
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Bioeng. Biotechnol. , 30 March 2023
Sec. Bioprocess Engineering
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1180431
This article is part of the Research Topic Biotechnology of Phosphate Solubilizing Microorganisms for Metabolites regulation: Present and Future View all 10 articles
Lead (Pb) is one of the most common heavy metal pollutants in the environment, which can indirectly or directly threaten human health. Lead immobilization by apatite can reduce the effectiveness of Pb cations via the formation of pyromorphite (Pyro). However, the formation of Pyro is always depending on the release of phosphorus (P) from apatite. Phosphate-solubilizing fungi (PSF) can secrete large amounts of organic acid to promote the release of P from apatite. Although the combination of PSF and apatite has shown a huge potential in Pb remediation, this pathway needs to be more attention, especially for organic acid secretion by PSF. This research mainly reviews the possible pathway to strengthen Pb immobilization by PSF and apatite. Meanwhile, the limitation of this approach is also reviewed, with the aim of a better stabilizing effect of Pb in the environment and promoting the development of these remediation technologies.
Lead (Pb) is one of the most important heavy metal pollutions in the environment, which has strong biological toxicity, wide distribution, and strong accumulation capacity (Arduini et al., 2010). The completely remove of Pb cations from soil is relatively long and complex due to the hidden and lagging performance (Shen et al., 2015). In-suit immobilization of Pb is an efficient pathway to reduce the toxicity of Pb in soil (Chen et al., 2006). Phosphate can effectively transfer Pb cations to highly insoluble Pb minerals via the phosphorus (P) release (Li et al., 2016b; Tian et al., 2018). However, the process of P release is unsustainable and easily chelates with metal cations in soil, e.g., Ca2+, Fe3+, etc (Tian et al., 2021a). The combination of phosphate solubilizing fungi (PSF) and phosphate is an effective and sustainable pathway in Pb in suit immobilization (Shao et al., 2021). As a new approach in Pb remediation, this technology needs to be more attention nowadays.
In current, phosphate is generally recognized as an excellent material in Pb remediation. The P released from phosphate can react with Pb to form highly insoluble pyromorphite (Pyro) Figure 1. Pyro is highly stable and has a low Ksp value (<10–85), which can significantly reduce Pb toxicity and mobility in soil (Arnich et al., 2003; Chaturvedi et al., 2007; Cheyns et al., 2012). In engineering, field and indoor leaching simulation tests, phosphates can convert Pb from high-activity forms to insoluble forms (John et al., 2001; Chen et al., 20231). The application of phosphate can reduce 12%–92% available Pb content in soil, and the toxicity characteristic leaching procedure Pb (TCLP-Pb) concentration can decrease from 82 mg/L to less than 5 mg/L (Melamed et al., 2003; Cao et al., 2009; Cui et al., 2010). The reaction formula between P and Pb is as follows (Park and Bolan, 2013):
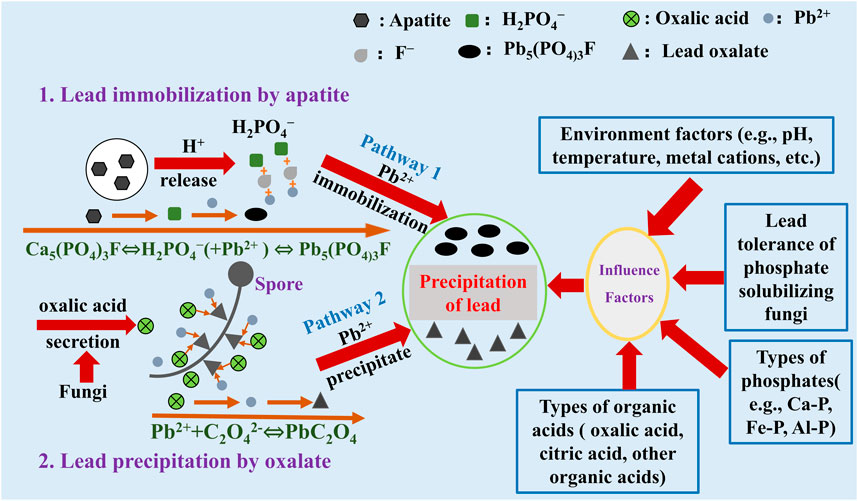
FIGURE 1. Lead remeditaion by phosphate solubilizing fungi and phosphate via the secretion of organic acid.
An acidic environment can significantly enhance phosphate dissolution and improve the release of P (Whitelaw, 1999; Singh and Reddy, 2011). However, the addition of chemical acid (e.g., sulfuric acid) is unsustainable and harmful to soil health. Oxalic acid is more efficient than sulfuric acid in phosphate dissolution (Mendes et al., 2020). Therefore, the utilization of oxalic acid and phosphate is a better choice in Pb remediation. Phosphate solubilizing fungi (PSF) can secrete large amounts of oxalic acid and promote the release of P from insoluble phosphate (Li et al., 2016a). Compare with bacteria, PSF not only maintains the ability of oxalic acid secretion but also can extend in soil via the mycelium. For example, the PSF of Aspergillus niger can promote the dissolution of FAp and carbonate in soil via the hypha extension and oxalic acid secretion (Tian et al., 2021b). In addition, the PSF of A. niger, Penicillium oxalicum, and Penicillium aurantiogriseum, etc., also has a strong ability to secrete oxalic acid (Tian et al., 2021a; Hu et al., 2022; Wang et al., 2022). Therefore, the combination of PSF and phosphate is a considerable pathway in Pb remediation.
The application of PSF and phosphate have been successfully applied in Pb remediation. A. niger and P. oxalicum combined with FAp can significantly remove more than 90% Pb cations in an aqueous solution via the formation of lead oxalate and Pyro (Li et al., 2016b; Tian et al., 2018). Meanwhile, Pb remediation in soil by this combination not only promotes the formation of lead oxalate but also increased the soil available P content (Tian et al., 2022b; Meng et al., 2022). In addition, the released P can be also isolated by PSF and not easily absorbed by plants, promoting the Pb remediation process (Menezes-Blackburb et al., 2016). However, the secretion of oxalic acid by PSF is usually influenced by different factors, such as pH, nutrients, phosphate types, and Pb concentration (Tian et al., 2019; Feng et al., 2022). Therefore, the appropriate technology and conditions are needed in Pb remediation by PSF and phosphate.
Pb remediation by PSF is usually affected by different Pb toxicity. The excessive concentration of Pb cations can limit the growth of fungi and reduce their bioremediation efficiency (Ye et al., 2018). However, the tolerance of Pb toxicity in PSF is different. A. niger has a higher Pb tolerance than P. oxalicum (Tian et al., 2018; Tian et al., 2019). A. niger can survive under 1,500 mg/L Pb concentration and maintain the ability of oxalic acid secretion (Tian et al., 2019). However, the PSF of P. oxalicum only resists 1,000 mg/L Pb concentration, and the secretion of oxalic acid is almost lost under 1,500 mg/L Pb concentration (Tian et al., 2019). Therefore, A. niger has a high Pb tolerance and is efficient in Pb remediation.
The type of phosphate affects the efficiency of Pb remediation mainly due to the P release capacity (Tian et al., 2021a). Hence selecting an appropriate phosphate is important in Pb remediation. The use of phosphates in Pb remediation usually contains water-soluble phosphates (WSP) and insoluble phosphates (IPs), including potassium dihydrogen phosphate, sodium dihydrogen phosphate and hydroxyapatite, fluorapatite bioapatite, etc. WSP has a high solubility of P and is efficient in Pb remediation. However, the use of WSP is easy to cause eutrophication of water and the excessive P can be fixed by metal cations in soil. Compared with WSP, IPs are more stable and need to mix with PSF in Pb remediation (Li et al., 2016b). PSF combined with IP can promote the continuous release of P via the secretion of organic acid and is suitable for long-term Pb remediation. However, the different IPs can affect the secretion of organic acid by PSF. For example, calcium phosphate (Ca-P) can stimulate A. niger to secrete more oxalic acid (Tian et al., 2021a). In addition, the dissolution of Ca-P is more efficient than Fe-P by A. niger. Therefore, Ca-P is the best choice in Pb remediation by PSF.
The different nutrients can significantly influence the secretion of organic acid by PSF and hence affect Pb remediation by phosphate. In the case of oxalate, the secretion of oxalate by PSF is affected by different environmental factors, such as carbon (C) source, nitrogen (N) source, environmental pH, etc (Palmieri et al., 2019). Nitrogen is a key factor affecting the metabolism of A. niger and the dissolution of phosphate rock (Paulo et al., 1988; Tian et al., 2018). Compare with ammonium and urea, nitrate can significantly increase the secretion of oxalate by A. niger and reduce the Pb concentration in Pb remediation with Ca-P (Feng et al., 2022). For nitrogen, nitrate is the suitable resource in Pb remediation by PSF and phosphate.
PSF and phosphate complex have been used to produce phosphate-based biofertilizers, which not only increase the P content in the soil but also function in Pb remediation (da Silva et al., 2017). The application of PSM biofertilizer can significantly increase crop yield and soil available P content, reducing the 50% phosphate fertilizer input (Fitriatin et al., 2017). Phosphate rock combined with PSF (P. oxalicum) can replace chemical fertilizers, and increase crop yield. In addition, the application of PSM biofertilizer and phosphate can also reduce the Pb concentrations in soil. For example, the combination of phosphogypsum (PG) and biofertilizer (containing A. niger) can reduce soil Pb concentration from 365 mg/kg to 302 mg/kg (Meng et al., 2022). PG not only provide a sufficient P source for the growth of A. niger in highly contaminated soils but also strengthens the formation of insoluble Pb minerals. Therefore, adding phosphate and PSF as fertilizer is an effective attempt at long-term Pb remediation.
Nitrogen sources can significantly affect the secretion of organic acids of A. niger, which could affect phosphate dissolution and Pb remediation (Gadd et al., 2014). The decomposition of inputted urea can produce carbon dioxide and form carbonates, which inhibits the growth of A. niger and the secretion of organic acids (Cinthya et al., 2006; Su et al., 2021). Ammonium and nitrates are more efficient in Pb remediation by A. niger and phosphate (Feng et al., 2022). In addition, calcium can stimulate A. niger to secrete more organic acids, hence the calcium-based nitrogen fertilizer is more suitable for Pb remediation by PSF and phosphate (Tian et al., 2021a). In addition, other microorganisms such as Rhodotorula mucilaginosa (Rho) can secrete large amounts of extracellular polymers (EPS) to form EPS-Pb in Pb toxicity resistance (Li et al., 2019). The addition of phosphate can significantly promote the secretion of EPS by Rho (Tian et al., 2022a). The Pb remove ratio in Rho and phosphate reached 99.9% (Tian et al., 2022a). In addition, the polysaccharides and other nutrients contained in EPS can support the growth of PSF. Therefore, EPS can be applied as a synergist in Pb remediation by PSF and phosphate.
In summary, the combination of PSF and phosphate in Pb remediation is an effective way in current research. On the one hand, PSF can secrete oxalic acid to promote the release of P from phosphate, and the released P can react with Pb cations to form highly insoluble pyromorphite. On the other hand, the secreted oxalic acid by PSF can also react with Pb to form insoluble lead oxalate Figure 1. However, this pathway is also limited due to the long-time dissolution of phosphate and the formation of insoluble Pb minerals. Increasing the secretion of oxalic acid by PSF is the key factor in Pb remediation by the combination of phosphate. Hence, the ability of oxalic acid secretion by PSF should be considered in a different environment. In the future, the enhancement of the micro-interface process in Pb remediation by PSF and phosphate should be explored, especially in strengthening the participation of oxalic acid. Improving the production of oxalic acid via the different pathways can promote Pb remediation faster and completely to reduce Pb toxicity. In addition, to obtain the best Pb remediation purpose in the environment, choosing the suitable PSF and phosphate are needed in practical application.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
DT and XZ wrote the manuscript. LW, MH, CZ and XY assisted in the data collection. DT, CZ, and XY conceived the idea, revised the manuscript and led the project. All authors contributed to the article and approved the submitted version.
This work was supported by the Science and Technology Major Project of Anhui Province (202103a06020012), the Program at the Department of Natural Resources of Anhui Province (No. 2021-K-4 and 2021-K-11), the National Natural Science Foundation of China (No. 42007030), the Natural Science Foundation of Anhui Province (No. 2008085QD187) and the Program (No. yj 2019-20) at Anhui Agricultural University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arduini, F., Calvo, J. Q., Palleschi, G., Moscone, D., and Amine, A. (2010). Bismuth-modified electrodes for lead detection. TrAC Trends Anal. Chem. 29, 1295–1304. doi:10.1016/j.trac.2010.08.003
Arnich, N., Lanhers, M. C., Laurensot, F., Podor, R., Montiel, A., and Burnel, D. (2003). In vitro and in vivo studies of lead immobilization by synthetic hydroxyapatite. Environ. Pollut. 124, 139–149. doi:10.1016/s0269-7491(02)00416-5
Cao, X., Wahbi, A., Ma, L., Li, B., and Yang, Y. (2009). Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 164, 555–564. doi:10.1016/j.jhazmat.2008.08.034
Chaturvedi, P. K., Seth, C. S., and Misra, V. (2007). Selectivity sequences and sorption capacities of phosphatic clay and humus rich soil towards the heavy metals present in zinc mine tailing. J. Hazard. Mater. 147, 698–705. doi:10.1016/j.jhazmat.2007.01.064
Chen, S. B., Zhu, Y. G., Ma, Y. B., and McKay, G. (2006). Effect of bone char application on Pb bioavailability in a Pb-contaminated soil. Environ. Pollut. 139, 433–439. doi:10.1016/j.envpol.2005.06.007
Chen, H. M., Min, F. F., Hu, X., Ma, D. H., and Huo, Z. (2023). Biochar assists phosphate solubilizing bacteria to resist combined Pb and Cd stress by promoting acid secretion and extracellular electron transfer 452, 131176.
Cheyns, K., Peeters, S., Delcourt, D., and Smolders, E. (2012). Lead phytotoxicity in soils and nutrient solutions is related to lead induced phosphorus deficiency. Environ. Pollut. 164, 242–247. doi:10.1016/j.envpol.2012.01.027
Cinthya, B. B., Gener, T. P., and Ely, N. (2006). Solubilization of CaHPO4 and AlPO4 by Aspergillus Niger in culture media with different carbon and nitrogen sources. Braz. J. Microbiol. 37, 434–438. doi:10.1590/S1517-83822006000400006
Cui, Y. S., Du, X., Weng, L. P., and Van Riemsdijk, W. H. (2010). Assessment of in situ immobilization of lead (Pb) and arsenic (as) in contaminated soils with phosphate and iron: Solubility and bioaccessibility. Water Air Soil Pollut. 213, 95–104. doi:10.1007/s11270-010-0370-8
da Silva, V. N., de Souza Fernandes da Silva, L. E., da Silva, A. J. N., Stamford, N. P., and de Macedo, G. R. (2017). Solubility curve of rock powder inoculated with microorganisms in the production of biofertilizers. Agric. Nat. Resour. 51, 142–147. doi:10.1016/j.anres.2017.01.001
Feng, Y., Zhang, L. L., Li, X., Wang, L. Y., Yusef, K. K., Gao, H. J., et al. (2022). Remediation of lead contamination by Aspergillus Niger and phosphate rocks under different nitrogen sources. Agronomy-Basel 12, 1639. doi:10.3390/agronomy12071639
Fitriatin, B. N., Suryatmana, P., Yuniarti, A., and Istifadah, N. (2017). The application of phosphate solubilizing microbes biofertilizer to increase soil P and yield of maize on Ultisols Jatinangor. KnE Life Sci. 2, 179. doi:10.18502/kls.v2i6.1037
Gadd, G. M., Bahri-Esfahani, J., Li, Q. W., Rhee, Y. J., Wei, Z., Fomina, M., et al. (2014). Oxalate production by fungi: Significance in geomycology, biodeterioration and bioremediation. Fungal Biol. Rev. 28, 36–55. doi:10.1016/j.fbr.2014.05.001
Hu, J., Wang, L. Y., Zhang, L. L., Gao, H. J., and Tian, D. (2022). A study of phosphate solubilizing capacity by penicillium aurantiogriseum under different carbon and nitrogen resources. E3S Web Conf. 350, 03002. doi:10.1051/e3sconf/202235003002
John, Y., David, E. M., Stan, W. C., and Robert, W. B. (2001). Lead immobilization using phosphoric acid in a smelter-contaminated urban soil. Environ. Sci. Technol. 35, 3553–3559. doi:10.1021/es001770d
Li, J., Jiang, Z., Chen, S., Wang, T., Jiang, L., Wang, M., et al. (2019). Biochemical changes of polysaccharides and proteins within EPS under Pb(II) stress in Rhodotorula mucilaginosa. Ecotoxicol. Environ. Saf. 174, 484–490. doi:10.1016/j.ecoenv.2019.03.004
Li, Z., Bai, T., Dai, L., Wang, F., Tao, J., Meng, S., et al. (2016a). A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 6, 25313. doi:10.1038/srep25313
Li, Z., Wang, F., Bai, T., Tao, J., Guo, J., Yang, M., et al. (2016b). Lead immobilization by geological fluorapatite and fungus Aspergillus Niger. J. Hazard. Mater. 320, 386–392. doi:10.1016/j.jhazmat.2016.08.051
Melamed, R., Cao, X., Chen, M., and Ma, L. Q. (2003). Field assessment of lead immobilization in a contaminated soil after phosphate application. Sci. Total Environ. 305, 117–127. doi:10.1016/S0048-9697(02)00469-2
Mendes, G. D., Murta, H. M., Valadares, R. V., da Silveira, W. B., da Silva, I. R., and Costa, M. D. (2020). Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization. Miner. Eng. 155, 106458. doi:10.1016/j.mineng.2020.106458
Menezes-Blackburn, D., Paredes, C., Zhang, H., Giles, C. D., Darch, T., Stutter, M., et al. (2016). Organic acids regulation of chemical-microbial phosphorus transformations in soils. Environ. Sci. Technol. 50, 11521–11531. doi:10.1021/acs.est.6b03017
Meng, L., Pan, S., Zhou, L., Santasup, C., Su, M., Tian, D., et al. (2022). Evaluating the survival of Aspergillus Niger in a highly polluted red soil with addition of Phosphogypsum and bioorganic fertilizer. Environ. Sci. Pollut. Res. 29, 76446–76455. doi:10.1007/s11356-022-21243-5
Palmieri, F., Estoppey, A., House, G. L., Lohberger, A., Bindschedler, S., Chain, P. S. G., et al. (2019). Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. Adv. Appl. Microbiol. 106, 49–77. doi:10.1016/bs.aambs.2018.10.001
Park, J. H., and Bolan, N. (2013). Lead immobilization and bioavailability in microbial and root interface. J. Hazard. Mater. 261, 777–783. doi:10.1016/j.jhazmat.2013.02.010
Paulo, C. C., Ely, N., and David, A. B. (1988). Soluble phosphate accumulation by Aspergillus Niger from fluorapatite. Appl. Microbiol. Biotechnol. 29, 501–505. doi:10.1007/BF00269076
Shao, X., Hao, W., Konhauser, K. O., Gao, Y., Tang, L., Su, M., et al. (2021). The dissolution of fluorapatite by phosphate-solubilizing fungi: A balance between enhanced phosphorous supply and fluorine toxicity. Environ. Sci. Pollut. Res. 28, 69393–69400. doi:10.1007/s11356-021-15551-5
Shen, Z., Jin, F., Wang, F., McMillan, O., and Al-Tabbaa, A. (2015). Sorption of lead by Salisbury biochar produced from British broadleaf hardwood. Bioresour. Technol. 193, 553–556. doi:10.1016/j.biortech.2015.06.111
Singh, H., and Reddy, M. S. (2011). Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur. J. Soil Biol. 47, 30–34. doi:10.1016/j.ejsobi.2010.10.005
Su, M., Meng, L. Z., Zhao, L., Tang, Y. K., Qiu, J. J., Tian, D., et al. (2021). Phosphorus deficiency in soils with red color: Insights from the interactions between minerals and microorganisms. Geoderma 404, 115311. doi:10.1016/j.geoderma.2021.115311
Tian, D., Cheng, X. H., Wang, L. Y., Hu, J., Zhou, N. N., Xia, J. J., et al. (2022a). Remediation of lead-contaminated water by red yeast and different types of phosphate. Front. Bioeng. Biotechnol. 10, 775058. doi:10.3389/fbioe.2022.775058
Tian, D., Jiang, Z., Jiang, L., Su, M., Feng, Z., Zhang, L., et al. (2019). A new insight into lead (II) tolerance of environmental fungi based on a study of Aspergillus Niger and Penicillium oxalicum. Environ. Microbiol. 21, 471–479. doi:10.1111/1462-2920.14478
Tian, D., Su, M., Zou, X., Zhang, L. L., Tang, L. Y., Geng, Y. Y., et al. (2021b). Influences of phosphate addition on fungal weathering of carbonate in the red soil from karst region. Sci. Total Environ. 755, 142570. doi:10.1016/j.scitotenv.2020.142570
Tian, D., Wang, L. Y., Hu, J., Zhang, L. L., Zhou, N. N., Xia, J. J., et al. (2021a). A study of P release from Fe-P and Ca-P via the organic acids secreted by Aspergillus Niger. J. Microbiol. 59, 819–826. doi:10.1007/s12275-021-1178-5
Tian, D., Wang, W., Su, M., Zheng, J., Wu, Y., Wang, S., et al. (2018). Remediation of lead-contaminated water by geological fluorapatite and fungus Penicillium oxalicum. Environ. Sci. Pollut. Res. 25, 21118–21126. doi:10.1007/s11356-018-2243-4
Tian, D., Xia, J. J., Zhou, N. N., Xu, M. Y., Li, X., Zhang, L. L., et al. (2022b). The utilization of phosphogypsum as a sustainable phosphate-based fertilizer by Aspergillus Niger. Agronomy-Basel 12, 646. doi:10.3390/agronomy12030646
Wang, L. Y., GuanHu, H. J., Feng, Y., Li, X., Yusef, K. K., Gao, H. J., et al. (2022). Aspergillus Niger enhances organic and inorganic phosphorus release from wheat straw by secretion of degrading enzymes and oxalic acid. J. Agric. Food Chem. 70, 10738–10746. doi:10.1021/acs.jafc.2c03063
Whitelaw, M. A. (1999). Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv. Agron. 69, 99–151. doi:10.1016/S0065-2113(08)60948-7
Keywords: lead remediation, apatite, phosphate solubilizing fungi, organic acid, phosphorus release
Citation: Tian D, Zhang X, Wang L, Han M, Zhang C and Ye X (2023) Lead remediation is promoted by phosphate-solubilizing fungi and apatite via the enhanced production of organic acid. Front. Bioeng. Biotechnol. 11:1180431. doi: 10.3389/fbioe.2023.1180431
Received: 06 March 2023; Accepted: 21 March 2023;
Published: 30 March 2023.
Edited by:
Haoming Chen, Nanjing University of Science and Technology, ChinaReviewed by:
Li Mingyu, Yanbian University, ChinaCopyright © 2023 Tian, Zhang, Wang, Han, Zhang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaochun Zhang, emhhbmdjY0BjYXUuZWR1LmNu; Xinxin Ye, eWV4eEBhaGF1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.