- 1School of Bioengineering Dalian University of Technology, Dalian, China
- 2Dalian Institute of Chemical Physics Chinese Academy of Sciences, Dalian, China
- 3Molekulare Biotechnologie and Systembiologie, TUKaiserslautern, Kaiserslautern, Germany
Editorial on the Research Topic
Metabolic engineering for bioresources and bioenergies production from microalgae
Microalgae are a group of photosynthetic microorganisms that can utilize atmospheric CO2 as a carbon source. They are regarded as promising green cell factories for the production of biofuels and highly valuable products. However, the production of biofuels and other valuable products is currently not economically viable in microalgae, due to the low yield. Therefore, rational metabolic engineering of microalgae to obtain robust microalgal strains is required, and a comprehensive understanding of lipid metabolism is also essential. Moreover, state-of-the-art molecular genetic tools including synthetic biological strategies will also facilitate potentiation of microalgae as cell factories for bioresource and bioenergy production. Thus, it seems timely to launch this Research Topic to collect recent achievements on metabolic or process engineering in microalgae that can enhance the production of biofuels and other valuable products.
This Research Topic includes eight research articles/original reviews on microalgal biology and biotechnology. These studies include strategic transgene selection, enzyme engineering, proteomic analysis, cultivation mode comparation, and mathematical modeling of growth. In addition, one review discussed the synthetic biology perspective on bioengineering tools for microalgae. Fluorescent proteins (FP) are useful reporters to monitor gene expression and subcellular localization. Gutiérrez et al. Demonstrated that a broad set of optimized synthetic FP can be used as fusion partners to monitor transgene expression in the eukaryotic model microalga Chlamydomonas reinhardtii (Figure 1A). This technical advance will facilitate high-throughput screening of algal transformants to identify high transgenes expression or trait stacking cells, since the target recombinant-FP fusion protein can be visualized in situ after electrophoresis. In the green microalga Haematococcus pluvialis, a novel bifunctional wax ester synthase (HpWS), involved in early triacylglycerol (TAG) accumulation under high light conditions (HL), was identified (Figure 1B) by Ma et al. Heterologous expression of HpWS in TAG-deficient Saccharomyces cerevisiae can restore the capability of wax and TAG biosynthesis. In addition, the overexpression of HpWS in Chlamydomonas reinhardtii enhanced TAG production (Ma et al.) This study provides a new molecular target for genetic manipulation to increase TAG and wax accumulation in microalgae. Dan et al. reported that overexpression of GlgP (glycogen phosphorylase) promoted effective glycogen degradation and increased sucrose production in the prokaryotic microalga Synechococcus elongatus (Figure 1C). This study avoided physiological and metabolic impairments caused by targeting of the glycogen synthesis pathway and provided novel insights into metabolic engineering approaches for efficient photosynthetic biosynthesis. Chen et al. investigated the proteomic responses of the dark-adapted unicellular eukaryotic microalga Euglena gracilis (Euglena) and the bleached mutant against light stimuli (Figure 1D). This study provided useful information about the unknown functions of residual plastids in bleached Euglena mutants. In addition, the same group reviewed the progress of molecular genetic engineering approaches in Euglena. The authors summarized various methods for transgene delivery, gene silencing, and genome editing, such as overexpression, RNA interference, and CRISPR/Cas9 systems (Chen et al.). The tools and knowledge summarized will pave the way for developing a synthetic biological approach to produce useful bioproducts in Euglena. Microalgae can utilize both organic and inorganic carbon sources to power their growth. The effects of different cultivation modes on the biomass productivity and biocomponent contents in Chlorella sp. Were examined by Yun et al. The authors found that mixotrophic cultivation of Chlorella sp. Can improve the yields of biomass, lipid, protein, and pigment in comparison to photoautotrophic and heterotrophic conditions (Figure 1E) (Yun et al.). Interestingly, this team also found that microbial communities composed of algae improved biofloc technology, including the nitrogen-related material cycle in Litopenaeus vannamei farms (Yun et al.). To realize large-scale cultivation of microalgae, photobioreactors have been commonly used. The self-shading effects on microalgal growth during continuous cultivation in photobioreactors were studied by Saccardo et al. The authors found that a strong shading effect occurs when the thicknesses of the algae is above 8 mm. To highlight the effect of mixing cycles, the study proposed an analogy between small-scale operations using continuous light and photobioreactors using pulsed light, based on characteristic parameters obtained from mathematical modeling of the growth (Figure 1F) (Saccardo et al.).
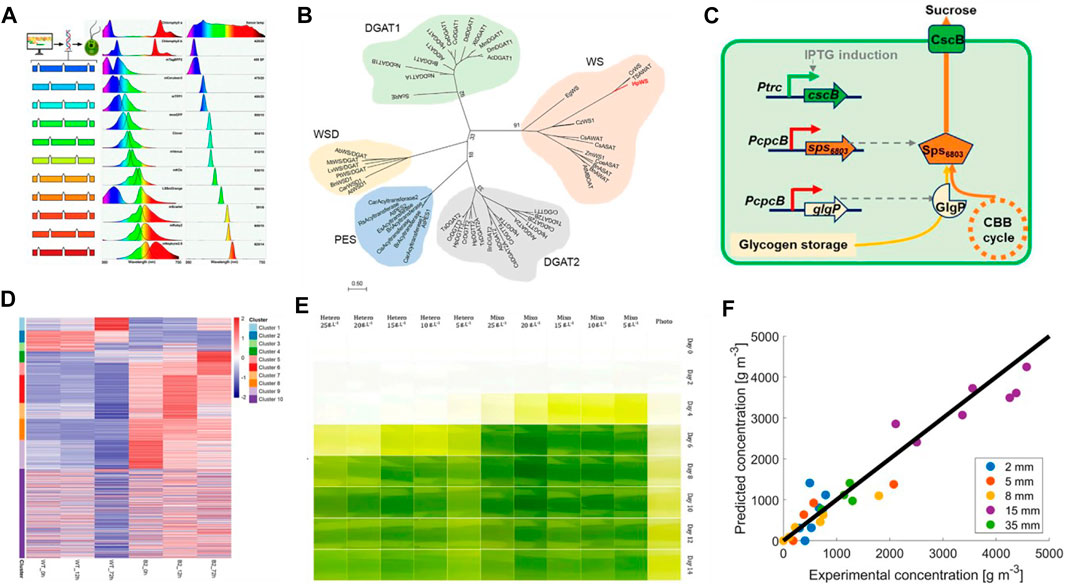
FIGURE 1. Representative articles in this Research Topic. (A) Technical advances for transformant selection in C. reinhardtii (Gutiérrez et al.). (B) Novel bifunctional wax ester synthase (HpWS), identified in H. pluvialis, and heterogeneous expression of HpWS in C. reinhardtii for triacylglycerol accumulation (Ma et al.). (C) Overexpression of GlgP (glycogen phosphorylase) increased sucrose production in S. elongatus (Dan et al.). (D) Proteomic analysis of dark-adapted E. gracilis and the bleached mutant against light stimuli (Chen et al.) (E) Effects of different cultivation modes on the biomass productivity and biocomponent contents in Chlorella sp (Yun et al.). (F) Self-shading effects on microalgal growth during continuous cultivation in photobioreactors (Saccardo et al.).
The articles in this Research Topic demonstrate that strategies such as synthetic biology, metabolic engineering, proteomic analysis, and process engineering will contribute to the production of biofuels and other valuable biocompounds from microalgae at industrial levels.
Author contributions
FK wrote and revised the manuscript. XC and MS-R provided suggestions and comments and revised the manuscript. All authors approved this manuscript for publication.
Acknowledgments
The editors thank all contributors, reviewers, and the editorial offce for their contributions to this Research Topic. The Topic Editors’ research was supported by the National Natural Science Foundation of China (31900221), and the Fundamental Research Funds from the Central Universities (DUT21LK14).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: metabolic engineering, metabolites, bioresource, bioenergy, microalgae
Citation: Kong F, Cao X and Schulz-Raffelt M (2023) Editorial: Metabolic engineering for bioresources and bioenergies production from microalgae. Front. Bioeng. Biotechnol. 10:1114854. doi: 10.3389/fbioe.2022.1114854
Received: 03 December 2022; Accepted: 19 December 2022;
Published: 05 January 2023.
Edited and reviewed by:
Manfred Zinn, HES-SO Valais-Wallis, SwitzerlandCopyright © 2023 Kong, Cao and Schulz-Raffelt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fantao Kong, a29uZ2ZhbnRhb0BkbHV0LmVkdS5jbg==
 Fantao Kong
Fantao Kong Xupeng Cao
Xupeng Cao Miriam Schulz-Raffelt
Miriam Schulz-Raffelt