
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Bioeng. Biotechnol. , 13 May 2022
Sec. Biomaterials
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.853436
This article is part of the Research Topic Recent Advancements in the Dental Biomaterials Applied in Various Diagnostic, Restorative, Regenerative and Therapeutic Procedures View all 13 articles
Enamel demineralization, as a type of frequently-occurring dental problem that affects both the health and aesthetics of patients, is a concern for both dental professionals and patients. The main chemical composition of the enamel, hydroxyapatite, is easy to be dissolved under acid attack, resulting in the occurrence of enamel demineralization. Among agents for the preventing or treatment of enamel demineralization, amorphous calcium phosphate (ACP) has gradually become a focus of research. Based on the nonclassical crystallization theory, ACP can induce the formation of enamel-like hydroxyapatite and thereby achieve enamel remineralization. However, ACP has poor stability and tends to turn into hydroxyapatite in an aqueous solution resulting in the loss of remineralization ability. Therefore, ACP needs to be stabilized in an amorphous state before application. Herein, ACP stabilizers, including amelogenin and its analogs, casein phosphopeptides, polymers like chitosan derivatives, carboxymethylated PAMAM and polyelectrolytes, together with their mechanisms for stabilizing ACP are briefly reviewed. Scientific evidence supporting the remineralization ability of these ACP agents are introduced. Limitations of existing research and further prospects of ACP agents for clinical translation are also discussed.
Enamel demineralization is one of the most common dental problems which could appear as white spot lesions (WSLs) in the early stage and even progress into cavities if effective interventions are not taken in time (Julien et al., 2013). In the normal oral environment, hydroxyapatite on the enamel surface contacts saliva and maintains the balance of dissolution and redeposition (Sollböhmer et al., 1995; Featherstone, 2004), hydroxyapatite could be dissolved into calcium and phosphorus ions while calcium and phosphorus ions in saliva could crystallize directionally and orderly, forming the enamel-like hydroxyapatite structure on the surface of the enamel (Dorozhkin, 1997). When the oral hygiene condition is poor, plaque biofilms form and adhere onto the enamel surface decomposing sugars, producing organic acids, and resulting in an acidic pH environment around the enamel. Under this circumstance, the dissolution-redeposition balance of hydroxyapatite is broken. The dissolution of hydroxyapatite occurs faster than the deposition of calcium and phosphorus ions, which eventually leads to the occurrence of enamel demineralization.
Based on the etiology of enamel demineralization, the strategies to prevent or treat enamel demineralization include: 1) Using antibacterial agents such as mouthwash or toothpaste containing antibacterial drugs (Hefti and Huber, 1987; Afennich et al., 2011; Hossainian et al., 2011; Rösing et al., 2017; Ahmed et al., 2019; Bijle et al., 2019; Guven et al., 2019; Karadağlıoğlu et al., 2019; Shang et al., 2020) which could inhibit the accumulation and adhesion of cariogenic bacteria on the enamel surface to reduce the acid production from plaque biofilms; 2) Using fluorinated agents such as fluoride mouthwash (Chow et al., 2000; Songsiripradubboon et al., 2014; Larsson et al., 2020) and fluoride varnish (Gontijo et al., 2007; Marinho, 2009; Perrini et al., 2016), which could not only inhibit cariogenic bacteria but release fluorine, co-crystallize with calcium and phosphorus ions to form fluorapatite on the enamel surface (Margolis and Murphy, 1986; Zandim-Barcelos et al., 2011).
In addition to the above strategies, the enamel biomimetic remineralization strategy, which bases on the natural enamel crystallization process (Cölfen and Mann, 2003), is being studied extensively due to its biomimetic mineralization capability (Chen et al., 2015; Wang et al., 2017). According to the nonclassical crystallization theory, the crystallization processes of natural enamel could be interpreted as the following steps: 1) Calcium and phosphorus ions aggregating together to form amorphous calcium phosphate (ACP); 2) Amelogenin stabilizing ACP into clusters; 3) ACP then directionally arranging to form bundles of hydroxyapatite, then gradually forming enamel crystal, and finally forming enamel prism (Beniash et al., 2009; Yang et al., 2010; Kwak et al., 2016). To mimic the crystallization process of natural enamel and to achieve remineralization of demineralized enamel, ACP needs to be stabilized and then crystallizes directionally and orderly to form the enamel-like hydroxyapatite. In this review, we mainly focused on how different agents stabilize ACP and their remineralization effects on enamel demineralization.
Amelogenin (Amel) plays an important role in the formation of natural enamel (Wright et al., 2011; Moradian-Oldak, 2012; Ruan and Moradian-Oldak, 2015). Amelogenin could interact with calcium and phosphorus ions through the tyrosine enrichment segment on its N-terminal and stabilize calcium and phosphorus ions to an amorphous state (Figure 1A). The C-terminal of Amel could guide ACP to crystallize into hydroxyapatite directionally (Tsiourvas et al., 2015). There are studies using chitosan to load amelogenin and form the chitosan-amelogenin gel (CS-Amel gel) and using this gel system for reconstruction of demineralized enamel. The CS-Amel gel could stabilize calcium and phosphorus ions into ACP, guide ACP to form the enamel-like crystals which bind closely with natural enamel crystals (Ruan et al., 2013; Ruan et al., 2014). In addition to the direct application of amelogenin, there are studies focused on the remineralization effect of amelogenin analogs. Zhong et al., 2021 self-assembled the N-terminal tyrosine segment of amelogenin to form leucine-rich amelogenin peptide (LRAP) and evaluated the stabilizing and directional guiding abilities of LRAP to calcium and phosphorus ions in mineralizing solutions. LRAP could stabilize calcium and phosphorus ions into ACP effectively and guide ACP to grow along its C-axis into bundles of hydroxyapatite crystals. Wang combined a phase conversion lyase (PTL) which mimics the function of the N-terminal of amelogenin with a synthetic peptide chain which has the function of the C-terminal of amelogenin to form amyloid amelogenin analog (PTL/C-AMG) (Wang Y et al., 2020). The PTL/C-AMG could combine calcium and phosphorus ions to form hydroxyapatite and promote the extension growth of hydroxyapatite crystals on the surface of natural enamel and eventually forms a highly ordered hydroxyapatite structure with mechanical properties similar to that of natural enamel. Lv and colleagues synthesized a short-chain polypeptide (QP5) based on the amino sequence of amelogenin and proved the stabilizing ability of QP5 to calcium and phosphorus ions. They verified the remineralization ability of QP5 to initial enamel demineralization in an in vitro enamel demineralization model and further confirmed its remineralization ability and potential for clinical transformation in a rat caries model (Lv et al., 2015; Han et al., 2017).
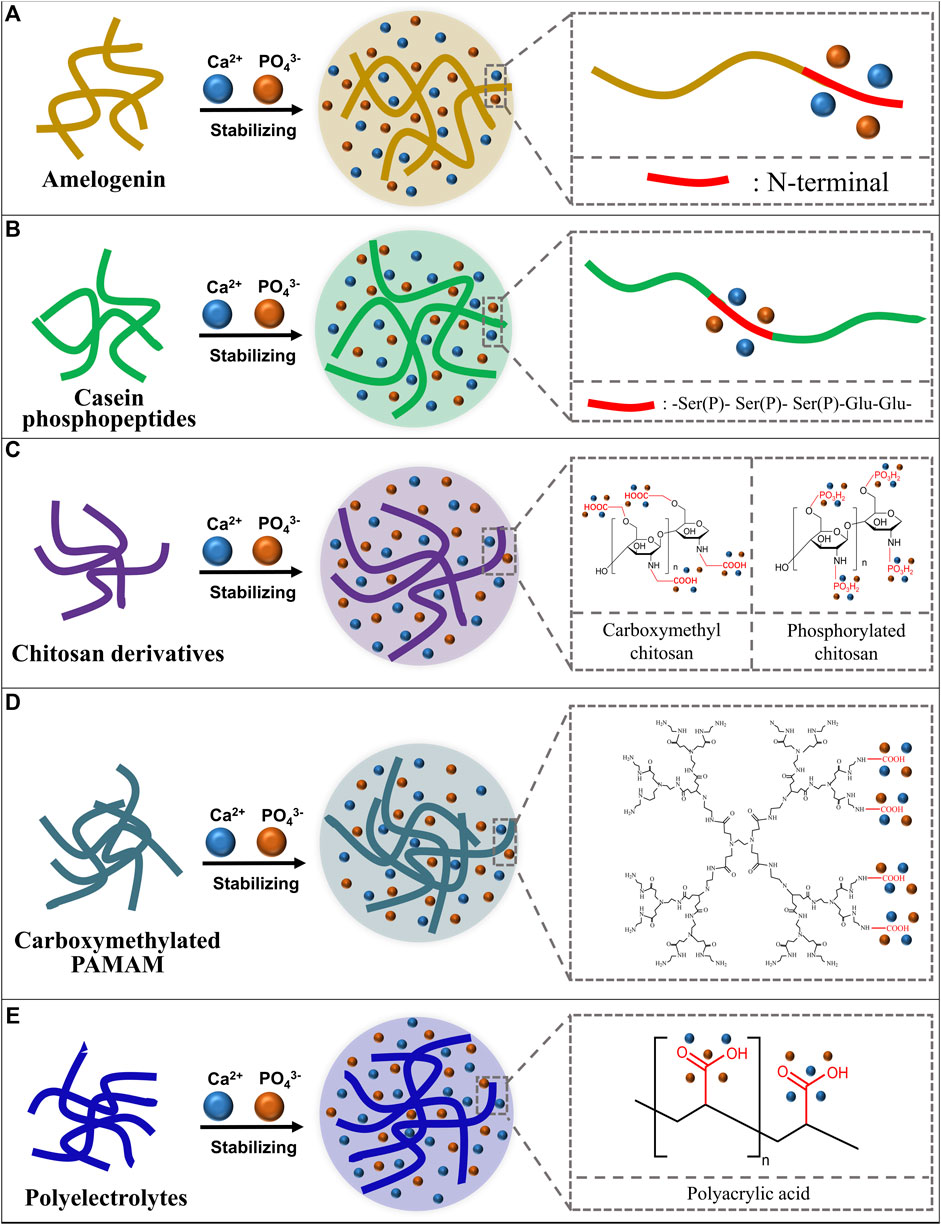
FIGURE 1. Schematic diagram of ACP stabilizers and how they stabilize the ACP. (A) Amelogenin stabilizes calcium and phosphorus ions with its N-terminal; (B) Casein phosphopeptides stabilize calcium and phosphorus ions with the -Ser(P)- Ser(P)- Ser(P)-Glu-Glu- sequence; (C) Chitosan derivatives stabilize calcium and phosphorus ions with functional groups; (D) Carboxymethylated poly-amidoamine (PAMAM) stabilizes calcium and phosphorus ions with carboxyl groups; (E) Polyelectrolytes stabilize calcium and phosphorus ions with functional groups.
Casein phosphopeptides (CPP) are casein extracts from milk which could markedly increase the apparent solubility of calcium phosphate ions by forming ACP (Reeves and Latour, 1958). Researchers found that the main active sequence of CPP, the phosphoserine - glutamate cluster (-Ser(P)- Ser(P)- Ser(P)-Glu-Glu-), could stabilize calcium and phosphate ions and form the CPP stabilized ACP complex (CPP-ACP) (Adamson and Reynolds, 1996) to avoid the spontaneously crystallizing, phase conversing and precipitating of calcium and phosphorus ion (Shen et al., 2001) (Figure 1B). Reynolds soaked artificially demineralized enamel in CPP-ACP solution and found that CPP-ACP could remineralize the subsurface demineralization of enamel effectively. The mechanism may be that CPP can maintain a high concentration of calcium and phosphorus ions in the solution to infiltrate into the subsurface lesion area to achieve efficient enamel remineralization (Reynolds, 1997). The team further validated the preventive effect of CPP-ACP on enamel demineralization in a rat caries model (Reynolds et al., 1995). With the U.S. Food and Drug Administration and other regulatory agencies confirming the biosafety of CPP-ACP (Cochrane et al., 2010), CPP-ACP is added into oral health care products such as Tooth Mousse (GC, Tokyo, Japan) (Rees et al., 2007) and Tooth Mousse Plus (CPP-ACPF, GC, Tokyo, Japan) (Hamba et al., 2011; Bataineh et al., 2017; Olgen et al., 2021). These agents have been gradually used in clinical practice and have been studied in a number of clinical trials (Sitthisettapong et al., 2015; Güçlü et al., 2016; Munjal et al., 2016; Thierens et al., 2019). However, the remineralization ability of CPP-ACP and CPP-ACPF for WSLs remains unknown. Researchers suggested that CPP-ACP and CPP-ACPF may have the ability to prevent and treat WSLs, but their effects are not significantly greater than using fluoride agent alone (Pithon et al., 2019; Wang D et al., 2020). In addition, casein related allergy in certain populations also limits the clinical use of CPP-ACP and CPP-ACPF.
In addition to the aforementioned amelogenin and its analogs and CPP, some kinds of polymers can also stabilize calcium and phosphate ions, including chitosan derivatives, poly-amidoamine and polyelectrolytes.
Chitosan derivatives, such as carboxymethyl chitosan (CMC) and phosphorylated chitosan (Pchi) could bind calcium ions through chelation reaction of carboxyl groups and calcium ions and then bind phosphate ions to form ACP (Figure 1C). The recrystallization of demineralization enamel is realized by the ordered crystallization of ACP to form enamel-like hydroxyapatite crystals (Zhang et al., 2014; Zhang et al., 2018). Zhu combined carboxymethyl chitosan (CMC) and lysozyme (LYZ) to stabilize ACP and formed the CMC/LYZ-ACP nano-gel, which can regenerate prism-like remineralized enamel layer on the surface of eroded enamel to realize the remineralization (Zhu et al., 2021). Song successively added CaCl2 and K2HPO4 into Pchi solution to construct the Pchi-ACP nano-complex. X-ray diffraction and selective electron diffraction results confirmed the amorphous state of the nano-complex. And the results of scanning electron microscopy and micro-CT proved that the Pchi-ACP nano-complex could realize the remineralization of demineralized enamel (Song et al., 2021).
Poly-amidoamine (PAMAM) was first synthesized by Tomalia in the 1980s (Tomalia et al., 1985). PAMAM contains a large number of amide groups that have the similar function to peptide bonds so that PAMAM could simulate functions of a variety of proteins and peptides (Svenson and Tomalia, 2012). PAMAM can have mineralization property through the modification of carboxyl groups. The carboxyl-modified PAMAM (PAMAM-COOH) could combine calcium ions through carboxyl groups and further attract phosphate ions to stabilize calcium and phosphate ions into ACP (Khopade et al., 2002; Zhou et al., 2007; Zhou et al., 2013) (Figure 1D). ACP could form enamel-like hydroxyapatite orderly on the surface of demineralized enamel through the crystallization guidance of PAMAM-COOH (Chen et al., 2013). Another study found that PAMAM-COOH can induce calcium and phosphorus ions to grow and crystallize along the z-axis on the surface of demineralized enamel, and the microhardness of the remineralized enamel is comparable to that of the natural enamel (Chen M et al., 2014).
Polyelectrolytes are a class of polymorphs with ionizable units, which could ionize into charged polymorphs and counter-ions with opposite charge in aqueous solution (Koetz and Kosmella, 2007) such as polyacrylic acid (PAA), polyallylamine (PAH), polyaspartic acid (PASP), et al. PAA has rich carboxyl groups to combine with calcium ions to form the -COO-/Ca2+ structure (Huang et al., 2008), so that PAA can stabilize ACP (Gower, 2008; Dey et al., 2010) (Figure 1E). Qi added calcium and phosphorus ions into the PAA solution to construct the PAA-ACP complex and verified the stability of the PAA-ACP complex by solution turbidity analysis and dynamic light scattering. Scanning electron microscopy, transmission electron microscopy, infrared spectroscopy and X-ray diffraction analyses proved the remineralization ability of PAA-ACP (Qi et al., 2018). Our group used PAA to stabilize amorphous calcium phosphate, and then loaded PAA-ACP with aminoated mesoporous silicon nanoparticle (aMSN) to form the PAA-ACP@aMSN delivery system. The PAA-ACP@aMSN was proved to have the ability to promote enamel remineralization and surface microhardness analysis and X-ray diffraction analysis showed that the remineralization layer induced by PAA-ACP@aMSN had comparable mechanical property and crystal texture to natural enamel (Hua et al., 2020). PAA-ACP could also act as a dental adhesive filler to endow adhesives with enamel remineralization ability (Wang et al., 2018). Other polyelectrolytes like polyallylamine (Niu et al., 2017; Yang et al., 2017), polyaspartic acid (Zhou, et al., 2021), polyglutamic acid (Sikirić, et al., 2009; Terauchi, et al., 2019) could alsostabilize calcium and phosphorus ions but the treatment or prevention effect of these polyelectrolyte-stabilized ACPs for enamel demineralization remains to be further investigated.
As a kind of amorphous substance, ACP is easy to spontaneously transform into apatite crystal in an aqueous solution from the thermodynamics point of view (Eanes et al., 1965; Chow et al., 1998). Therefore, in addition to the application of stabilizers to stabilize ACP in an amorphous state, another way to stabilize ACP is to store the prepared ACP in an anhydrous dry granular state to form ACP particles. Since the 1990s, ACP particle has been gradually used as a bioactive additive in the studies of tooth remineralization (Skrtic and Eanes, 1996). ACP particle could act as a bioactive filler of dental filling resin to endow the filling resin with the ability of continuous releasing of calcium and phosphorus ions to promote the formation of hydroxyapatite (Skrtic et al., 2004). However, the uncontrollable agglomeration of ACP particles in the resin affects the mechanical properties of resin such as bonding strength and bending strength, so that ACP particles are only suitable for materials with low requirements on mechanical properties, such as pit and fissure sealant (Skrtic et al., 2004; Dunn, 2007). In 2011, Xu synthesized nano ACP (NACP) by spray drying method for the first time and mixed it into dental resin as filler. The NACP modified dental resin could release calcium and phosphorus ions in an acidic environment, and the mechanical properties of the resin are even better than commercial dental resin materials (Xu et al., 2011). Since then, a large number of studies added NACP to dental materials such as orthodontic bonding resins, sealants, resin-modified glass ions and other materials, and verified their calcium and phosphorus ion release ability and enamel remineralization ability (Chen C et al., 2014; Ma et al., 2017; Liu et al., 2018; Xie et al., 2019; Gao et al., 2020; Ibrahim et al., 2020).
In addition to ACP agents, there are many other enamel remineralization agents such as fluorine containing agents, hydroxyapatite preparations and tricalcium phosphate. In vitro and in vivo studies have been conducted to compare the remineralization performance of ACP agents and other agents (Table 1). However, the conclusion varied among these studies. Some studies found that ACP agents have better remineralization effect than other agents, while others suggested that the remineralization effect of ACP agents is similar to or no better than other agents. Whether the ACP agents have better remineralization properties than other agents needs to be further investigated in the future research.
ACP agents have outstanding preventive and therapeutic capacity to enamel demineralization due to their ability to form the enamel-like hydroxyapatite on the surface of demineralized enamel (Kwak et al., 2016). However, Since ACP is easy to agglomerate and is unstable in an aqueous solution (Chow et al., 1998), the main challenge in applying the ACP for enamel remineralization is its stabilization. Many different materials that could stabilize calcium and phosphorus ions, including amelogenin and its analogs (Tsiourvas et al., 2015; Wang Y et al., 2020), casein phosphopeptides (Cross et al., 2005), polymers like chitosan derivatives (Zhu et al., 2021), carboxymethylated PAMAM (Chen et al., 2013) and polyelectrolytes (Hua et al., 2020), have been used in studies to stabilize calcium and phosphorus ions into ACP. Another strategy is to store the ACP in a water-free state so that ACP particles and NACP particles are formed (Betts et al., 1975; Xu et al., 2011). The remineralization abilities of these ACP agents have been confirmed in previous studies. However, except for CPP-ACP and CPP-ACPF which has been commercialized (Reise et al., 2021), most of the other ACP agents are still at in vitro experimental stage. Tt is still uncertain whether these ACP agents can achieve the remineralization of demineralized enamel in vivo. In addition, most of the studies evaluated the remineralization ability of ACP agents by measuring the hardness recovery of demineralized enamel (Gokkaya et al., 2020), observing the mineral deposition on demineralized enamel (Hua et al., 2020), or measuring the lesion depth (Soares-Yoshikawa et al., 2021). None of the above-mentioned evaluation methods can directly confirm whether ACP agents could form the enamel-like hydroxyapatite. The biomimetic remineralization ability of ACP agents needs further investigation. To further promote the translation of ACP agents into clinical application, basic studies with adequate evaluation methods as well as relevant in vivo studies are still needed. In addition, whether ACP agents have better remineralization effects compared to other agents remains to be further explored.
ACP complexes are in the amorphous state of the liquid phase (Chen et al., 2013; Niu et al., 2017; Qi et al., 2018; Song et al., 2021), and ACP particles (Skrtic et al., 2004; Xu et al., 2011) are solid powders. Neither the liquid nor the solid form is convenient for storage and direct application in the oral environment. Studies has been conducted to address the storage and application challenges of ACP agents:
1) Mouthwash. Studies used carriers like chitosan (Ruan et al., 2013) and carboxymethyl chitosan (Zhu et al., 2021) to load ACP agents and these delivery systems can be applied in the oral environment in the form of mouthwash.
2) Toothpaste and tooth desensitizer. Another form of application of the ACP agents is to make them into pastes. Our group used mesoporous silicon nanoparticles to load ACP agents to achieve the enrichment and storage of ACP and this delivery system can be applied as the filler of toothpaste (Hua et al., 2020). CPP-ACP agents can be used as desensitizers in the form of pastes (Pei et al., 2013; Chandavarkar and Ram, 2015; Yang et al., 2018).
3) Resin product. Particulate forms of ACP have been incorporated into resin products, like adhesives (Wang et al., 2018), pit and fissure sealants (Utneja et al., 2018), varnishes (Schemehorn et al., 2011) to achieve convenient applications that do not depend on patient compliance.
Mouthwash form of the ACP agent is convenient to use, but the relatively low concentration of ACP and its inability to persist on the enamel surface for long periods lead to the limited effectiveness of ACP agents to enamel remineralization. The paste-like application form could effectively enhance the concentration of ACP and could maintain a high concentration of ACP on the enamel surface during application, but like mouthwash, it still has a short duration of hydroxyapatite formation due to the effect of saliva flushing. ACP agents modified resin products can release ACP on the enamel surface for a long period thus achieving the long-term prevention or treatment of enamel demineralization. However, the effect of ACP agent incorporation on the performance of these products like mechanical performance and biocompatibility needs further exploration. And the long-term stability of the ACP release from these products should be considered in future studies.
Herein we summarize the strategies of stabilizing ACP. Calcium and phosphorus ions can be stabilized to the ACP state using a variety of methods, but the preventive and therapeutic effects of these ACP agents on enamel demineralization still await further investigation. There are three main forms of storage and application of ACP agents, namely mouthwash, toothpaste/tooth desensitizer, resin product. However, due to the shortcomings of the above-mentioned forms of ACP agents, more easy-to-use and long-lasting forms of ACP agents remain to be further explored.
JY drafted the manuscript. HY and TL revised the manuscript. FH and HH designed the work and revised the manuscript. All authors approved the final version to be published.
This work was financially supported by the National Natural Science Foundation of China (No. 81901044), Chinese Stomatological Association COS Basic Research Fund (No. COS-B2021-08), and Wuhan Young and Middle-aged Medical Talents Training Program (No. (2019)87).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamson, N. J., and Reynolds, E. C. (1996). Characterization of Casein Phosphopeptides Prepared Using Alcalase: Determination of Enzyme Specificity. Enzyme Microb. Technol. 19, 202–207. doi:10.1016/0141-0229(95)00232-4
Afennich, F., Slot, D., Hossainian, N., and Van der Weijden, G. (2011). The Effect of Hexetidine Mouthwash on the Prevention of Plaque and Gingival Inflammation: a Systematic Review. Int. J. Dent. Hyg. 9, 182–190. doi:10.1111/j.1601-5037.2010.00478.x
Ahmed, F., Prashanth, S., Sindhu, K., Nayak, A., and Chaturvedi, S. (2019). Antimicrobial Efficacy of Nanosilver and Chitosan against Streptococcus Mutans, as an Ingredient of Toothpaste Formulation: An In Vitro Study. J. Indian. Soc. Pedod. Prev. Dent. 37, 46–54. doi:10.4103/jisppd.jisppd_239_18
Akin, M., and Basciftci, F. A. (2012). Can White Spot Lesions Be Treated Effectively? Angle Orthod. 82 (5), 770–775. doi:10.2319/090711.578.1
Aras, A., Celenk, S., and Atas, O. (2020). Comparison of Traditional and Novel Remineralization Agents: A Laser Fluorescence Study. J. Oral Health Oral Epidemiol. 9 (1), 38–44. doi:10.22122/johoe.v9i1.1063
Bataineh, M., Malinowski, M., Duggal, M. S., and Tahmassebi, J. F. (2017). Comparison of the Newer Preventive Therapies on Remineralisation of Enamel In Vitro. J. Dent. 66, 37–44. doi:10.1016/j.jdent.2017.08.013
Behrouzi, P., Heshmat, H., Hoorizad Ganjkar, M., Tabatabaei, S. F., and Kharazifard, M. J. (2020). Effect of Two Methods of Remineralization and Resin Infiltration on Surface Hardness of Artificially Induced Enamel Lesions. J. Dent. (Shiraz) 21 (1), 12–17. doi:10.30476/dentjods.2019.77864
Beniash, E., Metzler, R. A., Lam, R. S. K., and Gilbert, P. U. P. A. (2009). Transient Amorphous Calcium Phosphate in Forming Enamel. J. Struct. Biol. 166, 133–143. doi:10.1016/j.jsb.2009.02.001
Betts, F., Blumenthal, N. C., Posner, A. S., Becker, G. L., and Lehninger, A. L. (1975). Atomic Structure of Intracellular Amorphous Calcium Phosphate Deposits. Proc. Natl. Acad. Sci. U.S.A. 72, 2088–2090. doi:10.1073/pnas.72.6.2088
Bhadoria, N., Gunwal, M. K., Kukreja, R., Maran, S., Devendrappa, S. N., and Singla, S. (2020). An In Vitro Evaluation of Remineralization Potential of Functionalized Tricalcium Phosphate Paste and CPP-ACPF on Artificial White Spot Lesion in Primary and Permanent Enamel. Int. J. Clin. Pediatr. Dent. 13 (6), 579–584. doi:10.5005/jp-journals-10005-1813
Bijle, M. N. A., Ekambaram, M., Lo, E. C. M., and Yiu, C. K. Y. (2019). The Combined Antimicrobial Effect of Arginine and Fluoride Toothpaste. Sci. Rep. 9, 8405. doi:10.1038/s41598-019-44612-6
Bröchner, A., Christensen, C., Kristensen, B., Tranæus, S., Karlsson, L., and Sonnesen, L. (2011). Treatment of Post-orthodontic White Spot Lesions with Casein Phosphopeptide-Stabilised Amorphous Calcium Phosphate. Clin. Oral. Investig. 15 (3), 369–373. doi:10.1007/s00784-010-0401-2
Chandavarkar, S. M., and Ram, S. M. (2015). A Comparative Evaluation of the Effect of Dentin Desensitizers on the Retention of Complete Cast Metal Crowns. Contemp. Clin. Dent. 6, S45–S50. doi:10.4103/0976-237x.152937
Chen, L., Liang, K., Li, J., Wu, D., Zhou, X., and Li, J. (2013). Regeneration of Biomimetic Hydroxyapatite on Etched Human Enamel by Anionic PAMAM Template In Vitro. Arch. Oral. Biol. 58, 975–980. doi:10.1016/j.archoralbio.2013.03.008
Chen, Z., Cao, S., Wang, H., Li, Y., Kishen, A., Deng, X., et al. (2015). Biomimetic Remineralization of Demineralized Dentine Using Scaffold of CMC/ACP Nanocomplexes in an In Vitro Tooth Model of Deep Caries. PLoS. One. 10, e0116553. doi:10.1371/journal.pone.0116553
ChenC, ., Weir, M., Cheng, L., Lin, N., Lin-Gibson, S., Chow, L., et al. (2014). Antibacterial Activity and Ion Release of Bonding Agent Containing Amorphous Calcium Phosphate Nanoparticles. Dent. Mat. 30, 891–901. doi:10.1016/j.dental.2014.05.025
ChenM, ., Yang, J., Li, J., Liang, K., He, L., Lin, Z., et al. (2014). Modulated Regeneration of Acid-Etched Human Tooth Enamel by a Functionalized Dendrimer that Is an Analog of Amelogenin. Acta. Biomater. 10, 4437–4446. doi:10.1016/j.actbio.2014.05.016
Chow, L. C., Takagi, S., Carey, C. M., and Sieck, B. A. (2000). Remineralization Effects of a Two-Solution Fluoride Mouthrinse: an In Situ Study. J. Dent. Res. 79, 991–995. doi:10.1177/00220345000790041601
Chow, L. C., Takagi, S., and Vogel, G. L. (1998). Amorphous Calcium Phosphate: the Contention of Bone. J. Dent. Res. 77, 6. doi:10.1177/00220345980770010901
Cochrane, N. J., Cai, F., Huq, N. L., Burrow, M. F., and Reynolds, E. C. (2010). New Approaches to Enhanced Remineralization of Tooth Enamel. J. Dent. Res. 89 (11), 1187–1197. doi:10.1177/0022034510376046
Cölfen, H., and Mann, S. (2003). Higher-Order Organization by Mesoscale Self-Assembly and Transformation of Hybrid Nanostructures. Angew. Chem. Int. Ed. Engl. 42, 2350–2365. doi:10.1002/anie.200200562
Cross, K. J., Huq, N. L., Palamara, J. E., Perich, J. W., and Reynolds, E. C. (2005). Physicochemical Characterization of Casein Phosphopeptide-Amorphous Calcium Phosphate Nanocomplexes. J. Biol. Chem. 280, 15362–15369. doi:10.1074/jbc.M413504200
Dey, A., Bomans, P. H., Müller, F. A., Will, J., Frederik, P. M., de With, G., et al. (2010). The Role of Prenucleation Clusters in Surface-Induced Calcium Phosphate Crystallization. Nat. Mat. 9, 1010–1014. doi:10.1038/nmat2900
Dorozhkin, S. (1997). Surface Reactions of Apatite Dissolution. J. Colloid. Interface. Sci. 191, 489–497. doi:10.1006/jcis.1997.4942
Dunn, W. (2007). Shear Bond Strength of an Amorphous Calcium-Phosphate-Containing Orthodontic Resin Cement. Am. J. Orthod. Dentofac. Orthop. 131, 243–247. doi:10.1016/j.ajodo.2005.04.046
Eanes, E. D., Gillessen, I. H., and Posner, A. S. (1965). Intermediate States in the Precipitation of Hydroxyapatite. Nature 208, 365–367. doi:10.1038/208365a0
Farzanegan, F., Ameri, H., Miri Soleiman, I., Khodaverdi, E., and Rangrazi, A. (2018). An In Vitro Study on the Effect of Amorphous Calcium Phosphate and Fluoride Solutions on Color Improvement of White Spot Lesions. Dent. J. (Basel). 6 (3). doi:10.3390/dj6030024
Farzanegan, F., Morteza-Saadat-Mostafavi, S., Ameri, H., and Khaki, H. (2019). Effects of Fluoride versus Amorphous Calcium Phosphate Solutions on Enamel Microhardness of White Spot Lesions: An Iin-Vvitro Study. J. Clin. Exp. Dent. 11 (3), e219–e224. doi:10.4317/jced.54448
Featherstone, J. (2004). The Continuum of Dental Caries--Evidence for a Dynamic Disease Process. J. Dent. Res. 83, C39–C42. doi:10.1177/154405910408301S08
Gao, Y., Liang, K., Weir, M. D., Gao, J., Imazato, S., Tay, F. R., et al. (2020). Enamel Remineralization via Poly(amido Amine) and Adhesive Resin Containing Calcium Phosphate Nanoparticles. J. Dent. 64, 58–67. doi:10.1016/j.jdent.2019.103262
Gokkaya, B., Ozbek, N., Guler, Z., Akman, S., Sarac, A. S., and Kargul, B. (2020). Effect of a Single Application of CPP-ACPF Varnish on the Prevention of Erosive Tooth Wear: An AAS, AFM and SMH Study. Oral. Health. Prev. Dent. 18, 311–318. doi:10.3290/j.ohpd.a43365
Gontijo, L., Cruz, R. d. A., and Brandao, P. R. G. (2007). Dental Enamel Around Fixed Orthodontic Appliances after Fluoride Varnish Application. Braz. Dent. J. 18, 49–53. doi:10.1590/s0103-64402007000100011
Gower, L. (2008). Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and its Role in Biomineralization. Chem. Rev. 108, 4551–4627. doi:10.1021/cr800443h
Güçlü, Z. A., Alaçam, A., and Coleman, N. J. (2016). A 12-Week Assessment of the Treatment of White Spot Lesions with CPP-ACP Paste And/or Fluoride Varnish. Biomed. Res. Int. 2016, 8357621. doi:10.1155/2016/8357621
Guven, Y., Ustun, N., Tuna, E. B., and Aktoren, O. (2019). Antimicrobial Effect of Newly Formulated Toothpastes and a Mouthrinse on Specific Microorganisms: An In Vitro Study. Eur. J. Dent. 13, 172–177. doi:10.1055/s-0039-1695655
Hamba, H., Nikaido, T., Inoue, G., Sadr, A., and Tagami, J. (2011). Effects of CPP-ACP with Sodium Fluoride on Inhibition of Bovine Enamel Demineralization: A Quantitative Assessment Using Micro-computed Tomography. J. Dent. 39, 405–413. doi:10.1016/j.jdent.2011.03.005
Han, S., Fan, Y., Zhou, Z., Tu, H., Li, D., Lv, X., et al. (2017). Promotion of Enamel Caries Remineralization by an Amelogenin-Derived Peptide in a Rat Model. Arch. Oral. Biol. 73, 66–71. doi:10.1016/j.archoralbio.2016.09.009
Hefti, A. F., and Huber, B. (1987). The Effect on Early Plaque Formation, Gingivitis and Salivary Bacterial Counts of Mouthwashes Containing Hexetidine/zinc, Aminefluoride/tin or Chlorhexidine. J. Clin. Periodontol. 14, 515–518. doi:10.1111/j.1600-051x.1987.tb00992.x
Hossainian, N., Slot, D. E., Afennich, F., and Van der Weijden, G. A. (2011). The Effects of Hydrogen Peroxide Mouthwashes on the Prevention of Plaque and Gingival Inflammation: a Systematic Review. Int. J. Dent. Hyg. 9, 171–181. doi:10.1111/j.1601-5037.2010.00492.x
Hua, F., Yan, J., Zhao, S., Yang, H., and He, H. (2020). In Vitro remineralization of Enamel White Spot Lesions with a Carrier-Based Amorphous Calcium Phosphate Delivery System. Clin. Oral. Investig. 24, 2079–2089. doi:10.1007/s00784-019-03073-x
Huang, G. J., Roloff-Chiang, B., Mills, B. E., Shalchi, S., Spiekerman, C., Korpak, A. M., et al. (2013). Effectiveness of MI Paste Plus and PreviDent Fluoride Varnish for Treatment of White Spot Lesions: a Randomized Controlled Trial. Am. J. Orthod. Dentofac. Orthop. 143 (1), 31–41. doi:10.1016/j.ajodo.2012.09.007
Huang, S.-C., Naka, K., and Chujo, Y. (2008). Effect of Molecular Weights of Poly(acrylic Acid) on Crystallization of Calcium Carbonate by the Delayed Addition Method. Polym. J. 40, 154–162. doi:10.1295/polymj.PJ2007162
Ibrahim, M. S., Balhaddad, A. A., Garcia, I. M., Collares, F. M., Weir, M. D., Xu, H. H. K., et al. (2020). pH-Responsive Calcium and Phosphate-Ion Releasing Antibacterial Sealants on Carious Enamel Lesions In Vitro. J. Dent. 97, 103323. doi:10.1016/j.jdent.2020.103323
Jo, S. Y., Chong, H. J., Lee, E. H., Chang, N. Y., Chae, J. M., Cho, J. H., et al. (2014). Effects of Various Toothpastes on Remineralization of White Spot Lesions. Korean. J. Orthod. 44 (3), 113–118. doi:10.4041/kjod.2014.44.3.113
Julien, K., Buschang, P., and Campbell, P. (2013). Prevalence of White Spot Lesion Formation during Orthodontic Treatment. Angle. Orthod. 83, 641–647. doi:10.2319/071712-584.1
Kamath, P., Nayak, R., Kamath, S. U., and Pai, D. (2017). A Comparative Evaluation of the Remineralization Potential of Three Commercially Available Remineralizing Agents on White Spot Lesions in Primary Teeth: An In Vitro Study. J. Indian. Soc. Pedod. Prev. Dent. 35 (3), 229–237. doi:10.4103/JISPPD.JISPPD_242_16
Karadağlıoğlu, Ö., Ulusoy, N., Başer, K. H. C., Hanoğlu, A., and Şık, İ. (2019). Antibacterial Activities of Herbal Toothpastes Combined with Essential Oils against Streptococcus Mutans. Pathogens 8, 20. doi:10.3390/pathogens8010020
Khopade, A., Khopade, S., and Jain, N. (2002). Development of Hemoglobin Aquasomes from Spherical Hydroxyapatite Cores Precipitated in the Presence of Half-Generation Poly(Amidoamine) Dendrimer. Int. J. Pharm. 241, 145–154. doi:10.1016/S0378-5173(02)00235-1
Kwak, S.-Y., Yamakoshi, Y., Simmer, J., and Margolis, H. C. (2016). MMP20 Proteolysis of Native Amelogenin Regulates Mineralization In Vitro. J. Dent. Res. 95, 1511–1517. doi:10.1177/0022034516662814
Larsson, K., Stime, A., Hansen, L., Birkhed, D., and Ericson, D. (2020). Salivary Fluoride Concentration and Retention after Rinsing with 0.05 and 0.2% Sodium Fluoride (NaF) Compared with a New High F Rinse Containing 0.32% NaF. Acta. Odontol. Scand. 78, 609–613. doi:10.1080/00016357.2020.1800085
Liu, Y., Zhang, L., Niu, L. N., Yu, T., Xu, H. H. K., Weir, M. D., et al. (2018). Antibacterial and Remineralizing Orthodontic Adhesive Containing Quaternary Ammonium Resin Monomer and Amorphous Calcium Phosphate Nanoparticles. J. Dent. 72, 53–63. doi:10.1016/j.jdent.2018.03.004
Lv, X., Yang, Y., Han, S., Li, D., Tu, H., Li, W., et al. (2015). Potential of an Amelogenin Based Peptide in Promoting Reminerlization of Initial Enamel Caries. Arch. Oral. Biol. 60, 1482–1487. doi:10.1016/j.archoralbio.2015.07.010
Ma, Y., Zhang, N., Weir, M. D., Bai, Y., and Xu, H. H. K. (2017). Novel Multifunctional Dental Cement to Prevent Enamel Demineralization Near Orthodontic Brackets. J. Dent. 64, 58–67. doi:10.1016/j.jdent.2017.06.004
Margolis, H., and Murphy, B. (1986). Effect of Low Levels of Fluoride in Solution on Enamel Demineralization In Vitro. J. Dent. Res. 65, 23–29. doi:10.1177/00220345860650010301
Marinho, V. C. C. (2009). Cochrane Reviews of Randomized Trials of Fluoride Therapies for Preventing Dental Caries. Eur. Arch. Paediatr. Dent. 10, 183–191. doi:10.1007/bf03262681
Moradian-Oldak, J. (2012). Protein-mediated Enamel Mineralization. Front. Biosci. 17, 1996–2023. doi:10.2741/4034
Munjal, D., Garg, S., Dhindsa, A., Sidhu, G. K., and Sethi, H. S. (2016). Assessment of White Spot Lesions and In-Vivo Evaluation of the Effect of CPP-ACP on White Spot Lesions in Permanent Molars of Children. J. Clin. Diagn. Res. 10 (5), ZC149–ZC154. doi:10.7860/jcdr/2016/19458.7896
Niu, L. N., Jee, S. E., Jiao, K., Tonggu, L., Li, M., Wang, L., et al. (2017). Collagen Intrafibrillar Mineralization as a Result of the Balance between Osmotic Equilibrium and Electroneutrality. Nat. Mat. 16, 370–378. doi:10.1038/nmat4789
Olgen, I. C., Sonmez, H., and Bezgin, T. (2021). Effects of Different Remineralization Agents on MIH Defects: a Randomized Clinical Study. Clin. Oral. Investig. doi:10.1007/s00784-021-04305-9
Oliveira, G. M., Ritter, A. V., Heymann, H. O., Swift, E., Donovan, T., Brock, G., et al. (2014). Remineralization Effect of CPP-ACP and Fluoride for White Spot Lesions In Vitro. J. Dent. 42 (12), 1592–1602. doi:10.1016/j.jdent.2014.09.004
Pei, D., Liu, S., Huang, C., Du, X., Yang, H., and Wang, Y. (2013). Effect of Pretreatment with Calcium-Containing Desensitizer on the Dentine Bonding of Mild Self-Etch Adhesives. Eur. J. Oral. Sci. 121, 204–210. doi:10.1111/eos.12047
Perrini, F., Lombardo, L., Arreghini, A., Medori, S., and Siciliani, G. (2016). Caries Prevention during Orthodontic Treatment: In-Vvivo Assessment of High-Fluoride Varnish to Prevent White Spot Lesions. Am. J. Orthod. Dentofac. Orthop. 149, 238–243. doi:10.1016/j.ajodo.2015.07.039
Pithon, M. M., Baião, F. S., Sant'Anna, L. I. D., Tanaka, O. M., and Cople-Maia, L. (2019). Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate-Containing Products in the Prevention and Treatment of White Spot Lesions in Orthodontic Patients: A Systematic Review. J. Investig. Clin. Dent. 10 (2), e12391. doi:10.1111/jicd.12391
Qi, Y., Ye, Z., Fok, A., Holmes, B. N., Espanol, M., Ginebra, M. P., et al. (2018). Effects of Molecular Weight and Concentration of Poly(Acrylic Acid) on Biomimetic Mineralization of Collagen. Acs. Biomater. Sci. Eng. 4, 2758–2766. doi:10.1021/acsbiomaterials.8b00512
Rees, J., Loyn, T., and Chadwick, B. (2007). Pronamel and Tooth Mousse: an Initial Assessment of Erosion Prevention In Vitro. J. Dent. 35, 355–357. doi:10.1016/j.jdent.2006.10.005
Reeves, R. E., and Latour, N. G. (1958). Calcium Phosphate Sequestering Phosphopeptide from Casein. Science 128, 472. doi:10.1126/science.128.3322.472
Reise, M., Kranz, S., Heyder, M., Jandt, K. D., and Sigusch, B. W. (2021). Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Compared to Fluoride Products in an In-Vitro Demineralization Model. Mater. (Basel). 14, 5974. doi:10.3390/ma14205974
Reynolds, E. C., Cain, C. J., Webber, F. L., Black, C. L., Riley, P. F., Johnson, I. H., et al. (1995). Anticariogenicity of Calcium Phosphate Complexes of Tryptic Casein Phosphopeptides in the Rat. J. Dent. Res. 74, 1272–1279. doi:10.1177/00220345950740060601
Reynolds, E. C. (1997). Remineralization of Enamel Subsurface Lesions by Casein Phosphopeptide-Stabilized Calcium Phosphate Solutions. J. Dent. Res. 76, 1587–1595. doi:10.1177/00220345970760091101
Rösing, C. K., Cavagni, J., Gaio, E. J., Muniz, F., Ranzan, N., Oballe, H. J. R., et al. (2017). Efficacy of Two Mouthwashes with Cetylpyridinium Chloride: a Controlled Randomized Clinical Trial. Braz. Oral. Res. 31, e47. doi:10.1590/1807-3107BOR-2017.vol31.0047
Ruan, Q., and Moradian-Oldak, J. (2015). Amelogenin and Enamel Biomimetics. J. Mat. Chem. B 3, 3112–3129. doi:10.1039/C5TB00163C
Ruan, Q., Siddiqah, N., Li, X., Nutt, S., and Moradian-Oldak, J. (2014). Amelogenin-chitosan Matrix for Human Enamel Regrowth: Effects of Viscosity and Supersaturation Degree. Connect. Tissue. Res. 55, 150–154. doi:10.3109/03008207.2014.923856
Ruan, Q., Zhang, Y., Yang, X., Nutt, S., and Moradian-Oldak, J. (2013). An Amelogenin-Chitosan Matrix Promotes Assembly of an Enamel-like Layer with a Dense Interface. Acta. Biomater. 9, 7289–7297. doi:10.1016/j.actbio.2013.04.004
Schemehorn, B. R., Wood, G. D., McHale, W., and Winston, A. E. (2011). Comparison of Fluoride Uptake Into Tooth Enamel From Two Fluoride Varnishes Containing Different Calcium Phosphate Sources. J. Clin. Dent. 22 (2), 51–54.
Shang, Q., Gao, Y., Qin, T., Wang, S., Shi, Y., and Chen, T. (2020). Interaction of Oral and Toothbrush Microbiota Affects Oral Cavity Health. Front. Cell. Infect. Microbiol. 10, 17. doi:10.3389/fcimb.2020.00017
Shen, P., Cai, F., Nowicki, A., Vincent, J., and Reynolds, E. C. (2001). Remineralization of Enamel Subsurface Lesions by Sugar-free Chewing Gum Containing Casein Phosphopeptide-Amorphous Calcium Phosphate. J. Dent. Res. 80, 2066–2070. doi:10.1177/00220345010800120801
Sikirić, M. D., Gergely, C., Elkaim, R., Wachtel, E., Cuisinier, F. J., and Füredi-Milhofer, H. (2009). Biomimetic Organic-Inorganic Nanocomposite Coatings for Titanium Implants. J. Biomed. Mat. Res. A 89, 759–771. doi:10.1002/jbm.a.32021
Singh, S., Singh, S. P., Goyal, A., Utreja, A. K., and Jena, A. K. (2016). Effects of Various Remineralizing Agents on the Outcome of Post-orthodontic White Spot Lesions (WSLs): a Clinical Trial. Prog. Orthod. 17 (1), 25. doi:10.1186/s40510-016-0138-9
Singla, S., Maran, S., Bhadoria, N., Gunwal, M. K., Kukreja, R., and Devendrappa, S. N. (2020). An In Vitro Evaluation of Remineralization Potential of Functionalized Tricalcium Phosphate Paste and CPP-ACPF on Artificial White Spot Lesion in Primary and Permanent Enamel. Int. J. Clin. Pediatr. Dent. 13 (6), 579–584. doi:10.5005/jp-journals-10005-1813
Sitthisettapong, T., Doi, T., Nishida, Y., Kambara, M., and Phantumvanit, P. (2015). Effect of CPP-ACP Paste on Enamel Carious Lesion of Primary Upper Anterior Teeth Assessed by Quantitative Light-Induced Fluorescence: A One-Year Clinical Trial. Caries. Res. 49 (4), 434–441. doi:10.1159/000434728
Skrtic, D., Antonucci, J. M., Eanes, E. D., and Eidelman, N. (2004). Dental Composites Based on Hybrid and Surface-Modified Amorphous Calcium Phosphates. Biomaterials 25, 1141–1150. doi:10.1016/j.biomaterials.2003.08.001
Skrtic, D., and Eanes, E. (1996). Improved Properties of Amorphous Calcium Phosphate Fillers in Remineralizing Resin Composites. Dent. Mat. 12, 295–301. doi:10.1016/S0109-5641(96)80037-6
Soares-Yoshikawa, A. L., Varanda, T., Iwamoto, A. S., Kantovitz, K. R., Puppin-Rontani, R. M., and Pascon, F. M. (2021). Fluoride Release and Remineralizing Potential of Varnishes in Early Caries Lesions in Primary Teeth. Microsc. Res. Tech. 84, 1012–1021. doi:10.1002/jemt.23662
Sollböhmer, O., May, K. P., and Anders, M. (1995). Force Microscopical Investigation of Human Teeth in Liquid. Thin. Solid. Films. 264, 176–183. doi:10.1016/0040-6090(95)05847-8
Song, J., Li, T., Gao, J., Li, C., Jiang, S., and Zhang, X. (2021). Building an Aprismatic Enamel-like Layer on a Demineralized Enamel Surface by Using Carboxymethyl Chitosan and Lysozyme-Encapsulated Amorphous Calcium Phosphate Nanogels. J. Dent. 107, 103599. doi:10.1016/j.jdent.2021.103599
Songsiripradubboon, S., Hamba, H., Trairatvorakul, C., and Tagami, J. (2014). Sodium Fluoride Mouthrinse Used Twice Daily Increased Incipient Caries Lesion Remineralization in an In Situ Model. J. Dent. 42, 271–278. doi:10.1016/j.jdent.2013.12.012
Svenson, S., and Tomalia, D. A. (2012). Dendrimers in Biomedical Applications—Reflections on the Field. Adv. Drug. Deliv. Rev. 64, 102–115. doi:10.1016/j.addr.2005.09.01810.1016/j.addr.2012.09.030
Tahmasbi, S., Mousavi, S., Behroozibakhsh, M., and Badiee, M. (2019). Prevention of White Spot Lesions Using Three Remineralizing Agents: An In Vitro Comparative Study. J. Dent. Res. Dent. Clin. Dent. Prospects. 13 (1), 36–42. doi:10.15171/joddd.2019.006
Terauchi, M., Tamura, A., Tonegawa, A., Yamaguchi, S., Yoda, T., and Yui, N. (2019). Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates. Polym. (Basel) 11, 1327. doi:10.3390/polym11081327
Thierens, L. A. M., Moerman, S., Elst, C. V., Vercruysse, C., Maes, P., Temmerman, L., et al. (2019). The In Vitro Remineralizing Effect of CPP-ACP and CPP-ACPF after 6 and 12 Weeks on Initial Caries Lesion. J. Appl. Oral. Sci. 27, e20180589. doi:10.1590/1678-7757-2018-0589
Tomalia, D. A., Baker, H., Dewald, J., Hall, M., Kallos, G., Martin, S., et al. (1985). A New Class of Polymers: Starburst-Dendritic Macromolecules. Poly. J. 17, 117–132. doi:10.1295/polymj.17.117
Tsiourvas, D., Tsetsekou, A., Kammenou, M.-I., and Boukos, N. (2015). Biomimetic Synthesis of Ribbon-like Hydroxyapatite Employing Poly(l-Arginine). Mat. Sci. Eng. C. Mat. Biol. Appl. 58, 1225–1231. doi:10.1016/j.msec.2015.09.076
Utneja, S., Talwar, S., Nawal, R. R., Sapra, S., Mittal, M., Rajain, A., et al. (2018). Evaluation of Remineralization Potential and Mechanical Properties of Pit and Fissure Sealants Fortified with Nano-Hydroxyapatite and Nano-Amorphous Calcium Phosphate Fillers: An In Vitro Study. J. Conserv. Dent. 21, 681–690. doi:10.4103/jcd.jcd_31_18
Wang, D., Deng, J., Deng, X., Fang, C., Zhang, X., and Yang, P. (2020). Controlling Enamel Remineralization by Amyloid-like Amelogenin Mimics. Adv. Mat. 32, 2002080. doi:10.1002/adma.202002080
Wang, H., Xiao, Z., Yang, J., Lu, D., Kishen, A., Li, Y., et al. (2017). Oriented and Ordered Biomimetic Remineralization of the Surface of Demineralized Dental Enamel Using HAP@ACP Nanoparticles Guided by Glycine. Sci. Rep. 7, 40701. doi:10.1038/srep40701
Wang, Y., Hua, F., and Jiang, H. (2020). CPP-ACP May Be Effective, but Not Significantly Greater Than Using Fluorides Alone, in Preventing and Treating White Spot Lesions Around Orthodontic Brackets. J. Evid. Based. Dent. Pract. 20 (1), 101416. doi:10.1016/j.jebdp.2020.101416
Wang, Z., Ouyang, Y., Wu, Z., Zhang, L., Changyu, S., Fan, J., et al. (2018). A Novel Fluorescent Adhesive-Assisted Biomimetic Mineralization. Nanoscale 18, 18980–18987. doi:10.1039/C8NR02078G
Wright, J. T., Li, Y., Suggs, C., Kuehl, M. A., Kulkarni, A. B., and Gibson, C. W. (2011). The Role of Amelogenin during Enamel-Crystallite Growth and Organization In Vivo. Eur. J. Oral. Sci. 119 (Suppl. 1), 65–69. doi:10.1111/j.1600-0722.2011.00883.x
Xie, X., Wang, L., Xing, D., Qi, M., Li, X., Sun, J., et al. (2019). Novel Rechargeable Calcium Phosphate Nanoparticle-Filled Dental Cement. Dent. Mat. J. 38, 1–10. doi:10.4012/dmj.2017-420
Xu, H. H., Moreau, J. L., Sun, L., and Chow, L. C. (2011). Nanocomposite Containing Amorphous Calcium Phosphate Nanoparticles for Caries Inhibition. Dent. Mat. 27, 762–769. doi:10.1016/j.dental.2011.03.016
Yadav, P., Desai, H., Patel, K., Patel, N., and Iyengar, S. (2019). A Comparative Quantitative & Qualitative Assessment in Orthodontic Treatment of White Spot Lesion Treated with 3 Different Commercially Available Materials - In Vitro Study. J. Clin. Exp. Dent. 11 (9), e776–e782. doi:10.4317/jced.56044
Yang, H., Chen, Z., Yan, H., and Huang, C. (2018). Effects of Calcium-Containing Desensitizers on the Bonding Stability of an Etch-And-Rinse Adhesive against Long-Term Water Storage and pH Cycling. Dent. Mat. J. 37, 122–129. doi:10.4012/dmj.2017-006
Yang, H., Niu, L., Sun, J. l., Huang, X., Pei, D., Cui, H., et al. (2017). Biodegradable Mesoporous Delivery System for Biomineralization Precursors. Int. J. Nanomed. 12, 839–854. doi:10.2147/IJN.S128792
Yang, X., Wang, L., Qin, Y., Sun, Z., Henneman, Z. J., Moradian-Oldak, J., et al. (2010). How Amelogenin Orchestrates the Organization of Hierarchical Elongated Microstructures of Apatite. J. Phys. Chem. B 114, 2293–2300. doi:10.1021/jp910219s
Zandim-Barcelos, D., Tschoppe, P., Sampaio, J., and Kielbassa, A. (2011). Effect of Saliva Substitutes in Combination with Fluorides on Remineralization of Subsurface Dentin Lesions. Support. Care. Cancer. 19, 1143–1149. doi:10.1007/s00520-010-0924-8
Zhang, J., Lynch, R., Watson, T., and Banerjee, A. (2018). Remineralisation of Enamel White Spot Lesions Pre-treated with Chitosan in the Presence of Salivary Pellicle. J. Dent. 72, 21–28. doi:10.1016/j.jdent.2018.02.004
Zhang, X., Li, Y., Sun, X., Kishen, A., Deng, X., Yang, X., et al. (2014). Biomimetic Remineralization of Demineralized Enamel with Nano-Complexes of Phosphorylated Chitosan and Amorphous Calcium Phosphate. J. Mat. Sci. Mat. Med. 25, 2619–2628. doi:10.1007/s10856-014-5285-2
Zhong, X., Lai, T. T., Chen, L., and Tian, K. (2021). Self-assembly and Mineralization of Full-Length Human Amelogenin and its Functional Fragments In Vitro. West. China. J. Stomatol. 32, 2002080. doi:10.7518/hxkq.2021.04.007
Zhou, Y., Yang, J., Lin, Z., Li, J., Liang, K., Yuan, H., et al. (2013). Triclosan-loaded Poly(amido Amine) Dendrimer for Simultaneous Treatment and Remineralization of Human Dentine. Colloids. Surf. B. Biointerfaces. 115, 237–243. doi:10.1016/j.colsurfb.2013.11.045
Zhou, Z. H., Zhou, P. L., Yang, S. P., Yu, X. B., and Yang, L. Z. (2007). Controllable Synthesis of Hydroxyapatite Nanocrystals via a Dendrimer-Assisted Hydrothermal Process. Mat. Res. Bull. 42, 1611–1618. doi:10.1016/j.materresbull.2006.11.041
Zhou, Z., Zhang, L., Li, J., Shi, Y., Wu, Z., Zheng, H., et al. (2021). Polyelectrolyte-calcium Complexes as a Pre-precursor Induce Biomimetic Mineralization of Collagen. Nanoscale 13, 953–967. doi:10.1039/d0nr05640e
Keywords: enamel, demineralization, remineralization, amorphous calcium phosphate, hydroxyapatite
Citation: Yan J, Yang H, Luo T, Hua F and He H (2022) Application of Amorphous Calcium Phosphate Agents in the Prevention and Treatment of Enamel Demineralization. Front. Bioeng. Biotechnol. 10:853436. doi: 10.3389/fbioe.2022.853436
Received: 12 January 2022; Accepted: 15 April 2022;
Published: 13 May 2022.
Edited by:
Kumar Chandan Srivastava, Al Jouf University, Saudi ArabiaReviewed by:
Shanshan Liu, The first affiliated hospital of bengbu medical college, ChinaCopyright © 2022 Yan, Yang, Luo, Hua and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Hua, aHVhZmFuZ0B3aHUuZWR1LmNu; Hong He, ZHJoZWhvbmdAd2h1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.