
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol., 22 June 2022
Sec. Biomaterials
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.927248
This article is part of the Research TopicBiomaterials In AsiaView all 36 articles
 Xiaoqiong Ding1,2†
Xiaoqiong Ding1,2† Yangnan Hu3†
Yangnan Hu3† Hong Cheng3†
Hong Cheng3† Xiaoli Zhang4
Xiaoli Zhang4 Ling Lu4
Ling Lu4 Song Gao4
Song Gao4 Cheng Cheng4
Cheng Cheng4 Lifen Wang2
Lifen Wang2 Xiaoyun Qian4
Xiaoyun Qian4 Chen Zhang5
Chen Zhang5 Renjie Chai2,3,4,5,6,7,8*‡
Renjie Chai2,3,4,5,6,7,8*‡ Xia Gao1*‡
Xia Gao1*‡ Zhichun Huang2*‡
Zhichun Huang2*‡The ideal treatment for sensory hearing loss is to regenerate inner ear hair cells (HCs) through stem cell therapy, thereby restoring the function and structure of the cochlea. Previous studies have found that Lgr5+ supporting cells (SCs) in the inner ear can regenerate HCs, thus being considered inner ear progenitor cells. In addition to traditional biochemical factors, physical factors such as electrical conductivity also play a crucial role in the regulation of stem cell proliferation and differentiation. In this study, the graphene substrates were used to culture Lgr5+ progenitor cells and investigated their regulatory effects on cells. It was demonstrated that the graphene substrates displayed great cytocompatibility for Lgr5+ progenitors and promoted their sphere-forming ability. Moreover, more Myosin7a+ cells were found on the graphene substrates compared with tissue culture polystyrene (TCPS). These results suggest that graphene is an efficient interface that can promote the differentiation of Lgr5+ progenitors into HCs, which is great significance for its future application in combination with Lgr5+ cells to regenerate HCs in the inner ear.
Inner ear sensory hair cells (HCs) mainly function for transduction of mechanical stimuli into electrical signals and are mechanoreceptors for sound recognition (Li et al., 2018; Liu et al., 2019; Zhang et al., 2019). Aging, ototoxic drugs, trauma, inflammation, and other factors can all contribute to hair cell damage, resulting in sensorineural hearing loss (Liu et al., 2016; Zhou et al., 2020). Sensorineural hearing loss is a common sensory disease caused by damage or loss of HCs, affecting millions of people worldwide. Supporting cells (SCs) in the auditory and vestibular systems of birds and fish have been reported to have the capability to regenerate HCs in response to the damage to HCs (Corwin and Cotanche, 1988; Balak et al., 1990; Warchol, 2011). However, studies have shown that HC damage in adult mammals is irreparable, which lead to permanent hearing loss (Rubel et al., 2013). Recently, several studies reported that Lgr5+ SCs in the inner ear can regenerate HCs and considered inner ear progenitor cells, which bringing new possibilities for the regeneration of HCs in adult mammals (Bermingham-McDonogh and Reh, 2011; Chai et al., 2011; Chai et al., 2012; Shi et al., 2013; Bramhall et al., 2014; Waqas et al., 2016).
The most ideal treatment for sensory hearing loss is to regenerate inner ear HCs to restore the structure and function of the cochlea (Wang et al., 2015). The behaviors of cochlear of progenitor cells including proliferation and differentiation are regulated by numerous biochemical and physical factors. Investigating the regulatory strategies that promote the differentiation of cochlear progenitor cells into mature HCs is critical for HC regeneration and hearing reconstruction. Several studies have shown that some conductive materials such as graphene and MXenes can regulate the behavior of stem cells, including proliferation and differentiation (Guo et al., 2016; Farokhi et al., 2021; Yao et al., 2021; Guo et al., 2022). Lgr5+ progenitors have characteristics similar to adult stem cells. The detailed regulatory effects of these conductive materials on Lgr5+ progenitors’ behavior and their potential in the treatment of sensorineural hearing loss have not been investigated.
Herein, in this work, we fabricated graphene substrates and studied their regulatory effects on the proliferation and differentiation. Graphene has been reported to significantly promote neuronal differentiation of neural stem cells (NSCs). Several studies have reported their potential for applications in the biomedical field, including drug delivery (Song et al., 2020; Sattari et al., 2021), photothermal therapy (de Melo-Diogo et al., 2019; Palmieri et al., 2020; Wang et al., 2020), and nerve regeneration (Aydin et al., 2018; Grijalvo and Díaz, 2021). Therefore, the current study focus on the effects of graphene substrate on the survival, proliferation and differentiation into HCs in Lgr5+ progenitors, which is of great significance for the combined therapy of physical stimulation and stem cell transplantation for the treatment of sensorineural hearing loss.
Lgr5-EGFP-IRES-creERT2 mice (stock no. 008875) were purchased from The Jackson Laboratory. All animal experiments were performed in accordance with protocols that were approved by the Animal Care and Use Committee of Southeast University (Approved No. 20200402025) and the National Institute of Health’s Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and to prevent their suffering.
For genotyping of transgenic mice, the tail tips were first collected, and genomic DNA was obtained by adding 180 uL 50 mM NaOH and incubating at 98°C for 1 h, followed by adding 20 uL 1 M Tris-HCl (pH 7.0). The tube was then vortexed vigorously for 1 min for complete tissue dispersion. The primers used for genotyping are as follows; wild-type (R) ATA CCC CAT CCC TTT TGA GC; Lgr5: (F) CTG CTC TCT GCT CCC AGT CT; mutant (R) GAA CTT CAG GGT CAG CTT GC.
The graphene substrates were purchased from Nanjing MKNANO Tech. Co., Ltd. Specifically, the graphene was prepared by chemical vapor deposition (CVD) and then transferred to the surface of coverslips to obtain a graphene substrate. For cell culture, the graphene substrates were immersed in 75% alcohol for 1 h, then washed three times with sterilized water and irradiated under ultraviolet light overnight.
The surface of graphene substrates was observed by Scanning Electron Microscope (SEM) (Zeiss, Ultra Plus). The graphene substrates were then characterized by a Raman microscopy (Renishaw inVia) and an X-ray diffractometer (XRD) (PANalytical Empyrean).
The cochleae of Lgr5-EGFP-creERT2 mice (postnatal day 1–3, P1-3) were dissected out and collected in a tube. Subsequently, the collected cochleae were digested by trypsin (0.125%, Invitrogen) at 37°C for 8 min, followed by adding equal volume of soybean trypsin inhibitor (Worthington Biochem) to terminate the digestion. Cochleae tissue were dissociated into single cell suspensions by blunt tips (Eppendorf) and then filtered with a 40 μm cell strainer (BD Biosciences). Finally, the obtained single cells were sorted by a BD FACS Aria III (BD Biosciences) through GFP channel. Finally, the sorted cells were cultured on graphene substrates and tissue culture polystyrene (TCPS), respectively.
The cells sorted by FAC were cultured in DMEM/F12 medium supplemented with N2 (1%, Invitrogen), B27 (2% dilution, Invitrogen), EGF (20 ng/ml, Sigma), bFGF (10 ng/ml, Sigma), and IGF-1 (50 ng/ml, Sigma), heparin sulfate (50 ng/ml, Sigma) (full medium), and ampicillin (0.1%, Beyotime). For sphere-forming assay, the sorted cells were seeded on TCPS or graphene substrates with 200 cells per well for 5 days. Subsequently, we measured the number and diameter of the formed spheres to evaluate their proliferation capacity. For differentiation assay, the sorted single cells and formed spheres were differentiated separately. The cells or spheres were cultured in a 4-well dish for 10 days, and immunofluorescence staining was then applied to analyze the differentiation of Lgr5+ cells. As described in previous reports, cell aggregation with more than 5 cells were considered a sphere or colony.
After culture, the cells grown on different substrates were fixed with 4% paraformaldehyde for 1 h at room temperature and then washed with PBS containing 0.1% Triton X-100 (PBST). The cells were then blocked with PBS containing 1% BSA for 1 h at room temperature. Afterwards, the primary antibody anti-Myosin7a (Proteus Bioscience) was diluted and incubated with these cells at 4°C overnight. After that, the cells were washed with PBST and then incubated with secondary antibody along with 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) for 1 h at room temperature. Finally, the cells cultured on different substrates were washed and then covered with coverslips in fluorescence mounting medium (DAKO). The cells were observed and captured with a laser scanning confocal microscope (Zeiss, LSM 700).
All data were analyzed with GraphPad Prism6 software and presented as mean ± SD. Two-tailed, unpaired Student’s t-tests were used to determine statistical significance when comparing two groups, and a value of p < 0.05 was considered statistically significant. At least three individual experiments were conducted for all experiments.
Graphene substrates used in our work were fabricated by a CVD method and then transferred to the surface of coverslips. The morphology of the graphene substrates was observed from the SEM image (Figure 1A). Figure 1B displays the XRD pattern of graphene substrates. It was suggested that the graphene has a distinct peak at 27.5°, which can be indexed to (002) diffraction plane. The Raman spectra displayed the characteristic peaks of 2D and G bands, and the intensity ratio indicated that the graphene substrates composed of a few layers of graphene sheets (Figure 1C).
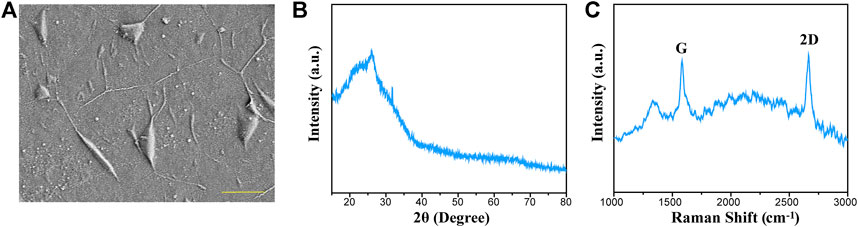
FIGURE 1. Characterization of graphene substrates prepared by the CVD method. (A) Representative SEM image of the graphene substrates. (B) XRD spectra for graphene substrates. (C) Raman spectra for graphene substrates.
To evaluate the cytocompatibility of graphene substrates, we isolated Lgr5+ cells from Lgr5-EGFP- CreERT2 mice by FAC sorting and cultured the sorted cells on graphene substrates and TCPS for different times. The flow cytometry plots displayed that about 5.8% of the whole cochlear cell population were Lgr5+ progenitors (Figures 2A,B). After culture, a CCK-8 assay was conducted to analyze the influence of graphene substrates on the viability of Lgr5+ cells (Figure 2C). It was suggested that cells cultured on graphene substrates for different times had similar viability to cells cultured on TCPS, indicating our prepared graphene substrates are nontoxic and cytocompatible. Therefore, we further investigated the effects of graphene substrates on the proliferation and differentiation of Lgr5+ progenitors.
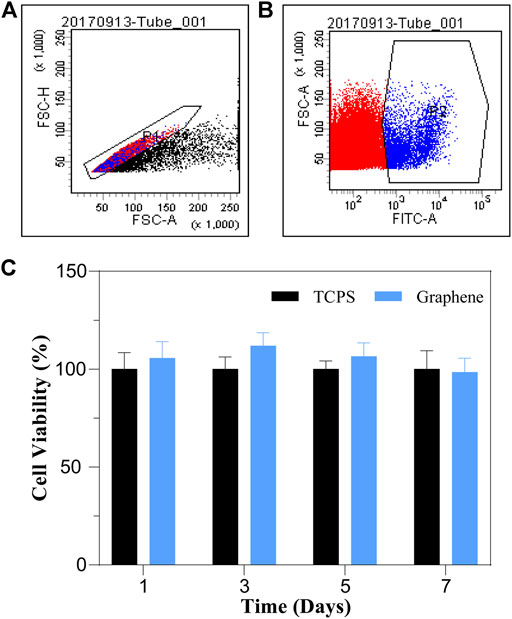
FIGURE 2. FAC sorting plots and the effects of graphene substrates on the cell viability of Lgr5+ progenitors. (A,B) FAC sorting plots. (C) Cell viability results from CCK-8 assay.
The capacity of self-renew is an important characteristic of stem cells and progenitor cells. Therefore, we cultured the sorted cells on graphene substrates and TCPS for 5 days to form spheres, respectively, to determine their proliferation capacity. Figures 3A,B showed images of spheres formed from Lgr5+ progenitors cultured on different substrates. It was suggested that the Lgr5+ progenitors cultured on graphene substrates formed more spheres than those cultured on TCPS (Figure 3C). However, there was no significant difference in the diameter of the formed spheres (Figure 3D). These results demonstrated that our prepared graphene substrates could promote the sphere-forming ability of Lgr5+ progenitors.
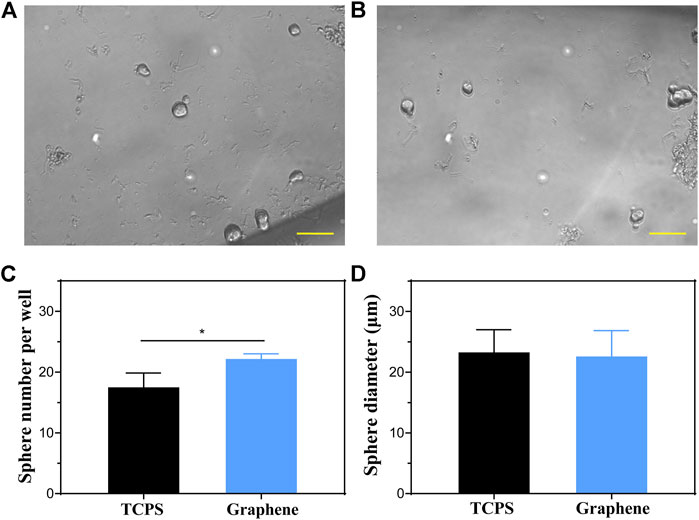
FIGURE 3. Sphere-forming assay of Lgr5+ progenitors. (A,B) The spheres formed by Lgr5+ progenitors cultured on TCPS (A) and graphene substrates (B). (C,D) The sphere number (C) and diameter (D) of Lgr5+ spheres cultured on different substrates. The scale bars are 50 μm in (A,B).
To investigate the HC regeneration capability of the formed spheres on different substrates, we performed differentiation assay for 10 days after 5 days of sphere-forming assay, as shown in Figure 4A. After differentiation, the spheres were stained with HC marker Myosin7a (Figures 4B,C). Subsequently, we counted the number of Myosin7a+ cells generated from each sphere and total Myosin7a+ cells per well. It was suggested that each sphere cultured on graphene generated more Myosin7a+ HCs than sphere cultured on TCPS (Figure 4D), and the total spheres on graphene also generated more Myosin7a+ HCs than those cultured on TCPS (Figure 4E).
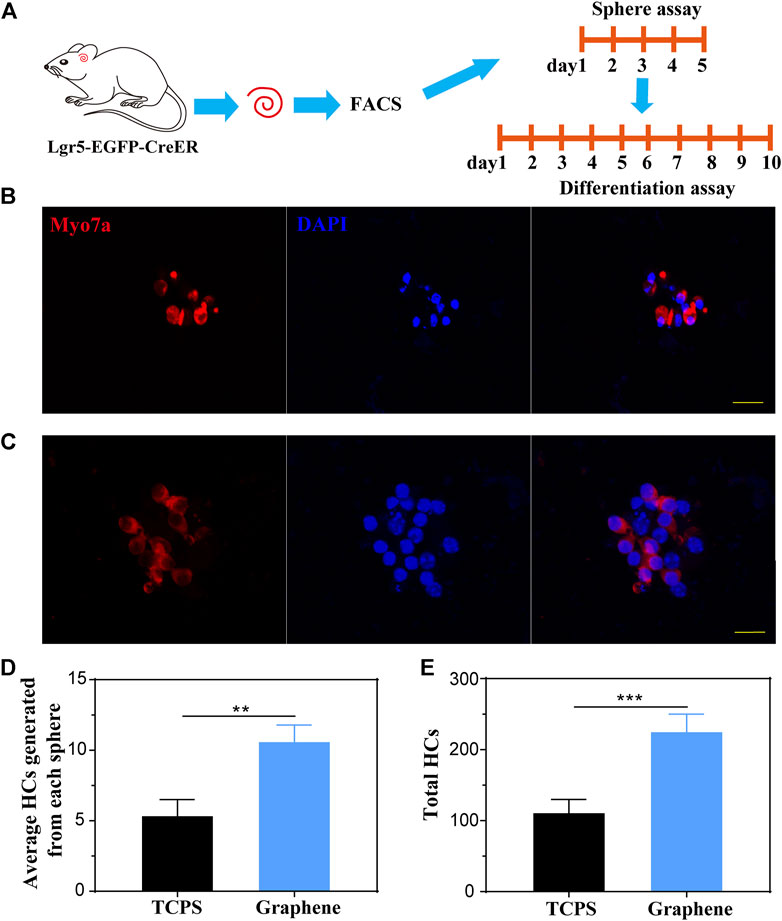
FIGURE 4. Lgr5+ spheres cultured on graphene substrates generate more HCs compared to those on TCPS. (A) Lgr5+ progenitors were cultured on different substrates for 5 days of sphere assay and 10 days of differentiation assay. (B,C) An Lgr5+ sphere cultured on TCPS (B) and graphene substrates (C) stained with the Myosin7a (red) and DAPI (blue). (D) Quantification of the average number of HCs differentiated from each sphere. (E) Quantification of the total number of HCs differentiated from Lgr5+ progenitors per well. The scale bars are 20 μm in (B,C).
To further explore the HC regeneration capability, we cultured the sorted Lgr5+ cells on laminin-coated graphene or TCPS substrates for 10 days of differentiation (Figure 5A). The cells were next stained with Myosin7a after 10 days of differentiation (Figures 5B,C). The results demonstrated that the cells cultured on graphene substrates formed significantly more Myosin7a+ colonies and total colonies than those cultured on TCPS (Figure 5D). It is worth noting that the Myosin7a+ cells inside the colony are mitotically regenerated HCs, and those outside the colony are directly differentiated HCs. The Myosin7a+ cells both inside and outside the colony were both counted. and the results suggested that the Lgr5+ progenitors cultured on graphene substrates differentiated more Myosin7a+ HCs both inside and outside the colony than those cultured on TCPS (Figure 5E). Overall, our findings suggest that graphene could promote the differentiation of Lgr5+ progenitors into HCs.
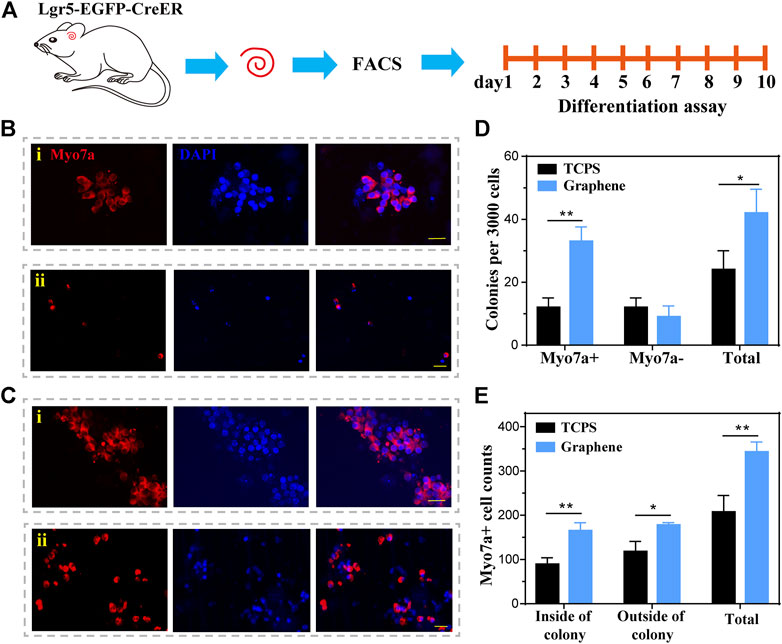
FIGURE 5. The differentiation of Lgr5+ progenitors. (A) The Lgr5+ progenitors were cultured on graphene or TCPS for 10 days of differentiation. (B) Immunofluorescence images of Lgr5+ progenitors cultured on TCPS after 10 days of differentiation, both inside (i) and outside (ii) the colony. (C) Immunofluorescence images of Lgr5+ progenitors cultured on graphene substrates after 10 days of differentiation, both inside (i) and outside (ii) the colony. (D) The number of colonies per 3,000 cells. (E) Quantification of Myosin7a+ cells. The scale bars are 20 μm in (B,C).
HCs in the mammalian cochlea mainly function for transduction of mechanical stimuli into electrical signals, thus play an important role in sound recognition. Irreversible damage or loss of HCs due to aging, ototoxic drugs, trauma, inflammation and other stress can lead to permanent hearing loss. In recent years, great progress has been made in HC regeneration, many studies have successfully induced embryonic stem cells and pluripotent stem cells to differentiate into hair cell-like cells in vitro (Roccio et al., 2018). Recently, several studies have reported that Lgr5+ SCs in the inner ear maintain the ability to generate HCs and SCs, and thereby considered inner ear progenitor cells. Lgr5 is a target gene of Wnt and is considered a stem cell marker in a variety of adult tissues (Barker et al., 2007; Jaks et al., 2008).
Recently, Smith-Cortinez et al. reported the long-term presence of Lgr5+ SCs in the adult mouse cochlea (Smith-Cortinez et al., 2021). These inner ear progenitor cells are able to survive in the cochlea even after severe ototoxic injury. Therefore, they have great potential in the treatment of sensorineural hearing loss caused by HC damage or loss. However, the efficiency of proliferation and regenerating HCs from Lgr5+ progenitors are low. Many studies have focused on the regulation of the proliferation and differentiation capacity of inner ear progenitor cells by different methods. With the development of tissue engineering technology, several novel therapeutic systems combining biomaterials with stem cells have been widely studied (Tong et al., 2015; Kim et al., 2018). In this work, we introduced graphene as a cell culture substrate to investigate its regulation on the behaviors of inner ear Lgr5+ progenitors, including proliferation and differentiation.
Graphene substrates have been shown to have great cytocompatibility and electrical conductivity, and have been extensively studied in the biomedical field. Various studies have suggested that graphene substrates could support cell culture and regulate the proliferation and differentiation of different types of stem cells (Park et al., 2011; Guo et al., 2016; Kenry et al., 2018). The regulation of stem cell differentiation by graphene opens a new horizon for its applications in regenerative medicine. However, its potential in the field of auditory field is remain exploring. Therefore, in this work, we investigated the in vitro regulation of graphene on inner ear progenitors, especially their proliferation and differentiation.
The sphere-forming assay suggested that Lgr5+ progenitors cultured on graphene substrates generated more spheres than those cultured on TCPS in vitro. However, there was no statistical difference in the diameter of the formed spheres. The results indicate that graphene can promote the sphere-forming ability of Lgr5+ progenitors. The results of differentiation assay suggested that Lgr5+ progenitors could differentiate into Myosin7a+ cells, and the number of Myosin7a+ cells on graphene was larger than TCPS, indicating that our prepared graphene substrates could promote the generation of HCs from Lgr5+ progenitors. Recent studies have found that the regeneration of HCs from inner ear progenitors were regulated by several genes, including Pou4f3, Atoh1, Cdh23, Jag2, Skp2 (Waqas et al., 2016). We speculate that graphene substrates may promote the differentiation of Lgr5+ progenitors into HCs by regulating the expression of related genes. We will carry out in-depth research on the underlying mechanism in the future. What’s important, we will develop more graphene-based 3D scaffolds with superior properties and combine them with stem cell transplantation for the regeneration of inner ear HCs.
In summary, we prepared graphene substrates by depositing graphene onto coverslips. We found that the graphene substrates promoted the sphere-forming and HC regeneration capabilities of cochlea Lgr5+ progenitors. The results suggest that graphene is an efficient interface that can promote the differentiation of Lgr5+ progenitor cells into HCs, which is essential for its future application in combination with Lgr5+ cells to regenerate HCs in the inner ear. In future work, we will continue to explore better graphene-based scaffolds to advance the application of graphene in stem cell therapy. In addition, we plan to further explore the specific mechanism by which graphene substrates regulate cell proliferation and differentiation. Understanding the specific mechanism of the interaction between graphene and inner ear Lgr5+ progenitors may provide more information for promoting HC regeneration through tissue engineering approaches.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by animal care and welfare committee of Southeast University.
XD, YH, and HC carried out the studies, participated in collecting data, and drafted the manuscript. XZ, LL, and SG performed the statistical analysis. CC, LW, XQ, and CZ participated in its design. RC, XG, and ZH designed the study and helped to draft the manuscript. All authors read and approved the final manuscript.
This work was supported by grants from the National Basic Research Program of China (2017YFA0105201), the National Natural Science Foundation of China (Nos. 81771019, 81970884, 82071059, 81900944, 81900941).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2022.927248/full#supplementary-material
Aydin, T., Gurcan, C., Taheri, H., and Yilmazer, A. (2018). Graphene Based Materials in Neural Tissue Regeneration. Adv. Exp. Med. Biol. 1107, 129–142. doi:10.1007/5584_2018_221
Balak, K., Corwin, J., and Jones, J. (1990). Regenerated Hair Cells Can Originate from Supporting Cell Progeny: Evidence from Phototoxicity and Laser Ablation Experiments in the Lateral Line System. J. Neurosci. 10 (8), 2502–2512. doi:10.1523/jneurosci.10-08-02502.1990
Barker, N., van Es, J. H., Kuipers, J., Kujala, P., van den Born, M., Cozijnsen, M., et al. (2007). Identification of Stem Cells in Small Intestine and Colon by Marker Gene Lgr5. Nature 449 (7165), 1003–1007. doi:10.1038/nature06196
Bermingham-McDonogh, O., and Reh, T. A. (2011). Regulated Reprogramming in the Regeneration of Sensory Receptor Cells. Neuron 71 (3), 389–405. doi:10.1016/j.neuron.2011.07.015
Bramhall, N. F., Shi, F., Arnold, K., Hochedlinger, K., and Edge, A. S. B. (2014). Lgr5-positive Supporting Cells Generate New Hair Cells in the Postnatal Cochlea. Stem Cell. Rep. 2 (3), 311–322. doi:10.1016/j.stemcr.2014.01.008
Chai, R., Kuo, B., Wang, T., Liaw, E. J., Xia, A., Jan, T. A., et al. (2012). Wnt Signaling Induces Proliferation of Sensory Precursors in the Postnatal Mouse Cochlea. Proc. Natl. Acad. Sci. U.S.A. 109 (21), 8167–8172. doi:10.1073/pnas.1202774109
Chai, R., Xia, A., Wang, T., Jan, T. A., Hayashi, T., Bermingham-McDonogh, O., et al. (2011). Dynamic Expression of Lgr5, a Wnt Target Gene, in the Developing and Mature Mouse Cochlea. Jaro 12 (4), 455–469. doi:10.1007/s10162-011-0267-2
Corwin, J. T., and Cotanche, D. A. (1988). Regeneration of Sensory Hair Cells after Acoustic Trauma. Science 240 (4860), 1772–1774. doi:10.1126/science.3381100
de Melo-Diogo, D., Lima-Sousa, R., Alves, C. G., and Correia, I. J. (2019). Graphene Family Nanomaterials for Application in Cancer Combination Photothermal Therapy. Biomater. Sci. 7 (9), 3534–3551. doi:10.1039/c9bm00577c
Farokhi, M., Mottaghitalab, F., Saeb, M. R., Shojaei, S., Zarrin, N. K., Thomas, S., et al. (2021). Conductive Biomaterials as Substrates for Neural Stem Cells Differentiation towards Neuronal Lineage Cells. Macromol. Biosci. 21 (1), 2000123. doi:10.1002/mabi.202000123
Grijalvo, S., and Díaz, D. D. (2021). Graphene-based Hybrid Materials as Promising Scaffolds for Peripheral Nerve Regeneration. Neurochem. Int. 147, 105005. doi:10.1016/j.neuint.2021.105005
Guo, R., Xiao, M., Zhao, W., Zhou, S., Hu, Y., Liao, M., et al. (2022). 2D Ti3C2TxMXene Couples Electrical Stimulation to Promote Proliferation and Neural Differentiation of Neural Stem Cells. Acta Biomater. 139, 105–117. doi:10.1016/j.actbio.2020.12.035
Guo, R., Zhang, S., Xiao, M., Qian, F., He, Z., Li, D., et al. (2016). Accelerating Bioelectric Functional Development of Neural Stem Cells by Graphene Coupling: Implications for Neural Interfacing with Conductive Materials. Biomaterials 106, 193–204. doi:10.1016/j.biomaterials.2016.08.019
Jaks, V., Barker, N., Kasper, M., van Es, J. H., Snippert, H. J., Clevers, H., et al. (2008). Lgr5 Marks Cycling, yet Long-Lived, Hair Follicle Stem Cells. Nat. Genet. 40 (11), 1291–1299. doi:10.1038/ng.239
Kenry, , Lee, W. C., Loh, K. P., and Lim, C. T. (2018). When Stem Cells Meet Graphene: Opportunities and Challenges in Regenerative Medicine. Biomaterials 155, 236–250. doi:10.1016/j.biomaterials.2017.10.004
Kim, H., Kim, S.-H. L., Choi, Y.-H., Ahn, Y.-H., and Hwang, N. S. (2018). Biomaterials for Stem Cell Therapy for Cardiac Disease. Adv. Exp. Med. Biol. 1064, 181–193. doi:10.1007/978-981-13-0445-3_11
Li, A., You, D., Li, W., Cui, Y., He, Y., Li, W., et al. (2018). Novel Compounds Protect Auditory Hair Cells against Gentamycin-Induced Apoptosis by Maintaining the Expression Level of H3K4me2. Drug Deliv. 25 (1), 1033–1043. doi:10.1080/10717544.2018.1461277
Liu, L., Chen, Y., Qi, J., Zhang, Y., He, Y., Ni, W., et al. (2016). Wnt Activation Protects against Neomycin-Induced Hair Cell Damage in the Mouse Cochlea. Cell. Death Dis. 7 (3)–e2136. doi:10.1038/cddis.2016.35
Liu, Y., Qi, J., Chen, X., Tang, M., Chu, C., Zhu, W., et al. (2019). Critical Role of Spectrin in Hearing Development and Deafness. Sci. Adv. 5 (4), eaav7803. doi:10.1126/sciadv.aav7803
Palmieri, V., Spirito, M. D., and Papi, M. (2020). Graphene-based Scaffolds for Tissue Engineering and Photothermal Therapy. Nanomedicine 15 (14), 1411–1417. doi:10.2217/nnm-2020-0050
Park, S. Y., Park, J., Sim, S. H., Sung, M. G., Kim, K. S., Hong, B. H., et al. (2011). Enhanced Differentiation of Human Neural Stem Cells into Neurons on Graphene. Adv. Mat. 23 (36), H263–H267. doi:10.1002/adma.201101503
Roccio, M., Perny, M., Ealy, M., Widmer, H. R., Heller, S., and Senn, P. (2018). Molecular Characterization and Prospective Isolation of Human Fetal Cochlear Hair Cell Progenitors. Nat. Commun. 9 (1), 4027. doi:10.1038/s41467-018-06334-7
Rubel, E. W., Furrer, S. A., and Stone, J. S. (2013). A Brief History of Hair Cell Regeneration Research and Speculations on the Future. Hear. Res. 297, 42–51. doi:10.1016/j.heares.2012.12.014
Sattari, S., Adeli, M., Beyranvand, S., and Nemati, M. (2021). Functionalized Graphene Platforms for Anticancer Drug Delivery. Ijn Vol. 16, 5955–5980. doi:10.2147/ijn.S249712
Shi, F., Hu, L., and Edge, A. S. B. (2013). Generation of Hair Cells in Neonatal Mice by β-catenin Overexpression in Lgr5-Positive Cochlear Progenitors. Proc. Natl. Acad. Sci. U.S.A. 110 (34), 13851–13856. doi:10.1073/pnas.1219952110
Smith-Cortinez, N., Yadak, R., Hendriksen, F. G. J., Sanders, E., Ramekers, D., Stokroos, R. J., et al. (2021). LGR5-Positive Supporting Cells Survive Ototoxic Trauma in the Adult Mouse Cochlea. Front. Mol. Neurosci. 14, 729625. doi:10.3389/fnmol.2021.729625
Song, S., Shen, H., Wang, Y., Chu, X., Xie, J., Zhou, N., et al. (2020). Biomedical Application of Graphene: From Drug Delivery, Tumor Therapy, to Theranostics. Colloids Surfaces B Biointerfaces 185, 110596. doi:10.1016/j.colsurfb.2019.110596
Tong, Z., Solanki, A., Hamilos, A., Levy, O., Wen, K., Yin, X., et al. (2015). Application of Biomaterials to Advance Induced Pluripotent Stem Cell Research and Therapy. Embo J. 34 (8), 987–1008. doi:10.15252/embj.201490756
Wang, C., Wang, X., Chen, Y., and Fang, Z. (2020). In-vitro Photothermal Therapy Using Plant Extract Polyphenols Functionalized Graphene Sheets for Treatment of Lung Cancer. J. Photochem. Photobiol. B Biol. 204, 111587. doi:10.1016/j.jphotobiol.2019.111587
Wang, T., Chai, R., Kim, G. S., Pham, N., Jansson, L., Nguyen, D.-H., et al. (2015). Lgr5+ Cells Regenerate Hair Cells via Proliferation and Direct Transdifferentiation in Damaged Neonatal Mouse Utricle. Nat. Commun. 6, 6613. doi:10.1038/ncomms7613
Waqas, M., Guo, L., Zhang, S., Chen, Y., Zhang, X., Wang, L., et al. (2016). Characterization of Lgr5+ Progenitor Cell Transcriptomes in the Apical and Basal Turns of the Mouse Cochlea. Oncotarget 7 (27), 41123–41141. doi:10.18632/oncotarget.8636
Warchol, M. E. (2011). Sensory Regeneration in the Vertebrate Inner Ear: Differences at the Levels of Cells and Species. Hear. Res. 273 (1-2), 72–79. doi:10.1016/j.heares.2010.05.004
Yao, X., Yan, Z., Wang, X., Jiang, H., Qian, Y., and Fan, C. (2021). The Influence of Reduced Graphene Oxide on Stem Cells: a Perspective in Peripheral Nerve Regeneration. Regen. Biomater. 8 (4), rbab032. doi:10.1093/rb/rbab032
Zhang, Y., Li, W., He, Z., Wang, Y., Shao, B., Cheng, C., et al. (2019). Pre-treatment with Fasudil Prevents Neomycin-Induced Hair Cell Damage by Reducing the Accumulation of Reactive Oxygen Species. Front. Mol. Neurosci. 12, 264. doi:10.3389/fnmol.2019.00264
Keywords: graphene, sensorineural hearing loss, hair cell regeneration, proliferation, differentiation
Citation: Ding X, Hu Y, Cheng H, Zhang X, Lu L, Gao S, Cheng C, Wang L, Qian X, Zhang C, Chai R, Gao X and Huang Z (2022) Graphene Substrates Promote the Differentiation of Inner Ear Lgr5+ Progenitor Cells Into Hair Cells. Front. Bioeng. Biotechnol. 10:927248. doi: 10.3389/fbioe.2022.927248
Received: 24 April 2022; Accepted: 03 June 2022;
Published: 22 June 2022.
Edited by:
Mingqiang Li, Third Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Wenguo Cui, Shanghai Jiao Tong University, ChinaCopyright © 2022 Ding, Hu, Cheng, Zhang, Lu, Gao, Cheng, Wang, Qian, Zhang, Chai, Gao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renjie Chai, cmVuamllY0BzZXUuZWR1LmNu; Xia Gao, eGlhZ2FvZ2FvQGhvdG1haWwuY29t; Zhichun Huang, aHVhbmcxOTM2MThAc29odS5jb20=
†These authors share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.