- 1School of Ophthalmology and Optometry and Eye Hospital, Wenzhou Medical University, Wenzhou, Zhejiang, China
- 2Eye Institute and Department of Ophthalmology, Eye & ENT Hospital, Fudan University, Key Laboratory of Myopia, Chinese Academy of Medical Sciences, Shanghai, China
- 3Department of Ophthalmology, Singleton Hospital, Swansea Bay University Health Board, Swansea, United Kingdom
- 4Department of Ophthalmology, Royal Gwent Hospital, Aneurin Bevan University Health Board, Newport, United Kingdom
- 5Department Ophthalmology, Yiwu Central Hospital, Yiwu, Zhejiang, China
- 6Shanghai Research Center of Ophthalmology and Optometry, Shanghai, China
Purpose: To investigate the relationship between corneal biomechanical and ocular biometric parameters, and to explore biomechanical asymmetry between anisometropic eyes using the corneal visualization Scheimpflug technology device (Corvis ST).
Methods: 180 anisometropic participants were included. Participants were divided into low (1.00≤△Spherical equivalent (SE) < 2.00D), moderate (2.00D≤△SE < 3.00D) and high (△SE ≥ 3.00D) anisometropic groups. Axial length (AL), keratometry, anterior chamber depth (ACD) and corneal biomechanical parameters were assessed using the OA-2000 biometer, Pentacam HR and Corvis ST, respectively.
Results: The mean age of participants was 16.09 ± 5.64 years. Stress-Strain Index (SSI) was positively correlated with SE (r = 0.501, p < 0.001) and negatively correlated with AL (r = -0.436, p < 0.001). Some other Corvis ST parameters had weak correlation with SE or AL. Corneal biomechanical parameters except for time of first applanation (A1T), length of second applanation (A2L), deformation amplitude (DA), first applanation stiffness parameter (SPA1) and ambrosia relational thickness-horizontal (ARTh) were correlated with ametropic parameters (SE or AL) in multiple regression analyses. A1T, velocity of first applanation (A1V), time of second applanation (A2T), A2L, velocity of second applanation (A2V), corneal curvature radius at highest concavity (HCR), peak distance (PD), DA, deformation amplitude ratio max (2 mm) (DAR), SPA1, integrated radius (IR), and SSI showed significant differences between fellow eyes (p < 0.05). There was no significant difference in asymmetry of corneal biomechanics among the three groups (p > 0.05). Asymmetry of some biomechanical parameters had weak correlation with asymmetry of mean corneal curvatures and ACD. However, asymmetry of corneal biomechanical parameters was not correlated with asymmetry of SE or AL (p > 0.05).
Conclusion: More myopic eyes had weaker biomechanical properties than the contralateral eye in anisometropia. However, a certain linear relationship between anisometropia and biomechanical asymmetry was not found.
Introduction
Anisometropia is a significant difference of 1.00D or more in refractive error between the eyes (Vincent et al., 2014). The prevalence of anisometropia was 5.3% in a Chinese elementary schoolchildren population and the prevalence and severity of anisometropia increased with refractive error (Lee C. et al., 2017). Anisometropia can affect binocular function and stereopsis (Birch et al., 2019; Atchison et al., 2020; Singh et al., 2021). In particular, anisometropia in early childhood can also lead to amblyopia (Smith et al., 2017). However, the mechanism of anisometropia has been not clear. Mechanical factors may play important roles in anisometropia (Vincent et al., 2014). The cornea, a crucial part of the ocular optical system, has complex biomechanical properties such as anisotropy, nonlinearity, and viscoelasticity (Esporcatte et al., 2020; Baptista et al., 2021; Chong and Dupps, 2021).
Corneal biomechanics is one of the factors that potentially affects corneal response to orthokeratology (González-Méijome et al., 2008; Wu et al., 2021). The viscoelasticity of the cornea was not only associated with the response but also the recovery of orthokeratology (González-Méijome et al., 2008). What’s more, corneal refractive surgery would change corneal biomechanics and even might lead to corneal ectasia (MA et al., 2016; H et al., 2019) and the corneal biomechanical parameters can predict postoperative refractive error (Wang et al., 2018). Thus, a better understanding of corneal biomechanics in anisometropia will be useful in clinical practice.
At present, there have been lots of studies focused on corneal biomechanical properties for different refractive states of eyes from different individuals (Lee et al., 2016; Long et al., 2019; Sedaghat et al., 2020; Tubtimthong et al., 2020). Multiple confounding factors are implicated in corneal biomechanical measurements in vivo. Researchers tried to minimize confounding factors such as age, gender, intraocular pressure (IOP), and central corneal thickness (CCT) and found corneal biomechanical properties are impaired with increasing myopia (Sedaghat et al., 2020; Yu et al., 2020; Ma et al., 2021). However, several factors such as hormonal levels, environmental conditions and corneal hydration status are still hard to eliminate among individuals (Kling and Hafezi, 2017). Anisometropia has fellow eyes with different refractive conditions from the same individual, which are ideal objects to carry out control studies minimizing confounding factors.
Previous studies using the ocular response analyzer (ORA) found that more myopic eyes had lower corneal hysteresis (CH) compared with the fellow eyes in high anisometropia, indicating more myopic eyes had weaker corneal biomechanical properties (Xu et al., 2010). ORA, the first clinical device to measure corneal biomechanics has some limitations, for example, the real meaning of ORA parameters is not clear and CH does not have a direct relationship with stiffness parameters (such as Young’s modulus) (Baptista et al., 2021). The corneal visualization Scheimpflug technology device (Corvis ST), using dynamic Scheimpflug imaging technology can display the dynamic process of corneal deformation under external force in real-time (Esporcatte et al., 2020), maybe a better choice to evaluate corneal biomechanics in vivo (Pinero and Alcon, 2015; Jędzierowska and Koprowski, 2019). Its continuously updated parameters help us to assess the corneal biomechanical properties more comprehensively. To the best of our knowledge, there is a lack of studies comparing the corneal biomechanical properties of anisometropia eyes using the Corvis ST.
The purpose of the current study was to investigate the relationship between corneal biomechanical parameters and ocular biometrics in anisometropia with the Corvis ST, and to explore biomechanical asymmetry between anisometropic fellow eyes.
Materials and methods
Study population
The study was conducted under the Declaration of Helsinki and approved by the Ethics Committee of the Eye Hospital at Wenzhou Medical University (2021–012-K-09). This was a cross-sectional study. A total of 180 anisometropic patients in the Department of optometry or refractive surgery center of Eye Hospital of Wenzhou Medical University from January 2021 to January 2022 participated in this study. All patients signed and informed consent form.
In our study, anisometropic patients over 10 years old were included. Subjects with any of the following conditions will be excluded: 1) History of corneal surgery (such as refractive surgery, pterygium excision, etc.), 2) History of intraocular surgery, 3) History of ocular trauma, 4) Suffering from corneal or eye diseases (such as keratoconus, corneal ulcer, corneal ectasia, glaucoma, etc.), 5) Suffering from systemic diseases that affect corneal biomechanics (such as diabetes, connective tissue disease, etc.), 6) Pregnant or in menstruation, 7) Recently use of drugs that affect corneal biomechanics (such as prostaglandins), 8) Recently wearing of contact lenses (soft contact lens within 1 week; RGP lens within 1 month; orthokeratology within 3 months), 9) Poor fixation, (10) Accommodative dysfunction. According to the degree of anisometropia, subjects were divided into three subgroups: 1) low anisometropia group: 1.00≤△Spherical equivalent (SE) < 2.00D, 2) moderate anisometropia group: 2.00D≤△SE < 3.00D, 3) high anisometropia group: △SE ≥ 3.00D (asymmetry of parameters were calculated by relative hyperopic value minus relative myopic value and “△” represented binocular asymmetry).
Methods
Subjects underwent routine eye examination, including subjective refraction, best-corrected visual acuity, slit-lamp and fundus examination. Axial lengths (AL) were measured with OA-2000 biometer (Tomey, Nagoya, Japan). Anterior segment parameters including corneal curvatures and anterior chamber depth (ACD) were measured with the Pentacam HR (Oculus, Wetzlar, Germany). Mean corneal curvatures (Km) were used for analysis. Corneal biomechanical parameters were obtained from the Corvis ST (Oculus, Wetzlar, Germany, 1.6r2187). To avoid corneal deformation affecting the measurement accuracy, the Corvis ST measurement was performed last in each patient. Both eyes of all participants were measured three times using the Corvis ST respectively, with the interval between the adjacent measurements longer than 1 min to restore the cornea to normal. The first eye to be measured was determined randomly. Only measurements with an “OK” quality were used for analysis. If the quality did not achieve an “OK” assessment, the examination was repeated. If the quality of multiple attempts did not achieve an “OK” assessment, the subject was excluded. All examinations were performed by an experienced ophthalmologist.
Corvis ST and SSI
The Corvis ST consists of a non-contact intraocular pressure measuring instrument and an ultra-high-speed Scheimpflug camera, which can display the dynamic process of corneal deformation under an external force in real-time. After every measurement, a series of corneal biomechanical parameters are generated. The dynamic corneal response parameters (DCR) consist of first applanation (A1) parameters, second applanation (A2) parameters, highest concavity (HC) parameters, Vinciguerra screening parameters. Supplementary Table S1 provides more detail about each parameter.
Stress-Strain Index (SSI) is a new Corvis ST parameter, based on finite element models. SSI is estimated according to numerical modeling with SP-HC, bIOP, and central corneal thickness (CCT) (Eliasy et al., 2019). Different from other parameters, SSI is a corneal stiffness index that can estimate corneal stiffness independent of bIOP and CCT (Eliasy et al., 2019; Liu et al., 2020).
Statistical analysis
Descriptive data were analyzed using SPSS software (IBM SPSS Statistics for Windows, Version 25.0.0, IBM, Armonk, New York, USA). First, one eye of participants was selected randomly to test the correlation between the corneal biomechanical parameters and ocular biometric parameters using Pearson correlation coefficients. Multiple linear regression with the stepwise method was applied to assess the relationship between corneal biomechanical parameters with SE, AL, ACD, Km, biomechanically corrected intraocular pressure (bIOP), CCT, age and gender. Second, a two-tailed paired t-test was used to compare corneal biomechanical properties of the binocular eyes. An analysis of variance (ANOVA) was used to compare corneal biomechanical asymmetry among different anisometropia groups. Pearson correlation coefficients were used to explore correlations between the degree of anisometropia and the asymmetry of corneal biomechanics. A p-value less than 0.05 was considered statistically significant.
Results
180 anisometropic participants were included, 95 (52.8%) males. The mean (± standard deviation) age was 16.09 ± 5.64 years (range: 10–38 years).
Correlation between corneal biomechanical parameters and ocular biometric parameters
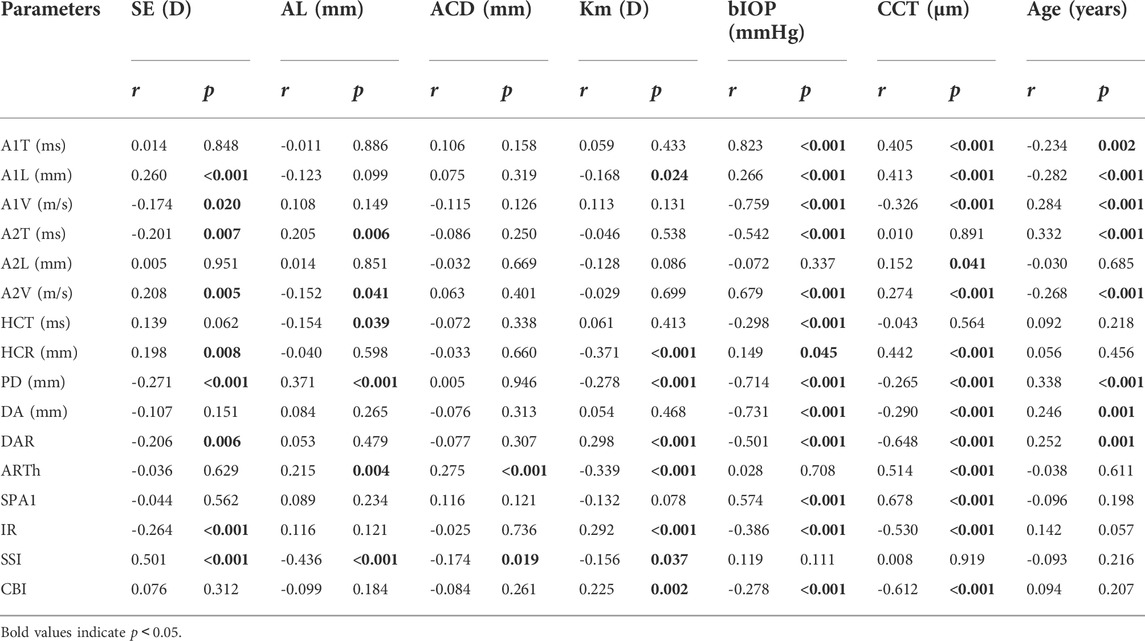
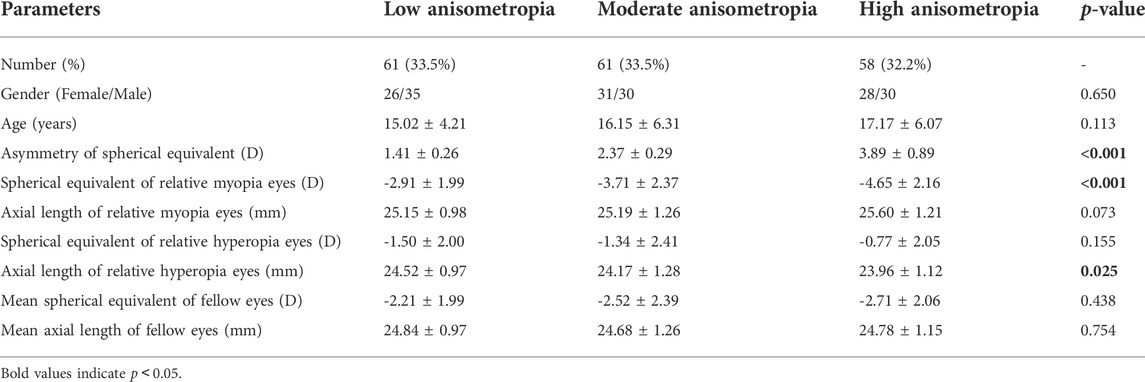
The mean SE, AL, ACD and Km were -2.44 ± 2.72D (range: 12.88D∼6.75D), 24.75 ± 1.34 mm (range: 21.21–29.27 mm), 3.29 ± 0.30 mm (range: 2.47–4.25 mm), and 42.97 ± 1.38D (range: 39.70D∼47.38D). SE had significant correlations with A1L (r = 0.260, p < 0.001), A1V (r = -0.174, p = 0.020), A2T (r = -0.201, p = 0.007), A2V (r = 0.208, p = 0.005), HCR (r = 0.198, p = 0.008), PD (r = -0.271, p < 0.001), DAR (r = -0.206, p = 0.006), IR (r = -0.264, p < 0.001) and SSI (r = 0.501, p < 0.001) (Figures 1A–I). AL was significantly correlated with A2T (r = 0.205, p = 0.006), A2V (r = -0.152, p = 0.041), HCT (r = -0.154 p = 0.039), PD (r = 0.371, p < 0.001), ARTh (r = 0.215, p = 0.004) and SSI (r = -0.436, p < 0.001) (Figures 2A–F). The correlations between corneal biomechanical parameters and other ocular parameters are displayed in Table 1.
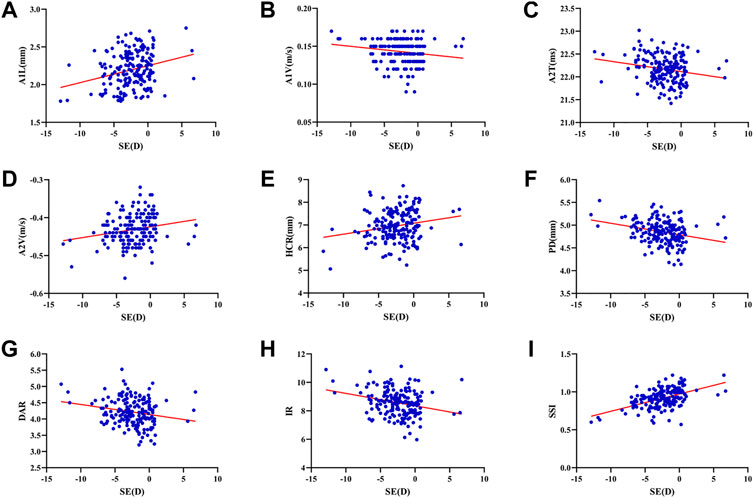
FIGURE 1. Corneal biomechanical parameters were significantly correlated with the refractive error. SE had significant correlations with A1L (r = 0.260, p < 0.001) (A), A1V (r = -0.174, p = 0.020) (B), A2T (r = -0.201, p = 0.007) (C), A2V (r = 0.208, p = 0.005) (D), HCR (r = 0.198, p = 0.008) (E), PD (r = -0.271, p < 0.001) (F), DAR (r = -0.206, p = 0.006) (G), IR (r = -0.264, p < 0.001) (H) and SSI (r = 0.501, p < 0.001) (I) (Panel 1A–I).
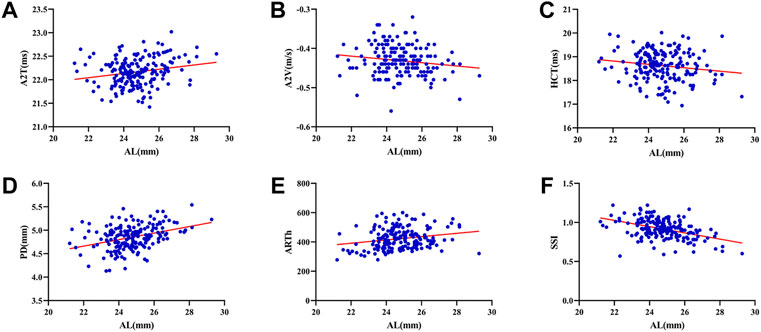
FIGURE 2. Corneal biomechanical parameters were significantly correlated with the refractive error. AL was significantly correlated with A2T (r = 0.205, p = 0.006) (A), A2V (r = -0.152, p = 0.041) (B), HCT (r = -0.154 p = 0.039) (C), PD (r = 0.371, p < 0.001) (D), ARTh (r = 0.215, p = 0.004) (E) and SSI (r = -0.436, p < 0.001) (F) (Panel 2A–F).
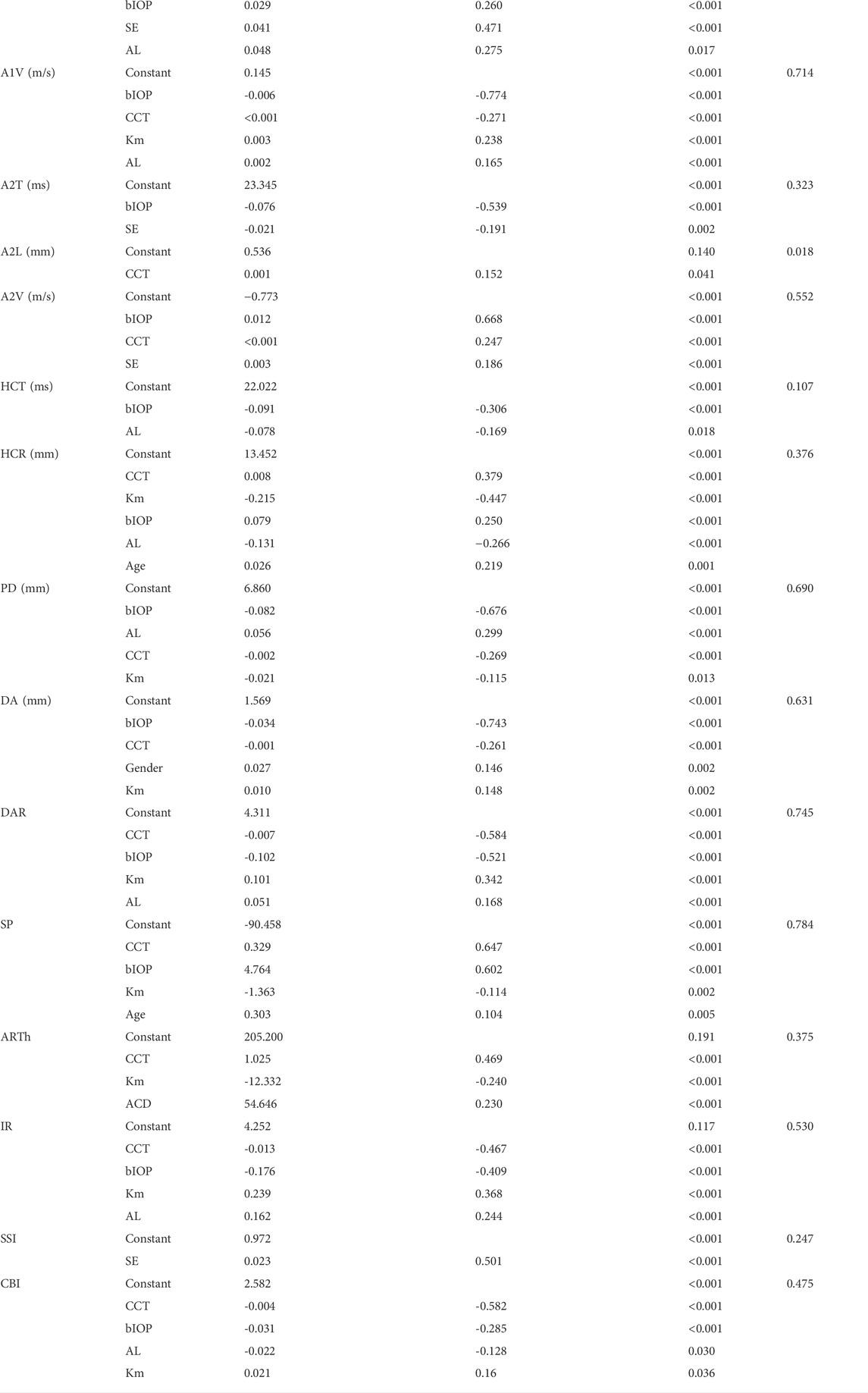
Multiple regression analysis for variables predicting corneal biomechanical parameters
Table 2 displays the regression equation for multivariate regression analysis. Other than A1T, A2L, DA, SP and ARTh, corneal biomechanical parameters had a correlation with ametropic parameters (SE or AL). CCT and bIOP had a greater impact on most corneal biomechanical parameters with larger Standardized
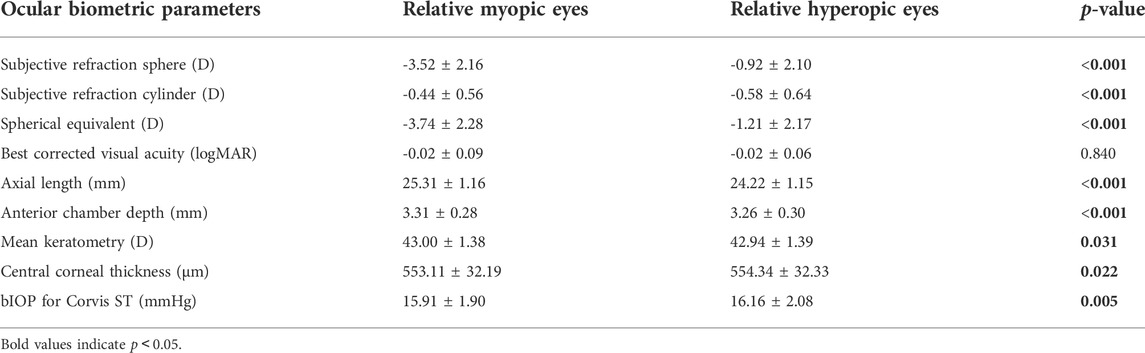
Comparison of ocular biometric and corneal biomechanical parameters between anisometropic fellow eyes
The SE was -3.52 ± 2.16D in relative myopic eyes and -0.92 ± 2.10D in the contralateral eyes. The mean
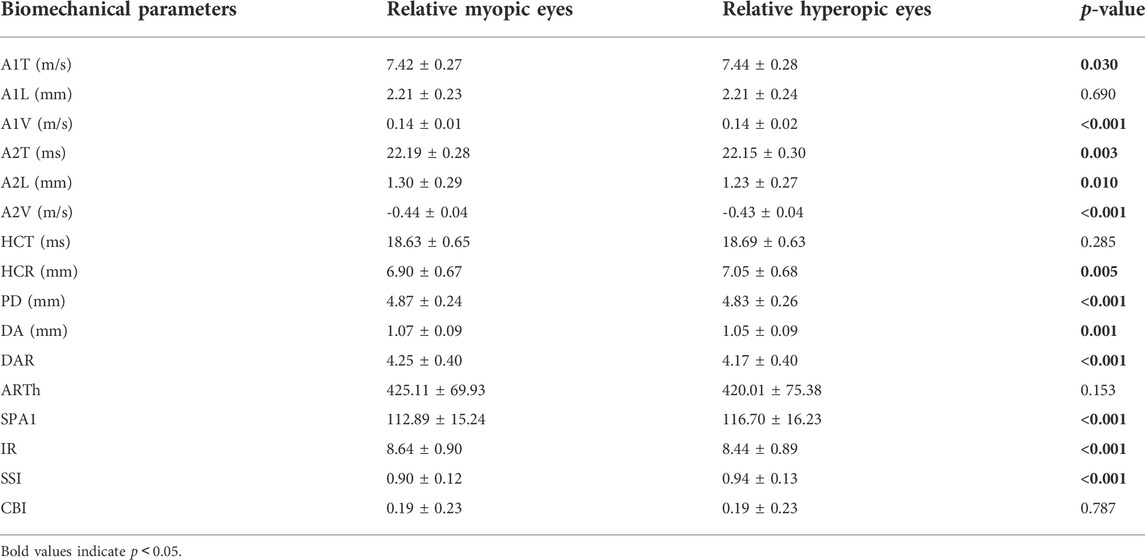
FIGURE 3. Histogram comparison of A1T, A1V, A2T, A2L, A2V, HCR, PD, DA, DAR, SPA1, IR, and SSI (A–L) in anisometropia fellow eyes. *means p < 0.05, **means p < 0.01 ***means p < 0.001.
Comparison of ocular biometric and corneal biomechanical asymmetry in varying severities of anisometropia
The number of participants in low, moderate and high anisometropia were 61 (33.5%), 61 (33.5%), 58 (32.2%), respectively. Mean ∆SE of three groups were 1.41 ± 0.26D, 2.37 ± 0.29D and 3.89 ± 0.89D. There were no statistically significant differences regarding the age, gender, mean SE or AL of fellow eyes among three groups (p > 0.05). More details were shown in Table 5. There were significant differences in asymmetry of SE, AL and ACD (p < 0.05) but not in corneal biomechanical asymmetry (p > 0.05) between anisometropic fellow eyes among three groups (Supplementary Table S2).
Correlation between corneal biomechanical asymmetry and degrees of anisometropia
△ACD was statistically significant correlated with △HCT (r = 0.161, p = 0.031) and △CBI (r = 0.200, p = 0.007). △Km was negatively associated with △SSI (r = -0.153, p = 0.041). There was no significant difference between the asymmetry of corneal biomechanical parameters and △SE or △AL (p > 0.05). More details showed in Supplementary Table S3.
Discussion
This was the first study to evaluate corneal biomechanics in anisometropia using the Corvis ST. The current study found that several corneal biomechanical parameters based on Corvis ST were slightly correlated with SE or AL (Table 1 and Figure 1 and Figure 2). PD was negatively correlated with SE (Figure 1F) and positively correlated with AL (Figure 2D). HCR was positively correlated with SE (Figure 1E). These findings were in agreement with those from Sedaghat et al. (Sedaghat et al., 2020), Lu et al. (Lu et al., 2022) and Wang et al. (Wang et al., 2015). Deformable parameters (HC parameters) indicated the elastic property of corneal collagen fibers (He et al., 2017). A smaller HCR and higher PD are associated with less resistance to deformation (Long et al., 2019; Esporcatte et al., 2020). We also found DAR and IR were negatively correlated with SE (Figures 1G,H), and positively correlated with AL in the regression analysis (Table 2). Kenia et al. (Kenia et al., 2020) found similar correlations between DAR, IR and SE. Vinciguerra screening parameters are a series of newly developed parameters based on an attempt to differentiate normal corneas from keratoconus corneas (Han et al., 2021). Smaller DAR and IR values indicated a softer cornea (Han et al., 2021). However, CCT and bIOP had a greater impact on most corneal deformation parameters (Table 2), which was similar to previous studies (Sedaghat et al., 2020; Yu et al., 2020). There have been remained a challenge that it is difficult to separate corneal biomechanical parameters from the effects of IOP and CCT in vivo measurement (Lee H. et al., 2017).
SSI is a new parameter SSI free of the influences of IOP and CCT (either IOP or CCT was not correlated with SSI (p > 0.05)) (Eliasy et al., 2019; Liu et al., 2020). We found SSI had no correlation with CCT and bIOP (Tables 1, 2). However, SSI was weakly correlated with bIOP (r = 0.23, p < 0.01) in another study (Han et al., 2020a). Although the results in different studies were not consistent, they also indicated SSI reduced the influence of bIOP and CCT on corneal biomechanical measurement. What’s more, SSI is the first standard mechanic metric in vivo which provides the whole stress-strain curve (Esporcatte et al., 2020), regardless of the level of the load (Eliasy et al., 2019). It helps us to assess cornea (a nonlinear viscoelasticity tissue) biomechanics better (Kling and Hafezi, 2017; Chong and Dupps, 2021). The current study indicated that SSI was correlated with SE (r = 0.501, p < 0.001) (Figure 1I) and AL (r = -0.436, p < 0.001) (Figure 2F). In line with our study, Liu et al. (Liu et al., 2021) indicated SSI was negatively correlated with AL (r = -0.476, p < 0.001). Han et al. (Han et al., 2020b) found SSI had a positive correlation with SE (r = 0.313, p < 0.01). Compared to other Corvis ST parameters, the result of SSI was more convincing to show that corneal biomechanical properties became weaker with the increase in myopia. High myopia is associated with abnormal scleral collagen fiber orientation and reduced diameter of fibers, which cause scleral biomechanics to weaken (Markov et al., 2018). Both the cornea and sclera derive from the same mesoderm, so the cornea may have similar changes to the sclera with myopia progression (He et al., 2017). A study based on form-deprivation myopia chicks showed myopic corneal tangent modulus became lower (Kang et al., 2018). Another study about the biophysical properties of corneal cells indicated that F-actin and microtubule content was changing with myopia inducing and recovering in the chick model. F-actin and microtubule content may affect corneal biomechanics (Xin et al., 2021). On the other hand, softer sclera in myopia will weaken the restrictive effect on corneal deformation (Sedaghat et al., 2020).
Age is one of the important factors in corneal biomechanics. Similar to previous studies (Celebi et al., 2018; Eliasy et al., 2019; Liu et al., 2020; Baptista et al., 2021), several Corvis ST parameters were correlated with age, indicating the cornea became stiffer with increasing age (Table 1 and Table 2). More glycation-induced (Kling and Hafezi, 2017) and sun-related (Baptista et al., 2021) cross-linking contribute to this change. However, there was no significant correlation between SSI and age (Table 1 and Table 2). Liu et al. found SSI was relatively stable in a young population and increased with age after the age of 35 (Liu et al., 2020). Most participants in our study were young (mean age was 16.09 ± 5.64 years). Thus, there was no correlation between SSI and age in our study.
Compared with traditional controlled research (comparison among emmetropes and various degrees of ametropia), the assessment of corneal biomechanics in anisometropic eyes would be more convenient to control confounding factors, such as hormonal level, environmental factors, and genetic factors. The current study found relative myopic eyes had greater A1V, A2T, A2L, A2V, PD, DA DAR, IR, and smaller A1T, HCR, SPA1, and SSI than contralateral eyes (p < 0.05) (Figures 3A–L), indicating corneal biomechanics was weaker in relative myopic eyes. But the difference was slight. Vincent and others did not find any differences in ORA parameters between anisometropic eyes (11.35 ± 1.37 vs. 11.30 ± 1.41, p > 0.05;
There is a hypothesis that increased IOP results in axial elongation and IOP may be one of the potential mechanical factors leading to myopia and anisometropia (Vincent et al., 2014). However, previous studies found IOP was symmetrical in anisometropic eyes using cornea-corrected IOP from the ORA (Xu et al., 2010; Vincent et al., 2011). The bIOP based on the Corvis ST can reduce the effect of corneal stiffness (Maklad et al., 2020). Relative myopic eyes had significant but not clinically meaningful lower bIOP than contralateral eyes in the current study (Table 3). It did not support this hypothesis and indicated that anisometropia developed with similar IOPs.
Scleral biomechanics were closely related to ocular elongation (Markov et al., 2018). It is supposed that scleral biomechanical changes can be transmitted to the cornea (Nguyen et al., 2020). According to this hypothesis, the first part of our study and previous studies found significant correlations between corneal biomechanics and the severity of myopia. However, our result showed this hypothesis may not make sense in anisometropia. In the current study, there was no significant difference of corneal biomechanical asymmetry among three groups (Supplementary Table S2), and corneal biomechanical asymmetry was no significantly correlated with the asymmetry of AL or SE (Supplementary Table S3). There are several possible explanations as follow. Firstly, the participants were healthy without corneal disease such as corneal ectasia, their corneal biomechanical properties were within a healthy range. The primary biometric basis of anisometropia is the asymmetry in axial length which is related to the sclera (Vincent et al., 2014). When anisometropia becomes severe, scleral biomechanical asymmetry is increasing but asymmetry in corneal biomechanics may remain within a normal range. Secondly, several factors play an important role in anisometropia, such as stochastic factors (variability that comes about from randomness or noise) (Flitcroft et al., 2021) and asymmetric visual experience between fellow eyes (Vincent et al., 2014). But the corneal biomechanical asymmetry between anisometropic eyes may be limited by the same genetic background. Thirdly, even though cornea and scleral are a whole, their biomechanical behaviors are still different when stress is applied (Bronte-Ciriza et al., 2021), thus corneal biomechanical measures may not fully represent the scleral biomechanics.
Significant correlations were found between the asymmetry of several corneal deformation parameters and anterior parameters (ACD and Km) (Supplementary Table S3). The anterior chamber will resist deformation when the cornea moves into anterior chamber with the air pulse (Tejwani et al., 2019). Corneal biomechanical measurements reflect anterior rather than whole ocular biomechanics in anisometropia. However, anisometropia is weaker correlated with anterior chamber depth than AL (the Pearson correlation was 0.183 vs. 0.735) (Hashemi et al., 2013), indicating the weak role of anterior segment factors in anisometropia. Longitudinal studies are needed to further observe the impact of anisometropia and myopia progression on biomechanics. Besides, novel in vivo devices that directly assess scleral biomechanics is needed to study the biomechanical mechanisms of anisometropia.
In conclusion, the relative myopia eyes had slightly weaker biomechanical properties than the contralateral eye in anisometropia. However, a certain linear relationship between anisometropia and biomechanical asymmetry was not found. Future studies including scleral biomechanics in anisometropia using novel devices are warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Eye Hospital to Wenzhou Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
All authors conceptualized the study, RG and YR: Conceptualization, Investigation, Visualization, Writing—original draft; YR, SL and JY: Investigation, Data curation; CM: Visualization, Formal analysis; HX and XL: Data curation; CM: Writing—review and editing; EP: Writing—review and editing; JH: Funding acquisition, Supervision; JH and JY: Methodology, Visualization, Writing—original draft; JH: Conceptualization, Funding acquisition, Supervision.
Funding
This work was supported in part by Project of Shanghai Science and Technology (22S11900200); Major Scientific and Technological Innovation Project of Wenzhou (ZY2021016); Zhejiang Provincial & Ministry of Health Research Fund for Medical Sciences (WKJ-ZJ-2134); Natural Science Foundation of Zhejiang Province (LY21H120003, LQ22H120005); and the EYE & ENT Hospital of Fudan University High-level Talents Program (2021318). The sponsor or funding organization had no role in the design or conduct of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2022.994353/full#supplementary-material
References
Atchison, D. A., Lee, J., Lu, J., Webber, A. L., Hess, R. F., Baldwin, A. S., et al. (2020). Effects of simulated anisometropia and aniseikonia on stereopsis. Ophthalmic Physiol. Opt. 40 (3), 323–332. doi:10.1111/opo.12680
Baptista, P. M., Ambrosio, R., Oliveira, L., Meneres, P., and Beirao, J. M. (2021). Corneal biomechanical assessment with ultra-high-speed Scheimpflug imaging during non-contact tonometry: A prospective review. Clin. Ophthalmol. 15, 1409–1423. doi:10.2147/OPTH.S301179
Birch, E. E., Jost, R. M., Wang, Y. Z., Kelly, K. R., and Giaschi, D. E. (2019). Impaired fellow eye motion perception and abnormal binocular function. Invest. Ophthalmol. Vis. Sci. 60 (10), 3374–3380. doi:10.1167/iovs.19-26885
Bronte-Ciriza, D., Birkenfeld, J. S., de la Hoz, A., Curatolo, A., Germann, J. A., Villegas, L., et al. (2021). Estimation of scleral mechanical properties from air-puff optical coherence tomography. Biomed. Opt. Express 12 (10), 6341–6359. doi:10.1364/BOE.437981
Celebi, A. R. C., Kilavuzoglu, A. E., Altiparmak, U. E., and Cosar Yurteri, C. B. (2018). Age-related change in corneal biomechanical parameters in a healthy Caucasian population. Ophthalmic Epidemiol. 25 (1), 55–62. doi:10.1080/09286586.2017.1351997
Chong, J., and Dupps, W. J. (2021). Corneal biomechanics: Measurement and structural correlations. Exp. Eye Res. 205, 108508. doi:10.1016/j.exer.2021.108508
Eliasy, A., Chen, K. J., Vinciguerra, R., Lopes, B. T., Abass, A., Vinciguerra, P., et al. (2019). Determination of corneal biomechanical behavior in-vivo for healthy eyes using CorVis ST tonometry: Stress-strain index. Front. Bioeng. Biotechnol. 7, 105. doi:10.3389/fbioe.2019.00105
Esporcatte, L. P. G., Salomao, M. Q., Lopes, B. T., Vinciguerra, P., Vinciguerra, R., Roberts, C., et al. (2020). Biomechanical diagnostics of the cornea. Eye Vis. (Lond). 7, 9. doi:10.1186/s40662-020-0174-x
Flitcroft, I., Mccullough, S., and Saunders, K. J. T. B. j. o. o. (2021). Br. J. Ophthalmol. 105 (9), 1211–1215. doi:10.1136/bjophthalmol-2020-316406
González-Méijome, J. M., Villa-Collar, C., Queirós, A., Jorge, J., and Parafita, M. A. (2008). Pilot study on the influence of corneal biomechanical properties over the short term in response to corneal refractive therapy for myopia. Cornea 27 (4), 421–426. doi:10.1097/ICO.0b013e318164e49d
H, G., Sm, H.-M., and ophthalmology, H. W. J. B. (2019). Corneal biomechanical properties after SMILE versus FLEX, LASIK, LASEK, or PRK: A systematic review and meta-analysis. BMC Ophthalmol. 19 (1), 167. doi:10.1186/s12886-019-1165-3
Han, F., Li, M., Wei, P., Ma, J., Jhanji, V., and Wang, Y. (2020a). Effect of biomechanical properties on myopia: A study of new corneal biomechanical parameters. BMC Ophthalmol. 20 (1), 459. doi:10.1186/s12886-020-01729-x
Han, F., Li, M., Wei, P., Ma, J., Jhanji, V., and Wang, Y. J. B. o. (2020b). Effect of biomechanical properties on myopia: A study of new corneal biomechanical parameters. BMC Ophthalmol. 20 (1), 459. doi:10.1186/s12886-020-01729-x
Han, S. B., Liu, Y. C., Mohamed-Noriega, K., and Mehta, J. S. (2021). Advances in imaging technology of anterior segment of the eye. J. Ophthalmol. 2021, 1–9. doi:10.1155/2021/9539765
Hashemi, H., Khabazkhoob, M., Emamian, M., Shariati, M., Abdolahi-nia, T., and Fotouhi, A. J. T. B. j. o. o. (2013). All biometric components are important in anisometropia, not just axial length. Br. J. Ophthalmol. 97 (12), 1586–1591. doi:10.1136/bjophthalmol-2013-303939
He, M., Wang, W., Ding, H., and Zhong, X. (2017). Corneal biomechanical properties in high myopia measured by dynamic Scheimpflug imaging technology. Optom. Vis. Sci. 94 (12), 1074–1080. doi:10.1097/OPX.0000000000001152
Jędzierowska, M., and Koprowski, R. (2019). Novel dynamic corneal response parameters in a practice use: A critical review. Biomed. Eng. OnLine 18 (1), 17. doi:10.1186/s12938-019-0636-3
Kang, B., Wang, L., Zheng, Y., Guggenheim, J., Stell, W., and Kee, C. J. P. o. (2018). High myopia induced by form deprivation is associated with altered corneal biomechanical properties in chicks. PLoS ONE 13 (11), e0207189. doi:10.1371/journal.pone.0207189
Kenia, V. P., Kenia, R. V., and Pirdankar, O. H. (2020). Association between corneal biomechanical parameters and myopic refractive errors in young Indian individuals. Taiwan J. Ophthalmol. 10 (1), 45–53. doi:10.4103/tjo.tjo_15_19
Kling, S., and Hafezi, F. (2017). Corneal biomechanics - a review. Ophthalmic Physiol. Opt. 37 (3), 240–252. doi:10.1111/opo.12345
Lee, C., Fang, S., Tsai, D., Huang, N., Hsu, C., Chen, S., et al. (2017a). Prevalence and association of refractive anisometropia with near work habits among young schoolchildren: The evidence from a population-based study. PLoS ONE 12 (3), e0173519. doi:10.1371/journal.pone.0173519
Lee, H., Kang, D. S., Ha, B. J., Choi, J. Y., Kim, E. K., Seo, K. Y., et al. (2017b). Biomechanical properties of the cornea measured with the dynamic Scheimpflug analyzer in young healthy adults. Cornea 36 (1), 53–58. doi:10.1097/ICO.0000000000001001
Lee, R., Chang, R. T., Wong, I. Y., Lai, J. S., Lee, J. W., and Singh, K. (2016). Assessment of corneal biomechanical parameters in myopes and emmetropes using the Corvis ST. Clin. Exp. Optom. 99 (2), 157–162. doi:10.1111/cxo.12341
Liu, G., Rong, H., Pei, R., Du, B., Jin, N., Wang, D., et al. (2020). Age distribution and associated factors of cornea biomechanical parameter stress-strain index in Chinese healthy population. BMC Ophthalmol. 20 (1), 436. doi:10.1186/s12886-020-01704-6
Liu, G., Rong, H., Zhang, P., Xue, Y., Du, B., Wang, B., et al. (2021). The effect of axial length elongation on corneal biomechanical property. Front. Bioeng. Biotechnol. 9, 777239. doi:10.3389/fbioe.2021.777239
Long, W., Zhao, Y., Hu, Y., Li, Z., Zhang, X., Zhao, W., et al. (2019). Characteristics of corneal biomechanics in Chinese preschool children with different refractive status. Cornea 38 (11), 1395–1399. doi:10.1097/ICO.0000000000001971
Lu, L. L., Hu, X. J., Yang, Y., Xu, S., Yang, S. Y., Zhang, C. Y., et al. (2022). Correlation of myopia onset and progression with corneal biomechanical parameters in children. World J. Clin. Cases 10 (5), 1548–1556. doi:10.12998/wjcc.v10.i5.1548
Ma, J., Wang, Y., Li, M., and Jhanji, V. (2021). Association between severity of myopia and deformation characteristics of the cornea based on propensity score matching analysis. J. Refract. Surg. 37 (5), 344–350. doi:10.3928/1081597x-20210222-02
Ma, W., Jb, R., and clinics, W. M. J. I. o. (2016). Complications of refractive surgery: Ectasia after refractive surgery. Int. Ophthalmol. Clin. 56 (2), 127–139. doi:10.1097/iio.0000000000000102
Maklad, O., Eliasy, A., Chen, K. J., Wang, J., Abass, A., Lopes, B. T., et al. (2020). Fluid-structure interaction based algorithms for IOP and corneal material behavior. Front. Bioeng. Biotechnol. 8, 970. doi:10.3389/fbioe.2020.00970
Markov, P. P., Eliasy, A., Pijanka, J. K., Htoon, H. M., Paterson, N. G., Sorensen, T., et al. (2018). Bulk changes in posterior scleral collagen microstructure in human high myopia. Mol. Vis. 24, 818–833.
Nguyen, B. A., Reilly, M. A., and Roberts, C. J. (2020). Biomechanical contribution of the sclera to dynamic corneal response in air-puff induced deformation in human donor eyes. Exp. Eye Res. 191, 107904. doi:10.1016/j.exer.2019.107904
Pinero, D. P., and Alcon, N. (2015). Corneal biomechanics: A review. Clin. Exp. Optom. 98 (2), 107–116. doi:10.1111/cxo.12230
Sedaghat, M. R., Momeni-Moghaddam, H., Azimi, A., Fakhimi, Z., Ziaei, M., Danesh, Z., et al. (2020). Corneal biomechanical properties in varying severities of myopia. Front. Bioeng. Biotechnol. 8, 595330. doi:10.3389/fbioe.2020.595330
Singh, P., Bergaal, S. K., Sharma, P., Agarwal, T., Saxena, R., and Phuljhele, S. (2021). Effect of induced anisometropia on stereopsis and surgical tasks in a simulated environment. Indian J. Ophthalmol. 69 (3), 568–572. doi:10.4103/ijo.IJO_1540_20
Smith, E. L., Hung, L. F., Arumugam, B., Wensveen, J. M., Chino, Y. M., and Harwerth, R. S. (2017). Observations on the relationship between anisometropia, amblyopia and strabismus. Vis. Res. 134, 26–42. doi:10.1016/j.visres.2017.03.004
Tejwani, S., Francis, M., Dinakaran, S., Kamath, V., Tilva, B., Das, R. K., et al. (2019). Influence of anterior biometry on corneal biomechanical stiffness of glaucomatous eyes treated with chronic medication or filtration surgery. J. Glaucoma 28 (7), 626–632. doi:10.1097/IJG.0000000000001247
Tubtimthong, A., Chansangpetch, S., Ratprasatporn, N., Manassakorn, A., Tantisevi, V., Rojanapongpun, P., et al. (2020). Comparison of corneal biomechanical properties among axial myopic, nonaxial myopic, and nonmyopic eyes. Biomed. Res. Int. 2020, 1–7. doi:10.1155/2020/8618615
Vincent, S. J., Collins, M. J., Read, S. A., and Carney, L. G. (2014). Myopic anisometropia: Ocular characteristics and aetiological considerations. Clin. Exp. Optom. 97 (4), 291–307. doi:10.1111/cxo.12171
Vincent, S. J., Collins, M. J., Read, S. A., Carney, L. G., and Yap, M. K. (2011). Interocular symmetry in myopic anisometropia. Optom. Vis. Sci. 88 (12), 1454–1462. doi:10.1097/OPX.0b013e318233ee5f
Wang, J., Li, Y., Jin, Y., Yang, X., Zhao, C., and Long, Q. (2015). Corneal biomechanical properties in myopic eyes measured by a dynamic Scheimpflug analyzer. J. Ophthalmol. 2015, 1–8. doi:10.1155/2015/161869
Wang, M., Zhang, Y., Wu, W., Young, J., Hatch, K., Pineda, R., et al. (2018). Predicting refractive outcome of small incision lenticule extraction for myopia using corneal properties. Transl. Vis. Sci. Technol. 7 (5), 11. doi:10.1167/tvst.7.5.11
Wu, J., Fang, W., Xu, H., Liu, X., Zhao, D., and Rong, Q. (2021). The biomechanical response of the cornea in orthokeratology. Front. Bioeng. Biotechnol. 9, 743745. doi:10.3389/fbioe.2021.743745
Xin, Y., Kang, B., Zheng, Y., Shan, S., Kee, C., and Tan, Y. J. B. j. (2021). Biophysical properties of corneal cells reflect high myopia progression. Biophys. J. 120 (16), 3498–3507. doi:10.1016/j.bpj.2021.05.010
Xu, S., Xu, A., Tao, A., Wang, J., Fan, F., and Lu, F. (2010). Corneal biomechanical properties and intraocular pressure in high myopic anisometropia. Eye Contact Lens 36 (4), 204–209. doi:10.1097/ICL.0b013e3181e4a60a
Yu, A. Y., Shao, H., Pan, A., Wang, Q., Huang, Z., Song, B., et al. (2020). Corneal biomechanical properties in myopic eyes evaluated via Scheimpflug imaging. BMC Ophthalmol. 20 (1), 279. doi:10.1186/s12886-020-01530-w
Keywords: anisometropia, refractive error, myopia, corneal biomechanics, Scheimpflug technology, Corvis ST
Citation: Gao R, Ren Y, Li S, Xu H, Lin X, McAlinden C, Ye J, Huang J and Yu J (2022) Assessment of corneal biomechanics in anisometropia using Scheimpflug technology. Front. Bioeng. Biotechnol. 10:994353. doi: 10.3389/fbioe.2022.994353
Received: 14 July 2022; Accepted: 12 September 2022;
Published: 04 October 2022.
Edited by:
Yang Liu, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Yan Wang, Tianjin Eye Hospital, ChinaGuihua Liu, Tianjin Medical University Eye Hospital, China
Copyright © 2022 Gao, Ren, Li, Xu, Lin, McAlinden, Ye, Huang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinjin Yu, amp5MDkxMjg0QDE2My5jb20=; Jinhai Huang, dmlwOTk5dmlwQDE2My5jb20=
†These authors contributed equally to this work and share first authorship
 Rongrong Gao1†
Rongrong Gao1† Yuecheng Ren
Yuecheng Ren Xuanqiao Lin
Xuanqiao Lin Colm McAlinden
Colm McAlinden Jinhai Huang
Jinhai Huang