- The Reproductive Medical Center, China-Japan Union Hospital of Jilin University, Changchun, China
Titanium dioxide nanoparticles (nano-TiO2) are widely used in food, textiles, coatings and personal care products; however, they cause environmental and health concerns. Nano-TiO2 can accumulate in the reproductive organs of mammals in different ways, affect the development of the ovum and sperm, damage reproductive organs and harm the growth and development of offspring. The oxidative stress response in germ cells, irregular cell apoptosis, inflammation, genotoxicity and hormone synthesis disorder are the main mechanisms of nano-TiO2 toxicity. Possible measures to reduce the harmful effects of nano-TiO2 on humans and nontarget organisms have emerged as an underexplored topic requiring further investigation.
1 Introduction
Nanomaterials, as a kind of ultrafine particle material, have the characteristics of the nanosize effect, the surface effect and the macroscopic quantum tunnelling effect. Nanomaterials are widely used in biological engineering, the medical field, ceramics, cosmetics, electronic sensors and other fields; nanomaterials include nanoparticle materials, nanomagnetic liquid materials, nanosolid materials, and nanofilm materials (Chen et al., 2020). Although nanomaterials are widely used, the toxicity studies of nanomaterials are not comprehensive and in depth. Titanium dioxide nanoparticles (nano-TiO2, <100 nm) are widely used in technology, industry, and consumer products (Luo et al., 2020; Cornu et al., 2022) due to their desirable physicochemical characteristics, including high reactivity, ultraviolet (UV) shielding function, large specific surface area, photocatalytic activity and unique quantum and electron-tunnelling effects (Ali et al., 2018). However, subsequent studies found that exposure to high levels of nano-TiO2 caused lung tumours in rats (Shi et al., 2013), and the International Agency for Research on Cancer (IARC) classified titanium dioxide as a Group 2B carcinogen (suspected carcinogen). In 2022, the European Commission (EC) clarified the definition of nanomaterials to support and align legislation across all sectors in a new recommendation. The use of TiO2 as a food additive was recently deemed unsafe by the European Food Safety Authority (EFSA) (Additives et al., 2021), and the EC announced the decision to ban its use. Therefore, the biological toxicity of nano-titanium dioxide has been a concern. A large number of studies have shown that after inhalation or ingestion of nano-TiO2, it accumulates in the lungs, digestive tract, heart, liver, kidney, spleen and reproductive organs, with different effects on the various organs (Gojznikar et al., 2022).
TiO2 is a natural oxide of titanium metal with a diameter of less than 100 mm. It has high thermal stability, hydrophilicity and chemical stability, as well as low toxicity and few biological effects. Brookite, anatase and rutile are the main polymorphs of nano-TiO2. A commonly noted property of nano-TiO2 is its photocatalytic ability, enabling it to stimulate the generation of free radicals, which can then react further with other compounds (Noman et al., 2019). Therefore, as a new green and efficient photocatalytic material, nano-TiO2 is widely used in every aspect of daily life, such as air purification, sewage treatment, and sterilization during environmental remediation. Nano-TiO2 is also added to cosmetics, toothpaste, ceramics and food additives. The wide application of nano-TiO2 increases human exposure, mainly through the respiratory tract, oesophagus and skin into the human body; furthermore, nano-TiO2 also accumulates in tissues and organs along with the circulatory system. Therefore, nano-TiO2 can affect human health through occupational exposure and the use of nano-TiO2-containing products directly and through environmental exposure to unintentionally released nano-TiO2 indirectly (Shi et al., 2013; Cornu et al., 2022). This review focuses on the effects of nano-TiO2 on the mammalian reproductive system.
2 Application of nano-TiO2 in medical diagnosis and treatment
Nano-TiO2 is used as a photosensitizer in cancer therapy and for photodynamic inactivation of antibiotic-resistant bacteria. This is possible because these nanoparticles have high biocompatibility and excellent photochemical properties. In photodynamic therapy, nano-TiO2 photosensitizers can be activated to produce cytotoxic ROS in response to specific wavelengths of light, thus killing tumour cells. Although UV-activated titanium dioxide nanoparticles have prospects for PDT therapy, this strategy appears to be ineffective in treating certain types of cancer and has limited clinical application. Limited UV penetration limits the technique to surface cancers such as skin cancer, nasopharyngeal cancer and oral cancer. At the same time, the duration of UV-mediated ROS production is not long enough to provide continuous and long-term anticancer effects. This limitation has led to the creation of composites containing nano-TiO2. The combinations of nano-TiO2 with carbon-based nanomaterials and inorganic dopants were studied for anticancer and antimicrobial PDT (Ni et al., 2017). Nano-TiO2 was applied inter alia in the synthesis of bioconjugates with cell-specific monoclonal antibodies to treat malignant tumours, while in the antimicrobial therapy it can be prepared of black nano-TiO2. Another application of nano-TiO2 is as a drug carrier (Liu et al., 2022). It allows drugs to reach diseased areas of the body while keeping healthy tissues unharmed (Gao et al., 2016). Antibodies or markers can be labelled on the surface of nano-TiO2 to design drug delivery to selected, diseased areas (Ghaderi et al., 2011; Jia and Jia, 2012). The proper drug delivery systems used for photosensitizers allows PDT to be performed in specific tissues. As a result, much attention should be paid to minimizing side effects and developing novel formulations that allow the direct delivery of active substances to target cells.
3 Effect of nano-TiO2 on the reproductive system
3.1 Accumulation of nano-TiO2 in genital organs
Nano-TiO2 can be ingested in a variety of ways and accumulates mainly in the lungs, liver, spleen, kidney, nervous system and other organs, as well as in the genital organs. In recent studies, mice were subjected to long-term exposure to nano-TiO2 through intragastric administration. At the end of exposure, pathological observation and Raman spectrum identification were performed, and the accumulation of Ti was detected in the ovaries of female mice (Hong et al., 2017) and the testes of male mice. This indicates that nano-TiO2 can penetrate the blood-testis barrier to cross into the testicular tissue and impair testicular function. Hong et al. investigated that maternal exposure to nano-TiO2 affect foetal development. The study shows that Ti concentrations were increased in maternal serum, placenta, and foetus in nano-TiO2-exposed mice. Furthermore, the number of both dead foetuses and foetuses were increased caused by Ti that were resorbed (Hong et al., 2017). Kyjovska et al. investigated the effect of maternal airway exposure to nano-TiO2 on the function of male reproductive system in the two following generations. Maternal exposure of nano-TiO2 tended to reduce sperm counts, although did not affect daily sperm production (DSP) significantly in the F1 generation. Overall, the time-to-first F2 litter increased with decreasing sperm production (Kyjovska et al., 2013). These studies demonstrated that nano-TiO2 can penetrate the placental barrier, as well as the blood-testis barrier. However, the transport mechanism of nano-TiO2 penetrating the blood-testis barrier and placental barrier remains unclear.
3.2 Effect of nano-TiO2 on the female reproductive system
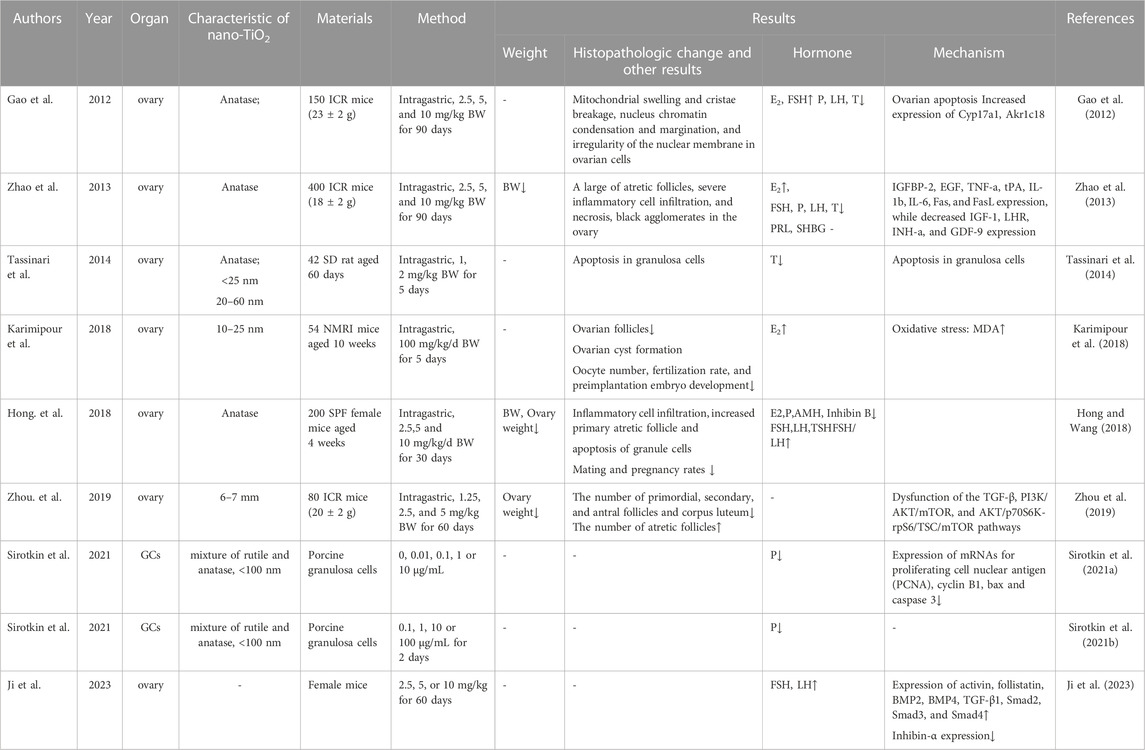
Nano-TiO2 has been shown to accumulate in the ovaries, but it has been relatively poorly studied in female mammals. Studies have shown that the body weight, ovarian weight and ovarian index of female mice were significantly decreased after long-term exposure to low-dose nano-TiO2(Zhao et al., 2013; Zhou et al., 2019). Several pathological changes were observed in the nano-TiO2 group, including reduction in the number of ovarian follicles, ovarian cyst formation, and follicle development impairment, suggesting that ovaries were damaged by nano-TiO2 exposure (Karimipour et al., 2018; Zhou et al., 2019). Karimipour. et al. found that nano-TiO2 caused a significant reduction in oocyte number, fertilization rate, preimplantation embryo development, pregnancy rates and number of births (Karimipour et al., 2018). Recent studies have demonstrated that chronic exposure to nano-TiO2 resulted in a reduction in fertility and follicle development. Follicle development and fertility are associated with the levels of sex hormones. The present study demonstrated that exposure to nano-TiO2 significantly decreased the serum levels of progesterone (P) and testosterone (T) and increased the concentration of estradiol (E2) (Gao et al., 2012; Zhao et al., 2013; Tassinari et al., 2014; Karimipour et al., 2018). However, the effects of nano-TiO2 on the levels of follicle stimulating hormone (FSH) and luteinizing hormone (LH) are controversial. Gao et al. found that after 90 days of exposure to nano-TiO2 in mice, serum FSH levels increased, LH levels decreased, and prolactin (PRL) and sexual hormone binding globulin (SHBG) levels did not change significantly (Gao et al., 2012). Zhao et al. found that under the same exposure, both serum FSH and LH levels in mice decreased significantly (Zhao et al., 2013). Interestingly, in the latest study, Ji et al. found that the serum levels of FSH and LH were significantly increased in mice exposed to the same dose of nano-TiO2 for 60 days (Ji et al., 2023). The different results may be related to the species line of experimental animals, the dose of nano-TiO2, exposure time, detection method, and the mall sample size, which need to be verified by further studies. Progesterone release was diminished after the addition of nano-TiO2 during porcine granulosa cell incubation (Sirotkin et al., 2021a; Sirotkin et al., 2021b). Nano-TiO2 may cause the disturbance of steroidogenesis, which results in a reduction in fertility and follicle development. The basic details of the involved studies are shown in Table 1.
3.3 Effect of nano-TiO2 on the male reproductive system
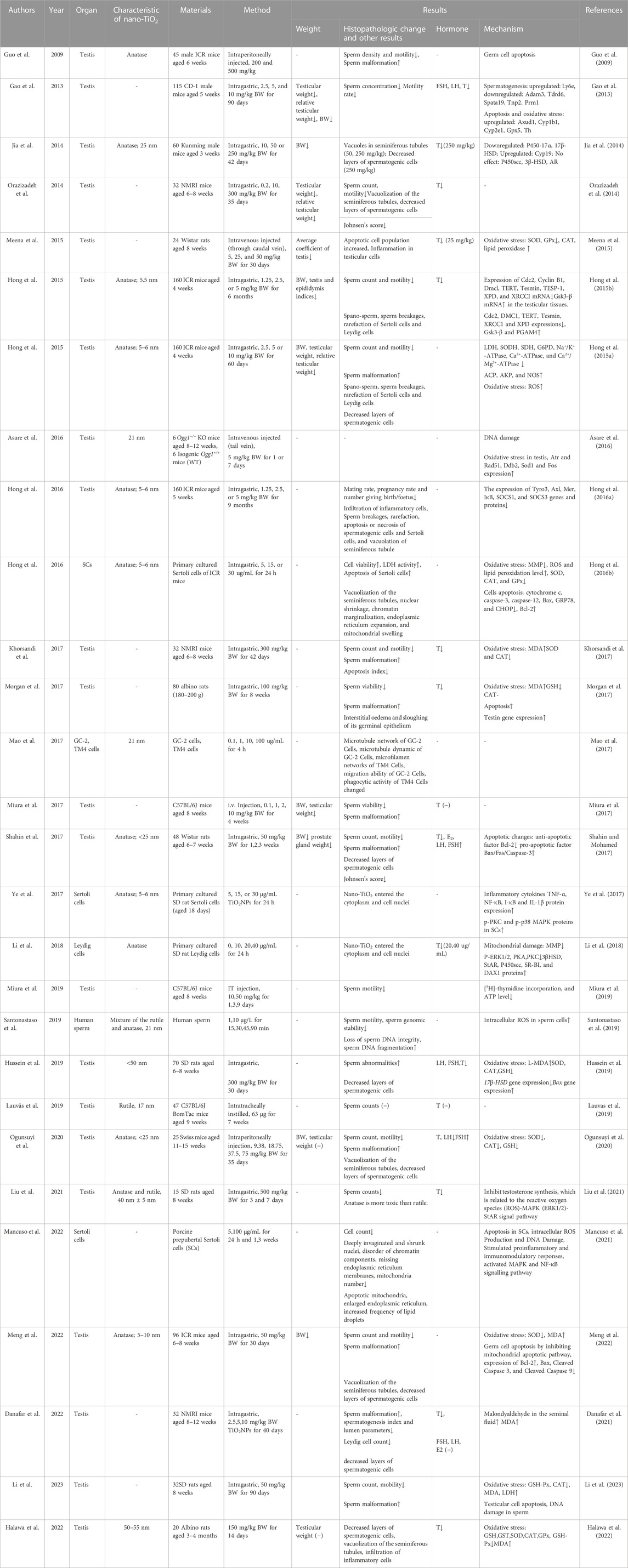
Nano-TiO2 can accumulate in the testis through the blood-testis barrier and have side effects on the male reproductive system. However, the specific mechanism remains unknown. Santonastaso et al. provided data on the evaluation of the potential genotoxicity of nano-TiO2 in vitro on human sperm cells. The results showed that nano-TiO2 can reduce sperm motility, induce the loss of sperm DNA integrity, cause sperm DNA fragmentation, decrease sperm genomic stability and increase intracellular reactive oxygen species (ROS) in sperm cells (Santonastaso et al., 2019). In animal experiments, the body weight, testicular weight and relative testicular weight decreased significantly after intragastric exposure to nano-TiO2, and the reduction was dose-dependent (Gao et al., 2013; Jia et al., 2014; Orazizadeh et al., 2014; Hong et al., 2015a; Hong et al., 2016a; Shahin and Mohamed, 2017; Lauvas et al., 2019; Meng et al., 2022; Li et al., 2023). Shahin et al. found that the weight of the rats prostates decreased significantly after exposure to nano-TiO2(Shahin and Mohamed, 2017). Several studies have demonstrated that nano-TiO2-treated rats clearly exhibited loss of normal architecture, degeneration of the seminiferous tubules, reduction in the number of spermatogenic cells, and infiltration of inflammatory cells in the testis (Meena et al., 2015; Hong et al., 2016a). Nano-TiO2 could migrate to Sertoli cells (SCs) and Leydig cells (LCs), which induced intracellular vacuoles, endoplasmic reticulum dilation, mitochondrial oedema, and chromatin distribution abnormalities in spermatogenic cells (Jia et al., 2014; Orazizadeh et al., 2014; Hong et al., 2015b; Hong et al., 2016a; Hong et al., 2016b; Shahin and Mohamed, 2017; Hussein et al., 2019; Lauvas et al., 2019; Ogunsuyi et al., 2020).
Nano-TiO2 affects the parameters of sperm in males. A number of in vivo studies in mice or rats demonstrated that nano-TiO2 is able to cross the blood-testis barrier and accumulate in the testis, resulting in a reduction in sperm numbers and motility and an increase in sperm morphological abnormalities, resulting in a reduction in the mating rate, fertility and number of offspring (Komatsu et al., 2008; Guo et al., 2009; Gao et al., 2013; Orazizadeh et al., 2014; Hong et al., 2015a; Hong et al., 2015b; Hong et al., 2016a; Khorsandi et al., 2017; Miura et al., 2017; Morgan et al., 2017; Shahin and Mohamed, 2017; Hussein et al., 2019; Lauvas et al., 2019; Miura et al., 2019; Santonastaso et al., 2019; Ogunsuyi et al., 2020; Additives et al., 2021; Danafar et al., 2021; Meng et al., 2022; Li et al., 2023). This may be caused by dysfunction of steroidogenesis and spermatogenesis after nano-TiO2 exposure. Several studies have demonstrated a marked decrease in serum T after chronic exposure to nano-TiO2(Gao et al., 2013; Jia et al., 2014; Orazizadeh et al., 2014; Hong et al., 2015b; Khorsandi et al., 2017; Morgan et al., 2017; Shahin and Mohamed, 2017; Li et al., 2018; Hussein et al., 2019; Lauvas et al., 2019; Ogunsuyi et al., 2020; Danafar et al., 2021; Liu et al., 2021; Halawa et al., 2022). Li. et al. cultured primary SD rat LCs in vitro and exposed them to different concentrations of nano-TiO2 for 24 h; the result clearly showed that T production in LCs was significantly lowered following simultaneous nano-TiO2 treatment (Li et al., 2018). However, individual studies have not found significant effects of nano-TiO2 on serum T levels (Miura et al., 2017; Lauvas et al., 2019). Regarding the effects of nano-TiO2 on serum FSH and LH levels, the results are not consistent. Gao et al. observed that intragastric injection with 2.5, 5, and 10 mg/kg BW nano-TiO2 for 90 days could reduce the serum FSH and LH levels (Gao et al., 2013). Hussein et al. obtained similar results in male SD rats after 30 days of intragastric injection with 300 mg/kg BW nano-TiO2(Hussein et al., 2019). However, Shahin et al. found that intragastric injection with 50 mg/kg BW nano-TiO2 for 3 weeks could significantly increase the serum FSH and LH levels (Shahin and Mohamed, 2017). Ogunsuyi et al. treated male Swiss rats with 75 mg/kg BW nano-TiO2 by gavage for 35 days, and the serum FSH level increased and the LH level decreased (Orazizadeh et al., 2014). In conclusion, exposure to nano-TiO2 can lead to disruption of steroidogenesis and decrease serum T levels, but there is no clear result on the effects on serum FSH and LH level, which may be related to the species line of experimental animals, the dose of nano-TiO2, or the exposure time, which need to be verified by further studies. The basic details of the involved studies are shown in Table 2.
4 Mechanism of reproductive toxicity of nano-TiO2
4.1 Oxidative stress
As a photocatalytic material, nano-TiO2 induces reproductive toxicity directly related to intracellular oxidative stress induced by high-efficiency photocatalysis. Previous studies have suggested that sperm DNA damage caused by nano-TiO2 may be related to the direct effect of ROS on genetic material through oxidative stress reactions in cells (Gao et al., 2012; Hong et al., 2015a; Meena et al., 2015; Asare et al., 2016; Hong et al., 2016b; Khorsandi et al., 2017; Morgan et al., 2017; Karimipour et al., 2018; Hussein et al., 2019; Santonastaso et al., 2019; Hong and Zhou, 2020; Ogunsuyi et al., 2020; Danafar et al., 2021; Liu et al., 2021; Mancuso et al., 2021; Halawa et al., 2022; Meng et al., 2022). It has been shown that nano-TiO2 can increase the synthesis of 8-oxo-2′-deoxyguanosine, which is a component of DNA, thus causing genetic damage. Gao et al. found mitochondrial swelling, irregularity of the nuclear membrane, nuclear chromatin condensation and margination after exposure to nano-TiO2. ROS production increased significantly in the ovary. The results suggest that ovarian apoptosis after nano-TiO2 exposure, and ROS accumulation led to apoptosis and DNA peroxidation in the ovary under nano-TiO2-induced toxicity(Gao et al., 2012). Karimipour et al. found that the serum malonaldehyde (MDA) level increased significantly in female mice after exposure to nano-TiO2(Lauvas et al., 2019). Santonastaso et al. exposed human sperm to nano-TiO2 cultured in vitro and found that ROS levels were significantly increased in sperm cells (Santonastaso et al., 2019). It was demonstrated that the expression of Axud1, Cyp1b1, Cyp2e1, Gpx5 and Th related to oxidative stress and apoptosis was upregulated after nano-TiO2 exposure in mouse testicular tissue (Gao et al., 2013). Recent studies found that ROS, SOD, CAT, GSH, GSH-Px and GST levels were decreased after exposure to nano-TiO2 in testicular cells, while MDA levels were increased (Gao et al., 2013; Hong et al., 2015a; Meena et al., 2015; Hong et al., 2016b; Khorsandi et al., 2017; Morgan et al., 2017; Shahin and Mohamed, 2017; Hussein et al., 2019; Hong and Zhou, 2020; Ogunsuyi et al., 2020; Danafar et al., 2021; Mancuso et al., 2021; Halawa et al., 2022; Meng et al., 2022; Li et al., 2023). Oral antioxidants (such as quercetin and chitosan) can reverse the damage of nano-TiO2, suggesting that oxidative stress may play an important role in the damage caused by nano-TiO2 in the male reproductive system (Khorsandi et al., 2017; Halawa et al., 2022). See in Figure 1A and Figure 2A.
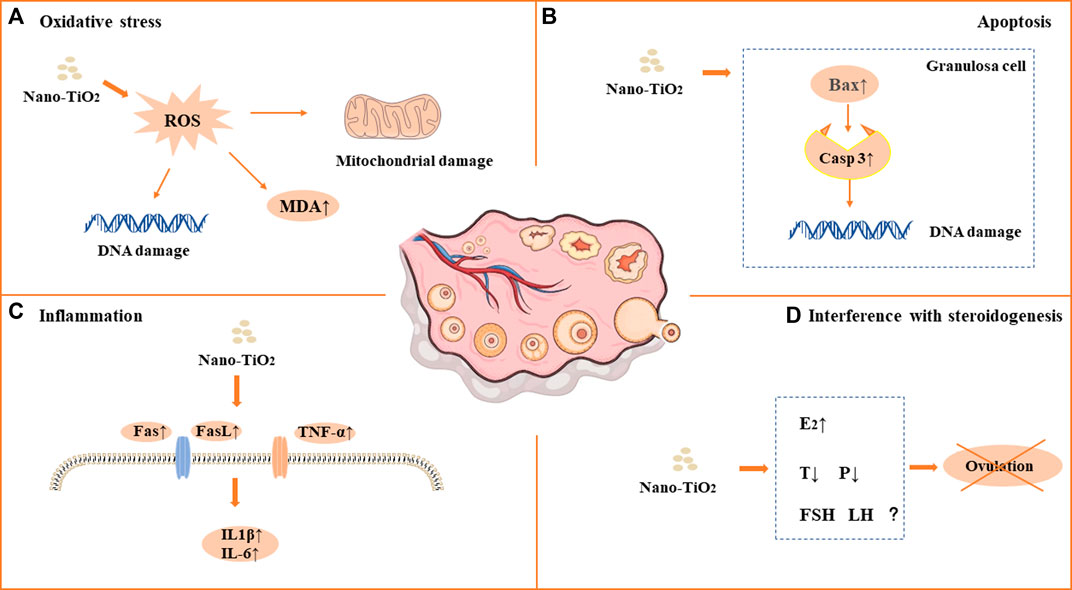
FIGURE 1. Mechanism of toxicity of nano-TiO2 on reproduction in female mammals. (A) Oxidative stress. (B) Apoptosis. (C) Inflammation. (D) Interference with steroidogenesis.
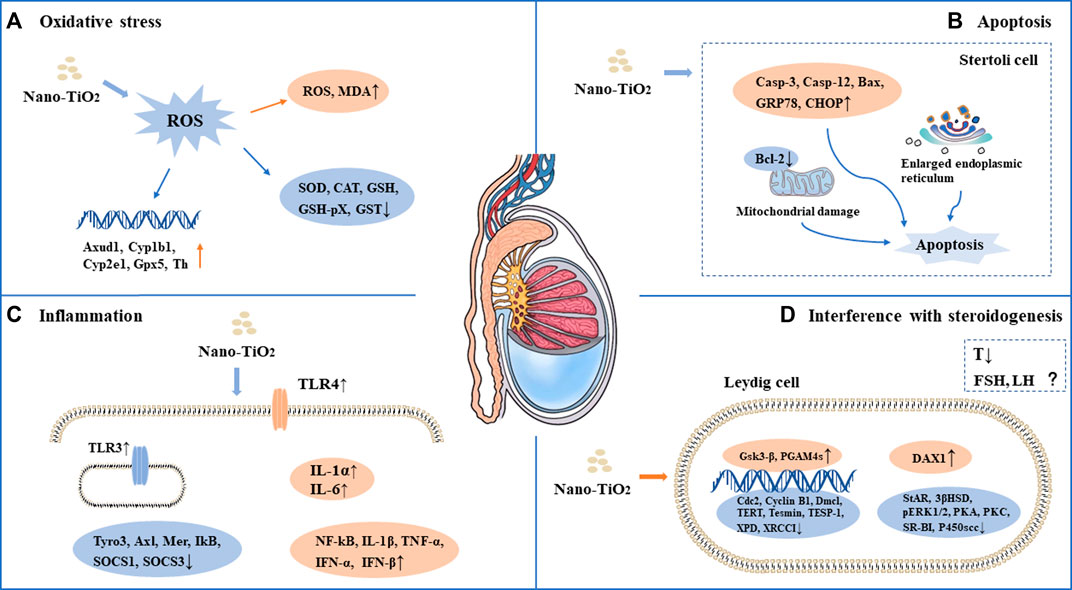
FIGURE 2. Mechanism of toxicity of nano-TiO2 on reproduction in male mammals. (A) Oxidative stress. (B) Apoptosis. (C) Inflammation. (D) Interference with steroidogenesis.
4.2 Apoptosis
Apoptosis plays an important role in male spermatogenesis by helping maintain the proper ratio of germ cells to surrounding supporting cells. Studies have shown that nano-TiO2 may interrupt the apoptosis process of germ cells. Tassnari et al. found the incidence of apoptosis increased significantly in granulosa cells in the ovary with an increase in the exposure dose of nano-TiO2(Tassinari et al., 2014). However, the mechanism was not clarified. Sirotkin et al. demonstrated the inhibitory action of nano-TiO2 on markers of mitochondrial/cytoplasmic apoptosis, caspase 3 and bax. This observation suggested that nano-TiO2 can directly inhibit ovarian granulosa cell cytoplasmic apoptosis (Sirotkin et al., 2021b). Moreover, suppression of both proliferation and apoptosis indicated that nano-TiO2 can suppress ovarian cell turnover directly. Guo et al. showed that intraperitoneal injection of 500 mg/kg/d BW nano-TiO2 in male rats could induce germ cell apoptosis (Guo et al., 2009). Hong et al. exposed primary cultured Sertoli cells of ICR mice to different concentrations of nano-TiO2 for 24 h and found that the apoptosis rate and death rate of Sertoli cells increased significantly. Upregulation of caspase-3, cytochrome c, caspase-12, Bax, C/EBP homologous protein, and glucose-regulated protein 78 expression and downregulation of bcl-2 protein expression in primary cultured SCs were induced by nano-TiO2 treatment (Hong et al., 2016b). Mancuso et al. exposed porcine prepubertal SCs to 100 μg/mL nano-TiO2 and noted that several large vacuoles were present in SCs, probably as a result of increased frequency of lipid droplets and/or enlarged endoplasmic reticulum and/or apoptotic mitochondria (Mancuso et al., 2021). At the same time, the caspase-3 pathway was activated, which cleaved p53 into active fragments of p19 kDa, inducing apoptosis. Several studies have shown that long-term exposure to nano-TiO2 at low doses can increase the apoptosis rate of SCs and stromal cells in spermatogenic tubules in male testes (Meena et al., 2015; Hong et al., 2016a; Khorsandi et al., 2017; Mao et al., 2017; Shahin and Mohamed, 2017; Li et al., 2023). In conclusion, the induction of apoptosis is one of the important reasons for the damage to the reproductive system caused by nano-TiO2. See in Figure 1B and Figure 2B.
4.3 Inflammation
Infection or non-infection and inflammation have harmful effects on reproduction within the male reproductive system, which usually manifest as lowered sperm numbers, reduced androgen production, and temporary loss of fertility. Toll-like receptors (TLRs) are expressed in SCs, which play an important role in the innate responses in the testis. Nano-TiO2 can induce the release of inflammatory cytokines through the natural immune response receptor family (TLR), thereby mediating chronic inflammation (Hong et al., 2016a). Therefore, the activation of nano-TiO2 and TLR receptors should be given sufficient attention. Zhao et al. found that after exposure to nano-TiO2, the expression levels of TNF-α, IL-1β, IL-6, Fas and FasL were significantly increased, while the expression levels of IGF-1, LHR, INH-α and GFF-9 were significantly decreased, resulting in chronic inflammation in the ovary (Zhao et al., 2013). Meena et al. found that the average coefficient of testis decreased and inflammatory cell infiltration occurred in testicular tissue after exposure to nano-TiO2 for 30 days (Meena et al., 2015). Hong et al. demonstrated that after long-term exposure to nano-TiO2 (9 months), a large number of inflammatory cells infiltrated mouse testicular tissue, and the expression of TLR3 and TLR4 was significantly increased, while the expression of Axl, Tyro3, IkB, Mer, SOCS1 and SOCS3 genes and proteins was significantly decreased (Hong et al., 2016a). Nano-TiO2 upregulated the expression of TNF-α, IL-1β, NF-kB, IFN-α and IFN-β and caused inflammation in primary cultured SD rat SCs(Ye et al., 2017). In an in vitro study, exposure of porcine prepubertal SCs to nano-TiO2 induced upregulated expression of the IL-1α and IL-6 genes and stimulated inflammatory and immunomodulatory responses (Mancuso et al., 2021). See in Figure 1C and Figure 2C.
4.4 Interference with steroidogenesis
Nano-TiO2 exposure interrupts androgen synthesis in male testis. Androgen is synthesized in LCs in the testis. Li et al. demonstrated that nano-TiO2 crosses the membrane into the nucleus or cytoplasm, triggering nuclear condensation and cellular vacuolization. LC viability decreased at the same nano-TiO2 concentration in a time-dependent manner, and nano-TiO2 treatment decreased mitochondrial membrane potential (MMP), testosterone levels, StAR, 3βHSD, pERK1/2, PKA, PKC, SR-BI, and P450scc and upregulated DAX1 in primary cultured rat LCs(Li et al., 2018). Furthermore, Hong et al. found that individual expression of the mRNAs and proteins of testis-specific genes, including TESP-1, Cyclin B1,Cdc2, TERT, Dmcl, Tesmin, XRCCI and XPD, was significantly decreased, whereas PGAM4 and Gsk3-β expression was greatly elevated in testis, which can reduce spermatogenesis in the altered testis-specific gene expression in nano-TiO2 exposed male mice (Hong et al., 2015b). See in Figure 1D and Figure 2D.
5 Conclusion and perspective
The existing and still growing evidence demonstrates the potential toxic effects of nano-TiO2 particles in humans through different exposure ways, including ingestion, injection and inhalation. Human exposure to nano-TiO2 relates to, environmental pollution, occupational settings, or certain consumer goods. It may lead to the aggravation of several chronic diseases, such as the neurodegenerative disease Alzheimer’s disease and glomerulonephritis; hence, nano-TiO2 may increase the risk of developing tumours or the progression of pre-existing processes of cancer. We can list the recent Commission Regulation (EU) 2022/63 (Official Journal of the European Union, L11/1, 18 January 2022), which has withdrawn TiO2 (E 171) as a food additive due to safety concerns to support this. Human exposure to nano-TiO2, whether associated with occupational conditions, environmental pollution, or certain consumer products, may affect reproductive function. Studies have shown that nano-TiO2 can accumulate in the reproductive organs or tissues through different pathways, affect the development of ovum and sperm and transmit to the next-generation through biological barriers such as the blood-testosterone barrier and the placental barrier (Kyjovska et al., 2013; Hong et al., 2017; Guillard et al., 2020). However, the transport mechanism by which nano-TiO2 penetrates biological barriers remains poorly understood. Studies have proven that TLR receptors are expressed in tissues of the human reproductive system, such as the ovary and testis (Hong et al., 2016a). Therefore, studies on the activation of nano-TiO2 and TLR receptors should be considered. The accumulation and toxicity of nano-TiO2 in germ cells and tissues may be related to particle size, surface coating, exposure concentration and exposure time. Only one study has explored the toxicity of different morphologies of nano-TiO2 on the male reproductive system (Liu et al., 2021). This result demonstrated that anatase is more toxic than rutile. Further studies are needed to explore the effects of different morphologies and particle sizes of nano-TiO2 on toxicity in the reproductive system to find a method to decrease the toxicity of nano-TiO2. In conclusion, the main causes of nano-TiO2 toxicity in the reproductive system include oxidative stress, apoptosis, inflammation and interference with steroidogenesis. Further strategies to minimize the environmental and health impacts of nano-TiO2 should include the development of environmentally friendly alternatives to nano-TiO2 and its efficient recycling. Due to the potential toxicity of nano-TiO2, it is necessary to systematically evaluate the toxicity of nano-TiO2 in the human reproductive system through large-scale epidemiological studies to further understand its distribution and accumulation in the reproductive system and transport mechanism through biological barriers. Further studies are needed to explore the mechanisms of nano-TiO2 deeply, to develop strategies to alleviate cellular damage and to provide theoretical guidance and a basis for safety evaluation and development of nano-TiO2 products.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Development Project of Jilin Province (Grant Nos. 20200801080GH, 20200801079 GH), the Science and Technology Research Project of Department of Education of Jilin Province (Grant No. JJKH20211200KJ), the Chunhui Program from the Ministry of Education of China (2020703), Natural Science Foundation of Jilin province (20210101252JC), the Scientific Research Project of Wu Jieping Medical Foundation (320.6750.2021-19-1), and the Bethune Plan of Jilin University (2023B24).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Additives, E. P. O. F., FlavouringsYounes, M., Aquilina, G., Castle, L., Engel, K. H., Fowler, P., et al. (2021). Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 19, e06585. doi:10.2903/j.efsa.2021.6585
Ali, I., Suhail, M., Alothman, Z. A., and Alwarthan, A. (2018). Recent advances in syntheses, properties and applications of TiO(2) nanostructures. RSC Adv. 8, 30125–30147. doi:10.1039/c8ra06517a
Asare, N., Duale, N., Slagsvold, H. H., Lindeman, B., Olsen, A. K., Gromadzka-Ostrowska, J., et al. (2016). Genotoxicity and gene expression modulation of silver and titanium dioxide nanoparticles in mice. Nanotoxicology 10, 312–321. doi:10.3109/17435390.2015.1071443
Chen, Y., Du, M., Yu, J., Rao, L., Chen, X., and Chen, Z. (2020). Nanobiohybrids: A synergistic integration of bacteria and nanomaterials in cancer therapy. BIO Integr. 1, 25–36. doi:10.15212/bioi-2020-0008
Cornu, R., Beduneau, A., and Martin, H. (2022). Ingestion of titanium dioxide nanoparticles: A definite health risk for consumers and their progeny. Arch. Toxicol. 96, 2655–2686. doi:10.1007/s00204-022-03334-x
Danafar, A., Khoradmehr, A., Hosseini Bondarabadi, M., Mazaheri, F., Tamadon, A., Pourmasoumi, S., et al. (2021). Impairment of sperm efficiency in mice following short-term nano-titanium dioxide exposure: An experimental study. Int. J. Reprod. Biomed. 19, 1045–1058. doi:10.18502/ijrm.v19i12.10055
Gao, G., Ze, Y., Li, B., Zhao, X., Zhang, T., Sheng, L., et al. (2012). Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J. Hazard Mater 243, 19–27. doi:10.1016/j.jhazmat.2012.08.049
Gao, G., Ze, Y., Zhao, X., Sang, X., Zheng, L., Ze, X., et al. (2013). Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J. Hazard Mater 258-259, 133–143. doi:10.1016/j.jhazmat.2013.04.046
Gao, Y., Jiang, M., Ma, Y., Wu, S., Li, W., Yang, X., et al. (2016). Nanoparticle-mediated delivery of multinuclear platinum(IV) prodrugs with enhanced drug uptake and the activity of overcoming drug resistance. Anticancer Drugs 27, 77–83. doi:10.1097/cad.0000000000000302
Ghaderi, S., Ramesh, B., and Seifalian, A. M. (2011). Fluorescence nanoparticles "quantum dots" as drug delivery system and their toxicity: A review. J. Drug Target 19, 475–486. doi:10.3109/1061186x.2010.526227
Gojznikar, J., Zdravkovic, B., Vidak, M., Leskosek, B., and Ferk, P. (2022). TiO(2) nanoparticles and their effects on eukaryotic cells: A double-edged sword. Int. J. Mol. Sci. 23, 12353. doi:10.3390/ijms232012353
Guillard, A., Gaultier, E., Cartier, C., Devoille, L., Noireaux, J., Chevalier, L., et al. (2020). Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiO(2) nanoparticles in an ex vivo placental perfusion model. Part Fibre Toxicol. 17, 51. doi:10.1186/s12989-020-00381-z
Guo, L. L., Liu, X. H., Qin, D. X., Gao, L., Zhang, H. M., Liu, J. Y., et al. (2009). Effects of nanosized titanium dioxide on the reproductive system of male mice. Zhonghua Nan Ke Xue 15, 517–522.
Halawa, A. A., Elshopakey, G. E., Elmetwally, M. A., El-Adl, M., Lashen, S., Shalaby, N., et al. (2022). Impact of chitosan administration on titanium dioxide nanoparticles induced testicular dysfunction. Sci. Rep. 12, 19667. doi:10.1038/s41598-022-22044-z
Hong, F., Si, W., Zhao, X., Wang, L., Zhou, Y., Chen, M., et al. (2015a). TiO2 nanoparticle exposure decreases spermatogenesis via biochemical dysfunctions in the testis of male mice. J. Agric. Food Chem. 63, 7084–7092. doi:10.1021/acs.jafc.5b02652
Hong, F., and Wang, L. (2018). Nanosized titanium dioxide-induced premature ovarian failure is associated with abnormalities in serum parameters in female mice. Int. J. Nanomedicine 13, 2543–2549. doi:10.2147/ijn.s151215
Hong, F., Wang, Y., Zhou, Y., Zhang, Q., Ge, Y., Chen, M., et al. (2016a). Exposure to TiO2 nanoparticles induces immunological dysfunction in mouse testitis. J. Agric. Food Chem. 64, 346–355. doi:10.1021/acs.jafc.5b05262
Hong, F., Zhao, X., Chen, M., Zhou, Y., Ze, Y., Wang, L., et al. (2016b). TiO2 nanoparticles-induced apoptosis of primary cultured Sertoli cells of mice. J. Biomed. Mater Res. A 104, 124–135. doi:10.1002/jbm.a.35548
Hong, F., Zhao, X., Si, W., Ze, Y., Wang, L., Zhou, Y., et al. (2015b). Decreased spermatogenesis led to alterations of testis-specific gene expression in male mice following nano-TiO2 exposure. J. Hazard Mater 300, 718–728. doi:10.1016/j.jhazmat.2015.08.010
Hong, F., and Zhou, Y. (2020). Spermatogenic apoptosis and the involvement of the Nrf2 pathway in male mice following exposure to nano titanium dioxide. J. Biomed. Nanotechnol. 16, 373–381. doi:10.1166/jbn.2020.2895
Hong, F., Zhou, Y., Zhao, X., Sheng, L., and Wang, L. (2017). Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int. J. Nanomedicine 12, 6197–6204. doi:10.2147/ijn.s143598
Hussein, M. M. A., Gad, E., Ahmed, M. M., Arisha, A. H., Mahdy, H. F., Swelum, A. A., et al. (2019). Amelioration of titanium dioxide nanoparticle reprotoxicity by the antioxidants morin and rutin. Environ. Sci. Pollut. Res. Int. 26, 29074–29084. doi:10.1007/s11356-019-06091-0
Ji, J., Zhou, Y., Li, Z., Zhuang, J., Ze, Y., and Hong, F. (2023). Impairment of ovarian follicular development caused by titanium dioxide nanoparticles exposure involved in the TGF -β/BMP/S mad pathway. Environ. Toxicol. 38, 185–192. doi:10.1002/tox.23676
Jia, F., Sun, Z., Yan, X., Zhou, B., and Wang, J. (2014). Effect of pubertal nano-TiO2 exposure on testosterone synthesis and spermatogenesis in mice. Arch. Toxicol. 88, 781–788. doi:10.1007/s00204-013-1167-5
Jia, X., and Jia, L. (2012). Nanoparticles improve biological functions of phthalocyanine photosensitizers used for photodynamic therapy. Curr. Drug Metab. 13, 1119–1122. doi:10.2174/138920012802850074
Karimipour, M., Zirak Javanmard, M., Ahmadi, A., and Jafari, A. (2018). Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int. J. Reprod. Biomed. 16, 397–404. doi:10.29252/ijrm.16.6.397
Khorsandi, L., Orazizadeh, M., Moradi-Gharibvand, N., Hemadi, M., and Mansouri, E. (2017). Beneficial effects of quercetin on titanium dioxide nanoparticles induced spermatogenesis defects in mice. Environ. Sci. Pollut. Res. Int. 24, 5595–5606. doi:10.1007/s11356-016-8325-2
Komatsu, T., Tabata, M., Kubo-Irie, M., Shimizu, T., Suzuki, K., Nihei, Y., et al. (2008). The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol Vitro 22, 1825–1831. doi:10.1016/j.tiv.2008.08.009
Kyjovska, Z. O., Boisen, A. M., Jackson, P., Wallin, H., Vogel, U., and Hougaard, K. S. (2013). Daily sperm production: Application in studies of prenatal exposure to nanoparticles in mice. Reprod. Toxicol. 36, 88–97. doi:10.1016/j.reprotox.2012.12.005
Lauvas, A. J., Skovmand, A., Poulsen, M. S., Kyjovska, Z. O., Roursgaard, M., Goericke-Pesch, S., et al. (2019). Airway exposure to TiO(2) nanoparticles and quartz and effects on sperm counts and testosterone levels in male mice. Reprod. Toxicol. 90, 134–140. doi:10.1016/j.reprotox.2019.07.023
Li, L., Mu, X., Ye, L., Ze, Y., and Hong, F. (2018). Suppression of testosterone production by nanoparticulate TiO<sub>2</sub> is associated with ERK1/2-PKA-PKC signaling pathways in rat primary cultured Leydig cells. Int. J. Nanomedicine 13, 5909–5924. doi:10.2147/ijn.s175608
Li, Y., Zhong, M., He, X., Zhang, R., Fu, Y., You, R., et al. (2023). The combined effect of titanium dioxide nanoparticles and cypermethrin on male reproductive toxicity in rats. Environ. Sci. Pollut. Res. Int. 30, 22176–22187. doi:10.1007/s11356-022-23796-x
Liu, J., Bao, X., Kolesnik, I., Jia, B., Yu, Z., Xing, C., et al. (2022). Enhancing the in vivo stability of polycation gene carriers by using PEGylated hyaluronic acid as a shielding system. BIO Integr. 3, 103–111. doi:10.15212/bioi-2021-0033
Liu, S., Tang, Y., Chen, B., Zhao, Y., Aguilar, Z. P., Tao, X., et al. (2021). Inhibition of testosterone synthesis induced by oral TiO(2) NPs is associated with ROS-MAPK(ERK1/2)-StAR signaling pathway in SD rat. Toxicol. Res. (Camb) 10, 937–946. doi:10.1093/toxres/tfab077
Luo, Z., Li, Z., Xie, Z., Sokolova, I. M., Song, L., Peijnenburg, W., et al. (2020). Rethinking nano-TiO(2) safety: Overview of toxic effects in humans and aquatic animals. Small 16, e2002019. doi:10.1002/smll.202002019
Mancuso, F., Arato, I., Di Michele, A., Antognelli, C., Angelini, L., Bellucci, C., et al. (2021). Effects of titanium dioxide nanoparticles on porcine prepubertal sertoli cells: An "in vitro" study. Front. Endocrinol. (Lausanne) 12, 751915. doi:10.3389/fendo.2021.751915
Mao, Z., Yao, M., Xu, B., Ji, X., Jiang, H., Han, X., et al. (2017). Cytoskeletons of two reproductive germ cell lines response differently to titanium dioxide nanoparticles mediating vary reproductive toxicity. J. Biomed. Nanotechnol. 13, 409–416. doi:10.1166/jbn.2017.2360
Meena, R., Kajal, K., and R, P. (2015). Cytotoxic and genotoxic effects of titanium dioxide nanoparticles in testicular cells of male wistar rat. Appl. Biochem. Biotechnol. 175, 825–840. doi:10.1007/s12010-014-1299-y
Meng, X., Li, L., An, H., Deng, Y., Ling, C., Lu, T., et al. (2022). Lycopene alleviates titanium dioxide nanoparticle-induced testicular toxicity by inhibiting oxidative stress and apoptosis in mice. Biol. Trace Elem. Res. 200, 2825–2837. doi:10.1007/s12011-021-02881-1
Miura, N., Ohtani, K., Hasegawa, T., Yoshioka, H., and Hwang, G. W. (2019). Biphasic adverse effect of titanium nanoparticles on testicular function in mice. Sci. Rep. 9, 14373. doi:10.1038/s41598-019-50741-9
Miura, N., Ohtani, K., Hasegawa, T., Yoshioka, H., and Hwang, G. W. (2017). High sensitivity of testicular function to titanium nanoparticles. J. Toxicol. Sci. 42, 359–366. doi:10.2131/jts.42.359
Morgan, A. M., Ibrahim, M. A., and Noshy, P. A. (2017). Reproductive toxicity provoked by titanium dioxide nanoparticles and the ameliorative role of Tiron in adult male rats. Biochem. Biophys. Res. Commun. 486, 595–600. doi:10.1016/j.bbrc.2017.03.098
Ni, W., Li, M., Cui, J., Xing, Z., Li, Z., Wu, X., et al. (2017). 808nm light triggered black TiO(2) nanoparticles for killing of bladder cancer cells. Mater Sci. Eng. C Mater Biol. Appl. 81, 252–260. doi:10.1016/j.msec.2017.08.020
Noman, M. T., Ashraf, M. A., and Ali, A. (2019). Synthesis and applications of nano-TiO(2): A review. Environ. Sci. Pollut. Res. Int. 26, 3262–3291. doi:10.1007/s11356-018-3884-z
Ogunsuyi, O. M., Ogunsuyi, O. I., Akanni, O., Alabi, O. A., Alimba, C. G., Adaramoye, O. A., et al. (2020). Alteration of sperm parameters and reproductive hormones in Swiss mice via oxidative stress after co-exposure to titanium dioxide and zinc oxide nanoparticles. Andrologia 52, e13758. doi:10.1111/and.13758
Orazizadeh, M., Khorsandi, L., Absalan, F., Hashemitabar, M., and Daneshi, E. (2014). Effect of beta-carotene on titanium oxide nanoparticles-induced testicular toxicity in mice. J. Assist. Reprod. Genet. 31, 561–568. doi:10.1007/s10815-014-0184-5
Santonastaso, M., Mottola, F., Colacurci, N., Iovine, C., Pacifico, S., Cammarota, M., et al. (2019). In vitro genotoxic effects of titanium dioxide nanoparticles (n-TiO(2)) in human sperm cells. Mol. Reprod. Dev. 86, 1369–1377. doi:10.1002/mrd.23134
Shahin, N. N., and Mohamed, M. M. (2017). Nano-sized titanium dioxide toxicity in rat prostate and testis: Possible ameliorative effect of morin. Toxicol. Appl. Pharmacol. 334, 129–141. doi:10.1016/j.taap.2017.08.014
Shi, H., Magaye, R., Castranova, V., and Zhao, J. (2013). Titanium dioxide nanoparticles: A review of current toxicological data. Part Fibre Toxicol. 10, 15. doi:10.1186/1743-8977-10-15
Sirotkin, A. V., Alexa, R., Stochmalova, A., and Scsukova, S. (2021a). Plant isoflavones can affect accumulation and impact of silver and titania nanoparticles on ovarian cells. Endocr. Regul. 55, 52–60. doi:10.2478/enr-2021-0007
Sirotkin, A. V., Bauer, M., Kadasi, A., Makovicky, P., and Scsukova, S. (2021b). The toxic influence of silver and titanium dioxide nanoparticles on cultured ovarian granulosa cells. Reprod. Biol. 21, 100467. doi:10.1016/j.repbio.2020.100467
Tassinari, R., Cubadda, F., Moracci, G., Aureli, F., D'amato, M., Valeri, M., et al. (2014). Oral, short-term exposure to titanium dioxide nanoparticles in sprague-dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology 8, 654–662. doi:10.3109/17435390.2013.822114
Ye, L., Hong, F., Ze, X., Li, L., Zhou, Y., and Ze, Y. (2017). Toxic effects of TiO2 nanoparticles in primary cultured rat sertoli cells are mediated via a dysregulated Ca2+/PKC/p38 MAPK/NF-κB cascade: Toxic Mechanisms of TIO2 NPS in Sertoli Cells. J. Biomed. Mater Res. A 105, 1374–1382. doi:10.1002/jbm.a.36021
Zhao, X., Ze, Y., Gao, G., Sang, X., Li, B., Gui, S., et al. (2013). Nanosized TiO2-induced reproductive system dysfunction and its mechanism in female mice. PLoS One 8, e59378. doi:10.1371/journal.pone.0059378
Keywords: titanium dioxide nanoparticles, nano-TiO2, reproductive, toxicty, mammal
Citation: Minghui F, Ran S, Yuxue J and Minjia S (2023) Toxic effects of titanium dioxide nanoparticles on reproduction in mammals. Front. Bioeng. Biotechnol. 11:1183592. doi: 10.3389/fbioe.2023.1183592
Received: 10 March 2023; Accepted: 27 April 2023;
Published: 12 May 2023.
Edited by:
Mingqiang Li, Third Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Ekaterina Naumenko, Kazan Federal University, RussiaQuan Zhou, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Minghui, Ran, Yuxue and Minjia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Minjia, c2hlbmdtakBqbHUuZWR1LmNu
†These authors have contributed equally to this work
 Fan Minghui†
Fan Minghui† Sheng Minjia
Sheng Minjia