- 1School of Aeronautics, Northwestern Polytechnical University, Xi’an, China
- 2Center of Special Environmental Biomechanics; Biomedical Engineering, Northwestern Polytechnical University, Xi’an, China
- 3School of Life Science, Northwestern Polytechnical University, Xi’an, China
- 4School of Civil Aviation, Northwestern Polytechnical University, Xi’an, China
- 5Joint International Research Laboratory of Impact Dynamic and Its Engineering Application, Xi’an, China
Changes in the mechanical properties of single cells are related to the physiological state and fate of cells. The construction of cell constitutive equations is essential for understanding the material characteristics of single cells. With the help of atomic force microscopy, bio-image processing algorithms, and other technologies, research investigating the mechanical properties of cells during static/quasi-static processes has developed rapidly. A series of equivalent models, such as viscoelastic models, have been proposed to describe the constitutive behaviors of cells. The stress-strain relations under dynamic processes are essential to completing the constitutive equations of living cells. To explore the dynamic mechanical properties of cells, we propose a novel method to generate a controllable dynamical compression shear coupling stress on living cells. A CFD model was established to visualize this method and display the theories, as well as assess the scope of the application. As the requirements or limitations are met, researchers can adjust the details of this model according to their lab environment or experimental demands. This micro-flow channel-based method is a new tool for approaching the dynamic mechanical properties of cells.
Introduction
During the processes of cell development, differentiation, physiology, and disease, cells receive not only chemical signals but also mechanical signals from the extracellular matrix and surrounding environment (Hamed, 2020). Mechanical forces are experienced by cells and may be interpreted as a signal to induce phenotypic and functional responses or pathways, such as gene expression cascades, protein synthesis, proliferation, and movement; these responses can temporarily or even permanently change the cellular state (Desprat et al., 2005; Patterson et al., 2019). Moreover, the mechanobiological responses of biological cells had been extensively studied also, e.g., the responses of mesenchymal stem cells and chondrocytes to mechanical stimuli (Zhang et al., 2008; Zhang et al., 2012; Ganadhiepan et al., 2019). From a mechanical perspective, cells present a special material that is far more complex than ordinary materials, such as metal and glass. It is worth noting that the mechanical properties of cells remain unstable in most pathological processes, such as metastasis, asthma, sickle cell anemia, and apoptosis (Alberts et al., 2002; Desprat et al., 2005; Hao et al., 2020). Thus, understanding the mechanical behaviors of cells can provide a useful perspective on describing disease progression and revealing the fundamental mechanisms of the working of biomaterials (Bao and Suresh, 2003; Moeendarbary and Harris, 2014).
In exploring the mechanical behaviors of cells and establishing stress-strain relationships in them, it is a challenge to properly apply a controllable force on the tissue/monolayer/cell and capture its real-time strain at a single cell scale (i.e.,
In conjunction with atomic force microscopy (AFM), the bio-image processing algorithm (Dudani et al., 2013), micropipette aspiration (MA) (Evans and Yeung, 1989), and microfluidic platforms (Urbanska et al., 2020) are the most common and effective mechanical tools to apply compression/tensile or shear stress on cells. In addition, to improve accuracy and collect more information, some modified techniques and methods have been designed, such as magnetic twisting cytometry (MTC) (Trepat et al., 2004) and uniaxial stretching rheometer (USR) (Desprat et al., 2005). Through these static or quasi-static mechanical experiments, it is believed that the mechanical behavior of cells is likely to resemble that of viscoelastic material (Katti et al., 2019). However, experiments exploring the stress-strain relationship of isolated living cells did not reach dynamic conditions or higher strain rates (
In the present work, we propose a novel method to apply combined dynamic compression-shear loading on isolated living cells under normal conditions; in addition, we extend the range of stiffness tensor of single biological cell piecewise function to higher strain rates (
Methods
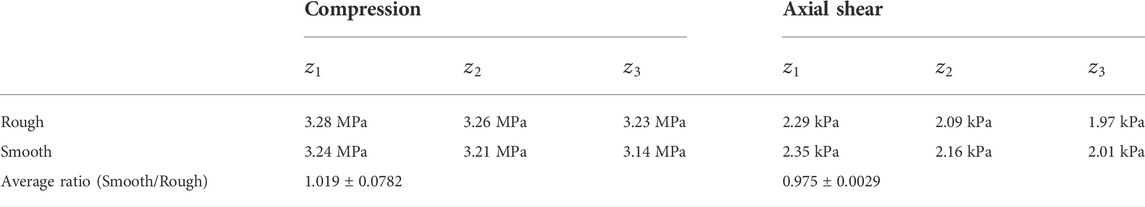
To illustrate the dynamic and coupled compression-shear loading method, a simple schematic diagram of the model is presented in Figures 1A,B. The model exhibits a gas gun, projectile, and a microfluidic chip with a rectangular conduit channel, where cells can be seeded on the bottom wall. In this mode, a projectile was accelerated to an initial velocity (
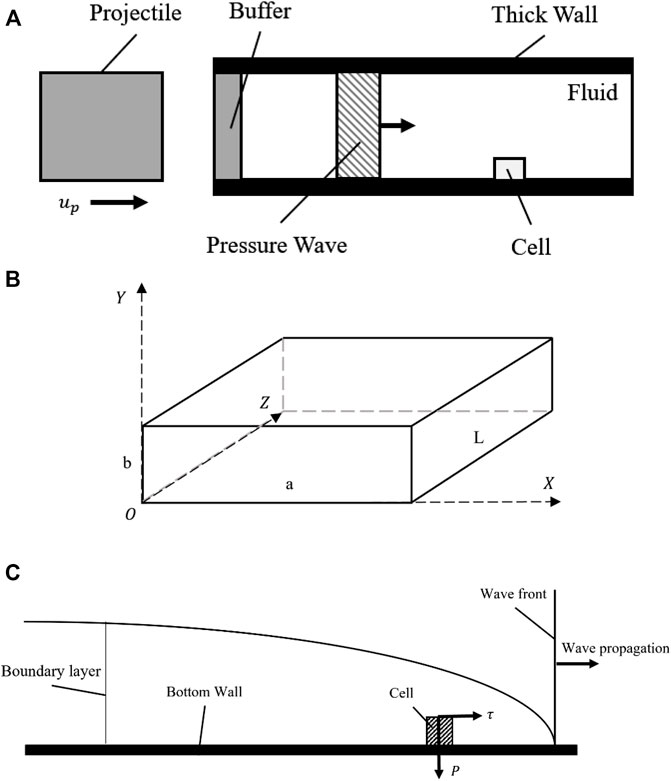
FIGURE 1. A schematic diagram showing the basics of the proposed model. (A) The schematic diagram of the geometry of the model; (B) The sketch of the rectangular channel; (C) The stress analysis of cells during wave propagation.
Navier-Stokes equations
To allow the pressure waves to propagate, the fluid used in this experiment was compressible with constant viscosity. Therefore, the equations of continuity and momentum (Navier-Stokes equations) used to describe the motion of the fluid are written as:
Where
Where
Where
Wave propagation celerity in a rectangular conduit channel
A general solution for the celerity of pressure wave propagating in the fluid has been derived by Hutarew and can be applied for a conduit of any cross-section (Hutarew, 1973). The form is:
Where K is the fluid bulk modulus,
Where a and b are the lengths of the long and short sides of the rectangular cross-section; e is the wall thickness;
Nevertheless, the properties of solids involved played an important role in wave propagation as per the wave celerity equation. An evaluation of the fluid-solid coupling effect was needed to examine the limitations when the motion of the channel was rigid. A dimensional parameter “
Where
Pressure wave generation by projectile impact
To describe the fluid suitable here with equations, the Tait and Tammann equations of state, which apply to a wide range of liquids, were used (Dymond and Malhotra, 1988; Hoang et al., 2015). The Tait equation can be written as a function of pressure
Where
As the whole process was assumed to be isentropic, the adiabatic exponent
Where γ is the volume Upon substituting volume γ by density ρ, we obtained:
The definition of acoustic velocity is:
Upon integration of Eq. 16, a function of pressure and wave speed describing the fluid density was obtained, which could be used to correct the density in CFD, as the fluid was considered compressible.
The acoustic velocity is derived from Eq. 15.
As the compressibility of the fluid was limited, an approximation of the acoustic velocity under normal conditions could be written as:
The local acoustic velocity could be written as:
Figure 2A displays the shock wave jumps conditions with a coordinate fixed at the shock wave front. The equations of conservation (mass; momentum; energy) could be written as:
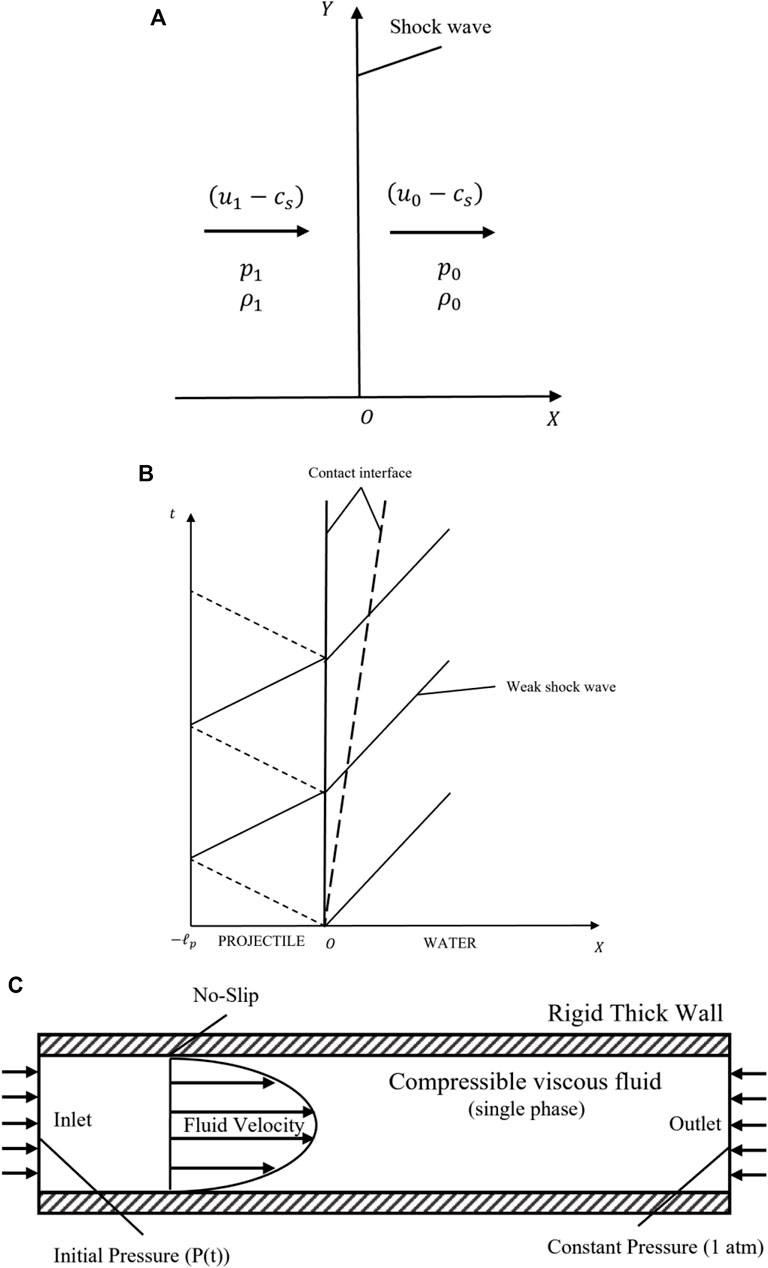
FIGURE 2. The isolation of boundary conditions and analysis of the model. (A) The weak shock wave jumps conditions under a coordinate fixed with the shock wave front; (B) The weak shock wave generated by projectile impact analysis; (C) A schematic diagram illustrating the micro-channel boundary conditions in CFD modeling.
These equations are usually known as the Rankine-Hugoniot equations (Smith, 1973). Here, we neglected the thickness of the shock front, and thus, the particle velocity,
The particle velocity could then be rewritten with Eq. 19.
or
Substituting by Eq. 20, gave:
The Taylor expanded form of Eq. 29 thus obtained is:
As the relationship between acoustic velocity and particle velocity was obtained, the differential form was:
As no cavitation or fluid column separation occurred and no cross-section changes were recorded along the channel, the Joukowsky equation was a perfect approximation to predict the maximum pressure in a water-hammer impact situation (Joukowsky, 1900; Walters and Leishear, 2018). This impact occurs at
Where
After the conditions of the fluid were solved, the impact condition obtained is shown in Figure 2B. The shock wave generation due to the projectile impact was a discrete process. In this study, since the buffer was assumed to be as thin as possible, it could be neglected given that it had the same impendence as the projectile. This model could be simplified to a projectile impacting the fluid column directly. Analysis of the projectile motion was performed according to Newton’s second law.
Where
Meanwhile, Eq. 12 was rewritten in terms of local acoustic velocity
The partial differential form of Eq. 33 is:
In our model, the pressure was too small as compared with the pressure constant, which is
The normal conditions here were
Upon integration of Eq. 36, a simplified function of pressure was gained.
Where the time constant was as follows:
Boundary layer induced by a very weak shock wave
Once a weak shock wave enters a static fluid boundary through the wall, a boundary layer begins to appear at the interaction point of the weak shock wave and the wall (Ackroyd, 1967; Davies and Bernstein, 1969). A study of the laminar compressible boundary layer induced by this weak shock wave was solved by H. Mirels’ in 1955, who provided theoretical evidence to prove that the weak shock waves generated by projectile impact indeed induce a boundary layer (Mirels, 1955).
Considering a possible turbulent boundary layer, R. E Melnik and B. Grossman developed an asymptotic theory within the limit of weak shock waves (Melnik and Grossman, 1974). Their three-layer description of the boundary layer was a natural extension of the asymptotic theories of Mellor (Mellor, 1972), Yajnik (Yajnik, 1970), Bush and Fendell (Bush and Fendell, 1972; Bush and Fendell, 1973) for incompressible boundary layers, as well as the theory by Afzal (Afzal, 1973) for compressible non-interacting turbulent boundary layers. FLUENT is a reliable and acceptable CFD software employed for relevant numerical simulations. To simulate our model, the k-ε model was utilized to analyze turbulent flow (Versteeg and Malalasekera, 2007; Nikpour et al., 2014).
Geometry definition
In the present work, the geometry definition consisted of a volume occupied by the flow with a specified shape of the physical boundaries. Here are several important criteria to limit the size of the channel, although, in the beginning, we planned to put the ‘lab’ into just one small chip to make this as convenient as possible. A fundamental restriction applied was the continuous flow in the fluid domain to ensure that the theories being considered were effective. A dimensionless parameter, the Knudsen number, was used to describe this problem (Guo et al., 2013); this number is defined as
Where
According to the requirements above, we established a simple CFD model to describe our method. The Cartesian coordinates system was created as shown in Figure 1B, and a
Mesh generation and solver settings
Transient simulation is strongly dependent on the quality of the mesh. For most water-hammer models to accurately capture the near-wall velocity, the mesh density near the wall should be concentrated. During the cross-section meshing process, the boundary layer neighboring the wall was divided into 20 layers with the first layer having a thickness of
Where
In the CFD solver setup, boundary settings comprise the physical description of the fluid flow as shown in Figure 2C. The pressure applied on the inlet boundary has been described as a function of time,
ANSYS Fluent® (2019R3) was utilized to obtain all the simulation results presented in this paper. In FLUENT, the numerical tech is a finite volume method (FVM). The whole process is transient. As the fluid is considered to be viscous, compressible, isothermal (no heat transfer), isotropic, and single-phase (no cavitation), the semi-implicit method for pressure-linked equations (SIMPLE) can be used as a flow solver. Convergence was defined to be
Results
Sample numerical results
A mesh independence analyses were performed using different element size (the original element size was proposed in Section 2.6) by the simulation of pressure and shear stress on bottom wall. The total mesh count for testing the independence were about
FIGURE 3. Mesh independence analysis and sample comparison results. The total mesh count for testing the independence were about
We choose a classical water hammer experiment (Inaba and Shepherd, 2010) to assess the modeling method in our work. In this example (shot 62), researchers accelerated a steel projectile (
In Figure 3C, we compared the experimental results (black line; shot 62, gauge g1) with the numerical results (blue line) calculated with the modeling method in Section 2. The maximum pressure of
Visualization of pressure wave propagation
To visualize and analyze the wave propagation process in detail, as well as the profile of the coupled compression and shear stresses in our method, a representative case with a water-filled channel and a projectile made of polymethyl methacrylate (PMMA) was designed; a practical size was determined as described in Section 2.5. The correlation between the projectile parameters (velocity and length) and the input pressure was assessed, and the results are shown in Figure 4. The maximum value and the duration of the pressure input were adjustable by changing the length and initial velocity of the projectile. This detailed relationship was derived in Section 2.3 (Joukowsky, 1900; Versteeg and Malalasekera, 2007).
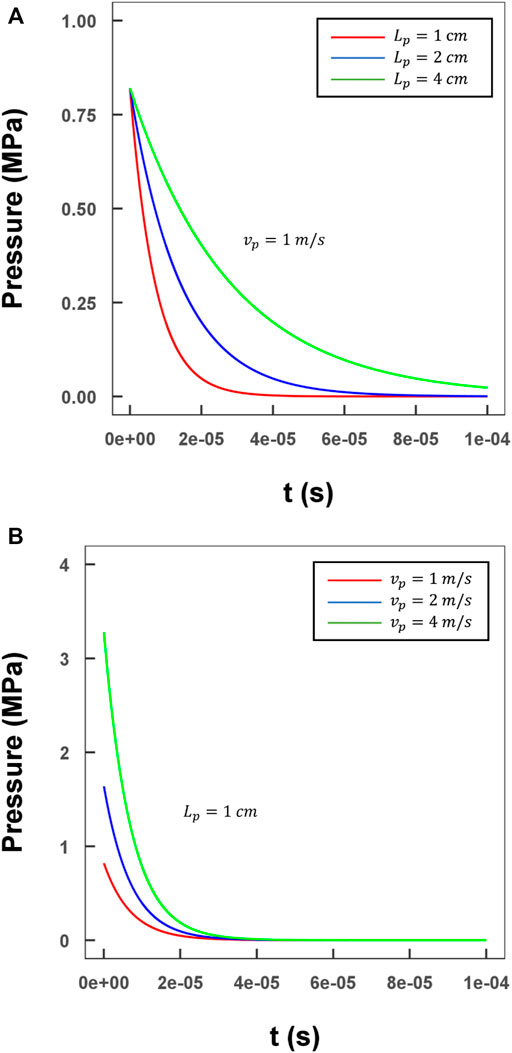
FIGURE 4. Evaluation of the relationship between various lengths/velocities of the projectile and the input pressure profile. (A) Three different lengths of projectile with a 1 m/s velocity (green line: 4 cm; blue line: 2 cm; red line: 1 cm); (B) Three different lengths of projectile with a 1 cm length (green line: 4 m/s; blue line: 2 m/s; red line: 1 m/s).
As the inlet boundary condition, maximum pressure of
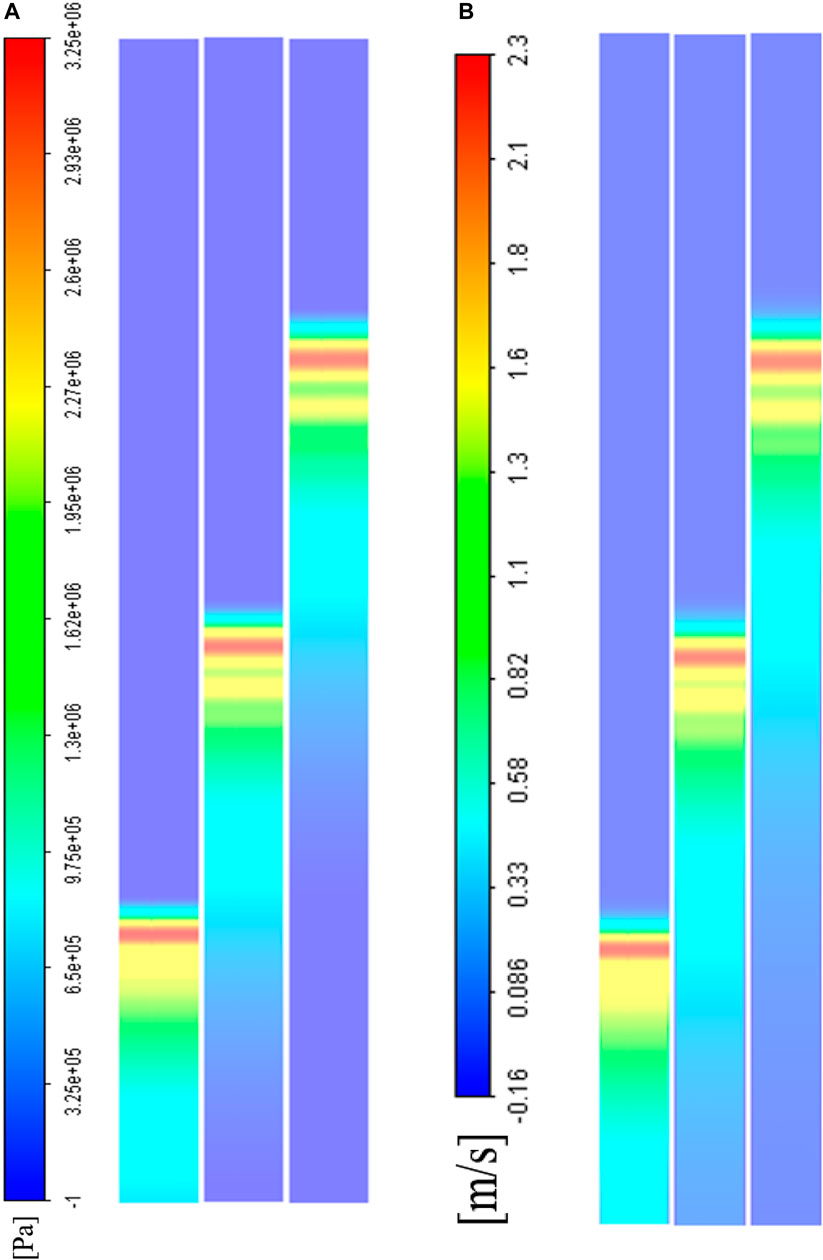
FIGURE 5. A CFD visualization of weak shock wave propagation in the middle axial cross-section at different time steps (
Stress distribution analysis of wall/cell cultured area
The bottom wall was defined as a smooth wall area for cell culture. The results at
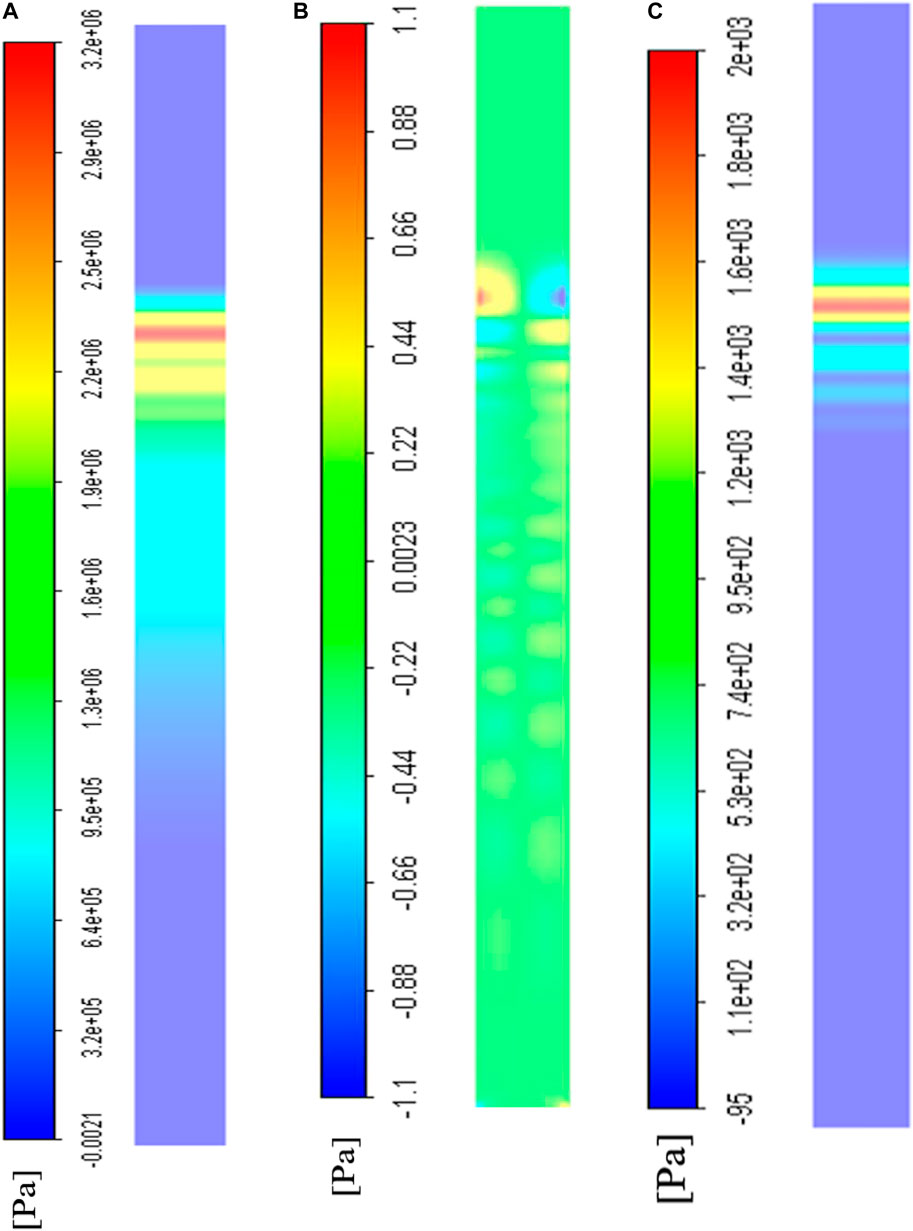
FIGURE 6. The CFD visualization of stress distribution on the bottom wall where cells were cultured at
Effect of cells on stress spatial distribution
To evaluate the influence of cultured cells on the spatial distribution of stress on the bottom wall, the relationship between the two distributions and the density and height of the cells could be written as:
Where
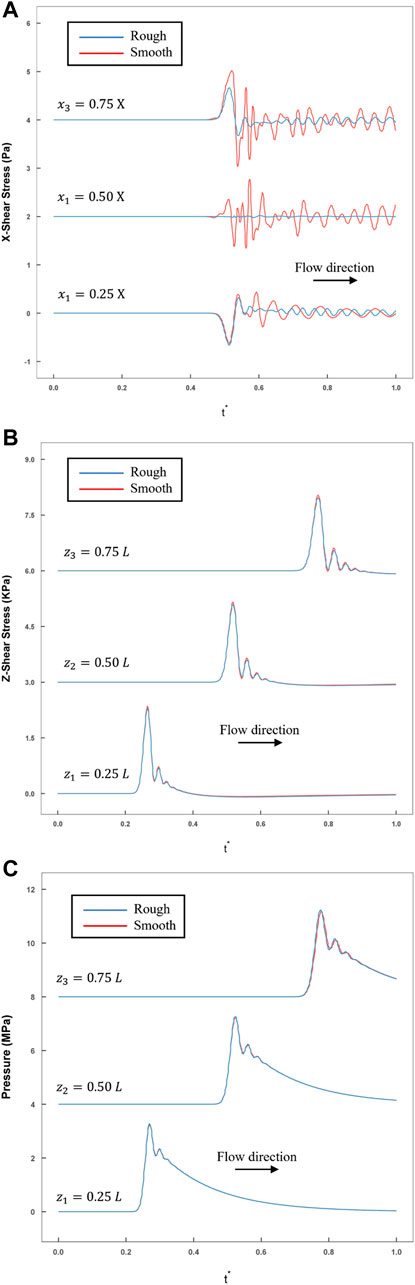
FIGURE 7. The comparison of rough and smooth model historical variations in stress were recorded by monitoring the points calculated by FLUENT (blue line: rough model; red line: smooth model). (A) The transverse shear stress recorded at three monitoring points (
In addition, the temporal variations in
Table 1 shows the attenuation of pressure and axial shear stress along the axial direction. In this model, the average reduction was within 1% in every
In addition, the temporal variations in the transverse direction are shown in Figure 8, where the monitoring points were set in the middle cross-section (
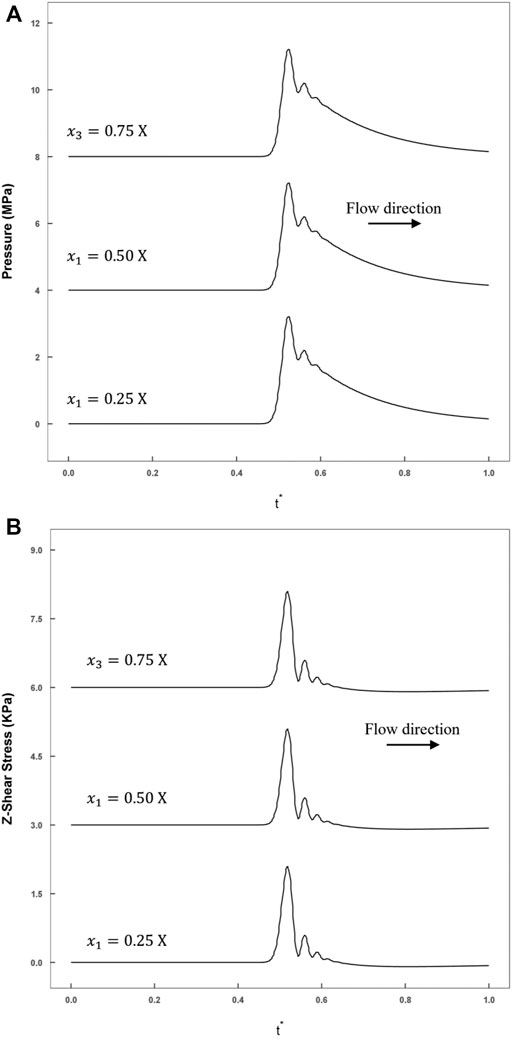
FIGURE 8. The smooth model historical variations in stress recorded by monitoring points calculated by FLUENT (blue line: rough model; red line: smooth model). (A) The axial shear stress recorded at three monitoring points (
Discussion
In the past, many studies have focused on revealing the mechanical properties of cells with the assistance of interdisciplinary techniques, such as solid mechanics, image processing, and fluid mechanics. Such works have implied that as compared to static or quasi-static loadings, the mechanical properties of cells are likely to show a distinct behavior difference under dynamic loading (
In this paper, we have proposed a new method for the application of dynamic loading that couple’s compression and shear stress synchronously on isolated cells under normal culture conditions. This method describes a case, where an impact loading from a projectile was employed to generate a pressure wave, together with corresponding shear stress due to the fluid viscosity. At the same time, the strain variations in the cell could be captured by a high-speed camera. Eventually, data on the essential stress and strain on the cells were collected to explore the mechanical properties of cells. We established a representative model (rectangular channel filled with water) as shown in Figures 1A,B. With this model, we explained two main questions as elaborated on in Section 2 and Section 3 on the working of this method and its known limitations:
1) How can the weak shock wave generated by projectile impact be calculated? Does the solid structure (channel) affect the flow and how can it reduce the fluid-structure interaction (FSI)?
2) Does the shear stress keep the same phase as the compression stress? Does the existence of cells have a strong influence on stress distribution? How is this related to the positions along the axial and transverse directions?
The basic purpose was to explore the biomechanical mechanism of single cell response to dynamic mechanical loading, where the length scale was focused on
The Doppler effect is commonly used to detect the flow velocity of a flowing fluid, but experimental techniques may render it less reliable on small velocities and the changing profile near the walls (Riasi et al., 2009). Several sophisticated numerical models have been established to more accurately explain the details of the transient flow (Ghidaoui and Kolyshkin, 2001; Martins et al., 2018). It has been proven that CFD performs very well in modeling the pressure wave traveling processes (Mandair, 2020). Therefore, we established the CFD model to help us evaluate the proposed method, and we will consider these evaluation results as a very important reference for future experimental work.
The boundary conditions should be comprehensively considered in CFD modeling as we have described in detail in Section 2 of this article. The criteria of channel size design were not too strict, which allowed us to adjust the details freely according to the actual lab environment, provided that the size details obeyed the above-mentioned limitations of the Knudson number to continuously maintain the fluid. As for the projectile, its material should be kept the same as that of the buffer. Figure 4 shows the means of controlling the amplitude and decay time constant of the initial pressure by the projectile.
The water hammer is a well-known problem. The weak shock wave generated in this problem would induce a sudden pressure jump corresponding to the low flow velocity. Accordingly, we described the stress wave propagation in total pressure and the flow velocity forms; we also visualized this process in the middle axial section (Figure 5). In this process, the dissipation effect was mainly due to the fluid viscosity and could not be neglected. We selected several points in the
Conclusions
In this paper, we focused on the application of dynamic loadings on single cells and revealed their mechanical response. Based on the Water-Hammer theories, we have developed a novel experimental method with a corresponding CFD model to help investigate cell mechanics under dynamic loadings. In this method, cells were normally cultured inside a microchannel. After impact, the stress wave generated applied a coupled compression-shear stress on an isolated living cell inside the microchannel. The results from an example model showed that the stress conditions could be easily controlled by controlling the velocity or length of the projectile. In addition, this method will allow researchers to adjust various design elements of their channels, such as the size, materials, etc., according to their lab’s environmental and actual needs, if the new design meets the relevant criteria presented in this study. This model offers repeatability, as a wide area is available for cell strain capturing, where cells suffer from nearly the same stress loadings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YL, HY, NZ, and DX conceived and designed the research. DX, SW, and NZ derived the theoretical process and the CFD modeling process. DX, NZ, and PZ wrote the paper. All authors reviewed and edited the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (12002285, 11722220, 61927810), Natural Science Basic Research Program of Shaanxi (2020JZ-11, 2020JQ-126), Fundamental Research Funds for the Central Universities (3102020smxy003), and the Program of Introducing Talents of Discipline to Universities (the 111 project) (No. BP0719007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ackroyd, J. (1967). On the laminar compressible boundary layer with stationary origin on a moving flat wall. Math. Proc. Camb. Phil. Soc. 63, 871–888. doi:10.1017/s0305004100041840
Afzal, N. (1973). A higher order theory for compressible turbulent boundary layers at moderately large Reynolds number. J. Fluid Mech. 57, 1–25. doi:10.1017/s0022112073000996
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., and Walters, P. (2002). Molecular biology of the cell. 4th edn. New York: Garland Science.
Bao, G., and Suresh, S. (2003). Cell and molecular mechanics of biological materials. Nat. Mater 2, 715–725. doi:10.1038/nmat1001
Ben-Dor, G., Igra, O., and Elperin, T. (2000). Handbook of shock waves, three volume set. San Diego, CA: Elsevier.
Bush, W. B., and Fendell, F. E. (1972). Asymptotic analysis of turbulent channel and boundary-layer flow. J. Fluid Mech. 56, 657–681. doi:10.1017/s0022112072002599
Bush, W. B., and Fendell, F. E. (1973). Asymptotic analysis of turbulent channel flow for mean turbulent energy closures. Phys. Fluids 16, 1189–1197. doi:10.1063/1.1694497
Davies, W., and Bernstein, L. (1969). Heat transfer and transition to turbulence in the shock-induced boundary layer on a semi-infinite flat plate. J. Fluid Mech. 36, 87–112. doi:10.1017/s0022112069001534
Deshpande, V. S., Heaver, A., and Fleck, N. A. (2006). An underwater shock simulator. Proc. R. Soc. A 462, 1021–1041. doi:10.1098/rspa.2005.1604
Desprat, N., Richert, A., Simeon, J., and Asnacios, A. (2005). Creep function of a single living cell. Biophysical J. 88, 2224–2233. doi:10.1529/biophysj.104.050278
Dudani, J. S., Gossett, D. R., Tse, T., and Di Carlo, D. (2013). Pinched-flow hydrodynamic stretching of single-cells. Lab. Chip 13, 3728–3734. doi:10.1039/c3lc50649e
Dymond, J., and Malhotra, R. (1988). The Tait equation: 100 years on. Int. J. Thermophys. 9, 941–951. doi:10.1007/bf01133262
Evans, E., and Yeung, A. (1989). Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophysical J. 56, 151–160. doi:10.1016/s0006-3495(89)82660-8
Ganadhiepan, G., Miramini, S., Patel, M., Mendis, P., and Zhang, L. (2019). Bone fracture healing under Ilizarov fixator: Influence of fixator configuration, fracture geometry, and loading. Int. J. Numer. Meth Biomed. Engng 35, (6), e3199. doi:10.1002/cnm.3199
Ghidaoui, M. S., and Kolyshkin, A. A. (2001). Stability analysis of velocity profiles in water-hammer flows. J. Hydraul. Eng. 127, 499–512. doi:10.1061/(asce)0733-9429(2001)127:6(499)
Guo, Z., Xu, K., and Wang, R. (2013). Discrete unified gas kinetic scheme for all Knudsen number flows: Low-speed isothermal case. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 88, 033305. doi:10.1103/PhysRevE.88.033305
Hao, Y., Cheng, S., Tanaka, Y., Hosokawa, Y., Yalikun, Y., and Li, M. (2020). Mechanical properties of single cells: Measurement methods and applications. Biotechnol. Adv. 45, 107648. doi:10.1016/j.biotechadv.2020.107648
Hoang, H., Galliero, G., Montel, F., and Bickert, J. (2015). Tait equation in the extended corresponding states framework: Application to liquids and liquid mixtures. Fluid Phase Equilibria 387, 5–11. doi:10.1016/j.fluid.2014.12.008
Inaba, K., and Shepherd, J. E. (2010). Flexural waves in fluid-filled tubes subject to axial impact. J. Press. Vessel Technol. 132, 021302. doi:10.1115/1.4000510
Joukowsky, N. (1900). Über den hydraulischen Stoss in Wasserleitungsröhren, Impériale des Sciences de St.Pétersbourg, Mémoires de lʾAcadémie.
Kandlikar, S., Garimella, S., Li, D., Colin, S., and King, M. R. (2005). Heat transfer and fluid flow in minichannels and microchannels. New York, NY: Elsevier.
Katti, D. R., Katti, K. S., Molla, S., and Kar, S. (2019). Biomechanics of cells as potential biomarkers for diseases: A new tool in mechanobiology. Encycl. Biomed. Eng. 13, 1–21. doi:10.1016/b978-0-12-801238-3.99938-0
Korteweg, V. D. (1878). Ueber die Fortpflanzungsgeschwindigkeit des Schalles in elastischen Röhren. Ann. Phys. Chem. 241, 525–542. doi:10.1002/andp.18782411206
Lennon, F. (1994). Shock wave propagation in water. Manchester, UK: Manchester Metropolitan University.
Mandair, S. (2020). 1D and 3D Water-Hammer Models: The energetics of high friction pipe flow and hydropower load rejection. Canada: University of Toronto.
Martins, N. M., Carriço, N. J., Ramos, D., and Covas, H. (2014). Velocity-distribution in pressurized pipe flow using CFD: Accuracy and mesh analysis. Comput. Fluids 105, 218–230. doi:10.1016/j.compfluid.2014.09.031
Martins, N. M. C., Brunone, B., Meniconi, S., Ramos, H. M., and Covas, D. I. C. (2018). Efficient computational fluid dynamics model for transient laminar flow modeling: Pressure wave propagation and velocity profile changes. J. Fluids Eng. 140, 011102. doi:10.1115/1.4037504
Martins, N. M., Soares, A. K., Ramos, H. M., and Covas, D. I. (2016). CFD modeling of transient flow in pressurized pipes. Comput. Fluids 126, 129–140. doi:10.1016/j.compfluid.2015.12.002
Mellor, G. L. (1972). The large Reynolds number, asymptotic theory of turbulent boundary layers. Int. J. Eng. Sci. 10, 851–873. doi:10.1016/0020-7225(72)90055-9
Melnik, R., and Grossman, B. (1974). Analysis of the interaction of a weak normal shock wave with a turbulent boundary layer. in 7th Fluid and PlasmaDynamics Conference, Palo Alto,CA,U.S.A., 598.doi:10.2514/6.1974-598
Moeendarbary, E., and Harris, A. R. (2014). Cell mechanics: Principles, practices, and prospects. WIREs Mech. Dis. 6, 371–388. doi:10.1002/wsbm.1275
Nagayama, K., Mori, Y., Shimada, K., and Nakahara, M. (2002). Shock Hugoniot compression curve for water up to 1 GPa by using a compressed gas gun. J. Appl. Phys. 91, 476–482. doi:10.1063/1.1421630
Nikpour, M., Nazemi, A., Dalir, A. H., Shoja, F., and Varjavand, P. (2014). Experimental and numerical simulation of water hammer. Arab. J. Sci. Eng. 39, 2669–2675. doi:10.1007/s13369-013-0942-1
Patterson, L. H. (2020). The MicroHammer: Investigating cellular response to impact with a microfluidic mems device. Santa Barbara: University of California.
Patterson, L. H., Walker, J. L., Rodriguez-Mesa, E., Shields, K., Foster, J. S., Valentine, M. T., et al. (2019). Investigating cellular response to impact with a microfluidic MEMS device. J. Microelectromechanical Syst. 29, 14–24.
Pelham, R. J., and Wang, Y.-L. (1997). Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U.S.A. 94, 13661–13665. doi:10.1073/pnas.94.25.13661
Pinnington, R. (1997). The axisymmetric wave transmission properties of pressurized flexible tubes. J. sound Vib. 204, 271–289. doi:10.1006/jsvi.1997.0938
Rapp, B. E. (2016). Microfluidics: Modeling, mechanics and mathematics. Cambridge, US: William Andrew.
Riasi, A., Nourbakhsh, A., and Raisee, M. (2009). Unsteady velocity profiles in laminar and turbulent water hammer flows. J. fluids Eng. 131, 121202. doi:10.1115/1.4000557
Shepherd, J. E., and Inaba, K. (2009). Shock loading and failure of fluid-filled tubular structures. Dyn. Fail. Mater. Struct. 5, 153–190. doi:10.1007/978-1-4419-0446-1_6
Thorley, A., and Guymer, C. (1976). Pressure surge propagation in thick-walled conduits of rectangular cross section.
Trepat, X., Grabulosa, M., Puig, F., Maksym, G. N., Navajas, D., and Farré, R. (2004). Viscoelasticity of human alveolar epithelial cells subjected to stretch. Am. J. Physiology-Lung Cell. Mol. Physiology 287, L1025–L1034. doi:10.1152/ajplung.00077.2004
Urbanska, M., Muñoz, H. E., Shaw Bagnall, J. S., Otto, O., Manalis, S. R., Di Carlo, D., et al. (2020). A comparison of microfluidic methods for high-throughput cell deformability measurements. Nat. Methods 17, 587–593. doi:10.1038/s41592-020-0818-8
Versteeg, H. K., and Malalasekera, W. (2007). An introduction to computational fluid dynamics: The finite method. London, UK: Pearson education.
Walters, T. W., and Leishear, R. A. (2018). When the Joukowsky equation does not predict maximum water hammer pressures, in Pressure Vessels and Piping Conference, Prague, Czech Republic: American Society of Mechanical Engineers ASME. doi:10.1115/pvp2018-84050
Yajnik, K. S. (1970). Asymptotic theory of turbulent shear flows. J. Fluid Mech. 42, 411–427. doi:10.1017/s0022112070001350
Zhang, L., Gardiner, B. S., Smith, D. W., Pivonka, P., and Grodzinsky, A. (2008). A fully coupled poroelastic reactive-transport model of cartilage. Mol. Cell Biomech. 5 (2), 133–153. doi:10.3970/mcb.2008.005.133
Keywords: dynamic compression-shear coupling stress, Device development, cell mechanics, CFD model, weak shock wave
Citation: Xu D, Zhang N, Wang S, Zhang P, Li Y and Yang H (2022) A method for generating dynamic compression shear coupled stress loading on living cells. Front. Bioeng. Biotechnol. 10:1002661. doi: 10.3389/fbioe.2022.1002661
Received: 25 July 2022; Accepted: 24 August 2022;
Published: 21 September 2022.
Edited by:
Guang-Kui Xu, Xi’an Jiaotong University, ChinaCopyright © 2022 Xu, Zhang, Wang, Zhang, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulong Li, bGl5dWxvbmdAbndwdS5lZHUuY24=; Hui Yang, a2l0dHl5aEBud3B1LmVkdS5jbg==
†These authors have contributed equally to this work
 Dasen Xu1,2†
Dasen Xu1,2† Nu Zhang
Nu Zhang Sijie Wang
Sijie Wang Pan Zhang
Pan Zhang Hui Yang
Hui Yang