- 1Institute for Medical Statistics and Informatics, Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 2Institute of Molecular Genetics and Genetic Engineering, University of Belgrade, Belgrade, Serbia
- 3Center for Molecular Biology, University of Vienna, Vienna, Austria
- 4Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 5Clinic for Gynecology and Obstetrics Narodni Front, Belgrade, Serbia
- 6Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, United States
Introduction: Preeclampsia (PE) is a pregnancy-associated, multi-organ, life-threatening disease that appears after the 20th week of gestation. The aim of this study was to perform a systematic review and meta-analysis to determine whether women with PE have disrupted miRNA expression compared to women who do not have PE.
Methods: We conducted a systematic review and meta-analysis of studies that reported miRNAs expression levels in placenta or peripheral blood of pregnant women with vs. without PE. Studies published before October 29, 2021 were identified through PubMed, EMBASE and Web of Science. Two reviewers used predefined forms and protocols to evaluate independently the eligibility of studies based on titles and abstracts and to perform full-text screening, data abstraction and quality assessment. Standardized mean difference (SMD) was used as a measure of effect size.
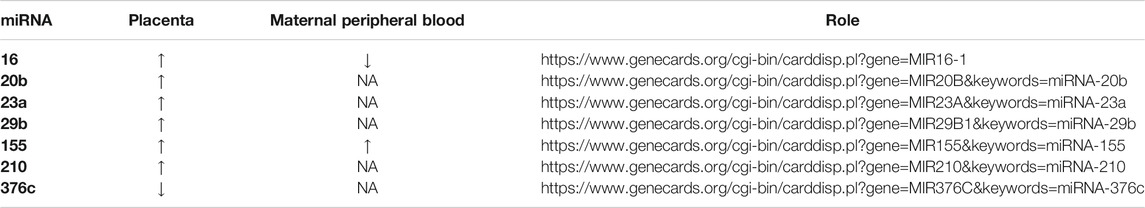
Results: 229 publications were included in the systematic review and 53 in the meta-analysis. The expression levels in placenta were significantly higher in women with PE compared to women without PE for miRNA-16 (SMD = 1.51,95%CI = 0.55–2.46), miRNA-20b (SMD = 0.89, 95%CI = 0.33–1.45), miRNA-23a (SMD = 2.02, 95%CI = 1.25–2.78), miRNA-29b (SMD = 1.37, 95%CI = 0.36–2.37), miRNA-155 (SMD = 2.99, 95%CI = 0.83–5.14) and miRNA-210 (SMD = 1.63, 95%CI = 0.69–2.58), and significantly lower for miRNA-376c (SMD = –4.86, 95%CI = –9.51 to –0.20). An increased level of miRNK-155 expression was found in peripheral blood of women with PE (SMD = 2.06, 95%CI = 0.35–3.76), while the expression level of miRNA-16 was significantly lower in peripheral blood of PE women (SMD = –0.47, 95%CI = –0.91 to –0.03). The functional roles of the presented miRNAs include control of trophoblast proliferation, migration, invasion, apoptosis, differentiation, cellular metabolism and angiogenesis.
Conclusion: miRNAs play an important role in the pathophysiology of PE. The identification of differentially expressed miRNAs in maternal blood creates an opportunity to define an easily accessible biomarker of PE.
Introduction
Preeclampsia (PE) has been shown to affect 1–7.5% of all pregnancies, making it one of the leading causes of maternal and fetal morbidity and mortality worldwide (Abalos et al., 2013; Witcher, 2018; Garovic et al., 2020). PE is a multi-factorial, multi-systemic pregnancy specific condition found typically after 20 weeks of gestation or early post-delivery (American College of Obstetricians and Gynecologists, 2019). Although clinical symptoms appear relatively late in pregnancy, PE pathology begins early, making the identification of potential biomarkers during the first trimester a possible strategy for identifying predictors of PE (McElrath et al., 2020). Several potential biomarkers already have been evaluated: C reactive protein (CRP), cytokines (IL-6, IL-8, TNF-α), microparticle proteins (C1RL, GP1BA, VTNC, and ZA2G), oxidative stress markers (malondialdehyde - MDA), and genetic factors (PAI-1 4G/5G polymorphism) (Black and Horowitz, 2018; Giannakou et al., 2018; Taravati and Tohidi, 2018; McElrath et al., 2020). There are few known biomarkers, however, that can accurately predict the risk for PE. The use of combinations of several biomarkers previously has been proposed as a diagnostic or predictive parameter, such as the ratio of soluble fms-like tyrosine kinase-1 to placental growth factor ratio (sFlt-1/PlGF) (Lecarpentier and Tsatsaris, 2016). A study by Garovic et al. reported podocyturia, defined as the presence of podocin-positive cells in urine sampled ≤24 h of delivery, as a 100% sensitive and specific diagnostic marker for PE (Garovic et al., 2007).
Significant progress has been made in the past decade in the assessment of epigenetic mechanisms that might be involved in the pathophysiology of PE, and which aim to identify potential diagnostic and/or predictive epigenetic markers of PE. More specifically, short non-coding microRNAs (miRNAs) are involved in post-transcriptional gene expression and play a role in numerous diseases, modulating regulatory pathways that control development, differentiation, and organ function. MiRNAs are single-stranded RNA molecules consisting of 19–24 nucleotides, and their mode of action is primarily by degrading targeted mRNA transcripts or inhibiting translation of mRNA into a protein product (Hombach and Kretz, 2016). It is also known that miRNA molecules are involved in the physiological regulation of major processes of placentation (Mouillet et al., 2015). It might therefore be anticipated that dysfunction of miRNA expression could be important for the development of PE. Studies recently published explored a possible causal relationship between miRNA expression and PE (Youssef and Marei, 2019; Hemmatzadeh et al., 2020). It has been demonstrated that expression levels of miRNAs in different tissues play a role in physiological pregnancy as regulators of trophoblast proliferation, migration, invasion, apoptosis, differentiation, cellular metabolism and placental angiogenesis (Hayder et al., 2018). The placenta is one of the main sources of miRNAs, but they also can be found in the circulation (Mouillet et al., 2015). Placental miRNA-210 expression has been the most studied in PE and other pregnancy related complications, and increased levels have been demonstrated (Muralimanoharan et al., 2012; Awamleh and Han, 2020). Results from evaluations of other frequently analyzed miRNAs, such as miRNA-155, -223, -126, -183, -182, -281b, -154, -139-5p, -29b, -181a, -15b (Mayor-Lynn et al., 2011; Yang et al., 2011; Zhao et al., 2013; Sheikh et al., 2016; Hemmatzadeh et al., 2020), suggest that miRNA expression differs according to the severity of PE (Jairajpuri et al., 2017), and also differs throughout the course of normal pregnancy (Cai et al., 2017). While some research has been done to investigate the association between miRNA expression levels and PE, there is still a lack of evidence to support the common use of miRNAs as functional biomarkers related to PE. The aim of this study was to perform a systematic review and meta-analysis to determine whether women with PE have disrupted miRNA expressions compared to women without PE.
Materials and Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and MOOSE guidelines (Stroup et al., 2000; Liberati et al., 2009).
Study Selection
Publications were screened for inclusion in the systematic review in two phases, and all disagreements were resolved by discussion at each stage with inclusion of a third reviewer. We included studies that compared miRNA expression levels between women with and without PE. Studies were eligible for inclusion if the miRNA expression levels were measured in both groups. Studies were excluded if they: 1) investigated other outcomes, 2) did not make comparisons between PE and control groups, 3) examined other populations (animal, cell lines), 4) assessed other epigenetic markers, 5) were abstracts, or 6) were not original articles.
Database Search
Two biostatisticians with expertise in conducting systematic reviews and meta-analyses (NM, AC) developed the search strategy. A systematic review of peer-reviewed publications was performed through searches of PubMed, Web of Science (WoS) and embase electronic databases until October 29, 2021. Search queries differed according to the database. Key words for the PubMed search were: preeclampsia and (epigenetic or epigenetics or miRNA or microRNA or DNA methylation or DNA methylation or long non coding RNA); for Wos: TS = *eclampsia and TS= (epigenetic* or microRNA or DNA methylation or gene imprinting or long non coding RNA), and for embase: preeclampsia and (epigenetics or microRNA or DNA methylation or genome imprinting or long untranslated RNA). Only publications in English were considered. In addition, reference lists of articles identified through electronic retrieval were manually searched, as well as relevant reviews and editorials. Experts in the field were contacted to identify other potentially relevant articles.
Authors of relevant articles were contacted to obtain missing data. Studies with combined data of gestational hypertension and/or chronic hypertension in pregnancy and PE were only eligible if data for the subset of women who developed preeclampsia were available.
Article Screening and Selection
Two reviewers (AC, JML) independently evaluated the eligibility of all titles and abstracts. Studies were included in the full text screening if either reviewer identified the study as being potentially eligible, or if the abstract and title did not include sufficient information. Studies were eligible for full text screening if they included comparisons of miRNA expression levels between women with and without PE. Preeclampsia included more severe, less severe, and not specified forms. The same reviewers independently performed full text screening to select articles for inclusion according to the criteria listed under Inclusion and Exclusion Criteria. Disagreements were resolved by consensus (AC, JML) or arbitration (NM, DS).
Data Abstraction and Quality Assessment
Two reviewers independently abstracted the following data: author(s), country of research, year of publication, study design, sample size, study population, maternal age, preeclampsia definitions, disease severity (more severe, less severe or not-specified PE), inclusion and exclusion criteria used in the original articles, sample type and time of sampling, matching, evaluated miRNAs, method for miRNA expression quantification, miRNA expression value, housekeeping gene for internal control, conclusion in original article. Each reviewer independently evaluated the quality of selected manuscripts using an adapted version of the Newcastle-Ottawa tool for observational studies (Wells et al., 2014). Reviewers used a standardized previously defined miRNA protocol when selecting and abstracting data. All detailed information about quality assessment, data extraction, variables, miRNA expression quantification methods and housekeeping gene for internal normalization are available at https://osf.io/g42ze/.
Statistical Analysis
The primary outcome was expression levels of miRNAs, presented as means with standard deviation. GetData Graph Digitizer version 2.26.0.20 was used to read miRNA values when figures presenting miRNA expression levels were available (Digitize graphs and plots, 2013). Median was used as an approximation of the arithmetic mean, and IQR/1.35 was used as an approximation of standard deviation. If standard error was used in the original article, standard deviation was calculated as sd = se*√n, and if the range was presented, standard deviation was estimated as (max-min)/4.
Methodologies for measuring miRNA expression levels varied; therefore, the standardized mean difference (SMD) was used as a measure of effect size to examine differences between the preeclampsia and non-preeclampsia groups. SMD expresses the difference between group means in units of standard deviation and was estimated by pooling individual trial results using random-effects models via the Der Simonian-Laird method. Heterogeneity was assessed using the Chi-square Q and I2 statistic. I2 presents the inconsistency between the study results and quantifies the proportion of observed dispersion that is real, i.e., due to between-study differences and not due to random error. The categorization of heterogeneity was based on the Cochrane Handbook (Higgins et al., 2019) and states that I2<30%, 30–60% or >60%, correspond to low, moderate and high heterogeneity, respectively. Forest plots were constructed for each analysis showing the SMD (box), 95% confidence interval (lines), and weight (size of box) for each trial. The overall effect size was represented by a diamond. Meta-analysis was performed for all miRNAs with available data from at least three relevant studies.
Sensitivity analyses were conducted to examine the effects of: 1) replacement of studies that measured miRNA expression levels in the chorionic plate with studies exploring the basal plate, 2) inclusion of measurements performed in more severe, less severe or not-specified PE forms only (instead of all PE forms), 3) replacement of miRNA expression levels obtained in term controls with miRNA expression levels in preterm controls, 4) inclusion of studies exploring miRNA expression levels in moderate or mild proteinuria PE groups, instead of severe proteinuria as in the PE group in the first analysis. A p value < 0.05 was statistically significant. Analyses were performed using Review Manager Version 5.4 (Cochrane, 2021).
Results
Systematic Review
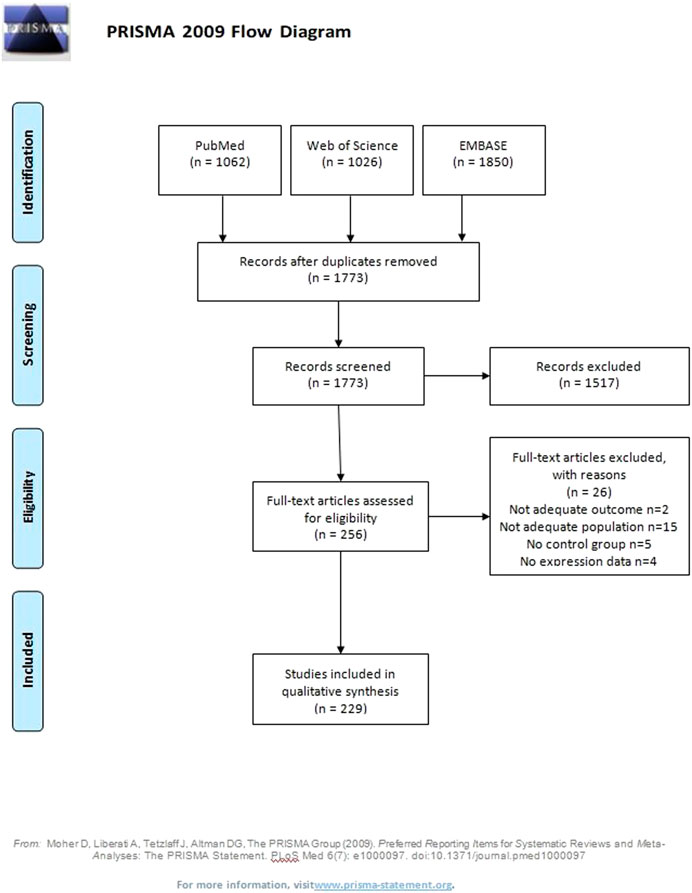
A total of 1773 potentially eligible articles were found. 1,517 articles were excluded because they were duplicates, not original articles, were without PE as the outcome, did not compare PE and control groups, examined populations other than women (animals, cell lines), did not explore miRNA expression levels, or were abstracts. Of the 256 reviewed full text articles, 229 were selected for inclusion in the systematic review. A flow diagram illustrating this selection process is presented in Figure 1.
Characteristics of all 229 publications included in the systematic review are presented in detail in Table 1. They were published between 2007 and 2021, with a total of 13043 participants; 6,459 women with and 6584 without PE. The minimum sample size of the PE group was four, and a minimum of one for the control group. The maximum sample size was 200 in PE and 321 in the control group. Four publications did not report the number of participants. 139 studies were cross-sectional, 64 were case-control, 11 were nested case-control studies, while only six were prospectively followed cohorts. Five studies included two or three sub-studies with the same or different study designs. In eighteen publications, the study design was not clearly stated. Most studies were from China (138), United States (19), and Czech Republic (8). Study groups were matched in 73 (32%) of all articles, and gestational age at the time of delivery was the most used variable for matching (in 53 of 73 publications). Maternal age at the time of delivery was used for matching in 35 publications. Other matching variables were BMI at the time of delivery, parity, race and/or ethnicity, gravidity, delivery, fetal gender, family history of PE, smoking history, additional comorbidities, systolic blood pressure at the time of inclusion, diastolic blood pressure at the time of inclusion, proteinuria at the time of inclusion, infant weight, pre-pregnancy indices, duration of storage of plasma samples, and maternal body weight at the time of delivery. Regression analysis was used to account for confounders in 14 publications. Ethnicity was reported in eight and race in eleven publications. Fetal gender was reported in 25 publications. The expression levels of miRNA were explored according to fetal gender in just three studies, and a regression model was adjusted for fetal gender in one publication. The most examined source of miRNAs was placenta, reported in 155/229 publications. Ninety-eight studies used maternal peripheral blood: plasma in 46, serum in 28, plasma exosomes in 9, mononuclear cells in 2, serum exosomes in 2, whole blood in 2, and leukocytes and buffy coat in one study each. Twelve studies analyzed miRNA expression levels in umbilical cord cell populations: mesenchymal stem cells in 4, and HUVECs, vein cells, maternal blood, exosomes, endothelial progenitor cells, serum, fetal blood, and umbilical cord tissue in one study each. Other rarely sampled tissues were myometrium, urine, maternal subcutaneous fat tissue endothelium, and placental blood vessel endothelium. Tissue was sampled at the time of delivery in 149 (65%) studies. In 60 studies, sampling was done prior to delivery and, in two studies, after delivery; 1 year after (Murphy et al., 2015), and 3–11 years after delivery (Hromadnikova et al., 2019b). Time of sampling was not reported in 34 (15%) publications. Most articles did not differentiate the type of PE (70%). Inclusion and exclusion criteria were not reported in most studies assessing miRNA in preeclamptic pregnancies. Only primiparous women were included in six studies, only non-smokers in 17, and only women without chronic hypertension in 89 publications. Detailed additional inclusion and exclusion criteria are presented in Supplementary Table S1. The presence of renal disease was the most common (50/229). The presence of diabetes mellitus (49/229) and the presence of cardiovascular disease (32/229) were reported less often. The presence of obesity was reported in six and preeclampsia in the previous gestation in five publications.
Disease severity was reported in 70/229 publications. Details regarding PE definitions and the diagnostic criteria used in the original articles are presented in Supplementary Tables S2, S3. qRT-PCR as the detection method with U6 as an internal control was utilized in almost all studies, and the details regarding quantification methods and housekeeping genes used are presented in Supplementary Table S4. A list of all explored miRNAs from the included publications according to PE severity (more severe, less severe, and not-specified PE) is presented in Supplementary Tables S5–S7.
Meta-Analysis
A meta-analysis was performed for the following fourteen miRNAs: miRNA-16, miRNA-17, miRNA-17-5p, miRNA-20b, miRNA-23a, miRNA-29a-3p, miRNA-29b, miRNA-30a-3p, miRNA-155, miRNA-155-5p, miRNA-181a, miRNA-195, miRNA-210, and miRNA-376c.
The expression levels were significantly higher in the placentas of women with PE compared to women without PE for miRNA-16 (SMD = 1.51, 95%CI = 0.55–2.46, p = 0.002) (Figure 2), miRNA-20b (SMD = 0.89, 95%CI = 0.33–1.45, p = 0.002) (Figure 3), miRNA-23a (SMD = 2.02, 95%CI = 1.25–2.78, p < 0.001) (Figure 4), miRNA-29b (SMD = 1.37, 95%CI = 0.36–2.37, p = 0.008) (Figure 5), miRNA-155 (SMD = 2.99, 95%CI = 0.83–5.14, p = 0.007) (Figure 6) and miRNA-210 (SMD = 1.63, 95%CI = 0.69–2.58, p < 0.001) (Figure 7). Subgroup analysis showed increased levels of miRNA-210 expression in placentas of women with more severe (SMD = 2.01, 95%CI = 0.31–3.71, p = 0.020), but not in women with a less severe form of PE (SMD = 0.39, 95%CI = –8.14 = 8.92, p = 0.930), compared to women without PE (Figure 7). The expression levels in placenta were significantly lower in women with PE compared to women without PE for miRNA-376c (SMD = –4.86, 95%CI = –9.51 to –0.20, p = 0.040) (Figure 8).
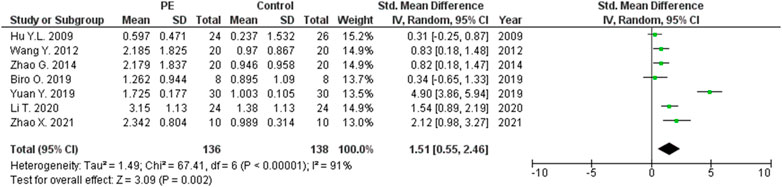
FIGURE 2. Meta-analysis of differences in expression level of miRNA-16 in placenta between women with vs. without preeclampsia.
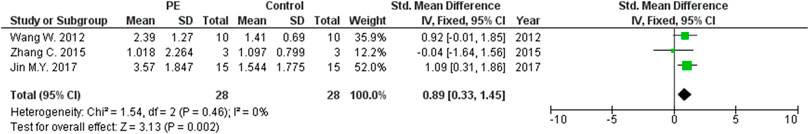
FIGURE 3. Meta-analysis of differences in expression level of miRNA-20b in placenta between women with vs. without preeclampsia.
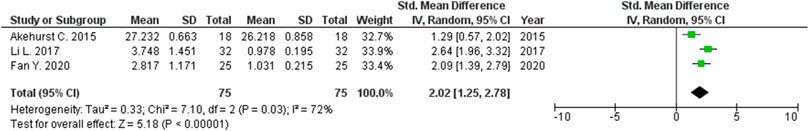
FIGURE 4. Meta-analysis of differences in expression level of miRNA-23a in placenta between women with vs. without preeclampsia.
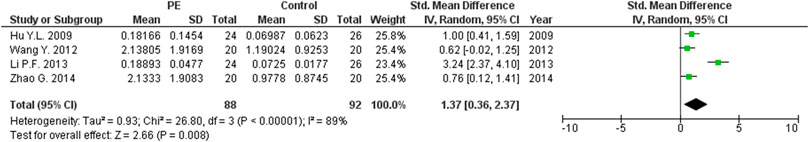
FIGURE 5. Meta-analysis of differences in expression level of miRNA-29b in placenta between women with vs. without preeclampsia.
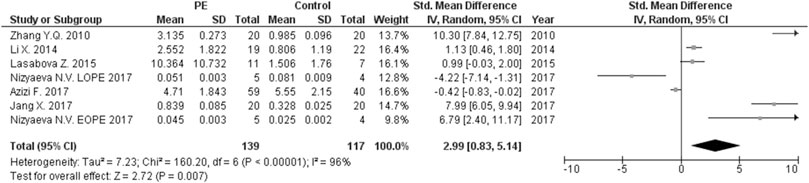
FIGURE 6. Meta-analysis of differences in expression level of miRNA-155 in placenta between women with vs. without preeclampsia.
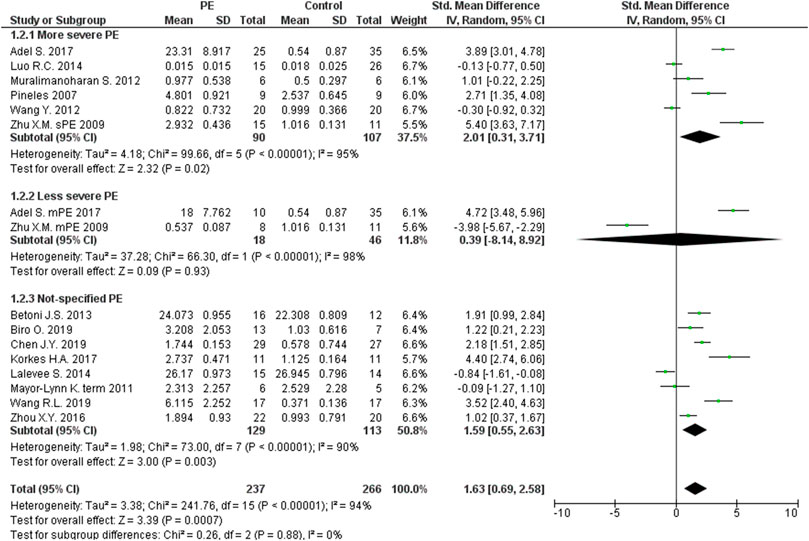
FIGURE 7. Meta-analysis of differences in expression level of miRNA-210 in placenta between women with vs. without preeclampsia.
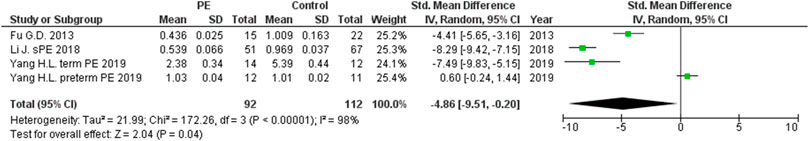
FIGURE 8. Meta-analysis of differences in expression level of miRNA-376c in placenta between women with vs. without preeclampsia.
The expression level was significantly higher in the maternal peripheral blood of women with PE compared to women without PE for miRNA-155 (SMD = 2.06, 95CI = 0.35–3.76, p = 0.020) (Figure 9), but it was lower for miRNA-16 (SMD = –0.47, 95%CI = –0.91 to –0.03, p = 0.040) (Figure 10).
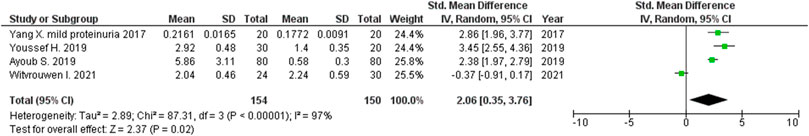
FIGURE 9. Meta-analysis of differences in expression level of miRNA-155 in peripheral blood between women with vs. without preeclampsia.
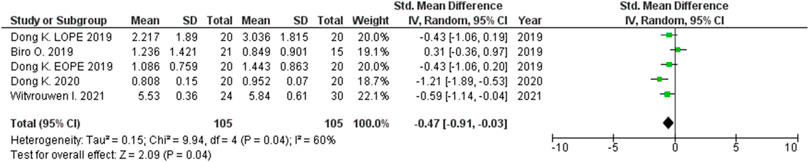
FIGURE 10. Meta-analysis of differences in expression level of miRNA-16 in peripheral blood between women with vs. without preeclampsia.
The functional roles of all significant miRNAs are presented in detail in Table 2. Although the roles of the evaluated miRNAs are confusing, special emphasis should be placed on the interpretation of the miRNAs known roles in controlling trophoblast proliferation, migration, invasion, apoptosis, differentiation, cellular metabolism, and angiogenesis.
Placental expression levels were not significantly different in women with PE compared to women without PE for miRNA-17 (SMD = 0.22, 95%CI = -1.35 to –1.79, p = 0.790) (Supplementary Figure S1), miRNA-30a-3p (SMD = 1.00, 95%CI = –0.50–2.50, p = 0.190) (Supplementary Figure S2), miRNA-181a (SMD = 0.05, 95%CI = –0.99–1.08, p = 0.930) (Supplementary Figure S3), and miRNA-195 (SMD = –0.16, 95%CI = –1.35–1.02, p = 0.780) (Supplementary Figure S4). The expression level was not significantly different in maternal peripheral blood in women with PE compared to women without PE for miRNA-17-5p (SMD = 0.08, 95%CI = –0.74–0.90, p = 0.850) (Supplementary Figure S5), miRNA-29a-3p (SMD = –0.29, 95%CI = –1.22–0.64, p = 0.540) (Supplementary Figure S6), miRNA-155-5p (SMD = –0.37, 95%CI = –1.07–0.33, p = 0.300) (Supplementary Figure S7), miRNA-181a (SMD = 0.22, 95%CI = –0.42–0.86, p = 0.500) (Supplementary Figure S8), and miRNA-210 (SMD = 0.48, 95%CI = –0.66–1.62, p = 0.410) (Supplementary Figure S9).
The same results were obtained when sensitivity analyses were performed to exclude studies with unspecified types of PE, to replace expression data obtained from the chorionic plate with those obtained from the basal plate, including/excluding different forms (more/less severe) of PE where possible (Supplementary Figures S10–S22).
Discussion
We identified in this study seven differentially expressed miRNAs in the placentas of women with vs without PE. miRNA-16, miRNA-20b, miRNA-23a, miRNA-29b, miRNA-155, and miRNA-210 were significantly increased in the placentas of PE women, while the levels of miRNA-376c were significantly decreased in PE placentas. We found no differences in the expression levels of miRNA-17, miRNA-30a-3p, miRNA-181a, and miRNA-195 in placentas of PE vs. non-PE women. A meta-analysis of the miRNA expression levels in the peripheral blood of PE women compared to women without PE was performed for miRNA-16, miRNA-17-5p, miRNA-29a-3p, miRNA-155, miRNA-155-5p, miRNA-181a and miRNA-210. A significant decrease in miRNA-16 expression levels in maternal peripheral blood of PE women was found, and no differences were found for other evaluated miRNAs. A sensitivity analysis did not change the results of the primary analysis.
Placentation is thought to be the basis for normal physiological pregnancy and is required for fetal growth and development, as well as the expectation of term labor. Several sensitive, precisely dictated, vascular processes involving angiogenesis at the fetal-maternal interface and adequate cytotrophoblast invasion with spiral-artery remodeling are essential for placentation (Weedon-Fekjær et al., 2014). At the very beginning of a pregnancy in which PE will develop, the transformation of proliferative endothelium into invasive endothelium is absent, and the expected extensive invasion of cytotrophoblasts into the spiral arteries does not occur. This results in pathologic remodeling of the placental arterioles, which become narrow, with reduced flow and sclerotic changes in the arteriolar walls (Mouillet et al., 2015). Placental ischemia promotes an inflammatory state that is characterized by increased production of inflammatory cytokines by pro-inflammatory T cells, and a decrease in regulatory and anti-inflammatory cytokines (Hanna et al., 2000). Decreased levels of anti-inflammatory cytokines (IL-10, IL-4) and increased pro-inflammatory cytokines (TNF-α, IL-6) in the circulation and placental tissue support the inflammatory background of preeclampsia (Keiser et al., 2009; Spence et al., 2021). These processes lead to placental malnutrition, and subsequent development of PE. The placenta is known to be an organ in which a large number of miRNAs are expressed (Mouillet et al., 2015). Several miRNAs contribute to the processes of trophoblast proliferation, invasion, and differentiation. miRNA-125b-1-3p and miRNA-210 inhibit trophoblast proliferation and invasion, while miRNA-155 inhibits trophoblast invasion only. In contrast, miRNA-376c enhances trophoblast proliferation and invasion (Mouillet et al., 2015). Fu et al. demonstrated that miR-376c promotes trophoblast cell proliferation, survival, migration, and invasion, and postulated that inhibition of Nodal and TGF-β signaling by miR-376c is important for adequate placentation (Fu et al., 2013). Primate-specific C19MC miRNAs, which are almost exclusively expressed in placenta, were described as important factors influencing adequate trophoblast invasion and arterial remodeling (Hromadnikova et al., 2013; Mouillet et al., 2015). As knowledge of the functional importance of miRNAs in adequate placentation and the development of PE increases (Hayder et al., 2018), it becomes important to determine whether miRNA expression levels are disrupted in PE, and which specific miRNA contributes predominantly to disease pathogenesis.
Meta-analysis in this study revealed significantly higher miRNA-16 expression in the placentas of women with PE compared to those without PE. Confirmation of the possible association between altered expression of miRNA-16 and PE was first described by Hu et al. who showed that there is increased expression of miRNA-16 in the placentas of women with severe PE (Hu et al., 2009). This was followed by Vu et al. who found increased expression of miRNA-16 in the sera of women with PE compared with healthy controls (Wu et al., 2012). The pathologic significance of miRNA-16 lies in its function in regulating the cell cycle. Liu et al. have shown that miRNA-16 stops the cell cycle in G1 phase by regulating the expressions of the CCND3, CCNE1 and CDK6 genes. Based on these physiological roles, it is supposed that miRNA-16 acts as a tumor suppressor (Yan X. et al., 2013). It also is known that the target of miRNA-16 is the Vascular Endothelial Growth Factor (VEGF) gene, whose product is an extremely important protein that initiates vasculogenesis in the placenta and induces proliferation and migration of endothelial cells in blood vessels (Wang and Zhao, 2010). In a study by Wang et al., miRNA-16 was found to have the potential to inhibit proliferation, migration and angiogenesis in mesenchymal stem cells (Wang Y. et al., 2012). The significantly lower miRNA-16 expression levels in the maternal peripheral blood of women with PE compared to those without PE led epigenetic analysis in another direction. It is proposed, but not proven, that miRNA-16 plays a significant role in the progression of human cardiac cell injury in ischemic dilated cardiomyopathy through endoplasmic reticulum stress, inflammation, autophagy, and apoptosis (Calderon-Dominguez et al., 2021). Down regulation of this miRNA, known as an anti-apoptotic factor, also was registered in ischemic myocardial cells, as a reaction to hypoxia in order to protect the tissue (Zhang H. J. et al., 2019). Therefore, miRNA-16 may play a role in both ischemic cardiomyopathy and preeclampsia, which similarly represent hypoxia induced pathological states. Original research articles have reported differing results regarding miRNA-16 levels in pregnancy complications. miRNA-16 levels were elevated in fetal macrosomia, but decreased in severe preeclampsia (Wu et al., 2012; Ge et al., 2015).
Increased expressions of miRNA-20b and miRNA-29b in the placentas of women with PE compared to women without PE were also found in our study. It is well known that the target gene for both miRNA-20 and miRNA-16 is VEGF, thus affecting placental vasculogenesis (Hayder et al., 2018). miRNA-20b binds to the Ephrin Type-B Receptor 4 (EPHB4) and Ephrin Type-B Receptor 2 (EPHB2), important receptors for intercellular communication, which have functions in the regulation of cellular morphology, binding, migration, proliferation, differentiation, and survival. These processes are assumed to be involved in the miRNA-20b contribution to placental blood vessel remodeling (Pasquale, 2005; Lisabeth et al., 2013). miRNA-29b is involved in the processes of trophoblast proliferation and invasion (Harapan and Andalas, 2015). miRNA-29b contributes to preeclampsia through dysregulation of the extracellular signal-regulated protein kinase and focal adhesion kinase (ERK/FAK) signaling pathway that allows the expression of matrix metalloproteinase-2 (MMP2), which is in turn an important factor for migration and invasion of trophoblast cells. Increased expression of miRNA-29b in severe PE has been previously shown to be associated with reduced expressions of MMP2 and integrin β1(ITGβ1) (Li H. et al., 2013).
The increased miRNA-23a levels in PE placentas support previously reported results that the level of this miRNA is upregulated in conditions related to abnormal angiogenesis (Chhabra et al., 2010). The main role of miRNA-23a, as part of the miR-23a∼27a∼24–2 cluster, is to mediate blood vessel genesis. It is included, except in PE, in pathological states such as muscle atrophy, cardiac hypertrophy, and cancers (Chhabra et al., 2010). Data in vitro, as well as in vivo, indicate that miRNA-23a and miR-23b may have opposite roles, with the former regulating angiogenesis and cellular junctions, and hence inhibiting vascular permeability, while miRNA-23b promotes permeability (Li et al., 2016).
MiRNA-155 expression levels were significantly increased in the placentas and maternal peripheral blood of women with PE compared to those without PE. The increased expression of miRNA-155 and resultant lower levels of cysteine-rich protein 61 (CYR61) and cyclin D1, have been associated with the inhibition of trophoblast invasion (Zhang et al., 2010; Dai et al., 2012). It also has been previously demonstrated that a significant increase in miRNA-155 decreases endothelial nitric oxide synthase (eNOS) expression and thus contributes to development of severe PE (Li X. et al., 2014). This result is consistent with findings from previous studies (Zhang et al., 2010; Gan et al., 2017). This immunomodulatory miRNA, induced in activated T lymphocytes, B lymphocytes and macrophages (Bernstein et al., 2003), is also disrupted in maternal peripheral blood. Its increased expression level was associated with a decreased level of pro-angiogenic factor, VEGF, in an experimental rat model of PE (Cheng et al., 2011). Newly performed studies have reported significantly higher levels of miRNA-155 in the maternal peripheral blood of women with compared to women without PE (Ayoub et al., 2019; Youssef and Marei, 2019; Witvrouwen et al., 2021).
MiRNA-210 has been the most evaluated small non-coding RNA. It is known that miRNA-210 is induced under hypoxic conditions which exist prior to, as well as during the clinical manifestations of PE. Hypoxia stimulates the production of NF-kB 1 (nuclear factor kappa-B 1) and HIF-1A (hypoxia inducible factor 1 α), which induce the expression of miRNA-210 (Muralimanoharan et al., 2012). Previous research has confirmed significantly increased expression of miRNA-210 in both the placentas and sera of women with PE and suggests that miRNA-210 obtained from serum may be a useful biomarker even months before diagnosis (Anton et al., 2013). Micro RNA-210 plays a role in several processes, such as inhibition of cytotrophoblast migration and invasion, differentiation, apoptosis, inflammation, angiogenesis, as well as in the regulation of cellular metabolism. miRNA-210 partially inhibits trophoblast invasion via the ERK/MAPK signaling pathway (Anton et al., 2012). Cell metabolism is dictated by miRNA-210 in that increased expression leads to decreased mitochondrial respiration and vice versa (Hayder et al., 2018). miRNA-210 also plays a role as a suppressor of EFNA3, a member of the ephrin ligand family which is important for cell migration, and HOXA9, an important angiogenesis regulator (Zhang et al., 2012; Luo et al., 2014). Overall, inadequate trophoblast invasion and impaired cellular metabolism are confirmed factors that can lead to the development of PE. Anton et al. found that for each 5-U increase in miR-210 in sera of previously healthy women at the beginning of the second trimester, the odds of PE development later in pregnancy increased fourfold (Anton et al., 2013).
MiRNA-376c plays a role in trophoblast proliferation and differentiation (Hayder et al., 2018). We found significantly lower levels of expression in the placentas of women with PE compared to women without PE, which is consistent with the findings of other studies (Fu et al., 2013; Yang H.-l. et al., 2019). Only Yang et al. showed no significant difference in the levels of miRNA-376c expression in the placentas of women with preterm preeclampsia and gestational age matched controls without PE (Yang H.-l. et al., 2019). Fu at al. showed that a decrease in miR-376c expression results in excessive apoptosis, insufficient cell proliferation, and shallow invasion of trophoblasts in the uterus in preeclampsia (Fu et al., 2013).
In summary, our results clearly identify a subset of miRNAs that are dysregulated in preeclampsia and clearly point towards the underlying mechanisms that may be contributing to the pathophysiology of preeclampsia. Our results set the stage for several venues for future research with an overall goal to facilitate early diagnosis and optimize fetal and maternal outcomes. First, given the clinical heterogeneity of preeclampsia (severe vs. mild, late vs. early, and “placental” vs. “maternal”), adequately designed and powered studies may detect differences in miRNA and related specific underlying mechanisms responsible for specific clinical subtypes. Second, clinical studies may identify a marker (or set of markers) with either predictive or diagnostic role. Third, further discovery of signaling pathways affected by miRNA may lead to mechanism-based therapies.
Our study has several limitations. They originate from the unavailability of all/some data from the original publications, uninformative figures presented in the articles, and selection of the housekeeping gene used for internal controls. The consequences of the data unavailability are possible exclusion of relevant data and a smaller number of included studies, as well as miRNAs, in the meta-analysis that may lead to an overestimation/underestimation of the effects of miRNA expression level on PE development. The importance of adequate selection of the housekeeping gene should be emphasized to standardize miRNA evaluation methodology and to provide comparability between studies. The definition of PE is not the same in each of the included studies which may lead to inclusion of heterogeneous cases that can change the assessment of the effect. Through the systematic review, it was realized that cases and controls were rarely matched for gestational age at the time of sampling. It is necessary to highlight the importance of comparing matched groups because it is known that there are physiological changes in miRNAs expression levels throughout pregnancy. The miRNA source in plasma may be maternal, fetal, or both, yet only a small number of studies reported these data.
Conclusion
MiRNAs play an important role in the pathophysiology of PE. The functional roles of the microRNAs found to be disrupted in preeclamptic pregnancies include control of trophoblast proliferation, migration, invasion, apoptosis, differentiation, cellular metabolism, and angiogenesis. The identification of differentially expressed miRNAs in maternal blood creates an opportunity to define an easily accessible biomarker of PE. A better understanding of the role of microRNAs in the development of PE offers great potential for developing diagnostic and therapeutic targets for PE.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
Conceptualization: AC, VG, ZM, DS, NM; Data curation: AC, JM, MS, NR, JK, NA, TS, VP, DS, NM; Formal analysis: AC, JM, MS, NR, DS, NM; Investigation: AC, VG, JM, OM, MS, NR, JK, NA, NM, TS, VP, DS, NM; Methodology: AC, VG, JM, DS, NM; Project administration: VG, DS, NM; Supervision: VG, DS, NM; Visualization: AC, JM, MS, DS, NM; Writing – original draft: AC, VG, JM, OM, MS, NR, NA, TS, JK, VP, DS, NM; Writing – review & editing: AC, VG, JM, OM, MS, NR, NA, NM, TS, JK, VP, DS, NM. All authors read and approved the final manuscript.
Funding
Funding: NIH R01-HL136348 (VG).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.782845/full#supplementary-material
References
Abalos, E., Cuesta, C., Grosso, A. L., Chou, D., and Say, L. (2013). Global and Regional Estimates of Preeclampsia and Eclampsia: A Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 170, 1–7. doi:10.1016/j.ejogrb.2013.05.005
Adel, S., Mansour, A., Louka, M., Matboli, M., Elmekkawi, S. F., and Swelam, N. (2017). Evaluation of MicroRNA-210 and Protein Tyrosine Phosphatase, Non-receptor Type 2 in Pre-eclampsia. Gene 596, 105–109. doi:10.1016/j.gene.2016.10.014
Akehurst, C., Small, H. Y., Sharafetdinova, L., Forrest, R., Beattie, W., Brown, C. E., et al. (2015). Differential Expression of microRNA-206 and its Target Genes in Preeclampsia. J. Hypertens. 33, 2068–2074. doi:10.1097/HJH.0000000000000656
Akgör, U., Ayaz, L., and Çayan, F. (2021). Expression Levels of Maternal Plasma microRNAs in Preeclamptic Pregnancies. J. Obstet. Gynaecol. 41, 910–914. doi:10.1080/01443615.2020.1820465
Ali, Z., Zafar, U., Zaki, S., Ahmad, S., Khaliq, S., and Lone, K. P. (2021). Expression Levels of MiRNA-16, SURVIVIN and TP53 in Preeclamptic and Normotensive Women. J. Pak. Med. Assoc. 71, 2208–2213. doi:10.47391/JPMA.1171
American College of Obstetricians and Gynecologists, (2019). ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 133, e1. doi:10.1097/AOG.0000000000003018
Anton, L., Brown, A. G., Parry, S., and Elovitz, M. A. (2012). Lipopolysaccharide Induces Cytokine Production and Decreases Extravillous Trophoblast Invasion through a Mitogen-Activated Protein Kinase-Mediated Pathway: Possible Mechanisms of First Trimester Placental Dysfunction. Hum. Reprod. 27, 61–72. doi:10.1093/humrep/der362
Anton, L., Olarerin-George, A. O., Hogenesch, J. B., and Elovitz, M. A. (2015). Placental Expression of miR-517a/b and miR-517c Contributes to Trophoblast Dysfunction and Preeclampsia. PLoS One 10, e0122707. doi:10.1371/journal.pone.0122707
Anton, L., Olarerin-George, A. O., Schwartz, N., Srinivas, S., Bastek, J., Hogenesch, J. B., et al. (2013). MiR-210 Inhibits Trophoblast Invasion and Is a Serum Biomarker for Preeclampsia. Am. J. Pathol. 183, 1437–1445. doi:10.1016/j.ajpath.2013.07.021
Awamleh, Z., Gloor, G. B., and Han, V. K. M. (2019). Placental microRNAs in Pregnancies with Early Onset Intrauterine Growth Restriction and Preeclampsia: Potential Impact on Gene Expression and Pathophysiology. BMC Med. Genomics 12, 91. doi:10.1186/s12920-019-0548-x
Awamleh, Z., and Han, V. K. M. (2020). Identification of miR-210-5p in Human Placentae from Pregnancies Complicated by Preeclampsia and Intrauterine Growth Restriction, and its Potential Role in the Pregnancy Complications. Pregnancy Hypertens. 19, 159–168. doi:10.1016/j.preghy.2020.01.002
Ayoub, S. E., Shaker, O. G., Abdelwahed, M. Y., Ahmed, N. A., Abdelhameed, H. G., Bosilah, A. H., et al. (2019). Association of MicroRNA-155rs767649 Polymorphism with Susceptibility to Preeclampsia. Int. J. Mol. Cel Med 8, 247–257. doi:10.22088/IJMCM.BUMS.8.4.247
Azizi, F., Saleh Gargari, S., Asadi Shahmirzadi, S., Dodange, F., Amiri, V., Mirfakhraie, R., et al. (2017). Evaluation of Placental Mir-155-5p and Long Non-coding RNA sONE Expression in Patients with Severe Pre-eclampsia. Int. J. Mol. Cel Med 6, 22–30.
Bai, Y., Yang, W., Yang, H.-x., Liao, Q., Ye, G., Fu, G., et al. (2012). Downregulated miR-195 Detected in Preeclamptic Placenta Affects Trophoblast Cell Invasion via Modulating ActRIIA Expression. PLoS One 7, e38875. doi:10.1371/journal.pone.0038875
Bernstein, E., Kim, S. Y., Carmell, M. A., Murchison, E. P., Alcorn, H., Li, M. Z., et al. (2003). Dicer Is Essential for Mouse Development. Nat. Genet. 35, 215–217. doi:10.1038/ng1253
Betoni, J. S., Derr, K., Pahl, M. C., Rogers, L., Muller, C. L., Packard, R. E., et al. (2013). MicroRNA Analysis in Placentas from Patients with Preeclampsia: Comparison of New and Published Results. Hypertens. Pregnancy 32, 321–339. doi:10.3109/10641955.2013.807819
Biró, O., Fóthi, Á., Alasztics, B., Nagy, B., Orbán, T. I., and Rigó, J. (2019). Circulating Exosomal and Argonaute-Bound microRNAs in Preeclampsia. Gene 692, 138–144. doi:10.1016/j.gene.2019.01.012
Black, K. D., and Horowitz, J. A. (2018). Inflammatory Markers and Preeclampsia. Nurs. Res. 67, 242–251. doi:10.1097/NNR.0000000000000285
Brkić, J., Dunk, C., O’Brien, J., Fu, G., Nadeem, L., Wang, Y.-l., et al. (2018). MicroRNA-218-5p Promotes Endovascular Trophoblast Differentiation and Spiral Artery Remodeling. Mol. Ther. 26, 2189–2205. doi:10.1016/j.ymthe.2018.07.009
Brodowski, L., Schröder-Heurich, B., von Hardenberg, S., Richter, K., von Kaisenberg, C. S., Dittrich-Breiholz, O., et al. (2021). Microrna Profiles of Maternal and Neonatal Endothelial Progenitor Cells in Preeclampsia. Ijms 22, 5320. doi:10.3390/ijms22105320
Cai, H., Li, D., Wu, J., and Shi, C. (2021). MiR-519d Downregulates LEP Expression to Inhibit Preeclampsia Development. Open Med. 16, 1215–1227. doi:10.1515/med-2021-0244
Cai, M., Kolluru, G. K., and Ahmed, A. (2017). Small Molecule, Big Prospects: MicroRNA in Pregnancy and its Complications. J. Pregnancy 2017, 1–15. doi:10.1155/2017/6972732
Calderon-Dominguez, M., Mangas, A., Belmonte, T., Quezada-Feijoo, M., Ramos, M., and Toro, R. (2021). Ischemic Dilated Cardiomyopathy Pathophysiology through microRNA-16-5p. Revista Española de Cardiología (English Edition) 74, 740–749. doi:10.1016/j.rec.2020.08.012
Campos, C. B., Marques, T. M., Pereira, R. W., and Sandrim, V. C. (2014). Reduced Circulating miR-196b Levels Is Associated with Preeclampsia. Pregnancy Hypertens. Int. J. Women's Cardiovasc. Health 4, 11–13. doi:10.1016/j.preghy.2013.10.002
Cao, G., Cui, R., Liu, C., and Zhang, Z. (2019). MicroRNA Regulation of Transthyretin in Trophoblast Biofunction and Preeclampsia. Arch. Biochem. Biophys. 676, 108129. doi:10.1016/j.abb.2019.108129
Chen, J., Zhao, L., Wang, D., Xu, Y., Gao, H., Tan, W., et al. (2019). Contribution of Regulatory Tcells to Immune Tolerance and Association of microRNA-210 and Foxp3 in P-reeclampsia. Mol. Med. Rep. 19, 1150–1158. doi:10.3892/mmr.2018.9733
Chen, S., Zhao, G., Miao, H., Tang, R., Song, Y., Hu, Y., et al. (2015). MicroRNA-494 Inhibits the Growth and Angiogenesis-Regulating Potential of Mesenchymal Stem Cells. FEBS Lett. 589, 710–717. doi:10.1016/j.febslet.2015.01.038
Chen, Y.-S., Shen, L., Mai, R.-Q., and Wang, Y. (2014). Levels of microRNA-181b and Plasminogen Activator Inhibitor-1 Are Associated with Hypertensive Disorders Complicating Pregnancy. Exp. Ther. Med. 8, 1523–1527. doi:10.3892/etm.2014.1946
Chhabra, R., Dubey, R., and Saini, N. (2010). Cooperative and Individualistic Functions of the microRNAs in the miR-23a∼27a∼24-2 Cluster and its Implication in Human Diseases. Mol. Cancer 9, 232. doi:10.1186/1476-4598-9-232
Chi, Z., and Zhang, M. (2018). Exploration of the Regulation and Control Mechanisms of miR-145 in T-rophoblast C-ell P-roliferation and I-nvasion. Exp. Ther. Med. 16, 5298–5304. doi:10.3892/etm.2018.6890
Choi, S.-Y., Yun, J., Lee, O.-J., Han, H.-S., Yeo, M.-K., Lee, M.-A., et al. (2013). MicroRNA Expression Profiles in Placenta with Severe Preeclampsia Using a PNA-Based Microarray. Placenta 34, 799–804. doi:10.1016/j.placenta.2013.06.006
Chu, X., Gu, Y., Sheng, W., Sun, J., Morgan, J. A., Lewis, D. F., et al. (2021). Downregulation of miR-126-3p Expression Contributes to Increased Inflammatory Response in Placental Trophoblasts in Preeclampsia. J. Reprod. Immunol. 144, 103281. doi:10.1016/j.jri.2021.103281
Cochrane (2021). RevMan 5 Download | Cochrane Training. Available at: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download (Accessed August 26, 2021).
Dai, X., and Cai, Y. (2018). Retracted: Down‐regulation of microRNA Let‐7d Inhibits the Proliferation and Invasion of Trophoblast Cells in Preeclampsia. J. Cel. Biochem. 119, 1141–1151. doi:10.1002/jcb.26282
Dai, Y., Qiu, Z., Diao, Z., Shen, L., Xue, P., Sun, H., et al. (2012). MicroRNA-155 Inhibits Proliferation and Migration of Human Extravillous Trophoblast Derived HTR-8/SVneo Cells via Down-Regulating Cyclin D1. Placenta 33, 824–829. doi:10.1016/j.placenta.2012.07.012
Demirer, S., Hocaoglu, M., Turgut, A., Karateke, A., and Komurcu-Bayrak, E. (2020). Expression Profiles of Candidate microRNAs in the Peripheral Blood Leukocytes of Patients with Early- and Late-Onset Preeclampsia versus normal Pregnancies. Pregnancy Hypertens. 19, 239–245. doi:10.1016/j.preghy.2019.11.003
Devor, E., Santillan, D., Scroggins, S., Warrier, A., and Santillan, M. (2020). Trimester-specific Plasma Exosome microRNA Expression Profiles in Preeclampsia. J. Maternal-Fetal Neonatal Med. 33, 3116–3124. doi:10.1080/14767058.2019.1569614
Digitize graphs and plots, (2013). GetData Graph Digitizer - Graph Digitizing Software - Download. Available at: http://getdata-graph-digitizer.com/download.php (Accessed August 26, 2021).
Ding, J., Huang, F., Wu, G., Han, T., Xu, F., Weng, D., et al. (2015). MiR-519d-3p Suppresses Invasion and Migration of Trophoblast Cells via Targeting MMP-2. PLoS One 10, e0120321. doi:10.1371/journal.pone.0120321
Dong, D., Khoong, Y., Ko, Y., and Zhang, Y. (2020). microRNA-646 I-nhibits A-ngiogenesis of E-ndothelial P-rogenitor C-ells in P-re-eclamptic P-regnancy by T-argeting the VEGF-A/HIF-1α axis. Exp. Ther. Med. 20, 1879–1888. doi:10.3892/etm.2020.8929
Dong, K., Zhang, X., Ma, L., Gao, N., Tang, H., Jian, F., et al. (2019). Downregulations of Circulating miR-31 and miR-21 Are Associated with Preeclampsia. Pregnancy Hypertens. 17, 59–63. doi:10.1016/j.preghy.2019.05.013
Doridot, L., Houry, D., Gaillard, H., Chelbi, S. T., Barbaux, S., and Vaiman, D. (2014). miR-34A Expression, Epigenetic Regulation, and Function in Human Placental Diseases. Epigenetics 9, 142–151. doi:10.4161/epi.26196
Eghbal‐Fard, S., Yousefi, M., Heydarlou, H., Ahmadi, M., Taghavi, S., Movasaghpour, A., et al. (2019). The Imbalance of Th17/Treg axis Involved in the Pathogenesis of Preeclampsia. J. Cel. Physiol. 234, 5106–5116. doi:10.1002/jcp.27315
Enquobahrie, D. A., Abetew, D. F., Sorensen, T. K., Willoughby, D., Chidambaram, K., and Williams, M. A. (2011). Placental microRNA Expression in Pregnancies Complicated by Preeclampsia. Am. J. Obstet. Gynecol. 204, 178e12–178. e21. doi:10.1016/j.ajog.2010.09.004
Fan, Y., Dong, Z., Zhou, G., Fu, J., Zhan, L., Gao, M., et al. (2020). Elevated miR-23a Impairs Trophoblast Migration and Invasiveness through HDAC2 Inhibition and NF-Κb Activation. Life Sci. 261, 118358. doi:10.1016/j.lfs.2020.118358
Fang, M., Du, H., Han, B., Xia, G., Shi, X., Zhang, F., et al. (2017). Hypoxia-inducible microRNA-218 Inhibits Trophoblast Invasion by Targeting LASP1: Implications for Preeclampsia Development. Int. J. Biochem. Cel Biol. 87, 95–103. doi:10.1016/j.biocel.2017.04.005
Fang, Y., Huang, Z., Tan, W., Zhang, Q., and Wu, J. (2018). High Expression of miR-182-5p Promotes Preeclampsia Progression. Eur. Rev. Med. Pharmacol. Sci. 22, 6583–6590. doi:10.26355/eurrev_201810_16132
Fu, G., Ye, G., Nadeem, L., Ji, L., Manchanda, T., Wang, Y., et al. (2013). MicroRNA-376c Impairs Transforming Growth Factor-β and Nodal Signaling to Promote Trophoblast Cell Proliferation and Invasion. Hypertension 61, 864–872. doi:10.1161/HYPERTENSIONAHA.111.203489
Gan, L., Liu, Z., Wei, M., Chen, Y., Yang, X., Chen, L., et al. (2017). MIR-210 and miR-155 as Potential Diagnostic Markers for Pre-eclampsia Pregnancies. Med. (United States 96, e7515. doi:10.1097/MD.0000000000007515
Gao, S., Wang, Y., Han, S., and Zhang, Q. (2017). Up-regulated microRNA-300 in Maternal Whole Peripheral Blood and Placenta Associated with Pregnancy-Induced Hypertension and Preeclampsia. Int. J. Clin. Exp. Pathol. 10, 4232–4242.
Gao, X., Li, H., and Wei, J. X. (2018a). MiR-4421 Regulates the Progression of Preeclampsia by Regulating CYP11B2. Eur. Rev. Med. Pharmacol. Sci. 22, 1533–1540. doi:10.26355/eurrev_201803_14557
Gao, Y., She, R., Wang, Q., Li, Y., and Zhang, H. (2018b). Up-regulation of miR-299 Suppressed the Invasion and Migration of HTR-8/SVneo Trophoblast Cells Partly via Targeting HDAC2 in Pre-eclampsia. Biomed. Pharmacother. 97, 1222–1228. doi:10.1016/j.biopha.2017.11.053
Garovic, V. D., Wagner, S. J., Turner, S. T., Rosenthal, D. W., Watson, W. J., Brost, B. C., et al. (2007). Urinary Podocyte Excretion as a Marker for Preeclampsia. Am. J. Obstet. Gynecol. 196, 320e1–320. e7. doi:10.1016/j.ajog.2007.02.007
Garovic, V. D., White, W. M., Vaughan, L., Saiki, M., Parashuram, S., Garcia-Valencia, O., et al. (2020). Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J. Am. Coll. Cardiol. 75, 2323–2334. doi:10.1016/j.jacc.2020.03.028
Ge, Q., Zhu, Y., Li, H., Tian, F., Xie, X., and Bai, Y. (2015). Differential Expression of Circulating miRNAs in Maternal Plasma in Pregnancies with Fetal Macrosomia. Int. J. Mol. Med. 35, 81–91. doi:10.3892/ijmm.2014.1989
Giannakou, K., Evangelou, E., and Papatheodorou, S. I. (2018). Genetic and Non-genetic Risk Factors for Pre-eclampsia: Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. Ultrasound Obstet. Gynecol. 51, 720–730. doi:10.1002/uog.18959
Gong, F., Chai, W., Wang, J., Cheng, H., Shi, Y., Cui, L., et al. (2020). miR-214-5p Suppresses the Proliferation, Migration and Invasion of Trophoblast Cells in Pre-eclampsia by Targeting Jagged 1 to Inhibit Notch Signaling Pathway. Acta Histochem. 122, 151527. doi:10.1016/j.acthis.2020.151527
Gunel, T., Hosseini, M. K., Gumusoglu, E., Kisakesen, H. I., Benian, A., and Aydinli, K. (2017). Expression Profiling of Maternal Plasma and Placenta microRNAs in Preeclamptic Pregnancies by Microarray Technology. Placenta 52, 77–85. doi:10.1016/j.placenta.2017.02.019
Gunel, T., Kamali, N., Hosseini, M. K., Gumusoglu, E., Benian, A., and Aydinli, K. (2020). Regulatory Effect of miR-195 in the Placental Dysfunction of Preeclampsia. J. Maternal-Fetal Neonatal Med. 33, 901–908. doi:10.1080/14767058.2018.1508439
Gunel, T., Zeybek, Y. G., Akçakaya, P., Kalelioglu, I., Benian, A., Ermis, H., et al. (2011). Serum microRNA Expression in Pregnancies with Preeclampsia. Genet. Mol. Res. 10, 4034–4040. doi:10.4238/2011.November.8.5
Guo, L., Liu, Y., Guo, Y., Yang, Y., and Chen, B. (2018). MicroRNA-423-5p Inhibits the Progression of Trophoblast Cells via Targeting IGF2BP1. Placenta 74, 1–8. doi:10.1016/j.placenta.2018.12.003
Guo, L., Tsai, S. Q., Hardison, N. E., James, A. H., Motsinger-Reif, A. A., Thames, B., et al. (2013). Differentially Expressed microRNAs and Affected Biological Pathways Revealed by Modulated Modularity Clustering (MMC) Analysis of Human Preeclamptic and IUGR Placentas. Placenta 34, 599–605. doi:10.1016/j.placenta.2013.04.007
Guo, L., Yang, Q., Lu, J., Li, H., Ge, Q., Gu, W., et al. (2011). A Comprehensive Survey of miRNA Repertoire and 3′ Addition Events in the Placentas of Patients with Pre-eclampsia from High-Throughput Sequencing. PLoS One 6, e21072. doi:10.1371/journal.pone.0021072
Guo, M., Zhao, X., Yuan, X., and Li, P. (2017). Elevated microRNA-34a Contributes to Trophoblast Cell Apoptosis in Preeclampsia by Targeting BCL-2. J. Hum. Hypertens. 31, 815–820. doi:10.1038/jhh.2017.65
Han, L., Luo, Q. q., Peng, M. g., Zhang, Y., and Zhu, X. h. (2021). miR ‐483 Is D Ownregulated in Pre‐eclampsia via Targeting Insulin‐like Growth Factor 1 ( IGF1 ) and Regulates the PI3K/Akt/mTOR Pathway of Endothelial Progenitor Cells. J. Obstet. Gynaecol. Res. 47, 63–72. doi:10.1111/jog.14412
Han, L., Zhao, Y., Luo, Q. Q., Liu, X. X., Lu, S. S., and Zou, L. (2017). The Significance of miR-145 in the Prediction of Preeclampsia. Bll 118, 523–528. doi:10.4149/BLL_2017_101
Hanna, N., Hanna, I., Hleb, M., Wagner, E., Dougherty, J., Balkundi, D., et al. (2000). Gestational Age-dependent Expression of IL-10 and its Receptor in Human Placental Tissues and Isolated Cytotrophoblasts. J. Immunol. 164, 5721–5728. doi:10.4049/jimmunol.164.11.5721
Harapan, H., and Andalas, M. (2015). The Role of microRNAs in the Proliferation, Differentiation, Invasion, and Apoptosis of Trophoblasts during the Occurrence of Preeclampsia-A Systematic Review. Tzu Chi Med. J. 27, 54–64. doi:10.1016/j.tcmj.2015.05.001
Hayder, H., Fu, G., Nadeem, L., O’Brien, J. A., Lye, S. J., and Peng, C. (2021). Overexpression of Mir-210-3p Impairs Extravillous Trophoblast Functions Associated with Uterine Spiral Artery Remodeling. Ijms 22, 3961. doi:10.3390/ijms22083961
Hayder, H., O’Brien, J., Nadeem, U., and Peng, C. (2018). MicroRNAs: Crucial Regulators of Placental Development. Reproduction 155, R259–R271. doi:10.1530/REP-17-0603
Hemmatzadeh, M., Shomali, N., Yousefzadeh, Y., Mohammadi, H., Ghasemzadeh, A., and Yousefi, M. (2020). MicroRNAs: Small Molecules with a Large Impact on Pre‐eclampsia. J. Cel. Physiol. 235, 3235–3248. doi:10.1002/jcp.29286
Higgins, J. P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons. doi:10.1002/9781119536604
Hocaoglu, M., Demirer, S., Senturk, H., Turgut, A., and Komurcu-Bayrak, E. (2019). Differential Expression of Candidate Circulating microRNAs in Maternal Blood Leukocytes of the Patients with Preeclampsia and Gestational Diabetes Mellitus. Pregnancy Hypertens. 17, 5–11. doi:10.1016/j.preghy.2019.04.004
Hombach, S., and Kretz, M. (2016). Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 937, 3–17. doi:10.1007/978-3-319-42059-2_1
Hong, F., Li, Y., and Xu, Y. (2014). Decreased Placental miR-126 Expression and Vascular Endothelial Growth Factor Levels in Patients with Pre-eclampsia. J. Int. Med. Res. 42, 1243–1251. doi:10.1177/0300060514540627
Hromadnikova, I., Dvorakova, L., Kotlabova, K., and Krofta, L. (2019a). The Prediction of Gestational Hypertension, Preeclampsia and Fetal Growth Restriction via the First Trimester Screening of Plasma Exosomal C19MC microRNAs. Ijms 20, 2972. doi:10.3390/ijms20122972
Hromadnikova, I., Kotlabova, K., Doucha, J., Dlouha, K., and Krofta, L. (2012). Absolute and Relative Quantification of Placenta-specific MicroRNAs in Maternal Circulation with Placental Insufficiency-Related Complications. J. Mol. Diagn. 14, 160–167. doi:10.1016/j.jmoldx.2011.11.003
Hromadnikova, I., Kotlabova, K., Dvorakova, L., and Krofta, L. (2019b). Postpartum Profiling of microRNAs Involved in Pathogenesis of Cardiovascular/cerebrovascular Diseases in Women Exposed to Pregnancy-Related Complications. Int. J. Cardiol. 291, 158–167. doi:10.1016/j.ijcard.2019.05.036
Hromadnikova, I., Kotlabova, K., Hympanova, L., and Krofta, L. (2015a). Cardiovascular and Cerebrovascular Disease Associated microRNAS Are Dysregulated in Placental Tissues Affected with Gestational Hypertension, Preeclampsia and Intrauterine Growth Restriction. PLoS One 10, e0138383. doi:10.1371/journal.pone.0138383
Hromadnikova, I., Kotlabova, K., Hympanova, L., and Krofta, L. (2016). Gestational Hypertension, Preeclampsia and Intrauterine Growth Restriction Induce Dysregulation of Cardiovascular and Cerebrovascular Disease Associated microRNAs in Maternal Whole Peripheral Blood. Thromb. Res. 137, 126–140. doi:10.1016/j.thromres.2015.11.032
Hromadnikova, I., Kotlabova, K., Ivankova, K., Vedmetskaya, Y., and Krofta, L. (2017). Profiling of Cardiovascular and Cerebrovascular Disease Associated microRNA Expression in Umbilical Cord Blood in Gestational Hypertension, Preeclampsia and Fetal Growth Restriction. Int. J. Cardiol. 249, 402–409. doi:10.1016/j.ijcard.2017.07.045
Hromadnikova, I., Kotlabova, K., Ondrackova, M., Kestlerova, A., Novotna, V., Hympanova, L., et al. (2013). Circulating C19MC MicroRNAs in Preeclampsia, Gestational Hypertension, and Fetal Growth Restriction. Mediators Inflamm. 2013, 1–12. doi:10.1155/2013/186041
Hromadnikova, I., Kotlabova, K., Ondrackova, M., Pirkova, P., Kestlerova, A., Novotna, V., et al. (2015b). Expression Profile of C19MC microRNAs in Placental Tissue in Pregnancy-Related Complications. DNA Cel Biol. 34, 437–457. doi:10.1089/dna.2014.2687
Hu, E., Ding, L., Miao, H., Liu, F., Liu, D., Dou, H., et al. (2015). MiR-30a Attenuates Immunosuppressive Functions of IL-1β-elicited Mesenchymal Stem Cells via Targeting TAB3. FEBS Lett. 589, 3899–3907. doi:10.1016/j.febslet.2015.11.001
Hu, S., Li, J., Tong, M., Li, Q., Chen, Y., Lu, H., et al. (2019). MicroRNA-144-3p M-ay P-articipate in the P-athogenesis of P-reeclampsia by T-argeting Cox-2. Mol. Med. Rep. 19, 4655–4662. doi:10.3892/mmr.2019.10150
Hu, T.-X., Guo, X., Wang, G., Gao, L., He, P., Xia, Y., et al. (2017). MiR133b Is Involved in Endogenous Hydrogen Sulfide Suppression of sFlt-1 Production in Human Placenta. Placenta 52, 33–40. doi:10.1016/j.placenta.2017.02.012
Hu, T.-X., Wang, G., Guo, X.-J., Sun, Q.-Q., He, P., Gu, H., et al. (2016). MiR 20a,-20b and -200c Are Involved in Hydrogen Sulfide Stimulation of VEGF Production in Human Placental Trophoblasts. Placenta 39, 101–110. doi:10.1016/j.placenta.2016.01.019
Hu, Y., Li, P., Hao, S., Liu, L., Zhao, J., and Hou, Y. (2009). Differential Expression of microRNAs in the Placentae of Chinese Patients with Severe Pre-eclampsia. Clin. Chem. Lab. Med. 47, 923–929. doi:10.1515/CCLM.2009.228
Huang, J., Zheng, L., Kong, H., Wang, F., Su, Y., and Xin, H. (2020). miR-139-5p Promotes the Proliferation and Invasion of Trophoblast Cells by Targeting sFlt-1 in Preeclampsia. Placenta 92, 37–43. doi:10.1016/j.placenta.2020.02.003
Huang, X., Wu, L., Zhang, G., Tang, R., and Zhou, X. (2019). Elevated MicroRNA-181a-5p Contributes to Trophoblast Dysfunction and Preeclampsia. Reprod. Sci. 26, 1121–1129. doi:10.1177/1933719118808916
Ishibashi, O., Ohkuchi, A., Ali, M. M., Kurashina, R., Luo, S.-S., Ishikawa, T., et al. (2012). Hydroxysteroid (17-β) Dehydrogenase 1Is Dysregulated byMir-210andMir-518cThat Are Aberrantly Expressed in Preeclamptic Placentas. Hypertension 59, 265–273. doi:10.1161/HYPERTENSIONAHA.111.180232
Jairajpuri, D. S., Malalla, Z. H., Mahmood, N., and Almawi, W. Y. (2017). Circulating microRNA Expression as Predictor of Preeclampsia and its Severity. Gene 627, 543–548. doi:10.1016/j.gene.2017.07.010
Jairajpuri, D. S., Malalla, Z. H., Sarray, S., and Mahmood, N. (2021). Analysis of Differential Expression of Hypoxia-Inducible microRNA-210 Gene Targets in Mild and Severe Preeclamptic Patients. Non-coding RNA Res. 6, 51–57. doi:10.1016/j.ncrna.2021.03.001
Jelena, M., Sopić, M., Joksić, I., Zmrzljak, U. P., Karadžov-Orlić, N., Košir, R., et al. (2020). Placenta-specific Plasma miR518b Is a Potential Biomarker for Preeclampsia. Clin. Biochem. 79, 28–33. doi:10.1016/j.clinbiochem.2020.02.012
Jiang, F., Li, J., Wu, G., Miao, Z., Lu, L., Ren, G., et al. (2015). Upregulation of microRNA-335 and microRNA-584 Contributes to the Pathogenesis of Severe Preeclampsia through Downregulation of Endothelial Nitric Oxide Synthase. Mol. Med. Rep. 12, 5383–5390. doi:10.3892/mmr.2015.4018
Jiang, L., Long, A., Tan, L., Hong, M., Wu, J., Cai, L., et al. (2017). Elevated microRNA-520g in Pre-eclampsia Inhibits Migration and Invasion of Trophoblasts. Placenta 51, 70–75. doi:10.1016/j.placenta.2017.02.001
Jin, M., Li, H., Xu, H., Huo, G., and Yao, Y. (2017). MicroRNA-20b Inhibits Trophoblast Cell Migration and Invasion by Targeting MMP-2. Int. J. Clin. Exp. Pathol. 10, 10901–10909.
Kamali Simsek, N., Benian, A., Sevgin, K., Ergun, Y., Goksever Celik, H., Karahuseyinoglu, S., et al. (2021). Microrna Analysis of Human Decidua Mesenchymal Stromal Cells from Preeclampsia Patients. Placenta 115, 12–19. doi:10.1016/j.placenta.2021.09.004
Keiser, S. D., Veillon, E. W., Parrish, M. R., Bennett, W., Cockrell, K., Fournier, L., et al. (2009). Effects of 17-Hydroxyprogesterone on Tumor Necrosis Factor- -Induced Hypertension during Pregnancy. Am. J. Hypertens. 22, 1120–1125. doi:10.1038/ajh.2009.149
Khaliq, O. P., Murugesan, S., Moodley, J., and Mackraj, I. (2018). Differential Expression of miRNAs Are Associated with the Insulin Signaling Pathway in Preeclampsia and Gestational Hypertension. Clin. Exp. Hypertens. 40, 744–751. doi:10.1080/10641963.2018.1431257
Kim, S., Lee, K.-S., Choi, S., Kim, J., Lee, D.-K., Park, M., et al. (2018). NF-κB-responsive miRNA-31-5p Elicits Endothelial Dysfunction Associated with Preeclampsia via Down-Regulation of Endothelial Nitric-Oxide Synthase. J. Biol. Chem. 293, 18989–19000. doi:10.1074/jbc.RA118.005197
Kim, S., Park, M., Kim, J.-Y., Kim, T., Hwang, J., Ha, K.-S., et al. (2020). Circulating miRNAs Associated with Dysregulated Vascular and Trophoblast Function as Target-Based Diagnostic Biomarkers for Preeclampsia. Cells 9, 2003. doi:10.3390/cells9092003
Kolkova, Z., Holubekova, V., Grendar, M., Nachajova, M., Zubor, P., Pribulova, T., et al. (2021). Association of Circulating Mirna Expression with Preeclampsia, its Onset, and Severity. Diagnostics 11, 476. doi:10.3390/diagnostics11030476
Korkes, H. A., De Oliveira, L., Sass, N., Salahuddin, S., Karumanchi, S. A., and Rajakumar, A. (2017). Relationship between Hypoxia and Downstream Pathogenic Pathways in Preeclampsia. Hypertens. Pregnancy 36, 145–150. doi:10.1080/10641955.2016.1259627
Kumar, P., Luo, Y., Tudela, C., Alexander, J. M., and Mendelson, C. R. (2013). The C-Myc-Regulated MicroRNA-17∼92 (miR-17∼92) and miR-106a∼363 Clusters Target hCYP19A1 and hGCM1 to Inhibit Human Trophoblast Differentiation. Mol. Cel. Biol. 33, 1782–1796. doi:10.1128/MCB.01228-12
Lalevée, S., Lapaire, O., and Bühler, M. (2014). MiR455 Is Linked to Hypoxia Signaling and Is Deregulated in Preeclampsia. Cell Death Dis 5, e1408. doi:10.1038/cddis.2014.368
Lasabová, Z., Vážan, M., Zibolenová, J., Švecová, I., and Lasabová, A. Z. (2015). Overexpression of miR-21 and miR-122 in Preeclamptic Placentas. Neuro Endocrinol. Lett. 36, 695–699.
Lázár, L., Nagy, B., Molvarec, A., Szarka, A., and Rigó, J. (2012). Role of Hsa-miR-325 in the Etiopathology of Preeclampsia. Mol. Med. Rep. 6, 597–600. doi:10.3892/mmr.2012.954
Lecarpentier, E., and Tsatsaris, V. (2016). Angiogenic Balance (sFlt-1/PlGF) and Preeclampsia. Ann. d'Endocrinologie 77, 97–100. doi:10.1016/j.ando.2016.04.007
Li, H., Ge, Q., Guo, L., and Lu, Z. (2013a). Maternal Plasma miRNAs Expression in Preeclamptic Pregnancies. Biomed. Res. Int. 2013, 1–9. doi:10.1155/2013/970265
Li, H. Q., Fan, J. J., Li, X. H., and Bao, D. (2020a). MiR-507 Inhibits the Growth and Invasion of Trophoblasts by Targeting CAMK4. Eur. Rev. Med. Pharmacol. Sci. 24, 5856–5862. doi:10.26355/eurrev_202006_21477
Li, J., Du, J., Wang, Z., Wang, C., Bai, J., and Zhang, S. (2018). Expression of miR-376 in B-lood of P-regnant W-omen with P-reeclampsia and its E-ffect on 25-hydroxyvitamin D. Exp. Ther. Med. 16, 1701–1706. doi:10.3892/etm.2018.6394
Li, J., Zhao, Y., Lu, Y., Ritchie, W., Grau, G., Vadas, M. A., et al. (2016). The Poly-Cistronic miR-23-27-24 Complexes Target Endothelial Cell Junctions: Differential Functional and Molecular Effects of miR-23a and miR-23b. Mol. Ther. - Nucleic Acids 5, e354. doi:10.1038/mtna.2016.62
Li, L., Hou, A., Gao, X., Zhang, J., Zhang, L., Wang, J., et al. (2017a). Lentivirus-mediated miR-23a Overexpression Induces Trophoblast Cell Apoptosis through Inhibiting X-Linked Inhibitor of Apoptosis. Biomed. Pharmacother. 94, 412–417. doi:10.1016/j.biopha.2017.07.082
Li, P., Guo, W., Du, L., Zhao, J., Wang, Y., Liu, L., et al. (2013b). MicroRNA-29b Contributes to Pre-eclampsia through its Effects on Apoptosis, Invasion and Angiogenesis of Trophoblast Cells. Clin. Sci. 124, 27–40. doi:10.1042/CS20120121
Li, Q., Han, Y., Xu, P., Yin, L., Si, Y., Zhang, C., et al. (2020b). Elevated microRNA-125b Inhibits Cytotrophoblast Invasion and Impairs Endothelial Cell Function in Preeclampsia. Cell Death Discov. 6, 35. doi:10.1038/s41420-020-0269-0
Li, Q., Long, A., Jiang, L., Cai, L., Xie, L., Gu, J. A., et al. (2015). Quantification of Preeclampsia-Related microRNAs in Maternal Serum. Biomed. Rep. 3, 792–796. doi:10.3892/br.2015.524
Li, Q., Pan, Z., Wang, X., Gao, Z., Ren, C., and Yang, W. (2014a). MiR-125b-1-3p Inhibits Trophoblast Cell Invasion by Targeting Sphingosine-1-Phosphate Receptor 1 in Preeclampsia. Biochem. Biophysical Res. Commun. 453, 57–63. doi:10.1016/j.bbrc.2014.09.059
Li, R., Wang, N., Xue, M., Long, W., Cheng, C., Mi, C., et al. (2019). A Potential Regulatory Network Among WDR86-AS1, miR-10b-3p, and LITAF Is Possibly Involved in Preeclampsia Pathogenesis. Cell Signal. 55, 40–52. doi:10.1016/j.cellsig.2018.12.006
Li, T., Zhou, B., He, Y., Liu, J., and Li, Y. (2020c). Expression and Clinical Diagnostic Value of miR-383 in Patients with Severe Preeclampsia. Cel Mol Biol (Noisy-le-grand) 66, 92–100. doi:10.14715/cmb/2020.66.3.14
Li, W., Yu, N., Fan, L., Chen, S.-H., and Wu, J.-L. (2020d). Circ_0063517 Acts as ceRNA, Targeting the miR-31-5p-ETBR axis to Regulate Angiogenesis of Vascular Endothelial Cells in Preeclampsia. Life Sci. 244, 117306. doi:10.1016/j.lfs.2020.117306
Li, X., Li, C., Dong, X., and Gou, W. (2014b). MicroRNA-155 Inhibits Migration of Trophoblast Cells and Contributes to the Pathogenesis of Severe Preeclampsia by Regulating Endothelial Nitric Oxide Synthase. Mol. Med. Rep. 10, 550–554. doi:10.3892/mmr.2014.2214
Li, X., Song, Y., Liu, D., Zhao, J., Xu, J., Ren, J., et al. (2017b). MiR-495 Promotes Senescence of Mesenchymal Stem Cells by Targeting Bmi-1. Cell. Physiol. Biochem. 42, 780–796. doi:10.1159/000478069
Liao, G., Cheng, D., Li, J., and Hu, S. (2021). Clinical Significance of microRNA-320a and Insulin-like Growth Factor-1 Receptor in Early-Onset Preeclampsia Patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 263, 164–170. doi:10.1016/j.ejogrb.2021.06.032
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P. A., et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. Plos Med. 6, e1000100. doi:10.1371/journal.pmed.1000100
Licini, C., Avellini, C., Picchiassi, E., Mensà, E., Fantone, S., Ramini, D., et al. (2021). Pre-eclampsia Predictive Ability of Maternal miR-125b: a Clinical and Experimental Study. Translational Res. 228, 13–27. doi:10.1016/j.trsl.2020.07.011
Lip, S. V., Boekschoten, M. V., Hooiveld, G. J., van Pampus, M. G., Scherjon, S. A., Plösch, T., et al. (2020). Early-onset Preeclampsia, Plasma microRNAs, and Endothelial Cell Function. Am. J. Obstet. Gynecol. 222, 497e1–497. e12. doi:10.1016/j.ajog.2019.11.1286
Lisabeth, E. M., Falivelli, G., and Pasquale, E. B. (2013). Eph Receptor Signaling and Ephrins. Cold Spring Harbor Perspect. Biol. 5, a009159. doi:10.1101/cshperspect.a009159
Liu, B., Liu, L., Cui, S., Qi, Y., and Wang, T. (2021). Expression and Significance of microRNA ‐126 and VCAM ‐1 in Placental Tissues of Women with Early‐onset Preeclampsia. J. Obstet. Gynaecol. Res. 47, 2042–2050. doi:10.1111/jog.14732
Liu, E., Liu, Z., Zhou, Y., Chen, M., Wang, L., and Li, J. (2019a). MicroRNA-142-3p I-nhibits T-rophoblast C-ell M-igration and I-nvasion by D-isrupting the TGF-β1/Smad3 S-ignaling P-athway. Mol. Med. Rep. 49, 3775–3782. doi:10.3892/mmr.2019.9997
Liu, F., Wu, K., Wu, W., Chen, Y., Wu, H., Wang, H., et al. (2018). miR-203 C-ontributes to P-re-eclampsia via I-nhibition of VEGFA E-xpression. Mol. Med. Rep. 17, 5627–5634. doi:10.3892/mmr.2018.8558
Liu, J. J., Zhang, L., Zhang, F. F., Luan, T., Yin, Z. M., Rui, C., et al. (2019b). Influence of miR-34a on Preeclampsia through the Notch Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 23, 923–931. doi:10.26355/eurrev_201902_16978
Liu, L., Wang, Y., Fan, H., Zhao, X., Liu, D., Hu, Y., et al. (2012). MicroRNA-181a Regulates Local Immune Balance by Inhibiting Proliferation and Immunosuppressive Properties of Mesenchymal Stem Cells. Stem Cells 30, 1756–1770. doi:10.1002/stem.1156
Liu, W., Liu, T., Jiang, F., Liu, C., Zhao, X., Gao, Y., et al. (2011). microRNA-155 Regulates Angiotensin II Type 1 Receptor Expression in Umbilical Vein Endothelial Cells from Severely Pre-eclamptic Pregnant Women. Int. J. Mol. Med. 27, 393–399. doi:10.3892/ijmm.2011.598
Liu, Z., Zhao, X., Shan, H., Gao, H., and Wang, P. (2019c). microRNA-520c-3p Suppresses NLRP3 Inflammasome Activation and Inflammatory cascade in Preeclampsia by Downregulating NLRP3. Inflamm. Res. 68, 643–654. doi:10.1007/s00011-019-01246-8
Lou, C. X., Zhou, X. T., Tian, Q. C., Xie, H. Q., and Zhang, J. Y. (2018). Low Expression of microRNA-21 Inhibits Trophoblast Cell Infiltration through Targeting PTEN. Eur. Rev. Med. Pharmacol. Sci. 22, 6181–6189. doi:10.26355/eurrev_201810_16023
Lu, T.-M., Lu, W., and Zhao, L.-J. (2017). MicroRNA-137 Affects Proliferation and Migration of Placenta Trophoblast Cells in Preeclampsia by Targeting ERRα. Reprod. Sci. 24, 85–96. doi:10.1177/1933719116650754
Luizon, M. R., Conceição, I. M. C. A., Viana-Mattioli, S., Caldeira-Dias, M., Cavalli, R. C., and Sandrim, V. C. (2021). Circulating MicroRNAs in the Second Trimester from Pregnant Women Who Subsequently Developed Preeclampsia: Potential Candidates as Predictive Biomarkers and Pathway Analysis for Target Genes of miR-204-5p. Front. Physiol. 12, 678184. doi:10.3389/fphys.2021.678184
Luo, R., Shao, X., Xu, P., Liu, Y., Wang, Y., Zhao, Y., et al. (2014). MicroRNA-210 Contributes to Preeclampsia by Downregulating Potassium Channel Modulatory Factor 1. Hypertension 64, 839–845. doi:10.1161/HYPERTENSIONAHA.114.03530
Luo, S., Cao, N., Tang, Y., and Gu, W. (2017a). Identification of Key microRNAs and Genes in Preeclampsia by Bioinformatics Analysis. PLoS One 12, e0178549. doi:10.1371/journal.pone.0178549
Luo, S., Li, H., Cao, N., Tang, Y., and Gu, W. (2017b). MicroRNA-148a Affects Functions of Placental Trophoblast Cells in Preeclampsia by Regulating HLA-G. Int. J. Clin. Exp. Pathol. 10, 5205–5212.
Luque, A., Farwati, A., Crovetto, F., Crispi, F., Figueras, F., Gratacós, E., et al. (2014). Usefulness of Circulating microRNAs for the Prediction of Early Preeclampsia at First-Trimester of Pregnancy. Sci. Rep. 4, 4882. doi:10.1038/srep04882
Lv, Y., Lu, X., Li, C., Fan, Y., Ji, X., Long, W., et al. (2019). miR-145-5p Promotes Trophoblast Cell Growth and Invasion by Targeting FLT1. Life Sci. 239, 117008. doi:10.1016/j.lfs.2019.117008
Lykoudi, A., Kolialexi, A., Lambrou, G. I., Braoudaki, M., Siristatidis, C., Papaioanou, G. K., et al. (2018). Dysregulated Placental microRNAs in Early and Late Onset Preeclampsia. Placenta 61, 24–32. doi:10.1016/j.placenta.2017.11.005
Ma, H. Y., Cu, W., Sun, Y. H., and Chen, X. (2020). MiRNA-203a-3p Inhibits Inflammatory Response in Preeclampsia through Regulating IL24. Eur. Rev. Med. Pharmacol. Sci. 24, 5223–5230. doi:10.26355/eurrev_202005_21304
Ma, R., Lu, Y., Dou, C., and Gu, Q. (2019). Clinical Significance of miR-133a and miR-206 in Pregnant Women with Preeclampsia and Correlation with Pregnancy Outcomes. Int. J. Clin. Exp. Med. 12, 7383–7391.
Mao, Y., Hou, B., Shan, L., Sun, X., and Wang, L. (2021). Aberrantly Up-Regulated miR-142-3p Inhibited the Proliferation and Invasion of Trophoblast Cells by Regulating FOXM1. Placenta 104, 253–260. doi:10.1016/j.placenta.2021.01.002
Martinez-Fierro, M. L., Carrillo-Arriaga, J. G., Luevano, M., Lugo-Trampe, A., Delgado-Enciso, I., Rodriguez-Sanchez, I. P., et al. (2019). Serum Levels of miR-628-3p and miR-628-5p during the Early Pregnancy Are Increased in Women Who Subsequently Develop Preeclampsia. Pregnancy Hypertens. 16, 120–125. doi:10.1016/j.preghy.2019.03.012
Martinez-Fierro, M. L., and Garza-Veloz, I. (2021). Analysis of Circulating Microrna Signatures and Preeclampsia Development. Cells 10, 1003. doi:10.3390/cells10051003
Martinez-Fierro, M. L., Garza-Veloz, I., Gutierrez-Arteaga, C., Delgado-Enciso, I., Barbosa-Cisneros, O. Y., Flores-Morales, V., et al. (2018). Circulating Levels of Specific Members of Chromosome 19 microRNA Cluster Are Associated with Preeclampsia Development. Arch. Gynecol. Obstet. 297, 365–371. doi:10.1007/s00404-017-4611-6
Mavreli, D., Lykoudi, A., Lambrou, G., Papaioannou, G., Vrachnis, N., Kalantaridou, S., et al. (2020). Deep Sequencing Identified Dysregulated Circulating MicroRNAs in Late Onset Preeclampsia. In Vivo 34, 2317–2324. doi:10.21873/invivo.12044
Mayor-Lynn, K., Toloubeydokhti, T., Cruz, A. C., and Chegini, N. (2011). Expression Profile of microRNAs and mRNAs in Human Placentas from Pregnancies Complicated by Preeclampsia and Preterm Labor. Reprod. Sci. 18, 46–56. doi:10.1177/1933719110374115
McElrath, T. F., Cantonwine, D. E., Gray, K. J., Mirzakhani, H., Doss, R. C., Khaja, N., et al. (2020). Late First Trimester Circulating Microparticle Proteins Predict the Risk of Preeclampsia. Sci. Rep. 10, 17353. doi:10.1038/s41598-020-74078-w
Mei, Z., Huang, B., Zhang, Y., Qian, X., Mo, Y., and Deng, N. (2019). Histone Deacetylase 6 Negatively Regulated microRNA-199a-5p Induces the Occurrence of Preeclampsia by Targeting VEGFA In Vitro. Biomed. Pharmacother. 114, 108805. doi:10.1016/j.biopha.2019.108805
Meng, H.-X., Xu, L.-N., Jing, G., Qian, L., and Qi, M.-G. (2017). MiR-223 Promotes Trophoblast Cell Survival and Invasion by Targeting STAT3 in Preeclampsia. Int. J. Clin. Exp. Med. 10, 6577–6585.
Miura, K., Higashijima, A., Murakami, Y., Tsukamoto, O., Hasegawa, Y., Abe, S., et al. (2015). Circulating Chromosome 19 miRNA Cluster microRNAs in Pregnant Women with Severe Pre-eclampsia. J. Obstet. Gynaecol. Res. 41, 1526–1532. doi:10.1111/jog.12749
Motawi, T. M. k., Sabry, D., Maurice, N. W., and Rizk, S. M. (2018). Role of Mesenchymal Stem Cells Exosomes Derived microRNAs; miR-136, miR-494 and miR-495 in Pre-eclampsia Diagnosis and Evaluation. Arch. Biochem. Biophys. 659, 13–21. doi:10.1016/j.abb.2018.09.023
Mouillet, J.-F., Ouyang, Y., Coyne, C. B., and Sadovsky, Y. (2015). MicroRNAs in Placental Health and Disease. Am. J. Obstet. Gynecol. 213, S163–S172. doi:10.1016/j.ajog.2015.05.057
Munaut, C., Tebache, L., Blacher, S., Noël, A., Nisolle, M., and Chantraine, F. (2016). Dysregulated Circulating miRNAs in Preeclampsia. Biomed. Rep. 5, 686–692. doi:10.3892/br.2016.779
Muralimanoharan, S., Maloyan, A., Mele, J., Guo, C., Myatt, L. G., and Myatt, L. (2012). MIR-210 Modulates Mitochondrial Respiration in Placenta with Preeclampsia. Placenta 33, 816–823. doi:10.1016/j.placenta.2012.07.002
Murphy, M. S.-Q., Casselman, R. C., Tayade, C., and Smith, G. N. (2015). Differential Expression of Plasma microRNA in Preeclamptic Patients at Delivery and 1 Year Postpartum. Am. J. Obstet. Gynecol. 213, 367e1–367. e9. doi:10.1016/j.ajog.2015.05.013
Nejad, R. M. A., Saeidi, K., Gharbi, S., Salari, Z., and Saleh-Gohari, N. (2019). Quantification of Circulating miR-517c-3p and miR-210-3p Levels in Preeclampsia. Pregnancy Hypertens. 16, 75–78. doi:10.1016/j.preghy.2019.03.004
Niu, Z.-r., Han, T., SunluanLuanxia, X., Luan, L.-x., Gou, W.-l., and Zhu, X.-m. (2018). MicroRNA-30a-3p Is Overexpressed in the Placentas of Patients with Preeclampsia and Affects Trophoblast Invasion and Apoptosis by its Effects on IGF-1. Am. J. Obstet. Gynecol. 218, 249e1–249. e12. doi:10.1016/j.ajog.2017.11.568
Nizyaeva, N. V., Kulikova, G. V., Nagovitsyna, M. N., Kan, N. E., Prozorovskaya, K. N., and Shchegolev, A. I. (2018). Change in OncomicroRNA Expression in the Placenta during Preeclampsia. Bull. Exp. Biol. Med. 165, 793–797. doi:10.1007/s10517-018-4267-7
Nizyaeva, N. V., Kulikova, G. V., Nagovitsyna, M. N., Kan, N. E., Prozorovskaya, K. N., Shchegolev, A. I., et al. (2017). Expression of MicroRNA-146a and MicroRNA-155 in Placental Villi in Early- and Late-Onset Preeclampsia. Bull. Exp. Biol. Med. 163, 394–399. doi:10.1007/s10517-017-3812-0
Ospina-Prieto, S., Chaiwangyen, W., Herrmann, J., Groten, T., Schleussner, E., Markert, U. R., et al. (2016). MicroRNA-141 Is Upregulated in Preeclamptic Placentae and Regulates Trophoblast Invasion and Intercellular Communication. Translational Res. 172, 61–72. doi:10.1016/j.trsl.2016.02.012
Pasquale, E. B. (2005). Eph Receptor Signalling Casts a Wide Net on Cell Behaviour. Nat. Rev. Mol. Cel Biol. 6, 462–475. doi:10.1038/nrm1662
Peng, P., Song, H., Xie, C., Zheng, W., Ma, H., Xin, D., et al. (2021). miR-146a-5p-mediated Suppression on Trophoblast Cell Progression and Epithelial-Mesenchymal Transition in Preeclampsia. Biol. Res. 54, 30. doi:10.1186/s40659-021-00351-5
Pillay, P., Vatish, M., Duarte, R., Moodley, J., and Mackraj, I. (2019). Exosomal microRNA Profiling in Early and Late Onset Preeclamptic Pregnant Women Reflects Pathophysiology. Ijn 14, 5637–5657. doi:10.2147/IJN.S208865
Pineles, B. L., Romero, R., Montenegro, D., Tarca, A. L., Han, Y. M., Kim, Y. M., et al. (2007). Distinct Subsets of microRNAs Are Expressed Differentially in the Human Placentas of Patients with Preeclampsia. Am. J. Obstet. Gynecol. 196, 261e1–261. e6. doi:10.1016/j.ajog.2007.01.008
Qian, S., and Liu, R. (2019). miR-30b Facilitates Preeclampsia through Targeting MXRA5 to Inhibit the Viability, Invasion and Apoptosis of Placental Trophoblast Cells. Int. J. Clin. Exp. Pathol. 12, 4057–4065.
Salomon, C., Guanzon, D., Scholz-Romero, K., Longo, S., Correa, P., Illanes, S. E., et al. (2017). Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs across Gestation. J. Clin. Endocrinol. Metab. 102, 3182–3194. doi:10.1210/jc.2017-00672
Sandrim, V. C., Dias, M. C., Bovolato, A. L. d. C., Tanus‐Santos, J. E., Deffune, E., and Cavalli, R. C. (2016a). Plasma from Pre‐eclamptic Patients Induces the Expression of the Anti‐angiogenic miR‐195‐5p in Endothelial Cells. J. Cel. Mol. Med. 20, 1198–1200. doi:10.1111/jcmm.12767
Sandrim, V., Luizon, M., Palei, A., Tanus-Santos, J., and Cavalli, R. (2016b). Circulating microRNA Expression Profiles in Pre-eclampsia: Evidence of Increased miR-885-5p Levels. Bjog: Int. J. Obstet. Gy 123, 2120–2128. doi:10.1111/1471-0528.13903
Sekar, D., Lakshmanan, G., Mani, P., and Biruntha, M. (2019). Methylation-dependent Circulating microRNA 510 in Preeclampsia Patients. Hypertens. Res. 42, 1647–1648. doi:10.1038/s41440-019-0269-8
Shao, X., Liu, Y., Liu, M., Wang, Y., Yan, L., Wang, H., et al. (2017). Testosterone Represses Estrogen Signaling by Upregulating miR-22. Hypertension 69, 721–730. doi:10.1161/HYPERTENSIONAHA.116.08468
Sheikh, A. M., Small, H. Y., Currie, G., and Delles, C. (2016). Systematic Review of Micro-RNA Expression in Pre-eclampsia Identifies a Number of Common Pathways Associated with the Disease. PLoS One 11, e0160808. doi:10.1371/journal.pone.0160808
Shen, L., Li, Y., Li, R., Diao, Z., Yany, M., Wu, M., et al. (2018). Placenta-associated S-erum E-xosomal miR-155 D-erived from P-atients with P-reeclampsia I-nhibits eNOS E-xpression in H-uman U-mbilical V-ein E-ndothelial C-ells. Int. J. Mol. Med. 41, 1731–1739. doi:10.3892/ijmm.2018.3367
Sheng, C., Zhao, Y., and Zhu, L. (2020). Down-regulation of EDN1 Gene Expression by Circulating miR-206 Is Associated with Risk of Preeclampsia. Medicine (Baltimore) 99, e20319. doi:10.1097/MD.0000000000020319
Shi, Z., She, K., Li, H., Yuan, X., Han, X., and Wang, Y. (2019). MicroRNA-454 Contributes to Sustaining the Proliferation and Invasion of Trophoblast Cells through Inhibiting Nodal/ALK7 Signaling in Pre-eclampsia. Chemico-Biological Interactions 298, 8–14. doi:10.1016/j.cbi.2018.10.012
Singh, K., Williams, J., Brown, J., Wang, E. T., Lee, B., Gonzalez, T. L., et al. (2017). Up-regulation of microRNA-202-3p in First Trimester Placenta of Pregnancies Destined to Develop Severe Preeclampsia, a Pilot Study. Pregnancy Hypertens. 10, 7–9. doi:10.1016/j.preghy.2017.04.002
Song, H., Wang, X., Li, J. C., and Lv, Y. H. (2020). MiR-655-3p Inhibits Growth and Invasiveness of Trophoblasts via Targeting PBX3 and Thus Deteriorates Preeclampsia. Eur. Rev. Med. Pharmacol. Sci. 24, 10346–10351. doi:10.26355/eurrev_202010_23383
Spence, T., Allsopp, P. J., Yeates, A. J., Mulhern, M. S., Strain, J. J., and McSorley, E. M. (2021). Maternal Serum Cytokine Concentrations in Healthy Pregnancy and Preeclampsia. J. Pregnancy 2021, 1–33. doi:10.1155/2021/6649608
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 283, 2008–2012. doi:10.1001/jama.283.15.2008
Sun, M., Chen, H., Liu, J., Tong, C., and Meng, T. (2015). MicroRNA-34a Inhibits Human Trophoblast Cell Invasion by Targeting MYC. BMC Cel Biol 16, 21. doi:10.1186/s12860-015-0068-2
Tang, Q., Gui, J., Wu, X., and Wu, W. (2019). Downregulation of miR-424 in Placenta Is Associated with Severe Preeclampsia. Pregnancy Hypertens. 17, 109–112. doi:10.1016/j.preghy.2019.05.017
Tao, J., Xia, L.-Z., Liang, L., Chen, Y., Wei, D., Meng, J., et al. (2020). MiR-124-3p Promotes Trophoblast Cell HTR-8/SVneo Pyroptosis by Targeting Placental Growth Factor. Placenta 101, 176–184. doi:10.1016/j.placenta.2020.08.011
Taravati, A., and Tohidi, F. (2018). Comprehensive Analysis of Oxidative Stress Markers and Antioxidants Status in Preeclampsia. Taiwanese J. Obstet. Gynecol. 57, 779–790. doi:10.1016/j.tjog.2018.10.002
Timofeeva, A. V., Gusar, V. A., Kan, N. E., Prozorovskaya, K. N., Karapetyan, A. O., Bayev, O. R., et al. (2018). Identification of Potential Early Biomarkers of Preeclampsia. Placenta 61, 61–71. doi:10.1016/j.placenta.2017.11.011
Truong, G., Guanzon, D., Kinhal, V., Elfeky, O., Lai, A., Longo, S., et al. (2017). Oxygen Tension Regulates the miRNA Profile and Bioactivity of Exosomes Released from Extravillous Trophoblast Cells - Liquid Biopsies for Monitoring Complications of Pregnancy. PLoS One 12, e0174514. doi:10.1371/journal.pone.0174514
Tsai, P.-Y., Li, S.-H., Chen, W.-N., Tsai, H.-L., and Su, M.-T. (2017). Differential miR-346 and miR-582-3p Expression in Association with Selected Maternal and Fetal Complications. Ijms 18, 1570. doi:10.3390/ijms18071570
Ura, B., Feriotto, G., Monasta, L., Bilel, S., Zweyer, M., and Celeghini, C. (2014). Potential Role of Circulating microRNAs as Early Markers of Preeclampsia. Taiwanese J. Obstet. Gynecol. 53, 232–234. doi:10.1016/j.tjog.2014.03.001
Vashukova, E. S., Glotov, A. S., Fedotov, P. V., Efimova, O. A., Pakin, V. S., Mozgovaya, E. V., et al. (2016). Placental microRNA Expression in Pregnancies Complicated by Superimposed Pre-eclampsia on Chronic Hypertension. Mol. Med. Rep. 14, 22–32. doi:10.3892/mmr.2016.5268
Wang, C. Y., Tsai, P. Y., Chen, T. Y., Tsai, H. L., Kuo, P. L., and Su, M. T. (2019a). Elevated miR‐200a and miR‐141 Inhibit Endocrine Gland‐derived Vascular Endothelial Growth Factor Expression and Ciliogenesis in Preeclampsia. J. Physiol. 597, 3069–3083. doi:10.1113/JP277704
Wang, F., and Yan, J. (2018). MicroRNA-454 Is Involved in Regulating Trophoblast Cell Proliferation, Apoptosis, and Invasion in Preeclampsia by Modulating the Expression of Ephrin Receptor B4. Biomed. Pharmacother. 107, 746–753. doi:10.1016/j.biopha.2018.08.055
Wang, H., Zhang, L., Guo, X., Bai, Y., Li, Y.-X., Sha, J., et al. (2018a). MiR-195 Modulates Oxidative Stress-Induced Apoptosis and Mitochondrial Energy Production in Human Trophoblasts via Flavin Adenine Dinucleotide-dependent Oxidoreductase Domain-Containing Protein 1 and Pyruvate Dehydrogenase Phosphatase Regulatory Subunit. J. Hypertens. 36, 306–318. doi:10.1097/HJH.0000000000001529
Wang, N., Feng, Y., Xu, J., Zou, J., Chen, M., He, Y., et al. (2018b). miR-362-3p Regulates Cell Proliferation, Migration and Invasion of Trophoblastic Cells under Hypoxia through Targeting Pax3. Biomed. Pharmacother. 99, 462–468. doi:10.1016/j.biopha.2018.01.089
Wang, N., Li, R., and Xue, M. (2019b). Potential Regulatory Network in the PSG10P/miR-19a-3p/IL1RAP Pathway Is Possibly Involved in Preeclampsia Pathogenesis. J. Cel. Mol. Med. 23, 852–864. doi:10.1111/jcmm.13985
Wang, R., Liu, W., Liu, X., Liu, X., Tao, H., Wu, D., et al. (2019c). MicroRNA‐210 Regulates Human Trophoblast Cell Line HTR‐8/SVneo Function by Attenuating Notch1 Expression: Implications for the Role of microRNA‐210 in Pre‐eclampsia. Mol. Reprod. Dev. 86, 896–907. doi:10.1002/mrd.23154
Wang, S., Wang, X., Weng, Z., Zhang, S., Ning, H., and Li, B. (2017). Expression and Role of microRNA 18b and Hypoxia Inducible Factor-1α in P-lacental T-issues of P-reeclampsia P-atients. Exp. Ther. Med. 14, 4554–4560. doi:10.3892/etm.2017.5067
Wang, W., Feng, L., Zhang, H., Hachy, S., Satohisa, S., Laurent, L. C., et al. (2012a). Preeclampsia up-regulates angiogenesis-associated microRNA (i.e., miR-17, -20a, and -20b) that target ephrin-B2 and EPHB4 in human placenta. J. Clin. Endocrinol. Metab. 97, E1051–E1059. doi:10.1210/jc.2011-3131
Wang, Y., Cheng, K., Zhou, W., Liu, H., Yang, T., Hou, P., et al. (2019d). miR-141-5p Regulate ATF2 via Effecting MAPK1/ERK2 Signaling to Promote Preeclampsia. Biomed. Pharmacother. 115, 108953. doi:10.1016/j.biopha.2019.108953
Wang, Y., Dong, Q., Gu, Y., and Groome, L. J. (2016a). Up-regulation of miR-203 Expression Induces Endothelial Inflammatory Response: Potential Role in Preeclampsia. Am. J. Reprod. Immunol. 76, 482–490. doi:10.1111/aji.12589
Wang, Y., Fan, H., Zhao, G., Liu, D., Du, L., Wang, Z., et al. (2012b). MiR-16 Inhibits the Proliferation and Angiogenesis-Regulating Potential of Mesenchymal Stem Cells in Severe Pre-eclampsia. FEBS J. 279, 4510–4524. doi:10.1111/febs.12037
Wang, Y., Lumbers, E. R., Arthurs, A. L., De Meaultsart, C. C., Mathe, A., Avery-Kiejda, K. A., et al. (2018c). Regulation of the Human Placental (Pro)renin Receptor-Prorenin-Angiotensin System by microRNAs. Mol. Hum. Reprod. 24, 453–464. doi:10.1093/molehr/gay031
Wang, Y. P., Zhao, P., Liu, J. Y., Liu, S. M., and Wang, Y. X. (2020). MicroRNA-132 Stimulates the Growth and Invasiveness of Trophoblasts by Targeting DAPK-1. Eur. Rev. Med. Pharmacol. Sci. 24, 9837–9843. doi:10.26355/eurrev_202010_23193
Wang, Y., Yang, X., Yang, Y., Wang, W., Zhao, M., Liu, H., et al. (2016b). High-throughput Deep Screening and Identification of Four Peripheral Leucocyte microRNAs as Novel Potential Combination Biomarkers for Preeclampsia. J. Perinatol. 36, 263–267. doi:10.1038/jp.2015.192
Wang, Y., Zhang, Y., Wang, H., Wang, J., Zhang, Y., Wang, Y., et al. (2014). Aberrantly Up-Regulated miR-20a in Pre-eclampsic Placenta Compromised the Proliferative and Invasive Behaviors of Trophoblast Cells by Targeting Forkhead Box Protein A1. Int. J. Biol. Sci. 10, 973–982. doi:10.7150/ijbs.9088
Wang, Y., and Zhao, S. (2010). Vascular Biology of the Placenta. Review. doi:10.4199/C00016ED1V01Y201008ISP009
Wang, Z., Wang, P., Wang, Z., Qin, Z., Xiu, X., Xu, D., et al. (2019e). MiRNA‐548c‐5p Downregulates Inflammatory Response in Preeclampsia via Targeting PTPRO. J. Cel. Physiol. 234, 11149–11155. doi:10.1002/jcp.27758
Weedon-Fekjær, M. S., Sheng, Y., Sugulle, M., Johnsen, G. M., Herse, F., Redman, C. W., et al. (2014). Placental miR-1301 Is Dysregulated in Early-Onset Preeclampsia and Inversely Correlated with Maternal Circulating Leptin. Placenta 35, 709–717. doi:10.1016/j.placenta.2014.07.002
Wei, J., Blenkiron, C., Tsai, P., James, J. L., Chen, Q., Stone, P. R., et al. (2017). Placental Trophoblast Debris Mediated Feto-Maternal Signalling via Small RNA Delivery: Implications for Preeclampsia. Sci. Rep. 7, 14681. doi:10.1038/s41598-017-14180-8
Wells, G., Shea, B., O’connell, D., Peterson, J., and Welch, V. (2014). The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses.
Whigham, C.-A., MacDonald, T. M., Walker, S. P., Hiscock, R., Hannan, N. J., Pritchard, N., et al. (2020). MicroRNAs 363 and 149 Are Differentially Expressed in the Maternal Circulation Preceding a Diagnosis of Preeclampsia. Sci. Rep. 10, 18077. doi:10.1038/s41598-020-73783-w
Winger, E. E., Reed, J. L., and Ji, X. (2014). First Trimester Pbmc Microrna Predicts Adverse Pregnancy Outcome. Am. J. Reprod. Immunol. 72, 515–526. doi:10.1111/aji.12287
Winger, E. E., Reed, J. L., and Ji, X. (2015). First-trimester Maternal Cell microRNA Is a superior Pregnancy Marker to Immunological Testing for Predicting Adverse Pregnancy Outcome. J. Reprod. Immunol. 110, 22–35. doi:10.1016/j.jri.2015.03.005
Winger, E. E., Reed, J. L., Ji, X., and Nicolaides, K. (2018). Peripheral Blood Cell microRNA Quantification during the First Trimester Predicts Preeclampsia: Proof of Concept. PLoS One 13, e0190654. doi:10.1371/journal.pone.0190654
Witcher, P. M. (2018). Preeclampsia: Acute Complications and Management Priorities. AACN Adv. Crit. Care 29, 316–326. doi:10.4037/aacnacc2018710
Witvrouwen, I., Mannaerts, D., Ratajczak, J., Boeren, E., Faes, E., van Craenenbroeck, A. H., et al. (2021). MicroRNAs Targeting VEGF Are Related to Vascular Dysfunction in Preeclampsia. Biosci. Rep. 41, BSR20210874. doi:10.1042/BSR20210874
Wu, D., Shi, L., Hong, L., Chen, X., and Cen, H. (2020a). MiR-135a-5p Promotes the Migration and Invasion of Trophoblast Cells in Preeclampsia by Targeting β-TrCP. Placenta 99, 63–69. doi:10.1016/j.placenta.2020.07.028
Wu, G.-M., Jin, Y., Cao, Y.-M., and Li, J.-Y. (2020b). The Diagnostic Value and Regulatory Mechanism of miR-200a Targeting ZEB1 in Pregnancy-Induced Hypertension. Hypertens. Pregnancy 39, 243–251. doi:10.1080/10641955.2020.1757700
Wu, L., Zhou, H., Lin, H., Qi, J., Zhu, C., Gao, Z., et al. (2012). Circulating microRNAs Are Elevated in Plasma from Severe Preeclamptic Pregnancies. Reproduction 143, 389–397. doi:10.1530/REP-11-0304
Xiao, J., Tao, T., Yin, Y., Zhao, L., Yang, L., and Hu, L. (2017). miR-144 May Regulate the Proliferation, Migration and Invasion of Trophoblastic Cells through Targeting PTEN in Preeclampsia. Biomed. Pharmacother. 94, 341–353. doi:10.1016/j.biopha.2017.07.130
Xiaobo, Z., Qizhi, H., Zhiping, W., and Tao, D. (2019). Down-regulated miR-149-5p Contributes to Preeclampsia via Modulating Endoglin Expression. Pregnancy Hypertens. 15, 201–208. doi:10.1016/j.preghy.2019.01.002
Xie, N., Jia, Z., and Li, L. (2019). miR-320a U-pregulation C-ontributes to the D-evelopment of P-reeclampsia by I-nhibiting the G-rowth and I-nvasion of T-rophoblast C-ells by T-argeting I-nterleukin 4. Mol. Med. Rep. 20, 3256–3264. doi:10.3892/mmr.2019.10574
Xu, H., and Zhang, X. (2017). Abnormal Expression of microRNA-370 Regulates Endoglin Expression in Preeclampsia. Int. J. Clin. Exp. Med. 10, 4943–4949.
Xu, J., Gu, Y., Lewis, D. F., Cooper, D. B., McCathran, C. E., and Wang, Y. (2019). Downregulation of Vitamin D Receptor and miR‐126‐3p Expression Contributes to Increased Endothelial Inflammatory Response in Preeclampsia. Am. J. Reprod. Immunol. 82, e13172. doi:10.1111/aji.13172
Xu, P., Zhao, Y., Liu, M., Wang, Y., Wang, H., Li, Y.-x., et al. (2014). Variations of MicroRNAs in Human Placentas and Plasma from Preeclamptic Pregnancy. Hypertension 63, 1276–1284. doi:10.1161/HYPERTENSIONAHA.113.02647
Xu, Q., Song, Y., and Lu, L. (2021). Overexpression of Let‐7d Explains Down‐regulated KDM3A and ENO2 in the Pathogenesis of Preeclampsia. J. Cel. Mol. Med. 25, 8127–8139. doi:10.1111/jcmm.16299
Xue, F., Yang, J., Li, Q., and Zhou, H. (2019). Down-regulation of microRNA-34a-5p Promotes Trophoblast Cell Migration and Invasion via Targetting Smad4. Biosci. Rep. 39, BSR20181631. doi:10.1042/BSR20181631
Xueya, Z., Yamei, L., Sha, C., Dan, C., Hong, S., Xingyu, Y., et al. (2020). Exosomal Encapsulation of miR-125a-5p Inhibited Trophoblast Cell Migration and Proliferation by Regulating the Expression of VEGFA in Preeclampsia. Biochem. Biophysical Res. Commun. 525, 646–653. doi:10.1016/j.bbrc.2020.02.137
Yan, T., Liu, Y., Cui, K., Hu, B., Wang, F., and Zou, L. (2013a). MicroRNA-126 Regulates EPCs Function: Implications for a Role of miR-126 in Preeclampsia. J. Cel. Biochem. 114, 2148–2159. doi:10.1002/jcb.24563
Yan, X., Liang, H., Deng, T., Zhu, K., Zhang, S., Wang, N., et al. (2013b). The Identification of Novel Targets of miR-16 and Characterization of Their Biological Functions in Cancer Cells. Mol. Cancer 12, 1–11. doi:10.1186/1476-4598-12-92
Yang, H.-l., Zhang, H.-z., Meng, F.-r., Han, S.-y., and Zhang, M. (2019a). Differential Expression of microRNA-411 and 376c Is Associated with Hypertension in Pregnancy. Braz. J. Med. Biol. Res. 52, e7546. doi:10.1590/1414-431X20197546
Yang, Q., Lu, J., Wang, S., Li, H., Ge, Q., and Lu, Z. (2011). Application of Next-Generation Sequencing Technology to Profile the Circulating microRNAs in the Serum of Preeclampsia versus normal Pregnant Women. Clinica Chim. Acta 412, 2167–2173. doi:10.1016/j.cca.2011.07.029
Yang, S., Li, H., Ge, Q., Guo, L., and Chen, F. (2015). Deregulated microRNA Species in the Plasma and Placenta of Patients with Preeclampsia. Mol. Med. Rep. 12, 527–534. doi:10.3892/mmr.2015.3414
Yang, W., Wang, A., Zhao, C., Li, Q., Pan, Z., Han, X., et al. (2016). MiR-125b Enhances IL-8 Production in Early-Onset Severe Preeclampsia by Targeting Sphingosine-1-Phosphate Lyase 1. PLoS One 11, e0166940. doi:10.1371/journal.pone.0166940
Yang, X., and Guo, F. (2019). miR-342-3p S-uppresses C-ell M-igration and I-nvasion in P-reeclampsia by T-argeting P-latelet-derived G-rowth F-actor R-eceptor α. Mol. Med. Rep. 20, 1772–1780. doi:10.3892/mmr.2019.10372
Yang, X., and Meng, T. (2019). MicroRNA-431 Affects Trophoblast Migration and Invasion by Targeting ZEB1 in Preeclampsia. Gene 683, 225–232. doi:10.1016/j.gene.2018.10.015
Yang, X., and Meng, T. (2020). miR‐215‐5p Decreases Migration and Invasion of Trophoblast Cells through Regulating CDC6 in Preeclampsia. Cell Biochem. Funct. 38, 472–479. doi:10.1002/cbf.3492
Yang, X., Zhang, J., and Ding, Y. (2017). Association of microRNA-155, Interleukin 17A, and Proteinuria in Preeclampsia. Med. (United States 96, e6509. doi:10.1097/MD.0000000000006509
Yang, Y., Li, H., Ma, Y., Zhu, X., Zhang, S., and Li, J. (2019b). MiR-221-3p Is Down-Regulated in Preeclampsia and Affects Trophoblast Growth, Invasion and Migration Partly via Targeting Thrombospondin 2. Biomed. Pharmacother. 109, 127–134. doi:10.1016/j.biopha.2018.10.009
Yang, Y., Xi, L., Ma, Y., Zhu, X., Chen, R., Luan, L., et al. (2019c). The lncRNA Small Nucleolar RNA Host Gene 5 Regulates Trophoblast Cell Proliferation, Invasion, and Migration via Modulating miR‐26a‐5p/N‐cadherin axis. J. Cel. Biochem. 120, 3173–3184. doi:10.1002/jcb.27583
Yang, Z., Shan, N., Deng, Q., Wang, Y., Hou, Y., Mei, J., et al. (2021). Extracellular Vesicle‐derived microRNA‐18b Ameliorates Preeclampsia by Enhancing Trophoblast Proliferation and Migration via Notch2/TIM3/mTORC1 axis. J. Cel. Mol. Med. 25, 4583–4595. doi:10.1111/jcmm.16234
Youssef, H. M. G., and Marei, E. S. (2019). Association of MicroRNA-210 and MicroRNA-155 with Severity of Preeclampsia. Pregnancy Hypertens. 17, 49–53. doi:10.1016/j.preghy.2019.05.010
Yu, Z., Zhang, Y., Zheng, H., Gao, Q., and Wang, H. (2021). LncRNA SNHG16 Regulates Trophoblast Functions by the miR-218-5p/LASP1 axis. J. Mol. Histol. 52, 1021–1033. doi:10.1007/s10735-021-09985-x
Yuan, Y., Wang, X., Sun, Q., Dai, X., and Cai, Y. (2020). MicroRNA‐16 Is Involved in the Pathogenesis of Pre‐eclampsia via Regulation of Notch2. J. Cel. Physiol. 235, 4530–4544. doi:10.1002/jcp.29330
Zhang, C., Li, Q., Ren, N., Li, C., Wang, X., Xie, M., et al. (2015). Placental miR-106a∼363 Cluster Is Dysregulated in Preeclamptic Placenta. Placenta 36, 250–252. doi:10.1016/j.placenta.2014.11.020
Zhang, H. J., Zhang, Y. N., and Teng, Z. Y. (2019a). Downregulation of miR-16 P-rotects H9c2(2-1) C-ells against H-ypoxia/reoxygenation D-amage by T-argeting CIAPIN1 and R-egulating the NF-κB P-athway. Mol. Med. Rep. 20, 3113–3122. doi:10.3892/mmr.2019.10568
Zhang, W. M., Cao, P., Xin, L., Zhang, Y., Liu, Z., Yao, N., et al. (2019b). Effect of miR-133 on Apoptosis of Trophoblasts in Human Placenta Tissues via Rho/ROCK Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 23, 10600–10608. doi:10.26355/eurrev_201912_19755
Zhang, Y., Diao, Z., Su, L., Sun, H., Li, R., Cui, H., et al. (2010). MicroRNA-155 Contributes to Preeclampsia by Down-Regulating CYR61. Am. J. Obstet. Gynecol. 202, 466e1–466. e7. doi:10.1016/j.ajog.2010.01.057
Zhang, Y., Fei, M., Xue, G., Zhou, Q., Jia, Y., Li, L., et al. (2012). Elevated Levels of Hypoxia-Inducible microRNA-210 in Pre-eclampsia: New Insights into Molecular Mechanisms for the Disease. J. Cel. Mol. Med. 16, 249–259. doi:10.1111/j.1582-4934.2011.01291.x
Zhang, Y., Huang, G., Zhang, Y., Yang, H., Long, Y., Liang, Q., et al. (2017). MiR-942 Decreased before 20 Weeks Gestation in Women with Preeclampsia and Was Associated with the Pathophysiology of Preeclampsia In Vitro. Clin. Exp. Hypertens. 39, 108–113. doi:10.1080/10641963.2016.1210619
Zhao, G., Zhou, X., Chen, S., Miao, H., Fan, H., Wang, Z., et al. (2014). Differential Expression of microRNAs in Decidua-Derived Mesenchymal Stem Cells from Patients with Pre-eclampsia. J. Biomed. Sci. 21, 81. doi:10.1186/s12929-014-0081-3
Zhao, X., Liu, F., Zhang, J., Zhang, J., Zhang, L., and Chen, L. (2021a). LINC01128 - miR-16 Interaction Regulates the Migration and Invasion of Human Chorionic Trophoblast Cells. Hypertens. Pregnancy 40, 152–161. doi:10.1080/10641955.2021.1917602
Zhao, X., Zhang, X., Wu, Z., Mei, J., Li, L., and Wang, Y. (2021b). Up-regulation of microRNA-135 or Silencing of PCSK6 Attenuates Inflammatory Response in Preeclampsia by Restricting NLRP3 Inflammasome. Mol. Med. 27, 82. doi:10.1186/s10020-021-00335-x
Zhao, Y.-H., Liu, Y.-L., Fei, K.-L., and Li, P. (2020). Long Non-coding RNA HOTAIR Modulates the Progression of Preeclampsia through Inhibiting miR-106 in an EZH2-dependent Manner. Life Sci. 253, 117668. doi:10.1016/j.lfs.2020.117668
Zhao, Z., Moley, K. H., and Gronowski, A. M. (2013). Diagnostic Potential for miRNAs as Biomarkers for Pregnancy-specific Diseases. Clin. Biochem. 46, 953–960. doi:10.1016/j.clinbiochem.2013.01.026
Zheng, W., Chen, A., Yang, H., and Hong, L. (2020). MicroRNA-27a I-nhibits T-rophoblast C-ell M-igration and I-nvasion by T-argeting SMAD2: Potential R-ole in P-reeclampsia. Exp. Ther. Med. 20, 2262–2269. doi:10.3892/etm.2020.8924
Zhong, Y., Zhu, F., and Ding, Y. (2019). Differential microRNA Expression Profile in the Plasma of Preeclampsia and normal Pregnancies. Exp. Ther. Med. 18, 826–832. doi:10.3892/etm.2019.7637
Zhou, F., Sun, Y., Gao, Q., and Wang, H. (2020). microRNA-21 R-egulates the P-roliferation of P-lacental C-ells via FOXM1 in P-reeclampsia. Exp. Ther. Med. 20, 1871–1878. doi:10.3892/etm.2020.8930
Zhou, X., Li, Q., Xu, J., Zhang, X., Zhang, H., Xiang, Y., et al. (2016). The Aberrantly Expressed miR-193b-3p Contributes to Preeclampsia through Regulating Transforming Growth Factor-β Signaling. Sci. Rep. 6, 19910. doi:10.1038/srep19910
Zhu, H., Niu, X., Li, Q., Zhao, Y., Chen, X., and Sun, H. (2020). Circ_0085296 Suppresses Trophoblast Cell Proliferation, Invasion, and Migration via Modulating miR-144/e-Cadherin axis. Placenta 97, 18–25. doi:10.1016/j.placenta.2020.06.002
Zhu, L., and Liu, Z. (2021). Serum from Patients with Hypertension Promotes Endothelial Dysfunction to Induce Trophoblast Invasion through the miR-27b-3p/ATPase P-lasma M-embrane Ca2+ T-ransporting 1 axis. Mol. Med. Rep. 23, 319. doi:10.3892/mmr.2021.11958
Zhu, X.-m., Han, T., Sargent, I. L., Yin, G.-w., and Yaoqing, Y.-q. (2009). Differential Expression Profile of microRNAs in Human Placentas from Preeclamptic Pregnancies vs normal Pregnancies. Am. J. Obstet. Gynecol. 200, 661e1–661. e7. doi:10.1016/j.ajog.2008.12.045
Zolfaghari, M. A., Motavalli, R., Soltani-Zangbar, M. S., Parhizkar, F., Danaii, S., Aghebati-Maleki, L., et al. (2021). A New Approach to the Preeclampsia Puzzle; MicroRNA-326 in CD4+ Lymphocytes Might Be as a Potential Suspect. J. Reprod. Immunol. 145, 103317. doi:10.1016/j.jri.2021.103317
Zou, A.-X., Chen, B., Li, Q.-X., and Liang, Y.-C. (2018). MiR-134 Regulates the Progression of Preeclampsia. Eur. Rev. Med. Pharmacol. Sci. 22, 2199–2206. doi:10.26355/eurrev_201803_14557
Keywords: epigenetics, miRNA, preeclampsia, pathophysiology, meta-analysis
Citation: Cirkovic A, Stanisavljevic D, Milin-Lazovic J, Rajovic N, Pavlovic V, Milicevic O, Savic M, Kostic Peric J, Aleksic N, Milic N, Stanisavljevic T, Mikovic Z, Garovic V and Milic N (2021) Preeclamptic Women Have Disrupted Placental microRNA Expression at the Time of Preeclampsia Diagnosis: Meta-Analysis. Front. Bioeng. Biotechnol. 9:782845. doi: 10.3389/fbioe.2021.782845
Received: 24 September 2021; Accepted: 22 November 2021;
Published: 24 December 2021.
Edited by:
Umberto Galderisi, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Liv Cecilie Vestrheim Thomsen, University of Bergen, NorwayMartin Mueller, University Hospital Bern, Switzerland
Copyright © 2021 Cirkovic, Stanisavljevic, Milin-Lazovic, Rajovic, Pavlovic, Milicevic, Savic, Kostic Peric, Aleksic, Milic, Stanisavljevic, Mikovic, Garovic and Milic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vesna Garovic, Z2Fyb3ZpYy52ZXNuYUBtYXlvLmVkdQ==
†These authors share first authorship
 Andja Cirkovic
Andja Cirkovic Dejana Stanisavljevic1†
Dejana Stanisavljevic1† Jelena Kostic Peric
Jelena Kostic Peric Vesna Garovic
Vesna Garovic Natasa Milic
Natasa Milic