
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol. , 26 May 2023
Sec. Biomaterials
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1149662
This article is part of the Research Topic Advanced Oral Disease Therapy: Approaches, Biotechnology, and Bioactive Materials, Volume II View all 13 articles
Oral squamous cell carcinoma (OSCC) is the most aggressive oral and maxillofacial malignancy with a high incidence and low survival rate. OSCC is mainly diagnosed by tissue biopsy, which is a highly traumatic procedure with poor timeliness. Although there are various options for treating OSCC, most of them are invasive and have unpredictable therapeutic outcomes. Generally, early diagnosis and noninvasive treatment cannot be always satisfied simultaneously in OSCC. Extracellular vesicles (EVs) are involved in intercellular communication. EVs facilitate disease progression and reflect the location and status of the lesions. Therefore, EVs are relatively less invasive diagnostic tools for OSCC. Furthermore, the mechanisms by which EVs are involved in tumorigenesis and tumor treatment have been well studied. This article dissects the involvement of EVs in the diagnosis, development, and treatment of OSCC, providing new insight into the treatment of OSCC by EVs. Different mechanisms, such as inhibiting EV internalization by OSCC cells and constructing engineered vesicles, with potential applications for treating OSCC will be discussed in this review article.
Oral squamous cell carcinoma (OSCC) is a malignant tumor mainly originating from squamous epithelial cells. In 2018, there were 354,864 new cases and 177,384 deaths of OSCC (Bray et al., 2018). OSCC is usually diagnosed in advanced stages, which leads to a poor prognosis. Researchers attempted to find new methods for early diagnosis of OSCC. Currently, the main treatment of OSCC is still surgery combined with chemotherapy, and the main chemotherapy regimen includes 5-fluorouracil (5-FU) and cisplatin (Longley et al., 2003). However, 5-year survival remained low, mainly due to chemoresistance (Epstein et al., 2012; Chi et al., 2015). Therefore, it is crucial to improve the diagnostic approach and treatment method in OSCC.
EVs have been a main focus of research in recent years. They are membrane-derived vesicles secreted by different cells and widely found in body fluids. According to their biogenesis and releasing mode, EVs can be divided into microvesicles, exosomes, and apoptotic bodies. They carry DNA, RNA, protein, miRNA, and other substances (Doyle and Wang, 2019) (Figure 1). EVs widely exist in tumor microenvironment (TME) and participate in cellular communication. EVs have the potential to be used for minimally invasive diagnosis because they are involved in tumorigenesis and they contain is a biologic sample from tumor cells. EVs exist in various body fluids and are easy to obtain. In addition, EVs regulate cell function by delivering their vesicular substances, thus participating in the development of OSCC (van Niel et al., 2022). More and more attention has been recently paid to the role of EVs in tumorigenesis (Brinton et al., 2015; Bebelman et al., 2018; Urabe et al., 2020). EVs are involved in many important processes in OSCC (Kaiser, 2016), such as angiogenesis and epithelial-mesenchymal transformation (EMT). Tumor-associated macrophages (TAM) are the main cell in TME and promote tumor development. EVs can regulate the phenotypic transition of macrophages. EVs are internalized by macrophages, and their signaling molecules regulate the polarization of macrophages.
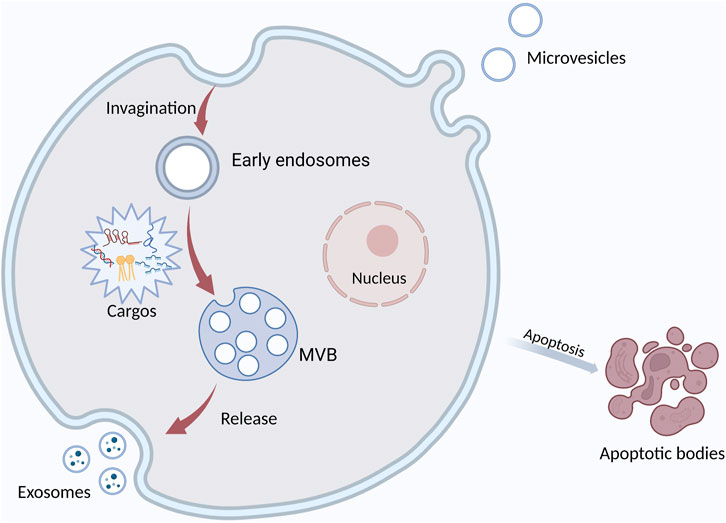
FIGURE 1. The formation and secretion of EVs. Extracellular vesicles are mainly classified into three main types: exosomes, microvesicles, and apoptotic bodies. Exosomes are formed by the fusion of multivesicular bodies (MVBs) with the plasma membrane, MVs form directly from the plasma membrane to the outward bud, and apoptotic bodies are released by the cells after apoptosis. It contains DNA, miRNA, protein, and other cargos.
In this review, we have discussed how EVs are involved in the diagnosis and development of OSCC. By understanding the role of EVs in the development of OSCC, it would be possible to design more effective therapeutic regimens. Moreover, by modifying the surface characteristics and contents of EVs, their targeting ability and treatment efficiency can improve (Herrmann et al., 2021). Therefore, producing manipulated EVs is a promising method for treating OSCC. So far, few review articles comprehensively analyzed the efficacy of EVs in the diagnosis, tumorigenesis, and treatment of OSCC, and most of them only analyzed some of these topics. Therefore, we summarized the role of EVs in the development, diagnosis, and treatment of OSCC. In addition, whether EVs can improve the survival rate of OSCC patients is also discussed.
EVs contain several types of biological molecules, including proteins, lipids, DNA, and RNA, which play important roles in the development and progression of OSCC. Particularly, they are involved in several biological processes such as proliferation, apoptosis, DNA repair, metabolism, angiogenesis, and immune response (Becker et al., 2016; Ciardiello et al., 2016; Almeria et al., 2019; Kalluri and LeBleu, 2020). Some constituents of EVs are upregulated and others are downregulated to contribute to tumor cell proliferation and invasion. Non-coding RNAs, including miRNA, lncRNA, and circ RNA, along with proteins, have been increasingly investigated as diagnostic and prognostic markers for OSCC. Apart from tissue specimens, diagnostic EVs are most often collected from body fluids, such as saliva and serum, or cell culture supernatants. Body fluid-derived EVs can be easily used for minimally invasive early diagnosis and prognosis (Li et al., 2021).
Exosomal RNAs are heavily involved in the interaction between tumor cells and TME (Table 1). Carcinoma-derived exosomal RNAs can promote TME remodeling, and exosomal RNAs originating from TME can also affect tumor cell behavior. By regulating tumor cell-TME crosstalk, exosomal RNAs can facilitate tumorigenesis, tumor growth, and metastasis (Fang et al., 2020; Tan et al., 2020; Forder et al., 2021; Lu et al., 2021; Villegas-Pineda et al., 2021; Zhao et al., 2022). Therefore, RNAs in EVs have increasingly received attention for OSCC diagnosis.
miRNAs, also known as small non-coding RNAs, have 19–24 nucleotides and post-transcriptionally regulate gene expression in several physiological and pathological processes (Lu et al., 2021). Circulatory miRNAs exist in three forms: free circulating miRNAs, exosomal miRNAs, and conjugated with argonaute proteins (Ago2) (Sahu and Routray, 2021). Free miRNAs in saliva, serum, and plasma can accelerate the less-invasiveness diagnosis of OSCC and improve patients’ compliance compared with conventional methods like tissue biopsy and mucosal scraping. Therefore, they received significant attention as a diagnostic method for oral cancer in recent years (He L. et al., 2020). Exosomes contain complex payloads encapsulated by a lipid bilayer, which improves the stability of miRNAs and allows them to resist nucleases (Sahu and Routray, 2021). Recent studies have elucidated that exosomes are deeply involved in the regulation of intercellular communication (Kalluri and LeBleu, 2020). Exosomal miRNAs modulate various disease progression by delivering signaling molecules and miRNAs. Oncogenic miRNAs (oncomiRs) and tumor-suppressing miRNAs (TS-miRNAs) are two subtypes of miRNAs that can be distinguished based on their regulatory roles in oncogenesis (Chen and Huang, 2021).
Exosomal miRNAs with differential expression in OSCC cells and normal cells have been the focus of research in recent years. Salivary exosomes from OSCC patients and exosomes derived from OSCC tissues had significantly higher levels of miR-24-3p compared with normal controls. The ROC curve revealed that the area under the curve (AUC) for salivary exosomes was 0.738 (He L. et al., 2020). Therefore, miR-24-3p in salivary exosomes can potentially help the early diagnosis of OSCC (He L. et al., 2020). As a potential diagnostic biomarker, miR-30a was significantly overexpressed in exosomes and OSCC tissues, with an AUC value of 0.812. A high level of miR-30a also predicted shorter recurrence-free survival (He T. et al., 2021). Similarly, miR-210 had significantly higher levels in plasma-derived EVs from OSCC patients than in plasma-derived EVs from control samples (AUC = 0.951) (Bigagli et al., 2021). miR-365 was expressed in OSCC cell lines and transferred into EVs produced by OSCC cell lines (Coon et al., 2020). Similar to miR-365, miR-23a-3p was significantly upregulated in OSCC cell lines (SCC-9 and CAL-27). Intriguingly, exosomes derived from OSCC cell lines contained higher levels of miR-23a-3p compared with OSCC cells. miR-23a-3p-rich tumor-derived exosomes (TEX) can induce macrophage polarization toward the M2 subtype, associated with tumor growth and invasion (Cai and Qiao, 2019). A miRNA array confirmed that compared with conjugated normal cells, oncogenic miR-155 and miR-21 were upregulated by two to five folds in exosomes derived from primary OSCC cells. They promote cell proliferation and invasion by downregulating PTEN and Bcl-6 tumor suppressor genes in OSCC cells (Chen and Huang, 2021). miR-21-5p is the most abundant microRNA (miR) in EVs released from OSCC cells. miR-21-5p promotes tumor cell stemness and migration (Chen et al., 2019). Two key oncomiRs, miR-342-3p and miR-1246, are highly expressed in OSCC-derived exosomes. They were transferred to HOC313-P cell and enhanced its motility by exosomes derived from HOC313-LM cell, a type of human OSCC cell line with high metastatic capacity (Sakha et al., 2016). miR-302b-3p and miR-517b-3p were only detected in salivary EVs from OSCC patients. miR-512-3p and miR-412-3p had higher expression levels in OSCC patients (Gai et al., 2018). In addition, the oxygen concentration in TME can affect the contents of OSCC-derived exosomes. OSCC-derived exosomes can enhance the metastasis and invasiveness of OSCC cells under hypoxic conditions (Li and Zhu, 2016). miRNA sequencing explains how exosomes from OSCCs in normoxic and hypoxic environments differently express miRNAs. Of 108 differentially expressed miRNAs in OSCCs, miR-21 exhibited the greatest difference. It was upregulated in OSCCs and induced EMT, which is needed for tumor migration and invasion. Exosomal miR-21 level may be used as a diagnostic marker in OSCC (Li and Zhu, 2016). Various onco-miRNAs are upregulated in OSCC-derived EVs, which can serve as diagnostic markers.
Although exosomal miRNAs have several advantages as OSCC biomarkers, their application is still controversial because the sensitivity of isolation methods alters the composition of exosomes. Furthermore, smoking, alcohol abuse, and HPV infection can upregulate exosomal miRNAs in the serum samples of healthy participants. Moreover, exosomal miRNAs can be upregulated in other malignant tumors like breast cancer, bladder cancer, hepatocellular carcinoma, and Hodgkin’s lymphoma (He L. et al., 2020).
On the other hand, exosomal miR-126, a TS-miRNA, is downregulated in OSCC. miR-126 downregulates epidermal growth factor-like domain multiple 7 (EGFL7), a tumor-suppressor in OSCC, thereby regulating vascular endothelial growth factor (VEGF), Notch, and Wnt signaling pathways. (Chen and Huang, 2021). Previously, it was found that miR-101-3p has significantly higher expression in normal tissues compared with adjacent malignant tissues. Similarly, it was shown that miR-101-3p is downregulated in several OSCC cell lines such as CAL27, SCC9, and TCA8113 (Xie et al., 2019a, 101-3). According to in-vitro and in-vivo experiments, exosomes transfer miR-101-3p from hBMSCs to OSCC cells to prevent OSCC progression (Xie et al., 2019a). Although TS-miRNAs have not yet been investigated comprehensively, they are promising for both diagnosis and treatment of OSCC.
It is worth noting that other exosomal non-coding RNAs always function through interaction with miRNAs. Bioinformatics analysis revealed that exosomal circ_0000199, a circRNA originating from the end of an RNA molecule, sponges particular miRNAs, such as miR-145-5p and miR-29b-3p, to upregulate oncoproteins in OSCC cells. Researchers found that exosomal circ_0000199 expression is associated with TNM stage, tumor size, and lymphatic metastasis and that circ_0000199 overexpression shortens the survival of OSCC patients (HR 3.57; 95% CI 2.48-6.24, p = 0.0035) (Luo et al., 2020). M1 macrophage-derived exosomal lncRNA HOTTIP can decrease proliferation, induce apoptosis, and enhance M1 phenotype differentiation in head and neck squamous cell carcinomas (HNSCC) (Jiang et al., 2022). Exosomal lncRNA LBX1-AS1 is released by RBPJ-OE macrophages. It competes with miR-182-5p to prevent the growth of tumor cells by regulating Forkhead Box O (FOXO) expression (Ai et al., 2021, 1). lncRNA Lnc-CAF is remarkably upregulated in HSC3 cell-derived exosomes. It can be absorbed by cancer-associated fibroblasts (CAFs) to upregulate Lnc-CAF. This forms a positive feedback loop and causes Lnc-CAF co-expression with IL-3, which promotes OSCC growth (Ding et al., 2018). Similar to miRNAs, other non-coding RNAs can help in the early detection of OSCC. However, there are only a few studies investigating the role of other types of exosomal non-coding RNAs in OSCC. circ IGHG is highly expressed in OSCC and induces EMT, suggesting a promising potential for theranostics, but still, further studies are needed (Liu et al., 2021).
Previous articles have reviewed several proteins used as hallmarks of OSCC prognosis (Sasahira and Kirita, 2018); nevertheless, exosomal proteins are extensively diverse and our knowledge about their functions is rapidly growing. Intriguingly, it was unfolded that PTEN, a crucial tumor suppressor protein for angiogenesis, is downregulated by miR-130b-3p in OSCC cells; however, PTEN mRNA level did not significantly change compared with adjacent healthy tissues (Yan et al., 2021). It has been suggested that PTEN and PDCD4 both downregulate STAT3 (Chen et al., 2019). STAT3 silencing reduced miR-21-5p level (Chen et al., 2019), which has been introduced as an oncomiR in previous sections. SWATH-MS analysis between healthy, OSCC_NLNM, and OSCC_LNM groups, showed that small proline-rich protein 3 (SPRR3) is significantly upregulated in the salivary small extracellular vesicles (S/SEVs) of OSCC-free participants (Fontana et al., 2021). Recently findings indicated how low expression of SPRR3 is involved in OSCC progression (Yu et al., 2020). In addition, the SWATH-MS analysis uncovered that some proteins (like lipocalin-2, LCN2, and S100) related to anti-microbial defense and inflammatory response are enriched in the S/SEVs of OSCC patients with lymph node metastasis (Fontana et al., 2021). Other studies also unveiled that they can serve as diagnostic and prognostic biomarkers of OSCC (Raffat et al., 2018; Rahimi et al., 2020). Laminin-332 was upregulated in EVs produced by OSCC cell lines (LN1-1) and in plasma EVs derived from OSCC patients with lymph node metastasis. After being absorbed by lymphatic endothelial cells (LECs), Laminin-332 promotes LEC migration and tube formation (Wang et al., 2019). ApoA1, CXCL7, PF4V1, and F13A1 have potential application as novel circulating biomarkers of OSCC with LNM (Li L. et al., 2019). CKLF-like MARVEL transmembrane domain-containing 6 (CMTM6) was highly expressed in OSCC specimens. Besides, it was revealed that exosomal CMTM6 can be transferred to macrophages and regulate macrophage polarization (Pang et al., 2021). HSP90ɑ and HSP90β, as molecular chaperones, were markedly increased in EVs from metastatic cell lines and predicted poor prognosis of OSCC (Ono et al., 2018). Matrix metallopeptidases (MMPs) facilitate OSCC metastasis by degrading ECMs (Khan et al., 2020). MMP9 was strongly upregulated in exosomes from tumor cells (Qadir et al., 2018). Mechanistically, these results imply that proteins in EVs can serve as useful biomarkers of OSCC progression and metastasis. Apart from cargo proteins in EVs, some surface markers can also help with diagnosis. Despite CD63, CD81, and CD9 are downregulated in patients with oral cancer (OC) (Zlotogorski-Hurvitz et al., 2016). It was unveiled that life expectancy after surgery was correlated with the plasma level of exosomal CD63 (Rodríguez Zorrilla et al., 2019). Centrosomal protein (CEP55) was found on tumor-derived exosomes in HNCC, but not in primary human oral keratinocytes (Qadir et al., 2018). However, the specific mechanisms by which CD molecules and other transmembrane proteins are involved in OSCC progression remain unclear and further studies are warranted.
Taken together, these biomarkers have great potential for the diagnosis and prognosis of OSCC. Although our information about exosomal proteins, especially about exosomal surface proteins, is limited. CD markers lack stability and enough specificity. Future studies on exosomal proteins are needed to improve early diagnosis and prognosis in OSCC.
Since the constituents of EVs can be used as diagnostic markers in OSCC, EVs may be involved in the development of OSCC. Herein, we review key components of OSCC development (Figure 2), such as TAM, polarization, angiogenesis, and EMT, and enumerate the role of EVs in these processes. In addition, we also elucidate the mechanism by which EVs promote chemotherapy resistance in OSCC and more comprehensively dissect the role of EVs in OSCC development.
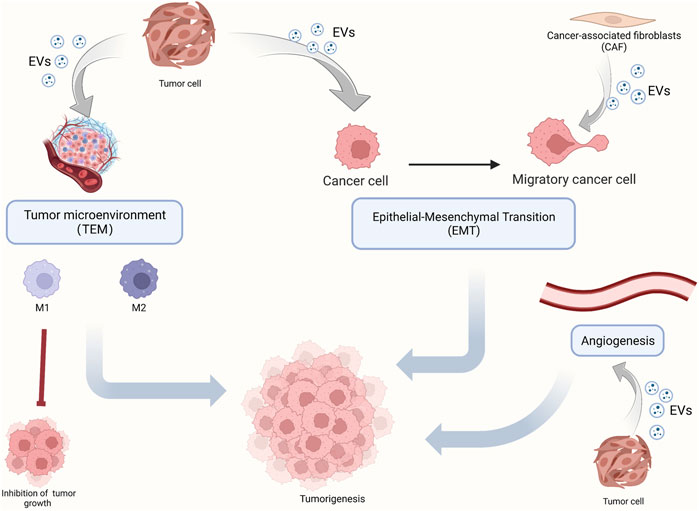
FIGURE 2. The role of EVs in OSCC tumorigenesis. OSCC tumorigenesis mainly includes the polarization of macrophages in the tumor microenvironment, epithelium-mesenchymal transformation, and angiogenesis. EVs carry molecular signals which play a regulating role.
TME supports the growth of tumor cells. TME is composed of immune cells, stromal cells, extracellular matrix, and other components. The interaction between tumor cells and TME is crucial for tumor growth (Joyce and Pollard, 2009). TAMs are the main cells in TME and contribute to tumor development to varying degrees (Ruffell et al., 2012; Coussens et al., 2013; Mantovani et al., 2017). Recent studies have indicated that TAMs include M1 (classically activated macrophage) and M2 (alternatively activated macrophage) subtypes (Laviron and Boissonnas, 2019). OSCC cells and macrophages can release EVs that regulate TME. M1 macrophages have been described as the pro-inflammatory phenotype and play an important role in anti-microbial defense. M2 macrophages have been described as the anti-inflammatory phenotype and facilitate post-inflammatory tissue repair (Baig et al., 2020).
Cancer cells release various EVs (Akers et al., 2013; Doyle and Wang, 2019). Several products of cancer cells can regulate macrophage polarization through the paracrine mechanism. EVs released by cancer cells can be internalized by macrophages and modulate the phenotypes of macrophages (Bellmunt et al., 2019; Baig et al., 2020). Many studies have suggested that M1 macrophages are antitumor phenotypes (Ai et al., 2021). M1 exosomal lncRNA HOTTIP inhibits HNSCC progression by competitively absorbing miRNA-19a-3p and miRNA-19b-3p and activating TLR5/NF-κB signaling pathway (Jiang et al., 2022). miRNA-9 is a key miRNA for tumor growth, metastasis, immunity, and radiosensitivity. Exosomes secreted by HPV + HNSCC contain miRNA-9, which downregulates PPARδ in macrophages, thereby inducing M1 polarization and increasing radiosensitivity in HNSCC (Tong et al., 2020). Recent studies have shown that M1-like cells also promote tumor development. The high invasiveness of M1-like TAM has been associated with the aggressive characteristics of some cancers. Similarly, M1-like TAM promoted tumor cell migration in OSCC (Gao et al., 2018; He Y. et al., 2020; Oshi et al., 2020). OSCC cells secrete EVs containing THBS1 that promotes macrophage polarization into M1-like cells. M1-like macrophages secrete IL-6, which activates Jak/STAT3 pathway in OSCC cells and promotes EMT, OSCC stemness, and THBS1 transcription (Xiao et al., 2018; You et al., 2022). In conclusion, the positive feedback loop between M1-like TAM and OSCC cells regulates EMT and cancer stemness (You et al., 2022). THBS1 is the most abundant protein secreted by OSCC. It is a multifunctional protein and an effective regulator of macrophage activation (Pal et al., 2016). In summary, M1-like macrophages are no longer considered the tumor-resistant phenotype, and they can promote tumor progression in certain circumstances.
EVs provide a new method of information exchange between cells. Tumor cells interact with other cells in the TME by miRNA-riched exosomes (Brinton et al., 2015). TEM is mainly composed of macrophages, whose function can be regulated by OSCC-derived exosomes. M2-like macrophages promote tumor development (Baig et al., 2020). Endoplasmic reticulum stress was positively correlated with poor survival in OSCC patients. Many studies showed that programmed cell death-ligand 1 (PD-L1) prevents T cell activation and contributes to tumor immune escape (Xie F. et al., 2019; Daassi et al., 2020). Studies have shown that endoplasmic reticulum stress enhances PD-L1-rich exosome secretion by OSCC cells, thereby promoting M2 polarization of macrophages. M2-like macrophages impair the cytotoxic response of CD8+ T cell immune response and promote tumorigenesis (Yuan et al., 2022). CMTM6 is a key regulator of immune response in cancer. OSCC cells can release CMTM6-rich exosomes to activate ERK1/2 signaling pathway in macrophages, induce an M2-like phenotype, and promote tumor progression (Pang et al., 2021). The study also proved that CMTM6 deletion can reduce the proliferation and migration of OSCC cells, providing a new idea for treating OSCC.
EVs contain proteins, DNA, and miRNA. They precisely regulate tumor cell communication with neighboring cells and distant cells. Tumor cells can use EVs to reprogram signaling pathways in target cells and promote angiogenesis (Qadir et al., 2018). EVs can carry tumor antigens and specific proteins involved in vesicle formation and transportation (Baietti et al., 2012). Angiogenesis is essential for tumor development. Neovascularization provides oxygen supply and nutrients for the tumor cells. Increased angiogenesis and upregulation of VEGF reduce the overall survival rate of patients with OSCC (Kyzas et al., 2005). Therefore, understanding the mechanism of angiogenesis helps the targeted therapy in OSCC. Among 45 OSCC patients, higher levels of circulating microparticles (MPs) were significantly associated with tumor size, lymph node metastasis classification, vascular density, and VEGF expression. Circulating MPs isolated from OSCC patients can be internalized by human umbilical vein endothelial cells (HUVEC) and promote endothelial cell proliferation, migration, invasion, angiogenesis, and expression of pro-angiogenic factors (Ren et al., 2016). Studies have shown that EVs isolated from HNSCC cells can induce angiogenesis. EPHB2 carried by EVs can promote angiogenesis by inducing ephrin reverse signal transduction, thereby promoting tumor cell survival and metastasis (Sato et al., 2019). Many studies have shown that miRNAs carried by EVs can regulate angiogenesis in HNSCC (Panvongsa et al., 2022). AS a tumor suppressor gene, PTEN plays an important role in tumorigenesis. Exosomes carrying miRNA-130B-3P can promote angiogenesis by downregulating PTEN expression (Yan et al., 2021). OSCC-derived exosomes carry miRNA-221, which downregulates PIK3R1 and promotes HUVEC migration and duct formation (He S. et al., 2021). Many studies attempted to inhibit angiogenesis, thereby preventing OSCC development (Rosenberger et al., 2019). It has been suggested that exosomes of human deciduous stem cells carry miRNA100-5P and miRNA-1246, which inhibit angiogenesis (Liu P. et al., 2022).
EMT is a critical process in tumorigenesis. EMT is characterized by reduced expression of cell adhesion molecules and transformation of the cytoskeleton from keratin to vimentin. Epithelial cells are closely connected; however, mesenchymal cells have different morphologies, providing a higher invasion and migration capacity (Yeung and Yang, 2017). EMT is divided into three types. Type three EMT has been a research hotspot in recent years, as it is related to tumorigenesis. Identification and inhibition of key molecules in EMT is an important prerequisite for controlling tumor development. Tumor-derived exosomes (TDEs) can promote EMT, thereby enhancing invasion and migration capacity (Syn et al., 2016). CAFs also play a critical role in EMT. Many studies have shown that CAFs can interact with tumor cells via exosomes, and exosomal miRNAs can participate in oncogenesis (Yang et al., 2017). Transcription factors can directly modulate gene expression in EMT. Changes in RNA expression can also regulate EMT. The initiation and progression of EMT need different signaling pathways, and these pathways interact with each other as a network (Lamouille et al., 2014). As a bidirectional regulatory process, the interaction between EVs and tumor cells markedly differs based on the constituents of EVs. For instance, fibroblasts transfer exosomal miRNA-34a-5p to OSCC cells and may be involved in OSCC progression via the AKT/GSK-3β/β-catenin/Snail signaling pathway. These findings can help the treatment of OSCC. miRNA-34a-5p/AXL axis inhibitors may treat OSCC (Li et al., 2018). Currently, many studies are investigating EMT-based interventions to inhibit tumorigenesis.
Chemoresistance can markedly undermine the efficacy of cisplatin and 5-FU, as the main chemotherapeutic agents for OSCC (Longley et al., 2003).
EVs can impair chemosensitivity and induce chemoresistance in OSCC through various mechanisms, thereby lowering the survival rate among OSCC patients. The constituents of exosomes, drug efflux by EVs, changes in vesicular pH, the anti-apoptotic signal transmitted by EVs, regulation of DNA repair mechanisms, immune response, and induction of cancer stemness and EMT by EVs are all involved in such outcomes (Law et al., 2021).
Many studies have shown that miRNAs mediate chemoresistance in tumor cells (Chen et al., 2014a; Chen et al., 2014b). OSCC cells with 5-FU resistance secreted APCDD1L-AS1-rich exosomes, which targeted miRNA-1224-5p and regulated miR-1224-5p/nuclear receptor binding SET domain protein 2 (NSD2) to induce 5-FU resistance (Li et al., 2021c). EMT enhances chemoresistance (Wang et al., 2016). Cells with acquired chemoresistance (cisRes90-OSCC) release exosomal miRNA-155, which targets FOXO3a and regulates EMT in OSCC cells (Kirave et al., 2020). Studies have shown that cisplatin-resistant cell lines (HSC-3-R, SCC-9-R) can transfer miRNA-21a to OSCC cells through exosomes. miRNA-21a induces cisplatin resistance by targeting PTEN and programmed cell death 4 (PDCD4) (Liu et al., 2017). Stromal cells such as macrophages can modulate the sensitivity of tumor cells to chemotherapeutic agents. It has been shown that macrophage-derived exosomes can reduce the sensitivity of OSCC cells to chemotherapeutic agents by activating the AKT/GSK-3β signaling pathway (Tomita et al., 2020).
The combination of surgery and chemotherapy is the most common and efficient therapeutic approach for patients with OSCC. In addition to chemotherapy resistance, which is common in OSCC, chemotherapeutics also cause major side effects, such as bone marrow depression, nephrotoxicity, gastrointestinal discomfort, skin and tongue lesions, and severe weight loss (Wang et al., 2015; Huang et al., 2020). Therefore, alternative therapeutic approaches are needed.
EVs from other cells may modulate some pathophysiological processes such as macrophage polarization, angiogenesis, and EMT through paracrine mechanism (Ohno et al., 2013; Kamerkar et al., 2017). Recently, inhibiting the ability of OSCC cells to endocytose EVs from TME has demonstrated satisfactory therapeutic efficacy (Fujiwara et al., 2018; Sasabe et al., 2022) (Figure 3).
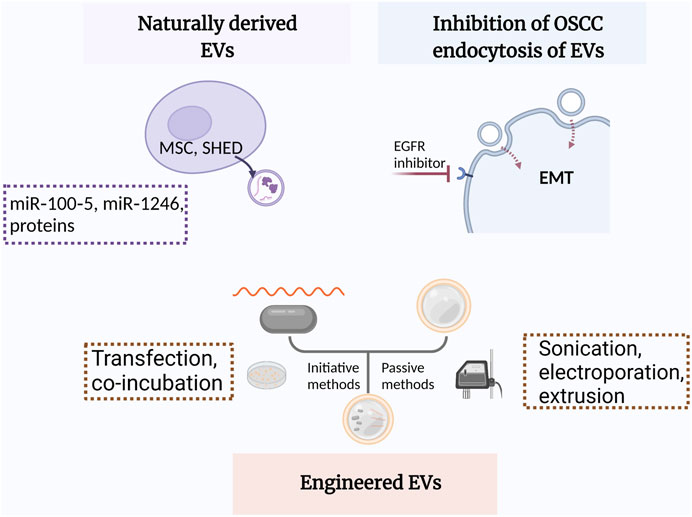
FIGURE 3. Schematic illustration of EVs applied in OSCC treatment. MSC, mesenchymal stem cells; SHEDs, stem cells of human exfoliated deciduous teeth; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transition.
Mesenchymal stem cells (MSCs) can either suppress or promote immune response (Li et al., 2012; Liang et al., 2021). MSC-derived EVs can be used in cancer treatment. miR-16 from MSC-derived EVs can inhibit breast cancer growth by downregulating VEGF and subsequent angiogenesis (Lee et al., 2013). Similarly, exosomes derived from human exfoliated deciduous teeth (SHEDs) can attenuated angiogenesis in OSCC. A xenograft transplantation model indicated that miR-100-5p and miR-1246 carried by SHED-Exos can inhibited angiogenesis (Liu P. et al., 2022). However, MSC derived EVs are regarded as a double-edged sword due to promoting tumor progression. More studies are needed to overcome this issue.
Nanotechnology, particularly the development of different types of nanoparticles such as liposomes and biomimetic nanoparticles, has considerably improved the targeted treatment of tumors (Zhang et al., 2020). After being modified through initiative (transfection and co-incubation) or passive methods (freeze–thaw cycles, sonication, electroporation, and extrusion), nanoparticles can be loaded with chemotherapeutic agents, specific miRNAs, or other cargoes (Ebnoether and Muller, 2020; Zhang et al., 2021).
Modified EVs applied in other diseases can be similarly used for treating OSCC. Engineered EVs are now extensively used as drug carriers. We have previously constructed engineered neutrophil-derived apoptotic bodies (eNABs) loaded with hexyl 5-aminolevulinate hydrochloride (HAL) to ameliorate cardiac infarction (Bao et al., 2022). We also produced chimeric apoptotic bodies (cABs) preloaded with microRNA-21 to modulate inflammatio (Dou et al., 2020). As cancer immunotherapeutic drug carriers, dendritic cell-derived exosomes (DCs-Exo) loaded with antigens have been used for treating malignant melanoma (Nikfarjam et al., 2020). For specifically targeting cancer cells and preventing rapid clearance of drugs, specialized exosomes were constructed using iEXO-OXA platform and MSC-derived exosomes with superficial alteration with oxaliplatin (OXA) (Zhou et al., 2021) Through surface modification, CD44 facilitates lipid-mimetic-chains-grafted HA-modified EVs penetration into tumoral tissues and DOX delivery to tumor cells (Liu et al., 2019). Future improvements in EV design can improve the targeted therapy of OSCC. Different sources of biomimetic EVs have been used in previous studies to improve the efficiency of engineered EVs. Intriguingly, engineered bovine milk exosomes linked to doxorubicin via a special bond were packed with endoperoxides and chlorin e6 (Ce6) through a series of reactions. Producing exosomes by this method can overcome the low efficiency and ethical issues caused by using natural vesicles (Zhang et al., 2020).
In addition to promoting the selective uptake of therapeutic EVs by tumor cells, it is feasible to inhibit EVs endocytosis by phagocytic cells. Both upregulation of CD47 (Mathieu et al., 2019) and downregulation of integrins α6β4 and αvβ5 on the surface of exosomes can prevent tumor cell metastasis (Hoshino et al., 2015).
Although much experience has been provided about engineered EVs in other cancers, few studies have been conducted in OSCC. Some researchers have found that the constituents of EVs can inhibit OSCC growth. Exosomal miR-34a-5p targets AXL, thereby inhibiting the proliferation and motility of OSCC cells through AKT/GSK-3β/β-catenin/Snail signaling pathway. But miR-34a-5p has low expression in OSCC cells. It suggests that EVs can be loaded with inhibitors of the miR-34a-5p/AXL axis to treat OSCC (Li, 2018). In fact, some miRs are enriched in exosomes to function efficiently. For instance, γδT cells infected with lenti-miR138 virus can produce miR-138-rich γδTDEs, which can be used in the treatment of OSCC. miR-138 targets PD-1 and CTLA-4 in CD8+ T cells and promotes anti-tumor immunity (L et al., 2019). Apart from miRs, some onco-miR inhibitors can be transported by EVs. Exosomes transfected with calcium chloride, a miR-155 suppressor, can upregulate FOXO3a and induce mesenchymal-epithelial transformation (MET) in cisplatin-resistant OSCC spheroids and in vivo, thereby reversing chemoresistance (Sayyed et al., 2021).
As mentioned previously, exosomes play a crucial role in the development of OSCC. Identifying the mechanism of exosome uptake can be promising in the treatment of OSCC. Epithelial growth factor receptor (EGFR) inhibition and knockdown both can inhibit exosome uptake by OSCC cells, thereby impeding OSCC invasion and progression. Similarly, OSCC development is influenced by the inhibitor of micropinocytosis (EIPA) (Sasabe et al., 2022). For example, cetuximab is assumed to be a therapeutic antibody for OSCC because it blocks EMT in EGFR-rich OSCC-EVs (Fujiwara et al., 2018).
Although most researchers believe that EVs are biocompatible even when administered repeatedly, their effects on other cells are still unclear. There are still many challenges, such as purification issues and immune properties, to be addressed before the clinical application of engineered EVs (Ketabat et al., 2019). Still, more progress in the field of engineered EVs is needed before their clinical application in OSCC.
Currently, EVs can be classified by their origins, including cell culture-derived EVs, body fluid-derived EVs, and tissue-derived EVs (Ti-EVs). Cells lose their heterogeneity after several in-vitro passages, which can mask their original biological characteristics. In particular, a two-dimensional (2D) cultural environment can barely stimulate the in-vivo microenvironment (Jensen and Teng, 2020). Furthermore, EVs extracted at a specific time obscured the temporal nature of their actions, which could not reflect the course of the disease. In addition, EVs cannot be easily purified from the body fluid, and they are commonly contaminated with various proteins, such as serum proteins. However, Ti-EVs contain less impurities than body fluid-derived EVs. Studies have shown that Ti-EVs promote the development and metastasis of tumors (Costa-Silva et al., 2015; Hoshino et al., 2015; Liu and Cao, 2016). As mentioned previously, TEM-derived EVs are involved in the development of OSCC by regulating macrophage polarization, angiogenesis, and EMT (Xie et al., 2019b). Therefore, more attention should be paid to Ti-EVs in the future stage. There are still some challenges to be solved in the extraction of Ti-EVs, such as its challenges regarding their isolation and purification. It is necessary to develop standardized extraction methods, characterization methods, and titer evaluation methods to improve the reliability of Ti-EV (Li et al., 2021).
As previously mentioned, EVs can carry substances that contribute to the development and resistance of OSCC, making them novel diagnostic tools for OSCC (Han et al., 2018). Due to their presence in various body fluids, EVs are easy to acquire. Particularly, salivary EVs can be obtained at different stages of OSCC, without causing any discomfort to patients. This accelerates the use of EVs for diagnosing OSCC, following its progression, and developing more effective treatment methods.
However, there are still many problems to be solved before using EV as a new diagnostic method. The first problem is the lack of uniform diagnostic criteria. The contents of EVs derived from various cells are different. For instance, EVs derived from tumor parenchyma and those derived from immune cells in TEM are different (Liu P. et al., 2022). Secondly, most articles focus on the role of a single molecule. However, EVs have a variety of markers on their surface and carry various signaling molecules. Whether a single molecule or a combination of multiple molecules can be used as a diagnostic method needs to be clarified in the future. This also suggests that EVs can simultaneously target several signaling pathways.
Recently, some studies have shown that nano-flow cytometer (nFCM) can be used to detect DNA in EVs, which can be a more comprehensive method to determine the source of EVs for tumor diagnosis (Liu H. et al., 2022). In addition, it has been reported that microfluidic devices can be used to analyze and characterize single EVs to determine their exact physiological effects. It can become a new method of minimally invasive diagnosis of diseases (Bordanaba-Florit et al., 2021).
EVs are increasingly recognized as a promising drug delivery system due to their unique physiological characteristics, including excellent biocompatibility, biodegradability, non-immunogenicity, and targeted delivery (Ha et al., 2016). However, EVs from various sources have their advantages and limitations. For instance, immune cell-derived EVs are highly targeted but not lethal; MSC-derived EVs have only anti-inflammatory and regenerative properties, and macrophage-derived EVs can regulate TEM and stimulate T cell activation. Therefore, engineering EVs, which can enhance their advantages and improve their limitations, has received much attention. Engineered EVs can be modified in an active or passive manner to enhance their therapeutic effects (Zhang et al., 2021). For example, car-T through the chimeric antigen receptor, improves the targeting specificity of T cells. Therefore, EVs can from car-T cells may become a new method of tumor treatment.
There are several challenges associated with EV-based treatment, including: 1) time-consuming and low-yield extraction process through ultra-centrifugation. 2) low efficiency of drug loading. 3) short duration of action in vivo. These issues necessitate further research and development, with a focus on constructing more effective engineered EVs.
EVs have emerged as a significant players in the diagnosis, development, drug resistance, and treatment of OSCC, and the precise mechanisms remain incompletely understood. This article aimed to review the involvement of EVs in the diagnosis, prognosis, tumorigenesis, drug resistance, and treatment of OSCC and outline the challenges associated with the use of EVs.
Recent studies indicated that EVs can be used as new diagnostic tools. As EVs can be easily obtained from saliva, blood, and other body fluids, the identification of their content is the least invasive diagnostic method. EVs are involved in many processes during OSCC development. They can regulate TAM polarization and promote or inhibit tumor development. EVs can also interact with tumor cells to promote angiogenesis, which is essential during tumorigenesis. Furthermore, EVs promote EMT, thereby facilitating tumor progression and invasion. EVs are also involved in drug resistance, leading to poor prognosis in OSCC. This article discussed natural EVs and engineered EVs. It also explained how to inhibit OSCC development by inhibiting EV endocytosis by tumor cells. Engineered vesicles can improve the treatment of OSCC.
In summary, EVs play a crucial role in the pathogenesis and progression of OSCC. Standardizing the diagnostic pattern and producing engineered vesicles for treating OSCC require more attention in the future. By addressing these challenges, we can accelerate the clinical translation of this promising biotechnology.
YZ and FJ contributed to the conception and design of the study. YZ and JL designed and wrote the whole manuscript sections. YZ and JL designed all Figures of the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (grant number 82170988), the National Key Research and Development Program of China (2022YFA11044003), and Chinese Stomatological Association Youth Clinical Research Foundation for Orthodontics (grant number CSA-02020-01).
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ai, Y., Wei, H., Wu, S., Tang, Z., Li, X., and Zou, C. (2021). Exosomal LncRNA LBX1-AS1 derived from RBPJ overexpressed-macrophages inhibits oral squamous cell carcinoma progress via miR-182-5p/FOXO3. Front. Oncol. 11, 605884. doi:10.3389/fonc.2021.605884
Akers, J. C., Gonda, D., Kim, R., Carter, B. S., and Chen, C. C. (2013). Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol 113, 1–11. doi:10.1007/s11060-013-1084-8
Almeria, C., Weiss, R., Roy, M., Tripisciano, C., Kasper, C., Weber, V., et al. (2019). Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front. Bioeng. Biotechnol. 7, 292. doi:10.3389/fbioe.2019.00292
Baietti, M. F., Zhang, Z., Mortier, E., Melchior, A., Degeest, G., Geeraerts, A., et al. (2012). Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677–685. doi:10.1038/ncb2502
Baig, M. S., Roy, A., Rajpoot, S., Liu, D., Savai, R., Banerjee, S., et al. (2020). Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 69, 435–451. doi:10.1007/s00011-020-01318-0
Bao, L., Dou, G., Tian, R., Lv, Y., Ding, F., Liu, S., et al. (2022). Engineered neutrophil apoptotic bodies ameliorate myocardial infarction by promoting macrophage efferocytosis and inflammation resolution. Bioact. Mater. 9, 183–197. doi:10.1016/j.bioactmat.2021.08.008
Bebelman, M. P., Smit, M. J., Pegtel, D. M., and Baglio, S. R. (2018). Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 188, 1–11. doi:10.1016/j.pharmthera.2018.02.013
Becker, A., Thakur, B. K., Weiss, J. M., Kim, H. S., Peinado, H., and Lyden, D. (2016). Extracellular vesicles in cancer: Cell-to-Cell mediators of metastasis. Cancer Cell 30, 836–848. doi:10.1016/j.ccell.2016.10.009
Bellmunt, À. M., López-Puerto, L., Lorente, J., and Closa, D. (2019). Involvement of extracellular vesicles in the macrophage-tumor cell communication in head and neck squamous cell carcinoma. PLoS ONE 14, e0224710. doi:10.1371/journal.pone.0224710
Bigagli, E., Locatello, L. G., Di Stadio, A., Maggiore, G., Valdarnini, F., Bambi, F., et al. (2021). Extracellular vesicles miR-210 as a potential biomarker for diagnosis and survival prediction of oral squamous cell carcinoma patients. J. Oral Pathol. Med. 51, 350. doi:10.1111/jop.13263
Bordanaba-Florit, G., Royo, F., Kruglik, S. G., and Falcón-Pérez, J. M. (2021). Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles. Nat. Protoc. 16, 3163–3185. doi:10.1038/s41596-021-00551-z
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 68, 394–424. doi:10.3322/caac.21492
Brinton, L. T., Sloane, H. S., Kester, M., and Kelly, K. A. (2015). Formation and role of exosomes in cancer. Cell. Mol. Life Sci. 72, 659–671. doi:10.1007/s00018-014-1764-3
Cai, J., and Qiao, B. (2019). Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am. J. Physiol. Cell Physiol. 316, C731–C740. doi:10.1152/ajpcell.00366.2018
Chen, C.-M., and Huang, C.-C. (2021). Exosome-derived microRNAs in oral squamous cell carcinomas impact disease prognosis. Oral Oncol. 120, 105402. doi:10.1016/j.oraloncology.2021.105402
Chen, J.-H., Wu, A. T. H., Bamodu, O. A., Yadav, V. K., Chao, T.-Y., Tzeng, Y.-M., et al. (2019). Ovatodiolide suppresses oral cancer malignancy by down-regulating exosomal mir-21/STAT3/β-catenin cargo and preventing oncogenic transformation of normal gingival fibroblasts. Cancers (Basel) 12, E56. doi:10.3390/cancers12010056
Chen, W., Cai, Y., Lv, M., Chen, L., Zhong, S., Ma, T., et al. (2014a). Exosomes from docetaxel-resistant breast cancer cells alter chemosensitivity by delivering microRNAs. Tumor Biol. 35, 9649–9659. doi:10.1007/s13277-014-2242-0
Chen, W., Liu, X., Lv, M., Chen, L., Zhao, J., Zhong, S., et al. (2014b). Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of MicroRNAs. PLoS ONE 9, e95240. doi:10.1371/journal.pone.0095240
Chi, A. C., Day, T. A., and Neville, B. W. (2015). Oral cavity and oropharyngeal squamous cell carcinoma-an update: Oral and Oropharyngeal Cancer Update. CA A Cancer J. Clin. 65, 401–421. doi:10.3322/caac.21293
Ciardiello, C., Cavallini, L., Spinelli, C., Yang, J., Reis-Sobreiro, M., de Candia, P., et al. (2016). Focus on extracellular vesicles: New Frontiers of cell-to-cell communication in cancer. Int. J. Mol. Sci. 17, 175. doi:10.3390/ijms17020175
Coon, J., Kingsley, K., and Howard, K. M. (2020). miR-365 (microRNA): Potential biomarker in oral squamous cell carcinoma exosomes and extracellular vesicles. Int. J. Mol. Sci. 21, E5317. doi:10.3390/ijms21155317
Costa-Silva, B., Aiello, N. M., Ocean, A. J., Singh, S., Zhang, H., Thakur, B. K., et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826. doi:10.1038/ncb3169
Coussens, L. M., Zitvogel, L., and Palucka, A. K. (2013). Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 339, 286–291. doi:10.1126/science.1232227
Daassi, D., Mahoney, K. M., and Freeman, G. J. (2020). The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 20, 209–215. doi:10.1038/s41577-019-0264-y
Ding, L., Ren, J., Zhang, D., Li, Y., Huang, X., Hu, Q., et al. (2018). A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis 39, 397–406. doi:10.1093/carcin/bgy006
Dou, G., Tian, R., Liu, X., Yuan, P., Ye, Q., Liu, J., et al. (2020). Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci. Adv. 6, eaba2987. doi:10.1126/sciadv.aba2987
Doyle, L., and Wang, M. (2019). Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8, 727. doi:10.3390/cells8070727
Ebnoether, E., and Muller, L. (2020). Diagnostic and therapeutic applications of exosomes in cancer with a special focus on head and neck squamous cell carcinoma (HNSCC). IJMS 21, 4344. doi:10.3390/ijms21124344
Epstein, J. B., Thariat, J., Bensadoun, R.-J., Barasch, A., Murphy, B. A., Kolnick, L., et al. (2012). Oral complications of cancer and cancer therapy: From cancer treatment to survivorship. CA A Cancer J. Clin. 62, 400–422. doi:10.3322/caac.21157
Fang, Z., Xu, J., Zhang, B., Wang, W., Liu, J., Liang, C., et al. (2020). The promising role of noncoding RNAs in cancer-associated fibroblasts: An overview of current status and future perspectives. J. Hematol. Oncol. 13, 154. doi:10.1186/s13045-020-00988-x
Fontana, S., Mauceri, R., Novara, M. E., Alessandro, R., and Campisi, G. (2021). Protein cargo of salivary small extracellular vesicles as potential functional signature of oral squamous cell carcinoma. Int. J. Mol. Sci. 22, 11160. doi:10.3390/ijms222011160
Forder, A., Hsing, C.-Y., Trejo Vazquez, J., and Garnis, C. (2021). Emerging role of extracellular vesicles and cellular communication in metastasis. Cells 10, 3429. doi:10.3390/cells10123429
Fujiwara, T., Eguchi, T., Sogawa, C., Ono, K., and Murakami, J. S. (2018). Ibaragi Carcinogenic epithelial-mesenchymal transition initiated by oral cancer exosomes is inhibited by anti-EGFR antibody cetuximab. Oral Oncol. 86. 251, doi:10.1016/j.oraloncology.2018.09.030
Gai, C., Camussi, F., Broccoletti, R., Gambino, A., Cabras, M., Molinaro, L., et al. (2018). Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 18, 439. doi:10.1186/s12885-018-4364-z
Gao, S., Hu, J., Wu, X., and Liang, Z. (2018). PMA treated THP-1-derived-IL-6 promotes EMT of SW48 through STAT3/ERK-dependent activation of Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 108, 618–624. doi:10.1016/j.biopha.2018.09.067
Ha, D., Yang, N., and Nadithe, V. (2016). Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 6, 287–296. doi:10.1016/j.apsb.2016.02.001
Han, Y., Jia, L., Zheng, Y., and Li, W. (2018). Salivary exosomes: Emerging roles in systemic disease. Int. J. Biol. Sci. 14, 633–643. doi:10.7150/ijbs.25018
He, L., Cheng, B., and Xia, J. (2020a). Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed. Pharmacother. 121, 109553. doi:10.1016/j.biopha.2019.109553
He, S., Zhang, W., Li, X., Wang, J., Chen, X., Chen, Y., et al. (2021a). Oral squamous cell carcinoma (OSCC)-derived exosomal MiR-221 targets and regulates phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) to promote human umbilical vein endothelial cells migration and tube formation. Bioengineered 12, 2164–2174. doi:10.1080/21655979.2021.1932222
He, T., Guo, X., Li, X., Liao, C., Wang, X., and He, K. (2021b). Plasma-derived exosomal microRNA-130a serves as a noninvasive biomarker for diagnosis and prognosis of oral squamous cell carcinoma. J. Oncol. 2021, 5547911. doi:10.1155/2021/5547911
He, Y., Du, J., and Dong, Z. (2020b). Myeloid deletion of phosphoinositide-dependent kinase-1 enhances NK cell-mediated antitumor immunity by mediating macrophage polarization. OncoImmunology 9, 1774281. doi:10.1080/2162402X.2020.1774281
Herrmann, I. K., Wood, M. J. A., and Fuhrmann, G. (2021). Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759. doi:10.1038/s41565-021-00931-2
Hoshino, A., Costa-Silva, B., Shen, T.-L., Rodrigues, G., Hashimoto, A., Tesic Mark, M., et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. doi:10.1038/nature15756
Huang, Z., Zhang, Q., Wang, Y., Chen, R., Wang, Y., Huang, Z., et al. (2020). Inhibition of caspase-3-mediated GSDME-derived pyroptosis aids in noncancerous tissue protection of squamous cell carcinoma patients during cisplatin-based chemotherapy. Am. J. Cancer Res. 10, 4287–4307.
Jensen, C., and Teng, Y. (2020). Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 7, 33. doi:10.3389/fmolb.2020.00033
Jiang, H., Zhou, L., Shen, N., Ning, X., Wu, D., Jiang, K., et al. (2022). M1 macrophage-derived exosomes and their key molecule lncRNA HOTTIP suppress head and neck squamous cell carcinoma progression by upregulating the TLR5/NF-κB pathway. Cell Death Dis. 13, 183. doi:10.1038/s41419-022-04640-z
Joyce, J. A., and Pollard, J. W. (2009). Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252. doi:10.1038/nrc2618
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367, eaau6977. doi:10.1126/science.aau6977
Kamerkar, S., LeBleu, V. S., Sugimoto, H., Yang, S., Ruivo, C. F., Melo, S. A., et al. (2017). Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503. doi:10.1038/nature22341
Ketabat, F., Pundir, M., Mohabatpour, F., Lobanova, L., Koutsopoulos, S., Hadjiiski, L., et al. (2019). Controlled drug delivery systems for oral cancer treatment—current status and future perspectives. Pharmaceutics 11, 302. doi:10.3390/pharmaceutics11070302
Khan, T., Relitti, N., Brindisi, M., Magnano, S., Zisterer, D., Gemma, S., et al. (2020). Autophagy modulators for the treatment of oral and esophageal squamous cell carcinomas. Med. Res. Rev. 40, 1002–1060. doi:10.1002/med.21646
Kirave, P., Gondaliya, P., Kulkarni, B., Rawal, R., Garg, R., Jain, A., et al. (2020). Exosome mediated miR-155 delivery confers cisplatin chemoresistance in oral cancer cells via epithelial-mesenchymal transition. Oncotarget 11, 1157–1171. doi:10.18632/oncotarget.27531
Kyzas, P. A., Cunha, I. W., and Ioannidis, J. P. A. (2005). Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: A meta-analysis. Clin. Cancer Res. 11, 1434–1440. doi:10.1158/1078-0432.CCR-04-1870
Lamouille, S., Xu, J., and Derynck, R. (2014). Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196. doi:10.1038/nrm3758
Laviron, M., and Boissonnas, A. (2019). Ontogeny of tumor-associated macrophages. Front. Immunol. 10, 1799. doi:10.3389/fimmu.2019.01799
Law, Z.-J., Khoo, X. H., Lim, P. T., Goh, B. H., Ming, L. C., Lee, W.-L., et al. (2021). Extracellular vesicle-mediated chemoresistance in oral squamous cell carcinoma. Front. Mol. Biosci. 8, 629888. doi:10.3389/fmolb.2021.629888
Lee, J.-K., Park, S.-R., Jung, B.-K., Jeon, Y.-K., Lee, Y.-S., Kim, M.-K., et al. (2013). Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLOS ONE 8, e84256. doi:10.1371/journal.pone.0084256
Li, C., Zhou, Y., Liu, J., Su, X., Qin, H., Huang, S., et al. (2019b). Potential markers from serum-purified exosomes for detecting oral squamous cell carcinoma metastasis. Cancer Epidemiol. Biomarkers Prev. 28, 1668. doi:10.1158/1055-9965.EPI-18-1122
Li, L., Lu, S., Liang, X., Cao, B., Wang, S., Jiang, J., et al. (2019a). δTDEs: An efficient delivery system for miR-138 with anti-tumoral and immunostimulatory roles on oral squamous cell carcinoma. Mol. Ther. Nucleic acids 14. doi:10.1016/j.omtn.2018.11.009
Li, L., and Zhu, G. (2016). Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 76, 1770–1780. doi:10.1158/0008-5472.CAN-15-1625
Li, S., Man, Q., Gao, X., Lin, H., Wang, J., Su, F., et al. (2021). Tissue-derived extracellular vesicles in cancers and non-cancer diseases: Present and future. J Extracell. Vesicle 10, e12175. doi:10.1002/jev2.12175
Li, S., Shi, Z., Fu, S., Li, Q., Li, B., Sang, L., et al. (2021c). Exosomal-mediated transfer of APCDD1L-AS1 induces 5-fluorouracil resistance in oral squamous cell carcinoma via miR-1224-5p/nuclear receptor binding SET domain protein 2 (NSD2) axis. Bioengineered 12, 7177–7193. doi:10.1080/21655979.2021.1979442
Li, W., Ren, G., Huang, Y., Su, J., Han, Y., Li, J., et al. (2012). Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 19, 1505–1513. doi:10.1038/cdd.2012.26
Li, Y.-Y. (2018). Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 36, 209–220. doi:10.1016/j.ebiom.2018.09.006
Li, Y., Tao, Y., Gao, S., Li, P., Zheng, J., Zhang, S., et al. (2018). Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 36, 209–220. doi:10.1016/j.ebiom.2018.09.006
Liang, W., Chen, X., Zhang, S., Fang, J., Chen, M., Xu, Y., et al. (2021). Mesenchymal stem cells as a double-edged sword in tumor growth: Focusing on MSC-derived cytokines. Cell Mol. Biol. Lett. 26, 3. doi:10.1186/s11658-020-00246-5
Liu, H., Tian, Y., Xue, C., Niu, Q., Chen, C., and Yan, X. (2022a). Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J Extracell. Vesicle 11, e12206. doi:10.1002/jev2.12206
Liu, J., Jiang, X., Zou, A., Mai, Z., Huang, Z., Sun, L., et al. (2021). circIGHG-induced epithelial-to-mesenchymal transition promotes oral squamous cell carcinoma progression via miR-142-5p/IGF2BP3 signaling. Cancer Res. 81, 344–355. doi:10.1158/0008-5472.CAN-20-0554
Liu, J., Ye, Z., Xiang, M., Chang, B., Cui, J., Ji, T., et al. (2019). Functional extracellular vesicles engineered with lipid-grafted hyaluronic acid effectively reverse cancer drug resistance. Biomaterials 223, 119475. doi:10.1016/j.biomaterials.2019.119475
Liu, P., Zhang, Q., Mi, J., Wang, S., Xu, Q., Zhuang, D., et al. (2022b). Exosomes derived from stem cells of human deciduous exfoliated teeth inhibit angiogenesis in vivo and in vitro via the transfer of miR-100-5p and miR-1246. Stem Cell Res. Ther. 13, 89. doi:10.1186/s13287-022-02764-9
Liu, T., Chen, G., Sun, D., Lei, M., Li, Y., Zhou, C., et al. (2017). Exosomes containing miR-21 transfer the characteristic of cisplatin resistance by targeting PTEN and PDCD4 in oral squamous cell carcinoma. Acta Biochimica Biophysica Sinica 49, 808–816. doi:10.1093/abbs/gmx078
Liu, Y., and Cao, X. (2016). Organotropic metastasis: Role of tumor exosomes. Cell Res. 26, 149–150. doi:10.1038/cr.2015.153
Longley, D. B., Harkin, D. P., and Johnston, P. G. (2003). 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 3, 330–338. doi:10.1038/nrc1074
Lu, Y., Zheng, Z., Yuan, Y., Pathak, J. L., Yang, X., Wang, L., et al. (2021). The emerging role of exosomes in oral squamous cell carcinoma. Front. Cell Dev. Biol. 9, 628103. doi:10.3389/fcell.2021.628103
Luo, Y., Liu, F., Guo, J., and Gui, R. (2020). Upregulation of circ_0000199 in circulating exosomes is associated with survival outcome in OSCC. Sci. Rep. 10, 13739. doi:10.1038/s41598-020-70747-y
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L., and Allavena, P. (2017). Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416. doi:10.1038/nrclinonc.2016.217
Mathieu, M., Martin-Jaular, L., Lavieu, G., and Théry, C. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17. doi:10.1038/s41556-018-0250-9
Nikfarjam, S., Rezaie, J., Kashanchi, F., and Jafari, R. (2020). Dexosomes as a cell-free vaccine for cancer immunotherapy. J. Exp. Clin. Cancer Res. 39, 258. doi:10.1186/s13046-020-01781-x
Ohno, S., Takanashi, M., Sudo, K., Ueda, S., Ishikawa, A., Matsuyama, N., et al. (2013). Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 21, 185–191. doi:10.1038/mt.2012.180
Ono, K., Eguchi, T., Sogawa, C., Calderwood, S. K., Futagawa, J., Kasai, T., et al. (2018). HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell Biochem. 119, 7350–7362. doi:10.1002/jcb.27039
Oshi, M., Tokumaru, Y., Asaoka, M., Yan, L., Satyananda, V., Matsuyama, R., et al. (2020). M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 10, 16554. doi:10.1038/s41598-020-73624-w
Pal, S. K., Nguyen, C. T. K., Morita, K., Miki, Y., Kayamori, K., Yamaguchi, A., et al. (2016). THBS1 is induced by TGFB1 in the cancer stroma and promotes invasion of oral squamous cell carcinoma. J. Oral Pathol. Med. 45, 730–739. doi:10.1111/jop.12430
Pang, X., Wang, S., Zhang, M., Jiang, J., Fan, H., Wu, J., et al. (2021). OSCC cell-secreted exosomal CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling pathway. Cancer Immunol. Immunother. 70, 1015–1029. doi:10.1007/s00262-020-02741-2
Panvongsa, W., Pegtel, D. M., and Voortman, J. (2022). More than a bubble: Extracellular vesicle microRNAs in head and neck squamous cell carcinoma. Cancers 14, 1160. doi:10.3390/cancers14051160
Qadir, F., Aziz, M. A., Sari, C. P., Ma, H., Dai, H., Wang, X., et al. (2018). Transcriptome reprogramming by cancer exosomes: Identification of novel molecular targets in matrix and immune modulation. Mol. Cancer 17, 97. doi:10.1186/s12943-018-0846-5
Raffat, M. A., Hadi, N. I., Hosein, M., Mirza, S., Ikram, S., and Akram, Z. (2018). S100 proteins in oral squamous cell carcinoma. Clin. Chim. Acta 480, 143–149. doi:10.1016/j.cca.2018.02.013
Rahimi, S., Roushandeh, A. M., Ahmadzadeh, E., Jahanian-Najafabadi, A., and Roudkenar, M. H. (2020). Implication and role of neutrophil gelatinase-associated lipocalin in cancer: lipocalin-2 as a potential novel emerging comprehensive therapeutic target for a variety of cancer types. Mol. Biol. Rep. 47, 2327–2346. doi:10.1007/s11033-020-05261-5
Ren, J. G., Zhang, W., Liu, B., Man, Q. W., Xiong, X. P., Li, C., et al. (2016). Clinical significance and roles in angiogenesis of circulating microparticles in oral cancer. J. Dent. Res. 95, 860–867. doi:10.1177/0022034516641037
Rodríguez Zorrilla, S., Pérez-Sayans, M., Fais, S., Logozzi, M., Gallas Torreira, M., and García García, A. (2019). A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers (Basel) 11, E429. doi:10.3390/cancers11030429
Rosenberger, L., Ezquer, M., Lillo-Vera, F., Pedraza, P. L., Ortúzar, M. I., González, P. L., et al. (2019). Stem cell exosomes inhibit angiogenesis and tumor growth of oral squamous cell carcinoma. Sci. Rep. 9, 663. doi:10.1038/s41598-018-36855-6
Ruffell, B., Affara, N. I., and Coussens, L. M. (2012). Differential macrophage programming in the tumor microenvironment. Trends Immunol. 33, 119–126. doi:10.1016/j.it.2011.12.001
Sahu, S., and Routray, S. (2021). Assessing the analytical efficacy of TEX in diagnosing oral cancer using a systematic review approach. J. Oral Pathol. Med. 50, 123–128. doi:10.1111/jop.13126
Sakha, S., Muramatsu, T., Ueda, K., and Inazawa, J. (2016). Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci. Rep. 6, 38750. doi:10.1038/srep38750
Sasabe, E., Tomomura, A., Liu, H., Sento, S., Kitamura, N., and Yamamoto, T. (2022). Epidermal growth factor/epidermal growth factor receptor signaling blockage inhibits tumor cell-derived exosome uptake by oral squamous cell carcinoma through macropinocytosis. Cancer Sci. 113, 609. doi:10.1111/cas.15225
Sasahira, T., and Kirita, T. (2018). Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int. J. Mol. Sci. 19, 2413. doi:10.3390/ijms19082413
Sato, S., Vasaikar, S., Eskaros, A., Kim, Y., Lewis, J. S., Zhang, B., et al. (2019). EPHB2 carried on small extracellular vesicles induces tumor angiogenesis via activation of ephrin reverse signaling. JCI Insight 4, e132447. doi:10.1172/jci.insight.132447
Sayyed, A. A., Gondaliya, P., Mali, M., Pawar, A., Bhat, P., Khairnar, A., et al. (2021). MiR-155 inhibitor-laden exosomes reverse resistance to cisplatin in a 3D tumor spheroid and xenograft model of oral cancer. Mol. Pharm. 18, 3010–3025. doi:10.1021/acs.molpharmaceut.1c00213
Syn, N., Wang, L., Sethi, G., Thiery, J.-P., and Goh, B.-C. (2016). Exosome-Mediated metastasis: From epithelial–mesenchymal transition to escape from immunosurveillance. Trends Pharmacol. Sci. 37, 606–617. doi:10.1016/j.tips.2016.04.006
Tan, S., Xia, L., Yi, P., Han, Y., Tang, L., Pan, Q., et al. (2020). Exosomal miRNAs in tumor microenvironment. J. Exp. Clin. Cancer Res. 39, 67. doi:10.1186/s13046-020-01570-6
Tomita, R., Sasabe, E., Tomomura, A., and Yamamoto, T. (2020). Macrophage-derived exosomes attenuate the susceptibility of oral squamous cell carcinoma cells to chemotherapeutic drugs through the AKT/GSK-3β pathway. Oncol. Rep. 44, 1905. doi:10.3892/or.2020.7748
Tong, F., Mao, X., Zhang, S., Xie, H., Yan, B., Wang, B., et al. (2020). HPV + HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett. 478, 34–44. doi:10.1016/j.canlet.2020.02.037
Urabe, F., Kosaka, N., Ito, K., Kimura, T., Egawa, S., and Ochiya, T. (2020). Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiology-Cell Physiology 318, C29–C39. doi:10.1152/ajpcell.00280.2019
van Niel, G., Carter, D. R. F., Clayton, A., Lambert, D. W., Raposo, G., and Vader, P. (2022). Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 23, 369–382. doi:10.1038/s41580-022-00460-3
Villegas-Pineda, J. C., Lizarazo-Taborda, M. D. R., de-Arellano, A. R., and Pereira-Suárez, A. L. (2021). Exosomal miRNAs and lncRNAs: The modulator keys of cancer-associated fibroblasts in the genesis and progression of malignant neoplasms. Front. Cell Dev. Biol. 9, 717478. doi:10.3389/fcell.2021.717478
Wang, J., Wei, Q., Wang, X., Tang, S., Liu, H., Zhang, F., et al. (2016). Transition to resistance: An unexpected role of the EMT in cancer chemoresistance. Genes and Dis. 3, 3–6. doi:10.1016/j.gendis.2016.01.002
Wang, S.-H., Liou, G.-G., Liu, S.-H., Chang, J. S., Hsiao, J.-R., Yen, Y.-C., et al. (2019). Laminin γ2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin α3-dependent uptake by lymphatic endothelial cells. Int. J. Cancer 144, 2795–2810. doi:10.1002/ijc.32027
Wang, Z.-Q., Liu, K., Huo, Z.-J., Li, X.-C., Wang, M., Liu, P., et al. (2015). A cell-targeted chemotherapeutic nanomedicine strategy for oral squamous cell carcinoma therapy. J. Nanobiotechnol 13, 1–10. doi:10.1186/s12951-015-0116-2
Xiao, M., Zhang, J., Chen, W., and Chen, W. (2018). M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 37, 143. doi:10.1186/s13046-018-0815-2
Xie, C., Du, L.-Y., Guo, F., Li, X., and Cheng, B. (2019a). Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol. Cell Biochem. 458, 11–26. doi:10.1007/s11010-019-03526-7
Xie, C., Ji, N., Tang, Z., Li, J., and Chen, Q. (2019b). The role of extracellular vesicles from different origin in the microenvironment of head and neck cancers. Mol. Cancer 18, 83. doi:10.1186/s12943-019-0985-3
Xie, F., Xu, M., Lu, J., Mao, L., and Wang, S. (2019c). The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol. Cancer 18, 146. doi:10.1186/s12943-019-1074-3
Yan, W., Wang, Y., Chen, Y., Guo, Y., Li, Q., and Wei, X. (2021). Exosomal miR-130b-3p promotes progression and tubular formation through targeting PTEN in oral squamous cell carcinoma. Front. Cell Dev. Biol. 9, 616306. doi:10.3389/fcell.2021.616306
Yang, F., Ning, Z., Ma, L., Liu, W., Shao, C., Shu, Y., et al. (2017). Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol. Cancer 16, 148. doi:10.1186/s12943-017-0718-4
Yeung, K. T., and Yang, J. (2017). Epithelial-mesenchymal transition in tumor metastasis. Mol. Oncol. 11, 28–39. doi:10.1002/1878-0261.12017
You, Y., Tian, Z., Du, Z., Wu, K., Xu, G., Dai, M., et al. (2022). 回路M1-like tumor-associated macrophages cascade a mesenchymal/stem-like phenotype of oral squamous cell carcinoma via the IL6/Stat3/THBS1 feedback loop. J. Exp. Clin. Cancer Res. 41, 10. doi:10.1186/s13046-021-02222-z
Yu, L., Yang, Z., Liu, Y., Liu, F., Shang, W., Shao, W., et al. (2020). Identification of SPRR3 as a novel diagnostic/prognostic biomarker for oral squamous cell carcinoma via RNA sequencing and bioinformatic analyses. PeerJ 8, e9393. doi:10.7717/peerj.9393
Yuan, Y., Jiao, P., Wang, Z., Chen, M., Du, H., Xu, L., et al. (2022). Endoplasmic reticulum stress promotes the release of exosomal PD-L1 from head and neck cancer cells and facilitates M2 macrophage polarization. Cell Commun. Signal 20, 12. doi:10.1186/s12964-021-00810-2
Zhang, M., Liang, J., Yang, Y., Liang, H., Jia, H., and Li, D. (2020). Current trends of targeted drug delivery for oral cancer therapy. Front. Bioeng. Biotechnol. 8, 618931. doi:10.3389/fbioe.2020.618931
Zhang, X., Zhang, H., Gu, J., Zhang, J., Shi, H., Qian, H., et al. (2021). Engineered extracellular vesicles for cancer therapy. Adv. Mater. 33, 2005709. doi:10.1002/adma.202005709
Zhao, S., Mi, Y., Zheng, B., Wei, P., Gu, Y., Zhang, Z., et al. (2022). Highly-metastatic colorectal cancer cell released miR-181a-5p-rich extracellular vesicles promote liver metastasis by activating hepatic stellate cells and remodelling the tumour microenvironment. J. Extracell. Vesicles 11, e12186. doi:10.1002/jev2.12186
Zhou, S., Zhu, Y., Li, Z., Zhu, Y., He, Z., and Zhang, C. (2021). Exosome-derived long non-coding RNA ADAMTS9-AS2 suppresses progression of oral submucous fibrosis via AKT signalling pathway. J. Cell Mol. Med. 25, 2262–2273. doi:10.1111/jcmm.16219
Keywords: oral squamous cell carcinoma, extracellular vesicles, diagnosis, tumorigenesis, treatment
Citation: Zhang Y, Liu J, Liu S, Yu L, Liu S, Li M and Jin F (2023) Extracellular vesicles in oral squamous cell carcinoma: current progress and future prospect. Front. Bioeng. Biotechnol. 11:1149662. doi: 10.3389/fbioe.2023.1149662
Received: 22 January 2023; Accepted: 19 May 2023;
Published: 26 May 2023.
Edited by:
Yuan Yin, Fourth Military Medical University, ChinaReviewed by:
Dali Zheng, Fujian Medical University, ChinaCopyright © 2023 Zhang, Liu, Liu, Yu, Liu, Li and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Jin, amluZmFuZ0BmbW11LmVkdS5jbg==; Meng Li, MTE5MzQwODgxQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.