- 1Department of Molecular Biomedical Sciences and Comparative Medicine Institute, North Carolina State University, Raleigh, NC, United States
- 2Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, North Carolina State University, Raleigh, NC, United States
Cardiac regenerative medicine faces big challenges such as a lack of adult cardiac stem cells, low turnover of mature cardiomyocytes, and difficulty in therapeutic delivery to the injured heart. The interaction of bioengineering and cardiac regenerative medicine offers innovative solutions to this field. For example, cell reprogramming technology has been applied by both direct and indirect routes to generate patient-specific cardiomyocytes. Various viral and non-viral vectors have been utilized for gene editing to intervene gene expression patterns during the cardiac remodeling process. Cell-derived protein factors, exosomes, and miRNAs have been isolated and delivered through engineered particles to overcome many innate limitations of live cell therapy. Protein decoration, antibody modification, and platelet membranes have been used for targeting and precision medicine. Cardiac patches have been used for transferring therapeutics with better retention and integration. Other technologies such as 3D printing and 3D culture have been used to create replaceable cardiac tissue. In this review, we discuss recent advancements in bioengineering and biotechnologies for cardiac regenerative medicine.
Introduction
Cardiovascular disease (CVD) is the leading cause of death in both developed and developing countries. According to the World Health Organization (WHO), 17.9 million people across the globe (31%) die due to CVD, of which 85% die from myocardial infarction (MI) (World Health Organization, 2021). In adult hearts post-MI, injured heart muscle cells are replaced by fibrotic tissue. During this maladaptive remodeling process, activated cardiac fibroblasts turn into myofibroblasts, causing stiffness and fibrosis, which is, in turn, associated with poor prognosis and heart failure. Though cardiac fibroblasts provide structural integrity to the heart after MI, it also causes a non-contracting scar. Such events hamper cardiac perfusion and pumping capacity and leads to cardiac remodeling further toward depravation to cardiac dysfunction, myocardium loss, and, eventually, heart failure (Xin et al., 2013; Saparov et al., 2017; Tallquist and Molkentin, 2017; Bo et al., 2020; Nelson and Brunt, 2021).
Conventional treatments for MI include coronary artery bypass, coronary reperfusion therapy, and fibrinolytic therapy, which are mainly for acute symptom relief rather than to promote repair and regeneration of the damaged myocardium (Awada et al., 2015). A heart transplant or a left ventricular assist device (LVAD) (Rose et al., 2001) is the final treatment option for heart failure patients. However, prognosis varies due to the complexity of the required highly invasive transplant surgery and its subsequent acute/chronic immune rejections (White and Chew, 2008; Wilhelm, 2015). Although pharmacological treatments of β-blockers and angiotensin-converting enzyme (ACE) inhibitors (Packer et al., 2001; McMurray et al., 2014) are beneficial to MI patients, these existing approaches make it necessary to explore new methods of treatment that aim at regenerating the infarcted myocardium as well as becoming implementable in the clinical practices (Raziyeva et al., 2020).
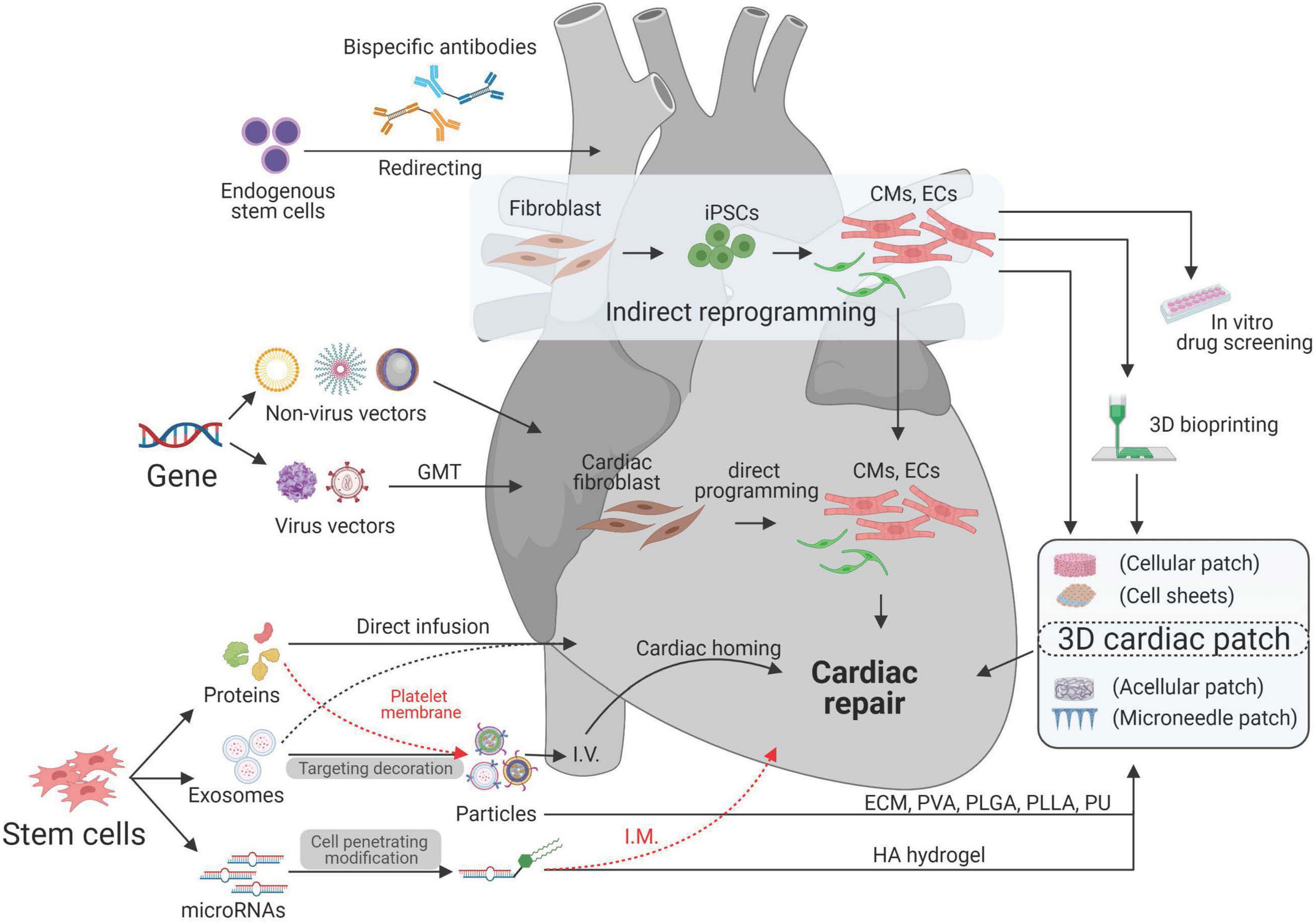
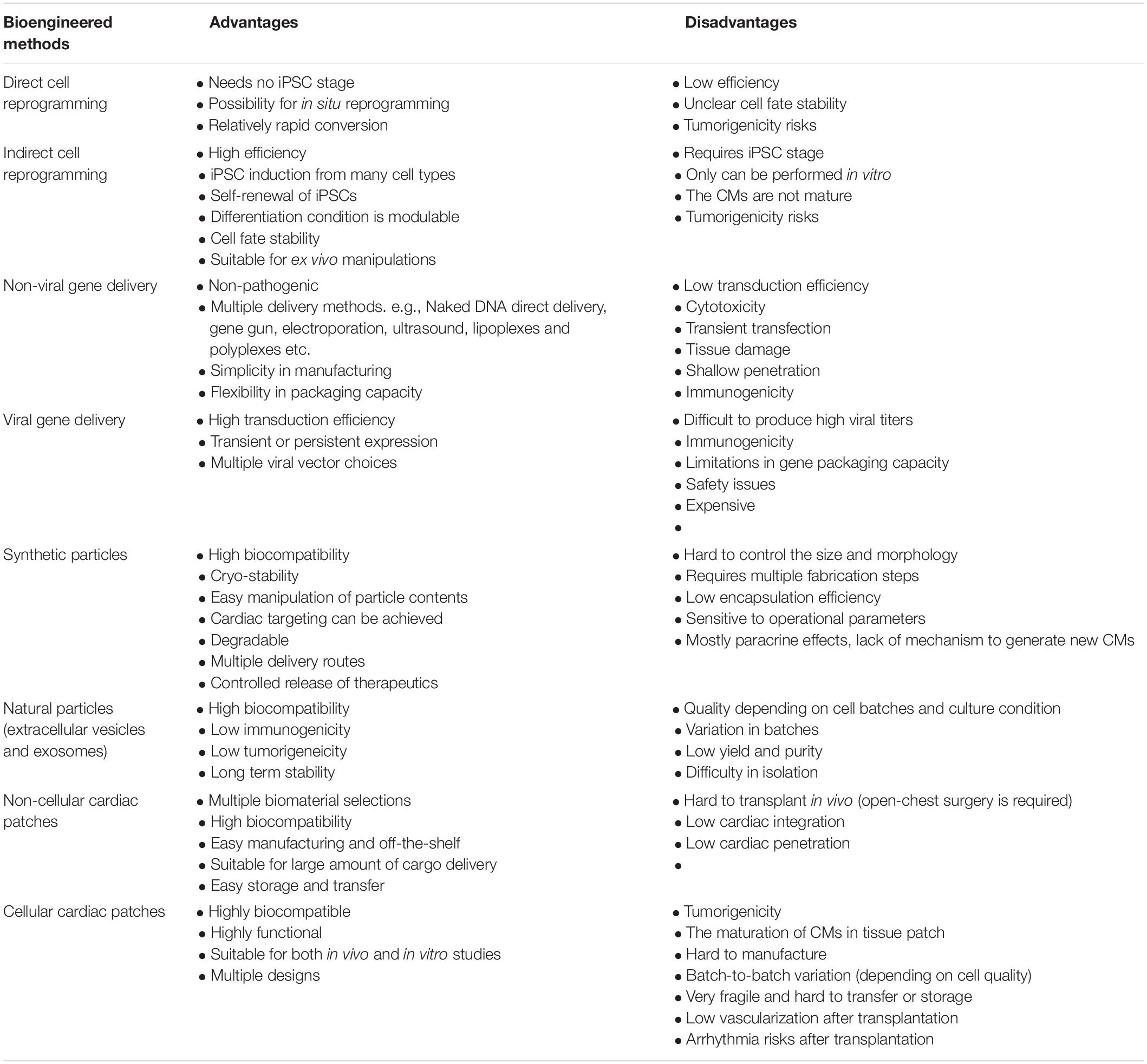
Biomedical engineering seeks to close the gap between engineering and medicine by combining the design and problem-solving skills of engineering with medical and biological sciences. Biomedical engineering hopes to advance health care treatment, including diagnosis, monitoring, and therapy. It has been transforming the cardiac regenerative approaches into potential treatments for CVD (Lee and Walsh, 2016). Treatments for ischemic/reperfusion-damaged or infarcted myocardium have been designed by using multifarious biotechnologies based on the purpose of treatment. With the idea of using autologous cells for cardiac treatment, patient-specific cardiomyocytes (CMs) were generated through cell reprogramming technologies (Wang et al., 2021). To improve the regenerative capability of CMs, various viral and non-viral vectors have been used for gene editing to intervene with gene expression during cardiac remodeling process after MI (Rincon et al., 2015; Kohama et al., 2020). To overcome the retention, fragile, tumorigenicity, and immunogenicity limitations of cell therapy (Tang et al., 2018c), cell-derived protein factors, exosomes, and miRNAs have been isolated and delivered through micro- (Huang et al., 2020) or nanosized particles (Su et al., 2018b). For better targeting, scientists have used proteins, antibodies, and platelet membranes to decorate their therapeutics. For better retention and integration, cardiac patches have been designed by transfer therapeutics in vehicles made of various biomaterials (Mei and Cheng, 2020). Additionally, 3D printing (Maiullari et al., 2018) and 3D culture (Jackman et al., 2018) technologies were utilized to create replaceable cardiac tissue (Figure 1 and Table 1). In this review, we will discuss current biotechnologies for cardiac repair.
Cell Reprogramming
Cell reprogramming is a powerful tool that converts the somatic cell lineage into pluripotent stem cells (iPSCs) (Rao and Malik, 2012), CMs (Fu et al., 2015) or endothelial cells (ECs) (Lee C. S. et al., 2017). Generally, this tool is used both in vitro and in vivo for cardiac injury site repair (Patel et al., 2016), cardiac disease modeling, or drug screening (Ebert et al., 2012; Chen and Vunjak-Novakovic, 2018). In the process of changing cell fate, an intermediary pluripotent state is key to differentiate direct and indirect reprogramming (Wang et al., 2021).
Indirect Cell Reprogramming
Indirect cell reprogramming from adult somatic cells to iPSC-derived CMs (iPSC-CMs) is a well-established process (Tai et al., 2018). iPSCs from multiple origins are now commercially available. This reprogramming method is widely used not only due to the difficulty of culturing human primary CMs in vitro but also because they contain patient-specific genomic information and could be used for autologous cardiac regenerative medicine (Martins et al., 2014). Commonly, adult fibroblasts are reprogrammed into iPSCs through the activation of alkaline phosphatase, silencing of somatic-specific expression, expression of SSEA1, and progressive silencing of exogenous genes with upregulation of Oct4 and Nanog (Teshigawara et al., 2017). However, these CMs are closer to an immature stage in terms of marker expression, ultrastructural features, metabolic signature, and electrophysiological properties (Tang, 2020). First, the origin of somatic cells is a determinant of iPSC-CM maturation (Pianezzi et al., 2020). Comparison of iPSC-CMs derived from cardiac-derived mesenchymal progenitor cells (CPCs), bone marrow-derived mesenchymal stem cells (BMCs), and human dermal fibroblasts (HDFs) that comes from the same patient showed the cardiac somatic cell’s enhanced capacity for cardiac re-differentiation due to upregulated cardiac genes (MYH6, TNNI3, KCNQ1, KCNE1) (Altomare et al., 2016; Pianezzi et al., 2020). Additionally, the application of iPSC-CMs is highly affected by the purification process because tumors can form during in vitro culture of iPSCs that increase the malignant risks to in vivo application (Tohyama et al., 2013). To overcome the purification obstacles, a distinct metabolic flow technology has been designed to enable large-scale purification through glucose depletion and lactate supplementation because the mature iPSC-CMs have a higher oxygen consumption rate with increased mitochondrial maturity (Tohyama et al., 2013; Tang, 2020).
Direct Cell Reprogramming
Direct cell reprograming is a process of transforming of somatic cells to a desired cell fate without a pluripotent or multipotent state (Wang et al., 2021). Ideally, direct cell reprogramming is more suitable for in vivo cardiac tissue repair by generating reprogrammed cells in situ in the diseased heart (Wang et al., 2021); however, it is still challenging to perform it in vivo due to the low transforming efficiency. For example, direct reprogramming of transcriptional factors like Gata4, Oct4, Tbx5, Sox2, and Klf4 were delivered directly into the damaged heart to initiate regeneration (Ieda et al., 2010; Chen et al., 2017; Hashimoto et al., 2018). Six core transcriptional factors Gata4, Hand2, Mef2c, Mesp1, Nkx2.5, and Tbx5 were examined for their cardiac linage reprograming capability (Li et al., 2015; Wang et al., 2015). Retroviruses were used to express these transcription factors in fibroblasts that were derived from adult mice (Pasumarthi and Field, 2002; Ieda et al., 2010; Song et al., 2012). Another study reported in vitro formation of CMs from fibroblasts by expressing transcriptional factors Gata4, Mef2c, and Tbx5 (GMT) and thereby functionally repopulating the scar (Qian et al., 2012; Wang et al., 2015). Although direct cell reprogramming bypasses early developmental stages (Barreto et al., 2019) such as the cardiac progenitor stages, the tumorigenic risks may not lower than indirect reprogramming because the small molecules cannot be guaranteed, which can also produce iPSCs (Chen et al., 2017). Most importantly, the fate of transduced cells in vivo is still debated, although single-cell transcriptomics have been done to discover the mechanism of the fate conversion from fibroblast to CMs (Liu et al., 2017). Additionally, miRNAs have the ability to regulate various signaling pathways at the same time, which makes them a promising alternative (Sandmaier and Telugu, 2015). Researchers provided evidence that direct administration of miRNAs through lipid-based transfection at the target site successfully converted fibroblasts into cardiomyocytes in vivo (Elmén et al., 2008; Sridharan and Plath, 2011). Cardiac reprogramming through miRNAs (miR-1 miR-133, miR208, and miR499) was enhanced when combined with JAK inhibitor I treatment (Jayawardena et al., 2012).
Gene Editing
Many genes are essential for CM proliferation and cardiac repair. For example, ERBB2 has been reported triggering mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation through YAP activation and EMT (epithelial–mesenchymal transition)-like processes (D’Uva et al., 2015; Aharonov et al., 2020). Cyclin A2 or CCNA2, a gene normally silenced after birth, has been demonstrated as a key cell cycle regulatory gene to induce cardiac repair by mediating both the G1–S and G2–M transitions of the cell cycle (Shapiro et al., 2014). So, an efficient delivery of the desired gene to heart is important for cardiac gene therapy.
Non-viral Gene Delivery
In cardiac gene therapy, efficient delivery of the desired gene to target tissue is important. Non-viral gene delivery methods, such as needle or jet injection, hydrodynamic gene transfer, electroporation, and cationic lipids make use of natural or synthetic compounds or physical forces to deliver the gene of interest to the target site. It is an important technology for tissue engineering with many features, including low toxicity, easy modification, high productivity, and cell specificity (Wu et al., 2018). A needleless liquid jet methodology using a jet device with micro jets has been used to expand or generate cell pores for cardiac gene delivery. However, it is not suitable for cardiac applications due to the jet’s piercing with high force (Fargnoli et al., 2017). Additionally, hydrophilic naked DNA could be consumed by cells (Al-Dosari and Gao, 2009); however, only a small percentage of target cells express the delivered genes, thus, making them inefficient.
Viral Gene Delivery
Recombinant viral vectors such as adenoviruses, lentiviruses, and adeno-associated viruses are generally used for gene delivery (Wolfram and Donahue, 2013). Adenoviral gene delivery systems have been extensively used in gene-based therapies and cell-based therapies. Adenoviral vectors have shown a high transduction rate in both dividing and non-dividing cells (Lee S. et al., 2017). The knockdown of lncRNA through adenovirus-mediated lncRNA approaches lead to the reduction in macrophage infiltration and cardiomyocyte apoptosis in the heart after MI (Wang K. et al., 2017). Although adenovirus vectors were used in clinical trials due to their large tropism profile (Jessup et al., 2011), adenoviral-mediated gene delivery triggers immunogenicity in humans and lacks integration with the host genome, making it an unfavorable choice (Vilaysane and Muruve, 2009; Wasala et al., 2011). Lentiviral vectors are suitable for long-term transgene expression, which integrates their genome into the hosts with a preference for transcriptionally active sites (Bulcha et al., 2021). Lentiviral vectors can also elicit relatively weak immune responses through vector design (Bulcha et al., 2021). Lentiviral-mediated CCND2 gene transfection enhanced the regenerative potency of iPSCs-CMs (Zhu et al., 2018). Additionally, enhanced expression of Gata-4 and Nkz2.5 by lentiviral-mediated prodynorphin vectors resulted in a drastic increase in the CMs’ beating activity (Rincon et al., 2015). However, lentiviral vectors are limiting for cardiovascular disease treatments due to their low transduction rate in the myocardium in vivo (Rincon et al., 2015). The adeno-associated virus (AVV), as a cardiotropic vector, can be designed as a viral therapeutic to promote cardiac repair after MI (Yoo et al., 2018). AAV6 was reported as the most effective vector to transduce CMs (Ambrosi et al., 2019). For example, AAV6 was designed to deliver miR-199a to a pig model of MI (Gabisonia et al., 2019). Although the results showed cardiac improvement after 1 month, all pigs died due to subsequent uncontrolled expression of miR-199a that resulted in sudden arrhythmia.
Particle Design
Stem cell therapy has been a promising approach mediated through paracrine effects (Maghin et al., 2020; Sid-Otmane et al., 2020). However, engraftment of transplanted cells and their low retention limit its therapeutic efficacy. Also, other concerns such as tumorigenicity, immunogenicity, and product stability need to be taken into account (Tang et al., 2018c). Since stem cells secrete a plethora of molecules like cytokines, soluble proteins, and extracellular vesicles that help in cardiac repair and regeneration (Tao et al., 2018), analysis of the cell secretome has gained importance in elucidating therapeutic mechanisms (Ellison-Hughes and Madeddu, 2017).
Synthetic Particles
Micro-sized synthetic stem cells (SSCs) have been fabricated to mimic the paracrine effects of a live cell through a cell-mimicking microparticle (CMMP) technology (Tang et al., 2017; Huang et al., 2020). Briefly, poly lactic-co-glycolic acid (PLGA) is used to encapsulate the stem cell-derived secretomes. Afterward, microparticles are coated with cell membranes to become SSCs (Tang et al., 2017). This technology not only overcomes the multiple inherent limitations of live-cell therapy (Huang et al., 2020) but also holds a high applicability for different cell lines, for example, both cardiac stromal cells (CSCs) and mesenchymal stem cells (MSCs) have been synthetically created (Luo et al., 2017; Tang et al., 2017; Huang et al., 2020). Additionally, to transfer miR21 into macrophages and ECs, positively charged mesoporous silica nanoparticles (MSNs) are designed to encapsulate miR21 and are injected into the ischemic heart of pigs for treatment (Li Y. et al., 2021). MSNs are reported as a highly biocompatible and transfection-effective nanoparticle that are generated through a classical CTAB-templated, base-catalyzed sol–gel method (Li Y. et al., 2021). These microparticles exert their beneficial effects mainly through mimicking cell paracrine of protein factors and membrane-based cell–cell interaction with injured cells (Luo et al., 2017; Tang et al., 2017). Also, the nanoparticles that are consumed by CMs and fibroblasts release the enveloped miRNAs and proteins to regulate gene expression (Hodgkinson et al., 2015). To fully understand the mechanism, the combination of cell secretomes have to be further studied because the cardiac beneficial results may come from the combination or any of the secretome contents, including miRNAs, protein factors, exosomes, extracellular vesicles, and so on. Moreover, cell secretome is not standardized due to the variation of cell lines, culture conditions, and purity, which makes it even harder to uncover the veil of cell therapy.
Natural Particles
Exosomes, a type of EVs, are bilipid layered nanovesicles with a diameter of around 35–150 nm that carry encapsulated proteins, membrane-bound proteins, and miRNAs and are capable of triggering various complex function-altering pathways (Li et al., 2017; Sun et al., 2018). Their small size allows them to pass through small capillaries, giving them more access to tissues than transplanted cells (Verweij et al., 2019; Dinh et al., 2020). Exosomes can be extracted and purified through techniques such as immunoaffinity capture, polymeric precipitation, tangential flow ultracentrifugation and size exclusion. Exosomes isolated from several cell lines including MSCs (Huang P. et al., 2019; Hu et al., 2021), iPSCs (Gao et al., 2020), cardiosphere-derived cells (CDCs) (Vandergriff et al., 2015; Gallet et al., 2017), and CPCs (Barile et al., 2017) have demonstrated cardiac protection through neovascularization and anti-inflammation (Teng et al., 2015; Huang P. et al., 2019). MSC-derived exosomes also contain miRNAs (miR-30b, let-7f, miR-424, and miR-30c) that promote angiogenesis For example, MSC-derived exosomal miR-21-5p heightens cardiac contractile strength and calcium handling through PI3K signaling (Mayourian et al., 2018; Qiao et al., 2019).
Cardiac Targeting
Heart stem cell therapy is usually administered intramyocardially via open-chest or percutaneous coronary intervention (PCI) (Malliaras and Marbán, 2011). As the invasive nature of this drug delivery is not effective and appealing, researchers focus on developing therapeutics with targeting ability to the injured myocardium. This concept allows drugs to interact specifically with the infracted region and impart therapeutic benefits. Various targeting strategies, such as cardiotropic vector selection, peptide decoration, magnetic reactive carrier, or antibody editing have been employed for efficient drug delivery (Cores et al., 2015; Weinberger and Eschenhagen, 2021).
Cardiotropic Vector Selection
Cardiac gene therapy requires viral vectors to safely access the heart specifically. So, it is essential to find a cardiotropic vector for gene delivery. For example, although multiple strains of AAVs from serotypes 1 to 9 have been isolated, only AAV9 (Inagaki et al., 2006) and AAV6 (Bish et al., 2008; Gao et al., 2011) showed a higher transduction efficiency. However, these vectors require intrapericardial or intramyocardial administration. Since there is a difference in host specificity (Asokan and Samulski, 2013), it is even harder to evaluate AAV vectors in preclinical models and clinical translation.
Peptide Targeting
Peptide-based targeting has been achieved by decorating peptide (Kanki et al., 2011) onto therapeutics. A 12-amino acid non-naturally occurring peptide NH2-APWHLSSQYSRT-COOH was reported as a cardiomyocyte-targeting peptide (Feldman and Zahid, 2020). The peptide motif CSTSMLKAC was also identified as a potential tool for heart homing (Shelke et al., 2008; Kanki et al., 2011). For example, CDC-derived exosomes were designed to target the heart injury site via cardiac homing peptide CSTSMLKAC through a dioleoylphosphatidylethanolamine N-hydroxysuccinimide (DOPE-NHS) linker on the exosomal membrane (Shelke et al., 2008). Since the prostaglandin E2 (PGE2) receptors are overexpressed in the pathological cardiac microenvironment after ischemic/reperfusion injury (Kim et al., 2008; Zhang et al., 2015), researchers decorated PGE2 on nanoparticles through EDC/NHS coupling chemistry to increase cardiac homing capability (Su et al., 2018b).
Platelet Targeting
Platelet-based targeting has been designed based on the inherent properties of platelets. Platelet surface receptors such as the GPIb/IX/V complex, GPVI and α2β1, β1, and β3 integrins, are responsible for interactions with exposed injury sites (Li et al., 2018). The platelet membrane has been isolated and coated on either cell (Shen et al., 2016; Tang et al., 2018a), micro- (Li et al., 2020) or nano- (Su et al., 2018b) particles to endow cardiac injury site homing capability (Li et al., 2018). To increase targeting and cellular uptake of nanoparticles on coronary artery stents, scientists activated ECs through binding P-selectin to platelet glycoprotein Ibα (GP Ibα) on platelet-mimicking nanoparticles (Lin et al., 2010).
Antibody Targeting
There are many cardiac targeting approaches designed to utilize the innate specific binding capability of antibodies. Bispecific antibodies (BsAbs) have been designed to bind two different targets simultaneously by combining variable domains of desired monoclonal antibodies into an integrated structure (Huang et al., 2019a). BsAbs are generated by chemical conjugation, hybridoma fusion, or genetic engineering such as recombinant DNA technology (Parashar et al., 2011). To redirect endogenous bone marrow stem cells (BMSCs) to the injured heart, BsAbs were designed to link F(ab′)2 fragments from monoclonal anti-CD34 and anti-cardiac myosin heavy chain through chemical cycloaddition of AZ-PEG-NHS or DBCO-PEG-NHS on those F(ab′)2 fragments (Huang et al., 2019b). In this study, after G-CSF stimulation, the administration of BsAbs redirected circulating BMSCs to the injured myocardium (Huang et al., 2019b). Additionally, an inhalable platelet-targeting bispecific antibody (PT-BsAb) was designed by linking of CD34 (HSC binding) and CD42b (platelet binding) to redirect stem cells from the lungs to the heart for repair (Liu M. et al., 2021).
To increase the targeting capability of the injury site, a poly (N-isopropylacrylamide) nanogel with tissue plasminogen activator (tPA) and cell contractility inhibitor Y-27632 coupled anti-fibrin antibodies on the outside of nanoparticles through an EDC/sulfo-NHA method (Mihalko et al., 2018). The injected nanoparticles were directed by anti-fibrin antibodies to the fibrin-rich site post-MI and released tPA and Y-27632 to the site of injury (Mihalko et al., 2018; Huang et al., 2019b). Besides targeting, antibodies were also decorated on platelet-inspired cardiac-targeting microparticles to neutralize inflammatory cytokines. For example, since IL-1β has been demonstrated as a primary pro-inflammatory cytokine during cardiac remodeling, IL-1β antibodies were linked to platelet membrane on microparticles through 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly (ethylene glycol) (DSPE-PEG) (Li et al., 2020).
Cardiac Patch Design
Cardiac patches have been devised to ameliorate the cardiac function post-MI (Mei and Cheng, 2020). A cardiac patch is typically composed of substrate and active therapeutic agents (Yang et al., 2013). Cardiac patches can have therapeutic ingredients ranging from cells, such as iPSCs, myoblasts and MSCs, to bioactive molecules, such as miRNAs, exosomes, and microparticles (Mei et al., 2010; Squillaro et al., 2016; Wang L. L. et al., 2017; Giacomelli et al., 2020).
Cellular to Non-cellular Patches
Cell-based cardiac patches have been designed to increase the survival ratio of the embedded cells and to ensure cellular retention (Braunwald, 2018; Zhu et al., 2021). For example, a recently developed cardiac patch fabricated with biomimetic microvessels and CSCs in fibrin gel enhanced angiogenesis, CSC retention, and survival rate after heart transplantation (Gao et al., 2018; Su et al., 2018a). Furthermore, non-cellular cardiac patches have been designed to overcome the limitations of live cellular cardiac patches (Tang et al., 2018b; Li Z. et al., 2021). For example, a specific miRNA patch was designed to modulate gene expression during cardiac remodeling (Wang L. L. et al., 2017). For miR-302 cardiac delivery, researchers designed a system by using a cholesterol molecule to decorate miR-302 mimics and using adamantane or cyclodextrin to modify hydrogels. Since the modified hydrogel showed self-assembly into shear-thinning and self-healing gels, the cholesterol on miR-302 mimics interacts with cyclodextrin to achieve sustained release (Wang L. L. et al., 2017; Huang et al., 2019a). To enhance clinical feasibility, an off-the-shelf cardiac patch was designed by embedding synthetic cardiac stromal cells into a decellularized extracellular matrix through a vacuum filtration method (Huang et al., 2020). In addition, exosomes derived from MSCs were integrated into hydrogels, providing a minimally invasive delivery method through intrapericardial injection. This injectable patch revolutionized the delivery of cardiac patches, which normally needs a traumatic open-chest surgery (Zhu and Cheng, 2021; Zhu et al., 2021).
Tissue Patches
Cardiac tissue patch is one important type of cell-based patches that has been created by culture of embryonic stem cell-derived cardiomyocytes (ESC-CMs) (Chong et al., 2014), iPSC-CMs, or neonatal rodent cardiomyocytes (NRCMs) into a 3D scaffold to form a functional 3D structure (Pomeroy et al., 2020). For example, cardiac tissue patches cultured with multiple layers of iPSC-CMs or NRCMs within hydrogels or other porous polymer scaffolds form a randomly oriented and electromechanically integrated cardiac tissue patch (Shadrin et al., 2017; Pomeroy et al., 2020). In a window chamber model and a rodent MI model, the iPSC-CM 3D cardiac patch showed a preserved structure, electrical function, and successful vascularization (Shadrin et al., 2017; Cui et al., 2020). Implantation of this engineered cardiac tissue patch to the injured heart in vivo showed improved vascularization in the infarct region and reduced fibrosis (Wendel et al., 2014; Iseoka et al., 2018) (Uygun et al., 2010; Dvir et al., 2011; Fleischer et al., 2017).
3D bioprinting enables the production of 3D tissue constructs with precise architecture and integration of various cell types. The microenvironment of printed tissue accurately resembles native conditions, which, in turn, helps promote complex tissue formation in vitro (Gu et al., 2020). Different cellular techniques such as inkjet, stereolithography, and extrusion bioprinting are used for the development of cardiovascular tissues (Jana and Lerman, 2015; Duan, 2017). Common cell types used for cardiac tissue printing include MSCs, CSCs, ESCs, iPSCs, and cardiac fibroblasts (Cui et al., 2017). The laser-induced transfer (LIFT)-based cell bioprinting has been used to fabricate EC- and MSC-laden polyester urethane urea (PEUU) cardiac patches (Cui et al., 2018). When compared with non-patterned cardiac patches, patterned patches increased angiogenesis in the border zone of the infarction, as well as preservation of cardiac function after acute MI (Shengjie et al., 2009). 3D bioprinting could facilitate the development of the therapeutic potential of stem cells, which would play an important role in regenerative medicine (Wang et al., 2018; Pomeroy et al., 2020).
Conclusion and Future Directions
Cellular reprogramming is a new paradigm in cell biology and provides a unique and efficient way to generate cell types of interest for cardiac repair by changing one cell fate to another (Wang et al., 2021). Usually, the indirect reprogramming routes require an in vitro engineered 3D tissue and then transplant in vivo (Querdel et al., 2021). The direct reprogramming bypass early developmental stages and administer the cardiac transcriptional factors directly by viral vectors. From a translational perspective, the technology of direct reprogramming holds great potential as a treatment due to its features including fast turnaround time and feasibility for in vivo applications (Wang et al., 2021). However, a large scale of somatic cells could be converted through indirect preprogramming to create a paradigm for in vitro CRISPR–Cas9 screening, drug screening, and disease modeling (Wang et al., 2021). Although cell reprogramming showed a potential strategy for the cardiac repair, there are few advantages and disadvantages in both reprogramming routes. Direct reprogramming rarely produces beating CMs after a long culture period (Fu et al., 2013; Nam et al., 2013). In comparison, indirect reprogramming is robust to produce beating CMs that are not in a mature stage. In addition, indirect reprogramming achieves a high conversion efficiency around 70 to even 90% (Pomeroy et al., 2020); however, direct reprogramming has a low conversion efficiency (4.8%) (Engel and Ardehali, 2018) due to the presence of epigenetic barriers such as Bmi1 (Zhou et al., 2016). This low conversion efficiency remains a major hurdle for direct reprogramming, and even the process was already improved by administering cardiac transcriptional factors along with epigenetic modifiers, inhibitors, cytokines, and miRNAs (Engel and Ardehali, 2018).
The goal of gene therapy for cardiac repair is to modify a gene or genetic pathway. Safety and efficacy are important to develop tolerance and ease administration that may be translated to the clinic (Wolfram and Donahue, 2013). For example, adenoviral vectors may trigger acute inflammation, which impacts gene transfer efficacy and may cause host morbidity (Liu and Muruve, 2003). Also, gene delivery through the myocardium or coronary injections has a low cardiac transfection outcome due to the neutralization by existing endogenous antibodies (Jessup et al., 2011). As previously mentioned, long-term expression of target genes may also cause sudden death in pig studies (Gabisonia et al., 2019).
Different types of stem cells have been studied as potential candidates for cardiac regenerative medicine. However, live stem cell delivery has many inherent limitations such as tumorigenicity, immunogenicity, cell death, and low retention after transplantation (Tang et al., 2018c). So, multiple cell-derived secretomes, exosomes, and miRNAs have been engineered as alternatives for heart repair by mimicking paracrine effects of the cell or manipulating gene expression during cardiac remodeling after MI. These cell-derived therapeutics have been combined with different biomaterials to overcome the limitations of low cardiac engraftment/retention, low miRNA stability, and delivery difficulties (Huang et al., 2019a).
Non-targeted cardiac therapeutics with an intravenous delivery usually affects multiple systems, which may cause systemic side effects. Targeted therapeutics, on the other hand, are designed for precise cardiac treatment with one or multiple intravenous injections. The targeting technology is achieved mainly through decorating a cardiac homing molecule on nanosized particles, which are safe in circulation with minimal chance of stimulating coagulations (Dobrovolskaia et al., 2009). Additionally, endogenous stem cells may be stimulated and redirected by BsAbs (Huang et al., 2019b; Liu M. et al., 2021). However, all targeting methods have to be further studied due to the low targeting capability and treatment efficacy.
3D cardiac patches are a promising method in cardiac repair and are either cellular or non-cellular. The cellular patches are generated through seeding of different live cells into various 3D scaffolds. To enhance the survival of the transplanted live cells, the patches have been engineered with mimetic blood vessels (Su et al., 2018a, 2020) or cocultured with ECs (Shadrin et al., 2017). For better integration, the scaffold has been engineered with microneedles (Tang et al., 2018b). Additionally, researchers have manipulated cell growth and differentiation conditions through culture medium optimization to enhance the maturity of iPSC-CMs on tissue patches (Machiraju and Greenway, 2019). Non-cellular patches are generated by seeding different cell derivatives into various 3D scaffolds. Compared with cellular cardiac patches, these patches have better stability, biocompatibility, modifiability, and low tumorigenicity and immunogenicity. 3D bioprinting technology has been widely utilized in cardiac repair by integrating biomaterials with different cell types to precisely pattern a cardiac structure (Liu N. et al., 2021). However, this technology is still in the early stage and needs to be improved (Liu N. et al., 2021).
Additional biotechnologies not mentioned in this review such as cardiac spheroids, single ventricles, bundles, regenerative gene expression, and design of biomaterials also play an important role in the field. Although there are plenty of technologies, it is not easy to get past the bottleneck of heart regenerative medicine, for example, the maturity of iPSC-CMs, the optimized cell protein factor combination, the detailed miRNA regulation mechanism, the key gene for cardiomyocyte regeneration, creation of large-sized cardiac tissue (Gao et al., 2018), and control of drug delivery-caused trauma. To overcome these obstacles, future interdisciplinary cooperation will be the key in the research area. For example, engineering of an injectable material that have controlled gelation speed, biocompatibility, degradative ability, and temperature sensitivity will be essential to create an injectable cardiac patch. Also, screening and designing of cardiotropic viral vectors through structure evolution of capsid variants (Tse et al., 2017) would enhance cardiac gene therapy. Moreover, natural exosomes are not clinically feasible due to many inherent limitations that could be overcome through cell-based pre-isolation exosome engineering and post-isolation exosome engineering (Huang P. et al., 2019; Jafari et al., 2020). Although mainly practiced in research labs today, innovative experimental bioengineering technologies will revolutionize heart repair field in the future.
Author Contributions
MC, DZ, and KH wrote the text of this review article with guidance from KC and KH. All authors have reviewed the final version and approved the content in this manuscript.
Funding
This work was supported by grants from the National Institutes of Health (R01 HL123920, HL137093, HL144002, HL146153, and HL147357 to KC) and the American Heart Association (18TPA34230092 and 19EIA34660286 to KC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aharonov, A., Shakked, A., Umansky, K. B., Savidor, A., Genzelinakh, A., Kain, D., et al. (2020). ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat. Cell Biol. 22, 1346–1356. doi: 10.1038/s41556-020-00588-4
Al-Dosari, M. S., and Gao, X. (2009). Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 11, 671–681.
Altomare, C., Pianezzi, E., Cervio, E., Bolis, S., Biemmi, V., Benzoni, P., et al. (2016). Human-induced pluripotent stem cell-derived cardiomyocytes from cardiac progenitor cells: effects of selective ion channel blockade. Europace 18(Suppl. 4), iv67–iv76.
Ambrosi, C. M., Sadananda, G., Han, J. L., and Entcheva, E. (2019). Adeno-associated virus mediated gene delivery: implications for scalable in vitro and in vivo cardiac optogenetic models. Front. Physiol. 10:168. doi: 10.3389/fphys.2019.00168
Asokan, A., and Samulski, R. J. (2013). An emerging adeno-associated viral vector pipeline for cardiac gene therapy. Hum. Gene Ther. 24, 906–913. doi: 10.1089/hum.2013.2515
Awada, H. K., Hwang, M. P., and Wang, Y. (2015). Towards comprehensive cardiac repair and regeneration after myocardial infarction: aspects to consider and proteins to deliver. Biomaterials 82, 94–112. doi: 10.1016/j.biomaterials.2015.12.025
Barile, L., Moccetti, T., Marbán, E., and Vassalli, G. (2017). Roles of exosomes in cardio protection. Eur. Heart J. 38, 1372–1379.
Barreto, S., Hamel, L., Schiatti, T., Yang, Y., and George, V. (2019). Cardiac progenitor cells from stem cells: learning from genetics and biomaterials. Cells 8:1536. doi: 10.3390/cells8121536
Bish, L. T., Sleeper, M. M., Brainard, B., Cole, S., Russell, N., Withnall, E., et al. (2008). Percutaneous trans endocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in Canines. Mol. Ther. 16, 1953–1959. doi: 10.1038/mt.2008.202
Bo, B., Zhou, Y., Zheng, Q., Wang, G., Zhou, K., and Wei, J. (2020). The molecular mechanisms associated with aerobic exercise-induced cardiac regeneration. Biomolecules 11:19. doi: 10.3390/biom11010019
Braunwald, E. (2018). Cell-based therapy in cardiac regeneration: an overview. Circ. Res. 123, 132–137. doi: 10.1161/circresaha.118.313484
Bulcha, J. T., Wang, Y., Ma, H., Tai, P. W. L., and Gao, G. (2021). Viral vector platforms within the gene therapy landscape. Signal Transduct. Target Ther. 6:53.
Chen, T., and Vunjak-Novakovic, G. (2018). In vitro models of ischemia-reperfusion injury. Regen. Eng. Transl. Med. 4, 142–153.
Chen, Y., Yang, Z., Zhao, Z.-A., and Shen, Z. (2017). Direct reprogramming of fibroblasts into cardiomyocytes. Stem Cell Res. Ther. 8:118.
Chong, J. J. H., Yang, X., Don, C. W., Minami, E., Liu, Y.-W., Weyers, J. J., et al. (2014). Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510:273.
Cores, J., Caranasos, G. T., and Cheng, K. (2015). Magnetically targeted stem cell delivery for regenerative medicine. J. Funct. Biomater. 6, 526–546. doi: 10.3390/jfb6030526
Cui, H., Liu, C., Esworthy, T., Huang, Y., Yu, Z. X., Zhou, X., et al. (2020). 4D physiologically adaptable cardiac patch: a 4-month in vivo study for the treatment of myocardial infarction. Sci Adv. 6:eabb5067. doi: 10.1126/sciadv.abb5067
Cui, H., Miao, S., Esworthy, T., Zhou, X., Lee, S.-J., Liu, C., et al. (2018). 3D bioprinting for cardiovascular regeneration and pharmacology. Adv. Drug Deliv. Rev. 132, 252–269.
Cui, H., Nowicki, M., Fisher, J. P., and Zhang, L. G. (2017). 3D bioprinting for organ regeneration. Adv Healthc Mater. 6:10.1002/adhm.201601118 doi: 10.1002/adhm.201601118
Dinh, P. U. C., Paudel, D., Brochu, H., et al. (2020). Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 11:1064.
Dobrovolskaia, M. A., Patri, A. K., Zheng, J., Clogston, J. D., Ayub, N., Aggarwal, P., et al. (2009). Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine 5, 106–117. doi: 10.1016/j.nano.2008.08.001
Duan, B. (2017). State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann. Biomed. Eng. 45, 195–209. doi: 10.1007/s10439-016-1607-5
D’Uva, G., Aharonov, A., Lauriola, M., Kain, D., Yahalom-Ronen, Y., Carvalho, S., et al. (2015). ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 17, 627–638. doi: 10.1038/ncb3149
Dvir, T., Timko, B. P., Kohane, D. S., and Langer, R. (2011). Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 6, 13–22. doi: 10.1038/nnano.2010.246
Ebert, A. D., Liang, P., and Wu, J. C. (2012). Induced pluripotent stem cells as a disease modeling and drug screening platform. J. Cardiovasc. Pharmacol. 60, 408–416. doi: 10.1097/fjc.0b013e318247f642
Ellison-Hughes, G. M., and Madeddu, P. (2017). Exploring pericyte and cardiac stem cell secretome unveils new tactics for drug discovery. Pharmacol. Ther. 171, 1–12. doi: 10.1016/j.pharmthera.2016.11.007
Elmén, J., Lindow, M., Schütz, S., Lawrence, M., Petri, A., Obad, S., et al. (2008). LNA-mediated microRNA silencing in non-human primates. Nature 452, 896–899. doi: 10.1038/nature06783
Engel, J. L., and Ardehali, R. (2018). Direct cardiac reprogramming: progress and promise. Stem Cells Int. 2018:1435746.
Fargnoli, A. S., Katz, M. G., and Bridges, C. R. (2017). A needleless liquid jet injection delivery approach for cardiac gene therapy. Methods Mol. Biol. 1521, 219–226. doi: 10.1007/978-1-4939-6588-5_15
Feldman, K. S., and Zahid, M. (2020). In Vivo imaging of transduction efficiencies of cardiac targeting peptide. J. Vis. Exp. 11:160.
Fleischer, S., Feiner, R., and Dvir, T. (2017). Cutting-edge platforms in cardiac tissue engineering. Curr. Opin. Biotechnol. 47, 23–29. doi: 10.1016/j.copbio.2017.05.008
Fu, J.-D., Stone, N. R., Liu, L., Spencer, C. I., Qian, L., Hayashi, Y., et al. (2013). Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 1, 235–247. doi: 10.1016/j.stemcr.2013.07.005
Fu, Y., Huang, C., Xu, X., Gu, H., Ye, Y., Jiang, C., et al. (2015). Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Res. 25, 1013–1024. doi: 10.1038/cr.2015.99
Gabisonia, K., Prosdocimo, G., Aquaro, G. D., Carlucci, L., Zentilin, L., Secco, I., et al. (2019). MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 569, 418–422. doi: 10.1038/s41586-019-1191-6
Gallet, R., Dawkins, J., Valle, J., Simsolo, E., de Couto, G., Middleton, R., et al. (2017). Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodeling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38, 201–211.
Gao, G., Bish, L. T., Sleeper, M. M., Mu, X., Sun, L., Lou, Y., et al. (2011). Trans endocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in Rhesus Macaques. Hum. Gene Ther. 22, 979–984. doi: 10.1089/hum.2011.042
Gao, L., Gregorich, Z. R., Zhu, W., Mattapally, S., Oduk, Y., Lou, X., et al. (2018). Large cardiac muscle patches engineered from human induced-pluripotent stem cell–derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 137, 1712–1730. doi: 10.1161/circulationaha.117.030785
Gao, L., Wang, L., Wei, Y., Krishnamurthy, P., Walcott, G. P., Menasché, P., et al. (2020). Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci. Transl. Med. 12:eaay1318. doi: 10.1126/scitranslmed.aay1318
Giacomelli, E., Meraviglia, V., Campostrini, G., Cochrane, A., Cao, X., van Helden, R. W. J., et al. (2020). Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell 26, 862.e11–879.e11.
Gu, Z., Fu, J., Lin, H., and He, Y. (2020). Development of 3D bioprinting: from printing methods to biomedical applications. Asian J. Pharm. Sci. 15, 529–557. doi: 10.1016/j.ajps.2019.11.003
Hashimoto, H., Olson, E. N., and Bassel-Duby, R. (2018). Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 15, 585–600. doi: 10.1038/s41569-018-0036-6
Hodgkinson, C. P., Kang, M. H., Dal-Pra, S., Mirotsou, M., and Dzau, V. J. (2015). MicroRNAs and cardiac regeneration. Circ. Res. 116, 1700–1711.
Hu, S., Li, Z., Shen, D., Zhu, D., Huang, K., Su, T., et al. (2021). Exosome-eluting stents for vascular healing after ischaemic injury. Nat. Biomed. Eng. doi: 10.1038/s41551-021-00705-0
Huang, K., Hu, S., and Cheng, K. (2019a). A new era of cardiac cell therapy: opportunities and challenges. Adv. Healthcare Mater. 8, 1–18. doi: 10.1111/nph.13457
Huang, K., Li, Z., Su, T., Shen, D., Hu, S., and Cheng, K. (2019b). Bispecific antibody therapy for effective cardiac repair through redirection of endogenous stem cells. Adv. Ther. 2:1900009. doi: 10.1002/adtp.201900009
Huang, K., Ozpinar, E. W., Su, T., Tang, J., Shen, D., Qiao, L., et al. (2020). An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 12:eaat9683. doi: 10.1126/scitranslmed.aat9683
Huang, P., Wang, L., Li, Q., Tian, X., Xu, J., Xu, J., et al. (2019). Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc. Res. 116, 353–367.
Ieda, M., Fu, J. D., Delgado-Olguin, P., Vedantham, V., Hayashi, Y., Bruneau, B. G., et al. (2010). Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386. doi: 10.1016/j.cell.2010.07.002
Inagaki, K., Fuess, S., Storm, T. A., Gibson, G. A., Mctiernan, C. F., Kay, M. A., et al. (2006). Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 14, 45–53. doi: 10.1016/j.ymthe.2006.03.014
Iseoka, H., Miyagawa, S., Fukushima, S., Saito, A., Masuda, S., Yajima, S., et al. (2018). Pivotal role of non-cardiomyocytes in electromechanical and therapeutic potential of induced pluripotent stem cell-derived engineered cardiac tissue. Tissue Eng. Part A 24, 287–300. doi: 10.1089/ten.tea.2016.0535
Jackman, C. P., Ganapathi, A. M., Asfour, H., Qian, Y., Allen, B. W., Li, Y., et al. (2018). Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 159, 48–58. doi: 10.1016/j.biomaterials.2018.01.002
Jafari, D., Shajari, S., Jafari, R., Mardi, N., Gomari, H., Ganji, F., et al. (2020). Designer exosomes: a new platform for biotechnology therapeutics. BioDrugs 34, 567–586. doi: 10.1007/s40259-020-00434-x
Jana, S., and Lerman, A. (2015). Bioprinting a cardiac valve. Biotechnol. Adv. 33, 1503–1521. doi: 10.1016/j.biotechadv.2015.07.006
Jayawardena, T. M., Egemnazarov, B., Finch, E. A., Zhang, L., Payne, J. A., Pandya, K., et al. (2012). MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 110, 1465–1473. doi: 10.1161/circresaha.112.269035
Jessup, M., Greenberg, B., Mancini, D., Cappola, T., Pauly, D. F., Jaski, B., et al. (2011). Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID). Circulation 124, 304–313. doi: 10.1161/circulationaha.111.022889
Kanki, S., Jaalouk, D. E., Lee, S., Yu, A. Y. C., Gannon, J., and Lee, R. T. (2011). Identification of targeting peptides for ischemic myocardium by in vivo phage display. J. Mol. Cell Cardiol. 50, 841–848. doi: 10.1016/j.yjmcc.2011.02.003
Kim, S. H., Jeong, J. H., Ou, M., Yockman, J. W., Kim, S. W., and Bull, D. A. (2008). Cardiomyocyte-targeted siRNA delivery by prostaglandin E(2)-Fas siRNA polyplexes formulated with reducible poly(amido amine) for preventing cardiomyocyte apoptosis. Biomaterials 29, 4439–4446. doi: 10.1016/j.biomaterials.2008.07.047
Kohama, Y., Higo, S., Masumura, Y., Shiba, M., Kondo, T., Ishizu, T., et al. (2020). Adeno-associated virus-mediated gene delivery promotes S-phase entry-independent precise targeted integration in cardiomyocytes. Sci. Rep. 10, 1–13.
Lee, C. S., Bishop, E. S., Zhang, R., Yu, X., Farina, E. M., Yan, S., et al. (2017). Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 4, 43–63. doi: 10.1016/j.gendis.2017.04.001
Lee, R. T., and Walsh, K. (2016). The future of cardiovascular regenerative medicine. Circulation 133, 2618–2625. doi: 10.1161/circulationaha.115.019214
Lee, S., Kim, J. E., Johnson, B., Al, Andukuri, A., and Yoon, Y.-S. (2017). Direct reprogramming into endothelial cells: a new source for vascular regeneration. Regen. Med. 12, 317–320. doi: 10.2217/rme-2017-0022
Li, P., Kaslan, M., Lee, S. H., Yao, J., and Gao, Z. (2017). Progress in exosome isolation techniques. Theranostics 7, 789–804. doi: 10.7150/thno.18133
Li, X.-H., Li, Q., Jiang, L., Deng, C., Liu, Z., Fu, Y., et al. (2015). Generation of functional human cardiac progenitor cells by high-efficiency protein transduction. Stem Cells Transl. Med. 4, 1415–1424. doi: 10.5966/sctm.2015-0136
Li, Y., Chen, X., Jin, R., Chen, L., Dang, M., Cao, H., et al. (2021). Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 7:eabd6740. doi: 10.1126/sciadv.abd6740
Li, Z., Hu, S., and Cheng, K. (2018). Platelets and their biomimetics for regenerative medicine and cancer therapies. J. Mater. Chem. B. 6, 7354–7365. doi: 10.1039/c8tb02301h
Li, Z., Hu, S., Huang, K., Su, T., Cores, J., and Cheng, K. (2020). Targeted anti-IL-1β platelet microparticles for cardiac detoxing and repair. Sci. Adv. 6:eaay0589. doi: 10.1126/sciadv.aay0589
Li, Z., Zhu, D., Hui, Q., Bi, J., Yu, B., Huang, Z., et al. (2021). Injection of ROS-responsive hydrogel loaded with basic fibroblast growth factor into the pericardial cavity for heart repair. Adv. Funct. Mater. 2021:2004377. doi: 10.1002/adfm.202004377
Lin, A., Sabnis, A., Kona, S., Nattama, S., Patel, H., Dong, J.-F., et al. (2010). Shear-regulated uptake of nanoparticles by endothelial cells and development of endothelial-targeting nanoparticles. J. Biomed. Mater. Res. A 93, 833–842.
Liu, M., Lutz, H., Zhu, D., Huang, K., Li, Z., Dinh, P.-U. C., et al. (2021). Bispecific antibody inhalation therapy for redirecting stem cells from the lungs to repair heart injury. Adv. Sci. 8:2002127. doi: 10.1002/advs.202002127
Liu, N., Ye, X., Yao, B., Zhao, M., Wu, P., Liu, G., et al. (2021). Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 6, 1388–1401. doi: 10.1016/j.bioactmat.2020.10.021
Liu, Q., and Muruve, D. (2003). Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 10, 935–940. doi: 10.1038/sj.gt.3302036
Liu, Z., Wang, L., Welch, J. D., Ma, H., Zhou, Y., Vaseghi, H. R., et al. (2017). Single-cell transcriptomics reconstructs fate conversion from fibroblast to cardiomyocyte. Nature 551, 100–104. doi: 10.1038/nature24454
Luo, L., Tang, J., Nishi, K., Yan, C., Dinh, P. U., Cores, J., et al. (2017). Fabrication of synthetic mesenchymal stem cells for the treatment of acute myocardial infarction in mice. Circ. Res. 120, 1768–1775. doi: 10.1161/circresaha.116.310374
Machiraju, P., and Greenway, S. C. (2019). Current methods for the maturation of induced pluripotent stem cell-derived cardiomyocytes. World J. Stem Cells 11, 33–43. doi: 10.4252/wjsc.v11.i1.33
Maghin, E., Garbati, P., Quarto, R., Piccoli, M., and Bollini, S. (2020). Young at heart: combining strategies to rejuvenate endogenous mechanisms of cardiac repair. Front. Bioeng. Biotechnol. 8:447. doi: 10.3389/fbioe.2020.00447
Maiullari, F., Costantini, M., Milan, M., Pace, V., Chirivì, M., Maiullari, S., et al. (2018). A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 8, 1–15.
Malliaras, K., and Marbán, E. (2011). Cardiac cell therapy: where we’ve been, where we are, and where we should be headed. Br. Med. Bull. 98, 161–185. doi: 10.1093/bmb/ldr018
Martins, A. M., Vunjak-Novakovic, G., and Reis, R. L. (2014). The current status of iPS cells in cardiac research and their potential for tissue engineering and regenerative medicine. Stem Cell Rev. Rep. 10, 177–190. doi: 10.1007/s12015-013-9487-7
Mayourian, J., Ceholski, D. K., Gorski, P. A., Mathiyalagan, P., Murphy, J. F., Salazar, S. I., et al. (2018). Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ. Res. 122, 933–944. doi: 10.1161/circresaha.118.312420
McMurray, J. J., Packer, M., Desai, A. S., Gong, J., Lefkowitz, M. P., Rizkala, A. R., et al. (2014). Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004.
Mei, X., and Cheng, K. (2020). Recent development in therapeutic cardiac patches. Front. Cardiovasc. Med. 7:610364. doi: 10.3389/fcvm.2020.610364
Mei, Y., Saha, K., Bogatyrev, S. R., Yang, J., Hook, A. L., Kalcioglu, Z. I., et al. (2010). Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 9, 768–778. doi: 10.1038/nmat2812
Mihalko, E., Huang, K., Sproul, E., Cheng, K., and Brown, A. C. (2018). Targeted treatment of ischemic and fibrotic complications of myocardial infarction using a dual-delivery microgel therapeutic. ACS Nano 12, 7826–7837. doi: 10.1021/acsnano.8b01977
Nam, Y.-J., Song, K., and Olson, E. N. (2013). Heart repair by cardiac reprogramming. Nat. Med. 19, 413–415. doi: 10.1038/nm.3147
Nelson, V. L., and Brunt, K. R. (2021). Cutting the molecular brakes to achieve cardiac regeneration. Cell Death Differ. 28, 1126–1129. doi: 10.1038/s41418-020-00681-z
Packer, M., Coats, A. J., Fowler, M. B., Katus, H. A., Krum, H., Mohacsi, P., et al. (2001). Carvedilol prospective randomized cumulative survival study group. Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 344, 1651–1658.
Parashar, A., Sarkar, S., Ganguly, A., Sharma, S. K., and Suresh, M. R. (2011). Bispecific antibodies for diagnostic applications. Bispec. Antibodies 349–367. doi: 10.1007/978-3-642-20910-9_19
Pasumarthi, K. B. S., and Field, L. J. (2002). Cardiomyocyte cell cycle regulation. Circ. Res. 90, 1044–1054. doi: 10.1161/01.res.0000020201.44772.67
Patel, V., Mathison, M., Singh, V. P., Yang, J., and Rosengart, T. K. (2016). Direct cardiac cellular reprogramming for cardiac regeneration. Curr. Treat. Options Cardiovasc. Med. 18:58.
Pianezzi, E., Altomare, C., Bolis, S., Balbi, C., Torre, T., Rinaldi, A., et al. (2020). Role of somatic cell sources in the maturation degree of human induced pluripotent stem cell-derived cardiomyocytes. Biochim. Biophys. Acta Mol. Cell Res. 1867:118538. doi: 10.1016/j.bbamcr.2019.118538
Pomeroy, J. E., Helfer, A., and Bursac, N. (2020). Biomaterializing the promise of cardiac tissue engineering. Biotechnol. Adv. 42:107353. doi: 10.1016/j.biotechadv.2019.02.009
Qian, L., Huang, Y., Spencer, C. I., Foley, A., Vedantham, V., Liu, L., et al. (2012). In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598. doi: 10.1038/nature11044
Qiao, L., Hu, S., Liu, S., Zhang, H., Ma, H., Huang, K., et al. (2019). microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Invest. 129, 2237–2250. doi: 10.1172/jci123135
Querdel, E., Reinsch, M., Castro, L., Köse, D., Bähr, A., Reich, S., et al. (2021). Human engineered heart tissue patches remuscularize the injured heart in a dose-dependent manner. Circulation. 120:047904.
Rao, M. S., and Malik, N. (2012). Assessing iPSC reprogramming methods for their suitability in translational medicine. J. Cell. Biochem. 113, 3061–3068. doi: 10.1002/jcb.24183
Raziyeva, K., Smagulova, A., Kim, Y., Smagul, S., Nurkesh, A., and Saparov, A. (2020). Preconditioned and genetically modified stem cells for myocardial infarction treatment. Int. J. Mol. Sci. 21:7301. doi: 10.3390/ijms21197301
Rincon, M. Y., VandenDriessche, T., and Chuah, M. K. (2015). Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation. Cardiovasc. Res. 108, 4–20. doi: 10.1093/cvr/cvv205
Rose, E. A., Gelijns, A. C., Moskowitz, A. J., Heitjan, D. F., Stevenson, L. W., Dembitsky, W., et al. (2001). Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 345, 1435–1443.
Sandmaier, S. E., and Telugu, B. P. (2015). MicroRNA-mediated reprogramming of somatic cells into induced pluripotent stem cells. Methods Mol. Biol. 1330, 29–36. doi: 10.1007/978-1-4939-2848-4_3
Saparov, A., Ogay, V., Nurgozhin, T., Chen, W. C. W., Mansurov, N., Issabekova, A., et al. (2017). Role of the immune system in cardiac tissue damage and repair following myocardial infarction. Inflamm. Res. 66, 739–751. doi: 10.1007/s00011-017-1060-4
Shadrin, I. Y., Allen, B. W., Qian, Y., Jackman, C. P., Carlson, A. L., Juhas, M. E., et al. (2017). Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 8:1825.
Shapiro, S. D., Ranjan, A. K., Kawase, Y., Cheng, R. K., Kara, R. J., Bhattacharya, R., et al. (2014). Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci. Transl. Med. 6:224ra27. doi: 10.1126/scitranslmed.3007668
Shelke, A. R., Roscoe, J. A., Morrow, G. R., Colman, L. K., Banerjee, T. K., and Kirshner, J. J. (2008). Effect of a nausea expectancy manipulation on chemotherapy-induced nausea: a university of Rochester cancer center community clinical oncology program study. J. Pain Symptom Manage. 35, 381–387. doi: 10.1016/j.jpainsymman.2007.05.008
Shen, D., Tang, J., Hensley, M. T., Li, T., Caranasos, T. G., Zhang, T., et al. (2016). Effects of matrix metalloproteinases on the performance of platelet fibrin gel spiked with cardiac stem cells in heart repair. Stem Cells Transl. Med. 5, 793–803. doi: 10.5966/sctm.2015-0194
Shengjie, L., Xiong, Z., Wang, X., Yan, Y., Liu, H., and Zhang, R. (2009). Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J. Bioact. Compat. Polym. 24, 249–265. doi: 10.1177/0883911509104094
Sid-Otmane, C., Perrault, L. P., and Ly, H. Q. (2020). Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J. Transl. Med. 18:336.
Song, K., Nam, Y. J., Luo, X., Qi, X., Tan, W., Huang, G. N., et al. (2012). Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604. doi: 10.1038/nature11139
Squillaro, T., Peluso, G., and Galderisi, U. (2016). Clinical trials with mesenchymal stem cells: an update. Cell Transpl. 25, 829–848. doi: 10.3727/096368915x689622
Sridharan, R., and Plath, K. (2011). Small RNAs loom large during reprogramming. Cell Stem Cell 8, 599–601. doi: 10.1016/j.stem.2011.05.009
Su, T., Huang, K., Daniele, M. A., Hensley, M. T., Young, A. T., Tang, J., et al. (2018a). Cardiac stem cell patch integrated with microengineered blood vessels promotes cardiomyocyte proliferation and neovascularization after acute myocardial infarction. ACS Appl. Mater. Interfaces 10, 33088–33096. doi: 10.1021/acsami.8b13571
Su, T., Huang, K., Ma, H., Liang, H., Dinh, P.-U., Chen, J., et al. (2018b). Platelet-inspired nanocells for targeted heart repair after ischemia/reperfusion injury. Adv. Funct. Mater. 0, 1803567. doi: 10.1002/adfm.201803567
Su, T., Huang, K., Mathews, K. G., Scharf, V. F., Hu, S., Li, Z., et al. (2020). Cardiac stromal cell patch integrated with engineered microvessels improves recovery from myocardial infarction in rats and pigs. ACS Biomater. Sci. Eng. 6, 6309–6320. doi: 10.1021/acsbiomaterials.0c00942
Sun, Z., Shi, K., Yang, S., Liu, J., Zhou, Q., Wang, G., et al. (2018). Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer 17, 1–19. doi: 10.1007/978-3-642-27841-9_7227-1
Tai, Y.-L., Chen, K.-C., Hsieh, J.-T., and Shen, T.-L. (2018). Exosomes in cancer development and clinical applications. Cancer Sci. 109, 2364–2374. doi: 10.1111/cas.13697
Tallquist, M. D., and Molkentin, J. D. (2017). Redefining the identity of cardiac fibroblasts. Nat. Rev. Cardiol. 14, 484–491. doi: 10.1038/nrcardio.2017.57
Tang, J., Shen, D., Caranasos, T. G., Wang, Z., Vandergriff, A. C., Allen, T. A., et al. (2017). Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat. Commun. 8, 1–9. doi: 10.1155/2015/765846
Tang, J., Su, T., Huang, K., Dinh, P. U., Wang, Z., Vandergriff, A., et al. (2018a). Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat. Biomed. Eng. 2, 17–26. doi: 10.1038/s41551-017-0182-x
Tang, J., Wang, J., Huang, K., Ye, Y., Su, T., Qiao, L., et al. (2018b). Cardiac cell–integrated microneedle patch for treating myocardial infarction. Sci. Adv. 4:eaat9365. doi: 10.1126/sciadv.aat9365
Tang, J., Cores, J., Huang, K., Cui, X.-L., Luo, L., Zhang, J.-Y., et al. (2018c). Concise review: is cardiac cell therapy dead? embarrassing trial outcomes and new directions for the future. Stem Cells Transl. Med. 0, 354–359. doi: 10.1002/sctm.17-0196
Tao, S. C., Guo, S. C., and Zhang, C. Q. (2018). Modularized extracellular vesicles: the dawn of prospective personalized and precision medicine. Adv. Sci. 5:1700449. doi: 10.1002/advs.201700449
Teng, X., Chen, L., Chen, W., Yang, J., Yang, Z., and Shen, Z. (2015). Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol. Biochem. 37, 2415–2424. doi: 10.1159/000438594
Teshigawara, R., Cho, J., Kameda, M., and Tada, T. (2017). Mechanism of human somatic reprogramming to iPS cell. Lab. Investig. 97, 1152–1157. doi: 10.1038/labinvest.2017.56
Tohyama, S., Hattori, F., Sano, M., Hishiki, T., Nagahata, Y., Matsuura, T., et al. (2013). Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127–137. doi: 10.1016/j.stem.2012.09.013
Tse, L. V., Klinc, K. A., Madigan, V. J., Castellanos Rivera, R. M., Wells, L. F., Havlik, L. P., et al. (2017). Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc. Natl. Acad. Sci. U.S.A. 114, E4812–E4821.
Uygun, B., Soto-Gutierrez, A., Yagi, H., et al. (2010). Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 16, 814–820. doi: 10.1038/nm.2170
Vandergriff, A. C., Andrade, J. B., Tang, J., Hensley, M. T., Piedrahita, J. A., Caranasos, T. G., et al. (2015). Intravenous cardiac stem cell-derived exosomes ameliorate cardiac dysfunction in doxorubicin induced dilated cardiomyopathy. Stem Cells Int. 2015:960926.
Verweij, F. J., Revenu, C., Arras, G., Dingli, F., Loew, D., Pegtel, D. M., et al. (2019). Live tracking of inter-organ communication by endogenous exosomes in vivo. Dev. Cell. 48, 573.e4–589.e4.
Vilaysane, A., and Muruve, D. A. (2009). The innate immune response to DNA. Semin. Immunol. 21, 208–214.
Wang, H., Yang, Y., Liu, J., et al. (2021). Direct cell reprogramming: approaches, mechanisms and progress. Nat. Rev. Mol. Cell Biol. [Epub ahead of print]. doi: 10.1038/s41580-021-00335-z
Wang, K., Gan, T. Y., Li, N., Liu, C. Y., Zhou, L. Y., Gao, J. N., et al. (2017). Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 24, 1111–1120. doi: 10.1038/cdd.2017.61
Wang, L., Liu, Z., Yin, C., Asfour, H., Chen, O., Li, Y., et al. (2015). Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ. Res. 116, 237–244. doi: 10.1161/circresaha.116.305547
Wang, L. L., Liu, Y., Chung, J. J., Wang, T., Gaffey, A. C., Lu, M., et al. (2017). Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat. Biomed. Eng. 1, 983–992. doi: 10.1038/s41551-017-0157-y
Wang, Z., Lee, S. J., Cheng, H. J., Yoo, J. J., and Atala, A. (2018). 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 70, 48–56. doi: 10.1016/j.actbio.2018.02.007
Wasala, N. B., Shin, J. H., and Duan, D. (2011). The evolution of heart gene delivery vectors. J. Gene Med. 13, 557–565. doi: 10.1002/jgm.1600
Weinberger, F., and Eschenhagen, T. (2021). Cardiac regeneration: new hope for an old dream. Annu. Rev. Physiol. 83, 1–23.
Wendel, J. S., Ye, L., Zhang, P., Tranquillo, R. T., and Zhang, J. J. (2014). Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng. Part A 20, 1325–1335. doi: 10.1089/ten.tea.2013.0312
Wilhelm, M. J. (2015). Long-term outcome following heart transplantation: current perspective. J. Thorac. Dis. 7, 549–551.
Wolfram, J. A., and Donahue, J. K. (2013). Gene therapy to treat cardiovascular disease. J. Am. Heart Assoc. 2:e000119.
World Health Organization (2021). Global Action Plan for the Prevention and Control of NCDs 2013-2020. Geneva: World Health Organization.
Wu, P., Chen, H., Jin, R., Weng, T., Ho, J. K., You, C., et al. (2018). Non-viral gene delivery systems for tissue repair and regeneration. J. Transl. Med. 16:29.
Xin, M., Olson, E., and Bassel-Duby, R. (2013). Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 14, 529–541. doi: 10.1038/nrm3619
Yang, S. Y., O’Cearbhaill, E. D., Sisk, G. C., Park, K. M., Cho, W. K., Villiger, M., et al. (2013). A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 4, 2–11.
Yoo, S. Y., Jeong, S.-N., Kang, J.-I., and Lee, S.-W. (2018). Chimeric adeno-associated virus-mediated cardiovascular reprogramming for ischemic heart disease. ACS Omega 3, 5918–5925. doi: 10.1021/acsomega.8b00904
Zhang, Y., Desai, A., Yang, S. Y., Bae, K. B., Antczak, M. I., Fink, S. P., et al. (2015). Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 348:aaa2340. doi: 10.1126/science.aaa2340
Zhou, Y., Wang, L., Vaseghi, H. R., Liu, Z., Lu, R., Alimohamadi, S., et al. (2016). Bmi1 is a key epigenetic barrier to direct cardiac reprogramming. Cell Stem Cell 18, 382–395. doi: 10.1016/j.stem.2016.02.003
Zhu, D., and Cheng, K. (2021). Cardiac cell therapy for heart repair: should the cells be left out? Cells 10:641. doi: 10.3390/cells10030641
Zhu, D., Li, Z., Huang, K., Caranasos, T. G., Rossi, J. S., and Cheng, K. (2021). Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 12:1412.
Keywords: bioengineering, cardiac repair, cell reprogramming, exosome, cardiac patch, targeting
Citation: Chingale M, Zhu D, Cheng K and Huang K (2021) Bioengineering Technologies for Cardiac Regenerative Medicine. Front. Bioeng. Biotechnol. 9:681705. doi: 10.3389/fbioe.2021.681705
Received: 17 March 2021; Accepted: 12 April 2021;
Published: 03 June 2021.
Edited by:
Jianyi Zhang, University of Alabama at Birmingham, United StatesReviewed by:
Lei Ye, National Heart Centre Singapore, SingaporeZhen Ma, Syracuse University, United States
Shijun Hu, Soochow University, China
Copyright © 2021 Chingale, Zhu, Cheng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Cheng, a2VfY2hlbmdAbmNzdS5lZHU=; Ke Huang, a2h1YW5nN0BuY3N1LmVkdQ==
 Mira Chingale
Mira Chingale Dashuai Zhu
Dashuai Zhu Ke Cheng
Ke Cheng Ke Huang
Ke Huang