- 1Department of Pharmacology, Xiangnan University, Chenzhou, China
- 2Affiliated Hospital of Xiangnan University, Chenzhou, China
- 3Department of Trauma and Reconstructive Surgery, Rheinisch-Westfälische Technische Hochschule Aachen University Hospital, Aachen, Germany
Schistosomiasis has been a fatal obstinate disease that threatens global human health, resulting in the granulomatous inflammation and liver fibrosis.
Objective:The aim of this study was to evaluate the therapeutic effects and mechanisms of hydroxyasiaticoside combined with praziquantel in the treatment of schistosomiasis-induced liver fibrosis.
Methods:Mice were randomly distributed into four experimental groups: normal control group, model group, praziquantel group, praziquantel + hydroxyasiaticoside group. Except for the normal control group, they were infected with Schistosomia cercariae through the abdominal skin to induce liver fibrosis. In the intervention group, mice were administered with the respective drugs by gavage after 8 weeks of infection. At the end of the treatment, mice were sacrificed to collect blood for the determination of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) serum levels. Moreover, the liver was excised, weighed, and liver indices were calculated. Histopathological examination was performed to assess liver morphology. Besides, the expression of collagen type I and III in liver was determined; the mRNA expression levels of IL-6 and TNF-α in liver tissues were measured using Real-time PCR while ELISA and western blotting were performed on liver tissue homogenate to determine the protein expression of IL-6 and TNF-α.
Results:The combination of praziquantel and hydroxyasiaticoside lowered the pathological scores of schistosomiasis-induced hepatic fibrosis, the liver indice, serum AST and ALT levels, improved liver morphology, downregulated the expression levels of hepatic type I and III collagen, inhibited the mRNA expression levels of pro-inflammatory factors (IL-6 and TNF-α) in the liver of mice relative to the praziquantel alone.
Conclusion:The combination of hydroxyasiaticoside and praziquantel is a potential therapeutic option for schistosomiasis-induced hepatic fibrosis. Notably, this combination noticeably suppresses the protein and mRNA expression levels of pro-inflammatory factors (TNF-α and IL-6) in the liver.
Introduction
In terms of public health impact, schistosomiasis is the second most important parasitic disease in the world (Tucker et al., 2013). Every year, 120 million symptomatic (Chitsulo et al., 2004), 70 million disability-adjusted (King et al., 2005) and schistosomiasis preferentially occurs in developing countries (Steinmann et al., 2006). Liver fibrosis is a wound-healing process that is aimed at restoring organ integrity after mechanical stress, autoimmune reactions and infections induced severe injury. The solicitation of this process is pathogenic and a pathognomonic feature of diseases like schistosomiasis (Campana and Iredale, 2017; Aydin and Akçali, 2018; Parola and Pinzani, 2019). Currently, there are still no efficacious therapeutic options for schistosomiasis-induced liver fibrosis.
In the past decades, traditional Chinese medicine has attracted significant attention due to its broad-spectrum anti-inflammatory and anti-fibrosis activities (Zhou et al., 2016; Yao et al., 2019). Centella asiatica is the whole dry grass of C. asiatica of umbelliferae used for clearing heat, dampness, detoxification and detumescence (Bylka et al., 2014). Hydroxyasiaticoside is a triterpenoid saponin that is isolated from C. asiatica. It has been reported that hydroxyasiaticoside has numerous significant biological effects, such as anti-inflammation, anti-oxidation, anti-depression, anti-tumor, and anti-scar proliferation (Fitri et al., 2018a,b; Song et al., 2018; He et al., 2019). However, it has not been established whether hydroxy asiaticoside can alleviate schistosomiasis-induced fibrosis.
To further demonstrate our hypothesis, in our study, we successfully established a mice model of schistosomiasis-induced liver fibrosis that were utilized to assess the therapeutic efficacies and mechanisms of a combination of hydroxyasiaticoside and praziquantel. Results have shown that the combination of hydroxyasiaticoside and praziquantel not only consistently improved the liver fibrosis extent and lowered the pathological scores but also efficaciously reversed the rising level of AST and ALT released by the damaged liver cells. Impressively, this combination also remarkably inhibited the pre-inflammatory mediators release of IL-6 and TNF-α, which provides a specific target in the screening of anti-inflammatory drugs for the treatment of schistosomiasis-induced liver fibrosis. Herein, we effectively evaluate the significant roles of a combination of hydroxyasiaticoside and praziquantel, which open up new avenues for exploring alternative therapeutic options in treating schistosomiasis-associated liver fibrosis.
Materials and Methods
Experimental Animal
Eighty male Kunming mice weighing about 18–22 g were purchased from Dongchuang Experimental Animal Science and Technology Service Department. The mice were allowed 3 days to acclimatize to the environment before experimentation. Mice were freely provided with drinking water and standard pellet feeds. Positive snails were purchased from Hunan Institute of Schistosomiasis Control. Cercariae were acquired by routine methods at 24–28°C in the laboratory.
Drugs and Materials
Centella asiatica with standard purity of 98.93% (HPLC), batch number: MUST-19032915, were purchased from Chengdu Manster Biotechnology Co., Ltd. The chromatographic conditions used were as follows: Shimadzu InertSustain C18 column (4.6 × 250 mm, 5 μm); Mobile phase: acetonitrile: methanol: water=26: 24: 50; Flow rate: 0.8 ml/min; Detection wavelength: 204 nm; Injection volume: 10 μL. Praziquantel was purchased from Nanjing Pharmaceutical Co., Ltd. Aspartate aminotransferase (AST), alanine aminotransferase (ALT) kits, and other regents were obtained from Nanjing Jiancheng Biotechnology Co., Ltd.
Experimental Method
Animal Model Preparation and Experimental Set-Up
Mice were randomly distributed into four groups: normal control group, model group, praziquantel group, and praziquantel + hydroxyasiaticoside group. To successfully establish mice models of schistosomiasis-induced fibrosis, mice were fixed to a plate, and their hair on a small skin area of the lower abdomen was shaved. Subsequently, the exposed naked skin was rinsed with normal saline twice. Twenty five cercariae were microscopically counted on the cover slides. Except for the normal control group, the cover slides were then applied to the exposed skin of mice for about 10 min. Appearance of several hyperemic spots on the skin indicated the successful cercariae infection.
Experimental Animal Administration
Mice in the normal control group were not infected with the cercariae of Schistosoma japonicum. For the experimental control group, mice infected with the cercariae were intragastrically administered with 0.5 mL of normal saline each day for consecutive 8 weeks. Mice in the praziquantel group were intragastrically administered with praziquantel at a dose of 500 mg/kg (0.5 mL) for consecutive 2 days after the cercariae infection, followed by the administration of 0.5 mL of saline. For the praziquantel + hydroxyasiaticoside group, mice after the cercariae infection were intragastrically administered with praziquantel at the equal dose for 2 days, followed by hydroxyasiaticoside administration with a dose of 0.5 mL of 40 mg/kg for consecutive 8 weeks. At the end 16th week, mice were fasted for 16 h and then weighed. Finally, mice in different groups were anesthetized, blood samples were then collected from eyeballs for further tests.
Liver Index Calculation and Histochemical Staining
The obtained blood was centrifuged at 3,000 rpms for 10 min after which serum was obtained. Serum ALT and AST levels were determined using ELISA kits. Subsequently, mice were sacrificed by cervical dislocation. Their livers were excised, and weighed to calculate the liver indices (the number of milligrams of liver per gram body weight). The middle lobe of the liver tissue was fixed in 4% paraformaldehyde and then gradient-dehydrated via various concentrations of ethanol. These samples were embedded in liquid paraffin (Zhang et al., 2019b). It was then sectioned, stained with H&E and Masson reagents, and cover slipped. Pathological changes of the liver tissue were visualized and observed under a light microscope. The degree of liver fibrosis was divided into 4 grades (Parola and Pinzani, 2019): Grade 0 (20.1 points)–normal; grade I (21.2 points)–collagen fibers wrapped around the granuloma and inserted into it; grade II (22.2 points)–the existence of intense fibrosis in the portal area and only a small amount of fibrosis between lobules; grade III (2308 points)–a large amount of fibrous tissue extending into the interlobular. Moreover, the type I and III collagen levels in the liver were also determined. Briefly, the immunohistochemically stained sections of the liver were observed under *20 visual field of objective lens. Twenty visual fields were randomly selected in each group, and the average absorbance of brown granules in cells were measured by automatic color image analysis system.
ELISA and Western Blot Analysis of Protein Levels of TNF-α and IL-6
In the ELISA experiments, the known antigen was firstly diluted to 1–10 μg/ml with coating buffer and add 0.1 ml of diluted antigen solution to each well overnight. The samples were then washed thrice on the next day. Afterwards, we added 0.1 ml of the diluted sample to each reaction well and incubated for 1 h. Subsequently, 0.1 ml of freshly diluted enzyme-labeled secondary antibody was introduced and incubated at 37°C for 30 min. At last, 0.1 ml of the temporarily prepared TMB substrate solution and 0.05 ml of 2 M sulfuric acid was added to each reaction well and reacted for 30 min. The OD value was determined by enzyme labeling at 450 nm using an ELISA tester after coloring.
For western blot experiments, the BCA protein assay kit was used to determine the protein concentration of each sample. Equal amounts of protein samples were then scraped into SDS-PAGE gel electrophoresis, followed by electro-transfer to PVDF membrane. These membranes were incubated with primary antibodies at 4°C overnight and horseradish peroxidase-conjugated secondary antibodies for 2 h. The immune complexes of each group were detected by ECL WB detection system.
Real-Time Quantitative PCR Analysis
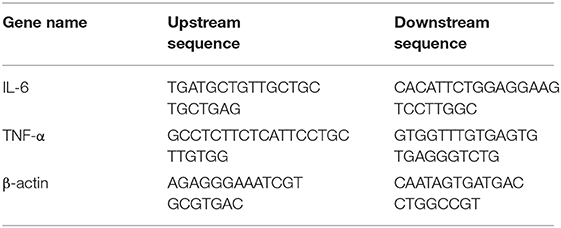
mRNA expression levels of samples in different groups were measured using Real-time PCR. Total RNA was isolated from liver tissues using a TRIzol Reagent (ThermoFisher, U.S.A). Approximately 1 ug of RNA was reverse-transcribed into cDNA, which was utilized as a template for real-time PCR detection via SYBR Premix Ex Taq reagent kit (TaKaRa, Japan). Expression levels were calculated using the 2−ΔCT method. Primers used for RT-PCR analysis are shown following Table 1.
Statistical Processing
SPSS 21.0 software was utilized to analyze the experimental data. Mean differences between groups were compared with ANOVA and Student's test. All data are expressed as mean ± standard deviation. p ≤ 0.05 was set as the threshold for statistical significance.
Results
Characterization of Centella asiatica Extract
Hydroxyasiaticoside was extracted from C. asiatica by ethanol reflux method. Then it was separated and purified. Finally, it was characterized using a high-performance liquid chromatography (HPLC). The purity of hydroxyasiaticoside was found to be 90.9%. The required concentration was prepared by dilution in normal saline. The regression equation of the standard curve of hydroxyasiaticoside: Y = 282.96X-23.171, R2 = 0.9998, and the standard curve are shown in Supplementary Figure 1.
Comparison of Liver Morphology and Liver Index
For mice in the normal control group, the liver surface appeared smooth, ruddy, soft, with sharp edges and an intact capsule. Additionally, no pathological features of liver fibrosis were observed. On the contrary, the livers of model group mice were dark-brown, enlarged, brittle, and accompanied with the tense capsule and even distribution of granular processes. Although liver appearances in the praziquantel and praziquantel + hydroxyasiaticoside groups were slightly different with compared to those of the normal control group, they were noticeably improved compared to the model group.
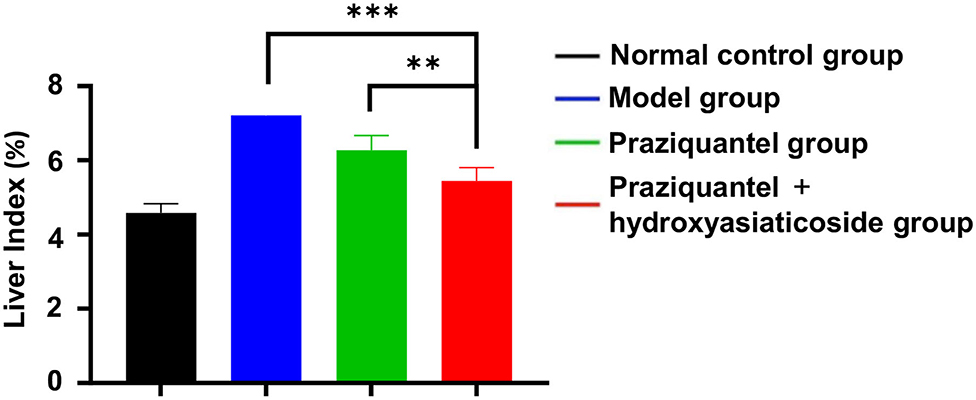
Figure 1 shows that the liver index of model group was significantly higher than that of the normal control group. Furthermore, the praziquantel group exhibited a decrease in liver index when compared to the model group. This was attributed to a certain degree of attenuation effect from praziquantel treatment. Notably, praziquantel + hydroxyasiaticoside group exhibited the lowest liver index among all treatment groups, demonstrating that the combination of praziquantel and hydroxyasiaticoside exerted a desirable curative impact on the liver index.
H and E and Masson Staining of Liver Tissues and Liver Fibrosis Scores
It is noteworthy that no significant liver pathological changes were observed in the normal control group. However, substantial chronic egg granulomas surrounded by spindle cells and collagen fibers were found in the model group, praziquantel group, and praziquantel + hydroxyasiaticoside treatment group, as shown in Figure 2. A small amount of acute egg granuloma and a large quantity of inflammatory cells were observed in the portal area, and the collagen fiber bundles around the granuloma and venules surrounded the hepatic lobules. As given in Figures 2A,B, the extent of liver tissue lesion in the praziquantel + hydroxyasiaticoside group was significantly low than that of the praziquantel group. This finding confirmed that a combination of praziquantel and hydroxyasiaticoside was more effective in ameliorating the lesion compared to praziquantel alone. The liver fibrosis scores in various treatment groups was calculated based on the grade standard of liver fibrosis. As shown in Figure 2C, the praziquantel group and praziquantel + hydroxyasiaticoside group exhibited noticeably lower hepatic fibrosis scores relative to the model group (p < 0.001). However, the difference in scores between these two treatment groups was not significant (p > 0.05).
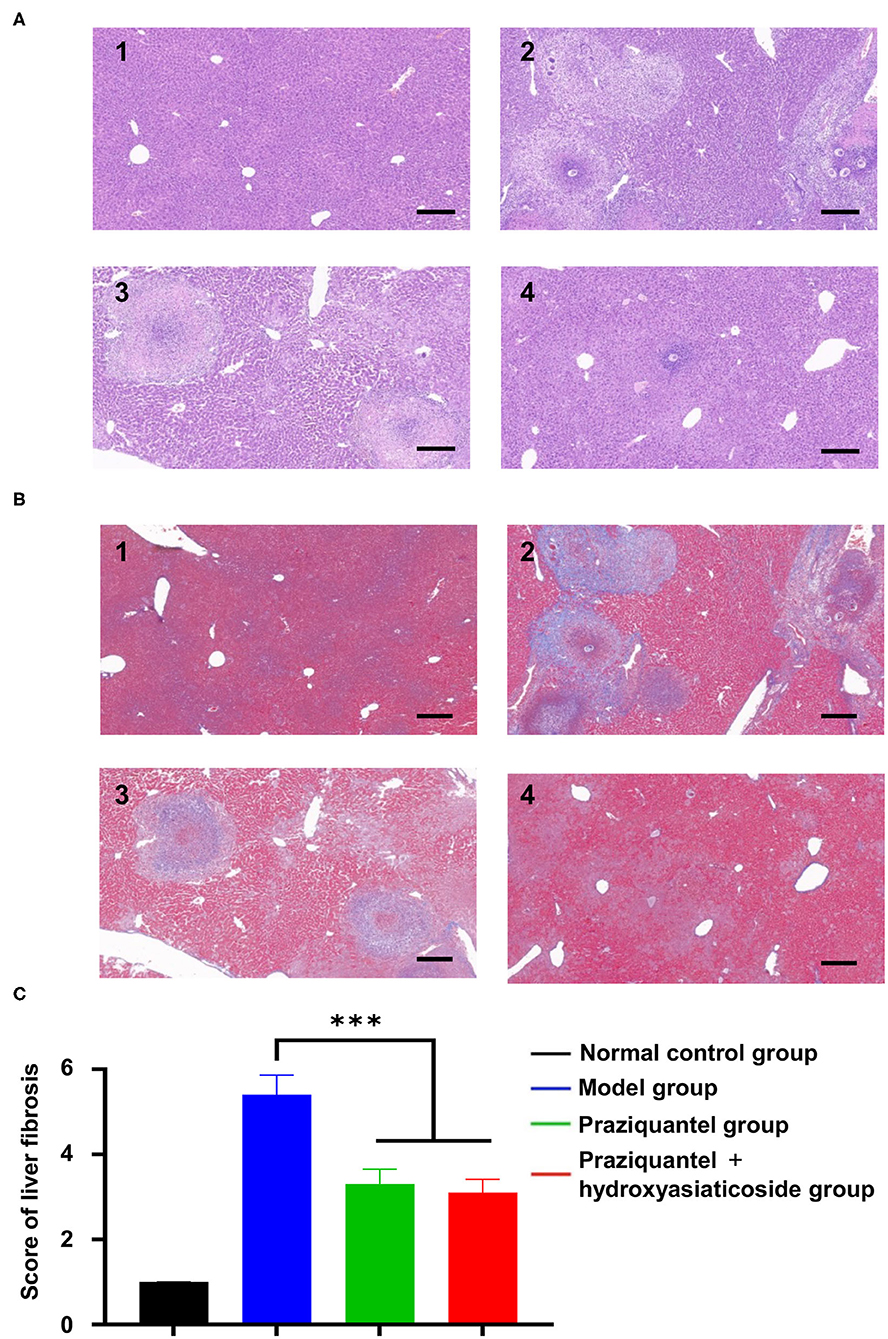
Figure 2. H&E (A) and Masson (B) staining observation of morphological changes of liver tissue sections of mice in different treatment groups. Scale bar: 200 μm. 1: normal control group; 2: model group; 3: praziquantel group; 4: praziquantel + hydroxyasiaticoside group. (C) Comparisons of the liver fibrosis scores in various groups. ***p < 0.001.
Serum of ALT and AST Levels
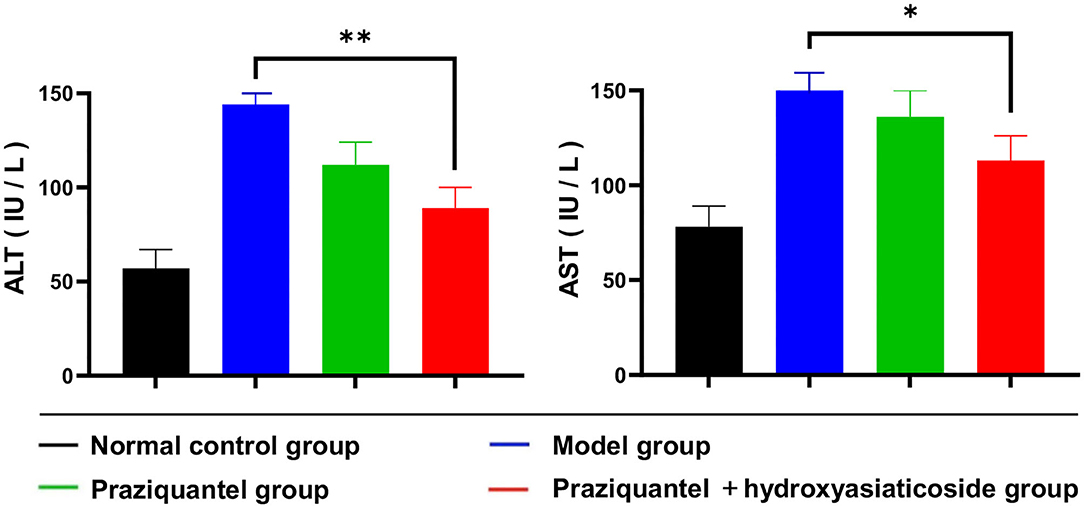
Schistosoma japonicum infection elevated serum ALT and AST levels. Figure 3 shown that serum ALT and AST levels in the praziquantel group were remarkably low than those of the model group. Nevertheless, ALT and AST levels in the praziquantel + hydroxyasiaticoside group continued to decrease and remained relatively low when compared to the praziquantel group. This validated that a combination of praziquantel + hydroxyasiaticoside is an efficacious approach for ameliorating S. japonicum-induced elevated serum ALT and AST levels. The numerical variation of serum ALT and AST levels in the different treatment groups was as shown in Supplementary Table 1.
Immunohistochemical Staining and Analysis of Type I and III Collagen Expression
Figures 4A,B displayed that hepatic type I and III collagen appeared brown, dense flaky, and were mainly distributed in egg granuloma and portal areas, especially in the model group. In the normal control group, there was no observable collagen staining. Besides, the brown hepatic type I and III collagen appeared to be improved to a certain extent after subjecting to the praziquantel or praziquantel + hydroxyasiaticoside treatments. As indicated in Figures 4C,D, the expression of type I and III collagen in the model group was noticeably higher than in the normal control group. Compared to either model group or praziquantel group, a combination of praziquantel and hydroxyasiaticoside given rise to significantly low hepatic type I and type III collagen levels in liver tissues (p < 0.001). This revealed that a combination of praziquantel and hydroxyasiaticoside had obvious superiorities in suppressing collagen levels compared to praziquantel alone. The numerical variation of hepatic type I and type III collagen levels in the various groups were shown in Supplementary Table 2.
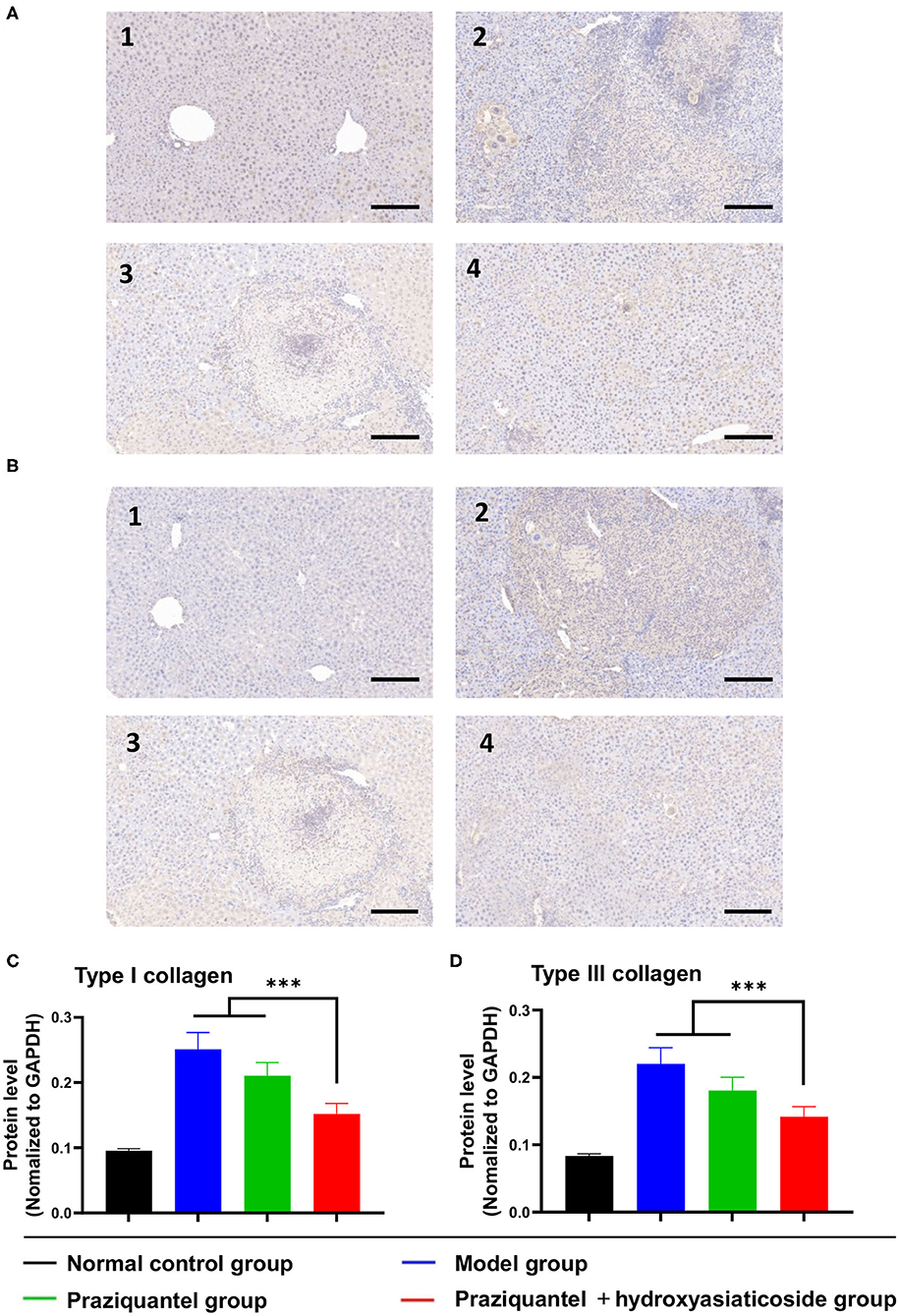
Figure 4. Immunohistochemistry analysis of the expression levels of type I (A) and III (B) collagen in liver tissues. Scale bar: 200 μm. Quantitative detection of the expression levels of type I (C) and III (D) collagen among different groups. 1: normal control group; 2: model group; 3: praziquantel group; 4: praziquantel + hydroxyasiaticoside group. ***p < 0.001.
Protein Expression Levels of TNF-α and IL-6
Cytokines, such as TNF-α and IL-6, play an essential role in the progression of the schistosomiasis. Schistosoma japonicum infection inevitably results in a noticeable elevation of the expression levels of TNF- α and IL-6 proteins in damaged liver tissues of mice. Mice in the model group exhibited high TNF-α and IL-6 protein levels owing to schistosomiasis-induced liver tissue damage (Figure 5A). Of note, the expression levels of TNF-α and IL-6 proteins in the praziquantel group was lower than those of the model group from western blotting observation. Notably, ELISA analysis shown that the praziquantel + hydroxyasiaticoside group exhibited the lowest expression levels of TNF-α and IL-6 (Figures 5B,C), revealing a favorable inhibition effect of praziquantel + hydroxyasiaticoside on the pro-inflammatory factors. They could be exploited as therapeutic drug combinations for inhibiting inflammation cytokines secretion, therefore, improving overall curative performance.
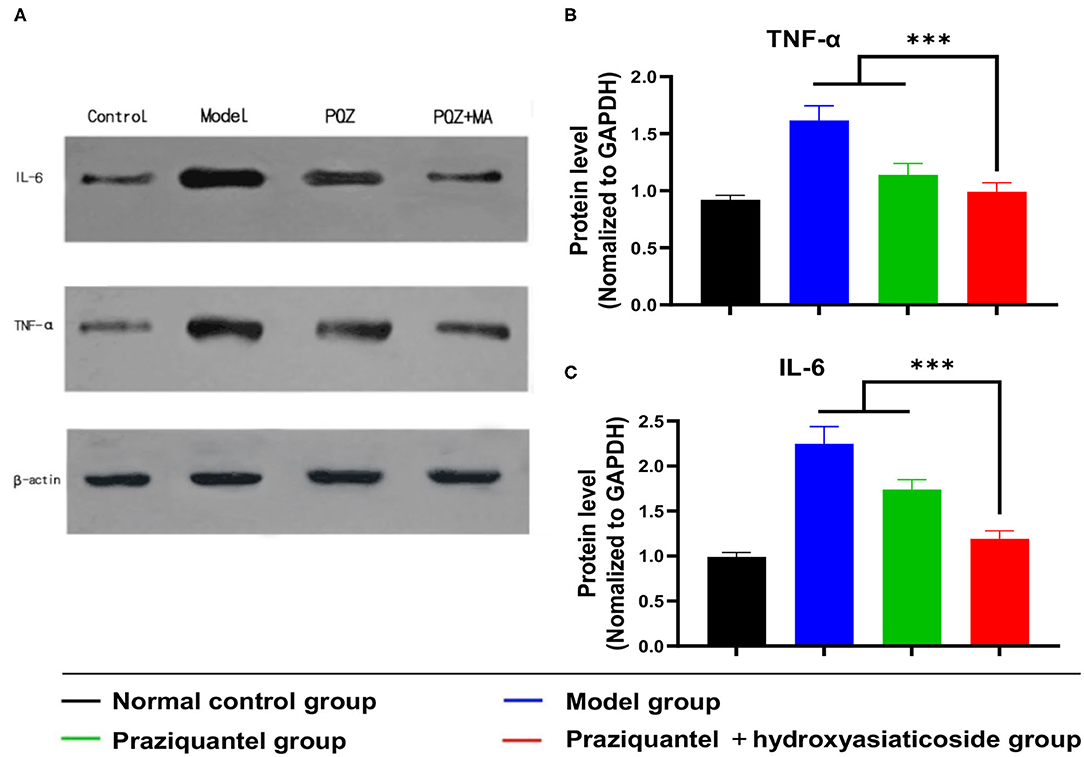
Figure 5. (A) Western blot analysis of protein levels of IL-6 and TNF-α in liver tissues. Quantitative ELISA detection analysis of protein levels of TNF-α (B) and IL-6 (C). ***p < 0.001.
mRNA Expression of TNF-α and IL-6 in Liver Tissues
Afterwards, we further investigated the mRNA expression levels of TNF-α and IL-6 in liver tissues using RT-PCR, and the obtained results are presented in Figures 6A,B. It was found that the S. japonicum infection remarkably elevated mRNA expression levels of pro-inflammatory factors (TNF-α and IL-6) in the model group. Compared to the model group, the praziquantel group exhibited a certain inhibitory effect on the mRNA expression levels of TNF-α and IL-6. Impressively, there was a lowest mRNA expression levels of TNF-α and IL-6 in praziquantel + hydroxyasiaticoside group, which was slightly higher than the normal control group. This clearly demonstrated the satisfactory inhibitory effect on the mRNA expression of pro-inflammatory factors.
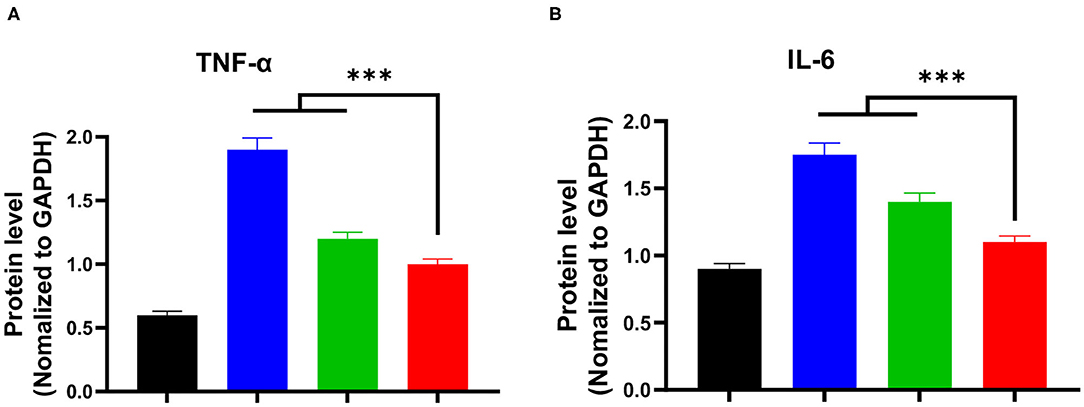
Figure 6. The mRNA expression levels of TNF-α (A) and IL-6 (B) in the liver among various groups. ***p < 0.001.
Discussion
Schistosomiasis is a fatal disease that threatens human health, and more than 150 million people are infected with schistosomiasis globally (Bylka et al., 2014; Zhou et al., 2016; Fitri et al., 2018a). Previous studies showed that liver fibrosis symptoms due to S. japonicum can persist for a long time after complete disinfestation (Fitri et al., 2018b; Song et al., 2018; He et al., 2019). Patients with advanced schistosomiasis-induced liver fibrosis gradually develop cirrhosis due to egg deposition, and often die of gastrointestinal bleeding and liver failure caused by portal hypertension (Barnett, 2018; Chuah et al., 2019; Lewis and Tucker, 2019). Numerous studies have documented that liver fibrosis can be reversed, but liver cirrhosis is totally irreversible (Kamdem et al., 2018; Cai et al., 2019; Liu et al., 2019). Therefore, it is of importance to develop strategies for preventing or treating liver fibrosis.
Hydroxyasiaticoside has anti-inflammatory, antioxidant, and anti-tumor effects (Filgueira et al., 2018; Chen et al., 2019). However, it had not been established whether hydroxyasiaticoside can improve S. japonicum induced liver fibrosis. In this study, we found that 16 weeks after infection with the cercariae of S. japonicum, mice developed morphological and pathological features of liver fibrosis, and simultaneously, there was a significant decrease of liver indices. After 8 weeks of treatment with praziquantel, the extent of liver fibrosis in mice infected with cercariae was lower relative to the model group. It was noted that the liver fibrosis extent in mice after treatment with hydroxyasiaticoside for another 8 weeks consistently improved and relieved due to the role of hydroxyasiaticoside. This indicated that hydroxyasiaticoside is effective as a therapeutic option for early liver fibrosis-induced by schistosomiasis. It is well-acknowledged that the damaged liver cells could release some enzymes and other substances into the serum. In this study, we found that S. japonicum infection remarkably elevated serum ALT and AST levels. However, the treatment with hydroxyasiaticoside efficaciously lowered and reversed the rising ALT and AST expression levels. This clearly indicated that hydroxyasiaticoside had an effective hepatoprotective effects on liver cells.
Fibrosis is a self-repairing response after tissue damage. It is mediated by inflammation and persistent liver inflammation is a prerequisite for the formation of liver fibrosis (Zhou et al., 2014; Lurie et al., 2015; Campana and Iredale, 2017; Zhao et al., 2017b; Fernández-Ruiz and Aguado, 2018; Šibíková et al., 2018; Song et al., 2019). Abnormal cytokine production during inflammation can lead to the synthesis/degradation of the extracellular matrix (ECM) of liver. Cytokines such as TNF-α, IL-6, and IL-1 β, which are significant mediators of tissue damage, play an essential role in the progression of schistosomiasis (Qi et al., 2014; Yukinori and David, 2017; Jones and Jenkins, 2018; Li et al., 2019). TNF-α is induced by a wide range of pathogenic stimuli, and exerts a specific impact on the inflammatory response (Feng et al., 2019; Zhang et al., 2019a). IL-6 regulates neutrophil transport by coordinating chemokine production and leukocyte apoptosis (Ghasemi, 2018; Giudice and Gangestad, 2018; Tseng et al., 2019; Wang et al., 2020; Yu et al., 2020). Therefore, inhibition of pro-inflammatory mediators provides a specific target and direction in the screening of anti-inflammatory drugs. These results collectively indicate that hydroxyasiaticoside noticeably inhibits the expression levels of TNF-α and IL-6, and simultaneously improves schistosomiasis-induced liver fibrosis by regulating inflammatory response factors in vivo.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by Ethics Committee of Chenzhou University.
Author Contributions
All authors wrote the manuscript and have given approval to the final version of the manuscript.
Funding
This work was supported by grants from the Scientific Research Fund of Hunan Provincial Education Department (18A458), Natural Science Foundation of Hunan Province (2016 J J6141), technological innovation projects of science and Technology Bureau of Chenzhou (zdyf201916).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2020.613784/full#supplementary-material
References
Aydin, M. M., and Akçali, K. C. (2018). Liver fibrosis. Turk. J. Gastroenterol. 29, 14–21. doi: 10.5152/tjg.2018.17330
Bylka, W., Znajdek-Awizeń, P., Studzińska-Sroka, E., Dańczak-Pazdrowska, A., and Brzezińska, M. (2014). Centella asiatica in dermatology: an overview. Phytother. Res. 28, 1117–1124. doi: 10.1002/ptr.5110
Cai, P. F., Mu, Y., Olveda, R. M., Ross, A. G., Olveda, D. U., and McManus, D. P. (2019). Circulating miRNAs as footprints for liver fibrosis grading in schistosomiasis. EBioMedicine 37, 334–343. doi: 10.1016/j.ebiom.2018.10.048
Campana, L., and Iredale, J. P. (2017). Regression of liver fibrosis. Semin. Liver Dis. 37, 1–10. doi: 10.1055/s-0036-1597816
Chen, Q. L., Zhang, J. Q., Zheng, T., Chen, H., Nie, H., Zheng, B., et al. (2019). The role of microRNAs in the pathogenesis, grading and treatment of hepatic fibrosis in schistosomiasis. Parasit. Vectors 30, 611–617. doi: 10.1186/s13071-019-3866-0
Chitsulo, L., Loverde, P., and Engels, D. (2004). Schistosomiasis. Nat. Rev. Microbiol. 12:3. doi: 10.1038/nrmicro801
Chuah, C., Gobert, G. N., Latif, B., Heo, C. C., and Leow, C. Y. (2019). Schistosomiasis in malaysia: a review. Acta Trop. 190, 137–143. doi: 10.1016/j.actatropica.2018.11.012
Feng, X. R., Xu, W. G., Li, Z. M., Song, W. T., Ding, J. X., and Chen, X. S. (2019). Immunomodulatory nanosystems. Adv. Sci. 6:1900101. doi: 10.1002/advs.201900101
Fernández-Ruiz, M., and Aguado, J. M. (2018). Risk of infection associated with anti-TNF-α therapy. Expert Rev. Anti Infect. Ther. 16, 939–956. doi: 10.1080/14787210.2018.1544490
Filgueira, N. A., Saraiva, C. M. d. A, Juc,á, N. T., Bezerra, M. F., and Lacerda, C. M. (2018). Schistosomal liver fibrosis and hepatocellular carcinoma-case series of patients submitted to liver transplantation. Braz. J. Infect. Dis. 22, 352–354. doi: 10.1016/j.bjid.2018.06.001
Fitri, A. R., Pavasant, P., Chamni, S., and Sumrejkanchanakij, P. (2018b). Determination and quantification of asiaticoside in endophytic fungus from Centella asiatica (L.) urban. World J. Microbiol. Biotechnol. 6, 111–116. doi: 10.1007/s11274-018-2493-9
Fitri, A. R., Pavasant, P., Chamni, S, and Sumrejkanchanakij, P. (2018a). Asiaticoside induces osteogenic differentiation of human periodontal ligament cells through the wnt pathway. J. Periodontol. 89, 596–605. doi: 10.1002/JPER.17-0471
Ghasemi, H. (2018). Roles of IL-6 in ocular inflammation: a review. Ocul. Immunol. Inflamm. 26, 37–50. doi: 10.1080/09273948.2016.1277247
Giudice, M. D., and Gangestad, S. W. (2018). Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 70, 61–75. doi: 10.1016/j.bbi.2018.02.013
He, L. L., Hong, G. J., Zhou, L., Zhang, J. G., Fang, J., He, W., et al. (2019). Asiaticoside, a component of Centella asiatica attenuates RANKL-induced osteoclastogenesis via NFATc1 and NF-κB signaling pathways. J. Cell Physiol. 234, 4267–4276. doi: 10.1002/jcp.27195
Jones, S. A., and Jenkins, B. J. (2018). Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 18, 773–789. doi: 10.1038/s41577-018-0066-7
Kamdem, S. D., Moyou-Somo, R., Brombacher, F., and Nono, J. K. (2018). Host regulators of liver fibrosis during human schistosomiasis. Front. Immunol. 28:2781. doi: 10.3389/fimmu.2018.02781
King, C. H., Dickman, K., and Tisch, D. J. (2005). Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 365, 1561–1569. doi: 10.1016/S0140-6736(05)66457-4
Lewis, F. A., and Tucker, M. S. (2019). Schistosomiasis. Adv. Exp. Med. Bio. 1154, 45–70. doi: 10.1007/978-1-4939-0915-5_3
Li, Y. C., Wang, H. H., Zhang, R., Zhang, G. J., Yang, Y., and Liu, Z. G. (2019). Antitumor activity of asiaticoside against multiple myeloma drug-resistant cancer cells is mediated by autophagy induction, activation of effector caspases, and inhibition of cell migration, invasion, and STAT-3 signaling pathway. Med. Sci. Monit. 20, 1355–1361. doi: 10.12659/MSM.913397
Liu, J. C., Zhou, B. Q., Chen, D. P., Zhou, C., Shi, Q., Zheng, C. S., et al. (2019). Transjugular intrahepatic portosystemic shunt placement in patients with schistosomiasis-induced liver fibrosis. Cardiovasc. Intervent. Radiol. 42, 1760–1770. doi: 10.1007/s00270-019-02295-6
Lurie, Y., Webb, M., Cytter-Kuint, R., Shteingart, S., and Lederkremer, G. Z. (2015). Non-invasive diagnosis of liver fibrosis and cirrhosis. World J. Gastroenterol. 7, 11567–11583. doi: 10.3748/wjg.v21.i41.11567
Parola, M., and Pinzani, M. (2019). Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med. 65, 37–55. doi: 10.1016/j.mam.2018.09.002
Qi, F. Y., Yang, L., Tian, Z., Zhao, M. G., Liu, S. B., and An, J. Z. (2014). Neuroprotective effects of asiaticoside. Neural Regen Res. 1, 1275–1282. doi: 10.4103/1673-5374.137574
Šibíková, M., Živný, J., and Janota, J. (2018). Cell membrane-derived microvesicles in systemic inflammatory response. Folia Biol. 64, 113–124.
Song, D. Q., Jiang, X., Liu, Y. L., Sun, Y. H., Cao, S. S., and Zhang, Z. (2018). Asiaticoside attenuates cell growth inhibition and apoptosis induced by Aβ 1-42 via inhibiting the TLR4/NF-κB signaling pathway in human brain microvascular endothelial cells. Front. Pharmacol. 30, 28–35. doi: 10.3389/fphar.2018.00028
Song, L. G., Zhang, B. B., Liu, J. H., Wang, M., Ma, X. H., Wang, L. F., et al. (2019). Reversal of liver fibrosis after splenectomy in a patient with advanced schistosomiasis japonica: a case report with 4-year follow-up. PLoS Negl. Trop. Dis. 11:e0007174. doi: 10.1371/journal.pntd.0007174
Steinmann, P., Keiser, J., Bos, R., Tanner, M., and Utzinger, J. (2006). Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6, 411–425. doi: 10.1016/S1473-3099(06)70521-7
Tseng, Y. J., Dong, L., Liu, Y. F., Xu, N., Ma, W., Weng, S. Q., et al. (2019). Role of autophagy in chronic liver inflammation and fibrosis. Curr. Protein Pept. Sci. 20, 817–822. doi: 10.2174/1389203720666190305165203
Tucker, M. S., Karunaratne, L. B., Lewis, F. A., Freitas, T. C., and Liang, Y. S. (2013). Schistosomiasis. Curr. Protoc. Immunol. 103, 19.1.1–19.1.58. doi: 10.1002/0471142735.im1901s103
Wang, J., Li, Z., Wang, Z., Yu, Y., Li, D., Li, B., Ding, J., et al. (2020). Nanomaterials for combinational radio–immuno oncotherapy. Adv. Funct. Mater. 30:1910676. doi: 10.13702/j.1000-0607.180204
Yao, L., Li, J., Li, L. L., Li, X. X., Zhang, R., Zhang, Y. J., et al. (2019). Coreopsis tinctoria Nutt ameliorates high glucose-induced renal fibrosis and inflammation via the TGF-β1/SMADS/AMPK/NF-κB pathways. BMC Complement Altern. Med. 10, 14–20. doi: 10.1186/s12906-018-2410-7
Yu, L., He, P., Xu, Y. C., Kou, X. Y., Yu, Z. Q., Xie, X. B., et al. (2020). Manipulations of DNA four-way junction architecture and DNA modified Fe3O4@Au nanomaterials for the detection of miRNA. Sensors Actuators B Chem. 313:15. doi: 10.1016/j.snb.2020.128015
Yukinori, K., and David, A. B. (2017). Liver inflammation and fibrosis. J. Clin. Inves. 3, 55–64. doi: 10.1172/JCI88881
Zhang, X., Xi, Z. Q., Machuki, J. O., Luo, J. J., Yang, D. Z., Li, J. J., et al. (2019b). Gold cube-in-cube based oxygen nanogenerator: a theranostic nanoplatform for modulating tumor microenvironment for precise chemo-phototherapy and multimodal imaging. ACS Nano 13, 5306–5325. doi: 10.1021/acsnano.8b09786
Zhang, X. F., Wu, X. W., Hu, Q. Y., Wu, J., Wang, G. F., Hong, Z. W., et al. (2019a). Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 1:116464. doi: 10.1016/j.lfs.2019.05.020
Zhao, Y. P., Wang, W. H., Wu, X. H., Ma, X. Q., Qu, R. Z., Chen, X. M., et al. (2017). Mangiferin antagonizes TNF-α-mediated inflammatory reaction and protects against dermatitis in a mice model. Int. Immunopharmacol. 45, 174–179. doi: 10.1016/j.intimp.2017.02.014
Zhou, W. C., Zhang, Q. B., and Qiao, L. (2014). Pathogenesis of liver cirrhosis. World J. Gastroenterol. 21, 7312–7324. doi: 10.3748/wjg.v20.i23.7312
Keywords: praziquantel, madecassoside, Schistosoma japonicum, liver fibrosis, pro-inflammatory factors
Citation: Fang H, Yu L, You D, Peng N, Guo W, Wang J and Zhang X (2021) In vivo Therapeutic Effects and Mechanisms of Hydroxyasiaticoside Combined With Praziquantel in the Treatment of Schistosomiasis Induced Hepatic Fibrosis. Front. Bioeng. Biotechnol. 8:613784. doi: 10.3389/fbioe.2020.613784
Received: 03 October 2020; Accepted: 10 December 2020;
Published: 22 January 2021.
Edited by:
Jianxun Ding, Chinese Academy of Sciences, ChinaReviewed by:
Yao Wang, Renmin Hospital of Wuhan University, ChinaXiaohui Li, Central University of South Bihar, India
Huizhou Fan, Rutgers University, The State University of New Jersey, United States
Copyright © 2021 Fang, Yu, You, Peng, Guo, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Wang, NDc1OTExNDAzQHFxLmNvbQ==; Xing Zhang, emhhbmd4aW5nMDUyOWRxd2NAb3V0bG9vay5jb20=
 Huilong Fang1
Huilong Fang1 Xing Zhang
Xing Zhang