
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol. , 02 October 2023
Sec. Biomaterials
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1250804
This article is part of the Research Topic Novel Biomaterials to Improve the Biological Effects of Electromagnetic and Particle Radiation View all 6 articles
Photodynamic therapy (PDT) has been under development for at least 40 years. Multiple studies have demonstrated significant anti-tumor efficacy with limited toxicity concerns. PDT was expected to become a major new therapeutic option in treating localized cancer. However, despite a shifting focus in oncology to aggressive local therapies, PDT has not to date gained widespread acceptance as a standard-of-care option. A major factor is the technical challenge of treating deep-seated and large tumors, due to the limited penetration and variability of the activating light in tissue. Poor tumor selectivity of PDT sensitizers has been problematic for many applications. Attempts to mitigate these limitations with the use of multiple interstitial fiberoptic catheters to deliver the light, new generations of photosensitizer with longer-wavelength activation, oxygen independence and better tumor specificity, as well as improved dosimetry and treatment planning are starting to show encouraging results. Nanomaterials used either as photosensitizers per se or to improve delivery of molecular photosensitizers is an emerging area of research. PDT can also benefit radiotherapy patients due to its complementary and potentially synergistic mechanisms-of-action, ability to treat radioresistant tumors and upregulation of anti-tumoral immune effects. Furthermore, recent advances may allow ionizing radiation energy, including high-energy X-rays, to replace external light sources, opening a novel therapeutic strategy (radioPDT), which is facilitated by novel nanomaterials. This may provide the best of both worlds by combining the precise targeting and treatment depth/volume capabilities of radiation therapy with the high therapeutic index and biological advantages of PDT, without increasing toxicities. Achieving this, however, will require novel agents, primarily developed with nanomaterials. This is under active investigation by many research groups using different approaches.
Cancer research is crowded with therapeutic options that promise higher efficacy with less toxicity. Several new targeted biologic agents intended to combat tumor directly or indirectly via modulation of the immune or microvascular systems were predicted to be paradigm-shifting (Baudino, 2015) and have led some to question if there is a future role for traditional therapies such as radiation, surgery and chemotherapy (Arruebo et al., 2011). However, while clinical trials with some of these agents have demonstrated promise, many have failed (Mallarkey and Coombes, 2013; Maeda and Khatami, 2018; Sabnis and Bivona, 2019; Wong et al., 2019). In comparison, renewed interest in aggressive local therapies are leading to more radical surgeries and stereotactic radiotherapy for loco-regional disease (Weichselbaum and Hellman, 2011; Cheung et al., 2019). Even in the metastatic setting, surgical excision and stereotactic radiotherapy for treating the primary tumor bulk have demonstrated significantly increased progression-free and overall survival (Iyengar et al., 2018; Gomez et al., 2019; Palma et al., 2019). These benefits also exist with more advanced targeted drug therapies and perhaps may even synergistically, e.g., immunotherapy agents, for better overall treatment outcomes (Demaria et al., 2016; Weichselbaum et al., 2017), especially using localized therapies with potential abscopal effects.
A significant continuing challenge is to limit the toxicities of these aggressive local therapies. Surgical oncology has made significant advances in recent decades through improved and minimally-invasive techniques (Chang and Rattner, 2019). Further advances may arise through robotic-assisted surgeries but, despite many years of use, this has yet to translate into clear clinical benefit (Seigne et al., 2019). Combination therapies, including neoadjuvant and adjuvant chemotherapy, have demonstrated benefit in advanced and difficult-to-treat tumors (Forbes, 1982). Factors in individual patients that limit more aggressive management include comorbidities, advanced age, disease-related decline in performance status and long-term side effects from prior cancer therapies.
Radiotherapy has made great advances in local dose escalation without undue toxicities, mainly through advances in technologies for dose delivery. High-precision radiotherapy has existed for some time in machines such as the Gamma Knife, Cyber Knife, Tomotherapy, and linear accelerators (LINACs) with motion-tracking and beam modulation. The addition of inverse planning-based intensity-modulated radiotherapy (IMRT) has also advanced the field. These technologies allow high spatial precision that can rival surgery with less acute toxicity (Chang et al., 2015). This has proven useful in managing primary as well as oligometastatic disease (Palma et al., 2019). Clinical implementation has hit its stride in the past decade but added benefits of further refinements in dose delivery may be limited. Thus, we are approaching sub-millimeter expansion for planning target volumes (PTVs), whereby the area under treatment is intentionally expanded beyond the known clinical disease to accommodate for motion and setup error, but every last mm and fraction of a mm gained will likely yield diminishing clinically significant returns. New modalities such as ultra-high dose-rate FLASH radiotherapy may provide some additional benefit in maximizing tumor treatment efficacy while minimizing toxicity (Bourhis et al., 2019) but this remains to be proven. Further refinements in radiation dosing and fractionation, and novel clinical applications such as in oligometastatic disease may continue to advance the field but, in the absence of newer methods of safe dose escalation, we will eventually reach the limits of contemporary radiotherapy.
A variety of other biophysical modalities for treating cancers locally have been developed in the past few decades to improve the therapeutic index and deliver higher treatment efficacy with lower toxicity. Techniques such as radiofrequency ablation (RFA), high-intensity focused ultrasound (HIFU), endovascular embolization, hyperthermal therapy and cryotherapy have aimed to increase local tumor control and, in some cases, also possibly generate an immunologic response (Goode and Matson, 2004; Glazer and Curley, 2011; Nishikawa and Osaki, 2013). These various techniques have met with different degrees of success: some have established new niches but none have been widely transformative to date. Their limitations, including the invasiveness, lack of intrinsic tumor specificity and collateral damage to surrounding normal tissue, have limited their applications.
One form of local therapy that has demonstrated high anti-tumor activity with low toxicity is photodynamic therapy (PDT). First described over 100 years ago, this modality re-emerged in the early 1980s, driven by the availability of lasers as activating light sources and by the development of potent photosensitizers. The first regulatory approval, in 1993 in Canada, was for treatment of refractory superficial bladder cancer (van Straten et al., 2017) and PDT has since been approved in multiple countries for a range of tumor types and stages; from curative treatment of premalignant lesions to palliation of advanced disease. In its early modern phase, PDT was widely projected to be the next paradigm shift in local cancer management (Santos et al., 2019). Currently, it is well accepted for treating superficial cancers, including endoscopically-accessible lesions (Santos et al., 2019). It has also proven to be effective for tumors that are refractory to radiotherapy (Hatogai et al., 2016). However, PDT has failed to gain broader acceptance. A major technical factor is the difficulty of using PDT for deep-seated and larger solid tumors, due primarily to the limited penetration of the red/near-infrared activating light: in the simplest case of external light source irradiation or interstitial fiberoptic light sources, effective treatment depths or radii of 5–10 mm are typical. This is further compounded by these deeper penetrating, but longer, wavelength photons possessing lower amounts of energy needed for the photochemical reaction to generate singlet, with the theoretical maximum wavelength being about 800 nm to successfully generate singlet oxygen. Some of the most common cancers, such as lung, prostate, breast and gastrointestinal, are mainly deep-seated and are difficult or at least technically complex to treat for complete tumor destruction (Siegel et al., 2019). This limitation may be changing and eventually allow effective treatment anywhere in the body. One such evolution involves overlap with radiation oncology, whereby the precise targeting of modern X-ray technologies may provide the excitation energy to effect PDT (Ren et al., 2018; Cline et al., 2019), as discussed below.
PDT uses a two-step activation process to induce cytotoxic tissue damage. Visible or near-infrared (NIR) light provides the energy to activate photosensitizing molecules (PS), thereby generating cytotoxic reactive oxygen species (ROS) (Debele et al., 2015). The light and photosensitizer alone are each essentially non-toxic with no therapeutic activity. There is minimal heat generated in the tissue, distinguishing PDT form photothermal therapy where higher-power (laser) light is used to destroy tumor tissue through photocoagulation. However, as recently discussed by Wilson and Weersink (Wilson and Weersink, 2020), these two modalities do share many common technical features and the optimal choice between them can depend on the tumor size and location.
The most commonly used PSs in patients are porphyrin ring-based molecules that were “re-discovered” in the 1960s, following a much longer history as both phototherapeutic and photodiagnostic (fluorescence imaging) agents (Castano et al., 2004). They are analogous to endogenous porphyrin ring structures that are precursors in the heme biosynthetic pathway (Castano et al., 2004). This molecular structure confers important photophysical and photochemical; properties that make them efficient PDT agents. The key structure is a tetrapyrrole backbone that is also found in chlorins and phthalocyanines. The photodynamic and pharmacokinetic properties may be “fine-tuned” by the altering the specific molecular structure (Zhang et al., 2018).
The most commonly used light sources for clinical PDT are either diode lasers or light emitting diodes (LEDs): the former allows efficient light delivery through small diameter (typically, 200–400 μm) optical fibers for endoscopic or interstitial use, while the latter are relatively inexpensive and can be configured in different shapes and sizes to treat different tumor sites. Wavelength-filtered lamp-based systems are also used for skin malignancies or, more commonly, benign skin conditions (Brancaleon and Moseley, 2002). These various light sources produce energy continuously (CW) and treatments typically take minutes to tens of minutes to deliver sufficient local energy density (∼100 J/cm2) at a low enough lower power density (<∼100 mW/cm2) to avoid tissue heating and/or photochemical depletion of tissue oxygenation. The wavelength used in most clinical trials to date are in the 630–730 nm range to achieve usable tissue penetration while still providing sufficient photon energy to activate the photosensitizer. For more superficial lesions (e.g., skin, bladder), blue or green light are also used. Preclinically, ultrashort NIR laser pulses have been investigated to perform 2-photon activation, which may provide somewhat deeper tissue penetration and, more importantly, allow very precise (diffraction-limited) control over the three-dimensional activation volume. This comes with the drawback that 2-photon PDT needs a specific photosensitizer with a good absorption cross-section to both wavelengths and the specific molecular structure to convert their energies into singlet oxygen. The potential applications have been mainly in ophthalmology and dermatology (Wan et al., 2016; Bolze et al., 2017a): one report (Bolze et al., 2017b) has suggested effective treatment depths of several cm can be achieved in solid tumor, but the interpretation of these results is controversial. Ultrasound has also been investigated as the means to activate PSs (sonoluminescence) but the underlying biophysical mechanisms are not well understood and it has proven difficult to make this a robust approach (Wan et al., 2016; Bolze et al., 2017a).
As illustrated in Figure 1, upon absorption of a photon of suitable wavelength (energy), the ground-state photosensitizer, 1PS (S0), is excited to an electronic singlet state, 1PS* (S1). This can return to the ground state either via non-radiative internal conversion or by fluorescence emission, which is widely used for diagnostics and image-guided surgery (Daneshmand et al., 2018a; Ottolino-Perry et al., 2021; Sutton et al., 2023), or it can undergo intersystem crossing to a triplet state, 3PS* (T1) (Castano et al., 2004). This can decay back to the ground state either non-radiatively or by phosphorescence emission, or by reacting with a suitable molecular substrate via so-called Type I or Type II pathways. In Type I the triplet state reacts directly with biomolecules such as lipid membranes or water to form free radical species (Castano et al., 2004). In the Type II pathway, the excess energy is transferred to ground-state molecular oxygen, 3O2, to form highly-reactive singlet oxygen, 1O2. The Type II pathway is believed to be the dominant process with most (Castano et al., 2005a) but not all (Neuschmelting et al., 2018) clinical photosensitizers. One consequence is that the efficacy depends on the available molecular oxygen in the local tumor environment (see below). As a result of its high reactivity, the lifetime of 1O2 in cells/tissues is short (<1 μs), so that it diffuses only tens of nm before interacting and causing damage. Hence, the subcellular localization of the PS is critical in determining the organelle(s) damaged and, thereby, the resulting cell-death pathway and level of cytotoxicity: for example, the same amount of singlet oxygen is ∼10-fold more cytotoxic if the PS is localized to mitochondria rather than the plasma membrane (Mahalingam et al., 2018). This confers a high degree of biological control. For example, if the treatment light is delivered while the PS is still in the circulation, then vascular endothelial cell death and ischemia will be the primary route of tumor destruction rather than direct killing of the tumor cells. This has been exploited in some clinical studies (Azzouzi et al., 2017). The use of oxygen-independent photosensitizers is also an option (Larue et al., 2021), although this does not circumvent the need to deliver the PS, which may be compromised in poorly-vascularized regions of tumor.
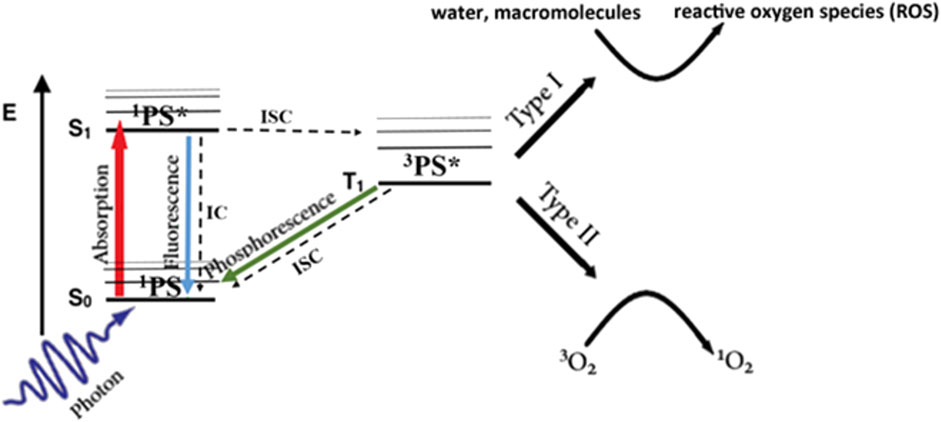
FIGURE 1. Simplified Jablonski energy diagram of photodynamic activation. Radiative transitions are shown as colored arrows and non-radiative transitions as black/dashed arrows.
The net effect of PDT is mainly to promote oxidative stress, which manifests in different processes, at the level of cellular, tumor and immune-system responses. In the cancer cell, the 1O2 can react with macromolecules, particularly lipids. Depending on the subcellular localization of the PS, this leads to plasma membrane damage and/or disruption of lysosomes, mitochondria or endoplasmic reticulum, inducing stress responses that lead to cell necrosis, apoptosis or autophagy (Castano et al., 2005a). Similar processes may occur in vascular endothelial cells (Castano et al., 2005b). The dying cells also release cytokine signals that promote death of nearby cells (the bystander effect) (Wang et al., 2018). Lastly, an important contributor to PDT’s clinical efficacy is its ability to “prime” the immune system, an effect that was observed initially in preclinical murine studies in which PDT generated durable cures in immune-competent animals (Korbelik et al., 1996), but has also been observed in patients (Thong et al., 2007; Thong et al., 2008; Beltrán Hernández et al., 2020).
It is well established that PDT-mediated damage via lipid peroxidation of the plasma membrane and cell organelles elicit strong pro-inflammatory signalling and results in a robust innate immune response leading to immunological cell death of cancer cells (Castano et al., 2005a). This also potentiates an adaptive response for long-term control. The PDT oxidative stress causes vascular injury-induced tumor ischemia, as well as strong direct cytotoxicity via lipid membrane disruption, cell death and spillage of cytoplasmic contents that act as damage-associated molecular patterns (DAMPs) to activate multiple signaling pathways such as Toll-like receptors (TLRs) and RIG-I-like receptors (RLRs) on dendritic cells, heat shock protein (HSP) pathways, nuclear factor kappa B (NFkB), tumor necrosis factor alpha (TNFα) and activator protein 1 (AP-1). DAMPs also activate CD8+ cytotoxic T lymphocytes through similar mechanisms as in radiotherapy, leading to multiple pro-inflammatory interleukins, chemokines, interferons and macrophage inflammatory proteins (MIP). In turn, the innate and adaptive immune response activates T lymphocytes, macrophages, natural killer (NK) cells and neutrophil infiltration to kill tumor cells directly, and also produces further pro-inflammatory activity, immune cell activating mediators and complement cascade activation (Panzarini et al., 2013; Chen et al., 2017a). Overall then, an acute and powerful inflammatory response and induction of early and late immune responses mediates the potential abscopal effects of PDT that have been seen in a range of preclinical and clinical studies (Moore et al., 1993; Castano et al., 2005b; Chen and Zhang, 2006; Takahashi and Misawa, 2007; Liu et al., 2008; Zou et al., 2014; Debele et al., 2015; Retif et al., 2015). These effects are recognized increasingly as significantly contributing to the efficacy of PDT, in terms of both primary tumor response and reduced tumor progression and metastatic risk and represent a clear advantage over other local biophysical therapies.
Ionizing radiation has also been investigated to potentiate immunotherapy drugs, particularly in the scenario of poor response to immune checkpoint inhibition (Panzarini et al., 2013; Weichselbaum et al., 2017). The main proposed mechanisms are via DAMPs in the microenvironment that lead to pro-inflammatory cytokines such as interleukin-1β (IL-1β), transforming growth factor β (TGF-β), fibroblast growth factor (FGF) and TNFα. In addition, cytosolic DNA ingested by dendritic cells from radiation-damaged tumor cells produce cyclic guanine monophosphate-adenosine monophosphate (cGAMP) via cGAMP synthase (cGAS) and stimulate interferon genes (STING) to transcribe type I interferon (IFN). ICD dampening simultaneously exists through TGF-β and IL-10 mediated regulatory T lymphocytes (Treg) and myeloid-derived suppressor cell (MDSC) activation, which act to suppress cytotoxic T lymphocyte activity (Vanpouille-Box et al., 2017). These competing ICD promoting and dampening pathways with radiotherapy can lead to a spectrum of responses such as tumor elimination, equilibrium, dormancy or escape. In practice, the clinical response to combined radiotherapy and immunotherapy has not been conclusively positive (Kwon et al., 2014; Asna et al., 2018).
Some of the immune-priming mechanisms are shared between radiotherapy and PDT, but key differences do exist. Both can generate abscopal effects in isolation via systemic spread of T and B lymphocytes sensitized to tumor neoantigens. Both rely on DAMP-mediated antigen-presenting cell (APC) activity, leading to recruitment and activation of effector T lymphocytes (Teff), and both cause chemokine and chemotaxin release to promote T lymphocyte infiltration and inflammation (Panzarini et al., 2013; Weichselbaum et al., 2017). A key feature of PDT is that it can elicit a strong innate immune response causing a high degree of tumor inflammation that tips the balance of immune-priming and immune-dampening towards the former. ROS-mediated damage to tumor cells favours necrosis and a high load of DAMPs from the cytosol being directly released into the extracellular environment. This induces strong cytokine/chemokine activation for the innate immune response and produces many neoantigens for the adaptive immune response.
In radiation therapy DNA-mediated cell death, together with DAMP presentation and cGAS-STING activation, does not produce as profound inflammatory and innate immune responses as PDT (Lee et al., 2009; Chen et al., 2017a) that contribute to the clinical outcomes. We note also that there is evidence of a negative immune response from surgical resection of tumors, both in animal models and in patients (Salo, 1992; Onuma et al., 2020), so that using PDT in the adjuvant setting with radiotherapy and/or surgery may be advantageous. Interestingly, the immune-stimulating profile of PDT and radiotherapy have not been directly compared to our knowledge, although the expectation is that PDT will be the more powerful immune stimulator given its central role in clinical PDT and the known mechanism of action.
Mechanistically, the cellular and, hence, tumor response to PDT is much more rapid (hours-days) than radiotherapy, since it depends on killing the tumor cells rather than reducing their proliferative capacity. This is due primarily to the initial targets for PDT damage being extra-nuclear, which accounts also for the extremely low level of induced resistance observed with PDT (Desmettre et al., 2004; Casas et al., 2011) and the fact that PDT is effective even in radiation-resistant tumors (Stewart et al., 1998a; Oniszczuk et al., 2016). It is known, however, that active targeting of photosensitizer to the cell nuclear can increase tumor cell kill by more than 1,000-fold (Long et al., 2022), although this has not been exploited clinically to data.
The tumor response to PDT may exceed what the direct, short-range ROS-mediated cancer cell killing alone would predict (Castano et al., 2005a). As indicated above, bystander and induced immunological effects can induce wider cell death via the above cell signalling factors (Castano et al., 2005a). Normal tissue is more resistant than tumors to these secondary indirect effects. Hence, in addition to preferential PS uptake/retention and geometric confinement of the treatment light, this contributes to the high cancer selectivity of PDT (Castano et al., 2005a). These indirect effects have parallels to spatially-fractionated radiotherapy, a modality that focuses high-dose radiation in a grid-like pattern on the target achieving tumor responses with less acute radiotoxicity. However, this has major logistical challenges and the efficacy is still limited by radioresistance and long-term dose-related toxicity (Billena and Khan, 2019).
A key difference between PDT and radiotherapy is the toxicity profile of each. The short-term toxicities with PDT have related primarily to skin photosensitivity. With the first-generation PSs such as Photofrin this required that the patient avoid direct sunlight and bright artificial light for several weeks. This issue is much less severe with current PSs for which the skin photosensitivity is short lived, requiring precautions for typically only a few days. Photosensitizers also have minimal systemic toxicities that are easily managed or avoided. Local pain during light treatment has been reported in skin lesions with aminolaevulinic acid-PPIX (ALA-PPIX) mediated PDT but is transient and can be managed by local cooling, analgesics or other conservative measures (Warren et al., 2009). As far as is known, PDT has no significant long-term toxicity, which contrast with radiotherapy where both acute and late off-target effects are seen, including fibrosis, lymphatic damage, organ dysfunction and increased rate of secondary malignancies.
A further advantage of PDT is that the normal host tissues show excellent healing responses, due to the non-thermal nature of PDT avoiding damage to collagen that preserves the tissue architecture. This is obviously important in treating skin lesions for good cosmesis but is also critical in treating tumors of hollow organs in order to maintain the organ integrity. There is also evidence that PDT is nerve sparing, which is highly relevant in sites such as the head and neck and prostate (Wright et al., 2006). Finally, while PDT is usually administered as a single acute dose, in the event of incomplete tumor response or local recurrence after treatment, it can be repeated without loss of efficacy or increased risk to achieve complete responses: this has been demonstrated, for example, in prostate cancer that is recurrent after radiotherapy (Nathan et al., 2002). Repeat treatment may involve either re-administration of both PS and light or, with PSs having long circulation/residence times, light only.
The modern era of PDT in clinical cancer therapy started in 1993 when a PPIX derivative called Porfimer sodium (Photofrin) gained regulatory approval in Canada for use in refractory superficial bladder cancer (van Straten et al., 2017). Approvals followed for several other PSs in many different countries. To date several hundred trials have been conducted with PDT, the majority of which showed positive outcomes (Santos et al., 2019). These trials have included PDT as monotherapy and in combination with radiation, surgery, chemotherapy or, more recently, targeted drug therapy. The oncological indications have included primary and recurrent cancers in the bladder, skin (mainly basal cell carcinoma), eye, lung, pancreas, head and neck, esophagus, stomach, biliary canal, anal canal, brain, breast, cervix and prostate. Metastatic disease has generally not been a target for PDT, with some exceptions such as intraperitoneal (colon, ovarian metastases) and intrapleural (mesothelioma) treatments. Clinical intent has ranged from cure of premalignant (dysplastic) and early-stage lesions through to salvage therapy and palliation. Trials have ranged from Phase I to Phase III, although relatively few large-scale randomized Phase III trials have been reported. The delivery of PS has been primarily intravenous for most solid tumors, topically for skin and cervical lesions and by instillation for bladder cancers. There have also been limited trials of intra-arterial embolization (Moore et al., 2008).
As mentioned above, light delivery systems have ranged from natural sunlight to lamps, LEDs (including “wearable” devices) and, particularly for endoscopic and interstitial light delivery, diode lasers. Depending on the particular tumor site and stage, treatment may be given on an out-patient or ambulatory basis, during endoscopy or intraoperatively, with the time between PS and light administration varying between minutes and days, depending on the PS pharmacokinetics.
The two-step activation and the various mechanisms-of-action of PDT confer a degree of “biochemical localization” of the PS to the site of disease, further enabled by the spatial localization of the activating light. This allows PDT to have a high anti-tumor activity with low damage to adjacent host tissue or surrounding organs. For example, skin malignancies are often treated with PDT with high efficacy and minimal damage or long-term cosmetic effect on the normal skin (Lui et al., 2004), and similar high therapeutic index is seen generally with PDT. This has led to implementation in salvage esophageal, head and neck, and other cancers recurring after definitive chemoradiation (Yano et al., 2012a; Vander Poorten et al., 2015). In these settings, PDT has demonstrated encouragingly high complete response rates (Kahaleh et al., 2008; Usuda et al., 2010; de Visscher et al., 2013).
Despite these multiple advantages, PDT has not been adopted as standard of care for many cancer indications, particularly in the United States. The National Comprehensive Cancer Network (NCCN) currently recommends considering PDT in basal cell carcinoma or cutaneous squamous cell carcinoma only when the disease is low-risk and superficial, and where surgery and radiotherapy is contraindicated (N.C.C. Network, 2020a; N.C.C. Network, 2020b). There are mentions in other settings such as for mesothelioma, where its use in combination with surgery is considered experimental (N.C.C. Network, 2020c). A summary of the recommended uses of PDT per NCCN guidelines is presented in Table 1. By comparison, other localized therapies such as RFA that were developed around the same time as PDT, have become front-line standard-of-care in US guidelines for multiple cancer sites (Friedman et al., 2004). A major challenge for PDT has been how to effectively treat deep-seated and larger solid tumors.
Second- and third-generation PSs have been developed with significantly improved tumor specificity, targeting upregulated pathways in cancerous cells (Castano et al., 2004) and, thereby, enabling selective tumor destruction in the target volume with minimal off-target toxicity. This feature is especially advantageous in a theranostic approach, using fluorescence imaging of the PS to localize the target tissue with high precision and sensitivity (Daneshmand et al., 2018b).
Nevertheless, for deep-seated and large solid tumors, delivering the activating light to and throughout the target volume remains challenging. As mentioned above, the limited depth penetrance of typical red/NIR activating light gives an effective treatment depth (or radius in the case of interstitial PDT: see below) of only 5–10 mm, depending on the tissue. The inhomogeneity in optical absorption and scattering of tumor tissue also makes it difficult to ensure that the complete target volume is fully treated (Dickey et al., 2004) and intratumoral heterogeneity of PS is also an issue. In addition, other factors, including the local light fluence rate, constant versus pulsed light delivery, variations in PS concentration in the target tissue, PS photobleaching (Dolmans et al., 2003) and low tumor oxygenation (baseline and PDT-induced) (Moore et al., 1993), can significantly affect the clinical outcomes (Xiao et al., 2007; van Straten et al., 2017). Light dosimetry modeling and fractionation techniques have been undertaken to address some of these challenges. For example, Figure 2 illustrates the use of standardized icosahedral light catheter placement along with switched pulse light delivery to deposit light homogenously, prevent photobleaching and rapid oxygen depletion.
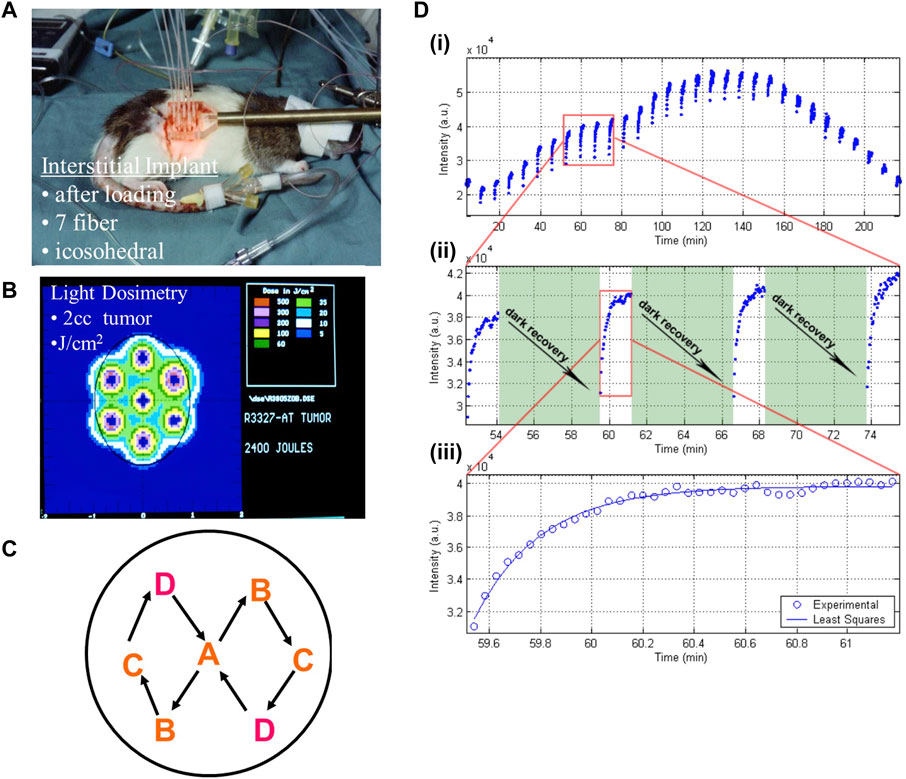
FIGURE 2. Methods of interstitial light delivery combined with spatial and temporal fractionation for optimizing PDT effect. A Dunning R3327 rat prostate cancer model was implanted with fiberoptics for light delivery in a standardized icosahedral layout (A), adopted for ease of light dosimetry calculation and geometric expansion to larger tumors (B). The fibers were activated sequentially in a specific geometric pattern (C) to allow fractionated therapy (D). This deposits light (measured in arbitrary units, a.u., by the detecting light catheter/photodiode arrangement) in short bursts (each blue point) at regular intervals either continuously (top) or with pauses to allow for recovery of the photosensitizer (middle). This allows more homogenously and controls the rate of photobleaching and oxygen depletion in the treatment field, which leads to higher therapeutic yield. Image adapted from Xiao et al. (2007) with permission. a.u. = arbitrary units.
The use of interstitial diffusing optical fibers for light delivery (iPDT) (Wilson and Patterson, 2008) has been employed clinically in multiple cancer sites, including prostate, pancreas, head and neck, brain and sites of metastases (Figure 2B) (Huang, 2005). However, Phase I, II and II clinical trials in these tumor groups have had varying levels of success. For example, Azzouzi et al. used the PS padaleporfin together with transperineal interstitial optical fibers and demonstrated significantly improved progression-free survival with minimal toxicity compared with active surveillance in low/intermediate-risk prostate cancer. iPDT with meta-tetra(hydroxyphenyl)chlorine (mTHPC) was employed successfully as a salvage treatment in a phase I/II trial of head & neck cancers that had recurred after chemoradiation (Lou et al., 2004a), achieving a 20% complete response rate, significant partial responses in almost all patients, some long-term survivors and no significant PS toxicities. Two patients did experience carotid rupture within 2 weeks of PDT due to disease invasion into the carotid, which speaks to the need for careful patient selection, given the strong and rapid necrotic tissue response to PDT (Lou et al., 2004a). Another phase I/II trial with verteporfin and MRI-guided iPDT in unresectable pancreatic cancer gave positive results in terms of tumor response and improved overall survival (Huggett et al., 2014), but the diameter of the treatment-induced tumor necrosis was variable, likely due to heterogeneities in light propagation, which speaks to the need for in situ dosimetry. The single interstitial fiber light delivery created up to a 12 mm diameter necrotic zone, so that treating larger tumors would have required the use of multiple sources. The latter approach has been most widely investigated in whole-organ targeted prostate cancer, where, for example, Davidson et al. (2009) used up to six cylindrically-diffusing fibers to treat recurrent cancer post radiation therapy and demonstrated a clear light-dose response threshold, above which 62% of the prostate was ablated, with minimal risk to normal tissues and ability to retreat if required. In metastatic disease, PDT has also proven effective for local control and symptom relief. For example, a recent Phase I trial for pathologic vertebral compression fractures in breast cancer patients with spinal metastases showed that PDT was safe, technically feasible and produced significant pain relief as an adjuvant to enable mechanical bone stabilization of the spine by destroying the space-occupying tumor mass (Fisher et al., 2019). Interstitial light delivery through vertobroplasty needles caused no short or long-term effects on the spinal cord that would have been of concern had the kyphoplasty been consolidated with palliative radiotherapy or stereotactic body radiotherapy (SBRT).
Despite positive clinical results with iPDT, accurately predicting the light dose distribution is significantly more susceptible to tissue heterogeneity than is the case with ionizing radiation, where soft tissues are uniformly “water-equivalent” (Wilson and Patterson, 2008). One strategy is to include interstitial light sensing fibers to monitor the distribution and fluence of the exciting light (Wilson and Patterson, 2008). This approach can provide feedback on calculated dosimetry, as well as real-time adjustment of light delivery during treatment to optimize standardization and therapeutic dose. The PS fluorescence can also be monitored in the same way. Clearly, this introduces an additional level of technical complexity to the treatment workflow and, to date, there has been a lack of consensus on the optimal approach: for example, using a few cylindrically-diffusing fibers or many point-diffusing sources to cover the target volume. Different models of light propagation in the target tissues have also been employed (Azzouzi et al., 2015). What does appear to be the case is that, at least for treating larger tumor volumes with curative intent, PDT treatment planning, energy delivery and dosimetry need to reach at least the same level of sophistication as has been achieved in radiation therapy, including brachytherapy with which it shares common features. The challenges are greater, however, because of PDT being a PS + light combination and tumor heterogeneity. Additional dosimetry tools, such as biophysical models and devices that integrate light, PS and oxygen levels (Wilson et al., 2003; Jarvi et al., 2012; Kosik et al., 2022), fluorescence monitoring of PS photobleaching (Sharwani and Alharbi, 2014), and direct measurement of 1O2 generation (Liang et al., 2012; Kim et al., 2016), show promise to address the challenge of achieving reliable individualized treatments.
A first approach to effective PDT treatment of larger tumor volumes is to use PSs with strong light absorption and high 1O2 quantum yield at NIR wavelengths between about 700 and 850 nm, where there is the best trade-off between light penetration and PS activation efficiency (1O2 quantum yield). PSs such as chlorins, bacteriochlorins, phthalocyanines and hypocrellins can have quantum yields of 0.9 or greater (Yoon et al., 2013) and NIR activation can double the effective treatment depth (or radius for iPDT) in some tissues (Abrahamse and Hamblin, 2016) compared with the first-generation porphyrin-based Photofrin that is activated at 630 nm. These newer PSs come with their own pharmacokinetics and biodistribution/localization profiles (Menter et al., 1998) and some show a variable degree of skin photosensitivity (Dougherty et al., 1990). NIR activation also often leads to lower quantum yields of singlet oxygen, so requiring higher light doses and, without prolonging treatment time, also higher power input (up to 40 W/cm2) to compensate (Veeranarayanan et al., 2018). Furthermore, the accuracy of irradiating with NIR light at depth also becomes a challenge as potentially larger spot sizes are needed to achieve deeper penetration, which limits the precision achievable for the activating light source (Ash et al., 2017). A variant of NIR photosensitization is the use of upconverting nanoparticles that absorb multiple NIR photons to generate visible light to activate traditional PSs. However, these require high NIR intensity (approaching 10 W/cm2) and so may be limited by potential thermal damage to normal tissue (Cho et al., 2009; Qiu et al., 2018). These issues of accuracy and thermal loading can be improved with 2-photon PDT, where the PS is excited when it absorbs two different wavelengths, which also gives better spatial localization at the intersection of the two light beams (Castano et al., 2004). See Table 2 for a comparison of the modalities. Overall, however, moving to the near-infrared and improving PS specificity will not eliminate the need to use iPDT to treat larger, deep-seated and endoscopically inaccessible tumors.
As mentioned above, a different strategy to treat deep/large tumors is to use ultrasound rather than light for PS activation. Sonodynamic therapy (SDT) has been demonstrated in various preclinical models (Yan et al., 2020) and a few small-scale clinical trials have shown potential benefit (Zha et al., 2023; D'Ammando et al., 2021). Most studies seem to use levels of PS and ultrasound that are close to their individual toxic doses, which is of concern. The ultimate safety and efficacy of SDT compared to PDT is still under investigation (Weber, 2018) and the underlying biophysical and biological mechanism of action are not well understood. SDT may also have problems with tissue heterogeneity and with effectively treating tumors deeper than a few cm without using more invasive techniques (Pfeiffer et al., 2015).
Beyond light delivery approaches, another way to maximize the therapeutic effect is to deliver the PS in a nanoparticle formulation, with or without active targeting, in so-called third-generation agents (Huang et al., 2012). These PDT nanoparticles aim to overcome biocompatibility issues with free PS, improve the kinetics and distribution characteristics and/or enable multimodal therapy. These issues can be particularly important with newer organic or inorganic PS constructs in order to trade biocompatibility and efficacy (Shim et al., 2022). Although some advantages can be achieved through conjugation of standard molecular PSs with antibodies, folate moieties or other small molecules, the majority of third-generation PDT approaches use nanoparticle systems (Dinakaran et al., 2023).
For nanoparticle-based PDT, the predominant constructs are with lipid nanoparticles (LNP) and their derivatives. LNPs here refer to a family of lipid-based nanoparticles such as micellar structures (single layer phospholipid with hydrophobic interior), liposomes (bi-layer phospholipids with physiologic interior environment), solid LNPs (admixture of lipids along with payload) and similar carriers. These are an attractive carrier system for PS because of the extensive research that has made them stable, biocompatible, have favorable distribution properties and drug release kinetics, provide passive targeting through the enhanced permeability and retention (EPR) effect or allow active targeting via aptamers, allow simultaneous co-delivery of other cancer drugs, and make even natively toxic drugs feasible to use in humans (Avci et al., 2014; Dinakaran et al., 2023). Beyond this obvious advantage, further development of LNP-based PDT agents has resulted in some new strategies in leveraging nanoparticles for PDT.
One optically active LNP agent was first reported by Lovell et al. (2011) with self-assembling porphyrin-lipid nanoparticles (Porphysomes) that exhibited high biocompatibility, minimal systemic toxicity (up to 1,000 mg/kg in mice), photothermal activation in the intact state and photodynamic activation when dissociated after cell uptake (Guidolin et al., 2021). Other iterations of Porphysomes have demonstrated theranostic capabilities with fluorescence and photoacoustic imaging (Muhanna et al., 2015). These examples show that LNP systems for PDT can not only gives the expected biocompatibility and distribution benefits as with chemotherapeutic payloads but can also present opportunities for unique new applications. Some drawbacks can still exist, however, namely, challenges in synthesis to achieve appropriate characteristics of size, polydispersity and encapsulation efficiency (Hou et al., 2021). Another issue is the tendency for LNP encapsulated PS to self-quench, which is why systems such as Porphysomes are not photodynamically active until dissociated into porphyrin-lipid monomers in vitro or in vivo (Muhanna et al., 2015).
Beyond LNPs, the next most common nanocarrier platform for PSs is polymeric nanoparticles such as poly-lactic-co-glycolic acid (PLGA). PLGA also has a track record of preclinical and clinical use to carry chemotherapeutic agents, albeit less developed than LNPs (Dinakaran et al., 2023). For the PLGA may have some advantages over LNP for photosensitizer delivery. PLGA is particularly effective at encapsulating strongly hydrophobic drugs such as PSs derived from porphyrins, hypocrellins and pthalocyanates (Kou et al., 2017). PLGA also typically has higher stability and slower drug-release kinetics than LNPs due to thicker walls, increased rigidity and lower permeability (Avci et al., 2014). This may be a hindrance for achieving slow-release with chemotherapeutics but can be beneficial for PDT where the PS can remain stable and in tissue for longer before light activation at the site of disease. These factors have helped PLGA achieve success in many preclinical PDT studies, such as with hypericin-loaded PLGA that has shown higher photoactivity and therapeutic index in ovarian cancer cells compared to PS alone (Zeisser-Labouèbe et al., 2006).
In addition to the dominant LNP and polymeric nanoparticle platforms, many other PS-nanoparticle constructs have been investigated preclinically, including inorganic systems such as gold nanoclusters (Maiolino et al., 2015), nanosilica (Karges et al., 2021), dendrimers (Avci et al., 2014) and fullerenes (Hamblin, 2018). While showing some favorable characteristics, currently none possess the balance of biocompatibility, biodistribution, toxicity and clinical implementation data enjoyed by LNPs and polymeric nanoparticles. The application of nanoparticles to PDT is still actively under research and one particularly exciting possibility is light-independent activation, such as with ionizing radiation (Dinakaran et al., 2020).
As a widely used modality, significant investment has been made to advance radiotherapy and maximize the therapeutic ration of anti-cancer effect-to-toxicity. This has included refinement of radiobiology-based strategies in the 1970s–2000s (Kirsch et al., 2018) and the increasing precision of radiation delivery (Pacelli et al., 2019). A new wave of interest in combination therapies, such as chemotherapy or targeted biologics plus immunotherapy has met with success in some, but not all, cancer types (Patrick et al., 2017; Nakhoda and Olszanski, 2020; Gong et al., 2021; Lin et al., 2022; Lu et al., 2022). Further advancements in combining radiotherapy with systemic agents are still underway with more bespoke, radiation-specific agents, and a variety of nanoparticle-based agents significantly contribute to this.
Nanoparticle-mediated radiosensitizers can be split into two groups: metallic and non-metallic. The best known metallic radiosensitizers are the high-Z materials such as gold nanoparticles (Kwatra et al., 2013). These are intended to increase the physical X-ray dose deposition in the target tumor and can enhance the efficacy by typically ∼2-fold (Kwatra et al., 2013). The mechanism is higher energy conversion through photoelectric effect and Compton effects, pair production and Auger electron generation (Kwatra et al., 2013). The dose enhancement is particularly pronounced below about 1 MeV photon energy, mediated by the photoelectric effect, increasing the probability of photon interaction and electron production by the Z3 (Almahwasi, 2016). The most clinically successful of these metallic nanoparticles is hafnium oxide, which effectively doubled complete responses in advanced soft tissue sarcoma, with minimal additional toxicity, as shown in Phase II/III clinical trials (Bonvalot et al., 2019). However, this required intratumoral injection, which limits its homogeneous intratumoral distribution and the clinical practicality for many sites (Holback and Yeo, 2011).
Efforts to use newer metallic nanoparticles designs aim to address some of these practical issues and further improve the efficacy. A common approach is by “decorating” the nanoparticles with moieties aimed at increasing biocompatibility or enhancing tumor-specific uptake. For example, coating metal nanoparticles in polyethylene glycol (PEG) facilitates intravenous injection, enhancing the pharmacologic properties and tumor uptake via the aforementioned EPR effect (Chattopadhyay et al., 2013). Other active-targeting strategies, e.g., conjugation with targeting antibodies against Human Epidermal growth factor Recepter-2 (HER-2) tumor antigens, can improve tumor accumulation and enhance treatment efficacy. Thus, HER-2 conjugated gold nanoparticles to treat breast cancer increase tumor cytotoxicity by 50% over untargeted radiosensitization alone (Chattopadhyay et al., 2013).
Although quite successfully preclinically, metal-based nanoparticles have limitations in clinical use due to the different photon energies used clinical radiotherapies. Typically, clinical linear accelerators produce photons with 6 MV or greater energy, where the dominant Compton effect is only weakly Z-dependent, greatly reducing the dose enhancement seen preclinically using lower energy irradiators where the photoelectric effect dominates (Mesbahi, 2010).
Alternative mechanisms of non-metallic nanoparticle radiosensitization have been explored that are not susceptible to this limitation, such as polymeric nanoparticles that deliver established chemotherapy-based radiosensitizers. Specific examples include Genexol-PM, paclitaxel-based nanoparticles that produced comparable radiosensitization in xenograft tumors models as paclitaxel alone but with less toxicity (Werner et al., 2013). Similar results have also been seen with free vs. nanoparticle-delivered doxorubicin (Werner et al., 2011). Another class of non-metallic radiosensitizers produces direct cellular toxicity under irradiation. Thus, superparamagnetic iron oxide nanoparticles (SPION) can amplify the effectiveness of the ROS generated by ionizing radiation, increasing the cytotoxicity by 2–3 fold (Huang et al., 2013; Kwatra et al., 2013). SPIONs have the added benefit of being visible on MRI and can be actively targeted using external magnetic fields. Other agents that are earlier in development, such as silica-based nanoparticles and fullerene structures, can produce direct cell membrane and organelle damage during irradiation (Kwatra et al., 2013).
Beyond these approaches, future nanotechnology-based strategies included nanoparticlized oxygen mimetics that radiosensitize through irreparable fixation of radiation-mediated DNA damage, but significantly reduce the toxicity of typical oxygen mimetics (Meng et al., 2018). An evolving field is the use of oncologic therapies with cytotoxic mechanisms that are complementary to radiation effects, with ROS-based agents being of particular interest (Howard et al., 2020). In this respect the mechanisms of PDT cell-kill that have been known for many years are particularly complementary to radiation (Stewart et al., 1998b). Advances in nanotechnology in the last 20 two decades has allowed realization of radiation-PDT combinations that exploit this complementarity (Viswanath and Won, 2022).
Some of the advantages and limitations of PDT are in part complementary to those of radiotherapy, and there is a degree of shared knowledge and understanding of some of the technical and practical issues, for example, around delivery to the tumor target, avoidance of collateral damage to normal tissues, and EM energy propagation and dosimetry (see Table 3). Beyond such parallels, there are emerging potential roles for PDT to advance radiation oncology and vice versa.
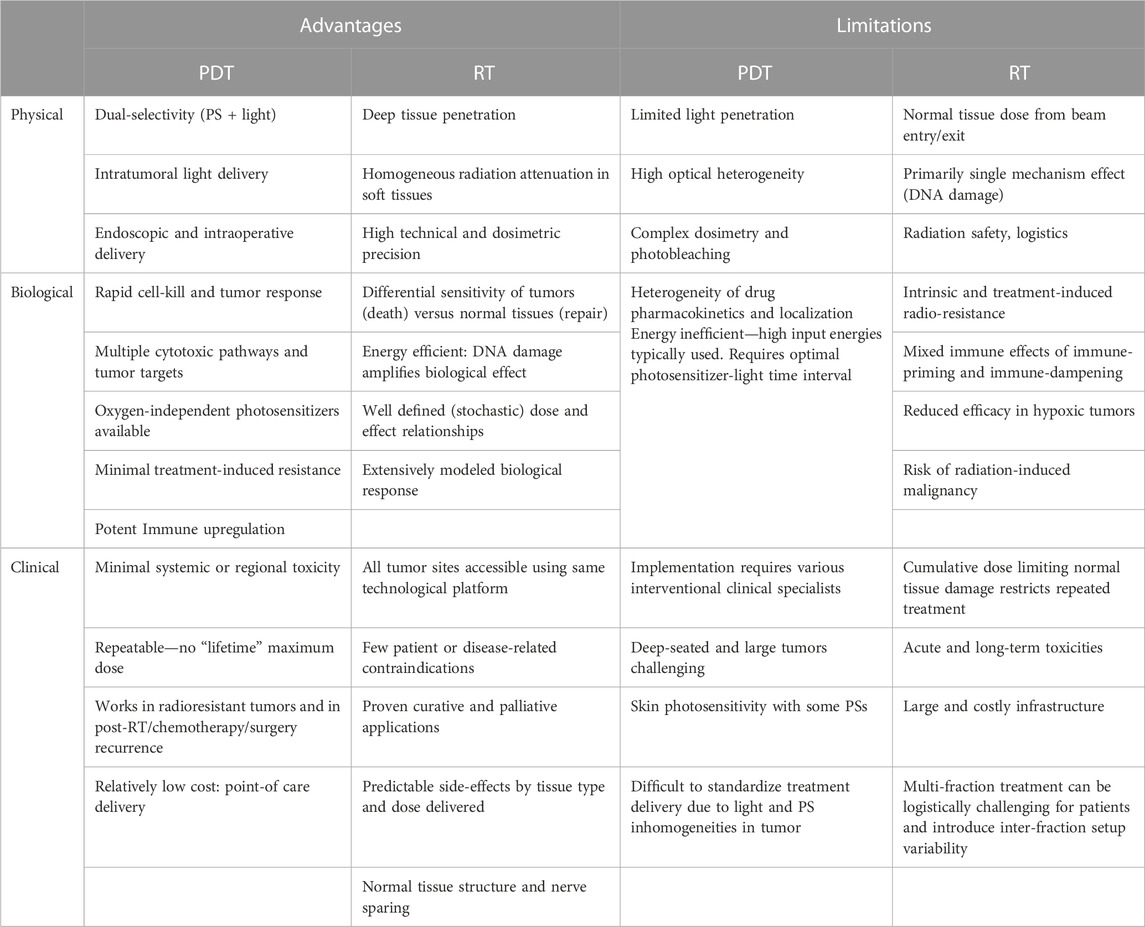
TABLE 3. Comparison of advantages and limitations of photodynamic therapy (PDT) and radiotherapy (RT) as currently practiced.
The most direct approach is simply to combine PDT and radiotherapy as distinct modalities, each optimized separately in individual patients in whom the single modalities may be sub-curative, because of inadequate tumor response and/or dose-limiting normal tissue toxicities. Importantly, the primary mechanisms of (tumor) cell death are distinct between the modalities, with radiotherapy (external beam or brachytherapy) being mediated by DNA damage leading to proliferative cell death or cytostasis (Baskar et al., 2014), and PDT acting via through ROS-mediated damage in extra-nuclear cell structures (e.g., mitochondria) to tumor cells and/or vascular endothelial cells, and also possibly leading to immune modulation. These complementary mechanisms of action may synergize the anti-tumor efficacy without greatly increasing collateral toxicity. Most preclinical combination studies have demonstrated at least additive effects (Allman et al., 2000; Bulin et al., 2019) and some have demonstrated possible synergy: e.g., MCF-7 breast cancer cells treated in vitro with X-rays and PDT showed a much greater decrease in cell viability than either treatment alone, even when doses were de-escalated for both modalities (Santos et al., 2019). Potential synergy was also seen by Nakano et al. (2011) in treating patients with recurrent Bowen’s Disease with PDT and electron radiotherapy, where complete response rates of 80%–100% were achieved with no additional normal tissue toxicity. Similarly encouraging results have also been seen with endobronchial tumors, sarcomas and esophageal cancers (Sanfilippo et al., 2001; Freitag et al., 2004; Kusuzaki et al., 2005).
Even without mechanistic synergy, an important characteristic of PDT is that it can be safely and effectively used either before or after radiotherapy, without causing excess toxicity or inducing resistance. Thus, PDT has been used in several patient cohorts with locally-recurrent disease following radiotherapy (Lou et al., 2004b; Wildeman et al., 2009; Yano et al., 2012b; Gangloff et al., 2012). The advantage is that, even in patients whose normal tissues cannot tolerate more radiation treatment, PDT can be given and produce good responses without added toxicity: in part, this works because (due to the limited penetration of light and the use of interstitial light delivery) there is little spread of PDT damage beyond the target tumor volume and in part because of the different cellular targets, so that even cells that are intrinsically radioresistant can be killed by PDT, as shown definitively in vitro (Stewart et al., 1998a; Oniszczuk et al., 2016). Radiation can also be delivered safely post PDT treatment, an example being in glioma where PDT is used immediately post-surgery to selectively kill surviving mesoscopic disease in the vicinity of the surgical bed and chemoradiation is then used to kill residual microscopic and disseminated disease (Rigual et al., 2013; Schipmann et al., 2021).
An emerging strategy is to directly combine radiotherapy with PDT by using X-rays or radionuclides as the energy source to activate the PS, thereby accessing deep-seated and large tumors non-invasively. This technique is known variously as “radioPDT,” “X-ray PDT,” “radiodynamic therapy” and others (Ren et al., 2018; Cline et al., 2019; Dinakaran et al., 2020). One exemplary technique would be the use high-energy X-rays as delivered, for example, in clinical radiotherapy using a LINAC but at a low, well-tolerated radiation dose (typically, <10 Gy). The preclinical studies of this technique have frequently exploited different nanoparticle formulations, either as X-ray activatable materials and/or as delivery vehicles for molecular photosensitizers (Dinakaran et al., 2023). Several specific molecular radioPDT agents are also being developed (Larue et al., 2018), as well as an emerging approach is the use of nanoclusters, whose small number (∼10–30) of gold or silver atoms confers molecule-like quantum energy levels that result in singlet oxygen generation under X-ray exposure (van de Looij et al., 2022).
Three different and possibly complementary physical activation pathways have been investigated: direct photosensitizer activation mediated by high-energy secondary electrons, radioscintillation light emitted from nanocrystals co-delivered with the photosensitizer within, typically polymeric, nanoparticles, and activation by the Cherenkov light generated in tissue by high-energy (>MeV) secondary electrons. For any of these nano materials and activation pathways, and depending on the PS localization, the subsequent photophysics, photochemistry and photobiology should be similar to conventional PDT using external light sources.
The potential for X-rays to elicit photodynamic response was shown first by Chen et al. (2006). X-rays induced luminescence from nanoscintillators and excited a photosensitizer via Förster resonance energy transfer (FRET) (Chen and Zhang, 2006; Retif et al., 2015). This was significantly more cytotoxicity in tumor cells in vitro than radiation alone and was mediated not only by DNA damage but also through cytoplasmic organelle stress. Multiple subsequent studies, particularly over the last 10 years, have evolved novel iterations of radioPDT nanoparticles aimed at increasing the therapeutic yield, optimizing the physical characteristic and/or targeting of specific cellular structures (Retif et al., 2015; Clement et al., 2016). If this can be successfully translated to the clinical setting, radioPDT could address shortcomings of both conventional PDT and radiotherapy, since it combines the high tissue penetrance, precise targeting and accurate dosimetry of modern radiotherapy with the superior tumor-control capability (both cytotoxicity and secondary immune upregulation) and low toxicity of PDT. In addition, tumor targeting of the PS, with or without the use of nanoparticles, could further increase the therapeutic index (Moore et al., 2008).
Despite promising results, several significant challenges remain for clinical translation. The first is the need to increase the efficiency, i.e., the cell kill per Gy radiation dose: although the principle of radioPDT has been well demonstrated in vitro, the level of tumor cell kill at sub-10 Gy dose is not large, so that many in vivo studies have resorted to intra-tumoral injection of the photosensitizer/nanoparticles to achieve a useful degree of tumor control. There are two primary strategies being pursued to address this limitation, based on improving either the radiation-optical physics and/or the radio-photobiology. The first requires consideration of the multiple steps in the activation pathway. For example, in using scintillation activation, this includes i) increasing the X-ray cross-section and quantum yield of light emission by the scintillation nanocrystals, ii) maximizing the spectral overlap and minimizing the separation of the scintillator and photosensitizer in order to maximum FRET, which is more efficient than 2-step scintillator luminescence emission and its subsequent absorption by the PS, and iii) increasing the 1O2 quantum yield of the PS. (Chen and Zhang, 2006; Clement et al., 2016). Further refinements of the nanoscintillator used can also lead to tuned characteristics with the photosensitizer, such as persistently luminescent nanoparticles (Liu et al., 2021) that can build on the light dosimetry work (Figure 2) to prevent quenching of higher potency photosensitizers (Xiao et al., 2007). Similarly, in Cherenkov-mediated radioPDT the main requirement is to maximize the spectral overlap of the photosensitizer with that of the Cherenkov light after it has propagated through the tissue, which is strongly tissue- and wavelength-dependent given that the Cherenkov spectrum is mainly in the UV and short-wavelength visible range.
The other option of increasing the biological efficiency of radioPDT, requires that a given cellular concentration of 1O2 is maximally cytotoxic, either directly in tumor cells or indirectly through vascular endothelial damage. A major factor in determining this intrinsic sensitivity is the subcellular localization of the PS. In particular, several studies with conventional (i.e., external light-activated) PDT have shown that the cytotoxicity can be increased by 3 orders-of-magnitude or more if the PS is localized in the cell nucleus rather than in cytosolic organelles, so that the resulting damage is primarily to DNA (Yu et al., 2016; Niculescu and Grumezescu, 2021; Long et al., 2022). This could be done by suitable targeting of the PS (and, if used, the nanoscintillator), for example, using TAT peptides. Note, however, that purely DNA damage-mediated radioPDT would likely result in loss of the advantageous secondary immune effects of PDT: this is probably not a major concern, since nuclear targeting is far from 100% efficient. DNA damage from radioPDT over and above the “background” level due to the ionizing radiation itself has been demonstrated using the clinical photosensitizer verteporfin (Clement et al., 2021). It was also shown using Cherenkov-light activation of psoralens that are routinely used in psoralen-ultraviolet A (PUVA) treatment of benign skin diseases (Vangipuram and Feldman, 2016) and have a degree of intrinsic (i.e., passive) nuclear localization. In one study, Radiation Enhanced with Cherenkov photo-Activation (RECA) demonstrated ∼10%–20% improvement in in vitro cytotoxicity over radiation alone (Yoon et al., 2018) and resulted in tumor growth inhibition in vivo (Oldham et al., 2016).
The requirement of achieving a large “photodynamic enhancement factor,” i.e., increased cell kill for a given radiation dose, to use radioPDT as a stand-alone treatment, is substantially reduced by using radioPDT as a novel form of radiosensitization for conventional fractionated radiotherapy, operating through novel photophysical processes rather than through radiochemistry as in traditional agents (Wang et al., 2016; Berry and Fan, 2021; Cockshott, 2021; Gong et al., 2021). This has been demonstrated using X-ray Cherenkov activation of psoralens, where ∼1%/Gy decrease in survival of B16 melanoma cells was achieved. While an important proof-of-principle, this level of sensitization would not translate into a biologically significant increase in efficacy over typical fractionated doses (ex: 60 Gy over 30 fractions), whereas about 10%/Gy decrease in cell survival gives 2–3 logs of cell kill and would be clinically significant. Interesting, in addition to the increased cell kill, psoralens also upregulated the expression of upregulated Major Histocompatibility Complex-I (MHC-I) up to 450% (Yoon et al., 2018). It will be interesting to see if this increase in immunogenic markers also confers an immune response through the non-nuclear component of the PS localization.
The second major challenge for radioPDT is that many of the proposed agents utilize novel, often exotic, structures and components that raise concerns for biocompatibility, in vivo toxicity, tumor localization and pharmacokinetics. In part, these factors arise from the nanoparticulate formulation itself as used in some studies, while scintillation-mediated radioPDT has the added factor of potentially toxic rare-earth-based nanocrystals (e.g., CeF3). Addition of antibodies, peptides or other tumor cell- or organelle-targeting moieties adds further complexity to the formulation (Clement et al., 2018; Deng et al., 2020; Clement et al., 2021).
Another important factor in radioPDT is the dependence on molecular oxygen that both PDT and radiotherapy share. In the sequential PDT-radiation or radiation-PDT approaches considered above, there is an opportunity for a degree of tumor re-oxygenation to occur between modalities, thereby increasing the efficacy, which is not the case with stand-alone radioPDT. Of note, due to the low rate of energy deposition rate used radiotherapy vs. PDT, it is unlikely that the photochemical depletion of molecular oxygen that is seen with conventional PDT at high light fluence rates will occur in radioPDT. An exception may be if FLASH irradiation is used, where the dose deposition rate is orders of magnitude higher (Matuszak et al., 2022). Many solid tumors contain a subset of hypoxic cells with pO2 <4% (Milosevic et al., 2012), which is in the range of the 50% sensitization point for oxygen enhancement factors in mammalian cell kill for PDT and radiotherapy of ∼0.5%–2% (Moore et al., 2007) and ∼3%–4% (Grimes et al., 2017), respectively. Hence, a detailed understanding of the performance of radioPDT under low pO2 conditions is needed in order to minimize the potential negative impact of tumor hypoxia. To date, only one study has systematically evaluated this: the cytotoxic effect was indeed reduced under highly hypoxic conditions but was preserved, at least in vitro, for pO2 >1% by optimizing the concentration of the PEG-PLGA encapsulated LaF3:Ce3+ nanoscintillator and PpIX photosensitizer nanoparticles and the radiation dose (Dinakaran et al., 2019); e.g., the cytotoxicity benefit of radioPDT over radiation alone was as high as 50% even under hypoxic conditions. Other groups have demonstrated the use of high quantum efficiency metallo-organic photosensitizer complexes (Azad et al., 2023) that are efficient in normoxia and hypoxia as well as oxygen-independent inorganic photosensitizers such as psolarens (Pathak, 1984; Zhang et al., 2015a), although delivery of these to poorly vascularized tumors may still be limiting. Use of the Type I reaction pathway, which relies on electron transfer to generate oxy and hydroxy radicals, is a further potential strategy to mitigate the effect of tumor hypoxic (Zhang et al., 2015b) and has demonstrated efficacy both in vitro and in vivo at physiologic tumor environments, including hypoxia (Dinakaran et al., 2019).
Notwithstanding these challenges, the potential theranostic utility, especially through the use of multifunctional nanoformulations, may facilitate clinical translation and impact of radioPDT. To date, much of the focus of theranostics, i.e., image-guided therapies, has been in radionuclide-based systems (Turner, 2018), with other operational modes such as photoacoustic, fluorescence and MRI-based systems emerging rapidly (Wang et al., 2020; Brito et al., 2021; Sarbadhikary et al., 2021). The fluorescence emission of the photosensitizer during radioPDT irradiation could be used in treatment planning and dosimetry, while theranostic radioPDT agents may be of value also in image-guided radiation therapy (IGRT). For example, some proposed radioPDT nanoparticles also show X-ray CT (or MRI) contrast (Chen et al., 2017b; Dinakaran et al., 2018; Xu et al., 2019) which would allow tracking them using, e.g., onboard imaging devices built into a LINAC. This would aid in ensuring adequate tumor uptake and optimal intra-tumoral localization of the radioPDT agent and to compensate for variations in pharmacokinetics. In particular, if the concentration in the tumor can tracked and quantified, then, combined with accurate radiation dosimetry, the effective radioPDT therapeutic yield could be predicted. This would enable better standardization of treatments and reduce variability arising from pharmacokinetic differences between patients or even in the same patient over successive fractions, thereby improving the overall quality and outcome of IGRT. Several radioPDT variants leverage nanotechnology to allow for such theranostic capabilities and there are many other targeting, biocompatibility and multimodal treatment strategies that could be developed (Chen et al., 2017c; Dinakaran et al., 2020; Clement et al., 2021; He et al., 2022).
PDT has potential benefits in oncology as an alternative stand-alone or adjuvant treatment modality. While many preclinical studies and a variety of clinical trials have demonstrated long-term tumor control, manageable short-term toxicity and virtually no long-term toxicity, PDT has not yet gained widespread clinical adoption in oncology beyond relatively limited specific applications. We have argued above that significant factors in this are the technical complexity and variable responses, particularly in treating large tumor masses and deep-seated tumors that are not endoscopically accessible. While there is certainly benefit in applying some of the treatment planning and dosimetry concepts developed for radiotherapy, and these are being implemented in some current clinical trials, the fundamental challenge is the limited and strong tissue- and wavelength-dependence of the penetration of visible and, albeit to a lesser extent, near-infrared light in tissues. On the other hand, there are distinct and clinically-significant biological advantages of PDT’s mechanism of action.
The interplay between PDT and radiation therapy to maximize the therapeutic efficacy and minimise side effects has been underexploited to date, despite the well-established absence of contraindications between the two modalities and their complementary mechanisms of action that yield potential synergism. A clear example is the efficacy of PDT in radioresistant tumors that, as described above, PDT can effectively salvage. This is a missed opportunity for both communities.
Closer integration may result from overlapping interest in radioPDT, used either as a stand-alone “acute” modality (possibly in combination with conventional radiation treatment) or as a novel form of radiation sensitization. Like conventional PDT itself (Akasov et al., 2019), its mechanisms-of-action through ROS generation can be tailored to maximize either direct or, via microvascular shutdown, indirect tumor cell death. In either case, immune upregulation should confer an additional level of local tumor control, with possible impact also on tumor progression and risk of metastatic spread. This field is still at an early stage of preclinical development, so that there are many avenues to be explored. The complementary basic and clinical perspectives, and over a century of experience in both optical and radiation biosciences, could accelerate this development and ensure that ultimately the modality will be translated into oncologic practice. Through radioPDT, the ability of advanced radiotherapy techniques to target lesions in the body can be interfaced with PDT’s biological effectiveness. This can advance the field of radiation oncology by providing greater therapeutic effect even in radioresistant tumors, without additional significant toxicity.
DD contributed to researching the topic, generating figures and tables and writing the manuscript. BW supervised the manuscript generation and contributed to revisions. All authors contributed to the article and approved the submitted version.
DD’s research in radioPDT is supported by the Canadian Institute of Health Research (CIHR); BW’s by the Canadian Institutes of Health Research, the Princess Margaret Foundation (IiR program), and the New Frontiers in Research Fund (C2MCI program).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abrahamse, H., and Hamblin, M. R. (2016). New photosensitizers for photodynamic therapy. Biochem. J. 473, 347–364. doi:10.1042/bj20150942
Akasov, R. A., Sholina, N. V., Khochenkov, D. A., Alova, A. V., Gorelkin, P. V., Erofeev, A. S., et al. (2019). Photodynamic therapy of melanoma by blue-light photoactivation of flavin mononucleotide. Sci. Rep. 9, 9679. doi:10.1038/s41598-019-46115-w
Allman, R., Cowburn, P., and Mason, M. (2000). Effect of photodynamic therapy in combination with ionizing radiation on human squamous cell carcinoma cell lines of the head and neck. Br. J. cancer 83, 655–661. doi:10.1054/bjoc.2000.1328
Almahwasi, A. (2016). Does hadron therapy offer enough effectiveness in treating cancer to be worth the cost?
Arruebo, M., Vilaboa, N., Sáez-Gutierrez, B., Lambea, J., Tres, A., Valladares, M., et al. (2011). Assessment of the evolution of cancer treatment therapies. Cancers 3, 3279–3330. doi:10.3390/cancers3033279
Ash, C., Dubec, M., Donne, K., and Bashford, T. (2017). Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 32, 1909–1918. doi:10.1007/s10103-017-2317-4
Asna, N., Livoff, A., Batash, R., Debbi, R., Schaffer, P., Rivkind, T., et al. (2018). Radiation therapy and immunotherapy—a potential combination in cancer treatment. Curr. Oncol. 25, 454–460. doi:10.3747/co.25.4002
Avci, P., Erdem, S. S., and Hamblin, M. R. (2014). Photodynamic therapy: one step ahead with self-assembled nanoparticles. J. Biomed. Nanotechnol. 10, 1937–1952. doi:10.1166/jbn.2014.1953
Azad, A. K., Lilge, L., Usmani, N. H., Lewis, J. D., Cole, H. D., Cameron, C. G., et al. (2023). High quantum efficiency ruthenium coordination complex photosensitizer for improved radiation-activated Photodynamic Therapy. Front. Oncol. 13, 1244709. doi:10.3389/fonc.2023.1244709
Azzouzi, A.-R., Lebdai, S., Benzaghou, F., and Stief, C. (2015). Vascular-targeted photodynamic therapy with TOOKAD® soluble in localized prostate cancer: standardization of the procedure. World J. Urol. 33, 937–944. doi:10.1007/s00345-015-1535-2
Azzouzi, A. R., Vincendeau, S., Barret, E., Cicco, A., Kleinclauss, F., van der Poel, H. G., et al. (2017). Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol. 18, 181–191. doi:10.1016/s1470-2045(16)30661-1
Baskar, R., Dai, J., Wenlong, N., Yeo, R., and Yeoh, K.-W. (2014). Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 1, 24. doi:10.3389/fmolb.2014.00024
Basset-Seguin, N., Ibbotson, S. H., Emtestam, L., Tarstedt, M., Morton, C., Maroti, M., et al. (2008). Topical methyl aminolaevulinate photodynamic therapy versus cryotherapy for superficial basal cell carcinoma: a 5 Year randomized trial. Eur. J. dermatology EJD 18, 547–553. doi:10.1684/ejd.2008.0472
Baudino, T. A. (2015). Targeted cancer therapy: the next generation of cancer treatment. Curr. Drug Discov. Technol. 12, 3–20. doi:10.2174/1570163812666150602144310
Beltrán Hernández, I., Yu, Y., Ossendorp, F., Korbelik, M., and Oliveira, S. (2020). Preclinical and clinical evidence of immune responses triggered in oncologic photodynamic therapy: clinical recommendations. J. Clin. Med. 9, 333. doi:10.3390/jcm9020333
Berry, M. R., and Fan, T. M. (2021). Target-based radiosensitization strategies: concepts and companion animal model outlook. Front. Oncol. 11, 768692. doi:10.3389/fonc.2021.768692
Billena, C., and Khan, A. J. (2019). A current review of spatial fractionation: back to the future? Int. J. Radiat. Oncol. Biol. Phys. 104, 177–187. doi:10.1016/j.ijrobp.2019.01.073
Bolze, F., Jenni, S., Sour, A., and Heitz, V. (2017a). Molecular photosensitisers for two-photon photodynamic therapy. ChemComm 53, 12857–12877. doi:10.1039/c7cc06133a
Bolze, F., Jenni, S., Sour, A., and Heitz, V. (2017b). Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. 53, 12857–12877. doi:10.1039/c7cc06133a
Bonvalot, S., Rutkowski, P. L., Thariat, J., Carrère, S., Ducassou, A., Sunyach, M.-P., et al. (2019). NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 20, 1148–1159. doi:10.1016/s1470-2045(19)30326-2
Bourhis, J., Montay-Gruel, P., Gonçalves Jorge, P., Bailat, C., Petit, B., Ollivier, J., et al. (2019). Clinical translation of FLASH tadiotherapy: why and how? Radiother. Oncol. 139, 11–17. doi:10.1016/j.radonc.2019.04.008
Brancaleon, L., and Moseley, H. (2002). Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 17, 173–186. doi:10.1007/s101030200027
Brito, B., Price, T. W., Gallo, J., Bañobre-López, M., and Stasiuk, G. J. (2021). Smart magnetic resonance imaging-based theranostics for cancer. Theranostics 11, 8706–8737. doi:10.7150/thno.57004
Bulin, A.-L., Broekgaarden, M., Simeone, D., and Hasan, T. (2019). Low dose photodynamic therapy harmonizes with radiation therapy to induce beneficial effects on pancreatic heterocellular spheroids. Oncotarget 10, 2625–2643. doi:10.18632/oncotarget.26780
Burger, M., Grossman, H. B., Droller, M., Schmidbauer, J., Hermann, G., Dragoescu, O., et al. (2013). Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: A meta-analysis of detection and recurrence based on raw data. Eur. Urol. 64, 846–854. doi:10.1016/j.eururo.2013.03.059
Casas, A., Di Venosa, G., Hasan, T., and Al, B. (2011). Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 18, 2486–2515. doi:10.2174/092986711795843272
Castano, A. P., Demidova, T. N., and Hamblin, M. R. (2004). Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 1, 279–293. doi:10.1016/s1572-1000(05)00007-4
Castano, A. P., Demidova, T. N., and Hamblin, M. R. (2005b). Mechanisms in photodynamic therapy: part three-photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn. Ther. 2, 91–106. doi:10.1016/s1572-1000(05)00060-8
Castano, A. P., Demidova, T. N., and Hamblin, M. R. (2005a). Mechanisms in photodynamic therapy: part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn. Ther. 2, 1–23. doi:10.1016/s1572-1000(05)00030-x
Chang, J., and Rattner, D. W. (2019). History of minimally invasive surgical oncology. Surg. Oncol. Clin. N. Am. 28, 1–9. doi:10.1016/j.soc.2018.07.001
Chang, J. Y., Senan, S., Paul, M. A., Mehran, R. J., Louie, A. V., Balter, P., et al. (2015). Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 16, 630–637. doi:10.1016/s1470-2045(15)70168-3
Chattopadhyay, N., Cai, Z., Kwon, Y. L., Lechtman, E., Pignol, J.-P., and Reilly, R. M. (2013). Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res. Treat. 137, 81–91. doi:10.1007/s10549-012-2338-4
Chen, H., Sun, X., Wang, G. D., Nagata, K., Hao, Z., Wang, A., et al. (2017b). LiGa5O8:Cr-Based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Mater Horiz. 4, 1092–1101. doi:10.1039/c7mh00442g
Chen, H., Sun, X., Wang, G. D., Nagata, K., Hao, Z., Wang, A., et al. (2017c). LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Mater. Horizons 4, 1092–1101. doi:10.1039/c7mh00442g
Chen, K., Liu, J., and Cao, X. (2017a). cGAS-STING pathway in senescence-related inflammation. Natl. Sci. Rev. 2017, nwx146. doi:10.1093/nsr/nwx146
Chen, W., and Zhang, J. (2006). Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 6, 1159–1166. doi:10.1166/jnn.2006.327
Cheung, F. P., Alam, N. Z., and Wright, G. M. (2019). The past, present and future of pulmonary metastasectomy: A review article. Ann. Thorac. Cardiovasc. Surg. 25, 129–141. doi:10.5761/atcs.ra.18-00229
Cho, S., Shin, M. H., Kim, Y. K., Seo, J.-E., Lee, Y. M., Park, C.-H., et al. (2009). Effects of infrared radiation and heat on human skin aging in vivo. J. Invest. Derm. Symp. P 14, 15–19. doi:10.1038/jidsymp.2009.7
Clement, S., Anwer, A. G., Pires, L., Campbell, J., Wilson, B. C., and Goldys, E. M. (2021). Radiodynamic therapy using TAT peptide-targeted verteporfin-encapsulated PLGA nanoparticles. Int. J. Mol. Sci. 22, 6425. doi:10.3390/ijms22126425
Clement, S., Chen, W., Deng, W., and Goldys, E. M. (2018). X-ray radiation-induced and targeted photodynamic therapy with folic acid-conjugated biodegradable nanoconstructs. Int. J. Nanomedicine 13, 3553–3570. doi:10.2147/ijn.s164967
Clement, S., Deng, W., Camilleri, E., Wilson, B. C., and Goldys, E. M. (2016). X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: determination of singlet oxygen Quantum yield. Sci. Rep. 6, 19954. doi:10.1038/srep19954
Cline, B., Delahunty, I., and Xie, J. (2019). Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 11, e1541. doi:10.1002/wnan.1541
Cockshott, A. (2021). Radiodynamic therapy promises improving cancer treatment. Scilight 2021, 2021. doi:10.1063/10.0008949
D'Ammando, A., Raspagliesi, L., Gionso, M., Franzini, A., Porto, E., Di Meco, F., et al. (2021). Sonodynamic therapy for the treatment of intracranial gliomas. J. Clin. Med. 10, 1101. doi:10.3390/jcm10051101
Daneshmand, S., Bazargani, S. T., Bivalacqua, T. J., Holzbeierlein, J. M., Willard, B., Taylor, J. M., et al. (2018b). Blue light cystoscopy for the diagnosis of bladder cancer: results from the US prospective multicenter registry. Urol. Oncol. 36, 361.e1–361.e6. doi:10.1016/j.urolonc.2018.04.013
Daneshmand, S., Patel, S., Lotan, Y., Pohar, K., Trabulsi, E., Woods, M., et al. (2018a). Efficacy and safety of blue light flexible cystoscopy with hexaminolevulinate in the surveillance of bladder cancer: a phase III, comparative, multicenter study. J. urology 199, 1158–1165. doi:10.1016/j.juro.2017.11.096
Davidson, S. R. H., Weersink, R. A., Haider, M. A., Gertner, M. R., Bogaards, A., Giewercer, D., et al. (2009). Treatment planning and dose analysis for interstitial photodynamic therapy of prostate cancer. Phys. Med. Biol. 54, 2293–2313. doi:10.1088/0031-9155/54/8/003
de Visscher, S. A., Melchers, L. J., Dijkstra, P. U., Karakullukcu, B., Tan, I. B., Hopper, C., et al. (2013). mTHPC-mediated photodynamic therapy of early stage oral squamous cell carcinoma: A comparison to surgical treatment. Ann. Surg. Oncol. 20, 3076–3082. doi:10.1245/s10434-013-3006-6
Debele, T. A., Peng, S., and Tsai, H. C. (2015). Drug carrier for photodynamic cancer therapy. Int. J. Mol. Sci. 16, 22094–22136. doi:10.3390/ijms160922094
Demaria, S., Coleman, C. N., and Formenti, S. C. (2016). Radiotherapy: changing the game in immunotherapy. Trends cancer 2, 286–294. doi:10.1016/j.trecan.2016.05.002
Deng, W., McKelvey, K. J., Guller, A., Fayzullin, A., Campbell, J. M., Clement, S., et al. (2020). Application of mitochondrially targeted nanoconstructs to neoadjuvant X-ray-induced photodynamic therapy for rectal cancer. ACS Central Sci. 6, 715–726. doi:10.1021/acscentsci.9b01121
Desmettre, T., Quentel, G., Benchaboune, M., Cohen, S. Y., Mordon, S., and Gaudric, A. (2004). Photodynamic therapy follow-up: when to retreat, when to observe, or when to propose an alternative treatment? J. Fr. Ophtalmol. 27, 291–298. doi:10.1016/s0181-5512(04)96133-5
Dickey, D. J., Partridge, K., Moore, R. B., and Tulip, J. (2004). Light dosimetry for multiple cylindrical diffusing sources for use in photodynamic therapy. Phys. Med. Biol. 49, 3197–3208. doi:10.1088/0031-9155/49/14/013
Dinakaran, D., Azad, A. K., and Wilson, B. C. (2023). “Chapter 13 - photodynamic and photothermal therapy using PLGA nanoparticles,” in Poly(lactic-co-glycolic acid) (PLGA) nanoparticles for drug delivery. Editor P. Kesharwani (Amsterdam, Netherlands: Elsevier), 357–391.
Dinakaran, D., Chen, H., Warkentin, B., Kumar, P., Usmani, N. H., Narain, R., et al. (2018). Novel radiation-activated photodynamic (radioPDT) nanoparticles for treating deep-seated tumors. Int. J. Radiat. Oncol. Biol. Phys. 102, e181–e182. doi:10.1016/j.ijrobp.2018.07.668
Dinakaran, D., Chen, H., Wuest, M., Sonke-Jans, H., Wuest, F., Murray, D., et al. (2019). Novel X-ray activated photodynamic (radioPDT) nanoparticles for deep-seated tumors. Int. J. Radiat. Oncol. Biol. Phys. 105, E676. doi:10.1016/j.ijrobp.2019.06.991
Dinakaran, D., Sengupta, J., Pink, D., Raturi, A., Chen, H., Usmani, N., et al. (2020). PEG-PLGA nanospheres loaded with nanoscintillators and photosensitizers for radiation-activated photodynamic therapy. Acta Biomater. 117, 335–348. doi:10.1016/j.actbio.2020.09.029
Dolmans, D. E. J. G. J., Fukumura, D., and Jain, R. K. (2003). Photodynamic therapy for cancer. Nat. Rev. Cancer 3, 380–387. doi:10.1038/nrc1071
Du, K. L., Both, S., Friedberg, J. S., Rengan, R., Hahn, S. M., and Cengel, K. A. (2010). Extrapleural pneumonectomy, photodynamic therapy and intensity modulated radiation therapy for the treatment of malignant pleural mesothelioma. Cancer Biol. Ther. 10, 425–429. doi:10.4161/cbt.10.5.12616
Fisher, C., Ali, Z., Detsky, J., Sahgal, A., David, E., Kunz, M., et al. (2019). Photodynamic therapy for the treatment of vertebral metastases: A phase I clinical trial. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 25, 5766–5776. doi:10.1158/1078-0432.ccr-19-0673
Forbes, J. F. (1982). Multimodality treatment of cancer. Aust. N. Z. J. Surg. 52, 341–346. doi:10.1111/j.1445-2197.1982.tb06005.x
Freitag, L., Ernst, A., Thomas, M., Prenzel, R., Wahlers, B., and Macha, H.-N. (2004). Sequential photodynamic therapy (PDT) and high dose brachytherapy for endobronchial tumour control in patients with limited bronchogenic carcinoma. Thorax 59, 790–793. doi:10.1136/thx.2003.013599
Friedberg, J. S., Mick, R., Stevenson, J. P., Zhu, T., Busch, T. M., Shin, D., et al. (2004). Phase II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 22, 2192–2201. doi:10.1200/jco.2004.07.097
Friedman, M., Mikityansky, I., Kam, A., Libutti, S. K., Walther, M. M., Neeman, Z., et al. (2004). Radiofrequency ablation of cancer. J. Vasc. Interv. Radiol. 27, 427–434. doi:10.1007/s00270-004-0062-0
Gangloff, P., Phulpin, B., Cortese, S., Gallet, P., Bravetti, P., Mastronicola, R., et al. (2012). Photodynamic therapy as salvage treatment for recurrent head and neck cancer. Med. Buccale Chir. Buccale 18, 325–331. doi:10.1051/mbcb/2012033
Glazer, E. S., and Curley, S. A. (2011). The ongoing history of thermal therapy for cancer. Surg. Oncol. Clin. N. Am. 20, 229–235. doi:10.1016/j.soc.2010.11.001
Gomez, D. R., Tang, C., Zhang, J., Blumenschein, G. R., Hernandez, M., Lee, J. J., et al. (2019). Local consolidative therapy vs. Maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 37, 1558–1565. doi:10.1200/jco.19.00201
Gong, L., Zhang, Y., Liu, C., Zhang, M., and Han, S. (2021). Application of radiosensitizers in cancer radiotherapy. Int. J. Nanomedicine 16, 1083–1102. doi:10.2147/ijn.s290438
Goode, J. A., and Matson, M. B. (2004). Embolisation of cancer: what is the evidence? Cancer Imaging 4, 133–141. doi:10.1102/1470-7330.2004.0021
Grimes, D. R., Warren, D. R., and Warren, S. (2017). Hypoxia imaging and radiotherapy: bridging the resolution gap. Br. J. Radiol. 90, 20160939. doi:10.1259/bjr.20160939
Guidolin, K., Ding, L., Chen, J., Wilson, B. C., and Zheng, G. (2021). Porphyrin-lipid nanovesicles (Porphysomes) are effective photosensitizers for photodynamic therapy. Nanophotonics 10, 3161–3168. doi:10.1515/nanoph-2021-0220
Hamblin, M. R. (2018). Fullerenes as photosensitizers in photodynamic therapy: pros and cons. Photochem. Photobiological Sci. 17, 1515–1533. doi:10.1039/c8pp00195b
Han, R., Zhao, M., Wang, Z., Liu, H., Zhu, S., Huang, L., et al. (2020). Super-efficient in vivo two-photon photodynamic therapy with a gold nanocluster as a type I photosensitizer. ACS Nano 14, 9532–9544. doi:10.1021/acsnano.9b05169
Hatogai, K., Yano, T., Kojima, T., Onozawa, M., Daiko, H., Nomura, S., et al. (2016). Salvage photodynamic therapy for local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Gastrointest. Endosc. 83, 1130–1139.e3. doi:10.1016/j.gie.2015.11.016
He, L., Yu, X., and Li, W. (2022). Recent progress and trends in X-ray-induced photodynamic therapy with low radiation doses. ACS Nano 16, 19691–19721. doi:10.1021/acsnano.2c07286
Holback, H., and Yeo, Y. (2011). Intratumoral drug delivery with nanoparticulate carriers. Pharm. Res. 28, 1819–1830. doi:10.1007/s11095-010-0360-y
Hou, X., Zaks, T., Langer, R., and Dong, Y. (2021). Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater 6, 1078–1094. doi:10.1038/s41578-021-00358-0
Howard, D., Sebastian, S., Le, Q. V.-C., Thierry, B., and Kempson, I. (2020). Chemical mechanisms of nanoparticle radiosensitization and radioprotection: A review of structure-function relationships influencing reactive oxygen species. Int. J. Mol. Sci. 21, 579. doi:10.3390/ijms21020579
Huang, G., Chen, H., Dong, Y., Luo, X., Yu, H., Moore, Z., et al. (2013). Superparamagnetic iron oxide nanoparticles: amplifying ROS stress to improve anticancer drug efficacy. Theranostics 3, 116–126. doi:10.7150/thno.5411
Huang, Y. Y., Sharma, S. K., Dai, T., Chung, H., Yaroslavsky, A., Garcia-Diaz, M., et al. (2012). Can nanotechnology potentiate photodynamic therapy? Nanotechnol. Rev. 1, 111–146. doi:10.1515/ntrev-2011-0005
Huang, Z. (2005). A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 4, 283–293. doi:10.1177/153303460500400308
Huggett, M. T., Jermyn, M., Gillams, A., Illing, R., Mosse, S., Novelli, M., et al. (2014). Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. cancer 110, 1698–1704. doi:10.1038/bjc.2014.95
Iyengar, P., Wardak, Z., Gerber, D. E., Tumati, V., Ahn, C., Hughes, R. S., et al. (2018). Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 4, e173501. doi:10.1001/jamaoncol.2017.3501
Jarvi, M. T., Patterson, M. S., and Wilson, B. C. (2012). Insights into photodynamic therapy dosimetry: simultaneous singlet oxygen luminescence and photosensitizer photobleaching measurements. Biophysical J. 102, 661–671. doi:10.1016/j.bpj.2011.12.043
Kahaleh, M., Mishra, R., Shami, V. M., Northup, P. G., Berg, C. L., Bashlor, P., et al. (2008). Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin. gastroenterology hepatology 6, 290–297. doi:10.1016/j.cgh.2007.12.004
Karges, J., Díaz-García, D., Prashar, S., Gómez-Ruiz, S., and Gasser, G. (2021). Ru(II) polypyridine complex-functionalized mesoporous silica nanoparticles as photosensitizers for cancer targeted photodynamic therapy. ACS Appl. Bio Mater. 4, 4394–4405. doi:10.1021/acsabm.1c00151
Karges, J., Kuang, S., Maschietto, F., Blacque, O., Ciofini, I., Chao, H., et al. (2020). Rationally designed ruthenium complexes for 1- and 2-photon photodynamic therapy. Nat. Commun. 11, 3262. doi:10.1038/s41467-020-16993-0
Kim, M. M., Penjweini, R., Gemmell, N. R., Veilleux, I., McCarthy, A., Buller, G. S., et al. (2016). A comparison of singlet oxygen explicit dosimetry (SOED) and singlet oxygen luminescence dosimetry (SOLD) for photofrin-mediated photodynamic therapy. Cancers 8, 109. doi:10.3390/cancers8120109
Kirsch, D. G., Diehn, M., Kesarwala, A. H., Maity, A., Morgan, M. A., Schwarz, J. K., et al. (2018). The future of radiobiology. J. Natl. Cancer Inst. 110, 329–340. doi:10.1093/jnci/djx231
Korbelik, M., Krosl, G., Krosl, J., and Dougherty, G. J. (1996). The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 56, 5647–5652.
Kosik, I., Weersink, R., and Wilson, B. (2022). Photoacoustic methods for direct monitoring of nanoparticle enhanced photothermal ablation in biological tissues: Visualizing scattering, absorption, and thermal effects. France: SPIE.
Kou, J., Dou, D., and Yang, L. (2017). Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 8, 81591–81603. doi:10.18632/oncotarget.20189
Kusuzaki, K., Murata, H., Matsubara, T., Miyazaki, S., Okamura, A., Seto, M., et al. (2005). Clinical trial of photodynamic therapy using acridine orange with/without low dose radiation as new limb salvage modality in musculoskeletal sarcomas. Anticancer Res. 25, 1225–1235.
Kwatra, D., Venugopal, A., and Anant, S. (2013). Nanoparticles in radiation therapy: a summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2, 330–342. doi:10.3978/j.issn.2218-676X.2013.08.06
Kwon, E. D., Drake, C. G., Scher, H. I., Fizazi, K., Bossi, A., van den Eertwegh, A. J., et al. (2014). Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 700–712. doi:10.1016/s1470-2045(14)70189-5
Larue, L., Ben Mihoub, A., Youssef, Z., Colombeau, L., Acherar, S., André, J. C., et al. (2018). Using X-rays in photodynamic therapy: an overview. Photochem. Photobiol. Sci. 17, 1612–1650. doi:10.1039/c8pp00112j
Larue, L., Moussounda Moussounda Koumba, T., Le Breton, N., Vileno, B., Arnoux, P., Jouan-Hureaux, V., et al. (2021). Design of a targeting and oxygen-independent platform to improve photodynamic therapy: A proof of concept. ACS Appl. Bio Mater. 4, 1330–1339. doi:10.1021/acsabm.0c01227
Lee, J. E., Park, H. S., Jung, S. S., Kim, S. Y., and Kim, J. O. (2009). A case of small cell lung cancer treated with chemoradiotherapy followed by photodynamic therapy. Thorax 64, 637–639. doi:10.1136/thx.2008.112912
Li, X., Lovell, J. F., Yoon, J., and Chen, X. (2020). Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674. doi:10.1038/s41571-020-0410-2
Liang, X., Wang, K. K.-h., and Zhu, T. (2012). Singlet oxygen dosimetry modeling for photodynamic therapy. France: SPIE.
Lin, X., Kong, D., and Chen, Z.-S. (2022). Editorial: chemo-radiation-resistance in cancer therapy. Front. Pharmacol. 13, 904063. doi:10.3389/fphar.2022.904063
Liu, N., Chen, X., Sun, X., Sun, X., and Shi, J. (2021). Persistent luminescence nanoparticles for cancer theranostics application. J. Nanobiotechnology 19, 113. doi:10.1186/s12951-021-00862-z
Liu, Y., Chen, W., Wang, S., and Joly, A. G. (2008). Investigation of water-soluble X-ray luminescence nanoparticles for photodynamic activation. App Phys. Lett. 92, 043901. doi:10.1063/1.2835701
Long, X., Zhang, X., Chen, Q., Liu, M., Xiang, Y., Yang, Y., et al. (2022). Nucleus-targeting phototherapy nanodrugs for high-effective anti-cancer treatment. Front. Pharmacol. 13, 905375. doi:10.3389/fphar.2022.905375
Lou, P. J., Jager, H. R., Jones, L., Theodossy, T., Bown, S. G., and Hopper, C. (2004a). Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br. J. cancer 91, 441–446. doi:10.1038/sj.bjc.6601993
Lou, P. J., Jäger, H. R., Jones, L., Theodossy, T., Bown, S. G., and Hopper, C. (2004b). Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br. J. cancer 91, 441–446. doi:10.1038/sj.bjc.6601993
Lovell, J. F., Jin, C. S., Huynh, E., Jin, H., Kim, C., Rubinstein, J. L., et al. (2011). Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 10, 324–332. doi:10.1038/nmat2986
Lu, L., Zhan, M., Li, X. Y., Zhang, H., Dauphars, D. J., Jiang, J., et al. (2022). Clinically approved combination immunotherapy: current status, limitations, and future perspective. Curr. Res. Immunol. 3, 118–127. doi:10.1016/j.crimmu.2022.05.003
Lui, H., Hobbs, L., Tope, W. D., Lee, P. K., Elmets, C., Provost, N., et al. (2004). Photodynamic therapy of multiple nonmelanoma skin cancers with verteporfinand red light–emitting diodes: two-year results evaluating tumor response and cosmetic outcomes. AMA Arch. Derm. 140, 26–32. doi:10.1001/archderm.140.1.26
Maeda, H., and Khatami, M. (2018). Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 7, 11. doi:10.1186/s40169-018-0185-6
Mahalingam, S. M., Ordaz, J. D., and Low, P. S. (2018). Targeting of a photosensitizer to the mitochondrion enhances the potency of photodynamic therapy. ACS Omega 3, 6066–6074. doi:10.1021/acsomega.8b00692
Maiolino, S., Moret, F., Conte, C., Fraix, A., Tirino, P., Ungaro, F., et al. (2015). Hyaluronan-decorated polymer nanoparticles targeting the CD44 receptor for the combined photo/chemo-therapy of cancer. Nanoscale 7, 5643–5653. doi:10.1039/c4nr06910b
Mallarkey, G., and Coombes, R. C. (2013). Targeted therapies in medical oncology: successes, failures and next steps. Ther. Adv. Med. Oncol. 5, 5–16. doi:10.1177/1758834012467829
Matuszak, N., Suchorska, W. M., Milecki, P., Kruszyna-Mochalska, M., Misiarz, A., Pracz, J., et al. (2022). FLASH radiotherapy: an emerging approach in radiation therapy. Rep. Pract. Oncol. Radiother. 27, 343–351. doi:10.5603/rpor.a2022.0038
Meng, L., Cheng, Y., Tong, X., Gan, S., Ding, Y., Zhang, Y., et al. (2018). Tumor oxygenation and hypoxia inducible factor-1 functional inhibition via a reactive oxygen species responsive nanoplatform for enhancing radiation therapy and abscopal effects. ACS Nano 12, 8308–8322. doi:10.1021/acsnano.8b03590
Menter, J. M., Hollins, T. D., Sayre, R. M., Etemadi, A. A., Willis, I., and Hughes, S. N. (1998). Protection against photodynamic therapy (PDT)-Induced photosensitivity by fabric materials. Photodermatol. Photoimmunol. Photomed. 14, 154–159. doi:10.1111/j.1600-0781.1998.tb00034.x
Mesbahi, A. (2010). A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Rep. Pract. Oncol. Radiotherapy 15, 176–180. doi:10.1016/j.rpor.2010.09.001
Milosevic, M., Warde, P., Menard, C., Chung, P., Toi, A., Ishkanian, A., et al. (2012). Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin. cancer Res. 18, 2108–2114. doi:10.1158/1078-0432.ccr-11-2711
Moore, R. B., Chapman, J. D., Mercer, J. R., Mannan, R. H., Wiebe, L. I., McEwan, A. J., et al. (1993). Measurement of PDT-induced hypoxia in dunning prostate tumors by iodine-123-iodoazomycin arabinoside. J. Nucl. Med. official Publ. Soc. Nucl. Med. 34, 405–411.
Moore, R. B., Xiao, Z., Owen, R. J., Ashforth, R., Dickey, D., Helps, C., et al. (2008). Photodynamic therapy of the canine prostate: intra-arterial drug delivery. Cardiovasc. interventional radiology 31, 164–176. doi:10.1007/s00270-007-9213-4
Moore, R. B., Xiao, Z., Tulip, J., and Chapman, J. D. (2007). A comparison of susceptibility to photodynamic treatment between endothelial and tumor cells in vitro and in vivo. Photodiagnosis Photodyn. Ther. 4, 160–169. doi:10.1016/j.pdpdt.2006.12.003
Morton, C., Horn, M., Leman, J., Tack, B., Bedane, C., Tjioe, M., et al. (2006). Comparison of topical methyl aminolevulinate photodynamic therapy with cryotherapy or fluorouracil for treatment of squamous cell carcinoma in situ: results of a multicenter randomized trial. Archives dermatology 142, 729–735. doi:10.1001/archderm.142.6.729
Muhanna, N., Jin, C. S., Huynh, E., Chan, H., Qiu, Y., Jiang, W., et al. (2015). Phototheranostic porphyrin nanoparticles enable visualization and targeted treatment of head and neck cancer in clinically relevant models. Theranostics 5, 1428–1443. doi:10.7150/thno.13451
Nakano, A., Watanabe, D., Akita, Y., Kawamura, T., Tamada, Y., and Matsumoto, Y. (2011). Treatment efficiency of combining photodynamic therapy and ionizing radiation for Bowen’s disease. J. Eur. Acad. Dermatol Venereol. 25, 475–478. doi:10.1111/j.1468-3083.2010.03757.x
Nakhoda, S. K., and Olszanski, A. J. (2020). Addressing recent failures in immuno-oncology trials to guide novel immunotherapeutic treatment strategies. Pharm. Med. 34, 83–91. doi:10.1007/s40290-020-00326-z
Nathan, T. R., Whitelaw, D. E., Chang, S. C., Lees, W. R., Ripley, P. M., Payne, H., et al. (2002). Photodynamic therapy for prostate cancer recurrence after radiotherapy: A phase I study. J. Urology 168, 1427–1432. doi:10.1016/s0022-5347(05)64466-7
Neuschmelting, V., Kim, K., Malekzadeh-Najafabadi, J., Jebiwott, S., Prakash, J., Scherz, A., et al. (2018). WST11 vascular targeted photodynamic therapy effect monitoring by multispectral optoacoustic tomography (MSOT) in mice. Theranostics 8, 723–734. doi:10.7150/thno.20386
Niculescu, A.-G., and Grumezescu, A. M. (2021). Photodynamic therapy—an up-to-date review. Appl. Sci. 11, 3626. doi:10.3390/app11083626
Nishikawa, H., and Osaki, Y. (2013). Comparison of high-intensity focused ultrasound therapy and radiofrequency ablation for recurrent hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2, 168–170. doi:10.3978/j.issn.2304-3881.2013.03.01
Ogawa, K., and Kobuke, Y. (2008). Recent advances in two-photon photodynamic therapy. Anti-Cancer Agents Med. Chem. 8, 269–279. doi:10.2174/187152008783961860
Oldham, M., Yoon, P., Fathi, Z., Beyer, W. F., Adamson, J., Liu, L., et al. (2016). X-ray psoralen activated cancer therapy (X-PACT). PLoS One 11, e0162078. doi:10.1371/journal.pone.0162078
Oniszczuk, A., Wojtunik-Kulesza, K. A., Oniszczuk, T., and Kasprzak, K. (2016). The potential of photodynamic therapy (PDT)—experimental investigations and clinical use. Biomed. Pharmacother. 83, 912–929. doi:10.1016/j.biopha.2016.07.058
Onuma, A. E., Zhang, H., Gil, L., Huang, H., and Tsung, A. (2020). Surgical stress promotes tumor progression: A focus on the impact of the immune response. J. Clin. Med. 9, 4096. doi:10.3390/jcm9124096
Ottolino-Perry, K., Shahid, A., DeLuca, S., Son, V., Sukhram, M., Meng, F., et al. (2021). Intraoperative fluorescence imaging with aminolevulinic acid detects grossly occult breast cancer: a phase II randomized controlled trial. Breast Cancer Res. 23, 72. doi:10.1186/s13058-021-01442-7
Pacelli, R., Caroprese, M., Palma, G., Oliviero, C., Clemente, S., Cella, L., et al. (2019). Technological evolution of radiation treatment: implications for clinical applications. Seminars Oncol. 46, 193–201. doi:10.1053/j.seminoncol.2019.07.004
Palma, D. A., Olson, R., Harrow, S., Gaede, S., Louie, A. V., Haasbeek, C., et al. (2019). Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet (London, Engl. 393, 2051–2058. doi:10.1016/s0140-6736(18)32487-5
Panzarini, E., Inguscio, V., and Dini, L. (2013). Immunogenic cell death: can it be exploited in PhotoDynamic therapy for cancer? BioMed Res. Int. 2013, 1–18. doi:10.1155/2013/482160
Pathak, M. A. (1984). Mechanisms of psoralen photosensitization reactions. Natl. Cancer Inst. Monogr. 66, 41–46.
Patrick, A. O., Hodi, F. S., Howard, L. K., Jon, M. W., and Jedd, D. W. (2017). Combination immunotherapy: a road map. J. Immunother. Cancer 5, 16. doi:10.1186/s40425-017-0218-5
Pfeiffer, D., Berger, J., and Gross, A. (2015). Single application of high-intensity focused ultrasound as primary therapy of localized prostate cancer: treatment-related predictors of biochemical outcomes. Asian J. Urol. 2, 46–52. doi:10.1016/j.ajur.2014.08.009
Qiu, H., Tan, M., Ohulchanskyy, T. Y., Lovell, J. F., and Chen, G. (2018). Recent progress in upconversion photodynamic therapy. Nanomater. (Basel) 8, 344. doi:10.3390/nano8050344
Ren, X.-D., Hao, X.-Y., Li, H.-C., Ke, M.-R., Zheng, B.-Y., and Huang, J.-D. (2018). Progress in the development of nanosensitizers for X-ray-induced photodynamic therapy. Drug Discov. Today 23, 1791–1800. doi:10.1016/j.drudis.2018.05.029
Retif, P., Pinel, S., Toussaint, M., Frochot, C., Chouikrat, R., Bastogne, T., et al. (2015). Nanoparticles for radiation therapy enhancement: the key parameters. Theranostics 5, 1030–1044. doi:10.7150/thno.11642
Rigual, N. R., Shafirstein, G., Frustino, J., Seshadri, M., Cooper, M., Wilding, G., et al. (2013). Adjuvant intraoperative photodynamic therapy in head and neck cancer. JAMA Otolaryngol. Head. Neck Surg. 139, 706–711. doi:10.1001/jamaoto.2013.3387
Sabnis, A. J., and Bivona, T. G. (2019). Principles of resistance to targeted cancer therapy: lessons from basic and translational cancer biology. Trends Mol. Med. 25, 185–197. doi:10.1016/j.molmed.2018.12.009
Salo, M. (1992). Effects of anaesthesia and surgery on the immune response. Acta Anaesthesiol. Scand. 36, 201–220. doi:10.1111/j.1399-6576.1992.tb03452.x
Sanfilippo, N. J., Hsi, A., DeNittis, A. S., Ginsberg, G. G., Kochman, M. L., Friedberg, J. S., et al. (2001). Toxicity of photodynamic therapy after combined external beam radiotherapy and intraluminal brachytherapy for carcinoma of the upper aerodigestive tract. Lasers Surg. Med. 28, 278–281. doi:10.1002/lsm.1051
Santos, A. l., Almeida, D., Terra, L., Baptista, M., and Labriola, L. (2019). Photodynamic therapy in cancer treatment - an update review. JCMT 2019, 25. doi:10.20517/2394-4722.2018.83
Sarbadhikary, P., George, B. P., and Abrahamse, H. (2021). Recent advances in photosensitizers as multifunctional theranostic agents for imaging-guided photodynamic therapy of cancer. Theranostics 11, 9054–9088. doi:10.7150/thno.62479
Schipmann, S., Müther, M., Stögbauer, L., Zimmer, S., Brokinkel, B., Holling, M., et al. (2021). Combination of ALA-induced fluorescence-guided resection and intraoperative open photodynamic therapy for recurrent glioblastoma: case series on a promising dual strategy for local tumor control. J. Neurosurg. JNS 134, 426–436. doi:10.3171/2019.11.jns192443
Seigne, J. D., Cass, I., and Wong, S. L. (2019). Requiem for robotic cancer surgery? Not so fast. Ann. Surg. Oncol. 26, 3425–3427. doi:10.1245/s10434-019-07669-1
Sharwani, A., and Alharbi, F. A. (2014). Monitoring of photobleaching in photodynamic therapy using fluorescence spectroscopy. Gulf J. Oncol. 1, 79–83.
Shim, G., Jeong, S., Oh, J. L., and Kang, Y. (2022). Lipid-based nanoparticles for photosensitive drug delivery systems. J. Pharm. Investigation 52, 151–160. doi:10.1007/s40005-021-00553-9
Siegel, R. L., Miller, K. D., and Jemal, A. (2019). Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34. doi:10.3322/caac.21551
Simone, C. B., and Cengel, K. A. (2014). Photodynamic therapy for lung cancer and malignant pleural mesothelioma. Semin. Oncol. 41, 820–830. doi:10.1053/j.seminoncol.2014.09.017
Stewart, F., Baas, P., and Star, W. (1998a). What does photodynamic therapy have to offer radiation oncologists (or their cancer patients)? Radiother. Oncol. 48, 233–248. doi:10.1016/s0167-8140(98)00063-2
Stewart, F., Baas, P., and Star, W. (1998b). What does photodynamic therapy have to offer radiation oncologists (or their cancer patients)? Radiotherapy Oncol. 48, 233–248. doi:10.1016/s0167-8140(98)00063-2
Stummer, W., Pichlmeier, U., Meinel, T., Wiestler, O. D., Zanella, F., and Reulen, H. J. (2006). Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 7, 392–401. doi:10.1016/s1470-2045(06)70665-9
Sutton, P. A., van Dam, M. A., Cahill, R. A., Mieog, S., Polom, K., Vahrmeijer, A. L., et al. (2023). Fluorescence-guided surgery: comprehensive review. BJS Open 7, zrad049. doi:10.1093/bjsopen/zrad049
Takahashi, J., and Misawa, M. (2007). Analysis of potential radiosensitizing materials for X-ray-induced photodynamic therapy. Nanobiotechnol 3, 116–126. doi:10.1007/s12030-008-9009-x
Tao, K., and Sun, K. (2020). “Chapter 6 - upconversion nanoparticles: a toolbox for biomedical applications,” in Photonanotechnology for therapeutics and imaging. Editor S. k. Choi (Amsterdam, Netherlands: Elsevier), 147–176.
Thong, P. S., Olivo, M., Kho, K. W., Bhuvaneswari, R., Chin, W. W., Ong, K. W., et al. (2008). Immune response against angiosarcoma following lower fluence rate clinical photodynamic therapy. J. Environ. Pathol. Toxicol. Oncol. 27, 35–42. doi:10.1615/jenvironpatholtoxicoloncol.v27.i1.40
Thong, P. S., Ong, K. W., Goh, N. S., Kho, K. W., Manivasager, V., Bhuvaneswari, R., et al. (2007). Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 8, 950–952. doi:10.1016/s1470-2045(07)70318-2
Dougherty, T. J., Cooper, M. T., and Mang, T. S., Cutaneous phototoxic occurrences in patients receiving Photofrin®. Lasers Surg. Med. 10 (1990) 485–488. doi:10.1002/lsm.1900100514
Turner, J. H. (2018). Recent advances in theranostics and challenges for the future. Br. J. Radiol. 91, 20170893. doi:10.1259/bjr.20170893
Usuda, J., Ichinose, S., Ishizumi, T., Hayashi, H., Ohtani, K., Maehara, S., et al. (2010). Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 16, 2198–2204. doi:10.1158/1078-0432.ccr-09-2520
van de Looij, S. M., Hebels, E. R., Viola, M., Hembury, M., Oliveira, S., and Vermonden, T. (2022). Gold nanoclusters: imaging, therapy, and theranostic roles in biomedical applications. Bioconjugate Chem. 33, 4–23. doi:10.1021/acs.bioconjchem.1c00475
van Straten, D., Mashayekhi, V., de Bruijn, H. S., Oliveira, S., and Robinson, D. J. (2017). Oncologic photodynamic therapy: basic principles, current clinical status and future directions. Cancers 9, 19. doi:10.3390/cancers9020019
Vander Poorten, V., Meulemans, J., Nuyts, S., Clement, P., Hermans, R., Hauben, E., et al. (2015). Postoperative photodynamic therapy as a new adjuvant treatment after robot-assisted salvage surgery of recurrent squamous cell carcinoma of the base of tongue. World J. Surg. Oncol. 13, 214. doi:10.1186/s12957-015-0630-6
Vangipuram, R., and Feldman, S. R. (2016). Ultraviolet phototherapy for cutaneous diseases: a concise review. Oral Dis. 22, 253–259. doi:10.1111/odi.12366
Vanpouille-Box, C., Alard, A., Aryankalayil, M. J., Sarfraz, Y., Diamond, J. M., Schneider, R. J., et al. (2017). DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618. doi:10.1038/ncomms15618
Veeranarayanan, S., Mohamed, M. S., Poulose, A. C., Rinya, M., Sakamoto, Y., Maekawa, T., et al. (2018). Photodynamic therapy at ultra-low NIR laser power and X-ray imaging using Cu(3)BiS(3) nanocrystals. Theranostics 8, 5231–5245. doi:10.7150/thno.25286
Viswanath, D., and Won, Y.-Y. (2022). Combining radiotherapy (RT) and photodynamic therapy (PDT): clinical studies on conventional RT-PDT approaches and novel nanoparticle-based RT-PDT approaches under preclinical evaluation. ACS Biomaterials Sci. Eng. 8, 3644–3658. doi:10.1021/acsbiomaterials.2c00287
Wan, G.-Y., Liu, Y., Chen, B.-W., Liu, Y.-Y., Wang, Y.-S., Zhang, N., et al. (2016). Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 13, 325–338. doi:10.20892/j.issn.2095-3941.2016.0068
Wang, G. D., Nguyen, H. T., Chen, H., Cox, P. B., Wang, L., Nagata, K., et al. (2016). X-ray induced photodynamic therapy: A combination of radiotherapy and photodynamic therapy. Theranostics 6, 2295–2305. doi:10.7150/thno.16141
Wang, H., An, L., Tao, C., Ling, Z., Lin, J., Tian, Q., et al. (2020). A smart theranostic platform for photoacoustic and magnetic resonance dual-imaging-guided photothermal-enhanced chemodynamic therapy. Nanoscale 12, 5139–5150. doi:10.1039/c9nr10039c
Wang, R., Zhou, T., Liu, W., and Zuo, L. (2018). Molecular mechanism of bystander effects and related abscopal/cohort effects in cancer therapy. Oncotarget 9, 18637–18647. doi:10.18632/oncotarget.24746
Warren, C. B., Karai, L. J., Vidimos, A., and Maytin, E. V. (2009). Pain associated with aminolevulinic acid-photodynamic therapy of skin disease. J. Am. Acad. Dermatol 61, 1033–1043. doi:10.1016/j.jaad.2009.03.048
Weber, M. (2018). New developments in photodynamic and sonodynamic cancer therapy. J. Glob. Oncol. 4, 222s. doi:10.1200/jgo.18.89900
Weichselbaum, R. R., and Hellman, S. (2011). Oligometastases revisited. Nat. Rev. Clin. Oncol. 8, 378–382. doi:10.1038/nrclinonc.2011.44
Weichselbaum, R. R., Liang, H., Deng, L., and Fu, Y. X. (2017). Radiotherapy and immunotherapy: A beneficial liaison? Nature reviews. Clin. Oncol. 14, 365–379. doi:10.1038/nrclinonc.2016.211
Werner, M. E., Copp, J. A., Karve, S., Cummings, N. D., Sukumar, R., Li, C., et al. (2011). Folate-targeted polymeric nanoparticle formulation of docetaxel is an effective molecularly targeted radiosensitizer with efficacy dependent on the timing of radiotherapy. ACS Nano 5, 8990–8998. doi:10.1021/nn203165z
Werner, M. E., Cummings, N. D., Sethi, M., Wang, E. C., Sukumar, R., Moore, D. T., et al. (2013). Preclinical evaluation of genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int. J. Radiat. Oncology*Biology*Physics 86, 463–468. doi:10.1016/j.ijrobp.2013.02.009
Wildeman, M. A., Nyst, H. J., Karakullukcu, B., and Tan, B. I. (2009). Photodynamic therapy in the therapy for recurrent/persistent nasopharyngeal cancer. Head. Neck Oncol. 1, 40. doi:10.1186/1758-3284-1-40
Wilson, B. C., and Patterson, M. S. (2008). The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 53, R61–R109. doi:10.1088/0031-9155/53/9/r01
Wilson, B. C., Weersink, R. A., and Lilge, L. (2003). “Fluorescence in photodynamic therapy dosimetry,” in Handbook of biomedical fluorescence (United States: CRC Press).
Wilson, B. C., and Weersink, R. A. (2020). The yin and yang of PDT and PTT. Photochem. Photobiol. 96, 219–231. doi:10.1111/php.13184
Wong, C. H., Siah, K. W., and Lo, A. W. (2019). Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286. doi:10.1093/biostatistics/kxx069
Wright, K., Liniker, E., MacRobert, A., Brown, R., Saffrey, M. J., and Phillips, J. (2006). Investigating the effect of photodynamic therapy on nerves using tissue engineered culture models. Buckinghamshire: The Open University.
Xiao, Z., Halls, S., Dickey, D., Tulip, J., and Moore, R. B. (2007). Fractionated versus standard continuous light delivery in interstitial photodynamic therapy of dunning prostate carcinomas. Clin. Cancer Res. 13, 7496–7505. doi:10.1158/1078-0432.ccr-07-1561
Xu, H., Ohulchanskyy, T. Y., Yakovliev, A., Zinyuk, R., Song, J., Liu, L., et al. (2019). Nanoliposomes Co-encapsulating CT imaging contrast agent and photosensitizer for enhanced, imaging guided photodynamic therapy of cancer. Theranostics 9, 1323–1335. doi:10.7150/thno.31079
Xu, M., Zhou, L., Zheng, L., Zhou, Q., Liu, K., Mao, Y., et al. (2021). Sonodynamic therapy-derived multimodal synergistic cancer therapy. Cancer Lett. 497, 229–242. doi:10.1016/j.canlet.2020.10.037
Yan, P., Liu, L.-H., and Wang, P. (2020). Sonodynamic therapy (SDT) for cancer treatment: advanced sensitizers by ultrasound activation to injury tumor. ACS Appl. Bio Mater. 3, 3456–3475. doi:10.1021/acsabm.0c00156
Yano, T., Muto, M., Yoshimura, K., Niimi, M., Ezoe, Y., Yoda, Y., et al. (2012a). Phase I study of photodynamic therapy using talaporfin sodium and diode laser for local failure after chemoradiotherapy for esophageal cancer. Radiat. Oncol. Lond. Engl. 7, 113. doi:10.1186/1748-717x-7-113
Yano, T., Muto, M., Yoshimura, K., Niimi, M., Ezoe, Y., Yoda, Y., et al. (2012b). Phase I study of photodynamic therapy using talaporfin sodium and diode laser for local failure after chemoradiotherapy for esophageal cancer. Radiat. Oncol. 7, 113. doi:10.1186/1748-717x-7-113
Yoon, I., Li, J. Z., and Shim, Y. K. (2013). Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 46, 7–23. doi:10.5946/ce.2013.46.1.7
Yoon, S. W., Tsvankin, V., Shrock, Z., Meng, B., Zhang, X., Dewhirst, M., et al. (2018). Enhancing radiation therapy through Cherenkov light-activated phototherapy. Int. J. Radiat. Oncol. Biol. Phys. 100, 794–801. doi:10.1016/j.ijrobp.2017.11.013
Yu, Z., Pan, W., Li, N., and Tang, B. (2016). A nuclear targeted dual-photosensitizer for drug-resistant cancer therapy with NIR activated multiple ROS. Chem. Sci. 7, 4237–4244. doi:10.1039/c6sc00737f
Zeisser-Labouèbe, M., Lange, N., Gurny, R., and Delie, F. (2006). Hypericin-loaded nanoparticles for the photodynamic treatment of ovarian cancer. Int. J. Pharm. 326, 174–181. doi:10.1016/j.ijpharm.2006.07.012
Zha, B., Yang, J., Dang, Q., Li, P., Shi, S., Wu, J., et al. (2023). A phase I clinical trial of sonodynamic therapy combined with temozolomide in the treatment of recurrent glioblastoma. J. Neurooncol 162, 317–326. doi:10.1007/s11060-023-04292-9
Zhang, C., Zhao, K., Bu, W., Ni, D., Liu, Y., Feng, J., et al. (2015a). Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. 54, 1770–1774. doi:10.1002/anie.201408472
Zhang, C., Zhao, K., Bu, W., Ni, D., Liu, Y., Feng, J., et al. (2015b). Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. 54, 1770–1774. doi:10.1002/anie.201408472
Zhang, J., Jiang, C., Figueiró Longo, J. P., Azevedo, R. B., Zhang, H., and Muehlmann, L. A. (2018). An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 8, 137–146. doi:10.1016/j.apsb.2017.09.003
Keywords: radiation, photodynamic therapy (PDT), radioPDT, X-ray PDT, XPDT, immune activation
Citation: Dinakaran D and Wilson BC (2023) The use of nanomaterials in advancing photodynamic therapy (PDT) for deep-seated tumors and synergy with radiotherapy. Front. Bioeng. Biotechnol. 11:1250804. doi: 10.3389/fbioe.2023.1250804
Received: 30 June 2023; Accepted: 22 September 2023;
Published: 02 October 2023.
Edited by:
Yun Sun, Shanghai Proton and Heavy Ion Center (SPHIC), ChinaReviewed by:
Sundus Erbas Cakmak, Konya Food and Agriculture University, TürkiyeCopyright © 2023 Dinakaran and Wilson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deepak Dinakaran, ZGVlcGFrLmRpbmFrYXJhbkBzdW5ueWJyb29rLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.