
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol., 07 October 2022
Sec. Tissue Engineering and Regenerative Medicine
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.1019437
This article is part of the Research TopicCell-Based Therapeutics for Intervertebral Disc DegenerationView all 7 articles
Intervertebral disc degeneration (IVDD) is a main cause of lower back pain, leading to psychological and economic burdens to patients. Physical therapy only delays pain in patients but cannot eliminate the cause of IVDD. Surgery is required when the patient cannot tolerate pain or has severe neurological symptoms. Although surgical resection of IVD or decompression of the laminae eliminates the diseased segment, it damages adjacent normal IVD. There is also a risk of re-protrusion after IVD removal. Cell therapy has played a crucial role in the development of regenerative medicine. Cell transplantation promotes regeneration of degenerative tissue. However, owing to the lack of vascular structure in IVD, sufficient nutrients cannot be provided for transplanted mesenchymal stem cells (MSCs). In addition, dead cells release harmful substances that aggravate IVDD. Extracellular vesicles (EVs) have been extensively studied as an emerging therapeutic approach. EVs generated by paracrine MSCs retain the potential of MSCs and serve as carriers to deliver their contents to target cells to regulate target cell activity. Owing to their double-layered membrane structure, EVs have a low immunogenicity and no immune rejection. Therefore, EVs are considered an emerging therapeutic modality in IVDD. However, they are limited by mass production and low loading rates. In this review, the structure of IVD and advantages of EVs are introduced, and the application of MSC-EVs in IVDD is discussed. The current limitations of EVs and future applications are described.
Intervertebral disc degeneration (IVDD) is among the most common spinal degenerative diseases and the main cause of chronic low back pain (LBP) in clinical patients (Zhang et al., 2021a). Approximately 40–50% of clinical LBP cases is caused by disc degeneration (Geurts et al., 2018). IVDD is a process of aging and damage caused by a series of complex molecular mechanisms and usually caused by extracellular matrix (ECM) breakdown and anabolic disturbances, leading to IVD bulging and loss of water content in the nucleus pulposus (NP) and subsequent loss of disc height (Kirnaz et al., 2022). IVDD causes partial or complete rupture of the annulus fibrosus (AF); the NP protrudes backward from the rupture and irritates or compresses the nerve root, thus causing low back and leg pain (Adams et al., 2015). LBP seriously endangers the physical and mental health of patients and usually results in a serious economic burden. In the United States alone, the cost of LBP treatment exceeds $100 billion per year (Katz, 2006). The prevalence of IVDD is increasing yearly due to risk factors, such as aging, obesity, chronic stress, and smoking (Kirnaz et al., 2022).
Clinical treatment for IVDD mainly includes drug therapy, bed rest, traction, acupuncture, massage, and electromagnetic or electrothermal therapy (Xin et al., 2022). Although these conservative treatments may relieve symptoms and reduce pain, they do not reverse IVDD (Oichi et al., 2020). Patients who cannot tolerate pain usually undergo surgical removal of the IVD or IVD fusion therapy (Xin et al., 2022). Surgical treatment eliminates the radicular pain caused by IVDD, as it fundamentally eliminates the source of LBP. However, surgical resection of the IVD may lead to reherniation, and reherniation rate after lumbar disc herniation was as high as 21.2% (Ran et al., 2015). In the long term, IVD fusion eliminates the mobility of adjacent cones, increasing the load and stress on the surrounding tissues and IVD, which leads to degeneration of other IVDs in adjacent segments (Kumar et al., 2001). Many therapeutic strategies for IVDD have been developed in tissue engineering and regenerative medicine. However, these modalities are only in the preclinical stage.
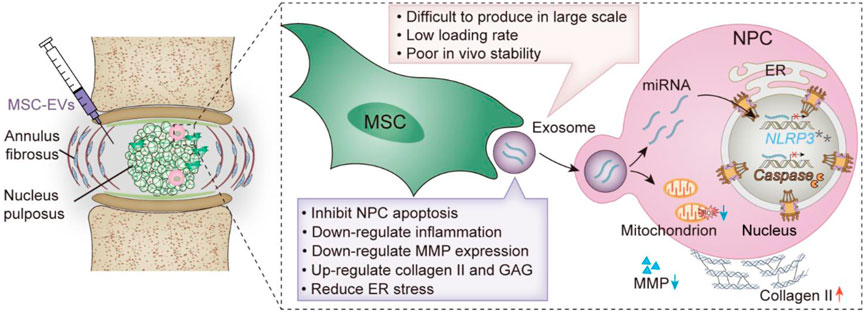
SCHEME 1. Schematic illustration of MSC-EVs promoting IVDD repair. Advantages and disadvantages of MSC-EVs.
With advances in cell therapy, stem cell repair of IVDD appears to be a good strategy. Based on the tissue engineering, stem cells have been widely used in regenerative medicine owing to their advantages of self-proliferation and directed differentiation at degeneration sites (Moeinabadi-Bidgoli et al., 2021). Mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), and adipose stem cells can differentiate into NP cells (NPCs) and synthesize new ECM for IVDD regeneration (Baldia et al., 2021; Ekram et al., 2021). Moreover, stem cells also reduce the inflammatory environment around IVDD through paracrine secretion of anti-inflammatory factors and chemokines (Zhang et al., 2022). However, the IVD is an avascular structure; the stem cells delivered around the IVDD cannot absorb enough nutrients from the surrounding tissue and cannot fully exert their effect of regenerating the NP and AF (Hajiesmailpoor et al., 2022). Stem cells delivered to the area surrounding IVDD die in large numbers due to the inability to take up nutrients; the inflammatory factors [interleukin (IL)-1, IL-6, and IL-8] released by dead cells will also cause surrounding inflammatory responses, which are usually not conducive to the survival of surrounding cells (Yuan et al., 2021a).
With the development of tissue engineering and regenerative medicine, extracellular vesicles (EVs) have made great progress as active biological substances for intercellular communication owing to their ability to promote tissue regeneration (Wang et al., 2022). MSC-EVs have therapeutic potential for the treatment of IVDD by promoting cell proliferation and tissue regeneration, modulating inflammatory responses, and reducing apoptosis (Bhujel et al., 2022). EVs originate from intracellular multivesicular bodies (MVBs), vesicular structures that are released from cells in a paracrine fashion into the extracellular matrix. EVs have a bilayer lipid membrane structure and have the advantages of low immunogenicity and good cytocompatibility in vivo. EVs contain cytokines, proteins, lipids, and non-coding RNAs. The transfer of EV content between cells inhibits the apoptosis of NPCs and promotes ECM synthesis to delay IVDD (Wang et al., 2022). In addition, EVs circumvent the adverse effects of stem cell therapy for IVDD, making it a promising therapeutic strategy for IVDD (DiStefano et al., 2022). In this review, the composition of IVDD is first introduced. Second, the generation and advantages of EVs and their applications in IVDD are discussed (schematic illustration and Table 1). Finally, the limitations of EV applications are mentioned, and future research directions are enumerated.
The IVD is a fibrocartilaginous pad between two adjacent cones that maintains the spine for slight movements (Newell et al., 2017). The IVD is mainly composed of three parts, namely NP, AF, and fibrocartilaginous endplate (CEP), that are distributed concentrically around the NP. The NP is a gel-like structure composed of water, type II collagen, chondrocyte-like cells, and proteoglycans, accounting for 40–50% of the volume of an adult IVD (Iatridis et al., 1996). Water accounts for 70–90% of the total weight of the NP, and the water content of the NP decreases with age (Antoniou et al., 1996). Fluid pressure within the IVD changes with decreasing water content, leading to a derangement of molecular anabolism in the IVD (decreased proteoglycans, glycosaminoglycans [GAG], aggrecans, and type II collagen and increased type I collagen) (Cao et al., 2022). The increase in matrix metalloproteinases (MMPs) in the ECM disrupts the normal equilibrium structure of the IVD and accelerates IVDD. MMP-3 and MMP-13 are considered key factors in ECM degradation in IVDD (Yao et al., 2016; Liang et al., 2022). There is no neurovascular structure around NPCs, and it is difficult to heal it once damaged. After AF rupture, NP prominence exposes the immune system to an otherwise immune IVD (Ye et al., 2022). Recruitment of inflammatory factors [IL-1β and tumor necrosis factor (TNF)] and immune cells (T cells, macrophages, and natural killer cells) to the IVD accelerates IVDD progression by destroying ECM components while clearing necrotic tissues (Risbud and Shapiro, 2014; Sun et al., 2020; Francisco et al., 2022). Maintaining the integrity of NPCs may avoid the massive destruction of the ECM. Therefore, treatment of damaged NPCs is a potential therapeutic target to delay IVDD progression. Replacing or repairing aging NPCs is the main mechanism by which EVs reverse IVDD (Collin et al., 2011).
The AF is a highly fibrotic tissue surrounding the NP and is mainly composed of a concentric fibrous structure comprising type II collagen near the NP and type I collagen outside (Peredo et al., 2020). In addition to this collagen difference, the tension of the outer AF is 2–3 times greater than that of the inner AF (Eyre and Muir, 1976). The AF protects the integrity of the NP and resists spinal stress. Owing to its avascular nature, low cellularity, and dense ECM composition, the AF rarely heals itself after tissue injury. Therefore, when the AF is damaged, it cannot withstand the pressure of the NP, and the NP protrudes into the spinal canal and compresses adjacent nerve roots, causing radicular pain (Peredo et al., 2020). Excessive mechanical load is the main cause of IVDD, with increased expression of the inflammatory factors IL-1β and TNF-α, which may further accelerate IVDD (Gonçalves et al., 2021; Chang et al., 2022). Induction of AF repair is considered an effective strategy for the treatment of IVDD.
The CEP is a thin layer of hyaline cartilage that separates the IVD from the upper and lower vertebral body bones. Unlike that of the AF and NP, the vascular structure within the CEP is the main source of IVD nutrition (Holm et al., 1981; Feng et al., 2022). Nonetheless, there are currently no reports of IVDD reversal by modulating the CEP in IVDD treatment (Lakstins et al., 2021). IVD regression is associated with inflammation, immune cell infiltration, and neovascularization (Krut et al., 2021). This organizing process exacerbates radicular pain in the microenvironment of the degenerative disc (Freemont et al., 1997). In addition, degenerated NPCs aggravate the degeneration of the CEP through EV formation (Feng et al., 2022). CEP denaturation may impede nutrient transport, resulting in failure to maintain material and metabolic balance in the NP (Wang et al., 2018). Therefore, regulation of the NP, AF, and CEP may provide potential targets for IVDD treatment.
Owing to the unique structure of avascular and tightly surrounded by AF around NPs, oral or systemic administration does not enable drug transport into NPCs. Although stem cells exist around IVD, the microenvironment around IVD cannot effectively induce stem cells to differentiate into NPCs (Li et al., 2021a). EVs inherit the advantages of their parental MSCs, which can modulate inflammatory and immune responses, and exert proliferative and antioxidant effects (Priyadarshani et al., 2016). Intra-IVD injection of MSC-EVs can transfer miRNAs and other substances carried by them to NPCs, replacing or repairing damaged NPCs and regulating the ECM components around NPCs to promote their growth (Collin et al., 2011).
EVs are a heterogeneous population of membrane vesicles secreted by all living cells and are grouped into three broad categories based on their subcellular origin and size: apoptotic bodies, membrane vesicles (MVs), and EVs (Veerman et al., 2019). EVs transfer cell membrane and cytoplasmic proteins, lipids and RNAs, and play an important role in maintaining cellular function and tissue homeostasis in multicellular organisms (Raposo and Stoorvogel, 2013; van Niel et al., 2018). EVs represent a new form of intercellular communication, promoting cell proliferation, differentiation, and angiogenesis and inhibiting apoptosis and inflammation to mediate tissue repair (Chang et al., 2021). Cells release different types of endosome- and plasma-membrane-derived membrane vesicles, termed EVs and MVs, respectively, into the extracellular environment (Raposo and Stoorvogel, 2013). MVs are 100–1,000 nm in diameter and are released from cells by direct budding from the plasma membrane (Lee and Kim, 2021). EVs and apoptotic bodies originate from nanoscale vesicles formed by inward budding of the endosomal membrane and are secreted into the extracellular milieu when MVBs fuse with the plasma membrane (Urabe et al., 2020). EVs and MVBs are produced by healthy cells as part of regular membrane turnover and exocytosis. In contrast, apoptotic bodies arise from outer membrane blebbing in cells undergoing apoptosis (El Andaloussi et al., 2013). Apoptotic bodies are 100–5,000 nm in diameter, whereas EVs are much smaller vesicles, approximately 30–150 nm in diameter (Li et al., 2021b). Once EVs are released by cells, they may be taken up by recipient cells to deliver molecules, such as proteins, lipids, and RNA, that they carry to target cells (Veerman et al., 2019). Internalization of EVs by recipient cells relies on a fusion mechanism between the vesicle and plasma membranes and is mediated mainly by clathrin-mediated endocytosis, caveolin-dependent endocytosis, micropinocytosis, and phagocytosis (Kwok et al., 2021). Membrane proteins and membrane receptors also play important roles in EV transmission. Owing to the size and number of MVs and exosomes, relatively pure single structures are usually not available for these vesicles. Therefore, the International Society for Extracellular Vesicles has uniformly named these membrane structures EVs (Théry et al., 2018).
EVs are vesicles produced by cells. Compared to polymer biomaterials, EVs have unique advantages as carriers. Their negatively charged surface is intrinsically stable in the circulatory, while enabling EVs to escape attack by monocytes owing to the surface expression of CD47 (Elsharkasy et al., 2020). Additionally, EVs are membrane structures that change their shape to pass through natural tissue and cellular barriers, such as the blood–brain barrier (Chen et al., 2016). The contents of an EV may be affected by factors, such as the type of cell source, physiological state of donor cells, and external stimuli (Kwok et al., 2021). Packaging of goods by EVs is mainly via exogenous and endogenous loading (Gupta et al., 2021). Endogenous loading mainly relies on genetic engineering technology to transform cells before EVs are produced and express or incorporate the mRNA or protein of the cargo into cells. When cells produce EVs, the cargo is wrapped and encapsulated into EVs as the EVs are secreted (Aubertin et al., 2021). This mode of delivery protects the cargo from degradation during transportation and ensures EV integrity. The cargo encapsulation efficiency and loading capacity of EVs depends on the active components of the cargo and its physicochemical properties (Piffoux et al., 2021). However, endogenous loading is only suitable for biomolecule encapsulation, not for the reproducible incorporation of chemically synthesized drugs (Rankin-Turner et al., 2021). Exogenous loading typically involves incorporation of cargo into EVs by various methods, including co-incubation, sonication, thermal shock, and electroporation (Armstrong et al., 2017). Co-incubation is a simple method of encapsulation, and mother cells may be passively loaded by co-incubation with molecular organisms. Packaging efficiency is related to the surface charge and characteristics of the carrier (Rankin-Turner et al., 2021). Sonication results in higher encapsulation efficiency than co-incubation. Sonication relies on ultrasonic waves to temporarily destroy the membrane structure, enabling the encapsulation of cargo into EVs regardless of surface charge and cargo properties (Sato et al., 2016). Electroporation enhances membrane permeability and enables passage of exogenous molecules by applying a transient electric field; in this method, loading efficiency is inversely proportional to the size of the loaded molecules (Heiser, 2000). EVs are secreted by cells in vivo and have natural advantages over polymer materials as carriers.
Although EVs are produced by cells and widely present in body fluids, intravenous EVs are cleared in the liver and spleen (Lener et al., 2015). Transformation of EVs results in EV chemotaxis to different locations in vivo. This is attributed to the presence of lipids and proteins on the EV surface that are acquired from the cell of origin during their biogenesis (Ou et al., 2021). EV distribution in vivo is mainly determined by different cell sources, routes of administration, and targeting (Wiklander et al., 2015). Cells of different origins have cell homing at different locations in the body; for example, immune cells preferentially target immunologically active sites, and this homing is species-independent (Wiklander et al., 2015). Macrophage-secreted EVs target areas of inflammation in vivo (Kalluri and LeBleu, 2020). Exosomes expressing the Tspan8-α4 complex are most readily taken up by endothelial and pancreatic cells (Rana et al., 2012). EVs are internalized by receptor cells dependent on ligand-receptor binding (Kwok et al., 2021). Therefore, engineering EVs to express specific ligands on their surfaces is beneficial to improve their targeting properties (Yong et al., 2020). Chemical modification and genetic engineering are widely used methods for EV membrane modification (Zhang et al., 2021b). Inserting a gene encoding a targeting protein into a donor cell, and secretion of protein in exosomes by the donor cell may achieve the corresponding targeting ability (Luan et al., 2017). Elevated expression of chemokine (C-C motif) ligand 18 on tumor cell-derived EVs promoted EV internalization by tumor cells expressing chemokine (C‒C motif) receptor 8 (Berenguer et al., 2018). Chemical modifications also endow EVs with unique targeting capabilities, whereas the biocompatibility of EVs reduces the risk of systemic toxicity commonly found in other nanomaterials.
The ability of stem cells for self-proliferation and direct differentiation has played an important role in tissue engineering and regenerative medicine (Hade et al., 2021; Scala et al., 2021). However, clinical application of stem cells have certain challenges. For example, inadequate retention and survival of MSCs at the site of administration hinders their therapeutic efficacy, as demonstrated in an osteoarthritis study (Arshi et al., 2020). Exogenous stem cells are not conducive to survival and proliferation in the ischemia and inflammatory microenvironment and may even lead to the death of many cells in the body (Zhao et al., 2021). Inflammatory factors released by dead cells also cause peripheral inflammatory responses that are often detrimental to the survival of peripheral cells (Yuan et al., 2021a). Changes in the microenvironment induce dramatic changes in stem cell behavior (Li et al., 2021c). EVs are produced by cells in a paracrine manner, and their production is a normal physiological process. Compared to stem cells, EVs avoid the side effects of cell therapy and play additional roles as carriers, remodeling, and signaling between cells. Stem cell-secreted EVs may replace stem cell-based therapies in various injury and disease models (Hade et al., 2021). MSC-EVs themselves exhibit anti-inflammatory effects, promote the polarization of macrophages to the M2 type, and provide basis for tissue repair (Arabpour et al., 2021). Therefore, EVs reduce adverse reactions by retaining the regenerative ability of stem cells. Additionally, EVs have a low immunogenicity compared to stem cells and will not cause immune rejection in the body (Elahi et al., 2020). Therefore, the development of EVs for tissue engineering and regenerative medicine applications is of great significance.
MSC transplantation has shown great promise in regenerative medicine (Liu et al., 2021). MSCs are multipotent adult stromal cells obtained from the bone marrow, umbilical cord, fat, and placenta (Herman et al., 2021). The application of bone marrow-derived mesenchymal stem cells (BMSCs) in IVDD has been reported. BMSC transplantation into the IVD resulted in the proliferation and differentiation into NP-like cells, a significant increase in viability and autophagy levels, a decrease in rat NPC apoptosis, and the subsequent induction of synthesis of new ECM (Loibl et al., 2019; Shi et al., 2021). Intravenous infusion of MSCs only transports a small number of MSCs to the damaged site, and most MSCs are cleared by the body (Mezey, 2011). EVs produced by MSCs via a paracrine manner are the most important mediators of the therapeutic effects of MSCs (Witwer et al., 2019).
Excessive oxidative stress and inflammation are the main causes of early IVDD. Mitochondrial production of reactive oxygen species (ROS) increases the number of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasomes around the IVDD (Zhang et al., 2017). The NLRP3 inflammasome is an essential component of the innate immune system, mediating caspase-1 activation and IL-1β secretion that results in cellular damage (Kelley et al., 2019). Apoptosis of NPCs is an important factor causing IVDD; apoptosis of NPCs causes water loss, decreased osmotic pressure, and changes in the quantity and distribution of collagen (Yang et al., 2009). Xia et al. demonstrated that BMSC-EVs inhibited ROS production in NPCs and NLRP3 inflammasome activation and attenuated NPC apoptosis in a rabbit IVDD model (Xia et al., 2019). BMSC cells were cultured in vitro, and the supernatant was collected after centrifugation to obtain MSC-EVs. The diameter of MSC-EVs was approximately 50–130 nm, and they were internalized by NPCs. MSC-EVs provided mitochondria-related proteins to NPCs to promote the recovery of mitochondrial function. Additionally, MSC-EVs slowed IVDD progression by reducing the expression of matrix metallopeptidase 13 (MMP-13) in the ECM. To further demonstrate that MSC-EVs reduce the expression of NLRP3 bodies and caspase-1 in NPCs, NPCs were treated with the proinflammatory factor TNF-α to increase the expression of NLRP3 bodies and caspase-1 in NPCs in vitro (Liao et al., 2021). Liao et al. have engineered modified MSC-EVs using gene-editing technology to increase their internalization by NPCs (Liao et al., 2021). The vector-expressing Cavin-2 was transfected into the Lamp2b gene in MSCs (the Lamp2b protein in the EV membrane) to express Cavin-2 in the EV membrane (Figure 1A). Cavin-2 is involved in caveolae-mediated endocytosis, which increases EV internalization by damaged NPCs. The effects of Cavin-2-modified EVs on NPCs were validated in an alginate hydrogel-simulated IVD in vitro (Figure 1B). Injection of EVs into the IVD at 2 and 4 weeks demonstrated that EVs promoted the repair of broken AF in the IVD (Figures 1C,D). EVs also significantly reduced the amount of apoptosis (Figure 1E). In addition to inflammatory factors, reduction of mitochondrial membrane potential by compression caused NP apoptosis (Ding et al., 2012). Hu et al. demonstrated that BMSCs-EVs alleviated compression-mediated NP apoptosis by inhibiting oxidative stress (Hu et al., 2021). In vitro experiments demonstrated that BMSCs-EVs had a protective effect on mitochondrial membrane potential and avoided compression-induced mitochondrial damage in NPCs. ROS levels of NPCs in the BMSCs-EV group were significantly lower than those in the compression group alone. Additionally, the accumulation of advanced glycation end products (AGEs) in the NP induced inflammation, metabolic dysfunction, and endoplasmic reticulum (ER) stress and promoted apoptosis of NPCs (Song et al., 2018). MSC-EVs alleviated AGE-induced ER stress by activating the protein kinase B (AKT) and extracellular signal-regulated kinase signaling pathways in vitro, thereby reducing NPC apoptosis in IVD progression (Liao et al., 2019; Xiao et al., 2022).
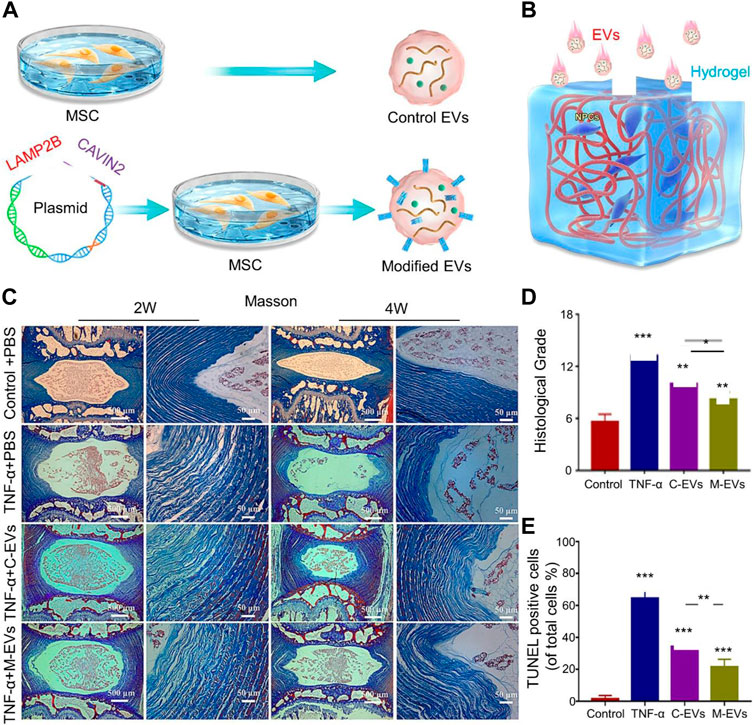
FIGURE 1. (A) Schematic diagram of the extracellular vesicle (EV) engineering process modified by Cavin-2. (B) Schematic illustration of EV treatment of intervertebral disc degeneration (IVDD) in an in vitro alginate hydrogel model. (C) Masson staining histological analysis at 2 and 4 weeks of treatment shows the morphology of IVDs in different treatment groups. (D) Histological grade based on the scale of disc degeneration in different groups. (E) Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) staining shows the apoptosis of different groups. Reproduced with permission from (Liao et al., 2021).
Autophagy is different from apoptosis. Apoptosis is a type of programmed cell death, whereas autophagy is a protective mechanism whereby cellular components are sequestered into lysosomes, which can digest these substrates and recycle them to generate new cellular structures (D’Arcy, 2019). In an in vitro-simulated IVD experiment, Zhou et al. co-incubated BMSCs and NPCs in a medium depleted of oxygen and glucose to mimic the oxygen-deficient state of IVD (Shi et al., 2021). BMSC-EVs also provided nutrients to NPCs and increased the expression of miR-155, thereby promoting the expression of autophagy marker proteins LC3 and Beclin-1 and formation of autophagosomes (Chen et al., 2019; Shi et al., 2021). MiR-155 inhibited apoptosis of NPCs by inhibiting the expression of caspase-3 (Wang et al., 2011; Zheng et al., 2013). Decreased autophagy capacity of NPCs was detected after knockdown of miR-155. miRNA expression was reduced in apoptotic NPCs, whereas EVs contained abundant miRNAs. Therefore, using EVs to provide miRNA to NPCs to inhibit apoptosis is a main mechanism by which EVs reverse IVDD (Chen et al., 2010). Cheng et al. demonstrated that BMSCs-exosomes inhibited apoptosis of NPCs by activating signaling pathways (Cheng et al., 2018). MiR-21 in MSC-EVs inhibits NPCS apoptosis by targeting phosphatase and tensin homolog (PTEN) through the phosphatidylinositol 3-kinase (PI3K)-AKT pathway, a phenomenon not observed in fibroblast-EVs; this indicates that EVs derived from different mother cells have different functions. Bone marrow MSC-EVs deliver miRNAs through different pathways to inhibit NPC apoptosis (Table 1) (Zhu et al., 2020a; Zhu et al., 2020b; Cui and Zhang, 2021; Wen et al., 2021; Yu et al., 2022a; Yu et al., 2022b; Sun et al., 2022). Another study showed that the pH in degenerated IVD decreased from the normal 7.0–7.2 range to 6.5–5.7. Compared to that in normal IVD pH conditions, the number of cells in BM-MSCs was reduced by 40 and 80% after 3 days of culture at pH 6.8 and 6.5, respectively (Lyu, 2022). Therefore, the acidic environment in aged NPCs is not conducive to the growth of MSC-EVs.
In IVDD, BMSCs are the most studied intervention among MSCs from other sources. Cytokine profiling of BMSCs, however, revealed intensive production of proinflammatory cytokines IL-6 and IL-8 (Kiselevskii et al., 2021), which may contradict the report that MSCs induce anti-inflammatory effects (Marimuthu and Pushpa Rani, 2021). In addition, BMSCs aid the expression of vascular endothelial growth factor (VEGF) to promote angiogenesis under hypoxic conditions (Archacka et al., 2021). These factors are not conducive to IVDD recovery. However, there is currently no report on VEGF following IVDD treatment with BMSC-EVs.
PLMSCs have more unlimited differentiation potential and a lower immunogenicity than BMSCs (Miao et al., 2006). Overexpression of chondrogenic transcription factors in PLMSCs accelerated their differentiation potential into chondrogenic progenitors, resulting in better homing, integration, and differentiation into NPCs of IVD (Khalid et al., 2022). miR-4450 targets NPCs to induce apoptosis, and its overexpression increases apoptosis and the inflammatory responses of NPCs and reduces ECM components to promote IVDD progression (Yuan et al., 2020). Zhang et al. collected placentas immediately after cesarean section and transfected the anti-miR-4450 gene (antagomiR-4450) into PLMSCs for culture (Yuan et al., 2020). After purification by ultracentrifugation, PLMSCs-EVs with inhibited miR-4450 gene expression were obtained, with diameters ranging from 30 to 150 nm. NPCs were co-cultured with PLMSCs-EVs, which transferred antagomiR-4450 into NPCs, to alleviate the NPC damage. The decreased expression of caspase-3 and MMP-13 and increased gait frequency were detected on the 42nd day in mice. Gait frequency is a powerful tool to evaluate exercise capacity in laboratory animals and clinical patients (Jarchi et al., 2018). The increase in gait frequency illustrates the recovery of motor capacity in mice. In conclusion, PLMSCs-EVs attenuated NPCS injury and delayed IVDD injury in vivo.
N6-methyladenosine is the most common endogenous RNA modification (Huang et al., 2018). Methyltransferase (METT) removes miRNA methylation and reduces miRNA expression. METTL14 exists in NPCs and stabilizes the expression of NLRP3 mRNA. NLRP3 induces inflammation in IVDD and accelerates the apoptosis of NPCs and progression of IVDD (Yuan et al., 2021b). Yuan et al. used a lentiviral vector that targets human METTL14 to carry miR-26a-5p for transfection into human umbilical cord mesenchymal stem cells (HUCMSCs) (Yuan et al., 2021b). HUCMSC-EVs were 65 ± 15 nm in diameter. HUCMSC-EVs provided miR-26a-5p to prevent NPC apoptosis by targeting METTL14 and inhibiting the production of METTL14 and NLRP3. Additionally, HUCMSC-EVs promoted the expression of collagen II and aggrecan in the ECM to delay IVDD progression (Qi et al., 2019).
PLMSCs-EVs release angiopoietin to stimulate angiogenesis (Komaki et al., 2017). While IVDs are avascular structures, and angiogenesis is not conducive to maintaining IVD structures, whether PLMSCs-EVs induce angiogenesis in IVD has not been reported. Furthermore, compared to BMSCs, PLMSCs and UC-MSCs are not easily available and have ethical issues that limit their application.
The main advantages of ADSCs over other sources of MSCs include abundance of tissue sources, ease of tissue collection and cell isolation, and their therapeutic potential (Bunnell, 2021). ADSCs are accessible, and there is an abundance of tissue that may be obtained in large quantities without affecting body function. Compared with other MSCs, ADSC-derived extracellular nanovesicles significantly promoted the mRNA expression of chondrocyte genes (collagen-II, aggrecan and Sox-9) in human NP cells (Zhang et al., 2021c). ADSC-EVs enter NPCs and the AF to reduce the expression of inflammatory factors and MMPs in vitro (González-Cubero et al., 2022). Zhang et al. demonstrated that ADSCs-EVs increased the migration and proliferation of NPCs and suppressed their inflammatory activity (Zhang et al., 2021c). ADSC-EVs detected the expression of the collagen-II gene in NPCs on the first day of co-incubation with NPCs. Decreased levels of inflammatory factors IL-1α, IL-1β, and TNF-α were detected on the seventh day. However, the low number of EVs generated from ADSCs with a short duration of action cannot sustainably support IVDD treatment. Xing et al. developed a decellularized extracellular matrix (dECM) hydrogel using porcine NP matrix for the delivery of ADSC-EVs (Figure 2A) (Xing et al., 2021). Porcine NP matrix was regenerative, and dECM@exo had a porous structure that retains glycosaminoglycans (GAGs) to remove 99% of DNA components. ADSC-EVs, with a diameter of 30–150 nm and a zeta potential of −32.7 mV, were stably bound to negatively charged groups in the ECM. In situ injection of dECM@exo supplemented ECM leakage in NPCs and provided an environment for the growth of NPCs. In vitro experiments confirmed that dECM@exo was able to gradually release most EVs for up to 28 days at 37°C. dECM@exo downregulated MMP-13 (Figure 2B) and upregulated collagen II (Figure 2C) to promote ECM synthesis and maintain the stability of IVDD. Additionally, dECM@exo reduced the expression of the NLRP3 inflammasome and suppressed the inflammatory response of IVD (Figure 2D).
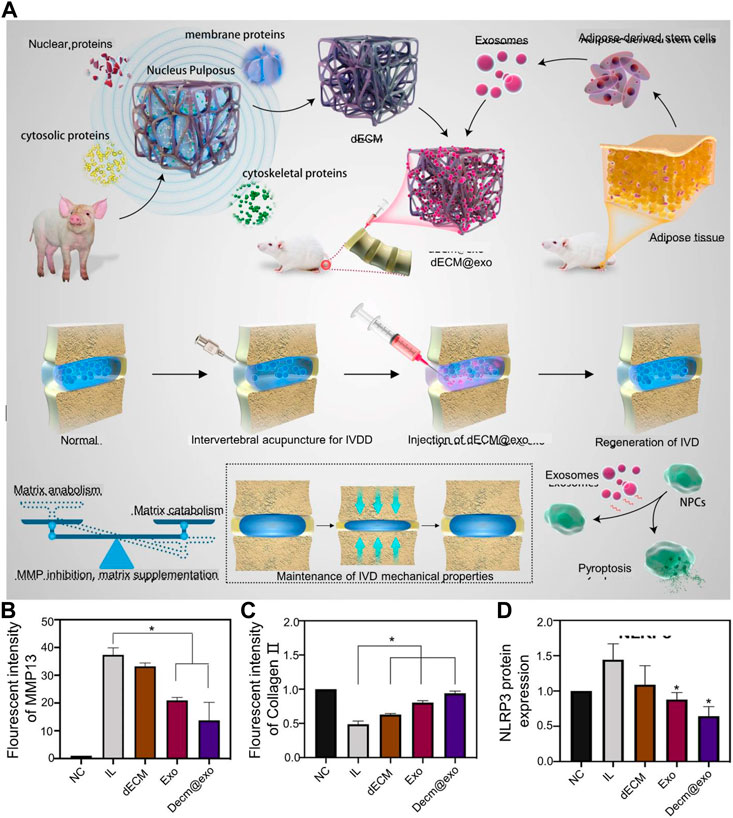
FIGURE 2. (A) Schematic diagram of the preparation mechanism of dECM@exo. (B) Quantitative analysis of the fluorescence intensity of matrix metallopeptidase 13 (MMP-13). (C) Quantitative analysis of the fluorescence intensity of collagen 11 in each group. (D) Quantification of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) expression. Reproduced with permission from (Xing et al., 2021).
Although MSC-EVs have made progress in relieving NPC aging and promoting ECM synthesis, harvesting MSCs from bone marrow and adipose tissues is invasive. In addition, the decreased proliferative potential and therapeutic efficacy of MSCs during in vitro expansion limits the application of MSC-EVs (Larson et al., 2010). iPSCs are a subset of stem cells that can be reprogrammed from any tissue type in the body (Cartwright et al., 2005). They have a unique ability to proliferate indefinitely and show totipotency in vitro. Compared with MSCs, EVs can be obtained in large quantities from iMSCs. iPSCs have a high proliferation rate and may be cultured for 40 passages in vitro and reprogrammed from any tissue type in vivo (Ohnuki and Takahashi, 2015). Senescence of NPCs plays a critical role in the pathological progression of IVDD (Jiang et al., 2013); aged NPCs have a decreased ability to increase proliferation and decompose ECM components, thus aggravating IVDD progression. The expression level of Sirt6 was reduced in senescent NPCs, and Sirt6 repaired broken DNA duplexes to protect cells from senescence (Tian et al., 2019). Sun et al. used EVs isolated from iPSCs for the treatment of IVDD (Sun et al., 2021). The diameter of iMSCs-sEVs was 80–200 nm iMSCs-sEVs were injected into a rat IVDD model, and miR-105-5p from iMSCs-sEVs activated the Sirt6 pathway in aging NPCs at the eighth week, thereby delaying the growth of NPCs, aging, and IVDD progression. iPSCs differentiated into NP-like cells in vitro in the presence of a specific medium and addition of growth factors (Chen et al., 2013; Tang et al., 2018). This study addressed the pitfalls of low availability of autologous NPCs in adults. However, this study only conducted in vitro experiments, and further confirmation of whether the NP-like cells synthesized in vitro may inhibit NPC degeneration and induce NPC regeneration in vivo is needed.
NP-MSCs have been found in degenerative and normal IVD tissues. NP-MSCs were significantly less inhibited under acidic in vitro conditions and expressed significantly higher levels of proteoglycans and collagen II than other MSCs (Zhang et al., 2021d). Forkhead-box F1 (FOXF1) is rarely expressed in degenerated NPCs. Therefore, transferring the FOXF1 transcriptional gene into degenerated NPCs to convert degenerated NPCs to healthy NPCs may be a therapeutic modality for IVD (Zhang et al., 2021d). FOXF1 is a healthy NP-specific marker involved in the regulation of cell differentiation, growth, and proliferation (Tuteja and Kaestner, 2007). Tang et al. transfected FOXF1 into a plasmid and transfected the plasmid into NP-EVs through electroporation (Figure 3A) (Tang et al., 2021). A total of 95% EVs containing FOXF1 were endocytosed by NPCs after 7 days (Figures 3B,D) and rapidly expressed in NPCs (Figure 3E). During in vitro culture for 4 weeks, transfection of FOXF1 did not affect cell survival (Figure 3C). After FOXF1-containing EVs were endocytosed by NPCs, upregulated expression of GAG in the IVD was detected (Figure 3F). NPMSCs may be isolated from degenerated IVD and non-degenerative IVD; however, non-degenerative IVD-derived NPMSCs have a stronger clonogenicity, higher proliferation rate, and higher migration rate than metamorphically derived NPMSCs (Jia et al., 2017). NPMSCs transplanted into IVDD that differentiate into NPCs to replace damaged NPCs are the best treatment method for IVDD. However, hypoxia regulation in degenerated IVD is not conducive to maintaining the number of NPMSCs (Tian et al., 2020). Compared to other types of MSCs, NPMSCs can upregulate the expression of hypoxia-inducible factor (HIF), collagen II, and proteoglycan genes (Marimuthu and Pushpa Rani, 2021). HIF is a transcription factor of cellular response to hypoxia, and NPCs express HIF-1α to maintain cellular energy metabolism and the ECM (Tran et al., 2013). HIF also reduces oxidative stress in the endoplasmic reticulum in degenerated discs (Novais et al., 2021). miR-15a targets and downregulates MMP-3 through the PI3K/AKT and Wnt3/β-catenin signaling pathways and increases collagen II and aggrecan levels (Zhang et al., 2021d). Zhang et al. isolated NPMSCs from NP tissues to regulate MMP-3 components in the ECM. EVs were isolated from NPs, and miR-15a was transfected into NP-EVs to provide miR-15a to NPMSCs (Zhang et al., 2021d). EVs containing miR-15a have a diameter of 102 ± 8 nm and increased the proliferation ability of NPMSCs after being taken up by NPMSCs. This study confirmed that anti-miR-15a inhibited collagen II and promoted the expression of MMP-3. Therefore, miR-15a derived from NP-EVs interacted with NP-MSCs to maintain the stability of IVDD.
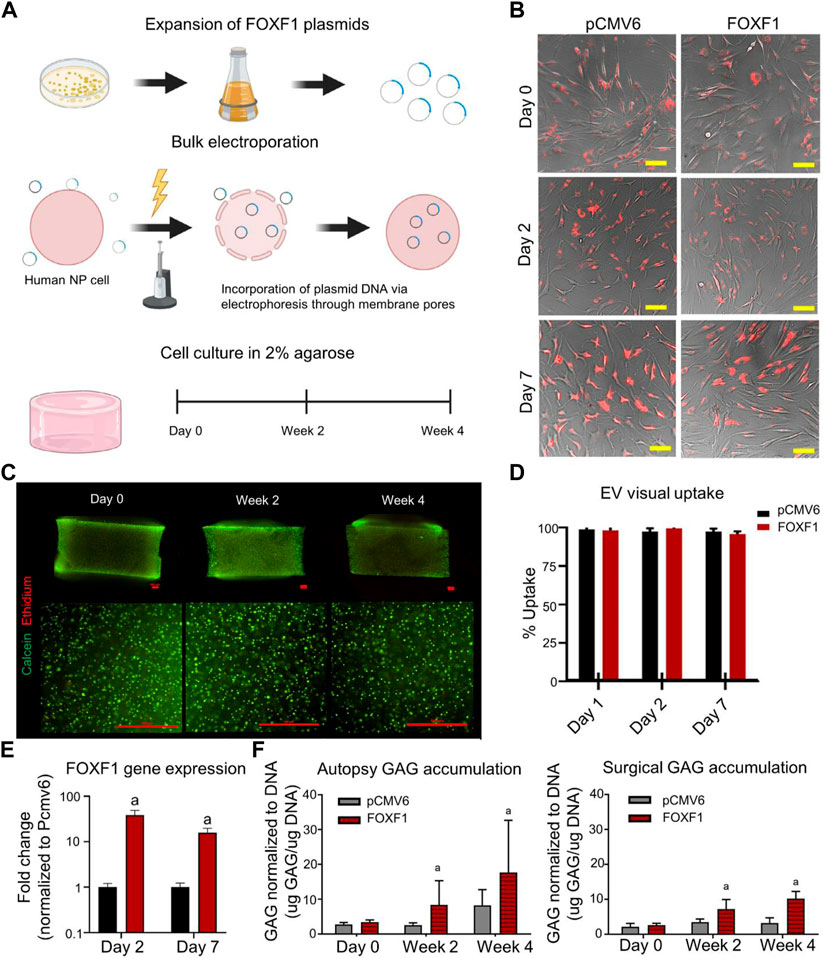
FIGURE 3. (A) Schematic representation of in vitro reprogramming of human nucleus pulposus (NP) cells by bulk electroporation of Forkhead-FOXF1). (B) Brightfield images of NP cells co-cultured in monolayers with PKH26-stained pCMV6 and FOXF1 extracellular vesicles (EVs, red), respectively, at 0, 2, and 7 d. (C) Representative images (bottom) of 4 × (top) and 10 × images (bottom) of cell-embedded three-dimensional (3D) agarose gels at days 0, 2, and 4 in culture (scale bar: 500 μm). Live cells are stained green (calcein-AM) and dead cells are stained red (ethidium-homodimer). (D) Quantified visual uptake by the percentage of cells with EVs over the total number of cells. (E) FOXF1 expression of FOXF1-EV-treated human NP cells in monolayers normalized to pCMV6-EV-treated cells. (F) Normalized to DNA content of autopsy and surgical human NP cells in 3D culture at 0, 2, and 4 weeks. Reproduced with permission from (Tang et al., 2021).
Approximately 5% of MSCs cells in the IVD are produced by AF, NP, and CEP cells (Harris et al., 2018). The distribution of spine-derived MSCs varies widely, with 18,500–61,875 MSCs isolated from 0.8 mm of CEP. CESCs are cell populations isolated from the CEP that differentiate into osteoblasts, adipocytes, and chondrocytes (Luo et al., 2021a). Compared to MSCs from other sources, CESCs are better at osteogenesis and chondrogenesis (Liu et al., 2011). They activate the HIF-1α/Wnt pathway via autocrine EVs to promote CESC migration and transdifferentiation into NPCs (Luo et al., 2021b). Normal tissue-derived CESCs are better at attenuating NP apoptosis by activating autophagy via the PI3K/AKT pathway than degenerating tissue-derived CESCs (Luo et al., 2021a). This effect was attenuated after blocking the PI3K/AKT pathway. Dong et al. secreted EVs from CESCs to carry miR-125-5p for targeting NPs (Chen and Jiang, 2022). Entry of miR-125-5p into NPCs downregulated the expression of caspase three and MMP-13 and upregulated that of GAG and collagen II. To increase the stability of EVs in vivo, Luo et al. developed a costal cartilage ECM hydrogel to deliver CESCs and transfected the Sphk2 gene into EVs using a lentiviral vector (Figure 4A) (Luo et al., 2022). Overexpression of Sphk2, a native protein, enhanced autophagic flux and suppressed NPC senescence. The hydrogels doped with the ECM of the costal cartilage had large voids, which were favorable for the growth of CESCs (Figure 4B). CESCs in the hydrogels released CESC-EVs and penetrated the AF within 72 h. The released EVs delivered Sphk2 to NPCs, enhanced autophagic flux, and inhibited NPC senescence. These EVs also reduced expression of IL-6 and MMP-13 in the IVD. In a mouse tail IVD model, Lenti-Sphk2-CESC-loaded ECM-Gels inhibited IVDD (Figure 4C). Although the hydrogel delivery of CESCs provides a theoretical basis for the treatment of IVDD, many questions remain to be resolved. For example, the properties of hydrogels and the time they may be maintained in vivo have not been confirmed, nor has the ability of EVs released from CESCs to be maintained in vivo. Additionally, Sphk2 is mainly expressed in the cytoplasm, which may inhibit senescence and promote proliferation, whereas its expression in the nucleus has the opposite effect (Wang et al., 2019). Stromal cell-derived factor-1 that is released in NPCs and degenerated CEPs also aggravates CESCs and ECM damage, thus promoting IVDD (Zhang et al., 2018).

FIGURE 4. (A) Flowchart of exosome extraction and treatment of intervertebral disc degeneration (IVDD). (B) Scanning electron microscopy images of hydrogels with or without extracellular matrix (ECM) modification of costal cartilage. (C) Morphology of IVD tissue at 3 and 6 weeks after treatment with ECM gel and Lenti-Sphk2-extracellular vesicles (EVs) in the control group. Reproduced with permission from (Luo et al., 2022).
USCs are stem cells obtained from human urine with multi-differentiation potential. Compared to MSCs from other sources, USCs are safe, non-invasive, widely sourced, and most importantly, do not involve ethical issues (Ji et al., 2017). Although their origin is unknown, USCs are likely derived from glomerular parietal cells (Wu et al., 2021). The lack of costimulatory molecules and MHC-II markers on USCs suggests a lower immunogenicity. During cell proliferation, telomeres gradually shorten and decrease, which reduces their proliferative capacity. Telomerase maintains the length of telomeres and the ability of the cell to proliferate. USCs have abundant telomerase activity and maintain their proliferation rate (Huang et al., 2022). Matrilin 3 (MATN3), a member of the matrilin protein family, is mainly distributed in chondrocytes and plays an important role in the synthesis of cartilage ECM. A reduction in its expression level may lead to IVDD. Zhang et al. demonstrated that USC-EVs enriched in MATN3 protein promoted NPC proliferation and ECM synthesis, thereby alleviating IVDD (Guo et al., 2021). NPCs could be endocytosed by NPCs within 3 h of co-incubation with USCs-EVs. USCs-EVs provide MATN3 to NPCs and promote ECM synthesis. After MATN3 knockout, USCs-EVs promoted the proliferation of NPCs and decreased ECM synthesis. Additionally, USCs-EVs changed the conformation of the transforming growth factor β (TGF-β) precursor complex by secreting MATN3, thereby promoting TGF-β activation. Activation of the TGF-β/SMAD pathway promoted collagen type II expression in the ECM. In another study, USCs-EVs inhibited IVDD by ameliorating endoplasmic reticulum stress (Xiang et al., 2020).
Owing to their uncertain origin, the safety of USCs is an important issue limiting their application. USCs express 571 genes responsible for neuromuscular diseases (Falzarano et al., 2021). The biological characteristics of USCs are not fully understood. USCs may be differentially expressed in various media owing to their different surface markers (Bento et al., 2020). In addition, the growth and proliferation ability of USCs from various aged sources differed. Therefore, side effects from USCs should be examined before their clinical application.
Although EVs play a role in delaying IVDD through intercellular signaling, EVs from various sources have different phenotypes, and EV secretion may be artificially induced by adjusting the composition of the medium. However, some issues remain to be resolved. At present, the mass and stable production of EVs are the main limitations of their clinical application (Luan et al., 2017). The most direct way to scale up production is to increase the number of EVs produced by a single cell, such as through three-dimensional (3D) culture methods or physical or chemical stimulation (Grangier et al., 2021). Compared to 2D culture technology, such as the use of hyperflasks or roller bottles as supports, 3D culture technology minimizes operation time, culture period, and cost by increasing the available surface area (Grangier et al., 2021). Obtained EVs are then subjected to ultracentrifugation for purification. The production of EVs may be considered a response of cells to their microenvironment (Staubach et al., 2021). Thus, it is unknown whether changes in the composition of the medium after large-scale culture and physicochemical stimulation may cause EVs to be secreted in a direction that deviates from what is needed. Different EV subpopulations emerge even within EV populations. Even current state-of-the-art techniques cannot completely separate EVs from subpopulations (Qin et al., 2021). Ultracentrifugation is the gold standard for the isolation of EVs; however, it is time consuming and cannot separate larger protein complexes (Momen-Heravi, 2017). Precipitation separates EVs by adding anionic complexes for coprecipitation with positive charges on the EV surface (Rider et al., 2016). However, contamination by co-precipitation of larger proteins and EVs cannot be resolved. Commercial size-exclusion chromatography (SEC) columns have been widely used recently. SEC-separated EVs have intact membrane structure and less contaminants (Tesovnik et al., 2021). However, there is currently no standard for the functional characterization of EVs obtained using this method. In other words, it is unknown whether EVs obtained from mass production undermine their therapeutic effect in vivo.
Another major role of EVs is as a carrier to transport substances to target cells. EVs carry DNA, various RNAs, proteins, and lipids of the parent cell after paracrine secretion. EVs are endocytosed by target cells and release their contents into target cells to regulate their function. The membrane structure of EVs makes them a safe delivery carrier. However, the low loading efficiency of EVs limits their application (Luan et al., 2017). At present, the loading methods for EVs include co-incubation, sonication, decompression, and electroporation. However, the loading efficiency of co-incubation is low, and the membrane structure of EVs is disrupted by ultrasonic electroporation. Likewise, there is currently no method for mass loading. Furthermore, how long post-production EVs may be stored in vitro and their in vivo stability after storage remain unknown. Therefore, it will take some time for EVs to be used clinically.
As the most common degenerative disease of the spine, IVDD seriously affects the physical and mental health of patients. For patients with IVDD, physical therapy only relieves pain but cannot eliminate the root cause of IVDD. After IVDD causes severe pain and neurological symptoms, chiropractors usually remove the IVD or decompress the spinal canal. However, after surgery, IVD remains at a high risk of herniation while aggravating damage to adjacent segments. As a result, surgery causes the patient to endure pain, often despite its high cost. Biomaterial-related tissue engineering and regenerative medicine experiments in animals have reported delay in IVDD progression. The 3D printed bracket provides a certain local pressure. Hydrogels modulate the immune-inflammatory microenvironment around the IVD to slow IVDD progression. However, it will take time before their clinical application.
EVs are an emerging therapeutic modality for IVDD treatment that may address immune rejection and the ethical shortcomings of cell therapy while maintaining its advantages. EVs have been fully demonstrated in recent years as a method of signal transmission between cells and substance carriers to target cells. Furthermore, the role of EVs in regenerative medicine is becoming increasingly prominent. EVs deliver anti-apoptotic mRNAs to NPCs to maintain NP activity, target the ECM to degrade MMPs, and promote collagen II and GAG synthesis to delay IVDD progression. EVs also deliver mitochondria-associated proteins to NPCs to inhibit NLRP3 activation. Methods of blocking apoptosis of NPCs have been demonstrated in preclinical studies. The subpopulation of MSCs-EVs is large, the components are complex, and the differences across batches are difficult to determine; thus, it is difficult to prepare a standard dosage for a particular disease treatment, and individual reactions are unpredictable. Although there is no clinical report on the use of EVs in IVDD, their applications for the treatment of acute coronary syndromes, Parkinson’s disease, and cancer have been explored. We believe that future EVs will contribute to a major breakthrough in slowing the progression of IVDD.
All authors read and approved the final manuscript. YX wrote the initial manuscript. CF and RY contributed new ideas. YH created the figures. YL and HW created Table 1. YX and JZ revised the manuscript. YX, YH, and YL revised the manuscript and approved the final version.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82071391), and the Achievement Transformation Fund of the First Hospital of Jilin University (Grant No. JDYYZH-2102052).
We would like to express our appreciation to everyone who was involved in the drafting and preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, M. A., Lama, P., Zehra, U., and Dolan, P. (2015). Why do some intervertebral discs degenerate, when others (in the same spine) do not? Clin. Anat. 28, 195–204. doi:10.1002/ca.22404
Antoniou, J., Steffen, T., Nelson, F., Winterbottom, N., Hollander, A. P., Poole, R. A., et al. (1996). The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Invest.. 98, 996–1003. doi:10.1172/jci118884
Arabpour, M., Saghazadeh, A., and Rezaei, N. (2021). Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 97, 107823. doi:10.1016/j.intimp.2021.107823
Archacka, K., Grabowska, I., Mierzejewski, B., Graffstein, J., Górzyńska, A., Krawczyk, M., et al. (2021). Hypoxia preconditioned bone marrow-derived mesenchymal stromal/stem cells enhance myoblast fusion and skeletal muscle regeneration. Stem Cell Res. Ther. 12, 448. doi:10.1186/s13287-021-02530-3
Armstrong, J. P. K., Holme, M. N., and Stevens, M. M. (2017). Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 11, 69–83. doi:10.1021/acsnano.6b07607
Arshi, A., Petrigliano, F. A., Williams, R. J., and Jones, K. J. (2020). Stem cell treatment for knee articular cartilage defects and osteoarthritis. Curr. Rev. Musculoskelet. Med. 13, 20–27. doi:10.1007/s12178-020-09598-z
Aubertin, K., Piffoux, M., Sebbagh, A., Gauthier, J., Silva, A. K. A., and Gazeau, F. (2021). Applications thérapeutiques des vésicules extracellulaires. Med. Sci. 37, 1146–1157. doi:10.1051/medsci/2021207
Baldia, M., Mani, S., Walter, N., Kumar, S., Srivastava, A., and Prabhu, K. (2021). Bone marrow-derived mesenchymal stem cells augment regeneration of intervertebral disc in a reproducible and validated mouse intervertebral disc degeneration model. Neurol. India 69, 1565–1570. doi:10.4103/0028-3886.333531
Bento, G., Shafigullina, A. K., Rizvanov, A. A., Sardão, V. A., Macedo, M. P., and Oliveira, P. J. (2020). Urine-derived stem cells: Applications in regenerative and predictive medicine. Cells 9, 573. doi:10.3390/cells9030573
Berenguer, J., Lagerweij, T., Zhao, X. W., Dusoswa, S., van der Stoop, P., Westerman, B., et al. (2018). Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J. Extracell. Vesicles 7, 1446660. doi:10.1080/20013078.2018.1446660
Bhujel, B., Shin, H. E., Choi, D. J., and Han, I. (2022). Mesenchymal stem cell-derived exosomes and intervertebral disc regeneration: Review. Int. J. Mol. Sci. 23, 7306. doi:10.3390/ijms23137306
Bunnell, B. A. (2021). Adipose tissue-derived mesenchymal stem cells. Cells 10, 3433. doi:10.3390/cells10123433
Cao, G., Yang, S., Cao, J., Tan, Z., Wu, L., Dong, F., et al. (2022). The role of oxidative stress in intervertebral disc degeneration. Oxidative Med. Cell. Longev. 2022, 1–16. doi:10.1155/2022/2166817
Cartwright, P., McLean, C., Sheppard, A., Rivett, D., Jones, K., and Dalton, S. (2005), LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132, 885–896. doi:10.1242/dev.01670
Chang, H. I., Chen, C. N., and Huang, K. Y. (2022). Mechanical stretch-induced NLRP3 inflammasome expression on human annulus fibrosus cells modulated by endoplasmic reticulum stress. Int. J. Mol. Sci. 23, 7951. doi:10.3390/ijms23147951
Chang, W. H., Cerione, R. A., and Antonyak, M. A. (2021)., 2174. Clifton, 143–170. doi:10.1007/978-1-0716-0759-6_10Extracellular vesicles and their roles in cancer progression.Methods Mol. Biol.N.J
Chen, C. C., Liu, L., Ma, F., Wong, C. W., Guo, X. E., Chacko, J. V., et al. (2016). Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell. Mol. Bioeng. 9, 509–529. doi:10.1007/s12195-016-0458-3
Chen, D., and Jiang, X. (2022). Exosomes-derived miR-125-5p from cartilage endplate stem cells regulates autophagy and ECM metabolism in nucleus pulposus by targeting SUV38H1. Exp. Cell Res. 414, 113066. doi:10.1016/j.yexcr.2022.113066
Chen, J., Lee, E. J., Jing, L., Christoforou, N., Leong, K. W., and Setton, L. A. (2013). Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PloS one 8, e75548. doi:10.1371/journal.pone.0075548e75548
Chen, J., Lin, Z., Deng, K., Shao, B., and Yang, D. (2019). Tension induces intervertebral disc degeneration via endoplasmic reticulum stress-mediated autophagy. Biosci. Rep. 39, BSR20190578. doi:10.1042/bsr20190578
Chen, T. S., Lai, R. C., Lee, M. M., Choo, A. B., Lee, C. N., and Lim, S. K. (2010). Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic acids Res. 38, 215–224. doi:10.1093/nar/gkp857
Cheng, X., Zhang, G., Zhang, L., Hu, Y., Zhang, K., Sun, X., et al. (2018). Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell. Mol. Med. 22, 261–276. doi:10.1111/jcmm.13316
Collin, E. C., Grad, S., Zeugolis, D. I., Vinatier, C. S., Clouet, J. R., Guicheux, J. J., et al. (2011). An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials 32, 2862–2870. doi:10.1016/j.biomaterials.2011.01.018
Cui, S., and Zhang, L. (2021). microRNA-129-5p shuttled by mesenchymal stem cell-derived extracellular vesicles alleviates intervertebral disc degeneration via blockade of LRG1-mediated p38 MAPK activation. J. Tissue Eng. 12, 204173142110216. doi:10.1177/20417314211021679
D’Arcy, M. S. (2019). Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 43, 582–592. doi:10.1002/cbin.11137
Ding, F., Shao, Z. W., Yang, S. H., Wu, Q., Gao, F., and Xiong, L. M. (2012). Role of mitochondrial pathway in compression-induced apoptosis of nucleus pulposus cells. Apoptosis 17, 579–590. doi:10.1007/s10495-012-0708-3
DiStefano, T. J., Vaso, K., Danias, G., Chionuma, H. N., Weiser, J. R., and Iatridis, J. C. (2022). Extracellular vesicles as an emerging treatment option for intervertebral disc degeneration: Therapeutic potential, translational pathways, and regulatory considerations. Adv. Healthc. Mat. 11, 2100596. doi:10.1002/adhm.202100596
Ekram, S., Khalid, S., Salim, A., and Khan, I. (2021). Regulating the fate of stem cells for regenerating the intervertebral disc degeneration. World J. Stem Cells 13, 1881–1904. doi:10.4252/wjsc.v13.i12.1881
El Andaloussi, S., Mäger, I., Breakefield, X. O., and Wood, M. J. A. (2013). Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357. doi:10.1038/nrd3978
Elahi, F. M., Farwell, D. G., Nolta, J. A., and Anderson, J. D. (2020). Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem cells Dayt. Ohio) 38, 15–21. doi:10.1002/stem.3061
Elsharkasy, O. M., Nordin, J. Z., Hagey, D. W., de Jong, O. G., Schiffelers, R. M., Andaloussi, S. E., et al. (2020). Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 159, 332–343. doi:10.1016/j.addr.2020.04.004
Eyre, D. R., and Muir, H. (1976). Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 157, 267–270. doi:10.1042/bj1570267Biochem J
Falzarano, M. S., Rossi, R., Grilli, A., Fang, M., Osman, H., Sabatelli, P., et al. (2021). Urine-derived stem cells express 571 neuromuscular disorders causing genes, making them a potential in vitro model for rare genetic diseases. Front. Physiol. 12, 716471. doi:10.3389/fphys.2021.716471
Feng, X., Li, Y., Su, Q., and Tan, J. (2022). Degenerative nucleus pulposus cells derived exosomes promoted cartilage endplate cells apoptosis and aggravated intervertebral disc degeneration. Front. Mol. Biosci. 9, 835976. doi:10.3389/fmolb.2022.835976
Francisco, V., Pino, J., González-Gay, M., Lago, F., Karppinen, J., Tervonen, O., et al. (2022). A new immunometabolic perspective of intervertebral disc degeneration. Nat. Rev. Rheumatol. 18, 47–60. doi:10.1038/s41584-021-00713-z
Freemont, A. J., Peacock, T. E., Goupille, P., Hoyland, J. A., O'Brien, J., and Jayson, M. I. (1997). Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 350, 178–181. doi:10.1016/s0140-6736(97)02135-1
Geurts, J. W., Willems, P. C., Kallewaard, J. W., van Kleef, M., and Dirksen, C. (2018). The impact of chronic discogenic low back pain: Costs and patients' burden. Pain Res. Manag. 2018, 1–8. doi:10.1155/2018/4696180
Gonçalves, R. M., Saggese, T., Yong, Z., Ferreira, J. R., Ignatius, A., Wilke, H. J., et al. (2021). Interleukin-1β more than mechanical loading induces a degenerative phenotype in human annulus fibrosus cells, partially impaired by anti-proteolytic activity of mesenchymal stem cell secretome. Front. Bioeng. Biotechnol. 9, 802789. doi:10.3389/fbioe.2021.802789
González-Cubero, E., González-Fernández, M. L., Olivera, E. R., and Villar-Suárez, V. (2022). Extracellular vesicle and soluble fractions of adipose tissue-derived mesenchymal stem cells secretome induce inflammatory cytokines modulation in an in vitro model of discogenic pain. Spine J. 22, 1222–1234. doi:10.1016/j.spinee.2022.01.012
Grangier, A., Branchu, J., Volatron, J., Piffoux, M., Gazeau, F., Wilhelm, C., et al. (2021). Technological advances towards extracellular vesicles mass production. Adv. Drug Deliv. Rev. 176, 113843. doi:10.1016/j.addr.2021.113843
Guo, Z., Su, W., Zhou, R., Zhang, G., Yang, S., Wu, X., et al. (2021). Exosomal MATN3 of urine-derived stem cells ameliorates intervertebral disc degeneration by antisenescence effects and promotes NPC proliferation and ECM synthesis by activating TGF-β. Oxidative Med. Cell. Longev. 2021, 5542241. doi:10.1155/2021/5542241
Gupta, D., Zickler, A. M., and El Andaloussi, S. (2021). Dosing extracellular vesicles. Adv. Drug Deliv. Rev. 178, 113961. doi:10.1016/j.addr.2021.113961
Hade, M. D., Suire, C. N., and Suo, Z. (2021). Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine. Cells 10, 1959. doi:10.3390/cells10081959
Hajiesmailpoor, A., Mohamadi, O., Emami, P., Farzanegan, R., and Ghorbani, M. (2022). Overview of stem cell therapy in intervertebral disc disease: Clinical perspectives. Curr. Stem Cell Res. Ther. 17. doi:10.2174/1574888x17666220628123912
Harris, L., and Vangsness, C. T. (2018). Mesenchymal stem cell levels of human spinal tissues. Spine 43, E545–E550. doi:10.1097/brs.0000000000002401
Heiser, W. C. (2000)., 130. Clifton, 117–134. doi:10.1385/1-59259-686-x:117Optimizing electroporation conditions for the transformation of mammalian cells.Methods Mol. Biol.N.J
Herman, S., Fishel, I., and Offen, D. (2021). Intranasal delivery of mesenchymal stem cells-derived extracellular vesicles for the treatment of neurological diseases. Stem cells Dayt. Ohio) 39, 1589–1600. doi:10.1002/stem.3456
Holm, S., Maroudas, A., Urban, J. P., Selstam, G., and Nachemson, A. (1981). Nutrition of the intervertebral disc: Solute transport and metabolism. Connect. tissue Res. 8, 101–119. doi:10.3109/03008208109152130
Hu, Y., Tao, R., Wang, L., Chen, L., Lin, Z., Panayi, A. C., et al. (2021). Exosomes derived from bone mesenchymal stem cells alleviate compression-induced nucleus pulposus cell apoptosis by inhibiting oxidative stress. Oxidative Med. Cell. Longev. 2021, 1–12. doi:10.1155/2021/2310025
Huang, H., Weng, H., Sun, W., Qin, X., Shi, H., Wu, H., et al. (2018). Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 20, 285–295. doi:10.1038/s41556-018-0045-z
Huang, Y.-Z., He, T., Cui, J., Jiang, Y.-L., Zeng, J.-F., Zhang, W.-Q., et al. (2022). Urine-derived stem cells for regenerative medicine: Basic biology, applications, and challenges. Tissue Eng. Part B Rev.. doi:10.1089/ten.teb.2021.0142
Iatridis, J. C., Weidenbaum, M., Setton, L. A., and Mow, V. C. (1996). Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc. Spine 21, 1174–1184. doi:10.1097/00007632-199605150-00009
Jarchi, D., Pope, J., Lee, T. K. M., Tamjidi, L., Mirzaei, A., and Sanei, S. (2018). A review on accelerometry-based gait analysis and emerging clinical applications. IEEE Rev. Biomed. Eng. 11, 177–194. doi:10.1109/rbme.2018.2807182
Ji, X., Wang, M., Chen, F., and Zhou, J. (2017). Urine-derived stem cells: The present and the future. Stem Cells Int. 2017, 1–8. doi:10.1155/2017/4378947
Jia, Z., Yang, P., Wu, Y., Tang, Y., Zhao, Y., Wu, J., et al. (2017). Comparison of biological characteristics of nucleus pulposus mesenchymal stem cells derived from non-degenerative and degenerative human nucleus pulposus. Exp. Ther. Med. 13, 3574–3580. doi:10.3892/etm.2017.4398
Jiang, L., Zhang, X., Zheng, X., Ru, A., Ni, X., Wu, Y., et al. (2013). Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: Implications for diabetic intervertebral disc degeneration. J. Orthop. Res. 31, 692–702. doi:10.1002/jor.22289
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367, eaau6977. doi:10.1126/science.aau6977
Katz, J. N. (2006). Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J. Bone Jt. Surg. 88, 21–24. doi:10.2106/jbjs.e.01273
Kelley, N., Jeltema, D., Duan, Y., and He, Y. (2019). The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20, 3328. doi:10.3390/ijms20133328
Khalid, S., Ekram, S., Salim, A., Chaudhry, G. R., and Khan, I. (2022). Transcription regulators differentiate mesenchymal stem cells into chondroprogenitors, and their in vivo implantation regenerated the intervertebral disc degeneration. World J. Stem Cells 14, 163–182. doi:10.4252/wjsc.v14.i2.163
Kirnaz, S., Capadona, C., Wong, T., Goldberg, J. L., Medary, B., Sommer, F., et al. (2022). Fundamentals of intervertebral disc degeneration. World Neurosurg. 157, 264–273. doi:10.1016/j.wneu.2021.09.066
Kiselevskii, M. V., Vlasenko, R. Y., Stepanyan, N. G., Shubina, I. Z., Sitdikova, S. M., Kirgizov, K. I., et al. (2021). Secretome of mesenchymal bone marrow stem cells: Is it immunosuppressive or proinflammatory? Bull. Exp. Biol. Med. 172, 250–253. doi:10.1007/s10517-021-05371-5
Komaki, M., Numata, Y., Morioka, C., Honda, I., Tooi, M., Yokoyama, N., et al. (2017). Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res. Ther. 8, 219. doi:10.1186/s13287-017-0660-9
Krut, Z., Pelled, G., Gazit, D., and Gazit, Z. (2021). Stem cells and exosomes: New therapies for intervertebral disc degeneration. Cells 10, 2241. doi:10.3390/cells10092241
Kumar, M. N., Jacquot, F., and Hall, H. (2001). Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur. Spine J. 10, 309–313. doi:10.1007/s005860000207
Kwok, Z. H., Wang, C., and Jin, Y., (2021). Extracellular vesicle transportation and uptake by recipient cells: A critical process to regulate human diseases. Processes. 9 273. doi:10.3390/pr9020273
Lakstins, K., Arnold, L., Gunsch, G., Flanigan, D., Khan, S., Gadde, N., et al. (2021). Characterization of the human intervertebral disc cartilage endplate at the molecular, cell, and tissue levels. J. Orthop. Res. 39, 1898–1907. doi:10.1002/jor.24854
Larson, B. L., Ylostalo, J., Lee, R. H., Gregory, C., and Prockop, D. J. (2010). Sox11 is expressed in early progenitor human multipotent stromal cells and decreases with extensive expansion of the cells. Tissue Eng. Part A 16, 3385–3394. doi:10.1089/ten.tea.2010.0085
Lee, J. Y., and Kim, H. S. (2021). Extracellular vesicles in regenerative medicine: Potentials and challenges. Tissue Eng. Regen. Med. 18, 479–484. doi:10.1007/s13770-021-00365-w
Lener, T., Gimona, M., Aigner, L., Börger, V., Buzas, E., Camussi, G., et al. (2015). Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles 4, 30087. doi:10.3402/jev.v4.30087
Li, B., Yang, Y., Wang, L., and Liu, G. (2021). Stem cell therapy and exercise for treatment of intervertebral disc degeneration. Stem Cells Int. 2021, 1–10. doi:10.1155/2021/7982333
Li, S. R., Man, Q. W., Gao, X., Lin, H., Wang, J., Su, F. C., et al. (2021). Tissue-derived extracellular vesicles in cancers and non-cancer diseases: Present and future. J. Extracell. Vesicles 10, e12175. doi:10.1002/jev2.12175
Li, Z., Huang, Z., and Bai, L. (2021). Cell interplay in osteoarthritis. Front. Cell Dev. Biol. 9, 720477. doi:10.3389/fcell.2021.720477
Liang, H., Luo, R., Li, G., Zhang, W., Song, Y., and Yang, C. (2022). The proteolysis of ECM in intervertebral disc degeneration. Int. J. Mol. Sci. 23, 1715. doi:10.3390/ijms23031715
Liao, Z., Liu, H., Ma, L., Lei, J., Tong, B., Li, G., et al. (2021). Engineering extracellular vesicles restore the impaired cellular uptake and attenuate intervertebral disc degeneration. ACS Nano 15, 14709–14724. doi:10.1021/acsnano.1c04514
Liao, Z., Luo, R., Li, G., Song, Y., Zhan, S., Zhao, K., et al. (2019). Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 9, 4084–4100. doi:10.7150/thno.33638
Liu, L. T., Huang, B., Li, C. Q., Zhuang, Y., Wang, J., and Zhou, Y. (2011). Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PloS one 6, e26285. doi:10.1371/journal.pone.0026285e26285
Liu, W., Tang, P., Wang, J., Ye, W., Ge, X., Rong, Y., et al. (2021). Extracellular vesicles derived from melatonin-preconditioned mesenchymal stem cells containing USP29 repair traumatic spinal cord injury by stabilizing NRF2. J. Pineal Res. 71, e12769. doi:10.1111/jpi.12769e12769
Loibl, M., Wuertz-Kozak, K., Vadala, G., Lang, S., Fairbank, J., and Urban, J. P. (2019). Controversies in regenerative medicine: Should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine 2, e1043. doi:10.1002/jsp2.1043e1043
Luan, X., Sansanaphongpricha, K., Myers, I., Chen, H., Yuan, H., and Sun, D. (2017). Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 38, 754–763. doi:10.1038/aps.2017.12
Luo, L., Gong, J., Wang, Z., Liu, Y., Cao, J., Qin, J., et al. (2022). Injectable cartilage matrix hydrogel loaded with cartilage endplate stem cells engineered to release exosomes for non-invasive treatment of intervertebral disc degeneration. Bioact. Mater. 15, 29–43. doi:10.1016/j.bioactmat.2021.12.007
Luo, L., Gong, J., Zhang, H., Qin, J., Li, C., Zhang, J., et al. (2021). Cartilage endplate stem cells transdifferentiate into nucleus pulposus cells via autocrine exosomes. Front. Cell Dev. Biol. 9, 648201. doi:10.3389/fcell.2021.648201
Luo, L., Jian, X., Sun, H., Qin, J., Wang, Y., Zhang, J., et al. (2021). Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy. Stem cells Dayt. Ohio) 39, 467–481. doi:10.1002/stem.3322
Lyu, F. J. (2022), Impact of microenvironmental changes during degeneration on intervertebral disc progenitor cells: A comparison with mesenchymal stem cells. Bioengineering 9, 148. doi:10.3390/bioengineering9040148
Marimuthu, C., and Pushpa Rani, V. (2021). Elucidating the role of cell-mediated inflammatory cytokines on allogeneic mouse-derived nucleus pulposus mesenchymal stem cells. J. Food Biochem. 45, e13681. doi:10.1111/jfbc.13681e13681
Mezey, E. (2011). The therapeutic potential of bone marrow-derived stromal cells. J. Cell. Biochem. 112, 2683–2687. doi:10.1002/jcb.23216
Miao, Z., Jin, J., Chen, L., Zhu, J., Huang, W., Zhao, J., et al. (2006). Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 30, 681–687. doi:10.1016/j.cellbi.2006.03.009
Moeinabadi-Bidgoli, K., Babajani, A., Yazdanpanah, G., Farhadihosseinabadi, B., Jamshidi, E., Bahrami, S., et al. (2021). Translational insights into stem cell preconditioning: From molecular mechanisms to preclinical applications. Biomed. Pharmacother. 142, 112026. doi:10.1016/j.biopha.2021.112026
Momen-Heravi, F. (2017). Isolation of extracellular vesicles by ultracentrifugation. Methods Mol. Biology,Extracellular Vesicles, 25–32. doi:10.1007/978-1-4939-7253-1_3
Newell, N., Little, J. P., Christou, A., Adams, M. A., Adam, C. J., and Masouros, S. D. (2017). Biomechanics of the human intervertebral disc: A review of testing techniques and results. J. Mech. Behav. Biomed. Mater. 69, 420–434. doi:10.1016/j.jmbbm.2017.01.037
Novais, E. J., Choi, H., Madhu, V., Suyama, K., Anjo, S. I., Manadas, B., et al. (2021). Hypoxia and hypoxia-inducible factor-1α regulate endoplasmic reticulum stress in nucleus pulposus cells: Implications of endoplasmic reticulum stress for extracellular matrix secretion. Am. J. Pathol. 191, 487–502. doi:10.1016/j.ajpath.2020.11.012
Ohnuki, M., and Takahashi, K. (2015). Present and future challenges of induced pluripotent stem cells. Phil. Trans. R. Soc. B 370, 20140367. doi:10.1098/rstb.2014.0367
Oichi, T., Taniguchi, Y., Oshima, Y., Tanaka, S., and Saito, T. (2020). Pathomechanism of intervertebral disc degeneration. JOR Spine 3, e1076. doi:10.1002/jsp2.1076e1076
Ou, Y.-H., Liang, J., Czarny, B., Wacker, M. G., Yu, V., Wang, J.-W., et al. (2021). Extracellular Vesicle (EV) biohybrid systems for cancer therapy: Recent advances and future perspectives. Seminars cancer Biol. 74, 45–61. doi:10.1016/j.semcancer.2021.02.006
Peredo, A. P., Gullbrand, S. E., Smith, H. E., and Mauck, R. L. (2020). Putting the pieces in place: Mobilizing cellular players to improve annulus fibrosus repair. Tissue Eng. Part B Rev. 27, 295–312. doi:10.1089/ten.teb.2020.0196
Piffoux, M., Volatron, J., Cherukula, K., Aubertin, K., Wilhelm, C., Silva, A. K. A., et al. (2021). Engineering and loading therapeutic extracellular vesicles for clinical translation: A data reporting frame for comparability. Adv. Drug Deliv. Rev. 178, 113972. doi:10.1016/j.addr.2021.113972
Priyadarshani, P., Li, Y., and Yao, L. (2016). Advances in biological therapy for nucleus pulposus regeneration. Osteoarthr. Cartil. 24, 206–212. doi:10.1016/j.joca.2015.08.014
Qi, L., Wang, R., Shi, Q., Yuan, M., Jin, M., and Li, D. (2019). Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. J. Bone Min. Metab. 37, 455–466. doi:10.1007/s00774-018-0953-9
Qin, B., Hu, X.-m., Su, Z.-h., Zeng, X.-b., Ma, H.-y., and Xiong, K. (2021). Tissue-derived extracellular vesicles: Research progress from isolation to application. Pathology - Res. Pract. 226, 153604. doi:10.1016/j.prp.2021.153604
Ran, J., Hu, Y., Zheng, Z., Zhu, T., Zheng, H., Jing, Y., et al. (2015). Comparison of discectomy versus sequestrectomy in lumbar disc herniation: A meta-analysis of comparative studies. PloS one 10, e0121816. doi:10.1371/journal.pone.0121816e0121816
Rana, S., Yue, S., Stadel, D., and Zöller, M. (2012). Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 44, 1574–1584. doi:10.1016/j.biocel.2012.06.018
Rankin-Turner, S., Vader, P., O'Driscoll, L., Giebel, B., Heaney, L. M., and Davies, O. G. (2021). A call for the standardised reporting of factors affecting the exogenous loading of extracellular vesicles with therapeutic cargos. Adv. Drug Deliv. Rev. 173, 479–491. doi:10.1016/j.addr.2021.04.012
Raposo, G., and Stoorvogel, W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. doi:10.1083/jcb.201211138
Rider, M. A., Hurwitz, S. N., and Meckes, D. G. (2016). ExtraPEG: A polyethylene glycol-based method for enrichment of extracellular vesicles. Sci. Rep. 6, 23978. doi:10.1038/srep23978
Risbud, M. V., and Shapiro, I. M. (2014). Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 10, 44–56. doi:10.1038/nrrheum.2013.160
Sato, Y. T., Umezaki, K., Sawada, S., Mukai, S.-a., Sasaki, Y., Harada, N., et al. (2016). Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 6, 21933. doi:10.1038/srep21933
Scala, P., Rehak, L., Giudice, V., Ciaglia, E., Puca, A. A., Selleri, C., et al. (2021). Stem cell and macrophage roles in skeletal muscle regenerative medicine. Int. J. Mol. Sci. 22, 10867. doi:10.3390/ijms221910867
Shi, M., Zhao, Y., Sun, Y., Xin, D., Xu, W., and Zhou, B. (2021). Therapeutic effect of co-culture of rat bone marrow mesenchymal stem cells and degenerated nucleus pulposus cells on intervertebral disc degeneration. Spine J. 21, 1567–1579. doi:10.1016/j.spinee.2021.05.007
Song, Y., Li, S., Geng, W., Luo, R., Liu, W., Tu, J., et al. (2018). Sirtuin 3-dependent mitochondrial redox homeostasis protects against AGEs-induced intervertebral disc degeneration. Redox Biol. 19, 339–353. doi:10.1016/j.redox.2018.09.006
Staubach, S., Bauer, F. N., Tertel, T., Börger, V., Stambouli, O., Salzig, D., et al. (2021). Scaled preparation of extracellular vesicles from conditioned media. Adv. Drug Deliv. Rev. 177, 113940. doi:10.1016/j.addr.2021.113940
Sun, Y., Zhang, W., and Li, X. (2021). Induced pluripotent stem cell-derived mesenchymal stem cells deliver exogenous miR-105-5p via small extracellular vesicles to rejuvenate senescent nucleus pulposus cells and attenuate intervertebral disc degeneration. Stem Cell Res. Ther. 12, 286. doi:10.1186/s13287-021-02362-1
Sun, Z., Liu, B., and Luo, Z.-J. (2020). The immune privilege of the intervertebral disc: Implications for intervertebral disc degeneration treatment. Int. J. Med. Sci. 17, 685–692. doi:10.7150/ijms.42238
Sun, Z., Tang, X., Li, Q., Wang, H., Sun, H., and Tian, J. (2022). Mesenchymal stem cell extracellular vesicles-derived microRNA-194-5p delays the development of intervertebral disc degeneration by targeting TRAF6. Regen. Ther. 19, 88–96. doi:10.1016/j.reth.2021.12.001
Tang, R., Jing, L., Willard, V. P., Wu, C. L., Guilak, F., Chen, J., et al. (2018). Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells. Stem Cell Res. Ther. 9, 61. doi:10.1186/s13287-018-0797-1
Tang, S., Salazar-Puerta, A., Richards, J., Khan, S., Hoyland, J. A., Gallego-Perez, D., et al. (2021). Non-viral reprogramming of human nucleus pulposus cells with FOXF1 via extracellular vesicle delivery: An in vitro and in vivo study. Eur. Cell. Mat. 41, 90–107. doi:10.22203/ecm.v041a07
Tesovnik, T., Jenko Bizjan, B., Šket, R., Debeljak, M., Battelino, T., and Kovač, J. (2021). Technological approaches in the analysis of extracellular vesicle nucleotide sequences. Front. Bioeng. Biotechnol. 9, 787551. doi:10.3389/fbioe.2021.787551
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1461450. doi:10.1080/20013078.2018.1461450
Tian, D., Liu, J., Chen, L., Zhu, B., and Jing, J. (2020). The protective effects of PI3K/Akt pathway on human nucleus pulposus mesenchymal stem cells against hypoxia and nutrition deficiency. J. Orthop. Surg. Res. 15, 29. doi:10.1186/s13018-020-1551-9
Tian, X., Firsanov, D., Zhang, Z., Cheng, Y., Luo, L., Tombline, G., et al. (2019). SIRT6 is responsible for more efficient DNA double-strand break repair in long-lived species. Cell 177, 622–638.e22. doi:10.1016/j.cell.2019.03.043
Tran, C. M., Fujita, N., Huang, B.-L., Ong, J. R., Lyons, K. M., Shapiro, I. M., et al. (2013). Hypoxia-inducible factor (HIF)-1α and CCN2 form a regulatory circuit in hypoxic nucleus pulposus cells: CCN2 suppresses HIF-1α level and transcriptional activity. J. Biol. Chem. 288, 12654–12666. doi:10.1074/jbc.m112.448860
Tuteja, G., and Kaestner, K. H. (2007). SnapShot:Forkhead transcription factors I. Cell 130, 1160.e1–1160.e2. doi:10.1016/j.cell.2007.09.005
Urabe, F., Kosaka, N., Ito, K., Kimura, T., Egawa, S., and Ochiya, T. (2020). Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiology-Cell Physiology 318, C29–c39. doi:10.1152/ajpcell.00280.2019
van Niel, G., D'Angelo, G., and Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228. doi:10.1038/nrm.2017.125
Veerman, R. E., Güçlüler Akpinar, G., Eldh, M., and Gabrielsson, S. (2019). Immune cell-derived extracellular vesicles - functions and therapeutic applications. Trends Mol. Med. 25, 382–394. doi:10.1016/j.molmed.2019.02.003
Wang, C., Guo, S., Gu, Q., Wang, X., Long, L., Xiao, C., et al. (2022). Exosomes: A promising therapeutic strategy for intervertebral disc degeneration. Exp. Gerontol. 163, 111806. doi:10.1016/j.exger.2022.111806
Wang, G., Huang, K., Dong, Y., Chen, S., Zhang, J., Wang, J., et al. (2018). Lycorine suppresses endplate-chondrocyte degeneration and prevents intervertebral disc degeneration by inhibiting NF-κB signalling pathway. Cellular physiology and biochemistry : International journal of experimental cellular physiology, biochemistry. Cell. Physiol. biochem. 45, 1252–1269. doi:10.1159/000487457
Wang, H.-Q., Yu, X.-D., Liu, Z.-H., Cheng, X., Samartzis, D., Jia, L.-T., et al. (2011). Deregulated miR-155 promotes Fas-mediated apoptosis in human intervertebral disc degeneration by targeting FADD and caspase-3. J. Pathol. 225, 232–242. doi:10.1002/path.2931
Wang, P., Yuan, Y., Lin, W., Zhong, H., Xu, K., and Qi, X. (2019). Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 19, 295. doi:10.1186/s12935-019-1014-8
Wen, T., Wang, H., Li, Y., Lin, Y., Zhao, S., Liu, J., et al. (2021), Bone mesenchymal stem cell-derived extracellular vesicles promote the repair of intervertebral disc degeneration by transferring microRNA-199a. Cell cycle 20 256–270. doi:10.1080/15384101.2020.1863682
Wiklander, O. P., Nordin, J. Z., O'Loughlin, A., Gustafsson, Y., Corso, G., Mäger, I., et al. (2015). Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4, 26316. doi:10.3402/jev.v4.26316
Witwer, K. W., Van Balkom, B. W. M., Bruno, S., Choo, A., Dominici, M., Gimona, M., et al. (2019). Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 8, 1609206. doi:10.1080/20013078.2019.1609206
Wu, R., Soland, M., Liu, G., Shi, Y., Zhang, C., Tang, Y., et al. (2021). Functional characterization of the immunomodulatory properties of human urine-derived stem cells. Transl. Androl. Urol. 10, 3566–3578. doi:10.21037/tau-21-506
Xia, C., Zeng, Z., Fang, B., Tao, M., Gu, C., Zheng, L., et al. (2019). Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic. Biol. Med. 143, 1–15. doi:10.1016/j.freeradbiomed.2019.07.026
Xiang, H., Su, W., Wu, X., Chen, W., Cong, W., Yang, S., et al. (2020). Exosomes derived from human urine-derived stem cells inhibit intervertebral disc degeneration by ameliorating endoplasmic reticulum stress. Oxidative Med. Cell. Longev. 2020, 1–21. doi:10.1155/2020/6697577
Xiao, Q., Zhao, Z., Teng, Y., Wu, L., Wang, J., Xu, H., et al. (2022). BMSC-derived exosomes alleviate intervertebral disc degeneration by modulating AKT/mTOR-Mediated autophagy of nucleus pulposus cells. Stem Cells Int. 2022, 1–12. doi:10.1155/2022/9896444
Xin, J., Wang, Y., Zheng, Z., Wang, S., Na, S., and Zhang, S. (2022). Treatment of intervertebral disc degeneration. Orthop. Surg. 14, 1271–1280.
Xing, H., Zhang, Z., Mao, Q., Wang, C., Zhou, Y., Zhou, X., et al. (2021). Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J. Nanobiotechnology 19, 264. doi:10.1186/s12951-021-00991-5
Yang, F., Leung, V. Y., Luk, K. D., Chan, D., and Cheung, K. M. (2009). Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol. Ther. 17, 1959–1966. doi:10.1038/mt.2009.146
Yao, Z., Nie, L., Zhao, Y., Zhang, Y., Liu, Y., Li, J., et al. (2016). Salubrinal suppresses IL-17-induced upregulation of MMP-13 and extracellular matrix degradation through the NF-kB pathway in human nucleus pulposus cells. Inflammation 39, 1997–2007. doi:10.1007/s10753-016-0435-y
Ye, F., Lyu, F. J., Wang, H., and Zheng, Z. (2022). The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine 5, e1196. doi:10.1002/jsp2.1196e1196
Yong, T., Wang, D., Li, X., Yan, Y., Hu, J., Gan, L., et al. (2020). Extracellular vesicles for tumor targeting delivery based on five features principle. J. Control. Release 322, 555–565. doi:10.1016/j.jconrel.2020.03.039
Yu, X. J., Liu, Q. K., Lu, R., Wang, S. X., Xu, H. R., Wang, Y. G., et al. (2022). Bone marrow mesenchymal stem cell-derived extracellular vesicles carrying circ_0050205 attenuate intervertebral disc degeneration. Oxidative Med. Cell. Longev. 2022, 1–27. doi:10.1155/2022/8983667
Yu, X., Xu, H., Liu, Q., Wang, Y., Wang, S., Lu, R., et al. (2022). circ_0072464 shuttled by bone mesenchymal stem cell-secreted extracellular vesicles inhibits nucleus pulposus cell ferroptosis to relieve intervertebral disc degeneration. Oxidative Med. Cell. Longev. 2022, 1–21. doi:10.1155/2022/2948090
Yuan, Q., Wang, X., Liu, L., Cai, Y., Zhao, X., Ma, H., et al. (2020). Exosomes derived from human placental mesenchymal stromal cells carrying AntagomiR-4450 alleviate intervertebral disc degeneration through upregulation of ZNF121. Stem Cells Dev. 29, 1038–1058. doi:10.1089/scd.2020.0083
Yuan, X., Li, T., Shi, L., Miao, J., Guo, Y., and Chen, Y. (2021). Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Mol. Med. 27, 91. doi:10.1186/s10020-021-00355-7
Yuan, X., Yuan, W., Ding, L., Shi, M., Luo, L., Wan, Y., et al. (2021). Cell-adaptable dynamic hydrogel reinforced with stem cells improves the functional repair of spinal cord injury by alleviating neuroinflammation. Biomaterials 279, 121190. doi:10.1016/j.biomaterials.2021.121190
Zhang, B., Xu, L., Zhuo, N., and Shen, J. (2017). Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem. Biophys. Res. Commun. 493, 373–381. doi:10.1016/j.bbrc.2017.09.015
Zhang, G. Z., Liu, M. Q., Chen, H. W., Wu, Z. L., Gao, Y. C., Ma, Z. J., et al. (2021). NF-κB signalling pathways in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 54, e13057. doi:10.1111/cpr.13057e13057
Zhang, H., Zhu, T., Zhang, L., and Wu, Q. (2018). Stromal cell-derived factor-1 induces matrix metalloproteinase expression in human endplate chondrocytes, cartilage endplate degradation in explant culture, and the amelioration of nucleus pulposus degeneration in vivo. Int. J. Mol. Med. 41, 969–976. doi:10.3892/ijmm.2017.3278
Zhang, Q., Shen, Y., Zhao, S., Jiang, Y., Zhou, D., and Zhang, Y. (2021). Exosomes miR-15a promotes nucleus pulposus-mesenchymal stem cells chondrogenic differentiation by targeting MMP-3. Cell. Signal. 86, 110083. doi:10.1016/j.cellsig.2021.110083
Zhang, W., Sun, T., Li, Y., Yang, M., Zhao, Y., Liu, J., et al. (2022). Application of stem cells in the repair of intervertebral disc degeneration. Stem Cell Res. Ther. 13, 70. doi:10.1186/s13287-022-02745-y
Zhang, X., Zhang, H., Gu, J., Zhang, J., Shi, H., Qian, H., et al. (2021). Engineered extracellular vesicles for cancer therapy. Adv. Mat. 33, 2005709. doi:10.1002/adma.202005709
Zhang, Z., Zhang, L., Yang, J., Huang, J., Cai, J., Zhang, S., et al. (2021). Influence of extracellular nanovesicles derived from adipose-derived stem cells on nucleus pulposus cell from patients with intervertebral disc degeneration. Exp. Ther. Med. 22, 1431. doi:10.3892/etm.2021.10866
Zhao, X., Li, Q., Guo, Z., and Li, Z. (2021). Constructing a cell microenvironment with biomaterial scaffolds for stem cell therapy. Stem Cell Res. Ther. 12, 583. doi:10.1186/s13287-021-02650-w
Zheng, S. R., Guo, G. L., Zhai, Q., Zou, Z. Y., and Zhang, W. (2013). Effects of miR-155 antisense oligonucleotide on breast carcinoma cell line MDA-MB-157 and implanted tumors. Asian Pac. J. cancer Prev. 14, 2361–2366. doi:10.7314/apjcp.2013.14.4.2361
Zhu, G., Yang, X., Peng, C., Yu, L., and Hao, Y. (2020). Exosomal miR-532-5p from bone marrow mesenchymal stem cells reduce intervertebral disc degeneration by targeting RASSF5. Exp. Cell Res. 393, 112109. doi:10.1016/j.yexcr.2020.112109
Zhu, L., Shi, Y., Liu, L., Wang, H., Shen, P., and Yang, H. (2020), Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: Therapeutic potential for intervertebral disc degenerative diseases. Cell cycle 19 1727–1739. doi:10.1080/15384101.2020.1769301
Keywords: extracellular vesicles, intervertebral disc degeneration, mesenchymal stem cells, cartilage endplate stem cells, extracellular matrix
Citation: Xia Y, Yang R, Hou Y, Wang H, Li Y, Zhu J and Fu C (2022) Application of mesenchymal stem cell-derived exosomes from different sources in intervertebral disc degeneration. Front. Bioeng. Biotechnol. 10:1019437. doi: 10.3389/fbioe.2022.1019437
Received: 15 August 2022; Accepted: 26 September 2022;
Published: 07 October 2022.
Edited by:
Zhen Li, AO Research Institute, SwitzerlandCopyright © 2022 Xia, Yang, Hou, Wang, Li, Zhu and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changfeng Fu, ZnVjZkBqbHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.