
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol., 20 December 2021
Sec. Synthetic Biology
Volume 9 - 2021 | https://doi.org/10.3389/fbioe.2021.768490
This article is part of the Research TopicEngineering Corynebacterium glutamicum Chassis for Synthetic Biology, Biomanufacturing, and BioremediationView all 20 articles
Hyaluronic acid (HA) is composed of alternating d-glucuronic acid and N-acetyl-d-glucosamine, with excellent biocompatibility and water retention capacity. To achieve heterologous biosynthesis of HA, Corynebacterium glutamicum, a safe GRAS (generally recognized as safe) host, was utilized and metabolically engineered previously. In this work, to achieve further enhancement of HA yield, four strategies were proposed and performed separately first, i.e., (1) improvement of glucose uptake via iolR gene knockout, releasing the inhibition of transporter IolT1/IolT2 and glucokinases; (2) intensification of cardiolipin synthesis through overexpression of genes pgsA1/pgsA2/cls involved in cardiolipin synthesis; (3) duly expressed Vitreoscilla hemoglobin in genome, enhancing HA titer coupled with more ATP and improved NAD+/NADH (>7.5) ratio; and (4) identification of the importance of glutamine for HA synthesis through transcriptome analyses and then enhancement of the HA titer via its supplement. After that, we combined different strategies together to further increase the HA titer. As a result, one of the optimal recombinant strains, Cg-dR-CLS, yielded 32 g/L of HA at 60 h in a fed-batch culture, which was increased by 30% compared with that of the starting strain. This high value of HA titer will enable the industrial production of HA via the engineered C. glutamicum.
Hyaluronic acid (HA), composed of alternating β-1,3-N-acetyl-d-glucosamine (GlcNAc) and β-1,4-d-glucuronic acid (GlcUA), belongs to the glycosaminoglycan family (Westbrook et al., 2018; Cheng et al., 2019b; Wang et al., 2020) and mainly exists in animal tissues such as chicken crowns. HA has already been applied in the clinical, cosmetic, pharmaceutical, and food industry due to its excellent biocompatibility and extraordinary water-retaining properties (Stecco et al., 2011; Graca et al., 2020; Yu et al., 2020). Generally, HAs with different molecular weights (MWs) have different applications (Qiu et al., 2021). High-molecular-weight HA (HMW-HA, MW ≥ 1 × 106 Da) is mainly used for joint cavity injection and cartilage degeneration repair, owing to its good viscoelasticity, moisture retention, and lubrication properties. Low-molecular-weight HA (LMW-HA, 1 × 104–1 × 106 Da) usually plays an important role in the cosmetics field (Qiu et al., 2021), since it can improve skin elasticity and reduce wrinkles (Pavicic et al., 2011), as well as regulate skin metabolism and delay aging (Camacho et al., 2016). In addition, HA oligosaccharides (MW ≤ 1 × 104 Da) may have significant application prospects in the food health field (Boltje et al., 2009), as they have been widely used in fruit juice, soy milk, jelly, and other food. In the future, HAs with different MWs will have more and better development prospects in different fields (Qiu et al., 2021).
Nowadays, industrial HA production has already been achieved through fermentation of group C Streptococcus (Streptococcus equisimilis and Streptococcus zooepidemicus) (Liu et al., 2011) and has gradually replaced the traditional animal issue extraction methods. However, Streptococcus sp. may produce some exotoxins and immunogens during HA production (Cheng et al., 2019b; Wang et al., 2020). Considering the potential hazards, adoption of GRAS (generally recognized as safe) strains to produce HA is required urgently. Up to now, HA has been biosynthesized successfully in Escherichia coli (Yu and Stephanopoulos 2008; Mao et al., 2009; Woo et al., 2019), Lactococcus lactis (Prasad et al., 2010; Sunguroglu et al., 2018; Jeeva et al., 2019), Bacillus subtilis (Westbrook et al., 2018a; Westbrook et al., 2018b; Li et al., 2019), Agrobacterium sp. (Mao and Chen 2007), and Corynebacterium glutamicum (Hoffmann and Altenbuchner 2014; Cheng et al., 2016; Cheng et al., 2017; Cheng et al., 2019b; Wang et al., 2020; Zheng et al., 2020). Even though the HA titers obtained from heterologous hosts are in general still lower than those achieved by natural, pathogenic producers. In the biosynthesis of HA, HA polymer is synthesized by an enzyme called HA synthase (HAS), which is grouped into two classes (Agarwal et al., 2019). Class I HAS is a kind of integral membrane protein containing a single domain, while Class II HAS is a soluble/membrane anchored protein with two domains (Weigel 2015). Most of the HAS found so far belong to Class I, such as HAS from S. equisimilis (seHAS) and Streptococcus pyogenes (spHAS); however, Class II enzyme is found only in Pasteurella multocida (pmHAS). It was reported that seHAS and spHAS contained a single HAS protein associated with an additional component with a mass of about 23 kDa, which was identified as cardiolipin (CL), one of the common bacterial membrane phospholipids (Weigel 2002). Thus, the active HAS enzyme contains a HAS protein monomer and about 14–18 CL molecules as a complex, in which CL is essential for enzymatic activity. It has not been studied whether enhanced CL synthesis would have an impact on HA production.
In order to meet the requirements of industrial production, researchers have made lots of efforts to increase the yield of HA in recombinant strains. Some hosts naturally harbor an almost complete metabolic route for HA synthesis, just lacking the HAS gene (hasA), such as B. subtilis, E. coli, and C. glutamicum (Manfrao-Netto et al., 2021). Based on the heterologous expression of hasA, different artificial has operons containing a combination of different genes have been constructed in various hosts to increase HA titer. Examples are spABC and sseAB in E. coli (Yu and Stephanopoulos 2008), pmhasA-tuaD-gtaB (operon hasABC) in B. subtilis 168 (Jia et al., 2013), and ssehasA-hasB operon in C. glutamicum (Cheng et al., 2016), which in general obtained about 1–7 g/L of HA. Besides, engineering metabolic pathways promoted the yield of HA as well in different strains (Jin et al., 2016; Westbrook et al., 2018a). Wang et al. (2020) coupled HA degradation with HA production through adding leech hyaluronidase, which led to 74.1 g/L of HA accumulation with super-low MW (∼53 kDa). In addition, some researchers also found that the cell-morphology engineering strategies, for example, downregulating or overexpressing the cell division initiator protein FtsZ in B. subtilis or C. glutamicum, can further enhance the HA titer (Westbrook et al., 2016; Zheng et al., 2020). For the overexpression of Vitreoscilla sp. hemoglobin (VHb), however, contrary results were reported in different strains: in B. subtilis, expression of VHb improved the HA titer from 0.9 to 1.8 g/L (Chien and Lee 2007); but in C. glutamicum, co-expression of the VHb gene (vgb) with hasA lowered HA yield by about 1.5-fold (Hoffmann and Altenbuchner 2014). In view of this, the effect of VHb varies case by case, which needs to be further investigated. Except for the genetic strategies, some researchers also enhanced the HA yield through optimization of the culture medium. Important functions of trace element and different kinds of carbon and nitrogen sources such as corn syrup powder and glucose were highlighted (Cheng et al., 2016; Chahuki et al., 2019).
Herein, we proposed four indirect pathway metabolic strategies to further enhance the HA titer in engineered C. glutamicum, i.e., enhancing the carbon substrate uptake via genetically activating the PTS-independent uptake system; regulating HA synthesis through the intensified synthesis of CL (an auxiliary molecule of HAS); duly expressing VHb via integration of the gene vgb into the genome of C. glutamicum, thereby promoting cell oxygen transfer, energy metabolism, and finally HA synthesis; and lastly finding the key inorganic nitrogen source (glutamine) through transcriptome analyses and then enhancing the HA titer via its supplementation. Combination strategies were further evaluated, and HA titer was significantly enhanced by optimal combinations.
DNA amplification was performed using 2× Phanta Max Master Mix, purchased from Vazyme (Nanjing, China). A DNA Gel Extraction Kit and Plasmid Miniprep Kit were purchased from Omega (Norcross, GA, United States). Gibson Assembly Reaction Kits were used, purchased from Clonesmarter Technologies (Scottsdale, AZ, United States). An ATP Assay Kit was obtained from Solarbio (Beijing, China), and a NAD+/NADH Assay Kit (WST-8 method) was purchased from Beyotime (Shanghai, China).
All the strains and plasmids used in this study were listed in Table 1, and the primers used for gene amplifications were listed in Supplementary Table S1. E. coli trans10 (TransGen Biotech Co., LTD) was used to construct and amplify the recombinant plasmids. Luria-Bertani broth (LB) medium (tryptone 10 g/L, yeast extract 5 g/L, and NaCl 10 g/L) was used for culturing E. coli strains. If necessary, 50 μg/ml kanamycin was added. C. glutamicum ATCC13032 was the starting host strain for subsequent C. glutamicum engineered strains. Cg-0 was constructed previously in our laboratory, and its genotype was shown in Table 1 (Cheng et al., 2019b). For flask culture, all of the engineered C. glutamicum strains were cultured in a 500 ml flask containing 50 ml fermentation medium (40 g/L glucose, 20 g/L (NH4)2SO4, 1 g/L K2HPO3, 0.5 g/L KH2PO3, 5 g/L MgSO4·7H2O, 0.01 g/L FeSO4·7H2O, and 0.01 g/L MnSO4, pH = 7.2) at 28°C, 200 rpm. The pH of the medium was adjusted to 7.2 every 12 h, and glucose was supplemented every 24 h.
Plasmid pK18mobsacB containing the sacB gene was used to conduct genome editing (such as iolR gene deletion) via double-crossover homologous recombination driven by sucrose selection (Schafer et al., 1994). Gene vgb was cloned from a plasmid in a previous study (Wang et al., 2018). The recombinant plasmid pK18mobsacB-Δldh::vgb was constructed and used for vgb integration into the genome. The genes relating to synthesis of CL (pgsA1, pgsA2, and cls) were amplified from the C. glutamicum genome and were fused into the plasmid APdapBBaF, resulting in the plasmid APdapBB-pgsA1/pgsA2/cls-aF. Competent cells of Cg-ΔLACPZ were used for construction of strain Cg-dR and Cg-VHb by homologous recombination via pK18mobsacB. Engineered strains Cg-dR, Cg-VHb, Cg-pgsA1, Cg-pgsA2, Cg-CLS, Cg-dR-VHb, Cg-dR-CLS, Cg-VHb-CLS and Cg-dR-VHb-CLS were constructed and utilized for studies in this work.
During flask cultivation, 1.5 ml broth sample was withdrawn at 24, 48, and 72 h, to determine the OD600 and HA titer of different recombinant strains. The HA titer was determined as follows: 3 ml ethanol was added into 1 ml fermentation broth, stored at 4°C for 2 h. The precipitation was collected by centrifugation (10,000 rpm, 3 min). Then 1 ml distilled water was added, and the HA yield was measured by the modified CTAB method, as previously described.
Cells of the two recombinant C. glutamicum strains (Cg-0 and Cg-0-half) cultured for 24 h were collected and centrifuged at room temperature for 10 min at 10,000 rpm and then stored at −70°C. The frozen cells were sent to Beijing Novogene to determine the transcriptome data.
The broth samples of Cg-0 and Cg-VHb were collected at 24 h in parallel culture. After centrifugation at 13,000 rpm for 5 min, cell pellets were harvested and washed twice with phosphate buffer solution (PBS, 20 mM, pH = 7.2). Then the cell suspensions in PBS were sent for MALDI-TOF-MS analyses, using an ABI 4800 Plus analyzer (Applied Biosystems, Foster City, CA, United States).
ATP and NAD+/NADH results of Cg-0 or Cg-VHb were measured by an ATP Assay Kit (Solarbio) and NAD+/NADH Assay Kit (Beyotime), respectively. For both assays, 1 ml broth sample at 24 or 48 h with a cell concentration of 1 OD was withdrawn and centrifuged at 13,000 rpm for 5 min. The pellet was washed twice and resuspended with 1 ml PBS (pH = 7.2, 20 mM) and then used for ATP assay and NAD+/NADH measurement with the standard protocol of the kit.
The fed-batch culture was conducted in a 10 L fermenter (Sartorius), containing 4 L fermentation medium as described above supplemented with 2 g/L glutamine. The aeration was set as 1 vvm, and agitation was set as 600 rpm, at 28°C. After 48 h of fermentation, the agitation was set as 800 rpm. The pH of the medium was retained at 7.2 by 8 M NaOH and 6 M HCl. The concentration of glucose was measured every 2 h after 8 h of fermentation. And 800 g/L glucose was added when its concentration dropped below 10 g/L, ensuring that the residual glucose concentration remained between 8 and 15 g/L.
As mentioned above, various efforts have been made to promote HA production in recombinant C. glutamicum, for example, optimization of has operon, knocking out competitive metabolic pathways, membrane engineering to enlarge the availability of cell membrane, and coupling HA synthesis with HA hydrolysis. Besides these, here, we tried to find out some strategies, indirect, non-regular, but effective as well, to further improve the HA titer in engineered C. glutamicum. Figure 1 showed the overview of the indirect metabolic strategies to promote HA production in this work.
As illustrated in Figure 1, Strategy 1 highlighted the promotion of glucose uptake via transport regulation. Carbon sources are essential factors for cell growth and target product synthesis. For most industrial fermentation processes, glucose is always the main carbon source due to its low price and high utilization efficiency. There are two glucose transport systems in C. glutamicum: phosphoenolpyruvate-dependent glucose phosphorylation via the phosphotransferase system (PTSGlc) and PTS-independent glucose uptake system (non-PTSGlc), such as the coupling system of myo-inositol permease and glucokinase (IPGS), and the coupling system of beta-glucoside-PTS permease and glucokinase (GPGS) (Ruan et al., 2020). For the IPGS pathway, glucoses are firstly transported into cells by myo-inositol permeases (IolT1/IolT2) and then phosphorylated by glucokinases (Glk and PpgK). The transcription of iolT1/iolT2/glk/ppkg, however, is repressed by a GntT-type regulator IolR in C. glutamicum (Klaffl et al., 2013). Deletion of the regulator IolR can strongly activate the non-PTS system and enhance the glucose uptake rate (Zhang B. et al., 2019). Therefore, deleting the IolR gene, thereby improving the glucose uptake efficiency, was proposed to be the first strategy to enhance HA titer.
As described in Table 1, the HA producer Cg-0 was utilized as the starting strain for subsequent studies, which could yield 6.2 g/L of HA in flask culture and 24.5 g/L of HA in fed-batch culture (Cheng et al., 2019b). The engineered Cg-dR was obtained after successful deletion of the gene iolR (Supplementary Figure S2). Effects of IolR deletion on cell growth and HA synthesis were evaluated via parallel flask culture of Cg-0 and Cg-dR. As shown in Figure 2, the OD600 of Cg-dR showed a litter difference with that of Cg-0, while the HA titer of Cg-dR was 6.9% higher than that of Cg-0 at 48 h, reaching 8.48 g/L.
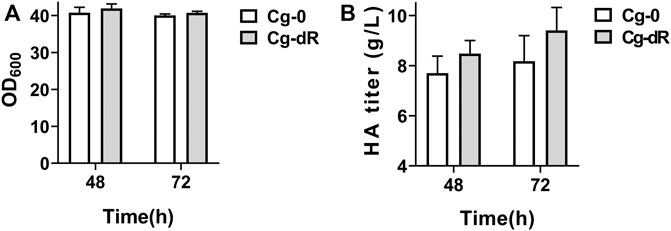
FIGURE 2. Cell growth and HA titer comparison of Cg-dR and control Cg-0. (A) OD600; (B) HA titer. Experiments were performed in triplicate.
Strategy 2 focused on the auxiliary factor for maintaining the high activity of HAS, which is of great importance for HA biosynthesis. It was reported that CL was the activator for seHAS (Tlapak-Simmons et al., 1998; Tlapak-Simmons et al., 1999). The active Streptococcal HAS contains a single HAS monomer and multiple CL molecules (14–18 molecules of CL) (Tlapak-Simmons et al., 1998); and the exogenous CL could rescue the HAS activity (Kakizaki et al., 2002). CL, also known as diphospholipin, is synthesized from the intermediate metabolite cytosine diphosphate-diacylglycerol (CDP-DAG) by phosphatidylglycerophosphate synthase (PGS), phosphatidylglycerophosphate phosphatase (PTPMT), and CL synthase (CLS). Among them, the key enzyme phosphatidylglycerol phosphate synthase has two copies in the C. glutamicum genome, which are annotated as pgsA1 and pgsA2, and the CLS is annotated as cls. In this study, the CL metabolic pathway was intensified via overexpression of pgsA1/pgsA2/cls genes, thereby indirectly enhancing the HAS activity and HA production.
The hypothetical “HAS–CL” complex model is shown in Figure 3A, in which CL molecules bind with different domains of HAS to form a “pore” for HA transportation. Three key enzymes for CL synthesis were figured out and labeled in Figure 1. Three engineered strains, Cg-pgsA1, Cg-pgsA2, and Cg-CLS overexpressing the above enzymes separately, were constructed and were evaluated in flask culture for 72 h (Figures 3C,D). For HA titer, pgsA2 and cls both enhanced the HA synthesis by ∼10% (reaching 9.2 and 9.5 g/L, respectively) with respect to that of the control Cg-0 (8.6 g/L), although pgsA1 reduced the HA titer by 14%.
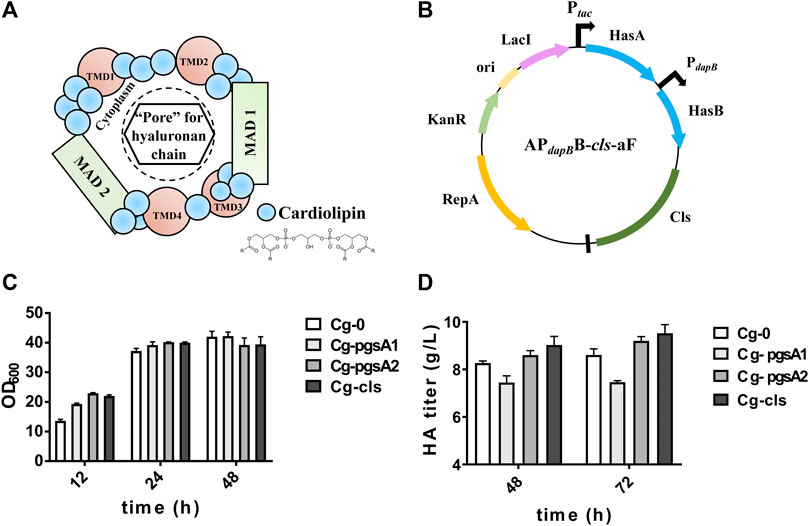
FIGURE 3. Cell growth and HA titer results after intensification of CL synthesis. (A) Hypothetical model of the active CLS–HAS complex: the scheme depicts the close association of CL molecules (blue dots) with one HAS protein to create an active enzyme. The growing HA chain would be transferred through this pore-like opening. The membrane domains of the HAS are labeled as transmembrane domains (TMDs) or membrane-associated domains (MADs) (Tlapak-Simmons et al., 1999). (B) Plasmid map of APdapBB-cls-aF. (C) OD600 of the engineered strains Cg-pgsA1, Cg-pgsA2, and Cg-CLS. (D) HA titer of the same engineered strains. Strain Cg-0 was the control. Experiments were performed in triplicate.
HA synthesis is a high-energy-demand process, in which many reactions need UTP and NAD+. Besides, acetyl-CoA is also required. For example, 1 mol of glucose-1-P is transformed to UDP-glucose, consuming 1 mol of UTP; after that, 1 mol of UDP-glucose is converted into 1 mol of UDP-glucuronic acid, consuming 2 mol of NAD+ and releasing 2 mol of NADH. The metabolism or recycle of these co-factors and also cell growth all need oxygen. To meet the high-oxygen and high-energy requirements of HA production, increasing dissolved oxygen (DO) is regarded as an effective strategy for both native producers and recombinant strains. Traditional stirring or aeration-rate optimization usually causes high energy consumption and physical damage to the cells (Galaction et al., 2004). VHb, found in the obligate aerobic bacteria Vitreoscilla, can improve respiration and energy metabolism under oxygen-limited conditions (Orii and Webster 1986; Zhao et al., 2017). To duly express VHb via a genome-integrated way to enhance oxygen transfer and cell intake was proposed as Strategy 3 for enhanced HA production.
Engineered Cg-VHb was constructed by inserting the gene vgb into the site of the lactate dehydrogenase gene (ldh) (Supplementary Figure S3). MALDI-TOF-MS was adopted to verify expression of VHb, as shown in Figure 4A. In comparison with Cg-0, a new peak occurred in Cg-VHb at around 15 kDa, which was the expressed VHb. Flask culture results showed that although cell growth was not obviously changed (Figure 4B), the HA titer of Cg-VHb was highly increased by 26% at 72 h (11.56 g/L) compared with that of Cg-0 (Figure 4C).
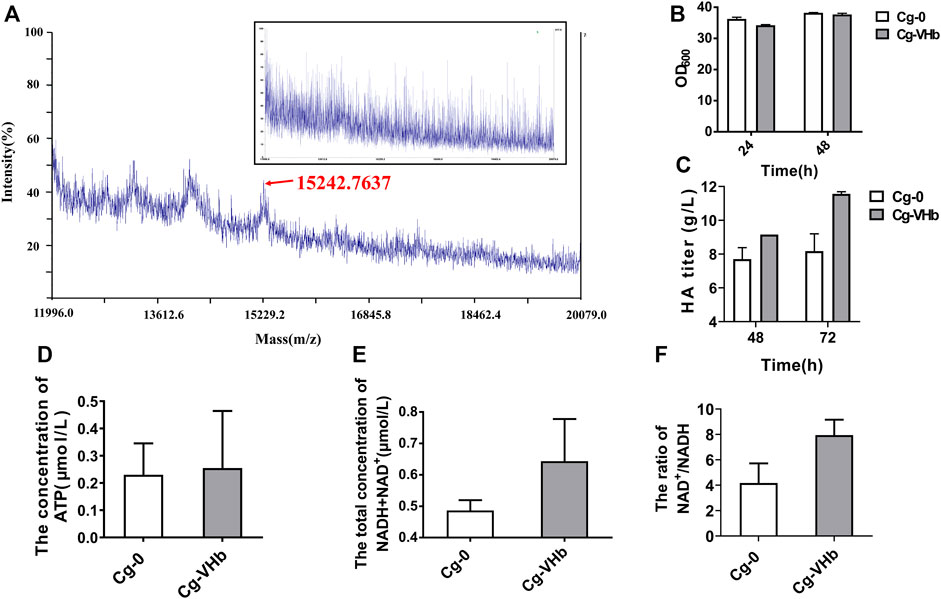
FIGURE 4. Enhancing HA synthesis via duly expressed VHb in genome. (A) MALDI-TOF-MS. (B) OD600. (C) HA titer. (D) ATP concentration. (E) Total concentration of NAD+ and NADH. (F) The ratio of NAD+ to NADH. In (D), Cg-VHb and Cg-0 were cultured in a flask for 24 h, and in (E,F), Cg-VHb and Cg-0 were cultured in a flask for 48 h. All of the measurements were performed in triplicate.
The ATP content was further assayed for both Cg-0 and Cg-VHb. As shown in Figure 4D, intracellular ATP concentration of Cg-VHb was higher than that of Cg-0 by 10.7%. At the same time, the intracellular NAD+/NADH ratio was investigated as well. It could be seen that the total concentration of NAD+ and NADH in Cg-VHb was higher than that of Cg-0 by 32.3% and that Cg-VHb generated more NAD+, resulting in a higher NAD+/NADH (>7.5) ratio of Cg-VHb than that of Cg-0. These results indicated that introduction of VHb indeed intensified uptake of oxygen, especially in the late stage of fermentation, thereby leading to more ATP and promoting the energy metabolism.
With combination of the above genetic strategies, we further constructed four engineered strains, i.e., Cg-dR-VHb, Cg-dR-CLS, Cg-VHb-CLS, and Cg-dR-CLS-VHb. We assayed the cell concentrations (Figure 5A) and HA titers (Figure 5B) at 24 and 48 h in flask culture for different strains. At 48 h, Cg-dR-VHb showed the highest HA yield, reaching 9.43 g/L. But surprisingly, the triple-strategy strain Cg-dR-VHb-CLS behaved even worse than the control, which should be further investigated.
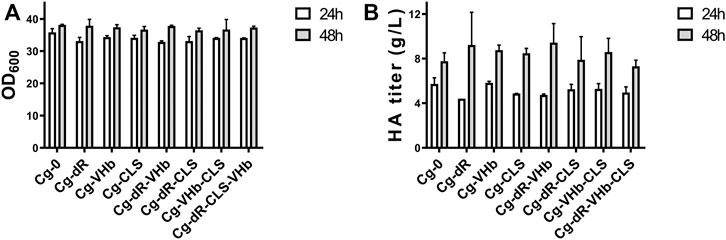
FIGURE 5. Combination of different indirect genetic strategies on cell growth and HA synthesis. (A) OD600 of different strains. (B) The HA titer of different strains. Strain Cg-0 was the control. Experiments were performed in triplicate.
According to the synthesis pathway of HA in recombinant C. glutamicum (Supplementary Figure S1), one molecule of glutamine is required together with UTP, NAD+, and acetyl-CoA (Figure 1). Glutamine provides an amino group for fructose 6-phosphate (F6P), which is converted into GlcN-6P by glutamine-fructose-6-phosphate aminotransferase, GlmS. Obviously, lots of glutamines will be consumed during HA synthesis. Thereby, we investigated the importance of glutamine for HA synthesis via both transcriptional analysis and glutamine supplementation, which was regarded as the fourth strategy.
To ensure the significance of glutamine on HA synthesis, transcriptome analysis was specifically performed with two controls, Cg-0 and Cg-0-half (the HA titer was only half of Cg-0). In total, 242 upregulated genes were identified. Not considering the putative protein genes, artificially expressed genes, and structural protein genes, five genes, cgl2910, proB, cgl0453, hisH, and cgl0448, were found to be related with glutamate/glutamine metabolism, as listed in Table 2.

TABLE 2. Identified key proteins with significant transcription difference in Cg-0 and Cg-0-half transcriptome.
It can be found that the products of these five genes are just the enzymes involved in the metabolism of glutamic acid and glutamine, as shown in Figure 6A. So next, we added 2 g/L of glutamine into the medium and conducted flask culture for 48 h to test the cell growth and HA accumulation characteristics. As shown in Figures 6B,C, both OD600 and HA synthesis were significantly affected by glutamine addition, especially for Cg-dR-CLS and Cg-VHb-CLS. The highest HA titer, 10.66 g/L, was achieved by Cg-dR-CLS with glutamine, while the control Cg-0 only accumulated 8.26 g/L HA under the same conditions. Further, we performed fed-batch culture in a 10 L fermenter by Cg-dR-CLS, and the time profiles were shown in Figure 7. It can be seen that the HA titer of Cg-dR-CLS at 60 h reached 32 g/L, HA yield on glucose was 0.28 g/g, and HA productivity was 0.53 g/L/h. These results were higher than those of the superior strain CgHA25 we previously reported (Cheng et al., 2019b). This indicated that the strategies in this work performed well during the scale-up process.
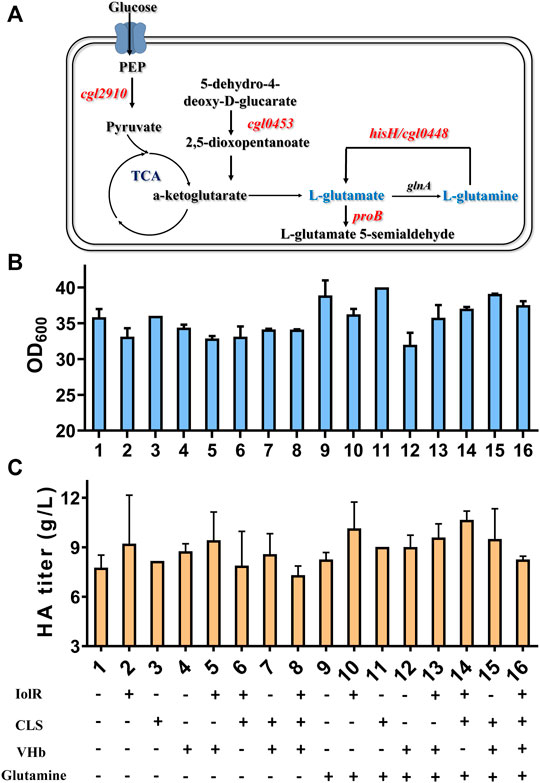
FIGURE 6. Glutamine metabolic pathway and effects of combined four strategies on cell growth and HA synthesis. (A) The key enzymes identified by transcriptome analysis are involved in glutamate or glutamine synthesis. (B) OD600 results of eight engineered strains under 16 different conditions. (C) HA titer of eight engineered strains under 16 different conditions.
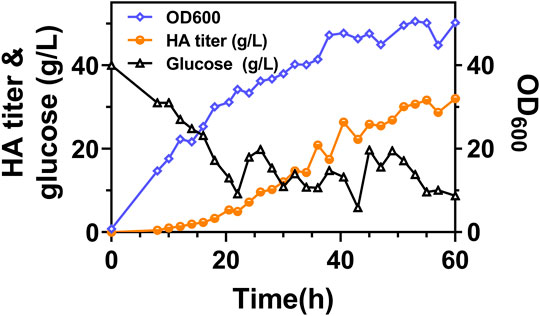
FIGURE 7. The time profiles of fed-batch fermentation by Cg-dR-CLS. Glucose feeding started at 22 h.
In this study, we strengthened the biosynthesis of HA in recombinant C. glutamicum through four indirect, non-regular metabolic strategies. We assumed the HA-producing strain as a “whole block factory” and enhanced the product synthesis via intensified bioprocesses, such as substrate uptake, HAS activity improvement, and oxygen transfer.
Firstly, glucose uptake was intensified by IolR knockout, leading to a 6.9% increase of HA titer. This is a common effective strategy for enhancing the titer of different products synthetized by C. glutamicum. For example, deletion of IolR and overexpression of IolT1 also increased the yield of l-ornithine by 10% (Zhang B. et al., 2019). Similarly, Zhang X. et al. (2019) found enhanced cell growth and l-serine production of 3.9-fold and 5.9-fold, respectively, via inactivation of IolR. Based on the same metabolic regulation strategy, some other alternative strategies can also be tried to strengthen carbon utilization and thereby enhance the product yield, such as overexpression of the glucose transporters IolT1/T2 and EIIglc.
CLs can bind HAS to form complexes and play an essential role in maintaining the biological activity of HAS. In a cell-free system, Weigel et al. reported that addition of CL into purified HAS increased the Km for UDP-GlcUA and decreased the Km for UDP-GlcNAc, finally giving an overall stimulation of Vmax. Both the seHAS and spHAS could maintain ∼60% initial activity by addition of bovine CL after being stored at −80°C for 2 months (Tlapak-Simmons et al., 1999). Based on the literature, we analyzed the CL synthesis pathway in C. glutamicum and focused on three key enzymes, PgsA1, PgsA2, and Cls.
We found that overexpression of pgsA1/pgsA2/cls can all accelerate cell growth to some degree, especially in the first 24 h. As reported previously, the pgsA gene codes for phosphatidylglycerophosphate synthase, which catalyzes the committed step of biosynthesis of phosphatidylglycerol (Dowhan 1997). And the cls gene codes for CLS, which condenses two molecules of phosphatidylglycerol to form CL in the prokaryote. As the major acidic phospholipids of the organism, phosphatidylglycerol and CL play important roles in bacterial cell structure and diverse physiological processes, such as DNA replication, cell division, respiration, and osmotic stress response (Wood 2018). Yasuhiro Shiba et al. (2004) reported that a pgsA-null mutation is lethal for E. coli. Xia et al. confirmed that E. coli are dependent on phosphatidylglycerol for cell growth, which cannot be substituted with phosphatidylinositol (Xia and Dowhan 1995). It can be seen that phosphatidylglycerol is essential for cell growth. As for CL, Kazuhisa Sekimizu et al. found that CL could activate the dnaA protein, which serves as the initiation of the protein of replication (Sekimizu and Kornberg 1988). Amer H. Asseri et al. (2021) reported that CL can also enhance the enzymatic activity of cytochrome bd for C. glutamicum, which is a terminal oxidase of respiratory pathways and can also enhance the oxygen consumption activity by twofold. Satomi Nishijima et al. (1988) came to the conclusion that the cls gene may confer growth or survival advantages for E. coli. In summary, pgsA1/pgsA2/cls genes are vital for cell growth and other physiological processes, and overexpression of these genes may promote cell growth to a certain degree.
In addition, we also found that pgsA1 overexpression had a negative effect on HA titer, while pgsA2 and cls could significantly promote the HA synthesis. We measured the concentration of free CLs in Cg-0 and Cg-CLS (Supplementary Table S2) and found that overexpression of cls led to an obvious increase of intracellular CLs. As for the negative effect of pgsA1 on the HA titer, we assumed that pgsA1 and pgsA2 might have different activities towards conversion of CDP-DAG, thus leading to different concentrations of CL. In addition, Triscott and Vanderijn (1986) reported that the optimal activity of HAS occurred at a CL/protein ratio (μg/μg) of 5:1. In the future, we can clone pgsA1/pgsA2/cls and HAS under different inducible promoters and then accurately adjust the ratio of CL/HAS, thereby further enhancing the HA synthesis.
The HA synthesis process requires high-oxygen and high-energy conditions. It was observed that a higher HA titer was achieved in aerobic conditions than in anaerobic conditions (Huang et al., 2006; Prasad et al., 2010). According to our previous study, the recombinant C. glutamicum can produce HA with Mw ranging from 0.2 to 0.3 MDa (Cheng et al., 2019b). It was reported that when the Mw of HA was above 5.7 × 104 Da, the broth viscosity increased with the HA concentration increasing, which would result in a great obstacle for nutrient and oxygen transfer. Nowadays, the industrialized HA titer was 6–10 g/L with a Mw of 2 MDa in Streptococcus (Cheng et al., 2019b). The high viscosity of fermentation media made the elevation of the HA titer in Streptococcus much more difficult. Therefore, to duly express VHb is probably an effective solution for this. Zhao et al. (2017) found that expression of VHb in E. coli resulted in a 94.4% increase of trans-4-hydroxy-l-proline production in a 100 ml shaking flask culture compared to the same strain without VHb expression. Wang et al. (2018) cloned and expressed VHb in a surfactin-producing strain B. subtilis THY-15, leading to a 24% increase in flask. But surprisingly, in C. glutamicum, co-expression of the VHb gene (vgb) with hasA based on plasmids lowered HA yield by 1.5-fold (Hoffmann and Altenbuchner 2014). Therefore, we deduced that duly expressing VHb is important for its positive function. We introduced VHb into the host via a genome-integrated strategy. After introduction of VHb, intracellular ATP and NAD+ increased dramatically, indicating that the energy metabolism was improved during fermentation. And it was assumed that more oxygen strengthened the oxidative phosphorylation process, thus improving NAD+ replenishment as well as the ratio of NAD+/NADH (Garrigues et al., 1997; Lan et al., 2006; Guez et al., 2008).
Besides, expression of the vgb gene increased the total concentration of NADH and NAD+. This could be attributed to the increased activity of the tricarboxylic acid (TCA) cycle (Pablos et al., 2014). It was reported that VHb can capture oxygen and transfer it to the terminal oxidases, and the dissociation rate constant of VHb is significantly higher than other hemoglobins. E. coli-expressing vgb would direct a higher fraction of glucose through the pentose phosphate pathway (ppp) and channel less acetyl-CoA through TCA than the wild-type strain, which generated an excess amount of NADPH, and resulted in a transhydrogenation reaction, leading to an H+-flux from NADPH to NAD+. To sum up, the effective delivery of oxygen to the cytochromes would regenerate NAD+ faster, activating the TCA cycle as well. During the dynamic balance of NADH and NAD+, the generation rate of NAD+ got faster, which probably led to the increase of the total concentration of NAD+ and NADH. As for the HA titer, we found that the HA titer of Cg-VHb was highly increased by 26%. Therefore, we supposed that integration of the VHb gene into the host genome is a promising strategy for industrialized HA bioproduction.
Finally, we investigated the influence of another important factor, amino group carrier (glutamine), on HA synthesis. We analyzed the transcriptome differences between HA high-producing strain and HA low-producing strain and identified that five genes relating to the metabolism of glutamate or glutamine were upregulated significantly. This indicated that when producing HA, the bacteria would enhance their native glutamic acid and glutamine synthesis to enhance the supplement of inorganic nitrogen for HA synthesis. To confirm this deduction, we tested the effectiveness of the exogenous addition of glutamine. Correspondingly, we found that the titer of HA was increased significantly. These results also implied that deficient supplement of glutamine will limit HA bioproduction to some degree. Aside from addition of glutamate/glutamine into the medium, we can also strengthen the synthesis of glutamine or glutamic acid in the engineered strains, for example, by overexpression of glutamine or glutamate synthase.
To conclude, four indirect metabolic engineering strategies for HA titer enhancement in engineered C. glutamicum were proposed and investigated in this work. In general, the enhancement of carbon uptake efficiency (specifically glucose), the auxiliary factor titer of HAS (CL), oxygen-transfer efficiency via duly expressing VHb, and supplement of glutamine all played positive and significant roles for enhanced HA synthesis. Combination strategies further elevated the HA titer, and the optimal strains showed a 30% increase in HA production under present conditions. It can be expected that after overall optimization and accurate regulation of different strategies next, the HA titer will be further increased significantly and thereby enable the scaled-up production of HA via the engineered C. glutamicum.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
YD, FC, MW, and CX performed the experiments and processed the data; YD wrote the paper; and HY planned and supervised the project.
This work was supported by the National Key R&D Program of China (2018YFA0902200), Natural Science Foundation of China (Nos. 21776157 and 22078173) and Shandong Province Key R&D Program of China (2020CXGC010602).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.768490/full#supplementary-material
Agarwal, G., K V, K., Prasad, S. B., Bhaduri, A., and Jayaraman, G. (2019). Biosynthesis of Hyaluronic Acid Polymer: Dissecting the Role of Sub Structural Elements of Hyaluronan Synthase. Sci. Rep. 9, 12510. doi:10.1038/s41598-019-48878-8
Asseri, A. H., Godoy-Hernandez, A., Goojani, H. G., Lill, H., Sakamoto, J., McMillan, D. G. G., et al. (2021). Cardiolipin Enhances the Enzymatic Activity of Cytochrome Bd and Cytochrome Bo3 Solubilized in Dodecyl-Maltoside. Sci. Rep. 11 (1), 8006. doi:10.1038/s41598-021-87354-0
Boltje, T. J., Buskas, T., and Boons, G. J. (2006). Opportunities and Challenges in Synthetic Oligosaccharide and Glycoconjugate Research. Nat. Chem. 1 (8), 611–622. doi:10.1038/nchem.399
Camacho, K. M., Menegatti, S., and Mitragotri, S. (2016). Low-molecular-weight Polymer-Drug Conjugates for Synergistic Anticancer Activity of Camptothecin and Doxorubicin Combinations. Nanomedicine 11 (9), 1139–1151. doi:10.2217/nnm.16.33
Chahuki, F. F., Aminzadeh, S., Jafarian, V., Tabandeh, F., and Khodabandeh, M. (2019). Hyaluronic Acid Production Enhancement via Genetically Modification and Culture Medium Optimization in Lactobacillus Acidophilus. Int. J. Biol. Macromolecules 121, 870–881. doi:10.1016/j.ijbiomac.2018.10.112
Cheng, F., Luozhong, S., Guo, Z., Yu, H., and Stephanopoulos, G. (2017). Enhanced Biosynthesis of Hyaluronic Acid Using Engineered Corynebacterium Glutamicum via Metabolic Pathway Regulation. Biotechnol. J. 12 (10), 1–8. doi:10.1002/biot.201700191
Cheng, F., Gong, Q., Yu, H., and Stephanopoulos, G. (2016). High-titer Biosynthesis of Hyaluronic Acid by recombinantCorynebacterium Glutamicum. Biotechnol. J. 11 (4), 574–584. doi:10.1002/biot.201500404
Cheng, F., Luozhong, S., Yu, H., and Guo, Z. (2019a). Biosynthesis of Chondroitin in Engineered Corynebacterium Glutamicum. J. Microbiol. Biotechnol. 29 (3), 392–400. doi:10.4014/jmb.1810.10062
Cheng, F., Yu, H., and Stephanopoulos, G. (2019b). Engineering Corynebacterium Glutamicum for High-Titer Biosynthesis of Hyaluronic Acid. Metab. Eng. 55, 276–289. doi:10.1016/j.ymben.2019.07.003
Chien, L. J., and Lee, C. K. (2007). Enhanced Hyaluronic Acid Production in Bacillus Subtilis by Coexpressing Bacterial Hemoglobin. Biotechnol. Prog. 23 (5), 1017–1022. doi:10.1021/bp070036w
Dowhan, W. (1997). Molecular Basis for Membrane Phospholipid Diversity: Why Are There So many Lipids? Annu. Rev. Biochem. 66, 199–232. doi:10.1146/annurev.biochem.66.1.199
Galaction, A. I., Cascaval, D., Oniscu, C., and Turnea, M. (2004). Enhancement of Oxygen Mass Transfer in Stirred Bioreactors Using Oxygen-Vectors. 1. Simulated Fermentation Broths. Bioproc. Biosyst Eng. 26 (4), 231–238. doi:10.1007/s00449-004-0353-5
Garrigues, C., Loubiere, P., Lindley, N. D., and Cocaign-Bousquet, M. (1997). Control of the Shift from Homolactic Acid to Mixed-Acid Fermentation in Lactococcus Lactis: Predominant Role of the NADH/NAD+ Ratio. J. Bacteriol. 179 (17), 5282–5287. doi:10.1128/jb.179.17.5282-5287.1997
Graça, M. F. P., Miguel, S. P., Cabral, C. S. D., and Correia, I. J. (2020). Hyaluronic Acid-Based Wound Dressings: A Review. Carbohydr. Polym. 241, 116364. doi:10.1016/j.carbpol.2020.116364
Guez, J. S., Müller, C. H., Danze, P. M., Büchs, J. P., and Jacques, P. (2008). Respiration Activity Monitoring System (RAMOS), an Efficient Tool to Study the Influence of the Oxygen Transfer Rate on the Synthesis of Lipopeptide by Bacillus Subtilis ATCC6633. J. Biotechnol. 134 (1-2), 121–126. doi:10.1016/j.jbiotec.2008.01.003
Hoffmann, J., and Altenbuchner, J. (2014). Hyaluronic Acid Production with Corynebacterium Glutamicum : Effect of media Composition on Yield and Molecular Weight. J. Appl. Microbiol. 117 (3), 663–678. doi:10.1111/jam.12553
Huang, W.-C., Chen, S.-J., and Chen, T.-L. (2006). The Role of Dissolved Oxygen and Function of Agitation in Hyaluronic Acid Fermentation. Biochem. Eng. J. 32 (3), 239–243. doi:10.1016/j.bej.2006.10.011
Jeeva, P., Shanmuga Doss, S., Sundaram, V., and Jayaraman, G. (2019). Production of Controlled Molecular Weight Hyaluronic Acid by Glucostat Strategy Using Recombinant Lactococcus Lactis Cultures. Appl. Microbiol. Biotechnol. 103 (11), 4363–4375. doi:10.1007/s00253-019-09769-0
Jia, Y., Zhu, J., Chen, X., Tang, D., Su, D., Yao, W., et al. (2013). Metabolic Engineering of Bacillus Subtilis for the Efficient Biosynthesis of Uniform Hyaluronic Acid with Controlled Molecular Weights. Bioresour. Tech. 132, 427–431. doi:10.1016/j.biortech.2012.12.150
Jin, P., Kang, Z., Yuan, P., Du, G., and Chen, J. (2016). Production of Specific-Molecular-Weight Hyaluronan by Metabolically Engineered Bacillus Subtilis 168. Metab. Eng. 35, 21–30. doi:10.1016/j.ymben.2016.01.008
Kakizaki, I., Takagaki, K., Endo, Y., Kudo, D., Ikeya, H., Miyoshi, T., et al. (2002). Inhibition of Hyaluronan Synthesis inStreptococcus equiFM100 by 4-methylumbelliferone. Eur. J. Biochem. 269 (20), 5066–5075. doi:10.1046/j.1432-1033.2002.03217.x
Klaffl, S., Brocker, M., Kalinowski, J., Eikmanns, B. J., and Bott, M. (2013). Complex Regulation of the Phosphoenolpyruvate Carboxykinase Gene Pck and Characterization of its Gntr-type Regulator Iolr as a Repressor of Myo-Inositol Utilization Genes in Corynebacterium Glutamicum. J. Bacteriol. 195 (18), 4283–4296. doi:10.1128/jb.00265-13
Lan, C. Q., Oddone, G., Mills, D. A., and Block, D. E. (2006). Kinetics ofLactococcus Lactis Growth and Metabolite Formation under Aerobic and Anaerobic Conditions in the Presence or Absence of Hemin. Biotechnol. Bioeng. 95 (6), 1070–1080. doi:10.1002/bit.21070
Li, Y., Li, G., Zhao, X., Shao, Y., Wu, M., and Ma, T. (2019). Regulation of Hyaluronic Acid Molecular Weight and Titer by Temperature in Engineered Bacillus Subtilis. 3 Biotech. 9 (6), 225. doi:10.1007/s13205-019-1749-x
Liu, L., Liu, Y., Li, J., Du, G., and Chen, J. (2011). Microbial Production of Hyaluronic Acid: Current State, Challenges, and Perspectives. Microb. Cel Fact 10, 99. doi:10.1186/1475-2859-10-99
Manfrao-Netto, J. H. C., Queiroz, E. B., de Oliveira Junqueira, A. C., Gomes, A. M. V., de Morais, D. G., Paes, H. C., et al. (2021). Genetic Strategies for Improving Hyaluronic Acid Production in Recombinant Bacterial Culture. J. Appl. Microbiol 00, 1–19.
Mao, Z., and Chen, R. R. (2007). Recombinant Synthesis of Hyaluronan by Agrobacterium Sp. Biotechnol. Prog. 23 (5), 1038–1042. doi:10.1021/bp070113n
Mao, Z., Shin, H.-D., and Chen, R. (2009). A Recombinant E. coli Bioprocess for Hyaluronan Synthesis. Appl. Microbiol. Biotechnol. 84 (1), 63–69. doi:10.1007/s00253-009-1963-2
Nishijima, S., Asami, Y., Uetake, N., Yamagoe, S., Ohta, A., and Shibuya, I. (1988). Disruption of the Escherichia coli Cls Gene Responsible for Cardiolipin Synthesis. J. Bacteriol. 170 (2), 775–780. doi:10.1128/jb.170.2.775-780.1988
Orii, Y., and Webster, D. A. (1986). Photodissociation of Oxygenated Cytochrome O(s) (Vitreoscilla) and Kinetic Studies of Reassociation. J. Biol. Chem. 261 (8), 3544–3547. doi:10.1016/s0021-9258(17)35680-6
Pablos, T. E., Sigala, J. C., Le Borgne, S., and Lara, A. R. (2014). Aerobic Expression ofVitreoscillahemoglobin Efficiently Reduces Overflow Metabolism inEscherichia Coli. Biotechnol. J. 9 (6), 791–799. doi:10.1002/biot.201300388
Pavicic, T., Gauglitz, G. G., Lersch, P., Schwach-Abdellaoui, K., Malle, B., Korting, H. C., et al. (2011). Efficacy of Cream-Based Novel Formulations of Hyaluronic Acid of Different Molecular Weights in Anti-wrinkle Treatment. J. Drugs Dermatol. 10 (9), 990–1000.
Prasad, S. B., Jayaraman, G., and Ramachandran, K. B. (2010). Hyaluronic Acid Production Is Enhanced by the Additional Co-expression of UDP-Glucose Pyrophosphorylase in Lactococcus Lactis. Appl. Microbiol. Biotechnol. 86 (1), 273–283. doi:10.1007/s00253-009-2293-0
Qiu, Y., Ma, Y., Huang, Y., Li, S., Xu, H., and Su, E. (2021). Current Advances in the Biosynthesis of Hyaluronic Acid with Variable Molecular Weights. Carbohydr. Polym. 269, 118320. doi:10.1016/j.carbpol.2021.118320
Ruan, H., Yu, H., and Xu, J. (2020). The Glucose Uptake Systems in Corynebacterium Glutamicum: a Review. World J. Microbiol. Biotechnol. 36 (9), 126. doi:10.1007/s11274-020-02898-z
Schäfer, A., Tauch, A., Jäger, W., Kalinowski, J., Thierbach, G., and Pühler, A. (1994). Small Mobilizable Multi-Purpose Cloning Vectors Derived from the Escherichia coli Plasmids pK18 and pK19: Selection of Defined Deletions in the Chromosome of Corynebacterium Glutamicum. Gene 145 (1), 69–73. doi:10.1016/0378-1119(94)90324-7
Sekimizu, K., and Kornberg, A. (1988). Cardiolipin Activation of dnaA Protein, the Initiation Protein of Replication in Escherichia coli. J. Biol. Chem. 263 (15), 7131–7135. doi:10.1016/s0021-9258(18)68615-6
Shiba, Y., Yokoyama, Y., Aono, Y., Kiuchi, T., Kusaka, J., Matsumoto, K., et al. (2004). Activation of the Rcs Signal Transduction System Is Responsible for the Thermosensitive Growth Defect of an Escherichia coli Mutant Lacking Phosphatidylglycerol and Cardiolipin. J. Bacteriol. 186 (19), 6526–6535. doi:10.1128/jb.186.19.6526-6535.2004
Stecco, C., Stern, R., Porzionato, A., Macchi, V., Masiero, S., Stecco, A., et al. (2011). Hyaluronan within Fascia in the Etiology of Myofascial Pain. Surg. Radiol. Anat. 33 (10), 891–896. doi:10.1007/s00276-011-0876-9
Sunguroglu, C., Sezgin, D. E., Celik, P. A., and Cabuk, A. (2018). Higher Titer Hyaluronic Acid Production in Recombinant Lactococcus Lactis. Prep. Biochem. Biotechnol. 48 (8), 734–742. doi:10.1080/10826068.2018.1508036
Tlapak-Simmons, V. L., Baggenstoss, B. A., Clyne, T., and Weigel, P. H. (1999). Purification and Lipid Dependence of the Recombinant Hyaluronan Synthases from Streptococcus Pyogenes andStreptococcus Equisimilis. J. Biol. Chem. 274 (7), 4239–4245. doi:10.1074/jbc.274.7.4239
Tlapak-Simmons, V. L., Kempner, E. S., Baggenstoss, B. A., and Weigel, P. H. (1998). The Active Streptococcal Hyaluronan Synthases (HASs) Contain a Single HAS Monomer and Multiple Cardiolipin Molecules. J. Biol. Chem. 273 (40), 26100–26109. doi:10.1074/jbc.273.40.26100
Triscott, M. X., and van de Rijn, I. (1986). Solubilization of Hyaluronic Acid Synthetic Activity from Streptococci and its Activation with Phospholipids. J. Biol. Chem. 261 (13), 6004–6009. doi:10.1016/s0021-9258(17)38485-5
Wang, Q., Yu, H., Wang, M., Yang, H., and Shen, Z. (2018). Enhanced Biosynthesis and Characterization of Surfactin Isoforms with Engineered Bacillus Subtilis through Promoter Replacement and Vitreoscilla Hemoglobin Co-expression. Process Biochem. 70, 36–44. doi:10.1016/j.procbio.2018.04.003
Wang, Y., Hu, L., Huang, H., Wang, H., Zhang, T., Chen, J., et al. (2020). Eliminating the Capsule-like Layer to Promote Glucose Uptake for Hyaluronan Production by Engineered Corynebacterium Glutamicum. Nat. Commun. 11 (1), 3120. doi:10.1038/s41467-020-16962-7
Weigel, P. H. (2015). Hyaluronan Synthase: The Mechanism of Initiation at the Reducing End and a Pendulum Model for Polysaccharide Translocation to the Cell Exterior. Int. J. Cel Biol 2015, 367579. doi:10.1155/2015/367579
Weigel, P. H. (2002). Functional Characteristics and Catalytic Mechanisms of the Bacterial Hyaluronan Synthases. IUBMB Life (International Union Biochem. Mol. Biol. Life) 54 (4), 201–211. doi:10.1080/15216540214931
Westbrook, A. W., Moo-Young, M., and Chou, C. P. (2016). Development of a CRISPR-Cas9 Tool Kit for Comprehensive Engineering of Bacillus Subtilis. Appl. Environ. Microbiol. 82 (16), 4876–4895. doi:10.1128/aem.01159-16
Westbrook, A. W., Ren, X., Moo-Young, M., and Chou, C. P. (2018a). Engineering of Cell Membrane to Enhance Heterologous Production of Hyaluronic Acid in Bacillus Subtilis. Biotechnol. Bioeng. 115 (1), 216–231. doi:10.1002/bit.26459
Westbrook, A. W., Ren, X., Oh, J., Moo-Young, M., and Chou, C. P. (2018b). Metabolic Engineering to Enhance Heterologous Production of Hyaluronic Acid in Bacillus Subtilis. Metab. Eng. 47, 401–413. doi:10.1016/j.ymben.2018.04.016
Woo, J. E., Seong, H. J., Lee, S. Y., and Jang, Y. S. (2019). Metabolic Engineering of Escherichia coli for the Production of Hyaluronic Acid from Glucose and Galactose. Front. Bioeng. Biotechnol. 7, 351. doi:10.3389/fbioe.2019.00351
Wood, J. M. (2018). Perspective: Challenges and Opportunities for the Study of Cardiolipin, a Key Player in Bacterial Cell Structure and Function. Curr. Genet. 64 (4), 795–798. doi:10.1007/s00294-018-0811-2
Xia, W., and Dowhan, W. (1995). Phosphatidylinositol Cannot Substitute for Phosphatidylglycerol in Supporting Cell Growth of Escherichia coli. J. Bacteriol. 177 (10), 2926–2928. doi:10.1128/jb.177.10.2926-2928.1995
Yang, P., Liu, W., Cheng, X., Wang, J., Wang, Q., and Qi, Q. (2016). A New Strategy for Production of 5-aminolevulinic Acid in Recombinant Corynebacterium Glutamicum with High Yield. Appl. Environ. Microbiol. 82 (9), 2709–2717. doi:10.1128/aem.00224-16
Yu, H., and Stephanopoulos, G. (2008). Metabolic Engineering of Escherichia coli for Biosynthesis of Hyaluronic Acid. Metab. Eng. 10 (1), 24–32. doi:10.1016/j.ymben.2007.09.001
Yu, L.-M., Liu, T., Ma, Y.-L., Zhang, F., Huang, Y.-C., and Fan, Z.-H. (2020). Fabrication of Silk-Hyaluronan Composite as a Potential Scaffold for Tissue Repair. Front. Bioeng. Biotechnol. 8, 1–9. doi:10.3389/fbioe.2020.578988
Zhang, B., Gao, G., Chu, X.-H., and Ye, B.-C. (2019a). Metabolic Engineering of Corynebacterium Glutamicum S9114 to Enhance the Production of L-Ornithine Driven by Glucose and Xylose. Bioresour. Tech. 284, 204–213. doi:10.1016/j.biortech.2019.03.122
Zhang, X., Lai, L., Xu, G., Zhang, X., Shi, J., Koffas, M. A. G., et al. (2019b). Rewiring the central Metabolic Pathway for High-Yield L-Serine Production in Corynebacterium Glutamicum by Using Glucose. Biotechnol. J. 14 (6), e1800497. doi:10.1002/biot.201800497
Zhao, T.-X., Li, M., Zheng, X., Wang, C.-H., Zhao, H.-X., Zhang, C., et al. (2017). Improved Production of Trans-4-hydroxy-L-proline by Chromosomal Integration of the Vitreoscilla Hemoglobin Gene into Recombinant Escherichia coli with Expression of Proline-4-Hydroxylase. J. Biosci. Bioeng. 123 (1), 109–115. doi:10.1016/j.jbiosc.2016.07.018
Keywords: hyaluronic acid, engineered Corynebacterium glutamicum, IolR deletion, cardiolipin (CL), Vitreoscilla hemoglobin (VHb), glutamine
Citation: Du Y, Cheng F, Wang M, Xu C and Yu H (2021) Indirect Pathway Metabolic Engineering Strategies for Enhanced Biosynthesis of Hyaluronic Acid in Engineered Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 9:768490. doi: 10.3389/fbioe.2021.768490
Received: 31 August 2021; Accepted: 22 November 2021;
Published: 20 December 2021.
Edited by:
Yu Wang, Tianjin Institute of Industrial Biotechnology (CAS), ChinaReviewed by:
Yu-Sin Jang, Gyeongsang National University, South KoreaCopyright © 2021 Du, Cheng, Wang, Xu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huimin Yu, eXVobUB0c2luZ2h1YS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.