
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 04 January 2023
Sec. Industrial Biotechnology
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.1100533
This article is part of the Research Topic Anaerobic Digestion of Waste Organics: Toxicity and Management View all 5 articles
 Nozha Abid1*
Nozha Abid1* Fatma Karray1
Fatma Karray1 Imen Kallel2
Imen Kallel2 Mariam Slim1
Mariam Slim1 Abdellatif Barakat3,4
Abdellatif Barakat3,4 Najla Mhiri1
Najla Mhiri1 Mohamed Chamkha1
Mohamed Chamkha1 Sami Sayadi5*
Sami Sayadi5*The current research work attempted to investigate, for the first time, the impact of biochar addition, on anaerobic digestion of olive mill wastewater with different initial chemical oxygen demand loads in batch cultures (10 g/L, 15 g/L, and 20 g/L). Methane yields were compared by applying one-way analysis of variance (ANOVA) followed by post-hoc Tukey’s analysis. The results demonstrated that adding at 5 g/L biochar to olive mill wastewater with an initial chemical oxygen demand load of 20 g/L increased methane yield by 97.8% and mitigated volatile fatty acid accumulation compared to the control batch. According to the results of microbial community succession revealed by the Illumina amplicon sequencing, biochar supplementation significantly increased diversity of the microbial community and improved the abundance of potential genera involved in direct interspecies electron transfer, including Methanothrix and Methanosarcina. Consequently, biochar can be a promising alternative in terms of the recovery of metabolic activity during anaerobic digestion of olive mill wastewater at a large scale.
Cultivation of olive trees and oil production are vital activities, mainly in Mediterranean countries. However, uncontrolled olive mill wastewater (OMW) discharge into the environment may beget serious problems owing to its high pollution degree, acidic pH and polyphenols compounds generating antimicrobial effects which involve the inhibition of natural biodegradability of organic load in natural water bodies (Sayadi et al., 2000). Among the numerous processes proposed for the effluent detoxification, biological treatments were considered less expensive and environmental friendly (McNamara et al., 2008). It has been reported that anaerobic digestion (AD) is more efficient than aerobic processes referring basically to the plausibility to treat effluents with high organic load and energy potential like OMW (Gelegenis et al., 2007). In addition, it displays several merits like operational economy, reduction in energy consumption, generation of biogaz and use of the stabilized digestate as a fertilizer (Dareioti et al., 2009).
However, AD effectiveness is limited by the slow metabolism between syntrophs and archaea (Zhao et al., 2016). In fact, some reactor operational modifications may cause volatile fatty acids (VFAs) or hydrogen (H2) accumulation that might be toxic to methanogens and acetogenic bacteria respectively, which triggers souring of anaerobic reactors and leads as a matter of fact to the process failure.
Particularly, for OMW, AD was affected chiefly by inhibitory substances such as phenolic compounds as well as long chain fatty acids (Beccari et al., 1999) as they inhibit anaerobic microorganisms. For these reasons, many researchers have been particularly oriented towards improving OMW AD efficiency through dilution, physico-chemical pretreatments, co-digestion or integrated treatments (Khoufi et al., 2015; Vavouraki et al., 2020). Khoufi et al. (2007) proved that OMW pretreatment with electrocoagulation followed by sedimentation led to removal of 76.2% of phenolic compounds and a chemical oxygen demand COD reduction of 43%. After this pretreatment, anaerobic biomethanization was conducted with high methane yield at a loading rate of 6 g COD L−1 day−1 compared to raw OMW which was toxic to microorganisms. The working mode for syntrophic metabolism during anaerobic methanogenesis was commonly reported as interspecies hydrogen transfer (IHT) (Boone et al., 1999), where H2 acts as a diffusive electron shuttle to mediate electron transfer from secondary fermenting bacteria to methanogens. However, the production of hydrogen catalysed by secondary fermenting bacteria is thermodynamically feasible (i.e., ΔG < 0) uniquely if hydrogen concentrations are quite low (H2 < 10–4 atm (Logan et al., 2002)). Since this condition is accomplished through the consumption of hydrogen by hydrogenotrophic methanogens, the syntrophic metabolism between oxidizing bacteria and archaea is crucial (Mcinerney et al., 2010). Yet, H2 diffusion between H2 producers and H2-consuming methanogens is slow (Stams et al., 2006), which reduces the methane formation rate during AD. Therefore, this syntrophic metabolism network has been confirmed to be metabolically low-efficient.
Over the last years, a new electron transfer pathway, referred to direct interspecies electron transfer (DIET), has been set forward as an alternative network which is more efficient than IHT (Xu et al., 2019). DIET may occur through biological electrical connections such as pili and outer surface c-type cytochromes (Xu et al., 2019). Moreover, several electrically conductive materials, such as graphene (Zhang and Tremblay, 2020) and carbon cloth (Zhao et al., 2016), served as additives for DIET enhancement between syntrophic microorganisms. However, excessive costs as well as the environmental risks of these materials like graphene might limit their use (Kang et al., 2007). Thus, recently, various researchers have investigated biochar as an effective additive to enhance AD. Biochar is an amorphous and a porous carbon-rich material produced by pyrolysis of biomass varieties in the absence or presence of a little amount of oxygen (Cantrell et al., 2012). It is reported that during AD, biochar increases buffering capacity (Zhang et al., 2014), immobilizes microbial cell and improves the methane production rate (Cai et al., 2016). Recently, Wang et al. (2020) have asserted that biochar derived from biowaste promoted DIET to accelerate syntrophic phenol oxidation during AD. The authors suggested a probable shift of syntrophic phenol metabolism from indirect transfer via H2 to direct interspecies electron transfer.
This work corresponds to a pioneering research that focuses on the impact of the biochar supplementation on AD of OMW. The chief objective of this research work was: Firstly, to examine the impact of different biochar concentrations on biochemical methane potential assays of OMW at different increasing COD loads: 10 g/L, 15 g/L and 20 g/L. Secondly, the prokaryotic community structure, both in the suspended solution and those integrated with biochar surface, were investigated by Illumina to explore their potential implication in DIET.
This work would provide new findings on the effect of biochar supplementation upon the production of methane from OMW which has an important implication for potential application at large scales.
Raw OMW invested in this study was produced by a continuous olive oil mill situated in Sfax (Tunisia). In order to separate suspended solids before use, the samples of OMW were centrifuged by Universal 320 R at 6,000 rpm for10 min. The microbial inoculum was supplied by a semi-pilot anaerobic bioreactor treating OMW and operating in a mesophilic regime.
The used biochar was derived from olive mill wastewater sludge from evaporation ponds in Sfax (Tunisia) and was produced via pyrolysis at a temperature of 450 °C. The parameters of the pyrolysis were recently described by Abid et al. (2022). The physico-chemical characteristics of the biochar were: pH = 10.8 ± 0.05; EC = 11 mS/cm; BET surface area (m2/g) = 2.77; element contents (oven dry basis): C = 45% +/2.8; N = 2.45% ± 0.13; H = 2.26% ± 0.05; S = 1.23% ± 0.32; O = 19.47% ± 0.3.
The study centered around the impact of the biochar concentration on the AD of OMW at different COD concentrations: 10 g/L, 15 g/L and 20 g/L. Based on previous studies (Luo et al., 2014; Altamirano-Corona et al., 2021), two biochar concentrations were used (5 g/L and 10 g/L).
Batch anaerobic digestion tests were performed in 100 ml batches with a working volume of 60 ml. The inoculum was mixed with a substrate, keeping a volatile solids (VS) ratio (VS substrate to VS inocula) at 1:1(Khoufi et al., 2015). Batches containing inoculum were conducted as blanks to subtract the background gas production. The pH of each batch was adjusted to approximately pH = 7.2 with NaOH (5 M) or HCl (5 M) after which nitrogen gaz was used to purge the system for 3 min so as to remove excess of O2 and thus ensure anaerobic conditions. Subsequently, the batches were placed in mesophilic conditions at 37°C.
The volume of methane is measured with a gas trapping device. This device consists of a syringe inserted into the batch through the septum and connected by a flexible tube, to an inverted vial containing a solution of NaOH (3 M) to fix CO2.
The tests were set up in triplicate and conducted during an incubation period. The mean values of methane production were calculated. The methane yield was expressed as mL CH4/g COD introduced and computed through dividing the cumulative volume of methane produced by the mass of COD introduced into the batch at the start–up. At the end of the incubations, the digestates were separated from the biochars for subsequent analyzes.
Characterization of OMW as well as the digestates included the following parameters: Total solids content (TS), VS., electrical conductivity (EC), pH, biological oxygen demand (BOD5), COD, total polyphenols, and VFAs. pH and EC were measured respectively with a pH meter type Néo Met/Ph- 220 L and a conductivimeter type WTW. TS was measured after oven drying at 105°C by weighing the sample before and after. Afterwards, the retained residues were dried at 105°C. VS. were analyzed by loss on ignition at 550°C for 2 h. COD was determined referring the standard procedure following the American Public Health Association (APHA, 2012). BOD5 was specified using the manometric method. Total polyphenols were determined using the Folin-Ciocalteu assay, as reported by Aliakbarian et al. (2015). VFAs were measured by HPLC according to the protocol described by Mechichi and Sayadi (2005).
The physico-chemical characteristics of OMW were pH = 4.9; EC = 14.7 mS/cm; TS = 42.6± 0.46 g/L; VS = 30.3± 0.5 g/L; COD = 47.4± 2.8 g/L; BOD5 = 1.75± 0.07 g/L; total polyphenols = 3.4± 0.12 g/L.
Sampling of microbes in the batches was undertaken at the end of methanization according to the method used by (Luo et al., 2014). To investigate the prokaryotic communities in the bottles, three fractions were distinguished as follows: suspended solution, loosely combined with biochar and tightly integrated with biochar surface. Total DNA from all fractions were extracted using DNeasy Power Soil Kit (QIAGEN).
The mixtures of 16 S rRNA gene amplicons (bTEFAP®) were generated through the use of a 515F/806R primer set, as previously reported by Dowd et al. (2008) and were sequenced with the MiSeq Illumina (paired-end 2 × 150 bp) platform of the Molecular Research Laboratory (Texas, USA). QIIME 1.9.1 was used to analyze raw data as described by Caporaso et al. (2010). In short, the raw reads were checked for adapter, chimera and low quality sequences. The trimmed reads were clustered into operational taxonomic units (OTUs) using a 97% sequence identity threshold with UCLUST (Edgar, 2010). The Green genes 13.8 database was used to perform taxonomic assignments. Relative abundance of archeal genus were calculated from all archeal sequences. Sequences from all archael OTUs and selected bacterial dominant OTUs (>1% of total sequences) were compared with related sequences retrieved from NCBI databases using BLAST algorithm (Altschul et al., 1990). QIIME software (version 1.9.1) was used to determine the Shannon and Simpson’s diversity indices, the observed species, the Chao1 richness estimator and the phylogenetic diversity index. Venn diagrams were constructed using the VENN DIAGRAM PLOTTER program (http://omics.pnl.gov/software/VennDiagramPlotter.php). The heat map was constructed by the ‘aheatmap’ function in the ‘NMF’ package of R (http://nmf.r-forge.r-project.org/aheatmap.html.
16 S rRNA raw reads from biochar free group (R0) and 5 g/L biochar supplemented group (R1), including suspended fraction of R0 (S0) and R1 (S1), cells loosly (L1) and tightly cells bound to biochar (T1), were deposited in the Short Read Archive of NCBI under project no. PRJNA856838.
The experimental values (n = 3) were presented in terms of the means ± standard deviation (SD). To determine the significant differences between the cumulative methane productions and methane yields in the batches (n = 3), three steps were undertaken. Firstly, the Shapiro-wilk test was used to analyze the normality distribution of variables. Secondly, the one-way ANOVA test was implemented. Thirdly, Tukey’s post hoc test was adopted. The significant test was fixed at p < 0.05. All statistical analyses were conducted with Statistical Package for the Social Sciences (SPSS) V 20.
This study purports to assess the influence of biochar concentrations (5 g/L and 10 g/L) on anaerobic digestion of OMW with different increasing COD loads (10 g/L, 15 g/L and 20 g/L) over an incubation period of 56 days. Statistical differences in methane production and methane yields were specified using one-way ANOVA and post hoc Tukey’s test analysis with a significance level of 0.05. Figure 1A depicts the cumulative methane production during OMW methanisation at a COD load of 10 g/L.
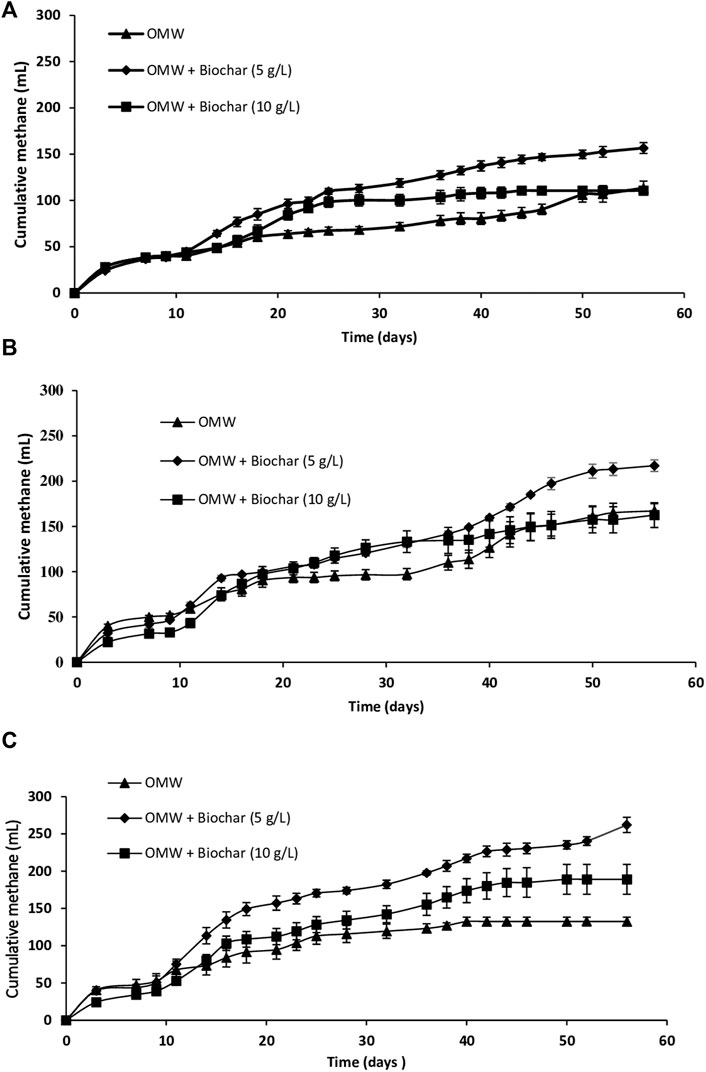
FIGURE 1. Cumulative methane production during AD of OMW at COD loads of (A) 10 g/L (B) 15 g/L and (C) 20 g/L.
Over the first 9 days, similar trends in cumulative methane production were observed in all the batches with a low CH4 production on day 3 followed by a plateau lasting 6 days which corresponded to the lag time. Subsequently, after 32 days, significant higher cumulative methane production was achieved in batches with 5 g/L and 10 g/L biochar (p < 0.05) compared to the control. However, the cumulative CH4 production for batches with 5 g/L biochar was significantly higher than that for batches with 10 g/L biochar (p < 0.05). At the end, the best significant methane yield (p < 0.05) was recorded for the batches with 5 g/L biochar (261.1 ml CH4/g COD introduced), with a yield improvement of 38.1% compared to the control.
Figure 1B exhibits the cumulative methane production during OMW methanization at a COD load of 15 g/L. As can be inferred, CH4 production started after 9 days of lag time in all the batches. Subsequently, the methane production was detected from day 9 to day 18 for the control before it plateaued until day 32; then, it reincreased till the end. This can be assigned to the slow syntrophic degradation during AD of OMW containing slow biodegraded organics (Khoufi et al., 2006). However, on day 32, cumulative CH4 productions in cultures with 5 g/L and 10 g/L biochar addition were significantly higher (p < 0.05) than the control. However, no significant difference was detected between them (p > 0.05). Yield improvements levels compared to the control amounted to 34.6% and 36.9% respectively for batches with 5 g/L and 10 g/L of biochar. From this point onward, a higher increase of methane production was detected in batches with 5 g/L biochar compared to those with 10 g/L till the end of the fermentation. Eventually, after 56 days, a significant higher methane yield was recorded in the batches with 5 g/L biochar (p < 0.05) (241.2 ml CH4/g COD introduced) compared to the control (Table 1) with a yield improvement of 30%.

TABLE 1. One-way ANOVA and post hoc Tukey’s test analysis on CH4 yields (mL/g COD introduced) at the end of anaerobic digestion of OMW with initial COD loads of 10 g/L, 15 g/L and 20 g/L.
Figure 1C displays cumulative methane production during AD of OMW with 20 g/L of COD load. As demonstrated, after a similar lag time of 9 days, methane production increased in all batches. Afterwards, it plateaued, after 25 and 42 days for the control batch and batch with 10 g/L of biochar, respectively. However, it continued to rise up to 56 days for batch with 5 g/L biochar. After 32 days, biochar supplementation at concentrations of 10 g/L and 5 g/L improved significantly (p < 0.05) the methane production compared to the control by 18.6% and 51.9%, respectively. Moreover, cultures with 5 g/L biochar presented significant higher methane production than those with 10 g/L biochar (p < 0.05). Likewise, significant yield improvements of 42.7% and 97.8% (p < 0.05) were reached at the end of digestion (56 days) for batches with 10 g/L and 5 g/L biochar respectively compared to the control. Consequently, a significant (p < 0.05) higher yield (218.4 ml/g COD introduced) was achieved in cultures with 5 g/L biochar compared to the control and culture with 10 g/L biochar (Table 1). As expected, a low yield of methane production was recorded during anaerobic digestion in the control batch (110.4 ml CH4/g COD introduced). This could be assigned to the high COD load and polyphenols contents which entail toxicity leading to a decrease in methane yield (Khoufi et al., 2007).
Variation of VFAs has been recognized as key indicators for the unbalance process during AD of OMW (Mechichi and Sayadi, 2005). Final VFAs as well as pH are highlighted in Figure 2. It can be noticed that all treatments except OMW with 20 g/L COD load (control), exhibited suitable pH values between 7.6 and 7.9 and null VFAs accumulation, which reflected the process stability during the methanization. However, in the control cultures with the highest COD load (20 g/L), final acidic pH of 5.92 along with high final accumulation of acetic acid (9.4 g/L) revealed AD inhibition which coincided with the observed lower methane yield of 110.4 ml/g COD introduced.
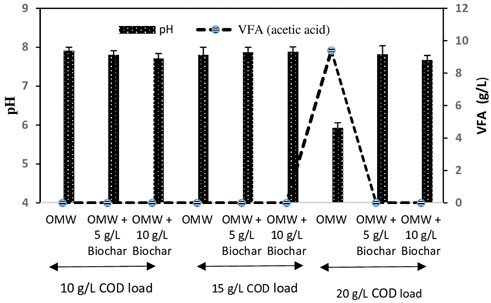
FIGURE 2. pH and volatile fatty acids concentrations at the end of anaerobic digestion of OMW at different COD loads.
After 56 days, polyphenols removal ratios were 52%, 47.1% and 19.5% in control cultures with COD loads of 10 g/L, 15 g/L and 20 g/L, respectively. However, biochar supplementation increased polyphenols removal efficiencies by 8, 8.6 and 27.5 percentage points, respectively (Figure 3).

FIGURE 3. Polyphenols removal efficiencies at the end of anaerobic digestion of OMW at different COD loads.
Additionnally, COD removal efficiencies after 56 days in control cultures with COD loads of 10 g/L, 15 g/L and 20 g/L were 55.33%, 54.37% and 32.98%, respectively. Yet, they increased by 21.6, 16.8 and 32.6 percentage points, respectively compared to controls after 5 g/L biochar supplementation. (Supplementary Figure S1).
Microbial communities (bacteria and archaea) were analyzed at the end of operation (56 days) in the cultures with an initial COD load of 20 g/L based on the 16 S rRNA gene amplicon sequencing using high-throughput sequency on an Illumina Miseq platform. Microbial communities of biochar free group (R0) and 5 g/L biochar supplemented group (R1), included suspended fractions of R0 (S0) and R1 (S1), loosly cells (L1) and tightly ones bound to biochar (T1).
As plotted in the venn diagram (Figure 4A), 557 OTUs were shared among all samples.
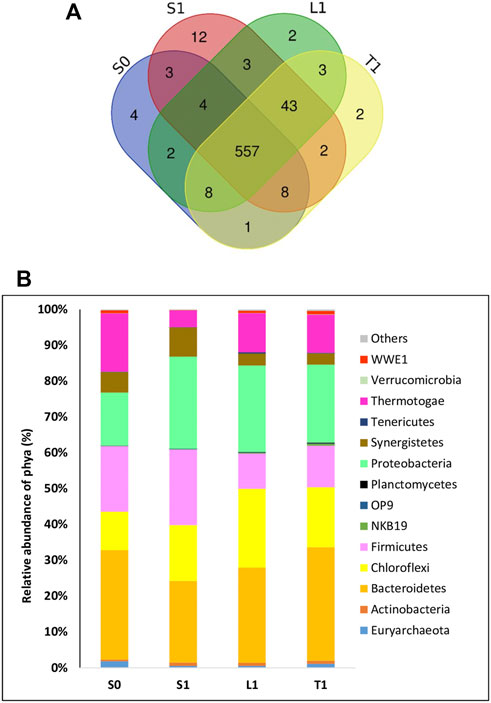
FIGURE 4. Analysis of microbial communities at the end of AD with initial COD load of 20 g/L in biochar free group (SO) and 5 g/L biochar supplemented group (Sl, Ll, Tl) (A) OTUs-VENN diagrams based on high-throughput sequencing analysis (B) Major phyla (relative abundance> 1% of all sequences).
A high number of unique OTUs was identified in the biochar supplemented samples (S1, L1 and T1) (12 + 2+2 OTUs) comparing to the biochar free group (S0) (4 OTUs).
Chao1, Shannon and Simpson indexes were performed to compare the prokaryotic richness and diversity between the biochar free (S0) and supplemented group (S1, L1 and T1). The diversity and richness estimators obtained from the Next-Generation Sequencing (NGS) data, are presented in Table 2. The prokaryotic diversity based on Shannon and Simpson indexes of the S0 group were about 5.258 and 0.936, respectively. However, in the biochar supplemented group (S1, L1 and T1), an increase in Shannon and Simpson indexes was recorded yielding an average of 5.960 and 0.959, respectively. Moreover, the R1 group showed higher Chao1 species richness estimator index (626 average OTUs) compared to the S0 group (587 OTUs). These findings indicated that microbial community structure and diversity changed following biochar addition.

TABLE 2. Diversity indexes of microbial community in biochar free group (S0) and biochar supplemented group (S1, L1, T1).
The microbial community composition at phylum level is portrayed in Figure 4B. All detected OTUs belong to 14 important phyla whose relative abundance > 0.1%. In the suspended fraction of the biochar free group (S0), the major detected phyla were: Bacteroidetes, Firmicutes, Thermotogae, Proteobacteria, Chloroflexi, Synergistetes and Euryarchaeota accounting for 30.55%, 18.29%, 16.29%, 14.83%, 10.73%, 5.74% and 1.82% respectively. Compared to the suspended fraction, an increase in relative abundance of Proteobacteria and Chloroflexi (14.83% and 10.73% respectively for S0) were recorded in the biochar supplemented groups, amounting respectively to 25.72% and 15.58% in the suspension (S1), 24.15% and 21.98% respectively in lously-bound biomasses (L1) and 21.78% and 16.73% respectively in tightly-bound cells (T1). However, a significant decrease in relative abundance of Thermotogae was noticed in all biomass fractions of biochar supplemented samples (S1 = 4.68%, L1 = 10.91%, T1 = 10.62%) compared to the biochar free groups (S0 = 16.29%).
The members of phyla Bacteroidetes and Firmicutes have important roles during anaerobic digestion in the generation of short chain fatty acids for methane production during hydrolysis and acidogenesis (Pan et al., 2019). Proteobacteria are syntrophic bacteria responsible for the cellulose and protein degradation and are also involved in the degradation of organic acids (Ma et al., 2019). The Chloroflexi phylum is a common fermenting group described in AD reactors. Moreover, Chloroflexi is also known as a phylum of electroactive bacteria (Hoareau et al., 2021). These results suggest that biochar addition improved the rate of hydrolytic as well as electroactive bacteria.
As for the bacterial community, taxa displaying a mean proportion of 1% were considered as the most abundant. The taxonomic classification at genus level (Figure 5) disclosed that the most dominant groups in the suspended fraction of the biochar-free group (S0), were Defluviitoga, Petrimonas, Soehngenia, Leptolinea, Thiocapsa, Flavilitoribacter, Fermentimonas, Methylocapsa, Pseudomonas, Aminobacterium, Cloacibacillus and Saccharicrinis amounting respectively to 16.22%, 15.92%, 11.02%, 9.5%, 9.06%, 5.23%, 2.12%, 1.62%, 1.51%, 1.48%, 1.33% and 1.09% of the sequence reads. The biochar supplemented groups demonstrated changes in the community compositions.
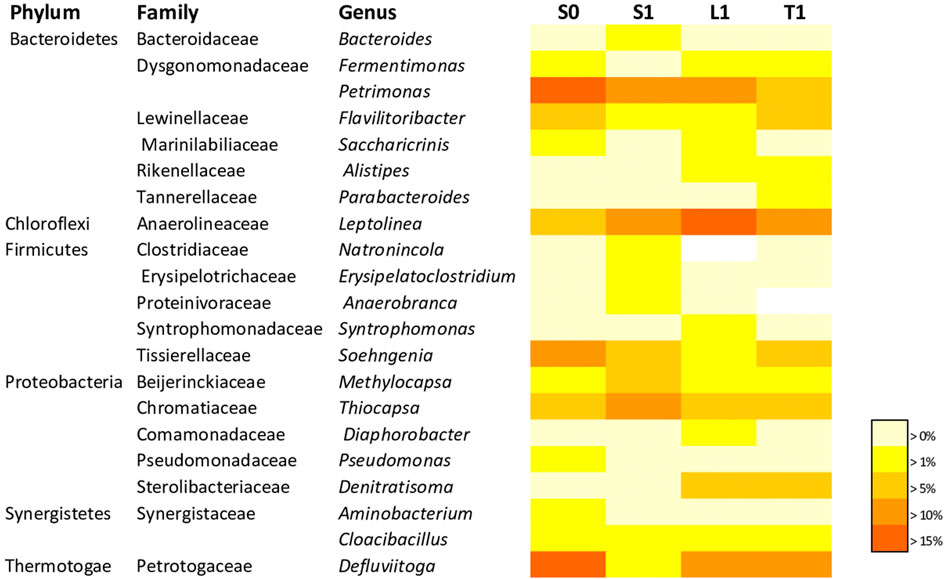
FIGURE 5. Heat map showing the relative abundance of dominant bacterial genera (> 1% of all sequences) related to the end of AD with initial COD load of 20 g/L in biochar free group. (S0) and 5 g/L biochar supplemented group (S1, L1, T1). The color intensityfor each panel corresponds to the genus abundance; white (0%) indicates low relative abundance, through yellow ( >1%) to red (> 15%) indicate a high level of relative abundance.
Notably, the genus Bacteroides from Bacteroidaceae family tended to be enriched in the suspended fraction in the biochar supplemented reactor (3.37% for S1, 0.27% for L1, 0.65% for T1) compared to the suspended fraction of R0 (S0 = 0.05%). Similar pattern was observed for Clostridiacea with genus Natronincola, which was exclusively enriched in the suspended fraction of the biochar supplemented reactor (1.9% for S1, 0% for L1, 0% for T1) while it was absent in the suspended fraction of R0 (S0 = 0%).
Likewise, Erysipelotrichaceae with the genus group of Erysipelatoclostridium (Erysipelatoclostridium ramosum species) (Supplementary Table S1) were enriched in the suspended fraction of the biochar supplemented reactor (3.48% for S1, 0.17% for L1, 0.13% for T1) compared to the suspended fraction of R0 (S0 = 0.68%).
Proteinivoraceae with the genus of Anaerobranca were exclusively enriched in the suspended fraction of the biochar supplemented sample (1.3% for S1, 0% for L1, 0% for T1) while being absent in the suspended fraction of R0 (S0 = 0%). It is well known that it corresponds to be an acidogenic bacteria (Yin et al., 2018). The abundance of Cloacibacillus from Synergistaceae family increased by about 3.43 folds in the suspended fraction of the biochar supplemented samples (4.58% for S1, 1.55% for L1, 1.07% for T1) compared to R0 (S0 = 1.33%). Anaerolineaceae with the genus of Leptolinea was boosted in the biochar supplemented samples (13.19% for S1, 17.94% for L1, 13.82% for T1) compared to S0 (9.50%). More importanly, Rikenellaceae family with the genus of Alistipes was distinctly more detected in the fixed biomass (2.08% for L1, 1.46% for T1) while it was not dominant in the suspended fractions (0.03% for S0 and 0.81% for S1). In this respect, the abundance of Parabacteroides from Tannerellaceae family increased exclusively in the loosly fixed biomass by 7.8 folds and in the tightly fixed biomass by 35.6 folds compared to S0 (0.05% for S0, 0.06% for S1, 0.4% for L1 and 1.79% for T1).
As illustrated in Figure 5, heterotrophic denitrifying bacteria were distincly enriched in the fixed biomass. Indeed, the relative percentage of Denitratisoma from Sterolibacteriaceae family was higher in the biochar fixed biomass (6.35% for T1, 5.81% for L1) while it was not dominant in the suspended fractions (0.06% for S0 and 0.28% for S1). It seemed that biochar served as a habitat for Denitratisoma which are heterotrophic denitrifying bacteria participating in nitrogen removal. Lu et al. (2021) reported that the relative abundance of Denitratisoma increased in the granulated activated carbon supplemented up-flow anaerobic sludge blanket reactors compared to the control (without activated carbon). Within this framework, the relative percentage of the genus of Diaphorobacter from Comamonadaceae family which is a facultative heterotrophic denitrifier was higher in the biochar fixed biomass and particularly in the loosly–bound fraction (S1 = 0.53%; L1 = 1.3%; T1 = 0.61%) than that in suspended fraction of R0 (S0 = 0.1%). As it has been proven that DIET is also involved in denitrification (Xie, 2006), Denitratisoma oestradiolicum and Diaphorobacter polyhydroxybutyrativorans (Supplementary Table S1) stand for potential exoelectrogens.
As far as the archaeal community is concerned, the taxonomic classification at genus level revealed that the most groups detected in biochar-free group (S0) include Methanomassiliicoccus, Methanoculleus, Methanobacterium and Methanothrix (Figure 6).
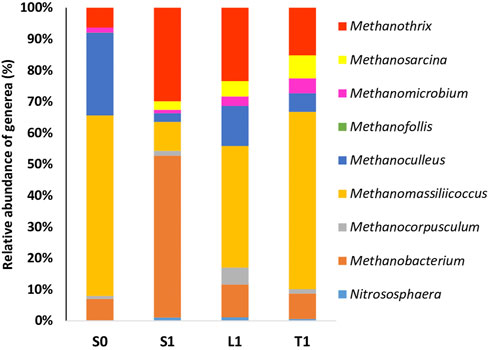
FIGURE 6. Relative abudance of archaeal community at genus level at the end of AD with initial COD load of 20 g/L in biochar free group (S0) and 5 g/L biochar supplemented group (S1, L1, T1).
Lower relative abundance of hydrogenetrophic methanogens such as Methanoculleus and Methanomassiliicoccus in S1 (2.81% and 9.24%, respectively) were detected compared to S0 (26.38% and 57.68%, respectively). However, Methanobacterium genus seemed to be enriched in the suspended fraction of biochar treatment (6.83% in S0, 51.76% in S1). A more diverse archaeal diversity was recorded in biochar supplemented groups compared to the control. Basically, Methanosarcina barkeri (Supplementary Table S2) was only identified in biochar supplemented groups and was particularly tightly attached to biochar (relative abundance of 7.36% in T1 compared to 4.92% in L1 and 2.72 in S1). Similar pattern was observed with Methanothrix which were enriched in biochar treatment samples and particularly in the suspended fraction (relative abundance of 29.84% in S1 compared to 6.26% in S0, 23.38% in L1 and 15.12% in T1).
In the current work, we attempt to assess the influence of biochar concentrations (5 g/L and 10 g/L) on anaerobic digestion of OMW with different increasing COD loads (10 g/L, 15 g/L and 20 g/L) over an incubation period of 56 days. Statistical differences in methane production and methane yields per Gram of CODintroduced were determined. When examining the final CH4 yield (mL/g COD introduced) for all the treatments, ANOVA analysis revealed a significant difference among the various COD loads treatments (Table 1). However, the Tukey’s post hoc test highlighted these points: Firstly, a significant lower yield was reached in raw OMW cultures with 20 g/L COD compared to cultures with 10 g/L COD. However, after methane production enhancement with 5 g/L biochar addition, final yield became significantly higher than that achieved in batch with 10 g/L COD load. Hence, we deduce that biochar supplementation during AD of OMW may entail promising results in terms of the recovery of metabolic activity when operating at high organic load rate at large scale applications. Furthemore, for all the treatments, 5 g/L biochar addition improved significantly methane yield compared to the control and treatments with 10 g/L biochar. These results suggested that, for all initial loads of COD, the addition of 5 g/L of biochar was more effective than the 10 g/L dose in increasing yield methane production.
Biochar supplementation may enhance methane production by adsorbing polyphenols which inhibit methanogens. This likelihood may be excluded since a higher concentration of biochar is needed for polyphenols elimination (Abid et al., 2022). It would be highly useful to analyze the microbial communities (bacteria and archaea) at the end of operation (56 days) in the cultures with initial COD load of 20 g/L to get insights into the microbial community response during AD with biochar supplementation. Microbial communities of biochar free group (R0) and 5 g/L biochar supplemented group (R1), involving suspended fraction of R0 (S0) and R1 (S1), loosly cells (L1) and tightly ones bound to biochar (T1) were analyzed. Results from high-throughput sequencing revealed that biochar supplementation improved the abundance of hydrolytic bacteria, mainly in the suspended fraction, such as Bacteroidaceae, as well as acidogenic bacteria such as Clostridiacea, Proteinivoraceae and fermentative genus group such as Erysipelatoclostridium. Bacteroidaceae play a key role in organic matter depolymerisation during acidogenesis phase thanks to several hydrolyzing enzymes (Wang et al., 2017). They seemed to be part of bacterial key players during AD exposed to high concentrations of phenol reaching 2 g/L (Poirier et al., 2016). Consequently, they were involved in the hydrolysis step to enhance polyphenols degradation. Our results go in good consistency with those of Pytlak et al. (2020) who asserted that biochar addition to a fermentation sludge containing sugar beet pulp leads to an enrichment of Bacteroidales. On the other side, it was emphasized that Bacteroides are probably able for the extracellular electron transfer since some species were enriched on the anode of a bio-electrolysis cell system and were able to transfer electrons directly to ferric iron (Wang et al., 2010). Clostridiaceae were known not only as acidogenic bacteria (Yin et al., 2018) but also as exoelectrogens. In particular, Natronincola peptidivorans was reported as an interesting new potential electro-synthesizing bacterium in the Clostridiaceae family capable of direct electron transfer in a microbial electrolysis cell (Quéméner et al., 2019). Erysipelatoclostridium were known for their ability to ferment several carbohydrates such as acetate, propionate and butyrate (Yutin and Galperin, 2013).
Stimulated CH4 after 5 g/L biochar addition to OMW with initial COD load of 20 g/L may be explained by the bacterial and archaeal communities shift with a significant enrichment of microbes involved in DIET. In this line, our results demonstrated the enrichement of Anaerolineaceae which were known for their capacities for extracellular transfer of electrons using fulvic acids as electron acceptors (Dang et al., 2016). These results proved to be consistent with the findings of Wang et al. (2018) who reported that biochar supplementation during AD of complex organic wastes entailed the enrichment of Anaerolineaceae. In this respect, Cloacibacillus species which were enriched in the suspended fraction of biochar supplemented group proved to be syntrophic amino-acid-oxydizing bacteria (Zhao et al., 2017). Recently, Yang et al. (2021) have argued that biochar addition during anaerobic digestion of swine manure enriched Cloacibacillus which might participate in DIET with Methanothrix.
More importantly, our results revealed that Rikenellaceae and Parabacteroides were distinctly more enriched in the fixed biomass of biochar. Recently, Rikenellaceae were identified as potential syntrophic bacteria capable of establishing magnetite-mediated direct electron transfer with methanogens to accelerate VFAs degradation (Lee et al., 2019). Parabacteroides are fermentative species. However, many researchers have recently reported the abundant growth of Parabacteroides under DIET-simulated conditions (Baek et al., 2019), which is suggestive that this group may potentially be involved in electro-syntrophic interactions during anaerobic digestion. Consequently, our results go in good conformity with previous researchers’ findings indicating that these groups may possibly be involved in electro-syntrophic interactions during anaerobic digestion.
Lower relative abundance of hydrogenetrophic methanogens such as Methanoculleus and Methanomassiliicoccus in S1 compared to S0 proved that IHT wan’t enhanced by biochar. Methanoculleus emergence has been reported as an early indicator which portends phenol inhibition towards microbial community during AD exposed to phenol concentrations between 1 and 2 g/L (Poirier et al., 2016). Hence, the rigorous hydrogenotrophic Methanoculleus seemed to play a key role to maintain AD via IHT in R0. However, Methanobacterium seemed to be enriched in the suspended fraction for the biochar treatment. Methanobacterium are hydrogenotrophic methanogens, which had been broadly detected in the AD of phenol culture (Na et al., 2016; Poirier et al., 2016). Consequently, positive effects of biochar addition on polyphenols alleviation can be attributed to Methanobacterium enrichement. They were recognized also as syntrophic microbes which participated to DIET and proved to be enriched in anaerobic digestion of sewage sludge process with biochar addition (Wu et al., 2019). Recently, they have been reported to be able to change the primary working mode of the syntrophic metabolism from IHT to DIET during anareobic digestion of swine manure with biochar addition (Yang et al., 2021).
Additionally, the enrichment of genera of archaea like Methanothrix and Methanosarcina is suggestive that DIET would be accelerated in the biochar treatment samples. Methanosarcina barkeri is known as a methanogen that is able to participate to DIET (Rotaru et al., 2014). Methanothrix can use both acetoclastic methanogenesis and DIET-CO2 reduction during AD with the addition of biochar (Wang C et al., 2018). Thus, the stimulation of methane production rate seems to be associated with the increase in abundance of these genera with independence of the syntrophy between bacteria and archaea via IHT. To sum up, several putative exoelectrogenic microbes were enriched in biochar treatment samples and particularly in suspended fractions such as Bacteroides, Natronincola and Cloacibacillus. More importantly, since the potential exoelectrogenic bacteria like Parabacteroides Rikenellaceae and Anaerolineaceae were enriched exclusively on the biochar, they could be directly interacting with archaea of Methanothrix and Methanosarcina via DIET. These archaea species which were enriched on the biochar, proved to be responsible for DIET. A Conceptual illustration of DIET possible mechanism in anaerobic digestion of OMW with biochar is described in Figure 7.
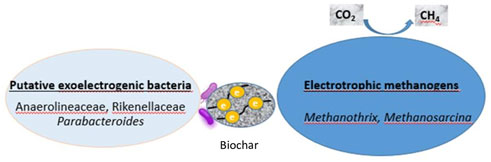
FIGURE 7. Conceptual illustration mechanism of enhancing anaerobic digestion of OMW by biochar via DIET.
As a conductive material, biochar can serve as an electron acceptor as well as a donor and allows DIET to take place (Park et al., 2018). When compared to IHT, DIET has been suggested as faster and energetically more effective than IHT (Xu et al., 2019) since it does not require energy to produce H2. As electrical conductivity plays an important role in terms of promoting DIET (Zhao et al., 2017), the used biochar in our study, which was derived from olive mill waste water sludge, would stand for a good candidate as it possesses high electrical conductivity (11 m/cm).
The inhibition of OMW polypenols towards methanogens begets the imbalance in the relationship between bacteria and methanogens, which results in significant VFAs accumulation and AD inhibition in the control cultures with the highest COD load (20 g/L). Furthermore, biochar supplementation at 5 g/L to OMW with initial COD load of 20 g/L mitigated VFAs accumulation. Our findings go in good correlation with the results of Wang G et al. (2018). These authors disclosed that biochar derived from sawdust alleviated VFAs accumulation during AD of complex organic wastes. Positive effects of biochar addition on the VFAs alleviation can be interpreted as follows: Our study revealed that biochar enhanced the growth of bacteria known for their ability to ferment several carbohydrates like acetate, propionate and butyrate (Yutin and Galperin, 2013) such as Erysipelatoclostridium. Consequently, enhanced degradation of VFAs was probably ascribed to the improved solubilization of OMW following the bacteria enrichement. Besides, our study demonstrated that biochar addition suppressed IHT and enabled DIET to take place. This pathway enhances the electron transport rates when compared to IHT. Hence, the H2 and formate concentrations are lower than during IHT. Therfore, syntrophic VFAs oxydation becomes thermodynamically feasible (Capson-Tojo et al., 2018) .
This study addresses the impact of biochar addition during AD of OMW in batch at different COD increasing loads. AD of OMW with initial COD load of 20 g/L led to process unbalance owing to VFAs accumulation. However, biochar addition at 5 g/L increased methane yield by 97.8%, mitigated VFAs accumulation and enriched prokaryotic as well as methanogenic communities.
Results indicated that biochar displayed a great potential in terms of enhancing VFAs degradation referring to the improved solubilization of OMW through microbial hydrolysis with increased abundance of hydrolytic bacteria such as Bacteroidaceae, as well as acidogenic bacteria such as Clostridiaceae, Proteinivoraceae. In addition, the enrichment of genera like Methanothrix and Methanosarcina may indicate the establishment of DIET in the biochar treatment samples.
This work provides new findings on the effect of biochar supplementation upon the production of methane from OMW which has an important implication for potential applications at large scales.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article.
NA: Conceptualization, Methodology, experimental work, Investigation, Software, Writing—Original Draft, Review and Editing FK: Methodology, Software, Review and Editing IK: Formal analysis MS: experimental work NM: experimental work AB: Review and Editing MC: Supervision SS: Funding acquisition, Supervision, Review and Editing All authors contributed to the article and approved the submitted version.
This study was supported by financial aids from ARIMNet2 PYRODI-GEST project under grant agreement no. 618127.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2022.1100533/full#supplementary-material
Abid, N., Masmoudi, M. A., Megdiche, M., Barakat, A., Ellouze, M., Chamkha, M., et al. (2022). Biochar from olive mill solid waste as an eco-friendly adsorbent for the removal of polyphenols from olive mill wastewater. Chem. Eng. Res. Des. 181, 384–398. doi:10.1016/j.cherd.2022.02.029
Aliakbarian, B., Casazza, A. A., and Perego, P. (2015). Kinetic and Isotherm Modelling of the Adsorption of Phenolic Compounds from Olive Mill Wastewater onto Activated Carbon. Food Technol. Biotechnol. 53, 207–214. doi:10.17113/ftb.53.02.15.3790
Altamirano-Corona, M. F., Anaya-Reza, O., and Durán-Moreno, A. (2021). Biostimulation of food waste anaerobic digestion supplemented with granular activated carbon, biochar and magnetite: A comparative analysis. Biomass Bioenergy 149, 106105. doi:10.1016/j.biombioe.2021.106105
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi:10.1016/S0022-2836(05)80360-2
APHA (2012). Standard methods for the examination of water and wastewater. 22th ed. DC, USA: Am. Public Heal. Assoc. Washingt.
Baek, G., Kim, J., Kim, J., and Lee, C. (2019). Individual and combined effects of magnetite addition and external voltage application on anaerobic digestion of dairy wastewater. Bioresour. Technol. 122443, 122443. doi:10.1016/j.biortech.2019.122443
Beccari, M., Carucci, G., Majone, M., and Torrisi, L. (1999). Role of lipids and phenolic compounds in the anaerobic treatment of olive oil mill effluents. Environ. Technol. (United Kingdom) 20, 105–110. doi:10.1080/09593332008616799
Boone, D. R., Johnson, R. L., and Liu, Y. (1999). Diffusion of the interspecies electron carriers H 2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake. Boone DR al 55, 1735–1741. doi:10.1128/aem.55.7.1735-1741.1989
Cai, J., He, P., Wang, Y., Shao, L., and Lü, F. (2016). Effects and optimization of the use of biochar in anaerobic digestion of food wastes. Waste Manag. Res. 34, 409–416. doi:10.1177/0734242X16634196
Cantrell, K. B., Hunt, P. G., Uchimiya, M., Novak, J. M., and Ro, K. S. (2012). Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 107, 419–428. doi:10.1016/j.biortech.2011.11.084
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Publ. Gr. 7, 335–336. doi:10.1038/nmeth.f.303
Capson-Tojo, G., Moscoviz, R., Ruiz, D., Santa-Catalina, G., Trably, E., Rouez, M., et al. (2018). Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 260, 157–168. doi:10.1016/j.biortech.2018.03.097
Dang, Y., Lei, Y., Liu, Z., Xue, Y., Sun, D., Wang, L. Y., et al. (2016). Impact of fulvic acids on bio-methanogenic treatment of municipal solid waste incineration leachate. Water Res. 106, 71–78. doi:10.1016/j.watres.2016.09.044
Dareioti, M. A., Dokianakis, S. N., Stamatelatou, K., Zafiri, C., and Kornaros, M. (2009). Biogas production from anaerobic co-digestion of agroindustrial wastewaters under mesophilic conditions in a two-stage process. Desalination 248, 891–906. doi:10.1016/j.desal.2008.10.010
Dowd, S. E., Callaway, T. R., Wolcott, R. D., Sun, Y., McKeehan, T., Hagevoort, R. G., et al. (2008). Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 8, 125. doi:10.1186/1471-2180-8-125
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. doi:10.1093/bioinformatics/btq461
Gelegenis, J., Georgakakis, D., Angelidaki, I., Christopoulou, N., and Goumenaki, M. (2007). Optimization of biogas production from olive-oil mill wastewater, by codigesting with diluted poultry-manure. Appl. Energy 84, 646–663. doi:10.1016/j.apenergy.2006.12.001
Hoareau, M., Erable, B., and Bergel, A. (2021). Oxygen-reducing bidirectional microbial electrodes: A mini-review. Electrochem. Commun. 123, 106930. doi:10.1016/j.elecom.2021.106930
Kang, S., Pinault, M., Pfefferle, L. D., and Elimelech, M. (2007). Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 23, 8670–8673. doi:10.1021/la701067r
Khoufi, S., Aloui, F., and Sayadi, S. (2006). Treatment of olive oil mill wastewater by combined process electro-Fenton reaction and anaerobic digestion. Water Res. 40, 2007–2016. doi:10.1016/j.watres.2006.03.023
Khoufi, S., Feki, F., and Sayadi, S. (2007). Detoxification of olive mill wastewater by electrocoagulation and sedimentation processes. J. Hazard. Mat. 142, 58–67. doi:10.1016/j.jhazmat.2006.07.053
Khoufi, S., Louhichi, A., and Sayadi, S. (2015). Optimization of anaerobic co-digestion of olive mill wastewater and liquid poultry manure in batch condition and semi-continuous jet-loop reactor. Bioresour. Technol. 182, 67–74. doi:10.1016/j.biortech.2015.01.092
Lee, J., Koo, T., Yulisa, A., and Hwang, S. (2019). Magnetite as an enhancer in methanogenic degradation of volatile fatty acids under ammonia-stressed condition. J. Environ. Manage. 241, 418–426. doi:10.1016/j.jenvman.2019.04.038
Logan, B. E., Oh, S. E., Kim, I. S., and Van Ginkel, S. (2002). Biological hydrogen production measured in batch anaerobic respirometers. Environ. Sci. Technol. 36, 2530–2535. doi:10.1021/es015783i
Lu, G., Ma, Y., Zang, L., Sun, Y., Yu, F., and Xue, R. (2021). Effects of granular activated carbon and Fe-modified granular activated carbon on anammox process start-up. RSC Adv. 11, 10625–10634. doi:10.1039/d1ra00384d
Luo, C., Lu, F., Shao, L., and He, P. (2014). Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 68, 710–718. doi:10.1016/j.watres.2014.10.052
Ma, J., Pan, J., Qiu, L., Wang, Q., and Zhang, Z. (2019). Biochar triggering multipath methanogenesis and subdued propionic acid accumulation during semi-continuous anaerobic digestion. Bioresour. Technol. 293, 122026. doi:10.1016/j.biortech.2019.122026
Mcinerney, M. J., Sieber, J. R., and Gunsalus, R. P. (2010). Syntrophy in anaerobic global carbon cycles. NIH Public Access 20, 623–632. doi:10.1016/j.copbio.2009.10.001
McNamara, C. J., Anastasiou, C. C., O’Flaherty, V., and Mitchell, R. (2008). Bioremediation of olive mill wastewater. Int. Biodeterior. Biodegr. 61, 127–134. doi:10.1016/j.ibiod.2007.11.003
Mechichi, T., and Sayadi, S. (2005). Evaluating process imbalance of anaerobic digestion of olive mill wastewaters. Eval. process imbalance Anaerob. Dig. olive mill wastewaters 40, 139–145. doi:10.1016/j.procbio.2003.11.050
Na, J., Lee, M., Yun, Y., Moon, C., Kim, M., and Kim, D. (2016). Microbial community analysis of anaerobic granules in phenol-degrading UASB by next generation sequencing. Biochem. Eng. J. 112, 241–248. doi:10.1016/j.bej.2016.04.030
Pan, J., Ma, J., Zhai, L., and Liu, H. (2019). Enhanced methane production and syntrophic connection between microorganisms during semi-continuous anaerobic digestion of chicken manure by adding biochar. J. Clean. Prod. 240, 118178. doi:10.1016/j.jclepro.2019.118178
Park, J. H., Kang, H. J., Park, K. H., and Park, H. D. (2018). Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 254, 300–311. doi:10.1016/j.biortech.2018.01.095
Poirier, S., Bize, A., Bureau, C., Bouchez, T., and Chapleur, O. (2016). Community shifts within anaerobic digestion microbiota facing phenol inhibition: Towards early warning microbial indicators? Water Res. 100, 296–305. doi:10.1016/j.watres.2016.05.041
Pytlak, A., Kasprzycka, A., Szafranek-nakonieczna, A., Kubaczy, A., Proc, K., Onopiuk, P., et al. (2020). Biochar addition reinforces microbial interspecies cooperation in methanation of sugar beet waste (pulp). Sci. Total Environ. 730, 138921.
Quéméner, E. D., Bridier, A., Tian, J., Madigou, C., Bureau, C., Qi, Y., et al. (2019). Biorefinery for heterogeneous organic waste using microbial electrochemical technology. Bioresour. Technol. 292, 121943. doi:10.1016/j.biortech.2019.121943
Rotaru, A., Shrestha, M., Liu, F., Markovaite, B., Chen, S., Nevin, K. P., et al. (2014). Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 80, 4599–4605.
Sayadi, S., Allouche, N., Jaoua, M., and Aloui, F. (2000). Detrimental effects of high molecular-mass polyphenols on olive mill wastewater biotreatment. Proc. Biochem. 35, 725–735.
Stams, A. J. M., De Bok, F. A. M., Plugge, C. M., Van Eekert, M. H. A., Dolfing, J., and Schraa, G. (2006). Exocellular electron transfer in anaerobic microbial communities. Environ. Microbiol. 8, 371–382. doi:10.1111/j.1462-2920.2006.00989.x
Vavouraki, A. I., Zakoura, M. V., Dareioti, M. A., and Kornaros, M. (2020). Biodegradation of polyphenolic compounds from olive mill wastewaters (OMW) during two-stage anaerobic Co-digestion of agro-industrial mixtures. Waste Biomass Valorization 11, 5783–5791. doi:10.1007/s12649-019-00887-4
Wang, A., Liu, L., Sun, D., Ren, N., and Lee, D. (2010). Isolation of Fe ( III ) -reducing fermentative bacterium Bacteroides sp . W7 in the anode suspension of a microbial electrolysis cell ( MEC ). Int. J. Hydrogen Energy 35, 3178–3182. doi:10.1016/j.ijhydene.2009.12.154
Wang, G., Gao, X., Li, Q., Zhao, H., Liu, Y., Wang, X. C., et al. (2020). Redox-based electron exchange capacity of biowaste-derived biochar accelerates syntrophic phenol oxidation for methanogenesis via direct interspecies electron transfer. J. Hazard. Mat. 390, 121726121726. doi:10.1016/j.jhazmat.2019.121726
Wang, S., Hou, X., and Su, H. (2017). Exploration of the relationship between biogas production and microbial community under high salinity conditions. Sci. Rep. 7, 1149. doi:10.1038/s41598-017-01298-y
Wang, C, C., Liu, Y., Gao, X., Chen, H., Xu, X., and Zhu, L. (2018). Role of biochar in the granulation of anaerobic sludge and improvement of electron transfer characteristics. Bioresour. Technol. 268, 28–35. doi:10.1016/j.biortech.2018.07.116
Wang, G, G., Li, Q., Gao, X., and Wang, X. C. (2018). Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 250, 812–820. doi:10.1016/j.biortech.2017.12.004
Wu, B., Yang, Q., Yao, F., Chen, S., He, L., Hou, K., et al. (2019). Bioresource Technology Evaluating the e ff ect of biochar on mesophilic anaerobic digestion of waste activated sludge and microbial diversity, 294. doi:10.1016/j.biortech.2019.122235
Xie, Y. (2006). Photoelectrochemical application of nanotubular titania photoanode. Electrochim. Acta 51, 3399–3406. doi:10.1016/j.electacta.2005.10.003
Xu, H., Chang, J., Wang, H., Liu, Y., Zhang, X., Liang, P., et al. (2019). Enhancing direct interspecies electron transfer in syntrophic-methanogenic associations with (semi)conductive iron oxides: Effects and mechanisms. Sci. Total Environ. 695, 133876. doi:10.1016/j.scitotenv.2019.133876
Yang, S., Chen, Z., and Wen, Q. (2021). Impacts of biochar on anaerobic digestion of swine manure: Methanogenesis and antibiotic resistance genes dissemination. Bioresour. Technol. 324, 124679. doi:10.1016/j.biortech.2021.124679
Yin, C., Shen, Y., Zhu, N., Huang, Q., Lou, Z., and Yuan, H. (2018). Anaerobic digestion of waste activated sludge with incineration bottom ash: Enhanced methane production and CO2 sequestration. Appl. Energy 215, 503–511. doi:10.1016/j.apenergy.2018.02.056
Yutin, N., and Galperin, M. Y. (2013). A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. clostridia 15, 2631–2641. doi:10.1111/1462-2920.12173
Zhang, J., Wang, Q., Zheng, P., and Wang, Y. (2014). Anaerobic digestion of food waste stabilized by lime mud from papermaking process. Bioresour. Technol. 170, 270–277. doi:10.1016/j.biortech.2014.08.003
Zhang, T., and Tremblay, P. (2020). iScience ll graphene : An antibacterial agent or a promoter of bacterial proliferation. ISCIENCE 23, 101787. doi:10.1016/j.isci.2020.101787
Zhao, Z., Li, Y., Quan, X., and Zhang, Y. (2017). Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 115, 266–277. doi:10.1016/j.watres.2017.02.067
Zhao, Z., Zhang, Y., Holmes, D. E., Dang, Y., Woodard, T. L., Nevin, K. P., et al. (2016). Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 209, 148–156. doi:10.1016/j.biortech.2016.03.005
Keywords: biochar, direct interspecies electron transfer, anaerobic digestion, prokaryotic communities, olive mill wastewater
Citation: Abid N, Karray F, Kallel I, Slim M, Barakat A, Mhiri N, Chamkha M and Sayadi S (2023) Role of biochar in anaerobic microbiome enrichment and methane production enhancement during olive mill wastewater biomethanization. Front. Bioeng. Biotechnol. 10:1100533. doi: 10.3389/fbioe.2022.1100533
Received: 16 November 2022; Accepted: 08 December 2022;
Published: 04 January 2023.
Edited by:
Fang Zhang, Fujian Agriculture and Forestry University, ChinaReviewed by:
Xiaodong Xin, Dongguan University of Technology, ChinaCopyright © 2023 Abid, Karray, Kallel, Slim, Barakat, Mhiri, Chamkha and Sayadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nozha Abid, bm96aGEuYWJpZEBmc3MudXNmLnRu; Sami Sayadi, c2FtaS5zYXlhZGlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.