attain successful remediation, i.e., chemical, physical, and biological procedures; yet many of these techniques are expensive and/or may have negative impacts on the surroundings. Recycling agricultural wastes still represents the most promising economical, safe, and successful approach to achieving a healthy and sustainable environment. Briefly, biochar acts as an efficient biosorbent for many PTEs in soils and waters. Furthermore, biochar can considerably reduce concentrations of herbicides in solutions. This review article explains the main reasons for the increasing levels of potentially toxic elements in the environment and their negative impacts on the ecosystem. Moreover, it briefly describes the advantages and disadvantages of using conventional methods for soil and water remediation then clarifies the reasons for using biochar in the clean-up practice of polluted soils and waters, either solely or in combination with other methods such as phytoremediation and soil washing technologies to attain more efficient remediation protocols for the removal of some PTEs, e.g., Cr and As from soils and water.
1 Introduction
Pollution is a global challenge that negatively affects life on Earth (Huang et al., 2019; Chen et al., 2020; Zheng et al., 2020). It is responsible for spreading many diseases and approximately 16% of premature death worldwide (Münzel et al., 2022). Since soil is the main terrestrial ecosystem (Qi et al., 2023) then soil pollution can threaten its biodiversity (Lu et al., 2020). Saving soil is essential to save the whole Earth (Gautam et al., 2023). This may take place via monitoring levels of contaminants in the environment and following up effective remediation routes to attain better environmental conditions.
Many contaminants undergo biodegradation while others are relatively stable in soil and water such as potentially toxic elements (PTEs) (Matin et al., 2020). Thus, these contaminants persist in soils for years (Zhang et al., 2020; Zhong et al., 2020) and can have devastating consequences on human health and the surrounding ecosystem (Gui et al., 2023), particularly on children (Egendorf et al., 2020). A point to note is that PTEs may further have negative impacts on female fertility and reproduction (Rashtian et al., 2019).
Environmental risks related to soil pollutants with PTEs should not be appraised only through soil screening levels but also by assessing their bio-available contents in soil (Galán et al., 2019). Mobile fractions of PTEs find their way to the groundwater (Farid et al., 2020) and transfer long distances via the hydraulic continuity of groundwater over vast areas to reach new lands which are not directly subjected to soil pollutants (Bassouny et al., 2020; Farid et al., 2020). Thus, following effective remediation methods could eliminate further environmental contamination with PTEs (Liu et al., 2020). These procedures include physical and chemical remediation methods, e.g., soil washing, encapsulation, soil replacement electrokinetic methods (Chen et al., 2020), amending soils with iron nanomaterials (Baragaño et al., 2020) or hydroxyapatite (Ibrahim et al., 2020)
Water pollution is also of growing concern (Kumar et al., 2019; Dar and Bhat, 2020) because it is a vital resource for all living organisms (Saini et al., 2020). Its decontamination is a requirement to attain better environmental conditions (Singh et al., 2020) following effective and safe remediation procedures (Sahoo and Swain, 2020), e.g., membrane filtration, reverse osmosis, and chemical precipitation (Saini et al., 2020). In spite of that, many of these methods are expensive (Koffi and Okabe, 2020). Otherwise, introducing low-cost materials of high sorptivity might be the optimum choice for water decontamination (Tauqeer et al., 2020). For example, biochar (Zheng et al., 2020) can effectively remove PTEs from contaminated waters within short time periods (Senthilkumar et al., 2020). Its mode of action is via 1) decreasing the solubility of inorganic pollutant ions in soil (Zheng et al., 2020) and water (Shaheen et al., 2019b) because of its alkaline nature (Shi et al., 2020) and it may also form metal ion-chelators (Naveed et al., 2020) of high solubility (Elshony et al., 2019); 2) binding contaminants with the functional groups of biochar to become less mobile or even immobile (Bandara et al., 2020); 3) increasing glomalin-related soil protein (GRSP) content in soil (Dubey et al., 2020) which sustains soil quality and minimizes contaminants transfer from soil to aquatic ecosystems (Wang et al., 2020); and 4) stimulating the activity of soil bacteria (Lévesque et al., 2020), especially endophytes (Waqas et al., 2017), to assist host plants to survive under high levels of organic and inorganic pollutants in soil (He et al., 2020).
More details on the advantages and disadvantages of the conventional physical and chemical remediation techniques that are used in decontaminating soils and waters are discussed further. This review also addresses the feasibility of using biochar as a safe organic resource to remediate contaminated soils and water and possible challenges that may affect PTEs binding with biochar to attain successful remediation procedures.
2 Environment
The environment is defined as “the sum of all surroundings, including natural resources and other factors that may affect growth and development of living organisms. It is the place (soils, water, air and food) that needs to be protected and restored.” However, unmanaged handling of the environmental resources has resulted in their contamination with PTEs (Abdelhafez and Li, 2014; Abdelhafez and Li, 2015; Abdelhafez et al., 2016; ElShazly et al., 2019a; ElShazly et al., 2019b; Ali et al., 2023; Farid et al., 2023).
2.1 Environmental contamination with PTEs
The term “environmental contamination” signifies the existence of unwanted constituents (contaminants) of any type from industrial, municipal, and agricultural wastes in the natural environment (Katayama et al., 2010). They usually originate from anthropogenic sources. Heavy metal “is a general collective term, which refers to the group of metals and metalloids of atomic density greater than 4,000 kg m-3, or in other terms their densities are five times more than water” (Nagajyoti et al., 2010). These contaminants are not biodegradable and thus adversely affect the environment (Jinping et al., 2010; Abbas and Abdelhafez, 2013; Abdelhafez and Li, 2014). Generally, most heavy metals are non-essential, e.g., Pb, Cd, Cr, Hg, and As while others, e.g., Fe, Cu, and Zn, are essential for several organisms (known as trace elements). Thus, the term “heavy metals” is vague and meaningless with no chemical or toxicological basis (Duffus, 2002). Alternatively, the term “Potentially Toxic Elements, PTEs” is in use, which is applicable only to the non-essential elements, e.g., Pb and Cd (Nagajyoti et al., 2010).
2.2 Sources of contamination with PTEs
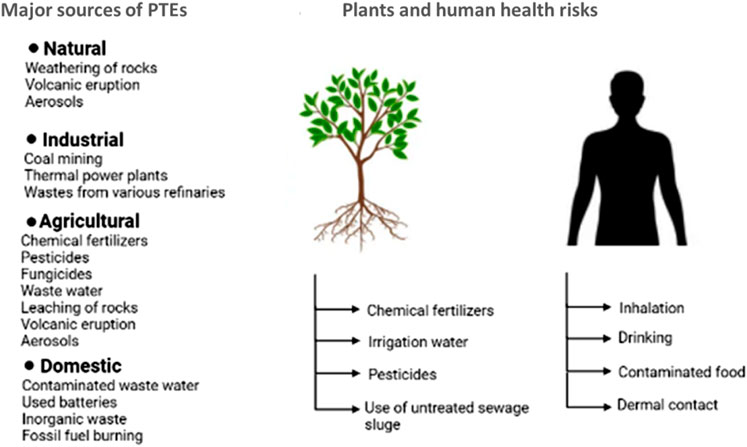
The major sources of environmental pollution are probably anthropogenic activities that result from unmanaged practices (Yaron et al., 2012; Abdelhafez and Li, 2014).
2.2.1 Natural sources of PTEs
During rock weathering, many contaminants find their way to surface water and/or groundwater hence possessing potential threats to the surroundings (Ma et al., 2019).
2.2.2 Agricultural practices and PTEs
Agricultural agrochemicals for fertilization and pesticides are widely used worldwide in food production (Abdelhafez et al., 2012) to satisfy the needs of the growing population (Abbas and Meharg, 2008; Abdelhafez et al., 2012; Eid et al., 2019; Mohamed et al., 2019; Abdelhafez et al., 2021). These agrochemicals contaminate agricultural soils with PTEs (Nagajyoti et al., 2010), representing potential ecological risk factors. Likewise, organic fertilizers such as animal manures and sewage sludge enrich soils with Mn, Zn, Cu, Co, Cr, Pb, Ni, and Cd upon their extensive use as fertilizers or amendments (Verklejim, 1993).
2.2.3 Industrial sources of PTEs
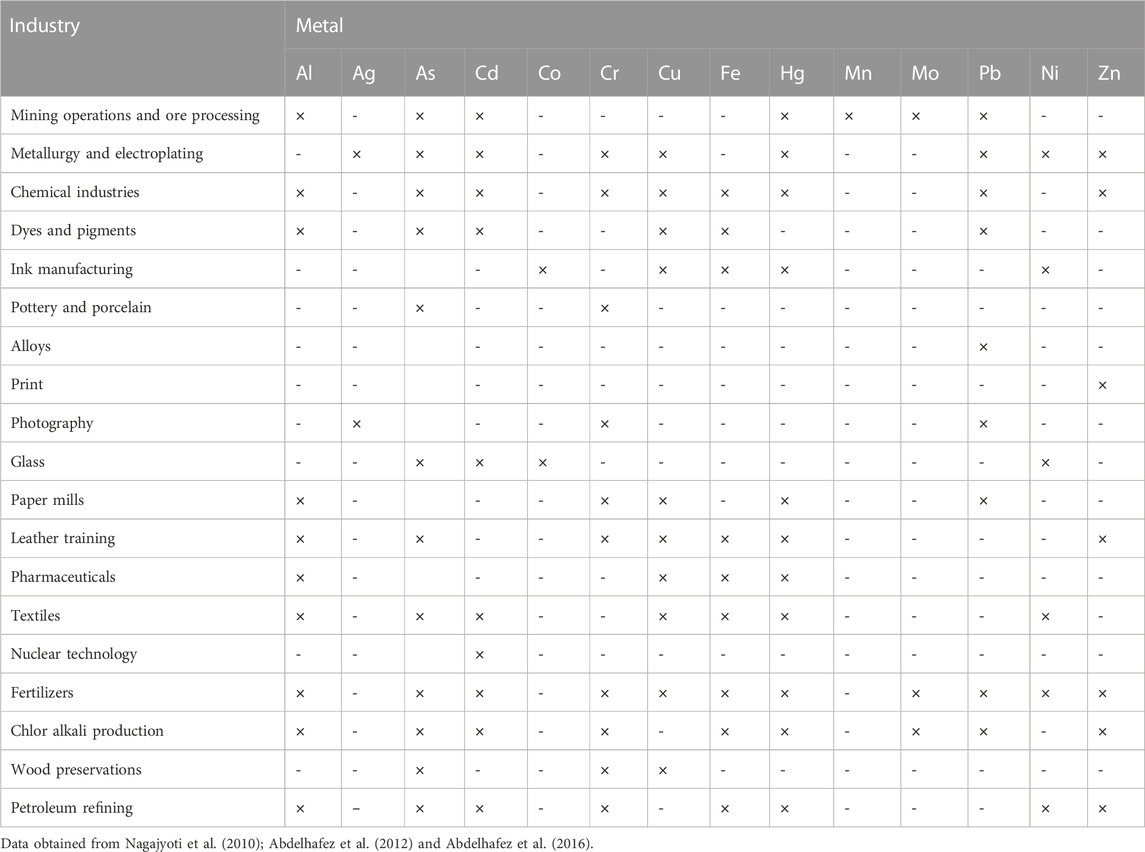
Rapid urbanization and industrialization, particularly in developing countries discharge PTEs into rivers and soils. These effluents may change the physical, chemical, and biological conditions of water bodies (Sangodoyin, 1991) while increasing the potential risk associated with using these waters. In the Jinxi River in China, anthropogenic activities were the major source of contamination of water streams with PTEs (Abdelhafez and Li, 2014; Abdelhafez and Li, 2015). Table 1 shows the abundance of metals in effluents from different industrial activities (Abdelhafez et al., 2009; Abdelhafez et al., 2010).
Other activities such as mining, refining, smelting, and metal grinding may bring considerable concentrations of PTEs to the surrounding environment (Herawati et al., 2000; Yanqun et al., 2005; Abdelhafez et al., 2016; Mohamed et al., 2018).
Metal ions may be emitted into the atmosphere in the forms of particulates and vapor when subjected to high temperatures and then react with water vapors forming aerosols which finally find their way to soil and water through dry deposition (dispersion by wind) or wet deposition (precipitated in rainfall). In shooting range and smelting operation soils, the levels of Pb sometimes exceeded 1% (Abdelhafez et al., 2014; Abdelhafez et al., 2016).
2.2.4 Soil pollution in relation to domestic and industrial effluents
Many water streams have become contaminated with PTEs via the discharge of industrial and domestic wastes. These contaminants find their way to the topsoil of the surrounding arable lands. Once they come in contact with soil particles, they become sorbed and this process is controlled by diffusion (Abbas and Bassouny, 2018). Considerable amounts of PTEs may go deeper into the soil through common agricultural practices, e.g., plowing and tillage (Hashim et al., 2017). Moreover, hydraulic continuity that exists between ground waters transfers contaminants to locations not directly irrigated with wastewater (Farid et al., 2020).
2.2.5 Aerosols and PTEs
Tiny solid or liquid particles suspended in the Earth’s atmosphere are known as aerosols (Seinfeld and Pandis, 2016). Generally, aerosols are of special importance on a global scale. In this concern, volcanic eruptions are a geothermal source of atmospheric contamination (Gudmundsson et al., 2019). The transportation and deposition of these aerosols increase the potentiality of PTE dispersion in the environment (Soltani et al., 2017). The transmitted fine particulates may be blown over a great distance and accelerated by downpours or snowfall (Behera et al., 2015; ElShazly et al., 2019a).
2.2.6 Other sources of environmental pollution with PTEs
Burning, landfills, incineration, and transportation (automobiles, diesel-powered vehicles, and aircraft) are additional sources of environmental pollution that add Cd, Co, Zn, Cr, Cu, Pb, Hg, Mn, Ni, Al, Fe, and Ti to the environment (Verklejim, 1993; Al-Hiyaly et al., 1998; Hashim et al., 2017). Chromated copper arsenate (CCA) treated wood structures are another source of PTEs when CCA is used as a wood preservative against bacteria, fungi, and termites (Abdelhafez et al., 2009; Abdelhafez et al., 2010).
3 Plant response to PTEs
Plants stop growing or even die when grown on soils highly contaminated with PTEs. High levels of PTEs increase the formation of free radicals and reactive oxygen species that cause oxidative stress and cellular damage in plants (Goyal et al., 2020). To survive under such stressful conditions, plants secrete low molecular mass substances such as organic acids and glutathione that bind with PTEs and lessen their mobility in soil. Also, pectin in plant cell walls limits PTE absorption by plants (Feng et al., 2021). Once contaminants enter plant cells, they become sequestered within cellular compartments such as vacuoles and limit their translocation to areal plant parts (Goyal et al., 2020). Tolerant or even hyperaccumulator plants display further mechanisms for controlling these contaminants, nevertheless, they exhibit very slow growth rates and small biomasses (Khan, 2020). Instead, using plant growth-promoting bacteria and mycorrhizae can further improve plant-based remediation strategies (Khan, 2020). Bacteria such as Alcaligenes faecalis, Bacillus cereus, and A. faecalis (Zainab et al., 2021) stimulate the activities of anti-oxidative enzymes such as catalase, peroxidase, and superoxide dismutase (El-Meihy et al., 2019) which scavenge reactive oxygen species (Kaur et al., 2021) and thus help plants to cope with PTE stress and enhance plant growth (Zainab et al., 2021). Non-enzymatic antioxidants, e.g., ascorbate, and metal-binding peptides may also help to lessen metal toxicity within plants (Kaur et al., 2021). Mycorrhizae also retain contaminants in roots and decrease their translocation within plants (Adeyemi et al., 2021).
Phytohormones are chemical messengers that sustain plant growth under PTE stress (Sytar et al., 2019). For example, indole acetic acid (IAA) increases energy trapping capacity in photosystem II (PSII) reaction centers (Ouzounidou and Ilias, 2005). Salicylic acid decreases the levels of free oxygen radicals while increasing plant chlorophyll content (Sytar et al., 2019).
4 Impact of PTEs on human health
4.1 PTEs exposure pathways
Humans are exposed to PTEs through different routes: i) ingestion (oral), which includes drinking water, intake of fruit, vegetables, meat and dairy products, and fish and shellfish; ii) inhalation of dust and chemicals volatilized in the air; and iii) dermal contact between human skin and chemicals or soil (Abdelhafez and Li, 2015; Megido et al., 2017). According to Chan et al. (1995), PTEs transmit to humans mainly through inhalation and ingestion routes.Figure 1.
Ingestion is a common exposure route to PTEs (Abdelhafez et al., 2012; Abdelhafez and Li, 2015). It is worth noting that previous studies did not include the distribution pattern of PTEs within the fine fractions of agricultural soil, which presents potential hazards for human health. In this context, fine soil particles of diameters of 10 or 2.5 µm may adhere easily to the skin, carrying PTEs to the human body (Madrid et al., 2008; Kong et al., 2012; Abdelhafez and Li, 2015). These contaminants settle in the higher respiratory tract and the alveolar areas of the lungs (Ajmone-Marsana et al., 2008).
4.2 Health effects of PTEs on human health
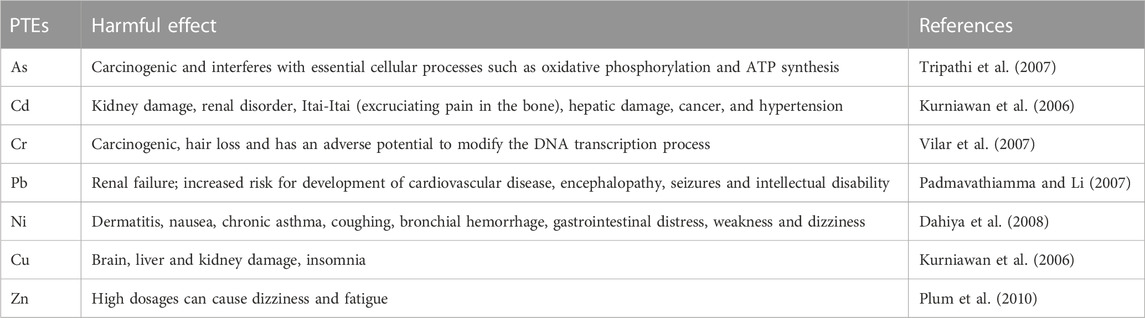
When these contaminants enter the food chain they have negative implications even at very low levels (Memon and Schröder, 2009). For more details see Table 2. The most problematic PTEs for human health are As, Cr, Cd, Cu, Pb, Zn, Cu, Hg, and Sn (Ghosh, 2010). In particular, As and Cr cause cancer, and Cd, Pb, and Ni lead to kidney failure and other symptoms (Kurniawan et al., 2006; Tripathi et al., 2007; Abbas and Bassouny, 2018). Accordingly, proper remediation protocols should be followed to improve and sustain the environment.
5 Remediation technologies of PTEs-contaminated water and soils
5.1 Remediation technologies of PTEs-contaminated water
There are several remediation protocols that can be followed for decontaminating wastewater, i.e., chemical (chemical precipitation and ion exchange and adsorption), physical (filtration and clarification), and biological (biosorption, biodegradation, and phytoremediation) remediation technologies. These techniques should be applied before water disposal from industries and municipalities into the surrounding environment.
5.1.1 Chemical remediation
Chemical precipitation protocols are broadly utilized for decontaminating wastewater containing high levels of PTEs. These procedures change the soluble contaminants into insoluble forms, thereby enabling their subsequent removal from the liquid phase by physical means, such as clarification and filtration (Arora et al., 2008). For instance, coagulants and flocculants enable the formation of particulate-sized aggregates, and their quantities depend on the pH and alkalinity of the treated water (Nomanbhay and Palanisamy, 2005). Granulated lime and calcium carbonate are efficient coagulants for the removal of As, Ni, Zn, and Cd from groundwater (Song et al., 2005; Lee et al., 2007). In addition, clay minerals can be used effectively to decontaminate aqueous solutions (ElShazly et al., 2019b).
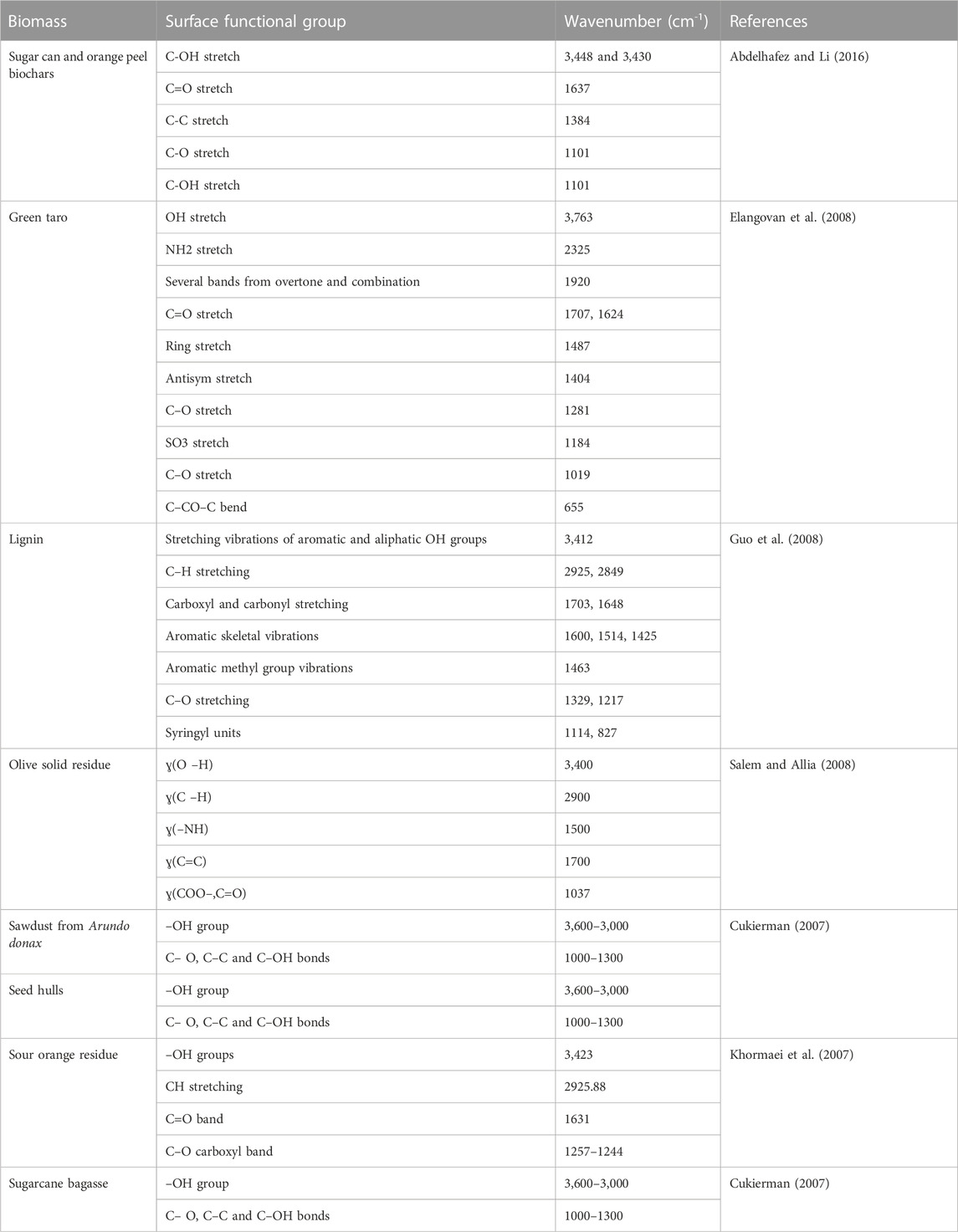
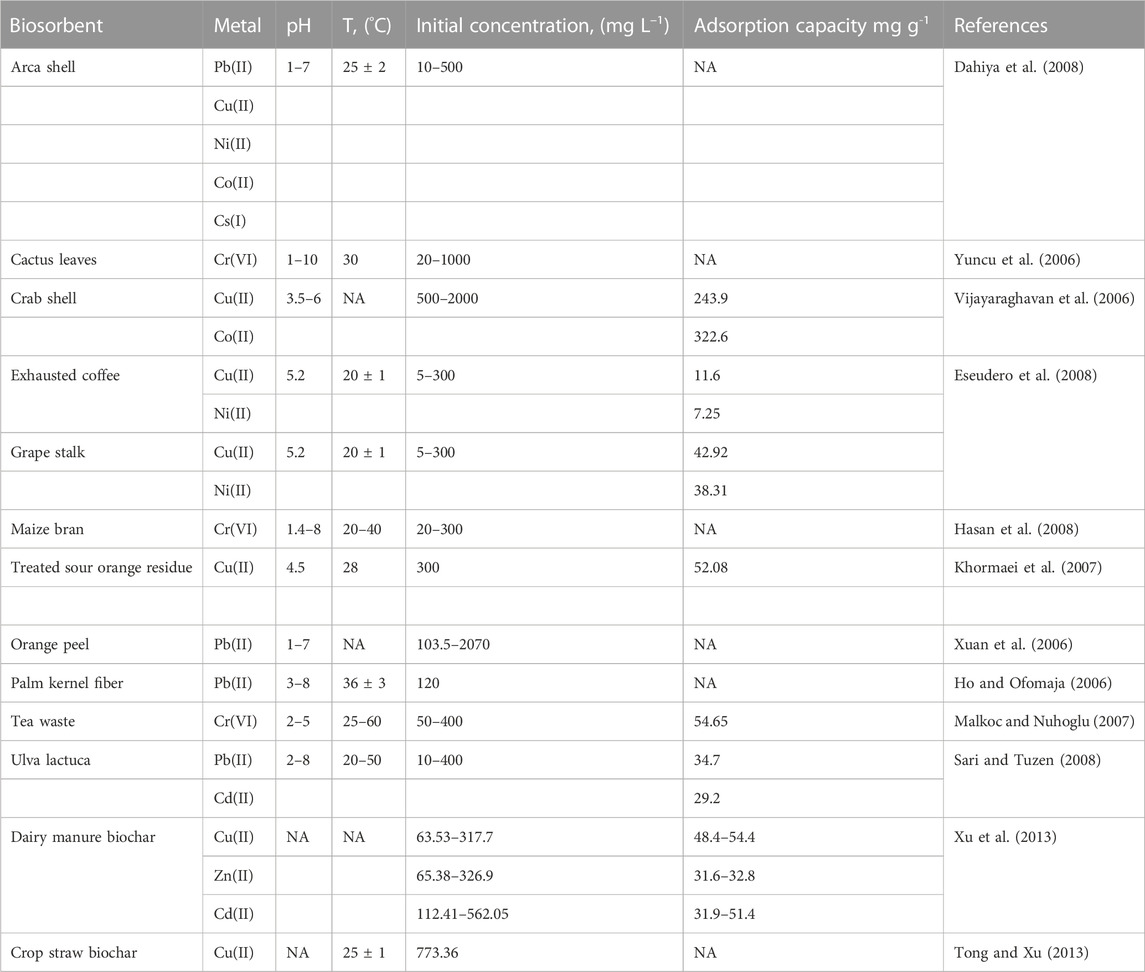
Surface functional groups play an important role in removing metal ions from water by using specific sorbent materials. Table 3 shows some of these functional groups. Herein, more natural and artificial biosorbent materials are examined as adsorbents for the removal of different PTEs from aqueous solutions. Table 4 presents the adsorbent capacities of different biosorbents for PTEs. The adsorption efficiency depends on the pH, sorbent dosage, contact time, temperature, and concentration of metal ions (Abdelhafez and Li, 2016; ElShazly et al., 2019b). Under low pH value, H+ competes with metal ions on surface functional groups of the sorbent material hence the removal efficiencies of metal ions decrease considerably (Arief et al., 2008).
5.1.2 Physical remediation
Water decontamination can take place via using filtration, air stripping, granular activated carbon absorption, or their combination (Wilson and Clarke, 1993). However, more attention should be paid when using washing technology to remove PTEs due to the leachability of major nutrients (N, P, and K).
5.1.3 Biological remediation
The use of biological remediation technologies is thought to be the optimum tool for remediating contaminated waters/soils.
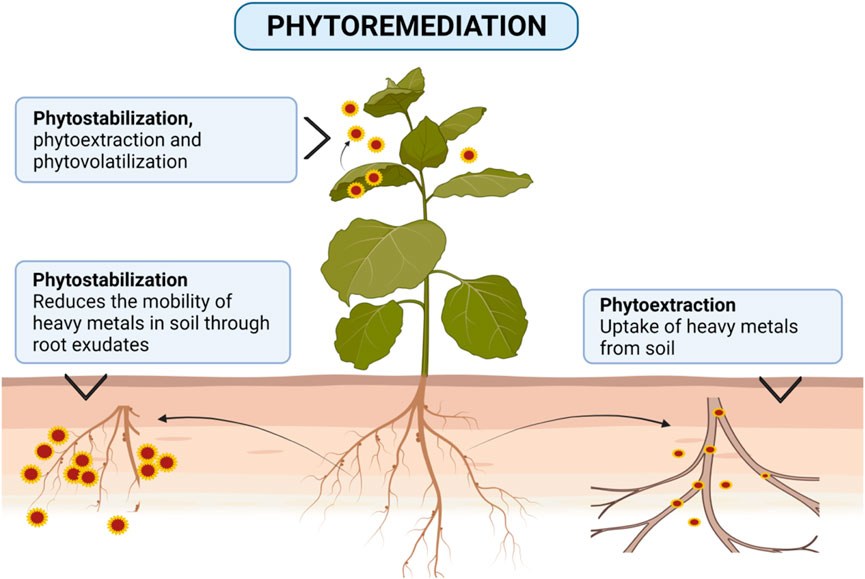
In this regard, the use of bacteria, fungi, and algae is economical, eco-friendly, and gives good results (Valls and Lorenzo, 2002). These microbes remove contaminants from water in their bodies (Ozdemir et al., 2003; Zouboulis et al., 2004; Congeevaram et al., 2007). Also, plant-induced phytoremediation can degrade or eliminate PTEs in contaminated water/soil. Phytoremediation exploits the plant’s innate biological mechanisms for removing PTEs or eliminates its adverse effects through different mechanisms (Ghosh and Singh, 2005) (Figure 2) as follows.
i) Phytoextraction: the ability to grow plants to absorb and accumulate toxic metals from water
ii) Phytovolatilization: evaporating certain metals through the above-ground parts of the plant
iii) Rhizofiltration: the use of plant roots to remove PTEs from contaminated waters.
5.2 Soil remediation technologies
Soil remediation is performed to achieve one of the following goals: 1) removal/extraction of the PTEs from contaminated soils by electrokinetic and/or washing procedures, which is an expensive procedure and might not be applicable for decontaminating vast areas of contaminated soils (Ko et al., 2006; Dermont et al., 2008) or 2) reducing metal mobility with “in situ” technologies such as stabilization by different amendments (organic or inorganic) (Chen et al., 2006; Sunarso and Ismadji, 2009) but the contaminants still exist in the soil. Overall, in situ soil remediation technologies are directed toward reducing the risk of PTEs in soils and can be classified into four main categories.
5.2.1 Excavation
Excavation is the oldest remediation technology for decontaminating soils, in which contaminated soil layers are replaced by clean ones (Lanphear et al., 2003). However, this method leads to the transfer of contaminants from one place to another, the spread of dust particles, and the transport of contaminated soil to other regions. As a matter of fact, excavation is considered the most expensive method of soil remediation (Lambert et al., 2014; González-Martínez et al., 2019).
5.2.2 Soil washing
Soil washing is a common technique for remediating soils contaminated with PTEs (Khan et al., 2004) in the presence of synthetic complexing agents, using chelators such as ethylene di amine tetra acetic acid (EDTA) and nitrilotriacetate (NTA) to enhance further removal efficiencies of soil contaminants (Arwidsson et al., 2010). However, the low decomposition rates of chelators in soil may cause toxicity and stress to soil biota (Nowack, 2002).
5.2.3 Phytoremediation
Some plants can take up and accumulate contaminants in their aboveground parts (Ebrahimbabaie et al., 2020; Lee et al., 2021), thus limiting their negative consequences to the surroundings (Tusher et al., 2021). This green technology is preferable to other conventional methods because it preserves substrate fertility and, at the same time, reduces the costs of remediation (Riaz et al., 2022). Moreover, it is a suitable eco-friendly solution for remediating large areas, besides being economical (Saxena et al., 2019). The major techniques of phytoremediation are phytostabilization, phytoextraction, and phytovolatilization.
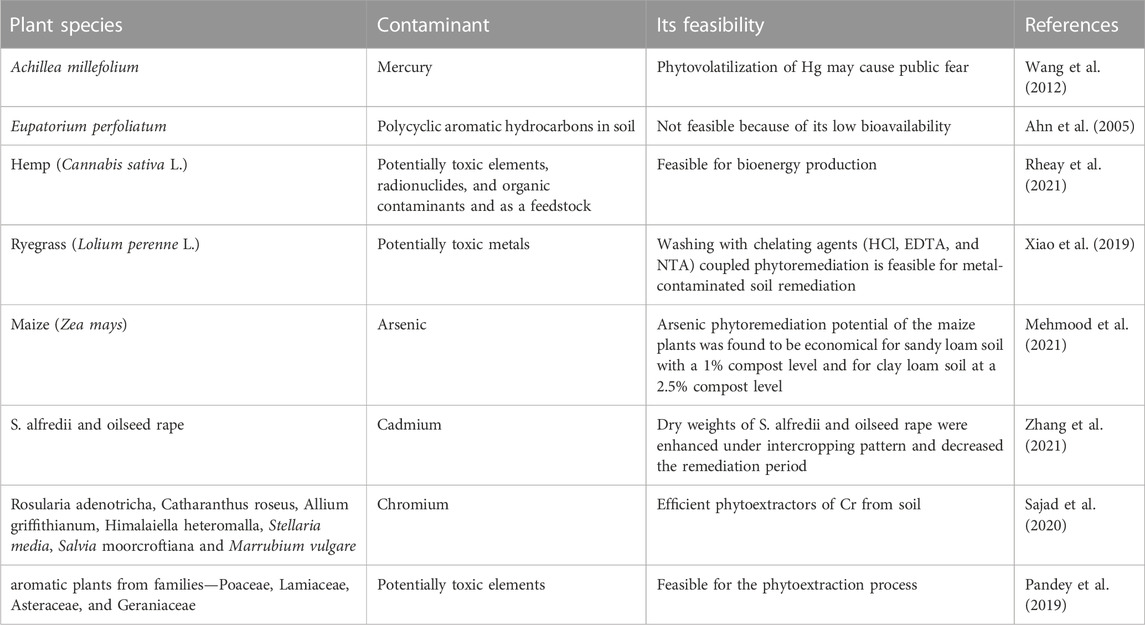
Many plant species have been shown to be efficient in remediating soils and waters contaminated with inorganic (Saxena et al., 2019; Edgar et al., 2021) and organic pollutants (Karaś et al., 2021) (Table 5) yet this process requires long time periods to lessen contaminants to attain acceptable public levels (Mustafa et al., 2022). Adding chelation agents could help in improving the efficiency of this process (Gavrilescu, 2022). Generally, edible crops are not suitable as phytoextractors for potentially toxic elements from contaminated sites (Saxena et al., 2019). Alternatively, aromatic plants can absorb and accumulate high concentrations of PTEs in the harvestable foliage while their oil is free from the risk of PTE accumulation (Lajayar et al., 2017). Also, plants grown for biofuel production are guaranteed for the phytoextraction process of PTEs from soils (Edgar et al., 2021; Rheay et al., 2021).
The removal of PTEs from soil takes place by selecting tolerant plants which have the ability to accumulate PTEs within their aboveground tissues (shoots) (Ali et al., 2013), at concentrations exceeding 0.1% for Cu, Cr, Ni, or Pb, or >1% for Mn or Zn (Yoon et al., 2006). PTEs may also be physically stabilized in soil and this method lessens their translocations to areal plant parts (phytostabilization). Otherwise, PTEs can be transformed into a gaseous form via leaves (phytovolatilization). The main mechanisms of the phytoremediation technique for remediating PTEs contaminated soils are shown in Figure 2.
5.3 Stabilization/solidification (S/S)
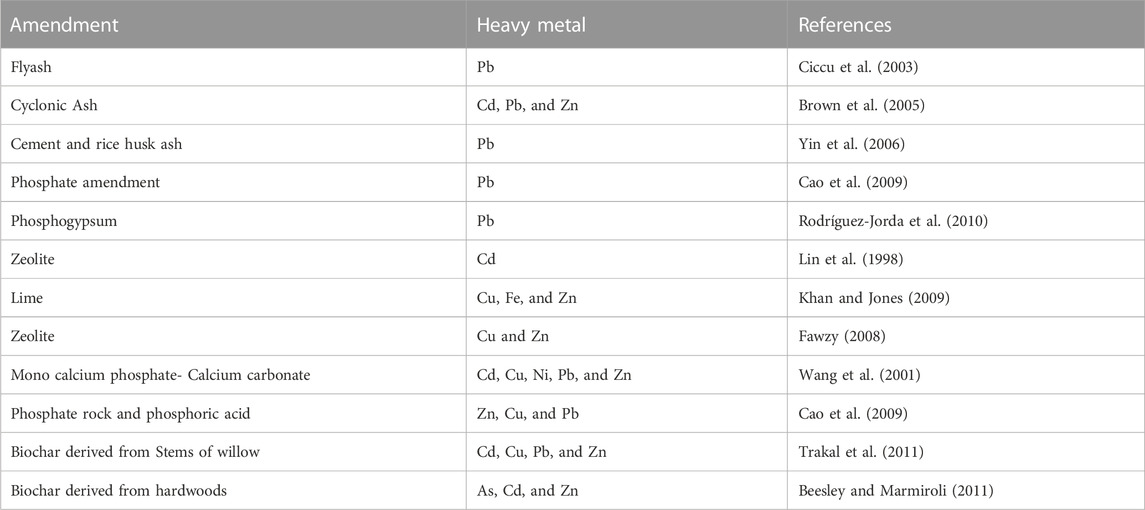
The stabilization/solidification method is used to lessen the solubility of PTEs using non-toxic materials (organic or inorganic) (Chen et al., 2006; Sunarso and Ismadji, 2009; Abdelhafez et al., 2014), especially in land with high contamination levels. Sorption and/or precipitation are the main routes for decreasing PTE bioavailability in soil (Basta and McGowen, 2004). These amendments include organic additives, phosphates, alkaline agents, and biosolids (Table 6).
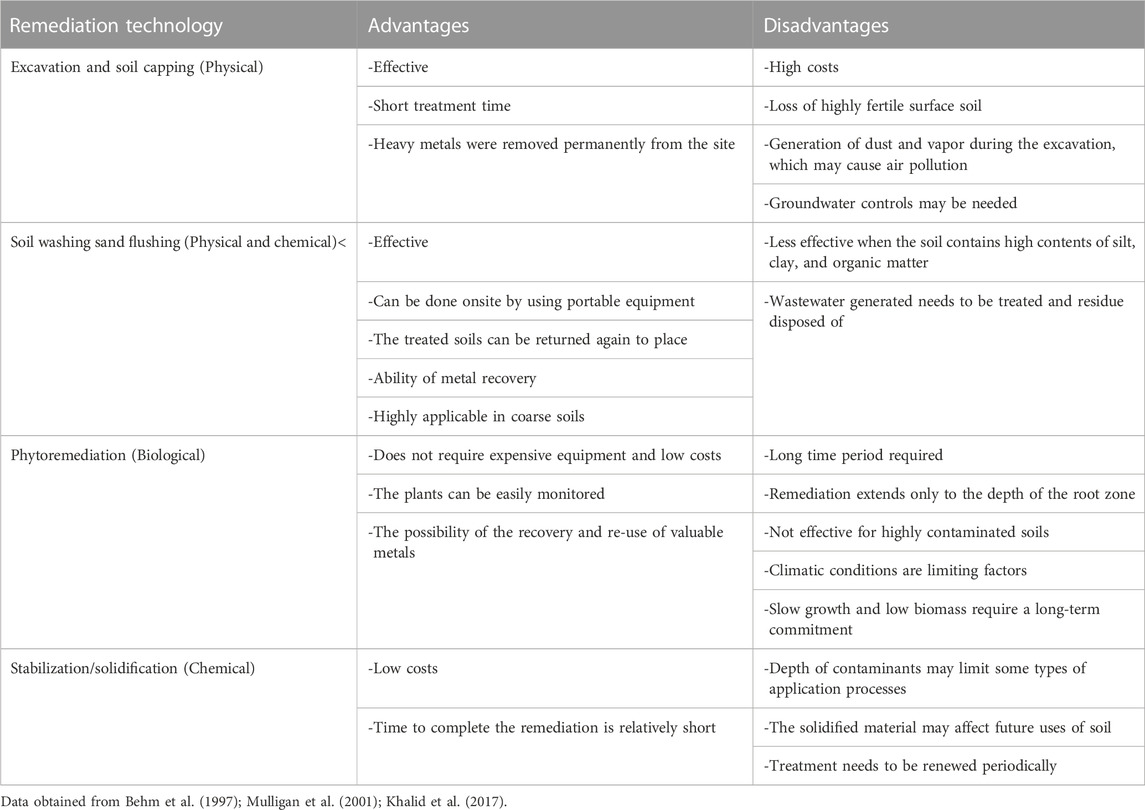
Table 7 shows a comparison between the conventional remediation technologies. Clearly, the stabilization/solidification (S/S) technique seems to be one of the most efficient methods because it is a cost-effective method that has rapid outcomes (USEPA, 2004). It is therefore recognized as the “best demonstrated available technology (BDTA)” by the USEPA for land disposal of most PTEs (Singh and Pant, 2005) in highly contaminated soil. From the aforementioned information, it seems obvious that the reuse of organic wastes is essential to remediate the PTE-contaminated water and soils.
6 Organic wastes and biochar
Every year, a huge amount of organic waste is produced annually without being properly recycled, especially in developing countries. For example, the amount of sugar cane and orange waste which is produced annually in China is estimated to be 123 and 32.7 million mega-grams (Abdelhafez et al., 2016). The corresponding amounts produced annually in Egypt exceed 44.0 million mega-grams. These residues should be recycled to be used in sustaining the environment rather than polluting it. In particular, biochar is a carbon-rich material product manufactured through pyrolysis of plant residues, i.e., wood or plant leaves at a relatively low temperature (<700°C) in the absence of oxygen or under limited oxygen conditions (Abdelhafez et al., 2014; Lehmann and Joseph, 2015; Abdelhafez et al., 2016; Abdelhafez and Li, 2016; Farid et al., 2022; Khalil et al., 2023).
6.1 Biochar for CO2 mitigation and improving soil fertility
Biochar has gained significant attention within the last few years because of its positive role in lessening CO2 emissions when used as an amendment to improve soil quality (Jeffery et al., 2011; Kookana et al., 2011; Abdelhafez et al., 2014; Abdelhafez et al., 2016). It is thought that biochar significantly reduces the readily available C fraction to microbes, thus, it slightly or insignificantly induces the activities of microbes and soil enzymes. This, in turn, enhances long-term carbon sequestration. Also, the dominance of aromatic organic carbon, which is very stable in the environment, guarantees its long-term existence in soil (Lehmann, 2007; Abdelhafez et al., 2017). For years, extensive human activities have caused degradation in soil quality and fertility. This negatively affects food production in many regions around the world. Accordingly, improving soil characteristics is necessary to overcome the lack of food production, especially in sub-Saharan Africa and South Asia, where the malnutrition percentages ranged from 32% to 22% of the total population, respectively (FAO, 2019). The solution is biochar as it can be used successfully to restore soil fertility and improve the soil’s physical, chemical, and hydrological properties (Novak et al., 2009; Free et al., 2010).
6.2 The potentiality of biochar for remediating PTE-contaminated water and soils
The role of biochar in improving soil fertility is not well-identified and is still being intensively studied. Only limited studies have investigated the potentiality of biochar derived from different organic sources in remediating soil and water contaminated with PTEs. Because of its porous structure (Abdelhafez et al., 2017), high cation exchange sites density, and net negative charge (Jing et al., 2019) biochar has a high capability to sorb PTEs (Jing et al., 2019) which diffuse into its micropores (Nguyen et al., 2008). This may further contribute to PTE precipitation in soils (Jing et al., 2019). The stabilization of PTEs in soil owing to biochar application can be attributed to the alkaline nature of biochar (Abdelhafez et al., 2017; Buss et al., 2019) which allows the functional groups of biochar to protonate and dissociate, replacing H+ in the solution with cationic PTEs (e.g., Pb and Cd) (Shaheen et al., 2019b). Also, increasing pH decreases the solubility and mobility of PTEs in soil (Shaheen et al., 2019b). With time, the exchangeable forms of PTEs co-precipitate in the form of inner-sphere complexes (Abdelhafez et al., 2016; Abdelhafez et al., 2017; Penido et al., 2019; Yuan et al., 2019) and change into less labile organic and residual fractions (Mohamed et al., 2018; Matin et al., 2020).
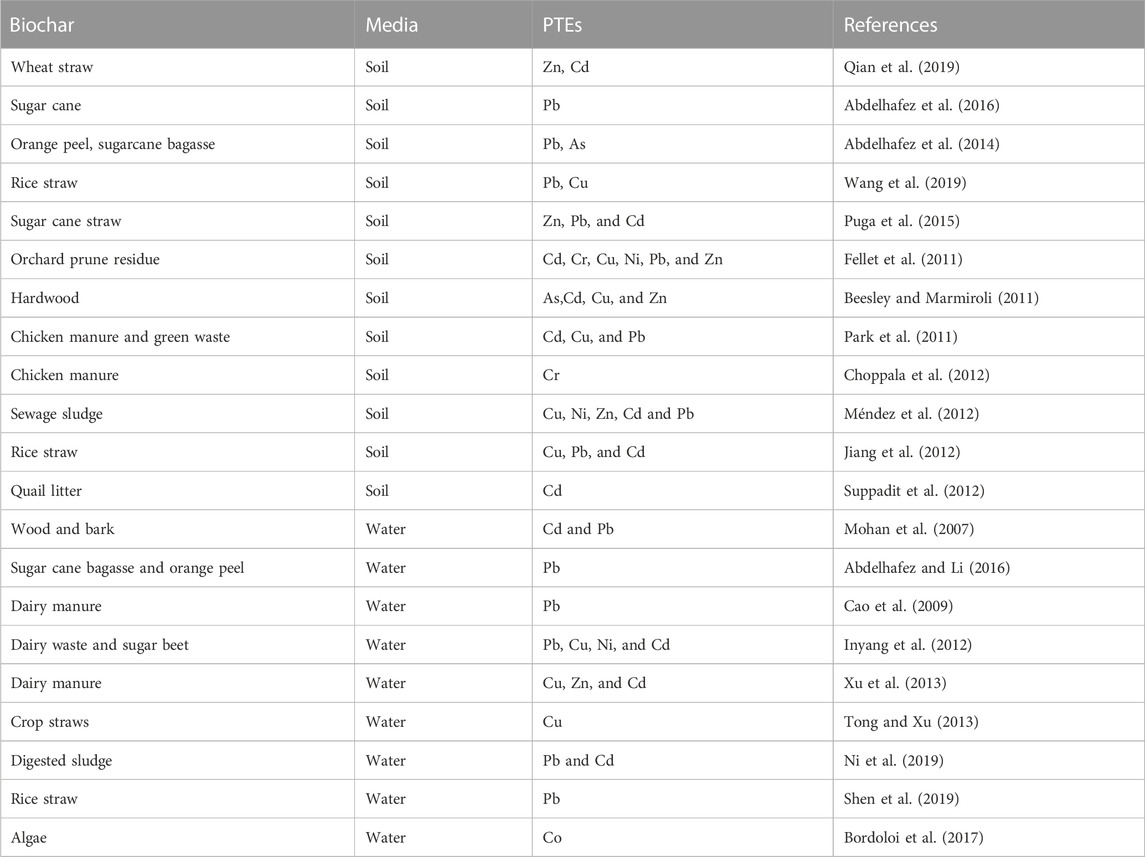
Although, this organic source may contain PTEs, the elevated pyrolysis temperature transforms PTEs into more stable and less toxic forms (de Souza et al., 2019). Thus, biochar acts as an efficient biosorbent for PTEs in contaminated soil (Mohamed et al., 2018) and water (Shaheen et al., 2019b). Biochar can also remove high amounts of herbicides from solutions by coating the dissolvable surfaces. It can therefore be used effectively to boost the health and nutrient status of the soil, particularly in the arid calcareous soil. Recent studies have shown the success of utilizing biochars in remediating water and soils contaminated with PTEs (Table 8).
The effect of biochar application on mobilizing metal ions in soil is confusing, for example, Abdelhafez et al. (2010; 2016) found that biochar increased the availability of Cu and As in biochar-treated soil. In addition, Alaboudi et al. (2019) explained that the addition of wood biomass biochar led to the transformation of Cr(III) into Cr(VI) due to increasing soil pH; consequently, its uptake was increased by maize plants. Furthermore, Shaheen et al. (2019a) reported that biochar applications increased the mobility of some PTEs in soil, such as Cu and As, through association with dissolved organic carbon. However, Lomaglio, et al. (2016) found that the addition of biochar decreased the labile concentration of Pb while increasing As and Sb solubility. Therefore, the role of biochar in stabilizing PTEs is still not well understood.
The degree of biochar stability depends mainly on the dose of applied biochar in addition to its mode of action period (Wang et al., 2019). In this regard, microbial and enzymatic activities (dehydrogenase, acidic and alkaline phosphatase, and urease) were higher in soils mixed with aged biochar than in fresh biochar soil (Yadav et al., 2019).
A point to note is that application of biochar not only increases the non-enzymatic antioxidants (soluble phenolic compounds and free proline) that increase plant tolerance to PTE stress (Kumar et al., 2022) but also stimulates the activities of metal-tolerant plant growth promoting rhizobacteria (Zhou et al., 2022) and mycorrhizae (Ortaş, 2016). Moreover, biochar increases plant growth promoting hormones to alleviate salt stress (Farhangi-Abriz and Torabian, 2018). This could be useful to increase the phytohormones which are responsible for alleviating PTE stress in plants.
Thus, future studies are required to investigate the effects of aging (from fresh to old) on the physiochemical properties of biochars in soils (differing in types) under field conditions. In addition, the effect of biochar on PTE solubility, especially, Cr, Cu, and As, is still a matter of concern.
7 Feasibility of the biochar/phytoremediation technique as a sustainable approach to manage PTEs polluted soils
Phytoremediation utilizes the natural ability of plants to uptake and accumulate contaminants from the media. Plants can hyperaccumulate PTEs, and certain species have shown remarkable tolerance and efficacy in remediating contaminated soils (Zheng et al., 2020). With the application of biochar, the efficiency of the phytoremediation process increases, e.g., its application enhanced plant growth, and increased metal sequestration. The biochar/phytoremediation technique operates through various mechanisms. Biochar improves soil properties by enhancing water retention, increasing nutrient availability, and stabilizing soil pH (Park et al., 2011). It acts as a sorbent for PTEs, reducing their mobility and bioavailability. In combination with plants, biochar provides a stable environment for root development and facilitates the uptake and translocation of PTEs by plants. It is worth noting that the biochar/phytoremediation technique offers several environmental benefits. It promotes carbon sequestration, as biochar remains longer in soils. This helps mitigate climate change by reducing greenhouse gas emissions. Additionally, the technique minimizes soil erosion, enhances soil fertility, and promotes biodiversity by creating a favorable habitat for soil organisms. Despite its promise, the biochar/phytoremediation technique faces certain challenges. The selection of suitable plant species, biochar properties, and application rates requires careful consideration (Cao et al., 2009). Long-term monitoring is essential to evaluate the persistence of remediation effects. Furthermore, the economic feasibility and scalability of the technique need to be assessed to encourage its widespread implementation.
8 Precautions while selecting appropriate remediation technology for PTEs
The selection of the appropriate remediation method is a function of several factors as follows.
i) Soil pH is a very important factor affecting the bioavailability of PTEs which decrease under alkaline conditions (Abdelhafez et al., 2012). In addition, soil texture and organic matter contents play significant roles in this concern, i.e., the higher the fine particles (clay and silt) contents in soil, the harder the metal extraction, since extracted PTEs might be adsorbed by iron-manganese oxides and located on the surfaces of those soil particles (Bradl, 2004). Furthermore, site conditions such as bedrock, large boulders clays, moisture content, and oily patches affect the solidification/stabilization and vitrification remediation technologies (Mulligan et al., 2001).
ii) Types of contaminants to be removed (organic/inorganic): some metals such as arsenic (As), chromium (Cr-VI), and mercury (Hg) do not form hydroxides (less soluble). Therefore, solidification/stabilization seems to not be appropriate for ameliorating soils contaminated with these types of PTEs (Mulligan et al., 2001). Furthermore, the high levels of Pb concentrations in shooting range and metal smelter-contaminated soils, which may exceed 1% (Yanqun et al., 2005; Levonmaki et al., 2006; Hashimoto et al., 2009), decrease the efficiency of remediating such soils by using the phytoremediation approach. The vitrification method is probably more suitable in areas containing low volatile metals with high glass solubility such as Pb, Cr, As, Zn, Cd, and Cu-contaminated soils (Smith et al., 1995). Unlike solid metals, Hg is characterized by its high volatility and low glass solubility, therefore, the vitrification method is unsuitable for remediating Hg-contaminated soils owing to the toxic gasses emitted during the vitrification process (Mulligan et al., 2001).
iii) The end use of contaminated soil: the future use of the soil should be considered before the remediation process to avoid unnecessary expenditures. Ok et al. (2010) showed that the pH of soil increased up to 12.5 when amended by calcined oyster shell powder in order to stabilize Cd and Pb. These types of remediated soils become unsuitable for agricultural purposes due to their high soil pH which limits the availability of nutritive elements.
9 Future outlook and conclusion
Potentially toxic metals are released into the environment mainly through anthropogenic activities as well as geological sources. These contaminants are responsible for spreading many diseases and almost 16% of premature deaths worldwide. A number of remediation techniques can therefore be followed to ameliorate PTE-contaminated soil and water, among which the immobilization technique is considered the best approach due to its easy availability and cost-effectiveness. In particular, the immobilization or removal of PTEs from soil and water with biochar has several advantages owing to its specific surface area, porous structure, and high selectivity for all the PTEs. We have reviewed more than 200 articles to compare the efficiency of existing technologies and biochar application in the remediation of contaminated soils and waters. Generally, the major mechanisms involved in PTE binding with biochar are complexation, precipitation, and adsorption.
Biochar acts as an efficient biosorbent for many PTEs in soil and water. It may, however, increase the mobility of other PTEs such as Cu and As via association with dissolved organic carbon. The degree of stability of biochar-PTEs in soil depends on the dose of applied biochar as well as its aging. More research is therefore needed to clarify this relationship in both soil and water. Furthermore, biochar can remove high amounts of herbicides from solutions. Thus, future studies should focus on the role of functional groups of biochar in the PTE remediation process, considering successive applications and long-term field investigations. The combination of different immobilizing agents in improving the phytoremediation efficiency of PTEs with biochar and also their consequences on the growth of plants by adding the required essential elements could be a matter of concern in future research.
Overall, the biochar/phytoremediation technique could have a significant impact as a sustainable approach for managing PTEs-polluted soils Its synergistic effects enhance PTE immobilization, reduce environmental risks, and promote ecosystem restoration. Although challenges exist, ongoing research and technological advancements are expected to address these limitations, further improving the feasibility and effectiveness of this technique.
Author contributions
XZ: Writing–original draft. GZ: Supervision, Writing–review and editing. HC: Writing–review and editing. ZS: Writing–review and editing. YZ: Writing–review and editing. MA: Writing–original draft. BA: Writing–review and editing. LZ: Writing–review and editing. AA: Supervision, Writing–original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Shanghai Agriculture Applied Technology Development Program, China (No. T20210104), the National Key Research and Development Program of China (No. 2021YFC3201503), the National S&T cooperation Program of Science and Technology Commission of Shanghai Municipality, China (No. 22015821200), and the Shanghai Sailing Program (No. 21YF1440900). This article was technically supported by the National Committee of Soil Science, Academy of Scientific Research and Technology, Egypt.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, M. H. H., and Abdelhafez, A. A. (2013). Role of EDTA in arsenic mobilization and its uptake by maize grown on an As-polluted soil. Chemosphere 90, 588–594. doi:10.1016/j.chemosphere.2012.08.042
Abbas, M. H. H., and Bassouny, M. (2018). Implications of long term irrigation with wastewater on the contents and retention kinetics of potentially toxic elements in Typic Torripsamment soils. Egypt J. Soil Sci. 58 (4), 0–357. doi:10.21608/ejss.2018.4232.1183
Abbas, M. H. H., and Meharg, A. A. (2008). Arsenate, arsenite and dimethyl arsinic acid (DMA) uptake and tolerance in maize (Zea mays L). Plant Soil 304 (1), 277–289. doi:10.1007/s11104-008-9549-9
Abdelhafez, A. A., Abbas, H. H., Abd-El-Aal, R. S., Kandil, N. F., Li, J., and Mahmoud, W. (2012). Environmental and health impacts of successive mineral fertilization in Egypt. Clean. (Weinh) 40 (4), 356–363. doi:10.1002/clen.201100151
Abdelhafez, A. A., Abbas, M. H. H., and Hamed, M. H. (2016). “Biochar: A solution for soil lead (Pb) pollution,” in The 8th int. Conference for development and the environment in the arab world (Egypt: Assiut University).
Abdelhafez, A. A., Abbas, M. H. H., and Li, J. (2017). “Biochar: the black diamond for soil sustainability, contamination control and agricultural production,” in Engineering applications of biochar (London, United Kingdom: IntechOpen), 7–27.
Abdelhafez, A. A., Abbas, M. H. H., and Li, L. (2014). Feasibility of biochar manufactured from organic wastes on the stabilization of heavy metals in a metal smelter contaminated soil. Chemosphere 117, 66–71. doi:10.1016/j.chemosphere.2014.05.086
Abdelhafez, A. A., Awad, Y. M., Abd El-Azeem, S. A. M., Kim, M. S., Ham, K. J., Lim, K. J., et al. (2010). Leaching of chromium, copper and arsenic in soils and rapid identification of CCA-treated woods using modified PAN stain. Korean J. Soil. Sci. Fert. 43 (1), 60–67. https://api.semanticscholar.org/CorpusID:138161890.
Abdelhafez, A. A., Awad, Y. M., Kim, M. S., Ham, K. J., Lim, K. L., Joo, J. H., et al. (2009). Environmental monitoring of heavy metals and arsenic in soils adjacent to CCA-treated wood structures in Gangwon Province, South Korea. Korean J. Environ. Agric. 28 (4), 340–346. doi:10.5338/kjea.2009.28.4.340
Abdelhafez, A. A., Eid, K. E., El-Abeid, S. E., Abbas, M. H. H., Ahmed, N., Mansour, R. R. M. E., et al. (2021). Application of soil biofertilizers to a clayey soil contaminated with Sclerotium rolfsii can promote production, protection and nutritive status of Phaseolus vulgaris. Chemosphere 271, 129321. doi:10.1016/j.chemosphere.2020.129321
Abdelhafez, A. A., and Li, J. (2015). Environmental monitoring of heavy metal status and human health risk assessment in the agricultural soils of the Jinxi River area, China. Hum. Ecol. Risk Assess. 21 (4), 952–971. doi:10.1080/10807039.2014.947851
Abdelhafez, A. A., and Li, J. (2014). Geochemical and statistical evaluation of heavy metal status in the region around Jinxi River, China. Soil Sediment. Contam. 23 (8), 850–868. doi:10.1080/15320383.2014.887651
Abdelhafez, A. A., and Li, L. (2016). Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J. Taiwan Inst. Chem. Eng. 61, 367–375. doi:10.1016/j.jtice.2016.01.005
Adeyemi, N. O., Atayese, M. O., Sakariyawo, O. S., Azeez, J. O., Abayomi Sobowale, S. P., Olubode, A., et al. (2021). Alleviation of heavy metal stress by arbuscular mycorrhizal symbiosis in Glycine max (L) grown in copper, lead and zinc contaminated soils. Rhizosphere 18, 100325. doi:10.1016/j.rhisph.2021.100325
Ahn, S., Werner, D., and Luthy, R. G. (2005). Physicochemical characterization of coke-plant soil for the assessment of polycyclic aromatic hydrocarbon availability and the feasibility of phytoremediation. Environ. Toxicol. Chem. 24, 2185–2195. doi:10.1897/04-564R.1
Ajmone-Marsana, F., Biasiolia, M., Kraljb, T., Grčmanb, H., Davidsonc, C. M., Hursthoused, A. S., et al. (2008). Metals in particle-size fractions of the soils of five European cities. Environ. Pollut. 152 (1), 73–81. doi:10.1016/j.envpol.2007.05.020
Al-Hiyaly, S. A., McNeilly, T., and Bradshaw, A. D. (1998). The effects of zinc contamination from electricity pylons - evolution in a replicated situation. New Phytol. 110 (4), 571–580. doi:10.1111/j.1469-8137.1988.tb00297.x
Alaboudi, K. A., Ahmed, B., and Brodie, G. (2019). Effect of biochar on Pb, Cd and Cr availability and maize growth in artificial contaminated soil. Ann. Agric. Sci. 64 (1), 95–102. doi:10.1016/j.aoas.2019.04.002
Ali, A., Farid, I. M., and Abbas, M. H. H. (2023). Evaluating the removal efficiency of potentially toxic elements (PTEs) from a shale deposit by citric acid. Egypt. J. Soil Sci. 63 (2), 0–150. doi:10.21608/ejss.2023.148878.1567
Ali, H., Khan, E., and Sajad, M. A. (2013). Phytoremediation of heavy metals—concepts and applications. Chemosphere 81 (7), 869–881. doi:10.1016/j.chemosphere.2013.01.075
Arief, V. O., Trilestari, K., Sunarso, J., Indraswati, N., and Ismadji, S. (2008). Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: characterization, biosorption parameters and mechanism studies. Clean. (Weinh) 36 (12), 937–962. doi:10.1002/clen.200800167
Arora, M., Kiran, B., Rani, S., Rani, A., Kaur, B., and Mitta, N. (2008). Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 111 (4), 811–815. doi:10.1016/j.foodchem.2008.04.049
Arwidsson, Z., Elgh-Dalgren, K., Kronhelm, T. V., Sjöberg, R., Allard, B., and van Hees, B. (2010). Remediation of heavy metal contaminated soil washing residues with amino polycarboxylic acids. J. Hazard Mater 173 (1-3), 697–704. doi:10.1016/j.jhazmat.2009.08.141
Bandara, T., Franks, A., Xu, J., Bolan, N., Wang, H., and Tang, C. (2020). Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit. Rev. Environ. Sci. Technol. 50 (9), 903–978. doi:10.1080/10643389.2019.1642832
Baragaño, D., Alonso, J., Gallego, J. R., Lobo, M. C., and Gil-Díaz, M. (2020). Zero valent iron and goethite nanoparticles as new promising remediation techniques for As-polluted soils. Chemosphere 238, 124624. doi:10.1016/j.chemosphere.2019.124624
Bassouny, M., Abbas, M., and Mohamed, I. (2020). Environmental risks associated with the leakage of untreated wastewater in industrial areas. Egypt J. Soil Sci. 60 (2), 0–128. doi:10.21608/ejss.2019.18787.1319
Basta, N. T., and McGowen, S. L. (2004). Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ. Pollut. 127 (1), 73–82. doi:10.1016/S0269-7491(03)00250-1
Beesley, L., and Marmiroli, M. (2011). The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 159 (2), 474–480. doi:10.1016/j.envpol.2010.10.016
Behera, S. N., Cheng, J., Huang, X., Zhu, Q., Liu, P., and Balasubramanian, R. (2015). Chemical composition and acidity of size-fractionated inorganic aerosols of 2013-14 winter haze in Shanghai and associated health risk of toxic elements. Atmos. Environ. 122, 259–271. doi:10.1016/j.atmosenv.2015.09.053
Behm, E., Gross, M., and Quesenberry, D. (1997). Groundwater pollution primer in situ vitrification. Available At: https://www.des.nh.gov/organization/divisions/water/dwgb/wrpp/documents/primer_chapter4.pdf.
Bordoloi, N., Goswami, R., Kumar, M., and Kataki, R. (2017). Biosorption of Co (II) from aqueous solution using algal biochar: kinetics and isotherm studies. Bioresour. Technol. 244, 1465–1469. doi:10.1016/j.biortech.2017.05.139
Bradl, H. B. (2004). Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 277 (1), 1–18. doi:10.1016/j.jcis.2004.04.005
Brown, S., Christensen, B., Lombi, E., McLaughlin, M., McGrath, S., Colpaert, J., et al. (2005). An inter-laboratory study to test the ability of amendments to reduce the availability of Cd, Pb, and Zn in situ. Environ. Pollut. 138 (1), 34–45. doi:10.1016/j.envpol.2005.02.020
Buss, W., Jansson, S., and Mašek, O. (2019). Unexplored potential of novel biochar-ash composites for use as organo-mineral fertilizers. J. Clean. Prod. 208, 960–967. doi:10.1016/j.jclepro.2018.10.189
Cao, X., Ma, L., Gao, B., and Harris, W. (2009). Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 43 (9), 3285–3291. doi:10.1021/es803092k
Chan, H. M., Kim, C., Khoda, K., Receveur, O., and Kuhnlein, H. V. (1995). Assessment of dietary exposure to trace metals in Baffin Inuit food. Environ. Health Perspect. 103 (7-8), 740–746. doi:10.1289/ehp.95103740
Chen, S. B., Zhu, Y. G., Ma, Y. B., and McKay, G. (2006). Effect of bone char application on Pb bioavailability in a Pb-contaminated soil. Environ. Pollut. 139 (3), 433–439. doi:10.1016/j.envpol.2005.06.007
Chen, X., Kumari, D., Cao, C. J., Plaza, G., and Achal, V. (2020). A review on remediation technologies for nickel-contaminated soil. Hum. Ecol. Risk Assess. Int. J. 26 (3), 571–585. doi:10.1080/10807039.2018.1539639
Choppala, G. K., Bolan, N. S., Mallavarapu, M., Chen, Z., and Naidu, R. (2012). The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. J. Environ. Qual. 41 (4), 1175–1184. doi:10.2134/jeq2011.0145
Ciccu, R., Ghiani, M., Serci, A., Fadda, S., Peretti, R., and Zucca, A. (2003). Heavy metal immobilization in the mining-contaminated soils using various industrial wastes. Min. Eng. 16 (3), 187–192. doi:10.1016/S0892-6875(03)00003-7
Congeevaram, S., Dhanarani, S., Park, J., Dexilin, M., and Thamaraiselvi, K. (2007). Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J. Hazard Mater 146 (1-2), 270–277. doi:10.1016/j.jhazmat.2006.12.017
Cukierman, A. L. (2007). Metal ion biosorption potential of lignocellulosic biomasses and marine algae for wastewater treatment. Adsorp Sci. Technol. 25 (3-4), 227–244. doi:10.1260/026361707782398182
Dahiya, S., Tripathi, R. M., and Hegde, A. G. (2008). Biosorption of heavy metals and radionuclide from aqueous solutions by pretreated arca shell biomass. J. Hazard Mater 150 (2), 376–386. doi:10.1016/j.jhazmat.2007.04.134
Dar, S. A., and Bhat, R. A. (2020). “Aquatic pollution stress and role of biofilms as environment cleanup technology,” in Fresh water pollution dynamics and remediation. Editors H. Qadri, R. Bhat, M. Mehmood, and G. Dar (Singapore: Springer), 293–318.
de Souza, E. S., Dias, Y. N., da Costa, H. S. C., Pinto, D. A., de Oliveira, D. M., Falção, N. P. D., et al. (2019). Organic residues and biochar to immobilize potentially toxic elements in soil from a gold mine in the Amazon. Ecotox Environ. Safe 169, 425–434. doi:10.1016/j.ecoenv.2018.11.032
Dermont, G., Bergeron, M., Mercier, G., and Richer-Lafleche, M. (2008). Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard Mater 152 (1), 1–31. doi:10.1016/j.jhazmat.2007.10.043
Dubey, R. K., Dubey, P. K., Chaurasia, R., Singh, H. B., and Abhilash, P. C. (2020). Sustainable agronomic practices for enhancing the soil quality and yield of Cicer arietinum L. under diverse agroecosystems. J. Environ. Manage 2, 110284. doi:10.1016/j.jenvman.2020.110284
Duffus, J. H. (2002). Heavy metals-a meaningless term. Pure Appl. Chem. 74 (5), 793–807. doi:10.1351/pac200274050793
Ebrahimbabaie, P., Meeinkuirt, W., and Pichtel, J. (2020). Phytoremediation of engineered nanoparticles using aquatic plants: mechanisms and practical feasibility. J. Environ. Sci. 93, 151–163. doi:10.1016/j.jes.2020.03.034
Edgar, V. N., Fabián, F. L., Mario, P. C. J., and Ileana, V. R. (2021). Coupling plant biomass derived from phytoremediation of potential toxic-metal-polluted soils to bioenergy production and high-value by-products—a review. Appl. Sci. 11 (7), 2982. doi:10.3390/app11072982
Egendorf, S. P., Gailey, A. D., Schachter, A. E., and Mielke, H. W. (2020). Soil toxicants that potentially affect children's health. Curr. Prob Pediatr. Ad 50 (1), 100741. doi:10.1016/j.cppeds.2019.100741
Eid, K. E., Abbas, M. H. H., Mekawi, E. M., ElNagar, M. M., Abdelhafez, A. A., Amin, B. H., et al. (2019). Arbuscular mycorrhiza and environmentally biochemicals enhance the nutritional status of Helianthus tuberosus and induce its resistance against Sclerotium rolfsii. Ecotox Environ. Safe 186, 109783. doi:10.1016/j.ecoenv.2019.109783
El-Meihy, R. M., Abou-Aly, H. E., Youssef, A. M., Tewfike, T. A., and El-Alkshar, E. A. (2019). Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ. Exp. Bot. 162, 295–301. doi:10.1016/j.envexpbot.2019.03.005
Elangovan, R., Philip, L., and Chandraraj, K. (2008). Biosorption of chromium species by aquatic weeds: kinetics and mechanism studies. J. Hazard Mater 152 (1), 100–112. doi:10.1016/j.jhazmat.2007.06.067
ElShazly, A. A. A., Abbas, M. H. H., Farid, I. M., Rizk, M., Abdelhafez, A. A., Abbas, H. H., et al. (2019a). Depthprofile distribution of Cs and its toxicity for canola plants grown on arid rainfed soils as affected by increasing K-inputs. Ecotox Environ. Safe 183, 109529. doi:10.1016/j.ecoenv.2019.109529
ElShazly, A. A. A., Abbas, M. H. H., Farid, I. M., Rizk, M., Mohamed, I., Abbas, H. H., et al. (2019b). Feasibility of using natural mineral ores for removing Cs and Sr from contaminated water. Ecotox Environ. Safe 175, 173–180. doi:10.1016/j.ecoenv.2019.03.044
Elshony, M., Farid, I., Alkamar, F., Abbas, M. H. H., and Abbas, H. (2019). Ameliorating a sandy soil using biochar and compost amendments and their implications as slow release fertilizers on plant growth. Egypt J. Soil Sci. 59 (4), 0–322. doi:10.21608/ejss.2019.12914.1276
Eseudero, C., Gabaldon, C., Marzal, P., and Villaescusa, I. (2008). Effect of EDTA on divalent metal adsorption onto grape stalk and exhausted coffee wastes. J. Hazard Mater 152 (2), 476–485. doi:10.1016/j.jhazmat.2007.07.013
FAO (2019). The state of food insecurity in the world 2019. Rome: United Nations Food and Agriculture Organization, FAO. Available At: www.fao.org/docrep/009/a0750e/a0750e00.htmm (Accessed June 16, 2019).
Farhangi-Abriz, S., and Torabian, S. (2018). Biochar increased plant growth-promoting hormones and helped to alleviates salt stress in common bean seedlings. J. Plant Growth Regul. 37 (2), 591–601. doi:10.1007/s00344-017-9756-9
Farid, I., Abbas, M., Bassouny, M., Gameel, A., and Abbas, H. (2020). Indirect impacts of irrigation with low-quality water on the environmental safety. Egypt. J. Soil Sci. 60 (1), 0–15. doi:10.21608/ejss.2019.15434.1294
Farid, I. M., Ahmed, M., Abbas, M. H. H., and Elshazly, A. (2023). The efficiency of using Na EDTA and DTPA to extract different fractions of soil strontium. Egypt. J. Soil Sci. 63 (3), 0–286. doi:10.21608/ejss.2023.208549.1589
Farid, I. M., Siam, H. S., Abbas, M. H. H., Mohamed, I., Mahmoud, S. A., Tolba, M., et al. (2022). Co-Composted biochar derived from rice straw and sugarcane bagasse improved soil properties, carbon balance, and zucchini growth in a sandy soil: A trial for enhancing the health of low fertile arid soils. Chemosphere 292, 133389. doi:10.1016/j.chemosphere.2021.133389
Fawzy, E. M. (2008). Soil remediation usingin situimmobilisation techniques. Chem. Ecol. 24 (2), 147–156. doi:10.1080/02757540801920154
Fellet, G., Marchiol, L., Delle Vedove, G., and Peressotti, A. (2011). Application of biochar on mine tailings: effects and perspectives for land reclamation. Chemosphere 83 (9), 1262–1267. doi:10.1016/j.chemosphere.2011.03.053
Feng, Z., Ji, S., Ping, J., and Cui, D. (2021). Recent advances in metabolomics for studying heavy metal stress in plants. TrAC Trends Anal. Chem. 143, 116402. doi:10.1016/j.trac.2021.116402
Free, H. F., McGill, C. R., Rowarth, J. S., and Hedley, M. J. (2010). The effect of biochar on maize (Zea mays) germination. New Zeal J. Agr. Res. 53 (1), 1–4. doi:10.1080/00288231003606039
Galán, E., Romero-Baena, A. J., Aparicio, P., and González, I. (2019). A methodological approach for the evaluation of soil pollution by potentially toxic trace elements. J. Geochem Explor 203, 96–107. doi:10.1016/j.gexplo.2019.04.005
Gautam, K., Sharma, P., Dwivedi, S., Singh, A., Gaur, V. K., Varjani, S., et al. (2023). A review on control and abatement of soil pollution by heavy metals: emphasis on artificial intelligence in recovery of contaminated soil. Environ. Res. 225, 115592. doi:10.1016/j.envres.2023.115592
Gavrilescu, M. (2022). Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 74, 21–31. doi:10.1016/j.copbio.2021.10.024
Ghosh, M., and Singh, S. P. (2005). A review on phytoremediation of heavy metals and utilization of its byproducts. Appl. Ecol. Environ. Res. 3 (1), 1–18. doi:10.15666/aeer/0301_001018
Ghosh, S. (2010). Wetland macrophytes as toxic metal accumulators. Int. J. Environ. Sci. 1 (4), 523–528. https://api.semanticscholar.org/CorpusID:87051767.
González-Martínez, A., de Simón-Martín, M., López, R., Táboas-Fernández, R., and Bernardo-Sánchez, A. (2019). Remediation of potential toxic elements from wastes and soils: analysis and energy prospects. Sustainability 11 (12), 3307. doi:10.3390/su11123307
Goyal, D., Yadav, A., Prasad, M., Singh, T. B., Shrivastav, P., Ali, A., et al. (2020). “Effect of heavy metals on plant growth: an overview,” in Contaminants in agriculture: sources, impacts and management. Editors M. Naeem, A. A. Ansari, and S. S. Gill (Cham: Springer International Publishing), 79–101.
Gudmundsson, G., Finnbjornsdottir, R. G., Johannsson, T., and Rafnsson, V. (2019). Air pollution in Iceland and the effects on human health. Review. Laeknabladid 105 (10), 443–452. doi:10.17992/lbl.2019.10.252
Gui, H., Yang, Q., Lu, X., Wang, H., Gu, Q., and Martín, J. D. (2023). Spatial distribution, contamination characteristics and ecological-health risk assessment of toxic heavy metals in soils near a smelting area. Environ. Res. 222, 115328. doi:10.1016/j.envres.2023.115328
Guo, X., Zhang, S., and Shan, X. (2008). Adsorption of metal ions on lignin. J. Hazard Mater 151 (1), 134–142. doi:10.1016/j.jhazmat.2007.05.065
Hasan, S. H., Singh, K. K., Prakash, O., Talat, M., and Ho, Y. S. (2008). Removal of Cr(VI) from aqueous solutions using agricultural waste ‘maize bran. J. Hazard Mater 152 (1), 356–365. doi:10.1016/j.jhazmat.2007.07.006
Hashim, T. A., Abbas, H. H., Farid, I. M., El-Husseiny, O. H. M., and Abbas, M. H. H. (2017). Accumulation of some heavy metals in plants and soils adjacent to Cairo – Alexandria agricultural highway. Egypt. J. Soil Sci. 57 (2), 0–232. doi:10.21608/ejss.2016.281.1047
Hashimoto, Y., Taki, T., and Sato, T. (2009). Extractability and leachability of Pb in a shooting range soil amended with poultry litter ash: investigations for immobilization potentials. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 44 (6), 583–590. doi:10.1080/10934520902784617
He, W., Megharaj, M., Wu, C. Y., Subashchandrabose, S. R., and Dai, C. C. (2020). Endophyte-assisted phytoremediation: mechanisms and current application strategies for soil mixed pollutants. Crit. Rev. Biotechnol. 40 (1), 31–45. doi:10.1080/07388551.2019.1675582
Herawati, N., Suzuki, S., Hayashi, K., Rivai, I. F., and Koyoma, H. (2000). Cadmium, copper and zinc levels in rice and soil of Japan, Indonesia and China by soil type. Bull. Environ. Contam. Toxicol. 64 (1), 33–39. doi:10.1007/s001289910006
Ho, Y. S., and Ofomaja, A. E. (2006). Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J. Hazard Mater 129 (1-3), 137–142. doi:10.1016/j.jhazmat.2005.08.020
Huang, Y., Wang, L., Wang, W., Li, T., He, Z., and Yang, X. (2019). Current status of agricultural soil pollution by heavy metals in China: A meta-analysis. Sci. Total Environ. 651 (2), 3034–3042. doi:10.1016/j.scitotenv.2018.10.185
Ibrahim, M., Labaki, M., Giraudon, J. M., and Lamonier, J. F. (2020). Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard Mater 383, 121139. doi:10.1016/j.jhazmat.2019.121139
Inyang, M., Gao, B., Yao, Y., Xue, Y., Zimmerman, A. R., Pullammanappallil, P., et al. (2012). Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 110, 50–56. doi:10.1016/j.biortech.2012.01.072
Jeffery, S., Verheijen, F. G. A., van der Veldea, M., and Bastos, A. C. (2011). A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 144 (1), 175–187. doi:10.1016/j.agee.2011.08.015
Jiang, J., Xu, R. K., Jiang, T. Y., and Li, Z. (2012). Immobilization of Cu (II), Pb (II) and Cd (II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J. Hazard Mater 229-230, 145–150. doi:10.1016/j.jhazmat.2012.05.086
Jing, F., Yang, Z., Chen, X., Liu, W., Guo, B., Lin, G., et al. (2019). Potentially hazardous element accumulation in rice tissues and their availability in soil systems after biochar amendments. J. Soils Sediments 19, 2957–2970. doi:10.1007/s11368-019-02296-5
Jinping, J., Longhua, W., Na, L., Yongming, L., Ling, L., Qiguo, Z., et al. (2010). Effects of multiple heavy metal contamination and repeated phytoextraction by Sedum plumbizincicola on soil microbial properties. Eur. J. Soil Biol. 46 (1), 18–26. doi:10.1016/j.ejsobi.2009.10.001
Karaś, M. A., Wdowiak-Wróbel, S., and Sokołowski, W. (2021). Selection of endophytic strains for enhanced bacteria-assisted phytoremediation of organic pollutants posing a public health hazard. Int. J. Mol. Sci. 22 (17), 9557. doi:10.3390/ijms22179557
Katayama, A., Bhula, R., Burns, G. R., Carazo, E., Felsot, A., Hamilton, D., et al. (2010). Bioavailability of xenobiotics in the soil environment. Rev. Environ. Contam. Toxicol. 204, 1–86. doi:10.1007/978-1-4419-1352-4_1
Kaur, R., Das, S., Bansal, S., Singh, G., Sardar, S., Dhar, H., et al. (2021). Heavy metal stress in rice: uptake, transport, signaling, and tolerance mechanisms. Physiol. Plant. 173 (1), 430–448. doi:10.1111/ppl.13491
Khalid, S., Shahid, M., Niazi, N. K., Murtaza, B., Bibi, I., and Dumat, C. (2017). A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem Explor 182, 247–268. doi:10.1016/j.gexplo.2016.11.021
Khalil, F. W., Abdel-Salam, M., Abbas, M. H. H., and Abuzaid, A. S. (2023). Implications of acidified and non-acidified biochars on N and K availability and their uptake by maize plants. Egypt. J. Soil Sci. 63 (1), 0–112. doi:10.21608/ejss.2023.184654.1560
Khan, A. G. (2020). Promises and potential of in situ nano-phytoremediation strategy to mycorrhizo-remediate heavy metal contaminated soils using non-food bioenergy crops (Vetiver zizinoides and Cannabis sativa). Int. J. Phytoremediation 22 (9), 900–915. doi:10.1080/15226514.2020.1774504
Khan, F. I., Husain, T., and Hejazi, H. (2004). An overview and analysis of site remediation technologies. J. Environ. Manage 71 (2), 95–122. doi:10.1016/j.jenvman.2004.02.003
Khan, M. J., and Jones, D. L. (2009). Effect of composts, lime and diammonium phosphate on the phytoavailability of heavy metals in a copper mine tailing soil. Pedosphere 19 (5), 631–641. doi:10.1016/S1002-0160(09)60158-2
Khormaei, M., Nasernejad, B., Edrisi, M., and Eslamzadeh, T. (2007). Copper biosorption from aqueous solutions by sour orange residue. J. Hazard Mater 149 (2), 269–274. doi:10.1016/j.jhazmat.2007.03.074
Ko, I. W., Lee, C. H., Lee, K. P., Lee, S. W., and Kim, K. W. (2006). Remediation of soil contaminated with arsenic, zinc, and nickel by pilot-scale soil washing. Environ. Prog. Sustain Energy 25 (1), 39–48. doi:10.1002/ep.10101
Koffi, N. J., and Okabe, S. (2020). Domestic wastewater treatment and energy harvesting by serpentine up-flow MFCs equipped with PVDF-based activated carbon air-cathodes and a low voltage booster. Chem. Eng. J. 380, 122443. doi:10.1016/j.cej.2019.122443
Kong, S., Lu, B., Ji, Y., Zhao, X., Bai, Z., Xu, Y., et al. (2012). Risk assessment of heavy metals in road and soil dusts within PM2.5, PM10 and PM100 fractions in Dongying city, Shandong Province, China. J. Environ. Monit. 14 (3), 791–803. doi:10.1039/c1em10555h
Kookana, R. S., Sarmah, A. K., Van Zwieten, L., Krull, E., and Singh, B. (2011). Chapter three-biochar application to soil: agronomic and environmental benefits and unintended consequences. Adv. Agron. 112, 103–143. doi:10.1016/B978-0-12-385538-1.00003-2
Kumar, A., Borisova, G., Maleva, M., TriptiShiryaev, G., and Tugbaeva, A. (2022). Biofertilizer based on biochar and metal-tolerant plant growth promoting rhizobacteria alleviates copper impact on morphophysiological traits in Brassica napus L. Microorganisms 10 (11), 2164. doi:10.3390/microorganisms10112164
Kumar, V., Parihar, R. D., Sharma, A., Bakshi, P., Sidhu, G. P. S., Bali, A. S., et al. (2019). Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 236, 124364. doi:10.1016/j.chemosphere.2019.124364
Kurniawan, T. A., Chan, G. Y. S., Lo, W., and Babel, S. (2006). Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 366 (2-3), 409–426. doi:10.1016/j.scitotenv.2005.10.001
Lajayar, B. A., Ghorbanpourb, M., and Nikabadi, S. (2017). Heavy metals in contaminated environment: destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol. Environ. Saf. 145, 337–390. doi:10.1016/j.ecoenv.2017.07.035
Lambert, M., Leven, B. A., and Green, R. M. (2014). New methods of cleaning up heavy metal in soils and water. U. S. Pat. Appl. 13/200, 968. https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.files/fileID/14295.
Lanphear, B. P., Succop, P., Roda, S., and Henningsen, G. (2003). The effect of soil abatement on blood lead levels in children living near a former smelting and milling operation. Public Health Rep. 118 (2), 83–91. doi:10.1016/S0033-3549(04)50223-6
Lee, J., Kaunda, R. B., Sinkala, T., Workman, C. F., Bazilian, M. D., and Clough, G. (2021). Phytoremediation and phytoextraction in sub-saharan Africa: addressing economic and social challenges. Ecotoxicol. Environ. Saf. 226, 112864. doi:10.1016/j.ecoenv.2021.112864
Lee, M., Paik, I. S., Kim, I., Kang, H., and Lee, S. (2007). Remediation of heavy metal contaminated groundwater originated from abandoned mine using lime and calcium carbonate. J. Hazard Mater 144 (1-2), 208–214. doi:10.1016/j.jhazmat.2006.10.007
Lehmann, J., and Joseph, S. (2015). “Biochar for environmental management: an introduction,” in Biochar for environmental management-Science, technology and implementation. Editors J. Lehmann, and S. Joseph (London: Routledge), 1–14.
Lévesque, V., Jeanne, T., Dorais, M., Ziadi, N., Hogue, R., and Antoun, H. (2020). Biochars improve tomato and sweet pepper performance and shift bacterial composition in a peat-based growing medium. Appl. Soil Ecol. 153, 103579. doi:10.1016/j.apsoil.2020.103579
Levonmaki, M., Hartikainen, H., and Kairesalo, T. (2006). Effect of organic amendment and plant roots on the solubility and mobilization of lead in soils at a shooting range. J. Environ. Qual. 35 (4), 1026–1031. doi:10.2134/jeq2005.0354
Lin, C. F., Lo, S. S., Lin, H. Y., and Lee, Y. (1998). Stabilization of cadmium contaminated soils using synthesized zeolite. J. Hazard Mater 60, 217–226. doi:10.1016/S0304-3894(98)00092-2
Liu, G., Shi, Y., Guo, G., Zhao, L., Niu, J., and Zhang, C. (2020). Soil pollution characteristics and systemic environmental risk assessment of a large-scale arsenic slag contaminated site. J. Clean. Prod. 251, 119721. doi:10.1016/j.jclepro.2019.119721
Lomaglio, T., Hattab-Hambli, N., Bret, A., Miard, F., Trupiano, D., Scippa, G. S., et al. (2016). Effect of biochar amendments on the mobility and (bio) availability of As, Sb and Pb in a contaminated mine technosol. J. Geochem. Explor. 182 (B), 138–148. doi:10.1016/j.gexplo.2016.08.007
Lu, Q., Wang, S., Bai, X., Liu, F., Li, C., Deng, Y., et al. (2020). Quantitative assessment of human health risks under different land uses based on soil heavy metal pollution sources. Hum. Ecol. Risk Assess. 27 (2), 327–343. doi:10.1080/10807039.2019.1710811
Ma, Q., Han, L., Zhang, J., Zhang, Y., Lang, Q., Li, F., et al. (2019). Environmental risk assessment of metals in the volcanic soil of Changbai mountain. Int. J. Environ. Res. Public Health 16 (11), 2047. doi:10.3390/ijerph16112047
Madrid, F., Díaz-Barrientos, E., and Madrid, L. (2008). Availability and bio-accessibility of metals in the clay fraction of urban soils of Sevilla. Environ. Pollut. 156 (3), 605–610. doi:10.1016/j.envpol.2008.06.023
Malkoc, E., and Nuhoglu, Y. (2007). Potential of tea factory waste for chromium(VI) removal from aqueous solutions: thermodynamic and kinetic studies. Sep. Purif. Technol. 54 (3), 291–298. doi:10.1016/j.seppur.2006.09.017
Matin, N. H., Jalali, M., and Buss, W. (2020). Synergistic immobilization of potentially toxic elements (PTEs) by biochar and nanoparticles in alkaline soil. Chemosphere 241, 124932. doi:10.1016/j.chemosphere.2019.124932
Megido, L., Suárez-Peña, B., Negral, L., Castrillón, L., and Fernández-Nava, Y. (2017). Suburban air quality: human health hazard assessment of potentially toxic elements in PM10. Chemosphere 177, 284–291. doi:10.1016/j.chemosphere.2017.03.009
Mehmood, T., Liu, C., Niazi, N. K. N., Gaurav, G. K., Ashraf, A., and Bibi, I. (2021). Compost-mediated arsenic phytoremediation, health risk assessment and economic feasibility using Zea mays L. in contrasting textured soils. Int. J. Phytoremediation 23 (9), 899–910. doi:10.1080/15226514.2020.1865267
Memon, A. R., and Schröder, P. (2009). Implications of metal accumulation mechanisms to phytoremediation. Environ. Sci. Pollut. Res. Int. 16 (2), 162–175. doi:10.1007/s11356-008-0079-z
Méndez, A., Gómez, A., Paz-Ferreiro, J., and Gascó, G. (2012). Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 89 (11), 1354–1359. doi:10.1016/j.chemosphere.2012.05.092
Mohamed, I., Ali, M., Ahmed, N., Abbas, M. H. H., Abdelsalam, M., Azab, A., et al. (2018). Cow manure-loaded biochar changes Cd fractionation and phytotoxicity potential for wheat in a natural acidic contaminated soil. Ecotox Environ. Safe 162, 348–353. doi:10.1016/j.ecoenv.2018.06.065
Mohamed, I., Eid, K. E., Abbas, M. H. H., Salem, A. A., Ahmed, N., Ali, M., et al. (2019). Use of plant growth promoting Rhizobacteria (PGPR) and mycorrhizae to improve the growth and nutrient utilization of common bean in a soil infected with white rot fungi. Ecotoxicol. Environ. Saf. 171, 539–548. doi:10.1016/j.ecoenv.2018.12.100
Mohan, D., Pittman, C. U., Bricka, M., Yancey, B., and Mohammad, J. (2007). Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 310 (1), 57–73. doi:10.1016/j.jcis.2007.01.020
Mulligan, C. N., Yong, R. N., and Gibbs, B. F. (2001). Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng. Geol. 60 (1-4), 193–207. doi:10.1016/S0013-7952(00)00101-0
Münzel, T., Hahad, O., Daiber, A., and Landrigan, P. J. (2022). Soil and water pollution and human health: what should cardiologists worry about? Cardiovasc. Res. 119 (2), 440–449. doi:10.1093/cvr/cvac082
Mustafa, H. M., Hayder, G., and Mustapa, S. I. M. (2022). Circular economy framework for energy recovery in phytoremediation of domestic wastewater. Energies 15 (9), 3075. doi:10.3390/en15093075
Nagajyoti, P. C., Lee, K. D., and Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 8, 199–216. doi:10.1007/s10311-010-0297-8
Naveed, M., Ramzan, N., Mustafa, A., Samad, A., Niamat, B., Yaseen, M., et al. (2020). Alleviation of salinity induced oxidative stress in Chenopodium quinoa by Fe biofortification and biochar—endophyte interaction. Agronomy 10, 168. doi:10.3390/agronomy10020168
Nguyen, B. T., Lehmann, J., Kinyangi, J., Smernik, R., Riha, S. J., and Engelhard, M. H. (2008). Long-term black carbon dynamics in cultivated soil. Biogeochemistry 89, 295–308. doi:10.1007/s10533-008-9220-9
Ni, B., Huang, Q., Wang, C., Ni, T., Sun, J., and Wei, W. (2019). Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 219, 351–357. doi:10.1016/j.chemosphere.2018.12.053
Nomanbhay, S. M., and Palanisamy, K. (2005). Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron J. Biotechn 8 (1), 43–53. doi:10.2225/vol8-issue1-fulltext-7
Novak, J. M., Larid, D. L., Ahmedna, M., Watts, D. W., and Niandou, M. A. S. (2009). Impact of biochar amendment on fertility of a Southeastern Coastal Plain soil. Soil Sci. 174 (2), 105–112. doi:10.1097/SS.0b013e3181981d9a
Nowack, B. (2002). Environmental chemistry of aminopolycarboxylate chelating agents. Environ. Sci. Technol. 36 (19), 4009–4016. doi:10.1021/es025683s
Ok, Y. S., Oh, S., Ahmad, M., Hyun, S., Kim, K., Moon, D. H., et al. (2010). Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ. Earth Sci. 61 (6), 1301–1308. doi:10.1007/s12665-010-0674-4
Ortaş, İ. (2016). “The role of mycorrhizae and biochar in plant growth and soil quality,” in Biochar: A regional supply chain approach in view of climate change mitigation. Editors B. B. Uzun, E. Apaydın Varol, J. Liu, and V. J. Bruckman (Cambridge: Cambridge University Press), 336–350.
Ouzounidou, G., and Ilias, I. (2005). Hormone-induced protection of sunflower photosynthetic apparatus against copper toxicity. Biol. Plant. 49 (2), 223–228. doi:10.1007/s10535-005-3228-y
Ozdemir, G., Ozturk, T., Ceyhan, N., Isler, R., and Cosar, T. (2003). Heavy metal biosorption by biomass of Ochrobactrum anthropi producing exopolysaccharide in activated sludge. Bioresour. Technol. 90 (1), 71–74. doi:10.1016/s0960-8524(03)00088-9
Padmavathiamma, P. K., and Li, L. Y. (2007). Phytoremediation technology: hyperaccumulation metals in plants. Water Air Soil Pollut. 184 (1-4), 105–126. doi:10.1007/s11270-007-9401-5
Pandey, J., Verma, R. K., and Singh, S. (2019). Suitability of aromatic plants for phytoremediation of heavy metal contaminated areas: A review. Int. J. Phytoremediation 21 (5), 405–418. doi:10.1080/15226514.2018.1540546
Park, J. H., Choppala, G. K., Bolan, N. S., Chung, J. W., and Chuasavathi, T. (2011). Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348 (1-2), 439–451. doi:10.1007/s11104-011-0948-y
Penido, E. S., Martins, G. C., Mendes, T. B. M., Melo, L. C. A., Guimarães, L. R. G., and Guilherme, L. R. G. (2019). Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotoxicol. Environ. Saf. 172, 326–333. doi:10.1016/j.ecoenv.2019.01.110
Plum, L. M., Rink, L., and Haase, H. (2010). The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health 7 (4), 1342–1365. doi:10.3390/ijerph7041342
Puga, A. P., Abreu, C. A., Melo, L. C. A., and Beesley, L. (2015). Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manage 159, 86–93. doi:10.1016/j.jenvman.2015.05.036
Qi, Z., Han, Y., Afrane, S., Liu, X., Zhang, M., Crittenden, J., et al. (2023). Patent mining on soil pollution remediation technology from the perspective of technological trajectory. Environ. Pollut. 316, 120661. doi:10.1016/j.envpol.2022.120661
Qian, T., Wu, P., Qin, Q., Huang, Y., and Wang, Y. (2019). Screening of wheat straw biochars for the remediation of soils polluted with Zn (II) and Cd (II). J. Hazard Mater 362, 311–317. doi:10.1016/j.jhazmat.2018.09.034
Rashtian, J., Chavkin, D. E., and Merhi, Z. (2019). Water and soil pollution as determinant of water and food quality/contamination and its impact on female fertility. Reprod. Biol. Endocrinol. 17, 5. doi:10.1186/s12958-018-0448-5
Rheay, H. T., Omondi, E. C., and Brewer, C. E. (2021). Potential of hemp (Cannabis sativa L) for paired phytoremediation and bioenergy production. GCB Bioenergy 13, 525–536. doi:10.1111/gcbb.12782
Riaz, U., Athar, T., Mustafa, U., and Iqbal, R. (2022). “Chapter 23 - economic feasibility of phytoremediation,” in Phytoremediation. Editors R. A. Bhat, T. M. P. Tonelli, G. H. Dar, and K. Hakeem (Amsterdam, Netherland), 481–502.
Rodríguez-Jorda, M. P., Gariddo, F., and García-González, M. T. (2010). Potential use of gypsum and lime rich industrial by-products for induced reduction of Pb, Zn and Ni leachability in an acid soil. J. Hazard Mater 175 (1-3), 762–769. doi:10.1016/j.jhazmat.2009.10.074
Sahoo, M. M., and Swain, J. B. (2020). Modified heavy metal Pollution index (m-HPI) for surface water Quality in river basins, India. Environ. Sci. Pollut. Res. 27, 15350–15364. doi:10.1007/s11356-020-08071-1
Saini, S., Gill, J. K., Kaur, J., Saikia, H. R., Singh, N., Kaur, I., et al. (2020). “Biosorption as environmentally friendly technique for heavy metal removal from wastewater,” in Fresh water pollution dynamics and remediation. Editors H. Qadri, R. Bhat, M. Mehmood, and G. Dar (Singapore: Springer), 167–181.
Sajad, M. A., Khan, M. S., Bahadur, S., Naeem, A., Ali, H., Batool, F., et al. (2020). Evaluation of chromium phytoremediation potential of some plant species of Dir Lower, Khyber Pakhtunkhwa, Pakistan. Acta Ecol. Sin. 40 (2), 158–165. doi:10.1016/j.chnaes.2019.12.002
Salem, Z., and Allia, K. (2008). Cadmium biosorption on vegetal biomass. Int. J. Chem. React. Eng. 6 (1), 1542–6580. doi:10.2202/1542-6580.1448
Sangodoyin, A. Y. (1991). Groundwater and surface water pollution by open refuse dump in Ibadan, Nigeria. J. Discov. Innov. 3 (1), 37–43.
Sari, A., and Tuzen, M. (2008). Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard Mater 152 (1), 302–308. doi:10.1016/j.jhazmat.2007.06.097
Saxena, G., Purchase, D., Mulla, S. I., Saratale, G. D., and Bharagava, R. N. (2019). “Phytoremediation of heavy metal-contaminated sites: eco-environmental concerns, field studies, sustainability issues, and future prospects,” in Reviews of environmental contamination and toxicology. Editor P. de Voogt (Cham: Springer), 71–131.
Seinfeld, J. H., and Pandis, S. N. (2016). Atmospheric chemistry and physics: From air pollution to climate change. New Jersey: John Wiley and Sons,Inc.
Senthilkumar, R., Reddy Prasad, D. M., Govindarajan, L., Saravanakumar, K., and Naveen Prasad, B. S. (2020). Synthesis of green marine algal-based biochar for remediation of arsenic(V) from contaminated waters in batch and column mode of operation. Int. J. Phytoremediation 22 (3), 279–286. doi:10.1080/15226514.2019.1658710
Shaheen, S. M., El-Naggar, A., Wang, J., Hassan, N. E. E., Niazi, N. K., Wang, H., et al. (2019a). “Biochar as an (Im)mobilizing agent for the potentially toxic elements in contaminated soils,” in Biochar from biomass and waste. Editors Y. S. Ok, D. C. W. Tsang, N. Bolan, and J. M. Novak (Amsterdam, Netherland), 255–274.
Shaheen, S. M., Niazi, N. K., Hassan, N. E. E., Bibi, I., Wang, H., Tsang, D. C. W., et al. (2019b). Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 64 (4), 216–247. doi:10.1080/09506608.2018.1473096
Shen, Z., Hou, D., Jin, F., Shi, J., Fan, X., Tsang, D. C. W., et al. (2019). Effect of production temperature on lead removal mechanisms by rice straw biochars. Sci. Total Environ. 655, 751–758. doi:10.1016/j.scitotenv.2018.11.282
Shi, R. Y., Ni, N., Nkoh, J. N., Dong, N., Zhao, W. R., Pan, X. Y., et al. (2020). Biochar retards Al toxicity to maize (Zea mays L) during soil acidification: the effects and mechanisms. Sci. Total Environ. 719, 137448. doi:10.1016/j.scitotenv.2020.137448
Singh, J., Yadav, P., Pal, A. K., and Mishra, V. (2020). “Water pollutants: origin and status,” in Sensors in water pollutants monitoring: Role of material. Advanced functional materials and sensors. Editors D. Pooja, P. Kumar, P. Singh, and S. Patil (Singapore: Springer), 5–20.
Singh, T. S., and Pant, K. K. (2005). Solidification/stabilization of arsenic containing solid wastes using Portland cement, fly ash and polymeric materials. J. Hazard Mater 131 (1-3), 29–36. doi:10.1016/j.jhazmat.2005.06.046
Smith, L. A., Means, J. L., Chen, A., Alleman, B., Chapma, C. C., Tixier, J. S., et al. (1995). Remedial option for metals-contaminated sites. Boca Raton, FL: CRC Press.
Soltani, N., Keshavarzi, B., Moore, F., Sorooshian, A., and Ahmadi, M. R. (2017). Distribution of potentially toxic elements (PTEs) in tailings, soils, and plants around Gol-E-Gohar iron mine, a case study in Iran. Environ. Sci. Pollut. Res. 24, 18798–18816. doi:10.1007/s11356-017-9342-5
Song, N., Lee, Y., and Lee, M. (2005). Remediation process by using lime and calcium carbonate for heavy metal contaminated groundwater originated from landfills. Econ. Environ. Geol. 38, 273–284. in Korean. https://www.kseeg.org/journal/download_pdf.php?spage=273andvolume=38andnumber=3.
Sunarso, J., and Ismadji, S. (2009). Decontamination of hazardous substances from solid matrices and liquids using supercritical fluids extraction: A review. J. Hazard Mater 161 (1), 1–20. doi:10.1016/j.jhazmat.2008.03.069
Suppadit, T., Phubphol, A., and Neumnoi, P. (2012). Effect of quail litter biochar on productivity of four new physic nut varieties planted in cadmium-contaminated soil. Chil. J. Agr. Res. 72 (1), 125–132. doi:10.4067/S0718-58392012000100020
Sytar, O., Kumari, P., Yadav, S., Brestic, M., and Rastogi, A. (2019). Phytohormone priming: regulator for heavy metal stress in plants. J. Plant Growth Regul. 38 (2), 739–752. doi:10.1007/s00344-018-9886-8
Tauqeer, M., Ahmad, M. S., Mohammad, A., and Baig, M. T. (2020). “Nanocomposite materials for wastewater decontamination,” in Modern age waste water problems. Editors M. Oves, M. Ansari, M. Zain Khan, and M. Shahadat (Cham: Springer), 23–46.
Tong, X., and Xu, R. (2013). Removal of Cu(II) from acidic electroplating effluent by biochars generated from crop straws. J. Environ. Sci. 25 (4), 652–658. doi:10.1016/S1001-0742(12)60118-1
Trakal, L., Komárek, M., Száková, J., Zemanová, V., and Tlustoš, P. (2011). Biochar application to metal contaminated soil: evaluating of Cd, Cu, Pb and Zn sorption behavior using single- and multi-element sorption experiment. Plant Soil Environ. 57 (8), 372–380. doi:10.17221/155/2011-PSE
Tripathi, R. D., Srivastava, S., Mishra, S., Singh, N., Tuli, R., Gupta, D. K., et al. (2007). Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol. 25 (4), 158–165. doi:10.1016/j.tibtech.2007.02.003
Tusher, T. R., Sarker, M. E., Nasrin, S., Kormoker, T., Proshad, R., Islam, M. S., et al. (2021). Contamination of toxic metals and polycyclic aromatic hydrocarbons (PAHs) in rooftop vegetables and human health risks in Bangladesh. Toxin Rev. 40 (4), 736–751. doi:10.1080/15569543.2020.1767650
Valls, M., and Lorenzo, V. D. (2002). Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol. Ecol. 26 (4), 338–327. doi:10.1111/j.1574-6976.2002.tb00618.x
Verklejim, J. A. S. (1993). “The effects of heavy metals stress on higher plants and their use as bio monitors,” in Plant as bioindicators: Indicators of heavy metals in the terrestrial environment. Editor B. Markert (New York: VCH), 415–424.
Vijayaraghavan, K., Palanivelu, K., and Velan, M. (2006). Biosorption of copper(II) and cobalt(II) from aqueous solutions by crab shell particles. Bioresour. Technol. 97 (12), 1411–1419. doi:10.1016/j.biortech.2005.07.001
Vilar, V. J. P., Botelho, C. M. S., and Boaventura, R. A. R. (2007). Chromium and zinc uptake by algae gelidium and agar extraction algal waste: kinetics and equilibrium. J. Hazard Mater 149 (3), 643–649. doi:10.1016/j.jhazmat.2007.04.023
Wang, J., Feng, X., Anderson, C. W. N., Xing, Y., and Shang, L. (2012). Remediation of mercury contaminated sites - a review. J. Hazard Mater 221-222, 1–18. doi:10.1016/j.jhazmat.2012.04.035
Wang, M., Ren, L., Wang, D., Cai, Z., Xia, X., and Ding, A. (2019). Assessing the capacity of biochar to stabilize copper and lead in contaminated sediments using chemical and extraction methods. J. Environ. Sci. 79, 91–99. doi:10.1016/j.jes.2018.11.007
Wang, Q., Lu, H., Chen, J., Jiang, Y., Williams, M. A., Wu, S., et al. (2020). Interactions of soil metals with glomalin-related soil protein as soil pollution bioindicators in mangrove wetland ecosystems. Sci. Total Environ. 709, 136051. doi:10.1016/j.scitotenv.2019.136051
Wang, Y. M., Chen, T. C., Yeh, K. J., and Shue, M. F. (2001). Stabilization of an elevated heavy metal contaminated site. J. Hazard Mater 88 (1), 63–74. doi:10.1016/S0304-3894(01)00289-8
Waqas, M., Kim, Y. H., Khan, A. L., Shahzad, R., Asaf, S., Hamayun, M., et al. (2017). Additive effects due to biochar and endophyte application enable soybean to enhance nutrient uptake and modulate nutritional parameters. J. Zhejiang Univ. Sci. B 18 (2), 109–124. doi:10.1631/jzus.B1500262
Wilson, D. J., and Clarke, A. N. (1993). “Soil vapor stripping,” in Hazardous waste site soil remediation: Theory and application of innovative technologies. Editors D. J. Wilson, and A. N. Clarke (New York, NY: CRC Press), 171–242.
Xiao, R., Ali, A., Wang, P., Li, R., Tian, X., and Zhang, Z. (2019). Comparison of the feasibility of different washing solutions for combined soil washing and phytoremediation for the detoxification of cadmium (Cd) and zinc (Zn) in contaminated soil. Chemosphere 230, 510–518. doi:10.1016/j.chemosphere.2019.05.121
Xu, X., Cao, X., Zhao, L., Wang, H., Yu, H., and Gao, B. (2013). Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. Int. 20 (1), 358–368. doi:10.1007/s11356-012-0873-5
Xuan, Z., Tang, Y., Li, X., Liu, Y., and Luo, F. (2006). Study on the equilibrium, kinetics and isotherm of biosorption of lead ions onto pretreated chemically modified orange peel. Biochem. Eng. J. 31 (2), 160–164. doi:10.1016/j.bej.2006.07.001
Yadav, V., Jain, S., Mishra, P., Khare, P., Shukla, A. K., Karak, T., et al. (2019). Amelioration in nutrient mineralization and microbial activities of sandy loam soil by short term field aged biochar. Appl. Soil Ecol. 138, 144–155. doi:10.1016/j.apsoil.2019.01.012
Yanqun, Z., Yuan, L., Jianjun, C., Haiyan, C., Li, Q., and Schratz, C. (2005). Hyper accumulation of Pb, Zn and Cd in herbaceous grown on lead-zinc mining area in Yunnan, China. Environ. Int. 31 (5), 755–762. doi:10.1016/j.envint.2005.02.004
Yaron, B., Dror, I., and Berkowitz, B. (2012). “Chapter 3, properties and behavior of selected inorganic and organometallic contaminants,” in Soil-Subsurface change, Chemical pollutants impacts (Berlin, Heidelberg: Springer-Verlag), 39–74.
Yin, C., Mahmud, H. B., and Shaaban, M. G. (2006). Stabilization/solidification of lead-contaminated soil using cement and rice husk ash. J. Hazard Mater 137 (3), 1758–1764. doi:10.1016/j.jhazmat.2006.05.013
Yoon, J., Cao, X., Zhou, Q., and Ma, L. Q. (2006). Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 368 (1-3), 456–464. doi:10.1016/j.scitotenv.2006.01.016
Yuan, P., Wang, J., Pan, Y., Shen, B., and Wu, C. (2019). Review of biochar for the management of contaminated soil: preparation, application and prospect. Sci. Total Environ. 659, 473–490. doi:10.1016/j.scitotenv.2018.12.400
Yuncu, B., Sanin, F. D., and Yetis, U. (2006). An investigation of heavy metal biosorption in relation to C/N ratio of activated sludge. J. Hazard Mater 137 (2), 990–997. doi:10.1016/j.jhazmat.2006.03.020
Zainab, N., Amna, S., Khan, A. A., Azeem, M. A., Ali, B., Wang, T., et al. (2021). PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. In industrially contaminated soils. Agronomy 11 (9), 1820. doi:10.3390/agronomy11091820
Zhang, J., Cao, X., Yao, Z., Lin, Q., Yan, B., Cui, X., et al. (2021). Phytoremediation of Cd-contaminated farmland soil via various sedum alfredii-oilseed rape cropping systems: efficiency comparison and cost-benefit analysis. J. Hazard. Mater. 419, 126489. doi:10.1016/j.jhazmat.2021.126489
Zhang, W., Zeng, Z., Liu, Z., Huang, J., Xiao, R., Shao, B., et al. (2020). Effects of carbon nanotubes on biodegradation of pollutants: positive or negative? Ecotox Environ. Safe 189, 109914. doi:10.1016/j.ecoenv.2019.109914
Zheng, H., Zhang, C., Liu, B., Liu, G., Zhao, M., Luo, X., et al. (2020). “Biochar for water and soil remediation: production, characterization, and application,” in A new paradigm for environmental chemistry and toxicology. Editors G. Jiang, and X. Li (Singapore: Springer), 163–196.
Zhong, C., Zhao, J., Chen, W., Wu, D., and Cao, G. (2020). Biodegradation of hydrocarbons by microbial strains in the presence of Ni and Pb. 3 Biotech. 10, 18. doi:10.1007/s13205-019-2011-2
Zhou, X., Zhang, X., Ma, C., Wu, F., Jin, X., Dini-Andreote, F., et al. (2022). Biochar amendment reduces cadmium uptake by stimulating cadmium-resistant PGPR in tomato rhizosphere. Chemosphere 307, 136138. doi:10.1016/j.chemosphere.2022.136138
Keywords: biochar, soil, water, potentially toxic elements (PTEs), remediation technologies
Citation: Zhang X, Zou G, Chu H, Shen Z, Zhang Y, Abbas MHH, Albogami BZ, Zhou L and Abdelhafez AA (2023) Biochar applications for treating potentially toxic elements (PTEs) contaminated soils and water: a review. Front. Bioeng. Biotechnol. 11:1258483. doi: 10.3389/fbioe.2023.1258483
Received: 14 July 2023; Accepted: 03 August 2023;
Published: 17 August 2023.
Edited by:
Sedky Hassan, Sultan Qaboos University, OmanReviewed by:
Khalid Abdallah Hussein, Assiut University, EgyptNaveed Ahmed Qambrani, Mehran University of Engineering and Technology, Pakistan
Copyright © 2023 Zhang, Zou, Chu, Shen, Zhang, Abbas, Albogami, Zhou and Abdelhafez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoyan Zou, em91Z3VveWFuQDI2My5uZXQ=; Ahmed A. Abdelhafez, YWhtZWQuYXppekBhZ3IubnZ1LmVkdS5lZw==
 Xu Zhang
Xu Zhang Guoyan Zou1,3*
Guoyan Zou1,3* Zheng Shen
Zheng Shen Yalei Zhang
Yalei Zhang Mohamed H. H. Abbas
Mohamed H. H. Abbas Ahmed A. Abdelhafez
Ahmed A. Abdelhafez