- 1UWA School of Agriculture and Environment, The University of Western Australia, Perth, WA, Australia
- 2UWA School of Human Sciences, The University of Western Australia, Perth, WA, Australia
The isoflavonoid derivatives, pterocarpans and coumestans, are explored for multiple clinical applications as osteo-regenerative, neuroprotective and anti-cancer agents. The use of plant-based systems to produce isoflavonoid derivatives is limited due to cost, scalability, and sustainability constraints. Microbial cell factories overcome these limitations in which model organisms such as Saccharomyces cerevisiae offer an efficient platform to produce isoflavonoids. Bioprospecting microbes and enzymes can provide an array of tools to enhance the production of these molecules. Other microbes that naturally produce isoflavonoids present a novel alternative as production chassis and as a source of novel enzymes. Enzyme bioprospecting allows the complete identification of the pterocarpans and coumestans biosynthetic pathway, and the selection of the best enzymes based on activity and docking parameters. These enzymes consolidate an improved biosynthetic pathway for microbial-based production systems. In this review, we report the state-of-the-art for the production of key pterocarpans and coumestans, describing the enzymes already identified and the current gaps. We report available databases and tools for microbial bioprospecting to select the best production chassis. We propose the use of a holistic and multidisciplinary bioprospecting approach as the first step to identify the biosynthetic gaps, select the best microbial chassis, and increase productivity. We propose the use of microalgal species as microbial cell factories to produce pterocarpans and coumestans. The application of bioprospecting tools provides an exciting field to produce plant compounds such as isoflavonoid derivatives, efficiently and sustainably.
1 Introduction
Bioprospecting enables researchers to explore biodiversity, and identify novel molecules, enzymes and microbes relevant to research and industrial applications (Beattie et al., 2011; AfifaHussain et al., 2022). Bioprospecting strategies can be sub-divided into microorganisms (metagenomics), enzymes (transcriptomics/proteomics), or individual molecules (metabolomics). Microbial bioprospecting explores vastly diverse microorganisms, which constitute more than two-third of global life forms, present in a diverse range of environments including extreme ones (Becker and Wittmann, 2020). Metagenomics tools provide information from even non-culturable microbes, being a rich source of novel metabolic pathways, enzymes and their related catalyzed products. The combination of omics-based technologies allows researchers to link genes with enzymatic pathways and metabolite biosynthesis, fuelling the discovery of novel molecules for diverse applications (Bansal et al., 2022). To understand how these molecules are produced, the whole enzymatic pathway needs to be identified, including the genes involved in the synthesis and its regulatory framework. In silico enzyme bioprospecting allows the identification of novel homologous sequences, and the possibility to model and trial enzymatic activities. After this in silico approach, heterologous biosynthetic pathways can be incorporated into model microorganisms using a combination of the most efficient enzymes through synthetic biology tools. Optimized microbial chassis are proven to increase yield and reduce the production’s environmental impact towards a circular bioeconomy model (Krüger et al., 2020). Bioprospecting strategies for enzymes and chassis offer an unprecedented number of novel biocatalysts for a more efficient and sustainable production.
The use of microbes such as Saccharomyces cerevisiae and Escherichia coli is one possible strategy to overcome the limitations of plant-based production systems. As the isoflavonoids derivatives pterocarpans and coumestans are mainly produced in plant species, microbes provide an interesting alternative for boosting production titer, sustainably. Additionally, bioprospecting microbes that naturally produce isoflavonoids allow the diversification of production chassis, moving away from model organisms and allowing the implementation of even more sustainable solutions.
Primary metabolites such as amino acids, carbohydrates and lipids are essential for the growth and development of living organism. Secondary metabolites are typically modified chemical derivatives of these metabolites; such modifications include methylation, glycosylation, and hydroxylation (Twaij and Hasan, 2022). Though not essential, secondary metabolites have significant influence in the survivability of organisms and, in the case of plants, are quite often vital for plant-biome interactions.
The secondary metabolites, pterocarpans and coumestans, have clinical potential in both plants and humans. These isoflavonoid derivatives are associated with antimicrobial and antifungal properties (considered a phytoalexin), and its action is explored to deal with plant diseases such as fungal infections (Gupta et al., 2022). Furthermore, they have shown some promise for the treatment diseases of Alzheimer’s disease, osteoporosis, and cancer (Dixit et al., 2015; Li et al., 2021; Tu et al., 2021). Thus, isoflavonoid derivatives merit further exploration with respect to not only its clinical effects but also its method of production.
This review summarizes research efforts to identify the biosynthetic pathways and microbial-based production systems for these plant secondary metabolites, identifying knowledge gaps and providing insights into unexplored production systems. The scope for this review is limited to the most cited coumestans and pterocarpan molecules: medicarpin, pisatin, maackiain, glyceollins, coumestrol, wedelolactone, psoralidin, and glycyrol. The main purpose of this review is to consolidate the link between the current knowledge of pterocarpans and coumestans biosynthesis (including gaps and limitations) and bioprospecting strategies to identify enzymes (address gaps) and novel production systems. We also discuss microbial and enzyme bioprospecting solutions to enhance the sustainable production of pterocarpans and coumestans using microbial-based production systems.
2 Isoflavonoids: A key family of phenolics
Phenolics are vastly diverse molecules distributed within several plant species (Tohge et al., 2013). They contain an aromatic ring and at least one hydroxyl group, and are derivatives of the aromatic amino acids, phenylalanine and tyrosine, through the shikimate or acetate pathways (Bravo, 1998). They are related to pigmentation and astringency, but also have diverse roles in plant defense against pathogens (Durazzo et al., 2019). The phenolics biosynthetic pathway starts from the amino acids phenylalanine and tyrosine, from which the enzymes phenylalanine ammonia-lyase (PAL) and tyrosine ammonia-lyase (TAL) generate cinnamate. Subsequently, a subfamily of 4-coumarate ligases produces different CoA-esters, including p-Coumaroyl-CoA, a key member of polyphenols biosynthetic pathways (Vogt, 2010). The phenolic family comprises phenolic acids, tannins, stilbenes, lignans, and flavonoids. Multiple applications are investigated for all these groups from the pharmaceutical, food, and cosmetic industries (Albuquerque et al., 2021).
Flavonoids are the largest family of polyphenols, ubiquitous in land plants and some algal species, potentially sharing a pathway from a common ancestor (Yonekura-Sakakibara et al., 2019). Epidemiological and meta-analysis studies show flavonoids exert positive human health effects with antioxidant, hepatoprotective, antibacterial, anti-inflammatory, antiviral, and anti-cancer properties (Kumar and Pandey, 2013; Amawi et al., 2017; Jiang et al., 2019). The substitution of chemical groups in the flavonoid backbone is related to its biological and chemical properties (Teng and Chen, 2019). Further chemical modifications such as O- and C-methylation and O- and C-glycosylation alter pharmacokinetic variables such as solubility and chemical stability, shifting the molecule’s bioavailability (Sajid et al., 2021a). Seven subclasses are categorized according to the degree of oxidation: flavanones, flavonols, flavones, flavanols, chalcones, anthocyanidins, and isoflavonoids.
Isoflavonoids main structure is composed of three rings, two benzene rings (A and B) linked by a pyran ring (C). Isoflavonoids is the only subclass of flavonoids with the B ring in position 3 instead of position 2 (Figure 1). More than 2,400 isoflavonoids are reported, of which isoflavones are the most extensively cited group, predominantly found in legume species such as soy, alfalfa and chickpea (Dixon, 1999). During biosynthesis of isoflavonoids, p-Coumaroyl-CoA is converted to a chalcone product by chalcone synthase (CHS), and is then catalyzed into the precursors naringenin and liquiritigenin by the chalcone isomerase (CHI). From these precursors, the isoflavonoids, genistein and daidzein, are generated due to the action of the isoflavone synthase (IFS) (Nabavi et al., 2020). Genistein and daidzein, along with the methylated biochanin A and formononetin, are widely described with multiple osteogenic, anticancer and antioxidant properties (Brodowska, 2017; Sajid et al., 2021b). A large range of isoflavonoid derivatives is produced from these molecules, including pterocarpans and coumestans.
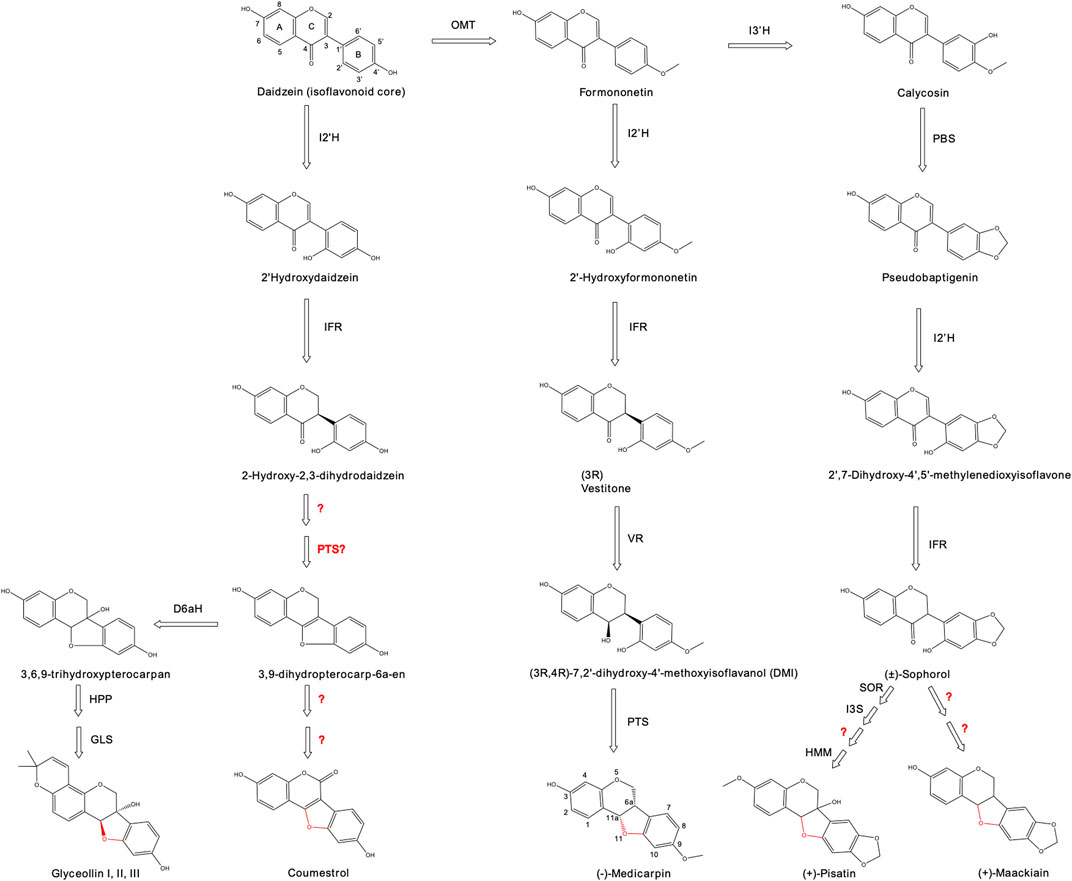
FIGURE 1. Biosynthetic pathway for the production of coumestans and pterocarpans from the precursor, daidzein. Numbers for identifying carbon positions are shown for daidzein (isoflavonoid core) and medicarpin (carbon numbering varies with the formation of the fourth ring). The formation of a fourth ring between carbons 6 and 11 is highlighted in red. Abbreviations: OMT = isoflavanone 4′-O-methyltransferase; I3′H = isoflavone 3′-hydroxylase; I2′H = isoflavone 2′-hydroxylase; IFR = isoflavone reductase; PBS = pseudobaptigenin synthase; VR = Vestitone reductase; PTS = pterocarpan synthase; SOR = sophorol reductase; I3S = isoflav-3-ene synthase; HMM = (+)-6a-hydroxymaackiain 3-O-methyltransferase; DHPH = 3,9-dihydroxypterocarpan 6a-hydroxylase; GLS = glyceollin synthase; HPP = trihydroxypterocarpan prenyltransferase; D6aH = 3,9-dihydroxypterocarpan 6a-hydroxylase. Question marks indicates unknown enzymatic reactions.
3 Pterocarpans and coumestans: Multipurpose molecules with incomplete biosynthetic pathways
Pterocarpans and coumestans are derivatives from the isoflavonoid daidzein. They share the differential feature of a formation of a fourth ring between C and B rings, presenting two asymmetric carbons at positions C-6a and C-11a. The chemical structure of relevant pterocarpans and coumestans is reported in Figure 1.
3.1 Production of pterocarpans
Pterocarpans are an extensive group of more than 400 isoflavonoid derivatives with described beneficial human health effects (Jiménez-González et al., 2008; Goel et al., 2013). There are two possible configurations, levorotatory (−) pterocarpans (configuration 6aR, 11aR) and dextrorotatory (+) pterocarpans with the opposite configuration. Within this classification, multiple legume species produce (−) pterocarpans, while just a few plant species, such as peanut (Arachis hypogaea), produce (+) pterocarpans (Strange et al., 1985).
Medicarpin is a pterocarpan widely cited due to its diverse range of applications. It is primarily found in legume species, being the main isoflavone in alfalfa (Medicago sativa L.) (Du et al., 2010). Medicarpin chemical structure is composed of a pterocarpan core with a hydroxyl group on position C-3 and a methyl group on position C-9. Besides its function as a natural antioxidant, medicarpin is a promising neuroprotective agent for Alzheimer’s disease, and also as a bone regeneration agent to treat osteoporosis (Dixit et al., 2015; Li et al., 2021; Kim et al., 2022). Medicarpin also has potential uses in the agricultural sector. As it is a phytoalexin with antimicrobial effects, medicarpin confers powdery mildew resistance to alfalfa species (Gupta et al., 2022). The first enzyme involved in the biosynthesis of medicarpin is the isoflavanone 4′-O-methyltransferase (OMT). It is a single-step reaction that incorporates a methyl group on the C- 4′ (ring B) of the precursor daidzein, resulting in formononetin as the product. The next step is the formation of 2′-hydroxyformononetin by the enzyme isoflavone 2′ hydroxylase (I2′H), which performs an oxidation at the C-6′ position (ring B). This enzyme belongs to the cytochrome P450 superfamily (class CYP81E1/E7) and produces a set of 2′ hydroxyflavones using different acceptor molecules. It was identified in chickpea, alfalfa and licorice species (Hinderer et al., 1987; Akashi et al., 1998; Liu et al., 2003). The following step in medicarpin synthesis involves the enzyme isoflavone reductase (IFR) that reduces 2′-hydroxyformononetin to the (3R)-2′-hydroxyisoflavanone, reported also as (3R)-vestitone (Paiva et al., 1991). This enzyme is particularly interesting because it introduces a chiral centre using an achiral precursor. IFR was first reported in alfalfa, but homologous sequences were identified from several legume species (Cheng et al., 2015). Vestitone reductase (VR) is the next enzymatic step, which reduces the keto group from position C-4 (ring C) creating the alcohol derivative 7, 2′-dihydroxy4′-methoxyisoflavanol (DMI). VR was first described in M. sativa and lately in the pea Pisum sativum (Guo et al., 1994a; DiCenzo and VanEtten, 2006). The final step utilizes the enzyme pterocarpan synthase (PTS) to create the characteristic fourth ring between positions C-6a and C-11a, using the two hydroxyl residues as substrates (Fischer et al., 1990). This reaction, which produces (−)-medicarpin from DMI, was described in alfalfa (Guo et al., 1994b). PTS is a dirigent, or stereochemistry-altering, enzyme that also catalyzes the formation of (+)-medicarpin, where the configuration of the hydrogen attached to the C-3 position determines which enantiomer (+ or −) will be produced.
Pisatin and maackiain are phytoalexins from the pterocarpans family that present multiple clinical effects. Maackiain was demonstrated as a potent anti-oxidant and anti-inflammatory agent with anti-sepsis, anti-allergies and neuroprotective actions, among others (Mizuguchi et al., 2015; Tsai et al., 2020; Bai et al., 2022). Besides its antimicrobial activities, pisatin has not been extensibly explored for clinical applications. Pisatin was the first (+)-pterocarpan identified, coming from the pea P. sativum, and maackiain was isolated from the Chinese medicinal herb Sophora flavescens (Cruickshank and Perrin, 1960; He et al., 2015). The enzyme isoflavone 3′-hydroxylase (I3′H) adds a hydroxyl group at the position C-3′ of the precursor formononetin, as the first step for both pisatin and maackiain biosynthesis. The metabolite produced, named calycosin, is then converted into pseudobaptigenin by the enzyme pseudobaptigenin synthase (PBS) (Liu et al., 2003). The pathway continues with the already mentioned I2′H and IFR enzymes, which introduces chirality to obtain the precursor compounds (+)-sophorol and (−)-sophorol, of different enantiomers. From there, the biosynthetic steps for the synthesis of maackiain are not fully identified yet, but it is believed that downstream enzymes from (+)-pisatin may be involved (DiCenzo and VanEtten, 2006). The production of (+)-pisatin involves the enzyme sophorol reductase (SOR) that produces (3R and 4R)-7, 2′-dihydroxy-4′5′-methylenedioxyisoflavanol (DiCenzo and VanEtten, 2006). The next step is catalyzed by isoflav-3-ene synthase (I3S) that converts the precursor into 7, 2′-dihydroxy-4′, 5′-methylenedioxyisoflav-3-ene (DMDIF). The subsequent enzymatic reaction is still not identified, but it produces the molecule (+)-6a-hydroxymaackiain using DMDIF as a precursor. The final step requires the action of the enzyme (+)-6a-hydroxymaackiain 3-O-methyltransferase (HMM), which adds a methyl group at the C-3 position to generate the final metabolite, (+)-pisatin (Wu et al., 1997).
Glyceollins are a set of soybean-specific pterocarpans with a described set of human health benefits (Pham T. H. et al., 2019). They have an anti-estrogenic effect that competes with endogenous estrogens, and are being tested as a suppressor of breast and ovarian tumorigenesis (Yamamoto et al., 2018). Glyceollin presents the distinctive feature of a fifth prenylated ring linked to the A ring (isoflavonoid core). The first steps from daidzein involve the already discussed I2′H and IFR enzymes to produce 2-hydroxy-2, 3-dihydrodaidzein. From there the enzyme PTS generates the fourth ring to obtain the molecule 3, 9-dihydroterocarp-6a-en, also reported as glycinol (Fischer et al., 1990). Subsequently, a set of prenyltransferases (PTs) convert glycinol into a whole set of precursors, where the prenylated site determines the glyceollin type (I, II or III) (Sukumaran et al., 2018). The last step is the cyclization of the fifth ring by glyceollin synthase, creating up to six different glyceollins depending on their prenyl position (Welle and Grisebach, 1988).
3.2 Production of coumestans
Coumestans is another important subgroup of isoflavonoid derivatives. They are usually reported as a separate group, although some authors refer to them as oxidized products of the pterocarpanoid subfamily (Goel et al., 2013). They are characterized by the addition of a keto group at position C-6, and a double bond between positions 6a and 11a. To date, more than 120 coumestans have been reported with potential clinical effects (Tu et al., 2021). The most studied compound within this family is coumestrol, which is present in soy leaves. Intake of coumestrol is associated with reduced risk for breast cancer, skin photoaging protection, and neuroprotection (Hedelin et al., 2008; Castro et al., 2014; Park et al., 2015). Coumestrol biosynthesis starts from daidzein where the previously mentioned I2′H enzyme adds a hydroxyl group to produce of 2′hydroxydaidzein. The next metabolic step is the reduction of the double bond between C-2 and C-3 (ring C) by the action of the IFR enzyme. The product obtained, 2-hydroxy-2, 3-dihydrodaidzein, is then converted into 3, 9-dihydroterocarp-6a-en by at least two unknown catalytic reactions. A combination of the VR enzyme plus the effect of the isoflav-3-ene synthase (I3S) are predicted to generate the isoflav-3-ene precursor for the characteristic fourth ring formation (Uchida et al., 2020). Finally, another two to three reactions are needed to add the keto group to C-2 of the isoflavonoid core, the distinctive feature of the coumestans subgroup. A transcriptomic study identified that up to 14 genes are associated for coumestrol biosynthesis from the precursor daidzein (Ha et al., 2019). The full metabolic pathway for coumestrol remains incomplete despite efforts to identify the genes involved on its synthesis.
Apart from coumestrol, other relevant coumestans with potential applications for human health are wedelolactone, psoralidin and glycyrol, although their biosynethic pathways are incomplete. Wedelolactone, first reported in the plant Wedelia calendulacea, is a coumestan skeleton with hydroxyl groups at positions 1, 8, and 9; and a methoxyl group at position 3. This metabolite has been studied for multiple clinical applications, such as anti-inflammation, anti-oxidative, inhibits breast cancer, and neuroprotective against Parkinsonism (Hsieh et al., 2015; Zhu et al., 2019; Sharma et al., 2021). Psoralidin, a coumestan first isolated from the legume Psoralea corylifolia, has the chemical characteristic of two hydroxyl groups at positions 3 and 9 and a prenyl group at position 2. Multiple pre-clinical studies have demonstrated its anticancer, antiosteoporotic, anti-inflammatory, anti-vitiligo, antibacterial, antiviral, and antidepressant-like effects (Sharifi-Rad et al., 2020). Lastly, glycyrol, a coumestan isolated from the Leguminosae species Glycyrrhiza sp., presents a distinctive feature of an O-methylation at position C-1 and a prenylation at position C-2. Glycyrol demonstrates multiple clinical uses as anti-cancer, anti-inflammatory, hepatoprotective, antimicrobial, and anti-viral agents (Tu et al., 2021). Overall, several pterocarpans and coumestans are described with clinical effects; their metabolic pathway is fully reported for some compounds (medicarpin, glyceollin), and is incompletely understood for others (coumestrol, maackiain). The identification of the missing enzymes, as well as the identification of the best production systems, are the first steps for boosting the biosynthesis of pterocarpans and coumestans.
4 Bioprospecting strategies
4.1 Exploring biodiversity
Microbial bioprospecting is a new terminology, but humans have used microbes for their benefit for many years, from the implementation of yeast for bread-making to the discovery of new molecules to treat diseases. The recent emergence of metagenomics analysis has unleashed the potential of microbial bioprospecting. It is estimated that there are more than 1 × 1016 microbes in just 1 ton of soil, of which 85%–99% are unculturable (Curtis and Sloan, 2005; Lok, 2015). Analysis of this massive data set led to the discovery of several new strains for both human (therapeutic, food production) and environmental (sustainable industries, bioremediation) applications.
Different approaches for microbial in silico bioprospecting are being already applied using bioinformatic resources. The two main approaches for the identification of microbial diversity are mining data from publicly available databases, or using raw data from different sampling sites following metagenomic pipelines (Vuong et al., 2022b). Within the available datasets, online trustworthy repositories are associated with the International Nucleotide Sequence Database: the DNA Databank of Japan (DDBJ; http://www.ddbj.nig.ac.jp/), the European Molecular Biology Laboratory’s European Bioinformatics Institute (EMBL-EBI; http://www.ebi.ac.uk/ena/) the National Center for Biotechnology Information (NCBI; https://www.ncbi.nlm.nih.gov/), and the Joint Genome Institute (JGI; https://img.jgi.doe.gov). For environmental metagenomic data, global sequencing initiatives such as Tara Oceans (http://oceanmicrobiome.embl.de/), the Earth Microbiome Project (https://earthmicrobiome.org/), and the Malaspina Gene Database are excellent sources of publicly available information (Acinas et al., 2021). Multiple tools are available for exploring metagenomic data to identify protein sequences and rebuild metabolic pathways. Bioinformatic tools such as QIIME (http://qiime.org/) allow the analysis of metagenomic raw data, and once individual genomes are segregated, tools such as Prodigal (https://github.com/hyattpd/Prodigal) predict genes and link them with metabolic pathways.
Different environments have been explored for novel microbes and metabolic pathways following a metagenomic approach (Quince et al., 2017). Marine samples are a rich source of new microbes, that are bio-prospected as a source of novel genes (Paoli et al., 2022). Extreme environments from polar to volcanic regions, provide a source of extremophiles that are investigated for human health applications using a multi-omics approach (Hedlund et al., 2014). Additionally, not only natural environments are considered a rich source of microorganisms. For instance, industrial effluents are targeted for microbes that degrade lipids, a key activity for biotechnological applications (Peil et al., 2016). The enzyme lipase is then recovered using liquid biphasic flotation, efficiently and sustainably (Sankaran et al., 2018). Overall, microbial bioprospecting generates a rich source of microorganisms for a diverse range of applications, including isoflavonoids production. The following section gives an example of how microalgal species can be targeted to produce isoflavonoid derivatives.
4.2 Employing microalgae
Microbes are an abundant source of new nutraceutical and pharmaceutical products. As phototrophic microbes (prokaryotic cyanobacteria and eukaryotic microalgae) share biosynthetic pathways with plants, scientists are exploring these organisms as chassis to produce plant metabolites. Microalgae are photosynthetic unicellular or colonial microorganisms with the ability to grow in different soil and underwater environments. Phototrophic microbes are divided into two prokaryotic divisions: Cyanophyta and Prochlorophyta, and nine eukaryotic divisions: Glaucophyta, Rhodophyta, Heterokontophyta, Haptophyta, Cryptophyta, Dinophyta, Euglenophyta, Chlorarachniophyta and Chlorophyta (Hemaiswarya et al., 2013). Decades ago, the species Dunaeliella salina was successfully implemented as a novel sustainable production system for β-carotene as it naturally produces up to 10% of its dry weight as β-carotene (Harvey and Ben-Amotz, 2020). Since then, microalgal species are bio-prospected for farming (aquaculture), biomanufacturing (nutraceutical, pharmaceutical, functional foods, biofuels), and environmental (bioremediation) applications (Choong et al., 2016; Mobin and Alam, 2017).
Using microalgal species as a sustainable production chassis to produce plant metabolites present several benefits. As a 3rd generation biorefinery, microalgae utilize renewable energy (sunlight) and CO2 and hence microalgae are a key global contributor to CO2 sequestration (Liu Z. et al., 2020; Prasad et al., 2021). Some microalgal species also have the potential to use wastewater as a source of nutrients, adding another positive impact on the environment as a remediation tool (Goswami et al., 2022). Additionally, similar to the yeast S. cerevisiae, some microalgal species are Generally Recognized As Safe (GRAS), so they can be consumed as food (Villarruel-Lopez et al., 2017; Wells et al., 2017; Caporgno and Mathys, 2018; Khemiri et al., 2020). Even though, conducting a food safety risk assessment is imperative to ensure a high-quality product for human consumption (Wu et al., 2022).
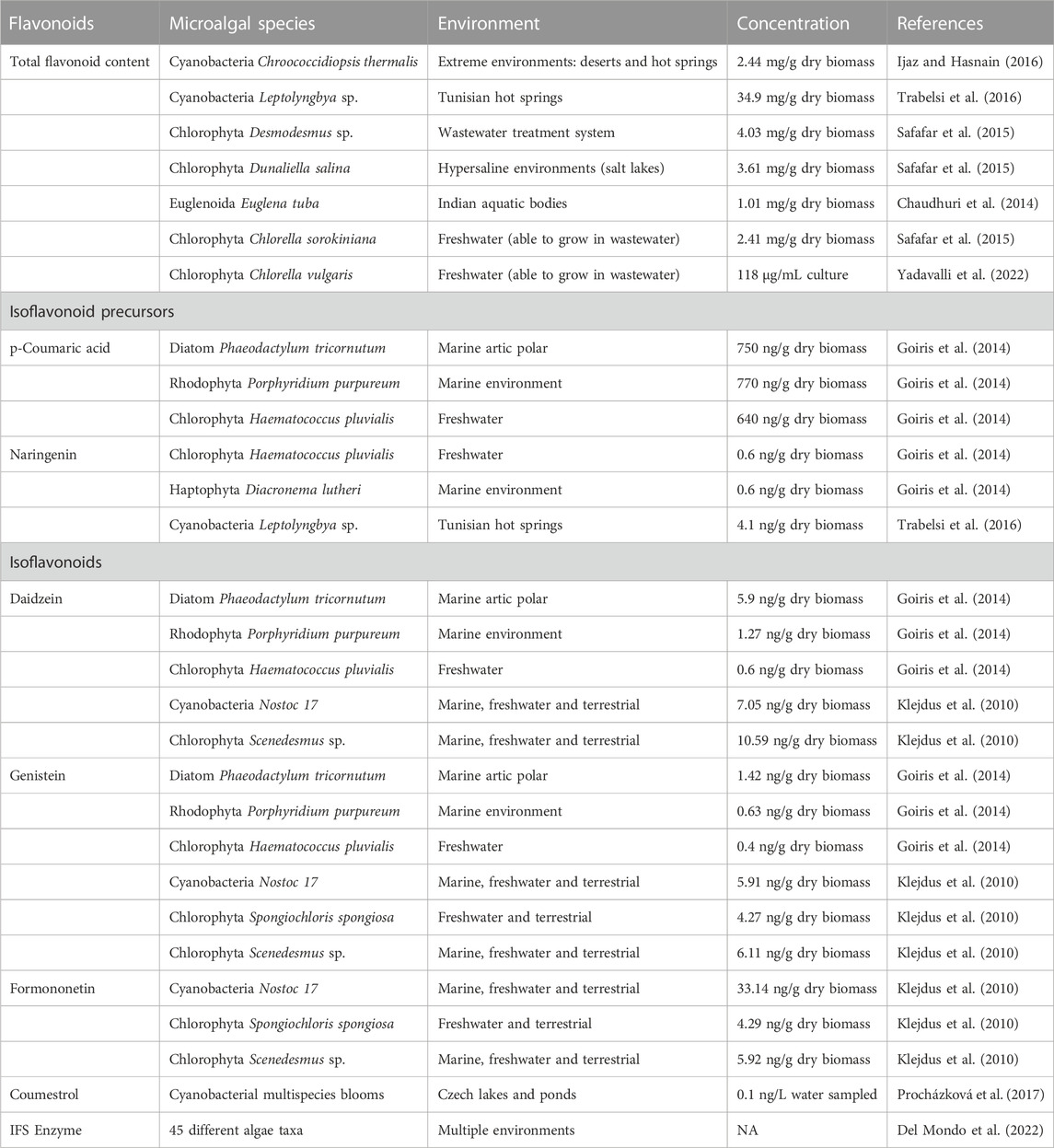
Isoflavonoids derivatives and their associated enzymes were recently identified in algal species (Del Mondo et al., 2022). A summary of these findings is represented in Table 1. Microalgal species share similar metabolic pathways with plants probably due to a primordial ancestor, creating an exciting avenue as isoflavonoid sustainable producers (Klejdus et al., 2010; Del Mondo et al., 2021). The isoflavonoids genistein and daidzein were found in the prokaryotic Cyanobacteria Nostoc, as well as in eukaryotes divisions such as Chlorophyta and Rhodophyta (Goiris et al., 2014; Del Mondo et al., 2021; Ferdous and Balia Yusof, 2021). Massive microalgal blooms may have a negative impact on the environment and human health, but those species can be explored in confined laboratory conditions to source valuable genes, enzymes, and molecules. The IFS, a key protein for the biosynthesis of isoflavonoids have been found in 45 of the 47 algal taxa analyzed according to sequence alignments (BLASTp) (Del Mondo et al., 2022). The IFS plus other 28 phenylpropanoid core enzymes were screened using a multistep in silico analysis, that demonstrates the presence of isoflavonoid-related genes across microalgal databases (Del Mondo et al., 2022).
Besides the identification of microalgal species that can naturally produce isoflavonoids, strategies should be followed to boost metabolite levels to generate a commercially viable solution. In recent years, engineering tools have been developed for some phototrophic microorganisms such as cyanobacteria, chlorophytes, diatoms, and eustigmatophytes. For these organisms, culture conditions and genetic edition tools were optimized (Vavitsas et al., 2021). Phototrophic unicellular microorganisms have already shown immense potential as sustainable production platforms, and the yet unexplored field of microalgal isoflavonoid production may soon become a reality.
4.3 Optimizing metabolic pathways
Enzymes with enhanced activity is a particularly effective strategy for boosting metabolite production. The approach is slightly different to microbial bioprospecting, where instead of using metagenomic data from the environment, an organism’s protein database (proteomics) is combined with its metabolic expression for discovering and optimizing enzymatic reactions. A diagram representing both microbial and enzyme bioprospecting strategies is shown in Figure 2.
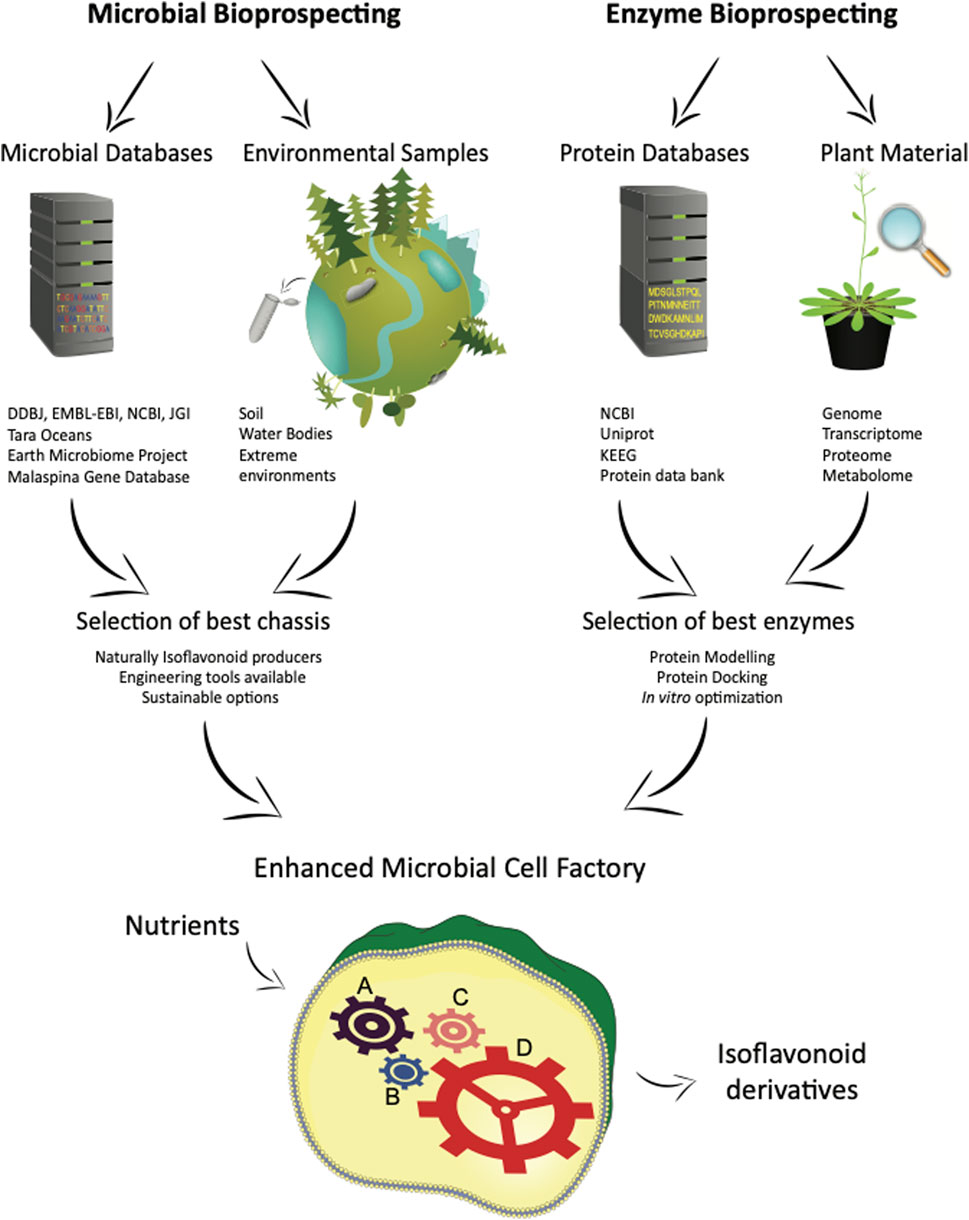
FIGURE 2. Bioprospecting strategies for microbial and enzyme bioprospecting. Microbial bioprospecting allows the identification of best chassis, whereas enzyme bioprospecting identifies the best enzymes for the production of isoflavonoid derivatives using microbial cell factories.
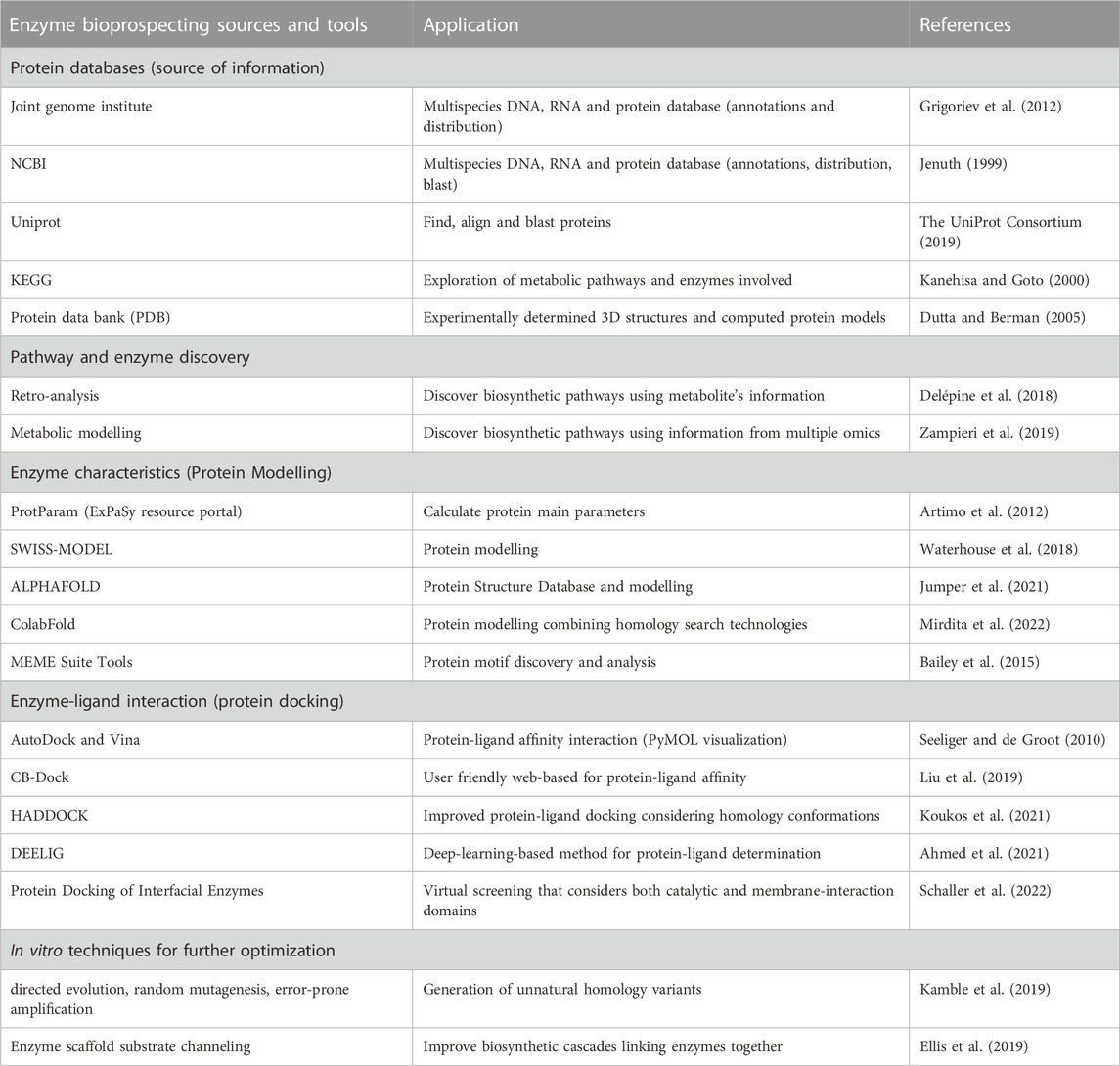
The identification of the state-of-the-art is the first step for the discovery of enzymes and whole metabolic pathways. As multiple steps are required for the discovery of the best enzymes, a summary of enzyme bioprospecting tools divided by stages is detailed in Table 2. To identify biosynthetic gaps, the presence of metabolites and their precursors can provide information about enzymatic reactions. Metabolite detection using High-Performance Liquid Chromatography (HPLC) or Mass Spectrometry (MS) analysis evaluates chemo-structural diversity to build enzymatic pathways (Santana et al., 2021). Nuclear magnetic resonance (NMR) spectroscopy is another approach that allows the identification of potentially bioactive compounds using the whole plant metabolomic set (Augustijn et al., 2021). The biosynthetic pathway of the enzymes involved in the generation of those metabolites can be predicted using retro-analysis tools, linking the precursors with the final product (Delépine et al., 2018). A machine learning approach allows not only to predict one metabolic pathway but the whole organism’s metabolomics, using genomic, transcriptomic and proteomic information altogether (Zampieri et al., 2019). A different approach is followed when the metabolic information is already known. The database KEGG is the most cited source of already fully described metabolic pathways and compound relationships. Multiple public protein databases provide information about proteins, such as Joint Genome Institute (JGI; https://jgi.doe.gov/), Uniprot (https://www.uniprot.org) and Protein Data Bank (PDB; https://www.rcsb.org/). The NCBI database, as well as Uniprot, allow the short-listing of homologous proteins as potential candidates for biosynthetic pathways.
After identifying the target enzyme within a specific biosynthetic pathway, the first step for boosting its activity is to model its 3D structure. Protein modelling is performed using tools such as ProtParam software (https://web.expasy.org/protparam/), SWISS-MODEL (https://swissmodel.expasy.org/) and Alphafold (https://alphafold.ebi.ac.uk/) to predict the protein conformation, kinetic parameters and physicochemical properties (extinction coefficient, estimated half-life, instability index, and aliphatic index). The search is usually based on full protein homology and domain structures, and motif analysis tools such as the MEME Suite Tool can provide an extra layer of enzymatic information (Bailey et al., 2015). Molecular docking is the next step that allows an in silico test of protein-ligand interactions. This virtual screening estimates the ligand position within the modeled protein cavity and the potential amino acids involved in the enzymatic reaction. Several tools are described in the literature for applying this technology to discover novel enzymes with improved activity, considering rigid and flexible dockings (Fan et al., 2019). Within these tools, the popular molecule-viewing software PyMOL is widely used with the docking suite AutoDock and Vina (Seeliger and de Groot, 2010). A rectangular box defines the binding ligand-protein site, from where multiple binding runs are performed between the ligand and the specific amino acids, to determine the binding energies. Cutting-edge techniques like High Ambiguity Driven DOCKing (HADDOCK) incorporate the factor of ligand conformation, adding an additional layer of information in the dynamic landscape of this field (Koukos et al., 2021). There are also user-friendly and web-based tools, such as CB-Dock, that retain the power of protein docking (Autodck Vina) without compromising the quality of the results (Liu Y. et al., 2020). In the last few years, new approaches have been developed using the power of deep learning and convolutional neural networks to “train” the model for the characterization of multiple protein-ligand affinity interactions. As an example, the deep-learning-based approach (DEELIG) uses this method (Ahmed et al., 2021). For interfacial enzymes, the protein motifs (catalytic and membrane binding) are analyzed separately, to build homology models and docking activity data (Schaller et al., 2022). The implementation of all these strategies allows researchers to predict protein-ligand binding affinity and overall enzymatic activity to select the more suitable enzymes for a target reaction.
Presently, in silico enzyme bioprospecting is positively impacting the way where novel enzymes are screened, optimized, and tested, in a cost-effective manner. Nevertheless, an in vitro approach is necessary to confirm the enzyme’s pharmacokinetic and predicted activity. Conventional enzyme engineering tools such as directed evolution, random mutagenesis, and error-prone amplification can add variability to the discovery of novel enzymes (Kamble et al., 2019). Once every enzymatic step is optimized for the best activity, multienzyme scaffolds can also contribute to improving biosynthetic cascades through substrate channeling, linking all the enzymes involved in a concise subcellular space (Ellis et al., 2019). Altogether, in silico and in vitro strategies enhance the biosynthetic pathway efficiency and positively contribute to obtain higher production titers.
5 Approaches for the production of pterocarpans and coumestans
Plant-based systems (homologous biosynthesis) are the main source of coumestans and pterocarpans, but have several limitations. First, plant-based systems are unsustainable due to the consumption of environmental resources (land, water, fertilizers). Second, the target plant biomass usually requires long growing periods to reach the harvest stage. Third, the concentration of coumestans and pterocarpans is low when compared with other isoflavones, and difficult to isolate and purify from the plant tissue. As an example, coumestrol from Glycine max cultivar “Santa rosa” (high coumestrol concentration) showed 1.85 μg/g dry material, while the same cultivar contains 560 μg/g dry material of the precursor daidzein (Mazur et al., 1998). Some of the plant-based production limitations can be solved by plant-tissue culturing. Coumestrol biosynthesis was obtained through adventitious soybean root cultivation, but it is still an unsustainable and cost-ineffective solution (Lee et al., 2022). Another production strategy is chemical synthesis, where the metabolite can be solely synthesized using chemical approaches. This process usually uses hazardous and expensive reagents, as is the case for the full chemical production of medicarpin (Yang et al., 2017). To some extent, a chemical reaction can be used to further modify plant metabolites, adding methyl or glycosyl groups to enhance bioavailability. This has been achieved for flavonoid production, where bioavailability, anti-inflammatory, and anticancer activities were enhanced by chemically adding methyl groups (Wen et al., 2017).
5.1 Evaluating microbial hosts
The application of microbial-based production systems (heterologous biosynthesis), empowered by bioprospecting, may provide an alternative solution for overcoming the limitations previously described by plant-based and chemical approaches. This lab-based production method does not require arable land, is scalable, sustainable, and cost-effective depending on the chassis (microbe) utilized. Granted, there are challenges that the utilization of microbial-based systems for plant natural products still need to overcome. The access distribution to this technology is mainly dominated by the United States, and the patenting of plant biosynthetic pathways may threaten small-acre producers from developing countries (French, 2019). A global regulatory framework is essential to guarantee fair access to this technology, and to protect the original sources of natural products.
The chassis selection plays a central role in the production of plant metabolites. The commonly used model species, Escherichia coli and S. cerevisiae, are largely employed as there are numerous of engineering tools available (Cravens et al., 2019). Between these two organisms, S. cerevisiae is more suitable for plant metabolites as it naturally contains the plant machinery (Cytochrome P450 enzymes and others) required for plant metabolite production. S. cerevisiae is also Generally Recognized As Safe (GRAS), being an excellent candidate to produce food supplements and other nutraceutical derivatives (Singh and Gaur, 2021). Other GRAS yeast species such as Aspergillus sp. and Hansenula polymorpha have been used for the expression of plant products (Pham J. V. et al., 2019). Bioprospecting strategies have already been implemented to identify bacterial host systems compatible for large-scale cultivation. Poorly investigated microorganisms such as the Gram-positive Streptomyces sp., Bacillus sp., and Lactococcus lactis are also promising cell factories for the production of produce plant chemicals. Different microbial production strategies have been explored for the production of isoflavonoids (Chouhan et al., 2017; Sajid et al., 2021b). Table 3 summarizes the current reports for coumestans, pterocaptans precursors and other isoflavonoid derivatives. The microbial-based production of the precursors p-coumaric acid, liquiritigenin and daidzein have already been achieved using both bacterial and yeast species, but the biosynthesis of coumestans and pterocarpans is incompletely described in the literature. In the case of coumestans (coumestrol), a heterologous production is still not feasible as its biosynthetic pathway is not fully described. Similarly, the de novo biosynthesis of medicarpin is complex and difficult to optimize as it requires several enzymatic reactions.
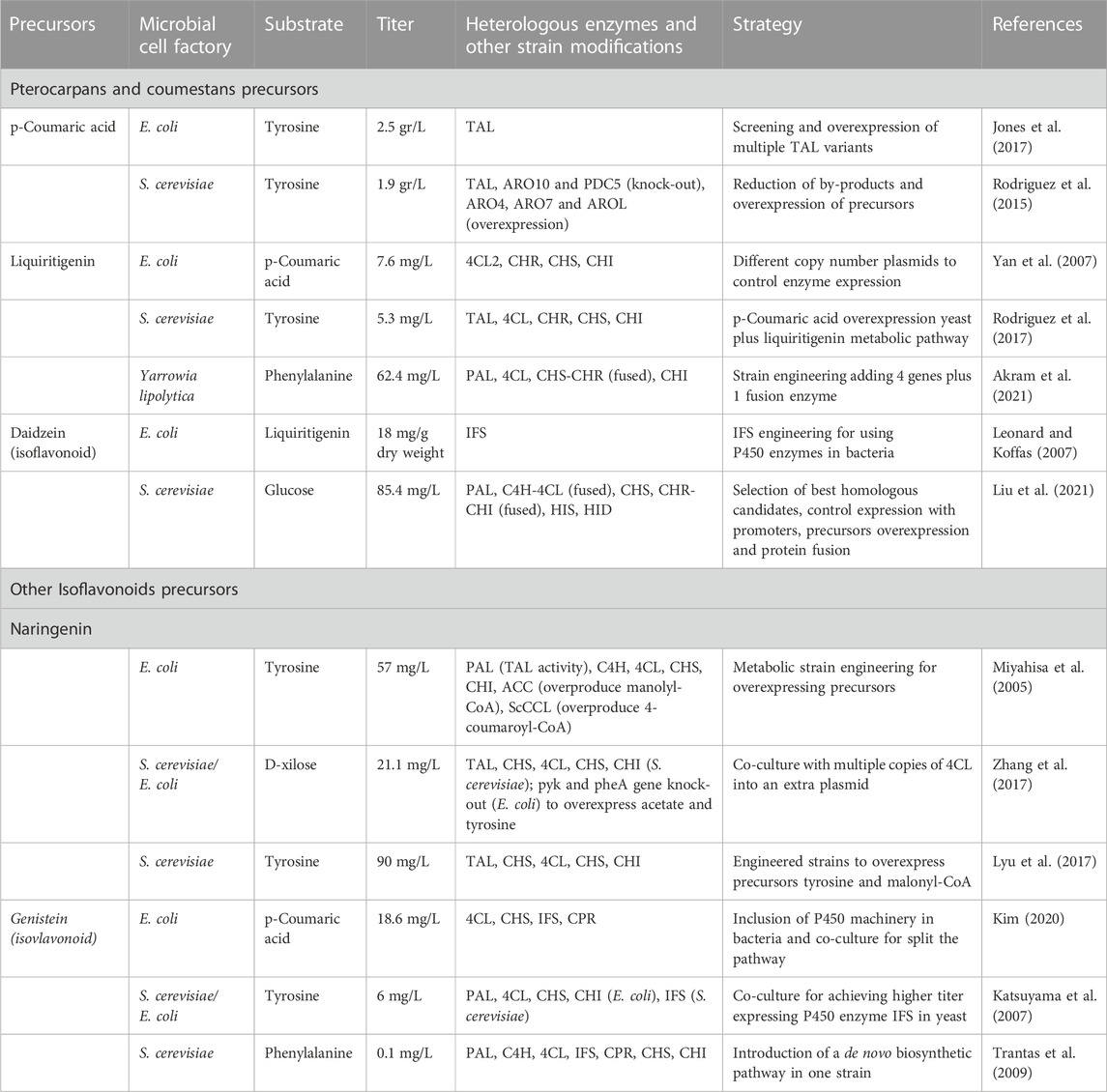
TABLE 3. Successful examples of heterologous biosynthesis of isoflavonoid precursors using different cell factories. Abbreviations: ARO10, phenylpyruvate decarboxylase; PDC5, pyruvate decarboxylase; TAL, tyrosine ammonia-lyase; ARO4, 3-deoxy-D-arabino-heptulosonic acid synthase; ARO7, chorismate mutase; AROL, shikimate kinase II; 4CL, 4-coumaroylCoA ligase; CHS, chalcone synthase; CHI, chalcone isomerase; CHR, chalcone reductase; IFS, isoflavone synthase; HIS, 2-hydroxyisoflavanone synthase; HID, 2-hydroxyisoflavanone dehydratase; PAL, phenylalanine ammonia lyase; C4H, cinnamate 4-hydroxylase; ACC, acetyl-CoA carboxylase; CCL, cinnamate/coumarate-CoA ligase; CPR, cytochrome P450 reductase.
A “divide and conquer” strategy may ease the metabolic burden of incorporating multiple genes in one strain. This co-culture strategy also allows the exploration of branched molecules combining different enzyme cascades. The selected metabolic pathway is separated and integrated into different strains, which can be the same or different species (Chen et al., 2019). As an example, an E. coli bi-culture was used to produce the flavonoid catechin using one strain to obtain naringenin (upstream precursor) while the second strain uses the precursor to generate the target metabolite (Jones et al., 2016). For isoflavonoids, up to 6 mg/L of the metabolite genistein was produced by dividing the pathway between bacterial and yeast species (Katsuyama et al., 2007). The use of polycultures is an efficient strategy for the biosynthesis of complex molecules. For instance, multiple enzymatic reactions were divided into several strains of E. coli to produce anthocyanins from simple sugars (Jones et al., 2017). Also, a symbiotic relationship can be established by forming a consortium where one species provides substrates to the other one and vice versa. This is the case of co-culturing S. cerevisiae with microalgal species, in which the algae produce essential nutrients and the yeast provides extra CO2 (Nguyen et al., 2020; Alam et al., 2022). Overall, the use of bioprospecting strategies for sourcing better enzymes and microbial chassis offers a promising strategy for boosting plant metabolite production.
Apart from the model species, E. coli and S. Cerevisiae, other microbes need be explored as production chassis for the production of pterocarpans and coumastans. The presence of pterocarpans and coumastans in cyanobacterial blooms indicates that the full metabolic pathway is already present in phototrophic bacteria (Procházková et al., 2017). Daidzein, a precursor of pterocarpans and coumastans, was found both in prokaryotic and eukaryotic microalgal species (Klejdus et al., 2010; Goiris et al., 2014). The addition of three to four enzymatic steps will allow the production of pterocarpans and coumastans from those microalgal species. Furthermore, the activation of promoters through nuclease-dead Cas9 systems can help to overexpress key enzymes of the pathway, as was demonstrated in rice plants (Gong et al., 2020). Lastly, the use of co-culture between yeast and microalgal species presents a strategy for enhancing secondary metabolite production, not yet been explored for pterocarpans and coumestans. Scientific advances in recent years provide an excellent set of tools to unleash the production of pterocarpans and coumastans. Filling the enzyme characterization gaps, and selecting the best production chassis would help to meet the escalating demand for these isoflavonoid derivatives.
6 Evaluating enzyme orthologs
Key enzymes for pterocarpans and coumenstans production such as OMT, I2H, IFR, VR, and PTS were originally identified from enzyme characterization studies. The OMT enzyme, key for the conversion of daidzein to formononetin, was first characterized in the legumes Medicago truncatula and Glycyrrhiza echinate (Akashi et al., 2003; Liu et al., 2006; Li et al., 2016). More recently, another enzyme from the species Pueraria lobata (PIOMT9) was identified as an alternative route for formononetin biosynthesis (Li et al., 2016). The membrane-bound CYP450 protein I2′H, key to hydroxylate isoflavones, was cloned and expressed in E. coli to test its ability to act on formononetin. The subtype sequence from Lotus japonicus generated 8.4 mg/L of hydroxyformononetin, while the G. echinate variety failed to produce that compound (Uchida et al., 2015). An ortholog from Astragalus membranaceus was also cloned and purified, but its functional activity was not fully reported (Chen et al., 2015). The IFR enzyme from G. max was purified and characterized to detect its activity in vitro, and overexpressed in soybean seeds to confirm the overproduction of glyceollins (Cheng et al., 2015). The IFR subtype from Medicago sativa was successfully implemented to produce vestitone (Uchida et al., 2017). The VR orthologous sequence from M. sativa was tested using an E. coli chassis, and its activity was tested to produce DMI (Guo and Paiva, 1995). The activity of PTS, a key enzyme for pterocarpan biosynthesis, was characterized for Glycyrrhiza echinata, L. japonicus, and G. max species. From that study, the G. echinate ortholog was identified as the most efficient for medicarpin production (Uchida et al., 2017).
Enzyme characterization using in silico tools is a useful approach for enhancing the heterologous production of pterocarpans and coumenstans. Flavones and flavonol synthases have been bio-prospected using some of the tools previously described. In an interesting approach, researchers curated 44 enzymes using PyMOL and AutoDock Vina for protein docking and based on the instability index and conserved domain data selected the best candidates (Wang et al., 2021). In a recent publication, the IFS, a critical enzyme for the biosynthesis of isoflavonoids, was analyzed by considering its interaction with the molecules, liquiritigenin and naringenin. The use of Alphafold and Swiss-Model for the modelling, and AutoDock tools for the docking, allowed the authors to determine in silico the IFS from Trifolium pretense as the best candidate for liquiritigenin conversion (Sajid et al., 2022). For pterocarpans and coumestans production, the enzyme VR has previously been crystallized, modelled and docked in silico to test its ability to produce DMI (Shao et al., 2007). The enzyme PTS, key in the cycling stage for producing the fourth ring structure, was recently analyzed using protein docking with the ligand DMI. The PTS enzyme from licorice (G. echinata) was determined as the best dirigent protein for the formation of both (−) and (+)-medicarpins (Meng et al., 2020). Besides these analyses, no other efforts have been reported so far for modelling and docking enzymes related to pterocarpans and coumestans biosynthesis.
7 Challenges and prospects
The main challenge that the scientific community will face shortly is how to apply sustainable production systems to cope with the increasing global demand for isoflavonoids. The discovery of a novel production chassis through microbial bioprospecting may help to identify more efficient and sustainable solutions. Bioprospecting allows the discovery of novel microorganisms that produce isoflavonoid derivatives, besides plant species. Phototropic microbes such as chlorophyta and cyanobacteria may become an alternative chassis, boosting metabolite production, and conserving the environment at the same time. Notably, the use of these species are potentially beneficial to the environment on account of their capacity to act as carbon-sinks and wastewater remediators, in line with the United Nations Sustainable Developments Goals (Vuong et al., 2022a). Efforts should be implemented to develop more engineering tools for algal species. A genetic toolkit is available for diatoms and cyanobacteria species, but gene delivery, transformation, and selection of recombinant microalgal strains require significant improvement to replace model species S. saccharomyces and E. Coli (Lee et al., 2023).
A full comprehension of the metabolic pathway is essential to determine the enzymes involved in pterocarpans and coumestans biosynthesis. Enzyme bioprospecting allows the identification and selection of the best enzyme homologs, using in silico bioinformatic tools for modelling and docking simulations. Although some pterocarpans and coumestans metabolic enzymes were analyzed, such as the IFS and PTS, further contributions should deliver more information about the best homologous enzymes for the whole pathway. Once the best production chassis and enzymes are identified, microbial-based systems should be implemented to boost pterocarpans and coumestans expression. The employment of a symbiotic poly-culture strategy may positively contribute to the target molecule production, and ease the optimization of fermentation conditions. Other tools that can help to boost production titer besides the incorporation of the metabolic pathway are: a) determining the perfect expression ratio of all the enzymes involved through techniques such as multiplex automatic genome engineering (MAGE) (Wang et al., 2009), b) implementation of strains with an increased expression of precursors and cofactors (Akhtar and Jones, 2014), c) applying substrate channeling through enzyme scaffolds and protein fusions (Kang et al., 2018; Choi et al., 2019). Overall, a link between researchers with metabolic pathway/enzymes background, and engineering specialists who work on microbial sustainable production systems may overcome the isoflavonoid production bottleneck.
8 Conclusion
Isoflavonoids derivatives such as pterocarpans and coumestans have applications in multiple fields. Diseases such as breast cancer, osteoporosis and neurodegenerative syndromes can be targeted using these molecules to improve human health and wellbeing. Nevertheless, further experimental contributions are needed to enhance their production, purification and overall implementation. Though efforts have been implemented to identify the biosynthetic pathways of some pterocarpans and coumestans, others remain unclear. A holistic bioprospecting strategy that considers both pathway identification (enzymes) and microbial discovery (production systems) is very much required. We describe the “state of the art” microbial-based systems for pterocarpans and coumestans production, and propose the implementation of microalgae species as sustainable production platforms. This strategy is not limited to isoflavonoid derivatives, and can be applied to other plant natural product such as terpenes, steroids, other phenolics. The shared knowledge contributed by the scientific community regarding the bioprospecting of enzymes and microbes will be invaluable for the development of production platforms for the isoflavonoid derivatives, pterocarpans and coumestans.
Author contributions
FP conceived, designed and wrote the initial draft of this review article. JP and PK reviewed and edited the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Funding
PK is supported by the University of Western Australia with additional research funding from ExPlanta Pty Ltd., towards Biosynthesis of Isoflavones—Formononetin (FMN) Proof of Concept project. JP is supported by an NHMRC Investigator Grant (GNT1196188).
Acknowledgments
FPR acknowledges the University of Western Australia for providing international fee-offset scholarship and the University Postgraduate Award.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acinas, S. G., Sánchez, P., Salazar, G., Cornejo-Castillo, F. M., Sebastián, M., Logares, R., et al. (2021). Deep ocean metagenomes provide insight into the metabolic architecture of bathypelagic microbial communities. Commun. Biol. 4, 604–615. doi:10.1038/s42003-021-02112-2
AfifaHussain, N., Baqar, Z., Mumtaz, M., El-Sappah, A. H., Show, P. L., Iqbal, H. M. N., et al. (2022). Bioprospecting fungal-derived value-added bioproducts for sustainable pharmaceutical applications. Sustain. Chem. Pharm. 29, 100755. doi:10.1016/j.scp.2022.100755
Ahmed, A., Mam, B., and Sowdhamini, R. (2021). Deelig: A deep learning approach to predict protein-ligand binding affinity. Bioinforma. Biol. Insights 15, 117793222110303. doi:10.1177/11779322211030364
Akashi, T., Aoki, T., and Ayabe, S. (1998). CYP81E1, a cytochrome P450 cDNA of licorice (Glycyrrhiza echinataL.), encodes isoflavone 2′-hydroxylase. Biochem. Biophys. Res. Commun. 251, 67–70. doi:10.1006/bbrc.1998.9414
Akashi, T., Sawada, Y., Shimada, N., Sakurai, N., Aoki, T., and Ayabe, S. (2003). cDNA cloning and biochemical characterization of S-Adenosyl-l-Methionine: 2,7,4′-Trihydroxyisoflavanone 4′-O-methyltransferase, a critical enzyme of the legume isoflavonoid phytoalexin pathway. Plant Cell Physiol. 44, 103–112. doi:10.1093/pcp/pcg034
Akhtar, M. K., and Jones, P. R. (2014). Cofactor engineering for enhancing the flux of metabolic pathways. Front. Bioeng. Biotechnol. 2, 30. doi:10.3389/fbioe.2014.00030
Akram, M., Rasool, A., An, T., Feng, X., and Li, C. (2021). Metabolic engineering of Yarrowia lipolytica for liquiritigenin production. Chem. Eng. Sci. 230, 116177. doi:10.1016/j.ces.2020.116177
Alam, Md. A., Wan, C., Tran, D. T., Mofijur, M., Ahmed, S. F., Mehmood, M. A., et al. (2022). Microalgae binary culture for higher biomass production, nutrients recycling, and efficient harvesting: A review. Environ. Chem. Lett. 20, 1153–1168. doi:10.1007/s10311-021-01363-z
Albuquerque, B. R., Heleno, S. A., Oliveira, M. B. P. P., Barros, L., and Ferreira, I. C. F. R. (2021). Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 12, 14–29. doi:10.1039/d0fo02324h
Amawi, H., Ashby, C. R., and Tiwari, A. K. (2017). Cancer chemoprevention through dietary flavonoids: what’s limiting? Chin. J. Cancer 36, 50. doi:10.1186/s40880-017-0217-4
Artimo, P., Jonnalagedda, M., Arnold, K., Baratin, D., Csardi, G., de Castro, E., et al. (2012). ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 40, W597–W603. doi:10.1093/nar/gks400
Augustijn, D., de Groot, H. J. M., and Alia, A. (2021). HR-MAS NMR applications in plant metabolomics. Molecules 26, 931. doi:10.3390/molecules26040931
Bai, X., Zhu, Y., Jie, J., Li, D., Song, L., and Luo, J. (2022). Maackiain protects against sepsis via activating AMPK/Nrf2/HO-1 pathway. Int. Immunopharmacol. 108, 108710. doi:10.1016/j.intimp.2022.108710
Bailey, T. L., Johnson, J., Grant, C. E., and Noble, W. S. (2015). The MEME suite. Nucleic Acids Res. 43, W39–W49. doi:10.1093/nar/gkv416
Bansal, M., Tiwari, N., and Sharma, J. G. (2022). “Chapter 3 - revolution in microbial bioprospecting via the development of omics-based technologies,” in Bioprospecting of microbial diversity. Editors P. Verma, and M. P. Shah (Netherlands: Elsevier).
Beattie, A. J., Hay, M., Magnusson, B., de Nys, R., Smeathers, J., and Vincent, J. F. V. (2011). Ecology and bioprospecting. Austral Ecol. 36, 341–356. doi:10.1111/j.1442-9993.2010.02170.x
Becker, J., and Wittmann, C. (2020). Microbial production of extremolytes — High-value active ingredients for nutrition, health care, and well-being. Curr. Opin. Biotechnol. 65, 118–128. doi:10.1016/j.copbio.2020.02.010
Bravo, L. (1998). Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 56, 317–333. doi:10.1111/j.1753-4887.1998.tb01670.x
Brodowska, K. M. (2017). Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 7, 108–123. doi:10.5281/zenodo.545778
Caporgno, M. P., and Mathys, A. (2018). Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 5, 58. doi:10.3389/fnut.2018.00058
Castro, C. C., Pagnussat, A. S., Moura, N., da Cunha, M. J., Machado, F. R., Wyse, A. T. S., et al. (2014). Coumestrol treatment prevents Na+, K+-ATPase inhibition and affords histological neuroprotection to male rats receiving cerebral global ischemia. Neurol. Res. 36, 198–206. doi:10.1179/1743132813Y.0000000286
Chaudhuri, D., Ghate, N. B., Deb, S., Panja, S., Sarkar, R., Rout, J., et al. (2014). Assessment of the phytochemical constituents and antioxidant activity of a bloom forming microalgae Euglena tuba. Biol. Res. 47, 24. doi:10.1186/0717-6287-47-24
Chen, J., Yuan, H., Zhang, L., Pan, H., Xu, R., Zhong, Y., et al. (2015). Cloning, expression and purification of isoflavone-2′-hydroxylase from Astragalus membranaceus Bge. Var. mongolicus (Bge.) Hsiao. Protein Expr. Purif. 107, 83–89. doi:10.1016/j.pep.2014.11.010
Chen, T., Zhou, Y., Lu, Y., and Zhang, H. (2019). Advances in heterologous biosynthesis of plant and fungal natural products by modular co-culture engineering. Biotechnol. Lett. 41, 27–34. doi:10.1007/s10529-018-2619-z
Cheng, Q., Li, N., Dong, L., Zhang, D., Fan, S., Jiang, L., et al. (2015). Overexpression of soybean isoflavone reductase (GmIFR) enhances resistance to phytophthora sojae in soybean. Front. Plant Sci. 6, 1024. doi:10.3389/fpls.2015.01024
Choi, K. R., Jang, W. D., Yang, D., Cho, J. S., Park, D., and Lee, S. Y. (2019). Systems metabolic engineering strategies: Integrating systems and synthetic biology with metabolic engineering. Trends Biotechnol. 37, 817–837. doi:10.1016/j.tibtech.2019.01.003
Choong, W. P., Tan, C. H., Show, P. L., Lam, H. L., Mohamad Annuar, M. S. B., Juan, J. C., et al. (2016). Efficient enzyme-catalysed transesterification of microalgal biomass from Chlamydomonas sp. Energy 116, 1370–1373. doi:10.1016/j.energy.2016.06.032
Chouhan, S., Sharma, K., Zha, J., Guleria, S., and Koffas, M. A. G. (2017). Recent advances in the recombinant biosynthesis of polyphenols. Front. Microbiol. 8, 2259. doi:10.3389/fmicb.2017.02259
Cravens, A., Payne, J., and Smolke, C. D. (2019). Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 10, 2142. doi:10.1038/s41467-019-09848-w
Cruickshank, I. a. M., and Perrin, D. R. (1960). Isolation of a phytoalexin from Pisum sativum L. Nature 187, 799–800. doi:10.1038/187799b0
Curtis, T. P., and Sloan, W. T. (2005). Exploring microbial diversity--A vast below. Science 309, 1331–1333. doi:10.1126/science.1118176
Del Mondo, A., Sansone, C., and Brunet, C. (2022). Insights into the biosynthesis pathway of phenolic compounds in microalgae. Comput. Struct. Biotechnol. J. 20, 1901–1913. doi:10.1016/j.csbj.2022.04.019
Del Mondo, A., Smerilli, A., Ambrosino, L., Albini, A., Noonan, D. M., Sansone, C., et al. (2021). Insights into phenolic compounds from microalgae: Structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol. 41, 155–171. doi:10.1080/07388551.2021.1874284
Delépine, B., Duigou, T., Carbonell, P., and Faulon, J.-L. (2018). RetroPath2.0: A retrosynthesis workflow for metabolic engineers. Metab. Eng. 45, 158–170. doi:10.1016/j.ymben.2017.12.002
DiCenzo, G. L., and VanEtten, H. D. (2006). Studies on the late steps of (+) pisatin biosynthesis: Evidence for (-) enantiomeric intermediates. Phytochemistry 67, 675–683. doi:10.1016/j.phytochem.2005.12.027
Dixit, M., Raghuvanshi, A., Gupta, C. P., Kureel, J., Mansoori, M. N., Shukla, P., et al. (2015). Medicarpin, a natural pterocarpan, heals cortical bone defect by activation of notch and wnt canonical signaling pathways. PLOS ONE 10, e0144541. doi:10.1371/journal.pone.0144541
Dixon, R. A. (1999). “1.28 - isoflavonoids: Biochemistry, molecular biology, and biological functions,” in Comprehensive natural products chemistry. Editors S. D. Barton, K. Nakanishi, and O. Meth-Cohn (Oxford: Pergamon), 773–823. doi:10.1016/B978-0-08-091283-7.00030-8
Du, H., Huang, Y., and Tang, Y. (2010). Genetic and metabolic engineering of isoflavonoid biosynthesis. Appl. Microbiol. Biotechnol. 86, 1293–1312. doi:10.1007/s00253-010-2512-8
Durazzo, A., Lucarini, M., Souto, E. B., Cicala, C., Caiazzo, E., Izzo, A. A., et al. (2019). Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. PTR 33, 2221–2243. doi:10.1002/ptr.6419
Dutta, S., and Berman, H. M. (2005). Large macromolecular complexes in the protein Data Bank: A status report. Structure 13, 381–388. doi:10.1016/j.str.2005.01.008
Ellis, G. A., Klein, W. P., Lasarte-Aragonés, G., Thakur, M., Walper, S. A., and Medintz, I. L. (2019). Artificial multienzyme scaffolds: Pursuing in vitro substrate channeling with an overview of current progress. ACS Catal. 9, 10812–10869. doi:10.1021/acscatal.9b02413
Fan, J., Fu, A., and Zhang, L. (2019). Progress in molecular docking. Quant. Biol. 7, 83–89. doi:10.1007/s40484-019-0172-y
Ferdous, U. T., and Balia Yusof, Z. N. (2021). Insight into potential anticancer activity of algal flavonoids: Current status and challenges. Molecules 26, 6844. doi:10.3390/molecules26226844
Fischer, D., Ebenau-Jehle, C., and Grisebach, H. (1990). Purification and characterization of pterocarpan synthase from elicitor-challenged soybean cell cultures. Phytochemistry 29, 2879–2882. doi:10.1016/0031-9422(90)87096-D
French, K. E. (2019). Harnessing synthetic biology for sustainable development. Nat. Sustain. 2, 250–252. doi:10.1038/s41893-019-0270-x
Goel, A., Kumar, A., and Raghuvanshi, A. (2013). Synthesis, stereochemistry, structural classification, and chemical reactivity of natural pterocarpans. Chem. Rev. 113, 1614–1640. doi:10.1021/cr300219y
Goiris, K., Muylaert, K., Voorspoels, S., Noten, B., De Paepe, D., E Baart, G. J., et al. (2014). Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 50, 483–492. doi:10.1111/jpy.12180
Gong, X., Zhang, T., Xing, J., Wang, R., and Zhao, Y. (2020). Positional effects on efficiency of CRISPR/Cas9-based transcriptional activation in rice plants. aBIOTECH 1, 1–5. doi:10.1007/s42994-019-00007-9
Goswami, R. K., Mehariya, S., Karthikeyan, O. P., Gupta, V. K., and Verma, P. (2022). Multifaceted application of microalgal biomass integrated with carbon dioxide reduction and wastewater remediation: A flexible concept for sustainable environment. J. Clean. Prod. 339, 130654. doi:10.1016/j.jclepro.2022.130654
Grigoriev, I. V., Nordberg, H., Shabalov, I., Aerts, A., Cantor, M., Goodstein, D., et al. (2012). The genome portal of the department of energy Joint genome Institute. Nucleic Acids Res. 40, D26–D32. doi:10.1093/nar/gkr947
Guo, L., Dixon, R. A., and Paiva, N. L. (1994a). Conversion of vestitone to medicarpin in alfalfa (Medicago sativa L.) is catalyzed by two independent enzymes. Identification, purification, and characterization of vestitone reductase and 7,2’-dihydroxy-4’-methoxyisoflavanol dehydratase. J. Biol. Chem. 269, 22372–22378. doi:10.1016/s0021-9258(17)31799-4
Guo, L., Dixon, R. A., and Paiva, N. L. (1994b). The ‘pterocarpan synthase” of alfalfa: Association and co-induction of vestitone reductase and 7,2′-dihydroxy-4′-methoxy-isoflavanol (DMI) dehydratase, the two final enzymes in medicarpin biosynthesis. FEBS Lett. 356, 221–225. doi:10.1016/0014-5793(94)01267-9
Guo, L., and Paiva, N. L. (1995). Molecular cloning and expression of alfalfa (Medicago sativa L.) vestitone reductase, the penultimate enzyme in medicarpin biosynthesis. Arch. Biochem. Biophys. 320, 353–360. doi:10.1016/0003-9861(95)90019-5
Gupta, A., Awasthi, P., Sharma, N., Parveen, S., Vats, R. P., Singh, N., et al. (2022). Medicarpin confers powdery mildew resistance in Medicago truncatula and activates the salicylic acid signalling pathway. Mol. Plant Pathol. 23, 966–983. doi:10.1111/mpp.13202
Ha, J., Kang, Y.-G., Lee, T., Kim, M., Yoon, M. Y., Lee, E., et al. (2019). Comprehensive RNA sequencing and co-expression network analysis to complete the biosynthetic pathway of coumestrol, a phytoestrogen. Sci. Rep. 9, 1934. doi:10.1038/s41598-018-38219-6
Harvey, P. J., and Ben-Amotz, A. (2020). Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cis β-carotene production. Algal Res. 50, 102002. doi:10.1016/j.algal.2020.102002
He, X., Fang, J., Huang, L., Wang, J., and Huang, X. (2015). Sophora flavescens Ait. Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 172, 10–29. doi:10.1016/j.jep.2015.06.010
Hedelin, M., Löf, M., Olsson, M., Adlercreutz, H., Sandin, S., and Weiderpass, E. (2008). Dietary phytoestrogens are not associated with risk of overall breast cancer but diets rich in coumestrol are inversely associated with risk of estrogen receptor and progesterone receptor negative breast tumors in Swedish women. J. Nutr. 138, 938–945. doi:10.1093/jn/138.5.938
Hedlund, B. P., Dodsworth, J. A., Murugapiran, S. K., Rinke, C., and Woyke, T. (2014). Impact of single-cell genomics and metagenomics on the emerging view of extremophile “microbial dark matter. Extremophiles 18, 865–875. doi:10.1007/s00792-014-0664-7
Hemaiswarya, S., Raja, R., Ravikumar, R., and Carvalho, I. (2013). Microalgae taxonomy and breeding. Biofuel Crops Prod. Physiol. Genet., 44–53.
Hinderer, W., Flentje, U., and Barz, W. (1987). Microsomal isoflavone 2′- and 3′-hydroxylases from chickpea (Cicer arietinum L.) cell suspensions induced for pterocarpan phytoalexin formation. FEBS Lett. 214, 101–106. doi:10.1016/0014-5793(87)80021-2
Hsieh, C.-J., Kuo, P.-L., Hou, M.-F., Hung, J.-Y., Chang, F.-R., Hsu, Y.-C., et al. (2015). Wedelolactone inhibits breast cancer-induced osteoclastogenesis by decreasing Akt/mTOR signaling. Int. J. Oncol. 46, 555–562. doi:10.3892/ijo.2014.2769
Ijaz, S., and Hasnain, S. (2016). Antioxidant potential of indigenous cyanobacterial strains in relation with their phenolic and flavonoid contents. Nat. Prod. Res. 30, 1297–1300. doi:10.1080/14786419.2015.1053088
Jenuth, J. P. (1999). “The NCBI,” in Bioinformatics Methods and protocols methods in molecular BiologyTM. Editors S. Misener, and S. A. Krawetz (Totowa, NJ: Humana Press), 301–312. doi:10.1385/1-59259-192-2:301
Jiang, D., Rasul, A., Batool, R., Sarfraz, I., Hussain, G., Mateen Tahir, M., et al. (2019). Potential anticancer properties and mechanisms of action of formononetin. Biomed. Res. Int. 2019, 1–11. doi:10.1155/2019/5854315
Jiménez-González, L., Álvarez-Corral, M., Muñoz-Dorado, M., and Rodríguez-García, I. (2008). Pterocarpans: Interesting natural products with antifungal activity and other biological properties. Phytochem. Rev. 7, 125–154. doi:10.1007/s11101-007-9059-z
Jones, J. A., Vernacchio, V. R., Collins, S. M., Shirke, A. N., Xiu, Y., Englaender, J. A., et al. (2017). Complete biosynthesis of anthocyanins using E. coli polycultures. mBio 8, e00621–17. doi:10.1128/mBio.00621-17
Jones, J. A., Vernacchio, V. R., Sinkoe, A. L., Collins, S. M., Ibrahim, M. H. A., Lachance, D. M., et al. (2016). Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab. Eng. 35, 55–63. doi:10.1016/j.ymben.2016.01.006
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. doi:10.1038/s41586-021-03819-2
Kamble, A., Srinivasan, S., and Singh, H. (2019). In-silico bioprospecting: Finding better enzymes. Mol. Biotechnol. 61, 53–59. doi:10.1007/s12033-018-0132-1
Kanehisa, M., and Goto, S. (2000). Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi:10.1093/nar/28.1.27
Kang, S.-Y., Heo, K. T., and Hong, Y.-S. (2018). Optimization of artificial curcumin biosynthesis in E. coli by randomized 5′-UTR sequences to control the multienzyme pathway. ACS Synth. Biol. 7, 2054–2062. doi:10.1021/acssynbio.8b00198
Katsuyama, Y., Miyahisa, I., Funa, N., and Horinouchi, S. (2007). One-pot synthesis of genistein from tyrosine by coincubation of genetically engineered Escherichia coli and Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 73, 1143–1149. doi:10.1007/s00253-006-0568-2
Khemiri, S., Khelifi, N., Nunes, M. C., Ferreira, A., Gouveia, L., Smaali, I., et al. (2020). Microalgae biomass as an additional ingredient of gluten-free bread: Dough rheology, texture quality and nutritional properties. Algal Res. 50, 101998. doi:10.1016/j.algal.2020.101998
Kim, B.-G. (2020). Biological synthesis of genistein in Escherichia coli. J. Microbiol. Biotechnol. 30, 770–776. doi:10.4014/jmb.1911.11009
Kim, J.-H., Kang, D.-M., Cho, Y.-J., Hyun, J.-W., and Ahn, M.-J. (2022). Medicarpin increases antioxidant genes by inducing NRF2 transcriptional level in HeLa cells. Antioxidants 11, 421. doi:10.3390/antiox11020421
Klejdus, B., Lojková, L., Plaza, M., Snóblová, M., and Stěrbová, D. (2010). Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 1217, 7956–7965. doi:10.1016/j.chroma.2010.07.020
Koukos, P. I., Réau, M., and Bonvin, A. M. J. J. (2021). Shape-restrained modeling of protein–small-molecule complexes with high ambiguity driven DOCKing. J. Chem. Inf. Model. 61, 4807–4818. doi:10.1021/acs.jcim.1c00796
Krüger, A., Schäfers, C., Busch, P., and Antranikian, G. (2020). Digitalization in microbiology - paving the path to sustainable circular bioeconomy. New Biotechnol. 59, 88–96. doi:10.1016/j.nbt.2020.06.004
Kumar, S., and Pandey, A. K. (2013). Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 1–16. doi:10.1155/2013/162750
Lee, E. J., Song, M. C., and Rha, C.-S. (2022). Mass biosynthesis of coumestrol derivatives and their isomers via soybean adventitious root cultivation in bioreactors. Front. Plant Sci. 13, 923163. doi:10.3389/fpls.2022.923163
Lee, T.-M., Lin, J.-Y., Tsai, T.-H., Yang, R.-Y., and Ng, I.-S. (2023). Clustered regularly interspaced short palindromic repeats (crispr) technology and genetic engineering strategies for microalgae towards carbon neutrality: A critical review. Bioresour. Technol. 368, 128350. doi:10.1016/j.biortech.2022.128350
Leonard, E., and Koffas, M. A. G. (2007). Engineering of artificial plant cytochrome P450 enzymes for synthesis of isoflavones by Escherichia coli. Appl. Environ. Microbiol. 73, 7246–7251. doi:10.1128/AEM.01411-07
Li, D., Cai, C., Liao, Y., Wu, Q., Ke, H., Guo, P., et al. (2021). Systems pharmacology approach uncovers the therapeutic mechanism of medicarpin against scopolamine-induced memory loss. Phytomedicine 91, 153662. doi:10.1016/j.phymed.2021.153662
Li, J., Li, C., Gou, J., Wang, X., Fan, R., and Zhang, Y. (2016). An alternative pathway for formononetin biosynthesis in Pueraria lobata. Front. Plant Sci. 7, 861. doi:10.3389/fpls.2016.00861
Liu, C.-J., Deavours, B. E., Richard, S. B., Ferrer, J.-L., Blount, J. W., Huhman, D., et al. (2006). Structural basis for dual functionality of isoflavonoid O-methyltransferases in the evolution of plant defense responses. Plant Cell 18, 3656–3669. doi:10.1105/tpc.106.041376
Liu, C.-J., Huhman, D., Sumner, L. W., and Dixon, R. A. (2003). Regiospecific hydroxylation of isoflavones by cytochrome P450 81E enzymes from Medicago truncatula. Plant J. 36, 471–484. doi:10.1046/j.1365-313X.2003.01893.x
Liu, L., Liu, H., Zhang, W., Yao, M., Li, B., Liu, D., et al. (2019). Engineering the biosynthesis of caffeic acid in Saccharomyces cerevisiae with heterologous enzyme combinations. Engineering 5, 287–295. doi:10.1016/j.eng.2018.11.029
Liu, Q., Liu, Y., Li, G., Savolainen, O., Chen, Y., and Nielsen, J. (2021). De novo biosynthesis of bioactive isoflavonoids by engineered yeast cell factories. Nat. Commun. 12, 6085. doi:10.1038/s41467-021-26361-1
Liu, Y., Grimm, M., Dai, W., Hou, M., Xiao, Z.-X., and Cao, Y. (2020a). CB-dock: A web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol. Sin. 41, 138–144. doi:10.1038/s41401-019-0228-6
Liu, Z., Wang, K., Chen, Y., Tan, T., and Nielsen, J. (2020b). Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nat. Catal. 3, 274–288. doi:10.1038/s41929-019-0421-5
Lyu, X., Ng, K. R., Lee, J. L., Mark, R., and Chen, W. N. (2017). Enhancement of naringenin biosynthesis from tyrosine by metabolic engineering of Saccharomyces cerevisiae. J. Agric. Food Chem. 65, 6638–6646. doi:10.1021/acs.jafc.7b02507
Mazur, W. M., Duke, J. A., Wähälä, K., Rasku, S., and Adlercreutz, H. (1998). Isoflavonoids and Lignans in Legumes: Nutritional and Health Aspects in Humans 11The method development and synthesis of the standards and deuterium-labelled compounds was supported by National Institutes of Health Grants No. 1 R01 CA56289-01 and No. 2 R01 CA56289-04, and analytical work by the EU research contract FAIR-CT95-0894. J. Nutr. Biochem. 9, 193–200. doi:10.1016/S0955-2863(97)00184-8
Meng, Q., Moinuddin, S. G. A., Kim, S.-J., Bedgar, D. L., Costa, M. A., Thomas, D. G., et al. (2020). Pterocarpan synthase (PTS) structures suggest a common quinone methide–stabilizing function in dirigent proteins and proteins with dirigent-like domains. J. Biol. Chem. 295, 11584–11601. doi:10.1074/jbc.RA120.012444
Mirdita, M., Schütze, K., Moriwaki, Y., Heo, L., Ovchinnikov, S., and Steinegger, M. (2022). ColabFold: Making protein folding accessible to all. Nat. Methods 19, 679–682. doi:10.1038/s41592-022-01488-1
Miyahisa, I., Kaneko, M., Funa, N., Kawasaki, H., Kojima, H., Ohnishi, Y., et al. (2005). Efficient production of (2S)-flavanones by Escherichia coli containing an artificial biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 68, 498–504. doi:10.1007/s00253-005-1916-3
Mizuguchi, H., Nariai, Y., Kato, S., Nakano, T., Kanayama, T., Kashiwada, Y., et al. (2015). Maackiain is a novel antiallergic compound that suppresses transcriptional upregulation of the histamine H1 receptor and interleukin-4 genes. Pharmacol. Res. Perspect. 3, e00166. doi:10.1002/prp2.166
Mobin, S., and Alam, F. (2017). Some promising microalgal species for commercial applications: A review. Energy Procedia 110, 510–517. doi:10.1016/j.egypro.2017.03.177
Nabavi, S. M., Šamec, D., Tomczyk, M., Milella, L., Russo, D., Habtemariam, S., et al. (2020). Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 38, 107316. doi:10.1016/j.biotechadv.2018.11.005
Nguyen, T. M. N., Nguyen, T. K. A., and Vu, T. K. L. (2020). Symbiotic photobioreactor using immobilized microalgae-yeast consortium for saccharomyces cerevisiae and chlorella vulgaris biomass production. J. Tech. Educ. Sci., 17–29.
Paiva, N. L., Edwards, R., Sun, Y. J., Hrazdina, G., and Dixon, R. A. (1991). Stress responses in alfalfa (Medicago sativa L.) 11. Molecular cloning and expression of alfalfa isoflavone reductase, a key enzyme of isoflavonoid phytoalexin biosynthesis. Plant Mol. Biol. 17, 653–667. doi:10.1007/BF00037051
Paoli, L., Ruscheweyh, H.-J., Forneris, C. C., Hubrich, F., Kautsar, S., Bhushan, A., et al. (2022). Biosynthetic potential of the global ocean microbiome. Nature 607, 111–118. doi:10.1038/s41586-022-04862-3
Park, G., Baek, S., Kim, J.-E., Lim, T., Lee, C. C., Yang, H., et al. (2015). Flt3 is a target of coumestrol in protecting against UVB-induced skin photoaging. Biochem. Pharmacol. 98, 473–483. doi:10.1016/j.bcp.2015.08.104
Peil, G. H. S., Kuss, A. V., Rave, A. F. G., Villarreal, J. P. V., Hernandes, Y. M. L., and Nascente, P. S. (2016). Bioprospecting of lipolytic microorganisms obtained from industrial effluents. An. Acad. Bras. Ciênc. 88, 1769–1779. doi:10.1590/0001-3765201620150550
Pham, J. V., Yilma, M. A., Feliz, A., Majid, M. T., Maffetone, N., Walker, J. R., et al. (2019a). A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 10, 1404. doi:10.3389/fmicb.2019.01404
Pham, T. H., Lecomte, S., Efstathiou, T., Ferriere, F., and Pakdel, F. (2019b). An update on the effects of glyceollins on human health: Possible anticancer effects and underlying mechanisms. Nutrients 11, 79. doi:10.3390/nu11010079
Prasad, R., Gupta, S. K., Shabnam, N., Oliveira, C. Y. B., Nema, A. K., Ansari, F. A., et al. (2021). Role of microalgae in global CO2 sequestration: Physiological mechanism, recent development, challenges, and future prospective. Sustainability 13, 13061. doi:10.3390/su132313061
Procházková, T., Sychrová, E., Javůrková, B., Večerková, J., Kohoutek, J., Lepšová-Skácelová, O., et al. (2017). Phytoestrogens and sterols in waters with cyanobacterial blooms - analytical methods and estrogenic potencies. Chemosphere 170, 104–112. doi:10.1016/j.chemosphere.2016.12.006
Quince, C., Walker, A. W., Simpson, J. T., Loman, N. J., and Segata, N. (2017). Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 35, 833–844. doi:10.1038/nbt.3935
Rodriguez, A., Kildegaard, K. R., Li, M., Borodina, I., and Nielsen, J. (2015). Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab. Eng. 31, 181–188. doi:10.1016/j.ymben.2015.08.003
Rodriguez, A., Strucko, T., Stahlhut, S. G., Kristensen, M., Svenssen, D. K., Forster, J., et al. (2017). Metabolic engineering of yeast for fermentative production of flavonoids. Bioresour. Technol. 245, 1645–1654. doi:10.1016/j.biortech.2017.06.043
Safafar, H., van Wagenen, J., Møller, P., and Jacobsen, C. (2015). Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 13, 7339–7356. doi:10.3390/md13127069
Sajid, M., Channakesavula, C., Stone, S., and Kaur, P. (2021a). Synthetic biology towards improved flavonoid pharmacokinetics. Biomolecules 11, 754. doi:10.3390/biom11050754
Sajid, M., Stone, S., and Kaur, P. (2021b). Recent advances in heterologous synthesis paving way for future green-modular bioindustries: A review with special reference to isoflavonoids. Front. Bioeng. Biotechnol. 9, 673270. doi:10.3389/fbioe.2021.673270
Sajid, M., Stone, S. R., and Kaur, P. (2022). Phylogenetic analysis and protein modelling of isoflavonoid synthase highlights key catalytic sites towards realising new bioengineering endeavours. Bioengineering 9, 609. doi:10.3390/bioengineering9110609
Sankaran, R., Show, P. L., Yap, Y. J., Tao, Y., Ling, T. C., and Tomohisa, K. (2018). Green technology of liquid biphasic flotation for enzyme recovery utilizing recycling surfactant and sorbitol. Clean. Technol. Environ. Policy 20, 2001–2012. doi:10.1007/s10098-018-1523-5
Santana, K., do Nascimento, L. D., Lima e Lima, A., Damasceno, V., Nahum, C., Braga, R. C., et al. (2021). Applications of virtual screening in bioprospecting: Facts, shifts, and perspectives to explore the chemo-structural diversity of natural products. Front. Chem. 9, 662688. doi:10.3389/fchem.2021.662688
Schaller, K. S., Molina, G. A., Kari, J., Schiano-di-Cola, C., Sørensen, T. H., Borch, K., et al. (2022). Virtual bioprospecting of interfacial enzymes: Relating sequence and kinetics. ACS Catal. 12, 7427–7435. doi:10.1021/acscatal.2c02305
Seeliger, D., and de Groot, B. L. (2010). Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 24, 417–422. doi:10.1007/s10822-010-9352-6
Shao, H., Dixon, R. A., and Wang, X. (2007). Crystal structure of vestitone reductase from alfalfa (Medicago sativa L.) J. Mol. Biol. 369, 265–276. doi:10.1016/j.jmb.2007.03.040
Sharifi-Rad, J., Kamiloglu, S., Yeskaliyeva, B., Beyatli, A., Alfred, M. A., Salehi, B., et al. (2020). Pharmacological activities of psoralidin: A comprehensive review of the molecular mechanisms of action. Front. Pharmacol. 11, 571459. doi:10.3389/fphar.2020.571459
Sharma, S., Trivedi, S., Pandey, T., Ranjan, S., Trivedi, M., and Pandey, R. (2021). Wedelolactone mitigates parkinsonism via alleviating oxidative stress and mitochondrial dysfunction through NRF2/SKN-1. Mol. Neurobiol. 58, 65–77. doi:10.1007/s12035-020-02080-4
Singh, N., and Gaur, S. (2021). “GRAS fungi: A new horizon in safer food product,” in Fungi in sustainable food production fungal biology. Editors X. Dai, M. Sharma, and J. Chen (Cham: Springer International Publishing), 27–37. doi:10.1007/978-3-030-64406-2_3
Strange, R. N., Ingham, J. L., Cole, D. L., Cavill, M. E., Edwards, C., Cooksey, C. J., et al. (1985). Isolation of the phytoalexin medicarpin from leaflets of Arachis hypogaea and related species of the tribe aeschynomeneae. Z. Für Naturforsch. C 40, 313–316. doi:10.1515/znc-1985-5-605
Sukumaran, A., McDowell, T., Chen, L., Renaud, J., and Dhaubhadel, S. (2018). Isoflavonoid-specific prenyltransferase gene family in soybean: GmPT01, a pterocarpan 2-dimethylallyltransferase involved in glyceollin biosynthesis. Plant J. 96, 966–981. doi:10.1111/tpj.14083
Teng, H., and Chen, L. (2019). Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 59, 2040–2051. doi:10.1080/10408398.2018.1437023
The UniProt Consortium (2019). UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515. doi:10.1093/nar/gky1049
Tohge, T., Watanabe, M., Hoefgen, R., and Fernie, A. R. (2013). The evolution of phenylpropanoid metabolism in the green lineage. Crit. Rev. Biochem. Mol. Biol. 48, 123–152. doi:10.3109/10409238.2012.758083
Trabelsi, L., Mnari, A., Abdel-Daim, M. M., Abid-Essafi, S., and Aleya, L. (2016). Therapeutic properties in Tunisian hot springs: First evidence of phenolic compounds in the cyanobacterium leptolyngbya sp. biomass, capsular polysaccharides and releasing polysaccharides. BMC Complement. Altern. Med. 16, 515. doi:10.1186/s12906-016-1492-3
Trantas, E., Panopoulos, N., and Ververidis, F. (2009). Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab. Eng. 11, 355–366. doi:10.1016/j.ymben.2009.07.004
Tsai, R.-T., Tsai, C.-W., Liu, S.-P., Gao, J.-X., Kuo, Y.-H., Chao, P.-M., et al. (2020). Maackiain ameliorates 6-hydroxydopamine and SNCA pathologies by modulating the PINK1/parkin pathway in models of Parkinson’s disease in Caenorhabditis elegans and the SH-SY5Y cell line. Int. J. Mol. Sci. 21, 4455. doi:10.3390/ijms21124455
Tu, Y., Yang, Y., Li, Y., and He, C. (2021). Naturally occurring coumestans from plants, their biological activities and therapeutic effects on human diseases. Pharmacol. Res. 169, 105615. doi:10.1016/j.phrs.2021.105615
Twaij, B. M., and Hasan, M. N. (2022). Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 13, 4–14. doi:10.3390/ijpb13010003
Uchida, K., Akashi, T., and Aoki, T. (2015). Functional expression of cytochrome P450 in Escherichia coli: An approach to functional analysis of uncharacterized enzymes for flavonoid biosynthesis. Plant Biotechnol. 32, 205–213. doi:10.5511/plantbiotechnology.15.0605a
Uchida, K., Akashi, T., and Aoki, T. (2017). The missing link in leguminous pterocarpan biosynthesis is a dirigent domain-containing protein with isoflavanol dehydratase activity. Plant Cell Physiol. 58, 398–408. doi:10.1093/pcp/pcw213
Uchida, K., Aoki, T., Suzuki, H., and Akashi, T. (2020). Molecular cloning and biochemical characterization of isoflav-3-ene synthase, a key enzyme of the biosyntheses of (+)-pisatin and coumestrol. Plant Biotechnol. advpub 20, 301–310. doi:10.5511/plantbiotechnology.20.0421a
Vavitsas, K., Kugler, A., Satta, A., Hatzinikolaou, D. G., Lindblad, P., Fewer, D. P., et al. (2021). Doing synthetic biology with photosynthetic microorganisms. Physiol. Plant. 173, 624–638. doi:10.1111/ppl.13455
Villarruel-Lopez, A., Ascencio, F., and Nuño, K. (2017). Microalgae, a potential natural functional food source- A review. Pol. J. Food Nutr. Sci. 67, 251–263. doi:10.1515/pjfns-2017-0017
Vogt, T. (2010). Phenylpropanoid biosynthesis. Phenylpropanoid Biosynth. Mol. Plant 3, 2–20. doi:10.1093/mp/ssp106
Vuong, P., Chong, S., and Kaur, P. (2022a). The little things that matter: How bioprospecting microbial biodiversity can build towards the realization of united Nations sustainable development Goals. Npj Biodivers. 1, 4–5. doi:10.1038/s44185-022-00006-y
Vuong, P., Wise, M. J., Whiteley, A. S., and Kaur, P. (2022b). Small investments with big returns: Environmental genomic bioprospecting of microbial life. Crit. Rev. Microbiol. 48, 641–655. doi:10.1080/1040841X.2021.2011833
Wang, H. H., Isaacs, F. J., Carr, P. A., Sun, Z. Z., Xu, G., Forest, C. R., et al. (2009). Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898. doi:10.1038/nature08187
Wang, Z., Huang, X., Liu, J., Xiao, F., Tian, M., Ding, S., et al. (2021). Screening and heterologous expression of flavone synthase and flavonol synthase to catalyze hesperetin to diosmetin. Biotechnol. Lett. 43, 2161–2183. doi:10.1007/s10529-021-03184-0
Waterhouse, A., Bertoni, M., Bienert, S., Studer, G., Tauriello, G., Gumienny, R., et al. (2018). SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303. doi:10.1093/nar/gky427
Welle, R., and Grisebach, H. (1988). Induction of phytoalexin synthesis in soybean: Enzymatic cyclization of prenylated pterocarpans to glyceollin isomers. Arch. Biochem. Biophys. 263, 191–198. doi:10.1016/0003-9861(88)90627-3
Wells, M. L., Potin, P., Craigie, J. S., Raven, J. A., Merchant, S. S., Helliwell, K. E., et al. (2017). Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 29, 949–982. doi:10.1007/s10811-016-0974-5
Wen, L., Jiang, Y., Yang, J., Zhao, Y., Tian, M., and Yang, B. (2017). Structure, bioactivity, and synthesis of methylated flavonoids. Ann. N. Y. Acad. Sci. 1398, 120–129. doi:10.1111/nyas.13350
Wu, G., Zhuang, D., Chew, K. W., Ling, T. C., Khoo, K. S., Van Quyen, D., et al. (2022). Current status and future trends in removal, control, and mitigation of algae food safety risks for human consumption. Mol. Basel Switz. 27, 6633. doi:10.3390/molecules27196633
Wu, Q., Preisig, C. L., and VanEtten, H. D. (1997). Isolation of the cDNAs encoding (+)6a-hydroxymaackiain 3-O-methyltransferase, the terminal step for the synthesis of the phytoalexin pisatin in Pisum sativum. Plant Mol. Biol. 35, 551–560. doi:10.1023/a:1005836508844
Yadavalli, R., Ratnapuram, H., Motamarry, S., Reddy, C. N., Ashokkumar, V., and Kuppam, C. (2022). Simultaneous production of flavonoids and lipids from Chlorella vulgaris and Chlorella pyrenoidosa. Biomass Convers. Biorefinery 12, 683–691. doi:10.1007/s13399-020-01044-x
Yamamoto, T., Sakamoto, C., Tachiwana, H., Kumabe, M., Matsui, T., Yamashita, T., et al. (2018). Endocrine therapy-resistant breast cancer model cells are inhibited by soybean glyceollin I through Eleanor non-coding RNA. Sci. Rep. 8, 15202. doi:10.1038/s41598-018-33227-y
Yan, Y., Huang, L., and Koffas, M. A. G. (2007). Biosynthesis of 5-deoxyflavanones in microorganisms. Biotechnol. J. 2, 1250–1262. doi:10.1002/biot.200700119
Yang, X., Zhao, Y., Hsieh, M.-T., Xin, G., Wu, R.-T., Hsu, P.-L., et al. (2017). Total synthesis of (+)-Medicarpin. J. Nat. Prod. 80, 3284–3288. doi:10.1021/acs.jnatprod.7b00741
Yonekura-Sakakibara, K., Higashi, Y., and Nakabayashi, R. (2019). The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 10, 943. doi:10.3389/fpls.2019.00943
Zampieri, G., Vijayakumar, S., Yaneske, E., and Angione, C. (2019). Machine and deep learning meet genome-scale metabolic modeling. PLOS Comput. Biol. 15, e1007084. doi:10.1371/journal.pcbi.1007084
Zhang, W., Liu, H., Li, X., Liu, D., Dong, X.-T., Li, F.-F., et al. (2017). Production of naringenin from D-xylose with co-culture of E. coli and S. cerevisiae. Eng. Life Sci. 17, 1021–1029. doi:10.1002/elsc.201700039
Keywords: microbial bioprospecting, enzyme bioprospecting, microbial-based production, isoflavonoids, pterocarpans and coumestans
Citation: Perez Rojo F, Pillow JJ and Kaur P (2023) Bioprospecting microbes and enzymes for the production of pterocarpans and coumestans. Front. Bioeng. Biotechnol. 11:1154779. doi: 10.3389/fbioe.2023.1154779
Received: 31 January 2023; Accepted: 18 April 2023;
Published: 28 April 2023.
Edited by:
M. Kalim Akhtar, United Arab Emirates University, United Arab EmiratesReviewed by:
Pau Loke Show, University of Nottingham Malaysia Campus, MalaysiaRufeng Wang, Shanghai University of Traditional Chinese Medicine, China
Copyright © 2023 Perez Rojo, Pillow and Kaur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernando Perez Rojo, ZmVybmFuZG8ucGVyZXpyb2pvQHJlc2VhcmNoLnV3YS5lZHUuYXU=; Parwinder Kaur, cGFyd2luZGVyLmthdXJAdXdhLmVkdS5hdQ==
 Fernando Perez Rojo1*
Fernando Perez Rojo1* J. Jane Pillow
J. Jane Pillow Parwinder Kaur
Parwinder Kaur