- 1Department of Mechanical and Aerospace Engineering, The University of Texas at Arlington, Arlington, TX, United States
- 2Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
In this manuscript, we have studied the microstructure of the axonal cytoskeleton and adopted a bottom-up approach to evaluate the mechanical responses of axons. The cytoskeleton of the axon includes the microtubules (MT), Tau proteins (Tau), neurofilaments (NF), and microfilaments (MF). Although most of the rigidity of the axons is due to the MT, the viscoelastic response of axons comes from the Tau. Early studies have shown that NF and MF do not provide significant elasticity to the overall response of axons. Therefore, the most critical aspect of the mechanical response of axons is the microstructural topology of how MT and Tau are connected and construct the cross-linked network. Using a scanning electron microscope (SEM), the cross-sectional view of the axons revealed that the MTs are organized in a hexagonal array and cross-linked by Tau. Therefore, we have developed a hexagonal Representative Volume Element (RVE) of the axonal microstructure with MT and Tau as fibers. The matrix of the RVE is modeled by considering a combined effect of NF and MF. A parametric study is done by varying fiber geometric and mechanical properties. The Young’s modulus and spacing of MT are varied between 1.5 and 1.9 GPa and 20–38 nm, respectively. Tau is modeled as a 3-parameter General Maxwell viscoelastic material. The failure strains for MT and Tau are taken to be 50 and 40%, respectively. A total of 4 RVEs are prepared for finite element analysis, and six loading cases are inspected to quantify the three-dimensional (3D) viscoelastic relaxation response. The volume-averaged stress and strain are then used to fit the relaxation Prony series. Next, we imposed varying strain rates (between 10/sec to 50/sec) on the RVE and analyzed the axonal failure process. We have observed that the 40% failure strain of Tau is achieved in all strain rates before the MT reaches its failure strain of 50%. The corresponding axonal failure strain and stress vary between 6 and 11% and 5–19.8 MPa, respectively. This study can be used to model macroscale axonal aggregate typical of the white matter region of the brain tissue.
Introduction
Under dynamic loading (e.g., rotational acceleration), the human brain tissue experiences shear deformation in different length scales (Wright and Ramesh, 2012), (Hasan et al., 2021a). The tissue level deviatoric deformation leads to cellular level stretching. The degree of stretching depends on the orientation and location of the individual axons. The rotational acceleration plane, the interface between white matter and gray matter, and the presence of stiff membranes can cause significant stress concentration at the cellular level (Smith and Meaney, 2000). This type of loading condition may lead to mechanical failure of the axonal microstructure and cause the most common pathological feature of Traumatic Brain Injury (TBI) called Diffuse Axonal Injury (DAI) (al Mahmud et al., 2020)– (Hasan et al., 2021b). However, we cannot directly measure or investigate DAI with the current technology (Wright, 2012). It is crucial to understand how the mechanical load is transferred from macro to microscale and to identify injury thresholds based on the cytoskeletal component failure. A multiscale computational approach is needed to bridge the injury information to measurable means. To understand how continuum scale tissue damage is correlated to the cellular microstructure, in this manuscript, we have studied and characterized the mechanical behavior of the axonal cytoskeletal core.
The brain is primarily classified into two regions at the tissue level: 1) gray matter and 2) white matter (see Figure 1A). The neuronal cells are randomly distributed in the gray matter giving its isotropic mechanical response (see Figure 1B); (Koser et al., 2015). The short-term shear modulus of the gray matter varies from (Kleiven, 2007)- (Shamloo et al., 2015) varies from 10–34 kPa (Zhang et al., 2004)– (Taylor and Ford, 2009). Neuronal cells, bundled together to form fiber tracts, are more organized in the white matter region. Therefore, the mechanical behavior is anisotropic in the white matter (Eskandari et al., 2021). The anisotropy can be introduced to the materials modeling of the white matter using diffusion tensor imaging (DTI) (Wright, 2012). One typical feature of white matter is that the glial cells extend their lipid bilayer and wrap the neurons called the myelin sheaths. Due to this intercellular network of the glial cells, white matter shows higher stiffness than gray matter (Weickenmeier et al., 2016). Coarse grain molecular dynamic simulations were performed to estimate the bulk modulus and Poisson’s ratio of lipid bilayer in the range of 21–54 MPa and 0.11–0.44, respectively. (el Sayed et al., 2008).-0.44, respectively (Jadidi et al., 2014), (Ayton et al., 2002). The excessive presence of the lipid bilayer in the myelin sheath could be another reason for higher stiffness in the white matter. The typical short-term shear modulus of the white matter region varies between (WATANABE et al., 2009)- (Chen et al., 1992) varies between 12–42 kPa (Zhang et al., 2004)– (Taylor and Ford, 2009). Several studies focused on characterizing white matter tissue properties in a bottom-up multiscale approach. In those studies, axon, and extracellular matrix (ECM) properties were used as input parameters, and a micromechanical method was used to estimate the effective properties (Arbogast and Margulies, 1999), (Yousefsani et al., 2018). In another study, Samad et al. (2013) used a top-down approach. The effective properties of axon and ECM were estimated using the white matter tissue-level properties from the relaxation tests (Javid et al., 2014). They have reported the short-term shear modulus of axon and ECM as 12.86 and 4.29 kPa, respectively. Similar stiffness of axon was also reported by Robert et al. (2007), who used the microneedle technique to stretch the PC12 neurites and found the elastic modulus to be 12 kPa (Bernal et al., 2007).
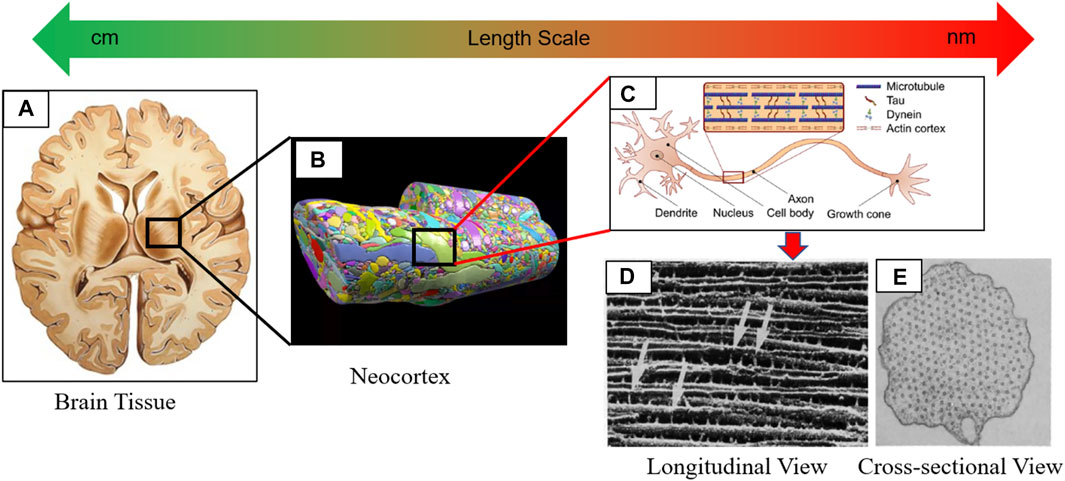
FIGURE 1. Depicting multiscale hierarchical structure of the brain tissue ranging from macroscale tissue to microscale neuronal cells. (A) Cross-sectional view of the brain tissue (gray and white matter) in the horizontal plane. (B) Reconstruction of the neocortex shows the heterogeneity posed by neuronal cells and ECM (Kasthuri et al., 2015). (C) Anatomy of the neuronal cell (de Rooij et al., 2017). (D) SEM image shows how axially oriented MT are cross-linked with the Tau. (C) A cross-sectional view of the transverse plane shows the hexagonal orientation of MT. (C–E) are adapted from (Chen et al., 1992)).
The inherent mechanical stiffness of the axon is due to its cytoskeletal components consisting of microtubule (MT), microfilament (MF), and neurofilament (NF) (see Figure 1C). Notably, a hexagonal array of MTs constructs a cross-linked network of viscoelastic nature with microtubule-associated protein tau (Tau) (see Figures 1D,E). Further study on the contribution of the axonal cytoskeleton components to the elastic properties of the axon was performed by Ouyang et al. (2013) (Ouyang et al., 2013). They carefully disrupted the individual cytoskeletal members by using Nocodazole, Cytochalasin D, and Acrylamide to disrupt MT, MF, and NF, respectively. When no drug was used, the transverse elastic modulus of the axon was found to be 9.5 kPa. The axonal elastic modulus was calculated from the atomic force microscopy (AFM) as 1.4, 5.7, and 3.4 kPa, respectively, by separately disrupting the MT, MF, and NF. It was concluded that the maximum stiffness of the axon came from the contribution of the MT cross-linked network. However, when all drugs were used, the elastic modulus of the axon was estimated to be 2 kPa which was very close to the computational estimation of the axonal membrane stiffness of 4.23 kPa (Zhang et al., 2017). Several studies were directed toward studying mechanical responses of axonal cytoskeleton components and reviewed in ref. (Khan et al., 2020), (Khan et al., 2021a). The viscoelastic response of MT, MF, NF, and Tau was studied in ref. (Adnan et al., 2018).– (Ishak Khan et al., 2021). Special attention was given to the MT-Tau network response in various loading conditions (e.g., tension, torsion) (Tang‐Schomer et al., 2010)– (Wu et al., 2019). Besides the structural failure, a cascade of complex molecular events follows the mechanical insult. The effect of phosphorylation initiates MT-Tau network disintegration much earlier than the fibers (e.g., MT and Tau) reach their failure strain (Khan et al., 2021c).
As discussed here, we can see that the study on the brain tissue mechanics using the top-down multiscale approach ends at the tissue to cellular level bridging. The high-fidelity full head models used the most refined mesh of 1–5.2 mm in size to study TBI using the finite element method (FEM) (Taylor and Ford, 2009), (Panzer et al., 2013)– (Chafi et al., 2010). Even in the lowest volume fraction, there could be thousands of axons within 1 mm mesh which must be resolved. Moreover, experimental observations indicated that the local cellular level strain was about 25% of the tissue level strain (Tamura et al., 2007). The heterogeneity and the interplay of the cellular microstructure is thought to be the reason of the inter-level mismatch (Montanino and Kleiven, 2018). Therefore, the threshold strain at the tissue level related to the TBI cannot be directly used to predict the DAI.
On the other hand, numerous studies were performed at the sub-cellular level. However, there are gaps between the sub-cellular level to the cellular level, and no attempts were made to use the bottom-up approach to connect these two levels. There is a need for bridging the cytoskeletal component responses to the effective properties of neuronal axons. The bridging will allow us to model the intermediate level between the cellular and tissue level, the axon aggregates. Understanding how cellular level strain is transmitted to the cytoskeletal sub-cellular level is also critical. Therefore, in this manuscript, we have addressed these two issues.
In the first part of this manuscript, we performed three-dimensional (3D) micromechanical viscoelastic characterization of the axon cytoskeletal core. We have developed the representative volume element (RVE) of the axonal microstructure for the micromechanical study. As discussed earlier, the primary fibers that carry the mechanical load and give stiffness to the axons are MTs. The MTs are packed unidirectionally in the hexagonal orientation and cross-linked with Tau (Chen et al., 1992). The axoplasm surrounding the MT-Tau cross-linked network is modeled as the matrix of the RVE. We have prepared four RVEs to perform the parametric study on the geometric variables (e.g., Tau radius, Tau spacing, and MT spacing). We have also varied the stiffness of the MT, and comparisons are discussed. Since there are three mutually orthogonal planes of symmetry in the RVE, the 3D mechanical response is orthotropic, and a total of nine viscoelastic relaxation functions are required to fully characterize the axonal core (Naik et al., 2008). As we have planned to use the relaxation functions as input parameters to develop the higher-level axon aggregate model, it is suitable for us to express the relaxation function as the Prony series. Six loading cases have been implemented on the RVE for the nine relaxation functions. Three of them are normal, and the rest are shear loading conditions (Wang et al., 2017). The homogenization is done by calculating the volume-averaged stress and strain of the RVE using the FEM (Sun and Vaidya, 1996a); (Barbero). The nonlinear regression fit is done between the volume-averaged stress and the hereditary integral expression of the Prony series coefficients by using the Levenberg-Marquardt algorithm (Gavin, 2019a), (Tzikang and Chen, 2000).
In the second part of this manuscript, we evaluated the axonal cytoskeletal damage threshold by varying the loading rates. The criteria for damage threshold are set to the MT and Tau failure strain of 50 and 40%, respectively (Janmey et al., 1991)– (Wegmann et al., 2011). Based on the previous studies, we have varied the strain rate from 10/s to 50/s with a 10/s increment in the direction of MT orientation (Elkin and Morrison, 2007)– (Morrison et al., 2003). The corresponding volume-averaged failure stress and strain of the axonal cytoskeletal core have been calculated and summarized.
Material and geometric properties of axon
We have taken a bottom-up approach from the multiscale point of view to develop the axon’s representative volume element (RVE) and characterize the mechanical properties. In doing so, we have modeled the detailed cytoskeletal components of the axon. The long stem-like part of the neuronal cell is the axon, and the main mechanical property is due to its microstructural components. Microtubules (MT) are the primary cytoskeletal component from which significant rigidity is contributed (Ouyang et al., 2013). Axially oriented MTs are cross-linked by the tau protein (Tau) of viscoelastic nature. The time-dependent viscoelastic nature of the axon is generally coming from the Tau. Figures 1C–E shows the detail of the microstructural components and their orientation.
The cross-sectional view of the axon indicates that the MTs are oriented in a hexagonal array, and most axonal microstructure studies model the MT bundle as such. Figures 2A,B shows how the MT bundles are cross-linked with Tau.
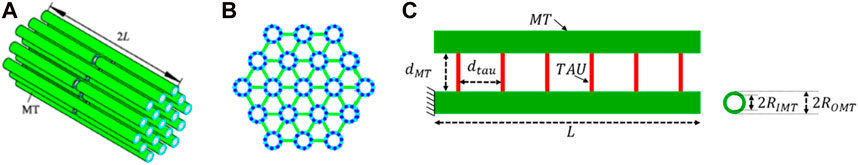
FIGURE 2. (A,B) Axonal microstructural model for analysis (adapted from (Wu et al., 2019)). (C) Geometric properties of the MT-Tau cross-linked network.
Just like any other microstructure of biomaterials, axonal microstructural parameters vary within a wide range. Figure 2C shows the geometric definition we have used as parameters. Table 1 summarizes those parameters and refers to the literature. The length of the MTs (
The Tau protein, on the other hand, shows viscoelastic mechanical behavior and is usually modeled as the Kelvin-Voigt model. The radius of Tau
As mentioned, the available material data for Tau protein is for the Kelvin-Voigt (KV) model. However, we have used ANSYS Mechanical for the viscoelastic characterization, and ANSYS only accepts viscoelastic parameters for General Maxwell (GM) model (Citation: “Ansys® Academic Research Mechanical, Release 18.1, Help System, Ansys APDL Theory Guide, ANSYS, Inc.”). Another challenge is that the relaxation modulus must be input for shear and bulk modulus. Therefore, we have first estimated the GM parameters for Young’s modulus by fitting creep responses of KV and GM. Then Alfrey’s correspondence principle was utilized to evaluate the shear relaxation parameters for Tau. Figure 3A shows the two steps of finding the parameters.
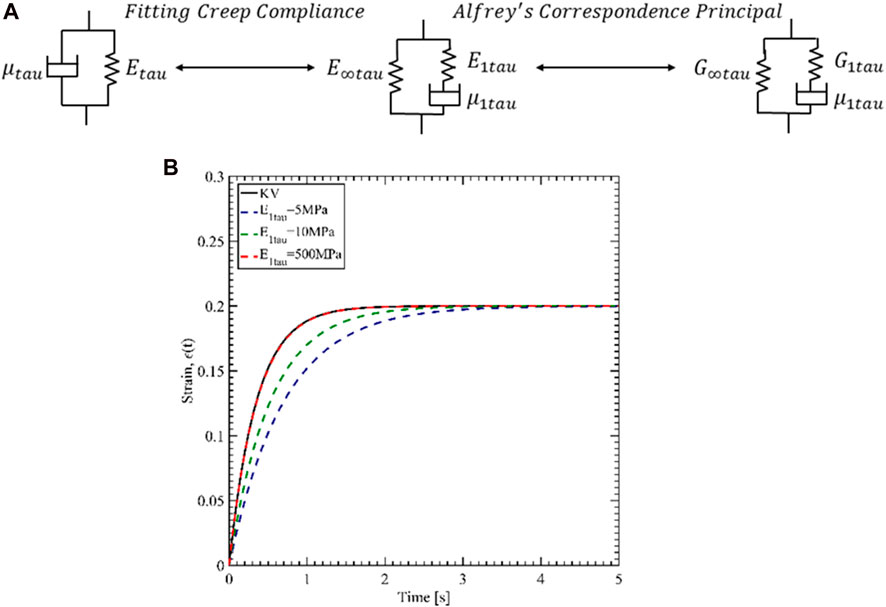
FIGURE 3. (A) Procedure of defining the Tau properties for ANSYS. (B) Creep response fit of 3-parameter General Maxwell to Kelvin-Voigt.
The constitutive relation between the stress (
Eqs 1, 2 are solved for creep loading (
Where
Once we have estimated the values of the 3-parameter GM model for Young’s modulus, we determined the shear modulus values. Alfrey’s correspondence principle states that the viscoelastic modulus is related to the Hookean linear elastic material in the frequency domain (Brinson and Brinson, 2008). In the linear elastic theory, the shear modulus and Young’s modulus is related by,
In the above equation, G, E, and
Where,
Considering constant Poisson’s ratio for Tau,
Where long term shear modulus,
Finally, the matrix is modeled as compressible Neo-Hookean material. The strain energy density function is given as,
Where,
Composite RVE modeling
The composite RVE of the axon is prepared for the finite element (FEM) micromechanical model. 4 RVEs are modeled by varying the geometric properties. The base RVE is called the RVE-1, and it is modeled for
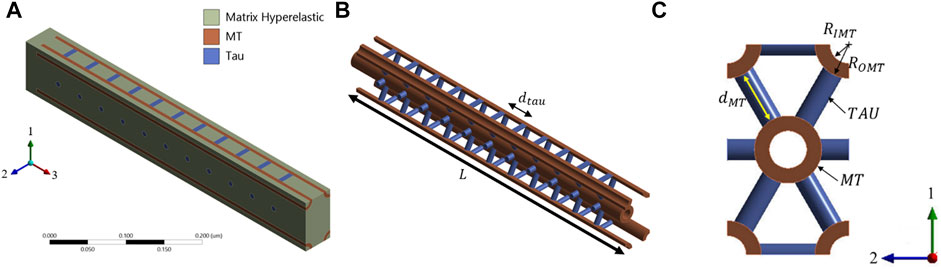
FIGURE 4. (A) Base RVE (RVE-1) for MT and Tau volume fractions of 0.185 and 0.036, respectively. MT is oriented in direction 3. (B) MT-Tau crosslinked network is shown with matrix hidden. (C) The unit cell of hexagonal array of MT is shown on the 1-2 plane.
Linear viscoelastic theory
To fully characterize the viscoelastic material properties of the axon, we have conducted relaxation tests. The relaxation test is defined as the time response of the stress as the strain remains constant. Since the viscoelastic material response is a combination of both viscous and elastic behaviors, the time-dependent stress is a function of the strain and time as,
Hereditary integral is used to represent the constitutive relation between the stress and strain under small deformation assumption as,
In the above equation,
For an anisotropic material
Due to the tensor symmetry, 9 independent relaxation functions are,
Where.
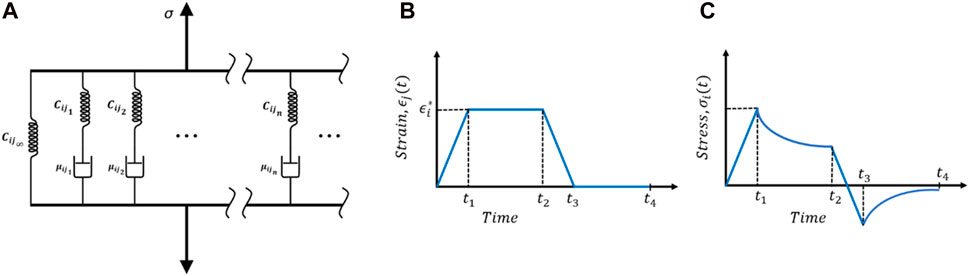
FIGURE 5. (A) General Maxwell viscoelastic model. Typical relaxation test: (B) time history of volume-averaged strain and (C) stress response.
The coefficients are defined as,
The relaxation time for each Maxwell element is,
And the short time modulus is related to the long-term modulus as,
Figures 5B,C show a typical relaxation loading condition. Ideally, the strain needed to be step-increased and kept constant for enough long time for the stress to relax. However, in practice, this is not possible; rather, strain is increased in a short period of time until
In the above two equations
Where,
Once the relaxation function is realized, then a nonlinear regression analysis is done to curve fit the coefficients of Eq. 13. For
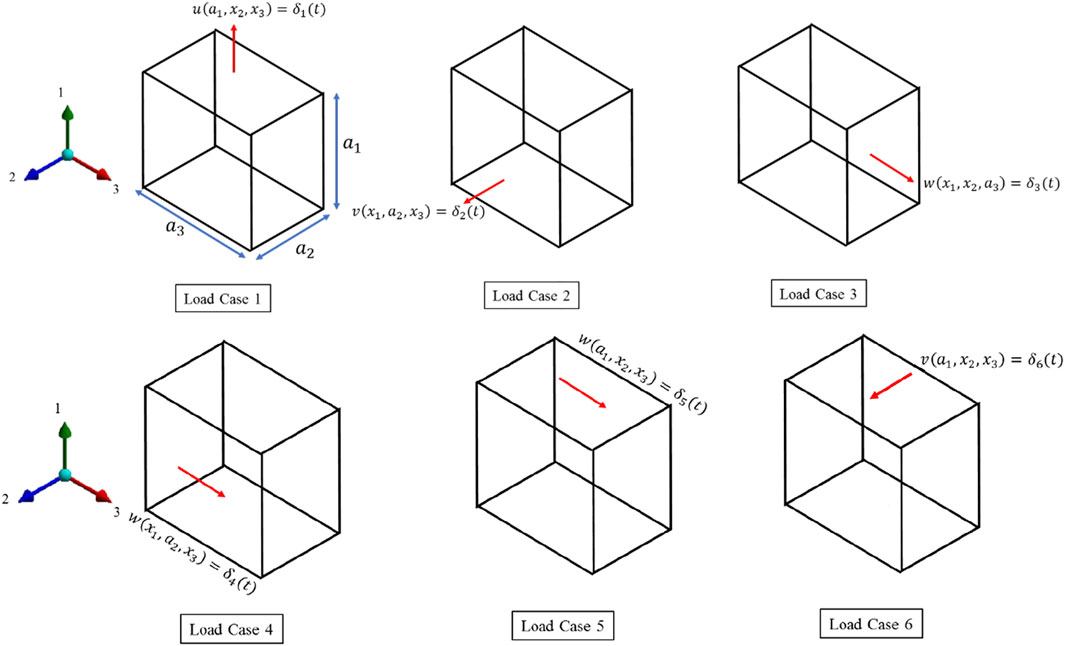
Load cases
As discussed in the previous section, 6 load cases are required for complete viscoelastic material characterization of the axon RVE. Figures 5B,C show a typical loading function and for all cases,
Load Case 1, 2 and 3
For the first three load cases, the face at
In the above equation,
Load Case 1
Load Case 2
Load Case 3
Load Case 4,5 and 6
Three shearing deformation is implemented as.
Load Case 4
Load Case 5
Load Case 6
Prony series fit to the time-dependent strain
Once we have the simulation data for the volume-averaged stress and strain (Eqs 16, 17) for different load cases, we can utilize a formulation that can be used to fit the Prony series parameters given in Eq. 13. The hereditary integral in Eq. 18 will be used by substituting the strain function (Eqs 14, 15) and the Prony kernel (Eq. 13).
Mathematical formulation
For the multiple loading process, the hereditary integral is done for the four steps of loading shown in Figures 5B,C. Considering
Step 2:
Step 3:
Step 4:
We have found that
Nonlinear regression for the curve fitting
The Prony series coefficients defined in Eqs 26–29 will be estimated using the nonlinear regression method. The Marquardt-Levenberg method has been implemented for the data fit (Press et al., 1989). The error function
Where,
Results and discussion
Viscoelastic characterization of axon
A total of 30 simulations have been carried out for 4 different RVEs. In the first 6 simulations, the Young’s Modulus of MT is set to 1.5 GPa and then increased to 1.9 GPa for another set of 6 simulations with RVE-1. Then rest of the 18 simulations are performed for three RVEs (RVE-2, RVE-3, and RVE-4) with
Load case 1, 2 and 3
The coefficients of the Prony series for the relaxation function
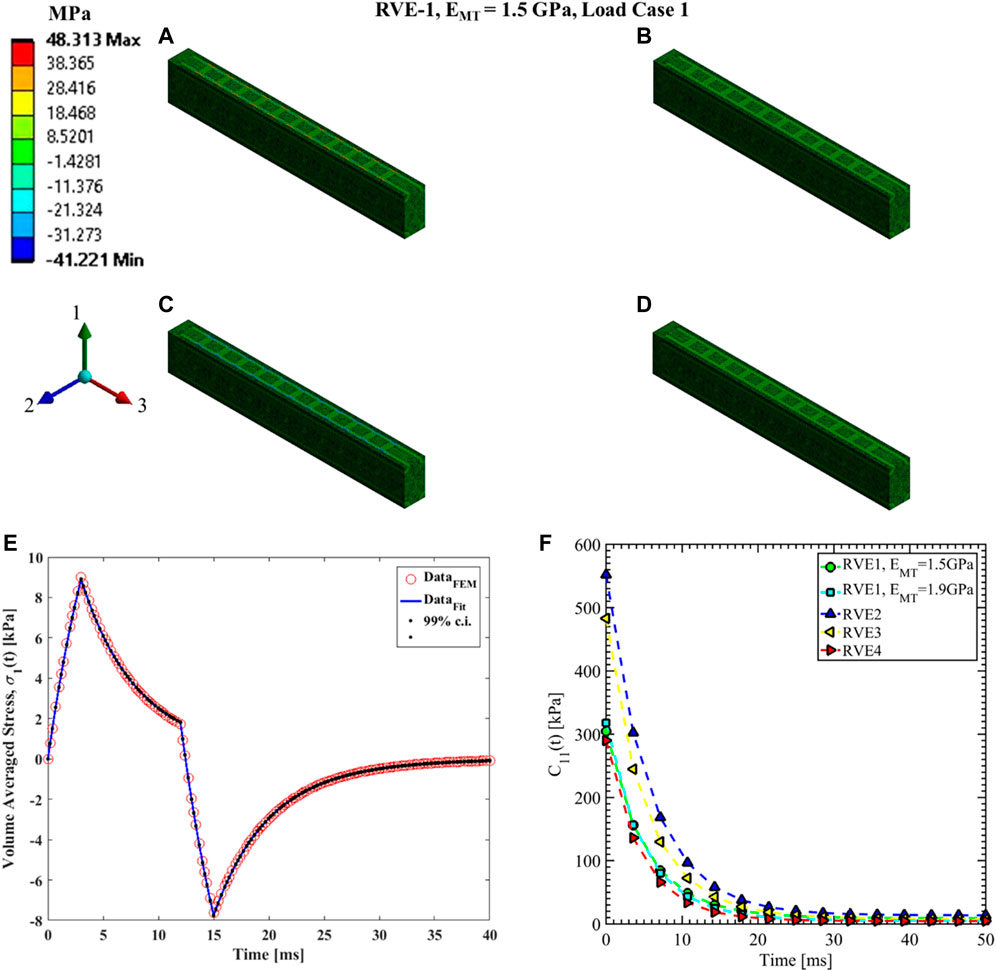
FIGURE 7. Contour plot of the element normal stress in direction 1 (
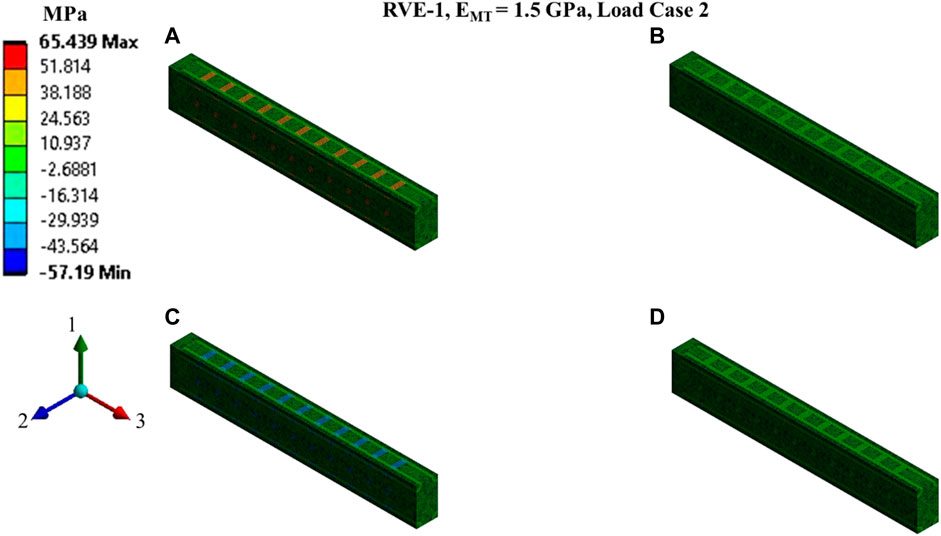
FIGURE 8. Contour plot of the element normal stress in direction 2 (
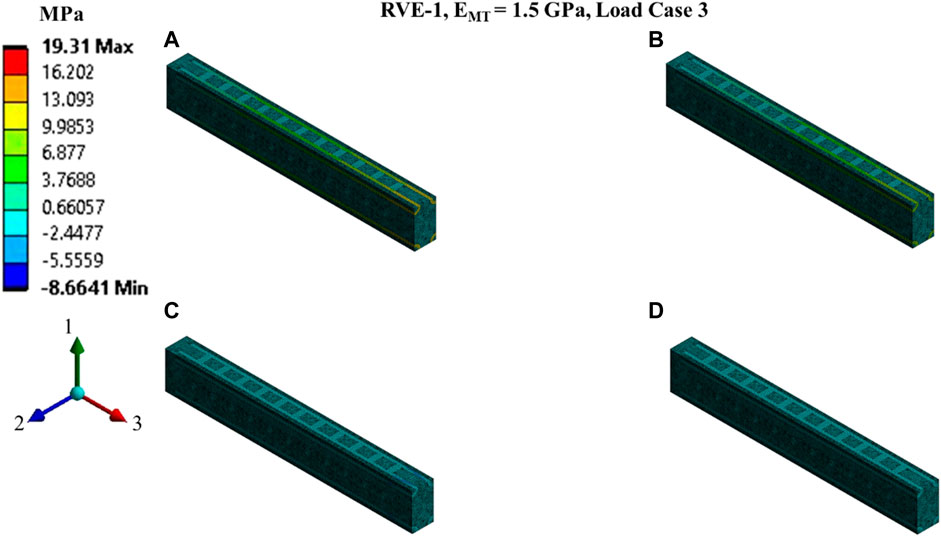
FIGURE 9. Contour plot of the element normal stress in direction 3 (
Nonlinear regression data fit for the Prony series coefficients using the volume-averaged stress from FEM simulations and Eqs 26–29 are shown in Figures 7E, 10A,C, 11A,C,E for load case 1 to 3, respectively. In those figures, DataFEM and DataFit corresponds to
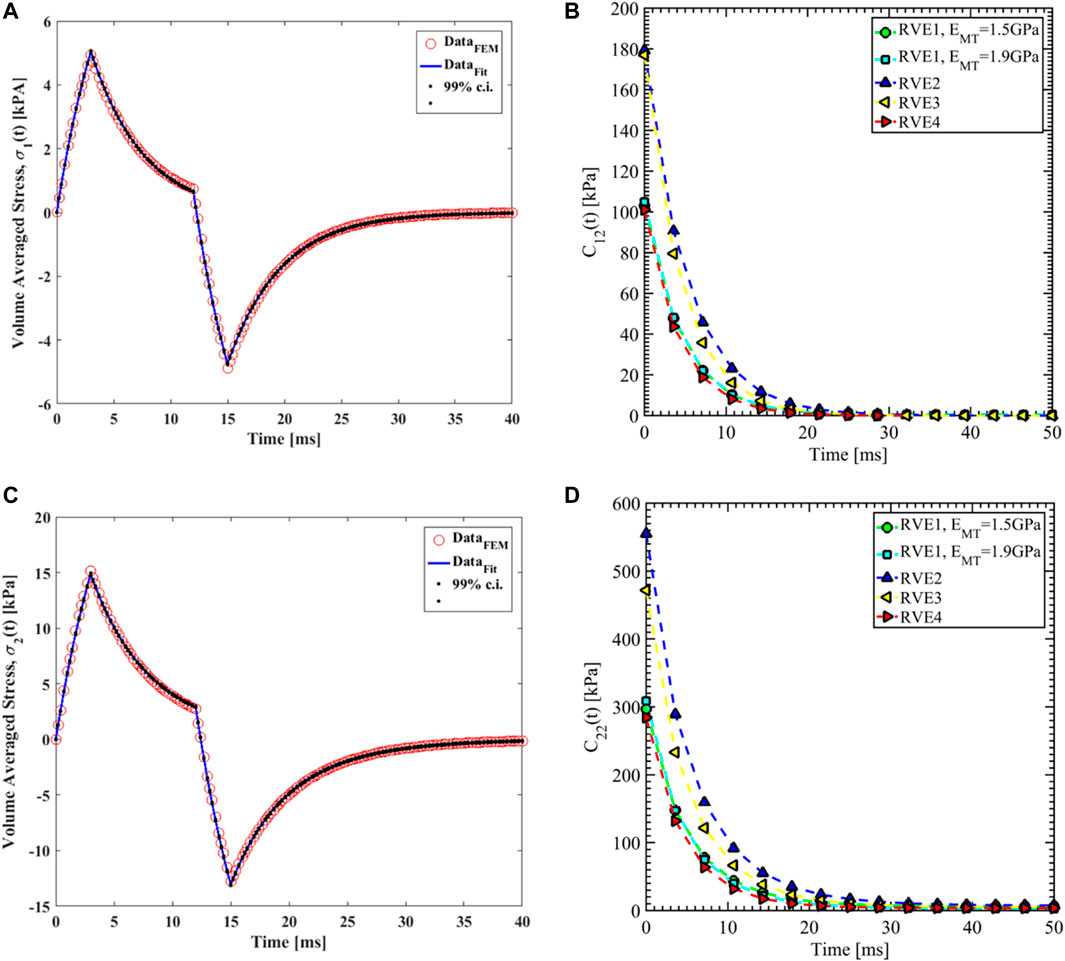
FIGURE 10. (A) Nonlinear regression data fit with 99% confidence interval (c.i.) is shown for volume averaged normal stress in direction 1 for RVE-1,
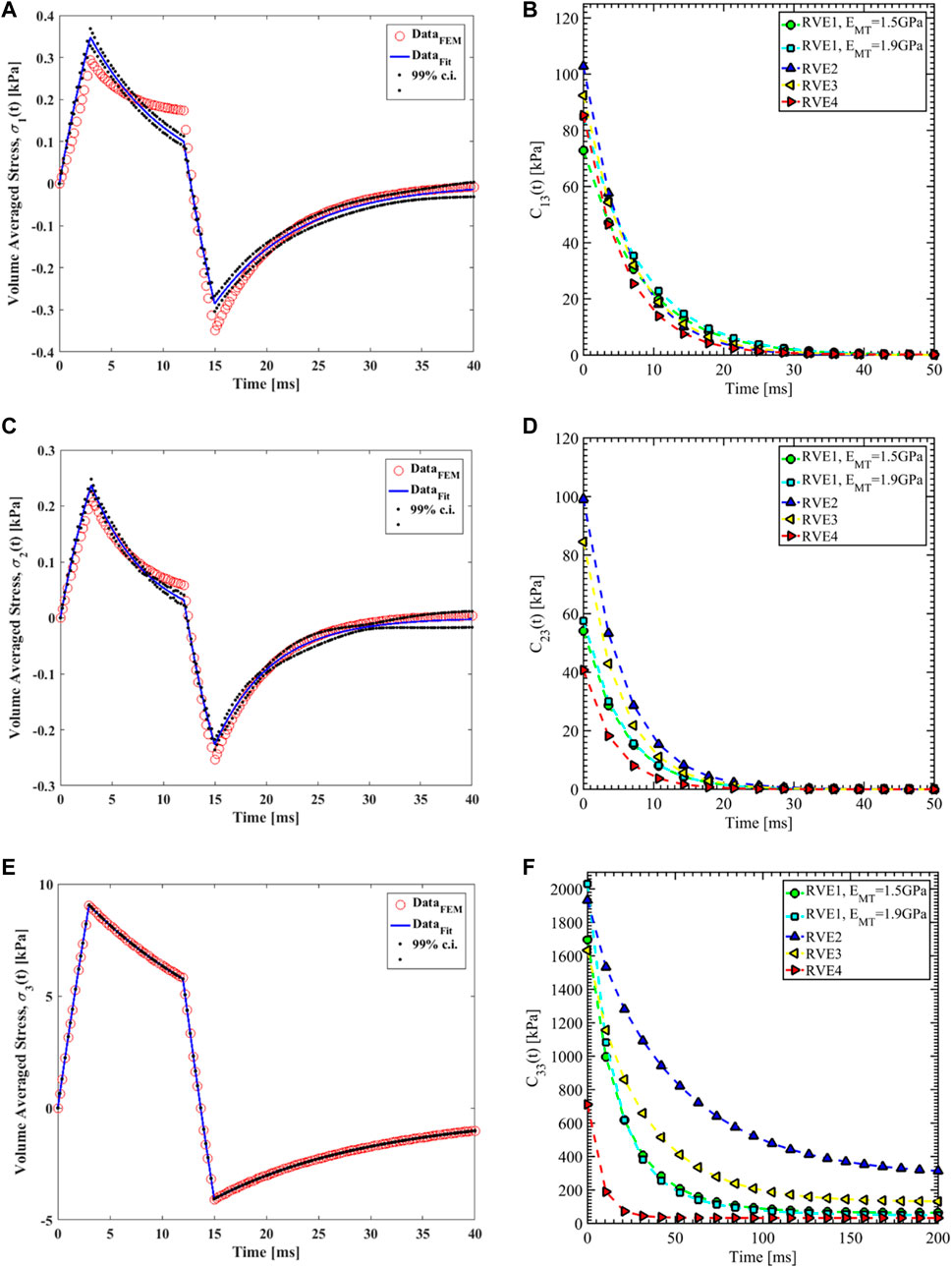
FIGURE 11. (A) Nonlinear regression data fit with 99% confidence interval (c.i.) is shown for volume averaged normal stress in direction 1 for RVE-1,
Since the orientation of the MT is in direction 3, Young’s modulus of MT has no significant effects on the relaxation functions in the transverse plane (
The volume fraction of Tau (
In RVE-4, the
Load case 4, 5 and 6
The shear relaxation functions are estimated using load cases 4, 5, and 6. Figures 12–14 show the elemental shear stress contour plots for the mentioned load cases on the RVE-1. Nonlinear regression data fit shown in Figures 12E, 13E, 14E, while the comparison of the relaxation function is shown in Figures 12F, 13F, 14F, respectively. Young’s modulus of MT has no significant effects on the shear relaxation functions. As we have seen earlier, the increasing volume fraction of Tau increases the short-term modulus in RVE-2 and RVE-3, respectively. Similarly, decreasing volume fraction of MT showed lower short-term modulus and short relaxation time. From Table 3, it is evident that shearing relaxation functions (
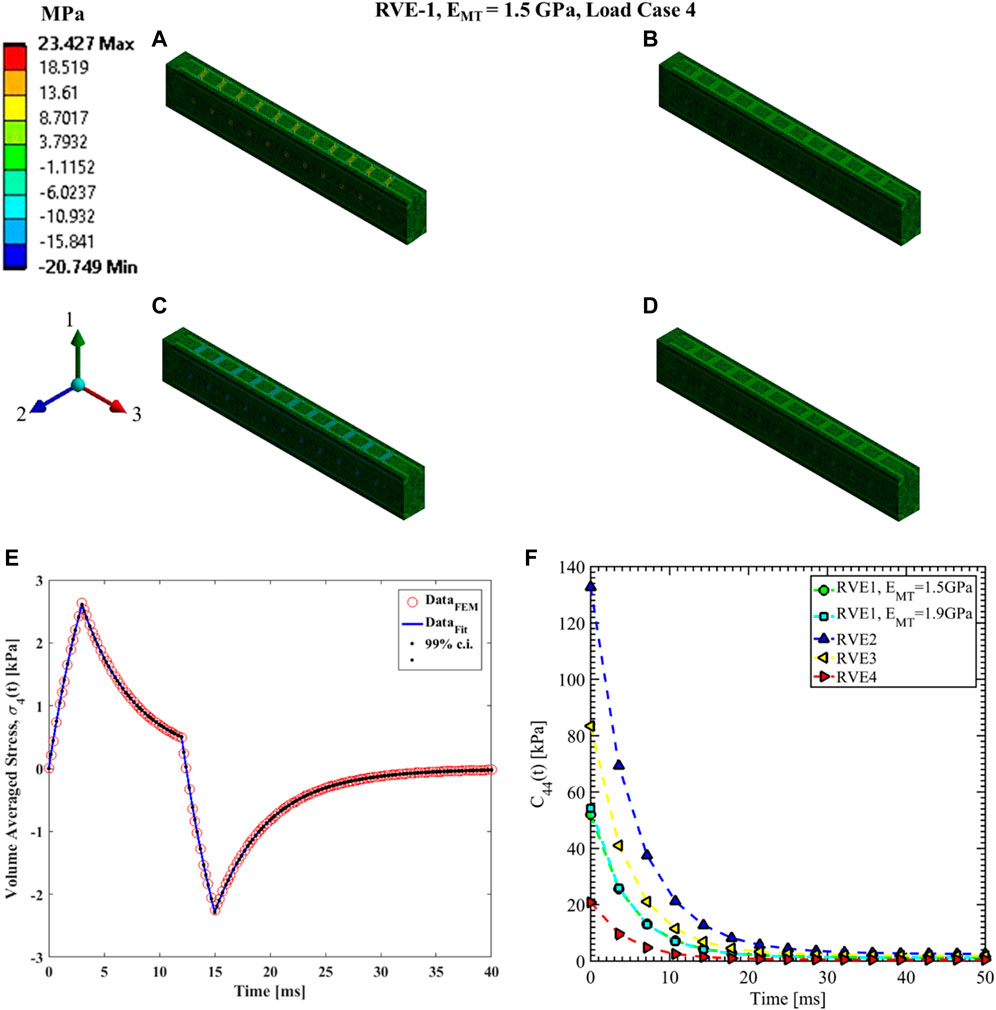
FIGURE 12. Contour plot of the element shear stress in directions 2 and 3 (
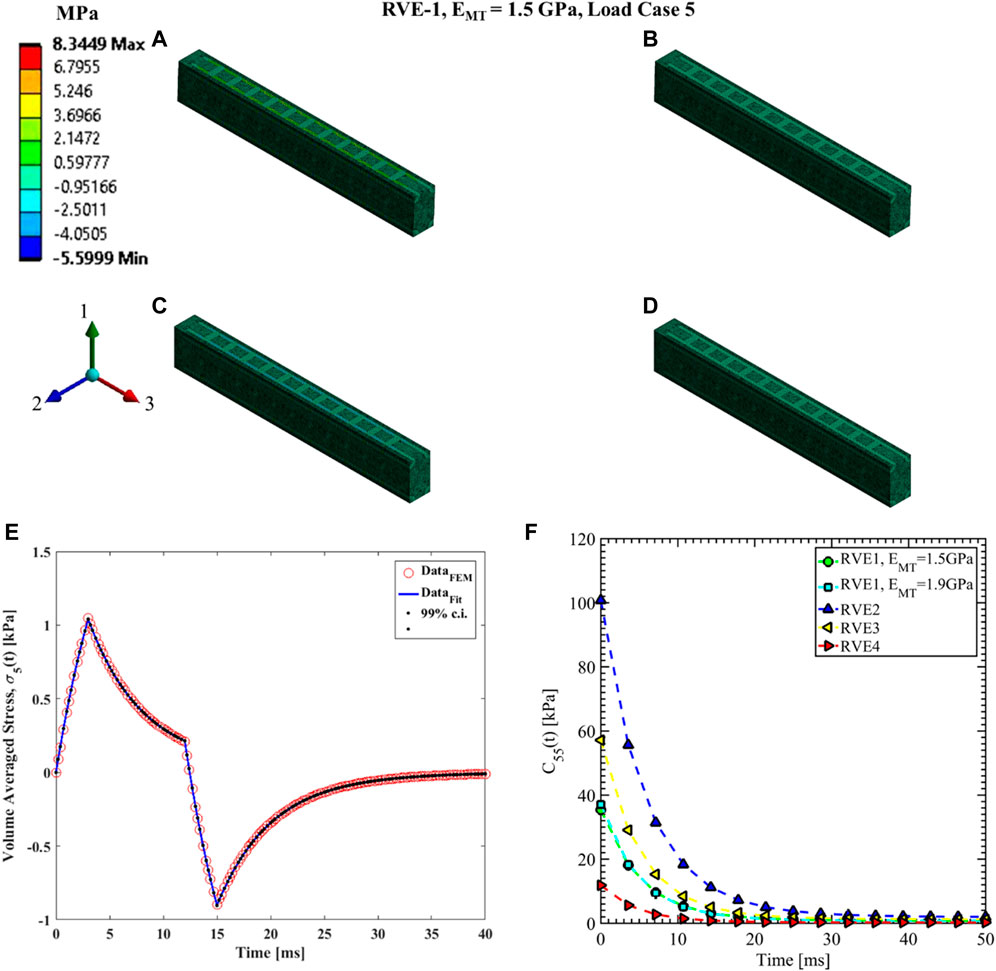
FIGURE 13. Contour plot of the element shear stress in directions 1 and 3 (
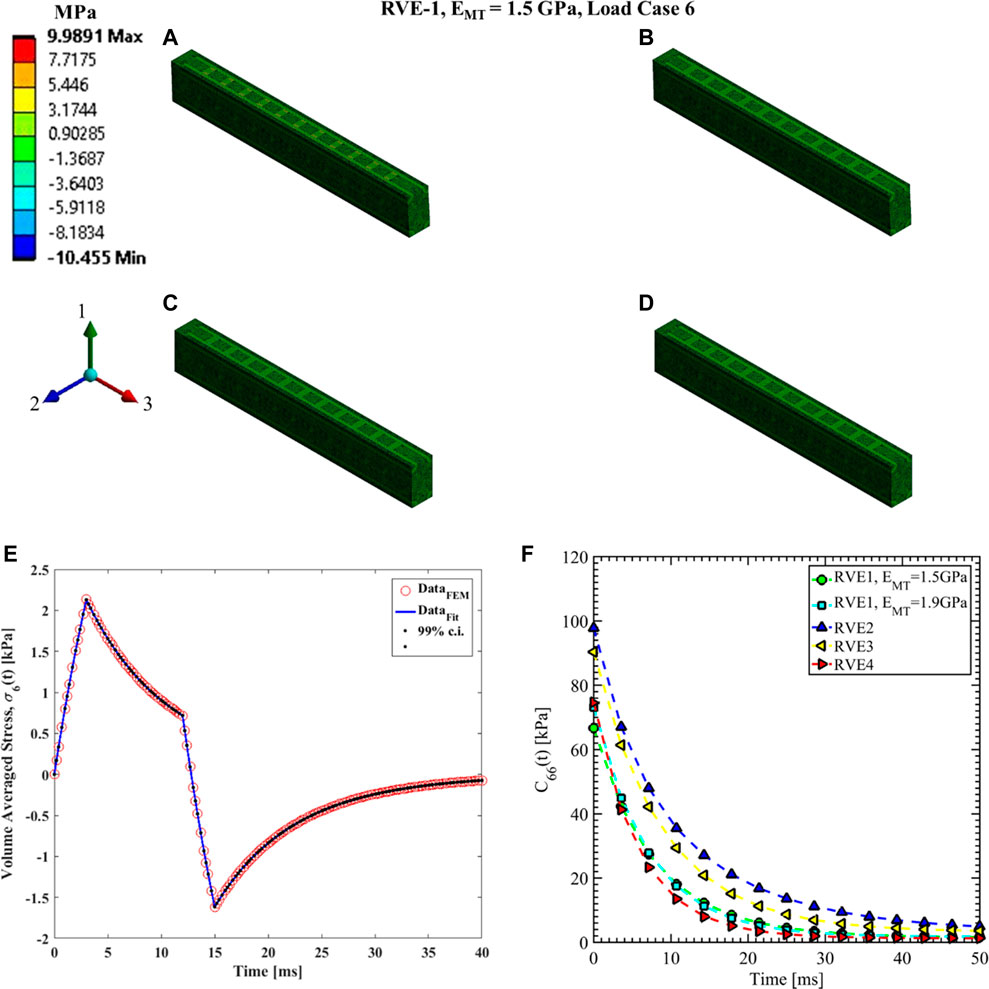
FIGURE 14. Contour plot of the element shear stress in directions 1 and 2 (
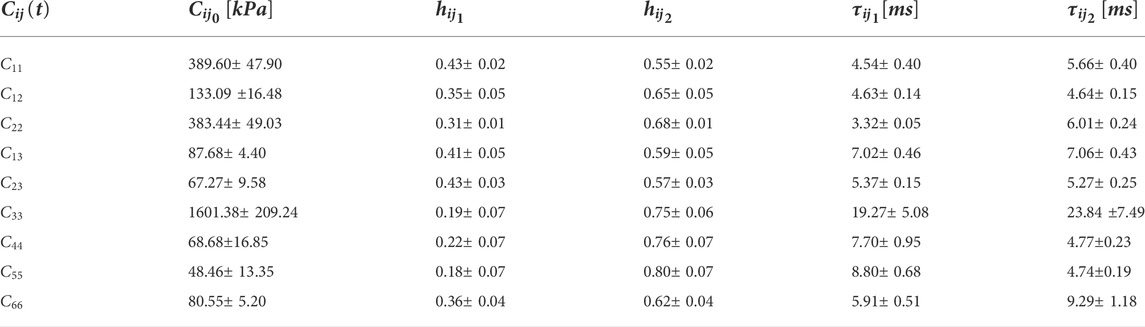
In Table 3, we have summarized the mean value of the Prony series coefficients for all 5 observations along with the standard error defined as
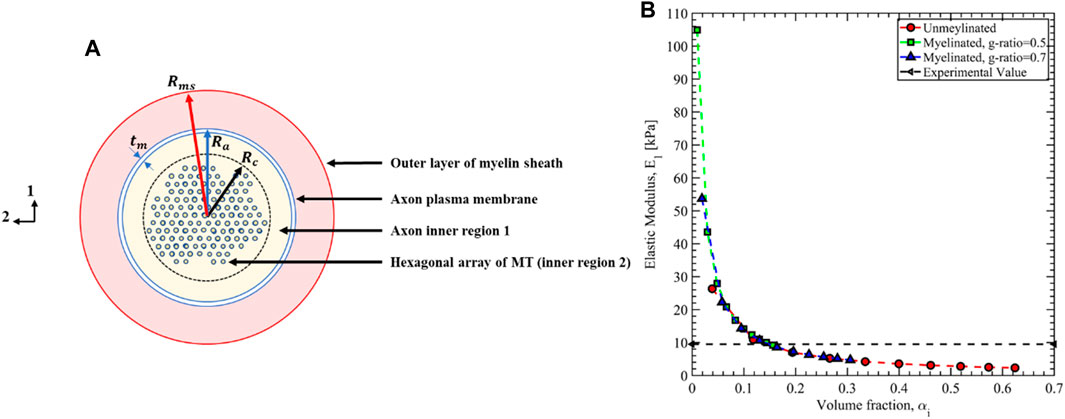
FIGURE 15. (A) Cross-sectional view of the axon microstructure. (B) The axon’s effective transverse elastic modulus is estimated using the inverse rule of mixture based on the composite theory.
For the unmyelinated axon
Damage Criteria of Axon Based on the Cytoskeletal Component Failure
In the second part of this manuscript, we have studied the axonal damage threshold based on the cytoskeletal component failure for different loading rates. As mentioned earlier, the load path of a mechanical insult extends from the tissue level to the cellular level and finally to the cytoskeletal level. It is experimentally verified that the maximum local strain in the axon is about only 25% of the tissue level strain (Tamura et al., 2007). This is perhaps due to the undulation of the neuronal axons (Wright, 2012) and the heterogeneity of the cellular microstructure (Montanino and Kleiven, 2018). In this manuscript, we have directly evaluated the cellular level stress and strain by calculating the volume-averaged stress and strain of the RVEs and correlating the cytoskeletal failure as the damage threshold criteria. We have considered the failure strain of the MT and Tau as 50 and 40%, respectively (Janmey et al., 1991)– (Wegmann et al., 2011). A total of 5 loading rates have been introduced in the direction of the MT orientation (direction 3). The strain rates are varied from 10/s to 50/s with a 10/s increment. A total of 25 simulations have been performed for 5 RVE cases (e.g., RVE-1 with
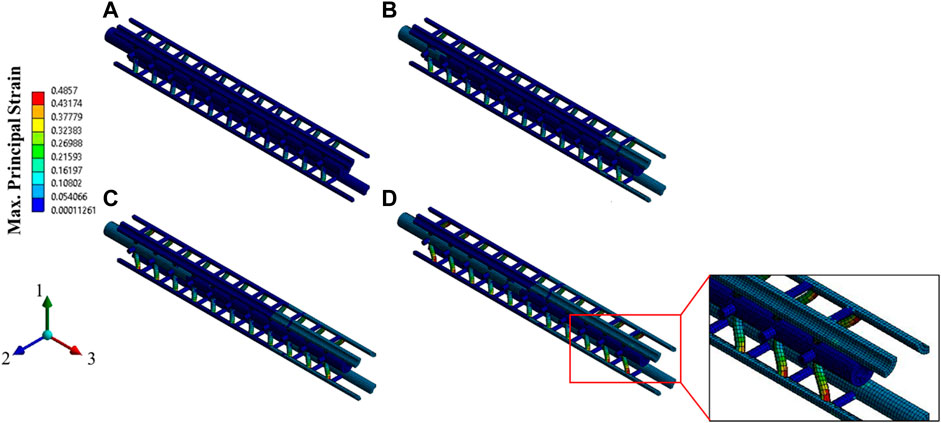
FIGURE 16. Contour plot of the maximum principal strain (elemental mean) for the strain rate of 5/sec, at (A) 2.5% strain, (B) 5% strain, (C) 7.5% strain, and (D) 10% strain (the matrix is hidden to show the strain on the Tau proteins).
In Figure 17A, we have plotted the strain vs. strain rate curve for different RVEs. For the Base RVE (i.e., RVE-1 with
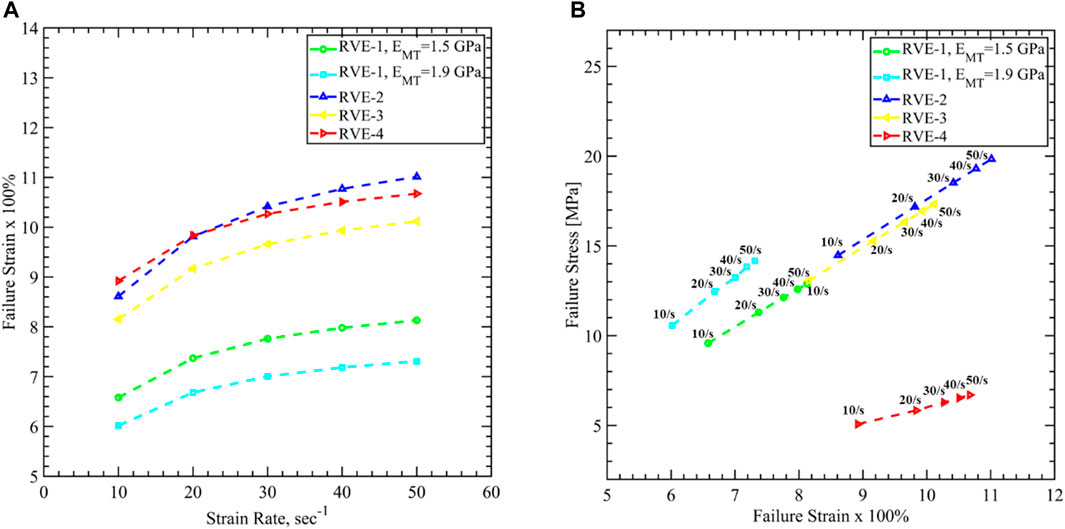
FIGURE 17. Axon cytoskeletal damage evaluation by mapping damage threshold values for (A) axon volume averaged failure strain vs. strain rate, and (B) axon volume averaged failure stress vs. axon volume averaged failure strain for different RVEs. 40% strain is taken as the failure strain of Tau, and corresponding axonal stress and strain are plotted for different loading rates.
Finally, in Figure 17B, we have plotted the failure stress vs. failure strain for different RVEs. In experimental observations, both in-vitro and in-vivo, cellular level stress cannot be identified. The current work can address this issue using the FEM analysis, and the failure stress value can be used for further study in larger FEM models. For the base RVE, the failure stress varies between 9.5 and 12.8 MPa as the strain rate increases. Increasing stiffness of MT shows higher failure stress from 10.5 to 14.1 MPa while decreasing
Conclusion
We have studied the cytoskeletal microstructure of the neuronal axon and developed the RVE to characterize the viscoelastic response. A total of six load cases are needed to estimate nine independent relaxation moduli, and they are summarized in the manuscript for further use in the axon aggregate model. The short time modulus
In the second part, we have studied axon damage based on the cytoskeletal component failure. The loading rate is varied between 10/s to 50/s to be consistent with the previous studies. The axon failure strain varies between 6.01 and 11.01%, considering Tau fails at 40% strain. Due to the limitation of achieving conversed FEM solution for the large deformations, all simulations are carried out up to 12% strain of the RVEs for all strain rates. We have not observed the MT reach its failure strain of 50% in this range of stretch. However, Tau failure is a good indication of the structural damage of the axon cytoskeleton, which can lead to functional damage as well. A recent study showed that axon failed at 7.3% strain via Tau failure when subjected to the loading rate <100/s, which agrees well with the results shown in this work (Estrada et al., 2021). The failure stress is also estimated and varies between 5 and 19.8 MPa.
As mentioned earlier, previous studies based on neuronal injury had identified both strain and strain rate threshold values; however, Wright (2012) identified that it is difficult to determine the combined effects of strain and strain rate from the available literature (Wright, 2012). Not to mention that it is also challenging to identify the stress level of the damaged axon experimentally in-vitro and in-vivo. Therefore, this work can address this issue by correlating the axon’s strain to strain rate and the failure stress level at the cellular level, which can be utilized in further studies of axon aggregates using FEM models. However, we need to address that due to the lack of data related to the Tau failure strain as a function of strain rate, we have used a fixed failure strain of 40%. Other than that, the cross-linked network of MT and Tau can also disintegrate by Tau detachment due to phosphorylation. The interfacial strength of Tau binding sites with MT is not included; instead, a perfect bonded condition is used in the FEM study. This also leads to higher stiffness along the MT direction. Other than the structural damage of the cytoskeletal microstructure, there are other forms of event that can lead to diffuse axonal injury (DAI) due to a mechanical insult (Montanino et al., 2021). Morphological damage due to the axonal swelling, mechanoporation through the plasma membrane, and glial cells damage can initiate a complex cascade of molecular events that can further cause cytoskeletal damage, which is also not investigated.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
FH: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Writing—Original Draft. KM: Resources, Writing—Review & Editing. MK: Resources, Visualization. AA: Conceptualization, Methodology, Validation, Writing—Review & Editing, Funding acquisition.
Funding
This work has been funded by the Force Health Protection (FHP) program through the Office of Naval Research (ONR) (Awards #N00014-21-1-2051, N00014-21-1-2043 and N00014-18-1-2082- Dr. Timothy Bentley, Program Manager).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adnan, A., Qidwai, S., and Bagchi, A. (2018). On the atomistic-based continuum viscoelastic constitutive relations for axonal microtubules. J. Mech. Behav. Biomed. Mat. 86, 375–389. doi:10.1016/j.jmbbm.2018.06.031
Ahmadzadeh, H., Smith, D. H., and Shenoy, V. B. (2014). Viscoelasticity of tau proteins leads to strain rate-dependent breaking of microtubules during axonal stretch injury: Predictions from a mathematical model. Biophys. J. 106 (5), 1123–1133. doi:10.1016/j.bpj.2014.01.024
al Mahmud, K. A. H., Hasan, F., Khan, M. I., and Adnan, A. (2020). On the molecular level cavitation in soft gelatin hydrogel. Sci. Rep. 10 (1), 9635. doi:10.1038/s41598-020-66591-9
Arbogast, K. B., and Margulies, S. S. (1999). A fiber-reinforced composite model of the viscoelastic behavior of the brainstem in shear. J. Biomechanics 32 (8), 865–870. doi:10.1016/S0021-9290(99)00042-1
Ayton, G., Smondyrev, A. M., Bardenhagen, S. G., McMurtry, P., and Voth, G. A. (2002). Calculating the bulk modulus for a lipid bilayer with nonequilibrium molecular dynamics simulation. Biophys. J. 82 (3), 1226–1238. doi:10.1016/S0006-3495(02)75479-9
Bain, A. C., and Meaney, D. F. (2000). Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J. Biomech. Eng. 122, 615–622. doi:10.1115/1.1324667
Bernal, R., Pullarkat, P. A., and Melo, F. (2007). Mechanical properties of axons. Phys. Rev. Lett. 99 (1), 018301–018309. doi:10.1103/PhysRevLett.99.018301
Brinson, H. F., and Brinson, L. C. (2008). Polymer engineering science and viscoelasticity. Boston, MA: Springer. doi:10.1007/978-0-387-73861-1
Chafi, M. S., Karami, G., and Ziejewski, M. (2010). Biomechanical assessment of brain dynamic responses due to blast pressure waves. Ann. Biomed. Eng. 38 (2), 490–504. doi:10.1007/s10439-009-9813-z
Chełminiak, P., Dixon, J. M., and Tuszyński, J. A. (2010). Torsional elastic deformations of microtubules within continuous sheet model. Eur. Phys. J. E 31, 215–227. doi:10.1140/epje/i2010-10562-x
Chen, J., Kanai, Y., Cowan, N. J., and Hirokawa, N. (1992). Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature 360, 674–677. doi:10.1038/360674a0
Clyne, T. W., and Hull, D. (2019). An introduction to composite materials. Cambridge, United Kingdom: Cambridge University Press. doi:10.1017/9781139050586
de Rooij, R., Miller, K. E., and Kuhl, E. (2017). Modeling molecular mechanisms in the axon. Comput. Mech. 59 (3), 523–537. doi:10.1007/s00466-016-1359-y
Duval, T., Saliani, A., Nami, H., Nanci, A., Stikov, N., Leblond, H., et al. (2019). Axons morphometry in the human spinal cord. Neuroimage 185, 119–128. doi:10.1016/j.neuroimage.2018.10.033
el Sayed, T., Mota, A., Fraternali, F., and Ortiz, M. (2008). Biomechanics of traumatic brain injury. Comput. Methods Appl. Mech. Eng. 197, 4692–4701. 51–52. doi:10.1016/j.cma.2008.06.006
Elkin, B. S., and Morrison, B. (2007). Region-specific tolerance criteria for the living brain. USA: Stapp Car Crash J.
Eskandari, F., Shafieian, M., Aghdam, M. M., and Laksari, K. (2021). Structural anisotropy vs. Mechanical anisotropy: The contribution of axonal fibers to the material properties of brain white matter. Ann. Biomed. Eng. 49 (3), 991–999. doi:10.1007/s10439-020-02643-5
Estrada, J. B., Cramer, H. C., Scimone, M. T., Buyukozturk, S., and Franck, C. (2021). Neural cell injury pathology due to high-rate mechanical loading. Brain Multiphys. 2, 100034. doi:10.1016/j.brain.2021.100034
Galbraith, J. A., Thibault, L. E., and Matteson, D. R. (1993). Mechanical and electrical responses of the squid giant axon to simple elongation. J. Biomech. Eng. 115 (1), 13–22. doi:10.1115/1.2895464
Gavin, H. P. (2019). The levenberg-marquardt algorithm for nonlinear least squares curve-fitting problems. USA: Duke University.
Gavin, H. P. (2019). The levenburg-marqurdt algorithm for nonlinear least squares curve-fitting problems. USA: Duke University.
Gray, J. A. B., and Ritchie, J. M. (1954). Effects of stretch on single myelinated nerve fibres. J. Physiol. 124, 84–99. doi:10.1113/jphysiol.1954.sp005087
Hasan, F., al Mahmud, K. A. H., Khan, M. I., Kang, W., and Adnan, A. (2021). Effect of random fiber networks on bubble growth in gelatin hydrogels. Soft Matter 17, 9293–9314. doi:10.1039/d1sm00587a
Hasan, F., al Mahmud, K. A. H., Khan, M. I., Patil, S., Dennis, B. H., and Adnan, A. (2021). Cavitation induced damage in soft biomaterials. Multiscale Sci. Eng. 3, 67–87. doi:10.1007/s42493-021-00060-x
Hirokawa, N., Shiomura, Y., and Okabe, S. (1988). Tau proteins: The molecular structure and mode of binding on microtubules. J. Cell Biol. 107, 1449–1459. doi:10.1083/jcb.107.4.1449
Inc., A. (2021). ANSYS Academic Research Mechanical APDL, Help System. R2 ed., R2.Element reference.
Ishak Khan, M., Gilpin, K., Hasan, F., Abu Hasan Al Mahmud, K., and Adnan, A. (2021). Effect of strain rate on single tau, dimerized tau and tau-microtubule interface: A molecular dynamics simulation study. Biomolecules 11, 1308. doi:10.3390/biom11091308
Jadidi, T., Seyyed-Allaei, H., Tabar, M. R. R., and Mashaghi, A. (2014). Poisson’s ratio and Young’s modulus of lipid bilayers in different phases. Front. Bioeng. Biotechnol. 2. doi:10.3389/fbioe.2014.00008
Janmey, P. A., Euteneuer, U., Traub, P., and Schliwa, M. (1991). Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol. 113, 155–160. doi:10.1083/jcb.113.1.155
Javid, S., Rezaei, A., and Karami, G. (2014). A micromechanical procedure for viscoelastic characterization of the axons and ECM of the brainstem. J. Mech. Behav. Biomed. Mater. 30, 290–299. doi:10.1016/j.jmbbm.2013.11.010
Kasthuri, N., Burns, Randal, Lewis Sussman, Daniel, Eldin Priebe, Carey, Pfister, Hanspeter, Lichtman, Jeff William, et al. (2015). Saturated reconstruction of a volume of neocortex. Cell 62, 648. doi:10.1016/j.cell.2015.06.054
Khan, M. I., Ferdous, S. F., and Adnan, A. (2012). Mechanical behavior of actin and spectrin subjected to high strain rate: A molecular dynamics simulation study. Comput. Struct. Biotechnol. J. 19, 1738–1749. doi:10.1016/j.csbj.2021.03.026
Khan, M. I., Ferdous, S. F., and Adnan, A. (2021). Mechanical behavior of axonal actin, spectrin, and their periodic structure: A brief Review. Multiscale Sci. Eng. 3, 185–204. 3–4. doi:10.1007/s42493-021-00069-2
Khan, M. I., Hasan, F., al Mahmud, K. A. H., and Adnan, A. (2020). Recent computational approaches on mechanical behavior of axonal cytoskeletal components of neuron: A brief Review. Multiscale Sci. Eng. 2, 199–213. doi:10.1007/s42493-020-00043-4
Khan, M. I., Hasan, F., al Mahmud, K. A. H., and Adnan, A. (2021). Viscoelastic response of neurofilaments: An atomistic simulation approach. Biomolecules 11, 540. doi:10.3390/biom11040540
Khan, M. I., Hasan, F., Hasan Al Mahmud, K. A., and Adnan, A. (2021). Domain focused and residue focused phosphorylation effect on tau protein: A molecular dynamics simulation study. J. Mech. Behav. Biomed. Mat. 113, 104149. doi:10.1016/j.jmbbm.2020.104149
Kleiven, S. (2007). Predictors for traumatic brain injuries evaluated through accident reconstructions. USA: Stapp Car Crash J.
Koser, D. E., Moeendarbary, E., Hanne, J., Kuerten, S., and Franze, K. (2015). CNS cell distribution and axon orientation determine local spinal cord mechanical properties. Biophys. J. 108 (9), 2137–2147. doi:10.1016/j.bpj.2015.03.039
LaPlaca, M. C., Cullen, D. K., McLoughlin, J. J., and Cargill, R. S. (2005). High rate shear strain of three-dimensional neural cell cultures: A new in vitro traumatic brain injury model. J. Biomech. 38 (5), 1093–1105. doi:10.1016/j.jbiomech.2004.05.032
Lazarus, C., Soheilypour, M., and Mofrad, M. R. K. (2015). Torsional behavior of axonal microtubule bundles. Biophys. J. 109 (2), 231–239. doi:10.1016/j.bpj.2015.06.029
Lee, H. H., Yaros, K., Veraart, J., Pathan, J. L., Liang, F. X., Kim, S. G., et al. (2019). Along-axon diameter variation and axonal orientation dispersion revealed with 3D electron microscopy: Implications for quantifying brain white matter microstructure with histology and diffusion MRI. Brain Struct. Funct. 224 (4), 1469–1488. doi:10.1007/s00429-019-01844-6
Mahmud, K. A. H. A., Hasan, F., Khan, M. I., and Adnan, A. (2021). Shock-induced damage mechanism of perineuronal nets. Biomolecules 2022 12), 10. doi:10.3390/biom12010010
Montanino, A., and Kleiven, S. (2018). Utilizing a structural mechanics approach to assess the primary effects of injury loads onto the axon and its components. Front. Neurol. 9, 643. doi:10.3389/fneur.2018.00643
Montanino, A., Li, X., Zhou, Z., Zeineh, M., Camarillo, D., and Kleiven, S. (2021). Subject-specific multiscale analysis of concussion: From macroscopic loads to molecular-level damage. Brain Multiphys. 2, 100027. doi:10.1016/j.brain.2021.100027
Morrison, B., Wang, Christopher C-B., Thomas, Fay C., Hung, Clark T., Ateshian, Gerard A., Sundstrom, Lars E., et al. (2003). A tissue level tolerance criterion for living brain developed with an in vitro model of traumatic mechanical loading. Stapp Car Crash J. 47, 103. doi:10.4271/2003-22-0006
Naik, A., Abolfathi, N., Karami, G., and Ziejewski, M. (2008). Micromechanical viscoelastic characterization of fibrous composites. J. Compos. Mat. 42 (12), 1179–1204. doi:10.1177/0021998308091221
Nyein, M. K., Jason, A. M., Yu, L., Pita, C. M., Joannopoulos, J. D., Moore, D. F., et al. (2010). In silico investigation of intracranial blast mitigation with relevance to military traumatic brain injury. Proc. Natl. Acad. Sci. U. S. A. 107, 20703–20708. doi:10.1073/pnas.1014786107
Ouyang, H., Nauman, E., and Shi, R. (2013). Contribution of cytoskeletal elements to the axonal mechanical properties. J. Biol. Eng. 7 (1), 21. doi:10.1186/1754-1611-7-21
Pampaloni, F., Lattanzi, G., Jonáš, A., Surrey, T., Frey, E., and Florin, E. L. (2006). Thermal fluctuations of grafted microtubules provide evidence of a length-dependent persistence length. Proc. Natl. Acad. Sci. U. S. A. 103, 10248–10253. doi:10.1073/pnas.0603931103
Panzer, M. B., Myers, B. S., and Bass, C. R. (2013). Mesh considerations for finite element blast modelling in biomechanics. Comput. Methods Biomech. Biomed. Engin. 16 (6), 612–621. doi:10.1080/10255842.2011.629615
Peter, S. J., and Mofrad, M. R. K. (2012). Computational modeling of axonal microtubule bundles under tension. Biophys. J. 102 (4), 749–757. doi:10.1016/j.bpj.2011.11.4024
Peter, S. J., and Mofrad, M. R. K. (2012). Computational modeling of axonal microtubule bundles under tension. Biophys. J. 102 (4), 749–757. doi:10.1016/j.bpj.2011.11.4024
Peters, A., Proskauer, C. C., and Kaiserman-Abramof, I. R. (1968). The small pyramidal neuron of the rat cerebral cortex. The axon hillock and initial segment. J. Cell Biol. 39 (3), 604–619. doi:10.1083/jcb.39.3.604
Press, W. H., Flannery, B. P., Teukolsky, S. A., and Vetterling, W. T. (1989). others, “Numerical recipes. Cambridge: Cambridge University Press.
Rosenberg, K. J., Ross, J. L., Feinstein, H. E., Feinstein, S. C., and Israelachvili, J. (2008). Complementary dimerization of microtubule-associated tau protein: Implications for microtubule bundling and tau-mediated pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 105, 7445–7450. doi:10.1073/pnas.0802036105
Saatman, K. E., Thibault, L. E., and Meaney, D. F. (1992). Biomechanics of isolated myelinated nerve as related to brain injury.
Sarangapani, K. K., Akiyoshi, B., Duggan, N. M., Biggins, S., and Asbury, C. L. (2013). Phosphoregulation promotes release of kinetochores from dynamic microtubules via multiple mechanisms. Proc. Natl. Acad. Sci. U. S. A. 110, 7282–7287. doi:10.1073/pnas.1220700110
Shamloo, A., Manuchehrfar, F., and Rafii-Tabar, H. (2015). A viscoelastic model for axonal microtubule rupture. J. Biomech. 48 (7), 1241–1247. doi:10.1016/j.jbiomech.2015.03.007
Smith, D. H., and Meaney, D. F. (2000). Axonal damage in traumatic brain injury. Neuroscientist 6, 483–495. doi:10.1177/107385840000600611
Soheilypour, M., Peyro, M., Peter, S. J., and Mofrad, M. R. K. (2015). Buckling behavior of individual and bundled microtubules. Biophys. J. 108 (7), 1718–1726. doi:10.1016/j.bpj.2015.01.030
Spillantini, M. G., and Goedert, M. (1998). Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 21, 428–433. doi:10.1016/S0166-2236(98)01337-X
Sun, C. T., and Vaidya, R. S. (1996). Prediction of composite properties from a representative volume element. Compos. Sci. Technol. 56 (2), 171–179. doi:10.1016/0266-3538(95)00141-7
Tamura, A., Nagayama, K., Matsumoto, T., and Hayashi, S. (2007). Variation in nerve fiber strain in brain tissue subjected to uniaxial stretch. Stapp Car Crash J. 51, 139–154. doi:10.4271/2007-22-0006
Tang‐Schomer, M. D., Patel, A. R., Baas, P. W., and Smith, D. H. (2010). Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. FASEB J. 24 (5), 1401–1410. doi:10.1096/fj.09-142844
Taylor, P. A., and Ford, C. C. (2009). Simulation of blast-induced early-time intracranial wave physics leading to traumatic brain injury. J. Biomech. Eng. 131, 061007–61016. doi:10.1115/1.3118765
Thibault, L. E., Galbraith, J. A., Thompson, C. J., and Gennarelli, T. A. (1981). Effects of high strain rate uniaxial extension on the electrophysiology of isolated neural tissue.
Tzikang, C., and Chen, T. (2000). Determining a Prony series for a viscoelastic material from time varying strain data. USA: Nasa.
Wang, R., Zhang, L., Hu, D., Liu, C., Shen, X., Cho, C., et al. (2017). A novel approach to impose periodic boundary condition on braided composite RVE model based on RPIM. Compos. Struct. 163, 77–88. doi:10.1016/j.compstruct.2016.12.032
Watanabe, D., Yuge, K., Nishimoto, T., Murakami, S., and Takao, H. (2009). Development of a human head FE model and impact simulation on the focal brain injury. J. Comput. Sci. Technol. 3 (1), 252–263. doi:10.1299/jcst.3.252
Wegmann, S., Schöler, J., Bippes, C. A., Mandelkow, E., and Muller, D. J. (2011). Competing interactions stabilize pro- and anti-aggregant conformations of human Tau. J. Biol. Chem. 286, 20512–20524. doi:10.1074/jbc.M111.237875
Weickenmeier, J., de Rooij, R., Budday, S., Steinmann, P., Ovaert, T. C., and Kuhl, E. (2016). Brain stiffness increases with myelin content. Acta Biomater. 42, 265–272. doi:10.1016/j.actbio.2016.07.040
Wright, R. M. (2012). A computational model for traumatic brain injury based on an axonal injury criterion. USA: The Johns Hopkins University.
Wright, R. M., and Ramesh, K. T. (2012). An axonal strain injury criterion for traumatic brain injury. Biomech. Model. Mechanobiol. 11 (1–2), 245–260. doi:10.1007/s10237-011-0307-1
Wu, J., Yuan, H., Li, L. y., Li, B., Fan, K., Li, S., et al. (2019). Mathematical modelling of microtubule-tau protein transients: Insights into the superior mechanical behavior of axon. Appl. Math. Model. 71, 452–466. doi:10.1016/j.apm.2019.02.030
Wu, J. Y., Yuan, H., and Li, L. Y. (2018). Mathematical modelling of axonal microtubule bundles under dynamic torsion. Appl. Math. Mech. 39 (6), 829–844. doi:10.1007/s10483-018-2335-9
Wu, Y. T., and Adnan, A. (2018). Damage and failure of axonal microtubule under extreme high strain rate: An in-silico molecular dynamics study. Sci. Rep. 8 (1), 12260. doi:10.1038/s41598-018-29804-w
Xu, K., Zhong, G., and Zhuang, X. (1979). Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456. doi:10.1126/science.1232251
Yousefsani, S. A., Shamloo, A., and Farahmand, F. (2018)., 80. January, 194–202. Micromechanics of brain white matter tissue: A fiber-reinforced hyperelastic model using embedded element techniqueJ. Mech. Behav. Biomed. Mat. doi:10.1016/j.jmbbm.2018.02.002
Yu, W., and Baas, P. W. (1994). Changes in microtubule number and length during axon differentiation. J. Neurosci. 14 (5 I), 2818–2829. doi:10.1523/jneurosci.14-05-02818.1994
Zhang, L., Yang, K. H., and King, A. I. (2004). A proposed injury threshold for mild traumatic brain injury. J. Biomech. Eng. 126 (2), 226–236. doi:10.1115/1.1691446
Keywords: mechanical behavior of axon, neuron, cytoskeleton, traumatic brain injury, finite element analysis, representative volume element (RVE), composite materials, mechanical characterization
Citation: Hasan F, Mahmud KA, Khan MI and Adnan A (2022) Viscoelastic damage evaluation of the axon. Front. Bioeng. Biotechnol. 10:904818. doi: 10.3389/fbioe.2022.904818
Received: 25 March 2022; Accepted: 22 August 2022;
Published: 06 October 2022.
Edited by:
Svein Kleiven, Royal Institute of Technology, SwedenReviewed by:
Yuan Feng, Shanghai Jiao Tong University, ChinaPatrick Alford, University of Minnesota Twin Cities, United States
Jin Zhang, Harbin Institute of Technology, China
Copyright © 2022 Hasan, Mahmud, Khan and Adnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashfaq Adnan, YWFkbmFuQHV0YS5lZHU=
 Fuad Hasan
Fuad Hasan KAH Al Mahmud1
KAH Al Mahmud1 Ashfaq Adnan
Ashfaq Adnan