- 1Department of Nuclear Medicine, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 2Key Laboratory of Radiopharmaceuticals, Ministry of Education, Beijing Normal University, Beijing, China
- 3Fujian Provincial Key Laboratory of Precision Medicine for Cancer, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
Purpose: This prospective trial aimed to evaluate the safety, dosimetry, and biodistribution of a novel theranostic probe 68Ga-DOTA-DiPSMA. Also, we have performed the first preliminary application with 68Ga-DOTA-DiPSMA in prostate cancer (PCa) patients.
Methods: Five healthy volunteers and ten PCa patients were injected with an intravenous bolus of 68Ga-DOTA-DiPSMA. They received serial whole-body PET scans from the time of injection up to 60 min post-injection, with a second PET/CT scanning at 120 min post-injection. In PCa patients, low-dose CT scan and whole-body PET were performed with 2 min per bed position in 40 min post-injection. Absorbed organ doses and effective doses were calculated using OLINDA/EXM. Normal organ uptake and tumor lesion uptake were measured. A lesion-by-lesion analysis was performed.
Results: 68Ga-DOTA-DiPSMA administration was safe and well-tolerated. The kidneys received the highest absorbed dose (114.46 ± 29.28 μSv/MBq), followed by the urinary bladder wall (100.82 ± 46.22 μSv/MBq) in accordance with the expected Prostate-Specific Membrane Antigen (PSMA) renal excretion of the tracer. The mean effective dose was 19.46 ± 1.73 μSv/MBq. The SUVmax of 68Ga-DOTA-DiPSMA PET/CT for PCa lesions, bone metastases, and lymph node metastases was 4.41 ± 2.72, 2.95 ± 1.11, and 3.26 ± 1.20, respectively.
Conclusion: Injection of 68Ga-DOTA-DiPSMA is safe and associated with low absorbed and effective doses. 68Ga-DOTA-DiPSMA shows favorable kinetics and imaging characteristics in patients who warrant further head-to-head comparison to validate 68Ga-DOTA-DiPSMA as an alternative for gallium-68-labeled PSMA clinical PET. Low nonspecific uptake in normal organs of 68Ga-DOTA-DiPSMA indicates potential radioligand therapy (RLT) application when labeled with 177Lu, 90Y, or 225Ac.
Introduction
Prostate cancer (PCa) is one of the most frequently diagnosed cancers in men and the lethal malignant diseases leading to male cancer-related death worldwide (Attard et al., 2016). The accurate presence and location of primary or recurrent tumors are critical for planning effective patient management (Mottet et al., 2021).
The diagnostic capability of conventional anatomic imaging such as MRI and CT to determine PCa is limited in metastases and specificity (Vos et al., 2013). Only prostate biopsy is the definitive way to confirm PCa (Attard et al., 2016). Multiple needle biopsies will increase the positive rate of lesion determination significantly. However, it is difficult to determine distant metastases and increase the risk of complications resulting from biopsy operation (Attard et al., 2016). There has been an unmet need for more advanced imaging modalities to determine primary and metastatic lesions that can be helpful to PCa patient management (observation, salvage local therapy, and systemic therapy). PET with 18F-FDG is effective for most malignant tumors, but it lacks sensitivity for PCa. Therefore, it is urgent to discover new nuclear medicine imaging agents with more specificity for PCa.
Prostate-Specific Membrane Antigen (PSMA) is a transmembrane glycoprotein enzyme selectively overexpressed in PCa cells, with its expression increasing in higher-grade malignancy (Bouchelouche et al., 2010). PET imaging with PSMA probes targeting various PCa-specific markers will provide additional molecular information to facilitate lesion detection and staging (Perera et al., 2020).
Recently, a relatively new nuclear imaging modality 68Ga-PSMA PET/CT imaging with good PCa diagnosis and staging performance has become increasingly utilized to evaluate PCa aggressiveness, especially in patients with biochemical recurrence after surgery (Sachpekidis et al., 2016; Koerber et al., 2017; Wang et al., 2020). PSMA can be coupled with different chelators and labeled with corresponding radionuclides for different purposes. The most widely used PSMA ligands in the clinical examination are PSMA-11 and PSMA-617 containing different linkers and chelators. According to the previously published papers, 68Ga-PSMA-11 and 177Lu-PSMA-617 are a molecular pair in metastatic castration-resistant PCa (mCRPC) diagnosis and radioligand therapy (RLT) (Rahbar et al., 2017; Sun et al., 2020; Violet et al., 2020). However, considering the high nonspecific uptake in the salivary, kidneys, and bone marrow of 68Ga-PSMA-11 and 177Lu-PSMA-617, novel PSMA tracers with lower accumulation in normal organs are urgently needed.
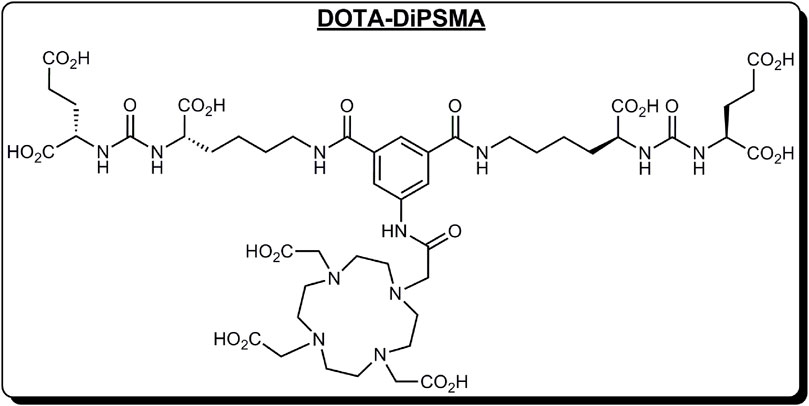
We have discovered a new PSMA dimer (DOTA-DiPSMA, Figure 1). The Prof. Cui group from Beijing Normal University will discuss the discovery and preclinical experiments, which will be published elsewhere. Preclinical experiments proved its good imaging ability and low unspecific uptake in normal organs including the liver, kidneys, spleen, and salivary glands. In addition, the affinity of DiPSMA-DOTA-COOH to the PSMA receptor can reach 1.56 nM. This was the first study in humans, following the abovementioned preclinical studies. In this study, we aimed to evaluate the safety, biodistribution, and dosimetry of 68Ga-DOTA-DiPSMA in healthy volunteers and its diagnostic efficacy in PCa patients.
Methods
Healthy Volunteers and Patients
This study was approved by the Independent Ethics Committee of First Affiliated Hospital of Fujian Medical University [No. MRCTA, ECFAH of FMU (2019)293]. All subjects gave written informed consent and were registered at ClinicalTrials.gov (NCT04525612). Five healthy volunteers and ten patients were enrolled in this study. Five healthy volunteers [5 men, age range 42–76 years (mean age ± SD, 59.83 ± 11.65 years); weight range, 55.0–78.0 kg (mean weight ± SD, 70.27 ± 13.05 kg)] were enrolled to validate the safety, biodistribution, and radiation dosimetry of 68Ga-DOTA-DiPSMA in this study. Exclusion criteria consisted of mental illness conditions, severe liver or kidney disease with serum creatinine greater than 3.0 mg/dl, or any hepatic enzyme level 5 times or more than the standard upper limit. Participants were also excluded if they were known to have severe allergy or hypersensitivity to intravenous radiographic contrast or claustrophobia during PET/CT scanning.
A total of 10 patients who were newly diagnosed as having PCa by sextant core-needle biopsy and had not received any prior therapy were enrolled with written informed consent. The inclusion criteria were those aged between 40 and 80 years, who have a prostate neoplasm identified by ultrasound or MRI, and were diagnosed by needle biopsy as having PCa. The exclusion criteria included claustrophobia, kidney or liver failure, and inability to fulfill the study. The demographics of healthy volunteers and patients are listed in Table 1.
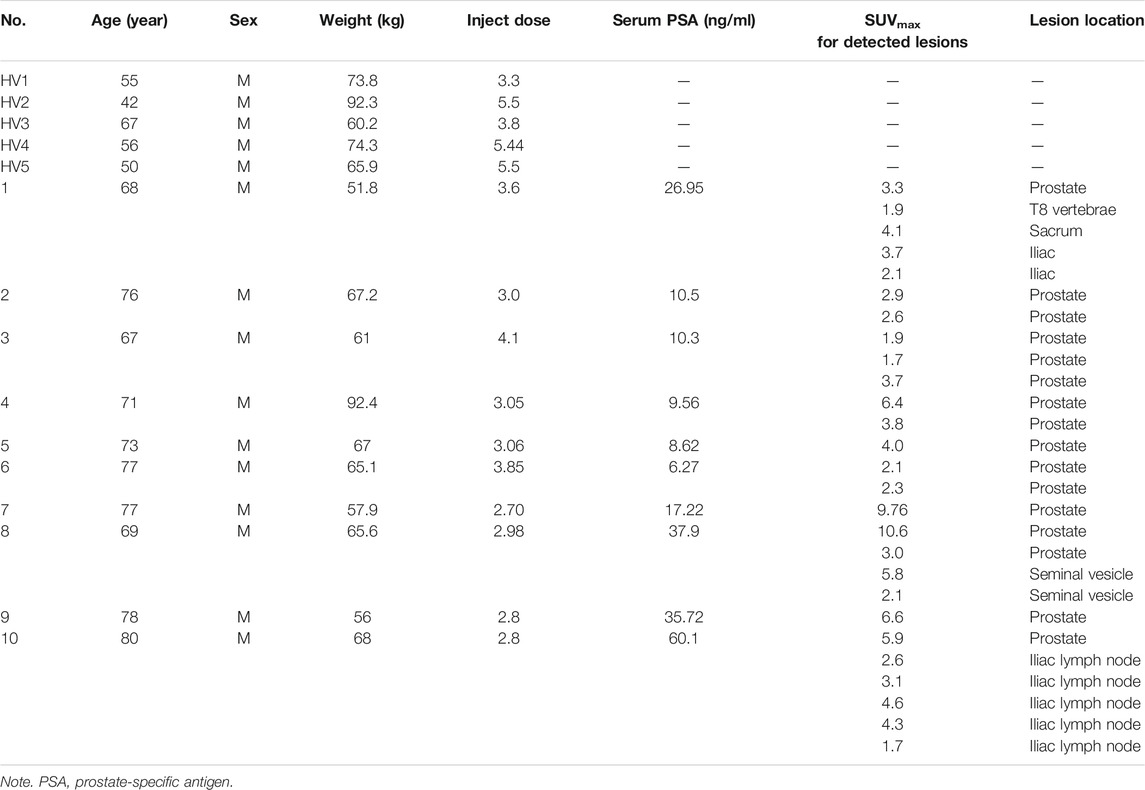
TABLE 1. Summary of the healthy volunteer and patient characteristics and PET findings in ten newly diagnosed prostate cancer patients with 68Ga-DOTA-DiPSMA.
Subject age averaged 73.6 ± 4.6 years (median 74.5 years; range 67–80 years), and body mass averaged 65.2 ± 11.0 kg (median 65.4 kg; range 51.8–92.4 kg). Serum prostate-specific antigen (PSA) values averaged 22.3 ± 17.6 ng/ml (median 13.9 ng/ml; range 6.27–60.1 ng/ml). The reported serum PSA levels were the most recent clinical values at the time of PET/CT imaging. Subjects were numbered chronologically in the order of imaging with 68Ga-DOTA-DiPSMA.
Safety Assessment
Patient safety was assessed and graded according to Common Terminology Criteria for Adverse Events (version 5.0), electrocardiograms, physical examination, and vital signs (blood pressure, respiratory rate, heart rate, and body temperature). Within the first 72 h after 68Ga-DOTA-DiPSMA injection, the research team kept phone contact with each subject, monitoring their adverse event (AE) responses.
Radiopharmaceutical Preparation
Precursors were supplied by Prof. Cui from Key Laboratory of Radiopharmaceuticals, Ministry of Education, Beijing Normal University. Radiolabeling of DiPSMA-DOTA-COOH was performed in a sterile hot cell manually. 68Ga3+ was eluted from a 68Ge/68Ga generator (JSC Isotope, Moscow, Russia) using 0.1 M of HCl. The clinical doses of DOTA-DiPSMA (30 μg) were compounded in 1.25 M of NaOAc buffer to adjust pH to around 4.0 and labeled with an average of 585.34 ± 177.97 MBq (15.82 ± 4.81 mCi) of 68Ga3+ using a reaction temperature of 95°C for 10 min. Our protocol permits the radiochemical purity of the product 68Ga-DOTA-DiPSMA to exceed 99% so that we omit the purification step. The final product will pass through a sterile filter membrane (Millipore, Billerica, MA, United States) and then be diluted to 5 ml in a sterile syringe for injection. The total time required for completion of radiolabeling and quality control averaged approximately 30 min. Quality control items are shown in Table 2.
Examination Procedures
For healthy volunteers, the blood pressure, pulse, respiratory frequency, and temperature were measured; and routine blood and urine tests, liver function, and renal function were examined immediately before and 24 h after the scan. In addition, any possible side effects during 68Ga-DOTA-DiPSMA PET/CT scanning and within 1 week after the examination were collected and analyzed. No specific subject preparation was requested on the day of 68Ga-DOTA-DiPSMA PET/CT. For the volunteers, after the whole-body low-dose CT scan, 111–222 MBq (3–6 mCi) of 68Ga-DOTA-DiPSMA were injected intravenously, followed by serial whole-body PET acquisitions. The whole body (from the top of the skull to the middle of the femur) of each volunteer was covered by 6 bed positions. The acquisition duration was 2 min/bed position at 5, 15, 30, 45, 60, and 120 min after injection.
For the patients, 68Ga-DOTA-DiPSMA PET/CT scanning was performed at 40 min after tracer administration. For each patient, 103.6–151.7 MBq (2.8–4.1 mCi) of 68Ga-DOTA-DiPSMA was injected intravenously. After a low-dose CT scan, whole-body PET was performed with 2 min per bed position (5–6 bed positions depending on the patient’s height). The emission data were corrected for randomness, dead time, scattering, and attenuation. The conventional reconstruction algorithm was used, and the images were zoomed with a factor of 1.2. The images were transferred to an MMWP workstation (Siemens, Erlangen, Germany) for analysis.
Biodistribution Assessment and Dosimetry
Image analysis was performed using MIM v6.9.4 (MIM Software Inc., Cleveland, OH, United Ststes). The volume of interests (VOIs) were drawn over healthy organs on all 68Ga-DOTA-DiPSMA PET images, and SUVmean in these VOIs was determined to obtain the biodistribution of this tracer. Tumor lesions were evaluated in consensus by two nuclear medicine physicians.
All source organs with relevant detectable activity were delineated on the PET images with CT guidance for the healthy volunteers, using MIM software v6.9.4. Time-integrated activity coefficients (normalized cumulated activity (NCA)) were calculated for each source organ by integrating their time–activity curves through curve fitting and normalizing the cumulated activity to the injected activity. Based on the time-integrated activity coefficients, individual absorbed organ doses and the effective dose were determined using OLINDA/EXM v1.1 (Vanderbilt University, Nashville, TN, United States). Calculations were performed with modeling of urinary bladder voiding. Parameters representing the fraction leaving the body via urine and biologic half-time were obtained from the fit and used to model urinary bladder voiding. Urinary bladder voiding models with voiding intervals of 1 h were applied. The 70-kg adult male models were used. Organ-absorbed doses, effective doses, and effective dose equivalents were calculated as mean ± SD across subjects. SPSS 23.0 Software (IBM SPSS, Chicago, IL, United States) was used for statistical analyses.
Result
Patient Safety
68Ga-DOTA-DiPSMA was found to be safe and well-tolerated in all subjects. No AEs or serious AEs occurred after 68Ga-DOTA-DiPSMA injection for all the healthy volunteers and patients. No apparent changes in vital signs or clinical laboratory tests were found before and after the injection of 68Ga-DOTA-DiPSMA.
Biodistribution
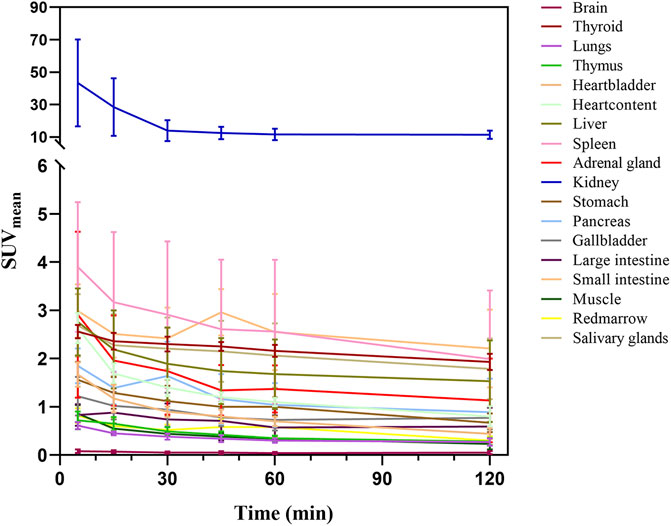
Figures 2, 3 illustrate the biodistribution of 68Ga-DOTA-DiPSMA as a function of time in healthy volunteers. The whole-body background of 68Ga-DOTA-DiPSMA was low. The highest uptake was observed in the kidney with a SUVmean of 43.4 ± 26.8 at 5 min p.i. and further decreased to 11.4 ± 6.5 at 120 min p.i. The spleen, liver, salivary gland, and small intestine showed moderate uptake, with SUVmean of 2.90 ± 1.5, 1.89 ± 0.75, 2.30 ± 0.87, and 2.42 ± 0.64 at 30 min after injection, respectively. Low background uptakes were observed in the brain, lungs, muscle, red marrow, heart, thyroid, gall bladder, pancreas, stomach, bone, and large intestine. The rapid presence in the kidneys, followed by a passage toward the urinary bladder, illustrated the tracer’s fast and mainly renal excretion.

FIGURE 2. A maximum intensity projection PET images at several time points post-injection of a 56-year-old male healthy volunteer.
Dosimetry
The average estimated absorbed organ in healthy volunteers is summarized in Table 3. The highest absorbed dose was received by the kidneys (114.46 ± 29.28 μSv/MBq), followed by the urinary bladder wall (100.82 ± 46.22 μSv/MBq). The mean effective dose was 19.46 ± 1.73 μSv/MBq.
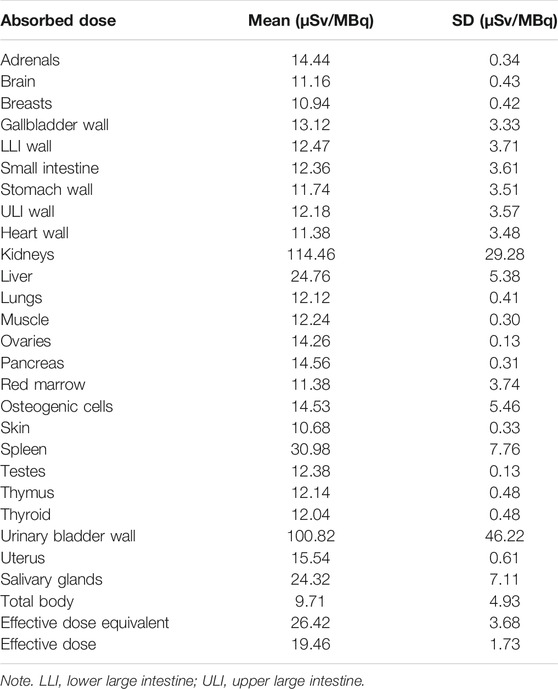
TABLE 3. Estimated absorbed organ doses and effective dose for 68Ga-DOTA-DiPSMA in healthy volunteers.
Detection of Primary Prostate Cancer
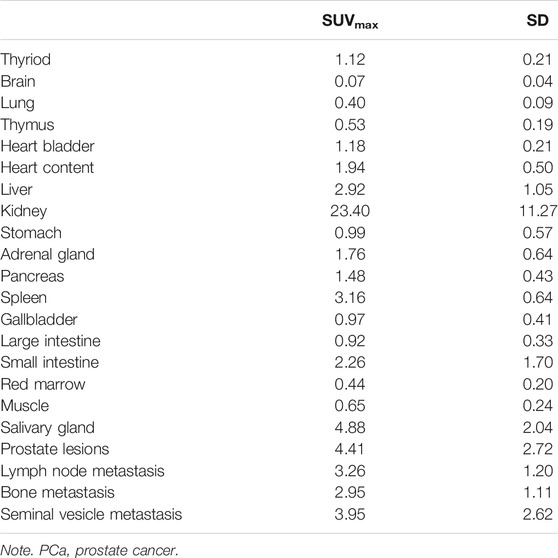
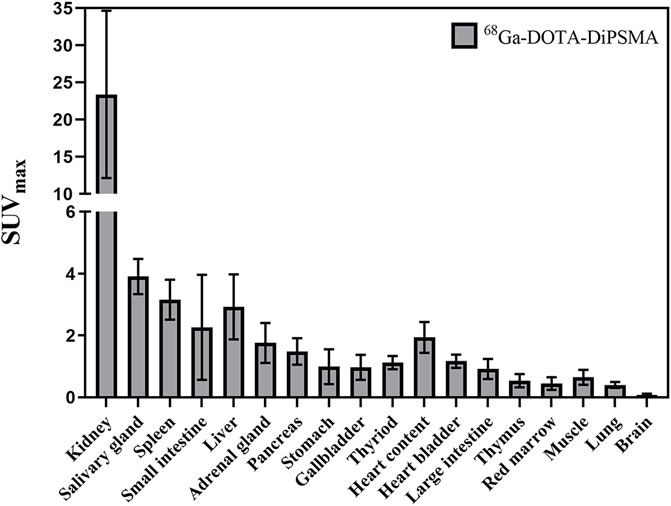
For the 10 patients with primary PCa, 68Ga-DOTA-DiPSMA PET/CT showed 27 positive findings, including 16 prostate lesions, 4 bone metastases, 5 lymph node metastases, and 2 seminal vesicle metastases. The primary lesions were confirmed by needle biopsy. SUVmax for prostate lesions, bone metastases, and lymph node metastases were 4.41 ± 2.72, 2.95 ± 1.11, and 3.26 ± 1.20, respectively (Table 1; Figure 4). Low background uptake was observed in 68Ga-DOTA-DiPSMA (the salivary glands SUVmax 4.88 ± 2.04, liver SUVmax 2.92 ± 1.05, kidneys SUVmax 23.40 ± 11.27, and spleen SUVmax 3.16 ± 0.64) (Table 4; Figure 5).
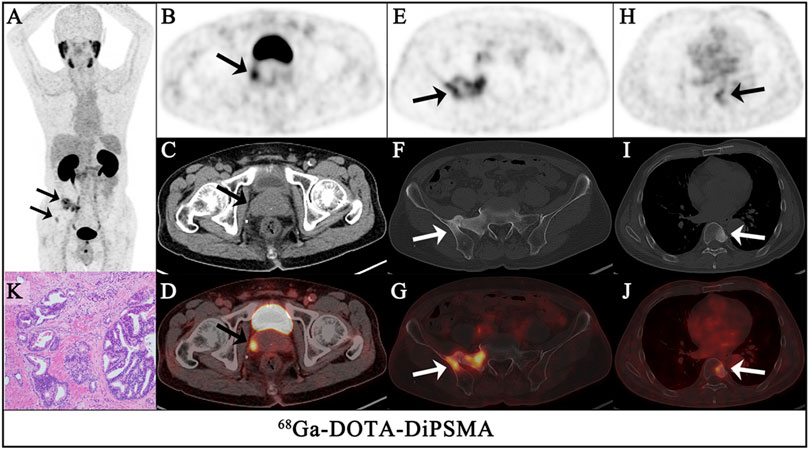
FIGURE 4. A 68-year-old-man had confirmed prostate cancer after a needle biopsy of the prostate for 1 week. The maximum intensity projection (MIP) of 68Ga-DOTA-DiPSMA PET/CT [(A), arrows] showed significantly abnormal uptake in the image. Axial views of the prostate (top, PET; middle, CT; bottom, fusion image) show intense uptake (SUVmax 3.3) in the isodense nodule of the prostate [(B–D), arrows]. In the other level axial views, increased 68Ga-DOTA-DiPSMA uptake was observed in the sacrum and iliac [(E–G), arrows] and the 8th thoracic vertebrae lesions [(H–J), arrows], which were concomitant with bone density increased. Postoperative pathology confirmed it as adenocarcinoma of the prostate (K).
Discussion
To our knowledge, this was the first human study to evaluate the novel tracer 68Ga-DOTA-DiPSMA in healthy volunteers and patients with PCa. This tracer is a new type of 68Ga-labeled dimer PSMA imaging agent with a simple structure, easy synthesis, and low synthesis cost. 68Ga-DOTA-DiPSMA can be prepared by a one-step labeling reaction in a high yield greater than 95% between 68Ga3+ ions eluted from a germanium–gallium generator and the precursor DiPSMA-DOTA-COOH.
Here, we presented the results of an independently performed first clinical evaluation of 68Ga-DOTA-DiPSMA in five healthy volunteers, including biodistribution, dosimetry, and safety. Also, we have performed the first initial application with 68Ga-DOTA-DiPSMA in PCa patients. The results showed that this tracer displayed favorable biodistribution and dosimetry features and was well-tolerated in all patients. 68Ga-DOTA-DiPSMA showed high PSMA affinity. The biodistribution of 68Ga-DOTA-DiPSMA was similar to that of 68Ga-PSMA-11 (Pfob et al., 2016). The rapid presence in the kidneys, followed by a passage toward the urinary bladder, illustrates the tracer’s fast and mainly renal excretion. The highest uptake was observed in the kidneys and rapidly cleared through the urinary system in both tracers, consistent with the published 68Ga-PSMA-11 results (Afshar-Oromieh et al., 2016; Zamboglou et al., 2016; Chen et al., 2019; Sandgren et al., 2019). However, 68Ga-DOTA-DiPSMA observed SUVmean values at 60 min for the kidneys, liver, spleen, and parotids (11.93 ± 3.54, 1.68 ± 0.72, 2.56 ± 1.49, and 2.16 ± 0.89, respectively) were in general lower than 68Ga-PSMA-11 (30.1 ± 6.6, 3.3 ± 0.6, 5.2 ± 2.5, and 9.4 ± 2.0, respectively) (Green et al., 2017).
The dosimetry data of 68Ga-DOTA-DiPSMA showed a little lower yet comparable effective dose than 68Ga-PSMA-11 (0.019 vs. 0.022/0.023 mSv/MBq) (Afshar-Oromieh et al., 2016; Sandgren et al., 2019), salivary glands (0.024 vs. 0.089 mSv/MBq), kidney (0.114 vs. 0.240 mSv/MBq), liver (0.0240 vs. 0.053 mSv/MBq), and spleen (0.031 vs. 0.046 mSv/MBq) (Sandgren et al., 2019). We thought that the lower liver and spleen dose of 68Ga-DOTA-DiPSMA might be attributed to the dosimetry methodology.
It is crucial to reduce the radiation dose of nonspecific organs and tissues in the field of radionuclide therapy (RLT) (ICRP, 2002). 177Lu-PSMA-617 RLT is a promising option for patients with mCRPC (Delker et al., 2016; Kratochwil et al., 2016; Paganelli et al., 2020; Rasul et al., 2020). Based on the lower nonspecific uptake and effective dose of 68Ga-DOTA-DiPSMA, the radiation dosimetry in normal organs seemed to be reduced when DOTA-DiPSMA was labeled with 177Lu for RLT. Low background uptakes were observed in the brain, lungs, muscle, red marrow, heart, thyroid, gall bladder, pancreas, stomach, bone, and large intestine. The low uptake of the dimer DOTA-DiPSMA in the parotid glands and the clearance in the kidneys were impressive, which could be an advantage for RLT.
A critical finding of our study is the high tumor accumulation of 68Ga-DOTA-DiPSMA, which showed high tumor uptakes with the highest SUVmax up to 10.6 on 68Ga-DOTA-DiPSMA. The primary lesions showed the highest uptake (SUVmax 4.41 ± 2.72). For metastasis lesions, the highest uptake was shown in a seminal vesicle (SUVmax 3.95 ± 2.61), followed by the iliac lymph node (SUVmax 3.26 ± 1.20), and the lowest uptake was observed in the bone (SUVmax 2.95 ± 1.11). However, the lower uptake of 68Ga-DOTA-DiPSMA in normal organs may be its advantage (Afshar-Oromieh et al., 2015; Fendler et al., 2017). The study on 68Ga-DOTA-DiPSMA provided a new radiotracer targeting PSMA to diagnose PCa. It was conducive to the accuracy of PCa staging. The small lesion near the urinary bladder would be more apparent with this relatively low background. The mechanism of DOTA-DiPSMA in reducing the uptake in the salivary gland and kidney was still unknown, which needs further studies to confirm.
The primary limitation of our study is the sample size, which did not enable accurate multivariate regression analysis in comparing the diagnosis efficacy of 68Ga-DOTA-DiPSMA with 68Ga-PSMA-11, which is the next work in our research group. Besides, neither blood nor urine samples were collected in our study, which will allow for the stability test in vivo. Further detailed and head-to-head comparison studies are required.
Conclusion
68Ga-DOTA-DiPSMA is safe and well-tolerated and shows favorable dosimetry and biodistribution in healthy volunteers and detection performances in PCa patients. The low uptake of the dimer DOTA-DiPSMA in the parotid glands and the clearance in the kidneys were impressive. The lower background of 68Ga-DOTA-DiPSMA showed its potential application for RLT when labeled with 177Lu. DOTA-DiPSMA is a promising novel theranostic tracer for both PCa patient diagnosis and RLT. Further validation by head-to-head comparison is warranted.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Independent Ethics Committee of First Affiliated Hospital of Fujian Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MC, WM, and SY: research design. JZ, ZL, XZ, and RL: acquisition of data. All were involved in the analysis and/or interpretation of data and drafting the article or revising it critically. All approved the submitted and final version.
Funding
This study was funded in part by the Joint Funds for the Innovation of Science and Technology, Fujian Province (2020Y9101), National Natural Science Foundation of China (82171982), Natural Science Foundation of Fujian (2020J05249), and Fujian Provincial Health Technology Project (2020GGA045).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afshar-Oromieh, A., Avtzi, E., Giesel, F. L., Holland-Letz, T., Linhart, H. G., Eder, M., et al. (2015). The Diagnostic Value of PET/CT Imaging with the 68Ga-labelled PSMA Ligand HBED-CC in the Diagnosis of Recurrent Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging. 42, 197–209. doi:10.1007/s00259-014-2949-6
Afshar-Oromieh, A., Hetzheim, H., Kübler, W., Kratochwil, C., Giesel, F. L., Hope, T. A., et al. (2016). Radiation Dosimetry of 68Ga-PSMA-11 (HBED-CC) and Preliminary Evaluation of Optimal Imaging Timing. Eur. J. Nucl. Med. Mol. Imaging. 43, 1611–1620. doi:10.1007/s00259-016-3419-0
Attard, G., Parker, C., Eeles, R. A., Schröder, F., Tomlins, S. A., Tannock, I., et al. (2016). Prostate Cancer. The Lancet. 387, 70–82. doi:10.1016/S0140-6736(14)61947-4
Bouchelouche, K., Choyke, P. L., and Capala, J. (2010). Prostate Specific Membrane Antigen- a Target for Imaging and Therapy with Radionuclides. Discov. Med. 9, 55–61. PMID: 20102687.
Chen, M., Zhang, Q., Zhang, C., Zhao, X., Marra, G., Gao, J., et al. (2019). Combination of 68Ga-PSMA PET/CT and Multiparametric MRI Improves the Detection of Clinically Significant Prostate Cancer: A Lesion-By-Lesion Analysis. J. Nucl. Med. 60, 944–949. doi:10.2967/jnumed.118.221010
Delker, A., Fendler, W. P., Kratochwil, C., Brunegraf, A., Gosewisch, A., Gildehaus, F. J., et al. (2016). Dosimetry for 177Lu-DKFZ-PSMA-617: a New Radiopharmaceutical for the Treatment of Metastatic Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging. 43, 42–51. doi:10.1007/s00259-015-3174-7
Fendler, W. P., Eiber, M., Beheshti, M., Bomanji, J., Ceci, F., Cho, S., et al. (2017). 68Ga-PSMA PET/CT: Joint EANM and SNMMI Procedure Guideline for Prostate Cancer Imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging. 44, 1014–1024. doi:10.1007/s00259-017-3670-z
Green, M. A., Eitel, J. A., Fletcher, J. W., Mathias, C. J., Tann, M. A., Gardner, T., et al. (2017). Estimation of Radiation Dosimetry for 68Ga-HBED-CC (PSMA-11) in Patients with Suspected Recurrence of Prostate Cancer. Nucl. Med. Biol. 46, 32–35. doi:10.1016/j.nucmedbio.2016.11.002
ICRP (2002). Basic Anatomical and Physiological Data for Use in Radiological protection: Reference Values. A Report of Age- and Gender-Related Differences in the Anatomical and Physiological Characteristics of Reference Individuals. ICRP Publication 89. Ann. ICRP. 8932, 5–265. PMID: 14506981. doi:10.3109/14653249.2010.487901
Koerber, S. A., Utzinger, M. T., Kratochwil, C., Kesch, C., Haefner, M. F., Katayama, S., et al. (2017). 68Ga-PSMA-11 PET/CT in Newly Diagnosed Carcinoma of the Prostate: Correlation of Intraprostatic PSMA Uptake with Several Clinical Parameters. J. Nucl. Med. 58, 1943–1948. doi:10.2967/jnumed.117.190314
Kratochwil, C., Giesel, F. L., Stefanova, M., Benešová, M., Bronzel, M., Afshar-Oromieh, A., et al. (2016). PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 57, 1170–1176. doi:10.2967/jnumed.115.171397
Mottet, N., van den Bergh, R. C. N., Briers, E., Van den Broeck, T., Cumberbatch, M. G., De Santis, M., et al. (2021). EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 79, 243–262. doi:10.1016/j.eururo.2020.09.042
Paganelli, G., Sarnelli, A., Severi, S., Sansovini, M., Belli, M. L., Monti, M., et al. (2020). Dosimetry and Safety of 177Lu PSMA-617 Along with Polyglutamate Parotid Gland Protector: Preliminary Results in Metastatic Castration-Resistant Prostate Cancer Patients. Eur. J. Nucl. Med. Mol. Imaging. 47, 3008–3017. doi:10.1007/s00259-020-04856-1
Perera, M., Papa, N., Roberts, M., Williams, M., Udovicich, C., Vela, I., et al. (2020). Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-Specific Membrane Antigen-Avid Lesions: A Systematic Review and Meta-Analysis. Eur. Urol. 77, 403–417. doi:10.1016/j.eururo.2019.01.049
Pfob, C. H., Ziegler, S., Graner, F. P., Köhner, M., Schachoff, S., Blechert, B., et al. (2016). Biodistribution and Radiation Dosimetry of 68Ga-PSMA HBED CC—a PSMA Specific Probe for PET Imaging of Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging. 43, 1962–1970. doi:10.1007/s00259-016-3424-3
Rahbar, K., Ahmadzadehfar, H., Kratochwil, C., Haberkorn, U., Schäfers, M., Essler, M., et al. (2017). German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 58, 85–90. doi:10.2967/jnumed.116.183194
Rasul, S., Hacker, M., Kretschmer-Chott, E., Leisser, A., Grubmüller, B., Kramer, G., et al. (2020). Clinical Outcome of Standardized 177Lu-PSMA-617 Therapy in Metastatic Prostate Cancer Patients Receiving 7400 MBq Every 4 Weeks. Eur. J. Nucl. Med. Mol. Imaging. 47, 713–720. doi:10.1007/s00259-019-04584-1
Sachpekidis, C., Kopka, K., Eder, M., Hadaschik, B. A., Freitag, M. T., Pan, L., et al. (2016). 68Ga-PSMA-11 Dynamic PET/CT Imaging in Primary Prostate Cancer. Clin. Nucl. Med. 41, e473–e479. doi:10.1097/RLU.0000000000001349
Sandgren, K., Johansson, L., Axelsson, J., Jonsson, J., Ögren, M., Ögren, M., et al. (2019). Radiation Dosimetry of [68Ga]PSMA-11 in Low-Risk Prostate Cancer Patients. EJNMMI Phys. 6, 2. doi:10.1186/s40658-018-0239-2
Sun, M., Niaz, M. O., Nelson, A., Skafida, M., and Niaz, M. J. (2020). Review of 177Lu-PSMA-617 in Patients With Metastatic Castration-Resistant Prostate Cancer. Cureus. 12, e8921. doi:10.7759/cureus.8921
Violet, J., Sandhu, S., Iravani, A., Ferdinandus, J., Thang, S.-P., Kong, G., et al. (2020). Long-Term Follow-Up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of 177Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 61, 857–865. doi:10.2967/jnumed.119.236414
Vos, E. K., Litjens, G. J. S., Kobus, T., Hambrock, T., Kaa, C. A. H.-v. d., Barentsz, J. O., et al. (2013). Assessment of Prostate Cancer Aggressiveness Using Dynamic Contrast-Enhanced Magnetic Resonance Imaging at 3 T. Eur. Urol. 64, 448–455. doi:10.1016/j.eururo.2013.05.045
Wang, B., Gao, J., Zhang, Q., Fu, Y., Liu, G., Shi, J., et al. (2020). Diagnostic Value of 68Ga-PSMA PET/CT for Detection of Phosphatase and Tensin Homolog Expression in Prostate Cancer: A Pilot Study. J. Nucl. Med. 61, 873–880. doi:10.2967/jnumed.119.236059
Keywords: prostate cancer, 68Ga-DOTA-DiPSMA, biodistribution, dosimetry, PET/CT
Citation: Zhang J, Lin Z, Zhang X, Lin R, Cui M, Miao W and Yao S (2022) 68Ga-DOTA-DiPSMA PET/CT Imaging: Biodistribution, Dosimetry, and Preliminary Application in Prostate Cancer. Front. Bioeng. Biotechnol. 9:811972. doi: 10.3389/fbioe.2021.811972
Received: 09 November 2021; Accepted: 17 December 2021;
Published: 28 January 2022.
Edited by:
Lu Wang, First Affiliated Hospital of Jinan University, ChinaReviewed by:
Shaoyu Liu, First Affiliated Hospital of Guangzhou Medical University, ChinaGanghua Tang, Southern Medical University, China
Hongguang Liu, Northeastern University, China
Copyright © 2022 Zhang, Lin, Zhang, Lin, Cui, Miao and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaobo Yao, eWFvc2hhb2JvMDA4QDE2My5jb20=
†These authors have contributed equally to this work
 Jiaying Zhang1†
Jiaying Zhang1† Xiaojun Zhang
Xiaojun Zhang Shaobo Yao
Shaobo Yao