
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 20 February 2023
Sec. Synthetic Biology
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1141272
This article is part of the Research Topic Efficient Biomanufacturing via Microbial Cell Factories, volume II View all 15 articles
Taxadiene is an important precursor in taxol biosynthesis pathway, but its biosynthesis in eukaryotic cell factories is limited, which seriously hinders the biosynthesis of taxol. In this study, it is found that there was the catalysis compartmentalization between two key exogenous enzymes of geranylgeranyl pyrophosphate synthase and taxadiene synthase (TS) for taxadiene synthesis progress, due to their different subcellular localization. Firstly, the enzyme-catalysis compartmentalization was overcome by means of the intracellular relocation strategies of taxadiene synthase, including N-terminal truncation of taxadiene synthase and enzyme fusion of GGPPS-TS. With the help of two strategies for enzyme relocation, the taxadiene yield was increased by 21% and 54% respectively, among them the GGPPS-TS fusion enzyme is more effective. Further, the expression of GGPPS-TS fusion enzyme was improved via the multi-copy plasmid, resulting that the taxadiene titer was increased by 38% to 21.8 mg/L at shake-flask level. Finally, the maximum taxadiene titer of 184.2 mg/L was achieved by optimization of the fed-batch fermentation conditions in 3 L bioreactor, which is the highest reported titer of taxadiene biosynthesis accomplished in eukaryotic microbes. This study provides a successful example for improving biosynthesis of complex natural products by solving the critical problem of multistep enzymes catalysis compartmentalization.
Taxol is one of the best natural anti-tumor drugs on the market. It is considered to be one of the most effective anti-cancer drugs for human beings in the next 20 years (Yang et al., 2020; Huang et al., 2021). The main source of taxol is derived from taxus plants, which is limited by the taxol abundance and the natural resources of taxus plants. On natural conditions, the growth speed of taxus plants is slow, and regeneration ability is poor, thus the extracted taxol has been unable to meet the demand of clinical from taxus plants (Goldspiel, 1997; Htay and Liu, 2005; Miller et al., 2008). At present, the production of taxol mainly as and semi-synthetic raw material using the natural precursor 10-deacetylbaccatin III. However, the acquisition of 10-deacetylbaccatin III has some limitations, such as low extraction efficiency, high production cost and dependence of raw materials on taxus plants (Cragg, 1998; Baloglu and Kingston, 1999; Frense, 2007). With the help of microbial metabolic engineering and synthetic biology technology, constructing a suitable heterologous biosynthesis system to produce taxol gradually became a research hotspot (Choi et al., 2019; Zhang et al., 2022). In recent years, the biosynthesis of taxadiene, an important intermediate of taxol, has made some progress (Gallego-Jara et al., 2020; Sabzehzari et al., 2020; Hu et al., 2021).
Taxadiene is a diterpenoid compound derived from cyclized geranyl pyrophosphate (GGPP) substrate of taxadiene synthase (TS) (Hefner et al., 1998; Williams et al., 2000; Walker and Croteau, 2001). GGPP is a common precursor produced from MEP or MVA pathway and generated by geranyl diphosphate synthase (GGPPS) (Vranová et al., 2013; Liao et al., 2016; Chatzivasileiou et al., 2019). To date, taxadiene biosynthesis has been realized and modified in different microbial systems (Moser and Pichler, 2019; Navale et al., 2021). In 2010, Ajikumar et al. gained the yield of taxadiene of 300 mg/L in shake flask fermentation through engineering multi-module metabolic pathway and optimizing the metabolic balance of taxadiene synthesis. And then the yield of taxadiene was further increased to 1,020 mg/L by modifying fed-batch fermentation (Ajikumar et al., 2010). To realize the biosynthesis of taxol, taxadiene also needs to undergo a series of complex reactions such as hydroxylation, acetylation and epoxidation (Jennewein and Croteau, 2001; Hu et al., 2021). But the complete intimal system and protein post-translational modification system were lack in the prokaryotic system, which severely limits biosynthesis of taxol using prokaryotic system (Bheri et al., 2020; Ramazi and Zahiri, 2021). Thus, in the past decade, there has been no major breakthrough in the heterosynthesis of taxol in E. coli (E. coli). And the eukaryotic system was regarded as a potential candidate for heterosynthesis of taxol, since the eukaryotic system was suitable for the expression of multiple heterologous P450 hydroxylases and their reductase, which are necessary for taxol synthesis (Hauser and Matthes, 2017; Jia et al., 2022). Therefore, in recent years, eukaryotic systems have been increasingly favored by researchers in taxadiene biosynthesis. Engels et al. screened the sources of GGPPS enzyme, engineered the isoprene pathway and optimized the TS gene, achieving the yield of taxadiene of 8.7 mg/L, which laid a foundation for the production of taxol in Saccharomyces cerevisiae (S. cerevisiae) (Engels et al., 2008). Behnaz et al. enhanced the expression of TS by introducing the solubilizing tags to improve taxadiene titre to 57 mg/L at 30°C. Meanwhile, Behnaz et al. also found the temperature-dependant phenomenon of TS, according to that, a maximum taxadiene titre of 129 mg/L was obtained by manipulating the fermentation temperature at 20°C (Nowrouzi et al., 2020). In addition, Li et al. introduced taxadiene synthase, and taxadiene-5α-hydroxylase in Nicotiana benthamiana through chloroplastic compartmentalized metabolic engineering to produce taxadiene (56.6 μg/g FW) and taxadiene-5α-ol (1.3 μg/g FW). This also was an alternative eukaryotic platform for taxol production (Li et al., 2019). Previous studies have shown that the accumulation of GGPP is abundant enough to supply downstream metabolic pathways for the production of taxadiene and its derivatives in both prokaryotic and eukaryotic systems (Ober, 2010; Zhou et al., 2012). However, the synthesis efficiency of taxadiene in the eukaryotic system still needs to be further improved compared to that in the prokaryotic system. The eukaryotic cells have abundant subcellular organelle structures, which may lead to different localization of the heterologous proteins for complex natural products (Luo et al., 2015; Du and Li, 2021). Thus, we speculate that key enzymes catalysis compartmentalization may be an important factor to limit the taxadiene biosynthesis in eukaryotic system.
Focusing on the problem of enzymes catalysis compartmentalization, many researchers have done a lot of work and made great achievements. Dawid et al. truncated the N-terminal mitochondrial location sequences of valine biosynthesis enzymes Ilv2, Ilv5 and Ilv3 to relocate the truncated enzymes into the cytoplasm, resulting in the increase of isobutanol production (Brat et al., 2012). Jiang et al. employed synthetic protein scaffolds to colocalize the sequential enzymes of Idi1, GES-Erg20 and IS to eliminate enzymes catalysis compartmentalization, and the citronellol titer was incerased to 8.30 g/L (Jiang et al., 2021). In addition, protein fusion is also an important means to realize target gene relocation and eliminate enzymes catalysis compartmentalization. Ma et al. introduced the peroxisome targeting sequence SKL to fusion proteins CrtW-CrtZ, causing fusion proteins CrtW-CrtZ to relocate to suitable organelle of peroxisome, and the astaxanthin titer was increased to 58.7 mg/L (Ma et al., 2021). In addition, Shi et al. fused protopanaxadiol synthase (PPDS) to lipid droplet membrane protein Pln1 for closing the spatial distance to substrate dammarenediol-II (DD) accumulated in lipid droplet, leading to the final product ginsenoside Compound K yield of 5 g/L (Shi et al., 2021).
In our previous study, we successfully constructed a high-yield strain of GGPP by screening the metabolic pathway gene and regulating the promoters of critical genes, which provided sufficient precursors for the biosynthesis of taxadiene (Song et al., 2017). Herein, we verified the catalysis compartmentalization of two key exogenous enzymes GGPPS and TS in taxadiene synthesis pathway (Figure 1). Next, we reconstructed the subcellular localization of TS in S. cerevisiae to eliminate enzymes (enzyme) catalysis compartmentalization using the methods of N-terminal signal peptide truncation of TS and enzyme fusion of GGPPS-t60TS, and the taxadiene yield was increased by 21% and 54% respectively. Especially, the GGPPS-TS fusion enzyme is more effective. And then, we improved the expression of GGPPS-TS fusion enzyme via the multi-copy plasmid, leading to the higher taxadiene titer of 21.8 mg/L at the shake-flask level. Finally, the taxadiene titer was increased to 184.2 mg/L for 144 h on the optimized fed-batch fermentation conditions in 3 L bioreactor, which is approximately increased to 17.8-fold compared with the initial strain yZCL010. This study is a successful example of improving the biosynthesis of complex natural products by overcoming multistep enzyme catalysis compartmentalization in the eukaryotic microbial cell.
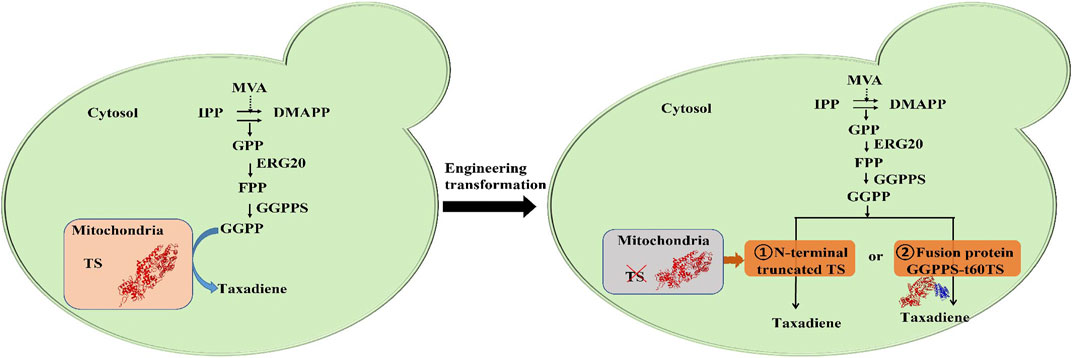
FIGURE 1. The biosynthesis pathway and metabolic engineering transformation of taxadiene. MVA, mevalonate; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; GPP, geranyl diphosphate; FPP, farnesyl diphosphate; GGPPS, geranylgeranyl diphosphate synthase; GGPP, geranylgeranyl diphosphate; TS, taxadiene synthase.
All the yeast strains used in this study were listed in Table 1. E. coli Top10 (TransGen Biotech, Beijing, China) was used for plasmids construction and amplification. BY4742 used for construction of taxadiene producing strains (listed in Table 1), was obtained from EUROSCARF (Frankfurt, Germany) (Entian and Kotter, 2007).
LB medium (0.5% yeast extract, 1% tryptone and 1% NaCl) was used to culture E. coli Top 10 for the propagation of recombinant plasmids, supplemented with 50 μg/mL kanamycin or 100 μg/mL ampicillin at 37°C. Yeast cells were routinely cultured in YPD medium (1% yeast extract, 2% peptone, 2% glucose) or synthetic complete (SC) drop-out medium (2% dextrose, 0.67% yeast nitrogen base, 0.2% amino acid mix lacking selected amino acids) at 30°C. The selected plasmids carrying nutritional screening tags were introduced into S. cerevisiae cells and cultured in a synthetic complete medium without corresponding amino acids. All the medium formulations for yeast culture was the same as our previous work (Zeng et al., 2020).
All the plasmids are summarized in Supplementary Table S1, and the primers used during plasmid construction are summarized in Supplementary Table S2. The yeast expression plasmid pRS415 and pRS425 were purchased from ADDGENE (American) and the ampicillin resistant gene of the plasmid was substituted by kanamycin resistant gene to construct pRS415K and pRS425K. SaGGPPS gene (ACCESSION P39464) and TbTS gene (ACCESSION Q41594) were codon optimized for expression in S. cerevisiae and synthesized by GenScript,Inc. (China) (Supplementary Table S3). The basic gene expression cassette was assembled by overlap extension PCR (OE-PCR) (pZCL026; pZCL027; pZCL041) (Supplementary Figure S2). The OE-PCR products were digested by BamH I/Not I and inserted into the corresponding sites of expression plasmid pRS415k, obtaining a series of plasmids for the construction of target enzymes localization strains. Then the plasmid with N-terminal truncated TbTS or fusion protein GGPPS-TS (including pZCL063; pZCL064; pZCL071) were constructed based on pZCL041(Supplementary Table S1). The plasmids were transformed of into yeast strains by the LiAc/ssDNA carrier DNA/PEG3350 method. The S. cerevisiae strains constructed in this study were listed in Table 1(Gietz and Woods, 2006).
To prepare seed vials, single isolates of each strain from agar plates were grown for SD Agar plate at 30°C and 200 rpm. And the preculture was transferred to a 250 mL flask filled with 50 mL fresh culture medium, the initial OD600 was 0.2. And then preculture was transferred into 250 mL flasks with 50 mL the same fresh medium with initial OD600 of 0.2 and cultivated at 30°C, 250 rpm for 10–12 h until the exponential phase. Finally, the third-grade seeds were transferred to a shake flask and fermented for 120 h. The cultured tertiary seeds were then transferred to 200 mL fresh SC medium for 10 h until OD600 reached about 6.0. Then the seed culture was transferred to a bioreactor containing a 2.7 L fermentation medium. The initial fermenter medium was YPD containing 20 g/L glucose. The 10% (v/v) n-dodecane (Sigma-Aldrich) was added to the culture at the beginning of the fermentation to enrich taxadiene, which could minimize the loss of taxadiene the product and protect the cells from phase the toxicity brought by taxadiene (Brennan et al., 2012).
For fed-batch fermentation, the inoculation amount in the fermenter is 10% (v/v), and the seed preparation conditions are the same as those in the shaking flask fermentation. The stirring speed is 300 rpm. The temperature was controlled at 30°C. Air flow is used to supply oxygen to the fermentation tank at 2 vvm, and the pH value in the fermentor was controlled at 6.0 with 3 M NaOH. In addition, the biomass in fermentation and the accumulation of taxadiene in a later stage were increased by nitrogen supplemental. In addition, 5 g/L nitrogen yeast extract was added three times at the initial stage of culture every 7 h during the beginning 48 h of cultivation. After 48 h of fermentation, the temperature dropped to 20°C, and 20% (v/v) dodecane was added for two-phase extractive fermentation, and Galactose inducer was added until the final concentration was 20 g/L. Cell growth and glucose concentration were continuously monitored during fermentation. The cell growth and the glucose concentration were constantly monitored during the fermentation process. 500 g/L glucose was fed periodically into the fermentation to keep the glucose concentration under 1.0 g/L. And the organic layer was harvested for taxadiene analysis by centrifugation of the fermentation broth at 12,000 rpm for 10 min. The GGOH (98% purity) was prepared to construct a standard curve to determine the GGOH yield.
GC-TOF/MS analyzed taxadiene and GGOH in fermentation products. The target product extracted from n-dodecane was diluted with n-hexane, then 1 μL sample was injected into Shimazu GC-2030 using a Shimazu GCMS-QP2020 automatic sampler. The sample was detected using a quartz capillary column (30 m × 0.25 mm, 0.25 mm DB-5MS, J&W Scientific, Folsom). Design the relevant parameters of the sample detection method. The injector temperature was set at 260°C. The column effluents were introduced into the ion source (250°C) of TOF/MS. And ions were generated by 40 mA ionization current of a 70 eV electron beam. The mass scan range was 50–800 m/z.
For GC-TOF/MS analysis of taxadiene and GGOH, the column chamber temperature was first kept constant at 70°C for 1 min, then increased to 200°C at a rate of 30°C/min for 1 min. Next it increased to 265°C at a rate of 12°C/min and kept for 3 min. The total run time was 14.75 min. Taxadiene was identified by mass fragments 109 m/z and 122 m/z, and the peak time was 10.32 min. GGOH was identified by mass fragments of 69 m/z, 93 m/z, and 119 m/z, and the peak time was 11.35 min.
Fluorescence microscopy was used to observe the distribution of fluorescent signals of target proteins in the subcellular to determine the subcellular localization of target proteins. A single colony of inverters extracted from an SC-U-L agar plate was first cultured into a 20 mL tube containing 5 mL SC-U-L medium and cultured at 30°C and 220 rpm for 20–24 h to achieve the exponential phase. The pre-cultured cells were then transferred to 20 mL test tubes and 5 mL identical fresh medium, initially OD600, and cultured at 30°C and 220 rpm for 48 h. Fluorescence image was observed under a fluorescence microscope and treated with fluorescence microscopy software FCSnap.
In this study, Geranylgeranyl diphosphate synthase from Sulfolobus acidocaldarius (SaGGPPS) and sequence-optimized TS from Taxus brevifolia (Tb t60TS) was introduced into the high-yield GGPP strain to construct the initial taxadiene production strain yZCL010 (Figure 2A). And the taxadiene titer of 10.2 mg/L and the geranylgeraniol (GGOH) titer of 214.2 mg/L were detected in the production strain yZCL010 (Figure 2B). According to the large accumulation of by-product GGOH in the synthesis of taxadiene, we speculated that the synthesis process of taxadiene with key enzymes of GGPPS and TS is limited, which should be the main reason for the low taxadiene production.
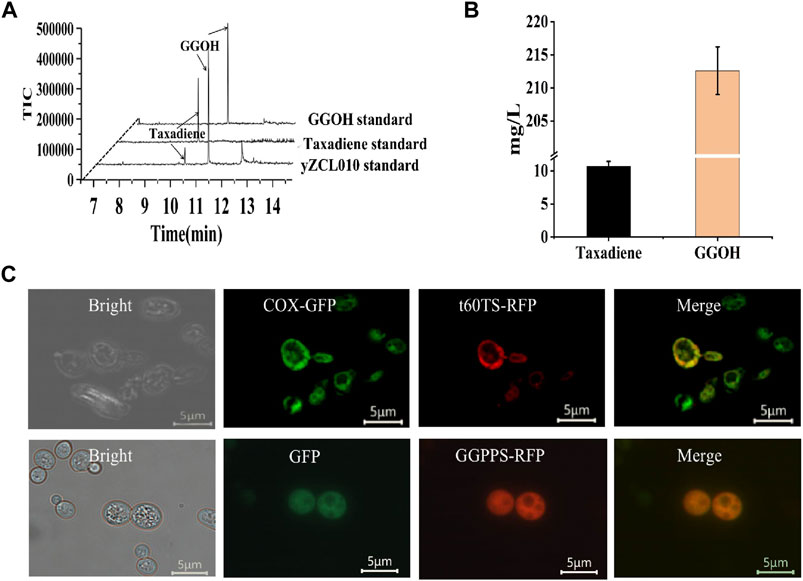
FIGURE 2. Construction of the initial taxadiene production strain and subcellular localization of key exogenous enzymes. (A) GC-MS results for the initial strain for taxadiene and GGOH production. The taxadiene and GGOH eluted at about 10.3 min and 11.3 min. (B) The titers of taxadiene and GGOH in strain yZCL010. (C) Subcellular localization of t60TS and GGPPS in S. cerevisiae.
Whereafter, the subcellular localizations of two key enzymes Tbt60TS and SaGGPPS for the taxadiene synthesis were detected. We firstly added red fluorescent protein (RFP) tag to the C-terminus of each target enzyme, and co-expressed them with fluorescent protein labeled endogenous subcellular localization protein, such as COXIII-GFP for mitochondrial localization characterization and sole expressed GFP for cytoplasmic localization characterization (Rodrigues et al., 2001; Naithani et al., 2003). Fluorescent microscopic image analysis of RFP and GFP signals showed that SaGGPPS was located in cytoplasm, while t60TS was located in mitochondria (Figure 2C). These results indicate that GGPPS and t60TS localized at different subcellular, which would result in the problem of catalytic compartmentalization between the two sequent enzymes GGPPS and t60TS. Therefore the large amount of GGPP generated in the cytoplasm was difficult to contact with TS to produce taxadiene, due to GGPP hardly is transported through the plastid membrane with significant efficiency (Bick and Lange, 2003; Vranová et al., 2012). Instead, accumulated GGPP was converted into by-product GGOH. In our constructed the initial taxadiene production strain yZCL010, only less than 5% of the precursor GGPP was converted to the target product taxadiene, while almost all the rest was produced into by-product GGOH, which is a great metabolic drain. According to the above key enzymes localization analysis and product detection results, the catalytic compartmentalization of enzymes would reduce the availability of substrates and intermediates, leading to large accumulation of by-product and low yield of the target product.
From the above study, SaGGPPS was located in the cytoplasm, which may lead to the accumulation of GGPP in the cytoplasm. Thus, Tbt60TS with mitochondria localization will hardly contact with the substrate GGPP to effectively generate taxadiene. Therefore, we attempted to relocate TS from mitochondria into the cytoplasm to improve conversion from GGPP to taxadiene. Here, the proper truncation position for TbTS overexpressed in S. cerevisiae was attempted to relocate TS. Based on the predicted results on the secondary structure by https://bioinf.cs.ucl.ac.uk/psipred/ (Supplementary Figure S1A) (McGuffin et al., 2000), N-terminus of TbTS was truncated at other two different positions (R84 and N97) besides M60 (t60TS), according to a certain gradient with slightly change, named t84TS and t97TS respectively (Supplementary Figure S1B).
To determine the subcellular localization of the t84TS and t97TS in S. cerevisiae, RFP tag was applied to N-terminal truncated TbTS and cytoplasmic localization protein GFP were also co-expressed (Refer to Section 3.1). Fluorescent microscopic image analysis showed that t84TS was located in the cytoplasm successfully (Figure 3A). In addition, t97TS were also located in the cytoplasm (data not shown).
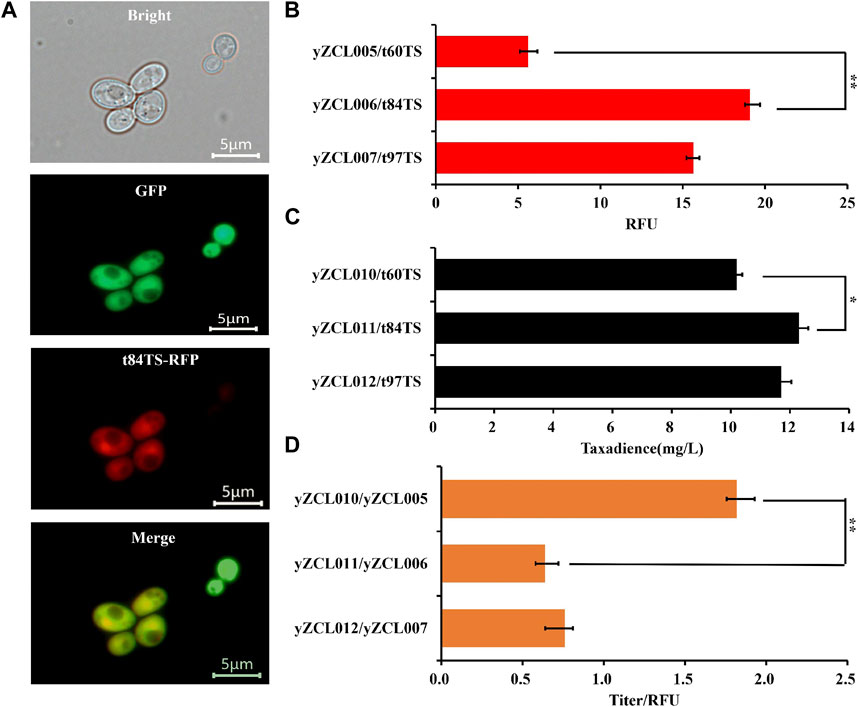
FIGURE 3. Effect of N-terminal truncation on subcellular localization and performance of TS in S. cerevisiae. (A) Subcellular localization of t84TS in S. cerevisiae. (B) Effect of N-terminal truncation on the expression of TS. (C) Effect of N-terminal truncation of TS on the yield of taxadiene. (D) Effect of N-terminal truncation on unit enzyme activity of TS. The error bars represent the means ± SD from three biological replicates. **p < 0.01, *p < 0.05, student’s t-test.
Meanwhile, N-terminal truncated TbTS (t84TS and t97TS) was introduced into the high-yield GGPP strain to construct the taxadiene production strain of yZCL011, and yZCL012, respectively. As shown in Figure 3B, notably t84TS and t97TS both led to an over 2.5-fold increase in protein soluble expression compared with that of t60TS, in view of the different fluorescence intensity detection. However, the titers of taxadiene only were slightly increased by 20.5% to 12.3 mg/L and by 14.3% to 11.7 mg/L in the production strains of yZCL011 and yZCL012 (Figure 3C), which indicated the taxadiene yield was not correspondingly increased with the enhanced expression of TS. In the case, we further investigated the effect of TS truncation on unit enzyme activity. It is finding that the enzyme activities of t84TS and t97TS with N-terminal deep truncation were either significantly decreased, even less than 50% (Figure 3D). Thus, the deep N-terminal truncation is an undesired strategy for optimizing the function of TS enzyme, even though TbTS was relocate and increased on soluble expression.
Since the catalysis compartmentalization between GGPPS and TS is the limiting step for taxadiene synthesis in S. cerevisiae, fusion expression of GGPPS and TS would overcome the problem of catalysis compartmentalization to improve sequential catalysis efficiency of GGPPS and TS. Here, the forward and reverse fusion of GGPPS and TS were adopted, and these two proteins were fused with the short flexible linker GSG (Ajikumar et al., 2010). Among them, in the forward fusion, SaGGPPS pulled Tbt60TS enzyme to localize in the cytoplasm of S. cerevisiae (Figure 4A). Conversely, Tbt60TS would traction SaGGPPS to localize in the mitochondria of S. cerevisiae in the reverse fusion.
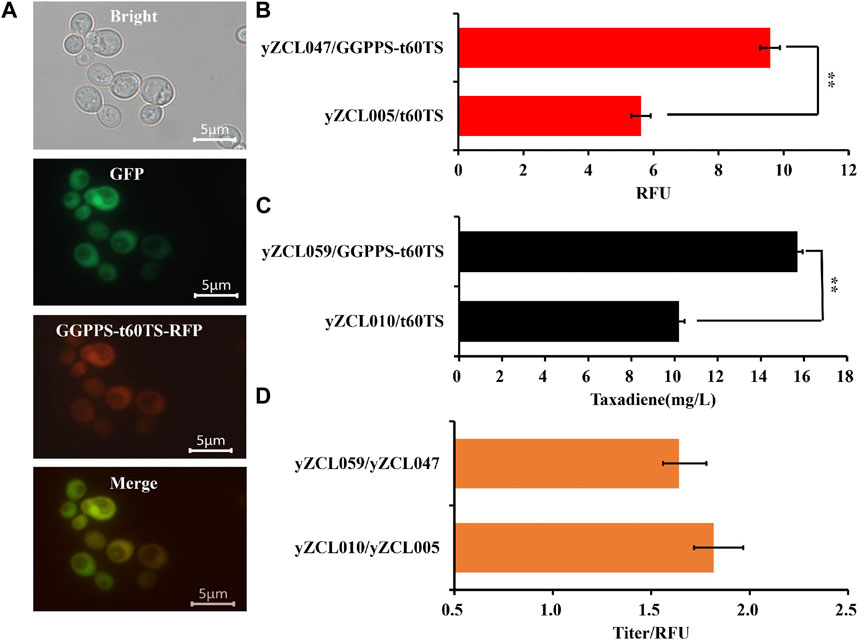
FIGURE 4. Effect of fusion protein GGPPS-t60TS on subcellular localization and performance of TS in S. cerevisiae. (A) Subcellular localization of fusion protein GGPPS-t60TS in S. cerevisiae. (B) Effect of fusion protein GGPPS-t60TS on the expression of TS. (C) Effect of fusion protein GGPPS-t60TS on the yield of taxadiene. (D) Effect of fusion protein GGPPS-t60TS on unit enzyme activity of TS. The error bars represent the means ± SD from three biological replicates. **p < 0.01, *p < 0.05, student’s t-test.
Next, the forward and reverse fusion protein of GGPPS-t60TS and t60TS-GGPPS were introduced into the high-yield GGPP strain to construct the taxadiene production strain yZCL059 and yZCL060, respectively. In the two taxadiene production strains, the yields of taxadiene have been signally improved, reaching to 15.7 mg/L and 13.8 mg/L respectively, relying on the introduction of forward and reverse fusion proteins of GGPPS-t60TS and t60TS-GGPPS (Supplementary Figure S3). Moreover, applying the forward fusion protein of GGPPS-t60TS achieved more significant effect in enhancing taxadiene biosynthesis, and the taxadiene titer of strain yZCL059 was increased by 54%, compared with that of the initial strain yZCL010. To sum up, the forward and reverse fusion protein of GGPPS-t60TS and t60TS-GGPPS were located in cytoplasm and mitochondria of S. cerevisiae respectively, and the fusion protein of GGPPS-t60TS in cytoplasm had a greater advantage. It suggested that the cytoplasmic microenvironment seemed be more conducive to the synthesis pathway of taxadiene than mitochondria microenvironment, due to the membrane barrier effect of mitochondria restricting the transfer of intermediates. Moreover, the upstream MVA and FPP synthesis pathways are mainly distributed in the cytoplasm (Vranová E et al., 2013), which will Provide sufficient precursors for the synthesis of GGPP and taxadiene. This may also partly explain the reason of the high production of taxadiene in prokaryotes at present.
In addition, the soluble expression of GGPPS-t60TS was enhanced by about 60% than that of sole t60TS (Figure 4B), which indicates that GGPPS also contributed to the soluble expression of TS, similar to the soluble protein tag effect, in the forward fusion protein. This is a desirable outcome, since the heterologous expression of TS has not been satisfactory in the present study. As well, enzymes fusion in sequential catalysis progress (GGPPS and TS in this study) might minimize the distance between them for higher catalytic activity through enhancing GGPP accessibility for TS (Albertsen et al., 2011). According to the above two favorable effects, the GGPPS-t60TS raised the maximum yield of taxadiene of 15.7 mg/L in strain yZCL059 (Figure 4C). Meanwhile, there was no significant difference in unit enzyme activity between the fusion protein of GGPPS-t60TS and the initial t60TS (Figure 4D), which takes into account the intrinsic low enzyme activity of TS (Soliman and Tang, 2015). Therefore, the forward fusion protein of GGPPS-t60TS demonstrated the significant advantages in the efficient synthesis of taxadiene in the eukaryotic system.
The forward fusion protein GGPPS-t60TS was proved to be more benefit for taxadiene production. According to the poor soluble expression of TS in yeast on previous studies (Nowrouzi et al., 2020), the expression of the fusion protein GGPPS-t60TS module was enhanced using a multi-copy plasmid to improve the conversion rate of substrate GGPP to taxadiene. We firstly inserted the gene of fusion protein GGPPS-t60TS into the multi-copy plasmid PRS425K at restriction enzymes sites of BamH I and Not I, and then, the constructed multi-copy plasmid PRS425K -GGPPS-t60TS was introduced into the high-yield GGPP strain to obtained the taxadiene production strain yZCL061. As shown in Figure 5, the taxadiene yield of stain yZCL061was increased by 38.9% than that of stain yZCL059, benefiting from protein expression improvement by multiple-copy plasmid, and taxadiene titer reached to 21.8 mg/L in the shake flask. On the contrary, the by-product GGOH yield was also increased by 4% in the stain yZCL061 compared with that in the stain yZCL059, which did not show statistical significance. Ultimately, the titer ratio of taxadiene to GGOH is enhanced by over 108%, from the titer ratio of 4.7% in initial production strain yZCL010 to 9.8% in stain yZCL061 (Figure 2B, Figure 5). Therefore, the fusion protein of GGPPS-t60TS significantly promoted the conversion efficiency of target product taxadiene.
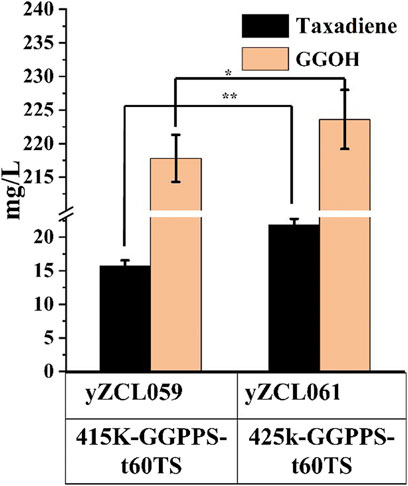
FIGURE 5. The effect of overexpression of fusion protein GGPPS-t60TS on the titers of taxadiene and GGOH. 415K incited single-copy plasmid, 425K incited multi-copy plasmid. The error bars represent the means ± SD from three biological replicates. **p < 0.01, *p < 0.05, student’s t-test.
To evaluate the performance of the best taxadiene biosynthesis strain (yZCL061), fed-batch fermentations were performed in a 5 L bioreactor using YPD as the batch medium. As shown in Figure 6, a total titer of 184.2 mg/L taxadiene with the maximal biomass at OD600 = 110.3 was achieved, which is the highest reported titer in eukaryotic cells. The fermentation process was divided into two stages. The first stage was the cell growth stage, in which the cultivation temperature was controlled at 30°C for 48 h until the cell density OD600 reached 72.8 (Figure 6). The initial glucose concentration was set as 20 g/L. The supplemental carbon source glucose was strictly controlled below 1 g/L due to the carbon source restriction strategy. The second stage taxadiene producing stage was initialized after the temperature was decreased to 20°C and 20 g/L galactose was added at 48 h (Nowrouzi et al., 2020). The supplemental carbon source was changed into ethanol because the existence of glucose would inhibit the expression of the GAL promoters. With fermentation proceeding, the taxadiene production also increased steadily. After 144 h cultivation, the taxadiene titer reached 184.2 ± 0.56 mg/L (Figure 6), which was eight times more than the output at the shake flask level. Even though the titer is still lower than that in E. coli (Ajikumar et al., 2010), increasing the yeast cell tolerance to taxadiene would be an efficient solution to minimize the taxadiene production gap between E. coli and yeast.
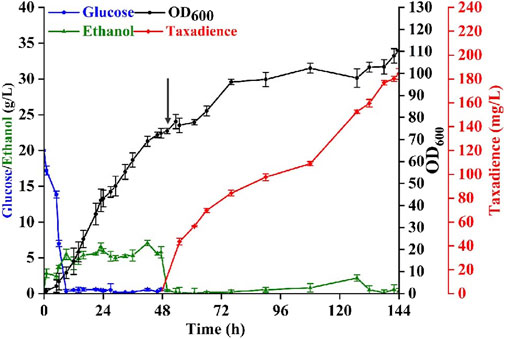
FIGURE 6. Taxadiene production in fed-batch fermentation. Fed-batch fermentations were performed at a 3.0 L scale, using YPD medium and by the engineered S. cerevisiae strain yZCL061. Black arrows represented the addition of n-dodecane and galactose into the medium. The error bars represent the means ± SD from three biological replicates.
In this work, it is found that the catalysis compartmentalization between two key enzymes of GGPPS and TS limited taxadiene production. Applying the intracellular relocation strategies for TS eliminated catalysis compartmentalization via N-terminal truncation of TS and enzyme fusion of GGPPS-TS. With the help of forward fusion protein of GGPPS-t60TS, the taxadiene yield was increased by 54% to 15.7 mg/L. Subsequently, the taxadiene titer was further enhanced to 21.8 mg/L at shake-flask level by over-expressing fusion enzyme of GGPPS-t60TS in multi-copy plasmid. Eventually, a highest reported titer of 184.2 mg/L taxadiene in eukaryotic cells was achieved in 3 L fed-batch fermentation. Our study provides an effective strategy to eliminate multistep enzymes catalysis compartmentalization for improving biosynthesis of complex natural products.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
MY and WX conceived the study. CZ and WC participated in strain construction. MY and CZ carried out results data analysis. CZ and TD participated in fed-batch fermentation. CZ and WC carried out the chemical analysis. BL, WX, and YW helped to draft the manuscript. BL and WX participated in design and coordination of the study. MY supervised the whole research and revised the manuscript. All the authors read and approved the final manuscript.
This work was supported by the Key-Area Research and Development Program of Guangdong Province (2020B0303070002), the National Key Research and Development Program of China (2018YFA0900702) and the National Natural Science Foundation of China (21621004 and 21676192).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2023.1141272/full#supplementary-material
Ajikumar, P. K., Xiao, W. H., Tyo, K. E., Wang, Y., Simeon, F., Leonard, E., et al. (2010). Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330 (6000), 70–74. doi:10.1126/science.1191652
Albertsen, L., Chen, Y., Bach, L. S., Rattleff, S., Maury, J., Brix, S., et al. (2011). Diversion of flux toward sesquiterpene production in Saccharomyces cerevisiae by fusion of host and heterologous enzymes. Appl. Environ. Microbiol. 77 (3), 1033–1040. doi:10.1128/AEM.01361-10
Baloglu, E., and Kingston, D. G. (1999). A new semisynthesis of paclitaxel from baccatin III. J. Nat. Prod. 62 (7), 1068–1071. doi:10.1021/np990040k
Bheri, M., Mahiwal, S., Sanyal, S. K., and Pandey, G. K. (2020). Plant protein phosphatases: What do we know about their mechanism of action? FEBS J. 288 (3), 756–785. doi:10.1111/febs.15454
Bick, J. A., and Lange, B. M. (2003). Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: Unidirectional transport of intermediates across the chloroplast envelope membrane. Archives Biochem. biophysics 415 (2), 146–154. doi:10.1016/s0003-9861(03)00233-9
Brat, D., Weber, C., Lorenzen, W., Bode, H. B., and Boles, E. (2012). Cytosolic re-localization and optimization of valine synthesis and catabolism enables increased isobutanol production with the yeast Saccharomyces cerevisiae. Biotechnol. biofuels 5 (1), 65–16. doi:10.1186/1754-6834-5-65
Brennan, T. C., Turner, C. D., Krömer, J. O., and Nielsen, L. K. (2012). Alleviating monoterpene toxicity using a two-phase extractive fermentation for the bioproduction of jet fuel mixtures in Saccharomyces cerevisiae. Biotechnol. Bioeng. 109 (10), 2513–2522. doi:10.1002/bit.24536
Chatzivasileiou, A. O., Ward, V., Edgar, S. M., and Stephanopoulos, G. (2019). Two-step pathway for isoprenoid synthesis. Proc. Natl. Acad. Sci. 116 (2), 506–511. doi:10.1073/pnas.1812935116
Choi, K. R., Jang, W. D., Yang, D., Cho, J. S., Park, D., and Lee, S. Y. (2019). Systems metabolic engineering strategies: Integrating systems and synthetic biology with metabolic engineering. Trends Biotechnol. 37 (8), 817–837. doi:10.1016/j.tibtech.2019.01.003
Cragg, G. M. (1998). Paclitaxel (Taxol®): A success story with valuable lessons for natural product drug discovery and development. Med. Res. Rev. 18 (5), 315–331. doi:10.1002/(SICI)1098-1128(199809)18:5<315::AID-MED3>3.0.CO;2
Du, L., and Li, S. (2021). Compartmentalized biosynthesis of fungal natural products. Curr. Opin. Biotechnol. 69, 128–135. doi:10.1016/j.copbio.2020.12.006
Engels, B., Dahm, P., and Jennewein, S. (2008). Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab. Eng. 10 (3-4), 201–206. doi:10.1016/j.ymben.2008.03.001
Entian, K. D., and Kötter, P. (2007). 25 yeast genetic strain and plasmid collections. Methods Microbiol. 36, 629–666. doi:10.1016/S0580-9517(06)36025-4
Frense, D. (2007). Taxanes: Perspectives for biotechnological production. Appl. Microbiol. Biotechnol. 73 (6), 1233–1240. doi:10.1007/s00253-006-0711-0
Gallego-Jara, J., Lozano-Terol, G., Sola-Martínez, R. A., Cánovas-Díaz, M., and de Diego Puente, T. (2020). A compressive review about taxol®: History and future challenges. Molecules 25 (24), 5986. doi:10.3390/molecules25245986
Gietz, R. D., and Woods, R. A. (2006). Yeast transformation by the LiAc/SS Carrier DNA/PEG method. Methods Mol. Biol. Clift. N.J.) 313, 107–120. doi:10.1385/1-59259-958-3:107
Goldspiel, B. R. (1997). Clinical overview of the taxanes. Pharmacotherapy 17 (52), 110s–125s. doi:10.1002/j.1875-9114.1997.tb03813.x
Hauser, K., and Matthes, J. (2017). Medical students’ medication communication skills regarding drug prescription a qualitative analysis of simulated physician-patient consultations. Eur. J. Clin. Pharmacol. 73 (4), 429–435. doi:10.1007/s00228-016-2192-0
Hefner, J., Ketchum, R. E., and Croteau, R. (1998). Cloning and functional expression of a cDNA encoding geranylgeranyl diphosphate synthase fromtaxus canadensisand assessment of the role of this prenyltransferase in cells induced for taxol production. Archives Biochem. biophysics 360 (1), 62–74. doi:10.1006/abbi.1998.0926
Htay, T., and Liu, M. W. (2005). Drug-eluting stent: A review and update. Vasc. Health Risk Manag. 1 (4), 263–276. doi:10.2147/vhrm.2005.1.4.263
Hu, Z., Liu, X., Tian, M., Ma, Y., Jin, B., Gao, W., et al. (2021). Recent progress and new perspectives for diterpenoid biosynthesis in medicinal plants. Med. Res. Rev. 41 (6), 2971–2997. doi:10.1002/med.21816
Huang, M., Lu, J. J., and Ding, J. (2021). Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospecting 11 (1), 5–13. doi:10.1007/s13659-020-00293-7
Jennewein, S., and Croteau, R. (2001). Taxol: Biosynthesis, molecular genetics, and biotechnological applications. Appl. Microbiol. Biotechnol. 57 (1), 13–19. doi:10.1007/s002530100757
Jia, Q., Brown, R., Köllner, T. G., Fu, J., Chen, X., Wong, G. K., et al. (2022). Origin and early evolution of the plant terpene synthase family. Proc. Natl. Acad. Sci. 119 (15), e2100361119. doi:10.1073/pnas.2100361119
Jiang, G., Yao, M., Wang, Y., Xiao, W., and Yuan, Y. (2021). A "push-pull-restrain" strategy to improve citronellol production in Saccharomyces cerevisiae. Metab. Eng. 66, 51–59. doi:10.1016/j.ymben.2021.03.019
Li, J., Mutanda, I., Wang, K., Yang, L., Wang, J., and Wang, Y. (2019). Chloroplastic metabolic engineering coupled with isoprenoid pool enhancement for committed taxanes biosynthesis in Nicotiana benthamiana. Nat. Commun. 10 (1), 4850–4912. doi:10.1038/s41467-019-12879-y
Liao, P., Hemmerlin, A., Bach, T. J., and Chye, M. L. (2016). The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 34 (5), 697–713. doi:10.1016/j.biotechadv.2016.03.005
Luo, Y., Li, B. Z., Liu, D., Zhang, L., Chen, Y., Jia, B., et al. (2015). Engineered biosynthesis of natural products in heterologous hosts. Chem. Soc. Rev. 44 (15), 5265–5290. doi:10.1039/c5cs00025d
Ma, Y., Li, J., Huang, S., and Stephanopoulos, G. (2021). Targeting pathway expression to subcellular organelles improves astaxanthin synthesis in Yarrowia lipolytica. Metab. Eng. 68, 152–161. doi:10.1016/j.ymben.2021.10.004
McGuffin, L. J., Bryson, K., and Jones, D. T. (2000). The PSIPRED protein structure prediction server. Bioinformatics 16 (4), 404–405. doi:10.1093/bioinformatics/16.4.404
Miller, K., Neilan, B., and Sze, D. M. (2008). Development of Taxol and other endophyte produced anti-cancer agents. Recent Pat. Anti-Cancer Drug Discov. 3 (1), 14–19. doi:10.2174/157489208783478685
Moser, S., and Pichler, H. (2019). Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 103 (14), 5501–5516. doi:10.1007/s00253-019-09892-y
Naithani, S., Saracco, S. A., Butler, C. A., and Fox, T. D. (2003). Interactions amongCOX1,COX2, andCOX3mRNA-specific translational activator proteins on the inner surface of the mitochondrial inner membrane ofSaccharomyces cerevisiae. Saccharomyces cerevisiae. Mol. Biol. Cell. 14 (1), 324–333. doi:10.1091/mbc.e02-08-0490
Navale, G. R., Dharne, M. S., and Shinde, S. S. (2021). Metabolic engineering and synthetic biology for isoprenoid production in Escherichia coli and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 105 (2), 457–475. doi:10.1007/s00253-020-11040-w
Nowrouzi, B., Li, R. A., Walls, L. E., d’Espaux, L., Malcı, K., Liang, L., et al. (2020). Enhanced production of taxadiene in Saccharomyces cerevisiae. Microb. Cell. factories 19 (1), 200–212. doi:10.1186/s12934-020-01458-2
Ober, D. (2010). Gene duplications and the time thereafter–examples from plant secondary metabolism. Plant Biol. 12 (4), 570–577. doi:10.1111/j.1438-8677.2009.00317.x
Ramazi, S., and Zahiri, J. (2021). Post-translational modifications in proteins: Resources, tools and prediction methods. Database 2021, baab012. doi:10.1093/database/baab012
Rodrigues, F., van Hemert, M., Steensma, H. Y., Côrte-Real, M., and Leão, C. (2001). Red fluorescent protein (DsRed) as a reporter in Saccharomyces cerevisiae. J. Bacteriol. 183 (12), 3791–3794. doi:10.1128/jb.183.12.3791-3794.2001
Sabzehzari, M., Zeinali, M., and Naghavi, M. R. (2020). Alternative sources and metabolic engineering of Taxol: Advances and future perspectives. Biotechnol. Adv. 43, 107569. doi:10.1016/j.biotechadv.2020.107569
Shi, Y., Wang, D., Li, R., Huang, L., Dai, Z., and Zhang, X. (2021). Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides. Metab. Eng. 67, 104–111. doi:10.1016/j.ymben.2021.06.002
Soliman, S., and Tang, Y. (2015). Natural and engineered production of taxadiene with taxadiene synthase. Biotechnol. Bioeng. 112 (2), 229–235. doi:10.1002/bit.25468
Song, T. Q., Ding, M. Z., Zhai, F., Liu, D., Liu, H., Xiao, W. H., et al. (2017). Engineering Saccharomyces cerevisiae for geranylgeraniol overproduction by combinatorial design. Sci. Rep. 7 (1), 14991–15011. doi:10.1038/s41598-017-15005-4
Vranová, E., Coman, D., and Gruissem, W. (2013). Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 64 (1), 665–700. doi:10.1146/annurev-arplant-050312-120116
Vranová, E., Coman, D., and Gruissem, W. (2012). Structure and dynamics of the isoprenoid pathway network. Mol. plant 5 (2), 318–333. doi:10.1093/mp/sss015
Walker, K., and Croteau, R. (2001). Taxol biosynthetic genes. Phytochemistry 58 (1), 1–7. doi:10.1016/s0031-9422(01)00160-1
Williams, D. C., Carroll, B. J., Jin, Q., Rithner, C. D., Lenger, S. R., Floss, H. G., et al. (2000). Intramolecular proton transfer in the cyclization of geranylgeranyl diphosphate to the taxadiene precursor of taxol catalyzed by recombinant taxadiene synthase. Chem. Biol. 7 (12), 969–977. doi:10.1016/s1074-5521(00)00046-6
Yang, Y. H., Mao, J. W., and Tan, X. L. (2020). Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 18 (12), 890–897. doi:10.1016/S1875-5364(20)60032-2
Zeng, B. X., Yao, M. D., Wang, Y., Xiao, W. H., and Yuan, Y. J. (2020). Metabolic engineering of Saccharomyces cerevisiae for enhanced dihydroartemisinic acid production. Front. Bioeng. Biotechnol. 8, 152. doi:10.3389/fbioe.2020.00152
Zhang, G., Wang, H., Zhang, Z., Verstrepen, K. J., Wang, Q., and Dai, Z. (2022). Metabolic engineering of yarrowia lipolytica for terpenoids production: Advances and perspectives. Crit. Rev. Biotechnol. 42 (4), 618–633. doi:10.1080/07388551.2021.1947183
Keywords: taxadiene, catalysis compartmentalization, enzyme fusion, relocalization, Saccharomyces cerevisiae
Citation: Zhang C, Chen W, Dong T, Wang Y, Yao M, Xiao W and Li B (2023) Elimination of enzymes catalysis compartmentalization enhancing taxadiene production in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 11:1141272. doi: 10.3389/fbioe.2023.1141272
Received: 10 January 2023; Accepted: 06 February 2023;
Published: 20 February 2023.
Edited by:
Wei Luo, Jiangnan University, ChinaReviewed by:
Hui-Min Qin, Tianjin University of Science and Technology, ChinaCopyright © 2023 Zhang, Chen, Dong, Wang, Yao, Xiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingdong Yao, bWluZ2RvbmcueWFvQHRqdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.