
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Bioeng. Biotechnol. , 09 May 2022
Sec. Synthetic Biology
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.914280
This article is part of the Research Topic Synthetic Biology approaches for the production of next generation chemicals: from natural products to new-to-nature molecules View all 4 articles
Arbutin is a hydroquinone glucoside and a natural product present in various plants. Arbutin potently inhibits melanin formation. This property has been exploited in whitening cosmetics and pharmaceuticals. Arbutin production relies mainly on chemical synthesis. The multi-step and complicated process can compromise product purity. With the increasing awareness of sustainable development, the current research direction prioritizes environment-friendly, biobased arbutin production. In this review, current strategies for arbutin production are critically reviewed, with a focus on plant extraction, chemical synthesis, biotransformation, and microbial fermentation. Furthermore, the bottlenecks and perspectives for future direction on arbutin biosynthesis are discussed.
Arbutin is a hydroquinone (HQ) glucoside natural product with two different configurations: alpha (α)- and beta (β)-arbutin (Couteau & Coiffard, 2016). The formation of the two isomers is based on the binding state of HQ and the anomeric carbon atom in the glucose molecule (Hazman et al., 2021). Beneficial properties attributed to the isomers include anti-oxidative (Yu et al., 2015), anti-inflammatory (Lee & Kim, 2012), anti-microbial (Jurica et al., 2017), and anti-cancer (Su et al., 2020) effects. The list of potential applications of arbutin is growing (Saeedi et al., 2021). Particularly, α- and β-arbutin are widely used in pharmaceutical and cosmetic industries due to their potent inhibition of tyrosinase activity (Liu et al., 2016). The biosynthesis of α- and β-arbutin is dependent on the glycosylation of hydroquinone using glycosidases as catalyst and activated sugar as glycosyl donor. Currently, the species and activity of α-glycosidases were significantly higher than that of β-glycosidases, which probably makes α-arbutin more active on tyrosinase than β-arbutin. The tyrosinase inhibition by α-arbutin was 10-times greater than that of β-arbutin, indicating the value of α-arbutin for the cosmetic industry (Funayama et al., 1995).
Increased standards of living have prompted increased demand for cosmetics. This has increased the demand for arbutin. To meet this demand, arbutin production has become the research focus. β-arbutin production mainly depends on plant extraction, chemical synthesis, and microbial fermentation (Figure 1). In contrast, α-arbutin production relies on biotransformation. In this review, we summarize the four synthetic methods of arbutin and systematically analyze their strengths and weaknesses.
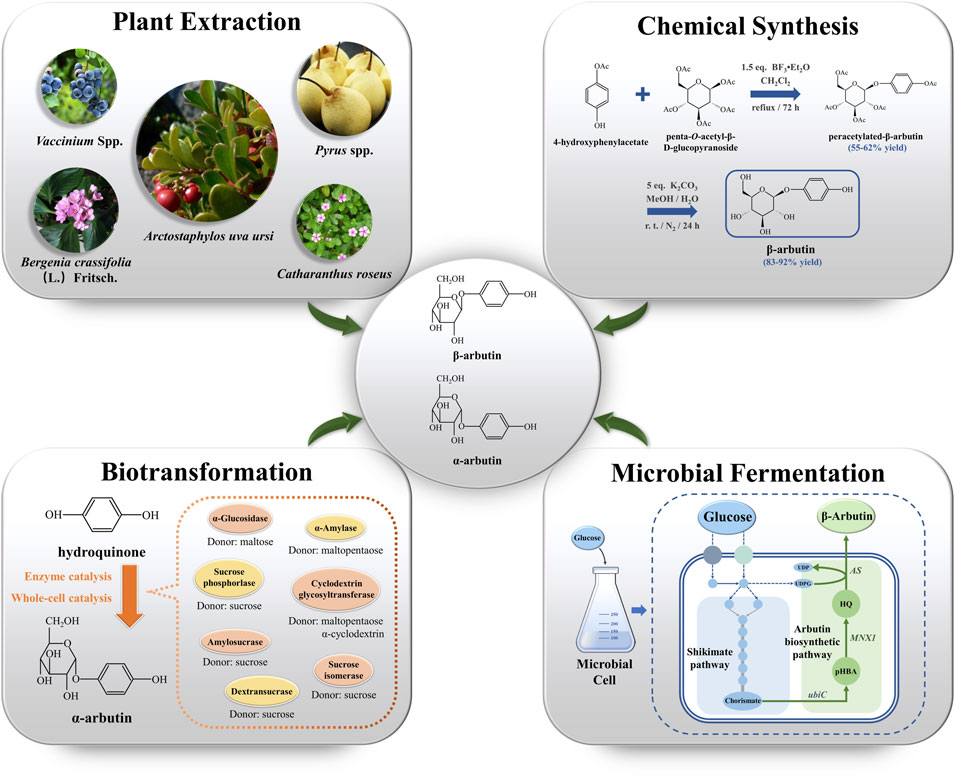
FIGURE 1. The production of arbutin via plant extraction, chemical synthesis, biotransformation, and microbial fermentation. ubiC encodes chorismate lyase; MNX1 encodes 4-hydroxybenzoate 1-hydroxylase; AS encodes arbutin synthase. UDPG: uridine diphosphate glucose; UDP: uridine diphosphate; pHBA: p-hydroxybenzoic acid; HQ: hydroquinone.
The primary natural source of β-arbutin is bearberry leaves (Arctostaphylos uvaursi) (Asensio et al., 2020). It is also found in pears (Cui et al., 2005), wheat, coffee, and tea (Migas & Krauze-Baranowska, 2015) (Figure 1). Although β-arbutin is widely available in plants, its extraction from plants is hindered by its low content, the complicated extraction process, and low purity of the extracted product. To overcome these drawbacks, plant cell culture techniques have been explored. In this approach, the glycosylation ability of plant cells is harnessed to convert exogenous HQ to β-arbutin. HQ catalyzed β-arbutin synthesis in Catharanthus roseus cells yielded 9.2 g/L β-arbutin (Inomata et al., 1991). Additionally, hairy roots of Brugmansia candida (Casas et al., 1998), Capsicum annuum (Lubitz et al., 2016), and Polygonum multiflorum have been used as plant cell reactors for β-arbutin synthesis (Table 1). Compared to plant extraction, plant cell culture techniques have the advantage of being independent of climate and other environmental factors in β-arbutin production. However, disadvantages of this method include the long production cycle, low yield, and difficulty in isolation and purification. The HQ addition time must be finally controlled since it has a significant influence on β-arbutin production. Currently, β-arbutin synthesis by plant cell culture method still cannot meet the requirements for industrial-scale production.
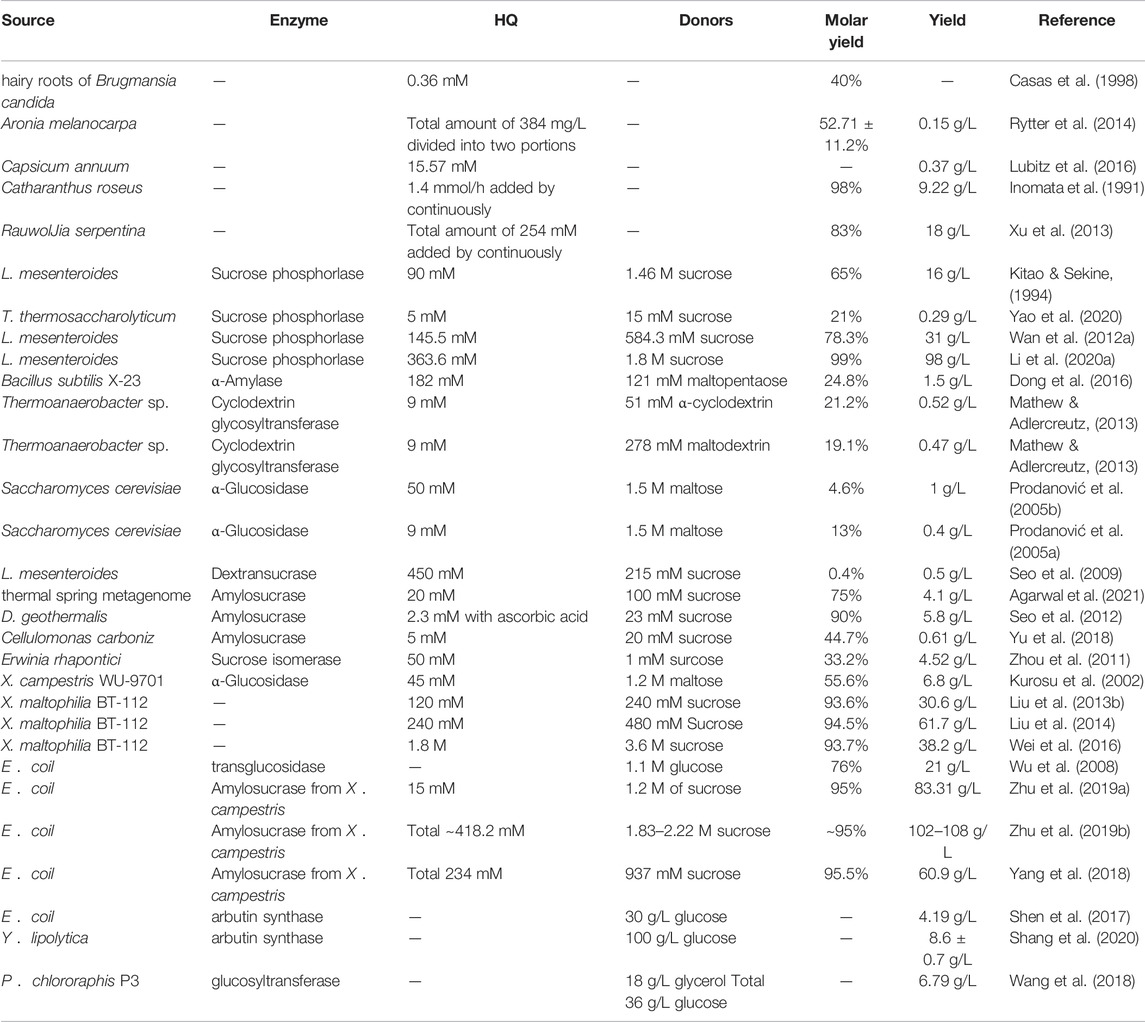
TABLE 1. Production of arbutin by plant cell reactors, enzymatic synthesis, and whole-cell catalysis.
Chemical synthesis is based on glucose with a protecting group and HQ that catalyze glycosylation. This is followed by the removal of the protecting group to obtain arbutin. Glycosylation is the crucial step in arbutin synthesis. The chemical synthesis of β-arbutin was first studied in 1912 (Zhou et al., 2019b). In the absence of solvent, glucose is treated with excess acetyl bromide. Next, the glycosyl bromide is treated with HQ under an alkaline condition to obtain tetra-acetyl arbutin. Finally, treatment with a barium hydroxide solution and acidification by CO2 yields β-arbutin.
Subsequent studies on the chemical synthesis of α-arbutin have been reported. Based on the different glycosyl donors used, chemical synthesis can be divided into the Koenigs-Knorr glycosylation, which uses bromo glucoside as the glycosyl donor, and the Helferich method, using an acyl group. The Helferich method dominates arbutin production due to its simplicity, low cost, and high product purity (Qiao et al., 2013). Tetra-O-benzyl-a-D-glucopyranosyl trichloroacetimidate as glucosyl donor with HQ reportedly obtained α-arbutin with a yield of 65% in a two-step reaction catalyzed by trimethylsilyl trifluoromethanesulfonate (Wang et al., 2006). The use of penta-O-acetyl-β-D-glucopyranoside and 4-hydroxyphenylacetate as aglycone donors for β-arbutin synthesis has also been studied (Figure 1). The substitution of penta-O-acetyl-α-D-glucopyranoside for penta-O-acetyl-β-D-glucopyranoside resulted in the production of unnatural α-arbutin without the production of its isomers (Chen et al., 2015). The use of 4-hydroxyphenylacetate is hindered by the lack of commercial availability; it must be synthesized through additional chemical steps. Although chemical synthesis can overcome the many disadvantages of natural extraction, it has a few drawbacks, such as the pronounced HQ toxicity, the labor-intensive nature of the process, strict reaction conditions, and low isomer selectivity. Thus, research has tended to focus on exploring arbutin production methods that are efficient, simple, and environment-friendly.
Biotransformation is an environment-friendly and mild synthesis approach that utilizes enzymes or whole cells as catalysts to convert HQ to arbutin. Compared with the chemical synthesis approach, arbutin production via biotransformation has the advantages of low energy consumption, reduced pollution, and high specificity.
Enzyme catalysis is the main route to synthesize α-arbutin. Glycosyltransferases (GTs) are used to produce α-arbutin from HQ and diverse glycosyl donors. GTs catalyze the formation of glycosidic bonds between glycosyl donors and acceptors. GTs are classified into two types, Leloir and non-Leloir, based on the catalytic properties (Xu et al., 2016). The Leloir type of GTs is most common. These use activated donor substrates, such as uridine diphosphate glucose, uridine diphosphate galactose, and uridine diphosphate rhamnose, for α-arbutin biosynthesis (Bungaruang et al., 2013). Non-Leloir type GTs do not require co-factors or activated substrates. Instead, they directly utilize the free energy from the cleavage of glycosyl donors, such as sucrose, starch, and their hydrolysis products, for α-arbutin biosynthesis (Plou et al., 2002). Currently, various GTs, including sucrose phosphorylase, α-amylase, cyclodextrin glycosyltransferase, α-glucosidase, dextransucrase, amylosesucrase, and sucrose isomerase, derived from different sources are used for α-arbutin production (Zhu et al., 2018) (Table 1) (Figure 1).
Sucrose phosphorylase (SPase), primarily derived from Leuconostoc sp., was the first GT demonstrated to be capable of transferring phosphorylated glucose or glucose groups produced by sucrose decomposition to other receptors to produce α-arbutin (Sugimoto et al., 2008). In 1994, the SPase of Leuconostoc mesenteroides was first demonstrated to be capable of catalyzing α-arbutin biosynthesis (Kitao & Sekine, 1994). The complex metabolic regulation mechanism of wild-type L. mesenteroides strains leads to limited SPase activity and low yields of α-arbutin. To improve the enzymatic activity and stability of SPase, a molecular biological technology approach was applied to construct a robust heterogeneous host to express SPase (Wan et al., 2012b). In this approach, SPase was overexpressed in Escherichia coli BL21 (DE3), and 98 g/L α-arbutin was synthesized by the purified recombinant enzyme under optimal conditions (Li et al., 2020b). In addition, co-expression of SPase from Thermoanaerobacterium thermosaccharolyticum (TtSPase) and molecular chaperone pG-TF2 (GroES-GroEL-Tig) resulted in 21% molar conversion of α-arbutin (Yao et al., 2020).
Cyclodextrin glucosyltransferase (CGTase), primarily derived from Thermoanaerobacter sp., is an extracellular enzyme that catalyzes the breakdown of starch and linear maltodextrin to produce cyclodextrins. CGTase catalyzes the transglycosylation of carbohydrates and non-carbohydrate compounds using its disproportionation activity. CGTase from Thermoanaerobacter sp. catalyzes transglycosylation of HQ and maltodextrin or α-cyclodextrin to produce α-arbutin. When α-cyclodextrin was used as a donor, the molar yield of α-arbutin was 21.2%. In addition, the use of a two-step enzymatic reaction system consisting of CGTase and amyloglucosidase increased the molar yield of α-arbutin to 30% (Mathew & Adlercreutz, 2013).
Amylosucrase (ASase), primarily derived from Deinococcus sp., is a transglucosidase that uses sucrose as the only glycosyl donor. ASase has superior catalytic efficiency for arbutin production (Tian et al., 2018). For instance, a novel ASase (ASmet) identified from the microflora metagenome of a thermal aquatic habitat displayed a 70% conversion efficiency from HQ to α-arbutin (Agarwal et al., 2019; Agarwal et al., 2021). Investigation of an ASase from Deinococcus geothermalis described that HQ in the reaction mixture was unstable, and the production yield was only 1.3%. However, the yield reached 90% with the addition of 0.2 mM ascorbic acid and a 10:1 M ratio of sucrose and HQ molecules (Seo et al., 2012). This study demonstrated that the oxidation of HQ was the main barrier in the catalytic reaction and that the addition of antioxidants could effectively inhibit oxidation and improve the yield of α-arbutin.
The greatest advantage of enzyme catalysis is that it can exclusively synthesize α-arbutin using reaction conditions that are milder and faster than chemical synthesis. However, the yield of α-arbutin varies with the type or source of enzyme, glycosyl donor, reaction time, and reaction temperature. Simultaneously, the toxic of HQ and high cost of activated substrates such as UDP-containing glycosyl donor restricted the application of enzyme catalysis. To address these problems, it is feasible to adopt flow addition strategy to control the concentration of HQ and develop whole cell catalysis for the synthesis of active glycosylated donors using endogenous metabolic pathways.
Microbial cells can also be directly used as catalysts. Cell lysis and enzyme purification are not required. Furthermore, the process can easily be scaled-up to produce valuable products (Wachtmeister & Rother, 2016). Recently, wild-type non-model and engineered model microorganisms have been used as cellular catalysts for the bioconversion of α-arbutin. For instance, lyophilized cells of Xanthomonas campestris WU-9701 harboring α-glycosidase have been utilized as biocatalysts to produce 42 mM α-arbutin from HQ in the presence of 1.2 mM maltose (Kurosu et al., 2002; Sato et al., 2012). Notably, mutant Xanthomonas maltophilia BT-112 produced α-arbutin at a titer of 61.7 g/L during dissolved oxygen-control pulse fed-batch fermentation. This result represents the highest reported α-arbutin titer using wild-type non-model microorganisms (Liu C.-Q. et al., 2013; Liu et al., 2014; Wei et al., 2016; Zhou et al., 2019a). The essence of whole-cell catalytic α-arbutin synthesis is using enzyme-catalyzed reactions in microorganisms. Although α-arbutin can be synthesized by whole-cell catalysis of wild-type strains, there are still some disadvantages that restrict its application include low GT expression and the unknown genetic background of the strains. To address this problem, recombinant E. coil harboring heterogenous GTs were employed as whole-cell catalysts to produce α-arbutin (Wu et al., 2008; Yang et al., 2018). Interestingly, batch-feeding whole-cell catalytic synthesis of α-arbutin using recombinant E. coli expressing ASase from X. campestris pv. 8004 (Amy-1) generated 306 mM of α-arbutin in 15 h with a 95% conversion rate of HQ (Zhu et al., 2019a). This production process was scaled-up in a 5000 L reactor and achieved 108 g/L of α-arbutin with a 95% molar conversion rate (Zhu et al., 2019b).
These results highlight the remarkable progress in generating and utilizing recombinant microorganisms harboring highly-expressed GTs for the whole-cell catalytic synthesis of α-arbutin. However, α-arbutin synthesis using whole-cell catalysis has certain limitations. The biosynthesis pathway of uridine diphosphate glucose (UDPG) in microorganisms are uncontrollable, and GT introduction frequently leads to unsatisfactory results. Modifying the biosynthesis pathway of UDPG to enhance GT catalytic efficiency is an active area of research.
α-arbutin production via biocatalysis has the advantages of single product composition and easy product separation. Nevertheless, excess HQ can lead to apoptosis of cells, limiting the substrate conversion efficiency, and affecting α-arbutin synthesis. To address this problem, a slow-release system was constructed by immobilizing a substrate capable of inhibiting cellular and enzymatic activities on a carrier that effectively reduces toxic substrate concentrations. Immobilizing HQ to the H107 resin reportedly improved the maximum tolerated concentration to 254 mM HQ in X. maltophilia BT-112. The α-arbutin productivity was 526% higher than that obtained using free HQ (Liu C. et al., 2013). In addition, immobilizing HQ to chitosan-coated magnetic nanoparticles allowed the production of 7.85 g/L α-arbutin during the catalysis of X. maltophilia BT-112 cells (He et al., 2021). In addition to immobilized substrates, immobilization approaches also have been developed to improve the stability and efficiency of biocatalysts for α-arbutin production. The ASase from Deinococcus geothermalis DSM 11300 (DgAS) could catalyze 400 mM HQ to produce 88.6 g/L α-arbutin, with 81% of 400 mM HQ converted in 30 min (Lee et al., 2018). Immobilization of DgAS on Amicogen LKZ118 beads resulted in the production of 95 g/L α-arbutin from 400 mM HQ, with the yield maintained between 85 and 90% in a single cycle (Lee et al., 2018).
Although high yields of α-arbutin have been obtained using biotransformation, the addition of HQ and extraction of GTs increases the cost of the process. Excess HQ added to improve the yield of arbutin can be toxic to cells, thereby inhibiting the catalytic activity of cells or enzymes. To address these problems, engineering high-performance arbutin-producing strains that produce arbutin via microbial fermentation is being explored as a promising approach (Figure 1).
To construct arbutin-producing microorganisms, the synthetic arbutin pathway must be elucidated. In 1997, a flavin adenine dinucleotide (FAD)-dependent 4-hydroxybenzoate 1-hydroxylase encoded by MNX1 was identified in Candida parapsilosis CBS604 (Figure 1). The enzyme catalyzes HQ synthesis from p-hydroxybenzoic acid (pHBA) (Eppink et al., 1997). In addition, arbutin synthase encoded by AS is a novel member of Class IV GTs belonging to the nucleotide recognition domain type 1β family (NRD1β) (Eberhardt et al., 2014) was discovered in Rauvolfa serpentina (Figure 1). This enzyme was demonstrated to catalyze β-arbutin synthesis from HQ using uridine diphosphate glucose as the glycosyl donor (Arend et al., 2001). The discovery of these two enzymes has made it possible to synthesize arbutin from glucose. MNX1 and AS were introduced into E. coli; the resulting bacteria were capable of synthesizing 54.71 mg/L of β-arbutin from glucose (Shen et al., 2017). This anabolic pathway was also shown to be feasible in Yarrowia lipolytica. Since the chorismate pyruvate lyase (UbiC), which can catalyze pHBA from chorismate, is unavailable, UbiC from E. coli should be introduced along with MNX1 and AS in Y. lipolytica. β-arbutin titer produced by engineered Y. lipolytica was 72.3 ± 2.1 mg/L after 72 h of fermentation (Shang et al., 2020). Additionally, a plasmid- and inducer-independent β-arbutin synthesis pathway involving MNX1, AS, and XanB2 (encoding chorismate pyruvate lyase), was constructed in P. chlororaphis P3. This pathway produced β-arbutin at a titer of 130 mg/L (Wang et al., 2018). These collective findings suggest that arbutin synthesis pathways from plants can be assembled into microorganisms.
Due to the insufficient supplementation of precursors, only a low yield of β-arbutin results from the assimilation of the arbutin synthesis pathway. Metabolic engineering strategies have been widely used to construct high-performance microbial strains to produce natural and non-natural compounds (Huccetogullari et al., 2019). Chorismate, the precursor in the arbutin biosynthetic pathway, is synthesized by the shikimate metabolic pathway. Modifying this pathway by metabolic engineering has been demonstrated as an effective approach to increase arbutin production. To improve β-arbutin production, overexpression of aroL (which encodes shikimate kinase II), ppsA (which encodes phosphoenolpyruvate synthetase), and tktA (which encodes transketolase) genes in the upstream pathway, and feedback inhibition resistant aroGfbr (which encodes 2-dehydro-3-deoxyphosphoheptonate aldolase) in E. coli SXL92 increased β-arbutin yield by 60-fold, with production reaching 3.29 g/L (Shen et al., 2017). Similarly, overexpression of DHS1 and DHS2 (which encode 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthase) in engineered Y. lipolytica increased β-arbutin yield to 819.6 mg/L (Shang et al., 2020). The collective results demonstrate that modifying the upstream pathway is beneficial in increasing β-arbutin production.
Since glucose is both a carbon source for cell growth and a substrate for β-arbutin production, optimizing the glucose supply is critical in improving β-arbutin production. Providing 30 g/L glucose was optimal for β-arbutin production by engineered E. coli; β-arbutin production reached 4.19 g/L in 48 h (Shen et al., 2017). Similarly, 100 g/L glucose proved to be optimal for engineered Y. lipolytica, with β-arbutin production reaching 8.6 ± 0.7 g/L, the highest yield reported to date (Shang et al., 2020). In addition, a mixture of glucose and pHBA supplemented by batch fermentation increased β-arbutin production in engineered P. chlororaphis P3 to 6.79 g/L (Wang et al., 2018).
Industrial arbutin production relies on chemical synthesis, which is hindered by the complicated operation steps, poor product stereoselectivity, and environmental concerns. In contrast, arbutin production through biotransformation has the advantages of easy access to raw materials, moderate fermentation conditions, short production period, high catalytic capacity, and environmental friendliness. Arbutin obtained by biotransformation is also easily purified as a single structure, which is crucial for industrial applications. The most effective and industrial-scale α-arbutin production has been achieved in recombinant E. coli expressing ASase from X. campestris. However, arbutin synthesis via biotransformation requires excellent biocatalysts, including high-performance GTs and recombinant microbial cells. These excellent biocatalysts could be obtained through directed evolution or protein structure-based targeted mutation of existing GTs. Furthermore, high-performing whole-cell catalysts can be achieved by optimizing GT expression, increasing the metabolic flux of the UDPG biosynthesis pathway, and co-expressing molecular chaperones.
Microbial fermentation provides a new candidate for direct arbutin synthesis from renewable carbon sources. This approach does not require additional HQ supplementation and enzyme extraction steps. The arbutin biosynthetic pathway has been assembled in various microorganisms, including E. coil, Y. lipolytica, and P. chlororaphis. However, the β-arbutin production titer is typically low. There are bottlenecks, such as tolerance, precursor supplement, and biosecurity, in the biosynthesis of aromatic compounds in E. coli and Y. lipolytica. To promote the microbial fermentation of arbutin, better microbial platforms must be developed. In recent years, inspired by the application of synthetic biology tools, a multitude of “generally regarded as safe” strains, such as Corynebacterium glutamicum and Bacillus subtilis, have been engineered to utilize the shikimate pathway to produce high value-added products. For example, recombinant C. glutamicum can produce 141 g/L shikimate (Kogure et al., 2016) and 82.7 g/L protocatechuic acid (Kogure et al., 2021). These yields are significantly higher than the yields obtained by other microorganisms. The biosynthesis of arbutin is derived from the shikimate pathway, like shikimate and protocatechuic acid. Thus, C. glutamicum has superior potential for arbutin biosynthesis.
KX planned and wrote the first manuscript. MX prepared the figure and wrote chapters, ZL and BY modified this manuscript. BZ supervised and finalized the manuscript. All authors read and approved the final manuscript.
This work was supported by grants from Major Discipline Academic and Technical Leaders Training Program of Jiangxi Province (No. 20212BCJ23012) Jiangxi Provincial Natural Science Foundation (No. 20202BAB213023), National Natural Science Foundation of China (No. 32000057). Jiangxi Province Postgraduate Innovation Special Fund Project (No. YC2021-S331).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Editage (www.editage.cn) for English language editing.
(HQ), hydroquinone ; (GTs), Glycosyltransferases ; (SPase), Sucrose phosphorylase ; (CGTase), Cyclodextrin glucosyltransferase ; (ASase), Amylosucrase ; (FAD), adenine dinucleotide ; (NRD1β), nucleotide recognition domain type 1β family ; (pHBA), p-hydroxybenzoic acid ; (UDPG), uridine diphosphate glucose.
Agarwal, N., Narnoliya, L. K., and Singh, S. P. (2019). Characterization of a Novel Amylosucrase Gene from the Metagenome of a Thermal Aquatic Habitat, and its Use in Turanose Production from Sucrose Biomass. Enzyme Microb. Technol. 131, 109372. doi:10.1016/j.enzmictec.2019.109372
Agarwal, N., Rai, A. K., and Singh, S. P. (2021). Biotransformation of Hydroquinone into α-arbutin by Transglucosylation Activity of a Metagenomic Amylosucrase. 3 Biotech. 11, 362. doi:10.1007/s13205-021-02909-2
Arend, J., Warzecha, H., Hefner, T., and Stöckigt, J. (2001). Utilizing Genetically Engineered Bacteria to Produce Plant-specific Glucosides. Biotechnol. Bioeng. 76 (2), 126–131. doi:10.1002/bit.1152
Asensio, E., Vitales, D., Pérez, I., Peralba, L., Viruel, J., Montaner, C., et al. (2020). Phenolic Compounds Content and Genetic Diversity at Population Level across the Natural Distribution Range of Bearberry (Arctostaphylos Uva-ursi, Ericaceae) in the Iberian Peninsula. Plants 9, 1250. doi:10.3390/plants9091250
Bungaruang, L., Gutmann, A., and Nidetzky, B. (2013). Leloir Glycosyltransferases and Natural Product Glycosylation: Biocatalytic Synthesis of theC-Glucoside Nothofagin, a Major Antioxidant of Redbush Herbal Tea. Adv. Synth. Catal. 355 (14-15), 2757–2763. doi:10.1002/adsc.201300251
Casas, D. A., Pitta-Alvarez, S. I., and Giulietti, A. M. (1998). Biotransformation of Hydroquinone by Hairy Roots ofBrugmansia candida and Effect of Sugars and Free-Radical Scavengers. Appl. Biochem. Biotechnol. 69 (2), 127–136. doi:10.1007/BF02919394
Chen, M., Chen, X., Wan, F., Zhang, B., Chen, J., and Xiong, Y. (2015). Effect of Tween 40 and DtsR1 on L-Arginine Overproduction in Corynebacterium Crenatum. Microb. Cell Fact. 14, 119. doi:10.1186/s12934-015-0310-9
Couteau, C., and Coiffard, L. (2016). Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 3, 27. doi:10.3390/cosmetics3030027
Cui, T., Nakamura, K., Ma, L., Li, J.-Z., and Kayahara, H. (2005). Analyses of Arbutin and Chlorogenic Acid, the Major Phenolic Constituents in Oriental Pear. J. Agric. Food Chem. 53, 3882–3887. doi:10.1021/jf047878k
Dong, X., Zhao, Y., Hu, J., Li, Y., and Wang, X. (2016). Attenuating L -lysine Production by Deletion of Ddh and lysE and Their Effect on L -threonine and L -isoleucine Production in Corynebacterium Glutamicum. Enzyme Microb. Technol. 93-94, 70–78. doi:10.1016/j.enzmictec.2016.07.013
Eberhardt, D., Jensen, J. V. K., and Wendisch, V. F. (2014). L-citrulline Production by Metabolically Engineered Corynebacterium Glutamicum from Glucose and Alternative Carbon Sources. Amb. Expr. 4, 85. doi:10.1186/s13568-014-0085-0
Eppink, M. H., Boeren, S. A., Vervoort, J., and van Berkel, W. J. (1997). Purification and Properties of 4-hydroxybenzoate 1-hydroxylase (Decarboxylating), a Novel Flavin Adenine Dinucleotide-dependent Monooxygenase from Candida Parapsilosis CBS604. J. Bacteriol. 179 (21), 6680–6687. doi:10.1128/jb.179.21.6680-6687.1997
Funayama, M., Arakawa, H., Yamamoto, R., Nishino, T., Shin, T., and Murao, S. (1995). Effects Ofα- Andβ-Arbutin on Activity of Tyrosinases from Mushroom and Mouse Melanoma. Biosci. Biotechnol. Biochem. 59 (1), 143–144. doi:10.1271/bbb.59.143
Hazman, Ö., Sarıova, A., Bozkurt, M. F., and Ciğerci, İ. H. (2021). The Anticarcinogen Activity of β-arbutin on MCF-7 Cells: Stimulation of Apoptosis through Estrogen Receptor-α Signal Pathway, Inflammation and Genotoxicity. Mol. Cell Biochem. 476 (1), 349–360. doi:10.1007/s11010-020-03911-7
He, J., Wei, M., Zhao, P., Xu, T., and Liu, C. (2021). Chitosan-coated Magnetic Nanoparticles Used as Substrate Immobilization Carrier for α-arbutin Biosynthesis Process. Colloid Interface Sci. Commun. 42, 100391. doi:10.1016/j.colcom.2021.100391
Huccetogullari, D., Luo, Z. W., and Lee, S. Y. (2019). Metabolic Engineering of Microorganisms for Production of Aromatic Compounds. Microb. Cell Fact. 18 (1), 1–29. doi:10.1186/s12934-019-1090-4
Inomata, S., Yokoyama, M., Seto, S., and Yanagi, M. (1991). High-level Production of Arbutin from Hydroquinone in Suspension Cultures of Catharanthus Roseus Plant Cells. Appl. Microbiol. Biotechnol. 36, 315–319. doi:10.1007/BF00208148
Jurica, K., Gobin, I., Kremer, D., Čepo, D. V., Grubešić, R. J., Karačonji, I. B., et al. (2017). Arbutin and its Metabolite Hydroquinone as the Main Factors in the Antimicrobial Effect of Strawberry Tree ( Arbutus Unedo L.) Leaves. J. Herb. Med. 8, 17–23. doi:10.1016/j.hermed.2017.03.006
Kitao, S., and Sekine, H. (1994). α-D-Glucosyl Transfer to Phenolic Compounds by Sucrose Phosphorylase fromLeuconostoc Mesenteroidesand Production of α-Arbutin. Biosci. Biotechnol. Biochem. 58 (1), 38–42. doi:10.1271/bbb.58.38
Kogure, T., Kubota, T., Suda, M., Hiraga, K., and Inui, M. (2016). Metabolic Engineering of Corynebacterium Glutamicum for Shikimate Overproduction by Growth-Arrested Cell Reaction. Metab. Eng. 38, 204–216. doi:10.1016/j.ymben.2016.08.005
Kogure, T., Suda, M., Hiraga, K., and Inui, M. (2021). Protocatechuate Overproduction by Corynebacterium Glutamicum via Simultaneous Engineering of Native and Heterologous Biosynthetic Pathways. Metab. Eng. 65, 232–242. doi:10.1016/j.ymben.2020.11.007
Kurosu, J., Sato, T., Yoshida, K., Tsugane, T., Shimura, S., Kirimura, K., et al. (2002). Enzymatic Synthesis of α-arbutin by α-anomer-selective Glucosylation of Hydroquinone Using Lyophilized Cells of Xanthomonas Campestris Wu-9701. J. Biosci. Bioeng. 93 (3), 328–330. doi:10.1263/jbb.93.32810.1016/s1389-1723(02)80037-8
Lee, H.-J., and Kim, K.-W. (2012). Anti-inflammatory Effects of Arbutin in Lipopolysaccharide-Stimulated BV2 Microglial Cells. Inflamm. Res. 61 (8), 817–825. doi:10.1007/s00011-012-0474-2
Lee, H., Kim, T.-S., Parajuli, P., Pandey, R. P., and Sohng, J. K. (2018). Sustainable Production of Dihydroxybenzene Glucosides Using Immobilized Amylosucrase from Deinococcus Geothermalis. J. Microbiol. Biotechnol. 28 (9), 1447–1456. doi:10.4014/jmb.1805.05054
Li, X., Xia, Y., Shen, W., Yang, H., Cao, Y., and Chen, X. (2020a). Characterization of a Sucrose Phosphorylase from Leuconostoc Mesenterides for the Synthesis of Alpha-Arbutin (In Chinese). Chin. J. Biotechnol. 36 (8), 1546–1555. doi:10.13345/j.cjb.190498
Liu, C.-Q., Deng, L., Zhang, P., Zhang, S.-R., Liu, L., Xu, T., et al. (2013b). Screening of High α-arbutin Producing Strains and Production of α-arbutin by Fermentation. World J. Microbiol. Biotechnol. 29 (8), 1391–1398. doi:10.1007/s11274-013-1302-8
Liu, C., Deng, L., Zhang, P., Zhang, S., Xu, T., Wang, F., et al. (2013a). Toward a Cost-Effective Method for α-arbutin Production by Using Immobilized Hydroquinone as a Glucosyl Acceptor. Process Biochem. 48 (10), 1447–1452. doi:10.1016/j.procbio.2013.03.005
Liu, C., Zhang, P., Zhang, S., Xu, T., Wang, F., and Deng, L. (2014). Feeding Strategies for the Enhanced Production of α-arbutin in the Fed-Batch Fermentation of Xanthomonas Maltophilia BT-112. Bioprocess Biosyst. Eng. 37 (2), 325–329. doi:10.1007/s00449-013-0980-9
Liu, L., Duan, X., and Wu, J. (2016). Modulating the Direction of Carbon Flow in Escherichia coli to Improve L -tryptophan Production by Inactivating the Global Regulator FruR. J. Biotechnol. 231, 141–148. doi:10.1016/j.jbiotec.2016.06.008
Lubitz, D., Jorge, J. M. P., Pérez-García, F., Taniguchi, H., and Wendisch, V. F. (2016). Roles of Export Genes cgmA and lysE for the Production of L-Arginine and L-Citrulline by Corynebacterium Glutamicum. Appl. Microbiol. Biotechnol. 100 (19), 8465–8474. doi:10.1007/s00253-016-7695-1
Mathew, S., and Adlercreutz, P. (2013). Regioselective Glycosylation of Hydroquinone to α-arbutin by Cyclodextrin Glucanotransferase from Thermoanaerobacter Sp. Biochem. Eng. J. 79, 187–193. doi:10.1016/j.bej.2013.08.001
Migas, P., and Krauze-Baranowska, M. (2015). The Significance of Arbutin and its Derivatives in Therapy and Cosmetics. Phytochem. Lett. 13, 35–40. doi:10.1016/j.phytol.2015.05.015
Plou, F. J., Martín, M. T., de Segura, A. G., Alcalde, M., and Ballesteros, A. (2002). Glucosyltransferases Acting on Starch or Sucrose for the Synthesis of Oligosaccharides. Can. J. Chem. 80 (6), 743–752. doi:10.1139/V02-104
Prodanović, R., Milosavić, N., Sladić, D., Zlatović, M., Božić, B., Veličković, T. Ć., et al. (2005a). Transglucosylation of Hydroquinone Catalysed by α-glucosidase from Baker's Yeast. J. Mol. Catal. B Enzym. 35 (4-6), 142–146. doi:10.1016/j.molcatb.2005.06.011
Prodanović, R. M., Milosavić, N. B., Sladić, D., Veličković, T. Ć., and Vujčić, Z. (2005b). Synthesis of Hydroquinone-α-Glucoside by α-glucosidasefrom Baker's Yeast. Biotechnol. Lett. 27 (8), 551–554. doi:10.1007/s10529-005-2880-9
Qiao, J.-q., Xu, D., Lian, H.-z., and Ge, X. (2013). Analysis of Related Substances in Synthetical Arbutin and its Intermediates by HPLC-UV and LC-ESI-MS. Res. Chem. Intermed. 41 (2), 691–703. doi:10.1007/s11164-013-1221-1
Rytter, J. V., Helmark, S., Chen, J., Lezyk, M. J., Solem, C., and Jensen, P. R. (2014). Synthetic Promoter Libraries for Corynebacterium Glutamicum. Appl. Microbiol. Biotechnol. 98 (6), 2617–2623. doi:10.1007/s00253-013-5481-x
Saeedi, M., Khezri, K., Seyed Zakaryaei, A., and Mohammadamini, H. (2021). A Comprehensive Review of the Therapeutic Potential of α‐arbutin. Phytotherapy Res. 35 (8), 4136–4154. doi:10.1002/ptr.7076
Sato, T., Hasegawa, N., Saito, J., Umezawa, S., Honda, Y., Kino, K., et al. (2012). Purification, Characterization, and Gene Identification of an α-glucosyl Transfer Enzyme, a Novel Type α-glucosidase from Xanthomonas Campestris Wu-9701. J. Mol. Catal. B Enzym. 80, 20–27. doi:10.1016/j.molcatb.2012.04.014
Seo, D.-H., Jung, J.-H., Ha, S.-J., Cho, H.-K., Jung, D.-H., Kim, T.-J., et al. (2012). High-yield Enzymatic Bioconversion of Hydroquinone to α-arbutin, a Powerful Skin Lightening Agent, by Amylosucrase. Appl. Microbiol. Biotechnol. 94 (5), 1189–1197. doi:10.1007/s00253-012-3905-7
Seo, E.-S., Kang, J., Lee, J.-H., Kim, G.-E., Kim, G. J., and Kim, D. (2009). Synthesis and Characterization of Hydroquinone Glucoside Using Leuconostoc Mesenteroides Dextransucrase. Enzyme Microb. Technol. 45 (5), 355–360. doi:10.1016/j.enzmictec.2009.07.011
Shang, Y., Wei, W., Zhang, P., and Ye, B.-C. (2020). Engineering Yarrowia Lipolytica for Enhanced Production of Arbutin. J. Agric. Food Chem. 68 (5), 1364–1372. doi:10.1021/acs.jafc.9b07151
Shen, X., Wang, J., Wang, J., Chen, Z., Yuan, Q., and Yan, Y. (2017). High-level De Novo Biosynthesis of Arbutin in Engineered Escherichia coli. Metab. Eng. 42, 52–58. doi:10.1016/j.ymben.2017.06.001
Su, Y., Sun, X., Wu, R., Zhang, X., and Tu, Y. (2020). Molecular Spectroscopic Behaviors of Beta-Arbutin in Anti-skin Cancer. Spectrosc. Lett. 53 (3), 172–183. doi:10.1080/00387010.2020.1715441
Sugimoto, K., Nomura, K., Nishiura, H., Ohdan, K., Nishimura, T., Hayashi, H., et al. (2008). Sucrose Phosphorylases Catalyze Transglycosylation Reactions on Carboxylic Acid Compounds. Biologia 63 (6), 1015–1019. doi:10.2478/s11756-008-0161-5
Tian, Y., Xu, W., Zhang, W., Zhang, T., Guang, C., and Mu, W. (2018). Amylosucrase as a Transglucosylation Tool: From Molecular Features to Bioengineering Applications. Biotechnol. Adv. 36 (5), 1540–1552. doi:10.1016/j.biotechadv.2018.06.010
Wachtmeister, J., and Rother, D. (2016). Recent Advances in Whole Cell Biocatalysis Techniques Bridging from Investigative to Industrial Scale. Curr. Opin. Biotechnol. 42, 169–177. doi:10.1016/j.copbio.2016.05.005
Wan, Y., Ma, J., Xu, R., He, A., Jiang, M., Chen, K., et al. (2012a). Properties of Sucrose Phosphorylase from Recombinant Escherichia coli and Enzymatic Synthesis of α-arbutin (In Chinese). Chin. J. Biotechnol. 28 (12), 1450–1459. doi:10.13345/j.cjb.2012.12.003
Wang, S., Fu, C., Bilal, M., Hu, H., Wang, W., and Zhang, X. (2018). Enhanced Biosynthesis of Arbutin by Engineering Shikimate Pathway in Pseudomonas Chlororaphis P3. Microb. Cell Fact. 17, 174. doi:10.1186/s12934-018-1022-8
Wang, Z.-X., Shi, X.-X., Chen, G.-R., Ren, Z.-H., Luo, L., and Yan, J. (2006). A New Synthesis of α-arbutin via Lewis Acid Catalyzed Selective Glycosylation of Tetra-O-Benzyl-α-D-Glucopyranosyl Trichloroacetimidate with Hydroquinone. Carbohydr. Res. 341 (11), 1945–1947. doi:10.1016/j.carres.2006.04.022
Wei, M., Ren, Y., Liu, C., Liu, R., Zhang, P., Wei, Y., et al. (2016). Fermentation Scale up for α-arbutin Production by Xanthomonas BT-112. J. Biotechnol. 233, 1–5. doi:10.1016/j.jbiotec.2016.05.022
Wu, P.-H., Nair, G. R., Chu, I.-M., and Wu, W.-T. (2008). High Cell Density Cultivation of Escherichia coli with Surface Anchored Transglucosidase for Use as Whole-Cell Biocatalyst for α-arbutin Synthesis. J. Ind. Microbiol. Biotechnol. 35 (2), 95–101. doi:10.1007/s10295-007-0270-0
Xu, L., Qi, T., Xu, L., Lu, L., and Xiao, M. (2016). Recent Progress in the Enzymatic Glycosylation of Phenolic Compounds. J. Carbohydr. Chem. 35 (1), 1–23. doi:10.1080/07328303.2015.1137580
Xu, M., Rao, Z., Yang, J., Dou, W., and Xu, Z. (2013). The Effect of a LYSE Exporter Overexpression on L-Arginine Production in Corynebacterium Crenatum. Curr. Microbiol. 67 (3), 271–278. doi:10.1007/s00284-013-0358-x
Yang, C., Fan, W., Zhang, R., Shi, J., Knežević-Jugović, Z., and Zhang, B. (2018). Study on Transglucosylation Properties of Amylosucrase from Xanthomonas Campestris Pv. Campestris and its Application in the Production of α-Arbutin. Catalysts 9, 5. doi:10.3390/catal9010005
Yao, D., Fan, J., Han, R., Xiao, J., Li, Q., Xu, G., et al. (2020). Enhancing Soluble Expression of Sucrose Phosphorylase in Escherichia coli by Molecular Chaperones. Protein Expr. PurificationPurif 169, 105571. doi:10.1016/j.pep.2020.105571
Yu, S., Wang, Y., Tian, Y., Xu, W., Bai, Y., Zhang, T., et al. (2018). Highly Efficient Biosynthesis of α-arbutin from Hydroquinone by an Amylosucrase from Cellulomonas Carboniz. Process Biochem. 68, 93–99. doi:10.1016/j.procbio.2018.02.012
Yu, X., Jin, H., Liu, W., Wang, Q., and Qi, Q. (2015). Engineering Corynebacterium Glutamicum to Produce 5-aminolevulinic Acid from Glucose. Microb. Cell Fact. 14, 183. doi:10.1186/s12934-015-0364-8
Zhou, H., Wang, B., Wang, F., Yu, X., Ma, L., Li, A., et al. (2019a). Chemo- and Regioselective Dihydroxylation of Benzene to Hydroquinone Enabled by Engineered Cytochrome P450 Monooxygenase. Angew. Chem. Int. Ed. 58 (3), 764–768. doi:10.1002/anie.201812093
Zhou, H., Zhao, J., Li, A., and Reetz, M. T. (2019b). Chemical and Biocatalytic Routes to Arbutin †. Molecules 24, 3303. doi:10.3390/molecules24183303
Zhou, X., Zheng, Y., Wei, X., Yang, K., Yang, X., Wang, Y., et al. (2011). Sucrose Isomerase and its Mutants from Erwinia Rhapontici Can Synthesise α-Arbutin. Ppl 18 (10), 1028–1034. doi:10.2174/092986611796378774
Zhu, L., Jiang, D., Zhou, Y., Lu, Y., Fan, Y., and Chen, X. (2019a). Batch-feeding Whole-Cell Catalytic Synthesis of α-arbutin by Amylosucrase from Xanthomonas Campestris. J. Ind. Microbiol. Biotechnol. 46 (6), 759–767. doi:10.1007/s10295-019-02143-z
Zhu, L., Xu, M., Lu, C., Chen, L., Xu, A., Fang, J., et al. (2019b). Optimization of Whole-Cell Biotransformation for Scale-Up Production of α-arbutin from Hydroquinone by the Use of Recombinant Escherichia coli. Amb. Expr. 9, 94. doi:10.1186/s13568-019-0820-7
Keywords: arbutin, plant extraction, chemical synthesis, biotransformation, microbial fermentation
Citation: Xu K-X, Xue M-G, Li Z, Ye B-C and Zhang B (2022) Recent Progress on Feasible Strategies for Arbutin Production. Front. Bioeng. Biotechnol. 10:914280. doi: 10.3389/fbioe.2022.914280
Received: 06 April 2022; Accepted: 19 April 2022;
Published: 09 May 2022.
Edited by:
Gilles Truan, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Francisco Solano, University of Murcia, SpainCopyright © 2022 Xu, Xue, Li, Ye and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Zhang, emhhbmdiaW4yOTE5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.