- 1Department of Pathology, Quanzhou Women’s and Children’s Hospital, Quanzhou, China
- 2Department of Pathology, Qingyuan Maternal and Child Health Hospital, Qingyuan, China
- 3School of Pharmaceutical Sciences, Southern Medical University, Guangzhou, China
- 4State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen, China
Drug resistance is the most significant causes of cancer chemotherapy failure. Various mechanisms of drug resistance include tumor heterogeneity, tumor microenvironment, changes at cellular levels, genetic factors, and other mechanisms. In recent years, more attention has been paid to tumor resistance mechanisms and countermeasures. Nanomedicine is an emerging treatment platform, focusing on alternative drug delivery and improved therapeutic effectiveness while reducing side effects on normal tissues. Here, we reviewed the principal forms of drug resistance and the new possibilities that nanomaterials offer for overcoming these therapeutic barriers. Novel nanomaterials based on tumor types are an excellent modality to equalize drug resistance that enables gain more rational and flexible drug selectivity for individual patient treatment. With the emergence of advanced designs and alternative drug delivery strategies with different nanomaterials, overcome of multidrug resistance shows promising and opens new horizons for cancer therapy. This review discussed different mechanisms of drug resistance and recent advances in nanotechnology-based therapeutic strategies to improve the sensitivity and effectiveness of chemotherapeutic drugs, aiming to show the advantages of nanomaterials in overcoming of drug resistance for tumor chemotherapy, which could accelerate the development of personalized medicine.
Introduction
The biggest barrier to cancer therapy is the inevitable emergence of drug resistance. Drug resistance can be divided into two categories according to the behind factors: primary and secondary (Longley and Johnston, 2005). Primary resistance indicates those factors present in the cancer cell or tissue itself before chemotherapy and reduces the efficacy of chemotherapy. However, because of various adaptive responses, such as increased expression of therapeutic targets and activation of alternative signaling pathways, secondary or acquired resistance can develop during therapeutic periods that are initially sensitive to cytotoxic agents (Lackner et al., 2012). Continuously activated proliferative signaling, inactivated growth suppressors, and activated metastasis factors are classical hallmarks of some tumors, which lead to drug resistance to chemotherapy. With the deepening of mechanism research, tumor heterogeneity, tumor microenvironment, and cancer stem cells (CSCs) were closely associated with the occurrence and development of drug resistance in chemotherapy. Besides, the characteristic of cell motility acquired from epithelial-mesenchymal transition (EMT), genetic factors of drug transport, especially the multiple drug resistance (MDR) is also considered to be vital factors for resistance development.
Considering the drug resistance is a complex mechanism, many dexterous solutions appeared in anticancer therapies to overcome drug resistance and increase the efficacy of treatment. Among these, nanomaterials as a promising drug delivery are being investigated for solving several forms of drug resistance (Markman et al., 2013; Da Silva et al., 2017). Compared with “free” drugs, nanomaterials have remarkable advantages containing higher bioavailability, slower drug release, lower drug usage, and better treatment outcomes. Statistically, there are more than 40 therapeutic nanomaterials approved for clinical use worldwide and at least 200 nanoparticles undergoing clinical trials (Schütz et al., 2013; van Der Meel et al., 2013; Bobo et al., 2016). The classical enhanced permeability and retention (EPR) effect in solid tumors promote the selective distribution of nanomaterials, further increase pharmacodynamics and reduce systemic side effects (Fang et al., 2011; Kobayashi et al., 2013). A possible concern is that the nanomaterials may be inclined to accumulate in drug disposal organs such as the liver and kidney, leading to severe organ toxicity. To avoid the side effects introduced by nanomaterials, researchers have developed lots of methods including changing the physicochemical properties of nanomaterial, PEG polymer chains modifications, and so on (Milla et al., 2012; Zhu et al., 2013). At present, more and more novel nanomaterial-based formulations are emerging in-clinic research, many of them win success especially in solving the therapeutic impediments in cancer treatment.
Here, in this review, we mainly described the mechanisms of drug resistance and focused on recent innovation of dexterous and sophisticated nano-strategies in overcoming drug resistance, providing ideas for tumor chemotherapy and related combination therapies (Scheme 1).
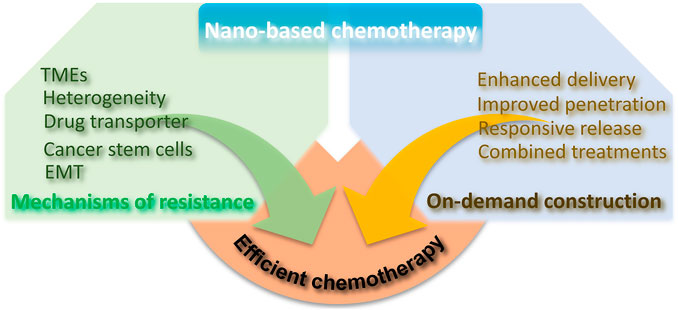
SCHEME 1. Illustration of chemotherapy resistance mechanism and the advantages of nano-based chemotherapy to achieve efficient chemotherapy.
Mechanism of Drug Resistance
The mechanism of drug resistance at tumor tissue level is extremely complicated. Commonly, tumor heterogeneity, tumor microenvironment (TME), drug transporter and multidrug resistance, cancer stem cells (CSCs), epithelial-mesenchymal transition (EMT) and tumor metastasis are known as the major drug resistance factors (Figure 1), which contribute to the off-target effect in the practice of chemotherapy. And drug transporters-mediated drug efflux impairs the cellular chemotherapeutics delivery, leading to the low therapeutic dose (Cheng et al., 2018a; Fan et al., 2018; Ke et al., 2021). Besides, low pH, hypoxic tumor microenvironment, and other anti-apoptotic molecules could enhance the survival compensation effect (Li and Kataoka, 2021). Except for the five drug resistance reasons we mentioned, other resistant reasons containing gene mutations and genomic instability, epigenetic changes such as DNA methylation and protein acetylation, inhibition of apoptotic signaling and overexpression of anti-apoptotic molecules (Min et al., 2017; Vasan et al., 2019). To comprehensively explain the innovative design and prospects of nanomedicine in overcoming of tumor resistance, this review mainly focuses on several classic resistance mechanisms. Clinical practice shows serious resistance against chemotherapeutic drugs significantly impairs the therapeutical effect and contributes to poor prognosis for cancer patients. Therefore, full understanding the mechanism of drug resistance at the molecular level might bring novel treatment strategies, which in turn will lead to deeper and longer-lasting cancer treatments.
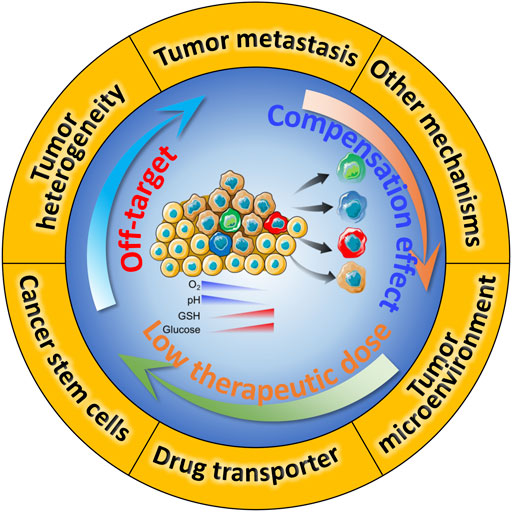
FIGURE 1. Schematic illustration of drug resistance in chemotherapy, and the underlying crosstalk between these mechanisms.
Tumor Heterogeneity
Heterogeneity is a distinct characteristic of tumor tissues from normal tissues and is represented by different morphological profiles. The heterogeneity may cause discrepant distribution of tumor cell subpopulations across different regions of the primary tumor (spatial heterogeneity) or variations in the molecular makeup of a single lesion over time (temporal heterogeneity). Cancer cells have the powerful capacity of self-renewal and differentiation, which give rise to abundant tumor types causing heterogeneity of the tumors (Marusyk and Polyak, 2010; Dagogo-Jack and Shaw, 2018). The driving forces behind tumor heterogeneity contain heritable and non-heritable mechanisms, either as a result of a natural progression of tumor or exposure to pressures created by clinical interventions. A substantial fraction of heterogeneity commonly results from environmental factors during tumor development and is therefore non-heritable. Tumor heterogeneity can be regarded as a process of Darwinian evolution theory (Polyak, 2014; Zellmer and Zhang, 2014). Tumor heterogeneity affects drug sensitivity and provides fuel for drug resistance in cancer therapy. The example of drug response influenced by temporal heterogeneity comes from a cellular barcoding experiment results that resistant clones often arise from the selective expansion of pre-existing populations during drug treatment (Bhang et al., 2015). Spatial heterogeneity has a greater impact on initial drug treatment response. However, analyses involving single-site biopsy sampling might result in underestimation of the degree of spatial heterogeneity. Instead, noninvasive “liquid biopsies” extracted from patient blood are a promising strategy for addressing the shortcomings of tissue sampling (Nagrath et al., 2007; Chabon et al., 2016). Recently, single-cell sequencing technology is revolutionizing biomedical research. Using single-cell genomics enables to characterize tumor heterogeneity by quantifying the gene expression of thousands of individual cells (Villani et al., 2017).
Tumor Microenvironment
The reprogramming of cellular metabolism is a common feature of cancer cells, one of which is known as the Warburg effect (Vaupel et al., 2019). Cancer cells would prefer to adopt aerobic glycolysis for growth rather than oxidative phosphorylation (OXPHOS), which strongly upregulate glucose uptake and aerobic glycolysis to increase intermediate glycolytic metabolites and the production of lactic acid (Lu et al., 2015). On the one hand, the glycolytic intermediates provide essential anabolic energy for cell proliferation and tumor growth. On the other hand, the increased by-product of lactic acid results in the accumulation of intracellular protons. The accumulation of protons in the intracellular environment activates the proton excludes (Na+/H+ exchanger, and carbonic anhydrase) and consequently changes the pH gradient in the tumor microenvironment (Izumi et al., 2003). This new biophysical setting profoundly influences the capability of drug uptake and pharmacological process in cancer cells. The lower pH value in tumor tissues gives rise to the protonation and neutralization of various chemotherapeutic drugs such as doxorubicin by preventing them from entering the targeted locus (Miraglia et al., 2005; Spugnini et al., 2015). Moreover, alteration of pH gradient easily promotes the formation of cytoplasmic acidic vesicles in tumor cells, which sequestrates chemical molecules in cell organelles eventually leading to the elimination of the drug through vesicle degranulation (Taylor et al., 2015).
Hypoxia is another common microenvironment feature in nearly all solid tumors. The uncontrolled proliferation of tumors limits the availability of oxygen supplied from the tumor vascular system, thus exposing them to low oxygen environments. On average, the oxygen level in tumor tissues is around 1–2% or below (McKeown, 2014). Hypoxia is also a key factor in cancer therapy resistance by regulating the tumor microenvironment (Rohwer and Cramer, 2011; Jing et al., 2019). The transcription factor hypoxia-inducible factor (HIF) usually serves as the major mediator of hypoxia response and is highly expressed in many cancers. Numerous evidence has shown that the inhibition of HIF-1α in cancer cells significantly enhanced sensitivity towards chemotherapeutic drugs (Brown et al., 2006; Liu et al., 2008). However, the underlying molecular mechanism of HIF-1α’s contributions to drug resistance is complicated, multiple, and specific in some tumor types. Some studies found that HIF-1 can induce the expression of the multidrug resistance 1 (MDR1) gene in a hypoxic tumor environment (Comerford et al., 2002). The membrane-located P-glycoprotein (P-gp) is the translation product of the MDR1 gene, which belongs to the family of ATP-binding cassette (ABC) transporters. Acting as a drug efflux pump, P-gp could decrease the intracellular concentration of chemotherapy agents such as paclitaxel, vinca alkaloids (Gottesman et al., 2002). Many chemotherapeutic agents such as 5-fluorouracil (5-FU), cisplatin, and DNA damage inducer triggering cell apoptosis largely depend on p53 pathway activation. Other important findings are that HIF-1 may function as an antagonist of p53-mediated cell death upon treatment with the chemotherapeutic agent (Hao et al., 2008; Rohwer et al., 2010). Besides, other reasons of HIF-1 contribute to chemotherapeutic drug resistance involving DNA damage repair, metabolic reprogramming, and immune microenvironment (Wirthner et al., 2008; Semenza, 2010; Vaupel and Multhoff, 2018).
Drug Transporter and Multidrug Resistance
Drug transporters are integral membrane proteins that are involved in drug disposition by affecting absorption, distribution, metabolism, and excretion (ADME). Clinical pharmacokinetic drug-drug interactions (DDIs) studies have shown that transporters mainly participate in the drug ADME process together with metabolic enzymes (Zhang et al., 2008). Based on their structure and mechanism, membrane transporters can be divided into two major superfamilies: the ATP-binding cassette (ABC) and solute-linked carrier (SLC) superfamily (Giacomini et al., 2010; Nigam, 2015). One of the drug transporter so far received the greatest attention is ABC family transporters namely ABCB1, also known as P-glycoprotein or MDR1. Tumor cells can achieve resistance to a broad range of anti-cancer drugs via activating drug efflux pumps of membrane-resident P-glycoprotein (P-gp). These hydrophobic drug substrates such as anthracyclines, vinca alkaloids, taxane, epipodophyllotoxins, topotecan, as well as several pharmaceuticals agents are presented to the transporter directly from the lipid bilayer. Such self-defense effect can occur in ATP-dependent or ATP-independent manners regardless of the concentration gradients of drugs (Pérez-Tomás, 2006; Robey et al., 2018). Research has suggested that the expression of P-gp is usually upregulated in a variety of tumors including the colon, kidney, liver, and pancreas. When exposed to chemotherapy drugs, the mRNA levels of MDR1 can be rapidly induced in tumor cells (Abolhoda et al., 1999). A more important discovery is that transporter protects against cell death not only by forcing out drugs but also via direct interference with the caspase-dependent pathway, which is called as efflux pump-independent MDR effect (Pallis et al., 2002). For the above reasons, it seems that P-gp inhibition is a clever therapeutic way to reverse the MDR phenotype. However, many of the P-gp modulators were substrates for other transporters or metabolic enzymes resulting in unpredictable pharmacokinetic interactions. For this reason, considerable efforts should be made to develop agents specifically inhibiting P-gp function in MDR-mediated drug resistance research.
Existence of Cancer Stem Cells
It is widely accepted that cancers develop from a small subset of stem cells with exclusive ability to self-renew and differentiate into the heterogeneous cancer cell types (Clarke et al., 2006; Beck and Blanpain, 2013). These intrinsic cancer stem cells or tumor-initiating cells constitute a population that is mainly responsible for cancer aggressiveness, drug resistance, and tumor relapse. There is no universal molecular feature of CSCs, although some cell-surface markers such as CD133, CD44 were associated with CSC phenotype in different types of tumors (Grosse-Gehling et al., 2013; Yan et al., 2015). Sometimes, it is assumed that the expression of these surface markers may reflect the CSC frequency rather than exact content in certain tumors. CSCs are rarely ranging from 1 per 1,000 to 1 per 100,000 in human tumors (Ishizawa et al., 2010). Nevertheless, when transplanted into the immunodeficient mice, these CSCs quickly enable the formation of secondary tumors. Chemotherapeutic drugs typically affect dividing cells, while CSCs are primarily quiescent cells with enhanced DNA repair mechanisms, always not influenced (Vitale et al., 2017). Besides, CSCs also express high levels of specific ABC drug transporters containing ABCB1, ABCG2, and ABCC1, which are known MDR genes in tumor cells, allowing for increased survival (Moitra, 2015). Although chemotherapy kills the majority of tumor cells, the resistant CSCs may still survive and expand the tumor again with self-renewing cells and differentiated offspring (Dean et al., 2005).
EMT and Tumor Metastasis
Epithelial-mesenchymal transition (EMT) is a process that epithelial cells lose their apical-basal polarity and cell-cell adhesive properties, acquire migratory and invasive properties of mesenchymal cells. These undergoing EMT cells exhibit decreased expression levels of epithelial markers (E-cadherin) and increased expression levels of mesenchymal genes (N-cadherin and vimentin) (Lamouille et al., 2014). In most cases, EMT leads to cell morphological changes and the gain of stem cell-like features. EMT is extensively involved in multiple biological pathological processes, especially in many aspects of cancer progression, including tumor metastasis and drug resistance (Du and Shim, 2016; Du et al., 2017). In diverse types of cancers, these undergoing EMT cells frequently overexpressed ABC family transporters and showed drug resistance phenotype similar to CSCs (Mani et al., 2008; Jiang et al., 2017). Studies have demonstrated that the promoters of ABC transporters contain several binding sites for EMT-related transcription factors. Overexpression of transcription factors such as Twist and Snail could increase the promoter activity and expression of ABC transporters in cancer cells, causing drug resistance in cancer treatment (Saxena et al., 2011). Besides, matrix metalloproteinases (MMPs) are involved in the regulation of EMT and other metastasis-related molecules, the combination of specific inhibitors against MMPs is an effective strategy to prevent tumor metastasis and improve tumor chemotherapy (Chen X. et al., 2021).
Nanomaterial Therapeutics Overcome Drug Resistance
Nanotechnology is initially defined as the “intentional design, characterization, production, and applications of materials, structures, devices, and systems by controlling their size and shape in the nanoscale from 1 to 100 nm range.” (N Engl J Med. 2010 Dec 16; 363 (25):2434–43) Nanotechnology has potential applications in medical applications because nanomaterials are similar in scale to biomolecules and systems, and can be designed to perform different functions. In cancer treatment, nanomaterials are mainly designed to aid in the transport of anticancer drugs through biological barriers. The aims of cancer nanomedicine are to use the properties and physical characteristics of nanomaterials for achieving more effective and safer cancer treatment (Nat Rev Cancer. 2017 Jan; 17 (1):20–37). In recent years, emerging evidence demonstrated that some nanomaterials have distinct advantages in reducing side effects and overcoming drug resistance. The following will focus on recent advancements in nanotherapeutic approaches to tackle drug resistance.
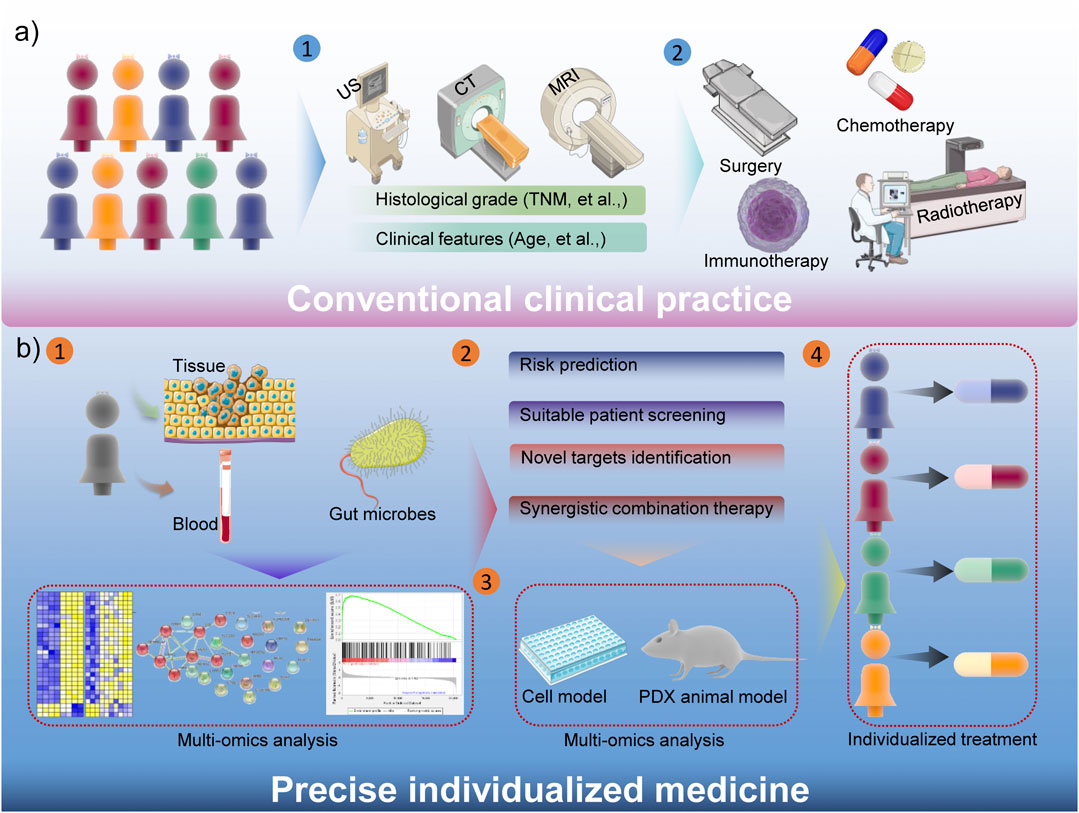
Precise Individualized Strategies for Overcoming Tumor Heterogeneity
Tumor heterogeneity is an important hallmark for some cancers, which contribute to the unsatisfactory treatment outcome (Liu et al., 2019). Traditional tumor treatments are mostly based on ultrasound (US), magnetic resonance imaging (MRI), computed tomography (CT) and other examinations to stage cancer patients, and then formulate treatment strategies based on histological grade (such as TNM staging, cancer thrombus formation, et al.) and some clinical characteristics of the disease, etc. (Lord et al., 2021). And then surgery, chemotherapy, radiotherapy, immunotherapy and other treatments could be chosen by median’s experience. Obviously, the consistency of conventional tumor treatments is poor, and this general treatments have a certain therapeutic effect on some patients, but due to the characteristics of tumor heterogeneity and other reasons, many patients have not benefited. The inhibitor against kirsten rat sarcoma virus (KRAS) oncogene is a good example. Due to the gene mutation of KRAS in many cancer patients, the clinical performance is not satisfactory (Kim et al., 2020). Another specific example is that sex also plays a critical role in the drug response, toxicity and other aspects (Hajipour et al., 2021). Therefore, precision medicine has received more and more attention in recent years, which could significantly compensate for the shortcomings of conventional strategies (Figure 2). Simply, patient’s sample-based multi-omics analysis could comprehensively demonstrate the underlying risk, suitable patients, novel drug targets, and promising combination therapies (Zhang and Yu, 2020). Not only that, precision medicine will also accelerate the process of clinical transformation, the cells and patient-derived xenograft (PDX)-based drug screening could enhance the efficacy of clinical trials (Wang et al., 2018a). Therefore, the precision medicine could guide the choice of clinical medication and treatment options, and more evidence also confirms that it is beneficial to improve the prognosis of cancer patients. Considering the better performance of nanomedicine in cancer therapy, the diverse functionality of nanomedicine remarkably facilitates precision nanomedicine, bringing revolutionary progress to tumor treatment.
Exploiting Novel pH-Responsive Nanoparticles Based on Tumor Acidity
Recognizing that tumors tend to be acidic environments, a class of pH-responsive nano-structures is developed and such systems could enhance uptake by tumor cells through acidic microenvironment-triggered drug release. These nanoparticles were reported as following. Self-assembled PEG-detachable polymeric micelles DOX-PMs (Xu et al., 2019), zwitterionic charge-switchable polymers S-NP/DOX (Yuan et al., 2012), pH and redox dual responsive nanoparticle RPDSG/DOX (Kc et al., 2012), pH-sensitive DSF/PTX co-loaded micelles (Huo et al., 2017), dual-pH responsive BCP-DOX micelles (Mao et al., 2016), which were designed to combat chemotherapy resistance based on the low pH tumor microenvironment, and the results have implied that these pH-responsive micelles have significant potential for efficiently combating chemotherapeutic agents (DOX and PTX) resistance in cancer treatment especially in co-delivery applications (Yu et al., 2015; Wang et al., 2017). Liu et al. reported a pH-sensitive DSF/PTX co-delivery systems work in drug-resistant tumor cells model. Briefly, the micelles were composed of succinic anhydride-modified PTX (SA-PTX) and disulfiram (DSF), a P-glycoprotein (P-gp) inhibitor, was simultaneously encapsulated into the hydrophobic core of poly (ethylene glycol)-block-poly (l-lysine) (PEG-b-PLL). Such a drug carrier was characterized as follows: 1) the micelles remain negative surface charge under blood circulation, while quickly changes to positive under acidic tumor tissues owing to the hydrolysis of anhydride group. 2) the endocytosis effect increases the uptake of micelles. 3) the internalized carriers could quickly release DSF accompanied by deformation of micelles, and then PTX drugs are released in acidic organelles in a pH-dependent sustained manner. 4) Because P-gp was inhibited by DSF, the PTX could accumulate at a higher concentration and effectively kill resistant tumor cells (Huo et al., 2017). Another good example is the combined PD173074 chemotherapeutics and chloroquine (CQ) to block the autophagy and fibroblast growth factor receptor 1 (FGFR1) mediated AZD9291-resistance (Figure 3). In details, a pH-sensitive nanoparticle system with shell-core structure was designed to encapsulate FGFR1 inhibitor PD173074 and autophagy inhibitor CQ to overcome the resistance of tyrosine kinase inhibitors (TKIs) AZD9291 in non-small cell lung cancer (NSCLC). FGFR1 signaling was described to contribute to EMT-associated acquired resistance of EGFR-TKI in NSCLC treatment. Therefore, NSCLC cells with EGFR-TKI resistance is sensitive to FGFR1 inhibitors (Wang et al., 2016). Further, the FGFR1 signaling could regulate cell autophagy, and autophagy inhibition is beneficial to enhance the anticancer effects of FGFR1 inhibitor (Lee et al., 2016). The novelty of this study is the application of pharmacological effects for innovative nanotheranostic. Furthermore, the biomineralization with CaP favour the nanoparticles with pH-sensitive to achieve the lysosome escape and realize the pH-responsive drug release, which could be applied by the low pH tumor microenvironment. The specific pH-activation drug release is fully studied, and many combined strategies were also developed based on other tumor microenvironments, such as higher glucose, low oxygen and higher Glutathione (GSH) concentration (Li et al., 2013; Li et al., 2017; Cheng et al., 2021). These pH-responsive nanoparticles not only can realize the escape of lysosome and enhance the pharmacological concentration, also achieve the responsive drug release to decrease the toxic side effects.
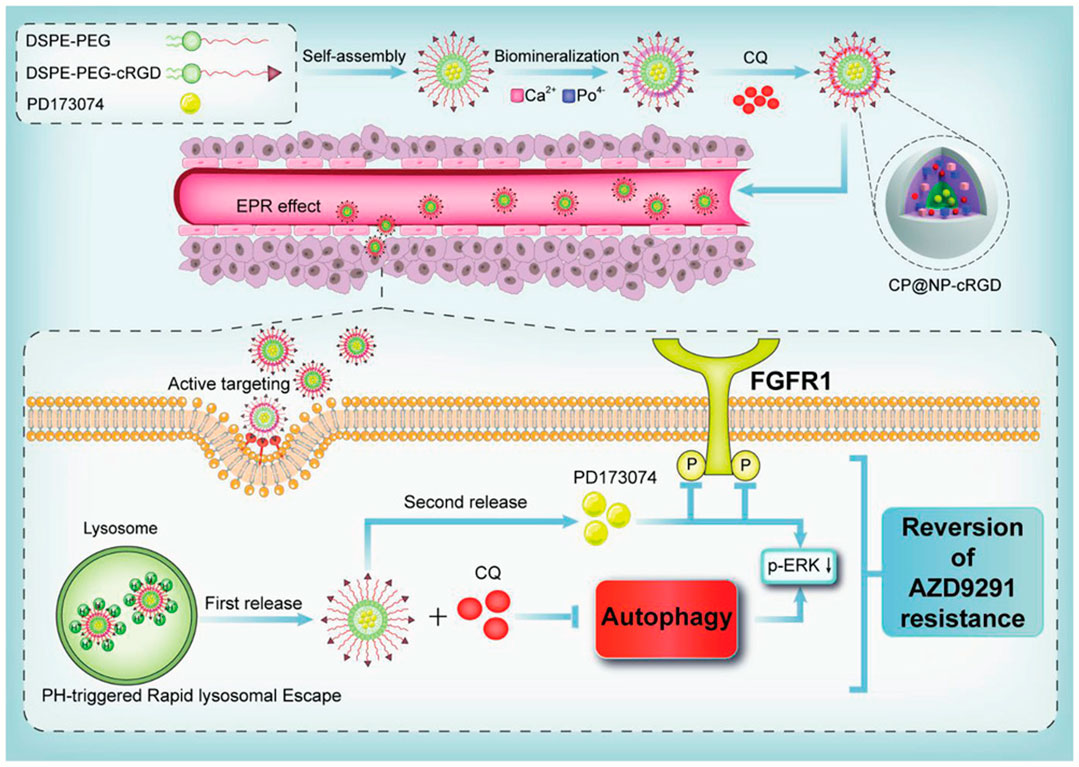
FIGURE 3. Schematic model of a pH-responsive shell-core nanoparticles to overcome AZD9291 drug resistance. Reproduced with permission (Gu et al., 2021). Copyright 2021, Wiley.
Nanoparticles Targeting the Hypoxic Tumor Microenvironment
Evidence has revealed that hypoxia in the tumor microenvironment has a crucial role in the clinical resistance of chemotherapy through a variety of signal transduction pathways and molecular changes (Pastorek and Pastorekova, 2015; Wang D. et al., 2021; Xu et al., 2021). The development of advanced nanomaterial delivery methods for reversing the resistance of hypoxic tumors is therefore of great interest but remains a considerable challenge. In general, strategies modulating hypoxia via nanomaterials that have been investigated can be divided into three categories including countering hypoxia, disregarding hypoxia, and exploiting hypoxia. The countering hypoxia strategy is a traditional targeting method. For example, the recent study designed a versatile tumor hypoxia-directed nanoparticle loaded with Acetazolamide (ATZ) specifically targeting the marker of tumor hypoxia marker carbonic anhydrase IX (CA IX) for reversing Sorafenib resistance in RCC treatment (Alsaab et al., 2018). Based on the biocompatibility and oxygen dissolving ability of perfluorocarbon (PFC), others developed an innovative nano-PFC as an oxygen shuttle for ultrasound-triggered tumor-specific delivery of oxygen (Song et al., 2016). This kind of efficient oxygen-enhanced nanomaterial may also provide a promising strategy to overcome the hypoxia-associated resistance in cancer treatment (Tian et al., 2017). Interestingly, some material scientists tend to explore and make use of the hypoxic feature in drug-resistant tumor cells that is called as exploiting hypoxia strategy. Numerous studies have indicated that hypoxia-triggered DNA damage, mitochondrial activity, autophagy, and drug efflux are treatment obstacles in platinum-based clinical chemotherapy. For example, the xeroderma pigmentosum group F (XPF), an DNA self-repairing protein could be regulated by hypoxia, which causes the acquired resistance of cancer cells to cisplatin (Angew Chem Int Ed Engl. 2016 Dec 12; 55 (50):15564–15568). Recently, researchers proposed a novel nano-chemotherapy strategy that is to build a liposome nanodrug containing glucose oxidase (GOx), tirapazamine (TPZ), and platinum (IV) prodrug (Chen J. et al., 2021). The nanodrug can not only be fully utilized but also further aggravate intracellular hypoxia of cisplatin-resistant tumor cells, thus fully activating TPZ drug activity. Activated TPZ effectively inhibited the expression level of XPF protein promoting DNA repair in tumor cells, thus achieving synergistically enhanced anti-tumor therapy with platinum drug (Figure 4).
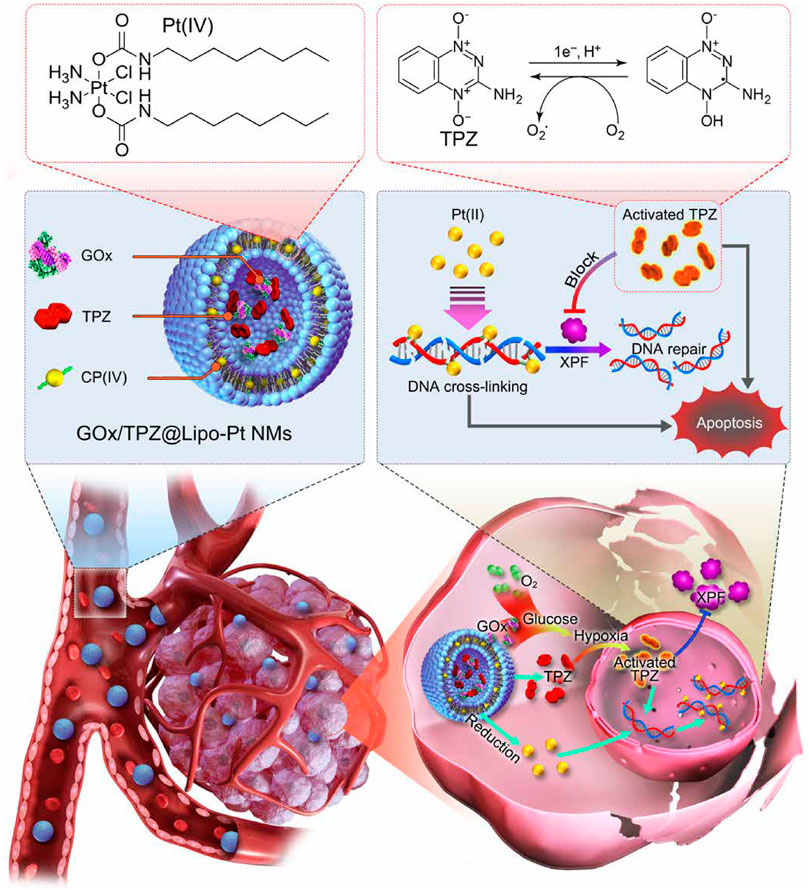
FIGURE 4. Schematic illustration of the GOx/TPZ@Lipo-Pt nanomaterial and hypoxia-induced reversal of cisplatin resistance. Reproduced with permission (Chen J. et al., 2021). Copyright 2021, American Chemical Society.
Typical Nanocarriers for Overcome of Drug Pump Efflux
With the development of nanotechnology, more and more new-style nanostructures were discovered. Among these, gold nanoparticles (AuNPs) are being widely used to deliver small molecules due to their unique optical properties and surface plasmon resonance effect. And PEG can be attached to the surface of gold nanoparticles to increase the stability and circulation time of chemotherapy drugs. Moreover, the surface plasmon band (SPR) of the nanoparticle will be modified by changing the shape of AuNPs from spheres to rods. One of the advantages of gold nanorods is the shift of the SPR allows for near-infrared (NIR) absorption at the cross-sections permitting a deeper penetration into living tissues (Arvizo et al., 2010). Many results have demonstrated these AuNPs or gold nanorods could be used for the circumvention of drug resistance (Song et al., 2017; Wang et al., 2018b). One notable work is the study that Vishwakarma SK et al. developed a stable colloidal suspension of sorafenib-gold nanoconjugate (SF-GNP) in an aqueous medium for reverting the drug resistance in HCC cells in a 3D model system. Owing to the potential of highly biocompatible, SF-GNP nanoconjugates significantly reduced the growth and proliferation of SF resistant tumor cells with very least or no side effect after intra-peritoneal administration of SF-GNP nanoconjugates in animals (Vishwakarma et al., 2017).
Except for gold nanoparticles, another representative nanomaterial is the nitric oxide (NO)-stimulated nanosystem for multidrug resistance cancer therapy. This system reversing the MDR effect attribute to the principle that gaseous signaling molecules nitric oxide (NO) can be used as ABC transporter inhibitors to downregulate the expression level of P-gp, thus creating a favorable microenvironment for the treatment of drug-resistant cancer cells (Pieretti et al., 2020). Interestingly, Wang et al. designed a sophisticated nanosystem containing NO-responsive liposome, encapsulate l-arginine (l-Arg)/Dox-loaded gold@copper sulfide yolk-shell nanoparticles (ADAu@CuS YSNPs) to programmable release Dox in Dox-resistant cancer. Various faceted Au NRs were embedded in CuS HNPs to optimize Au@CuS YSNPs. The finally formed ADLAu@CuS YSNPs can convert L-Arg into NO upon NIR laser irradiation, leading to hydrolysis of o-phenylenediamine-containing lipid and programmable release of NO and Dox. Once internalized into MCF-7/ADR cells, the early stage NO release of ADLAu@CuS YSNPs can inhibit P-gp expression and create a favorable microenvironment for the latter stage Dox accumulation, beneficial for MDR cancer therapy (Wang L. et al., 2019). This NO and Dox sequential release of ADLAu@CuS YSNPs presenting a potential direction for drug-resistant cancer therapy (Figure 5).
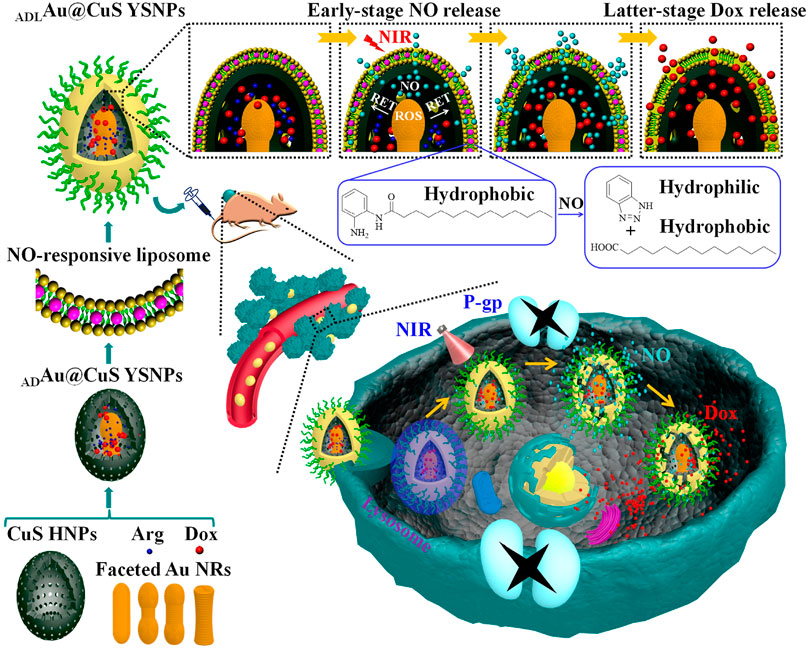
FIGURE 5. Illustration of the NO and Dox programmable released ADLAu@CuS YSNPs in MDR cancer therapy. Reproduced with permission (Wang L. et al., 2019). Copyright 2019, American Chemical Society.
The combination of chemotherapy and photothermal therapy is a promising strategy for cancer treatment. Despite the advantages in overcoming drug resistance, more studies have demonstrated the promising applications of these gold nanoparticles or NO-releasing nanomaterials in combined chemo-photothermal therapy (Zhang et al., 2016; Wei G. et al., 2019; Wang J. et al., 2021).
Co-Delivery Nano-Formulations for Reversal of Resistance Mechanism
The co-delivery system of chemo-pharmaceutical agents and siRNA is an excellent nano-therapeutic strategy for the effective killing of tumor cells (Xiao et al., 2017; Li et al., 2019). Since the major mechanisms of drug resistance are the overexpression of drug-resistant associated genes such as drug transporters, one approach for overcoming drug resistance is to use the co-delivery strategy that utilizes small interfering RNA (siRNA) to silence the expression of drug-resistant associated genes together with chemotherapeutic drugs. Reports have demonstrated that these co-delivery nanocarrier systems still potently reverses the multi-drug resistance effect in tumor tissues even under hypoxic and acidic conditions (Butt et al., 2016; Zeng et al., 2017; Marson et al., 2019; Joshi et al., 2020). The expression of the multidrug resistance 1 (MDR1) gene is a major obstacle that hinders the treatment of numerous cancer. Notably, the targeted resistant genes loaded in nanoparticles not only contain the MDR1 (P-gp) gene but also other genes including the STC2 gene in liver cancer, K-Ras gene in lung cancer, and Bcl2 gene in breast cancer (Jeong et al., 2017; Wen et al., 2017; Cheng et al., 2018b). Recently, Zhang et al. designed a multifunctional pH-sensitive drug delivery system loaded with siRNA and DOX for drug-resistant breast cancer treatment. The nanocarrier is comprised of EphA10 antibody-conjugated pH-sensitive doxorubicin (DOX), co-loaded with MDR1-siRNA liposome (shortened as DOX + siRNA/ePL). Results from the intracellular study indicated that DOX + siRNA/ePL possessed the ability for incremental cellular uptake and rapid endosomal escape in a time-dependent manner. Meanwhile, the animal experiments suggested that DOX + siRNA/ePL could inhibit the proliferation, induce apoptosis, and downregulate the P-gp expression in vivo (Zhang et al., 2018). Altogether, DOX + siRNA/ePL was expected to be a suitable co-delivery system for overcoming drug-resistant effects in breast cancer treatment (Figure 6).
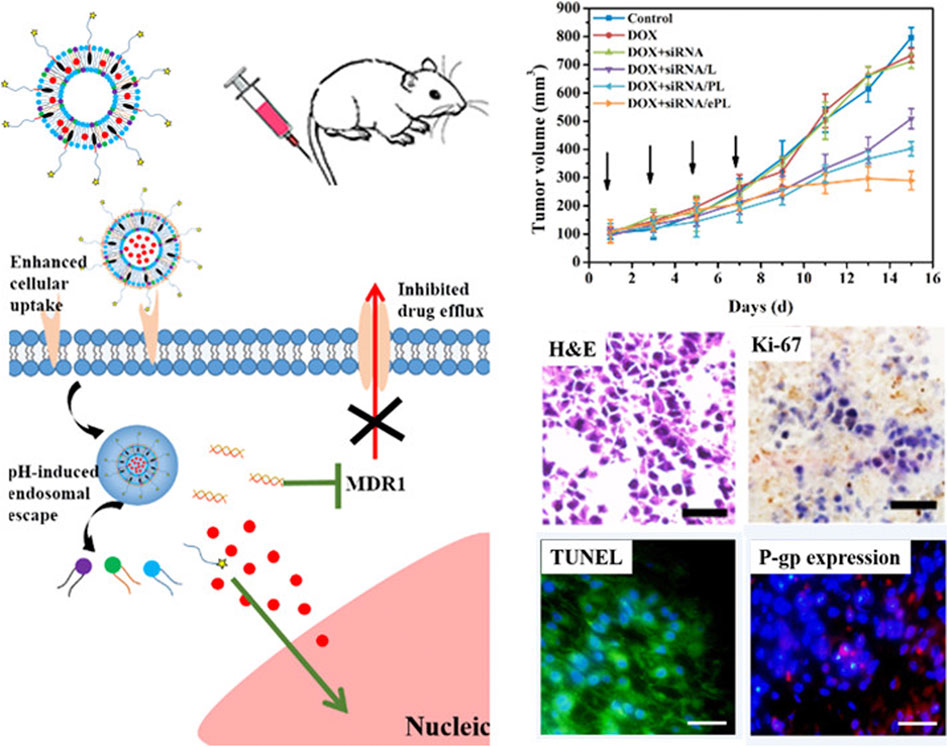
FIGURE 6. Schematic illustration of multifunctional DOX + siRNA/ePL lipoplexes to overcome MDR effect. Reproduced with permission (Zhang et al., 2018). Copyright 2018, American Chemical Society.
Enhanced Drug Delivery by Antibody-Modified Active Targeting
The low drug therapeutic dose is an important factor of acquired resistance in cancer treatment, efficient drug delivery could improve the pharmaceutic effect and prevent the occurrence of drug resistance. Sorafenib (SFB) is a common chemotherapeutical drug targeting vascular endothelial growth factor receptor (VEGFR), which is universally overexpressed in many tumors (Gounder et al., 2018). The therapeutic efficacy of sorafenib (SFB) in the clinic was greatly limited due to its short half-life as well as drug resistance (Wu et al., 2019). To solve these problems, Gan et al. developed a novel SFB-loaded polymeric nanoparticle for targeted therapy of liver cancer. This nanoparticle was fabricated from self-assembly of biodegradable block copolymers and drug SFB, followed by conjugating the anti-GPC3 antibody, referred to NP-SFB-Ab (Gan et al., 2018). Results have shown that NP-SFB-Ab greatly inhibits the tumor growth of xenograft tumors without obvious side effects. Other antibody-conjugated nanoparticles include HER2-antibody conjugated nanocarrier for multi-drug-resistant breast cancer therapy, ICAM-1 as well as Trop2 antibody-mediated nanoparticles for triple-negative breast cancer (TNBC) treatment (Vivek et al., 2014; Son et al., 2018; Wang M. et al., 2019). Gu et al. explored Pluronic P123-conjugated polypropylenimine (PPI) dendrimer (named as P123-PPI) to deliver shRNA against MDR1 in breast cancer cells (Gu et al., 2015). In detailed, anti-CD44 monoclonal antibody was also included to form nanocomplex, achieving the efficient gene delivery, enhanced tumor targeting and longer blood circulation. Based on the above design, the nanocomplexes significantly inhibited the P-gp expression, simultaneously drug resistant cells showed the increased cellular adriamycin (ADR) accumulation (Figure 7).
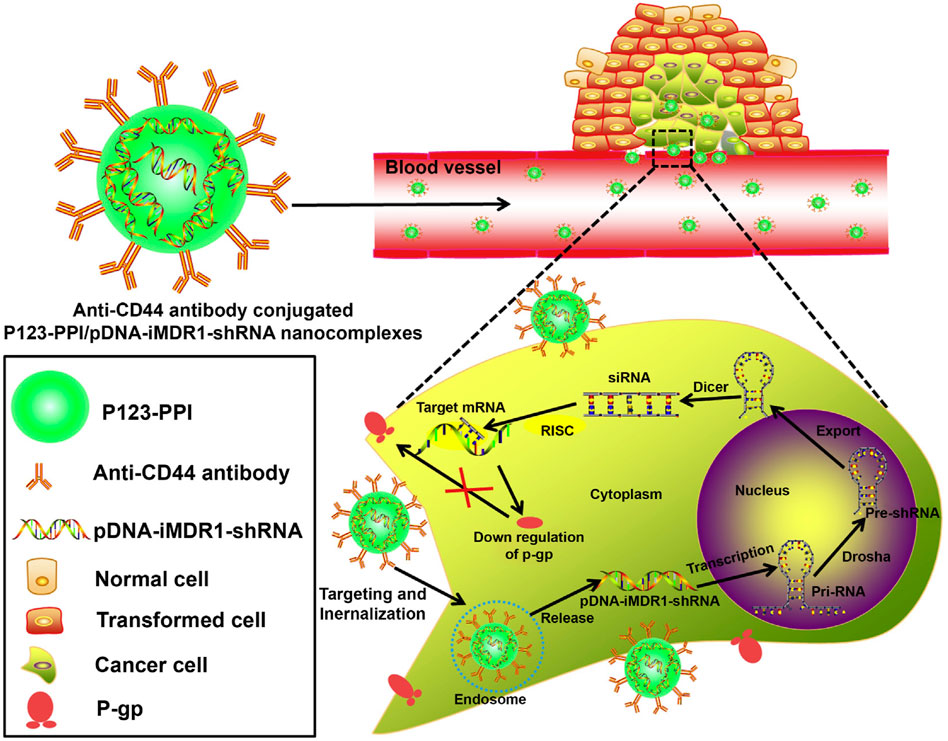
FIGURE 7. Schematic representation of the gene-silencing system of anti-CD44-P123-PPI/pDNA-iMDR1-shRNA nanocomplexes. Reproduced with permission (Gu et al., 2015). Copyright 2015, ELSEVIER.
Combined Strategies in Reversal of Drug Resistance
The combinatorial strategy of chemo-chemo, chemo-immune, and chemo-photothermal treatments, usually called “cocktail therapy”, have been reported to be effective solutions to overcome drug resistance. For example, Zhang et al. developed an iRGD peptide-modified lipid-polymer hybrid nanosystem (LPN), co-loaded with paclitaxel (PTX) and tetrandrine (TET). As expected, the PTX + TET/iRGD LPNs significantly suppressed P-gp expression, promoted ROS production and apoptosis in PTX-resistant tumors (Zhang et al., 2017). Like “Domino-effect”, the chemotherapy combined with immunotherapy could amplify the efficacy of anti-tumor treatment. Studies have demonstrated that many advanced nanoparticles are competent to break through barriers in combinatorial chemo-immune treatment (Wei X. et al., 2019; He et al., 2021; Yan et al., 2021). Notably, the latest study reveals the phenomenon that some nanomaterials possess the inherent biological effect on immunotherapy. These nonfunctional nanomaterials can overcome tumor resistance to PD-1 antibody and sensitize the therapeutic effect of PD-1 antibody, thus presenting a promising prospect in chemo-immune therapy (Sun et al., 2021).
Herein, the following picture simply illustrated a case that chemo-photothermal treatment applied in overcoming drug resistance (Figure 8). Wang et al. found that irradiation of Au@SiO2 resistant cells with femtosecond pulses at a lower intensity than photothermal therapy produced a unique photothermal effect, which induced intracellular heat shock factor (HSF-1) expression, inhibited the expression of p-glycoprotein (P-gp) on the cell membrane, and the NF-κB pathway. In addition, it can successfully induce the degradation of p53 protein mutated in drug-resistant cells, sensitize to chemotherapy drugs, and finally effectively overcome tumor drug resistance. Therefore, fs-pulsed laser irradiation provided a novel and promising strategy to combat drug resistance with the aid of a multifunctional nanocarrier (Au@SiO2) and triggered photothermal effects (Wang et al., 2014).
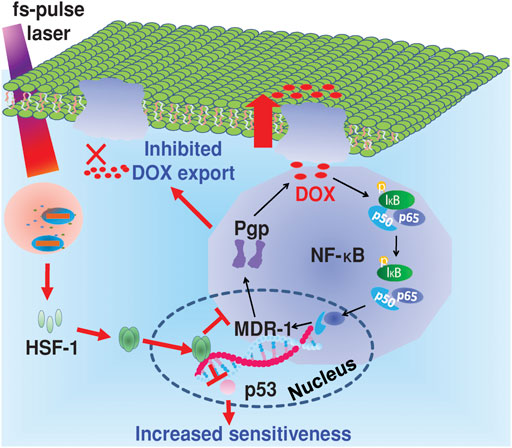
FIGURE 8. Mechanism of the reversal of drug resistance in cancer cells under fs-pulse laser irradiation. Reproduced with permission (Wang et al., 2014). Copyright 2014, Wiley.
Interaction of Chemotherapeutics and Metastasis-Related Inhibitors
Tumor metastasis is a major risk factor for poor prognosis of cancer patients, which is the most concerned issue in surgical treatment. Moreover, chemotherapy has little benefit in the treatment of multiple metastasis (He and Shi, 2014). Herein, the efficient inhibition of tumor metastasis is an urgent work to enhance the pharmaceutical effect of chemotherapeutics. Li et al. reported an enzymatically polymer to simultaneously deliver colchicine chemotherapeutics and marimastat, which is a potent MMPs inhibitor (Figure 9). Based on the efficient drug delivery, this nanosystem could disrupt the microtubules of tumor cells by colchicine, and simultaneously prevent tumor metastasis microenvironment, which could reduce the primary post-surgical recurrence and distant metastasis significantly (Li et al., 2021). To be noted that, MMPs are commonly activated in some tumor tissues, some nanosystem were developed to release chemotherapeutics in MMP-responsive manner (Yao et al., 2018; Vaghasiya et al., 2020), displaying an enhanced chemotherapeutical response.
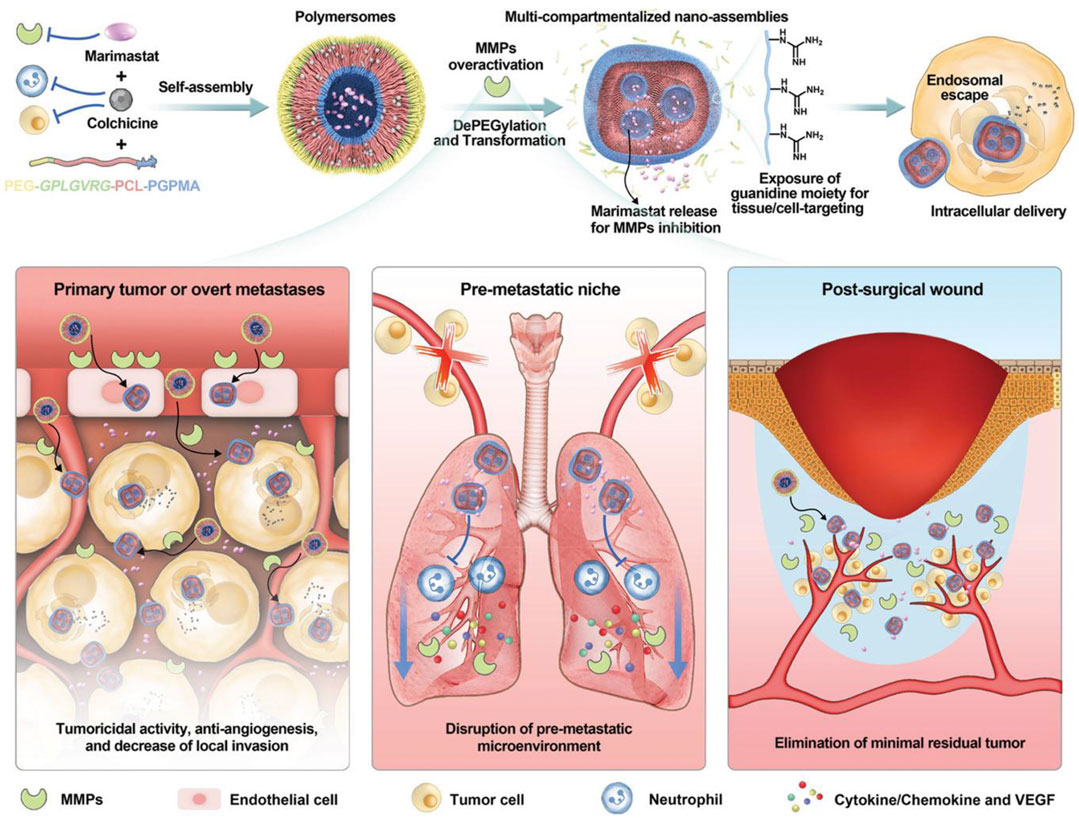
FIGURE 9. Schematic illustration of MMPs inhibition to eliminate tumor metastasis. Reproduced with permission (Li et al., 2021). Copyright 2021, Wiley.
Conclusion and Perspectives
Tumors are composed of mutually interacting tumor cells and tumor microenvironment. Consequently, internal cancer cells along with external environmental factors, extracellular matrix (ECM), and immune cells eventually determine the tumor fate. Tumor heterogeneity, the existence of CSCs, and the occurrence of EMT process in tumor cells are the inner driving force in chemotherapy resistance. However, alterative external pressure including hypoxia, acidic environment, and tumor-associated macrophage (TAM) directly affect drug kinetics and favor the selection of more malignant, therapy-resistant tumor cell subpopulations. Since the complexity of tumor heterogeneity and microenvironment, the causes of drug resistance may differ from individual patients and within a single tumor. Thus, the therapeutic strategies based on a single and fixed chemotherapy drug are inevitably suboptimal. In contrast, flexible and diverse drug delivery materials provided a window into the availability of better treatment. What’s more, multiple nanoparticles applied in chemotherapy regimens could be effectively combined with photothermal therapy, immunotherapy, and CRISPR-mediated gene edit, thus synergistically acquiring better therapeutic efficacy in cancer treatment (Cheng et al., 2020). Considering the complexity and the heterogeneity of tumors, it is necessary to distinguish which nanotechnology strategy is most likely effective from a given context. This is just like the targeted therapy in individual patients or as we usually called precision medicine (Mitchell et al., 2021). Of course, one of approaches is to replace the traditional small molecule drugs with more smart drugs such as anti-cancer peptides (ACPs). Compared with chemical drugs, peptide-based drug delivery strategy has obvious advantages in cancer treatment, especially in overcoming drug resistance. In addition, the conjugation of peptides to nanoparticles results in advanced materials for treatment of cancer, whose properties can be adjusted to maximal efficacy for a given application (Scarberry et al., 2008; Olson et al., 2010).
In recent years, immunotherapy-combined nanoformulations have been developed based on studies of tumor escape mechanism, transforms immunosuppressive tumors into immunostimulatory phenotypes and overcomes the pathways leading to tumor escape. The therapeutic strategy has the potential to induce durable antitumor immune responses in hematologic and solid malignancies and thus has become treatment algorithms for multiple tumor types (Bhatia and Kumar, 2014). Cancer immunotherapy expands potential targets from tumors to the entire immune system, which is an area worthy of further exploration. Therapeutic approaches to manipulate various aspects of the immune system have been widely investigated including immune checkpoint inhibitors, CAR T cell adoptive immunotherapy, oncolytic viruses and vaccines (Yang, 2015). Also, increasing efforts are attempting to employ combination therapy to engage different parts of the immune system, implying the importance of this holistic approach. Through integrating with immunotherapy, nanomedicine improves the delivery of immune stimulators to create a close dialogue between tumors and immune system, which provides unique opportunities to complement each other. In turn, immunotherapy may weaken the effect of biological barriers for nanomedicine, and sometimes may not require large-scale targeted tumor killing activity because activating a small number of immune cells in the tumor or surrounding tissue can spread an immune response (Li and Kataoka, 2021). Compared with solely chemotherapeutic method, immuno-oncological or immuno-chemical nanomedicine strategies have the great potential to improve the efficiency toward tumor therapy. In our personal view, tumor immunotherapy can be an excellent way to overcome chemotherapeutic drug resistance because this strategy directly targets the tumor microenvironment rather than the heterogeneous tumor cells to achieve efficacy. Furthermore, nano-immunotherapy strategy could also prevent the function of cancer stem cells (CSCs), which could significantly enhance the sensitivity of chemotherapy and tumor recurrence (Lang et al., 2019). All of these evidence shows the promising outcome of nanotherapeutics with combination of immunotherapy and chemotherapy.
It is challenging to design nanomaterials to share all desirable characteristics for overcoming all barriers. A concrete design should mainly depend on the specific drug cargo and tumor target. An ideal drug delivery system should adequately protect drug cargo, minimize nonspecific interactions with biological species, and allow efficient aggregation to target sites. In addition, the drug delivery system, once accumulated in tumor tissue, should provide sufficient penetration to deliver the drug throughout the tumor tissue, resulting in mass apoptosis of tumor cells (Li et al., 2014; Li et al., 2015; Li et al., 2021). For a long time, we have been tried to design more complex nanomaterials in many circumstances. However, the design itself is flawed to some extent. Besides, exploring the function of nanomaterial itself in vivo and its interaction with biological system at the tissue, cellular, and molecular levels are always ignored. We strongly suggest that researchers should pay more attention on the material itself biological functions and its pharmacokinetics in vivo. What’s more, many efforts should be made to provide definitive evidence for its functional mechanism instead of creating many new complicated nanoparticle systems for similar concept.
At present, nanoparticles are trying to open a new era for tumor treatment. Though nanomedicine is still at an early stage, the reported results in cancer treatment especially in reversing drug resistance are surprising, and importantly, these advanced antitumor concepts are of great references for chemists, materials scientists, biologists, and may provide new insights into cancer and even a breakthrough in other fields.
Author Contributions
LC and YZ contributed equally to this review. SZ, LC, and HC wrote the article. SZ and YZ conducted the literature analysis. WW, GW, SZ, and HC revised the article. All authors have read and agreed to the published version of the article.
Funding
This work was funded by Science and Technology Project of Quanzhou (2018Z155 and 2021N092S). The article processing charge was supported by Quanzhou Women’s and Children’s Hospital.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abolhoda, A., Wilson, A. E., Ross, H., Danenberg, P. V., Burt, M., and Scotto, K. W. (1999). Rapid Activation of MDR1 Gene Expression in Human Metastatic Sarcoma after In Vivo Exposure to Doxorubicin. Clin. Cancer Res. 5, 3352–3356.
Alsaab, H. O., Sau, S., Alzhrani, R. M., Cheriyan, V. T., Polin, L. A., Vaishampayan, U., et al. (2018). Tumor Hypoxia Directed Multimodal Nanotherapy for Overcoming Drug Resistance in Renal Cell Carcinoma and Reprogramming Macrophages. Biomaterials 183, 280–294. doi:10.1016/j.biomaterials.2018.08.053
Arvizo, R., Bhattacharya, R., and Mukherjee, P. (2010). Gold Nanoparticles: Opportunities and Challenges in Nanomedicine. Expert Opin. Drug Deliv. 7, 753–763. doi:10.1517/17425241003777010
Beck, B., and Blanpain, C. (2013). Unravelling Cancer Stem Cell Potential. Nat. Rev. Cancer 13, 727–738. doi:10.1038/nrc3597
Bhang, H.-e. C., Ruddy, D. A., Krishnamurthy Radhakrishna, V., Caushi, J. X., Zhao, R., Hims, M. M., et al. (2015). Studying Clonal Dynamics in Response to Cancer Therapy Using High-Complexity Barcoding. Nat. Med. 21, 440–448. doi:10.1038/nm.3841
Bhatia, A., and Kumar, Y. (2014). Cellular and Molecular Mechanisms in Cancer Immune Escape: a Comprehensive Review. Expert Rev. Clin. Immunol. 10, 41–62. doi:10.1586/1744666x.2014.865519
Bobo, D., Robinson, K. J., Islam, J., Thurecht, K. J., and Corrie, S. R. (2016). Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 33, 2373–2387. doi:10.1007/s11095-016-1958-5
Brown, L. M., Cowen, R. L., Debray, C., Eustace, A., Erler, J. T., Sheppard, F. C. D., et al. (2006). Reversing Hypoxic Cell Chemoresistance In Vitro Using Genetic and Small Molecule Approaches Targeting Hypoxia Inducible Factor-1. Mol. Pharmacol. 69, 411–418. doi:10.1124/mol.105.015743
Butt, A. M., Amin, M. C. I. M., Katas, H., Abdul Murad, N. A., Jamal, R., and Kesharwani, P. (2016). Doxorubicin and siRNA Codelivery via Chitosan-Coated pH-Responsive Mixed Micellar Polyplexes for Enhanced Cancer Therapy in Multidrug-Resistant Tumors. Mol. Pharmaceutics 13, 4179–4190. doi:10.1021/acs.molpharmaceut.6b00776
Chabon, J. J., Simmons, A. D., Lovejoy, A. F., Esfahani, M. S., Newman, A. M., Haringsma, H. J., et al. (2016). Circulating Tumour DNA Profiling Reveals Heterogeneity of EGFR Inhibitor Resistance Mechanisms in Lung Cancer Patients. Nat. Commun. 7, 11815. doi:10.1038/ncomms11815
Chen, J., Wang, X., Yuan, Y., Chen, H., Zhang, L., Xiao, H., et al. (2021a). Exploiting the Acquired Vulnerability of Cisplatin-Resistant Tumors with a Hypoxia-Amplifying DNA Repair-Inhibiting (HYDRI) Nanomedicine. Sci. Adv. 7, eabc5267. doi:10.1126/sciadv.abc5267
Chen, X., Zou, J., Zhang, K., Zhu, J., Zhang, Y., Zhu, Z., et al. (2021b). Photothermal/matrix Metalloproteinase-2 Dual-Responsive Gelatin Nanoparticles for Breast Cancer Treatment. Acta Pharmaceutica Sinica B 11, 271–282. doi:10.1016/j.apsb.2020.08.009
Cheng, H., Fan, X., Wang, X., Ye, E., Loh, X. J., Li, Z., et al. (2018a). Hierarchically Self-Assembled Supramolecular Host-Guest Delivery System for Drug Resistant Cancer Therapy. Biomacromolecules 19, 1926–1938. doi:10.1021/acs.biomac.7b01693
Cheng, H., Fan, X., Ye, E., Chen, H., Yang, J., Ke, L., et al. (2021). Dual Tumor Microenvironment Remodeling by Glucose-Contained Radical Copolymer for MRI Guided Photo-Immunotherapy. Adv. Mater. Online ahead of print, e2107674. doi:10.1002/adma.202107674
Cheng, H., Wu, Z., Wu, C., Wang, X., Liow, S. S., Li, Z., et al. (2018b). Overcoming STC2 Mediated Drug Resistance through Drug and Gene Co -delivery by PHB-PDMAEMA Cationic Polyester in Liver Cancer Cells. Mater. Sci. Eng. C. 83, 210–217. doi:10.1016/j.msec.2017.08.075
Cheng, Q., Wei, T., Farbiak, L., Johnson, L. T., Dilliard, S. A., and Siegwart, D. J. (2020). Selective Organ Targeting (SORT) Nanoparticles for Tissue-specific mRNA Delivery and CRISPR-Cas Gene Editing. Nat. Nanotechnol. 15, 313–320. doi:10.1038/s41565-020-0669-6
Clarke, M. F., Dick, J. E., Dirks, P. B., Eaves, C. J., Jamieson, C. H. M., Jones, D. L., et al. (2006). Cancer Stem Cells-Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 66, 9339–9344. doi:10.1158/0008-5472.can-06-3126
Comerford, K. M., Wallace, T. J., Karhausen, J., Louis, N. A., Montalto, M. C., and Colgan, S. P. (2002). Hypoxia-inducible Factor-1-dependent Regulation of the Multidrug Resistance (MDR1) Gene. Cancer Res. 62, 3387–3394.
Da Silva, C. G., Peters, G. J., Ossendorp, F., and Cruz, L. J. (2017). The Potential of Multi-Compound Nanoparticles to Bypass Drug Resistance in Cancer. Cancer Chemother. Pharmacol. 80, 881–894. doi:10.1007/s00280-017-3427-1
Dagogo-Jack, I., and Shaw, A. T. (2018). Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 15, 81–94. doi:10.1038/nrclinonc.2017.166
Dean, M., Fojo, T., and Bates, S. (2005). Tumour Stem Cells and Drug Resistance. Nat. Rev. Cancer 5, 275–284. doi:10.1038/nrc1590
Du, B., and Shim, J. S. (2016). Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 21, 965. doi:10.3390/molecules21070965
Du, F., Liu, H., Lu, Y., Zhao, X., and Fan, D. (2017). Epithelial-to-Mesenchymal Transition: Liaison between Cancer Metastasis and Drug Resistance. Crit. Rev. Oncog. 22, 275–282. doi:10.1615/critrevoncog.2018024855
Fan, X., Cheng, H., Wang, X., Ye, E., Loh, X. J., Wu, Y.-L., et al. (2018). Thermoresponsive Supramolecular Chemotherapy by "V"-Shaped Armed β-Cyclodextrin Star Polymer to Overcome Drug Resistance. Adv. Healthc. Mater. 7, 1701143. doi:10.1002/adhm.201701143
Fang, J., Nakamura, H., and Maeda, H. (2011). The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev. 63, 136–151. doi:10.1016/j.addr.2010.04.009
Gan, H., Chen, L., Sui, X., Wu, B., Zou, S., Li, A., et al. (2018). Enhanced Delivery of Sorafenib with Anti-GPC3 Antibody-Conjugated TPGS-B-PCL/Pluronic P123 Polymeric Nanoparticles for Targeted Therapy of Hepatocellular Carcinoma. Mater. Sci. Eng. C. 91, 395–403. doi:10.1016/j.msec.2018.05.011
Giacomini, K. M., Giacomini, K. M., Huang, S. M., Tweedie, D. J., Benet, L. Z., Brouwer, K. L., et al. (2010). Membrane Transporters in Drug Development. Nat. Rev. Drug Discov. 9, 215–236. doi:10.1038/nrd3028
Gottesman, M. M., Fojo, T., and Bates, S. E. (2002). Multidrug Resistance in Cancer: Role of ATP-dependent Transporters. Nat. Rev. Cancer 2, 48–58. doi:10.1038/nrc706
Gounder, M. M., Mahoney, M. R., Van Tine, B. A., Ravi, V., Attia, S., Deshpande, H. A., et al. (2018). Sorafenib for Advanced and Refractory Desmoid Tumors. N. Engl. J. Med. 379, 2417–2428. doi:10.1056/nejmoa1805052
Grosse-Gehling, P., Fargeas, C. A., Dittfeld, C., Garbe, Y., Alison, M. R., Corbeil, D., et al. (2013). CD133 as a Biomarker for Putative Cancer Stem Cells in Solid Tumours: Limitations, Problems and Challenges. J. Pathol. 229, 355–378. doi:10.1002/path.4086
Gu, J., Fang, X., Hao, J., and Sha, X. (2015). Reversal of P-Glycoprotein-Mediated Multidrug Resistance by CD44 Antibody-Targeted Nanocomplexes for Short Hairpin RNA-Encoding Plasmid DNA Delivery. Biomaterials 45, 99–114. doi:10.1016/j.biomaterials.2014.12.030
Gu, Y., Lai, S., Dong, Y., Fu, H., Song, L., Chen, T., et al. (2021). AZD9291 Resistance Reversal Activity of a pH‐Sensitive Nanocarrier Dual‐Loaded with Chloroquine and FGFR1 Inhibitor in NSCLC. Adv. Sci. 8, 2002922. doi:10.1002/advs.202002922
Hajipour, M. J., Aghaverdi, H., Serpooshan, V., Vali, H., Sheibani, S., and Mahmoudi, M. (2021). Sex as an Important Factor in Nanomedicine. Nat. Commun. 12, 2984. doi:10.1038/s41467-021-23230-9
Hao, J., Song, X., Song, B., Liu, Y., Wei, L., Wang, X., et al. (2008). Effects of Lentivirus-Mediated HIF-1α Knockdown on Hypoxia-Related Cisplatin Resistance and Their Dependence on P53 Status in Fibrosarcoma Cells. Cancer Gene Ther. 15, 449–455. doi:10.1038/cgt.2008.4
He, Q., and Shi, J. (2014). MSN Anti-cancer Nanomedicines: Chemotherapy Enhancement, Overcoming of Drug Resistance, and Metastasis Inhibition. Adv. Mater. 26, 391–411. doi:10.1002/adma.201303123
He, Y., Lei, L., Cao, J., Yang, X., Cai, S., Tong, F., et al. (2021). A Combinational Chemo-Immune Therapy Using an Enzyme-Sensitive Nanoplatform for Dual-Drug Delivery to Specific Sites by cascade Targeting. Sci. Adv. 7, eaba0776. doi:10.1126/sciadv.aba0776
Huo, Q., Zhu, J., Niu, Y., Shi, H., Gong, Y., Li, Y., et al. (2017). pH-triggered Surface Charge-Switchable Polymer Micelles for the Co-delivery of Paclitaxel/disulfiram and Overcoming Multidrug Resistance in Cancer. Ijn 12, 8631–8647. doi:10.2147/ijn.s144452
Ishizawa, K., Rasheed, Z. A., Karisch, R., Wang, Q., Kowalski, J., Susky, E., et al. (2010). Tumor-initiating Cells Are Rare in many Human Tumors. Cell Stem Cell 7, 279–282. doi:10.1016/j.stem.2010.08.009
Izumi, H., Torigoe, T., Ishiguchi, H., Uramoto, H., Yoshida, Y., Tanabe, M., et al. (2003). Cellular pH Regulators: Potentially Promising Molecular Targets for Cancer Chemotherapy. Cancer Treat. Rev. 29, 541–549. doi:10.1016/s0305-7372(03)00106-3
Jeong, H., Lee, S. H., Hwang, Y., Yoo, H., Jung, H., Kim, S. H., et al. (2017). Multivalent Aptamer-RNA Conjugates for Simple and Efficient Delivery of Doxorubicin/siRNA into Multidrug-Resistant Cells. Macromol Biosci. 17, 1600343. doi:10.1002/mabi.201600343
Jiang, Z.-S., Sun, Y.-Z., Wang, S.-M., and Ruan, J.-S. (2017). Epithelial-mesenchymal Transition: Potential Regulator of ABC Transporters in Tumor Progression. J. Cancer 8, 2319–2327. doi:10.7150/jca.19079
Jing, X., Yang, F., Shao, C., Wei, K., Xie, M., Shen, H., et al. (2019). Role of Hypoxia in Cancer Therapy by Regulating the Tumor Microenvironment. Mol. Cancer 18, 157. doi:10.1186/s12943-019-1089-9
Joshi, U., Filipczak, N., Khan, M. M., Attia, S. A., and Torchilin, V. (2020). Hypoxia-sensitive Micellar Nanoparticles for Co-delivery of siRNA and Chemotherapeutics to Overcome Multi-Drug Resistance in Tumor Cells. Int. J. Pharmaceutics 590, 119915. doi:10.1016/j.ijpharm.2020.119915
Kc, R. B., Thapa, B., and Xu, P. (2012). pH and Redox Dual Responsive Nanoparticle for Nuclear Targeted Drug Delivery. Mol. Pharm. 9, 2719–2729. doi:10.1021/mp300274g
Ke, L., Li, Z., Fan, X., Loh, X. J., Cheng, H., Wu, Y.-L., et al. (2021). Cyclodextrin-Based Hybrid Polymeric Complex to Overcome Dual Drug Resistance Mechanisms for Cancer Therapy. Polymers 13, 1254. doi:10.3390/polym13081254
Kim, D., Xue, J. Y., and Lito, P. (2020). Targeting KRAS(G12C): From Inhibitory Mechanism to Modulation of Antitumor Effects in Patients. Cell 183, 850–859. doi:10.1016/j.cell.2020.09.044
Kobayashi, H., Watanabe, R., and Choyke, P. L. (2013). Improving Conventional Enhanced Permeability and Retention (EPR) Effects; what Is the Appropriate Target? Theranostics 4, 81–89. doi:10.7150/thno.7193
Lackner, M. R., Wilson, T. R., and Settleman, J. (2012). Mechanisms of Acquired Resistance to Targeted Cancer Therapies. Future Oncol. 8, 999–1014. doi:10.2217/fon.12.86
Lamouille, S., Xu, J., and Derynck, R. (2014). Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cel Biol. 15, 178–196. doi:10.1038/nrm3758
Lang, T., Liu, Y., Zheng, Z., Ran, W., Zhai, Y., Yin, Q., et al. (2019). Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv. Mater. 31, e1903844. doi:10.1002/adma.201903844
Lee, J. E., Park, H. S., Lee, D., Yoo, G., Kim, T., Jeon, H., et al. (2016). Hippo Pathway Effector YAP Inhibition Restores the Sensitivity of EGFR-TKI in Lung Adenocarcinoma Having Primary or Acquired EGFR-TKI Resistance. Biochem. Biophysical Res. Commun. 474, 154–160. doi:10.1016/j.bbrc.2016.04.089
Li, J., Dirisala, A., Ge, Z., Wang, Y., Yin, W., Ke, W., et al. (2017). Therapeutic Vesicular Nanoreactors with Tumor‐Specific Activation and Self‐Destruction for Synergistic Tumor Ablation. Angew. Chem. 129, 14213–14218. doi:10.1002/ange.201706964
Li, J., Ge, Z., Toh, K., Liu, X., Dirisala, A., Ke, W., et al. (2021). Enzymatically Transformable Polymersome-Based Nanotherapeutics to Eliminate Minimal Relapsable Cancer. Adv. Mater. Online ahead of print, e2105254. doi:10.1002/adma.202105254
Li, J., Han, Y., Chen, Q., Shi, H., Ur Rehman, S., Siddiq, M., et al. (2014). Dual Endogenous Stimuli-Responsive Polyplex Micelles as Smart Two-step Delivery Nanocarriers for Deep Tumor Tissue Penetration and Combating Drug Resistance of Cisplatin. J. Mater. Chem. B. 2, 1813–1824. doi:10.1039/c3tb21383h
Li, J., and Kataoka, K. (2021). Chemo-physical Strategies to Advance the In Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 143, 538–559. doi:10.1021/jacs.0c09029
Li, J., Ke, W., Li, H., Zha, Z., Han, Y., and Ge, Z. (2015). Endogenous Stimuli-Sensitive Multistage Polymeric Micelleplex Anticancer Drug Delivery System for Efficient Tumor Penetration and Cellular Internalization. Adv. Healthc. Mater. 4, 2206–2219. doi:10.1002/adhm.201500379
Li, J., Shu, Y., Hao, T., Wang, Y., Qian, Y., Duan, C., et al. (2013). A Chitosan-Glutathione Based Injectable Hydrogel for Suppression of Oxidative Stress Damage in Cardiomyocytes. Biomaterials 34, 9071–9081. doi:10.1016/j.biomaterials.2013.08.031
Li, L., He, S., Yu, L., Elshazly, E. H., Wang, H., Chen, K., et al. (2019). Codelivery of DOX and siRNA by Folate-Biotin-Quaternized Starch Nanoparticles for Promoting Synergistic Suppression of Human Lung Cancer Cells. Drug Deliv. 26, 499–508. doi:10.1080/10717544.2019.1606363
Liu, L., Ning, X., Sun, L., Zhang, H., Shi, Y., Guo, C., et al. (2008). Hypoxia-inducible Factor-1 Alpha Contributes to Hypoxia-Induced Chemoresistance in Gastric Cancer. Cancer Sci. 99, 121–128. doi:10.1111/j.1349-7006.2007.00643.x
Liu, Q., Cai, J., Zheng, Y., Tan, Y., Wang, Y., Zhang, Z., et al. (2019). NanoRNP Overcomes Tumor Heterogeneity in Cancer Treatment. Nano Lett. 19, 7662–7672. doi:10.1021/acs.nanolett.9b02501
Longley, D., and Johnston, P. (2005). Molecular Mechanisms of Drug Resistance. J. Pathol. 205, 275–292. doi:10.1002/path.1706
Lord, A. C., D'souza, N., Shaw, A., Rokan, Z., Moran, B., Abulafi, M., et al. (2021). MRI-diagnosed Tumour Deposits and EMVI Status Have superior Prognostic Accuracy to Current Clinical TNM Staging in Rectal Cancer. Ann. Surg. Online ahead of print. doi:10.1097/SLA.0000000000004499
Lu, J., Tan, M., and Cai, Q. (2015). The Warburg Effect in Tumor Progression: Mitochondrial Oxidative Metabolism as an Anti-metastasis Mechanism. Cancer Lett. 356, 156–164. doi:10.1016/j.canlet.2014.04.001
Mani, S. A., Guo, W., Liao, M.-J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., et al. (2008). The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 133, 704–715. doi:10.1016/j.cell.2008.03.027
Mao, J., Li, Y., Wu, T., Yuan, C., Zeng, B., Xu, Y., et al. (2016). A Simple Dual-pH Responsive Prodrug-Based Polymeric Micelles for Drug Delivery. ACS Appl. Mater. Inter. 8, 17109–17117. doi:10.1021/acsami.6b04247
Markman, J. L., Rekechenetskiy, A., Holler, E., and Ljubimova, J. Y. (2013). Nanomedicine Therapeutic Approaches to Overcome Cancer Drug Resistance. Adv. Drug Deliv. Rev. 65, 1866–1879. doi:10.1016/j.addr.2013.09.019
Marson, D., Laurini, E., Aulic, S., Fermeglia, M., and Pricl, S. (2019). Evolution from Covalent to Self-Assembled PAMAM-Based Dendrimers as Nanovectors for siRNA Delivery in Cancer by Coupled In Silico-Experimental Studies. Part I: Covalent siRNA Nanocarriers. Pharmaceutics 11, 351. doi:10.3390/pharmaceutics11070351
Marusyk, A., and Polyak, K. (2010). Tumor Heterogeneity: Causes and Consequences. Biochim. Biophys. Acta (Bba) - Rev. Cancer 1805, 105–117. doi:10.1016/j.bbcan.2009.11.002
Mckeown, S. R. (2014). Defining Normoxia, Physoxia and Hypoxia in Tumours-Implications for Treatment Response. Bjr 87, 20130676. doi:10.1259/bjr.20130676
Milla, P., Dosio, F., and Cattel, L. (2012). PEGylation of Proteins and Liposomes: a Powerful and Flexible Strategy to Improve the Drug Delivery. Cdm 13, 105–119. doi:10.2174/138920012798356934
Min, H.-Y., Lee, S.-C., Woo, J. K., Jung, H. J., Park, K. H., Jeong, H. M., et al. (2017). Essential Role of DNA Methyltransferase 1-mediated Transcription of Insulin-like Growth Factor 2 in Resistance to Histone Deacetylase Inhibitors. Clin. Cancer Res. 23, 1299–1311. doi:10.1158/1078-0432.ccr-16-0534
Miraglia, E., Viarisio, D., Riganti, C., Costamagna, C., Ghigo, D., and Bosia, A. (2005). Na+/H+ Exchanger Activity Is Increased in Doxorubicin-Resistant Human colon Cancer Cells and its Modulation Modifies the Sensitivity of the Cells to Doxorubicin. Int. J. Cancer 115, 924–929. doi:10.1002/ijc.20959
Mitchell, M. J., Billingsley, M. M., Haley, R. M., Wechsler, M. E., Peppas, N. A., and Langer, R. (2021). Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 20, 101–124. doi:10.1038/s41573-020-0090-8
Moitra, K. (2015). Overcoming Multidrug Resistance in Cancer Stem Cells. Biomed. Res. Int. 2015, 635745. doi:10.1155/2015/635745
Nagrath, S., Sequist, L. V., Maheswaran, S., Bell, D. W., Irimia, D., Ulkus, L., et al. (2007). Isolation of Rare Circulating Tumour Cells in Cancer Patients by Microchip Technology. Nature 450, 1235–1239. doi:10.1038/nature06385
Nigam, S. K. (2015). What Do Drug Transporters Really Do? Nat. Rev. Drug Discov. 14, 29–44. doi:10.1038/nrd4461
Olson, E. S., Jiang, T., Aguilera, T. A., Nguyen, Q. T., Ellies, L. G., Scadeng, M., et al. (2010). Activatable Cell Penetrating Peptides Linked to Nanoparticles as Dual Probes for In Vivo Fluorescence and MR Imaging of Proteases. Proc. Natl. Acad. Sci. 107, 4311–4316. doi:10.1073/pnas.0910283107
Pallis, M., Turzanski, J., Higashi, Y., and Russell, N. (2002). P-glycoprotein in Acute Myeloid Leukaemia: Therapeutic Implications of its Association with Both a Multidrug-Resistant and an Apoptosis-Resistant Phenotype. Leuk. Lymphoma 43, 1221–1228. doi:10.1080/10428190290026277
Pastorek, J., and Pastorekova, S. (2015). Hypoxia-induced Carbonic Anhydrase IX as a Target for Cancer Therapy: from Biology to Clinical Use. Semin. Cancer Biol. 31, 52–64. doi:10.1016/j.semcancer.2014.08.002
Pérez-Tomás, R. (2006). Multidrug Resistance: Retrospect and Prospects in Anti-cancer Drug Treatment. Cmc 13, 1859–1876. doi:10.2174/092986706777585077
Pieretti, J. C., Pelegrino, M. T., Nascimento, M. H. M., Tortella, G. R., Rubilar, O., and Seabra, A. B. (2020). Small Molecules for Great Solutions: Can Nitric Oxide-Releasing Nanomaterials Overcome Drug Resistance in Chemotherapy? Biochem. Pharmacol. 176, 113740. doi:10.1016/j.bcp.2019.113740
Polyak, K. (2014). Tumor Heterogeneity Confounds and Illuminates: a Case for Darwinian Tumor Evolution. Nat. Med. 20, 344–346. doi:10.1038/nm.3518
Robey, R. W., Pluchino, K. M., Hall, M. D., Fojo, A. T., Bates, S. E., and Gottesman, M. M. (2018). Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 18, 452–464. doi:10.1038/s41568-018-0005-8
Rohwer, N., and Cramer, T. (2011). Hypoxia-mediated Drug Resistance: Novel Insights on the Functional Interaction of HIFs and Cell Death Pathways. Drug Resist. Updates 14, 191–201. doi:10.1016/j.drup.2011.03.001
Rohwer, N., Dame, C., Haugstetter, A., Wiedenmann, B., Detjen, K., Schmitt, C. A., et al. (2010). Hypoxia-Inducible Factor 1α Determines Gastric Cancer Chemosensitivity via Modulation of P53 and NF-Κb. PLoS One 5, e12038. doi:10.1371/journal.pone.0012038
Saxena, M., Stephens, M. A., Pathak, H., and Rangarajan, A. (2011). Transcription Factors that Mediate Epithelial-Mesenchymal Transition lead to Multidrug Resistance by Upregulating ABC Transporters. Cell Death Dis. 2, e179. doi:10.1038/cddis.2011.61
Scarberry, K. E., Dickerson, E. B., Mcdonald, J. F., and Zhang, Z. J. (2008). Magnetic Nanoparticle−Peptide Conjugates for In Vitro and In Vivo Targeting and Extraction of Cancer Cells. J. Am. Chem. Soc. 130, 10258–10262. doi:10.1021/ja801969b
Schütz, C. A., Juillerat-Jeanneret, L., Mueller, H., Lynch, I., and Riediker, M. (2013). Therapeutic Nanoparticles in Clinics and under Clinical Evaluation. Nanomedicine 8, 449–467. doi:10.2217/nnm.13.8
Semenza, G. L. (2010). HIF-1: Upstream and Downstream of Cancer Metabolism. Curr. Opin. Genet. Dev. 20, 51–56. doi:10.1016/j.gde.2009.10.009
Son, S., Shin, S., Rao, N. V., Um, W., Jeon, J., Ko, H., et al. (2018). Anti-Trop2 Antibody-Conjugated Bioreducible Nanoparticles for Targeted Triple Negative Breast Cancer Therapy. Int. J. Biol. Macromolecules 110, 406–415. doi:10.1016/j.ijbiomac.2017.10.113
Song, L., Jiang, Q., Liu, J., Li, N., Liu, Q., Dai, L., et al. (2017). DNA Origami/gold Nanorod Hybrid Nanostructures for the Circumvention of Drug Resistance. Nanoscale 9, 7750–7754. doi:10.1039/c7nr02222k
Song, X., Feng, L., Liang, C., Yang, K., and Liu, Z. (2016). Ultrasound Triggered Tumor Oxygenation with Oxygen-Shuttle Nanoperfluorocarbon to Overcome Hypoxia-Associated Resistance in Cancer Therapies. Nano Lett. 16, 6145–6153. doi:10.1021/acs.nanolett.6b02365
Spugnini, E. P., Sonveaux, P., Stock, C., Perez-Sayans, M., De Milito, A., Avnet, S., et al. (2015). Proton Channels and Exchangers in Cancer. Biochim. Biophys. Acta (Bba) - Biomembranes 1848, 2715–2726. doi:10.1016/j.bbamem.2014.10.015
Sun, M., Gu, P., Yang, Y., Yu, L., Jiang, Z., Li, J., et al. (2021). Mesoporous Silica Nanoparticles Inflame Tumors to Overcome Anti-PD-1 Resistance through TLR4-Nfκb axis. J. Immunother. Cancer 9, e002508. doi:10.1136/jitc-2021-002508
Taylor, S., Spugnini, E. P., Assaraf, Y. G., Azzarito, T., Rauch, C., and Fais, S. (2015). Microenvironment Acidity as a Major Determinant of Tumor Chemoresistance: Proton Pump Inhibitors (PPIs) as a Novel Therapeutic Approach. Drug Resist. Updates 23, 69–78. doi:10.1016/j.drup.2015.08.004
Tian, H., Luo, Z., Liu, L., Zheng, M., Chen, Z., Ma, A., et al. (2017). Cancer Cell Membrane-Biomimetic Oxygen Nanocarrier for Breaking Hypoxia-Induced Chemoresistance. Adv. Funct. Mater. 27, 1703197. doi:10.1002/adfm.201703197
Vaghasiya, K., Ray, E., Sharma, A., Katare, O. P., and Verma, R. K. (2020). Matrix Metalloproteinase-Responsive Mesoporous Silica Nanoparticles Cloaked with Cleavable Protein for "Self-Actuating" On-Demand Controlled Drug Delivery for Cancer Therapy. ACS Appl. Bio Mater. 3, 4987–4999. doi:10.1021/acsabm.0c00497
Van Der Meel, R., Vehmeijer, L. J. C., Kok, R. J., Storm, G., and Van Gaal, E. V. B. (2013). Ligand-targeted Particulate Nanomedicines Undergoing Clinical Evaluation: Current Status. Adv. Drug Deliv. Rev. 65, 1284–1298. doi:10.1016/j.addr.2013.08.012
Vasan, N., Baselga, J., and Hyman, D. M. (2019). A View on Drug Resistance in Cancer. Nature 575, 299–309. doi:10.1038/s41586-019-1730-1
Vaupel, P., and Multhoff, G. (2018). Hypoxia-/HIF-1α-Driven Factors of the Tumor Microenvironment Impeding Antitumor Immune Responses and Promoting Malignant Progression. Adv. Exp. Med. Biol. 1072, 171–175. doi:10.1007/978-3-319-91287-5_27
Vaupel, P., Schmidberger, H., and Mayer, A. (2019). The Warburg Effect: Essential Part of Metabolic Reprogramming and central Contributor to Cancer Progression. Int. J. Radiat. Biol. 95, 912–919. doi:10.1080/09553002.2019.1589653
Villani, A. C., Satija, R., Reynolds, G., Sarkizova, S., Shekhar, K., Fletcher, J., et al. (2017). Single-cell RNA-Seq Reveals New Types of Human Blood Dendritic Cells, Monocytes, and Progenitors. Science 356, eaah4573. doi:10.1126/science.aah4573
Vishwakarma, S. K., Sharmila, P., Bardia, A., Chandrakala, L., Raju, N., Sravani, G., et al. (2017). Use of Biocompatible Sorafenib-Gold Nanoconjugates for Reversal of Drug Resistance in Human Hepatoblatoma Cells. Sci. Rep. 7, 8539. doi:10.1038/s41598-017-08878-y
Vitale, I., Manic, G., De Maria, R., Kroemer, G., and Galluzzi, L. (2017). DNA Damage in Stem Cells. Mol. Cel 66, 306–319. doi:10.1016/j.molcel.2017.04.006
Vivek, R., Thangam, R., Nipunbabu, V., Rejeeth, C., Sivasubramanian, S., Gunasekaran, P., et al. (2014). Multifunctional HER2-Antibody Conjugated Polymeric Nanocarrier-Based Drug Delivery System for Multi-Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Inter. 6, 6469–6480. doi:10.1021/am406012g
Wang, D., Zhao, C., Xu, F., Zhang, A., Jin, M., Zhang, K., et al. (2021a). Cisplatin-resistant NSCLC Cells Induced by Hypoxia Transmit Resistance to Sensitive Cells through Exosomal PKM2. Theranostics 11, 2860–2875. doi:10.7150/thno.51797
Wang, J., Wu, C., Qin, X., Huang, Y., Zhang, J., Chen, T., et al. (2021b). NIR-II Light Triggered Nitric Oxide Release Nanoplatform Combined Chemo-Photothermal Therapy for Overcoming Multidrug Resistant Cancer. J. Mater. Chem. B 9, 1698–1706. doi:10.1039/d0tb02626c
Wang, L., Chang, Y., Feng, Y., Li, X., Cheng, Y., Jian, H., et al. (2019a). Nitric Oxide Stimulated Programmable Drug Release of Nanosystem for Multidrug Resistance Cancer Therapy. Nano Lett. 19, 6800–6811. doi:10.1021/acs.nanolett.9b01869
Wang, L., Lin, X., Wang, J., Hu, Z., Ji, Y., Hou, S., et al. (2014). Novel Insights into Combating Cancer Chemotherapy Resistance Using a Plasmonic Nanocarrier: Enhancing Drug Sensitiveness and Accumulation Simultaneously with Localized Mild Photothermal Stimulus of Femtosecond Pulsed Laser. Adv. Funct. Mater. 24, 4229–4239. doi:10.1002/adfm.201400015
Wang, M., Liu, W., Zhang, Y., Dang, M., Zhang, Y., Tao, J., et al. (2019b). Intercellular Adhesion Molecule 1 Antibody-Mediated Mesoporous Drug Delivery System for Targeted Treatment of Triple-Negative Breast Cancer. J. Colloid Interf. Sci. 538, 630–637. doi:10.1016/j.jcis.2018.12.032
Wang, Y., Sun, S., Zhang, Z., and Shi, D. (2018a). Nanomaterials for Cancer Precision Medicine. Adv. Mater. 30, 1705660. doi:10.1002/adma.201705660
Wang, Y., Wang, F., Liu, Y., Xu, S., Shen, Y., Feng, N., et al. (2018b). Glutathione Detonated and pH Responsive Nano-Clusters of Au Nanorods with a High Dose of DOX for Treatment of Multidrug Resistant Cancer. Acta Biomater. 75, 334–345. doi:10.1016/j.actbio.2018.06.012
Wang, Z., Li, X., Wang, D., Zou, Y., Qu, X., He, C., et al. (2017). Concurrently Suppressing Multidrug Resistance and Metastasis of Breast Cancer by Co-delivery of Paclitaxel and Honokiol with pH-Sensitive Polymeric Micelles. Acta Biomater. 62, 144–156. doi:10.1016/j.actbio.2017.08.027
Wang, Z., Xu, Z., and Zhu, G. (2016). A Platinum(IV) Anticancer Prodrug Targeting Nucleotide Excision Repair to Overcome Cisplatin Resistance. Angew. Chem. Int. Ed. 55, 15564–15568. doi:10.1002/anie.201608936
Wei, G., Yang, G., Wei, B., Wang, Y., and Zhou, S. (2019a). Near-infrared Light Switching Nitric Oxide Nanoemitter for Triple-Combination Therapy of Multidrug Resistant Cancer. Acta Biomater. 100, 365–377. doi:10.1016/j.actbio.2019.10.002
Wei, X., Liu, L., Li, X., Wang, Y., Guo, X., Zhao, J., et al. (2019b). Selectively Targeting Tumor-Associated Macrophages and Tumor Cells with Polymeric Micelles for Enhanced Cancer Chemo-Immunotherapy. J. Controlled Release 313, 42–53. doi:10.1016/j.jconrel.2019.09.021
Wen, Z.-M., Jie, J., Zhang, Y., Liu, H., and Peng, L.-P. (2017). A Self-Assembled Polyjuglanin Nanoparticle Loaded with Doxorubicin and Anti-kras siRNA for Attenuating Multidrug Resistance in Human Lung Cancer. Biochem. Biophysical Res. Commun. 493, 1430–1437. doi:10.1016/j.bbrc.2017.09.132
Wirthner, R., Wrann, S., Balamurugan, K., Wenger, R. H., and Stiehl, D. P. (2008). Impaired DNA Double-Strand Break Repair Contributes to Chemoresistance in HIF-1 -deficient Mouse Embryonic Fibroblasts. Carcinogenesis 29, 2306–2316. doi:10.1093/carcin/bgn231
Wu, H., Wang, C., Sun, J., Sun, L., Wan, J., Wang, S., et al. (2019). Self-Assembled and Self-Monitored Sorafenib/Indocyanine Green Nanodrug with Synergistic Antitumor Activity Mediated by Hyperthermia and Reactive Oxygen Species-Induced Apoptosis. ACS Appl. Mater. Inter. 11, 43996–44006. doi:10.1021/acsami.9b18086
Xiao, B., Ma, L., and Merlin, D. (2017). Nanoparticle-mediated Co-delivery of Chemotherapeutic Agent and siRNA for Combination Cancer Therapy. Expert Opin. Drug Deliv. 14, 65–73. doi:10.1080/17425247.2016.1205583
Xu, M., Zhang, C. Y., Wu, J., Zhou, H., Bai, R., Shen, Z., et al. (2019). PEG-detachable Polymeric Micelles Self-Assembled from Amphiphilic Copolymers for Tumor-Acidity-Triggered Drug Delivery and Controlled Release. ACS Appl. Mater. Inter. 11, 5701–5713. doi:10.1021/acsami.8b13059
Xu, R., Luo, X., Ye, X., Li, H., Liu, H., Du, Q., et al. (2021). SIRT1/PGC-1α/PPAR-γ Correlate with Hypoxia-Induced Chemoresistance in Non-small Cell Lung Cancer. Front. Oncol. 11, 682762. doi:10.3389/fonc.2021.682762
Yan, J., Zhang, Z., Zhan, X., Chen, K., Pu, Y., Liang, Y., et al. (2021). In Situ injection of Dual-Delivery PEG Based MMP-2 Sensitive Hydrogels for Enhanced Tumor Penetration and Chemo-Immune Combination Therapy. Nanoscale 13, 9577–9589. doi:10.1039/d1nr01155c
Yan, Y., Zuo, X., and Wei, D. (2015). Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cell Transl Med 4, 1033–1043. doi:10.5966/sctm.2015-0048
Yang, Y. (2015). Cancer Immunotherapy: Harnessing the Immune System to Battle Cancer. J. Clin. Invest. 125, 3335–3337. doi:10.1172/jci83871
Yao, Q., Kou, L., Tu, Y., and Zhu, L. (2018). MMP-responsive 'Smart' Drug Delivery and Tumor Targeting. Trends Pharmacol. Sci. 39, 766–781. doi:10.1016/j.tips.2018.06.003
Yu, P., Yu, H., Guo, C., Cui, Z., Chen, X., Yin, Q., et al. (2015). Reversal of Doxorubicin Resistance in Breast Cancer by Mitochondria-Targeted pH-Responsive Micelles. Acta Biomater. 14, 115–124. doi:10.1016/j.actbio.2014.12.001
Yuan, Y.-Y., Mao, C.-Q., Du, X.-J., Du, J.-Z., Wang, F., and Wang, J. (2012). Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 24, 5476–5480. doi:10.1002/adma.201202296
Zellmer, V. R., and Zhang, S. (2014). Evolving Concepts of Tumor Heterogeneity. Cell Biosci 4, 69. doi:10.1186/2045-3701-4-69
Zeng, Y., Yang, Z., Li, H., Hao, Y., Liu, C., Zhu, L., et al. (2017). Multifunctional Nanographene Oxide for Targeted Gene-Mediated Thermochemotherapy of Drug-Resistant Tumour. Sci. Rep. 7, 43506. doi:10.1038/srep43506
Zhang, J., Du, Z., Pan, S., Shi, M., Li, J., Yang, C., et al. (2018). Overcoming Multidrug Resistance by Codelivery of MDR1-Targeting siRNA and Doxorubicin Using EphA10-Mediated pH-Sensitive Lipoplexes: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Inter. 10, 21590–21600. doi:10.1021/acsami.8b01806
Zhang, J., Wang, L., Fai Chan, H., Xie, W., Chen, S., He, C., et al. (2017). Co-delivery of Paclitaxel and Tetrandrine via iRGD Peptide Conjugated Lipid-Polymer Hybrid Nanoparticles Overcome Multidrug Resistance in Cancer Cells. Sci. Rep. 7, 46057. doi:10.1038/srep46057
Zhang, L., Zhang, Y., Strong, J. M., Reynolds, K. S., and Huang, S.-M. (2008). A Regulatory Viewpoint on Transporter-Based Drug Interactions. Xenobiotica 38, 709–724. doi:10.1080/00498250802017715
Zhang, P.-Y., and Yu, Y. (2020). Precise Personalized Medicine in Gynecology Cancer and Infertility. Front. Cel Dev. Biol. 7, 382. doi:10.3389/fcell.2019.00382
Zhang, W., Wang, F., Wang, Y., Wang, J., Yu, Y., Guo, S., et al. (2016). pH and Near-Infrared Light Dual-Stimuli Responsive Drug Delivery Using DNA-Conjugated Gold Nanorods for Effective Treatment of Multidrug Resistant Cancer Cells. J. Controlled Release 232, 9–19. doi:10.1016/j.jconrel.2016.04.001
Keywords: tumor chemotherapy, drug resistance, tumor microenvironment, nanomedical strategy, combination therapy
Citation: Cao L, Zhu Y, Wang W, Wang G, Zhang S and Cheng H (2021) Emerging Nano-Based Strategies Against Drug Resistance in Tumor Chemotherapy. Front. Bioeng. Biotechnol. 9:798882. doi: 10.3389/fbioe.2021.798882
Received: 20 October 2021; Accepted: 19 November 2021;
Published: 07 December 2021.
Edited by:
Junjie Li, Innovation Centre of NanoMedicine (iCONM), JapanReviewed by:
Panyue Wen, University of Science and Technology of China, ChinaAmit Ranjan Maity, Amity University Kolkata, India
Yu Han, Hefei Normal University, China
Copyright © 2021 Cao, Zhu, Wang, Wang, Zhang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaoxiong Wang, d2FuZ2dhb3hpb25nMjAxM0AxNjMuY29t; Shuaishuai Zhang, emhhbmdzaHVhaTJAc211LmVkdQ==; Hongwei Cheng, aG9uZ3dlaTEwMjZAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Lei Cao1†
Lei Cao1† Hongwei Cheng
Hongwei Cheng