
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol., 07 November 2022
Sec. Bioprocess Engineering
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.1054757
This article is part of the Research TopicIsolation, Modification, and Characterization of the Constituents in Biomass and Their Bio-based Applications, Volume IIView all 14 articles
The soil’s rhizosphere is a highly active place where the exchange of substances and information occurs among plants, soils, and microorganisms. The microorganisms involved are crucial to the activities of plant growth and development, metabolism, and reproduction. Fritillaria L. medicinal plants are unique Chinese medicinal ingredients, but the continuous cropping obstacles formed in the artificial planting process is severely harmful to the growth and development of these medicinal plants. In this review, we summarized the current species and distribution of Fritillaria L. in China, and analyzed the changes in microbial diversity (mainly among bacteria and fungi) in the rhizosphere of these plants under long-term continuous cropping. The fungi showed an increasing trend in the soil rhizosphere, resulting in the transition of the soil from the high-fertility “bacterial type” to the low-fertility “fungal type” as planting years increased. Furthermore, the interaction between Fritillaria L. medicinal plants and the rhizosphere microorganisms was reviewed, and promising applications for the rhizosphere microbiome in the cultivation of Fritillaria L. medicinal plants were suggested. It is expected that this review will facilitate the in-depth understanding of rhizosphere microorganisms in the growth, accumulation of active ingredients, and disease control of Fritillaria L.
Fritillaria L., a valued traditional Chinese herbal medicine, contains diterpenoids, steroids, alkaloids, polysaccharides, which have broad applications in health care products, food, Chinese herbal formulas, and everyday chemical industries. This plant has the benefits of reducing phlegm, relieving coughs, reducing heat, moistening the lungs, improving blood stasis, and dispersing knots (Chen et al., 2020). It is also one of the main ingredients in cough- and phlegm-relieving Chinese patented medicines. However, the sources of Fritillaria medicinal plants in the wild are becoming increasingly reduced due to its small output, long resource regeneration cycle, overexploitation, and habitat destruction. In 2021, it was listed as a secondary protected plant among the national key protected wild plants (Cunningham et al., 2018). Moreover, the quantity and quality of Fritillaria medicinal materials on the market are inconsistent because of the wide variety of Fritillaria medicinal plants, the wide production area, and the lack of standardized processing and operation processes in planting and production. There are problems in concerning the medicinal and edible safety of Fritillaria medicinal materials, which are restricting the development of the Fritillaria medicinal plant industry (Chen et al., 2021). Therefore, developing artificial and standardized cultivation methods for Fritillaria medicinal plants is the most effective way to solve the contradiction between the current market demand for medicinal materials and its resource protection.
The rhizosphere is a unique ring-shaped zone mainly characterized by the interaction between the root system and soil microorganisms. A large number of microorganisms, such as bacteria, Actinomycetes, fungi, and soil animals, gathered around the plant root system, which is a part of the soil microenvironment that presenting special physical, chemical, and biological properties (Garcia and Kao-Kniffin, 2018; Mojicevic et al., 2019; Shao et al., 2021). As the most active component in the soil ecosystem, soil microbes mainly participate in the cycling of nutrients, such as carbon, nitrogen, phosphorus, and sulfur in the soil (Hemkemeyer 2021). Rhizosphere microbes improves the utilization rate of nutrients in the soil, thereby affecting the growth and development of plants and stress resistance, and promoting good plant health and soil quality. Therefore, it is of great significance to perform research on the rhizosphere microorganisms of medicinal plants (Mendes et al., 2013; Tian et al., 2018). However, few reports exists on the rhizosphere microorganisms of Fritillaria medicinal plants (Qiu, 2010; Pan et al., 2010a,Pan et al., 2010b; Mu et al., 2019a,Mu et al., 2019b,Mu et al., 2019c; Wu et al., 2021b).
This review article systematically describes the research results on the rhizosphere microorganisms associated with Fritillaria medicinal plants in detail. The words “Fritillaria,” “rhizosphere microorganisms,” and “Microbiome” were used as keywords to search the literature in the Chinese National Knowledge Infrastructure (CNKI) and Web of Science. Fifty-seven literature was searched in CNKI, and 57.9% of which was published from 2016 to 2021, six literature was searched in Web of Science. It is expected that this review will contribute to further understand the involvement of rhizosphere microorganisms in the growth of Fritillaria medicinal plants and the accumulation of alkaloids, which are the most important active ingredients of Fritillaria.
The medicinal quality and market price of Fritillaria L. from different places of origin have varied in recent years. To meet the requirements of the market, it is imperative to cultivate Fritillaria L. medicinal plants artificially. Five types of Fritillaria L. plants present in the Chinese Pharmacopoeia (Volume I, 2020 Edition) are collected; these include Fritillaria ussuriensis Maxim, Fritillaria pallidiflora Schrenk, Fritillaria thunbergia Miq., Fritillaria hupehensis Hsiao et K. C. Hsia, and Fritillaria cirrhosa D. Don. The specific growth environment and distribution of different Fritillaria medicinal plants are shown in Figure 1. The various Fritillaria medicinal plants have wide distribution range and high adaptability. It has been proven that it is feasible to breed wild Fritillaria medicinal plants (Huang et al., 2018; Luo M. et al., 2021a; Luo S. et al., 2021b). Five types of these Fritillaria plants all have the function of reducing heat, relieving coughs, reducing phlegm, and there are moistening lung function of F. ussuriensis and F. pallidiflora, detoxification, dissipating mass and eliminating carbuncle function of F. thunbergia, dissipating mass function of Fritillaria hupehensis, moistening lung, dissipating mass and eliminating carbuncle function of F. cirrhosa (Chinese Pharmacopoeia Commission, 2020).
As the most active component of the soil ecosystem, rhizosphere microorganisms play an important role in plant growth, nutrient circulation, and improving yields (Mendes et al., 2013). The diversity and colonization ability of soil microbial communities in different microhabitats affect the growth rate of pathogens and also play an important role in improving plant health (Li et al., 2013). The abundance and diversity of soil microbial community decreased in the field evaluation of root rot disease in Fritillaria ussuriensis, while the population of pathogens in the healthy soil sample was quite low, indicating that the microbial community structure affected the health of F. ussuriensis (Song et al., 2016). The diversity and structure of the microbial community were closely related to the health level of F. ussuriensis, this can lay a foundation for the systematic study on the interaction between microbial genera in the pathological process of F. ussuriensis (Jiao et al., 2022).
As the most abundant and widely distributed group of rhizosphere soil microorganisms, bacteria account for 70–90% of the total number of rhizosphere microorganisms, which are sensitive indicators of nutrient changes in rhizosphere soil (Yu et al., 2019). Researches about the relationship between the number and diversity of bacteria and Fritillaria medicinal plants were carried out. Liao (2011) found that the number of culturable bacteria of rhizosphere soil of F. thunbergii was decreased with the planting year increaced, which is related with the monocropping obstacle of F. thunbergii. In agricultural production, a variety of issues, such as disease accumulation, impaired growth, and quality decline, appeared in the third year after planting Fritillaria taipaiensis P. Y. Li seedlings (Mu et al., 2019a). This seriously affected the yield and quality of F. taipaiensis. The number of culturable rhizosphere microorganisms associated with wild and cultivated F. taipaiensis in different growth years and production areas was measured. It was found that the rhizosphere microorganisms of F. taipaiensis were abundant, and the number of bacteria was 2 × 106 cfu·g−1–3×107 cfu·g−1, when the total number of microorganisms was 3.8 × 107 cfu·g−1, but which decreaced alongwith the planting years of cultivated F. taipaiensis and growth year of wild F. taipaiensi (Mu, 2019b).
Using the high-throughput sequencing technology, from the phylum level analysis, it can be seen that the bacterial community within the soil rhizosphere was mainly composed of Proteobacteria Acidobacteria, Bacteroidetes, Verrucomicrobia, Firmicutes, Gemmatimonadetes, Actinobacteria, Planctomycetes, Nitrospirae, and Chloroflexi (Mu, 2019). Among them, Proteobacteria belonged to the dominant phylum with the largest relative abundance (54.82%). As planting years increased, the relative abundances of the genera Lactobacillus, Gemmatimonas, Bryobacter, and Bacteroides gradually decreased, whereas the relative abundances of the genera Methylotera, Sphingobacterium, and Pseudomonas gradually increased (Mu, 2019). Wu (2021a,Wu 2021b) reported that the composition of phyla and genera within soil bacterial communities changed alongwith the growth years of F. kansuensis. Among them, for the relative abundance, Actinomycetes (23.58%–32.08%), Proteobacteria (22.00%–28.80%), Acidobacteria (13.82%–20.86%) and Chloroflexi (8.43%–15.08%) belonging to the dominant phyla, and the continuous planting of Fritillaria kansuensis for 5 years would significantly reduce the diversity of bacterial communities in the soil rhizosphere. Tang et al. (2021) discovered that the bacterial diversity in the rhizosphere gradually decreased as the growth of Fritillaria thunbergii.
The bacteria that presented decreased relative abundance in rhizosphere soil included potassium-solubilizing bacteria (PSB), phosphate-solubilizing bacteria, and nitrogen-fixing bacteria, which may because of the increase in the relative abundance of saprophytic fungi. Phosphate solubilizing bacteria can convert insoluble phosphorus into effective phosphorus that can be absorbed and subsequently used by plants. According to the different substrates of phosphate-solubilizing bacteria, they could be divided into organic phosphorus bacteria and inorganic phosphorus bacteria (Chi et al., 2021), which is a type of bacteria isolated from soil that can decompose phosphorus-containing minerals. PSB, also known as potassium bacteria or silicate bacteria, convert mineral potassium into potassium that is available for plant absorption and utilization by adhering to the mineral surface and releasing acidic substances. These are a type of bacteria isolated from the soil that can decompose potassium-containing minerals (Han et al., 2022).
The isolation and screening of organophosphate-solubilizing bacteria, inorganic phosphate-solubilizing bacteria, and PSB from the rhizosphere soil of F. taipaiensis in the previous report revealed that these varied with different growth years (Table 1). As the planting years of F. taipaiensis increased, there was an increase and subsequent decrease in the total number of organophosphate-solubilizing bacteria, Actinomycetes, inorganic phosphate-solubilizing bacteria, as well as other bacteria and microorganisms in the rhizosphere. However, there was a decrease in the number of PSB. It was found that fertilization and continuous cropping significantly affected the community composition and function of rhizosphere microorganisms associated with F. taipaiensis.
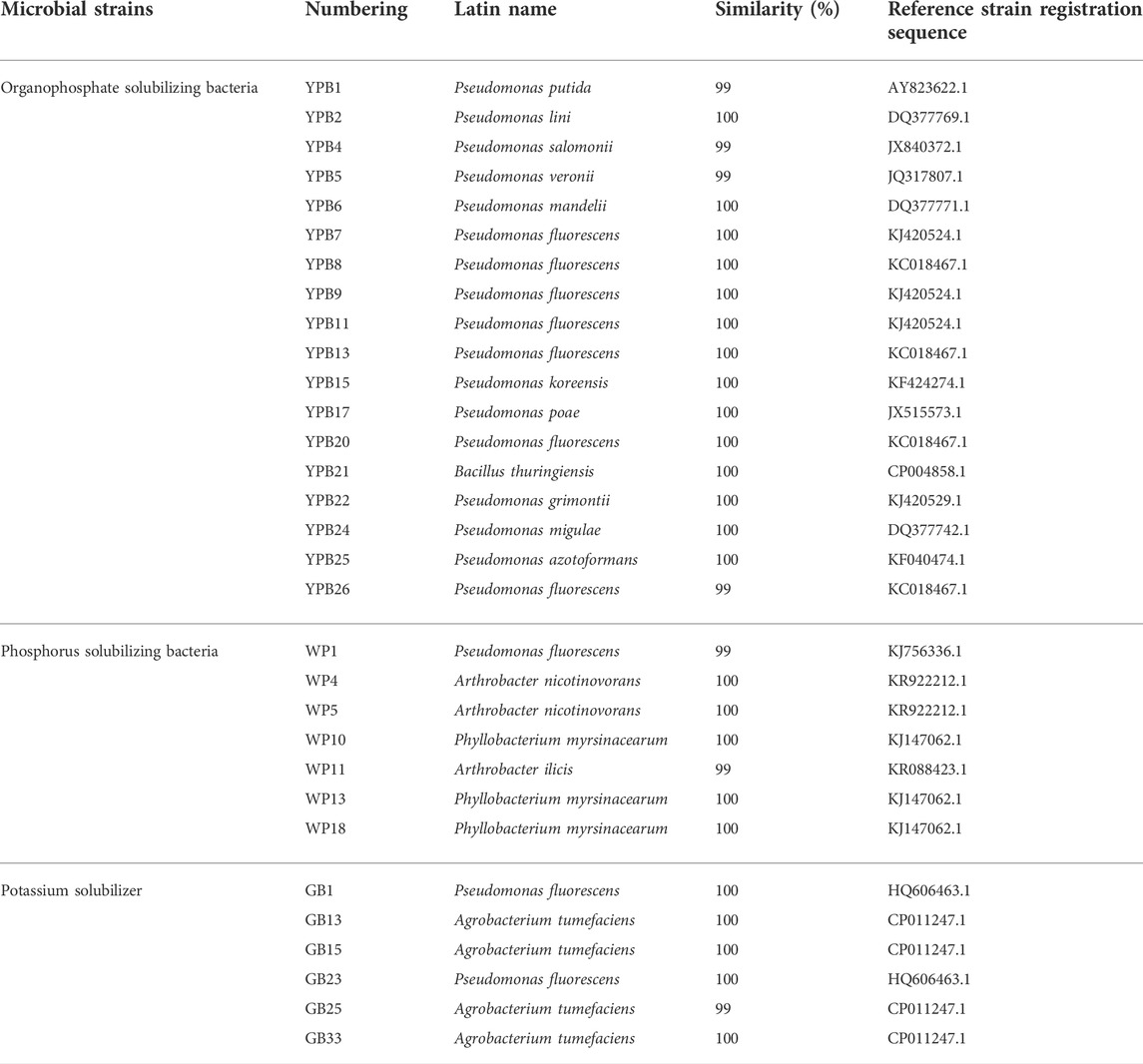
TABLE 1. Diversity of potassium solubilizing bacteria and phosphate solubilizing bacteria in the rhizosphere of Fritillaria L. medicinal plants.
It was discovered that the symbiosis between F. Taipaiensis and AM fungi was very common; different AM fungi infected the root system of F. Taipaiensis to different degrees; and the inoculation of AM fungi significantly increased the mycorrhizal infestation rate, and, in turn, the biomass of F. taipaiensis (Zhang, 2020). AM fungi could increase the content of inorganic elements in the rhizome and soil of Fritillaria taipaiensis P. Y. Li.; enhance the enrichment ability of inorganic elements, thus improving the growth and development of F. taipaiensis P. Y. Li.; and promote the accumulation of nutrients in medicinal material (Wei et al., 2021). Fritillaria thunbergii Miq. is a crop that is continually cropped; thus, it is necessary to change the land after 1 year of artificial planting (Yu and Wu, 2017). When the four planting years increased, there was a linear increase in the number of cultivable fungi in the soil from the root zone of F. thunbergii Miq., whereas the total amount of cultivable Actinomycetes, bacteria decreased linearly (Liao et al., 2011). In general, fungi prefer acidic environments, Actinomycetes prefer neutral to alkaline environments, while other bacteria mainly live in neutral environments. The continuous cropping of F. thunbergii Miq. reduced the pH value of the soil, resulting in the soil being slightly acidic. This, being conducive to the propagation of fungi, results in the transition of the soil from a high fertility “bacterial type” to a low fertility “fungal type” (Liao et al., 2011). As the planting years of F. taipaiensis increased, so did the fungi in the rhizosphere. This indicates that long-term continuous cropping would lead to a reduction in the numbers of beneficial microorganisms, and increase the numbers of pathogenic microorganisms in the rhizosphere of F. taipaiensis (Mu et al., 2019b; Gu et al., 2020), which is not conducive to the growth of F. taipaiensis.
Non-culture methods can more accurately reflect the community structure of rhizosphere microorganisms compared with traditional culture methods. The results of high-throughput sequencing of ITS sequence showed that fungal community in the rhizosphere of F. taipaiensis was mainly composed of Ascomycota, Zygomycota, Basidiomycota, Glomeromycota, Neocallistigomycota, and Chytridiomycota. And Ascomycota fungi were the most dominant population. With the increase in planting years, the relative abundance of Pseudogymnomyces in the soil rhizosphere gradually decreased, along with the relative abundance of populations such as pathogenic fungi in Fusarium, Gibberella, Rhizopus, Colletotrichum, and Peziza (Mu, 2019; Zhou et al., 2021).
The richness and diversity of rhizosphere microorganisms plays a key role in regulating ecological functions, such as organic matter decomposition, nutrient cycling, and soil carbon dynamics (Sasse et al., 2018). In nature, plants release and secrete various compounds into the environment through a variety of ways, such as via leaf litter, root residues, and root exudates. This occurrence provides vital carbon sources for the formation of aggregates, and affects the physical and chemical properties of community of rhizosphere microorganisms (Vezzani et al., 2018; Vives-Peris et al., 2020). Conversely, the biological, physical, and chemical characteristics of rhizosphere microorganisms affects host plants and their coexisting plants (Ehrenfeld et al., 2005). Root exudates are important factors in the formation of rhizosphere microorganisms, because the various primary metabolites and secondary metabolites secreted by roots can play the role of shaping, interfering, or transmitting signals to change the rhizosphere microflora, recruit and promote beneficial microorganisms, and resist harmful microorganisms (Venturi and Keel, 2016). Generally, the rhizosphere microorganism of plant are more abundance, active and rich in diversity than the no-rhizosphere microorganism.
Root exudates provided abundant nutrients and energy for the growth of rhizosphere microorganisms, and also affects the distribution, species, and quantity of rhizosphere microorganisms (Wang et al., 2007). The root exudates of Fritillaria medicinal plants include organic acids, amino acids, soluble sugars, total phenolic acids, and organic substances (Guo et al., 2013). Various organic substances, such as ethers, olefins, acids, aldehydes and ketones, esters, alkanes, ureas, phenols, and alcohols (Wang et al., 2009) have been found in root exudates. Among them, are high contents of aldehydes and ketones. Phenolic acids secreted by roots promote the growth of black spot fungus and Botrytis cinerea (Guo et al., 2013). Phenol and 1,3,5-triallyl-1,3,5-triazine-2,4,6 (1H, 3h, 5H) trione, being the main root exudates of Fritillaria, had a significant inhibitory effect on the growth of its seedlings (Wang, 2010; Wang et al., 2010a,b). Generally, the secondary metabolites produced by the roots of medicinal plants? were easily released into the soil, thereby promoting the colonization and shaping of the rhizosphere microorganism community, and driving the feedback effect of plant soil on defense and growth (Hu et al., 2018). This results in changes in the population structure of plant rhizosphere microorganisms. It should be noted that there are great differences in the species, dominant species and quantity of rhizosphere microorganisms in different growth stages of the same plant or in the same growth stage of different plants. This is due to the variety and quantity of plant root exudates, which vary with growth stage or plant variety (Brimecombe et al., 2001).
The metabolism of rhizosphere microorganisms either directly promotes or inhibits the nutrient absorption and growth of roots, which plays a key role in the secondary metabolism of plants. Therefore, in recent years, much attention has been paid to the interpretation of the quality formation, change, and mechanism of traditional Chinese medicine from the perspective of microecology, to clarify the influence of rhizosphere microorganisms on the growth of medicinal plants and medicinal ingredients. It has been confirmed that rhizosphere microorganisms improve the yield and medicinal components of medicinal plants. Zhang et al. (2020) inoculated four types of arbuscular mycorrhizal fungi (AMF) including Glomus constrictum (GC), Glomus versiforme (GV), Glomus mossae (GM), and Glomus aggregatum (GA). AMF alone could significantly increase the fresh weight, dry weight, and drying rate of F. taipaiensis bulbs, and increase the contents of Fritillarin A, sibelline glycoside, Fritillarin B, Fritillarin, and alkaloids in the bulb. AM fungi could increase the content of inorganic elements in the rhizome and soil of F. ussuriensis, and also enhance the enrichment ability of inorganic elements, thereby improving the growth and development of F. ussuriensis, and promoting the accumulation of nutrients in medicinal materials (Wei et al., 2021). Sun et al. (2022a) found that medicinal plants had a great diversity of rhizosphere AM fungi. Fungal colonization improved plant growth performance and root morphology, and significantly increased the content of most disaccharides, but either reduced or did not change the content of most monosaccharides. AM fungi significantly increased the concentration of the medicinal components (chrysophanol, physion, polydatin, and resveratrol) in the root of P. cuspidatum, and upregulated the expression of related synthase genes (Sun et al., 2022b). Therefore, AM fungi are beneficial to the growth and nutrient absorption of medicinal plants, thereby accelerating the accumulation of medicinal ingredients.
Pan et al. (2010a) used Bacillus subtilis to spray leaves and irrigate roots at different growth stages of F. pallidiflora, which increased the yield by 16.8%. The correlation analysis between rhizosphere microorganisms and the content of Siberian in the growth stage of F. pallidiflora showed that the fungi in rhizosphere soil had a significant positive correlation with the content of Siberian, while Actinomycetes and bacteria had a positive correlation with the content of Siberian (Pan et al., 2010b). These results are similar with those reported by Qiu (2010), and have the potential to be included in applications to further develop the resources of beneficial fungi in the rhizosphere of F. pallidiflora and improve its medicinal quality. Zhao et al. (2021) isolated 20 endophytic bacteria from 3-year-old F. przewalskii Maxim. plants, which were distributed in the three phyla of Proteobacteria, Firmicutes, and Actinomycetes. Among them, strains related to Bacillus, Rhizobium, and Pseudomonas were the dominant growth-promoting bacteria. Foliar spraying of the Firmicutes Bacillus, Rhizobium, and Pseudomonas compound promoted the growth of F. przewalskii Maxim. and significantly increased its yield. Tang et al. (2021) found that with the advancement of the growth process of F. thunbergii Miq., the content of alkaloids (Fritillarin A and Fritillarin B) in the bulb first increased and then decreased. Correlation analysis showed that the Chao1 index and Shannon index among the bacteria were positively correlated with the content of monomer alkaloids and total alkaloids, indicating that the soil bacterial community was closely related to the content of alkaloids. Furthermore, the secondary metabolites produced by Fritillaria cirrhosa D. Don displayed high antioxidant activity. For example, the low polar substances extracted by petroleum ether had weak ABTS free radical scavenging activity, whereas the high polar substances extracted by solvents such as n-butanol and ethyl acetate had high ABTS free radical scavenging activity (Pan et al., 2017). These results indicated that it was feasible to use beneficial rhizosphere microorganisms to improve the yield and quality of F. taipaiensis in artificial cultivation. During the long-term artificial cultivation of medicinal plants, agricultural management measures (Tan et al., 2013; Geng et al., 2018), such as the chemical fertilizer, pesticide spraying, and planting method caused a change in the rhizosphere microbial biomass and its community structure, which was mediated by soil physical and chemical properties. This resulted in varying degrees of differences in the medicinal value between wild medicinal materials and cultivated medicinal materials, which further increased the variation in quality of the same type of Chinese medicinal materials from different origins (Schmidt et al., 2019).
Microbial fertilizers are a type of live microbial preparation widely used in agricultural production. These play an irreplaceable role in future advancements in agriculture due to their contributions to environmental protection, soil improvement, fertility improvement, output increase, and quality improvement. For example, the exogenous inoculation of AMF, phosphorus-dissolving bacteria, potassium-dissolving bacteria, and other microbial fertilizers increased the alkaloid content of 3-year-old F. taipaiensis plants (Ma, 2021). Based on the results of the content of secondary metabolites, and relevant physical and chemical properties of rhizosphere soil, the quality of F. taipaiensis had a great relationship with rhizosphere microorganisms. However, the growth potential of seedlings co-cultured with a single strain was relatively low, and some even exhibited yellowing. After mixed inoculation of AMF, phosphorus-dissolving bacteria, and potassium-dissolving bacteria, the alkaloid content in bulbs was lower than that in F. taipaiensis plants that were co-cultured with single strains. This may be because of the limitation of a single strain, the unstable interaction between rhizosphere promoters and host plants (Rainer and Florian, 2018), or it may be due to the effect of unknown ecological functions (Lebeis et al., 2012). Therefore, the rhizosphere microorganisms of plants may not exist alone, but instead play a role in the active ingredients of F. taipaiensis in the form of a specific flora. In addition, the exogenous application of microbial agents increased the number of Actinomycetes and bacteria in the rhizosphere soil of 2-year-old F. taipaiensis plants, but had no obvious effect on the number of fungi. It has also significantly improved the activities of soil protease, acid phosphatase, urease, and sucrase, thereby playing an important role in the sustainable utilization and stability of soil nutrients (Dong, 2018). The exogenous application of different doses of microbial fertilizer significantly increased the height of the plant, the fresh weight of the plant and bulb, and the yield of F. pallidiflora. Therefore, microbial fertilizer was commonly applied in the cultivation of F. pallidiflora. It was more beneficial to increase the production and efficiency of F. pallidiflora by opening ditches and applying the microbial fertilizer during the growth period (Chen et al., 2017). Therefore, microbial fertilizer can improve the structure and density of the microbial population in the rhizosphere soil, as well as the quality.
Fritillaria Chinese herbal medicines generally take 4–6 years to grown from a germinating seed to flowering and bearing, and take at least 3 years for commercial medicinal materials. However, with the increase in the growth years of Fritillaria Chinese herbal medicines, conditions that are favorable for outbreaks of harmful rhizosphere microorganisms have been created in the process of artificial planting. Coupled with poor field management, the quality of medicinal materials gradually decreased. Common soil borne diseases, such as root rot disease, sclerotium disease, ray mold disease, rust disease, and yellow rot rust, occurred frequently and caused serious harm (Wang, 2010). This made it very easy for Fritillaria to develop bulb rot and lose its medicinal value (Liu et al., 2020; Wu, 2021a). Using morphological identification method, the pathogen of sclerotium disease and root rot disease of F. pallidiflora was identified as Stromatinia rapulum (Supi et al., 2012) and Fusarium solani var. coeruleum (Supi et al., 2015), respectively. But Sclerotium denigrans and Sclerotinia sclerotiorum caused the sclerotium disease of F. ussuriensis with molecular biology methods (Song et al., 2016a). The pathogens of root rot disease in F. thunbergia were Fusarium oxysporum and F. incarnatum, the pathogen of black spot disease was Alternaria alternata, and Phoma sp. could cause leaf spot disease (Wang, 2017). But the pathogens of root rot disease of F. przewalskii were F. oxysporum, F, tricinctum, Bionectria ochroleuca and Clonostachys rosea (Wu et al., 2021a). Botrytis cinerea was identified as the pathogen of ray mold disease of F. thunbergia, and not the Botrytis elliptica which was generally considered as the pathogen in the past (Li et al., 2022).
The plant microbiome, also known as the pan-genome, refers to the study of the role of the rhizosphere microbial community in the growth of host plants in a holistic manner. It plays an irreplaceable role in the ecological environment and agricultural production (Bulgarelli et al., 2013; Andezej and Philip, 2015), which is recognized as an important determinant of healthy plant growth (Berg et al., 2014). Combining the core strategy of “whole microbiome association analysis,” the microbiome accurately decoded the expression spectrum, composition spectrum, and function spectrum of the community/flora, discovered key organisms and their markers, and then clarified the complex causal chain and interaction mechanism between the “microbiome plant soil” (Claire, 2017).
According to the varied distribution of microorganisms on the root system, the rhizosphere microbiome could be divided into the three categories of rhizosphere microbiome, root surface microbiome, and root internal microbiome (Tripathi et al., 2018). Due to the limitations of research technology, these three aspects had been involved in previous studies, but they were not specifically analyzed as a whole to evaluate the importance of these rhizosphere microbiomes to plant growth. In the study of plant rhizosphere microbiome, it is necessary to pay attention to the different sampling methods of different microbiomes. After the plant roots were taken out of the soil, 1 mm-thick soil was collected from around the roots by shaking and washing. In this way, all the soil on the root surface was placed in a phosphate buffer solution, and the microorganisms close to the root surface were separated for rhizosphere microbiome analysis (Edwards et al., 2015). Because there were few microorganisms on the root surface and it was difficult to collect them, the cleaned roots were put into phosphate buffer solution for ultrasonic treatment for 30 s, and the small amount of plant soil residues in the buffer solution were considered the microbial components on the root surface. After the cleaned roots were chemically treated with sodium hypochlorite and ethanol, or all the microorganisms on the root surface were removed by continuous sonication performed twice, the roots were then crushed by adding glass beads, which was considered the microbial components in the roots (Lundberg et al., 2012; Reinhold-Hurek et al., 2015).
A large number of studies have indicated that the rhizosphere microbiome played an important role in promoting the growth and tolerance of host plants. These are mainly divided into two methods of negative interaction such as rhizosphere microorganisms infecting the host to cause diseases or competing with the host for nutrition, and positive interaction such as rhizosphere microorganisms promoting growth, stress resistance, and disease resistance (Bais et al., 2006). This interaction process exerts a strong selection pressure on both the rhizosphere microbiome and host plants, thus forming the mode and rate of rhizosphere microbial evolution and affecting the formation of the rhizosphere microbiome (Cosetta and Wolfe, 2019). An in-depth exploration of the selection mechanism of plants for the rhizosphere microbiome could guide the recombination and improvement of the rhizosphere microbiome in practical applications (Sun et al., 2021). It also improved the understanding of plant microbe interaction in theory, which is of great significance for future agricultural production. For example, using Indica rice and Japonica rice as experimental materials, Zhang et al. (2019) found that Indica rice often showed higher nitrogen utilization efficiency than that of Japonica rice. Furthermore, the diversity of the rhizosphere microbiome of Indica rice was significantly higher than that of Japonica rice, which directly confirmed the relationship between the rhizosphere microbiome and the nitrogen utilization efficiency of plants. Utilizing beneficial members of the rhizosphere microbiome and making microbial fertilizer is a very promising application within fertilizer preparation (Hu et al., 2016). Unfortunately, the current research on the development of the rhizosphere microbiome into microbial fertilizer is still at the experimental stage. Nevertheless, microbial fertilizers have potential use in the natural agricultural environment.
Yuan et al. (2018) found that when plants were infected by pathogenic bacteria, they could recruit and enrich certain beneficial microorganisms by sending specific signals. This phenomenon was the famous “cry for help hypothesis.” These beneficial recruited microorganisms in the rhizosphere improved the disease resistance of plants in four ways: competition, parasitism, antibiosis, and the induction of systemic resistance (Mendes et al., 2013). For example, the antibacterial metabolite 2,4-diacetyl-phloroglucinol secreted by Pseudomonas fluorescens in rhizosphere microorganisms inhibited Sclerotium rolfsii by more than 75% (Asadhi et al., 2013). P. fluorescens (WCS417r) that colonized the Arabidopsis rhizosphere could upregulate the expression level of defense-related genes of the host plant pathogen (Pseudomonas syringae pv tomato) (Wees et al., 1999). In addition, AMF established a good symbiotic system with most terrestrial plants, promoted its external hyphal network to accelerate the absorption of nutrients and water, and enhanced the resistance of host plants (Li et al., 2019). It could be seen that no matter how the rhizosphere microorganisms interact with plants, the successful colonization of microorganisms in the rhizosphere was of great importance to plants. This is because the different secretions secreted by plant roots were signals recognized by rhizosphere microorganisms, and also vectors for mutual communication between rhizosphere microorganisms. Therefore, these signals could, in-turn, be used for the colonization of rhizosphere microorganisms. Consequently, root exudates are considered to be important mediators in the communication between rhizosphere microorganisms and host plants, due to the diversity and complexity of their components. Currently, there is little research on the rhizosphere microbiome of Fritillaria plants; thus, the research on the rhizosphere microbiome will facilitate the recruitment of beneficial bacteria, and facilitate the development of Fritillaria resources.
In this review article, rhizosphere microorganisms were found to be closely related to the propagation, metabolism, and growth of Fritillaria medicinal plants. Therefore, the rhizosphere microorganisms of Fritillaria plants have received much attention, which not only helps to solve the practical problems of disease prevention among Fritillaria medicinal plants, but also improves the yield and quality of these plants. It also helps to clarify the interaction mechanism between Fritillaria medicinal plants and beneficial or harmful microorganisms in the rhizosphere. Based on the authors’ knowledge, future research on rhizosphere microorganisms of Fritillaria medicinal plants should focus on the following aspects.
Presently, the traditional method used on rhizosphere microorganisms is the pure culture method. With the development of molecular biology technology, the free culture method is widely used in the research of rhizosphere microorganisms of medicinal plants. However, the free culture method cannot obtain live strains, nor provide strains for the research of growth-promoting bacteria in the rhizosphere. Therefore, in the research of rhizosphere microbial diversity of Fritillaria, it is suggested to combine the pure culture method and free culture method. Moreover, there are few reports on the diversity of the rhizosphere microbial population of Fritillaria medicinal plants. Because most of the reports are only at the pure culture stage, in the future more attention should be carried out to explore the impact of different Fritillaria medicinal plant varieties, growth years, environmental conditions, and climate factors on the rhizosphere microbial diversity of these plants. The relationship between the rhizosphere microorganisms of Fritillaria medicinal plants and their growth should be understood in detail.
The mechanism of interaction between Fritillaria medicinal plants and rhizosphere microorganisms is not clearly understood. In the future, more attention should be paid to the growth-promoting mechanism and the mechanism of the continuous cropping obstacle of Fritillaria medicinal plants. Moreover, the development of biocontrol agents should be strengthened. The perennial Fritillaria medicinal plants have suffered from widespread and serious underground diseases and insect pests, resulting in a decline in the quality of Fritillaria Chinese medicinal materials. Therefore, microbial agents that are safe, efficient, and pollution-free should be developed, which can improve the soil environment, and the output and quality of Fritillaria medicinal plants (Dong, 2018).
At present, pathogens of main diseases of Fritillary medicinal plants have been reported (Supi et al., 2012; Supi et al., 2015; Song et al., 2016a; Wang, 2017; Wu et al., 2021; Li et al., 2022), but some pathogens are only identified by morphological identification method, and need to be further identified by molecular biology method. The pathogen of a certain plant of Fritillaria may be speculated from the incidence of other Fritillaria plants, but the pathogen of the same disease in different Fritillaria plants are not exactly the same. Therefore, it is necessary to strengthen the research on the pathogenic microorganisms of diseases and insect pests of Fritillaria. Moreover, it is necessary to strengthen the research on rhizosphere microorganisms of diseased plants and healthy plants, explore which changes of rhizosphere microorganisms are related to the occurrence of medicinal plant diseases of Fritillaria, and screen biocontrol strains from rhizosphere microorganisms. Moreover, it is necessary to strengthen the research on rhizosphere microorganisms of diseased plants and healthy plants, explore which changes of rhizosphere microorganisms are related to the occurrence of medicinal plant diseases of Fritillaria, and screen biocontrol strains from rhizosphere microorganisms.
Currently, it would be more meaningful to develop more field applications for rhizospheric microbes rather than potted plants. The research methods on the rhizosphere microorganisms of Fritillaria medicinal plants are relatively isolated, thus it is necessary to learn from the research methods between other medicinal plants and rhizosphere microorganisms to understand the interaction mechanism between Fritillaria medicinal plants and rhizosphere microorganisms. In summary, it is expected that this review article will facilitate the development of Fritillaria medicinal plants.
NZ, C-MM, J-JZ, M-GM, and W-DL: investigation. NZ, W-DL, and M-GM: supervision. NZ, C-MM, J-JZ, and M-GM: writing—original draft. NZ, M-GM, and W-DL: writing—review and editing. All authors contributed to the article and approved the submitted version.
The financial support from National Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0115), Natural Science Foundation of Chongqing Municipal Education Commission (KJZD-K202101201) and Science and Technology Innovation of Wanzhou District, Chongqing (wzstc20210216) are gratefully acknowledged.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Andezej, T., and Philip, P. P. (2015). Role of root microbiota in plant productivity. J. Exp. Bot. 66 (8), 2167–2175. doi:10.1093/jxb/erv157
Asadhi, S., Reddy, B. V., Sivaprasad, Y., Prathyusha, M., Murali Krishna, T., Vijay Krishna Kumar, K., et al. (2013). Characterisation, genetic diversity and antagonistic potential of 2, 4-diacetylphloroglucinol producingPseudomonas fluorescensisolates in groundnut-based cropping systems of Andhra Pradesh, India. Archives Phytopathology Plant Prot. 46 (16), 1966–1977. doi:10.1080/03235408.2013.782223
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., and Vivanco, J. M. (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266. doi:10.1146/annurev.arplant.57.032905.105159
Berg, G., Grube, M., Schloter, M., and Smalla, K. (2014). The plant microbiome and its importance for plant and human health. Front. Microbiol. 5, 491–492. doi:10.3389/fmicb.2014.00491
Brimecomve, M., Lelj, F. D., and Lynch, J. (2001). The effect of root exudates on rhizosphere microbial populations. Rhizosphere 361, 717–729. doi:10.1201/9780849384974-10
Bulgarelli, D., Schlaeppi, K., Spaepen, S., Ver, E., Themaat, L. V., and Schulza-Lefert, P. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64, 807–838. doi:10.1146/annurev-arplant-050312-120106
Chen, B. Y., Ma, H. H., Yang, T., Cheng, Z. M., Niu, X. X., and Ma, X. W. (2017). Effect of different microbial fertilizer dosage on the elements of Fritillaria pallidiflora Schvek. Xinjiang Agric. Sci. 54 (05), 871–877. doi:10.6048/j.issn.1001-4330.2017.05.010
Chen, D. Y., Zhang, Z. Z., Li, R. X., Chen, J. R., Dai, X. F., Kong, Z. Q., et al. (2021). Research progress on quality safety status and analytical methods of Bulbus fritillaria. J. Food Saf. Qual. 12 (23), 9242–9250. doi:10.19812/j.cnki.jfsq11-5956/ts.2021.23.036
Chen, T., Zhong, F., Yao, C., Chen, J., Ma, Y., Dong, J., et al. (2020). A systematic review on traditional uses, sources, phytochemistry, pharmacology, pharmacokinetics, and toxicity of Fritillariae cirrhosae bulbus. Evidence-Based Complementary Altern. Med. 2020 (5), 1–26. doi:10.1155/2020/1536534
Chi, J. L., Hao, M., Wang, Z. X., and Li, Y. (2021). Advances in research and application of phosphorus-solubilizing microorganism. J. Microbiol. 41 (01), 1–7. doi:10.3969/j.issn.1005-7021.2021.01.001
Chinese Pharmacopoeia Commission, (2020). Pharmacopoeia of people’s Republic of China: One edition. Beijing, China: China Medical Science Press, 126–127.
Cosetta, C. M., and Wolfe, B. E. (2019). Causes and consequences of biotic interactions within microbiomes. Curr. Opin. Microbiol. 50, 35–41. doi:10.1016/j.mib.2019.09.004
Cuningham, A. B., Brinckmann, J. A., Pei, S., Luo, P., Schippann, U., Long, X., et al. (2018). High altitude species, high profits: Can the trade in wild harvested Fritillaria cirrhosa (Liliaceae) be sustained? J. Ethnopharmacol. 223, 142–151. doi:10.1016/j.jep.2018.05.004
Dong, T. W. (2018). The effect of combined application of nitrogen phosphorus fertilizer and microbial fertilizer on growth and quality of two-years-old Fritillaria Taipaiensis. Xianyang, China, Northwestern Agriculture and Forestry University.
Edwards, J., Johnson, C., Santos-Medellin, C., Lurie, E., Sundaresan, V., Bhatnagar, S., et al. (2015). Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. U. S. A. 112 (8), 911–920. doi:10.1073/pnas.1414592112
Ehrenfeld, J. G., Ravit, B., and Elgersma, K. (2005). Feedback in the plant-soil system. Annu. Rev. Environ. Resour. 30 (1), 75–115. doi:10.1146/annurev.energy.30.050504.144212
Garcia, J., and Kao-Kniffin, J. (2018). Microbial group dynamics in plant rhizospheres and their implications on nutrient cycling. Front. Microbiol. 9, 1516. doi:10.3389/fmicb.2018.01516
Geng, Z., Liu, Y. F., Gou, Y., Zhou, Q. M., He, C. J., Guo, L., et al. (2018). Metabolomics study of cultivated bulbus Fritillariae cirrhosae at different growth stages using UHPLC-QTOF-MS coupled with multivariate data analysis. Phytochem. Anal. 29 (3), 290–299. doi:10.1002/pca.2742
Gu, W. C., Mu, M. J., Yang, M., Guo, D. Q., and Zhou, N. (2020). Correlation analysis between bulb quality and rhizosphere soil factors of Fritillaria taibaiensis. Chin. J. Exp. Traditional Med. Formulae 26 (7), 165–177. doi:10.13422/j.cnki.syfjx.20200713
Guo, X. Y., Yang, Q., Chen, Z. H., Cai, R. X., Fang, F., and Chen, W. R. (2013). Allelopathic effects of Fritillary thunberbg Miqwa root exudate to its seedling. J. Shanxi Agric. Sci. 41 (11), 1197–1201. doi:10.3969/j.issn.1002-2481.2013.11.12
Han, M., Zhu, X. Y., Chen, G. W., Wan, X. M., and Wang, G. (2022). Advances on potassium-solubilizing bacteria and their microscopic potassium solubilizing mechanisms. Acta Pedol. Sin. 59 (2), 334–348. doi:10.11766/trxb202009190525
Hemkemeyer, M., Schwalb, S. A., Heinze, S., Joergensen, R. G., and Wichern, F. (2021). Functions of elements in soil microorganisms. Microbiol. Res. 252 (11), 126832. doi:10.1016/j.micres.2021.126832
Hu, J., Wei, Z., Friman, V. P., Gu, S. h., Wang, X. f., Eisenhauer, N., et al. (2016). Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. MBIO 7 (6), 017900–e1816. doi:10.1128/mBio.01790-16
Hu, L. F., Rbert, C. A. M., Cadot, S., Zhang, X., Ye, M., Li, B. B., et al. (2018). Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 9 (1), 2738. doi:10.1038/s41467-018-05122-7
Huang, Y. S., Liu, H. M., Fang, C. X., Yu, Y., Chen, H. Z., Zhang, S. Y., et al. (2018). Comparative study on the pharmacodynamic differences of the anti-tussive and anti-inflammatory effects of the alkaloids from different varieties of Fritillariae cirrhosae bulbus. Traditional Chin. Drug Res. Clin. Pharmacol. 29 (1), 19–22. doi:10.19378/j.issn.1003-9783.2018.01.004
Jiao, N., Song, X., Song, R., Yin, D., and Deng, X. (2022). Diversity and structure of the microbial community in rhizosphere soil of Fritillaria ussuriensis at different health levels. PeerJ 10, e12778. doi:10.7717/peerj.12778
Lebeis, S. L., Rott, M., Dangl, J. L., and Schulze-Lefert, P. (2012). Culturing a plant microbiome community at the cross-Rhodes. New Phytol. 196 (2), 341–344. doi:10.1111/j.1469-8137.2012.04336.x
Li, F., Hao, Z. P., and Chen, B. D. (2019). Molecular mechanism for the adaption of arbuscular mycorrhizal symbiosis to phosphorus deficiency. J. Plant Nutr. Fertilizers 25 (11), 1989–1997. doi:10.11674/zwyf.18490
Li, F. L., Liu, M., Li, Z. P., Jiang, C. Y., Han, F. X., and Che, Y. P. (2013). Changes in soil microbial biomass and functional diversity with a nitrogen gradient in soil columns. Appl. Soil Ecol. 64, 1–6. doi:10.1016/j.apsoil.2012.10.006
Li, J. E., Wen, S. S., Zhang, Y. J., Jin, L. J., and Zhao, W. C. (2022). Identification of pathogenic fungi species of gray mold disease of Fritillaria thunbergii. Plant protection 48 (02), 151–156. doi:10.16688/j.zwbh.2021091
Liao, H. B., Li, Y. X., Shao, J. J., Fang, F., Guo, W. D., and Chen, W. R. (2011). Impacts of continuous cropping on Fritillaria thunbergii Miq. growth and rhizosphere soil properties. Chin. J. Ecol. 30 (10), 2203–2208. doi:10.13292/j.1000-4890.2011.0292
Liu, L. J., Wei, X. T., Liu, Y. H., and Yu, K. G. (2020). Screening of bio-control strain for rhizosphere soil of Fritillaria ussuriensis maxim. J. Jilin Teach. Inst. Eng. Technol. 36 (06), 89–91. doi:10.3969/j.issn.1009-9042.2020.06.028
Lundberg, D. S., Lebeis, S. L., Paredes, S. H., Yourtone, S., Gehring, J., Malfatti, S., et al. (2012). Defining the core arabidopsis thaliana root microbiome. Nature 488 (7409), 86–90. doi:10.1038/nature11237
Luo, M., Deng, C. F., Li, P. M., Tan, Q. S., Luo, Y., Xu, G., et al. (2021a). Research progress in medicinal plant Fritillaria taipaiensis P. Y. Li. Chin. Wild Plant Resour. 40 (2), 4256–4345. doi:10.3969/j.issn.1006-9690
Luo, S., Deng, C. F., Tan, Q. S., Luo, M., and Zhang, W. W. (2021b). Research progress on extraction, purification, structural characterization and biological activity of polysaccharides from Fritillaria taipaiensis P. Y. Li. Agric. Eng. Technol. 41 (14), 9496. doi:10.16815/j.cnki.11-5436/s.2021.14.059
Ma, M., Mu, M., Yang, M., and Zhou, Y. (2021). The effect of microbial fertilizer on the growth, rhizospheric environment and medicinal quality of Fritillaria taipaiensis. Horticulturae 7 (11), 500. doi:10.3390/horticulturae7110500
Mendes, R., Gareva, P., and Raaijmakers, J. M. (2013). The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37 (5), 634–663. doi:10.1111/1574-6976.12028
Mojicevic, M., D'agostino, P. M., Nikodinovic-Runic, J., Vasiljevic, B., Gulder, T., and Voinovic, S. (2019). Antifungal potential of bacterial rhizosphere isolates associated with three ethno-medicinal plants (poppy, chamomile, and nettle). Int. Microbiol. 22 (3), 343–353. doi:10.1007/s10123-019-00054-8
Mu, M. J., and Li, P. Y. (2019c). Study on effects of continuous cropping obstruction of Fritilliaria taipaiensis and its meitigation measures. Dali, China, Dali University. doi:10.27811/d.cnki.gdixy.2019.000161
Mu, M. J., Nie, S. Y., Wang, Q., Yang, M., Zhang, G., Guo, D. Q., et al. (2019a). Variation pattern of rhizospheric soil nutrient for Fritillaria taipaiensis. Chin. J. Exp. Traditional Med. Formulae 25 (7), 189–194. doi:10.13422/j.cnki.syfjx.20190711
Mu, M. J., Zhang, D. G., Zhang, H., Yang, M., Guo, D. Q., and Zhou, N. (2019b). Correlation between rhizospheric microorganisms distribution and alkaloid content of Fritillaria taipaiensis. Chin. J. Chin. Mat. Med. 44 (11), 2231–2235. doi:10.19540/j.cnki.cjcmm.20190301.013
Pan, F., Su, T. J., Deng, K. L., Wu, W., and Wu, W. (2017). Antioxidant activities and metabolic constituents of endophytic Fusarium tricinctum CBY11 isolated from Fritillaria cirrhosa. Mycosystema 36 (6), 752–765. doi:10.13346/j.mycosystema.160190
Pan, H. X., Cheng, Z. M., Mou, S. Y., Qi, X. L., and Bao, Q. (2010a). Distribution of rhizosphere soil microbes of Fritillaria pallidiflora and their correlation with imperialine content. Microbiol. China 37 (8), 1253–1257. doi:10.13344/j.microbiol.china.2010.08.024
Pan, H. X., Cheng, Z. M., Qi, X. L., Bao, Q., and Mou, S. Y. (2010b). Influence of rhizosphere useful microbe on the yield of cultivated Fritillaria pallidiflora. Arid. Land Geogr. 33 (6), 917–922. doi:10.13826/j.cnki.cn65-1103/x.2010.06.010
Qiu, B. S. (2010). Rhizosphere soil microbes of Fritillaria pallidiflora. Microbiology China 37 (08), 1252. doi:10.13344/j.microbiol.china.2010.08.023
Rainer, G. J., and Florian, W. (2018). Alive and kicking: Why dormant soil microorganisms matter. Soil Biol. Biochem. 116, 419–430. doi:10.1016/j.soilbio.2017.10.022
Reinhold-Hurek, B., Buenger, W., Burbano, C. S., Sabale, M., and Hurek, T. (2015). Roots shaping their microbiome: Global hotspots for microbial activity. Annu. Rev. Phytopathol. 53 (1), 403–424. doi:10.1146/annurev-phyto-082712-102342
Sasse, J., Martinoia, E., and Northen, T. (2018). Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 23 (1), 25–41. doi:10.1016/j.tplants.2017.09.003
Schmidt, J. E., Kent, A. D., Brisson, V. L., and Gaudin, A. (2019). Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 7 (1), 146. doi:10.1186/s40168-019-0756-9
Shao, Q. Y., Dong, C. B., Han, Y. F., and Liang, Z. Q. (2021). Advances in the study of plant rhizosphere microorganisms groups. J. Plant Nutr. Fertilizer 27 (01), 144–152. doi:10.11674/zwyf.20203
Song, X. S., Yu, W. J., Yin, D. C., Zhou, Q., and Deng, X. (2016a). Pathogen identification and bionomics of the black rot of Fritillaria ussuriensis. For. Pest Dis. 35 (3), 7–11. doi:10.3969/j.issn.1671-0886.2016.03.002
Song, X. S., Yu, W. J., Zhou, Q., and Deng, X. (2016b). Microbial ecological study about the black rot of Fritillaria ussuriensis and its biological control bacteria screening. For. Sci. Technol. 041 (006), 18. doi:10.3969/j.issn.1001-9499.2016.06.006
Sun, R. T., Feng, X. C., Zhang, Z. Z., Zhou, N., Feng, H. D., Liu, Y. M., et al. (2022a). Root endophytic fungi regulate changes in sugar and medicinal compositions of Polygonum cuspidatum. Front. Plant Sci. 13, 818909. doi:10.3389/fpls.2022.818909
Sun, R. T., Zhang, Z. Z., Liu, M. Y., Feng, X. C., Zhou, N., Feng, H. D., et al. (2022b). Arbuscular mycorrhizal fungi and phosphorus supply accelerate main medicinal component production of Polygonum cuspidatum. Front. Microbiol. 13, 1006140. doi:10.3389/fmicb.2022.1006140
Sun, Y., Chang, J. J., and Tian, C. J. (2021). Ecological functions of the bacterial chemotaxis systems in rhizosphere microbiome. Acta Ecol. Sin. 41 (24), 9963–9969. doi:10.5846/stx202009292520
Supi, R. Z. G. L., Li, K. M., and ShaduhaxiSulaiman, K. S. (2012). Primary study of sclerotiniose on Fritillaria pallidiflora schvek in xinjiang. Tianjin Agric. Sci. 18 (3), 147–148. doi:10.3969/j.issn.1006-6500.2012.03.036
Supi, R. Z. G. L., Sulaiman, K. S., and Aybiek, (2015). Research on identification of pathogeny and biological characteristics of bulbus fritillaria root rot. Tianjin Agric. Sci. 21 (3), 118–121. doi:10.3969/j.issn.1006-6500.2015.03.025
Tan, Y., Wang, Q. Q., Gao, T. T., Ma, Y., Shao, H. B., and Shao, C. Y. (2013). Effects of cultivation years on effective constituent content of Fritillaria pallidiflora Schernk. Plant Biosyst. - Int. J. Deal. all Aspects Plant Biol. 147 (4), 1184–1190. doi:10.1080/11263504.2013.859181
Tang, Y. J., Huang, X. G., Yan, X. N., and Yuan, X. F. (2021). Accumulation dynamics of alkaloids during the growth of Fritillaria thunbergii Miq. and its correlation with rhizosphere microenvironment. J. Zhejiang Chin. Med. Univ. 45 (08), 816–823. doi:10.16466/j.issn1005-5509.2021.08.002
Tian, Q., Qiang, Y., Chen, K. K., and Wang, J. Z. (2018). Research progress on the rare and endangered medicinal plant Fritillaria taipaiensis P. Y. Li. . Shaanxi J. Agric. Sci. 64 (9), 96–98. doi:10.3969/j.issn.0488-5368.2018.09.028
Tripathi, A., Mcdonald, D., Zhu, Q., Quinn, R. A., Taylor, B. C., Kosciolek, T., et al. (2018). Best practices for analysing microbiomes. Nat. Rev. Microbiol. 16 (7), 410–422. doi:10.1038/s41579-018-0029-9
Venturi, V., and Keel, C. (2016). Signaling in the rhizosphere. Trends Plant Sci. 21 (3), 187–198. doi:10.1016/j.tplants.2016.01.005
Vezzani, F. M., Andeson, C., Meenken, E., Gillespie, R., Peterson, M., and Bare, M. H. (2018). The importance of plants to development and maintenance of soil structure, microbial communities and ecosystem functions. Soil Tillage Res. 175, 139–149. doi:10.1016/j.still.2017.09.002
Vives-Peris, V., Ollas, C., Gomez-Cadenas, A., and Perez-Clemente, R. M. (2020). Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 39 (1), 3–17. doi:10.1007/s00299-019-02447-5
Wang, D. Y. (2017). Identification of main pathogenic fungus species isolated from Fritillaria thunbergu Miq. and their sensitivity to fungicides. Hangzhou, China: Zhejiang Chinese Medical University.
Wang, R. H., Zhang, Q. F., Zhou, B. L., Liao, H., and Ma, G. S. (2007). Analysis on the interaction between root exudates and rhizosphere microbes. Chin. J. Soil Sci. 38 (1), 167–172. doi:10.19336/j.cnki.trtb.2007.01.037
Wang, Y. (2010). Autotoxicity of Fritillaria pallidiflora in cropping continuous. Ürümqi, China, Xinjiang Normal University.
Wang, Y., Kaisar, S., Li, J., Zhang, Y. L., and Zhu, G. Q. (2010b). Autotoxicity of root exudates of Fritillaria pallidiflora Schvek. Crops 8 (1), 25–28. doi:10.16035/j.issn.1001-7283.2010.01.009
Wang, Y., Kaisar, S., Li, J., Zhang, Y. L., Zhu, G. Q., and Ayi, B. K. (2010a). Autotoxicity of root exudates of Fritillaria pallidiflora Schvek. Bull. Botanical Res. 30 (2), 248–252. doi:10.7525/j.issn.1673-5102.2010.02.021
Wang, Y., Kaisar, S., Li, J., Zhu, G. Q., Song, T. S., and Liu, L. (2009). Analysis of components in root exudates of Fritillaria pallidiflora Schvek seedlings at different ages by gas chromatography-mass spectrometry. Acta Bot. Boreal. Occident. Sin. 29 (2), 384–389. doi:10.3321/j.issn:1000-4025.2009.02.028
Wees, M. V., Luijendijk, M., Smoorenburg, I., van Loon, L. C., and Pieterse, C. M. (1999). Rhizobacteria mediated induced systemic resistance (ISR) in Arabidopsis is not associated with a direct effect on expression of known defense-related genes but stimulates the expression of the jasmonate-inducible gene Atvsp upon challenge. Plant Mol. Biol. 41 (4), 537–549. doi:10.1023/a:1006319216982
Wei, Z. C., Pan, X. J., Huang, X. L., Li, H. L., Guo, D. Q., and Zhou, N. (2021). Effects of inoculation of Fritillaria Taipaiensis P. Y. Li with growth-promoting bacteria on inorganic elements in rhizosphere soil. Environ. Chem. 40 (4), 1254–1262. doi:10.7524/j.issn.0254-6108.2020093003
Wu, R., Chen, H., Guo, F. X., Zhou, Y., and Jiao, X. S. (2021b). Effects of growth years on soil bacterial community structure in rhizosphore soil of Fritillaria przewalskii. Agric. Res. Arid Areas, 39(06), 153–161. doi:10.7606/j.issn.1000-7601
Wu, R. (2021). Mechanism of selection for stubbles and their adaptation in domesticated Fritillaria przewalskii maxim. Lanzhou, China, Gansu Agricultural University. doi:10.27025/d.cnki.ggsnu.2021.000054
Yu, H. P., and Wu, Z. J. (2017). Experimental study on continuous cropping and fallow cultivation of Fritillaria fritillary. Mod. Agric. Technol. 12 (04), 45–48. doi:10.3969/j.issn.1007-5739.2017.04.030
Yu, Z. H., Jin, J., Li, Y. S., Yang, Y., Zhao, Y., Liu, C. K., et al. (2019). Distinct effects of short-term reconstructed topsoil on soya bean and corn rhizosphere bacterial abundance and communities in Chinese Mollisol. R. Soc. open Sci. 6 (1), 181054. doi:10.1098/rsos.181054
Yuan, J., Zhao, J., Wen, T., Zhao, M., Li, R., Pim, G., et al. (2018). Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 6 (1), 156. doi:10.1186/s40168-018-0537-x
Zhang, J. H., Feng, B. B., and Wu, C. S. (2020). Effects of arbuscular mycorrhizal fungi on the growth and quality markers of Fritillaria P. Y. Li. Chin. J. Inf. Traditional Chin. Med. 27 (07), 88–93. doi:10.3969/j.issn.1005-5304.201908134
Zhang, J. Y., Liu, Y. X., Zhang, N., Hu, B., Jin, T., Xu, H. R., et al. (2019). NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 37 (6), 676–684. doi:10.1038/s41587-019-0104-4
Zhao, J., Liang, S. J., Yang, T., Wu, X. L., Qi, P. N., Wang, S. W., et al. (2021). Isolation of growth-promoting bacteria and effect of compound bacteria on yield of Fritillaria przewalskii. Chin. J. Exp. Traditional Med. Formulae 27 (24), 163–170. doi:10.13422/j.cnki.syfjx.20211512
Keywords: Fritillaria L., soil, rhizosphere microbes, microbiome, research progress
Citation: Zhou N, Mei C-M, Zhu X-Y, Zhao J-J, Ma M-G and Li W-D (2022) Research progress of rhizosphere microorganisms in Fritillaria L. medicinal plants. Front. Bioeng. Biotechnol. 10:1054757. doi: 10.3389/fbioe.2022.1054757
Received: 27 September 2022; Accepted: 25 October 2022;
Published: 07 November 2022.
Edited by:
Xin Zhou, Nanjing Forestry University, ChinaReviewed by:
Kunming Qin, Jiangsu Ocean University, ChinaCopyright © 2022 Zhou, Mei, Zhu, Zhao, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Guo Ma, bWdfbWFAYmpmdS5lZHUuY24=; Wei-Dong Li, bGl3ZWlkb25nMDgwMUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.