
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 06 December 2022
Sec. Biomechanics
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.1012544
This article is part of the Research Topic Individual’s Mechanics, Movement and Kinematics Post-Stroke View all 10 articles
 Michela Goffredo1
Michela Goffredo1 Stefania Proietti2,3
Stefania Proietti2,3 Sanaz Pournajaf1*
Sanaz Pournajaf1* Daniele Galafate1
Daniele Galafate1 Matteo Cioeta1
Matteo Cioeta1 Domenica Le Pera1
Domenica Le Pera1 Federico Posteraro4
Federico Posteraro4 Marco Franceschini1,3
Marco Franceschini1,3Background: The literature on upper limb robot-assisted therapy showed that robot-measured metrics can simultaneously predict registered clinical outcomes. However, only a limited number of studies correlated pre-treatment kinematics with discharge motor recovery. Given the importance of predicting rehabilitation outcomes for optimizing physical therapy, a predictive model for motor recovery that incorporates multidirectional indicators of a patient’s upper limb abilities is needed.
Objective: The aim of this study was to develop a predictive model for rehabilitation outcome at discharge (i.e., muscle strength assessed by the Motricity Index of the affected upper limb) based on multidirectional 2D robot-measured kinematics.
Methods: Re-analysis of data from 66 subjects with subacute stroke who underwent upper limb robot-assisted therapy with an end-effector robot was performed. Two least squares error multiple linear regression models for outcome prediction were developed and differ in terms of validation procedure: the Split Sample Validation (SSV) model and the Leave-One-Out Cross-Validation (LOOCV) model. In both models, the outputs were the discharge Motricity Index of the affected upper limb and its sub-items assessing elbow flexion and shoulder abduction, while the inputs were the admission robot-measured metrics.
Results: The extracted robot-measured features explained the 54% and 71% of the variance in clinical scores at discharge in the SSV and LOOCV validation procedures respectively. Normalized errors ranged from 22% to 35% in the SSV models and from 20% to 24% in the LOOCV models. In all models, the movement path error of the trajectories characterized by elbow flexion and shoulder extension was the significant predictor, and all correlations were significant.
Conclusion: This study highlights that motor patterns assessed with multidirectional 2D robot-measured metrics are able to predict clinical evalutation of upper limb muscle strength and may be useful for clinicians to assess, manage, and program a more specific and appropriate rehabilitation in subacute stroke patients.
Most stroke survivors experience upper limb motor impairments that negatively influence Activities of Daily Living (ADL) (Nichols-Larsen et al., 2005). Over the past two decades, robotic devices have been shown to provide intensive and highly repeatable therapy, enrich the sensorimotor experience, and offer customizable and repeatable support during treatment (Mehrholz et al., 2018; Morone et al., 2020; Gandolfi et al., 2021). In particular, Mehrholz et al. evidenced the efficacy of upper limb Robot-assisted Therapy (ulRT) in improving ADL, arm function, and arm muscle strength (Mehrholz et al., 2018) in stroke patients.
Recently, robots have been recognised not only as a rehabilitation device but also as a measurement tool, suggesting that they can provide a standardized and objective measure of a patient’s motor control and improve research knowledge on treatment effects and stroke recovery (Agrafiotis et al., 2021). In this regard, studies on ulRT have analyzed the Robot-Measured Kinematic (RMK) data to assess ulRT-induced biomechanical changes and patient progress over time (Dipietro et al., 2011; Balasubramanian et al., 2012; Panarese et al., 2012; Mazzoleni et al., 2013; Tran et al., 2018; Goffredo et al., 2019). Furthermore, RMK data have also been shown to be able to capture relevant aspects of goal-directed movements that may reveal pathological motor synergies in stroke survivors (Dipietro et al., 2011; Panarese et al., 2012; Goffredo et al., 2019). RMK metrics were also found to correlate with motor impairment as measured by Fugl-Meyer upper limb assessment (Colombo et al., 2005; Duret et al., 2016) and to be representative of pathological motor synergies when different directions of movement were analyzed (Panarese et al., 2012; Goffredo et al., 2019). The latter is in accordance with the kinematic approach to identify how the central nervous system represents and implements the motor control strategies necessary to obtain the movements in stroke patients (Micera et al., 2005). RMK data were also processed to predict the clinical assessment outcomes (Krebs et al., 2014; Duret et al., 2019; Agrafiotis et al., 2021; Goffredo et al., 2021; Grimm et al., 2021; Moretti et al., 2021), considering the importance of biomarkers of neurorehabilitation outcomes for evidence-based practice (Langhorne et al., 2009; Scott and &Dukelow, 2011; Duret et al., 2015; Franceschini et al., 2018). In this context, the Predict REcovery Potential (PREP2) tool is the predominant predictor of upper limb functional outcomes from clinical assessment, Magnetic Resonance Imaging (MRI) and Transcranial Magnetic Stimulation (TMS) biomarkers, with an impact on rehabilitation planning and realistic treatment goal setting (Stinear et al., 2017). Although PREP2 predicts correctly approximately 70% of patients, the major limitation is that TMS is not readily available in many clinical settings (Connell et al., 2021). Therefore, in rehabilitation hospitals equipped with robots for ulRT, an alternative, ecological robot-based method to predict rehabilitation outcome could be the analysis of RMK data at baseline. However, to the best of our knowledge there is no evidence in literature on the RMK-based predictors of clinical outcomes at discharge that account for motor synergies at baseline.
Considering the importance of quantitative indicators of upper limb function, Krebs et al. and Moretti et al. found that RMK metrics from a 2D robot can be biomarkers of clinical outcomes registered on the same day (Krebs et al., 2014; Moretti et al., 2021). Their findings were consistent with those of Grimm et al. who analyzed exoskeleton-based kinematics (Grimm et al., 2021). On the other hand, Agrafiotis et al., developed RMK-based models of clinical outcomes with the aim of removing inter- and intra-rater variability and reducing the sample size in stroke clinical trials (Agrafiotis et al., 2021). To our knowledge, only Duret et al. predicted upper limb recovery at the end of ulRT (Duret et al., 2019). They found that selected RMK parameters, calculated from the total end-effector trajectory did not predict the upper limb Fugl-Meyer Assessment. In our previous paper (Goffredo et al., 2021), we analyzed RMK metrics calculated from reaching movements with different directions. Specifically, the movement path error, the mean movement speed, and the number of speed peaks of each point-to-point trajectory were analyzed. Then, a generalized linear analysis was applied to estimate the relationships between the RMK measures at baseline and the (clinical and RMK) data at the end of ulRT, considering each direction of movement separately. The analysis revealed that a subset of the RMK metrics was correlated with the probability of rising one class in the Motricity Index at the end of ulRT. Our multidirectional 2D analysis of RMK metrics showed that pre-treatment kinematic data are representative of pathological motor synergies in stroke survivors, i.e., the ability to perform movements of shoulder (ab-adduction, internal and external rotation) and elbow (flexion-extension) is representative of flexor synergy in stroke patients (Goffredo et al., 2021). However, despite the importance of predicting rehabilitation outcomes for clinician decision-making and treatment optimization, there is a paucity of literature on post-ulRT rehabilitation outcomes based on patients’ upper limb abilities and motor synergies at baseline.
The aim of this study is to re-analyze the retrospective data from our previous study to develop a multidirectional 2D RMK-based predictive model for rehabilitation outcome (i.e., muscle strength assessed by the Motricity Index of the affected upper limb) at the end of ulRT. Potential predictors included patient demographics, stroke characteristics, and pre-treatment RMK metrics calculated considering upper limb movements which are representative of the stroke flexor synergy. In order to reliably predict the discharge rehabilitation outcome, we compared two least squares error multiple linear regression models that differ in terms of the output validation procedure.
This is a re-analysis of data acquired and processed by the IRCCS San Raffaele Roma (Rome, Italy) in an observational retrospective study (Ethical approval no. 06/17; 22/02/2017) on stroke inpatients who underwent ulRT in addition to the conventional therapy (Goffredo et al., 2021).
Sixty-six stroke patients who were trained for 20 sessions (5 times/week; 45 min per session) with the In Motion 2 robot (Bionik Laboratories, Watertown, MA, United States), were included in the study. The persons were inpatients admitted to the IRCCS San Raffaele Roma (Rome, Italy) between January 2011 and December 2017 who satisfied the following inclusion criteria: age between 18 and 80 years; first event of unilateral hemiparetic stroke; subacute phase (RT started within 30 ± 7 days post stroke); upper limb Chedoke-McMaster scores between 2 and 5; Motricity Index affected upper limb<100; ulRT for 20 sessions. Subjects were excluded from the study if they had bilateral impairment; chronic phase; ulRT for less than 20 sessions; interruption of the ulRT for more than three consecutive days; presence of other severe medical conditions; incomplete data in the database. More detailed information on the ulRT conducted by the patients is available in the previous papers of the authors (Goffredo et al., 2019; Goffredo et al., 2021).
The following demographic and clinical data have been collected at baseline: age, gender, affected side, stroke onset time, and etiology. Moreover, the following clinical outcomes were recorded before (T1) and after (T2) the ulRT: Motricity Index of the affected Upper Limb (MIUL) (Bohannon, 1999), Motricity Index sub-item assessing the elbow flexion (MIELBOW), and Motricity Index sub-item assessing the shoulder abduction (MISHOULDER). The MIUL is a discrete scale measuring the muscle strength of the paretic upper extremity. Three actions were separately assessed: pinch grasp, elbow flexion, and shoulder abduction. Each action was scored (0–33) with a MI sub-item composed of six classes, as defined by Wade (Wade, 1989). The total upper extremity score (MIUL) was calculated by adding one to the sum of the three sub-items (maximum possible score = 100). The MI sub-item related to the pinch grip was not considered in this study because the InMotion2-based ulRT typically involves the elbow and shoulder joints.
The RMK metrics were calculated from the trajectories (200 Hz) recorded by the robot, considering each movement direction separately (Goffredo et al., 2021). Figure 1 shows the reference system (coinciding with the lesion side) used to calculate the RMK metrics. Considering the results of our previous analysis of kinematic biomarkers for upper-limb motor recovery (Goffredo et al., 2021), we extracted the features that were correlated with muscle strength at discharge, i.e.: Movement Path Error direction A (MPEA), direction C (MPEC), and direction D (MPED); and mean Movement Speed direction B (MSB).
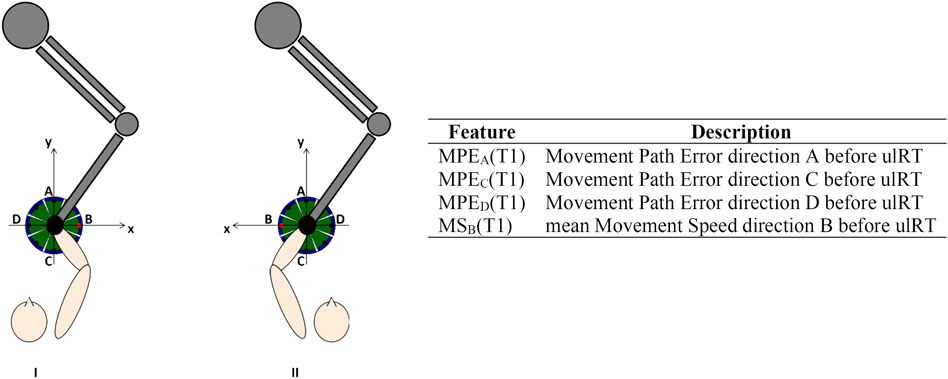
FIGURE 1. Experimental setup, reference system in case of right (I) or left (II) affected limb, and description of the features considered for the models’ development.
The MPE is a measure of accuracy (the value is 0 if the trajectory lies exactly on a straight line connecting the initial and the final target): it is computed as the mean value of the distance between each point of the actual path travelled by the subject from the ideal one (i.e., the straight line connecting the central target and the peripheral one). Since the considered peripheral targets are along the x and y axes, the MPE computation is the following:
where N is the number of samples for each trajectory, identified by the coordinates x[k] and y[k] in the xy plane. The MS has been computed from the discrete-time velocity signals vx[k] and vy[k] along the x and y axes, respectively as the mean value of the resultant velocities in the xy plane:
All RMK metrics were recorded at T1 and showed good test-retest reliability (Koeppel and Pila, 2020). Prior to modeling, age, stroke onset time, and RMK metrics have been standardized by subtracting the mean and scaling to unit variance. Figure 1 includes the list of features used for model development.
All statistical analyses were performed using the R statistical package system v. 4.2.0 (R Foundation for Statistical Computing, Austria). A significance level of 5% (p-value ≤ 0.05) was assumed. Demographic and clinical data were reported with frequencies and percentages if they were categorical variables, while continuous variables were expressed with mean and standard deviation, median, and interquartile range.
The study compared two types of outcome prediction models that differed in terms of the validation procedure. The first model was the least squares error multiple linear regression model with the Split Sample Validation procedure (SSV model). The second model was the least squares error multiple linear regression with the Leave-One-Out Cross-Validation procedure (LOOCV model). In both models, the outputs were MIELBOW (T2), MISHOULDER (T2), and MIUL (T2) discharge scores (adjusted for age, sex, stroke onset time, and clinical assessment scores admission), whereas the inputs were the RMK metrics at admission.
For the SSV models, 75% of the data were randomly separated for model training, whereas the remaining 25% were set aside for model validation (Bosecker et al., 2010). The Chi-square test for categorical variables, and ANalysis Of VAriance (ANOVA) for continuous variables when normally distributed, and otherwise Mann Whitney test, confirmed that the training and validation data sets were not significantly different. Moreover, the data set was randomly divided into four groups (25% data each), and the Kruskal Wallis test (Bonferroni’s correction) confirmed that there was no significant difference (p-value>0.05) between any combination of these groups in training and validation sets.
For the LOOCV models, one of the 66 records was removed, and the remaining were used to build the model. The resulting model was then used to make predictions about the record set aside (Bishop, 1995). This was repeated for each of the 66 cases.
Each prediction model was described by the percentage of variance explained (R2), the adjusted R2 (Radj2), the Mean Absolute Error (MAE), Root Means Squared Error (RMSE), and normalized RMSE (RMSEn). The normalized MAE (MAEn) was calculated to compare the performance of the model for the different dependent variables.
Results were assessed by correlation between therapist-assigned values and corrected predicted values (Spearman’s rank correlation coefficient, r, and associated p values). Since the MIUL is a discrete variable, each predicted value was corrected using a nearest neighbor procedure by assigning the score with the minimum Euclidean distance from the valid scores. Results were assessed by correlation between therapist-assigned scores and corrected predicted scores (Spearman’s rank correlation coefficient, r, and associated p-values). The strength of the correlations calculated in the analyses was interpreted as follows; |r|=0–0.3 very weak, |r|=0.31–0.5 weak, |r|=0.51–0.7 moderate, and |r|=0.71–1.0 strong (Moore et al., 2013).
Since March 2012, the Italian Data Protection Authority (Garante per la protezione dei dati personali) declared that IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico - Institute for scientific research and healthcare) is authorized to perform retrospective studies without the approval of the local Ethical Committee, and mandatory formal communication is sufficient. Such communication relative to this study was registered by the Ethical Committee of the IRCCS San Raffaele Roma (Rome, Italy) on 22/02/2017 (code number: 06/17).
In this study, we compared outcome predictors obtained by two modeling procedures differing from one another by output validation.
Table 1 shows the patient’s demographics and clinical characteristics at baseline: data are shown for all recruited subjects, training set, and validation set separately. No significant differences (p-value>0.05) were registered between the training and the validation sets.
Predictive models for muscle strength at T2 of each clinical outcome were developed using the least squares error multiple linear regression on the training set. In all models, the movement path errors in the C (MPEC) and D (MPED) directions were significant predictors (p-value<0.05). Specifically, MPEC provided larger contribution (MIELBOW: β = −5.16; MISHOULDER: β = −5.02; MIUL: β = −13.52) than MPED (MIELBOW: β = 3.37; MISHOULDER: β = 3.37; MIUL: β = 8.26).
The training and validation results of the SSV models for estimating the MIELBOW (T2), MISHOULDER (T2), and MIUL (T2) are shown in Table 2. All correlations were significant at p-value < 0.05. The residual plots had no significant patterns, indicating that no underlying trends in the data were missed and that the model was fit. The MIELBOW registered a moderate correlation in the training set (R2 = 0.683), but a weak one in the validation set (R2 = 0.481). This decrease is more remarkable in MISHOULDER, which is characterized by an R2 of 0.640 in the training set and a very weak level of correlation in the validation one (R2 = 0.200). The MIUL showed the highest R2 value in both datasets. The resulting models explained 64%–76% of the variance in discharge scores, in the training set. For predicting the clinical outcomes of a patient in the validation set, the average error was 6.098 points for the MIELBOW model (range 0–33), 8.357 points for the MISHOULDER model (range 0–33), and 17.265 points for the MIUL model (range 1–100).
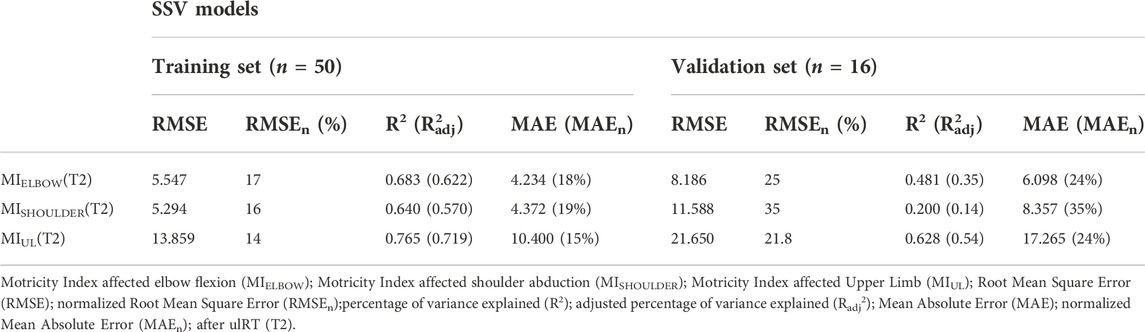
TABLE 2. SSV predictive models for the clinical outcomes (MIELBOW, MISHOULDER, MIUL) at the end of ulRT (T2). Results of the training and validation sets are depicted separately.
To illustrate the MI score predictions, the actual score of each patient was plotted together with the predictions generated by the models (Figure 2). The correlation between the therapist-assigned scores and predicted scores was moderate for MIELBOW (r = 0.794; p-value < 0.001) and MISHOULDER (r = 0.627; p-value < 0.001), and strong for MIUL (r = 0.839; p-value < 0.001).
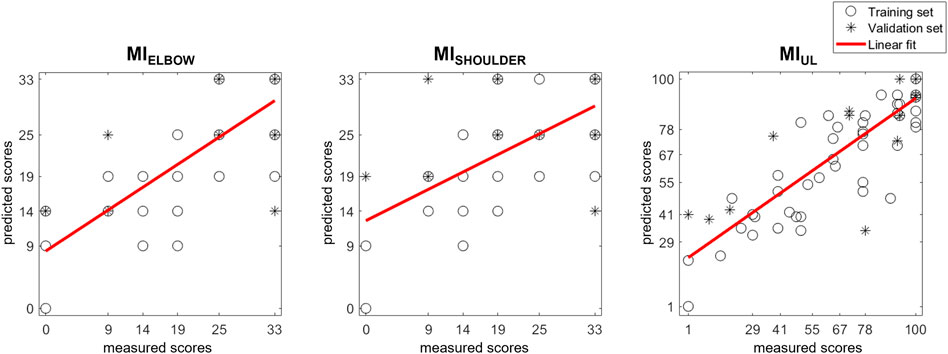
FIGURE 2. Scatter plot of MI scores prediction with SSV models. Each patient’s actual score was plotted together with the predictions generated by the models. Training and validation sets are depicted separately. The results of a linear polynomial fitting are also shown.
The following LOOCV predictive models of discharge MIELBOW, MISHOULDER, and MIUL were obtained:
The generalizability of each model was evaluated by testing its ability to predict scores of patients who were not involved in the development of the model (Table 3). In all models, the MPEC (i.e., the error of the trajectory towards the body) was the significant predictor, and all correlations were significant (p-value<0.05). The residual plots did not have any significant correlations. The resulting models explained 46.4%–67.6% of the variance in discharge scores, and the normalized RMSE ranged from 17% to 22%.
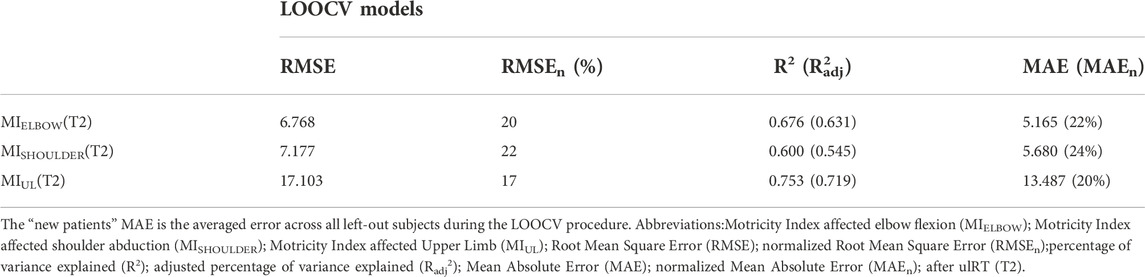
TABLE 3. Results from the predictive models for the discharge clinical outcomes (MIELBOW, MISHOULDER, MIUL)by applying the Leave-One-Out Cross-Validation (LOOCV) procedure.
Figure 3 depicts the actually measured score with the predicted ones generated by the models. The correlation coefficients between the therapist-assigned scores and the model’s output evidences a significant correlation in all outcomes: MIELBOW (r = 0.747; p-value < 0.001), MISHOULDER (r = 0.653; p-value < 0.001), and MIUL (r = 0.813; p-value < 0.001).
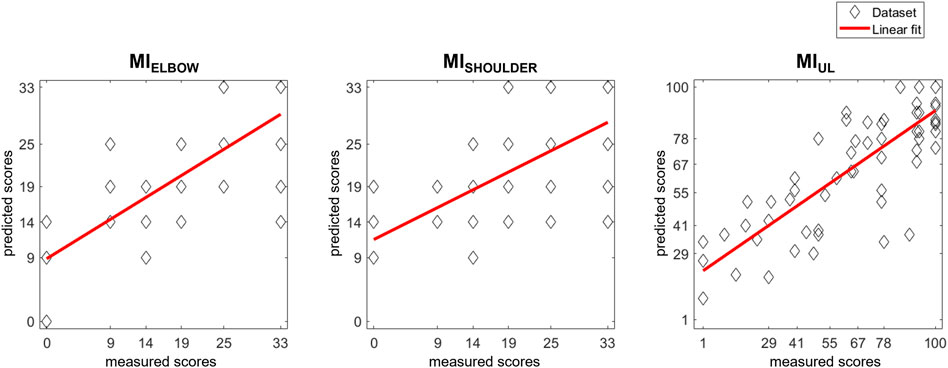
FIGURE 3. Scatter plot of MI scores prediction with LOOCV models. Each patient’s actual score was plotted together with the predictions generated by the models. The results of a linear polynomial fitting are also shown.
Predicting rehabilitation outcomes based on pre-treatment characteristics would be of great benefit to clinicians in setting realistic rehabilitation goals, personalizing treatment activities, and supporting patient discharge in an appropriate setting (Stinear et al., 2017). In the present article, we examined the predictive abilities of two types of multiple linear regression models to reliably predict the rehabilitation outcome at discharge using RMK-based features. Specifically, both SSV and LOOCV validation procedures were considered. Since the ability to perform point-to-point reaching movements in different directions is considered in the literature to be representative of different synergies involved in the performance of reaching tasks (Panarese et al., 2012; Goffredo et al., 2019), pre-treatment RMK metrics were considered as predictive features for rehabilitation outcomes at discharge in the models developed in this study, with each direction of movement assessed separately.
The procedure consisted of a re-analysis of 66 inpatients with subacute stroke who were included in a 4-week ulRT and whose data had been examined in our previous studies with different aims (Goffredo et al., 2019; Goffredo et al., 2021). In particular, in Goffredo et al. (Goffredo et al., 2021), a relationship was found between RMK measures at baseline and (clinical and RMK) data at the end of ulRT, considering each direction of movement separately (Goffredo et al., 2021), and revealing that a subset of kinematic parameters was correlated with the probability of rising one class in the Motricity Indexes (when considered as categorical variables). In contrast, in the present study, we developed models to predict Motricity Indexes at discharge from selected RMK features and found that the SSV and LOOCV validation procedures explained 54% and 71% of the variance in clinical scores at discharge respectively. Normalized errors ranged from 22% to 35% in the SSV models, and from 20% to 24% in the LOOCV ones.
Our findings are in agreement with the studies of Agrafiotis et al. (Agrafiotis et al., 2021) and Moretti et al. (Moretti et al., 2021), although they analyzed chronic stroke patients. Both studies found a significant correlation between RMK metrics and clinical outcomes (upper limb Fugl-Meyer Assessment (Agrafiotis et al., 2021; Moretti et al., 2021), Motor Power (Agrafiotis et al., 2021), NIH stroke scale (Agrafiotis et al., 2021), modified Rankin scale (Agrafiotis et al., 2021), Wolf Motor Function Test (Moretti et al., 2021), Barthel Index (Moretti et al., 2021), and Medical Research Council score (Moretti et al., 2021) demonstrating that traditional stroke assessment scales can be accurately reproduced by robotic measurements. The outcomes of these studies pave the way for the use of RMK data to reduce the sample size needed for future clinical trials on chronic stroke patients (Agrafiotis et al., 2021) and to objectively, quantitatively, and rapidly assess impairments in body function (Moretti et al., 2021). However, most published studies on this topic examined the relationships between technology-based metrics and clinical assessment outcomes measured close in time (e.g., concurrently) (Krebs et al., 2014; Grimm et al., 2021; Olesh et al., 2014; Wang et al., 1109). Conversely, our findings showed that baseline data collected from a rehabilitation robotic device are able to predict clinical outcomes at discharge with statistically significant accuracy. Mostafavi et al. (Mostafavi et al., 2013) showed results similar to ours although the RMK metrics derived from an exoskeleton robot and the predicted rehabilitation outcome at discharge was an overall measure of disability in ADL (i.e., the Functional Independence Measure).
Data analysis showed that the significant features for predicting the discharge Motricity Indexes were the errors of the trajectories towards the body, which were strongly influenced by the typical postural patterns of the upper extremity after stroke (Raghavan, 2015): the trajectory towards the body is performed by flexing the elbow and extending the shoulder and is representative of upper limb spastic co-contraction (Bensmail et al., 2010) and the typical pathological flexor strategy of stroke survivors (Hefter et al., 2012; McMorland et al., 2015). In our analysis, the error of the trajectory towards the body was a significant predictor in both SSV and LOOCV models, showing that the less accurate and controlled the trajectory towards the pathological patterns is, the smaller muscle strength, assessed with the Motricity Index, is at the end of the ulRT. Our findings agree partly with Gialanella & Santoro (Gialanella and Santoro, 2015), who showed that at the end of rehabilitation, the motor score of the functional independence measure was lower in patients having at admission, the only flexor synergy of the affected limbs. Similarly, the systematic review by Coupar et al. (Coupar et al., 2012) found strong evidence that less impairment at baseline is associated with better upper limb recovery. Conversely, the outcomes of our model are not in accordance with Welmer et al. (Welmer et al., 2006) who found that stroke patients with typical pathological synergies had significantly better functioning scores.
In our previous analysis of the data (Goffredo et al., 2021), the error of the trajectory towards the body negatively affects the probability of increasing one class in the discharge Motricity Indexes. However, in the re-analysis of data with least squares multiple linear regression, we found that the error of the trajectory towards the body was a significant predictor of Motricity Indexes, which strongly correlate (r > 0.8 for the MIUL) with the therapist-assigned ones (Figures 2, 3). The comparison between the SSV and the LOOCV models showed that the error of the trajectory towards the body was a significant predictor in both models, whereas the same metric towards target D appeared in the SSV models.
In the literature, the most widely recognized predictor of upper limb functional outcome is the PREP2 tool (Stinear et al., 2017; Connell et al., 2021), which is based on clinical, MRI, and TMS biomarkers. Despite its high accuracy, the major characteristic of PREP2 is that TMS is not readily available in many clinical settings. Therefore, in rehabilitation hospitals equipped with robots for ulRT, our ecological, quantitative, objective analysis of baseline RMK data could be a valuable alternative to predict the rehabilitation outcome. Furthermore, since RMK metrics are representative of the ability to perform goal-directed movements, RMK biomarkers are able to predict rehabilitation outcomes according to the motor synergies at the baseline.
This study has the following limitations due to its retrospective design: a limited number of subjects; lack of an ICF-based assessment (considering body function, activity, and participation); and lack of RMK data from able-bodied subjects. Considering the SSV models, the training and validation datasets differed with respect to the time of stroke onset: although no statistical significance was found between groups (p-value>0.05), it may have influenced the presence of stereotypic movement synergies. Although the analysis normalized the time of stroke onset before modelling, future studies with a more homogeneous sample of patients would be interesting. The limitation of the study in terms of database size can be partially overcome considering that LOOCV models are more reliable and unbiased than SSV ones (Bishop, 1995) and seem particularly suitable when the dataset is small and an accurate estimation of model performance is required. In addition, the LOOCV models confirmed the outcomes of the SSV procedure with a correlation of up to R2 = 0.753 (RMSEn = 17%). However, the future research agenda should consider large longitudinal studies including different categories of robots for upper limb rehabilitation (Gandolfi et al., 2021), evaluating the patients with an ICF-based assessment, and comparing the outcomes with a control group composed of able-bodied subjects.
Nevertheless, the study highlights that in stroke ulRT, the motor patterns assessed with RMK metrics strongly relate to discharge rehabilitation outcome and that the accuracy in performing elbow flexion movement is a significant predictor of outcome. The developed models, thus, are able to predict the clinical assessment of upper limb muscle strength and can be useful to clinicians to assess, manage, and program a more specific and appropriate rehabilitation in subacute stroke patients.
Multidirectional 2D RMK-based predictive models were developed and validated for discharge rehabilitation outcomes in sixty-six subacute stroke patients who performed ulRT with a planar end-effector robot. Accuracy in performing elbow flexion and shoulder extension movements was found to be a significant predictor of muscle strength at the end of ulRT: patients with a pathological upper limb flexor strategy were less likely to increase muscle strength at discharge.
Since the potential recovery of motor function depends on the synergies that occur after stroke, quantitative and measurable knowledge of upper limb function before initiation of physical therapy could be useful for an accurate and individualized prognosis, allowing more realistic expectations for recovery and helping to set realistic goals with a personalized rehabilitation program. In this respect, the results of this study suggest that subacute stroke patients with a marked flexor strategy tend to have a worse rehabilitation outcome at discharge: in these cases, physical therapy should focus on developing beneficial health synergies and avoid reinforcing pathological patterns.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical Committee of the IRCCS San Raffaele Roma (Rome, Italy) on 22/02/2017 (code number: 06/17). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MG has made substantial contributions to conception and design. SaP, DG, and MC participated in the enrolment phase, carried out the treatment, and carried out the clinical and kinematic assessments. MG and StP designed the algorithm for data analysis. MG participated in the study design and coordination and statistical analysis. MF, FP, SaP, StP, and DL participated in the manuscript revisions. MG gave the final approval of the version. All authors contributed to the article and approved the submitted version.
MG, StP, SaP, DG, MC, DLP, and MF were supported by the Italian Ministry of Health (Ricerca corrente).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agrafiotis, D. K., Yang, E., Littman, G. S., Byttebier, G., Dipietro, L., DiBernardo, A., et al. (2021). Accurate prediction of clinical stroke scales and improved biomarkers of motor impairment from robotic measurements. Plos one 16 (1), e0245874. doi:10.1371/journal.pone.0245874
Balasubramanian, S., Colombo, R., Sterpi, I., Sanguineti, V., and Burdet, E. (2012). Robotic assessment of upper limb motor function after stroke. Am. J. Phys. Med. Rehabilitation 91 (11), S255–S269. doi:10.1097/phm.0b013e31826bcdc1
Bensmail, D., Robertson, J. V., Fermanian, C., and Roby-Brami, A. (2010). Botulinum toxinto treat upper-limb spasticity in hemiparetic patients: Analysis of functionand kinematics of reaching movements. Neurorehabil. Neural Repair 24, 273–281. doi:10.1177/1545968309347682
Bishop, C. M. (1995). Neural networks for pattern recognition. Oxford, United Kingdom: Oxford University Press.
Bohannon, R. W. (1999). Motricity index scores are valid indicators of paretic upper extremity strength following stroke. J. Phys. Ther. Sci. 11 (2), 59–61. doi:10.1589/jpts.11.59
Bosecker, C., Dipietro, L., Volpe, B., and Igo Krebs, H. (2010). Kinematic robot-based evaluation scales and clinical counterparts to measure upper limb motor performance in patients with chronic stroke. Neurorehabil. Neural Repair 24 (1), 62–69. doi:10.1177/1545968309343214
Colombo, R., Pisano, F., Micera, S., Mazzone, A., Delconte, C., Carrozza, M., et al. (2005). Robotic techniques for Upper Limb Evaluation and rehabilitation of stroke patients. IEEE Trans. Neural Syst. Rehabil. Eng. 13 (3), 311–324. doi:10.1109/tnsre.2005.848352
Connell, L. A., Chesworth, B., Ackerley, S., Smith, M. C., and Stinear, C. M. (2021). Implementing the PREP2 algorithm to predict upper limb recovery potential after stroke in clinical practice: A qualitative study. Phys. Ther. 101 (5), pzab040. doi:10.1093/ptj/pzab040
Coupar, F., Pollock, A., Rowe, P., Weir, C., and Langhorne, P. (2012). Predictors of upper limb recovery after stroke: A systematic review and meta-analysis. Clin. Rehabil. 26 (4), 291–313. doi:10.1177/0269215511420305
Dipietro, L., Krebs, H. I., Volpe, B. T., Stein, J., Bever, C., Mernoff, S. T., et al. (2011). Learning, not adaptation, characterizes stroke motor recovery: Evidence from kinematic changes induced by robot-assisted therapy in trained and untrained task in the same workspace. IEEE Trans. Neural Syst. Rehabil. Eng. 20 (1), 48–57. doi:10.1109/tnsre.2011.2175008
Duret, C., Hutin, E., Lehenaff, L., and Gracies, J. M. (2015). Do all sub acute stroke patients benefit from robot-assisted therapy? A retrospective study. Restor. neurology Neurosci. 33 (1), 57–65. doi:10.3233/rnn-140418
Duret, C., Pila, O., Grosmaire, A. G., and Koeppel, T. (2019). Can robot-based measurements improve prediction of motor performance after robot-assisted upper-limb rehabilitation in patients with moderate-to-severe sub-acute stroke? Restor. Neurol. Neurosci. 37 (2), 119–129. doi:10.3233/rnn-180892
Duret, C., Courtial, O., and Grosmaire, A. G. (2016). Kinematic measures for upper limb motor assessment during robot-mediated training in patients with severe sub-acute stroke. Restor. Neurol. Neurosci. 34 (2), 237–245. doi:10.3233/rnn-150565
Franceschini, M., Goffredo, M., Pournajaf, S., Paravati, S., Agosti, M., De Pisi, F., et al. (2018). Predictors of activities of daily living outcomes after upper limb robot-assisted therapy in subacute stroke patients. PloS one 13 (2), e0193235. doi:10.1371/journal.pone.0193235
Gandolfi, M., Valè, N., Posteraro, F., Morone, G., Dell'orco, A., Botticelli, A., et al. (2021). State of the art and challenges for the classification of studies on electromechanical and robotic devices in neurorehabilitation: A scoping review. Eur. J. Phys. Rehabil. Med. 57, 831–840. doi:10.23736/s1973-9087.21.06922-7
Gialanella, B., and Santoro, R. (2015). Prediction of functional outcomes in stroke patients: The role of motor patterns according to limb synergies. Aging Clin. Exp. Res. 27 (5), 637–645. doi:10.1007/s40520-015-0322-7
Goffredo, M., Mazzoleni, S., Gison, A., Infarinato, F., Pournajaf, S., Galafate, D., et al. (2019). Kinematic parameters for tracking patient progress during upper limb robot-assisted rehabilitation: An observational study on subacute stroke subjects. Appl. Bionics Biomech. 20, 1–12. doi:10.1155/2019/4251089
Goffredo, M., Pournajaf, S., Proietti, S., Gison, A., Posteraro, F., and Franceschini, M. (2021). Retrospective robot-measured upper limb kinematic data from stroke patients are novel biomarkers. Front. Neurol. 12, 803901. doi:10.3389/fneur.2021.803901
Grimm, F., Kraugmann, J., Naros, G., and Gharabaghi, A. (2021). Clinical validation of kinematic assessments of post-stroke upper limb movements with a multi-joint arm exoskeleton. J. Neuroeng. Rehabil. 18 (1), 92–11. doi:10.1186/s12984-021-00875-7
Hefter, H., Jost, W. H., Reissig, A., Zakine, B., Bakheit, A. M., and Wissel, J. (2012). Classification of posture in poststroke upper limb spasticity: A potential decision tool for botulinum toxin A treatment? Int. J. Rehabilitation Res. 35 (3), 227–233. doi:10.1097/mrr.0b013e328353e3d4
Koeppel, T., and Pila, O. (2020). Test-retest reliability of kinematic assessments for upper limb robotic rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 28 (9), 2035–2042. doi:10.1109/tnsre.2020.3013705
Krebs, H. I., Krams, M., Agrafiotis, D. K., Di Bernardo, A., Chavez, J. C., Littman, G. S., et al. (2014). Robotic measurement of arm movements after stroke establishes biomarkers of motor recovery. Stroke 45 (1), 200–204. doi:10.1161/strokeaha.113.002296
Langhorne, P., Sandercock, P., and Prasad, K. (2009). Evidence-based practice for stroke. Lancet Neurol. 8 (4), 308–309. doi:10.1016/s1474-4422(09)70060-2
Mazzoleni, S., Sale, P., Franceschini, M., Bigazzi, S., Carrozza, M. C., Dario, P., et al. (2013). Effects of proximal and distal robot-assisted upper limb rehabilitation on chronic stroke recovery. NeuroRehabilitation 33 (1), 33–39. doi:10.3233/nre-130925
McMorland, A. J., Runnalls, K. D., and Byblow, W. D. (2015). A neuroanatomical framework for upper limb synergies after stroke. Front. Hum. Neurosci. 9, 82. doi:10.3389/fnhum.2015.00082
Mehrholz, J., Pohl, M., Platz, T., Kugler, J., and Elsner, B. (2018). Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 9, CD006876. doi:10.1002/14651858.CD006876.pub5
Micera, S., Carpaneto, J., Posteraro, F., Cenciotti, L., Popovic, M., and Dario, P. (2005). Characterization of upper arm synergies during reaching tasks in able-bodied and hemiparetic subjects. Clin. Biomech. 20 (9), 939–946. doi:10.1016/j.clinbiomech.2005.06.004
Moore, D. S., Notz, W. I., and Flinger, M. A. (2013). The basic practice of statistics. New York: WH Freeman and Company, 138.
Moretti, C. B., Hamilton, T., Edwards, D. J., Peltz, A. R., Chang, J. L., Cortes, M., et al. (2021). Robotic kinematic measures of the arm in chronic stroke: Part 2–strong correlation with clinical outcome measures. Bioelectron. Med. 7 (1), 21–13. doi:10.1186/s42234-021-00082-8
Morone, G., Cocchi, I., Paolucci, S., and Iosa, M. (2020). Robot-assisted therapy for arm recovery for stroke patients: State of the art and clinical implication. Expert Rev. Med. devices 17 (3), 223–233. doi:10.1080/17434440.2020.1733408
Mostafavi, S. M., Glasgow, J. I., Dukelow, S. P., Scott, S. H., and Mousavi, P. IEEE, 1–6.Prediction of stroke-related diagnostic and prognostic measures using robot-based evaluationProceedings of the 2013 IEEE 13th International Conference on Rehabilitation Robotics (ICORR)24-26 June 2013
Nichols-Larsen, D. S., Clark, P. C., Zeringue, A., Greenspan, A., and Blanton, S. (2005). Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke 36 (7), 1480–1484. doi:10.1161/01.STR.0000170706.13595.4f
Olesh, E. V., Yakovenko, S., and Gritsenko, V. (2014). Automated assessment of upper extremity movement impairment due to stroke. PloS one 9 (8), e104487. doi:10.1371/journal.pone.0104487
Panarese, A., Colombo, R., Sterpi, I., Pisano, F., and Micera, S. (2012). Tracking motor improvement at the subtask level during robot-aided neurorehabilitation of stroke patients. Neurorehabil. Neural Repair 26 (7), 822–833. doi:10.1177/1545968311431966
Raghavan, P. (2015). Upper limb motor impairment after stroke. Phys. Med. Rehabil. Clin. N. Am. 26, 599–610. doi:10.1016/j.pmr.2015.06.008
Scott, S. H., and Dukelow, S. P. (2011). Potential of robots as next-generation technology for clinical assessment of neurological disorders and upper-limb therapy. J. Rehabilitation Res. Dev. 48 (4), 335. doi:10.1682/jrrd.2010.04.0057
Stinear, C. M., Byblow, W. D., Ackerley, S. J., Smith, M. C., Borges, V. M., and Barber, P. A. (2017). PREP2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann. Clin. Transl. Neurol. 4 (11), 811–820. doi:10.1002/acn3.488
Tran, V. D., Dario, P., and Mazzoleni, S. (2018). Kinematic measures for upper limb robot-assisted therapy following stroke and correlations with clinical outcome measures: A review. Med. Eng. Phys. 53, 13–31. doi:10.1016/j.medengphy.2017.12.005
Wade, D. T. (1989). Measuring arm impairment and disability after stroke. Int. Disabil. Stud. 11, 89–92. doi:10.3109/03790798909166398
Wang, J., Yu, L., Wang, J., Guo, L., Gu, X., and Fang, Q. IEEE, 1–4. doi:10.1109/ISBB.2014.6820907Automated Fugl-Meyer assessment using SVR modelProceedings of the 2014 IEEE International Symposium on Bioelectronics and Bioinformatics (IEEE ISBB 2014)11-14 April 2014Chung Li, Taiwan
Keywords: robot-assisted therapy, stroke, motor recovery, upper extremity, kinematics, biomarkers, predictors
Citation: Goffredo M, Proietti S, Pournajaf S, Galafate D, Cioeta M, Le Pera D, Posteraro F and Franceschini M (2022) Baseline robot-measured kinematic metrics predict discharge rehabilitation outcomes in individuals with subacute stroke. Front. Bioeng. Biotechnol. 10:1012544. doi: 10.3389/fbioe.2022.1012544
Received: 05 August 2022; Accepted: 16 November 2022;
Published: 06 December 2022.
Edited by:
Yih-Kuen Jan, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Yingchun Zhang, University of Houston, United StatesCopyright © 2022 Goffredo, Proietti, Pournajaf, Galafate, Cioeta, Le Pera, Posteraro and Franceschini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanaz Pournajaf, cy5wb3VybmFqYWZAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.