- 1First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 2Department of Oncology, The First Affiliated Hospital of Xinxiang Medical University, Weihui, China
- 3Hemay Zhihui Science and Technology Co. Ltd, Tianjin, China
Endometrial injury is the main fact leading to infertility. Current treatments of endometrial injury present many problems, such as unable to achieve desired effects due to low retention and the inherent potential risk of injury. Besides, it is important to the development of bioinspired material that can mimic the natural tissue and possess native tissue topography. Hydrogel is a kind of bioinspired superhydrophilic materials with unique characteristics, such as excellent biocompatibility, biodegradability, porosity, swelling, and cross-linkage. These unique physiochemical properties of bioinspired hydrogels enable their promising application as novel delivery platform and alternative therapies for endometrial injury. In this mini review, we summarize the recent advances in bioinispred hydrogel-based delivery system for endometrial repair, including as a post-operative physical barrier and therapeutic delivery system. In addition, present status, limitations, and future perspectives are also discussed.
1 Introduction
Endometrium is the innermost layer of the uterus. This layer comprises epithelial and stroma components that are responsible for cell proliferation, differentiation, and shedding—which is deterministic of a woman’s viability for embryo implantation (Lessey, 2000). The normal endometrium can repair and regenerate itself and plays a pivotal role in female physiology and reproductive function. However, severe endocrine disorders, intrauterine infection and intrauterine surgery can lead to endometrial injury. When the uterine cavity lacks endometrial coverage, fibrosis, scarring and adhesion may occur, which result in decreased reception, loss, or reduced regenerative ability (Deans and Abbott, 2010; Liao et al., 2021; Lv et al., 2021), and those are the common cause of infertility worldwide (Ma et al., 2021).
Traditionally, there are three main treatments for endometrium repair, including surgical separation of the adherent endometrium, estrogen and cytokines therapy, and stem cell therapy (Yu et al., 2008; Evans-Hoeker and Young, 2014; Hooker et al., 2016)). For surgical treatment, the re-adhesion rate is high. For drug and stem cell therapy, the retention to the sites of endometrium is quite low (Abomaray et al., 2016; Liu et al., 2016; Xie et al., 2017; Mao et al., 2020). Moreover, the stem cells easily flow with blood and body fluids, making them difficult to be delivered to the damaged endometrium (Ma et al., 2021). Contrary, recent study shed light on the potential efficiency of constructed uterus-inspired niche for the efficient developmental events during the early stage of organogenesis (Gu et al., 2022). Knowledge of the above assertion prompts a need for a bio-material to provide a favorable environment for stem cell adhesion, controlled drug release and post-operation anti-adhesive barrier for effective repair of the endometrium.
Hydrogel is a kind of bioinspired superhydrophilic materials, which have similar compositional and structural features with natural tissues (Chen et al., 2021). Their polymeric three-dimentional (3D) network enables them to absorb and retain a significant amount of water. The water content of hydrogel can be as high as 90% (Zhang et al., 2019). Moreover, bioinspired hydrogel possesses excellent properties, such as high water absorbability, biocompatibility, low interfacial tension and degradability (Table 1). What’s more, the above mentional properties of bioinspired hydrogel can be tuned both physically and chemically, gaining great interests in a variety of applications such as tissue engineering, drug release, and 3D cell culture (Lim et al., 2014). All in all, hydrogel is a perfect platform for encapsulating and delivery of novel intrauterine therapies (Ma et al., 2018; Tang et al., 2018; Marycz et al., 2019), and serve as a promising biomaterial for endometrium repair (Townsend et al., 2019).
2 Application of bioinspired hydrogel in endometrium injury
Hydrogels have unique properties that make them enable for application in regenerative medicine. These properties include excellent biocompatibility and biodegradability, strong water-binding capacity, which enables them to double their size while swelling, an ability to incorporate therapeutic components for controlled release as well as, the ability to encapsulate seed cells at optimum physiological conditions (Wang et al., 2021). For endometrial injury, hydrogels are applied as a physical anti-adhesion barrier and as a delivery mechanism for therapeutics. The physical and biological properties of hydrogels enable them to influence the uterine microenvironment, biological behaviors, proliferation and angiogenesis. Swelling property makes hydrogel capable of compressing the bleeding point. The interactions between blood cells and the charged amino groups on the hydrogels confer aggregation, adhesion and blood clotting (Gu et al., 2010; Wen et al., 2016). The amino group, in association with the hydroxyl group of hydrogels also reacts with the oxygen free radicals to reduce the reactive oxygen-mediated damage of endometrium (Castro Marin et al., 2019; Wei et al., 2019). In addition, hydrogels can inhibit the release of TNF-α and IL-6 and increase the production of IL-8. These IL-8 induce an anti-inflammatory response on one hand and also combine with the neutral granulocytes surface receptors CXCR1/2 to produce active components against bacteria.
2.1 Application of bioinspired hydrogel as a post operative Physical Barrier
Conventional post-transcervical resection of adhesion (TCRA) anti-adhesion physical barriers such as intrauterine devices (IUD) and Foley catheter balloons pose the risk of recurring adhesion owing to a limited surface area (Bhandari et al., 2015; Chi et al., 2018). This implies that they are unable to cover the anterior and posterior walls of the uterus effectively. Another underlying issue is the risk of infection during their removal and their inability to promote pregnancy. Biomedical researches indicate the application of optimized hydrogels to be efficient in this regard (Xiao et al., 2015). Hyaluronic acid (HA) hydrogel is biocompatible, biodegradable as well as non-toxic to the cells and tissues. In fact, HA hydrogels were proven to reduce the risk of the reformation of intrauterine adhesions (IUAs) in women with an endometrial injury who undergo resection surgery (Can et al., 2018; Lee et al., 2020a; Tafti et al., 2021). However, studies found that hydrogel combined treatment strategy with the insertion of a urinary catheter or IUD had a more satisfactory effect in regaining an adhesion-free uterine cavity (Xiao et al., 2015; Li et al., 2019; Pabuccu et al., 2019). A meta-analysis indicated that Hyaluronic acid (HA) hydrogel platforms and chitosan platforms used after TCRA facilitate excellent tissue repair, and reduce adhesion recurrence but did not affect on postoperative pregnancy rate among patients (Fei et al., 2019). The latter is consistent with the research findings of Mao et al. (2020) which suggest HA hydrogels could enhance endometrial receptivity and clinical pregnancy rates in moderate IUA patients. Although many studies proved IUD combined with hydrogels reduce IUA recurrence, in some cases, the pregnancy rate remains lower in these patients. Furthermore, due to rapid degradation, it cannot stay for a prolonged duration inside the uterine cavity (Azumaguchi et al., 2019). Also, following hysteroscopic adhesiolysis, auto-cross-linked HA gel was found inefficient to reduce the recurrence rate of adhesion (Zhou et al., 2021b). This can be explained by, the use of concurrent adjuvant therapy which can mask the beneficial effect of hydrogels in reducing adhesion recurrence. Furthermore, the severity of pre-existing adhesions and the types of the gel used in different studies were also different. Whatever, the results suggesting the necessity of in-depth studies to explore the appropriate application of hydrogels to improve patients outcomes.
2.2 Application of bioinspired hydrogel as a Therapeutic Delivery System
Novel treatment emerged as a postoperative mechanism after TCRA and hysteroscopic adhesiolysis to facilitate the repair and regeneration of the endometrium and reduce the recurrence of adhesion. In the case of drug treatment for endometrial injuries, drugs may be administered via oral means, transvaginal or intravenous injections. Albeit, complications resulting from these drug administration methods, such as damage to liver and blood tissues (Lin et al., 2020), claim the requirement of other efficient modes of delivery of the drugs to the injured site. Also, stem therapy options facilitated the possibility of substitution or replacement of injured cells to aid in endometrium regeneration (Tan et al., 2016; Gan et al., 2017; Yin et al., 2019). Both therapies result in limited therapeutic effect due to the low retention to the sites of injury (Abomaray et al., 2016). For optimum therapeutic effect, the materials (drug or 3D stem cells) should be delivered and retained to the injured site to prevent bacterial infection. Hydrogel used in endometrial regeneration and repair is highly biocompatible, biodegradable and has a porous structure for encapsulation as well as the sustained release of materials. Therapeutic materials to be delivered include; estrogen, cytokines, stem cells and exosomes.
2.2.1 Application of Hydrogel as a Drug Delivery System
Hydrogel presents distinctive properties that interest its application for drug delivery in the repair and regeneration of endometrial injury. First, the porous structure enables the loading and control release of treatment drugs. Drugs such as β-estradiol can be delivered by hydrogel scaffolds for various purposes including endometrial regeneration (Zhang et al., 2017). Furthermore, the use of stimuli-responsive hydrogels brings many possibilities to drug delivery systems. Poloxamer hydrogel is a thermosensitive hydrogel with better fluidity. When optimized via a polycondensation reaction into heparin-modified poloxamer hydrogel, the half-life of its growth factors is extended and fluidity improved. It covers the injured site completely and perfectly becomes solid at equilibrium temperature (normal body temperature). This prevents bacterial infection and loss of the drug. Thus, injecting the thermosensitive hydrogel (E2-HPhydrogel) with the encapsulation of 17β-estradiol into the injured uterine cavity eliminates the weaknesses of exogenous administration of 17β-estradiol including water solubility, limited half-life time, and low concentrations at the injured area (Baghersad et al., 2018). Hormones such as estrogen, cytokine, exosomes, etc. could also be delivered efficiently and effectively while capitalizing on hydrophilic polymer chain linkage in hydrogels. With regards to estrogen release, the application of poloxamer hydrogel as a carrier does not prolong retention time. Aloe has traits that make it an ideal organic component to mix with poloxamer to form a more biologically friendly thermosensitive hydrogel system (Baghersad et al., 2018; Yao et al., 2020). Aloe-poloxamer (AP) hybrid hydrogel has been fabricated for treating endometrial injury and achieved a better therapeutic effect with a prolonged retention time (Figure 1A). Yao et al. designed a nano-composite aloe/poloxamer hydrogel containing E2 with an additional benefit to enhance the therapeutic effects of estrogen on endometrial regeneration by upregulating estrogen receptors, reducing the likelihood that high-dose estrogen would increase the risk of thrombosis and malignancy. Hydrogel as drug delivery system may be applied as in situ vaginal administration in a low viscous form at room temperature, further functionalization, such as with amino group, of poloxamer-based hydrogel prolong intravaginal residence (Figure 1B). Studies suggested that the large surface area and high vascularized nature of the vaginal area enable excellent drug absorption (Liu et al., 2017b; Ci et al., 2017). Again, vaginal administration has no first-pass effect and has low enzyme activity; therefore, drugs can perform a localized activity, and can also enter the systemic circulation. When thermosensitive hydrogel is administered vaginally, the hydrogel spreads rapidly into the folded area of the vaginal mucosa and forms a gel at body temperature (Andrzejewska et al., 2019). Drug delivery system through injectable hydrogels, as another option, provides other benefits including shortened duration of treatment, decreased risk of infection and prevention of scarring (Asai et al., 2012).
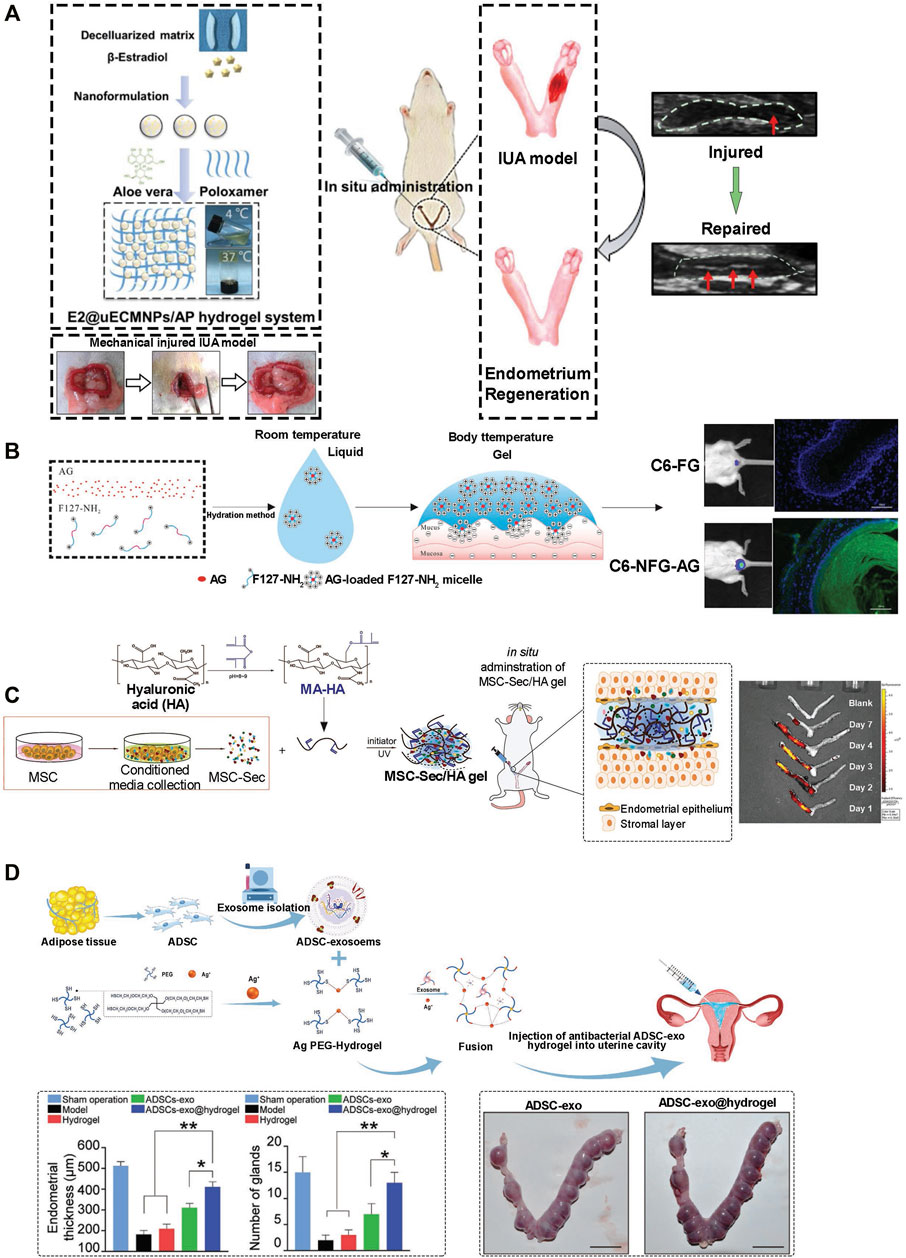
FIGURE 1. Application of hydrogel in endometrium injury. (A) E2@uECMNPs/AP hydrogel system for B-estradiol. Following establishment of rat intrauterine adhesion (IUA) model, and treatment the theragnostic ultrasound test was employed to compare the images of the injured IUA with or without E2@uECMNPs treatment (Yao et al., 2020). (B) Poloxamer 407 (F127)-based in situ hydrogel for the delivery of acetate gossypol (AG) as a model drug. Intravaginal retention of NFG (acetate gossypol-loaded aminated poloxamer 407-based temperature-sensitive hydrogel) and FG (F127 gel) was evaluated. Photographs and fluorescence microscopy showed NFG prolonged intravaginal residence (Ci et al., 2017). (C) HA-Hydrogel integrated with mesenchymal stem cell (MSC) to treat endometrial injury in a rat model. Schematic showing the synthesis of MSC-Sec-loaded, crosslinked HA gel. MSC-Sec/HA gel was injected in rodent model of endometrium injury and ex vivo fluorescent imaging of rat uteri showed the crosslinked HA can stay in the uterine cavity for roughly two estrous cycles (Liu et al., 2019). (D) Schematic overview of the development of an exosome secreted by adipose-derived stem cell (ADSC-exo) hydrogel for endometrial regeneration. Increase of endothelial thickness with a concomitant increase of gland numbers affirmed the ability of ADSC-exo hydrogel to promote tissue regeneration. Beside, no significantly higher pregnancy and implantation rates in the ADSC-exo and ADSC-exo hydrogel groups evidant normal endometrial formation and function (Lin et al., 2021).
2.2.2 Application of bioinspired Hydrogel as a Three-dimensional Cell Delivery and Culture System
Recent advances in the treatment of endometrium injury suggest cell therapy as a promising alternative to drug therapy. For example, Yi et al. stated that similar to normal rats, rats with injured uterus achieve nearly complete recovery, following treatment with uterus-derived extracellular matrix and seeded chorionic villi mesenchymal stem cells-combined reconstructable uterus-derived materials (RUMs) (Yi et al., 2022). Transplanting Mesenchymal Stem Cells (MSC) via hydrogel as a delivery system showed good potential for endometrial repair (Figure 1C) (Nelson et al., 2009). There have been several sources of stem cells that have been proposed for tissue regeneration. Bone marrow-derived MSC (BM-MSC) has been the most promising cell source of regeneration due to ease in acquisition, self-renewal ability, multi potential differentiation and weak immunogenicity (Ding et al., 2014; Zhou et al., 2021a). Other sources of stem cells include; Human umbilical cord mesenchymal stem cell (UCMSC), Endometrial stromal cell (EMSC), Endometrial perivascular cell (ENPSC) and Bone mononuclear cell (BMNC). Interestingly, hydrogels can be designed as culture systems with a tunable stiffness that encompasses a physiological range owing to their distinguishable properties. Normally hydrogels used for such applications have high-water content, excellent porosity and soft consistency. Also, such hydrogels can closely simulate natural living tissue. Collagen, a natural hydrogel, is widely used in conjunction with the BM-MSC for healing wounds and tissue regeneration as evidence shown in a research study by Ding et al. (Liu et al., 2017a). Again, other synthetic hydrogels used in conjunction with the stem cells include Pluronic F-127 (PF-127). However, it has been found that these synthetic models may have toxic side effects on the cells and thus, there is a need for antioxidants within the scaffold. Commonly, Vitamin C reduces the cytotoxic effect of PF127, and promotes the survival and growth of cells by influencing ECM and collagen homeostasis, exerting anti-inflammatory functions by downregulating the secretion of proinflammatory cytokines such as TNF-a and interleukin-6 (IL-6). Another high-quality carrier for BM-MSC is the photo-crosslinked PRP hydrogel (HNPRP) (Wenbo et al., 2020). The material can be quickly prepared in situ to form a strong scaffold and was demonstrated to achieve controlled release of growth factors and reduce tissue adhesion. All the above exogenous substances have potential safety problems such as immunoreaction risk. As such other research studies choose endogenous stem cell migration (Han et al., 2016). In situ delivery of stromal cell derivative-1α (SDF-1α) in a controlled release could accelerate the regeneration of multiple tissues.
2.2.3 Application of bioinspired Hydrogel as Exosome Delivery System
Exosomes are extracellular vesicles with a size range of 40–160 nm in diameter. Exosomes, both on their surfaces or inside of them, mainly contain cargo, such as lipids, proteins and nucleic acids, including DNA, mRNA and miRNA (Mathivanan et al., 2010; D'Asti et al., 2012). Under normal physiological conditions, exosomes can originate from endothelial cells, immune cells, tumor cells and mesenchymal stem cells (MSCs) and mimic the extracellular matrix or act as regulators of intercellular communication and immune response (Huang et al., 2021). Exosomes from MSCs are capable of functions similar to MSCs including tissue regeneration and repair (Askenase, 2020). However, exosomes showed several advantages over MSCs, including reduced risk of immune rejection and malignant growth, longer stability and readily circulatory capacity through capillaries (Wang et al., 2021). Owing to all these advantageous characteristics which make exosomes a current research hotspot, the use of purified exosomes can be limited by their rapid clearance from the host after being absorbed by the endothelial system. This limitation can be overcome by hydrogels that can protect exosomes acting as a carrier and delivery depot at the target site to achieve well-regulated cellular secretions more stable therapeutic effect (Figure 1D). Because of the unique physiochemical characteristic of hydrogel and the ability to controlled-release its embedded molecule, encapsulation of exosomes with hydrogel provide an outstanding candidate in plenty of treatments including bone and cartilage regeneration, cardiovascular diseases, spinal cord injury, periodontal and corneal repairs (Xie et al., 2022). Exosome has immense potential of treating injured endometrium by regulating EMT (Yao et al., 2019; Feng et al., 2020), miRNA (Tan et al., 2020; Shi et al., 2021), proliferation (Lv et al., 2020) and endometrium angiogenesis (Zhang et al., 2022). However, all these effects are achieved via the suboptimal approach of direct injection which restricts the activity of exosomes by local tissue irritation or reduced self-life. The ideal repair of endometrium by exosomes can be achieved by using the biocompatibility and physically tunable properties of hydrogels. The exosome-hydrogel system proved to be a safe, noninvasive and convenient method for repairing injured endometrium and can exert excellent effects by promoting angiogenesis, inhibiting local tissue fibrosis and increasing endometrial receptivity (Lin et al., 2021). Although the results were promising, the exosome-hydrogel also has the disadvantage that the bioscaffolds lack the native tissue topography to mimic the natural tissues.
3 Present status and future outlook
Although the damaged endometrium cells can be self-healing and self-regenerated in patients, treatment options such as drugs (estrogen, cytokines, 17β-estradiol, cytokines, etc.) and stem cell therapy aid in the repair and regenerative process. However, it has been found that these therapies usually have low retention to the sites of injury and thus, the desired therapeutic effect is not achieved (Zhao et al., 2021). Bioinspired hydrogel is a 3D hydrophilic polymer with excellent biocompatibility and biodegradability features. It have been identified as having the ability to transport and deliver these therapeutics to the sites of injury owing to their porous, biodegradable and biocompatible features. Coupled with its other distinct features, the material finds varied applications in endometrial regeneration and repair. It serves as an excellent anti-adhesion physical barrier, drug delivery system, three-dimensional cell delivery, and exosome delivery system. While multiresponsive hydrogels that respond to redox, pH and temperature are being proved effective in physiological environments (Lou et al., 2015). Hence, the development of multiresponsive hydrogels to adapt to the intrauterine environment is a future meaningful subject. In addition to physiologically relevant stimuli, the response of these polymers needs to measure in the presence of prevalent biomedical external stimuli such as magnetic field and UV light. To develop an innovative delivery system with excellent biocompatibility, the future direction of research needs compilation of the responsive properties of hydrogels in nearly every conceivable arrangement and minimize the synthesis complexity of these multi-responsive hydrogels. Hydrogels may be optimized to be sensitive to physiological environmental changes to facilitate their specificity and controlled release of treatment. This demonstrates the potential benefits of the encapsulation of cells or other therapeutic substances and underlines the importance of hydrogels in endometrial repair and regeneration. However, to achieve desired and effective therapeutic effects, it is recommended that future studies consider the extending application of multiresponsive hydrogel delivery system to endometrial injury treatment.
The hydrophobic and electrostatic interactions in hydrogels aid the supramolecular assembly of amphiphiles that hold a large amount of water. As a result, hydrogels are potential drug reservoirs and capable of maintaining slow and sustained release. Utilizing this property, researches entrapped two or more drugs in a biodegradable hydrogel to produce a synergistic effect against different ailments including cancer (Medatwal et al., 2020). Therefore, optimization of hydrogels by using rational molecular design to carry multiple drugs for emdometrium regeneration should be considered as this would improve the overall repair and regenerative process of the injured endometrium (An et al., 2021). This will aid in resolving infertility in patients.
Gene editing technology is considered as the driver of modifying genes in fundamental research and facilitating gene therapies in clinical developments. The invention of CRISPR (clustered regularly interspaced short palindromic repeats) Cas (CRISPR-associated) toolbox is a remarkable breakthrough for gene editing. The system contains a complex of the guide RNA (gRNA) that recognizes its complementary target DNA and the Cas nuclease that cut the dsDNA (Knott and Doudna, 2018; Es et al., 2019). Though the system is simple and flexible, the efficiency for targeted delivery of both gRNA and Cas9 needs thoughtful consideration (Lindsay-Mosher and Su, 2016). Biocompatible DNA hydrogels, harboring the crosslinked structure and programmable property, can be harnessed for the targeted transport of CRISPR biomaterials. Although gene therapy is not common for IUA, future research may be directed to using a CRISPR-DNA hydrogel system for the improvement of endometrium recovery. In recent years, bioprinting has gained significant attention not only for the fabrication of biomimetic tissue constructs but also in pharmaceuticals, improving drug screening, disease research, and controlled drug-delivery systems. Hydrogels, being the most common bioink for bioprinting, provide a supportive hydrated environment for cells and preserve the shape of printed materials (Wang et al., 2017). Through various crosslinking, hydrogels are capable of achieving different tissue engineering applications as bioinks and support baths (Zhou et al., 2022). Hydrogel bioinks that can respond to external stimuli, such as gelatin methacrylate (GelMA) polymer, photo-cross linked HA-hydrogels or polyethylene glycol and alginate hydrogel, proved to be outstanding materials for 3D bioprinting of human organoid including vascularized soft tissues, cardiac muscle, cartilage-like tissue constructs and components of the human heart (de Melo et al., 2019; Lee et al., 2019; Lee et al., 2020b; Kupfer et al., 2020). On the other hand, cell-laden hydrogels are the most frequently used and attractive choice to mimic the native niche (Mirdamadi et al., 2019; Spencer et al., 2019; Mancha Sanchez et al., 2020). Possible applications of cross-linked hydrogels as bioink of bath for bioprinting in IUA are foreseen for the repair of damaged endometrium, regenerative drugs or cell delivery. However, bioinspired hydrogels cannot become the first-line therapy in endometrial regeneration mainly because, the use of hydrogels as a single without a traditional IUD anti-adhesion barrier is less satisfactory. Increasing the mechanical strength can make hydrogel capable to meet the requirement of a culture system for the formation of normal functional endometrium for embryo implantation. In addition, for the clinically relevant applications, focus is needed toward possible chemical crosslinking and mechanical intigrety to withstand the conventional sterilization methods as terminal sterilization is difficult and time-consuming because of the hydrated nature of hydrogels (Lima et al., 2020). All these questions are still worth the continued efforts in the future.
Author contributions
RD, SM, and XZ prepared the manuscript, BW, MR, and LY prepared the Figures and Tables, TX and YL revised the manuscript, All authors approve the version of the manuscript to be published.
Funding
This work was financially supported by the Joint Fund Project of Xinxiang Medical University (No. LHGJ20200505).
Acknowledgments
We would like to thank Lily Zhang for her kindly support.
Conflict of interest
LY was employed by Hemay Zhihui Science and Technology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abomaray, F. M., Al Jumah, M. A., Alsaad, K. O., Jawdat, D., Al Khaldi, A., Alaskar, A. S., et al. (2016). Phenotypic and functional characterization of mesenchymal stem/multipotent stromal cells from decidua basalis of human term placenta. Stem Cells Int. 2016, 5184601–5184618. doi:10.1155/2016/5184601
An, H. W., Mamuti, M., Wang, X., Yao, H., Wang, M. D., Zhao, L., et al. (2021). “Rationally designed modular drug delivery platform based on intracellular peptide self‐assembly,” in Exploration (Wiley Online Library). 20210153.
Andrzejewska, A., Lukomska, B., and Janowski, M. (2019). Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells 37, 855–864. doi:10.1002/stem.3016
Asai, D., Xu, D., Liu, W., Garcia Quiroz, F., Callahan, D. J., Zalutsky, M. R., et al. (2012). Protein polymer hydrogels by in situ, rapid and reversible self-gelation. Biomaterials 33, 5451–5458. doi:10.1016/j.biomaterials.2012.03.083
Askenase, P. W. (2020). COVID-19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: Do the exosomes in convalescent plasma antagonize the weak immune antibodies? J. Extracell. Vesicles 10, e12004. doi:10.1002/jev2.12004
Azumaguchi, A., Henmi, H., and Saito, T. (2019). Efficacy of silicone sheet as a personalized barrier for preventing adhesion reformation after hysteroscopic adhesiolysis of intrauterine adhesions. Reprod. Med. Biol. 18, 378–383. doi:10.1002/rmb2.12294
Baghersad, S., Hajir Bahrami, S., Mohammadi, M. R., Mojtahedi, M. R. M., and Milan, P. B. (2018). Development of biodegradable electrospun gelatin/aloe-vera/poly(ε‑caprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Mater. Sci. Eng. C 93, 367–379. doi:10.1016/j.msec.2018.08.020
Bhandari, S., Bhave, P., Ganguly, I., Baxi, A., and Agarwal, P. (2015). Reproductive outcome of patients with asherman's syndrome: A saims experience. J. Reprod. Infertil. 16, 229–235.
Can, S., Kirpinar, G., Dural, O., Karamustafaoglu, B. B., Tas, I. S., Yasa, C., et al. (2018). Efficacy of a new crosslinked hyaluronan gel in the prevention of intrauterine adhesions. JSLS 22, e201800036. doi:10.4293/jsls.2018.00036
Castro Marin, A., Culcasi, M., Cassien, M., Stocker, P., Thetiot-Laurent, S., Robillard, B., et al. (2019). Chitosan as an antioxidant alternative to sulphites in oenology: EPR investigation of inhibitory mechanisms. Food Chem. x. 285, 67–76. doi:10.1016/j.foodchem.2019.01.155
Chen, Z., Lv, Z., Zhang, Z., Weitz, D. A., Zhang, H., Zhang, Y., et al. (2021). “Advanced microfluidic devices for fabricating multi‐structural hydrogel microsphere,” in Exploration (Wiley Online Library). 20210036.
Chi, Y., He, P., Lei, L., Lan, Y., Hu, J., Meng, Y., et al. (2018). Transdermal estrogen gel and oral aspirin combination therapy improves fertility prognosis via the promotion of endometrial receptivity in moderate to severe intrauterine adhesion. Mol. Med. Rep. 17, 6337–6344. doi:10.3892/mmr.2018.8685
Ci, L., Huang, Z., Liu, Y., Liu, Z., Wei, G., and Lu, W. (2017). Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: Preparation, characterization and application in a vaginal drug delivery system. Acta Pharm. Sin. B 7, 593–602. doi:10.1016/j.apsb.2017.03.002
D'asti, E., Garnier, D., Lee, T. H., Montermini, L., Meehan, B., and Rak, J. (2012). Oncogenic extracellular vesicles in brain tumor progression. Front. Physiol. 3, 294. doi:10.3389/fphys.2012.00294
De Melo, B. a. G., Jodat, Y. A., Mehrotra, S., Calabrese, M. A., Kamperman, T., Mandal, B. B., et al. (2019). 3D printed tissues: 3D printed cartilage‐like tissue constructs with spatially controlled mechanical properties (adv. Funct. Mater. 51/2019). Adv. Funct. Mat. 29, 1970350. doi:10.1002/adfm.201970350
Deans, R., and Abbott, J. (2010). Review of intrauterine adhesions. J. Minim. Invasive Gynecol. 17, 555–569. doi:10.1016/j.jmig.2010.04.016
Ding, L., Li, X., Sun, H., Su, J., Lin, N., Peault, B., et al. (2014). Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials 35, 4888–4900. doi:10.1016/j.biomaterials.2014.02.046
Es, I., Gavahian, M., Marti-Quijal, F. J., Lorenzo, J. M., Mousavi Khaneghah, A., Tsatsanis, C., et al. (2019). The application of the CRISPR-Cas9 genome editing machinery in food and agricultural science: Current status, future perspectives, and associated challenges. Biotechnol. Adv. 37, 410–421. doi:10.1016/j.biotechadv.2019.02.006
Evans-Hoeker, E. A., and Young, S. L. (2014). Endometrial receptivity and intrauterine adhesive disease. Semin. Reprod. Med. 32, 392–401. doi:10.1055/s-0034-1376358
Fei, Z., Bin, Z., Xin, X., Fei, H., and Yuechong, C. (2019). Meta-analysis on the use of hyaluronic acid gel to prevent recurrence of intrauterine adhesion after hysteroscopic adhesiolysis. Taiwan. J. Obstet. Gynecol. 58, 731–736. doi:10.1016/j.tjog.2019.09.002
Feng, Y., Zhan, F., Zhong, Y., and Tan, B. (2020). Effects of human umbilical cord mesenchymal stem cells derived from exosomes on migration ability of endometrial glandular epithelial cells. Mol. Med. Rep. 22, 715–722. doi:10.3892/mmr.2020.11137
Gan, L., Duan, H., Xu, Q., Tang, Y. Q., Li, J. J., Sun, F. Q., et al. (2017). Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy 19, 603–616. doi:10.1016/j.jcyt.2017.02.003
Gu, R., Sun, W., Zhou, H., Wu, Z., Meng, Z., Zhu, X., et al. (2010). The performance of a fly-larva shell-derived chitosan sponge as an absorbable surgical hemostatic agent. Biomaterials 31, 1270–1277. doi:10.1016/j.biomaterials.2009.10.023
Gu, Z., Guo, J., Zhai, J., Feng, G., Wang, X., Gao, Z., et al. (2022). A uterus-inspired niche drives blastocyst development to the early organogenesis. Adv. Sci., e2202282. doi:10.1002/advs.202202282
Han, Y., Liu, S., Mao, H., Tian, L., and Ning, W. (2016). Synthesis of novel temperature- and pH-sensitive ABA triblock copolymers P(DEAEMA-co-MEO(2)MA-co-OEGMA)-b-PEG-b-P(DEAEMA-co-MEO(2)MA-co-OEGMA): Micellization, Sol(-)Gel transitions, and sustained BSA release. Polym. (Basel) 8, 367. doi:10.3390/polym8110367
Hooker, A., Fraenk, D., Brolmann, H., and Huirne, J. (2016). Prevalence of intrauterine adhesions after termination of pregnancy: A systematic review. Eur. J. Contracept. Reprod. Health Care 21, 329–335. doi:10.1080/13625187.2016.1199795
Huang, J., Xiong, J., Yang, L., Zhang, J., Sun, S., and Liang, Y. (2021). Cell-free exosome-laden scaffolds for tissue repair. Nanoscale 13, 8740–8750. doi:10.1039/d1nr01314a
Knott, G. J., and Doudna, J. A. (2018). CRISPR-Cas guides the future of genetic engineering. Science 361, 866–869. doi:10.1126/science.aat5011
Kupfer, M. E., Lin, W. H., Ravikumar, V., Qiu, K., Wang, L., Gao, L., et al. (2020). In situ expansion, differentiation, and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ. Res. 127, 207–224. doi:10.1161/circresaha.119.316155
Lee, A., Hudson, A. R., Shiwarski, D. J., Tashman, J. W., Hinton, T. J., Yerneni, S., et al. (2019). 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487. doi:10.1126/science.aav9051
Lee, D. Y., Lee, S. R., Kim, S. K., Joo, J. K., Lee, W. S., Shin, J. H., et al. (2020a). A new thermo-responsive hyaluronic acid sol-gel to prevent intrauterine adhesions after hysteroscopic surgery: A randomized, non-inferiority trial. Yonsei Med. J. 61, 868–874. doi:10.3349/ymj.2020.61.10.868
Lee, S., Sani, E. S., Spencer, A. R., Guan, Y., Weiss, A. S., and Annabi, N. (2020b). Human-recombinant-elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv. Mat. 32, e2003915. doi:10.1002/adma.202003915
Lessey, B. A. (2000). The role of the endometrium during embryo implantation. Hum. Reprod. 15 (6), 39–50.
Li, X., Wu, L., Zhou, Y., Fan, X., Huang, J., Wu, J., et al. (2019). New crosslinked hyaluronan gel for the prevention of intrauterine adhesions after dilation and curettage in patients with delayed miscarriage: A prospective, multicenter, randomized, controlled trial. J. Minim. Invasive Gynecol. 26, 94–99. doi:10.1016/j.jmig.2018.03.032
Liao, Z., Liu, C., Wang, L., Sui, C., and Zhang, H. (2021). Therapeutic role of mesenchymal stem cell-derived extracellular vesicles in female reproductive diseases. Front. Endocrinol. 12, 665645. doi:10.3389/fendo.2021.665645
Lim, H. L., Hwang, Y., Kar, M., and Varghese, S. (2014). Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2, 603–618. doi:10.1039/c3bm60288e
Lima, C. S. A., Balogh, T. S., Varca, J., Varca, G. H. C., Lugao, A. B., Bucio, E., et al. (2020). An updated review of macro, micro, and nanostructured hydrogels for biomedical and pharmaceutical applications. Pharmaceutics 12, 970. doi:10.3390/pharmaceutics12100970
Lin, J., Wang, Z., Huang, J., Tang, S., Saiding, Q., Zhu, Q., et al. (2021). Fertility restoration: Microenvironment‐protected exosome‐hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring (small 11/2021). Small 17, 2170049. doi:10.1002/smll.202170049
Lin, Y., Dong, S., Zhao, W., Hu, K. L., Liu, J., Wang, S., et al. (2020). Application of hydrogel-based delivery system in endometrial repair. ACS Appl. Bio Mat. 3, 7278–7290. doi:10.1021/acsabm.0c00971
Lindsay-Mosher, N., and Su, C. (2016). Cancer gene therapy: Innovations in therapeutic delivery of CRISPR-cas9. Drug Discov. Today Dis. Models 21, 17–21. doi:10.1016/j.ddmod.2017.02.009
Liu, A. Z., Zhao, H. G., Gao, Y., Liu, M., and Guo, B. Z. (2016). Effectiveness of estrogen treatment before transcervical resection of adhesions on moderate and severe uterine adhesion patients. Gynecol. Endocrinol. 32, 737–740. doi:10.3109/09513590.2016.1160375
Liu, F., Hu, S., Yang, H., Li, Z., Huang, K., Su, T., et al. (2019). Hyaluronic acid hydrogel integrated with mesenchymal stem cell-secretome to treat endometrial injury in a rat model of asherman's syndrome. Adv. Healthc. Mat. 8, e1900411. doi:10.1002/adhm.201900411
Liu, X., Yang, Y., Niu, X., Lin, Q., Zhao, B., Wang, Y., et al. (2017a). An in situ photocrosslinkable platelet rich plasma - complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 62, 179–187. doi:10.1016/j.actbio.2017.05.023
Liu, Y., Yang, F., Feng, L., Yang, L., Chen, L., Wei, G., et al. (2017b). In vivo retention of poloxamer-based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 7, 502–509. doi:10.1016/j.apsb.2017.03.003
Lou, S., Gao, S., Wang, W., Zhang, M., Zhang, J., Wang, C., et al. (2015). Galactose-functionalized multi-responsive nanogels for hepatoma-targeted drug delivery. Nanoscale 7, 3137–3146. doi:10.1039/c4nr06714b
Lv, C. X., Duan, H., Wang, S., Gan, L., and Xu, Q. (2020). Exosomes derived from human umbilical cord mesenchymal stem cells promote proliferation of allogeneic endometrial stromal cells. Reprod. Sci. 27, 1372–1381. doi:10.1007/s43032-020-00165-y
Lv, H., Wu, B., Song, J., Wu, W., Cai, W., and Xu, J. (2021). Hydrogel, a novel therapeutic and delivery strategy, in the treatment of intrauterine adhesions. J. Mat. Chem. B 9, 6536–6552. doi:10.1039/d1tb01005k
Ma, J., Zhan, H., Li, W., Zhang, L., Yun, F., Wu, R., et al. (2021). Recent trends in therapeutic strategies for repairing endometrial tissue in intrauterine adhesion. Biomater. Res. 25, 40. doi:10.1186/s40824-021-00242-6
Ma, Y., Lin, M., Huang, G., Li, Y., Wang, S., Bai, G., et al. (2018). 3D spatiotemporal mechanical microenvironment: A hydrogel-based platform for guiding stem cell fate. Adv. Mat. 30, e1705911. doi:10.1002/adma.201705911
Mancha Sanchez, E., Gomez-Blanco, J. C., Lopez Nieto, E., Casado, J. G., Macias-Garcia, A., Diaz Diez, M. A., et al. (2020). Hydrogels for bioprinting: A systematic review of hydrogels synthesis, bioprinting parameters, and bioprinted structures behavior. Front. Bioeng. Biotechnol. 8, 776. doi:10.3389/fbioe.2020.00776
Mao, X., Zhang, J., Cai, R., Tao, Y., Gao, H., Kuang, Y., et al. (2020). Therapeutic role of granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with persistent thin endometrium: A prospective and randomized study. Int. J. Gynecol. Obstet. 150, 194–199. doi:10.1002/ijgo.13152
Marycz, K., Smieszek, A., Trynda, J., Sobierajska, P., Targonska, S., Grosman, L., et al. (2019). Nanocrystalline hydroxyapatite loaded with resveratrol in colloidal suspension improves viability, metabolic activity and mitochondrial potential in human adipose-derived mesenchymal stromal stem cells (hASCs). Polym. (Basel) 11, 92. doi:10.3390/polym11010092
Mathivanan, S., Ji, H., and Simpson, R. J. (2010). Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics 73, 1907–1920. doi:10.1016/j.jprot.2010.06.006
Medatwal, N., Ansari, M. N., Kumar, S., Pal, S., Jha, S. K., Verma, P., et al. (2020). Hydrogel-mediated delivery of celastrol and doxorubicin induces a synergistic effect on tumor regression via upregulation of ceramides. Nanoscale 12, 18463–18475. doi:10.1039/d0nr01066a
Mirdamadi, E., Muselimyan, N., Koti, P., Asfour, H., and Sarvazyan, N. (2019). Agarose slurry as a support medium for bioprinting and culturing freestanding cell-laden hydrogel constructs. 3D Print. Addit. Manuf. 6, 158–164. doi:10.1089/3dp.2018.0175
Nelson, T. J., Behfar, A., Yamada, S., Martinez-Fernandez, A., and Terzic, A. (2009). Stem cell platforms for regenerative medicine. Clin. Transl. Sci. 2, 222–227. doi:10.1111/j.1752-8062.2009.00096.x
Pabuccu, E. G., Kovanci, E., Sahin, O., Arslanoglu, E., Yildiz, Y., and Pabuccu, R. (2019). New crosslinked hyaluronan gel, intrauterine device, or both for the prevention of intrauterine adhesions. JSLS 23, e201800108. doi:10.4293/jsls.2018.00108
Shi, Q., Wang, D., Ding, X., Yang, X., and Zhang, Y. (2021). Exosome-shuttled miR-7162-3p from human umbilical cord derived mesenchymal stem cells repair endometrial stromal cell injury by restricting APOL6. Arch. Biochem. Biophys. 707, 108887. doi:10.1016/j.abb.2021.108887
Spencer, A. R., Shirzaei Sani, E., Soucy, J. R., Corbet, C. C., Primbetova, A., Koppes, R. A., et al. (2019). Bioprinting of a cell-laden conductive hydrogel composite. ACS Appl. Mat. Interfaces 11, 30518–30533. doi:10.1021/acsami.9b07353
Tafti, S. Z. G., Javaheri, A., Firoozabadi, R. D., Ashkezar, S. K., and Abarghouei, H. F. (2021). Role of hyaluronic acid intrauterine injection in the prevention of Asherman's syndrome in women undergoing uterine septum resection: An RCT. Int. J. Reprod. Biomed. 19, 339–346. doi:10.18502/ijrm.v19i4.9060
Tan, J., Li, P., Wang, Q., Li, Y., Li, X., Zhao, D., et al. (2016). Autologous menstrual blood-derived stromal cells transplantation for severe Asherman's syndrome. Hum. Reprod. 31, 2723–2729. doi:10.1093/humrep/dew235
Tan, Q., Xia, D., and Ying, X. (2020). miR-29a in exosomes from bone marrow mesenchymal stem cells inhibit fibrosis during endometrial repair of intrauterine adhesion. Int. J. Stem Cells 13, 414–423. doi:10.15283/ijsc20049
Tang, J. N., Cores, J., Huang, K., Cui, X. L., Luo, L., Zhang, J. Y., et al. (2018). Concise review: Is cardiac cell therapy dead? Embarrassing trial outcomes and new directions for the future. Stem Cells Transl. Med. 7, 354–359. doi:10.1002/sctm.17-0196
Townsend, J. M., Beck, E. C., Gehrke, S. H., Berkland, C. J., and Detamore, M. S. (2019). Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 91, 126–140. doi:10.1016/j.progpolymsci.2019.01.003
Wang, J., Yang, C., Xie, Y., Chen, X., Jiang, T., Tian, J., et al. (2021). Application of bioactive hydrogels for functional treatment of intrauterine adhesion. Front. Bioeng. Biotechnol. 9, 760943. doi:10.3389/fbioe.2021.760943
Wang, X., Ao, Q., Tian, X., Fan, J., Tong, H., Hou, W., et al. (2017). Gelatin-Based Hydrogels for Organ 3D Bioprinting, 9.Polym. (Basel)
Wei, L., Tan, W., Wang, G., Li, Q., Dong, F., and Guo, Z. (2019). The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 226, 115256. doi:10.1016/j.carbpol.2019.115256
Wen, J., Weinhart, M., Lai, B., Kizhakkedathu, J., and Brooks, D. E. (2016). Reversible hemostatic properties of sulfabetaine/quaternary ammonium modified hyperbranched polyglycerol. Biomaterials 86, 42–55. doi:10.1016/j.biomaterials.2016.01.067
Wenbo, Q., Lijian, X., Shuangdan, Z., Jiahua, Z., Yanpeng, T., Xuejun, Q., et al. (2020). Controlled releasing of SDF-1α in chitosan-heparin hydrogel for endometrium injury healing in rat model. Int. J. Biol. Macromol. 143, 163–172. doi:10.1016/j.ijbiomac.2019.11.184
Xiao, S., Wan, Y., Zou, F., Ye, M., Deng, H., Ma, J., et al. (2015). Prevention of intrauterine adhesion with auto-crosslinked hyaluronic acid gel: A prospective, randomized, controlled clinical study. Zhonghua Fu Chan Ke Za Zhi 50, 32–36.
Xie, Y., Guan, Q., Guo, J., Chen, Y., Yin, Y., and Han, X. (2022). Hydrogels for exosome delivery in biomedical applications. Gels 8, 328. doi:10.3390/gels8060328
Xie, Y., Zhang, T., Tian, Z., Zhang, J., Wang, W., Zhang, H., et al. (2017). Efficacy of intrauterine perfusion of granulocyte colony-stimulating factor (G-CSF) for infertile women with thin endometrium: A systematic review and meta-analysis. Am. J. Reprod. Immunol. 78, e12701. doi:10.1111/aji.12701
Yao, Q., Zheng, Y. W., Lan, Q. H., Wang, L. F., Huang, Z. W., Chen, R., et al. (2020). Aloe/poloxamer hydrogel as an injectable beta-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment. Eur. J. Pharm. Sci. 148, 105316. doi:10.1016/j.ejps.2020.105316
Yao, Y., Chen, R., Wang, G., Zhang, Y., and Liu, F. (2019). Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther. 10, 225. doi:10.1186/s13287-019-1332-8
Yi, X., Liu, F., Gao, K., Chen, F., Wang, Y., Li, H., et al. (2022). Reconstructable uterus-derived materials for uterus recovery toward efficient live births. Adv. Mat. 34, e2106510. doi:10.1002/adma.202106510
Yin, M., Zhou, H. J., Lin, C., Long, L., Yang, X., Zhang, H., et al. (2019). CD34(+)KLF4(+) stromal stem cells contribute to endometrial regeneration and repair. Cell Rep. 27, 2709–2724.e3. e2703. doi:10.1016/j.celrep.2019.04.088
Yu, D., Wong, Y. M., Cheong, Y., Xia, E., and Li, T. C. (2008). Asherman syndrome--one century later. Fertil. Steril. 89, 759–779. doi:10.1016/j.fertnstert.2008.02.096
Zhang, L., Yu, Z., Qu, Q., Li, X., Lu, X., and Zhang, H. (2022). Exosomal lncRNA HOTAIR promotes the progression and angiogenesis of endometriosis via the miR-761/HDAC1 Axis and activation of STAT3-mediated inflammation. Int. J. Nanomedicine 17, 1155–1170. doi:10.2147/ijn.s354314
Zhang, P., Zhao, C., Zhao, T., Liu, M., and Jiang, L. (2019). Recent advances in bioinspired gel surfaces with superwettability and special adhesion. Adv. Sci. (Weinh). 6, 1900996. doi:10.1002/advs.201900996
Zhang, S. S., Xia, W. T., Xu, J., Xu, H. L., Lu, C. T., Zhao, Y. Z., et al. (2017). Three-dimensional structure micelles of heparin-poloxamer improve the therapeutic effect of 17β-estradiol on endometrial regeneration for intrauterine adhesions in a rat model. Int. J. Nanomedicine 12, 5643–5657. doi:10.2147/ijn.s137237
Zhao, Y., Zhang, Z., Pan, Z., and Liu, Y. (2021). Advanced bioactive nanomaterials for biomedical applications. Exploration 1, 20210089. doi:10.1002/exp.20210089
Zhou, J., Zhang, Z., Joseph, J., Zhang, X., Ferdows, B. E., Patel, D. N., et al. (2021a). Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 1, 20210011. doi:10.1002/exp.20210011
Zhou, K., Sun, Y., Yang, J., Mao, H., and Gu, Z. (2022). Hydrogels for 3D embedded bioprinting: A focused review on bioinks and support baths. J. Mat. Chem. B 10, 1897–1907. doi:10.1039/d1tb02554f
Keywords: bioinspired, hydrogel, uterine, endometrial, reproductive
Citation: Dong R, Ma S, Zhao X, Wang B, Roy M, Yao L, Xia T and Liu Y (2022) Recent progress of Bioinspired Hydrogel-based delivery system for endometrial repair. Front. Bioeng. Biotechnol. 10:1013217. doi: 10.3389/fbioe.2022.1013217
Received: 06 August 2022; Accepted: 22 August 2022;
Published: 09 September 2022.
Edited by:
Pengchao Zhang, Wuhan University of Technology, ChinaReviewed by:
Xin Han, Nanjing University of Chinese Medicine, ChinaChunmei Ding, Sichuan University, China
Copyright © 2022 Dong, Ma, Zhao, Wang, Roy, Yao, Xia and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian Xia, eGlhdGlhbjc2QDE2My5jb20=; Yanting Liu, ZHIueWFudGluZ2xpdUBmb3htYWlsLmNvbQ==
 Rong Dong1
Rong Dong1 Mridul Roy
Mridul Roy Yanting Liu
Yanting Liu