
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol., 13 September 2022
Sec. Biomaterials
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.985881
This article is part of the Research TopicBioactive Bone Regenerative Materials and Bionic Prosthesis InterfacesView all 22 articles
Dental caries, a chronic and irreversible disease caused by caries-causing bacteria, has been listed as one of the three major human diseases to be prevented and treated. Therefore, it is critical to effectively stop the development of enamel caries. Remineralization treatment can control the progression of caries by inhibiting and reversing enamel demineralization at an early stage. In this process, functional materials guide the deposition of minerals on the damaged enamel, and the structure and hardness of the enamel are then restored. These remineralization materials have great potential for clinical application. In this review, advanced materials for enamel remineralization were briefly summarized, furthermore, an outlook on the perspective of remineralization materials were addressed.
The enamel, consisting of 96–97 wt% inorganic hydroxyapatite (HA, Ca10(PO4)6(OH)2), 3wt% water and 1wt% organic material, is the hardest tissue in the human body (Bowen et al., 2018; Harper et al., 2021). However, enamel is susceptible to acid, causing enamel demineralization and even developing cavities (Pitts et al., 2017). Currently, hundreds of millions of people in the world is under the enamel damage (Peres et al., 2019). It is difficult to repair enamel on its own due to the lack of sufficient calcium and phosphate ions in saliva (Lawn et al., 2010; Lacruz et al., 2017). Therefore, artificial materials such as resin, metal or bioglass are commonly used for clinical repair of cavities (Dorri et al., 2017). In terms of composition, mechanical properties, and appearance, these composites differ significantly from enamel. By comparison, enamel remineralization can be an effective clinical method for restoring the natural properties and structure of enamel while avoiding the problems associated with filling materials. Remineralization requires replacing minerals lost during the early stages of demineralization to restore enamel hardness or structure.
Remineralized materials are essential to enamel repair. Functional materials can promote and arrange the deposition of calcium and phosphate ions or alter the solubility of the HA. They can be divided into inorganic materials, organic materials, and polymeric materials (Figure 1). These functional materials are designed to rebuild remineralized tissue on damaged enamel surfaces, thereby preventing disease progression while also improving aesthetics and mechanical strength. Therefore, materials for enamel remineralization have a bright future in clinic. Although several reviews of remineralized materials have been published (Cochrane et al., 2010; Ding et al., 2017; Pandya and Diekwisch, 2019), enamel remineralized materials have been innovated and developed. As a result, it is critical to review the relevant research progress in time for the construction and upgrading of the enamel remineralization system. In this review, the characteristics and working mechanism of remineralized materials are briefly summarized. The specific functions of various functional materials will be clarified by category, with reference opinions provided for future material design and synthesis.
Functional inorganic materials can induce the formation of apatite layers or release ions, which can promote the remineralization of enamel. When the remineralized layer forms, calcium phosphates (CaPs) provide exogenous ions to compensate minerals lost by enamel, while fluoride and magnesium ions can exchange with calcium ions in HA, changing the solubility and mechanical properties of ion-doped HA. Therefore, the ability of inorganic materials to release ions and the change in enamel properties caused by their participation in HA are the primary focal point of researches.
CaPs can provide ions to reconstruct damaged enamel. Remineralization solutions containing calcium and phosphorus ions are usually used in remineralization experiments, which must be replaced or replenished on a regular basis. Some stable CaPs materials can provide ions required for an extended time. Amorphous calcium phosphate (ACP), tricalcium phosphate (TCP), and nano-hydroxyapatite (nHA) are common CaPs materials used for remineralization. The type and size of the CaPs crystals can influence the ion supply capacity and the depth of ion entry into the lesion. Therefore, the mineralization effects of these materials are differrent.
ACP, the precursor phase of biogenic HA of bone and tooth, is the basic mineralization unit in the biological mineralization process (Gelli et al., 2019). Aqueous ACP solutions contain abundant Ca2+ and PO43− ions, which form highly hydrated clusters. The structure and composition of the crystalline phase change after further aggregation of clusters until the thermodynamically stable crystalline HA (alkaline conditions) or carbon brushes (acidic conditions) formed (He et al., 2020). Usually, such reaction time is fast in the absence of external interference. Only ACP solutions failed to restore enamel (Shao et al., 2019). Therefore, enamel remineralization requires ensuring the stability of ACP in solution and prolonging its phase transition time. Acidic groups, such as carboxyl and phosphoric groups, can bind calcium ions in solution, preventing Ca2+ and PO43− from aggregating. Organic compounds with carboxyl or phosphate groups are the most common ACP stabilizers. It is a good method to use amino acids such as aspartate (Asp), glutamate (Glu), citrate (Delgado-López et al., 2014; Iafisco et al., 2015), and the phosphate stabilizer triethylamine (Shao et al., 2019) to maintain the size of ACP particles, ensuring ion supply in the subsequent mineralization process. In addition, the casein phosphopeptide (CPP) that containing four to seven phosphate groups can attach to ACP nanoclusters, forming CPP-ACP. CPP-ACP complexes have been used as common additives for caries prevention. Furthermore, CPP-ACP in combination with fluoride show advantages in remineralization of existing lesions (Bijle et al., 2018; Tao et al., 2018). However, CPP-containing products should be used with caution in individuals with lactose intolerance issues.
TCP can be divided into α-TCP and β-TCP according to the crystal form. β-TCP is often used in dental materials because of its great biodegradability and biocompatibility. When exposed to acid, β-TCP degrades to release ions for enamel restoration. After surface functionalization by carboxylic acid and surfactants, functionalized TCP (fTCP) can prevent fluoride from binding with calcium ions on the enamel surface prematurely to build a low-dose fluoride release system (Karlinsey and Pfarrer, 2012; Shen et al., 2018; Viana et al., 2020). After combining with fumaric acid, fTCP can show significantly higher calcium bioavailability than β-TCP and better remineralization of subsurface enamel damage (Karlinsey et al., 2010).
nHA is a bioactive and biocompatible material with a small particle size of 10–20 nm in diameter and 60–80 nm in length (Huang et al., 2011). The nanometer size enables nHA to penetrate deeper lesion layers through large lesion pores and repair enamel damage (Juntavee et al., 2018; Bossu et al., 2019; Memarpour et al., 2019). However, high-concentrating nHA tend to self-aggregate into large-sized nHA, which can affect the amount and depth of nHA entering the lesion (Huang et al., 2009). As a carrier, the gel effectively extends the contact time between the active ingredient and the enamel, allowing nHA to fill the small holes and depressions. Both silica-based glycerol hydrogel (Khonina et al., 2020) and carbomer-based gel (Sari et al., 2021) containing nHA can repair damaged enamel.
Fluorinated compounds have been commonly utilized since the previous century to reverse or prevent enamel defects from spreading. Consequently, the global incidence rate of dental caries has decreased dramatically (Jokstad, 2016; Clark et al., 2020). Fluoride reduces demineralization by altering enamel solubility (Lynch et al., 2004). Fluoride and calcium ions are more strongly bound than hydroxyl groups. Therefore fluoride can replace hydroxyl to form fluorapatite (FAP), which has high acid resistance and poor solubility (Clark et al., 2020). Fluorides, on the other hand, can promote remineralization by encouraging Ca2+ in saliva to attach to the tooth surface. In addition, fluoride can reduce the adhesion and growth of germs by blocking the activities of numerous enzymes.
Fluoride is primarily ingested through drinking water (75%). Fluoridation of home water is a typical measure to prevent dental caries in many countries, and it can successfully reduce the incidence of dental caries. Fluoride can also be found in a variety of oral care treatments and dental materials, including sodium fluoride (NaF), stannous fluoride, silver diamine fluoride, acidulated phosphate fluoride, ammonium fluoride, and others (Barrera-Ortega et al., 2020). Fluoride sustained-release ability could be altered by combining fluoride ions with different cations and complexing it with different organic molecules. In toothpaste and rinses, polyvalent fluorides with tin and titanium as cations exhibit excellent corrosion resistance (Zanatta et al., 2020). This is due to the fact that they can not only produce CaF2 on the enamel surface, but can also generate metallic precipitates on the enamel surface, which contributes to the reduction of calcium ion loss when subjected to external erosion.
However, fluorides have caused certain issues when they are used. Fluoride tends to develop a disordered layered structure of remineralization layer, which is considerably different from natural enamel. The mechanical characteristics of the remineralized layer can be weakened by these disordered formations. Organic compounds like amelogenin can help minimize the occurrence of disordered structures in the reaction system (Yu et al., 2019) (Figure 2). Moreover, excessive fluoride use can result in hazardous effects like dental fluorosis and skeletal fluorosis (Philip, 2019). It also has the possibility to make cariogenic bacteria resistant, diminishing the effectiveness of follow-up prophylaxis (Liao et al., 2017). Fluorinated hydroxyapatite is rapidly formed in the superficial enamel layer of very concentrated F− solutions, preventing Ca2+ and PO43− from penetrating deeper into the lesion. As a result, subsurface enamel lesions can fail to mineralize adequately. Therefore, fluoride slow-release systems made of copolymer acrylic reservoirs and glass ionomer cement can be great alternatives for promoting enamel mineralization by extending the trailing effect (Chong et al., 2018).
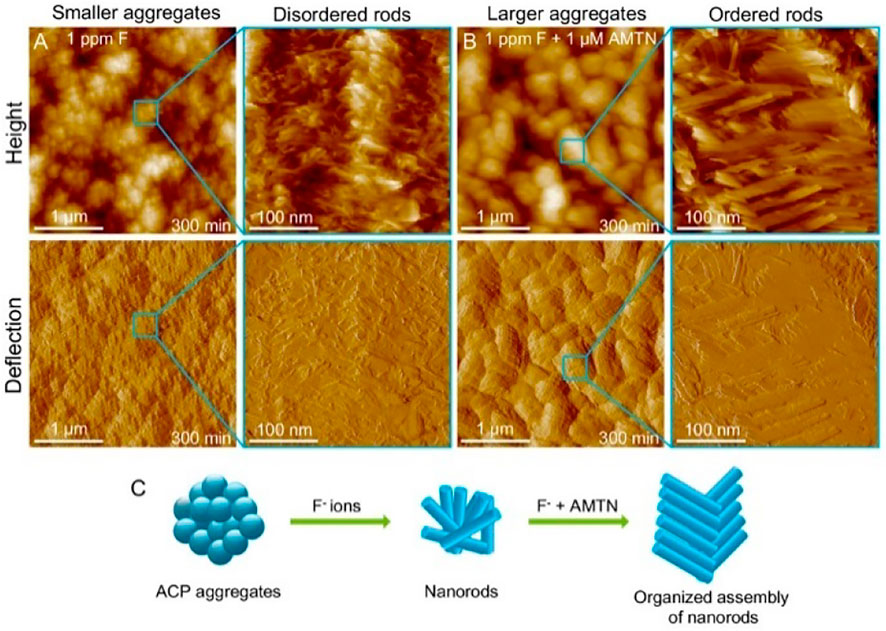
FIGURE 2. AFM height and deflection images of NPF solution in the presence of (A) 1 ppm F and (B) 1 ppm F and 1 μM AMTN for 300 min. (C) Schematic representation of nanorod tissue assembly in the presence of F ions and AMTN. Reprinted with permission from [ (Yu et al., 2019) ]. Copyright © 2019 American Chemical Society.
Fluoride compounds, as traditional enamel remineralization materials, have a relatively well-studied mineralization mechanism, which facilitates the development of novel fluoride-mediated remineralization systems. However, the functions of fluoride compounds are still need to be improved, and thus, blends or composites of fluoride compounds, which can combine multifunction together to achieve satisfactory clinical results, are greatly needed in the future.
Magnesium presents in the hard tissues of the body. In enamel, the content of Mg2+ is ranging from 0.2 to 0.5 wt%. Mg2+ is present near the grain boundaries as an intergranular phase of Mg substituted amorphous calcium phosphate (Mg-ACP) (La Fontaine et al., 2016). Such amorphous phases have been proved to make a significant impact on the mechanical characteristics and wear resistance of enamel (Gordon et al., 2015). Mg2+ slows crystal growth by competing with calcium ions at the growth point during mineralization, affecting the production of apatite (Ren et al., 2010; Abdallah et al., 2016). As a result, Mg2+ can act as a competitive inhibitor to guide narrower crystal columns, which promotes a highly ordered arrangement and increases mineralized tissue hardness. As the concentration of Mg2+ on the enamel surface increases, the nano-hardness of the enamel rises dramatically (Kis et al., 2021). Layer by layer mineralization process is used to create multilayer arrays of enamel-like FAP/polymer nanocomposites controlled by Mg2+ (FPN-M) at room temperature (Li Y. et al., 2021) (Figure 3). In the presence of Mg2+, the single nanorods are refined in size and a highly compact array is formed, eventually, (FPN-M)n exhibits excellent mechanical strength and transparency. The present researches have demonstrated Mg2+ have great importance during the process of enamel remineralization, therefore, more and more attention should be paid to Magnesium related materials. Besides, further understanding of the relationship between Mg2+ and biomineralization can help develop strategies to improve the mechanical properties of mineralized tissues and improve the functions of repaired tooth enamel.
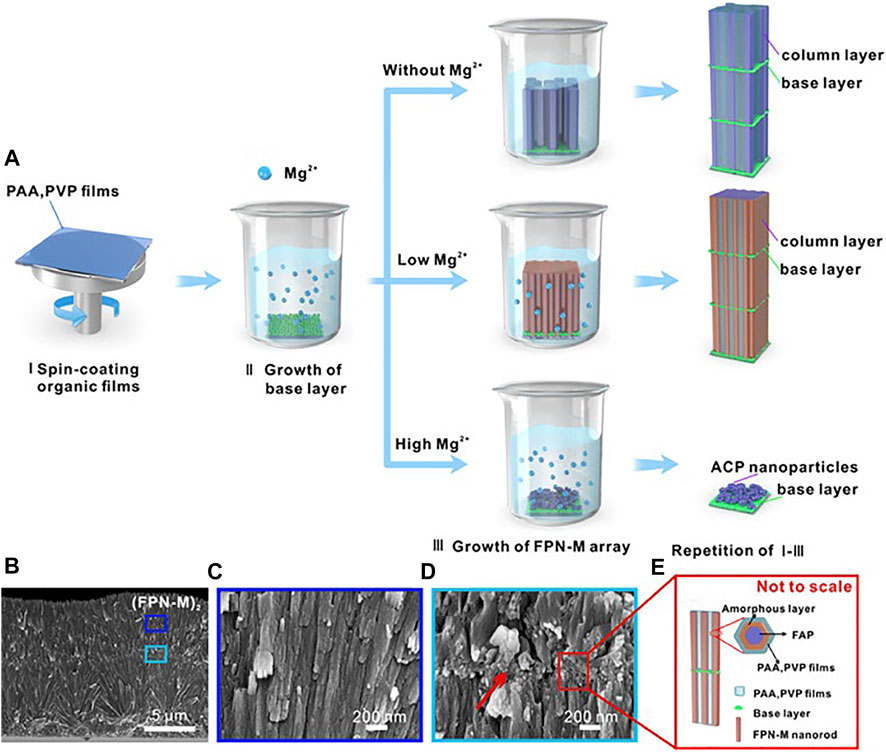
FIGURE 3. (A) The schematic diagram of the synthetic multilayer FAP/polymer nanocomposite controlled by Mg2+. (B)—(C) Cross-sectional SEM images of (FPN-M)2 with corresponding details. (D) Granular interlaminar base layer (indicated by red arrows). (E) Interlayer structure and amorphous-reinforced architecture schematic diagram. Reprinted with permission from [ (Li Y. et al., 2021) ]. Copyright© 2021 American Chemical Society.
Inorganic matter production and growth require a relatively constant environment, which organic materials can offer. Organic molecules are rich in acidic functional groups such as carboxyl, phosphate, and sulfonic acid. These functional groups can induce inorganic compound nucleation, inhibit overgrowth, or interact with hydroxyapatite on the surface of the enamel to increase adsorption capacity. Understanding the specific role of these organic compounds can help to clarify the mechanism of enamel mineralization and provide ideas for future remineralized material design.
Amino acid molecules contain different amounts of amino and carboxyl groups. Depending on the isoelectric point, amino acids can be classified as acidic, neutral, and basic amino acids. Among them, acidic amino acids are negatively charged in weakly acidic solutions, which can influence the nucleation, crystallization, growth, and crystal transformation of HA. Glu and Asp can operate as soft templates, connecting two calcium ions diagonally to generate ordered HA crystals that parallel to the enamel column while stabilizing calcium ions in the solution. The crystals on the enamel surface can grow more ordered with the amino acid concentration rises. Asp and Glu is used to deposit the CaCO3 layer as a sacrificial template on the enamel’s surface (Wu et al., 2015). The acidic amino acids then absorbed phosphate and carbonate ions, depositing HA into the CaCO3 layer to form the rod crystal. Glycine (Gly), a highly hydrophilic amino acid, can also be used as a biological additive to create enamel-like structures (Tao et al., 2007). According to the molecular dynamics experiment, Gly exhibits the same adsorption abilities and coverage in all directions of the crystal surface, which maintains HA’s c-axis propensity (Pan et al., 2007). In the carboxymethyl chitosan-stabilized ACP remineralization system, a rod-shaped crystal layer is successfully produced in artificial caries when Gly is introduced to the system, whereas the system without Gly fail (Wang et al., 2017). Arginine (Arg), a basic amino acid, positively affects pH homeostasis, bacterial ecology, and pathogenicity. Arg is metabolized in oral biofilms to produce ammonia mainly through the internal arginine deiminase system (ADS) of bacteria (Streptococcus sanguis and Streptococcus). Ammonia produced under this pathway has a significant pH-raising effect, while inhibiting tooth demineralization by neutralizing acids in the peripheral environment (Bijle et al., 2021b). It also facilitates the formation of arginine-friendly microorganisms while disrupting the internal homeostasis of caries-causing bacteria (Nascimento et al., 2019). The combination of Arg and fluoride can create a pH-responsive fluoride pool that inhibit acid production and has potential synergistic effects in maintaining a healthy oral microbial balance (Agnello et al., 2017; Bijle et al., 2021a). The pool can also significantly improve the fluoride uptake and surface hardness of damaged enamel compared to fluoride alone (Zheng et al., 2015; Bijle et al., 2020).
Enamel matrix proteins (EMPs) and proteases control the formation of enamel (Jia et al., 2020; Shin et al., 2020). EMPs govern the parallelism between the glazing columns and organize them in a dense and slender hexagonal prism structure at the micro-level by regulating the creation and structure of HA crystals (Bartlett, 2013; Uskokovic, 2015; Bai et al., 2020). These highly co-oriented glaze columns give enamel its remarkable shear strength and make it resistant to everyday abrasion (Yeom et al., 2017). Over 90% of the EMPs consists of amelogenin. Amelogenin can be enzymatically processed into different peptides. These peptides undergo a change in spatial conformation, manifested by α-helix unraveling and β-sheet and β-turns formation, at which point amyloid-like aggregation occur in the proteins (Carneiro et al., 2016; Bai et al., 2020). Then, they self-assemble into oligomers and nanospheres (Fang et al., 2011; Engelberth et al., 2018; Bai et al., 2020). These oligomeric nanospheres further form nanochains that concentrate Ca2+ and PO43− in the peripheral matrix, generating mineralized precursors during enamel development, which then serve as templates to guide the crystal phase transition, eventually generating HA (Gil-Bona and Bidlack, 2020) (Figure 4). The enamel columns then elongate in one direction to form a hexagonal prismatic structure (Jokisaari et al., 2019).
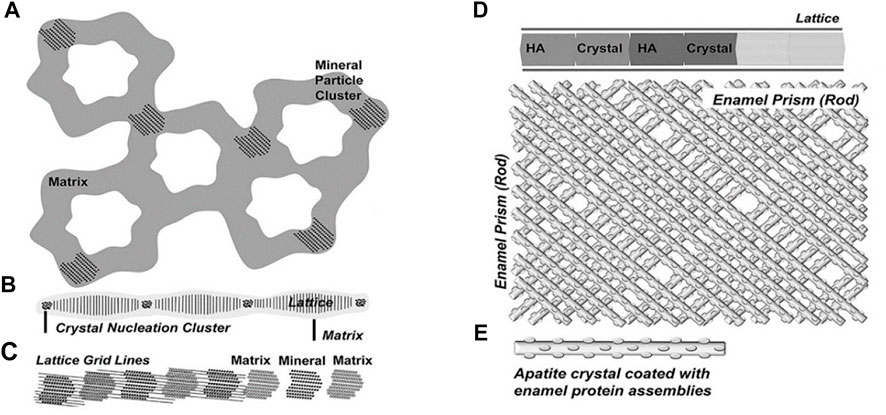
FIGURE 4. Five successive stages of enamel crystal precipitation and extension. (A) Stage of calcium phosphate particles precipitating and adhering to the organized enamel protein matrix, (B) Formation of initial crystal needle through single nucleation sites, (C) Extension of enamel crystals by lattice-guided alignment of individual apatite crystals, (D) Stage of crystal further extension and growing, (E) Enamel prisms (rods) are formed by the cross arrangement of single enamel crystals. Reprinted with permission from [ (Jokisaari et al., 2019) ]. Copyright © 2019 American Chemical Society.
Enzymes are critical requirements for enamel biomineralization. Enzymes activate the biological function of amelogenin and degrade organic matter in the matrix until a sufficiently hard tissue formed (Prajapati et al., 2016). Matrix metalloproteinase 20 (MMP-20) cleaves amelogenin, and the product peptide controls the lengthening and growth of crystal nucleus and induces HA mineralization (Fukae et al., 1998; Nagano et al., 2009; Gil-Bona and Bidlack, 2020). Addition of MMP-20 to full-length porcine amelogenin can promote neatly aligned bundles of enamel-like HA, whereas in the absence of MMP-20, only ACP particles seen (Kwak et al., 2016). Another important enzyme is Kallikrein-related peptidase 4 (KLK4). During enamel maturation, KLK4 degrades the organic matrix in the mineral (Smith et al., 2017; Sari et al., 2021). The width and thickness of the microcrystals can increase when proteins are removed from mature enamel. If the enzyme is deficient, the enamel will undergo hypoplasia (Simmer et al., 2009; Smith et al., 2017).
In-depth studies of the enzymatic cleavage products revealed three major functional domains of the amelogenin (Mukherjee et al., 2019; Dissanayake et al., 2020). N-terminal: a hydrophobic tyrosine-rich N-terminal region, known as tyrosine-rich amelogenin peptide (TRAP), is critical in the directed assembly of amelogenin (Buchko et al., 2018). The central region: the central hydrophobic proline-rich region is mainly composed of X-Y-proline (X and Y are usually glutamine) repeat motifs, which is rich in β-sheets and β-turns. The C-terminal: a highly hydrophilic domain contains a large number of acidic amino acid residues. These residues could combine with Ca2+ to provide nucleation sites and bind to the (100) face of octacalcium phosphate (OCP), the intermediate sub-stable phase in early enamel, thereby govern the direction in which the enamel column extends (Wu et al., 2017) (Figure 5). Leucine-rich amelogenin peptide (LRAP), which contains two self-assembled domains of full-length amelogenin, is the most common alternative splicing product of amelogenin (Xia et al., 2016; Green et al., 2019). It is discovered that depending on the phosphorylated version of the peptide on serine 16, LRAP can perform distinct activities. Phosphorylated LRAP (+P) inhibits calcium phosphate crystallization and stabilizes ACP, whereas LRAP (–P) directs the production of aligned enamel crystals (Yamazaki et al., 2017; Le Norcy et al., 2018). In vitro, LARP and PPi are used to remineralize the eroded enamel, and acicular HA crystals are successfully regenerated on the surface (Kwak et al., 2017). Inspired by these structural domains, amelogenin analogs are designed and synthesized to induce in vitro bionic remineralization. After grafting different fragments, these synthetic functional peptides can be easier to obtain and show certain functional enhancements, such as adsorption ability.
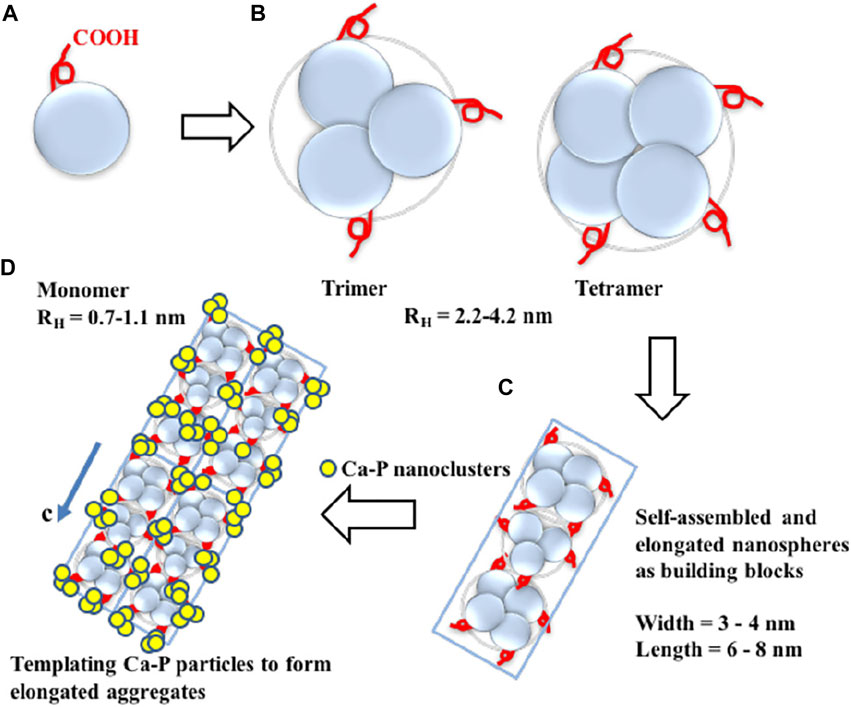
FIGURE 5. Diagram of amelogenin C-terminal peptides self-assembly to guide the extension of OCP crystallization. (A) The monomeric structure of amelogenin’s C-terminal peptide. The red curve represents the -COOH terminus. (B) Hydrophobic interactions lead to the formation of oligomeric amelogenin’s C-terminal peptides. (C) Nanorod structures as building blocks are formed by the association of oligomers. (D) Elongated organic-inorganic complex aggregates formed by the building blocks nanorods and CaP nanoclusters. Reprinted with permission from [ (Wu et al., 2017) ]. Copyright © 2017 American Chemical Society.
Non-amelogenins work in early enamel formation, including enamelin and tuftelin. Enamelin acts as a transport and nucleation protein that affects amelogenin to regulate early enamel development (Bartlett et al., 2006; Lacruz et al., 2017; Yan et al., 2017). Tuftelin is an acidic protein produced by ameloblasts during the early stages of enamel formation. It is concentrated near the dentin-enamel intersection, in which enamel mineralization begins. The tuftelin-derived peptide (TDP) is created based on the structure of tuftelin. The group repaired by TDP demonstrate comparable enamel hardness and lesion depth healing results after pH cycling to NaF groups (Ding et al., 2020).
Functional peptides inspired by bioproteins can in some ways replicate the unique functions of these bioproteins, as well as easier access. Assembly of these peptides with different functions can produce multifunctional peptides, such as peptides with high enamel binding and remineralization capacity or peptides with antibacterial and remineralization activities (Table 1).
Amelogenin analogs are created by mimicking the functional domain of amelogenin. These synthetic peptides outperform full-length amelogenin in synthesis, purification, and retention (Gungormus et al., 2012; Dogan et al., 2018). The focus of recreating enamel structure and function in vitro is inducing columns growth and elongation directly, which is predominantly regulated by the C-tail. Therefore, peptides with C-terminal can stimulate remineralization in vitro. Amelogenin inspired peptides of 26 and 32 amino acid residues (P26 and P32) with hydrophilic inner N- and C-terminal are produced to mimic the “nanosphere” structure in the enamel matrix (Mukherjee et al., 2018). A firm mineralized layer is successfully produced on the enamel surface after 7 days of in-situ culturing with polypeptide solution. C-axis oriented nanorods are generated on the enamel surface by repeating the peptide application process. P32 can restore the hardness of etched enamel better because the crystals created by P26 are smaller than those produced by P32. A chimeric peptide is created by grafting the C-terminal onto HA6-1, which can be selectively attached to the enamel surface (Xiao et al., 2017). The C-terminals of the chimeric peptide increases the peptide adsorption and facilitates the formation of a mechanically strong remineralized layer. QP5 is consisting of five highly conserved Gln-Pro-X repeat sequence in the center region and a hydrophilic C-tail (Lv et al., 2015; Chu et al., 2018; Ren et al., 2018; Li et al., 2020). When compared to amino acids, QP5 has a better remineralization impact, which effectively restored enamel surface hardness and reduced surface roughness value (Li et al., 2020; Wang Y. et al., 2020). Moreover, QP5 can enhance remineralization in a complicated oral environment, as demonstrated by the rat caries model (Han et al., 2017). Shortened amelogenin derived peptide 5 (shADP5) is employed as an active ingredient to generate a mineralized layer in solution. The enamel surface is healed after 1 h of mineralization, and the average hardness and elastic modulus are higher than control samples, with the hardness of 2.23 ± 0.23 GPa vs. 2.10 ± 0.26 GPa and elastic modulus of 58.6 ± 4.7 GPa vs. 55.1 ± 4.3 GPa (Dogan et al., 2018). A phase transfer lysozyme (PTL) membrane can be used to simulate the N-terminal of amelogenin (Wang D. et al., 2020). After the occurrence of amyloid aggregation, the internal structure of lysozyme is changed: the α-helixes unravel and the β-sheets is formed through hydrophobic interactions, which is similar to the spatial phase shift of amelogenin self-assembling. At the liquid/solid interface, those β-sheet-rich proteins are quickly organized into nanoparticles, forming a nanofilm that could be adsorbed on the enamel surface and serve as a scaffold for subsequent remineralization. The hydrophilic C-tail is then grafted onto PTL to produce PTL/C-AMG, which can guide HA growing in a direction. A 2.0–2.8 μm thick remineralization layer is produced after applying 1 mg/ml PTL/C-AMG to demineralized tooth slices for 7 days. These remineralized layers have similar properties to natural enamel with a “fish-scale” structure.
Statherin, a tyrosine-rich peptide with 43 amino acid residues, is a salivary protein that is found in the oral acquired membrane. Because of a unique combination of high negative charged domains on the N-terminal and enamel surface, statherin can securely cling to the enamel surface (Raj et al., 1992; Gururaja and Levine, 1996). To replicate the property of high HA binding, several peptides derived from statherin are created (Shuturminska et al., 2017; Luo et al., 2019; Carvalho et al., 2020). Separating the N-terminal of statherin can yield the peptide SN15 (Dodds et al., 2005; Shimotoyodome et al., 2006; Luo et al., 2019). Grafting SN15 onto PAMAM can improve its absorption on enamel surface (Gao et al., 2020). The statherin stimulated peptide and tannic acid are used to make SAP-TA (Yang et al., 2017). Polyphenol groups in TA can grab Ca2+ and trigger HA crystal renewal. Iron ions work in tandem with SAP-TA to generate a thick layer that boosts adsorption capacity. Therefore, SAP-TA/Fe (III) can improve the adhesion and mechanical properties of the remineralization interface (surface microhardness recovery >80%, binding force 64.85 N). Peptide-7 is designed and synthesized with a significant number of carboxyl groups on its side chain to help in firmly interacting with HA and directional elongation of HA crystals (Liu et al., 2018). Under the guidance of Peptide 7, a dense mineralized crystal layer with tight adhesion was formed.
Bioactive peptides have antibacterial and remineralization properties, which can be obtained by grafting units capable of promoting remineralization onto the active sequences of antimicrobial peptides. This bioactive peptide can protect enamel against demineralization while also promoting self-healing regeneration in a remineralized environment. P-113 is the smallest antibacterial unit of histatin 5, which is a type of natural antimicrobial peptide (Zhou et al., 2020). A study coupled dopamine (DA), SpSp (DPS) domains, and binding peptide binding peptide SKHKGGKHKGGKHKG on P-113 to find the most cost-effective peptide (Zhou et al., 2021). The experiment has discovered that P-113-DPS show similar antibacterial effect to Sp-H5 and can kill the majority of Streptococcus mutans (S. mutans) at low concentrations. After a 24-hour remineralization experiment, an 8.5 μm thick needle-like remineralization layer is formed on the enamel surface in the P -113-DPS group, twice as thick as the control group (4 μm) (Figure 6). The low-complexity protein segments (LCPSs) 37SYSGYS42 in the fused in sarcoma protein is capable of forming nucleation structures that form reversible amyloid fibrils (Hughes et al., 2018). LCPSs are highly hydrophilic and structurally flexible. Due to weak multivalent interactions, proteins are entangled and subsequently form web-like structures. LCPSs containing a phosphate or phosphonate group is named LCPS-OP and LCPS-CP. These acidified polypeptides can bind calcium ions and acts as soft templates to induce HA formation. At the same time, the hydrophilic negatively charged peptide coating can reduce the bacterial adhesion of caries-causing bacteria by virtue of the negative electric mutual repulsion (Chang et al., 2022). Given that this bioactive peptide may more effectively repair damaged enamel while inhibiting further erosion of dental cariogenic bacteria, it may be an ideal material for the prevention of dental caries. In addition, some antimicrobial materials have been added to the remineralization system to promote enamel remineralization. The first type of materials can cover the enamel surface with an antifouling layer, and the second type can be used to destroy the bacterial biomass through the positive charges. Table 2 summarizes these materials that combine antimicrobial and remineralization functions and Figure 7 shows their modes.
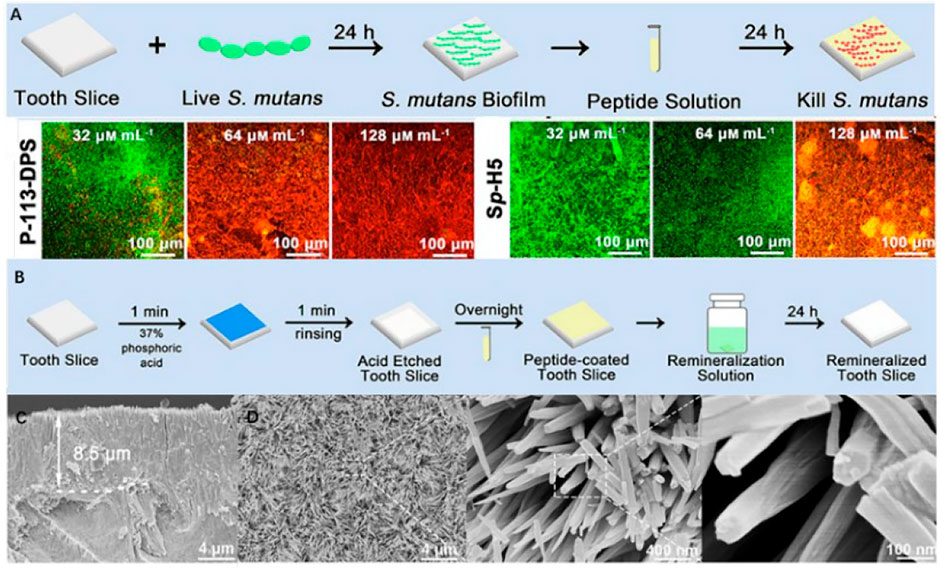
FIGURE 6. (A) Schematic diagram of anti-S. mutans biofilm on the enamel surface and fluorescence images (×20) of S. mutans biofilm in various concentrations of P-113-DPS and Sp-H5 solutions. (B) Remineralization experiment schematic. (C) 8.5 μm needle-like remineralized layers formed by P-113- remineralized layers and (D) its FE-SEM micrographs (×5000, ×50,000, and ×200,000). Reprinted with permission from [ (Zhou et al., 2021) ]. Copyright © 2021 American Chemical Society.
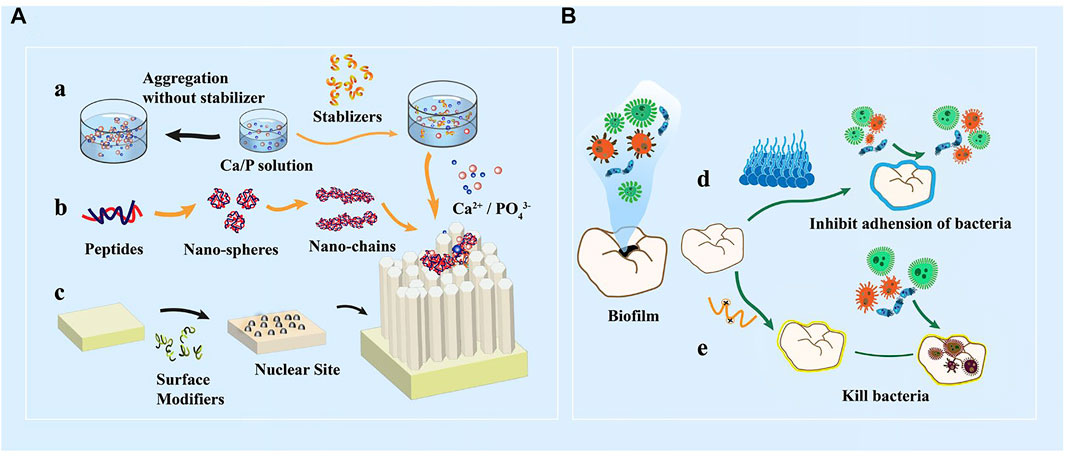
FIGURE 7. (A) In vitro peptide-induced bionic remineralization process. a) Organic matter stabilized calcium and phosphorus solution provides sufficient amount of ions b) Self-assembly of peptides into nanospheres, nano-chain structures orderly guide the deposition of calcium and phosphorus ions and extension of ordered orientation c) Organic matter modifies the substrate surface to form nucleation sites. (B) Mechanism of action of antibacterial anti-caries materials. d) Enamel surface coating reduces microbial adhesion; e) Positively charged peptides direct killing of caries-causing bacteria.
Dentin phosphoprotein, made up of a significant amount of aspartate serine repeat sequences that have a strong affinity for HA, can act as a nucleation template in the process of dentin mineralization (He and George, 2004). Therefore, inspired by dentin phosphoprotein, the peptide 8DSS containing eight DSS repetitions is synthesized. 8DSS can capture Ca2+ and act as a diffusion barrier that prevent CaP from dissolving. In the repair of both surface cavities and deep lesions, 8DSS demonstrated equal remineralization ability to NaF (Yang et al., 2014; Liang et al., 2015; Zheng et al., 2019). In addition, 8DSS is able to resist hydrolase assault and sustain its action in the mouth due to the short peptide chain length, which is conductive to clinical application.
Self-assembly peptide P11-4 is a well-studied small molecule peptide. When activated by external stimuli, P11-4 can self-assemble through intermolecular hydrogen bonds between peptide backbones and form three-dimensional scaffolds in lesions (Alkilzy et al., 2018a). At this time, the negative group formed by 4 Glu-residues on P11-4 can attract calcium ions and induce mineralization. According to µCT imaging, the mineralization of the samples treated by P11-4 increase by 68% in 14 days (Kind et al., 2017). It is worth noting that P11-4 guided remineralization occurs in the subsurface of lesions, which can compensate for the shortcomings of fluoride. As a result, the combination of P11-4 and fluoride varnish can produce good results in clinical applications. In an experiment on children over 5 years old with obvious active early caries, P11-4 + fluoride varnish are superior to fluoride in terms of vision and safety (Alkilzy et al., 2018b). In some other clinical trials, P11-4 successfully treated white spot lesions caused by enamel demineralization and significantly improved the appearance (Doberdoli et al., 2020; Jablonski-Momeni et al., 2020; Sedlakova Kondelova et al., 2020).
Alendronate (ALN), a powerful bone resorption inhibitor with a high affinity for HA, is used to treat and prevent osteoporosis (Chen S. et al., 2020). The phosphate of ALN exchanges with the phosphate of HA in enamel, forming coordination chains that tightly bind it to the enamel surface (Palazzo et al., 2007). Therefore, ALN can act as a “glue” in the mineralization system to increase the adsorption of materials. ALN modified poly (amino amine) dendrimers (Wu et al., 2013) and carboxymethyl chitosan (Wang et al., 2017) can significantly increase their absorption on the enamel surface. In addition, after forming a HA layer around the ALN-modified polyacrylic acid (PAA), the outer layer of HA is zinc-modified to synthesize ZHA@ALN (Xu et al., 2020). Once ZHA@ALN dissolved by acid, a substantial number of calcium, phosphorus, and zinc ions can be released for remineralization and sterilizing. The inner layer of ALN-PAA adheres quickly to the enamel surface due to the ALN. Then, PAA serves as an antifouling layer with 75.05% bacteriostatic efficiency.
Polymer materials have complex side groups and spatial structures, which enable them to mimic the enamel matrix and induce mineralization. Some are used to keep ions stable, and some can be made into gel carriers to transport functional peptides while forming a protective layer on the enamel surface. Due to their high biocompatibility and adaptability, polymer materials can be an ideal choice for promoting enamel remineralization.
Non-collagenous proteins (NCPs) stabilize crystal precursors during dentin and bone collagen mineralization. NCP analogs, such as polyaspartic acid (pAsp), polyglutamic acid (PGA), and biocompatible polymers polyacrylic acid (PAA), contain a large number of carboxyl groups. These polymers can be used to create induced liquid precursors by stabilizing calcium ions. PGA and pAsp can also bind to calcium ions on the enamel surface, strengthening adhesion and providing nucleation sites (Ustriyana et al., 2020a; Ustriyana et al., 2020b). It has been discovered that the α-helical of pAsp or PGA can promote HA crystal nucleation by templating Ca2+ distribution. The HPO and -COO- can work together to attract Ca2+ and form stable Ca2+ triangles, which can develop into the nucleation core of ACP (Zeng et al., 2021). PAA can also chelate with Ca2+ while maintaining liquid phase stability and transporting ions continuously for subsequent biomineralization (Chen R. et al., 2020; Xu et al., 2020; Li N. et al., 2021). Furthermore, PAA can direct the transformation of ACP to form acicular microcrystals (Wang et al., 2018).
Poly (amino amine) (PAMAM) is a kind of synthetic protein with a dendritic structure. PAMAM can self-assemble into nanospheres, nanochains, and nanoribbons (Yang et al., 2015). Grafting different groups such as -NH2, -COOH and -OH onto PAMAM can improve its ability to bind Ca2+or promote its adsorption on the enamel surface. The mineralization effects decrease in the order of -NH2 > -COOH > -OH (Fan et al., 2020). This is because positive charged PAMAM-NH2 can be more adsorbed on the negatively charged enamel surface. In addition, the adherence of S. mutans is also evaluated. Both PAMAM-COOH and PAMAM-NH2 are shown to be effective in forming a smooth remineralized layer and minimizing S. mutans adherence (Jia et al., 2020). Grafted with SN15, SN15-PAMAM can increase adsorption on the enamel surface and achieve 90% higher mineralization effect than the control group. (Gao et al., 2020).
Polydopamine (PDA), the polymer of dopamine that rich in amino and phenolic groups, shows great hydrophilicity. It has been used as a functional agent to increase the wettability and biocompatibility of substrate (Barclay et al., 2017; Ghorbani et al., 2019). After being immersed in PDA solution, a dense film can be formed on the surface of the material in a short time (Kaushik et al., 2020). The film contains a large number of charged groups, to which calcium and phosphorus ions will be attracted and form a stable bond (Ryu et al., 2010; Murari et al., 2020). It is also observed that the HA crystals on the PDA modified enamel surface accumulated more closely, suggesting that PDA might help in inducing uniform crystal nucleation (Zhou et al., 2012). This may be because PDA can increase surface hydrophilicity, decrease the interfacial energy, and accelerate crystallization speed of HA (Qu et al., 2020). Based on the super adhesion of PDA, HA layer can be synthesized on the subsurface of different materials after being modified (Chen et al., 2019; Wong et al., 2022).
Biopolymers, including proteins and polysaccharides, have been used for the bionic formation of HA. Most of these polymers are mostly used in mineralized systems in the form of gels. All of these biopolymers show excellent non-immune and biocompatible properties, meanwhile with the advantages of easy storage and clinic application.
Chitosan (CS), a cationic polysaccharide, can rarely produce allergic or inflammatory reactions in humans (Younes and Rinaudo, 2015). Therefore, CS has been used to construct organic templates and scaffolds, which can ensure the bioactivity of peptides for mineralization guidance (Ruan et al., 2016; Ren et al., 2018). Furthermore, CS is also able to prevent bacteria from adhering and reproducing. The adherence of S. mutans may be decreased by 94.91% by employing CS alone (Ren et al., 2019). This is because chitosan is positively charged. When CS comes into touch with the negatively charged bacterial wall, the structure of the bacterial wall would be disrupted. Simultaneously, positive charged CS can adhere to the negatively charged enamel surface, preventing acid erosion (He et al., 2019; Boda et al., 2020). In addition, the antibacterial function can be enhanced when CS paired with fluoride (Wassel and Khattab, 2017; Ren et al., 2019). Carboxymethyl chitosan (CMC), formed by CS carboxylation, also has excellent ACP stabilization and can promote the formation of enamel remineralization layers. (Chen et al., 2015; Xiao et al., 2017). The mineralization system using chitosan as a gel carrier is summarized in Table 3.
Agarose is a natural polysaccharide with -OH groups that can form a thermally reversible gel. Agarose aqueous gel is widely used in medical systems such as mineral regeneration and drug delivery (Zarrintaj et al., 2018). The abundant hydroxyl groups in agarose molecules have a strong mutual attraction with Ca2+, allowing agarose to control the formation of nano ACP precursors and act as an ion reservoir to transport mineral precursors to the enamel surface for mineral mesomorphic transformation. The average elastic modulus and nano hardness of enamel increased significantly to 89.46 ± 11.82 and 3.04 ± 0.75 GPA after 6 days of the interaction of agarose gel with 500 ppm F (Cao et al., 2014a). When chitosan is added to the agarose aqueous gel, the groups between the two gels are cross-linked with each other, forming a fiber structure together with calcium ions, which can further simulate the protein matrix for enamel repair. The regeneration layer is 7.5–8.5 μm thick and regained 77.4% of the natural enamel’s microhardness (Musat et al., 2021). Agarose can also be combined with amelogenin to form oriented hexagonal prism enamel columns on the enamel surface (Cao et al., 2014b).
Gelatin is a peptide molecular polymer. Gel peptides in gelatin can form salt bonds with phosphate groups on the surface of apatite, causing enamel-like minerals to regenerate. The limited directional diffusion of ions in the gel environment promotes heterogeneous nucleation, resulting in a crystal with a clear structure (Zhang et al., 2010). Using the bionic double-layer gel system assisted by anodic aluminum oxide, it is possible to successfully prepare HA crystals with good orientation (Chen et al., 2019).
Silk fibroin (SF) governs the synthesis of calcium carbonate in mollusks and the creation of animal shells. SF contains a large number of β-sheets, which are rich in acid aspartic acid and have a high affinity for calcium ions. In the rotary thermal evaporation approach, SF serves as a template to guide heterogeneous nucleation of HA and mineral layers with natural enamel-like shape, organization, and mechanical characteristics swiftly formed (Wang S. et al., 2020).
Abalone water-soluble matrix (AWSM) plays an important role in the formation abalone shells. The proportion of organic matter and inorganic minerals in abalone shells is very similar to enamel (95% calcium carbonate and less than 5% organic matter). Therefore, AWSM can promote the formation of crystals. High AWSM concentration can increase the content of calcium and phosphate in the mineralized layer and promote to form a parallel, dense and highly ordered structure (Wen et al., 2016).
Carboxy betaine (CB) polymers are amphoteric polymers with functional carboxyl and quaternary ammonium groups. The carboxyl groups can serve as Ca2+ and PO43− deposition sites, whereas the positive quaternary amino group has bactericidal properties (Xu et al., 2018). Furthermore, CB can resist bacterial adhesion via electrostatically induced hydration. ACP can be stabilized by amphoteric ionic poly (carboxy betaine acrylamide) (PCBAA). PCBAA/ACP nanocomposites contribute to the growth of HAP in the damaged sublayer of enamel by blocking spontaneous ion conversion on the enamel surface while releasing sufficient ions (He et al., 2022). Thus, PCBAA/ACP nanocomposites performed admirably in both remineralization (10.08 μm thick remineralized layer in mice intraoral for 14 days) and antimicrobial experiments (almost no bacterial adherence). ACP and poly (vinylpyrrolidone) nanofibers are mixed, and making electrospun mats. The mats can be hydrated to form a gel in the savila containing fluoride. Then, calcium and phosphorus ions crystallize under the guidance of fluorine ions to form HA (Fletcher et al., 2011).
Enamel caries have been common problem in our daily life, great efforts have been paid to design new materials and realize the remineralization of enamel. However, it is still a great challenge to repair the defect enamel and restore its functions, as for the emerging materials for enamel remineralization, there is still a long way to go to satisfy the clinic applications. Firstly, most of the current materials used for the remineralization still need a long time, from several days to more than 10 days, secondly, the stability and mechanical properties are not satisfying enough, in addition, most of the remineralization systems depends a lot on the solution or concentration of mineralization medium. Therefore, it is critical important to design advanced materials that can be used in enamel remineralization and solve the clinic problems.
In the next decades, materials, both inorganic materials or polymers that can promote the mineralization speed, especially which could tune the alignment of mineralized apatite along the native mineral structure or composition, should be a charming field, besides, on considering the complicated oral environment, bacterial infections also threaten the treatment of dental health, therefore, materials with multifunction should also be designed and may pave the way of enamel remineralization. In addition, further researches in the remineralization mechanisms are also much important, which may be helpful to direct the design of new materials and their final applications.
Conceptualization, JW and ZL; writing-original draft preparation, JX, HS, and JL; writing—review and editing, JW, HS, HY, PW; supervision, ZL; project administration, ZL and JW; funding acquisition, ZL and JW. All authors are involved in the revision and approved the submitted manuscript.
This work is supported by the financial support from the National Natural Science Foundation of China (No.52163016&81960205), Jiangxi Key Research and Development Program (20202BBG73012), Jiangxi Provincial program for the academic and technological leaders of main subjects (20213BCJL22051), and the Technological Plan of Health Commission of Jiangxi Province of China (SKJP520204072).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdallah, M. N., Eimar, H., Bassett, D. C., Schnabel, M., Ciobanu, O., Nelea, V., et al. (2016). Diagenesis-inspired reaction of magnesium ions with surface enamel mineral modifies properties of human teeth. Acta Biomater. 37, 174–183. doi:10.1016/j.actbio.2016.04.005
Agnello, M., Cen, L., Tran, N. C., Shi, W., McLean, J. S., and He, X. (2017). Arginine improves pH homeostasis via metabolism and microbiome modulation. J. Dent. Res. 96 (8), 924–930. doi:10.1177/0022034517707512
Alkilzy, M., Santamaria, R. M., Schmoeckel, J., and Splieth, C. H. (2018a). Treatment of carious lesions using self-assembling peptides. Adv. Dent. Res. 29 (1), 42–47. doi:10.1177/0022034517737025
Alkilzy, M., Tarabaih, A., Santamaria, R. M., and Splieth, C. H. (2018b). Self-assembling peptide P11-4 and fluoride for regenerating enamel. J. Dent. Res. 97 (2), 148–154. doi:10.1177/0022034517730531
Bai, Y., Yu, Z., Ackerman, L., Zhang, Y., Bonde, J., Li, W., et al. (2020). Protein nanoribbons template enamel mineralization. Proc. Natl. Acad. Sci. U. S. A. 117 (32), 19201–19208. doi:10.1073/pnas.2007838117
Barclay, T. G., Hegab, H. M., Clarke, S. R., and Ginic-Markovic, M. (2017). Versatile surface modification using polydopamine and related polycatecholamines: Chemistry, structure, and applications. Adv. Mat. Interfaces 4 (19), 1601192. doi:10.1002/admi.201601192
Barrera-Ortega, C. C., Vázquez-Olmos, A. R., Sato-Berrú, R. Y., and Araiza-Téllez, M. A. (2020). Study of demineralized dental enamel treated with different fluorinated compounds by Raman spectroscopy. J. Biomed. Phys. Eng. 10 (5), 635–644. doi:10.31661/jbpe.v0i0.2003-1089
Bartlett, J. D. (2013). Dental enamel development: Proteinases and their enamel matrix substrates. ISRN Dent. 2013, 1–24. doi:10.1155/2013/684607
Bartlett, J. D., Ganss, B., Goldberg, M., Moradian-Oldak, J., Paine, M. L., Snead, M. L., et al. (2006). 3. Protein-protein interactions of the developing enamel matrix. Curr. Top. Dev. Biol. 74, 57–115. doi:10.1016/S0070-2153(06)74003-0
Bijle, M. N., Abdalla, M. M., Ashraf, U., Ekambaram, M., and Yiu, C. K. Y. (2021a). Enamel remineralization potential of arginine-fluoride varnish in a multi-species bacterial pH-cycling model. J. Dent. 104, 103528. doi:10.1016/j.jdent.2020.103528
Bijle, M. N. A., Yiu, C. K. Y., and Ekambaram, M. (2018). Calcium-based caries preventive agents: A meta-evaluation of systematic reviews and meta-analysis. J. Evid. Based Dent. Pract. 18 (3), 203–217.e4. doi:10.1016/j.jebdp.2017.09.003
Bijle, M. N., Ekambaram, M., Lo, E. C., and Yiu, C. K. Y. (2020). The enamel remineralization potential of fluoride varnishes containing arginine. J. Dent. (Shiraz). 99, 103411. doi:10.1016/j.jdent.2020.103411
Bijle, M. N., Pichika, M. R., Mak, K. K., Parolia, A., Babar, M. G., Yiu, C., et al. (2021b). Concentration-dependent multi-potentiality of L-arginine: Antimicrobial effect, hydroxyapatite stability, and MMPs inhibition. Molecules 26 (21), 6605. doi:10.3390/molecules26216605
Boda, S. K., Fischer, N. G., Ye, Z., and Aparicio, C. (2020). Dual oral tissue adhesive nanofiber membranes for pH-responsive delivery of antimicrobial peptides. Biomacromolecules 21 (12), 4945–4961. doi:10.1021/acs.biomac.0c01163
Bossu, M., Saccucci, M., Salucci, A., Di Giorgio, G., Bruni, E., Uccelletti, D., et al. (2019). Enamel remineralization and repair results of biomimetic hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotechnology 17 (1), 17. doi:10.1186/s12951-019-0454-6
Bowen, W. H., Burne, R. A., Wu, H., and Koo, H. (2018). Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26 (3), 229–242. doi:10.1016/j.tim.2017.09.008
Buchko, G. W., Jayasinha Arachchige, R., Tao, J., Tarasevich, B. J., and Shaw, W. J. (2018). Identification of major matrix metalloproteinase-20 proteolytic processing products of murine amelogenin and tyrosine-rich amelogenin peptide using a nuclear magnetic resonance spectroscopy based method. Arch. Oral Biol. 93, 187–194. doi:10.1016/j.archoralbio.2018.06.001
Cao, Y., Mei, M. L., Li, Q. L., Lo, E. C., and Chu, C. H. (2014a). Agarose hydrogel biomimetic mineralization model for the regeneration of enamel prismlike tissue. ACS Appl. Mat. Interfaces 6 (1), 410–420. doi:10.1021/am4044823
Cao, Y., Mei, M. L., Li, Q. L., Lo, E. C., and Chu, C. H. (2014b). Enamel prism-like tissue regeneration using enamel matrix derivative. J. Dent. (Shiraz). 42 (12), 1535–1542. doi:10.1016/j.jdent.2014.08.014
Carneiro, K. M., Zhai, H., Zhu, L., Horst, J. A., Sitlin, M., Nguyen, M., et al. (2016). Amyloid-like ribbons of amelogenins in enamel mineralization. Sci. Rep. 6, 23105. doi:10.1038/srep23105
Carvalho, T. S., Araujo, T. T., Ventura, T. M. O., Dionizio, A., Camara, J. V. F., Moraes, S. M., et al. (2020). Acquired pellicle protein-based engineering protects against erosive demineralization. J. Dent. (Shiraz). 102, 103478. doi:10.1016/j.jdent.2020.103478
Chang, R., Liu, Y. J., Zhang, Y. L., Zhang, S. Y., Han, B. B., Chen, F., et al. (2022). Phosphorylated and phosphonated low‐complexity protein segments for biomimetic mineralization and repair of tooth enamel. Adv. Sci. 9 (6), 2103829. doi:10.1002/advs.202103829
Chen, R., Jin, R., Li, X., Fang, X., Yuan, D., Chen, Z., et al. (2020). Biomimetic remineralization of artificial caries dentin lesion using Ca/P-PILP. Dent. Mat. 36, 1397–1406. doi:10.1016/j.dental.2020.08.017
Chen, S., Guo, R., Xie, C., Liang, Q., and Xiao, X. (2020). Biomimetic mineralization of nanocrystalline hydroxyapatites on aminated modified polylactic acid microspheres to develop a novel drug delivery system for alendronate. Mater. Sci. Eng. C 110, 110655. doi:10.1016/j.msec.2020.110655
Chen, Z., Cao, S., Wang, H., Li, Y., Kishen, A., Deng, X., et al. (2015). Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries. PLoS One 10 (1), e0116553. doi:10.1371/journal.pone.0116553
Chen, Z., Miao, Z., Zhang, P., Xiao, H., Liu, H., Ding, C., et al. (2019). Bioinspired enamel-like oriented minerals on general surfaces: Towards improved mechanical properties. J. Mat. Chem. B 7 (34), 5237–5244. doi:10.1039/c9tb00676a
Chong, L. Y., Clarkson, J. E., Dobbyn-Ross, L., and Bhakta, S. (2018). Slow-release fluoride devices for the control of dental decay. Cochrane Database Syst. Rev. 3, CD005101. doi:10.1002/14651858.CD005101.pub4
Chu, Q., Gao, Y., Gao, X., Dong, Z., Song, W., Xu, Z., et al. (2018). Ablation of Runx2 in ameloblasts suppresses enamel maturation in tooth development. Sci. Rep. 8 (1), 9594. doi:10.1038/s41598-018-27873-5
Clark, M. B., Keels, M. A., Slayton, R. L., Section On Oral, H., Fisher-Owens, S. A., Huff, Q. A., et al. (2020). Fluoride use in caries prevention in the primary care setting. Pediatrics 146 (6), e2020034637. doi:10.1542/peds.2020-034637
Cochrane, N. J., Cai, F., Huq, N. L., Burrow, M. F., and Reynolds, E. C. (2010). New approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 89 (11), 1187–1197. doi:10.1177/0022034510376046
Delgado-López, J. M., Frison, R., Cervellino, A., Gómez-Morales, J., Guagliardi, A., and Masciocchi, N. (2014). Crystal size, morphology, and growth mechanism in bio-inspired apatite nanocrystals. Adv. Funct. Mat. 24 (8), 1090–1099. doi:10.1002/adfm.201302075
Ding, C., Chen, Z., and Li, J. (2017). From molecules to macrostructures: Recent development of bioinspired hard tissue repair. Biomater. Sci. 5 (8), 1435–1449. doi:10.1039/c7bm00247e
Ding, L., Han, S., Peng, X., Wang, K., Zheng, S., Li, H., et al. (2020). Tuftelin-derived peptide facilitates remineralization of initial enamel caries in vitro. J. Biomed. Mat. Res. 108 (8), 3261–3269. doi:10.1002/jbm.b.34663
Dissanayake, S. S. M., Ekambaram, M., Li, K. C., Harris, P. W. R., and Brimble, M. A. (2020). Identification of Key functional motifs of native amelogenin protein for dental enamel remineralisation. Molecules 25 (18), 4214. doi:10.3390/molecules25184214
Doberdoli, D., Bommer, C., Begzati, A., Haliti, F., Heinzel-Gutenbrunner, M., and Juric, H. (2020). Randomized clinical trial investigating self-assembling peptide P11-4 for treatment of early occlusal caries. Sci. Rep. 10 (1), 4195. doi:10.1038/s41598-020-60815-8
Dodds, M. W., Johnson, D. A., and Yeh, C. K. (2005). Health benefits of saliva: A review. J. Dent. (Shiraz). 33 (3), 223–233. doi:10.1016/j.jdent.2004.10.009
Dogan, S., Fong, H., Yucesoy, D. T., Cousin, T., Gresswell, C., Dag, S., et al. (2018). Biomimetic tooth repair: Amelogenin-derived peptide enables in vitro remineralization of human enamel. ACS Biomater. Sci. Eng. 4 (5), 1788–1796. doi:10.1021/acsbiomaterials.7b00959
Dorri, M., Martinez-Zapata, M. J., Walsh, T., Marinho, V. C., Sheiham Deceased, A., and Zaror, C. (2017). Atraumatic restorative treatment versus conventional restorative treatment for managing dental caries. Cochrane Database Syst. Rev. 12, CD008072. doi:10.1002/14651858.CD008072.pub2
Engelberth, S. A., Bacino, M. S., Sandhu, S., Li, W., Bonde, J., and Habelitz, S. (2018). Progression of self-assembly of amelogenin protein supramolecular structures in simulated enamel fluid. Biomacromolecules 19 (10), 3917–3924. doi:10.1021/acs.biomac.8b00808
Fan, M., Zhang, M., Xu, H. H. K., Tao, S., Yu, Z., Yang, J., et al. (2020). Remineralization effectiveness of the PAMAM dendrimer with different terminal groups on artificial initial enamel caries in vitro. Dent. Mat. 36 (2), 210–220. doi:10.1016/j.dental.2019.11.015
Fang, P. A., Conway, J. F., Margolis, H. C., Simmer, J. P., and Beniash, E. (2011). Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc. Natl. Acad. Sci. U. S. A. 108 (34), 14097–14102. doi:10.1073/pnas.1106228108
Fletcher, J., Walsh, D., Fowler, C. E., and Mann, S. (2011). Electrospun mats of PVP/ACP nanofibres for remineralization of enamel tooth surfaces. CrystEngComm 13 (11), 3692. doi:10.1039/c0ce00806k
Fukae, M., Tanabe, T., Uchida, T., Lee, S. K., Ryu, O. H., Murakami, C., et al. (1998). Enamelysin (matrix metalloproteinase-20): Localization in the developing tooth and effects of pH and calcium on amelogenin hydrolysis. J. Dent. Res. 77 (8), 1580–1588. doi:10.1177/00220345980770080501
Gao, Y., Liang, K., Weir, M. D., Gao, J., Imazato, S., Tay, F. R., et al. (2020). Enamel remineralization via poly(amido amine) and adhesive resin containing calcium phosphate nanoparticles. J. Dent. 92, 103262. doi:10.1016/j.jdent.2019.103262
Gelli, R., Ridi, F., and Baglioni, P. (2019). The importance of being amorphous: Calcium and magnesium phosphates in the human body. Adv. Colloid Interface Sci. 269, 219–235. doi:10.1016/j.cis.2019.04.011
Ghorbani, F., Zamanian, A., Behnamghader, A., and Joupari, M. D. (2019). A facile method to synthesize mussel-inspired polydopamine nanospheres as an active template for in situ formation of biomimetic hydroxyapatite. Mater. Sci. Eng. C 94, 729–739. doi:10.1016/j.msec.2018.10.010
Gil-Bona, A., and Bidlack, F. B. (2020). Tooth enamel and its dynamic protein matrix. Int. J. Mol. Sci. 21 (12), 4458. doi:10.3390/ijms21124458
Gordon, L. M., Cohen, M. J., MacRenaris, K. W., Pasteris, J. D., Seda, T., and Joester, D. (2015). Amorphous intergranular phases control the properties of rodent tooth enamel. Science 347 (6223), 746–750. doi:10.1126/science.1258950
Green, D. R., Schulte, F., Lee, K. H., Pugach, M. K., Hardt, M., and Bidlack, F. B. (2019). Mapping the tooth enamel proteome and amelogenin phosphorylation onto mineralizing porcine tooth crowns. Front. Physiol. 10, 925. doi:10.3389/fphys.2019.00925
Gungormus, M., Oren, E. E., Horst, J. A., Fong, H., Hnilova, M., Somerman, M. J., et al. (2012). Cementomimetics-constructing a cementum-like biomineralized microlayer via amelogenin-derived peptides. Int. J. Oral Sci. 4 (2), 69–77. doi:10.1038/ijos.2012.40
Gururaja, T. L., and Levine, M. J. (1996). Solid-phase synthesis and characterization of human salivary statherin: A tyrosine-rich phosphoprotein inhibitor of calcium phosphate precipitation. Pept. Res. 9 (6), 283–289.
Han, S., Fan, Y., Zhou, Z., Tu, H., Li, D., Lv, X., et al. (2017). Promotion of enamel caries remineralization by an amelogenin-derived peptide in a rat model. Arch. Oral Biol. 73, 66–71. doi:10.1016/j.archoralbio.2016.09.009
Harper, R. A., Shelton, R. M., James, J. D., Salvati, E., Besnard, C., Korsunsky, A. M., et al. (2021). Acid-induced demineralisation of human enamel as a function of time and pH observed using X-ray and polarised light imaging. Acta Biomater. 120, 240–248. doi:10.1016/j.actbio.2020.04.045
He, G., and George, A. (2004). Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J. Biol. Chem. 279 (12), 11649–11656. doi:10.1074/jbc.M309296200
He, J., Bao, Y., Li, J., Qiu, Z., Liu, Y., and Zhang, X. (2019). Nanocomplexes of carboxymethyl chitosan/amorphous calcium phosphate reduce oral bacteria adherence and biofilm formation on human enamel surface. J. Dent. (Shiraz). 80, 15–22. doi:10.1016/j.jdent.2018.11.003
He, J., Yang, J., Li, M., Li, Y., Pang, Y., Deng, J., et al. (2022). Polyzwitterion manipulates remineralization and antibiofilm functions against dental demineralization. ACS Nano 16 (2), 3119–3134. doi:10.1021/acsnano.1c10812
He, K., Sawczyk, M., Liu, C., Yuan, Y., Song, B., Deivanayagam, R., et al. (2020). Revealing nanoscale mineralization pathways of hydroxyapatite using in situ liquid cell transmission electron microscopy. Sci. Adv. 6 (47), eaaz7524. doi:10.1126/sciadv.aaz7524
Hou, A., Luo, J., Zhang, M., Li, J., Chu, W., Liang, K., et al. (2020). Two-in-one strategy: A remineralizing and anti-adhesive coating against demineralized enamel. Int. J. Oral Sci. 12 (1), 27. doi:10.1038/s41368-020-00097-y
Huang, S. B., Gao, S. S., and Yu, H. Y. (2009). Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed. Mat. 4 (3), 034104. doi:10.1088/1748-6041/4/3/034104
Huang, S., Gao, S., Cheng, L., and Yu, H. (2011). Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 45 (5), 460–468. doi:10.1159/000331207
Hughes, M. P., Sawaya, M. R., Boyer, D. R., Goldschmidt, L., Rodriguez, J. A., Cascio, D., et al. (2018). Atomic structures of low-complexity protein segments reveal kinked beta sheets that assemble networks. Science 359 (6376), 698–701. doi:10.1126/science.aan6398
Iafisco, M., Ramírez-Rodríguez, G. B., Sakhno, Y., Tampieri, A., Martra, G., Gómez-Morales, J., et al. (2015). The growth mechanism of apatite nanocrystals assisted by citrate: Relevance to bone biomineralization. CrystEngComm 17 (3), 507–511. doi:10.1039/c4ce01415d
Jablonski-Momeni, A., Nothelfer, R., Morawietz, M., Kiesow, A., and Korbmacher-Steiner, H. (2020). Impact of self-assembling peptides in remineralisation of artificial early enamel lesions adjacent to orthodontic brackets. Sci. Rep. 10 (1), 15132. doi:10.1038/s41598-020-72185-2
Jia, L., Tao, S., Yang, J., Liang, K., Yu, Z., Gao, Y., et al. (2020). Adhesion of Streptococcus mutans on remineralized enamel surface induced by poly(amido amine) dendrimers. Colloids Surfaces B Biointerfaces 197, 111409. doi:10.1016/j.colsurfb.2020.111409
Jokisaari, J. R., Wang, C., Qiao, Q., Hu, X., Reed, D. A., Bleher, R., et al. (2019). Particle-attachment-mediated and matrix/lattice-guided enamel apatite crystal growth. ACS Nano 13 (3), 3151–3161. doi:10.1021/acsnano.8b08668
Jokstad, A. (2016). Secondary caries and microleakage. Dent. Mat. 32 (1), 11–25. doi:10.1016/j.dental.2015.09.006
Juntavee, N., Juntavee, A., and Plongniras, P. (2018). Remineralization potential of nano-hydroxyapatite on enamel and cementum surrounding margin of computer-aided design and computer-aided manufacturing ceramic restoration. Int. J. Nanomedicine 13, 2755–2765. doi:10.2147/IJN.S165080
Karlinsey, R. L., Mackey, A. C., Walker, E. R., and Frederick, K. E. (2010). Preparation, characterization and in vitro efficacy of an acid-modified beta-TCP material for dental hard-tissue remineralization. Acta Biomater. 6 (3), 969–978. doi:10.1016/j.actbio.2009.08.034
Karlinsey, R. L., and Pfarrer, A. M. (2012). Fluoride plus functionalized beta-TCP: A promising combination for robust remineralization. Adv. Dent. Res. 24 (2), 48–52. doi:10.1177/0022034512449463
Kaushik, N., Nhat Nguyen, L., Kim, J. H., Choi, E. H., and Kumar Kaushik, N. (2020). Strategies for using polydopamine to induce biomineralization of hydroxyapatite on implant materials for bone tissue engineering. Int. J. Mol. Sci. 21 (18), 6544. doi:10.3390/ijms21186544
Khonina, T. G., Chupakhin, O. N., Shur, V. Y., Turygin, A. P., Sadovsky, V. V., Mandra, Y. V., et al. (2020). Silicon-hydroxyapatiteglycerohydrogel as a promising biomaterial for dental applications. Colloids Surfaces B Biointerfaces 189, 110851. doi:10.1016/j.colsurfb.2020.110851
Kind, L., Stevanovic, S., Wuttig, S., Wimberger, S., Hofer, J., Muller, B., et al. (2017). Biomimetic remineralization of carious lesions by self-assembling peptide. J. Dent. Res. 96 (7), 790–797. doi:10.1177/0022034517698419
Kis, V. K., Sulyok, A., Hegedus, M., Kovacs, I., Rozsa, N., and Kovacs, Z. (2021). Magnesium incorporation into primary dental enamel and its effect on mechanical properties. Acta Biomater. 120, 104–115. doi:10.1016/j.actbio.2020.08.035
Kwak, S. Y., Litman, A., Margolis, H. C., Yamakoshi, Y., and Simmer, J. P. (2017). Biomimetic enamel regeneration mediated by leucine-rich amelogenin peptide. J. Dent. Res. 96 (5), 524–530. doi:10.1177/0022034516688659
Kwak, S. Y., Yamakoshi, Y., Simmer, J. P., and Margolis, H. C. (2016). MMP20 proteolysis of native amelogenin regulates mineralization in vitro. J. Dent. Res. 95 (13), 1511–1517. doi:10.1177/0022034516662814
La Fontaine, A., Zavgorodniy, A., Liu, H., Zheng, R., Swain, M., and Cairney, J. (2016). Atomic-scale compositional mapping reveals Mg-rich amorphous calcium phosphate in human dental enamel. Sci. Adv. 2 (9), e1601145. doi:10.1126/sciadv.1601145
Lacruz, R. S., Habelitz, S., Wright, J. T., and Paine, M. L. (2017). Dental enamel formation and implications for oral health and disease. Physiol. Rev. 97 (3), 939–993. doi:10.1152/physrev.00030.2016
Lawn, B. R., Lee, J. J. W., and Chai, H. (2010). Teeth: Among nature's most durable biocomposites. Annu. Rev. Mat. Res. 40 (1), 55–75. doi:10.1146/annurev-matsci-070909-104537
Le Norcy, E., Lesieur, J., Sadoine, J., Rochefort, G. Y., Chaussain, C., and Poliard, A. (2018). Phosphorylated and non-phosphorylated leucine rich amelogenin peptide differentially affect ameloblast mineralization. Front. Physiol. 9, 55. doi:10.3389/fphys.2018.00055
Li, N., Cui, W., Cong, P., Tang, J., Guan, Y., Huang, C., et al. (2021). Biomimetic inorganic-organic hybrid nanoparticles from magnesium-substituted amorphous calcium phosphate clusters and polyacrylic acid molecules. Bioact. Mat. 6 (8), 2303–2314. doi:10.1016/j.bioactmat.2021.01.005
Li, Y., Ping, H., Wei, J., Zou, Z., Zhang, P., Xie, J., et al. (2021). Bioprocess-inspired room-temperature synthesis of enamel-like fluorapatite/polymer nanocomposites controlled by magnesium ions. ACS Appl. Mat. Interfaces 13 (21), 25260–25269. doi:10.1021/acsami.1c04575
Li, Z., Ren, Q., Cui, J., Hu, D., Tian, T., He, T., et al. (2020). Comparing the efficacy of hydroxyapatite nucleation regulated by amino acids, poly-amino acids and an amelogenin-derived peptide. CrystEngComm 22 (22), 3814–3823. doi:10.1039/c9ce01925a
Liang, K., Xiao, S., Shi, W., Li, J., Yang, X., Gao, Y., et al. (2015). 8DSS-promoted remineralization of demineralized dentin in vitro. J. Mat. Chem. B 3 (33), 6763–6772. doi:10.1039/c5tb00764j
Liao, Y., Brandt, B. W., Li, J., Crielaard, W., Van Loveren, C., and Deng, D. M. (2017). Fluoride resistance in Streptococcus mutans: A mini review. J. Oral Microbiol. 9 (1), 1344509. doi:10.1080/20002297.2017.1344509
Liu, Y., Ding, C., He, L., Yang, X., Gou, Y., Xu, X., et al. (2018). Bioinspired heptapeptides as functionalized mineralization inducers with enhanced hydroxyapatite affinity. J. Mat. Chem. B 6 (13), 1984–1994. doi:10.1039/c7tb03067c
Luo, M., Gao, Y., Yang, S., Quan, X., Sun, D., Liang, K., et al. (2019). Computer simulations of the adsorption of an N-terminal peptide of statherin, SN15, and its mutants on hydroxyapatite surfaces. Phys. Chem. Chem. Phys. 21 (18), 9342–9351. doi:10.1039/c9cp01638d
Lv, X., Yang, Y., Han, S., Li, D., Tu, H., Li, W., et al. (2015). Potential of an amelogenin based peptide in promoting reminerlization of initial enamel caries. Arch. Oral Biol. 60 (10), 1482–1487. doi:10.1016/j.archoralbio.2015.07.010
Lynch, R. J., Navada, R., and Walia, R. (2004). Low-levels of fluoride in plaque and saliva and their effects on the demineralisation and remineralisation of enamel; role of fluoride toothpastes. Int. Dent. J. 54 (5), 304–309. doi:10.1111/j.1875-595x.2004.tb00003.x
Memarpour, M., Shafiei, F., Rafiee, A., Soltani, M., and Dashti, M. H. (2019). Effect of hydroxyapatite nanoparticles on enamel remineralization and estimation of fissure sealant bond strength to remineralized tooth surfaces: An in vitro study. BMC Oral Health 19 (1), 92. doi:10.1186/s12903-019-0785-6
Mukherjee, K., Ruan, Q., and Moradian-Oldak, J. (2019). Peptide-mediated biomimetic regrowth of human enamel in situ. Methods Mol. Biol. 1922, 129–138. doi:10.1007/978-1-4939-9012-2_13
Mukherjee, K., Ruan, Q., Nutt, S., Tao, J., De Yoreo, J. J., and Moradian-Oldak, J. (2018). Peptide-based bioinspired approach to regrowing multilayered aprismatic enamel. ACS Omega 3 (3), 2546–2557. doi:10.1021/acsomega.7b02004
Murari, G., Bock, N., Zhou, H., Yang, L., Liew, T., Fox, K., et al. (2020). Effects of polydopamine coatings on nucleation modes of surface mineralization from simulated body fluid. Sci. Rep. 10 (1), 14982. doi:10.1038/s41598-020-71900-3
Musat, V., Anghel, E. M., Zaharia, A., Atkinson, I., Mocioiu, O. C., Busila, M., et al. (2021). A chitosan-agarose polysaccharide-based hydrogel for biomimetic remineralization of dental enamel. Biomolecules 11 (8), 1137. doi:10.3390/biom11081137
Nagano, T., Kakegawa, A., Yamakoshi, Y., Tsuchiya, S., Hu, J. C. C., Gomi, K., et al. (2009). Mmp-20 and Klk4 cleavage site preferences for amelogenin sequences. J. Dent. Res. 88 (9), 823–828. doi:10.1177/0022034509342694
Nascimento, M. M., Alvarez, A. J., Huang, X., Browngardt, C., Jenkins, R., Sinhoreti, M. C., et al. (2019). Metabolic profile of supragingival plaque exposed to arginine and fluoride. J. Dent. Res. 98 (11), 1245–1252. doi:10.1177/0022034519869906
Palazzo, B., Iafisco, M., Laforgia, M., Margiotta, N., Natile, G., Bianchi, C. L., et al. (2007). Biomimetic hydroxyapatite-drug nanocrystals as potential bone substitutes with antitumor drug delivery properties. Adv. Funct. Mat. 17 (13), 2180–2188. doi:10.1002/adfm.200600361
Pan, H., Tao, J., Xu, X., and Tang, R. (2007). Adsorption processes of Gly and Glu amino acids on hydroxyapatite surfaces at the atomic level. Langmuir 23 (17), 8972–8981. doi:10.1021/la700567r
Pandya, M., and Diekwisch, T. G. H. (2019). Enamel biomimetics-fiction or future of dentistry. Int. J. Oral Sci. 11 (1), 8. doi:10.1038/s41368-018-0038-6
Peres, M. A., Macpherson, L. M. D., Weyant, R. J., Daly, B., Venturelli, R., Mathur, M. R., et al. (2019). Oral diseases: A global public health challenge. Lancet 394 (10194), 249–260. doi:10.1016/S0140-6736(19)31146-8
Philip, N. (2019). State of the art enamel remineralization systems: The next frontier in caries management. Caries Res. 53 (3), 284–295. doi:10.1159/000493031
Pitts, N. B., Zero, D. T., Marsh, P. D., Ekstrand, K., Weintraub, J. A., Ramos-Gomez, F., et al. (2017). Dental caries. Nat. Rev. Dis. Prim. 3, 17030. doi:10.1038/nrdp.2017.30
Prajapati, S., Ruan, Q., Mukherjee, K., Nutt, S., and Moradian-Oldak, J. (2018). The presence of MMP-20 reinforces biomimetic enamel regrowth. J. Dent. Res. 97 (1), 84–90. doi:10.1177/0022034517728504
Prajapati, S., Tao, J., Ruan, Q., De Yoreo, J. J., and Moradian-Oldak, J. (2016). Matrix metalloproteinase-20 mediates dental enamel biomineralization by preventing protein occlusion inside apatite crystals. Biomaterials 75, 260–270. doi:10.1016/j.biomaterials.2015.10.031
Qu, Y., Gu, T., Du, Q., Shao, C., Wang, J., Jin, B., et al. (2020). Polydopamine promotes dentin remineralization via interfacial control. ACS Biomater. Sci. Eng. 6 (6), 3327–3334. doi:10.1021/acsbiomaterials.0c00035
Raj, P. A., Johnsson, M., Levine, M. J., and Nancollas, G. H. (1992). Salivary statherin. Dependence on sequence, charge, hydrogen bonding potency, and helical conformation for adsorption to hydroxyapatite and inhibition of mineralization. J. Biol. Chem. 267 (9), 5968–5976. doi:10.1016/s0021-9258(18)42650-6
Ren, F., Leng, Y., Xin, R., and Ge, X. (2010). Synthesis, characterization and ab initio simulation of magnesium-substituted hydroxyapatite. Acta Biomater. 6 (7), 2787–2796. doi:10.1016/j.actbio.2009.12.044
Ren, Q., Ding, L., Li, Z., Wang, X., Wang, K., Han, S., et al. (2019). Chitosan hydrogel containing amelogenin-derived peptide: Inhibition of cariogenic bacteria and promotion of remineralization of initial caries lesions. Arch. Oral Biol. 100, 42–48. doi:10.1016/j.archoralbio.2019.02.004
Ren, Q., Li, Z., Ding, L., Wang, X., Niu, Y., Qin, X., et al. (2018). Anti-biofilm and remineralization effects of chitosan hydrogel containing amelogenin-derived peptide on initial caries lesions. Regen. Biomater. 5 (2), 69–76. doi:10.1093/rb/rby005
Ruan, Q., Liberman, D., Bapat, R., Chandrababu, K. B., Phark, J. H., and Moradian-Oldak, J. (2016). Efficacy of amelogenin-chitosan hydrogel in biomimetic repair of human enamel in pH-cycling systems. J. Biomed. Eng. Inf. 2 (1), 119–128. doi:10.5430/jbei.v2n1p119
Ryu, J., Ku, S. H., Lee, H., and Park, C. B. (2010). Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mat. 20 (13), 2132–2139. doi:10.1002/adfm.200902347
Sari, M., Ramadhanti, D. M., Amalina, R., ChotimahAna, I. D., and Yusuf, Y. (2021). Development of a hydroxyapatite nanoparticle-based gel for enamel remineralization -A physicochemical properties and cell viability assay analysis. Dent. Mat. J. 41, 68–77. doi:10.4012/dmj.2021-102
Sedlakova Kondelova, P., Mannaa, A., Bommer, C., Abdelaziz, M., Daeniker, L., di Bella, E., et al. (2020). Efficacy of P11-4 for the treatment of initial buccal caries: A randomized clinical trial. Sci. Rep. 10 (1), 20211. doi:10.1038/s41598-020-77057-3
Shao, C., Jin, B., Mu, Z., Lu, H., Zhao, Y., Wu, Z., et al. (2019). Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 5 (8), eaaw9569. doi:10.1126/sciadv.aaw9569
Shen, P., Walker, G. D., Yuan, Y., Reynolds, C., Stanton, D. P., Fernando, J. R., et al. (2018). Importance of bioavailable calcium in fluoride dentifrices for enamel remineralization. J. Dent. (Shiraz). 78, 59–64. doi:10.1016/j.jdent.2018.08.005
Shimotoyodome, A., Kobayashi, H., Tokimitsu, I., Matsukubo, T., and Takaesu, Y. (2006). Statherin and histatin 1 reduce parotid saliva-promoted Streptococcus mutans strain MT8148 adhesion to hydroxyapatite surfaces. Caries Res. 40 (5), 403–411. doi:10.1159/000094286
Shin, N. Y., Yamazaki, H., Beniash, E., Yang, X., Margolis, S. S., Pugach, M. K., et al. (2020). Amelogenin phosphorylation regulates tooth enamel formation by stabilizing a transient amorphous mineral precursor. J. Biol. Chem. 295 (7), 1943–1959. doi:10.1074/jbc.RA119.010506
Shuturminska, K., Tarakina, N. V., Azevedo, H. S., Bushby, A. J., Mata, A., Anderson, P., et al. (2017). Elastin-like protein, with statherin derived peptide, controls fluorapatite formation and morphology. Front. Physiol. 8, 368. doi:10.3389/fphys.2017.00368
Simmer, J. P., Hu, Y., Lertlam, R., Yamakoshi, Y., and Hu, J. C. (2009). Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J. Biol. Chem. 284 (28), 19110–19121. doi:10.1074/jbc.M109.013623
Smith, C. E. L., Poulter, J. A., Antanaviciute, A., Kirkham, J., Brookes, S. J., Inglehearn, C. F., et al. (2017). Amelogenesis imperfecta; genes, proteins, and pathways. Front. Physiol. 8, 435. doi:10.3389/fphys.2017.00435
Song, J., Li, T., Gao, J., Li, C., Jiang, S., and Zhang, X. (2021). Building an aprismatic enamel-like layer on a demineralized enamel surface by using carboxymethyl chitosan and lysozyme-encapsulated amorphous calcium phosphate nanogels. J. Dent. (Shiraz). 107, 103599. doi:10.1016/j.jdent.2021.103599
Tao, J., Pan, H., Zeng, Y., Xu, X., and Tang, R. (2007). Roles of amorphous calcium phosphate and biological additives in the assembly of hydroxyapatite nanoparticles. J. Phys. Chem. B 111 (47), 13410–13418. doi:10.1021/jp0732918
Tao, S., Zhu, Y., Yuan, H., Tao, S., Cheng, Y., Li, J., et al. (2018). Efficacy of fluorides and CPP-ACP vs fluorides monotherapy on early caries lesions: A systematic review and meta-analysis. PLoS One 13 (4), e0196660. doi:10.1371/journal.pone.0196660
Uskokovic, V. (2015). When 1+1>2: Nanostructured composites for hard tissue engineering applications. Mater. Sci. Eng. C 57, 434–451. doi:10.1016/j.msec.2015.07.050
Ustriyana, P., Harmon, E., Chen, K., Michel, F. M., and Sahai, N. (2020a). Oligo(l-glutamic acids) in calcium phosphate precipitation: Chain length effect. J. Phys. Chem. B 124 (29), 6278–6287. doi:10.1021/acs.jpcb.0c01689
Ustriyana, P., Michel, F. M., Wilson, M. C., Harmon, E., Chen, J., Liu, T., et al. (2020b). Oligo(l-glutamic acids) in calcium phosphate precipitation: Mechanism of delayed phase transformation. J. Phys. Chem. B 124 (29), 6288–6298. doi:10.1021/acs.jpcb.0c01690
Viana, Í. E. L., Lopes, R. M., Silva, F. R. O., Lima, N. B., Aranha, A. C. C., Feitosa, S., et al. (2020). Novel fluoride and stannous -functionalized β-tricalcium phosphate nanoparticles for the management of dental erosion. J. Dent. 92, 103263. doi:10.1016/j.jdent.2019.103263
Wang, D., Deng, J., Deng, X., Fang, C., Zhang, X., and Yang, P. (2020). Controlling enamel remineralization by amyloid-like amelogenin mimics. Adv. Mat. 32 (31), e2002080. doi:10.1002/adma.202002080
Wang, H., Xiao, Z., Yang, J., Lu, D., Kishen, A., Li, Y., et al. (2017). Oriented and ordered biomimetic remineralization of the surface of demineralized dental enamel using HAP@ACP nanoparticles guided by Glycine. Sci. Rep. 7, 40701. doi:10.1038/srep40701
Wang, S., Zhang, L., Chen, W., Jin, H., Zhang, Y., Wu, L., et al. (2020). Rapid regeneration of enamel-like-oriented inorganic crystals by using rotary evaporation. Mater. Sci. Eng. C 115, 111141. doi:10.1016/j.msec.2020.111141
Wang, Y., Hu, D., Cui, J., Zeng, Y., Gan, X., Chen, Z., et al. (2020). Unraveling the mechanism for an amelogenin-derived peptide regulated hydroxyapatite mineralization via specific functional domain identification. J. Mat. Chem. B 8, 10373–10383. doi:10.1039/d0tb00949k
Wang, Z., Ouyang, Y., Wu, Z., Zhang, L., Shao, C., Fan, J., et al. (2018). A novel fluorescent adhesive-assisted biomimetic mineralization. Nanoscale 10 (40), 18980–18987. doi:10.1039/c8nr02078g
Wassel, M. O., and Khattab, M. A. (2017). Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J. Adv. Res. 8 (4), 387–392. doi:10.1016/j.jare.2017.05.006
Wen, Z., Chen, J., Wang, H., Zhong, S., Hu, Y., Wang, Z., et al. (2016). Abalone water-soluble matrix for self-healing biomineralization of tooth defects. Mater. Sci. Eng. C 67, 182–187. doi:10.1016/j.msec.2016.05.015
Wong, H. M., Zhang, Y. Y., and Li, Q. L. (2022). An enamel-inspired bioactive material with multiscale structure and antibacterial adhesion property. Bioact. Mat. 7, 491–503. doi:10.1016/j.bioactmat.2021.05.035
Wu, D., Yang, J., Li, J., Chen, L., Tang, B., Chen, X., et al. (2013). Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 34 (21), 5036–5047. doi:10.1016/j.biomaterials.2013.03.053
Wu, S., Yu, M., Li, M., Wang, L., Putnis, C. V., and Putnis, A. (2017). In situ atomic force microscopy imaging of octacalcium phosphate crystallization and its modulation by amelogenin’s C-terminus. Cryst. Growth & Des. 17 (4), 2194–2202. doi:10.1021/acs.cgd.7b00129
Wu, X., Zhao, X., Li, Y., Yang, T., Yan, X., and Wang, K. (2015). In situ synthesis carbonated hydroxyapatite layers on enamel slices with acidic amino acids by a novel two-step method. Mater. Sci. Eng. C 54, 150–157. doi:10.1016/j.msec.2015.05.006
Xia, Y., Ren, A., and Pugach, M. K. (2016). Truncated amelogenin and LRAP transgenes improve Amelx null mouse enamel. Matrix Biol. 52-54, 198–206. doi:10.1016/j.matbio.2015.11.005
Xiao, Z., Que, K., Wang, H., An, R., Chen, Z., Qiu, Z., et al. (2017). Rapid biomimetic remineralization of the demineralized enamel surface using nano-particles of amorphous calcium phosphate guided by chimaeric peptides. Dent. Mat. 33 (11), 1217–1228. doi:10.1016/j.dental.2017.07.015
Xu, M., Ji, F., Qin, Z., Dong, D., Tian, X., Niu, R., et al. (2018). Biomimetic mineralization of a hydroxyapatite crystal in the presence of a zwitterionic polymer. CrystEngComm 20 (17), 2374–2383. doi:10.1039/c8ce00119g
Xu, X., Wang, N., Wu, M., Wang, J., Wang, D., Chen, Z., et al. (2020). Programmed antibacterial and mineralization therapy for dental caries based on zinc-substituted hydroxyapatite/alendronate-grafted polyacrylic acid hybrid material. Colloids Surfaces B Biointerfaces 194, 111206. doi:10.1016/j.colsurfb.2020.111206
Yamazaki, H., Beniash, E., Yamakoshi, Y., Simmer, J. P., and Margolis, H. C. (2017). Protein phosphorylation and mineral binding affect the secondary structure of the leucine-rich amelogenin peptide. Front. Physiol. 8, 450. doi:10.3389/fphys.2017.00450
Yan, W. J., Ma, P., Tian, Y., Wang, J. Y., Qin, C. L., Feng, J. Q., et al. (2017). The importance of a potential phosphorylation site in enamelin on enamel formation. Int. J. Oral Sci. 9 (11), e4. doi:10.1038/ijos.2017.41
Yang, X., Shang, H., Ding, C., and Li, J. (2015). Recent developments and applications of bioinspired dendritic polymers. Polym. Chem. 6 (5), 668–680. doi:10.1039/c4py01537a
Yang, X., Yang, B., He, L., Li, R., Liao, Y., Zhang, S., et al. (2017). Bioinspired peptide-decorated tannic acid for in situ remineralization of tooth enamel: In vitro and in vivo evaluation. ACS Biomater. Sci. Eng. 3 (12), 3553–3562. doi:10.1021/acsbiomaterials.7b00623
Yang, Y., Lv, X. P., Shi, W., Li, J. Y., Li, D. X., Zhou, X. D., et al. (2014). 8DSS-promoted remineralization of initial enamel caries in vitro. J. Dent. Res. 93 (5), 520–524. doi:10.1177/0022034514522815
Yeom, B., Sain, T., Lacevic, N., Bukharina, D., Cha, S. H., Waas, A. M., et al. (2017). Abiotic tooth enamel. Nature 543 (7643), 95–98. doi:10.1038/nature21410
Younes, I., and Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 13 (3), 1133–1174. doi:10.3390/md13031133
Yu, M., Wang, L., Zhang, W., and Ganss, B. (2019). Organized assembly of fluorapatite nanorods controlled by amelotin: Implications for enamel regeneration. ACS Appl. Nano Mat. 2 (12), 7566–7576. doi:10.1021/acsanm.9b01661
Zanatta, R. F., Caneppele, T. M. F., Scaramucci, T., El Dib, R., Maia, L. C., Ferreira, D., et al. (2020). Protective effect of fluorides on erosion and erosion/abrasion in enamel: A systematic review and meta-analysis of randomized in situ trials. Arch. Oral Biol. 120, 104945. doi:10.1016/j.archoralbio.2020.104945
Zarrintaj, P., Manouchehri, S., Ahmadi, Z., Saeb, M. R., Urbanska, A. M., Kaplan, D. L., et al. (2018). Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 187, 66–84. doi:10.1016/j.carbpol.2018.01.060
Zeng, J., Yang, S., Yu, H., Xu, Z., Quan, X., and Zhou, J. (2021). Simulation insight into the synergic role of citrate and polyaspartic peptide in biomineralization. Langmuir 37 (11), 3410–3419. doi:10.1021/acs.langmuir.0c03626
Zhang, J., Jiang, D., Zhang, J., Lin, Q., and Huang, Z. (2010). Synthesis of dental enamel-like hydroxyapaptite through solution mediated solid-state conversion. Langmuir 26 (5), 2989–2994. doi:10.1021/la9043649
Zheng, W., Ding, L., Wang, Y., Han, S., Zheng, S., Guo, Q., et al. (2019). The effects of 8DSS peptide on remineralization in a rat model of enamel caries evaluated by two nondestructive techniques. J. Appl. Biomater. Funct. Mat. 17 (1), 228080001982779. doi:10.1177/2280800019827798
Zheng, X., Cheng, X., Wang, L., Qiu, W., Wang, S., Zhou, Y., et al. (2015). Combinatorial effects of arginine and fluoride on oral bacteria. J. Dent. Res. 94 (2), 344–353. doi:10.1177/0022034514561259
Zhou, L., Li, Q. L., and Wong, H. M. (2021). A novel strategy for caries management: Constructing an antibiofouling and mineralizing dual-bioactive tooth surface. ACS Appl. Mat. Interfaces 13 (26), 31140–31152. doi:10.1021/acsami.1c06989
Zhou, L., Wong, H. M., Zhang, Y. Y., and Li, Q. L. (2020). Constructing an antibiofouling and mineralizing bioactive tooth surface to protect against decay and promote self-healing. ACS Appl. Mat. Interfaces 12 (2), 3021–3031. doi:10.1021/acsami.9b19745
Keywords: advanced materials, enamel caries, demineralization, remineralization, hydroxyapatite
Citation: Xu J, Shi H, Luo J, Yao H, Wang P, Li Z and Wei J (2022) Advanced materials for enamel remineralization. Front. Bioeng. Biotechnol. 10:985881. doi: 10.3389/fbioe.2022.985881
Received: 04 July 2022; Accepted: 22 August 2022;
Published: 13 September 2022.
Edited by:
He Liu, Jilin University, ChinaReviewed by:
Lunguo Xia, Shanghai Jiao Tong University, ChinaCopyright © 2022 Xu, Shi, Luo, Yao, Wang, Li and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihua Li, bGl6aGlodWFAbmN1LmVkdS5jbg==; Junchao Wei, d2VpanVuY2hhb0BuY3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.