- 1Institute of Genetics and Biophysics (IGB), National Research Council (CNR), Naples, Italy
- 2Institute of Experimental Endocrinology and Oncology (IEOS), National Research Council (CNR), Naples, Italy
Introduction
As early as the second half of the 19th century, Charles Darwin hypothesized as a part of his pangenesis theory that every cell type in the body could generate minute size “gemmules” full of molecules to communicate with other cell types (Darwin, 1868). This seminal intuition fell unnoticed for more than 150 years until contemporary scientists may recognize extracellular vesicles (EVs) in Darwin’s gemmules (Liu and Chen, 2018; Margolis and Sadovsky, 2019; Bergese et al., 2020). Nowadays it has been clearly shown that cells from different organisms, including eukaryotes, both animals (from yeast to mammals) and plants, but also prokaryotic cells, have been demonstrated to release vesicles into the extracellular environment either constitutively or following cell stimulation. EVs have also been isolated from diverse body fluids, including blood, urine, saliva, breast milk, amniotic fluid, cerebrospinal fluid, bile, and semen (Keller et al., 2011; Kalluri and LeBleu, 2020). All EVs are lipid-membrane encapsulated particles filled of cellular content, comprising proteins, metabolites, nucleic acids, lipids, and even entire organelles, some of them specifically sorted and enriched in EV populations with a pattern reflective of cell functions and conditions (Raposo and Stoorvogel, 2013; Yáñez-Mó et al., 2015; Meldolesi, 2018; Van Niel et al., 2018). Very far from representing a tool to eliminate waste material, as hypothesized at the beginning, EVs are able to target specific cells and deliver molecules that induce specific cell response (Van Niel et al., 2018; Kalluri and LeBleu, 2020; Mantile et al., 2020). Therefore, EV-based cell communication has become an extremely intriguing mechanism that attracted a lot of scientists for its great potential in basic as well as applied research.
Challenges and Initiatives in Extracellular Vesicle Investigation
Due to their interesting features, including high stability in body fluids, capacity to cross the blood brain boundaries, cross-communication among species, EVs have been emerging as key tools for a plethora of applications. Interestingly, EVs are a promising source of new biomarkers for liquid biopsy to be used in the diagnosis, prognosis and treatment of different pathologies, including cancer, immune, inflammatory, cardiovascular and neurodegenerative diseases (Kalluri and LeBleu, 2020; Lin et al., 2020; Tamura et al., 2021; Vozel et al., 2021). Moreover, EVs definitely are a valid alternative to synthetic nanocarriers for drug delivery and for the development of pioneering new therapeutic approaches (Zipkin, 2019; Zarovni et al., 2021).
Thanks also to the outgrowth of new technologies, recent decades have seen a sharp increase in the number of scientific publications focusing on EVs. However, the expanding interest in EV research had also raised some vexing problems, including misleading nomenclature, unknown influence of pre-analytical variables, extreme heterogeneity in the procedures adopted to separate and properly characterize EVs, poor definition of the methodologies themselves, lack of sample quality control and in general of dedicated reference materials and relative standards. As a consequence, this uncertainty slowed down the acceptance of EV potential from scientists of other fields, regulatory agencies, politicians and investors (Roy et al., 2018; Nieuwland et al., 2020; Bazzan et al., 2021). To address these issues, the EV community joined in a common effort and strongly committed itself to rigor and standardization of procedures and reproducibility of results. In 2011, during a meeting in Paris, the International Society for Extracellular Vesicles (ISEV) was founded and in 2012, its official journal, the Journal of Extracellular Vesicles was launched (Lötvall et al., 2012). Since then, ISEV annual meetings and workshops have been regularly organized throughout the world, giving rise to a series of position papers providing guidance on important topics, such as standardization of sample collection and processing (Witwer et al., 2013), RNA analysis (Hill et al., 2013; Mateescu et al., 2017), and diagnostic and therapeutic uses of EVs (Lener et al., 2015; Reiner et al., 2017; Clayton et al., 2018, 2019). In 2014, the ISEV board members provided researchers with the first “Minimal Information for the Study of EVs” (MISEV), a set of biochemical, biophysical and functional standards that should be used to attribute any specific biological cargo or functions to EVs (Lötvall et al., 2014). The MISEV2014 were revised through a community guidance survey (Witwer et al., 2017) and updated as the 382-author “MISEV2018” (Théry et al., 2018), both manuscripts having a strong impact on EV community, as revealed by the extremely high number of citations (more than 1,400 and 2,800 Scopus citations, respectively, by January 2022). MISEV are expected to be further updated according to comments and suggestions of ISEV members, collected through a recent survey (Witwer et al., 2021).
More recently, an ISEV subcommittee specifically devoted to rigor and standardization in EV studies (https://www.isev.org/rigor-standardization) has been created, including several task forces, each one focusing on specific key topics in the field (Nieuwland et al., 2020). Other societies have also been contributing to increase rigor and standardization in EV studies. The International Society on Thrombosis and Hemostasis (ISTH) addressed standardization of EV fluo cytometry (Lacroix et al., 2010; Lacroix et al., 2013; Cointe et al., 2017), and recently collaborated in a transversal working group with ISEV and the International Society for the Advancement of Cytometry (ISAC) to develop guidelines and best practices for EV fluo cytometric experiments, named MIFloCyt-EV (Welsh et al., 2020, 2021; Van Der Pol et al., 2021). Another collaboration has been established between ISEV and the International Society for Cell and Gene Therapy (ISCT) to consider carefully the key issues to address before exploiting the potential of EVs in therapeutic approaches against coronavirus disease-19 (Borger et al., 2020). Meanwhile, several national societies for scientists studying EVs have also been formed and specific EV databases, such as Exocarta (http://www.exocarta.org), Vesiclepedia (Kalra et al., 2012), EVpedia (Kim et al., 2013), exRNA Atlas (Subramanian et al., 2015), and ExoRBase 2.0 (Lai et al., 2022) have been developed to collect the increasing amount of data produced on EV cargo identification. A data depository platform, the EV-track (https://www.evtrack.org; Van Deun et al., 2017), was also created for recording the experimental parameters of EV-related studies providing higher research quality and transparency.
However, the level of adherence to MISEV guidelines and exploitation of additional voluntary online reporting platforms is still unclear. A recent study using a text mining approach on 5,096 accessible EV papers published between 2012 and 2020 has shown that the awareness of investigators to better characterize their EV preparations using a combination of several methods was significantly rised, especially in the studies citing the MISEV position statements (Poupardin et al., 2021). On the other hand, feedback collection on the methods used for EV separation and characterization through more than 600 voluntary ISEV Rigor and Standardization surveys revealed still the lack of sample quality controls, and at the same time the recognized need to give more attention to these topics (Royo et al., 2020). Very recently, the ISEV Board promoted a survey within the ISEV community to understand actual engagement with MISEV, determine how the guidelines could be improved, and define the relationship with other rigor initiatives (Witwer et al., 2021). More than 700 feedbacks were received and analyzed, most of them assessing a strong impact of MISEV on the overall quality of EV studies, but, interestingly about one-third of respondents did not follow the guidelines or did not publish EV studies after MISEV 2018. In a minority of cases, MISEV2018 were perceived too restrictive and long or neglecting key topics. Moreover, the majority of the respondents had not used yet the EV-TRACK platform or were unfamiliar with it (Witwer et al., 2021). From this emerging scenario, the importance of promoting quality culture, improving guideline definition and adherence, and implementing more tools, among which scientist may choose for better and easier standardization of their research, comes fully to light.
Quality Management at a Glance
The birth of “modern standardization” has been identified at the end of the 18th century with the first Industrial Revolution and the scaling up of production (Kolb and Hoover, 2012; Hellman and Liu, 2013). In the early 1900s, Frederick Winslow Taylor formulated a new approach to factory management, called scientific management, dividing the planning function from the production, focusing on the efficiency and productivity, and introducing pioneer ideas, still valid nowadays, such as employee training and implementation of standardized best practices (Taylor, 2003). In the 1930s, Walter Shewhart of the Bell Telephone Laboratories implemented the Statistical Quality Control of product variables, demonstrating that by eliminating the variation of the process a good standard of end product could be achieved (Shewhart, 1940). At the end of the second World War, thanks to the contribution of Armand Vallin Feigenbaum, Edward Deming, Joseph Moses Juran and Philip Crosby, modern Quality was born, based on the prevention of accidents through the design and implementation of a formal Quality system. Meanwhile, with the very beginning of globalization, the need for standards became internationally recognized. The first international standardizing body with general competence, was the International Standardization Association, created in 1930 and then substituted by the International Organization for Standardization (ISO) founded in 1947. In 1987, ISO published the series of Quality standards which is now known as ISO 9000, widely accepted as “gold standards” in Quality Management (QM), and successively revised in 1994, 2000, 2008, and 2015 (Ziegel and Lamprecht, 1993; Hadjicostas, 2010; ISO, 2019). A QM System is defined as a formalized system that documents processes, procedures and responsibilities, to support the organization activities in meeting their own objectives, customer and regulatory requirements as well as improving their performance on a continuous basis (Ziegel and Lamprecht, 1993; Hadjicostas, 2010; ISO, 2019). In the context of research institutes, a structured approach to QM has a great potential to improve the rigor, reproducibility, reliability and ultimately value and technological transfer potential of scientific research (Bongiovanni et al., 2015; Lanati, 2018; Molinéro-Demilly et al., 2018; Gregory, 2020; Hewera et al., 2020; Liguori and Kisslinger, 2020; Lovrenčić Mikelić, 2020; Hollmann et al., 2021). In addition, the implementation of a QM approach may support the development and execution of research projects, especially in an interdisciplinary, multi-site and high-risk context, providing a roadmap toward improved harmonization and standardization of procedures as well as reliability of results (Dehouck et al., 2019; Liguori and Kisslinger, 2020).
Quality Management Tools at the Service of EV Research: Fostering Rigor, Standardization, Reproducibility and Technology Transfer
The implementation of QM tools can be extremely valid for scientists to successfully overcome the EV challenges (Rovira et al., 2017; Ayers et al., 2019). Here we report a list of methodologies that can be applied in the different phases of a study, from the initial set-up to the full development of a process, and the relative examples of applications for EV research (Figure 1A).
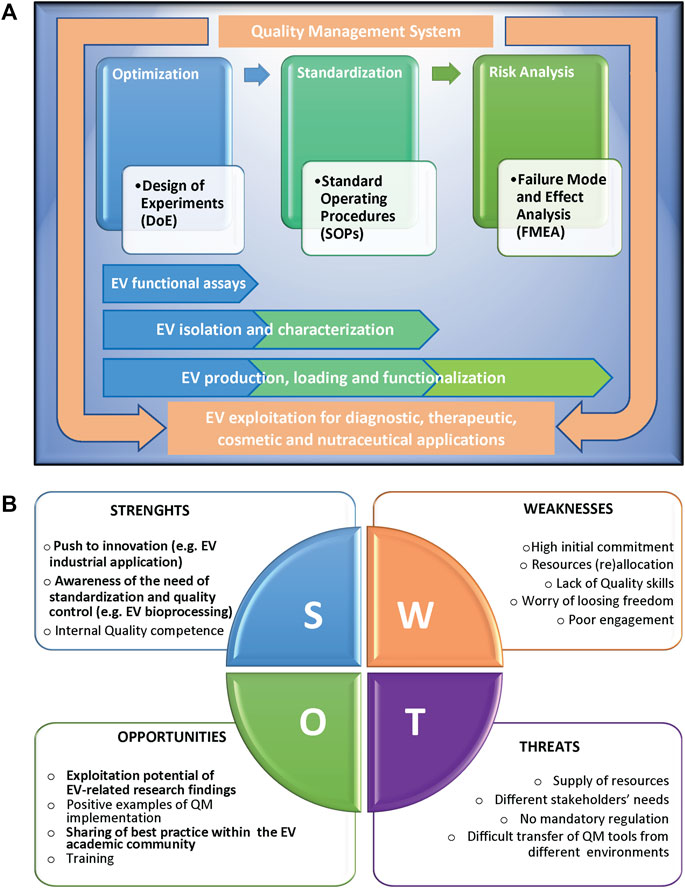
FIGURE 1. (A) Scheme representing the Quality methodologies supporting the different phases of a complex scientific process, from the initial set-up and optimization, to the successive standardization and risk management, with the relative applications to extracellular vesicle (EV) studies. The different methodologies can be applied singularly or can converge in an overall Quality Management System, in compliance with international standards, to support EV exploitation. (B) SWOT analysis of the implementation of Quality Management tools in an academic research environment, highlighting the relative Strenghts (S), Weaknesses (W), Opportunities (O), and Threats (T). Factors particularly relevant in the context of EV research are in bold.
Optimization
To find the optimal configurations of variables (factors) which maximize the output(s) of a defined process, traditional experimentation has typically used a one-factor-at-a-time (OFAT) approach, in which every factor is kept constant except for the one under investigation. The OFAT method, however, neglects the complexity of biological processes, that requires instead the simultaneous examination of the factors to be controlled. To overcome this limitation, a Design of Experiments (DoE) statistical approach can be applied to multivariable processes to identify the optimal combination of factors and model their interaction, leading to major benefits in both product performance as well as management of resources (Mandenius and Brundin, 2008; Montgomery, 2012; Weissman and Anderson, 2015; Lipsitz et al., 2016; Myers et al., 2016; Politis et al., 2017; Grangeia et al., 2020). In recent years, the DoE approach is emerging in many fields of scientific research, including cell biology, biochemistry and nanotechnologies (Mancinelli et al., 2015, 2021; Durakovic, 2017; Toms et al., 2017; Lanati, 2018; Narenderan et al., 2019; Papaneophytou, 2019; Xu et al., 2020; Esteban et al., 2021; Papaneophytou et al., 2021; Tavares Luiz et al., 2021). In the EV context, DoE has been successfully implemented to model the effect of different factors, such as time, EV dose, EV type (exosomes or microvesicles) on different parameters related to EV uptake and cargo delivery, fundamental for therapeutic application (Xu et al., 2020; Dave et al., 2021).
Standardization
Once a process is optimized, the definition of clear work instructions, such as guidelines and standard operating procedures (SOPs) is highly recommended for controlling activities, assuring reliability and reproducibility of results and last but not least training of new personnel and transfer of competence, and know-how (Hattemer-Apostel, 2001; Gough and Hamrell, 2010; Digilio et al., 2016). SOPs are also very useful for mitigating both health and safety risk as well as instrument damage or erroneous utilization (Akyar, 2012). Definition of SOPs is fundamental for multisite consortia that share results, samples and methodologies as well as for transfer of knowledge and/or technologies to applicative fields. In the EV field, the importance of standard procedures has been specially highlighted for the scalable production of quality standard nanosized EVs from different natural sustainable sources to be used for drug delivery applications (Liguori and Kisslinger, 2020; Buschmann et al., 2021; Paterna et al., 2022).
Risk Analysis
Risk management improves the reproducibility of any research process, reducing sources of errors, causing, when intercepted, reworking as well as time and money waste, or, when remain hidden, procedure inaccuracy, high variability, and non-reproducibility of research results. Failure Mode and Effect Analysis (FMEA) is a systematic approach for identifying all possible failures in a design, process, product or service (Tague, 2004; Lanati, 2018). Recently, FMEA has been successfully applied as a tool for risk assessment in biomedical research, biopharmaceutical manufacturing processes, analytical procedures and clinical trials (Zimmermann and Hentschel, 2011; Lee et al., 2017; Mascia et al., 2020; Petretta et al., 2021). Risk analysis is an extremely valid tool to implement in EV basic and even more applicative studies, for the identification and assessment of causes and consequences, and the definition of suitable controls (Reiner et al., 2017).
Quality Management System
All the tools described can be used alone or can be implemented in the context of a QM system, in compliance with ISO 9001 standards. Recent examples of the application of QM system in public research institutions have indicated many advantages in terms of governance, control, efficiency, and results (Biasini, 2012; Bongiovanni et al., 2015; Poli et al., 2015; Molinéro-Demilly et al., 2018; Hewera et al., 2020). An ISO-like QM System, slim, flexible and research-oriented has been implemented for the FET Open project entitled VES4US “Extracellular vesicles from a natural source for tailor-made nanomaterials,” conceived since the early beginning with a strong commitment towards Quality culture and principles. The implementation of such QM System supported the project activities, the standardization and sharing of procedures among the different research sites and the achievement of the final objectives (Liguori and Kisslinger, 2020; Adamo et al., 2021; Picciotto et al., 2021).
Discussion and Future Perspectives
The application of QM to scientific research can positively impact the academic research work by implementing interoperability and activities coordination, improving management of resources and data, increasing reliability and reproducibility of results, and ultimately performance, in term of publications, patents, success in grants applications and/or technology transfer, and overall scientific reputation. As shown by the SWOT analysis in Figure 1B, internal obstacles (weaknesses) to implementation of QM tools include a strong initial commitment, the difficulties to dedicate internal resources, in terms of both money and personnel, the average lack of academic researchers’ specific skills in Quality, and last but not least, the scientists’ worry that such tools might affect research freedom and creativity. Moreover, external threats are the difficulties in supplying resources combined to different needs of stakeholders, in many cases the absence of clear mandatory rules, and the objective obstacles in the transfer of QM models from companies or services to public research environments. All these issues make it hard to motivate scientists to approach Quality culture and implement QM tools. On the other side, positive internal factors (strengths) for QM implementation in academic research are the strong push of several research projects to innovation and to having an impact on society challenges, the growing awareness of standardization relevance, and the availability of internal research staff trained in Quality that can guide QM implementation. Key external factors (opportunities) are the increase of exploitation potential of research findings, of positive examples of QM tools implementation in research institutions, of sharing of best practice and QM models, and of training chances closer to research expectations.
EV community is strongly committed to face important themes such as quality control of EV preparations, standardization of isolation and characterization methodologies, and reproducibility of the results, and then might be very prone to adopt Quality principles and methods in their research activities. Cross contamination among EV research and development and QM might produce new tools and methodologies specifically tailored on the challenges and needs of EV research and researchers, that can really support the field, with the minimum impact on flexibility. The pioneering examples here summarized are showing that the use of general Quality principles and processes, alone or in combinations, is not only a viable option but rather the cornerstone in fostering rigor, standardization and reproducibility to fully access to the high potential of EV findings for medical, industrial and environmental applications.
Author Contributions
Both GLL and AK conceived the idea of this article. GLL wrote the first draft and prepared the Figure. GLL and AK revised the article and approved the submitted version.
Funding
This work was supported by grants from Regione Campania (PO FESR 2014–2020 SATIN) to GLL, and the European Union’s Horizon 2020 research and innovation programme (VES4US project, grant agreement No 801338) to AK and GLL.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge Antonella Bongiovanni for critical reading of the manuscript, Mariarosaria Aletta for bibliographic support and Anna Maria Aliperti for English language editing.
References
Adamo, G., Fierli, D., Romancino, D. P., Picciotto, S., Barone, M. E., Aranyos, A., et al. (2021). Nanoalgosomes: Introducing Extracellular Vesicles Produced by Microalgae. J. Extracellular Vesicles 10, e12081. doi:10.1002/jev2.12081
Akyar, I. (2012). “Standard Operating Procedure (What Are They Good for),” in Latest Research into Quality Control. Editor I. Akyar. doi:10.5772/50439
Ayers, L., Pink, R., Carter, D. R. F., and Nieuwland, R. (2019). Clinical Requirements for Extracellular Vesicle Assays. J. Extracellular Vesicles 8, 1593755. doi:10.1080/20013078.2019.1593755
Bazzan, E., Tinè, M., Casara, A., Biondini, D., Semenzato, U., Cocconcelli, E., et al. (2021). Critical Review of the Evolution of Extracellular Vesicles' Knowledge: From 1946 to Today. Ijms 22, 6417. doi:10.3390/ijms22126417
Bergese, P., Bongiovanni, A., Chiari, M., Vainio, S., and Sales, G. (2020). EV Manifesto. Available at: http://www.fetfx.eu/wp-content/uploads/2020/03/EVs-Manifesto_final.pdf.
Biasini, V. (2012). Implementation of a Quality Management System in a Public Research centre. Accred Qual. Assur. 17, 621–626. doi:10.1007/s00769-012-0936-9
Bollin, F., Dechavanne, V., and Chevalet, L. (2011). Design of experiment in CHO and HEK Transient Transfection Condition Optimization. Protein Expr. Purif. 78, 61–68. doi:10.1016/j.pep.2011.02.008
Bongiovanni, A., Colotti, G., Liguori, G. L., Di Carlo, M., Digilio, F. A., Lacerra, G., et al. (2015). Applying Quality and Project Management Methodologies in Biomedical Research Laboratories: a Public Research Network's Case Study. Accred Qual. Assur. 20, 203–213. doi:10.1007/s00769-015-1132-5
Borger, V., Weiss, D. J., Anderson, J. D., Borr, F. E., Verena, B., Bussolati, B., et al. (2020). International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy Statement on Extracellular Vesicles from Mesenchymal Stromal Cells and Other Cells: Considerations for Potential Therapeutic Agents to Suppress Coronavirus Diseases. Cytotherapy 42, 482–485. doi:10.1016/j.jcyt.2020.05.002
Buschmann, D., Mussack, V., and Byrd, J. B. (2021). Separation, Characterization, and Standardization of Extracellular Vesicles for Drug Delivery Applications. Adv. Drug Deliv. Rev. 174, 348–368. doi:10.1016/j.addr.2021.04.027
Clayton, A., Boilard, E., Buzas, E. I., Cheng, L., Falcón-Perez, J. M., Gardiner, C., et al. (2019). Considerations towards a Roadmap for Collection, Handling and Storage of Blood Extracellular Vesicles. J. Extracellular Vesicles 8, 1647027. doi:10.1080/20013078.2019.1647027
Clayton, A., Buschmann, D., Byrd, J. B., Carter, D. R. F., Cheng, L., Compton, C., et al. (2018). Summary of the ISEV Workshop on Extracellular Vesicles as Disease Biomarkers, Held in Birmingham, UK, during December 2017. J. Extracellular Vesicles 7, 1473707. doi:10.1080/20013078.2018.1473707
Cointe, S., Judicone, C., Robert, S., Mooberry, M. J., Poncelet, P., Wauben, M., et al. (2017). Standardization of Microparticle Enumeration across Different Flow Cytometry Platforms: Results of a Multicenter Collaborative Workshop. J. Thromb. Haemost. 15, 187–193. doi:10.1111/jth.13514
Dave, K. M., Zhao, W., Hoover, C., D’Souza, A., and S Manickam, D. (2021). Extracellular Vesicles Derived from a Human Brain Endothelial Cell Line Increase Cellular ATP Levels. AAPS PharmSciTech 22, 1–27. doi:10.1208/s12249-020-01892-w
Dehouck, P., Koeber, R., Scaravelli, E., and Emons, H. (2019). The Integration of Quality Management Systems in Testing Laboratories: a Practitioner's Report. Accred Qual. Assur. 24, 151–156. doi:10.1007/s00769-018-1365-1
Digilio, F. A., Lanati, A., Bongiovanni, A., Mascia, A., Di Carlo, M., Barra, A., et al. (2016). Quality-based Model for Life Sciences Research Guidelines. Accred Qual. Assur. 21, 221–230. doi:10.1007/s00769-016-1205-0
Durakovic, B. (2017). Design of Experiments Application, Concepts, Examples: State of the Art. Pen 5, 421–439. doi:10.21533/pen.v5i3.145
Esteban, P. P., Patel, H., Veraitch, F., and Khalife, R. (2021). Optimization of the Nutritional Environment for Differentiation of Human‐induced Pluripotent Stem Cells Using Design of Experiments-A Proof of Concept. Biotechnol. Prog. doi:10.1002/btpr.3143
Gough, J., and Hamrell, M. (2010). Standard Operating Procedures (SOPs): How to Write Them to Be Effective Tools. Drug Inf. J 44, 463–468. doi:10.1177/009286151004400410
Grangeia, H. B., Silva, C., Simões, S. P., and Reis, M. S. (2020). Quality by Design in Pharmaceutical Manufacturing: A Systematic Review of Current Status, Challenges and Future Perspectives. Eur. J. Pharmaceutics Biopharmaceutics 147, 19–37. doi:10.1016/j.ejpb.2019.12.007
Gregory, C. W. (2020). Building a Quality Management System in a Core Facility: A Genomics Core Case Study. J. Biomol. Tech. 31, 20–3102. doi:10.7171/jbt.20-3102-004
Hadjicostas, E. (2010). ISO 9000 Quality Management System. in Quality Assurance in Analytical Chemistry: Training and Teaching, 45–71. doi:10.1007/978-3-642-13609-2_3
Hattemer-Apostel, R. (2001). Standard Operating Procedures - A Novel Perspective. Qual. Assur. J. 5, 207–219. doi:10.1002/qaj.155
Hellman, P., and Liu, Y. (2013). Development of Quality Management Systems: How Have Disruptive Technological Innovations in Quality Management Affected Organizations? QIP J. 17, 104–119. doi:10.12776/qip.v17i1.154
Hewera, M., Nickel, A.-C., Knipprath, N., Muhammad, S., Fan, X., Steiger, H.-J., et al. (2020). An Inexpensive and Easy-To-Implement Approach to a Quality Management System for an Academic Research Lab. F1000Res 9, 660. doi:10.12688/f1000research.24494.2
Hill, A. F., Pegtel, D. M., Lambertz, U., Leonardi, T., O'Driscoll, L., Pluchino, S., et al. (2013). ISEV Position Paper: Extracellular Vesicle RNA Analysis and Bioinformatics. J. Extracellular Vesicles 2, 22859. doi:10.3402/jev.v2i0.22859
Hollmann, S., Kremer, A., Baebler, Š., Trefois, C., Gruden, K., Rudnicki, W. R., et al. (2021). The Need for Standardisation in Life Science Research - an Approach to Excellence and Trust. F1000Res 9, 1398. doi:10.12688/f1000research.27500.1
Kalluri, R., and LeBleu, V. S. (2020). The Biology , Function , and Biomedical Applications of Exosomes. Science 367. doi:10.1126/science.aau6977
Kalra, H., Simpson, R. J., Ji, H., Aikawa, E., Altevogt, P., Askenase, P., et al. (2012). Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. Plos Biol. 10, e1001450. doi:10.1371/journal.pbio.1001450
Keller, S., Ridinger, J., Rupp, A.-K., Janssen, J. W., and Altevogt, P. (2011). Body Fluid Derived Exosomes as a Novel Template for Clinical Diagnostics. J. Transl. Med. 9. doi:10.1186/1479-5876-9-86
Kim, D.-K., Kang, B., Kim, O. Y., Choi, D.-s., Lee, J., Kim, S. R., et al. (2013). EVpedia: An Integrated Database of High-Throughput Data for Systemic Analyses of Extracellular Vesicles. J. Extracellular Vesicles 2, 20384. doi:10.3402/jev.v2i0.20384
Lacroix, R., Judicone, C., Mooberry, M., Boucekine, M., Key, N. S., Dignat-George, F., et al. (2013). Standardization of Pre-analytical Variables in Plasma Microparticle Determination: Results of the International Society on Thrombosis and Haemostasis SSC Collaborative Workshop. J. Thromb. Haemost. 11, 1190–1193. doi:10.1111/jth.12207
Lacroix, R., Robert, S., Poncelet, P., Kasthuri, R. S., Key, N. S., and Dignat-George, F. (2010). Standardization of Platelet-Derived Microparticle Enumeration by Flow Cytometry with Calibrated Beads: Results of the International Society on Thrombosis and Haemostasis SSC Collaborative Workshop. J. Thromb. Haemost. 8, 2571–2574. doi:10.1111/j.1538-7836.2010.04047.x
Lai, H., Li, Y., Zhang, H., Hu, J., Liao, J., Su, Y., et al. (2022). exoRBase 2.0: an Atlas of mRNA, lncRNA and circRNA in Extracellular Vesicles from Human Biofluids. Nucleic Acids Res. 50, D118–D128. doi:10.1093/nar/gkab1085
Lanati, A. (2018). Quality Management in Scientific Research. Springer International Publishing. doi:10.1007/978-3-319-76750-5
Lee, H., Lee, H., Baik, J., Kim, H., and Kim, R. Y. (2017). Failure Mode and Effects Analysis Drastically Reduced Potential Risks in Clinical Trial Conduct. Dddt Vol. 11, 3035–3043. doi:10.2147/DDDT.S145310
Lener, T., Gimona, M., Aigner, L., Börger, V., Buzas, E., Camussi, G., et al. (2015). Applying Extracellular Vesicles Based Therapeutics in Clinical Trials - an ISEV Position Paper. J. Extracellular Vesicles 4, 30087. doi:10.3402/jev.v4.30087
Liguori, G. L., and Kisslinger, A. (2021). Standardization and Reproducibility in EV Research: the Support of a Quality Management System Biological Membrane Vesicles: Scientific, Biotechnological and Clinical Considerations. Adv. Biomembranes Lipid Self-Assembly (Elsiever), 175–206. doi:10.1016/bs.abl.2020.05.005
Lin, Y., Anderson, J. D., Rahnama, L. M. A., Gu, S. V., and Knowlton, A. A. (2020). Exosomes in Disease and Regeneration: Biological Functions, Diagnostics, and Beneficial Effects. Am. J. Physiology-Heart Circulatory Physiol. 319, H1162–H1180. doi:10.1152/AJPHEART.00075.2020
Lipsitz, Y. Y., Timmins, N. E., and Zandstra, P. W. (2016). Quality Cell Therapy Manufacturing by Design. Nat. Biotechnol. 34, 393–400. doi:10.1038/nbt.3525
Liu, Y., and Chen, Q. (2018). 150 Years of Darwin's Theory of Intercellular Flow of Hereditary Information. Nat. Rev. Mol. Cel Biol 19, 749–750. doi:10.1038/s41580-018-0072-4.150
Lötvall, J., Hill, A. F., Hochberg, F., Buzás, E. I., Di Vizio, D., Gardiner, C., et al. (2014). Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracellular Vesicles 3, 26913. doi:10.3402/jev.v3.26913
Lötvall, J., Rajendran, L., Gho, Y.-S., Thery, C., Wauben, M., Raposo, G., et al. (2012). The Launch of Journal of Extracellular Vesicles (JEV), the Official Journal of the International Society for Extracellular Vesicles - about Microvesicles, Exosomes, Ectosomes and Other Extracellular Vesicles. J. Extracellular Vesicles 1, 18514. doi:10.3402/jev.v1i0.18514
Lovrenčić Mikelić, I. (20202030). Evolution of Quality Management System at the Ruđer Bošković institute. 17th IMEKO TC 10 EUROLAB Virtual Conf. “Global Trends Testing, Diagn. Insp, 61–66.
Mancinelli, S., Turcato, A., Kisslinger, A., Bongiovanni, A., Zazzu, V., Lanati, A., et al. (2021). Design of Transfections: Implementation of Design of Experiments for Cell Transfection fine Tuning. Biotech. Bioeng. 118, 4488–4502. doi:10.1002/bit.27918
Mancinelli, S., Zazzu, V., Turcato, A., Lacerra, G., Digilio, F. A., Mascia, A., et al. (2015). Mathematical Models in Biology. Springer International Publishing, 45–63. doi:10.1007/978-3-319-23497-7_4Applying Design of Experiments Methodology to PEI Toxicity Assay on Neural Progenitor Cells
Mandenius, C.-F., and Brundin, A. (2008). Bioprocess Optimization Using Design-Of-Experiments Methodology. Biotechnol. Prog. 24, 1191–1203. doi:10.1002/btpr.67
Mantile, F., Franco, P., Stoppelli, M. P., and Liguori, G. L. (2021). Biological Role and Clinical Relevance of Extracellular Vesicles as Key Mediators of Cell Communication in Cancer Biological Membrane Vesicles: Scientific, Biotechnological and Clinical Considerations. Adv. Biomembranes Lipid Self-Assembly (Elsiever), 37–117. doi:10.1016/bs.abl.2020.05.006
Margolis, L., and Sadovsky, Y. (2019). The Biology of Extracellular Vesicles: The Known Unknowns. Plos Biol. 17, e3000363. doi:10.1371/journal.pbio.3000363
Mascia, A., Cirafici, A. M., Bongiovanni, A., Colotti, G., Lacerra, G., Di Carlo, M., et al. (2020). A Failure Mode and Effect Analysis (FMEA)-based Approach for Risk Assessment of Scientific Processes in Non-regulated Research Laboratories. Accred Qual. Assur. 25, 311–321. doi:10.1007/s00769-020-01441-9
Mateescu, B., Kowal, E. J. K., van Balkom, B. W. M., Bartel, S., Bhattacharyya, S. N., Buzás, E. I., et al. (2017). Obstacles and Opportunities in the Functional Analysis of Extracellular Vesicle RNA - an ISEV Position Paper. J. Extracellular Vesicles 6, 1286095. doi:10.1080/20013078.2017.1286095
Meldolesi, J. (2018). Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 28, R435–R444. doi:10.1016/j.cub.2018.01.059
Molinéro-Demilly, V., Charki, A., Jeoffrion, C., Lyonnet, B., O'Brien, S., and Martin, L. (2018). An Overview of Quality Management System Implementation in a Research Laboratory. Int. J. Metrol. Qual. Eng. 9, 2. doi:10.1051/ijmqe/2017025
Myers, R. H., Montgomery, D. C., and Christine, Anderson-Cook. (2016). Response Surface Methodology: Process and Product Optimization Using Designed Experiments. 4th ed. Wileys.
Narenderan, S. T., Meyyanathan, S. N., and Karri, V. V. S. R. (2019). Experimental Design in Pesticide Extraction Methods: A Review. Food Chem. 289, 384–395. doi:10.1016/j.foodchem.2019.03.045
Nieuwland, R., Falcón‐Pérez, J. M., Théry, C., and Witwer, K. W. (2020). Rigor and Standardization of Extracellular Vesicle Research: Paving the Road towards Robustness. J. Extracellular Vesicles 10. doi:10.1002/jev2.12037
N. Politis, S., Colombo, P., Colombo, G., and M. Rekkas, D. (2017). Design of Experiments (DoE) in Pharmaceutical Development. Drug Dev. Ind. Pharm. 43, 889–901. doi:10.1080/03639045.2017.1291672
Papaneophytou, C. (2019). Design of Experiments as a Tool for Optimization in Recombinant Protein Biotechnology: From Constructs to Crystals. Mol. Biotechnol. 61, 873–891. doi:10.1007/s12033-019-00218-x
Papaneophytou, C., Zervou, M.-E., and Theofanous, A. (2021). Optimization of a Colorimetric Assay to Determine Lactate Dehydrogenase B Activity Using Design of Experiments. SLAS DISCOVERY: Advancing Sci. Drug Discov. 26, 383–399. doi:10.1177/2472555220956589
Paterna, A., Rao, E., Adamo, G., Raccosta, S., Picciotto, S., Romancino, D., et al. (2022). Isolation of Extracellular Vesicles Form Microalgae: A Renewable and Scalable Bioprocess. Front. Bioeng. Biotechnol.. doi:10.3389/fbioe.2022.836747
Petretta, M., Desando, G., Grigolo, B., and Roseti, L. (2021). Cartilage Tissue Engineering by Extrusion Bioprinting: Process Analysis, Risk Evaluation, and Mitigation Strategies. Materials 14, 3528. doi:10.3390/ma14133528
Picciotto, S., Barone, M. E., Fierli, D., Aranyos, A., Adamo, G., Božič, D., et al. (2021). Isolation of Extracellular Vesicles from Microalgae: towards the Production of Sustainable and Natural Nanocarriers of Bioactive Compounds. Biomater. Sci. 9, 2917–2930. doi:10.1039/D0BM01696A
Poli, M., Pardini, S., Passarelli, I., Citti, I., Cornolti, D., and Picano, E. (2015). The 4A's Improvement Approach: a Case Study Based on UNI EN ISO 9001:2008. Total Qual. Manag. Business Excell. 26, 1113–1130. doi:10.1080/14783363.2014.912456
Poupardin, R., Wolf, M., and Strunk, D. (2021). Adherence to Minimal Experimental Requirements for Defining Extracellular Vesicles and Their Functions. Adv. Drug Deliv. Rev. 176, 113872. doi:10.1016/j.addr.2021.113872
Raposo, G., and Stoorvogel, W. (2013). Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cel Biol. 200, 373–383. doi:10.1083/jcb.201211138
Reiner, A. T., Witwer, K. W., van Balkom, B. W. M., de Beer, J., BrodieLi., C., Corteling, R. L., et al. (20172017). Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. STEMCELLS Transl. Med. 6, 1730–1739. doi:10.1002/sctm.17-0055
Rovira, J., Diekmann, F., Campistol, J. M., and Ramírez-Bajo, M. J. (2017). Therapeutic Application of Extracellular Vesicles in Acute and Chronic Renal Injury. Nefrología (English Edition) 37, 126–137. doi:10.1016/j.nefroe.2017.03.004
Roy, S., Hochberg, F. H., and Jones, P. S. (2018). Extracellular Vesicles: the Growth as Diagnostics and Therapeutics; a Survey. J. Extracellular Vesicles 7, 1438720. doi:10.1080/20013078.2018.1438720
Royo, F., Théry, C., Falcón-Pérez, J. M., Nieuwland, R., and Witwer, K. W. (2020). Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 9, 1955. doi:10.3390/cells9091955
Subramanian, S. L., Kitchen, R. R., Alexander, R., Carter, B. S., Cheung, K.-H., Laurent, L. C., et al. (2015). Integration of Extracellular RNA Profiling Data Using Metadata, Biomedical Ontologies and Linked Data Technologies. J. Extracellular Vesicles 4, 27497. doi:10.3402/jev.v4.27497
Tamura, T., Yoshioka, Y., Sakamoto, S., Ichikawa, T., and Ochiya, T. (2021). Extracellular Vesicles as a Promising Biomarker Resource in Liquid Biopsy for Cancer. Evcna. doi:10.20517/evcna.2021.06
Tavares Luiz, M., Santos Rosa Viegas, J., Palma Abriata, J., Viegas, F., Testa Moura de Carvalho Vicentini, F., Lopes Badra Bentley, M. V., et al. (2021). Design of Experiments (DoE) to Develop and to Optimize Nanoparticles as Drug Delivery Systems. Eur. J. Pharmaceutics Biopharmaceutics 165, 127–148. doi:10.1016/j.ejpb.2021.05.011
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): a Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles. doi:10.1080/20013078.2018.1535750
Toms, D., Deardon, R., and Ungrin, M. (2017). Climbing the Mountain: Experimental Design for the Efficient Optimization of Stem Cell Bioprocessing. J. Biol. Eng. 11. doi:10.1186/s13036-017-0078-z
Van Der Pol, E., Welsh, J. A., and Nieuwland, R. (2021). Minimum Information to Report about a Flow Cytometry experiment on Extracellular Vesicles: Communication from the ISTH SSC Subcommittee on Vascular Biology. J. Thromb. Haemost 20, 245–251. doi:10.1111/jth.15540
Van Deun, J., Van Deun, J., Mestdagh, P., Agostinis, P., Akay, Ö., Anand, S., et al. (2017). EV-TRACK: Transparent Reporting and Centralizing Knowledge in Extracellular Vesicle Research. Nat. Methods 14, 228–232. doi:10.1038/nmeth.4185
Van Niel, G., D'Angelo, G., and Raposo, G. (2018). Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cel Biol. 19, 213–228. doi:10.1038/nrm.2017.125
Vozel, D., Božič, D., Jeran, M., Jan, Z., Pajnič, M., Pađen, L., et al. (2021). Autologous Platelet- and Extracellular Vesicle-Rich Plasma Is an Effective Treatment Modality for Chronic Postoperative Temporal Bone Cavity Inflammation: Randomized Controlled Clinical Trial. Front. Bioeng. Biotechnol. 9, 1–11. doi:10.3389/fbioe.2021.677541
Weissman, S. A., and Anderson, N. G. (2015). Design of Experiments (DoE) and Process Optimization. A Review of Recent Publications. Org. Process. Res. Dev. 19, 1605–1633. doi:10.1021/op500169m
Welsh, J. A., Tang, V. A., Pol, E., and Görgens, A. (2021). MIFlowCyt‐EV: The Next Chapter in the Reporting and Reliability of Single Extracellular Vesicle Flow Cytometry Experiments. Cytometry 99, 365–368. doi:10.1002/cyto.a.24268
Welsh, J. A., Van Der Pol, E., Arkesteijn, G. J. A., Bremer, M., Brisson, A., Coumans, F., et al. (2020). MIFlowCyt‐EV: a Framework for Standardized Reporting of Extracellular Vesicle Flow Cytometry Experiments. J. Extracellular Vesicles 9, 1713526. doi:10.1080/20013078.2020.1713526
Witwer, K. W., Buzás, E. I., Bemis, L. T., Bora, A., Lässer, C., Lötvall, J., et al. (2013). Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracellular Vesicles 2, 20360. doi:10.3402/jev.v2i0.20360
Witwer, K. W., Goberdhan, D. C., O'Driscoll, L., Théry, C., Welsh, J. A., Blenkiron, C., et al. (2021). Updating MISEV: Evolving the Minimal Requirements for Studies of Extracellular Vesicles. J. Extracellular Vesicle 10. doi:10.1002/jev2.12182
Witwer, K. W., Soekmadji, C., Hill, A. F., Wauben, M. H., Buzás, E. I., Di Vizio, D., et al. (2017). Updating the MISEV Minimal Requirements for Extracellular Vesicle Studies: Building Bridges to Reproducibility. J. Extracellular Vesicles 6, 1396823. doi:10.1080/20013078.2017.1396823
Xu, L., Faruqu, F. N., Liam‐or, R., Abu Abed, O., Li, D., Venner, K., et al. (2020). Design of experiment (DoE)‐driven In Vitro and In Vivo Uptake Studies of Exosomes for Pancreatic Cancer Delivery Enabled by Copper‐free Click Chemistry‐based Labelling. J. Extracellular Vesicles 9, 1779458. doi:10.1080/20013078.2020.1779458
Yáñez-Mó, M., Siljander, P. R.-M., Andreu, Z., Bedina Zavec, A., Borràs, F. E., Buzas, E. I., et al. (2015). Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracellular Vesicles 4, 27066. doi:10.3402/jev.v4.27066
Zarovni, N., Loria, F., Zenatelli, R., Mladenovic, D., Paolini, L., Adamo, G., et al. (2021). CHAPTER 12. Standardization and Commercialization of Extracellular Vesicles. Extracellular Vesicles: Appl. Regenerative Med. Ther. Diagn., 303–335. doi:10.1039/9781839164552-00303
Zimmermann, H. F., and Hentschel, N. (2011). Proposal on How to Conduct a Biopharmaceutical Process Failure Mode and Effect Analysis (FMEA) as a Risk Assessment Tool. PDA J. Pharm. Sci. Tech. 65, 506–512. doi:10.5731/pdajpst.2011.00784
Keywords: extracellular vesicles, quality management (QM), optimization, standardization, risk analysis, design of experiments (DoE), standard operating procedure (SOP), failure mode and effect analysis (FMEA)
Citation: Liguori GL and Kisslinger A (2022) Quality Management Tools on the Stage: Old but New Allies for Rigor and Standardization of Extracellular Vesicle Studies. Front. Bioeng. Biotechnol. 10:826252. doi: 10.3389/fbioe.2022.826252
Received: 30 November 2021; Accepted: 31 January 2022;
Published: 10 March 2022.
Edited by:
Marco P. Monopoli, Royal College of Surgeons in Ireland, IrelandReviewed by:
Aleksandra Leszczynska, University of California, San Diego, United StatesAnnalisa Radeghieri, University of Brescia, Italy
Copyright © 2022 Liguori and Kisslinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanna L. Liguori, Z2lvdmFubmEubGlndW9yaUBpZ2IuY25yLml0
 Giovanna L. Liguori
Giovanna L. Liguori Annamaria Kisslinger
Annamaria Kisslinger