- Department of Chemical Engineering, Hongik University, Seoul, South Korea
Ferredoxins are metalloproteins that deliver electrons to several redox partners, including [FeFe] hydrogenases that are potentially a component of biological H2 production technologies. Reduced ferredoxins can also lose electrons to molecular oxygen, which may lower the availability of electrons for cellular or synthetic reactions. Ferredoxins thus play a key role in diverse kinds of redox biochemistry, especially the enzymatic H2 production catalyzed by [FeFe] hydrogenases. We investigated how the yield of anaerobic and aerobic H2 production vary among the four different types of ferredoxins that are used to deliver electrons extracted from NADPH within the synthetic, fermentative pathway. We also assessed the electron loss due to O2 reduction by reduced ferredoxins within the pathway, for which the difference was as high as five-fold. Our findings provide valuable insights for further improving biological H2 production technologies and can also facilitate elucidation of mechanisms governing interactions between Fe–S cluster(s) and molecular oxygen.
Introduction
Ferredoxins (Fd) are redox proteins that mediate electron metabolism in numerous kinds of cells across diverse organisms. The [Fe–S] cluster(s) in ferredoxins are responsible for electron receipt and transfer to redox partners. The type and number of [Fe–S] clusters in a ferredoxin can vary, which in turn affect the rate at which electrons are delivered, as well as redox potential of the protein (Guan et al., 2018). For example, Synechocystis sp. PCC 6803 ferredoxin (SynFd) has one [2Fe–2S] cluster while Clostridium pasteurianum ferredoxin (CpFd) harbors two [4Fe–4S] clusters. The midpoint redox potentials for the two are −412 and −387 mV, respectively (Bottin and Lagoutte, 1992; Brereton et al., 1999).
One of ferredoxins’ redox partners is a metalloprotein called hydrogenase. Hydrogenases can receive electrons from reduced ferredoxins (Fdred) and combine them with protons to produce H2. The enzymatic activity of hydrogenases, especially the [FeFe] subtype proficient in H2 production such as the one from the C. pasteurianum (CpI), can be exploited for biological H2 production (Lu and Koo, 2019); wide deployment of the technology can contribute to reduction of CO2 emission while supplying H2. Toward this aim, researchers have successfully developed both fermentative and photosynthetic pathways for reducing ferredoxins, which can subsequently be used for the enzymatic H2 production (Smith et al., 2011; Yacoby et al., 2011). To date, however, people have tested H2 production from these pathways only in an anaerobic reactor due to the high O2 sensitivity of [FeFe] hydrogenases.
O2 is a byproduct of photosynthesis and also an effective reagent for regenerating adenosine triphosphate (ATP) during fermentation. As such, it is an unavoidable constituent of the aforementioned biological H2 production processes; its influence on electron flow and H2 production need to be understood. Previous work showed that Fdred is capable of reducing O2 into superoxide and peroxide (Allen, 1975), which is known as the Mehler reaction within the photosynthetic pathways. Hosein and Palmer also reported the Fd oxidation by O2 and its autocatalytic nature (Hosein and Palmer, 1983). Since Fdred is the source of electrons for the enzymatic H2 production, presence of O2 results in a lower H2 yield via decreasing the amount of electrons delivered to the hydrogenases (Benemann et al., 1973; Koo and Swartz, 2018). O2 also drives inactivation of the hydrogenases, which is irreversible and fast (within minutes under the atmospheric [O2]) for most [FeFe] kinds (Lu and Koo, 2019). By fusing ferredoxin to an [FeFe] hydrogenase, Eilenberg et al. (2016) successfully increased the photosynthetic H2 production in the presence of O2, proposing that Fdred delayed inactivation of the hydrogenase at the expense of electrons. The authors also confirmed the increase in aerobic H2 production by fusing different types of ferredoxin and hydrogenase (Koo, 2020).
We developed physiological assays and analytical methods for characterizing O2 sensitivity of [FeFe] hydrogenases during H2 production (Koo et al., 2016). Using this method, the authors studied the enzymatic H2 production in the presence of O2 at a greater detail to better understand its implications for biological H2 production and photosynthesis. First, we used isotopically labeled O2, (18O2) to confirm O2 reduction by Fdred. We then analyzed how the rates of O2 reduction vary with varying concentrations of reactants, as well as with different types of ferredoxins harboring different kind and number of Fe–S clusters. Lastly, we studied how electron loss from Fdred to O2 varies among different combinations of ferredoxin NADP+ reductases (FNR) and ferredoxins with the goal of identifying the most effective combination in minimizing the electron leakage during the enzymatic H2 production.
Materials and Methods
Protein Expression and Purification
SynFd, CpFd, Zea mays Fd (ZmFd), Anabaena variablis Fd (AnFd), Synechocystis sp. PCC 6803 FNR (SynFNR), and Rice root FNR (RrFNR) were expressed in vivo in Escherichia coli and purified as described previously (Lu et al., 2015). CpI was also expressed heterologously in E. coli and purified as reported in the previous work (Koo and Swartz, 2018).
Reagent Preparation
Nicotinamide adenine dinucleotide phosphate (NADPH), dithionite, glucose-6-phosphate (G6P), G6P dehydrogenase (G6PD), superoxide dismutase (SOD) and catalase were purchased from Sigma-Aldrich.
O2 Reduction and Aerobic H2 Production Measurements
The O2 reduction experiments were conducted in 8.4 mL crimp vials where 840 μL reaction mixtures contained the following (unless stated otherwise): 50 mM Tris buffer pH 7.0, 10 mM G6P, 4 units of G6PD, 5.0 mM NADPH, 5.0 or 50 μM FNR, and 5.0 μM of Fd. The reaction mixtures were prepared inside a N2-only glovebox (CHOA Engineering). Before sealing the vials with rubber septa, magnetic stir bars were added for mixing. After removal from the glovebox, the sealed vials were placed on a stir plate to initiate mixing at 300 rpm. O2 was introduced to the headspace at t = 0 min (or 10 min when examining various pairs of FNR and Fd) by using a syringe with a 20-gage needle (Daehan Sciences); air or a gas-tight handheld tank filled with O2 was used as a source. O2 and H2 concentrations were measured by sampling 200 μL of the headspace with a valved 23-gage needle (Daehan Sciences), and using gas chromatography (GC 6500, YL Instrument). The H2 production experiments were done in the same manner with the addition of 10 nM CpI to the aforementioned reaction mixtures inside the anaerobic glovebox.
18O2 Reduction Experiments
The amount of H218O formed by the reaction mixtures (50 mM Tris buffer pH 7.0, 10 mM G6P, 4 units of G6PD, 5.0 mM NADPH, 5.0 or 50 μM FNR, 5.0 μM of Fd, 4.2 U SOD and 4.2 U catalase) were measured as follows. The 8.4 mL crimp vials (Thermofisher Scientific) were first opened using a decapper, and the reaction buffer were immediately put in the 80 °C water bath (Daehan Sciences) for the following hour to terminate any ongoing enzymatic reactions. Next, the reaction buffer was distilled at 120 °C and only the water vapor was collected by condensation. The collected samples were mixed with distilled H2O at two different ratios (2- and 10-fold dilution) and submitted together with a sample containing only distilled H2O to the mass spectrometry (National Center for Inter-University Research Facilities, Seoul) for analysis of H218O content.
Results and Discussion
Confirmation of 18O2 Reduction by Fdred
We have previously reported O2 consumption by SynFdred during fermentative H2 production where electrons are extracted from NADPH and delivered to hydrogenases via RrFNR and SynFd (Koo et al., 2016). In this study, we replaced the source of O2 from air to 10 vol% 18O2. This allowed us to directly measure accumulation of H218O in the buffer using mass spectrometry. Since Fdred was reported to be capable of only uni- and divalent reduction of O2 (Allen, 1975), we modified the biochemical reaction as described in Figure 1. Instead of adding the hydrogenase enzyme for the NADPH-driven H2 production (blue arrows from Fdred), SOD and catalase were added in the absence of the hydrogenase (red arrows from Fdred) to increase the likelihood that any reactive oxygen species (ROS) generated by Fdred would be converted into H218O for isotopic detection.
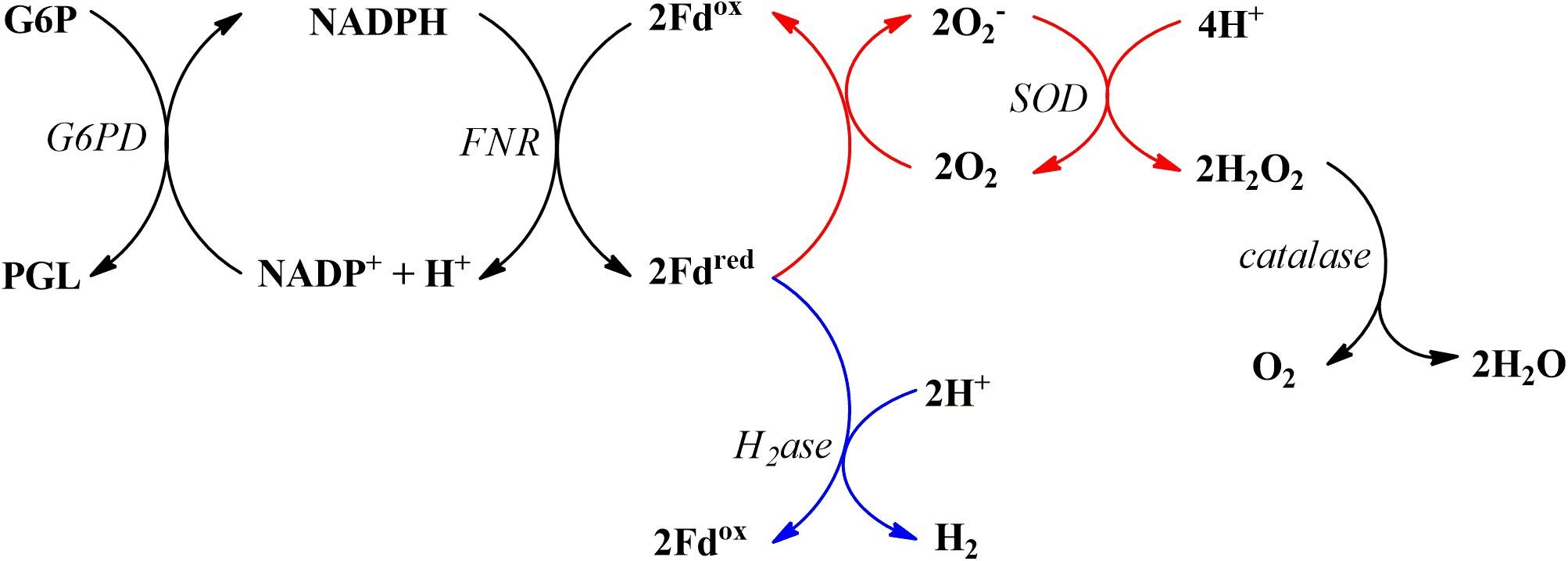
Figure 1. The biochemical reaction series for NADPH-driven reduction of Fd, putative subsequent reduction of O2 into O2 radical, and the final conversion into H2O and O2 via SOD and catalase (red arrows); the electron flow for subsequent H2 production are shown in blue arrows. Abbreviations are as follows: glucose-6-phosphate (G6P), glucose-6-phosphate dehydrogenase (G6PD), 6-phosphoglucono-D-lactone (PGL), hydrogenase (H2ase).
Four reaction mixtures were prepared, incubated with 10 vol% 18O2 for an hour, and analyzed to determine (1) consumption of the headspace 18O2 and (2) formation of H218O. The concentrations of reagents were as follows: 10 mM G6P, 4 U G6PD, 5.0 mM NADPH, 5.0 μM RrFNR, 5.0 μM SynFd, 4 U SOD, and 4 U catalase in 50 mM Tris buffer, pH 7.0. Incremental addition of reagents in reaction sequence (Figure 1) starting at SynFd clearly confirmed our previous finding (Koo et al., 2016): SynFdred is responsible for O2 consumption (Figure 2). The results also suggested that SynFdred is not capable of reducing O2 fully into H2O. This was evident from the observation that a significant amount of H218O was formed only in the presence of both SOD and catalase. The fact that Synechocystis sp. cells have native SOD and catalase is consistent with our interpretation in that these enzymes can rapidly remove harmful O2 radicals generated by SynFdred.
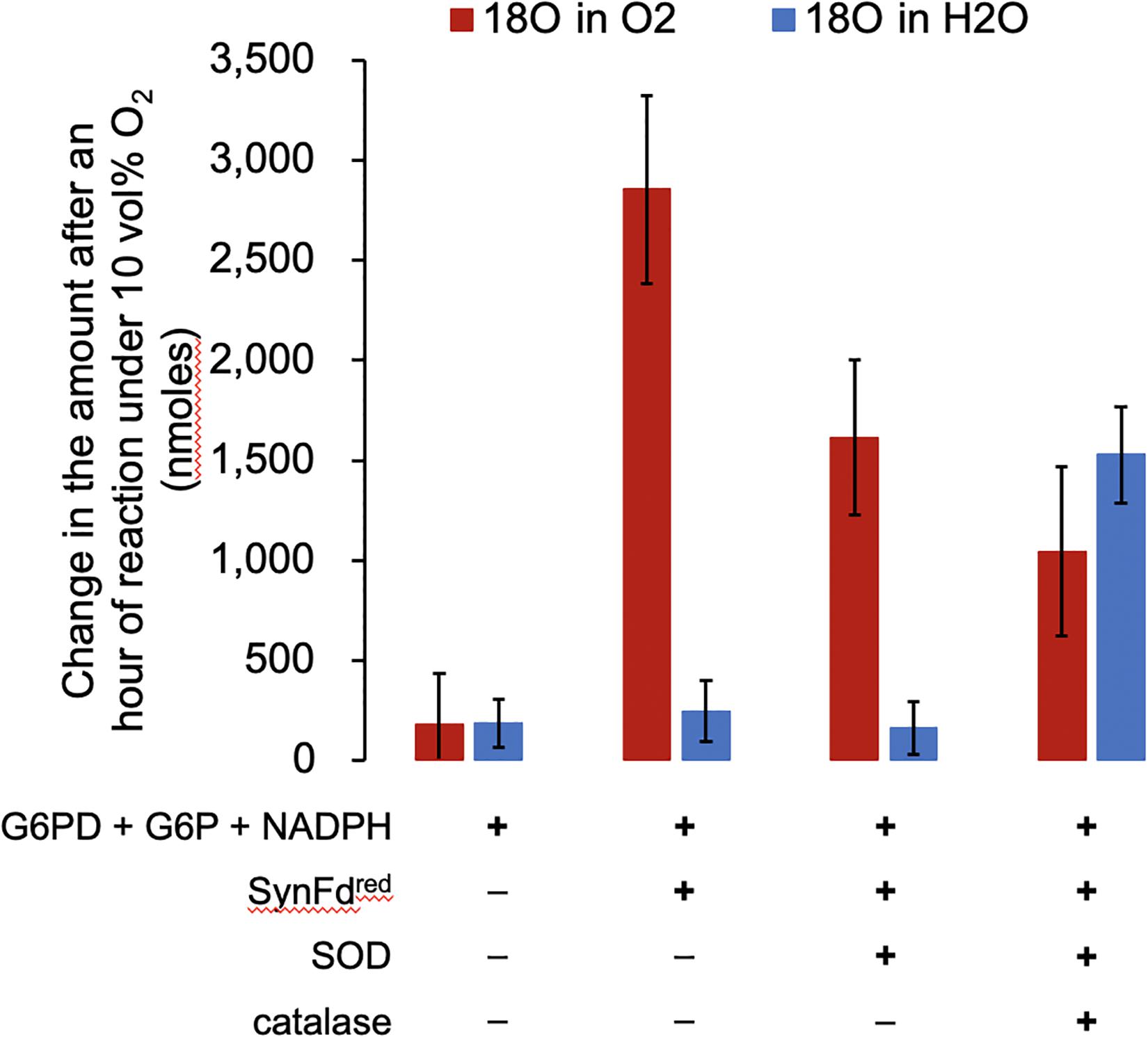
Figure 2. The amount of 18O2 consumed and H218O generated by SynFdred over an hour with 10 vol% O2 in the headspace. The error bars represent standard deviation (n = 2).
Assuming that SOD do not impact the O2 reduction activity of SynFdred, we expected to see the decrease in headspace O2 reduce by half (Figure 1, following the red arrows only from Fdred) in the mixture containing only up to SOD. This is indeed what we observed: The loss of headspace O2 after an hour decreased from roughly 2,900 to 1,600 nmoles upon addition of only SOD, approximately 50% reduction considering experimental error. We also expected to see a further reduction in the amount by which headspace O2 decreases upon addition of catalase to the mixture containing SOD (Figure 1) since O2 is regenerated in the presence of both. Indeed, the loss of headspace O2 after an hour further decreased to roughly 1,000 nmoles. The water molecules containing 18O were only formed when both SOD and catalase were added to the reaction mixture. Furthermore, the amount of H218O produced, 1,500 nmoles, is similar to the stoichiometrically expected value of 1,300 nmoles: Each peroxide molecule generated by SOD will be converted into a water molecule by catalase. Based on the headspace O2 loss, the average turnover number (TON) of O2 reduction by SynFd was 11 per minute per reduced ferredoxin. It is important to note that this TON is not physiologically relevant. Fd mostly delivers electrons for NADPH regeneration inside cells while, in our experiments, the SynFd molecules were reduced using the electrons extracted from NADPH to consume O2.
Kinetic Study on O2 Reduction by Fdred
We next examined how the rate of electron loss to O2 varies with respect to O2 partial pressure in the headspace. As discussed in the previous section, SOD and catalase affect the apparent O2 consumption by regenerating about half of the putative O2 radicals back to O2. We thus conducted the following experiments in the absence of SOD and catalase. The concentrations of reagents inside the sealed glass vials were as usual except for RrFNR, which was increased by 10-fold to 50 μM RrFNR. The higher [RrFNR] was chosen in order to enhance electron flux to Fd (KM of 18 or 39 μM for SynFd and CpFd, respectively) and thereby increase changes in the O2 peaks for more accurate analysis.
The results summarized in Figure 3 and Supplementary Figure 1 indicated that the O2 reduction rate, or equivalently the electron loss rate is directly proportional to the headspace [O2]. A linear relationship between the two was observed for both types of Fd from 1 to 21 vol% O2 in the headspace of the reactor vial. Within this range, the observed O2 consumption rate varied from 21 to 255 or 46 to 479 nmoles/min for SynFd and CpFd, respectively. The corresponding specific rate of electron loss to O2 ranged between 5 and 61 per minute for SynFd (assuming only univalent reduction of O2) and approximately 1.9-fold higher for CpFd. As expected, increasing the [RrFNR] from 5 to 50 μM increased the specific rate of electron loss, but only by about three-fold.
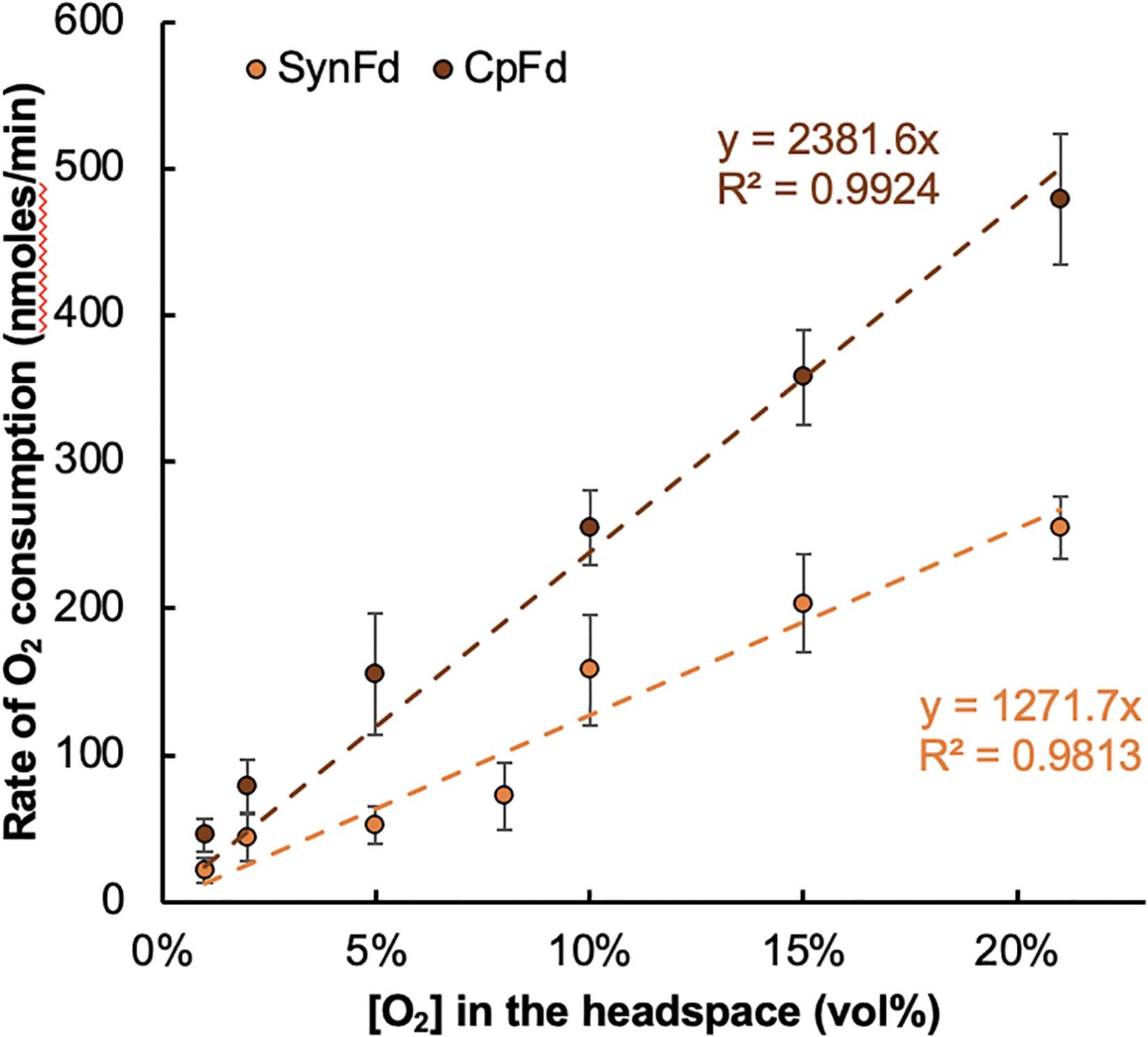
Figure 3. Changes in the rate of O2 reduction by SynFdred and CpFdred versus the headspace [O2] inside the reactor vials. Each rate was measured by calculating the linear slope from the time-course of [O2] changes in the headspace over the course of 30 min (time interval of 10 min).
Based on these observations, we analyzed the kinetic parameters for the reductive O2 consumption. The following rate law (Eq. 1a) was proposed where the consumption is modeled as a non-enzymatic biochemical reaction between Fdred and O2:
where [O2] refers to the concentration of O2 in the aqueous buffer solution. For the cases where [Fdred] is fixed, the rate law further simplifies into the equation only dependent on [O2] (Eq. 1b). This will be true if the electron flux from FNR to Fd increases as [O2] increases to maintain [Fdred] approximately constant. We observe a linear fit for the plot of dO2/dt versus [O2] (Figure 3), which suggests that the O2 reduction by Fdred follows a pseudo first order rate law under the experimental conditions. The rate constant of this kinetic model (eqn. 2, b = 1), namely is calculated to be 0.0013 and 0.0025 per minute for SynFd and CpFd, respectively.
A multitude of factors may be responsible for the differences in the O2 reduction activity of the two types of Fd. To begin with, SynFd harbors one [2Fe–2S] cluster while there are two [4Fe–4S] clusters in CpFd (Bertini et al., 1995; Van Den Heuvel et al., 2003). The former is known to be capable of transitioning between [2Fe–2S]2+ and [2Fe–2S]+ while the latter can undergo changes among three states—[4Fe–4S]3+, [4Fe–4S]2+, and [4Fe–4S]+ (Yao et al., 2012). The midpoint redox potentials (RE) are similar: −412 and −387 mV for SynFd and CpFd, respectively (Grzyb et al., 2018). The dissociation constants with RrFNR and CpI are also not significantly different. In this regard, the difference in the range and nature of oxidation states appear to be more important with respect to the Fd’s O2 reduction activity. The results in the subsequent section suggests that the local environment surrounding the Fe–S clusters also matter.
Aerobic H2 Production From Various Pairs of FNR and Fd
We have previously reported that the rate of O2 consumption is different when SynFd versus CpFd is used for the NADPH-driven H2 production pathway (Koo et al., 2016). The rate was approximately twice as fast with CpFd, meaning that more electrons are lost to O2 reduction instead of being used for H2 production by the hydrogenase. The choice of CpFd over SynFd would therefore result in a lower NADPH-driven H2 production efficiency. In this manner, the choice of Fd can impact the efficiency and overall yield of aerobic, biological H2 production when Fd molecules deliver electrons to the hydrogenase enzyme.
We examined various combinations of Fd and FNR to study how the electron loss to O2 differs during H2 production (Figure 1, co-existence of both the blue and red arrow pathways) under 5.0 vol% O2. Different FNRs were evaluated as well since the electron flux rate through the FNR could influence the steady state [Fdred] (Bingham et al., 2012; Lu et al., 2015), which in turn would affect the rate of electron loss to O2. The concentrations of the reagents were as follows: 10 mM G6P, 1 U G6PD, 5 mM NADPH, 5 μM FNR, 5 μM Fd, and 10 nM WT CpI in 50 mM Tris–HCl buffer pH 7.0. The reaction lasted for an hour, and we analyzed the data with respect to (1) anaerobic FNR TON during H2 production, (2) relative aerobic H2 yield, and (3) the overall change in the headspace [O2].
The results (Table 1) indicated that electrons in Fdred are lost to O2 at a faster rate with a higher (anaerobic) FNR TON within the NADPH-driven H2 production (r = 0.82). This was expected since a higher FNR TON means a greater electron flux to Fd. In contrast, there was insignificant correlation between the anaerobic FNR TON and the relative aerobic H2 yield of the reaction (r = −0.25). This confirmed our previous finding from the CpI mutagenesis study where we reported insignificant relationship between the two variables (Koo and Swartz, 2018). This observation suggests that it may be possible to improve both the electron flux and aerobic H2 yield by engineering FNR and/or Fd. Table 1 also revealed that AnFd is the most efficient in delivering electrons to the hydrogenase in the presence of O2. In contrast, CpFd lost the most electrons to O2 among the four types of Fd studied in this experiment. The difference in efficiency between the two ferredoxins was striking: The overall amount of O2 consumed by CpFdred over an hour was as much as four-fold of the amount by AnFdred.
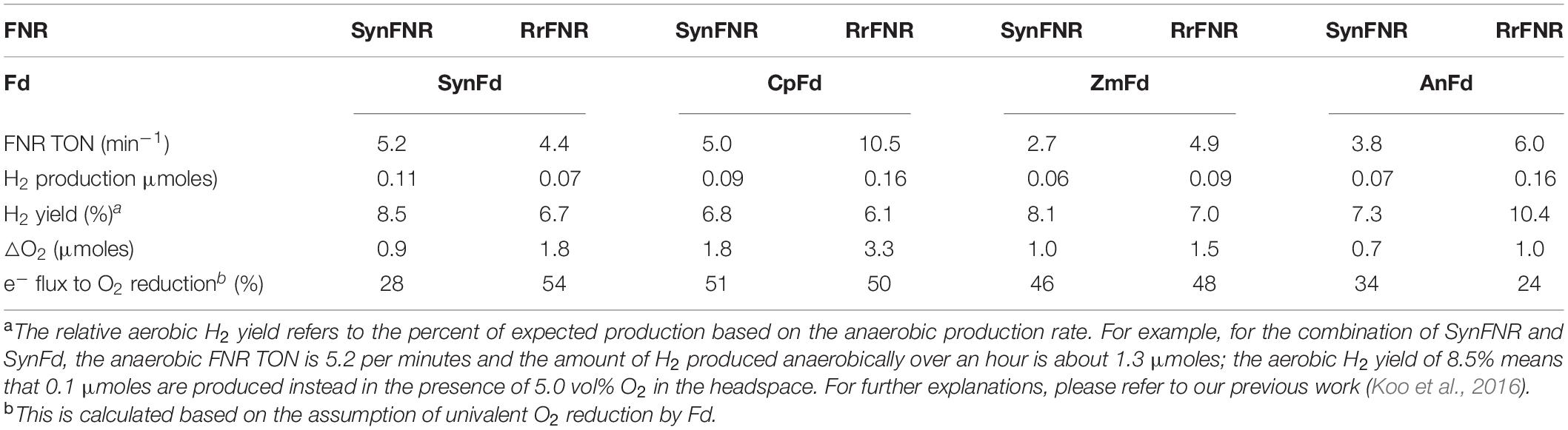
Table 1. The anaerobic FNR TON, the relative aerobic H2 yield, and the amount of reductive O2 consumption over 1 hour for various combinations of FNR-Fd under 5.0% O2.
AnFd harbors one [2Fe–2S] cluster with the midpoint RE potential of −405 mV (Jacobson et al., 1993). This is not much different from ZmFd, for example, which also features one [2Fe–2S] cluster and the midpoint RE potential of −390 mV (Shinohara et al., 2017). The difference in terms of electron leakage to O2 reduction during H2 production, however, is nearly two-fold. The slightly higher midpoint RE cannot explain this difference since SynFd loses more electrons to O2 in spite of the lower midpoint RE potential (−412 mV) than AnFd (Table 1). These results indicate that other factors affect the partitioning of the electron flux between H2 production and O2 reduction. Given the substantial difference for the same type of Fd when a different FNR is used, we suspect that the binding dynamics is one of the factors influencing the partitioning. The reported binding affinities between native Fds and FNRs used in this study range between 15 to 50 μM (Lu et al., 2015; Shiigi, 2015). No obvious relationships between these affinities and the FNR TON or electron flux to O2 reduction were observed, likely owing to the complex nature of multi-component interactions in our assay (Figure 1). A fully exhaustive study on the binding interactions between each pair within the assay may help to elucidate the electron partitioning phenomena.
Conclusion
We studied how the choice of ferredoxin affects the aerobic, biological H2 production catalyzed by the [FeFe] hydrogenase molecules. The results revealed that the electron flux from the reduced ferredoxin can partition between O2 and the hydrogenase and that the ratio of partitioning can vary among the different types of ferredoxin. We compared the results obtained by combining two different types of FNR with four different types of Fd. The differences in the amount of O2 reduced and H2 produced over an hour were as large as 4.7- and 2.3-fold of the lowest set. Our findings suggest that it is possible to reduce the electron loss to O2 by varying Fd and/or FNR within the biological H2 production pathway. The results also hint the possibility of simultaneously improving the TON of H2 production (which is limited by FNR TON in the NADPH-driven reaction). Since the two redox proteins are commonly found across many forms of life, large combinatorial libraries may be screened to find even better pair to enhance biological H2 production beyond the current state of the art.
Data Availability Statement
The original contributions presented in the study are included in the article/entary Material, further inquiries can be directed to the corresponding author.
Author Contributions
JK planned and conducted research. He also wrote the manuscript, analyzed data. YC conducted part of the experiments and also contributed to analysis of some of the data. Both authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Research Foundation of Korea (NRF-2019R1C1C1002642).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors, JK.
Acknowledgments
We deeply thank Prof. James R. Swartz (Stanford University) for the plasmids and cell lines.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.641305/full#supplementary-material
References
Allen, J. F. (1975). A two-step mechanism for the photosynthetic reduction of oxygen by ferredoxin. Biochem. Biophys. Res. Commun. 66, 36–43. doi: 10.1016/s0006-291x(75)80291-9
Benemann, J. R., Berenson, J. A., Kaplan, N. O., and Kamen, M. D. (1973). Hydrogen evolution by a chloroplast-ferredoxin-hydrogenase system. Proc. Natl. Acad. Sci. U.S.A. 70, 2317–2320. doi: 10.1073/pnas.70.8.2317
Bertini, I., Donaire, A., Feinberg, B. A., Luchinat, C., Picciolp, M., and Yuan, H. (1995). Solution structure of the oxidized 2[4Fe-4S] ferredoxin from Clostridium pasteurianum. Eur. J. Biochem. 232, 192–205. doi: 10.1111/j.1432-1033.1995.tb20799.x
Bingham, A. S., Smith, P. R., and Swartz, J. R. (2012). Evolution of an [FeFe] hydrogenase with decreased oxygen sensitivity. Int. J. Hydrogen Energy 37, 2965–2976. doi: 10.1016/j.ijhydene.2011.02.048
Bottin, H., and Lagoutte, B. (1992). Ferrodoxin and flavodoxin from the cyanobacterium Synechocystis sp PCC 6803. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1101, 48–56. doi: 10.1016/0167-4838(92)90465-p
Brereton, P. S., Maher, M. J., Tregloan, P. A., and Wedd, A. G. (1999). Investigation of the role of surface residues in the ferredoxin from Clostridium pasteurianum. Biochim. Biophys. Acta - Protein Struct. Mol. Enzymol. 1429, 307–316. doi: 10.1016/s0167-4838(98)00197-6
Eilenberg, H., Weiner, I., Ben-Zvi, O., Pundak, C., Marmari, A., Liran, O., et al. (2016). The dual effect of a ferredoxin-hydrogenase fusion protein in vivo: successful divergence of the photosynthetic electron flux towards hydrogen production and elevated oxygen tolerance. Biotechnol. Biofuels 9, 1–10.
Grzyb, J., Gieczewska, K., Łabuz, J., and Sztatelman, O. (2018). Detailed characterization of Synechocystis PCC 6803 ferredoxin:NADP+ oxidoreductase interaction with model membranes. Biochim. Biophys. Acta Biomembr. 1860, 281–291. doi: 10.1016/j.bbamem.2017.10.012
Guan, X., Chen, S., Voon, C. P., Wong, K. B., Tikkanen, M., and Lim, B. L. (2018). FdC1 and leaf-type ferredoxins channel electrons from photosystem i to different downstream electron acceptors. Front. Plant Sci. 9:410. doi: 10.3389/fpls.2018.00410
Hosein, B., and Palmer, G. (1983). The kinetics and mechanism of oxidation of reduced spinach ferredoxin by molecular oxygen and its reduced products. Biochim. Biophys. Acta 723, 383–390. doi: 10.1016/0005-2728(83)90045-2
Jacobson, B. L., Rayment, I., Holden, H. M., Chae, Y. K., and Markley, J. L. (1993). Molecular structure of the oxidized, recombinant, heterocyst [2Fe-2S] ferredoxin from anabaena 7120 determined to 1.7-Å resolution. Biochemistry 32, 6788–6793. doi: 10.1021/bi00077a033
Koo, J. (2020). Enhanced aerobic H2 production by engineering an [FeFe] hydrogenase from Clostridium pasteurianum. Int. J. Hydrogen Energy 45, 10673–10679. doi: 10.1016/j.ijhydene.2020.01.239
Koo, J., Shiigi, S., Rohovie, M., Mehta, K., and Swartz, J. R. (2016). Characterization of [FeFe] hydrogenase O2 sensitivity using a new, physiological approach. J. Biol. Chem. 291, 21563–21570. doi: 10.1074/jbc.m116.737122
Koo, J., and Swartz, J. R. (2018). System analysis and improved [FeFe] hydrogenase O2 tolerance suggest feasibility for photosynthetic H2 production. Metab. Eng. 49, 21–27. doi: 10.1016/j.ymben.2018.04.024
Lu, F., Smith, P. R., Mehta, K., and Swartz, J. R. (2015). Development of a synthetic pathway to convert glucose to hydrogen using cell free extracts. Int. J. Hydrogen Energy 40, 9113–9124. doi: 10.1016/j.ijhydene.2015.05.121
Lu, Y., and Koo, J. (2019). O2 sensitivity and H2 production activity of hydrogenases — a review. Biotechnol. Bioeng. 116, 3124–3135. doi: 10.1002/bit.27136
Shiigi, S. (2015). Engineering In Vitro Photobiological H2 Production. Ph.D. thesis, Stanford University, Stanford, CA.
Shinohara, F., Kurisu, G., Hanke, G., Bowsher, C., Hase, T., and Kimata-Ariga, Y. (2017). Structural basis for the isotype-specific interactions of ferredoxin and ferredoxin: NADP+ oxidoreductase: an evolutionary switch between photosynthetic and heterotrophic assimilation. Photosynth. Res. 134, 281–289. doi: 10.1007/s11120-016-0331-1
Smith, P. R., Bingham, A. S., and Swartz, J. R. (2011). Generation of hydrogen from NADPH using an [FeFe] hydrogenase. Int. J. Hydrogen Energy 37, 2977–2983. doi: 10.1016/j.ijhydene.2011.03.172
Van Den Heuvel, R. H. H., Svergun, D. I., Petoukhov, M. V., Coda, A., Curti, B., Ravasio, S., et al. (2003). The active conformation of glutamate synthase and its binding to ferredoxin. J. Mol. Biol. 330, 113–128. doi: 10.1016/s0022-2836(03)00522-9
Yacoby, I., Pochekailov, S., Toporik, H., Ghirardi, M. L., King, P. W., and Zhang, S. (2011). Photosynthetic electron partitioning between [FeFe] - hydrogenase and ferredoxin: NADP þ-oxidoreductase (FNR) enzymes in vitro. Proc. Natl. Acad. Sci. U.S.A. 108, 9396–9401.
Keywords: ferredoxin, biohydrogen, metalloprotein, redox biochemistry, oxidation
Citation: Koo J and Cha Y (2021) Investigation of the Ferredoxin’s Influence on the Anaerobic and Aerobic, Enzymatic H2 Production. Front. Bioeng. Biotechnol. 9:641305. doi: 10.3389/fbioe.2021.641305
Received: 14 December 2020; Accepted: 10 February 2021;
Published: 26 February 2021.
Edited by:
Yuan Lu, Tsinghua University, ChinaReviewed by:
Klaas J. Jan Hellingwerf, University of Amsterdam, NetherlandsPaul W. King, National Renewable Energy Laboratory (DOE), United States
Copyright © 2021 Koo and Cha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jamin Koo, jaminkoo@alumni.stanford.edu
 Jamin Koo
Jamin Koo Yeeun Cha
Yeeun Cha