
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol., 02 March 2021
Sec. Nanobiotechnology
Volume 9 - 2021 | https://doi.org/10.3389/fbioe.2021.629832
This article is part of the Research TopicApplication For Nanotechnology For the Treatment of Brain Diseases and Disorders View all 9 articles
 Ebenezeri Erasto Ngowi1,2,3†
Ebenezeri Erasto Ngowi1,2,3† Yi-Zhen Wang1†
Yi-Zhen Wang1† Lei Qian1†
Lei Qian1† Yasmeen Ahmed Saleheldin Hassan Helmy1,2
Yasmeen Ahmed Saleheldin Hassan Helmy1,2 Bright Anyomi4
Bright Anyomi4 Tao Li1
Tao Li1 Meng Zheng5
Meng Zheng5 En-She Jiang1,6
En-She Jiang1,6 Shao-Feng Duan1,7
Shao-Feng Duan1,7 Jian-She Wei1,4
Jian-She Wei1,4 Dong-Dong Wu1,8*
Dong-Dong Wu1,8* Xin-Ying Ji1,9*
Xin-Ying Ji1,9*Brain is by far the most complex organ in the body. It is involved in the regulation of cognitive, behavioral, and emotional activities. The organ is also a target for many diseases and disorders ranging from injuries to cancers and neurodegenerative diseases. Brain diseases are the main causes of disability and one of the leading causes of deaths. Several drugs that have shown potential in improving brain structure and functioning in animal models face many challenges including the delivery, specificity, and toxicity. For many years, researchers have been facing challenge of developing drugs that can cross the physical (blood–brain barrier), electrical, and chemical barriers of the brain and target the desired region with few adverse events. In recent years, nanotechnology emerged as an important technique for modifying and manipulating different objects at the molecular level to obtain desired features. The technique has proven to be useful in diagnosis as well as treatments of brain diseases and disorders by facilitating the delivery of drugs and improving their efficacy. As the subject is still hot, and new research findings are emerging, it is clear that nanotechnology could upgrade health care systems by providing easy and highly efficient diagnostic and treatment methods. In this review, we will focus on the application of nanotechnology in the diagnosis and treatment of brain diseases and disorders by illuminating the potential of nanoparticles.
Brain diseases and disorders refer to a large group of health conditions affecting the brain including injuries, infections, tumors, and neurological disorders. Based on the 2015 statistics, brain diseases and disorders are the main cause of disabilities and the second leading cause of mortality with more than 250.7 million disability-adjusted life-years and 9.4 million deaths (GBD 2015 Neurological Disorders Collaborator Group, 2017). By definition, the term “brain diseases” encompasses a group of medical conditions that are usually transmittable and commonly caused by external forces such as viruses, bacteria, and so on (Ghosh and Higgins, 2018), whereas “brain disorders” include non-transmittable but commonly inheritable medical conditions caused by the disruption of the normal body structure and functioning as a result of birth defects or genetic malfunctions (Borsche et al., 2020). Brain diseases include viruses/bacteria/fungi/parasite-caused brain infections (BIs), whereas disorders include conditions such as multiple sclerosis (MS), autism spectrum disorder (ASD), and Alzheimer disease (AD). Despite their differences, the two terms are regularly used interchangeably. The most notable features of brain diseases and disorders include deterioration of cognitive, motor, and behavioral functions resulting from the impairment of neurological activities. The treatment of these conditions has been hindered by the complexity and sensitivity of the organ. Some of the diseases including bacterial and fungal BI can be cured by specific antibiotics if discovered in initial states, or vaccines can be applied to prevent their onset (Baccarini et al., 2020); however, others such as neurodegenerative disorders have no exact cures. The physical, chemical, and electric barriers prevent the entrance of materials including most drugs into the brain (Janzer and Raff, 1987; Butt and Jones, 1992; Boulton et al., 2002). Previously, potential drugs used to be dissolved in the solvents that could disrupt the blood–brain barrier (BBB) such as ethanol, polysorbate 80 (PS-80), and dimethyl sulfoxide in order to increase their penetration and sensitivity (Hanig et al., 1972; Azmin et al., 1985; Butt and Jones, 1992). In recent years, nanoparticles (NPs)–based treatments have emerged as the potential therapy for brain diseases and disorders due to easy transportability across the BBB, a credit of their unique features such as small size, selectivity, less toxicity, biodegradability, and solubility (Broadwell et al., 1982; Rabanel et al., 2020).
NPs refer to smallest particles usually within the size range of 1–100, at most less than 1,000 nm (Narayanan and Sakthivel, 2011). The particles are formed from natural or artificial manipulation of compounds or metals. So far, different kinds of NPs have been produced such as metal and metal oxides, liposomal, polymeric, fullerenes, nanoemulsions, solid–lipid (SL), polylactide-co-glycoside (PLGA) NPs, and so on, with varying physical and chemical properties (Figure 1; Narayanan and Sakthivel, 2011; Djordjevic et al., 2015; Ding et al., 2016; Lee et al., 2016; Dong et al., 2018; Rasouli and Tabrizian, 2019; Matsuno et al., 2020). The synthesized NPs have been applied in different fields such as cosmetics, agriculture, and medicines (Lu et al., 2015; Karny et al., 2018; Hydbring and Du, 2019; Katebi et al., 2019). In medicines, NPs are used in the diagnosis and treatments of different diseases especially the ones that are deeply seated such as metastatic cancers, brain tumor, and neurodegenerative disorders (Figure 2; Zhang et al., 2016; Li et al., 2019a). Because of the challenges facing the effective and efficient delivery of drugs in such conditions, NPs appear to be an important discovery that may enhance the effectiveness and efficiency of potential drugs. In response to the current rise in number of NPs that have shown enormous potential in the treatment of brain diseases and disorders, this review will summarize the application of NPs in the treatment of brain diseases and disorders, as well as the challenges facing this novel discovery. Hereby, the therapeutic potential of several NPs including metal, lipid, polymeric, coffee, SL, chitosan (CS), magnetic, rare-earth (RE), fullerenes, poly(butyl cyanoacrylate) (PBCA), PLGA, betulinic, and liposomal NPs will be discussed.
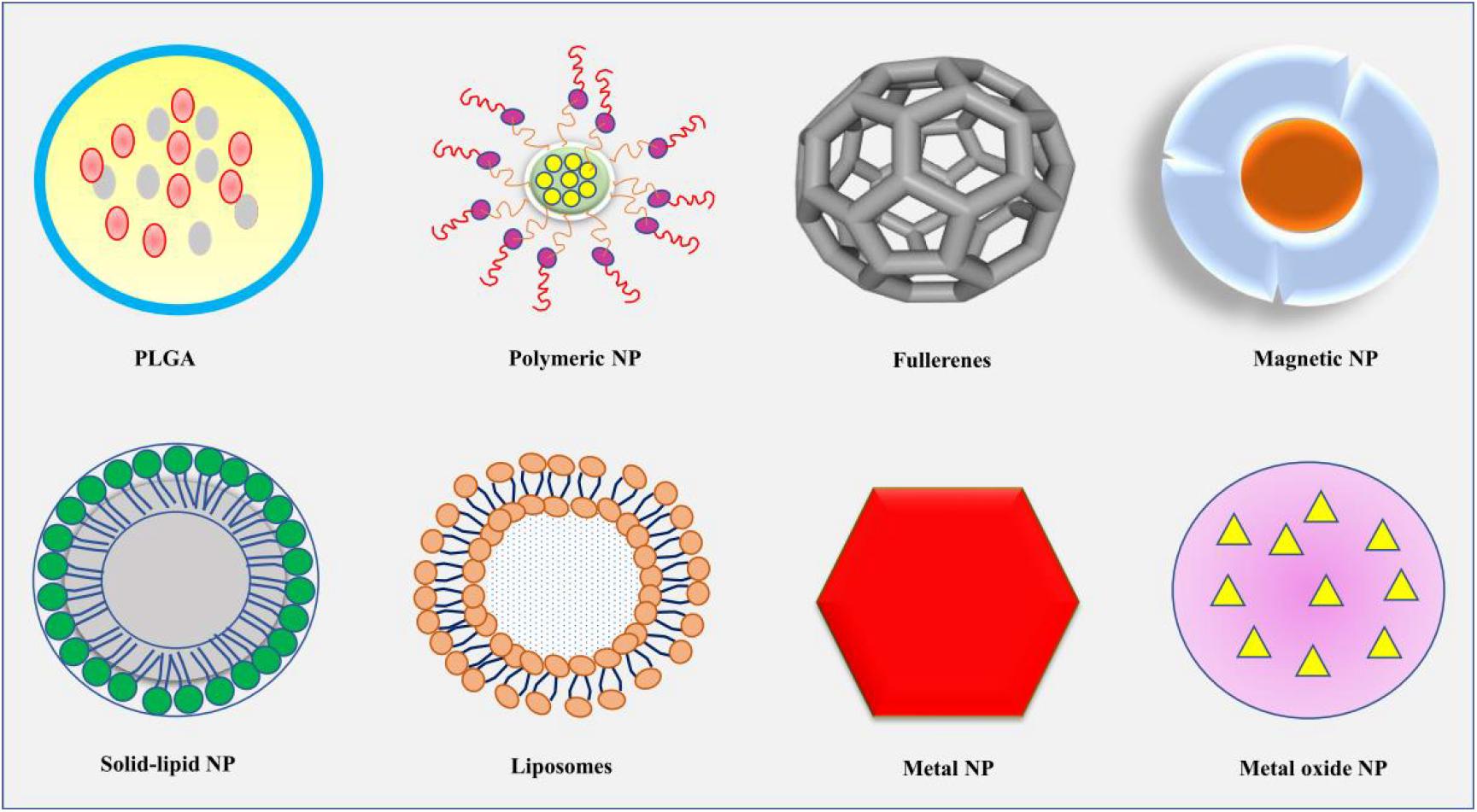
Figure 1. The diagrammatic presentation of some of the common shapes of NPs, including PLGA, polymeric NP, fullerenes, magnetic NP, solid-lipid NP, liposomes, metal NP, and metal oxide NP. NP, nanoparticle; PLGA, polylactide-co-glycoside.
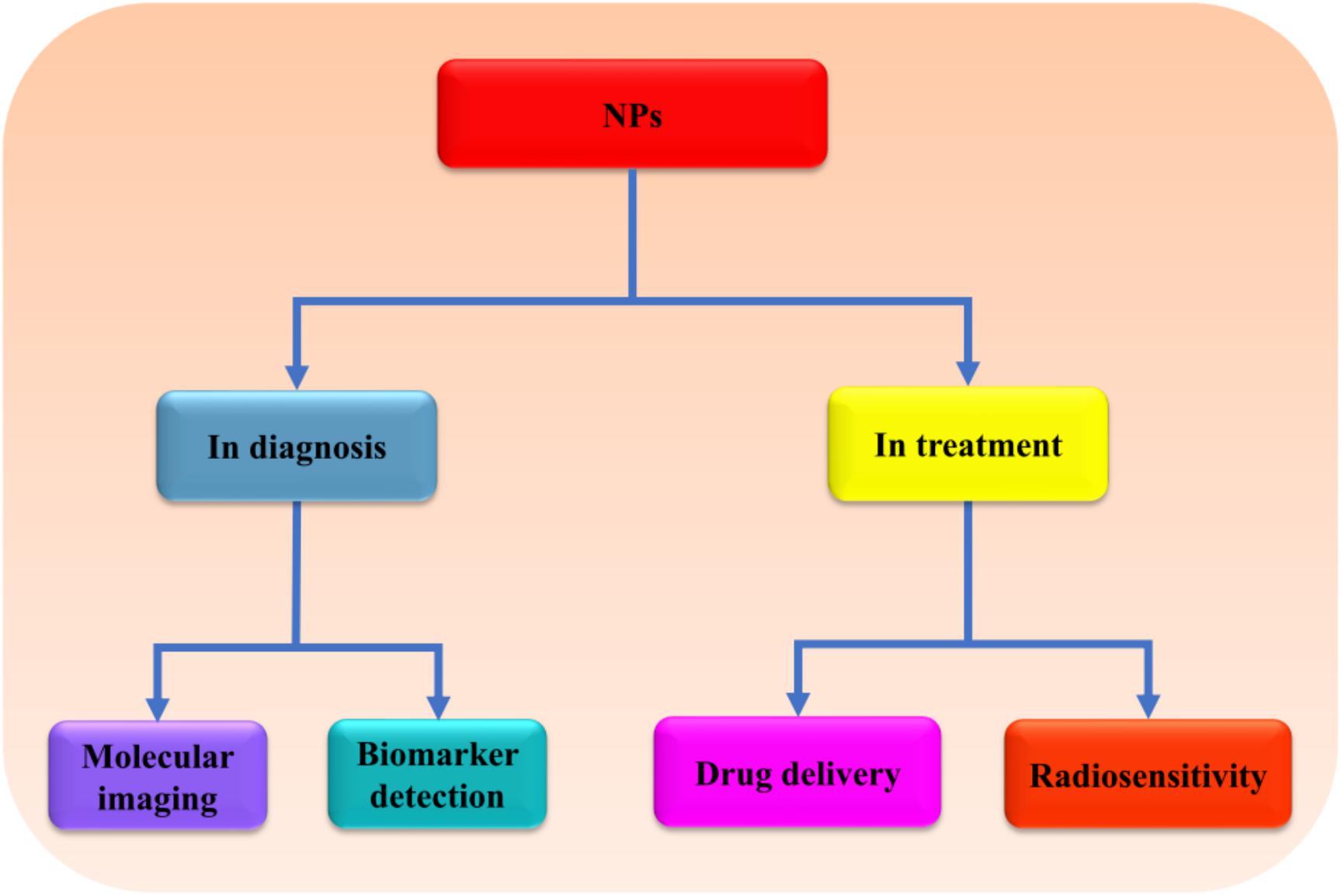
Figure 2. The possible application of NPs in brain therapy. NPs can be used in diagnosis as well as treatment of brain diseases and disorders due to their high sensitivity, specificity, and ability to cross BBB. NPs, nanoparticles; BBB, blood–brain barrier.
BBB is a physical barrier formed by endothelial cells (ECs) with the main role of maintaining and regulating the movement of nutrients and other essential materials to the brain, thereby protecting its integrity. The ECs are located on the outer and inner sides of the closely packaged tight junctions that touch the outer EC membranes and prevent easy penetration of materials (Reese and Karnovsky, 1967). Some of the main functions of the BBB include the regulation of the flow of materials in and out of the brain, ionic balance, and protection from the diffusion of circulating agents, neurotransmitters, xenobiotics, and other substances that can affect the integrity of the brain (Abbott et al., 2010). Studies show that poor permeability of molecules across the BBB is significantly associated with high electrical and chemical [P-glycoproteins (P-gp)] resistance (Crone and Christensen, 1981; Butt et al., 1990; Li et al., 2018). Some of the vital regulators of tight junctions’ activities identified are cyclic adenosine monophosphate and astrocytes (Rubin et al., 1991; Hurst and Clark, 1998). In brain diseases and disorders, the BBB is highly disrupted, resulting into unregulated diffusion of molecules, leading to further brain damage (Dallasta et al., 1999; Algotsson and Winblad, 2007). Because the BBB prevents the entrance of materials basing on their size and solubility, most of the potential drugs fail to penetrate because they do not meet the required criteria (Pardridge, 2012). One of the common techniques used to improve the transportation of drugs across the barrier is the temporal disruption using focused ultrasound (McDannold et al., 2012), although the mechanism involved and the effect of the technique on an already disrupted barrier are yet to be elucidated. Otherwise, the search for a non-disruptive technique for transportation of drugs to the brain has also been given a high priority, and in recent times, NPs have proven to be efficient in fulfilling the role.
Crossing the BBB and blood–cerebrospinal fluid (CSF) barrier has been the main challenges hindering the treatment of brain diseases and disorders. The efflux of materials across BBB is carefully mediated by P-gp; hence, its downregulation is implicated with the progression of neurodegenerative disorders and tumor (Henson et al., 1992; van Assema et al., 2012; Jeynes and Provias, 2013). The inhibition of P-gp improves the penetration of drugs across the BBB and their subsequent effects (Jablonski et al., 2014). NPs of PBCA have been reported to suppress P-gp-mediated phenytoin resistance in rats (Fang et al., 2016). Moreover, a recent study shows that encapsulation of andrographolide (a neuroprotective drug) into SL NPs increases its permeability to the BBB compared to free drug (Graverini et al., 2018). In summary, the above data indicate that NPs can enhance the penetration of potential drugs and increase their target ability by regulating p-gp (Figure 3).
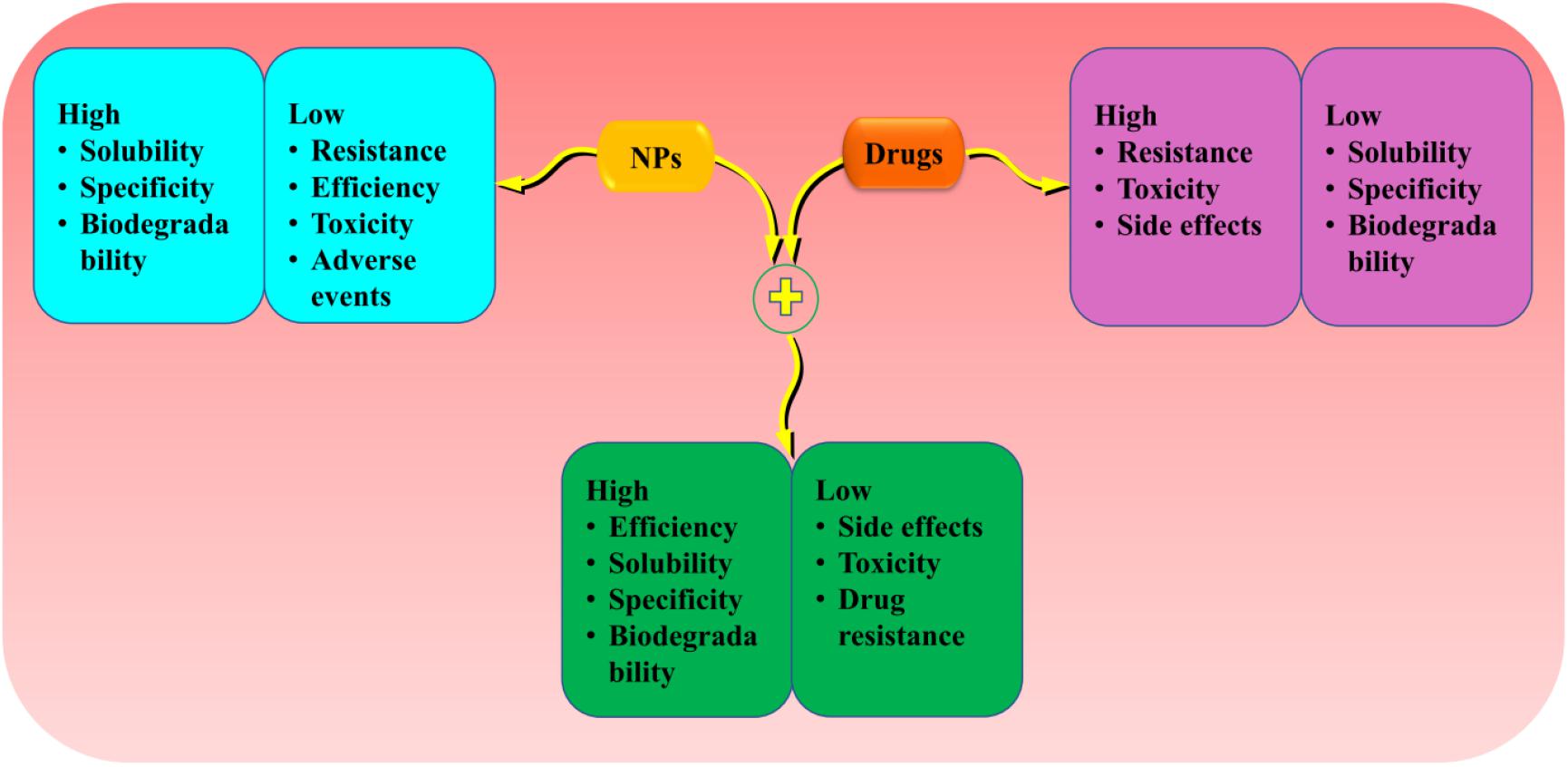
Figure 3. The advantage of loading drugs into the NPs compared to individual treatments. Encapsulation of potential drugs offers advantages into the NPs and drugs features to provide high effectiveness and efficiency. NPs, nanoparticles.
The therapeutic efficacy of most drugs is affected by their cytotoxicity. The brain toxicity induced by NPs is much less compared to conventional therapy. For example, in cerebral ischemia/reperfusion model, intranasal administration of PLGA NPs is reported to be highly effective in transporting a mitoNEET ligand inhibitor NL-1 with no toxicity (Saralkar et al., 2020). Further studies have also shown that the encapsulation of cytotoxic drug such as amphotericin B (antifungal drug), thioridazine (antipsychotic drug), and sorafenib (anticancer drug) into NPs markedly improves their toxicity index by enhancing drug solubility, bioavailability, and sustained release (Tang et al., 2015; Vibe et al., 2016; Li et al., 2020).
Alternatively, NPs can also increase the cytotoxicity of conventional drugs in their targeted area, e.g., tumor cells. In a recent study, treatment with polyethylene glycol (PEG)-modified silica (Si) NPs has been shown to increase the cytotoxicity of anticancer drug, 3N-cyclopropylmethyl-7-phenyl-pyrrolo-quinolinone as compared to free drug in an in vitro model (Morillas-Becerril et al., 2020). Despite the observed potential of NPs, some of these compounds can also result in cytotoxicity, including Si NPs whose effect is shown to be influenced by the porosity and size of the particles (Mohammadpour et al., 2019). Apart from the use of particle sizes and shapes that show less toxicity, another method that can be used to improve the efficiency of NPs is the addition of PEG also known as PEGylation (Mendonça et al., 2016; Abakumov et al., 2018). In summary, the evidences above indicate significant reduction in drug toxicity when loaded into some NPs as compared to when administered freely; although in some cases the encapsulation can result in increased cytotoxicity, the event can be reduced with PEGylation or alteration of particle size and porosity.
Other parameters that are essential in determining the efficacy of a drug is solubility and bioavailability. Solubility is the ability of the drug to dissolve, whereas bioavailability is the extent to which the drug can reach the systemic blood circulation and subsequently the targeted site (Chow, 2014; Alany, 2017). Unlike solubility, the factors affecting drug bioavailability can be drug-related or body-related. Some of them include age, sex, gut pH, genetics, drug dosage, and formulation. Because of the importance of these parameters, improving both of them can lead to better drug efficacy and ultimately treatment of the disease. It has been reported that silver (Ag) NPs can significantly enhance the solubility of methane and ethane in water, with the solubility shown to increase with NPs mass loading (Rahmati-Abkenar and Manteghian, 2020). A recent study indicates that the loading of hydrophobic drug, carvedilol, into CS-sodium tripolyphosphate (STPP) NPs increases its bioavailability and promotes slow and sustained release of the drug (Sharma et al., 2019). Similarly, the oral bioavailability and solubility of curcumin (a polyphenol and turmeric compound) can be improved by loading into PEGylated SL NPs (Ban et al., 2020). The bioavailability and solubility of many other potential drugs such as astilbin, sorafenib, apigenin, and astaxanthin also have been reported to be improved following the encapsulation into NPs (Huang et al., 2019; Liu et al., 2019; Park et al., 2019; Zheng and Zhang, 2019). Together, the data above suggest that NPs can potentially increase the solubility and bioavailability of less-soluble drugs, therefore improving their efficacy.
The specificity and biocompatibility of the drug ensure effective delivery to the targeted site. Incorporating drugs into NPs help to substantially enhance these parameters. A recent evidence shows that chimeric antigen receptor T-cell membrane–encapsulated NPs have high specificity in targeting tumor cells by recognizing glycan-3 proteins, which are highly expressed in hepatocellular carcinoma cells with good biocompatibility and safety in normal cells (Ma et al., 2020). Biomimetic gold (Au) NPs stabilized by seaweed extracts have also been reported to be lethal in breast cancer cells MDA-MB-231 at the dose of less than 45 μg/mL while showing no effects on human embryonic kidney cells at 150 μg/mL, which confirmed the high biocompatibility and selectivity of the NPs (Jeyarani et al., 2020). In addition, specific antibody-loaded iron oxide (IO) NPs have shown high sensitivity and specificity, greater than 95 and 90%, respectively, in capturing amyloid β (Aβ) and Tau proteins in the serum and CSF-mimicking samples and about 80–90% in human whole blood samples as compared to the common antibody-conjugated magnetic micron beads, which show approximately only 20% specificity and sensitivity, suggesting the potential of the technique as a biomarker for dementias (Li et al., 2019b). Overall, the above data imply that NPs are highly specific and biocompatible and therefore can be used to deliver drugs to the targeted sites more efficiently.
MI is an important field in biomedical science associated with the analysis of pathogenesis or body functioning at the molecular level. The imaging techniques provide easy visualization, characterization, and quantification of activity of interest in the body with high sensitivity and specificity (Weissleder and Mahmood, 2001). It involves the use of advanced techniques of different capabilities including microscopy, bioluminescence imaging, ultrasound, X-ray radiography, magnetic resonance imaging, positron emission tomography, and single-photon emission computed tomography. MI techniques have proven to be useful in analysis and characterization of different brain diseases ranging from infections to brain tumors and neurological disorders (Mankoff, 2007; Aldossary et al., 2019; Bocan et al., 2019). The specificity of MI is enhanced by the use of contrast beacons known as probes (Schocke et al., 2002). Probes that bind to specific targets are called targetable probes, whereas the ones that react with specific indicators on their targets to produce a visible signal are termed as activatable probes. A previous study suggests that oligopeptides NPs can act as activatable probes because of their ability to produce fluorescence as a result of the stimulation by low pH of tumor microenvironment (Massoud and Gambhir, 2003). Besides, it has been shown that PS-80–coated PBCA dextran polymeric NPs can be used to transport targetable probes across the BBB, thereby facilitating the visualization of Aβ plaques in AD model (Zhao et al., 2014). A recent study also reports that sulfated dextran-coated IO NPs can effectively improve bioimaging of the activated microglia-induced brain inflammation by binding to the highly expressed class A scavenger receptors (Tang et al., 2018). In addition, it has also been shown that RE-doped NPs can be used in fluorescence imaging to facilitate the emission of short-wave infrared light after binding to integrin α Vβ3 (Naczynski et al., 2018). In summary, the information above indicates that NPs can be used to improve MI by delivering bioimaging probes or acting as probes themselves, confirming their importance in diagnosis of deep-seated tumor and brain diseases.
Biomarker is simply a detectable substance/indicator that is directly associated with a certain condition or state. The effectiveness of the biomarker to differentiate between healthy and unhealthy individuals and its specificity in characterization of the disease stage are key in management of diseases. Different biomarkers have been identified in brain diseases and disorders; however, their application is hindered by the lack of suitable techniques. NPs have shown to be useful in detecting key biomarkers of brain diseases and disorders with great efficiency. In traumatic brain injury (TBI) patients, plasma levels of ubiquitin-C-terminal hydrolase-L1 (UCH-L1) have been identified to be significantly elevated as compared to healthy individuals and therefore could serve as a potential biomarker for the condition (Posti et al., 2016). A recent study demonstrates that a novel method involving surface plasmon resonance of Au NPs can rapidly and effectively detect UCH-L1 biomarker in TBI patients with 100% sensitivity and specificity (Singh et al., 2018). Besides, Aβ levels have been markedly correlated with dementia and associated diseases (van Steenoven et al., 2019). Studies have shown that modified magnetic NPs can effectively and safely detect Aβ plaques in the mouse model of AD (Cheng et al., 2015; Zeng et al., 2018). Anticholesterol antibody-bound magnetic NPs have further been shown to be effective in detecting elevated cholesterol levels, which is also a key marker for AD (Fernández-Cabada and Ramos-Gómez, 2019). Moreover, a recent study indicates fluorescent NPs can be used to detect AD biomarkers including Aβ, inflammatory cytokines, and Tau proteins (Sun et al., 2021). Collectively, these data suggest that NPs are quick and effective in detecting biomarkers for brain diseases and disorders.
Delivery of the potential drugs in the brain is one of the main challenges facing the treatment of brain diseases and disorders. The drug is supposed to be able to cross the BBB and reach the designated target without causing serious short- or long-term damage into the brain. The size and number of hydrogen bonds are among the factors preventing the transportation of the drugs across the BBB (Pardridge and Mietus, 1979; Pardridge, 2012). In recent years, NPs have gained a lot of attention due to their ability to cross the BBB and serve as a carrier for potential drugs. NPs show enhanced BBB penetrating capabilities and can be loaded with potential brain-targeting drugs (Lin et al., 2016; He et al., 2019; Sadegh Malvajerd et al., 2019). In addition, Au NPs have also been shown to be effective in delivering antibody across the BBB by binding to transferrin receptors, although the effect depends on the affinity and valency of the conjugated antibody (Johnsen et al., 2018). Intranasal delivery of huperzine A with lactoferrin-conjugated N-trimethylated CS-modified PLGA increases its bioavailability and retention time in the mouse model of AD (Meng et al., 2018). Together, these data imply that NPs can effectively and efficiently deliver different drugs across the BBB.
The resistance to potential drugs is one of the main challenges in treatment of chronic and progressive diseases. However, radiosensitization offers a promising solution to the situation. It involves the sensitization of the tissues/cells to the radiation by physical, chemical, or pharmacological means (Gallez, 2015). It has been reported that the treatment of glioblastoma mouse model with a folate-targeted NP-mediated kringle 1 domain of hepatocyte growth factor gene can significantly induce antitumor effects and improve the sensitivity to ionizing radiation by promoting checkpoint kinase-1–induced cell cycle arrest and inhibiting the activation of tyrosine kinase receptors, ataxia telangiectasia mutated−checkpoint kinase-2 pathway, and Ki-67 expression (Figure 4; Zhang et al., 2018). Similarly, NPs made of lipid–poly(hypoxic radiosensitized polyprodrug), IO conjugated with epidermal growth factor receptor (EGFR), and RE have also been reported to enhance the sensitivity of radiotherapy in glioma cells by increasing the oxidative stress (Bouras et al., 2015; Hua et al., 2018; Lu et al., 2019). The cotreatment of PEGylated-Au NPs with radiation improve sensitivity, resulting in enhanced DNA damage (Joh et al., 2013). However, compared to Au NPs, Ag NPs have more sensitization effect mediated through the promotion of autophagy (Liu et al., 2016). In brief, NPs enhance the radiosensitivity of brain cells by promoting autophagy and hypoxia-induced oxidative stress, suggesting that this combination could be effective in treatment of brain diseases.
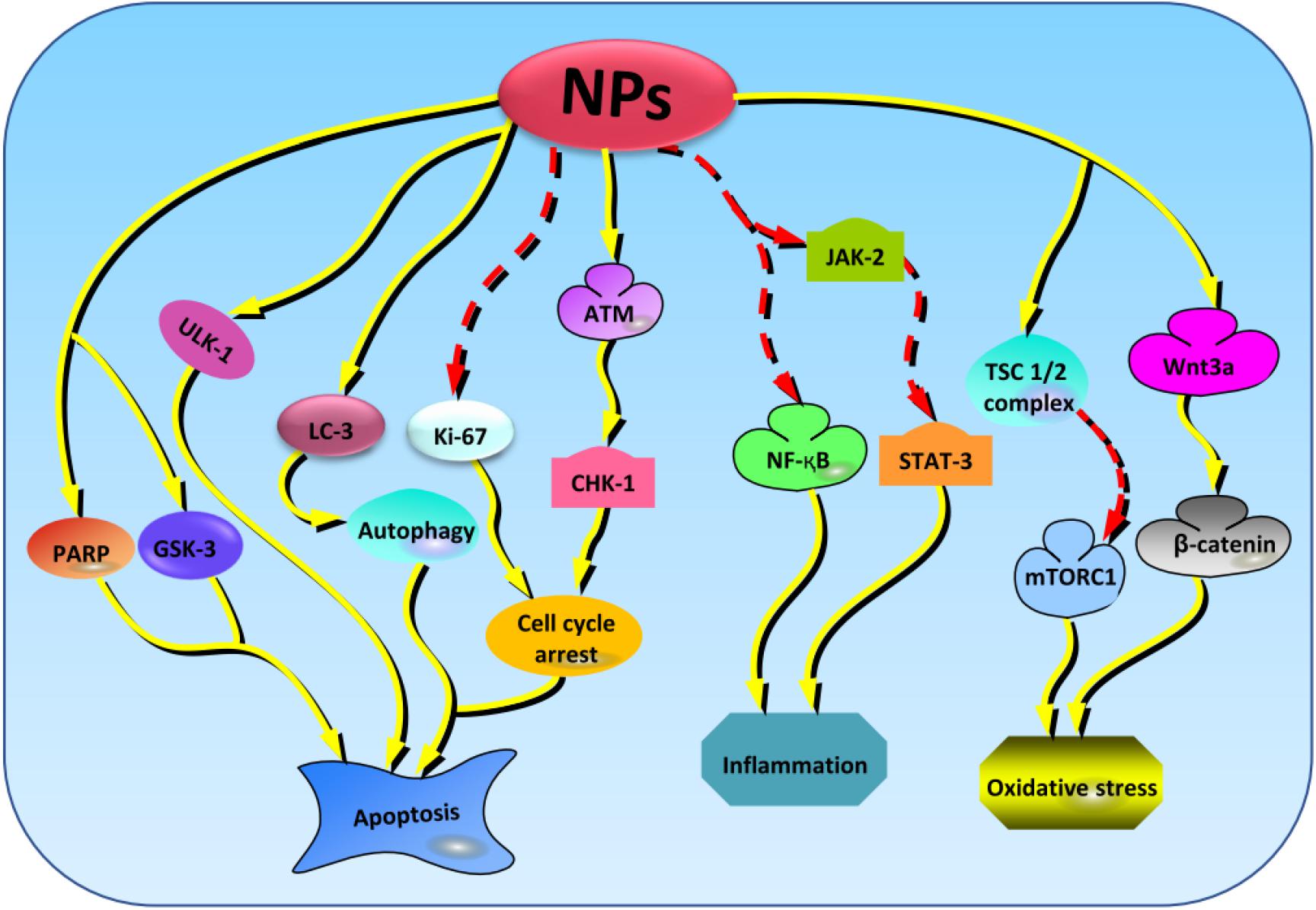
Figure 4. The key cellular markers targeted by NPs in regulating the oxidative stress, inflammation, and apoptosis activities. From the left to right; NPs stabilize the levels of PARP, GSK-3, and ULK-1 to regulate apoptosis. Next, NPs amplifies the expression of LC-3 to induce protective autophagy and regulate apoptosis. NPs also suppress the expressions of Ki-67 and promotes the activation of ATM/CHK-1 cascades resulting in promotion of apoptosis. NPs can also enhance the inhibition of NF-κB and JAK-2/STAT-3 signaling pathways to regulate inflammation. Besides, NPs elevate the levels of TSC1/2 complex and Wnt3a, thereby promoting the inhibition and activation of mTORC1 and β-catenin, respectively, and resulting in the stimulation of antioxidant activities. NPs, nanoparticles; PARP, poly(ADP-ribose) polymerase; GSK-3, glycogen synthase kinase-3; ULK-1, unc-51 like autophagy activating kinase-1; ATM/CHK-1, ataxia telangiectasia mutated/checkpoint kinase-1; NF-κB, nuclear factor κB; JAK-2/STAT-3, janus kinase 2/signal transducer and activator of transcription 3; TSC1/2, tuberous sclerosis protein complex 1/2; mTORC1, mammalian target of rapamycin complex 1.
Brain tumor involves malignant and benign types of tumor that affect the brain. Because of the complexity of the brain, not only the metastatic but also the growth of benign tumor can have detrimental outcomes. According to the 2018 report, there were more than 298,000 new cases of brain tumor worldwide (Bray et al., 2018). The progression of the disease is associated with cognitive dysfunction (Cramer et al., 2019). The pathology of the disease is not well classified, and its treatment is still uncertain. However, several studies have reported the therapeutic advantage of NPs in delivering potential antitumor drugs (Nance et al., 2014; Feng et al., 2017; Chen et al., 2018). The delivery of small interfering RNA by targeting several genes including sodium–potassium (Na-K)–chloride cotransporter 1, yes-associated protein 1, roundabout homolog 1, EGFR, and survivin using polymeric NPs can significantly reduce the growth and migration of glioblastoma cells in a selective manner (Kozielski et al., 2019). It has been shown that modified polymeric NPs loaded with herpes simplex virus type 1 thymidine kinase combined with ganciclovir can markedly reduce the viability of glioma cells and increase the survival of tumor-bearing mice (Mangraviti et al., 2015). The above data confirm the use of NPs for gene and drug delivery to target brain tumors.
BIs consist of rare but deadly infections caused by microorganisms such as bacteria, viruses, fungi, and parasites that trigger inflammation in the brain or surrounding tissues (Sarrazin et al., 2012). Bacterial and viral infections are the most common. These infections are characterized by acute to chronic inflammations, oxidative stress, and subsequently neuronal impairment. A report shows that treatment with nerolidol-loaded NPs can efficiently improve memory defects and stabilize the levels of reactive oxygen species (ROS) and activities of Na-K ATPase and acetylcholine esterase previously altered by Trypanosoma evansi infection in mice (Baldissera et al., 2017). Also, the encapsulation of elvitegravir drug into PLGA NPs improves its inhibitory effects on human immunodeficiency virus 1 (HIV-1)–infected human monocyte-derived microglia-like cells and mouse model without affecting the integrity of the BBB (Gong et al., 2020). The clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9/gRNA-loaded magnetoelectric NPs have also been reported to inhibit HIV-1 infection in microglia cells, indicating the potential of NPs in transporting gene therapy (Kaushik et al., 2019). Studies also show that NPs can increase the bioavailability of anti-HIV drugs such as darunavir, indinavir, and efavirenz, thereby improving their ability to cross BBB and targetability of viral infections in the brain (Desai and Thakkar, 2018; Karami et al., 2019; Martins et al., 2019). Besides, cobalt phosphate and hydroxide NPs have also shown potential inhibitory effects on parasite-induced toxicity of granulomatous amoebic encephalitis caused by Acanthamoeba castellanii belonging to the T4 genotype (Anwar et al., 2019a). The conjugation of the antidiabetic drugs glimepiride, repaglinide, and vildagliptin with Ag NPs can significantly inhibit the A. castellanii–mediated BI by preventing encystation and cytotoxicity (Anwar et al., 2019b). Further studies indicate that the treatment with Au and zinc oxide (ZnO) NPs can effectively improve oxidant/antioxidant status and neuronal impairment by regulating different genes altered following Schistosoma mansoni infection in mice (Dkhil et al., 2015; Bauomy, 2020). Similarly, Au NPs have been shown to reduce herpes simplex virus-1 infection–associated neurological defects by attenuating Aβ peptides aggregation and β-secretase activities (I et al., 2020). Otherwise, drug delivery with NPs has also shown enormous potential in treating cerebral tuberculosis, encephalitis virus infection, and amoebic meningoencephalitis infections in mouse models (Marcianes et al., 2017; Rajendran et al., 2017; LaBauve et al., 2018). Overall, the use of NPs in BI could reduce the cytotoxic effects and improve the efficiency of the treatments.
TBI is the leading cause of trauma-associated disabilities and deaths worldwide, estimated to affect more than 69 million people each year (Dewan et al., 2018). Accidents, sports, and gunshot are among the common causes of the TBI. The condition is associated with elevated levels of melanin in CSF that results in the promotion of oxidative stress and metabolic defects (Seifman et al., 2008). TBI patients have high levels of inflammatory markers in the CSF, which can be correlated with the severity of their condition (Kerr et al., 2018). TBI has been identified to be among the risk factors for neurodegenerative diseases including AD and Parkinson disease (PD) (Guo et al., 2000; Fleminger et al., 2003). Encapsulation of brain-delivered neutrophic factor, stromal cell–derived factor-1, cerebrolysin, and ROS-reactive agents into the NPs have been reported to be effective in improving neurological impairments resulting from TBI in mouse models (Ruozi et al., 2015; Khalin et al., 2016; Yoo et al., 2017; Zamproni et al., 2017). A recent study reports that treatment of TBI rat model with cerium oxide (CeO2) NPs can significantly reduce brain damage by restoring the cognitive abilities and promoting antioxidant properties (Bailey et al., 2020). It has been reported that the administration of immunomodulatory NPs can markedly improve motor impairments and reduce inflammatory and edema in TBI mouse model (Sharma et al., 2020). A risk analysis study reports that consumption of coffee in midlife reduces the risk of development of AD later in life, indicating that coffee has neuroprotective properties (Eskelinen et al., 2009). The NPs of coffee can be synthesized by boiling the coffee in water, filtrating to remove oil and large particles, followed by sonication (Ratliff et al., 2019). In TBI mouse model, treatment with nano-coffee can effectively improve behavioral characteristics and stabilize the levels of glycogen synthase kinase-3 (GSK-3) and poly(ADP-ribose) polymerase, the key biomarkers for apoptosis and cellular damage (Ratliff et al., 2019). In summary, NPs offer a crucial option for delivering drugs and reduce TBI-associated neurological damage by inhibiting apoptosis, inflammation, and oxidative stress.
Stroke is one of the primary causes of death and disability worldwide. IS is the most common type of stroke accounting for more than 79% of all stroke cases reported in 2017 (Virani et al., 2020). Almost 53% of new cases of IS reported in 2016 occurred in people between the ages of 44 and 70 years (Lindsay et al., 2019). The condition is characterized by the blockage of blood vessel as a result of blood clot or fat deposition. The progression of the disease is associated with several mechanisms including excitotoxicity, oxidative stress, and inflammation, which causes damage to cells and tissues (Castillo et al., 1999; Suwanwela et al., 2006; Kelly et al., 2008; Chehaibi et al., 2016). A recent study indicates that treatment of murine models of IS with selenium (Se) NPs can efficiently suppress neurodegenerative properties by regulating autophagy, inflammation, and oxidative stress through the upregulation of Unc-51–like autophagy activating kinase-1 and Wnt3a and suppression of Jack2/Stat3 and mTORC1 signaling cascades (Amani et al., 2019). Another study demonstrates that betulinic NPs improve the transportation of glyburide in BBB, resulting in enhanced antiedema and antioxidant properties in IS mice (Deng et al., 2019). Moreover, AMB3100-conjugated, size-shrinkable NPs have also been reported to facilitate the delivery efficiency of glyburide and reduce its toxicity (Guo et al., 2018). Besides, it has also been shown that the administration of polyhydroxylated fullerene NPs can significantly suppress brain damage by alleviating antioxidant status and nitric contents in IS mouse model (Vani et al., 2016). The protective effect of fullerene NPs is also linked with the downregulation of aquaporin-1 protein resulting in inhibition of edema (Darabi and Mohammadi, 2017). It has also been reported that treatment of IS rat model with melanin NPs can significantly inhibit the ROS and reactive nitrogen species–induced brain damage (Liu et al., 2017). So et al. (2019) showed that the administration of acetate-loaded liposomal NPs can markedly reduce microglial stimulation and chronic inflammation without affecting oxidative stress, apoptosis, and neurogenesis processes. Overall, the above information suggests that NPs can help in treatment of IS by acting as a delivery vehicle for drugs and by directly affecting the mechanism leading to the progression of the disease.
Amnesia is a medical condition characterized by memory loss, which results from either brain diseases or injury. Most common risk factors for the disease are substance abuse, toxicity, brain diseases, head injury, and blood loss (Langer, 2019). Currently, there are no specific drug treatments for the disease. Similar to other brain diseases, targeting amnesia is challenged by the drugs-associated protection mechanisms of the brain. A previous study reports a significant increase in memory recovery rate following the treatment with PS-80–coated rivastigmine CS NPs in mouse model of scopolamine (SC)–induced amnesia (Nagpal et al., 2013b). Similarly, nerve growth factor–loaded PBCA NPs modified with PS-80 reduce amnesic activities and improve cognitive functions in amnesia rat models (Basel et al., 2005). Besides, treatment with galantamine-loaded thiolated CS NPs can also restore memory defects in amnesia animal model (Sunena et al., 2019). In contrast, the gallic acid–loaded CS NPs coated with PS-80 and ZnO NPs show no significant improvement in memory loss induced by SC treatment in mice as compared to the administration of their corresponding pure drugs; however, these NPs can be used to enhance brain delivery of potential drugs (Nagpal et al., 2013a; Yadav et al., 2018). Collectively, NPs have shown improved drug-delivery efficiency; however, more studies are needed to investigate their effects in the treatment of amnesia.
ASD is a developmental disorder characterized by behavioral and communication difficulties. Individuals with ASD have poor communication skills as well as limited and repetitive behavioral patterns and interests (Bejerot and Nordin, 2014). The symptoms are usually seen in early childhood and progress to adulthood; however, with proper support and interventions, some difficulties can be camouflaged (American Psychiatric Association, 2013). The prevalence of the disorder in United States is 1/59 for 8 year-old children (Baio et al., 2018). In Norway, the prevalence of the ASD has been reported to increase, with more effect observed in preschool children ≤ 5 years old compared to school children aged 6–16 years (Özerk and Cardinal, 2020). Despite the lack of global data, there is a need for developing novel treatment options for the disorder. In valproic-induced ASD rats, treatment with nano-hesperetin could restore behavioral defects and inhibit inflammation and oxidative stress activities (Khalaj et al., 2018). Alternatively, prenatal exposure of mice with titanium dioxide (TiO2) NPs induces ASD-like behavioral impairment in offspring; however, the compound has no physiological effects (Notter et al., 2018). In brief, the above information indicates that NPs have potential in delivering drugs; however, more studies are needed to assess the side effects of the NPs.
ALS is a group of progressive neurons-targeting degenerative diseases that affect the central nervous system (CNS), resulting in motor malfunctions and paralysis. The disease mostly affects children from 2 to 5 years, and in most cases, the death occurs within 5 years as a result of respiratory paralysis (Brown and Al-Chalabi, 2017). Currently, there are no cures for the disease. Compared to healthy individuals, ALS patients have a high rate of oxidative stress (Chico et al., 2018), neurotoxicity (Lam et al., 2016), and inflammation (Keizman et al., 2009). It has been reported that treatment with CeO2 NPs can effectively improve muscle activities and survival of ALS-induced mouse models by reducing oxidative stress–induced damage (DeCoteau et al., 2016). Similarly, treatment with Au NPs loaded with an inhibitor of hypoxia-inducible factor FM19G11 has been shown to promote the differentiation and proliferation of epidermal stem progenitor cells by elevating the associated genes in ALS mouse model (Marcuzzo et al., 2019). Moreover, treatment with adapalene-loaded poly(lactic acid)–poly(ethylene glycol) NPs induces neuroprotection and improves survival and motor functioning in ALS mice by stimulating retinoid signaling pathway (Medina et al., 2020). Together, these data imply that NPs have a great potential in improving the efficiency and transporting ASL drugs.
AD is a common type of dementia characterized by aging-related progressive degeneration of neurons resulting in reduced cognitive ability and other neuropathological features. According to the 2016 data, at least one person develops the disease after each 66 seconds in America, and the number is expected to increase abruptly in the coming years (Alzheimer’s Association, 2016). The accumulation of Tau proteins is among the pathological features associated with the progression of neurodegenerative diseases including AD (Nam et al., 2020; Tagai et al., 2020). A recent study shows that protein-capped cadmium sulfide and IO NPs can effectively inhibit the polymerization and fibrillization of Tau proteins with the inhibition rates of 63 and 49%, respectively (Sonawane et al., 2019). Another pathological feature of AD is the accumulation of Aβ, which results into the reduced Aβ-binding capacity and formation of plaques (Hansson et al., 2009; Esparza et al., 2013). GSK-3, a serine/threonine kinase, has been shown to participate in the production of Aβ and hyperphosphorylation of Tau proteins and subsequently the progression of AD (Qu et al., 2014). Further evidences indicate that GSK-3 works with histamine deacetylase (HDAC) proteins to regulate neuronal activities (Chen et al., 2010; Bardai et al., 2012). Correspondingly, the inhibitors of GSK-3 and HDAC have been reported to be effective in suppressing AD (Green et al., 2008; De Simone et al., 2019; Soares Romeiro et al., 2019). The loading of nicotinamide, an HDAC inhibitor into the SL NPs, can significantly reduce cognitive impairment associated with AD by reducing the phosphorylation of Tau proteins in rat model (Vakilinezhad et al., 2018). Alternatively, the treatment of 5XFAD mice with vitamin D–binding protein–loaded PLGA NPs attenuates cognitive defects by inhibiting Aβ binding and accumulation (Jeon et al., 2019). Au NPs have also been reported to induce cytoprotective effects in AD rat model by promoting anti-inflammatory responses and improving antioxidant status (Dos Santos Tramontin et al., 2020). Furthermore, surface-coated Au NPs have also been shown to reduce Aβ aggregation, with the effect varying with the diameter and surface chemistry of the NPs (Moore et al., 2017). It has been reported that Au NPs with negative surface potential significantly reduce Aβ fibrillization and associated neurotoxicity in AD model (Liao et al., 2012). Furthermore, a recent study suggests that Au NPs with smaller size are more effective in suppressing Aβ fibrillization compared to the larger ones (Gao et al., 2017). Collectively, the data above signify that NPs can be used to deliver drugs targeting peptides dysregulated in AD such as Aβ more efficiently and effectively.
PD is one of the most common types of neurodegenerative disorder with high prevalence in adults older than 50 years. Data show that more than 6.1 million people had PD in 2016, which is an increase of 2.4-fold from 1990 (GBD 2016 Parkinson’s Disease Collaborators, 2018). The disease is characterized by the loss of substantia nigra dopaminergic neurons and formation of Lewy bodies (LBs) and symptomized by motor and non-motor defects (Forno, 1996). Both genetics and environmental factors play a crucial role in the progression of the disease. The LBs contain α-synuclein aggregates (Spillantini et al., 1997, 1998), which contribute to the progression of the disease by facilitating neuronal loss and sensitivity to stresses (Cooper et al., 2018). α-Synuclein further participates in the promotion of apoptosis (Lee et al., 2001), inflammation (Chatterjee et al., 2020), and suppression of neuronal stem cell differentiation (Oliveira et al., 2015). A recent study indicates that α-synuclein is highly expressed in plasma and serum of PD patients compared to healthy individuals and suggests the possibility of using the protein as a diagnosis indicator for the disease (Chang et al., 2020). With respect to NPs, it has been reported that treatment with α-synuclein short-hairpin RNA-loaded magnetic IO NPs coated with oleic acid can efficiently improve motor dysfunction in PD mouse model by reversing α-synuclein–mediated elevation of apoptotic markers Bcl-2–associated X protein and p53 and suppression of B-cell lymphoma 2 (Niu et al., 2017). Study also shows that microRNA-124–loaded polymeric NPs are effective in repairing motor defects and alleviating PD symptoms (Saraiva et al., 2016). Alternatively, ceria NPs can also reduce ROS levels in PD mouse model (Kwon et al., 2018). Besides, treatment with iron (Fe) chelation NPs modified with zwitterionic poly(2-methacryloyloxyethyl phosphorylcholine) and HIV-1–transactivating transcriptor to delay its saturation in blood and increase its in vivo lifetime can reverse PD symptoms more effectively compared to individual treatments (Wang et al., 2017). Further study reveals that treatment of alkaline reserpine–induced PD mouse model with Au NPs can significantly reverse behavioral defects and improve antioxidant status and neuronal survival (da Silva Córneo et al., 2020). Moreover, the treatment of PD-induced mouse model with nanodopamine drugs also improves motor defects with low toxicity as compared to pure levodopa, a primary drug used for the treatment of PD (Vong et al., 2020). Likewise, metformin-loaded polydopamine NPs promote anti-inflammatory, antiapoptotic, and antioxidative properties associated with the proteolytic degradation of phosphorylated serine 129 of α-synuclein protein induced by targeting a histone-lysine N-methyltransferase enzyme known as the enhancer of zeste homolog 2 (Sardoiwala et al., 2020). Other NPs and nanodrugs that have been reported to have significant potential in the treatment of PD by regulating oxidative stress and inflammation including vitamin E–loaded naringenin nanoemulsions (Gaba et al., 2019), selegiline CS NPs (Sridhar et al., 2018), borneol and lactoferrin comodified NPs (Tang et al., 2019), resveratrol NPs (Palle and Neerati, 2018), and Cerium NPs (Hegazy et al., 2017). In summary, NPs and nanodrugs have great potential in treatment of PD because of their role in the regulation of inflammation, oxidative stress, apoptosis, α-synuclein activities, and the downstream effects in motor and non-motor dysfunctions.
HD is a progressive neurodegenerative disease of autosomal dominant origin characterized by motor, cognitive, and psychiatric impairments. Genetically, the disease occurs because of the mutation in huntingtin gene indicated by the extension of polyglutamate repeats in exon-1, the event that leads to the posttranslational-mediated functional defects of its downstream protein (Langbehn et al., 2004). High rate of tryptophan metabolism, inflammation, oxidative stress, excitotoxicity, and gene dysregulation has been established as key molecular processes associated with the progression of the disease in patients and animal model (Augood et al., 1997; Shin et al., 2005; Stoy et al., 2005; Sánchez-López et al., 2012; Hsiao et al., 2013). The analyses of brain autopsy from HD patients indicate a significant reduction of Se, an essential metal with protective properties against cytotoxicity and redox imbalance (Lu et al., 2014). Alternatively, a recent study reports that Se, iron, and chromium are among the essential elements that are considerably elevated in the blood samples of HD patients compared to normal individuals (Squadrone et al., 2020). In Caenorhabditis elegans, treatment with low doses of Se NPs reverses brain condition by improving oxidative status and inhibiting the aggregation of huntingtin proteins, suggesting the potential of the compound in the treatment of HD (Cong et al., 2019). Similarly, evidence shows that TiO2 NPs have the ability to catalyze the oxidation of methionine on the N-terminal domain of the mutant huntingtin protein, thereby forming a sulfoxide and preventing the aggregation of the protein (Ceccon et al., 2019). It has also been shown that the loading of thymoquinone into the SL NPs markedly suppresses the progression of HD by increasing the activity of ATPase enzymes and reducing the production of inflammatory markers and the nuclear translocation of phosphorylated nuclear factor κB in rat model (Ramachandran and Thangarajan, 2018). Moreover, the encapsulation of peptide-based polyglutamate aggregation inhibitors into PLGA NPs can enhance their protective effects in Neuro 2A and PC12 cellular models as well as its biocompatibility in Drosophila model of HD (Joshi et al., 2019). In both neuronal cell and mouse model, poly(trehalose) NPs have also been reported to be extremely efficient in inhibiting the progression HD by suppressing the accumulation of mutant huntingtin protein (Debnath et al., 2017). The alteration of cholesterol metabolism has also been reported in the animal model of the disease (Marullo et al., 2012). Specifically, HD is linked with the alteration in the levels of 24S-hydroxycholesterol, a vital cholesterol metabolite produced by the hydroxylation reaction catalyzed by cholesterol-24 hydrolase (Leoni et al., 2013). A recent study shows that the elevation of the enzyme is crucial for the treatment of the disease as it facilitates the proteasomal and autophagy-mediated clearance of mutant huntingtin aggregates (Kacher et al., 2019). Besides, evidence shows that treatment with cholesterol-loaded glycopeptide-modified polymeric NPs can reverse behavioral and cognitive defects in HD mice (Valenza et al., 2015). In analyzing the nose to brain delivery, Passoni et al. (2020) reveal that liposomal NPs are effective in delivering cholesterol via this route in HD mouse model, confirming its potential in the treatment of HD. In brief, these above evidences confirm the neuroprotective role of NPs and their potential in the treatment of HD by targeting key mechanisms involved in the progression of the disease.
MS is a neurological disorder of the CNS and a common cause of disability in young adults. The disease affects more than 2.2 million people worldwide, and its prevalence has increased significantly in many regions (GBD 2016 Multiple Sclerosis Collaborators, 2019). Currently, there are no effective cures for the disease; however, several drugs are used to treat/reduce the symptoms of the disease especially in initial stages. The main features of the disease reported to occur in early patients include cortical demyelination and meningeal inflammation (Bø et al., 2003; Lucchinetti et al., 2011). Recent studies demonstrate that SL NPs and CS NPs can potentially increase the bioavailability and neuroprotective effects of a relapsing-MS drug dimethyl fumarate in rat model (Ojha and Kumar, 2018; Ojha et al., 2019). Another study also suggests that glucocorticoids and inorganic–organic hybrid NPs can also be used to treat MS (Montes-Cobos et al., 2017). Moreover, the encapsulation of chondroitinase ABC 1 into porous silicon NPs counteracts the neuronal damage by facilitating remyelination in MS mouse model (Rezaei et al., 2020). Together, these data suggest that NPs can be used to improve the efficiency and bioavailability of potential MS drugs.
According to the International League Against Epilepsy, epilepsy is regarded as a brain disease characterized by (i) two unprovoked or reflex seizures occurring over 24 h apart; (ii) one unprovoked/reflex seizure and at least 60% probability of further seizures to occur over the next 10 years, after two unprovoked seizures; and (iii) the diagnosis of epilepsy syndromes (Fisher et al., 2014). The disease can occur at all ages; however, it is common in children and adults. The activation of astrocytes and microglia plays a key role in the progression of the disease (Shapiro et al., 2008; Najjar et al., 2011; Bedner et al., 2015). The treatment of epilepsy has been hindered by the low bioavailability and the delivery of the drugs to the brain. Curcumin has proven to be potential in the treatment of epilepsy because of its ability to suppress cognitive deficit and glial activation and promote antioxidant and anti-inflammatory properties (Kaur et al., 2015). In a mouse model of chronic epilepsy, the incorporation of curcumin with CS-alginate STPP NPs significantly increases its corresponding effects on cell death, cognitive defects, and glial activation, consistently with the solubility of the compound (Hashemian et al., 2017). Curcumin-loaded SL NPs induce neuroprotective effect by reducing apoptosis via upregulation of erythropoietin and klotho, reduction of tumor necrosis factor-α (TNF-α), and the subsequent activation of P38 MAPK pathways (Mansoor et al., 2018; Huang et al., 2020). It has also been shown that treatment with piperine-loaded CS-STPP NPs strongly inhibits the progression of epileptic symptoms by suppressing cell death and astrocyte stimulation compared to non-loaded piperine-treated mice (Anissian et al., 2018). A previous study also reports that treatment of epileptic rat model with pluronic P85-coated PBCA NPs alleviates the effects of P-gp in phenyltoin resistance and increases its bioavailability (Fang et al., 2016). The loading of carbamazepine (an anticonvulsant drug used to treat epilepsy) into the poloxamer 188–coated PLGA NPs also improves the drug effect in isoniazid-induced epilepsy rat model compared to the administration of free drug (Zybina et al., 2018). Further evidence suggests that the treatment with quercetin-conjugated IO-β-cyclodextrin NPs can markedly enhance the therapeutic effect of quercetin in epileptic mouse model (Hashemian et al., 2019). Together, the data above suggest that the incorporation of potential epilepsy drugs into NPs improve their sensitivity and efficiency.
The success of NPs in clinical trials is evidenced by more than 250 US Food and Drug Administration–approved nanodrugs available on the market. Some of the interesting drugs include Doxil (doxorubicin HCL liposome injection), Invega Sustenna (paliperidone palmitate), DepoCyt (liposomal cytarabine), and Plegridy (PEGylated interferon β-1a) used for the treatment of multiple myeloma, schizophrenia, lymphomatous meningitis, and MS, respectively (Ventola, 2017). Liposomal formulation is the most common nanodrug available in the market so far, implicating more than 33% of drugs (D’Mello et al., 2017). In addition, numerous clinical trials have been conducted to identify the applicability of NMs in clinical settings. Previous study reports that glioblastoma patients treated with magnetic NPs and reduced radiotherapy have improved overall survival compared to conventional therapy–treated counterparts (Maier-Hauff et al., 2011). Besides, NPs also help to reduce toxicity caused by conventional drug (Eckes et al., 2011). It is worth noting that magnetic NPs also show 70% chemotherapy (temozolomide)–delivering capability and distribution in intracranial tumor region in pet dogs (Young et al., 2018). Moreover, recent studies reveal that treatment with omega-3 fatty acids and curcumin NPs significantly reduces inflammation in migraine patients by suppressing the expressions of TNF-α, intercellular adhesive molecule 1, and cooxygenase-2/inducible nitric oxide synthase (Abdolahi et al., 2017, 2019; Soveyd et al., 2018; Table 1). Together, the above evidences indicate that NPs have enormous potential in brain diseases by facilitating drug delivery, inducing synergistic effects, and reducing drug toxicity.
The incorporation of nanotechnology in medical field helps to improve the diagnosis and treatment of different diseases by increasing the sensitivity of equipment and different parameters of the drugs, thereby enhancing their efficacy. Because of the ability of NPs to cross the BBB, these compounds provide a potential option for diagnosing and treating the brain diseases and disorders, which have proven to be challenging for many years. However, to ensure the effectiveness and efficiency of these particles, further studies are needed to determine their toxicity and bioaccumulation in clinical settings. Some of these NPs cause deterioration of the brain functioning and increase oxidative stress (Table 2). Therefore, the use the NPs with therapeutic usefulness and low toxicity should be prioritized to achieve high outcome and prevent further damage to the brain. In addition, it is important to enhance their sensitivity to target specific biomarkers by improving the formulation with specific antibodies. After further exploration, the potential of nanotechnology in the treatment of brain diseases and disorders will be limitless.
EN, Y-ZW, LQ, D-DW, S-FD, and X-YJ: conceptualization. EN, Y-ZW, and LQ: data curation. X-YJ and D-DW: funding acquisition. EN, Y-ZW, LQ, YH, BA, TL, MZ, and E-SJ: writing–original draft. S-FD, J-SW, D-DW, and X-YJ: visualization and supervision. EN, Y-ZW, LQ, and D-DW: editing. All authors contributed to the article and approved the submitted version.
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81802718, 81670088, and U1504817), the Foundation of Science and Technology Department of Henan Province, China (Nos. 202102310480, 182102310335, and 192102310151), the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038), and the Science Foundation for Young Talents of Henan University College of Medicine, China (No. 2019013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
BBB, blood–brain barrier; NPs, nanoparticles; P-gp, p-glycoproteins; SL, solid lipid; CS, chitosan; PEG, polyethylene glycol; IO, iron oxide; Ag, silver; STPP, sodium tripolyphosphate; Au, gold; A β, amyloid β; MI, molecular imaging; PS-80, polysorbate 80; RE, rare-earth; UCH-L1, ubiquitin-C-terminal hydrolase-L; EGFR, epidermal growth factor receptor; CNS, central nervous system; ROS, reactive oxygen species; Se, selenium; CuO, copper oxide; ZnO, zinc oxide; CeO2, cerium oxide; BI, brain infections; TBI, traumatic brain injury; CSF, cerebral spinal fluid; IS, ischemic stroke; SC, scopolamine; ASD, autism spectrum disorder; TiO2, titanium dioxide; ALS, amyotrophic lateral sclerosis; AD, Alzheimer disease; GSK-3, glycogen synthase kinase-3; HDAC, histamine deacetylase; PD, Parkinson disease; LB, Lewy bodies; Na-K, sodium–potassium; PBCA, poly(butyl cyanoacrylate).
Abakumov, M. A., Semkina, A. S., Skorikov, A. S., Vishnevskiy, D. A., Ivanova, A. V., Mironova, E., et al. (2018). Toxicity of iron oxide nanoparticles: size and coating effects. J. Biochem. Mol. Toxicol. 32:e22225. doi: 10.1002/jbt.22225
Abbott, N. J., Patabendige, A. A., Dolman, D. E., Yusof, S. R., and Begley, D. J. (2010). Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25. doi: 10.1016/j.nbd.2009.07.030
Abdolahi, M., Jafarieh, A., Sarraf, P., Sedighiyan, M., Yousefi, A., Tafakhori, A., et al. (2019). The neuromodulatory effects of ω-3 fatty acids and nano-curcumin on the COX-2/iNOS network in migraines: a clinical trial study from gene expression to clinical symptoms. Endocr. Metab. Immune Disord. Drug Targets 19, 874–884. doi: 10.2174/1871530319666190212170140
Abdolahi, M., Tafakhori, A., Togha, M., Okhovat, A. A., Siassi, F., Eshraghian, M. R., et al. (2017). The synergistic effects of ω-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-α gene expression and serum level in migraine patients. Immunogenetics 69, 371–378. doi: 10.1007/s00251-017-0992-8
Alany, R. (2017). Solid state characterization, solid dispersions, solubility enhancement, drug dissolution and drug release. Pharm. Dev. Technol. 22:1. doi: 10.1080/10837450.2017.1275305
Aldossary, N. M., Kotb, M. A., and Kamal, A. M. (2019). Predictive value of early MRI findings on neurocognitive and psychiatric outcomes in patients with severe traumatic brain injury. J. Affect. Disord. 243, 1–7. doi: 10.1016/j.jad.2018.09.001
Algotsson, A., and Winblad, B. (2007). The integrity of the blood-brain barrier in Alzheimer’s disease. Acta Neurol. Scand. 115, 403–408. doi: 10.1111/j.1600-0404.2007.00823.x
Alzheimer’s Association (2016). Alzheimer’s disease facts and figures. Alzheimers Dement. 12, 459–509. doi: 10.1016/j.jalz.2016.03.001
Amani, H., Habibey, R., Shokri, F., Hajmiresmail, S. J., Akhavan, O., Mashaghi, A., et al. (2019). Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci. Rep. 9:6044. doi: 10.1038/s41598-019-42633-9
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Washington, DC: American Psychiatric Association Publishing.
Anissian, D., Ghasemi-Kasman, M., Khalili-Fomeshi, M., Akbari, A., Hashemian, M., Kazemi, S., et al. (2018). Piperine-loaded chitosan-STPP nanoparticles reduce neuronal loss and astrocytes activation in chemical kindling model of epilepsy. Int. J. Biol. Macromol. 107(Pt A), 973–983. doi: 10.1016/j.ijbiomac.2017.09.073
Anwar, A., Numan, A., Siddiqui, R., Khalid, M., and Khan, N. A. (2019a). Cobalt nanoparticles as novel nanotherapeutics against Acanthamoeba castellanii. Parasites Vectors 12:280. doi: 10.1186/s13071-019-3528-2
Anwar, A., Siddiqui, R., Raza Shah, M., and Khan, N. A. (2019b). Antidiabetic drugs and their nanoconjugates repurposed as novel antimicrobial agents against Acanthamoeba castellanii. J. Microbiol. Biotechnol. 29, 713–720. doi: 10.4014/jmb/1903.03009
Augood, S. J., Faull, R. L., and Emson, P. C. (1997). Dopamine D1 and D2 receptor gene expression in the striatum in Huntington’s disease. Ann. Neurol. 42, 215–221. doi: 10.1002/ana.410420213
Azmin, M. N., Stuart, J. F., and Florence, A. T. (1985). The distribution and elimination of methotrexate in mouse blood and brain after concurrent administration of polysorbate 80. Cancer Chemother. Pharmacol. 14, 238–242. doi: 10.1007/BF00258124
Baccarini, C. I., Simon, M. W., Brandon, D., Christensen, S., Jordanov, E., and Dhingra, M. S. (2020). Safety and immunogenicity of a quadrivalent meningococcal conjugate vaccine in healthy meningococcal-naïve children 2-9 years of age: a phase III, randomized study. Pediatr. Infect. Dis. J. 39, 955–960. doi: 10.1097/INF.0000000000002832
Bailey, Z. S., Nilson, E., Bates, J. A., Oyalowo, A., Hockey, K. S., Sajja, V., et al. (2020). Cerium oxide nanoparticles improve outcome after in vitro and in vivo mild traumatic brain injury. J. Neurotrauma 37, 1452–1462. doi: 10.1089/neu.2016.4644
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., Warren, Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. MMWR Surveil. Summ. 67, 1–23. doi: 10.15585/mmwr.ss6706a1
Baldissera, M. D., Souza, C. F., Grando, T. H., Moreira, K. L., Schafer, A. S., Cossetin, L. F., et al. (2017). Nerolidol-loaded nanospheres prevent behavioral impairment via ameliorating Na+, K+-ATPase and AChE activities as well as reducing oxidative stress in the brain of Trypanosoma evansi-infected mice. Naunyn Schmiedebergs Arch. Pharmacol. 390, 139–148. doi: 10.1007/s00210-016-1313-8
Ban, C., Jo, M., Park, Y. H., Kim, J. H., Han, J. Y., Lee, K. W., et al. (2020). Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 302:125328. doi: 10.1016/j.foodchem.2019.125328
Bardai, F. H., Price, V., Zaayman, M., Wang, L., and D’Mello, S. R. (2012). Histone deacetylase-1 (HDAC1) is a molecular switch between neuronal survival and death. J. Biol. Chem. 287, 35444–35453. doi: 10.1074/jbc.M112.394544
Basel, A. A., Petrov, V. E., Trofimov, S. S., Voronina, T. A., and Aliautdin, R. N. (2005). [Antiamnesic activity of nerve growth factor adsorbed on poly(butyl) cyanoacrylate nanoparticles coated with polysorbate-80]. Eksp. Klin. Farmakol. 68, 3–8.
Bauomy, A. A. (2020). Zinc oxide nanoparticles and L-carnitine effects on neuro-schistosomiasis mansoni induced in mice. Environ. Sci. Pollut. Res. Int. 27, 18699–18707. doi: 10.1007/s11356-020-08356-5
Bedner, P., Dupper, A., Hüttmann, K., Müller, J., Herde, M. K., Dublin, P., et al. (2015). Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138(Pt 5), 1208–1222. doi: 10.1093/brain/awv067
Bejerot, S., and Nordin, V. (2014). Autismspektrumsyndrom ersätter Aspergers syndrom och autism [Autism spectrum syndrome replaces Asperger syndrome and autism]. Lakartidningen 111, 1660–1663.
Bø, L., Vedeler, C. A., Nyland, H. I., Trapp, B. D., and Mørk, S. J. (2003). Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J. Neuropathol. Exp. Neurol. 62, 723–732. doi: 10.1093/jnen/62.7.723
Bocan, T. M., Stafford, R. G., Brown, J. L., Akuoku Frimpong, J., Basuli, F., Hollidge, B. S., et al. (2019). Characterization of brain inflammation, apoptosis, hypoxia, blood-brain barrier integrity and metabolism in venezuelan equine encephalitis virus (VEEV TC-83) exposed mice by in vivo positron emission tomography imaging. Viruses 11:1052. doi: 10.3390/v11111052
Borsche, M., König, I. R., Delcambre, S., Petrucci, S., Balck, A., Brüggemann, N., et al. (2020). Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain 143, 3041–3051. doi: 10.1093/brain/awaa246
Boulton, D. W., DeVane, C. L., Liston, H. L., and Markowitz, J. S. (2002). In vitro P-glycoprotein affinity for atypical and conventional antipsychotics. Life Sci. 71, 163–169. doi: 10.1016/s0024-3205(02)01680-6
Bouras, A., Kaluzova, M., and Hadjipanayis, C. G. (2015). Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J. Neurooncol. 124, 13–22. doi: 10.1007/s11060-015-1807-0
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Broadwell, R. D., Salcman, M., and Kaplan, R. S. (1982). Morphologic effect of dimethyl sulfoxide on the blood-brain barrier. Science 217, 164–166. doi: 10.1126/science.7089551
Brown, R. H., and Al-Chalabi, A. (2017). Amyotrophic lateral sclerosis. N. Engl. J. Med. 377, 162–172. doi: 10.1056/NEJMra1603471
Butt, A. M., and Jones, H. C. (1992). Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain Res. 569, 100–105. doi: 10.1016/0006-8993(92)90374-i
Butt, A. M., Jones, H. C., and Abbott, N. J. (1990). Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J. Physiol. 429, 47–62. doi: 10.1113/jphysiol.1990.sp018243
Castillo, J., Dávalos, A., and Noya, M. (1999). Aggravation of acute ischemic stroke by hyperthermia is related to an excitotoxic mechanism. Cerebrovasc. Dis. 9, 22–27. doi: 10.1159/000015891
Ceccon, A., Tugarinov, V., and Clore, G. M. (2019). TiO2 nanoparticles catalyze oxidation of huntingtin Exon 1-derived peptides impeding aggregation: a quantitative NMR study of binding and kinetics. J. Am. Chem. Soc. 141, 94–97. doi: 10.1021/jacs.8b11441
Chang, C. W., Yang, S. Y., Yang, C. C., Chang, C. W., and Wu, Y. R. (2020). Plasma and serum Alpha-Synuclein as a biomarker of diagnosis in patients with Parkinson’s disease. Front. Neurol. 10:1388. doi: 10.3389/fneur.2019.01388
Chatterjee, K., Roy, A., Banerjee, R., Choudhury, S., Mondal, B., Halder, S., et al. (2020). Inflammasome and α-synuclein in Parkinson’s disease: a cross-sectional study. J. Neuroimmunol. 338:577089. doi: 10.1016/j.jneuroim.2019.577089
Chehaibi, K., Trabelsi, I., Mahdouani, K., and Slimane, M. N. (2016). Correlation of oxidative stress parameters and inflammatory markers in ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 25, 2585–2593. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.042
Chen, E. M., Quijano, A. R., Seo, Y. E., Jackson, C., Josowitz, A. D., Noorbakhsh, S., et al. (2018). Biodegradable PEG-poly(ω-pentadecalactone-co-p-dioxanone) nanoparticles for enhanced and sustained drug delivery to treat brain tumors. Biomaterials 178, 193–203. doi: 10.1016/j.biomaterials.2018.06.024
Chen, S., Owens, G. C., Makarenkova, H., and Edelman, D. B. (2010). HDAC6 regulates mitochondrial transport in hippocampal neurons. PLoS One 5:e10848. doi: 10.1371/journal.pone.0010848
Cheng, K. K., Chan, P. S., Fan, S., Kwan, S. M., Yeung, K. L., Wáng, Y. X., et al. (2015). Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 44, 155–172. doi: 10.1016/j.biomaterials.2014.12.005
Chico, L., Ienco, E. C., Bisordi, C., Lo Gerfo, A., Petrozzi, L., Petrucci, A., et al. (2018). Amyotrophic lateral sclerosis and oxidative stress: a double-blind therapeutic trial after Curcumin supplementation. CNS Neurol. Disord. Drug Targets 17, 767–779. doi: 10.2174/1871527317666180720162029
Chow, S. C. (2014). Bioavailability and bioequivalence in drug development. Wiley Interdiscip. Rev. Comput. Stat. 6, 304–312. doi: 10.1002/wics.1310
Cong, W., Bai, R., Li, Y. F., Wang, L., and Chen, C. (2019). Selenium nanoparticles as an efficient nanomedicine for the therapy of Huntington’s disease. ACS Appl. Mater. Interf. 11, 34725–34735. doi: 10.1021/acsami.9b12319
Cooper, J. F., Spielbauer, K. K., Senchuk, M. M., Nadarajan, S., Colaiácovo, M. P., and Van Raamsdonk, J. M. (2018). α-synuclein expression from a single copy transgene increases sensitivity to stress and accelerates neuronal loss in genetic models of Parkinson’s disease. Exp. Neurol. 310, 58–69. doi: 10.1016/j.expneurol.2018.09.001
Cramer, C. K., McKee, N., Case, L. D., Chan, M. D., Cummings, T. L., Lesser, G. J., et al. (2019). Mild cognitive impairment in long-term brain tumor survivors following brain irradiation. J. Neurooncol. 141, 235–244. doi: 10.1007/s11060-018-03032-8
Crone, C., and Christensen, O. (1981). Electrical resistance of a capillary endothelium. J. Gen. Physiol. 77, 349–371. doi: 10.1085/jgp.77.4.349
da Silva Córneo, E., de Bem Silveira, G., Scussel, R., Correa, M., da Silva Abel, J., Luiz, G. P., et al. (2020). Effects of gold nanoparticles administration through behavioral and oxidative parameters in animal model of Parkinson’s disease. Coll. Surf. B Biointerf. 196:111302. doi: 10.1016/j.colsurfb.2020.111302
Da̧browska-Bouta, B., Sulkowski, G., Strużyński, W., and Strużyński, L. (2019). Prolonged exposure to silver nanoparticles results in oxidative stress in cerebral myelin. Neurotox. Res. 35, 495–504. doi: 10.1007/s12640-018-9977-0
Dallasta, L. M., Pisarov, L. A., Esplen, J. E., Werley, J. V., Moses, A. V., Nelson, J. A., et al. (1999). Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 155, 1915–1927. doi: 10.1016/S0002-9440(10)65511-3
Darabi, S., and Mohammadi, M. T. (2017). Fullerenol nanoparticles decrease ischaemia-induced brain injury and oedema through inhibition of oxidative damage and aquaporin-1 expression in ischaemic stroke. Brain Injury 31, 1142–1150. doi: 10.1080/02699052.2017.1300835
De Simone, A., La Pietra, V., Betari, N., Petragnani, N., Conte, M., Daniele, S., et al. (2019). Discovery of the first-in-class GSK-3β/HDAC dual inhibitor as disease-modifying agent to combat Alzheimer’s disease. ACS Med. Chem. Lett. 10, 469–474. doi: 10.1021/acsmedchemlett.8b00507
Debnath, K., Pradhan, N., Singh, B. K., Jana, N. R., and Jana, N. R. (2017). Poly(trehalose) nanoparticles prevent amyloid aggregation and suppress polyglutamine aggregation in a huntington’s disease model mouse. ACS Appl. Mater. Interf. 9, 24126–24139. doi: 10.1021/acsami.7b06510
DeCoteau, W., Heckman, K. L., Estevez, A. Y., Reed, K. J., Costanzo, W., Sandford, D., et al. (2016). Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine 12, 2311–2320. doi: 10.1016/j.nano.2016.06.009
Deng, G., Ma, C., Zhao, H., Zhang, S., Liu, J., Liu, F., et al. (2019). Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics 9, 6991–7002. doi: 10.7150/thno.35791
Desai, J., and Thakkar, H. (2018). Darunavir-loaded lipid nanoparticles for targeting to HIV reservoirs. AAPS PharmSciTech 19, 648–660. doi: 10.1208/s12249-017-0876-0
Dewan, M. C., Rattani, A., Gupta, S., Baticulon, R. E., Hung, Y. C., Punchak, M., et al. (2018). Estimating the global incidence of traumatic brain injury. J. Neurosurg. doi: 10.3171/2017.10.JNS17352 [Epub ahead of print],
Ding, Y., Zheng, J., Zhang, F., and Kan, J. (2016). Synthesis and characterization of retrograded starch nanoparticles through homogenization and miniemulsion cross-linking. Carbohydr. Polym. 151, 656–665. doi: 10.1016/j.carbpol.2016.06.007
Djordjevic, A., Ignjatovic, N., Seke, M., Jovic, D., Uskokovic, D., and Rakocevic, Z. (2015). Synthesis and characterization of hydroxyapatite/fullerenol nanocomposites. J. Nanosci. Nanotechnol. 15, 1538–1542. doi: 10.1166/jnn.2015.8671
Dkhil, M. A., Bauomy, A. A., Diab, M. S., Wahab, R., Delic, D., and Al-Quraishy, S. (2015). Impact of gold nanoparticles on brain of mice infected with Schistosoma mansoni. Parasitol. Res. 114, 3711–3719. doi: 10.1007/s00436-015-4600-2
D’Mello, S. R., Cruz, C. N., Chen, M. L., Kapoor, M., Lee, S. L., and Tyner, K. M. (2017). The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 12, 523–529. doi: 10.1038/nnano.2017.67
Dong, J., Liu, M., Jiang, R., Huang, H., Wan, Q., Wen, Y., et al. (2018). Synthesis and biological imaging of cross-linked fluorescent polymeric nanoparticles with aggregation-induced emission characteristics based on the combination of RAFT polymerization and the Biginelli reaction. J. Colloid Interface Sci. 528, 192–199. doi: 10.1016/j.jcis.2018.05.043
Dos Santos Tramontin, N., da Silva, S., Arruda, R., Ugioni, K. S., Canteiro, P. B., de Bem Silveira, G., et al. (2020). Gold nanoparticles treatment reverses brain damage in Alzheimer’s disease model. Mol. Neurobiol. 57, 926–936. doi: 10.1007/s12035-019-01780-w
Eckes, J., Schmah, O., Siebers, J. W., Groh, U., Zschiedrich, S., Rautenberg, B., et al. (2011). Kinetic targeting of pegylated liposomal doxorubicin: a new approach to reduce toxicity during chemotherapy (CARL-trial). BMC Cancer 11:337. doi: 10.1186/1471-2407-11-337
Eskelinen, M. H., Ngandu, T., Tuomilehto, J., Soininen, H., and Kivipelto, M. (2009). Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J. Alzheimers Dis. 16, 85–91. doi: 10.3233/JAD-2009-0920
Esparza, T. J., Zhao, H., Cirrito, J. R., Cairns, N. J., Bateman, R. J., Holtzman, D. M., et al. (2013). Amyloid-β oligomerization in Alzheimer dementia versus high-pathology controls. Ann. Neurol. 73, 104–119. doi: 10.1002/ana.23748
Fang, Z., Chen, S., Qin, J., Chen, B., Ni, G., Chen, Z., et al. (2016). Pluronic P85-coated poly(butylcyanoacrylate) nanoparticles overcome phenytoin resistance in P-glycoprotein overexpressing rats with lithium-pilocarpine-induced chronic temporal lobe epilepsy. Biomaterials 97, 110–121. doi: 10.1016/j.biomaterials.2016.04.021
Feng, Q., Shen, Y., Fu, Y., Muroski, M. E., Zhang, P., Wang, Q., et al. (2017). Self-assembly of gold nanoparticles shows microenvironment-mediated dynamic switching and enhanced brain tumor targeting. Theranostics 7, 1875–1889. doi: 10.7150/thno.18985
Fernández-Cabada, T., and Ramos-Gómez, M. (2019). A novel contrast agent based on magnetic nanoparticles for cholesterol detection as Alzheimer’s disease biomarker. Nanoscale Res. Lett. 14:36. doi: 10.1186/s11671-019-2863-8
Fisher, R. S., Acevedo, C., Arzimanoglou, A., Bogacz, A., Cross, J. H., Elger, C. E., et al. (2014). ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55, 475–482. doi: 10.1111/epi.12550
Fleminger, S., Oliver, D. L., Lovestone, S., Rabe-Hesketh, S., and Giora, A. (2003). Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial rhe evidence 10 years on; a partial replication. J. Neurol. Neurosurg. Psychiatry 74, 857–862. doi: 10.1136/jnnp.74.7.857
Forno, L. S. (1996). Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 55, 259–272. doi: 10.1097/00005072-199603000-00001
Gaba, B., Khan, T., Haider, M. F., Alam, T., Baboota, S., Parvez, S., et al. (2019). Vitamin E loaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-OHDA Parkinson’s disease model. BioMed Res. Int. 2019:2382563. doi: 10.1155/2019/2382563
Gallez, B. (2015). “Radiosensitization,” in Encyclopedia of Cancer, ed. M. Schwab (Berlin: Springer), doi: 10.1007/978-3-642-27841-9_4924-2
Gao, G., Zhang, M., Gong, D., Chen, R., Hu, X., and Sun, T. (2017). The size-effect of gold nanoparticles and nanoclusters in the inhibition of amyloid-β fibrillation. Nanoscale 9, 4107–4113. doi: 10.1039/c7nr00699c
GBD 2015 Neurological Disorders Collaborator Group (2017). Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 16, 877–897. doi: 10.1016/S1474-4422(17)30299-5
GBD 2016 Multiple Sclerosis Collaborators (2019). Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 269–285. doi: 10.1016/S1474-4422(18)30443-5
GBD 2016 Parkinson’s Disease Collaborators (2018). Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953.
Ghosh, P., and Higgins, D. E. (2018). Listeria monocytogenes infection of the brain. J. Vis. Exp. 140:58723. doi: 10.3791/58723
Gong, Y., Zhi, K., Nagesh, P., Sinha, N., Chowdhury, P., Chen, H., et al. (2020). An elvitegravir nanoformulation crosses the blood-brain barrier and suppresses HIV-1 replication in microglia. Viruses 12:564. doi: 10.3390/v12050564
Grauer, O., Jaber, M., Hess, K., Weckesser, M., Schwindt, W., Maring, S., et al. (2019). Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J. Neurooncol. 141, 83–94. doi: 10.1007/s11060-018-03005-x
Graverini, G., Piazzini, V., Landucci, E., Pantano, D., Nardiello, P., Casamenti, F., et al. (2018). Solid lipid nanoparticles for delivery of andrographolide across the blood-brain barrier: in vitro and in vivo evaluation. Coll. Surf. B Biointerf. 161, 302–313. doi: 10.1016/j.colsurfb.2017.10.062
Green, K. N., Steffan, J. S., Martinez-Coria, H., Sun, X., Schreiber, S. S., Thompson, L. M., et al. (2008). Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci. 28, 11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008
Guo, X., Deng, G., Liu, J., Zou, P., Du, F., Liu, F., et al. (2018). Thrombin-responsive, brain-targeting nanoparticles for improved stroke therapy. ACS Nano 12, 8723–8732. doi: 10.1021/acsnano.8b04787
Guo, Z., Cupples, L. A., Kurz, A., Auerbach, S. H., Volicer, L., Chui, H., et al. (2000). Head injury and the risk of AD in the MIRAGE study. Neurology 54, 1316–1323. doi: 10.1212/wnl.54.6.1316
Hanig, J. P., Morrison, J. M. Jr., and Krop, S. (1972). Ethanol enhancement of blood-brain barrier permeability to catecholamines in chicks. Eur. J. Pharmacol. 18, 79–82. doi: 10.1016/0014-2999(72)90134-3
Hansson, S. F., Andréasson, U., Wall, M., Skoog, I., Andreasen, N., Wallin, A., et al. (2009). Reduced levels of amyloid-beta-binding proteins in cerebrospinal fluid from Alzheimer’s disease patients. J. Alzheimers Dis. 16, 389–397. doi: 10.3233/JAD-2009-0966
Hashemian, M., Anissian, D., Ghasemi-Kasman, M., Akbari, A., Khalili-Fomeshi, M., Ghasemi, S., et al. (2017). Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Prog. Neuro Psychopharmacol. Biol. Psychiatry 79(Pt B), 462–471. doi: 10.1016/j.pnpbp.2017.07.025
Hashemian, M., Ghasemi-Kasman, M., Ghasemi, S., Akbari, A., Moalem-Banhangi, M., Zare, L., et al. (2019). Fabrication and evaluation of novel quercetin-conjugated Fe3O4-β-cyclodextrin nanoparticles for potential use in epilepsy disorder. Int. J. Nanomed. 14, 6481–6495. doi: 10.2147/IJN.S218317
He, H., Yao, J., Zhang, Y., Chen, Y., Wang, K., Lee, R. J., et al. (2019). Solid lipid nanoparticles as a drug delivery system to across the blood-brain barrier. Biochem. Biophys. Res. Commun. 519, 385–390. doi: 10.1016/j.bbrc.2019.09.017
Hegazy, M. A., Maklad, H. M., Samy, D. M., Abdelmonsif, D. A., El Sabaa, B. M., and Elnozahy, F. Y. (2017). Cerium oxide nanoparticles could ameliorate behavioral and neurochemical impairments in 6-hydroxydopamine induced Parkinson’s disease in rats. Neurochem. Int. 108, 361–371. doi: 10.1016/j.neuint.2017.05.011
Heidari, Z., Mohammadipour, A., Haeri, P., and Ebrahimzadeh-Bideskan, A. (2019). The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra. Iran. J. Basic Med. Sci. 22, 745–751. doi: 10.22038/ijbms.2019.33611.8018
Henson, J. W., Cordon-Cardo, C., and Posner, J. B. (1992). P-glycoprotein expression in brain tumors. J. Neurooncol. 14, 37–43. doi: 10.1007/BF00170943
Hsiao, H. Y., Chen, Y. C., Chen, H. M., Tu, P. H., and Chern, Y. (2013). A critical role of astrocyte-mediated nuclear factor-κB-dependent inflammation in Huntington’s disease. Hum. Mol. Genet. 22, 1826–1842. doi: 10.1093/hmg/ddt036
Hua, L., Wang, Z., Zhao, L., Mao, H., Wang, G., Zhang, K., et al. (2018). Hypoxia-responsive lipid-poly-(hypoxic radiosensitized polyprodrug) nanoparticles for glioma chemo- and radiotherapy. Theranostics 8, 5088–5105. doi: 10.7150/thno.26225
Huang, R., Zhu, Y., Lin, L., Song, S., Cheng, L., and Zhu, R. (2020). Solid lipid nanoparticles enhanced the neuroprotective role of curcumin against epilepsy through activation of Bcl-2 family and P38 MAPK pathways. ACS Chem. Neurosci. 11, 1985–1995. doi: 10.1021/acschemneuro.0c00242
Huang, Y., Zhao, X., Zu, Y., Wang, L., Deng, Y., Wu, M., et al. (2019). Enhanced solubility and bioavailability of apigenin via preparation of solid dispersions of mesoporous silica nanoparticles. Iran. J. Pharm. Res. 18, 168–182.
Hurst, R. D., and Clark, J. B. (1998). Alterations in transendothelial electrical resistance by vasoactive agonists and cyclic AMP in a blood-brain barrier model system. Neurochem. Res. 23, 149–154. doi: 10.1023/a:1022420606634
Hydbring, P., and Du, J. (2019). “Nanoparticle interaction with immune cells for nanoparticle-mediated (Anticancer) immunotherapy,” in Theranostic Bionanomaterials, eds W. Cui and X. Zhao (Amsterdam: Elsevier), 55–73. doi: 10.1016/B978-0-12-815341-3.00003-1
I, R. I., Mj, S., R, G., Fj, M., Mj, B., and Ma, M. F. (2020). Gold nanoparticles crossing blood-brain barrier prevent HSV-1 infection and reduce herpes associated amyloid-βsecretion. J. Clin. Med. 9:155. doi: 10.3390/jcm9010155
Jablonski, M. R., Markandaiah, S. S., Jacob, D., Meng, N. J., Li, K., Gennaro, V., et al. (2014). Inhibiting drug efflux transporters improves efficacy of ALS therapeutics. Ann. Clin. Transl. Neurol. 1, 996–1005. doi: 10.1002/acn3.141
Janzer, R. C., and Raff, M. C. (1987). Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325, 253–257. doi: 10.1038/325253a0
Jeon, S. G., Cha, M. Y., Kim, J. I., Hwang, T. W., Kim, K. A., Kim, T. H., et al. (2019). Vitamin D-binding protein-loaded PLGA nanoparticles suppress Alzheimer’s disease-related pathology in 5XFAD mice. Nanomedicine 17, 297–307. doi: 10.1016/j.nano.2019.02.004
Jeyarani, S., Vinita, N. M., Puja, P., Senthamilselvi, S., Devan, U., Velangani, A. J., et al. (2020). Biomimetic gold nanoparticles for its cytotoxicity and biocompatibility evidenced by fluorescence-based assays in cancer (MDA-MB-231) and non-cancerous (HEK-293) cells. J. Photochem. Photobiol. B Biol. 202:111715. doi: 10.1016/j.jphotobiol.2019.111715
Jeynes, B., and Provias, J. (2013). P-glycoprotein altered expression in Alzheimer’s disease: regional anatomic variability. J. Neurodegen. Dis. 2013:257953. doi: 10.1155/2013/257953
Joh, D. Y., Sun, L., Stangl, M., Al Zaki, A., Murty, S., Santoiemma, P. P., et al. (2013). Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS One 8:e62425. doi: 10.1371/journal.pone.0062425
Johnsen, K. B., Bak, M., Kempen, P. J., Melander, F., Burkhart, A., Thomsen, M. S., et al. (2018). Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics 8, 3416–3436. doi: 10.7150/thno.25228
Joshi, A. S., Singh, V., Gahane, A., and Thakur, A. K. (2019). Biodegradable nanoparticles containing mechanism based peptide inhibitors reduce polyglutamine aggregation in cell models and alleviate motor symptoms in a Drosophila model of Huntington’s disease. ACS Chem. Neurosci. 10, 1603–1614. doi: 10.1021/acschemneuro.8b00545
Kacher, R., Lamazière, A., Heck, N., Kappes, V., Mounier, C., Despres, G., et al. (2019). CYP46A1 gene therapy deciphers the role of brain cholesterol metabolism in Huntington’s disease. Brain 142, 2432–2450. doi: 10.1093/brain/awz174
Karami, Z., Saghatchi Zanjani, M. R., Rezaee, S., Rostamizadeh, K., and Hamidi, M. (2019). Neuropharmacokinetic evaluation of lactoferrin-treated indinavir-loaded nanoemulsions: remarkable brain delivery enhancement. Drug Dev. Ind. Pharm 45, 736–744. doi: 10.1080/03639045.2019.1569039
Karny, A., Zinger, A., Kajal, A., Shainsky-Roitman, J., and Schroeder, A. (2018). Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 8:7589. doi: 10.1038/s41598-018-25197-y
Katebi, S., Esmaeili, A., Ghaedi, K., and Zarrabi, A. (2019). Superparamagnetic iron oxide nanoparticles combined with NGF and quercetin promote neuronal branching morphogenesis of PC12 cells. Int. J. Nanomed. 14, 2157–2169. doi: 10.2147/IJN.S191878
Kaur, H., Patro, I., Tikoo, K., and Sandhir, R. (2015). Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 89, 40–50. doi: 10.1016/j.neuint.2015.07.009
Kaushik, A., Yndart, A., Atluri, V., Tiwari, S., Tomitaka, A., Gupta, P., et al. (2019). Magnetically guided non-invasive CRISPR-Cas9/gRNA delivery across blood-brain barrier to eradicate latent HIV-1 infection. Sci. Rep. 9:3928. doi: 10.1038/s41598-019-40222-4
Keizman, D., Rogowski, O., Berliner, S., Ish-Shalom, M., Maimon, N., Nefussy, B., et al. (2009). Low-grade systemic inflammation in patients with amyotrophic lateral sclerosis. Acta Neurol. Scand. 119, 383–389. doi: 10.1111/j.1600-0404.2008.01112.x
Kelly, P. J., Morrow, J. D., Ning, M., Koroshetz, W., Lo, E. H., Terry, E., et al. (2008). Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the biomarker evaluation for antioxidant therapies in stroke (BEAT-Stroke) study. Stroke 39, 100–104. doi: 10.1161/STROKEAHA.107.488189
Kerr, N., Lee, S. W., Perez-Barcena, J., Crespi, C., Ibañez, J., Bullock, M. R., et al. (2018). Inflammasome proteins as biomarkers of traumatic brain injury. PLoS One 13:e0210128. doi: 10.1371/journal.pone.0210128
Khalaj, R., Hajizadeh Moghaddam, A., and Zare, M. (2018). Hesperetin and it nanocrystals ameliorate social behavior deficits and oxido-inflammatory stress in rat model of autism. Int. J. Dev. Neurosci. 69, 80–87. doi: 10.1016/j.ijdevneu.2018.06.009
Khalin, I., Alyautdin, R., Wong, T. W., Gnanou, J., Kocherga, G., and Kreuter, J. (2016). Brain-derived neurotrophic factor delivered to the brain using poly (lactide-co-glycolide) nanoparticles improves neurological and cognitive outcome in mice with traumatic brain injury. Drug Deliv. 23, 3520–3528. doi: 10.1080/10717544.2016.1199609
Kozielski, K. L., Ruiz-Valls, A., Tzeng, S. Y., Guerrero-Cázares, H., Rui, Y., Li, Y., et al. (2019). Cancer-selective nanoparticles for combinatorial siRNA delivery to primary human GBM in vitro and in vivo. Biomaterials 209, 79–87. doi: 10.1016/j.biomaterials.2019.04.020
Kwon, H. J., Kim, D., Seo, K., Kim, Y. G., Han, S. I., Kang, T., et al. (2018). Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in Parkinson’s disease. Angew. Chem. 57, 9408–9412. doi: 10.1002/anie.201805052
LaBauve, A. E., Rinker, T. E., Noureddine, A., Serda, R. E., Howe, J. Y., Sherman, M. B., et al. (2018). Lipid-coated mesoporous silica nanoparticles for the delivery of the ML336 antiviral to inhibit encephalitic alphavirus infection. Sci. Rep. 8:13990. doi: 10.1038/s41598-018-32033-w
Lackner, P., Beer, R., Broessner, G., Helbok, R., Galiano, K., Pleifer, C., et al. (2008). Efficacy of silver nanoparticles-impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalus. Neurocrit. Care 8, 360–365. doi: 10.1007/s12028-008-9071-1
Lam, L., Chin, L., Halder, R. C., Sagong, B., Famenini, S., Sayre, J., et al. (2016). Epigenetic changes in T-cell and monocyte signatures and production of neurotoxic cytokines in ALS patients. FASEB J. 30, 3461–3473. doi: 10.1096/fj.201600259RR
Langbehn, D. R., Brinkman, R. R., Falush, D., Paulsen, J. S., Hayden, M. R., and International Huntington’s Disease Collaborative Group (2004). A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 65, 267–277. doi: 10.1111/j.1399-0004.2004.00241.x
Langer, K. G. (2019). Early history of amnesia. Front. Neurol. Neurosci. 44:64–74. doi: 10.1159/000494953
Lee, F. J., Liu, F., Pristupa, Z. B., and Niznik, H. B. (2001). Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 15, 916–926. doi: 10.1096/fj.00-0334com
Lee, J. H., Shin, Y., Lee, W., Whang, K., Kim, D., Lee, L. P., et al. (2016). General and programmable synthesis of hybrid liposome/metal nanoparticles. Sci. Adv. 2:e1601838. doi: 10.1126/sciadv.1601838
Leoni, V., Long, J. D., Mills, J. A., Di Donato, S., Paulsen, J. S., and Predict-Hd study group. (2013). Plasma 24S-hydroxycholesterol correlation with markers of Huntington disease progression. Neurobiol. Dis. 55, 37–43. doi: 10.1016/j.nbd.2013.03.013
Li, H. Y., Liu, F., and Wang, H. R. (2018). Correlation between Nurr1 expression and drug resistance in the brain of rats with epilepsy. Eur. Rev. Med. Pharmacol. Sci. 22, 1506–1513. doi: 10.26355/eurrev_201803_14500
Li, N., Chen, Y., Sun, H., Huang, T., Chen, T., Jiang, Y., et al. (2020). Decreasing acute toxicity and suppressing colorectal carcinoma using Sorafenib-loaded nanoparticles. Pharm. Dev. Technol. 25, 556–565. doi: 10.1080/10837450.2020.1718704
Li, Y., Hao, L., Liu, F., Yin, L., Yan, S., Zhao, H., et al. (2019a). Cell penetrating peptide-modified nanoparticles for tumor targeted imaging and synergistic effect of sonodynamic/HIFU therapy. Int. J. Nanomed. 14, 5875–5894. doi: 10.2147/IJN.S212184
Li, Y., Lim, E., Fields, T., Wu, H., Xu, Y., Wang, Y. A., et al. (2019b). Improving sensitivity and specificity of amyloid-β peptides and tau protein detection with antibiofouling magnetic nanoparticles for liquid biopsy of alzheimer’s disease. ACS Biomater. Sci. Eng. 5, 3595–3605. doi: 10.1021/acsbiomaterials.9b00086
Liao, Y. H., Chang, Y. J., Yoshiike, Y., Chang, Y. C., and Chen, Y. R. (2012). Negatively charged gold nanoparticles inhibit Alzheimer’s amyloid-β fibrillization, induce fibril dissociation, and mitigate neurotoxicity. Small 8, 3631–3639. doi: 10.1002/smll.201201068
Lin, T., Zhao, P., Jiang, Y., Tang, Y., Jin, H., Pan, Z., et al. (2016). Blood-brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano 10, 9999–10012. doi: 10.1021/acsnano.6b04268
Lindsay, M. P., Norrving, B., Sacco, R. L., Brainin, M., Hacke, W., Martins, S., et al. (2019). World Stroke Organization (WSO): global stroke fact sheet 2019. Int. J. Stroke 14, 806–817. doi: 10.1177/1747493019881353
Liu, C., Zhang, S., McClements, D. J., Wang, D., and Xu, Y. (2019). Design of Astaxanthin-loaded core-shell nanoparticles consisting of chitosan oligosaccharides and poly (lactic- co-glycolic acid): enhancement of water solubility, stability, and bioavailability. J. Agric. Food Chem. 67, 5113–5121. doi: 10.1021/acs.jafc.8b06963
Liu, H., Yang, H., Fang, Y., Li, K., Tian, L., Liu, X., et al. (2020a). Neurotoxicity and biomarkers of zinc oxide nanoparticles in main functional brain regions and dopaminergic neurons. Sci Total Environ. 705:135809. doi: 10.1016/j.scitotenv.2019.135809
Liu, H., Zhang, W., Fang, Y., Yang, H., Tian, L., Li, K., et al. (2020b). Neurotoxicity of aluminum oxide nanoparticles and their mechanistic role in dopaminergic neuron injury involving p53-related pathways. J. Hazard. Mater. 392:122312. doi: 10.1016/j.jhazmat.2020.122312
Liu, P., Jin, H., Guo, Z., Ma, J., Zhao, J., Li, D., et al. (2016). Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomed. 11, 5003–5014. doi: 10.2147/IJN.S115473
Liu, Y., Ai, K., Ji, X., Askhatova, D., Du, R., Lu, L., et al. (2017). Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 139, 856–862. doi: 10.1021/jacs.6b11013
Lu, P. J., Huang, S. C., Chen, Y. P., Chiueh, L. C., and Shih, D. Y. (2015). Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 23, 587–594. doi: 10.1016/j.jfda.2015.02.009
Lu, V. M., Crawshay-Williams, F., White, B., Elliot, A., Hill, M. A., and Townley, H. E. (2019). Cytotoxicity, dose-enhancement and radiosensitization of glioblastoma cells with rare earth nanoparticles. Artif. Cells Nanomed. Biotechnol. 47, 132–143. doi: 10.1080/21691401.2018.1544564
Lu, Z., Marks, E., Chen, J., Moline, J., Barrows, L., Raisbeck, M., et al. (2014). Altered selenium status in Huntington’s disease: neuroprotection by selenite in the N171-82Q mouse model. Neurobiol. Dis. 71, 34–42. doi: 10.1016/j.nbd.2014.06.022
Lucchinetti, C. F., Popescu, B. F., Bunyan, R. F., Moll, N. M., Roemer, S. F., Lassmann, H., et al. (2011). Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188–2197. doi: 10.1056/NEJMoa1100648
Ma, W., Zhu, D., Li, J., Chen, X., Xie, W., Jiang, X., et al. (2020). Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics 10, 1281–1295. doi: 10.7150/thno.40291
Maier-Hauff, K., Ulrich, F., Nestler, D., Niehoff, H., Wust, P., Thiesen, B., et al. (2011). Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 103, 317–324. doi: 10.1007/s11060-010-0389-0
Mangraviti, A., Tzeng, S. Y., Kozielski, K. L., Wang, Y., Jin, Y., Gullotti, D., et al. (2015). Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. ACS Nano 9, 1236–1249. doi: 10.1021/nn504905q
Manickam, V., Dhakshinamoorthy, V., and Perumal, E. (2019). Iron oxide nanoparticles affects behaviour and monoamine levels in mice. Neurochem. Res. 44, 1533–1548. doi: 10.1007/s11064-019-02774-9
Mansoor, S. R., Hashemian, M., Khalili-Fomeshi, M., Ashrafpour, M., Moghadamnia, A. A., and Ghasemi-Kasman, M. (2018). Upregulation of klotho and erythropoietin contributes to the neuroprotection induced by curcumin-loaded nanoparticles in experimental model of chronic epilepsy. Brain Res. Bull. 142, 281–288. doi: 10.1016/j.brainresbull.2018.08.010
Marcianes, P., Negro, S., García-García, L., Montejo, C., Barcia, E., and Fernández-Carballido, A. (2017). Surface-modified gatifloxacin nanoparticles with potential for treating central nervous system tuberculosis. Int. J. Nanomed. 12, 1959–1968. doi: 10.2147/IJN.S130908
Marcuzzo, S., Isaia, D., Bonanno, S., Malacarne, C., Cavalcante, P., Zacheo, A., et al. (2019). FM19G11-loaded gold nanoparticles enhance the proliferation and self-renewal of ependymal stem progenitor cells derived from ALS mice. Cells 8:279. doi: 10.3390/cells8030279
Martins, C., Araújo, F., Gomes, M. J., Fernandes, C., Nunes, R., Li, W., et al. (2019). Using microfluidic platforms to develop CNS-targeted polymeric nanoparticles for HIV therapy. Eur. J. Pharm. Biopharm. 138, 111–124. doi: 10.1016/j.ejpb.2018.01.014
Marullo, M., Valenza, M., Leoni, V., Caccia, C., Scarlatti, C., De Mario, A., et al. (2012). Pitfalls in the detection of cholesterol in Huntington’s disease models. PLoS Curr. 4:e505886e9a1968. doi: 10.1371/505886e9a1968
Massoud, T. F., and Gambhir, S. S. (2003). Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 17, 545–580. doi: 10.1101/gad.1047403
Matsuno, J., Kanamaru, T., Arai, K., Tanaka, R., Lee, J. H., Takahashi, R., et al. (2020). Synthesis and characterization of nanoemulsion-mediated core crosslinked nanoparticles, and in vivo pharmacokinetics depending on the structural characteristics. J. Controlled Release 324, 405–412. doi: 10.1016/j.jconrel.2020.05.035
McDannold, N., Arvanitis, C. D., Vykhodtseva, N., and Livingstone, M. S. (2012). Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 72, 3652–3663. doi: 10.1158/0008-5472.CAN-12-0128
Medina, D. X., Chung, E. P., Teague, C. D., Bowser, R., and Sirianni, R. W. (2020). Intravenously administered, retinoid activating nanoparticles increase lifespan and reduce neurodegeneration in the SOD1G93A mouse model of ALS. Front. Bioeng. Biotechnol. 8:224. doi: 10.3389/fbioe.2020.00224
Mendonça, M. C., Soares, E. S., de Jesus, M. B., Ceragioli, H. J., Batista, ÂG., Nyúl-Tóth, Á, et al. (2016). PEGylation of reduced graphene oxide induces toxicity in cells of the blood-brain barrier: an in vitro and in vivo study. Mol. Pharm. 13, 3913–3924. doi: 10.1021/acs.molpharmaceut.6b00696
Meng, Q., Wang, A., Hua, H., Jiang, Y., Wang, Y., Mu, H., et al. (2018). Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 13, 705–718. doi: 10.2147/IJN.S151474
Mohammadpour, R., Yazdimamaghani, M., Cheney, D. L., Jedrzkiewicz, J., and Ghandehari, H. (2019). Subchronic toxicity of silica nanoparticles as a function of size and porosity. J. Control. Release 304, 216–232. doi: 10.1016/j.jconrel.2019.04.041
Montes-Cobos, E., Ring, S., Fischer, H. J., Heck, J., Strauß, J., Schwaninger, M., et al. (2017). Targeted delivery of glucocorticoids to macrophages in a mouse model of multiple sclerosis using inorganic-organic hybrid nanoparticles. J. Control. Release 245, 157–169. doi: 10.1016/j.jconrel.2016.12.003
Moore, K. A., Pate, K. M., Soto-Ortega, D. D., Lohse, S., van der Munnik, N., Lim, M., et al. (2017). Influence of gold nanoparticle surface chemistry and diameter upon Alzheimer’s disease amyloid-β protein aggregation. J. Biol. Eng. 11:5. doi: 10.1186/s13036-017-0047-6
Morillas-Becerril, L., Peta, E., Gabrielli, L., Russo, V., Lubian, E., Nodari, L., et al. (2020). Multifunctional, CD44v6-targeted ORMOSIL nanoparticles enhance drugs toxicity in cancer cells. Nanomaterials 10:298. doi: 10.3390/nano10020298
Naczynski, D. J., Stafford, J. H., Türkcan, S., Jenkins, C., Koh, A. L., Sun, C., et al. (2018). Rare-earth-doped nanoparticles for short-wave infrared fluorescence bioimaging and molecular targeting of αVβ3-expressing tumors. Mol. Imaging 17:1536012118799131. doi: 10.1177/1536012118799131
Nagpal, K., Singh, S. K., and Mishra, D. N. (2013a). Nanoparticle mediated brain targeted delivery of gallic acid: in vivo behavioral and biochemical studies for protection against scopolamine-induced amnesia. Drug Deliv. 20, 112–119. doi: 10.3109/10717544.2013.779330
Nagpal, K., Singh, S. K., and Mishra, D. N. (2013b). Optimization of brain targeted chitosan nanoparticles of Rivastigmine for improved efficacy and safety. Int. J. Biol. Macromol. 59, 72–83. doi: 10.1016/j.ijbiomac.2013.04.024
Najjar, S., Pearlman, D., Miller, D. C., and Devinsky, O. (2011). Refractory epilepsy associated with microglial activation. Neurologist 17, 249–254. doi: 10.1097/NRL.0b013e31822aad04
Nam, E., Lee, Y. B., Moon, C., and Chang, K. A. (2020). Serum tau proteins as potential biomarkers for the assessment of Alzheimer’s disease progression. Int. J. Mol. Sci. 21:5007. doi: 10.3390/ijms21145007
Nance, E., Zhang, C., Shih, T. Y., Xu, Q., Schuster, B. S., and Hanes, J. (2014). Brain-penetrating nanoparticles improve paclitaxel efficacy in malignant glioma following local administration. ACS Nano 8, 10655–10664. doi: 10.1021/nn504210g
Narayanan, K. B., and Sakthivel, N. (2011). Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv. Colloid Interface Sci. 169, 59–79. doi: 10.1016/j.cis.2011.08.004
Niu, S., Zhang, L. K., Zhang, L., Zhuang, S., Zhan, X., Chen, W. Y., et al. (2017). Inhibition by multifunctional magnetic nanoparticles loaded with alpha-synuclein RNAi plasmid in a Parkinson’s disease model. Theranostics 7, 344–356.
Notter, T., Aengenheister, L., Weber-Stadlbauer, U., Naegeli, H., Wick, P., Meyer, U., et al. (2018). Prenatal exposure to TiO2 nanoparticles in mice causes behavioral deficits with relevance to autism spectrum disorder and beyond. Transl. Psychiatry 8:193. doi: 10.1038/s41398-018-0251-2
Ojha, S., and Kumar, B. (2018). Preparation and statistical modeling of solid lipid nanoparticles of dimethyl fumarate for better management of multiple sclerosis. Adv. Pharm. Bull. 8, 225–233. doi: 10.15171/apb.2018.027
Ojha, S., Kumar, B., and Chadha, H. (2019). Neuroprotective potential of dimethyl fumarate-loaded polymeric nanoparticles against multiple sclerosis. Indian J. Pharm. Sci. 81, 496–502. doi: 10.36468/pharmaceutical-sciences.535
Oliveira, L. M., Falomir-Lockhart, L. J., Botelho, M. G., Lin, K. H., Wales, P., Koch, J. C., et al. (2015). Elevated α-synuclein caused by SNCA gene triplication impairs neuronal differentiation and maturation in Parkinson’s patient-derived induced pluripotent stem cells. Cell Death Dis. 6:e1994. doi: 10.1038/cddis.2015.318
Ouni, S., Askri, D., Jeljeli, M., Abdelmalek, H., Sakly, M., and Amara, S. (2020). Toxicity and effects of copper oxide nanoparticles on cognitive performances in rats. Arch. Environ. Occup. Health 75, 384–394. doi: 10.1080/19338244.2019.1689376
Özerk, K., and Cardinal, D. (2020). Prevalence of Autism/ASD among preschool and school-age children in Norway. Contemp. Sch. Psychol. 24, 419–428. doi: 10.007/s40688-020-00302-z
Palle, S., and Neerati, P. (2018). Improved neuroprotective effect of resveratrol nanoparticles as evinced by abrogation of rotenone-induced behavioral deficits and oxidative and mitochondrial dysfunctions in rat model of Parkinson’s disease. Naunyn Schmiedebergs Arch. Pharmacol. 391, 445–453. doi: 10.1007/s00210-018-1474-8
Pardridge, W. M. (2012). Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 32, 1959–1972. doi: 10.1038/jcbfm.2012.126
Pardridge, W. M., and Mietus, L. J. (1979). Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J. Clin. Invest. 64, 145–154. doi: 10.1172/JCI109433
Park, S. Y., Kang, Z., Thapa, P., Jin, Y. S., Park, J. W., Lim, H. J., et al. (2019). Development of sorafenib loaded nanoparticles to improve oral bioavailability using a quality by design approach. Int. J. Pharm. 566, 229–238. doi: 10.1016/j.ijpharm.2019.05.064
Passoni, A., Favagrossa, M., Colombo, L., Bagnati, R., Gobbi, M., Diomede, L., et al. (2020). Efficacy of cholesterol nose-to-brain delivery for brain targeting in Huntington’s disease. ACS Chem. Neurosci. 11, 367–372. doi: 10.1021/acschemneuro.9b00581
Posti, J. P., Takala, R. S., Runtti, H., Newcombe, V. F., Outtrim, J., Katila, A. J., et al. (2016). The levels of glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 during the first week after a traumatic brain injury: correlations with clinical and imaging findings. Neurosurgery 79, 456–464. doi: 10.1227/NEU.0000000000001226
Qu, Z. S., Li, L., Sun, X. J., Zhao, Y. W., Zhang, J., Geng, Z., et al. (2014). Glycogen synthase kinase-3 regulates production of amyloid-β peptides and tau phosphorylation in diabetic rat brain. ScientificWorldJournal 2014:878123. doi: 10.1155/2014/878123
Rabanel, J. M., Piec, P. A., Landri, S., Patten, S. A., and Ramassamy, C. (2020). Transport of PEGylated-PLA nanoparticles across a blood brain barrier model, entry into neuronal cells and in vivo brain bioavailability. J. Control. Release 328, 679–695. doi: 10.1016/j.jconrel.2020.09.042
Rahmati-Abkenar, M., and Manteghian, M. (2020). Effect of silver nanoparticles on the solubility of methane and ethane in water. J. Nat. Gas Sci. Eng. 82:103505. doi: 10.1016/j.jngse.2020.103505
Rajendran, K., Anwar, A., Khan, N. A., and Siddiqui, R. (2017). Brain-eating amoebae: silver nanoparticle conjugation enhanced efficacy of anti-amoebic drugs against Naegleria fowleri. ACS Chem. Neurosci. 8, 2626–2630.
Ramachandran, S., and Thangarajan, S. (2018). Thymoquinone loaded solid lipid nanoparticles counteracts 3-Nitropropionic acid induced motor impairments and neuroinflammation in rat model of Huntington’s disease. Metab. Brain Dis. 33, 1459–1470. doi: 10.1007/s11011-018-0252-0
Rasouli, M. R., and Tabrizian, M. (2019). An ultra-rapid acoustic micromixer for synthesis of organic nanoparticles. Lab Chip 19, 3316–3325. doi: 10.1039/c9lc00637k
Ratliff, W. A., Saykally, J. N., Mervis, R. F., Lin, X., Cao, C., and Citron, B. A. (2019). Behavior, protein, and dendritic changes after model traumatic brain injury and treatment with nanocoffee particles. BMC Neurosci. 20:44. doi: 10.1186/s12868-019-0525-5
Reese, T. S., and Karnovsky, M. J. (1967). Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 34, 207–217. doi: 10.1083/jcb.34.1.207
Rezaei, S., Dabirmanesh, B., Zare, L., Golestani, A., Javan, M., and Khajeh, K. (2020). Enhancing myelin repair in experimental model of multiple sclerosis using immobilized chondroitinase ABC I on porous silicon nanoparticles. Int. J. Biol. Macromol. 146, 162–170. doi: 10.1016/j.ijbiomac.2019.12.258
Rubin, L. L., Hall, D. E., Porter, S., Barbu, K., Cannon, C., Horner, H. C., et al. (1991). A cell culture model of the blood-brain barrier. J. Cell Biol. 115, 1725–1735. doi: 10.1083/jcb.115.6.1725
Ruozi, B., Belletti, D., Sharma, H. S., Sharma, A., Muresanu, D. F., Mössler, H., et al. (2015). PLGA nanoparticles loaded Cerebrolysin: studies on their preparation and investigation of the effect of storage and serum stability with reference to traumatic brain injury. Mol. Neurobiol. 52, 899–912. doi: 10.1007/s12035-015-9235-x
Sadegh Malvajerd, S., Azadi, A., Izadi, Z., Kurd, M., Dara, T., Dibaei, M., et al. (2019). Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: preparation, optimization, and pharmacokinetic evaluation. ACS Chem. Neurosci. 10, 728–739. doi: 10.1021/acschemneuro.8b00510
Saleh, A., Schroeter, M., Ringelstein, A., Hartung, H. P., Siebler, M., Mödder, U., et al. (2007). Iron oxide particle-enhanced MRI suggests variability of brain inflammation at early stages after ischemic stroke. Stroke 38, 2733–2737. doi: 10.1161/STROKEAHA.107.481788
Sánchez-López, F., Tasset, I., Agüera, E., Feijóo, M., Fernández-Bolaños, R., Sánchez, F. M., et al. (2012). Oxidative stress and inflammation biomarkers in the blood of patients with Huntington’s disease. Neurol. Res. 34, 721–724. doi: 10.1179/1743132812Y.0000000073
Saraiva, C., Paiva, J., Santos, T., Ferreira, L., and Bernardino, L. (2016). MicroRNA-124 loaded nanoparticles enhance brain repair in Parkinson’s disease. J. Control. Release 235, 291–305. doi: 10.1016/j.jconrel.2016.06.005
Saralkar, P., Arsiwala, T., and Geldenhuys, W. J. (2020). Nanoparticle formulation and in vitro efficacy testing of the mitoNEET ligand NL-1 for drug delivery in a brain endothelial model of ischemic reperfusion-injury. Int. J. Pharm. 578:119090. doi: 10.1016/j.ijpharm.2020.119090
Sardoiwala, M. N., Srivastava, A. K., Kaundal, B., Karmakar, S., and Choudhury, S. R. (2020). Recuperative effect of metformin loaded polydopamine nanoformulation promoting EZH2 mediated proteasomal degradation of phospho-α-synuclein in Parkinson’s disease model. Nanomedicine 24:102088. doi: 10.1016/j.nano.2019.102088
Sarrazin, J. L., Bonneville, F., and Martin-Blondel, G. (2012). Brain infections. Diagn. Intervent. Imaging 93, 473–490. doi: 10.1016/j.diii.2012.04.020
Schocke, M. F., Seppi, K., Esterhammer, R., Kremser, C., Jaschke, W., Poewe, W., et al. (2002). Diffusion-weighted MRI differentiates the Parkinson variant of multiple system atrophy from PD. Neurology 58, 575–580. doi: 10.1212/wnl.58.4.575
Seifman, M. A., Adamides, A. A., Nguyen, P. N., Vallance, S. A., Cooper, D. J., Kossmann, T., et al. (2008). Endogenous melatonin increases in cerebrospinal fluid of patients after severe traumatic brain injury and correlates with oxidative stress and metabolic disarray. J. Cereb. Blood Flow Metab. 28, 684–696. doi: 10.1038/sj.jcbfm.9600603
Shapiro, L. A., Wang, L., and Ribak, C. E. (2008). Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia 49(Suppl. 2) 33–41. doi: 10.1111/j.1528-1167.2008.01491.x
Sharma, M., Sharma, R., Jain, D. K., and Saraf, A. (2019). Enhancement of oral bioavailability of poorly water-soluble carvedilol by chitosan nanoparticles: optimization and pharmacokinetic study. Int. J. Biol. Macromol. 135, 246–260. doi: 10.1016/j.ijbiomac.2019.05.162
Sharma, S., Ifergan, I., Kurz, J. E., Linsenmeier, R. A., Xu, D., Cooper, J. G., et al. (2020). Intravenous immunomodulatory nanoparticle treatment for traumatic brain injury. Ann. Neurol. 87, 442–455. doi: 10.1002/ana.25675
Shin, J. Y., Fang, Z. H., Yu, Z. X., Wang, C. E., Li, S. H., and Li, X. J. (2005). Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J. Cell Biol. 171, 1001–1012. doi: 10.1083/jcb.200508072
Singh, G. P., Nigam, R., Tomar, G. S., Monisha, M., Bhoi, S. K., Arulselvi, S., et al. (2018). Early and rapid detection of UCHL1 in the serum of brain-trauma patients: a novel gold nanoparticle-based method for diagnosing the severity of brain injury. Analyst 143, 3366–3373. doi: 10.1039/c8an00533h
So, P. W., Ekonomou, A., Galley, K., Brody, L., Sahuri-Arisoylu, M., Rattray, I., et al. (2019). Intraperitoneal delivery of acetate-encapsulated liposomal nanoparticles for neuroprotection of the penumbra in a rat model of ischemic stroke. Int. J. Nanomed. 14, 1979–1991. doi: 10.2147/IJN.S193965
Soares Romeiro, L. A., da Costa Nunes, J. L., de Oliveira Miranda, C., Simões Heyn Roth Cardoso, G., de Oliveira, A. S., Gandini, A., et al. (2019). Novel sustainable-by-design HDAC inhibitors for the treatment of Alzheimer’s disease. ACS Med. Chem. Lett. 10, 671–676. doi: 10.1021/acsmedchemlett.9b00071
Sonawane, S. K., Ahmad, A., and Chinnathambi, S. (2019). Protein-capped metal nanoparticles inhibit tau aggregation in Alzheimer’s disease. ACS omega. 4, 12833–12840. doi: 10.1021/acsomega.9b01411
Soveyd, N., Abdolahi, M., Djalali, M., Hatami, M., Tafakhori, A., Sarraf, P., et al. (2018). The combined effects of ω -3 fatty acids and nano-curcumin supplementation on intercellular adhesion molecule-1 (ICAM-1) gene expression and serum levels in migraine patients. CNS Neurol. Disord. Drug Targets 16, 1120–1126. doi: 10.2174/1871527317666171213154749
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M., and Goedert, M. (1998). alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473. doi: 10.1073/pnas.95.11.6469
Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., and Goedert, M. (1997). Alpha-synuclein in Lewy bodies. Nature 388, 839–840. doi: 10.1038/42166
Squadrone, S., Brizio, P., Abete, M. C., and Brusco, A. (2020). Trace elements profile in the blood of Huntington’ disease patients. J. Trace Elem. Med. Biol. 57, 18–20. doi: 10.1016/j.jtemb.2019.09.006
Sridhar, V., Gaud, R., Bajaj, A., and Wairkar, S. (2018). Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in Parkinson’s disease. Nanomedicine 14, 2609–2618. doi: 10.1016/j.nano.2018.08.004
Stoy, N., Mackay, G. M., Forrest, C. M., Christofides, J., Egerton, M., Stone, T. W., et al. (2005). Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J. Neurochem. 93, 611–623. doi: 10.1111/j.1471-4159.2005.03070.x
Sun, L., Liu, D., Fu, D., Yue, T., Scharre, D., and Zhang, L. (2021). Fluorescent peptide nanoparticles to detect amyloid-beta aggregation in cerebrospinal fluid and serum for Alzheimer’s disease diagnosis and progression monitoring. Chem. Eng. J. 405:126733. doi: 10.1016/j.cej.2020.126733
Sunena, Singh, S. K., and Mishra, D. N. (2019). Nose to brain delivery of galantamine loaded nanoparticles: in-vivo pharmacodynamic and biochemical study in mice. Curr. Drug Deliv. 16, 51–58. doi: 10.2174/1567201815666181004094707
Suwanwela, N. C., Chutinet, A., and Phanthumchinda, K. (2006). Inflammatory markers and conventional atherosclerotic risk factors in acute ischemic stroke: comparative study between vascular disease subtypes. J. Med. Assoc. Thai. 89, 2021–2027.
Tagai, K., Ono, M., Kubota, M., Kitamura, S., Takahata, K., Seki, C., et al. (2020). High-contrast in vivo imaging of tau pathologies in Alzheimer’s and non-Alzheimer’s disease tauopathies. Neuron 109, 42–58.e8. doi: 10.1016/j.neuron.2020.09.0423
Tang, S., Wang, A., Yan, X., Chu, L., Yang, X., Song, Y., et al. (2019). Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv. 26, 700–707. doi: 10.1080/10717544.2019.1636420
Tang, T., Valenzuela, A., Petit, F., Chow, S., Leung, K., Gorin, F., et al. (2018). In vivo MRI of functionalized iron oxide nanoparticles for brain inflammation. Contrast Media Mol. Imaging 2018:3476476. doi: 10.1155/2018/3476476
Tang, X., Liang, Y., Zhu, Y., Xie, C., Yao, A., Chen, L., et al. (2015). Anti-transferrin receptor-modified amphotericin B-loaded PLA-PEG nanoparticles cure Candidal meningitis and reduce drug toxicity. Int. J. Nanomed. 10, 6227–6241. doi: 10.2147/IJN.S84656
Vakilinezhad, M. A., Amini, A., Akbari Javar, H., Baha’addini Beigi Zarandi, B. F., Montaseri, H., and Dinarvand, R. (2018). Nicotinamide loaded functionalized solid lipid nanoparticles improves cognition in Alzheimer’s disease animal model by reducing Tau hyperphosphorylation. DARU J. Pharm. Sci. 26, 165–177. doi: 10.1007/s40199-018-0221-5
Valenza, M., Chen, J. Y., Di Paolo, E., Ruozi, B., Belletti, D., Ferrari Bardile, C., et al. (2015). Cholesterol-loaded nanoparticles ameliorate synaptic and cognitive function in Huntington’s disease mice. EMBO Mol. Med. 7, 1547–1564. doi: 10.15252/emmm.201505413
van Assema, D. M., Lubberink, M., Bauer, M., van der Flier, W. M., Schuit, R. C., Windhorst, A. D., et al. (2012). Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain 135(Pt 1), 181–189. doi: 10.1093/brain/awr298
van Steenoven, I., van der Flier, W. M., Scheltens, P., Teunissen, C. E., and Lemstra, A. W. (2019). Amyloid-β peptides in cerebrospinal fluid of patients with dementia with Lewy bodies. Alzheimers Res. Ther. 11:83. doi: 10.1186/s13195-019-0537-5
Vani, J. R., Mohammadi, M. T., Foroshani, M. S., and Jafari, M. (2016). Polyhydroxylated fullerene nanoparticles attenuate brain infarction and oxidative stress in rat model of ischemic stroke. EXCLI J. 15, 378–390. doi: 10.17179/excli2016-309
Ventola, C. L. (2017). Progress in nanomedicine: approved and investigational nanodrugs. P T 42, 742–755.
Vibe, C. B., Fenaroli, F., Pires, D., Wilson, S. R., Bogoeva, V., Kalluru, R., et al. (2016). Thioridazine in PLGA nanoparticles reduces toxicity and improves rifampicin therapy against mycobacterial infection in zebrafish. Nanotoxicology 10, 680–688. doi: 10.3109/17435390.2015.1107146
Virani, S. S., Alonso, A., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2020). Heart disease and stroke statistics— 2020 update: a report from the American Heart Association. Circulation 141, e139–e596. doi: 10.1161/CIR.0000000000000757
Vong, L. B., Sato, Y., Chonpathompikunlert, P., Tanasawet, S., Hutamekalin, P., and Nagasaki, Y. (2020). Self-assembled polydopamine nanoparticles improve treatment in Parkinson’s disease model mice and suppress dopamine-induced dyskinesia. Acta Biomater. 109, 220–228. doi: 10.1016/j.actbio.2020.03.021
Wang, N., Jin, X., Guo, D., Tong, G., and Zhu, X. (2017). Iron chelation nanoparticles with delayed saturation as an effective therapy for Parkinson disease. Biomacromolecules 18, 461–474. doi: 10.1021/acs.biomac.6b01547
Weissleder, R., and Mahmood, U. (2001). Molecular imaging. Radiology 219, 316–333. doi: 10.1148/radiology.219.r01ma19316
Yadav, E., Singh, D., Yadav, P., and Verma, A. (2018). Comparative evaluation of Prosopis cineraria (L.) druce and its ZnO nanoparticles on scopolamine induced Amnesia. Front. Pharmacol. 9:549. doi: 10.3389/fphar.2018.00549
Yaqub, A., Faheem, I., Anjum, K. M., Ditta, S. A., Yousaf, M. Z., Tanvir, F., et al. (2020). Neurotoxicity of ZnO nanoparticles and associated motor function deficits in mice. Appl. Nanosci. 10, 177–185. doi: 10.1007/s13204-019-01093-3
Yoo, D., Magsam, A. W., Kelly, A. M., Stayton, P. S., Kievit, F. M., and Convertine, A. J. (2017). Core-cross-linked nanoparticles reduce neuroinflammation and improve outcome in a mouse model of traumatic brain injury. ACS Nano 11, 8600–8611. doi: 10.1021/acsnano.7b03426
Young, J. S., Bernal, G., Polster, S. P., Nunez, L., Larsen, G. F., Mansour, N., et al. (2018). Convection-enhanced delivery of polymeric nanoparticles encapsulating chemotherapy in canines with spontaneous supratentorial tumors. World Neurosurg. 117, e698–e704. doi: 10.1016/j.wneu.2018.06.114
Zamproni, L. N., Mundim, M. V., Porcionatto, M. A., and des Rieux, A. (2017). Injection of SDF-1 loaded nanoparticles following traumatic brain injury stimulates neural stem cell recruitment. Int. J. Pharm. 519, 323–331. doi: 10.1016/j.ijpharm.2017.01.036
Zeng, J., Wu, J., Li, M., and Wang, P. (2018). A novel magnetic nanoparticle for early detection of amyloid plaques in Alzheimer’s disease. Arch. Med. Res. 49, 282–285. doi: 10.1016/j.arcmed.2018.09.005
Zhang, M., Viennois, E., Prasad, M., Zhang, Y., Wang, L., Zhang, Z., et al. (2016). Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 101, 321–340. doi: 10.1016/j.biomaterials.2016.06.018
Zhang, W., Duan, R., Zhang, J., Cheung, W., Gao, X., Zhang, R., et al. (2018). H1/pHGFK1 nanoparticles exert anti-tumoural and radiosensitising effects by inhibition of MET in glioblastoma. Br. J. Cancer 118, 522–533. doi: 10.1038/bjc.2017.461
Zhang, Y., Tu, B., Jiang, X., Xu, G., Liu, X., Tang, Q., et al. (2019). Exposure to carbon black nanoparticles during pregnancy persistently damages the cerebrovascular function in female mice. Toxicology 422, 44–52. doi: 10.1016/j.tox.2019.04.014
Zhao, Y., Ji, T., Wang, H., Li, S., Zhao, Y., and Nie, G. (2014). Self-assembled peptide nanoparticles as tumor microenvironment activatable probes for tumor targeting and imaging. J. Control. Release 177, 11–19. doi: 10.1016/j.jconrel.2013.12.037
Zheng, D., and Zhang, Q. F. (2019). Bioavailability enhancement of astilbin in rats through Zein-Caseinate nanoparticles. J. Agric. Food Chem. 67, 5746–5753. doi: 10.1021/acs.jafc.9b00018
Keywords: nanotechnology, brain diseases and disorders, diagnosis, treatment, nanoparticles
Citation: Ngowi EE, Wang Y-Z, Qian L, Helmy YASH, Anyomi B, Li T, Zheng M, Jiang E-S, Duan S-F, Wei J-S, Wu D-D and Ji X-Y (2021) The Application of Nanotechnology for the Diagnosis and Treatment of Brain Diseases and Disorders. Front. Bioeng. Biotechnol. 9:629832. doi: 10.3389/fbioe.2021.629832
Received: 16 November 2020; Accepted: 25 January 2021;
Published: 02 March 2021.
Edited by:
Roberto Molinaro, University of Urbino Carlo Bo, ItalyReviewed by:
Jianbo Jia, Guangzhou University, ChinaCopyright © 2021 Ngowi, Wang, Qian, Helmy, Anyomi, Li, Zheng, Jiang, Duan, Wei, Wu and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Dong Wu, ZGR3dWJpb21lZDIwMTBAMTYzLmNvbQ==; Xin-Ying Ji, MTAxOTAwOTZAdmlwLmhlbnUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.