- 1School of Information Engineering, Xijing University, Xi’an, China
- 2College of Grassland and Environment Science, Xinjiang Agricultural University, Urumqi, China
The prediction of protein–protein interactions (PPIs) in plants is vital for probing the cell function. Although multiple high-throughput approaches in the biological domain have been developed to identify PPIs, with the increasing complexity of PPI network, these methods fall into laborious and time-consuming situations. Thus, it is essential to develop an effective and feasible computational method for the prediction of PPIs in plants. In this study, we present a network embedding-based method, called DWPPI, for predicting the interactions between different plant proteins based on multi-source information and combined with deep neural networks (DNN). The DWPPI model fuses the protein natural language sequence information (attribute information) and protein behavior information to represent plant proteins as feature vectors and finally sends these features to a deep learning–based classifier for prediction. To validate the prediction performance of DWPPI, we performed it on three model plant datasets: Arabidopsis thaliana (A. thaliana), mazie (Zea mays), and rice (Oryza sativa). The experimental results with the fivefold cross-validation technique demonstrated that DWPPI obtains great performance with the AUC (area under ROC curves) values of 0.9548, 0.9867, and 0.9213, respectively. To further verify the predictive capacity of DWPPI, we compared it with some different state-of-the-art machine learning classifiers. Moreover, case studies were performed with the AC149810.2_FGP003 protein. As a result, 14 of the top 20 PPI pairs identified by DWPPI with the highest scores were confirmed by the literature. These excellent results suggest that the DWPPI model can act as a promising tool for related plant molecular biology.
Introduction
Prediction of protein–protein interactions (PPIs) in plants is of great biological importance (Fukao, 2012). Cells receive endogenous signals to regulate their gene expression under a special signaling pathway. In this process, proteins play an essential role in regulating and mediating the biological activities of plant cells (Lehti-Shiu and Shiu, 2012). In addition, the identification of PPIs not only helps understand how proteins perform their biological functions but also provides essential information for rational drug design. Traditional biological experimental methods, such as mass spectrometry (Woods et al., 2011), tandem affinity purification (Rohila et al., 2009), and yeast-two hybrid (Fang et al., 2002) were used. Nevertheless, these conventional approaches are costly, time-consuming, and prone to high false-positive rates. Thus, the development of novel computational models to identify potential PPIs would be of enormous value to plant genomics and genetics.
Recently, many bioinformatic methods have been proposed for identifying PPIs. These approaches can be roughly split into three categories: docking-based methods (Yan et al., 2017), structure-based methods (Hayashi et al., 2018), and sequence-based methods (Pan et al., 2021). Typically, the first two techniques perform better than the sequence-based methods. However, docking- and structure-based methods usually need the structural details of proteins. Problems arise when these prior data do not exist. Moreover, with the evolution of genome sequencing technology, a vast number of protein sequences have been discovered. Against this backdrop, the sequence-based approaches have attracted increasing attention. Most of the computational approaches adopt the machine learning algorithms such as support vector machine (Guo et al., 2008; Romero-Molina et al., 2019; Chakraborty et al., 2021), random forest (Li et al., 2012; Wang et al., 2019; Yang et al., 2020), and K-nearest neighbor (Ambert and Cohen, 2011; Ning et al., 2019). Some studies have also combined machine learning techniques with feature descriptors of protein sequences to predict PPIs, such as the Moran and Geary autocorrelation descriptor (Chen et al., 2020), conjoint Triad descriptor (Shen et al., 2007), and multi-scale local feature descriptors (You et al., 2015). These feature descriptors aim to summarize the information of 20 canonical amino acid sequences for PPI prediction.
Unlike the traditional machine learning approaches, deep learning-based approaches can not only extract high-dimensional features directly from the primary sequence (Ekbal et al., 2016; Zeng et al., 2020; Wang J. et al., 2021) but also capture their non-linear dependencies to increase prediction accuracy. Therefore, deep learning algorithms have been widely applied to predict associations between different biomolecules in recent years. For example, Czibula et al. (2021) introduced a method called AutoPPI to predict PPIs that used two autoencoders, which correspond to three kinds of neural network architectures. Qiang et al. (2020) presented an approach named CPPred-FL that used multiple feature descriptors to identify cell-penetrating peptides. CPPred-FL introduced a novel feature representation learning scheme to capture features from different perspectives. Huang et al. (2021) proposed a method, called MVMTMDA, for predicting microRNA–disease associations (MDAs). This model creates a multi-view representation of microRNAs that can predict MDAs via an end-to-end multitasking technique. Yuan et al. (2021) developed a deep graph-based framework named GraphPPIS for identifying PPIs. GraphPPIS transformed the prediction problem of PPI sites as a graph node classification task, which can be solved via deep learning techniques.
Recently, some studies have indicated that the information of network data is useful in prediction problems, including position, degree, and neighboring nodes in the graph. For example, Lim et al. (2019) presented a graph neural network, which used a distance-aware graph attention technique to predict drug-target interactions. Zhao et al. (2020) predict PPIs that combined the spatial relationship of protein sequence with the potential sequential feature of the ontological annotation semantics. Xu et al. (2020) developed a method called PPI-GE, which predicts PPIs by combining the contact graph energy and physicochemical graph energy. Xiao and Deng (2020) proposed a new node embedding approach to predict PPIs that captures the topological information from higher-order neighborhoods of PPI network nodes. Li et al. (2021) built a novel model called GAEMDA that used a graph neural network-based encoder to detect the miRNA-disease associations. Wang L. et al. (2021) presented a novel framework named NMFCDA to identify CircRNA-disease association by combining kernel similarity information, disease semantic information, and protein sequence information. Zheng et al. (2019) built a model called MLMDA to predict MDAs. This model combined miRNA functional similarity, Gaussian interaction profile kernel similarity information and disease semantic similarity with deep auto-encoder neural network and random forest classifier for the MDAs prediction. Guo et al. (2019) proposed a computational approach named LDASR to identify potential associations between lncRNAs and diseases. The method abstracted feature vectors for lncRNA and disease from multiple similarity matrices and the rotational forest algorithm is used for carrying the prediction.
Inspired by these graph embedding methods, we propose a novel efficient computational approach called DWPPI to predict potential protein–protein interactions in plants. This model employed two critical information: the original attribute information of the protein sequence, and the behavior information of the PPI graph network. To be specific, we first constructed a plants protein–protein bipartite graph to summarize the associations between these proteins, in which each plant protein is represented by a node, and each link represents their association. Then, we employed a graph embedding algorithm method, Deepwalk, to capture behavior information from the links, and used a word embedding algorithm, word2vec to encode the protein sequence for extracting attribute information. Thirdly, the behavior and attribute information were combined to form the fusion matrix, which is finally fed into a deep neural network (DNN) to predict potential plant-protein pairs. For evaluating the performance of the proposed method, we tested it on three model plant PPI datasets (including Arabidopsis thaliana, Zea mays, and Oryza sativa) based on fivefold cross validation (5-fold CV). As a result, DWPPI obtained 89.47, 95.00, and 85.63% prediction accuracy with the AUC of 0.9548, 0.9867, and 0.9213 on the three datasets, respectively. In comparison with different feature descriptors and machine learning-based classifiers, DWPPI also yields good predictive performance. Besides, we also tested a case study on the AC149810.2_FGP003 protein of the Zea mays dataset. Finally, 14 of the top 20 plant–protein interaction pairs with the highest prediction scores were confirmed in the published literature. These experimental results further demonstrated that our model brings new insights for discovering and exploring the intermolecular interactions.
Results
Evaluation Metrics
In this article, 5-fold CV was used to access the predictive performance of the DWPPI model. First, all the plant–protein pairs were randomly divided into five parts, which were disjoint and roughly equal. Second, four of the parts were used as the training set to train DWPPI, and the remaining one was adopted as the test set to yield the prediction results. Lastly, different sections were selected in turn as the training set, and step 2 was repeated until all sections were taken once and only once as the test set. The final experimental results were obtained by averaging the performance of five replicates. In this work, five parameters such as accuracy (Acc), Sensitivity (Sen), Specificity (Spec), Precision (PR.), and Matthew correlation coefficient (MCC) were performed to assess the predictive performance, which can be defined as:
In the above formulas,
Performance Evaluation Using Fivefold Cross Validation
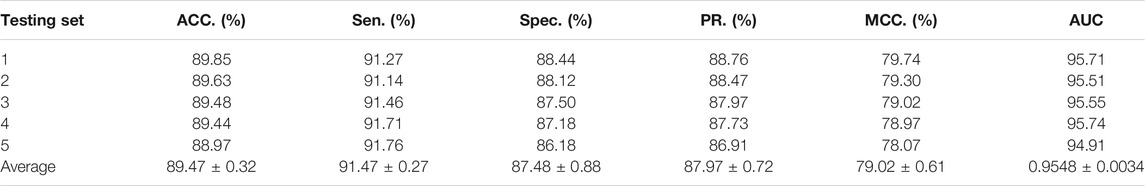
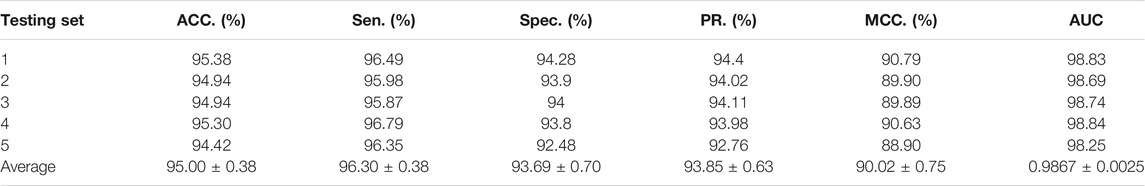
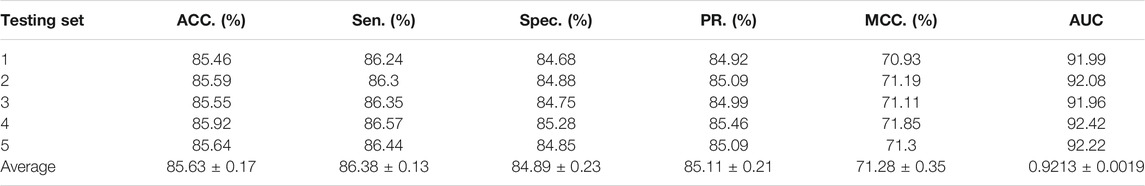
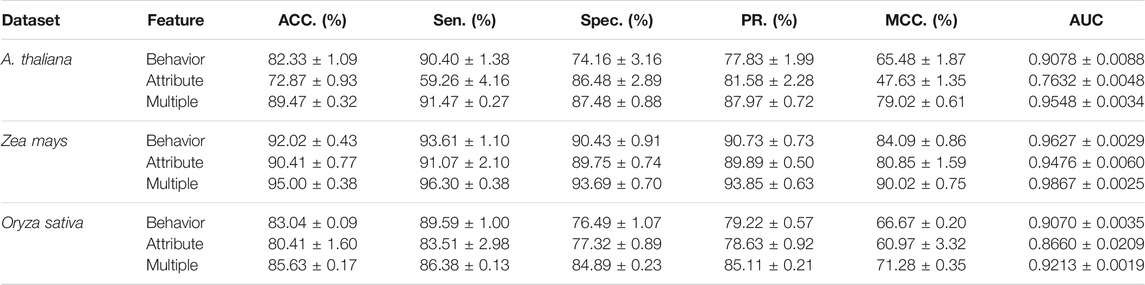
To access the capabilities of DWPPI, we performed it on the A. thaliana, Zea mays, and Oryza sativa datasets, respectively. To obtain better predictive stability and accuracy, we combined the behavior feature and attribute feature as the multiple feature to predict PPIs in plants. Table 1 summarizes the experimental results on the A. thaliana dataset, from which we can observe that the average ACC of fivefold CV method is 89.47%, the Sen is 91.47%, the Spec is 87.48%, the PR is 87.97%, the MCC is 79.02%, and AUC value is 0.9548, respectively. Their standard deviations are 0.32, 0.27, 0.88, 0.72, and 0.61% and 0.0034, respectively. Among the five sets of predictive performance, the lowest accuracy rate came to 88.97% and the best result rate of up to 89.85%. The experimental results of 5-fold CV on the Zea mays dataset are listed in Table 2. Here, it can be observed that the average ACC, Sen, Spec, PR, MCC, and AUC value obtained by DWPPI are 95.00, 96.30, 93.69, 93.85, 90.02% and 0.9867, respectively. The standard deviations are 0.38, 0.38, 0.70, 0.63, 0.75% and 0.0025, respectively. Table 3 lists the prediction results of the Oryza sativa dataset. The average ACC, Sen, Spec, PR, MCC and AUC value by 5-fold CV are 85.63, 86.38, 84.89, 85.11, 71.28%, and 0.9213, respectively. Their standard deviations are 0.17, 0.13, 0.23, 0.21, 0.35% and 0.0019, respectively. Figures 1–3 show the ROC and PR curves generated by the DWPPI model on the A. thaliana, Zea mays, and Oryza sativa PPI datasets, respectively.
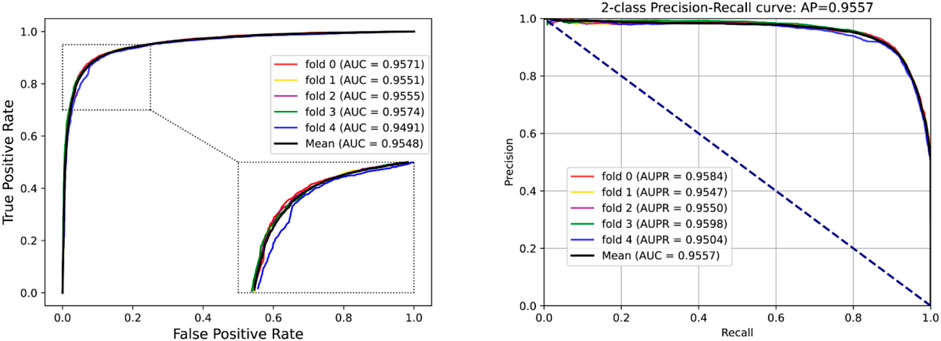
FIGURE 1. ROC and PR curves yielded by the DWPPI model on the A. thaliana dataset with the multiple feature.
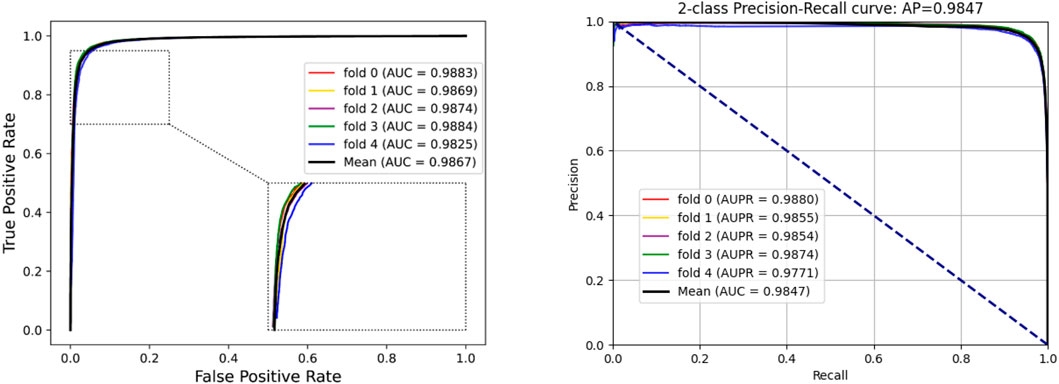
FIGURE 2. ROC and PR curves yielded by the DWPPI model on the Zea mays dataset with the multiple feature.
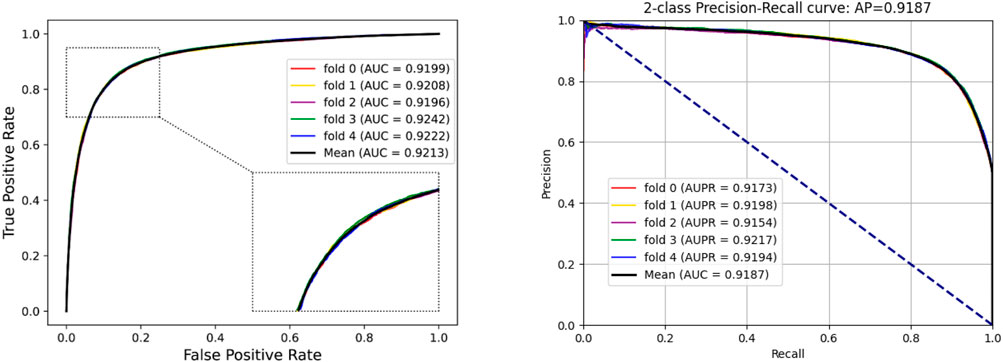
FIGURE 3. ROC and PR curves yielded by the DWPPI model on the Oryza sativa dataset with the multiple feature.
Performance Comparison of Different Classifiers on DWPPI
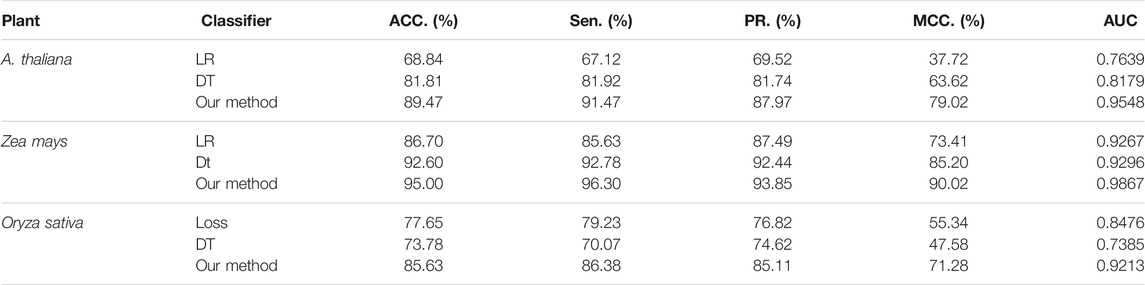
In the prediction framework of the DWPPI model, we adopted the deep neural network (DNN) to classify the interaction between different plant proteins. In order to validate the effect of DNN on the DWPPI model, we made a comparison of the DNN model with some different classifier models. More concretely, we keep the multiple feature of the DWPPI model unchanged and experimented with some different classifiers instead of DNN, including logistic regression (LR) and decision trees (DT). The experimental results produced by these classifiers on the three plant PPI datasets are summarized in Table 4. It can be observed from Table 4 that the proposed model with the DNN as the classifier obtained significantly higher ACC and AUC values compared to other classifier models. For visual comparison, we present the ACC and AUC values as a histogram in Figure 4. These results indicated that DNN classifiers are applicable for the plant PPI prediction. The main reason for this performance is that the proposed deep learning framework can effectively mine the deep information embedded in the PPI network and significantly help increase the model performance.
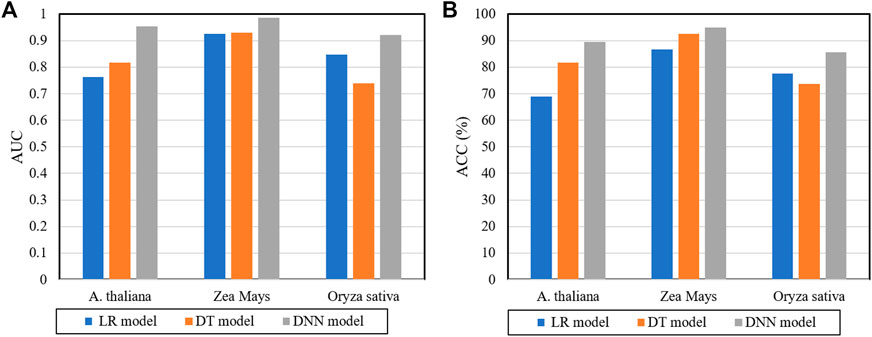
FIGURE 4. Comparison results of different classifiers with the DWPPI model (A) is the predicted AUC values of different classifiers on three plants PPIs datasets. (B) is the predicted ACC values of different classifiers on the three plants PPIs datasets.
Comparison of the Multiple Feature With the Attribute Feature and Behavior Feature
To further evaluate the efficiency of the proposed feature representation, we also performed experiments on the DNN model that only used the signal behavior or attribute feature via 5-fold CV. Table 5 provides the comparison results of the multiple feature with the feature that only used the behavior or attribute feature. In detail, the average predictive accuracy using the behavior information on A. thaliana, Zea mays, and Oryza sativa datasets was 82.33, 92.02, and 83.04%, and the yielded AUC values were 0.9078, 0.9627, and 0.9070, respectively. The average prediction results of using the attribute information on these datasets were 72.87, 90.41, and 80.41%; the obtained AUC values were 0.7632, 0.9476, and 0.8660, respectively. Taking the A. thaliana dataset as an example, the ACC gap between multiple and behavior features is 7.14%. Similarity, the ACC gap between multiple and attribute features is 16.6%. Compared with the results obtained by the multiple feature, we can conclude that employing the behavior or attribute feature alone cannot obtain better prediction results. All these experimental results demonstrated that the proposed multiple feature could help predict potential interaction between plant–protein pairs.
Case Study
To further evaluate the predictive ability of DWPPI, we performed a case study based on the Zea mays dataset. In the experiment, the AC149810.2_FGP003 protein was chosen to construct the case study, and all known protein–protein interactions provided by the Zea mays dataset were used to train DWPPI. The testing set was the PPI pairs consisting of the AC149810.2_FGP003 protein. After yielding the predicted results, we verified the top 20 PPI pairs with the highest predicted scores in the newly published literature. As shown in Table 6, 14 of the top 20 predicted proteins are verified in the experimental data provided by the PPIM dataset. The point to note is that the other six protein pairs of the unknown interaction are not proved by the literature, and there is no denying the possibility of interaction between them.
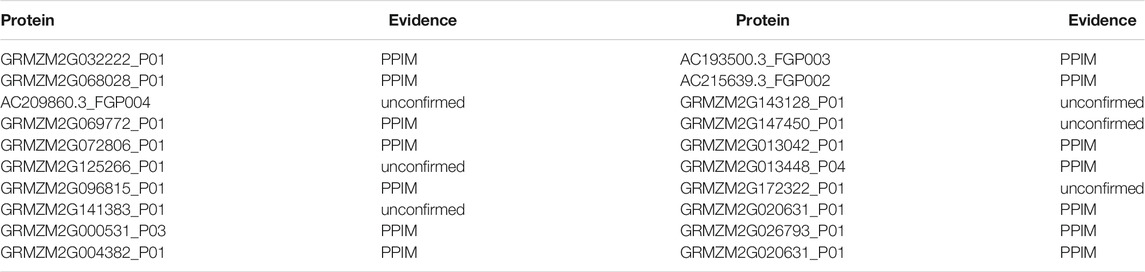
TABLE 6. Prediction of the top 14 predicted proteins based on known interactions on the Zea mays dataset.
Materials and Methods
Data Collection
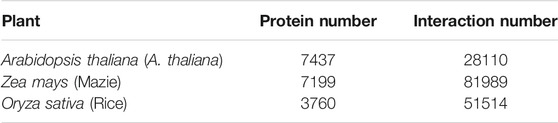
To evaluate the predictive performance of the DWPPI model, we applied it on three publicly available and widely used model plant datasets, Arabidopsis thaliana (A. thaliana), maize (Zea mays), and rice (Oryza sativa). Concretely, A. thaliana holds an esteemed position in the field of plant research and it makes a major contribution to the development of the plant protection, and increases the production of crops. The A. thaliana dataset was collected from public databases including IntAct (Kerrien et al., 2012), TAIR (Rhee et al., 2003), and BioGRID (Oughtred et al., 2019). After discarding the redundant PPIs, we yielded 28,110 PPI pairs from 7,437 different A. thaliana proteins. Although some negative sampling schemes had been developed previously, there is no single gold standard for constructing the non-interaction samples. The most widespread method is to select pairs randomly from non-interacted samples. The number of possible non-interaction pairs is 55,280,859 (7437 × 7437 − 28110), and we randomly selected 28,110 pairs as the negative samples for the A. thaliana dataset. Consequently, the whole A. thaliana dataset consisted of whole 56,220 protein pairs. We also tested DWPPI on the maize (Zea mays) and rice (Oryza sativa) datasets, which are two of world’s most economically important crops. For the Zea mays dataset, we collected 81,989 positive samples covering 7,199 different maize proteins from the PPIM database (Zhu et al., 2016). Similarly, we randomly selected 81,989 protein pairs from different subcellular localizations as the negative samples. Finally, Oryza sativa was constructed by 103,028 samples covering 3,760 types of rice proteins from the PRIN database (Gu et al., 2011). The number of proteins and interactions for these three model plant datasets are summarized in Table 7.
Behavior Information
As a widely used graph-embedding approach, Deepwalk (Perozzi et al., 2014) was applied in the plant interaction network to represent the potential relationship of the vertices. In this work, let
where
The skip-gram algorithm was used to iterate over all detected matches of the sequence in window
where
The embedding matrix
where
Attribute Representation
In the DWPPI model, the word2vec algorithm (Mikolov et al., 2013a) was used to embed the protein sequence for capturing the attribute information of plant proteins. There are two main models in word2vec: 1) continuous bag-of-words model (CBOW) and 2) continuous skip-gram model (Skip-Gram). The difference between the CBOW and Skip-Gram model is that CBOW uses the context to predict the current words, while Skip-Gram applies the current word to predict the context. If the training data are not very big, the Skip-Gram method will be more efficient. In our experiment, considering the size of our plant PPI dataset, we selected the CBOW model of the word2vec algorithm to learn more frequent words and speed up the training time.
The amino acid sequences of these plant proteins were encoded as matrixes via the word2vec algorithm to extract the attribute information of plant nodes. The k-mers (k consecutive amino acids) method was used to regard the sequence as a word, and each protein sequence will be expressed as multiple k-mers. As shown in Figure 5, given a sequence MNLLLFFL, the unit of the 4-mers are MNLL, NLLL, LLLF, LLFF, and LFFL. To speed up the computation, the CBOW-based word2vec algorithm was selected to study the appearance pattern of the k-mers. Here, the protein sequences and k-mers correspond to the sentences and words in a natural language, respectively. In this work, the trained word2vec model will generate 64-dimensional embedding vectors in each k-mer to construct the embedding matrix of each protein. In the previous study, the 4-mer had been proved that it can achieve the optimal prediction accuracy via the 5-fold CV method.
Deep Neural Network
Deep learning supports highly flexible architectures. In recent years, deep learning-based techniques have been widely used in the field of bioinformatics, such as recurrent neural network (RNN) (Kavuluru et al., 2017), deep belief network (DBN) (Wen et al., 2017), convolutional neural network (CNN) (Rifaioglu et al., 2020), and so on. Different deep learning architectures are appropriate for different problems. For example, RNN is suitable for exploring the sequential information, DBN is always used to account for high-dimensional correlations of biological data, and CNN is capable of extracting input complex features at different spatial scales (Lecun et al., 2015). Considering the interactions in plant proteins and the possible high dimension of behavior and attribute information, we used DNN as the architecture to predict potential PPIs in plants.
DNN is composed of an input layer, multiple hidden layers, and an output layer. Typically, the neural networks are fed data from the input layer, and then they will be transformed through the hidden layers in a non-linear way and the final result will be calculated to the output layer. The neurons in the hidden and output layers will be linked to all neurons in the previous layer. Each neuron computes a weighted sum of its inputs and utilizes a nonlinear activation function to derive its outputs
where
Conclusion
Predicting protein–protein interactions in plants help study the gene function of plans and also help understand essential roles thatthey play in a variety of biological processes. Systematically predicting potential plant–protein pairs will help increase crop yields. Compared to traditional wet experimental approaches, the dry experimental methods based on soft computing help analyze large-scale genetic data to detect new interactions between them. Thanks to the development of computing and storage capabilities of computers, the computational method helps quickly achieve scientific research results without the need for cell staining and pipettes. Moreover, the computational approaches effectively remove false positive signals, reduce unreliable results, and increase the chance of finding real but weak signals.
In this work, we used a natural language processing algorithm to describe the attribute information of protein nodes, and a graph embedding technique was used to represent the behavior information of protein links. Then, we combined the behavior and attribute information as the multiple feature to further improve the prediction power of the DWPPI model. The deep learning-based DNN classifier was adopted to train and predict these features. The presented DWPPI model integrates these algorithms organically and takes full advantage of their superiority, thus yielding excellent results in the experiment. In the 5-fold CV experiment, when performed on the model plant PPI datasets, Arabidopsis thaliana, Zea mays, and Oryza sativa, the proposed model obtains 89.47, 95.00, and 85.63% prediction accuracy with 0.9548, 0.9867, and 0.9213 AUC values, respectively. In further studies, we will investigate more natural language processing methods for problems of potential protein–protein interaction prediction in plants.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: Publicly available datasets were analyzed in this study. This data can be found here: http://arabidopsis.org/; http://www.ebi.ac.uk/intact; http://www.thebiogrid.org/; http://comp-sysbio.org/ppim; http://bis.zju.edu.cn/prin/.
Author Contributions
Conceptualization, methodology, and software, JP; validation, formal analysis, L-PL; investigation, W-ZH; resources, J-XG; data curation and visualization, C-QY; writing—original draft preparation, JP; writing—review and editing, L-PW; supervision, JP; project administration, Z-YZ; funding acquisition, Z-HY. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Science and Technology Innovation 2030-New Generation Artificial Intelligence Major Project (No.2018AAA0100103), and in part by the NSFC Program, under Grant 61873212, 62072378, and 62002297.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank all anonymous reviewers for their constructive comments.
References
Ambert, K. H., and Cohen, A. M. (2011). K-information Gain Scaled Nearest Neighbors: a Novel Approach to Classifying Protein-Protein Interaction-Related Documents. Ieee/acm Trans. Comput. Biol. Bioinform 9, 305–310. doi:10.1109/TCBB.2011.32
Angermueller, C., Pärnamaa, T., Parts, L., and Stegle, O. (2016). Deep Learning for Computational Biology. Mol. Syst. Biol. 12, 878. doi:10.15252/msb.20156651
Chakraborty, A., Mitra, S., De, D., Pal, A. J., Ghaemi, F., Ahmadian, A., et al. (2021). Determining Protein-Protein Interaction Using Support Vector Machine: A Review. IEEE Access.
Chen, C., Zhang, Q., Yu, B., Yu, Z., Lawrence, P. J., Ma, Q., et al. (2020). Improving Protein-Protein Interactions Prediction Accuracy Using XGBoost Feature Selection and Stacked Ensemble Classifier. Comput. Biol. Med. 123, 103899. doi:10.1016/j.compbiomed.2020.103899
Czibula, G., Albu, A.-I., Bocicor, M. I., and Chira, C. (2021). AutoPPI: An Ensemble of Deep Autoencoders for Protein-Protein Interaction Prediction. Entropy 23, 643. doi:10.3390/e23060643
Ekbal, A., Saha, S., and Bhattacharyya, P. (2016). “A Deep Learning Architecture for Protein-Protein Interaction Article Identification,” in 2016 23rd International Conference On Pattern Recognition (ICPR): IEEE), 3128
Fang, Y., Macool, D., Xue, Z., Heppard, E., Hainey, C., Tingey, S., et al. (2002). Development of a High-Throughput Yeast Two-Hybrid Screening System to Study Protein-Protein Interactions in Plants. Mol. Gen. Genomics 267, 142–153. doi:10.1007/s00438-002-0656-7
Fukao, Y. (2012). Protein-Protein Interactions in Plants. Plant Cel Physiol. 53, 617–625. doi:10.1093/pcp/pcs026
Gu, H., Zhu, P., Jiao, Y., Meng, Y., and Chen, M. (2011). PRIN: a Predicted rice Interactome Network. BMC bioinformatics 12.1 (2011), 1–13. doi:10.1186/1471-2105-12-161
Guo, Y., Yu, L., Wen, Z., and Li, M. (2008). Using Support Vector Machine Combined with Auto Covariance to Predict Protein-Protein Interactions from Protein Sequences. Nucleic Acids Res. 36, 3025–3030. doi:10.1093/nar/gkn159
Guo, Z.-H., You, Z.-H., Wang, Y.-B., Yi, H.-C., and Chen, Z.-H. (2019). A Learning-Based Method for LncRNA-Disease Association Identification Combing Similarity Information and Rotation forest. IScience 19, 786–795. doi:10.1016/j.isci.2019.08.030
Hayashi, T., Matsuzaki, Y., Yanagisawa, K., Ohue, M., and Akiyama, Y. (2018). MEGADOCK-web: an Integrated Database of High-Throughput Structure-Based Protein-Protein Interaction Predictions. BMC bioinformatics 19, 62–72. doi:10.1186/s12859-018-2073-x
Huang, Y. A., Chan, K. C. C., You, Z. H., Hu, P., Wang, L., and Huang, Z. A. (2021). Predicting microRNA-Disease Associations from lncRNA-microRNA Interactions via Multiview Multitask Learning. Brief Bioinform 22, bbaa133. doi:10.1093/bib/bbaa133
Kavuluru, R., Rios, A., and Tran, T. (2017). Extracting Drug-Drug Interactions with Word and Character-Level Recurrent Neural Networks", in: 2017 IEEE International Conference on Healthcare Informatics (ICHI): IEEE).doi:10.1109/ichi.2017.15
Kerrien, S., Aranda, B., Breuza, L., Bridge, A., Broackes-Carter, F., Chen, C., et al. (2012). The IntAct Molecular Interaction Database in 2012. Nucleic Acids Res. 40, D841–D846. doi:10.1093/nar/gkr1088
Kingma, D. P., and Ba, J. (2014). Adam: A Method for Stochastic Optimization. arXiv preprint arXiv:1412.6980.
Lecun, Y., Bengio, Y., and Hinton, G. (2015). Deep Learning. nature 521, 436–444. doi:10.1038/nature14539
Lehti-Shiu, M. D., and Shiu, S.-H. (2012). Diversity, Classification and Function of the Plant Protein Kinase Superfamily. Phil. Trans. R. Soc. B 367, 2619–2639. doi:10.1098/rstb.2012.0003
Li, B.-Q., Feng, K.-Y., Chen, L., Huang, T., and Cai, Y.-D. (2012). Prediction of Protein-Protein Interaction Sites by Random forest Algorithm with mRMR and IFS.
Li, Z., Li, J., Nie, R., You, Z. H., and Bao, W. (2021). A Graph Auto-Encoder Model for miRNA-Disease Associations Prediction. Brief Bioinform 22. doi:10.1093/bib/bbaa240
Lim, J., Ryu, S., Park, K., Choe, Y. J., Ham, J., and Kim, W. Y. (2019). Predicting Drug-Target Interaction Using a Novel Graph Neural Network with 3D Structure-Embedded Graph Representation. J. Chem. Inf. Model. 59, 3981–3988. doi:10.1021/acs.jcim.9b00387
Mikolov, T., Chen, K., Corrado, G., and Dean, J. (2013a). Efficient Estimation of Word Representations in Vector Space. arXiv preprint arXiv:1301.3781.
Mikolov, T., Sutskever, I., Chen, K., Corrado, G. S., and Dean, J. (2013b). “Distributed Representations of Words and Phrases and Their Compositionality,” in Advances in Neural Information Processing Systems), 3111
Nair, V., and Hinton, G. E. (2010). “Rectified Linear Units Improve Restricted Boltzmann Machines,” in Icml.
Ning, Q., Ma, Z., and Zhao, X. (2019). dForml(KNN)-PseAAC: Detecting Formylation Sites from Protein Sequences Using K-Nearest Neighbor Algorithm via Chou's 5-step Rule and Pseudo Components. J. Theor. Biol. 470, 43–49. doi:10.1016/j.jtbi.2019.03.011
Oughtred, R., Stark, C., Breitkreutz, B.-J., Rust, J., Boucher, L., Chang, C., et al. (2019). The BioGRID Interaction Database: 2019 Update. Nucleic Acids Res. 47, D529–D541. doi:10.1093/nar/gky1079
Pan, J., Li, L.-P., Yu, C.-Q., You, Z.-H., Ren, Z.-H., and Tang, J.-Y. (2021). FWHT-RF: A Novel Computational Approach to Predict Plant Protein-Protein Interactions via an Ensemble Learning Method. Scientific Programming.
Perozzi, B., Al-Rfou, R., and Skiena, S. (2014). “Deepwalk: Online Learning of Social Representations,” in Proceedings of the 20th ACM SIGKDD international conference on Knowledge discovery and data mining), 701–710.
Qiang, X., Zhou, C., Ye, X., Du, P.-F., Su, R., and Wei, L. (2020). CPPred-FL: a Sequence-Based Predictor for Large-Scale Identification of Cell-Penetrating Peptides by Feature Representation Learning. Brief. Bioinformatics 21, 11–23.
Rhee, S. Y., Beavis, W., Berardini, T. Z., Chen, G., Dixon, D., Doyle, A., et al. (2003). The Arabidopsis Information Resource (TAIR): a Model Organism Database Providing a Centralized, Curated Gateway to Arabidopsis Biology, Research Materials and Community. Nucleic Acids Res. 31, 224–228. doi:10.1093/nar/gkg076
Rifaioglu, A. S., Nalbat, E., Atalay, V., Martin, M. J., Cetin-Atalay, R., and Doğan, T. (2020). DEEPScreen: High Performance Drug-Target Interaction Prediction with Convolutional Neural Networks Using 2-D Structural Compound Representations. Chem. Sci. 11, 2531–2557. doi:10.1039/c9sc03414e
Rohila, J. S., Chen, M., Chen, S., Chen, J., Cerny, R. L., Dardick, C., et al. (2009). Protein-protein Interactions of Tandem Affinity Purified Protein Kinases from rice. PloS one 4, e6685. doi:10.1371/journal.pone.0006685
Romero‐Molina, S., Ruiz‐Blanco, Y. B., Harms, M., Münch, J., and Sanchez‐Garcia, E. (2019). PPI‐detect: A Support Vector Machine Model for Sequence‐based Prediction of Protein–Protein Interactions. J. Comput. Chem. 40, 1233–1242.
Shen, J., Zhang, J., Luo, X., Zhu, W., Yu, K., Chen, K., et al. (2007). Predicting Protein-Protein Interactions Based Only on Sequences Information. Proc. Natl. Acad. Sci. 104, 4337–4341. doi:10.1073/pnas.0607879104
Wang, J., Zou, Q., and Lin, C. (2021a). A Comparison of Deep Learning-Based Pre-processing and Clustering Approaches for Single-Cell RNA Sequencing Data. Brief. Bioinform. doi:10.1093/bib/bbab345
Wang, L., You, Z.-H., Zhou, X., Yan, X., Li, H.-Y., and Huang, Y.-A. (2021b). NMFCDA: Combining Randomization-Based Neural Network with Non-negative Matrix Factorization for Predicting CircRNA-Disease Association. Appl. Soft Comput. 110, 107629. doi:10.1016/j.asoc.2021.107629
Wang, X., Yu, B., Ma, A., Chen, C., Liu, B., and Ma, Q. (2019). Protein-protein Interaction Sites Prediction by Ensemble Random Forests with Synthetic Minority Oversampling Technique. Bioinformatics 35, 2395–2402. doi:10.1093/bioinformatics/bty995
Wen, M., Zhang, Z., Niu, S., Sha, H., Yang, R., Yun, Y., et al. (2017). Deep-Learning-Based Drug-Target Interaction Prediction. J. Proteome Res. 16, 1401–1409. doi:10.1021/acs.jproteome.6b00618
Woods, A. G., Sokolowska, I., Yakubu, R., Butkiewicz, M., Lafleur, M., Talbot, C., et al. (2011). “Blue Native page and Mass Spectrometry as an Approach for the Investigation of Stable and Transient Protein-Protein Interactions,” in Oxidative Stress: Diagnostics, Prevention, and Therapy (American Chemical Society), 341–367. doi:10.1021/bk-2011-1083.ch012
Xiao, Z., and Deng, Y. (2020). Graph Embedding-Based Novel Protein Interaction Prediction via Higher-Order Graph Convolutional Network. PloS one 15, e0238915. doi:10.1371/journal.pone.0238915
Xu, B., Wang, N., Chen, T., and Li, M. (2015). Empirical Evaluation of Rectified Activations in Convolutional Network. arXiv preprint arXiv:1505.00853.
Xu, D., Xu, H., Zhang, Y., Chen, W., and Gao, R. (2020). Protein-protein Interactions Prediction Based on Graph Energy and Protein Sequence Information. Molecules 25, 1841. doi:10.3390/molecules25081841
Yan, Y., Zhang, D., Zhou, P., Li, B., and Huang, S.-Y. (2017). HDOCK: a Web Server for Protein-Protein and Protein-DNA/RNA Docking Based on a Hybrid Strategy. Nucleic Acids Res. 45, W365–W373. doi:10.1093/nar/gkx407
Yang, X., Yang, S., Li, Q., Wuchty, S., and Zhang, Z. (2020). Prediction of Human-Virus Protein-Protein Interactions through a Sequence Embedding-Based Machine Learning Method. Comput. Struct. Biotechnol. J. 18, 153–161. doi:10.1016/j.csbj.2019.12.005
You, Z.-H., Chan, K. C. C., and Hu, P. (2015). Predicting Protein-Protein Interactions from Primary Protein Sequences Using a Novel Multi-Scale Local Feature Representation Scheme and the Random forest. PloS one 10, e0125811. doi:10.1371/journal.pone.0125811
Yuan, Q., Chen, J., Zhao, H., Zhou, Y., and Yang, Y. (2021). Structure-aware Protein–Protein Interaction Site Prediction Using Deep Graph Convolutional Network. Bioinformatics.
Zeng, M., Zhang, F., Wu, F. X., Li, Y., Wang, J., and Li, M. (2020). Protein-protein Interaction Site Prediction through Combining Local and Global Features with Deep Neural Networks. Bioinformatics 36, 1114–1120. doi:10.1093/bioinformatics/btz699
Zhang, C., and Woodland, P. C. (2016). “DNN Speaker Adaptation Using Parameterised Sigmoid and ReLU Hidden Activation Functions,” in 2016 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP): IEEE), Shanghai, China, March 20–25, 2016, 5300–5304. doi:10.1109/icassp.2016.7472689
Zhao, L., Wang, J., Hu, Y., and Cheng, L. (2020). Conjoint Feature Representation of GO and Protein Sequence for PPI Prediction Based on an Inception RNN Attention Network. Mol. Ther. - Nucleic Acids 22, 198–208. doi:10.1016/j.omtn.2020.08.025
Zheng, K., You, Z. H., Wang, L., Zhou, Y., Li, L. P., and Li, Z. W. (2019). MLMDA: a Machine Learning Approach to Predict and Validate MicroRNA-Disease Associations by Integrating of Heterogenous Information Sources. J. Transl Med. 17, 260–314. doi:10.1186/s12967-019-2009-x
Keywords: plant, protein-protein interaction, network embedding, multi-source information, deep neural networks
Citation: Pan J, You Z-H, Li L-P, Huang W-Z, Guo J-X, Yu C-Q, Wang L-P and Zhao Z-Y (2022) DWPPI: A Deep Learning Approach for Predicting Protein–Protein Interactions in Plants Based on Multi-Source Information With a Large-Scale Biological Network. Front. Bioeng. Biotechnol. 10:807522. doi: 10.3389/fbioe.2022.807522
Received: 02 November 2021; Accepted: 25 February 2022;
Published: 21 March 2022.
Edited by:
Ratul Chowdhury, Harvard Medical School, United StatesCopyright © 2022 Pan, You, Li, Huang, Guo, Yu, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Ping Li, Y3MyYmlvaW5mb3JtYXRpY3NAZ21haWwuY29t; Chang-Qing Yu, Z3VvanhfNTE2QDEyNi5jb20=
 Jie Pan
Jie Pan Zhu-Hong You
Zhu-Hong You Li-Ping Li
Li-Ping Li Wen-Zhun Huang1
Wen-Zhun Huang1 Zheng-Yang Zhao
Zheng-Yang Zhao