- 1Department of Clinical Laboratory, The First Affiliated Hospital of Guangxi University of Science and Technology, Liuzhou, Guangxi, China
- 2Department of Radiation Oncology, The First Affiliated Hospital, Air Force Medical University, Xi’an, China
Traditional tumor models cannot perfectly simulate the real state of tumors in vivo, resulting in the termination of many clinical trials. 3D tumor models’ technology provides new in vitro models that bridge the gap between in vitro and in vivo findings, and organoids maintain the properties of the original tissue over a long period of culture, which enables extensive research in this area. In addition, they can be used as a substitute for animal and in vitro models, and organoids can be established from patients’ normal and malignant tissues, with unique advantages in clinical drug development and in guiding individualized therapies. 3D tumor models also provide a promising platform for high-throughput research, drug and toxicity testing, disease modeling, and regenerative medicine. This report summarizes the 3D tumor model, including evidence regarding the 3D tumor cell culture model, 3D tumor slice model, and organoid culture model. In addition, it provides evidence regarding the application of 3D tumor organoid models in precision oncology and drug screening. The aim of this report is to elucidate the value of 3D tumor models in cancer research and provide a preclinical reference for the precise treatment of cancer patients.
Introduction
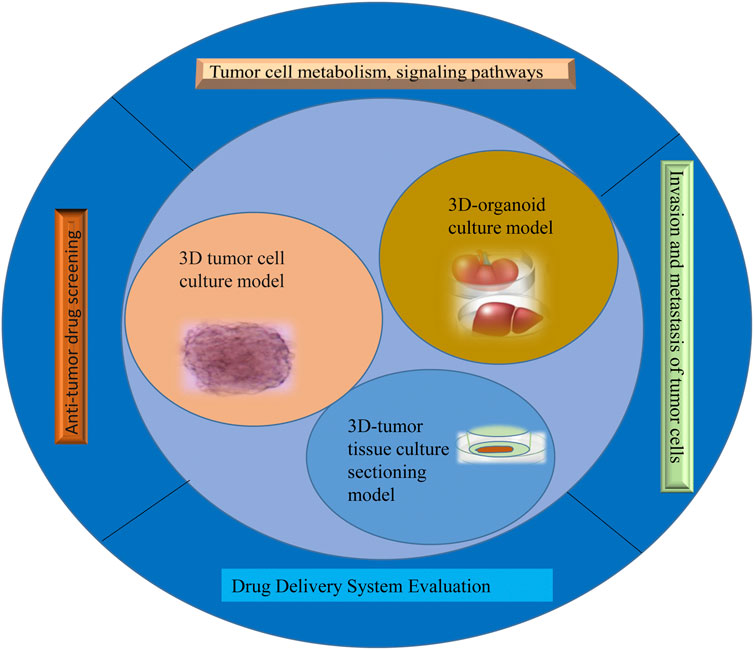
The success rate of the clinical development of antineoplastic drugs is much lower than that of other drugs. The reason is that tumors are a far more complex disease than is retained, and their occurrence, growth, and metastasis are related not only to tumor cells but also to their environment (Swann and Smyth, 2007). The tumor microenvironment is the ecological environment on which tumor cells depend for survival and development (Anderson and Simon, 2020). In this environment, tumor cells come into contact with each other and with immune cells (Sharonov et al., 2020), tumor-associated fibroblasts (Truffi et al., 2020), endothelial cells (Sobierajska et al., 2020), inflammatory cells (Turley et al., 2015), and noncellular components, which can significantly affect the biological properties of tumor cells, such as their polarity, structure, resistance, migration, and invasion (Turley et al., 2015). The traditional tumor monolayer cell culture model uses the nature of cell adherent growth to form a dense tumor monolayer cell structure; these culture methods are simple to operate with and low cost, but 2D cell culture does not reflect the in vivo environment in terms of morphology, structure, and function and cannot simulate the three-dimensional characteristics of tumor heterogeneity and the microenvironment (Tuveson and Clevers, 2019). Therefore, the single-layer planar culture model is insufficient for predicting the real condition of drugs in tumor tissue, and the experimental results are rarely consistent with the results of clinical trials, resulting in a low success rate in antitumor drug development (Xu et al., 2018a). This study summarizes the 3D tumor cell culture model, 3D tumor slice model, and organoid culture model and reviews their application in antitumor drug research (Figure 1). It aims at elucidating the value of 3D tumor models in cancer research and provides a preclinical reference for the precise treatment of cancer patients.
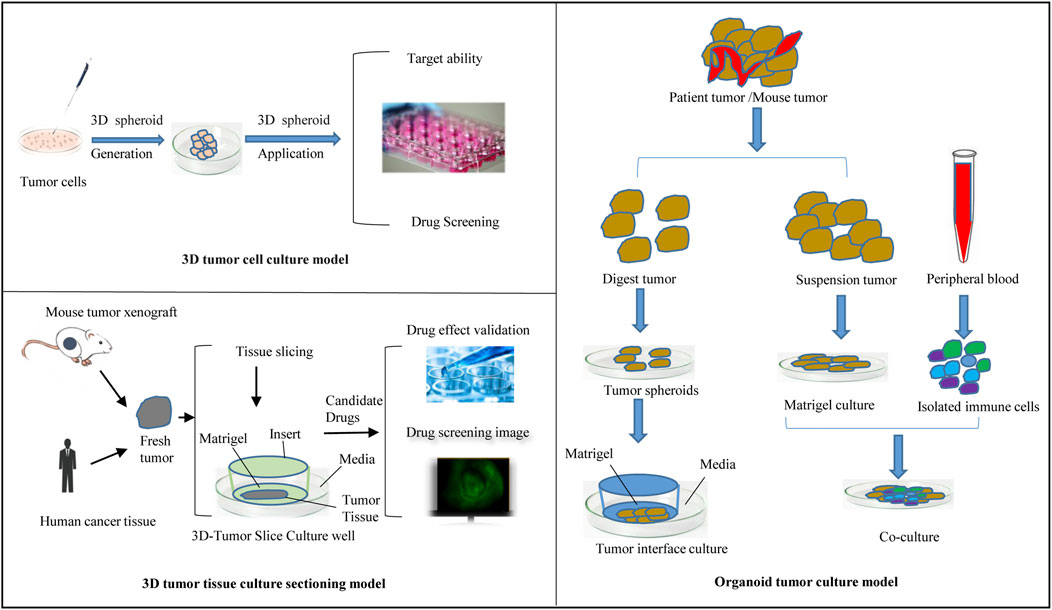
Culture to promote our understanding of complex biological processes, and address the limitations of many traditional 2D cell cultures. At present, organoid models derived from 3D cell culture are gradually being used in a variety of research applications, including cell biology, regeneration methods, precision medicine, and drug toxicity and efficacy testing, showing great application potential. The use of 3D cell culture as the main cell culture process in the future will undoubtedly become a major trend, but there are still some challenges for scientists to solve before this technology can be widely used (Neal et al., 2018). As cell culture media components, such as scaffolds and gels, continue to evolve, 3D tumor cell models can be used to simulate three-dimensional spaces and microenvironments similar to tumors in vivo. This technology has gradually become the most promising cell research model and is widely used in antitumor drug research. This section provides a brief introduction to commonly used 3D tumor cell models and their applications in antitumor drug research (Figure 2).
Multicellular tumor spheroid culture model
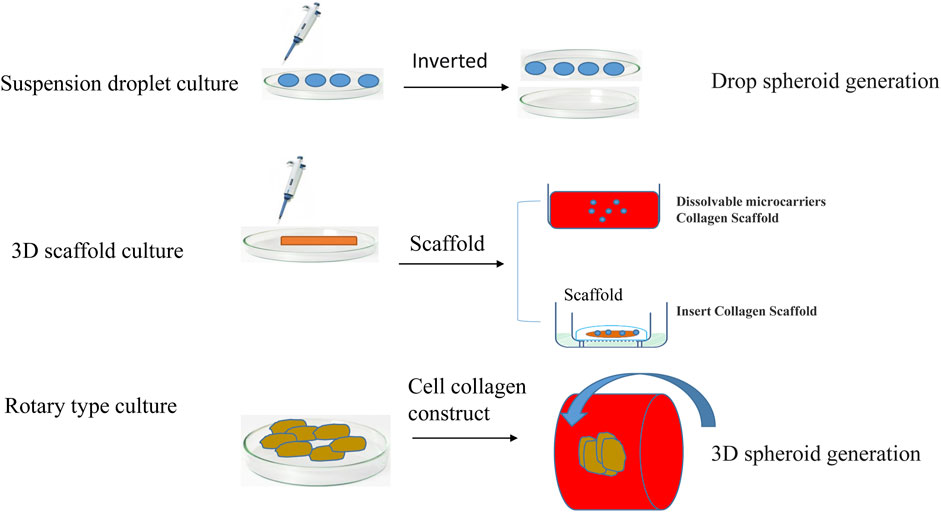
At present, the cell model that can be used to best characterize tumor organs is multicellular tumor spheroids (MCTSs). MCTSs are classified according to the culture method, mainly including suspension culture, rotary culture, and scaffold culture (Figure 3). Suspension culture occurs when a dish rich in cell droplets is flipped, and surface tension and gravity promote the formation of a suspension of cells that aggregate into tumor spheres (Tung et al., 2011). Suspension droplet culture does not require special equipment, the culture cost is low, and the size of the tumor ball is relatively uniform, but the traditional suspension drop method is not suitable for long-term culture due to evaporation; additionally, it is difficult to keep the original microenvironment features, the tumor spheroids need to metastasize after formation and then cultured, and the isolation and purification procedures are more complicated. Tumor cell aggregates are later cultured using 96-well or 384-well plates to more accurately control the size of the tumor spheres. Suspension culture promotes the spontaneous aggregation of tumor cells by reducing the effects of gravity during the formation of multi tumor cell spheroids (Lee et al., 2007). Suspension cultures are easy to perform and no special equipment is required, but the culture cycle is long, and the tumor bulb size is not easy to control.
By maintaining a certain stirring rate and promoting the transport of nutrients and metabolic waste, the interaction of the matrix is maintained. This method can be used to culture a wide variety of cell types but is complex and expensive until making it particularly unsuitable for shear-sensitive or low-adhesion cells. Multiple cell co-culture models based on scaffolds have been widely studied. Scaffolder-based 3D models are embedded outside the cells of the simulated tumor with cells or clusters of cells. Scaffold culture promotes the adherence of tumor cells to the scaffold fiber culture through the continuous division of cells; the cells fill the gaps in the scaffold and form a cell ball. The technique is simple, can be used to culture a variety of cell types, and can also utilize growth factors, cytokines and other features of the tumor cell microenvironment (Tsai et al., 2022). The most commonly used scaffold is collagen. The disadvantage of stent culture is that the stent is relatively expensive, and the scaffold component has a greater impact on cell culture. Stent culture is the most commonly used and well-studied method of tumor spheroid culture.
Since single tumor spheroid formation cannot reflect the tumor microenvironment (Pang et al., 2019), a hybrid system with different cells were applied in anti-tumor drug evaluation to overcome the limitation of tumor, and the effect on tumor cell properties was also studied. Generally, a hybrid co-culture system from different cells is difficult to establish. However, to some extent, the hybrid co-culture system reflects the interaction between tumor and its surrounding microenvironment in vitro. It can be used to screen drugs. Kim et al. (2012) developed cellular complexes containing human hepatocellular carcinoma cell line (Hep-G2) and rat insulin-secreting cell line (RIN-5F) by using a co-culture hybrid cellular spheroids model (HCSs). They found that the insulin and albumin levels in the HCSs were considerably higher than those in single tumor spheroid formation. Thus, a hybrid system had its advantage in immune effect in the whole tumor microenvironment.
Tumor-on-a-chip culture model
With the rapid development of chip-making technology, production materials, and detection methods in microelectronic processing technology, generating a culture model of cells and even “organs” on chips for screening drugs is of great importance (Albanese et al., 2013). The tumor on a chip fabrication and design are mainly constituted of a cell culture/tissue chamber and a channel for delivering the medium. Through adjusting microchannels on the chip to simulate the structure and state of solid tumor tissue, which can be used to evaluate and screening drugs. Aung et al. (2020) has implanted breast cancer cells in multilayer paper chips for perfusion culture, simulated the structure of 3D solid tumors, established a barrier for the exchange of materials between tumor cells and the external environment, simulated capillaries in vivo with microchannels in the chip, adjusted the perfusion speed of microchannels on the chip, and simulated the relatively insufficient state of vascular oxygenation in solid tumor tissue. After the perfusion culture was completed, the tumor tissue is decomposed by splitting the multilayer membrane and detecting the tumor metabolic state at different depths. Experiments have proven that this multilayer paper chip promotes fast external growth and slow internal growth in solid tumors, and internal hypoxia necrosis occurs in the presence of tumor microenvironments with low oxygen and low pH (Kang et al., 2016). Microfluidic cell culture (Huh et al., 2011; Mehling and Tay, 2014; Ng et al., 2015; Bale and Borenstein, 2018) on a chip is a technique for cell culture on a chip with a cell culture chamber and a channel for delivering media (Walsh et al., 2009). Microfluidics can be used to precisely control the perfusion speed of the injected and transported medium between cells and extracellular matrix simulation materials. Unlike traditional cell experiments, microfluidic chip culture can be used to reflect the interaction between cells, the cell microenvironment, and the concentration gradient formed by various cytokines, etc., with strong controllability, large data volume, and reliable results (Huh et al., 2011; Polidoro et al., 2021). Recently, using a 3D microfluidic system in the presence of fibronectin to explore the crosstalk between fibroblasts and breast cancer cells (MDA-MB-231), the results showed that the capability of the model to pinpoint the contribution of different components of the tumor microenvironment (TME) (Lugo-Cintrón et al., 2020). However, shortcomings, such as difficulties in chip production and application, have limited the promotion of this model.
The hydrogel-based 3D bioprinting tumor models
3D bioprinting is a 3D printing technology that uses bio-ink loaded with cells as a printing material to produce biologically active tissue and organ scaffolds and chips (Mandrycky et al., 2016; Gungor-Ozkerim et al., 2018; Heinrich et al., 2019; Murphy et al., 2020). At present, 3D bioprinting combined with cells has gradually become a research hotspot, which has a good expected application and prospect in tissue engineering regeneration, drug screening, disease treatment and other aspects (Annabi et al., 2014). Hydrogel materials contain a large amount of water, which requires mild conditions for printing. There are three main working principles applied to hydrogel, including inkjet bioprinting system based on materials and adhesives, extrusion nozzle bioprinting system and bioprinting system based on photopolymerization. The printing methods based on photopolymerization included stereo lithography, two-photon polymerization and laser-induced transfer (Murphy et al., 2020). 3D printing technologies with various working principles have been widely used in hydrogels. In addition, 3D bioprinting combined with microfluidic technology can create complex flow channels/chambers and functional biological structures with 3D heterostructures, cell placement, and tissue specificity to more closely resemble real tissues or organs (Heinrich et al., 2019). Colosi et al. (2016) used the mixture of alginate and GelMA to develop a bioink with low viscosity, combined with a microfluidic platform to form a microfluidic system with accurate and controlled deposition. It could promote the propagation and migration of cells inside biological structures. Based on this, they created a non-uniform 3D tissue model in vitro to simulate native tissues. It can be used in drug development.
3D tumor tissue culture sectioning model
The tumor tissue culture model is an aged tumor three-dimensional model in which a block of tumor tissue is placed on a dedicated porous culture plate, an appropriate medium is added, and the tumor tissue is cultured before it can be used for experimental studies (Huh et al., 2011; Polidoro et al., 2021) (Figure 2). Unlike traditional monolayer cell culture, this method can be used to accurately predict the sensitivity of tumor tissue to antitumor drugs while maintaining the original tumor structure and is used to screen antitumor drugs and guide personalized administration (Ravi et al., 2015; Zuppinger, 2019; De León et al., 2020; Jensen and Teng, 2020; Habanjar et al., 2021). The disadvantages of this model are the lack of reproducibility due to the natural heterogeneity of donor tissues and the difficulty of applying the required techniques, such as imaging and flow cytometry; thus, the application has limitations.
Three-dimensional tumor slide culture (3D-TSC) can be used to quickly and accurately reproduce the high complexity of tumors in vivo for drug screening, especially for immunotherapy drugs (Sivakumar et al., 2019; Nishida-Aoki et al., 2020). 3D-TSCs are produced by cutting slices of a fresh tumor without prior treatment while preserving the tumor structure, stroma, and TME. In our previous study (Huang and Zhao, 2020; Xing et al., 2021; Huang and Zhao, 2022; Peng et al., 2022), tumor slices from colon cancer and liver cancer patients generated after surgery were used, and nanomedicine combined with immunotherapy was applied to this platform to test toxicity and efficiency. Other results also showed that the components of the TME, including T cells and macrophages, could survive in the 3D-TSC platform for more than a week after 3D-TSC culture, thus allowing the study of the immune environment (Kenerson et al., 2021). The early drug screening results of 3D-TSC showed similar results to those of PDOs, and the generation of 3D-TSC can produce faster results, providing rapid and accurate guidance strategies for clinical patients during treatment decision-making, especially regarding the response of the tumor to various new immunotherapies (Ravi et al., 2015; Habanjar et al., 2021). Figure 2 summarizes the flow of the use of 3D-TSCs in a preclinical tumor model.
Organoid culture model
The development of organoid technology has laid the foundation for the cultivation of tumor organoids. Tumor organoids are mainly generated either by gene editing normal tissue-derived organoids or by culturing directly from tumor tissue (Lee et al., 2007; Li et al., 2018; Jensen and Teng, 2020). Tumor tissue extraction is less restrictive, and the procedure inolves surgery, puncture biopsy, circulating tumor cells, pleural effusion, and cell brushing. The method of establishing organoids from tumor tissue is summarized as follows: after obtaining surgical excision of tissue, first, fat and muscle tissue are removed from cancer tissue, followed by trypsin (and) or collagenase treatment to digest the tissue according to the characteristics of the tissue. The cell suspension is resuspended with stromal colloid after passing through the cell screen and finally injected into the culture plate, and the appropriate medium is added for subsequent culture. The composition of the medium varies according to the characteristics of different cancer species, which are usually based on several types of factors, including V82 signaling pathway activators, tyrosine receptor kinase ligands, and signaling pathway inhibitors (Neal et al., 2018; Nuciforo et al., 2018; Cattaneo et al., 2020; Lu et al., 2021). During organoid research, clonal drift can be avoided by passing the entire Petri dish, using earlier generations of organoids, and reducing the number of passages.
Tumor research requires identifying the model that is closest to the real state of the tumor in vivo as the object of study. The traditional tumor research models mainly include cell culture, transgenic mice, and human tumor xenotransplantation models, each with advantages and disadvantages (Neal et al., 2018; Cattaneo et al., 2020; Yuki et al., 2020; Zhang et al., 2022a). Cell culture cycles are short and inexpensive, but gene drift occurs after multiple passages, resulting in changes in cell phenotype and drug sensitivity. In addition, cell culture cannot be used to simulate the interaction between cancer cells and the microenvironment, and it is difficult to replicate the patient’s treatment response. A human tumor can be directly transplanted into an animal for modeling, which preserves the heterogeneity of the tumor, but exhibits the limitations of a long modeling cycle and high cost (van de Wetering et al., 2015; Yan et al., 2018; Tuveson and Clevers, 2019; Yoshida, 2020; Yuki et al., 2020; Xu et al., 2021). In addition, the inability to maintain the matrix composition of human tumors may lead to mouse-like evolution of the tumor tissue. Finally, due to species differences, transgenic mice cannot be used to fully reflect the genetic and proteomic complexity of human tumors, and the results of these experiments lack consistency with human disease progression and drug sensitivity, which weakens the application value of transgenic mouse models to a certain extent.
Organoids have the combined advantages of the above culture methods and have unique advantages in tumor research (Neal et al., 2018). First, the core advantage of tumor organoid research is that it preserves the heterogeneity of the original tumor. Second, tumor organoids enable the expansion of small tumor samples from different sources (e.g., from puncture biopsy, pleural effusion, circulating tumor cells), which can be used to model different stages of tumors. Unlike tumor cells cultured using traditional (Zhang et al., 2022a) methods, tumor organoids exhibit genomic and transcriptome stability, which result in the maintenance of the protein expression pattern of the original tissue. Tumor cell organoids are implanted in mice after culture, and the modeling speed and success rate are high. It is worth noting that organoid technology can be used to separately model cancerous tissue and normal tissue obtained from the same patient source, providing a reliable control during tumor research.
Application of 3D tumor organoids models
Antitumor drug screening
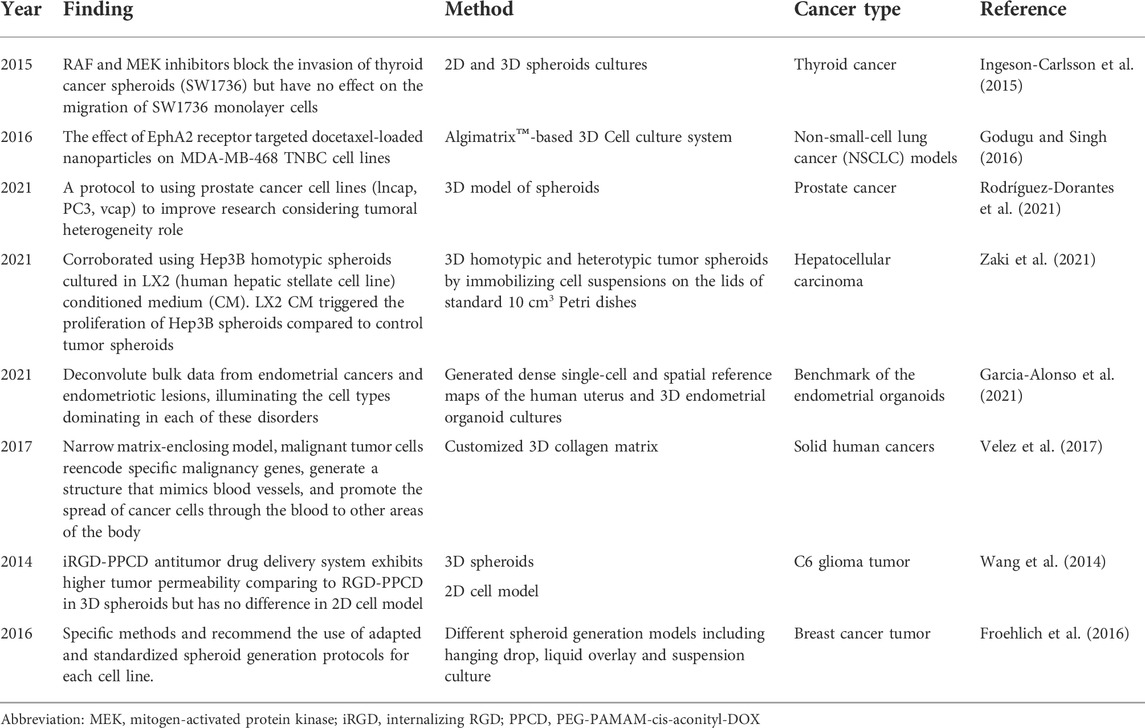
The tumor microenvironment significantly affects targeted drug therapy, and cell growth performed in traditional 2D culture models does not exhibit three-dimensional spatial structure, resulting in antitumor drugs with false-positive results entering clinical trials, with a high failure rate and a large time and energy costs. Part of the reason is that the early trials using monolayer planar cells as the subject of the study were poorly designed, and the screening efficiency of antitumor drugs was low. Ingeson-Carlsson et al. (2015) compared the effects of RAF and MEK inhibitors to BRAF inhibitors on thyroid cancer drug responses during experiments on tumor cell migration in 2D and 3D cultures. They have shown that RAF and MEK inhibitors block the invasion of thyroid cancer spheroids (SW1736) but have no effect on the migration of SW1736 monolayer cells. Other studies (Godugu et al., 2013; Godugu and Singh, 2016) showed that an in vitro 3D model of antineoplastic drug screening was developed with the AlgiMatrix™ scaffold, in which cytotoxicity can be determined by a cell proliferation test and the effectiveness of antineoplastic drugs can be evaluated based on the spheroid number and size distribution. By immunohistochemistry and RT‒PCR evaluation, the assessment of anti-apoptotic markers and the comparison of 3D model and 2D monolayer model results, the data showed that 3D in vitro trials of cultured antineoplastic drugs were more suitable for the screening and evaluation of antineoplastic drugs. Active tumor-stromal interactions in hepatocellular carcinoma showed weaker efficacy than in two-dimensional monolayer cultured cell and different potency in 3D spheroid models, demonstrating the great potential of 3D multicellular spheroid models in the discovery and development of anticancer drugs (Vinci et al., 2015; Nath and Devi, 2016; Rodríguez-Dorantes et al., 2021; Zaki et al., 2021). The antitumor drug screening application are summarized in Table 1.
Tumor cell metabolism and signaling pathways
Tumor signaling pathways and interventions do occur in monolayer planar cell culture (Rodenhizer et al., 2016; Flint et al., 2020; Garcia-Alonso et al., 2021; Yi et al., 2021); (Table 1). However, studies have shown that signaling pathway activation in 3D cultured multicellular tumor spheres is significantly different from that in monolayer 2D planar cells due to the death receptor DR4. One of the reasons for the difference in signaling pathways between 2D and 3D cultured cell models is the different tumor microenvironment. Extracellular matrix components, such as adhesin and fibronectin, provide key signals that affect cellular function by activating intracellular signaling pathways, and integrins located at the cell-matrix interface are also activated by changes in extracellular matrix composition (Rashidian and Luo, 2016; Rodenhizer et al., 2016; Yi et al., 2021). Hsu and Huang (2013) developed a dynamic 3D multicellular spheroid (MSCs) using a unique biomaterial, and its differentiation ability was observed to be transmitted with Wnt signaling. This finding is not observable in conventional monolayer culture cells; suitable 3D cell spheres can be used to detect the role of Wnt signal regulation in different extracellular environments and can be used to study the behavior of tumor stem cells. The function and properties of 3D tumor spheres are more similar to those of solid tumor tissue than monolayer planar cell cultures.
Invasion and metastasis of tumor cells
Invasion and metastasis of tumor cells, including the interaction of tumor cancer cells with the in-situ cell microenvironment and metastatic microenvironment, is an extremely complex process (Horie et al., 2012; Horie et al., 2015; Dwyer et al., 2016; Cattin et al., 2018; Du et al., 2018; Ilina et al., 2020; Colombo and Cattaneo, 2021). The main reason for the lack of recent research resides in the absence of an ideal model to simulate this complex physiological environment (Salgueiredo-Giudice et al., 2012; Schreiber-Brynzak et al., 2015; Rodenhizer et al., 2018). Velez et al. (2017) used a customized 3D collagen matrix to study the metastasis mechanisms of malignant cell tumors. They have shown that in a relatively narrow matrix-enclosing model, malignant tumor cells re-encode specific malignancy genes, generate a structure that mimics blood vessels, and promote the spread of cancer cells through the blood to other areas of the body, and this property of tumor cells has never been demonstrated in traditional monolayer cell culture methods; thus, the key to successful tumor cell experiments is to establish a 3D cell culture model that more accurately simulates the in vivo environment (De León et al., 2020; Garcia-Alonso et al., 2021). Metastasis of tumor cells model are summarized in Table 1.
Drug delivery system evaluation
Using tumor-targeted nanocarriers, such as liposomes, nanoparticles, or micelles, produces unique advantages for the delivery of antitumor drugs or genes, such as increasing the in vivo circulation time, increasing tumor site accumulation, and reducing toxicity to normal organs (Campisi et al., 2012; Shin et al., 2013; Li et al., 2018; Carey-Ewend et al., 2020; Nii et al., 2020; Bartusik-Aebisher et al., 2021; Borodina et al., 2021; Bromma et al., 2021; Foglietta et al., 2021). RGD peptides are a kind of peptide that contain Arg-Gly-Asp sequence, internalizing RGD peptide (iRGD) can increase drug penetration into extravascular tumor tissue. PEGylated PAMAM dendrimer (G4) with DOX conjugated by acid-sensitive cis-aconityl linkage (PEG-PAMAM-cis-aconityl-DOX, PPCD) was modified by a RGD cyclopeptide. Wang et al. (2014) used C6 glioma 3D spheroids to show that the iRGD-PPCD (internalizing RGD peptide with PEG-PAMAM-cis-aconityl-DOX, PPCD antitumor drug delivery system is similar to RGD-PPCD, which exhibits higher tumor permeability, and when this group of experiments was performed in a 2D cell model, there was no significant difference in the results of analyses of in vitro cytotoxicity and cell uptake using the two delivery systems. This finding shows that the 3D model has incomparable advantages in the evaluation of the permeability of antitumor drug delivery systems. Traditional 2D monolayer cell culture systems have many limitations, and 3D tumor cells can be used to mimic more complex cellular heterogeneity and interactions as well as tumor microenvironmental conditions (Wang et al., 2014; Xu et al., 2014; Wan et al., 2017; Tortorella et al., 2021). Although 3D models have incomparable advantages over 2D models, 3D cell culture models also have limitations that hinder their further application. First, not all tumor cells can be cultured into 3D cell models, and although many cell lines can form dense spheroids with the help of ECM substrates or scaffolds, cell lines such as SK-BR-3 and suspension cell lines remain difficult to use in the formation of spheroids (Froehlich et al., 2016). In addition, the culture and analysis protocols for 3D cell models have not been standardized; although 3D cell models can be established in large quantities, their formation method, initial cell number, the type and amount of cell-matrix used, and many other factors affect the formation process and lead to the uneven size of 3D cells (Xu et al., 2014; Wan et al., 2017).
Challenges and opportunities
The dilemma of tumor organoids
First, due to uncertainty in the growth factors required for some tumor tissues, it is difficult for the corresponding organoids to grow in vitro for a long time (Turco et al., 2017; Peng et al., 2018; Zhang et al., 2022b; Geng et al., 2022). Second, at present, tumor organoids are mainly derived from epithelial tumors, and methods for generating nonepithelial cell-derived organoids still need further research (Lombaert et al., 2017; Nikolić and Rawlins, 2017; Martignani et al., 2018). In addition, during organoid culture, growth factors or small molecule inhibitors need to be added to the culture medium, and the requirements for the culture medium are different due to the differences in gene expression in different tumor subtypes, which may affect the gene expression or signal transduction pathway of organoids, which in turn affects drug sensitivity and interferes with the results of the study.
Although organoid technology still has limitations, it provides a new model for tumor research and has great potential. To date, efficient organoid establishment has been achieved in a variety of tumors. Tumor organoids can be used to study the dynamic evolution of tumors and to perform preclinical efficacy evaluation, tumor microenvironment studies, and assessments of adjuvant immunotherapy. With the further development of organoid biobanks and chips, the future use of tumor organoid research is worth investigating during the development of preclinical experiments (Tatullo et al., 2020; Benitez et al., 2021; Zhou et al., 2021).
The first biobank of tumor organoids was established
Intestinal tumor organoids were the first to be established. Subsequently, the colorectal cancer tumor organoid biobank was established for the first time. Since then, the generation of biobanks of different tumor organoids has begun to develop, and the number has been expanding continuously (Pauli et al., 2017). The Tumor Organoid Biobank contains resources regarding tumor organoids with different pathological types and gene mutations, and tumor organoid studies conducted with large sample sizes can be used to further statistically clarify the relationship between specific gene mutations and drug sensitivity (Pauli et al., 2017; Yang et al., 2021; Ren et al., 2022). The Human Cancer Model Initiative is generating an organoid biobank that provides clinical and genetic information on existing organoids on its website.
Application in preclinical drug evaluation
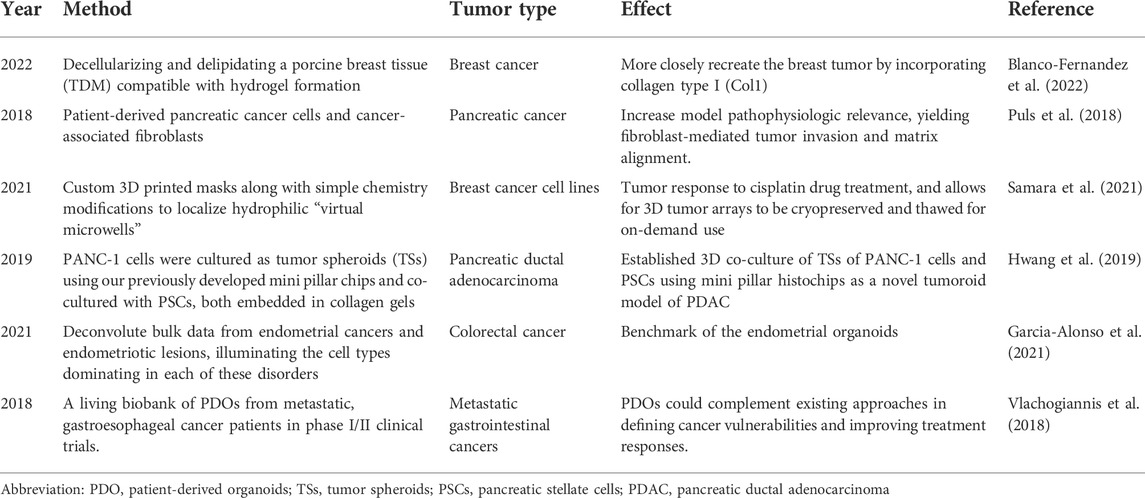
Preclinical drug trials focus on clarifying an understanding of drug efficacy and toxicity. Table 2 lists the represented tumor models and their effects. At present, the study of drug efficacy is mostly carried out in animal tumor models. Due to the lack of tumor heterogeneity and the existence of species differences, most drugs show different drug sensitivities in vivo and in vitro and even in different in vitro models (Weeber et al., 2017; Bleijs et al., 2019; Driehuis et al., 2020; Yoshida, 2020). Tumor organoids have high accuracy in predicting a patient’s response to treatment. In colorectal cancer, the patient’s organoid and patient response to the drug were compared, and the results showed that the positive predictive value of the tumor organoid’s response to the patient’s drug was DDA. The study suggests that organoids have shorter incubation times and higher predictive value than traditional models, which helps shorten the drug development cycle. Microfluidic platforms can be used to simulate capillary drug transmission in the tumor microenvironment, providing data regarding drug metabolism and response in cancer patients under physiological flow conditions (Ng et al., 2015). In recent years, a variety of tumor organoids have shown great potential in clinical drug screening, and tumor organoids exhibit drug responses that are consistent with the patient’s drug response and can be used to predict the patient’s treatment response to mitigate the shortcomings of traditional preclinical models in clarifying drug efficacy. It is worth noting that the combination of microfluidic technology and organoid technology can be used to carry out high-throughput drug screening, which greatly shortens the drug development cycle.
Application in immunotherapy
The development of immunotherapy is gradually changing the treatment strategy that is used in cancer patients. Studies (Jacob et al., 2020; Yuki et al., 2020; Forsythe et al., 2021; Qu et al., 2021) have found that neoantigens associated with tumor cells are key to stimulating an immune response. Insufficient tumor-associated antigens can weaken the proliferation of antitumor immune cells in vivo, and in patients with a low tumor mutation burden, in vitro activation to amplify immune cells and infusion is a good treatment strategy. Organoids have a highly similar heterogeneity to tumors in vivo, and coculture with peripheral blood lymphocytes can induce and enrich reactive S cells in peripheral blood without antigen agnosticism, which is highly targeted for specific individual tumors (Jacob et al., 2020; Yuki et al., 2020; Forsythe et al., 2021; Qu et al., 2021). In the future, chimeric antigen receptor (CAR)-T cell (CART-T) therapy and other cellular immunotherapies in the 3D tumor organoid model platform will be developed for antitumor drug screening (Xu et al., 2018b; Schnalzger et al., 2019; Klein et al., 2020; Yu and Huang, 2020).
In addition, multicellular tumor spheroid culture has 3D characteristics under quiescent culture conditions, but conventional tumor spheroids cannot be used to reflect vascular perfusion or other dynamic characteristics. Some researchers use microfluidic systems to culture multicellular tumor spheroids, but because of the high cost of this approach, it is not suitable for large-scale production, which hinders its application, and the culture method also needs to be further studied. Therefore, 3D tumor cell culture technology still needs to be developed, and when selecting antitumor drugs, it is necessary to consider the conditions and purposes of the experiments and to reasonably select 3D cell culture methods and support material or matrix according to cell type (Edmondson et al., 2014; Fang and Eglen, 2017; Davoudi et al., 2021; Sun et al., 2021). 3D tumor cells mimicking the 3D microenvironment are receiving increasing attention from researchers. In the future, with the continuous improvement of the functionality and controllability of biological materials, materials more suitable for 3D cell culture will likely be prepared according to different research purposes and tumor cell types, such as CloneSeq - Single-cell clonal 3D culture development (Sun et al., 2021). To address the limitations of current 3D tumor cell culture methods and to more accurately simulate the real microenvironment of tumor cells in vivo, scientific researchers should aim at obtaining more accurate scientific conclusions in preclinical research and improve antitumor drug screening.
Conclusion
This report summarizes the 3D tumor organoid model, including the 3D tumor cell culture model, 3D tumor slice model, and organoid culture model. In addition, it provides evidence of the application of the 3D tumor organoid model in precision oncology and drug screening. The aim of the report is to elucidate the value of 3D tumor models in cancer research and provide a preclinical reference for the precise treatment of cancer patients.
Author contributions
SH initiated and designed the study; XG and SH edited and approved the manuscript. All authors approved this version of the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albanese, A., Lam, A. K., Sykes, E. A., Rocheleau, J. V., and Chan, W. C. (2013). Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat. Commun. 4, 2718. doi:10.1038/ncomms3718
Anderson, N. M., and Simon, M. C. (2020). The tumor microenvironment. Curr. Biol. 30 (16), R921–r925. doi:10.1016/j.cub.2020.06.081
Annabi, N., Tamayol, A., Uquillas, J. A., Akbari, M., Bertassoni, L. E., Cha, C., et al. (2014). 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mat. 26 (1), 85–124. doi:10.1002/adma.201303233
Aung, A., Kumar, V., Theprungsirikul, J., Davey, S. K., and Varghese, S. (2020). An engineered tumor-on-a-chip device with breast cancer-immune cell interactions for assessing T-cell recruitment. Cancer Res. 80 (2), 263–275. doi:10.1158/0008-5472.can-19-0342
Bale, S. S., and Borenstein, J. T. (2018). Microfluidic cell culture platforms to capture hepatic physiology and complex cellular interactions. Drug Metab. Dispos. 46 (11), 1638–1646. doi:10.1124/dmd.118.083055
Bartusik-Aebisher, D., Chrzanowski, G., Bober, Z., and Aebisher, D. (2021). An analytical study of Trastuzumab-dendrimer-fluorine drug delivery system in breast cancer therapy in vitro. Biomed. Pharmacother. 133, 111053. doi:10.1016/j.biopha.2020.111053
Benitez, J. A., Finlay, D., Castanza, A., Parisian, A. D., Ma, J., Longobardi, C., et al. (2021). PTEN deficiency leads to proteasome addiction: A novel vulnerability in glioblastoma. Neuro. Oncol. 23 (7), 1072–1086. doi:10.1093/neuonc/noab001
Blanco-Fernandez, B., Rey-Vinolas, S., Bağcı, G., Rubi-Sans, G., Otero, J., Navajas, D., et al. (2022). Bioprinting decellularized breast tissue for the development of three-dimensional breast cancer models. ACS Appl. Mater. Interfaces. 14 (26), 29467–29482.
Bleijs, M., van de Wetering, M., Clevers, H., and Drost, J. (2019). Xenograft and organoid model systems in cancer research. Embo J. 38 (15), e101654. doi:10.15252/embj.2019101654
Borodina, T., Gileva, A., Akasov, R., Trushina, D., Burov, S., Klyachko, N., et al. (2021). Fabrication and evaluation of nanocontainers for lipophilic anticancer drug delivery in 3D in vitro model. J. Biomed. Mat. Res. 109 (4), 527–537. doi:10.1002/jbm.b.34721
Bromma, K., Alhussan, A., Perez, M. M., Howard, P., Beckham, W., and Chithrani, D. B. (2021). Three-dimensional tumor spheroids as a Tool for reliable investigation of combined gold nanoparticle and docetaxel treatment. Cancers (Basel) 13 (6), 1465. doi:10.3390/cancers13061465
Campisi, G., Giannola, L. I., Fucarino, A., Marino Gammazza, A., Pitruzzella, A., Marciano, V., et al. (2012). Medium-term culture of primary oral squamous cell carcinoma in a three- dimensional model: Effects on cell survival following topical 5-fluororacile delivery by drug-loaded matrix tablets. Curr. Pharm. Des. 18 (34), 5411–5420. doi:10.2174/138161212803307536
Carey-Ewend, A. G., Hagler, S. B., Bomba, H. N., Goetz, M. J., Bagó, J. R., Hingtgen, S. D., et al. (2020) Developing bioinspired three-dimensional models of brain cancer to evaluate tumor-homing neural stem cell therapy. Tissue Eng Part A. Epub ahead of print 2020/10/22. doi:10.1089/ten.tea.2020.0113
Cattaneo, C. M., Dijkstra, K. K., Fanchi, L. F., Kelderman, S., Kaing, S., van Rooij, N., et al. (2020). Tumor organoid-T-cell coculture systems. Nat. Protoc. 15 (1), 15–39. doi:10.1038/s41596-019-0232-9
Cattin, S., Ramont, L., and Rüegg, C. (2018). Characterization and in vivo validation of a three-dimensional multi-cellular culture model to study heterotypic interactions in colorectal cancer cell growth, invasion and metastasis. Front. Bioeng. Biotechnol. 6, 97. doi:10.3389/fbioe.2018.00097
Colombo, E., and Cattaneo, M. G. (2021). Multicellular 3D models to study tumour-stroma interactions. Int. J. Mol. Sci. 22 (4), 1633. doi:10.3390/ijms22041633
Colosi, C., Shin, S. R., Manoharan, V., Massa, S., Costantini, M., Barbetta, A., et al. (2016). Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mat. 28 (4), 677–684. doi:10.1002/adma.201503310
Davoudi, F., Ghorbanpoor, S., Yoda, S., Pan, X., Crowther, G. S., Yin, X., et al. (2021). Alginate-based 3D cancer cell culture for therapeutic response modeling. Star. Protoc. 2 (2), 100391. doi:10.1016/j.xpro.2021.100391
De León, S. E., Pupovac, A., and McArthur, S. L. (2020). Three-Dimensional (3D) cell culture monitoring: Opportunities and challenges for impedance spectroscopy. Biotechnol. Bioeng. 117 (4), 1230–1240. doi:10.1002/bit.27270
Driehuis, E., Kretzschmar, K., and Clevers, H. (2020). Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 15 (10), 3380–3409. doi:10.1038/s41596-020-0379-4
Du, Y., Herath, S. C. B., Wang, Q. G., Asada, H., and Chen, P. (2018). Determination of Green's function for three-dimensional traction force reconstruction based on geometry and boundary conditions of cell culture matrices. Acta Biomater. 67, 215–228. doi:10.1016/j.actbio.2017.12.002
Dwyer, A. R., Ellies, L. G., Holme, A. L., and Pixley, F. J. (2016). A three-dimensional co-culture system to investigate macrophage-dependent tumor cell invasion. J. Biol. Methods 3 (3), e49. doi:10.14440/jbm.2016.132
Edmondson, R., Broglie, J. J., Adcock, A. F., and Yang, L. (2014). Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay. Drug Dev. Technol. 12 (4), 207–218. doi:10.1089/adt.2014.573
Fang, Y., and Eglen, R. M. (2017). Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 22 (5), 456–472. doi:10.1177/1087057117696795
Flint, L. E., Hamm, G., Ready, J. D., Ling, S., Duckett, C. J., Cross, N. A., et al. (2020). Characterization of an aggregated three-dimensional cell culture model by multimodal mass spectrometry imaging. Anal. Chem. 92 (18), 12538–12547. doi:10.1021/acs.analchem.0c02389
Foglietta, F., Serpe, L., and Canaparo, R. (2021). The effective combination between 3D cancer models and stimuli-responsive nanoscale drug delivery systems. Cells 10 (12), 3295. doi:10.3390/cells10123295
Forsythe, S. D., Erali, R. A., Sasikumar, S., Laney, P., Shelkey, E., D'Agostino, R., et al. (2021). Organoid platform in preclinical investigation of personalized immunotherapy efficacy in appendiceal cancer: Feasibility study. Clin. Cancer Res. 27 (18), 5141–5150. doi:10.1158/1078-0432.ccr-21-0982
Froehlich, K., Haeger, J. D., Heger, J., Pastuschek, J., Photini, S. M., Yan, Y., et al. (2016). Generation of multicellular breast cancer tumor spheroids: Comparison of different protocols. J. Mammary Gland. Biol. Neoplasia 21 (3-4), 89–98. doi:10.1007/s10911-016-9359-2
Garcia-Alonso, L., Handfield, L. F., Roberts, K., Nikolakopoulou, K., Fernando, R. C., Gardner, L., et al. (2021). Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 53 (12), 1698–1711. doi:10.1038/s41588-021-00972-2
Geng, R., Harland, N., Montes-Mojarro, I. A., Fend, F., Aicher, W. K., Stenzl, A., et al. (2022). CD24: A marker for an extended expansion potential of urothelial cancer cell organoids in vitro? Int. J. Mol. Sci. 23 (10), 5453. doi:10.3390/ijms23105453
Godugu, C., Patel, A. R., Desai, U., Andey, T., Sams, A., and Singh, M. (2013). AlgiMatrix™ based 3D cell culture system as an in-vitro tumor model for anticancer studies. PLoS One 8 (1), e53708. doi:10.1371/journal.pone.0053708
Godugu, C., and Singh, M. (2016). AlgiMatrix™-Based 3D cell culture system as an in vitro tumor model: An important Tool in cancer research. Methods Mol. Biol. 1379, 117–128. doi:10.1007/978-1-4939-3191-0_11
Gungor-Ozkerim, P. S., Inci, I., Zhang, Y. S., Khademhosseini, A., and Dokmeci, M. R. (2018). Bioinks for 3D bioprinting: An overview. Biomater. Sci. 6 (5), 915–946. doi:10.1039/c7bm00765e
Habanjar, O., Diab-Assaf, M., Caldefie-Chezet, F., and Delort, L. (2021). 3D cell culture systems: Tumor application, advantages, and disadvantages. Int. J. Mol. Sci. 22 (22), 12200. doi:10.3390/ijms222212200
Heinrich, M. A., Liu, W., Jimenez, A., Yang, J., Akpek, A., Liu, X., et al. (2019). Bioprinting: 3D bioprinting: From benches to translational applications (small 23/2019). Small 15 (23), 1970126. doi:10.1002/smll.201970126
Horie, M., Saito, A., Mikami, Y., Ohshima, M., Morishita, Y., Nakajima, J., et al. (2012). Characterization of human lung cancer-associated fibroblasts in three-dimensional in vitro co-culture model. Biochem. Biophys. Res. Commun. 423 (1), 158–163. doi:10.1016/j.bbrc.2012.05.104
Horie, M., Saito, A., Yamaguchi, Y., Ohshima, M., and Nagase, T. (2015). Three-dimensional Co-culture model for tumor-stromal interaction. J. Vis. Exp. (96), 52469. Epub ahead of print 2015/03/06. doi:10.3791/52469
Hsu, S. H., and Huang, G. S. (2013). Substrate-dependent Wnt signaling in MSC differentiation within biomaterial-derived 3D spheroids. Biomaterials 34 (20), 4725–4738. doi:10.1016/j.biomaterials.2013.03.031
Huang, S., and Zhao, Q. (2020). Nanomedicine-combined immunotherapy for cancer. Curr. Med. Chem. 27 (34), 5716–5729. doi:10.2174/0929867326666190618161610
Huang, S., and Zhao, Q. (2022). The trend of immunotherapy combined with nanomedicine. Curr. Med. Chem. 29 (22), 3817–3818. doi:10.2174/0929867328666211117094947
Huh, D., Hamilton, G. A., and Ingber, D. E. (2011). From 3D cell culture to organs-on-chips. Trends Cell Biol. 21 (12), 745–754. doi:10.1016/j.tcb.2011.09.005
Hwang, H. J., Oh, M. S., Lee, D. W., and Kuh, H.-J. (2019). Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells. J. Exp. Clin. Cancer Res. 38 (1), 258.
Ilina, O., Gritsenko, P. G., Syga, S., Lippoldt, J., La Porta, C. A. M., Chepizhko, O., et al. (2020). Cell-cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nat. Cell Biol. 22 (9), 1103–1115. doi:10.1038/s41556-020-0552-6
Ingeson-Carlsson, C., Martinez-Monleon, A., and Nilsson, M. (2015). Differential effects of MAPK pathway inhibitors on migration and invasiveness of BRAF(V600E) mutant thyroid cancer cells in 2D and 3D culture. Exp. Cell Res. 338 (2), 127–135. doi:10.1016/j.yexcr.2015.08.003
Jacob, F., Ming, G. L., and Song, H. (2020). Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nat. Protoc. 15 (12), 4000–4033. doi:10.1038/s41596-020-0402-9
Jensen, C., and Teng, Y. (2020). Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 7, 33. doi:10.3389/fmolb.2020.00033
Kang, J., Lee, D. W., Hwang, H. J., Yeon, S. E., Lee, M. Y., and Kuh, H. J. (2016). Mini-pillar array for hydrogel-supported 3D culture and high-content histologic analysis of human tumor spheroids. Lab. Chip 16 (12), 2265–2276. doi:10.1039/c6lc00526h
Kenerson, H. L., Sullivan, K. M., Labadie, K. P., Pillarisetty, V. G., and Yeung, R. S. (2021). Protocol for tissue slice cultures from human solid tumors to study therapeutic response. Star. Protoc. 2 (2), 100574. doi:10.1016/j.xpro.2021.100574
Kim, J. Y., Kim, H. W., Bae, S. J., Joo, D., Huh, K., Fang, Y., et al. (2012). Hybrid cellular spheroids from hepatocellular carcinoma and insulin-secreting cell lines. Transpl. Proc. 44 (4), 1095–1098. doi:10.1016/j.transproceed.2012.02.016
Klein, E., Hau, A. C., Oudin, A., Golebiewska, A., and Niclou, S. P. (2020). Glioblastoma organoids: Pre-clinical applications and challenges in the context of immunotherapy. Front. Oncol. 10, 604121. doi:10.3389/fonc.2020.604121
Lee, G. Y., Kenny, P. A., Lee, E. H., and Bissell, M. J. (2007). Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 4 (4), 359–365. doi:10.1038/nmeth1015
Li, J., Zhou, Y., Chen, W., Yuan, Z., You, B., Liu, Y., et al. (2018). A novel 3D in vitro tumor model based on silk fibroin/chitosan scaffolds to mimic the tumor microenvironment. ACS Appl. Mat. Interfaces 10 (43), 36641–36651. doi:10.1021/acsami.8b10679
Lombaert, I., Movahednia, M. M., Adine, C., and Ferreira, J. N. (2017). Concise review: Salivary Gland regeneration: Therapeutic approaches from stem cells to tissue organoids. Stem Cells 35 (1), 97–105. doi:10.1002/stem.2455
Lu, Z., Nie, B., Zhai, W., and Hu, Z. (2021). Delineating the longitudinal tumor evolution using organoid models. J. Genet. Genomics 48 (7), 560–570. doi:10.1016/j.jgg.2021.06.010
Lugo-Cintrón, K. M., Gong, M. M., Ayuso, J. M., Tomko, L. A., Beebe, D. J., Virumbrales-Munoz, M., et al. (2020). Breast fibroblasts and ECM components modulate breast cancer cell migration through the secretion of MMPs in a 3D microfluidic Co-culture model. Cancers (Basel) 12 (5), 1173. doi:10.3390/cancers12051173
Mandrycky, C., Wang, Z., Kim, K., and Kim, D. H. (2016). 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 34 (4), 422–434. doi:10.1016/j.biotechadv.2015.12.011
Martignani, E., Accornero, P., Miretti, S., and Baratta, M. (2018). Bovine mammary organoids: A model to study epithelial mammary cells. Methods Mol. Biol. 1817, 137–144. doi:10.1007/978-1-4939-8600-2_14
Mehling, M., and Tay, S. (2014). Microfluidic cell culture. Curr. Opin. Biotechnol. 25, 95–102. doi:10.1016/j.copbio.2013.10.005
Murphy, S. V., De Coppi, P., and Atala, A. (2020). Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 4 (4), 370–380. doi:10.1038/s41551-019-0471-7
Nath, S., and Devi, G. R. (2016). Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 163, 94–108. doi:10.1016/j.pharmthera.2016.03.013
Neal, J. T., Li, X., Zhu, J., Giangarra, V., Grzeskowiak, C. L., Ju, J., et al. (2018). Organoid modeling of the tumor immune microenvironment. Cell 175 (7), 1972–1988.e16. doi:10.1016/j.cell.2018.11.021.1972
Ng, A. H., Li, B. B., Chamberlain, M. D., and Wheeler, A. R. (2015). Digital microfluidic cell culture. Annu. Rev. Biomed. Eng. 17, 91–112. doi:10.1146/annurev-bioeng-071114-040808
Nii, T., Kuwahara, T., Makino, K., and Tabata, Y. (2020). A Co-culture system of three-dimensional tumor-associated macrophages and three-dimensional cancer-associated fibroblasts combined with biomolecule release for cancer cell migration. Tissue Eng. Part A 26 (23-24), 1272–1282. doi:10.1089/ten.tea.2020.0095
Nikolić, M. Z., and Rawlins, E. L. (2017). Lung organoids and their use to study cell-cell interaction. Curr. Pathobiol. Rep. 5 (2), 223–231. doi:10.1007/s40139-017-0137-7
Nishida-Aoki, N., Bondesson, A. J., and Gujral, T. S. (2020). Measuring real-time drug response in organotypic tumor tissue slices. J. Vis. Exp. (159), e61036. Epub ahead of print 2020/05/19. doi:10.3791/61036
Nuciforo, S., Fofana, I., Matter, M. S., Blumer, T., Calabrese, D., Boldanova, T., et al. (2018). Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 24 (5), 1363–1376. doi:10.1016/j.celrep.2018.07.001
Pang, L., Ding, J., Ge, Y., Fan, J., and Fan, S. K. (2019). Single-cell-derived tumor-sphere formation and drug-resistance assay using an integrated microfluidics. Anal. Chem. 91 (13), 8318–8325. doi:10.1021/acs.analchem.9b01084
Pauli, C., Hopkins, B. D., Prandi, D., Shaw, R., Fedrizzi, T., Sboner, A., et al. (2017). Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 7 (5), 462–477. doi:10.1158/2159-8290.cd-16-1154
Peng, W. C., Logan, C. Y., Fish, M., Anbarchian, T., Aguisanda, F., Alvarez-Varela, A., et al. (2018). Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell 175 (6), 1607–1619.e15. e1615. doi:10.1016/j.cell.2018.11.012
Peng, Z., Lv, X., and Huang, S. (2022). Recent progress on the role of fibronectin in tumor stromal immunity and immunotherapy. Curr. Top. Med. Chem. 22, 647. Epub ahead of print 2022/06/17. doi:10.2174/1568026622666220615152647
Polidoro, M. A., Ferrari, E., Marzorati, S., Lleo, A., and Rasponi, M. (2021). Experimental liver models: From cell culture techniques to microfluidic organs-on-chip. Liver Int. 41 (8), 1744–1761. doi:10.1111/liv.14942
Puls, T. J., Tan, X., Husain, M., Whittington, C. F., Fishel, M., and Voytik-Harbin, S. L. (2018). Development of a novel 3D tumor-tissue invasion model for high-throughput, High-content phenotypic drug screening. Sci. Rep. 8 (1), 13039.
Qu, J., Kalyani, F. S., Liu, L., Cheng, T., and Chen, L. (2021). Tumor organoids: Synergistic applications, current challenges, and future prospects in cancer therapy. Cancer Commun. (Lond). 41 (12), 1331–1353. doi:10.1002/cac2.12224
Rashidian, J., and Luo, K. (2016). Three-dimensional mammary epithelial cell morphogenesis model for analysis of TGFß signaling. Methods Mol. Biol. 1344, 121–135. doi:10.1007/978-1-4939-2966-5_7
Ravi, M., Paramesh, V., Kaviya, S. R., Anuradha, E., and Solomon, F. P. (2015). 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 230 (1), 16–26. doi:10.1002/jcp.24683
Ren, X., Chen, W., Yang, Q., Li, X., and Xu, L. (2022). Patient-derived cancer organoids for drug screening: Basic technology and clinical application. J. Gastroenterol. Hepatol. 37, 1446–1454. Epub ahead of print 2022/07/01. doi:10.1111/jgh.15930
Rodenhizer, D., Gaude, E., Cojocari, D., Mahadevan, R., Frezza, C., Wouters, B. G., et al. (2016). A three-dimensional engineered tumour for spatial snapshot analysis of cell metabolism and phenotype in hypoxic gradients. Nat. Mat. 15 (2), 227–234. doi:10.1038/nmat4482
Rodenhizer, D., Dean, T., Xu, B., Cojocari, D., and McGuigan, A. P. (2018). A three-dimensional engineered heterogeneous tumor model for assessing cellular environment and response. Nat. Protoc. 13 (9), 1917–1957. doi:10.1038/s41596-018-0022-9
Rodríguez-Dorantes, M., Cruz-Hernandez, C. D., Cortés-Ramírez, S. A., Cruz-Burgos, J. M., Reyes-Grajeda, J. P., Peralta-Zaragoza, O., et al. (2021). Prostate cancer spheroids: A three-dimensional model for studying tumor heterogeneity. Methods Mol. Biol. 2174, 13–17. doi:10.1007/978-1-0716-0759-6_2
Salgueiredo-Giudice, F., Corrêa-Abrahão, A., Fornias-Sperandio, F., da-Costa-Dal-Vechio, A., and dos-Santos-Pinto-Junior, D. (2012). An in vitro study showing the three-dimensional microenvironment influence over the behavior of head and neck squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal 17 (3), e377–e382. doi:10.4317/medoral.17538
Samara, B., Deliorman, M., Sukumar, P., and Qasaimeh, M. A. (2021). Cryopreservable arrays of paper-based 3D tumor models for high throughput drug screening. Lab. Chip. 21 (5), 844–854.
Schnalzger, T. E., de Groot, M. H., Zhang, C., Mosa, M. H., Michels, B. E., Roder, J., et al. (2019). 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. Embo J. 38 (12), e100928. doi:10.15252/embj.2018100928
Schreiber-Brynzak, E., Klapproth, E., Unger, C., Lichtscheidl-Schultz, I., Goschl, S., Schweighofer, S., et al. (2015). Three-dimensional and co-culture models for preclinical evaluation of metal-based anticancer drugs. Invest. New Drugs 33 (4), 835–847. doi:10.1007/s10637-015-0260-4
Sharonov, G. V., Serebrovskaya, E. O., Yuzhakova, D. V., Britanova, O. V., and Chudakov, D. M. (2020). B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 20 (5), 294–307. doi:10.1038/s41577-019-0257-x
Shin, C. S., Kwak, B., Han, B., and Park, K. (2013). Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug. Mol. Pharm. 10 (6), 2167–2175. doi:10.1021/mp300595a
Sivakumar, R., Chan, M., Shin, J. S., Nishida-Aoki, N., Kenerson, H. L., Elemento, O., et al. (2019). Organotypic tumor slice cultures provide a versatile platform for immuno-oncology and drug discovery. Oncoimmunology 8 (12), e1670019. doi:10.1080/2162402x.2019.1670019
Sobierajska, K., Ciszewski, W. M., Sacewicz-Hofman, I., and Niewiarowska, J. (2020). “Endothelial cells in the tumor microenvironment,” in Tumor microenvironment: Non-hematopoietic cells. Editor A. Birbrair (Cham: Springer International Publishing), 71–86.
Sun, X., Bavli, D., Kozulin, C., Motzik, A., Buxboim, A., and Ram, O. (2021). CloneSeq - single-cell clonal 3D culture and analysis protocol. Star. Protoc. 2 (4), 100794. doi:10.1016/j.xpro.2021.100794
Swann, J. B., and Smyth, M. J. (2007). Immune surveillance of tumors. J. Clin. Invest. 117 (5), 1137–1146. doi:10.1172/jci31405
Tatullo, M., Marrelli, B., Benincasa, C., Aiello, E., Makeeva, I., Zavan, B., et al. (2020). Organoids in translational oncology. J. Clin. Med. 9 (9), 2774. doi:10.3390/jcm9092774
Tortorella, E., Palmieri, D., Piermarini, M., Gigante, D., Oddo, L., Toumia, Y., et al. (2021). Phase change dimethyldioctadecylammonium-shelled microdroplets as a promising drug delivery system: Results on 3D spheroids of mammalian tumor cells. J. Vis. Exp. (169), e62255. Epub ahead of print 2021/03/30. doi:10.3791/62255
Truffi, M., Sorrentino, L., and Corsi, F. (2020). “Fibroblasts in the tumor microenvironment,” in Tumor microenvironment: Non-hematopoietic cells. Editor A. Birbrair (Cham: Springer International Publishing), 15–29.
Tsai, K. K., Huang, S. S., Northey, J. J., Liao, W. Y., Hsu, C. C., Cheng, L. H., et al. (2022). Screening of organoids derived from patients with breast cancer implicates the repressor NCOR2 in cytotoxic stress response and antitumor immunity. Nat. Cancer 3 (6), 734–752. doi:10.1038/s43018-022-00375-0
Tung, Y-C., Hsiao, A. Y., Allen, S. G., Torisawa, Y. s., Ho, M., and Takayama, S. (2011). High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 136 (3), 473–478. doi:10.1039/c0an00609b
Turco, M. Y., Gardner, L., Hughes, J., Cindrova-Davies, T., Gomez, M. J., Farrell, L., et al. (2017). Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 19 (5), 568–577. doi:10.1038/ncb3516
Turley, S. J., Cremasco, V., and Astarita, J. L. (2015). Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 15 (11), 669–682. doi:10.1038/nri3902
Tuveson, D., and Clevers, H. (2019). Cancer modeling meets human organoid technology. Science 364 (6444), 952–955. doi:10.1126/science.aaw6985
van de Wetering, M., Francies, H. E., Francis, J. M., Bounova, G., Iorio, F., Pronk, A., et al. (2015). Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161 (4), 933–945. doi:10.1016/j.cell.2015.03.053
Velez, D. O., Tsui, B., Goshia, T., Chute, C. L., Han, A., Carter, H., et al. (2017). 3D collagen architecture induces a conserved migratory and transcriptional response linked to vasculogenic mimicry. Nat. Commun. 8 (1), 1651. doi:10.1038/s41467-017-01556-7
Vinci, M., Box, C., and Eccles, S. A. (2015). Three-dimensional (3D) tumor spheroid invasion assay. J. Vis. Exp. (99), e52686. Epub ahead of print 2015/05/21. doi:10.3791/52686
Vlachogiannis, G., Hedayat, S., Vatsiou, A., Jamin, Y., Fernández-Mateos, J., Khan, K., et al. (2018). Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Sci. 359(6378), 920–926.
Walsh, C. L., Babin, B. M., Kasinskas, R. W., Foster, J. A., McGarry, M. J., and Forbes, N. S. (2009). A multipurpose microfluidic device designed to mimic microenvironment gradients and develop targeted cancer therapeutics. Lab. Chip 9 (4), 545–554. doi:10.1039/b810571e
Wan, X., Ball, S., Willenbrock, F., Yeh, S., Vlahov, N., Koennig, D., et al. (2017). Perfused three-dimensional organotypic culture of human cancer cells for therapeutic evaluation. Sci. Rep. 7 (1), 9408. doi:10.1038/s41598-017-09686-0
Wang, K., Zhang, X., Liu, Y., Liu, C., Jiang, B., and Jiang, Y. (2014). Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates. Biomaterials 35 (30), 8735–8747. doi:10.1016/j.biomaterials.2014.06.042
Weeber, F., Ooft, S. N., Dijkstra, K. K., and Voest, E. E. (2017). Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chem. Biol. 24 (9), 1092–1100. doi:10.1016/j.chembiol.2017.06.012
Xing, F., Liu, Y. C., Huang, S., Lyu, X., Su, S. M., Chan, U. I., et al. (2021). Accelerating precision anti-cancer therapy by time-lapse and label-free 3D tumor slice culture platform. Theranostics 11 (19), 9415–9430. doi:10.7150/thno.59533
Xu, X., Farach-Carson, M. C., and Jia, X. (2014). Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 32 (7), 1256–1268. doi:10.1016/j.biotechadv.2014.07.009
Xu, H., Jiao, Y., Qin, S., Zhao, W., Chu, Q., and Wu, K. (2018a). Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Exp. Hematol. Oncol. 7, 30. doi:10.1186/s40164-018-0122-9
Xu, H., Lyu, X., Yi, M., Zhao, W., Song, Y., and Wu, K. (2018b). Organoid technology and applications in cancer research. J. Hematol. Oncol. 11 (1), 116. doi:10.1186/s13045-018-0662-9
Xu, R., Zhou, X., Wang, S., and Trinkle, C. (2021). Tumor organoid models in precision medicine and investigating cancer-stromal interactions. Pharmacol. Ther. 218, 107668. doi:10.1016/j.pharmthera.2020.107668
Yan, H. H. N., Siu, H. C., Law, S., Ho, S. L., Yue, S. S., Tsui, W. Y., et al. (2018). A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 23 (6), 882–897.e11. e811. doi:10.1016/j.stem.2018.09.016
Yang, H., Wang, Y., Wang, P., and Zhang, N. (2021). Tumor organoids for cancer research and personalized medicine. Cancer Biol. Med. 19 (3), 0–332. doi:10.20892/j.issn.2095-3941.2021.0335
Yi, C., Lai, S. L., Tsang, C. M., Artemenko, M., Shuen Tang, M. K., Pang, S. W., et al. (2021). A three-dimensional spheroid-specific role for Wnt-β-catenin and Eph-ephrin signaling in nasopharyngeal carcinoma cells. J. Cell Sci. 134 (16), jcs256461. doi:10.1242/jcs.256461
Yoshida, G. J. (2020). Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 13 (1), 4. doi:10.1186/s13045-019-0829-z
Yu, J., and Huang, W. (2020). The progress and clinical application of breast cancer organoids. Int. J. Stem Cells 13 (3), 295–304. doi:10.15283/ijsc20082
Yuki, K., Cheng, N., Nakano, M., and Kuo, C. J. (2020). Organoid models of tumor immunology. Trends Immunol. 41 (8), 652–664. doi:10.1016/j.it.2020.06.010
Zaki, M. Y. W., Shetty, S., Wilkinson, A. L., Patten, D. A., Oakley, F., and Reeves, H. (2021). A three-dimensional spheroid model to investigate the tumor-stromal interaction in hepatocellular carcinoma. J. Vis. Exp. (175), e62868. Epub ahead of print 2021/10/19. doi:10.3791/62868
Zhang, J., Tavakoli, H., Ma, L., Li, X., Han, L., and Li, X. (2022a). Immunotherapy discovery on tumor organoid-on-a-chip platforms that recapitulate the tumor microenvironment. Adv. Drug Deliv. Rev. 187, 114365. doi:10.1016/j.addr.2022.114365
Zhang, M., Lv, L., Cai, H., Li, Y., Gao, F., Yu, L., et al. (2022b). Long-term expansion of porcine intestinal organoids serves as an in vitro model for swine enteric coronavirus infection. Front. Microbiol. 13, 865336. doi:10.3389/fmicb.2022.865336
Zhou, L., Zhang, C., Zhang, Y., and Shi, C. (2021). Application of organoid models in prostate cancer research. Front. Oncol. 11, 736431. doi:10.3389/fonc.2021.736431
Keywords: 3D tumor cell culture, 3D tumor sectioning, antitumor drugs screening, tumor organoids, precision oncology
Citation: Guan X and Huang S (2022) Advances in the application of 3D tumor models in precision oncology and drug screening. Front. Bioeng. Biotechnol. 10:1021966. doi: 10.3389/fbioe.2022.1021966
Received: 18 August 2022; Accepted: 13 September 2022;
Published: 28 September 2022.
Edited by:
Gianni Ciofani, Italian Institute of Technology (IIT), ItalyReviewed by:
Elisa Cimetta, University of Padua, ItalyI-Chi Lee, National Tsing Hua University, Taiwan
Copyright © 2022 Guan and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigao Huang, aHVhbmdzaGlnYW8yMDEwQGFsaXl1bi5jb20=
†These authors share first authorship
 Xiaoyong Guan1†
Xiaoyong Guan1† Shigao Huang
Shigao Huang