
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 08 February 2023
Sec. Biosensors and Biomolecular Electronics
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1144463
This article is part of the Research Topic Point-of-Care Diagnostics Technology and Applications View all 21 articles
 Ying Chen1†
Ying Chen1† Lulu Sha1†
Lulu Sha1† Wenqing Li1
Wenqing Li1 Liuyan Zhou1
Liuyan Zhou1 Bing Pei2
Bing Pei2 Xinyu Bian1
Xinyu Bian1 Yongxin Ji3
Yongxin Ji3 Yiping Liu1
Yiping Liu1 Li Wang4*
Li Wang4* Huan Yang1*
Huan Yang1*Background: Klebsiella pneumoniae (KP, K. pneumoniae) is one of the most important nosocomial pathogens that cause severe respiratory infections. As evolutionary high-toxic strains with drug resistance genes increase year by year, the infections caused by it are often accompanied by high mortality, which may be fatal to infants and can cause invasive infections in healthy adults. At present, the traditional clinical methods for detecting K. pneumoniae are cumbersome and time-consuming, and the accuracy and sensitivity are not high. In this study, nanofluorescent microsphere (nFM)-based immunochromatographic test strip (ICTS) quantitative testing platform were developed for point-of-care testing (POCT) method of K. pneumoniae.
Methods: 19 clinical samples of infants were collected, the genus-specific gene of mdh was screened from K. pneumoniae. Polymerase chain reaction (PCR) combined with nFM-ICTS based on magnetic purification assay (PCR-ICTS) and strand exchange amplification (SEA) combined with nFM-ICTS based on magnetic purification assay (SEA-ICTS) were developed for the quantitative detection of K. pneumoniae. The sensitivity and specificity of SEA-ICTS and PCR-ICTS were demonstrated by the existing used classical microbiological methods, the real-time fluorescent quantitative PCR (RTFQ-PCR) and PCR assay based on agarose gel electrophoresis (PCR-GE).
Results: Under optimum working conditions, the detection limits of PCR-GE, RTFQ-PCR, PCR-ICTS and SEA-ICTS are 7.7 × 10−3, 2.5 × 10−6, 7.7 × 10−6, 2.82 × 10−7 ng/μL, respectively. The SEA-ICTS and PCR-ICTS assays can quickly identify K. pneumoniae, and could specifically distinguish K. pneumoniae samples from non-K. pneumoniae samples. Experiments have shown a diagnostic agreement of 100% between immunochromatographic test strip methods and the traditional clinical methods on the detection of clinical samples. During the purification process, the Silicon coated magnetic nanoparticles (Si-MNPs) were used to removed false positive results effectively from the products, which showed of great screening ability. The SEA-ICTS method was developed based on PCR-ICTS, which is a more rapid (20 min), low-costed method compared with PCR-ICTS assay for the detection of K. pneumoniae in infants. Only need a cheap thermostatic water bath and takes a short detection time, this new method can potentially serve as an efficient point-of-care testing method for on-site detection of pathogens and disease outbreaks without fluorescent polymerase chain reaction instruments and professional technicians operation.
Klebsiella pneumoniae (KP, K. pneumoniae) is one of the most harmful opportunistic bacteria and one of the main causes of nosocomial infections (Kong et al., 2018). It can cause bacterial pneumonia, liver abscesse (Chen et al., 2011; Nordmann and Poirel, 2014), sepsis and even other life-threatening consequences. In clinical investigations, drug-resistant K. pneumoniae was found to be highly contagious (Nordmann et al., 2012), especially in infants (Lee et al., 2020), because their immune system is fragile. The infections caused by K. pneumoniae are usually accompanied by high mortality (Arena et al., 2020), which could make severe challenges to clinical anti-infection treatment (Xu et al., 2017; Jia et al., 2022) and endanger infants’ health in developing countries (Zaidi et al., 2005; Li et al., 2021). At present, traditional microbial culture and drug resistance detection technologies consume a long time and are insensitive (Khan et al., 2014). Compared with the culture method, the molecular detection method is fast, sensitive and specific (Hooi et al., 2001). Among them, polymerase chain reaction (PCR) and immunochromatography are more commonly used. Traditional PCR detection methods based on agarose gel electrophoresis (PCR-GE) and real-time fluorescence quantitative PCR (RTFQ-PCR) (Chen et al., 2011; Nordmann and Poirel, 2014) have been widely used in the detection of pathogenic microorganism. However, PCR-GE can only be used for qualitative testing, and the following nucleic acid dyes in the agarose gel electrophoresis step are also toxic. RTFQ-PCR equipment is expensive (Glupczynski et al., 2016; Glupczynski et al., 2017; Song et al., 2020), which is not conducive to widespread clinical application.
In 2000, a study have developed a method, termed loop-mediated isothermal amplification (LAMP), that amplifies DNA under isothermal conditions (Notomi et al., 2000). This method employs a DNA polymerase and a set of four specially designed primers that recognize a total of six distinct sequences on the target DNA. Recently, a study using N.BspD6I nickel enzyme further explored the possible mechanism of DNA synthesis based on Bst DNA polymerase under non-template conditions (Zyrina et al., 2007). In 2016, strand exchange amplification (SEA) based on double-stranded DNA (dsDNA) degeneration bubbles was reported (Shi et al., 2016), which is the same with LAMP assay. However, the LAMP technology generally needs multiple pairs of primers and the primer design of LAMP is difficult, while SEA only uses one pair of primers. In SEA, a pair of primers (as shown in Figure 1) has been used to amplify DNA efficiently and steadily at a single temperature. As shown in the figure, a double-stranded DNA (dsDNA) exchanges one of its strands by a homologous single-stranded DNA (ssDNA) to form a heteroduplex product after completing the first primer expansion. DNA base have been added randomly by Bst DNA polymerase to the 3′ suspension due to its inherent nucleotide transferase activity. In the following rounds, more and more shift DNA strands with random 3′ suspension bases accumulate rapidly by the action of primers and Bst DNA polymerase. A new double strand can be formed if the newly synthesized strand has a 3′ drape DNA base, which complements the 3′ drape base of the previous replacement strand.
Immunochromatography test paper technology was first introduced in the 1980s. The design of immunochromatographic test strip (ICTS) can be currently used for pathogens’ nucleic acid detection, and is called “nucleic acid lateral flow immunoassay” (NALFIA) (Duong and Rhee, 2007; Chen et al., 2008; Chen et al., 2019; Zhuang et al., 2019; Chen et al., 2022). Immunochromatography test strip technology is currently widely used in fields requiring rapid antigen testing, especially in clinical medicine (Lin et al., 2022), agriculture (Dong et al., 2022; Lu et al., 2022), drug abuse (Suryoprabowo et al., 2021), food (Wang et al., 2022; Xing et al., 2022) and environmental semi-antigen pollutants (Ren et al., 2021), and microbial pollutants (Gao et al., 2021). In recent years, scientists have been working to develop ICTS methods for pathogen detection (Li et al., 2020a), but its low sensitivity has made it a bottleneck worldwide. With the advancement of nanotechnology, functional magnetic nanomaterials are gradually applied to biological detection due to their large specific surface area, superparamagnetism, and controllable size (Duong and Rhee, 2007; Chen et al., 2019). The magnetic beads enable the isolation or extraction of target molecule or substance due to the good biocompatibility and adequate functional groups for chemical fixation which have been applied to immunoassay. Silicon coated magnetic nanoparticles (Si-MNPs) we made can be used for specific bioaffinity capture of molecules. Moreover, nanofluorescent microsphere (nFM) have good thermal stability, dispersibility, biocompatibility, high fluorescence stability, surface modification, narrow particle size and high luminous efficiency, and nFM are rarely employed for the immunochromatography methods (Zhang et al., 2015; Chen et al., 2019; Zhuang et al., 2019; Chen et al., 2022; Chen et al., 2023).
In order to enable detection in situ or without expensive instrument conditions, we developed and validated a novel, rapid and sensitive immunoassay combined with strand exchange amplification (SEA) for isothermal and quantitative detection of K. pneumoniae in infants with severe infection disease by a point-of-care immunochromatographic technique based on nanofluorescent microspheres (SEA-ICTS) in this study. With the use of functional nanoparticles as signal amplifiers greatly increases the sensitivity of ICTS for fluorescence sensing, the entire process can be completed in 30 min and the results can be quantified by ICTS sensor reader without false positives or negatives.
The genus-specific gene of mdh (Sun et al., 2008; Thong et al., 2011) was screened from K. pneumoniae to investigate the application potential of new immune chromatography technology in early clinical detection as well as to compare the existing used classical microbiological methods, PCR-GE and the RTFQ-PCR method. The SEA-ICTS method was optimized based on PCR-ICTS we established in this study; However, the optimized SEA-ICTS method is a more rapid, low-costed method compared with PCR-ICTS assay for the detection of Klebsiella pneumoniae (KP, K. pneumoniae) in infants and can potentially serve as an efficient point-of-care testing (POCT) method for on-site detection of pathogens and disease outbreaks.
The bacterial strains used in this study included 19 clinical strains of Klebsiella pneumoniae (KP, K. pneumoniae) in infants collected from Nanjing Children’s Hospital (as shown in Table 1), one standard strain of K. pneumonia (ATCC 700603) and 14 clinical strains of non-Klebsiella pneumoniae (NKP) were provided from the Affiliated Hospital of Xuzhou Medical University and Xuzhou First People’s Hospital. Strains identification was performed by the traditional methods of bacteria culture and Vitek 2 Compact (BioMerieux, France), and all pathogenic strain DNA specimens are kept in the −80°C refrigerator. DNA extraction of all samples was carried out by TIANamp bacterial DNA kit (TIANGEN, Beijing, China) according to the manufacturer’s instructions. Ultraviolet spectrophotometer (Merinton SMA 1000, Beijing) was used to determine the ratio of A260/A280, and agarose electrophoresis was used to detect DNA integrity and evaluate DNA quality. The genome DNA (gDNA) of the above strain is stored at −80°C until it is thawed before analysis.
All oligonucleotides, including target-specific primer sets (PCR and SEA) were synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). ICTS consists of six parts: sample pad, nitrocellulose membrane (NC), conjugate pad, absorption pad, polystyrene support card and detection area with test (T), and control line (C). Streptavidin-modified fluorescent microspheres were added into 1 mL of 0.01 mol/L PBS solution (pH 7.4) containing 0.9% Nacl, 1% BSA and 0.09% Proclin, then the mixture was centrifugated at 14,000 rpm for 15 min at 4°C. The supernatant was discarded and the residue was reconstituted in 1 ml of resuspension by vortex mixing and sonication. Repeat the above cleaning steps three times. Finally, 10 μL of Streptavidin-modified Fluorescent Microspheres were reconstituted in 100 μL of resuspension.
The sample pad was first treated with PBS solution (pH 7.4) containing 6% trehalose, 0.5% (v/v) Tween 20, 1% BSA, 0.5% pvpk30, 0.5% Tetronic 1,307 and 0.05% proclin300 followed by drying at 37°C for 10 h. The conjugate pad was saturated with Streptavidin-modified Fluorescent Microspheres and then dried at 37°C for 2 h. To obtain the detection area, Biotin−BSA and Anti-digoxin antibodies were diluted to the proper concentrations, filtered, and dispensed at the test line (T line) and control line (C line) respectively. The strip was assembled by laminating the sample pad, conjugate pad, NC membrane, and absorbent pad onto a backing card. The conjugate pad and the absorbent pad were separately attached to the ends of the NC membrane with overlaps of 2 mm. The sample pad was pasted onto the conjugate pad with a 2 mm overlap. The assembled backing was cut into the width of 4 mm and placed in a sealed bag with desiccant. In the end, it should be kept away from light and stored at 4°C.
The genus-specific gene of mdh sequence of Klebsiella pneumoniae strain was obtained from GenBank nucleotide sequence database (http://www.ncbi.nlm.nih.gov). The specific primers for SEA (sF and sR) and PCR (F and R) were designed by NUPACK network software and Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA). Forward primer of sF and F were labeled with biotin at the 5′ end and reverse primer of sR and R were labeled with digoxigenin at the 3′ end. The primers above were synthesized by Sangon Biotech (Shanghai) Co., Ltd.
The SEA reaction was carried out within the final volume of 25 μL reaction system containing 7.5 μL primer, 2.5 μL DNA template, 4 μL buffer B of DNA polymerase and 11 μL buffer A of isothermal amplification buffer (Qingdao Naide Biotechnology Co., Ltd.) to form a reaction system, blow and mix well, and briefly centrifuge to remove bubbles. The product is visualized under ultraviolet light through 3% agarose gel electrophoresis. SEA’s identification and experimental procedures are auxiliary information. In order to optimize the reaction conditions, the fluorescence values of K. pneumoniae (ATCC 700603) genomic DNA (gDNA) were measured in a constant temperature of water bath of 60°C, 61°C, 62°C, 63°C, 64°C and 65°C. Each SEA reaction contains a positive and negative control, and all SEA experiments are repeated.
The PCR reaction was performed in a final volume of 25 µL containing 12.5 μL green Taq mixture (Vazyme, Nanjing), 1 μL forward primer (10 μmol/L), 1 μL reverse primer (10 μmol/L), 1 μL sample DNA and sterile deionized water. The PCR reaction was carried out on a Bio-Rad PCR system under the following conditions: 95°C 5 min, 30 cycles of 95°C 30 s, 58.5°C 30 s and 72°C 30 s, finally extend for 7 min at 72°C. The products were visualized under ultraviolet light through 1.5% (w/v) agarose gel electrophoresis (PCR-GE) to minimize the possibility of non-specific amplification.
Add 5 μL SEA or PCR products and 9 μL Si-MNPs, the mixture was hatched at room temperature (RT), and slowly mix for 5 min to fully adsorb DNA fragments. The Si-MNPs-DNA complex was then fixed with magnets for 2 min and washed twice with alcohol solution (85%). Suspend Si-MNPs with 15 μL of sterile deionized water, and then remove Si-MNPs from the nucleic acid products. Then the purified SEA or PCR products can be used for ICTS detection or stored at −20°C. The whole process is shown in Figure 2.
Add the reaction mixture of 100 μL containing purified 15 μL SEA or PCR products and 85 μL PBS (pH 7.4) to the reaction tube. Then, the mixed solution were directly added into our ICTS sample pad, which was the specific positions designated as the capture test lines on the strip, and 100 μL PBS solution was used as the blank control. The fluorescence value was read by ICTS sensor reader of Nanoeasy 1700 (Nanoeast, Nanjing, China) which we made after 2 min and the relevant image was recorded by fluorescence imager. The quantification of SEA or PCR products can be completed in 3 min.
The genus-specific gene of mdh was used to detect the specificity of microorganisms of K. pneumoniae, and other pathogenic samples (as shown in Table 1) of SEA-ICTS and PCR-ICTS methods as well as to evaluate the specificity of the test paper. The SEA amplification were carried out using 28.2 to 2.82 × 10−8 ng/μL standard DNA to evaluate the detection limits of SEA-GE and SEA-ICTS (Figure 7). The PCR amplification were carried out with standard DNA of 7.7 to 7.7 × 10−7 ng/μL to evaluate the detection limits of PCR-GE and PCR-ICTS (Figure 7). Sterile deionized water is used as a negative control, and the concentration is repeated three times per concentration. The ICTS sensor reader is used for detection. In the fluorescence threshold test, the signal value is defined as 500 (detection limit). If the signal value is greater than 500, the result is positive, otherwise the signal value is less than 500, which is negative.
K. pneumoniae standard strain (ATCC 700603), 19 clinical isolates and 14 non-Klebsiella pneumoniae reference strains were used to evaluate the specificity of SEA-ICTS and PCR-ICTS assays (as shown in Table 1). And sterile phosphate-buffered saline (PBS buffer, pH 7.4) was used as a negative control for DNA extraction. The reaction was carried out under optimized conditions. All samples were performed in duplicate.
RTFQ-PCR reaction was carried out on the StepOne PULS fluorescence quantitative PCR analyzer (ABI, Foster, CA, United States) using a 2X SG Fast qPCR Master Mix (High Rox, BBI, ABI). Each 20 μL reaction mixture contains 10 μL 2X SybrGreen qPCR main mixture (High Rox, B639273, BBI, ABI), 0.4 μL primer F (10 μmol/L), 0.4 μL primer R (10 μmol/L), 2 μL DNA template and sterile deionized water with a volume of up to 20 μL. The cyclic conditions are as follows: 95°C 3 min, 45 cycles of 95°C 5 s, 60°C 30 s. The sensitivity of RTFQ-PCR (mdh) was evaluated using gDNA (1.74 × 107–1.74 × 103 copies/μL) of different concentrations of K. pneumoniae strains. All processes were repeated three times, including negative and positive control.
Genomic DNA is extracted, and then performed to nucleic acid isothermal amplification of SEA and variable temperature amplification of PCR, respectively (Figure 3). The specific primers of genus-specific gene mdh for SEA (sF and sR) and PCR (F and R) were labelled on 5′-end with biotin and digoxigenin, respectively. After purification of SEA or PCR products, the reaction mixture was directly loaded on the immunochromatographic test strips based on nFM. One end of the enlarged products is marked with biotin and bound to FM-labeled streptavidin, and the other end is marked with digoxin, which binds to the anti-digoxin antibody on the test line (T), redundant streptavidin-coated nFM can be combined with biotin-labeled BSA on the control line (C), thereby a portable test paper reader of fluorescence detection system can receive fluorescence signal. Using the ICTS sensor reader to detect stronger fluorescence signals, and the results can be quantified and shared in real time through the network. In the presence of the target, the positive result appeared with two data at the T and C line positions on the strip, while in the absence of the target, the negative result with a very low value (value < 500) at the T line position on the strip. Negative control using PBS instead of SEA or PCR products did not result in any value on the T lines. So the SEA-ICTS and PCR-ICTS methods can be used to rapid and specific analysis of gDNA isolated from K. pneumoniae. No false-positive or false-negative results were ever seen, and the results were comparable to PCR-GE and RTFQ-PCR (as shown in Table 1).
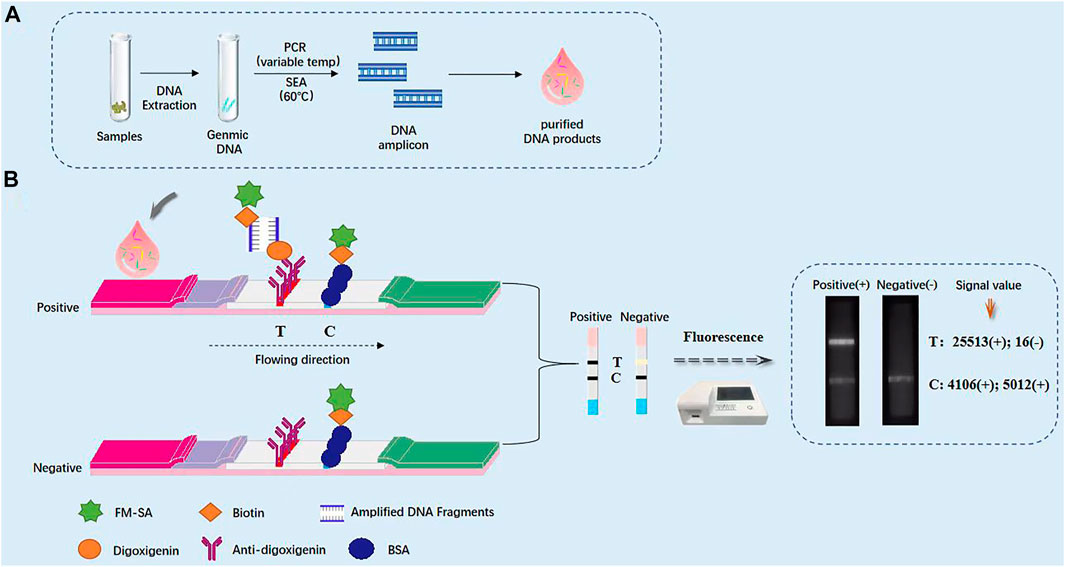
FIGURE 3. Schematic diagram of immunochromatographic test strip (ICTS). (A) Schematic diagram of immunochromatographic test strip for the detection of Klebsiella pneumoniae. (B) Photograph of the packaged ICTS and its internal structure, consisting of a sample pad, a conjugate pad, a detection region with test and control lines, and an absorbent pad.
The nanofluorescent microspheres-streptavidin (nFM-SA) conjugates was prepared based on coupling method and the corresponding characterization was performed. Figure 4A shows that the nFM have a standard spherical structure before and after SA modification. From the transmission electron microscopy image, compared with free-nFM, the surface of the nFM-SA microspheres was covered with a membrane-like structure. Both nFM and nFM-SA have good uniformity and dispersibility and are soluble in water and other common solvents. To confirm the success of cross-linking, fluorescence spectra and ultraviolet visible (UV-Vis) absorption were used. Compared with unmodified nFM, nFM-SA has obvious a protein characteristic peak near 280 nm in UV–visible absorption spectra, indicating that SA has successfully combined with nFM (Figure 4B). As shown the fluorescence spectroscopy in Figure 4C, the maximum emission wavelength of nFM is 676 nm, and the fluorescence intensity of nFM-SA decreased compared with that of the unmodified nFM. The main reason is attributed to the fact that the organic structure of SA wrapped on the surface of the fluorescent microspheres absorbs part of the fluorescence of the nFM (Shang et al., 2021). These data indicate that the nFM-SA complex were successfully prepared and fully met the test strip requirements.
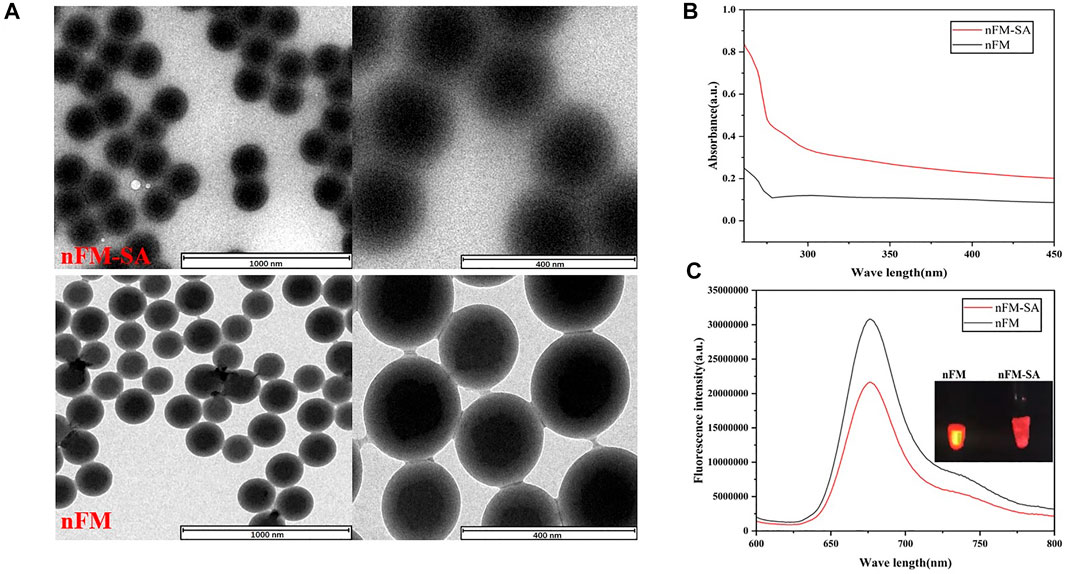
FIGURE 4. Characterization of nFM and nFM-SA conjugates. (A) Transmission electron microscopy (TEM) images of synthesized nFM and nFM-SA conjugates, (B) Ultraviolet visible (UV–vis) absorption, and (C) fluorescence emission spectra of free nFM and nFM-SA conjugates.
To test the feasibility of the SEA-ICTS and PCR-ICTS platform for nucleic acid detection, a genus-specific gene of mdh for K. pneumoniae was selected. The SEA and PCR products were visualized under ultraviolet light through agarose gel electrophoresis (GE), and compared to DNA Marker (TaKaRa, Dalian, China) to verify the products or the size of amplicons in order to minimize the possibility of non-specific amplification. The results showed that the PCR and SEA products were successfully amplified, while negative control did not show any amplification results (Figure 5).
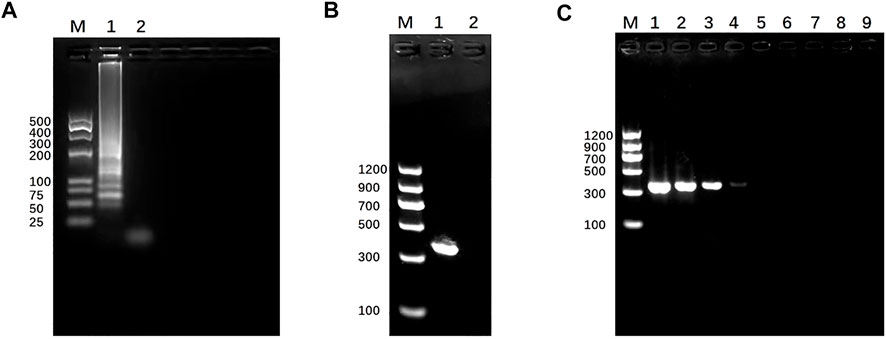
FIGURE 5. Establishment and identification of SEA and PCR. (A) SEA result. Lane M, DL 500 DNA marker. Lane 1, SEA product of mdh for Klebsiella pneumoniae by 3% (w/v) agarose gel electrophoresis. Lane 2, negative control. (B) PCR result. Lane M, DL 2000 DNA marker. Lane 1, PCR products of mdh for Klebsiella pneumoniae determined by 1.5% (w/v) agarose gel electrophoresis. Lane 2, negative control.(C) Detection sensitivity of PCR-GE using serially diluted Klebsiella pneumoniae (mdh); lane M, DL1200 marker; lanes 1–8, DNA concentration from 7.7 to 7.7 × 10−7 ng/μL. Lane 9, negative control. The size of the PCR products (mdh) was 364 bp.
In order to correctly detect SEA or PCR products, Si-MNPs were used as a purification and signal amplifier to remove false positive results. As shown in Table 2, the test line value in the dilution group (blank control) showed that the background signal value of the fluorescence intensity is relatively low, indicating that the test strip itself does not produce false positive. In the negative control groups, the fluorescence intensity of the pre-purification signal of the negative samples were 5,643 and 4,548, indicating that the amplification of the non-target band caused false positive results. The signal intensity after purification were reduced to 176 and 187, indicating that most non-target strips have been cleared during purification, and ICTS can be used to detect K. pneumoniae as an effective tool. There is no significant difference in signal values between S1, S2 and S3 positive samples before and after purification, which are similar to the results of P1, P2 and P3. These results are consistent with the results of agarose gel electrophoresis. Therefore, the ICTS strips had good repeatability and strong screening ability, which can eliminate false positive results.
In order to optimize the reaction conditions of temperature and time, the standard strain of K. pneumoniae was used as a target to optimize the reaction temperature in a constant temperature of water bath of 58°C, 59°C, 60°C, 61°C and 62°C. All SEA-ICTS experiments were repeated and contain negative control. As shown in Figure 6A, the optimum reaction temperature was 60°C. Then, the fluorescence values of SEA amplicons were collected every 5 min from 0 to 60 min under 60°C as the optimize the reaction temperature. As shown in Figure 6B, when the reaction time reached 20 min, the fluorescence values was 31,153, which was significantly different from the negative control. The SEA products were also visualized by agarose gel electrophoresis (SEA-GE), the results were consistent with SEA-ICTS (Figure 6C). Therefore, 60°C reaction for 20 min was determined as the optimal reaction conditions for SEA-ICTS assay.
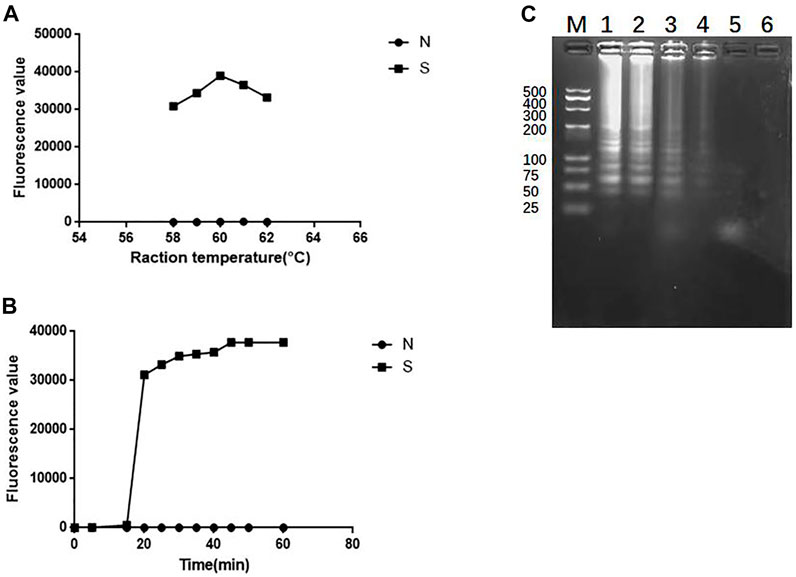
FIGURE 6. Optimize the reaction conditions of SEA. (A) Fluorescence values of standard strain of Klebsiella pneumonia at 58°C, 59°C, 60°C, 61°C and 62°C, respectively by SEA-ICTS; (B) Fluorescence values of parallel samples collected by SEA-ICTS every 5 min from 0 to 60 min at 60°C; (C) SEA product determined by 3% agarose gel electrophoresis. Lane M, DL 500 DNA marker. Lane 1, 35 min. Lane 2, 30 min. Lane 3, 25 min. Lane 4, 20 min. Lane 5, 15 min, Lane 6, negative control.
In SEA-ICTS method, the mdh gene was detected by using a concentration of 28.2 ng/μL and ten times continuously diluted. The results determine the sensitivity of SEA-ICTS. In SEA-ICTS, the fluorescence signal values of standard DNA dilution range from 51686.88 to 310.07, and the concentration of mdh decreased from 28.2 to 2.82 × 10−8 ng/μL. The relationship between different concentrations and the test value was observed {Y = 45286.1 − 44072.9/(1 + exp^[(x+2.4)/0.6])}, the correlation analysis shows that the Pearson correlation coefficient (Pearson’s r) between DNA concentration and ICTS results is R2 = 0.9396 (Figure 7A). The results showed that the sensitivity of SEA-ICTS for the detection of mdh was 2.82 × 10−7 ng/μL.
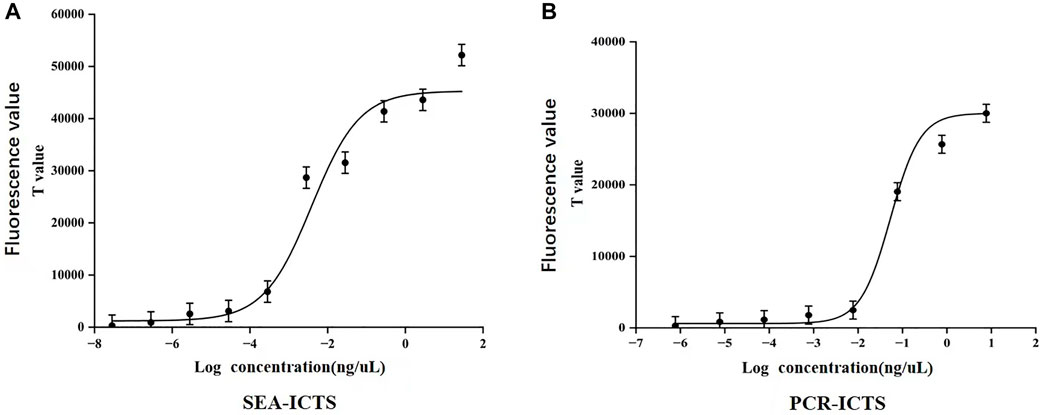
FIGURE 7. Sensitivity detection of Klebsiella pneumoniae. (A,B), The correlation curves were established with the logarithmic value of DNA concentration as the abscissa and the signal value (T value) of ICTS as the ordinate. Each value was derived from three independent experiments, and the error bars mean standard deviations.
In PCR-ICTS method, the mdh gene was detected by using a standard strain concentration of 7.7 ng/μL and diluted ten times continuously. The results determine the sensitivity of PCR-GE and PCR-ICTS (Figure 5C, 7). In PCR-ICTS, the fluorescence signal values of standard DNA dilution range from 286.52 to 30,462. The relationship between different concentrations and the test value was observed {Y = 30008.6 − 29388/(1 + exp^[(x + 1.3)/0.3])}, and mdh concentration decreased from 7.7 to 7 × 10−7ng/μL. Correlation analysis shows that the Pearson correlation coefficient (Pearson’s r) between DNA concentration and ICTS results is R2 = 0.9411. The results show that the detection limits of the mdh gene used by PCR-ICTS is 7.7 × 10−6 ng/μL, which is 1,000 times that measured by PCR-GE of 7.7 × 10−3 ng/μL (Figure 5C). However, the detection limits of the mdh gene used by SEA-ICTS is 2.82 × 10−7 ng/μL, which is 10 times that measured by PCR-ICTS.
To verify the specificity of new methods, Klebsiella pneumoniae (KP, K. pneumoniae) specimens from infants and 14 cases of non-Klebsiella pneumoniae (nKP) biopathogens were tested. The results showed that only K. pneumoniae strains showed characteristic amplification band, while nKP strains and negative control did not show any amplicon (Figure 8). Then, 19 cases Klebsiella pneumoniae (KP, K. pneumoniae) specimens from infants and 14 cases of non-Klebsiella pneumoniae (nKP) biopathogens were tested by SEA-ICTS and PCR-ICTS. The results showed that the detection of KP and nKP with ICTS technology were consistent with traditional detection methods, which showed a high degree of specificity (Table 3).
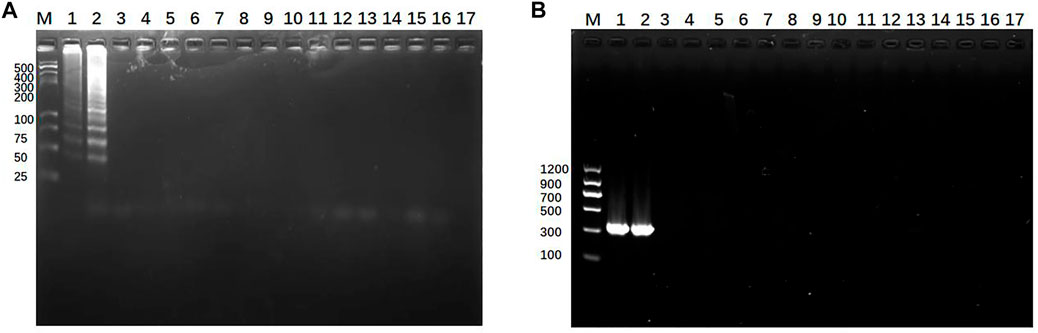
FIGURE 8. Specificity detection of Klebsiella pneumoniae. (A) Detection specificity of SEA-GE using Klebsiella pneumoniae (mdh) and non-Klebsiella pneumoniae: lane M, DL 500 DNA marker; lanes 1-2, Klebsiella pneumoniae with different DNA concentrations; lanes 3–16, non-Klebsiella pneumoniae, Lane 17, negative control. (B) Detection specificity of PCR-GE using Klebsiella pneumoniae and non-Klebsiella pneumoniae: lane M, DL 1200 DNA marker; lanes 1-2, Klebsiella pneumoniae; lanes 3–16, non-Klebsiella pneumoniae. Lane 17, negative control.
The genus-specific gene of mdh of K. pneumoniae was detected by the gold standard detection method of RTFQ-PCR. According to Figure 9A, there is a good linear relationship between the logarithm of DNA concentration and the periodic threshold (Ct) value of RTFQ-PCR. The linear equation is y = −3.211x + 38.869, and the R2 value exceeds 0.99. The minimum detection limit for plasmids containing the target gene is 67.3 copies/uL, which is converted to a DNA concentration with the sensitivity of 2.5 × 10−6 ng/uL. From Figure 9B, it can be judged that the detection results of different DNA concentrations of K. pneumoniae are very repetitive and sensitive.
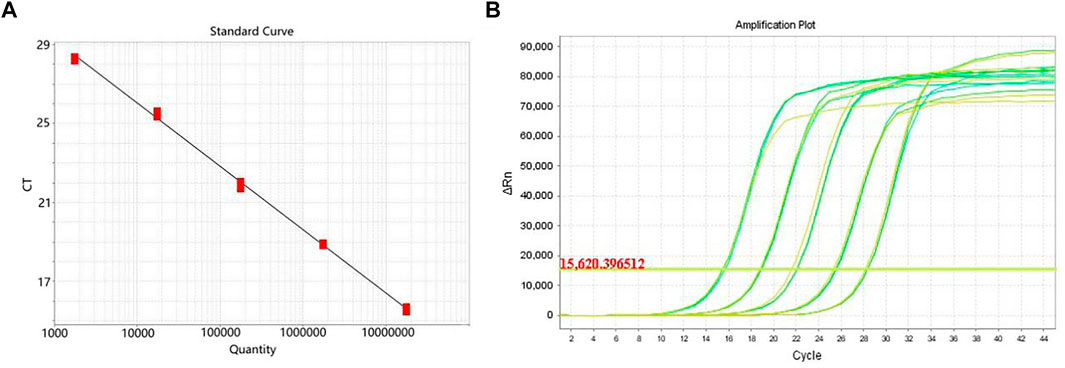
FIGURE 9. Detection sensitivity of RTFQ-PCR using standard strain of Klebsiella pneumoniae. (A) The standard curve of the RTFQ-PCR for the detection of 5 Klebsiella pneumoniae DNA samples from 5.8 × 10−2 to 5.8 × 10−6 ng/μL; (B) The amplification curves of RTFQ-PCR method of these 5 Klebsiella pneumoniae samples with different concentrations. Numbers 1–5 represent the different concentrations.
In recent years, due to long-term excessive and improper clinical use of carbapenem antibiotic etc., the detection rate of K. pneumoniae has been increasing, especially in hospital-born infants (Yu et al., 2016) with serious infectious diseases (Lôme et al., 2018; Porreca et al., 2018; Huang et al., 2020).
In this study, 19 samples used SEA-ICTS, PCR-ICTS, qualitative testing of agarose gel electrophoresis, and traditional clinical methods as well as the molecular gold standard technology of RTFQ-PCR for the detection of the genus-specific gene of K. pneumoniae from infants.
Under optimum working conditions, the detection limits of PCR-GE, RTFQ-PCR, PCR-ICTS and SEA-ICTS are 7.7 × 10−3, 2.5 × 10−6, 7.7 × 10−6, 2.82 × 10−7 ng/μL, respectively. The PCR-ICTS and the SEA-ICTS assays have detected K. pneumoniae, and could specifically distinguish K. pneumoniae samples from non-Klebsiella pneumoniae samples. Moreover, the fluorescence intensity by ICTS was higher than electrophoretic band in agarose gel electrophoresis detection. The results showed that SEA-ICTS and PCR-ICTS detection platform in this study have high sensitivity and strong screening ability, which can eliminate false negative results.
Immune chromatography can only detect specific bacterial antibodies based on monoclonal or polyclonal (Li et al., 2020b), and most test strips are only used for qualitative experiments. In this study, streptavidin-modified nFM were used to capture amplification products labeled with biotin. Compared with colloidal gold, nFM has higher signal values, a wider linear range, better interoperability and repeatability, and is also suitable for quantitative detection. In addition, the high degree of specific affinity and multi-stage amplification between biotin and nFM can be used to improve the sensitivity of detection lines to immune binding and trace analysis.
In recent years, new ICTS-based detection methods have emerged, which means that ICTS methods are practical in many fields. These methods reduce the detection workload and the possibility of bacterial contamination (Kuo et al., 2015; Vyas et al., 2015). The ICTS method, combined with some special equipment, can process large-volume samples in complex analytical processes. For example, Xu et al. (2013) proposed a new information and communication technology that uses multiclonal antibodies to label FMs to detect C. jejuni, which can only qualitatively detect clostridium difficile. Zhang et al. (2013) developed a plant pathogens (Cmn and Pss) detection method based on microsphere-based fluorescent immunoassay, which analysis time (1 h) was much shorter compared with ELISA (6–8 h). However, flow cytometry and more samples are required in this method. Blavikova et al. (2011) developed an ICTS detection method requiring special equipment and complex analytical processes (total analysis time is 16 h).
However, the ICTS quantitative testing platform developed in this study have advantages such as being simple to operate, low-cost, high sensitivity and specificity as well as rapid quantitative detection. It can achieve quantitative detection of target DNA sequences through low-costed and small-sized detection equipment. After purification process of magnetic beads, the false positive can also be reduced; Therefore, the ICTS quantitative testing platform are popularized and used in areas with poor clinical and medical equipment where vulnerable to K. pneumoniae pathogens. In contrast, traditional microbial methods usually take 2–3 days to obtain qualitative results, which is time-consuming and laborious. PCR assay based on agarose gel electrophoresis (PCR-GE) takes a relatively long time (220 min:30 + 100 + 90), but it has low sensitivity, toxic nucleic acid dyes used, and cannot obtain quantitative results; RTFQ-PCR method has advantages of faster (160 min: 30 + 130) and high sensitivity, however, this assay requires skilled technicians and expensive nucleic acid amplification instrument, which limited the application scope of this method (Table 4).
In fact, after purification of nucleic acid amplification products (SEA or PCR products), the ICTS detection time only need 2–3 min. In this article, we choose 30 min as a comparative benchmark for the whole process including purification and ICTS. Especially, in our ICTS quantitative testing platform, the SEA-ICTS method was optimized based on PCR-ICTS we established in this study, which is a more rapid, low-costed method compared with PCR-ICTS assay for the detection of Klebsiella pneumoniae (KP, K. pneumoniae) (KP) in infants. Only needs cheap thermostatic water bath and takes a short detection time, this new method can potentially serve as an efficient POCT method for on-site detection of pathogens and disease outbreaks without fluorescent PCR instruments and professional technicians operation.
In summary, this study aims to eliminate the application of gel electrophoresis in nucleic acid amplification product analysis and replace it with a faster, quantitative and cost-effective ICTS testing platform of SEA-ICTS and PCR-ICTS. The detection sensitivity of these two methods is nearly the same with RTFQ-PCR, but the cost is lower than that of RTFQ-PCR, which is cheaper and more suitable for testing clinical samples, especially in poor working conditions in hospitals. SEA-ICTS and PCR-ICTS methods can be used as alternatives to complement each other, according to the test working conditions. Therefore, due to its high sensitivity, strong specificity, simple operation, low-costed and strong detectability, the new ICTS quantitative testing platform can be used as an ideal tool to quantitatively and accurately determine whether patients, especially infants are infected with K. pneumoniae.
This paper introduces an nFM-based ICTS quantitative testing platform (SEA-ICTS and PCR-ICTS) that can detect Klebsiella pneumonia in infants as low as 2.82 × 10−7 ng/μL of SEA-ICTS method and 7.7 × 10−6 ng/μL of PCR-ICTS method. The results have showed that the new ICTS methods were fast, non-toxic, simple and low-costed. In particular, the SEA-ICTS method does not require special and expensive equipment and complex technologies to achieve high specificity and sensitivity, facilitate the rapid detection of K. pneumoniae, and may become an alternative fast and easy clinical sample detection tool to RTFQ-PCR and the traditional clinical detection methods.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements.
YC and HY: conception and design. YC and LW: administrative support; LW, BP, and YJ: provision of study materials or patients; LS, WL, and LZ: collection and assembly of data; LS, XB, and YL: data analysis and interpretation. All authors: manuscript writing and final approval of manuscript.
This work was supported by grants from “Qinglan Project” in Jiangsu Province, Xuzhou Science and Technology Planning Project (KC22115).
Author YJ was employed by Nanjing Nanoeast Biotech Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arena, F., Di Pilato, V., Vannetti, F., Fabbri, L., Antonelli, A., Coppi, M., et al. (2020). Population structure of KPC carbapenemase-producing Klebsiella pneumoniae in a long-term acute-care rehabilitation facility: identification of a new lineage of clonal group 101, associated with local hyperendemicity. Microb. Genom. 6 (1), e000308. doi:10.1099/mgen.0.000308
Blazkova, M., Javurkova, B., Fukal, L., and Rauch, P. (2011). Immunochromatographic strip test for detection of genus Cronobacter. Biosens. Bioelectron. 26 (6), 2828–2834. doi:10.1016/j.bios.2010.10.001
Chen, W. J., Tsai, P. J., and Chen, Y. C. (2008). Functional nanoparticle-based proteomic strategies for characterization of pathogenic bacteria. Anal. Chem. 80 (24), 9612–9621. doi:10.1021/ac802042x
Chen, L., Mediavilla, J. R., Endimiani, A., Rosenthal, M. E., Zhao, Y., Bonomo, R. A., et al. (2011). Multiplex real-time PCR assay for detection and classification of Klebsiella pneumoniae carbapenemase gene (bla KPC) variants. J. Clin. Microbiol. 49 (2), 579–585. doi:10.1128/JCM.01588-10
Chen, Y., Zhang, L., Xu, L., Guo, X., Yang, H., Zhuang, L., et al. (2019). Rapid and sensitive detection of Shigella flexneri using fluorescent microspheres as label for immunochromatographic test strip. Ann. Transl. Med. 7 (20), 565. doi:10.21037/atm.2019.09.46
Chen, Z., Zhao, K., He, Z., Luo, X., Qin, Z., Tan, Y., et al. (2022). Development and evaluation of a thermostatic nucleic acid testing device based on magnesium pyrophosphate precipitation for detecting Enterocytozoon hepatopenaei. Chin. Chem. Lett. 33 (8), 4053–4056. doi:10.1016/j.cclet.2022.01.072
Chen, H., Ma, X., Zhang, X., Hu, G., Deng, Y., Li, S., et al. (2023). Novel aerosol detection platform for SARS-CoV-2: Based on specific magnetic nanoparticles adsorption sampling and digital droplet PCR detection. Chin. Chem. Lett. 34 (1), 107701. doi:10.1016/j.cclet.2022.07.044
Dong, H., Xu, D., Wang, G., Meng, X., Sun, X., Yang, Q., et al. (2022). Broad-specificity time-resolved fluorescent immunochromatographic strip for simultaneous detection of various organophosphorus pesticides based on indirect probe strategy. Anal. Methods 14 (10), 1051–1059. doi:10.1039/D2AY00067A
Duong, H. D., and Rhee, J. I. (2007). Use of CdSe/ZnS core-shell quantum dots as energy transfer donors in sensing glucose. Talanta 73 (5), 899–905. doi:10.1016/j.talanta.2007.05.011
Gao, S., Wu, J., Wang, H., Hu, S., and Meng, L. (2021). Highly sensitive detection of Cronobacter sakazakii based on immunochromatography coupled with surface-enhanced Raman scattering. J. Dairy Sci. 104 (3), 2748–2757. doi:10.3168/jds.2020-18915
Glupczynski, Y., Evrard, S., Ote, I., Mertens, P., Huang, T. D., Leclipteux, T., et al. (2016). Evaluation of two new commercial immunochromatographic assays for the rapid detection of OXA-48 and KPC carbapenemases from cultured bacteria. J. Antimicrob. Chemother. 71 (5), 1217–1222. doi:10.1093/jac/dkv472
Glupczynski, Y., Jousset, A., Evrard, S., Bonnin, R. A., Huang, T.-D., Dortet, L., et al. (2017). Prospective evaluation of the OKN K-SeT assay, a new multiplex immunochromatographic test for the rapid detection of OXA-48-like, KPC and NDM carbapenemases. J. Antimicrob. Chemother. 72 (7), 1955–1960. doi:10.1093/jac/dkx089
Hooi, L. N., Looi, I., and Ng, A. J. (2001). A study on community acquired pneumonia in adults requiring hospital admission in Penang. Med. J. Malays. 56 (3), 275–284.
Huang, J., Chen, X., Zhao, Y., Shi, Y., Zhao, Z., Ding, H., et al. (2020). Outbreak of KPC-producing Klebsiella pneumoniae ST15 strains in a Chinese tertiary hospital: resistance and virulence analyses. J. Med. Microbiol. 71 (2). doi:10.1099/jmm.0.001494
Jia, X., Zhu, Y., Jia, P., Liu, X., Yu, W., Li, X., et al. (2022). Emergence of a superplasmid coharboring hypervirulence and multidrug resistance genes in Klebsiella pneumoniae poses new challenges to public health. Microbiol. Spectr. 10 (6), e0263422. doi:10.1128/spectrum.02634-22
Khan, F. Y., Abukhattab, M., AbuKamar, M., and Anand, D. (2014). Adult Klebsiella pneumoniae meningitis in Qatar: clinical pattern of ten cases. Asian Pac J. Trop. Biomed. 4 (8), 669–672. doi:10.12980/APJTB.4.201414B100
Kong, Z., Shen, J., Li, X., Kang, H., Sun, D., and Gu, B. (2018). Analysis of drug resistance genes of carbapenem-resistant Klebsiella pneumoniae in children. Chin. J. Nosocomiol. 28 (20). doi:10.11816/cn.ni.2018-182834
Kuo, Y. B., Li, Y. S., and Chan, E. C. (2015). Rapid identification of HPV 16 and 18 by multiplex nested PCR-immunochromatographic test. J. Virol. Methods 212, 8–11. doi:10.1016/j.jviromet.2014.10.009
Lee, J. H., Jang, Y. R., Ahn, S. J., Choi, S. J., and Kim, H. S. (2020). A retrospective study of pyogenic liver abscess caused primarily by Klebsiella pneumoniae vs. non-Klebsiella pneumoniae: CT and clinical differentiation. Abdom. Radiol. (NY) 45 (9), 2669–2679. doi:10.1007/s00261-019-02389-2
Li, G., Rong, Z., Wang, S., Zhao, H., Piao, D., Yang, X., et al. (2020). Rapid detection of brucellosis using a quantum dot-based immunochromatographic test strip. PLoS Neglected Trop. Dis. 14 (9), e0008557. doi:10.1371/journal.pntd.0008557
Li, C., He, X., Yang, Y., Gong, W., Huang, K., Zhang, Y., et al. (2020). Rapid and visual detection of African swine fever virus antibody by using fluorescent immunochromatography test strip. Talanta 219, 121284. doi:10.1016/j.talanta.2020.121284
Li, Y., Li, D., Xue, J., Ji, X., Shao, X., and Yan, J. (2021). The epidemiology, virulence and antimicrobial resistance of invasive Klebsiella pneumoniae at a Children's medical center in eastern China. Infect. Drug Resist 14, 3737–3752. doi:10.2147/IDR.S323353
Lin, L., Xu, X., Song, S., Xu, L., Zhu, Y., Kuang, H., et al. (2022). Immunological quantitative detection of dicofol in medicinal materials. Analyst 147 (15), 3478–3485. doi:10.1039/D2AN00462C
Lôme, V., Brunel, J. M., Pages, J. M., and Bolla, J. M. (2018). Multiparametric profiling for identification of chemosensitizers against gram-negative bacteria. Front. Microbiol. 9, 204. doi:10.3389/fmicb.2018.00204
Lu, Y., Shan, Y., Huang, H., Zhu, L., Li, B., Wang, S., et al. (2022). Quantum dot microsphere-based immunochromatography test strip enabled sensitive and quantitative on-site detections for multiple mycotoxins in grains. Food Chem. 376, 131868. doi:10.1016/j.foodchem.2021.131868
Nordmann, P., and Poirel, L. (2014). The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin. Microbiol. Infect. 20 (9), 821–830. doi:10.1111/1469-0691.12719
Nordmann, P., Gniadkowski, M., Giske, C. G., Poirel, L., Woodford, N., and Miriagou, V. (2012). Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Infect. 18 (5), 432–438. doi:10.1111/j.1469-0691.2012.03815.x
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., et al. (2000). Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28 (12), e63. doi:10.1093/nar/28.12.e63
Porreca, A. M., Sullivan, K. V., and Gallagher, J. C. (2018). The epidemiology, evolution, and treatment of KPC-producing organisms. Curr. Infect. Dis. Rep. 20 (6), 13. doi:10.1007/s11908-018-0617-x
Ren, S., Li, Q., Wang, J., Fan, B., Bai, J., Peng, Y., et al. (2021). Development of a fast and ultrasensitive black phosphorus-based colorimetric/photothermal dual-readout immunochromatography for determination of norfloxacin in tap water and river water. J. Hazard. Mater. 402, 123781. doi:10.1016/j.jhazmat.2020.123781
Shang, Y., Cai, S., Ye, Q., Wu, Q., Shao, Y., Qu, X., et al. (2021). Quantum dot nanobeads-labelled lateral flow immunoassay strip for rapid and sensitive detection of Salmonella Typhimurium based on strand displacement loop-mediated isothermal amplification. Engineering. doi:10.1016/j.eng.2021.03.024
Shi, C., Shang, F., Zhou, M., Zhang, P., Wang, Y., and Ma, C. (2016). Triggered isothermal PCR by denaturation bubble-mediated strand exchange amplification. Chem. Commun. 52 (77), 11551–11554. doi:10.1039/c6cc05906f
Song, W., Park, M. J., Jeong, S., Shin, D. H., Kim, J. S., Kim, H. S., et al. (2020). Rapid identification of OXA-48-like, KPC, NDM, and VIM carbapenemase-producing enterobacteriaceae from culture: Evaluation of the RESIST-4 O.K.N.V. Multiplex lateral flow assay. Ann. Lab. Med. 40 (3), 259–263. doi:10.3343/alm.2020.40.3.259
Sun, Z., Chen, Z., Hou, X., Li, S., Zhu, H., Qian, J., et al. (2008). Locked nucleic acid pentamers as universal PCR primers for genomic DNA amplification. PLoS ONE 3 (11), e3701. doi:10.1371/journal.pone.0003701
Suryoprabowo, S., Liu, L., Kuang, H., Cui, G., and Xu, C. (2021). Fluorescence based immunochromatographic sensor for rapid and sensitive detection of tadalafil and comparison with a gold lateral flow immunoassay. Food Chem. 342, 128255. doi:10.1016/j.foodchem.2020.128255
Thong, K. L., Lai, M. Y., Teh, C. S. J., and Chua, K. H. (2011). Simultaneous detection of methicillin-resistant,Staphylococcus aureus, Acinetobacter baumannii,Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa by multiplex PCR. Trop. Biomed. 28 (1), 21–31.
Vyas, S. S., Jadhav, S. V., Majee, S. B., Shastri, J. S., and Patravale, V. B. (2015). Development of immunochromatographic strip test using fluorescent, micellar silica nanosensors for rapid detection of B. abortus antibodies in milk samples. Biosens. Bioelectron. 70, 254–260. doi:10.1016/j.bios.2015.03.045
Wang, L., Sun, J., Ye, J., Wang, L., and Sun, X. (2022). One-step extraction and simultaneous quantitative fluorescence immunochromatography strip for AFB1 and Cd detection in grain. Food Chem. 374, 131684. doi:10.1016/j.foodchem.2021.131684
Xing, G., Sun, X., Li, N., Li, X., Wu, T., and Wang, F. (2022). New advances in lateral flow immunoassay (LFI) technology for food safety detection. Molecules 27 (19), 6596. doi:10.3390/molecules27196596
Xu, D., Wu, X., Li, B., Li, P., Ming, X., Chen, T., et al. (2013). Rapid detection of Campylobacter jejuni using fluorescent microspheres as label for immunochromatographic strip test. Food Sci. Biotechnol. 22, 585–591. doi:10.1007/s10068-013-0118-5
Xu, L., Sun, X., and Ma, X. (2017). Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 16 (1), 18. doi:10.1186/s12941-017-0191-3
Yu, J., Tan, K., Rong, Z., Wang, Y., Chen, Z., Zhu, X., et al. (2016). Nosocomial outbreak of KPC-2- and NDM-1-producing Klebsiella pneumoniae in a neonatal ward: a retrospective study. BMC Infect. Dis. 16 (1), 563. doi:10.1186/s12879-016-1870-y
Zaidi, A. K., Huskins, W. C., Thaver, D., Bhutta, Z. A., Abbas, Z., and Goldmann, D. A. (2005). Hospital-acquired neonatal infections in developing countries. Lancet 365 (9465), 1175–1188. doi:10.1016/S0140-6736(05)71881-X
Zhang, F., Li, J., Zou, M., Chen, Y., Wang, Y., and Qi, X. (2013). Simultaneous detection of Clavibacter michiganensis subsp. nebraskensis and Pantoea stewartii subsp. stewartii based on microsphere immunoreaction. J. Biomol. Screen. 18 (4), 474–480. doi:10.1177/1087057112467818
Zhang, X., Wu, C., Wen, K., Jiang, H., Shen, J., Zhang, S., et al. (2015). Comparison of fluorescent microspheres and colloidal gold as labels in lateral flow immunochromatographic assays for the detection of T-2 toxin. Molecules 21 (1), E27. doi:10.3390/molecules21010027
Zhuang, L., Ji, Y., Tian, P., Wang, K., Kou, C., Gu, N., et al. (2019). Polymerase chain reaction combined with fluorescent lateral flow immunoassay based on magnetic purification for rapid detection of canine parvovirus 2. BMC Vet. Res. 15 (1), 30. doi:10.1186/s12917-019-1774-3
Keywords: PCR, immunochromatographic test strip (ICTS), POCT, strand exchange amplification (SEA), Klebsiella pneumoniae, rapid detection, nanofluorescent microsphere (nFM)
Citation: Chen Y, Sha L, Li W, Zhou L, Pei B, Bian X, Ji Y, Liu Y, Wang L and Yang H (2023) Rapid quantitative detection of Klebsiella pneumoniae in infants with severe infection disease by point-of-care immunochromatographic technique based on nanofluorescent microspheres. Front. Bioeng. Biotechnol. 11:1144463. doi: 10.3389/fbioe.2023.1144463
Received: 14 January 2023; Accepted: 30 January 2023;
Published: 08 February 2023.
Edited by:
Yanqi Wu, Jamhuriya University of Science and Technology, SomaliaReviewed by:
Hui Chen, Hunan University of Technology, ChinaCopyright © 2023 Chen, Sha, Li, Zhou, Pei, Bian, Ji, Liu, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Yang, eWFuZ2h1YW5nMjAxNUB0bXUuZWR1LmNu; Li Wang, anN4endkbEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.