- 1The Key Laboratory of Industrial Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi, China
- 2School of Food Science and Technology, Jiangnan University, Wuxi, China
- 3State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, China
ε-poly-L-lysine (ε-PL) is a naturally occurring poly(amino acid) of varying polymerization degree, which possesses excellent antimicrobial activity and has been widely used in food and pharmaceutical industries. To provide new perspectives from recent advances, this review compares several conventional and advanced strategies for the discovery of wild strains and development of high-producing strains, including isolation and culture-based traditional methods as well as genome mining and directed evolution. We also summarize process engineering approaches for improving production, including optimization of environmental conditions and utilization of industrial waste. Then, efficient downstream purification methods are described, including their drawbacks, followed by the brief introductions of proposed antimicrobial mechanisms of ε-PL and its recent applications. Finally, we discuss persistent challenges and future perspectives for the commercialization of ε-PL.
Introduction
ε-poly-L-lysine (ε-PL) is a homopoly(amino acid) consisting of 25–35 L-lysine residues with amide linkages formed between the ε-amino and α-carboxy groups. Since it has many valuable characteristics such as bacteriostatic, soluble, biodegradable, edible, and non-toxic to humans and environment, ε-PL is widely used in food and medicine industries as food preservatives, dietary agents, and gene/drug/vaccine carriers (Shih et al., 2006; Yuan et al., 2018). In 1989, ε-PL was first approved as a natural food preservative by the Ministry of Health, Labour and Welfare in Japan. Then it has been used as a preservative in many countries such as South Korea, United States and China (Xu et al., 2016).
ε-PL can be synthesized chemically via many methods, such as solid-phase synthesis and ring-opening polymerization (Tao et al., 2015; Tao, 2016). However, these methods often contain complicated steps (e.g., protection and deprotection reactions) with low ε-PL yield, which may generate a lot of by-products and raise environmental issues. Compared with chemosynthesis, microbial production is a more attractive approach for ε-PL industrialization because it is more economic, practical, efficient and environmentally friendly. Currently, a total of only three companies around the world have been able to manufacture ε-PL at commercial scale, which are Chisso Co. (Tokyo, Japan), Okuno chemical industries Co. (Osaka, Japan) and SunBio Co. (Shizuoka, Japan). Among them, Chisso Co. was the most prominent ε-PL manufacturers, which established a production line with an annual output of 1,000 tons, using a mutant strain of Streptomyces albulus (Hirohara et al., 2007). These three companies have provided the majority of ε-PL in the world at a relatively high price (180 USD/kg). As a novel value-added product, the growing demand for ε-PL has facilitated related researches in biomanufacturing and industrial applications.
Figure 1 provides a holistic overview of ε-PL research beginning with the year when ε-PL was discovered. From 1977 through 2009, research efforts were relatively low and focused mostly on methods for isolation of ε-PL-producing strains. After that, research has increasingly focused on strain improvement and process engineering for enhancing the ε-PL production. It can also be noted that studies concerning downstream process have been few but constant during the last 7 yr. In addition, the literature on ε-PL applications also significantly increased since 2014, which may be related to the increasing number of breakthroughs in ε-PL biomanufacturing (e.g., the improved ε-PL production and the reduced cost).
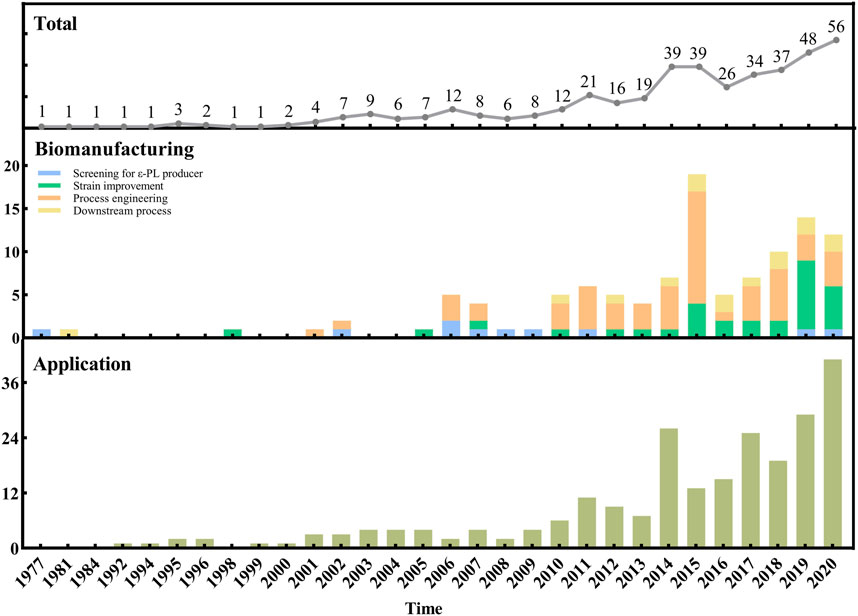
FIGURE 1. Evolution over the years of the number of references concerning the biomanufacturing and applications of ε-PL.
In recent years, substantial progress has been made in the biomanufacturing of ε-PL, including: 1) recent technologies (e.g., gene mining) developed for screening novel ε-PL producers, other than filamentous microorganisms; 2) some novel methods (e.g., gene swap strategy) for controlling the distribution of ε-PL molecular weight; 3) some recent strategies (e.g., ribosome engineering) and genetic engineering methods for directed evolution of robust ε-PL-producing strains; 4) economical and high efficiency techniques for ε-PL production, such as low-cost feedstocks for fermentation, solid-state fermentation, in situ product removal, and cell immobilization, and 5) different downstream extraction processes optimized for higher purity and recovery at relatively lower cost. Moreover, since many applications of ε-PL in food and pharmaceutical industries are dependent on its antimicrobial capacity, the understanding of the action mode of ε-PL on different microorganism is helpful in guiding its applications. This review first summarized and discussed the recent advances in ε-PL biomanufacturing, focusing on the strain improvement and process engineering. Subsequently, the latest studies concerning applications of ε-PL were briefly introduced, including its antimicrobial mechanisms. Finally, we conclude with discussion of the existing challenges and future perspectives for this burgeoning field. We hope this review can help to promote the development of ε-PL-producing strains and the integrated, large-scale production (and downstream) processes of ε-PL for various industrial applications.
Biosynthesis of ε-PL by Various Microorganisms
Typical ε-PL-producing Strains
In 1977, ε-PL was accidentally discovered as an extracellular secretion produced by S. albulus NBRC14147 (Shima and Sakai, 1977). Following this discovery, no ε-PL-producing microbe was isolated until a dye-based method was developed in 2002 (Nishikawa and Ogawa, 2002). A total of 10 ε-PL producers were isolated from 300 soil samples via this easy and efficient method, and were identified as members of the genera Streptomyces, Epichloë, Streptoverticillum, Kitasatospora.
Currently, increasing numbers of ε-PL-producing strains have been screened out as the development of novel screening methods. Efforts in ε-PL biomanufacturing research have mainly focused on Streptomyces, especially on S. albulus, which gives the highest reported ε-PL production (70 g/L) in liquid fermentation after strain evolution and fermentation process optimization (Wang et al., 2019). Some other actinomycetes, such as Kitasatospora, Streptomyces ahygroscopicus, Streptomyces griseofuscus and Streptomyces diastatochromogenes have also been investigated in some studies, with various degrees of success (Ouyang et al., 2006; Chen J. et al., 2019; Shu et al., 2019; Wang et al., 2021). Additionally, in 2012, a marine Bacillus subtilis SDNS was first discovered to produce a small amount of ε-PL (76.3 mg/L) under the optimized culture conditions (El-Sersy et al., 2012). Subsequently, some other bacteria such as Bacillus thuringiensis, Bacillus cereus, Bacillus licheniformis, Lactobacillus delbrueckiistrain, Lactococcus lactisstrain and Corynebacterium variabile were also identified to have ε-PL synthetases in their genomes, indicating that bacteria also have the potential to be industrial ε-PL producers (Shukla and Mishra, 2013; Chheda and Vernekar, 2014; Bhattacharya et al., 2017; Samadlouie et al., 2020; Jiang et al., 2021). Recent study also showed that ergot fungi (Epichloë festucae) can produce higher ε-PL production (69.9 mg/L) via genetic engineering (Purev et al., 2020). The discovery of ε-PL synthetases in these microbes suggests that ε-PL can be found naturally not only from soil samples under decayed plant matter, but also in marine water, entomo, plants, cheese, or even on human skin, and play a role in their ecologies (Chheda and Vernekar, 2014; Purev et al., 2020; Jiang et al., 2021). With the constant innovation of methods for isolation, it is believed that a greater variety of ε-PL producers will be discovered for industrial production, with a wider distribution.
Screening for ε-PL Microbes
Screening for ε-PL microbes has recently attracted considerable research attention. However, the proportion of these strains in nature is relatively small, and there are no distinct characteristics for direct identification of ε-PL-producing microbes. Historically, the conventional method for screening ε-PL producers is to analyze extracellular secretions, although this technique is unsuitable for large culture collections.
An efficient, commonly used method for screening isolates is by detecting the interaction between basic/acidic dyes and polycationic ε-PL secreted in culture broth. For example, Nishikawa and Ogawa (2012) incubated strains in a agar culture media containing either basic (methylene blue) or acidic dyes (Poly R-478), then picked potential ε-PL-producing isolates that formed haloes in the medium. This method has been used to obtain large numbers of new candidate isolates, especially members of two group of soil microflora, Streptomycete and ergot fungi (Nishikawa and Ogawa, 2002). Although such a method was determined to be accurate and sensitive, the toxicity of methylene blue can affect the detection rate of ε-PL-producing microbes. To address this issue, Li et al. (2011) proposed a “sandwich layer” method in which colonies from soil samples are first cultured on medium A, then the whole agar of medium A is lifted from the plate and transferred to the surface of medium B (containing the methylene blue indicator), thus forming a medium A-colonies- medium B “sandwich.” After incubation for 2 h at 40°C, strains forming haloes are then picked and purified. This method was successfully used to identify S. griseofuscus, which can yield 7.5 g/L ε-PL in fed-batch fermentation (Li et al., 2011).
“Two-stage culture” is another commonly used method for strain screening. Shima et al. (1983) proposed this method based on the discrepancy between the optimum pH of cell growth and ε-PL accumulation in S. albulus No. 346. Subsequently, Hirohara et al. (2006) developed the two-stage culture method in which each colony was first cultured in growth medium (pH 6.8) for 20–48 h (cell growth culture stage), then mycelium was collected, washed, resuspended, and incubated in production medium (ε-PL production culture stage) (Hirohara et al., 2006). The two-stage culture method is used extensively and many ε-PL producers have been successfully isolated and tested, such as Streptomyces sp. USE-11 and Kitasatospora sp. PL6-3 (Ouyang et al., 2006; Hirohara et al., 2007).
The “half-leaf” method is a recently reported protocol for screening ε-PL-producing actinomycete strains (Chen X. et al., 2019). The principle underlying this method is that ε-PL demonstrates an obvious protective and curative activity against Tobacco mosaic virus (TMV). Thus, the left side of a Nicotiana glutinosa leaf is treated with TMV and the secretion of Streptomyces strains, while the right side of the same leaf is treated only with TMV. By comparison of the numbers of local lesions on left and right side of N. glutinosa leaves, S. ahygroscopicus STZ was identified as an ε-PL producer.
Genome mining technique have also come into wide use for high-throughput screening for strains that carry genes encoding putative Pls. Homologs of Pls in different species can show low sequence similarity, and hence, some ε-PL coding sequences have been overlooked during the gene annotation. S. albulus Pls is the most thoroughly studied Pls, and its homologs in Streptomycetes are well conserved. Geng et al. (2014) revealed that pls gene sequences from S. albulus NK660 showed 99 and 84% similarity with that from two other Streptomycete strains (S. albulus NBRC14147 and S. roseoverticillatus MN-10). Recently, a gene encoding a Pls-like protein was discovered in the genome of C. variabile by NCBI BLAST search, and although the similarity between this protein and Pls from S. albulus is only 51%, they share highly similar domain architecture (Jiang et al., 2021). This method also facilitated identification of several Pls homologs in the genomes of Coryneform actinobacteria (i.e., Brevibacterium, Arthrobacter, etc.) and Ascomycota fungi (Purev et al., 2020; Jiang et al., 2021).
In summary, there are several methods for successful screening of ε-PL-producing strains, including dye-based indicators, two-stage culture, and genome mining. Dye-based methods are straight forward but laborious, while “two-stage culture” and genome mining are more user-friendly, but require several time-consuming steps of mycelium cultivation and product detection or DNA sequencing and BLAST analysis, respectively. A more direct and simpler method, such as detection by HPLC, may also serve as a viable option for identification of ε-PL-producing strains.
Key Enzymes in ε-PL Biosynthesis Pathway
ε-PL synthetase (Pls, encoded by pls gene) has been characterized as a membrane-bounded non-ribosomal peptide synthase (NRPS)-like enzyme which catalyzes the polymerization of L-lysine monomers into ε-PL (Yamanaka et al., 2008). This enzyme has a typical NRPS adenylation domain (A domain) for the activation of substrate to aminoacyl-O-AMP, and a thiolation domain (T domain) that mediates localization and transfer of the activated substrate. However, the N-terminus of Pls carries neither a condensation domain (C domain) nor a thioesterase domain (TE domain), as found in typical NRPSs, but instead harbors three tandem domains (C1-C3) related to acetyltransferase functions and six transmembrane domains (TM1-TM6) (Figure 2) (Kito et al., 2013; Jiang et al., 2021). As mentioned above, Pls have been identified in some Ascomycota fungi and Coryneform actinobacteria. Interestingly, despite the low amino acid similarity (∼50%) between the amino acid sequences of Strepromyces albulus Pls and E. festucae Pls, or S. albulus Pls and C. variabile Pls, these three enzymes share similar domain architectures (Purev et al., 2020; Jiang et al., 2021). Beyond this, study also revealed that the acidic pH conditions in the ε-PL fermentation process facilitate the accumulation of intracellular ATP and Pls-mediated activation of substrate to aminoacyl-O-AMP, which are essential to the biosynthesis of ε-PL (Yamanaka et al., 2010).

FIGURE 2. Domain architectures and catalytic mechanisms of typical non-ribosomal peptide synthase (A) and ε-PL synthetase (in S.albulus, E. festucae and C. variabile) (B) (Yamanaka et al., 2008; Xu et al., 2016; Purev et al., 2020; Jiang et al., 2021).
As is well-known, ɛ-PL can be synthesized by Pls at pH 4.0, while hydrolyzed by ε-PL degradation enzyme (Pld) with the increasing pH at the end of the fermentation process (Kahar et al., 2001). The biological function of Pld is to maintain normal growth of the strain under the pressure of ε-PL by reducing its toxicity for the strain itself (Kito et al., 2002; Hamano et al., 2006; Yamanaka et al., 2010). Many efforts have been made to determine whether Pld participates in the regulation of ε-PL synthesis and control of polymer length. In vitro assays have shown that the incubation of Pls with ATP and L-lysine directly produced ɛ-PL of 3–17 L-lysine units, suggesting that biosynthesis and chain length of ε-PL can be directly controlled by Pls (Yamanaka et al., 2008). At present, two types of ɛ-PL degradation enzymes, named Pld and PldII, have been identified in ε-PL-producing Streptomyces, with exo-type and endo-type peptidase activities, respectively (Yamanaka et al., 2010). Theoretically, deletion of these two enzymes should reduce the loss of ɛ-PL and improve its overall titer during the fermentation. However, the inactivation of Pld and PldII showed no effect on the production or polymerization degree of ε-PL, which suggests that the biosynthesis and polymer length of ε-PL appears to be directly controlled by Pls, rather than Plds (Yamanaka et al., 2010).
Factors Affecting ε-PL Molecular Weight During the Biosynthesis
In general, the biofunctionalization and practical application of biopolymers are tightly associated with its molecular weight (Sha et al., 2019). For instance, low molecular weight (<400 kDa) poly-gamma-glutamic acid (γ-PGA) is more functional than high molecular weight (>1000 kDa) for some bioengineering applications, such as a crop cryoprotectant or synergist for fertilizers (2 kDa), calcium absorption enhancer (11 kDa), and drug carrier (45–60 kDa) (Lee et al., 2018). Molecular weight is a main factor affecting the antimicrobial activity of ɛ-PL. However, the bitter flavor of commercial ε-PL products (3.2–4.5 kDa) limit their application in the food industry. This problem could be potentially resolved by appropriately reducing the chain length of the ε-PL polymers. Hence, understanding the key factors that affect ε-PL molecular weight can open new avenues for optimizing ε-PL biosynthesis, as well as for engineering the targeted synthesis of novel ɛ-PLs with desired chain lengths.
Polydispersity of ε-PL molecular weight is primarily determined by the ε-PL-producing strain (Takehara et al., 2010). The majority of obtained ε-PL polymers have nearly the same molecular weight as the first ones that were discovered (3.2–4.5 kDa), though some are much lower (Xu et al., 2019). Hirohara et al. (2007) found some actinomycetes produced ɛ-PLs with molecular weights of 4.1, 3.6, 3.2, 2.4, and 2.0 kDa. Notably, Kitasatospora sp. PL6-3 secretes an ε-PL with the highest reported molecular weight (5.01 kDa), although ɛ-PL production is poor in this strain (Ouyang et al., 2006).
As mentioned above, Pls is a main factor determining the degree of ε-PL polymerization (Yamanaka et al., 2010). Thus, one study used a “gene swap strategy” to integrate a pls gene from a short-chain ε-PL producer Kitasatospora aureofaciens into the long-chain ε-PL-high-yielding S. albulus, which resulted in high-yield synthesis of short-chain ε-PL (Xu et al., 2019). To further identify which functional domain determines ε-PL chain length, Hamano et al. (2014) carried out a random mutagenesis screen of C-terminal Pls domains by targeted error-prone PCR. The results showed that mutations (Pls-L883P and Pls-W646S) in the linker regions that connect the TM (1 and 2) and TM (3 and 4) domains leads to shorter chain length (from 21–32 to 10–23-mer and 9–23-mer, respectively). Saturation mutation of W646 and L883 further obtained a series of enzyme variants that produced short-chain ε-PL. Interestingly, no mutation in Pls was found to induce longer length in ε-PL chains (Hamano et al., 2014).
Intracellular microenvironments can affect the synthesis of metabolites and their associated primary and secondary metabolic pathways. It warrants mention that the polymerization degree of ε-PL is affected by the microenvironment of the host cell. Polymerization degree of ε-PL produced by S. albulus NK660 was found to be lower than that produced by NBRC14147, despite their respective pls genes sharing more than 99% similarity (Geng et al., 2014). E. festucae E437 is a novel ε-PL-producing strain that can reportedly produce 10.4 mg/L ε-PL with 28–34 L-lysine residues. Overexpression of the fungal Pls (from E. festucae E437) in E. festucae F11 (which produces negligible ε-PL) resulted in a lower degree of ε-PL polymerization than that in E437, suggesting that host cell have an effect on the degree of polymerization (Purev et al., 2020).
The addition of short-chain alcohols, including butanol, glycol, propanediol, or butanediol can also affect ε-PL molecular weight (Chen et al., 2018; Ding et al., 2018). Nishikawa and Ogawa (2002) were the first to find an unknown ε-PL derivative when glycerol was used as a carbon source in the fermentation. This ε-PL derivative was then identified as an ester formed between the terminal carboxyl group of ε-PL and the hydroxyl group of glycerol. These ε-PL-polyol esters were formed during the L-lysine polymerization process, rather than through an exogenous esterification reaction. Moreover, the quantity of L-lysine residues in the ε-PL ester decreased commensurately with increasing polyol concentration. Further research revealed that the addition of polyol lowered the ε-PL molecular weight through a terminal carboxyl esterification reaction between ε-PL and polyol which inhibits ε-PL elongation (Nishikawa and Ogawa, 2006). Glycerol is widely used as a carbon source for producing ε-PL, instead of glucose, which results in lower polymerization and higher productivity (Chen and Mao, 2013; Chen et al., 2018). Chemical modification of cyclodextrin, e.g., with sulfate, can further enhance the effects of glycerol, reducing the ε-PL molecular weight from 3.5 to 4.5 kDa to less than 2.5 kDa (Nishikawa, 2009).
In light of these studies, the “gene swap strategy” and the addition of short-chain alcohols both appear to be effective methods for increasing the production of ε-PL with lower molecular weight.
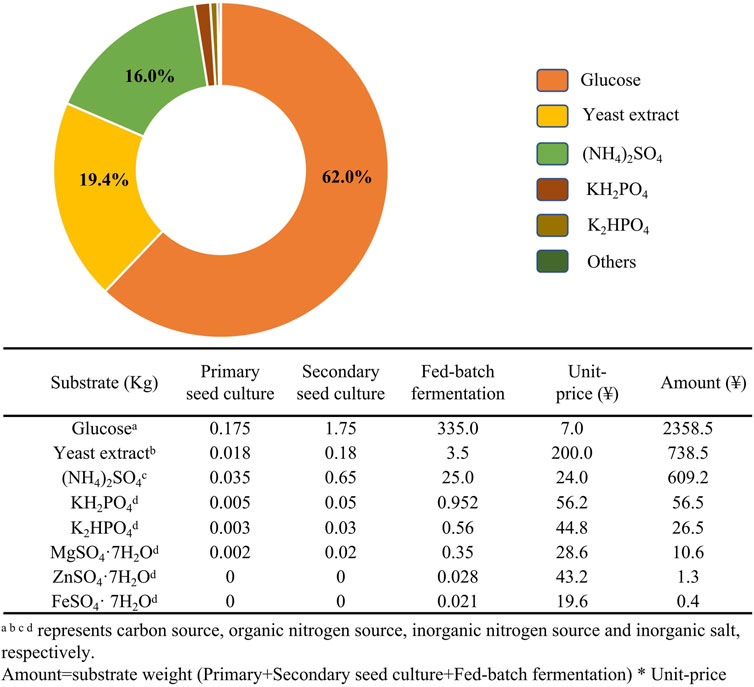
Strain Improvement
To promote the industrial adoption of ɛ-PL, many lines of research have focused on engineering strains with elevated capacity for ɛ-PL synthesis. Table 1 summarizes studies of cell engineering strategies and the corresponding levels of ɛ-PL obtained with different ɛ-PL producers, and the detailed information is discussed below.
Mutagenesis, Genome Shuffling and Ribosome Engineering
A strong ɛ-PL producer is the foundation of any commercially successful fermentation system, which can overcome the extremely slow growth rate and low ɛ-PL production of the wild-type strain. Due to the lack of effective genetic transformation methods for these recalcitrant bacterial strains, conventional mutagenesis served as the primary method for engineering new strains in the three decades following the initial discovery of ɛ-PL-producing strains (Shima and Sakai, 1977; Hamano et al., 2005). Since excess accumulation of L-lysine and L-glycine inhibit the activity of aspartokinase (Ask) in the L-lysine synthetic pathway, mutagenesis studies combined with screening for the “L-lysine analog (S-(2-aminoethyl)-L-cysteine (AEC))+glycine-resistant” mutants ultimately resulted in a mutant strain that could secrete 4-fold higher ɛ-PL than wild type (Hiraki et al., 1998). Currently, the utilization of atmospheric and room temperature plasma (ARTP) mutagenesis results in 18.46% improvement in ɛ-PL production, which may be attributable to an up-regulation in metabolite levels necessary for L-lysine synthesis and degradation pathways, based on metabolomic profiles (Xiang et al., 2020).
Carbon flux from substrate is another limiting factor for production. To increase substrate utilization, Li et al. (2012) used genome shuffling to obtain a recombinant strain with high glucose tolerance. Subsequently, genome shuffling was combined with interspecific hybridization of five ɛ-PL-producing Streptomyces to further improve the metabolic flow of the glycolysis pathway and TCA cycle, resulting in a strain that could produce 24.5 g/L ɛ-PL (Li et al., 2013). More recently, similar work facilitated the integration of traits from S. albulus and B. subtilis enabling ɛ-PL production 256.1% higher than that of the parent strain (Li et al., 2018). Additionally, since the auto-inhibition caused by the accumulation of ɛ-PL may limit cell growth and ɛ-PL biosynthesis, genome shuffling has been also used to rapidly increase the ɛ-PL tolerance and production (Zhou et al., 2015).
Another highly noteworthy strategy for semi-rational screening of high ɛ-PL producers is ribosome engineering. It has been reported that the introduction of resistance to some macrolide antibiotics can strongly activate the production of various secondary metabolites, including actinorhodin and salinomycin (Ochi, 2007; Zhang et al., 2019). Through this technique, mutations conferring resistance to streptomycin and five other antibiotics were successively induced in S. albulus to generate the S. albulus R6 mutant strain, which exhibited the highest reported ɛ-PL production of 70.3 g/L, 2.79-fold greater than the original strain, using an optimized acidic pH shock strategy (Wang et al., 2019). Mutations in protein S12 (Q856H) and RNA polymerase (R99P) of the R6 strain could be responsible for the increased transcription of the pls gene and elevated activities of key enzymes in ɛ-PL biosynthetic pathway, consequently increasing the level of Pls activity, as well as L-lysine and ATP concentrations available for ɛ-PL production (Wang et al., 2019). To explore the mechanism by which streptomycin resistance leads to higher productions, a high ɛ-PL-producing mutant was generated by continuous selection for streptomycin resistance (and hence increasing mutations) (Liu et al., 2019b). Comparative proteomics then revealed that up-regulated protein expression related to L-lysine metabolism, energy metabolism, transcriptional regulation and translation pathways together facilitated an intracellular metabolic environment conducive to secretion of ɛ-PL. Although ribosome engineering has rapidly and effectively enhanced ɛ-PL production, the regulatory relationship between the mutated ribosome and RNA polymerase and ɛ-PL metabolism remain unclear. Currently, the application of a high-throughput screen, or a combination of ribosome engineering with conventional screening and genome shuffling can together enable further improvement to the efficiency of generating high ɛ-PL-producing strains (Wang et al., 2016; Liu et al., 2017; Wang L. et al., 2017). However, these processes are time-consuming and labor-intensive, and it remains difficult to reroute metabolic flux towards ɛ-PL production through well-established methods.
Gene Engineering
Genetic information provided by whole genome sequencing can provide a comprehensive picture of intracellular physiology and metabolism. Comparative genome analysis, as well as proteomic and metabolomic approaches can also guide directed evolution of strains through rational genetic engineering (Wang et al., 2015b; Xiang et al., 2020). For example, genome analysis of fully sequenced ɛ-PL-producing S. albulus ZPM revealed the presence of more than 40 secondary metabolite biosynthetic gene clusters, approximately half of which were polyketide synthases (PKSs) or non-ribosomal peptide synthetases (NRPSs) (Wang et al., 2015b). This finding indicates the main challenges related to ɛ-PL production, i.e., low yield and low production, respectively arising due to co-products potentially generated by these biosynthetic gene clusters during fermentation (Figure 3), and unknown rate-limiting steps in the ɛ-PL biosynthetic pathway (Yamanaka et al., 2020). These issues together limit the application of genetic and metabolic engineering in ɛ-PL-producing strains.
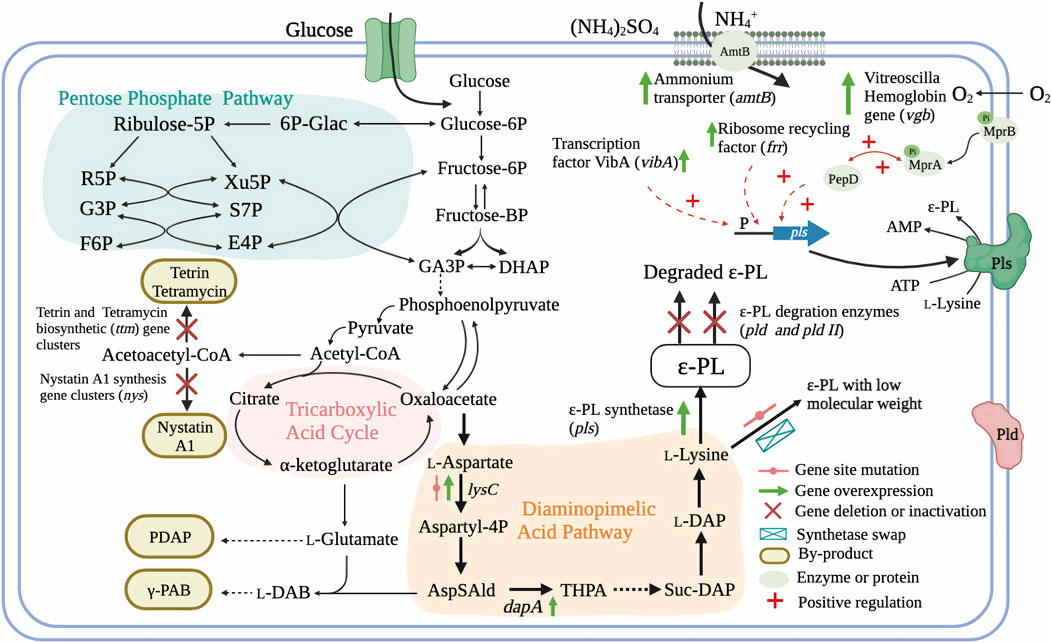
FIGURE 3. Genetic engineering strategies for the regulation of ɛ-PL production and molecular weight and in ɛ-PL-producing Streptomyces and endophytic fungi (Hamano et al., 2007; Takehara et al., 2008; Yamanaka et al., 2010; Xia et al., 2013; Hamano et al., 2014; Xu et al., 2015a; Xu T. et al., 2018; Liu et al., 2019a; Pan et al., 2019a; Xu et al., 2019; Purev et al., 2020; Wang A. et al., 2020; Yamanaka et al., 2020; Li et al., 2021). AmtB, ammonium transporter; MprA/B and PepD, a signal transduction system; GA3P, glyceratldehyde-3-phosphate; DHAP, dihydroxy acetone phosphate; L-DAB, L-diaminobutanoic acid; γ-PAB, γ -poly-L-diaminobutanoic acid; PDAP, poly-L-diaminopropionic acid; AspSAld, L-Asparte-Semialdehyde; THPA, Hydroxy-tetrahydrodipicolinate; Suc-DAP, Succinyl-diaminoheptanedioate; L-DAP, L-diaminoheptanedioate; ɛ-PL, ε-poly-L-lysine.
One commonly applied strategy for enhancing poly(amino acid) production such as γ-PGA and ɛ-PL involves increasing the intracellular availability of precursors (Cao et al., 2018). To construct a high-yield ɛ-PL producer, the first gene delivery system was developed for S. albulus (Hamano et al., 2005). Since wild-type Ask in S. albulus is subject to feedback inhibition by L-lysine and L-threonine, Ask was mutated (M68V) to eliminate this inhibitory effect and then homologously expressed in S. albulus, which showed increased biosynthesis of ɛ-PL (Hamano et al., 2007). More recently, dihydrodipicolinate synthase (DapA), a key enzyme in L-lysine biosynthesis pathway, has also been overexpressed for enhanced ɛ-PL production, which contributed to a 19.8% increase in ɛ-PL production by S. diastatochromogenes (Li et al., 2021).
L-lysine is the sole precursor of ɛ-PL, which is polymerized by membrane-associated Pls. Hence, enhancing Pls expression represents a viable strategy for increasing ɛ-PL productivity. One previous study showed that overexpression of the pls gene using the kasOp* promoter and ribosome binding site from phage Φ C31 capsid protein in a modified S. albulus strain resulted in 88.2% higher ɛ-PL production than that of wild type. Consequently, this strain achieved 20.1 g/L ɛ -PL in a 3 days fermentation (Wang A. et al., 2020). Similar results were observed in the endophytic fungus E. festucae E437 overexpressing the Epls (fungal ε-PL synthetase) gene, for which ɛ-PL production was enhanced 6.72-fold over that of wild type (Purev et al., 2020).
Since ε-PL is a secondary metabolite, its production is strictly regulated by intracellular regulatory mechanisms, as with other secondary metabolites. For instance, E. festucae F11 carries all of the genes necessary for synthesis of ε-PL, but these genes are not all transcribed and almost no ɛ-PL is produced. Introduction of the VibA transcription factor into E. festucae F11 led to induction of Pls activity and accumulation of ɛ-PL. Furthermore, overexpression of VibA in another E. festucae (E437) also led to a 3.7-fold increase in ε-PL production (Purev et al., 2020). Similarly, HrdD, a sigma factor mediating sensitive responses to pH change, was found to bind the pls gene promoter regions, suggesting the potential for pH-related regulation. A mutation in HrdD discovered through genomic comparisons between high- and low-yield strains provided a potential target site for engineering improved strains (Wang et al., 2015b). Moreover, proteomic analysis revealed that ribosome recycling factor is related to elevated expression of pls gene and ɛ-PL productivity in a hyper-yielding Streptomyces (Liu et al., 2019a). Transcriptome analysis of the acid tolerance response by S. albulus M-Z18 uncovered a signal transduction system (MprA/B and PepD) that can positively regulate the pls transcription, and thus could significantly enhance the production of ɛ-PL (Figure 3) (Pan et al., 2019a; Wang C. et al., 2020).
Elimination of metabolic pathways that divert a precursor or metabolite from the target molecule into unexpected byproducts, has often been reported to increase the concentration of target products. Most byproducts found in ɛ-PL-producing Streptomyces species were biopolymers or antibiotic compounds, such as poly(gamma-L-diaminobutanoic acid) (γ-PAB), γ-PGA, poly(L-diaminopropionic acid) (PDAP), and tetramycin A and B, among others (Takehara et al., 2008; Nishikawa and Kobayashi, 2009; Xia et al., 2013; Yamanaka et al., 2020). Interestingly, studies reporting low co-production of γ-PAB and PDAP with ɛ-PL, did not block or suppress these pathways in their efforts to increase ɛ-PL production. Recently, Yamanaka and colleagues revealed that genes for biosynthesis of five polyene macrolide antibiotics (tetramycin A and B, tetrin A and B, and a trace amount of nystatin A1) are also present in the genome of ɛ-PL-producing strain S. albulus 14147. Targeted inactivation of the four other antibiotics in a nystatin A1-deficient mutant led to enhanced ɛ-PL biosynthetic flux and an approximate 20% increase in ɛ-PL production (Yamanaka et al., 2020).
As a secondary metabolite, any enhancement to metabolic rates in the producer cell has the potential to improve ɛ-PL biosynthesis along with other metabolites. To this end, cellular metabolic activity and microbial biomass can be promoted in multiple ways. For instance, oxygen is a limiting factor for aerobic metabolism in submerged liquid fermentations, especially under high cell density. Oxygen supply is a well-known bottleneck in ɛ-PL fermentation because the increased ɛ-PL concentration and cell density result in heavy viscosity of the culture broth, consequently limiting oxygen transfer from the sterile gas to the growth medium. Vitreoscilla Hemoglobin (VHb, encoded by the vgb gene) can bind with oxygen at low concentrations and transport it to sites in the cell where greater oxygen supply is needed for metabolic processes. Expression of VHb has been historically used to enhance metabolic efficiency and biomass of microbial cells. For example, Xu et al. (2015b) introduced the vgb gene (GenBank No. M30794.1) into S. albulus PD-1, the mutant S. albulus PD-2 (containing pIB 139-vgb) presented an improved oxygen uptake rate, ATP levels, and transcription of the pls gene, which resulted in a 50.7% increase in ɛ-PL production compared to the wild type. Similarly, Gu et al. (2016) overexpressed vgb in S. albulus NK660, thereby leading to 26.7 and 14.6% increases in ɛ-PL production and cell dry weight, respectively, over that of wild type.
In addition to oxygen requirements, high nitrogen availability is also essential for L-lysine synthesis, since each L-lysine has two nitrogen atoms while most other amino acids contain only one nitrogen atom. To improve nitrogen translocation and utilization efficiency, the ammonium transporter gene amtB was overexpressed in S. albulus PD-1-amtB, resulting in an increase ε-PL production of 57.2% compared to the wild type (Xu D. et al., 2018). S-adenosylmethionine synthetase (SAM, encoded by the metK gene) is a methyl donor that provides a methyl group to several reactions necessary for primary and secondary metabolism. However, an S. albulus NK660 metK-overexpressing strain showed no discernible increase in ε-PL but had 9.79% more biomass than wild-type NK660 (Gu et al., 2016). Therefore, the combination of this strategy with other approach may redirect metabolic flux from cell growth to the biosynthesis of ε-PL. Collectively, although the transformation efficiency for gene editing of ε-PL-producing Streptomycete and endophytic fungi is still low, redirecting the metabolic pathways towards ε-PL biosynthesis has gradually become more feasible using genetic or metabolic engineering.
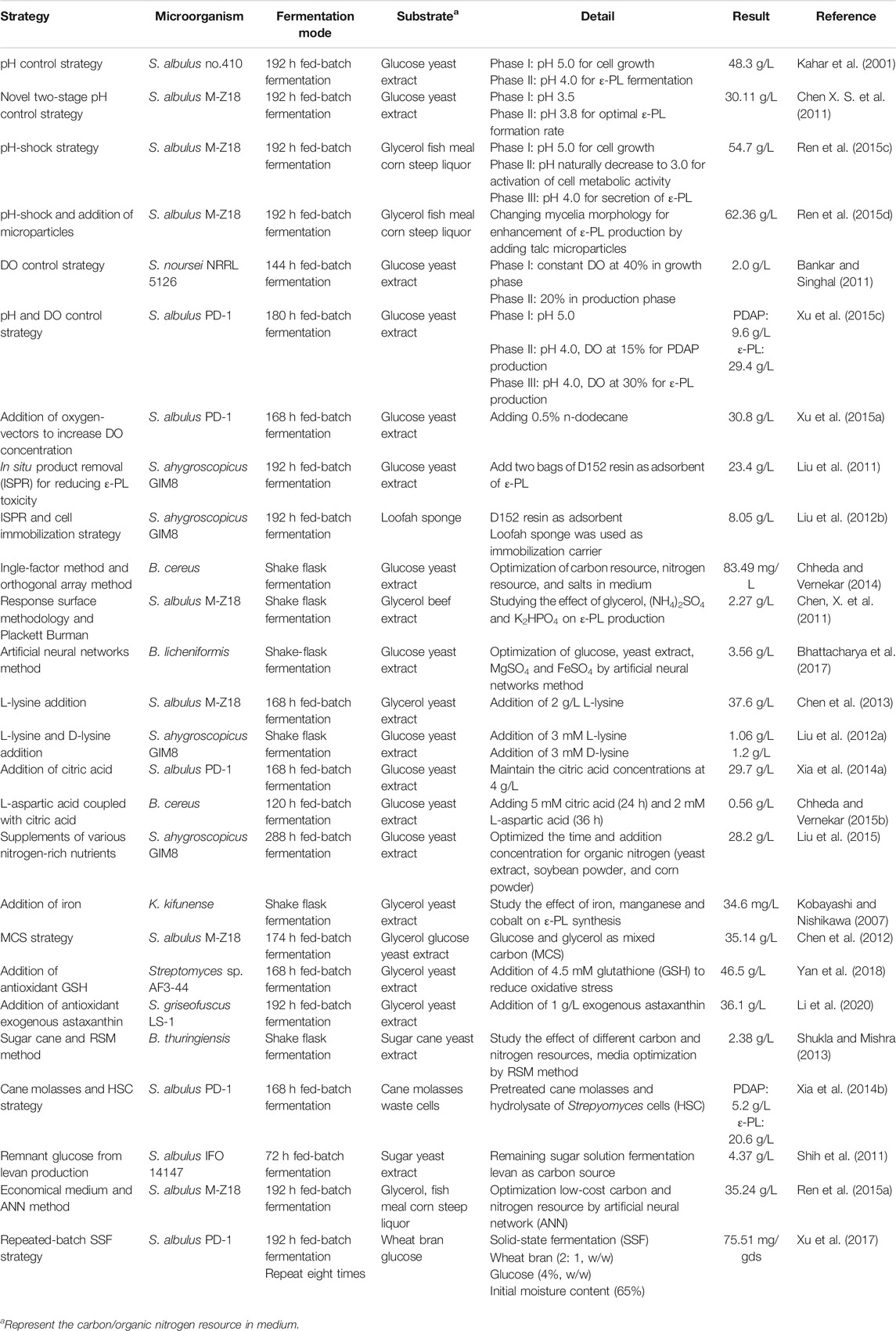
Process Engineering
Optimizing Environmental Conditions
In ɛ-PL fermentation, culture pH naturally decreases to about 3.0 if acidity is not controlled. In this case, the metabolism of ɛ-PL-producing strains can be seriously inhibited by fluctuations in the intracellular microenvironment, resulting in the low biomass and ɛ-PL production. Therefore, pH-control strategies through automatic feeding of an alkali solution (i.e., NaOH or NH4OH) is a reliable method for ensuring sufficient accumulation of high biomass. Kahar et al. (2001) found that pH 4.0 is optimal for ε-PL formation, while higher pH (>4.5) is better for cell growth. Therefore, a strategy to control pH was clearly advantageous in which pH was first maintained higher than 5.0 to increase biomass, then pH was allowed to naturally decrease to 4.0, where it was then maintained for accumulation of ɛ-PL. Under these conditions, ε-PL production was enhanced from 5.7 to 48.3 g/L. Through an analogous pH control strategy, Shih and Shen (2006) raised the pH for cell growth to 6.8 and observed a 258% increase in ε-PL production.
Recent research has shown that temporary (5–12 h) suppression of low pH in the exponential phase of S. albulus culture can contribute to higher cell viability and capacity for ε-PL synthesis. A two-stage pH control strategy was first proposed by Chen X. S. et al. (2011), with pH shifting from 3.5 to 3.8 at 36 h for optimal rates of ɛ-PL formation during the exponential and stationary phases, which ultimately tripled ε-PL production from 9.13 to 30.11 g/L. Based on this method, Ren et al. (2015c) divided the ε-PL fermentation into three stages and developed an acid pH-shock (PS) strategy, including 1) enrichment and cell culture at pH 5.0; 2) activation of cell metabolic activity at pH 3.0; and 3) secretion of ε-PL at pH 4.0. ε-PL production and accumulation rates were 54.7 and 6.84 g/L/day using this method, 52.50% higher than those of control (no pH shock). In addition to exceptional ε-PL production, biomass, mycelial viability, and intracellular ATP concentration were all significantly higher under PS condition than those of the control (Ren et al., 2015b). The underlying relationship between high ε-PL production, biomass, mycelial viability, ATP accumulation, and pH shock was further explained by transcriptomic profiling at the early, middle, and late stages of ε-PL fermentation, which showed up-regulation of transcriptional regulatory genes involved in nitrogen metabolism, energy metabolism, electron respiratory chain, and redox homeostasis (Pan et al., 2019a). Other work has shown that the MprA/B and PepD signal transduction system, which positively regulate the transcription and expression of pls gene, are also activated by pH shock. Deletion of mprA/B and pepD genes resulted in 83.5–93.2% and 52.1–60.6% respective reductions in pls transcription and ε-PL production (Pan et al., 2019b). Acid tolerance response by S. albulus has also been considered a contributing factor in high ε-PL production. Pan et al. (2017) found that induction of pH stress (pH 3.0) in the PS strategy enhanced acid tolerance by S. albulus. Transcriptomic analysis at different environmental pH levels (3.0, 4.0, and 5.0) further revealed that pH stress induced an overall acid tolerance response by S. albulus M-Z18, while concomitant ε-PL production was proposed to be a response mechanism to combat acid stress (Figure 4) (Wang A. et al., 2020).
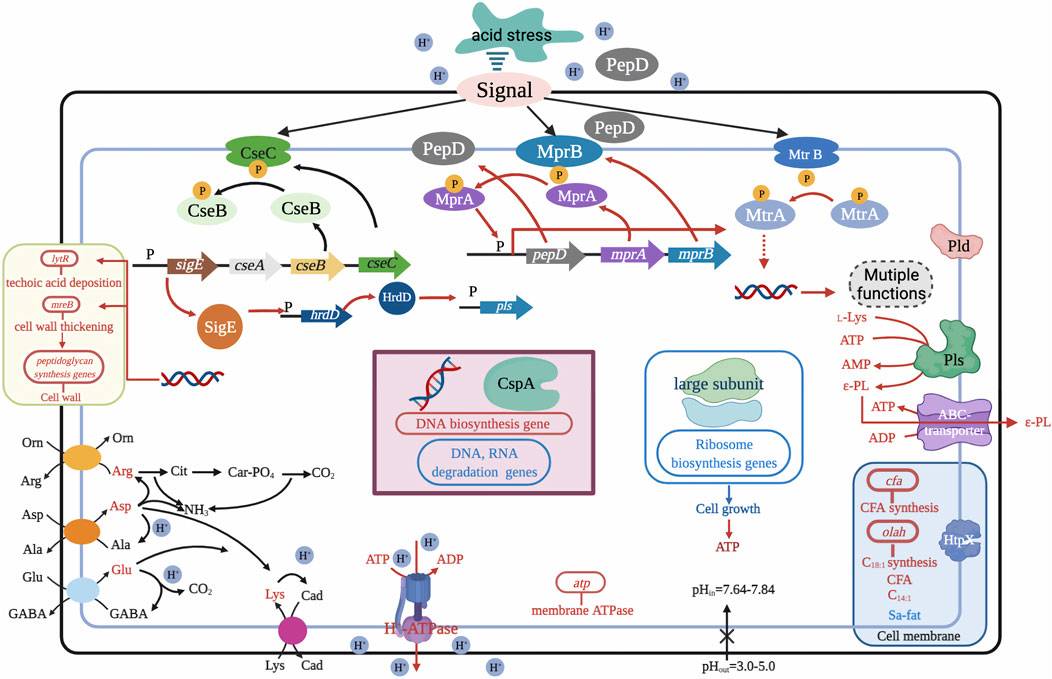
FIGURE 4. Possible mechanism for the acid tolerance response by S. albulus M-Z18. Figure modified from Wang C. et al. (2020). Arg, arginine; Asp, aspartate; L-Lys, L-lysine; C18:1, oleic acid; Orn, ornithine; Ala, alanine; Cad, cadaverine; C14:1, myristoleic acid; Cit, citrulline; Glu, glutamate; ε-PL, ε-Poly-L-lysine; Sa-fat, saturated fatty acid; Car-PO4, carbamoyl phosphate; GABA, γ-aminobutyrate; CFA, cyclopropane fatty acid.
Dissolved oxygen (DO) is also an important parameter in the ε-PL fermentation process for its effects on ε-PL biosynthesis, mycelial biomass, and substrate utilization rates. However, oxygen supply is often limited in ε-PL fermentation broth because of high oxygen consumption and cell density. Bankar and Singhal (2011) proposed a two-stage DO control strategy for increased biomass and ε-PL production in Streptomyces noursei NRRL 5126 through investigation of growth kinetics under different aeration, agitation, and DO levels. The most appropriate DO levels during the production phase differed depending on the desired products. An interesting strategy was then developed for co-producing PDAP and ε-PL by controlling pH and DO concentrations. In this strategy, pH control is similar to that of Kahar’s strategy, while DO was successively maintained at 15 and 30% for PDAP and ε-PL production, respectively. This method led to an increased yield of 21.8%, which is the highest reported yield among similar production systems (Xu et al., 2015c). However, the high agitation speeds and aeration require high power consumption and lead to undesirable impacts on mycelial morphology and ε-PL production.
Another method to solve the problem of oxygen limitation is the addition of an oxygen-vector. Xu et al. (2015a) improved oxygen supply and maintained DO concentration at >32% saturation by adding 0.5% n-dodecane in broth. The enhanced ε-PL and biomass concentrations (by 31.6 and 20.7%, respectively) under conditions of elevated oxygen were likely related to stronger carbon and energy metabolism. In addition, Vitreoscilla hemoglobin, mentioned above, can also help to enhance respiration and oxidative phosphorylation by binding and delivering the oxygen to terminal respiratory oxidase (Xu et al., 2015b; Gu et al., 2016).
Additionally, some novel strategies have also been proposed for increasing ε-PL production. Notably, in situ product removal (ISPR) is an integrated method that combines production and separation for avoiding end-product toxicity/feedback inhibition, thereby simultaneously enhancing the overall process efficiency. Liu et al. (2011) selected ion-exchange D152 resin as an effective adsorbent to develop a resin-based ISPR system, ultimately obtaining 3.62- and 6.22-fold increases in ε-PL production in shake-flask and 5-L fermenter, respectively. In other research, ISPR was combined with a cell immobilization technique using a loofah sponge as an immobilization carrier. Through this combination method, immobilized cells of S. ahygroscopicus GIM8 could be repeatedly used, resulting in a 10-fold increase in ε-PL production, from 0.82 to 8.05 g/L (Liu et al., 2012b).
Optimizing Nutrient Supply
Media composition can directly affect cell growth and biosynthesis by microorganisms. M3G medium (Figure 5) was the first reported medium for ε-PL fermentation and remains commonly used. Various approaches for optimizing medium have been designed based on this original culture medium, such as the single-factor method, orthogonal array method, response surface methodology (RSM), and artificial neural networks (ANN). These methods have facilitated significant (56.4–200%) improvements to ε-PL production (Chheda A. H. and Vernekar M. R., 2015; Bhattacharya et al., 2017; Guo et al., 2018). Among these studies, Plackett-Burman assays showed that sources of carbon (typically glucose and glycerol) and organic/inorganic nitrogen (i.e., yeast extract, peptone, and (NH4)2SO4) were major influencing factors in ε-PL production (Chen X. S. et al., 2011; Guo et al., 2018).
Providing nutrients that participate in the ε-PL metabolism is another effective method for enhancing ε-PL production. Chen et al. (2013) improved ε-PL production by 6.2% through the addition of L-lysine to broth, and determined that the addition of L-lysine directly contributed to ε-PL biosynthesis by L-[U-13C] lysine labeling. Similarly, Liu et al. (2012a) discovered that the addition of L-lysine or D-lysine could lead to greater ε-PL production and biomass, although these two amino acids participated in different metabolic pathways in the ε-PL-producing strain. Citric acid is an important TCA cycle intermediate, and the addition of citric acid strengthened ε-PL synthesis while reducing the PDAP by-product. The regulatory mechanism underlying this effect is potentially due to citric acid inhibition of the TCA cycle, which redirects the metabolic flux into the L-aspartate biosynthetic pathway, thereby increasing the ε-PL production from 21.7 to 29.7 g/L (Xia et al., 2014a). Based on the above evidence, feeding L-aspartate coupled with citric acid could further lead to higher productions in B. cereus and S. noursei by 6-fold and 5.5-fold, respectively (Chheda A. H. and Vernekar M. R., 2015; Chheda A. H. and Vernekar M. R., 2015). Analogously, supplementation with various nitrogen-rich feedstocks, such as beef extract, soybean powder, yeast extract, fish meal, and corn steep liquor can provide abundant L-aspartate, L-glutamate, L-arginine, and L-lysine, which can remarkably improve rates of cell growth and ε-PL synthesis (Liu et al., 2015). Iron, manganese, and cobalt are also reportedly involved in the expression of pls genes. Kobayashi and Nishikawa (2007) added the above metals in liquid culture medium, which enhanced the production of ε-PL by Kitasatospora kifunense.
Low-cost Agri-Industrial Wastes Fermentation
The price of medium represents a substantial proportion of the operating costs in ε-PL industrial fermentations. In 192 h S. albulus fermentation in a 50-L bioreactor, the cost of the carbon source (glucose) accounts for 62.0% the total substrate costs for M3G medium (Figure 5). Thus, lower cost agri-industrial waste products, such as crude glycerol and sugar cane have been tested as attractive potential alternative carbon sources for ε-PL fermentation.
Streptomyces dissimilates glycerol (a waste product of biodiesel production) via the phosphorylative pathway. In S. albulus, glycerol is converted into glycerol-3-phosphate by glycerol kinase (GK), then oxidized into dihydroxyacetone phosphate (DHAP) by glycerol-3-phosphate dehydrogenase (G-3-PDH), which then directly participates in the glycolysis pathway or enters the gluconeogenesis pathway after it is isomerized to glyceraldehyde-3-phosphate. Previous studies have demonstrated that the rate of ε-PL formation by S. albulus cells grown on glycerol was 40% higher compared with cells grown on glucose. This result may be attributable to the 25% higher flux of glycerol into ε-PL synthesis compared to glucose (Chen and Mao, 2013). Glycerol-derived ε-PL has a characteristically lower chain length and toxicity for ε-PL-producing strains, which could at least partially explain the enhanced production of ε-PL (Ding et al., 2018). In addition, the activities of GK and G-3-PDH, key enzymes in glycerol assimilation pathway, may be inhibited by a limited oxygen supply in later fermentation stages (Du et al., 2021). Hence, tests of a glucose-glycerol mixed carbon source (MCS) for culture S. albulus resulted in a substantially higher growth rate and ε-PL productivity (Chen et al., 2012; Zeng et al., 2014). Transcriptional analyses further showed that glucose and glycerol could be simultaneously utilized in a MCS with no mutual transcriptional suppression, thus providing more carbon skeletons for glycolysis pathway (Zeng et al., 2017). The overall strengthened central carbon metabolism, amino acid metabolism, energy metabolism, and Pls activity could all contribute to the accumulation of higher biomass and ε-PL production (Zhang et al., 2018; Zeng et al., 2019). However, the rate of ε-PL biosynthesis with MCS declined sharply in the late phase of fed-batch fermentations. Cell physiology and transcriptomic analyses showed that excessive oxidative stress induced by reactive oxygen species (ROS) could contribute to the decreased capacity for ε-PL synthesis (Zeng et al., 2015). This problem could be partly solved by the addition of reducing agents, such as antioxidant glutathione and exogenous astaxanthin, which could ameliorate excessive oxidative stress and help to maintain a high ε-PL formation rate during the fermentation (Yan et al., 2018; Li et al., 2020).
As a by-product of the sugar industry, sugar cane has also been applied in ε-PL fermentation by S. albulus and Bacillus sp. (Shukla and Mishra, 2013; Xia et al., 2014b). When pretreated cane molasses and hydrolysate of Streptomyces cells (HSC) were used as carbon and nitrogen sources, respectively, 20.6 g/L of ε-PL and 5.2 g/L of PDAP were obtained in 1-t fermenter (Xia et al., 2014b). Another method utilizing remnant glucose from levan production as an alternative carbon source produced 4.37 g/L of ε-PL, which is environmentally friendly and economical since it converts outdated feedstocks into high-value products (Shih et al., 2011).
The price of organic nitrogen sources (e.g., yeast and beef extracts) represents the second largest proportion (19.4%) of substrate costs. Corn powder (biowaste corncob residue), fish meal, and soybean powder are commonly used substitutes for organic nitrogen sources (Figure 5). Ren et al. (2015c) produced 54.7 g/L ε-PL by culturing S. albulus M-Z18 on glycerol and fish meal. These feedstocks combined with talc microparticles to alter mycelial morphology resulted in ε-PL production as high as 62.36 g/L, which is the highest reported production from agri-industrial wastes (Ren et al., 2015d). Recent work by Xu et al. (2017) in which a repeated-batch solid-state fermentation (SSF) was performed using a mixed carbon source of wheat bran (2:1, w/w), rapeseed cake, and glucose (4% w/w) showed a maximum ε-PL production of 86.62 mg/g substrate after eight repeated batches of SSF. Overall, several innovative strategies have been developed to increase ε-PL accumulation and reduce the cost of raw materials, with varying success (Table 2).
Downstream Processes
Downstream processing has been reported to occupy an important place in production, and its cost can reach 50–70% of the total production cost in some industrial biotechnology fields (Wei H. et al., 2018). Most ε-PL purification routes are based on ion-exchange strategies. The first ε-PL purification route consisted of cation ion-exchange adsorption, activated-carbon decoloration, organic-solvent precipitation, and gel chromatography (Shima and Sakai, 1981). Zhu et al. (2016) deveploped the purification process by applications of strong cation (HZB-3B) and weak cation (D155) resins, leading to high desorption efficiency and overall recovery of 97.57 and 94.49%, respectively. However, most ε-PL purification processes are for applications requiring chemical structure identification, and organic solvents used in the above methods could hide some dangers for future industrial production. In 2010, Jia et al. (2010) used an ultra-filtration technique with 2 and 5 kDa cut-offs to fractionate ε-PL, the largest advantage of this approach was that the ε-PL product could be controlled to ensure a relatively high molecular weight (3.2–4.5 kDa) product. However, the high cost of freeze drying remains a problem for industrial applications.
Combinatorial approaches for higher ε-PL purity and recovery have been developed in consideration of the strengths and weaknesses of the above methods. For example, Bankar et al. (2014) loaded culture broth sequentially onto a chromatographic column (Amberlite IRC 50), followed by ultrafiltration (20 and 5 kDa), solvent precipitation, and gel permeation chromatography, finally obtaining products with a purity of 97.58% and ε-PL yield of 90.42% under the optimal conditions. For large scale purification, Chen et al. (2018) sequentially used 30 kDa ultra-filtration, ion-exchange adsorption, macroporous resin and 1 kDa ultra-filtration for protein removal, purification, decolorization and desalting. To further improve the efficiency of ε-PL purification, cation ion-exchange resin (IRC-50) with NH4+ has been suggested to offer the highest adsorption capability and desorption ratio for ε-PL extraction, and ε-PL purity was enhanced from 90.2 to 97.10% under optimal conditions (Chen J. et al., 2019). Furthermore, another method combing ion-exchange resin (Amberlite IRC-50), macroporous adsorption resin (SX-8), and gel column chromatography (Sephadex G-25) can also extract ε-PL from the culture broth successfully (Chen X. et al., 2019).
Precipitation is a rapid and relatively simple method for high recovery of ε-PL. Katano et al. (2012) first used the tetraphenylborate anion to precipitate and separate ε-PL from a 100-ml fermentation broth, rapidly obtaining ε-PL at a 95% recovery rate. These workers subsequently developed the dipicrylamine anion (a yellow anionic dye) for precipitating polycationic ε-PL (Katano et al., 2015). Metal-chelate affinity precipitation was also used to separate and purify ε-PL, which afforded electrophoretic purity with recovery as high as 98.42% (Li et al., 2017a). Although precipitation processes can obtain high ε-PL purification from small volumes of fermentation broth containing ε-PL, the heavy use of acetone can introduce food-safety problems in industrial production.
Recently, aqueous two-phase extraction was developed as an environmentally friendly method for selective separation of ε-PL from fermentation broth. To this end, Sequeira et al. (2021) constructed four kinds of ionic liquid aqueous biphasic systems by using different ionic liquids with polypropylene glycol, consequently reaching 93.42% extraction efficiency for ε-PL in culture broth. An aqueous biphasic system can also be combined with ultra-filtration for large scale ε-PL purification. In this system, ε-PL from culture broth was subjected to triplicate extraction by ethanol/ammonium aqueous two-phase system, followed by desalting by ultra-filtration, which together provided new avenues for ε-PL purification (Chen et al., 2020). Although it resulted in a high purity (92.39%) ε-PL product at an acceptable recovery ratio (87.72%), this extraction method may cause some the environmental and economic problems.
Overall, many operations such as filtration, extraction, drying and sub-packaging are required for downstream ε-PL processing, which can cumulatively increase the cost. To date, the drawbacks in downstream processing have posed one of the major obstacles limiting the commercialization of ε-PL.
Recent Advances in Applications of ε-PL
At present, ε-PL is mainly used as an effective food preservative to control pathogenic microorganisms and food spoilage due to its safety and strong antimicrobial activity. Many recent applications of ε-PL in biomedicine industry, such as dressing material and suture, also depend on its antimicrobial capacity (Fursatz et al., 2018; Peng et al., 2020). As a consequence, the mechanisms underlying its antimicrobial effects has become a hot research topic in recent years (Shu et al., 2021). To elucidate its antimicrobial mechanism, we focus on describing the action mode of ε-PL on various microorganism, including its effect on the cell wall, cell membrane, cytoplasm, gene and protein expression as well as the metabolic pathways. On this basis, recent applications of ε-PL in food and pharmaceutical industries were briefly summarized.
Antimicrobial Property and Mechanisms of ε-PL
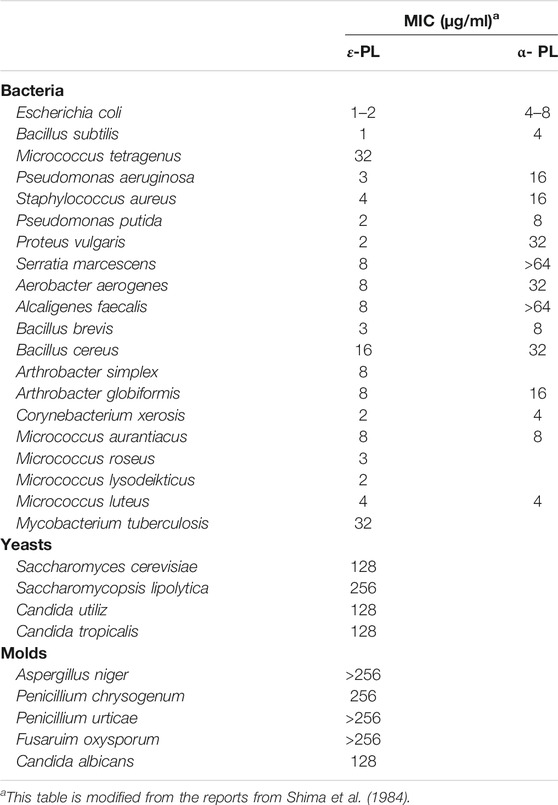
As a natural, cationic antimicrobial peptides, ε-PL is well known for its strong inhibition of bacteria, fungi, and viruses (Shima et al., 1984; Mookherjee et al., 2020). The strength of the inhibitory effect on cell growth by ε-PL is evaluated in Table 3. It is observed that in comparison with α-PL (6.4 kDa), ε-PL (3.2–3.8 kDa) was more effective against bacteria. Furthermore, both Gram-positive and Gram-negative bacteria have been found to be sensitive to naturally occurring ε-PL (1–8 μg/ml), but a similar inhibitory effect is only observed in yeast and filamentous fungi at much higher concentrations (>128 μg/ml).
Multiple reports have described that ε-PL inhibits the growth of various microorganisms by affecting the integrity and permeability of their cell walls and cell membranes (Hyldgaard et al., 2014; Wei M. et al., 2018; Lan et al., 2019; Su et al., 2019; Liu H. et al., 2020; Dou et al., 2021). For example, in Escherichia coli O157:H7 (Figure 6A), the interactions between ε-PL and phospholipid groups result in distorting and shriveling of the cell membrane in a non-specific, carpet-like mechanism, in which the membrane is forced to fold in on itself, contributing to vesicle/micelle and toroidal pore formation (Hyldgaard et al., 2014). Additionally, in Alternaria alternata and Botrytis cinerea, ε-PL was also observed to inhibit spore germination and germ tube elongation (Figure 6B), leading to abnormal morphological development (Jiao et al., 2020; Li et al., 2019; Liu H. et al., 2020).
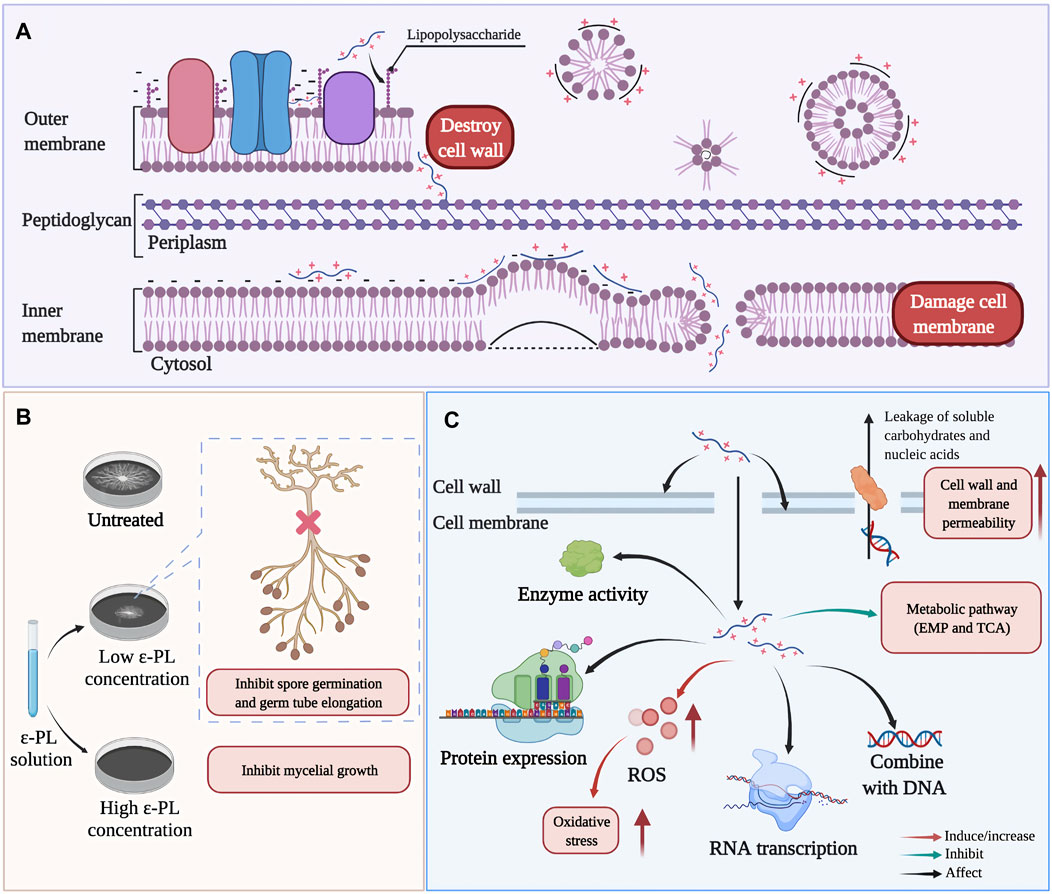
FIGURE 6. Possible antimicrobial mechanisms of ε-PL. (A) the action of ε-PL on cell structure of Escherichia coli O157:H7 (Ye et al., 2013). (B) The inhibition of ε-PL on spore germination and germ tube elongation (Liu Z. et al., 2020). (C) Summary of main antibacterial actions of ε-PL on different microorganism, such as E.coli, Shewanella putrefaciens, Staphylococcus aureus, Saccharomyces cerevisiae, Salmonella Typhimurium (Ye et al., 2013; Tan et al., 2019; Hou et al., 2020; Shen et al., 2020; Hou et al., 2021).
Deeper research has revealed that upon entry into the cytoplasm, ε-PL stimulates the production of ROS, and can affect cellular responses to oxidative stress and self-defense, ultimately retarding respiration, impeding cell viability, and thus potentially inducing cell death. For example, treatment with ε-PL can induce the accumulation of intracellular ROS and elevate expression of oxidative stress genes (sodA and oxyR) in E. coli O157:H7, while decreasing transcription of virulence genes (eaeA and espA) and SOS response genes (lexA) (Ye et al., 2013). In addition to killing some pathogens of humans, animals, or plants, ε-PL can also trigger host defense signaling in the infected organisms. For example, in jujube fruit infected with grey mold (caused by B. cinerea), the expression of defense response (respiratory burst oxidase homolog, RBOH) gene was induced by ε-PL treatment, while in tomato (Solanum lycopersicum), ε-PL treatment led to activation of specific antifungal defense-associated transcripts (β -1,3- glucanase, chitinase, and other PR proteins) (Sun et al., 2017; Li et al., 2019). Recently, high-throughput omics-based techniques greatly advanced our understanding of the impacts of ε-PL on cellular metabolism and related physiological effects. Proteomic screens have revealed that exposure to ε-PL can down-regulate the expression of protein-synthesis components in Shewanella putrefaciens (Hou et al., 2021). Similarly, genome-wide DNA microarray analysis showed that ε-PL could inhibit the ability of Salmonella typhimurium to colonize food by down-regulating the expression of genes necessary for biofilm formation (Shen et al., 2020). In addition, metabolomics analysis also demonstrated that ε-PL-induced stress resulted in the inhibition of primary metabolic pathways (mainly glycolysis and the tricarboxylic acid cycle (TCA cycle)) in Saccharomyces cerevisiae and Staphylococcus. aureus (Bo et al., 2014; Tan et al., 2019) (Figure 6C).
Recent Applications of ε-PL
Given the natural and highly effective cationic antimicrobial properties, ε-PL and its derivatives have various applications in food, biomedicine and biomaterial industries. Although naturally occurring ε-PL has a bitter flavor, ε-PL is widely used as food preservative to improve the quality and shelf life of numerous foods, including starch-based foods, seafood, dairy products, as well as fruits and vegetables (Tuohetisayipu et al., 2019). For instance, Li et al. (2017b) found that alginate-based edible coating containing 0.05% ε-PL significantly inhibited the growth of yeast and mould, while maintained the green color, total chlorophylls content, and antioxidant capacity of the fresh-cut kiwifruit. In addition, the combination of ε-PL with other food additives can further improve the antimicrobial effect (Sun et al., 2018). For example, minimal inhibitory concentration of ε-PL and nisin (another natural food preservative) towards spoilage Lactobacillus plantarum were and 75 and 0.468 mg/L, respectively, while the combined use of ε-PL and nisin at much smaller concentrations (9.375 and 0.05 mg/L) can completely inhibit the growth of spoilage L. plantarum (Bortolotto et al., 2021). In another research, the combined use of ε-PL (200 mg/L) and chitooligosaccharide (400 mg/L) also showed much stronger antifungal activity against B. cinerea than the use of ε-PL (200 mg/L) or chitooligosaccharide (400 mg/L) alone (Sun et al., 2018).
Furthermore, ε-PL has also been adopted as an antimicrobial emulsifier by conjugation with dextran through the Maillard reaction. The conjugate obtained from this process has characteristics superior to those of many commercial emulsifiers, such as Sunsoft SE-11 and Q-18S (Li et al., 2014). As a dietary agent, ε-PL can reportedly prevent high-fat diet-associated weight gain by inhibiting lipase activity, which results in suppression of dietary fat absorption from the small intestine (Tsujita et al., 2006; Tsujita and Takaku, 2009). The lipid-lowering effects and structural stability of ε-PL can be further improved by formation of a complex between cationic ε-PL and anionic pectin through electrostatic interactions (Song et al., 2017). Very recent research has shown that ε-PL could affect nutrient utilization (i.e., proteins, lipids, and fiber) by regulation of the gut microbiota in Ningxiang pigs (Zhang et al., 2020).
In the biomedical industry, ε-PL was originally used as an effective antitumor and antiviral adjuvant for introducing high levels of serum interferon in monkeys, chimpanzees, and humans (Levy et al., 1969; Wakamoto et al., 2007). However, recent studies have shown that ε-PL can serve as an effective drug carrier due to its high biocompatibility in target cells, high drug loading capacity, wide range of molecular sizes appropriate for specific needs, as well as non-toxicity and excellent biodegradability in target cells (Chen et al., 2021). For example, Wu et al. (2020) used metformin hydrochloride (MetHCl) as a model drug, and designed dissolving tablets consisting of a carboxymethylcellulose/poly-L-lysine/tripolyphosphate complex (CMC/PLL/TPP). This formulation showed a high drug loading efficiency (85.76%) and rapid drug dissolution and release profile (i.e., complete release within 10 min in simulated saliva) in the environment. These results implied that this ε-PL complex could be potentially used as a viable drug delivery system (Wu et al., 2020). In addition, ε-PL and its complexes, such as pluronic grafted dendritic α, ε-PLs (DPL-PF127) and ε-PL-sodium alginate nanoparticles, have been used as novel carriers for delivery of non-viral genes and vaccines (Dung et al., 2016; Yuan et al., 2018). Another recent report described a magnetic mesoporous silica/ε-PL nanomotor-based complex with strong potential for application in treatment of heavy metal poisoning due to its high capacity for removal of excessive lead ions from blood (Liu J. N. et al., 2020).
ε-PL has also been used in the production of other biomaterials, such as antimicrobial hydrogels for wound healing dressings, biosensor for direct detection of triglycerides in whole blood and as a fiber-based biosorbents for microbial inactivation and dyes removal (Wang R. et al., 2017; Xu T. et al., 2018; Jiang and Zhou, 2021). Furthermore, ε-PL has been reported to function as an ideal protectant for enzymes because its linear structure provides sufficient space for modification of enzyme surfaces by its amino groups, thus enhancing accessibility to the active site (Huang et al., 2019).
Conclusions and Future Prospects
ε-PL is an industrially attractive molecule due to its great potential as an antibacterial food preservative, therapeutic gene or drug carrier, enzyme stabilizer, and component of cosmetics. Since its discovery in 1977 by Shima and Sakai, research on ε-PL has spanned 44 yr (Shima and Sakai, 1977). Current research has enhanced the biosynthetic capabilities of ε-PL-producing strains through cell engineering approaches including mutagenesis, genome shuffling, ribosome engineering, and genetic engineering. Many studies have also attempted to enhance the efficiency of ε-PL biosynthesis and reduce costs through development of optimized industrial production strategies and utilization of agri-industrial wastes. Of course, many problems such as regulation of the complex metabolic networks, low productivity and conversion rate for ε-PL biosynthesis, have hindered its broad commercial adoption.
The development of different strategies is thus necessary to achieve stable, high-yielding strains and to enhance the biosynthesis of ε-PL. First, genetic and metabolic strategies can be used to enhance the biosynthetic pathway activity while inhibiting or eliminating competing pathways. Multi-omics comparative analyses between high-yield (obtained by conventional methods) and low-yield strains can also provide abundant candidate sites for targeted mutagenesis and directed strain evolution by metabolic engineering. In addition, with the rapid development of synthetic biology, the minimal genome required for ε-PL production could be determined and assembled, thus possibly enabling ε-PL biosynthesis in different or non-model microorganisms, facilitated by advanced tools for genetic modification. Industrial microorganisms are subject to a variety of environmental stress-inducing factors and adverse conditions, and adaptive evolution may be an effective strategy to improve their tolerance to these environmental stresses. ε-PL biosynthesis could also be potentially improved through directed evolution of ε-PL synthetase, enhancing its acid tolerance and catalytic efficiency under low pH. In addition, since high concentration of ATP is required for industrial ε-PL synthesis, it may be fruitful to regulate ε-PL biosynthesis through enhanced oxygen or ATP availability, through genetic engineering, or novel strategies for oxygen delivery, and new bioreactors. In view of its sophisticated ATP synthesis and metabolic capabilities, novel ε-PL-producing strains can be engineered with a Streptomyces chassis to produce other high-ATP-requirement products, such as hyaluronic acid (Yoshimura et al., 2015).
Overall, as a high-value product, ε-PL has a wide range of prospective industrial applications that could benefit from its safe and bioactive properties. With further understanding of its biomanufacturing process, more advanced biotechnologies will be used to enhance the biosynthetic capacity for this product, reducing the cost of biomanufacturing and accelerating its industrialization, ultimately promoting the future commercial application of ε-PL.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
LW, XC, ZR, and XX designed the framework of this article. LW, CZ, and JZ searched and summarized the literature, LW wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the following: National Key R&D Program of China (2020YFA0907700); National Natural Science Foundation of China (31901622 and 31671846); Natural Science Foundation of Jiangsu Province (BK20190585 and BK20191332); Program of the Key Laboratory of Industrial Biotechnology, Ministry of Education, China (KLIB-KF202007 and KLIB-KF202010); Program of Introducing Talents of Discipline to Universities (111-2-06).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bankar, S. B., and Singhal, R. S. (2011). Improved Poly-L-Lysine Biosynthesis Using Streptomyces Noursei NRRL 5126 by Controlling Dissolved Oxygen During Fermentation. J. Microbiol. Biotechnol. 21 (6), 652–658. doi:10.4014/jmb.1103.02007
Bankar, S. B., Chaudhary, S. A., and Singhal, R. S. (2014). Purification and Characterization of Poly-Epsilon-Lysine from Streptomyces Noursei NRRL 5126. J. Sci. Ind. Res. (India) 73, 33–40.
Bhattacharya, S., Dineshkumar, R., Dhanarajan, G., Sen, R., and Mishra, S. (2017). Improvement of ε-polylysine Production by Marine Bacterium Bacillus Licheniformis Using Artificial Neural Network Modeling and Particle Swarm Optimization Technique. Biochem. Eng. J. 126, 8–15. doi:10.1016/j.bej.2017.06.020
Bo, T., Liu, M., Zhong, C., Zhang, Q., Su, Q. Z., Tan, Z. L., et al. (2014). Metabolomic Analysis of Antimicrobial Mechanisms of ε-Poly-l-lysine on Saccharomyces cerevisiae. J. Agric. Food Chem. 62 (19), 4454–4465. doi:10.1021/jf500505n
Bortolotto, F. C. K., Farfan, M. H. d. R., Jede, N. C. K., Danielski, G. M., and Macedo, R. E. F. d. (2021). Nisin and ε-poly-L-lysine as Natural Antimicrobials Towards Spoilage-Associated Lactobacillus Plantarum. Cienc. Rural 51 (2), e20200423. doi:10.1590/0103-8478cr20200423
Cao, M., Feng, J., Sirisansaneeyakul, S., Song, C., and Chisti, Y. (2018). Genetic and Metabolic Engineering for Microbial Production of Poly-γ-Glutamic Acid. Biotechnol. Adv. 36 (5), 1424–1433. doi:10.1016/j.biotechadv.2018.05.006
Chen, X. S., and Mao, Z. G. (2013). Comparison of Glucose and Glycerol as Carbon Sources for ε-Poly-l-Lysine Production by Streptomyces Sp. M-Z18. Appl. Biochem. Biotechnol. 170 (1), 185–197. doi:10.1007/s12010-013-0167-5
Chen, X. S., Ren, X. D., Dong, N., Li, S., Li, F., Zhao, F.-L., et al. (2012). Culture Medium Containing Glucose and Glycerol as a Mixed Carbon Source Improves ε-poly-l-lysine Production by Streptomyces Sp. M-Z18. Bioproc. Biosyst. Eng. 35 (3), 469–475. doi:10.1007/s00449-011-0586-z
Chen, X. S., Ren, X. D., Zeng, X., Zhao, F. L., Tang, L., Zhang, H.-J., et al. (2013). Enhancement of ε-poly-l-lysine Production Coupled with Precursor L-Lysine Feeding in Glucose-Glycerol Co-fermentation by Streptomyces Sp. M-Z18. Bioproc. Biosyst Eng. 36 (12), 1843–1849. doi:10.1007/s00449-013-0958-7
Chen, X. S., Wang, K. F., Zheng, G.-C., Gao, Y., and Mao, Z. G. (2018). Preparation, Characterization and Antimicrobial Activity of ε-poly-l-lysine with Short Chain Length Produced from Glycerol by Streptomyces Albulus. Process Biochem. 68, 22–29. doi:10.1016/j.procbio.2018.03.001
Chen, X., Diao, W., Ma, Y., and Mao, Z. (2020). Extraction and Purification of ε-poly-l-lysine from Fermentation Broth Using an Ethanol/ammonium Sulfate Aqueous Two-phase System Combined with Ultrafiltration. RSC Adv. 10 (49), 29587–29593. doi:10.1039/d0ra04245e
Chen, S., Huang, S., Li, Y., and Zhou, C. (2021). Recent Advances in Epsilon-Poly-L-Lysine and L-Lysine-Based Dendrimer Synthesis, Modification, and Biomedical Applications. Front. Chem. 9, 659304. doi:10.3389/fchem.2021.659304
Chen, J., Liu, H., Xia, Z., Zhao, X., Wu, Y., and An, M. (2019). Purification and Structural Analysis of the Effective Anti-TMV Compound ε-Poly-l-lysine Produced by Streptomyces Ahygroscopicus. Molecules 24 (6), 1156. doi:10.3390/molecules24061156
Chen, X., Tang, L., Li, S., Liao, L., Zhang, J., and Mao, Z. (2011). Optimization of Medium for Enhancement of ε-Poly-L-Lysine Production by Streptomyces Sp. M-Z18 with Glycerol as Carbon Source. Bioresour. Technol. 102 (2), 1727–1732. doi:10.1016/j.biortech.2010.08.071
Chen, X., Li, Q., He, H., Zhang, J., and Mao, Z. (2019). Effect of Ion Form of the Ion-Exchange Resin on ε-poly-l-lysine Purification from Microbial Fermentation Broth. RSC Adv. 9 (21), 12174–12181. doi:10.1039/c9ra00493a
Chen, X. S., Li, S., Liao, L. J., Ren, X. D., Li, F., Tang, L., et al. (2011). Production of ε-poly-l-lysine Using a Novel Two-Stage pH Control Strategy by Streptomyces Sp. M-Z18 from Glycerol. Bioproc. Biosyst Eng. 34 (5), 561–567. doi:10.1007/s00449-010-0505-8
Chheda, A. H., and Vernekar, M. R. (2014). Improved Production of Natural Food Preservative ε-poly-l-lysine Using a Novel Producer Bacillus Cereus. Food Biosci. 7, 56–63. doi:10.1016/j.fbio.2014.05.005
Chheda, A. H., and Vernekar, M. R. (2015a). Optimization of Medium Components and Feeding Strategies for Epsilon Poly-L-Lysine Production by Streptomyces Noursei NRRL 5126. Int. J. Pharm. Sci. Res. 6 (5), 1982–1991. doi:10.13040/ijpsr.0975-8232.6(5).1982-91
Chheda, A. H., and Vernekar, M. R. (2015b). Enhancement of ε-poly-l-lysine (ε-PL) Production by a Novel Producer Bacillus Cereus Using Metabolic Precursors and Glucose Feeding. 3 Biotech. 5 (5), 839–846. doi:10.1007/s13205-015-0291-8
Ding, J., Liang, H., Fu, S., Liu, R., Deng, Z., and Liu, T. (2018). Modification of Ɛ-Poly-L-Lysine In Vivo to Reduce Self-Toxicity and Enhance Antibiotic Overproduction. Aiche J. 64 (12), 4187–4192. doi:10.1002/aic.16190
Dou, Y., Routledge, M. N., Gong, Y., Godana, E. A., Dhanasekaran, S., Yang, Q., et al. (2021). Efficacy of Epsilon-Poly-L-Lysine Inhibition of Postharvest Blue Mold in Apples and Potential Mechanisms. Postharvest Biol. Technol. 171, 111346. doi:10.1016/j.postharvbio.2020.111346
Du, F., Wang, Y. Z., Xu, Y. S., Shi, T. Q., Liu, W. Z., Sun, X. M., et al. (2021). Biotechnological Production of Lipid and Terpenoid from Thraustochytrids. Biotechnol. Adv. 48, 107725. doi:10.1016/j.biotechadv.2021.107725
Dung, T. H., Le, T. D., Eom, K. D., Kim, S. J., and Yoo, H. (2016). Preparation of Pluronic Grafted Dendritic α,ε-poly(L-lysine)s and Characterization as a Delivery Adjuvant of Antisense Oligonucleotide. J. Nanosci. Nanotechnol. 16 (2), 1370–1374. doi:10.1166/jnn.2016.11927
El-Sersy, N. A., Abdelwahab, A. E., Abouelkhiir, S. S., Abou-Zeid, D. M., and Sabry, S. A. (2012). Antibacterial and Anticancer Activity of ε-Poly-L-Lysine (ε-PL) Produced by a Marine Bacillus Subtilis sp. J. Basic Microbiol. 52 (5), 513–522. doi:10.1002/jobm.201100290
Fürsatz, M., Skog, M., Sivlér, P., Palm, E., Aronsson, C., Skallberg, A., et al. (2018). Functionalization of Bacterial Cellulose Wound Dressings with the Antimicrobial Peptide ε -Poly-L-Lysine. Biomed. Mater. 13 (2), 025014. doi:10.1088/1748-605X/aa9486
Geng, W., Yang, C., Gu, Y., Liu, R., Guo, W., Wang, X., et al. (2014). Cloning of ε‐poly‐ L ‐lysine (ε‐ PL ) Synthetase Gene from a Newly Isolated ε‐ PL ‐Producing Streptomyces Albulus NK 660 and its Heterologous Expression in Streptomyces Lividans. Microb. Biotechnol. 7 (2), 155–164. doi:10.1111/1751-7915.12108
Gu, Y., Wang, X., Yang, C., Geng, W., Feng, J., Wang, Y., et al. (2016). Effects of Chromosomal Integration of the Vitreoscilla Hemoglobin Gene (Vgb) and S-Adenosylmethionine Synthetase Gene (metK) on ε-Poly-l-Lysine Synthesis in Streptomyces Albulus NK660. Appl. Biochem. Biotechnol. 178 (7), 1445–1457. doi:10.1007/s12010-015-1958-7
Guo, F., Zheng, H., Cheng, Y., Song, S., Zheng, Z., and Jia, S. (2018). Medium Optimization for ε-poly-L-lysine Production by Streptomyces Diastatochromogenes Using Response Surface Methodology. Lett. Appl. Microbiol. 66 (2), 124–131. doi:10.1111/lam.12812
Hamano, Y., Nicchu, I., Hoshino, Y., Kawai, T., Nakamori, S., and Takagi, H. (2005). Development of Gene Delivery Systems for the Ɛ-Poly-L-Lysine Producer, Streptomyces Albulus. J. Biosci. Bioeng. 99 (6), 636–641. doi:10.1263/jbb.99.636
Hamano, Y., Yoshida, T., Kito, M., Nakamori, S., Nagasawa, T., and Takagi, H. (2006). Biological Function of the Pld Gene Product that Degrades -Poly-L-Lysine in Streptomyces Albulus. Appl. Microbiol. Biotechnol. 72 (1), 173–181. doi:10.1007/s00253-006-0396-4
Hamano, Y., Nicchu, I., Shimizu, T., Onji, Y., Hiraki, J., and Takagi, H. (2007). Ɛ-Poly-L-lysine Producer, Streptomyces Albulus, Has Feedback-Inhibition Resistant Aspartokinase. Appl. Microbiol. Biotechnol. 76 (4), 873–882. doi:10.1007/s00253-007-1052-3
Hamano, Y., Kito, N., Kita, A., Imokawa, Y., Yamanaka, K., Maruyama, C., et al. (2014). ε-Poly- L -Lysine Peptide Chain Length Regulated by the Linkers Connecting the Transmembrane Domains of ε-Poly- L -Lysine Synthetase. Appl. Environ. Microbiol. 80 (16), 4993–5000. doi:10.1128/aem.01201-14
Hiraki, J., Hatakeyama, M., Morita, H., and Izumi, Y. (1998). Improved Epsilon-Poly-L-Lysine Production of an S-(2-aminoethyl)-L-cysteine Resistant Mutant of Streptomyces Albulus. Seibutsu-Kogaku Kaishi (Tokyo) 76 (12), 487–493.
Hirohara, H., Takehara, M., Saimura, M., Masayuki, A., and Miyamoto, M. (2006). Biosynthesis of Poly(ɛ-L-Lysine)s in Two Newly Isolated Strains of Streptomycessp. Appl. Microbiol. Biotechnol. 73 (2), 321–331. doi:10.1007/s00253-006-0479-2
Hirohara, H., Saimura, M., Takehara, M., Miyamoto, M., and Ikezaki, A. (2007). Substantially Monodispersed Poly(ɛ-L-Lysine)s Frequently Occurred in Newly Isolated Strains of Streptomyces Sp. Appl. Microbiol. Biotechnol. 76 (5), 1009–1016. doi:10.1007/s00253-007-1082-x
Hou, Y., Wang, F., Tan, Z., Cui, J., and Jia, S. (2020). Antifungal Mechanisms of ε-poly-L-Lysine with Different Molecular Weights on Saccharomyces cerevisiae. Korean J. Chem. Eng. 37 (3), 482–492. doi:10.1007/s11814-019-0466-9
Hou, W., Zhang, Y., Zhang, Y., Yue, Q., Wang, L., Min, T., et al. (2021). Label‐free Proteomics Study on Shewanella Putrefaciens Regulated by ε ‐ Poly‐lysine Treatment. J. Appl. Microbiol. 131, 791–800. doi:10.1111/jam.14954
Huang, J., Zhuang, W., Ge, L., Wang, K., Wang, Z., Niu, H., et al. (2019). Improving Biocatalytic Microenvironment with Biocompatible ε-poly-l-lysine for One Step Gluconic Acid Production in Low pH Enzymatic Systems. Process Biochem. 76, 118–127. doi:10.1016/j.procbio.2018.10.018
Hyldgaard, M., Mygind, T., Vad, B. S., Stenvang, M., Otzen, D. E., and Meyer, R. L. (2014). The Antimicrobial Mechanism of Action of Epsilon-Poly- L -Lysine. Appl. Environ. Microbiol. 80 (24), 7758–7770. doi:10.1128/aem.02204-14
Jia, S., Fan, B., Dai, Y., Wang, G., Peng, P., and Jia, Y. (2010). Fractionation and Characterization of Ɛ-Poly-L-Lysine from Streptomyces Albulus CGMCC 1986. Food Sci. Biotechnol. 19 (2), 361–366. doi:10.1007/s10068-010-0051-9
Jiang, W., and Zhou, X. (2021). Enzymatic Preparation of Oxidized Viscose Fibers-Based Biosorbent Modified with ε-polylysine for Dyes Removal and Microbial Inactivation. Int. J. Biol. Macromol. 166, 509–520. doi:10.1016/j.ijbiomac.2020.10.208
Jiang, X., Radko, Y., Gren, T., Palazzotto, E., Jørgensen, T. S., Cheng, T., et al. (2021). Distribution of ε-Poly- L -Lysine Synthetases in Coryneform Bacteria Isolated from Cheese and Human Skin. Appl. Environ. Microbiol. 87 (10), e01841-20. doi:10.1128/aem.01841-20
Jiao, W., Liu, X., Chen, Q., Du, Y., Li, Y., Yue, F., et al. (2020). Epsilon-poly-l-lysine (ε-PL) Exhibits Antifungal Activity In Vivo and In Vitro Against Botrytis Cinerea and Mechanism Involved. Postharvest Biol. Technol. 168, 111270. doi:10.1016/j.postharvbio.2020.111270
Kahar, P., Iwata, T., Hiraki, J., Park, E. Y., and Okabe, M. (2001). Enhancement of ε-polylysine Production by Streptomyces Albulus Strain 410 Using pH Control. J. Biosci. Bioeng. 91 (2), 190–194. doi:10.1016/s1389-1723(01)80064-5
Katano, H., Yoneoka, T., Kito, N., Maruyama, C., and Hamano, Y. (2012). Separation and Purification of ε-Poly-L-lysine from the Culture Broth Based on Precipitation with the Tetraphenylborate Anion. Anal. Sci. 28 (12), 1153–1157. doi:10.2116/analsci.28.1153
Katano, H., Kasahara, Y., Ushimaru, K., Maruyama, C., and Hamano, Y. (2015). Separation and Purification of ε-Poly-L-lysine with its Colorimetric Determination Using Dipicrylamine. Anal. Sci. 31 (12), 1273–1277. doi:10.2116/analsci.31.1273
Kito, M., Takimoto, R., Yoshida, T., and Nagasawa, T. (2002). Purification and Characterization of an ε-poly- L -Lysine-Degrading Enzyme from an ε-poly- L -Lysine-Producing Strain of Streptomyces Albulus. Arch. Microbiol. 178 (5), 325–330. doi:10.1007/s00203-002-0459-6
Kito, N., Maruyama, C., Yamanaka, K., Imokawa, Y., Utagawa, T., and Hamano, Y. (2013). Mutational Analysis of the Three Tandem Domains of ε-poly-l-lysine Synthetase Catalyzing the L-Lysine Polymerization Reaction. J. Biosci. Bioeng. 115 (5), 523–526. doi:10.1016/j.jbiosc.2012.11.020
Kobayashi, K., and Nishikawa, M. (2007). Promotion of ε-poly-l-lysine Production by Iron in Kitasatospora Kifunense. World J. Microbiol. Biotechnol. 23 (7), 1033–1036. doi:10.1007/s11274-006-9318-y
Lan, W., Zhang, N., Liu, S., Chen, M., and Xie, J. (2019). ε-Polylysine Inhibits Shewanella Putrefaciens with Membrane Disruption and Cell Damage. Molecules 24 (20), 3727. doi:10.3390/molecules24203727
Lee, J. M., Kim, J.-H., Kim, K. W., Lee, B.-J., Kim, D.-G., Kim, Y.-O., et al. (2018). Physicochemical Properties, Production, and Biological Functionality of Poly-γ-D-Glutamic Acid with Constant Molecular Weight from Halotolerant Bacillus Sp. SJ-10. Int. J. Biol. Macromol. 108, 598–607. doi:10.1016/j.ijbiomac.2017.12.055
Levy, H. B., Law, L. W., and Rabson, A. S. (1969). Inhibition of Tumor Growth by Polyinosinic-Polycytidylic Acid. Proc. Natl. Acad. Sci. 62 (2), 357–361. doi:10.1073/pnas.62.2.357
Li, S., Tang, L., Chen, X., Liao, L., Li, F., and Mao, Z. (2011). Isolation and Characterization of a Novel ε-poly-l-lysine Producing Strain: Streptomyces Griseofuscus. J. Ind. Microbiol. Biotechnol. 38 (4), 557–563. doi:10.1007/s10295-010-0803-9
Li, S., Li, F., Chen, X. S., Wang, L., Xu, J., Tang, L., et al. (2012). Genome Shuffling Enhanced ε-Poly-l-Lysine Production by Improving Glucose Tolerance of Streptomyces Graminearus. Appl. Biochem. Biotechnol. 166 (2), 414–423. doi:10.1007/s12010-011-9437-2
Li, S., Chen, X., Dong, C., Zhao, F., Tang, L., and Mao, Z. (2013). Combining Genome Shuffling and Interspecific Hybridization Among Streptomyces Improved ε-Poly-l-Lysine Production. Appl. Biochem. Biotechnol. 169 (1), 338–350. doi:10.1007/s12010-012-9969-0
Li, C., Liu, F., Gong, Y., Wang, Y., Xu, H., Yuan, F., et al. (2014). Investigation into the Maillard Reaction between Ɛ-Polylysine and Dextran in Subcritical Water and Evaluation of the Functional Properties of the Conjugates. LWT - Food Sci. Technol. 57 (2), 612–617. doi:10.1016/j.lwt.2014.01.039
Li, S., Ding, Z., Liu, J., and Cao, X. (2017a). Metal-Chelate Affinity Precipitation with Thermo-Responsive Polymer for Purification of ε-Poly-l-Lysine. Appl. Biochem. Biotechnol. 183 (4), 1254–1264. doi:10.1007/s12010-017-2495-3
Li, S., Zhang, L., Liu, M., Wang, X., Zhao, G., and Zong, W. (2017b). Effect of Poly-ε-Lysine Incorporated into Alginate-Based Edible Coatings on Microbial and Physicochemical Properties of Fresh-Cut Kiwifruit. Postharvest Biol. Technol. 134, 114–121. doi:10.1016/j.postharvbio.2017.08.014
Li, S., Wang, N., Du, Z.-J., and Chen, G.-J. (2018). Intergeneric Hybridization between Streptomyces Albulus and Bacillus Subtilis Facilitates Production of ε-Poly-L-lysine from Corn Starch Residues. Biotechnol. Bioproc. E 23 (5), 580–587. doi:10.1007/s12257-018-0253-1
Li, H., He, C., Li, G., Zhang, Z., Li, B., and Tian, S. (2019). The Modes of Action of Epsilon-Polylysine (ε-PL) Against Botrytis Cinerea in Jujube Fruit. Postharvest Biol. Technol. 147, 1–9. doi:10.1016/j.postharvbio.2018.08.009
Li, S., Ji, J., Hu, S., and Chen, G. (2020). Enhancement of ε-poly-l-lysine Production in Streptomyces Griseofuscus by Addition of Exogenous Astaxanthin. Bioproc. Biosyst Eng. 43 (10), 1813–1821. doi:10.1007/s00449-020-02372-y
Li, W., Lv, J., Dong, T., Li, X., Li, X., Tan, Z., et al. (2021). Effects of Amino Acids and Overexpression of dapA Gene on the Production of ε-Poly-L-lysine by Streptomyces Diastatochromogenes Strains. Curr. Microbiol. 78 (7), 2640–2647. doi:10.1007/s00284-021-02510-z
Liu, S., Wu, Q., Zhang, J., and Mo, S. (2011). Production of ε-poly-l-lysine by Streptomyces Sp. Using Resin-Based, In Situ Product Removal. Biotechnol. Lett. 33 (8), 1581–1585. doi:10.1007/s10529-011-0616-6
Liu, S., Wu, Q., Zhang, J., and Mo, S. (2012a). Effects of L-Lysine and D-Lysine on Ɛ-Poly-L-Lysine Biosynthesis and Their Metabolites by Streptomyces Ahygroscopicus GIM8. Biotechnol. Bioproc. E 17 (6), 1205–1212. doi:10.1007/s12257-012-0296-7
Liu, S., Wu, Q., Zhang, J., Mo, S., Yang, X., and Xiao, C. (2012b). Enhanced ε-Poly-L-lysine Production from Streptomyces Ahygroscopicus by a Combination of Cell Immobilization and In Situ Adsorption. J. Microbiol. Biotechnol. 22 (9), 1218–1223. doi:10.4014/jmb.1111.11039
Liu, S. R., Wu, Q. P., Zhang, J. M., and Mo, S. P. (2015). Efficient Production of ε-Poly-L-Lysine by Streptomyces Ahygroscopicus Using One-Stage pH Control Fed-Batch Fermentation Coupled with Nutrient Feeding. J. Microbiol. Biotechnol. 25 (3), 358–365. doi:10.4014/jmb.1405.05069
Liu, Y. J., Chen, X. S., Zhao, J. J., Pan, L., and Mao, Z. G. (2017). Development of Microtiter Plate Culture Method for Rapid Screening of ε-Poly-L-Lysine-Producing Strains. Appl. Biochem. Biotechnol. 183 (4), 1209–1223. doi:10.1007/s12010-017-2493-5
Liu, Y., Chen, X., Pan, L., and Mao, Z. (2019a). Differential Protein Expression of a Streptomycin-resistantStreptomyces Albulusmutant in High Yield Production of ε-poly-l-lysine: A Proteomics Study. RSC Adv. 9 (42), 24092–24104. doi:10.1039/c9ra03156a
Liu, Y., Chen, X., Zhao, J., Li, Q., and Mao, Z. (2019b). Improvement of ε-poly-L-lysine Production of Streptomyces Albulus by Continuous Introduction of Streptomycin Resistance. Process Biochem. 82, 10–18. doi:10.1016/j.procbio.2019.04.006
Liu, H., Chen, J., Xia, Z., An, M., and Wu, Y. (2020). Effects of ε-poly-l-lysine on Vegetative Growth, Pathogenicity and Gene Expression of Alternaria alternata Infecting Nicotiana Tabacum. Pestic. Biochem. Physiol. 163, 147–153. doi:10.1016/j.pestbp.2019.11.005
Liu, J. N., Chang, S. L., Xu, P. W., Tan, M. H., Zhao, B., Wang, X. D., et al. (2020). Structural Changes and Antibacterial Activity of Epsilon-Poly-L-Lysine in Response to pH and Phase Transition and Their Mechanisms. J. Agric. Food Chem. 68 (4), 1101–1109. doi:10.1021/acs.jafc.9b07524
Liu, Z., Xu, T., Wang, M., Mao, C., and Chi, B. (2020). Magnetic Mesoporous Silica/ε-Polylysine Nanomotor-Based Removers of Blood Pb2+. J. Mater. Chem. B 8 (48), 11055–11062. doi:10.1039/d0tb02270e
Mookherjee, N., Anderson, M. A., Haagsman, H. P., and Davidson, D. J. (2020). Antimicrobial Host Defence Peptides: Functions and Clinical Potential. Nat. Rev. Drug Discov. 19 (5), 311–332. doi:10.1038/s41573-019-0058-8
Nishikawa, M., and Kobayashi, K. (2009). Streptomyces Roseoverticillatus Produces Two Different Poly(amino Acid)s: Lariat-Shaped γ-poly(l-glutamic Acid) and ϵ-poly(l-lysine). Microbiol-Sgm. 155, 2988–2993. doi:10.1099/mic.0.029694-0
Nishikawa, M., and Ogawa, K. i. (2002). Distribution of Microbes Producing Antimicrobial ε-Poly- L -Lysine Polymers in Soil Microflora Determined by a Novel Method. Appl. Environ. Microbiol. 68 (7), 3575–3581. doi:10.1128/aem.68.7.3575-3581.2002
Nishikawa, M., and Ogawa, K. i. (2006). Inhibition of Epsilon-Poly- L -Lysine Biosynthesis in Streptomycetaceae Bacteria by Short-Chain Polyols. Appl. Environ. Microbiol. 72 (4), 2306–2312. doi:10.1128/aem.72.4.2306-2312.2006
Nishikawa, M. (2009). Molecular Mass Control Using Polyanionic Cyclodextrin Derivatives for the Epsilon-Poly-L-Lysine Biosynthesis by Streptomyces. Enzyme Microb. Technol. 45 (4), 295–298. doi:10.1016/j.enzmictec.2009.03.011
Ochi, K. (2007). From Microbial Differentiation to Ribosome Engineering. Biosci. Biotechnol. Biochem. 71 (6), 1373–1386. doi:10.1271/bbb.70007
Ouyang, J., Xu, H., Li, S., Zhu, H., Chen, W., Zhou, J., et al. (2006). Production of ε-poly-L-lysine by Newly isolatedKitasatospora Sp. PL6-3. Biotechnol. J. 1 (12), 1459–1463. doi:10.1002/biot.200600079
Pan, L., Chen, X. S., Liu, M. M., Liu, Y. J., and Mao, Z. G. (2017). Efficient Production of ε-poly-l-lysine from Glucose by Two-Stage Fermentation Using pH Shock Strategy. Process Biochem. 63, 8–15. doi:10.1016/j.procbio.2017.08.008
Pan, L., Chen, X., Wang, K., and Mao, Z. (2019a). A Temporal Transcriptomic Dynamics Study Reveals the Reason of Enhanced ε-poly-L-lysine Production in Streptomyces Albulus M-Z18 by pH Shock. Process Biochem. 85, 1–11. doi:10.1016/j.procbio.2019.07.012
Pan, L., Chen, X., Wang, K., and Mao, Z. (2019b). Understanding High ε-poly-l-lysine Production by Streptomyces Albulus Using pH Shock Strategy in the Level of Transcriptomics. J. Ind. Microbiol. Biotechnol. 46 (12), 1781–1792. doi:10.1007/s10295-019-02240-z
Peng, X., Liu, G., Zhu, L., Yu, K., Qian, K., and Zhan, X. (2020). In Vitro and In Vivo Study of Novel Antimicrobial Gellan-Polylysine Polyion Complex Fibers as Suture Materials. Carbohydr. Res. 496, 108115. doi:10.1016/j.carres.2020.108115
Purev, E., Kondo, T., Takemoto, D., Niones, J. T., and Ojika, M. (2020). Identification of ε-Poly-L-lysine as an Antimicrobial Product from an Epichloë Endophyte and Isolation of Fungal ε-PL Synthetase Gene. Molecules 25 (5), 1032. doi:10.3390/molecules25051032
Ren, X. D., Chen, X. S., Tang, L., Sun, Q. X., Zeng, X., and Mao, Z. G. (2015a). Efficient Production of ε-poly-l-lysine from Agro-Industrial By-Products by Streptomyces Sp. M-Z18. Ann. Microbiol. 65 (2), 733–743. doi:10.1007/s13213-014-0913-0
Ren, X. D., Chen, X. S., Tang, L., Zeng, X., Wang, L., and Mao, Z. G. (2015b). Physiological Mechanism of the Overproduction of ε-poly-l-lysine by Acidic pH Shock in Fed-Batch Fermentation. Bioproc. Biosyst Eng. 38 (11), 2085–2094. doi:10.1007/s00449-015-1448-x
Ren, X. D., Chen, X. S., Zeng, X., Wang, L., Tang, L., and Mao, Z. G. (2015c). Acidic pH Shock Induced Overproduction of ε-poly-l-lysine in Fed-Batch Fermentation by Streptomyces Sp. M-Z18 from Agro-Industrial By-Products. Bioproc. Biosyst Eng. 38 (6), 1113–1125. doi:10.1007/s00449-015-1354-2
Ren, X. D., Xu, Y. J., Zeng, X., Chen, X. S., Tang, L., and Mao, Z. G. (2015d). Microparticle-enhanced Production of ε-poly-l-lysine in Fed-Batch Fermentation. RSC Adv. 5 (100), 82138–82143. doi:10.1039/c5ra14319e
Samadlouie, H. R., Gharanjik, S., and Tabatabaie, Z. B. (2020). Optimization of the Production of ε-Poly-L-Lysine by Novel Producer Lactic Acid Bacteria Isolated from Traditional Dairy Products. Biomed. Res. Int. 2020, 2145656. doi:10.1155/2020/2145656
Sequeira, R. A., Sharma, M., Pereira, M. M., Singh, N., Bhattacharya, S., Chudasama, N. A., et al. (2021). One Step Selective Partition of ε-polylysine Present in Broth Cultures in Ionic Liquid-Based Aqueous Biphasic Systems. Sep. Sci. Technol. 56 (3), 631–639. doi:10.1080/01496395.2020.1728323
Sha, Y., Zhang, Y., Qiu, Y., Xu, Z., Li, S., Feng, X., et al. (2019). Efficient Biosynthesis of Low-Molecular-Weight Poly-γ-Glutamic Acid by Stable Overexpression of PgdS Hydrolase in Bacillus Amyloliquefaciens NB. J. Agric. Food Chem. 67 (1), 282–290. doi:10.1021/acs.jafc.8b05485
Shen, C., Islam, M. T., Masuda, Y., Honjoh, K. I., and Miyamoto, T. (2020). Transcriptional Changes Involved in Inhibition of Biofilm Formation by ε-polylysine in Salmonella Typhimurium. Appl. Microbiol. Biotechnol. 104 (12), 5427–5436. doi:10.1007/s00253-020-10575-2
Shih, I. L., and Shen, M. H. (2006). Optimization of Cell Growth and Poly(ɛ-Lysine) Production in Batch and Fed-Batch Cultures by Streptomyces Albulus IFO 14147. Process Biochem. 41 (7), 1644–1649. doi:10.1016/j.procbio.2006.03.013
Shih, I., Shen, M., and Van, Y. (2006). Microbial Synthesis of Poly(ε-Lysine) and its Various Applications. Bioresour. Technol. 97 (9), 1148–1159. doi:10.1016/j.biortech.2004.08.012
Shih, I. L., Wang, T. C., Chou, S. Z., and Lee, G. D. (2011). Sequential Production of Two Biopolymers-Levan and Poly-ε-Lysine by Microbial Fermentation. Bioresour. Technol. 102 (4), 3966–3969. doi:10.1016/j.biortech.2010.11.066
Shima, S., and Sakai, H. (1977). Polylysine Produced by Streptomyces. Agric. Biol. Chem. 41, 1807–1809. doi:10.1271/bbb1961.41.1807
Shima, S., and Sakai, H. (1981). Poly-L-lysine Produced by Streptomyces. Part II. Taxonomy and Fermentation Studies. Agric. Biol. Chem. 45 (11), 2497–2502. doi:10.1080/00021369.1981.10864929
Shima, S., Oshima, S., and Sakai, H. (1983). Biosynthesis of ε-poly-L-lysine by Washed Mycelium of Streptomyces Albulus No. 346. J. Agric. Chem. Soc. Jpn. 57, 221–226. doi:10.1271/nogeikagaku1924.57.221
Shima, S., Matsuoka, H., Iwamoto, T., and Sakai, H. (1984). Antimicrobial Action of Epsilon-Poly-L-Lysine. J. Antibiot. 37 (11), 1449–1455. doi:10.7164/antibiotics.37.1449
Shu, C., Cui, K., Li, Q., Cao, J., and Jiang, W. (2021). Epsilon-poly-l-lysine (ε-PL) Exhibits Multifaceted Antifungal Mechanisms of Action that Control Postharvest Alternaria Rot. Int. J. Food Microbiol. 348, 109224. doi:10.1016/j.ijfoodmicro.2021.109224
Shukla, S. C., and Mishra, A. (2013). ε-PolyLysine Production from Sugar Cane Molasses by a New Isolates of Bacillus Sp. And Optimization of the Fermentation Condition. Ann. Microbiol. 63 (4), 1513–1523. doi:10.1007/s13213-013-0615-z
Song, M., Lopez-Pena, C. L., McClements, D. J., Decker, E. A., and Xiao, H. (2017). Safety Evaluation and Lipid-Lowering Effects of Food-Grade Biopolymer Complexes (ε-Polylysine-Pectin) in Mice Fed a High-Fat Diet. Food Funct. 8 (5), 1822–1829. doi:10.1039/c7fo00222j
Su, R., Li, T., Fan, D., Huang, J., Zhao, J., Yan, B., et al. (2019). The Inhibition Mechanism Of ϵ‐polylysine against Bacillus Cereus emerging in Surimi Gel during Refrigerated Storage. J. Sci. Food Agric. 99 (6), 2922–2930. doi:10.1002/jsfa.9505
Sun, G., Wang, H., Shi, B., Shangguan, N., Wang, Y., and Ma, Q. (2017). Control Efficiency and Expressions of Resistance Genes in Tomato Plants Treated with ε-poly-l-lysine against Botrytis Cinerea. Pestic. Biochem. Physiol. 143, 191–198. doi:10.1016/j.pestbp.2017.07.007j
Sun, G., Yang, Q., Zhang, A., Guo, J., Liu, X., Wang, Y., et al. (2018). Synergistic Effect of the Combined Bio-Fungicides ε-poly- L -lysine and Chitooligosaccharide in Controlling Grey Mould ( Botrytis Cinerea ) in Tomatoes. Int. J. Food Microbiol. 276, 46–53. doi:10.1016/j.ijfoodmicro.2018.04.006
Takehara, M., Saimura, M., Inaba, H., and Hirohara, H. (2008). Poly(γ-l-diaminobutanoic Acid), a Novel Poly(amino Acid), Coproduced with poly(É›-L-Lysine) by Two Strains of Streptomyces Celluloflavus. FEMS Microbiol. Lett. 286 (1), 110–117. doi:10.1111/j.1574-6968.2008.01261.x
Takehara, M., Hibino, A., Saimura, M., and Hirohara, H. (2010). High-yield Production of Short Chain Length Poly(ε-L-Lysine) Consisting of 5-20 Residues by Streptomyces Aureofaciens, and its Antimicrobial Activity. Biotechnol. Lett. 32 (9), 1299–1303. doi:10.1007/s10529-010-0294-9
Tan, Z., Shi, Y., Xing, B., Hou, Y., Cui, J., and Jia, S. (2019). The Antimicrobial Effects and Mechanism of ε-poly-lysine Against Staphylococcus aureus. Bioresour. Bioproc. 6, 11. doi:10.1186/s40643-019-0246-8
Tao, Y., Chen, X., Jia, F., Wang, S., Xiao, C., Cui, F., et al. (2015). New Chemosynthetic Route to Linear ε-poly-lysine. Chem. Sci. 6 (11), 6385–6391. doi:10.1039/c5sc02479j
Tao, Y. (2016). New Polymerization Methodology of Amino Acid Based on Lactam Polymerization. Acta Polym. Sin 9, 1151–1159. doi:10.11777/j.issn1000-3304.2016.16130
Tsujita, T., and Takaku, T. (2009). Inhibition by ε-Polylysine of Fat Digestion in the Stomach and Intestine of Rats. Biosci. Biotechnol. Biochem. 73 (3), 536–542. doi:10.1271/bbb.80587
Tsujita, T., Takaichi, H., Takaku, T., Aoyama, S., and Hiraki, J. (2006). Antiobesity Action of Ɛ-Polylysine, A Potent Inhibitor of Pancreatic Lipase. J. Lipid Res. 47 (8), 1852–1858. doi:10.1194/jlr.M600168-JLR200
Tuersuntuoheti, T., Wang, Z., Wang, Z., Liang, S., Li, X., and Zhang, M. (2019). Review of the Application of ε‐poly‐L‐lysine in Improving Food Quality and Preservation. J. Food Process. Preserv. 43 (10), e14153. doi:10.1111/jfpp.14153
Wakamoto, H., Matsuda, H., Kawamoto, K., and Makino, S.-i. (2007). Epsilon-polylysine Microparticle Adjuvant Drives Cytokine Production to Th1 Profile. J. Vet. Med. Sci. 69 (7), 717–723. doi:10.1292/jvms.69.717
Wang, A., Tian, W., Cheng, L., Xu, Y., Wang, X., Qin, J., et al. (2020). Enhanced ε-Poly-L-Lysine Production by the Synergistic Effect of ε-Poly-L-Lysine Synthetase Overexpression and Citrate in Streptomyces Albulus. Front. Bioeng. Biotechnol. 8, 288. doi:10.3389/fbioe.2020.00288
Wang, C., Ren, X., Yu, C., Wang, J., Wang, L., Zhuge, X., et al. (2020). Physiological and Transcriptional Responses of Streptomyces Albulus to Acid Stress in the Biosynthesis of ε-Poly-L-lysine. Front. Microbiol. 11, 1379. doi:10.3389/fmicb.2020.01379
Wang, L., Chen, X., Wu, G., Li, S., Zeng, X., Ren, X., et al. (2015a). Improved ε-poly-l-lysine Production of Streptomyces Sp. FEEL-1 by Atmospheric and Room Temperature Plasma Mutagenesis and Streptomycin Resistance Screening. Ann. Microbiol. 65 (4), 2009–2017. doi:10.1007/s13213-015-1039-8
Wang, L., Gao, C., Tang, N., Hu, S., and Wu, Q. (2015b). Identification of Genetic Variations Associated with Epsilon-Poly-Lysine Biosynthesis in Streptomyces Albulus ZPM by Genome Sequencing. Sci. Rep. 5, 9201. doi:10.1038/srep09201
Wang, L., Chen, X., Wu, G., Zeng, X., Ren, X., Li, S., et al. (2016). Genome Shuffling and Gentamicin-Resistance to Improve ε-Poly-l-Lysine Productivity of Streptomyces Albulus W-156. Appl. Biochem. Biotechnol. 180 (8), 1601–1617. doi:10.1007/s12010-016-2190-9
Wang, L., Li, S., Zhao, J., Liu, Y., Chen, X., Tang, L., et al. (2019). Efficiently Activated ε‐poly‐L‐lysine Production by Multiple Antibiotic‐resistance Mutations and Acidic pH Shock Optimization in Streptomyces Albulus. Microbiologyopen 8, e00728. doi:10.1002/mbo3.728
Wang, Z., Guo, F., Dong, T., Tan, Z., Abdelraof, M., Wang, Z., et al. (2021). Metabolomic Analysis of Biosynthesis Mechanism of ε-Polylysine Produced by Streptomyces Diastatochromogenes. Front. Bioeng. Biotechnol. 9, 698022. doi:10.3389/fbioe.2021.698022
Wang, L., Chen, X., Wu, G., Li, S., Zeng, X., Ren, X., et al. (2017). Enhanced ε-poly-l-lysine Production by Inducing Double Antibiotic-Resistant Mutations in Streptomyces Albulus. Bioproc. Biosyst Eng. 40 (2), 271–283. doi:10.1007/s00449-016-1695-5
Wang, R., Li, Q., Chi, B., Wang, X., Xu, Z., Xu, Z., et al. (2017). Enzyme-induced Dual-Network ε-poly-l-lysine-based Hydrogels with Robust Self-Healing and Antibacterial Performance. Chem. Commun. 53 (35), 4803–4806. doi:10.1039/c6cc09326d
Wei, H., Wang, L., Zhao, G., Fang, Z., Wu, H., Wang, P., et al. (2018). Extraction, Purification and Identification of Menaquinones from Flavobacterium Meningosepticum Fermentation Medium. Process Biochem. 66, 245–253. doi:10.1016/j.procbio.2018.01.007
Wei, M., Ge, Y., Li, C., Chen, Y., Wang, W., Duan, B., et al. (2018). Antifungal Activity of ε-poly-L-lysine on Trichothecium Roseum In Vitro and its Mechanisms. Physiol. Mol. Plant Pathol. 103, 23–27. doi:10.1016/j.pmpp.2018.04.005
Wu, Q. X., Wang, Z. D., Zheng, M. F., Su, T., Wang, X. H., Guan, Y. X., et al. (2020). Development of Metformin Hydrochloride Loaded Dissolving Tablets with Novel Carboxymethylcellulose/poly-L-Lysine/TPP Complex. Int. J. Biol. Macromolecules 155, 411–420. doi:10.1016/j.ijbiomac.2020.03.191
Xia, J., Xu, H., Feng, X., Xu, Z., and Chi, B. (2013). Poly(l-diaminopropionic Acid), a Novel Non-proteinic Amino Acid Oligomer Co-produced with Poly(ε-L-Lysine) by Streptomyces Albulus PD-1. Appl. Microbiol. Biotechnol. 97 (17), 7597–7605. doi:10.1007/s00253-013-4936-4
Xia, J., Xu, Z., Xu, H., Feng, X., and Bo, F. (2014a). The Regulatory Effect of Citric Acid on the Co-production of Poly(ε-Lysine) and Poly(l-Diaminopropionic Acid) in Streptomyces Albulus PD-1. Bioproc. Biosyst Eng. 37 (10), 2095–2103. doi:10.1007/s00449-014-1187-4
Xia, J., Xu, Z., Xu, H., Liang, J., Li, S., and Feng, X. (2014b). Economical Production of Poly(ε-L-Lysine) and Poly(l-Diaminopropionic Acid) Using Cane Molasses and Hydrolysate of Streptomyces Cells by Streptomyces Albulus PD-1. Bioresour. Technol. 164, 241–247. doi:10.1016/j.biortech.2014.04.078
Xiang, J., Yang, Y., Dabbour, M., Mintah, B. K., Zhang, Z., Dai, C., et al. (2020). Metabolomic and Genomic Profiles of Streptomyces Albulus with a Higher ε-polylysine Production through ARTP Mutagenesis. Biochem. Eng. J. 162, 107720. doi:10.1016/j.bej.2020.107720
Xu, D., Yao, H., Cao, C., Xu, Z., Li, S., Xu, Z., et al. (2018). Enhancement of ε-poly-l-lysine Production by Overexpressing the Ammonium Transporter Gene in Streptomyces Albulus PD-1. Bioproc. Biosyst Eng. 41 (9), 1337–1345. doi:10.1007/s00449-018-1961-9
Xu, Z., Bo, F., Xia, J., Sun, Z., Li, S., Feng, X., et al. (2015a). Effects of Oxygen-Vectors on the Synthesis of Epsilon-Poly-Lysine and the Metabolic Characterization of Streptomyces Albulus PD-1. Biochem. Eng. J. 94, 58–64. doi:10.1016/j.bej.2014.11.009
Xu, Z., Cao, C., Sun, Z., Li, S., Xu, Z., Feng, X., et al. (2015b). Construction of a Genetic System for Streptomyces Albulus PD-1 and Improving Poly(ε-L-Lysine) Production Through Expression of Vitreoscilla Hemoglobin. J. Microbiol. Biotechnol. 25 (11), 1819–1826. doi:10.4014/jmb.1506.06084
Xu, Z., Feng, X., Sun, Z., Cao, C., Li, S., Xu, Z., et al. (2015c). Economic Process to Co-produce Poly(ε- L -lysine) and Poly( L -diaminopropionic Acid) by a pH and Dissolved Oxygen Control Strategy. Bioresour. Technol. 187, 70–76. doi:10.1016/j.biortech.2015.03.101
Xu, Z., Xu, Z., Feng, X., Xu, D., Liang, J., and Xu, H. (2016). Recent Advances in the Biotechnological Production of Microbial Poly(ɛ-L-Lysine) and Understanding of its Biosynthetic Mechanism. Appl. Microbiol. Biotechnol. 100 (15), 6619–6630. doi:10.1007/s00253-016-7677-3
Xu, D., Yao, H., Xu, Z., Wang, R., Xu, Z., Li, S., et al. (2017). Production of ε-poly-lysine by Streptomyces Albulus PD-1 via Solid-State Fermentation. Bioresour. Technol. 223, 149–156. doi:10.1016/j.biortech.2016.10.032
Xu, D., Wang, R., Xu, Z., Xu, Z., Li, S., Wang, M., et al. (2019). Discovery of a Short-Chain ε-Poly-l-lysine and its Highly Efficient Production via Synthetase Swap Strategy. J. Agric. Food Chem. 67 (5), 1453–1462. doi:10.1021/acs.jafc.8b06019
Xu, T., Chi, B., Chu, M., Zhang, Q., Zhan, S., Shi, R., et al. (2018). Hemocompatible ε-Polylysine-Heparin Microparticles: A Platform for Detecting Triglycerides in Whole Blood. Biosens. Bioelectron. 99, 571–577. doi:10.1016/j.bios.2017.08.030
Yamanaka, K., Maruyama, C., Takagi, H., and Hamano, Y. (2008). ε-Poly-L-lysine Dispersity Is Controlled by a Highly Unusual Nonribosomal Peptide Synthetase. Nat. Chem. Biol. 4 (12), 766–772. doi:10.1038/nchembio.125
Yamanaka, K., Kito, N., Imokawa, Y., Maruyama, C., Utagawa, T., and Hamano, Y. (2010). Mechanism of ε-Poly- L -Lysine Production and Accumulation Revealed by Identification and Analysis of an ε-Poly- L -Lysine-Degrading Enzyme. Appl. Environ. Microbiol. 76 (17), 5669–5675. doi:10.1128/aem.00853-10
Yamanaka, K., Hamano, Y., and Oikawa, T. (2020). Enhancement of Metabolic Flux Toward ε-poly-l-lysine Biosynthesis by Targeted Inactivation of Concomitant Polyene Macrolide Biosynthesis in Streptomyces Albulus. J. Biosci. Bioeng. 129 (5), 558–564. doi:10.1016/j.jbiosc.2019.12.002
Yan, P., Sun, H., Lu, P., Liu, H., and Tang, L. (2018). Enhancement of ε-poly-l-lysine Synthesis in Streptomyces by Exogenous Glutathione. Bioproc. Biosyst Eng. 41 (1), 129–134. doi:10.1007/s00449-017-1849-0
Ye, R., Xu, H., Wan, C., Peng, S., Wang, L., Xu, H., et al. (2013). Antibacterial Activity and Mechanism of Action of ε-poly-l-lysine. Biochem. Biophys. Res. Commun. 439 (1), 148–153. doi:10.1016/j.bbrc.2013.08.001
Yoshimura, T., Shibata, N., Hamano, Y., and Yamanaka, K. (2015). Heterologous Production of Hyaluronic Acid in an ε-Poly- L -Lysine Producer, Streptomyces Albulus. Appl. Environ. Microbiol. 81 (11), 3631–3640. doi:10.1128/aem.00269-15
Yuan, J., Guo, L., Wang, S., Liu, D., Qin, X., Zheng, L., et al. (2018). Preparation of Self-Assembled Nanoparticles of ε-polylysine-sodium Alginate: A Sustained-Release Carrier for Antigen Delivery. Colloids Surf. B: Biointerf. 171, 406–412. doi:10.1016/j.colsurfb.2018.07.058
Zeng, X., Chen, X. S., Ren, X. D., Liu, Q. R., Wang, L., Sun, Q. X., et al. (2014). Insights into the Role of Glucose and Glycerol as a Mixed Carbon Source in the Improvement of ε-Poly-l-Lysine Productivity. Appl. Biochem. Biotechnol. 173 (8), 2211–2224. doi:10.1007/s12010-014-1026-8
Zeng, X., Chen, X. S., Gao, Y., Ren, X. D., Wang, L., and Mao, Z. G. (2015). Continuously High Reactive Oxygen Species Generation Decreased the Specific ϵ-poly- L -lysine Formation Rate in Fed-Batch Fermentation Using Glucose and Glycerol as a Mixed Carbon Source. Process Biochem. 50 (12), 1993–2003. doi:10.1016/j.procbio.2015.09.012
Zeng, X., Zhao, J., Chen, X., Mao, Z., and Miao, W. (2017). Insights into the Simultaneous Utilization of Glucose and Glycerol by Streptomyces Albulus M-Z18 for High ε-poly-l-lysine Productivity. Bioproc. Biosyst Eng. 40 (12), 1775–1785. doi:10.1007/s00449-017-1832-9
Zeng, X., Miao, W., Wen, B., Mao, Z., Zhu, M., and Chen, X. (2019). Transcriptional Study of the Enhanced ε-poly-l-lysine Productivity in Culture Using Glucose and Glycerol as a Mixed Carbon Source. Bioproc. Biosyst Eng. 42 (4), 555–566. doi:10.1007/s00449-018-2058-1
Zhang, J. H., Zeng, X., Chen, X. S., and Mao, Z. G. (2018). Metabolic Analyses of the Improved ε-poly-l-lysine Productivity Using a Glucose-Glycerol Mixed Carbon Source in Chemostat Cultures. Bioproc. Biosyst Eng. 41 (8), 1143–1151. doi:10.1007/s00449-018-1943-y
Zhang, K., Mohsin, A., Dai, Y., Chen, Z., Zhuang, Y., Chu, J., et al. (2019). Combinatorial Effect of ARTP Mutagenesis and Ribosome Engineering on an Industrial Strain of Streptomyces Albus S12 for Enhanced Biosynthesis of Salinomycin. Front. Bioeng. Biotechnol. 7, 212. doi:10.3389/fbioe.2019.00212
Zhang, X., Hou, Z., Xu, B., Xie, C., Wang, Z., Yu, X., et al. (2020). Dietary Supplementation of ε-Polylysine Beneficially Affects Ileal Microbiota Structure and Function in Ningxiang Pigs. Front. Microbiol. 11, 544097. doi:10.3389/fmicb.2020.544097
Zhou, Y. P., Ren, X. D., Wang, L., Chen, X. S., Mao, Z. G., and Tang, L. (2015). Enhancement of ε-poly-lysine Production in ε-poly-lysine-tolerant Streptomyces Sp. By Genome Shuffling. Bioproc. Biosyst Eng. 38 (9), 1705–1713. doi:10.1007/s00449-015-1410-y
Keywords: epsilon-poly-l-lysine, strain improvement, process engineering, downstream process, antimicrobial property
Citation: Wang L, Zhang C, Zhang J, Rao Z, Xu X, Mao Z and Chen X (2021) Epsilon-poly-L-lysine: Recent Advances in Biomanufacturing and Applications. Front. Bioeng. Biotechnol. 9:748976. doi: 10.3389/fbioe.2021.748976
Received: 28 July 2021; Accepted: 15 September 2021;
Published: 28 September 2021.
Edited by:
Bo Yu, Chinese Academy of Sciences, ChinaReviewed by:
Jiayang Qin, Binzhou Medical University, ChinaYouqiang Xu, Beijing Technology and Business University, China
K Madhavan Nampoothiri, National Institute for Interdisciplinary Science and Technology (CSIR), India
Copyright © 2021 Wang, Zhang, Zhang, Rao, Xu, Mao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xusheng Chen, Y2hlbnhzQGppYW5nbmFuLmVkdS5jbg==
 Liang Wang
Liang Wang Chongyang Zhang
Chongyang Zhang Jianhua Zhang1
Jianhua Zhang1 Zhiming Rao
Zhiming Rao Xueming Xu
Xueming Xu Zhonggui Mao
Zhonggui Mao Xusheng Chen
Xusheng Chen