- 1Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang, China
- 2China Medical University and Department of Pathology, Shenyang, China
Mesenchymal stem cells (MSCs), one of the most common types of stem cells, are involved in the modulation of the tumor microenvironment (TME). With the advancement of nanotechnology, exosomes, especially exosomes secreted by MSCs, have been found to play an important role in the initiation and development of tumors. In recent years, nanobiotechnology and bioengineering technology have been gradually developed to detect and identify exosomes for diagnosis and modify exosomes for tumor treatment. Several novel therapeutic strategies bioengineer exosomes to carry drugs, proteins, and RNAs, and further deliver their encapsulated cargoes to cancer cells through the properties of exosomes. The unique properties of exosomes in cancer treatment include targeting, low immunogenicity, flexibility in modification, and high biological barrier permeability. Nevertheless, the current comprehensive understanding of the roles of MSCs and their secreted exosomes in cancer development remain inadequate. It is necessary to better understand/update the mechanism of action of MSCs-secreted exosomes in cancer development, providing insights for better modification of exosomes through bioengineering technology and nanobiotechnology. Therefore, this review focuses on the role of MSCs-secreted exosomes and bioengineered exosomes in the development, progression, diagnosis, and treatment of cancer.
1 Introduction
Cancer is a life-threating disease and the leading cause of death in the world, with an estimated 19.3 million new diagnoses and 10 million deaths in 2020 (Sung et al., 2021). Benefiting from extensive research on cancer pathophysiology and molecular mechanisms, a variety of innovative approaches have been developed, such as targeted therapy, bacterial therapy, and immunotherapy (Waldman et al., 2020; Zhong L. et al., 2021; Gupta et al., 2021; Hou et al., 2022). Despite rapid advances in various approaches, most cancer remains incurable today. Many cancers develop drug resistance and continue to grow and metastasize, suggesting that effective control of tumor development and progression is still a long way off (Vasan et al., 2019; Saini and Twelves, 2021).
Current cancer diagnosis relies on symptoms, imaging, and biochemical indicators, all of which have various levels of sensitivity and specificity. Imaging examinations are usually not needed in individuals without symptoms of cancer, but the possibility of some cancers such as pancreatic cancer and colon cancer is easily overlooked because these cancers are often asymptomatic until they metastasize. Furthermore, benign or malignant nodules (e.g., thyroid nodules or lung nodules) are often inconclusive even with imaging examination and require further confirmation by invasive biopsy. Since delayed diagnosis leads to delayed treatments and a poorer prognosis (Aden et al., 2022; Barrios, 2022; Lone et al., 2022; Nicholson and Lyratzopoulos, 2023), detection of cancer-related biomarkers in a simple blood draw is the most important strategy for early detection and early treatment of cancer. Therefore, liquid biopsy is a recent trend in cancer screening and management (Handa et al., 2021; Lone et al., 2022) as it can quickly determine the levels of specific cancer-related biomarkers in body fluids, especially blood.
Cell-cell interactions in the tumor microenvironment (TME) play an important role in cancer initiation, progression, and metastasis. In addition to direct cell-cell interaction, these cancer cells, immune cells, and stromal cells present in the TME can also affect theire biological functions and phenotypes by secreting a variety of soluble factors through extracellular vesicles (EVs), such as exosomes and microvesicles (Walcher et al., 2020). EVs can carry macromolecules (e.g., DNA, proteins, RNAs, and lipids) to other cells in the TME, and can also reach distant sites to facilitate the formation of pre-metastatic niche. There is evidence that EVs in cancer cells contains distinct inclusions from normal cells, supporting the potential value of EVs as cancer biomarkers (van Niel et al., 2018; Shehzad et al., 2021). Of noted, engineered EVs can also be used as a delivery system to deliver specific substances to cancer cells (Elsharkasy et al., 2020).
Exosomes are a type of EVs with a bilayer membrane, which contain proteins, RNAs, lipids, metabolites, growth factors, cytokines, or other factors inside. Exosomes secreted by cells can deliver their cargo to other nearby cells or enter circulation to affect distant cells (Zhang Y. et al., 2020). Therefore, exosomes represent the current state of the cells that secrete them (Zhang Y. et al., 2020). Cancer cells are known to exhibit distinct transcriptomics and metabolomics from normal cells, and this difference can be reflected in the exosomes. Therefore, exosomes are attractive biomarker in liquid biopsy for early diagnosis of cancer and evaluation of treatment response (Handa et al., 2021; Lone et al., 2022). In addition to the potential use in cancer diagnosis, exosomes can be engineered to deliver payload consisting of microRNA (miRNA), small interfering RNA (siRNA), long non-coding RNA (lncRNA), proteins, and cytotoxic drugs to cancer cells (Walker et al., 2019; Dilsiz, 2020; Zhang et al., 2022).
Mesenchymal stem cells (MSCs), one of the main sources of stem cells in regenerative medicine, are often in the TME and play a critical promoting role in cancer development (Whiteside, 2018). MSCs are pluripotent cells with self-renewal capability and can also differentiate into osteoblasts, chondrocytes, and adipocytes. Given their potent immunomodulatory and immunosuppressive properties and tissue repair capabilities, MSCs have been considered as an attractive tool for the management of immune-related diseases such as inflammatory bowel disease and cancer (Kang et al., 2018). Inflammatory and immune responses in the TME are undoubtedly involved in cancer development (Greten and Grivennikov, 2019). Benefiting from immunosuppression and immune evasion in the TME, cancer cells continues to develop and progress under the surveillance of innate immunity (Kim and Cho, 2022). MSCs in the TME can not only produce extracellular matrix (ECM) components for cancer cells, but also secrete growth factors (Liang et al., 2021) to promote the polarization of M2 macrophages and expand myeloid-derived suppressor cells (MDSC), thereby facilitating immune evasion for cancer development (Ridge et al., 2017; Boutilier and Elsawa, 2021). The above regulation of cancer cells by MSCs is mainly achieved through paracrine activities, which is mediated by exosomes (Jafari et al., 2019).
Precision medicine provides breakthroughs in the detection of cancer-specific molecules and personalized precision therapy. Therefore, the purpose of this review is to comprehensively summarize the current application progress of MSCs-derived exosomes in cancer diagnosis and targeted therapy.
2 Structure and biogenesis of exosomes
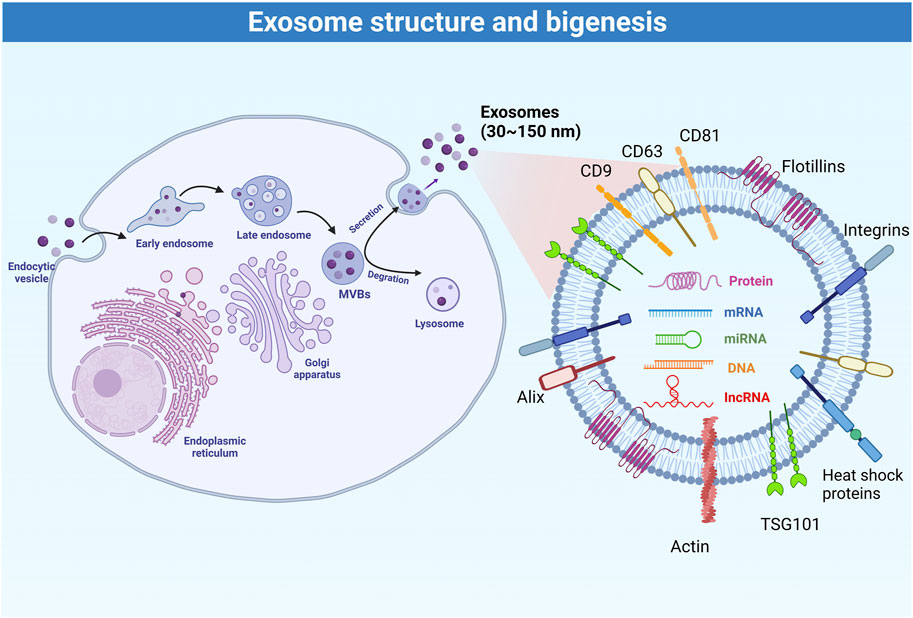
EVs can be divided into three categories based on their size and biogenesis: 1) exosomes derived from exocytosis, with a size 30–200 nm; 2) microvesicles produced by budding and blebbing from the plasma membrane, with a size 100–1,000 nm, and; 3) apoptotic bodies released by apoptotic cells, with a size >1,000 nm (Colombo et al., 2014). Exosomes are membrane-bound extracellular vesicles that carry proteins, DNA, RNAs, and metabolites (Bobrie et al., 2011), and the internal content reflects the nature and the status of the cells that secrete them (Zamani et al., 2019). Under the electron microscopy, natural exosomes have a spheroid shape, while artificial or engineered exosomes exhibit a bi-concave or cup shape (Yellon and Davidson, 2014). The main biomarkers of exosomes include CD9, CD63, CD81, Alix, TSG101, integrins, heat shock proteins, actin, and flotillins (Zhang et al., 2019). The rigid bilayer membrane of the exosomes contains lipid components such as sphingomyelin, cholesterol, and ceramides, which are functionally involved in exosome secretion, structure, and signaling (Skotland et al., 2019). Various types of DNA and RNA are also commonly found in exosomes (Mashouri et al., 2019). Among them, miRNAs represent the most abundant RNA species in exosomes (Huang et al., 2013; Zhang et al., 2019) and are functionally involved in exosome-mediated cellular communication (Zhang et al., 2019).
Figure 1 summarizes the process of exosome biogenesis. Multivesicular bodies and late endosomes are specialized endosomal compartments enriched in intraluminal vesicles that sequester specific proteins, lipids, and cytosolic components and are ultimately secreted as exosomes (Huang et al., 2013). Multivesicular bodies are transported by the cytoskeleton to the plasma membrane for exocytosis (Colombo et al., 2014), or to lysosomes or autophagosomes for degradation (Kalluri and LeBleu, 2020). Nevertheless, the molecular mechanisms regulating the secretion and/or degradation of multivesicular bodies remain poorly understood (White et al., 2006). It is also unclear whether specific transmembrane proteins or cargoes within the multivesicular bodies affect their secretion and degradation (Mashouri et al., 2019). It is now known that the biogenesis and secretion of intraluminal vesicles are driven by the endosomal sorting complex required for transport (ESCRT) machinery. ESCRT is a cytoplasmic multi-subunit system that is critical for membrane remodeling, multivesicular body sorting, and exosome secretion (Schmidt and Teis, 2012). Defects in any member of the ESCRT machinery may reduce exosome secretion (Colombo et al., 2013; Hoshino et al., 2013) or affect exosome composition (Baietti et al., 2012). Conversely, increasing the activity of ESCRT members such as the use of leptin could further increase exosome secretion (Giordano et al., 2019). Some viruses (e.g., hepatitis C virus) have also been found to affect the ESCRT machinery to promote the exosome-mediated transfer of viral RNAs (Dreux et al., 2012). Furthermore, ESCRT activity, exosome secretion, and exosome composition are also affected by ubiquitination (Putz et al., 2008), sphingolipid ceramide (Trajkovic et al., 2008), endosome-specific tetraspanins CD9, CD63 and CD81 (Perez-Hernandez et al., 2013), and Rab GTPases (Blanc and Vidal, 2018). Hence, the regulation of exosome secretion is a fine-tuned process that can respond to a variety of cellular and molecular factors. The board properties and characteristics of the exosome can be used to finely monitor physiological and pathological processes.
Exosomes are composed of various types of molecules, such as lipids on lipid bilayer (e.g., cholesterol, ceramides, sphingomyelin, phosphatidylinositol, phosphatidylserine, phosphatidylcholine, phosphatidylethanolamine, and gangliosides), glycoproteins (e.g., β-galactosidase, O-linked glycans, N-linked glycans), adhesion molecules (integrin-α, integrin-β, and P-selectin), tetraspanins (e.g., CD9, CD37, CD53, CD63, CD81, and CD82), antigen-presenting molecules (e.g., MHC Class I and MHC Class II), and other signaling receptors (e.g., FasL, TNF receptor, and TfR). These molecules are all involved in exosome biogenesis, cargo selection, secretion, release, targeting, and uptake. MHC molecules participate in the immune response through their antigen-presenting capabilities. Exosome components also include heat shock proteins (e.g., Hsp20, Hsp27, Hsp60, Hsp90, and Hsc70), cytoskeletal proteins (e.g., actin, cofilin, and tubulin), ESCRT machinery (e.g., Alix and TSG-101), and membrane transport and fusion proteins (e.g., GTPases, annexins, flotillin, Rabs, dynamin, and syntaxin), as well as a variety of growth factors, cytokines, and nucleic acids. Cargo within exosomes is thought to remain inert until delivered to the target cell, and then become active to regulate cellular metabolism (Gurung et al., 2021).
3 Mesenchymal stem cells (MSCs)
MSCs are adult stem cells with high differentiation potential and self-renewal capacity (Pittenger et al., 2019; Margiana et al., 2022). MSCs differentiation can be driven by a variety of factors, including cytokines, chemokines, extracellular vesicles, and inflammatory stimuli (Pittenger et al., 2019; Margiana et al., 2022). Benefiting from the characteristics of immune regulation and promoting cell survival, MSCs have been clinically used to treat various diseases such as metabolic abnormalities, inflammation, infection, immune disorders, and tissue injury damage (Yuan et al., 2021; Margiana et al., 2022). On the other hand, MSCs have dual characteristics of promoting and inhibiting tumorigenesis and progression. In generally, the innate immune system can recognize cancer cells and kill them. Therefore, a cancer cell must first escape the surveillance of the immune system before they grow into a mass of tumors (Prendergast, 2008), while the potent immunosuppressive function of MSCs helps cancer cells escape immune surveillance (Liang et al., 2021). In addition, MSCs are functionally involved in tumor angiogenesis, which is necessary to provide adequate nutrients to growing tumors (Li et al., 2016; Ghollasi et al., 2021). In aggressive tumors, cancer-associated fibroblasts (CAFs) can secrete a variety of pro-cancer cytokines and growth factors, which can further promote the differentiation of MSCs into CAFs (Kalluri, 2016). MSCs can also secrete growth factors to promote tumor development and invasion, which can serve as hallmarks of invasive cancer and metastatic spread (Wang and Zhou, 2013; Ribatti et al., 2020). Furthermore, MSCs can inhibit cancer stem cells (CSCs) apoptosis and promote their proliferation to further participate in cancer aggressiveness, drug resistance, metastasis, and recurrence (Walcher et al., 2020; Hayat et al., 2021).
In addition to promoting tumor growth, MSCs also have tumor suppressive properties (Ye et al., 2021). MSCs can suppress the proliferation of Kaposi’s sarcoma cells by inhibiting the activation of Akt signaling cascade (Khakoo et al., 2006), which is involved in the proliferation and survival of cancer cells (Madhunapantula et al., 2011). MSCs can also upregulate the expression of p21, a cell cycle inhibitor, in various cell lines including liver cancer, lymphoma, and insulinoma (Lu et al., 2008). Besides, MSCs can increase the infiltration of inflammatory cells, regulate cancer cell cycle, and inhibit angiogenesis (Chen X. et al., 2021). Nevertheless, the balance of the dual roles of MSCs on cancer cells and their functional switch remains to be elucidated.
4 Regulatory mechanisms of MSCs-derived exosome in cancer
Regarding the role of EVs in cancer, Zhu et al. (Zhu et al., 2012) showed that exosomes secreted by MSCs had the same angiogenesis-promoting effects as MSCs themselves in a xenograft mouse model of gastrointestinal cancer. A study by Lee et al. (Lee et al., 2013) reported an opposite role of MSCs in breast cancer cells and found that this difference may be attributed to the amount and types of miRNAs in exosomes, which was further supported by Pakravan et al. (Pakravan et al., 2017) and other studies (Alcayaga-Miranda et al., 2016; Rosenberger et al., 2019). In other words, MSCs exert their dual functions to enhance or inhibit tumor development in a paracrine manner (Ridge et al., 2017; Kang et al., 2018), which is closely related to tumor-associated miRNAs contained in MSC-derived exosomes. Several studies have investigated the effects of specific miRNAs in exosomes on cancer cells (Dilsiz, 2020; Li B. et al., 2021; Li et al., 2022). MSC-derived exosomes can promote tumor development in a variety of solid tumors through their internal miRNAs (Du et al., 2014; Zhao et al., 2019; Zhou et al., 2019), which appears to be associated with the activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway (Zhu et al., 2012). Furthermore, several miRNAs, proteins, and long non-coding RNAs (lncRNAs) in MSC-derived exosomes were found to suppress the cancer cell proliferation and promote cancer cell apoptosis (Bruno et al., 2013; Wu et al., 2013; Furuta et al., 2016; Reza et al., 2016; Takahara et al., 2016; Maffey et al., 2017; Zhang F. et al., 2020).
Cancer cells can invade surrounding tissues through three main patterns: amoeboid cell migration, mesenchymal cell migration, and collective cell migration. Moreover, TME and anticancer drugs can switch the invasive pattern of cancer cells, resulting in limited efficacy of anticancer drugs (Wu et al., 2021). From a cellular perspective, epithelial-to-mesenchymal transition (EMT) is a critical step in the invasion and metastatic spread of solid tumors. After EMT, cancer cells acquire migration and invasion capacities and reduce their adhesion to surrounding cells (Wang and Zhou, 2013; Ribatti et al., 2020). Mesenchymal cells are elongated cells that can move forward using cytoskeletal contractility (Wu et al., 2021). Studies have shown that MSC-derived exosomes can regulate EMT through the ERK pathway (Du et al., 2016; Maffey et al., 2017). In addition, exosomes secreted by MSCs can promote the transformation of macrophages into cancer-associated macrophages, thereby promoting EMT, cancer cell proliferation, migration and invasion, and distant tumorigenesis (Paskeh et al., 2022; Du et al., 2014; Shi et al., 2016; Dong et al., 2018; Zhao et al., 2019; Zhou et al., 2019). MSC-derived exosomes have also been reported to be involved in tumor dormancy (Lugano et al., 2020; Zheng X. et al., 2021; Zhong Y. et al., 2021), which is the ability of tumors to remain in a small number of undetectable tumor cells after primary tumor resection. Notably, tumor dormancy is associated with anticancer drug resistance, prolonged asymptomatic residual disease, and cancer recurrence (Gomis and Gawrzak, 2017). Breast cancer cells that migrated to the bone marrow were found to hide within MSCs populations and become dormant and chemoresistant until reactivated (Fornetti et al., 2018).
Resistance of cancer cells to anticancer drugs is a great challenge in the clinical treatment of cancer. Tumor cells can acquire resistance to specific drugs through mutations, polymorphisms, and splicing variations in the various genes during evolution process in response to drug toxicity or targeted metabolism (Lin et al., 2020). One of the most common resistance mechanisms is the overexpression of membrane transporters that actively pump these absorbed anticancer drugs out of cancer cell. Another mechanism is that the targets of anticancer drugs are mutated to reduce drug efficacy and toxicity. Anticancer drug resistance also includes the activation of mechanisms that favor cancer cell survival and decrease apoptosis (He et al., 2020). Overactivation of DNA repair mechanisms can also reduce the effectiveness of anticancer drugs that cause DNA damage (Zheng X. et al., 2021). Importantly, exosomes are involved in all of these mechanisms of anticancer drug resistance (Zheng X. et al., 2021; Zhong Y. et al., 2021). Another feature of tumor development is angiogenesis, which is a necessary process to ensure adequate nutrient and oxygen supply during cancer cell growth (Lugano et al., 2020). Nonetheless, the role of MSC-derived exosomes in angiogenesis remains controversial. Some studies reported that MSC-derived exosomes can induce angiogenesis through the Wnt pathway (Salomon et al., 2013; Lopatina et al., 2014; Gong et al., 2017; McBride et al., 2017; Olsen et al., 2017). However, some studies reported different findings that MSC-derived exosomes can reduce angiogenesis by downregulating VEGF and CD31 (Lee et al., 2013; Pakravan et al., 2017).
In addition to functioning as carriers of proteins, RNAs, and cytokines, exosomes can also act as antigen carriers to stimulate innate and adaptive immune responses (Thery et al., 2009). IDO-1-containing exosomes secreted by MSCs can reduce IFN-γ expression in dendritic cells and NK cells (He et al., 2018). Furthermore, MSC-derived exosomes can also induce IL-10 secretion, increase the numbers of regulatory T (Treg) cells, and suppress Th17 activation, thereby promoting immune escape (Favaro et al., 2016).
5 The role of mesenchymal stem cell derived exosomes RNA in cancer diagnosis and prognosis
Needle biopsies and surgical excision biopsies are most common methods to obtain cancer biospecimens. A needle biopsy is a procedure to obtain a small portion of cells, which may miss aggressive or biologically different lesions in case of tumor heterogeneity. Although surgical excision biopsy can examine the entire tumor, it cannot provide detailed information on disseminated tumor cells and metastases. Another concern is that the biopsy procedure is invasive, with potential risks of complications such as discomfort, hemorrhage, and infection. In contrast, liquid biopsies have received increasing attention in cancer diagnosis and monitoring due to the advantage of requiring only a simple blood draw (Handa et al., 2021; Lone et al., 2022). Liquid biopsies based on the detection of circulating cancer cells, miRNAs, EVs, cell-free DNA, and proteins. The advantages of liquid biopsy are that biomaterials can be obtained rapidly and easily, with minimal pain and risk for patients, and it also allows a comprehensive assessment of tumor burden. Moreover, liquid biopsy has several limitations, including challenging in the isolation of biomaterials, short half-life of the biomarkers in the biomaterials, and possible contamination by normal cells (Handa et al., 2021; Lone et al., 2022). Compared with other materials obtained by liquid biopsy, exosomes are of interest because they contain miRNAs derived from cancer cells and MSCs. Since specific miRNAs may be upregulated in cancer cells, monitoring specific miRNAs is an effective strategy for cancer screening, diagnosis, and monitoring (Handa et al., 2021; Lone et al., 2022). In addition, exosomal miRNAs are present in all human physiological fluids, such as plasma, serum, urine, saliva, bile, breast milk, and cerebrospinal fluid. Given the prevalence of exosomes in physiological fluids and the stability of miRNAs in exosome, it is feasible and practicable to use exosomal miRNAs as unique biomarkers for early cancer diagnosis.
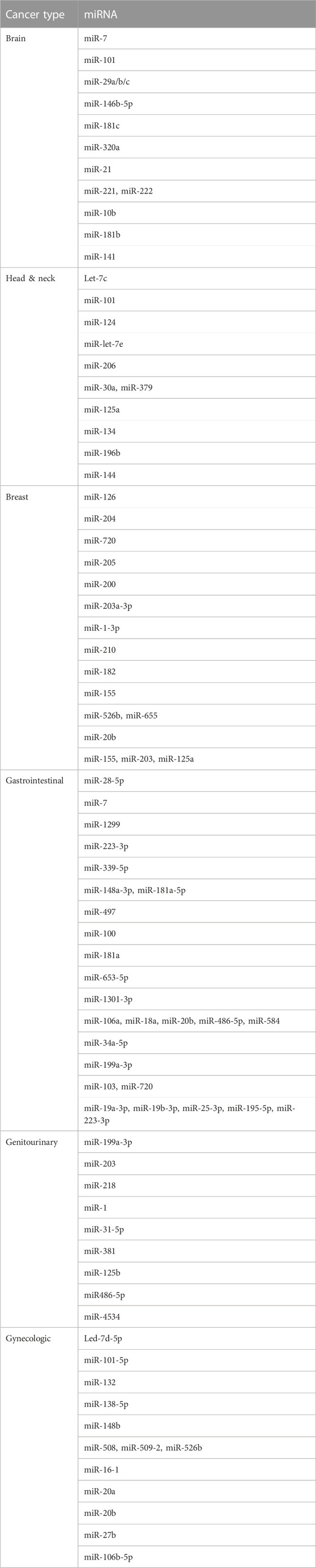
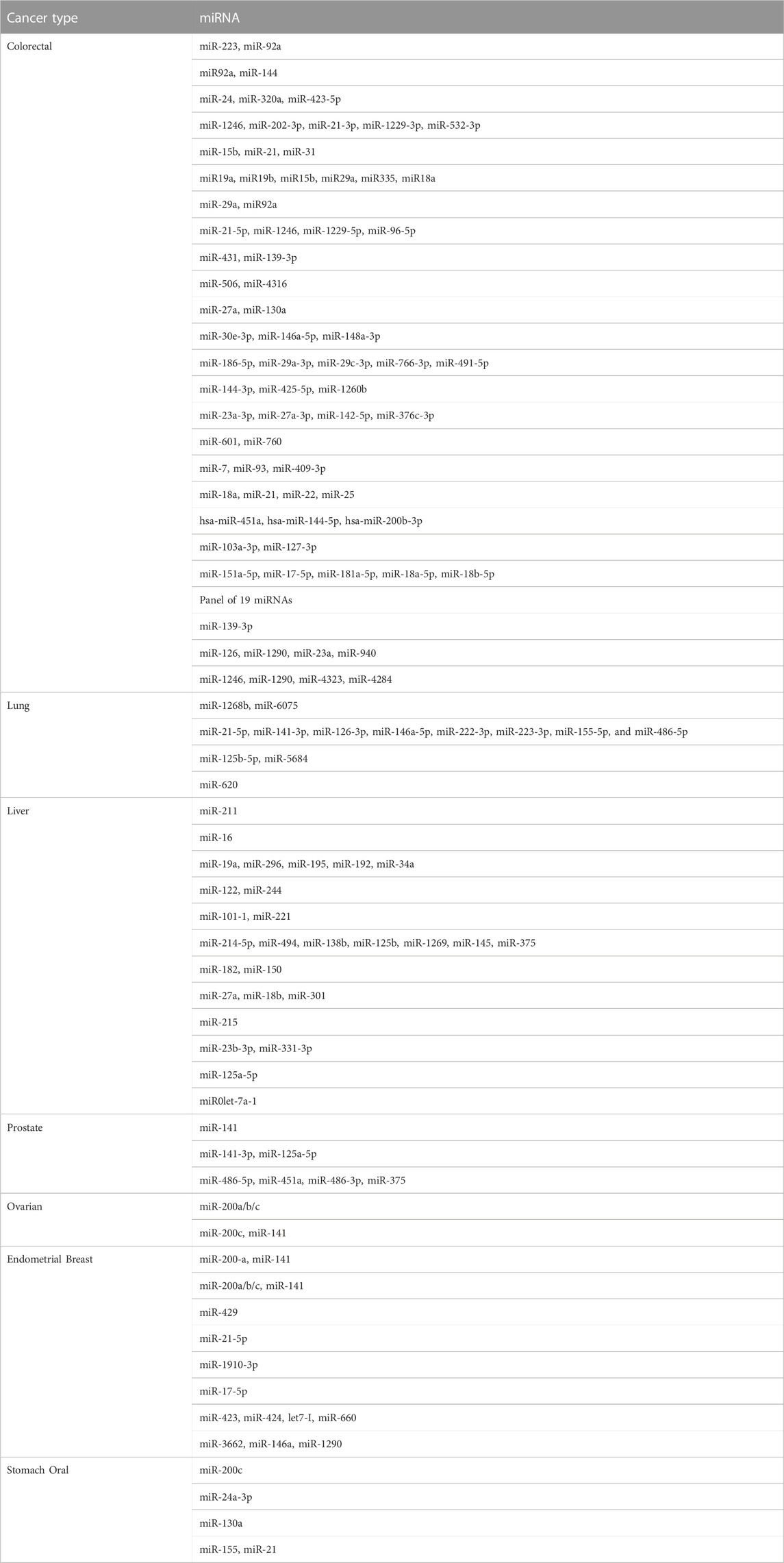
The number of detectable exosomes is increased in cancer patients compared with healthy controls (Suchorska and Lach, 2016). Therefore, exosomal RNA has been used for the diagnosis and prognosis of various cancers (Zhu et al., 2019). For example, the levels of miR-221 in peripheral blood has been suggested as a diagnostic marker for gastric cancer (Huang et al., 2018). Increased levels of exosomal miR-214, miR-221, and miR-222 are associated with the development of gastric cancer (Wang et al., 2014), and upregulation of miR-214 is associated with venous invasion and poor prognosis of gastric cancer (Ueda et al., 2010). In addition, miR-122 is associated with the diagnosis and prognosis of liver cancer (Lu et al., 2008; Coulouarn et al., 2009), while miR-1231 has diagnostic value for pancreatic cancer (Shang et al., 2019). Table 1 lists several dysregulated miRNAs in cancer cells, and Table 2 lists several miRNAs that have been confirmed to have accurate cancer diagnostic performance.
Lung cancer, one of the most common and deadly forms of cancers in the world, is often diagnosed at advanced stages (Sung et al., 2021). Wu et al. (Wu et al., 2020) identified a panel of eight exosomal miRNAs that can effectively detect stage I or II lung cancer with high accuracy. Li et al. (Li X. et al., 2021) found that miR3913-5p levels were associated with increased treatment resistance. The levels of miR-125b-5p and miR-5684 have diagnostic and prognostic values in lung cancer (Zhang Z. et al., 2020), while the level of miR-620 levels is significantly lower in patients with lung cancer (Tang et al., 2020). Furthermore exosomal miR-1246 was reported to be significantly associated with TNM stage (Huang and Qu, 2020), while a panel of six miRNAs was found to be associated with radioresistance (Zheng Q. et al., 2021).
Breast cancer is the most common cancer in women in the world and (Sung et al., 2021). Several miRNAs were identified as potential biomarkers for the diagnosis of breast cancer, such as miR-1910-3p (Wang et al., 2020), miR-17-5p (Lv et al., 2020), and a panel of four urinary exosomal miRNAs (Hirschfeld et al., 2020). Studies have shown that the sensitivity and specificity of exosomes in the diagnosis of breast cancer are 93% and 87%, respectively (Liu M. et al., 2021). In addition, some miRNAs have also identified to have prognostic value in breast cancer (Li D. et al., 2020; Wang X. et al., 2021; Xun et al., 2021).
With regard to prostate cancer, miRNAs in serum and urinary exosomes have also been identified as having diagnostic (Li W. et al., 2020; Li Z. et al., 2021) or prognostic (Guo et al., 2020; Kim et al., 2021; Rode et al., 2021) values. In addition, several miRNAs have been identified for use in the diagnosis and prognosis of various types of cancer (e.g., oral squamous cell carcinoma and colorectal cancer) (Preethi et al., 2022). Since serum contains a variety of exosomes in circulation, only one blood draw can simultaneously detect the profile of multiple miRNAs, which represent the characteristics of different tumors. In a clinical sense, detection of miRNAs from circulating exosomes could serve as a useful screening tool for cancer diagnosis to start early treatment strategies.
EVs can also be used to deliver short peptides to targeted cancer cells for cancer therapy. For example, delivery of GSK-J1 has good effects on carboplatin-resistant ovarian cancer showed promising therapeutic efficacy, including induction of cancer cell apoptosis, reduction of cell motility, and prevention of cell spheroids (Yang et al., 2022). Even for difficult-to-treat glioblastoma with poor prognosis, several exosome-based platforms have been established to overcome the blood–brain barrier (BBB) and showed promising results, such as zinc sulfide-based hybrid exosome-coated nanoplatform and HDX@YSN @ CCM@cRGD delivery system (Mo et al., 2022; Liu et al., 2023).
6 Therapeutic applications of MSC-derived exosomes
Since exosomes are involved in the paracrine signaling, MSC-secreted exosomes secreted by MSCs will be a good tool for cancer treatment. In fact, some studies have reported the promising results of exosomes in cancer therapy (Staufer et al., 2021; Ferreira et al., 2022). It still need to pay attention that MSCs themselves and the exosomes they secrete have dual characteristics that promote and inhibit cancer development (Weng et al., 2021). Nevertheless, exosomes have interesting characteristics for cancer therapy, such as targeting, low immunogenicity, modification flexibility, and high BBB permeability (Dalmizrak and Dalmizrak, 2022).
Various methods are currently available for purifying exosomes (Thery et al., 2018; Wang J. et al., 2021), but none guarantee the isolation of pure exosomes, and often require further identification and purification steps (Thery et al., 2018). Despite these effective methods, expensive equipment and large samples are necessary. In addition, and each step has a risk of contamination, resulting in low efficiency, high sample loss, and low exosome recovery rate, and low purity. The common methods used for exosome purification include gold standard ultracentrifugation, density gradient centrifugation, size exclusion chromatography, immunoaffinity, and polymer precipitation. Some novel techniques that only require smaller samples, shorter purification time and higher recovery efficiency have been developed, including TiO2-based exosome isolation, Fe3O4@TiO2-CD63 aptamer, ExoCAS-2, microvortex chips, acoustofluidic platform, acoustofluidic centrifugation, paper-based anionic isotachophoresis, microfluidic nanowire array, ExoDFF, raman assay chip, and lipid microarray (Chen J. et al., 2021). Although these new methods require specific advanced equipment, they are promising approaches to obtain high-purity exosomes.
Several studies investigated the effects of exosomes secreted by MSCs on different types of cancer cells in vitro and in vivo. In breast cancer, MSC-secreted exosomes carrying miR-16 can inhibit angiogenesis and tumor progression (Lee et al., 2013), and exosomes carrying miR-100 can inhibit angiogenesis as well as tumor proliferation and migration (Pakravan et al., 2017). In breast cancer metastatic to the bones, MSC-derived exosomes carrying miR-23b not only reduced tumor proliferation and invasion, but also increased dormancy of metastatic cancer cells and decreased sensitivity to docetaxel (Ono et al., 2014). In prostate cancer, MSC-derived exosomes carrying miR-145 reduced proliferation and promote apoptosis of cancer cells (Takahara et al., 2016). In lung cancer, let-7i-loaded exosomes have been demonstrated to inhibit proliferation and metastasis of lung cancer cells (Liu J. et al., 2021). In a phase I clinical trial, autologous ascites-derived exosomes combined with GM-CSF can induce immune response against colorectal cancer (Dai et al., 2008).
Exosomes can be isolated and purified from a variety of cells, and additional engineering of these exosomes further increases the therapeutic potential. Exosome engineering includes cargo/payload and surface engineering. Cargo/payload engineering allows the encapsulation of specific molecules (e.g., proteins, miRNA, lncRNA, etc.) within exosome. Furthermore, therapeutic drugs can also be loaded in the hydrophilic core or the lipophilic membrane of the exosomes (Haney et al., 2019; Walker et al., 2019). Surface modification engineering of exosomes can render them more targeted toward specific cells, especially different cancer cells (Liang et al., 2021). In other words, these exosomes loaded with RNAs, proteins, drugs, and other chemicals can target specific cells or cancer cells to exert anticancer effects. The engineering of exosomes mainly includes two strategies: engineering before exosome isolation and engineering after exosome isolation (Weng et al., 2021).
In the “engineering before exosome isolation” strategy, the exosome-providing cells are first modified to package interest therapeutic cargo in exosomes. A common strategy is to overexpress therapeutic RNAs and/or proteins, resulting in these overexpressed RNAs/proteins being encapsulated in exosomes (Herrmann et al., 2021). Another strategy is to incubate these exosome-providing cells with drugs to generate drug-loaded exosomes. Therefore, engineering before exosome isolation strategy usually preserves the native membrane of exosomes (Weng et al., 2021). An ongoing early phase I trial is investigating the clinical application value of personalized vaccines made from exosomes derived from patient-isolated tumor cells, dendritic cells, and macrophages in patients with recurrent/metastatic bladder cancer (ClinicalTrials.gov identifier: NCT05559177).
In the “engineering after exosome isolation” strategy, the isolated exosomes are passively or actively loaded with payloads. Lipophilic drugs can be passively absorbed by exosomes in a concentration gradient manner, while hydrophilic drugs can be loaded to exosomes by electroporation, sonication, freeze/thaw cycles, extrusion, and chemicals (Weng et al., 2021). Nonetheless, caution must be taken to avoid exosome aggregation, membrane damage, or loss of immunogenicity. Several new technologies are also being developed, such as the EXPLORs strategy to encapsulate anti-inflammatory peptides in exosomes (Yim et al., 2016), and protein-based sorting of miRNAs into exosomes (Villarroya-Beltri et al., 2013; Shurtleff et al., 2016). Surface molecules on the exosomal membrane can affect the selectivity of exosomes for specific target cells. Hence, modifying the surface molecules of exosomes can alter the biodistribution and tropism of the exosomes. In fact, the main goal of surface engineering is to increase the specificity of exosomes to specific targets, most of which target cancer cells to protect normal cells and reduce systemic treatment toxicity. Surface engineering is usually achieved through genetic engineering, chemical modification, and hybrid membrane engineering (Weng et al., 2021). Genetic engineering involves transfecting cells with plasmids to overexpress RNAs or proteins of interest, indirectly promoting exosome loading. For example, increasing the N-terminal portion of Lamp2b protein on the surface of exosomes can increase the binding affinity and selectivity for ligands (Alvarez-Erviti et al., 2011; Weng et al., 2021). Nevertheless, genetic engineering of exosome modification still has some questions to be solved. The main questions include the correct expression of the fusion protein, the accuracy of target recognition, the possibility of loss of function, and the loss of immunogenicity. The strategy of chemical modification involves the covalent bonding of molecules to the surface of exosomes to target specific cells.
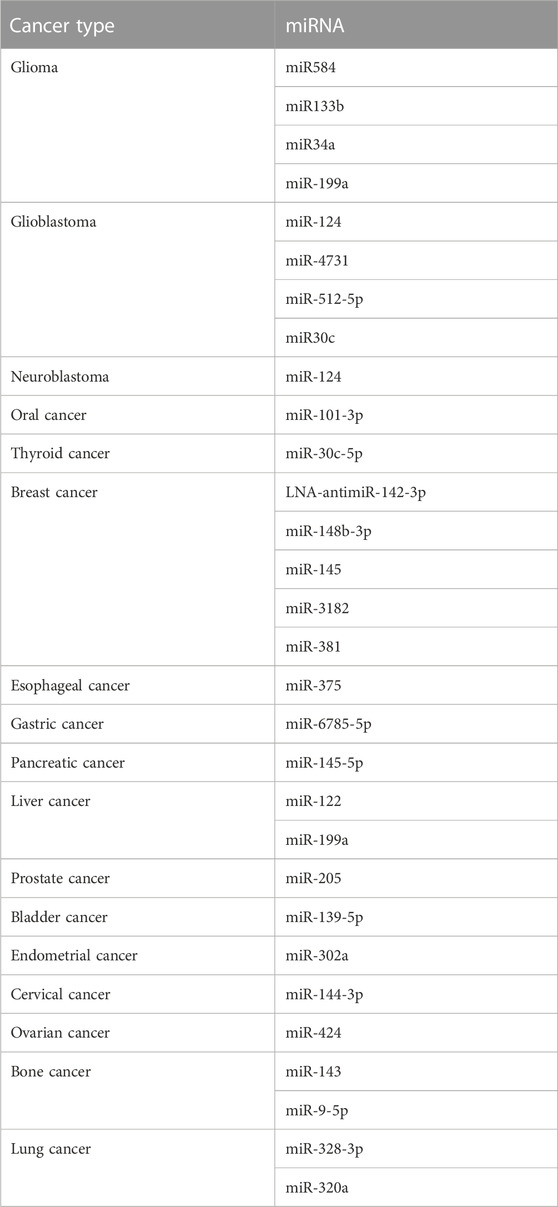
The ultimate goal of exosomes in cancer therapy is to precisely deliver cargo or payload to cancer cells, thereby reducing cancer cell proliferation and invasiveness, promoting cancer cell death, and/or increasing sensitivity to other therapeutic drugs. Regarding miRNAs as cargo for exosomes, encapsulation of miRNAs in exosomes is an suitable delivery strategy because free miRNAs are easily degraded in circulation (Zhang et al., 2022). There are two miRNA-based strategies for cancer therapy: miRNA suppression and miRNA replacement. The miRNA suppression strategy is used when the target miRNA suppresses the oncogene, while the miRNA replacement strategy can be used when the miRNA to be replaced is downregulated in the cancer cells and cannot inhibit the oncogene (Dalmizrak and Dalmizrak, 2022; Zhang and Farwell, 2008). Given the complexity of cancer mechanisms, simultaneous targeting of multiple genes is permissible (Baumann and Winkler, 2014). Furthermore, the advantage of using miRNAs as therapeutic tools is that most miRNAs can target and regulate multiple genes simultaneously (De Veirman et al., 2016; Li and Li, 2018). For example, exosomes carrying miR-122 can reduce the proliferation and increase the sensitivity of liver cancer cells to 5-fluorouracil (5-FU) and doxorubicin (Fornari et al., 2009; Zhang et al., 2013; Li X. et al., 2020). Exosomes loaded with miR199a-3p can downregulate the expression of YAP1, CD151, and mTOR and increase chemosensitivity (Fornari et al., 2010; Kim et al., 2016; Ren et al., 2016). Exosomes loaded with miR-379 suppress breast cancer growth by regulating COX-2 (O'Brien et al., 2018). In glioma, exosomes carrying miR-146b, miR-124a, or miR-34a have been shown to decrease tumor proliferation by inhibiting EGFR, NF-κB, FOXA2, and MYCN (Katakowski et al., 2013; Lang et al., 2018; Wang B. et al., 2019). Besides, many studies have also revealed the effects of miRNAs in exosomes in various cancers, such as breast cancer (O'Brien et al., 2018; Yuan et al., 2019), glioma (Katakowski et al., 2013; Lang et al., 2018), glioblastoma (Wang B. et al., 2019), colorectal cancer (Xu et al., 2019), prostate cancer (Jiang et al., 2019), endometrial cancer (Li et al., 2019), pancreatic cancer (Wu et al., 2019), cervical cancer (Zhang H. et al., 2020), and ovarian cancer (Meng et al., 2021). Table 3 lists several miRNAs with cancer therapeutic value. There is no doubt that all current studies support the effectiveness of exosomes in delivering miRNAs to fight cancer. However, there is a potential safety concern regarding exosome-based miRNAs delivery before clinical application.
In addition to miRNAs being attractive payloads for cancer therapy, other payloads also have potential. Zhou et al. (Zhou W. et al., 2021) proposed the use of MSC-derived exosomes to deliver oxaliplatin and siRNAs for the treatment of pancreatic cancer. Paclitaxel is a highly hydrophobic compound, and its traditional formulation relies on solvents and excipients known to cause toxicity (Fu et al., 2018; Oun et al., 2018). In addition, exosome-encapsulated paclitaxel could also decrease its treatment-related cytotoxicity (Wang P. et al., 2019). The effectiveness of paclitaxel-loaded exosomes has been confirmed in various cancers, such as pancreatic, breast, lung, and ovarian cancers using is also possible (Pascucci et al., 2014; Melzer et al., 2019). In particular, exosomes loaded with doxorubicin can cross the BBB, which is the main reason why brain metastases are difficult to treat (Yang et al., 2015). Besides, exosomes can effectively deliver other anticancer drugs, such as porphyrin, tirapazamine, docetaxel, and cisplatin (Zhang Y. et al., 2020). An attractive feature of exosome-based drug delivery system is that it is not limited to the intravenous route of administration, as subcutaneous, intraperitoneal, intratumoral, intranasal, and oral routes are also potential routes (Zhang Y. et al., 2020). An ongoing phase I clinical trial was conducted to investigate the effect of plant (grape)-derived exosomes loaded with curcumin for the treatment of colon cancer (ClinicalTrials.gov NCT01294072), but the step of engineered MSC-derived exosomes is still in progress.
7 Clinical applications of MSC-derived exosomes
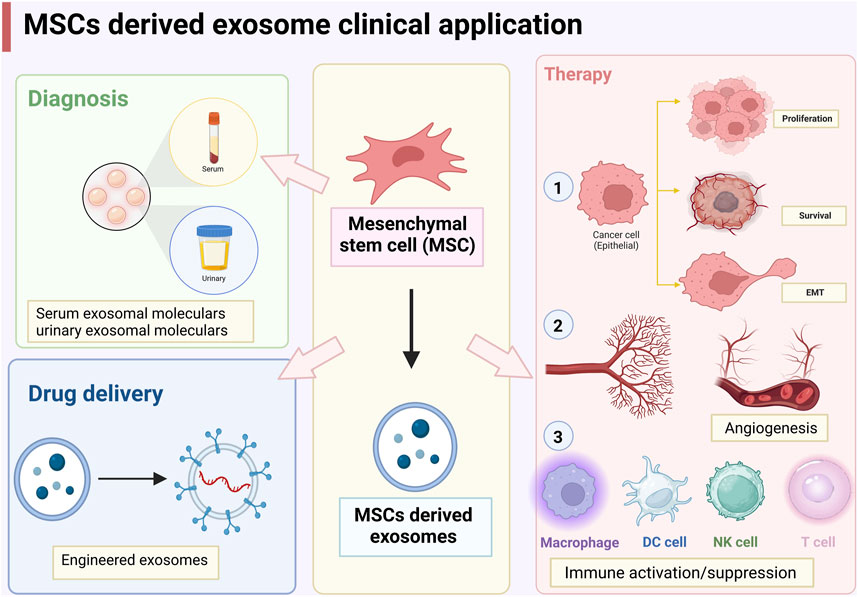
As mentioned above, MSC-derived exosomes can promote and/or inhibit cancer cell proliferation and apoptosis, EMT, angiogenesis, and immune activation (Figure 2). The dual role of MSCs in cancer development are mainly attributed to different proteins, miRNAs, lncRNAs, and cytokines encapsulated in exosomes. Challenges in using MSC-derived exosomes for clinical diagnosis and therapy include efficient and rapid exosome isolation techniques, long-term preservation of exosomes, and rapid quantification and identification of exosomes. In addition, the use of exosomes for therapeutic purposes also involves the large-scale production of engineered exosomes, the high specificity in targeting cancer cells, long-term safety, and the property of avoiding macrophage phagocytosis and destruction (Weng et al., 2021). Although all cells can secrete exosomes, MSCs are the most prolific exosome producers commercially and have been approved by the FDA for therapeutic purposes (Zhou T. et al., 2021). In addition to the advantage of modification and storage, MSC-derived exosomes are natural carriers, which are more biocompatible and less immunogenic than other nanocarriers such as liposomes (Weng et al., 2021). Therefore, MSC-derived exosomes have great potential in cancer therapy.
Current technologies for producing exosomes are insufficient to meet clinical needs and further improvement are needed, including reliable large-scale production, qualified and consistent exosome content, and avoidance of contamination. First of all, increasing the yield of MSCs from cell culture is the most important part. Currently, MSCs are usually cultured in two-dimensional plastic tissue Petri dish and flasks, which greatly limit the area for their growth. After all, the two-dimensional growth environment is not comparable to the three-dimensional environment in the body. Therefore, some synthetic biomaterial scaffolds have been designed to mimic the structure and function of the ECM. Some studies have shown that Avitene Ultrafoam collagen hemostat doubles the yield of exosomes released by BM-MSCs compared with those cultured in plastic tissue Petri dish (Tao et al., 2017). Second, the production of exosomes can be improved by regulating the lysosome pathway to affect the production, secretion and degradation process of exosomes. For example, activation of P2X7 receptors on the membrane can enhance exosome production by triggering membrane blistering, sorting of endosomal contents, and fusion with polyvesicles to accelerate exosome release (Qu and Dubyak, 2009).
In recent years, some studies have focused on methods for efficient and large-scale production of exosomes. Yang Zhaogang et al. developed a method of cell nanoporation that can generate large numbers of functional mRNA-encapsulating exosomes. Compared with other strategies for producing EVs, batch electroporation produced higher yields. Cellular nanoporation produced up to 50-fold increase in exosomes and more than 1000-fold increase in exosomal mRNA transcripts. Similar excellent results were also observed in cells with low basal levels of exosome secretion (Yang et al., 2020).
8 Application of exosome mimics as substitutes for MSCs-derived exosomes in cancer therapy
Despites the promise of exosomes as a drug delivery method in cancer therapy, the large-scale production of exosomes remains a challenging issue. In addition, the drug delivery efficiency of exosomes is also an unresolved problem. Once in the body, exosomes are easily cleared, and the therapeutic effect will be greatly reduced, especially for exosomes with low drug loading (Chen C. et al., 2021). Therefore, exosome mimetics (EMs), as a substitute for natural exosomes, are gradually becoming a more promising drug delivery platform due to their higher yields and the same biological functions as exosomes. EMs have many advantages as carriers for cancer treatment. First, the lipid bilayer of EMs can also fuse with the cell membrane, facilitating the internalization of packaged drugs. Second, the size of the EMs can be adjusted, which facilitates their infiltration into tumor blood vessels and spread into tumor tissue (Guo et al., 2021; García-Fernández and Fuente Freire, 2023).
Structurally, EMs have the same lipid bilayer structure as exosomes. In addition, EMs can utilize protein functionalized vesicle surfaces to regulate target cells to increase their circulation in the blood either by direct contact or by connecting hydrophilic molecules on the vesicle surface. EMs are mainly divided into liposomes, exosome-liposome nanoparticle hybrid system, exosome-inorganic/organic nanoparticle hybrid system, etc.
Liposomes, one of the most widely studied artificial EMs, are characterized by high permeability and retention. Therefore, liposomes can accumulate in tumors through vascular system. In addition, PEGylation based liposomes can reduce their interactions with blood proteins and immune cells, preventing them from being coated with blood proteins, recognized as foreign particles, and then cleared by macrophages (Mohamed et al., 2019). Currently, there are some marketed liposomal drug products as delivery systems, such as Inflexil ® V. Doxil ®, Lipusu ®, and DaunoXOme ®. In addition, some are already in clinical trials, such as Amikacin liposomes (Beltrán-Gracia et al., 2019; Boutilier and Elsawa, 2021).
Numerous studies have been conducted on the design of exosome-liposome hybrid structures to improve drug delivery systems. The extrusion method is the simplest and most effective method for preparing exosome-liposome hybrid structures. Wang et al. used extrusion method to prepare liposome-exosome hybrid structure from bone marrow stromal cells (BMSCs), and successfully encapsulated doxorubicin through ammonium sulfate gradient, and showed better tumor specificity and biocompatibility in vivo and in vitro (Wang J. et al., 2022). Cheng et al. also reported a membrane fusion technology for cancer immunotherapy by combining engineered exosomes with thermosensitive liposomes and showed substantial accumulation at tumor sites (Cheng et al., 2021).
The exosome-inorganic/organic nanoparticle hybrid system is a relatively novel technique. This hybrid system integrates the advantages of inorganic or organic components to enhance the characteristics of exosomes. Yong et al. loaded DOX into mesoporous silica nanoparticles (DOX-MPS), and found that DOX-MPS can enter tumor cells and cancer stem cell through endocytosis, thereby enhancing the accumulation and infiltration of tumor tissues (Yong et al., 2019). In addition, a bionic nanoparticle platform composed of a metal organic framework was developed and loaded exosomes secreted from MDA-MB-231 cells. This system not only exerts a high loading capacity of foreign proteins (94%) and a high efficiency of exosomes modification (97%), but also shows promising effect of tumor targeting therapy in vivo and in vitro (Cheng et al., 2018).
Due to the wide range of sources, low cost, stable physicochemical properties, and excellent biocompatibility, Bone marrows (BMs) may become a way for personalized nanodrug delivery in the future. Although some released nanovesicles have been approved, there is still a lot of work to be done (Wang X. et al., 2022), such as the optimal combination of BMs components, large-scale clinical production processes, and the reliability of in human application.
9 Future directions
Indeed, MSC-secreted exosomes represent an advanced technology utilized for cancer diagnosis and therapy. The efficacy of these exosomes, concerning sensitivity, specificity, and accuracy, can be influenced by the miRNAs they carry. However, it's worth noting that the current purification methods for exosomes still need continuous refinement and optimization to enhance their performance.
Despite some ongoing clinical trials, various challenges and unanswered questions must be addressed before exosomes can be utilized in humans. In order to produce clinical-grade MSC-derived exosomes without causing any significant toxicity and to achieve consistent and reproducible effects, it is crucial to develop reliable large-scale production methods. Furthermore, ensuring the safety of exosomes is an important issue. This includes preventing contamination with other MSC-derived and maintaining the integrity of exosome content throughout the production process. On the other hand, given the immunomodulatory capacities of MSCs, the extracellular vesicles secreted by MSCs could potentially serve as a viable therapy for graft-versus-host disease (GVHD), which is characterized by acute and chronic severe inflammation in multiple organs. A recent clinical study investigated the effect of human MSCs-derived EVs in acute GVHD mice and showed that MSCs-derived exosomes prolonged the survival of GVHD mice (Fujii et al., 2018). Another study by Zhu et al. explored the safety and effectiveness of aerosol inhalation of EVs derived from human adipose MSCs (hAMSC Exos) in patients with COVID-19. The results showed that lung lesions subsided after continuous inhalation of hAMSC Exos for 5 days, without adverse reactions and well tolerated (Zhu et al., 2022).
Exosomes can encapsulate various miRNAs, some of which may promote the proliferation and aggressiveness of cancer cells (Weng et al., 2021). Currently, producing therapeutic MSCs-derived exosomes involves a substantial workload. To address this, researchers are developing exosome-mimics as an alternative to overcome the production challenges of MSCs-derived exosomes (Lu and Huang, 2020). Another crucial consideration is standardization, which is essential for any therapeutic product.
10 Conclusion
This review summarizes the roles of MSCs and MSC-derived exosomes in cancer initiation, progression, diagnosis, and treatment. Absolutely, exosome-based therapeutic strategies show great promise in the fight against cancer. A deeper understanding of the mechanisms of MSCs-derived exosomes and their impact on cancer development is crucial. This knowledge would open up new therapeutic opportunities and innovative strategies to fight cancer.
Author contributions
YZ and YD are the first author who write the article. QS is the corresponding author. AZ and JW collected the references and prepared figures. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SL declared a shared parent affiliation with the authors at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aden, D., Zaheer, S., and Raj, S. (2022). Challenges faced in the cancer diagnosis and management-COVID-19 pandemic and beyond-Lessons for future. Heliyon 8 (12), e12091. doi:10.1016/j.heliyon.2022.e12091
Alcayaga-Miranda, F., Gonzalez, P. L., Lopez-Verrilli, A., Varas-Godoy, M., Aguila-Diaz, C., Contreras, L., et al. (2016). Prostate tumor-induced angiogenesis is blocked by exosomes derived from menstrual stem cells through the inhibition of reactive oxygen species. Oncotarget 7 (28), 44462–44477. doi:10.18632/oncotarget.9852
Alvarez-Erviti, L., Seow, Y., Yin, H., Betts, C., Lakhal, S., and Wood, M. J. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29 (4), 341–345. doi:10.1038/nbt.1807
Baietti, M. F., Zhang, Z., Mortier, E., Melchior, A., Degeest, G., Geeraerts, A., et al. (2012). Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14 (7), 677–685. doi:10.1038/ncb2502
Barrios, C. H. (2022). Global challenges in breast cancer detection and treatment. Breast 62 (1), S3–S6. doi:10.1016/j.breast.2022.02.003
Baumann, V., and Winkler, J. (2014). miRNA-based therapies: strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med. Chem. 6 (17), 1967–1984. doi:10.4155/fmc.14.116
Beltrán-Gracia, E., López-Camacho, A., Higuera-Ciapara, I., Velázquez-Fernández, J. B., and Vallejo-Cardona, A. A. (2019). Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. 10 (1), 11. doi:10.1186/s12645-019-0055-y
Blanc, L., and Vidal, M. (2018). New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 9 (1-2), 95–106. doi:10.1080/21541248.2016.1264352
Bobrie, A., Colombo, M., Raposo, G., and Thery, C. (2011). Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 12 (12), 1659–1668. doi:10.1111/j.1600-0854.2011.01225.x
Boutilier, A. J., and Elsawa, S. F. (2021). Macrophage polarization states in the tumor microenvironment. Int. J. Mol. Sci. 22 (13), 6995. doi:10.3390/ijms22136995
Bruno, S., Collino, F., Deregibus, M. C., Grange, C., Tetta, C., and Camussi, G. (2013). Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 22 (5), 758–771. doi:10.1089/scd.2012.0304
Chen, C., Sun, M., Wang, J., Su, L., Lin, J., and Yan, X. (2021c). Active cargo loading into extracellular vesicles: highlights the heterogeneous encapsulation behaviour. J. Extracell. Vesicles 10 (13), e12163. doi:10.1002/jev2.12163
Chen, J., Li, P., Zhang, T., Xu, Z., Huang, X., Wang, R., et al. (2021b). Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 9, 811971. doi:10.3389/fbioe.2021.811971
Chen, X., Zhang, S., Du, K., Zheng, N., Liu, Y., Chen, H., et al. (2021a). Gastric cancer-secreted exosomal X26nt increases angiogenesis and vascular permeability by targeting VE-cadherin. Cancer Sci. 112 (5), 1839–1852. doi:10.1111/cas.14740
Cheng, G., Li, W., Ha, L., Han, X., Hao, S., Wan, Y., et al. (2018). Self-Assembly of extracellular vesicle-like metal-organic framework nanoparticles for protection and intracellular delivery of biofunctional proteins. J. Am. Chem. Soc. 140 (23), 7282–7291. doi:10.1021/jacs.8b03584
Cheng, L., Zhang, X., Tang, J., Lv, Q., and Liu, J. (2021). Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials 275, 120964. doi:10.1016/j.biomaterials.2021.120964
Colombo, M., Moita, C., van Niel, G., Kowal, J., Vigneron, J., Benaroch, P., et al. (2013). Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 126 (24), 5553–5565. doi:10.1242/jcs.128868
Colombo, M., Raposo, G., and Thery, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289. doi:10.1146/annurev-cellbio-101512-122326
Coulouarn, C., Factor, V. M., Andersen, J. B., Durkin, M. E., and Thorgeirsson, S. S. (2009). Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene 28 (40), 3526–3536. doi:10.1038/onc.2009.211
Dai, S., Wei, D., Wu, Z., Zhou, X., Wei, X., Huang, H., et al. (2008). Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 16 (4), 782–790. doi:10.1038/mt.2008.1
Dalmizrak, A., and Dalmizrak, O. (2022). Mesenchymal stem cell-derived exosomes as new tools for delivery of miRNAs in the treatment of cancer. Front. Bioeng. Biotechnol. 10, 956563. doi:10.3389/fbioe.2022.956563
De Veirman, K., Wang, J., Xu, S., Leleu, X., Himpe, E., Maes, K., et al. (2016). Induction of miR-146a by multiple myeloma cells in mesenchymal stromal cells stimulates their pro-tumoral activity. Cancer Lett. 377 (1), 17–24. doi:10.1016/j.canlet.2016.04.024
Dilsiz, N. (2020). Role of exosomes and exosomal microRNAs in cancer. Future Sci. OA 6 (4), FSO465. doi:10.2144/fsoa-2019-0116
Dong, L., Pu, Y., Zhang, L., Qi, Q., Xu, L., Li, W., et al. (2018). Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 9 (2), 218. doi:10.1038/s41419-018-0323-5
Dreux, M., Garaigorta, U., Boyd, B., Decembre, E., Chung, J., Whitten-Bauer, C., et al. (2012). Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 12 (4), 558–570. doi:10.1016/j.chom.2012.08.010
Du, T., Ju, G., Wu, S., Cheng, Z., Cheng, J., Zou, X., et al. (2014). Microvesicles derived from human Wharton's jelly mesenchymal stem cells promote human renal cancer cell growth and aggressiveness through induction of hepatocyte growth factor. PLoS One 9 (5), e96836. doi:10.1371/journal.pone.0096836
Du, W. J., Chi, Y., Yang, Z. X., Li, Z. J., Cui, J. J., Song, B. Q., et al. (2016). Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res. Ther. 7 (1), 163. doi:10.1186/s13287-016-0418-9
Elsharkasy, O. M., Nordin, J. Z., Hagey, D. W., de Jong, O. G., Schiffelers, R. M., Andaloussi, S. E., et al. (2020). Extracellular vesicles as drug delivery systems: why and how? Adv. Drug Deliv. Rev. 159, 332–343. doi:10.1016/j.addr.2020.04.004
Favaro, E., Carpanetto, A., Caorsi, C., Giovarelli, M., Angelini, C., Cavallo-Perin, P., et al. (2016). Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 59 (2), 325–333. doi:10.1007/s00125-015-3808-0
Ferreira, J. V., da Rosa Soares, A., Ramalho, J., Maximo Carvalho, C., Cardoso, M. H., Pintado, P., et al. (2022). LAMP2A regulates the loading of proteins into exosomes. Sci. Adv. 8 (12), eabm1140. doi:10.1126/sciadv.abm1140
Fornari, F., Gramantieri, L., Giovannini, C., Veronese, A., Ferracin, M., Sabbioni, S., et al. (2009). MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 69 (14), 5761–5767. doi:10.1158/0008-5472.can-08-4797
Fornari, F., Milazzo, M., Chieco, P., Negrini, M., Calin, G. A., Grazi, G. L., et al. (2010). MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 70 (12), 5184–5193. doi:10.1158/0008-5472.can-10-0145
Fornetti, J., Welm, A. L., and Stewart, S. A. (2018). Understanding the bone in cancer metastasis. J. Bone Min. Res. 33 (12), 2099–2113. doi:10.1002/jbmr.3618
Fu, B., Wang, N., Tan, H. Y., Li, S., Cheung, F., and Feng, Y. (2018). Multi-component herbal products in the prevention and treatment of chemotherapy-associated toxicity and side effects: a review on experimental and clinical evidences. Front. Pharmacol. 9, 1394. doi:10.3389/fphar.2018.01394
Fujii, S., Miura, Y., Fujishiro, A., Shindo, T., Shimazu, Y., Hirai, H., et al. (2018). Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem Cells 36 (3), 434–445. doi:10.1002/stem.2759
Furuta, T., Miyaki, S., Ishitobi, H., Ogura, T., Kato, Y., Kamei, N., et al. (2016). Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 5 (12), 1620–1630. doi:10.5966/sctm.2015-0285
García-Fernández, J., and Fuente Freire, M. (2023). Exosome-like systems: nanotechnology to overcome challenges for targeted cancer therapies. Cancer Lett. 561, 216151. doi:10.1016/j.canlet.2023.216151
Ghollasi, M., Ghasembaglou, S., Rahban, D., Korani, M., Motallebnezhad, M., Asadi, M., et al. (2021). Prospects for manipulation of mesenchymal stem cells in tumor therapy: anti-angiogenesis property on the spotlight. Int. J. Stem Cells 14 (4), 351–365. doi:10.15283/ijsc20146
Giordano, C., Gelsomino, L., Barone, I., Panza, S., Augimeri, G., Bonofiglio, D., et al. (2019). Leptin modulates exosome biogenesis in breast cancer cells: an additional mechanism in cell-to-cell communication. J. Clin. Med. 8 (7), 1027. doi:10.3390/jcm8071027
Gomis, R. R., and Gawrzak, S. (2017). Tumor cell dormancy. Mol. Oncol. 11 (1), 62–78. doi:10.1016/j.molonc.2016.09.009
Gong, M., Yu, B., Wang, J., Wang, Y., Liu, M., Paul, C., et al. (2017). Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 8 (28), 45200–45212. doi:10.18632/oncotarget.16778
Greten, F. R., and Grivennikov, S. I. (2019). Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51 (1), 27–41. doi:10.1016/j.immuni.2019.06.025
Guo, P., Busatto, S., Huang, J., Morad, G., and Moses, M. A. (2021). A facile magnetic extrusion method for preparing endosome-derived vesicles for cancer drug delivery. Adv. Funct. Mater 31 (44), 2008326. doi:10.1002/adfm.202008326
Guo, T., Wang, Y., Jia, J., Mao, X., Stankiewicz, E., Scandura, G., et al. (2020). The identification of plasma exosomal miR-423-3p as a potential predictive biomarker for prostate cancer castration-resistance development by plasma exosomal miRNA sequencing. Front. Cell Dev. Biol. 8, 602493. doi:10.3389/fcell.2020.602493
Gupta, K. H., Nowicki, C., Giurini, E. F., Marzo, A. L., and Zloza, A. (2021). Bacterial-based cancer therapy (BBCT): recent advances, current challenges, and future prospects for cancer immunotherapy. Vaccines (Basel) 9 (12), 1497. doi:10.3390/vaccines9121497
Gurung, S., Perocheau, D., Touramanidou, L., and Baruteau, J. (2021). The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal 19 (1), 47. doi:10.1186/s12964-021-00730-1
Handa, T., Kuroha, M., Nagai, H., Shimoyama, Y., Naito, T., Moroi, R., et al. (2021). Liquid biopsy for colorectal adenoma: is the exosomal miRNA derived from organoid a potential diagnostic biomarker? Clin. Transl. Gastroenterol. 12 (5), e00356. doi:10.14309/ctg.0000000000000356
Haney, M. J., Klyachko, N. L., Harrison, E. B., Zhao, Y., Kabanov, A. V., and Batrakova, E. V. (2019). TPP1 delivery to lysosomes with extracellular vesicles and their enhanced brain distribution in the animal model of batten disease. Adv. Healthc. Mater 8 (11), e1801271. doi:10.1002/adhm.201801271
Hayat, H., Hayat, H., Dwan, B. F., Gudi, M., Bishop, J. O., and Wang, P. (2021). A concise review: the role of stem cells in cancer progression and therapy. Onco Targets Ther. 14, 2761–2772. doi:10.2147/ott.s260391
He, J. G., Xie, Q. L., Li, B. B., Zhou, L., and Yan, D. (2018). Exosomes derived from Ido1-overexpressing rat bone marrow mesenchymal stem cells promote immunotolerance of cardiac allografts. Cell Transpl. 27 (11), 1657–1683. doi:10.1177/0963689718805375
He, L., Chen, Y., Ke, Z., Pang, M., Yang, B., Feng, F., et al. (2020). Exosomes derived from miRNA-210 overexpressing bone marrow mesenchymal stem cells protect lipopolysaccharide induced chondrocytes injury via the NF-κB pathway. Gene 751, 144764. doi:10.1016/j.gene.2020.144764
Herrmann, I. K., Wood, M. J. A., and Fuhrmann, G. (2021). Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16 (7), 748–759. doi:10.1038/s41565-021-00931-2
Hirschfeld, M., Rucker, G., Weiss, D., Berner, K., Ritter, A., Jager, M., et al. (2020). Urinary exosomal MicroRNAs as potential non-invasive biomarkers in breast cancer detection. Mol. Diagn Ther. 24 (2), 215–232. doi:10.1007/s40291-020-00453-y
Hoshino, D., Kirkbride, K. C., Costello, K., Clark, E. S., Sinha, S., Grega-Larson, N., et al. (2013). Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 5 (5), 1159–1168. doi:10.1016/j.celrep.2013.10.050
Hou, J., He, Z., Liu, T., Chen, D., Wang, B., Wen, Q., et al. (2022). Evolution of molecular targeted cancer therapy: mechanisms of drug resistance and novel opportunities identified by CRISPR-cas9 screening. Front. Oncol. 12, 755053. doi:10.3389/fonc.2022.755053
Huang, D., and Qu, D. (2020). Early diagnostic and prognostic value of serum exosomal miR-1246 in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 13 (7), 1601–1607.
Huang, X., Yuan, T., Tschannen, M., Sun, Z., Jacob, H., Du, M., et al. (2013). Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 14, 319. doi:10.1186/1471-2164-14-319
Huang, Y., Liu, K., Li, Q., Yao, Y., and Wang, Y. (2018). Exosomes function in tumor immune microenvironment. Adv. Exp. Med. Biol. 1056, 109–122. doi:10.1007/978-3-319-74470-4_7
Jafari, D., Malih, S., Eslami, S. S., Jafari, R., Darzi, L., Tarighi, P., et al. (2019). The relationship between molecular content of mesenchymal stem cells derived exosomes and their potentials: opening the way for exosomes based therapeutics. Biochimie 165, 76–89. doi:10.1016/j.biochi.2019.07.009
Jiang, S., Mo, C., Guo, S., Zhuang, J., Huang, B., and Mao, X. (2019). Human bone marrow mesenchymal stem cells-derived microRNA-205-containing exosomes impede the progression of prostate cancer through suppression of RHPN2. J. Exp. Clin. Cancer Res. 38 (1), 495. doi:10.1186/s13046-019-1488-1
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. doi:10.1126/science.aau6977
Kalluri, R. (2016). The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16 (9), 582–598. doi:10.1038/nrc.2016.73
Kang, J., Zhang, L., Luo, X., Ma, X., Wang, G., Yang, Y., et al. (2018). Systematic exposition of mesenchymal stem cell for inflammatory bowel disease and its associated colorectal cancer. Biomed. Res. Int. 2018, 1–16. doi:10.1155/2018/9652817
Katakowski, M., Buller, B., Zheng, X., Lu, Y., Rogers, T., Osobamiro, O., et al. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 335 (1), 201–204. doi:10.1016/j.canlet.2013.02.019
Khakoo, A. Y., Pati, S., Anderson, S. A., Reid, W., Elshal, M. F., Rovira, , et al. (2006). Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J. Exp. Med. 203 (5), i7–i47. doi:10.1083/jcb1734oia7
Kim, J. H., Badawi, M., Park, J. K., Jiang, J., Mo, X., Roberts, L. R., et al. (2016). Anti-invasion and anti-migration effects of miR-199a-3p in hepatocellular carcinoma are due in part to targeting CD151. Int. J. Oncol. 49 (5), 2037–2045. doi:10.3892/ijo.2016.3677
Kim, M. Y., Shin, H., Moon, H. W., Park, Y. H., Park, J., and Lee, J. Y. (2021). Urinary exosomal microRNA profiling in intermediate-risk prostate cancer. Sci. Rep. 11 (1), 7355. doi:10.1038/s41598-021-86785-z
Kim, S. K., and Cho, S. W. (2022). The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front. Pharmacol. 13, 868695. doi:10.3389/fphar.2022.868695
Lang, F. M., Hossain, A., Gumin, J., Momin, E. N., Shimizu, Y., Ledbetter, D., et al. (2018). Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro Oncol. 20 (3), 380–390. doi:10.1093/neuonc/nox152
Lee, J. K., Park, S. R., Jung, B. K., Jeon, Y. K., Lee, Y. S., Kim, M. K., et al. (2013). Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 8 (12), e84256. doi:10.1371/journal.pone.0084256
Li, B., Cao, Y., Sun, M., and Feng, H. (2021a). Expression, regulation, and function of exosome-derived miRNAs in cancer progression and therapy. FASEB J. 35 (10), e21916. doi:10.1096/fj.202100294rr
Li, C., Zhou, T., Chen, J., Li, R., Chen, H., Luo, S., et al. (2022). The role of Exosomal miRNAs in cancer. J. Transl. Med. 20 (1), 6. doi:10.1186/s12967-021-03215-4
Li, D., Wang, J., Ma, L. J., Yang, H. B., Jing, J. F., Jia, M. M., et al. (2020a). Identification of serum exosomal miR-148a as a novel prognostic biomarker for breast cancer. Eur. Rev. Med. Pharmacol. Sci. 24 (13), 7303–7309. doi:10.26355/eurrev_202007_21889
Li, G. C., Zhang, H. W., Zhao, Q. C., Sun, L. I., Yang, J. J., Hong, L., et al. (2016). Mesenchymal stem cells promote tumor angiogenesis via the action of transforming growth factor β1. Oncol. Lett. 11 (2), 1089–1094. doi:10.3892/ol.2015.3997
Li, H., and Li, F. (2018). Exosomes from BM-MSCs increase the population of CSCs via transfer of miR-142-3p. Br. J. Cancer 119 (6), 744–755. doi:10.1038/s41416-018-0254-z
Li, W., Dong, Y., Wang, K. J., Deng, Z., Zhang, W., and Shen, H. F. (2020b). Plasma exosomal miR-125a-5p and miR-141-5p as non-invasive biomarkers for prostate cancer. Neoplasma 67 (6), 1314–1318. doi:10.4149/neo_2020_191130n1234
Li, X., Chen, C., Wang, Z., Liu, J., Sun, W., Shen, K., et al. (2021b). Elevated exosome-derived miRNAs predict osimertinib resistance in non-small cell lung cancer. Cancer Cell Int. 21 (1), 428. doi:10.1186/s12935-021-02075-8
Li, X., Li, C., Zhang, L., Wu, M., Cao, K., Jiang, F., et al. (2020c). The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol. Cancer 19 (1), 1. doi:10.1186/s12943-019-1085-0
Li, X., Liu, L. L., Yao, J. L., Wang, K., and Ai, H. (2019). Human umbilical cord mesenchymal stem cell-derived extracellular vesicles inhibit endometrial cancer cell proliferation and migration through delivery of exogenous miR-302a. Stem Cells Int. 2019, 1–11. doi:10.1155/2019/8108576
Li, Z., Li, L. X., Diao, Y. J., Wang, J., Ye, Y., and Hao, X. K. (2021c). Identification of urinary exosomal miRNAs for the non-invasive diagnosis of prostate cancer. Cancer Manag. Res. 13, 25–35. doi:10.2147/cmar.s272140
Liang, W., Chen, X., Zhang, S., Fang, J., Chen, M., Xu, Y., et al. (2021). Mesenchymal stem cells as a double-edged sword in tumor growth: focusing on MSC-derived cytokines. Cell Mol. Biol. Lett. 26 (1), 3. doi:10.1186/s11658-020-00246-5
Lin, Y., Luo, Y., Sun, Y., Guo, W., Zhao, X., Xi, Y., et al. (2020). Genomic and transcriptomic alterations associated with drug vulnerabilities and prognosis in adenocarcinoma at the gastroesophageal junction. Nat. Commun. 11 (1), 6091. doi:10.1038/s41467-020-19949-6
Liu, J., Feng, Y., Zeng, X., He, M., Gong, Y., and Liu, Y. (2021b). Extracellular vesicles-encapsulated let-7i shed from bone mesenchymal stem cells suppress lung cancer via KDM3A/DCLK1/FXYD3 axis. J. Cell Mol. Med. 25 (4), 1911–1926. doi:10.1111/jcmm.15866
Liu, M., Mo, F., Song, X., He, Y., Yuan, Y., Yan, J., et al. (2021a). Exosomal hsa-miR-21-5p is a biomarker for breast cancer diagnosis. PeerJ 9, e12147. doi:10.7717/peerj.12147
Liu, W., Wei, L., Li, M., and Mo, J. (2023). Zinc sulfide-based hybrid exosome-coated nanoplatform for targeted treatment of glioblastoma in an orthotopic mouse glioblastoma model. Mater. Today Adv. 17, 100327. doi:10.1016/j.mtadv.2022.100327
Lone, S. N., Nisar, S., Masoodi, T., Singh, M., Rizwan, A., Hashem, S., et al. (2022). Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 21 (1), 79. doi:10.1186/s12943-022-01543-7
Lopatina, T., Bruno, S., Tetta, C., Kalinina, N., Porta, M., and Camussi, G. (2014). Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal 12, 26. doi:10.1186/1478-811x-12-26
Lu, M., and Huang, Y. (2020). Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 242, 119925. doi:10.1016/j.biomaterials.2020.119925
Lu, Y. R., Yuan, Y., Wang, X. J., Wei, L. L., Chen, Y. N., Cong, C., et al. (2008). The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol. Ther. 7 (2), 245–251. doi:10.4161/cbt.7.2.5296
Lugano, R., Ramachandran, M., and Dimberg, A. (2020). Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 77 (9), 1745–1770. doi:10.1007/s00018-019-03351-7
Lv, S., Wang, Y., Xu, W., and Dong, X. (2020). Serum exosomal miR-17-5p as a promising biomarker diagnostic biomarker for breast cancer. Clin. Lab. 66 (9). doi:10.7754/clin.lab.2020.200127
Maffey, A., Storini, C., Diceglie, C., Martelli, C., Sironi, L., Calzarossa, C., et al. (2017). Mesenchymal stem cells from tumor microenvironment favour breast cancer stem cell proliferation, cancerogenic and metastatic potential, via ionotropic purinergic signalling. Sci. Rep. 7 (1), 13162. doi:10.1038/s41598-017-13460-7
Madhunapantula, S. V., Mosca, P. J., and Robertson, G. P. (2011). The Akt signaling pathway: an emerging therapeutic target in malignant melanoma. Cancer Biol. Ther. 12 (12), 1032–1049. doi:10.4161/cbt.12.12.18442
Margiana, R., Markov, A., Zekiy, A. O., Hamza, M. U., Al-Dabbagh, K. A., Al-Zubaidi, S. H., et al. (2022). Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res. Ther. 13 (1), 366. doi:10.1186/s13287-022-03054-0
Mashouri, L., Yousefi, H., Aref, A. R., Ahadi, A. M., Molaei, F., and Alahari, S. K. (2019). Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 18 (1), 75. doi:10.1186/s12943-019-0991-5
McBride, J. D., Rodriguez-Menocal, L., Guzman, W., Candanedo, A., Garcia-Contreras, M., and Badiavas, E. V. (2017). Bone marrow mesenchymal stem cell-derived CD63(+) exosomes transport Wnt3a exteriorly and enhance dermal fibroblast proliferation, migration, and angiogenesis in vitro. Stem Cells Dev. 26 (19), 1384–1398. doi:10.1089/scd.2017.0087
Melzer, C., Rehn, V., Yang, Y., Bahre, H., von der Ohe, J., and Hass, R. (2019). Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers (Basel) 11 (6), 798. doi:10.3390/cancers11060798
Meng, Q., Zhang, B., Zhang, Y., Wang, S., and Zhu, X. (2021). Human bone marrow mesenchymal stem cell-derived extracellular vesicles impede the progression of cervical cancer via the miR-144-3p/CEP55 pathway. J. Cell Mol. Med. 25 (4), 1867–1883. doi:10.1111/jcmm.15573
Mo, J., Chen, X., Li, M., Liu, W., Zhao, W., Lim, L. Y., et al. (2022). Upconversion nanoparticle-based cell membrane-coated cRGD peptide bioorthogonally labeled nanoplatform for glioblastoma treatment. ACS Appl. Mater Interfaces 14, 49454–49470. doi:10.1021/acsami.2c11284
Mohamed, M., Abu Lila, A. S., Shimizu, T., Alaaeldin, E., Hussein, A., Sarhan, H. A., et al. (2019). PEGylated liposomes: immunological responses. Sci. Technol. Adv. Mater 20 (1), 710–724. doi:10.1080/14686996.2019.1627174
Nicholson, B. D., and Lyratzopoulos, G. (2023). Progress and priorities in reducing the time to cancer diagnosis. Br. J. Cancer 128 (3), 468–470. doi:10.1038/s41416-022-02045-5
O'Brien, K. P., Khan, S., Gilligan, K. E., Zafar, H., Lalor, P., Glynn, C., et al. (2018). Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene 37 (16), 2137–2149. doi:10.1038/s41388-017-0116-9
Olsen, J. J., Pohl, S. O., Deshmukh, A., Visweswaran, M., Ward, N. C., Arfuso, F., et al. (2017). The role of Wnt signalling in angiogenesis. Clin. Biochem. Rev. 38 (3), 131–142.
Ono, M., Kosaka, N., Tominaga, N., Yoshioka, Y., Takeshita, F., Takahashi, R. U., et al. (2014). Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal 7 (332), ra63. doi:10.1126/scisignal.2005231
Oun, R., Moussa, Y. E., and Wheate, N. J. (2018). Correction: the side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 47 (23), 7848. doi:10.1039/c8dt90088d
Pakravan, K., Babashah, S., Sadeghizadeh, M., Mowla, S. J., Mossahebi-Mohammadi, M., Ataei, F., et al. (2017). MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol. (Dordr) 40 (5), 457–470. doi:10.1007/s13402-017-0335-7
Pascucci, L., Cocce, V., Bonomi, A., Ami, D., Ceccarelli, P., Ciusani, E., et al. (2014). Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J. Control Release 192, 262–270. doi:10.1016/j.jconrel.2014.07.042
Paskeh, M. D. A., Entezari, M., Mirzaei, S., Zabolian, A., Saleki, H., Naghdi, M. J., et al. (2022). Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 15 (1), 83. doi:10.1186/s13045-022-01305-4
Perez-Hernandez, D., Gutierrez-Vazquez, C., Jorge, I., Lopez-Martin, S., Ursa, A., Sanchez-Madrid, F., et al. (2013). The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 288 (17), 11649–11661. doi:10.1074/jbc.m112.445304
Pittenger, M. F., Discher, D. E., Peault, B. M., Phinney, D. G., Hare, J. M., and Caplan, A. I. (2019). Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 4, 22. doi:10.1038/s41536-019-0083-6
Preethi, K. A., Selvakumar, S. C., Ross, K., Jayaraman, S., Tusubira, D., and Sekar, D. (2022). Liquid biopsy: exosomal microRNAs as novel diagnostic and prognostic biomarkers in cancer. Mol. Cancer 21 (1), 54. doi:10.1186/s12943-022-01525-9
Prendergast, G. C. (2008). Immune escape as a fundamental trait of cancer: focus on ido. Oncogene 27 (28), 3889–3900. doi:10.1038/onc.2008.35
Putz, U., Howitt, J., Lackovic, J., Foot, N., Kumar, S., Silke, J., et al. (2008). Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J. Biol. Chem. 283 (47), 32621–32627. doi:10.1074/jbc.m804120200
Qu, Y., and Dubyak, G. R. (2009). P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal 5 (2), 163–173. doi:10.1007/s11302-009-9132-8
Ren, K., Li, T., Zhang, W., Ren, J., Li, Z., and Wu, G. (2016). miR-199a-3p inhibits cell proliferation and induces apoptosis by targeting YAP1, suppressing Jagged1-Notch signaling in human hepatocellular carcinoma. J. Biomed. Sci. 23 (1), 79. doi:10.1186/s12929-016-0295-7
Reza, A., Choi, Y. J., Yasuda, H., and Kim, J. H. (2016). Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci. Rep. 6, 38498. doi:10.1038/srep38498
Ribatti, D., Tamma, R., and Annese, T. (2020). Epithelial-mesenchymal transition in cancer: a historical overview. Transl. Oncol. 13 (6), 100773. doi:10.1016/j.tranon.2020.100773
Ridge, S. M., Sullivan, F. J., and Glynn, S. A. (2017). Mesenchymal stem cells: key players in cancer progression. Mol. Cancer 16 (1), 31. doi:10.1186/s12943-017-0597-8
Rode, M. P., Silva, A. H., Cisilotto, J., Rosolen, D., and Creczynski-Pasa, T. B. (2021). miR-425-5p as an exosomal biomarker for metastatic prostate cancer. Cell Signal 87, 110113. doi:10.1016/j.cellsig.2021.110113
Rosenberger, L., Ezquer, M., Lillo-Vera, F., Pedraza, P. L., Ortuzar, M. I., Gonzalez, P. L., et al. (2019). Stem cell exosomes inhibit angiogenesis and tumor growth of oral squamous cell carcinoma. Sci. Rep. 9 (1), 663. doi:10.1038/s41598-018-36855-6
Saini, K. S., and Twelves, C. (2021). Determining lines of therapy in patients with solid cancers: a proposed new systematic and comprehensive framework. Br. J. Cancer 125 (2), 155–163. doi:10.1038/s41416-021-01319-8
Salomon, C., Ryan, J., Sobrevia, L., Kobayashi, M., Ashman, K., Mitchell, M., et al. (2013). Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One 8 (7), e68451. doi:10.1371/journal.pone.0068451
Schmidt, O., and Teis, D. (2012). The ESCRT machinery. Curr. Biol. 22 (4), R116–R120. doi:10.1016/j.cub.2012.01.028
Shang, S., Wang, J., Chen, S., Tian, R., Zeng, H., Wang, L., et al. (2019). Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer Med. 8 (18), 7728–7740. doi:10.1002/cam4.2633
Shehzad, A., Islam, S. U., Shahzad, R., Khan, S., and Lee, Y. S. (2021). Extracellular vesicles in cancer diagnostics and therapeutics. Pharmacol. Ther. 223, 107806. doi:10.1016/j.pharmthera.2021.107806
Shi, S., Zhang, Q., Xia, Y., You, B., Shan, Y., Bao, L., et al. (2016). Mesenchymal stem cell-derived exosomes facilitate nasopharyngeal carcinoma progression. Am. J. Cancer Res. 6 (2), 459–472.
Shurtleff, M. J., Temoche-Diaz, M. M., Karfilis, K. V., Ri, S., and Schekman, R. (2016). Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife 5, e19276. doi:10.7554/elife.19276
Skotland, T., Hessvik, N. P., Sandvig, K., and Llorente, A. (2019). Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 60 (1), 9–18. doi:10.1194/jlr.r084343
Staufer, O., Dietrich, F., Rimal, R., Schroter, M., Fabritz, S., Boehm, H., et al. (2021). Bottom-up assembly of biomedical relevant fully synthetic extracellular vesicles. Sci. Adv. 7 (36), eabg6666. doi:10.1126/sciadv.abg6666
Suchorska, W. M., and Lach, M. S. (2016). The role of exosomes in tumor progression and metastasis (Review). Oncol. Rep. 35 (3), 1237–1244. doi:10.3892/or.2015.4507
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Takahara, K., Ii, M., Inamoto, T., Nakagawa, T., Ibuki, N., Yoshikawa, Y., et al. (2016). microRNA-145 mediates the inhibitory effect of adipose tissue-derived stromal cells on prostate cancer. Stem Cells Dev. 25 (17), 1290–1298. doi:10.1089/scd.2016.0093
Tang, Y., Zhang, Z., Song, X., Yu, M., Niu, L., Zhao, Y., et al. (2020). Tumor-derived exosomal miR-620 as a diagnostic biomarker in non-small-cell lung cancer. J. Oncol. 2020, 6691211–6691219. doi:10.1155/2020/6691211
Tao, S-C., Guo, S-C., Li, M., Ke, Q-F., Guo, Y-P., and Zhang, C-Q. (2017). Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl. Med. 6 (3), 736–747. doi:10.5966/sctm.2016-0275
Thery, C., Ostrowski, M., and Segura, E. (2009). Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9 (8), 581–593. doi:10.1038/nri2567
Thery, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7 (1), 1535750. doi:10.1080/20013078.2018.1535750
Trajkovic, K., Hsu, C., Chiantia, S., Rajendran, L., Wenzel, D., Wieland, F., et al. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319 (5867), 1244–1247. doi:10.1126/science.1153124
Ueda, T., Volinia, S., Okumura, H., Shimizu, M., Taccioli, C., Rossi, S., et al. (2010). Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 11 (2), 136–146. doi:10.1016/s1470-2045(09)70343-2
van Niel, G., D'Angelo, G., and Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19 (4), 213–228. doi:10.1038/nrm.2017.125
Vasan, N., Baselga, J., and Hyman, D. M. (2019). A view on drug resistance in cancer. Nature 575 (7782), 299–309. doi:10.1038/s41586-019-1730-1
Villarroya-Beltri, C., Gutierrez-Vazquez, C., Sanchez-Cabo, F., Perez-Hernandez, D., Vazquez, J., Martin-Cofreces, N., et al. (2013). Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980. doi:10.1038/ncomms3980
Wang, B., Wu, Z. H., Lou, P. Y., Chai, C., Han, S. Y., Ning, J. F., et al. (2019a). Retracted article: human bone marrow-derived mesenchymal stem cell-secreted exosomes overexpressing microRNA-34a ameliorate glioblastoma development via down-regulating MYCN. Cell Oncol. (Dordr). 42 (6), 783–799. doi:10.1007/s13402-019-00461-z
Walcher, L., Kistenmacher, A. K., Suo, H., Kitte, R., Dluczek, S., Strauss, A., et al. (2020). Cancer stem cells-origins and biomarkers: perspectives for targeted personalized therapies. Front. Immunol. 11, 1280. doi:10.3389/fimmu.2020.01280
Waldman, A. D., Fritz, J. M., and Lenardo, M. J. (2020). A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20 (11), 651–668. doi:10.1038/s41577-020-0306-5
Walker, S., Busatto, S., Pham, A., Tian, M., Suh, A., Carson, K., et al. (2019). Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 9 (26), 8001–8017. doi:10.7150/thno.37097
Wang, B., Mao, J. H., Wang, B. Y., Wang, L. X., Wen, H. Y., Xu, L. J., et al. (2020). Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-κB signaling pathway. Cancer Lett. 489, 87–99. doi:10.1016/j.canlet.2020.05.038
Wang, J., Chen, D., and Ho, E. A. (2021b). Challenges in the development and establishment of exosome-based drug delivery systems. J. Control Release 329, 894–906. doi:10.1016/j.jconrel.2020.10.020
Wang, J., Li, M., Jin, L., Guo, P., Zhang, Z., Zhanghuang, C., et al. (2022a). Exosome mimetics derived from bone marrow mesenchymal stem cells deliver doxorubicin to osteosarcoma in vitro and in vivo. Drug Deliv. 29 (1), 3291–3303. doi:10.1080/10717544.2022.2141921
Wang, M., Zhao, C., Shi, H., Zhang, B., Zhang, L., Zhang, X., et al. (2014). Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br. J. Cancer 110 (5), 1199–1210. doi:10.1038/bjc.2014.14
Wang, P., Wang, H., Huang, Q., Peng, C., Yao, L., Chen, H., et al. (2019b). Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics 9 (6), 1714–1727. doi:10.7150/thno.30716
Wang, X., Qian, T., Bao, S., Zhao, H., Chen, H., Xing, Z., et al. (2021a). Circulating exosomal miR-363-5p inhibits lymph node metastasis by downregulating PDGFB and serves as a potential noninvasive biomarker for breast cancer. Mol. Oncol. 15 (9), 2466–2479. doi:10.1002/1878-0261.13029
Wang, X., Zhao, X., Zhong, Y., Shen, J., and An, W. (2022b). Biomimetic exosomes: a new generation of drug delivery system. Front. Bioeng. Biotechnol. 10, 865682. doi:10.3389/fbioe.2022.865682
Wang, Y., and Zhou, B. P. (2013). Epithelial-mesenchymal transition---A hallmark of breast cancer metastasis. Cancer Hallm. 1 (1), 38–49. doi:10.1166/ch.2013.1004
Weng, Z., Zhang, B., Wu, C., Yu, F., Han, B., Li, B., et al. (2021). Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 14 (1), 136. doi:10.1186/s13045-021-01141-y
White, I. J., Bailey, L. M., Aghakhani, M. R., Moss, S. E., and Futter, C. E. (2006). EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 25 (1), 1–12. doi:10.1038/sj.emboj.7600759
Whiteside, T. L. (2018). Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin. Immunol. 35, 69–79. doi:10.1016/j.smim.2017.12.003
Wu, D. M., Wen, X., Han, X. R., Wang, S., Wang, Y. J., Shen, M., et al. (2019). Retracted: bone marrow mesenchymal stem cell-derived exosomal MicroRNA-126-3p inhibits pancreatic cancer development by targeting ADAM9. Mol. Ther. Nucleic Acids 16, 229–245. doi:10.1016/j.omtn.2019.02.022
Wu, J. S., Jiang, J., Chen, B. J., Wang, K., Tang, Y. L., and Liang, X. H. (2021). Plasticity of cancer cell invasion: patterns and mechanisms. Transl. Oncol. 14 (1), 100899. doi:10.1016/j.tranon.2020.100899
Wu, Q., Yu, L., Lin, X., Zheng, Q., Zhang, S., Chen, D., et al. (2020). <p>Combination of serum miRNAs with serum exosomal miRNAs in early diagnosis for non-small-cell lung cancer</p>. Cancer Manag. Res. 12, 485–495. doi:10.2147/cmar.s232383
Wu, S., Ju, G. Q., Du, T., Zhu, Y. J., and Liu, G. H. (2013). Microvesicles derived from human umbilical cord Wharton's jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS One 8 (4), e61366. doi:10.1371/journal.pone.0061366
Xu, Y., Shen, L., Li, F., Yang, J., Wan, X., and Ouyang, M. (2019). microRNA-16-5p-containing exosomes derived from bone marrow-derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. J. Cell Physiol. 234 (11), 21380–21394. doi:10.1002/jcp.28747
Xun, J., Du, L., Gao, R., Shen, L., Wang, D., Kang, L., et al. (2021). Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics 11 (14), 6847–6859. doi:10.7150/thno.51864
Yang, B., Liu, W., Li, M., and Mo, J. (2022). GSK-J1-loaded, hyaluronic acid-decorated metal-organic frameworks for the treatment of ovarian cancer. Front. Pharmacol. 13, 1023719. doi:10.3389/fphar.2022.1023719
Yang, T., Martin, P., Fogarty, B., Brown, A., Schurman, K., Phipps, R., et al. (2015). Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 32 (6), 2003–2014. doi:10.1007/s11095-014-1593-y
Yang, Z., Shi, J., Xie, J., Wang, Y., Sun, J., Liu, T., et al. (2020). Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 4 (1), 69–83. doi:10.1038/s41551-019-0485-1
Ye, H., Pan, J., Gong, E., Cai, X., Xu, C., Li, Y., et al. (2021). Inhibitory effect of immunologically activated mesenchymal stem cells on lung cancer cell growth and metastasis. Cancer Biother Radiopharm. 38, 322–335. doi:10.1089/cbr.2020.3855
Yellon, D. M., and Davidson, S. M. (2014). Exosomes: nanoparticles involved in cardioprotection? Circ. Res. 114 (2), 325–332. doi:10.1161/circresaha.113.300636
Yim, N., Ryu, S. W., Choi, K., Lee, K. R., Lee, S., Choi, H., et al. (2016). Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 7, 12277. doi:10.1038/ncomms12277
Yong, T., Zhang, X., Bie, N., Zhang, H., Zhang, X., Li, F., et al. (2019). Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 10 (1), 3838. doi:10.1038/s41467-019-11718-4
Yuan, J., Wei, Z., Xu, X., Ocansey, D. K. W., Cai, X., and Mao, F. (2021). The effects of mesenchymal stem cell on colorectal cancer. Stem Cells Int. 2021, 1–14. doi:10.1155/2021/9136583
Yuan, L., Liu, Y., Qu, Y., Liu, L., and Li, H. (2019). Exosomes derived from MicroRNA-148b-3p-overexpressing human umbilical cord mesenchymal stem cells restrain breast cancer progression. Front. Oncol. 9, 1076. doi:10.3389/fonc.2019.01076
Zamani, P., Fereydouni, N., Butler, A. E., Navashenaq, J. G., and Sahebkar, A. (2019). The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc Med. 29 (6), 313–323. doi:10.1016/j.tcm.2018.10.010
Zhang, B., and Farwell, M. A. (2008). Translational medicine: microRNAs: A new emerging class of players for disease diagnostics and gene therapy. J. Cell Mol. Med. 12 (1), 3–21. doi:10.1111/j.1582-4934.2007.00196.x
Zhang, F., Lu, Y., Wang, M., Zhu, J., Li, J., Zhang, P., et al. (2020b). Exosomes derived from human bone marrow mesenchymal stem cells transfer miR-222-3p to suppress acute myeloid leukemia cell proliferation by targeting IRF2/INPP4B. Mol. Cell Probes 51, 101513. doi:10.1016/j.mcp.2020.101513
Zhang, H., Chen, Z., Wang, X., Huang, Z., He, Z., and Chen, Y. (2013). Long non-coding RNA: a new player in cancer. J. Hematol. Oncol. 6, 37. doi:10.1186/1756-8722-6-37
Zhang, H., Wang, J., Ren, T., Huang, Y., Liang, X., Yu, Y., et al. (2020d). Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 490, 54–65. doi:10.1016/j.canlet.2020.07.008
Zhang, Y., Bi, J., Huang, J., Tang, Y., Du, S., and Li, P. (2020a). Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomedicine 15, 6917–6934. doi:10.2147/ijn.s264498
Zhang, Y., Liu, Q., Zhang, X., Huang, H., Tang, S., Chai, Y., et al. (2022). Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J. Nanobiotechnology 20 (1), 279. doi:10.1186/s12951-022-01472-z
Zhang, Y., Liu, Y., Liu, H., and Tang, W. H. (2019). Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 9, 19. doi:10.1186/s13578-019-0282-2
Zhang, Z., Tang, Y., Song, X., Xie, L., Zhao, S., and Song, X. (2020c). Tumor-derived exosomal miRNAs as diagnostic biomarkers in non-small cell lung cancer. Front. Oncol. 10, 560025. doi:10.3389/fonc.2020.560025
Zhao, W., Qin, P., Zhang, D., Cui, X., Gao, J., Yu, Z., et al. (2019). Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging (Albany NY) 11 (21), 9581–9596. doi:10.18632/aging.102406
Zheng, Q., Ding, H., Wang, L., Yan, Y., Wan, Y., Yi, Y., et al. (2021b). Circulating exosomal miR-96 as a novel biomarker for radioresistant non-small-cell lung cancer. J. Oncol. 2021, 5893981. doi:10.1155/2021/5893981
Zheng, X., Liu, J., Li, X., Tian, R., Shang, K., Dong, X., et al. (2021a). Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 497, 190–201. doi:10.1016/j.canlet.2020.10.009
Zhong, L., Li, Y., Xiong, L., Wang, W., Wu, M., Yuan, T., et al. (2021a). Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Target Ther. 6 (1), 201. doi:10.1038/s41392-021-00572-w
Zhong, Y., Li, H., Li, P., Chen, Y., Zhang, M., Yuan, Z., et al. (2021b). Exosomes: a new pathway for cancer drug resistance. Front. Oncol. 11, 743556. doi:10.3389/fonc.2021.743556
Zhou, T., Yuan, Z., Weng, J., Pei, D., Du, X., He, C., et al. (2021b). Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 14 (1), 24. doi:10.1186/s13045-021-01037-x
Zhou, W., Zhou, Y., Chen, X., Ning, T., Chen, H., Guo, Q., et al. (2021a). Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 268, 120546. doi:10.1016/j.biomaterials.2020.120546
Zhou, X., Li, T., Chen, Y., Zhang, N., Wang, P., Liang, Y., et al. (2019). Mesenchymal stem cell-derived extracellular vesicles promote the in vitro proliferation and migration of breast cancer cells through the activation of the ERK pathway. Int. J. Oncol. 54 (5), 1843–1852. doi:10.3892/ijo.2019.4747
Zhu, L., Li, J., Gong, Y., Wu, Q., Tan, S., Sun, D., et al. (2019). Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol. Cancer 18 (1), 74. doi:10.1186/s12943-019-1000-8
Zhu, W., Huang, L., Li, Y., Zhang, X., Gu, J., Yan, Y., et al. (2012). Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 315 (1), 28–37. doi:10.1016/j.canlet.2011.10.002
Keywords: mesenchymal stem cells, exosomes, biotechnology, cancer therapy, cancer diagnosis
Citation: Zhou Y, Dong Y, Zhang A, Wu J and Sun Q (2023) The role of mesenchymal stem cells derived exosomes as a novel nanobiotechnology target in the diagnosis and treatment of cancer. Front. Bioeng. Biotechnol. 11:1214190. doi: 10.3389/fbioe.2023.1214190
Received: 29 April 2023; Accepted: 03 August 2023;
Published: 17 August 2023.
Edited by:
Shenglong Li, China Medical University, ChinaReviewed by:
Yingnan Yang, Harbin Medical University, ChinaJingxin Mo, University of New South Wales, Australia
Copyright © 2023 Zhou, Dong, Zhang, Wu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Sun, MjYxMTQyMDY1QHFxLmNvbQ==
†These authors have contributed equally to this work
 You Zhou1†
You Zhou1† Aixue Zhang
Aixue Zhang Qiang Sun
Qiang Sun