- 1Chitkara College of Pharmacy, Chitkara University, Rajpura, India
- 2Yunnan Herbal Laboratory, College of Ecology and Environmental Sciences, Yunnan University, Kunming, China
- 3The International Joint Research Center for Sustainable Utilization of Cordyceps Bioresources in China and Southeast Asia, Yunnan University, Kunming, China
- 4Department of Biochemistry and Molecular Biology, Faculty of Life Science, Mawlana Bhashani Science and Technology University, Tangail, Bangladesh
- 5Department of Biosciences, COMSATS University Islamabad, Sahiwal, Pakistan
- 6Unit of Biochemistry, Faculty of Medicine, University Sultan Zainal Abidin, Kuala Terengganu, Malaysia
- 7Department of Pharmacy, Faculty of Allied Health Sciences, Daffodil International University, Dhaka, Bangladesh
- 8Department of Pharmacy, BGC Trust University Bangladesh, Chittagong, Bangladesh
- 9Faculty of Medicine and Pharmacy, University of Oradea, Oradea, Romania
Current advancements in nanotechnology and nanoscience have resulted in new nanomaterials, which may pose health and environmental risks. Furthermore, several researchers are working to optimize ecologically friendly procedures for creating metal and metal oxide nanoparticles. The primary goal is to decrease the adverse effects of synthetic processes, their accompanying chemicals, and the resulting complexes. Utilizing various biomaterials for nanoparticle preparation is a beneficial approach in green nanotechnology. Furthermore, using the biological qualities of nature through a variety of activities is an excellent way to achieve this goal. Algae, plants, bacteria, and fungus have been employed to make energy-efficient, low-cost, and nontoxic metallic nanoparticles in the last few decades. Despite the environmental advantages of using green chemistry-based biological synthesis over traditional methods as discussed in this article, there are some unresolved issues such as particle size and shape consistency, reproducibility of the synthesis process, and understanding of the mechanisms involved in producing metallic nanoparticles via biological entities. Consequently, there is a need for further research to analyze and comprehend the real biological synthesis-dependent processes. This is currently an untapped hot research topic that required more investment to properly leverage the green manufacturing of metallic nanoparticles through living entities. The review covers such green methods of synthesizing nanoparticles and their utilization in the scientific world.
Introduction
To lessen the risks associated with nanotechnology, the ideal option is to use green nanotechnology in manufacturing and implementation. One of the most significant advancements in nanotechnology and materials science is the creation of engineered nanomaterials (Mu et al., 2021; Sun et al., 2021). Nanotechnology has penetrated various fields such as drug delivery and other biomedical applications (Chopra et al., 2021a, 2021b, 2022; Singla et al., 2021; Bhattacharya et al., 2022). Moving these things out of the lab and into the real world is the only way to bring them to life. There are tens of thousands of these goods on the market, most found in daily personal care, cosmetics, and apparel. Commercializing successful disruptive technologies is essential for a wide range of human applications and worldwide progress, but critical attention is required in the materials’ potential, health evaluation, and environmental impact. There’s little doubt that nanoparticles (NPs) provide a health concern that has to be handled quickly, and their production and use are essentially unregulated, especially in the development of the Universe. While new chemical processes are designed with little risk in mind, hazardous compounds are minimized or eliminated via a collection of fundamentals. This is a crucial feature of the green chemistry developing industry (Hassan et al., 2021).
A large amount of time and effort has been devoted to developing acceptable synthetic methods for creating nanoparticles because of their physiochemical characteristics and many uses. However, environmental contamination produced by heavy metals restricts several physiochemical techniques to form metal nanoparticles. As a result, the manufacturing of nanoparticles by biological methods has emerged as a new trend in the industry due to its nontoxicity, repeatability, ease of scaling up, and well-defined shape. Researchers have found that novel resources such as microbes and plants have the most potential for producing nanoparticles (Shafey, 2020; Lahiri et al., 2021; Cuong et al., 2022; Devra, 2022; Ettadili et al., 2022; Lomelí-Rosales et al., 2022; Majeed et al., 2022; Mustapha et al., 2022; Najafi et al., 2022). Metal nanoparticles have been synthesized using a variety of microorganisms, including bacteria, fungus, and yeast, as well as plants. “Green synthesis” is necessary to prevent the generation of undesirable or dangerous by-products via the build-up of dependable, sustainable, and eco-friendly synthesis techniques. The usage of optimal solvent systems and natural resources (such as organic systems) is vital to attain this aim. Green production of metallic nanoparticles has been utilized to accommodate diverse biological components (e.g., bacteria, fungus, algae, and plant extracts) (e.g., bacteria, fungi, algae, and plant extracts). Among the current greenways of synthesis for metal/metal oxide nanoparticles, the use of plant extracts is a straight forward technique to generate nanoparticles at large scale in comparison to bacteria and/or fungal assisted synthesis. These compounds are known together as biogenic nanoparticles. Here, we present an update on recent breakthroughs in the synthesis of biological nanoparticles and outline their future development and potential uses (Abu Hajleh et al., 2021).
Green Synthesis of Nanoparticles
In recent studies, it has been proven that microorganisms and plants may be used to synthesize nanoparticles in a way that is both ecologically friendly and safe to use (Makarov et al., 2014; Gowramma et al., 2015). Microbes and plants have always been able to collect and store inorganic metallic ions from their environment. Because of their enticing properties, many living things have effective biological factories, minimizing pollution while also recovering metals from industrial waste. The capacity of a living creature to employ its metabolic processes to transform inorganic metallic ions into metal nanoparticles has opened the door to a relatively new and primarily untapped area of study (Baker et al., 2013). Since discovering microbes’ ability to interact with, remove, and gather metallic elements from their surroundings, several biotechnological applications, such as bioremediation and bioleaching (Vicas et al., 2019) have been developed. They can interact with their environment because of their lipid-based amphipathic membranes, which allow for various oxidation-reduction events to take place and promote biochemical transformations (Cavalu et al., 2020). Microorganisms grown in specific settings may also accelerate linked oxidation and reduction in nanoparticle formation (Belliveau et al., 1987; Kowshik et al., 2002; Durán et al., 2005; Lengke et al., 2006). Still, the oxidation-reduction mechanisms are unknown to humans. Much research is still needed to fully understand and explain the differences in nanoparticle size and form across different metals when they are created by the same microorganism (Bhattacharya and Gupta, 2005). Even when it comes to using plants to make nanoparticles, this is still true. There are several advantages to using plants instead of other ecologically friendly biological systems like bacteria and fungi, such as eliminating expensive and time-consuming preparation and isolation methods. Contrastingly, the use of plants or plant-derived extracts to create nanoparticles is usually regarded as safer and more efficient than the use of other biological systems for nanoparticle production. Another advantage of plant-based biosynthesis over different ways is that it is a straightforward process that can readily be scaled up for the large-scale manufacture of nanoparticles. This is a significant advantage over other alternatives.
Nanoparticle production is possible with each living organism’s specific biochemical processing abilities. Nanoparticles can only be synthesized by certain biological organisms because of their enzyme activity and metabolic processes. As a result, to produce nanoparticles with well-defined features such as size and form, it is necessary to carefully pick the appropriate biological entity. However, there are a few exceptions to the general rule that biological entities with a high capacity for heavy metal accumulation are more likely to synthesize metallic nanoparticles. When working with microorganisms, the methods used to cultivate them are essential. Many culturing parameters, including nutrition, light intensity, medium pH, temperature, mixing speed, and buffer strength, must be optimized to increase enzyme activity (Singh et al., 2010). An innovative alternative to standard chemical synthesis and the more challenging growth and isolation techniques necessary for many microorganisms has recently been discovered in the biological creation of nanoparticles using plants and plant extracts. A combination of compounds found in plant extracts has been shown to reduce and stabilize (cap) the formation of nanoparticles (Narayanan and Sakthivel, 2008; Sathishkumar et al., 2009; Singh et al., 2010). As a result of their complexity and non-toxicity, these biological molecules have become more popular.
Biosynthesis of Nanoparticles Using Plants
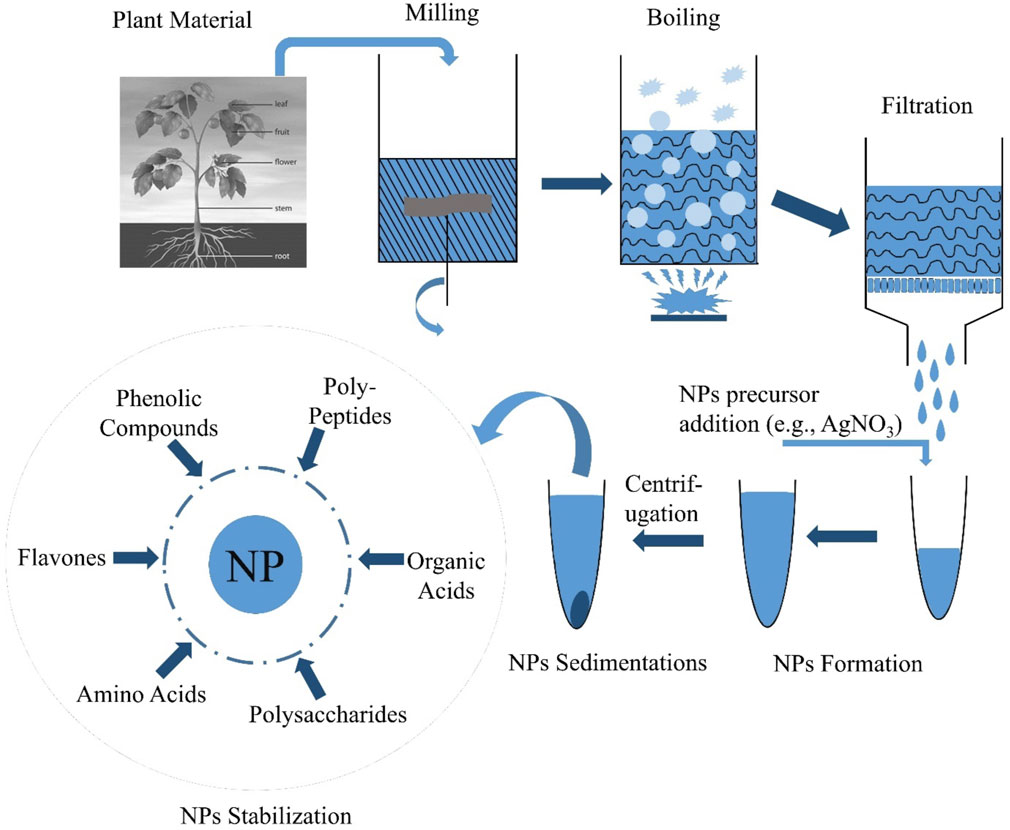
Plant nanotechnology has recently opened up new pathways for the production of nanoparticles and is an environmentally benign, simple, quick, and stable technique. Using water as a reducing solvent to synthesize nanoparticles has several benefits, including biocompatibility, scalability, and medicinal application (Noruzi, 2015). This means that plant-derived nanoparticles may meet the rising demand for nanoparticles with applications in biomedicine and the environment since they are made from easily accessible plant components and are not hazardous. Gold and silver nanoparticle synthesis utilizing Panax ginseng leaf and root extract has recently been shown to be possible using medicinal plants as sources of raw materials (Singh et al., 2016c; 2016b). In addition, metal nanoparticles have been synthesized using different plant components, such as the leaves, fruits, and stems, and their extracts. The pathway for biosynthesis of nanoparticles from plants has been shown in Figure 1.
Furthermore, it has been suggested that secondary metabolites such as flavonoids and alkaloids have important functions in metal salt reduction and capping and stabilizing agents for nanoparticles generated from proteins and amino acids (Duan et al., 2015). Corallina officinalis extract, for example, contains polyphenols and proteins with carbonyl groups that might help create and stabilize gold nanoparticles (El-Kassas and El-Sheekh, 2014). Silver and gold nanoparticles were synthesized and stabilized in Murraya koenigii leaf extract by Philip et al. (Philip et al., 2011).
According to the literature review, biologically synthesized NPs are more active than physicochemically synthesized NPs. Metallic NPs produced from plant extracts are stable and mono-dispersible when the pH, incubation time, mixing ratio, and temperature are all accurately regulated. Curry, mango, neem, turmeric, and guava have all been utilized to create Gold NPs. Plant extracts are rich in polyphenols, which hasten the breakdown of organic materials (Huston et al., 2021). Metal NPs may be harvested from plants with a great capacity to decrease metal ions both on their surface and in multiple organs and tissues distant from the ion penetration point. According to the metal bioaccumulation study, nanoparticles (NPs) are the most frequent metal deposit (Khalaj et al., 2020). If you look at extracts from plants and look for compounds like terpenoids and phenolic acids (Rahman et al., 2021c) as well as proteins in spectroscopic measurements, you’ll see that metal ions may be reduced to nanostructured forms. Shankar SS et al. employed geranium leaf extract to synthesize silver NPs extra-cellularly using the rapid reduction of silver ions in an aqueous silver nitrate solution. In solution, the particles produced quasilinear superstructures ranging in size from 16 to 40 nm, which were seen by transmission electron microscopy (TEM) and found to be very stable and crystalline (Shankar et al., 2003).
By allowing reduction processes to run using aqueous solutions of AgNO3 and chloroauric acid, researchers could produce pure metallic and bimetallic silver and gold nanoparticles using the broth of Neem (Azadirachta indica) leaves. During the examination, silver and gold nanoparticles were polydispersed and flat plate-like in shape. After being exposed to Au3+ and Ag + ions in solution, bimetallic Au core-Ag shell NPs were formed, which were verified by TEM analysis and further showed that the Ag NPs served as adsorbents onto the gold NPs, resulting in the core-shell structure (Shankar et al., 2004). Using Murraya koenigii leaf extract, Laura Christen and others were able to produce silver nanoparticles as part of their investigation on the impact of broth concentration on the reduction process and particle size. As previously mentioned, the broth was removed in the same way. Leaf broth content was investigated using reaction mixtures comprising 1:25, 1:50, 1:100, and 1:200 AgNO3 with 1:100 leaf broth and 1:250 leaf broth to 10−3 M AgNO3. The reduction process was investigated using UV analysis. At 435 nm, the absorbance peak was recorded at a range beyond the normal range (Christensen et al., 2011). The researchers found that the reduction rate and particle size decreased along with the agglomeration tendency when broth content increased.
FTIR investigations have indicated that polyphenols in green tea extract may be used as a capping agent as well as a reducer, as proven by researchers (KSV, 2017). Satoaki Onitsuka and others synthesized gold and silver nanoparticles (NPs) from the Camellia sinensis plant extracts. Precursors such as HAuCl4 or AgNO3 were used to react with the tea leaf extracts as catalysts (aqueous solutions). At low temperatures in ambient conditions, HAuCl4 or AgNO3 aqueous solutions were combined with aqueous NaHCO3 to create metal NP solutions, which were then tested. It has been revealed that the antimicrobial pigments included in Ag and Au NPs have diameters of 30 and 10 nm, respectively (Onitsuka et al., 2019).
In 2017, Roy created silver nanoparticles using neem leaf extracts (Roy et al., 2017). AgNO3 and neem extract solution was also stored at a low light level in a dark room as a precautionary measure. Silver nanoparticles showed antibacterial efficacy against E. coli and Gram-positive bacteria but were more effective than either. The UV absorption peak was discovered to be between 420 and 450 nm. According to the study’s results, Ag NPs may be produced at a lower concentration of plant extract (Rahman et al., 2021b) (Roy et al., 2017). In the production of silver NPs, the leaves of Azadirachta indica and Triphala have been employed. Neem leaves yielded Ag NPs of 43 nm in diameter, whereas Triphala leaves yielded 59 nm-diameter Triphala-derived Ag NPs. Neem and Triphala were shown to reduce the growth of gentamicin and ampicillin-resistant K. pneuomoniae, and similar findings were seen in S. Typhi resistant to gentamicin and piperacillin (Gavhane et al., 2012).
Calotropis procera, a member of the Asclepiadaceous family, was used to synthesize CuO NPs sustainably (Reddy, 2017). Because of their low bandgap, these NPs are frequently employed in numerous applications such as photocatalysis (Li J. et al., 2011). It is utilized to treat splenic, pilescausal, and tumor-related disorders. Fresh Calotropis leaves were cleaned in distilled water and dried in the Sun before being chopped into fine pieces and put in deionized water to boil until the solution became yellow, as described in this article. It was then added to the solution with cupric acid and heated until a blue-green paste was formed. At 700°C, this powder was calcined till the substance’s color became black.
By employing Punica granatum peels extract, Alaa Y. Ghidan et al. were able to green manufacture copper nanoparticles (Ghidan et al., 2016). Punica granatum’s fresh peels are picked and washed numerous times to eliminate any contaminants before being used in cooking. It was discovered that the color of the solution changed from white to yellow when the powdered peels were blended with distilled, sterile water and then boiled. CuO NPs were produced by dissolving copper acetate powder in water and stirring it with a magnetic stirrer for some time. It was discovered that after adding the P. granatum extract, the solution changed colorfrom green to brown, suggesting the formation of monodispersed Cu NPs in the solution.
Copper NPs were freshly synthesized from fresh Abutilon indicum leaves by Ijaz et al. (Ijaz et al., 2017). They were gently washed to remove dust and dry and shadowed sections from the fresh Abutilon indicum leaves. The leaves were ground up and sieved through a 200-nm screen to make fuel.
Abutilon indicum extract, Copper (II) nitrate trihydrate, and double-distilled water made CuO NPs. The solution was homogenized for 2–5 min using a magnetic stirrer. Next, a pre-heated muffle furnace was utilized to execute a combustion reaction on the mixture to yield CuO NPs at a temperature of 400 °C. The ash component of the plant extracts was removed by filtering the resulting combination. Methanol was used to eliminate contaminants from the solution after rinsing with distilled water. Researchers reported the synthesis of copper nanoparticles supported on sodium borosilicate glass using extract of Acalypha indica L. (Nasrollahzadeh et al., 2018b). The nanoparticles were able to reduce the 2,4-dinitrophenilhydrazine (2,4-DNPH), 4-nitrophenol (4-NP), methyl orange (MO), methylene blue (MB) and congo red (CR) by using NaBH4 in aqueous medium. Similarly copper nanoparticles synthesized from Plantago asiatica leaf extract, were reported to catalyze the direct cyanation of aldehyde using K4Fe(CN)6 (Nasrollahzadeh et al., 2017b).
Copper nanoparticles, were also reported to synthesize from, Centella asiatica L. leaf extract (Nasrollahzadeh et al., 2018c). The copper nanoparticles were further immobilized on the surface of manganese dioxide. The resulting nanoparticles were able to form the recycle catalyst that can be applied for the reduction of 2,4-DNPH and MB. Another, group of researchers prepared copper nanoparticles, using extracts of Euphorbia maculata aerial parts and reported their activity to reduce MB and RhB (Pakzad et al., 2019).
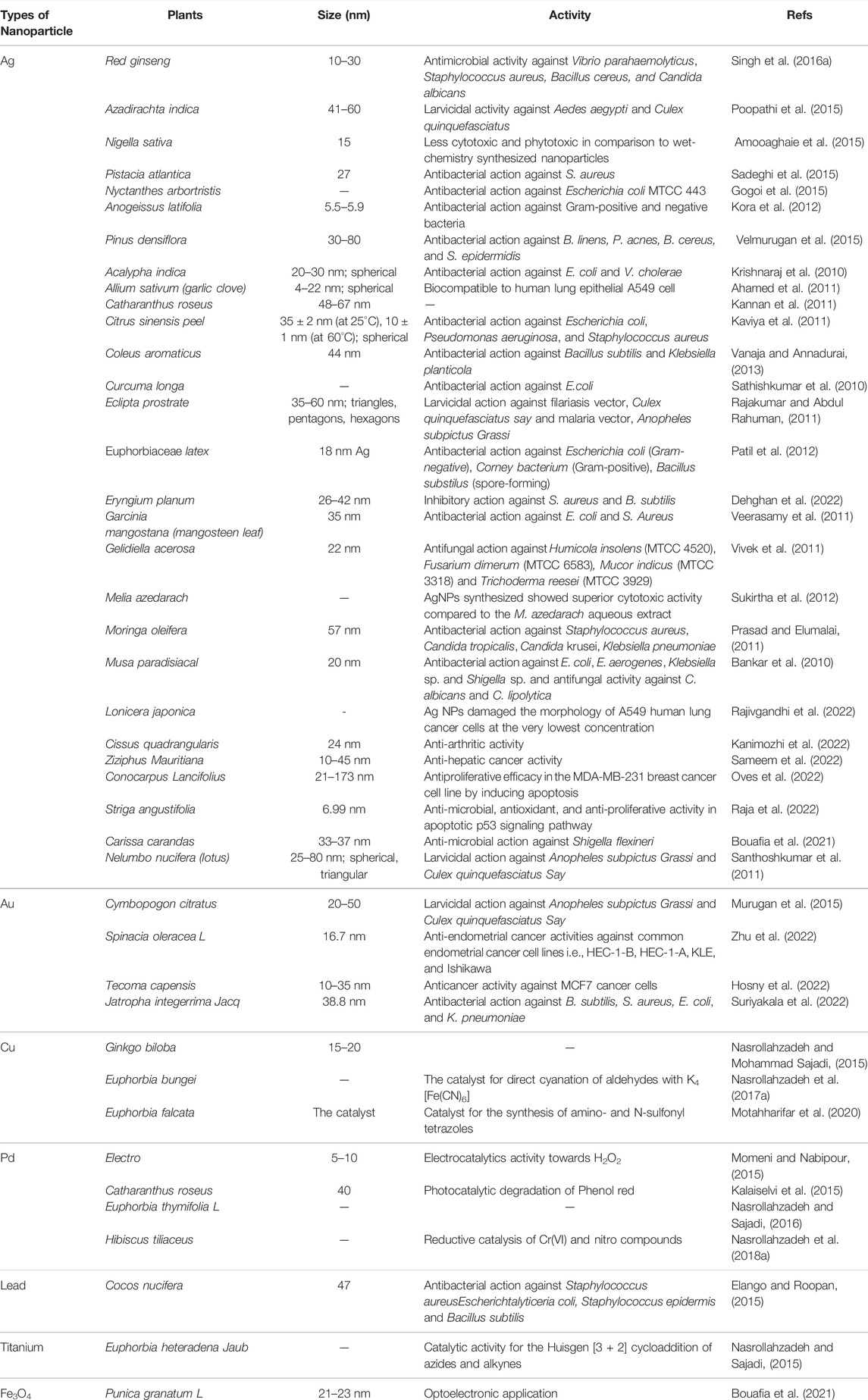
The bio-agent used in the synthesis of biological NPs significantly impacts the technology used to execute it. If you want to make metal NPs from plant extracts, for example, you’ll first need to gather, wash, and dry the plant component you want (such as a leaf or fruit), then crush it before extracting the resulting crystals from the solvent (Mohamad et al., 2014). Following this, the extract is filtered or centrifuged to remove the solid plant residue, and the metal precursor chemical is combined with the residue under certain circumstances. Several factors may affect the reaction time (and hence the properties of nanoparticles), such as the ratio of reactant concentrations and the temperature of the reaction, pH, light, ultrasound, or microwave heating (Varma, 2012; Mittal et al., 2013). Incubation is followed by high-speed centrifugation of the nanoparticles, which are entirely rinsed in water/solvent (e.g., ethanol, methanol) and collected (Singh et al., 2016d). The reaction conditions significantly impact the number and morphology (shape, size distribution) of the resultant nanoparticles. Additionally, the extracted source has a considerable impact since different plant extracts might range significantly in their concentration and mix of reducing and stabilizing biomolecules (Kumar and Yadav, 2009). Other plants contributing to the nanoparticle synthesis have been enlisted in Table 1.
Fungi Assisted Nanoparticle Synthesis
Fungal biomass and associated metabolites are used to synthesize NPs in a relatively young field of nanotechnology known as “myconanotechnology” (Gade et al., 2010). Micro- and macrofungi alike have several reducing enzymes and proteins, which provide a significant advantage in the production of NP. As opposed to bacteria, fungi generate a diverse spectrum of enzymes, which allows the transformation of metal salts into NPs to occur very quickly in contrast.
The bio-potential of the fungal cell wall is also assumed to be significant in the absorption and reduction of metal ions and the generation of metal NPs (Gade et al., 2010; Khan et al., 2018). It's still unclear precisely how NPs are generated or what biological components are involved in the process. It is suggested that fungus-mediated NPs arise either in vivo or in vitro. Most of the harmful transition metal ions are converted to a non-toxic form in the mycelia of the fungus during the in vivo method, which exploits this process to make NPs intracellularly. It is possible to directly use washed mushroom mycelia to produce NPs intracellularly in this method. Mycelia must undergo additional treatment to remove the NPs from the mycelia before being used again (Molnár et al., 2018).
In contrast, there are three approaches to make NPs from fungal cell-free extracts using the in vitro methodology. The first technique to produce NPs is to use the fermented fungus’s supernatant, which contains extracellular proteins and enzymes (Vágó et al., 2016). According to the second approach (Siddiqi and Husen, 2016). Bioengineered nanoparticles (e.g., AuNPs) may be generated by the intracellular components released into the medium due to the breakdown of cell walls. The use of fungal mycelia’s aqueous extract for the production of NPs is also an option. NPs may be produced by the autolysis of fungal cells, followed by the dissolution of membrane proteins and surface carbohydrates in the solution. The washing and re-suspension of fungal mycelia in a pathogen-free environment is a difficult and not always possible operation using this technique, as previously indicated (Kitching et al., 2016).
Most well-known NPs syntheses use Basidiomycota to produce edible mushrooms (Aygün et al., 2020). Oyster and Ganoderma species have been the subject of several studies in the last few years to learn more about the synthesis of NPs. The mushrooms produced through pure culture are non-pathogenic, non-toxic, and can create a wide variety of physiologically active proteins (Chopra et al., 2021c). Consequently, their higher than normal enzyme activity [108] can transform dangerous compounds into more minor toxic forms, create large amounts of biomass, and accumulate NPs in their mycelia and culture media. Since the discovery of NP synthesis by basidiomycetes, compared to the discovery of NP synthesis by other lower fungi and bacteria, more research into the process of NP synthesis is required.
As a result of its high bioactivity, Ganoderma sp. is one of the most investigated mushrooms for the production of NPs. Over 250 different varieties of this fungus have been identified. This includes G. lucidum, G. applanatum, G. capense, and G. tsugae. Pharmacological evaluation of Ganoderma sp. has demonstrated its antibacterial, anti-HIV, anti-inflammatory, anti-proliferative, anti-diabetic, anticancer, hypocholesterolemic, and hepato-protective potential (Sudheer et al., 2019; Mominur Rahman et al., 2021; Rahman et al., 2022). These mushrooms have also been proven to be helpful in the synthesis of NPs, particularly AgNPs. There are certain drawbacks when using mushrooms to synthesize metallic NPs, such as the need to maintain aseptic growing conditions, the possibility of contamination in samples, and the variability in NP size when using these mushrooms (Li X. et al., 2011). AgNPs were produced using G. sessiliforme and showed significant antibacterial and antioxidant activity (Mohanta et al., 2018). There are two prominent mushrooms, G. lucidum and G. applanatum, recognized for their bioactive components and antibacterial qualities in the culinary and medical worlds. To synthesize AgNPs, the mushrooms’ extracts were employed (Poudel et al., 2017; Al-Ansari et al., 2020).
There is evidence that an edible fungus called Volvariella volvacea’s aqueous extract contains one of the most effective agents for decreasing and capping the extracellular production of gold and silver nanoparticles (Au–Ag NPs) (Philip, 2009; Bhattacharya et al., 2022). Another long-term investigation used the immobilized fungus Coriolus versicolor to bioremediate cadmium salt and synthesize stable CdS nanoparticles in aqueous settings. Similar results were obtained. The immobilized fungus was shown to remove more than 90% of cadmium within 2 hours, whereas auto capped CdS NPs were produced under aqueous conditions, thereby providing a dual role in this investigation (Li X. et al., 2011).
The basidiomycete’s fungus was principally responsible for the production of AuNPs. The P. citrinopileatus, the P. eous, the P. cystidiosus, the P. ostreatus, P. eryngii, and the P. flabellatus were all examined. Tests for Pulmonarius were carried out as well (Madhanraj et al., 2017). Researchers found that the antioxidant properties of AuNPs synthesized by P. Pulmonarius were at their highest levels in the samples examined in antioxidant studies. Most AuNPs were synthesized from P. eous and P. florida, which had the most remarkable ability to reduce ferric oxide. A human colon cancer cell line, HCT-116, was tested with the AuNPs produced using Lentinus sajor-caju (Fr.) extract as the reducing agent (Chaturvedi et al., 2020). HCT-116s antiproliferative effects were dose-dependent and obvious right away. When the cancer cells were examined, they showed signs of cell integrity loss and DNA breakage.
To make AuNPs, researchers employed laccase extracted from P. ostreatus mushrooms (El-Batal et al., 2015). A greater amount of AuNPs was produced after the mushroom had been subjected to 5 kGy of gamma radiation. A radiolytic reduction is thought to have happened, in which radiolysis of the aqueous solution produced H3O+, H, OH, and H2O2 species, all of which reduced the metal salts into ionized form. Flammulina velutipes, a fungus, have also been demonstrated to produce AuNPs inside cells (Narayanan et al., 2015). The mycelium’s inner cell membrane was the primary location where AuNPs were found, which was not a surprise. An enzyme located on the inner surface of the cell membrane was thought to be responsible for reducing the gold precursors. Using phenolic compounds extracted from G. applanatum, an AuNP-based decolorization technique for methylene blue dye was developed recently, and the findings were reported in Nano Letters. An average of 18.7 nm-sized face-centered cubics AuNPs, capable of decolorizing methylene blue in 35 s, were spotted by our researchers using phenol-capped AuNPs (Abdul-Hadi et al., 2020). Another study found that A. bisporus-derived AuNPs with a size range of 10–50 nm had remarkable antifungal activity against the pathogenic fungus A. flavus (Eskandari-Nojedehi et al., 2018). Many other fungi responsible for synthesizing metallic nanoparticles have been enlisted in Table 2.
Bacterial Mediated Synthesis
Critical metals must permeate the cell wall into the cytoplasm and then return through the meshwork of the cell wall to be discharged into the environment. Since the cell wall’s peptidoglycans provide polyanions for the metal-to-chemical reactive group stoichiometric interaction, metal is deposited on the cell wall in an inorganic form. Many metal-binding sites on the wall may be changed using chemical methods to convert positive charge to negative charge (an essential step in the metal-binding process). Chemical change of peptidoglycan of Bacillus subtilis allowed for improved metal penetration and deposition with a large quantity of metal (Beveridge and Murray, 1980). A factor of 20,000 to 40,000 above the extracellular concentration of metals may be found in the cell. Bacteria may benefit from dipole moments created by metal deposition by aligning themselves with the geomagnetic field. A cell’s internal and exterior environments and bacteria species with different morphologies frequently influence the crystalline and non-crystalline phases of particle formation when particles are created.
In the silver-resistant bacterial strain, Pseudomonas stutzeri AG259, which was isolated from a silver mine, internal accumulation of silver NPs, as well as some silver sulphide, with sizes ranging from 35 to 46 nm, was observed (Slawson et al., 1992). When P. stutzeri AG259 was exposed to high concentrations of silver ions during growth, larger particles were produced, resulting in the intracellular synthesis of silver NPs ranging in size from a few nm to 200 nm (Klaus-Joerger et al., 2001). Silver detoxification was accomplished by the bacteria P. stutzeri AG259 through precipitation in the periplasmic space and bioreduction to elemental silver with a variety of crystal typologies, including hexagons and equilateral triangles, as well as three different types of particles: elemental crystalline silver, monoclinic silver sulphide (Ag2S), and a further undetermined structure (Klaus et al., 1999). The thickness of the crystals was regulated by the periplasmic space, but not their width, which might be rather large (100–200 nm) due to the presence of the periplasmic space.
Psychrophilic bacteria Phaeocystis antarctica, Pseudomonas meridiana, Arthrobacter kerguelensis, Arthrobacter gangotriensis, and two mesophilic bacteria, Bacillus indicus, and Bacillus cecembensis, were employed to biosynthesize silver nanoparticles (NPs) with sizes ranging from 6 to 13 nm. These NPs stayed steady in the dark over 8 months. The generation and stability of silver nanoparticles seemed to depend on the temperature, pH, or kind of bacteria from which the supernatant was obtained, among other factors. Researchers observed that A. kerguelensis supernatant could not produce silver nanoparticles at the same temperature that P. antarctica could produce the same nanoparticles. This work provided significant evidence that the components in cell-free culture supernatants that encouraged the production of silver NPs varied from one bacterial species to another and that this was true across all bacterial species (Shivaji et al., 2011).
It is possible to synthesize AgNPs using this method, in which bacteria break down Ag+ to its elemental form (Ag0) outside the cell. Several shapes and sizes of AgNPs may be found in extracellularly produced AgNPs. These include hexagonal, spherical, triangular, circular, and cuboidal, depending on the culture medium utilised for the growth of bacteria (Nanda and Saravanan, 2009; Elbeshehy et al., 2015; Otari et al., 2015). The reducing agent for the biogenic reduction of Ag+ to Ag0 is the proteins on the bacterial cell wall or tiny soluble secretory enzymes. Extracellular synthesis of AgNPs by many bacterial taxa has been functionally described in the natural environment (Islam et al., 2021). Aeromonas sp. SH10 dry cell mass decreases Ag+ to Ag0 in the medium (Fu et al., 2006). Outside the cell, Bacillus licheniformis, Bacillus pumilus, and Bacillus persicus create AgNPs with a size range of 72–92 nm (Elbeshehy et al., 2015). Extracellular synthesis makes use of high-speed centrifugation (10,000 to 12,000 rpm) to capture AgNPs in solution, which may then be re-suspended in a variety of solvents once they have been recovered. Because of this, they are widely used in various fields, including optoelectronics, electrical circuits and systems, bioimaging, and sensory technologies.
When AgNPs are synthesized inside bacteria, silver ions are transported by membrane proteins. As a result of reducing Ag + to Ag0, certain silver-resistant bacteria limit their toxicity by accumulating Ag0 in the cell wall or periplasmic space (Murugan et al., 2014). Some studies have discovered up to 25 percent of the mass of Ag0 in the bacterial cell wall. Pseudomonas stutzeri AG259 lowers the AgNO3 solution and produces AgNPs with a 200 nm size and a modest amount of monoclinic crystallised acanthite (Ag2S) (Murugan et al., 2014). AgNPs (varying in size from 10 to 15 nm) produced by Corynebacteria sp. SH09 also forms a diamine Ag complex on the cell wall (Zhang et al., 2005). AgNPs created within cells must be recovered by additional stages such as bacterial cell lysis via ultrasonication, heat or chemical methods such as salt and detergents (Fesharaki et al., 2010; Kalishwaralal et al., 2010). Extracellular and intracellular synthesis of AgNPs by Proteus mirablis and Vibrio alginolyticus, respectively, has been observed in different media and growth conditions (Samadi et al., 2009; Rajeshkumar and Malarkodi, 2014). A study by Pugazhenthiran et al. [52] found that Bacillus sp. synthesised AgNPs (5–15 nm) in the medium and periplasm (Pugazhenthiran et al., 2009).
Several reports of Shewanella species are Gram-negative, polar flagellated, rod-like, and found in aquatic or marine environments (Bowman et al., 1997; Venkateswaran et al., 1999). Most Shewanella species are mesophilic, psychrotolerant, and psychrophilic bacteria (Zhao et al., 2005). Shewanella alga is a Gram-negative bacillus that may be found in water and soil, where it thrives (Kim et al., 2006). S. algae was used by Konishi et al. (Konishi et al., 2007) to deposit gold nanoparticles. In the presence of lactate or H2 as an electron donor and ferric citrate (III) citrate as an electron acceptor, S. algae bacteria can grow anaerobically. When H2 gas was used as an electron source, they showed that S. algae resting cells could reduce ions (1 mM) into elemental gold in 30 min at 25°C throughout a pH range of 2.0–7.0 (Caccavo et al., 1992). It was found that the periplasmic area of S. algae cells was filled with biogenic gold nanoparticles (10–20 nm). Extracellular gold NPs were deposited when the pH of the solution fell to <2.8. There was a wide range of biogenic gold NPs (15–200 nm) on the bacterial cells at this pH. Biogenic gold NPs (20 nm) were coated on the bacterial cells, and bigger gold particles (350 nm) were deposited extracellularly in a solution with pH 2.0. So it may be inferred that pH significantly impacts the form and location of biogenic gold nanoparticles (Konishi et al., 2007). The quick degradation of soluble gold [Au(III)] into insoluble gold was shown to be the likely cause of the drop in soluble gold concentration. Lacking H2 gas, S. algae cells failed to use lactate as an alternate electron source to decrease Au (III). Furthermore, H2 gas did not chemically reduce Au (III) in a sterile control condition devoid of S. algal cells. Thus, in the presence of molecular H2 as an electron donor, S. algae resting cells converted soluble Au (III) into insoluble gold (Kashefi et al., 2001; Konishi et al., 2006).
The NADH-dependent reductase enzyme, which gives an electron and oxidizes to NAD, was used to produce Ag nanoparticles using Pseudomonas stutzeri AG259 bacteria. Biological reduction of Ag ions to nanoparticles occurs due to electron transfer (Ahmad et al., 2003). The extracellular manufacture of Au nanoparticles was achieved in Husseiny et al., who reduced Au ions using Pseudomonas aeruginosa (Husseiny et al., 2007). Other studies have also shown the lack of participation of biological enzymes. When Bacillus megaterium cells were dried, Liu and others synthesized Au nanoparticles (Liu et al., 2009). Sneha et al. conducted a similar investigation employing a Corynebacterium sp. and found that a nonenzymatic reduction mechanism was responsible for the creation of nanoparticles (Sneha et al., 2010). Many variables are thought to be involved in the decrease of nanoparticles.
For the first component, the cell wall contains organic functional groups that promote reduction, and for the second, the proper environmental conditions are required, including a suitable acidity and temperature (Lin et al., 2001). Lactobacillus sp. A09 and Bacillus megaterium D01, for example, may convert Ag ions to silver nanoparticles through the interaction of functional groups on the cell wall (Lin et al., 2005). The pH and temperature considerably impact nanoparticle size, shape, and content (Hulkoti and Taranath, 2014). Smaller particle sizes, for example, have more distinct physicochemical features than larger ones.
Consequently, it is necessary to tune the synthesis parameters throughout the nanoparticle production process to improve the overall particle qualities. Because these two characteristics are critical to nanoparticle production, choosing the suitable culture medium for certain bacteria and the right metallic salt is essential (Roh et al., 2001; Nair and Pradeep, 2002). It has been proven that the size and shape of particles may be affected by the concentration of metallic salts and the pH of the medium. Au nanoparticles ranging in size from 10 to 20 nm were formed at low concentrations of AuCl4 at pH 6. The salt content was increased to create Au nanowires at pH 6 (He et al., 2008). Changing the pH to four also produced spheres and triangular nanometer-scale plates when the salt concentrations were diluted. There is a definite correlation between pH and nanoparticle shape during production (Husseiny et al., 2007). Other researchers also synthesized metallic nanoparticles using bacterial species are enlisted in Table 3.
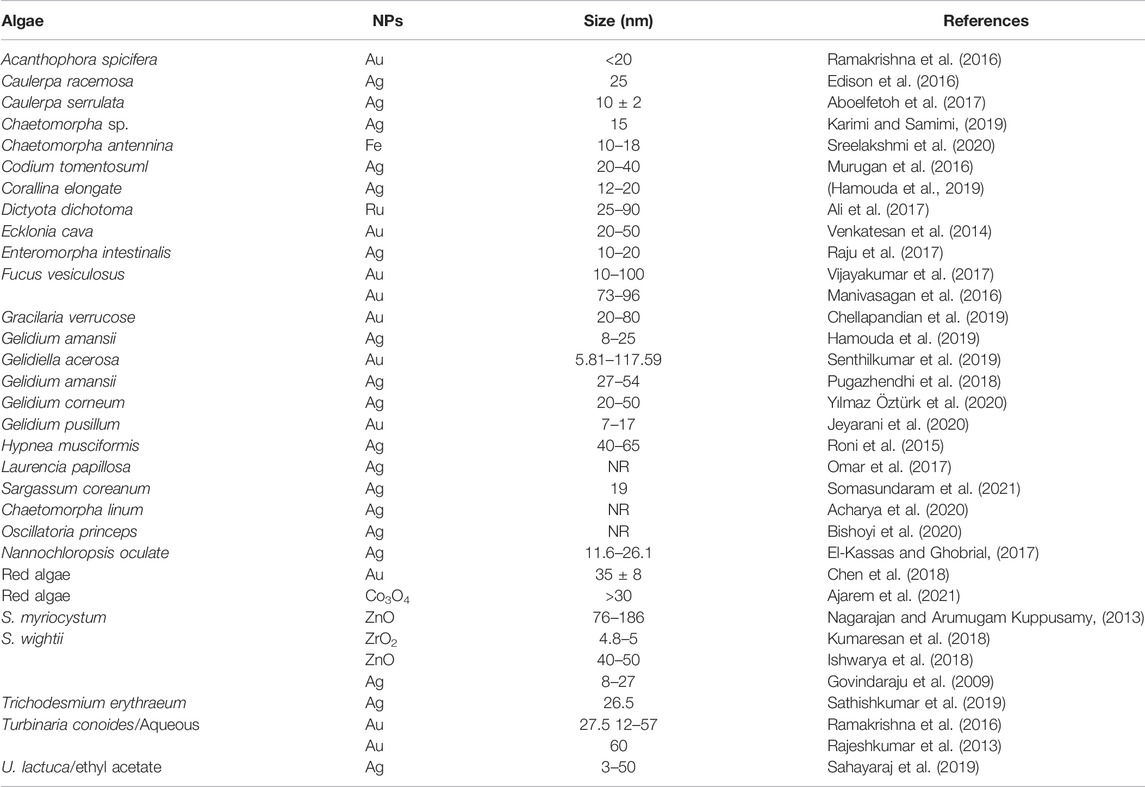
Algae Assisted Synthesis
Pterocladia capillacae, Jania rubins, Ulva faciata, and Colpmenia sinus (El-Rafie et al., 2013) algae specie’s have been used to synthesize silver nanoparticles. The NPs were 7–20 nm in diameter and spherical in shape. Researchers believe their antibacterial action is caused by a blockage of bacterial cell processes caused by their adhesion to the cell wall. Recent studies have shown the antimicrobial efficacy of Sargassum longifolium alga-derived silver nanoparticles (Rajeshkumar et al., 2014). After 1 h of mixing AgNO3 and aqueous algal extract, the reaction mixture becomes brown. The nanoparticles showed an absorption peak at 440 nm for polydispersed silver nanoparticles. The pH of the reaction mixture has been shown to significantly influence the production of silver nanoparticles. At a lower pH (6.2), the reaction mixture changed color more slowly. With the rise in pH, the reduction process became more visible. With increasing concentrations of silver nanoparticles, the antifungal activity against Aspergillus fumigatus, Candida albicans, and Fusarium sp. was observed to rise.
Pithophora oedogonia, a fresh water green alga, has been used to synthesize silver nanoparticles diameter 25–44 nm. Carbohydrates, saponins, steroids, and proteins were shown to decrease AgNO3 to silver nanoparticles by IR spectroscopy and quantitative analysis of the extract. Compared with Gram-positive bacteria, they were shown to be more efficacious (Sinha et al., 2015).
The production of silver nanoparticles from the marine alga Caulerpa racemosa and their antibacterial efficacy against human diseases have also been reported by Kathiraven et al. (Kathiraven et al., 2015). Staphylococcus aureus and Proteus mirabilis bacteria were killed at a low 5–15 L (5–25 nm) silver nanoparticles having face-centered cubic shape. Using a combination of 14 bacteria and microalgae, silver nanoparticles were created. Even in the dark, extracellular polysaccharides were producing nanoparticles. Silver nanoparticles of varying sizes and morphologies were found, ranging from species to species (Patel et al., 2015). Six harmful microorganisms were used to assess the antibacterial activity. The cell membrane is damaged due to the production of free radicals.
Living cells of the Euglena gracilis microalga to produce gold nanoparticles by Dahoumane and others (Dahoumane et al., 2016). Like other marine algae, the biomaterial in the alga acts as a reducing agent, capping agent, and catalyst. The yield of nanoparticles is influenced by several variables, including pH, reaction time, temperature, and concentration. Gold nanoparticle generation and release are thought to proceed in three steps: 1) absorption of Au+3; 2) reduction of Au+3 to Au0; and 3) release of gold nanoparticles into the solvent. This theory has been widely accepted. As a result of their distributed nature, they don’t collect in one place. Their dimensions range from 10 nm to several hundred nanometers. This shows that all algae have a tolerance limit and a certain ability to decrease metal ions to protect them from the poisonous impact of Au3+/Au0, since an AuCl3 concentration of 103 M is deadly to E. gracilis.
Stoechospermum marginatum biomass has been shown to produce gold nanoparticles biogenically (Arockiya Aarthi Rajathi et al., 2012). Within 10 min of adding HAuCl4, the brown extract became ruby red, displaying an absorption at 550 nm in the UV-vis spectrum due to SPR (Singaravelu et al., 2007). These polydispersed nanoparticles were cylindrical primarily, hexagonal, and triangular, with a diameter of 18.7–93.7 nm in the TEM pictures. On the other hand, SEM scans revealed the development of 40–85 nm gold nanoparticles. Algal extracts include terpenoids and phenols, which function as catalysts to convert gold ions into gold nanoparticles. A face-centered cubic gold structure was discovered using X-ray diffraction (Shankar et al., 2003).
In recent years, a limited number of researchers have reported the manufacture of Ag nanoparticles utilizing numerous kinds of seaweed. There have been reports of the biosynthesis of Ag nanoparticles using Padina tetrastromatica (brown seaweed), for example (Rajeshkumar et al., 2012). According to their research, the nanoparticles were spherical in form, with a mean particle size of 14 nm, and showed antibacterial properties. From Codium capitatum, the crystalline Ag nanoparticles were also biosynthesized in the range of 3–44 nm, with an average particle size of 30 nm (Kannan et al., 2013). Spirogyra insignis, another green alga, created spherical nanoparticles with a mean particle size of 30 nm (Castro et al., 2013), whereas Padina tetrastromatica, a macroalga, produced crystalline spherical nanoparticles with a size range of 5–35 nm (Gopinath, 2015). Although the antifungal, antibacterial, and anticancer effects of Ag nanoparticles generated by seaweeds have also been discovered. Biosynthesis of both Ag and Au nanoparticles has been achieved by Ramkumar Vijayan et al. using an aqueous solution containing an extract from Turbinaria conoides. The nanoparticles were also tested for their ability to inhibit the development of biofilms (Nag et al., 2021). Antimicrobial nanoparticles derived from a Sargassum plagiophyllum aqueous extract have also been demonstrated to be effective against bacterial pathogens such as Escherichia coli (Stalin Dhas et al., 2014).
Romero-González et al. found that de-alginate seaweed debris may be employed as a catalyst for reducing Au ions in solution to generate Au particles ranging in size from the nanometers to roughly 6 μm. It was discovered that the functions found in seaweed were effective in generating stable particles with a wide range of forms such as hexagonal and decahedral plates and rods, as well as irregular and decahedral rods (Romero-González et al., 2003). An example of an eco-friendly approach to extract Au from hydrometallurgical solutions was shown by Mata et al. in a similar investigation. Brown seaweed (Fucus vesiculosus) was used to biosorb and bioreduce Au, resulting in nanoparticles of varied sizes and morphologies (Mata et al., 2009). Sargassum wightii Greville, a marine alga, has also been used to produce Au nanoparticles by Singaravelu et al. (Singaravelu et al., 2007). Alga generated stable nanoparticles that ranged from 8 to 15 nm in diameter and were spherical. Researchers have been able to produce a wide range of stable nanoparticle sizes in similar studies by Luangpipat et al. using Chlorella vulgaris (Luangpipat et al., 2011), Rajasulochana et al. (Kappaphycus alvarezii) (Rajasulochana et al., 2010), and Stalin Dhas et al. (Sargassum myriocystum) (Stalin Dhas et al., 2012), while Senapati et al. have reported the biosynthesis of Au nanoparticles (Senapati et al., 2012). Green alga Spirogyra insignis and red alga Chondrus crispus were used by Castro et al. to synthesize Au nanoparticles (Castro et al., 2013). A brown alga, Stoechospermum marginatum, was used to biosynthesize gold nanoparticles by Arockiya Aarthi Rajathi et al. Their analysis found that the nanoparticles were crystallized and varied in size from 18.7 to 93.7 nm, with a limited number of hexagonal and triangular platelets in the mix. Diterpenoids in brown seaweed were discovered to be directly engaged in reducing Au by the hydroxyl groups. The nanoparticles also showed antibiotic efficacy against various bacterial pathogens (Arockiya Aarthi Rajathi et al., 2012). For example, brown seaweeds (Turbinaria ornate and Padina pavonica) exhibit biosynthesized Au nanoparticles ranging in size from 7 to 11 nm (Ashokkumar and Vijayaraghavan, 2016) and from 30 to 70 nm (Padina pavonica). The biosynthesis of gold nanoparticles by two freshwater algae species has also been shown (Sharma et al., 2014a; 2014b). These species are the green alga Prasiola crispa and the red alga Lemanea fluviatilis.
Abboud et al. reported a biosynthesis of copper oxide nanoparticles utilizing a brown alga extract (Bifurcaria bifurcata). Nanoparticles of cuprous oxide (Cu2O) and cupric oxide (CuO) were generated in a simple technique. A few nanoparticles were elongated, but the vast majority of the particles were spherical. An average particle size of 22.6 nm was discovered for the samples, which varied from 5 to 45 nm. Copper oxide nanoparticles were shown to be effective against both Enterobacter aerogenes and Staphylococcus aureus in subsequent antibacterial experiments (Abboud et al., 2014). The manufacture of copper cored copper oxide nanoparticles utilizing red seaweed extracts (Kappaphycus alvarezii) was also described in a recent work by Khanehzaei et al. Stabilized copper cored-cuprous oxide nanoparticles with a mean particle size of 53 nm were synthesized in the presence of seaweed. Nanoparticle surfaces were also discovered to be capped by pairs of electrons, some hydroxide and sulphur groups from the water-soluble sulphated polysaccharides present in seaweed cell walls (Khanehzaei et al., 2015).
One-step green biogenic synthesis of ferric oxide (Fe3O4) nanoparticles using brown seaweed was recently shown in work by Mahdavi and others (Sargassum muticum). To make Fe3O4 nanoparticles, an aqueous seaweed extract was combined with an aqueous ferric chloride solution. Reduction and capping are both accomplished by the amino, carboxy, and hydroxyl functional groups produced from the water-soluble polysaccharide cell walls (Mahdavi et al., 2013). The average size of the particles formed was 18 nm, crystalline, and cubic in shape. It was shown that the Fe3O4 nanoparticles generated by Namvar et al. have anticancer efficacy against human cancer cell lines, including leukemia, breast cancer, cervical cancer, and liver cancer, when used in vitro tests. The buildup of Fe3O4 nanoparticles in treated cells was shown to increase cell death in vitro tests and proved their potential utility in cancer therapy (Namvar et al., 2014).
Another work used the marine green alga Caulerpa serrulate to bio fabricate stable colloidal crystalline AgNPs. The manufactured NPs were found to be between 10 and 2 nm in diameter and spherical in form by TEM. The photocatalytic activity was shown, with 99 percent of Congo red dye degraded after only 6 min of incubation. They also showed antibacterial action against Gram-negative and Gram-positive bacteria, including Staphylococcus aureus, Shigella sp., Salmonella typhi, and Escherichia coli (Aboelfetoh et al., 2017). Another complex dye, methylene blue (MB), is hazardous to living beings, making its breakdown a critical concern for both the environment and biology. After 30 min of exposure to light and NaBH4, Edison et al. could bio generate AgNPs that could totally break down MB in the presence of the marine green alga Caulerpa racemosa (Edison et al., 2016).
Bioactive molecules for the production of AgNPs have been discovered by combining a sulfated polysaccharide obtained from the marine red alga Porphyra vietnamensis with silver nitrate. Antibacterial activity against Gram-negative and Gram-positive bacteria was shown by the production of NPs with an average diameter of 13 nm (Venkatpurwar and Pokharkar, 2011).
A chloroauric acid (HAuCl4) solution and an aqueous extract of marine microalgae (Tetraselmis suecica) were used to synthesize and characterize gold nanoparticles (AuNPs). There was a distinct band in the UV–Vis spectrum that corresponded to the formation of AuNPs. However, the most common 79 nm diameter with a polydispersed and crystalline structure (Shakibaie et al., 2010). Their diameter ranged from 51 to 120 nm. Polysaccharide hydroxyl groups from the algal polysaccharides were shown to have an essential role in the biosynthesis of AuNPs from Padina gymnospora. The generated NPs’ crystalline nature was verified by X-ray diffraction (XRD), and an AFM study showed that they were between 53 and 67 nm in size (Singh et al., 2013).
Ramakrishna and his colleagues used Sargassum tenerrimum and Turbinaria conoides as reducing agents for gold ions. Two extracts of the gold nanoparticles showed photocatalytic activity by degrading 4-nitrophenol and p-nitroaniline into their corresponding aminoarenes (4-aminophenol and p-phenylenediamine) and rendering naturally coloured solutions (Rhodamine B and Sulforhodamine) into colorless solution in the presence of NaBH4 as a catalyst (Ramakrishna et al., 2016). Because of the additive action of nanosilver and the wide range of phytoconstituents with intrinsic antimicrobial capabilities, silver nanoparticles with exceptional stability and environmental friendliness can be easily manufactured from plant extracts and demonstrate a broad spectrum of antimicrobial activities, anticancer activities and catalytic reduction of 4-nitrophenol (Bharadwaj et al., 2021). Nanoparticles synthesized using algae are enlisted in Table 4.
Factors Affecting Biosynthesis of Nanoparticles
There are a number of elements that influence the formation and shape of nanoparticles that have been developed. Researchers have linked these variances to the synthetic process’s choice of adsorbate and catalyst (Patra and Baek, 2014). Nanoparticles creation from biological extracts may also be affected by reaction conditions. Studies have shown that a reaction solution’s pH has a significant impact on the production of the nanoparticles that result. The form and size of the generated nanoparticles may be affected by changes in the reaction pH. When comparing lower acidic pH values to higher acidic pH values, bigger particles are produced. The bigger particles (25–85 nm) were generated at pH two whereas the smaller particles (5–20 nm) were created at pH three and four in a research using Avena sativa biomass (Armendariz et al., 2004). Particle aggregation may have been caused by the lack of functional groups at pH 2, according to the researchers. The bacteria Rhodopseudomonas capsulate was shown to produce gold nanoparticles in a similar manner. It was discovered that, with a pH rise of 7, spherical particles measuring 10–20 nm were present. Nanoplates were formed when the reaction pH was lowered to 4 (He et al., 2007).
Another researcher demonstrated that the pH of Saudi Dates extract had an impact on the shape, reaction rate, and size of biosynthesized Pt NPs(Al-Radadi, 2019). The reaction rate was found to be quicker when the dispersive medium’s hydroxyl content rose. The acidified media, on the other hand, created a variety of different-sized particles. Shape and size of synthesised Pt NPs are expected to be rod-shaped at pHs 1.5, 3.5, 5, and 7, with a diameter of 700.5 nm, spherical at sizes 5.0–5.4 nm, 2.5–13.8 nm, and rod-shaped at pHs 1.5–5.5 with 700.5 nm diameter.
Another key part of any synthesis is temperature. Temperature increase has showed catalytic behaviour by boosting the reaction rate and efficiency of nanoparticle synthesis while using biological entities to formulate nanoparticles. According to a research on neem leaf extracts and the production of AgNPs, temperature elevation (10–50 °C) was linked to an increase in the reduction of Ag+(Verma and Mehata, 2016). Smaller AgNPs were formed at 50°C in the same way as Kaviya et al. found in the generation of AgNPs from citrus peel extract using different temperatures (Kaviya et al., 2011). AgNPs were also produced in this manner from Escherichia coli wasted culture supernatants (Gurunathan et al., 2009). The scientists speculated that a critical enzyme involved in the creation of nanoparticles may have been affected by elevated temperatures. But the study’s findings showed that temperatures over 60°C favoured the creation of larger-sized particles, which was surprising. Molecular kinetics at high temperatures causes fast reduction of Ag+ (which aids reduction and nucleation) at the expense of secondary reduction on nascent particle surfaces, which is why this finding was made. At higher incubation temperatures. Saudi’s dates extract was used by Al-Radadi to study the effect of temperature on the biogenesis of Pt NPs. The average particle size was 3.4 nm at 20°C and 2.6 nm at 30°C, according to microscopy measurements (Al-Radadi, 2019). It has also been shown that temperature has an effect on the structure of nanoparticles as well. While AgNPs were generated at ambient temperature using Cassia fistula extracts, spherical AgNPs were created at higher temperatures (over 60 °C) (Lin et al., 2010). Plant macromolecules’ interactions with Ag faces were assumed to be altered by high temperatures in the research, which prevented the coalescence of nearby nanoparticles.
Applications of Biosynthesized Metallic Nanoparticles
Silver Nanoparticles
Silver has a long history of usage as an antibacterial, and its nanoforms are significantly superior and more biocompatible antimicrobial agents now than their conventional counterparts. Research on silver consumption in nanotechnology has been going on since the dawn of the age of nanotechnology, and this section covers a lot of that work. Silver nanoparticles have received a great deal of attention in recent years for their potential to combat infectious diseases by closing the gaps in current antimicrobial formulation techniques, eradicating drug-resistant microorganisms, and establishing a foothold for the emerging field of conjugated silver nanoparticles. This research could help us better understand the role of silver nanoparticles in future antimicrobial treatments. Because of the additive action of nanosilver and the large variety of phytoconstituents with intrinsic antimicrobial capabilities, silver nanoparticles may be readily manufactured from plant extracts with extraordinary stability and environmental friendliness and demonstrate a broad spectrum of antimicrobial activities.
In research by Loo et al., silver nanoparticles (4.06 nm) produced from Puerh tea leaves have been discovered to have high antibacterial efficacy against Gram-ve pathogenic pathogens such as Klebsiella pneumonia, Escherichia coli, and Salmonella typhimurium. Furthermore, the Minimum inhibitory concentrations (MICs) recorded were: 3.9, 3.9, 7.8, and 3.90 μg/ml (Loo et al., 2018). Silver nanoparticles (quasi-spherical and spherical; 5 nm in size) were synthesized by Garibo et al. using an aqueous extract of the perennial tree Lysiloma acapulcensis and were proven to be powerful antibacterial agents in their research (Garibo et al., 2020; Rahman et al., 2021a). Selim et al. studied the antimicrobial activity of silver nanoparticles with an average size of 50 nm on M. tuberculosis, MDR Mycobacterium tuberculosis strain, and clinical isolates in important research (M. tuberculosis and M. bovis). All of the investigated substances were strongly inhibited by the produced nanoparticles. M. tuberculosis MIC values were determined to be 4 μg/ml and 1 μg/ml for M. bovis and M. tuberculosis correspondingly. M. tuberculosis MDR strain has a MIC value of 16 μg per litre. However, MIC values of 1–16 μg/ml and 4–32 μg/ml have been recorded for M. tuberculosis and M. bovis clinical isolates, respectively. According to the findings of this investigation, silver nanoformulations may have the antitubercular potential (Selim et al., 2018). Using synthesized silver nanoparticles, Singh et al. found that antibacterial activity of bacteriogenic silver nanoparticles against the pathogenic nosocomial Acinetobacter baumannii was inhibited with a MIC value of 16 μg/ml, significantly lower than the MIC values of ampicillin (4,096 μg/ml), amoxicillin (2048 μg/ml), and erythromycin (64 μg/ml). This research reveals that AgNPs have more promise and effectiveness in treating hospital-acquired illnesses than traditional antibiotics (Singh et al., 2017).
Proton motive force strength generated by the ionic connection between AgNPs and the bacterial cell wall may interrupt the activity of enzymes containing thiol groups (Sereemaspun et al., 2008). When used against E. coli, Avicennia marina-based AgNPs were shown to have bactericidal effects because the proton motive force may have been dissipated (Gnanadesigan et al., 2012). The effect of AgNPs on the cell membrane and cell is dependent on the cell’s makeup. Using Argemone mexicana leaf extract and antibacterial experiments with E. coli and P. aeruginosa, Singh et al. synthesized green nanoparticles in their work (Singh et al., 2010). According to the research, both the bactericidal effects of the AgNPs and the polymer subunit’s potential to break membranes were shown to have antibacterial properties.
In comparison to Gram-negative bacteria, Gram-positive bacteria have a stronger cell wall due to a lower concentration of lipopolysaccharides, making them a more formidable barrier to the entry of AgNPs. Gram-negative bacteria’s cell walls and membranes are thinner due to more lipopolysaccharides and less peptidoglycan. They adhere to AgNPs due to their composition, stability, and negative charge. Because AgNPs have an electrical affinity to bacteria, they may be used to kill them, as was previously stated (Abbaszadegan et al., 2015). A change in the cell’s internal environment and membrane polarization is caused by this attraction and activity, resulting in cell death (Abalkhil et al., 2017). In contrast to bacteria that aren’t exposed to AgNPs, those that display morphological and physiological alterations, and their integrity is disrupted. The components of the bacterial cell, such as nucleic acid, proteins, enzymes, metabolites, and the bacterial cell’s energy supply, are released into the environment when the cell wall and membrane are ruptured (Gomaa, 2017).
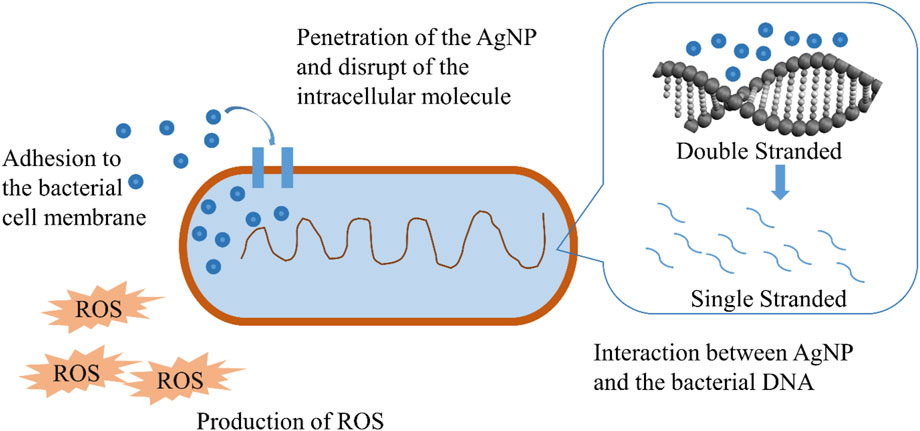
AgNPs attach to proteins and DNA in bacteria and induce conformational changes, resulting in less stable states that limit their ability to function (Bondarenko et al., 2013). According to studies, DNA degradation is facilitated by sulphur and other amino groups on the membrane surface. The bacteria are affected due to the interaction between the AgNP and the bacterium-infected macromolecules (DNA, protein, and lipids). AgNPs derived from Datura stramonium, for example, were shown to exhibit antibacterial action against E. coli by Gomathi et al. (Gomathi et al., 2017) (2017). According to the findings of this research, Ag ions from AgNPs enter bacterium cells, where they cause significant damage and eventually lead to cell death. In a similar vein, AgNPs generated from Urtica dioica interacted with bacterium cells and penetrated them, releasing Ag+ ions that inhibited DNA replication and eventually killed the bacteria, as described by Jyoti et al. (Jyoti et al., 2016).
Antimicrobials may cause oxidative stress in bacteria by introducing them to the cell. The generation of reactive oxidative species (ROS) in resistant bacteria is one method of inhibiting their proliferation. Antimicrobials are often used to raise ROS levels. However, the introduction of AgNPs to resistant microorganisms elevates ROS levels, leading to resistant species, according to a recent study. Based on the findings of Khan and Ali (Khan and Ali, 2020), it seems that the introduction of AgNPs to resistant microorganisms such as Xanthomonas citri, S. aureus, and Erwinia carotovora causes ROS levels to rise beyond critical levels. The study found that ROS levels rose when AgNPs were added to bacterial suspensions, resulting in bacterial suppression. AgNPs caused the rise in ROS when exposed to resistant strains of E. coli and S. aureus, according to Das et al. (2017). The research found that when these resistant strains are treated with AgNPs, the ROS levels in these bacteria rise, resulting in cellular inhibition (As shown in Figure 2). According to Kim et al. (2011), AgNPs generated ROS that damaged the cell membrane, protein, and DNA of E. coli and S. aureus (Kim et al., 2011).
Oyster mushroom AgNPs were shown to be superior to S. aureus in terms of antibacterial activity against E. coli and Pseudomonas aeruginosa (Nithya and Ragunathan, 2009). It was shown that P. ostreatus-AgNPs effectively against Gram-positive bacteria such as S. aureus when evaluated by disc diffusion technique (Mirunalini et al., 2012). It was found that the most significant inhibition zone of P. ostreatus-AgNPs was 8 mm. The inhibitory zone was smaller in oyster mushroom extracts than silver nanoparticles. Ag+'s antibacterial properties have not been fully elucidated; however, it may be due to the electrostatic attraction between nanoparticles’ positive and bacteria’s negative charges (Mirunalini et al., 2012). The antibacterial properties of AgNP made it a contender for use as an antimicrobial agent.
Syzygium cumini, also known as “Jammun” in Hindi, is a member of the plant family Myrtaceae and is sometimes referred to as “Indian blackberry.” Syzygium cumini, a common medicinal herb, is used to treat a broad range of ailments. Since ancient times, people have utilised the bark of this herb to cure a wide range of ailments, from sore throats and bronchitis to asthma and diarrhoea to ulcers and stomach ailments including diarrhea and biliousness. Syzygium cumini extract was used by Chakravarty et al. to synthesize silver nanoparticles with anti-inflammatory, antibacterial, and antioxidant properties (Chakravarty et al., 2022). DPPH free radical quenching characteristics of these green nanoparticles were found to be excellent. Anti-inflammatory silver nanoparticles that are green or bio-synthesized are likewise effective, with an 82.7 percent inhibition of albumin denaturation at a 1000-μg-per-liter concentration (Chakravarty et al., 2022).
Flowering plant and member of the Portulacaceae family Portulaca oleracea L. is often known as Purslane, the duckweed or small hogweed. Al-Otibi et al. created two types of green AgNPs, one by irradiating previously prepared AgNPs with 60Co γ-ray while utilising chitosan or by combining the aqueous extract of P. oleracea with silver nitrate (AgNO3) (normal AgNPs) (gamma-irradiated AgNPs) (Al-Otibi et al., 2022). Curvularia spicifera, Macrophomina phaseolina, and Bipolaris sp. were all shown to be plant pathogenic fungus. Compared to the regular AgNPs, the irradiation green AgNPs demonstrated a higher antifungal impact against all three of the tested fungal strains, with just a few outliers. There were noticeable changes in the fungal strains after exposure to the two AgNP formulations, including flaccid structures and compacted hyphae. Against C. spicifera, M. phaseolina, and Bipolaris species, the biosynthesized P. oleracea AgNPs seemed to exhibit antifungal activities. It is possible that these AgNPs might be used as a fungicide to protect a variety of plants against pathogens.
Gold Nanoparticles
C. aromaticus leaf extracts were utilised to create the Au NPs, then employed as antibacterial agents. Temperatures (at 30, 60, and 100°C) were used to control the generation of NPs. It was shown that C. aromaticus-prepared Au NPs had strong antibacterial activity (ABA) when tested against various strains of bacteria (Boomi et al., 2019). For this investigation, nigra leaves from A. nigra were also used to make AuNPs, and their ABA content was tested. Bacteria (Gram-positive and Gram-negative) could not grow in the presence of Au nanoparticles, measured to be 21.52 nm in size. A. nigra, according to the findings, may be able to produce nanoparticles of gold (Au NPs) that serve as antibacterial agents (Baruah et al., 2018). Cashew leaves extract was also employed to synthesis antibacterial agents, and it was shown that the biogenically produced Au NPs were quite effective against both B. subtilis and E. coli bacteria (Sunderam et al., 2019). Gold nanoparticles produced from M. koenigii leaf extract showed a significant level of ABA activity.
Au NPs were synthesized using green and black tea leaf extracts, and their ABA activity was investigated. Room-temperature-prepared Au NPs had a diameter of 10 nm and were influential in treating bacterial strains (Onitsuka et al., 2019). Au NPs may also be generated at room temperature using C. japonica leaf extract. To see whether it could compete with regular antibiotics against bacteria like K. pneumoniae and E. coli and the common cold and flu virus and Staphylococcus aureus and Candida albicans and Proteus mirabilis the Au NPs’ ABA was put to the test (Sharma et al., 2019). The aqueous leaf extract of G. superba was used to fabricate Au NPs, and the NPs were spherical and 20 nm in size (Gopinath et al., 2016). G. superba aqueous leaf extract was proposed to synthesize Au NPs to develop medications efficient in treating microbial toxins based on data showing great ABA against bacteria (Gram-positive and negative) (Gopinath et al., 2016).
Au NPs were prepared at low temperatures using P. atlantica leaf extract, and the antibacterial properties of the NPs were also tested. Particles with a diameter of 50–60 nm were used. Diffusion technique was employed to assess the antibacterial activity of Au NPs, and the broth dilution method was used to determine the MIC and Minimum Bactericidal Concentration (MBC). The ABA of the Au NPs was encouraging, and the MIC value was also relatively low, equivalent to that of a conventional antibiotic (Hamelian et al., 2018). The aqueous leaf extract of N. nouchali was utilized to synthesize Au NPs, and the same NPs were used for the ABA assessment against E. coli, as previously reported. N. nouchali leaf extract extracts were used to manufacture 54.7 nm-sized AuNPs, which showed promise in therapeutic effectiveness (Maji et al., 2019). Au NPS synthesis was also carried out using A. bettzickiana leaf extract. S. typhi, B. subtilis, P. aeroginosa, E. aerogenes, S. aureus, and M. luteus were tested against the ABA particles ranging from 80 to 120 nm in diameter (Nagalingam et al., 2018). The ABA of Au NPs generated by fresh/dry leaf extract of M. indica extracts was also tested by Philip (Philip, 2010). These particles were produced using a cost-effective, repeatable green approach with 17–20 nm average diameter. A survey of bacterial species demonstrated that the produced Au NPs had promising ABA.
There has been significant interest in gold nanoparticles because of their unusual inertness to the external environment. Biosensing and medication delivery might benefit significantly from its properties (Elahi et al., 2018). It is also used to destroy potentially hazardous dyes to demonstrate its photocatalytic properties. Methyl Blue and Methyl Orange colors were reduced after 12 min of adding Au NP solution, according to Kumar et al., While Direct Blue 24 took just 18 min (Bogireddy et al., 2015). The breakdown of environmentally harmful colours like Methylene Blue has been made possible with certain plants. The paper, textile, and rubber sectors employ this basic cationic dye. The gold NPs mediated by the floral extract of Plumeria alba efficiently eliminated all of the Methylene blue dye. Degradation takes 40 min for a 5 percent solution and 70 min for a 1 percent solution to be complete (Mata et al., 2016).
It was found that plant-AuNPs could be easily synthesized utilizing the leaf extracts of Paederia foetida Linn (Bhuyan et al., 2017). When decomposing rhodamine B in an aqueous solution with NaBH4, the AuNPs showed photocatalytic efficiency under Sun irradiation. At a wavelength of 554 nm, UV-vis spectroscopy revealed the whole process. During the photocatalytic process, the dye solution lost its bright pink hue. It became colorless, showing that the AuNPs played an essential role in producing structural alterations in the rhodamine B dye and deleting chromophoric groups linked to dye molecules. Adsorption of BH4 on the surface of AuNPs induced electron transfer from BH4 to rhodamine B through AuNPs, which was the catalytic process.
AuNPs-based sensors were created by reducing HAuCl4 using Solanum lycopersicums juice extract as a reducing agent. The plant-AuNPs had an SPR peak at 546 nm, red-shifted to 800 nm, and lost strength when Cu2+ was added to the mixture, as seen in the UV-vis spectrum (Duan et al., 2019). Purple had become blue in the reaction, indicating the presence of AuNPs and Cu2+ ions interacting. An SPR peak that appeared at 800 nm resulted from molecules adsorbing onto AuNPs and causing them to clump together. Bonds produced between the AuNPs containing Cu2+ and the biomolecules in the juice extract from Solanum lycopersicum were used as linkers to bind the biomolecules created. These materials might detect Cu2+ ions in water purification by aggregating AuNPs and changing their color further.
Smilax glabra rhizome extracts preserved AuNPs, and the therapeutic impact of the AuNPs produced from Smilax glabra on obese and diabetic rats was studied (Ansari et al., 2019). Results from histopathological studies showed plant-AuNPs therapy repaired the nuclei and membranes of diabetes cells, as well as the cells’ cytoplasm. As well as, AuNPs stabilized with extracts of Vetex negundo and Camellia sinensis leaves were utilised to treat acute myeloid leukemia in mice models and to test the efficacy of pro-apoptotic agents on human gastric cancer cells.
Zinc Nanoparticles
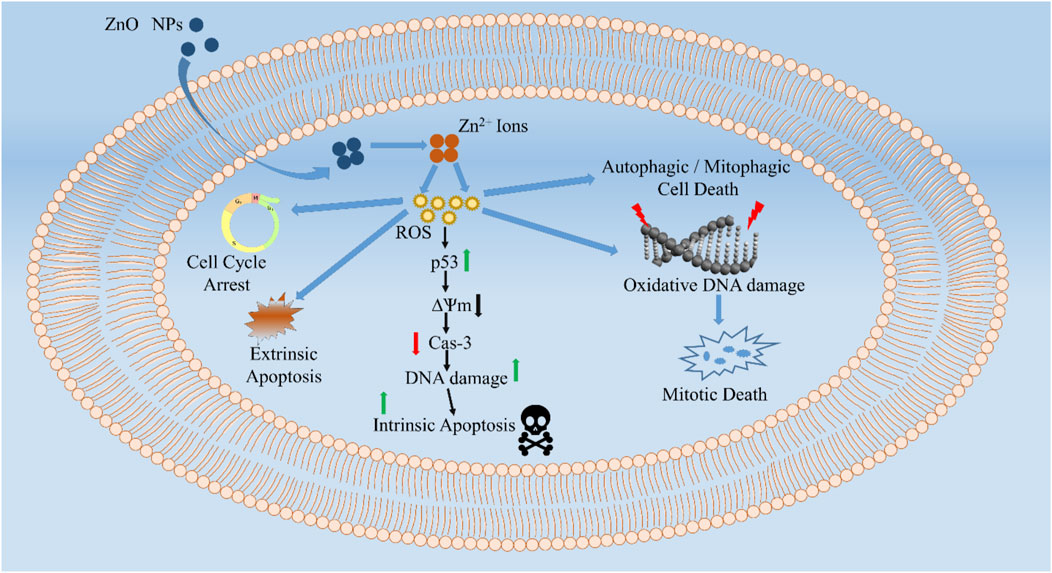
To study the cytotoxic impact of green ZnO NPs on MG-63 osteosarcoma cancer cells, the cells were exposed for 24 h at doses of 1–100 μg/ml (Sisubalan et al., 2018). A concentration of 10 μg/ml of ZnO NPs is sufficient to kill 50% of the cells. The release of reactive oxygen species (ROS) by ZnO NPs causes morphological damage and cell death. Non-melanoma skin cancer cells (A431) and normal Vero cells were tested for cytotoxicity of green produced ZnO spherical and sheet-like NPs, respectively (Jevapatarakul et al., 2020). Dose-dependent cytotoxicity of ZnO (30–150 g/ml) was seen in lines A431 at the highest dosage, resulting in cell death of 40–50% in 48 h without harming normal Vero cells. Targeted medication delivery using ZnO nanoparticles provides new options for cancer therapy that are both safer and more effective. Zinc oxide nanoparticles (ZnO) may be used as nanocarriers for various chemotherapeutic drugs that synergistically impact cancer cells (As shown in Figure 3). A new nanocomposite of Cur/PMMA-PEG/ZnO NPs was developed by Dhivya et al. to transport curcumin and improve its solubility and cytotoxicity. PMMA-PEG/ZnO nanocomposite with an average size of less than 80 nm was shown to release curcumin faster under acidic pH 5.8. The IC50 for human gastric cancer AGS cells was 0.01 g/ml for the Cur/PMMA-PEG/ZnO nanocomposite (Dhivya et al., 2017).
Anticancer Effect of Zinc
Nanoparticles with antifungal activities have been extensively researched and published. Among them, phytogenic ZnO NPs are the most widely studied because of their biocompatibility and flexibility. The antifungal agent ZnO NPs was produced utilizing surfactant extracted from seeds of Acacia concinna (Sharma et al., 2010). An aloe-broth-mediated ZnO NP was evaluated against various aspergilli such as Aspergillus flavus, Nidulans, Trichoderma harzianum, and Stolonifer. Fungicidal efficacy against the tested fungus strains was shown using ZnO NP suspensions with concentrations between 8 and 20 mM (Gunalan et al., 2012). To combat a wide range of fungus species found in a drinking water pipeline, ZnO NPs were synthesized using the stem extract of Boswellia ovalifoliolata. Meyerozyma caribbica, Aspergillus parvisclerotigenus, Meyerozyma guilliermondii, Rhizopus oryzae, Aspergillus oryzae, and Trichoderma asperellum were all successfully treated with ZnNPs (Supraja et al., 2016). L. aculeata extract was used to synthesize ZnO NPs that were well disseminated and showed potential antifungal efficacy against A. niger, F. oxysporum, and P. funiculosum (Narendhran and Sivaraj, 2016). To make biofuel-grade ZnO NPs, Moringa oleifera extract was used in the extraction process (Surendra et al., 2016). On Alternaria saloni and Sclerrotium rolfiistrains the particles demonstrated significant antifungal action. Tests were performed using the disc diffusion technique on biogenic ZnO NPs made from Murraya koeniggi and Azadirachta indica (L.) leaf extract (Elumalai et al., 2015). According to the findings, both ZnO NPs have significant antifungal activity. Candida albicans ATCC 2091, Candida glabrata NCIM3448, and Cryptococcus neoformans ATCC34664 were evaluated against green generated ZnO NPs using Ziziphus nummularia leaf extract by the minimum inhibitory concentration and time-kill assays, respectively (Padalia and Chanda, 2017). C. albicans, C. glabrata, and C. neoformans had MIC values of 1.25 mg/ml and 10 mg/ml, respectively. In the presence of Candida albicans, Pongamia pinnata seed extract-mediated ZnO NPs showed significant biofilm suppression efficacy at 50 mg/ml (Malaikozhundan et al., 2017). At a 100 μg/ml concentration, ZnO NPs produced using Cumin seed extract stopped 66% of Rhizoctonia solani fungus growth (Zare et al., 2017). Microwave aided the extraction of phytochemicals from Suaeda aegyptiaca to make ZnO NPs is a biogenic approach. The ZnO NPs generated by the former method had better inhibitory efficiency against Candida albicans and Aspergillus oryzae than those obtained by maceration. Increased concentration and irradiation power increased antifungal activity (Rajabi et al., 2017). Aspergillus flavus, Aspergillus niger, Aspergillus fumigatus, and Fusarium solani were evaluated against Silybum marianum extract-mediated ZnO nanoparticles. Every strain tested under 1 mg/ml showed significant inhibition (Hameed et al., 2019).
Miscellaneous
Antibacterial activity against E. coli, Klebsiella pneumonia, Salmonella choleraesuis, and Pseudomonas aeruginosa has been shown by Au-Pt NPs composites of between 2 and 3 nm in size. (Zhao et al., 2014). According to recent investigations, bacterial growth inhibition has been linked to ATP synthesis and mitochondrial membrane potential. Antibacterial activity against P. aeruginosa was also studied using polyvinylpyrrolidone-coated PtNPs of various shapes and sizes, ranging from 2 to 20 nm. The toxicity of nanoparticles depends on their size, as shown by the toxic effects of NPs less than 3 nm on P. aeruginosa, whereas NPs larger than 3 nm had no or reduced damaging impact (Gopal et al., 2013). E. coli and S. aureus were both killed by PtNPs coated in PVP. E. coli growth was suppressed by small-sized NPs, consistent with findings from prior research (Taglietti et al., 2012). Antibacterial activity against E. coli in the vicinity of the metal composition, the rGO matrix, and the bacteria was improved by beading Pt/Ag NPs on reduced graphene oxide (rGO) nanosheets containing holes. The bacteria on the porous rGO matrix may be tracked using Ag ions released from the nanocomposite directly and quickly. Antibacterial activity against E. coli, Lactococcus lactis, and Klebsiella pneumonia was maximized when polyvinylpyrrolidone (PVP) nanoparticles (PVP/PtNPs) were combined as nanocomposites (Ramkumar et al., 2017).
Recyclability and Reusability of Green-Synthesized Nanoparticles
The areas of materials engineering and nanotechnology are increasingly concerned with sustainability techniques, frameworks, and metrics in an attempt to mitigate environmental and health concerns connected with the manufacturing, use, and disposal of innovative nanomaterials (Dhingra et al., 2010). Veisi et al., synthesized Ag nanoparticles based on Thymbra spicata, the plant being rich source of thymol, carvacrol and myrcene (Veisi et al., 2018). In spite of the significant catalytic activity of Ag Nanoparticles/Thymbra. When Ag NPs/Thymbra were separated and reapplied in RhB and MB colour degradation, their recycling efficiency was determined correspondingly. No significant catalytic activity loss was seen when Ag NPs/Thymbra was recycled eight times for both dyes and 4-nitrophenol, indicating the great stability of Ag NPs/Thymbra. Moradnia et al., studied the synthesis of MgFeCrO4 based nanoparticles, using Tragacanth gel for the bioremediation of DB122 dye (Moradnia et al., 2020). Nanoparticles degraded DB122 dye with in 60 s and kinetics were compling with the pseudo first order kinetics. The photocatalyst was able to degrade the dye and didn’t showed any remarkable variation even after four runs. Nasrollahzadeh et al., studied the green synthesis of Palladium nanoparticles using barberry fruit extract and immobilized on reduced graphene oxide (Nasrollahzadeh et al., 2016). The reusability was tested on reduction of 4-nitrophenol with NaBH4 as model. The catalyst was recovered successfully after completion of activity without significance loss of its reducing activity. Similarly, researchers developed copper nanoparticles using Commersonia bartramia extract and immobilized using Al2O3 surface (Nasrollahzadeh et al., 2019). The catalyst was able to show significant changes upto 7th cycle, for reduction of 2,4-dinitrophenylhydrazine.
Biocompatibility and Toxicity
The possible toxicity of nanoparticles to biological creatures has sparked controversy. Although pollens, fine sand and dust, volcanic ash, ocean spray, and biological material like viruses have been present in humans and other organisms for a long time, worry about nanoparticles is relatively new, due mostly to anthropogenic, synthetic, and manufactured nanoparticles. The skin, lungs, and gastrointestinal system are all often exposed to the outside world, making them ideal entrance points for nanomaterials of any type. Skin, on the other hand, is rather impermeable, making it less susceptible to NPs than the lungs and gastrointestinal system. Post-entry NPs may move from the entry ports into the circulatory and lymphatic systems and eventually into the tissues and organs of the human body. The worry is with a select kind of nanoparticles that induce permanent cell damage through oxidative stress or organelle injury, despite the fact that many nanomaterials are harmless and even helpful to health.
The size and content of the NPs seem to have a significant impact on the degree of harm they cause. Surface area, chemistry, and coating and functionalization of metallic nanoparticles are only a few of the many aspects that might have an adverse influence on the health of those exposed to them. In order to gather correct information for future policy and regulatory procedures, it is vital to focus on the research of toxicology of each item (Das R. K. et al., 2017). Due to the difficulty in evaluating nanoparticles’ toxicities using conventional toxicology methodologies, the toxicity evaluation of nanomaterials is difficult. In part, this is owing to nanomaterials’ tiny size and distinctive surface characteristics, which make them behave differently from bulk materials. Existing exposure pathways may be widened due to their modest size. In terms of toxicokinetics, nanomaterial surface qualities vary from those of bulk materials. Because nanoparticles have a large surface area, estimates based on mass are generally incorrect. As a result, it is difficult to collect accurate and repeatable exposure and toxicity data owing to the lack of reliable physical data such as surface area, composition, surface characteristics, and aggregation state (Hulla et al., 2015). The biological activity of nanoparticles generated with various reducing agents has been reported. Toxicity levels may vary dramatically depending on the biological agent utilized to synthesize biogenic nanoparticles, which are more common than non-biogenic ones. Nanoparticle characteristics are influenced by a variety of parameters, including their composition, size, shape, surface charge, and capping molecules (Venil et al., 2016). When tested against the MCF-7 breast cancer cell line, the cytotoxicity of silver nanoparticles generated using flexirubin was substantially greater than that of AgNPs made chemically. With 6-carboxypullulan and pullulan, it was found that AgNPs generated with 6-carboxypullulan were smaller than those synthesised with pullulan. Antimicrobial activity of carboxypullulan-mediated AgNPs was enhanced due of their larger negative zeta potential values (Coseri et al., 2015). AgNPs made from green tea extract (GT-AgNP) and coffee extract (CAgNP) demonstrated outstanding antibacterial effects in another fascinating investigation. For this reason, antimicrobial chemotherapy cannot employ C-AgNPs as they are hazardous to mammalian cells, while GT-AgNPs are benign (Rónavári et al., 2017). Standardized guidelines have also been developed recently in this regard. Researchers in the United Kingdom and the United States have made reference materials for nanotoxicity testing accessible to the public. For the first time, the International Alliance for Nano Environment, Human Health and Safety Harmonization began producing test methods for nanotoxicity testing. High-throughput nanomaterial screening seems promising and not too far away in view of the toxicity testing in the twenty-first century recommended by the US National Research Council (NRC).
Challenges and Future Directions
Since the PK, biodistribution, and safety of nanoparticle-based therapies are heavily influenced by particle dimensions, the size and distribution of nanoparticles is generally acknowledged as a distinguishing property. The renal excretion of nanoparticles less than 20–30 nm is fast, while the mononuclear-phagocytic system (MPS; also known as reticuloendothelial systems) in the liver, spleen, and bone marrow is more effective in absorbing particles larger than 200 nm after administration (Moghimi et al., 2001) The liver and spleen are the primary sites of nanoparticles 150–300 nm in size (Gaumet et al., 2008), while colloids 200–400 nm in size are rapidly cleared by the liver (Douglas et al., 1987). Nanoparticles having a diameter of less than 200 nm may take advantage of the EPR effect for improved drug accumulation in tumours since tumour blood arteries have fenestrations ranging from 0.2 to 1.2 μm. When constructing a nanomedicine, the distribution of particle sizes must also be taken into consideration. While nanoparticles may theoretically have a wide range of sizes, if most of the particles are less than 200 nm in diameter, they may not have the full “benefits” associated with nanomedicine. The size and distribution of nanoparticles must be carefully managed throughout small-scale preparation and especially during larger-scale manufacture.
For nanoparticle behaviour and interaction with proteins and cells, surface characteristics of nanoparticles are crucial (Moghimi, 2003). For nanoparticles to remain stable and opsonize, several surface properties (such as charge, hydrophobicity, functional groups) must be present (Moghimi et al., 2001; Moghimi and Szebeni, 2003). These nanoparticles are coated with a variety of blood components in the process of opsonization; this triggers the complement pathway so that macrophages may remove them from circulation and prevent them from spreading (Gigli and Nelson, 1968). The biological synthesis of nanoparticles is a simple, low-risk method for producing them. The use of fungus to synthesise nanoparticles has been shown in a variety of domains. Smart medication delivery systems that deliver pharmaceuticals to the exact location where they are needed will aid in the early detection of sickness. Developing a biosensor and detection system that can protect crops from insects and diseases is a worthwhile endeavor.
In a nutshell, work on myconanotechnology is ongoing. The use of nanoparticles will continue to expand, but we must first investigate their toxicity, environmental buildup, and impact on human and animal health. Nanoparticles may also be utilized to cure a variety of serious ailments and open up new avenues in the biomedical area in the future, according to recent research. AgNPs might be used to power gadgets, which could help alleviate the current energy shortage. Nanoparticles have been extensively studied in vitro, but there is a dearth of data on their effects in vivo, as shown by the literature. There is still a lot of work that needs to be done in the subject of nanomaterials in order to make significant advancements across a variety of industries. However, despite the many advantages of using fungal-mediated metal nanoparticles, there are still a number of issues that must be addressed in order for this technology to become a reality on the market today.
Controlling nanostructure dispersity, which strongly influences electrical and optical characteristics, and isolating and purifying plural form are major issues in microbial nanobiosynthesis. The size distribution of a nanoparticle population is a critical feature that affects the particle’s behaviour in fluids. Methods including freeze-thawing, osmotic stress, and centrifugation may modify nanoparticle structures and cause aggregation and precipitation. Using appropriate methodologies might enhance microbial nanoparticle biosynthesis. The application of genetic engineering techniques and suitable microbial strains might assist overcome the disadvantages of slower production rate and polidispersity (relative to chemical-based nanomanufacturing) (Jeevanandam et al., 2018). A capping layer of biomolecules adsorbed on the surface of microbial biosynthetic nanoparticles acts as a stabilising agent and biological active layer (Ramya et al., 2015). The ability to identify capping agents (primarily peptides like glutathione, metallothioneins, membrane associated proteins, etc.) and purify nanoparticles (Jeevanandam et al., 2018) is critical for future in vivo medicinal applications.
Conclusion
Many efforts have developed novel green synthesis processes during the previous several decades. Living creatures have a lot of potential for making nanomaterials that may be used in various sectors, including biomedicine. To manufacture nano-objects of the appropriate size and form, organisms ranging from primary bacteria to very sophisticated eukaryotes may all be employed. Prokaryotes are the most basic biomasses, making them easier to genetically alter to create more desirable synthesis chemicals. However, bacterial culture and large-scale manufacturing remain difficult compared to alternative methods. Bacteria were investigated as the first nano-factories to manufacture noble metal nanoparticles as a first step. The poor synthesis rate and restricted number of size and form distributions accessible, on the other hand, steered the research towards fungi and algae.
Fungi may be used to produce green nanoparticles on a massive scale. They’re simple to work with in downstream processing, and they release a lot of enzymes that help with the reduction. They also have metal filament tolerance, a high binding capacity, and intracellular uptake. However, eukaryotes have a considerably more difficult time genetically manipulating individual enzymes to increase production.
Plant extracts have lately been the subject of several studies, and the number of research papers published in this area has exploded in the past 2 years due to their widespread availability, environmental friendliness, and cost-effectiveness. This green chemistry strategy of utilizing living entities is in stark contrast with traditional chemical and physical processes that commonly involve hazardous compounds that have the potential to cause environmental toxicity, cytotoxicity, and carcinogenicity. Whilst biological entities have been extensively used to produce nanoparticles, the use of plant sources offers a straightforward, clean, non-toxic, and robust procedure that does not need any special culture preparation or isolation techniques that are normally required for bacteria and fungi-based techniques. In particular, the utilization of plant extracts for manufacturing nanoparticles is affordable, readily scaled up, and environment-friendly. Plant extracts have the ability to generate nanoparticles with a specified size, shape and content. Plant produced nanoparticles have the potential to be extensively employed in current medical processes utilizing nanoparticles such as fluorescent labelling in immunoassays, targeted administration of therapeutic medications, tumour death by heating (hyperthermia), and as antibacterial agents in bandages. On another front, plant produced nanoparticles have the potential to be exploited for the delivery of anti-microbiological chemicals for use as insecticides for agricultural crops. Moreover, agricultural crop wastes and food industry wastes are also ideal prospects for delivering supplies of plant-based bio-chemicals with the ability to synthesis metallic nanoparticles and related products. Despite the environmental advantages of using green chemistry based biological synthesis over traditional methods as discussed in this article there are some unresolved issues such as particle size and shape consistency, reproducibility of the synthesis process, and understanding of the mechanisms involved in producing metallic nanoparticles via biological entities. Therefore, there is a need for further research to analyze and comprehend the real biological synthesis dependent processes. This is a vastly untapped subject that needs much more research investment to properly leverage the green manufacturing of metallic nanoparticles through living entities.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abalkhil, T. A., Alharbi, S. A., Salmen, S. H., and Wainwright, M. (2017). Bactericidal Activity of Biosynthesized Silver Nanoparticles against Human Pathogenic Bacteria. Biotechnol. Biotechnological Equipment 31, 411–417. doi:10.1080/13102818.2016.1267594
Abbaszadegan, A., Ghahramani, Y., Gholami, A., Hemmateenejad, B., Dorostkar, S., Nabavizadeh, M., et al. (2015). The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 1–8. doi:10.1155/2015/720654
Abboud, Y., Saffaj, T., Chagraoui, A., El Bouari, A., Brouzi, K., Tanane, O., et al. (2014). Biosynthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanoparticles (CONPs) Produced Using Brown Alga Extract (Bifurcaria Bifurcata). Appl. Nanosci. 4, 571–576. doi:10.1007/s13204-013-0233-x
Abdul-Hadi, S. Y., Owaid, M. N., Rabeea, M. A., Abdul Aziz, A., and Jameel, M. S. (2020). Rapid Mycosynthesis and Characterization of Phenols-Capped crystal Gold Nanoparticles from Ganoderma Applanatum, Ganodermataceae. Biocatal. Agric. Biotechnol. 27, 101683. doi:10.1016/j.bcab.2020.101683
Aboelfetoh, E. F., El-Shenody, R. A., and Ghobara, M. M. (2017). Eco-friendly Synthesis of Silver Nanoparticles Using green Algae (Caulerpa Serrulata): Reaction Optimization, Catalytic and Antibacterial Activities. Environ. Monit. Assess. 189, 349. doi:10.1007/s10661-017-6033-0
Abu Hajleh, M. N., Abu‐Huwaij, R., AL‐Samydai, A., Al‐Halaseh, L. K., and Al‐Dujaili, E. A. (2021). The Revolution of Cosmeceuticals Delivery by Using Nanotechnology: A Narrative Review of Advantages and Side Effects. J. Cosmet. Dermatol. 20, 3818–3828. doi:10.1111/JOCD.14441
Acharya, D., Satapathy, S., Somu, P., Parida, U. K., and Mishra, G. (2020). Apoptotic Effect and Anticancer Activity of Biosynthesized Silver Nanoparticles from Marine Algae Chaetomorpha Linum Extract against Human Colon Cancer Cell HCT-116. Biol. Trace Elem. Res. 199, 1812–1822. doi:10.1007/S12011-020-02304-7
Ahamed, M., Majeed Khan, M. A., Siddiqui, M. K. J., Alsalhi, M. S., and Alrokayan, S. A. (2011). Green Synthesis, Characterization and Evaluation of Biocompatibility of Silver Nanoparticles. Physica E: Low-dimensional Syst. Nanostructures 43, 1266–1271. doi:10.1016/j.physe.2011.02.014
Ahmad, A., Mukherjee, P., Senapati, S., Mandal, D., Khan, M. I., Kumar, R., et al. (2003). Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Oxysporum. Colloids Surf. B Biointerfaces 28. doi:10.1016/S0927-7765(02)00174-1
Ajarem, J. S., Maodaa, S. N., Allam, A. A., Taher, M. M., and Khalaf, M. (2021). Benign Synthesis of Cobalt Oxide Nanoparticles Containing Red Algae Extract: Antioxidant, Antimicrobial, Anticancer, and Anticoagulant Activity. J. Clust. Sci. 33, 717–728. doi:10.1007/s10876-021-02004-9
Akter, S., and Huq, M. A. (2020). Biologically Rapid Synthesis of Silver Nanoparticles by Sphingobium Sp. MAH-11T and Their Antibacterial Activity and Mechanisms Investigation against Drug-Resistant Pathogenic Microbes. Artif. Cell Nanomedicine, Biotechnol. 48, 672–682. doi:10.1080/21691401.2020.1730390
Akter, S., Lee, S.-Y., Siddiqi, M. Z., Balusamy, S. R., Ashrafudoulla, M., Rupa, E. J., et al. (2020). Ecofriendly Synthesis of Silver Nanoparticles by Terrabacter Humi Sp. Nov. And Their Antibacterial Application against Antibiotic-Resistant Pathogens. Ijms 21, 9746. doi:10.3390/ijms21249746
Al-Ansari, M. M., Dhasarathan, P., Ranjitsingh, A. J. A., and Al-Humaid, L. A. (2020). Ganoderma Lucidum Inspired Silver Nanoparticles and its Biomedical Applications with Special Reference to Drug Resistant Escherichia coli Isolates from CAUTI. Saudi J. Biol. Sci. 27, 2993–3002. doi:10.1016/j.sjbs.2020.09.008
Al-Bahrani, R., Raman, J., Lakshmanan, H., Hassan, A. A., and Sabaratnam, V. (2017). Green Synthesis of Silver Nanoparticles Using Tree Oyster Mushroom Pleurotus Ostreatus and its Inhibitory Activity against Pathogenic Bacteria. Mater. Lett. 186, 21–25. doi:10.1016/j.matlet.2016.09.069
Al-Otibi, F., Alfuzan, S. A., Alharbi, R. I., Al-Askar, A. A., AL-Otaibi, R. M., Al Subaie, H. F., et al. (2022). Comparative Study of Antifungal Activity of Two Preparations of green Silver Nanoparticles from Portulaca Oleracea Extract. Saudi J. Biol. Sci. 29, 2772–2781. doi:10.1016/J.SJBS.2021.12.056
Al-Radadi, N. S. (2019). Green Synthesis of Platinum Nanoparticles Using Saudi's Dates Extract and Their Usage on the Cancer Cell Treatment. Arabian J. Chem. 12, 330–349. doi:10.1016/j.arabjc.2018.05.008
Ali, M. Y. S., Anuradha, V., Abishek, R., Yogananth, N., and Sheeba, H. (2017). In Vitro Anticancer Activity of Green Synthesis Ruthenium Nanoparticle from Dictyota Dichotoma Marine Algae. Nanoworld J. 03, 1. doi:10.17756/nwj.2017-049
Amooaghaie, R., Saeri, M. R., and Azizi, M. (2015). Synthesis, Characterization and Biocompatibility of Silver Nanoparticles Synthesized from Nigella Sativa Leaf Extract in Comparison with Chemical Silver Nanoparticles. Ecotoxicology Environ. Saf. 120, 400–408. doi:10.1016/j.ecoenv.2015.06.025
Ansari, S., Bari, A., Ullah, R., Mathanmohun, M., Veeraraghavan, V. P., and Sun, Z. (2019). Gold Nanoparticles Synthesized with Smilax Glabra Rhizome Modulates the Anti-obesity Parameters in High-Fat Diet and Streptozotocin Induced Obese Diabetes Rat Model. J. Photochem. Photobiol. B: Biol. 201, 111643. doi:10.1016/j.jphotobiol.2019.111643
Armendariz, V., Herrera, I., Peralta-Videa, J. R., Jose-Yacaman, M., Troiani, H., Santiago, P., et al. (2004). Size Controlled Gold Nanoparticle Formation by Avena Sativa Biomass: Use of Plants in Nanobiotechnology. J. Nanoparticle Res. 6, 377–382. doi:10.1007/s11051-004-0741-4
Arockiya Aarthi Rajathi, F., Parthiban, C., Ganesh Kumar, V., and Anantharaman, P. (2012). Biosynthesis of Antibacterial Gold Nanoparticles Using Brown Alga, Stoechospermum Marginatum (Kützing). Spectrochimica Acta A: Mol. Biomol. Spectrosc. 99, 166–173. doi:10.1016/j.saa.2012.08.081
Ashokkumar, T., and Vijayaraghavan, K. (2016). Brown Seaweed-Mediated Biosynthesis of Gold Nanoparticles. J. Environ. Biotechnol. Res. 2, 1.
Aygün, A., Özdemir, S., Gülcan, M., Cellat, K., and Şen, F. (2020). Synthesis and Characterization of Reishi Mushroom-Mediated green Synthesis of Silver Nanoparticles for the Biochemical Applications. J. Pharm. Biomed. Anal. 178, 112970. doi:10.1016/j.jpba.2019.112970
Baker, S., Harini, B. P., Rakshith, D., and Satish, S. (2013). Marine Microbes: Invisible Nanofactories. J. Pharm. Res. 6, 383–388. doi:10.1016/j.jopr.2013.03.001
Bankar, A., Joshi, B., Kumar, A. R., and Zinjarde, S. (2010). Banana Peel Extract Mediated Novel Route for the Synthesis of Silver Nanoparticles. Colloids Surf. A: Physicochemical Eng. Aspects 368, 58–63. doi:10.1016/j.colsurfa.2010.07.024
Baruah, D., Goswami, M., Yadav, R. N. S., Yadav, A., and Das, A. M. (2018). Biogenic Synthesis of Gold Nanoparticles and Their Application in Photocatalytic Degradation of Toxic Dyes. J. Photochem. Photobiol. B: Biol. 186, 51–58. doi:10.1016/j.jphotobiol.2018.07.002
Belliveau, B. H., Starodub, M. E., Cotter, C., and Trevors, J. T. (1987). Metal Resistance and Accumulation in Bacteria. Biotechnol. Adv. 5, 101–127. doi:10.1016/0734-9750(87)90006-1
Beveridge, T. J., and Murray, R. G. (1980). Sites of Metal Deposition in the Cell wall of Bacillus Subtilis. J. Bacteriol. 141, 876–887. doi:10.1128/jb.141.2.876-887.1980
Bhainsa, K. C., and D'Souza, S. F. (2006). Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Aspergillus fumigatus. Colloids Surf. B: Biointerfaces 47, 160–164. doi:10.1016/j.colsurfb.2005.11.026
Bharadwaj, K. K., Rabha, B., Pati, S., Choudhury, B. K., Sarkar, T., Gogoi, S. K., et al. (2021). Green Synthesis of Silver Nanoparticles Using diospyros Malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-nitrophenol (4-np). Nanomaterials 11, 1999. doi:10.3390/nano11081999
Bhardwaj, A. K., Shukla, A., Maurya, S., Singh, S. C., Uttam, K. N., Sundaram, S., et al. (2018). Direct Sunlight Enabled Photo-Biochemical Synthesis of Silver Nanoparticles and Their Bactericidal Efficacy: Photon Energy as Key for Size and Distribution Control. J. Photochem. Photobiol. B: Biol. 188, 42–49. doi:10.1016/j.jphotobiol.2018.08.019
Bhat, R., Deshpande, R., Ganachari, S. V., Huh, D. S., and Venkataraman, A. (2011). Photo-Irradiated Biosynthesis of Silver Nanoparticles Using Edible MushroomPleurotus Floridaand Their Antibacterial Activity Studies. Bioinorganic Chem. Appl. 2011, 1–7. doi:10.1155/2011/650979
Bhattacharya, D., and Gupta, R. K. (2005). Nanotechnology and Potential of Microorganisms. Crit. Rev. Biotechnol. 25, 199–204. doi:10.1080/07388550500361994
Bhattacharya, T., Soares, G. A. B. E., Chopra, H., Rahman, M. M., Hasan, Z., Swain, S. S., et al. (2022). Applications of Phyto-Nanotechnology for the Treatment of Neurodegenerative Disorders. Materials 15, 804. doi:10.3390/MA15030804
Bhuyan, B., Paul, A., Paul, B., Dhar, S. S., and Dutta, P. (2017). Paederia Foetida Linn . Promoted Biogenic Gold and Silver Nanoparticles: Synthesis, Characterization, Photocatalytic and In Vitro Efficacy against Clinically Isolated Pathogens. J. Photochem. Photobiol. B: Biol. 173, 210–215. doi:10.1016/j.jphotobiol.2017.05.040
Bishoyi, A. K., Sahoo, C. R., Sahoo, A. P., and Padhy, R. N. (2020). Bio-synthesis of Silver Nanoparticles with the Brackish Water Blue-green Alga Oscillatoria Princeps and Antibacterial Assessment. Appl. Nanosci. 11 (11), 389–398. doi:10.1007/S13204-020-01593-7
Bogireddy, N. K. R., Hoskote Anand, K. K., and Mandal, B. K. (2015). Gold Nanoparticles - Synthesis by Sterculia Acuminata Extract and its Catalytic Efficiency in Alleviating Different Organic Dyes. J. Mol. Liquids 211, 868–875. doi:10.1016/j.molliq.2015.07.027
Bondarenko, O., Ivask, A., Käkinen, A., Kurvet, I., and Kahru, A. (2013). Particle-Cell Contact Enhances Antibacterial Activity of Silver Nanoparticles. PLoS One 8, e64060. doi:10.1371/journal.pone.0064060
Boomi, P., Ganesan, R. M., Poorani, G., Gurumallesh Prabu, H., Ravikumar, S., and Jeyakanthan, J. (2019). Biological Synergy of Greener Gold Nanoparticles by Using Coleus Aromaticus Leaf Extract. Mater. Sci. Eng. C 99, 202–210. doi:10.1016/j.msec.2019.01.105
Bouafia, A., Laouini, S. E., Tedjani, M. L., Ali, G. A., and Barhoum, A. (2021). Green Biosynthesis and Physicochemical Characterization of Fe3O4 Nanoparticles Using Punica Granatum L. Fruit Peel Extract for Optoelectronic Applications. Textile Res. J. 1, 004051752110066. doi:10.1177/00405175211006671
Bowman, J. P., McCammon, S. A., Nichols, D. S., Skerratt, J. H., Rea, S. M., Nichols, P. D., et al. (1997). Shewanella Gelidimarina Sp. Nov. And Shewanella Frigidimarina Sp. nov., Novel Antarctic Species with the Ability to Produce Eicosapentaenoic Acid (20:5 3) and Grow Anaerobically by Dissimilatory Fe(III) Reduction. Int. J. Syst. Bacteriol. 47, 1040–1047. doi:10.1099/00207713-47-4-1040
Caccavo, F., Blakemore, R. P., and Lovley, D. R. (1992). A Hydrogen-Oxidizing, Fe(III)-reducing Microorganism from the Great Bay Estuary, New Hampshire. Appl. Environ. Microbiol. 58, 3211–3216. doi:10.1128/aem.58.10.3211-3216.1992
Castro, L., Blázquez, M. L., Muñoz, J. A., González, F., and Ballester, A. (2013). Biological Synthesis of Metallic Nanoparticles Using Algae. IET nanobiotechnol. 7, 109–116. doi:10.1049/iet-nbt.2012.0041
Cavalu, S., Fritea, L., Brocks, M., Barbaro, K., Murvai, G., Costea, T. O., et al. (2020). Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties. Materials 13, 2077. doi:10.3390/ma13092077
Chakravarty, A., Ahmad, I., Singh, P., Ud Din Sheikh, M., Aalam, G., Sagadevan, S., et al. (2022). Green Synthesis of Silver Nanoparticles Using Fruits Extracts of Syzygium Cumini and Their Bioactivity. Chem. Phys. Lett. 1, 139493. doi:10.1016/J.CPLETT.2022.139493
Chaturvedi, V. K., Yadav, N., Rai, N. K., Ellah, N. H. A., Bohara, R. A., Rehan, I. F., et al. (2020). Pleurotus Sajor-Caju-Mediated Synthesis of Silver and Gold Nanoparticles Active against Colon Cancer Cell Lines: A New Era of Herbonanoceutics. Molecules 25, 3091. doi:10.3390/molecules25133091
Chellapandian, C., Ramkumar, B., Puja, P., Shanmuganathan, R., Pugazhendhi, A., and Kumar, P. (2019). Gold Nanoparticles Using Red Seaweed Gracilaria Verrucosa: Green Synthesis, Characterization and Biocompatibility Studies. Process Biochem. 80, 58–63. doi:10.1016/j.procbio.2019.02.009
Chen, X., Zhao, X., Gao, Y., Yin, J., Bai, M., and Wang, F. (2018). Green Synthesis of Gold Nanoparticles Using Carrageenan Oligosaccharide and Their In Vitro Antitumor Activity. Mar. Drugs 16, 277. doi:10.3390/md16080277
Chopra, H., Bibi, S., Islam, F., Ahmad, S. U., Olawale, O. A., Alhumaydhi, F. A., et al. (2022). Emerging Trends in the Delivery of Resveratrol by Nanostructures: Applications of Nanotechnology in Life Sciences. ene 4, 13. doi:10.1155/2022/3083728
Chopra, H., Dey, P. S., Das, D., Bhattacharya, T., Shah, M., Mubin, S., et al. (2021a). Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 26, 4998. doi:10.3390/molecules26164998
Chopra, H., Gandhi, S., Gautam, R. K., and Kamal, M. A. (2022b). Bacterial Nanocellulose Based Wound Dressings: Current and Future Prospects. Cpd 28, 570–580. doi:10.2174/1381612827666211021162828
Chopra, H., Mishra, A. K., Baig, A. A., Mohanta, T. K., Mohanta, Y. K., and Baek, K.-H. (2021c). Narrative Review: Bioactive Potential of Various Mushrooms as the Treasure of Versatile Therapeutic Natural Product. JoF 7, 728. doi:10.3390/JOF7090728
Christensen, L., Vivekanandhan, S., Misra, M., and Kumar Mohanty, A. (2011). Biosynthesis of Silver Nanoparticles Using Murraya Koenigii (Curry Leaf): An Investigation on the Effect of Broth Concentration in Reduction Mechanism and Particle Size. Adv. Mater. Lett. 2, 429–434. doi:10.5185/amlett.2011.4256
Coseri, S., Spatareanu, A., Sacarescu, L., Rimbu, C., Suteu, D., Spirk, S., et al. (2015). Green Synthesis of the Silver Nanoparticles Mediated by Pullulan and 6-carboxypullulan. Carbohydr. Polym. 116, 9–17. doi:10.1016/j.carbpol.2014.06.008
Cuong, H. N., Pansambal, S., Ghotekar, S., Oza, R., Thanh Hai, N. T., Viet, N. M., et al. (2022). New Frontiers in the Plant Extract Mediated Biosynthesis of Copper Oxide (CuO) Nanoparticles and Their Potential Applications: A Review. Environ. Res. 203, 111858. doi:10.1016/j.envres.2021.111858
Dahoumane, S. A., Yéprémian, C., Djédiat, C., Couté, A., Fiévet, F., Coradin, T., et al. (2016). Improvement of Kinetics, Yield, and Colloidal Stability of Biogenic Gold Nanoparticles Using Living Cells of Euglena Gracilis Microalga. J. Nanopart Res. 18, 1. doi:10.1007/s11051-016-3378-1
Das, B., Dash, S. K., Mandal, D., Ghosh, T., Chattopadhyay, S., Tripathy, S., et al. (2017a). Green Synthesized Silver Nanoparticles Destroy Multidrug Resistant Bacteria via Reactive Oxygen Species Mediated Membrane Damage. Arabian J. Chem. 10, 862–876. doi:10.1016/j.arabjc.2015.08.008
Das, R. K., Pachapur, V. L., Lonappan, L., Naghdi, M., Pulicharla, R., Maiti, S., et al. (2017b). Biological Synthesis of Metallic Nanoparticles: Plants, Animals and Microbial Aspects. Nanotechnol. Environ. Eng. 2, 1. doi:10.1007/s41204-017-0029-4
Dehghan, Z., Ranjbar, M., Govahi, M., and Khakdan, F. (2022). Green Synthesis of Ag/Fe3O4 Nanocomposite Utilizing Eryngium Planum L. Leaf Extract and its Potential Applications in Medicine. J. Drug Deliv. Sci. Technology 67, 102941. doi:10.1016/j.jddst.2021.102941
Devra, V. (2022). “Plant and Agri-Waste-Mediated Synthesis of Metal Nanoparticles,” in Agri-Waste and Microbes for Production of Sustainable Nanomaterials, 47–77. doi:10.1016/b978-0-12-823575-1.00030-5
Dhingra, R., Naidu, S., Upreti, G., and Sawhney, R. (2010). Sustainable Nanotechnology: Through green Methods and Life-Cycle Thinking. Sustainability 2, 3323–3338. doi:10.3390/su2103323
Dhivya, R., Ranjani, J., Bowen, P. K., Rajendhran, J., Mayandi, J., and Annaraj, J. (2017). Biocompatible Curcumin Loaded PMMA-PEG/ZnO Nanocomposite Induce Apoptosis and Cytotoxicity in Human Gastric Cancer Cells. Mater. Sci. Eng. C 80, 59–68. doi:10.1016/j.msec.2017.05.128
Douglas, S. J., Davis, S. S., and Illum, L. (1987). Nanoparticles in Drug Delivery. Crit. Rev. Ther. Drug Carrier Syst. 3, 233–261. Available at: http://europepmc.org/abstract/MED/3549008.
Du, L., Jiang, H., Liu, X., and Wang, E. (2007). Biosynthesis of Gold Nanoparticles Assisted by Escherichia coli DH5α and its Application on Direct Electrochemistry of Hemoglobin. Electrochemistry Commun. 9, 1165–1170. doi:10.1016/j.elecom.2007.01.007
Duan, B., Wang, M., Li, Y., Jiang, S., Liu, Y., and Huang, Z. (2019). Dual-emitting Zein-Protected Gold Nanoclusters for Ratiometric Fluorescence Detection of Hg2+/Ag+ Ions in Both Aqueous Solution and Self-Assembled Protein Film. New J. Chem. 43, 14678–14683. doi:10.1039/c9nj03524a
Duan, H., Wang, D., and Li, Y. (2015). Green Chemistry for Nanoparticle Synthesis. Chem. Soc. Rev. 44, 5778–5792. doi:10.1039/c4cs00363b
Duraisamy, S., Kasi, M., Balakrishnan, S., and Al-Sohaibani, S. (2014). Biosynthesis of Silver Nanoparticles Using Acacia Leucophloea Extract and Their Antibacterial Activity. Ijn 9, 2431. doi:10.2147/IJN.S61779
Durán, N., Marcato, P. D., Alves, O. L., De Souza, G. I., and Esposito, E. (2005). Mechanistic Aspects of Biosynthesis of Silver Nanoparticles by Several Fusarium Oxysporum Strains. J. Nanobiotechnol 3, 8. doi:10.1186/1477-3155-3-8
Edison, T. N. J. I., Atchudan, R., Kamal, C., and Lee, Y. R. (2016). Caulerpa Racemosa: a marine green Alga for Eco-Friendly Synthesis of Silver Nanoparticles and its Catalytic Degradation of Methylene Blue. Bioproc. Biosyst. Eng. 39, 1401–1408. doi:10.1007/s00449-016-1616-7
El-Batal, A. I., Elkenawy, N. M., Yassin, A. S., and Amin, M. A. (2015). Laccase Production by Pleurotus Ostreatus and its Application in Synthesis of Gold Nanoparticles. Biotechnol. Rep. 5, 31–39. doi:10.1016/j.btre.2014.11.001
El-Kassas, H. Y., and El-Sheekh, M. M. (2014). Cytotoxic Activity of Biosynthesized Gold Nanoparticles with an Extract of the Red Seaweed Corallina Officinalis on the MCF-7 Human Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 15, 4311–4317. doi:10.7314/APJCP.2014.15.10.4311
El-Kassas, H. Y., and Ghobrial, M. G. (2017). Biosynthesis of Metal Nanoparticles Using Three marine Plant Species: Anti-algal Efficiencies against "Oscillatoria Simplicissima". Environ. Sci. Pollut. Res. 24, 7837–7849. doi:10.1007/s11356-017-8362-5
El-Rafie, H. M., El-Rafie, M. H., and Zahran, M. K. (2013). Green Synthesis of Silver Nanoparticles Using Polysaccharides Extracted from marine Macro Algae. Carbohydr. Polym. 96, 403–410. doi:10.1016/j.carbpol.2013.03.071
Elahi, N., Kamali, M., and Baghersad, M. H. (2018). Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 184, 537–556. doi:10.1016/j.talanta.2018.02.088
Elango, G., and Roopan, S. M. (2015). Green Synthesis, Spectroscopic Investigation and Photocatalytic Activity of lead Nanoparticles. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 139, 367–373. doi:10.1016/j.saa.2014.12.066
Elbeshehy, E. K. F., Elazzazy, A. M., and Aggelis, G. (2015). Silver Nanoparticles Synthesis Mediated by New Isolates of Bacillus spp., Nanoparticle Characterization and Their Activity against Bean Yellow Mosaic Virus and Human Pathogens. Front. Microbiol. 6, 453. doi:10.3389/fmicb.2015.00453
Elumalai, K., Velmurugan, S., Ravi, S., Kathiravan, V., and Ashokkumar, S. (2015). Bio-fabrication of Zinc Oxide Nanoparticles Using Leaf Extract of Curry Leaf (Murraya Koenigii) and its Antimicrobial Activities. Mater. Sci. Semiconductor Process. 34, 365–372. doi:10.1016/j.mssp.2015.01.048
Eskandari-Nojedehi, M., Jafarizadeh-Malmiri, H., and Rahbar-Shahrouzi, J. (2018). Hydrothermal green Synthesis of Gold Nanoparticles Using Mushroom (Agaricus Bisporus) Extract: Physico-Chemical Characteristics and Antifungal Activity Studies. Green. Process. Synth. 7, 38–47. doi:10.1515/gps-2017-0004
Ettadili, F. E., Aghris, S., Laghrib, F., Farahi, A., Saqrane, S., Bakasse, M., et al. (2022). Recent Advances in the Nanoparticles Synthesis Using Plant Extract: Applications and Future Recommendations. J. Mol. Struct. 1248, 131538. doi:10.1016/j.molstruc.2021.131538
Fernández, J. G., Fernández-Baldo, M. A., Berni, E., Camí, G., Durán, N., Raba, J., et al. (2016). Production of Silver Nanoparticles Using Yeasts and Evaluation of Their Antifungal Activity against Phytopathogenic Fungi. Process Biochem. 51, 1306–1313. doi:10.1016/j.procbio.2016.05.021
Fesharaki, P. J., Nazari, P., Shakibaie, M., Rezaie, S., Banoee, M., Abdollahi, M., et al. (2010). Biosynthesis of Selenium Nanoparticles Using Klebsiella pneumoniae and Their Recovery by a Simple Sterilization Process. Braz. J. Microbiol. 41, 461–466. doi:10.1590/S1517-83822010000200028
Fu, M., Li, Q., Sun, D., Lu, Y., He, N., Deng, X., et al. (2006). Rapid Preparation Process of Silver Nanoparticles by Bioreduction and Their Characterizations. Chin. J. Chem. Eng. 14, 114–117. doi:10.1016/S1004-9541(06)60046-3
Gade, A., Ingle, A., Whiteley, C., and Rai, M. (2010). Mycogenic Metal Nanoparticles: Progress and Applications. Biotechnol. Lett. 32, 593–600. doi:10.1007/s10529-009-0197-9
Garibo, D., Borbón-Nuñez, H. A., de León, J. N. D., García Mendoza, E., Estrada, I., Toledano-Magaña, Y., et al. (2020). Green Synthesis of Silver Nanoparticles Using Lysiloma Acapulcensis Exhibit High-Antimicrobial Activity. Sci. Rep. 10, 12805. doi:10.1038/s41598-020-69606-7
Gaumet, M., Vargas, A., Gurny, R., and Delie, F. (2008). Nanoparticles for Drug Delivery: The Need for Precision in Reporting Particle Size Parameters. Eur. J. Pharmaceutics Biopharmaceutics 69, 1–9. doi:10.1016/j.ejpb.2007.08.001
Gavhane, A. J., Padmanabhan, P., Kamble, S. P., and Jangle, S. N. (2012). Synthesis of Silver Nanoparticles Using Extract of Neem Leaf and Triphala and Evaluation of Their Antimicrobial Activities. Int. J. Pharma Bio Sci. 3, P88–P100.
Ghidan, A. Y., Al-Antary, T. M., and Awwad, A. M. (2016). Green Synthesis of Copper Oxide Nanoparticles Using Punica Granatum Peels Extract: Effect on green Peach Aphid. Environ. Nanotechnology, Monit. Management 6, 95–98. doi:10.1016/j.enmm.2016.08.002
Gigli, I., and Nelson, R. A. (1968). Complement Dependent Immune Phagocytosis. I. Requirements for C'1, C'4, C'2, C'3. Exp. Cel Res. 51, 45–67. doi:10.1016/0014-4827(68)90158-4
Gnanadesigan, M., Anand, M., Ravikumar, S., Maruthupandy, M., Syed Ali, M., Vijayakumar, V., et al. (2012). Antibacterial Potential of Biosynthesised Silver Nanoparticles Using Avicennia marina Mangrove Plant. Appl. Nanosci. 2, 143–147. doi:10.1007/s13204-011-0048-6
Gogoi, N., Babu, P. J., Mahanta, C., and Bora, U. (2015). Green Synthesis and Characterization of Silver Nanoparticles Using Alcoholic Flower Extract of Nyctanthes Arbortristis and In Vitro Investigation of Their Antibacterial and Cytotoxic Activities. Mater. Sci. Eng. C 46, 463–469. doi:10.1016/j.msec.2014.10.069
Gomaa, E. Z. (2017). Silver Nanoparticles as an Antimicrobial Agent: A Case Study on Staphylococcus aureus and escherichia Coli as Models for Gram-Positive and Gram-Negative Bacteria. J. Gen. Appl. Microbiol. 63, 36–43. doi:10.2323/jgam.2016.07.004
Gomathi, M., Rajkumar, P. V., Prakasam, A., and Ravichandran, K. (2017). Green Synthesis of Silver Nanoparticles Using Datura Stramonium Leaf Extract and Assessment of Their Antibacterial Activity. Resource-Efficient Tech. 3, 280–284. doi:10.1016/j.reffit.2016.12.005
Gopal, J., Hasan, N., Manikandan, M., and Wu, H.-F. (2013). Bacterial Toxicity/compatibility of Platinum Nanospheres, Nanocuboids and Nanoflowers. Sci. Rep. 3, 1. doi:10.1038/srep01260
Gopinath, K. F. P. A. (2015). Eco-Friendly Synthesis and Characterization of Silver Nanoparticles Using Marine Macroalga Padina Tetrastromatica. Int. J. Sci. Res. 4, 1.
Gopinath, K., Kumaraguru, S., Bhakyaraj, K., Mohan, S., Venkatesh, K. S., Esakkirajan, M., et al. (2016). Green Synthesis of Silver, Gold and Silver/gold Bimetallic Nanoparticles Using the Gloriosa Superba Leaf Extract and Their Antibacterial and Antibiofilm Activities. Microb. Pathogenesis 101, 1–11. doi:10.1016/j.micpath.2016.10.011
Govindaraju, K., Kiruthiga, V., Kumar, V. G., and Singaravelu, G. (2009). Extracellular Synthesis of Silver Nanoparticles by a marine Alga, Sargassum Wightii Grevilli and Their Antibacterial Effects. j nanosci nanotechnol 9, 5497–5501. doi:10.1166/jnn.2009.1199
Gowramma, B., Keerthi, U., Rafi, M., and Muralidhara Rao, D. (2015). Biogenic Silver Nanoparticles Production and Characterization from Native Stain of Corynebacterium Species and its Antimicrobial Activity. 3 Biotech. 35, 195–201. doi:10.1007/s13205-014-0210-4
Gunalan, S., Sivaraj, R., and Rajendran, V. (2012). Green Synthesized ZnO Nanoparticles against Bacterial and Fungal Pathogens. Prog. Nat. Sci. Mater. Int. 22, 693–700. doi:10.1016/j.pnsc.2012.11.015
Gurunathan, S., Kalishwaralal, K., Vaidyanathan, R., Venkataraman, D., Pandian, S. R. K., Muniyandi, J., et al. (2009). Biosynthesis, Purification and Characterization of Silver Nanoparticles Using Escherichia coli. Colloids Surf. B: Biointerfaces 74, 328–335. doi:10.1016/j.colsurfb.2009.07.048
Hameed, S., Khalil, A. T., Ali, M., Numan, M., Khamlich, S., Shinwari, Z. K., et al. (2019). Greener Synthesis of ZnO and Ag-ZnO Nanoparticles Using Silybum marianum for Diverse Biomedical Applications. Nanomedicine 14, 655–673. doi:10.2217/nnm-2018-0279
Hamelian, M., Hemmati, S., Varmira, K., and Veisi, H. (2018). Green Synthesis, Antibacterial, Antioxidant and Cytotoxic Effect of Gold Nanoparticles Using Pistacia Atlantica Extract. J. Taiwan Inst. Chem. Eng. 93, 21–30. doi:10.1016/j.jtice.2018.07.018
Hamouda, R. A., Abd El-Mongy, M., and Eid, K. F. (2019). Comparative Study between Two Red Algae for Biosynthesis Silver Nanoparticles Capping by SDS: Insights of Characterization and Antibacterial Activity. Microb. Pathogenesis 129, 224–232. doi:10.1016/j.micpath.2019.02.016
Hassan, S. E.-D., Fouda, A., Radwan, A. A., Salem, S. S., Barghoth, M. G., Awad, M. A., et al. (2019). Endophytic Actinomycetes Streptomyces Spp Mediated Biosynthesis of Copper Oxide Nanoparticles as a Promising Tool for Biotechnological Applications. J. Biol. Inorg. Chem. 24, 377–393. doi:10.1007/s00775-019-01654-5
Hassan, T., Huang, X., Zhou, C., Sikander, M., Khan, G., and Saeed, S. (2021). Nanoparticles in Cancer Treatment: A Narrative Review. Proc. Pakistan Acad. Sci. B. Life Environ. Sci. 58, 1–18. doi:10.53560/PPASB
He, S., Guo, Z., Zhang, Y., Zhang, S., Wang, J., and Gu, N. (2007). Biosynthesis of Gold Nanoparticles Using the Bacteria Rhodopseudomonas Capsulata. Mater. Lett. 61, 3984–3987. doi:10.1016/j.matlet.2007.01.018
He, S., Zhang, Y., Guo, Z., and Gu, N. (2008). Biological Synthesis of Gold Nanowires Using Extract of Rhodopseudomonas Capsulata. Biotechnol. Prog. 24, 476–480. doi:10.1021/bp0703174
Hosny, M., Fawzy, M., El-Badry, Y. A., Hussein, E. E., and Eltaweil, A. S. (2022). Plant-assisted Synthesis of Gold Nanoparticles for Photocatalytic, Anticancer, and Antioxidant Applications. J. Saudi Chem. Soc. 26, 101419. doi:10.1016/J.JSCS.2022.101419
Hulkoti, N. I., and Taranath, T. C. (2014). Biosynthesis of Nanoparticles Using Microbes-A Review. Colloids Surf. B: Biointerfaces 121, 474–483. doi:10.1016/j.colsurfb.2014.05.027
Hulla, J., Sahu, S., and Hayes, A. (2015). Nanotechnology. Hum. Exp. Toxicol. 34, 1318–1321. doi:10.1177/0960327115603588
Huq, M. A., and Akter, S. (2021a). Bacterial Mediated Rapid and Facile Synthesis of Silver Nanoparticles and Their Antimicrobial Efficacy against Pathogenic Microorganisms. Materials 14, 2615. doi:10.3390/MA14102615
Huq, M. A., and Akter, S. (2021b). Biosynthesis, Characterization and Antibacterial Application of Novel Silver Nanoparticles against Drug Resistant Pathogenic klebsiella Pneumoniae and salmonella Enteritidis. Molecules 26, 5996. doi:10.3390/molecules26195996
Huq, M. A., and Akter, S. (2021). Characterization and Genome Analysis of Arthrobacter Bangladeshi Sp. Nov., Applied for the green Synthesis of Silver Nanoparticles and Their Antibacterial Efficacy against Drug-Resistant Human Pathogens. Pharmaceutics 13, 1691. doi:10.3390/pharmaceutics13101691
Huq, M. A., and Akter, S. (2021c). Chitinophaga Chungangae Sp. nov., Isolated from a Korean Grape Garden and its Potential to Biosynthesize Ginsenoside Rg2. Arch. Microbiol. 203, 5483–5489. doi:10.1007/s00203-021-02533-x
Huq, M. A. (2020). Biogenic Silver Nanoparticles Synthesized by Lysinibacillus Xylanilyticus MAHUQ-40 to Control Antibiotic-Resistant Human Pathogens Vibrio Parahaemolyticus and Salmonella Typhimurium. Front. Bioeng. Biotechnol. 8, 597502. doi:10.3389/fbioe.2020.597502
Husseiny, M. I., El-Aziz, M. A., Badr, Y., and Mahmoud, M. A. (2007). Biosynthesis of Gold Nanoparticles Using Pseudomonas aeruginosa. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 67, 1003–1006. doi:10.1016/j.saa.2006.09.028
Husseiny, S. M., Salah, T. A., and Anter, H. A. (2015). Biosynthesis of Size Controlled Silver Nanoparticles by Fusarium Oxysporum, Their Antibacterial and Antitumor Activities. Beni-Suef Univ. J. Basic Appl. Sci. 4, 225–231. doi:10.1016/j.bjbas.2015.07.004
Huston, M., Debella, M., Dibella, M., and Gupta, A. (2021). Green Synthesis of Nanomaterials. Nanomaterials 11, 2130. doi:10.3390/nano11082130
Ijaz, F., Shahid, S., Khan, S. A., Ahmad, W., and Zaman, S. (2017). Green Synthesis of Copper Oxide Nanoparticles Using Abutilon Indicum Leaf Extract: Antimicrobial, Antioxidant and Photocatalytic Dye Degradation Activitie. Trop. J. Pharm. Res. 16, 743. doi:10.4314/tjpr.v16i4.2
Ilahi, N., Haleem, A., Iqbal, S., Fatima, N., Sajjad, W., Sideeq, A., et al. (2021). Biosynthesis of Silver Nanoparticles Using Endophytic Fusarium Oxysporum Strain NFW16 and Their In Vitro Antibacterial Potential. Microsc. Res Tech. 1, 1. doi:10.1002/JEMT.24018
Ishwarya, R., Vaseeharan, B., Subbaiah, S., Nazar, A. K., Govindarajan, M., Alharbi, N. S., et al. (2018). Sargassum Wightii -synthesized ZnO Nanoparticles - from Antibacterial and Insecticidal Activity to Immunostimulatory Effects on the green Tiger Shrimp Penaeus Semisulcatus. J. Photochem. Photobiol. B: Biol. 183, 318–330. doi:10.1016/j.jphotobiol.2018.04.049
Islam, F., Bibi, S., Meem, A. F. K., Islam, M. M., Rahaman, M. S., Bepary, S., et al. (2021). Natural Bioactive Molecules: An Alternative Approach to the Treatment and Control of COVID-19. Ijms 22, 12638. doi:10.3390/IJMS222312638
Jeevanandam, J., Barhoum, A., Chan, Y. S., Dufresne, A., and Danquah, M. K. (2018). Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 9, 1050–1074. doi:10.3762/BJNANO.9.98
Jevapatarakul, D., T-Thienprasert, J., Payungporn, S., Chavalit, T., Khamwut, A., and T-Thienprasert, N. P. (2020). Utilization of Cratoxylum Formosum Crude Extract for Synthesis of ZnO Nanosheets: Characterization, Biological Activities and Effects on Gene Expression of Nonmelanoma Skin Cancer Cell. Biomed. Pharmacother. 130, 110552. doi:10.1016/j.biopha.2020.110552
Jeyarani, S., Vinita, N. M., Puja, P., Senthamilselvi, S., Devan, U., Velangani, A. J., et al. (2020). Biomimetic Gold Nanoparticles for its Cytotoxicity and Biocompatibility Evidenced by Fluorescence-Based Assays in Cancer (MDA-MB-231) and Non-cancerous (HEK-293) Cells. J. Photochem. Photobiol. B: Biol. 202, 111715. doi:10.1016/j.jphotobiol.2019.111715
John, M. S., Nagoth, J. A., Ramasamy, K. P., Mancini, A., Giuli, G., Natalello, A., et al. (2020). Synthesis of Bioactive Silver Nanoparticles by a pseudomonas Strain Associated with the Antarctic Psychrophilic Protozoon Euplotes Focardii. Mar. Drugs 18, 38. doi:10.3390/md18010038
Jyoti, K., Baunthiyal, M., and Singh, A. (2016). Characterization of Silver Nanoparticles Synthesized Using Urtica Dioica Linn. Leaves and Their Synergistic Effects with Antibiotics. J. Radiat. Res. Appl. Sci. 9, 217–227. doi:10.1016/j.jrras.2015.10.002
Kalaiselvi, A., Roopan, S. M., Madhumitha, G., Ramalingam, C., and Elango, G. (2015). Synthesis and Characterization of Palladium Nanoparticles Using Catharanthus Roseus Leaf Extract and its Application in the Photo-Catalytic Degradation. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 135, 116–119. doi:10.1016/j.saa.2014.07.010
Kalishwaralal, K., BarathManiKanth, S., Pandian, S. R. K., Deepak, V., and Gurunathan, S. (2010). Silver Nanoparticles Impede the Biofilm Formation by Pseudomonas aeruginosa and Staphylococcus Epidermidis. Colloids Surf. B: Biointerfaces 79, 340–344. doi:10.1016/j.colsurfb.2010.04.014
Kanimozhi, S., Durga, R., Sabithasree, M., Kumar, A. V., Sofiavizhimalar, A., Kadam, A. A., et al. (2022). Biogenic Synthesis of Silver Nanoparticle Using Cissus Quadrangularis Extract and its Invitro Study. J. King Saud Univ. - Sci. 34, 101930. doi:10.1016/J.JKSUS.2022.101930
Kannan, N., Mukunthan, K. S., and Balaji, S. (2011). A Comparative Study of Morphology, Reactivity and Stability of Synthesized Silver Nanoparticles Using Bacillus Subtilis and Catharanthus Roseus (L.) G. Don. Colloids Surf. B: Biointerfaces 86, 378–383. doi:10.1016/j.colsurfb.2011.04.024
Kannan, R. R. R., Stirk, W. A., and Van Staden, J. (2013). Synthesis of Silver Nanoparticles Using the Seaweed Codium Capitatum P.C. Silva (Chlorophyceae). South Afr. J. Bot. 86, 1–4. doi:10.1016/j.sajb.2013.01.003
Karimi, S., and Samimi, T. (2019). Green and Simple Synthesis Route of Ag@AgCl Nanomaterial Using green marine Crude Extract and its Application for Sensitive and Selective Determination of Mercury. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 222, 117216. doi:10.1016/j.saa.2019.117216
Kashefi, K., Tor, J. M., Nevin, K. P., and Lovley, D. R. (2001). Reductive Precipitation of Gold by Dissimilatory Fe(III)-Reducing Bacteria and Archaea. Appl. Environ. Microbiol. 67, 3275–3279. doi:10.1128/AEM.67.7.3275-3279.2001
Kathiraven, T., Sundaramanickam, A., Shanmugam, N., and Balasubramanian, T. (2015). Green Synthesis of Silver Nanoparticles Using marine Algae Caulerpa Racemosa and Their Antibacterial Activity against Some Human Pathogens. Appl. Nanosci. 5, 499–504. doi:10.1007/s13204-014-0341-2
Kaur, H., Dolma, K., Kaur, N., Malhotra, A., Kumar, N., Dixit, P., et al. (2015). Marine Microbe as Nano-Factories for Copper Biomineralization. Biotechnol. Bioproc. E 20, 51–57. doi:10.1007/s12257-014-0432-7
Kaviya, S., Santhanalakshmi, J., Viswanathan, B., Muthumary, J., and Srinivasan, K. (2011). Biosynthesis of Silver Nanoparticles Using Citrus Sinensis Peel Extract and its Antibacterial Activity. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 79, 594–598. doi:10.1016/j.saa.2011.03.040
Khalaj, M., Kamali, M., Costa, M. E. V., and Capela, I. (2020). Green Synthesis of Nanomaterials - A Scientometric Assessment. J. Clean. Prod. 267, 122036. doi:10.1016/j.jclepro.2020.122036
Khan, A. U., Malik, N., Khan, M., Cho, M. H., and Khan, M. M. (2018). Fungi-assisted Silver Nanoparticle Synthesis and Their Applications. Bioproc. Biosyst. Eng. 41, 1–20. doi:10.1007/s00449-017-1846-3
Khan, R., and Fulekar, M. H. (2016). Biosynthesis of Titanium Dioxide Nanoparticles Using Bacillus Amyloliquefaciens Culture and Enhancement of its Photocatalytic Activity for the Degradation of a Sulfonated Textile Dye Reactive Red 31. J. Colloid Interf. Sci. 475, 184–191. doi:10.1016/j.jcis.2016.05.001
Khan, T., and Ali, G. S. (2020). Variation in Surface Properties, Metabolic Capping, and Antibacterial Activity of Biosynthesized Silver Nanoparticles: Comparison of Bio-Fabrication Potential in Phytohormone-Regulated Cell Cultures and Naturally Grown Plants. RSC Adv. 10, 38831–38840. doi:10.1039/d0ra08419k
Khanehzaei, H., Ahmad, M. B., Shameli, K., and Ajdari, Z. (2015). Synthesis and Characterization of Cu@Cu2O Core Shell Nanoparticles Prepared in Seaweed Kappaphycus Alvarezii media. Int. J. Electrochem. Sci. 10.
Kim, D.-M., Kang, C.-I., Lee, C. S., Kim, H.-B., Kim, E.-C., Kim, N. J., et al. (2006). Treatment Failure Due to Emergence of Resistance to Carbapenem during Therapy for Shewanella Algae Bacteremia. J. Clin. Microbiol. 44, 1172–1174. doi:10.1128/JCM.44.3.1172-1174.2006
Kim, S. H., Lee, H. S., Ryu, D. S., Choi, S. J., and Lee, D. S. (2011). Antibacterial Activity of Silver-Nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 39, 1.
Kimber, R. L., Lewis, E. A., Parmeggiani, F., Smith, K., Bagshaw, H., Starborg, T., et al. (2018). Biosynthesis and Characterization of Copper Nanoparticles UsingShewanella Oneidensis: Application for Click Chemistry. Small 14, 1703145. doi:10.1002/smll.201703145
Kitching, M., Choudhary, P., Inguva, S., Guo, Y., Ramani, M., Das, S. K., et al. (2016). Fungal Surface Protein Mediated One-Pot Synthesis of Stable and Hemocompatible Gold Nanoparticles. Enzyme Microb. Technology 95, 76–84. doi:10.1016/j.enzmictec.2016.08.007
Klaus, T., Joerger, R., Olsson, E., and Granqvist, C.-G. (1999). Silver-based Crystalline Nanoparticles, Microbially Fabricated. Proc. Natl. Acad. Sci. U.S.A. 96, 13611–13614. doi:10.1073/pnas.96.24.13611
Klaus-Joerger, T., Joerger, R., Olsson, E., and Granqvist, C. (2001). Bacteria as Workers in the Living Factory: Metal-Accumulating Bacteria and Their Potential for Materials Science. Trends Biotechnol. 19, 15–20. doi:10.1016/S0167-7799(00)01514-6
Konishi, Y., Tsukiyama, T., Ohno, K., Saitoh, N., Nomura, T., and Nagamine, S. (2006). Intracellular Recovery of Gold by Microbial Reduction of AuCl4− Ions Using the Anaerobic Bacterium Shewanella Algae. Hydrometallurgy 81, 24–29. doi:10.1016/j.hydromet.2005.09.006
Konishi, Y., Tsukiyama, T., Tachimi, T., Saitoh, N., Nomura, T., and Nagamine, S. (2007). Microbial Deposition of Gold Nanoparticles by the Metal-Reducing Bacterium Shewanella Algae. Electrochimica Acta 53, 186–192. doi:10.1016/j.electacta.2007.02.073
Kora, A. J., Beedu, S. R., and Jayaraman, A. (2012). Size-controlled green Synthesis of Silver Nanoparticles Mediated by Gum Ghatti (Anogeissus Latifolia) and its Biological Activity. Org. Med. Chem. Lett. 2, 17. doi:10.1186/2191-2858-2-17
Kowshik, M., Deshmukh, N., Vogel, W., Urban, J., Kulkarni, S. K., and Paknikar, K. M. (2002). Microbial Synthesis of Semiconductor CdS Nanoparticles, Their Characterization, and Their Use in the Fabrication of an Ideal Diode. Biotechnol. Bioeng. 78, 583–588. doi:10.1002/bit.10233
Krishnaraj, C., Jagan, E. G., Rajasekar, S., Selvakumar, P., Kalaichelvan, P. T., and Mohan, N. (2010). Synthesis of Silver Nanoparticles Using Acalypha indica Leaf Extracts and its Antibacterial Activity against Water Borne Pathogens. Colloids Surf. B: Biointerfaces 76, 50–56. doi:10.1016/j.colsurfb.2009.10.008
Ksv, G. (2017). Green Synthesis of Iron Nanoparticles Using Green Tea Leaves Extract. J. Nanomedine. Biotherapeutic Discov. 07, 1. doi:10.4172/2155-983x.1000151
Kumar, V., and Yadav, S. K. (2009). Plant-mediated Synthesis of Silver and Gold Nanoparticles and Their Applications. J. Chem. Technol. Biotechnol. 84, 151–157. doi:10.1002/jctb.2023
Kumaresan, M., Vijai Anand, K., Govindaraju, K., Tamilselvan, S., and Ganesh Kumar, V. (2018). Seaweed Sargassum Wightii Mediated Preparation of Zirconia (ZrO2) Nanoparticles and Their Antibacterial Activity against Gram Positive and Gram Negative Bacteria. Microb. Pathogenesis 124, 311–315. doi:10.1016/j.micpath.2018.08.060
Lahiri, D., Nag, M., Sheikh, H. I., Sarkar, T., Edinur, H. A., Pati, S., et al. (2021). Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 12, 636588. doi:10.3389/fmicb.2021.636588
Lengke, M. F., Ravel, B., Fleet, M. E., Wanger, G., Gordon, R. A., and Southam, G. (2006). Mechanisms of Gold Bioaccumulation by Filamentous Cyanobacteria from Gold(III)−Chloride Complex. Environ. Sci. Technol. 40, 6304–6309. doi:10.1021/es061040r
Li, J., Sun, F., Gu, K., Wu, T., Zhai, W., Li, W., et al. (2011a). Preparation of Spindly CuO Micro-particles for Photodegradation of Dye Pollutants under a Halogen Tungsten Lamp. Appl. Catal. A: Gen. 406, 51–58. doi:10.1016/j.apcata.2011.08.007
Li, X., Xu, H., Chen, Z.-S., and Chen, G. (2011b). Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 1–16. doi:10.1155/2011/270974
Lin, L., Wang, W., Huang, J., Li, Q., Sun, D., Yang, X., et al. (2010). Nature Factory of Silver Nanowires: Plant-Mediated Synthesis Using Broth of Cassia Fistula Leaf. Chem. Eng. J. 162, 852–858. doi:10.1016/j.cej.2010.06.023
Lin, Z., Wu, J., Xue, R., and Yang, Y. (2005). Spectroscopic Characterization of Au3+ Biosorption by Waste Biomass of Saccharomyces cerevisiae. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 61, 761–765. doi:10.1016/j.saa.2004.03.029
Liu, S., Wei, L., Hao, L., Fang, N., Chang, M. W., Xu, R., et al. (2009). Sharper and Faster "Nano Darts" Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano 3, 3891–3902. doi:10.1021/nn901252r
Lomelí-Rosales, D. A., Zamudio-Ojeda, A., Reyes-Maldonado, O. K., López-Reyes, M. E., Basulto-Padilla, G. C., Lopez-Naranjo, E. J., et al. (2022). Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Capsicum Chinense Plant. Molecules 27, 1692. doi:10.3390/molecules27051692
Loo, Y. Y., Rukayadi, Y., Nor-Khaizura, M.-A. -R., Kuan, C. H., Chieng, B. W., Nishibuchi, M., et al. (2018). In Vitro antimicrobial Activity of green Synthesized Silver Nanoparticles against Selected Gram-Negative Foodborne Pathogens. Front. Microbiol. 9, 1555. doi:10.3389/fmicb.2018.01555
Luangpipat, T., Beattie, I. R., Chisti, Y., and Haverkamp, R. G. (2011). Gold Nanoparticles Produced in a Microalga. J. Nanopart Res. 13, 6439–6445. doi:10.1007/s11051-011-0397-9
Madhanraj, R., Eyini, M., and Balaji, P. (2017). Antioxidant Assay of Gold and Silver Nanoparticles from Edible Basidiomycetes Mushroom Fungi. Fra 7, 137–142. doi:10.5530/fra.2017.2.20
Mahdavi, M., Namvar, F., Ahmad, M., and Mohamad, R. (2013). Green Biosynthesis and Characterization of Magnetic Iron Oxide (Fe3O4) Nanoparticles Using Seaweed (Sargassum Muticum) Aqueous Extract. Molecules 18, 5954–5964. doi:10.3390/molecules18055954
Majeed, M., Hakeem, K. R., and Rehman, R. U. (2022). Synergistic Effect of Plant Extract Coupled Silver Nanoparticles in Various Therapeutic Applications- Present Insights and Bottlenecks. Chemosphere 288, 132527. doi:10.1016/j.chemosphere.2021.132527
Maji, A., Beg, M., Das, S., Chandra Jana, G., Jha, P. K., Islam, M. M., et al. (2019). Spectroscopic Study on Interaction of Nymphaea Nouchali Leaf Extract Mediated Bactericidal Gold Nanoparticles with Human Serum Albumin. J. Mol. Struct. 1179, 685–693. doi:10.1016/j.molstruc.2018.11.055
Makarov, V. V., Love, A. J., Sinitsyna, O. V., Makarova, S. S., Yaminsky, I. V., Taliansky, M. E., et al. (2014). "Green" Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Naturae 6, 35–44. doi:10.32607/20758251-2014-6-1-35-44
Malaikozhundan, B., Vaseeharan, B., Vijayakumar, S., Pandiselvi, K., Kalanjiam, M. A. R., Murugan, K., et al. (2017). Biological Therapeutics of Pongamia Pinnata Coated Zinc Oxide Nanoparticles against Clinically Important Pathogenic Bacteria, Fungi and MCF-7 Breast Cancer Cells. Microb. Pathogenesis 104, 268–277. doi:10.1016/j.micpath.2017.01.029
Manivasagan, P., Bharathiraja, S., Bui, N. Q., Jang, B., Oh, Y.-O., Lim, I. G., et al. (2016). Doxorubicin-loaded Fucoidan Capped Gold Nanoparticles for Drug Delivery and Photoacoustic Imaging. Int. J. Biol. Macromolecules 91, 578–588. doi:10.1016/j.ijbiomac.2016.06.007
Mata, R., Bhaskaran, A., and Sadras, S. R. (2016). Green-synthesized Gold Nanoparticles from Plumeria alba Flower Extract to Augment Catalytic Degradation of Organic Dyes and Inhibit Bacterial Growth. Particuology 24, 78–86. doi:10.1016/j.partic.2014.12.014
Mata, Y. N., Torres, E., Blázquez, M. L., Ballester, A., González, F., and Muñoz, J. A. (2009). Gold(III) Biosorption and Bioreduction with the Brown Alga Fucus Vesiculosus. J. Hazard. Mater. 166, 612–618. doi:10.1016/j.jhazmat.2008.11.064
Mirunalini, S., Arulmozhi, V., Deepalakshmi, K., and Krishnaveni, M. (2012). Intracellular Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Edible Mushrooms. Not. Sci. Biol. 4, 55–61. doi:10.15835/nsb448051
Mittal, A. K., Chisti, Y., and Banerjee, U. C. (2013). Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 31, 346–356. doi:10.1016/j.biotechadv.2013.01.003
Moghimi, S. M., Hunter, A. C., and Murray, J. C. (2001). Long-circulating and Target-specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 53, 283–318.
Moghimi, S. M. (2003). Modulation of Lymphatic Distribution of Subcutaneously Injected Poloxamer 407-coated Nanospheres: The Effect of the Ethylene Oxide Chain Configuration. FEBS Lett. 540, 241–244. doi:10.1016/S0014-5793(03)00273-4
Moghimi, S. M., and Szebeni, J. (2003). Stealth Liposomes and Long Circulating Nanoparticles: Critical Issues in Pharmacokinetics, Opsonization and Protein-Binding Properties. Prog. Lipid Res. 42, 463–478. doi:10.1016/S0163-7827(03)00033-X
Mohamad, N. A. N., Arham, N. A., Jai, J., and Hadi, A. (2014). “Plant Extract as Reducing Agent in Synthesis of Metallic Nanoparticles: A Review,” in Advanced Materials Research. doi:10.4028/www.scientific.net/AMR.832.350
Mohanta, Y., Nayak, D., Biswas, K., Singdevsachan, S., Abd_Allah, E., Hashem, A., et al. (2018). Silver Nanoparticles Synthesized Using Wild Mushroom Show Potential Antimicrobial Activities against Food Borne Pathogens. Molecules 23, 655. doi:10.3390/molecules23030655
Molnár, Z., Bódai, V., Szakacs, G., Erdélyi, B., Fogarassy, Z., Sáfrán, G., et al. (2018). Green Synthesis of Gold Nanoparticles by Thermophilic Filamentous Fungi. Sci. Rep. 8, 3943. doi:10.1038/s41598-018-22112-3
Momeni, S., and Nabipour, I. (2015). A Simple Green Synthesis of Palladium Nanoparticles with Sargassum Alga and Their Electrocatalytic Activities towards Hydrogen Peroxide. Appl. Biochem. Biotechnol. 176, 1937–1949. doi:10.1007/s12010-015-1690-3
Mominur Rahman, M., Islam, F., Saidur Rahaman, M., Sultana, N. A., Fahim, N. F., and Ahmed, M. (2021). Studies on the Prevalence of HIV/AIDS in Bangladesh Including Other Developing Countries. Adv. Tradit Med. (Adtm) 2021, 1–12. doi:10.1007/S13596-021-00610-6
Moradnia, F., Taghavi Fardood, S., Ramazani, A., and Gupta, V. K. (2020). Green Synthesis of Recyclable MgFeCrO4 Spinel Nanoparticles for Rapid Photodegradation of Direct Black 122 Dye. J. Photochem. Photobiol. A: Chem. 392, 112433. doi:10.1016/j.jphotochem.2020.112433
Motahharifar, N., Nasrollahzadeh, M., Taheri-Kafrani, A., Varma, R. S., and Shokouhimehr, M. (2020). Magnetic Chitosan-Copper Nanocomposite: A Plant Assembled Catalyst for the Synthesis of Amino- and N-Sulfonyl Tetrazoles in Eco-Friendly media. Carbohydr. Polym. 232, 115819. doi:10.1016/j.carbpol.2019.115819
Mu, S., Liu, Q., Kidkhunthod, P., Zhou, X., Wang, W., and Tang, Y. (2021). Molecular Grafting towards High-Fraction Active Nanodots Implanted in N-Doped Carbon for Sodium Dual-Ion Batteries. Natl. Sci. Rev. 8, 1. doi:10.1093/nsr/nwaa178
Murugan, K., Benelli, G., Panneerselvam, C., Subramaniam, J., Jeyalalitha, T., Dinesh, D., et al. (2015). Cymbopogon Citratus-Synthesized Gold Nanoparticles Boost the Predation Efficiency of Copepod Mesocyclops Aspericornis against Malaria and Dengue Mosquitoes. Exp. Parasitol. 153, 129–138. doi:10.1016/j.exppara.2015.03.017
Murugan, K., Panneerselvam, C., Subramaniam, J., Madhiyazhagan, P., Hwang, J.-S., Wang, L., et al. (2016). Eco-friendly Drugs from the marine Environment: Spongeweed-Synthesized Silver Nanoparticles Are Highly Effective on Plasmodium Falciparum and its Vector Anopheles stephensi, with Little Non-target Effects on Predatory Copepods. Environ. Sci. Pollut. Res. 23, 16671–16685. doi:10.1007/s11356-016-6832-9
Musa, S. F., Yeat, T. S., Kamal, L. Z. M., Tabana, Y. M., Ahmed, M. A., El Ouweini, A., et al. (2018). Pleurotus Sajor-Cajucan Be Used to Synthesize Silver Nanoparticles with Antifungal Activity againstCandida Albicans. J. Sci. Food Agric. 98, 1197–1207. doi:10.1002/jsfa.8573
Mustapha, T., Misni, N., Ithnin, N. R., Daskum, A. M., and Unyah, N. Z. (2022). A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Ijerph 19, 674. doi:10.3390/ijerph19020674
Nag, M., Lahiri, D., Sarkar, T., Ghosh, S., Dey, A., Edinur, H. A., et al. (2021). Microbial Fabrication of Nanomaterial and its Role in Disintegration of Exopolymeric Matrices of Biofilm. Front. Chem. 9, 1. doi:10.3389/fchem.2021.690590
Nagalingam, M., Kalpana, V. N., Rajeswari, V. D., and Panneerselvam., A. (2018). Biosynthesis, Characterization, and Evaluation of Bioactivities of Leaf Extract-Mediated Biocompatible Gold Nanoparticles from Alternanthera Bettzickiana. Biotechnol. Rep. 19, e00268. doi:10.1016/j.btre.2018.e00268
Nagarajan, S., and Arumugam Kuppusamy, K. (2013). Extracellular Synthesis of Zinc Oxide Nanoparticle Using Seaweeds of Gulf of Mannar, India. J. Nanobiotechnology 11, 39. doi:10.1186/1477-3155-11-39
Nair, B., and Pradeep, T. (2002). Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lactobacillus Strains. Cryst. Growth Des. 2, 293–298. doi:10.1021/cg0255164
Najafi, S., Razavi, S. M., Khoshkam, M., and Asadi, A. (2022). Green Synthesized of Sulfur Nanoparticles and its Application on Lettuce Plants Metabolic Profiling. BioNanoSci. 1, 1. doi:10.1007/s12668-021-00918-2
Namvar, F., Mohammad, R., Baharara, J., Mahdavi, M., Amini, E., Yeap, S. K., et al. (2014). Cytotoxic Effect of Magnetic Iron Oxide Nanoparticles Synthesized via Seaweed Aqueous Extract. Ijn 9, 2479. doi:10.2147/IJN.S59661
Nanda, A., and Saravanan, M. (2009). Biosynthesis of Silver Nanoparticles from Staphylococcus aureus and its Antimicrobial Activity against MRSA and MRSE. Nanomedicine: Nanotechnology, Biol. Med. 5, 452–456. doi:10.1016/j.nano.2009.01.012
Narayanan, K. B., Park, H. H., and Han, S. S. (2015). Synthesis and Characterization of Biomatrixed-Gold Nanoparticles by the Mushroom Flammulina Velutipes and its Heterogeneous Catalytic Potential. Chemosphere 141, 169–175. doi:10.1016/j.chemosphere.2015.06.101
Narayanan, K. B., and Sakthivel, N. (2008). Coriander Leaf Mediated Biosynthesis of Gold Nanoparticles. Mater. Lett. 62, 4588–4590. doi:10.1016/j.matlet.2008.08.044
Narendhran, S., and Sivaraj, R. (2016). Biogenic ZnO Nanoparticles Synthesized Using L. Aculeata Leaf Extract and Their Antifungal Activity against Plant Fungal Pathogens. Bull. Mater. Sci. 39, 1–5. doi:10.1007/s12034-015-1136-0
Nasrollahzadeh, M., Atarod, M., and Sajadi, S. M. (2017a). Biosynthesis, Characterization and Catalytic Activity of Cu/RGO/Fe3O4 for Direct Cyanation of Aldehydes with K4[Fe(CN)6]. J. Colloid Interf. Sci. 486, 153–162. doi:10.1016/j.jcis.2016.09.053
Nasrollahzadeh, M., Issaabadi, Z., and Sajadi, S. M. (2019). Green Synthesis of Cu/Al2O3 Nanoparticles as Efficient and Recyclable Catalyst for Reduction of 2,4-dinitrophenylhydrazine, Methylene Blue and Congo Red. Composites B: Eng. 166, 112–119. doi:10.1016/j.compositesb.2018.11.113
Nasrollahzadeh, M., Issaabadi, Z., and Sajadi, S. M. (2018a). Green Synthesis of Pd/Fe3O4 Nanocomposite Using Hibiscus tiliaceus L. Extract and its Application for Reductive Catalysis of Cr(VI) and nitro Compounds. Separation Purif. Technology 197, 253–260. doi:10.1016/j.seppur.2018.01.010
Nasrollahzadeh, M., and Mohammad Sajadi, S. (2015). Green Synthesis of Copper Nanoparticles Using Ginkgo Biloba L. Leaf Extract and Their Catalytic Activity for the Huisgen [3 + 2] Cycloaddition of Azides and Alkynes at Room Temperature. J. Colloid Interf. Sci. 457, 141–147. doi:10.1016/j.jcis.2015.07.004
Nasrollahzadeh, M., Mohammad Sajadi, S., Rostami-Vartooni, A., Alizadeh, M., and Bagherzadeh, M. (2016). Green Synthesis of the Pd Nanoparticles Supported on Reduced Graphene Oxide Using Barberry Fruit Extract and its Application as a Recyclable and Heterogeneous Catalyst for the Reduction of Nitroarenes. J. Colloid Interf. Sci. 466, 360–368. doi:10.1016/j.jcis.2015.12.036
Nasrollahzadeh, M., Momeni, S. S., and Sajadi, S. M. (2017b). Green Synthesis of Copper Nanoparticles Using Plantago Asiatica Leaf Extract and Their Application for the Cyanation of Aldehydes Using K4Fe(CN)6. J. Colloid Interf. Sci. 506, 471–477. doi:10.1016/j.jcis.2017.07.072
Nasrollahzadeh, M., and Sajadi, S. M. (2016). Green Synthesis of Pd Nanoparticles Mediated by Euphorbia Thymifolia L. Leaf Extract: Catalytic Activity for Cyanation of Aryl Iodides under Ligand-free Conditions. J. Colloid Interf. Sci. 469, 191–195. doi:10.1016/j.jcis.2016.02.024
Nasrollahzadeh, M., and Sajadi, S. M. (2015). Synthesis and Characterization of Titanium Dioxide Nanoparticles Using Euphorbia Heteradena Jaub Root Extract and Evaluation of Their Stability. Ceramics Int. 41, 14435–14439. doi:10.1016/j.ceramint.2015.07.079
Nasrollahzadeh, M., Sajjadi, M., Dasmeh, H. R., and Sajadi, S. M. (2018b). Green Synthesis of the Cu/sodium Borosilicate Nanocomposite and Investigation of its Catalytic Activity. J. Alloys Compounds 763, 1024–1034. doi:10.1016/j.jallcom.2018.05.012
Nasrollahzadeh, M., Sajjadi, M., and Mohammad Sajadi, S. (2018c). Biosynthesis of Copper Nanoparticles Supported on Manganese Dioxide Nanoparticles Using Centella asiatica L. Leaf Extract for the Efficient Catalytic Reduction of Organic Dyes and Nitroarenes. Cuihua Xuebao/chinese J. Catal. 39, 109–117. doi:10.1016/S1872-2067(17)62915-2
Nithya, R., and Ragunathan, R. (2009). Synthesis of Silver Nanoparticle Using Pleurotus Sajor Caju and its Antimicrobial Study. Dig. J. Nanomater. Biostructures 4, 1.
Noruzi, M. (2015). Biosynthesis of Gold Nanoparticles Using Plant Extracts. Bioproc. Biosyst. Eng. 38, 1–14. doi:10.1007/s00449-014-1251-0
Omar, H. H., S. Bahabri, F., and M. El-Gend, A. (2017). Biopotential Application of Synthesis Nanoparticles as Antimicrobial Agents by Using Laurencia Papillosa. Int. J. Pharmacol. 13, 303–312. doi:10.3923/ijp.2017.303.312
Onitsuka, S., Hamada, T., and Okamura, H. (2019). Preparation of Antimicrobial Gold and Silver Nanoparticles from tea Leaf Extracts. Colloids Surf. B: Biointerfaces 173, 242–248. doi:10.1016/j.colsurfb.2018.09.055
Otari, S. V., Patil, R. M., Ghosh, S. J., Thorat, N. D., and Pawar, S. H. (2015). Intracellular Synthesis of Silver Nanoparticle by Actinobacteria and its Antimicrobial Activity. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 136, 1175–1180. doi:10.1016/j.saa.2014.10.003
Oves, M., Ahmar Rauf, M., Aslam, M., Qari, H. A., Sonbol, H., Ahmad, I., et al. (2022). Green Synthesis of Silver Nanoparticles by Conocarpus Lancifolius Plant Extract and Their Antimicrobial and Anticancer Activities. Saudi J. Biol. Sci. 29, 460–471. doi:10.1016/j.sjbs.2021.09.007
Owaid, M. N., Raman, J., Lakshmanan, H., Al-Saeedi, S. S. S., Sabaratnam, V., and Abed, I. A. (2015). Mycosynthesis of Silver Nanoparticles by Pleurotus Cornucopiae Var. Citrinopileatus and its Inhibitory Effects against Candida Sp. Mater. Lett. 153, 186–190. doi:10.1016/j.matlet.2015.04.023
Padalia, H., and Chanda, S. (2017). Characterization, Antifungal and Cytotoxic Evaluation of green Synthesized Zinc Oxide Nanoparticles Using Ziziphus Nummularia Leaf Extract. Artif. Cell Nanomedicine, Biotechnol. 45, 1751–1761. doi:10.1080/21691401.2017.1282868
Pakzad, K., Alinezhad, H., and Nasrollahzadeh, M. (2019). Green Synthesis of Ni@Fe3O4 and CuO Nanoparticles Using Euphorbia Maculata Extract as Photocatalysts for the Degradation of Organic Pollutants under UV-Irradiation. Ceramics Int. 45, 17173–17182. doi:10.1016/j.ceramint.2019.05.272
Patel, V., Berthold, D., Puranik, P., and Gantar, M. (2015). Screening of Cyanobacteria and Microalgae for Their Ability to Synthesize Silver Nanoparticles with Antibacterial Activity. Biotechnol. Rep. 5, 112–119. doi:10.1016/j.btre.2014.12.001
Patil, R. S., Kokate, M. R., and Kolekar, S. S. (2012). Bioinspired Synthesis of Highly Stabilized Silver Nanoparticles Using Ocimum Tenuiflorum Leaf Extract and Their Antibacterial Activity. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 91, 234–238. doi:10.1016/j.saa.2012.02.009
Patra, J. K., and Baek, K.-H. (20142014). Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 1–12. doi:10.1155/2014/417305
Philip, D. (2009). Biosynthesis of Au, Ag and Au-Ag Nanoparticles Using Edible Mushroom Extract. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 73, 374–381. doi:10.1016/j.saa.2009.02.037
Philip, D. (2010). Rapid green Synthesis of Spherical Gold Nanoparticles Using Mangifera Indica Leaf. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 77, 807–810. doi:10.1016/j.saa.2010.08.008
Philip, D., Unni, C., Aromal, S. A., and Vidhu, V. K. (2011). Murraya Koenigii Leaf-Assisted Rapid green Synthesis of Silver and Gold Nanoparticles. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 78, 899–904. doi:10.1016/j.saa.2010.12.060
Poopathi, S., De Britto, L. J., Praba, V. L., Mani, C., and Praveen, M. (2015). Synthesis of Silver Nanoparticles from Azadirachta Indica-A Most Effective Method for Mosquito Control. Environ. Sci. Pollut. Res. 22, 2956–2963. doi:10.1007/s11356-014-3560-x
Poudel, M., Pokharel, R., and Pradhananga, R. (2017). Biosynthesis of Silver Nanoparticles Using Ganoderma Lucidum and Assessment of Antioxidant and Antibacterial Activity. Int. J. Appl. Sci. Biotechnol. 5, 523–531. doi:10.3126/ijasbt.v5i4.18776
Prasad, T. N., and Elumalai, E. K. (2011). Biofabrication of Ag Nanoparticles Using Moringa Oleifera Leaf Extract and Their Antimicrobial Activity. Asian Pac. J. Trop. Biomed. 1, 439–442. doi:10.1016/S2221-1691(11)60096-8
Pugazhendhi, A., Prabakar, D., Jacob, J. M., Karuppusamy, I., and Saratale, R. G. (2018). Synthesis and Characterization of Silver Nanoparticles Using Gelidium Amansii and its Antimicrobial Property against Various Pathogenic Bacteria. Microb. Pathogenesis 114, 41–45. doi:10.1016/j.micpath.2017.11.013
Pugazhenthiran, N., Anandan, S., Kathiravan, G., Udaya Prakash, N. K., Crawford, S., and Ashokkumar, M. (2009). Microbial Synthesis of Silver Nanoparticles by Bacillus Sp. J. Nanopart Res. 11, 1811–1815. doi:10.1007/s11051-009-9621-2
Qiao, Z.-P., Wang, M.-Y., Liu, J.-F., and Wang, Q.-Z. (2022). Green Synthesis of Silver Nanoparticles Using a Novel Endophytic Fungus Letendraea Sp. WZ07: Characterization and Evaluation of Antioxidant, Antibacterial and Catalytic Activities (3-in-1 System). Inorg. Chem. Commun. 138, 109301. doi:10.1016/J.INOCHE.2022.109301
Rahman, M., Islam, M., Islam, M., Harun-Or-rashid, M., Islam, M., Abdullah, S., et al. (2022). Stem Cell Transplantation Therapy and Neurological Disorders: Current Status and Future Perspectives. Biology 11, 147. doi:10.3390/BIOLOGY11010147
Rahman, M. M., Ferdous, K. S., Ahmed, M., Islam, M. T., Khan, M. R., Perveen, A., et al. (2021a). Hutchinson-Gilford Progeria Syndrome: An Overview of the Molecular Mechanism, Pathophysiology and Therapeutic Approach. Cgt 21, 216–229. doi:10.2174/1566523221666210303100805
Rahman, M. M., Rahaman, M. S., Islam, M. R., Hossain, M. E., Mannan Mithi, F., Ahmed, M., et al. (2021b). Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties. Antibiotics 10, 1076. doi:10.3390/ANTIBIOTICS10091076
Rahman, M. M., Rahaman, M. S., Islam, M. R., Rahman, F., Mithi, F. M., Alqahtani, T., et al. (2021c). Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 27, 233. doi:10.3390/MOLECULES27010233
Raja, K., Balamurugan, V., Selvakumar, S., and Vasanth, K. (2022). Striga Angustifolia Mediated Synthesis of Silver Nanoparticles: Anti-microbial, Antioxidant and Anti-proliferative Activity in Apoptotic P53 Signalling Pathway. J. Drug Deliv. Sci. Technology 67, 102945. doi:10.1016/J.JDDST.2021.102945
Rajabi, H. R., Naghiha, R., Kheirizadeh, M., Sadatfaraji, H., Mirzaei, A., and Alvand, Z. M. (2017). Microwave Assisted Extraction as an Efficient Approach for Biosynthesis of Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biological Properties. Mater. Sci. Eng. C 78, 1109–1118. doi:10.1016/j.msec.2017.03.090
Rajakumar, G., and Abdul Rahuman, A. (2011). Larvicidal Activity of Synthesized Silver Nanoparticles Using Eclipta Prostrata Leaf Extract against Filariasis and Malaria Vectors. Acta Tropica 118, 196–203. doi:10.1016/j.actatropica.2011.03.003
Rajasulochana, P., Dhamotharan, R., Murugakoothan, P., Murugesan, S., and Krishnamoorthy, P. (2010). Biosynthesis and Characterization of Gold Nanoparticles Using the Alga Kappaphycus Alvarezii. Int. J. Nanosci. 09, 511–516. doi:10.1142/S0219581X10007149
Rajeshkumar, S., Kannan, C., and Annadurai, G. (2012). Synthesis and Characterization of Antimicrobial Silver Nanoparticles Using marine Brown Seaweed Padina Tetrastromatica. Drug Invent. Today 4, 511.
Rajeshkumar, S., and Malarkodi, C. (2014). In Vitro Antibacterial Activity and Mechanism of Silver Nanoparticles against Foodborne Pathogens. Bioinorganic Chem. Appl. 2014, 1–10. doi:10.1155/2014/581890
Rajeshkumar, S., Malarkodi, C., Paulkumar, K., Vanaja, M., Gnanajobitha, G., and Annadurai, G. (2014). Algae Mediated Green Fabrication of Silver Nanoparticles and Examination of its Antifungal Activity against Clinical Pathogens. Int. J. Met. 2014, 1–8. doi:10.1155/2014/692643
Rajeshkumar, S., Malarkodi, C., Vanaja, M., Gnanajobitha, G., Paulkumar, K., Kannan, C., et al. (2013). Antibacterial Activity of Algae Mediated Synthesis of Gold Nanoparticles from Turbinaria Conoides. Der Pharma Chem. 5, 224.
Rajivgandhi, G. N., Chackaravarthi, G., Ramachandran, G., Manoharan, N., Ragunathan, R., Siddiqi, M. Z., et al. (2022). Synthesis of Silver Nanoparticle (Ag NPs) Using Phytochemical Rich Medicinal Plant Lonicera japonica for Improve the Cytotoxicity Effect in Cancer Cells. J. King Saud Univ. - Sci. 34, 101798. doi:10.1016/J.JKSUS.2021.101798
Raju, B., Muniyasamy, A., Prakash, S. G., Sundararaj, A. S., and Kesavachandran, U. (2017). Phycosynthesis of Nanostructured Silver Using Enteromorpha Intestinalis and Evaluation of its Inhibitory Effect on Human Bacterial and Fungal Pathogens. J. Clust. Sci. 28, 1739–1748. doi:10.1007/s10876-017-1166-4
Ramakrishna, M., Rajesh Babu, D., Gengan, R. M., Chandra, S., and Nageswara Rao, G. (2016). Green Synthesis of Gold Nanoparticles Using marine Algae and Evaluation of Their Catalytic Activity. J. Nanostruct Chem. 6, 1–13. doi:10.1007/s40097-015-0173-y
Raman, J., Reddy, G. R., Lakshmanan, H., Selvaraj, V., Gajendran, B., Nanjian, R., et al. (2015). Mycosynthesis and Characterization of Silver Nanoparticles from Pleurotus Djamor Var. Roseus and Their In Vitro Cytotoxicity Effect on PC3 Cells. Process Biochem. 50, 140–147. doi:10.1016/j.procbio.2014.11.003
Ramkumar, V. S., Pugazhendhi, A., Prakash, S., Ahila, N. K., Vinoj, G., Selvam, S., et al. (2017). Synthesis of Platinum Nanoparticles Using Seaweed Padina Gymnospora and Their Catalytic Activity as PVP/PtNPs Nanocomposite towards Biological Applications. Biomed. Pharmacother. 92, 479–490. doi:10.1016/j.biopha.2017.05.076
Ramya, S., Shanmugasundaram, T., and Balagurunathan, R. (2015). Biomedical Potential of Actinobacterially Synthesized Selenium Nanoparticles with Special Reference to Anti-biofilm, Anti-oxidant, Wound Healing, Cytotoxic and Anti-viral Activities. J. Trace Elem. Med. Biol. 32, 30–39. doi:10.1016/j.jtemb.2015.05.005
Raza, S., Ansari, A., Siddiqui, N. N., Ibrahim, F., Abro, M. I., and Aman, A. (2021). Biosynthesis of Silver Nanoparticles for the Fabrication of Non Cytotoxic and Antibacterial Metallic Polymer Based Nanocomposite System. Sci. Rep. 11, 1–15. doi:10.1038/s41598-021-90016-w
Reddy, K. R. (2017). Green Synthesis, Morphological and Optical Studies of CuO Nanoparticles. J. Mol. Struct. 1150, 553–557. doi:10.1016/j.molstruc.2017.09.005
Roh, Y., Lauf, R. J., McMillan, A. D., Zhang, C., Rawn, C. J., Bai, J., et al. (2001). Microbial Synthesis and the Characterization of Metal-Substituted Magnetites. Solid State. Commun. 118, 529. doi:10.1016/S0038-1098(01)00146-6
Romero-González, M. E., Williams, C. J., Gardiner, P. H. E., Gurman, S. J., and Habesh, S. (2003). Spectroscopic Studies of the Biosorption of Gold(III) by Dealginated Seaweed Waste. Environ. Sci. Technol. 37, 4163–4169. doi:10.1021/es020176w
Rónavári, A., Kovács, D., Igaz, N., Vágvölgyi, C., Boros, I., Kónya, Z., et al. (2017). Biological Activity of green-synthesized Silver Nanoparticles Depends on the Applied Natural Extracts: A Comprehensive Study. Ijn 12, 871–883. doi:10.2147/IJN.S122842
Roni, M., Murugan, K., Panneerselvam, C., Subramaniam, J., Nicoletti, M., Madhiyazhagan, P., et al. (2015). Characterization and Biotoxicity of Hypnea Musciformis-Synthesized Silver Nanoparticles as Potential Eco-Friendly Control Tool against Aedes aegypti and Plutella Xylostella. Ecotoxicology Environ. Saf. 121, 31–38. doi:10.1016/j.ecoenv.2015.07.005
Roy, P., Das, B., Mohanty, A., and Mohapatra, S. (2017). Green Synthesis of Silver Nanoparticles Using Azadirachta Indica Leaf Extract and its Antimicrobial Study. Appl. Nanosci. 7, 843–850. doi:10.1007/s13204-017-0621-8
Sadeghi, B., Rostami, A., and Momeni, S. S. (2015). Facile green Synthesis of Silver Nanoparticles Using Seed Aqueous Extract of Pistacia Atlantica and its Antibacterial Activity. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 134, 326–332. doi:10.1016/j.saa.2014.05.078
Sahayaraj, K., Rajesh, S., Rathi, J. A. M., and Kumar, V. (2019). Green Preparation of Seaweed‐based Silver Nano‐liquid for Cotton Pathogenic Fungi Management. IET nanobiotechnol. 13, 219–225. doi:10.1049/iet-nbt.2018.5007
Samadi, N., Golkaran, D., Eslamifar, A., Jamalifar, H., Fazeli, M. R., and Mohseni, F. A. (2009). Intra/Extracellular Biosynthesis of Silver Nanoparticles by an Autochthonous Strain of Proteus mirabilis Isolated fromPhotographic Waste. J. Biomed. Nanotechnology 5, 247–253. doi:10.1166/jbn.2009.1029
Sameem, S., Neupane, N. P., Saleh Ansari, S. M., Uzzaman Khan, M. M., Kumar, V., Pathak, P., et al. (2022). Phyto-fabrication of Silver Nanoparticles from Ziziphus Mauritiana against Hepatic Carcinoma via Modulation of Rho Family-Alpha Serine/threonine Protein Kinase. J. Drug Deliv. Sci. Technology 70, 103227. doi:10.1016/J.JDDST.2022.103227
Santhoshkumar, T., Rahuman, A. A., Rajakumar, G., Marimuthu, S., Bagavan, A., Jayaseelan, C., et al. (2011). Synthesis of Silver Nanoparticles Using Nelumbo nucifera Leaf Extract and its Larvicidal Activity against Malaria and Filariasis Vectors. Parasitol. Res. 108, 693–702. doi:10.1007/s00436-010-2115-4
Saravanan, M., Barik, S. K., MubarakAli, D., Prakash, P., and Pugazhendhi, A. (2018a). Synthesis of Silver Nanoparticles from Bacillus Brevis (NCIM 2533) and Their Antibacterial Activity against Pathogenic Bacteria. Microb. Pathogenesis 116, 221–226. doi:10.1016/j.micpath.2018.01.038
Saravanan, M., Gopinath, V., Chaurasia, M. K., Syed, A., Ameen, F., and Purushothaman, N. (2018b). Green Synthesis of Anisotropic Zinc Oxide Nanoparticles with Antibacterial and Cytofriendly Properties. Microb. Pathogenesis 115, 57–63. doi:10.1016/j.micpath.2017.12.039
Sathishkumar, M., Sneha, K., and Yun, Y.-S. (2010). Immobilization of Silver Nanoparticles Synthesized Using Curcuma Longa Tuber Powder and Extract on Cotton Cloth for Bactericidal Activity. Bioresour. Technology 101, 7958–7965. doi:10.1016/j.biortech.2010.05.051
Sathishkumar, M., Sneha, K., and Yun, Y. (2009). Palladium Nanocrystal Synthesis Using Curcuma Longa Tuber Extract. Int. J. Mater. Sci. 4, 1.
Sathishkumar, R. S., Sundaramanickam, A., Srinath, R., Ramesh, T., Saranya, K., Meena, M., et al. (2019). Green Synthesis of Silver Nanoparticles by Bloom Forming marine Microalgae Trichodesmium Erythraeum and its Applications in Antioxidant, Drug-Resistant Bacteria, and Cytotoxicity Activity. J. Saudi Chem. Soc. 23, 1180–1191. doi:10.1016/j.jscs.2019.07.008
Selim, A., Elhaig, M. M., Taha, S. A., and Nasr, E. A. (2018). Antibacterial Activity of Silver Nanoparticles against Field and Reference Strains of Mycobacterium tuberculosis, Mycobacterium Bovis and Multiple-Drug-Resistant Tuberculosis Strains. Rev. Sci. Tech. OIE 37, 823–830. doi:10.20506/rst.37.3.2888
Sen, I. K., Maity, K., and Islam, S. S. (2013). Green Synthesis of Gold Nanoparticles Using a Glucan of an Edible Mushroom and Study of Catalytic Activity. Carbohydr. Polym. 91, 518–528. doi:10.1016/j.carbpol.2012.08.058
Senapati, S., Syed, A., Moeez, S., Kumar, A., and Ahmad, A. (2012). Intracellular Synthesis of Gold Nanoparticles Using Alga Tetraselmis Kochinensis. Mater. Lett. 79, 116–118. doi:10.1016/j.matlet.2012.04.009
Senthilkumar, P., Surendran, L., Sudhagar, B., and Ranjith Santhosh Kumar, D. S. (2019). Facile green Synthesis of Gold Nanoparticles from marine Algae Gelidiella Acerosa and Evaluation of its Biological Potential. SN Appl. Sci. 1, 1. doi:10.1007/s42452-019-0284-z
Sereemaspu, A., Hongpitich, P., Rojanathan, R., Maneewatta, P., Ekgasit, S., and Warisnoich, W. (2008). Inhibition of Human Cytochrome P450 Enzymes by Metallic Nanoparticles: A Preliminary to Nanogenomics. Int. J. Pharmacol. 4, 492–495. doi:10.3923/ijp.2008.492.495
Shafey, A. M. E. (2020). Green Synthesis of Metal and Metal Oxide Nanoparticles from Plant Leaf Extracts and Their Applications: A Review. Green. Process. Synth. 9, 304–339. doi:10.1515/gps-2020-0031
Shakibaie, M., Forootanfar, H., Mollazadeh-Moghaddam, K., Bagherzadeh, Z., Nafissi-Varcheh, N., Shahverdi, A. R., et al. (2010). Green Synthesis of Gold Nanoparticles by the marine microalgaTetraselmis Suecica. Biotechnol. Appl. Biochem. 57, 71–75. doi:10.1042/ba20100196
Shankar, S. S., Ahmad, A., and Sastry, M. (2003). Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 19, 1627–1631. doi:10.1021/bp034070w
Shankar, S. S., Rai, A., Ahmad, A., and Sastry, M. (2004). Rapid Synthesis of Au, Ag, and Bimetallic Au Core-Ag Shell Nanoparticles Using Neem (Azadirachta indica) Leaf Broth. J. Colloid Interf. Sci. 275, 496–502. doi:10.1016/j.jcis.2004.03.003
Sharma, B., Purkayastha, D. D., Hazra, S., Gogoi, L., Bhattacharjee, C. R., Ghosh, N. N., et al. (2014a). Biosynthesis of Gold Nanoparticles Using a Freshwater green Alga, Prasiola Crispa. Mater. Lett. 116, 94–97. doi:10.1016/j.matlet.2013.10.107
Sharma, B., Purkayastha, D. D., Hazra, S., Thajamanbi, M., Bhattacharjee, C. R., Ghosh, N. N., et al. (2014b). Biosynthesis of Fluorescent Gold Nanoparticles Using an Edible Freshwater Red Alga, Lemanea Fluviatilis (L.) C.Ag. And Antioxidant Activity of Biomatrix Loaded Nanoparticles. Bioproc. Biosyst. Eng. 37, 2559–2565. doi:10.1007/s00449-014-1233-2
Sharma, D., Rajput, J., Kaith, B. S., Kaur, M., and Sharma, S. (2010). “Synthesis of ZnO Nanoparticles and Study of Their Antibacterial and Antifungal Properties,” in Thin Solid Films, 519, 1224–1229. doi:10.1016/j.tsf.2010.08.073Thin Solid Films
Sharma, T. S. K., Selvakumar, K., Hwa, K. Y., Sami, P., and Kumaresan, M. (2019). Biogenic Fabrication of Gold Nanoparticles Using Camellia Japonica L. Leaf Extract and its Biological Evaluation. J. Mater. Res. Technology 8, 1412–1418. doi:10.1016/j.jmrt.2018.10.006
Shivaji, S., Madhu, S., and Singh, S. (2011). Extracellular Synthesis of Antibacterial Silver Nanoparticles Using Psychrophilic Bacteria. Process Biochem. 46, 1800–1807. doi:10.1016/j.procbio.2011.06.008
Siddiqi, K. S., and Husen, A. (2016). Fabrication of Metal Nanoparticles from Fungi and Metal Salts: Scope and Application. Nanoscale Res. Lett. 11, 1. doi:10.1186/s11671-016-1311-2
Singaravelu, G., Arockiamary, J. S., Kumar, V. G., and Govindaraju, K. (2007). A Novel Extracellular Synthesis of Monodisperse Gold Nanoparticles Using marine Alga, Sargassum Wightii Greville. Colloids Surf. B: Biointerfaces 57, 97–101. doi:10.1016/j.colsurfb.2007.01.010
Singh, A., Jain, D., Upadhyay, M. K., Khandelwal, N., and Verma, H. N. (2010). Green Synthesis of Silver Nanoparticles Using Argemone Mexicana Leaf Extract and Evaluation of Their Antimicrobial Activities. Dig. J. Nanomater. Biostructures 5, 483.
Singh, M., Kalaivani, R., Manikandan, S., Sangeetha, N., and Kumaraguru, A. K. (2013). Facile green Synthesis of Variable Metallic Gold Nanoparticle Using Padina Gymnospora, a Brown marine Macroalga. Appl. Nanosci. 3, 145–151. doi:10.1007/s13204-012-0115-7
Singh, P., Kim, Y.-J., Zhang, D., and Yang, D.-C. (2016d). Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 34, 588–599. doi:10.1016/j.tibtech.2016.02.006
Singh, P., Kim, Y. J., Wang, C., Mathiyalagan, R., El-Agamy Farh, M., and Yang, D. C. (2016a). Biogenic Silver and Gold Nanoparticles Synthesized Using Red Ginseng Root Extract, and Their Applications. Artif. Cell Nanomedicine, Biotechnol. 44, 1–6. doi:10.3109/21691401.2015.1008514
Singh, P., Kim, Y. J., Wang, C., Mathiyalagan, R., and Yang, D. C. (2016b). The Development of a green Approach for the Biosynthesis of Silver and Gold Nanoparticles by usingPanax Ginsengroot Extract, and Their Biological Applications. Artif. Cell Nanomedicine, Biotechnol. 44, 1–8. doi:10.3109/21691401.2015.1011809
Singh, P., Kim, Y. J., and Yang, D. C. (2016c). A Strategic Approach for Rapid Synthesis of Gold and Silver Nanoparticles byPanax Ginsengleaves. Artif. Cell Nanomedicine, Biotechnol. 44, 1949–1957. doi:10.3109/21691401.2015.1115410
Singh, R., Vora, J., Nadhe, S. B., Wadhwani, S. A., Shedbalkar, U. U., and Chopade, B. A. (2018). Antibacterial Activities of Bacteriagenic Silver Nanoparticles against Nosocomial Acinetobacter Baumannii. J. Nanosci. Nanotechnol. 18, 3806–3815. doi:10.1166/jnn.2018.15013
Singla, R. K., Sai, C. S., Chopra, H., Behzad, S., Bansal, H., Goyal, R., et al. (2021). Natural Products for the Management of Castration-Resistant Prostate Cancer: Special Focus on Nanoparticles Based Studies. Front. Cell Dev. Biol. 9, 745177. doi:10.3389/FCELL.2021.745177
Sinha, S. N., Paul, D., Halder, N., Sengupta, D., and Patra, S. K. (2015). Green Synthesis of Silver Nanoparticles Using Fresh Water green Alga Pithophora Oedogonia (Mont.) Wittrock and Evaluation of Their Antibacterial Activity. Appl. Nanosci. 5, 703–709. doi:10.1007/s13204-014-0366-6
Sintubin, L., De Windt, W., Dick, J., Mast, J., Van Der Ha, D., Verstraete, W., et al. (2009). Lactic Acid Bacteria as Reducing and Capping Agent for the Fast and Efficient Production of Silver Nanoparticles. Appl. Microbiol. Biotechnol. 84, 741–749. doi:10.1007/s00253-009-2032-6
Sisubalan, N., Ramkumar, V. S., Pugazhendhi, A., Karthikeyan, C., Indira, K., Gopinath, K., et al. (2018). ROS-mediated Cytotoxic Activity of ZnO and CeO2 Nanoparticles Synthesized Using the Rubia Cordifolia L. Leaf Extract on MG-63 Human Osteosarcoma Cell Lines. Environ. Sci. Pollut. Res. 25, 10482–10492. doi:10.1007/s11356-017-0003-5
Slawson, R. M., Van Dyke, M. I., Lee, H., and Trevors, J. T. (1992). Germanium and Silver Resistance, Accumulation, and Toxicity in Microorganisms. Plasmid 27, 72–79. doi:10.1016/0147-619X(92)90008-X
Sneha, K., Sathishkumar, M., Mao, J., Kwak, I. S., and Yun, Y.-S. (2010). Corynebacterium Glutamicum-Mediated Crystallization of Silver Ions through Sorption and Reduction Processes. Chem. Eng. J. 162, 989–996. doi:10.1016/j.cej.2010.07.006
Somasundaram, C. K., Atchudan, R., Edison, T. N. J. I., Perumal, S., Vinodh, R., Sundramoorthy, A. K., et al. (2021). Sustainable Synthesis of Silver Nanoparticles Using Marine Algae for Catalytic Degradation of Methylene Blue. Catalysts 11, 1377. doi:10.3390/CATAL11111377
Sreelakshmi, B., Induja, S., Adarsh, P. P., Rahul, H. L., Arya, S. M., Aswana, S., et al. (2021). Drought Stress Amelioration in Plants Using green Synthesised Iron Oxide Nanoparticles. Mater. Today Proc. 41, 723–727. doi:10.1016/j.matpr.2020.05.801
Stalin Dhas, T., Ganesh Kumar, V., Karthick, V., Jini Angel, K., and Govindaraju, K. (2014). Facile Synthesis of Silver Chloride Nanoparticles Using marine Alga and its Antibacterial Efficacy. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 120, 416–420. doi:10.1016/j.saa.2013.10.044
Stalin Dhas, T., Ganesh Kumar, V., Stanley Abraham, L., Karthick, V., and Govindaraju, K. (2012). Sargassum Myriocystum Mediated Biosynthesis of Gold Nanoparticles. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 99, 97–101. doi:10.1016/j.saa.2012.09.024
Sudheer, S., Alzorqi, I., Manickam, S., and Ali, A. (2019). “Bioactive Compounds of the Wonder Medicinal Mushroom "Ganoderma Lucidum",” in Reference Series in Phytochemistry, 1863–1893. doi:10.1007/978-3-319-78030-6_45
Sukirtha, R., Priyanka, K. M., Antony, J. J., Kamalakkannan, S., Thangam, R., Gunasekaran, P., et al. (2012). Cytotoxic Effect of Green Synthesized Silver Nanoparticles Using Melia Azedarach against In Vitro HeLa Cell Lines and Lymphoma Mice Model. Process Biochem. 47, 273–279. doi:10.1016/j.procbio.2011.11.003
Sun, L., Wang, G., Zhang, C., Jin, Q., and Song, Y. (2021). On the Rheological Properties of Multi-Walled Carbon Nano-Polyvinylpyrrolidone/silicon-Based Shear Thickening Fluid. Nanotechnol. Rev. 10, 1339–1348. doi:10.1515/ntrev-2021-0087
Sunderam, V., Thiyagarajan, D., Lawrence, A. V., Mohammed, S. S. S., and Selvaraj, A. (2019). In-vitro Antimicrobial and Anticancer Properties of green Synthesized Gold Nanoparticles Using Anacardium Occidentale Leaves Extract. Saudi J. Biol. Sci. 26, 455–459. doi:10.1016/j.sjbs.2018.12.001
Supraja, N., Prasad, T. N. V. K. V., Krishna, T. G., and David, E. (2016). Synthesis, Characterization, and Evaluation of the Antimicrobial Efficacy of Boswellia Ovalifoliolata Stem Bark-Extract-Mediated Zinc Oxide Nanoparticles. Appl. Nanosci. 6, 581–590. doi:10.1007/s13204-015-0472-0
Surendra, T. V., Roopan, S. M., Al-Dhabi, N. A., Arasu, M. V., Sarkar, G., and Suthindhiran, K. (2016). Vegetable Peel Waste for the Production of ZnO Nanoparticles and its Toxicological Efficiency, Antifungal, Hemolytic, and Antibacterial Activities. Nanoscale Res. Lett. 11, 546. doi:10.1186/s11671-016-1750-9
Suriyakala, G., Sathiyaraj, S., Babujanarthanam, R., Alarjani, K. M., Hussein, D. S., Rasheed, R. A., et al. (2022). Green Synthesis of Gold Nanoparticles Using Jatropha Integerrima Jacq. Flower Extract and Their Antibacterial Activity. J. King Saud Univ. - Sci. 34, 101830. doi:10.1016/J.JKSUS.2022.101830
Taglietti, A., Diaz Fernandez, Y. A., Amato, E., Cucca, L., Dacarro, G., Grisoli, P., et al. (2012). Antibacterial Activity of Glutathione-Coated Silver Nanoparticles against Gram Positive and Gram Negative Bacteria. Langmuir 28, 8140–8148. doi:10.1021/la3003838
Vágó, A., Szakacs, G., Sáfrán, G., Horvath, R., Pécz, B., and Lagzi, I. (2016). One-step green Synthesis of Gold Nanoparticles by Mesophilic Filamentous Fungi. Chem. Phys. Lett. 645, 1–4. doi:10.1016/j.cplett.2015.12.019
Vanaja, M., and Annadurai, G. (2013). Coleus Aromaticus Leaf Extract Mediated Synthesis of Silver Nanoparticles and its Bactericidal Activity. Appl. Nanosci. 3, 217–223. doi:10.1007/s13204-012-0121-9
Varma, R. S. (2012). Greener Approach to Nanomaterials and Their Sustainable Applications. Curr. Opin. Chem. Eng. 1, 123–128. doi:10.1016/j.coche.2011.12.002
Veerasamy, R., Xin, T. Z., Gunasagaran, S., Xiang, T. F. W., Yang, E. F. C., Jeyakumar, N., et al. (2011). Biosynthesis of Silver Nanoparticles Using Mangosteen Leaf Extract and Evaluation of Their Antimicrobial Activities. J. Saudi Chem. Soc. 15, 113–120. doi:10.1016/j.jscs.2010.06.004
Veisi, H., Azizi, S., and Mohammadi, P. (2018). Green Synthesis of the Silver Nanoparticles Mediated by Thymbra Spicata Extract and its Application as a Heterogeneous and Recyclable Nanocatalyst for Catalytic Reduction of a Variety of Dyes in Water. J. Clean. Prod. 170, 1536–1543. doi:10.1016/j.jclepro.2017.09.265
Velmurugan, P., Park, J.-H., Lee, S.-M., Jang, J.-S., Lee, K.-J., Han, S.-S., et al. (2015). Synthesis and Characterization of Nanosilver with Antibacterial Properties Using Pinus Densiflora Young Cone Extract. J. Photochem. Photobiol. B: Biol. 147, 63–68. doi:10.1016/j.jphotobiol.2015.03.008
Venil, C. K., Sathishkumar, P., Malathi, M., Usha, R., Jayakumar, R., Yusoff, A. R. M., et al. (2016). Synthesis of Flexirubin-Mediated Silver Nanoparticles Using Chryseobacterium Artocarpi CECT 8497 and Investigation of its Anticancer Activity. Mater. Sci. Eng. C 59, 228–234. doi:10.1016/j.msec.2015.10.019
Venkatesan, J., Manivasagan, P., Kim, S.-K., Kirthi, A. V., Marimuthu, S., and Rahuman, A. A. (2014). Marine Algae-Mediated Synthesis of Gold Nanoparticles Using a Novel Ecklonia Cava. Bioproc. Biosyst. Eng. 37, 1591–1597. doi:10.1007/s00449-014-1131-7
Venkateswaran, K., Moser, D. P., Dollhopf, M. E., Lies, D. P., Saffarini, D. A., MacGregor, B. J., et al. (1999). Polyphasic Taxonomy of the Genus Shewanella and Description of Shewanella Oneidensis Sp. Nov. Int. J. Syst. Bacteriol. 49, 705–724. doi:10.1099/00207713-49-2-705
Venkatpurwar, V., and Pokharkar, V. (2011). Green Synthesis of Silver Nanoparticles Using marine Polysaccharide: Study of In-Vitro Antibacterial Activity. Mater. Lett. 65, 999–1002. doi:10.1016/j.matlet.2010.12.057
Verma, A., and Mehata, M. S. (2016). Controllable Synthesis of Silver Nanoparticles Using Neem Leaves and Their Antimicrobial Activity. J. Radiat. Res. Appl. Sci. 9, 109–115. doi:10.1016/j.jrras.2015.11.001
Vicas, S. I., Cavalu, S., Laslo, V., Tocai, M., Costea, T. O., and Moldovan, L. (2019). Growth, Photosynthetic Pigments, Phenolic, Glucosinolates Content and Antioxidant Capacity of Broccoli Sprouts in Response to Nanoselenium Particles Supply. Not Bot. Horti Agrobo 47, 1. doi:10.15835/nbha47311490
Vijayabharathi, R., Sathya, A., and Gopalakrishnan, S. (2018). Extracellular Biosynthesis of Silver Nanoparticles Using Streptomyces Griseoplanus SAI-25 and its Antifungal Activity against Macrophomina Phaseolina , the Charcoal Rot Pathogen of Sorghum. Biocatal. Agric. Biotechnol. 14, 166–171. doi:10.1016/j.bcab.2018.03.006
Vijayakumar, S., Vaseeharan, B., Malaikozhundan, B., Gobi, N., Ravichandran, S., Karthi, S., et al. (2017). A Novel Antimicrobial Therapy for the Control of Aeromonas Hydrophila Infection in Aquaculture Using marine Polysaccharide Coated Gold Nanoparticle. Microb. Pathogenesis 110, 140–151. doi:10.1016/j.micpath.2017.06.029
Vinay Gopal, J., Thenmozhi, M., Kannabiran, K., Rajakumar, G., Velayutham, K., and Rahuman, A. A. (2013). Actinobacteria Mediated Synthesis of Gold Nanoparticles Using Streptomyces Sp. VITDDK3 and its Antifungal Activity. Mater. Lett. 93, 360–362. doi:10.1016/j.matlet.2012.11.125
Vivek, M., Kumar, P. S., Steffi, S., and Sudha, S. (2011). Biogenic Silver Nanoparticles by Gelidiella Acerosa Extract and Their Antifungal Effects. Avicenna J. Med. Biotechnol. 3, 143–148.
Wang, D., Xue, B., Wang, L., Zhang, Y., Liu, L., and Zhou, Y. (2021). Fungus-mediated green Synthesis of Nano-Silver Using Aspergillus sydowii and its Antifungal/antiproliferative Activities. Sci. Rep. 11, 1. doi:10.1038/s41598-021-89854-5
Yehia, R. S., and Al-Sheikh, H. (2014). Biosynthesis and Characterization of Silver Nanoparticles Produced by Pleurotus Ostreatus and Their Anticandidal and Anticancer Activities. World J. Microbiol. Biotechnol. 30, 2797–2803. doi:10.1007/s11274-014-1703-3
Yılmaz Öztürk, B., Yenice Gürsu, B., and Dağ, İ. (2020). Antibiofilm and Antimicrobial Activities of green Synthesized Silver Nanoparticles Using marine Red Algae Gelidium Corneum. Process Biochem. 89, 208–219. doi:10.1016/j.procbio.2019.10.027
Zare, E., Pourseyedi, S., Khatami, M., and Darezereshki, E. (2017). Simple Biosynthesis of Zinc Oxide Nanoparticles Using Nature's Source, and It's In Vitro Bio-Activity. J. Mol. Struct. 1146, 96–103. doi:10.1016/j.molstruc.2017.05.118
Zhang, H., Li, Q., Lu, Y., Sun, D., Lin, X., Deng, X., et al. (2005). Biosorption and Bioreduction of Diamine Silver Complex byCorynebacterium. J. Chem. Technol. Biotechnol. 80, 285–290. doi:10.1002/jctb.1191
Zhao, J.-S., Manno, D., Beaulieu, C., Paquet, L., and Hawari, J. (2005). Shewanella Sediminis Sp. nov., a Novel Na+-Requiring and Hexahydro-1,3,5-Trinitro-1,3,5-Triazine-Degrading Bacterium from marine Sediment. Int. J. Syst. Evol. Microbiol. 55, 1511–1520. doi:10.1099/ijs.0.63604-0
Zhao, Y., Ye, C., Liu, W., Chen, R., and Jiang, X. (2014). Tuning the Composition of AuPt Bimetallic Nanoparticles for Antibacterial Application. Angew. Chem. Int. Ed. 53, 8127–8131. doi:10.1002/anie.201401035
Zhong-Yu, L., Fu, J. K., Jin-Kun, F., Jian-Ming, W., Yue-Ying, L., and Hu, C. (2001). Preliminary Study on the Mechanism of Nonenzymatic Bioreduction of Precious Metal Ions. Acta Phys. - Chim. Sin. 17, 477–480. doi:10.3866/pku.whxb20010520
Zhu, B., Xie, N., Yue, L., Wang, K., Bani-Fwaz, M. Z., Hussein Osman, H.-E., et al. (2022). Formulation and Characterization of a Novel Anti-human Endometrial Cancer Supplement by Gold Nanoparticles green-synthesized Using Spinacia Oleracea L. Leaf Aqueous Extract. Arabian J. Chem. 15, 103576. doi:10.1016/J.ARABJC.2021.103576
Keywords: nanoparticle, green nanotechnology, preparation, synthesis, application
Citation: Chopra H, Bibi S, Singh I, Hasan MM, Khan MS, Yousafi Q, Baig AA, Rahman MM, Islam F, Emran TB and Cavalu S (2022) Green Metallic Nanoparticles: Biosynthesis to Applications. Front. Bioeng. Biotechnol. 10:874742. doi: 10.3389/fbioe.2022.874742
Received: 12 February 2022; Accepted: 22 March 2022;
Published: 06 April 2022.
Edited by:
Md. Amdadul Huq, Chung-Ang University, South KoreaReviewed by:
Siddhartha Pati, Natnov Bioscience Pvt Ltd., IndiaMahmoud Nasrollahzadeh, University of Qom, Iran
Copyright © 2022 Chopra, Bibi, Singh, Hasan, Khan, Yousafi, Baig, Rahman, Islam, Emran and Cavalu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shabana Bibi, shabana_bibi@ynu.edu.cn; Talha Bin Emran, talhabmb@bgctub.ac.bd; Simona Cavalu, simona.cavalu@gmail.com
 Hitesh Chopra
Hitesh Chopra Shabana Bibi
Shabana Bibi Inderbir Singh
Inderbir Singh Mohammad Mehedi Hasan
Mohammad Mehedi Hasan Muhammad Saad Khan
Muhammad Saad Khan Qudsia Yousafi
Qudsia Yousafi Atif Amin Baig6
Atif Amin Baig6 Md. Mominur Rahman
Md. Mominur Rahman Fahadul Islam
Fahadul Islam Talha Bin Emran
Talha Bin Emran Simona Cavalu
Simona Cavalu