
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Bioeng. Biotechnol., 11 January 2022
Sec. Biomaterials
Volume 9 - 2021 | https://doi.org/10.3389/fbioe.2021.820468
This article is part of the Research TopicBiomaterials with the regulation of reactive oxygen/nitrogen species for biomedical applicationsView all 21 articles
Reactive oxygen species (ROS) are the key signaling molecules in many physiological signs of progress and are associated with almost all diseases, such as atherosclerosis, aging, and cancer. Bone is a specific connective tissue consisting of cells, fibers, and mineralized extracellular components, and its quality changes with aging and disease. Growing evidence indicated that overproduced ROS accumulation may disrupt cellular homeostasis in the progress of bone modeling and remodeling, leading to bone metabolic disease. Thus, ROS-responsive biomaterials have attracted great interest from many researchers as promising strategies to realize drug release or targeted therapy for bone-related diseases. Herein, we endeavor to introduce the role of ROS in the bone microenvironment, summarize the mechanism and development of ROS-responsive biomaterials, and their completion and potential for future therapy of bone-related diseases.
As an internal support system, bone is a dynamic connective tissue with highly mineralized architecture giving it substantial strength, providing a structural foundation for the human body and muscle (McEnery et al., 2016). Bone is in the dynamic modeling and remodeling processes by developing the activities of bone formation and resorption, for the healthy development of the skeleton (Florencio-Silva et al., 2015). With the increased longevity of human life and the aging of population, bone-related diseases, such as osteoporosis, osteosarcoma, bone metastasis, osteoarthritis, and osteomyelitis, cause significant pain and amplify the economic burden for millions of people worldwide. Growing evidence indicates that bone homeostasis is adversely affected by oxidative stress induced by reactive oxygen species (ROS), thus targeting ROS might be a vital approach for the treatment of bone metabolic disorders (Agidigbi and Kim, 2019). In the meantime, ROS are necessary for bone remodeling machinery as they can enhance the degradation of the mineralized matrix and affect the behaviors of all the cells involved in bone remodeling. ROS is normally divided into two categories: non-radical derivatives of oxygen and free chemically reactive oxygen radicals (Phaniendra et al., 2015). ROS production could lead to an increased level of oxidative stress, which increases the risk of mutations in mitochondrial and nuclear DNA (Russo et al., 2012). Besides, oxidative stress has been known as a major donor to the immune response and linked with the pathophysiology of almost all organs (Mittal et al., 2014). It has also been proved that oxidative stress play role in aging and lead to degenerative diseases with increasing age (Sharifi-Rad et al., 2020). Therefore, it is important to understand how ROS modulate bone biology and pathology.
In addition, for the therapy of bone-related diseases, researchers are developing various scaffolds, hydrogels, nanocarriers, and drugs (Yao et al., 2019; Yin et al., 2021; Zheng et al., 2021). Synthetic or natural biomaterials can enhance the restoration of bone structure and function. Implant materials make contact with the patient’s tissue, permitting interactions with endogenous bone. Among all kinds of treatments, developing novel biomaterials and drugs which targeted the high-level ROS should be promising solutions for bone-related diseases. The antioxidants or selected therapeutic compounds can be loaded with biomaterials and will be stimuli-responsive released with the presence of oxidation. Hubbel’s team was the first to use ROS-responsive biomaterials for drug delivery in 2001 (Napoli et al., 2001), and then this new strategy quickly spreads for different biomedical applications. However, the summary of the recently developed strategies about ROS-responsive biomaterial for bone-related diseases is still lacking. In this review, we will introduce the major findings of the regulatory role of ROS in osteoclast biology, including the influence of ROS on signaling, proliferation, and differentiation of bone cells. The review will also mention the ROS-responsive biomaterials which could be excellent options sense and respond ROS microenvironment. How targeting ROS may be a solution for the therapy of bone-related diseases, such as osteoarthritis, will be discussed. Finally, we propose a prospect for the trends and future development of ROS-responsive biomaterials for the application in bone tissue.
Bone is a dynamic organ to achieve the functions such as growth, movement, organ protection, or calcium/phosphate equilibrium (Hardy and Fernandez-Patron, 2020). Bone remodeling is mediated by the progress of formation and resorption led by the activities of osteoblast and osteoclast, allowing bone growth and tissue regeneration (Callaway and Jiang, 2015). In bone tissues, ROS generation is an essential signal in living organisms which could regulate cell functions, mediate inflammation, and affect the pathophysiology of tissues (Wauquier et al., 2009). Among all forms of ROS, the majority of forms related to the osteoclastogenesis are superoxide (O2−) and hydrogen peroxide (H2O2). Early research in the 1990s was about the first connecting ROS and osteoclast function by assessing the osteoclast activity after adding oxidants or antioxidant enzymes, indicating that the superoxide radical could enhance bone resorption (Garrett et al., 1990). ROS originated from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) were localized to the interface of bone-osteoclast, resulting in the production of superoxide within the osteoclast (Key et al., 1990). At the same time, other studies indicated that H2O2 was the main ROS to promote the formation and activity of osteoclast (Kim et al., 2006). However, different from these studies describing a direct role of ROS in bone resorption, subsequent studies suggested that ROS could promote osteoclast formation and activity via an indirect mechanism in activating signaling pathways (Hall et al., 1995). Specifically, ROS could influence several signaling pathways such as nuclear factor κB (NF-κB) and produce the following receptor activator of NF-κB ligand (RANKL).
Upon to the enhanced activity of osteoclast by ROS, H2O2 has shown an inhibition effect on the cell differentiation of osteoblast activities. Oxidative stress originated from H2O2 restrained the process of osteoblastic differentiation in the bone marrow stromal cells (BMSCs) of mouse and rabbit (Bai et al., 2004), showing a reduction in the alkaline phosphatase (ALP) activity. In addition to influencing the differentiation process, ROS also affects the lifespan of osteoblast (Linares et al., 2009). Thus, as shown in Figure 1, ROS not only directly promotes osteoclastogenesis but also inhibits the differentiation and growth of osteoblasts by stimulating RANKL-induced formation of osteoclast. ROS seems to perform an important role in bone by affecting both cell types. These findings also indicate that the treatment strategies for bone diseases by targeting ROS should be promising.
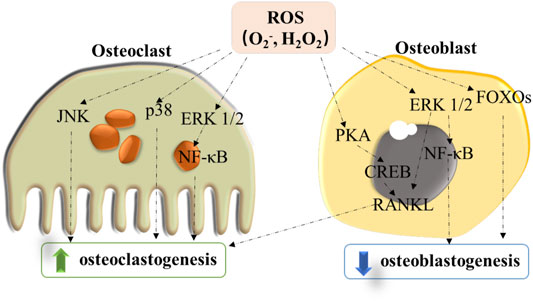
FIGURE 1. Modulation role of ROS for signaling pathways in inhibiting osteoblast differentiation and enhancing osteoclastogenesis.
ROS are overproduced in diseased cells, and this property has been employed to develop ROS-responsive biomaterials as an intelligent drug delivery system. In response to ROS, ROS-responsive biomaterials can release drugs, used as targeting delivery agents, imaging agents, and therapeutic agents for regulating the tissue microenvironments and tissue regeneration. As described in Figure 2, mechanisms of the responsive function for ROS-responsive biomaterials can be classified into two types: ROS affect the physical properties of biomaterials especially affect their solubility, or ROS mediate the chemical properties which will lead to the bond cleavage reaction.
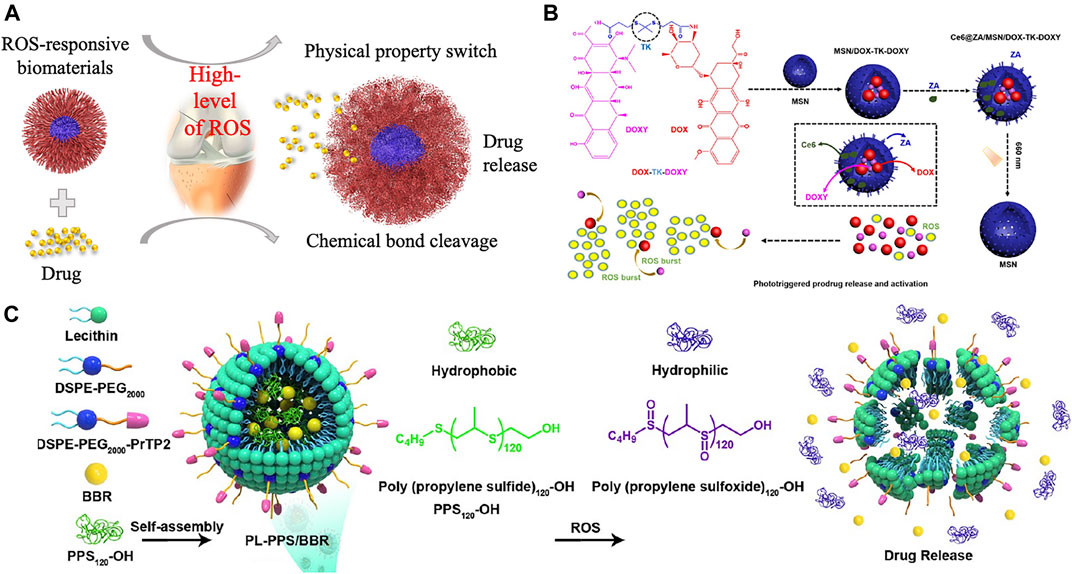
FIGURE 2. (A). Scheme of ROS-responsive drug-loaded biomaterials and its responsive mechanism for the treatment of bone-related diseases. (B). Prodrug DOX-TK-DOXY was loaded into the mesoporous silica nanoparticles (MSNs) and disrupt the TK linkage of the prodrug in an ROS environment. Reproduced with permission (Tong et al., 2020). Copyright 2020 American Chemical Society. (C) PPS120 converted to poly(propylene sulfoxide)120 and released drug in an ROS environment. Reproduced with permission (Zhao et al., 2021). Copyright 2021 American Chemical Society.
ROS can affect the physical property, especially the solubility of poly(propylene sulfide) (PPS), thioether-containing polymers, tellurium-, and selenium-containing polymers, favoring the drug release property (Gao and Xiong, 2021). In the presence of an oxidative environment, the hydrophobic sulfides can convert into hydrophilic sulfoxides and sulfones, contributing to their increased water solubility and potential application for drug delivery (Napoli et al., 2004). Poly(ethylene glycol) (PEG)-b-PPS and PPS-b-pyromellitic dianhydride (PMDA) copolymers are sensitized to H2O2, but shows no response against superoxide. While the PPS–PEG–superoxide dismutase (SOD) micelles are capable to turn into hydrophilic under both superoxide and hydrogen peroxide. In addition, thioether-based biomaterials also will be oxidized under the ROS environment. When susceptible to ROS (100 mM H2O2), the hydrophobic backbone of thioether containing biomaterials will be transformed to hydrophilic (Mahmoud et al., 2011). When simulated the ROS environment with 1 mM H2O2, a fast release of berberine (BBR) was observed from PL-PPS/BBR at 48 h (Figure 2C). During this progress, PPS120 scavenged ROS and converted to poly(propylene sulfoxide)120 when reacted with ROS to obtain the rapid release of BBR (Zhao et al., 2021). Thioether-containing polymers contribute an effective method for loading and delivering hydrophobic drugs especially for the application of cancer therapy. Selenium-containing copolymers show a similar solubility change under ROS as PPS, but more sensitive to ROS due to the bigger lower bond energy and larger radius of the selenium atom, making selenium-containing polymers a promising material for drug delivery. It was reported that the diselenide-containing micelles could react with ROS at a low concentration of H2O2 (0.01% v/v) and release drug in a short time (Xu et al., 2013).
In addition to the oxidant-induced solubility conversion, ROS can cleave the chemical bonds linkage of poly(thioketal) (TK), poly(proline), phenylboronic acid, and ester-containing polymers, giving rise to their degradation under the ROS environment. TK-containing polymers can be prepared by directly condensing polymerization using thiols for cancer therapy. With the degradation of thioketal linkages after exposure to 0.2 mM KO2, poly(1,4-phenylene-acetone dimethylenethioketal) (PPADT) has been used for the oral delivery of TNF-α-siRNA to the intestine of mice to treat ulcerative colitis (Wilson et al., 2010). Another ROS and pH dual-responsive PPADT-based biomaterial was developed for antiinflammatory therapy, showing 50% drug releasing in response to 1 mM H2O2 within 4 h (Pu et al., 2014). The poly(thioketal urethane) (PTK-URs) scaffolds are degraded in contact with the ROS released by cells, showing promising applications for wound repair (Martin et al., 2014) and bone regeneration (McEnery et al., 2016).
Among the various functional groups, phenyboronic acid and ester are unique because they are highly selectively and rapidly oxidized in responsive to H2O2 and generate phenol and boronic acid (Li et al., 2017). Being a polymer backbone or connected through an ether linkage were two strategies to include the groups in ROS sensitivity polymers which can be degraded in H2O2 (50–100 mM). An ROS-responsive nanocarrier (3I-NM@siRNA), which achieve the ROS-responsive property by adding the arylboronic ester group, was designed to carry small interfering RNA (siRNA) to improve long circulation stability of siRNA and increase their delivery efficiency (Zheng et al., 2019). Proline, as one of the amino acids, could also be cleaved by oxidation because of the metal-catalyzed oxidation of the proline segments, which make them a good candidate for ROS-responsive biomaterials (Amici et al., 1989).
Osteoarthritis (OA) is the most common form of arthritis, occurring most frequently in the population aged over 60 years (Shane Anderson and Loeser, 2010). OA was a degenerative joint disease and referred as an imbalance between the decay and formation of chondrocytes, extracellular matrix (ECM), and subchondral bone with a main feature of the destruction of cartilage (Sharma et al., 2013). Many factors could increase the risk of OA, including biomechanical forces, ROS, and auto-immunity (Corti, 2003). ROS is suspected in the pathological variation of microenvironments. The excessive ROS in OA will induce the damage of DNA and chondrocytes, and affect the production and turnover of ECM by stimulating matrix metalloproteinases (MMPs). In addition, the excess ECM production will stimulate immune cells to generate more ROS (Khan et al., 2017).
Since there is a positive relation between the high-level ROS and OA, many antioxidants which could scavenge the ROS are used to suppress inflammatory response in OA, including vitamin C, polysaccharides, or polymers without drugs. Polyphenols are derived from plants such as tea or grape, and have high antioxidant property, which have been extensively applied in bone regeneration and capsulated in a layer-by-layer coated gelatin nanoparticle (Shutava et al., 2009). To obtain a longer site-specific retention time, the ROS scavenger such as nitroxide radical compounds were loaded into triblock copolymers. Sponge polymeric microsphere (PPS-MS) was prepared to treat post-traumatic osteoarthritis (PTOA) by inhibiting the MMP activity and the destruction of articular cartilage (O’Grady et al., 2018).
Traditional therapy for OA requires frequent drug administration due to the quick removal speed. The drug delivery systems maintain the concentration of drugs and achieve specific release at the desirable site. The high concentration of ROS in OA can play as a trigger for the drug release from ROS-responsive biomaterials. For example, hollow poly(lactide-co-glycolide) (PLGA) microspheres were designed to treat OA for the delivery of antiinflammatory drug dexamethasone, FeCl2, sodium bicarbonate, and ethanol. PLGA shells allow the penetration of H2O2 and excessive H2O2 will active Fenton’s reaction, leading ethanol transform into acetic acid, thus making the sodium bicarbonate generate CO2 gas to breaks the PLGA shells and release dexamethasone. Compared to free dexamethasone, the responsive drug-loaded biomaterials show significant therapeutic effects (Chung et al., 2015).
Though the presence of ROS would worsen the condition in OA, ROS is required during the early phase of the antimicrobial (Ren et al., 2020a; Ji et al., 2021; Wang et al., 2021; Zhu et al., 2021) and anticancer (Ren et al., 2020b) progress for osteomyelitis and osteosarcomas. Particular emphasis is placed on the ROS-mediated photochemical and molecular mechanisms that give rise to the establishment of photothermal therapy as a treatment of highly resistant diseases, especially invasive and metastatic tumors. The inadequate ROS suppression could hinder the healing but the redox balance needs to be carefully maintained during the tissue regeneration phase.
The photodynamic therapy is a recently developed treatment that produces ROS from photosensitizers or photosensitizing agents by light activation to kill cancer cells or bacteria (Shi et al., 2019). Hu et al. (2019) reported a lipid-polymer hybrid nanocarrier with an ROS-responsive core consisted of homopolymer poly(thioketal phosphoester) (TK-PPE) to encapsulate chlorin e6 (Ce6) and DOX for the treatment of cancer. With the irradiation of 660 nm laser, the encapsulated Ce6 will generate ROS, inducing PDT for cancer treatment and rapidly degrades TK-PPE to burst release the loaded DOX, leading to an efficient combinational therapy of chemo-PDT to inhibit the growth of tumor in vitro and in vivo (Hu et al., 2019). Similarly, a size changeable HBPTK-Ce6 was designed by conjugating Ce6 onto the micelles containing thioketal units and loaded the anticancer drug camptothecin (CPT). The irradiation of 660 nm laser lead to the size reducing of HPBTK-Ce6@CPT and boost CPT release, which contribute a much deeper penetration in tumor to kill inner tumor cells compared with the original large-size micelles (Jin et al., 2019). More recently, bone-targeting prodrug mesoporous silica-based nanoreactor was developed to treat osteosarcoma. Upon laser irradiation, the loaded Ce6 produces in situ ROS and disrupt the TK linkage to release DOX and doxycycline (DOXY) from the prodrugs (Figure 2B) (Tong et al., 2020).
Many bone-related diseases such as osteoporosis, rheumatoid arthritis, and bone metastases have been linked to the states of higher ROS, thus understanding how ROS interacts with osteoclasts and other cells in the bone could enhance the development of novel therapeutic biomaterials to target ROS in the bone tissue. Antioxidant compounds may be beneficial to bone health, such as dried plum polyphenols (Bu et al., 2008) or simvastatin (Moon et al., 2011). Although it seems promising to use antioxidant agents to target ROS and treat bone diseases, ROS is present over the whole body. Therefore, the non-specificity ROS targeted compounds may affect other tissues, which are not limited to bone. To specifically treat the bone-related disease, biomaterials which could specifically target bone with ROS responsible property should be focused in the future. The combination with bone targeted drug such as bisphosphonates, oligopeptides, or tetracycline could be a prospective solution. In addition, adapter with ligand-receptor binding with the bone site could be decorated with the surface of ROS-responsive biomaterials. Moreover, multiple responsive carriers can be developed by the combination of ROS-responsive system with functional groups to achieve more accurate controlled release of drugs.
To sum up, ROS-responsive biomaterials have advantages when mediating oxidative stress-related diseases as drug delivery carriers or therapeutic agents, and thus should be a promising strategy for regulating bone microenvironments and bone regeneration.
XR, HL, and XW contributed equally. XW contributed to the conception. XR and HL drew the figures and wrote the article. WW, XW, and JS revised the manuscript. All authors read and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowledge the financial support from National Key R&D Program of China (2018YFC2001500); National Natural Science Foundation of China (91749204, 82172098, 81771491); Shanghai Municipal Health Commission General Program (201740237).
Agidigbi, T. S., and Kim, C. (2019). Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Ijms 20, 3576–3616. doi:10.3390/ijms20143576
Amici, A., Levine, R. L., Tsai, L., and Stadtman, E. R. (1989). Conversion of Amino Acid Residues in Proteins and Amino Acid Homopolymers to Carbonyl Derivatives by Metal-Catalyzed Oxidation Reactions. J. Biol. Chem. 264, 3341–3346. doi:10.1016/s0021-9258(18)94071-8
Bai, X.-c., Lu, D., Bai, J., Zheng, H., Ke, Z.-y., Li, X.-m., et al. (2004). Oxidative Stress Inhibits Osteoblastic Differentiation of Bone Cells by ERK and NF-Κb. Biochem. Biophysical Res. Commun. 314, 197–207. doi:10.1016/j.bbrc.2003.12.073
Bu, S. Y., Lerner, M., Stoecker, B. J., Boldrin, E., Brackett, D. J., Lucas, E. A., et al. (2008). Dried Plum Polyphenols Inhibit Osteoclastogenesis by Downregulating NFATc1 and Inflammatory Mediators. Calcif. Tissue Int. 82, 475–488. doi:10.1007/s00223-008-9139-0
Callaway, D. A., and Jiang, J. X. (2015). Reactive Oxygen Species and Oxidative Stress in Osteoclastogenesis, Skeletal Aging and Bone Diseases. J. Bone Miner. Metab. 33, 359–370. doi:10.1007/s00774-015-0656-4
Chung, M.-F., Chia, W.-T., Wan, W.-L., Lin, Y.-J., and Sung, H.-W. (2015). Controlled Release of an Anti-inflammatory Drug Using an Ultrasensitive ROS-Responsive Gas-Generating Carrier for Localized Inflammation Inhibition. J. Am. Chem. Soc. 137, 12462–12465. doi:10.1021/jacs.5b08057
Corti, M. C., and Rigon, C. (2003). Epidemiology of Osteoarthritis: Prevalence, Risk Factors and Functional Impact. Aging Clin. Exp. Res. 15, 359–363. doi:10.1007/BF03327356
Florencio-Silva, R., Sasso, G. R. D. S., Sasso-Cerri, E., Simões, M. J., and Cerri, P. S. (20152015). Biology of Bone Tissue: Structure, Function, and Factors that Influence Bone Cells. Biomed. Res. Int. 2015, 1–17. doi:10.1155/2015/421746
Gao, F., and Xiong, Z. (2021). Reactive Oxygen Species Responsive Polymers for Drug Delivery Systems. Front. Chem. 9, 1–17. doi:10.3389/fchem.2021.649048
Garrett, I. R., Boyce, B. F., Oreffo, R. O., Bonewald, L., Poser, J., and Mundy, G. R. (1990). Oxygen-derived Free Radicals Stimulate Osteoclastic Bone Resorption in Rodent Bone In Vitro and In Vivo. J. Clin. Invest. 85, 632–639. doi:10.1172/JCI114485
Hall, T. J., Schaeublin, M., Jeker, H., Fuller, K., and Chambers, T. J. (1995). The Role of Reactive Oxygen Intermediates in Osteoclastic Bone Resorption. Biochem. Biophysical Res. Commun. 207, 280–287. doi:10.1006/bbrc.1995.1184
Hardy, E., and Fernandez-Patron, C. (2020). Destroy to Rebuild: The Connection between Bone Tissue Remodeling and Matrix Metalloproteinases. Front. Physiol. 11, 1–24. doi:10.3389/fphys.2020.00047
Hu, L., Cao, Z., Ma, L., Liu, Z., Liao, G., Wang, J., et al. (2019). The Potentiated Checkpoint Blockade Immunotherapy by ROS-Responsive Nanocarrier-Mediated cascade Chemo-Photodynamic Therapy. Biomaterials 223, 119469. doi:10.1016/j.biomaterials.2019.119469
Ji, Y., Han, Z., Ding, H., Xu, X., Wang, D., Zhu, Y., et al. (2021). Enhanced Eradication of Bacterial/Fungi Biofilms by Glucose Oxidase-Modified Magnetic Nanoparticles as a Potential Treatment for Persistent Endodontic Infections. ACS Appl. Mater. Inter. 13, 17289–17299. doi:10.1021/acsami.1c01748
Jin, H., Zhu, T., Huang, X., Sun, M., Li, H., Zhu, X., et al. (2019). ROS-responsive Nanoparticles Based on Amphiphilic Hyperbranched Polyphosphoester for Drug Delivery: Light-Triggered Size-Reducing and Enhanced Tumor Penetration. Biomaterials 211, 68–80. doi:10.1016/j.biomaterials.2019.04.029
Key, L. L., Ries, W. L., Taylor, R. G., Hays, B. D., and Pitzer, B. L. (1990). Oxygen Derived Free Radicals in Osteoclasts: The Specificity and Location of the Nitroblue Tetrazolium Reaction. Bone 11, 115–119. doi:10.1016/8756-3282(90)90058-7
Khan, N. M., Haseeb, A., Ansari, M. Y., Devarapalli, P., Haynie, S., and Haqqi, T. M. (2017). Wogonin, a Plant Derived Small Molecule, Exerts Potent Anti-inflammatory and Chondroprotective Effects through the Activation of ROS/ERK/Nrf2 Signaling Pathways in Human Osteoarthritis Chondrocytes. Free Radic. Biol. Med. 106, 288–301. doi:10.1016/j.freeradbiomed.2017.02.041
Kim, H., Kim, I. Y., Lee, S. Y., and Jeong, D. (2006). Bimodal Actions of Reactive Oxygen Species in the Differentiation and Bone-Resorbing Functions of Osteoclasts. FEBS Lett. 580, 5661–5665. doi:10.1016/j.febslet.2006.09.015
Li, C., Pan, R., Li, P., Guan, Q., Ao, J., Wang, K., et al. (2017). Hydrogen Peroxide-Responsive Nanoprobe Assists Circulating Tumor Cell Identification and Colorectal Cancer Diagnosis. Anal. Chem. 89, 5966–5975. doi:10.1021/acs.analchem.7b00497
Linares, G. R., Xing, W., Govoni, K. E., Chen, S.-T., and Mohan, S. (2009). Glutaredoxin 5 Regulates Osteoblast Apoptosis by Protecting against Oxidative Stress. Bone 44, 795–804. doi:10.1016/j.bone.2009.01.003
Mahmoud, E. A., Sankaranarayanan, J., Morachis, J. M., Kim, G., and Almutairi, A. (2011). Inflammation Responsive Logic Gate Nanoparticles for the Delivery of Proteins. Bioconjug. Chem. 22, 1416–1421. doi:10.1021/bc200141h
Martin, J. R., Gupta, M. K., Page, J. M., Yu, F., Davidson, J. M., Guelcher, S. A., et al. (2014). A Porous Tissue Engineering Scaffold Selectively Degraded by Cell-Generated Reactive Oxygen Species. Biomaterials 35, 3766–3776. doi:10.1016/j.biomaterials.2014.01.026
McEnery, M. A. P., Lu, S., Gupta, M. K., Zienkiewicz, K. J., Wenke, J. C., Kalpakci, K. N., et al. (2016). Oxidatively Degradable Poly(thioketal Urethane)/ceramic Composite Bone Cements with Bone-like Strength. RSC Adv. 6, 109414–109424. doi:10.1039/c6ra24642g
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., and Malik, A. B. (2014). Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signaling 20, 1126–1167. doi:10.1089/ars.2012.5149
Moon, H.-J., Kim, S. E., Yun, Y. P., Hwang, Y.-S., Bang, J. B., Park, J.-H., et al. (2011). Simvastatin Inhibits Osteoclast Differentiation by Scavenging Reactive Oxygen Species. Exp. Mol. Med. 43, 605–612. doi:10.3858/emm.2011.43.11.067
Napoli, A., Tirelli, N., Kilcher, G., and Hubbell, A. (2001). New Synthetic Methodologies for Amphiphilic Multiblock Copolymers of Ethylene Glycol and Propylene Sulfide. Macromolecules 34, 8913–8917. doi:10.1021/ma0108057
Napoli, A., Valentini, M., Tirelli, N., Müller, M., and Hubbell, J. A. (2004). Oxidation-responsive Polymeric Vesicles. Nat. Mater 3, 183–189. doi:10.1038/nmat1081
O’Grady, K. P., Kavanaugh, T. E., Cho, H., Ye, H., Gupta, M. K., Madonna, M. C., et al. (2018). Drug-Free ROS Sponge Polymeric Microspheres Reduce Tissue Damage from Ischemic and Mechanical Injury. ACS Biomater. Sci. Eng. 4, 1251–1264. doi:10.1021/acsbiomaterials.6b00804
Phaniendra, A., Jestadi, D. B., and Periyasamy, L. (2015). Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 30, 11–26. doi:10.1007/s12291-014-0446-0
Pu, H.-L., Chiang, W.-L., Maiti, B., Liao, Z.-X., Ho, Y.-C., Shim, M. S., et al. (2014). Nanoparticles with Dual Responses to Oxidative Stress and Reduced pH for Drug Release and Anti-inflammatory Applications. ACS Nano 8, 1213–1221. doi:10.1021/nn4058787
Ren, X., Gao, R., Van Der Mei, H. C., Ren, Y., Peterson, B. W., and Busscher, H. J. (2020a). Eradicating Infecting Bacteria while Maintaining Tissue Integration on Photothermal Nanoparticle-Coated Titanium Surfaces. ACS Appl. Mater. Inter. 12, 34610–34619. doi:10.1021/acsami.0c08592
Ren, X., Yi, Z., Sun, Z., Ma, X., Chen, G., Chen, Z., et al. (2020b). Natural Polysaccharide-Incorporated Hydroxyapatite as Size-Changeable, Nuclear-Targeted Nanocarrier for Efficient Cancer Therapy. Biomater. Sci. 8, 5390–5401. doi:10.1039/d0bm01320j
Russo, G., Curcio, F., Bulli, G., Aran, L., Della-morte, D., Testa, G., et al. (2012). Oxidative Stress and Diseases. Oxidative Stress Dis, 757–772. doi:10.5772/2535
Shane Anderson, A., and Loeser, R. F. (2010). Why Is Osteoarthritis an Age-Related Disease? Best Pract. Res. Clin. Rheumatol. 24, 15–26. doi:10.1016/j.berh.2009.08.006
Sharifi-Rad, M., Anil Kumar, N. V., Zucca, P., Varoni, E. M., Dini, L., Panzarini, E., et al. (2020). Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 11, 1–21. doi:10.3389/fphys.2020.00694
Sharma, A., Jagga, S., Lee, S.-S., and Nam, J.-S. (2013). Interplay between Cartilage and Subchondral Bone Contributing to Pathogenesis of Osteoarthritis. Ijms 14, 19805–19830. doi:10.3390/ijms141019805
Shi, X., Zhang, C. Y., Gao, J., and Wang, Z. (2019). Recent Advances in Photodynamic Therapy for Cancer and Infectious Diseases. WIREs Nanomed Nanobiotechnol 11, e1560. doi:10.1002/wnan.1560
Shutava, T. G., Balkundi, S. S., Vangala, P., Steffan, J. J., Bigelow, R. L., Cardelli, J. A., et al. (2009). Layer-by-layer-coated Gelatin Nanoparticles as a Vehicle for Delivery of Natural Polyphenols. ACS Nano 3, 1877–1885. doi:10.1021/nn900451a
Tong, F., Ye, Y., Chen, B., Gao, J., Liu, L., Ou, J., et al. (2020). Bone-Targeting Prodrug Mesoporous Silica-Based Nanoreactor with Reactive Oxygen Species Burst for Enhanced Chemotherapy. ACS Appl. Mater. Inter. 12, 34630–34642. doi:10.1021/acsami.0c08992
Wang, Z., Mei, L., Liu, X., and Zhou, Q. (2021). Hierarchically Hybrid Biocoatings on Ti Implants for Enhanced Antibacterial Activity and Osteogenesis. Colloids Surf. B: Biointerfaces 204, 111802. doi:10.1016/j.colsurfb.2021.111802
Wauquier, F., Leotoing, L., Coxam, V., Guicheux, J., and Wittrant, Y. (2009). Oxidative Stress in Bone Remodelling and Disease. Trends Mol. Med. 15, 468–477. doi:10.1016/j.molmed.2009.08.004
Wilson, D. S., Dalmasso, G., Wang, L., Sitaraman, S. V., Merlin, D., and Murthy, N. (2010). Orally Delivered Thioketal Nanoparticles Loaded with TNF-α-siRNA Target Inflammation and Inhibit Gene Expression in the Intestines. Nat. Mater 9, 923–928. doi:10.1038/nmat2859
Xu, H., Cao, W., and Zhang, X. (2013). Selenium-containing Polymers: Promising Biomaterials for Controlled Release and Enzyme Mimics. Acc. Chem. Res. 46, 1647–1658. doi:10.1021/ar4000339
Yao, Y., Zhang, H., Wang, Z., Ding, J., Wang, S., Huang, B., et al. (2019). Reactive Oxygen Species (ROS)-responsive Biomaterials Mediate Tissue Microenvironments and Tissue Regeneration. J. Mater. Chem. B 7, 5019–5037. doi:10.1039/c9tb00847k
Yin, X., Hao, Y., Lu, Y., Zhang, D., Zhao, Y., Mei, L., et al. (2021). Bio‐Multifunctional Hydrogel Patches for Repairing Full‐Thickness Abdominal Wall Defects. Adv. Funct. Mater. 31, 2105614. doi:10.1002/adfm.202105614
Zhao, Z., Han, Z., Naveena, K., Lei, G., Qiu, S., Li, X., et al. (2021). ROS-responsive Nanoparticle as a Berberine Carrier for OHC-Targeted Therapy of Noise-Induced Hearing Loss. ACS Appl. Mater. Inter. 13, 7102–7114. doi:10.1021/acsami.0c21151
Zheng, M., Liu, Y., Wang, Y., Zhang, D., Zou, Y., Ruan, W., et al. (2019). ROS‐Responsive Polymeric siRNA Nanomedicine Stabilized by Triple Interactions for the Robust Glioblastoma Combinational RNAi Therapy. Adv. Mater. 31, 1903277–1903279. doi:10.1002/adma.201903277
Zheng, W., Hao, Y., Wang, D., Huang, H., Guo, F., Sun, Z., et al. (2021). Preparation of Triamcinolone Acetonide-Loaded Chitosan/fucoidan Hydrogel and its Potential Application as an Oral Mucosa Patch. Carbohydr. Polym. 272, 118493. doi:10.1016/j.carbpol.2021.118493
Keywords: bone remodeling, ROS-responsive biomaterials, bone-related diseases, bone regeneration, photodynamic therapy
Citation: Ren X, Liu H, Wu X, Weng W, Wang X and Su J (2022) Reactive Oxygen Species (ROS)-Responsive Biomaterials for the Treatment of Bone-Related Diseases. Front. Bioeng. Biotechnol. 9:820468. doi: 10.3389/fbioe.2021.820468
Received: 23 November 2021; Accepted: 10 December 2021;
Published: 11 January 2022.
Edited by:
Huihua Yuan, Nantong University, ChinaReviewed by:
Jingyao Li, University of Wisconsin-Madison, United StatesCopyright © 2022 Ren, Liu, Wu, Weng, Wang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weizong Weng, ZHJ3ZW5nd2Vpem9uZ0AxNjMuY29t; Xiuhui Wang, YmxhY2tyYWJiaXRAc2h1LmVkdS5jbg==; Jiacan Su, ZHJzdWppYWNhbkAxNjMuY29t
†These authors have contributed equally to this worky
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.