- 1Department of Nuclear Medicine, PET/CT-MRI Center, Center of Cyclotron and PET Radiopharmaceuticals, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 2Department of Neurology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Zhejiang, China
- 3Epilepsy Center, Guangdong 999 Brain Hospital, Guangzhou, China
- 4Department of Pathology, Guangdong 999 Brain Hospital, Guangzhou, China
Focal cortical dysplasia (FCD) type IIIa is an easily ignored cause of intractable temporal lobe epilepsy. This study aimed to analyze the clinical, electrophysiological, and imaging characteristics in FCD type IIIa and to search for predictors associated with postoperative outcome in order to identify potential candidates for epilepsy surgery. We performed a retrospective review including sixty-six patients with FCD type IIIa who underwent resection for drug-resistant epilepsy. We evaluated the clinical, electrophysiological, and neuroimaging features for potential association with seizure outcome. Univariate and multivariate analyses were conducted to explore their predictive role on the seizure outcome. We demonstrated that thirty-nine (59.1%) patients had seizure freedom outcomes (Engel class Ia) with a median postsurgical follow-up lasting 29.5 months. By univariate analysis, duration of epilepsy (less than 12 years) (p = 0.044), absence of contralateral insular lobe hypometabolism on PET/MRI (pLog-rank = 0.025), and complete resection of epileptogenic area (pLog-rank = 0.004) were associated with seizure outcome. The incomplete resection of the epileptogenic area (hazard ratio = 2.977, 95% CI 1.218–7.277, p = 0.017) was the only independent predictor for seizure recurrence after surgery by multivariate analysis. The results of past history, semiology, electrophysiological, and MRI were not associated with seizure outcomes. Carefully included patients with FCD type IIIa through a comprehensive evaluation of their clinical, electrophysiological, and neuroimaging characteristics can be good candidates for resection. Several preoperative factors appear to be predictive of the postoperative outcome and may help in optimizing the selection of ideal candidates to benefit from epilepsy surgery.
Introduction
Epilepsy is one of the most common severe chronic neurological disorders and is considered a severe public health concern (Blumcke et al., 2017). Drug-resistant epilepsy accounts for at least one-third of people with epilepsy (Montaz-Rosset et al., 2019). The most familiar developmental cortical malformation that leads to intractable epilepsy is focal cortical dysplasia (FCD) (Palmini and Holthausen, 2013). FCD is the most common histopathology diagnosis in children and the third most familiar etiology in adult patients undergoing epilepsy resection (Hauptman and Mathern, 2012). With the development of neuroimaging techniques, especially MRI and 18F-FDG PET (Tassi et al., 2002), more studies have shown that a higher incidence of FCD than previously diagnosed have enhanced the preoperative recognition and classification of these malformations (Widdess-Walsh et al., 2006). These patients have a serious seizure burden: more than 60% suffer from daily seizures, and a large number of FCD patients have onset of epilepsy by the age of 16 years (Chapman et al., 2005).
For these refractory epilepsy patients associated with FCD, the resection of epilepsy has become the most promising alternative therapeutic option to achieve seizure control in recent decades. Among the FCD patients with resection, postoperative seizure control ranged from 52% to 67% (D. W. Kim et al., 2009; Lerner et al., 2009). Incomplete resection of the epileptogenic area could lead to persistent seizures (Najm et al., 2013). These epilepsy individuals are confronted with difficulties because operative seizure outcomes cannot be predicted accurately before operation. Therefore, in patients with intractable epilepsy and FCD, the combination of clinical, electrophysiological data and multimodality neuroimaging features during a detailed preoperative assessment may help to precisely localize the epileptogenic zone (EZ) (Pittau et al., 2014) and predict seizure outcome after surgery (Mintzer and Sperling, 2008).
However, previous researches have focused on investigating the predictors of postoperative outcomes for isolated FCD patients or dual pathology using Palmini’s FCD classification (Chang et al., 2011); thus, the predictors of postoperative seizure outcome in FCD associated with other principal lesions according to new classification (Blumcke et al., 2011), especially newly classified FCD type IIIa [FCD type I coexisting with hippocampal sclerosis (HS) in the temporal lobe, which is also a common pathology and different from isolated FCD in temporal lobe epilepsy (TLE) (Marusic et al., 2007)], are less well discussed (Fauser et al., 2015). Although it is very critical to explore the predictors in identifying perfect candidates for epilepsy surgery and evaluating the postoperative outcome of individual patients, there have been only restricted data about the predictive factors involved in the surgical therapy of intractable FCD type IIIa.
In our study, the demographic, clinical, and electrophysiological characteristics, the neuroimaging features, and the postoperative outcome were reviewed in the patients with histologically confirmed FCD type IIIa in our epilepsy center. The goal of the current study was to determine the reliable predictive factors for seizure freedom outcome using the Kaplan–Meier (KM) survival analysis and multivariate Cox proportional hazards model in order to better recognize potential candidates for epilepsy surgery.
Materials and Methods
Study Subjects
Our study retrospectively analyzed consecutive individuals diagnosed with medically intractable TLE and FCD type IIIa as determined by histopathology who underwent resective epilepsy surgery at our epilepsy center from January 1, 2014, until April 31, 2019. Drug-resistant epilepsy was diagnosed according to the criteria proposed by International League Against Epilepsy (ILAE), which fulfilled the definition “failure of adequate trials of two tolerated and appropriately chosen anti-epileptic drugs (AEDs) schedules (whether as monotherapies or in combination) to achieve seizure freedom” (Kwan et al., 2010).
The inclusion criteria were as follows: 1) all FCD type IIIa (HS-related FCD type I in the temporal lobe) was diagnosed by histopathology results after surgery according to the 2011 ILAE classification system for FCD (Blumcke et al., 2011); 2) postoperative follow-up was at least 12 months after surgery; and 3) either adult or children patients were included.
Exclusion criteria were as follows: 1) isolated FCD type I and II; 2) FCD in other brain lobes or multiple brain lobes; 3) hemispheric dysplasia, tuberous sclerosis, and periventricular nodular heterotopias; 4) isolated HS; 5) HS associated with tumor, vascular malformation or other early lesions (e.g., trauma, ischemic injury, and encephalitis) in the temporal lobe.
Video Electroencephalogram and Stereoelectroencephalography
All patients underwent long-term scalp video electroencephalogram (VEEG) recording with 21 channels based on the international 10–20 system, with attached anterior temporal electrodes. Interictal and ictal scalp VEEG was recorded in each patient, and at least two habitual seizures during monitoring were reviewed for all patients. Ictal rhythm/interictal spike in the electrodes of an epileptogenic lobe or two adjacent electrodes was considered as a localizing pattern of ictal-onset rhythm/interictal spike.
Stereoelectroencephalography (SEEG) monitoring was recommended if the presurgical noninvasive evaluation was disconcordant, there was no visible causative lesion seen on MRI, or eloquent cortex was included. Some patients were implanted with electrodes in areas that could be associated with the EZ using robotic stereotactic assistance (ROSA) system. Long-term recordings were conducted after implanting electrodes utilizing VEEG monitoring system. And the EZ was defined as the regions covered by the electrodes that presented ictal discharge patterns that occurred either before or simultaneously with clinical behavior (Seo et al., 2011).
Imaging
All epilepsy patients underwent sequential PET/MRI, conducted with CT-based attenuation correction and MRI using a trimodality PET/CT-MRI system (full ring, time-of-flight discovery PET/CT 690, 3T Discovery MR 750, GE Healthcare, Chicago, IL, USA) in the PET/CT-MRI Center at The First Affiliated Hospital of Jinan University. MRI was conducted first on a 3.0-T MR scanner (GE Discovery 750, Milwaukee, WI, USA). The interictal PET/CT scans for all patients were conducted using a GE Discovery PET-CT 690 system. All patients had clinical seizures no less than 24 h before the PET scan to exclude possible effects on PET sensitivity, and VEEG monitoring was not performed simultaneously with the PET scan. 18F-FDG PET visual analysis was semiquantitative and performed by two experienced nuclear physicians on the GE AW 4.6 workstation during the preoperation evaluation and before invasive SEEG. 18F-FDG PET and MRI data were sent to a dedicated review workstation (GE Advantage workstation, version 4.6; GE Healthcare Life Sciences GE), also evaluated by two experienced nuclear physicians.
Surgery and Pathology
All patients underwent resection after the review of the presurgical evaluation information in a multidisciplinary case discussion. The resection range included the onset zone monitored by intracranial electrodes, the focal dysplastic area adjacent to the zone, and the hypometabolism region around the onset zone. Generally speaking, if the epileptogenic lesion is located in the mesial temporal lobe and temporal neocortex, the involved neocortex and mesial structures needed to be removed. We usually perform standardized anterior temporal lobe resection (ATLR) in our center. The temporal neocortex (4–5 cm on the dominant temporal cortex and 5–6 cm on the nondominant temporal cortex), in combination with the mesial temporal structures (consisting of the hippocampus, parahippocampal gyrus, and amygdala), was removed (Kemerdere et al., 2018). Some patients underwent extended temporal lobectomy and amygdalo-hippocampectomy on the basis of the results of SEEG. All patients underwent MRI to evaluate the completeness of resection after surgery. Complete excision was defined by resection of all of the MRI lesions and seizure onset zones defined by SEEG according to postoperative MRI. Moreover, almost all of these patients had changes in the medial structure, such as HS, which required complete resection. If postoperative MRI showed residual medial structure, it was considered incomplete resection.
The histopathological diagnosis was acquired by disposing of the surgical samples, as previously described (Chang et al., 2011). Histopathology of FCD was classified using ILAE 2011 classification guideline (Blumcke et al., 2011), as follows: FCD type IIIa refers to the temporal cortical dyslamination in combination with HS.
Surgical Outcomes
Seizure outcome for all patients after surgery was determined by a review of clinic visits or telephone contacts. All individuals were followed up until April 31, 2020. Seizure outcome classifications after operation were made using Engel’s classification (Engel, 1993): Engel class Ia, completely seizure free after surgery; and Engel class I, seizure free or auras only or convulsions with drug withdrawal only. Patients were considered seizure free only if they never experienced seizures or auras throughout the follow-up period (Engel class Ia outcome), and acute postoperative seizures within the first week were excluded (Cahill et al., 2019).
Statistical Analyses
The study population was divided into two groups including seizure freedom and seizure recurrence based on the prognosis at the last follow-up. For exploratory purposes, the continuous variables were compared between these two groups using the nonparametric Mann–Whitney U test due to non-normal distributions. Categorical variables were compared between two groups using a chi-square test or Fisher’s exact test, as appropriate.
KM survival curves were used to characterize the probability of remaining seizure free after surgery over time. First, KM survival analysis was performed to investigate the probability of seizure-free survival in the overall group. All cases lost to follow-up were censored at the date of last clinical contact. Then, the differences between groups in seizure freedom distributions were compared using the log-rank test for the univariate analysis. Variables with p < 0.1 in univariate analysis were then included in the multivariate Cox proportional regression model to analyze independent prognostic effect for seizure recurrence, Mantel–Haenszel hazard ratio (HR), and 95% CI were calculated.
All data analyses were conducted using the IBM SPSS 19.0 software package (SPSS Inc., Chicago, IL, USA) and GraphPad Prism, version 8.0 (GraphPad Software, La Jolla, CA, USA). A two-tailed p < 0.05 was considered statistically significant for all statistical tests.
Results
Patient Demographic and Clinical Characteristics
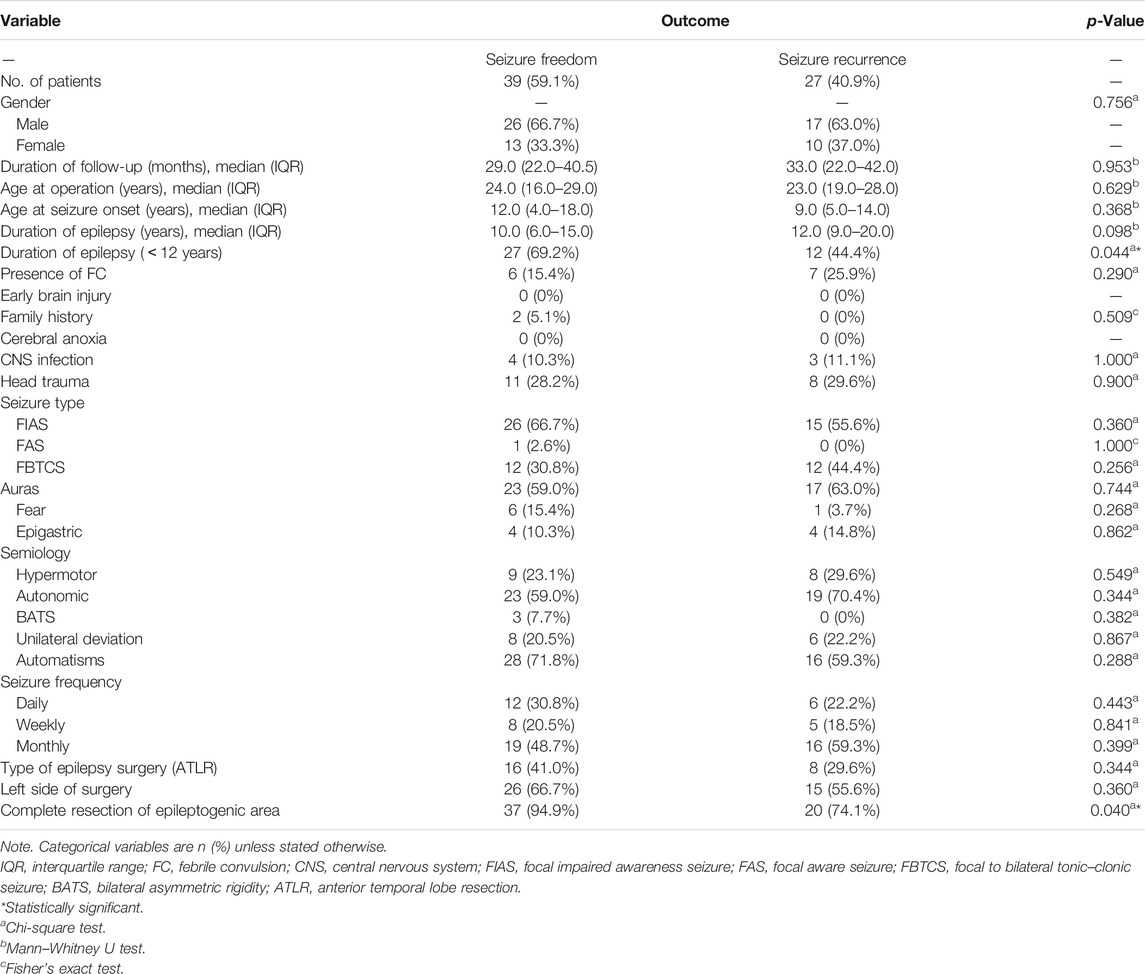
We first selected three hundred and forty-eight drug-resistant epilepsy patients who underwent resection in our epilepsy center. Then we excluded patients with other types of potentially epileptogenic lesions (n = 128), FCD in other brain lobes or multiple brain lobes (n = 120), other types of FCD in temporal lobe (n = 22), insufficient postoperative follow-up (n = 8), undetermined death (n = 2), and lost to follow-up (n = 2). Finally, sixty-six patients met the inclusion criteria (Figure 1). The patients’ basic demographics and clinical characteristics are summarized in Table 1.
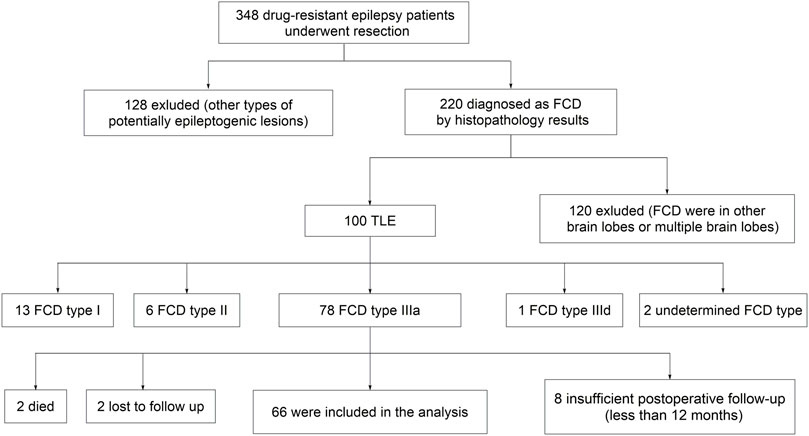
FIGURE 1. Flowchart of patient selection. FCD, focal cortical dysplasia; TLE, temporal lobe epilepsy.
The most common preoperative seizure type based on the new classification method of seizure types (Fisher et al., 2017) was focal impaired awareness seizure (FIAS) (62.1%), followed by focal to bilateral tonic–clonic seizure (FBTCS) (36.4%). The frequency of seizures before surgery was divided into three categories: daily seizures (≥1 seizure each day), weekly seizures (≥1 seizure each week), and monthly seizures (≥1 seizure each month) (Chang et al., 2011). Among these, eighteen (27.3%) patients had daily seizures. Finally, twenty-four patients (36.4%) underwent ATLR, and fifty-seven (86.4%) patients underwent complete resection of epileptogenic area.
Preoperative Electrophysiological and Neuroimaging Characteristics
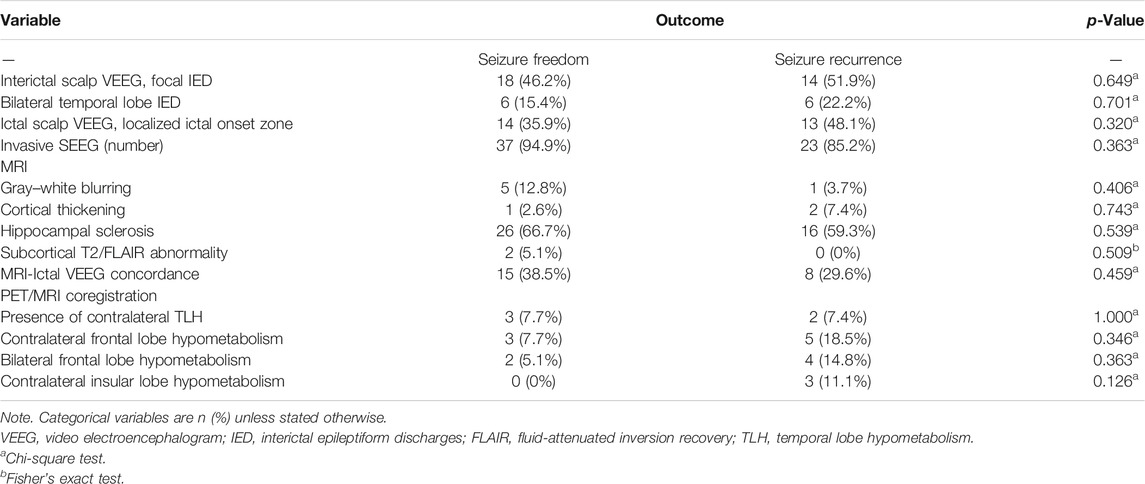
The electrophysiological characteristics of these patients are demonstrated in Table 2. Focal interictal epileptiform discharges (IEDs) on scalp VEEG were localized in thirty-two (48.5%) patients. Moreover, localized ictal onset zone on scalp EEG was clearly localized in twenty-seven (40.9%) patients. SEEG was conducted in sixty (90.9%) cases using the ROSA system (Medtech, Montpellier, France), confirming that ictal discharge patterns occurred confined to either the mesial temporal lobe or neocortical areas.
We also assessed the preoperative neuroimaging features in overall cases (Table 2). Structural MRI could only detect thirteen (19.7%) patients with FCD type IIIa. Approximately 63.6% of patients had typical HS lesions on structural MRI, whereas only six (9.1%) patients presented gray–white matter transition blurring. Moreover, cortical thickening and subcortical T2/fluid-attenuated inversion recovery (FLAIR) abnormality were significantly less frequent in only three (4.5%) and two (3%) patients, respectively. MRI findings were closely concordant with ictal scalp VEEG onset pattern in twenty-three (34.8%) patients.
Moreover, we further analyzed the metabolism pattern in these sixty-six patients using 18F-FDG PET/MRI coregistration. Contralateral temporal lobe hypometabolism (TLH) was observed in only five (7.6%) patients. Contralateral frontal lobe hypometabolism and bilateral frontal lobe hypometabolism were also found in about eight (12.1%) and six (9.1%) patients, respectively. We also demonstrated that contralateral insular lobe hypometabolism was relatively infrequent in three (4.5%) patients.
Postoperative Seizure Outcomes
The continuous Engel class Ia seizure freedom outcome after the operation is illustrated using KM curves (Figure 2). The cumulative probability of seizure freedom survival was 69.7% (95% CI, 58.6–80.8%), 62.3% (95% CI, 50.3–74.4%), 56.5% (95% CI, 43.0–70.0%), and 48.4% (95% CI, 29.8–67.1%) at 12, 24, 36, and 60 months or more after surgery, respectively.
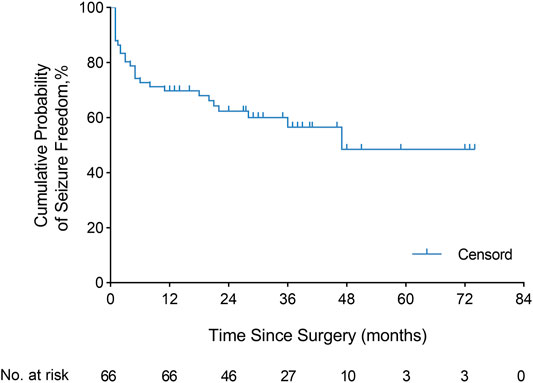
FIGURE 2. Engel class Ia seizure freedom. Kaplan–Meier curve demonstrated the seizure freedom outcome after resection in the overall population (66 patients). The cumulative probability of continuous seizure freedom was 69.7% (95% CI, 58.6–80.8%), 62.3% (95% CI, 50.3–74.4%), 56.5% (95% CI, 43.0–70.0%), and 48.4% (95% CI, 29.8–67.1%) at 12, 24, 36, and 60 months or more after surgery, respectively.
To evaluate in detail the seizure outcomes after surgery, we further investigated each Engel classification outcome distribution at different follow-up times (≥12 months) in sixty-six patients (Figure 3). At the last follow-up time (median, 29.5 months), thirty-nine patients (59.1%) were free of seizures and auras (Engel class Ia) after surgery, and one patient (1.5%) had Engel class Ib–d outcome. Seventeen patients (25.8%) had Engel class II outcome, three patients (4.5%) had Engel class III outcome, and six patients (9.1%) had an Engel class IV outcome.
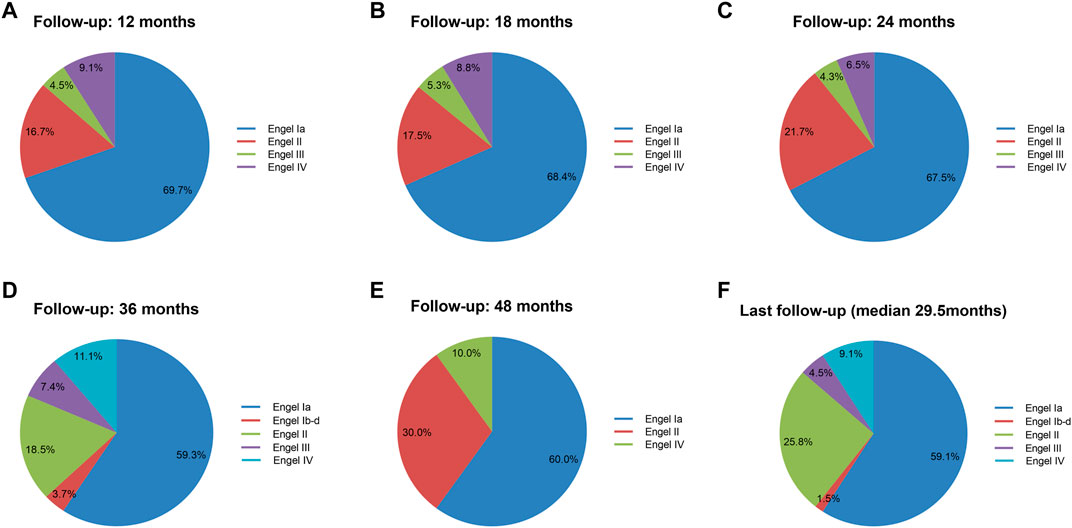
FIGURE 3. Engel classification outcome during postoperative follow-up. Engel classification outcomes at postoperative 12, 18, 24, 36, and 48 months and last follow-up. Total numbers of investigated cohorts and the rates of cohorts were illustrated for each Engel class outcome. The Engel class Ia outcome worsened with time.
Predictors of Seizure Freedom Outcome
We firstly performed a univariate analysis of the predictive factors influencing the seizure outcome (Table 1, 2), and the postoperative seizure outcome was classified as two groups including seizure freedom and seizure recurrence at last follow-up. The preoperative clinical, electrophysiological, and neuroimaging characteristics were explored. More patients with complete resection of the epileptogenic area were likely to obtain seizure freedom outcomes (94.9 versus 74.1%, p = 0.040). The patients with a duration of epilepsy less than 12 years had increased likelihood of seizure freedom after surgery (69.2 versus 44.4%, p = 0.044). The patients with ATLR were more likely to obtain seizure freedom (41.0 versus 29.6%) but did not reach significance (p = 0.344). Moreover, the presence of gray–white blurring or HS on MRI was also more likely to obtain seizure freedom but failed to reach significance (p = 0.406 and p = 0.539, respectively).
We further explored some univariate predictors utilizing the KM survival curves of seizure freedom outcome (Figures 4, 5). In our included patients, the influence of the two preoperative factors on seizure outcome was statistically significant, as follows (Figure 4D, Figure 5D): 1) the patients with complete resection of the epileptogenic area were significantly more likely to achieve seizure freedom (Engel class Ia) compared with patients with incomplete resection of epileptogenic area (pLog-rank = 0.004). 2) Patients without contralateral insular lobe hypometabolism were significantly more likely to achieve seizure freedom as compared with patients with contralateral insular lobe hypometabolism (pLog-rank = 0.025). We found that duration of epilepsy (more or less than 12 years), absence or presence of FC or FBTCS, and ATLR did not influence the Engel class Ia outcome (pLog-rank = 0.110, pLog-rank = 0.428, pLog-rank = 0.237, pLog-rank = 0.275, respectively) (Figures 4A–C,E). The KM survival curves demonstrated no significant effect of absence or presence of gray–white blurring on MRI on seizure freedom outcome (pLog-rank = 0.246) (Figure 5A). There was no significance on Engel class Ia outcome of concordance or discordance of preoperative MRI with preoperative ictal scalp VEEG (pLog-rank = 0.566) (Figure 5B). Moreover, the absence or presence of contralateral frontal lobe hypometabolism on PET/MRI did not impact seizure outcome (pLog-rank = 0.278) (Figure 5C).
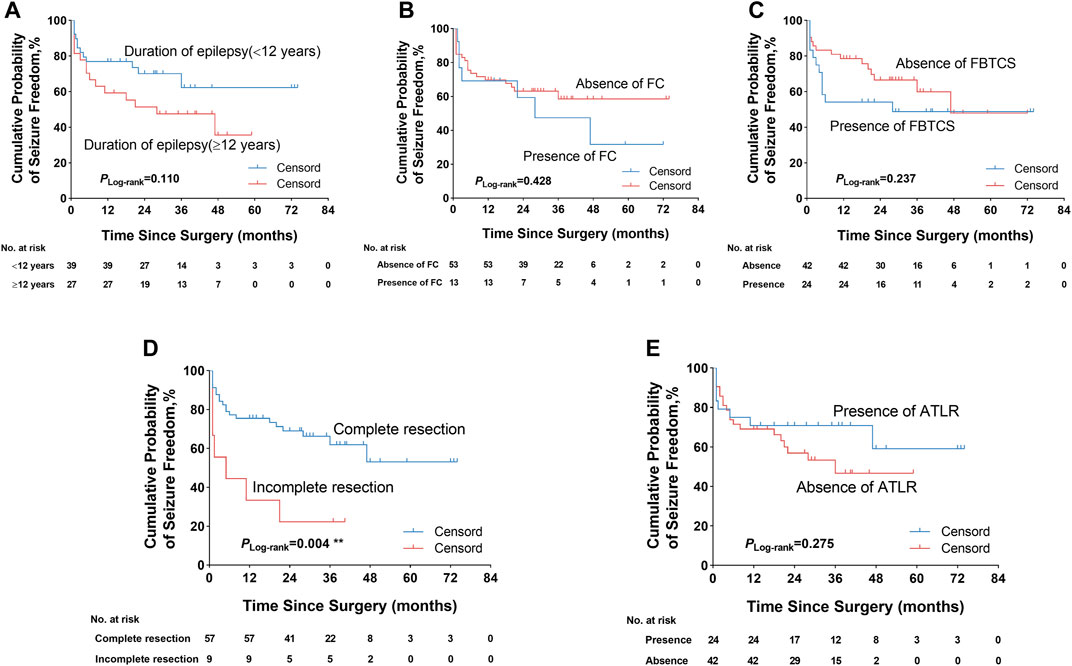
FIGURE 4. Kaplan–Meier survival curves of Engel class Ia seizure freedom depending on different outcome predictors. (A) Duration of epilepsy was not significantly associated with seizure freedom. (B) Absence or presence of FC did not significantly influence seizure outcome. (C) Absence or presence of FBTCS did not impact seizure outcome. (D) Complete resection of the epileptogenic area was significantly more likely to obtain seizure freedom. (E) ATLR was also not significantly associated with seizure outcome. **p < 0.01. FC, febrile convulsion; FBTCS, focal to bilateral tonic–clonic seizure; ATLR, anterior temporal lobe resection.
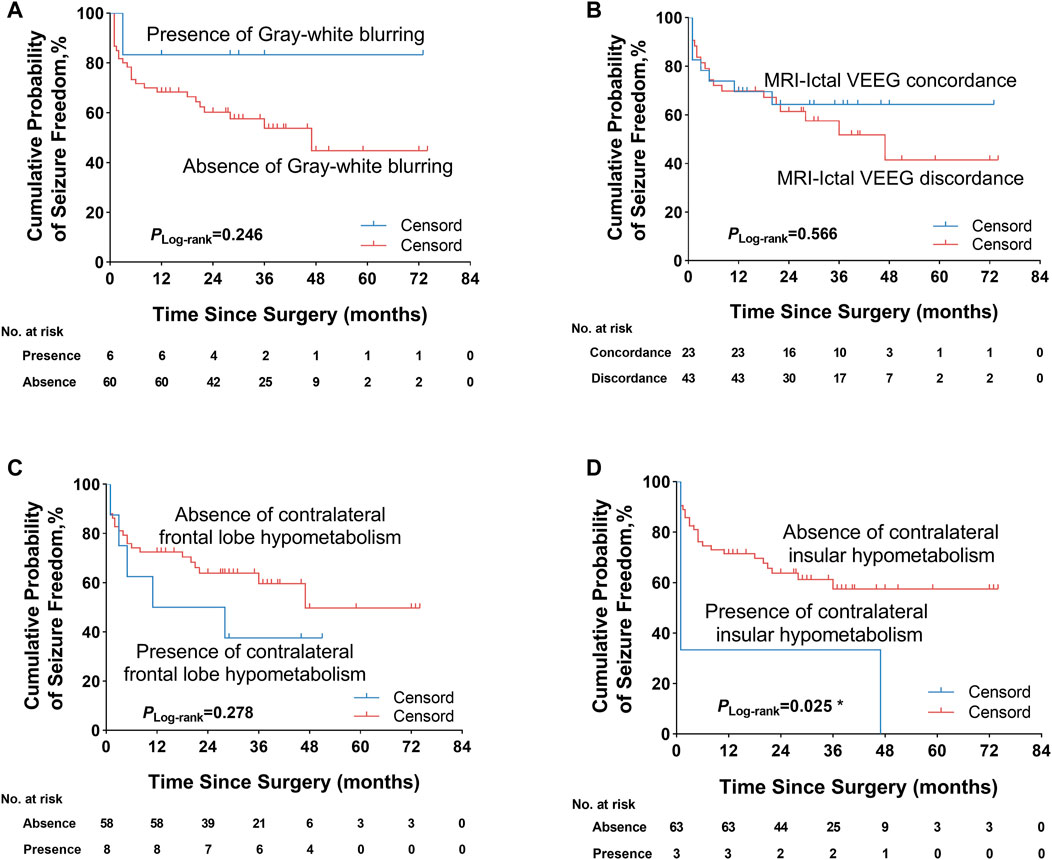
FIGURE 5. Kaplan–Meier survival curves of Engel class Ia seizure freedom since surgery. (A) Absence or presence of gray–white blurring on MRI was not significantly associated with seizure outcome. (B) Concordance or discordance of preoperative MRI with ictal scalp VEEG did not significantly influence seizure outcome. (C) Absence or presence of contralateral frontal lobe hypometabolism on PET/MRI was also not significantly correlated with seizure outcome. (D) Patients without contralateral insular lobe hypometabolism on PET/MRI were significantly more likely to obtain seizure freedom. *p < 0.05. VEEG, video electroencephalogram.
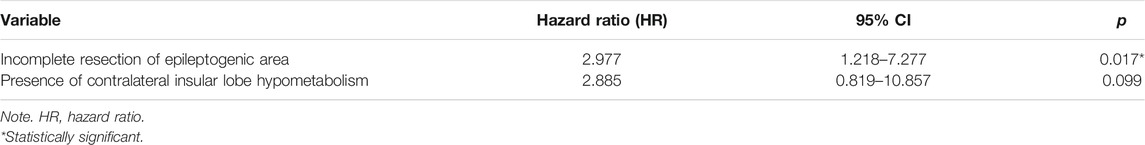
To further confirm the above univariable predictor identified as potentially significant in the KM survival curves analysis, we conducted a multivariable analysis utilizing a Cox proportional hazards regression model (Table 3). In the overall patients, the presence of incomplete resection of the epileptogenic area was the independent significant predictor of seizure recurrence and was correlated with an approximately three-fold increase in the risk of postoperative seizure recurrence (HR = 2.977, 95% CI 1.218–7.277, p = 0.017) as compared with patients who underwent complete resection. The predictive value of the presence of contralateral insular lobe hypometabolism on PET/MRI was also investigated, but the predictor failed to reach statistical significance (HR = 2.885, 95% CI 0.819–10.857, p = 0.099).
Discussion
Although some studies have investigated the electroclinical, imaging characteristics and predictive factors of postoperative outcomes in the patients with FCD, the features and predictive role for FCD type IIIa patients have yet to be evaluated. This is the first cohort including children and adults followed up 22.0–40.6 months after operative treatment of drug-resistant epilepsy caused by FCD type IIIa according to the new ILAE classification method. In this retrospective study, we demonstrated that duration of epilepsy (less than 12 years), absence of contralateral insular lobe hypometabolism on PET/MRI, and complete resection of the epileptogenic area were associated with seizure outcome by univariate analysis. Multivariate Cox regression analysis indicated that the incomplete resection of the epileptogenic area was the only independent predictor for seizure recurrence after surgery.
In the present study, we showed that FIAS was the most common seizure type in FCD type IIIa patients, followed by FBTCS. The most common symptoms were automatisms and autonomic behavior. A large number of patients (60.6%) with FCD type IIIa had auras. This result was less than 90% reported by Fauser et al. (2013). Moreover, we demonstrated that the patients with ATLR were more likely to obtain seizure freedom but did not reach significance, which was in accordance with the previous finding, which reported that patients with FCD type IIIa were more likely to achieve better seizure outcomes after ATLR compared with HS alone (Duhrsen et al., 2018). We also found that only a fraction of patients with FCD type IIIa had the manifestation of FCD on MRI including gray–white blurring, cortical thickening, and subcortical T2/FLAIR abnormality, which was concordant with the previous findings (D. W. Kim et al., 2012). Paradoxically, Fauser et al. reported that 88% of patients with FCD type IIIa presented with the gray–white blurring or subcortical signal intensity abnormality on MRI. This result also demonstrated that the presurgical identification of FCD type IIIa by MRI remained difficult and doubtful to a certain degree. These comprehensively clinical, electrophysiological and neuroimaging features could sufficiently help the clinician to diagnose and evaluate the patients with FCD type IIIa.
We reported that 59.1% of patients had the postoperative seizure freedom outcome at the last follow-up. This result aligned with the seizure freedom outcome reported by the previous findings (Fauser et al., 2013; Deleo et al., 2016) and worse than the favorable outcome reported by Fauser et al. (2015)and Jayalakshmi et al. (2019). We further investigated that the predictive factors of postoperative seizure freedom were complete resection of the EZ, absence of contralateral insular lobe hypometabolism on PET/MRI, and duration of epilepsy less than 12 years. However, we could not replicate previously reported predictors such as younger age at the surgery, absence of acute postoperative seizure, and intracranial electrode implantation (Kim et al., 2011; Jin et al., 2016; Andrews et al., 2019; Chen et al., 2019).
Our study first indicated that contralateral insular lobe hypometabolism on PET/MRI coregistration was correlated with seizure recurrence in patients with FCD type IIIa but was not an independent predictive factor for seizure outcome by multivariate Cox regression analysis. This was similar to the previous finding reported by Hur et al. (2013), who observed the trend towards a worse postoperative outcome in patients with insular hypometabolism, but the results did not reach significance. It was a sign that the insular cortex present dense interconnection neural network and allowed for the fast spread of ictal epileptic discharges. Such extensive involvement of the ictal discharges led to wider regions of hypometabolism in interictal PET/MRI imaging. Further studies are needed to explore the relationship between the hypometabolism pattern and postoperative outcome.
In accordance with the previous studies (D. W. Kim et al., 2009; Fauser et al., 2015; Oluigbo et al., 2015; Jin et al., 2016), our current study also demonstrated that complete resection of the EZ was the independent predictor of postoperative seizure outcome for FCD type IIIa. This study found that the patients with incomplete resection of the epileptogenic area had an approximately three-fold increase in the risk of seizure recurrence after surgery as compared with those with complete resection. However, our results also indicated that among the patients undergoing complete resection, 35.0% with complete resection of the epileptogenic area had seizure recurrence, which was higher than the previous finding (Jin et al., 2016). This phenomenon possibly resulted from the preoperative inaccurate recognition of the epileptogenic area and the development or maturation of a new EZ (Najm et al., 2013). Moreover, we indicated that the shorter duration of epilepsy (less than 12 years) was also a predictive factor of seizure freedom outcome after surgery, which was in accordance with some studies that duration of epilepsy was significantly associated with surgical outcome (Kral et al., 2003; Fauser et al., 2008; Fauser et al., 2015). This result highlighted that we should consider early epilepsy surgery in patients in whom drug-resistant epilepsy becomes obvious. However, in another study, Jin et al. (2016) did not observe the significant association of duration of epilepsy with surgical outcome.
In the current study, we showed that age at operation and seizure onset was not associated with the seizure outcome, which was in concordance with the previous studies that there was no significant influence of age at operation and seizure onset on postoperative seizure outcome (D. W. Kim et al., 2009; Krsek et al., 2009; Rowland et al., 2012; Jin et al., 2016). However, a large study about FCD including FCD type IIIa demonstrated that a younger age at operation could aid in obtaining seizure freedom (Fauser et al., 2015). In addition, Kim et al. (2011) also reported that younger age at the time of surgery was more likely to achieve good postoperative outcomes. This discordance may have resulted from the various criteria of patient selection and the different FCD subtypes. The relationship between the operation time and postoperative outcome for FCD, especially FCD type IIIa, was needed for further investigation.
Several limitations should be considered in the explanation of these results. Firstly, this is a retrospective study, so the selection bias is inherent to the study. We try to minimize bias as much as possible by performing blinded electrophysiological, imaging, and histopathology review utilizing novel classification methods. Secondly, this single-center and retrospective study limits its general relevance. Moreover, another limitation of our study is that we do not record EEG during the 18F-FDG PET scan and cannot exclude ictal scans.
In summary, postoperative seizure outcome in the surgical treatment of FCD type IIIa was in more than half of the patients. This underlines that surgery can be a critically effective therapy option for FCD type IIIa patients. More specifically, we comprehensively concluded the clinical, electrophysiological, and neuroimaging characteristics of FCD type IIIa, and we further investigated the prognostic factors of FCD type IIIa by surgical treatment. The surgical outcome of FCD type IIIa is affected by critical determinants, including duration of epilepsy, complete resection of the epileptogenic area, and contralateral insular lobe hypometabolism on PET/MRI. The complete resection of the epileptogenic area is the only independent predictor for seizure freedom outcome after surgery. With the advancement of both noninvasive multimodality imaging and comprehensive invasive assessment with SEEG, we will further improve preoperative evaluation and the postoperative outcome for patients with intractable epilepsy associated with FCD type IIIa.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee from Guangdong 999 Brain Hospital and conducted in accordance with the Declaration of Helsinki guidelines. Written informed consent was obtained from patients prior to surgery. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minors’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LZ, HZ, and WZ contributed to the writing of the manuscript. LZ, HZ, WZ, JG, and YT collected the original data. LZ and CZ contributed to the statistical analysis of the data and constructed the figures and tables. QT and XH contributed to the analysis of electrophysiological data. HL and BC contributed to the analysis of histopathological information. XL and YT contributed to the analysis of imaging information. QG and HX conceived the paper layout and modified the paper. All authors had read and approved the final version of the manuscript.
Funding
This work was financially supported by the Project of Innovative Team for the Guangdong Universities (No. 2018KCXTD001), the National Natural Science Foundation of China (No. 81701751, 81871383), and Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515011192).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Boston Professional Group (BPG) Editing for editing the paper.
References
Andrews, J. P., Gummadavelli, A., Farooque, P., Bonito, J., Arencibia, C., Blumenfeld, H., et al. (2019). Association of Seizure Spread with Surgical Failure in Epilepsy. JAMA Neurol. 76, 462–469. doi:10.1001/jamaneurol.2018.4316
Blumcke, I., Spreafico, R., Haaker, G., Coras, R., Kobow, K., Bien, C. G., et al. (2017). Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 377, 1648–1656. doi:10.1056/NEJMoa1703784
Blümcke, I., Thom, M., Aronica, E., Armstrong, D. D., Vinters, H. V., Palmini, A., et al. (2011). The Clinicopathologic Spectrum of Focal Cortical Dysplasias: A Consensus Classification Proposed by an Ad Hoc Task Force of the ILAE Diagnostic Methods Commission1. Epilepsia 52, 158–174. doi:10.1111/j.1528-1167.2010.02777.x
Cahill, V., Sinclair, B., Malpas, C. B., McIntosh, A. M., Chen, Z., Vivash, L. E., et al. (2019). Metabolic Patterns and Seizure Outcomes Following Anterior Temporal Lobectomy. Ann. Neurol. 85, 241–250. doi:10.1002/ana.25405
Chang, E. F., Wang, D. D., Barkovich, A. J., Tihan, T., Auguste, K. I., Sullivan, J. E., et al. (2011). Predictors of Seizure freedom after Surgery for Malformations of Cortical Development. Ann. Neurol. 70, 151–162. doi:10.1002/ana.22399
Chapman, K., Wyllie, E., Najm, I., Ruggieri, P., Bingaman, W., Lüders, J., et al. (2005). Seizure Outcome after Epilepsy Surgery in Patients with normal Preoperative MRI. J. Neurol. Neurosurg. Psychiatry 76, 710–713. doi:10.1136/jnnp.2003.026757
Chen, J., Huang, Z., Li, L., Ren, L., and Wang, Y. (2019). Histological Type of Focal Cortical Dysplasia Is Associated with the Risk of Postsurgical Seizure in Children and Adolescents. Tcrm 15, 877–884. doi:10.2147/TCRM.S203039
Deleo, F., Garbelli, R., Milesi, G., Gozzo, F., Bramerio, M., Villani, F., et al. (2016). Short- and Long-Term Surgical Outcomes of Temporal Lobe Epilepsy Associated with Hippocampal Sclerosis: Relationships with Neuropathology. Epilepsia 57, 306–315. doi:10.1111/epi.13277
Dührsen, L., Sauvigny, T., House, P. M., Stodieck, S., Holst, B., Matschke, J., et al. (2018). Impact of Focal Cortical Dysplasia Type IIIa on Seizure Outcome Following Anterior Mesial Temporal Lobe Resection for the Treatment of Epilepsy. J. Neurosurg. 128, 1668–1673. doi:10.3171/2017.2.JNS161295
Engel, J. V. N. P., Rasmussen, T. B., and Ojemann, L. M. (1993). “Outcome with Respect to Epileptic Seizures,” in Surgical Treatment of the Epilepsies. Editor J Engel. 2nd ed. (New York: Raven Press), 609–621.
Fauser, S., Bast, T., Altenmüller, D.-M., Schulte-Mönting, J., Strobl, K., Steinhoff, B. J., et al. (2008). Factors Influencing Surgical Outcome in Patients with Focal Cortical Dysplasia. J. Neurol. Neurosurg. Psychiatry 79, 103–105. doi:10.1136/jnnp.2007.116038
Fauser, S., Essang, C., Altenmüller, D.-M., Staack, A. M., Steinhoff, B. J., Strobl, K., et al. (2015). Long-term Seizure Outcome in 211 Patients with Focal Cortical Dysplasia. Epilepsia 56, 66–76. doi:10.1111/epi.12876
Fauser, S., Essang, C., Altenmüller, D. M., Staack, A., Steinhoff, B. J., Strobl, K., et al. (2013). Is There Evidence for Clinical Differences Related to the New Classification of Temporal Lobe Cortical Dysplasia? Epilepsia 54, 909–917. doi:10.1111/epi.12147
Fisher, R. S., Cross, J. H., D'Souza, C., French, J. A., Haut, S. R., Higurashi, N., et al. (2017). Instruction Manual for the ILAE 2017 Operational Classification of Seizure Types. Epilepsia 58, 531–542. doi:10.1111/epi.13671
Hauptman, J. S., and Mathern, G. W. (2012). Surgical Treatment of Epilepsy Associated with Cortical Dysplasia: 2012 Update. Epilepsia 53 (Suppl. 4), 98–104. doi:10.1111/j.1528-1167.2012.03619.x
Hur, J. A., Kang, J. W., Kang, H.-C., Kim, H. D., Kim, J. T., and Lee, J. S. (2013). The Significance of Insular Hypometabolism in Temporal Lobe Epilepsy in Children. J. Epilepsy Res. 3, 54–62. doi:10.14581/jer.13011
Jayalakshmi, S., Nanda, S. K., Vooturi, S., Vadapalli, R., Sudhakar, P., Madigubba, S., et al. (2019). Focal Cortical Dysplasia and Refractory Epilepsy: Role of Multimodality Imaging and Outcome of Surgery. AJNR Am. J. Neuroradiol 40, 892–898. doi:10.3174/ajnr.A6041
Jin, B., Wang, J., Zhou, J., Wang, S., Guan, Y., and Chen, S. (2016). A Longitudinal Study of Surgical Outcome of Pharmacoresistant Epilepsy Caused by Focal Cortical Dysplasia. J. Neurol. 263, 2403–2410. doi:10.1007/s00415-016-8274-1
Kemerdere, R., Ahmedov, M. L., Alizada, O., Yeni, S. N., Oz, B., and Tanriverdi, T. (2018). Effect of Temporal Neocortical Pathology on Seizure Freeness in Adult Patients with Temporal Lobe Epilepsy. World Neurosurg. 116, e801–e805. doi:10.1016/j.wneu.2018.05.095
Kim, D. W., Kim, S., Park, S.-H., Chung, C.-K., and Lee, S. K. (2012). Comparison of MRI Features and Surgical Outcome Among the Subtypes of Focal Cortical Dysplasia. Seizure 21, 789–794. doi:10.1016/j.seizure.2012.09.006
Kim, D. W., Lee, S. K., Chu, K., Park, K. I., Lee, S. Y., Lee, C. H., et al. (2009). Predictors of Surgical Outcome and Pathologic Considerations in Focal Cortical Dysplasia. Neurology 72, 211–216. doi:10.1212/01.wnl.0000327825.48731.c3
Kim, Y. H., Kang, H.-C., Kim, D.-S., Kim, S. H., Shim, K.-W., Kim, H. D., et al. (2011). Neuroimaging in Identifying Focal Cortical Dysplasia and Prognostic Factors in Pediatric and Adolescent Epilepsy Surgery. Epilepsia 52, 722–727. doi:10.1111/j.1528-1167.2010.02950.x
Kral, T., Clusmann, H., Blümcke, I., Fimmers, R., Ostertun, B., Kurthen, M., et al. (2003). Outcome of Epilepsy Surgery in Focal Cortical Dysplasia. J. Neurol. Neurosurg. Psychiatry 74, 183–188. doi:10.1136/jnnp.74.2.183
Krsek, P., Maton, B., Jayakar, P., Dean, P., Korman, B., Rey, G., et al. (2009). Incomplete Resection of Focal Cortical Dysplasia Is the Main Predictor of Poor Postsurgical Outcome. Neurology 72, 217–223. doi:10.1212/01.wnl.0000334365.22854.d3
Kwan, P., Arzimanoglou, A., Berg, A. T., Brodie, M. J., Allen Hauser, W., Mathern, G., et al. (2010). Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51, 1069–1077. doi:10.1111/j.1528-1167.2009.02397.x
Lerner, J. T., Salamon, N., Hauptman, J. S., Velasco, T. R., Hemb, M., Wu, J. Y., et al. (2009). Assessment and Surgical Outcomes for Mild Type I and Severe Type II Cortical Dysplasia: a Critical Review and the UCLA Experience. Epilepsia 50, 1310–1335. doi:10.1111/j.1528-1167.2008.01998.x
Marusic, P., Tomásek, M., Krsek, P., Krijtová, H., Zárubová, J., Zámecník, J., et al. (2007). Clinical Characteristics in Patients with Hippocampal Sclerosis with or without Cortical Dysplasia. Epileptic Disord. 9 (Suppl. 1), S75–S82. doi:10.1684/epd.2007.0152
Mintzer, S., and Sperling, M. R. (2008). When Should a Resection Sparing Mesial Structures Be Considered for Temporal Lobe Epilepsy? Epilepsy Behav. 13, 7–11. doi:10.1016/j.yebeh.2008.02.015
Montaz-Rosset, M.-S., Scholly, J., Voulleminot, P., Severac, F., Hirsch, E., Valenti-Hirsch, M. P., et al. (2019). Comparison of Functional Deficit Zone Defined by FDG PET to the Epileptogenic Zones Described in Stereo-Electroencephalograph in Drug-Resistant Epileptic Patients Treated by Surgery. Clin. Nucl. Med. 44, 526–531. doi:10.1097/RLU.0000000000002615
Najm, I., Jehi, L., Palmini, A., Gonzalez-Martinez, J., Paglioli, E., and Bingaman, W. (2013). Temporal Patterns and Mechanisms of Epilepsy Surgery Failure. Epilepsia 54, 772–782. doi:10.1111/epi.12152
Oluigbo, C. O., Wang, J., Whitehead, M. T., Magge, S., Myseros, J. S., Yaun, A., et al. (2015). The Influence of Lesion Volume, Perilesion Resection Volume, and Completeness of Resection on Seizure Outcome after Resective Epilepsy Surgery for Cortical Dysplasia in Children. J Neurosurg Pediatr 15, 644–650. doi:10.3171/2014.10.Peds14282
Palmini, A., and Holthausen, H. (2013). Focal Malformations of Cortical Development: a Most Relevant Etiology of Epilepsy in Children. Handb Clin. Neurol. 111, 549–565. doi:10.1016/b978-0-444-52891-9.00058-0
Pittau, F., Mã©gevand, P., Sheybani, L., Abela, E., Grouiller, F. d. r., Spinelli, L., et al. (2014). Mapping Epileptic Activity: Sources or Networks for the Clinicians? Front. Neurol. 5, 218. doi:10.3389/fneur.2014.00218
Rowland, N. C., Englot, D. J., Cage, T. A., Sughrue, M. E., Barbaro, N. M., and Chang, E. F. (2012). A Meta-Analysis of Predictors of Seizure freedom in the Surgical Management of Focal Cortical Dysplasia. Jns 116, 1035–1041. doi:10.3171/2012.1.Jns111105
Seo, J. H., Holland, K., Rose, D., Rozhkov, L., Fujiwara, H., Byars, A., et al. (2011). Multimodality Imaging in the Surgical Treatment of Children with Nonlesional Epilepsy. Neurology 76, 41–48. doi:10.1212/WNL.0b013e318204a380
Tassi, L., Colombo, N., Garbelli, R., Francione, S., Lo Russo, G., Mai, R., et al. (2002). Focal Cortical Dysplasia: Neuropathological Subtypes, EEG, Neuroimaging and Surgical Outcome. Brain 125, 1719–1732. doi:10.1093/brain/awf175
Keywords: characteristics, predictors, postoperative outcome, focal cortical dysplasia type IIIa, drug-resistant epilepsy
Citation: Zhang L, Zhou H, Zhang W, Ling X, Zeng C, Tang Y, Gan J, Tan Q, Hu X, Li H, Cheng B, Xu H and Guo Q (2022) Electroclinical and Multimodality Neuroimaging Characteristics and Predictors of Post-Surgical Outcome in Focal Cortical Dysplasia Type IIIa. Front. Bioeng. Biotechnol. 9:810897. doi: 10.3389/fbioe.2021.810897
Received: 08 November 2021; Accepted: 26 November 2021;
Published: 10 January 2022.
Edited by:
Jian Yang, Shanghai University, ChinaReviewed by:
Erik Dirk Gommer, Maastricht University Medical Centre, NetherlandsYingZhu Chen, Yangzhou University, China
Copyright © 2022 Zhang, Zhou, Zhang, Ling, Zeng, Tang, Gan, Tan, Hu, Li, Cheng, Xu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Xu, dHhoQGpudS5lZHUuY24=; Qiang Guo, Z3VvcWlhbmc5OTlicmFpbkAxNjMuY29t
 Lingling Zhang
Lingling Zhang Hailing Zhou
Hailing Zhou Wei Zhang3
Wei Zhang3 Hainan Li
Hainan Li Qiang Guo
Qiang Guo