- 1Department of Bioengineering, Imperial College London, London, United Kingdom
- 2Centre for Blast Injury Studies, Imperial College London, London, United Kingdom
- 3Department of Surgery and Cancer, Imperial College London, London, United Kingdom
Introduction: Due to loss in musculoskeletal capacity, there is an increased burden on the residual limbs of bilateral transfemoral and through-knee persons with limb loss. This reduced capacity is associated with an increased cost of walking that is detrimental to functionality. Compensatory gait strategies are adopted by this population. However, how these strategies relate to specific muscle recruitment is not known. The primary aim of this study is to characterize muscle recruitment during gait of this population. The secondary aim is to assess whether the measured kinematics can be actuated when the endurance of specific muscles is reduced and if this is the case, which alternative muscles facilitate this.
Methods: 3D gait data and high-resolution magnetic resonance images were acquired from six bilateral transfemoral and through-knee persons with limb loss. Subject-specific anatomical muscle models were developed for each participant, and a validated musculoskeletal model was used to quantify muscle forces in two conditions: during normal gait (baseline) and when muscles, which were identified as functioning above a “healthy” level at baseline, have a reduced magnitude of maximum force capacity (reduced endurance simulation). To test the hypothesis that there are differences in muscle forces between the baseline trials and the simulations with reduced muscular endurance, a Bonferroni corrected two-way ANOVA with repeated measures was completed between the two states.
Results: The baseline analysis showed that the hip flexors experience relatively high muscle activations during gait. The reduced endurance simulation found two scenarios. First, for 5 out of the 12 simulations, the baseline kinematics could not be reproduced with the reduced muscular capacity. Second, for 7 out of 12 cases where the baseline kinematics were achieved, this was possible with compensatory increased activation of some muscles with similar functions (p ≤ 0.003).
Discussion: Evidently, due to the loss of the ankle plantar flexors, gait imposes a high demand on the flexor muscle group of the residual limb. This study highlights how the elevated cost of gait in this population manifests in muscle recruitment. To enhance functionality, it is critical to consider the mechanical demand on the hip flexors and to develop rehabilitation interventions accordingly.
1 Introduction
The partial loss of a lower limb manifests as a loss in neuromusculoskeletal capacity. The central nervous system will adapt to this loss through the adoption of compensatory strategies which enable a person with limb loss (PWLL) to ambulate (Hoffman et al., 1997; Su et al., 2007; Visser et al., 2011; Visser et al., 2011; van der Kruk et al., 2021). High levels of functionality for lower-limb PWLLs are possible and have been observed in the ex-military population when comprehensive rehabilitation interventions were completed and state-of-the-art prosthetics used (Ladlow et al., 2015; Jarvis et al., 2017; Jarvis et al., 2021). However, gait remains more functionally demanding for bilateral transfemoral PWLLs compared to able-bodied persons (Hoffman et al., 1997), with lower scores found in 6 min walking tests (Linberg et al., 2013) and higher metabolic cost during gait (Jarvis et al., 2017) in the young ex-military population.
Compensatory gait strategies, emerging as a result of amputation, result in high muscle forces that lead to elevated hip joint contact forces (Su et al., 2007; Wentink et al., 2013). Increased muscle activations place a large physiological demand on the muscle of the residual limb, likely increasing energy expenditure and having a detrimental effect on endurance (Fang et al., 2007), rendering PWLLs less able to perform recreational activities and activities of daily life (Wong et al., 2015). However, how the compensatory gait strategies relate to specific muscle recruitment patterns is not known.
Muscular endurance is defined as the ability of a muscle to execute extended repetitions of muscle contractions (Kell et al., 2001). With repeated muscle contractions, as occurs during walking, there will be a reduction in muscle performance associated with a reduction in the muscle’s ability to produce force (Van Geel et al., 2021). Experiments on isolated muscle cells demonstrate that contractions near maximum capacity induce a reduction in the magnitude of the force of the contractions (Bishop, 2012). The reduction in maximum voluntary contraction (MVC) of intact muscles during tasks has been explained by metabolic accumulation and impaired motor control (Bishop, 2012). Muscular endurance of lower-limb muscle during gait has been quantified for healthy subjects by an observed reduction in the medium frequency of EMG signals (Fidalgo-Herrera et al., 2022). An experiment that implemented “fatigue protocols” on lower-limb muscles, consisting of a repeated sit-to-stand task, reported that muscle endurance affects spatiotemporal and kinematic features of gait in healthy subjects (Barbieri et al., 2013). These changes in gait characteristics increase the risk of falling (Mohd Safee and Abu Osman, 2021) and localized tissue injury (Kumar, 2001). Endurance is dependent on the intensity of muscle contractions (West et al., 1995). Overall, high-intensity muscle forces will limit a PWLL’s functionality as they will be detrimental to lower-limb muscular endurance during gait.
Musculoskeletal (MSK) modeling enables muscle and articular contact forces to be estimated. Inverse MSK modeling uses the observed kinematics and ground reaction forces to determine the net forces and moments at the joints and uses an optimization method to resolve the muscle force sharing. FreeBody is a segment-based MSK model (Cleather and Bull, 2015) that has been extensively validated for muscle activations using EMG experimental data for bilateral transfemoral amputees (Toderita et al., 2021), unilateral transtibial amputees (Ding et al., 2023), knee joint contact forces for osteoarthritis patients (Ding et al., 2016), and hip joint contact forces also using instrumented implants (Amiri and Bull, 2022).
Able-bodied walking is an optimized activity that minimizes muscle recruitment (Crowninshield and Brand, 1981). Therefore, this can provide a gold standard for the development of muscle coordination strategies for improved endurance. Muscle activation can be estimated using experimental EMG data and normalized to EMG at MVC. Healthy walking recruits lower-limb muscles generally to below or up to 50% of their MVC (Burden et al., 2003; Nishijima et al., 2010; Franz and Kram, 2013; Guan et al., 2019; Elsais et al., 2020), with many values in the literature being considerably lower than 50%. A range between 5%–64% has been reported for different muscles with an average value of ∼ 27%. The demand of gait on individuals has also been quantified by net torque actuated across joints as a percentage of maximal torque capacity. Two studies have quantified maximal torque produced in hip flexion and hip extension for self-selected walking. One study quantified this value to be below 30% in flexion and extension in young adults (n = 10) (Anderson and Madigan, 2014). The other found the percentage of maximum torque capacity produced during gait to be below 50% in the hip flexors and below 35% in the hip extensors for adults (n = 14) (Requiao et al., 2005). Both studies found that this metric of “functional capacity utilized” increased with increased cadence.
The first objective of this study was to quantify the muscle forces in bilateral TFTK PWLL gait. The second objective was to determine the impact of reduced endurance, simulated by reducing specific muscle force capacities, on this group’s ability to actuate their baseline kinematics and to identify potential compensatory muscle strategies. Both objectives aim to increase the understanding of muscle recruitment strategies in this population to inform future rehabilitation research.
2 Materials and methods
2.1 Participants
This study focused on the young ex-military population as a model of the highest potential functional outcome (Jarvis et al., 2021). They are young, have had comprehensive rehabilitation, and are provided with state-of-the-art prosthetics. As lower-limb anatomical and biomechanical factors are different between sexes, a mixed group should include equal numbers. This was not possible as this population has few female PWLLs. Therefore, this study looked at only male participants. A total of 6 male (aged 27–36 years) bilateral TFTK PWLLs due to trauma were included in this study. All subjects were fitted with their own state-of-the-art mechanical prosthetic feet and microprocessor prosthetic knees. Subject details are presented in Table 1. Ethical approval was received from the institutional ethics review board, and written informed consent was obtained from the participants.
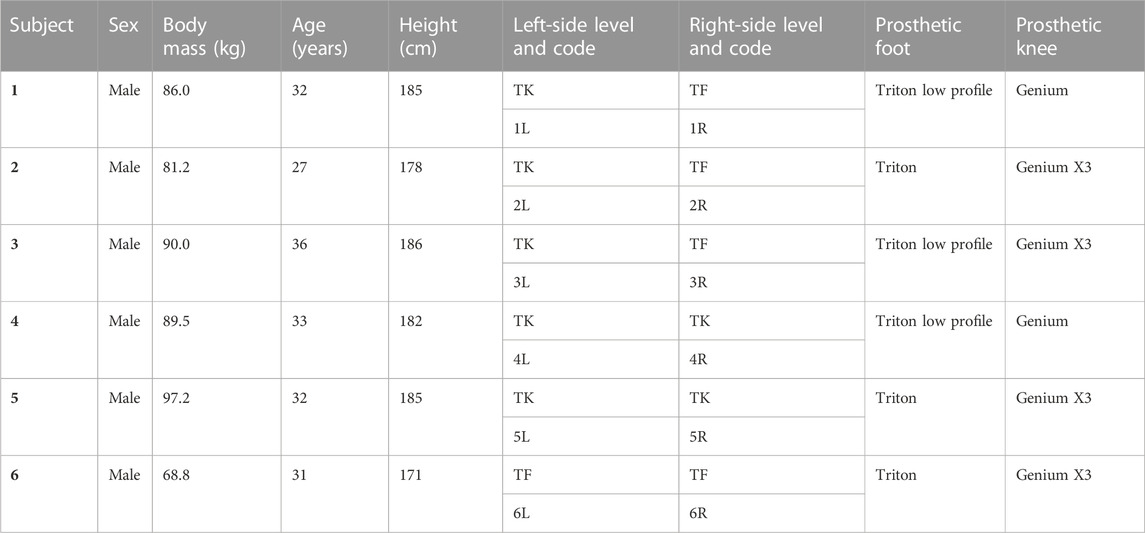
TABLE 1. Bilateral persons with limb loss participant details, TK = through-knee and TF = transfemoral.
2.2 Gait data collection
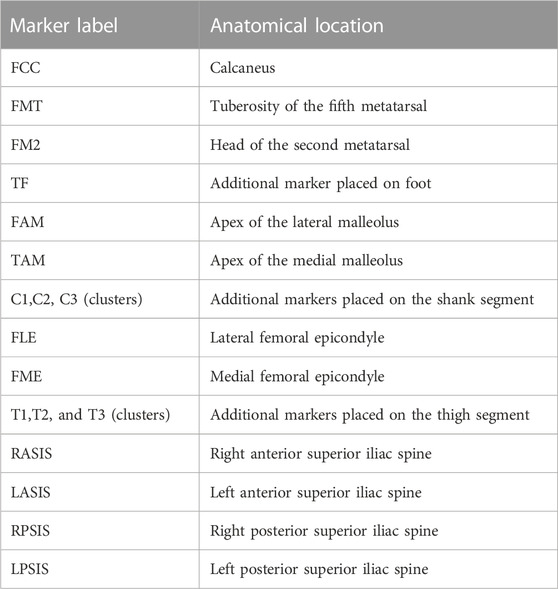
Three self-selected gait trials and one static calibration trial were collected per participant in a gait analysis laboratory consisting of a motion capture system with 10 cameras (VICON, Oxford Metrics Group, United Kingdom) and two force plates (Kistler Type 9286B, Kistler Instrumented AG, Winterthur, Switzerland). The marker 3D displacement and ground reaction forces were sampled at 120 and 960 Hz, respectively. Reflective markers were attached to 14 anatomical landmarks, as listed in Table 2, on both the left and right legs, as well as two clusters of markers on the thigh and calf of both limbs (Toderita et al., 2021).
2.3 Anatomical dataset
Anatomical datasets of all participants have previously been published (Toderita et al., 2021). In summary, these datasets were built from MRI scans acquired with a 3.0T MRI scanner (MAGNETOM Verio, Siemens, Germany) with a 3D T1-weighted spoiled gradient echo sequence set at a 450 × 450
2.4 Subject-specific MSK modeling
The FreeBody model (Cleather and Bull, 2015) was utilized to quantify the muscle forces during the gait trials. FreeBody consists of four segments: the foot, shank, thigh, and pelvis, which are connected through the ankle, hip, and knee joints. The residuum, socket, and liner are modeled as the thigh segment. The transfemoral PWLL MSK model contains 92 muscle elements, representing 21 muscles. The subject-specific geometry of muscle and bones was obtained from the MRI images, the details of which are outlined elsewhere (Toderita et al., 2021) and are described here briefly. For each leg, the MRI scans of the subject were used to render 3D shapes of bones and muscles. Subsequently, following the methodology of Horsman et al., 2007, the muscle origins, insertion and via points, hip joint rotation center, and a cylindrical wrapping surface (to represent iliopsoas muscle elements actions along superior pubic ramus of the pelvis) were digitized.
The physiological cross-sectional area (PSCA) of each muscle was calculated using the following equation:
where muscle volume (
The maximum force potential (
where
Quaternion algebra was used to perform inverse kinematic calculations, and inverse dynamics simulations were carried out using a wrench formulation to estimate the net forces and moments during the captured motion (Dumas et al., 2004). The model then ran a one-step constraint static optimization to estimate muscle and articular contact forces over the captured gait cycle. The load-sharing optimization minimized the sum of the cubed muscle activations, as shown in Eq 3, while satisfying the equations of motion of the MSK system and constraining the muscle forces to their maximum force potentials:
where J and
Two sets of simulations were performed: first, to identify overburdened muscles during gait (baseline) and then to quantify the consequences of the reduced muscular endurance of the overburdened muscles (reduced endurance).
2.4.1 Baseline PWLL gait simulations
Captured gait kinematics and kinetics, along with the subject-specific MSK geometry, were used to estimate muscle forces. Muscles whose mean activations (
2.4.2 Reduced muscular endurance PWLL gait simulations
Muscular endurance is defined by the ability of a muscle to maintain a level of muscle contraction over extended repetitions (Kell et al., 2001). A reduced muscular endurance can, therefore, be quantified by a reduced force production capacity during a task of repeated contractions (i.e., gait). A reduced muscular endurance was, therefore, simulated by re-running the optimizations with reduced maximum force potentials (
The reduced endurance simulations were carried out on one gait trial per subject; the trial selected was the trial with the highest maximum force of the ‘overburdened’ muscles from the baseline gait optimization. It was investigated whether the optimization was able to find a feasible solution (i.e., whether the reduced muscle capacity can reproduce the experimentally collected baseline kinematics). If a feasible solution could be found, it would likely be due to a compensatory muscle recruitment strategy (van der Kruk et al., 2021). It was hypothesized that muscles with a similar function would indicate a significantly greater muscle recruitment force in the reduced endurance condition compared to baseline.
Normal distribution of the maximum muscle activations (force/
3 Results
3.1 Example plot
The baseline muscle activations (F/
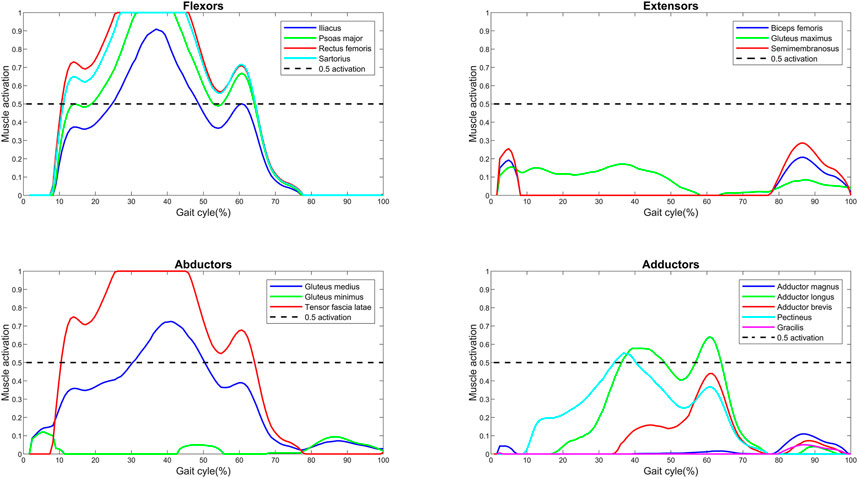
FIGURE 1. Muscle activations (force normalized to
3.2 Characterizing the muscle overloading
The maximum force generated by the muscles of the residual limb has been normalized to
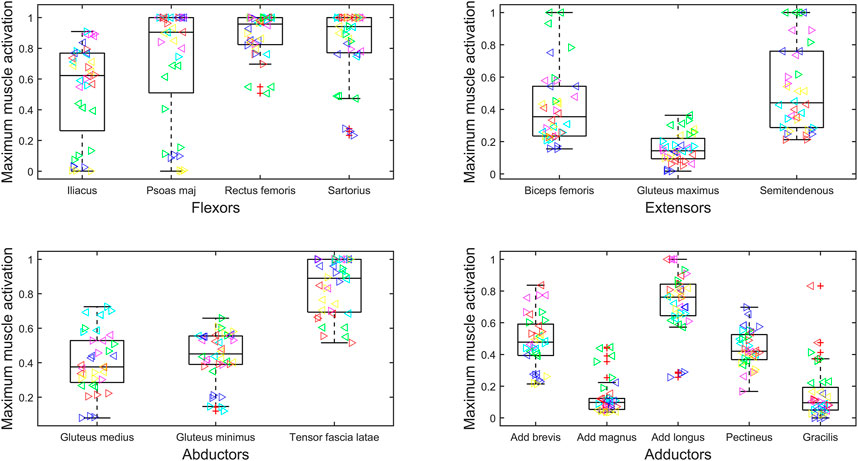
FIGURE 2. Maximum muscle activation (force normalized to
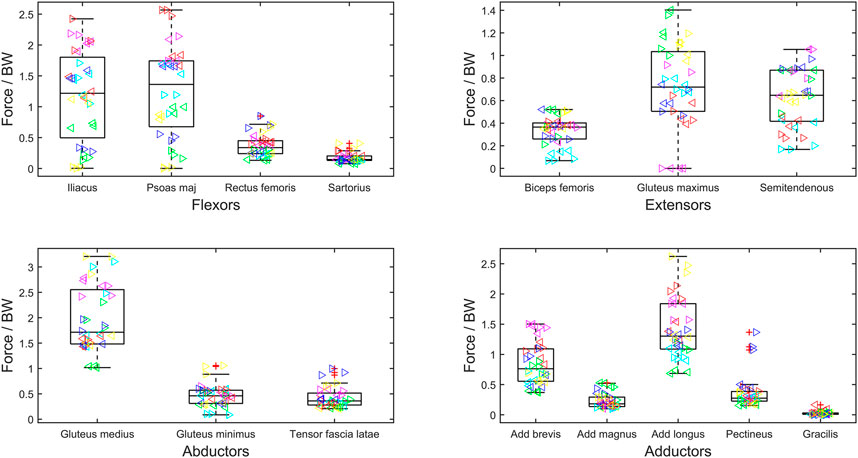
FIGURE 3. Maximum muscle force normalized to body weight (BW) during gait cycle of bilateral transfemoral persons with limb loss. Muscles are categorized as hip flexors, extensors, abductors, and adductors. The box plots show min, max, median, 25 and 75 percentiles, and the outliers. Each subject is plotted in a separate color, and the left legs are represented by ‘►’ and right by ‘◄’.
3.3 Reduced endurance simulation
Following the baseline characterization, the psoas major and the adductor longus were identified as muscles which would be particularly susceptible to experiencing reduced endurance (as they have high activations and high magnitudes); their
An example plot of the reduced endurance condition is presented in Figure 4. The leg plotted in this example is the left leg of subject 2 (2L). This example shows that the simulated reduced force capacity of the psoas major and the adductor longus increases the force contributions of the remaining muscles of the same primary function.
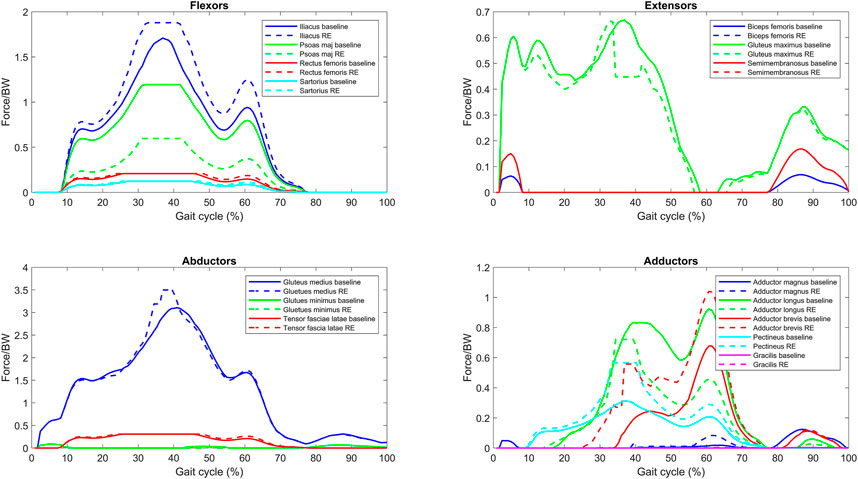
FIGURE 4. Normalized muscle force for one bilateral transfemoral person with limb loss for baseline and reduced endurance (RE) condition gait trial (reduce force capacity of the psoas major and adductor longus).
Figure 5 presents the overall effect of the reduced endurance condition on the maximum activations (force normalized to
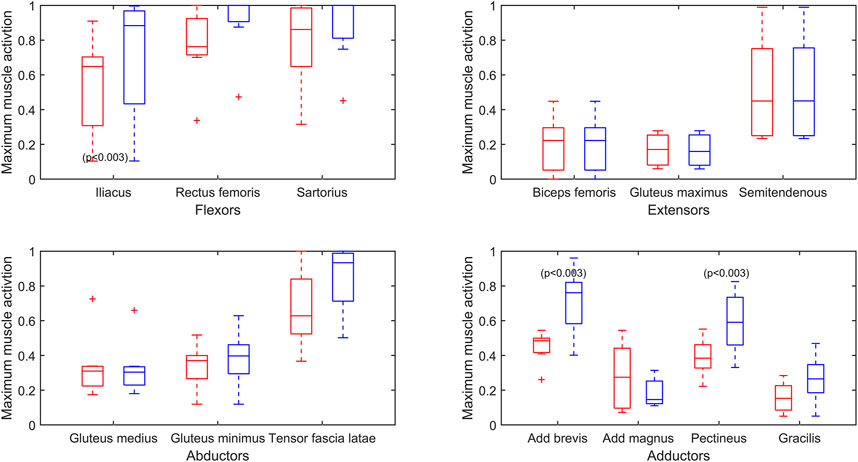
FIGURE 5. Maximum muscle activation (force/
4 Discussion
In agreement with the literature, this study has shown that bilateral TFTK PWLL gait results in highly burdened flexor muscles due to the loss of ankle propulsion (Mcnealy And Gard, 2008). Although not their primary function, the adductor longus and the tensor fascia latae provide some flexor action (Neumann, 2010), and these also have high activations in this study. This study has highlighted the high cost of gait on the residual MSK system of this young traumatic TFTK PWLL population, highlighting the importance of the preservation of all residual flexor action capacity in surgery and flexor functionality in rehabilitation, as well as its consideration with aging. This high cost is particularly significant for the psoas major and adductor longus, as their critical role in function is demonstrated in the reduced endurance simulation in this study, with 5 out of the 12 simulations not being able to find a feasible solution and preserve baseline kinematics with the reduced capacity of these two muscles. Their function should, therefore, be given emphasis in rehabilitation protocols.
Our estimations of muscle force found the average activation of the flexor muscles to be above 50% of their maximum force potential. Lower-limb PWLLs experience a loss in muscular capacity. Therefore, daily tasks are executed close to their functional capacity (van der Kruk et al., 2021). The effect of this specific loss in capacity on muscular endurance seems unexplored in published studies in the context of lower-limb PWLLs. However, this is comparable to the loss in muscular capacity of the elderly population, which has been much more comprehensively studied. In studies of the elderly population, muscle activation (EMG/MVC) has been taken as a measure of work relative to capacity. It is concluded that the elderly population has greater difficulty with performing daily activities due to experiencing early-onset fatigue because the activities require performance that is closer to their functional capacity than the younger population (Hortobágyi et al., 2003; Hahn et al., 2005; Hortobágyi et al., 2011). For example, through an EMG experimental set up, one study found that level walking required 80.2% MVC in the elderly group compared to 37.5% MVC in the younger group when quantifying the sum of muscle activation for the vastus lateralis, biceps femoris, tibialis anterior, and the lateral gastrocnemius (Hortobágyi et al., 2011). Another study by the same author highlights how this difference in muscle activity with respect to muscle capacity between the old and the young is true for other activities of daily life for example. Similar differences were found in ascending (62% and 28% MVC) and descending (79% and 33% MVC) stairs between the elderly and younger participants, respectively, when just studying the vastus lateralis (Hortobágyi et al., 2003). We have used a similar method of analyzing the comparative relative demands of walking, and these studies consolidate the need for a rehabilitation method to relieve the flexor muscles with respect to their capacity, to reduce the risk of detriment to muscular endurance and reflect how our findings may be applicable to other activities of daily life.
High inter-subject variability within muscle forces and high inter-subject variance in kinematics and, therefore, resultant loading has been reported previously in the bilateral population (Wright et al., 2008; Wentink et al., 2013). In our study, this is particularly evident when looking at the maximum muscle activations of the psoas major and iliacus (Figure 2). For some participants, the muscles appear to become saturated, and in other participants, they are only minimally recruited. This variation suggests the need for patient-specific prescription of interventions and highlights potential underlying differences in gait biomechanics.
Our baseline gait simulations found that the psoas major and the adductor longus function at comparatively high activations and magnitude. High-intensity muscle contractions will induce a reduced muscular performance (Kumar, 2001; Westerblad and Allen, 2002), so when selecting muscles that may experience reduced endurance, we chose the muscles with both high activations and high absolute forces as these will have a larger contribution to the moments of gait. Muscles that function at high activation but low relative absolute magnitude may experience a decline in performance, but this will be less detrimental to gait. Our simulations of reduced endurance of the psoas major and adductor longus demonstrated two scenarios. The first, which was true for half the subjects, is that the original kinematics were not possible with the reduced contribution of psoas major and adductor longus. This corroborates our findings that these muscles are essential for maintaining gait of bilateral TFTK PWLLs, and in these cases, a kinematic adaptation is required. The second is where a full solution could be found despite the simulated reduced capacity of the adductor longus and psoas major. This occurred in 7 out of 12 simulations, highlighting redundancy in the MSK system and therefore robustness to a reduced endurance of the overburdened muscles, as shown previously (Komura et al., 2004). This was achieved by compensating for the impaired muscles by the recruitment of muscles with similar functions. Muscle recruitment in the reduced endurance state requires high forces of the compensating muscles, which in turn increases the acute functional demand on these muscles. This study included both transfemoral PWLLs and through-knee PWLLs. It is interesting to observe that the distribution of TF and TK between the legs which could find a solution in the reduced endurance state (TF = 3, TF = 4) and the legs which could not find a solution (TF = 3, TK = 2) was fairly even. This is unexpected, as the TF legs will have a lower residual muscular capacity, suggesting that the properties of the more proximal flexor muscles (such as the iliacus and psoas major) are responsible for this distinction between the legs which could maintain kinematics in the reduced endurance state and the legs which could not.
There are limitations to this study, notably that the inverse dynamics model is limited to the recorded kinematics. This means that any kinematic changes that may occur due to the simulated reduction in force capacity in the reduced endurance condition were not included. A forward dynamics model could predict the changes in kinematics due to the potentially reduced endurance of certain muscles. A forward dynamics model could also predict optimal kinematics for reduced muscle activation and improved load sharing. Predicted optimal kinematics could be employed as a quantified aim in the design of a novel rehabilitation intervention. Forward dynamics could also be used to identify the cause of the variability in muscle activations observed in this study by modeling differences in anatomy (due to surgery, training, or natural variation) and biomechanics. Other limitations include the inherent assumptions and estimates of the MSK model. For example, although the muscle volumes were subject-specific, the fiber length to muscle length ratio, pennation angle, and sarcomere length were obtained from in vivo cadaveric studies (Toderita et al., 2021) as it is not possible to acquire these values from MRI imaging. Muscle force capacities are sensitive to such properties.
This study has developed a new approach to analyze muscular endurance using inverse dynamics modeling. It has highlighted the critical muscles that are likely to have reduced endurance and so limit PWLLs’ ability to walk. Research on clinical interventions to address this reduced endurance may focus on gait retraining, such as changing the kinematics or modifying the balance of muscle forces required to achieve a proscribed gait pattern.
Data availability statement
All data are available upon reasonable request, subject to the ethical approval and participant confidentiality. Requests to access these datasets should be directed to YS5iZW50b24yMEBpbXBlcmlhbC5hYy51aw==.
Ethics statement
The studies involving human participants were reviewed and approved by the Imperial College Research Ethics Committee and NHS REC. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AMB, PA, AMJB, and AHM conceived and designed the study and interpreted the results. AMB performed the modeling, data processing, and analysis. DPH and BS completed the data collection. AMB wrote the first draft of the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
Funding
This work was supported by the UK Engineering and Physical Sciences Research Council (EPSRC) Grant EP/S02249X/1 for the Center for Doctoral Training in Prosthetics and Orthotics and The Royal British Legion Center for Blast Injury Studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amann, M., and Dempsey, J. A. (2008). Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J. physiology 586, 161–173. doi:10.1113/jphysiol.2007.141838
Amiri, P., and Bull, A. M. (2022). Prediction of in vivo hip contact forces during common activities of daily living using a segment-based musculoskeletal model. Front. Bioeng. Biotechnol. 10, 995279. doi:10.3389/fbioe.2022.995279
Anderson, D. E., and Madigan, M. L. (2014). Healthy older adults have insufficient hip range of motion and plantar flexor strength to walk like healthy young adults. J. biomechanics 47, 1104–1109. doi:10.1016/j.jbiomech.2013.12.024
Anderson, F. C., and Pandy, M. G. (2001). Static and dynamic optimization solutions for gait are practically equivalent. J. Biomech. 34, 153–161.
Barbieri, F. A., Dos Santos, P. C. R., Vitório, R., Van Dieën, J. H., and Gobbi, L. T. B. (2013). Effect of muscle fatigue and physical activity level in motor control of the gait of young adults. Gait posture 38, 702–707. doi:10.1016/j.gaitpost.2013.03.006
Bishop, D. J. (2012). Fatigue during intermittent-sprint exercise. Clin. Exp. Pharmacol. Physiology 39, 836–841. doi:10.1111/j.1440-1681.2012.05735.x
Burden, A., Trew, M., and Baltzopoulos, V. (2003). Normalisation of gait EMGs: A re-examination. J. Electromyogr. Kinesiol. 13, 519–532. doi:10.1016/s1050-6411(03)00082-8
Cleather, D. J., and Bull, A. M. (2015). The development of a segment-based musculoskeletal model of the lower limb: Introducing FreeBody. R. Soc. open Sci. 2, 140449. doi:10.1098/rsos.140449
Crowninshield, R. D., and Brand, R. A. (1981). A physiologically based criterion of muscle force prediction in locomotion. J. biomechanics 14, 793–801. doi:10.1016/0021-9290(81)90035-x
de Groote, F., Kinney, A. L., Rao, A. V., and Fregly, B. J. (2016). Evaluation of direct collocation optimal control problem formulations for solving the muscle redundancy problem. Ann. Biomed. Eng. 44, 2922–2936. doi:10.1007/s10439-016-1591-9
Ding, Z., Henson, D., Sivapuratharasu, B., Mcgregor, A., and Bull, A. (2023). The effect of muscle atrophy in people with unilateral transtibial amputation for three activities: Gait alone does not tell the whole story. J. Biomechanics 149, 111484. doi:10.1016/j.jbiomech.2023.111484
Ding, Z., Nolte, D., Kit Tsang, C., Cleather, D. J., Kedgley, A. E., and Bull, A. M. (2016). In vivo knee contact force prediction using patient-specific musculoskeletal geometry in a segment-based computational model. J. Biomechanical Eng. 138, 021018. doi:10.1115/1.4032412
Dumas, R., Aissaoui, R., and de Guise, J. A. (2004). A 3D generic inverse dynamic method using wrench notation and quaternion algebra. Comput. methods biomechanics Biomed. Eng. 7, 159–166. doi:10.1080/10255840410001727805
Elsais, W. M., Preece, S. J., Jones, R. K., and Herrington, L. (2020). Between-day repeatability of lower limb EMG measurement during running and walking. J. Electromyogr. Kinesiol. 55, 102473. doi:10.1016/j.jelekin.2020.102473
Fang, L., Jia, X., and Wang, R. (2007). Modeling and simulation of muscle forces of trans-tibial amputee to study effect of prosthetic alignment. Clin. Biomech. 22, 1125–1131. doi:10.1016/j.clinbiomech.2007.07.017
Fidalgo-Herrera, A., Miangolarra-Page, J., and Carratalá-Tejada, M. (2022). Traces of muscular fatigue in the rectus femoris identified with surface electromyography and wavelets on normal gait. Physiother. Theory Pract. 38, 211–225. doi:10.1080/09593985.2020.1725945
Fraisse, N., Martinet, N., Kpadonou, T., Paysant, J., Blum, A., and André, J. (2008). Les muscles de l’amputé tibial Muscles of the below-knee amputees. Ann. de réadaptation de médecine physique 50, 218–227. doi:10.1016/j.annrmp.2008.01.012
Franz, J. R., and Kram, R. (2013). How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait posture 37, 378–384. doi:10.1016/j.gaitpost.2012.08.004
Guan, X., Kuai, S., Song, L., Liu, W., Liu, Y., Ji, L., et al. (2019). Effects of ankle joint motion on pelvis-hip biomechanics and muscle activity patterns of healthy individuals in knee immobilization gait. J. Healthc. Eng. 2019, 1–10. doi:10.1155/2019/3812407
Hahn, M. E., Lee, H.-J., and Chou, L.-S. (2005). Increased muscular challenge in older adults during obstructed gait. Gait posture 22, 356–361. doi:10.1016/j.gaitpost.2004.11.012
Henson, D. P., Edgar, C., Ding, Z., Sivapuratharasu, B., le Feuvre, P., Finnegan, M. E., et al. (2021). Understanding lower limb muscle volume adaptations to amputation. J. Biomechanics 125, 110599. doi:10.1016/j.jbiomech.2021.110599
Hoffman, M. D., Sheldahl, L. M., Buley, K. J., and Sandford, P. R. (1997). Physiological comparison of walking among bilateral above-knee amputee and able-bodied subjects, and a model to account for the differences in metabolic cost. Archives Phys. Med. rehabilitation 78, 385–392. doi:10.1016/s0003-9993(97)90230-6
Horsman, M. K., Koopman, H. F., Van der Helm, F. C., Prosé, L. P., and Veeger, H. (2007). Morphological muscle and joint parameters for musculoskeletal modelling of the lower extremity. Clin. Biomech. 22, 239–247. doi:10.1016/j.clinbiomech.2006.10.003
Hortobágyi, T., Finch, A., Solnik, S., Rider, P., and Devita, P. (2011). Association between muscle activation and metabolic cost of walking in young and old adults. Journals Gerontology Ser. A Biomed. Sci. Med. Sci. 66, 541–547. doi:10.1093/gerona/glr008
Hortobágyi, T., Mizelle, C., Beam, S., and Devita, P. (2003). Old adults perform activities of daily living near their maximal capabilities. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 58, M453–M460. doi:10.1093/gerona/58.5.m453
Jarvis, H. L., Bennett, A. N., Twiste, M., Phillip, R. D., Etherington, J., and Baker, R. (2017). Temporal spatial and metabolic measures of walking in highly functional individuals with lower limb amputations. Archives Phys. Med. rehabilitation 98, 1389–1399. doi:10.1016/j.apmr.2016.09.134
Jarvis, H. L., Reeves, N. D., Twiste, M., Phillip, R. D., Etherington, J., and Bennett, A. N. (2021). Can high-functioning amputees with state-of-the-art prosthetics walk normally? A kinematic and dynamic study of 40 individuals. Ann. Phys. Rehabilitation Med. 64, 101395. doi:10.1016/j.rehab.2020.04.007
Kell, R. T., Bell, G., and Quinney, A. (2001). Musculoskeletal fitness, health outcomes and quality of life. Sports Med. 31, 863–873. doi:10.2165/00007256-200131120-00003
Komura, T., Prokopow, P., and Nagano, A. (2004). Evaluation of the influence of muscle deactivation on other muscles and joints during gait motion. J. biomechanics 37, 425–436. doi:10.1016/j.jbiomech.2003.09.022
Kumar, S. (2001). Theories of musculoskeletal injury causation. Ergonomics 44, 17–47. doi:10.1080/00140130120716
Ladlow, P., Phillip, R., Etherington, J., Coppack, R., Bilzon, J., Mcguigan, M. P., et al. (2015). Functional and mental health status of United Kingdom military amputees Postrehabilitation. Archives Phys. Med. rehabilitation 96, 2048–2054. doi:10.1016/j.apmr.2015.07.016
Linberg, A. A., Roach, K. E., Campbell, S. M., Stoneman, P. D., Gaunaurd, I. A., Raya, M. A., et al. (2013). Comparison of 6-minute walk test performance between male Active Duty soldiers and servicemembers with and without traumatic lowerlimb loss. J. Rehabilitation Res. Dev. 50, 931–940. doi:10.1682/jrrd.2012.05.0098
Mcnealy, L. L., and Gard, A. S. (2008). Effect of prosthetic ankle units on the gait of persons with bilateral trans-femoral amputations. Prosthetics Orthot. Int. 32, 111–126. doi:10.1080/02699200701847244
Mohd Safee, M. K., and Abu Osman, N. A. (2021). Effect of lower limb muscle fatigue on fall risk for transfemoral amputee: A pilot study. Occup. Ther. Int. 2021, 1–6. doi:10.1155/2021/4357473
Neumann, D. A. (2010). Kinesiology of the hip: A focus on muscular actions. J. Orthop. Sports Phys. Ther. 40, 82–94. doi:10.2519/jospt.2010.3025
Nishijima, Y., Kato, T., Yoshizawa, M., Miyashita, M., and Iida, H. (2010). Application of the segment weight dynamic movement method to the normalization of gait EMG amplitude. J. Electromyogr. Kinesiol. 20, 550–557. doi:10.1016/j.jelekin.2009.07.006
Putz, C., Block, J., Gantz, S., Heitzmann, D., Dreher, T., Lehner, B., et al. (2017). Structural changes in the thigh muscles following trans-femoral amputation. Eur. J. Orthop. Surg. Traumatology 27, 829–835. doi:10.1007/s00590-017-1929-5
Requiao, L., Nadeau, S., Milot, M., Gravel, D., Bourbonnais, D., and Gagnon, D. (2005). Quantification of level of effort at the plantarflexors and hip extensors and flexor muscles in healthy subjects walking at different cadences. J. Electromyogr. Kinesiol. 15, 393–405. doi:10.1016/j.jelekin.2004.12.004
Su, P.-F., Gard, S. A., Lipschutz, R. D., and Kuiken, T. A. (2007). Gait characteristics of persons with bilateral transtibial amputations. J. rehabilitation Res. Dev. 44 (4), 491–501. doi:10.1682/jrrd.2006.10.0135
Toderita, D., Henson, D., Klemt, C., Ding, Z., and Bull, A. M. (2021). An anatomical atlas-based scaling study for quantifying muscle and hip joint contact forces in above and through-knee amputees using validated musculoskeletal modelling. IEEE Trans. Biomed. Eng. 68, 3447–3456. doi:10.1109/tbme.2021.3075041
Van der Kruk, E., Silverman, A. K., Koizia, L., Reilly, P., Fertleman, M., and Bull, A. M. (2021). Age-related compensation: Neuromusculoskeletal capacity, reserve & movement objectives. J. Biomechanics 122, 110385. doi:10.1016/j.jbiomech.2021.110385
Van Geel, F., Hvid, L. G., Van Noten, P., Eijnde, B. O., Dalgas, U., and Feys, P. (2021). Is maximal muscle strength and fatigability of three lower limb muscle groups associated with walking capacity and fatigability in multiple sclerosis? An exploratory study. Multiple Scler. Relat. Disord. 50, 102841. doi:10.1016/j.msard.2021.102841
Visser, J., Mccarthy, I., Marks, L., and Davis, R. (2011). Is hip muscle strength the key to walking as a bilateral amputee, whatever the level of the amputations? Prosthetics Orthot. Int. 35, 451–458. doi:10.1177/0309364611422268
Wentink, E. C., Prinsen, E. C., Rietman, J. S., and Veltink, P. H. (2013). Comparison of muscle activity patterns of transfemoral amputees and control subjects during walking. J. neuroengineering rehabilitation 10, 87–11. doi:10.1186/1743-0003-10-87
West, W., Hicks, A., Clements, L., and Dowling, J. (1995). The relationship between voluntary electromyogram, endurance time and intensity of effort in isometric handgrip exercise. Eur. J. Appl. physiology Occup. physiology 71, 301–305. doi:10.1007/bf00240408
Westerblad, H., and Allen, D. G. (2002). Recent advances in the understanding of skeletal muscle fatigue. Curr. Opin. rheumatology 14, 648–652. doi:10.1097/00002281-200211000-00003
Wong, D. W.-C., Lam, W. K., Yeung, L., and Lee, W. C. (2015). Does long-distance walking improve or deteriorate walking stability of transtibial amputees? Clin. Biomech. 30, 867–873. doi:10.1016/j.clinbiomech.2015.05.015
Wright, D., Marks, L., and Payne, R. (2008). A comparative study of the physiological costs of walking in ten bilateral amputees. Prosthetics Orthot. Int. 32, 57–67. doi:10.1080/03093640701669108
Keywords: transfemoral bilateral limb loss, muscle endurance, muscoskeletal modelling, gait, biomechanics
Citation: Benton AM, Amiri P, Henson DP, Sivapuratharasu B, Mcgregor AH and Bull AMJ (2023) Characterization of muscle recruitment during gait of bilateral transfemoral and through-knee persons with limb loss. Front. Bioeng. Biotechnol. 11:1128528. doi: 10.3389/fbioe.2023.1128528
Received: 20 December 2022; Accepted: 17 March 2023;
Published: 04 April 2023.
Edited by:
Yih-Kuen Jan, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Mohammad S. Shourijeh, Rice University, United StatesSiobhan Strike, University of Roehampton London, United Kingdom
Copyright © 2023 Benton, Amiri, Henson, Sivapuratharasu, Mcgregor and Bull. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alice M. Benton, YS5iZW50b24yMEBpbXBlcmlhbC5hYy51aw==
†Present Address: Pouya Amiri, School of Kinesiology and Health Studies, Queen’s University, Kingston, Canada
 Alice M. Benton
Alice M. Benton Pouya Amiri
Pouya Amiri David P. Henson
David P. Henson Biranavan Sivapuratharasu
Biranavan Sivapuratharasu Alison H. Mcgregor
Alison H. Mcgregor Anthony M. J. Bull
Anthony M. J. Bull